Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) is an unabated risk factor for end-stage liver diseases with no available therapies. Dysregulated immune responses are critical culprits of MASLD pathogenesis. Independent contributions from either the innate or adaptive arms of the immune system or their unidirectional interplay are commonly studied in MASLD. However, the bidirectional communication between innate and adaptive immune systems, and its impact on MASLD, remains insufficiently understood. Given that both innate and adaptive immune cells are indispensable for the development and progression of inflammation in MASLD, elucidating pathogenic contributions stemming from the bidirectional interplay between these two arms holds potential for development of novel therapeutics for MASLD. Here, we review the immune cell types and bidirectional pathways that influence the pathogenesis of MASLD, and highlight potential pharmacologic approaches to combat MASLD based on current knowledge of this bidirectional crosstalk.
Keywords: immune crosstalk, adaptive immunity, innate immunity, MASLD, MASH, NAFLD, NASH
eTOC blurb
To date, immunological studies of MASLD have focused on discrete immune regulators and unidirectional mechanisms. Here, Sawada et al. review the bidirectional immune pathways that influence the pathogenesis of MASLD and highlight potential pharmacologic approaches based on this crosstalk.
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) refers to a spectrum of liver disorders ranging from metabolic dysfunction-associated steatotic liver (MASL) to metabolic dysfunction-associated steatohepatitis (MASH)1,2. MASL is characterized by triglyceride deposition in hepatocytes with no or very minor inflammation and no hepatocyte ballooning, which is typically considered a reversible state. To be classified under the MASLD umbrella, steatosis is associated with at least one cardiometabolic risk factor such as obesity3, dyslipidemia, hypertension, and insulin resistance without excessive alcohol intake2. MASH, on the other hand, involves lobular inflammation, fibrosis, and hepatocyte ballooning, which can progress to irreversible fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)4,5.
The use of MASLD, MASL, and MASH was recently endorsed by pan-national liver associations (American Association for Study of Liver Disease [AASLD], European Association for Study of the Liver [EASL], and Asociación Latinoamericana para el Estudio del Hígado [ALEH]) via a Delphi process in replacement of non-alcoholic fatty liver disease (NAFLD), non-alcoholic fatty liver (NAFL), and non-alcoholic steatohepatitis (NASH), respectively, to reduce stigma and enhance disease awareness, understanding, and drug/biomarker development with the new nomenclature and diagnostic criteria2. Notably, because this change in nomenclature occurred during development of this review, all literature cited utilize NAFLD terminology and diagnostic criteria. However, a retrospective study found that 98% of individuals that fulfilled the criteria for NAFLD also fulfilled those for MASLD6, providing reasonable rationale to consider findings from older NAFLD studies as valid under the new MASLD definition. Thus, to avoid confusion we will use the new MASLD nomenclature when referencing cited literature.
Epidemiological studies, using NAFLD diagnostic classifications, found that 20–30% of adults with MASL develop MASH7, with 20–50% of individuals with MASH approximated to progress to cirrhosis8. Even in those that don’t progress to cirrhosis, about 13–49% of all HCCs develop in individuals with noncirrhotic MASH9. In pediatric populations diagnosed with MASLD via biopsy, 25–50% have MASH and 10–25% have advanced fibrosis at initial presentation10–14. MASH poses a significant risk for advanced liver diseases (the fastest growing cause of HCC15) and liver failure (the largest cause of liver transplant in women and the second largest in men16, and the fastest growing indication of the need for liver transplantation15,17), in addition to vascular (e.g., portal hypertension18, cardiovascular disease [CVD]19) and metabolic (e.g., type 2 diabetes [T2DM]20) complications. Although MASLD represents a significant clinical burden, approved pharmacological therapies to prevent or treat MASLD are not available21 despite the numerous potential avenues currently being explored22. Thus, vast efforts are underway to elucidate the mechanisms by which MASL progresses to MASH.
Dogmatically, the multi-hit hypothesis is believed to shape MASLD development and progression23,24. The initial hit, hepatocyte triglyceride accumulation, sensitizes and predisposes hepatocytes to subsequent hits that drive and regulate disease progression and pathogenicity. Lipotoxicity25, reactive oxygen species (ROS) production26,27, intestinal microbiome28, and induction of proinflammatory immune mediators are all proposed as mechanisms associated with MASLD pathogenesis. Because these “second hits” are not specific to the liver, MASLD is considered not only a hepatic but also a systemic inflammatory disease29,30. Moreover, it is recognized that non-liver tissues also significantly contribute to the pathogenesis of MASLD. Although critical in MASLD pathogenesis, mediators of systemic inflammation31, as well as contributions of adipose32,33 and muscle34,35 tissue inflammation to MASLD have been reviewed elsewhere31. Thus, here we focus specifically on immune responses that shape liver tissue inflammation in MASLD.
Different types of mouse models have been employed for the study of MASLD, notably those involving various dietary challenges including altered caloric content (e.g., high fat diet [HFD]36, MASH diet37) or deficient in specific nutrients (e.g., methionine-choline deficient [MCD] diet38, choline-deficient, L-amino acid defined [CDAA] diet39), and chemical perturbations (e.g., carbon tetrachloride [CCl4]40). Due to the wide scope of MASLD, each animal model recapitulates certain aspects of MASLD and not the entire disease spectra. For example, HFD feeding drives robust steatosis with minimal MASH/fibrosis41,42, while MCD diet induces hepatic inflammation, hepatocellular damage, and cirrhosis without obesity43. Of note, HFD feeding in combination with thermoneutral housing was recently shown to have a potential to unlock modeling of full MASLD spectra in mice43 and to uncover novel processes that instruct immune responses in MASLD progression.
The immune responses need to be tightly regulated in type, timing, and amplitude. Delayed or insufficient vigor of immune response can result in inadequate protection from bacterial, fungal, and viral infections. Conversely, too vigorous of a response can itself be harmful – which is seen, paradigmatically, in the development of inflammatory diseases (e.g., rheumatoid arthritis, type I diabetes, psoriasis, atopic dermatitis and systemic lupus erythematosus) (reviewed in 44). Further, the pathophysiology of these autoimmune diseases is linked to dysregulated proinflammatory cytokine (e.g., TNF, IL-6, IFNγ, IL-1β, IL-23, type I IFNs) production (reviewed in 45). Homeostatic production of these immune mediators is involved in the physiology of healthy liver, while an aberrant production is associated with both obesity and MASLD pathogenesis (e.g., hepatic inflammation, fibrosis and hepatocellular damage) (reviewed in 46). Thus, it is not surprising that research endeavors in the field have focused on identifying the key immune and non-immune cells that produce these proinflammatory immune mediators, the processes that control their production, and the mechanisms by which these mediators drive MASLD pathogenesis.
The immune mediators produced by resident liver parenchymal cells as well as resident and infiltrating immune cells can additively activate both innate and adaptive immune systems that in turn drive MASLD development and progression. Notably, studies conducted on immune responses in MASLD have traditionally focused on the role of innate immunity in disease development and progression. Recent reports, however, have highlighted the sufficiency of innate immunity to cause MASL while the adaptive arm is required for development and progression of MASH47 (Figure 1A). Hence, recent research directions have in part shifted towards improved understanding of the role of adaptive immunity in MASLD pathogenesis. Despite such efforts, the MASLD research has largely adopted a linear progression of inflammation, where innate immune responses unidirectionally instruct the function of adaptive immune responses (Figure 1B). However, such views largely omit the key novel discoveries in the field of immunology: the communication between innate and adaptive immune systems is in fact a bidirectional process48–50 – whereby the cells of the adaptive immune system also activate and instruct the function of innate immune responses51–53. Thus, understanding the contributions of the bidirectional communication may be important for unlocking the enigma of immune responses and immune cell function in MASLD that may aid in the development of novel therapeutics (Figure 1C). Here, based on this new knowledge, we review the innate and adaptive immune cells involved in the bidirectional crosstalk, the cellular/molecular mechanisms underlying this bidirectional immune communication, and speculate on the potential immune therapeutic approaches for MASLD via manipulation of the bidirectional crosstalk.
Figure 1. The contribution of innate and adaptive immune systems in MASLD and the role of bidirectional communication between the two arms.
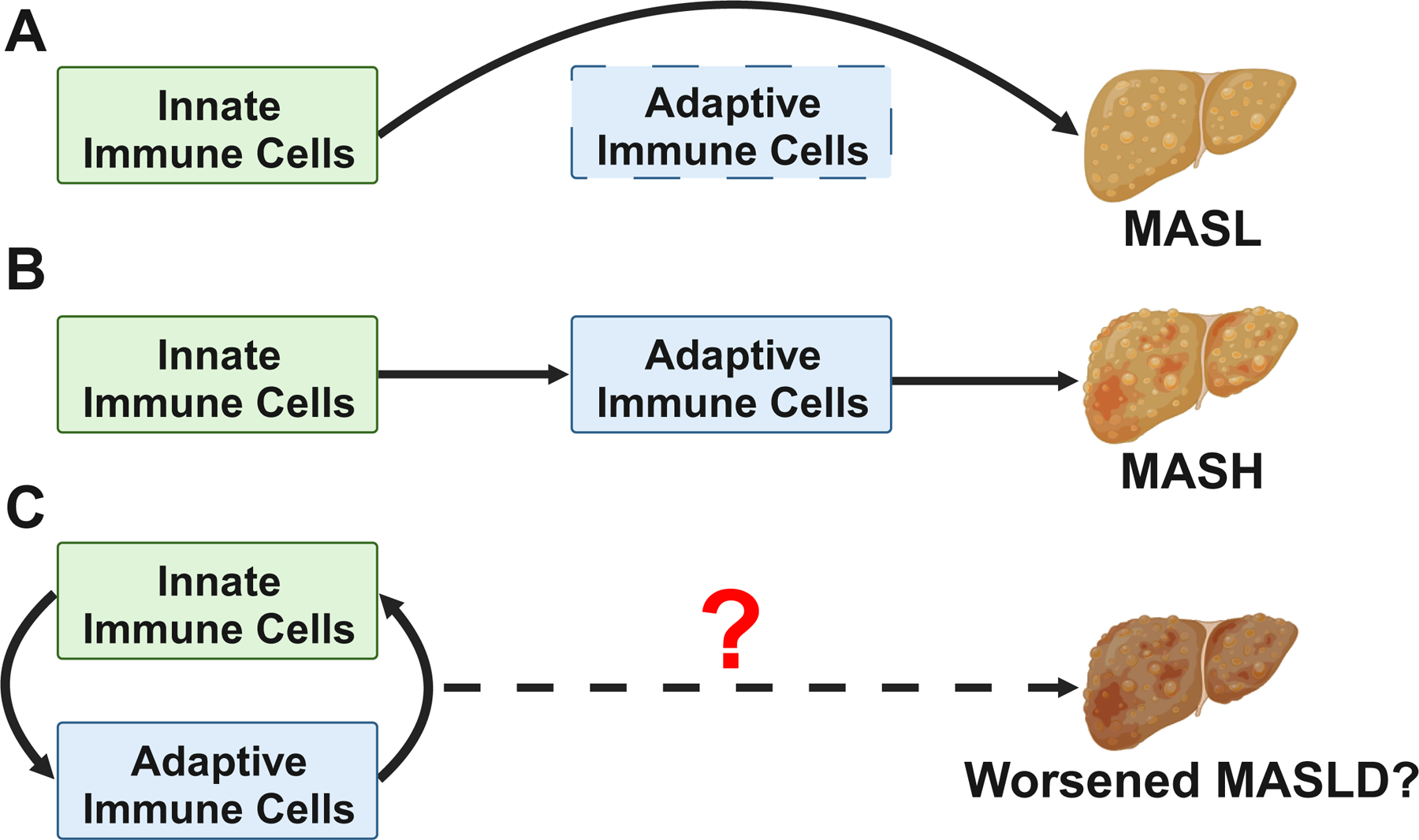
(A) Though the activities of innate immune cells are sufficient to drive the development of metabolic dysfunction-associated steatotic liver (MASL), ablation of adaptive immune cells (e.g., recombination activating 1 [Rag1]-knockout in mice) prevents the development of metabolic dysfunction-associated steatohepatitis (MASH). (B) Long-appreciated paradigm of MASLD disease progression, in which unidirectional innate activation of adaptive immune cells provides key pathogenic signals to promote the development of MASH. (C) Proposed model in which bidirectional immune crosstalk between innate and adaptive immune cells drives full-blown MASLD pathogenesis.
Immunological landscape in MASLD pathogenesis
Despite substantial research to understand the immune cell function in MASLD, additional in-depth investigations of cellular subsets and mechanisms relevant to MASLD pathogenesis are needed. A comprehensive review of the varied immune cell populations and their impact on the inflammatory progression of MASLD has been recently covered extensively elsewhere54. Thus, here we only provide an introductory overview of key innate and adaptive immune cells to MASLD that are critically involved in pathways of bidirectional communication introduced in the “Innate and Adaptive Immune Cell Crosstalk” section that follows (Figure 2).
Figure 2. Immunological landscape of MASLD pathogenesis.
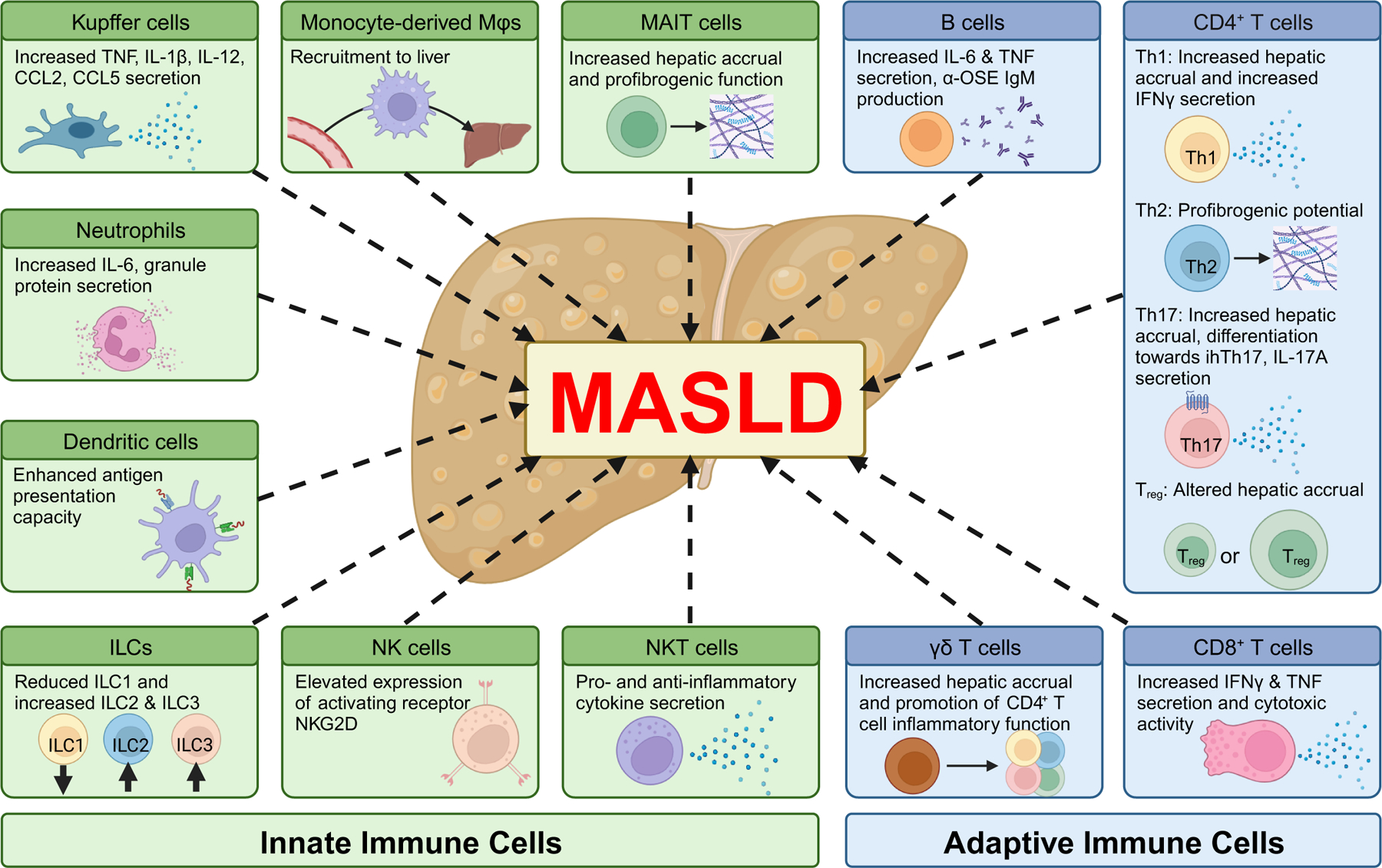
Hepatic immune cell function is reshaped during MASLD and contributes to disease pathogenesis. Within innate immune cells, Kupffer cells (KCs) exhibit increased activation leading to increased cytokine and chemokine secretion. However, the MASH environment increases KC death, and in turn the KC population is replaced via increased recruitment of circulating monocytes that differentiate into macrophages. KC activation also recruits neutrophils, which secrete IL-6 and granule proteins to further promote proinflammatory landscape in the liver. Dendritic cells (DCs) exhibit increased hepatic accrual and antigen presentation capacity in MASLD. Contributions of innate lymphoid cells (ILCs) are understudied, with knowledge being limited to changes in hepatic accrual – namely decreased ILC1 and increased ILC2 and ILC3. NK cells express increased level of activating receptor NKG2D and promote activation of other immune cells in the liver by increased secretion of IFNγ. The contributions of NKT cells are disease stage-dependent, secreting both pro and antiinflammatory cytokines that inhibit pathogenesis during early stages but promote disease progression in later stages. Mucosal associated invariant T (MAIT) cells exhibit increased hepatic accrual and proinflammatory/profibrogenic properties, although they have also been associated with suppression of inflammation in MASLD. Of the adaptive immune cells, the contributions of CD4+ T cells are the most studied. Among the canonical proinflammatory subsets, Th1 cells exhibit increased hepatic accrual and IFNγ secretion, and Th17 cells exhibit increased hepatic accrual and IL-17 secretion. Th17 cells are further differentiated towards a highly inflammatory CXCR3+ intrahepatic subset (ihTh17 cells) in MASH. The roles of Th2 and Treg cells are less defined in MASLD. Profibrogenic potential of Th2 cells have been implicated in progression to cirrhosis, while hepatic accrual (and potentially their contributions towards MASLD) of Treg cells varies depending on the disease model. CD8+ T cells secrete more IFNγ and TNF and exhibit higher cytotoxic activity. γδ T cells show increased hepatic accrual (only in mice) and promote CD4+ T cell function. B cells increase the production of proinflammatory cytokines (IL-6 and TNF) and anti-OSE antibodies.
Innate immunity
Liver is enriched with various subsets of innate immune cells, including the liver-resident macrophage subsets, and immune cell populations including neutrophils, dendritic cells (DCs), natural killer (NK) cells, and natural killer T (NKT) cells. Activation of these cells and subsequent dysregulated production of inflammatory cytokines amplify hepatic accrual of immune cells and exacerbate inflammation and hepatocellular damage55,56.
Macrophages:
Two major subsets of macrophages are present in the liver – liver resident macrophages or Kupffer cells (KCs), and monocyte-derived macrophages (Mo-Ms) recruited from circulation. Various mechanisms by which KCs and Mo-Ms contribute to MASLD, MASH and HCC progression are reviewed in detail in multiple recent literature57–59. In obesity, pathogen-associated molecular patterns (PAMPs; e.g., LPS, bacterial DNAs) are increased which directly activates KCs60,61. Specifically, in MASH, Toll-like receptor (TLR) 4 expression in KCs is higher compared to other TLRs62. Notably, LPS binding to TLR4 triggers MAPK, p38, NF-κB signaling63,64 to induce proinflammatory cytokine (e.g., TNF, IL-1β, IL-12) and chemokine (e.g., CCL2, CCL5) secretion that promotes local inflammation. Mo-Ms are recruited to the liver by injured hepatocytes or activated KCs65, adding on to the diversity of hepatic macrophage populations in MASLD66. Thus, in the MASLD/MASH mouse livers, the composition of the hepatic macrophage pool is altered, as recruited Mo-Ms and KCs derived from recruited monocytes replace embryonic KCs67–70. Mo-Ms exhibit proinflammatory phenotypes that augment liver injury and drive disease progression65, as mice lacking Mo-Ms (e.g., Ccr2−/− mice) show less CDAA diet-driven steatosis, hepatic inflammatory cell infiltration, and fibrosis71.
Neutrophils:
Neutrophils are one of the first leukocytes recruited to the liver following KC activation via PAMPs72,73. Neutrophil hepatic accrual instigates a proinflammatory environment by robust secretion of IL-6, which promotes tissue inflammation and fibrosis74,75. By recruiting additional Mo-Ms and interacting with other antigen presenting cells (APCs), neutrophils amplify the feed forward inflammatory cascade76. Additionally, the release of neutrophil granule proteins (e.g., myeloperoxidase, neutrophil elastase, proteinase 3) promotes ROS production and NETosis77–79, which cumulatively enhances inflammation, hepatocellular damage, and progression to HCC80. Notably, neutrophil-to-lymphocyte ratio is positively correlated with MASLD severity, and indicative of higher risk of advanced cirrhosis81 and HCC82 among individuals with MASLD, suggesting the key role neutrophils play in MASLD progression.
Dendritic cells (DC):
Hepatic DCs are heterogeneous population that can be grouped into plasmacytoid DCs (PDCA-1+; pDCs) and myeloid/classical DCs (PDCA-1-; mDCs), with further sub-groups83. Depending on the cellular subtypes and environmental cues, DCs can promote both proinflammatory84 and antiinflammatory85 responses. In healthy livers, DCs predominantly display an immature phenotype exemplified by a low capacity to endocytose antigens and stimulate T lymphocytes86. However, during hepatic injury or with increased cellular lipid content, DCs switch to an immunogenic phenotype with enhanced capacity to present antigens and increased proinflammatory cytokine production87. Of note, a recent study identified LKB1-AMPK/SIK signaling axis as a mechanism by which DCs limit Th17 polarization in the liver and play a protective role in MASLD88. Thus, given that the heterogeneity and divergent functional effects of hepatic DCs in MASLD are disease stage-dependent, their definitive role in MASLD pathogenesis is yet to be defined89,90.
Natural Killer (NK) cells:
Hepatic NK cells represent a heterogeneous population, which is further amplified during disease state91–93 and is likely responsible for the divergent findings in their role in MASLD (reviewed in 94). In individuals with MASH, increased circulating/conventional NK (cNK) cells are found in the liver95 along with elevated expressions of the activating receptor NKG2D and its ligands MIC A/B in the liver parenchyma96. Together, these processes suggest that activation of NK cells occurs in MASLD. Notably, NK cells convert toward ILC1-like phenotype and become less cytotoxic in obese livers of both humans and mice97. Such changes in NK cell population during MASLD may lead to differential outcomes of either promoting or preventing disease progression. These variable effects in disease progression may also depend on disease stage, as NK cell proinflammatory function is postulated to drive MASH but also hinder HCC98,99.
Natural Killer T (NKT) cells:
Depending on the mechanisms of activation, the type 1 or invariant natural killer T (iNKT) cells have both proinflammatory and antiinflammatory effector functions, accompanied by rapid production of cytokines in large amounts100. HFD-fed mice lacking iNKT cells show higher susceptibility to weight gain and steatosis101, along with increased hepatic inflammation, ALT levels, and fibrosis102, supporting protective/antiinflammatory roles of iNKT cells in MASLD. Mechanistically, iNKT cells contribute to obesity-driven hepatic immune balance by CD206-mediated crosstalk with an antiinflammatory, IL-10 producing KC subset (KC-1)103. In early stages of MASLD, iNKT cells are recruited to the liver and secrete IL-4, promoting the resolution of hepatic inflammation and aiding in liver injury repair104. Protective roles of iNKT cells were also suggested upon disease progression to HCC, via their antiinflammatory properties during oncogenic β-catenin-induced liver inflammation105. Notably, obesity-associated hepatic cholesterol accumulation was found to selectively suppress NKT cell antitumor surveillance in the liver106. On the other hand, pathogenic involvement of NKT cells in MASLD was also reported; accumulation of NKT cells is associated with exacerbated fibrosis in MASH107, and LIGHT secreted by NKT cells was shown to activate NF-κB signaling that facilitates steatosis and MASH to HCC transition47. These contradictory data may be attributable to their varying effector functions108,109, in addition to the variations in immunological landscape dependent on the disease stage110.
Other innate immune cell types:
In addition to those introduced above, innate lymphoid cells (ILCs) and mucosal associated invariant T (MAIT) cells have been suggested to contribute to MASH pathogenesis. Despite having overlapping effector functions with CD4+ T cells in obesity111–115, the literature on ILCs and MAIT cells in MASLD is somewhat limited. Specifically, reduced ILC1116 and increased ILC3117 numbers are reported in MASLD, while increase in ILC2 is seen in fibrotic liver118 and in the liver of individuals with HCC119. Meanwhile, hepatic MAIT cell numbers are increased in MASLD120 and in individuals with MASLD-related cirrhosis121, where they are believed to exhibit profibrogenic properties121. In contrast, mice with genetic ablation of MR1 (MHC class I-related protein; expression restricted to MAIT cells122) fed MCD diet develop severe steatosis and proinflammatory characteristics120.
Adaptive immunity
Adaptive immunity includes cell-mediated and humoral immunity, mediated principally by T and B lymphocytes, respectively. The major T lymphocytes involved in adaptive immunity include CD4+ T cells (further categorized into T helper [Th] 1, Th2, Th17, regulatory T [Treg] cells, etc.), CD8+ T cells, and γδ T cells123. B lymphocytes are similarly classified into different subsets including transitional, naïve, memory, double negative, regulatory, B1, and antibody secreting B cells124. Growing attention is being directed towards the role of adaptive immunity in MASLD, leading to ongoing discoveries about its involvement in MASH7.
CD4+ T cells:
CD4+ T cells are highly plastic immune cells, capable of shaping both pro- and antiinflammatory landscape. CD4+ T cells are grouped according to their cytokine production and transcription factor expression, including Th1 (IFNγ; Tbet), Th2 (IL-4; GATA3), Th17 (IL-17; RORγt), and Treg (IL-10/TGFβ; FOXP3)125,126 cells. Despite the divergent reports on the shift in the number of total hepatic CD4+ T cells in MASLD (e.g., progressive hepatic accrual in MASLD127,128 or decreased hepatic presence in transition to HCC129), published reports suggest that polarization of CD4+ T cells towards Th1130 and Th17128,131 subsets along with increased production of IFNγ and IL-17A132–136 contributes to MASLD progression129,137,138. Blocking integrin-mediated hepatic recruitment of CD4+ T cells attenuated hepatic inflammation and fibrosis in mouse model of MASLD, providing further evidence of the necessity of CD4+ T cells in MASLD pathogenesis139.
Th1 and Th2 cells are implicated in MASH pathogenesis by skewed balance of elevated proinflammatory Th1 responses relative to reduced antiinflammatory Th2 responses137. Accumulation of Th1 cells and increased systemic and hepatic IFNγ are reported in individuals with MASH140. In fact, IFNγ is considered a pathogenic contributor to MASLD progression, as genetic ablation of IFNγ in mice protects from MASH and hepatic fibrosis141. In contrast, the role of Th2 cells in MASLD is poorly understood. Although increased number of Th2 cells in the peripheral blood of individuals with MASLD are reported95, the implications of such alterations remain unclear. The antiinflammatory cytokines produced by Th2 cells may alleviate hepatic inflammation, while their high profibrogenic potential142 may contribute to progression towards cirrhosis.
Th17 cells, via amplification of proinflammatory signals that sustain tissue inflammation, are considered major contributors to MASLD pathogenesis, with IL-17A believed to be a key cytokine driving this process76,135,143,144. In MASLD, hepatic Th17 cell numbers are increased in mice36 and hepatic presence of IL-17A producing cells is associated with steatosis to MASH transition in humans131. Mechanistically, IL-17 induces the expression of chemokines (e.g., CXCL1, CCL2) that facilitate neutrophil and macrophage infiltration and activation and amplify tissue inflammation and fibrogenesis143,145. Recently, a subset of highly inflammatory hepatic Th17 cells that express CXCR3 and co-produce IL-17A, IFNγ, and TNF was identified as a critical contributor to MASLD pathogenesis128. The number of these inflammatory hepatic Th17 cells increases during MASLD progression in mice, and their presence correlates with MASLD severity in humans128.
Treg cells, via regulation of effector T cell activation, serve as critical immune regulators that prevent the excessive activation of pathogenic immune responses. However, the role of Treg cells in MASH progression remains incompletely defined. The frequency of hepatic Treg cells decreases in experimental models of HFD-driven MASLD129,146 and is associated with increased oxidative stress in liver microenvironment147. In addition, adoptive transfer of splenic Treg cells from lean mice into obese animals attenuated hepatocellular damage and inflammation, suggesting that Treg cells restrict MASLD progression147. However, in MASH models using choline-deficient diet with HFD feeding and diethylnitrosamine injections148 or high fat high carbohydrate diet149, increase in hepatic Treg cells was observed. Further, Treg cell depletion ameliorated the progression to HCC148 and adoptive transfer of Treg cells to animals with MASH exacerbated steatosis and liver damage149 in these models. Human studies on Treg cells in MASLD are similarly conflicting, with both increased138,150 and reduced131 frequency and numbers of intrahepatic Treg cells being reported.
CD8+ T cells:
The contributions of CD8+ T cells to MASLD pathogenesis are context dependent. CD8+ T cell numbers are increased in the liver of individuals with MASH47, and inhibition of CD8+ T cell function in animal model decreases hepatic steatosis and inflammation151. Hepatic accrual of activated CD8+ T cells152 amplifies the proinflammatory environment via increased production of IFNγ and TNF and induces cytotoxic activity-driven hepatocellular damage153. Upon reversal of disease progression, however, CD8+ T cells directly contribute to the resolution of hepatic inflammation and fibrosis154. Of note, MASH reduces CD8+ T cell mobility by inducing metabolic/mitochondrial dysfunction152 and impairs tumor antigen-specific CD8+ T cell response155, ultimately leading to HCC progression.
B cells:
Although limited, existing evidence supports a pathogenic role for B cells in MASLD156. Hepatic B cell accrual is seen in both humans157 and mice158 with MASLD and is accompanied by higher B cell expression of inflammatory mediators (e.g., IL-6, TNF). Whether B cell production of these mediators directly promotes MASLD pathogenesis, or indirectly amplifies hepatic inflammation via induction of CD4+ T cell differentiation towards Th1/Th17 cells in MASLD liver remains unknown158. In some individuals with MASLD, elevated levels of circulating IgA, IgM, and IgG were reported159. Specifically, circulating IgG levels against oxidative stress-derived epitopes (anti-OSE IgG) are increased in MASLD157,160,161. Of note, loss of IL-10 producing regulatory B cells in mice with MASLD has been reported162.
Innate and Adaptive Immune Cell Crosstalk
The bidirectional crosstalk between adaptive and innate immune cells has recently been linked with critical immune cell inflammatory functions163. Given the increased recognition on the roles of peripheral and intrahepatic adaptive immune cells in MASLD109,156, understanding the bidirectional crosstalk between innate and adaptive immune cells in MASLD pathogenesis is of high priority. In this section, we review receptor/ligand interactions known to play a role in the bidirectional crosstalk between innate and adaptive immune cells and discuss their potential roles in MASLD (summarized in Table 1).
Table 1.
Effects of dysregulated immune crosstalk pathways in MASLD mouse models
| Pathway | Model used | Diet Fed | Effects on MASLD (compared to WT) | Ref |
|---|---|---|---|---|
| CD28/B7.1, B7.2 | CD28−/− mice (C57BL/6) | HFD (60% kcal fat) |
↓hepatic steatosis; ↓hepatic inflammation; ↓hepatocellular damage |
181 |
| B7.1−/−B7.2−/− mice (C57BL/6) | HFD (60% kcal fat) |
↑hepatic steatosis; ↑hepatic inflammation; ↓hepatocellular damage |
182 | |
| Antibody-mediated depletion of B7.1 and B7.2 in wildtype mice (C57BL/6) | HFD (60% kcal fat) | ↓hepatic steatosis; ↓hepatic inflammation | 182 | |
| 4–1BB/4–1BBL | 4–1BB−/− mice (C57BL/6) | HFD (60% kcal fat) | ↓hepatic steatosis; ↓hepatic inflammation | 190 |
| OX40/OX40L | OX-40−/− mice (C57BL/6) | HFD (45% kcal fat) | ↓hepatic steatosis; ↓hepatic inflammation | 195 |
| Rag2−/−IL2rg−/− mice (C57BL/6) adoptively transferred with OX40-deficient T cells | HFD (45% kcal fat) | ↓hepatic steatosis; ↓hepatic inflammation | 195 | |
| CD40/CD40L | CD40−/− mice (C57BL/6) | HFD (55% kcal fat) | ↑hepatic steatosis; ↓hepatic inflammation | 201, 202 |
| wildtype mice (C57BL/6) adoptively transferred with Rag1−/− and CD40−/− BM cells | HFD (45% kcal fat) | ↑hepatic steatosis | 202 | |
| CD40fl/fl CD11c-Cre mice (C57BL/6) | HFD (54% kcal fat) |
↑hepatic steatosis; ↑hepatocellular damage; ↓hepatic Treg accrual |
200 | |
| CD40fl/fl CD11c-Cre mice (C57BL/6) | NASH diet (40% kcal fat, 20% kcal fructose, 2% kcal cholesterol) | ↓hepatic inflammation; ↑hepatocellular damage | 200 | |
| CD40L−/− mice (C57BL/6) | HFD (23% kcal fat) | ↑↓hepatic steatosis | 204, 205 | |
| CD40L−/− mice (BALB/c) | Olive oil administration (6.6 mL/kg of body weight, 3 times/week via oral gavage) | ↑hepatic steatosis | 203 | |
| Fas/FasL | Fasfl/fl Albumin-Cre mice (C57BL/6) | HFD (58% kcal fat) | ↓hepatic steatosis | 215 |
| IFN-I/IFNAR | IFNAR1−/− mice (C57BL/6) | HFD (60% kcal fat) | ↓hepatic steatosis | 228 |
| IFNARfl/fl Albumin-Cre mice (C57BL/6) | MCD diet | ↑hepatic steatosis; ↑hepatic inflammation | 229 | |
| IRF3−/− mice (C57BL/6) | HFD (61.6% kcal fat) | ↑hepatic steatosis; ↑hepatocellular damage | 231 | |
| IRF5flfl Lyz2-Cre mice (C57BL/6) | MCD diet |
↓hepatocellular damage; ↓hepatic inflammation; ↓cirrhosis |
232 | |
| IRF5flfl Lyz2-Cre mice (C57BL/6) | CCl4 (0.5 μL/g diluted 1:5 in olive oil; 2 times/week) |
↓hepatocellular damage; ↓hepatic inflammation; ↓cirrhosis |
232 | |
| IRF9−/− mice (C57BL/6) | HFD (61.6% kcal fat) | ↑hepatic steatosis | 230 | |
| APRIL, BAFF/BAFF-R, BCMA, TACI | APRIL−/− mice (C57BL/6) | HFD (60% kcal fat) | ↓hepatocellular damage | 251 |
| BAFF-transgenic mice (C57BL/6) | HFD (60% kcal fat) | ↓hepatocellular damage | 251 | |
| BAFF−/− mice (C57BL/6) | HFD (60% kcal fat) |
↓hepatic steatosis; ↓hepatic inflammation; ↓cirrhosis |
254 | |
| Antibody-mediated neutralization of BAFF in wildtype mice (C57BL/6) | MCD diet |
↓hepatic steatosis; ↓hepatic inflammation; ↓hepatocellular damage |
157 | |
| TACI-Ig mice (C57BL/6) | MCD diet | ↓hepatic inflammation | 157 | |
| TACI-Ig mice (C57BL/6) | CDAA diet | ↓cirrhosis | 157 | |
| IL-6/IL-6 receptor | Antibody-mediated neutralization of IL-6 receptor in wildtype mice (C57BL/6) | MCD diet | ↑hepatic steatosis; ↓hepatocellular damage | 266 |
HFD, high fat diet; MCD, methionine choline deficient; CDAA, choline deficient and amino acid defined; IFNAR, IFN-I receptor; IRF, interferon regulatory factor; BM, bone marrow. Red upwards arrows indicate augmentation of phenotype. Blue downwards arrows indicate attenuation of phenotype.
Receptor/ligand-driven communication pathways between innate and adaptive immune cells
Signal transduction through co-stimulatory molecules represents a key method of communication between the cells of the innate and adaptive immune systems. Although both antigen dependent and independent activation of the adaptive immune cells have been suggested to play a role in MASLD pathogenesis164,165, much of the research to date has focused on the processes dependent on antigen encounter and innate immune cell activation. For example, upon activation of the T cell receptor (TCR) signaling pathway, upregulated co-stimulatory molecules colocalize with TCR and subsequently engage with their respective ligands/receptors expressed on APCs and pathogen-experienced hematopoietic/non-hematopoietic cells to further promote immune cell activation and function166–169. While the implications of signal transduction downstream of the co-stimulatory receptor in T cells are relatively well understood, the capabilities of the ligands and their interaction with co-stimulatory receptors to “reverse signal” to the APCs to enhance their activation and/or function remain underappreciated170–173 (Figure 3A,B). Critically, recent reports demonstrate the direct ability of effector T cells to instruct innate immune cell functions52,53. Hence, here we discuss the key pathways of bidirectional communication between innate and adaptive immune cells and how they contribute to MASLD.
Figure 3. The bidirectional crosstalk between adaptive and innate immune cells and the downstream effects of each signaling pathway.
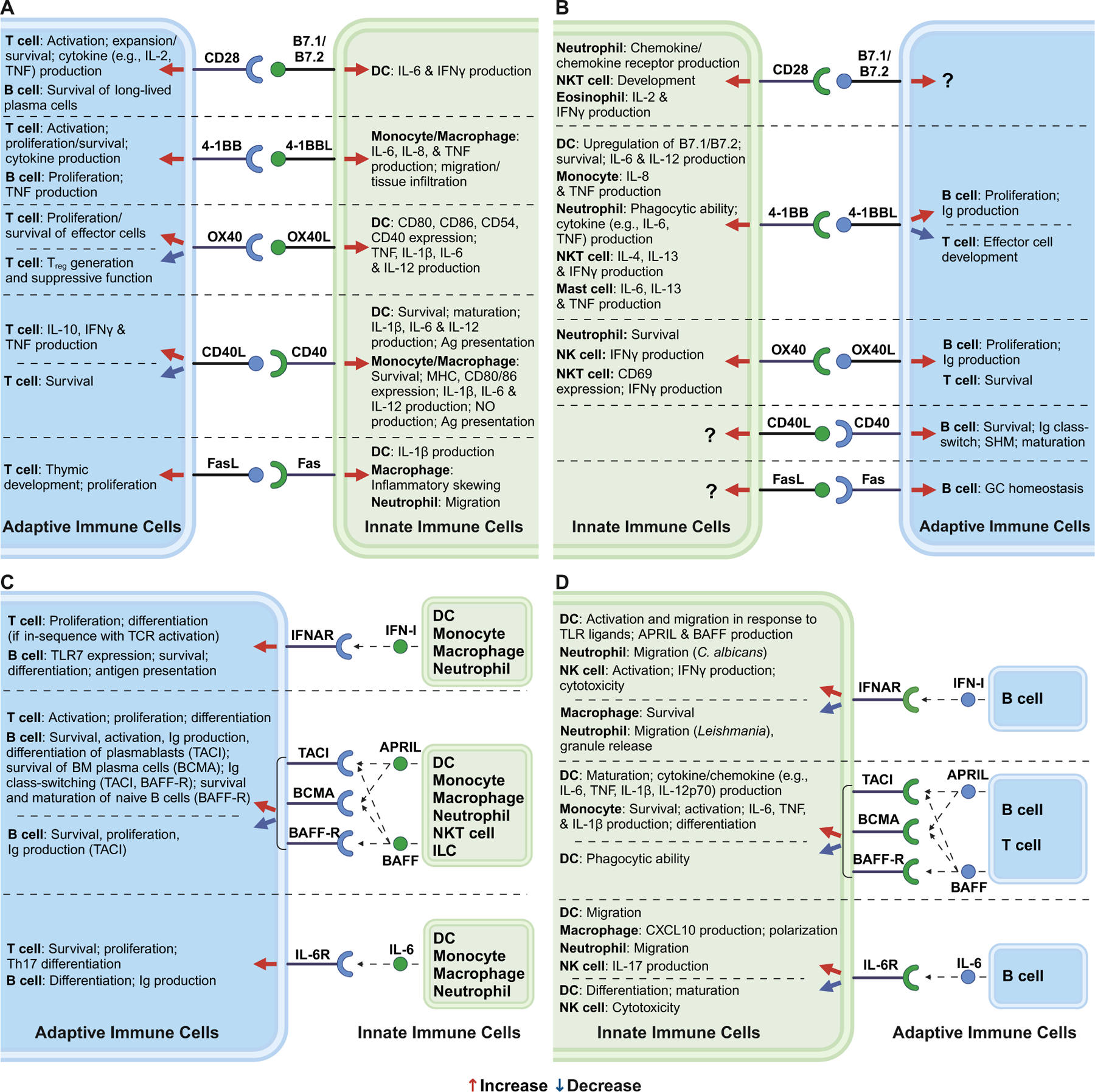
(A) Canonically appreciated receptor/ligand-driven communication pathways between adaptive and innate immune cells, and “reverse signaling” of these pathways via respective ligands. (B) Signaling pathways listed in (A) in which the receptors/ligands are expressed on the opposite arms of the immune system. (C) Canonically appreciated cytokine-driven communication pathways between adaptive and innate immune cells. (D) Signaling pathways listed in (C) in which the cytokines/receptors are expressed on the opposite arms of the immune system. Red arrow indicates increasing downstream effects. Blue arrow indicates decreasing downstream effects. Receptors, ligands, and cytokines denoted in blue indicate those expressed by adaptive immune cells. Receptors, ligands, and cytokines denoted in green indicate those expressed by innate immune cells. NO, nitric oxide; Ag, antigen; Ig, immunoglobulin; SHM, somatic hypermutation; GC, germinal center; BM, bone marrow.
CD28/B7.1 & B7.2 Signaling:
The function of CD28 expressed on T cells is best characterized in the context of T cell activation174. Though less appreciated, CD28 also promotes long lived plasma cell survival175, neutrophil chemokine and chemokine receptor expression176,177, NKT cell development178, and eosinophil cytokine production179. The interactions of B7.1 (CD80) and B7.2 (CD86) expressed on APCs180 with CD28 expressed on T cells induces DC-centric secretion of inflammatory mediators173, in turn initiating inflammatory responses in both T cells and APCs. Mice with genetic ablation of CD28 have lower hepatic triglyceride accumulation, inflammation, and hepatocellular damage in HFD driven MASLD181. The CD28 deficient mice also express lower hepatic levels of Foxp3, invoking the role of CD28 in maintaining hepatic Treg cell pool. Because Treg cells are canonically considered antiinflammatory, CD28 may indirectly counter MASLD progression via its effects on hepatic Treg cell accrual. Correspondingly, mice with genetic deletion of B7.1 and B7.2 exhibit augmented MASLD pathology and reduced Treg cell numbers when fed HFD182. These findings suggest that CD28/B7 interaction and signaling is critical for maintaining Treg cell population and could play a beneficial role in MASLD. However, blockade of B7 signaling via anti-B7.1/B7.2 antibodies ameliorates hepatic steatosis and inflammation without skewing Treg cell development and numbers182. Hence, these data support a pathogenic involvement of CD28/B7 signaling when its effects on Treg cells are masked. Combined, the existing data imply a divergent role of CD28/B7 signaling dependent on its effects on Treg cells. Unlike in mice, the role of CD28/B7 signaling has not been directly studied in human MASLD. A case study report demonstrated a dramatic improvement in insulin resistance in an individual treated with abatacept (CTLA-4 Ig, a fusion protein of cytotoxic T lymphocyte antigen 4 [CTLA-4] linked to IgG designed to inhibit CD28-B7 binding)183. Whether and how CD28 signaling, and more specifically the immune crosstalk mediated by this pathway, shapes MASLD pathogenesis in humans remains to be investigated.
4–1 BB/4–1BBL Signaling:
Initially identified as a specific co-stimulatory receptor expressed on activated T cells184, it is now appreciated that 4–1BB (CD137) is also expressed on B cells, NKT cells, DCs, neutrophils, and macrophages where it similarly promotes their effector functions168,185,186. 4–1BBL (CD137L), the ligand for 4–1BB, propagates the reverse signaling that promotes proinflammatory functions of B cells, DCs, and macrophages/monocytes187,188, while also limiting T cell effector functions189. In mice fed HFD, hepatic expression of 4–1BB and 4–1BBL is elevated compared to chow fed counterparts190. Further, 4–1BB-deficient mice exhibit attenuated HFD-driven hepatic steatosis and inflammation190. Analysis of 4–1BB expression in human HCC tumor microenvironment revealed that 4–1BB is almost exclusively expressed on tumor-infiltrating CD8+ T cells191. Because the individuals recruited for this study were not limited to those with underlying metabolic dysfunction, whether the 4–1BB upregulation is specific to HCC precipitated from MASLD remains to be elucidated.
OX40/OX40L Signaling:
OX40 (CD134), a co-stimulatory receptor induced in activated T cells192, also impacts the function and survival of NKT cells, NK cells, and neutrophils167. The reverse signaling through its ligand OX40L (CD252) promotes DC proinflammatory function188, B cell proliferation and antibody production193, and T cell survival194. Notably, individuals with MASH have higher plasma levels of soluble OX40 compared with healthy controls, and OX40 levels positively correlate with MASH severity195. In experimental models of MASLD, OX40 and OX40L expression within hepatic mononuclear cells and the plasma levels of soluble OX40 are increased195. In addition, OX40-deficiency in T cells is linked with lower hepatic immune cell (e.g., monocytes, Th1 and Th17 cells) accrual and proinflammatory function, and lower MASH severity195.
CD40/CD40L Signaling:
CD40 receptor is expressed specifically on APCs and enhances APC proinflammatory phenotype and function169. CD40L (CD154), the ligand for CD40, is expressed on activated T cells and enhances T cell effector functions while also inducing T cell apoptosis196–198. The involvement of CD40 signaling in MASLD pathogenesis is reviewed in detail elsewhere199. HFD fed CD40 deficient mice (both whole body and CD11c+ DC-specific knockout models200–202), despite amplified hepatic steatosis, do not exhibit hepatic inflammation. However, in MASH, despite similar hepatic steatosis, liver inflammation is reduced in mice lacking CD40 in CD11c+ cells compared to wild type controls200. The differential effects of the two diets were attributed to divergent hepatic Treg cell accrual. On the other hand, the data regarding the role of CD40L in MASLD pathogenesis is less consistent. CD40L-deficient mice exhibit varying severity of hepatic steatosis in experimental models of MASLD203–205, depending on the diet and genetic background of animals used. Given the divergent findings and the ability of CD40L to bind to two other receptors in addition to CD40206, the need for further in-depth ligand/receptor studies is highlighted.
Fas/FasL Signaling:
Canonically considered an inducer of apoptotic cell death of Fas (CD95)-expressing cells207, Fas signaling also contributes to the activation of both innate and adaptive immune responses. Specifically, FasL (CD178) is required for adequate CD8+ T cell proliferation upon TCR engagement208, in addition to contributing to T cell thymic development209, invoking a role of FasL as a costimulatory signal. Induction of Fas signaling in innate immune cells, by FasL expressed on CD4+ T cells, leads to pathogen-independent IL-1β production52. Further, Fas signaling at-large leads to macrophage polarization210, neutrophil migration211, and germinal center B cell homeostasis212. In individuals with MASH, levels of soluble Fas and FasL in serum as well as hepatic Fas expression are elevated compared to healthy individuals213,214. Similarly, mice with hepatocyte-specific ablation of Fas have reduced HFD-driven hepatic steatosis215.
Cytokine-driven communication pathways between innate and adaptive immune cells
In addition to the communication pathways through receptor/ligand interactions introduced above, communication between innate immune cells and adaptive immune cells, especially B cells, can also occur in the form of soluble factors216 (Figure 3C,D). Here we discuss the contributions of select mediators including type I interferons (IFN-I), B cell activation factor (BAFF)/a proliferation-inducing ligand (APRIL), and IL-6 in MASLD pathogenesis.
Type I Interferon (IFN-I) Signaling:
While almost all cells can produce and respond to IFN-I217–221, IFN-I produced by pDCs upregulate TLR7 expression, and sensitivity of TLR7-induced maturation, in naïve B cells216,222. Conversely, B cells produce IFN-I in response to TLR9 ligation223, and pDCs depend on IFN-I for their activation and migration224. Both TLR7 and TLR9 activation is viewed as a critical driver of MASLD225,226, with TLR9 signaling in intrahepatic B cells proposed to dominantly drive its inflammatory gene expression227. Further, highlighted by the necessity of IFN-1 receptor (IFNAR) expression to induce HFD-driven steatosis in mice228, IFN-I play a key role in MASLD development228–233 (reviewed extensively in 234), and pDC-driven IFN-I promotes induction of obesity235.
B cell activation factor (BAFF)/A proliferation-inducing ligand (APRIL) Signaling:
B cell survival and maturation in the periphery, including the liver, is dependent on signals induced by BAFF (CD257) and its close homolog APRIL (CD256)236–238. Both BAFF and APRIL are predominately expressed by innate immune cells in response to proinflammatory cytokines and TLR signaling activation239–241. Their cognate receptors are transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI or CD267), B-cell maturation antigen (BCMA or CD269), and BAFF receptor (BAFF-R or CD268)241–243. Both BAFF and APRIL bind to TACI and BCMA, while BAFF also binds to BAFF-R238. Activation of these receptors induces transcription of genes that shape inflammatory functions of B cells244–247, CD4+ T cells248, DCs249, and monocytes250, while it can also shape adipocyte function251. Although reverse signaling through BAFF/APRIL is understudied, signaling via membrane-bound BAFF and APRIL in macrophages induces expression of proinflammatory mediators187,252,253. Of note, BAFF serum levels are reduced in individuals with obesity and are negatively correlated with body mass index (BMI)251, which suggests that BAFF may contribute to aspects of MASLD pathogenesis that is divergent from those linked to metabolic derangements. In mouse models of MASLD, those deficient of BAFF display reduced hepatic steatosis157,254, while those with overexpressed BAFF are protected from hepatocellular damage251. In congruence with these results, APRIL deficient mice, with increased serum BAFF levels, exhibit reduced hepatocellular damage251. Whether the impact of BAFF/APRIL on MASLD pathogenesis is dependent on the expressing cell type or the receptor(s) they act through is unknown. To this end, inhibition of TACI signaling selectively depletes marginal zone and B2 B cells255, and in experimental mouse models of MASLD, TACI signaling inhibition reduces hepatic inflammation and fibrosis157,251,256.
Interleukin 6 (IL-6) Signaling:
IL-6, a pleotropic proinflammatory cytokine, is produced by both immune and non-immune cells257. Although first discovered for its stimulatory effects on B cells258, it is now appreciated that IL-6 regulates inflammatory functions of CD4+ T cells259, DCs260, macrophages261, neutrophils262, and NK cells263,264. In humans, increased circulating and hepatic IL-6 levels are reported in MASLD265. In mice, increased hepatic B cell accrual as well as increased production of IL-6 by hepatic B cells is reported in MASLD158. Interestingly, inhibition of IL-6 signaling via MR16–1 (an IL-6 receptor neutralizing antibody) in mice fed MCD diet enhanced hepatic steatosis but alleviated hepatocellular damage266. Whether IL-6 production by, and IL-6 receptor signaling in immune cells is sufficient/required for MASLD pathogenesis is yet to be elucidated.
Immune cell crosstalk modulation in MASLD: towards therapy
While considerable progress in elucidating the role of immune responses in pathogenesis of MASLD has been made, no specific immune therapies to MASLD exist. Although immune cell depletion therapies might restrict MASH progression, unwanted side effects that involve immunosuppression and toxicity warrant development of more selective therapies for MASLD. Thus, more discriminatory strategies that target specific immune cell recruitment and/or costimulatory pathways, including potential inhibition of the activation of both arms of the immune system through the bidirectional crosstalk, could represent a viable avenue for MASLD treatment.
Targeting cytokine signaling has proved useful in the treatment of various inflammatory diseases. For example, inhibitors of IL-17 and IL-23 signaling are FDA approved or have shown promising results in late-stage clinical trials for treatment of inflammatory diseases that share immunopathological features with MASLD/MASH267–275. To this end, clinical studies targeting cytokine-driven pathways of immune crosstalk, especially IFN-I, IL-6, and TNF, have shown strong potential for inflammatory disease treatment (Table 2). IFN-α kinoid (immunotherapeutic vaccine that induces the generation of IFN-neutralizing antibodies276) treatment lowered disease activity state in individuals with systemic lupus erythematosus (SLE) with further assessment in a phase III clinical trial announced276. Similarly, anifrolumab (IFN-α/β receptor blocking antibody) treatment improved clinical symptoms of SLE in another phase III trial277. Additionally, tocilizumab (IL-6 receptor antagonist) is approved by the FDA for treatment of rheumatoid arthritis and juvenile arthritis278, although its use has been associated with increased weight gain indicating a potential deleterious effect on MASLD279. However, targeting cytokine-driven pathways in MASLD treatment may prove challenging given that treatment of individuals with NASH using pentoxifylline (phosphodiesterase inhibitor that decreases TNF gene transcription) did not significantly improve transaminase levels, liver histology, and metabolic markers compared to the placebo group280. Such shortcomings highlight the potential need to target receptor/ligand-driven pathways of immune crosstalk, or possibly to utilize such new approaches in combination with cytokine inhibition.
Table 2.
Currently available pharmacologic agents with potential to be used for MASLD treatment by targeting pathways of the bidirectional immune crosstalk
| Drug Name | Target Pathway | Mechanism of Action | Effects on Inflammatory Disease | Ref |
|---|---|---|---|---|
| Abatacept | CD28/B7.1, B7.2 | CTLA4-Ig: prevention of T cell activation by blocking B7 binding to CD28 | Reduces inflammation in various forms of arthritis | 281-284 |
| Rocatinlimab | OX40/OX40L | antiOX40 antibody: inhibition and reduction of activated OX40-expressing T cells | Progressive improvements in atopic dermatitis | 296 |
| BI 655064 | CD40/CD40L | antiCD40 antibody: blockade of CD40/CD40L interaction | Improvement of clinical and biological markers of rheumatoid arthritis | 298 |
| IFN-α kinoid | IFN-I/IFNAR | Immunotherapeutic vaccine: induction of generation of IFN-neutralizing antibodies | Improvement of clinical signs/symptoms in SLE | 276 |
| Anifrolumab | IFN-I/IFNAR | IFNAR/IFNBR blocking antibody: blockade of IFNAR signaling | Improvement of clinical signs/symptoms in SLE | 277 |
| Tocilizumab | IL-6/IL-6 receptor | IL-6 receptor antagonist: blockade of IL-6 receptor signaling | FDA approved to treat rheumatoid arthritis and juvenile arthritis | 278 |
| Pentoxifylline | TNF/TNF receptor | Phosphodiesterase inhibitor: suppression of TNF gene expression | Improvement of liver enzymes and insulin resistance, and reduction in steatosis and lobular inflammation, in NASH | 280 |
SLE, systemic lupus erythematosus; TNF, tumor necrosis factor.
Preclinical studies that utilize immunotherapies targeting receptor/ligand-driven pathways have also shown promise in inflammatory disease treatment (Table 2). For example, abatacept, which binds to B7.1 and B7.2 with higher affinity than CD28 and blocks T cell activation, is in clinical use for rheumatoid arthritis281,282, psoriatic arthritis283, and juvenile idiopathic arthritis284. Given the clinical link between these inflammatory arthritic conditions and MASLD269, whether abatacept, or other pharmacologic agents targeting the CD28/B7 pathway285, can be efficacious in treating MASLD is yet to be shown. However, abatacept have been suggested to induce hepatocellular damage286, warranting further cell-specific mechanistic interrogation of the CD28/B7.1&B7.2 signaling pathway in MASLD for its therapeutic exploitation.
Similarly, modulation of 4–1BB/4–1BBL pathway shows promise given blockade of 4–1BB/4–1BBL interaction suppresses inflammation in mouse models of rheumatoid arthritis287, atherosclerosis288, and experimental autoimmune myocarditis289. However, potential deleterious effects of such interventions should be carefully considered, because 4–1BB deficient mice display altered myeloid progenitor cell growth and reduced adaptive immune responses290.
Blocking the OX40/OX40L pathway is similarly proven effective in counteracting several inflammatory diseases including asthma291,292, autoimmune encaphelomyelitis293, and type 1 diabetes294 in animal studies (reviewed in 295). Furthermore, a recent clinical trial revealed that rocatinlimab, an anti-OX40 antibody that blocks OX40/OX40L interaction, is effective in improving atopic dermatitis296. Notably, blockade of OX40/OX40L interaction preferentially inhibits effector T cells and restricts widespread immunosuppression. However, the value of therapeutic inhibition of OX40/OX40L axis in MASLD remains unknown.
Targeting CD40/CD40L signaling in mouse models of autoimmune cholangitis via anti-CD40L antibody treatment reduced liver inflammation and lowered autoantibody levels297. Further, BI 655064, an antagonistic anti-CD40 antibody that selectively binds to CD40 and blocks CD40/CD40L interaction, improved inflammatory markers in individuals with rheumatoid arthritis298, invoking a potential beneficial role of CD40/CD40L blockade in inflammatory diseases. The safety, tolerability, and pharmacodynamics of another anti-CD40 antibody, dacetuzumab/lucatumumab, were tested in individuals with primary biliary cirrhosis (Clinical Trial ID: NCT02193360). Although the results have not yet been published, the use of this antagonist in a liver inflammatory disease reinforces the notion that CD40/CD40L axis is a potential candidate for future therapeutic targeting of inflammation in MASLD.
Data on pharmacologic targeting of Fas/FasL signaling is limited, with one preclinical study showing that ONL1204, a small peptide antagonist of Fas, reduces clinical and inflammatory markers in mouse models of glaucoma299. Based on these observations, a clinical trial investigating its use for the treatment of age-related macular degeneration, a condition with inflammatory pathogenesis300, is currently being conducted (Clinical Trial ID: NCT04744662). Whether its use in humans can recapitulate the antiinflammatory effects observed in mice, and its efficacy in countering inflammatory diseases beyond ophthalmic conditions, remain to be investigated.
CD40, OX40, 4–1BB, and Fas are all members of the TNF receptor superfamily (TNFRSF)301, and despite the apparent potential, targeting other TNFRSF has shown limited clinical efficacy in the context of hepatic inflammation in alcoholic hepatitis302–304 and chronic hepatitis C305,306. Thus, improved understanding of how immune interactions regulate MASLD (e.g., specific cell types, tissues, and stage of disease development that these pathways impact) will aid in development of more efficacious therapies targeting such interactions. Further, given the heterogeneity of human MASLD54,307, and associated HCC308, the potential of combining the inhibition of cytokine- and receptor/ligand-driven pathways holds promise for development of personalized therapies.
Conclusion
Vast research endeavors to understand MASLD etiology, pathogenesis, and progression have uncovered the critical involvement of immune cells and inflammatory mediators in disease pathogenesis. Advancements in the field of immunology have enabled further dissection of individual immune components and their respective roles in MASLD pathology. Of note, the bidirectional communication between innate and adaptive immune systems was discovered not so long ago, and the contributions of such communication in MASLD is not yet fully appreciated. Although our discussion regarding innate and adaptive crosstalk was centered around those with activating effects, we acknowledge the potential contributions of those that invoke inhibitory effects (e.g., CTLA-4 [CD152]/B7 family [CD80/CD86]309 and PD-1 [CD279]/PD-L1 [CD274]310). Though studies have indicated their roles in MASLD311,312, further work would provide an improved basis for the discussion of potential benefits of limiting low level autoinflammation to restrict MASLD progression.
As more studies begin to interrogate bidirectional immune signaling pathways in MASLD, an exciting new avenue of potential therapeutic approaches has been recognized. Targeting these pathways can be especially effective, allowing both arms of the immune response contributing to disease pathogenesis to be modulated. Improved understanding of how these pathways contribute to MASLD pathogenesis would be required, however, for effective and precise therapeutic strategy. Specifically, some remaining knowledge gaps that will significantly advance the field forward if addressed include definition of: (1) key B and T cell subset(s) involved in the innate-adaptive immune cell crosstalk that drives MASLD pathogenesis, (2) innate immune cell involvement in these interactions, (3) critical liver microenvironment factors that promote or inhibit innate and adaptive immune cell crosstalk, (4) disease stage (e.g., MASH vs HCC308, early fibrosis vs late cirrhosis313; given immunological distinctions99) in which these pathways of bidirectional crosstalk play a significant role, and (5) impact of immune bidirectional crosstalk on MASLD-associated cardiometabolic complications (e.g., portal hypertension18, CVD19, T2DM20). Finally, given that diet-induced mouse models of MASLD employed by many studies highlighted in this review also simultaneously induce obesity, investigations on the regulatory functions of innate-adaptive bidirectional crosstalk in MASLD pathogenesis may uncover their contributions to other inflammatory sequalae associated with metabolic syndrome.
Acknowledgements/Funding Support:
This work was supported in part by NIH R01DK099222, Department of Defense (DoD) W81XWH2010392, American Diabetes Association (ADA) 1-18-IBS-100, CCHMC Pediatric Diabetes and Obesity Center, and CCRF Endowed Scholar Award (to S.D.); R01DK099222-02S1 (associated with S.D., and M.E.M.F); American Heart Association (AHA) 17POST33650045 and ADA 1-19-PMF-019 (to M.E.M.F); CCHMC Albert B. Sabin Fellowship (to K.S.); and PHS Grant P30 DK078392 Pathology of the Digestive Disease Research Core Center at CCHMC (associated with S.D.). All figures were created using BioRender.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interests.
REFERENCES
- 1.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, and Loomba R (2023). AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 77, 1797–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, et al. (2023). A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol, 101133.37364816 [Google Scholar]
- 3.Sarwar R, Pierce N, and Koppe S (2018). Obesity and nonalcoholic fatty liver disease: current perspectives. Diabetes Metab Syndr Obes 11, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinella ME (2015). Nonalcoholic fatty liver disease: a systematic review. JAMA 313, 2263–2273. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, and Loomba R (2015). Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clinical gastroenterology and hepatology 13, 643–654. e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song SJ, Che-To Lai J, Lai-Hung Wong G, Wai-Sun Wong V, and Cheuk-Fung Yip T (2023). Can we use old NAFLD data under the new MASLD definition? J Hepatol [DOI] [PubMed] [Google Scholar]
- 7.Sutti S, and Albano E (2020). Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol 17, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhai M, Liu Z, Long J, Zhou Q, Yang L, Zhou Q, Liu S, and Dai Y (2021). The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017. Sci Rep 11, 5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younes R, and Bugianesi E (2018). Should we undertake surveillance for HCC in patients with NAFLD? J Hepatol 68, 326–334. [DOI] [PubMed] [Google Scholar]
- 10.Goyal NP, and Schwimmer JB (2016). The Progression and Natural History of Pediatric Nonalcoholic Fatty Liver Disease. Clin Liver Dis 20, 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsinikos T, Mrowczynski-Hernandez P, and Kohli R (2021). Pediatric Nonalcoholic Fatty Liver Disease. Pediatr Clin North Am 68, 1309–1320. [DOI] [PubMed] [Google Scholar]
- 12.Jobira B, Frank DN, Silveira LJ, Pyle L, Kelsey MM, Garcia-Reyes Y, Robertson CE, Ir D, Nadeau KJ, and Cree-Green M (2021). Hepatic steatosis relates to gastrointestinal microbiota changes in obese girls with polycystic ovary syndrome. PLoS One 16, e0245219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Pai AK, and Putra J (2022). Paediatric non-alcoholic fatty liver disease: an approach to pathological evaluation. J Clin Pathol 75, 443–451. [DOI] [PubMed] [Google Scholar]
- 14.Vos MB, Van Natta ML, Blondet NM, Dasarathy S, Fishbein M, Hertel P, Jain AK, Karpen SJ, Lavine JE, Mohammad S, et al. (2022). Randomized placebo-controlled trial of losartan for pediatric NAFLD. Hepatology 76, 429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, Eguchi Y, Wong VW, Negro F, Yilmaz Y, et al. (2019). Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol 17, 748–755 e743. [DOI] [PubMed] [Google Scholar]
- 16.Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, Tran T, Ayoub WS, Lu SC, et al. (2018). NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol 113, 1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam R, Karam V, Cailliez V, JG OG, Mirza D, Cherqui D, Klempnauer J, Salizzoni M, Pratschke J, Jamieson N, et al. (2018). 2018 Annual Report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl Int 31, 1293–1317. [DOI] [PubMed] [Google Scholar]
- 18.Nababan SHH, and Lesmana CRA (2022). Portal Hypertension in Nonalcoholic Fatty Liver Disease: From Pathogenesis to Clinical Practice. J Clin Transl Hepatol 10, 979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anstee QM, Targher G, and Day CP (2013). Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 10, 330–344. [DOI] [PubMed] [Google Scholar]
- 20.Targher G, Corey KE, Byrne CD, and Roden M (2021). The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol 18, 599–612. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson D, and Finck BN (2021). Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol 17, 484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Masi A, Li X, Lee D, Jeon J, Wang Q, Baek S, Park O, Mottis A, Strotjohann K, Rapin A, et al. (2023). Cyclo(His-Pro): A further step in the management of steatohepatitis. JHEP Rep 5, 100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi S, Takahashi S, Sasaki T, Kumagai T, and Nagata K (2011). Progression of alcoholic and non-alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab Pharmacokinet 26, 30–46. [DOI] [PubMed] [Google Scholar]
- 24.Farrell GC, van Rooyen D, Gan L, and Chitturi S (2012). NASH is an Inflammatory Disorder: Pathogenic, Prognostic and Therapeutic Implications. Gut Liver 6, 149–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alkhouri N, Dixon LJ, and Feldstein AE (2009). Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol 3, 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delli Bovi AP, Marciano F, Mandato C, Siano MA, Savoia M, and Vajro P (2021). Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review. Front Med (Lausanne) 8, 595371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Lee G, Heo SY, and Roh YS (2021). Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease. Antioxidants (Basel) 11, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He LH, Yao DH, Wang LY, Zhang L, and Bai XL (2021). Gut Microbiome-Mediated Alteration of Immunity, Inflammation, and Metabolism Involved in the Regulation of Non-alcoholic Fatty Liver Disease. Front Microbiol 12, 761836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reccia I, Kumar J, Akladios C, Virdis F, Pai M, Habib N, and Spalding D (2017). Non-alcoholic fatty liver disease: A sign of systemic disease. Metabolism 72, 94–108. [DOI] [PubMed] [Google Scholar]
- 30.Fotbolcu H, and Zorlu E (2016). Nonalcoholic fatty liver disease as a multi-systemic disease. World J Gastroenterol 22, 4079–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marques P, Francisco V, Martinez-Arenas L, Carvalho-Gomes A, Domingo E, Piqueras L, Berenguer M, and Sanz MJ (2023). Overview of Cellular and Soluble Mediators in Systemic Inflammation Associated with Non-Alcoholic Fatty Liver Disease. Int J Mol Sci 24, 2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Rao H, Liu F, Wei L, Li H, and Wu C (2021). Recent Advances in Adipose Tissue Dysfunction and Its Role in the Pathogenesis of Non-Alcoholic Fatty Liver Disease. Cells 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordeiro A, Costa R, Andrade N, Silva C, Canabrava N, Pena MJ, Rodrigues I, Andrade S, and Ramalho A (2020). Does adipose tissue inflammation drive the development of non-alcoholic fatty liver disease in obesity? Clin Res Hepatol Gastroenterol 44, 394–402. [DOI] [PubMed] [Google Scholar]
- 34.Altajar S, and Baffy G (2020). Skeletal Muscle Dysfunction in the Development and Progression of Nonalcoholic Fatty Liver Disease. J Clin Transl Hepatol 8, 414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhanji RA, Narayanan P, Allen AM, Malhi H, and Watt KD (2017). Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 66, 2055–2065. [DOI] [PubMed] [Google Scholar]
- 36.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Graspeuntner S, Cappelletti M, Wu D, Mukherjee R, Chan CC, Lawson MJ, Klarquist J, et al. (2017). Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med 23, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, Guy CD, and Diehl AM (2015). Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One 10, e0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, and Green RM (2008). Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res 49, 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denda A, Kitayama W, Kishida H, Murata N, Tsutsumi M, Tsujiuchi T, Nakae D, and Konishi Y (2002). Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient, L-amino acid-defined diet. Jpn J Cancer Res 93, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boll M, Weber LW, Becker E, and Stampfl A (2001). Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Z Naturforsch C J Biosci 56, 649–659. [DOI] [PubMed] [Google Scholar]
- 41.Ito M, Suzuki J, Tsujioka S, Sasaki M, Gomori A, Shirakura T, Hirose H, Ito M, Ishihara A, Iwaasa H, et al. (2007). Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res 37, 50–57. [DOI] [PubMed] [Google Scholar]
- 42.Christ A, Lauterbach M, and Latz E (2019). Western Diet and the Immune System: An Inflammatory Connection. Immunity 51, 794–811. [DOI] [PubMed] [Google Scholar]
- 43.Oates JR, Sawada K, Giles DA, Alarcon PC, Damen M, Szabo S, Stankiewicz TE, Moreno-Fernandez ME, and Divanovic S (2023). Thermoneutral housing shapes hepatic inflammation and damage in mouse models of non-alcoholic fatty liver disease. Front Immunol 14, 1095132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson A, and Diamond B (2001). Autoimmune diseases. N Engl J Med 345, 340–350. [DOI] [PubMed] [Google Scholar]
- 45.O’Shea JJ, Ma A, and Lipsky P (2002). Cytokines and autoimmunity. Nat Rev Immunol 2, 37–45. [DOI] [PubMed] [Google Scholar]
- 46.Carter-Kent C, Zein NN, and Feldstein AE (2008). Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol 103, 1036–1042. [DOI] [PubMed] [Google Scholar]
- 47.Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, Ringelhan M, Simonavicius N, Egger M, Wohlleber D, et al. (2014). Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 26, 549–564. [DOI] [PubMed] [Google Scholar]
- 48.Gasteiger G, and Rudensky AY (2014). Interactions between innate and adaptive lymphocytes. Nat Rev Immunol 14, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strutt TM, McKinstry KK, and Swain SL (2011). Control of innate immunity by memory CD4 T cells. Adv Exp Med Biol 780, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swain SL, McKinstry KK, and Strutt TM (2012). Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol 12, 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soudja SM, Chandrabos C, Yakob E, Veenstra M, Palliser D, and Lauvau G (2014). Memory-T-cell-derived interferon-gamma instructs potent innate cell activation for protective immunity. Immunity 40, 974–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain A, Irizarry-Caro RA, McDaniel MM, Chawla AS, Carroll KR, Overcast GR, Philip NH, Oberst A, Chervonsky AV, Katz JD, et al. (2020). T cells instruct myeloid cells to produce inflammasome-independent IL-1beta and cause autoimmunity. Nat Immunol 21, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDaniel MM, Chawla AS, Jain A, Meibers HE, Saha I, Gao Y, Jain V, Roskin K, Way SS, and Pasare C (2022). Effector memory CD4(+) T cells induce damaging innate inflammation and autoimmune pathology by engaging CD40 and TNFR on myeloid cells. Sci Immunol 7, eabk0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huby T, and Gautier EL (2022). Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol 22, 429–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhan YT, and An W (2010). Roles of liver innate immune cells in nonalcoholic fatty liver disease. World J Gastroenterol 16, 4652–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maher JJ, Leon P, and Ryan JC (2008). Beyond insulin resistance: Innate immunity in nonalcoholic steatohepatitis. Hepatology 48, 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park SJ, Garcia Diaz J, Um E, and Hahn YS (2023). Major roles of kupffer cells and macrophages in NAFLD development. Front Endocrinol (Lausanne) 14, 1150118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu GX, Wei S, Yu C, Zhao SQ, Yang WJ, Feng YH, Pan C, Yang KX, and Ma Y (2023). Activation of Kupffer cells in NAFLD and NASH: mechanisms and therapeutic interventions. Front Cell Dev Biol 11, 1199519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barreby E, Chen P, and Aouadi M (2022). Macrophage functional diversity in NAFLD - more than inflammation. Nat Rev Endocrinol 18, 461–472. [DOI] [PubMed] [Google Scholar]
- 60.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, and Wallace M (2007). Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol 47, 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. (2012). Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye D, Li FY, Lam KS, Li H, Jia W, Wang Y, Man K, Lo CM, Li X, and Xu A (2012). Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut 61, 1058–1067. [DOI] [PubMed] [Google Scholar]
- 63.Kremer M, Thomas E, Milton RJ, Perry AW, van Rooijen N, Wheeler MD, Zacks S, Fried M, Rippe RA, and Hines IN (2010). Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology 51, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bieghs V, and Trautwein C (2013). The innate immune response during liver inflammation and metabolic disease. Trends Immunol 34, 446–452. [DOI] [PubMed] [Google Scholar]
- 65.Morinaga H, Mayoral R, Heinrichsdorff J, Osborn O, Franck N, Hah N, Walenta E, Bandyopadhyay G, Pessentheiner AR, Chi TJ, et al. (2015). Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes 64, 1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oates JR, McKell MC, Moreno-Fernandez ME, Damen M, Deepe GS Jr., Qualls JE, and Divanovic S (2019). Macrophage Function in the Pathogenesis of Non-alcoholic Fatty Liver Disease: The Mac Attack. Front Immunol 10, 2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reid DT, Reyes JL, McDonald BA, Vo T, Reimer RA, and Eksteen B (2016). Kupffer Cells Undergo Fundamental Changes during the Development of Experimental NASH and Are Critical in Initiating Liver Damage and Inflammation. PLoS One 11, e0159524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seidman JS, Troutman TD, Sakai M, Gola A, Spann NJ, Bennett H, Bruni CM, Ouyang Z, Li RZ, Sun X, et al. (2020). Niche-Specific Reprogramming of Epigenetic Landscapes Drives Myeloid Cell Diversity in Nonalcoholic Steatohepatitis. Immunity 52, 1057–1074 e1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Remmerie A, Martens L, Thone T, Castoldi A, Seurinck R, Pavie B, Roels J, Vanneste B, De Prijck S, Vanhockerhout M, et al. (2020). Osteopontin Expression Identifies a Subset of Recruited Macrophages Distinct from Kupffer Cells in the Fatty Liver. Immunity 53, 641–657 e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tran S, Baba I, Poupel L, Dussaud S, Moreau M, Gelineau A, Marcelin G, Magreau-Davy E, Ouhachi M, Lesnik P, et al. (2020). Impaired Kupffer Cell Self-Renewal Alters the Liver Response to Lipid Overload during Non-alcoholic Steatohepatitis. Immunity 53, 627–640 e625. [DOI] [PubMed] [Google Scholar]
- 71.Miura K, Yang L, van Rooijen N, Ohnishi H, and Seki E (2012). Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol 302, G1310–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kazankov K, Jorgensen SMD, Thomsen KL, Moller HJ, Vilstrup H, George J, Schuppan D, and Gronbaek H (2019). The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 16, 145–159. [DOI] [PubMed] [Google Scholar]
- 73.McDonald B, Jenne CN, Zhuo L, Kimata K, and Kubes P (2013). Kupffer cells and activation of endothelial TLR4 coordinate neutrophil adhesion within liver sinusoids during endotoxemia. Am J Physiol Gastrointest Liver Physiol 305, G797–806. [DOI] [PubMed] [Google Scholar]
- 74.Cho Y, and Szabo G (2021). Two Faces of Neutrophils in Liver Disease Development and Progression. Hepatology 74, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paquissi FC (2016). Immune Imbalances in Non-Alcoholic Fatty Liver Disease: From General Biomarkers and Neutrophils to Interleukin-17 Axis Activation and New Therapeutic Targets. Front Immunol 7, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giles DA, Moreno-Fernandez ME, and Divanovic S (2015). IL-17 Axis Driven Inflammation in Non-Alcoholic Fatty Liver Disease Progression. Curr Drug Targets 16, 1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pulli B, Ali M, Iwamoto Y, Zeller MW, Schob S, Linnoila JJ, and Chen JW (2015). Myeloperoxidase-Hepatocyte-Stellate Cell Cross Talk Promotes Hepatocyte Injury and Fibrosis in Experimental Nonalcoholic Steatohepatitis. Antioxid Redox Signal 23, 1255–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, and Clouston AD (2014). The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 59, 1393–1405. [DOI] [PubMed] [Google Scholar]
- 79.Zhao X, Yang L, Chang N, Hou L, Zhou X, Yang L, and Li L (2020). Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling. Cell Death Dis 11, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O’Doherty RM, Minervini MI, et al. (2018). Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 68, 1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, Zein NN, and Feldstein AE (2012). Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 32, 297–302. [DOI] [PubMed] [Google Scholar]
- 82.Thomas CE, Yu YC, Luu HN, Wang R, Paragomi P, Behari J, and Yuan JM (2023). Neutrophil-lymphocyte ratio in relation to risk of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Cancer Med 12, 3589–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bernsmeier C, and Albano E (2017). Liver dendritic cells and NAFLD evolution: a remaining open issue (Elsevier; ), pp. 1120–1122. [DOI] [PubMed] [Google Scholar]
- 84.Sutti S, Locatelli I, Bruzzi S, Jindal A, Vacchiano M, Bozzola C, and Albano E (2015). CX3CR1-expressing inflammatory dendritic cells contribute to the progression of steatohepatitis. Clin Sci (Lond) 129, 797–808. [DOI] [PubMed] [Google Scholar]
- 85.Henning JR, Graffeo CS, Rehman A, Fallon NC, Zambirinis CP, Ochi A, Barilla R, Jamal M, Deutsch M, Greco S, et al. (2013). Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology 58, 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomson AW, and Knolle PA (2010). Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol 10, 753–766. [DOI] [PubMed] [Google Scholar]
- 87.Heymann F, and Tacke F (2016). Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol 13, 88–110. [DOI] [PubMed] [Google Scholar]
- 88.van der Zande HJ, Brombacher EC, Lambooij JM, Pelgrom LR, Zawistowska-Deniziak A, Patente TA, Heieis GA, Otto F, Ozir-Fazalalikhan A, Yazdanbakhsh M, et al. (2023). Dendritic cell-intrinsic LKB1-AMPK/SIK signaling controls metabolic homeostasis by limiting the hepatic Th17 response during obesity. JCI Insight 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cai J, Zhang XJ, and Li H (2019). The Role of Innate Immune Cells in Nonalcoholic Steatohepatitis. Hepatology 70, 1026–1037. [DOI] [PubMed] [Google Scholar]
- 90.Bernsmeier C, and Albano E (2017). Liver dendritic cells and NAFLD evolution: A remaining open issue. J Hepatol 66, 1120–1122. [DOI] [PubMed] [Google Scholar]
- 91.Peng H, and Tian Z (2017). Diversity of tissue-resident NK cells. Semin Immunol 31, 3–10. [DOI] [PubMed] [Google Scholar]
- 92.Tian Z, Chen Y, and Gao B (2013). Natural killer cells in liver disease. Hepatology 57, 1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luci C, Vieira E, Perchet T, Gual P, and Golub R (2019). Natural Killer Cells and Type 1 Innate Lymphoid Cells Are New Actors in Non-alcoholic Fatty Liver Disease. Front Immunol 10, 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinez-Chantar ML, Delgado TC, and Beraza N (2021). Revisiting the Role of Natural Killer Cells in Non-Alcoholic Fatty Liver Disease. Front Immunol 12, 640869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Diedrich T, Kummer S, Galante A, Drolz A, Schlicker V, Lohse AW, Kluwe J, Eberhard JM, and Schulze Zur Wiesch J (2020). Characterization of the immune cell landscape of patients with NAFLD. PLoS One 15, e0230307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kahraman A, Schlattjan M, Kocabayoglu P, Yildiz-Meziletoglu S, Schlensak M, Fingas CD, Wedemeyer I, Marquitan G, Gieseler RK, Baba HA, et al. (2010). Major histocompatibility complex class I-related chains A and B (MIC A/B): a novel role in nonalcoholic steatohepatitis. Hepatology 51, 92–102. [DOI] [PubMed] [Google Scholar]
- 97.Cuff AO, Sillito F, Dertschnig S, Hall A, Luong TV, Chakraverty R, and Male V (2019). The Obese Liver Environment Mediates Conversion of NK Cells to a Less Cytotoxic ILC1-Like Phenotype. Front Immunol 10, 2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, and Korangy F (2009). Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50, 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koo SY, Park EJ, and Lee CW (2020). Immunological distinctions between nonalcoholic steatohepatitis and hepatocellular carcinoma. Exp Mol Med 52, 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui Y, and Wan Q (2019). NKT Cells in Neurological Diseases. Front Cell Neurosci 13, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martin-Murphy BV, You Q, Wang H, De La Houssaye BA, Reilly TP, Friedman JE, and Ju C (2014). Mice lacking natural killer T cells are more susceptible to metabolic alterations following high fat diet feeding. PLoS One 9, e80949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miyagi T, Takehara T, Uemura A, Nishio K, Shimizu S, Kodama T, Hikita H, Li W, Sasakawa A, Tatsumi T, et al. (2010). Absence of invariant natural killer T cells deteriorates liver inflammation and fibrosis in mice fed high-fat diet. J Gastroenterol 45, 1247–1254. [DOI] [PubMed] [Google Scholar]
- 103.Han M, Geng J, Zhang S, Rao J, Zhu Y, Xu S, Wang F, Ma F, Zhou M, and Zhou H (2023). Invariant natural killer T cells drive hepatic homeostasis in nonalcoholic fatty liver disease via sustained IL-10 expression in CD170(+) Kupffer cells. Eur J Immunol, e2350474. [DOI] [PubMed] [Google Scholar]
- 104.Liew PX, Lee WY, and Kubes P (2017). iNKT Cells Orchestrate a Switch from Inflammation to Resolution of Sterile Liver Injury. Immunity 47, 752–765 e755. [DOI] [PubMed] [Google Scholar]
- 105.Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, Yamagoe S, Colnot S, Viguier M, Perret C, et al. (2012). Oncogenic beta-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest 122, 586–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang W, Zhou J, Yang W, Feng Y, Wu H, Mok MTS, Zhang L, Liang Z, Liu X, Xiong Z, et al. (2022). Aberrant cholesterol metabolic signaling impairs antitumor immunosurveillance through natural killer T cell dysfunction in obese liver. Cell Mol Immunol 19, 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, Witek RP, Choi SS, Guy CD, Fearing CM, et al. (2010). Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology 51, 1998–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arrese M, Cabrera D, Kalergis AM, and Feldstein AE (2016). Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci 61, 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hirsova P, Bamidele AO, Wang H, Povero D, and Revelo XS (2021). Emerging Roles of T Cells in the Pathogenesis of Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Front Endocrinol (Lausanne) 12, 760860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao B, and Tsukamoto H (2016). Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology 150, 1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen H, Sun L, Feng L, Yin Y, and Zhang W (2022). Role of Innate lymphoid Cells in Obesity and Insulin Resistance. Front Endocrinol (Lausanne) 13, 855197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. (2015). Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eberl G, Colonna M, Di Santo JP, and McKenzie AN (2015). Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348, aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, et al. (2011). Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259. [DOI] [PubMed] [Google Scholar]
- 115.Toubal A, Kiaf B, Beaudoin L, Cagninacci L, Rhimi M, Fruchet B, da Silva J, Corbett AJ, Simoni Y, Lantz O, et al. (2020). Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat Commun 11, 3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tosello-Trampont AC, Krueger P, Narayanan S, Landes SG, Leitinger N, and Hahn YS (2016). NKp46(+) natural killer cells attenuate metabolism-induced hepatic fibrosis by regulating macrophage activation in mice. Hepatology 63, 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamaguchi M, Okamura T, Fukuda T, Nishida K, Yoshimura Y, Hashimoto Y, Ushigome E, Nakanishi N, Majima S, Asano M, et al. (2021). Group 3 Innate Lymphoid Cells Protect Steatohepatitis From High-Fat Diet Induced Toxicity. Front Immunol 12, 648754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gonzalez-Polo V, Pucci-Molineris M, Cervera V, Gambaro S, Yantorno SE, Descalzi V, Tiribelli C, Gondolesi GE, and Meier D (2019). Group 2 innate lymphoid cells exhibit progressively higher levels of activation during worsening of liver fibrosis. Ann Hepatol 18, 366–372. [DOI] [PubMed] [Google Scholar]
- 119.Xu X, Ye L, Zhang Q, Shen H, Li S, Zhang X, Ye M, and Liang T (2021). Group-2 Innate Lymphoid Cells Promote HCC Progression Through CXCL2-Neutrophil-Induced Immunosuppression. Hepatology 74, 2526–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y, Huang B, Jiang X, Chen W, Zhang J, Wei Y, Chen Y, Lian M, Bian Z, Miao Q, et al. (2018). Mucosal-Associated Invariant T Cells Improve Nonalcoholic Fatty Liver Disease Through Regulating Macrophage Polarization. Front Immunol 9, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hegde P, Weiss E, Paradis V, Wan J, Mabire M, Sukriti S, Rautou PE, Albuquerque M, Picq O, Gupta AC, et al. (2018). Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun 9, 2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Corbett AJ, Awad W, Wang H, and Chen Z (2020). Antigen Recognition by MR1-Reactive T Cells; MAIT Cells, Metabolites, and Remaining Mysteries. Front Immunol 11, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shuai Z, Leung MW, He X, Zhang W, Yang G, Leung PS, and Eric Gershwin M (2016). Adaptive immunity in the liver. Cell Mol Immunol 13, 354–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanz I, Wei C, Jenks SA, Cashman KS, Tipton C, Woodruff MC, Hom J, and Lee FE (2019). Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front Immunol 10, 2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raphael I, Nalawade S, Eagar TN, and Forsthuber TG (2015). T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 74, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saravia J, Chapman NM, and Chi H (2019). Helper T cell differentiation. Cell Mol Immunol 16, 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, and Albano E (2014). Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 59, 886–897. [DOI] [PubMed] [Google Scholar]
- 128.Moreno-Fernandez ME, Giles DA, Oates JR, Chan CC, Damen M, Doll JR, Stankiewicz TE, Chen X, Chetal K, Karns R, et al. (2021). PKM2-dependent metabolic skewing of hepatic Th17 cells regulates pathogenesis of non-alcoholic fatty liver disease. Cell Metab 33, 1187–1204 e1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, et al. (2016). NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531, 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kremer M, Hines IN, Milton RJ, and Wheeler MD (2006). Favored T helper 1 response in a mouse model of hepatosteatosis is associated with enhanced T cell-mediated hepatitis. Hepatology 44, 216–227. [DOI] [PubMed] [Google Scholar]
- 131.Rau M, Schilling AK, Meertens J, Hering I, Weiss J, Jurowich C, Kudlich T, Hermanns HM, Bantel H, Beyersdorf N, et al. (2016). Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J Immunol 196, 97–105. [DOI] [PubMed] [Google Scholar]
- 132.Chackelevicius CM, Gambaro SE, Tiribelli C, and Rosso N (2016). Th17 involvement in nonalcoholic fatty liver disease progression to non-alcoholic steatohepatitis. World J Gastroenterol 22, 9096–9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rolla S, Alchera E, Imarisio C, Bardina V, Valente G, Cappello P, Mombello C, Follenzi A, Novelli F, and Carini R (2016). The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin Sci (Lond) 130, 193–203. [DOI] [PubMed] [Google Scholar]
- 134.Gomes AL, Teijeiro A, Buren S, Tummala KS, Yilmaz M, Waisman A, Theurillat JP, Perna C, and Djouder N (2016). Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 30, 161–175. [DOI] [PubMed] [Google Scholar]
- 135.Harley IT, Stankiewicz TE, Giles DA, Softic S, Flick LM, Cappelletti M, Sheridan R, Xanthakos SA, Steinbrecher KA, Sartor RB, et al. (2014). IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology 59, 1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tang Y, Bian Z, Zhao L, Liu Y, Liang S, Wang Q, Han X, Peng Y, Chen X, Shen L, et al. (2011). Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol 166, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Van Herck MA, Weyler J, Kwanten WJ, Dirinck EL, De Winter BY, Francque SM, and Vonghia L (2019). The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front Immunol 10, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bertola A, Bonnafous S, Anty R, Patouraux S, Saint-Paul MC, Iannelli A, Gugenheim J, Barr J, Mato JM, Le Marchand-Brustel Y, et al. (2010). Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One 5, e13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rai RP, Liu Y, Iyer SS, Liu S, Gupta B, Desai C, Kumar P, Smith T, Singhi AD, Nusrat A, et al. (2020). Blocking integrin alpha(4)beta(7)-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J Hepatol 73, 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ferreyra Solari NE, Inzaugarat ME, Baz P, De Matteo E, Lezama C, Galoppo M, Galoppo C, and Chernavsky AC (2012). The role of innate cells is coupled to a Th1-polarized immune response in pediatric nonalcoholic steatohepatitis. J Clin Immunol 32, 611–621. [DOI] [PubMed] [Google Scholar]
- 141.Luo XY, Takahara T, Kawai K, Fujino M, Sugiyama T, Tsuneyama K, Tsukada K, Nakae S, Zhong L, and Li XK (2013). IFN-gamma deficiency attenuates hepatic inflammation and fibrosis in a steatohepatitis model induced by a methionine- and choline-deficient high-fat diet. Am J Physiol Gastrointest Liver Physiol 305, G891–899. [DOI] [PubMed] [Google Scholar]
- 142.Gieseck RL, Wilson MS, and Wynn TA (2018). Type 2 immunity in tissue repair and fibrosis. Nature Reviews Immunology 18, 62–76. [DOI] [PubMed] [Google Scholar]
- 143.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M, et al. (2012). Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143, 765–776 e763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Cappelletti M, Huppert SS, Iwakura Y, Dong C, Shanmukhappa SK, and Divanovic S (2016). Regulation of Inflammation by IL-17A and IL-17F Modulates Non-Alcoholic Fatty Liver Disease Pathogenesis. PLoS One 11, e0149783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, Wang X, Ryffel B, and Sun B (2013). IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol 191, 1835–1844. [DOI] [PubMed] [Google Scholar]
- 146.He B, Wu L, Xie W, Shao Y, Jiang J, Zhao Z, Yan M, Chen Z, and Cui D (2017). The imbalance of Th17/Treg cells is involved in the progression of nonalcoholic fatty liver disease in mice. BMC Immunol 18, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ma X, Hua J, Mohamood AR, Hamad AR, Ravi R, and Li Z (2007). A high-fat diet and regulatory T cells influence susceptibility to endotoxin-induced liver injury. Hepatology 46, 1519–1529. [DOI] [PubMed] [Google Scholar]
- 148.Wang L, Zhang H, Wang S, Chen X, and Su J (2021). Bone Marrow Adipocytes: A Critical Player in the Bone Marrow Microenvironment. Front Cell Dev Biol 9, 770705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dywicki J, Buitrago-Molina LE, Noyan F, Davalos-Misslitz AC, Hupa-Breier KL, Lieber M, Hapke M, Schlue J, Falk CS, Raha S, et al. (2022). The Detrimental Role of Regulatory T Cells in Nonalcoholic Steatohepatitis. Hepatol Commun 6, 320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Soderberg C, Marmur J, Eckes K, Glaumann H, Sallberg M, Frelin L, Rosenberg P, Stal P, and Hultcrantz R (2011). Microvesicular fat, inter cellular adhesion molecule-1 and regulatory T-lymphocytes are of importance for the inflammatory process in livers with non-alcoholic steatohepatitis. APMIS 119, 412–420. [DOI] [PubMed] [Google Scholar]
- 151.Bhattacharjee J, Kirby M, Softic S, Miles L, Salazar-Gonzalez RM, Shivakumar P, and Kohli R (2017). Hepatic Natural Killer T-cell and CD8+ T-cell Signatures in Mice with Nonalcoholic Steatohepatitis. Hepatol Commun 1, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wabitsch S, McCallen JD, Kamenyeva O, Ruf B, McVey JC, Kabat J, Walz JS, Rotman Y, Bauer KC, Craig AJ, et al. (2022). Metformin treatment rescues CD8(+) T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatol 77, 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dudek M, Pfister D, Donakonda S, Filpe P, Schneider A, Laschinger M, Hartmann D, Huser N, Meiser P, Bayerl F, et al. (2021). Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature 592, 444–449. [DOI] [PubMed] [Google Scholar]
- 154.Koda Y, Teratani T, Chu PS, Hagihara Y, Mikami Y, Harada Y, Tsujikawa H, Miyamoto K, Suzuki T, Taniki N, et al. (2021). CD8(+) tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat Commun 12, 4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McVey JC, Green BL, Ruf B, McCallen JD, Wabitsch S, Subramanyam V, Diggs LP, Heinrich B, Greten TF, and Ma C (2022). NAFLD indirectly impairs antigen-specific CD8(+) T cell immunity against liver cancer in mice. iScience 25, 103847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barrow F, Khan S, Wang H, and Revelo XS (2021). The Emerging Role of B Cells in the Pathogenesis of NAFLD. Hepatology 74, 2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bruzzi S, Sutti S, Giudici G, Burlone ME, Ramavath NN, Toscani A, Bozzola C, Schneider P, Morello E, Parola M, et al. (2018). B2-Lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD). Free Radic Biol Med 124, 249–259. [DOI] [PubMed] [Google Scholar]
- 158.Zhang F, Jiang WW, Li X, Qiu XY, Wu Z, Chi YJ, Cong X, and Liu YL (2016). Role of intrahepatic B cells in non-alcoholic fatty liver disease by secreting pro-inflammatory cytokines and regulating intrahepatic T cells. J Dig Dis 17, 464–474. [DOI] [PubMed] [Google Scholar]
- 159.McPherson S, Henderson E, Burt AD, Day CP, and Anstee QM (2014). Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol 60, 1055–1062. [DOI] [PubMed] [Google Scholar]
- 160.Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, and Day CP (2005). Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut 54, 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nobili V, Parola M, Alisi A, Marra F, Piemonte F, Mombello C, Sutti S, Povero D, Maina V, Novo E, et al. (2010). Oxidative stress parameters in paediatric non-alcoholic fatty liver disease. Int J Mol Med 26, 471–476. [DOI] [PubMed] [Google Scholar]
- 162.Karl M, Hasselwander S, Zhou Y, Reifenberg G, Kim YO, Park KS, Ridder DA, Wang X, Seidel E, Hovelmeyer N, et al. (2022). Dual roles of B lymphocytes in mouse models of diet-induced nonalcoholic fatty liver disease. Hepatology 76, 1135–1149. [DOI] [PubMed] [Google Scholar]
- 163.McDaniel MM, Meibers HE, and Pasare C (2021). Innate control of adaptive immunity and adaptive instruction of innate immunity: bi-directional flow of information. Curr Opin Immunol 73, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Unutmaz D, Pileri P, and Abrignani S (1994). Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med 180, 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Geginat J, Campagnaro S, Sallusto F, and Lanzavecchia A (2002). TCR-independent proliferation and differentiation of human CD4+ T cell subsets induced by cytokines. Adv Exp Med Biol 512, 107–112. [DOI] [PubMed] [Google Scholar]
- 166.Saito T, Yokosuka T, and Hashimoto-Tane A (2010). Dynamic regulation of T cell activation and co-stimulation through TCR-microclusters. FEBS Lett 584, 4865–4871. [DOI] [PubMed] [Google Scholar]
- 167.Croft M, So T, Duan W, and Soroosh P (2009). The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev 229, 173–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vinay DS, and Kwon BS (1998). Role of 4–1BB in immune responses. Semin Immunol 10, 481–489. [DOI] [PubMed] [Google Scholar]
- 169.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, and Noelle RJ (2009). Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 229, 152–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schwarz H (2005). Biological activities of reverse signal transduction through CD137 ligand. J Leukoc Biol 77, 281–286. [DOI] [PubMed] [Google Scholar]
- 171.Sun M, and Fink PJ (2007). A new class of reverse signaling costimulators belongs to the TNF family. J Immunol 179, 4307–4312. [DOI] [PubMed] [Google Scholar]
- 172.Croft M (2010). Control of immunity by the TNFR-related molecule OX40 (CD134). Annu Rev Immunol 28, 57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, et al. (2004). CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol 5, 1134–1142. [DOI] [PubMed] [Google Scholar]
- 174.Acuto O, and Michel F (2003). CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol 3, 939–951. [DOI] [PubMed] [Google Scholar]
- 175.Utley A, Chavel C, Lightman S, Holling GA, Cooper J, Peng P, Liu W, Barwick BG, Gavile CM, Maguire O, et al. (2020). CD28 Regulates Metabolic Fitness for Long-Lived Plasma Cell Survival. Cell Rep 31, 107815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Venuprasad K, Chattopadhyay S, and Saha B (2003). CD28 signaling in neutrophil induces T-cell chemotactic factor(s) modulating T-cell response. Hum Immunol 64, 38–43. [DOI] [PubMed] [Google Scholar]
- 177.Venuprasad K, Parab P, Prasad DV, Sharma S, Banerjee PR, Deshpande M, Mitra DK, Pal S, Bhadra R, Mitra D, et al. (2001). Immunobiology of CD28 expression on human neutrophils. I. CD28 regulates neutrophil migration by modulating CXCR-1 expression. Eur J Immunol 31, 1536–1543. [DOI] [PubMed] [Google Scholar]
- 178.Zheng X, Zhang H, Yin L, Wang CR, Liu Y, and Zheng P (2008). Modulation of NKT cell development by B7-CD28 interaction: an expanding horizon for costimulation. PLoS One 3, e2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Woerly G, Roger N, Loiseau S, Dombrowicz D, Capron A, and Capron M (1999). Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J Exp Med 190, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sharpe AH, and Freeman GJ (2002). The B7-CD28 superfamily. Nat Rev Immunol 2, 116–126. [DOI] [PubMed] [Google Scholar]
- 181.Poggi M, Morin SO, Bastelica D, Govers R, Canault M, Bernot D, Georgelin O, Verdier M, Burcelin R, Olive D, et al. (2015). CD28 deletion improves obesity-induced liver steatosis but increases adiposity in mice. Int J Obes (Lond) 39, 977–985. [DOI] [PubMed] [Google Scholar]
- 182.Chatzigeorgiou A, Chung KJ, Garcia-Martin R, Alexaki VI, Klotzsche-von Ameln A, Phieler J, Sprott D, Kanczkowski W, Tzanavari T, Bdeir M, et al. (2014). Dual role of B7 costimulation in obesity-related nonalcoholic steatohepatitis and metabolic dysregulation. Hepatology 60, 1196–1210. [DOI] [PubMed] [Google Scholar]
- 183.Ursini F, Mauro D, Naty S, Gagliardi D, and Grembiale RD (2012). Improvement in insulin resistance after short-term treatment with abatacept: case report and short review. Clin Rheumatol 31, 1401–1402. [DOI] [PubMed] [Google Scholar]
- 184.Kwon BS, and Weissman SM (1989). cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A 86, 1963–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vinay DS, and Kwon BS (2011). 4–1BB signaling beyond T cells. Cell Mol Immunol 8, 281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim DH, Chang WS, Lee YS, Lee KA, Kim YK, Kwon BS, and Kang CY (2008). 4–1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J Immunol 180, 2062–2068. [DOI] [PubMed] [Google Scholar]
- 187.Lee WH, Seo D, Lim SG, and Suk K (2019). Reverse Signaling of Tumor Necrosis Factor Superfamily Proteins in Macrophages and Microglia: Superfamily Portrait in the Neuroimmune Interface. Front Immunol 10, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Eissner G, Kolch W, and Scheurich P (2004). Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev 15, 353–366. [DOI] [PubMed] [Google Scholar]
- 189.Eun SY, Lee SW, Xu Y, and Croft M (2015). 4–1BB ligand signaling to T cells limits T cell activation. J Immunol 194, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kim CS, Kim JG, Lee BJ, Choi MS, Choi HS, Kawada T, Lee KU, and Yu R (2011). Deficiency for costimulatory receptor 4–1BB protects against obesity-induced inflammation and metabolic disorders. Diabetes 60, 3159–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim HD, Park S, Jeong S, Lee YJ, Lee H, Kim CG, Kim KH, Hong SM, Lee JY, Kim S, et al. (2020). 4–1BB Delineates Distinct Activation Status of Exhausted Tumor-Infiltrating CD8(+) T Cells in Hepatocellular Carcinoma. Hepatology 71, 955–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, and Williams AF (1987). Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol 24, 1281–1290. [DOI] [PubMed] [Google Scholar]
- 193.Wang Q, Chen Y, Ge Y, Sun J, Shi Q, Ju S, Dai J, Yu G, and Zhang X (2004). Characterization and functional study of five novel monoclonal antibodies against human OX40L highlight reverse signalling: enhancement of IgG production of B cells and promotion of maturation of DCs. Tissue Antigens 64, 566–574. [DOI] [PubMed] [Google Scholar]
- 194.Soroosh P, Ine S, Sugamura K, and Ishii N (2006). OX40-OX40 ligand interaction through T cell-T cell contact contributes to CD4 T cell longevity. J Immunol 176, 5975–5987. [DOI] [PubMed] [Google Scholar]
- 195.Sun G, Jin H, Zhang C, Meng H, Zhao X, Wei D, Ou X, Wang Q, Li S, Wang T, et al. (2018). OX40 Regulates Both Innate and Adaptive Immunity and Promotes Nonalcoholic Steatohepatitis. Cell Rep 25, 3786–3799 e3784. [DOI] [PubMed] [Google Scholar]
- 196.Bretscher PA (1999). A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A 96, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Blair PJ, Riley JL, Harlan DM, Abe R, Tadaki DK, Hoffmann SC, White L, Francomano T, Perfetto SJ, Kirk AD, et al. (2000). CD40 ligand (CD154) triggers a short-term CD4(+) T cell activation response that results in secretion of immunomodulatory cytokines and apoptosis. J Exp Med 191, 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Peng X, Kasran A, Warmerdam PA, de Boer M, and Ceuppens JL (1996). Accessory signaling by CD40 for T cell activation: induction of Th1 and Th2 cytokines and synergy with interleukin-12 for interferon-gamma production. Eur J Immunol 26, 1621–1627. [DOI] [PubMed] [Google Scholar]
- 199.Lepreux S, Villeneuve J, Dewitte A, Berard AM, Desmouliere A, and Ripoche J (2017). CD40 signaling and hepatic steatosis: Unanticipated links. Clin Res Hepatol Gastroenterol 41, 357–369. [DOI] [PubMed] [Google Scholar]
- 200.Aarts S, Reiche M, den Toom M, Gijbels M, Beckers L, Gerdes N, and Lutgens E (2019). Depletion of CD40 on CD11c+ cells worsens the metabolic syndrome and ameliorates hepatic inflammation during NASH. Scientific Reports 9, 14702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Guo CA, Kogan S, Amano SU, Wang M, Dagdeviren S, Friedline RH, Aouadi M, Kim JK, and Czech MP (2013). CD40 deficiency in mice exacerbates obesity-induced adipose tissue inflammation, hepatic steatosis, and insulin resistance. Am J Physiol Endocrinol Metab 304, E951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wolf D, Jehle F, Michel NA, Bukosza EN, Rivera J, Chen YC, Hoppe N, Dufner B, Rodriguez AO, Colberg C, et al. (2014). Coinhibitory suppression of T cell activation by CD40 protects against obesity and adipose tissue inflammation in mice. Circulation 129, 2414–2425. [DOI] [PubMed] [Google Scholar]
- 203.Villeneuve J, Lepreux S, Mulot A, Berard AM, Higa-Nishiyama A, Costet P, De Ledinghen V, Bioulac-Sage P, Balabaud C, Nurden AT, et al. (2010). A protective role for CD154 in hepatic steatosis in mice. Hepatology 52, 1968–1979. [DOI] [PubMed] [Google Scholar]
- 204.Wolf D, Jehle F, Ortiz Rodriguez A, Dufner B, Hoppe N, Colberg C, Lozhkin A, Bassler N, Rupprecht B, Wiedemann A, et al. (2012). CD40L deficiency attenuates diet-induced adipose tissue inflammation by impairing immune cell accumulation and production of pathogenic IgG-antibodies. PLoS One 7, e33026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Poggi M, Engel D, Christ A, Beckers L, Wijnands E, Boon L, Driessen A, Cleutjens J, Weber C, Gerdes N, et al. (2011). CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arterioscler Thromb Vasc Biol 31, 2251–2260. [DOI] [PubMed] [Google Scholar]
- 206.Takada YK, Yu J, Shimoda M, and Takada Y (2019). Integrin Binding to the Trimeric Interface of CD40L Plays a Critical Role in CD40/CD40L Signaling. J Immunol 203, 1383–1391. [DOI] [PubMed] [Google Scholar]
- 207.Peter ME, and Krammer PH (2003). The CD95(APO-1/Fas) DISC and beyond. Cell Death & Differentiation 10, 26–35. [DOI] [PubMed] [Google Scholar]
- 208.Suzuki I, and Fink PJ (1998). Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med 187, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Boursalian TE, and Fink PJ (2003). Mutation in fas ligand impairs maturation of thymocytes bearing moderate affinity T cell receptors. J Exp Med 198, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Isayama F, Moore S, Hines IN, and Wheeler MD (2016). Fas Regulates Macrophage Polarization and Fibrogenic Phenotype in a Model of Chronic Ethanol-Induced Hepatocellular Injury. Am J Pathol 186, 1524–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ottonello L, Tortolina G, Amelotti M, and Dallegri F (1999). Soluble Fas Ligand Is Chemotactic for Human Neutrophilic Polymorphonuclear Leukocytes. The Journal of Immunology 162, 3601–3606. [PubMed] [Google Scholar]
- 212.Hao Z, Duncan GS, Seagal J, Su YW, Hong C, Haight J, Chen NJ, Elia A, Wakeham A, Li WY, et al. (2008). Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity 29, 615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Alkhouri N, Alisi A, Okwu V, Matloob A, Ferrari F, Crudele A, De Vito R, Lopez R, Feldstein AE, and Nobili V (2015). Circulating Soluble Fas and Fas Ligand Levels Are Elevated in Children with Nonalcoholic Steatohepatitis. Dig Dis Sci 60, 2353–2359. [DOI] [PubMed] [Google Scholar]
- 214.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, and Gores GJ (2003). Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125, 437–443. [DOI] [PubMed] [Google Scholar]
- 215.Item F, Wueest S, Lemos V, Stein S, Lucchini FC, Denzler R, Fisser MC, Challa TD, Pirinen E, Kim Y, et al. (2017). Fas cell surface death receptor controls hepatic lipid metabolism by regulating mitochondrial function. Nat Commun 8, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Crampton SP, Voynova E, and Bolland S (2010). Innate pathways to B-cell activation and tolerance. Ann N Y Acad Sci 1183, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.McNab F, Mayer-Barber K, Sher A, Wack A, and O’Garra A (2015). Type I interferons in infectious disease. Nat Rev Immunol 15, 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Crouse J, Kalinke U, and Oxenius A (2015). Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol 15, 231–242. [DOI] [PubMed] [Google Scholar]
- 219.Kiefer K, Oropallo MA, Cancro MP, and Marshak-Rothstein A (2012). Role of type I interferons in the activation of autoreactive B cells. Immunol Cell Biol 90, 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang L, Jiang X, Pfau D, Ling Y, and Nathan CF (2021). Type I interferon signaling mediates Mycobacterium tuberculosis-induced macrophage death. J Exp Med 218, e20200887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Paolini R, Bernardini G, Molfetta R, and Santoni A (2015). NK cells and interferons. Cytokine Growth Factor Rev 26, 113–120. [DOI] [PubMed] [Google Scholar]
- 222.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, and Hartmann G (2005). Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol 174, 4043–4050. [DOI] [PubMed] [Google Scholar]
- 223.Akkaya M, Akkaya B, Miozzo P, Rawat M, Pena M, Sheehan PW, Kim AS, Kamenyeva O, Kabat J, Bolland S, et al. (2017). B Cells Produce Type 1 IFNs in Response to the TLR9 Agonist CpG-A Conjugated to Cationic Lipids. J Immunol 199, 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O’Garra A, Vicari A, and Trinchieri G (2005). Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med 201, 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kim S, Park S, Kim B, and Kwon J (2016). Toll-like receptor 7 affects the pathogenesis of non-alcoholic fatty liver disease. Sci Rep 6, 27849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Shepard CR (2020). TLR9 in MAFLD and NASH: At the Intersection of Inflammation and Metabolism. Front Endocrinol (Lausanne) 11, 613639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Deng CJ, Lo TH, Chan KY, Li X, Wu MY, Xiang Z, and Wong CM (2022). Role of B Lymphocytes in the Pathogenesis of NAFLD: A 2022 Update. Int J Mol Sci 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ghazarian M, Revelo XS, Nohr MK, Luck H, Zeng K, Lei H, Tsai S, Schroer SA, Park YJ, Chng MHY, et al. (2017). Type I Interferon Responses Drive Intrahepatic T cells to Promote Metabolic Syndrome. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wieser V, Adolph TE, Grander C, Grabherr F, Enrich B, Moser P, Moschen AR, Kaser S, and Tilg H (2018). Adipose type I interferon signalling protects against metabolic dysfunction. Gut 67, 157–165. [DOI] [PubMed] [Google Scholar]
- 230.Wang XA, Zhang R, Jiang D, Deng W, Zhang S, Deng S, Zhong J, Wang T, Zhu LH, Yang L, et al. (2013). Interferon regulatory factor 9 protects against hepatic insulin resistance and steatosis in male mice. Hepatology 58, 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang XA, Zhang R, She ZG, Zhang XF, Jiang DS, Wang T, Gao L, Deng W, Zhang SM, Zhu LH, et al. (2014). Interferon regulatory factor 3 constrains IKKbeta/NF-kappaB signaling to alleviate hepatic steatosis and insulin resistance. Hepatology 59, 870–885. [DOI] [PubMed] [Google Scholar]
- 232.Alzaid F, Lagadec F, Albuquerque M, Ballaire R, Orliaguet L, Hainault I, Blugeon C, Lemoine S, Lehuen A, Saliba DG, et al. (2016). IRF5 governs liver macrophage activation that promotes hepatic fibrosis in mice and humans. JCI Insight 1, e88689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chan CC, Damen M, Moreno-Fernandez ME, Stankiewicz TE, Cappelletti M, Alarcon PC, Oates JR, Doll JR, Mukherjee R, Chen X, et al. (2020). Type I interferon sensing unlocks dormant adipocyte inflammatory potential. Nat Commun 11, 2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mohlenberg M, Terczynska-Dyla E, Thomsen KL, George J, Eslam M, Gronbaek H, and Hartmann R (2019). The role of IFN in the development of NAFLD and NASH. Cytokine 124, 154519. [DOI] [PubMed] [Google Scholar]
- 235.Hannibal TD, Schmidt-Christensen A, Nilsson J, Fransen-Pettersson N, Hansen L, and Holmberg D (2017). Deficiency in plasmacytoid dendritic cells and type I interferon signalling prevents diet-induced obesity and insulin resistance in mice. Diabetologia 60, 2033–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pieper K, Grimbacher B, and Eibel H (2013). B-cell biology and development. J Allergy Clin Immunol 131, 959–971. [DOI] [PubMed] [Google Scholar]
- 237.Shulga-Morskaya S, Dobles M, Walsh ME, Ng LG, MacKay F, Rao SP, Kalled SL, and Scott ML (2004). B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J Immunol 173, 2331–2341. [DOI] [PubMed] [Google Scholar]
- 238.Mackay F, and Schneider P (2009). Cracking the BAFF code. Nat Rev Immunol 9, 491–502. [DOI] [PubMed] [Google Scholar]
- 239.Mackay F, Schneider P, Rennert P, and Browning J (2003). BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol 21, 231–264. [DOI] [PubMed] [Google Scholar]
- 240.Scapini P, Bazzoni F, and Cassatella MA (2008). Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol Lett 116, 1–6. [DOI] [PubMed] [Google Scholar]
- 241.Dillon SR, Gross JA, Ansell SM, and Novak AJ (2006). An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov 5, 235–246. [DOI] [PubMed] [Google Scholar]
- 242.Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, Thompson JS, Wheway J, Chtanova T, Groom J, et al. (2004). B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol 173, 807–817. [DOI] [PubMed] [Google Scholar]
- 243.Darce JR, Arendt BK, Wu X, and Jelinek DF (2007). Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol 179, 7276–7286. [DOI] [PubMed] [Google Scholar]
- 244.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, and Cerutti A (2002). DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol 3, 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, and Cerutti A (2004). Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol 172, 3268–3279. [DOI] [PubMed] [Google Scholar]
- 246.Huang X, Di Liberto M, Cunningham AF, Kang L, Cheng S, Ely S, Liou HC, Maclennan IC, and Chen-Kiang S (2004). Homeostatic cell-cycle control by BLyS: Induction of cell-cycle entry but not G1/S transition in opposition to p18INK4c and p27Kip1. Proc Natl Acad Sci U S A 101, 17789–17794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mackay F, and Schneider P (2008). TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev 19, 263–276. [DOI] [PubMed] [Google Scholar]
- 248.Chen M, Lin X, Liu Y, Li Q, Deng Y, Liu Z, Brand D, Guo Z, He X, Ryffel B, et al. (2014). The function of BAFF on T helper cells in autoimmunity. Cytokine Growth Factor Rev 25, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chang SK, Mihalcik SA, and Jelinek DF (2008). B lymphocyte stimulator regulates adaptive immune responses by directly promoting dendritic cell maturation. J Immunol 180, 7394–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chang SK, Arendt BK, Darce JR, Wu X, and Jelinek DF (2006). A role for BLyS in the activation of innate immune cells. Blood 108, 2687–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chan CC, Harley ITW, Pfluger PT, Trompette A, Stankiewicz TE, Allen JL, Moreno-Fernandez ME, Damen M, Oates JR, Alarcon PC, et al. (2021). A BAFF/APRIL axis regulates obesogenic diet-driven weight gain. Nat Commun 12, 2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Jeon ST, Kim WJ, Lee SM, Lee MY, Park SB, Lee SH, Kim IS, Suk K, Choi BK, Choi EM, et al. (2010). Reverse signaling through BAFF differentially regulates the expression of inflammatory mediators and cytoskeletal movements in THP-1 cells. Immunol Cell Biol 88, 148–156. [DOI] [PubMed] [Google Scholar]
- 253.Lee SM, Kim EJ, Suk K, and Lee WH (2011). BAFF and APRIL induce inflammatory activation of THP-1 cells through interaction with their conventional receptors and activation of MAPK and NF-kappaB. Inflamm Res 60, 807–815. [DOI] [PubMed] [Google Scholar]
- 254.Nakamura Y, Abe M, Kawasaki K, Miyake T, Watanabe T, Yoshida O, Hirooka M, Matsuura B, and Hiasa Y (2019). Depletion of B cell-activating factor attenuates hepatic fat accumulation in a murine model of nonalcoholic fatty liver disease. Sci Rep 9, 977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Schneider P, Takatsuka H, Wilson A, Mackay F, Tardivel A, Lens S, Cachero TG, Finke D, Beermann F, and Tschopp J (2001). Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med 194, 1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Liu L, Inouye KE, Allman WR, Coleman AS, Siddiqui S, Hotamisligil GS, and Akkoyunlu M (2018). TACI-Deficient Macrophages Protect Mice Against Metaflammation and Obesity-Induced Dysregulation of Glucose Homeostasis. Diabetes 67, 1589–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tanaka T, Narazaki M, and Kishimoto T (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6, a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Maeda K, Mehta H, Drevets DA, and Coggeshall KM (2010). IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production. Blood 115, 4699–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li B, Jones LL, and Geiger TL (2018). IL-6 Promotes T Cell Proliferation and Expansion under Inflammatory Conditions in Association with Low-Level RORgammat Expression. J Immunol 201, 2934–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Xu YD, Cheng M, Shang PP, and Yang YQ (2022). Role of IL-6 in dendritic cell functions. J Leukoc Biol 111, 695–709. [DOI] [PubMed] [Google Scholar]
- 261.Xu W, Joo H, Clayton S, Dullaers M, Herve MC, Blankenship D, De La Morena MT, Balderas R, Picard C, Casanova JL, et al. (2012). Macrophages induce differentiation of plasma cells through CXCL10/IP-10. J Exp Med 209, 1813–1823, S1811–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, and Jenkins BJ (2008). IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol 181, 2189–2195. [DOI] [PubMed] [Google Scholar]
- 263.Wu J, Gao FX, Wang C, Qin M, Han F, Xu T, Hu Z, Long Y, He XM, Deng X, et al. (2019). IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J Exp Clin Cancer Res 38, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cifaldi L, Prencipe G, Caiello I, Bracaglia C, Locatelli F, De Benedetti F, and Strippoli R (2015). Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol 67, 3037–3046. [DOI] [PubMed] [Google Scholar]
- 265.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, and Feldstein AE (2008). Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 103, 1372–1379. [DOI] [PubMed] [Google Scholar]
- 266.Yamaguchi K, Itoh Y, Yokomizo C, Nishimura T, Niimi T, Fujii H, Okanoue T, and Yoshikawa T (2010). Blockade of interleukin-6 signaling enhances hepatic steatosis but improves liver injury in methionine choline-deficient diet-fed mice. Lab Invest 90, 1169–1178. [DOI] [PubMed] [Google Scholar]
- 267.Ganzetti G, Campanati A, and Offidani A (2015). Non-alcoholic fatty liver disease and psoriasis: So far, so near. World J Hepatol 7, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kwon J, Lee C, Heo S, Kim B, and Hyun CK (2021). DSS-induced colitis is associated with adipose tissue dysfunction and disrupted hepatic lipid metabolism leading to hepatosteatosis and dyslipidemia in mice. Sci Rep 11, 5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Barbarroja N, Ruiz-Ponce M, Cuesta-Lopez L, Perez-Sanchez C, Lopez-Pedrera C, Arias-de la Rosa I, and Collantes-Estevez E (2022). Nonalcoholic fatty liver disease in inflammatory arthritis: Relationship with cardiovascular risk. Front Immunol 13, 997270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Choi D, Sheridan H, and Bhat S (2023). Risankizumab-rzaa: A New Therapeutic Option for the Treatment of Crohn’s Disease. Ann Pharmacother 57, 579–584. [DOI] [PubMed] [Google Scholar]
- 271.Blegvad C, Skov L, and Zachariae C (2019). Ixekizumab for the treatment of psoriasis: an update on new data since first approval. Expert Rev Clin Immunol 15, 111–121. [DOI] [PubMed] [Google Scholar]
- 272.Mease PJ, Helliwell PS, Hjuler KF, Raymond K, and McInnes I (2021). Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis 80, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Blair HA (2021). Secukinumab: A Review in Psoriatic Arthritis. Drugs 81, 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Miller J, Puravath AP, and Orbai AM (2021). Ixekizumab for Psoriatic Arthritis: Safety, Efficacy, and Patient Selection. J Inflamm Res 14, 6975–6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, et al. (2012). Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 366, 1181–1189. [DOI] [PubMed] [Google Scholar]
- 276.Paredes JL, and Niewold TB (2020). Type I interferon antagonists in clinical development for lupus. Expert Opin Investig Drugs 29, 1025–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, Bae SC, Brohawn PZ, Pineda L, Berglind A, et al. (2020). Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N Engl J Med 382, 211–221. [DOI] [PubMed] [Google Scholar]
- 278.Thompson CA (2010). FDA approves tocilizumab to treat rheumatoid arthritis. Am J Health Syst Pharm 67, 254. [DOI] [PubMed] [Google Scholar]
- 279.Choi IA, Sagawa A, Lee EY, Lee EB, and Song YW (2020). Tocilizumab Increases Body Weight and Serum Adipokine Levels in Patients with Rheumatoid Arthritis Independently of Their Treatment Response: a Retrospective Cohort Study. J Korean Med Sci 35, e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, and Rinella ME (2011). Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol 10, 277–286. [PubMed] [Google Scholar]
- 281.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, et al. (2005). Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 353, 1114–1123. [DOI] [PubMed] [Google Scholar]
- 282.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, Szechinski J, Li T, Ge Z, Becker JC, et al. (2006). Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 144, 865–876. [DOI] [PubMed] [Google Scholar]
- 283.Noisette A, and Hochberg MC (2018). Abatacept for the treatment of adults with psoriatic arthritis: patient selection and perspectives. Psoriasis (Auckl) 8, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kuemmerle-Deschner JB, and Benseler S (2008). Abatacept in difficult-to-treat juvenile idiopathic arthritis. Biologics 2, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chen R, Ganesan A, Okoye I, Arutyunova E, Elahi S, Lemieux MJ, and Barakat K (2020). Targeting B7–1 in immunotherapy. Med Res Rev 40, 654–682. [DOI] [PubMed] [Google Scholar]
- 286.Shah P, Sundaram V, and Bjornsson E (2020). Biologic and Checkpoint Inhibitor-Induced Liver Injury: A Systematic Literature Review. Hepatol Commun 4, 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, and Kwon BS (2004). 4–1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med 10, 1088–1094. [DOI] [PubMed] [Google Scholar]
- 288.Jeon HJ, Choi JH, Jung IH, Park JG, Lee MR, Lee MN, Kim B, Yoo JY, Jeong SJ, Kim DY, et al. (2010). CD137 (4–1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation 121, 1124–1133. [DOI] [PubMed] [Google Scholar]
- 289.Haga T, Suzuki J, Kosuge H, Ogawa M, Saiki H, Haraguchi G, Maejima Y, Isobe M, and Uede T (2009). Attenuation of experimental autoimmune myocarditis by blocking T cell activation through 4–1BB pathway. J Mol Cell Cardiol 46, 719–727. [DOI] [PubMed] [Google Scholar]
- 290.Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK, Koller BH, Wolisi G, Broxmeyer HE, and Vinay DS (2002). Immune responses in 4–1BB (CD137)-deficient mice. J Immunol 168, 5483–5490. [DOI] [PubMed] [Google Scholar]
- 291.Burrows KE, Dumont C, Thompson CL, Catley MC, Dixon KL, and Marshall D (2015). OX40 blockade inhibits house dust mite driven allergic lung inflammation in mice and in vitro allergic responses in humans. Eur J Immunol 45, 1116–1128. [DOI] [PubMed] [Google Scholar]
- 292.Gauvreau GM, Boulet LP, Cockcroft DW, FitzGerald JM, Mayers I, Carlsten C, Laviolette M, Killian KJ, Davis BE, Larche M, et al. (2014). OX40L blockade and allergen-induced airway responses in subjects with mild asthma. Clin Exp Allergy 44, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Nohara C, Akiba H, Nakajima A, Inoue A, Koh CS, Ohshima H, Yagita H, Mizuno Y, and Okumura K (2001). Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol 166, 2108–2115. [DOI] [PubMed] [Google Scholar]
- 294.Bresson D, Fousteri G, Manenkova Y, Croft M, and von Herrath M (2011). Antigen-specific prevention of type 1 diabetes in NOD mice is ameliorated by OX40 agonist treatment. J Autoimmun 37, 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Fu Y, Lin Q, Zhang Z, and Zhang L (2020). Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm Sin B 10, 414–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Guttman-Yassky E, Simpson EL, Reich K, Kabashima K, Igawa K, Suzuki T, Mano H, Matsui T, Esfandiari E, and Furue M (2023). An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: a multicentre, double-blind, placebo-controlled phase 2b study. Lancet 401, 204–214. [DOI] [PubMed] [Google Scholar]
- 297.Tanaka H, Yang GX, Iwakoshi N, Knechtle SJ, Kawata K, Tsuneyama K, Leung P, Coppel RL, Ansari AA, Joh T, et al. (2013). Anti-CD40 ligand monoclonal antibody delays the progression of murine autoimmune cholangitis. Clin Exp Immunol 174, 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Visvanathan S, Daniluk S, Ptaszynski R, Muller-Ladner U, Ramanujam M, Rosenstock B, Eleftheraki AG, Vinisko R, Petrikova A, Kellner H, et al. (2019). Effects of BI 655064, an antagonistic anti-CD40 antibody, on clinical and biomarker variables in patients with active rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase IIa study. Ann Rheum Dis 78, 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Krishnan A, Kocab AJ, Zacks DN, Marshak-Rothstein A, and Gregory-Ksander M (2019). A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J Neuroinflammation 16, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tan W, Zou J, Yoshida S, Jiang B, and Zhou Y (2020). The Role of Inflammation in Age-Related Macular Degeneration. Int J Biol Sci 16, 2989–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ward-Kavanagh LK, Lin WW, Sedy JR, and Ware CF (2016). The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity 44, 1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Spahr L, Rubbia-Brandt L, Frossard JL, Giostra E, Rougemont AL, Pugin J, Fischer M, Egger H, and Hadengue A (2002). Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol 37, 448–455. [DOI] [PubMed] [Google Scholar]
- 303.Tilg H, Jalan R, Kaser A, Davies NA, Offner FA, Hodges SJ, Ludwiczek O, Shawcross D, Zoller H, Alisa A, et al. (2003). Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol 38, 419–425. [DOI] [PubMed] [Google Scholar]
- 304.Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broet P, Emilie D, et al. (2004). A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 39, 1390–1397. [DOI] [PubMed] [Google Scholar]
- 305.Zein NN, and Etanercept Study G (2005). Etanercept as an adjuvant to interferon and ribavirin in treatment-naive patients with chronic hepatitis C virus infection: a phase 2 randomized, double-blind, placebo-controlled study. J Hepatol 42, 315–322. [DOI] [PubMed] [Google Scholar]
- 306.Chen YM, Chen HH, Chen YH, Hsieh TY, Hsieh CW, Hung WT, Lan JL, and Chen DY (2015). A comparison of safety profiles of tumour necrosis factor alpha inhibitors and rituximab therapy in patients with rheumatoid arthritis and chronic hepatitis C. Ann Rheum Dis 74, 626–627. [DOI] [PubMed] [Google Scholar]
- 307.Arrese M, Arab JP, Barrera F, Kaufmann B, Valenti L, and Feldstein AE (2021). Insights into Nonalcoholic Fatty-Liver Disease Heterogeneity. Semin Liver Dis 41, 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mandlik DS, Mandlik SK, and Choudhary HB (2023). Immunotherapy for hepatocellular carcinoma: Current status and future perspectives. World J Gastroenterol 29, 1054–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Alegre ML, Frauwirth KA, and Thompson CB (2001). T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol 1, 220–228. [DOI] [PubMed] [Google Scholar]
- 310.Sharpe AH, and Pauken KE (2018). The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18, 153–167. [DOI] [PubMed] [Google Scholar]
- 311.Lin SZ, and Fan JG (2022). Peripheral immune cells in NAFLD patients: A spyhole to disease progression. EBioMedicine 75, 103768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Lombardi R, Piciotti R, Dongiovanni P, Meroni M, Fargion S, and Fracanzani AL (2022). PD-1/PD-L1 Immuno-Mediated Therapy in NAFLD: Advantages and Obstacles in the Treatment of Advanced Disease. Int J Mol Sci 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Albhaisi S, and Noureddin M (2021). Current and Potential Therapies Targeting Inflammation in NASH. Front Endocrinol (Lausanne) 12, 767314. [DOI] [PMC free article] [PubMed] [Google Scholar]


