Abstract
Vascular cognitive impairment (VCI) represents the second most common cause of dementia after Alzheimer's disease, and pathological changes in cerebral vascular structure and function are pivotal causes of VCI. Cognitive impairment caused by arterial ischemia has been extensively studied the whole time; the influence of cerebral venous congestion on cognitive impairment draws doctors’ attention in recent clinical practice, but the underlying neuropathophysiological alterations are not completely understood. This study elucidated the specific pathogenetic role of cerebral venous congestion in cognitive-behavioral deterioration and possible electrophysiological mechanisms. Using cerebral venous congestion rat models, we found these rats exhibited decreased long-term potentiation (LTP) in the hippocampal dentate gyrus and impaired spatial learning and memory. Based on untargeted metabolomics, N-acetyl-L-cysteine (NAC) deficiency was detected in cerebral venous congestion rats; supplementation with NAC appeared to ameliorate synaptic deficits, rescue impaired LTP, and mitigate cognitive impairment. In a cohort of cerebral venous congestion patients, NAC levels were decreased; NAC concentration was negatively correlated with subjective cognitive decline (SCD) score but positively correlated with mini-mental state examination (MMSE) score. These findings provide a new perspective on cognitive impairment and support further exploration of NAC as a therapeutic target for the prevention and treatment of VCI.
Keywords: Vascular contributions to cognitive impairment and dementia (VCID), vascular cognitive impairment (VCI), vein, cerebral circulation, cerebral venous congestion
Introduction
Vascular contributions to cognitive impairment and dementia (VCID) represents the second most common form of dementia after Alzheimer's disease (AD), and pathological changes in cerebral vascular structure and function are pivotal causes of VCI.1,2 The role of arterial abnormalities in the pathophysiology of VCI is well understood; however, the relationship between venous drainage impairment, such as cerebral venous congestion, and VCI has not been systematically studied. The cerebral venous system (CVS) contains approximately 70% of the total cerebral blood volume and plays a fundamental role in maintaining central nervous system (CNS) homeostasis.3–8 To sustain the function properly of cerebral circulation, the automatic regulation of the brain blood vessel can prevent and protect the brain from cerebral overflow-induced injury. One of the key issues related to the rules of circulation is that arterial and venous blood flow must be in harmony, and the blood entering the brain from the arterial system is matched with the amount of blood exiting the brain via the venous system. Cerebral venous flow decreases, and venous pressure increases more than cerebrospinal fluid (CSF) pressure, causing venous dilation, microvesicles, and capillary leakage; thus, CSF pressure increases and arterial flow decreases, forming a vicious cycle. Previous studies have suggested the possible involvement of impaired cerebral veins in certain CNS disorders, such as multiple sclerosis (MS),3,9,10 AD,6,11–14 Parkinson’s disease (PD),15,16 transient global amnesia (TGA),17–19 migraine, 20 cerebral microhemorrhages (CMHs; microbleeds), 21 leukoaraiosis, 22 and cerebral small vessel disease (SVD).6,14,23 Another latest study highlighted the critical role of the cerebral veins as a neuroimmune interface and clarified age-related dysfunction and neuroinflammatory attacks. 24 Recently, cardiologists found that cognitive impairment is a crucial complication among heart failure patients, with an incidence ranging from 25% to 80%.25,26 In addition to decreased cardiac output, they thought that cerebral venous congestion (backward failure) also contributes significantly to the pathogenesis of cognitive decline associated with heart failure. Fulop et al.27,28 showed that, after cerebral venous congestion, mice exhibited impaired spatial learning and memory, altered motor coordination, and impaired gait function. Our previous study 29 found that subjective memory decline was present in 30.2% of cerebral venous congestion patients. Existing literature evaluating the association between cerebral venous congestion and cognitive impairment is limited and needs to be explored.
The purpose of this study was to determine whether cerebral venous congestion is associated with cognitive impairment using a validated cerebral venous congestion rat model by extracranial and internal jugular vein ligation (JVL)21,27,30 and to characterize the electrophysiological mechanism. We found a metabolite with potential value for the clinical diagnosis and prevention of cerebral venous congestion. These findings provide a reference for understanding the pathological mechanism of VCI and a new perspective for the prevention and treatment of cognitive impairment related to cerebral venous congestion.
Material and methods
Animals
In the present study, adult Sprague Dawley (SD) rats weighing 180–220 g were purchased from Vital River (Beijing, China). Animals were bred and housed in a group of three in standard laboratory conditions with free access to food and water throughout the study at Capital Medical University. Rats were supplemented daily with NAC (Sigma-Aldrich #A9165; dissolved in 0.9% NaCl, protected from light) in drinking water (exchanged every 3–4 days) at a dose of 100 mg/kg bodyweight for 1 month. All animal experimental protocols were approved by the Animal Care and Use Committee of Capital Medical University (permit no. AEEI-2018-121) and performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal data reporting followed the ARRIVE 2.0 guideline. 31
JVL rat model
Rats were deeply anesthetized with 4% isoflurane and subsequently maintained between 1.5–2% isoflurane during the surgical procedure using an isoflurane vaporizer (RWD, Shenzhen, China). Each rat was placed in a dorsally recumbent position under a dissection microscope, and the hair from the neck was removed. A cutaneous midline incision was made on the neck. Blunt dissection of the subcutaneous tissue was performed, and the dorsolateral salivary glands were separated. The internal jugular vein, located in the carotid sheath on both sides (above the internal carotid arteries next to the vagus nerve), was subsequently separated and exposed according to the published protocol of Auletta and co-workers, 30 making an oblique incision cross one-third point on the orbital line near the ear, retracting the temporalis muscle dorsally and the exorbital lacrimal gland ventrally, exposing and ligating the retroglenoid vein, which is the extracranial termination of the transverse sinus in rodents. Surgical ligations were performed with 8-0 nonabsorbable surgical sutures. Sham rats received the same skin incision and operation without surgical ligation under isoflurane anesthesia. Once the surgery was completed, the wounds were closed using a 6-0 surgical suture with a simple continuous pattern. Rats were allowed to recover under the heat lamp, and antibiotic ointment was applied over the skin incision, following five consecutive days. All efforts were made to minimize the number of animals used and their suffering.
Behavioral tests
All the rats used for behavioral tests were housed in groups. Rats were used for behavioral tests 1 month postoperatively.
Y maze test
YMT was performed to assess short-term spatial working memory by spontaneous alternation. The device is a level, three-armed maze (50 cm long × 18 cm wide × 25 cm high walls) in which the angle between each of the two adjacent arms is 120°. Rats were placed at the center of the three arms and allowed to explore freely for 8 min. The total arm entries and sequences of each arm were recorded.
Novel object recognition test
NOR was performed to test learning and memory exploratory preference. The device includes a small open field box (72 cm long × 72 cm wide × 45 cm high walls) and two objects of different shapes but of the same material. Rats were acclimated to the open field box without objects for 10 min on Day 1, and with two identical objects for 10 min on Day 2. On Day 3, one of the familiar objects was replaced with a new object, and the rats were allowed to explore two different objects for 5 min.
Open field test
OFT was conducted to measure locomotion and spontaneous activity. The device consists of a small, open-topped box known as the open field (100 cm long × 100 cm wide × 50 cm high walls). Rats were placed into the open field box to track position and locomotor activity for 10 min using a video tracking system (SuperMaze). The percentage of time spent in the center area and total distance were calculated.
Rotarod test
Motor coordination was assessed by using an automated four-lane rotarod. Rats were placed on the rotarod to adapt to 10 s at the initial speed of 5 rpm/min, then accelerated from 5 rpm/min to 40 rpm/min within 2 min, and maintained until 5 min. Rats were trained for 3 days to ensure that they could maintain themselves on the rod. The duration and speed to fall were recorded.
Measurements of ICP
To evaluate the ICP, a midline incision was made over the vertex following anesthesia, and then a whole caudal to the coronal suture was drilled 4 mm from the midline. The dura was punctured, and a microsensor for ICP was inserted intracranially. An ICP monitor was used to measure the ICP.
Measurements of CBF
CBF was evaluated by laser speckle contrast imaging (LSCI; RWD, Shenzhen, China) before JVL (baseline), immediately after JVL surgery, and finally at the respective endpoints of JVL. A drill was used to thin the skull and polish the area around the coronal line, and then to polish the surface of the thinned skull evenly and smoothly until the blood vessels on the dura were visible. Then, the rat was moved to the laser probe to monitor the CBF of the regions of interest (ROIs). The monitoring time of each rat was 5 min. Changes in CBF before and after JVL were measured by using a perfusion speckle imager.
NAC, ALCAR, NAM, and GABA measurements
The production of NAC, ALCAR, NAM, and GABA was measured using an N-acetylcysteine fluorometric assay kit (BioVision), ELISA Kit for Acetyl-L-carnitine (Cloud-Clone Corp), Nicotinamide (Rat/Human) ELISA Kit (J&L Biological), and QuickDetect™ GABA (Rat/Human) ELISA Kit (BioVision), respectively, following the manufacturer’s protocols.
Hippocampal slice preparation and maintenance
The general procedures for preparing hippocampal slices were similar to those described previously. 32 Rats were anesthetized and intracardially perfused with ice-cold, high-sucrose modified artificial CSF (aCSF) containing (in mM): 10 glucose, 213 sucrose, 3 KCl, 1 NaH2PO4·2H2O, 26 NaHCO3, 0.5 CaCl2·2H2O, 5 MgCl2·6H2O (pH 7.4). The brains were separated from the skull and rapidly removed into ice-cold high-sucrose aCSF. The brain was cut on a vibratome (Leica, Bensheim, Germany) in high-sucrose modified aCSF and immediately transferred to an incubation chamber containing aCSF (in mM): 10 glucose, 125 NaCl, 5 KCl, 1.2 NaH2PO4·2H2O, 26 NaHCO3, 2.6 CaCl2·2H2O, 1.3 MgCl2·6H2O (pH 7.4) (all reagents from Sigma). The 400 µm thick acute hippocampal slices were collected starting from the first slice that allowed the identification of separated dentate gyrus (DG) and all cornu ammonis (CA) regions and incubated to recover at 35 °C for 40 min before being allowed to equilibrate at room temperature for an additional 20 min. All slices were maintained in this interface chamber at room temperature until being transferred to the recording chamber of an upright microscope for electrophysiological recording. During slice preparation, maintenance, and recording, aCSF was continuously saturated with 95% O2 and 5% CO2.
Electrophysiological recording
A multielectrode dish (MED 64 planar microelectrodes; Panasonic, Osaka, Japan) was prepared as described previously. 32 Following incubation, the slices were transferred to a recording chamber, the perforant pathway was selected for stimulation, and the recording site was determined by the occurrence of the fEPSP response. After stabilizing synaptic responses for 15 min, an input-output curve was first determined for each group via the measurements of fEPSP amplitude or slope in response to a series of stimulation intensities starting at 10 μA. The 30–50% maximum of input-output curve stimulation intensity was determined for subsequent LTP recording. After 15 min, the stable baseline was established, and LTP was induced by TBS, which consisted of five bursts with intervals of 10 seconds. Each burst contained four pulses at 100 Hz with an inter-burst interval of 200 ms (5 × TBS). After 5 × TBS, the test stimulus was repeatedly delivered once every 30 seconds for 60 min to observe any changes in LTP magnitude and duration. PPF was assessed using inter-stimulus intervals of 20, 50, 100, 150, and 200 ms. The evoked fEPSPs were amplified by a 64-channel amplifier and then digitized at a 20 kHz sampling rate. Traces were obtained and analyzed using a MED 64 system software program (Alpha Med Science Inc., Osaka, Japan).
Tissue staining
The brain was collected at the indicated time course of each figure legend. After being deeply anesthetized, the animals were perfused through the heart with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). Then, brains were removed from the skull, fixed overnight in 4% PFA, and dehydrated in 20% and 30% sucrose in PFA solution for every 24 h until they sunk to the bottom. The brain tissue was embedded in paraffin and sliced into 4-μm-thick coronal sections. After dewaxing, hematoxylin and eosin (H&E) and Nissl staining of coronal brain sections was performed. The brain sections were observed and photographed under a light microscope.
Brain tissue samples
The brain was harvested after perfusion with ice-cold PBS to remove blood. After eliminating the brain stem and cerebellum, brain tissues were cut into 5 slices (approximately 4 mm thick per portion). Then, 20 mg samples were randomly extracted from each slice (the same location of brain tissues was collected for the sham and JVL groups) and mixed. All the samples were stored at −80°C until further processing.
Untargeted metabolomics analysis
The untargeted metabolomics analysis process was performed using the BGI-tech pipeline (BGI). Briefly, 25 mg of brain tissues was weighed and extracted by directly adding 800 µl of precooled extraction reagent, and internal standards mix 1 (IS1) and internal standards mix 2 (IS2) were added for quality control of sample preparation. After homogenization for 5 min using TissueLyser (JXFSTPRP, China), the samples were sonicated for 10 min and incubated for 1 hour at −20°C. Then, the samples were centrifuged for 15 min (25000 rpm, 4 °C), and the supernatant was transferred for vacuum freeze-drying. The metabolites were resuspended in 200 µl of 10% methanol and sonicated for 10 min at 4 °C, after centrifugation for 15 min at 25000 rpm, and the supernatants were transferred to autosampler vials for LC-MS analysis. A quality control (QC) sample was prepared by pooling the same volume of each sample to evaluate the reproducibility of the whole LC-MS analysis. This experiment used a Waters 2 D UPLC (Waters, USA) tandem Q Exactive HF high-resolution mass spectrometer (Thermo Fisher Scientific, USA) for the separation and detection of metabolites.
Patient identification and clinical data collection
All patients were recruited from Xuanwu Hospital, Capital Medical University (Beijing, China). Patients experiencing cerebral venous congestion who were enrolled in this registry between January 2020 and June 2021 were consecutively included in this study. We collected 72 plasma and CSF samples from 36 patients with cerebral venous congestion that were diagnosed by MRI+MRV, CT+CTV, or DSA (3.0 T MR maps were analyzed, including the sequences of axial T1WI, T2WI, DWI, FLAIR, ADC, and CE-MRV. 3 D-CT images of the brain and neck were used to assess patients with bone compression. Additionally, patients who were candidates for endovascular procedures also underwent DSA. Two experienced radiologists analyzed the above imaging data independently); aged ≥16 years; and from 36 volunteers who were recruited for the research (HC groups). Demographic and clinical data were collected from patients. Baseline data were collected at admission for all patients, such as age, sex, body mass index (BMI), and biochemical indicators, including glucose, total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C), in plasma. This study was approved by the Institutional Ethics Committee of Xuanwu Hospital, Capital Medical University ([2020]098). The consent forms of all patients were signed before information collection.
Statistical analysis
All the data were analyzed with SPSS 19.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 6 (GraphPad Software, La Jolla, CA). Baseline characteristics and outcome proportions were reported using descriptive statistics. All data were tested for normality by the Shapiro-Wilk test before the subsequent statistical analysis. Continuous variables are expressed as the mean ± standard deviation (SD) or median with interquartile range (IQR), and categorical variables are expressed as percentages. Spearman’s rank correlation test was used to analyze the correlation of different variables. The statistical significance of differences among more than two groups was tested by one-way ANOVA. Differences within the experimental groups were determined by two-way ANOVA and Student’s t-test. LTP curves and input-output curves were analyzed with repeated-measures analysis of variance. All tests were 2-sided, and P < 0.05 was set as statistically significant for all tests.
Results
Cerebral venous congestion induced spatial learning and memory impairments in rats
To study pathological events of cerebral venous congestion, we used a surgical technique involving bilateral JVL21,27,30 in rats to create a rodent cerebral venous congestion model (Figure 1(a) and (b)). It has been confirmed that venous outflow congestion contributes to the development of intracranial hypertension. 33 We measured intracranial pressure (ICP) 1 month after surgery and found that the average ICP value of the JVL group was significantly higher than that of the sham group (P < 0.001; Figure 1(c)). Cerebral blood flow (CBF) was recorded before JVL surgery as well as 0.5 hours postoperatively and 30 days postoperatively (Figure 1(d)), confirming that CBF was equivalent under basal conditions, increased immediately at 0.5 hours after JVL surgery (P = 0.001; Figure 1(e)), and then recovered slowly using LSCI (Figure 1(d) and (e)). At 30 days after JVL surgery, compared with the sham group, the average CBF of the JVL group decreased significantly (P < 0.001; Figure 1(e)).
Figure 1.
The influence of cerebral venous congestion on intracranial pressure, cerebral blood flow, cognitive performance, and motor function. (a, b) Schematic diagram of the cerebral venous drainage system and surgical intervention sites in rodents. (c) ICP values (n = 6). The color code (blue, Sham; red, JVL) is used for all results. (d, e) Representative contrast images of cerebral perfusion and CBF changes (presented as bar graphs) in rats before JVL (baseline), 30 min, and finally at 30 days after JVL (n = 6). (f) Spontaneous alternation in the Y-maze test (n = 6). (g) Exploratory preference in the NOR test (n = 6). (h, i) Percentage of time spent in the center area and total distance in the OFT (n = 6). (j, k) Duration and speed to fall in the RRT (n = 6). Dots depict individual samples in c, f–k. All data are shown as the mean ± SD. Statistical significance was calculated using a two-tailed unpaired Student’s t-test. ns, with no significance; *p < 0.05; **p < 0.01; ***p < 0.001. JVL: jugular vein ligation; ICP: intracranial pressure; CBF: cerebral blood flow; NOR: novel object recognition; OFT: open-field test; RRT: rotarod test.
Clinical and experimental studies have demonstrated that chronic cerebral hypoperfusion is an important factor that impairs cognitive functions. Behavioral tests, including the Y-maze and novel object recognition (NOR), are routinely used to measure different aspects of cognitive ability. Compared to sham rats, the Y-maze spontaneous alternation (P = 0.003; Figure 1(f)) and NOR exploratory preference (P < 0.001; Figure 1(g)) of JVL rats decreased significantly 1 month after surgery. To exclude potential confounders related to motor coordination or neuropsychological impairment, we performed the open-field test (OFT) and rotarod test (RRT). JVL rats did not exhibit reduced interest in exploring the central region (P = 0.262; Figure 1(h)) and locomotion (P = 0.382; Figure 1(i)) in the OFT; duration (P = 0.449; Figure 1(j)) and speed (P > 0.999; Figure 1(k)) to fall in the RRT. These results suggest that cerebral venous congestion rats exhibit impaired working and spatial recognition memory, which is closely related to the hippocampus.
Cerebral venous congestion decreased hippocampal LTP in rats
We aimed to uncover the structural and functional causes of spatial learning and memory impairments in cerebral venous congestion rats. Considering that the primary structural basis of memory function is morphology and altered synaptic function in the hippocampus, we examined the morphology, number, and electrophysiology of hippocampal neurons. Nissl staining (Figure 2(a)) revealed that the neurons were arranged compactly with normal cell morphology and no obvious damage, and there was no significant difference in hippocampal surface areas between the sham and JVL groups. LTP of the hippocampus is considered to be the electrophysiological basis of learning and memory. 34 Taking into account the behavioral results that found impaired hippocampus-dependent cognition in JVL rats, we examined LTP in the perforant pathway-DG circuit of rat hippocampal slices at the same age (Figure 2(b) to (i); Figure S1). First, we recorded and analyzed the input-output curve of field excitatory postsynaptic potential (fEPSP). Compared with sham rats, there was no significant difference in fEPSP amplitude (Figure S1A) or slope (Figure S1B) in response to a series of stimulation intensities in JVL rats, suggesting that the function of hippocampal basal synaptic transmission was not impaired in cerebral venous congestion rats.
Figure 2.
LTP was damaged in the hippocampus of cerebral venous congestion rats. (a) Representative Nissl-stained sections of the brain, cortex, hippocampus, CA3, CA1 and basal ganglia (n = 5). Scale bars (brain, 500 μm; hippocampus, 100 μm; others, 50 μm). (b, c) Summary plots of normalized fEPSP amplitude (%) and slope (%) during the recording period in the dentate gyrus. The fEPSP was induced by 5 × TBS (arrow). Inset traces: representative traces of fEPSP before (black curve) and after (blue curve for Sham; red curve for JVL) the induction of potentiation. (d-i) Normalized fEPSP amplitude and slope for the PTP (0–2 min post-TBS) (d, e), E-LTP (15–20 min post-TBS) (f, g), and last 10 min (h, i). All data are shown as the mean ± SD. Statistical significance was calculated using a two-tailed unpaired Student’s t-test (d-i) or repeated-measures analysis of variance (b, c). Data are representative of at least five independent experiments. ns, with no significance; *p < 0.05; **p < 0.01; ***p < 0.001. LTP: long-term potentiation; fEPSP: field excitatory postsynaptic potential; TBS: theta-burst stimulation; PTP: post-tetanic potentiation; E-LTP: early-LTP.
Next, LTP was induced by 5 × TBS after 15 min of stable baseline recording, and both the amplitude (Figure 2(b)) and the slope (Figure 2(c)) of fEPSP recorded in these two groups substantially increased over time. Hippocampal synaptic plasticity occurs in multiple phases that involve short-term and long-term changes at the synapse. We compared post-tetanic potentiation (PTP, 0–2 min post-TBS), as well as early (E-LTP, 15–20 min post-TBS) and late (L-LTP, last 10 min) phases of long-term plasticity, to determine which phases were particularly vulnerable to cerebral venous congestion. Normalized to baseline, the percentage of amplitude and slope values were lower in the JVL group than in the sham group in the PTP (P < 0.001 for amplitude, P < 0.001 for slope; Figure 2(d) and (e)), E-LTP (P < 0.001 for amplitude, P < 0.001 for slope; Figure 2(f) and (g)), and L-LTP (P < 0.001 for amplitude, P < 0.001 for slope; Figure 2(h) and (i)) phases, reflecting deficits in synaptic plasticity. The current findings indicate that presynaptic neurotransmitter release properties, modification of pre-existing proteins of the postsynaptic membrane, and new protein synthesis might be affected by cerebral venous congestion-related pathological factors.
Paired-pulse facilitation (PPF) is a form of short-term presynaptic plasticity defined as an increased postsynaptic response to the second stimuli of two close successive stimuli. 35 Paired-pulse ratios were measured across a range of inter-stimulus intervals (20–200 ms) under the conditions described above. Notably, there was no statistically significant difference in the PPF ratio of the two stimuli at either interval between hippocampal slices from the sham and JVL groups (Figure S1C and D). This result indicates that the attenuation of hippocampal LTP in cerebral venous congestion rats could not be attributed to alterations in basal synaptic transmission. Moreover, these findings suggest that long-term synaptic strength is more susceptible to the pathological context of cerebral venous congestion than basal synaptic function.
Cerebral venous congestion altered the metabolite profile of the brain
The homeostasis of memory networks depends on continuous blood perfusion to deliver oxygen and glucose to maintain a normal brain metabolic state, and any persistent disturbance of blood flow leads to disturbances in brain metabolism. To reveal the underlying metabolic perturbation of cerebral venous congestion-induced pathological changes, the metabolite profiles of brain tissue samples were analyzed using liquid chromatography tandem mass spectrometry (LC-MS/MS). From LC-MS, a total of 2752 ions in the positive ion mode and 988 ions in the negative ion mode were selected for statistical preprocessing. Partial least-squares discrimination analysis (PLS-DA) revealed that the brain tissue metabolomic profile of JVL rats was significantly different from that of sham rats. The t-test with false discovery ratio (FDR) correction was used for significant measurement of each metabolic signature between comparisons. With the selection of VIP > 1.0 and FDR-adjusted P value <0.05, 51 differential metabolic signatures in positive and 15 in negative modes were screened.
It is evident from the volcano plot that there were 12 higher and 54 lower metabolites in the JVL group (Figure 3(a)). Their relative levels were visualized in a heatmap based on cluster analysis (Figure 3(b)). According to the list of identified metabolites, 66 differential metabolites after JVL surgery were used for metabolite sets enrichment analysis using MetaboAnalyst software. As shown in Figure 3(c), metabolite sets analysis revealed that alterations in valine, leucine, and isoleucine biosynthesis; cysteine and methionine metabolism; butanoate metabolism; nicotinate and nicotinamide metabolism; pantothenate and CoA biosynthesis; and alanine, aspartate and glutamate metabolism had good hits. In this context, we also analyzed several metabolites of neurotransmitter amino acids and their derivatives, including γ-aminobutyric acid (GABA), nicotinamide (NAM), acetyl-L-carnitine (ALCAR), and NAC, and found that all decreased significantly in the JVL group (FC = 0.806, P = 0.045 for GABA; FC = 0.780, P = 0.008 for NAM; FC = 0.682, P = 0.007 for ALCAR; FC = 0.703, P = 0.028 for NAC; Figure 3(d)). Among the identified differential metabolites, the contents of the metabolites in the CSF (Figure 3(e); Figure S2A, E and I), cortex (Figure 3(f); Figure S2B, F and J), and hippocampus (Figure 3(g); Figure S2C, G and K) regions of JVL rats were significantly lower than those in sham rats, but the plasma (Figure 3(h); Figure S2D, H and L) showed no significant change.
Figure 3.
Characterization of metabolic differences between the sham and JVL groups. (a) Volcano plots of the sham and JVL groups. (b) Heatmap of the 66 differential metabolites identified from the comparison of the sham versus JVL groups (n = 3). The colors from green to red in the heatmap indicate the elevation of metabolites. (c) Metabolite sets enrichment analysis based on the altered metabolites. The color denotes the significance from highest (red) to lowest (yellow). (d) Significantly altered differential metabolites are presented as the ratio of the abundance in JVL rats to those in sham rats (n = 3). (e–h) Comparison of the concentration of NAC in the CSF (e), cortex (f), hippocampus (g), and plasma (h). All data are presented as the mean ± SD (two-tailed unpaired Student’s t-test) (e–h). Dots depict individual samples in e–h. Data are representative of at least five independent experiments in e–h. ns, with no significance; *p < 0.05; **p < 0.01; ***p < 0.001. GABA: γ-aminobutyric acid; NAM: nicotinamide; ALCAR: acetyl-L-carnitine; NAC: N-acetyl-L-cysteine.
NAC supplementation mitigated cognitive impairment and damage to hippocampal LTP in cerebral venous congestion rats
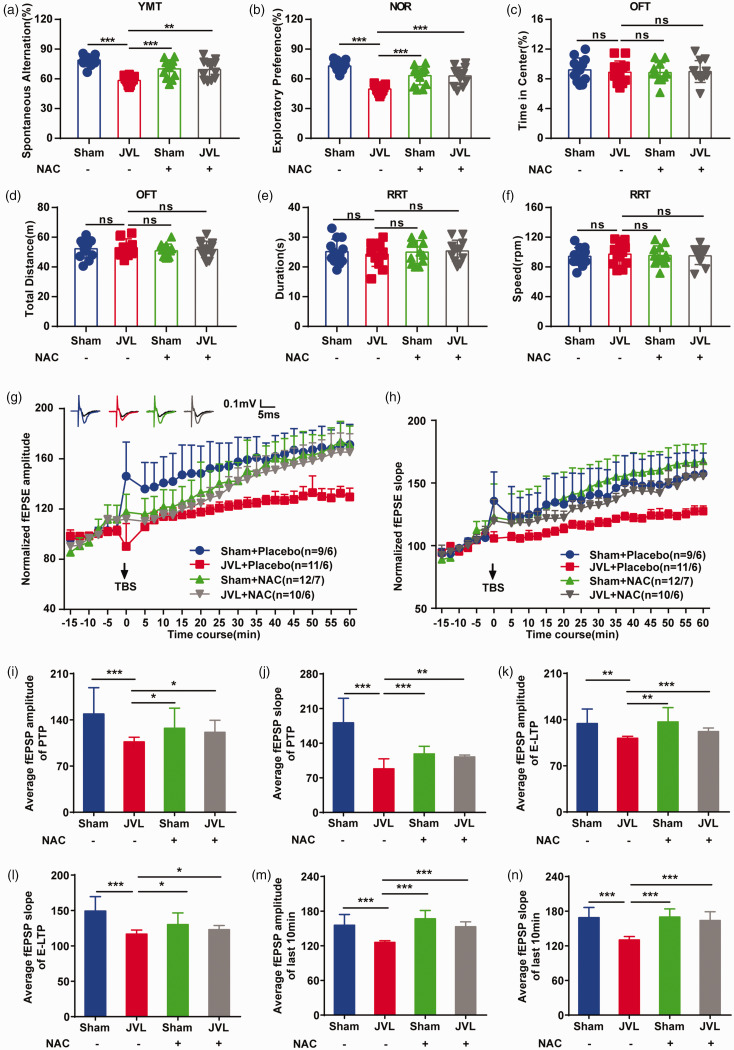
Metabolites, including GABA,36,37 NAM,38,39 ALCAR,40,41 and NAC,42,43 have been reported to have protective and therapeutic potential for neurological disorders. Thus, we examined their effects on learning and memory using behavioral tests in cerebral venous congestion rats. First, we measured concentration changes in the CSF, cortex, hippocampus, and plasma. A significant increase in NAC concentration (Figure S3A-D) was observed after NAC administration in every region (GABA, NAM, and ALCAR level data are not shown here). Then, we tested hippocampus-dependent spatial learning and memory behaviors. Both spontaneous alternation in the Y-maze and exploratory preference in the NOR of JVL rats were lower, and NAC, GABA, NAM, and ALCAR were able to mitigate the decrease to different extents (Figure 4(a) and (b); Figure S4A and B). Importantly, NAC could recover their reduction to a level closest to that in sham rats, demonstrating that NAC is the best option to ameliorate cognitive impairment induced by cerebral venous congestion. Meanwhile, to exclude the possibility that the improvement in cognitive performance by NAC, GABA, NAM, or ALCAR was due to the enhancement of movement ability, we used the OFT and RRT to detect spontaneous locomotor behavior activity and found no significant difference among the groups (Figure 4(c) to (f); Figure S4C–F), indicating that NAC, GABA, NAM, and ALCAR did not affect locomotor activity in cerebral venous congestion rats.
Figure 4.
NAC supplementation rescued impaired learning and memory ability and damaged hippocampal LTP in cerebral venous congestion rats. (a) Spontaneous alternation in the Y-maze (n = 12). (b) Exploratory preference in the NOR (n = 12). (c, d) Continued.Percentage of time spent in the center area and total distance in the center area in the OFT (n = 12). (e, f) Duration and speed to fall in the RRT (n = 12). (g, h) Summary plots of normalized fEPSP amplitude (%) and slope (%) during the recording period in the dentate gyrus. The fEPSP was induced by 5 × TBS (arrow). Inset traces: representative traces of fEPSP before (black curve) and after (blue curve for Sham + Placebo; red curve for JVL + Placebo; green curve for Sham + NAC; grey curve for JVL + NAC) the induction of potentiation. (i–n) Normalized fEPSP amplitude and slope for the PTP (0–2 min post-TBS) (i, j), E-LTP (15–20 min post-TBS) (k, l), and last 10 min (m, n). All data are presented as the mean ± SD. Statistical significance was calculated using a two-tailed unpaired Student’s t-test (a–f, i–n) or repeated-measures analysis of variance (g, h). Dots depict individual samples in a–f. Data are representative of at least five independent experiments. ns, with no significance; *p < 0.05; **p < 0.01; ***p < 0.001. NOR: novel object recognition; OFT: open-field test; RRT: rotarod test; LTP: long-term potentiation; fEPSP: field excitatory postsynaptic potential; TBS: theta-burst stimulation; PTP: post-tetanic potentiation; E-LTP: early-LTP; NAC: N-acetyl-L-cysteine.
Considering the profound effect of NAC on learning and memory behaviors, we determined whether the molecular mechanisms underlying synaptic plasticity are affected by NAC. After treating JVL rats with the NAC for 1 month, we examined the effect of NAC on long-term synaptic strength and found that the fEPSP amplitudes and slopes of both groups were slightly but nonsignificantly higher than those of the treated sham group (Figure 4(g) and (h)), while the average fEPSP amplitudes ([JVL + NAC] vs. [JVL +Placebo], P = 0.023; Figure 4(i)) and slopes ([JVL +NAC] vs. [JVL + Placebo], P = 0.002; Figure 4(j)) in the PTP; the average fEPSP amplitudes ([JVL +NAC] vs. [JVL + Placebo], P < 0.001; Figure 4(k)) and slopes ([JVL + NAC] vs. [JVL + Placebo], P = 0.023; Figure 4(l)) in the E-LTP; the average fEPSP amplitudes ([JVL + NAC] vs. [JVL + Placebo], P < 0.001; Figure 4(m)) and slopes ([JVL + NAC] vs. [JVL + Placebo], P < 0.001; Figure 4(n)) in the L-LTP were significantly higher in NAC-treated JVL rats than in control JVL rats. In addition, both the input-output curves of the field potential (Figure S3E and F) and the paired-pulse ratio (Figure S3G and H) were unchanged in hippocampal slices in all groups. Importantly, detrimental effects, such as toxic pathological changes in the brain, heart, liver, spleen, lung, and kidney, were not observed in the placebo and NAC treatment groups (Figure S5A-C). Overall, these results imply that NAC exhibits the ability to restore synaptic plasticity in the form of LTP in cerebral venous congestion rats.
Clinical characteristics of healthy individuals and cerebral venous congestion patients
Data from rodents suggested learning and memory impairments accompanied by alteration of the metabolite profiles in the brains of cerebral venous congestion rats. Importantly, among these metabolites, NAC supplementation can partially reverse memory impairment caused by cerebral venous congestion, suggesting that cerebral venous congestion may lead to learning and memory impairments by affecting NAC deficiency. However, what about cerebral venous congestion patients? To further validate the association of NAC concentration with learning and memory impairment in cerebral venous congestion patients. 36 cerebral venous congestion patients were diagnosed by MRI + MRV, CT + CTV, or DSA, and 36 healthy controls (HCs) matched for age, sex, and body mass index (BMI) were selected.
The baseline characteristics of these participants are shown in Table 1. There were no significant differences in age, sex, BMI, or biochemical indicators, including glucose, TC, HDL-C, and LDL-C, in plasma between the HCs and cerebral venous congestion patients. Additionally, the MMSE scores (P = 0.000) and SCD scores (P = 0.000) of HCs and cerebral venous congestion patients were significantly different.
Table 1.
Clinical characteristics of participants.
| Variable | Healthy control(n = 36) | Cerebral venous congestion patient (n = 36) | Z value | P value |
|---|---|---|---|---|
| Sex (female) | 62.29 ± 8.40 | 58.63 ± 9.00 | −0.035 | 0.972 |
| Age (IQR), year | 62.29 ± 8.40 | 58.63 ± 9.12 | −1.595 | 0.111 |
| BMI | 24.12 ± 3.04 | 23.86 ± 3.88 | −0.290 | 0.772 |
| SBP (mmHg) | 127.55 ± 19.55 | 121.89 ± 15.45 | −0.773 | 0.439 |
| DBP (mmHg) | 78.26 ± 12.45 | 73.32 ± 10.40 | −1.034 | 0.301 |
| WBC (109/L) | 6.53 ± 0.88 | 6.92 ± 0.75 | −0.290 | 0.772 |
| Tc (mmol/L) | 4.03 ± 0.69 | 4.31 ± 1.82 | −1.563 | 0.118 |
| Tg (mmol/L) | 1.35 ± 0.48 | 1.25 ± 0.38 | −1.406 | 0.160 |
| HDL (mmol/L) | 1.19 ± 0.27 | 1.12 ± 0.27 | −1.179 | 0.072 |
| LDL (mmol/L) | 2.39 ± 0.42 | 2.48 ± 0.44 | −0.525 | 0.600 |
| Glu (μmol/L) | 4.99 ± 0.53 | 4.88 ± 0.59 | −1.345 | 0.179 |
| NAC (mmol/L) | 1.06 ± 0.23 | 0.35 ± 0.12 | −7.045 | 0.000 |
| SCD score | 0.45 ± 0.96 | 3.08 ± 2.17 | −5.205 | 0.000 |
| MMSE score | 29.35 ± 1.14 | 27.61 ± 1.51 | −4.587 | 0.000 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; WBC: white blood cell; TC: total cholesterol; Tg: triglyceride; HDL: high-density lipoprotein; LDL: low-density lipoprotein; Glu: glucose; NAC: N-acetyl-L-cysteine; SCD: subjective cognitive decline; MMSE: mini-mental state examination.
Furthermore, the concentrations of GABA, NAM, ALCAR, and NAC were detected in plasma and CSF by ELISA in HCs and cerebral venous congestion patients. As shown in Figure 5(a) to (d), the contents of these metabolites in the patients were significantly lower than those in the HCs. Additionally, the concentration of NAC in CSF was negatively correlated with the SCD score (r = −0.528, P = 0.000; Figure 5(e)) and positively correlated with the MMSE score (r = 0.493, P = 0.000; Figure 5(f)). These results suggest that cerebral venous congestion associated with NAC deficiency may lead to learning and memory impairments.
Figure 5.
The abundance of differential metabolites in healthy controls and cerebral venous congestion patients, as well as the correlation between NAC concentration and cognitive domains. (a-d) Comparison of the concentrations of GABA (a), NAM (b), ALCAR (c), and NAC (d) in healthy controls and cerebral venous congestion patients (n = 36). (e, f) The correlation between NAC concentration and SCD score (e) or MMSE score (f). All data are shown as the mean ± SD (two-tailed unpaired Student’s t-test). Dots depict individual samples. Data are representative of at least five independent experiments in a–d. ns, with no significance; *p < 0.05; **p < 0.01; ***p < 0.001. GABA: γ-aminobutyric acid; NAM: nicotinamide; ALCAR: acetyl-L-carnitine; NAC: N-acetyl-L-cysteine.
Discussion
The results of this study verified that cerebral venous congestion significantly reduced learning and memory and illustrated the relevant electrophysiological mechanism in a validated rat model. Based on untargeted metabolomics, we also found a deficiency of NAC that occurred in cerebral venous congestion rats. Importantly, supplementation with NAC mitigated impaired LTP and synaptic function, as well as cognitive impairment.
The low-pressure, low-velocity, and large-volume venous circulation of the brain plays critical roles in the maintenance of CNS homeostasis.4,7,28,33 Cerebral venous congestion can lead to a wide range of neuropathological consequences, including blood-brain barrier (BBB) disruption, 27 neuroinflammation, 27 exacerbation of neurodegeneration, 33 development of cerebral microhemorrhages of venous origin, 21 altered production of cerebrospinal fluid,28,44 impaired function of the lymphatic system 6 and dysregulation of CBF. 14 Based on MRI assessment, recent clinical evidence has suggested that cerebral venous obstructions are closely related to neurodegenerative processes. Zhu's group45,46 elucidated the significant association between deep medullary veins (DMVs) disruption and brain atrophy. Zhang et al. 47 found that DMVs insufficiency may be one of the crucial pathogenic mechanisms of MRI-visible perivascular spaces in basal ganglia (BG-PVS), a common pathological phenomenon involved in dementia.
The jugular veins are considered to be the main pathways of cerebral venous drainage 33 and play an important role in regulating ICP, 48 as well as maintaining homeostasis of cerebral perfusion in physiological and pathological settings.33,48 In most cases, cerebral venous flow can be influenced by anatomical variations, incompetence of the jugular valve, and changes in central venous or intrathoracic pressure. 49 Previous studies have established a rodent model of cerebral venous congestion by internal and external JVL,21,27,30 which can be adapted to study the consequences of cerebral venous congestion as they relate to BBB disruption, cerebral venous hypertension, dysregulation of CBF, and impaired learning and memory. 27 Indeed, we observed a significant increase in ICP and a decrease in CBF after JVL surgery. Our results also expand on previous reports of the association between the extent of hypoperfusion in the brain parenchyma and the severity of cerebral venous congestion in patients. 50 Cognitive impairment can occur as a result of persistent cerebral hypoperfusion in the setting of venous obstruction. In this study, we found that hippocampal LTP in JVL rats was suppressed compared with sham rats during the whole LTP stage (JVL vs. sham, P < 0.001 for amplitude, P < 0.001 for slope), PTP (JVL vs. sham, P < 0.001 for amplitude, P < 0.001 for slope), E-LTP (JVL vs. sham, P < 0.001 for amplitude, P < 0.001 for slope), and L-LTP (JVL vs. sham, P < 0.001 for amplitude, P < 0.001 for slope) were damaged in terms of both the amplitude and the slope of LTP, suggesting that cerebral venous congestion is the potential cause of cognitive impairment.
Abundant reports have investigated the relationship between amino acid metabolism and cognitive function.51–54 In mild cognitive impairment, some amino acids increase, while others decrease as a result of their oxidation as neuronal energy sources when glucose catabolism becomes impaired. For example, the neuronal glutamate-glutamine cycle is disrupted in mild cognitive impairment, and excess proline has been implicated in the prevention of glutamate uptake in the brain. Other investigations have linked the modification of amino acids in the brain. To date, amino acids that have been reported to be associated with cognitive impairments are glutamine/glutamate, leucine, valine, tyrosine, lysine, and cysteine.51,52,55,56 NAC, an N-acetyl derivative of the natural amino acid L-cysteine, is reported to remain a strong candidate for adjunct treatment for psychiatric disorders,42,57–60 including depression,42,58,59 bipolar depression,42,58,59,61,62 autism,42,63 compulsive and grooming disorders,42,58,59,64 schizophrenia,42,58,59,65,66 AD and PD,42,43,60 with many clinical and preclinical trials underway. In this study, we used untargeted metabolomics of LC-MS/MS analysis to identify different metabolites associated with cerebral venous congestion and found several significantly changed metabolites, including NAC. As an effective non-specific antioxidant, NAC plays a pivotal role in oxidative stress homeostasis through the production of GSH, the cysteine/cystine cycle, or the action of the glutamate/cystine antiporter.67,68 In particular, the endogenously produced molecule NAC is critical for hippocampal function and several behavioral domains and is often cited as a master regulator of multiple neuropsychiatric disorders.42,57–59,69 According to the two-hit vascular hypothesis of AD,70–72 hypoxia-induced cognitive dysfunction in AD expressing elevated levels of the amyloidogenic proteins implicated, which can be reversed or ameliorated by NAC administration in a manner associated with reductions in oxidative damage. Converging evidence from our group has shown that supplementation with NAC can mitigate cognitive deficits. Such neuroprotective effects are relevant because NAC can ameliorate synaptic deficits and rescue the impaired LTP in the hippocampus, providing proof that NAC is involved in hippocampal plasticity processes.
Biomarkers have emerged as crucial components in the search for the cause of acute and chronic cerebrovascular diseases and are lynchpins in the work that will lead to pharmacotherapeutic breakthroughs. 73 Cerebral circulation dysregulation and cerebrovascular disease may lead to metabolic abnormalities in the brain, causing severe cerebral functional defects, and metabolites in a pathological state could provide useful insight into pathogenesis, identification of therapeutic targets, and biomarker discovery. 74 This study found that a deficiency of NAC occurred in cerebral venous congestion rats, and the CSF NAC concentration of the cerebral venous congestion group was significantly lower than that of the HC group. In addition, our results have identified a significant association between high CSF concentrations of NAC and lower scores on the neuropsychological SCD cognitive testing, or higher scores on the neuropsychological MMSE. These findings suggest that NAC may be a biomarker with high translational value for targeting learning and memory impairment due to cerebral venous congestion.
The findings of our study should be interpreted within its limitations. First, human cerebral venous outflow mainly relies on the internal jugular vein and vertebral vein plexus. In rodents, the external jugular vein is the main channel responsible for the drainage of intracranial blood. In the present study, the pathophysiological changes induced by cerebral venous congestion were investigated only in a rat model. Therefore, new investigations using primate animal models are needed to better understand the relationships between cerebral venous congestion and the pathogenesis of cognitive impairments in the future. Second, memory loss is likely mediated by defects in synaptic functionality. The structural adaptation of neurons is also an important form of synaptic plasticity. The loss of neurons, abnormal neuronal morphology, such as reduced dendritic spine density, and abnormal synaptic structure were all consistently associated with impaired LTP. Our observations were limited to the number and anatomical structure of neurons, neither of which was found to be altered. Studies investigating the dendritic length, complexity, and dendritic spine density of pyramidal neurons in the DG area are also warranted. Finally, this research focused on identifying metabolites that differ between cerebral venous congestion patients and healthy individuals and discovering potential biomarkers. We identified NAC as a promising novel diagnostic and therapeutic option for cerebral venous congestion. However, the precise link between NAC and cerebral venous congestion-related cognitive deficits is not yet clear. In future research, proteomics and genomics should be used in combination to explore and evaluate the specific mechanisms that underlie cerebral venous congestion-related physiological conditions and aberrant processes.
In this study, the pathological effects of cerebral venous congestion on cognitive impairment were confirmed by a validated rat model. Meanwhile, deficiency of NAC was detected by untargeted metabolomics; supplementation with NAC potentially alleviated cognitive impairments. Additionally, a correlation between NAC and cognitive scores was found in the cerebral venous congestion patient cohort. This study provides a new perspective for scientific understanding of the function of the cerebral venous system and the pathological mechanism of VCI disorders, as well as a new concept for the prevention and treatment of cognitive impairment related to cerebral venous congestion.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231182244 for Cerebral venous congestion alters brain metabolite profiles, impairing cognitive function by Huimin Wei, Huimin Jiang, Yifan Zhou, Xuechun Xiao, Chen Zhou and Xunming Ji in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Cheung Kong (Changjiang) Scholars Program (T2014251), the Pharmaceutical Collaboration Project of Beijing Science and Technology Commission (Z181100001918026), grants from National Natural Science Foundation of China (82271311).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: HW, CZ, and XJ designed research; HW, HJ, YZ, XX, and CZ performed research; HW, HJ, and CZ contributed and analyzed data; HW, CZ, and XJ wrote and edited the paper. XJ is responsible for the overall content as the guarantor.
ORCID iD: Xunming Ji https://orcid.org/0000-0003-0293-2744
Supplementary material: Supplemental material for this article is available online.
References
- 1.Dichgans M, Leys D. Vascular cognitive impairment. Circ Res 2017; 120: 573–591. [DOI] [PubMed] [Google Scholar]
- 2.Toyama K, Spin JM, Mogi M, et al. Therapeutic perspective on vascular cognitive impairment. Pharmacol Res 2019; 146: 104266. [DOI] [PubMed] [Google Scholar]
- 3.Haacke EM, Beggs CB, Habib C. The role of venous abnormalities in neurological disease. Rev Recent Clin Trials 2012; 7: 100–116. [DOI] [PubMed] [Google Scholar]
- 4.Zivadinov R, Chung C-P. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med 2013; 11: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simka M, Skuła M. Potential involvement of impaired venous outflow from the brain in neurodegeneration: lessons learned from the research on chronic cerebrospinal venous insufficiency. Rev Recent Clin Trials 2019; 14: 235–236. [DOI] [PubMed] [Google Scholar]
- 6.Ghali MGZ, Marchenko V, Yaşargil MG, et al. Structure and function of the perivascular fluid compartment and vertebral venous plexus: illumining a novel theory on mechanisms underlying the pathogenesis of Alzheimer's, cerebral small vessel, and neurodegenerative diseases. Neurobiol Dis 2020; 144: 105022. [DOI] [PubMed] [Google Scholar]
- 7.Zivadinov R. Is there a link between the extracranial venous system and Central nervous system pathology? BMC Med 2013; 11: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang T, Sun Y, Lu Z, et al. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev 2017; 34: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haacke EM, Ge Y, Sethi SK, et al. An overview of venous abnormalities related to the development of lesions in multiple sclerosis. Front Neurol 2021; 12: 561458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander JS, Prouty L, Tsunoda I, et al. Venous endothelial injury in central nervous system diseases. BMC Med 2013; 11: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung C-P, Beggs C, Wang P-N, et al. Jugular venous reflux and white matter abnormalities in Alzheimer's disease: a pilot study. J Alzheimers Dis 2014; 39: 601–609. [DOI] [PubMed] [Google Scholar]
- 12.Lai AY, Dorr A, Thomason LAM, et al. Venular degeneration leads to vascular dysfunction in a transgenic model of Alzheimer's disease. Brain 2015; 138: 1046–1058. [DOI] [PubMed] [Google Scholar]
- 13.Morrone CD, Bishay J, McLaurin J. Potential role of venular amyloid in Alzheimer's disease pathogenesis. IJMS 2020; 21: 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncombe J, Kitamura A, Hase Y, et al. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci (Lond) 2017; 131: 2451–2468. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Wu B, Wang X, et al. Vascular, flow and perfusion abnormalities in Parkinson's disease. Parkinsonism Relat Disord 2020; 73: 8–13. [DOI] [PubMed] [Google Scholar]
- 16.Fekete G, Tamás G, Novák L, et al. Globus pallidus internus deep brain stimulation for a patient with Parkinson's disease and cerebral developmental venous anomaly in the region of the basal ganglia. Neurol India 2022; 70: 457–458. [DOI] [PubMed] [Google Scholar]
- 17.Han K, Hu H-H, Chao AC, et al. Transient global amnesia linked to impairment of brain venous drainage: an ultrasound investigation. Front Neurol 2019; 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis SL. Aetiology of transient global amnesia. Lancet 1998; 352: 397–399. [DOI] [PubMed] [Google Scholar]
- 19.Chung CP, Hsu HY, Chao AC, et al. Detection of intracranial venous reflux in patients of transient global amnesia. Neurology 2006; 66: 1873–1877. [DOI] [PubMed] [Google Scholar]
- 20.Chung C-P, Chao AC, Hsu H-Y, et al. Decreased jugular venous distensibility in migraine. Ultrasound Med Biol 2010; 36: 11–16. [DOI] [PubMed] [Google Scholar]
- 21.Nyul-Toth A, Fulop GA, Tarantini S, et al. Cerebral venous congestion exacerbates cerebral microhemorrhages in mice. Geroscience 2022; 44: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moody DM, Brown WR, Challa VR, et al. Periventricular venous collagenosis: association with leukoaraiosis. Radiology 1995; 194: 469–476. [DOI] [PubMed] [Google Scholar]
- 23.Shaaban CE, Aizenstein HJ, Jorgensen DR, et al. In vivo imaging of venous side cerebral small-vessel disease in older adults: an MRI method at 7T. AJNR Am J Neuroradiol 2017; 38: 1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rustenhoven J, Drieu A, Mamuladze T, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 2021; 184: 1000–1016.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooghiemstra AM, Bertens AS, Leeuwis AE, et al. The missing link in the pathophysiology of vascular cognitive impairment: Design of the Heart-Brain study. Cerebrovasc Dis Extra 2017; 7: 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon JA, Moffitt P, Perez-Moreno AC, et al. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail 2017; 23: 464–475. [DOI] [PubMed] [Google Scholar]
- 27.Fulop GA, Ahire C, Csipo T, et al. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience 2019; 41: 575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulop GA, Tarantini S, Yabluchanskiy A, et al. Role of age-related alterations of the cerebral venous circulation in the pathogenesis of vascular cognitive impairment. Am J Physiol Heart Circ Physiol 2019; 316: H1124–H1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou D, Ding J, Asmaro K, et al. Clinical characteristics and neuroimaging findings in internal jugular venous outflow disturbance. Thromb Haemost 2019; 119: 308–318. [DOI] [PubMed] [Google Scholar]
- 30.Auletta L, Greco A, Albanese S, et al. Original research: feasibility and safety of two surgical techniques for the development of an animal model of jugular vein occlusion. Exp Biol Med (Maywood) 2017; 242: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab 2020; 40: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X-Y, Liu M-G, Yuan D-L, et al. Nociception-induced spatial and temporal plasticity of synaptic connection and function in the hippocampal formation of rats: a multi-electrode array recording. Mol Pain 2009; 5: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong L-S, Guo Z-N, Ou Y-B, et al. Cerebral venous collaterals: a new fort for fighting ischemic stroke? Prog Neurobiol 2018; 163–164: 172–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 1993; 361: 31–39. [DOI] [PubMed] [Google Scholar]
- 35.Luo C, Kuner T, Kuner R. Synaptic plasticity in pathological pain. Trends Neurosci 2014; 37: 343–355. [DOI] [PubMed] [Google Scholar]
- 36.Wong CGT, Bottiglieri T, Snead OC. GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol 2003; 54 Suppl 6: S3–12. [DOI] [PubMed] [Google Scholar]
- 37.Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 2019; 102: 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019; 572: 474–480. [DOI] [PubMed] [Google Scholar]
- 39.Tribble JR, Otmani A, Sun S, et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol 2021; 43: 101988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasca C, Bigio B, Lee FS, et al. Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proc Natl Acad Sci U S A 2018; 115: 8627–8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pennisi M, Lanza G, Cantone M, et al. Acetyl-L-carnitine in dementia and other cognitive disorders: a critical update. Nutrients 2020; 12: 1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berk M, Malhi GS, Gray LJ, et al. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci 2013; 34: 167–177. [DOI] [PubMed] [Google Scholar]
- 43.Tardiolo G, Bramanti P, Mazzon E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules 2018; 23: 3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aso T, Sugihara G, Murai T, et al. A venous mechanism of ventriculomegaly shared between traumatic brain injury and normal ageing. Brain 2020; 143: 1843–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ao D-H, Zhang D-D, Zhai F-F, et al. Brain deep medullary veins on 3-T MRI in a population-based cohort. J Cereb Blood Flow Metab 2021; 41: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Z-Y, Zhai F-F, Ao D-H, et al. Deep medullary veins are associated with widespread brain structural abnormalities. J Cereb Blood Flow Metab 2022; 42: 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang K, Zhou Y, Zhang W, et al. MRI-visible perivascular spaces in basal ganglia but not centrum semiovale or hippocampus were related to deep medullary veins changes. J Cereb Blood Flow Metab 2022; 42: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beggs CB. Venous hemodynamics in neurological disorders: an analytical review with hydrodynamic analysis. BMC Med 2013; 11: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall-Goebel K, Laurie SS, Alferova IV, et al. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open 2019; 2: e1915011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zamboni P, Menegatti E, Weinstock-Guttman B, et al. Hypoperfusion of brain parenchyma is associated with the severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: a cross-sectional preliminary report. BMC Med 2011; 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tynkkynen J, Chouraki V, van der Lee SJ, et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer's disease: a prospective study in eight cohorts. Alzheimers Dement 2018; 14: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toledo JB, Arnold M, Kastenmüller G, et al. Metabolic network failures in Alzheimer's disease: a biochemical road map. Alzheimers Dement 2017; 13: 965–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shao Y, Le W. Recent advances and perspectives of metabolomics-based investigations in Parkinson's disease. Mol Neurodegener 2019; 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao Y, Li T, Liu Z, et al. Comprehensive metabolic profiling of Parkinson's disease by liquid chromatography-mass spectrometry. Mol Neurodegener 2021; 16: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wuolikainen A, Andersen PM, Moritz T, et al. ALS patients with mutations in the SOD1 gene have an unique metabolomic profile in the cerebrospinal fluid compared with ALS patients without mutations. Mol Genet Metab 2012; 105: 472–478. [DOI] [PubMed] [Google Scholar]
- 56.Polis B, Samson AO. Role of the metabolism of branched-chain amino acids in the development of Alzheimer's disease and other metabolic disorders. Neural Regen Res 2020; 15: 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skvarc DR, Dean OM, Byrne LK, et al. The effect of N-acetylcysteine (NAC) on human cognition – a systematic review. Neurosci Biobehav Rev 2017; 78: 44–56. [DOI] [PubMed] [Google Scholar]
- 58.Raghu G, Berk M, Campochiaro PA, et al. The multifaceted therapeutic role of N-acetylcysteine (NAC) in disorders characterized by oxidative stress. Curr Neuropharmacol 2021; 19: 1202–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smaga I, Frankowska M, Filip M. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br J Pharmacol 2021; 178: 2569–2594. [DOI] [PubMed] [Google Scholar]
- 60.Slattery J, Kumar N, Delhey L, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev 2015; 55: 294–321. [DOI] [PubMed] [Google Scholar]
- 61.Berk M, Copolov DL, Dean O, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder – a double-blind randomized placebo-controlled trial. Biol Psychiatry 2008; 64: 468–475. [DOI] [PubMed] [Google Scholar]
- 62.Berk M, Dean O, Cotton SM, et al. The efficacy of N-acetylcysteine as an adjunctive treatment in bipolar depression: an open label trial. J Affect Disord 2011; 135: 389–394. [DOI] [PubMed] [Google Scholar]
- 63.Hardan AY, Fung LK, Libove RA, et al. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol Psychiatry 2012; 71: 956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith L, Tracy DK, Giaroli G. What future role might N-acetyl-cysteine have in the treatment of obsessive compulsive and grooming disorders?: a systematic review. J Clin Psychopharmacol 2016; 36: 57–62. [DOI] [PubMed] [Google Scholar]
- 65.Bulut M, Savas HA, Altindag A, et al. Beneficial effects of N-acetylcysteine in treatment resistant schizophrenia. World J Biol Psychiatry 2009; 10: 626–628. [DOI] [PubMed] [Google Scholar]
- 66.Lavoie S, Murray MM, Deppen P, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 2008; 33: 2187–2199. [DOI] [PubMed] [Google Scholar]
- 67.Venè R, Castellani P, Delfino L, et al. The cystine/cysteine cycle and GSH are independent and crucial antioxidant systems in malignant melanoma cells and represent druggable targets. Antioxid Redox Signal 2011; 15: 2439–2453. [DOI] [PubMed] [Google Scholar]
- 68.Iyer SS, Jones DP, Brigham KL, et al. Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury. Am J Respir Cell Mol Biol 2009; 40: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thakurta IG, Banerjee P, Bagh MB, et al. Combination of N-acetylcysteine, α-lipoic acid and α-tocopherol substantially prevents the brain synaptosomal alterations and memory and learning deficits of aged rats. Exp Gerontol 2014; 50: 19–25. [DOI] [PubMed] [Google Scholar]
- 70.Sweeney MD, Kisler K, Montagne A, et al. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 2018; 21: 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 2017; 18: 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hansson O. Biomarkers for neurodegenerative diseases. Nat Med 2021; 27: 954–963. [DOI] [PubMed] [Google Scholar]
- 74.Montaner J, Ramiro L, Simats A, et al. Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nat Rev Neurol 2020; 16: 247–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231182244 for Cerebral venous congestion alters brain metabolite profiles, impairing cognitive function by Huimin Wei, Huimin Jiang, Yifan Zhou, Xuechun Xiao, Chen Zhou and Xunming Ji in Journal of Cerebral Blood Flow & Metabolism