Abstract
Invasive fungal diseases are a public health problem. They affect a constantly increasing number of at-risk patients, and their incidence has risen in recent years. These opportunistic infections are mainly due to Candida sp. but less common or rare yeast infections should not be underestimated. These so-called “less common” yeasts include Ascomycota of the genera Candida (excluding the five major Candida species), Magnusiomyces/Saprochaete, Malassezia, and Saccharomyces, and Basidiomycota of the genera Cryptococcus (excluding the Cryptococcus neoformans/gattii complex members), Rhodotorula, and Trichosporon. The aim of this review is to (i) inventory the less common yeasts isolated in humans, (ii) provide details regarding the specific anatomical locations where they have been detected and the clinical characteristics of the resulting infections, and (iii) provide an update on yeast taxonomy. Of the total of 239,890 fungal taxa and their associated synonyms sourced from the MycoBank and NCBI Taxonomy databases, we successfully identified 192 yeasts, including 127 Ascomycota and 65 Basidiomycota. This repertoire allows us to highlight rare yeasts and their tropism for certain anatomical sites and will provide an additional tool for diagnostic management.
Keywords: rare yeasts, uncommon yeasts, repertoire
1. Introduction
Yeasts are unicellular eukaryotic organisms classified as members of the Kingdom Fungi. To date, more than 2000 species have been described, estimated to represent less than 1% of yeast species present on the Earth [1,2,3,4]. This number continues to grow with the widespread use of molecular taxonomy methods [5].
Yeast infections are among the top three healthcare-associated bloodstream infections in the United States, and they are in fourth place among all healthcare-associated infections, with Candida sp. leading the way [6]. With an attributable mortality rate of almost 40% in the case of systemic involvement, they represent a major public health issue [7]. Five species are responsible for ~90% of human fungal infections, namely, Candida albicans, C. tropicalis, C. glabrata, C. parapsilosis, and Pichia kudriavzevii (syn. C. krusei) [8,9]. Also, populations at risk of developing invasive fungal diseases are increasing, as are immunocompromised and severely ill patients [10], and the fungal repertoire is expanding to include less common or rare yeasts involved in human pathology [9,11]. The less common major pathogens include Ascomycota of the genera Candida (excluding the five major Candida species), Magnusiomyces/Saprochaete, Malassezia, and Saccharomyces, and Basidiomycota of the genera Cryptococcus (excluding the Cryptococcus neoformans/gattii complex members), Rhodotorula, and Trichosporon. Increasing attention is being paid to several emerging yeasts. One example is Candida auris, the involvement of which in human pathology has been increasingly reported since its first isolation in 2009, and the number of clinical cases rose from 329 in 2018 to 1012 in 2021 [12]. Others, such as Pseudozyma sp., although described inconsistently in the literature, may be responsible for systemic infections and interest in them should not be lost [13]. However, knowledge of the infections caused by these so-called “less common” or “rare” yeasts remains incomplete [10].
In this review, we offer an overview of less common or rare yeasts identified in humans as of 16 June 2020. Only publications reporting identification by culture and nucleotide analysis, whether or not they are associated with histopathology, were retained. We also provide information on the organs where these yeasts were isolated and on the semiology of the infections. As in our previous publication, our repertoire of the non-dermatophyte moulds [14] is divided into two. First, we describe the taxa of interest and their preferential site of infection, and then we describe which yeast species were involved at each major anatomical site.
2. Materials and Methods
2.1. Systematic Literature Review and Database Creation
We adopted the same approach to materials and methods as Menu et al. [14]. Briefly, all fungi names and synonyms were collected from both MycoBank (https://www.mycobank.org/ accessed on 15 November 2019) and NCBI Taxonomy (https://www.ncbi.nlm.nih.gov/taxonomy accessed on 15 November 2019). After aggregation and deduplication of these two listings, we obtained a single a list of 239,890 fungi taxa and any corresponding synonyms. For each fungus name in the list, a Python script version 3.7 and Biopython package version 1.74 [11] were used to query PubMed on 15 November 2019 to find bibliographical references that mentioned the fungus name or its synonyms, associated with the term “human” in the article title (TI), abstract (AB), author-supplied keywords (Other Term (OT)), or the Medical Subject Heading (MeSH) terms. The syntax of the queries was dynamically built using this pattern (fungi_name_or_synonyms [TIAB] OR fungi_name_or_synonyms [OT] OR fungi_name_or_synonyms [MeSH]) AND (“Human”[TIAB] OR “Human”[OT] OR “Humans”[MeSH]). A total of 7428 fungi taxa were found with at least one PubMed reference and incorporated in an MS AccessTM database (Access 2013, Microsoft, Redmond, WA, USA) created on 16 June 2020.
2.2. Manual Database Incrementation
As we previously described [14], the title and/or abstract and/or whole paper of each PubMed reference was manually analysed to ensure that it had been isolated from humans. As this process was laborious, a time limit of 16 June 2020 was set to ensure the same PubMed content for each fungal species. No start date limit was set.
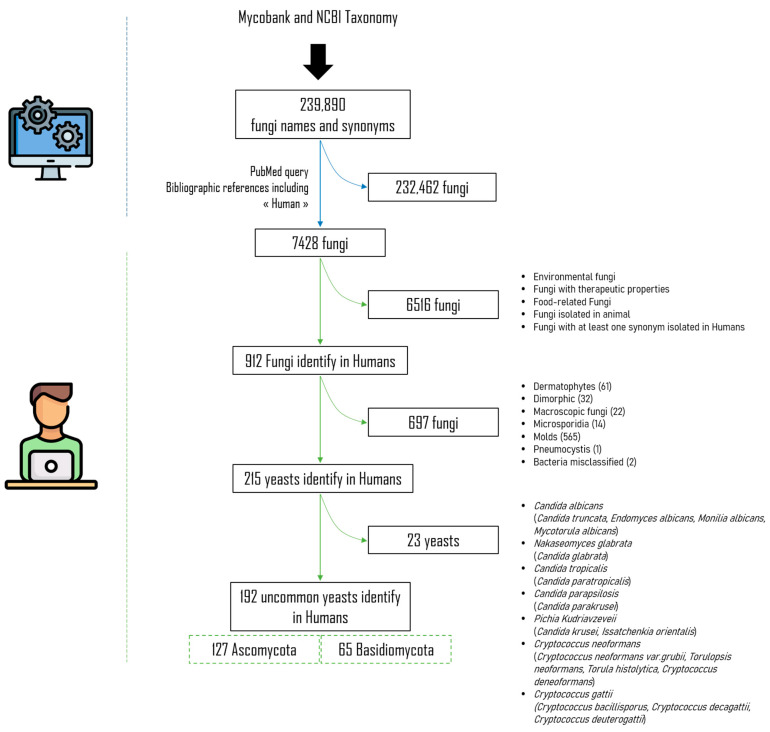
After analysis, 6516 fungal taxa that were ultimately not found in humans were excluded. Filamentous fungi (moulds and dermatophytes), microsporidia, dimorphic fungi, and Pneumocystis isolated in humans were also excluded. We also excluded the most common yeasts and focused on the less common species. Among the Ascomycota, we therefore chose to exclude the five Candida species that are most commonly implicated in human diseases, namely, Candida albicans, C. tropicalis, C. glabrata, C. parapsilosis, and Pichia kudriavzevii (syn. C. krusei) [8,9]. Similarly, among Basidiomycota, we chose to exclude species of the Cryptococcus neoformans and Cryptococcus gattii complexes (Figure 1).
Figure 1.
Systematic literature review flowchart.
We analysed the titles and/or abstracts and/or full paper and/or Supplementary Materials, when available, of 192 uncommon yeast fungal names and synonyms isolated in humans to complete the information on the anatomical site involved and the associated infection semiology by filling in the PubMed Unique Identifier (PMID) of the publication concerned. Only identifications by direct diagnosis were taken into account, including culture (followed by morphological, Matrix-Assisted Laser Desorption Ionization Time of Flight (MALDI-TOF) mass spectrometry, and DNA sequence-based identification), whether or not this was associated with histopathological findings and Polymerase Chain Reaction (PCR). Publications reporting a species-level diagnosis based solely on histopathological examination or indirect methods were excluded. The date of first publication, last publication, and the name used were also reported in the software. The anatomical sites included have previously been detailed by Menu et al. [14].
2.3. Data Analysis
The MS Access® database (Access 2013, Microsoft) was converted into two Excel files (Excel 2013, Microsoft). In the first file, the number of PMIDs per taxa was calculated by the anatomical site where the fungi were isolated. In the second, the number of PMIDs per fungus was given for the two main divisions (i.e., Ascomycota yeasts, Basidiomycota yeasts) and the subdivisions chosen by the authors correspond to the main yeast genera, namely, Candida, the Magnusiomyces/Saprochaete clade, Malassezia, and Saccharomyces, for the Ascomycota, and Cryptococcus, Rhodotorula, and Trichosporon, for the Basidiomycota, according to the infections associated with the isolation of the fungus, as reported by the authors. If more than one case was described in a publication with the same anatomical site, it counted for one publication due to a unique PMID.
2.4. Taxonomy
The taxonomy of yeasts has significantly evolved since the “one fungus, one name” nomenclature was adopted. We chose to divide the yeasts into Ascomycota and Basidiomycota. Within these major divisions, the subdivisions chosen by the authors correspond to the main genera, namely, Candida, the Magnusiomyces/Saprochaete clade, Malassezia, and Saccharomyces for the Ascomycota, and Cryptococcus, Rhodotorula, and Trichosporon for the Basidiomycota. We then used data from the literature to indicate the name of the species currently recommended, referred to as the “current name” [15,16]. We specified “Under classification” when the taxonomy had not yet been settled, as mentioned by Sugita et al. [16].
2.5. Synonyms
We referred to the “current name” in MycoBank and data in the literature to identify synonyms. The current name/synonym association was then checked by querying the PubMed database.
2.6. Figures
All figures were produced using the online tool WordArt version 4.17 (https://wordart.com/ accessed on 15 November 2019). The size of the name of each species was proportional to the number of times it occurred in the database. Ascomycota are represented in cold colours (Candida spp. in blue, Saccharomyces spp. in purple, the Magnusiomyces/Saprochaete clade in green, and others in grey-blue) and Basidiomycota in warm colours (Cryptococcus spp. In purple, Rhodotorula spp. In red, Trichosporon spp. In orange, and Malassezia spp. in pink).
3. Results
3.1. Fungal Location by Focusing on the Predominant Genus
In total, 2496 articles/PMIDs were included. This bibliographical research was divided in two groups respecting the two main divisions: Ascomycota and Basidiomycota. We identified 127 fungal species of 39 genera belonging to Ascomycota and 65 fungal species of 18 genera belonging to Basidiomycota, which had been reported at least once in humans.
3.1.1. Ascomycota
The list of the Ascomycota taxa is detailed in Table S1 with their former and current scientific name, if applicable. Briefly, there were 80 Candida spp., 10 of the Magnusiomyces/Saprochaete clade, 5 Saccharomyces spp., and 33 other yeasts species distributed in 24 genera. Each of these taxa results are presented below. Candida was the leading genus; 1954 publications reported the isolation of these Ascomycota at every anatomical site (a publication could be counted multiple times due to the possible report of multiple anatomical sites in the same publication). This was followed by the Saccharomyces genus, followed by the Magnusiomyces/Saprochaete clade. For all Ascomycota, systemic localisation was the most common.
Candida spp.
Despite having excluded from our analysis the five Candida species responsible for over 90% of human infections, namely, Candida albicans, C. tropicalis, C. glabrata, C. parapsilosis, and Pichia kudriavzevii (syn. C. krusei), the other Candida species were the most frequently isolated yeasts in human clinical samples [8,9]. Two species were strongly represented: Candida dubliniensis and Candida auris. In terms of the time lapse between first description and last publication, Candida auris eclipses Candida dubliniensis (11 years and 25 years, respectively). These species emerged since their first description in 1995 in oral candidiasis in an HIV patient and from the external ear in 2009 in a patient treated at a Japanese hospital [17,18], respectively. The number of publications for Candida dubliniensis in human pathologies is probably underestimated because it had been commonly identified as Candida albicans before 1995 [19]. This is followed by Candida lusitaniae and Candida guilliermondii (syn. Meyerozyma guilliermondii). Although Candida is one of the most commonly found species in the normal human microbiota, it can become an opportunistic pathogen in the presence of risk factors [20,21]. The spectrum of candidiasis is broad, ranging from cutaneous to systemic candidiasis [22]. In this repertoire, Candida were mainly isolated from the systemic level with a total of 669 publications. The next anatomical sites were the oto-rhino-laryngology (ORL) system (279 publications, of which 130 concerned C. dubliniensis), the urinary tract, and the skin system, probably linked to the mucosal commensal nature of Candida species. In less well-represented anatomical sites, such as the skeletal system, we find a majority of Candida auris species, followed by Candida lusitaniae, Candida guilliermondii, and Candida dubliniensis. The same four species were also found in the central nervous system. Interestingly, Candida auris was mainly reported from post-neurosurgery ventriculitis [23,24,25] while Candida dubliniensis was reported from meningitis secondary to haematogenous dissemination [26,27,28,29], suggesting a potential species-dependent invasiveness not to be overlooked. Finally, Kodamaea ohmeri (formerly Pichia ohmeri) stands out with a total of 56 publications. This yeast, used in the food industry for fermentation, is commonly isolated from the environment [30] and was isolated in the majority of cases at the systemic level (31 publications).
-
2.
Magnusiomyces/Saprochaete clade
The four species involved in human pathology forming the Magnusiomyces/Saprochaete clade are Magnusiomyces clavatus, Geotrichum candidum, and Magnusiomyces capitatus. The taxonomy has been revised many times [31]. These arthroconidia yeasts present in the environment are often responsible for opportunistic breakthrough infections in severely immunocompromised populations [32]. Systemic involvement stands out (95 publications), which is consistent with the literature, as bloodstream infections with or without pulmonary infections or skin lesions are the most frequent in at-risk patients [32,33]. The lung, gut, skin, ORL system, and urinary system also stand out, which may correspond to the ability of these yeasts to colonise these human anatomical sites [10].
-
3.
Saccharomyces spp.
Comprising only three species found in human pathology, Saccharomyces spp. represented a total of 321 publications. Saccharomyces cerevisiae, commonly known as brewer’s or baker’s yeast due to its wide use in the food industry, is the leading cause of bloodstream infections (91 publications) [34]. These infections often occur secondarily to the use of Saccharomyces boulardii orally administered probiotics in immunocompromised patients [35]. They are infrequently commensal of mucosal surfaces such as the gastro-intestinal tract, which explains its isolation within it [36]. This repertoire highlights the isolation of S. cerevisiae from the genitalia and ORL system.
-
4.
Others
Among the ascomycetous yeasts that were not classified within the previous three genera, Lodderomyces elongisporus stands out, with a total of ten publications. This rare yeast has mostly been isolated from bloodstream infections. Cardiac involvement has also been reported in the form of native valve endocarditis in an intravenous drug user, emphasising its potential invasiveness [37]. Pichia farinosa and Sporopachydermia cereana were found in systemic locations. Torulopsis magnoliae was isolated from the eye and was involved in endophthalmitis following cataract surgery [38].
3.1.2. Basidiomycota
After excluding the Cryptococcus neoformans species complex and Cryptococcus gattii species complex, the list of the Basidiomycota taxa is presented in Table S2 with their former and current scientific names, if applicable. Briefly, there were 16 Cryptococcus spp., 13 Malassezia spp., 7 Rhodotorula spp., 19 Trichosporon spp., and 11 other species distributed in six genera. Each of these taxa results is presented below. Trichosporon was the leading genus, with 809 reports of the isolation of these Basidiomycota at almost all anatomical sites. In second place were the Malassezia yeasts, followed by the Rhodotorula and uncommon Cryptococcus species. For all Basidiomycota, cutaneous involvement is the most common (525 publications), with Malassezia accounting for 60% of the publications. Systemic localisation ranks second, with 398 publications in total; but obviously for species belonging to the genera Cryptococcus, Rhodotorula, and Trichosporon, the skin remains the most common location.
Cryptococcus spp.
Cryptococcus species are basidiomycetous encapsulate yeasts, ubiquitous in the environment [39]. In this repertoire, we chose not to analyse species belonging to the Cryptococcus neoformans complex and Cryptococcus gattii complex in order to focus on uncommon Cryptococcus species. We found 14 species isolated in humans, with a predominance of Papiliotrema laurentii (formerly Cryptococcus laurentii) and Naganishia albida (formerly Cryptococcus albidus). Compared to Cryptococcus neoformans, we found a tropism of these both species for the bloodstream (19 and 13 publications, respectively), the lungs (7 publications each), the cutaneous system (9 and 12 publications, respectively), and the central nervous system (8 and 3 publications, respectively), also reported in the literature [40]. Two species were isolated from the osteo-articular system: Cryptococcus albidus without detail of its location [41] and Cryptococcus luteolus from a case of tenosynovitis [42]. Interestingly, ocular involvement was reported for Cryptococcus albidus, Cryptococcus laurentii, and Cryptococcus curvatus, and this was mostly of the keratitis type. No cardiac, hepatic, skeletal, endocrinal, placental, or dental involvement was reported.
-
2.
Malassezia spp.
Malassezia spp. are known to be major human skin commensals with pathogenicity limited to the skin [43]. This is reflected in our repertoire, with cutaneous localisation predominating with 316 publications of the 525 in total (60%). These Basidiomycota also inhabit the mucosal sites of humans, as shown by the ORL system, the digestive tract, and the genital localisation (13 publications each). However, among the Malassezia genus, three species have been isolated from bloodstream infections: Malassezia furfur, Malassezia pachydermatis, and Malassezia sympodialis, highlighting its potential invasiveness. Malassezia infections are probably underestimated due to their dependence on lipids, which makes its isolation by culture complicated in the absence of specific media [44]. Interestingly, 13 publications reported the isolation of Malassezia from the eye, with a majority with periocular involvement, namely, blepharitis and dacryocystitis, and a rare case of Malassezia restricta keratitis [45].
-
3.
Rhodotorula spp.
Rhodotorula spp. are widespread in the environment and are commonly part of the intestinal mycobiome [46]. In this repertoire, we found five species isolated in humans. Rhodotorula mucilaginosa (formerly Rhodotorula rubra) was the predominant species with a total of 105 publications, followed by Rhodotorula glutinis with a total of 23 publications and Cystobasidium minutum (formerly Rhodotorula minuta) with a total of 10 publications. These three species were mainly responsible for fungaemia in immunocompromised patients and were therefore isolated at the systemic level [47]. Interestingly, Rhodotorula mucilaginosa was isolated in the skin system (17 publications), responsible for superficial cutaneous involvement. It was the only Rhodotorula species responsible for onychomycosis [48,49,50,51,52], demonstrating a capability to degrade keratin, as demonstrated in the literature [52]. One publication reported liver involvement secondary to pulmonary infection, pointing to the pathogenicity of this species [53]. Interestingly, Rhodotorula toruloides was isolated once from the bloodstream, and the unique case of Rhodotorula pilimanae in humans was a native infective endocarditis.
-
4.
Trichosporon spp.
Trichosporon is widely represented in our repertoire. We found 19 species comprising two largely predominant species, Cutaneotrichosporon cutaneum (formerly Trichosporon beigelii, Trichosporon cutaneum, and Trichosporon cutaneum) and Trichosporon asahii. These species are found in an equivalent way in almost all locations except for the teeth, gums, and placenta. The two main localisations are systemic (90 and 86 publications, respectively) and cutaneous (55 publications for each), reflecting the vast pathogenic power of Trichosporon, from superficial skin damage known as white piedra to blood-borne dissemination occurring mostly in immunocompromised patients [47]. Our attention was drawn to the cardiac tropism of Cutaneotrichosporon cutaneum (26 publications), resulting in various types of infections, such as endocarditis mostly on prosthetic valves [54], and pericardium or myocardium involvement in disseminated cases [55,56]. Three other species appear to share this particular cardiac tropism, Trichosporon asahii, Trichosporon inkin, and Trichosporon mycotoxinivorans, which should not be ignored.
-
5.
Others
Among the Basidiomycota not classified in these four genera, Pseudozyma aphidis stands out, with a total of seven publications. This opportunistic pathogen has been described as an environmental yeast and is found at the systemic level, pulmonary level, and cutaneous level. As it has also not yet been isolated in the human digestive tract, gastro-intestinal translocation is presented as a probable source of infection [57]. Another rare fungus, Sporobolomyces salmonicolor, stands out for its isolation being limited solely to deep sites with systemic involvement, endophthalmitis-like ocular involvement, and two meningitis-like central nervous system involvements [58,59,60,61,62]. Likewise, the only reported isolation of Sporobolomyces roseus was from the cerebrospinal fluid of a patient with meningitis [63].
3.2. Fungal Location by Focusing on Anatomical Site
Within the 19 anatomical sites, the semiology of infection was detailed for the five major categories of fungi involved in human pathologies (Table 1).
Table 1.
Anatomical sites and nosological framework of the different taxa. CNS: central nervous system; ORL: oto-rhino-laryngology; the numbers in the table refer to PMIDs.
| Ascomycota | Basidiomycota | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida spp.1 | Magnusiomyces/Saprochaete clade | Others | Saccharomyces spp. | Total | Cryptococcus spp.2 | Malassezia spp. | Others | Rhodotorula spp. | Trichosporon spp. | Total | ||
| Systemic | 682 | 87 | 23 | 95 | 887 | 37 | 70 | 9 | 64 | 242 | 422 | 1309 |
| Anatomical site | ||||||||||||
| Unspecified | 11 | 2 | 1 | 14 | 3 | 4 | 7 | 21 | ||||
| Blood | 664 | 79 | 19 | 90 | 852 | 36 | 70 | 8 | 59 | 220 | 393 | 1245 |
| Bone marrow | 3 | 2 | 1 | 6 | 1 | 7 | 8 | 14 | ||||
| Lymph nodes | 4 | 2 | 3 | 2 | 11 | 1 | 1 | 1 | 7 | 10 | 21 | |
| Semiology | ||||||||||||
| Aortitis | 1 | 2 | 3 | 3 | ||||||||
| Vasculitis | 1 | 1 | 4 | 4 | 5 | |||||||
| CNS | 35 | 4 | 2 | 41 | 19 | 3 | 3 | 12 | 29 | 66 | 107 | |
| Anatomical site | ||||||||||||
| Unspecified | 15 | 1 | 1 | 17 | 2 | 1 | 2 | 8 | 13 | 30 | ||
| Specimen | ||||||||||||
| Brain abscess | 4 | 2 | 6 | 4 | 4 | 10 | ||||||
| Semiology | ||||||||||||
| Encephalitis | 1 | 1 | 1 | |||||||||
| Mass | 1 | 1 | 1 | 1 | 2 | |||||||
| Meningitis | 14 | 14 | 13 | 1 | 3 | 9 | 14 | 40 | 54 | |||
| Meningo-encephalitis | 2 | 2 | 4 | 1 | 1 | 2 | 8 | 10 | ||||
| Ocular | 54 | 8 | 1 | 2 | 65 | 9 | 14 | 1 | 6 | 9 | 39 | 104 |
| Anatomical site | ||||||||||||
| Unspecified | 10 | 1 | 11 | 2 | 4 | 1 | 2 | 9 | 20 | |||
| Conjunctival | 3 | 1 | 4 | 2 | 2 | 6 | ||||||
| Orbital | 4 | 4 | 4 | |||||||||
| Specimen | ||||||||||||
| Lacrimal fluid | 5 | 2 | 7 | 1 | 1 | 8 | ||||||
| Semiology | ||||||||||||
| Blepharitis | 6 | 6 | 6 | |||||||||
| Endophthalmitis | 16 | 1 | 1 | 18 | 1 | 1 | 3 | 5 | 10 | 28 | ||
| Keratitis | 16 | 3 | 2 | 21 | 6 | 1 | 2 | 2 | 11 | 32 | ||
| Auditory system | 35 | 1 | 36 | 1 | 11 | 8 | 20 | 56 | ||||
| Anatomical site | 0 | |||||||||||
| Unspecified | 23 | 23 | 10 | 5 | 15 | 38 | ||||||
| Semiology | ||||||||||||
| Otomycoses | 12 | 1 | 13 | 1 | 1 | 3 | 5 | 18 | ||||
| Dental and gums | 16 | 4 | 4 | 23 | 24 | |||||||
| Anatomical site | ||||||||||||
| Unspecified | 15 | 2 | 2 | 19 | 19 | |||||||
| Specimen | ||||||||||||
| Abscess | 1 | 1 | 2 | 2 | ||||||||
| Semiology | ||||||||||||
| Periodontitis | 1 | 2 | 3 | 3 | ||||||||
| ORL system | 302 | 21 | 3 | 50 | 376 | 7 | 15 | 7 | 51 | 80 | 456 | |
| Anatomical site | ||||||||||||
| Unspecified | 6 | 1 | 7 | 1 | 1 | 8 | ||||||
| Nasal | 14 | 1 | 2 | 17 | 2 | 2 | 2 | 6 | 23 | |||
| Oesophagus | 9 | 3 | 1 | 3 | 16 | 1 | 12 | 13 | 29 | |||
| Oral mucosa | 224 | 11 | 2 | 34 | 271 | 2 | 3 | 6 | 14 | 25 | 296 | |
| Pharyngeal | 12 | 1 | 13 | 1 | 1 | 1 | 3 | 16 | ||||
| Rhino-sinusitis | 3 | 3 | 1 | 3 | 2 | 6 | 9 | |||||
| Tongue | 7 | 1 | 2 | 10 | 1 | 2 | 3 | 13 | ||||
| Tracheal | 22 | 5 | 7 | 34 | 1 | 5 | 17 | 23 | 57 | |||
| Tonsil | 5 | 5 | 5 | |||||||||
| Lung | 215 | 66 | 13 | 37 | 331 | 23 | 8 | 5 | 5 | 143 | 184 | 515 |
| Anatomical site | ||||||||||||
| Unspecified | 6 | 7 | 1 | 14 | 3 | 3 | 4 | 10 | 24 | |||
| Lower respiratory tract | 68 | 23 | 4 | 17 | 112 | 10 | 5 | 1 | 58 | 74 | 186 | |
| Lymph nodes | 1 | 5 | 1 | 7 | 4 | 4 | 11 | |||||
| Mediastinum | 1 | 2 | 1 | 4 | 2 | 2 | 6 | |||||
| Parenchymal cavity | 2 | 2 | 2 | 2 | 4 | |||||||
| Pleura | 15 | 2 | 1 | 4 | 22 | 2 | 2 | 15 | 19 | 41 | ||
| Upper respiratory tract | 108 | 15 | 3 | 11 | 137 | 4 | 1 | 1 | 1 | 38 | 45 | 182 |
| Specimen | ||||||||||||
| Abscess | 1 | 1 | 2 | 2 | ||||||||
| Semiology | ||||||||||||
| Interstitial pneumonitis | 1 | 1 | 1 | |||||||||
| Invasive | 2 | 1 | 2 | 5 | 3 | 3 | 8 | |||||
| Pneumonia | 12 | 12 | 3 | 27 | 3 | 2 | 1 | 1 | 15 | 22 | 49 | |
| Breast | 1 | 2 | 3 | 2 | 1 | 3 | 6 | |||||
| Anatomical site | ||||||||||||
| Breast implant | 1 | 1 | 1 | |||||||||
| Nipples | 1 | 1 | 1 | |||||||||
| Specimen | ||||||||||||
| Milk | 1 | 2 | 3 | 1 | 1 | 4 | ||||||
| Heart | 31 | 4 | 1 | 6 | 42 | 2 | 4 | 40 | 46 | 88 | ||
| Anatomical site | ||||||||||||
| Unspecified | 2 | 1 | 1 | 4 | 1 | 17 | 18 | 22 | ||||
| Myocardium | 1 | 1 | 5 | 5 | 6 | |||||||
| Pericardium | 4 | 1 | 5 | 4 | 4 | 9 | ||||||
| Semiology | ||||||||||||
| Implanted device endocarditis | 7 | 2 | 9 | 2 | 11 | 13 | 22 | |||||
| Native valve endocarditis | 18 | 2 | 1 | 2 | 23 | 2 | 1 | 3 | 6 | 29 | ||
| Enteric | 155 | 35 | 7 | 60 | 257 | 8 | 15 | 12 | 89 | 124 | 381 | |
| Anatomical site | ||||||||||||
| Unspecified | 73 | 23 | 4 | 40 | 140 | 3 | 6 | 1 | 28 | 38 | 178 | |
| Appendix | 1 | 1 | 1 | |||||||||
| Biliary tract | 12 | 2 | 3 | 17 | 2 | 2 | 19 | |||||
| Colitis | 2 | 2 | 1 | 1 | 3 | |||||||
| Enteritis | 1 | 1 | 1 | |||||||||
| Gastric | 6 | 1 | 2 | 9 | 1 | 5 | 6 | 15 | ||||
| Pancreas | 1 | 1 | 2 | 1 | 4 | 5 | 7 | |||||
| Spleen | 6 | 5 | 2 | 13 | 2 | 24 | 26 | 39 | ||||
| Specimen | ||||||||||||
| Abscess | 25 | 2 | 27 | 27 | ||||||||
| Peritoneal fluid | 5 | 5 | 1 | 1 | 6 | |||||||
| Semiology | ||||||||||||
| Cholecystitis | 2 | 2 | 2 | |||||||||
| Peritonitis | 25 | 4 | 3 | 8 | 40 | 5 | 10 | 19 | 34 | 74 | ||
| Liver | 17 | 8 | 7 | 32 | 1 | 1 | 1 | 37 | 40 | 72 | ||
| Anatomical site | ||||||||||||
| Unspecified | 6 | 5 | 2 | 13 | 1 | 27 | 28 | 41 | ||||
| Specimen | ||||||||||||
| Abscess | 3 | 1 | 3 | 7 | 1 | 1 | 2 | 4 | 11 | |||
| Ascites fluid | 7 | 1 | 2 | 10 | 7 | 7 | 17 | |||||
| Semiology | ||||||||||||
| Hepatitis | 1 | 1 | 2 | 1 | 1 | 3 | ||||||
| Urinary tract | 199 | 20 | 1 | 24 | 244 | 3 | 9 | 1 | 108 | 121 | 365 | |
| Anatomical site | ||||||||||||
| Unspecified | 5 | 5 | 1 | 1 | 6 | |||||||
| Bladder | 1 | 1 | 1 | 1 | 2 | |||||||
| Kidney | 11 | 3 | 3 | 17 | 1 | 23 | 24 | 41 | ||||
| Prostate gland | 1 | 1 | 1 | |||||||||
| Specimen | ||||||||||||
| Urine | 162 | 14 | 17 | 193 | 3 | 8 | 1 | 58 | 70 | 263 | ||
| Semiology | 0 | |||||||||||
| Mass | 5 | 1 | 6 | 6 | ||||||||
| Pyelonephritis | 2 | 1 | 1 | 4 | 1 | 1 | 5 | |||||
| Urinary tract infection | 12 | 2 | 1 | 2 | 17 | 24 | 24 | 41 | ||||
| Genital | 110 | 3 | 55 | 168 | 3 | 13 | 4 | 11 | 31 | 199 | ||
| Anatomical site | ||||||||||||
| Unspecified | 8 | 2 | 10 | 1 | 1 | 4 | 2 | 8 | 18 | |||
| Endometrium | 1 | 1 | 1 | |||||||||
| Epididymis | 1 | 1 | 1 | |||||||||
| External genitalia | 1 | 2 | 3 | 3 | ||||||||
| Glans | 3 | 1 | 4 | 10 | 10 | 14 | ||||||
| Ovaries | 1 | 1 | 1 | |||||||||
| Vaginal mucosa | 98 | 53 | 151 | 2 | 1 | 5 | 8 | 159 | ||||
| Specimen | ||||||||||||
| Sperm | 1 | 1 | 1 | |||||||||
| Semiology | ||||||||||||
| Urethral infection | 1 | 1 | 1 | |||||||||
| Osteo-articular system | 47 | 7 | 2 | 56 | 2 | 4 | 16 | 22 | 78 | |||
| Anatomical site | ||||||||||||
| Unspecified | 12 | 12 | 1 | 1 | 2 | 4 | 16 | |||||
| Joint | 5 | 1 | 6 | 1 | 4 | 5 | 11 | |||||
| Specimen | ||||||||||||
| Synovial fluid | 5 | 1 | 6 | 1 | 2 | 3 | 9 | |||||
| Semiology | ||||||||||||
| Arthritis | 8 | 3 | 11 | 3 | 3 | 14 | ||||||
| Mass (including mycetoma) | 1 | 1 | 1 | |||||||||
| Osteomyelitis | 10 | 1 | 2 | 13 | 1 | 3 | 4 | 17 | ||||
| Prosthesis-associated Osteitis | 1 | 1 | 1 | |||||||||
| Spondylodiscitis | 6 | 1 | 7 | 2 | 2 | 9 | ||||||
| Skeletal muscles | 1 | 1 | 1 | 1 | 2 | |||||||
| Anatomical site | ||||||||||||
| Unspecified | 1 | 1 | 1 | 1 | 2 | |||||||
| Soft tissue | 4 | 4 | 5 | 5 | 9 | |||||||
| Anatomical site | ||||||||||||
| Unspecified | 4 | 4 | 5 | 5 | 9 | |||||||
| Skin | 204 | 30 | 5 | 7 | 246 | 26 | 355 | 5 | 18 | 183 | 587 | 833 |
| Anatomical site | ||||||||||||
| Unspecified | 10 | 1 | 2 | 13 | 3 | 6 | 8 | 17 | 30 | |||
| Nails | 48 | 1 | 3 | 2 | 54 | 1 | 9 | 5 | 28 | 43 | 97 | |
| Subcutaneous | 8 | 1 | 1 | 10 | 2 | 1 | 1 | 10 | 14 | 24 | ||
| Superficial cutaneous | 133 | 25 | 2 | 1 | 161 | 18 | 302 | 2 | 5 | 121 | 448 | 609 |
| Semiology | ||||||||||||
| Dermatitis | 1 | 1 | 43 | 1 | 44 | 45 | ||||||
| Mycetoma | 1 | 1 | 1 | 1 | 2 | |||||||
| Tinea capitis | 1 | 10 | 11 | 11 | ||||||||
| Tinea cruris | 1 | 1 | 1 | |||||||||
| Tinea genitalis | 1 | 1 | 1 | |||||||||
| Tinea pedis | 2 | 2 | 4 | 1 | 1 | 5 | ||||||
| Ulcer | 1 | 1 | 2 | 1 | 4 | 7 | 8 | |||||
| Endocrine gland | 1 | 15 | 16 | 16 | ||||||||
| Anatomical site | ||||||||||||
| Adrenal | 1 | 4 | 5 | 5 | ||||||||
| Thymus | 2 | 2 | 2 | |||||||||
| Thyroid | 9 | 9 | 9 | |||||||||
| Placental infection | 3 | 1 | 4 | 4 | ||||||||
| Anatomical site | ||||||||||||
| Placenta | 1 | 1 | 1 | |||||||||
| Semiology | ||||||||||||
| Chorioamnionitis | 3 | 3 | 3 | |||||||||
| Total | 2110 | 298 | 55 | 354 | 2816 | 138 | 519 | 24 | 138 | 988 | 1808 | 4624 |
1 Excluding Candida albicans, C. tropicalis, C. glabrata, C. parapsilosis, and P. kudriavzevii (syn. C. krusei). 2 Excluding Cryptococcus neoformans species complex and Cryptococcus gattii species complex.
3.2.1. Systemic
The systemic involvement is the anatomical site most affected by fungal yeast infections.
Regarding systemic involvement (including fungaemia, aortitis, vasculitis, lymph node infection, and bone marrow infection), there is a large majority of fungaemia, especially candidaemia. Almost all yeasts can cause fungaemia (Figure 2). Saccharomyces cerevisiae, Cutaneotrichosporon cutaneum, Candida auris, and Trichosporon asahii are the predominant species, with over 85 publications each. These emerging species are nosocomial opportunistic pathogens [64,65]. In this review, 41% (35/86) of Candida auris isolated in the bloodstream was related to positive catheter culture, which a key risk factor due to the potential for biofilm formation [65].
Figure 2.
WordCloud of the yeast species involved in systemic infections. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
Saccharomyces cerevisiae is the leading pathogen, with 91 publications (synonyms included). This yeast, widely used in the food industry in the fermentation process, can be used as a probiotic to modulate the digestive microbiota. Unfortunately, its use as probiotic is dangerous in severely immunosuppressed patients and can lead to fungaemia [66].
3.2.2. Central Nervous System
In most cases, damage to the central nervous system results in meningitis (54 publications) and, rarely, in meningo-encephalitis (10 publications) or brain abscess (10 publications). A single case of encephalitis was reported due to Geotrichum capitatum (new Magnusiomyces capitatus) infection [67].
The taxa Candida spp., followed in order by Trichosporon spp., Cryptococcus spp., and Rhodotorula spp., was predominant (Figure 3). The prevalence of Rhodotorula mucilaginosa and Rhodotorula glutinis in the CNS underlines the invasive potential of these ubiquitous yeasts, which are widespread in the environment. Also known to be less virulent than Cryptococcus sp. or Candida sp., they may be responsible for meningitis or ventriculitis in immunosuppressed individuals [68].
Figure 3.
WordCloud of the yeast species isolated from the central nervous system. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
On the species level, Cutaneotrichosporon cutaneum and Trichosporon asahii were predominant, followed by Candida dubliniensis, Papiliotrema laurentii, and Rhodotorula mucilaginosa (Table S1).
3.2.3. Eye
All categories of fungi can affect the ocular system. However, in this review, the Candida spp. taxa stands out (Figure 4). Ocular diseases due to Candida sp. can manifest as endophthalmitis or keratitis. Endogenous endophthalmitis was frequently encountered secondary to a bloodstream infection [69,70]. Fungal keratitis mainly developed secondary to keratoplasty [71,72,73,74], trauma [75,76,77], or immunosuppressive therapy [78]. As species level, Malassezia furfur was predominant with Candida dubliniensis, Wickerhamomyces anomalus, and Candida auris (Table S1).
Figure 4.
WordCloud of the yeast species isolated from the eye. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
Malassezia spp. were mainly isolated in periocular sites causing blepharitis, dacryocystitis, or conjunctivitis (Table 1). We found a rare case of Malassezia restricta keratitis secondary to soil contamination in a farm worker [45].
Surprisingly, less common Cryptococcus species were described in ocular involvement (nine publications) responsible for keratitis related to contact lens wear [79,80], post-surgery [81], or following plant trauma [82]. These are the species Cutaneotrichosporon curvatus, Naganishia albida, and Papiliotrema laurentii and synonyms.
3.2.4. Auditory System
As stated by Bojanović et al. [83], species of the genus Candida are predominant (Figure 5). Among the uncommon species, Candida auris is by far the majority, with 21 publications reporting it being isolated from the auditory system. It has a well-known tropism for the auditory system, as it was firstly isolated from the external ear in 2009 in a hospital in Japan [18]. Fewer cases report the isolation of Malassezia spp. from the auditory system, as in most cases it is part of the microbiota of the external ear canal [84]. However, one case of malignant otitis externa with Malassezia sympodialis in a diabetic patient has been reported, highlighting a potential pathogenic effect [85]. Similarly, Trichosporon species have more rarely been isolated from the auditory system and implicated in otomycoses (Table 1).
Figure 5.
WordCloud of the yeast species name isolated from the ears. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
Among the rarely represented genera, we found a unique case report of Geotrichum candidum isolation in otitis externa in Turkey [86]. Among the genus Cryptococcus, only one case of otomycosis due to Naganishia albida was reported with manipulation of the ear canal considered as a risk factor [87].
3.2.5. Oto-Rhino-Laryngology System
We found a majority of Ascomycota in the ORL system with 79% of Candida species (279/351) (Table S1; Figure 6). The predominant anatomical site was the oral mucosa, which is not surprising, as the Candida genus is known to colonise the oral cavity [88]. Yeasts then have the ability to go from a commensal to a pathogenic state in the presence of risk factors such as immunosuppression. The species most commonly implicated in oral candidiasis are those of the Candida genus, followed by species of the Saccharomyces genus [89].
Figure 6.
WordCloud of the yeast species isolated from the oto-rhino-laryngology system. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
3.2.6. Pulmonary System
All the different taxa for both Ascomycota and Basidiomycota are represented in the pulmonary system (Figure 7). We found a majority of Candida species and Trichosporon species. Colonisation of the upper and lower respiratory system is the prerequisite for an invasive infection. Real lung infections remain rare and include yeast pneumonia (49 publications), lung abscess (2 publications), and the lung cavity (4 publications). Among the species responsible for yeast pneumonia, two stand out, namely Cutaneotrichosporon cutaneum and Magnusiomyces capitatus. Other species may be encountered on an anecdotal basis with a single publication, such as Malassezia pachydermatis, Rhodotorula glutinis, Sporobolomyces salmonicolor, Cyberlindnera jadinii, Lachancea kluyveri, Naganishia adeliensis, Trichosporon asteroides, Metschnikowia pulcherrima, Candida duobushaemulonii, Pichia fermentans, Candida intermedia, Apiotrichum loubieri, Nakaseomyces bracarensis, Candida sake, Lachancea fermentati, Diutina pseudorugosa, Zygoascus hellenicus, Blastobotrys adeninivorans, Debaryomyces nepalensis, Fereydounia khargensis, Wallemia mellicola, Hannaella luteola, Kluyveromyces fragilis, Metschnikowia sinensis, and Torulopsis pintolopesii.
Figure 7.
WordCloud of the yeast species isolated from the pulmonary system. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
The lower respiratory tract is the most frequent localisation (186 publications) followed by the upper respiratory tract (182 publications).
3.2.7. Cardiac Involvement
Cardiac involvement was found in 12% (23 species/192) of the less common yeast species described in this repertoire. All the taxa belonging to the Ascomycota were represented in this localisation and were responsible for native or prosthetic valve endocarditis. Interestingly, among the Basidiomycota, only the taxa Trichosporon seems to have a cardiac tropism and, more rarely, Rhodotorula and Malassezia. Cutaneotrichosporon cutaneum (formerly Trichosporon beigelii and Trichosporon cutaneum) was the main species involved in this localisation (26 publications) with three other Trichosporon species, namely, Trichosporon asahii, Trichosporon inkin, and Trichosporon mycotoxinivorans (Figure 8). Cardiac involvement was mainly in the form of native valve endocarditis (23 publications) followed by prosthetic valve or implanted equipment endocarditis (15 publications). Focusing specifically on the sites concerned, the aortic valve (11 publications), mitral valve (10 publications), and tricuspid valve (7 publications) were the most frequently concerned (Table 2).
Figure 8.
WordCloud of the yeast species isolated from the heart. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
Table 2.
Details of cardiac sites affected by native valve endocarditis and associated species.
| Name * | Tricuspid Valve | Mitral Valve | Aortic Valve | Endocardium | Pulmonary Valve | Atrium | Ventricle | ND |
|---|---|---|---|---|---|---|---|---|
| Blastoschizomyces capitatus | 1 [90] | |||||||
| Candida colliculosa | 1 [91] | |||||||
| Candida dubliniensis | 2 [92,93] | 1 [94] | ||||||
| Candida guilliermondii | 1 [95] | |||||||
| Candida kefyr | 1 [96] | |||||||
| Candida lusitaniae | 2 [97,98] | |||||||
| Candida mycoderma | 1 [99] | |||||||
| Candida sake | 1 [100] | 1 [101] | ||||||
| Candida zeylanoides | 1 [102] | |||||||
| Geotrichum clavatum | 1 [103] | 1 [103] | ||||||
| Hansenula anomala | 1 [104] | |||||||
| Kodamaea ohmeri | 1 [105] | 1 [106] | ||||||
| Lodderomyces elongisporus | 1 [37] | |||||||
| Malassezia furfur | 1 [107] | 1 [107] | 1 [108] | |||||
| Pichia fabianii | 1 [109] | |||||||
| Pichia ohmeri | 1 [110] | |||||||
| Rhodotorula pilimanae | 1 [111] | 1 [111] | ||||||
| Saccharomyces cerevisiae | 1 [112] | 1 [113] | ||||||
| Trichosporon asahii | 1 [114] | 1 [114] | ||||||
| Trichosporon beigelii | 1 [115] | 1 [115] | 1 [115] | |||||
| Trichosporon cutaneum | 1 [116] | |||||||
| Yarrowia lipolytica | 1 [117] | |||||||
| Total | 7 | 10 | 11 | 1 | 1 | 1 | 2 | 4 |
ND: Not determined. * The species name in the table is that found in the cited publication.
3.2.8. Digestive System
Concerning the digestive system, yeasts are mostly isolated from the peritoneum and implicated in peritonitis. In this review, 82% (61/74) of the publications reported peritoneal dialysis-related peritonitis. When data were available, other risk factors for peritonitis were underlying neoplasia [118], gastric and duodenal ulcer perforation [119], recent digestive surgery [120,121,122,123,124], severe immunosuppression [125,126], and pancreatitis [127]. In some cases, infection of the peritoneum, as well as other unusual infection sites including the biliary tract (9 publications), gastric tract (12 publications), and spleen (19 publications), may also be a secondary condition to the haematogenous dissemination of yeasts [128].
The species mostly found were Saccharomyces cerevisiae, Cutaneotrichosporon cutaneum, Candida auris, Clavispora lusitaniae, Magnusiomyces capitatus, Trichosporon asahii, Geotrichum candidum, and Meyerozyma guilliermondii (Table S1; Figure 9).
Figure 9.
WordCloud of the yeast species isolated from the gut. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
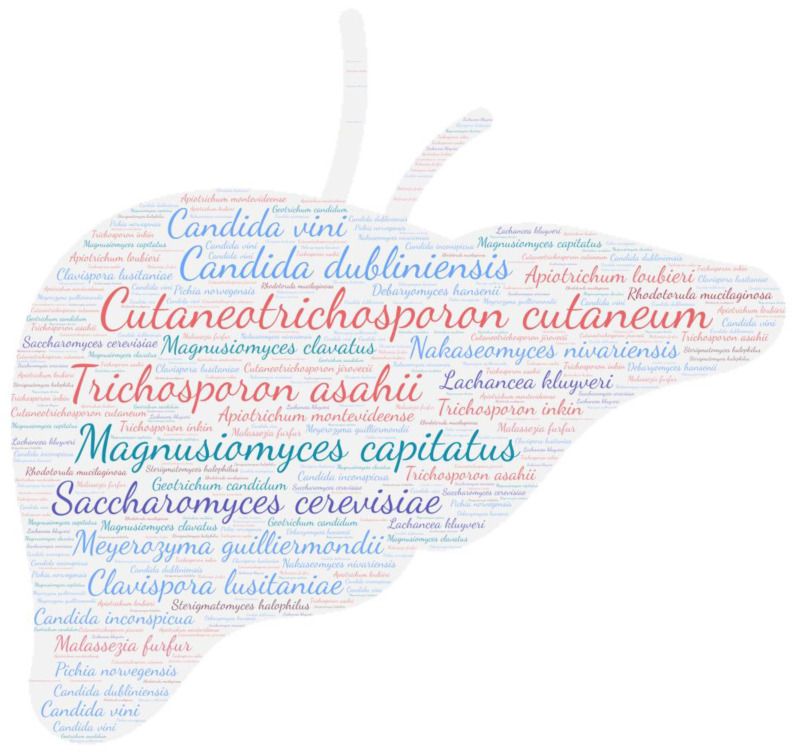
3.2.9. Liver Involvement
Liver involvement was predominantly described among the taxa Trichosporon and Candida, resulting in ascites, abscesses, and hepatitis (Figure 10). Less frequently, cases have also been reported for the Magnusiomyces/Saprochaete clade (nine publications); Saccharomyces spp. (seven publications), mainly Saccharomyces cerevisiae; Malassezia furfur (six publications); and Rhodotorula mucilaginosa (one publication). Interestingly, the only publication reporting isolation of Sterigmatomyces halophilus was a case of a liver abscess in an immunocompromised patient due to a marine-derived Basidiomycota [129].
Figure 10.
WordCloud of the yeast species isolated from the liver. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
3.2.10. Urinary Tract
In the urinary tract, yeasts were most frequently isolated in urine samples with a clear predominance of species of genus Candida (62%; 162/263) (Figure 11). Funguria are mainly nosocomial infections and urinary catheterisation plays an important role in urinary tract infections due to biofilm formation [130,131,132]. Unfortunately, due to a lack of data concerning the presence or otherwise of a urinary catheter, we were not able to assess the proportion of catheter infections in this review.
Figure 11.
WordCloud of the yeast species isolated from the urinary tract. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
Candida auris was the most isolated species followed closely by Trichosporon asahii. Trichosporon asahii is known to be the most frequent pathogen causing urinary tract infections among the genus Trichosporon. This site of infection is considered as “uncommon” [133]. Clavispora lusitaniae, Cutaneotrichosporon cutaneum, and Candida dubliniensis were also represented.
3.2.11. Osteo-Articular System
Among the osteo-articular diseases, yeasts can cause osteomyelitis (17 publications) as well as arthritis (14 publications) and, more rarely, spondylodiscitis (9 publications). Candida species are predominant, followed by Trichosporon species (Figure 12).
Figure 12.
WordCloud of the yeast species isolated from the osteo-articular system. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
Four Rhodotorula mucilaginosa osteo-articular diseases were reported, one post-operative persistent femoral non-union [134], one femoral prosthesis infection [135], one hip-joint prosthesis infection [136], and one case of an infection associated with multifocal skeletal tuberculosis [137]. Saccharomyces cerevisiae was responsible for two cases of osteomyelitis [138,139] and Cryptococcus luteolus was responsible for a case of tenosynovitis [42].
3.2.12. Skin System
Basidiomycota are predominant in cutaneous system involvement (587 publications), with Cryptococcus species as the leader (Figure 13). Cryptococcal skin lesions may be primary cutaneous lesions or sentinels for disseminated disease [140]. The second leading yeasts were Candida species. These Ascomycota may be a commensal on the skin but can also become pathogenic in the presence of predisposing factors such as impaired immunological status or skin barrier disruption [22,141]. However, deep cutaneous Candida infections are uncommon [142], as subcutaneous infections are rarely due to Candida auris [143,144], Candida duobushaemulonii [142], or Candida rugosa [145]. Trichosporon species appeared in third place. These too are part of the normal skin microbiota but can cause a common superficial infection in tropical and subtropical regions known as “the white piedra” [47,146].
Figure 13.
WordCloud of the yeast species isolated from the skin system. The size of the name of each species is proportional to the number of times it occurs in the repertoire.
In this repertoire, it is thus not surprising that we found a majority of superficial cutaneous lesions, followed distantly by onychomycosis (Table 1). A few subcutaneous involvements were described, caused by Candida auris, Candida duobushaemulonii, Candida rugosa, Papiliotrema laurentii, Cutaneotrichosporon debeurmannianum, Kodamaea ohmeri, Magnusiomyces capitatus, Malassezia furfur, Pichia ohmeri, Saccharomyces cerevisiae, Trichosporon asahii, Trichosporon cutaneum, Trichosporon inkin, Trichosporon montevideense, Trichosporon ovoides, and Wallemia sebi. Two yeasts, Candida guilliermondii and Pseudozyma aphidis, were isolated from mycetoma, but they were associated with the isolation of Madurella mycetomatis and Nocardia otitidiscaviarum, respectively [147,148].
3.2.13. Genital Sphere
Genital sphere involvement is mostly related to the Candida genera and Saccharomyces genera (Table S1) [130]. Saccharomyces cerevisiae is the leading species, followed by Candida dubliniensis, Clavispora lusitaniae, and Meyerozymaguilliermondii.
Genital sphere involvement is almost exclusively limited to the vaginal mucosa and is sometimes responsible for vaginitis (159 publications). It should be noted that the isolation of yeasts from the vaginal mucosa is not systematically pathological and can refer to colonisation [149]. Unusual female genital sphere infection sites were sporadically reported, as in a case of a tubo-ovarian abscess caused by Candida kefyr [150] and endometritis caused by Trichosporon beigelii [151].
Concerning the male genital sphere, yeast isolation is rarer and often related to glans colonisation (14 publications). Interestingly, it has been shown that yeast colonisation was more frequently observed among uncircumcised versus circumcised men [152,153]. Urethral involvement is rare [154] and one case of orchi-epididymitis post-dissemination of Geotrichum capitatum has been reported, but it remains exceptional [155].
3.2.14. Anatomical Sites Rarely Involved
Dental location. Only Ascomycota were reported in the dental location, probably due to the commensal nature of the oral mucosa. Yeasts were mainly found in the dental mycobiota or dental plaque. Two cases of dento-alveolar abscesses due to Meyerozyma guilliermondii (anc. Candida guilliermondii) [156] and Magnusiomyces capitatus (anc. Trichosporon capitatum) [157] were found.
Endocrine glands. Only Basidiomycota were reported among endocrine glands with 15 publications reporting Trichosporon spp. and 1 publication reporting Malassezia furfur. Trichosporon species were Cutaneotrichosporon cutaneum, Trichosporon asahii, Trichosporon inkin, and Trichosporon capitatum. All the cases were autopsy findings secondary to the disseminated infection.
Breast. Ascomyceta and Basidiomycota have rarely been isolated from this particular site and few infections have been reported. Long-term nipple discharge due to Pityrosporum orbiculare (now Malassezia furfur) has been described [158]. Interestingly, one case of Trichosporon beigelii (now Cutaneotrichosporon cutaneum) breast implant infection has been reported in an immunocompetent patient in Thailand [159]. Saccharomyces cerevisiae [160,161], Lodderomyces elongisporus [162], and Malassezia globosa [160] have all been isolated from milk samples.
Placental infection. Only Ascomycota were reported among placental infections. One cases of sepsis with chorioamnionitis caused by Kluyveromyces marxianus (formerly Candida kefyr) was reported with placental transmission to premature fraternal twins [163]. Clavispora lusitaniae was responsible for two cases of chorioamnionitis with foetal infections [164,165]. Saccharomyces cerevisiae has been isolated from placental samples [166].
4. Discussion
There is increasing clinical interest in less common yeasts implicated in infectious diseases in immunosuppressed patients. The aim of this repertoire was to catalogue as exhaustively as possible the uncommon yeasts identified in humans by culture and molecular biology, whether or not they were associated with histopathological findings.
We opted to exclude some yeast species categorised as “fungal priority pathogens” by the WHO [167]. These include, notably, the five prominent Candida species (Candida albicans, C. tropicalis, C. glabrata, C. parapsilosis, and P. kudriavzevii), along with those from the Cryptococcus neoformans/gattii complex. This deliberate choice aims to avoid overwhelming the repertoire with publications about these common yeasts, to ensure a more balanced understanding of fungal diversity, and to help uncover valuable information about rarer yeasts that might otherwise remain hidden. We found that 192 less common or rare yeasts, including 127 Ascomycota and 65 Basidiomycota, had been identified in humans. The specific anatomical locations or samples in which these fungi were detected, along with the characteristics of the infections, were clearly delineated. The first publication reporting the isolation of one of the less common yeasts dates back to 1947 and concerns Pityrosporum ovale (now Malassezia furfur) [168]. To ensure consistency, we chose to stop our analysis on 16 June 2020. The most recently described species in our repertoire is Rhodotorula toruloides, isolated from blood culture and identified by sequencing, presenting an emerging agent of bloodstream infection [169].
Systemic location was predominant with a total of 1309 publications. Interestingly, when synonyms and former names are taken into account, Saccharomyces cerevisiae is the predominant species (91 publications), followed by Cutaneotrichosporon cutaneum (90 publications) and with Candida auris in third place (86 publications). However, Candida auris is listed as a “fungal priority pathogen” [167], unlike the other two predominant pathogens. S. cerevisiae and Cutaneotrichosporon cutaneum do not attract as much attention, even though they present real emerging trends.
This fungal repertoire presents a useful tool for diagnostic management. Some less common or rare yeast species may be associated with invasive infections in high-risk patients, and their description could be used to pinpoint them. Considering that the most commonly isolated aetiological agents in mycotic endocarditis are Candida spp. and Aspergillus spp., there might be a propensity to focus solely on their detection, effectively excluding the possibility of other fungal origins [170]. However, this fungal repertoire highlights the cardiac tropism of Trichosporon spp., which may go unnoticed in comparison with much more common species, and must be considered [171]. Trichosporon yeasts are opportunistic agents leading to superficial-to-severe infections in at-risk populations [64]. This repertoire emphasises their invasive potential, as they are widely represented in deep-seated localisations. They are some of the less common yeasts most frequently found in the heart and liver. Similarly, they are second only to Candida species in systemic localisations, the central nervous system, the pulmonary system, where Trichosporon beigelii is the second most common cause of yeast pneumonia, and the digestive system, where they are mostly found in the spleen and involved in peritonitis. We also found some surprising localisations, such as for species of the Cryptococcus genus. In addition to the expected locations in the bloodstream, the lungs, the cutaneous system, and the CNS, we found less common Cryptococcus species in the ocular region, responsible for keratitis and endophthalmitis. A distinction between Ascomycota and Basidiomycota has also been made for certain rarer localisations. In this way, we saw that endocrine gland damage during dissemination is limited to autopsy findings for Basidiomycota. Similarly, placental damage is limited to Ascomycota and, more specifically, Candida kefyr and Candida lusitaniae, probably leading to their presence in the genital mucosa, and systematically resulting in foetal damage [163,164,165].
This repertoire also provides an update of the current taxonomy, bringing together the old and new names, taking into account the “one fungus, one name” unification, helping clinicians to identify rare yeast isolations and their synonyms [15,16]. Due to constantly evolving fungal taxonomy, some yeast species may be over- or underestimated, creating a bias, and classifications are bound to evolve. One well-known example is Candida dubliniensis, which was identified as Candida albicans until 1995 resulting in an underestimation of its involvement in human pathology [19].
One other bias of our study lies in the choice of query following the pattern (fungi_name_or_synonyms [TIAB] OR fungi_name_or_synonyms[OT] OR fungi_name_or_synonyms[MeSH]) AND (“Human”[TIAB] OR “Human”[OT] OR “Humans”[MeSH]). The title, abstract, authors’ keywords, and MeSH descriptors of a PubMed/MEDLINE bibliographic record are particularly important for understanding and grasping the content of an article, as they contain the semantics and main concepts addressed in the article. We choose the MeSH descriptor “Human(s)” as it is a “Check Tag” that is systematically added by the human indexer of the PubMed/MEDLINE database even if the word “human” does not appear in the text (see https://www.nlm.nih.gov/bsd/indexing/training/CHK_010.html accessed on 15 November 2019). However, it is possible that a few articles may not have been identified.
Finally, as for the non-dermatophytic mould repertoire [14], one limitation lies in its temporal scope. Only references which existed in PubMed up until 16 June 2020 were considered, ensuring uniformity across all fungal species. Nonetheless, medical mycology is dynamic, and newly emerging organisms constantly need to be taken into account by clinicians and microbiology laboratories [172]. It will therefore be necessary to update these data regularly.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jof9111099/s1, Table S1: Number of publications found by anatomical site and species for Ascomycota division. Several anatomical sites of isolation could be found in the same publication (PMID); Table S2: Number of publications found by anatomical site and species for the Basidiomycota yeast division. In the same publication (PMID), several anatomical sites of isolation could be found.
Author Contributions
Conceptualisation, S.R. and C.L.; methodology, E.M., Q.F. and J.-C.D.; software, Q.F. and J.-C.D.; formal analysis, E.M. and C.L.; data curation, E.M.; writing—original draft preparation, E.M., C.L. and J.-C.D.; supervision, S.R. and C.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kurtzman C.P., Piškur J. Taxonomy and Phylogenetic Diversity among the Yeasts. In: Sunnerhagen P., Piskur J., editors. Comparative Genomics: Using Fungi as Models. Springer; Berlin/Heidelberg, Germany: 2006. pp. 29–46. Topics in Current Genetics. [Google Scholar]
- 2.Segal-Kischinevzky C., Romero-Aguilar L., Alcaraz L.D., López-Ortiz G., Martínez-Castillo B., Torres-Ramírez N., Sandoval G., González J. Yeasts Inhabiting Extreme Environments and Their Biotechnological Applications. Microorganisms. 2022;10:794. doi: 10.3390/microorganisms10040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtzman C.P., Fell J.W., Boekhout T., Robert V. Chapter 7—Methods for Isolation, Phenotypic Characterization and Maintenance of Yeasts. In: Kurtzman C.P., Fell J.W., Boekhout T., editors. The Yeasts. 5th ed. Elsevier; London, UK: 2011. pp. 87–110. [Google Scholar]
- 4.Crous P.W., Gams W., Stalpers J.A., Robert V., Stegehuis G. MycoBank: An Online Initiative to Launch Mycology into the 21st Century. Stud. Mycol. 2004;50:19–22. [Google Scholar]
- 5.Kurtzman C.P., Fell J.W. Yeast Systematics and Phylogeny—Implications of Molecular Identification Methods for Studies in Ecology. In: Péter G., Rosa C., editors. Biodiversity and Ecophysiology of Yeasts. Springer; Berlin/Heidelberg, Germany: 2006. pp. 11–30. The Yeast Handbook. [Google Scholar]
- 6.Magill S.S., O’Leary E., Janelle S.J., Thompson D.L., Dumyati G., Nadle J., Wilson L.E., Kainer M.A., Lynfield R., Greissman S., et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018;379:1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lortholary O., Renaudat C., Sitbon K., Desnos-Ollivier M., Bretagne S., Dromer F. French Mycoses Study Group the Risk and Clinical Outcome of Candidemia Depending on Underlying Malignancy. Intensive Care Med. 2017;43:652–662. doi: 10.1007/s00134-017-4743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kullberg B.J., Arendrup M.C. Invasive Candidiasis. N. Engl. J. Med. 2015;373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S., Kumar A., Roudbary M., Mohammadi R., Černáková L., Rodrigues C.F. Overview on the Infections Related to Rare Candida Species. Pathogens. 2022;11:963. doi: 10.3390/pathogens11090963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S.C.-A., Perfect J., Colombo A.L., Cornely O.A., Groll A.H., Seidel D., Albus K., de Almedia J.N., Garcia-Effron G., Gilroy N., et al. Global Guideline for the Diagnosis and Management of Rare Yeast Infections: An Initiative of the ECMM in Cooperation with ISHAM and ASM. Lancet Infect. Dis. 2021;21:e375–e386. doi: 10.1016/S1473-3099(21)00203-6. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M., Chakrabarti A. Candidiasis and Other Emerging Yeasts. Curr. Fungal. Infect. Rep. 2023;17:15–24. doi: 10.1007/s12281-023-00455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanyaolu A., Okorie C., Marinkovic A., Abbasi A.F., Prakash S., Mangat J., Hosein Z., Haider N., Chan J. Candida auris: An Overview of the Emerging Drug-Resistant Fungal Infection. Infect. Chemother. 2022;54:236–246. doi: 10.3947/ic.2022.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pande A., Non L.R., Romee R., Santos C.A.Q. Pseudozyma and Other Non-Candida Opportunistic Yeast Bloodstream Infections in a Large Stem Cell Transplant Center. Transpl. Infect. Dis. 2017;19:e12664. doi: 10.1111/tid.12664. [DOI] [PubMed] [Google Scholar]
- 14.Menu E., Filori Q., Dufour J.-C., Ranque S., L’Ollivier C. A Repertoire of Clinical Non-Dermatophytes Moulds. J. Fungi. 2023;9:433. doi: 10.3390/jof9040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidd S.E., Abdolrasouli A., Hagen F. Fungal Nomenclature: Managing Change Is the Name of the Game. Open Forum. Infect. Dis. 2023;10:ofac559. doi: 10.1093/ofid/ofac559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takashima M., Sugita T. Taxonomy of Pathogenic Yeasts Candida, Cryptococcus, Malassezia, and Trichosporon. Med. Mycol. J. 2022;63:119–132. doi: 10.3314/mmj.22.004. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan D.J., Westerneng T.J., Haynes K.A., Bennett D.E., Coleman D.C. Candida dubliniensis Sp. Nov.: Phenotypic and Molecular Characterization of a Novel Species Associated with Oral Candidosis in HIV-Infected Individuals. (Pt 7)Microbiol. Read. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 18.Satoh K., Makimura K., Hasumi Y., Nishiyama Y., Uchida K., Yamaguchi H. Candida auris Sp. Nov., a Novel Ascomycetous Yeast Isolated from the External Ear Canal of an Inpatient in a Japanese Hospital. Microbiol. Immunol. 2009;53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 19.Ells R., Kock J.L.F., Pohl C.H. Candida albicans or Candida dubliniensis? Mycoses. 2011;54:1–16. doi: 10.1111/j.1439-0507.2009.01759.x. [DOI] [PubMed] [Google Scholar]
- 20.McCarty T.P., White C.M., Pappas P.G. Candidemia and Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2021;35:389–413. doi: 10.1016/j.idc.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Oliva A., De Rosa F.G., Mikulska M., Pea F., Sanguinetti M., Tascini C., Venditti M. Invasive Candida Infection: Epidemiology, Clinical and Therapeutic Aspects of an Evolving Disease and the Role of Rezafungin. Expert Rev. Anti. Infect. Ther. 2023;21:957–975. doi: 10.1080/14787210.2023.2240956. [DOI] [PubMed] [Google Scholar]
- 22.Lu H., Hong T., Jiang Y., Whiteway M., Zhang S. Candidiasis: From Cutaneous to Systemic, New Perspectives of Potential Targets and Therapeutic Strategies. Adv. Drug Deliv. Rev. 2023;199:114960. doi: 10.1016/j.addr.2023.114960. [DOI] [PubMed] [Google Scholar]
- 23.Sayeed M.A., Farooqi J., Jabeen K., Awan S., Mahmood S.F. Clinical Spectrum and Factors Impacting Outcome of Candida auris: A Single Center Study from Pakistan. BMC Infect. Dis. 2019;19:384. doi: 10.1186/s12879-019-3999-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatamzas E., Madder H., Jeffery K. Neurosurgical Device-Associated Infections Due to Candida auris—Three Cases from a Single Tertiary Center. J. Infect. 2019;78:409–421. doi: 10.1016/j.jinf.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz-Gaitán A., Moret A.M., Tasias-Pitarch M., Aleixandre-López A.I., Martínez-Morel H., Calabuig E., Salavert-Lletí M., Ramírez P., López-Hontangas J.L., Hagen F., et al. An Outbreak Due to Candida auris with Prolonged Colonisation and Candidaemia in a Tertiary Care European Hospital. Mycoses. 2018;61:498–505. doi: 10.1111/myc.12781. [DOI] [PubMed] [Google Scholar]
- 26.Yamahiro A., Lau K.H.V., Peaper D.R., Villanueva M. Meningitis Caused by Candida dubliniensis in a Patient with Cirrhosis: A Case Report and Review of the Literature. Mycopathologia. 2016;181:589–593. doi: 10.1007/s11046-016-0006-7. [DOI] [PubMed] [Google Scholar]
- 27.Andrew N.H., Ruberu R.P., Gabb G. The First Documented Case of Candida dubliniensis Leptomeningeal Disease in an Immunocompetent Host. BMJ Case Rep. 2011;2011:bcr0620114384. doi: 10.1136/bcr.06.2011.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez A., Jewtuchowicz V.M., Malzone M.C., Lopez-Daneri G., Saa G., Iovannitti C.A., Tokumoto M., Mujica M.T. Phenotypic and Genotypic Characterisation of Candida dubliniensis Isolate in a Patient with Disseminated Candidiasis. Mycoses. 2011;54:e602–e605. doi: 10.1111/j.1439-0507.2010.01917.x. [DOI] [PubMed] [Google Scholar]
- 29.van Hal S.J., Stark D., Harkness J., Marriott D. Candida dubliniensis Meningitis as Delayed Sequela of Treated C. dubliniensis Fungemia. Emerg. Infect. Dis. 2008;14:327–329. doi: 10.3201/eid1402.070985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou M., Li Y., Kudinha T., Xu Y., Liu Z. Kodamaea Ohmeri as an Emerging Human Pathogen: A Review and Update. Front. Microbiol. 2021;12:736582. doi: 10.3389/fmicb.2021.736582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan E., Al-Hatmi A.M.S., Ilkit M., Gerrits van den Ende A.H.G., Hagen F., Meis J.F., de Hoog G.S. Molecular Diagnostics of Arthroconidial Yeasts, Frequent Pulmonary Opportunists. J. Clin. Microbiol. 2018;56:e01427-17. doi: 10.1128/JCM.01427-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Principe M.I., Seidel D., Criscuolo M., Dargenio M., Rácil Z., Piedimonte M., Marchesi F., Nadali G., Koehler P., Fracchiolla N., et al. Clinical Features and Prognostic Factors of Magnusiomyces (Saprochaete) Infections in Haematology. A Multicentre Study of SEIFEM/Fungiscope. Mycoses. 2023;66:35–46. doi: 10.1111/myc.13524. [DOI] [PubMed] [Google Scholar]
- 33.Durán Graeff L., Seidel D., Vehreschild M.J.G.T., Hamprecht A., Kindo A., Racil Z., Demeter J., De Hoog S., Aurbach U., Ziegler M., et al. Invasive Infections Due to Saprochaete and Geotrichum Species: Report of 23 Cases from the FungiScope Registry. Mycoses. 2017;60:273–279. doi: 10.1111/myc.12595. [DOI] [PubMed] [Google Scholar]
- 34.Legras J.-L., Merdinoglu D., Cornuet J.-M., Karst F. Bread, Beer and Wine: Saccharomyces Cerevisiae Diversity Reflects Human History. Mol. Ecol. 2007;16:2091–2102. doi: 10.1111/j.1365-294X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- 35.Wombwell E., Bransteitter B., Gillen L.R. Incidence of Saccharomyces Cerevisiae Fungemia in Hospitalised Patients Administered Saccharomyces Boulardii Probiotic. Mycoses. 2021;64:1521–1526. doi: 10.1111/myc.13375. [DOI] [PubMed] [Google Scholar]
- 36.Enache-Angoulvant A., Hennequin C. Invasive Saccharomyces Infection: A Comprehensive Review. Clin. Infect. Dis. 2005;41:1559–1568. doi: 10.1086/497832. [DOI] [PubMed] [Google Scholar]
- 37.Daveson K.L., Woods M.L. Lodderomyces Elongisporus Endocarditis in an Intravenous Drug User: A New Entity in Fungal Endocarditis. J. Med. Microbiol. 2012;61:1338–1340. doi: 10.1099/jmm.0.047548-0. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeld S.I., Jost B.F., Litinsky S.M., Gelender H., Glatzer R.J., Flynn H.W. Persistent Torulopsis Magnoliae Endophthalmitis Following Cataract Extraction. Ophthalmic Surg. 1994;25:154–156. doi: 10.3928/1542-8877-19940301-06. [DOI] [PubMed] [Google Scholar]
- 39.Cano E.J., Yetmar Z.A., Razonable R.R. Cryptococcus Species Other Than Cryptococcus neoformans and Cryptococcus gattii: Are They Clinically Significant? Open Forum. Infect. Dis. 2020;7:ofaa527. doi: 10.1093/ofid/ofaa527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khawcharoenporn T., Apisarnthanarak A., Mundy L.M. Non-Neoformans Cryptococcal Infections: A Systematic Review. Infection. 2007;35:51–58. doi: 10.1007/s15010-007-6142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugita T., Takashima M., Ikeda R., Nakase T., Shinoda T. Intraspecies Diversity of Cryptococcus albidus Isolated from Humans as Revealed by Sequences of the Internal Transcribed Spacer Regions. Microbiol. Immunol. 2001;45:291–297. doi: 10.1111/j.1348-0421.2001.tb02621.x. [DOI] [PubMed] [Google Scholar]
- 42.Hunter-Ellul L., Schepp E.D., Lea A., Wilkerson M.G. A Rare Case of Cryptococcus Luteolus-Related Tenosynovitis. Infection. 2014;42:771–774. doi: 10.1007/s15010-014-0593-5. [DOI] [PubMed] [Google Scholar]
- 43.Ianiri G., LeibundGut-Landmann S., Dawson T.L. Malassezia: A Commensal, Pathogen, and Mutualist of Human and Animal Skin. Annu. Rev. Microbiol. 2022;76:757–782. doi: 10.1146/annurev-micro-040820-010114. [DOI] [PubMed] [Google Scholar]
- 44.Theelen B., Cafarchia C., Gaitanis G., Bassukas I.D., Boekhout T., Dawson T.L. Malassezia Ecology, Pathophysiology, and Treatment. Med. Mycol. 2018;56:S10–S25. doi: 10.1093/mmy/myx134. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki T., Hori N., Miyake T., Hori Y., Mochizuki K. Keratitis Caused by a Rare Fungus, Malassezia Restricta. Jpn. J. Ophthalmol. 2007;51:292–294. doi: 10.1007/s10384-007-0447-0. [DOI] [PubMed] [Google Scholar]
- 46.Hof H. Rhodotorula Spp. in the Gut-Foe or Friend? GMS Infect. Dis. 2019;7:Doc02. doi: 10.3205/id000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miceli M.H., Díaz J.A., Lee S.A. Emerging Opportunistic Yeast Infections. Lancet Infect. Dis. 2011;11:142–151. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 48.Pârvu M., Moţ C.A., Pârvu A.E., Mircea C., Stoeber L., Roşca-Casian O., Ţigu A.B. Allium sativum Extract Chemical Composition, Antioxidant Activity and Antifungal Effect against Meyerozyma guilliermondii and Rhodotorula mucilaginosa Causing Onychomycosis. Molecules. 2019;24:3958. doi: 10.3390/molecules24213958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moț A.C., Pârvu M., Pârvu A.E., Roşca-Casian O., Dina N.E., Leopold N., Silaghi-Dumitrescu R., Mircea C. Reversible Naftifine-Induced Carotenoid Depigmentation in Rhodotorula Mucilaginosa (A. Jörg.) F.C. Harrison Causing Onychomycosis. Sci. Rep. 2017;7:11125. doi: 10.1038/s41598-017-11600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou J., Chen M., Chen H., Pan W., Liao W. Rhodotorula Minuta as Onychomycosis Agent in a Chinese Patient: First Report and Literature Review. Mycoses. 2014;57:191–195. doi: 10.1111/myc.12143. [DOI] [PubMed] [Google Scholar]
- 51.Martini K., Müller H., Huemer H.P., Höpfl R. Nail Psoriasis Masqueraded by Secondary Infection with Rhodotorula mucilaginosa. Mycoses. 2013;56:690–692. doi: 10.1111/myc.12091. [DOI] [PubMed] [Google Scholar]
- 52.da Cunha M.M.L., dos Santos L.P.B., Dornelas-Ribeiro M., Vermelho A.B., Rozental S. Identification, Antifungal Susceptibility and Scanning Electron Microscopy of a Keratinolytic Strain of Rhodotorula Mucilaginosa: A Primary Causative Agent of Onychomycosis. FEMS Immunol. Med. Microbiol. 2009;55:396–403. doi: 10.1111/j.1574-695X.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 53.Fischer J., Hamacher L., Fries J., Hallek M., Cornely O.A., Kochanek M., Boell B. Rhodotorula Mucilaginosa as a Cause of Recurrent Pulmonary Infection and Liver Infiltration in a Patient with CLL. Ann. Hematol. 2016;95:1569–1570. doi: 10.1007/s00277-016-2726-7. [DOI] [PubMed] [Google Scholar]
- 54.Mooty M.Y., Kanj S.S., Obeid M.Y., Hassan G.Y., Araj G.F. A Case of Trichosporon Beigelii Endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 2001;20:139–142. doi: 10.1007/PL00011245. [DOI] [PubMed] [Google Scholar]
- 55.Walling D.M., McGraw D.J., Merz W.G., Karp J.E., Hutchins G.M. Disseminated Infection with Trichosporon beigelii. Rev. Infect. Dis. 1987;9:1013–1019. doi: 10.1093/clinids/9.5.1013. [DOI] [PubMed] [Google Scholar]
- 56.Gardella S., Nomdedeu B., Bombí J.A., Muñoz J., Puig de la Bellacasa J., Pumarola A., Rozman C. Fatal Fungemia with Arthritic Involvement Caused by Trichosporon beigelii in a Bone Marrow Transplant Recipient. J. Infect. Dis. 1985;151:566. doi: 10.1093/infdis/151.3.566. [DOI] [PubMed] [Google Scholar]
- 57.Telles J.P., Ribeiro V.S.T., Kraft L., Tuon F.F. Pseudozyma Spp. Human Infections: A Systematic Review. Med. Mycol. 2021;59:1–6. doi: 10.1093/mmy/myaa025. [DOI] [PubMed] [Google Scholar]
- 58.Tang H.-J., Lai C.-C., Chao C.-M. Central-Line-Associated Bloodstream Infection Caused by Sporobolomyces salmonicolor. Infect. Control Hosp. Epidemiol. 2015;36:1111–1112. doi: 10.1017/ice.2015.158. [DOI] [PubMed] [Google Scholar]
- 59.Sharma V., Shankar J., Kotamarthi V. Endogeneous Endophthalmitis Caused by Sporobolomyces salmonicolor. Eye. 2006;20:945–946. doi: 10.1038/sj.eye.6702051. [DOI] [PubMed] [Google Scholar]
- 60.Plazas J., Portilla J., Boix V., Pérez-Mateo M. Sporobolomyces Salmonicolor Lymphadenitis in an AIDS Patient. Pathogen or Passenger? AIDS. 1994;8:387–388. doi: 10.1097/00002030-199403000-00015. [DOI] [PubMed] [Google Scholar]
- 61.Bross J.E., Manning P., Kacian D., Talbot G.H. Pseudomeningitis Caused by Sporobolomyces Salmonicolor. Am. J. Infect. Control. 1986;14:220–223. doi: 10.1016/0196-6553(86)90121-5. [DOI] [PubMed] [Google Scholar]
- 62.Misra V.C., Randhawa H.S. Sporobolomyces Salmonicolor Var. Fischerii, a New Yeast. Arch. Microbiol. 1976;108:141–143. doi: 10.1007/BF00425104. [DOI] [PubMed] [Google Scholar]
- 63.McNicholas S., McDermott H., Power L., Johnson E.M., Moroney J., Humphreys H., Smyth E.G. Sporobolomyces Roseus in the Cerebrospinal Fluid of an Immunocompetent Patient—To Treat or Not to Treat? J. Med. Microbiol. 2012;61:295–296. doi: 10.1099/jmm.0.036293-0. [DOI] [PubMed] [Google Scholar]
- 64.Francisco E.C., Hagen F. JMM Profile: Trichosporon Yeasts: From Superficial Pathogen to Threat for Haematological-Neutropenic Patients. J. Med. Microbiol. 2022;71:001621. doi: 10.1099/jmm.0.001621. [DOI] [PubMed] [Google Scholar]
- 65.Watkins R.R., Gowen R., Lionakis M.S., Ghannoum M. Update on the Pathogenesis, Virulence, and Treatment of Candida auris. Pathog. Immun. 2022;7:46–65. doi: 10.20411/pai.v7i2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muñoz P., Bouza E., Cuenca-Estrella M., Eiros J.M., Pérez M.J., Sánchez-Somolinos M., Rincón C., Hortal J., Peláez T. Saccharomyces Cerevisiae Fungemia: An Emerging Infectious Disease. Clin. Infect. Dis. 2005;40:1625–1634. doi: 10.1086/429916. [DOI] [PubMed] [Google Scholar]
- 67.Yilmaz Karapinar D., Karadaş N., Önder Siviş Z., Yazici P., Duyu M., Metin D., Karapinar B., Aydinok Y. Rare Severe Mycotic Infections in Children Receiving Empirical Caspofungin Treatment for Febrile Neutropenia. Braz. J. Infect. Dis. 2015;19:549–552. doi: 10.1016/j.bjid.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menon S., Gupta H.R., Sequeira R., Chavan S., Gholape D., Amandeep S., Bhilave N., Chowdhary A.S. Rhodotorula Glutinis Meningitis: A Case Report and Review of Literature. Mycoses. 2014;57:447–451. doi: 10.1111/myc.12180. [DOI] [PubMed] [Google Scholar]
- 69.Słowik M., Biernat M.M., Urbaniak-Kujda D., Kapelko-Słowik K., Misiuk-Hojło M. Mycotic Infections of the Eye. Adv. Clin. Exp. Med. 2015;24:1113–1117. doi: 10.17219/acem/50572. [DOI] [PubMed] [Google Scholar]
- 70.Rosenberger E., Youssef D.A., Safdar S., Larzo C.R., Myers J. Third Case of Candida dubliniensis Endogenous Endophthalmitis in North America: Case Report and Review of the Literature. Int. Ophthalmol. 2014;34:945–950. doi: 10.1007/s10792-013-9880-x. [DOI] [PubMed] [Google Scholar]
- 71.Augustin V.A., Weller J.M., Kruse F.E., Tourtas T. Fungal Interface Keratitis After Descemet Membrane Endothelial Keratoplasty. Cornea. 2018;37:1366–1369. doi: 10.1097/ICO.0000000000001727. [DOI] [PubMed] [Google Scholar]
- 72.Kamoshita M., Matsumoto Y., Nishimura K., Katono Y., Murata M., Ozawa Y., Shimmura S., Tsubota K. Wickerhamomyces Anomalus Fungal Keratitis Responds to Topical Treatment with Antifungal Micafungin. J. Infect. Chemother. 2015;21:141–143. doi: 10.1016/j.jiac.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 73.Wessel J.M., Bachmann B.O., Meiller R., Kruse F.E. Fungal Interface Keratitis by Candida Orthopsilosis Following Deep Anterior Lamellar Keratoplasty. BMJ Case Rep. 2013;2013:bcr2012008361. doi: 10.1136/bcr-2012-008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lai C.-C., Lee M.-R., Hsiao C.-H., Tan C.-K., Lin S.-H., Liao C.-H., Huang Y.-T., Hsueh P.-R. Infections Caused by Candida lipolytica. J. Infect. 2012;65:372–374. doi: 10.1016/j.jinf.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Saud Al-Abbas A.H., Ling J.L., Muhammed J., Hussein A. Rare Kodamaea Ohmeri Keratitis Following a Trivial Vegetative Trauma. BMJ Case Rep. 2019;12:e229660. doi: 10.1136/bcr-2019-229660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sengupta J., Saha S., Khetan A., Ganguly A., Banerjee D. Candida Fermentati: A Rare Yeast Involved in Fungal Keratitis. Eye Contact Lens. 2013;39:e15–e18. doi: 10.1097/ICL.0b013e318255121f. [DOI] [PubMed] [Google Scholar]
- 77.Parentin F., Liberali T., Perissutti P. Polymicrobial Keratomycosis in a Three-Year-Old Child. Ocul. Immunol. Inflamm. 2006;14:129–131. doi: 10.1080/09273940500328487. [DOI] [PubMed] [Google Scholar]
- 78.Park K.-A., Ahn K., Chung E.-S., Chung T.-Y. Pichia Anomala Fungal Keratitis. Cornea. 2008;27:619–620. doi: 10.1097/ICO.0b013e318166c442. [DOI] [PubMed] [Google Scholar]
- 79.Obrubov A.S., Slonimskii A.Y. Contact lens-related keratitis and purulent corneal ulcers. Vestn. Oftalmol. 2018;134:17–24. doi: 10.17116/oftalma201813404117. [DOI] [PubMed] [Google Scholar]
- 80.Ritterband D.C., Seedor J.A., Shah M.K., Waheed S., Schorr I. A Unique Case of Cryptococcus laurentii Keratitis Spread by a Rigid Gas Permeable Contact Lens in a Patient with Onychomycosis. Cornea. 1998;17:115–118. doi: 10.1097/00003226-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 81.de Castro L.E.F., Sarraf O.A., Lally J.M., Sandoval H.P., Solomon K.D., Vroman D.T. Cryptococcus Albidus Keratitis after Corneal Transplantation. Cornea. 2005;24:882–883. doi: 10.1097/01.ico.0000157404.34774.1a. [DOI] [PubMed] [Google Scholar]
- 82.Huang Y.-H., Lin I.-H., Chang T.-C., Tseng S.-H. Early Diagnosis and Successful Treatment of Cryptococcus albidus Keratitis: A Case Report and Literature Review. Medicine. 2015;94:e885. doi: 10.1097/MD.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bojanović M., Stalević M., Arsić-Arsenijević V., Ignjatović A., Ranđelović M., Golubović M., Živković-Marinkov E., Koraćević G., Stamenković B., Otašević S. Etiology, Predisposing Factors, Clinical Features and Diagnostic Procedure of Otomycosis: A Literature Review. J. Fungi. 2023;9:662. doi: 10.3390/jof9060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cho O., Sugita T. Comprehensive Analysis of the Fungal Microbiota of the Human External Auditory Canal Using Pyrosequencing. Med. Mycol. J. 2017;58:E1–E4. doi: 10.3314/mmj.16-00023. [DOI] [PubMed] [Google Scholar]
- 85.Chai F.C., Auret K., Christiansen K., Yuen P.W., Gardam D. Malignant Otitis Externa Caused by Malassezia sympodialis. Head Neck. 2000;22:87–89. doi: 10.1002/(SICI)1097-0347(200001)22:1<87::AID-HED13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 86.Değerli K., Ecemiş T., Günhan K., Başkesen T., Kal E. Agents of otomycosis in Manisa region, Turkey, 1995–2011. Mikrobiyol. Bul. 2012;46:79–84. [PubMed] [Google Scholar]
- 87.Aboutalebian S., Mahmoudi S., Okhovat A., Khodavaisy S., Mirhendi H. Otomycosis Due to the Rare Fungi Talaromyces Purpurogenus, Naganishia Albida and Filobasidium Magnum. Mycopathologia. 2020;185:569–575. doi: 10.1007/s11046-020-00439-8. [DOI] [PubMed] [Google Scholar]
- 88.Szymańska J., Wójtowicz A., Malm A. Assessment of Candida Spp. Frequency in the Oral Cavity Ontocenosis of Healthy Individuals in Different Age Groups. J. Pre-Clin. Clin. Res. 2016;10:91–94. doi: 10.5604/18982395.1227563. [DOI] [Google Scholar]
- 89.Sangeorzan J.A., Bradley S.F., He X., Zarins L.T., Ridenour G.L., Tiballi R.N., Kauffman C.A. Epidemiology of Oral Candidiasis in HIV-Infected Patients: Colonization, Infection, Treatment, and Emergence of Fluconazole Resistance. Am. J. Med. 1994;97:339–346. doi: 10.1016/0002-9343(94)90300-X. [DOI] [PubMed] [Google Scholar]
- 90.Martino P., Venditti M., Micozzi A., Morace G., Polonelli L., Mantovani M.P., Petti M.C., Burgio V.L., Santini C., Serra P. Blastoschizomyces Capitatus: An Emerging Cause of Invasive Fungal Disease in Leukemia Patients. Rev. Infect. Dis. 1990;12:570–582. doi: 10.1093/clinids/12.4.570. [DOI] [PubMed] [Google Scholar]
- 91.Kaygusuz I., Mulazimoglu L., Cerikcioglu N., Toprak A., Oktay A., Korten V. An Unusual Native Tricuspid Valve Endocarditis Caused by Candida colliculosa. Clin. Microbiol. Infect. 2003;9:319–322. doi: 10.1046/j.1469-0691.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- 92.Shrestha A., Munankarmi R. Candida dubliniensis Tricuspid Valve Endocarditis. Medicine. 2019;72:550–551. [PubMed] [Google Scholar]
- 93.Tran C., Cometta A., Letovanec I., Jaton K., Wenger A., Ruchat P., Jaussi A. Candida dubliniensis in Recurrent Polymicrobial Tricuspid Endocarditis. Echocardiography. 2007;24:756–759. doi: 10.1111/j.1540-8175.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- 94.Carr M.J., Clarke S., O’Connell F., Sullivan D.J., Coleman D.C., O’Connell B. First Reported Case of Endocarditis Caused by Candida dubliniensis. J. Clin. Microbiol. 2005;43:3023–3026. doi: 10.1128/JCM.43.6.3023-3026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang C.-H., Huang M.-M., Yeih D.-F., Lu K.-C., Hou Y.-C. A Chronic Hemodialysis Patient with Isolated Pulmonary Valve Infective Endocarditis Caused by Non-Albicans Candida: A Rare Case and Literature Review. BMC Nephrol. 2017;18:286. doi: 10.1186/s12882-017-0706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chopra T., Bhargava A., Kumar S., Chopra A., Dhar S., Afonso L., Sobel J.D. Candida Kefyr Endocarditis in a Patient with Hypertrophic Obstructive Cardiomyopathy. Am. J. Med. Sci. 2010;339:188–189. doi: 10.1097/MAJ.0b013e3181c0d945. [DOI] [PubMed] [Google Scholar]
- 97.Patel A., Almuti W., Polenakovik H. Acute Limb Ischemia Due to Candida lusitaniae Aortic Valve Endocarditis. Heart Lung. 2014;43:338–340. doi: 10.1016/j.hrtlng.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 98.Hariya A., Naruse Y., Kobayashi T., Endo M., Ikeda Y., Yoshii T., Momomura S., Ishiwata S., Maehara A. Fungal endocarditis found at onset of lower limb acute aortic occlusion; report of a case. Kyobu Geka. 2005;58:831–834. [PubMed] [Google Scholar]
- 99.Ouyang H., Wu X., Zhang J. Giant Vegetation in the Right Ventricle Caused by Staphylococcus aureus and Candida mycoderma. Heart Surg. Forum. 2014;17:E7–E9. doi: 10.1532/HSF98.2013252. [DOI] [PubMed] [Google Scholar]
- 100.Anuradha S., Agarwal S.K., Prakash A., Singh N.P., Kaur R. Candida Sake—A Rare Cause of Fungal Endocarditis. Med. J. Malays. 2008;63:75–76. [PubMed] [Google Scholar]
- 101.Aggarwal N., Pannu H.S., Verma P., Saini D.M. Native Aortic Valve Endocarditis Caused by Candida Sake. J. Heart Valve Dis. 2008;17:194–196. [PubMed] [Google Scholar]
- 102.Whitby S., Madu E.C., Bronze M.S. Case Report: Candida Zeylanoides Infective Endocarditis Complicating Infection with the Human Immunodeficiency Virus. Am. J. Med. Sci. 1996;312:138–139. doi: 10.1016/S0002-9629(15)41781-1. [DOI] [PubMed] [Google Scholar]
- 103.Picard M., Cassaing S., Letocart P., Verdeil X., Protin C., Chauvin P., Iriart X., Cavalié L., Valentin A., Marchou B., et al. Concomitant Cases of Disseminated Geotrichum Clavatum Infections in Patients with Acute Myeloid Leukemia. Leuk. Lymphoma. 2014;55:1186–1188. doi: 10.3109/10428194.2013.820290. [DOI] [PubMed] [Google Scholar]
- 104.Nohinek B., Zee-Cheng C.S., Barnes W.G., Dall L., Gibbs H.R. Infective Endocarditis of a Bicuspid Aortic Valve Caused by Hansenula Anomala. Am. J. Med. 1987;82:165–168. doi: 10.1016/0002-9343(87)90400-1. [DOI] [PubMed] [Google Scholar]
- 105.Sundaram P.S., Bijulal S., Tharakan J.A., Antony M. Kodamaea Ohmeri Tricuspid Valve Endocarditis with Right Ventricular Inflow Obstruction in a Neonate with Structurally Normal Heart. Ann. Pediatr. Cardiol. 2011;4:77–80. doi: 10.4103/0974-2069.79632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ni B., Gu W., Mei Y., Miao K., Zhang S., Shao Y. A Rare Life-Threatening Kodamaea Ohmeri Endocarditis Associated with Hemophagocytic Lymphohistiocytosis. Rev. Esp. Cardiol. 2018;71:51–53. doi: 10.1016/j.recesp.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 107.Shek Y.H., Tucker M.C., Viciana A.L., Manz H.J., Connor D.H. Malassezia Furfur—Disseminated Infection in Premature Infants. Am. J. Clin. Pathol. 1989;92:595–603. doi: 10.1093/ajcp/92.5.595. [DOI] [PubMed] [Google Scholar]
- 108.Alpert G., Bell L.M., Campos J.M. Malassezia Furfur Fungemia in Infancy. Clin. Pediatr. 1987;26:528–531. doi: 10.1177/000992288702601007. [DOI] [PubMed] [Google Scholar]
- 109.Hamal P., Ostransky J., Dendis M., Horváth R., Ruzicka F., Buchta V., Vejsova M., Sauer P., Hejnar P., Raclavsky V. A Case of Endocarditis Caused by the Yeast Pichia Fabianii with Biofilm Production and Developed in Vitro Resistance to Azoles in the Course of Antifungal Treatment. Med. Mycol. 2008;46:601–605. doi: 10.1080/13693780802078180. [DOI] [PubMed] [Google Scholar]
- 110.João I., Duarte J., Cotrim C., Rodrigues A., Martins C., Fazendas P., Oliveira L.M., Diogo J., Carrageta M. Native Valve Endocarditis Due to Pichia Ohmeri. Heart Vessel. 2002;16:260–263. doi: 10.1007/s003800200034. [DOI] [PubMed] [Google Scholar]
- 111.Naveh Y., Friedman A., Merzbach D., Hashman N. Endocarditis Caused by Rhodotorula Successfully Treated with 5-Fluorocytosine. Br. Heart J. 1975;37:101–104. doi: 10.1136/hrt.37.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fiore N.F., Conway J.H., West K.W., Kleiman M.B. Saccharomyces Cerevisiae Infections in Children. Pediatr. Infect. Dis. J. 1998;17:1177–1179. doi: 10.1097/00006454-199812000-00022. [DOI] [PubMed] [Google Scholar]
- 113.Ruiz-Esquide F., Díaz M.C., Wu E., Silva V. Verrucous endocarditis secondary to Saccharomyces cerevisiae. A case report. Rev. Med. Chil. 2002;130:1165–1169. doi: 10.4067/S0034-98872002001000012. [DOI] [PubMed] [Google Scholar]
- 114.Izumi K., Hisata Y., Hazama S. A Rare Case of Infective Endocarditis Complicated by Trichosporon asahii Fungemia Treated by Surgery. Ann. Thorac. Cardiovasc. Surg. 2009;15:350–353. [PubMed] [Google Scholar]
- 115.Reyes C.V., Stanley M.M., Rippon J.W. Trichosporon Beigelii Endocarditis as a Complication of Peritoneovenous Shunt. Hum. Pathol. 1985;16:857–859. doi: 10.1016/S0046-8177(85)80262-8. [DOI] [PubMed] [Google Scholar]
- 116.Brahn E., Leonard P.A. Trichosporon Cutaneum Endocarditis: A Sequela of Intravenous Drug Abuse. Am. J. Clin. Pathol. 1982;78:792–794. doi: 10.1093/ajcp/78.5.792. [DOI] [PubMed] [Google Scholar]
- 117.Desnos-Ollivier M., Letscher-Bru V., Neuvéglise C., Dromer F. Yarrowia Lipolytica Causes Sporadic Cases and Local Outbreaks of Infections and Colonisation. Mycoses. 2020;63:737–745. doi: 10.1111/myc.13095. [DOI] [PubMed] [Google Scholar]
- 118.Virgilio E., Mercantini P., Ferri M., Cavallini M., Teggi A., Ziparo V. Coexistence of Diffuse Malignant Peritoneal Mesothelioma and Candida Norvegensis Peritonitis. Surg. Infect. 2014;15:660–661. doi: 10.1089/sur.2014.088. [DOI] [PubMed] [Google Scholar]
- 119.Sandven P., Nilsen K., Digranes A., Tjade T., Lassen J. Candida Norvegensis: A Fluconazole-Resistant Species. Antimicrob. Agents Chemother. 1997;41:1375–1376. doi: 10.1128/AAC.41.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Al Bshabshe A., Joseph M.R.P., Battayah E.S., Hamid M.E. Fungal Peritonitis Caused by Pichia Kudriavzevii Following Sleeve Gastrectomy. Ann. Saudi Med. 2019;39:205–208. doi: 10.5144/0256-4947.2019.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bulat O., Bulat C., Blaj M., Lupusoru I., Scripcariu V. A Rare Colonization in Peritoneum After Blunt Abdominal Trauma: S. putrefaciens and S. cerevisiae. Balkan Med. J. 2018;35:333–335. doi: 10.4274/balkanmedj.2017.0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Popiel K.Y., Wong P., Lee M.J., Langelier M., Sheppard D.C., Vinh D.C. Invasive Saccharomyces Cerevisiae in a Liver Transplant Patient: Case Report and Review of Infection in Transplant Recipients. Transpl. Infect. Dis. 2015;17:435–441. doi: 10.1111/tid.12384. [DOI] [PubMed] [Google Scholar]
- 123.Canafax D.M., Mann H.J., Dougherty S.H. Postoperative Peritonitis Due to Saccharomyces Cerivisiae—Treated with Ketoconazole. Drug Intell. Clin. Pharm. 1982;16:698–699. doi: 10.1177/106002808201600911. [DOI] [PubMed] [Google Scholar]
- 124.Dougherty S.H., Simmons R.L. Postoperative Peritonitis Caused by Saccharomyces Cerevisiae. Arch. Surg. 1982;117:248. doi: 10.1001/archsurg.1982.01380260114019. [DOI] [PubMed] [Google Scholar]
- 125.Bariş A., Gencer H., Dalgiç N., Şik G., Özakkaş F., Aktaş E. Magnusiomyces Capitatus Peritonitis in a Child with Acute Lymphocytic Leukemia as a Breakthrough Infection During Caspofungin Therapy. Pediatr. Infect. Dis. J. 2017;36:e351–e353. doi: 10.1097/INF.0000000000001720. [DOI] [PubMed] [Google Scholar]
- 126.Camus V., Thibault M.-L., David M., Gargala G., Compagnon P., Lamoureux F., Girault C., Michot J.-B., Stamatoullas A., Lanic H., et al. Invasive Geotrichum Clavatum Fungal Infection in an Acute Myeloid Leukaemia Patient: A Case Report and Review. Mycopathologia. 2014;177:319–324. doi: 10.1007/s11046-014-9746-4. [DOI] [PubMed] [Google Scholar]
- 127.D’Assumpcao C., Lee B., Heidari A. A Case of Magnusiomyces Capitatus Peritonitis Without Underlying Malignancies. J. Investig. Med. High Impact. Case Rep. 2018;6:2324709618795268. doi: 10.1177/2324709618795268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leuck A.-M., Rothenberger M.K., Green J.S. Fungemia Due to Lachancea Fermentati: A Case Report. BMC Infect. Dis. 2014;14:250. doi: 10.1186/1471-2334-14-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Imashioya T., Kodama Y., Ooka T., Nakagawa S., Nishikawa T., Tanabe T., Okamoto Y., Imuta N., Kirishima M., Tanimoto A., et al. Liver Abscess Due to Sterigmatomyces Halophilus in a Boy with Acute Lymphoblastic Leukemia. J. Infect. Chemother. 2019;25:1047–1049. doi: 10.1016/j.jiac.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 130.Kauffman C.A., Vazquez J.A., Sobel J.D., Gallis H.A., McKinsey D.S., Karchmer A.W., Sugar A.M., Sharkey P.K., Wise G.J., Mangi R., et al. Prospective Multicenter Surveillance Study of Funguria in Hospitalized Patients. The National Institute for Allergy and Infectious Diseases (NIAID) Mycoses Study Group. Clin. Infect. Dis. 2000;30:14–18. doi: 10.1086/313583. [DOI] [PubMed] [Google Scholar]
- 131.Yashavanth R., Shiju M.P., Bhaskar U.A., Ronald R., Anita K.B. Candiduria: Prevalence and Trends in Antifungal Susceptibility in a Tertiary Care Hospital of Mangalore. J. Clin. Diagn. Res. 2013;7:2459–2461. doi: 10.7860/JCDR/2013/6298.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sobel J.D., Fisher J.F., Kauffman C.A., Newman C.A. Candida Urinary Tract Infections—Epidemiology. Clin. Infect. Dis. 2011;52((Suppl. S6)):S433–S436. doi: 10.1093/cid/cir109. [DOI] [PubMed] [Google Scholar]
- 133.Acampora N., Frizza A., Brau F., Torelli R., Vella A., De Carolis E., Fantoni M. A Case of Trichosporon Asahii Urinary Tract Infection in a Frail Elderly Patient. Infez. Med. 2019;27:93–96. [PubMed] [Google Scholar]
- 134.Goyal R., Das S., Arora A., Aggarwal A. Rhodotorula Mucilaginosa as a Cause of Persistent Femoral Nonunion. J. Postgrad. Med. 2008;54:25–27. doi: 10.4103/0022-3859.39186. [DOI] [PubMed] [Google Scholar]
- 135.Savini V., Sozio F., Catavitello C., Talia M., Manna A., Febbo F., Balbinot A., Di Bonaventura G., Piccolomini R., Parruti G., et al. Femoral Prosthesis Infection by Rhodotorula mucilaginosa. J. Clin. Microbiol. 2008;46:3544–3545. doi: 10.1128/JCM.00873-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cutrona A.F., Shah M., Himes M.S., Miladore M.A. Rhodotorula Minuta: An Unusual Fungal Infection in Hip-Joint Prosthesis. Am. J. Orthop. Belle Mead NJ. 2002;31:137–140. [PubMed] [Google Scholar]
- 137.Thanos L., Mylona S., Kokkinaki A., Pomoni M., Tsiouris S., Batakis N. Multifocal Skeletal Tuberculosis with Rhodotorula Minuta Co-Infection. Scand J. Infect. Dis. 2006;38:309–311. doi: 10.1080/00365540500372648. [DOI] [PubMed] [Google Scholar]
- 138.Seng P., Cerlier A., Cassagne C., Coulange M., Legré R., Stein A. Saccharomyces Cerevisiae Osteomyelitis in an Immunocompetent Baker. IDCases. 2016;5:1–3. doi: 10.1016/j.idcr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hovi L., Saarinen U.M., Donner U., Lindqvist C. Opportunistic Osteomyelitis in the Jaws of Children on Immunosuppressive Chemotherapy. J. Pediatr. Hematol. Oncol. 1996;18:90–94. doi: 10.1097/00043426-199602000-00018. [DOI] [PubMed] [Google Scholar]
- 140.Akram S.M., Koirala J. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Cutaneous Cryptococcus. [Google Scholar]
- 141.Kim M.S., Lee S.M., Sung H.S., Won C.H., Chang S., Lee M.W., Choi J.-H., Moon K.-C. Clinical Analysis of Deep Cutaneous Mycoses: A 12-Year Experience at a Single Institution. Mycoses. 2012;55:501–506. doi: 10.1111/j.1439-0507.2012.02191.x. [DOI] [PubMed] [Google Scholar]
- 142.Fang S.-Y., Wei K.-C., Chen W.-C., Lee S.-J., Yang K.-C., Wu C.-S., Sun P.-L. Primary Deep Cutaneous Candidiasis Caused by Candida Duobushaemulonii in a 68-Year-Old Man: The First Case Report and Literature Review. Mycoses. 2016;59:818–821. doi: 10.1111/myc.12540. [DOI] [PubMed] [Google Scholar]
- 143.Roberts S.C., Zembower T.R., Bolon M.K., Kadakia A.R., Gilley J.H., Ko J.H., Clark J., Ward-Fore S., Taiwo B.O. Successful Treatment of a Candida auris Intra-Articular Infection. Emerg. Microbes Infect. 2019;8:866–868. doi: 10.1080/22221751.2019.1625287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Belkin A., Gazit Z., Keller N., Ben-Ami R., Wieder-Finesod A., Novikov A., Rahav G., Brosh-Nissimov T. Candida auris Infection Leading to Nosocomial Transmission, Israel, 2017. Emerg. Infect. Dis. 2018;24:801–804. doi: 10.3201/eid2404.171715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dubé M.P., Heseltine P.N., Rinaldi M.G., Evans S., Zawacki B. Fungemia and Colonization with Nystatin-Resistant Candida Rugosa in a Burn Unit. Clin. Infect. Dis. 1994;18:77–82. doi: 10.1093/clinids/18.1.77. [DOI] [PubMed] [Google Scholar]
- 146.Piérard G.E., Read D., Piérard-Franchimont C., Lother Y., Rurangirwa A., Arrese Estrada J. Cutaneous Manifestations in Systemic Trichosporonosis. Clin. Exp. Dermatol. 1992;17:79–82. doi: 10.1111/j.1365-2230.1992.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 147.Chen B., Zhu L., Xuan X., Wu L., Zhou T., Zhang X., Li B. Isolation of Both Pseudozyma Aphidis and Nocardia Otitidiscaviarum from a Mycetoma on the Leg. Int. J. Dermatol. 2011;50:714–719. doi: 10.1111/j.1365-4632.2010.04814.x. [DOI] [PubMed] [Google Scholar]
- 148.Rieth H. Candida Guilliermondii as an accompanying germ of Madurella mycetomi. Settling of yeast colonies in the depressions of the uneven black mycetoma plaques. Mykosen. 1970;13:315–316. doi: 10.1111/j.1439-0507.1970.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 149.Achkar J.M., Fries B.C. Candida Infections of the Genitourinary Tract. Clin. Microbiol. Rev. 2010;23:253–273. doi: 10.1128/CMR.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Okmen F., Ekici H., Ari S.A. Case Report of a Tubo-Ovarian Abscess Caused by Candida kefyr. J. Obstet. Gynaecol. Can. 2018;40:1466–1467. doi: 10.1016/j.jogc.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 151.Chan R.M., Lee P., Wroblewski J. Deep-Seated Trichosporonosis in an Immunocompetent Patient: A Case Report of Uterine Trichosporonosis. Clin. Infect. Dis. 2000;31:621. doi: 10.1086/313968. [DOI] [PubMed] [Google Scholar]
- 152.Iskit S., Ilkit M., Turç-Biçer A., Demirhindi H., Türker M. Effect of Circumcision on Genital Colonization of Malassezia Spp. in a Pediatric Population. Med. Mycol. 2006;44:113–117. doi: 10.1080/13693780500225919. [DOI] [PubMed] [Google Scholar]
- 153.Aridoğan I.A., Ilkit M., Izol V., Ates A. Malassezia and Candida Colonisation on Glans Penis of Circumcised Men. Mycoses. 2005;48:352–356. doi: 10.1111/j.1439-0507.2005.01144.x. [DOI] [PubMed] [Google Scholar]
- 154.Eng R.H., Drehmel R., Smith S.M., Goldstein E.J. Saccharomyces Cerevisiae Infections in Man. Sabouraudia. 1984;22:403–407. doi: 10.1080/00362178485380651. [DOI] [PubMed] [Google Scholar]
- 155.Mahul P., Piens M.A., Guyotat D., Godard J., Archimbaud E., Bui-Xuan B., Motin J. Disseminated Geotrichum Capitatum Infection in a Patient with Acute Myeloid Leukemia. Mycoses. 1989;32:573–577. doi: 10.1111/j.1439-0507.1989.tb02184.x. [DOI] [PubMed] [Google Scholar]
- 156.McManners J., Samaranayake L.P. Suppurative Oral Candidosis. Review of the Literature and Report of a Case. Int. J. Oral. Maxillofac. Surg. 1990;19:257–259. doi: 10.1016/S0901-5027(05)80413-8. [DOI] [PubMed] [Google Scholar]
- 157.Oelz O., Schaffner A., Frick P., Schaer G. Trichosporon Capitatum: Thrush-like Oral Infection, Local Invasion, Fungaemia and Metastatic Abscess Formation in a Leukaemic Patient. J. Infect. 1983;6:183–185. doi: 10.1016/S0163-4453(83)92942-0. [DOI] [PubMed] [Google Scholar]
- 158.Bertini B., Kuttin E.S., Beemer A.M. Cytopathology of Nipple Discharge Due to Pityrosporum Orbiculare and Cocci in an Elderly Woman. Acta Cytol. 1975;19:38–42. [PubMed] [Google Scholar]
- 159.Reddy B.T., Torres H.A., Kontoyiannis D.P. Breast Implant Infection Caused by Trichosporon beigelii. Scand. J. Infect. Dis. 2002;34:143–144. doi: 10.1080/00365540110026895. [DOI] [PubMed] [Google Scholar]
- 160.Dinleyici M., Pérez-Brocal V., Arslanoglu S., Aydemir O., Ozumut S.S., Tekin N., Vandenplas Y., Moya A., Dinleyici E.C. Human Milk Mycobiota Composition: Relationship with Gestational Age, Delivery Mode, and Birth Weight. Benef. Microbes. 2020;11:151–162. doi: 10.3920/BM2019.0158. [DOI] [PubMed] [Google Scholar]
- 161.Heisel T., Nyaribo L., Sadowsky M.J., Gale C.A. Breastmilk and NICU Surfaces Are Potential Sources of Fungi for Infant Mycobiomes. Fungal Genet. Biol. 2019;128:29–35. doi: 10.1016/j.fgb.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Amezcua López J.A., Solís Pacheco J.R., García Morales E., Gutiérrez Padilla J.A., Zepeda Morales A.S.M., Angulo Castellanos E., López Mincitar M., Flores Arévalo K.F., Rodríguez Arreola A., Aguilar Uscanga B.R. Influence of the diet of Mexican women on the nutritional quality and the presence of beneficial microorganisms in human milk. Nutr. Hosp. 2019;36:1139–1149. doi: 10.20960/nh.02477. [DOI] [PubMed] [Google Scholar]
- 163.Pineda C., Kaushik A., Kest H., Wickes B., Zauk A. Maternal Sepsis, Chorioamnionitis, and Congenital Candida Kefyr Infection in Premature Twins. Pediatr. Infect. Dis. J. 2012;31:320–322. doi: 10.1097/INF.0b013e31823eee1a. [DOI] [PubMed] [Google Scholar]
- 164.Huang M., Cham E.M., Eppes C.S., Gerber S.E., Reed K.D., Ernst L.M. Placental and Fetal Findings in Intrauterine Candida Lusitaniae Infection Following In Vitro Fertilization and Embryo Transfer. Pediatr. Dev. Pathol. 2012;15:127–131. doi: 10.2350/11-04-1019-CR.1. [DOI] [PubMed] [Google Scholar]
- 165.DiLorenzo D.J., Wong G., Ludmir J. Candida Lusitaniae Chorioamnionitis in a Bone Marrow Transplant Patient. Obstet. Gynecol. 1997;90:702–703. doi: 10.1016/S0029-7844(97)00304-9. [DOI] [PubMed] [Google Scholar]
- 166.Lager S., de Goffau M.C., Sovio U., Peacock S.J., Parkhill J., Charnock-Jones D.S., Smith G.C.S. Detecting Eukaryotic Microbiota with Single-Cell Sensitivity in Human Tissue. Microbiome. 2018;6:151. doi: 10.1186/s40168-018-0529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. [(accessed on 8 November 2022)]. Available online: https://www.who.int/publications-detail-redirect/9789240060241.
- 168.Cots J.S., Thygeson P., Waisman M. Observations on Pityrosporum Ovale in Seborrheic Blepharitis and Conjunctivitis. Am. J. Ophthalmol. 1947;30:1485–1494. doi: 10.1016/s0002-9394(47)91854-0. [DOI] [PubMed] [Google Scholar]
- 169.Brito-Santos F., Figueiredo-Carvalho M.H.G., Coelho R.A., de Oliveira J.C.A., Monteiro R.V., da Silva Chaves A.L., Almeida-Paes R. Molecular Identification and Antifungal Susceptibility Testing of Pucciniomycotina Red Yeast Clinical Isolates from Rio de Janeiro, Brazil. Braz. J. Microbiol. 2020;51:95–98. doi: 10.1007/s42770-019-00191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tattevin P., Revest M., Lefort A., Michelet C., Lortholary O. Fungal Endocarditis: Current Challenges. Int. J. Antimicrob. Agents. 2014;44:290–294. doi: 10.1016/j.ijantimicag.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 171.Mulè A., Rossini F., Sollima A., Lenzi A., Fumarola B., Amadasi S., Chiari E., Lorenzotti S., Saccani B., Van Hauwermeiren E., et al. Trichosporon Asahii Infective Endocarditis of Prosthetic Valve: A Case Report and Literature Review. Antibiotics. 2023;12:1181. doi: 10.3390/antibiotics12071181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wickes B.L., Wiederhold N.P. Molecular Diagnostics in Medical Mycology. Nat. Commun. 2018;9:5135. doi: 10.1038/s41467-018-07556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article.