Summary
Human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) hold promise for transplantation medicine. Diverse human leukocyte antigen (HLA) profiles necessitate autologous cells or multiple cell lines for therapeutics, incurring time and cost. Advancements in CRISPR-Cas9 and cellular therapies have led to the conceptualization of “off-the-shelf” universal cell donor lines, free of immune rejection. Overcoming immune rejection is a challenge. This review outlines strategies to modulate the major histocompatibility complex (MHC) to generate a universal cell donor line. Upon bypassing MHC mismatch, multifaceted approaches are required to generate foreign host-tolerated cells. Universal cells harbor risks, namely immune escape and tumor formation. To mitigate, we review safety mechanisms enabling donor cell inactivation or removal. Achieving a universal cell line would reduce treatment wait time, eliminate donor search, and reduce graft-versus-host disease risk without immunosuppression. The pursuit of universally tolerated cells is under way, ready to transform transplantation and regenerative medicine.
Human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) hold promise for transplantation medicine, with the potential to reduce treatment wait time, eliminate donor search, and reduce graft-versus-host disease risk without immunosuppression. This review outlines strategies to generate a universal cell donor line.
Introduction
Overcoming the complexity of the mammalian immune system has been a problem in the field of transplantation and regenerative medicine since the first solid organ transplant in 1954 (reviewed in Hughes, 2014). The introduction of immunosuppressive agents decades later allowed transplantation to become a viable treatment option for tissue and organ failure. The interplay between donor and recipient immune systems, logistical complexities, and organ donation shortages present a distinct set of challenges in solid organ transplantation. An impediment to successful organ transplantation is the adaptive immune responses that recognize foreign antigens on the cell surface, triggering rejection and leading to eventual destruction of the grafted tissue. Beyond immunosuppression, the scarcity of donor organs compounds the difficulties faced in transplantation medicine. Despite a 2-fold increase in transplants performed across the past 3 decades, waiting lists have grown 6-fold, highlighting the need for more donors (Kupiec-Weglinski, 2022). In 2021, the United States alone experienced a record high of 41,354 transplants, however, 116,566 patients remained on the waiting list with a further 6,564 deaths while waiting (United Network for Organ Sharing, 2021). Notably, these statistics do not include patients relying on other forms of care such as dialysis who may benefit from transplant.
Similar concerns arise in allogeneic hematopoietic stem cell transplantation. Despite use of immunosuppressive drugs, graft-versus-host-disease (GvHD) remains a non-trivial complication. Transplanted allogeneic T cells may attack the recipient’s tissue, causing a systemic T cell-mediated immune reaction. The lack of available hematopoietic stem cell donors exacerbates transplantation difficulties. Even with approximately 9 million registered donors in the United States, finding an appropriate match remains the primary challenge for patients. In 2021, a total of 22,827 hematopoietic stem cell transplants were performed in the United States alone, emphasizing the demand for the procedure (Health Resources and Services Administration, 2023). In Australia, despite more than 165,000 registered donors, more Australian patients receive donations from abroad; a trend that shifted slightly in 2021 due to the COVID-19 pandemic with more Australian recipients receiving transplants from unrelated Australian donors due to risk of importing cells (Australian Bone Marrow Donor Registry, 2022). Moreover, patients face side effects of conditioning regimens and an increased susceptibility to infection. These challenges form a barrier to patients requiring such procedures, increasing the logistical complexities associated with transplantation (Nakamura et al., 2020).
Recent papers have highlighted the feasibility of a universal cell donor line by using human induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) capable of evading immune recognition and subsequent rejection. The introduction of a universal cell donor line would be invaluable in the context of primary and secondary bone marrow failure where the only established treatment option is hematopoietic stem cell transplantation from an appropriately matched tissue donor. Additionally, the concept of universal cell donor lines and their differentiated derivatives could hold promise in addressing more prevalent challenges relating to organ dysfunction, where the primary critical treatment option is transplantation. This avenue of treatment remains limited by organ shortages. Here, we review the leading research in the generation of universal cell lines and give an overview of the safeguard considerations that need to be addressed.
Bypassing the MHC
The expression of highly polymorphic human leukocyte antigens (HLA) facilitates rejection of transplanted cells, tissues, and organs. These molecules are encoded by the major histocompatibility complex (MHC) genes on chromosome 6 that function to present antigenic peptides to T cells. Specifically, HLA class I molecules including HLA-A, HLA-B, and HLA-C are expressed on the surface of nucleated cells and present endogenous antigens. This functions to identify cells as belonging to self, or in the case of transplantation, as non-self. Expression of HLA class II molecules that include HLA-DR, HLA-DP, and HLA-DQ, are exclusive to antigen-presenting cells. Cytotoxic T cells (CD8+) and helper T cells (CD4+) then recognize peptides presented by HLA class I and II, respectively, ultimately leading to targeted killing or inflammatory T cell responses. The highly polymorphic nature of molecules within the MHC makes finding an appropriate HLA donor match difficult, thereby requiring systemic immunosuppression to mitigate this. However, suppression of immune function increases infection and cancer risks. Acute GvHD remains a significant adverse outcome, with approximately 30%–60% of patients undergoing allogeneic hematopoietic stem cell transplantation developing acute GvHD (Jagasia et al., 2012).
Modification of MHC class I to avoid immune detection
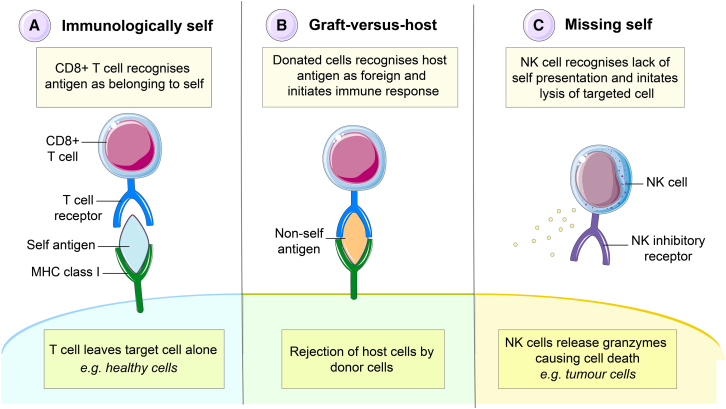
Beta 2 microglobulin (β2M) is a non-polymorphic gene located on chromosome 15 that encodes that subunit required for expression of HLA class I alpha chains (Li et al., 2016). Early animal studies showed that β2m deficiency resulted in defective assembly of MHC class I molecules on the cell surface and CD8+ T cell deficiencies (Koller et al., 2010; Zijlstra et al., 2010). Similar results were observed in later studies using human stem cells owing to the fact that HLA class I cannot be expressed in the absence of this molecule (Riolobos et al., 2013; Wang et al., 2015). It was demonstrated that less than 1% of CD8+ T cell-mediated killing was observed in the β2M deleted human ESCs after differentiation into alveolar lineage-specific cells (Wang et al., 2015). However, this approach of MHC class I deletion alone would have limited applicability, as the transplanted cells remain prone to lysis by natural killer (NK) cells, by activation of the missing-self response (Liao et al., 1991) (Figure 1).
Figure 1.
Overcoming the major histocompatibility class I complex
(A–C) The MHC class I functions to identify body cells as belonging to self. Cells that belong to self (A) are recognized as such by immune cells and are left alone. Cells of host origin including infected body cells (B) are recognized as immunologically foreign by cytotoxic T cells and targeted for removal. Removal of the MHC class I by genetic deletion (C) activates natural killer cells that target the cells for removal by lysis.
Using concepts in maternal-fetal tolerance to identify genes for hypo-immunogenic cells
To overcome NK-mediated lysis of MHC class I modified cells, expression of non-classical HLA class I molecules capable of binding NK cell inhibitory receptors has been investigated (Gornalusse et al., 2017). HLA-E is a minimally polymorphic HLA class I molecule that interacts with the CD94/NGK2A complex on the NK cell surface to inhibit lysis (Braud et al., 1998). This is observed during pregnancy where expression of HLA-E on extravillous trophoblasts facilitates evasion of NK cell-mediated cytotoxicity by interaction with the CD94/NGK2A complex (King et al., 2000). Similar to HLA-E, non-classical HLA-G is minimally polymorphic and involved in placental tolerance (Le Gal et al., 1999). HLA-G is expressed by trophoblast cells that are in direct contact with maternal tissues to establish immune tolerance at the maternal-fetal interface (Poehlmann et al., 2006). Specifically, HLA-G prevents NK cell and T cell cytotoxicity through functioning as a self-antigen (Le Gal et al., 1999; Poehlmann et al., 2006). Interestingly, studies have highlighted that reductions in the expression of HLA-G is observed in miscarriage, spontaneous abortions, premature birth, and preeclampsia, highlighting the importance of this molecule in immune tolerance (Aldrich et al., 2001; Goldman-Wohl et al., 2000; Wang et al., 2012).
These phenomena during pregnancy have prompted investigation aimed at exploiting the function of HLA-E and HLA-G in the tolerance of foreign antigens. Researchers previously generated a fusion β2M-HLA-E protein in human ESCs, where HLA-E was the only HLA class I molecule expressed (Gornalusse et al., 2017). The cells and their differentiated derivatives did not activate CD8+ T cells from an unmatched donor and were resistant to NK-mediated lysis by prevention of the “missing-self” response (Gornalusse et al., 2017). A similar study was completed using a β2M-HLA-G fusion protein that found eliminating surface expression of classic HLA class I protected the cells from NK and T cell-mediated cytotoxicity (Shi et al., 2020). Pregnancy highlights the role of non-classical HLA class I molecules in perhaps the most robust example of immune tolerance within mammalian species.
This aspect of immune tolerance has been observed clinically. Patients with functional renal transplants had higher levels of soluble HLA-G dimers in comparison with patients experiencing transplant rejection (Ezeakile et al., 2014). It is suggested that HLA-G has an inhibitory effect on both the activation and cytotoxic activity of CD8+ T cells by reducing expression of genes involved in the granzyme b pathway, subsequently minimizing the toxic potential of immune cells toward the graft cell or tissue. These studies show the promise of using these minimally polymorphic HLA molecules and have been combined with MHCII knockout strategies in recent successful teratoma studies that are discussed in more detail below.
Targeting MHC class II to force immune tolerance
Presentation of MHC class II is restricted to specialist antigen-presenting cells and, thus, research has focused on targeting the transcription factor required for MHC class II. Deletion of class II transactivator (Ciita) in mice showed a tissue-specific impairment of MHC class II expression and a reduction in CD4+ T cells (Chang et al., 1996). Further, the same studies found that knocking out CIITA in human and mouse ESCs allowed the engineered cells to evade CD4+ T cell-mediated responses within these mouse models (Chang et al., 1996; Chen et al., 2015); an important finding given that these cells are the primary effectors within transplanted cells. Nevertheless, the immune system itself is multifaceted in nature and thus, any attempt at exploiting this system must to be multifaceted.
Synergistic targeting of MHC class I and II to prevent rejection
Multiple studies have combined the approach of eliminating expression of MHC class I and II molecules to prevent immune rejection. In 2018, iPSCs with β2M and CIITA deletions were generated and were found to be resistant to cytotoxic effects of human T cells (Mattapally et al., 2018). Importantly, the engineered iPSCs retained their pluripotency and were able to be differentiated into cardiomyocytes that maintained their electrical properties. A similar system was investigated in mice with β2M and CIITA deletions using mouse and human iPSCs; however, with additional CD47 overexpression (Deuse et al., 2019). CD47 is highly expressed by syncytiotrophoblast cells lining the placental interface and thus, introduction of this molecule could assist in the creation of hypoimmunogenic cells, similarly to maternal-fetal tissues during pregnancy (Deuse et al., 2019). These engineered cells inhibited the activity of NK cells in vivo using allogeneic BALB/C mice for the mouse iPSC line and humanized CD34+ hematopoietic stem cell engrafted NSG-SGM3 mice for the human iPSC line (Deuse et al., 2019). This was demonstrated upon development of teratomas in isogenic and allogeneic mice upon transplantation of engineered iPSCs without immunosuppression (Deuse et al., 2019). The teratomas and differentiated derivatives were detectable within a week and were not rejected after 50 days. Mice were killed at the end of the 80-day observation period or if tumor development exceeded 1.5 cm3 (Deuse et al., 2019). Comparably, allogeneic mice injected with wild-type (WT) iPSCs cleared the teratoma within a month. Notably, the protective effect of CD47 was completely removed using a CD47 blocking antibody in mouse iPSCs (Deuse et al., 2019).
Expression of other immunomodulatory molecules has been performed to achieve tolerance in engineered cells. Expressing PD-L1 and CD47 in conjunction with replacement of HLA class I with HLA-G and removal of HLA class II molecules caused significantly less immune activation and clearance by T cells, NK cells, and macrophages in vitro and in vivo (Han et al., 2019). This specific cell line was transplanted into immunodeficient mice to allow teratoma formation over a period of 4 to 6 weeks. Experimental mice teratomas were protected from rejection demonstrated by reduced T cell infiltration and reduced NK cell cytotoxicity after incubation with allogeneic NK cells (Han et al., 2019).
While teratoma formation is a useful metric in studies of transplantation, it is not ideal as a therapeutic outcome. Although representative of successful engraftment, teratomas are an expansion of undifferentiated stem cells. Getting engineered cells past the immune system is important, but what is essential is the ability to differentiate these cell lines into the mature lineages required to have a therapeutic effect. This has been successfully demonstrated by (Feng et al. (2023), who have used CRISPR-Cas9 to knockout B2m and Ciita in WT iPSCs, which were then differentiated into oligodendrocyte progenitor cells (OPCs) and transplanted into the brains of immunodeficient CD (Nur7) mice that exhibit a Canavan disease-like phenotype due to a mutation in the aspatoacylase gene (ASPA). Harvesting of brains revealed widespread migration, survival, and remyelination of WT transplanted OPCs within the brain 6 months after transplantation (Feng et al., 2023). Crucially, the OPCs had similar ASPA enzymatic activity to WT mice, reduced NAA accumulation in the brain, and substantially improved motor function in CD mice (Feng et al., 2023). The successful transplantation of these universal iPSCs, which, after in vitro differentiation into functional OPCs, are capable of rescuing the disease phenotype, emphasizes the groundbreaking potential of these universal donor cells.
Modulating the local immune environment; a lesson from tumor evasion
Modifications to the MHC may not be the only way to circumvent rejection. Cancer research has demonstrated that tumor cells are able to resist cytolytic clearance and, subsequently, the immune system loses responsiveness to the tumor cells (Lanza et al., 2019). This concept is known as immune cloaking and leads to the idea that modulating various components within the immune system will allow the acceptance of foreign tissue and enforce donor tolerance.
Checkpoint inhibition has been a prominent topic in the field of cancer research and immunotherapy (La-Beck et al., 2015; Pardoll, 2012). Cytotoxic T lymphocyte-associated antigen (CTLA-4) contributes to the negative regulation of T cell responses (Greenwald et al., 2001; Takahashi et al., 2000). Recombinant CTLA-4 has been shown to inhibit immune responses and subsequently, transplant rejection demonstrating therapeutic potential (Grosso and Jure-Kunkel, 2013). The checkpoint inhibitor, PD-1 acts in concert with CTLA-4 and has also received notable attention. PD-1 contributes to negative regulation of T cell function upon interaction of this surface receptor with corresponding ligand PD-L1. A 2014 study forced expression of both CTLA-4 and PD-L1 in human ESCs and transplanted them into the hindleg of humanized mice (Rong et al., 2014). Mice were euthanized after a period of 6 to 8 weeks and teratomas were examined. The experimental mice demonstrated teratoma formation in 93% of subjects with 0% rejection of the tumor within the observation period in the absence of immunosuppression (Rong et al., 2014). Further, these cells maintained their differentiation ability into fibroblasts and cardiac myocytes (Rong et al., 2014). However, it is important to keep in mind the observation period was fixed at 6 to 8 weeks and thus, it cannot be ascertained if tumor regression would have occurred over a longer time frame.
Various strategies have been employed to generate cell lines that are tolerated within allogeneic environments (Deuse et al., 2019). The most promising seem to include removal of MHC components from the cell surface. Particularly, a multifactorial approach that counters various aspects of the immune system that are triggered in response to absence of MHC, such as NK cell-mediated cytotoxicity. Table S1 summarizes the different approaches taken to create a universal cell donor line. While overcoming the immunological barriers of allogeneic transplantation is a significant feat, the essential task lies in demonstrating the effective differentiation of transplanted cell products into the required mature lineage relevant to a given disease. This objective must not only overcome immune rejection but yield a therapeutic effect.
Pitfalls of MHC manipulation and concerns for development
Engineering cells to be universally tolerated overcomes immunological hurdles reviewed here, yet raises several clinical concerns. Manipulating the MHC mirrors escape strategies used by tumor cells; the deletion of MHC genes, thus this poses an additional risk of universal donor cells developing into tumors. There is danger in altering genes that identify us as self, potentially compromising surveillance of the immune environment. Beyond immune evasion causing neoplasm, or teratoma formation, the use of graft cells that have been virally infected and/or cultured contributes to clinical concerns about genetic instability and neoplastic changes.
As highlighted previously, knockout of the β2M gene results in non-functional HLA class I expression and cells escaping cytotoxic targeting (Hicklin et al., 1998). A similar mechanism is at play in the melanoma cell lines Me18105, Me9923, and Me1386 where naturally occurring mutations in the β2M gene cause complete loss of the HLA class I antigens (Hicklin et al., 1998). This alteration in the β2M gene is proposed to occur early in tumor progression and results in selection of HLA class I negative tumor cells that evade the immune system by escaping cytotoxic attack from CD8+ T cells (Hicklin et al., 1998). Failure to address these considerations during the development of a universal cell line could lead to malignant transformation. Alterations to HLA class I molecules can enhance immune evasion and cytotoxic resistance, further complicated by HLA-negative cells evading normal immune mechanisms (Lanza et al., 2019). As we pursue universal cell donor lines, diligent investigation of clinical concerns is required to ensure safety and efficacy. It is essential that any attempt at a universal cell line would feature a tightly regulated cell safety system. Further, compatibility of blood groups must be considered where erythroid differentiation is planned. A therapeutic cell line would need to be blood group O. This will avoid any potential rejection of cells with incompatible surface antigens given blood group O lacks A and B antigens on the cell surface and thus, enables a broader utility of the therapeutic product.
Establishing a cell safety system
To optimize cell-based therapies, the potential of treatment-related toxicities must be considered and minimized. Current molecular-based cell safety switches are listed in Table S2. Safety switches have come to the fore in the anti-leukaemic chimeric antigen receptor T cell (CAR-T cell) therapy field, and have been optimized to enhance therapeutic efficiency and reduce risk of adverse events (Zheng et al., 2021). For instance, FITC and folate can be conjugated to form a bifunctional safety switch whereby the molecules serve as an “on” switch. In this case, the CAR-T cell therapy is unable to be activated without the safety switch and thus, it is only after administration that the cell therapy is activated to target tumor cells (Zheng et al., 2021). Rapamycin is another example of a safety molecule that functions as an “on” switch. In the absence of Rapamycin, CAR-T cells are kept inactivated by way of disconnecting the antigen-binding and intracellular signaling subunits (Zheng et al., 2021). In its presence, Rapamycin assembles these two complexes into a complete construct after injection of cellular therapy into the patient (Zheng et al., 2021). The benefit of “on”-based safety switches is they allow a gradual titration of the therapy to the clinically beneficial level, while limiting adverse reactions.
In contrast to the “on” switches discussed, the herpes simplex virus thymidine kinase (HSV-TK) suicide gene system is a cell safety system used to control cell growth (Greenwald et al., 2001; Takahashi et al., 2000). The system works by transcriptionally linking cyclin-dependent kinase 1 (CDK1) and HSV-TK. This works because CDK1 is expressed in dividing cells blocking the G2 to M phase of the cell cycle and so, any dividing cell that co-expresses CDK1 and HSV-TK can be killed with ganciclovir (Liang et al., 2018). This has been demonstrated in studies with both iPSCs and ESCs where elimination of cells engineered with these suicide genes was induced upon ganciclovir administration (Cheng et al., 2012; Schuldiner et al., 2003). A major drawback of the HSV-TK suicide switch is that ganciclovir is used as first-line treatment against cytomegalovirus, a common adverse event in immunocompromised patients.
Inducible caspase 9 (iCasp9) is a suicide gene system composed of the intracellular portion of the caspase 9 protein that is fused to a drug-binding domain, derived from the FK506-binding protein and commonly expressed from the safe harbor, adeno-associated virus integration site 1 (AAVS1) locus (Straathof et al., 2005). Upon administration of chemical inducer of dimerization (CID) AP1903, caspase 9 dimerization occurs and it becomes activated leading to apoptotic signaling and subsequent apoptosis of the transduced cell (Gargett and Brown, 2014). Caspase 9 is a late-stage apoptotic marker and thus modification of this molecule prevents activation of engineered cells even in those with upregulation of anti-apoptotic molecules (Di Stasi et al., 2011). Just a single dose of CID at 10 nM can effectively kill over 99% of iCasp9 cells with high transgenic expression in both in vitro and in vivo models (Di Stasi et al., 2011).
iCasp9 is advantageous over the well-characterized HSV-TK as it has lower immunogenicity and is able to induce more rapid, complete cell death (Sahillioglu and Schumacher, 2022). In four patients with GvHD, activation of the suicide gene system eliminated upwards of 90% of engineered T cells without recurrence (Di Stasi et al., 2011). This was achieved in as little as 30 min, while HSV-TK systems can take several days to be effective (Sahillioglu and Schumacher, 2022). A more recent phase 1 clinical trial explored the minor histocompatibility antigen HA-1H T cell receptor (TCR) gene transfer in treatment of high-risk hematological malignancies after allogeneic stem cell transplantation (van Balen et al., 2020). T cells were harvested from HA-1H-negative stem cell donors before HA-1H TCR gene transfer into the cells to treat patients positive for the HA-1H allele HLA-A∗02:01 after allogeneic stem cell transplantation (van Balen et al., 2020). The trial showed no infusion-related toxicity or evidence of GvHD in any of the transplanted patients (van Balen et al., 2020). Further, expansion of the transduced T cells was demonstrated in three of five patients (van Balen et al., 2020). Notably, the efficacy in this trial was too low to warrant further investigation using this specific strategy.
Multiple clinical trials have assessed the safety profile of iCasp9 introduction into T cell products to treat a variety of diseases (NCT01494103, NCT00710892). While looking at engineering T cells to target different disease processes, the studies involve patients receiving iCasp9 cells intravenously after 30–90 days post stem cell transplantation. In patients receiving iCasp9 cells, and treated with AP1903, 85%–95% of the circulating introduced CD3+CD19+ iCasp9 T cells were eliminated within 30 min (Zhou et al., 2015.) Although the short-term safety studies show no concerns for these products, long-term safety of the insertion of iCasp9 over decades has not yet been determined.
Notably, suicide gene systems are irreversible in nature and consequently, activation of the cell safety switch results in loss of therapeutic activity. Although safety switches, such as iCasp9 are typically built into safe harbors, a 2018 study inserted the iCasp9 gene downstream of the endogenous OCT4 promoter in both mouse and human pluripotent stem cells (Liu et al., 2022). The cell lines were able to induce cell death after treatment with CID both in vitro and in vivo within 2 to 6 h (Liu et al., 2022). Upon differentiation, CID could no longer affect cells, highlighting its importance in removing undifferentiated cells in early teratoma formation while maintaining functional differentiated transplanted cells (Liu et al., 2022).
Other options to induce transplanted cell suicide involve engineering cells to express cell surface markers such as CD20 or a truncated form of the epidermal growth factor receptor (EGFRt), which then enable the use of monoclonal antibodies to delete the cells through antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity (Griffioen et al., 2009). Studies have used various cell surface markers to achieve this; an example of this work showing CAR-T cell depletion through expression of CD20 and a truncated version of CD34 where effective lysis of the CAR-T cells was demonstrated to occur both in vitro and in vivo, with a 96% depletion in the circulating CAR-T cells upon administration of the anti-CD20 antibody, Rituximab (Volger et al., 2010).
Ensuring universal donor cell systems have controlled growth without cellular escape is important. However, given that between 105 and 1010 cells are used for various cell therapies, cellular escape could pose a likely threat depending on the transplant. With that in mind, the use of a universal stem cell line may provide a temporary replacement for dysfunctional cells and thus, create a scaffold within a specific microenvironment for cellular regeneration.
Changing the face of transplantation medicine
Eliminating immune rejection risk upon transplantation of grafted cells would overcome the biggest hurdle facing transplant and regenerative medicine today. The potential for readily available therapeutic cell products would eliminate the need for patients to undergo systemic immune suppression, but also eliminate the waiting time required to find an appropriate donor. Identification of a novel combination of immune regulatory molecules could protect iPSC or ESC donor cells from rejection and elicit tolerance; however, elimination of MHC molecules could compromise the ability of the immune system to both survey and eliminate infected cells. Thus, redundancies and safeguards must be implemented to prevent malignant transformation of cells. No therapy is without risk, and the potential advent of universal cells would provide an indispensable tool for prospective cell therapies to treat a wide variety of diseases including diabetes, eye diseases, and hematological disorders, as well as the potential to be engineered into more complex tissue structures.
Acknowledgments
K.A.F. is supported by the Alex Gadomski Fellowship, funded by Maddie Riewoldt’s Vision. Additional grant support was provided by the National Health and Medical Research Council (GNT 2020517) and Leadership Fellowship (AWH).
A.S. is supported by the Alex Gadomski Postgraduate Scholarship in Medical Research funded by Maddie Riewoldt’s Vision and the Menzies Institute for Medical Research at the University of Tasmania.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.09.010.
Supplemental information
References
- Aldrich C.L., Stephenson M.D., Karrison T., Odem R.R., Branch D.W., Scott J.R., Schreiber J.R., Ober C. HLA-G genotypes and pregnancy outcome in couples with unexplained recurrent miscarriage. Mol. Hum. Reprod. 2001;7:1167–1172. doi: 10.1093/molehr/7.12.1167. [DOI] [PubMed] [Google Scholar]
- Australian Bone Marrow Donor Registry . 2022. Annual Bone Marrow Donor Registry Annual Report 2021-22.https://www.abmdr.org.au/wp-content/uploads/2022/05/ABMDR_Annual_Report_2021-22.pdf Accessed: 30 August 2023. Available at: [Google Scholar]
- Braud V.M., Allan D.S., O’Callaghan C.A., Söderström K., D’Andrea A., Ogg G.S., Lazetic S., Young N.T., Bell J.I., Phillips J.H., et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- Chang C.H., Guerder S., Hong S.C., van Ewijk W., Flavell R.A. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immun. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- Cheng F., Ke Q., Chen F., Cai B., Gao Y., Ye C., Wang D., Zhang L., Lahn B.T., Li W., Xiang A.P. Protecting against wayward human induced pluripotent stem cells with a suicide gene. Biomaterials. 2012;33:3195–3204. doi: 10.1016/j.biomaterials.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Chen H., Li Y., Lin X., Cui D., Cui C., Li H., Xiao L. Functional disruption of human leukocyte antigen II in human embryonic stem cell. Biol. Res. 2015;48:59. doi: 10.1186/s40659-015-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuse T., Hu X., Gravina A., Wang D., Tediashvili G., De C., Thayer W.O., Wahl A., Garcia J.V., Reichenspurner H., et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019;37:252–258. doi: 10.1038/s41587-019-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi A., Tey S.-K., Dotti G., Fujita Y., Kennedy-Nasser A., Martinez C., Straathof K., Liu E., Durett A.G., Grilley B., et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeakile M., Portik-Dobos V., Wu J., Horuzsko D.D., Kapoor R., Jagadeesan M., Mulloy L.L., Horuzsko A. HLA-G dimers in the prolongation of kidney allograft survival. J. Immunol. Res. 2014;2014 doi: 10.1155/2014/153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Chao J., Ye P., Luong Q., Sun G., Liu W., Cui Q., Flores S., Jackson N., Shayento A.N.H., et al. 2023. Developing Hypoimmunogenic Human iPSC-Derived Oligodendrocyte Progenitor Cells as an Off-The-Shelf Cell Therapy for Myelin Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett T., Brown M.P. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front. Pharmacol. 2014;5:235. doi: 10.3389/fphar.2014.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Wohl D.S., Ariel I., Greenfield C., Hochner-Celnikier D., Cross J., Fisher S., Yagel S. Lack of human leukocyte antigen-G expression in extravillous trophoblasts is associated with pre-eclampsia. Mol. Hum. Reprod. 2000;6:88–95. doi: 10.1093/molehr/6.1.88. [DOI] [PubMed] [Google Scholar]
- Gornalusse G.G., Hirata R.K., Funk S.E., Riolobos L., Lopes V.S., Manske G., Prunkard D., Colunga A.G., Hanafi L.-A., Clegg D.O., et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 2017;35:765–772. doi: 10.1038/nbt.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R.J., Boussiotis V.A., Lorsbach R.B., Abbas A.K., Sharpe A.H. CTLA-4 Regulates Induction of Anergy In Vivo. Immun. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- Griffioen M., van Egmond E.H.M., Kester M.G.D., Willemze R., Falkenburg J.H.F., Heemskerk M.H.M. Retroviral transfer of human CD20 as a suicide gene for adoptive T-cell therapy. Haematologica. 2009;94:1316–1320. doi: 10.3324/haematol.2008.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso J.F., Jure-Kunkel M.N. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- Han X., Wang M., Duan S., Franco P.J., Kenty J.H.-R., Hedrick P., Xia Y., Allen A., Ferreira L.M.R., Strominger J.L., et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2019;116:10441–10446. doi: 10.1073/pnas.1902566116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Resources and Services Administration Donation and Transplantation Statistics. 2023. https://bloodstemcell.hrsa.gov/data/donation-and-transplantation-statistics Accessed 24 August 2023. Available at:
- Hicklin D.J., Wang Z., Arienti F., Rivoltini L., Parmiani G., Ferrone S. beta2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J. Clin. Invest. 1998;101:2720–2729. doi: 10.1172/JCI498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L.D. The transplant patient and transplant medicine in family practice. J. Family Med. Prim. Care. 2014;3:345–354. doi: 10.4103/2249-4863.148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia M., Arora M., Flowers M.E.D., Chao N.J., McCarthy P.L., Cutler C.S., Urbano-Ispizua A., Pavletic S.Z., Haagenson M.D., Zhang M.-J., et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A., Allan D.S., Bowen M., Powis S.J., Joseph S., Verma S., Hiby S.E., McMichael A.J., Loke Y.W., Braud V.M. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol. 2000;30:1623–1631. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Koller B.H., Marrack P., Kappler J.W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. 1990. Journal Immunol. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Kupiec-Weglinski J.W. Grand Challenges in Organ Transplantation. Front. Transplant. 2022;1 doi: 10.3389/frtra.2022.897679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La-Beck N.M., Jean G.W., Huynh C., Alzghari S.K., Lowe D.B. Immune Checkpoint Inhibitors: New Insights and Current Place in Cancer Therapy. Pharmacotherapy. 2015;35:963–976. doi: 10.1002/phar.1643. [DOI] [PubMed] [Google Scholar]
- Lanza R., Russell D.W., Nagy A. Engineering universal cells that evade immune detection. Nat. Rev. Immunol. 2019;19:723–733. doi: 10.1038/s41577-019-0200-1. [DOI] [PubMed] [Google Scholar]
- Le Gal F.A., Riteau B., Sedlik C., Khalil-Daher I., Menier C., Dausset J., Guillet J.G., Carosella E.D., Rouas-Freiss N. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int. Immunol. 1999;11:1351–1356. doi: 10.1093/intimm/11.8.1351. [DOI] [PubMed] [Google Scholar]
- Liang Q., Monetti C., Shutova M.V., Neely E.J., Hacibekiroglu S., Yang H., Kim C., Zhang P., Li C., Nagy K., et al. Linking a cell-division gene and a suicide gene to define and improve cell therapy safety. Nature. 2018;563:701–704. doi: 10.1038/s41586-018-0733-7. [DOI] [PubMed] [Google Scholar]
- Liao N.S., Bix M., Zijlstra M., Jaenisch R., Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- Li L., Dong M., Wang X.-G. The Implication and Significance of Beta 2 Microglobulin: A Conservative Multifunctional Regulator. Chin. Med. J. 2016;129:448–455. doi: 10.4103/0366-6999.176084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Suo Y., Li C., Chen M., Zheng S., Li H., Tang C., Fan N., Lan T., et al. Inducible caspase-9 suicide gene under control of endogenous oct4 to safeguard mouse and human pluripotent stem cell therapy. Mol. Ther. Methods Clin. Dev. 2022;24:332–341. doi: 10.1016/j.omtm.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattapally S., Pawlik K.M., Fast V.G., Zumaquero E., Lund F.E., Randall T.D., Townes T.M., Zhang J. Human Leukocyte Antigen Class I and II Knockout Human Induced Pluripotent Stem Cell-Derived Cells: Universal Donor for Cell Therapy. J. Am. Heart Assoc. 2018;7:e010239. doi: 10.1161/JAHA.118.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Kageyama S., Kupiec-Weglinski J.W. Innate Immunity in Ischemia-reperfusion Injury and Graft Rejection. Curr. Opin. Organ Transplant. 2019;24:687–693. doi: 10.1097/MOT.0000000000000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlmann T.G., Schaumann A., Busch S., Fitzgerald J.S., Aguerre-Girr M., Le Bouteiller P., Schleussner E., Markert U.R. Inhibition of term decidual NK cell cytotoxicity by soluble HLA-G1. Am. J. Reprod. Immunol. 2006;56:275–285. doi: 10.1111/j.1600-0897.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Riolobos L., Hirata R.K., Turtle C.J., Wang P.-R., Gornalusse G.G., Zavajlevski M., Riddell S.R., Russell D.W. HLA engineering of human pluripotent stem cells. Mol. Ther. 2013;21:1232–1241. doi: 10.1038/mt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Z., Wang M., Hu Z., Stradner M., Zhu S., Kong H., Yi H., Goldrath A., Yang Y.-G., Xu Y., Fu X. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell. 2014;14:121–130. doi: 10.1016/j.stem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahillioglu A.C., Schumacher T.N. Safety switches for adoptive cell therapy. Curr. Opin. Immunol. 2022;74:190–198. doi: 10.1016/j.coi.2021.07.002. [DOI] [PubMed] [Google Scholar]
- Schuldiner M., Itskovitz-Eldor J., Benvenisty N. Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cell. 2003;21:257–265. doi: 10.1634/stemcells.21-3-257. [DOI] [PubMed] [Google Scholar]
- Shi L., Li W., Liu Y., Chen Z., Hui Y., Hao P., Xu X., Zhang S., Feng H., Zhang B., et al. Generation of hypoimmunogenic human pluripotent stem cells via expression of membrane-bound and secreted β2m-HLA-G fusion proteins. Stem Cell. 2020;38:1423–1437. doi: 10.1002/stem.3269. [DOI] [PubMed] [Google Scholar]
- Straathof K.C., Pulè M.A., Yotnda P., Dotti G., Vanin E.F., Brenner M.K., Heslop H.E., Spencer D.M., Rooney C.M. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. Immunologic Self-Tolerance Maintained by Cd25 Cd4 Regulatory T Cells Constitutively Expressing Cytotoxic T Lymphocyte–Associated Antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Network for Organ Sharing . 2021. All-Time Records Set in 2021 for Organ Transplants, Deceased Donor Donation.https://unos.org/news/2021-all-time-records-organ-transplants-deceased-donor-donation/ Accessed: 24 August 2022. Available at: [Google Scholar]
- van Balen P., Jedema I., van Loenen M.M., de Boer R., van Egmond H.M., Hagedoorn R.S., Hoogstaten C., Veld S.A.J., Hageman L., van Liempt P.A.G., et al. HA-1H T-Cell Receptor Gene Transfer to Redirect Virus-Specific T Cells for Treatment of Hematological Malignancies After Allogeneic Stem Cell Transplantation: A Phase 1 Clinical Study. Front. Immunol. 2020;11:1804. doi: 10.3389/fimmu.2020.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler I., Newrzela S., Hartmann S., Schneider N., von Laer D., Koehl U., Grez M. An improved bicistronic CD20/tCD34 vector for efficient purification and in vivo depletion of gene-modified T cells for adoptive immunotherapy. Mol. Ther. 2010;18:1330–1338. doi: 10.1038/mt.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Quan Y., Yan Q., Morales J.E., Wetsel R.A. Targeted Disruption of the β2-Microglobulin Gene Minimizes the Immunogenicity of Human Embryonic Stem Cells. Stem Cells Transl. Med. 2015;4:1234–1245. doi: 10.5966/sctm.2015-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li B., Wang J., Lei J., Liu C., Ma Y., Zhao H. Evidence that miR-133a causes recurrent spontaneous abortion by reducing HLA-G expression. Reprod. Biomed. Online. 2012;25:415–424. doi: 10.1016/j.rbmo.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Nandakumar K.S., Cheng K. Optimization of CAR-T Cell-Based Therapies Using Small-Molecule-Based Safety Switches. J. Med. Chem. 2021;64:9577–9591. doi: 10.1021/acs.jmedchem.0c02054. [DOI] [PubMed] [Google Scholar]
- Zhou X., Dotti G., Krance R.A., Martinez C.A., Naik S., Kamble R.T., Durett A.G., Dakhova O., Savoldo B., Di Stasi A., et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood. 2015;125:4103–4113. doi: 10.1182/blood-2015-02-628354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N.E., Loring J.M., Raulet D.H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. 1990. J. Immunol. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.