Abstract
Systems biology can be defined as “the study of a biological process in which all of the relevant components are investigated together in parallel to discover mechanism.” Although the approach is not new, it has come to the forefront as a result of genome sequencing projects circa 2000. It has elements of large-scale data acquisition (chiefly next-generation sequencing [NGS]-based methods and protein mass spectrometry) and large-scale data analysis (‘big data’ integration and Bayesian modeling). Here we discuss these methodologies and show how they can be applied to understand the downstream effects of GPCR signaling, specifically looking at how the neurohypophyseal peptide hormone vasopressin, working through the V2 receptor and PKA activation, regulates the water channel aquaporin-2. The emerging picture provides a detailed framework for understanding the molecular mechanisms involved in water balance disorders, pointing the way to improved treatment of both polyuric disorders and water-retention disorders causing dilutional hyponatremia.
Keywords: GPCR, protein mass spectrometry, next-generation DNA sequencing, diabetes insipidus, syndrome of inappropriate antidiuresis, hyponatremia, collecting duct, kidney
I. Background and Introduction
Disorders of water balance are common among patients hospitalized in tertiary care centers (1; 2). Most such disorders are associated with dysregulation of renal water excretion involving loss of homeostatic control of osmotic water transport across the epithelium of the renal collecting duct. These water balance disorders can be divided into two groups (Table 1): (a) polyuric disorders (diabetes insipidus syndromes) characterized by high rates of urinary water excretion and water intake with a tendency toward contraction of the extracellular fluid (ECF) volume (3; 4); and (b) dilutional hyponatremia due to excessive water retention including the syndrome of inappropriate antidiuresis (SIAD) (5; 6). Clinical management, including pharmacological therapy, of these disorders depends on understanding the physiological processes that control water transport by the collecting duct.
Table 1.
Classification of Water Balance Disorders
| A. Polyuric Disorders | ||
|---|---|---|
| 1. Central | a. Neurohypophyseal diabetes insipidus | |
| b. Primary polydipsia | ||
|
| ||
| 2. Nephrogenic Diabetes Insipidus | a. X-linked nephrogenic diabetes insipidus | |
| b. Autosomal nephrogenic diabetes insipidus | ||
| c. Acquired nephrogenic diabetes insipidus | ||
| i) Drug induced (e.g., lithium) | ||
| ii) Hypercalcemic | ||
| iii) Hypokalemic | ||
| d. Osmotic diuresis (e.g., diabetes mellitus) | ||
|
| ||
| 3. Gestational | ||
|
| ||
| B. Dilutional Hyponatremia | ||
|
| ||
| 1. Syndrome of Inappropriate Antidiuresis (SIAD) | a. Ectopic secretion of antidiuretic substances | |
| b. Drug induced (e.g., chlorpropamide) | ||
| c. CNS-related (e.g., brain tumor, head trauma) | ||
| d. Pulmonary (e.g., mechanical ventilation, tumors) | ||
|
| ||
| 2. Nephrogenic SIAD | ||
|
| ||
| 3. Edematous States | a. Congestive heart failure | |
| b. Hepatic cirrhosis | ||
| c. Nephrotic syndrome | ||
|
| ||
| 4. Exercise-Associated | ||
Viewed at a systemic level (Figure 1A) (1), regulation of water balance is largely dependent on a high-gain feedback mechanism that governs the secretion of the nine-amino-acid peptide hormone arginine vasopressin (AVP) into the general circulation via the hypothalamo-neurohypophyseal axis. Osmoreceptors in the hypothalamus sense plasma osmolality (7). When plasma osmolality increases above a physiological threshold (290–295 mOsm for most individuals), e.g., as a result of respiratory water losses or sweating, AVP secretion occurs, resulting in graded increases in AVP concentration in the blood. AVP in the circulation can bind to receptors in the kidney that decrease water excretion (Fig. 1A), returning a greater fraction of filtered water back to the blood, thus countering the increase in plasma osmolality that triggered AVP secretion. The half-life of AVP in the circulation is short, approximately 24 minutes in humans (8), owing to widespread expression of vasopressinase (Gene symbol: Lnpep) and excretion by the kidney. The short half-life allows for a rapid aquaretic (water excretion) response to water intake. The rate of water excretion can vary over a broad range in response to changes in plasma vasopressin levels without substantial changes in net solute excretion, thereby allowing for independent control of salt and water excretion (9). High osmolality also triggers thirst, which is important for restoring water deficits (10). Additional receptors in the oropharynx and stomach provide important signals that modulate drinking behavior and vasopressin secretion (11).
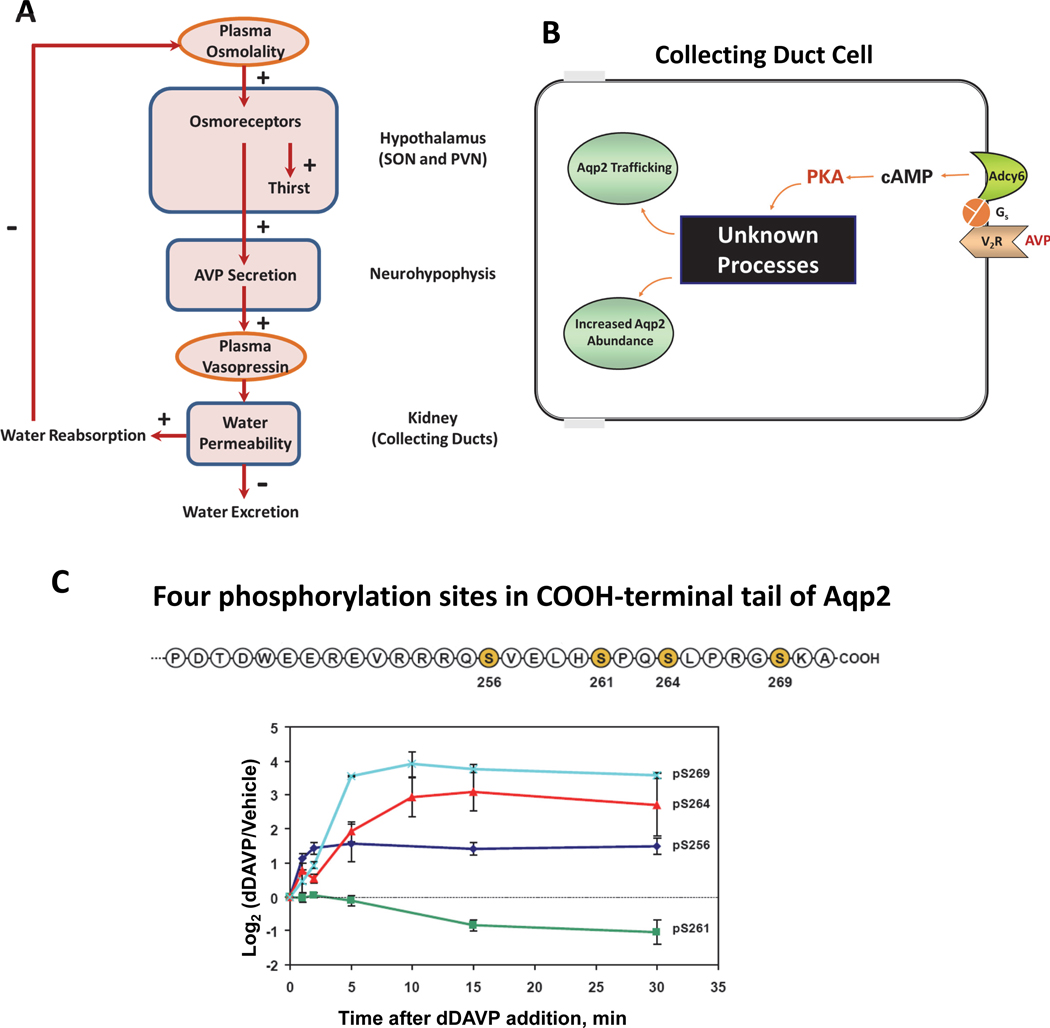
Figure 1. Regulation of water excretion by vasopressin.
(A) Feedback regulation of water excretion and thirst. Plasma osmolality is sensed in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) of the hypothalamus, regulating the secretion of arginine vasopressin (AVP) by the neurohypophysis (posterior pituitary) with concomitant regulation of thirst. The resulting increase in plasma AVP triggers actions in the kidney to increase water reabsorption from the collecting duct pro-urine to the blood, thereby lowering plasma osmolality. (B) AVP regulates water excretion by controlling osmotic water permeability in collecting duct principal cells. AVP binds to the vasopressin V2 receptor, which increases cyclic AMP production by activation of adenylyl cyclase 6 (Adcy6) via the heterotrimeric G-protein alpha subunit Gs. Elevation of cyclic AMP activates protein kinase A (PKA) that triggers increases in trafficking of the water channel aquaporin-2 (Aqp2) to the plasma membrane as well as increases in the total abundance of the Aqp2 protein. Identification of the ‘Unknown Processes’ that link PKA to regulation of Aqp2 is the main topic of this review. (C) A cluster of four phosphorylation sites in the carboxyl terminal tail of Aqp2 are regulated by vasopressin. Serines in positions 256, 261, 264, and 269 (shaded) were identified by Hoffert et al. (29). Immunoblotting with phospho-specific antibodies to each of the four sites showed that each is regulated by vasopressin although with differing time courses (30) (below).
AVP regulates renal water excretion chiefly through the control of the osmotic water permeability of collecting duct cells (Figure 1A). When AVP levels are high, the water permeability of the collecting duct principal cells is high, allowing water to move across the collecting duct epithelium from the pro-urine to the blood rather than to be excreted. This action of AVP occurs via its binding to the V2 subtype vasopressin receptor (Gene symbol: Avpr2), a G protein-coupled receptor (GPCR) that signals chiefly through Gαs and activation of adenylyl cyclase 6 (Adcy6) to produce cyclic AMP (cAMP, Figure 1B). (Terminology footnote here) V2 receptor activation also results in the mobilization of intracellular calcium (12), which generates calcium spikes that increase in frequency with addition of vasopressin (13; 14). However, vasopressin does not increase inositol 1,4,5-trisphosphate (ITP) levels or activate protein kinase C in collecting duct cells (15), suggesting that the calcium mobilization is not due to promiscuous coupling of the V2 receptor to the G-protein alpha subunit Gq/11. Instead, the calcium mobilization appears to result from effects of PKA-mediated phosphorylation of ITP receptors; this phosphorylation lowers the threshold for calcium-stimulated calcium release in the endoplasmic reticulum (16). Although other cAMP effectors are expressed in collecting duct cells, experiments involving CRISPR deletion of the two protein kinase A (PKA) catalytic genes in the mouse genome (Prkaca and Prkacb) have revealed that AVP actions in collecting duct cells are almost entirely dependent on PKA activation (16; 17), results that are consistent with the conventional view in the renal vasopressin field (18–20). In addition, the vasopressin V2 receptor can signal through the β-arrestin pathway (21), although we have questioned the physiological role of this pathway in collecting duct cells (18) based on the finding that vasopressin decreases active site phosphorylation of ERK1 and ERK2 in collecting duct cells (14; 22; 23) rather than increasing it as expected when β-arrestin signaling is activated (21). The downstream molecular target of vasopressin signaling is the water channel protein, aquaporin-2 (Aqp2).
II. The Aquaporin-2 Water Channel
The ability of vasopressin to increase the osmotic water permeability of renal collecting duct cells depends chiefly on regulation of the molecular water channel Aqp2 (Gene symbol: Aqp2) (24). Aqp2 is an integral membrane protein that forms water permeable pores in cell membranes, facilitating water movement across hydrophobic lipid bilayers. In collecting duct cells, it is responsible for water movement across the apical plasma membrane, whereas two other abundant channels, aquaporin-3 and aquaporin-4, mediate water movement across the basolateral plasma membrane (24). Under most circumstances water transport across the apical plasma membrane is rate-limiting for transepithelial movement of water, allowing control of Aqp2 to regulate transepithelial water permeability.
Physiological studies in animal models have revealed two basic modes of vasopressin-mediated regulation of the Aqp2 water channel: short-term regulation occurring over a few minutes and long-term regulation occurring over hours to days. The short-term regulation of Aqp2 occurs as a result of membrane trafficking of water channel-containing vesicles to and from the apical plasma membrane (25). Vasopressin-mediated translocation of Aqp2 to the apical plasma membrane occurs as a result of increased exocytic insertion into the plasma membrane and decreased endocytic internalization of Aqp2 (26; 27). The effect of vasopressin on Aqp2 trafficking is believed to be mediated by changes in phosphorylation at four sites in the cytosolic COOH-terminal tail of Aqp2 (28; 29) (Figure 1C). Vasopressin markedly increases Aqp2 phosphorylation at Ser269, which appears to be responsible for inhibition of Aqp2 endocytosis (30; 31), thereby increasing osmotic water permeability. Exocytosis of Aqp2 containing vesicles requires phosphorylation at a different site, Ser256 (32–34). Phosphorylation of Aqp2 at Ser256 has been shown to be necessary for phosphorylation at Ser269 and therefore it appears to be a keystone for regulation of both endocytosis and exocytosis of Aqp2 (30). In contrast, vasopressin decreases phosphorylation at Ser261 by decreasing the activity of ERK1/2 and p38 kinases (22; 35). Decreased phosphorylation of Aqp2 at Ser261 appears to increase the stability of the Aqp2 protein (35) but does not affect its trafficking (36).
Aside from the short-term action of vasopressin to regulate Aqp2 trafficking, the long-term effect increases the amount of the Aqp2 protein, thereby magnifying the short-term response (37; 38). Aqp2 protein abundance increases are paralleled by increases in the osmotic water permeability of the collecting ducts (37). Proteomics studies using metabolic labeling with stable isotopes showed that the increase in Aqp2 protein abundance in response to vasopressin occurs as a result of i increases in translation rate and protein stability (39). The increase in Aqp2 protein half-life was about 45% (from 9.8±1.3 to14.2±1.1 hours) with dDAVP treatment (39), while the Aqp2 translation rate was increased by more than 10-fold. The increase in translation rate is likely due to increased Aqp2 mRNA levels (Section V.).
Extensive studies of the role of Aqp2 in water balance disorders, both polyuric and hyponatremic syndromes (Table 1), have shown that the long-term regulation of Aqp2 abundance rather than the short-term regulation of Aqp2 trafficking is defective in almost all instances, the exception being polyuria due to hypercalcemia (24). Therefore, to understand and treat water balance disorders, one must be knowledgeable about the long-term action of vasopressin that increases the abundance of Aqp2 protein in the renal collecting duct.
III. Pharmacological Agents Targeting the V2 Vasopressin Receptor
The chief pharmacological agents in use currently for the treatment of water balance disorders are V2 receptor agonists and antagonists.
Vaptans.
The vaptans are nonpeptide antagonists of vasopressin receptors. Tolvaptan, one of them, is V2 receptor selective and widely used for treatment of hyponatremic disorders, including SIAD and the water retention that occurs in patients with severe hepatic cirrhosis or congestive heart failure (6) (Table 1). The clinical use of vaptans has recently expanded because of FDA-approval for use in autosomal dominant polycystic kidney disease (40), which is characterized by multiple renal epithelial cysts that grow in response to cAMP generation in association with V2 receptor activation (41; 42). Other vaptans are currently under development. Tolvaptan has become an important tool for investigation of the physiological actions of vasopressin in the renal collecting duct (43).
There are several alternatives to tolvaptan for the treatment of dilutional hyponatremia. Demeclocycline, a bacteriostatic tetracycline antibiotic, has been used to treat chronic hyponatremia in patients with SIAD and is a cheaper alternative to tolvaptan. Demeclocycline appears to reduce vasopressin-mediated cAMP generation through its reduction in abundance of adenylyl cyclase 5 and/or 6 protein (44), although the mechanism is not known. Oral urea is also used to treat dilutional hyponatremia (45) via its osmotic diuretic action, which increases the excretion of water that is largely free of non-urea solutes. Drug discovery projects have identified small molecules that inhibit the collecting duct urea transporter, Slc14a2, thereby enhancing urea-induced diuresis (46–49) but such agents have not yet been approved for clinical use. For chronic dilutional hyponatremia, fluid restriction remains a mainstay of treatment.
dDAVP (Desmopressin).
A V2R-selective vasopressin analog dDAVP (1-deamino-8-D-arginine vasopressin), often referred to as ‘desmopressin’ (50) is used for the treatment of central and gestational diabetes insipidus (Table 1). It exhibits prolonged antidiuretic action compared to AVP owing to enhanced resistance to vasopressinase (Gene symbol: Lnpep). dDAVP is not effective in heritable forms of nephrogenic diabetes insipidus (NDI), whether caused by mutations in the AVPR2 or the AQP2 gene. It may however have efficacy in some forms of acquired NDI. As it is a peptide, it cannot be administered orally, but can be absorbed if administered intranasally. dDAVP is widely used in physiological studies aimed at understanding V2 receptor signaling, including most of the studies described in this review. A variety of orally active anti-diuretic agents repurposed from other indications have been employed in the past for the treatment of NDI, although none have shown favorable therapeutic ratios. These include chlorpropamide, thiazide diuretics and indomethacin (51).
X-linked NDI that is caused by mutations in the V2 receptor is normally unresponsive to dDAVP treatment. Accordingly, there have been efforts to identify treatments that can potentially increase cAMP levels or activate its effectors in the collecting duct in the absence of a functional V2 receptor (52; 53). Of particular note, two Gαs-coupled GPCR, other than the V2 receptor, have been found to be selectively expressed in collecting duct principal cells: . the prostaglandin E2 (PGE2) EP4 receptor (Ptger4) (54) and the calcitonin receptor-like receptor (Calcrl) (55). Agonists for these GPCRs have the potential of producing antidiuresis in X-linked NDI patients. Indeed, an EP4 receptor agonist (ONO-AE1–329) increased cAMP and osmotic water permeability in isolated collecting ducts from rodents (54). EP4 receptor activation was also seen to increase Aqp2 trafficking to the plasma membrane in cultured collecting duct cells (56). Calcrl has alternative ligands depending on which receptor activity-modifying protein (RAMP) is co-expressed. Expression of RAMP3, a vasopressin-induced protein (57), in collecting duct predicts that either amylin or adrenomedullin is the relevant ligand. Adrenomedullin has been shown to stimulate cAMP production in isolated collecting duct segments (58). An amylin analog, pramlintide, has been approved for the treatment of type I and II diabetes mellitus (59), but we are unaware of studies investigating its effect on water excretion.
High-throughput screening has been utilized to identify small molecules that inhibit Aqp2 trafficking in response to forskolin, an activator of adenylyl cyclases, in a cell culture model (60). Among other targets, this study identified the fungicide fluconazole as a potential inhibitor of cAMP-mediated redistribution of Aqp2. However, in isolated, perfused mouse collecting ducts, fluconazole was found to increase, not decrease, osmotic water permeability, while reducing urinary output in tolvaptan-treated mice (61).
IV. The Systems Biology Concept
Because dysregulation of Aqp2 protein abundance is central to most water balance disorders (vide supra), we focus the remainder of this review on the question, “What are the molecular mechanisms by which AVP, acting through the V2 receptor and PKA activation, increases Aqp2 protein abundance in renal collecting duct cells?”. Answers to this question may aid in identifying new therapeutic approaches for water balance disorders. Beyond this, since PKA plays important roles in many other cell types, the answers may be informative with regard to treatment of disorders of other tissues and organs. The question posed above is complex and is perhaps best addressed by discovery approaches that comprise the field of systems biology.
Systems biology can be defined as “The study of a biological process in which all of the relevant components are studied together in parallel to discover mechanism (62)”. Systems biology is often contrasted with reductionist scientific approaches that break problems into their elements and then study them sequentially. Much of the bedrock in understanding of biological mechanisms comes from reductionist studies. However, with the advent of genome projects at the beginning of the century, which provided nearly comprehensive identification of genes and their nucleotide base sequences, systems level (“-omics”) methodologies such as protein mass spectrometry and next-generation DNA sequencing (NGS) have become feasible, facilitating wholesale discovery of genes and proteins involved in biological processes. Such discoveries represent new hypotheses that can be addressed more deeply by traditional reductionist approaches. In some cases, systems biology methods can identify so-called ‘emergent properties’ of complex systems that are not recognizable through studies of individual proteins (63). Such emergent properties may dominate in complex pathophysiological processes such as those involved in diabetes insipidus or hyponatremic syndromes. For such disorders, systems approaches can yield mechanistic understanding.
Systems biology investigations rely extensively on two methodologies, protein mass spectrometry (proteomics) and NGS. In previous reviews, we have discussed applications of proteomics and phosphoproteomics methodologies to study vasopressin signaling (18; 64). Here, we focus on NGS methodologies applied to the study of the role of vasopressin in the regulation of Aqp2 in the renal collecting duct in the setting of water-balance disorders. A concise summary of the key proteomics and NGS methodologies is presented in Table 2.
Table 2.
Omic Methods Discussed in this Paper and in Salhadar et al.(18)
| Modality | Method | Description | Application in Current Paper |
|---|---|---|---|
| Protein mass spectrometry | Quantitative comprehensive proteomics (SILAC) | Stable isotope-based metabolic labeling used to quantify abundance of every expressed protein in cultured cells | Identification of effects of vasopressin and PKA deletion on proteome of collecting duct cells |
| Quantitative comprehensive proteomics (TMT) | Multiplexed chemical labeling of tryptic peptides to quantify abundance of every expressed protein in cell or tissue | Identification of effects of vasopressin on proteome of native collecting ducts | |
| Dynamic SILAC | Pulse labeling of proteins with stable isotopes to measure half-lives and translation rates proteome-wide | Identification of effects of vasopressin on proteome of collecting duct cells | |
| Quantitative phospho-proteomics | Proteome-wide SILAC or TMT quantification of tryptic peptides followed by phosphopeptide enrichment using affinity chromatography | Identification of effects of vasopressin or PKA deletion on phosphoproteome in collecting duct | |
| Next generation sequencing (NGS) | RNA-seq | Detection and quantification of all mRNA species and non-coding RNAs | Identification of effects of vasopressin or PKA deletion on transcriptome in collecting duct |
| ChIP-seq for RNA polymerase II | Chromatin immunoprecipitation using antibody to RNA Polymerase II followed by NGS to mark active cis-regulatory elements and identify transcribed genes | Genome-wide identification of actively transcribed genes in collecting duct cells | |
| ChIP-seq for histone H3K27 acetylation | Chromatin immunoprecipitation using antibody to histone H3 acetylated at lysine 27 followed by NGS to mark active cis-regulatory elements | Genome-wide identification of effects of vasopressin on enhancer activity | |
| ATAC-seq | Tn5 transposase insertion of sequencing adapters into open chromatin regions of the genome followed by NGS | Genome-wide identification of effects of vasopressin on DNA accessibility | |
| ChIP-seq for individual transcription factors | Chromatin immunoprecipitation using antibody to transcription factor proteins followed by NGS to identify binding sites occupied by the transcription factor | Genome-wide identification of binding sites for CREB and C/EBPβ in collecting duct cells |
V. RNA-sequencing (RNA-seq): Transcriptome-Wide Effects of Vasopressin
RNA-seq, among the most widely used NGS techniques, uses NGS to identify and quantify RNA species in biological systems and especially for differential gene expression analysis (65; 66) (Figure 2A). With advances in RNA-seq technologies and associated computational methods, RNA-seq has become a ubiquitous tool for understanding genomic function and the complexity of biological systems. We have applied it to understand how gene expression is regulated in collecting duct cells by vasopressin and other factors (16; 67–72).
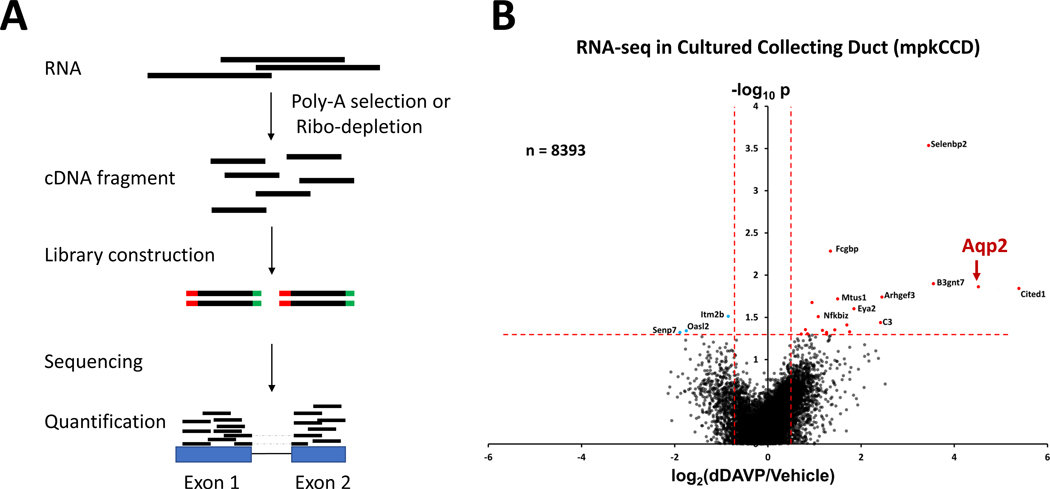
Figure 2. RNA-seq analysis of vasopressin signaling in mouse collecting duct cells (mpkCCD).
(A) A typical RNA-seq experiment starts with the selection of polyadenylated RNA transcripts using oligonucleotide (dT) primers (Poly-A selection) or depletion of high abundance ribosomal RNA (Ribo-depletion). The resulting RNA is then reverse transcribed to produce cDNA fragments, depending on the RNA-seq methods. Sequencing adapters (red and green bars) are then attached to the cDNA fragments to generate sequencing libraries through PCR amplification. The barcode libraries are deep sequenced using a high-throughput sequencing platform (e.g., Illumina) to obtain short sequencing reads. Sequencing reads, including exon and junction reads (reads with dashed lines), are subsequently aligned to the mouse reference genome to quantify gene expression. (B) Volcano plot for RNA-seq data for all 8393 detectable transcripts in dDAVP and vehicle-treated cells at 24 hrs. A relatively small number of transcripts, including Aqp2, were altered in abundance by vasopressin. The horizontal axis shows the mean log2(dDAVP/Vehicle) for 9 pairs of samples. The vertical axis shows −log10P for t-tests for each gene. Vertical dashed lines show 95% confidence interval for random variation based on Vehicle:Vehicle comparisons (2 × SD). Panel B was modified from Sandoval et al. (68).
The increase in Aqp2 protein synthesis (increased Aqp2 translation) in response to vasopressin described in Section II could be due either to an increase in Aqp2 mRNA abundance or selective regulation of Aqp2 translation. To determine the effects of vasopressin (dDAVP) on mRNA levels throughout the transcriptome, RNA-seq was carried out in cultured mouse mpkCCD cells after 24-hour exposure to dDAVP (Figure 2B) (68). Remarkably, there was a highly selective increase in Aqp2 mRNA. Of the 8393 transcripts quantified (https://helixweb.nih.gov/ESBL/Database/Vasopressin/), only a very small fraction showed significant changes in abundance, including Aqp2 which was increased more than 20-fold. The increase in Aqp2 mRNA in response to vasopressin was anticipated from prior results (73–75), although the marked selectivity of the response for Aqp2 was not anticipated. The data also pointed to two protein kinases, upregulated at an mRNA level, that hypothetically could be involved in vasopressin response: salt-inducible kinase 1 (Sik1) and PCTAIRE kinase 3 (Cdk18). No changes in transcripts that code for PKA catalytic subunits (Prkaca and Prkacb) were found, which is not surprising since PKA is regulated chiefly through changes in cAMP levels (76; 77).
Genome editing techniques have added a new dimension to systems level research and the development of CRISPR-Cas9 technology have been recognized with the 2020 Nobel Prizes in Chemistry (78). CRISPR-Cas9 was used to delete the two genes that code for the PKA catalytic subunits (Prkaca and Prkacb) in vasopressin-sensitive mpkCCD collecting duct cells. We then conducted RNA-seq to assess transcriptomic changes associated with loss of PKA signaling (16). Deletion of PKA in this way resulted in a virtually complete disappearance of Aqp2 mRNA in multiple CRISPR clones. Rescue of the cells by transfection of either PKA catalytic subunit restored Aqp2 mRNA expression. Thus, the ability of vasopressin to increase Aqp2 mRNA in collecting duct cells is critically dependent on PKA. Interestingly, unlike what occurs in PKA-intact cells, Aqp2 failed to translocate to the plasma membrane (as detected with immunofluorescence imaging) in response to dDAVP treatment of PKA-double knockout cells transfected with Aqp2. (16). Thus, it appears that both the short-term (Aqp2 trafficking) and long-term (Aqp2 abundance increase) effects of vasopressin in collecting duct cells are PKA-dependent. Unexpectedly, dDAVP treatment increased phosphorylation of Aqp2 at Ser256 (Figure 1C) in the PKA-double knockout cells, indicating that this phosphorylation event does not require PKA (17).
Expression atlases from RNA-seq studies.
An important and ambitious goal in mammalian biology is to catalog gene expression in all cell types of the body using RNA-seq methodology (79). Such resources can be explored at https://gtexportal.org/home/ and https://singlecell.broadinstitute.org/single_cell. These resources can furnish substantial aid in planning drug discovery efforts based on the following reasoning. Regulatory pathways typically consist of multiple proteins acting in series or in parallel. Among the best targets in such pathways are proteins that are selectively expressed in the target tissue or cell type. Gene expression atlases identify protein transcripts in a particular pathway that are selective for the target cells versus other cell types in the body, thereby pointing to molecular targets that have the best chances of having beneficial therapeutic selectivity. In vasopressin signaling in the collecting duct, two such proteins are the vasopressin V2 receptor and Aqp2. What additional proteins are expressed in collecting duct principal cells but not in other cell types? The renal tubule is made up of at least 16 different cell types in 14 tubule segments, each with different functional roles in the kidney (80). Identification of genes that are selectively expressed in each of the16 cell types cannot be readily ascertained from organ or tissue level RNA-seq analysis (71). To address this, two basic approaches utilizing RNA-seq have been employed: (a) RNA-seq applied to microdissected renal tubules corresponding to 14 distinct segments (67; 81); and (b) single-cell RNA-seq carried out in individual cells from cell suspensions derived from enzymatic digestion (70; 82–85)..
RNA-seq in microdissected renal tubule segments.
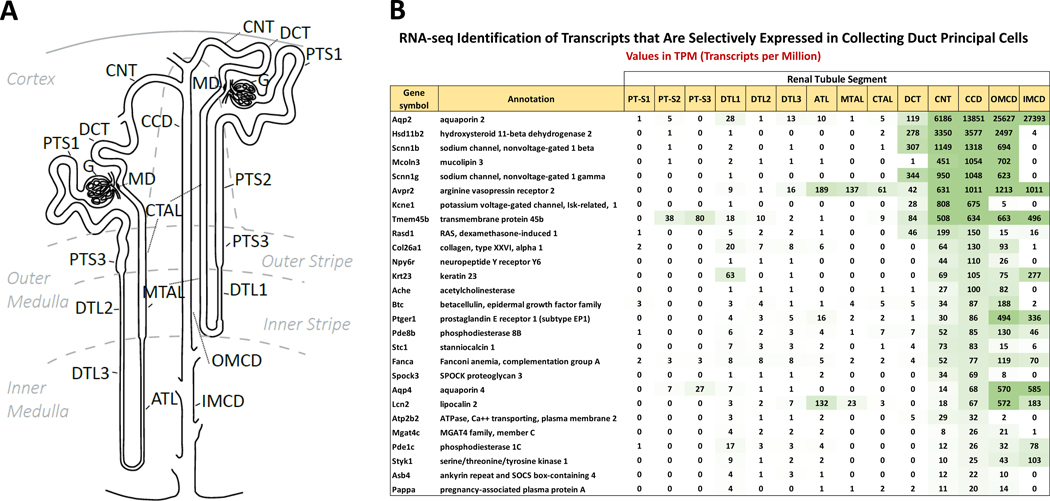
Small-sample RNA-seq has been carried out in each of 14 types of renal tubule epithelia microdissected from rat or mouse kidneys in a technique that we have called ‘single-tubule RNA-seq’ (67; 81). These microdissected-tubule samples typically contain 500–1000 cells that furnish more than enough mRNA to conduct deep transcriptomic profiling using RNA-seq. Figure 3 shows TPM (transcripts per million) values for transcripts found to be selectively expressed in principal cells of the mouse renal collecting duct using criteria defined in Chen et al (81). The list includes the V2 vasopressin receptor (Avpr2) and Aqp2 as expected, but also 25 additional collecting-duct selective gene products representing candidate genes that hypothetically could be targeted for collecting duct-selective drug actions. These include two GPCRs other than Avpr2 (the neuropeptide Y/pancreatic polypeptide receptor Npy6r and the prostaglandin E2 receptor Ptger1), two cyclic nucleotide phosphodiesterases (Pde1c and Pde8b), a protein kinase (Styk1), a growth factor (betacellulin), and multiple transporter proteins other than Aqp2 (the epithelial sodium channel subunits Scnn1b and Scnn1g, the potassium channel Kcne1, two calcium transport proteins [Atp2b2 and Mcoln3] and the water channel Aqp4).Some of these mRNAs code for proteins that are targeted by drugs in clinical use for purposes unrelated to renal function.
Figure 3. Small sample RNA-seq in microdissected renal tubules.
(A) Nomenclature for all 14 renal tubule segments. The scheme shows the connection of both a short-looped and a long-looped nephron to the collecting duct system. Definitions: PT-S1, the initial segment of the proximal tubule; PT-S2, proximal straight tubule in cortical medullary rays; PT-S3, last segment of the proximal straight tubule in the outer stripe of outer medulla; DTL1, the short descending limb of the loop of Henle; DTL2, long descending limb of the loop of Henle in the outer medulla; DTL3, long descending limb of the loop of Henle in the inner medulla; ATL, thin ascending limb of the loop of Henle; MTAL, medullary thick ascending limb of the loop of Henle; CTAL, cortical thick ascending limb of the loop of Henle; MD, macula densa; DCT, distal convoluted tubule; CNT, connecting tubule; CCD, cortical collecting duct; OMCD, outer medullary collecting duct; IMCD, inner medullary collecting duct. (B) Distributions of transcript abundances for collecting duct specific genes. TPM, transcripts per million. Panel A was redrawn from Chen et al. (80).
Recently, we applied single-tubule RNA-seq to identify pathophysiological mechanisms in lithium-induced nephrogenic diabetes insipidus (NDI) (72) and in the syndrome of inappropriate antidiuresis (SIADH) (69). Lithium-induced NDI occurs as a result of repression of Aqp2 gene expression due to lithium-induced ERK activation, which triggers an immediate early transcriptional response and principal cell dedifferentiation associated with activation of an inflammatory-like response (72). SIADH is a hyponatremic disorder that derives from unregulated high levels of AVP or other antidiuretic substances. This leads to increased osmotic transport of water from the lumen to the blood in renal collecting ducts, resulting in dilution of body fluids. The dilution triggers a compensatory response known as ‘vasopressin escape’ associated with loss of Aqp2 gene expression despite continued high circulating levels of vasopressin. The escape process therefore limits the degree of hyponatremia in SIADH. Single-tubule RNA-seq studies in rats showed that vasopressin escape is due to TGFβ signaling and partial epithelial-to-mesenchymal transition, resulting in dedifferentiation of collecting duct principal cells with loss of Aqp2 gene expression. The demonstrated AVP-independent regulation of Aqp2 in these studies therefore points to additional sets of potential drug targets for modification of water transport in collecting ducts (69; 72).
RNA-seq in single-cells from the renal tubule.
Single-cell RNA-seq (scRNA-seq) enables gene expression analysis at single-cell resolution, providing opportunities to resolve the cell state and identify novel cell types. Recent studies (70; 84; 85), including those from our laboratory (70; 82) have provided deep analyses of heterogeneous kidney cell types. In particular, by coupling cell enrichment methods with scRNA-seq, we have characterized the transcriptomes of major cell types in the collecting duct (i.e., principal cells and intercalated cells) (70) and identified minority cell types spanning the CTAL-DCT-CNT region (Figure 3A) of the renal tubule (82).
VI. ChIP-seq: RNA Polymerase II
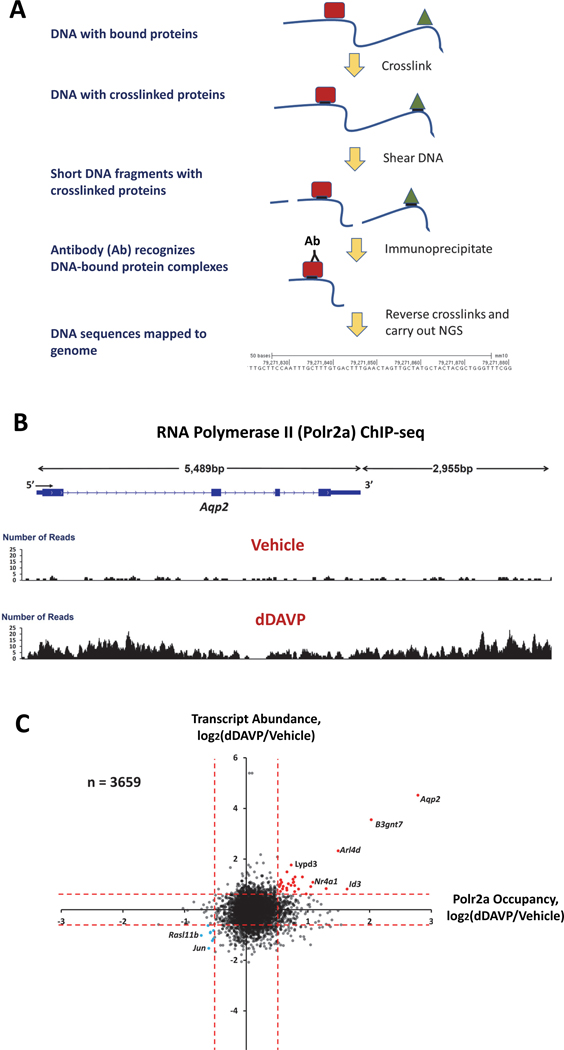
The increase in Aqp2 mRNA in collecting duct cells in response to vasopressin could either be due to an increase in Aqp2 gene transcription or to an increase in Aqp2 mRNA stability. To address the former possibility, we carried out ChIP-seq (chromatin-immunoprecipitation followed by DNA sequencing) using antibodies to the A subunit of the RNA polymerase II complex (Pol2ra). The ChIP-seq method is summarized in Figure 4A. If vasopressin increases Aqp2 transcription, RNA-polymerase II occupancy over the Aqp2 gene body should be increased. We detected a 7-fold increase in RNA-polymerase II occupancy in dDAVP-treated cells compared to vehicle controls (Figure 4B), supporting the view that vasopressin does indeed increase Aqp2 transcription (68). We also identified an increase in RNA-polymerase II binding to a >3000 bp region downstream from the Aqp2 gene, which is due to the presence of an enhancer in this region (Section VII.). As observed for Aqp2 transcript abundance, dDAVP treatment had a highly selective effect on RNA-polymerase II occupancy over the Aqp2 gene (Figure 4C), although a few genes showed smaller increases in both transcript abundance and RNA-polymerase II occupancy. We have proposed (68) that such high selectivity could only be due to cooperative regulation of multiple transcription factors, likely at least 4 or 5 acting in tandem. A major goal, therefore, is to use omic data to identify transcription factor candidates for regulation of Aqp2 gene transcription and test their roles using ChIP-seq for specific transcription factors and CRISPR-mediated deletion of the transcription factors. Identification of candidate transcription factors can be addressed using additional ChIP-seq modalities as well as a method called “ATAC-seq”.
Figure 4. ChIP-seq.
(A) ChIP-seq procedure. (B) ChIP-seq using an antibody to RNA Polymerase II (Polr2a) reports occupancy of RNA Polymerase II complex over Aqp2 gene in presence and absence of V2 receptor selective vasopressin analog dDAVP. (C) Plot of transcript abundance ratio (dDAVP/Vehicle) versus RNA Polymerase II occupancy ratio (dDAVP/Vehicle) shows that only a few genes are regulated transcriptionally in response to V2 vasopressin receptor binding.
VII. ATAC-Seq and Histone H3K27Ac ChIP-Seq: Finding Enhancers
Transcription factors bind to accessible regions of DNA called enhancers to transactivate target genes through an enhancer-to-promoter looping process that can indirectly regulate the RNA polymerase II complex to alter transcriptional initiation or elongation (86). A strategy for identification of candidate transcription factors for a particular gene consists of experimental identification of enhancer regions, followed by bioinformatic analysis to find motifs that are associated with particular transcription factor families. Members of these transcription factor families can be winnowed using RNA-seq or proteomic data to eliminate unexpressed transcription factor genes in the relevant tissue. Identification of enhancer regions depends on techniques to assess chromatin accessibility, i.e., regions whose histone structure has been loosened by post-transcriptional modifications to allow other proteins to bind DNA.
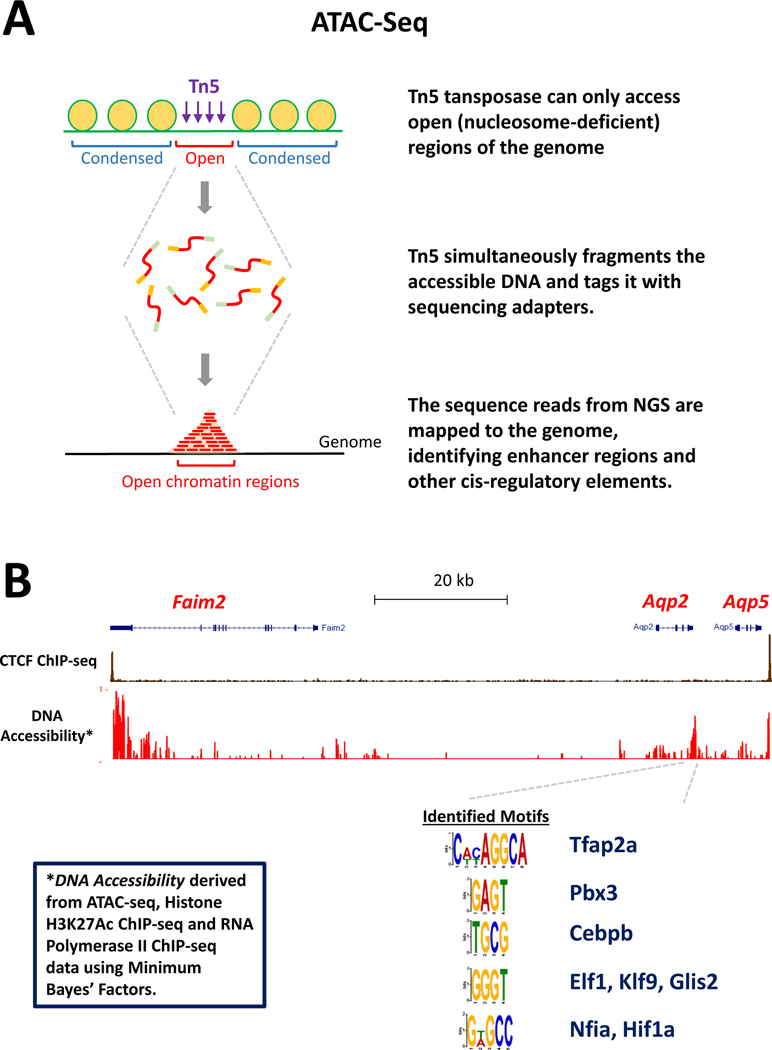
Chromatin accessibility can be mapped using a variety of techniques, including the classic DNase I hypersensitivity assay coupled to NGS (DNase-Seq). In recent years, two main tools have been employed for identification of enhancers, namely Histone H3K27 acetylation ChIP-seq and ATAC-seq (Assay for Transposase-Accessible Chromatin using Sequencing). Acetylation at lysine residues of histone proteins is an epigenetic marker for the status of chromatin and indicative of transcriptional activation (87). For example, histone H3 acetylation at lysine 27 (H3K27Ac) represents a dynamic chromatin status associated with transcriptional activation and is the most common modification assessed for identification of enhancers. Histone H3K27Ac can be quantified using the ChIP-seq procedure described in Figure 4A with the use of an antibody specific for Histone H3 acetylated at the lysine at position 27. The ATAC-seq method (88) is diagrammed in Figure 5A. It uses a modified Tn5 transposase, which accesses open (nucleosome-deficient) regions of the genome, to generate DNA fragments and to add sequencing adaptors that allow the fragments to be identified by next-generation sequencing.
Figure 5. Enhancer identification.
(A) Protocol for ATAC-seq identification of regions of open chromatin. (B) Mapping of open chromatin regions within the CTCF-loop surrounding the Aqp2 gene. Several potential transcription factors binding motifs were identified in a putative enhancer just 3’ to the Aqp2 gene. Candidate transcription factors are listed.
In vasopressin-responsive mpkCCD cells, the combination of ATAC-Seq, H3K27Ac ChIP-Seq and RNA Polymerase II ChIP-seq was used to identify open chromatin regions throughout the genome (89), including putative cis-regulatory regions in the CTCF loop encompassing the Aqp2 gene (89; 90) (Figure 5B). CTCF binding defines the boundaries of the region containing enhancers that regulate genes within that region (86). The DNA accessible regions in the Aqp2 CTCF loop are shown in Figure 5B. An identified accessible region of DNA just downstream from the Aqp2 gene is of particular interest. It contains nucleotide base sequences that are potential binding sites for several transcription factor families (Figure 5B), representing candidate transcription factors for regulation of Aqp2 gene transcription (16; 90). Roles for these candidate transcription factors can be investigated using a combination of CRISPR-Cas9 deletion of the transcription factors, CRISPR-mediated mutation of the putative binding sites in the enhancer region, and ChIP-seq to test whether the transcription factors actually bind to the predicted sites.
ATAC-seq has recently been extended to a single nucleus level in kidney (91), providing a resource that identifies chromatin accessible regions in multiple epithelial cell types including collecting duct principal cells and connecting tubule cells (http://susztaklab.com/developing_adult_kidney/igv/). This creates a vast untapped resource for investigation of regulation of gene expression including vasopressin-regulated genes other than Aqp2.
VIII. Transcription Factor ChIP-Seq: Evaluating Potential Roles of C/EBPβ and CREB in Aqp2 Gene Transcription
Among the transcription factors predicted to bind within the enhancer region downstream from the Aqp2 gene (Figure 5B) is Cebpb (aka C/EBPβ), a bZIP family transcription factor believed to act as a so-called “pioneer” transcription factor (92), which can bind to DNA regions that are relatively inaccessible and can recruit chromatin regulators for chromatin structure to create open regions adjacent to the binding site (93). Jung et al. (89) carried out ChIP-seq using the protocol described in Figure 4A with an antibody recognizing Cebpb to identify Cebpb binding sites throughout the genome in cultured vasopressin-responsive collecting duct cells (mpkCCD cells). This analysis confirmed several previously recognized binding sites but also identified a novel vasopressin-responsive binding site for Cebpb at the predicted site in the Aqp2-downstream enhancer (Figure 5B). Cebpb translocation to the nucleus in response to vasopressin was previously found in the same cell line using protein mass spectrometry (see https://hpcwebapps.cit.nih.gov/ESBL/Database/QuantNucProteomics/). Thus, Cebpb is a good candidate for regulation of Aqp2 transcription, but this role needs to be tested through genome editing techniques to delete Cebpb and its putative binding site.
Another well discussed candidate transcription factor is CREB (Creb1), which possesses a so-called p-KID domain that can be phosphorylated by PKA and other basophilic protein kinases. Several review articles tout Creb1 as a potential regulator of Aqp2 transcription but without direct evidence (3; 24; 94; 95). However, a recent ChIP-seq analysis in mpkCCD cells (89) demonstrated a lack of Creb1 binding anywhere within the CTCF loop that contains Aqp2 despite avid binding to multiple sites identified in prior studies. This finding suggests that Creb1 may not be directly involved in vasopressin-mediated Aqp2 gene transcription.
IX. Data Sharing and Large-Scale Data Integration
Data sharing.
The advent of genome sequencing projects around the turn of the 21st century spawned a revolution in biology referred to as ‘systems biology”. The information from genomic sequencing projects in humans and other species enabled development of methods to profile protein properties across the proteome (mostly using protein mass spectrometry) as well as DNA and RNA properties across the genome (mostly using NGS techniques). Systems level data, if made available, can be used by the scientific community, including the pharmacology community to guide further experimental studies aimed at drug discovery, characterization of drug mechanisms, prediction of adverse effects, etc. While data sharing is common, e.g., ProteomeXchange for proteomic data and Gene Expression Omnibus (GEO) for NGS data, these resources mostly focus on unprocessed data that are not immediately useful to users without a considerable amount of bioinformatics expertise. In general, there is no standard mechanism for sharing curated data. However, for kidney-specific data, we have developed the Kidney Systems Biology Project website (https://hpcwebapps.cit.nih.gov/ESBL/Database/) that allows users to browse or download curated data sets from many renal research laboratories. Much of the data described in this review is accessible there.
Big data integration.
An essential objective in the utilization of systems level (omic) data sets is to use techniques to integrate data from multiple data sets. “Big Data” refers to techniques that allow cross-interrogation of multiple large data sets to obtain multiple types of information about one individual. In everyday life, the concept is used, for example, in determining credit worthiness of individual consumers. In biology, we want to be able to pull data relevant to a given gene or protein out of multiple large data sets and integrate it with the existing literature of reductionist studies (96; 97). An important tool for big-data analysis is Bayesian modeling, the use of Bayes Theorem to integrate information from multiple sources to make predictions regarding causality (98–102). In general, progress in systems biology applications for pharmacology will likely depend as much on the development of computational methods for data management as much as on data generation.
X. Conclusion and Future Directions
In this review, we have described application of systems biology methods focusing mainly on the biological response of a single GPCR, the V2 vasopressin receptor. Going forward, it will be informative to extend multi-omic approaches to other Gαs-coupled GPCRs in other tissues in order to discover which molecular responses to vasopressin are general features of Gαs-mediated signaling and which responses are specific to the V2 vasopressin receptor in collecting duct cells. Beyond this, in collecting duct cells, it will be important to prioritize the observations described here to orchestrate a deeper analysis of vasopressin signaling using classical reductionist tools. The systems-derived data that we describe in this review provides a rich source of hypotheses for such studies. More generally, the multiple techniques and analyses described herein can be applied to other cellular responses and in particular, ones that are of pharmacological or toxicological interest. Such efforts offer the possibility of advancing the understanding of mechanisms of action of current drugs and the discovery of novel therapeutic targets.
Of note, although we present comprehensive omics methods, within that strategy we describe a reductionist approach in which we isolate renal tubule suspensions, microdissected tubules or cultured cells to allow assessment of the intrinsic behavior of the cells involved outside of the larger context of the animal. Obviously, it is important to relate the findings to those obtained in corresponding studies of intact animals, particularly with pathophysiological questions. For example, proteomics and RNA-Seq has been employed to identify the signaling pathways involved in lithium-induced nephrogenic diabetes insipidus (72; 103). These studies focus on the cellular mechanisms that explain the pronounced polyuria seen in response to lithium in multiple animal studies and in patients receiving lithium salts for manic-depressive disorder, which are well documented in the literature. Studies of the effects of lithium (72) and in a SIADH model (69) have shown the feasibility of using rapidly microdissected tubules to read out the regulatory state of the cells after in vivo perturbations. New modalities are on the horizon, viz. spatial proteomics (104; 105) and spatial transcriptomics (106) that may broaden the armamentarium for studies of in vivo states.
ACKNOWLEDGMENTS
The work was primarily funded by the Division of Intramural Research, National Heart, Lung, and Blood Institute (projects ZIA-HL001285 and ZIA-HL006129, M.A.K.). Authors declare that there is no conflict of interest.
Footnotes
Terminology Footnote in Background and Introduction Section
(Footnote text: Non-italicized gene symbols are used to designate proteins or transcripts. When referring specifically to the gene rather than the gene products, the gene symbols are italicized. Official gene symbols are archived by the Nomenclature Committee of the Human Genome Organization (HUGO) accessible at https://www.genenames.org/. In some cases, we use abbreviations to represent products of closely related genes where we do not know which specific gene is involved. An example is PKA (protein kinase A) which corresponds to two separate genes, Prkaca and Prkacb, coding for two alternative catalytic subunits of “PKA”.)
LITERATURE CITED:
- 1.Knepper MA, Kwon TH, Nielsen S. 2015. Molecular physiology of water balance. N Engl J Med 372:1349–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upadhyay A, Jaber BL, Madias NE. 2006. Incidence and prevalence of hyponatremia. Am J Med 119:S30–5 [DOI] [PubMed] [Google Scholar]
- 3.Bockenhauer D, Bichet DG. 2015. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol 11:576–88 [DOI] [PubMed] [Google Scholar]
- 4.Refardt J, Winzeler B, Christ-Crain M. 2020. Diabetes Insipidus: An Update. Endocrinol Metab Clin North Am 49:517–31 [DOI] [PubMed] [Google Scholar]
- 5.Verbalis JG. 2020. Commentary: Evidence-based Medicine for SIAD. The Journal of Clinical Endocrinology & Metabolism 106:e1042–e3 [DOI] [PubMed] [Google Scholar]
- 6.Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, et al. 2013. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 126:S1–42 [DOI] [PubMed] [Google Scholar]
- 7.Verney EB. 1947. The antidiuretic hormone and the factors which determine its release. Proc R Soc Lond B Biol Sci 135:25–106 [PubMed] [Google Scholar]
- 8.Baumann G, Dingman JF. 1976. Distribution, blood transport, and degradation of antidiuretic hormone in man. J Clin Invest 57:1109–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenton RA, Knepper MA. 2007. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87:1083–112 [DOI] [PubMed] [Google Scholar]
- 10.Bichet DG. 2019. Regulation of Thirst and Vasopressin Release. Annu Rev Physiol 81:359–73 [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman CA, Huey EL, Ahn JS, Beutler LR, Tan CL, et al. 2019. A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature 568:98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Star RA, Nonoguchi H, Balaban R, Knepper MA. 1988. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest 81:1879–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yip KP. 2002. Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol 538:891–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisitkun T, Jacob V, Schleicher SM, Chou CL, Yu MJ, Knepper MA. 2008. Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. Am J Physiol Renal Physiol 295:F1030–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou CL, Rapko SI, Knepper MA. 1998. Phosphoinositide signaling in rat inner medullary collecting duct. Am J Physiol 274:F564–72 [DOI] [PubMed] [Google Scholar]
- 16.Isobe K, Jung HJ, Yang CR, Claxton J, Sandoval P, et al. 2017. Systems-level identification of PKA-dependent signaling in epithelial cells. Proc Natl Acad Sci U S A 114:E8875-e84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta A, Yang CR, Limbutara K, Chou CL, Rinschen MM, et al. 2020. PKA-independent vasopressin signaling in renal collecting duct. Faseb j 34:6129–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salhadar K, Matthews A, Raghuram V, Limbutara K, Yang CR, et al. 2020. Phosphoproteomic Identification of Vasopressin/cAMP/PKA-Dependent Signaling in Kidney. Mol Pharmacol DOI: 10.1124/mol.120.119602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranieri M, Di Mise A, Tamma G, Valenti G. 2019. Vasopressin-aquaporin-2 pathway: recent advances in understanding water balance disorders. F1000Res 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung HJ, Kwon TH. 2016. Molecular mechanisms regulating aquaporin-2 in kidney collecting duct. Am J Physiol Renal Physiol 311:F1318–f28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, et al. 2003. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem 278:6258–67 [DOI] [PubMed] [Google Scholar]
- 22.Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, et al. 2010. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci U S A 107:3882–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta A, Yang CR, Salhadar K, Park E, Chou CL, et al. 2021. Phosphoproteomic identification of vasopressin-regulated protein kinases in collecting duct cells. Br J Pharmacol 178:1426–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA. 2002. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82:205–44 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. 1995. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A 92:1013–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen S, Knepper MA. 1993. Vasopressin activates collecting duct urea transporters and water channels by distinct physical processes. Am J Physiol 265:F204–13 [DOI] [PubMed] [Google Scholar]
- 27.Brown D. 2003. The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284:F893–901 [DOI] [PubMed] [Google Scholar]
- 28.Nishimoto G, Zelenina M, Li D, Yasui M, Aperia A, et al. 1999. Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue. Am J Physiol 276:F254–9 [DOI] [PubMed] [Google Scholar]
- 29.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. 2006. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci U S A 103:7159–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, et al. 2008. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283:24617–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moeller HB, Praetorius J, Rützler MR, Fenton RA. 2010. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci U S A 107:424–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsura T, Gustafson CE, Ausiello DA, Brown D. 1997. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol 272:F817–22 [PubMed] [Google Scholar]
- 33.Fushimi K, Sasaki S, Marumo F. 1997. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272:14800–4 [DOI] [PubMed] [Google Scholar]
- 34.van Balkom BW, Savelkoul PJ, Markovich D, Hofman E, Nielsen S, et al. 2002. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem 277:41473–9 [DOI] [PubMed] [Google Scholar]
- 35.Nedvetsky PI, Tabor V, Tamma G, Beulshausen S, Skroblin P, et al. 2010. Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J Am Soc Nephrol 21:1645–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu HJ, Matsuzaki T, Bouley R, Hasler U, Qin QH, Brown D. 2008. The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am J Physiol Renal Physiol 295:F290–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. 1994. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci U S A 91:8984–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kishore BK, Terris JM, Knepper MA. 1996. Quantitation of aquaporin-2 abundance in microdissected collecting ducts: axial distribution and control by AVP. Am J Physiol 271:F62–70 [DOI] [PubMed] [Google Scholar]
- 39.Sandoval PC, Slentz DH, Pisitkun T, Saeed F, Hoffert JD, Knepper MA. 2013. Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol 24:1793–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, et al. 2012. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367:2407–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masoumi A, Reed-Gitomer B, Kelleher C, Schrier RW. 2007. Potential pharmacological interventions in polycystic kidney disease. Drugs 67:2495–510 [DOI] [PubMed] [Google Scholar]
- 42.Sussman CR, Wang X, Chebib FT, Torres VE. 2020. Modulation of polycystic kidney disease by G-protein coupled receptors and cyclic AMP signaling. Cell Signal 72:109649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miranda CA, Lee JW, Chou CL, Knepper MA. 2014. Tolvaptan as a tool in renal physiology. Am J Physiol Renal Physiol 306:F359–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kortenoeven ML, Sinke AP, Hadrup N, Trimpert C, Wetzels JF, et al. 2013. Demeclocycline attenuates hyponatremia by reducing aquaporin-2 expression in the renal inner medulla. Am J Physiol Renal Physiol 305:F1705–18 [DOI] [PubMed] [Google Scholar]
- 45.Lockett J, Berkman KE, Dimeski G, Russell AW, Inder WJ. 2019. Urea treatment in fluid restriction-refractory hyponatraemia. Clin Endocrinol (Oxf) 90:630–6 [DOI] [PubMed] [Google Scholar]
- 46.Knepper MA, Miranda CA. 2013. Urea channel inhibitors: a new functional class of aquaretics. Kidney Int 83:991–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang S, Zhao Y, Wang S, Li M, Xu Y, et al. 2021. Discovery of novel diarylamides as orally active diuretics targeting urea transporters. Acta Pharm Sin B 11:181–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M, Zhang S, Yang B. 2020. Urea Transporters Identified as Novel Diuretic Drug Targets. Curr Drug Targets 21:279–87 [DOI] [PubMed] [Google Scholar]
- 49.Esteva-Font C, Anderson MO, Verkman AS. 2015. Urea transporter proteins as targets for small-molecule diuretics. Nat Rev Nephrol 11:113–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sawyer WH, Acosta M, Balaspiri L, Judd J, Manning M. 1974. Structural changes in the arginine vasopressin molecule that enhance antidiuretic activity and specificity. Endocrinology 94:1106–15 [DOI] [PubMed] [Google Scholar]
- 51.Verbalis JG. 2003. Diabetes insipidus. Rev Endocr Metab Disord 4:177–85 [DOI] [PubMed] [Google Scholar]
- 52.Sands JM, Klein JD. 2016. Physiological insights into novel therapies for nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 311:F1149–f52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bockenhauer D, Bichet DG. 2017. Nephrogenic diabetes insipidus. Curr Opin Pediatr 29:199–205 [DOI] [PubMed] [Google Scholar]
- 54.Li JH, Chou CL, Li B, Gavrilova O, Eisner C, et al. 2009. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest 119:3115–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagner S, Stahl U, Knoblauch B, McGregor GP, Lang RE. 2002. Calcitonin receptor-like receptor: identification and distribution in human peripheral tissues. Cell Tissue Res 310:41–50 [DOI] [PubMed] [Google Scholar]
- 56.Olesen ET, Moeller HB, Assentoft M, MacAulay N, Fenton RA. 2016. The vasopressin type 2 receptor and prostaglandin receptors EP2 and EP4 can increase aquaporin-2 plasma membrane targeting through a cAMP-independent pathway. Am J Physiol Renal Physiol 311:F935–f44 [DOI] [PubMed] [Google Scholar]
- 57.Robert-Nicoud M, Flahaut M, Elalouf JM, Nicod M, Salinas M, et al. 2001. Transcriptome of a mouse kidney cortical collecting duct cell line: effects of aldosterone and vasopressin. Proc Natl Acad Sci U S A 98:2712–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Owada A, Nonoguchi H, Terada Y, Marumo F, Tomita K. 1997. Microlocalization and effects of adrenomedullin in nephron segments and in mesangial cells of the rat. Am J Physiol 272:F691–7 [DOI] [PubMed] [Google Scholar]
- 59.Hay DL, Garelja ML, Poyner DR, Walker CS. 2018. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br J Pharmacol 175:3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bogum J, Faust D, Zühlke K, Eichhorst J, Moutty MC, et al. 2013. Small-molecule screening identifies modulators of aquaporin-2 trafficking. J Am Soc Nephrol 24:744–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vukićević T, Hinze C, Baltzer S, Himmerkus N, Quintanova C, et al. 2019. Fluconazole Increases Osmotic Water Transport in Renal Collecting Duct through Effects on Aquaporin-2 Trafficking. J Am Soc Nephrol 30:795–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knepper MA. 2015. Systems biology of diuretic resistance. J Clin Invest 125:1793–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mast FD, Ratushny AV, Aitchison JD. 2014. Systems cell biology. J Cell Biol 206:695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rinschen MM, Limbutara K, Knepper MA, Payne DM, Pisitkun T. 2018. From Molecules to Mechanisms: Functional Proteomics and Its Application to Renal Tubule Physiology. Physiol Rev 98:2571–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, Gerstein M, Snyder M. 2009. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stark R, Grzelak M, Hadfield J. 2019. RNA sequencing: the teenage years. Nat Rev Genet 20:631–56 [DOI] [PubMed] [Google Scholar]
- 67.Lee JW, Chou CL, Knepper MA. 2015. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J Am Soc Nephrol 26:2669–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandoval PC, Claxton JS, Lee JW, Saeed F, Hoffert JD, Knepper MA. 2016. Systems-level analysis reveals selective regulation of Aqp2 gene expression by vasopressin. Sci Rep 6:34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee JW, Alsady M, Chou CL, de Groot T, Deen PMT, et al. 2018. Single-tubule RNA-Seq uncovers signaling mechanisms that defend against hyponatremia in SIADH. Kidney Int 93:128–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen L, Lee JW, Chou CL, Nair AV, Battistone MA, et al. 2017. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci U S A 114:E9989–e98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clark JZ, Chen L, Chou CL, Jung HJ, Lee JW, Knepper MA. 2019. Representation and relative abundance of cell-type selective markers in whole-kidney RNA-Seq data. Kidney Int 95:787–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sung CC, Chen L, Limbutara K, Jung HJ, Gilmer GG, et al. 2019. RNA-Seq and protein mass spectrometry in microdissected kidney tubules reveal signaling processes initiating lithium-induced nephrogenic diabetes insipidus. Kidney Int 96:363–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. 1997. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8:861–7 [DOI] [PubMed] [Google Scholar]
- 74.Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, et al. 1997. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest 99:1852–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, et al. 2002. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem 277:10379–86 [DOI] [PubMed] [Google Scholar]
- 76.Taylor SS, Ilouz R, Zhang P, Kornev AP. 2012. Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol 13:646–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sassone-Corsi P. 2012. The cyclic AMP pathway. Cold Spring Harb Perspect Biol 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ledford H, Callaway E. 2020. Pioneers of revolutionary CRISPR gene editing win chemistry Nobel. Nature 586:346–7 [DOI] [PubMed] [Google Scholar]
- 79.Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, et al. 2017. The Human Cell Atlas. Elife 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L, Clark JZ, Nelson JW, Kaissling B, Ellison DH, Knepper MA. 2019. Renal-Tubule Epithelial Cell Nomenclature for Single-Cell RNA-Sequencing Studies. J Am Soc Nephrol 30:1358–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L, Chou CL, Knepper MA. 2021. A Comprehensive Map of mRNAs and Their Isoforms across All 14 Renal Tubule Segments of Mouse. J Am Soc Nephrol 32:898–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen L, Chou CL, Knepper MA. 2021. Targeted Single-Cell RNA-seq Identifies Minority Cell Types of Kidney Distal Nephron. J Am Soc Nephrol 32:887–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu H, Kirita Y, Donnelly EL, Humphreys BD. 2019. Advantages of Single-Nucleus over Single-Cell RNA Sequencing of Adult Kidney: Rare Cell Types and Novel Cell States Revealed in Fibrosis. J Am Soc Nephrol 30:23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park J, Shrestha R, Qiu C, Kondo A, Huang S, et al. 2018. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360:758–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ransick A, Lindström NO, Liu J, Zhu Q, Guo JJ, et al. 2019. Single-Cell Profiling Reveals Sex, Lineage, and Regional Diversity in the Mouse Kidney. Dev Cell 51:399–413.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hnisz D, Day DS, Young RA. 2016. Insulated Neighborhoods: Structural and Functional Units of Mammalian Gene Control. Cell 167:1188–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Z, Shilatifard A. 2019. Epigenetic modifications of histones in cancer. Genome Biol 20:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. 2013. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods 10:1213–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jung HJ, Raghuram V, Lee JW, Knepper MA. 2018. Genome-Wide Mapping of DNA Accessibility and Binding Sites for CREB and C/EBPβ in Vasopressin-Sensitive Collecting Duct Cells. J Am Soc Nephrol 29:1490–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wen B, Jung HJ, Chen L, Saeed F, Knepper MA. 2020. NGS-Integrator: An efficient tool for combining multiple NGS data tracks using minimum Bayes’ factors. BMC Genomics 21:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheng X, Ma Z, Wu J, Liu H, Qiu C, et al. 2020. Mapping the genetic architecture of human traits to cell types in the kidney identifies mechanisms of disease and potential treatments. bioRxiv:2020.11.09.375592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plachetka A, Chayka O, Wilczek C, Melnik S, Bonifer C, Klempnauer KH. 2008. C/EBPbeta induces chromatin opening at a cell-type-specific enhancer. Mol Cell Biol 28:2102–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zaret KS, Carroll JS. 2011. Pioneer transcription factors: establishing competence for gene expression. Genes Dev 25:2227–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kortenoeven ML, Fenton RA. 2014. Renal aquaporins and water balance disorders. Biochim Biophys Acta 1840:1533–49 [DOI] [PubMed] [Google Scholar]
- 95.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE. 2015. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10:135–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Y, Yang CR, Raghuram V, Parulekar J, Knepper MA. 2016. BIG: a large-scale data integration tool for renal physiology. Am J Physiol Renal Physiol 311:F787–f92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanghi A, Zaringhalam M, Corcoran CC, Saeed F, Hoffert JD, et al. 2014. A knowledge base of vasopressin actions in the kidney. Am J Physiol Renal Physiol 307:F747–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hwang JR, Chou CL, Medvar B, Knepper MA, Jung HJ. 2017. Identification of β-catenin-interacting proteins in nuclear fractions of native rat collecting duct cells. Am J Physiol Renal Physiol 313:F30–f46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xue Z, Chen JX, Zhao Y, Medvar B, Knepper MA. 2017. Data integration in physiology using Bayes’ rule and minimum Bayes’ factors: deubiquitylating enzymes in the renal collecting duct. Physiol Genomics 49:151–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LeMaire SM, Raghuram V, Grady CR, Pickering CM, Chou CL, et al. 2017. Serine/threonine phosphatases and aquaporin-2 regulation in renal collecting duct. Am J Physiol Renal Physiol 312:F84–f95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang CR, Raghuram V, Emamian M, Sandoval PC, Knepper MA. 2015. Deep proteomic profiling of vasopressin-sensitive collecting duct cells. II. Bioinformatic analysis of vasopressin signaling. Am J Physiol Cell Physiol 309:C799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bradford D, Raghuram V, Wilson JL, Chou CL, Hoffert JD, et al. 2014. Use of LC-MS/MS and Bayes’ theorem to identify protein kinases that phosphorylate aquaporin-2 at Ser256. Am J Physiol Cell Physiol 307:C123–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trepiccione F, Pisitkun T, Hoffert JD, Poulsen SB, Capasso G, et al. 2014. Early targets of lithium in rat kidney inner medullary collecting duct include p38 and ERK1/2. Kidney Int 86:757–67 [DOI] [PubMed] [Google Scholar]
- 104.Chaurand P, Stoeckli M, Caprioli RM. 1999. Direct profiling of proteins in biological tissue sections by MALDI mass spectrometry. Anal Chem 71:5263–70 [DOI] [PubMed] [Google Scholar]
- 105.Han G, Spitzer MH, Bendall SC, Fantl WJ, Nolan GP. 2018. Metal-isotope-tagged monoclonal antibodies for high-dimensional mass cytometry. Nat Protoc 13:2121–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stickels RR, Murray E, Kumar P, Li J, Marshall JL, et al. 2021. Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2. Nat Biotechnol 39:313–9 [DOI] [PMC free article] [PubMed] [Google Scholar]