Abstract
Background and Aims
Herein we analysed the influence of early life factors, including breast milk composition, on the development of the intestinal microbiota of infants born to mothers with and without IBD.
Methods
The MECONIUM [Exploring MEChanisms Of disease traNsmission In Utero through the Microbiome] study is a prospective cohort study consisting of pregnant women with or without IBD and their infants. Longitudinal stool samples were collected from babies and analysed using 16s rRNA sequencing and faecal calprotectin. Breast milk proteomics was profiled using Olink inflammation panel.
Results
We analysed gut microbiota of 1034 faecal samples from 294 infants [80 born to mothers with and 214 to mothers without IBD]. Alpha diversity was driven by maternal IBD status and time point. The major influencers of the overall composition of the microbiota were mode of delivery, feeding, and maternal IBD status. Specific taxa were associated with these exposures, and maternal IBD was associated with a reduction in Bifidobacterium. In 312 breast milk samples [91 from mothers with IBD], mothers with IBD displayed lower abundance of proteins involved in immune regulation, such as thymic stromal lymphopoietin, interleukin-12 subunit beta, tumour necrosis factor-beta, and C-C motif chemokine 20, as compared with control mothers [adjusted p = 0.0016, 0.049, 0.049, and 0.049, respectively], with negative correlations with baby´s calprotectin, and microbiome at different time points.
Conclusion
Maternal IBD diagnosis influences microbiota in their offspring during early life. The proteomic profile of breast milk of women with IBD differs from that of women without IBD, with distinct time-dependent associations with baby’s gut microbiome and feacal calprotectin.
Keywords: Early life, breast milk, microbiota, IBD
1. Introduction
The early life period, including prenatal development, is a crucial period for gut microbial acquisition and colonisation, driving immune system development and maturation, with long-lasting consequences on immune homeostasis, influencing susceptibility to diseases.1 During early life, microbiome communities are most sensitive to external factors such as mode of delivery, breastfeeding, diet, and exposure to antibiotics, among others.2–4 Besides, data show that the gut microbiome during pregnancy is the largest contributor of infant-acquired bacterial strains,5 implying that maternal microbiota can influence baby´s microbiome composition and therefore immune development.6,7
Inflammatory bowel diseases [IBD] are associated with a dysregulation of the intestinal microbiota, which may be transmitted to the offspring of pregnant patients with IBD.8 The MECONIUM [Exploring MEChanisms Of disease traNsmission In Utero through the Microbiome] study is a prospective cohort comprising pregnant women with or without IBD and their offspring.8 Initial findings have shown that babies born to mothers with IBD have higher faecal calprotectin levels compared with controls,9 and display lower bacterial diversity and more pro-inflammatory gut microbiota composition during the first 3 months of life, resulting in the development of an unbalanced immune system in germ-free mice colonised with their stool.8 Here, we aimed to explore the longer-term impact of maternal IBD status, alongside other important environmental and IBD-related exposures, on the microbiome composition of the offspring during the first 2 years of life. Given the importance of breastfeeding for microbiome and immune system development, we also aimed to assess the impact of maternal IBD status in breast milk proteomics.
2. Matherials and Methods
2.1. Study design
The MECONIUM cohort has been previously described .8 Briefly, pregnant women with a confirmed diagnosis of IBD, and age- and race-matched controls, were recruited between March 2015 and August 2018. Following delivery, mothers were asked to donate serial stool samples, as previously described.8 For the analysis presented here, samples obtained at Days 30, 60, and 90, and at 12, 18, and 24 months, were analysed for microbiome, and there were also samples obtained at 36 and 48 months with faecal calprotectin data available. Women were also asked to donate a breast milk sample at around 2–3 weeks postpartum. To be eligible for analysis it was not mandatory that all babies had donated samples throughout all time points. For each woman, demographics and relevant medical and obstetric history were collected; for women with IBD, a detailed clinical history was obtained, including disease phenotype,10 surgical history, medications, and disease activity at baseline and during pregnancy.11,12 Additional information was collected on the mother and newborn, including gestational age, mode of delivery, and baby’s sex. Neonates were considered preterm if delivery occurred at < 37 weeks of gestational age. Peripartum antibiotic usage [immediately before or during delivery] was recorded. Detailed information on infant’s feeding type, antibiotic use, and medical problems during follow-up was prospectively obtained at the time of sampling. Feeding practices were assessed at each time point and classified as exclusive formula feeding, exclusive breastfeeding, or mixed if both formula and breast milk feeding were used. For analysis purposes, feeding type and antibiotic usage were coded in a cumulative way, where prior exposures to formula feeding or antibiotics were carried forward in the next time point.
2.2. Sample collection and processing
Infant stool samples were shipped overnight to Mount Sinai on ice packs and stored at -20°C until aliquoted and transferred to -80°C within 48 h. Breast milk samples were collected in sterile tubes, shipped overnight on ice packs, and stored at -80°C.
2.2.1. 16S rRNA sequencing
Samples covering the first 2 years of life were sequenced for microbiota analysis. Total DNA was isolated from each stool specimen using a bead-beating method with the PowerSoil DNA Isolation Kit, following manufacturer’s protocol [Mobio, Carlsbad, CA]. Bacterial DNA isolation was performed as described previously.8 Dual-barcoded universal primers 347F/803R targeting the V3-V4 region of bacterial 16S rRNA gene were used for DNA amplification. Characterisation of the intestinal microbiota was performed with 16S rRNA paired-end sequencing with Illumina HiSeq2500 platform, using the fast-mode pair-end 250 protocol. Demultiplexing was done with fastq-multx from ea-utils. Demultiplexed fastq sequences were used in DADA2 pipeline. Taxonomic assignment was done in DADA2 with Silva v132 classifier. Amplicon sequence variant [ASV] table was rarefied to 5000 reads.
2.2.2. Faecal calprotectin
Faecal calprotectin [FC] concentration was measured by CALPROLAB™ Calprotectin ELISA kit [CALPRO AS, Lysaker, Norway] as previously described.9 The concentration of the biomarker was calculated using calibration curve that was built with calprotectin standards using GraphPad Prism 8.1.1 [GraphPad Software, San Diego, USA]. Values are presented as micrograms per gram.
2.2.3. Breast milk proteomics
Breast milk samples were analysed with an OLINK Proseek® multiplex assay, a proximity extension assay [PEA] technology with oligonucleotide-labelled antibody probe pairs that bind to their respective targets.13,14 Upon binding of antibody pairs to their respective targets, DNA reporters bound to the antibodies gave rise to new DNA amplicons with each ID-barcoding their respective antigens. The amplicons were sequentially quantified using a Fluidigm BioMarkTM HD real-time PCR platform. Breast milk samples were analysed using inflammation multiplex panel, providing a high-throughput, multiplex immunoassay enabling analysis of 92 inflammation-related protein biomarkers simultaneously.15
2.2.4. Statistical analysis
Participant characteristics were compared between maternal IBD groups using t test for continuous variables and chi square test for categorical variables.
2.2.4.1. Gut microbiota analysis
Microbiota richness [α-diversity] was estimated using the Simpson´s and Shannon Index of Diversity. Kruskal–Wallis test with post hoc Dunn test was used to estimate the difference in α-diversity and faecal calprotectin between the different time points. Additionally, a linear mixed model [lmer function, lme4 package] was used to infer the influence of the variables of interest on the alpha diversity dynamics. Time point was added to the model as a fixed effect and infant identifier as a random effect. Spearman correlation analysis was used to detect the association between alpha-diversity and faecal calprotectin at different time points.
Β-diversity [Bray–Curtis dissimilarity] was visualised with principal coordinates analysis plots. To identify which variables were associated with intestinal microbiota abundance or β-diversity [Bray–Curtis dissimilarity] over the first 2 years of life, permutational multivariate analysis of variance [PERMANOVA] were used as implemented in adonis2 function from vegan package version 2.5-7. R2 statistics were used to describe the variance explained by different variables. Sex, pre-term birth, delivery mode, feeding type [exclusive breastfeeding, exclusive formula feeding, or mixed feeding], antibiotic exposure, and maternal IBD diagnosis (control, Crohn’s disease [CD], or ulcerative colitis [UC]) were selected a priori as covariates. Βeta diversity was measured in the overall population, and thereafter the same variables were tested in a stratified analysis looking at babies born to control and to IBD mothers separately.
To further assess the effect of early life exposures and maternal IBD diagnosis on the relative abundance of particular bacterial taxa, multivariate analysis as implemented in MaAsLin2 R package [Maaslin2_0.99.1] was conducted.16 Analyses were conducted in all infants first, and thereafter were restricted to babies born to IBD mothers.
2.2.4.2. Breast milk proteomics analysis
Wilcoxon rank sum test was used to compare the abundance of proteins in breast milk of mothers with and without IBD. False-discovery rate [FDR] was used for multiple testing correction.17 Spearman correlation analysis was conducted between significantly different breast milk proteins and faecal calprotectin and microbiome. MaAsLin2 was used for multivariate analysis. Adjusted p <0.05 was considered statistically significant, except for MaAsLin2 [threshold for significance <0.25]. R statistical software [R version 3.6.0] was used.
3. Results
3.1. Participant characteristics
The main clinical features of the study participants with available microbiome [16S] data are described in Table 1.
Table 1.
Characteristics of infants stratified by maternal IBD status.
| Infants born to IBD mothers | Infants born to control mothers | p | |
|---|---|---|---|
| N | 80 | 216 | |
| Mother with CD/UC | 43/37 | - | |
| Baby´s gender male | 57.5% | 47.2% | 0.2 |
| Maternal age at delivery | 32.2 ± 3.8 | 32.8 ± 3.6 | 0.33 |
| Birthweight [kg] | 3.36 ± 0.58 | 3.42 ± 0.56 | 0.44 |
| Mean gestational age at delivery [weeks] | 39.2 ± 1.7 | 39.4 ± 1.7 | 0.30 |
| Delivery by caesarean section | 45.0% | 25.2% | 0.002 |
| Low birthweight | 5.0% | 3.7% | 0.88 |
| Preterm birth [<37 weeks] | 5.0% | 4.2% | 0,26 |
| NICU stay | 5.0% | 8.4% | 0.59 |
| Exclusive breastfeeding | 38% | 59% | <0.001 |
| Exclusive formula feeding | 10% | 2% | |
| Mixed feeding | 52% | 39% | |
| Exposure to antibiotics in the peripartum period | 53.3% | 39% | 0.09 |
| Exposure to antibiotics up to 3 months of life | 11% | 12% | 0.80 |
| Exposure to antibiotics up to 1st year of life | 34% | 34% | 0.99 |
| Exposure to antibiotics up to 18 months of life | 44% | 42% | 0.79 |
| Exposure to antibiotics up to 2nd year of life | 50% | 45% | 0.47 |
Feeding and antibiotic are categorised at the end of the participant follow-up in a cumulative way.
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; NICU, neonatal intensive care.
Babies born to IBD mothers were more frequently delivered by caesarean section [45% versus 25%, p = 0.002], and were less frequently exclusively breastfed [38% versus 59%, p <0.001]. There were no significant differences between IBD mothers and controls except for a higher proportion of participants of Jewish ancestry among IBD patients [37.2% versus 13.6%, p <0.001] [Supplementary Table S1]. Active disease during pregnancy was observed in 28.7% of the mothers with IBD and was mild in most cases. In the offspring, there were no significant differences in terms of gender distribution, mean gestational age at delivery, birthweight, need for neonatal intensive care, or antibiotic exposure. Among babies born to IBD mothers, 43 were born to mothers with CD and 37 to mothers with UC. The main features of case mothers and their babies, stratified by maternal IBD type, are displayed in Supplementary Table S2. Among mothers with IBD [Supplementary Table S2], those with CD had a more frequent history of surgery [30% versus 0%, p <0.001] and higher use of biologics as compared with those with UC [51% versus 32%, p = 0.047] Mothers with UC were more frequently treated with aminosalicylates [57% versus 21%, p = 0.001] and immunomodulators [30% versus 14%, p = 0.08] and were more likely to report active disease during pregnancy [41% versus 19%, p = 0.02]. More babies born to mothers with CD were born prematurely [9% versus 0%, p = 0.04] [Supplementary Table S2]. Otherwise, there were no other significant differences between the CD and UC groups. No baby developed IBD during follow-up.
3.2. Microbiota diversity
Given the prospective nature of the study, differing numbers of samples were available at various time points, as represented in Figure 1 and described in Supplementary Table S3. At the time of data analysis, there was a total of 294 infants from whom longitudinal stool samples were available for 16S sequencing analysis [80 born to mothers with IBD and 214 born to control mothers]. Overall, there were 1034 stool samples subjected to 16S sequencing, 276 from babies born to IBD mothers [Supplementary Table S3]. Of these, 290 babies had at least one measurement of faecal calprotectin available for up to 4 years of life.
Figure 1.
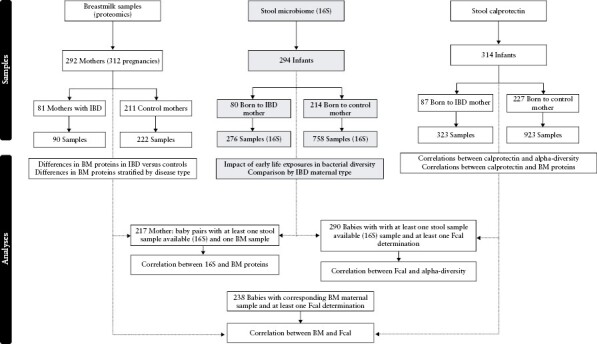
Participant and sample distribution. The core study was based on babies with 16S sequencing data available; to maximise sample number, breast milk samples from ongoing sample collection with available Olink proteomics data were included; faecal calprotectin samples from infants were included only if there was at least one sample with 16S available or a corresponding breast milk sample available to perform correlation analysis.
We started by looking into microbial diversity measures such as the α- and β-diversity. The bacterial α-diversity in infants significantly increased during the first 3 months of life and at later time points [Shannon and Simpson’s Diversity Index, both Kruskal–Wallis test p <0.001 Supplementary Table S4, Supplementary Figure S1]. The increase in bacterial diversity was accompanied by a decrease in faecal calprotectin [Spearman’s correlation rho = −0.22; p = 1.48e−10 [Supplementary Table S5, Supplementary Figure S2 and S3]. In the linear mixed model, both timepoint and maternal IBD status were significantly associated with the α-diversity dynamics [timepoint p = 5.57e−16, maternal diagnosis of IBD p = 0.03, Supplementary Figure S4]. Time point was also a main driver of the β-diversity [Supplementary Figure S5, Adonis p = 0.001].
We then looked into the contributions of offspring sex, maternal IBD diagnosis, gestational age, delivery mode, feeding behaviour, and antibiotics use to the β-diversity or overall microbiota composition during the first 2 years of life [Figure 2]. The variation of the intestinal microbiota composition was mainly explained by mode of delivery [caesarean section or vaginal delivery], feeding type [breastfeeding or formula feeding], and maternal IBD diagnosis [Supplementary Table S6, Supplementary Figures S6–S11]. In the first month, the intestinal microbiota composition was mainly driven by the mode of delivery [R2 = 0.035 p = 0.001], feeding type [R2 = 0.019, p = 0.001], and diagnosis of IBD in the mother [R2 = 0.011, p = 0.007]. Maternal IBD diagnosis was also a main driver of the intestinal microbiota composition of the infants at Month 2 [R2 = 0.014, p = 0.004], Month 3 [R2 = 0.009, p = 0.045], and Month 24 [R2 = 0.044, p = 0.041]. Mode of delivery was significantly associated with intestinal microbiota composition in Month 2 [R2 = 0.026, p = 0.001] and Month 3 [R2 = 0.030, p = 0.001] of life. The gender of the baby, antibiotic exposure, and preterm birth were not significantly associated with intestinal microbiome β-diversity.
Figure 2.
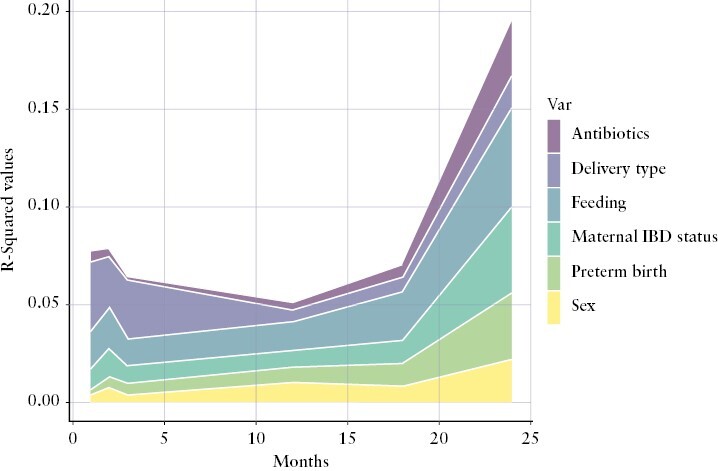
Early-life factors associated with β-diversity [Bray–Curtis dissimilarity] variation at different time points. R2 statistics were used to describe the variance explained by different variables. Variables are aligned with the graph colour-coding (sex is the bottom variabble, followed by preterm birth, maternal IBD status, feeding, delivery type and antibiotics in the top.)
We repeated these analyses by looking separately at control babies and babies born to IBD mothers, observing similar results [Supplementary Table S6].
3.3. The impact of early life exposures on the microbial taxa in the offspring
We next interrogated which specific bacterial taxa were associated with given early life exposures in all infants. Multivariate analysis [MaAsLin] with linear mixed model including feeding type, time point, IBD type, and delivery mode showed a lower abundance of Bifidobacterium in babies born to mothers with IBD [MaAsLin2 p = 0.02]. A higher abundance of Bacteroides and Parabacteroides was observed in babies born vaginally [MaAsLin2 p = 2 × 10−6 and 1.3 × 10−4, respectively] and higher abundance of Enterococcus was observed in babies born through caesarean section [MaAsLin2 p = 7.8 × 10−4]. Staphylococcus, a typical skin bacterium, was more abundant in infants that were exclusively breastfed compared with exclusively formula-fed infants [p = 3.4 × 10−6]; in contrast, Enterococcus was more abundant in those who were formula-fed [p = 0.04]. However, the most consistent finding throughout the first 3 months of life was an increased abundance of Intestinibacter in exclusively formula-fed infants [Supplementary Figure S12; MaAsLin2 p = 7.3 × 10−8], after adjusting for other variables as described above. No effect of feeding was seen at later time points
We repeated the multivariate analysis only in babies born to IBD mothers. Time point, specific diagnosis of IBD [CD or UC], delivery mode, feeding type [formula feeding, exclusively breastfeeding, or mixed], exposure to antibiotics up to Day 30, exposure to biologic therapy during pregnancy, and disease activity during pregnancy were introduced in the multivariate model [MaAsLin2]. We observed similar findings regarding Intestinibacter [MaAsLin2 p = 0.001] and Staphylococcus [MaAsLin2 p = 0.002] variation according to feeding type. In babies born to mothers with IBD through vaginal delivery, we observed a trend of Parabacteroides being more abundant than in babies born via caesarean section [MaAsLin p = 0.08].
3.4. Protein composition of the breast milk of mothers with and without IBD
Overall, 90 samples from 81 mothers with IBD [56 with CD and 34 with UC] and 222 samples from 211 control mothers collected on an average 18 days postpartum [25th and 75th percentile, 16 and 31 days, respectively] were used for the current analysis. From these, there were 217 mother-baby dyads, for whom breast milk and stool microbiome samples [16S] were available. We compared protein biomarkers between breast milk samples of mothers with and without IBD, given the important role of feeding in the intestinal microbiota composition of the offspring, and our results pointing to feeding type as a major determinant of bacterial diversity. We found 26 protein biomarkers that showed different levels based on maternal IBD status [Supplementary Table S7; Wilcoxon rank sum test, control vs IBD, uncorrected p <0.05]. After correction for multiple testing, breast milk from mothers with IBD showed significantly less abundance of thymic stromal lymphopoietin [TSLP], interleukin-12 subunit beta [IL-12β], tumour necrosis factor-beta [TNFβ], and C-C motif chemokine 20 [CCL20], as compared with the breast milk from control mothers [FDR p = 0.0016, 0.049, 0.049, and 0.049, respectively] [Figure 3, Supplementary Table S7].
Figure 3.
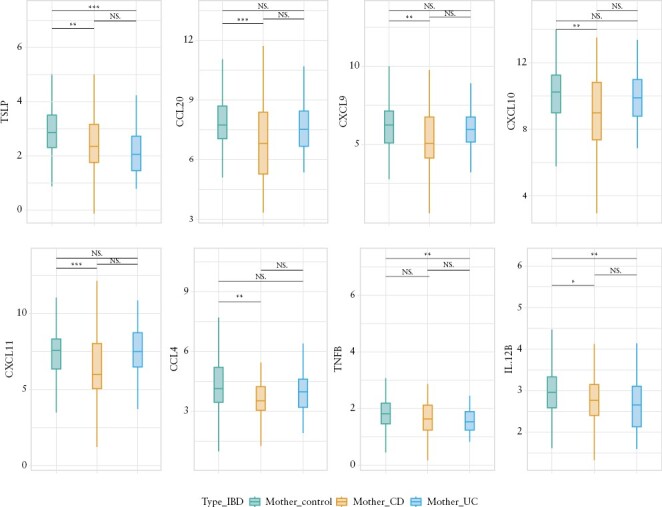
Comparison of protein concentration in the breast milk of mothers with Crohn’s disease [CD], ulcerative colitis [UC], and controls [corrected for multiple hypothesis testing].
Analysis was repeated taking into account IBD type of the mothers. The breast milk of mothers with CD had less CCL20, C-C motif chemokine 4 [CCL4], C-X-C motif chemokine [CXCL] 9, CXCL10, and CXCL11 as compared with control mothers [FDR p = 0.023, 0.033, 0.045, 0.033, and 0.023, respectively]. In contrast, the breast milk of mothers with UC had less TSLP than control mothers [FDR p = 0.004]. [Figure 3, Supplementary Table S7].
We further correlated the eight significantly different protein biomarkers [CCL20, CCL4, CXCL10, CXCL11, CXCL9, IL12β, TNFβ, and TSLP] with the faecal calprotectin of the infants. Overall, 238 babies had at least one faecal calprotectin measurement and matching breast milk sample from their mothers. The levels of TSLP in the breast milk correlated negatively with the faecal calprotectin of the infants at Month 12 [rho=−0.19, p-value = 0.014]. Similar negative correlations were observed between CCL20 and faecal calprotectin of the infants at Month 36 [rho= −0.24, p-value = 0.02], and between TNFβ and faecal calprotectin of the infants at Month 18 [rho= −0.24, p-value = 0.02] and at Month 36 [rho= −0.21, p-value = 0.048]. No significant correlations were observed between the other cytokines and faecal calprotectin at various time points [Figure 4].
Figure 4.
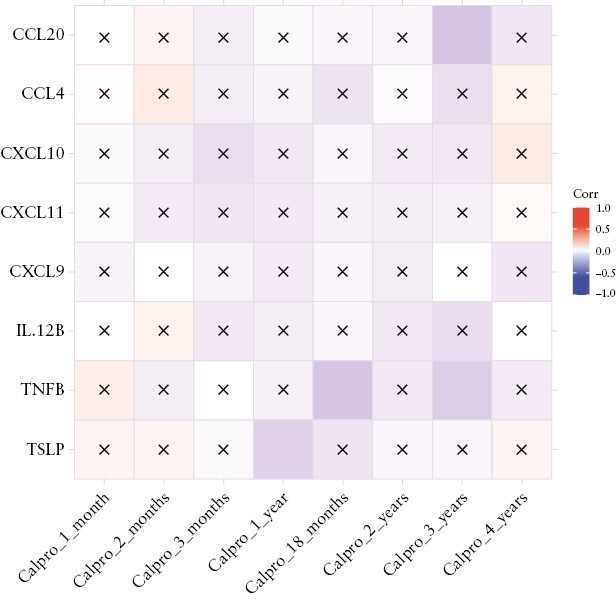
Significant correlation between the eight breast milk biomarkers in mothers with or without inflammatory bowel disease [CCL20, CCL4, CXCL10, CXCL11, CXCL9, IL12β, TNFβ, and TSLP] and faecal calprotectin of the infants at different time points. Boxes with crosses indicate non-significant correlations. Boxes without crosses indicate statistically significant correlations.
We then sought to explore possible correlations between breast milk protein biomarkers and the intestinal microbiota. No statistically significant correlations were found between the eight significantly different protein biomarkers [CCL20, CCL4, CXCL10, CXCL11, CXCL9, IL12β, TNFβ, and TSLP] and overall bacterial diversity at any time point. Nevertheless, different suggestive correlations were observed between these protein biomarkers and particular bacterial taxa. CCL4 [Month 2, Spearman’s rho=−0.16, unadjusted p = 0.021; Month 3, rho=−0.22, p = 0.003], CCL20 [Month 3, rho=−0.19, unadjusted p = 0.009], IL12β [Month 3, rho=−0.19, unadjusted p = 0.007], and TSLP [Month 12, rho=−0.17, unadjusted p = 0.04] negatively correlated with the relative abundance of Bifidobacterium at different time points [Supplementary Figures S13–S18].
4. Discussion
Herein we sought to explore the impact of early life exposures, including maternal IBD status and breast milk proteomics, on the microbiome composition of babies participating in the prospective MECONIUM study. In this study, we showed that bacterial richness, as measured by the α-diversity, continuously increased between the third and 24th months of life, and that multiple exposures affected overall microbiome composition [β-diversity] during early life. Moreover, early life exposures had varying impact at given time periods, the most important being mode of delivery, type of early life feeding, and maternal IBD status. Importantly, the contribution of the early life exposures examined in our study to the microbiome development did not differ between babies born to mothers with vs without IBD, emphasising the importance of prenatal IBD exposure on the microbiome composition during early life. We also demonstrated that the breast milk of mothers with IBD presented a different protein composition, with distinct associations found with baby’s gut microbiome and faecal calprotectin. Given the increasing recognition of early life as an important time period for microbiome development and modulation, we here explored how different factors affected microbial diversity during the first 2 years of life. Interestingly, we found that the contribution of important exposures such as mode of delivery and feeding was similar in babies born to mothers with and without IBD. In addition, we showed that taxonomic differences associated with vaginal vs caesarean section deliveries and various feeding behaviours were independent of maternal IBD diagnosis. We also found a significant reduction in specific proteins involved in the immune system regulation, namely CCL20, IL12β, TNFβ, and TSLP, in the breast milk of mothers with IBD compared with those without IBD, which correlated with faecal calprotectin and microbiome composition in babies at different periods of early life. Moreover, among babies born to IBD mothers, some taxonomic features correlated with the breast milk composition.
The first 2–3 years of life have been shown to be central to the establishment of the gut microbiome,18 when multiple exposures contribute to the shaping of the gut microbiome communities. Early life microbiome plays an essential role in immune system maturation, promotion of mucosal barrier integrity, and protection against pathogenic bacteria.18 Importantly, during this period, gut microbial composition rapidly changes and is most sensitive to external exposures, reinforcing the importance of healthy microbiome colonisation, as gut microbial disturbances can potentially lead to adverse health outcomes later in life.18 Mode of delivery, feeding patterns, antibiotic exposure, and preterm birth, as well as maternal health, chronic conditions or risky lifestyle,19 gut microbiota, and breast milk,20–22 among others, have all been shown to be the major drivers of microbiome acquisition and establishment.23–25
IBD results from a complex interaction between host genetics, mucosal immune system, environmental exposures, and microbiome. Increasing evidence suggests that dysbiosis precedes and contributes to disease susceptibility and onset.26 We had previously shown that microbiome diversity and composition in the first 3 months of life were largely driven by maternal IBD status, and that babies born to mothers with IBD demonstrated a lower bacterial diversity.8 Along the same lines, the large GEM cohort following first-degree relatives of probands with CD has recently shown that the offspring exposed to parental CD perinatally had a higher risk of developing CD than those exposed to parental CD later in life. Interestingly, they observed a dose-response effect where the earlier in life the offspring was exposed to mother’s CD, the higher the risk for own CD development they had, a trend not observed for paternal CD exposure.27 Herein, we have significantly expanded these results by analysing over 1000 stool samples from almost 300 babies over different early life periods of development. We showed that maternal IBD status remains a significant contributor to microbiome composition and richness up to the age of 2 years.7 We could also confirm that babies born to mothers with IBD have a lower relative abundance of bacteria from the Bifidobacterium genus.8
Delivery mode was a major determinant of microbiome in early months: being born vaginally was associated with higher abundance of Bacteroides during the first 3 months of life, which is in line with prior observations.28 Given the role of some Bacteroides species in regulating intestinal immunity,29,30 it has been suggested that the adverse health effects associated with caesarean section could be partly explained by the negative association with this bacterial taxon.31,32 Despite its major influence, the effect of mode of delivery disappeared with time in babies born both to control and to IBD mothers, which is consistent with prior studies.33–35 Solid food introduction and diversity, increasing social contact diversity, or other factors that were not captured in our study, are likely to become more important factors driving microbiome composition during these stages of development.
Feeding type, breast milk versus formula, also had a strong impact on microbiome composition after adjustment for maternal IBD diagnosis. Breastfed babies presented higher relative abundance of Staphylococcus, a typical skin bacterium, whereas exclusively formula-fed babies displayed higher relative abundance of Intestinibacter. Breast milk provides the newborn with bioactive factors required for immune maturation, organ development, and healthy microbial colonisation.36,37 It is thought that, at least in part, these benefits may be mediated through shaping microbial composition directly through maternal transmission of microbes, and indirectly through human milk oligosaccharides [HMOs] and other immune constituents.38 Several cytokines transferred through breast milk display immunomodulatory effects on cell subsets involved in developing the specific immune response of the child, acting in the prevention of allergies and hypersensitivities,39 even if the ability for these cytokines to survive the infant´s stomach and exert a biological effect remains poorly understood.40 A possible effect of these cytokines in modulating the oral-pharyngeal mucosal immune system should also be taken into account.41 Breast milk composition depends on stage of lactation, maternal health status, diet, environment, and genetics. Prior work conducted in 3- and 6-month breast milk samples has shown that the breast milk of women with IBD presented lower levels of IgA, lactose, and 2-aminobutyrate. In this prior work, there was also a trend observed for women with IBD to present with higher pro-inflammatory cytokines and lower anti-inflammatory cytokines at both Month 3 and Month 6 postpartum.42 Given the importance of breast milk in the postpartum period in our cohort and with an impact at later time points, we here compared breast milk proteomics around the 2nd and 3rd week postpartum, a period when lactation has been shown to be associated with high concentrations of proteins and peptides involved in immune development.43 We found significantly lower abundance of particular proteins, mostly involved in immune regulation, including CCL20, IL12β, TNFβ, and TSLP in mothers with IBD as compared with controls. TSLP was significantly reduced both in mothers with CD and in mothers with UC, whereas CCL20 was found to be lower in the breast milk of mothers with CD as compared with UC or healthy controls. TSLP is an epithelial-derived cytokine that acts as a lymphocyte growth factor.44 TSLP is expressed in the intestine, with highest expression noted in the colon, where it has a role in regulating inflammatory responses and protecting against colitis by promoting steady-state mutualistic T cell responses and Th2-dependent immune responses, limiting Th1- and Th17-driven intestinal inflammation and inducing a tolerogenic dendritic cell phenotype following intestinal bacterial colonisation.44–47
TSLP has been described in breast milk, where its relative concentration is highest during the earlier stages of lactation, but not in baby formula.48 CCL20 has a function in the formation and function of mucosal lymphoid tissue, recruiting immature dendritic cells and lymphocytes to target sites,49 and has been detected in higher concentrations in breast milk predominantly in the 1st and 2nd week postpartum, decreasing thereafter.50 Both TNFβ [significantly reduced in breast milk from UC mothers as compared with controls] and IL12β [significantly reduced in both CD and UC mothers as compared with controls] play a role in the regulation of immune response, particularly of the innate immune system. IL-12 can stimulate the growth and development of T lymphocytes and NK cells,51 and provided through the breast milk it may assist in addressing the balance between predisposed Th2-type responses and the Th1-type, cytokine-driven, cell-mediated responses in the infant.52 Interestingly, TSLP, CCL20, and TNFβ were negatively correlated with infant´s calprotectin at later months. We also observed various relationships between these cytokines and the infant´s microbiota at different time points, which may indicate a role of the breast milk composition in the development of the early life microbiome.
The strengths of our study include a unique prospective cohort of infants born to IBD mothers and controls, with access to a large sample collection obtained at specific time points the infant’s growth, coinciding with microbiome and immune system development, as well as detailed exposure data. We were able to investigate the role of maternal IBD status and IBD-related variables, alongside other relevant exposures, known to be major influencers of microbiome development in infants. Moreover, we analysed the breast milk proteomics of mothers with IBD and controls and assessed its impact on microbiome and calprotectin in babies, detecting differences in proteins known to be involved in immune system maturation.
Our study is not without limitations. Based on our study design, we could not accurately capture the effects of solid food introduction on microbiome colonisation. Most babies in the study reported solid food introduction by age 4 or 5 months, but there were no stool samples collected between 3 and 12 months. Likewise, we could not account for other potential exposures such as pets, social dwelling, or day care. Despite the observed differences in the breast milk composition and its correlation with the microbiome diversity and faecal calprotectin levels, our findings are associative as we cannot infer causality on what could be the immune-related consequences, if any, on the mucosal immune system of the infant, especially given the early time point at which breast milk was collected. Additionally, we acknowledge that the panel used for studying breast milk proteomics was biased towards proteins involved in inflammation, and therefore may not fully represent breast milk proteomics. Likewise, whether different patterns of microbiome may increase risk for IBD in babies exposed to IBD in utero remains unknown and would require a long-term follow-up. Finally, these findings are merely associative, and at the best open the door for mechanist studies exploring potential role of maternal microbiome and breast milk proteomics on the gut immunity of the offspring; they cannot and should not be used to drawn any specific recommendations for breastfeeding among mothers with IBD. Future studies should explore the early life as a period for disease modulation, and might benefit from using metagenomic sequencing and untargeted proteomics coupled with baby’s stool metabolomics to better understand potential risk transmission.
This hypothesis-generating prospective study suggests that maternal IBD status contributes to microbiome development in the offspring up to at least 2 years of age, which could at least in part be mediated through breast milk composition. Our findings lay the groundwork for future studies applying comprehensive omics data and mechanistic approaches to determine how early life factors affect the health of the infants exposed to IBD in utero. Understanding the critical events that take place in early life in at-risk populations could pave the way to the development of novel strategies aimed at promoting a healthy microbiome colonisation, with the ultimate goal of reducing future risk of IBD.
Study data will be made available upon reasonable request to the corresponding author.
Supplementary Material
Contributor Information
João Sabino, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA; Icahn School of Medicine at Mount Sinai, Division of Gastroenterology, Department of Medicine, New York, NY, USA; Department of Gastroenterology, University Hospitals of Leuven, Leuven, Belgium.
Leonid Tarassishin, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA.
Caroline Eisele, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA; College of Medicine, Pennsylvania State University College of Medicine, Hershey, PA, USA.
Kelly Hawkins, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA.
Amelie Barré, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA.
Nile Nair, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, Massachusetts.
Alexa Rendon, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA.
Anketse Debebe, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA.
Mellissa Picker, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA.
Manasi Agrawal, Icahn School of Medicine at Mount Sinai, Division of Gastroenterology, Department of Medicine, New York, NY, USA; Center for Molecular Prediction of IBD [PREDICT], Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
Joanne Stone, Gastroenterology Division, Icahn School of Medicine at Mount Sinai, Department of Obstetrics, Gynecology and Reproductive Sciences, New York, NY, USA.
James George, Icahn School of Medicine at Mount Sinai, Division of Gastroenterology, Department of Medicine, New York, NY, USA.
Peter Legnani, Icahn School of Medicine at Mount Sinai, Division of Gastroenterology, Department of Medicine, New York, NY, USA.
Elana Maser, Icahn School of Medicine at Mount Sinai, Division of Gastroenterology, Department of Medicine, New York, NY, USA.
Ching-Lynn Chen, Gastroenterology Division, Icahn School of Medicine at Mount Sinai, Department of Obstetrics, Gynecology and Reproductive Sciences, New York, NY, USA.
Anne Thjømøe, CALPRO AS, Lysaker, Norway.
Einar Mørk, CALPRO AS, Lysaker, Norway.
Marla Dubinsky, Icahn School of Medicine at Mount Sinai, Division of Pediatric Gastroenterology and Hepatology, New York, NY, USA.
Jianzhong Hu, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA.
Jean-Frederic Colombel, Icahn School of Medicine at Mount Sinai, Division of Gastroenterology, Department of Medicine, New York, NY, USA.
Inga Peter, Icahn School of Medicine at Mount Sinai, Department of Genetics and Genomic Sciences, New York, NY, USA.
Joanna Torres, Icahn School of Medicine at Mount Sinai, Division of Gastroenterology, Department of Medicine, New York, NY, USA; Gastroenterology Division, Hospital Beatriz Ângelo, Loures, Portugal; Gastroenterology Division, Hospital da Luz, Lisbon, Portugal; Faculdade de Medicina, Universidade de Lisboa, Portugal.
Funding
This study was funded by International Organization for the Study of Inflammatory Bowel Diseases, Crohn’s and Colitis Foundation, the Leona M. and Harry B. Helmsley Charitable Trust, and NIH/NIDDK [R21DK125906].
Conflict of Interest
JS: received speaker fees from Takeda, Falk, Abbvie, Ferring, Pfizer, and Janssen; and consultancy fees from Abbvie, Celltrion, Ferring, Fresenius, Pharmanovia, Pharmacosmos and Janssen; and research support from Galapagos and Viatris. JS is supported by a Senior Clinical Investigator grant from the Research Foundation—Flanders. JT received speaker fees from Janssen, Abbvie, Pfizer, and Galapagos; and advisory board fees from Janssen, Pfizer, Arena Pharmaceuticals, Abbvie, and Galapagos; and research support from Janssen and Abbvie. JFC has received personal fees from Abb-Vie, Amgen, Boehringer Ingelheim, Celgene, Celltrion, Enterome, Ferring,Genentech, Janssen–Johnson & Johnson, MedImmune, Merck, Pfizer, Protagonist, Second Genome, Seres, Shire, Takeda, Theradiag, and PPM Ser-vices; has received grant support from AbbVie, Janssen–Johnson & Johnson,and Takeda; and holds stock options in Genfit and Intestinal Biotech Development. MA reports no conflict of interest. MA is supported by the National Institute of Diabetes and Digestive and Kidney Diseases [K23DK129762-02].
Author contributions
Concept: JS, JFC, IP, and JT. Sample and data collection: CE, KH, AB, NN, AR, AD. Sample processing and analysis: LT, AT, EM, and JH. Formal analysis: JS and JT. Writing, review, and editing: all authors. Supervision: JFC, IP, and JT.
References
- 1. Torow N, Hornef MW.. The neonatal window of opportunity: setting the stage for life-long host-microbial interaction and immune homeostasis. J Immunol 2017;198:557–63. [DOI] [PubMed] [Google Scholar]
- 2. Sbihi H, Boutin RC, Cutler C, Suen M, Finlay BB, Turvey SE.. Thinking bigger: How early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease. Allergy 2019;74:2103–15. [DOI] [PubMed] [Google Scholar]
- 3. Agrawal M, Sabino J, Frias-Gomes C, et al. Early life exposures and the risk of inflammatory bowel disease: Systematic review and meta-analyses. EClinicalMedicine 2021;36:100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van den Elsen LWJ, Garssen J, Burcelin R, Verhasselt V.. Shaping the gut microbiota by breastfeeding: the gateway to allergy prevention? Front Pediatr 2019;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferretti P, Pasolli E, Tett A, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 2018;24:133–45.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science 2016;351:1296–302. [DOI] [PubMed] [Google Scholar]
- 7. Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG.. The infant microbiome development: mom matters. Trends Mol Med 2015;21:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres J, Hu J, Seki A, et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut 2020;69:42–51. [DOI] [PubMed] [Google Scholar]
- 9. Kim ES, Tarassishin L, Eisele C, et al.; Mount Sinai Road to Prevention Study Group. Longitudinal changes in fecal calprotectin levels among pregnant women with and without inflammatory bowel disease and their babies. Gastroenterology 2021;160:1118–30.e3. [DOI] [PubMed] [Google Scholar]
- 10. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19:5A–36A. [DOI] [PubMed] [Google Scholar]
- 11. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002;122:512–30. [DOI] [PubMed] [Google Scholar]
- 12. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH.. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fredriksson S, Gullberg M, Jarvius J, et al. Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol 2002;20:473–7. [DOI] [PubMed] [Google Scholar]
- 15. Huang J, Khademi M, Fugger L, et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc Natl Acad Sci U S A 2020;117:12952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mallick H, Rahnavard A, McIver LJ, et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol 2021;17:e1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995;57:289–300. [Google Scholar]
- 18. Kapourchali FR, Cresci GAM.. Early-life gut microbiome: the importance of maternal and infant factors in its establishment. Nutr Clin Pract 2020;35:386–405. [DOI] [PubMed] [Google Scholar]
- 19. Ma J, Prince AL, Bader D, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun 2014;5:3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu DM, Meyer KM, Prince AL, Aagaard KM.. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 2016;7:459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng J, Xiao X, Zhang Q, et al. The effects of maternal and post-weaning diet interaction on glucose metabolism and gut microbiota in male mice offspring. Biosci Rep 2016;36:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boudry G, Charton E, Le Huerou-Luron I, et al. The relationship between breast milk components and the infant gut microbiota. Front Nutr 2021;8:629740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tamburini S, Shen N, Wu HC, Clemente JC.. The microbiome in early life: implications for health outcomes. Nat Med 2016;22:713–22. [DOI] [PubMed] [Google Scholar]
- 24. O’Neill IJ, Sanchez Gallardo R, Saldova R, et al. Maternal and infant factors that shape neonatal gut colonization by bacteria. Expert Rev Gastroenterol Hepatol 2020;14:651–64. [DOI] [PubMed] [Google Scholar]
- 25. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 2016;8:343ra–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turpin W, Goethel A, Bedrani L, Croitoru Mdcm K.. Determinants of IBD heritability: genes, bugs, and more. Inflamm Bowel Dis 2018;24:1133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee SH, Qiu L, Olivera PA, Leibovitzh H, et al. Peri-natal exposure to parental Crohn’s disease is associated with impaired gut barrier, microbiome composition differences and increased risk of Crohn’s disease. 2023;17:i40–1. [Google Scholar]
- 28. Shaterian N, Abdi F, Ghavidel N, Alidost F.. Role of cesarean section in the development of neonatal gut microbiota: A systematic review. Open Med [Wars] 2021;16:624–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol 2004;5:104–12. [DOI] [PubMed] [Google Scholar]
- 30. An D, Oh SF, Olszak T, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 2014;156:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wernroth ML, Peura S, Hedman AM, et al. Development of gut microbiota during the first 2 years of life. Sci Rep 2022;12:9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sandall J, Tribe RM, Avery L, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 2018;392:1349–57. [DOI] [PubMed] [Google Scholar]
- 33. Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM.. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 2017;23:314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stokholm J, Thorsen J, Chawes BL, et al. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol 2016;138:881–9.e2. [DOI] [PubMed] [Google Scholar]
- 35. Rutayisire E, Huang K, Liu Y, Tao F.. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: a systematic review. BMC Gastroenterol 2016;16:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brenmoehl J, Ohde D, Wirthgen E, Hoeflich A.. Cytokines in milk and the role of TGF-beta. Best Pract Res Clin Endocrinol Metab 2018;32:47–56. [DOI] [PubMed] [Google Scholar]
- 37. Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr 2005;135:1–4. [DOI] [PubMed] [Google Scholar]
- 38. Cacho NT, Lawrence RM.. Innate immunity and breast milk. Front Immunol 2017;8:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palmeira P, Carneiro-Sampaio M.. Immunology of breast milk. Rev Assoc Med Bras 2016;62:584–93. [DOI] [PubMed] [Google Scholar]
- 40. Dawod B, Marshall JS.. Cytokines and soluble receptors in breast milk as enhancers of oral tolerance development. Front Immunol 2019;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu RQ, Zhang DF, Tu E, Chen Q-M, Chen WJ.. The mucosal immune system in the oral cavity-an orchestra of T cell diversity. Int J Oral Sci 2014;6:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meng X, Dunsmore G, Koleva P, et al. The profile of human milk metabolome, cytokines, and antibodies in inflammatory bowel diseases versus healthy mothers, and potential impact on the newborn. J Crohns Colitis 2019;13:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu J, Dingess KA, Mank M, Stahl B, Heck AJR.. Personalized profiling reveals donor- and lactation-specific trends in the human milk proteome and peptidome. J Nutr 2021;151:826–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Al-Shami A, Spolski R, Kelly J, et al. A role for thymic stromal lymphopoietin in CD4[+] T cell development. J Exp Med 2004;200:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taylor BC, Zaph C, Troy AE, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med 2009;206:655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeuthen LH, Fink LN, Frokiaer H.. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology 2008;123:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mosconi I, Geuking MB, Zaiss MM, et al. Intestinal bacteria induce TSLP to promote mutualistic T-cell responses. Mucosal Immunol 2013;6:1157–67. [DOI] [PubMed] [Google Scholar]
- 48. Macfarlane TV, Seager AL, Moller M, Morgan G, Thornton CA.. Thymic stromal lymphopoietin is present in human breast milk. Pediatr Allergy Immunol 2010;21:e454–6. [DOI] [PubMed] [Google Scholar]
- 49. Sibartie S, O’Hara AM, Ryan J, et al. Modulation of pathogen-induced CCL20 secretion from HT-29 human intestinal epithelial cells by commensal bacteria. BMC Immunol 2009;10:54–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lourenço AG, Komesu MC, Duarte G, et al. High levels of chemokine c-c motif ligand 20 in human milk and its production by oral keratinocytes. Breastfeed Med 2017;12:116–21. [DOI] [PubMed] [Google Scholar]
- 51. Bertagnolli MM, Lin BY, Young D, Herrmann SH.. IL-12 augments antigen-dependent proliferation of activated T lymphocytes. J Immunol 1992;149:3778–83. [PubMed] [Google Scholar]
- 52. Bryan D-L, Hawkes JS, Gibson RA.. Interleukin-12 in human milk. Pediatr Res 1999;45:858–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


