Abstract
This study aimed to evaluate the potential effects of choline chloride: glycerol-based natural deep eutectic solvent (NADES) as a plasticizer, NADES extract (NADESext) of lavender as both plasticizer and active ingredient, as well as the lyophilized extract (LE) of lavender at different concentrations (0.5 %, 1 %, and 2 %) on the physical, mechanical, optical, thermal, barrier, morphological, and antioxidant properties of pectin films. The properties of the films were compared to those of the neat pectin film and the film plasticized with glycerol. The addition of plasticizers and LE increased thickness, water vapor permeability, and elongation at break values of the films while decreasing tensile strength and young modulus. Pectin films plasticized with glycerol, NADES, and NADESext had a similar color property but a lower opacity. The use of LE decreased lightness and increased opacity compared to the films with plasticizers. The addition of plasticizers revealed a smoother surface than neat pectin film while LE triggered the formation of agglomerates on the films. Changes in the FTIR spectra of the films showed some interactions between pectin and polyphenols in LE. The plasticizers had an insignificant effect on the antioxidant capacity of films whereas LE improved antioxidant capacity depending on the concentration. In conclusion, the results suggested that pectin films with NADES and LE could be beneficially used to improve antioxidant packaging technology along with acceptable mechanical properties.
Keywords: Pectin, Edible film, Natural deep eutectic solvent, Lavender, Antioxidant capacity
1. Introduction
Food packaging is a substantial part of the food supply chain. It is not only a container for food products but also a protective barrier [1] against physical, chemical, and biological hazards [2]. For this, food packaging systems are essential for sustaining food safety and quality during transportation, distribution, and storage. Depending on the characteristics of the food to be packaged, some materials such as glass, paper, metal, and plastic are commonly used for packaging applications [1]. Among these basic packaging materials, plastics are preferred for short-term or single-use packaging applications due to their cost-effectiveness, easy processing, and favorable physical and mechanical properties. However, the majority of plastic packaging materials are derived from non-renewable petroleum-based resources. After the consumption of food packed with plastic material, the remaining plastic packaging material becomes waste and causes serious environmental pollution due to their limited biodegradation and recycling [1,3]. Therefore, there have been growing concerns about using petroleum-based plastics to package food products. In this context, numerous studies have been conducted on the production of food packaging films produced by renewable biopolymers as an alternative to plastic films [4].
Polysaccharides, proteins, and lipids are the most common biomaterials for the production of edible films. These biopolymer materials are biodegradable, safe, and annually renewable. Also, many of the biopolymers can be valorized from agri-food industry wastes or by-products for edible film applications [5,6]. For example, pectin as a potential film-forming material is one of the most abundant biopolymers in food processing by-products such as citrus peels and apple pomace [7,8]. The use of pectin for the development of biodegradable films has been studied to replace non-biodegradable ones. However, the brittleness arising from the strong interaction among pectin molecules has caused significant limitations in the food application of pectin films. The use of plasticizers makes pectin films more flexible by reducing intermolecular forces and increasing molecular mobility [9,10].
Natural deep eutectic solvents (NADESs) are tailor-made solvents composed of a eutectic mixture of natural compounds. In food applications, they are extensively used in the field of extraction of bioactive compounds. Many studies have shown their excellent extraction ability for bioactive compounds [11]. Beyond being an effective extraction medium, NADESs are also promising natural plasticizers for biodegradable films. Nowadays, the use of NADES as an alternative plasticizer has heightened interest in the plasticization of biopolymer-based films [[12], [13], [14], [15], [16], [17], [18], [19]]. These studies have shown that NADESs as plasticizers have shown good compatibility with biodegradable film. However, information on the plasticization of pectin-based films using NADESs is still limited in the literature [[20], [21], [22]].
Apart from flexibility, another issue with pectin films is to combine the benefits of natural plant extracts with pectin films to develop active films. The incorporation of plant extracts provides superior antioxidant and antimicrobial properties to the films compared to neat pectin films [[23], [24], [25]]. Lavender (Lavandula angustifolia) is a medicinal and aromatic plant belonging to the Lamiaceae family. Lavender extracts are an excellent source of polyphenols [26] which contribute to their bioactive properties. Hence, lavender extracts could be a suitable candidate as a functional ingredient to produce active pectin film. Despite the effective extraction performances of NADES, on the other hand, recovery of bioactive compounds from NADES media by evaporation after extraction is a challenge due to their very low vapor pressure [27]. Recently, the direct use of NADES extract (NADESext) in edible film production has been proposed to overcome this drawback [14].
To the best of our knowledge, no research has been carried out on the use of aqueous and NADES extracts of lavender in the production of active pectin films. Therefore, the objectives of this study were to (i) test the plasticizer potential of NADES and NADESext in pectin films and (ii) examine the effects of the incorporation of the lyophilized extract (LE) from lavender at different concentrations (0.5 %, 1 %, and 2 % w/v) on pectin films plasticized with NADES using the solvent casting method. In this sense, the films were characterized in terms of their physical, mechanical, optical, thermal, barrier, morphological, and antioxidant properties.
2. Materials and methods
2.1. Materials and chemicals
The commercial high methoxyl apple pectin (degree of esterification = 66.58–68.52 %) was purchased from Tito (Smart Kimya, İzmir, Türkiye). Lavender (L. angustifolia) flowers were harvested from the Research and Application Field of the Faculty of Agricultural Sciences and Technologies at Nigde Omer Halisdemir University (Türkiye). The freshly harvested flowers were dried, ground, and sieved before the extractions. Choline chloride (ChCl) and glycerol (Gly) were purchased from Acros Organics (Belgium) and Tekkim (Türkiye). 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid) (ABTS), and 1,1-diphenyl-2-picryhydrazyl (DPPH) were acquired from Sigma-Aldrich (St. Louis, MO, USA).
2.2. NADES preparation
In the NADES preparation, ChCl and Gly were used as hydrogen bond acceptors and hydrogen bond donors, respectively. ChCl was dried at 45 °C for 24 h before use. ChCl and Gly were weighed at a molar ratio of 1:2. These components of NADES were placed into a flask at 70 °C with constant stirring until a transparent and homogenous liquid formed.
2.3. Extractions
NADESext from lavender flowers was prepared according to a previous method [28]. Briefly, NADESext was obtained using ultrasound-assisted extraction based on 33.5 % water content in NADES at 60 % ultrasound amplitude and 60 °C temperature with a time of 17.5 min and the liquid-to-solid ratio of 31.7 mL/g. After extraction, the extract was centrifuged for 10 min at 10,000×g. The supernatant was collected and stored at 4 °C in the dark.
Along with NADES extraction, ultrasound-assisted aqueous extraction was also done in the above-mentioned conditions using distilled water as an extraction solvent to produce lyophilized extract (LE) from lavender flowers. The supernatant phase was collected after centrifugation at 7500×g for 10 min. The water-soluble solid content of the resulting supernatant was 1.57 ± 0.06 %. The liquid extract was transformed to powder form using a freeze-drying process and used for active film production.
2.4. Preparation of pectin films
Film-forming aqueous solutions were prepared by dissolving 2.00 g pectin in 100 mL of distilled water. The mixture was kept stirring overnight at room temperature (∼25 °C) to allow the dissolution of pectin. Gly, NADES, and NADESext as a plasticizer at 30 parts per 100 parts of the pectin was added into solutions under stirring (600 rpm) at room temperature. In the production of LE-activated films, LE was added into the film-forming solution at different concentrations of 0.5 %, 1 %, and 2 % w/v of the aqueous solution with constant stirring (600 rpm) for 20 min at room temperature. NADES as a plasticizer was separately added to these solutions at 30 parts per 100 parts of the pectin. A total of seven different types of pectin-based formulations were prepared. The control film (Pec) formulation was prepared without the addition of extract or plasticizer. The other fabricated films were designated as Pec-Gly, Pec-NADES, and Pec-NADESext depending on the plasticizer type as well as Pec-NADES-0.5 %, Pec-NADES-1%, and Pec-NADES-2% depending on the LE concentration. Each film-forming solution (40 mL) was poured onto the dishes to control the initial thickness. The film-forming solutions were poured into square plastic Petri dishes (120 mm × 120 mm) and dried in a controlled test cabinet (TK 120, Nüve, Ankara, Türkiye) at a relative humidity (RH) of 50 % and a temperature of 25 °C for at least 72 h before further measurements were taken.
2.5. Characterization of films
2.5.1. Thickness
The thickness of the films was measured at least seven random points using a digital micrometer (Dasqua, Italy) with a sensitivity of 0.001 mm. The mean values were used to calculate mechanical, optical, and permeability values.
2.5.2. Moisture content
The moisture content of the films was determined gravimetrically in a hot air oven at 105 °C until constant weight. The initial weight (M0) of the films was measured. After drying, the film was weighed and its weight was recorded as M1. These values (M0 and M1) were used to calculate the moisture contents of films according to Eq. (1):
| (1) |
2.5.3. Mechanical properties
Tensile strength (TS), elongation at break (EAB), and young modulus (YM) were measured using a texture analyzer (TA-XT2i, Stable Micro Systems, Surrey, England) equipped with tensile test grips according to the standard method D882-10 [29]. Using a scalpel, the films were cut into rectangular specimens with 10 mm × 80 mm dimensions. The texture analyzer was operated with an initial grip of 60 mm and a cross-head speed of 0.1 mm/s. The YM was determined from the linear slope of the stress versus strain curves. The TS was calculated by dividing the maximum force (Fmax) by the initial cross-sectional area (A) (Eq. (2). Besides, EAB was calculated by Eq. (3).
| (2) |
| (3) |
where, ΔL (mm) and L0 (mm) were the change in length and the initial length of the film, respectively.
2.5.4. Optical properties
The color of the film samples was determined by measuring the CIELAB color parameters: L* (lightness), a* (red to green), and b* (yellow to blue) using a colorimeter (CR-400, Konica, Minolta, Japan). Before measurements, the colorimeter was standardized with a white calibration plate. The total color difference (ΔE), whiteness index (WI), and yellowness index (YI) were calculated using the following Eqs. (4), (5), (6):
| (4) |
| (5) |
| (6) |
where L*std, a*std, and b*std are values of the standard white plate and L*, a*, and b* are values of the film sample.
The light transmittance of the films (10 mm × 40 mm) was recorded at various wavelengths between 200 and 800 nm using a UV–vis spectrophotometer (Evolution 300, Thermo Scientific, Waltham, USA). An empty quartz cuvette was used as a reference. For the evaluation of the opacity values, the absorbance of films was recorded at 600 nm using the spectrophotometer (Evolution 300, Thermo Scientific) with an empty quartz cuvette as a reference. The opacity values were calculated by Eq. (7):
| (7) |
2.5.5. Water vapor permeability (WVP)
Water vapor permeability (WVP) of the pectin films was gravimetrically measured using the water method according to the standard method E 96-95 [30]. The circular shapes (Area = 0.016 m2) of films were sealed on test cups filled with distilled water to achieve 100 % RH. The test cells were placed in a controlled test cabinet (TK 120, Nüve) at 25 °C and 50 % RH. The test cups were weighed at specific time intervals (i.e. 1, 2, 3, 4, 5, 6, 12, 18, 24, 30, 36, and 48 h periods). The weight loss of the test cup was plotted as a function of time. The slope of the curves was calculated by linear regression. The water vapor transmission rate (WVTR, g m−2 day−1) was determined according to Eq. (8):
| (8) |
WVP (g s−1 m−1 Pa−1) was then calculated using Eq. (9):
| (9) |
where is the film thickness average (m), is the saturation vapor pressure at 25 °C, is the relative humidity in the test cups, is the relative humidity in the air-conditioned chamber.
2.5.6. Scanning electron microscopy (SEM)
The surface morphology of the film samples was analyzed using a scanning electron microscope (Zeiss Sigma 300 Field Emission SEM, Oberkochen, Germany). Before analysis, the films were freeze-dried for 48 h and then coated with a thin layer of gold to make them conductors. Images were taken at 10.00 kV voltage and 5.00 or 10.00 K × magnifications.
2.5.7. Fourier transform infrared (FTIR) spectroscopy
The chemical structure analysis of the film samples was done by an FTIR spectrophotometer (Vertex 70, Bruker Optics, Germany) with a 4 cm−1 resolution. The FTIR spectra were taken in the wavenumber range of 4000–500 cm−1.
2.5.8. X-ray diffraction (XRD) analysis
The XRD was used to investigate the structural changes of the films using the X-ray diffractometer (PANalytical Empyrean, Netherlands) with Cu Kα radiation source working at 40 kV and 40 mA in the 2θ range of 10°–40°.
2.5.9. Thermogravimetric analysis (TGA)
A thermogravimetric analyzer (STA TG-DSC/DTA PT1600, Linseis, Germany) was used to examine the thermal stability of the films. The samples were heated from 25 °C to 600 °C at a constant temperature rate of 10 °C/min under a nitrogen atmosphere. Derivative thermogravimetric (DTG) curves were the first derivative of the TGA curves.
2.5.10. Bioactive properties
To prepare the film extract solution, film samples (100 mg) were mixed with 10 mL distilled water in a test tube and extracted in a water bath for 2 h at 25 °C. After centrifugation at 3000×g for 10 min, the resulting supernatant was used for the antioxidant capacity assays.
For the ABTS assay [31], 7 mM ABTS radical solution containing 2.45 mM potassium persulfate was prepared and incubated in the dark at room temperature for 12–16 h. Then, this solution was diluted with phosphate buffer saline (PBS, pH 7.4) until its absorbance at 734 nm was 0.700 (±0.02). A 20 μL extract and 1.98 mL of the ABTS radical solution were mixed in the cuvette. After incubation in the dark at room temperature for 6 min, the absorbance value of the solution was read at 734 nm using a UV–vis spectrophotometer (Evolution 300, Thermo Scientific). The results were expressed as mg Trolox equivalent per gram (mg TE/g) of film.
In the DPPH assay [32], 10 μL of each film solution was uniformly mixed with 2.9 mL of 0.1 mM ethanolic DPPH• solution, followed by shaking for 10 s. Then, this mixture was incubated for 30 min at room temperature in the dark. After incubation, the mixture was centrifuged at 3000×g for 10 min, the resulting supernatant was used for the analysis. The absorbance value was recorded at a wavelength of 517 nm. The results were expressed as mg Trolox equivalent per gram (mg TE/g) of film.
2.6. Statistical analysis
The experiment was a completely randomized design. The measured values were expressed as mean ± standard deviation and each measurement was done minimally in triplicate. The film samples were defined as the independent variable and the analysis results as the dependent variable. Statistical evaluations were done by one-way analysis of variance (ANOVA), followed by Duncan's multiple comparison test using the SPSS 24 package program for Windows (Chicago, IL, USA). Statistical significance was defined at a 95 % confidence level.
3. Results and discussion
3.1. Physical properties
The thickness, moisture content, WVTR and WVP of the films are presented in Table 1. The thickness of pectin films increased with the presence of plasticizers, probably due to the reduction in interactions between the pectin-pectin chains. Pec-NADES and Pec-NADESext films were thicker than Pec-Gly films. A similar result was found by Yu et al. [16], who tested the change in the thickness of chitosan films using Gly and different NADES plasticizers. Although the thickness of the NADESext film was higher than that of NADES film, there was no statistically significant difference (p > 0.05). Similarly, Chandra Roy et al. [14] reported that chitosan films plasticized with NADESext containing astaxanthin were thicker than the films plasticized with pure NADES. This observation was attributed to bioactive compounds in the NADESext by the authors. As for the variations with the addition of LE, the thicker films were obtained by increasing the solid matter amount in the film solutions. As expected, the thickest film was Pec-NADES-2%. These findings agreed with previous studies [24,33,34] showing that the incorporation of plant extracts resulted in thicker films.
Table 1.
Physical and mechanical properties of pectin films.
| Films | Thickness (μm) | Moisture Content (%) | Water vapor transmission rate (g m−2 day−1) | Water vapor permeability (x 10−10 g m−1 s−1 Pa−1) | Tensile strength (MPa) | Elongation at break (%) | Young modulus (MPa) |
|---|---|---|---|---|---|---|---|
| Pec | 32.11 ± 1.18a | 11.43 ± 0.46a | 265.65 ± 10.98a | 0.62 ± 0.03a | 68.27 ± 2.50f | 3.31 ± 0.45a | 94.57 ± 2.75d |
| Pec-Gly | 41.88 ± 2.50b | 22.52 ± 0.94f | 422.81 ± 89.30c | 1.29 ± 0.27b | 32.41 ± 2.41d | 19.38 ± 2.48d | 11.16 ± 0.75b |
| Pec-NADES | 45.33 ± 2.79c | 19.08 ± 0.78e | 430.91 ± 13.99c | 1.43 ± 0.05b | 30.81 ± 0.88d | 16.29 ± 1.83c | 12.36 ± 1.67c |
| Pec-NADESext | 47.23 ± 3.37cd | 16.78 ± 0.79b | 396.83 ± 15.93bc | 1.37 ± 0.05b | 39.62 ± 2.11e | 14.77 ± 1.16b | 12.66 ± 1.07c |
| Pec-NADES-0.5 % | 49.75 ± 4.80d | 18.64 ± 0.86de | 370.42 ± 4.86bc | 1.35 ± 0.02b | 21.28 ± 2.95c | 20.59 ± 2.58de | 12.16 ± 0.90bc |
| Pec-NADES-1% | 65.38 ± 4.48e | 17.33 ± 0.54bc | 362.55 ± 27.11bc | 1.73 ± 0.13c | 18.85 ± 2.84b | 22.04 ± 1.69e | 11.14 ± 0.58b |
| Pec-NADES-2% | 82.46 ± 6.29f | 17.78 ± 0.79cd | 343.96 ± 2.84b | 2.07 ± 0.02d | 14.15 ± 1.64a | 25.37 ± 3.31f | 8.74 ± 0.58a |
Different lowercase letters indicate statistically significant differences (p < 0.05) in the properties of pectin films.
Besides, a significant variation (p < 0.05) of the moisture content was measured in pectin films as affected by plasticizer and LE (Table 1). The water content of the films increased with the addition of plasticizers and LE compared to the Pec film. The highest moisture content was measured in the Pec-Gly films which were in good agreement with a previous study on the plasticization of starch-based films that reported higher moisture content in the film plasticized with Gly than films plasticized with ChCl: oxalic acid and ChCl: ascorbic acid as NADES plasticizers [19]. Besides, Pec-NADESext films had a lower moisture content than Pec-NADES films. This was attributed to the presence of 33.5 % (v/v) water in NADESext when compared to pure NADES. Although both NADES and NADESext were used at the same concentration (30 parts per 100 parts of pectin) in the production of films, the use of water reduced the amount of NADES in NADESext. Since the water evaporated easily during drying, the Pec-NADESext film had a lower water content. With the addition of LE, the moisture content of the active films decreased when compared to the Pec-NADES film. This decrease was probably due to the affinity between the polyphenolic compounds in LE and the hydroxyl groups of pectin, which limits the interaction of hydroxyl groups with water molecules [35].
The WVTR and WVP values of pectin films ranged from 265.65 to 430.91 g m−2 day−1 and 0.62 to 2.07 × 10−10 g m−1 s−1 Pa−1, respectively· (Table 1.). The WVTR and WVP values of films increased when a plasticizer was added to the film matrix. It was reported that the WVP of pectin films can vary based on the type, hydrophilic properties, and molecular weights of the plasticizers used [36]. Plasticizers such as Gly reduce polymer-polymer interactions and increase molecular mobility. This facilitates the diffusion of water vapors and leads to an increase in WVP [37,38]. However, there were no significant (p > 0.05) differences among the WVP of the pectin films plasticized with Gly, NADES, and NADESext. In other words, the type of plasticizer had an insignificant effect (p > 0.05) on WVP. Similarly, Pereira & Andrade [39] reported an insignificant effect (p > 0.05) of Gly and ChCl: Gly (1:2) on the WVP of a composite film composed of chitosan, microcrystalline cellulose, and curcumin. An opposite finding was reported by Gouveia et al. [22] studying on plasticization of pectin films produced by thermo-compression molding using Gly and ChCl: Gly (1:2). The difference between the results of this study and those of Gouveia et al. [22] can be attributed to the different film-forming methods used to produce the pectin films. In addition to the plasticizers, the use of LE also had a significant effect (p < 0.05) on the WVP of the films. Pec-NADES and Pec-NADES-0.5 % films showed similar WVP values while the WVP of pectin films significantly (p < 0.05) increased after the addition of 1 % and 2 % LE. This increase was ascribed to the hydrophilicity of LE.
3.2. Mechanical properties
The effects of plasticizer and LE on the TS, EAB, and YM values are presented in Table 1. The results showed that the addition of plasticizers and LE into the pectin films had a significant effect (p < 0.05) on the mechanical properties of the films. Pec film showed a higher TS and YM as well as a lower EAB compared to other ones, which can be explained by the strong interaction between polymer chains. Incorporating the Gly, NADES, and NADESext into pectin films decreased the TS and YM values and increased the EAB values. A similar finding was reported by Zdanowicz & Johansson [40] for the native potato starch films prepared with Gly and ChCl: Gly when compared to starch films prepared without a plasticizer. It is well known that incorporating a plasticizer into the film-forming solution increases molecular mobility, resulting in greater flexibility and extensibility [12]. In agreement with our findings, a previous study reported that when NADES (ChCl: Gly) and NADESext containing green tea polyphenols at 30 % was used as a plasticizer in chitosan films, the TS decreased and the EAB increased when compared to neat chitosan film [15]. In the present study, NADESext displayed a higher TS and a lower EAB value than NADES. This can be explained by the water content in NADESext. NADESs have been recognized as highly efficient solvents for the extraction of bioactive compounds. Nonetheless, the intrinsic viscosity of NADES can significantly influence their extraction performance [28]. Hence, the addition of water is deemed necessary to mitigate and modulate their viscosity levels. The water content in NADESext was 33.5 % for the effective extraction of lavender phenolics, however, no water was added to pure NADES. Despite the constant plasticizer content in all film samples, the presence of water in NADESext could have potentially restricted its efficacy as a plasticizer. Moreover, it is important to mention that the incorporation of LE into the films plasticized with NADES led to subsequent decreases in the TS and YM values, accompanied by a gradual increase in the EAB value. Similar results were also reported by Han & Song [23] for the watermelon rind pectin films containing kiwifruit peel extract.
3.3. Optical properties
The color parameters and opacity values of the films are presented in Table 2. The addition of plasticizers including Gly, NADES, and NADESext into the films had an insignificant effect (p > 0.05) on the color parameters including L*, a*, b*, ΔE, WI, and YI of the films. Contrary to these findings, it was reported by Kyriakidou et al. [41] that ChCl: Gly at molar ratio of 1:11 and ChCl: Gly containing pomegranate peel phenolics had different effects on the color properties of chitosan films. However, the color properties of the films were highly dependent on the amount of LE incorporated into the film-forming solution. With increasing concentration of LE, the L* value of the films decreased, presenting a darker color, while the b* value was increased, reflecting the increases in yellowness of the films containing LE. Similar behavior was also observed for the WI and YI. These changes in the film colors can be attributed to the original color of the extracts. A similar trend for L* and b* values was observed by Go & Song [34] after the incorporation of rambutan peel extract into pectin films.
Table 2.
Optical properties of pectin films.
| Films | L* | a* | b* | ΔE | WI | YI | Opacity (Abs600/mm) |
|---|---|---|---|---|---|---|---|
| Pec | 93.23 ± 0.28d | −0.88 ± 0.04c | 4.94 ± 0.22a | 2.04 ± 0.34a | 94.35 ± 0.21d | 7.56 ± 0.35a | 1.82 ± 0.11e |
| Pec-Gly | 93.32 ± 0.45d | −0.91 ± 0.03c | 5.04 ± 0.12a | 2.05 ± 0.37a | 94.27 ± 0.14d | 7.71 ± 0.21a | 1.23 ± 0.08b |
| Pec-NADES | 93.28 ± 0.26d | −0.91 ± 0.01c | 5.09 ± 0.20a | 2.10 ± 0.28a | 94.21 ± 0.18d | 7.80 ± 0.31a | 1.25 ± 0.07b |
| Pec-NADESext | 93.33 ± 0.31d | −0.89 ± 0.01c | 5.10 ± 0.09a | 2.08 ± 0.21a | 94.22 ± 0.08d | 7.80 ± 0.13a | 1.12 ± 0.03a |
| Pec-NADES-0.5 % | 87.98 ± 0.16c | −1.69 ± 0.05a | 24.69 ± 0.19b | 22.14 ± 0.21b | 75.01 ± 0.19c | 40.09 ± 0.36b | 1.43 ± 0.07c |
| Pec-NADES-1% | 85.33 ± 0.87b | −1.23 ± 0.26b | 32.57 ± 1.98c | 30.45 ± 1.95c | 67.18 ± 1.97b | 54.54 ± 3.44c | 1.56 ± 0.09d |
| Pec-NADES-2% | 71.54 ± 0.54a | 6.95 ± 0.38d | 53.51 ± 0.72d | 55.56 ± 0.87d | 45.78 ± 0.74a | 106.87 ± 2.14d | 2.16 ± 0.05f |
Different lowercase letters indicate statistically significant differences (p < 0.05) in the properties of pectin films.
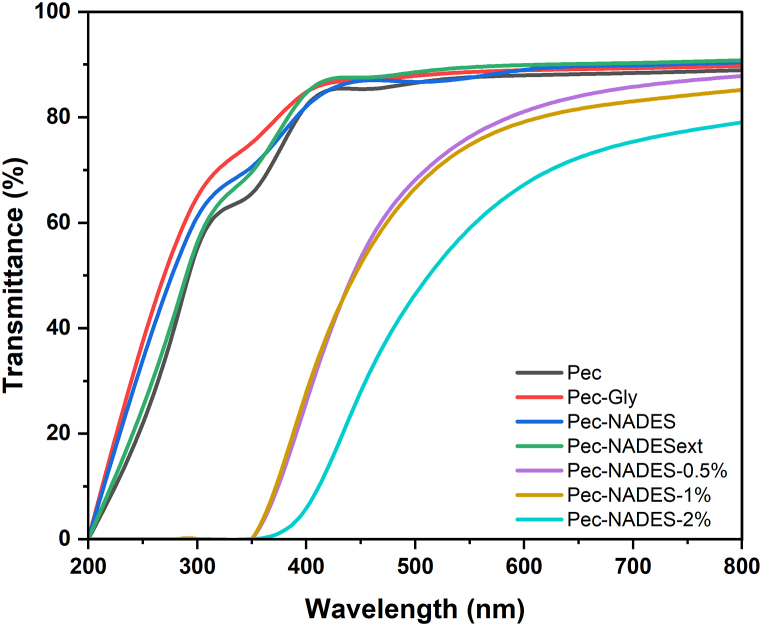
As for the light transmission characteristics, the opacity value of films significantly (p < 0.05) decreased after the addition of plasticizers (Table 2), which means that the films containing a plasticizer were more transparent. This was probably related to the dilution effect of plasticizers. On the other hand, the opacity value of films plasticized with NADES increased from 1.25 to 2.16 when different concentrations of LE were added, which can be related to the light absorption ability of the bioactive compounds in LE. A similar finding was also reported by Go & Song [34] for pectin films produced with rambutan peel extracts at different concentrations. Considering the light transmission spectra of the films (Fig. 1), it was observed that the use of LE dramatically reduced the transmission of UV light and visible light through the films. These findings indicated that pectin films with LE can be an excellent barrier to prevent lipid oxidation triggered by light.
Fig. 1.
Light transmission spectra of the pectin films.
3.4. SEM
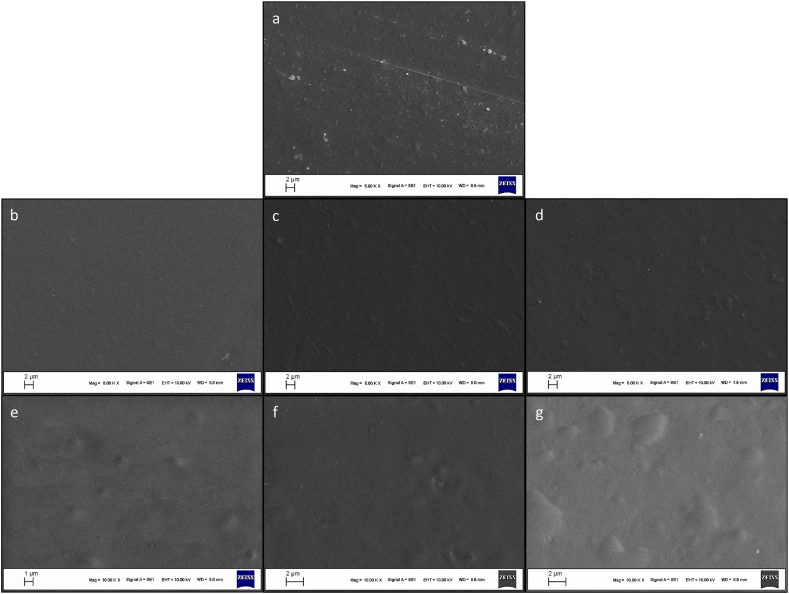
The SEM analysis was conducted to evaluate the surface structure of films at high magnification. The micrographs of the pectin film are presented in Fig. 2. There was no crack and phase separation in the surface areas of the films, suggesting a cohesive matrix. All film samples showed a continuous microstructure. The Pec film with a few solid particles had a rougher surface than the other films. Although some irregularities are observed on their surface, the images showed smoother surface morphology after the addition of plasticizers. Among the films added plasticizer, the use of Gly resulted in a smoother surface than NADES and NADESext. The addition of LE had an obvious effect on the surface structure of the film. The surface of the film containing LE displayed bulges, which could be due to the interaction between components in the LE and the pectin matrix. In general, SEM images of the films were indicative of compatible film structure when using NADES, NADESext, and LE.
Fig. 2.
SEM surface micrographs of (a) Pec, (b) Pec-Gly, (c) Pec-NADES, (d) Pec-NADESext, (e) Pec-NADES-0.5 %, (f) Pec-NADES-1%, (g) Pec-NADES-2%.
3.5. FT-IR analysis
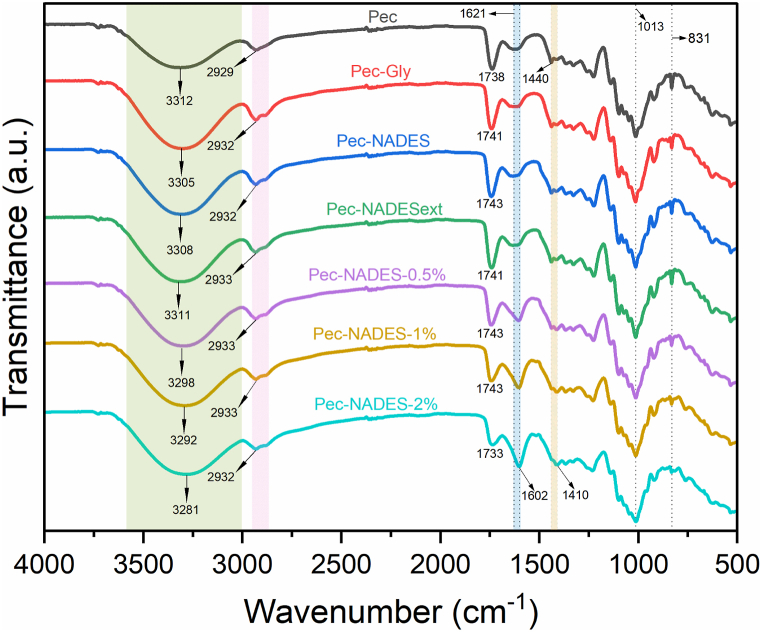
The FT-IR analysis was performed to evaluate the chemical structure of the films. The FT-IR spectra of pectin films are shown in Fig. 3. The spectrum of all pectin films displayed a broad absorption band from 3600 to 3000 cm−1, representing O–H stretching vibration [36]. The peak centered at 3312 cm−1 slightly broadened and shifted to a lower wavenumber with the addition of plasticizers and LE. This suggested that there were additional hydrogen bonds formed between the plasticizers and the LE and pectin matrix. The doublet peak around 2929 cm−1 corresponded to the C–H bond stretching vibrations of methyl and methylene groups in the films [24]. The peak around 1738 cm−1 was assigned to the C O stretching vibration of the methyl-esterified carbonyl groups (-COOCH3). Besides, the peaks at 1621 cm−1 and 1440 cm−1 represented asymmetric and symmetric stretching vibration of the free carboxyl (–COO−), respectively [42]. The intensity of the peak at 1738 cm−1 decreased with the incorporation of LE whereas the peak at 1621 cm−1 sharpened and shifted to 1602 cm−1. The changes in the peak intensities were proportional to the level of LE in the film matrix. This finding could be an indication of the de-methylation of carbonyl groups due to the interaction between the pectin chain and LE. This was supported by the increase in the intensity of the peak at 1621 cm−1, representing asymmetric stretching vibration of the free carboxyl. A similar observation was also reported by Han & Song [24] for pectin films containing sage leaf extract at different concentrations. In that study, higher peak intensity in the range of 1620–1631 cm−1 was attributed to the presence of amine and carbonyl groups in the sage leaf extract. In this study, the peak observed at 1013 cm−1 was attributed to the C–O–C stretching of the saccharide structure of the pectin [43]. Additionally, the peaks at 918 and 831 cm−1 were assigned to the absorption of d-glucopyranosyl and a-D- mannopyranose [44].
Fig. 3.
Fourier transforms infrared spectra of pectin films.
3.6. TGA
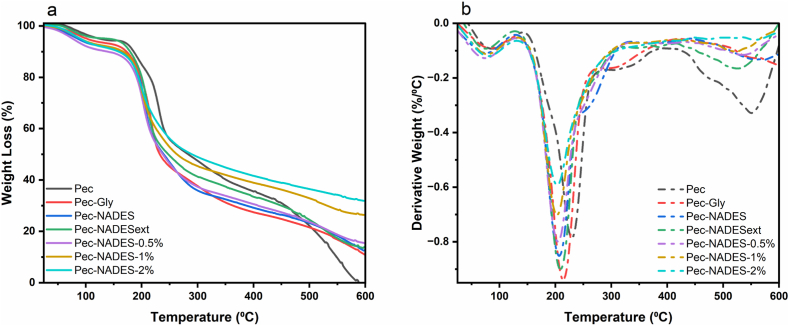
The thermal properties of the pectin films were evaluated by the TGA. The TGA and DTG curves are illustrated in Fig. 4. According to the TGA/DTG curves of pectin films, they displayed multiple stages of mass loss. The first stage occurred in the range of 41–143 °C due to the evaporation of free water, the bound water with pectin, and the loss of volatile compounds of LE. The subsequent weight loss that happened between 143 °C and 390 °C was attributed to the decomposition of plasticizers and the pectin backbone. The maximum weight loss temperature (Tmax) for Pec film was 229 °C. With the addition of plasticizers and LE, the Tmax of films moved to a lower temperature than that of Pec film. Tmax for Pec-Gly, Pec-NADES, Pec-NADESext, Pec-NADES-0.5 %, Pec-NADES-1%, and Pec-NADES-2% was 213 °C, 206 °C, 208 °C, 203 °C, 203 °C, and 203 °C, respectively. The results suggested that the use of NADES and NADESext as a plasticizer resulted in relatively lower thermal stability compared to Gly. A similar finding was also reported for chitosan films plasticized with ChCl: Gly as a NADES [16]. The addition of LE to Pec-NADES reduced the Tmax from 206 °C to 203 °C. However, increasing LE concentration did not affect the thermal properties of the films which suggests that depolymerizing occurred to a similar degree in these films. Besides, Pec film burned completely at 589 °C. The remaining weight was 10.57 %, 12.23 %, 13.20 %, 15.06 %, 25.98 %, and 31.43 % for Pec-Gly, Pec-NADES, Pec-NADESext, Pec-NADES-0.5 %, Pec-NADES-1%, and Pec-NADES-2% films, respectively. The final residual weight of the films containing 0.5 %, 1 %, and 2 % LE at 600 °C was higher than the other films. This observation could be attributed to the stable aromatic rings in the LE [24].
Fig. 4.
TGA (a) and DTG (b) curves of pectin films.
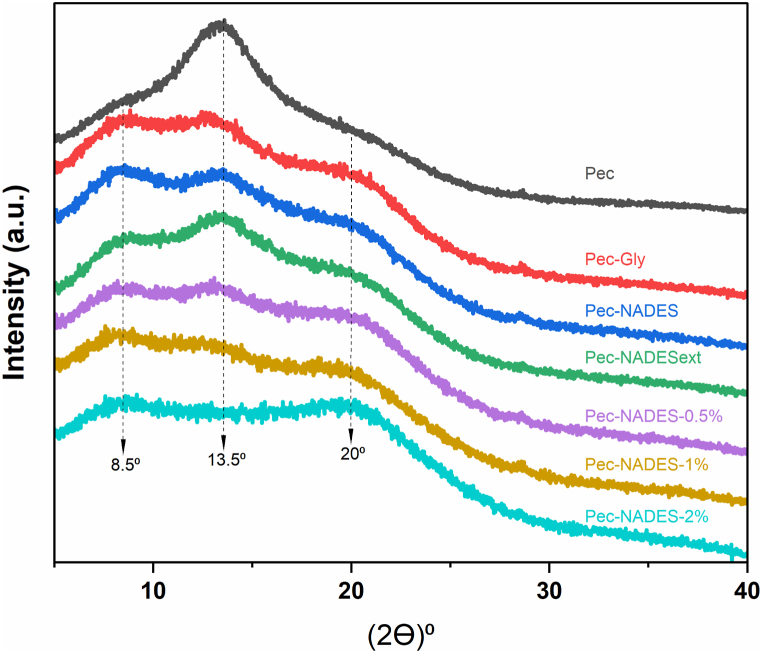
3.7. XRD analysis
The XRD patterns of the pectin films are presented in Fig. 5. The diffractograms of the films did not show sharp diffraction peaks while they displayed broad halo peaks. This indicated that all films had an amorphous structure and a low degree of crystallinity [45]. In Pec film, a broad diffraction peak was observed at 2θ ≈ 13.5°. In previous studies, it has been reported that pectin films have a characteristic peak at 12.4° [46], 15.72° [47], and 22.4° [48]. After adding the plasticizers, the intensity of the peak at 2θ ≈ 13.5° decreased. Moreover, the use of plasticizers caused the appearance of two new broad peaks at 2θ ≈ 8.5° and ≈ 20°. This observation could be related to molecular interactions between the pectin molecules and the plasticizers which weakened the hydrogen bonding between the pectin-pectin chains. In general, the diffraction pattern of the films produced using a plasticizer was similar to each other. On the other hand, the addition of LE at 0.5 % level showed a greater reduction in the intensity of the peak at 2θ ≈ 13.5° compared to pectin films produced without LE. A similar finding was reported in the study of Marangoni Júnior et al. [49] where an active pectin film was developed using green propolis extract. Moreover, this broad peak disappeared when the LE concentration increased up to 2 %. This observation showed that the films prepared with LE were more amorphous than the films without LE addition. Although some changes in peaks were detected, the incorporation of both plasticizers and LE into the pectin matrix did not affect its amorphous character. The results from the XRD analysis were in line with the results from the mechanical analysis showing that films containing plasticizers and LE with a lower TS and YM and a higher EAB (Table 1).
Fig. 5.
XRD patterns of pectin films.
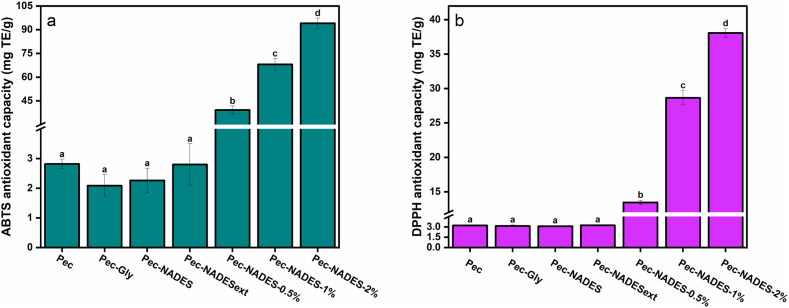
3.8. Antioxidant capacity
The antioxidant capacity of the pectin film was evaluated using ABTS and DPPH assays and the results are shown in Fig. 6. All films demonstrated an inhibitory effect against the radicals in both assays. There were no significant differences (p > 0.05) among the antioxidant capacities of Pec, Pec-Gly, Pec-NADES, and Pec-NADESext films. Similarly, an insignificant difference in the antioxidant capacity between neat pectin film and the pectin film plasticized with Gly was measured in the study of Meerasri & Sothornvit [50]. In a study by Yu et al. [16], in which the effects of deep eutectic solvent type and concentration on the antioxidant capacity of chitosan films were investigated, it was reported a slight decrease in the antioxidant capacity of the chitosan films when ChCl: Gly and Gly were used as plasticizers whereas ChCl: acetylsalicylic acid and ChCl: vitamin C displayed superior antioxidant capacities. Besides, the authors reported that increasing the concentration of ChCl: acetylsalicylic acid and ChCl: vitamin C from 20 % to 60 % (w/w) improved ABTS scavenging activity and Fe3+ reducing ability of the films [16]. In another study, Prajapati & Jadeja [51] performed NADES extraction of betanin from red dragon fruit peel using threedeep eutectic solvents including ChCl: urea, ChCl: Gly, and ChCl: ethylene glycol, and the resulting extracts were incorporated into the chitosan films as plasticizers and sources of antioxidant compounds. In that study, Prajapati & Jadeja [51] measured an insignificant effect on the antioxidant capacities of the films when compared to the control film as observed in the present study. In this study, statistically insignificant results observed in the antioxidant capacity of the films with the use of NADES and NADESext can be attributed to the type of the utilized NADES and the concentration of NADESext.
Fig. 6.
ABTS (a) and DPPH (b) antioxidant capacities of pectin films. (Different lowercase letters indicate statistically significant (p < 0.05) differences.).
As for the effect of LE addition, the inclusion of LE into the film matrix significantly (p < 0.05) increased the antioxidant capacity. Furthermore, the incorporation of LE showed a concentration-dependent increase in the antioxidant capacity of the films. As expected, the greatest antioxidant capacity was determined in the Pec-NADES-2% film. The improved antioxidant capacity of pectin films added with the LE was attributed to phenolic compounds in the aqueous extract of lavender [28]. These results were in line with those of previous studies [23,34,49] where it was reported the excellent antioxidant capacity of the pectin films was due to the incorporation of natural extracts. According to our findings, the LE from lavender could be a promising antioxidant source for the production of bioactive pectin films plasticized with NADES.
4. Conclusion
In this study, pectin films were fabricated with Gly, NADES, and NADESext as plasticizers. Additionally, LE at different concentrations was incorporated into pectin films plasticized with NADES to produce antioxidant films. The plasticizers including Gly, NADES, and NADESext increased thickness and water vapor permeability and decreased opacity. NADES and NADESext displayed the plasticizer effect by improving the flexibility of the films, which is important for packaging applications. Furthermore, the incorporation of LE resulted in a further increase in flexibility. On the other hand, the addition of NADESext to the film solution had an insignificant effect on the antioxidant capacity of the films when used both as a plasticizer and a source of bioactive compounds. However, the use of LE significantly improved the antioxidant capacity of the films plasticized with NADES. According to our results, NADES composed of ChCl: Gly can be used as a plasticizer to enhance the mechanical properties of pectin films. The results of this study can be utilized for further applications of antioxidant pectin films in the packaging industry.
Funding statement
This study was supported by the Unit of the Scientific Research Projects of Nigde Omer Halisdemir University (TGT2021/2-LUTEP).
CRediT authorship contribution statement
Hamza Alasalvar: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. Zeliha Yildirim: Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing, Resources. Metin Yildirim: Methodology, Resources, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Rhim J.W., Park H.M., Ha C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013;38:1629–1652. doi: 10.1016/J.PROGPOLYMSCI.2013.05.008. [DOI] [Google Scholar]
- 2.Khezerlou A., Zolfaghari H., Banihashemi S.A., Forghani S., Ehsani A. Plant gums as the functional compounds for edible films and coatings in the food industry: a review. Polym. Adv. Technol. 2021;32:2306–2326. doi: 10.1002/PAT.5293. [DOI] [Google Scholar]
- 3.Mohamed S.A.A., El-Sakhawy M., El-Sakhawy M.A.M. Polysaccharides, protein and lipid -based natural edible films in food packaging: a review. Carbohydr. Polym. 2020;238 doi: 10.1016/J.CARBPOL.2020.116178. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira-Costa B.E., Andrade C.T. Natural polymers used in edible food packaging—history, function and application trends as a sustainable alternative to synthetic plastic. Polysaccharides. 2022;3:32–58. doi: 10.3390/POLYSACCHARIDES3010002. 3 (2021) 32–58. [DOI] [Google Scholar]
- 5.Karimi Sani I., Masoudpour-Behabadi M., Alizadeh Sani M., Motalebinejad H., Juma A.S.M., Asdagh A., Eghbaljoo H., Khodaei S.M., Rhim J.W., Mohammadi F. Value-added utilization of fruit and vegetable processing by-products for the manufacture of biodegradable food packaging films. Food Chem. 2023;405 doi: 10.1016/J.FOODCHEM.2022.134964. [DOI] [PubMed] [Google Scholar]
- 6.Bayram B., Ozkan G., Kostka T., Capanoglu E., Esatbeyoglu T. Valorization and application of fruit and vegetable wastes and by-products for food packaging materials. Mol. 2021;26:4031. doi: 10.3390/MOLECULES26134031. 26 (2021) 4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva M.A., Bierhalz A.C.K., Kieckbusch T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009;77:736–742. doi: 10.1016/J.CARBPOL.2009.02.014. [DOI] [Google Scholar]
- 8.Mao G., Wu D., Wei C., Tao W., Ye X., Linhardt R.J., Orfila C., Chen S. Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: targeting rhamnogalacturonan I. Trends Food Sci. Technol. 2019;94:65–78. doi: 10.1016/J.TIFS.2019.11.001. [DOI] [Google Scholar]
- 9.Giancone T., Torrieri E., Di Pierro P., Cavella S., Giosafatto C.V.L., Masi P. Effect of surface density on the engineering properties of high methoxyl pectin-based edible films. Food Bioprocess Technol. 2011;4:1228–1236. doi: 10.1007/S11947-009-0208-9. [DOI] [Google Scholar]
- 10.Zhang P., Zhao Y., Shi Q. Characterization of a novel edible film based on gum ghatti: effect of plasticizer type and concentration. Carbohydr. Polym. 2016;153:345–355. doi: 10.1016/J.CARBPOL.2016.07.082. [DOI] [PubMed] [Google Scholar]
- 11.Mišan A., Nađpal J., Stupar A., Pojić M., Mandić A., Verpoorte R., Choi Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020;60:2564–2592. doi: 10.1080/10408398.2019.1650717. [DOI] [PubMed] [Google Scholar]
- 12.Grala D., Biernacki K., Freire C., Kuźniarska-Biernacka I., Souza H.K.S., Gonçalves M.P. Effect of natural deep eutectic solvent and chitosan nanoparticles on physicochemical properties of locust bean gum films. Food Hydrocoll. 2022;126 doi: 10.1016/J.FOODHYD.2021.107460. [DOI] [Google Scholar]
- 13.Zhang W., Shen J., Gao P., Jiang Q., Xia W. Sustainable chitosan films containing a betaine-based deep eutectic solvent and lignin: Physicochemical, antioxidant, and antimicrobial properties. Food Hydrocoll. 2022;129 doi: 10.1016/J.FOODHYD.2022.107656. [DOI] [Google Scholar]
- 14.Chandra Roy V., Ho T.C., Lee H.J., Park J.S., Nam S.Y., Lee H., Getachew A.T., Chun B.S. Extraction of astaxanthin using ultrasound-assisted natural deep eutectic solvents from shrimp wastes and its application in bioactive films. J. Clean. Prod. 2021;284 doi: 10.1016/J.JCLEPRO.2020.125417. [DOI] [Google Scholar]
- 15.Alves T.F.P., Teixeira N., Vieira J., Vicente A.A., Mateus N., de Freitas V., Souza H.K.S. Sustainable chitosan packaging films: Green tea polyphenolic extraction strategies using deep eutectic solvents. J. Clean. Prod. 2022;372 doi: 10.1016/J.JCLEPRO.2022.133589. [DOI] [Google Scholar]
- 16.Yu J., Xu S., Goksen G., Yi C., Shao P. Chitosan films plasticized with choline-based deep eutectic solvents: UV shielding, antioxidant, and antibacterial properties. Food Hydrocoll. 2023;135 doi: 10.1016/J.FOODHYD.2022.108196. [DOI] [Google Scholar]
- 17.Galvis-Sánchez A.C., Castro M.C.R., Biernacki K., Gonçalves M.P., Souza H.K.S. Natural deep eutectic solvents as green plasticizers for chitosan thermoplastic production with controlled/desired mechanical and barrier properties. Food Hydrocoll. 2018;82:478–489. doi: 10.1016/J.FOODHYD.2018.04.026. [DOI] [Google Scholar]
- 18.Zhao P., Wang J., Yan X., Cai Z., Fu L., Gu Q., Liu L., Jin H., Fu Y. Functional chitosan/zein films with Rosa roxburghii Tratt leaves extracts prepared by natural deep eutectic solvents. Food Packag. Shelf Life. 2022;34 doi: 10.1016/J.FPSL.2022.101001. [DOI] [Google Scholar]
- 19.de Sousa A.S.B., Lima R.P., da Silva M.C.A., Moreira D. das N., Pintado M.M.E., Silva S. de M. Natural deep eutectic solvent of choline chloride with oxalic or ascorbic acids as efficient starch-based film plasticizers. Polymer (Guildf) 2022;259 doi: 10.1016/J.POLYMER.2022.125314. [DOI] [Google Scholar]
- 20.Shafie M.H., Yusof R., Samsudin D., Gan C.Y. Averrhoa bilimbi pectin-based edible films: Effects of the linearity and branching of the pectin on the physicochemical, mechanical, and barrier properties of the films. Int. J. Biol. Macromol. 2020;163:1276–1282. doi: 10.1016/J.IJBIOMAC.2020.07.109. [DOI] [PubMed] [Google Scholar]
- 21.Shafie M.H., Yusof R., Naharudin I., Wong T.W., Zafarina Z., Gan C.Y. Effect of different molar ratios of choline chloride–citric acid monohydrate in deep eutectic solvents as plasticizers for Averrhoa bilimbi pectin films. J. Food Meas. Char. 2022;16:3832–3843. doi: 10.1007/S11694-022-01479-Y. [DOI] [Google Scholar]
- 22.Gouveia T.I.A., Biernacki K., Castro M.C.R., Gonçalves M.P., Souza H.K.S. A new approach to develop biodegradable films based on thermoplastic pectin. Food Hydrocoll. 2019;97 doi: 10.1016/j.foodhyd.2019.105175. [DOI] [Google Scholar]
- 23.Han H.S., Bin Song K. Antioxidant properties of watermelon (Citrullus lanatus) rind pectin films containing kiwifruit (Actinidia chinensis) peel extract and their application as chicken thigh packaging. Food Packag. Shelf Life. 2021;28 doi: 10.1016/J.FPSL.2021.100636. [DOI] [Google Scholar]
- 24.Han H.S., Bin Song K. Antioxidant activities of Mandarin (Citrus unshiu) peel pectin films containing sage (Salvia officinalis) leaf extract. Int. J. Food Sci. Technol. 2020;55:3173–3181. doi: 10.1111/IJFS.14581. [DOI] [Google Scholar]
- 25.Shivangi S., Dorairaj D., Negi P.S., Shetty N.P. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021;121 doi: 10.1016/J.FOODHYD.2021.107046. [DOI] [Google Scholar]
- 26.Héral B., Stierlin É., Fernandez X., Michel T. Phytochemicals from the genus Lavandula: a review. Phytochemistry Rev. 2020:1–21. doi: 10.1007/s11101-020-09719-z. [DOI] [Google Scholar]
- 27.Ruesgas-Ramón M., Figueroa-Espinoza M.C., Durand E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017;65:3591–3601. doi: 10.1021/ACS.JAFC.7B01054. [DOI] [PubMed] [Google Scholar]
- 28.Alasalvar H., Yildirim Z. Ultrasound-assisted extraction of antioxidant phenolic compounds from Lavandula angustifolia flowers using natural deep eutectic solvents: an experimental design approach. Sustain. Chem. Pharm. 2021;22 doi: 10.1016/J.SCP.2021.100492. [DOI] [Google Scholar]
- 29.ASTM . Annu. B. ASTM Stand. American Society for Testing and Materials; West Conshohocken, PA: 2010. Standard test method for tensile properties of thin plastic sheeting. [DOI] [Google Scholar]
- 30.ASTM . Annu. B. ASTM Stand. American Society for Testing and Materials; West Conshohocken, PA: 1995. Standard test method for water vapor transmission of materials. [Google Scholar]
- 31.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 32.Görgüç A., Bircan C., Yılmaz F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019;283:637–645. doi: 10.1016/j.foodchem.2019.01.077. [DOI] [PubMed] [Google Scholar]
- 33.Kumar N., Pratibha, Trajkovska Petkoska A., Khojah E., Sami R., Al-Mushhin A.A.M. Chitosan edible films enhanced with pomegranate peel extract: study on physical, biological, thermal, and barrier properties. Materials. 2021;14:3305. doi: 10.3390/MA14123305. 14 (2021) 3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go E.J., Bin Song K. Development and characterization of Citrus junos pomace pectin films incorporated with rambutan (Nephelium Lappaceum) peel extract. Coatings. 2020;10 doi: 10.3390/COATINGS10080714. [DOI] [Google Scholar]
- 35.Nguyen T.T., Thi Dao U.T., Thi Bui Q.P., Bach G.L., Ha Thuc C.N., Ha Thuc H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog. Org. Coating. 2020;140 doi: 10.1016/J.PORGCOAT.2019.105487. [DOI] [Google Scholar]
- 36.Pasini Cabello S.D., Takara E.A., Marchese J., Ochoa N.A. Influence of plasticizers in pectin films: Microstructural changes. Mater. Chem. Phys. 2015;162:491–497. doi: 10.1016/J.MATCHEMPHYS.2015.06.019. [DOI] [Google Scholar]
- 37.Ghasemlou M., Khodaiyan F., Oromiehie A. Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr. Polym. 2011;84:477–483. doi: 10.1016/J.CARBPOL.2010.12.010. [DOI] [Google Scholar]
- 38.Rodríguez M., Osés J., Ziani K., Maté J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Res. Int. 2006;39:840–846. doi: 10.1016/J.FOODRES.2006.04.002. [DOI] [Google Scholar]
- 39.Pereira P.F., Andrade C.T. Optimized pH-responsive film based on a eutectic mixture-plasticized chitosan. Carbohydr. Polym. 2017;165:238–246. doi: 10.1016/J.CARBPOL.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 40.Zdanowicz M., Johansson C. Mechanical and barrier properties of starch-based films plasticized with two- or three component deep eutectic solvents. Carbohydr. Polym. 2016;151:103–112. doi: 10.1016/J.CARBPOL.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 41.Kyriakidou A., Makris D.P., Lazaridou A., Biliaderis C.G., Mourtzinos I. Physical properties of chitosan films containing pomegranate peel extracts obtained by deep eutectic solvents. Foods. 2021;10:1262. doi: 10.3390/FOODS10061262. 10 (2021) 1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.jie Meng Y., ya Wang S., wei Guo Z., mei Cheng M., Li J., qiang Li D. Design and preparation of quaternized pectin-Montmorillonite hybrid film for sustained drug release. Int. J. Biol. Macromol. 2020;154:413–420. doi: 10.1016/J.IJBIOMAC.2020.03.140. [DOI] [PubMed] [Google Scholar]
- 43.Jahromi M., Niakousari M., Golmakani M.T., Mohammadifar M.A. Physicochemical and structural characterization of sodium caseinate based film-forming solutions and edible films as affected by high methoxyl pectin. Int. J. Biol. Macromol. 2020;165:1949–1959. doi: 10.1016/J.IJBIOMAC.2020.10.057. [DOI] [PubMed] [Google Scholar]
- 44.Younis H.G.R., Zhao G. Physicochemical properties of the edible films from the blends of high methoxyl apple pectin and chitosan. Int. J. Biol. Macromol. 2019;131:1057–1066. doi: 10.1016/J.IJBIOMAC.2019.03.096. [DOI] [PubMed] [Google Scholar]
- 45.Hosseini S., Parastouei K., Khodaiyan F. Simultaneous extraction optimization and characterization of pectin and phenolics from sour cherry pomace. Int. J. Biol. Macromol. 2020;158:911–921. doi: 10.1016/J.IJBIOMAC.2020.04.241. [DOI] [PubMed] [Google Scholar]
- 46.Norcino L.B., Mendes J.F., Natarelli C.V.L., Manrich A., Oliveira J.E., Mattoso L.H.C. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. 2020;106 doi: 10.1016/J.FOODHYD.2020.105862. [DOI] [Google Scholar]
- 47.Nisar T., Wang Z.C., Yang X., Tian Y., Iqbal M., Guo Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018;106:670–680. doi: 10.1016/J.IJBIOMAC.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 48.Almasi H., Azizi S., Amjadi S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020;99 doi: 10.1016/J.FOODHYD.2019.105338. [DOI] [Google Scholar]
- 49.Marangoni Júnior L., Gonçalves S. de Á., da Silva R.G., Martins J.T., Vicente A.A., Alves R.M.V., Vieira R.P. Effect of green propolis extract on functional properties of active pectin-based films. Food Hydrocoll. 2022;131 doi: 10.1016/J.FOODHYD.2022.107746. [DOI] [Google Scholar]
- 50.Meerasri J., Sothornvit R. Characterization of bioactive film from pectin incorporated with gamma-aminobutyric acid. Int. J. Biol. Macromol. 2020;147:1285–1293. doi: 10.1016/J.IJBIOMAC.2019.10.094. [DOI] [PubMed] [Google Scholar]
- 51.Prajapati R.A., Jadeja G.C. Optimization of ultrasound-assisted deep eutectic solvent extraction of betanin and its application in chitosan-based biofilm. Biomass Convers. Biorefinery. 2023 doi: 10.1007/s13399-023-03808-7. [DOI] [Google Scholar]