Abstract
Introduction
Timely diagnosis of interstitial lung disease (ILD) is limited by obstacles in the current patient pathway. Misdiagnosis and delays are common and may lead to a significant burden of diagnostic procedures and worse outcomes. This Delphi survey aimed to identify consensus on the key steps that facilitate the patient journey to an accurate ILD diagnosis and appropriate management in the US.
Methods
A modified Delphi analysis was conducted, comprising three online surveys based on a comprehensive literature search. The surveys spanned five domains (guidelines, community screening, diagnosis, management and specialist referral) and were completed by a panel of US physicians, including primary care physicians and pulmonologists practising in community or academic settings. A priori definitions of consensus agreement were median scores of 2–3 (agree strongly/agree), with an IQR of 0–1 for questions on a 7-point Likert scale from −3 to 3, or ≥80% agreement for binary questions.
Results
Forty-nine panellists completed the surveys and 62 statements reached consensus agreement. There was consensus agreement on what should be included in the primary care evaluation of patients with suspected ILD and the next steps following workup. Regarding diagnosis in community pulmonology care, consensus agreement was reached on the requisition and reporting of high-resolution CT scans and the appropriate circumstances for holding multidisciplinary discussions. Additionally, there was consensus agreement on which symptoms and comorbidities should be monitored, the frequency of consultations and the assessment of disease progression. Regarding specialist referral, consensus agreement was reached on which patients should receive priority access to ILD centres and the contents of the referral package.
Conclusions
These findings clarify the most common issues that should merit further evaluation for ILD and help define the steps for timely, accurate diagnosis and appropriate collaborative specialty management of patients with ILD.
Keywords: Interstitial Fibrosis, Imaging/CT MRI etc, Respiratory Measurement
Video Abstract.
Disclaimer: this video summarises a scientific article published by BMJ Publishing Group Limited (BMJ). The content of this video has not been peer-reviewed and does not constitute medical advice. Any opinions expressed are solely those of the contributors. Viewers should be aware that professionals in the field may have different opinions. BMJ does not endorse any opinions expressed or recommendations discussed. Viewers should not use the content of the video as the basis for any medical treatment. BMJ disclaims all liability and responsibility arising from any reliance placed on the content.
WHAT IS ALREADY KNOWN ON THIS TOPIC
The patient pathway towards diagnosis of interstitial lung disease (ILD) and appropriate disease management is currently lengthy with numerous obstacles, which can lead to worse outcomes for patients.
WHAT THIS STUDY ADDS
This modified Delphi survey provided consensus agreement among primary care providers, community pulmonologists and ILD specialists on over 95% of the surveys’ statements on guidelines, community screening, community diagnosis, community management and specialist referral.
These findings clarify the most common symptoms requiring further evaluation for ILD, steps for timely and accurate diagnosis and how to effectively collaborate between different medical specialties to manage patients with ILD.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Improving ILD disease recognition and knowledge of the appropriate next steps will reduce delays and improve the pathway to ILD diagnosis and management.
Introduction
Interstitial lung disease (ILD) is a large, diverse group of pulmonary disorders characterised by diffuse parenchymal lung infiltration.1 Reported prevalence of ILD in the USA is 74.3 per 100 000 people based on one study,2 and in those over the age of 65, prevalence is as high as 494 per 100 000 people.3 Mortality rates can be as high as 80% over 5 years, depending on the disease entity4; hence, timely diagnosis is important for delaying disease progression and ultimately prolonging survival.5 Given the growing availability of therapeutic interventions, including antifibrotics and immune modulators, for use in certain ILD subgroups, early and accurate ILD diagnosis is more clinically relevant than ever.
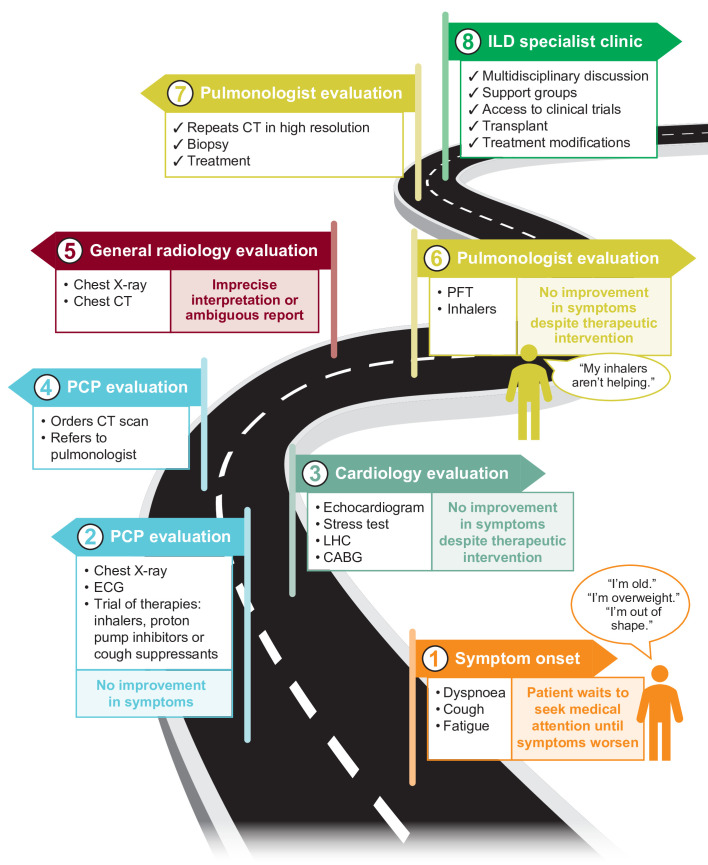
The patient pathway to ILD diagnosis and management can be circuitous, and opportunities for an early diagnosis are often missed due to lack of disease recognition, unfamiliarity with diagnostic procedures and/or delays in referral to specialist care (figure 1).6–8 Reports of diagnostic delays range from 7 months to >5 years from onset of symptoms.5 6 9–11 These delays may begin with patients explaining away symptoms—assuming they are associated with normal ageing, for example—and forgoing medical assessment.5 Once in primary care, symptoms are often initially attributed to more common respiratory or cardiovascular conditions; patients can be empirically prescribed ineffective therapies such as inhaled bronchodilators and high-dose corticosteroids,8 and referred for cardiac testing if symptoms do not improve. The presence of common comorbidities such as coronary artery disease and gastro-oesophageal reflux disease may mask the symptoms of ILD and contribute to delays in diagnosis.
Figure 1.
Current pathway to ILD diagnosis with examples of missed opportunities. CABG, coronary artery bypass graft; ILD, interstitial lung disease; LHC, left heart catheterisation; PCP, primary care physician; PFT, pulmonary function test.
Appropriate chest imaging, specifically CT, and pulmonology referral are essential steps in the ILD diagnostic pathway. Reporting of ILD findings using standardised terminology on chest CT has been shown to significantly increase the likelihood of prompt pulmonology referral7; however, ambiguous reporting and misinterpretation are common.12–14 Delays in referral to pulmonology and specialist ILD care are therefore widely reported.5 7 8 In a structured online survey of adults with a self-reported diagnosis of ILD, 30.4% reported more than four visits to their primary care physicians (PCPs) before referral to a pulmonary specialist.6 Even following pulmonologist evaluation, ILD diagnosis is frequently delayed according to Medicare claims data.15
This fraught, convoluted diagnostic process can have a detrimental effect on patient quality of life and can subject patients to multiple, unnecessary diagnostic procedures while still resulting in diagnostic uncertainty. It may also present a significant financial burden for patients undergoing repeated testing and taking time out of work to attend medical appointments.6 16 Delays may ultimately lead to worse outcomes for the patient,7 with longer delays shown to be associated with significantly increased risk of death.8 Improved guidance at each stage of the patient pathway may help to ensure that patients with ILD receive appropriate care and the best prognosis possible.
Delphi surveys are well established as a robust consensus technique for health-related cases in which clinical evidence is insufficient or contradictory.17 The aim of this study was to achieve consensus among PCPs and pulmonologists on the key steps that facilitate the patient journey to an accurate and expedited ILD diagnosis and appropriate management in the USA.
Methods
Identification of modified Delphi survey items
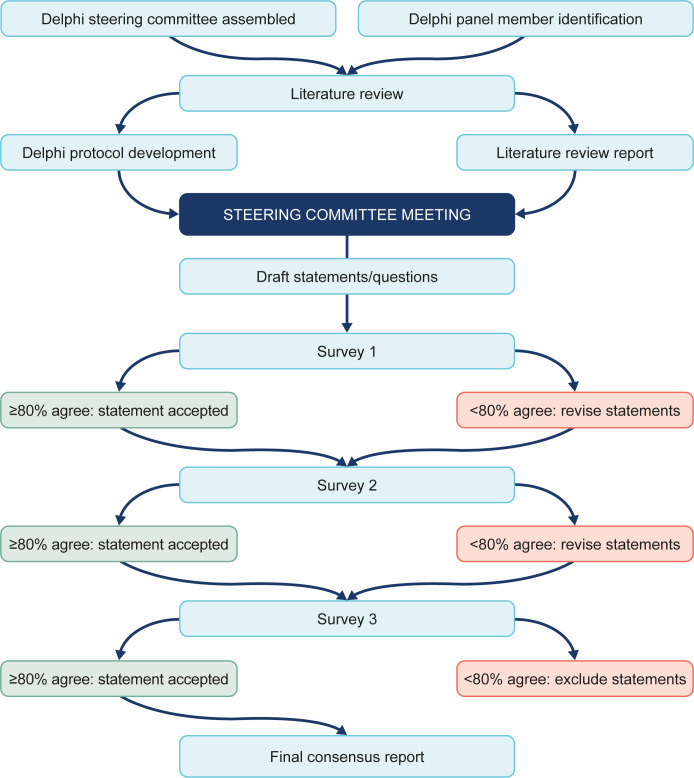
This study used a modified Delphi process (figure 2). To inform development of the Delphi survey, a comprehensive literature review was performed across five focus areas: (1) guidelines; (2) community screening; (3) community diagnosis; (4) community management; and (5) specialist referral. A Steering Committee of experts was assembled with AHC and JAdA as co-chairs. The Steering Committee included 10c ILD centres (AHC, JAdA, HJK, AJP and Timothy Whelan) and 5 from community centres (CR, DLH, MR, TK and SB)), one radiologist (MMS) and one internist (CT). The Steering Committee defined the topics for the Delphi analysis, the panel recruitment criteria, the definitions of consensus and identified a suitable panel.
Figure 2.
Modified Delphi process.
Selection of Delphi panel
Physicians were considered for inclusion in the panel if they were a practising US clinician at the time of recruitment and consented to participation in all three surveys. PCPs were required to have ≥5 years of clinical experience with patients experiencing undifferentiated shortness of breath. Pulmonologists were required to have ≥5 years of clinical experience diagnosing, treating or imaging ILDs.
Modified Delphi survey execution
We conducted a three-round, web-based (SurveyMonkey) survey between April 2021 and September 2021 following published standards of Delphi methodology.18 The surveys were divided into the five focus areas. Pulmonologists were required to complete all survey sections, while PCPs were required to complete the sections most relevant to their practice: (1) guidelines; and (2) community screening. Survey 1 was developed to gather information on panellists’ practices and primarily consisted of open-ended and multiple-choice questions. Surveys 2 and 3 included statements for which the panellists were asked to provide their level of agreement. Some statements were repeated in subsequent surveys to define the stability of the consensus achieved. Panel responses were kept anonymous and weighted equally. Panellists were encouraged to provide comments or reasoning for their responses and these, along with the quantitative results of each survey, were used to shape the next set of questions and statements. When completing subsequent surveys, panellists were given summaries of the previous responses given by the group for consideration.
Definitions of consensus
A priori thresholds of consensus were defined for this study. For statements rated using a 7-point Likert scale, consensus was predefined as a median score of 2–3 (agree to strongly agree) or –2 to –3 (disagree to strongly disagree) with an IQR of 0–1. Consensus for statements rated using a binary scale was predefined as ≥80% agreement or disagreement.
Patient and public involvement
None.
Results
In total, 53 pulmonologists and PCPs from the USA accepted the invitation to participate in the Delphi panel. Of these, 49 participated in the surveys: 48 (91%) completed Survey 1, 40 (75%) completed Survey 2 and 36 (68%) completed Survey 3. The participating panel comprised 43 pulmonologists and 6 PCPs. Twenty pulmonologists were based in academic or teaching hospitals and 23 were based in community centres. Of the PCPs, two were based in internal medicine and four in family medicine. The panellists have extensive clinical experience and dedicate a high percentage of their time to caring for patients with respiratory symptoms, including ILD (online supplemental table S1). The panel was presented with 65 statements and by the end of Survey 3, consensus was reached on 62 (95.4%) of them. We describe below the items that achieved consensus and those that did not. All consensus statements and a detailed description of the evolution of statements across the three surveys are presented in the online supplemental tables S2–S7.
bmjresp-2022-001594supp001.pdf (350.1KB, pdf)
Guidelines
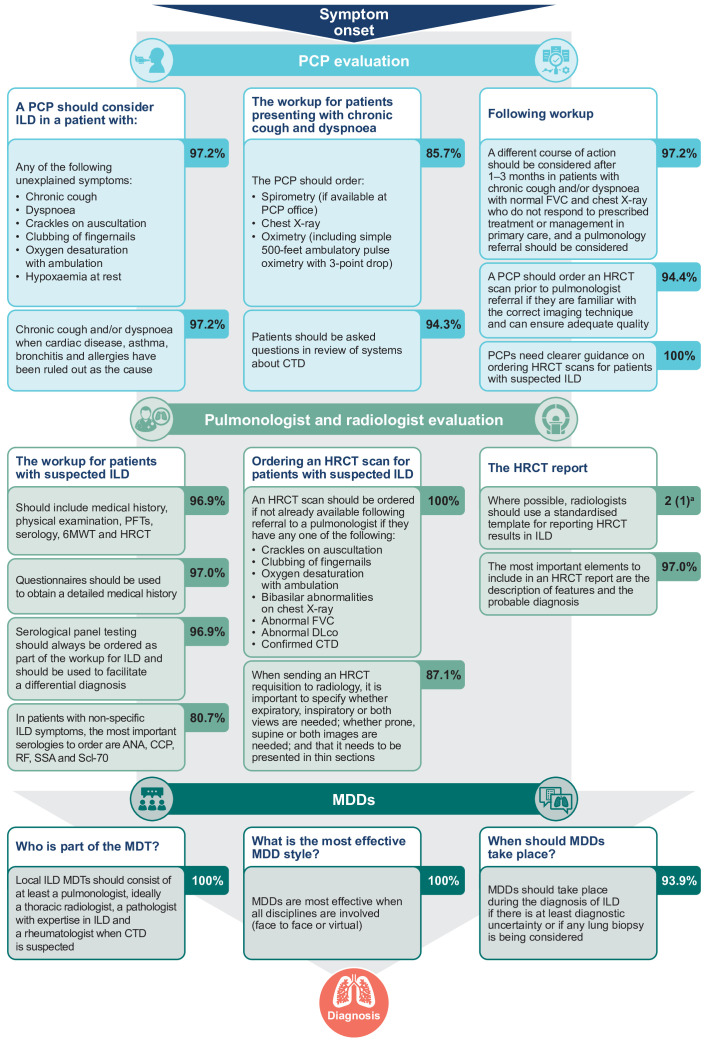
All eight items in the guidelines focus area reached consensus agreement by the third survey round (online supplemental table S2). The panel agreed that the current guidelines for the diagnosis and management of ILD are not completely clear, and consensus was stable across the second (82.5%) and third survey rounds (94.4%). There was also consensus agreement on the need for increased awareness and education around ILD for patients, PCPs (100% agreement for both), pulmonologists and rheumatologists (97.2% agreement for both). Unanimous consensus agreement was reached on the need for PCP guidelines; specifically, when to suspect ILD, when to refer patients to a pulmonologist and guidance on pulmonary function tests (PFTs). It was also agreed that ILD centres need improved guidance on multidisciplinary co-management of patients with ILD (97.2% agreement).
Recognition of ILD in primary care
Initial presentation of symptomatic patients
From 14 initial questions in Survey 1, 10 statements reached consensus agreement by Survey 3, with one line of questioning removed based on feedback from the panel (online supplemental table S2; figure 3). Consensus agreement was reached on when a PCP should suspect ILD, including which conditions should be ruled out in patients presenting with chronic cough and dyspnoea (cardiac disease, asthma, bronchitis and allergies; 97.2% agreement) and which unexplained symptoms may be a sign of ILD (97.2% agreement). There was also consensus agreement (94.3%) on what should be included in the workup of a symptomatic patient and what PCPs should do if ILD is still suspected following workup. It was agreed (94.4%) that a PCP should order a high-resolution CT (HRCT) scan prior to pulmonology referral if they are familiar with the correct imaging technique to request. However, there was also unanimous agreement (100%) that PCPs need clearer guidance on how to order an HRCT scan. For those who do not respond to initial treatment, the panel agreed (97.2%) that pulmonology referral should be considered after 1–3 months.
Figure 3.
Flow chart indicating the consensus on the patient pathway to diagnosis. aFor statements assessed on a 7-point Likert scale, the data are median (IQR). The scale was from –3 (strongly disagree) to +3 (strongly agree). 6MWT, 6-minute walk test; ANA, antinuclear antibodies; CCP, cyclic citrullinated peptide; CTD, connective tissue disease; DLco, diffusing capacity of the lung for carbon monoxide; FVC, forced vital capacity; HRCT, high-resolution CT; ILD, interstitial lung disease; MDD, multidisciplinary discussion; MDT, multidisciplinary team; PCP, primary care physician; PFT, pulmonary function test; RF, rheumatoid factor; SSA, Sjögren’s-syndrome-related antigen A.
For patients presenting in primary, rheumatology or cardiology care, the panel agreed (100%) that ILD should be considered in patients with a cough, dyspnoea and crackles on auscultation that are not explained by an initial workup. It was also agreed that these patients should be co-managed by a pulmonologist, and cardiologists should consider concurrent pulmonology workup or diagnostic testing (100% agreement).
Screening of asymptomatic patients
From nine initial questions in Survey 1, five statements reached consensus agreement by Survey 3 (online supplemental table S2). It was agreed that patients with connective tissue disease (CTD) should be screened for ILD at disease baseline and then every 12–18 months depending on the underlying disease (90.9%). Despite this, consensus was not reached (65.9% agreement) on whether asymptomatic patients with rheumatoid arthritis, specifically, should be screened for ILD and further research is needed to determine which patients would benefit from HRCT screening (94.3% agreement).
It was agreed that patients with a history of systemic sclerosis (94.2%) and patients aged >50 years with a family history of pulmonary fibrosis (83.9%) should be screened for ILD with at least full PFTs. However, consensus was not reached on the additional use of HRCT screening (72.7% and 69.7% agreement for patients with a history of systemic sclerosis and a family history of pulmonary fibrosis, respectively). For patients with one first-degree relative with pulmonary fibrosis or idiopathic interstitial pneumonia in the family, further research and guidance are needed to determine whether ILD screening is appropriate (97.2% agreement).
Specific diagnosis and appropriate management of ILD
Community diagnosis
From 16 initial questions in Survey 1, 14 statements reached consensus agreement by Survey 3, with one line of questioning halted at Survey 2 based on futility (online supplemental table S2; figure 3). There was strong consensus agreement (median=3, IQR=1) that it is important to make a specific ILD diagnosis whenever possible. The panel agreed (96.9%) on items that should be included in the pulmonologist workup of a patient with suspected ILD: medical history (using questionnaires (97.0% agreement)), physical examination, PFTs, serology, 6-minute walk test and HRCT. The most important serologies to order for patients with non-specific ILD symptoms are antinuclear antibodies, cyclic citrullinated peptide, rheumatoid factor, Sjögren’s-syndrome-related antigen A and topoisomerase I (80.7% agreement).
There was agreement on the requisition of HRCT scans and quality of HRCT reports. The panel agreed (100%) that pulmonologists should order an HRCT scan, if not already done by the referring physician, if patients present with cough and dyspnoea along with other key ILD indicators. The HRCT requisition should specify if expiratory images should be obtained in addition to standard inspiratory images; whether prone images should be done in addition to routine supine images; and should indicate that it needs to be presented with thin sections (87.1% agreement). The panel agreed that radiologists should use a standardised template for the HRCT report (median=2, IQR=1), and it should at least include the description of features and the most likely radiological diagnosis (97.0% agreement).
There was consensus that multidisciplinary discussions should take place if there is at least diagnostic uncertainty or if lung biopsy is being considered (93.9% agreement). The multidisciplinary team should consist of at least a pulmonologist, radiologist (ideally a thoracic radiologist), a pathologist with expertise in ILD and a rheumatologist when CTD is suspected (100% agreement).
Community management
All 10 items in the community management focus area reached consensus agreement by Survey 3 (table 1; online supplemental table S2). The panel agreed (97.0%) that patients with any CTD-ILD should be co-managed with a rheumatologist and pulmonologist. In terms of patient follow-up visits, there was unanimous agreement (100%) that patients with ILD should be seen by a pulmonologist every 3–6 months, depending on the diagnosis and disease severity. There was also agreement (100%) on what should be evaluated at follow-up visits, including which symptoms and common comorbidities should be regularly monitored. The panel agreed (96.9%) that ILD progression should be monitored using at least spirometry and measurement of diffusing capacity, with HRCT scans considered only if clinical deterioration is observed (96.9% agreement). It was also agreed that the decision to carry out PFTs depends on the specific diagnosis, disease severity and treatment (93.8% agreement).
Table 1.
Statements on the appropriate management of ILD in the community and specialist ILD centre settings with unanimous consensus agreement
| Consensus statement | Number of responses |
| Community management | |
| Pulmonologists should follow up with their patients with ILD every 3–6 months, depending on the diagnosis and disease severity. | 32 |
| At follow-up visits for patients with ILD, pulmonologists should evaluate disease progression, physical function, symptom severity, quality of life, suitability for clinical trials, suitability for lung transplant and side effects of medication. | 33 |
| Cough, dyspnoea, fatigue and the emotional well-being of patients should be monitored regularly in patients with ILD. | 33* |
| Common comorbidities that should be monitored/assessed in patients with ILD are pulmonary hypertension, GERD, sleep disordered breathing, COPD and lung cancer if appropriate. | 33* |
| It is important to discuss pulmonary rehabilitation; lung transplant and clinical trial opportunities; symptom management; advanced care planning and goals of care; and palliative care when managing patients with ILD. | 32 |
| Specialist referral | |
| Pulmonologists should consider referral of a patient with ILD to a specialist ILD or PFF centre if there is diagnostic or treatment uncertainty, the patient requests referral, the patient is a transplant or clinical trial candidate, or if there is disease progression despite treatment. | 32 |
| Patients with idiopathic pulmonary fibrosis should receive early referral to an ILD centre if they are young at disease onset, transplant eligible, or have rapid disease progression. | 33 |
| Patients who are potential transplant candidates or have rapidly progressing disease should be given priority access to an ILD centre on referral. | 33 |
| Referring physicians should always share all relevant patient medical records when referring to an academic centre. | 38 |
| When referring a patient with ILD to a specialist ILD or PFF centre, the referral package should ideally contain the PFT history, CT scan images and reports, biopsy results, serologies if available, pulmonary and rheumatology clinical notes and reasons for referral. | 32 |
| Telehealth should be made available in ILD centres. | 42 |
| Following referral to an ILD centre, patients should be co-managed by a community pulmonologist and ILD centre if possible. | 31 |
| Patients who are eligible for transplant, enrolled in clinical trials, have rapidly progressing or complex disease or are receiving specialised treatment should remain in the care of a pulmonologist at an ILD centre. | 31 |
*Statements were developed based on high/unanimous consensus agreement achieved on two related questions at Survey 2, which were combined but were not presented to the panel.
COPD, chronic obstructive pulmonary disease; CTD, connective tissue disease; GERD, gastro-oesophageal reflux disease; ILD, interstitial lung disease; PFF, Pulmonary Fibrosis Foundation; PFT, pulmonary function test.
In terms of discussions with patients at follow-up visits, the panel agreed (100%) on the importance of discussing non-pharmacological ILD management options including pulmonary rehabilitation; lung transplant; clinical trial opportunities; symptom management; advanced care planning and goals of care; and palliative care. In addition, there was strong agreement (median=3, IQR=0) on the importance of shared decision-making with patients when prescribing treatment.
Referral to an ILD centre
All 15 items in the specialist referral focus area reached consensus agreement by the third survey round (table 1; online supplemental table S2). The panel agreed (96.8%) on criteria for selecting an ILD centre for referral of a patient with ILD. There was also consensus agreement (100%) on which patients should be referred: patients with diagnostic or treatment uncertainty, disease progression despite treatment, transplant or clinical trial candidates and those who request a referral. The panel considered 4–6 weeks on referral to be a reasonable access time (96.9% agreement); however, priority access should be given to potential transplant candidates or those with rapidly progressing disease (100% agreement). For patients with idiopathic pulmonary fibrosis (IPF) specifically, early referral should be given to those who are young at disease onset, transplant eligible or have rapid disease progression (100% agreement).
Consensus agreement was reached on who is responsible for the referral package and what should be included. It should be the shared responsibility of the referring community physician, the pulmonologist at the ILD centre and the patient to ensure that the referral package is shared with the ILD centre (93.8% agreement) and it should not be the responsibility of the patient alone (93.8% agreement). The referral package should include all relevant patient medical records (100% agreement) and ideally include the PFT history, CT scan images and reports, biopsy results, serologies if available, pulmonary and rheumatology clinical notes and reasons for referral (100% agreement). It should also state whether the patient should be returned to community care after consultation (90.0% agreement). Regardless of the referral package, pulmonologists at ILD centres should review all records before carrying out any further diagnostic testing (median=3, IQR=1).
With regards to ongoing management, there was consensus agreement (100%) that telehealth should be made available at ILD centres. The panel also agreed (100%) that patients should be co-managed by a community pulmonologist and ILD centre where possible, and all relevant patient records should be shared with community physicians (median=3, IQR=1). Exceptions include patients eligible for transplant, enrolled in clinical trials, with rapidly progressing or complex disease, or receiving specialised treatment, who should remain in the care of a pulmonologist at an ILD centre (100% agreement).
Discussion
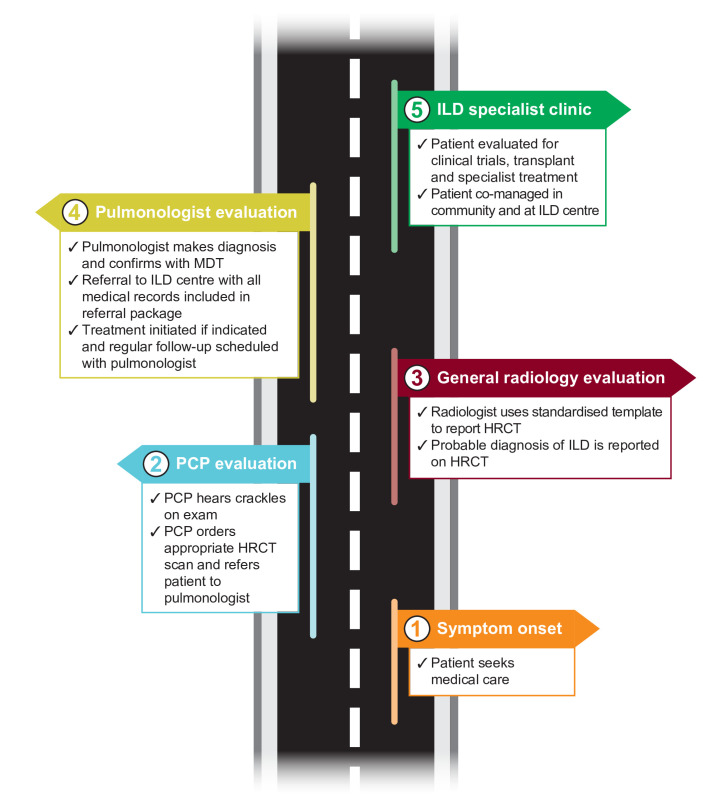
We conducted a Delphi survey involving a diverse panel of PCPs and pulmonologists in the USA and identified the key elements of the patient pathway towards early ILD diagnosis and appropriate management. Our analysis achieved consensus agreement in areas where there is currently uncertainty (figure 4).
Figure 4.
Ideal patient journey from symptom onset to early diagnosis and management based on Delphi consensus. HRCT, high-resolution CT; ILD, interstitial lung disease; MDT, multidisciplinary team; PCP, primary care physician.
We identified practical considerations for PCPs when working up patients with respiratory symptoms, including which clinical signs should prompt ILD suspicion, how they should be evaluated and when a patient should be referred to a pulmonologist. The consensus achieved in this area may provide a blueprint for PCPs who rarely see ILD in the clinic and is of particular significance given that our surveys also highlighted the need for increased awareness and education for PCPs on ILD. These findings are in line with other studies in which substantial delays to ILD diagnosis have been reported in primary care.5–7 In addition, consensus was obtained regarding the need for further research on which rheumatological patients would benefit from HRCT screening.
Robust consensus was achieved on the key elements that facilitate diagnosis of patients with ILD once they have been referred to a pulmonologist. An important aspect of this was the consensus reached on the quality of the radiology report. Together with the most important items to be included on the HRCT requisition, our surveys identified the basic requirements for the radiologist’s HRCT report, including the need for standardised templates. These recommendations are in agreement with previous reports,14 and may decrease the variability of reporting, reduce the need for repeated scans and increase the chances of making a specific ILD diagnosis.
We also identified key indicators that should prompt ILD centre referral, including criteria for early and priority access. Increased awareness of which patients would benefit from early referral may reduce delayed access to ILD centres, which is of importance given that referral delays are associated with a higher risk of death in IPF.8 It was also agreed that when referring a patient with ILD to specialist care, there should be an indication of whether the patient should be returned to community care after consultation.
Since the emergence of severe acute respiratory syndrome coronavirus 2, commonly known as coronavirus disease 2019 (COVID-19), it has become even more vital to delineate and shorten the pathway to accurate diagnosis and management of ILD. This is not only relevant for patients with ILD, whose outcomes could be complicated by contraction of the virus, but also patients whose access to care has been impacted by the pandemic. COVID-19 presents another complication to the patient pathway to ILD diagnosis since delays may be caused by assumptions that persistent cough is a result of post-COVID-19 sequela. Hence, the availability of telehealth at ILD centres is of particular relevance and is an area that reached unanimous consensus.
The Delphi panellists agreed, in some cases unanimously, that further guidance and disease awareness are needed for healthcare providers across specialties who manage patients with ILD. It is important to note that this Delphi survey and analysis were carried out prior to the publication of the 2022 American Thoracic Society clinical practice guidelines on IPF and progressive pulmonary fibrosis19; therefore, these updates were not considered by the panellists.
A key strength of this study is the diversity of the Delphi panel, which included multiple important stakeholders in the pathway to ILD diagnosis and management. Both academic and community pulmonologists participated, as well as PCPs, providing perspectives from different areas of expertise and experience.
Our study has the following limitations. First, there are no standard criteria for defining consensus in Delphi studies. In our study, consensus was predefined by the Steering Committee; however, our results have not been statistically evaluated and the consensus reached may not be generalisable. Second, the anonymity of the panellists during the Delphi process may lead to responses based on insufficient consideration, and it is difficult to qualify any possible external influences on the opinions of the individual experts. Although all panellists had the required relevant experience for participating in the Delphi analysis, the surveys spanned three different areas of expertise. Given that responses of the panellists were equally weighted, consensus may have been impacted by responses from panellists who are not specialists in that particular field. To minimise this bias, PCPs were not required to complete the sections aimed at pulmonologists and the panellists could opt out of answering any question by selecting ‘not applicable/unsure’. However, pulmonologists were able to provide their opinions on recognising ILD in primary care, since these were viewed as important insights. A further limitation was that, despite several topics exploring primary care practice, there was under-representation of PCPs in the Delphi analysis. This was a result of challenges associated with recruitment, with fewer PCPs responding to invitations to participate and qualifying as a panellist per the predefined inclusion criteria, compared with pulmonologists. Although there is no agreed minimum panel number for achieving consensus in the literature,20 there is likely a bias for pulmonologists’ perspectives on these topics. The panel was also limited to physicians practising in the USA and findings may not be generalisable for global practices. Finally, respondents may have been biased in their responses by knowing that the Delphi survey concerned ILD, particularly regarding how truly able PCPs are at identifying ILD in a general primary care clinic.
This modified Delphi approach was a collaborative process that led to robust consensus agreement among PCPs, community pulmonologists and ILD specialists on the key considerations that facilitate the patient pathway to diagnosis and management of ILD. These findings clarify the most common symptoms that should merit further evaluation for ILD and help define the steps for timely, accurate diagnosis in the PCP and outpatient pulmonary settings. They also illustrate how patients with ILD should transition between medical specialties and the collaboration needed between rheumatology, community pulmonologists and ILD specialists to ensure appropriate care. Increased disease recognition and knowledge of the next appropriate steps will reduce referral delays and improve the efficiency of the pathway to ILD diagnosis and management.
Acknowledgments
The Delphi process leading to this Consensus Statement was facilitated by Meditech Media, with support and funding from Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI had no influence on the participating experts’ opinions or final consensus. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and did not receive payment related to the development of this Consensus Statement. Hannah King, PhD, of Nucleus Global provided writing, editorial support and formatting assistance, which was contracted and funded by BIPI. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as potential intellectual property considerations. The authors thank Timothy Whelan for his contributions as a Steering Committee member during the Delphi analysis. The authors would also like to thank all physicians who participated in the Delphi panel for their valuable contributions: Ayodeji Adegunsoye, Kerri Aronson, Emily Balser, Rebecca Bascom, Sidney Braman, Charles A Brown, James Carswell, Laura Paige Clark, Sonia Vishin Compton, Nikita Desai, Alpa Desai, Sakshi Dua, David K Handshoe, Kevin C Harbour, David G Hill, Jared M Intaphan, Manvinder Kainth, Robert J Kaner, Sola Kim, Rachana Krishna, Joyce Lee, Praveen Mannam, Mark J Mayson, Aaron Milstone, Sydney Montesi, Emily Myers, Ankit Nahata, Kirana Narayana, Luke Neilans, Maria L Padilla, Suneet Pahwa, Luca Paoletti, Jane M Parks, Paul M Perry, Wilson A Quezada, David P Rodgers, Jeremy G Schenkein, Mary B Scholand, Boris Shkolnik, Lawrence D Shulman, Erin Snyder, Paul Strachan, Anumpama Tiwari, Christopher J Vaughan, Leslie Wilke, Jeffrey M Wolf, Paul Wolters, Nicholas Wysham, David Zhang.
Footnotes
Contributors: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). All authors contributed to the overall concept and design of the Delphi survey, as well as the methodology, which included input on the inclusion criteria for the expert panel participants. All authors had access to the Delphi questionnaire data and provided their analysis and interpretation of the survey results. All authors provided critical input throughout and participated in the review, revision and approval of this manuscript. AHC is responsible for the overall content as guarantor. The viewpoints expressed in this manuscript solely represent those of its authors. These viewpoints do not necessarily expressly reflect those of Boehringer Ingelheim.
Funding: This Delphi analysis was supported and funded by Boehringer Ingelheim Pharmaceuticals Inc. (BIPI). The Delphi process leading to this Consensus Statement was facilitated by Nucleus Global, with support and funding from BIPI. BIPI had no influence on the participating experts’ opinions or final consensus. Hannah King, PhD, of Nucleus Global provided writing, editorial support, and formatting assistance, which was contracted and funded by BIPI. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as potential intellectual property considerations.
Competing interests: AHC reports receiving research contracts from Boehringer Ingelheim, Roche-Genentech, United Therapeutics, Fibrogen, Veracyte, Galapagos, Pilant, Kadmon, Bristol Myers Squibb, Bellerophon Therapeutics, Pulmonary Fibrosis Foundation (PFF) and Galecto; consulting fees from Veracyte; speaker fees from Boehringer Ingelheim, Genentech, The France Foundation and Paradigm Medical; medical writing support from Boehringer Ingelheim, Veracyte and United Therapeutics; and reports involvement at the PFF as a Senior Medical Advisor, outside the submitted work. SB reports receiving speaker fees from Boehringer Ingelheim, outside the submitted work. DLH reports receiving clinical research grants from Boehringer Ingelheim, Galapagos, Bellerophon Therapeutics and Fibrogen, outside the submitted work. TK reports receiving speaker fees from Boehringer Ingelheim, outside the submitted work. HJK has nothing to disclose. AJP reports receiving grants from the American Lung Association and the National Heart, Lung and Blood Institute (NHLBI); consulting fees from Regeneron, Roche and Imvaria; speaker fees from the National Association for Continuing Education and DynaMed; and reports involvement in a Boehringer Ingelheim advisory board, outside the submitted work. MR reports involvement in a Boehringer Ingelheim advisory board, outside the submitted work. CR reports receiving speaker fees from Boehringer Ingelheim, outside the submitted work. MMS reports receiving grants, consulting fees and speaker fees from Boehringer Ingelheim and Genentech, outside the submitted work. CT reports receiving speaker fees from AstraZeneca, outside the submitted work. JAdA reports receiving speaker fees from Boehringer Ingelheim; participation on Data Safety Monitoring Boards for Respivant, Roche-Genentech and NHLBI; and involvement in the PFF as a Scientific Advisory Committee member, outside the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Olson A, Hartmann N, Patnaik P, et al. Estimation of the prevalence of progressive fibrosing interstitial lung diseases: systematic literature review and data from a physician survey. Adv Ther 2021;38:854–67. 10.1007/s12325-020-01578-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coultas DB, Zumwalt RE, Black WC, et al. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994;150:967–72. 10.1164/ajrccm.150.4.7921471 [DOI] [PubMed] [Google Scholar]
- 3. Raghu G, Chen S-Y, Yeh W-S, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med 2014;2:566–72. 10.1016/S2213-2600(14)70101-8 [DOI] [PubMed] [Google Scholar]
- 4. Gomer RH, Lupher ML. Investigational approaches to therapies for idiopathic pulmonary fibrosis. Expert Opin Investig Drugs 2010;19:737–45. 10.1517/13543784.2010.484018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoyer N, Prior TS, Bendstrup E, et al. Risk factors for diagnostic delay in idiopathic pulmonary fibrosis. Respir Res 2019;20:103. 10.1186/s12931-019-1076-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cosgrove GP, Bianchi P, Danese S, et al. Barriers to timely diagnosis of interstitial lung disease in the real world: the INTENSITY survey. BMC Pulm Med 2018;18:9. 10.1186/s12890-017-0560-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pritchard D, Adegunsoye A, Lafond E, et al. Diagnostic test interpretation and referral delay in patients with interstitial lung disease. Respir Res 2019;20:253. 10.1186/s12931-019-1228-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lamas DJ, Kawut SM, Bagiella E, et al. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med 2011;184:842–7. 10.1164/rccm.201104-0668OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hewson T, McKeever TM, Gibson JE, et al. Timing of onset of symptoms in people with idiopathic pulmonary fibrosis. Thorax 2018;73:683–5. 10.1136/thoraxjnl-2017-210177 [DOI] [PubMed] [Google Scholar]
- 10. Collard HR, Tino G, Noble PW, et al. Patient experiences with pulmonary fibrosis. Respir Med 2007;101:1350–4. 10.1016/j.rmed.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 11. Schoenheit G, Becattelli I, Cohen AH. Living with idiopathic pulmonary fibrosis: an in-depth qualitative survey of European patients. Chron Respir Dis 2011;8:225–31. 10.1177/1479972311416382 [DOI] [PubMed] [Google Scholar]
- 12. Filev PD, Little BP, Duong P-A. Second-opinion reads in interstitial lung disease imaging: added value of subspecialty interpretation. J Am Coll Radiol 2020;17:786–90. 10.1016/j.jacr.2019.12.016 [DOI] [PubMed] [Google Scholar]
- 13. Nathan SD, Pastre J, Ksovreli I, et al. HRCT evaluation of patients with interstitial lung disease: comparison of the 2018 and 2011 diagnostic guidelines. Ther Adv Respir Dis 2020;14:1753466620968496. 10.1177/1753466620968496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berkowitz EA, Bernheim A, Little BP. Introducing ILD-RADS: a pilot study of an interstitial lung disease standardized reporting template. J Am Coll Radiol 2019;16:1169–72. 10.1016/j.jacr.2019.02.032 [DOI] [PubMed] [Google Scholar]
- 15. Mooney J, Chang E, Lalla D, et al. Potential delays in diagnosis of idiopathic pulmonary fibrosis in Medicare beneficiaries. Ann Am Thorac Soc 2019;16:393–6. 10.1513/AnnalsATS.201806-376RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spagnolo P, Ryerson CJ, Putman R, et al. Early diagnosis of fibrotic interstitial lung disease: challenges and opportunities. Lancet Respir Med 2021;9:1065–76. 10.1016/S2213-2600(21)00017-5 [DOI] [PubMed] [Google Scholar]
- 17. Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2:i–iv. 10.3310/hta2030 [DOI] [PubMed] [Google Scholar]
- 18. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014;67:401–9. 10.1016/j.jclinepi.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 19. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022;205:e18–47. 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol 2021;11:116–29. 10.5662/wjm.v11.i4.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2022-001594supp001.pdf (350.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request.