Abstract
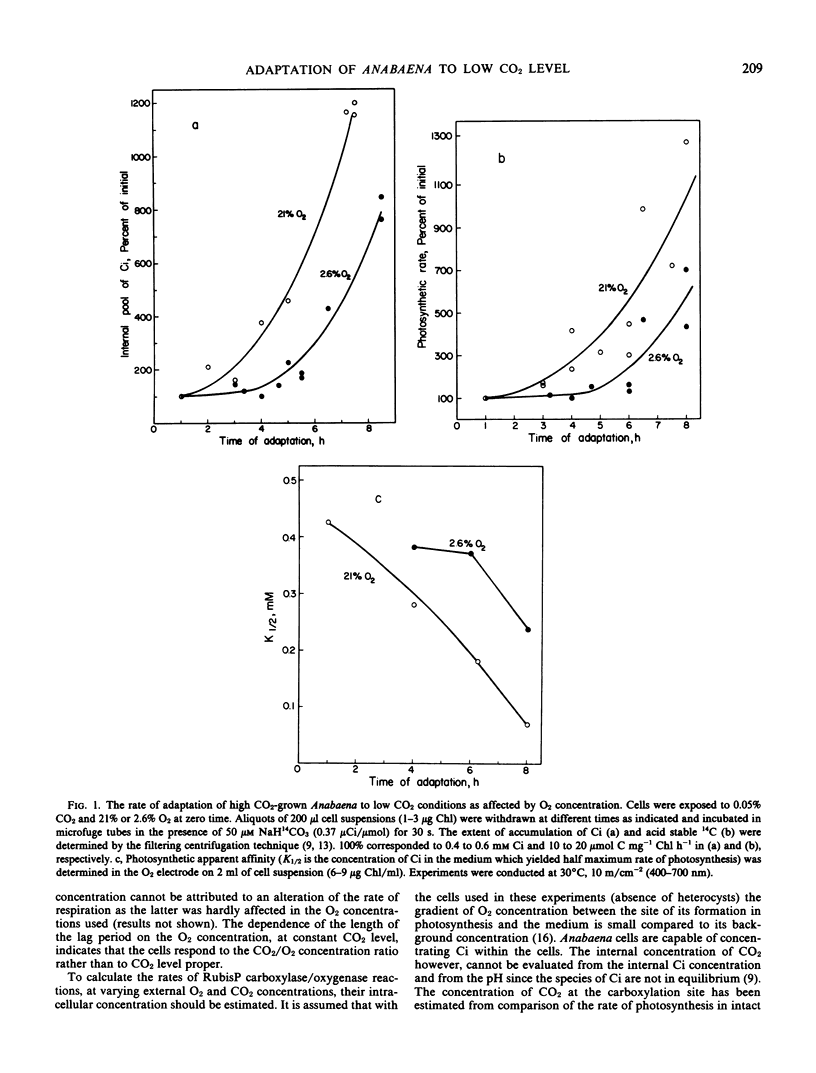
The rate of adaptation of high CO2 (5% v/v CO2 in air)-grown Anabaena to a low level of CO2 (0.05% v/v in air) was determined as a function of O2 concentration. Exposure of cells to low (2.6%) O2 concentration resulted in an extended lag in the adaptation to low CO2 concentration. The rate of adaptation following the lag was not affected by the concentration of O2. The length of the lag period is markedly affected by the O2/CO2 concentration ratio, indicating that the signal for adaptation to low CO2 may be related to the relative rate of ribulose-1,5-bisphosphate carboxylase/oxygenase activities, rather than to CO2 concentration proper. This suggestion is supported by the observed accumulation of phosphoglycolate following transfer of cells from high to low CO2 concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R. Kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase from Anabaena variabilis. Arch Biochem Biophys. 1980 Apr 15;201(1):247–254. doi: 10.1016/0003-9861(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Birmingham B. C., Colman B. Measurement of carbon dioxide compensation points of freshwater algae. Plant Physiol. 1979 Nov;64(5):892–895. doi: 10.1104/pp.64.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Berry J. A. Glycolate Excretion and the Oxygen to Carbon Dioxide Net Exchange Ratio during Photosynthesis in Chlamydomonas reinhardtii. Plant Physiol. 1981 Feb;67(2):229–232. doi: 10.1104/pp.67.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Zenvirth D., Reinhold L., Berry J. A. Involvement of a Primary Electrogenic Pump in the Mechanism for HCO(3) Uptake by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1982 Apr;69(4):978–982. doi: 10.1104/pp.69.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Zenvirth D., Harel E., Kaplan A. Induction of HCO(3) Transporting Capability and High Photosynthetic Affinity to Inorganic Carbon by Low Concentration of CO(2) in Anabaena variabilis. Plant Physiol. 1982 May;69(5):1008–1012. doi: 10.1104/pp.69.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. A. Nutrient transport in microalgae. Adv Microb Physiol. 1980;21:47–226. doi: 10.1016/s0065-2911(08)60356-2. [DOI] [PubMed] [Google Scholar]
- Shelp B. J., Canvin D. T. Photorespiration in Air and High CO(2)-Grown Chlorella pyrenoidosa. Plant Physiol. 1981 Dec;68(6):1500–1503. doi: 10.1104/pp.68.6.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]


