Abstract
This scoping review focuses on the essential role of models for causal inference in shaping actionable artificial intelligence (AI) designed to aid clinicians in decision-making. The objective was to identify and evaluate the reporting quality of studies introducing models for causal inference in intensive care units (ICUs), and to provide recommendations to improve the future landscape of research practices in this domain. To achieve this, we searched various databases including Embase, MEDLINE ALL, Web of Science Core Collection, Google Scholar, medRxiv, bioRxiv, arXiv, and the ACM Digital Library. Studies involving models for causal inference addressing time-varying treatments in the adult ICU were reviewed. Data extraction encompassed the study settings and methodologies applied. Furthermore, we assessed reporting quality of target trial components (i.e., eligibility criteria, treatment strategies, follow-up period, outcome, and analysis plan) and main causal assumptions (i.e., conditional exchangeability, positivity, and consistency). Among the 2184 titles screened, 79 studies met the inclusion criteria. The methodologies used were G methods (61%) and reinforcement learning methods (39%). Studies considered both static (51%) and dynamic treatment regimes (49%). Only 30 (38%) of the studies reported all five target trial components, and only seven (9%) studies mentioned all three causal assumptions. To achieve actionable AI in the ICU, we advocate careful consideration of the causal question of interest, describing this research question as a target trial emulation, usage of appropriate causal inference methods, and acknowledgement (and examination of potential violations of) the causal assumptions.
Subject terms: Machine learning, Statistical methods, Epidemiology
Introduction
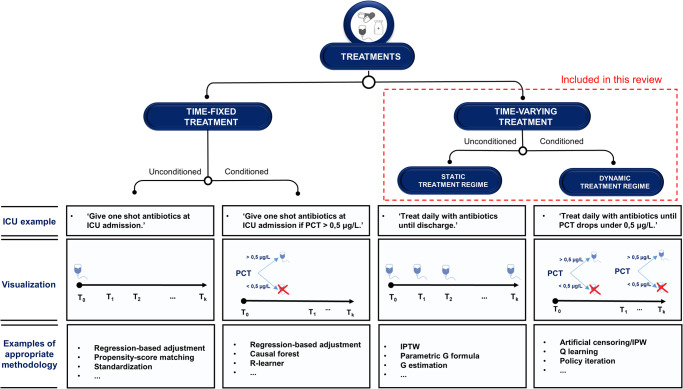
Many treatment choices in the intensive care unit (ICU) are made quickly, based on patient characteristics that are changing and monitored in real-time. Given this dynamic and data-rich environment, the ICU is pre-eminently a place where artificial intelligence (AI) holds the promise to aid clinical decision making1–3. So far, however, most AI models developed for the ICU remain within the prototyping phase4,5. One explanation for this may be that most models in the ICU are built for the task of prediction, i.e., mapping input data to (future) patient outcomes6. However, even a very accurate prediction of, for instance, sepsis7, does not tell a physician what to do in order to treat or prevent it. In other words, predictive AI is seldom actionable. More meaningful decision support in the ICU could be provided by models that assists clinicians in what to do (i.e., ‘actionable AI’8,9). To develop actionable AI, one needs to take into account cause and effect. Causal inference (CI) represents the task of estimating causal effects by comparing patient outcomes under multiple counterfactual treatments6,10. The most widely used method for CI is a randomized controlled trial (RCT). Through randomization (coupled with full compliance), the difference in outcome between treatment groups can be interpreted as a causal treatment effect. Because carrying out RCTs may be infeasible due to cost, time, and ethical constraints, observational studies are sometimes the only alternative. CI using observational data can be seen as an attempt to emulate the RCT that would have answered the question of interest (i.e., the ‘target trial’)11. With such an approach, however, treatment is not assigned randomly and extra adjustment for confounding is required. In the simple situation of a time-fixed (or ‘point’) treatment (Fig. 1, Box 1)12,13, this can be achieved by ‘standard methods’ like regression or propensity-score (PS) analyses14. However, ICU physicians are typically confronted with treatment decisions which occur at multiple time-points, i.e., time-varying treatments (Fig. 1, Box 1)12,13. Estimating the effect of time-varying treatments using observational data is often complicated by treatment-confounder feedback15, leading to ‘treatment-affected time-varying confounding’ (TTC, Box 1)13,16,17. Usage of standard methods in the presence of TTC leads to bias18,19. Inverse-probability-of-treatment weighting (IPTW), the parametric G formula and G estimation (collectively known as ‘G methods’, Box 1) were introduced by Robins20 to estimate causal effects in the presence of TTC, making them well-suited for CI in the ICU. Time-fixed treatments can be either unconditioned (e.g., ‘treat all patients at admission’) or conditioned on one or more covariates to make these more individualized (Fig. 1). Likewise, time-varying treatments can be either unconditioned, or tailored to more specific patient groups based on one or more (time-varying) covariates. We refer to these as static treatment regimes (STRs) and dynamic treatment regimes (DTRs), respectively (Fig. 1, Box 1)12. The latter type is most common in the ICU, as treatment choices are typically dynamically re-evaluated based on the evolving patient state. For example, rather than deciding upon admission to administer vasopressors daily, an ICU physician reconsiders giving this treatment throughout the ICU stay based on the patient’s response. Hence, the practical question of interest often requires a comparison of DTRs. Reinforcement learning (RL)21 is another class of methods which, like G methods, can be used to estimate optimal DTRs and have been increasingly applied to ICU data22. Partly due to the different language used to describe similar concepts (see Supplementary Table 1), studies applying G methods and RL may appear as completely separate disciplines. However, they show great similarities and can be used to build actionable AI models. The aim of this scoping review is to (1) outline how CI research is conducted concerning time-varying treatments in the ICU, (2) discuss quality of reporting, and (3) give recommendations to improve future research practice.
Fig. 1. Taxonomy of treatment types.
Treatments can be time-fixed or time-varying, and both these variants can be unconditioned, or conditioned on one or more (static and/or time-varying) patient characteristics, i.e., more ‘individualized’. Which methodology is appropriate to estimate causal effects of treatment using observational data, depends on the treatment type. The bottom row contains examples of some (not all) methodologies which could be used for the corresponding treatment type. Even when an appropriate method is used, satisfaction of the three causal assumptions (and hence, unbiased causal estimates) is not guaranteed. ICU intensive care unit, IPTW inverse-probability-of-treatment weighting, IPW inverse-probability weighting, PCT procalcitonin.
Box 1 Causal inference glossary.
Time-fixed treatment: a treatment that only occurs at the start of follow-up, or does not change over time.
Time-varying treatment: any treatment that is not time-fixed. Time-varying treatments can be sub-divided in static and dynamic treatment regimes.
Static treatment regime (STR): a treatment regime that is not tailored to evolving patient characteristics.
Dynamic treatment regime (DTR): a treatment regime where the treatment decisions depend on changing patient characteristics and/or treatment history.
Treatment-affected time-varying confounding (TTC): time-varying confounding in which one or more time-varying confounders are affected by previous treatment.
G methods: a class of methods proposed to adjust for TTC in the estimation of time-varying-treatment effects, including inverse-probability-of-treatment weighting, the parametric G formula, and G estimation.
Reinforcement learning (RL): a class of methods that deals with the problem of sequential decision making which returns an optimal treatment regime (or ‘policy’).
Off-policy evaluation (OPE): the task of estimating the value of a certain policy, using data from settings where patients were treated according to a different policy (i.e., observational data).
- Causal assumptions:
- • Conditional exchangeability: Exchangeability means equal risks in treated and untreated groups if patients in the untreated group were treated, and vice versa. Observational data often violates exchangeability due to confounding and/or selection bias, and, therefore, causal inference requires the assumption that all confounders are measured and adjusted for, to achieve exchangeability conditional on these confounders.
- • Positivity: To estimate a treatment’s causal effect, one must compare treated and untreated patient data. This requires having both treated and untreated patients in all subgroups (or ‘strata’) defined by different confounder values. In other words, treatment and non-treatment should occur in all confounder strata with some positive probability.
- • Consistency: Consistency assumes that the outcome for a given treatment will be the same, irrespective of the way treatments are ‘assigned’.
Results
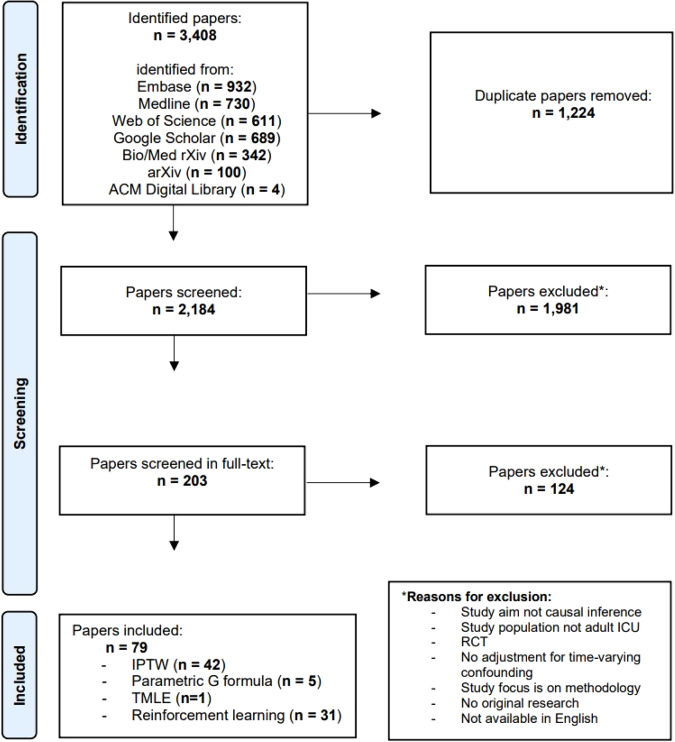
Through our search, we discovered 2184 articles that were unique. During the independent screening of titles and abstracts, we achieved a high agreement rate of 93%. Following the resolution of any disagreements, we excluded 1981 studies. Moving forward, we conducted independent full-text screening for the remaining 203 articles, resulting in an agreement rate of 86%. Once a consensus on eligibility was reached, we included 79 studies in the review (Fig. 2). The articles were published between 2005 and 2023, with a steadily growing number of articles per year starting around 2010 (Supplementary Fig. 1a). A reference list of all included studies and the list with collected items per study can be found in the Supplementary References and supplementary Table 2, respectively. Most studies applied G methods (n = 48, 61%), of which 42 (53%) used IPTW, five (6%) used the parametric G formula. One (1%) study23 used targeted minimum loss-based estimation (TMLE), a method which combines elements of IPTW and the parametric G formula24. 31 (39%) studies used RL methods (Table 1). The three most frequently studied treatment categories were anti-inflammatory drugs (n = 9, 11%), hospital-acquired complications (n = 8, 10%), and sedatives and analgesics (n = 8, 10%). Most studies (n = 46, 58%) considered mortality (at varying follow-up times) as the primary outcome. Thirty-six studies (46%) included data from at least two different ICUs. Studies that used RL generally included more patients than studies that used G methods, with a median of 9782 patients (IQR 5022–17,898) versus 1498 (IQR 606–3407) and relied more often on open-source ICU databases (74% vs 23%). In total, 34 (43%) of the studies used one or more open-source ICU database, among which the Medical Information Mart for Intensive Care (MIMIC)-III database25 was the most frequently used (n = 24, 30%). In contrast to RL studies (which inherently deal with DTRs), only eight26–33 of the 48 studies (17%) that used G methods considered DTRs and seven of these were published in or after 2020 (Supplementary Fig. 1b).
Fig. 2. Flowchart of study selection.
This flow diagram displays the screening strategy for the inclusion of studies in this scoping review.
Table 1.
Characteristics of the included studies grouped by used causal inference method.
| IPTW (N = 42) No (%) | Parametric G formula (N = 5) No (%) | TMLE (N = 1) No (%) | RL (N = 31) No (%) | All (N = 79) No (%) | |
|---|---|---|---|---|---|
| Exposure of interest (categorized) | |||||
| Vasopressors & intra-venous fluids | 0 (0) | 0 (0) | 0 (0) | 10 (32) | 10 (13) |
| Anti-inflammatory drugs | 7 (17) | 0 (0) | 0 (0) | 2 (6) | 9 (11) |
| Hospital-acquired complications | 8 (19) | 0 (0) | 0 (0) | 0 (0) | 8 (10) |
| Sedatives & analgesics | 1 (2) | 0 (0) | 0 (0) | 6 (19) | 7 (9) |
| Antimicrobials | 4 (10) | 0 (0) | 0 (0) | 0 (0) | 4 (5) |
| Mechanical ventilation | 1 (2) | 2 (40) | 0 (0) | 3 (10) | 6 (8) |
| Anticoagulants | 1 (2) | 0 (0) | 0 (0) | 2 (6) | 3 (4) |
| Diuretics | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 3 (4) |
| Renal replacement therapy | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 3 (4) |
| Sodium bicarbonate | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 3 (4) |
| Blood transfusion | 2 (5) | 0 (0) | 0 (0) | 1 (3) | 3 (4) |
| Other | 9 (21) | 3 (60) | 1 (100) | 7 (23) | 20 (25) |
| Primary outcome (categorized) | |||||
| Mortality | 32 (76) | 2 (40) | 1 (100) | 11 (35) | 46 (58) |
| Combined | 0 (0) | 0 (0) | 0 (0) | 13 (42) | 13 (16) |
| Maintenance of clinical target value | 0 (0) | 0 (0) | 0 (0) | 6 (19) | 6 (8) |
| Hospital-acquired complications | 1 (2) | 2 (40) | 0 (0) | 0 (0) | 3 (4) |
| Need for mechanical ventilation | 2 (5) | 0 (0) | 0 (0) | 0 (0) | 2 (3) |
| Other | 7 (17) | 1 (20) | 0 (0) | 1 (3) | 9 (11) |
| Number of included ICUsa | |||||
| 1 | 16 (38) | 2 (40) | 0 (0) | 20 (77) | 38 (51) |
| 2–4 | 5 (12) | 1 (20) | 1 (100) | 4 (15) | 11 (15) |
| 5–10 | 3 (7) | 1 (20) | 0 (0) | 0 (0) | 4 (5) |
| 11–20 | 4 (10) | 1 (20) | 0 (0) | 0 (0) | 5 (7) |
| 21–100 | 5 (12) | 0 (0) | 0 (0) | 0 (0) | 5 (7) |
| >100 | 9 (21) | 0 (0) | 0 (0) | 2 (8) | 11 (15) |
| Utilized open source databases | |||||
| MIMIC-II | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 1 (1) |
| MIMIC-III | 6 (14) | 0 (0) | 0 (0) | 18 (58) | 24 (30) |
| MIMIC-IV | 3 (7) | 1 (20) | 0 (0) | 3 (10) | 7 (9) |
| eICU | 2 (5) | 0 (0) | 0 (0) | 2 (6) | 4 (5) |
| AmsterdamUMCdb | 0 (0) | 1 (20) | 0 (0) | 2 (6) | 3 (4) |
| Study size (n patients)a | |||||
| 0–100 | 2 (5) | 0 (0) | 0 (0) | 0 (0) | 2 (3) |
| 100–500 | 7 (17) | 1 (20) | 0 (0) | 0 (0) | 8 (11) |
| 501–1000 | 9 (21) | 0 (0) | 1 (100) | 1 (4) | 11 (15) |
| 1001–5000 | 16 (38) | 3 (60) | 0 (0) | 6 (23) | 25 (34) |
| >5000 | 8 (19) | 1 (20) | 0 (0) | 19 (73) | 28 (38) |
| Type of exposure regime | |||||
| Static | 36 (86) | 3 (60) | 1 (100) | 0 (0) | 40 (51) |
| Dynamic | 6 (14) | 2 (40) | 0 (0) | 31 (100) | 39 (49) |
IPTW inverse probability of treatment weighting, TMLE targeted minimum loss-based estimation, RL reinforcement learning.
aFor the number of included ICUs and study size, the studies that used simulated patient data (n = 5) are not taken into account and therefore, the number of RL and all studies add up to 26 and 74, respectively.
Method-specific items
Among the studies that used IPTW (n = 43), eighteen applied stabilized weights, one applied weight truncation, and ten studies applied both weight stabilization and truncation. The remaining studies did not apply weight stabilization or truncation. Among studies that applied RL on real (i.e., not simulated) patient data (n = 26), fourteen studies used either an importance-sampling based34, model-based35,36, a doubly robust off-policy evaluation (OPE, Box 1) method37, or a combination of these. Thirteen studies used the so-called ‘U-curve method’38 (Box 1) and for nine of these, this was the only reported OPE method. Two studies did not report any evaluation of the optimized DTR (Supplementary Fig. 2).
Quality of reporting
A total of 1183 unique quality of reporting (QOR) items were assessed independently, encompassing the eight, eleven, and ten target trial subcomponents for the IPTW/TMLE, parametric G formula, and RL papers, respectively, along with six distinct items for the causal assumptions in all included studies. Initially, the reviewers agreed on 1012 items (86%), and any remaining disagreements were resolved through discussion until consensus was reached. As each subcomponent of the analysis plan component required for IPTW studies also applies to TMLE, we used the same subcomponents to judge the analysis plan component of the study that used TMLE23.
Target trial components
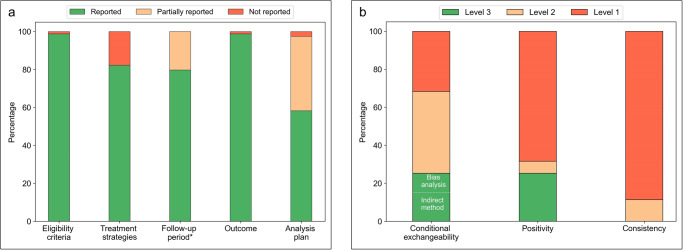
Both the ‘eligibility criteria’ and ‘outcome’ components were reported in 78 (99%) of the studies (Fig. 3a). We scored the ’treatment strategies’, ‘follow-up period’ and ‘analysis plan’ components as partially or not reported in respectively 14 (18%), 16 (20%) and 33 (42%) of the studies. All five target trial components were fully reported in only 30 (38%) studies. The reporting of the target trial components grouped by used CI method are summarized in Supplementary Figs. 3–5 and tabulated for each individual study in Supplementary Tables 3–5.
Fig. 3. Quality of Reporting summary plots.
a Reporting quality of the target trial components. *For the follow-up component, the studies that used simulated patient data (n = 5) are not taken into account. b Reporting quality of causal assumptions (level 1 = assumption not mentioned, level 2 = assumption mentioned, level 3 = attempt to check for potential violations of the assumption reported).
Causal assumptions
The conditional exchangeability assumption remained unmentioned in 25 (32%), was mentioned in 34 (43%), and an attempt to check for potential violations was reported in 20 studies (25%, Fig. 3b). Among the studies that reported a check for potential violations, eight studies31,33,39–44 performed a bias analysis. The positivity assumption remained unmentioned in 54 (68%), was mentioned in five (6%), and a check for potential violations was reported in 20 (25%) of the studies. The consistency assumption was mentioned in nine (11%) of the studies. All three assumptions were mentioned (or a check for potential violations was reported) in only seven (9%) studies (Supplementary Table 6). The reporting of assumptions grouped by CI method used are summarized in Supplementary Figs. 6–8 and individual results for all studies are tabulated in Supplementary Table 6. In general, the causal assumptions remained unmentioned more often in studies that applied RL, compared to those which applied G methods (Supplementary Figs. 6–8). All studies that reported a check for potential violations of the conditional exchangeability assumption also mentioned this assumption, whereas for the positivity assumption, eleven out of 20 studies that reported a check for potential violations did not explicitly mention positivity (Supplementary Table 6).
Adjusting for treatment-affected time-varying confounding
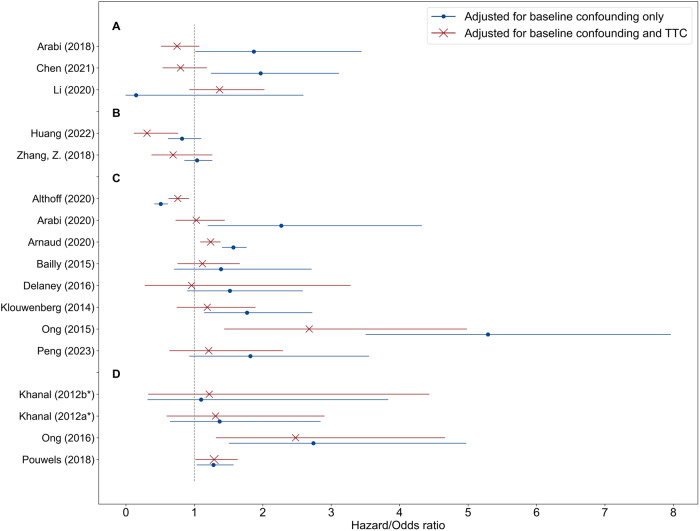
Seventeen studies (22%) estimated the treatment effect by adjusting for baseline confounding and by adjusting for baseline confounding and TTC. For most of these studies, the point estimates of the treatment effects varied substantially after adjusting for both baseline and TTC, moving the effect estimate towards or away from the null hypothesis, or even leading to opposite effect estimates (Fig. 4).
Fig. 4. Influence of adjusting for treatment-affected time-varying confounding.
Treatment effect estimates adjusted only for baseline confounding versus adjusted for baseline and treatment-affected time-varying confounding (TTC) reported by sixteen of the included studies. Treatment effects were reported in terms of odds or hazard ratios (including the reported 95% CIs). In three studies (A), the point estimates moved to the opposite direction, in two (B) and eight (C) studies, the estimates moved away from and towards the null hypothesis, respectively. In three studies (D), there was a marginal difference in point estimates. Pouwels et al. (2020) estimated treatment effect on length-of-stay (in terms of days) by adjusting for baseline confounding and by adjusting for baseline confounding and TTC, and found a marginal difference in point estimates. *Khanal et al. (2012) compared prolonged intermittent renal replacement therapy with two different alternative treatments.
Discussion
Our scoping review of 79 published studies revealed a diverse range of treatments being investigated. Despite the fact that most treatments of interest in the ICU setting are DTRs, we observed a dominant emphasis on STRs among studies that used G methods. Many studies had inadequate reporting of the components of target trials, with the ‘treatment strategies’, ‘follow-up’ and ‘analysis plan’ components being incompletely reported most frequently. The causal assumptions were frequently not specified, especially in studies utilizing RL methods, indicating a potential lack of awareness in this research field of the importance of these assumptions. The upcoming ‘Prediction of Counterfactuals Guideline’ (PRECOG)45 aims to provide guidance on the reporting of causal assumptions and model evaluation in CI studies using observational data, and we anticipate that its adoption may yield substantive enhancements in the quality of reporting in forthcoming CI studies utilizing observational ICU data. We decided not to employ the ROBINS-I tool46 for assessing bias in observational studies in our evaluation. We made this choice for two main reasons. Firstly, utilizing ROBINS-I would require verifying specific causal assumptions, which, in turn, would demand expert knowledge of the treatment-outcome relationships studied in each of the included articles. Given the extensive scope of our review and the fact that many studies did not explicitly address these causal assumptions, we found this task to be beyond the intended scope of our review. Consequently, we opted to evaluate studies based on their acknowledgment of these assumptions and their reported attempts to validate them. Secondly, when conducting causal inference with observational data, the potential for bias arises not only from the absence of randomization but also from potential biases stemming from incorrect study design choices47. Rather than assessing these design choices directly, we chose to evaluate studies based on the clarity and completeness of their reporting regarding these choices. To structure this evaluation, we used the target trial framework11, which is widely recognized and used as a conceptual benchmark to describe study design choices in causal inference studies using observational data across various medical domains48–52. G methods and RL methods are often perceived as separate disciplines, but show great similarities53. For example, Q-learning54 (an RL method, used by many of the included studies55–59) is very similar -and under certain conditions even algebraically equivalent- to G estimation (a G method)60. An important difference is that G methods are used for modeling both STRs and DTRs, while RL methods typically deal with DTRs. As both G methods and RL methods perform the same CI task (i.e., finding optimal treatment regimes), both rely on the same, strong causal assumptions, which should be acknowledged. While the consistency assumption is often plausible for treatments in the ICU, violations of the conditional exchangeability and positivity assumption are more likely and should be examined. Prior to examining violations of the causal assumptions, one needs a research question that is truly of interest in the ICU, a clear description of study design choices (e.g., using the target trial framework11), and usage of a CI method that is appropriate for the type of studied treatment. The results of our scoping review have led to five recommendations that build on widely accepted views and concepts in the field of CI to improve future research and move towards actionable AI in the ICU (Box 2).
Box 2 Summary of recommendations for future research.
-
Ask the right research question
When developing a model for causal inference, consider clinically relevant treatments. In the ICU, treatment decisions typically occur at multiple time point during admission (i.e., time-varying treatments) and often depend on the patient’s response to treatment (i.e., dynamic treatment regimes).
-
Describe the question as a target trial emulation
It is useful to imagine a randomized trial that would have answered the research question (i.e., the target trial). Even if performing the target trial is not feasible, describing its components using the target trial framework helps to identify flaws in the relevance of a research question and correctness of the analysis.
-
Use methods that suit the research question
Standard methods, like propensity-score analyses, are easy to implement and suffice for time-fixed treatments, but lead to biased estimates when used to adjust for treatment-affected time-varying confounding (TTC). Research questions concerning time-varying treatments are often complicated by TTC, and, therefore, require methods that can appropriately adjust for this.
-
Mind the conditional exchangeability assumption
Causal inference is not possible based on data only, and incorporation of expert knowledge is key to think about the causal structure between the treatment and outcome of interest. Representing this expert knowledge in causal diagrams is useful to visualize potential sources of bias. A bias analysis can be helpful to quantify how much bias unmeasured confounders could produce.
-
Mind the positivity assumption
This assumption is verifiable, but this is rather complex for time-varying treatments and violations are expected given the dynamic nature of the ICU. Violations could be minimized by increasing the sample size (e.g., by more usage of open-source ICU databases) and by studying treatment regimes that are (more) similar to those observed in the data. Examination of estimated inverse-probability-of-treatment weights is useful to detect (but not rule out) positivity violations.
Ask the right research question
Treatments of interest in the ICU are typically DTRs and, therefore, this type of treatments is expected to be the focus of CI research in the ICU. However, despite an upward trend in recent years (Supplementary Fig. 1b), still 83% of the studies that used G methods studied STRs. To illustrate that many of these studies are considering research questions that are not truly of interest in the ICU, we will explore some examples. Zhang and colleagues divided patients into two groups according to whether they received diuretics within the first two days of ICU admission or not61. Thus, the emulated target trial answers the question whether or not to administer diuretics at the start of ICU admission. However, we argue that the question an ICU physician is really interested in is when to administer diuretics throughout the whole ICU stay, taking into account changing patient characteristics such as fluid balance (especially at later ICU stages). In addition, many of the included studies emulated target trials comparing ‘giving treatment sometime during follow-up’ versus ‘never giving treatment’. For example, Bailly and colleagues studied the effect of systemic antifungal therapy, comparing a treated group (those who received antifungals during their ICU stay) with an untreated group (those who never received antifungals)62. As giving treatment ‘sometime during follow-up’ can be done in many ways, the estimated treatment effect is ill-defined and typically not truly of interest. In other words, both studies by Zhang61 and Bailly62 serve as examples of emulated RCTs that would never be conducted in the ICU. Conversely, Morzywołek and colleagues27 emulated a target trial that reflects a very relevant research question, i.e., what is the optimal moment to start renal replacement therapy in acute kidney injury? They identified an optimal DTR based on a combination of biomarkers lowered mortality without increasing the number of RRTs, offering valuable insights for planning a future confirmatory RCT. Wang and colleagues investigated the effectiveness of low tidal volume ventilation32, a DTR investigated in one of the most influential RCTs in the field of intensive care medicine63. This trial faced criticism (among other concerns) due to high non-adherence rates. Consequently, the target trial these authors emulated reflected the highly relevant question: what would the treatment effect have been under full compliance?
Describe the question as a target trial emulation
It can be beneficial to conceptualize a randomized trial that could have addressed the research query (i.e., the target trial). Even in cases where executing the exact target trial is unfeasible, outlining its constituent elements within the target trial framework11 aids in recognizing shortcomings in the appropriateness of the research question and accuracy of the analysis. Almost one fifth of the included studies lacked a clear description of the ‘treatment strategies’ component of the target trial, that is, which treatment regimes are compared in the target trial. For example, Arabi and colleagues64 used IPTW to study the effect of corticosteroid therapy for ICU patients with Middle East Respiratory Syndrome. However, it remains unclear which treatment regimes (e.g., ‘treat daily with corticosteroids’) are being compared. Moreover, more than one third of the included studies lacked a complete description of the ‘analysis plan’ component and therefore, are not reproducible. We advocate detailed description which allows reproduction of the used methodology, ideally accompanied with code and (example) data.
Use methods that suit the research question
Many studies were not included in this review because they modeled time-fixed treatments. As time-fixed treatments in the ICU are rare, we hypothesize that in many of these studies, the implicit treatment of interest is time-varying. Research questions concerning time-varying treatments may be reformulated into simplified, time-fixed versions, because standard methods are easier to implement or high-quality, longitudinal data is unavailable. One may argue that, if the bias introduced by TTC18,19 is negligible, standard methods suffice for CI in time-varying treatments as well. However, empirical results from studies included in this review suggest that adjusting for TTC can lead to substantial differences in effect estimates and sometimes even to opposite conclusions (Fig. 4). Hence, it is possible that many of the excluded studies that implicitly studied time-varying treatments but modeled these as if they are time-fixed, published biased effect estimates. We advocate adjustment for TTC in any CI study where the treatment of interest is time-varying. Modeling DTRs is slightly more complex than STRs (which may be a reason for the focus on STRs among the included studies) and therefore requires different approaches. Various methods exist to find optimal DTRs, either from a set of pre-specified regimes or directly from data (for an overview, we refer to the book by Chakraborty and Moodie65). Among the included studies in this review, for example, Shahn and colleagues28 used ‘artificial censoring/IPW’65–67 to estimate the optimal fluid-limiting treatment regime for sepsis patients among a pre-specified set of DTRs (i.e., ‘fluid caps’). RL methods and G estimation can be used to approximate optimal DTRs without a pre-specified set of regimes. In RL studies, finding the optimal treatment regime (typically referred to as the optimal ‘policy’, see Supplementary Table 1) is typically followed by a validation step to quantify the value of the optimized regime (i.e., OPE, Box 1). The ‘U-curve method’38, a frequently used OPE method among the included RL studies in this review (Supplementary Fig. 2), is based on associating the difference between the (observed) clinician’s treatment regime and the (estimated) optimal treatment regime with patient outcome. As it completely ignores the potential effect of confounders, we recommend avoiding this method.
Mind the conditional exchangeability assumption
Conditional exchangeability is never guaranteed using observational data as the absence of unmeasured confounders is not verifiable in the data. To think about residual confounding or selection bias, incorporation of subject-matter expertise is key. Directed acyclic graphs (DAGs)68 provide a simple and lucid approach for researchers dealing with observational data to showcase this expert knowledge, theories, and suppositions regarding the causal relationships among variables. For practical guidance on effective utilization of DAGs we refer to the work of Tennant and colleagues69. There are different approaches to quantify how potential violations of the conditional exchangeability would affect the study results70. Indirect approaches consider, for instance, the effect of adding additional confounders11. A ‘bias analysis’ (or sensitivity analysis)71 examines the characteristics of potential unmeasured confounders and can be useful to quantify how much bias it would produce as a function of those characteristics.
Mind the positivity assumption
The positivity assumption -on the contrary- is verifiable, although this is rather complex for time-varying treatments72 and, given its dynamic nature, violations are expected in the ICU setting. The intuition for this assumption is that one can only study a treatment regime using data of patients who have received treatment that conform to this regime. The number of patient treatment histories that match the treatment regime of interest (i.e., the ‘effective sample size’73) shrinks with the number of treatment decisions in the patient’s history (which tends to be high in the ICU). For example, Gottesman and colleagues38 applied RL to a dataset of 3855 patients to find an optimal treatment regime for sepsis, but the effective sample size for this regime was only a few dozen. A small effective sample size makes positivity222222 violations likely and leads to high uncertainties in estimated treatment effects. A straight-forward opportunity to tackle this challenge is increasing the sample size. Therefore, we advocate more usage (if appropriate) of the four currently available open-source ICU databases74. However, increasing the sample size does not guarantee increasing the effective sample size, as the patients in the extra dataset may not be treated according to the regime of interest. Hence, another opportunity to increase the effective sample size is to minimize the mismatches between the treatment regime(s) of interest and those observed in the data. Studies employing G methods may simply accomplish this by avoiding the modeling of treatment regimes which differ greatly from the treatment protocol in place. In contrast, studies utilizing RL typically do not pre-specify treatment regimes (as they optimize them through learning agents) and consequently, avoiding specific treatment regimes (or policies) is more challenging. In the RL literature, various approaches have emerged to address this issue, resulting in policies more closely aligned with physician practices75. These approaches can be categorized into ‘policy constraint’ and ‘uncertainty based’ methods, with the latter exemplified by the application of Conservative-Q Learning in two of the included RL studies76–78. Detection (but not ruling out) of violations of the positivity assumption can be facilitated through examination of the distribution of estimated (stabilized) inverse-probability-of-treatment (IPT) weights72, which was done in 20 of the 42 studies that utilized IPTW (Supplementary Table 6). This examination is recommended not only for IPTW but also for studies utilizing other CI methods. In studies using RL methods that employ importance-sampling34 (which is closely related to IPTW53) for OPE, analogous to the examination of IPT weights, it is recommended to examine the distribution of the importance weights38. In studies using IPTW, weight stabilization and truncation can be used to limit high uncertainties in the effect estimates. Weight stabilization can improve the precision of effect estimates without the introduction of bias. However, a model based on stabilized weights results in a slightly different effect estimate compared to non-stabilized weights79 and, therefore, should be interpreted carefully. Weight truncation also improves precision, but at the expense of bias. Examination of the influence of the introduced bias by checking the change of the effect estimates under progressive truncation of IPT weights is recommended80.
This review stresses the importance of causality for actionable AI and provides a contemporary overview of CI research in the ICU literature. We describe shortcomings of the identified studies in terms of reporting and, furthermore, provide handles for improving future CI research. These recommendations are not limited to the ICU but apply to medical CI research as a whole. Unlike other reviews on time-varying medical treatments22,81,82, we did not limit our focus to either G methods or RL, but rather acknowledge that both these method classes can be used to perform CI tasks and therefore, hold the promise to bring actionable AI to the bedside.
Our review has limitations. First, whereas efforts were made to ensure that the literature search was comprehensive, we could have missed studies for different reasons. Some research might have employed unconventional terminology to delineate their chosen CI method or utilized a CI approach that fell outside the scope of our search strategy. For example, dynamic weighted ordinary least squares (dWOLS)83,84 is a relatively new method which has been used to model DTRs in the ICU setting in several studies85,86. This method benefits from properties of both Q-learning (an RL method) and G-estimation (a G method) and may be an interesting direction for future research. Also, digital twin technology builds on causal inference and has been applied in the ICU setting87. Second, items that were not collected could be of interest for future investigation. For example, we did not differentiate RL further into specific RL methods.
Towards actionable AI in the ICU, we concur with the guidance of editors of critical care journals88,89 to change the focus of observational research in the ICU from prediction to causal inference. To unlock this potential in a trustworthy and responsible manner, we advocate development of models for CI focusing on clinically relevant treatments, using a description of the research question as a target trial emulation, choosing appropriate CI methods given the treatment of interest and acknowledging (and ideally examining potential violation of) the causal assumptions.
Methods
The study protocol was registered in the online PROSPERO database (CRD42022324014)90. The filled-in PRISMA Extension for Scoping Reviews (PRISMAScR) checklist91 can be found in Supplementary Table 7.
Search strategy
Candidate articles were identified through a comprehensive search in Embase, MEDLINE ALL, Web of Science Core Collection, Google Scholar, medRxiv, bioRxiv, arXiv and ACM Digital Library up to March 2023, with no start date. We developed a search strategy that could be modified for each database (Supplementary Table 8). Search terms included relevant controlled vocabulary terms and free text variations for CI, G methods, or common RL methods, combined with ICU related terms.
Eligibility criteria
We included any primary research article, conference proceedings or pre-print papers that present models for the task of CI concerning time-varying treatments in patients admitted to the adult ICU. Articles were not eligible if it modeled a time-fixed treatment, utilized data from an RCT (unless the treatment of interest was not the randomized treatment), it focused on the introduction of new methodology, or was an abstract-only, review, opinion, or survey. Duplicates and articles not written in English were also excluded.
Study selection
Duplicate removal and eligibility screening were performed using EndNote 20 (Clarivate Analytics, Philadelphia, PA, USA). We used a two-stage approach for screening: first by title and abstract and then by full article text. Two reviewers (JS and WK) independently screened the titles and abstracts. The two reviewers then independently performed full-text screening on all articles. Conflicts regarding the eligibility of studies during the screening process were resolved by consensus in regular sessions between the two reviewers.
Data extraction
Data were extracted by JS and confirmed by WK. The items for extraction were based on the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) checklist92, supplemented by method-specific items. We extracted the following items from all included studies: details of study variables (i.e., studied treatment and primary outcome), the number of included ICUs, usage of open-source database(s), number of participants included, studied treatment type (Fig. 1), and used CI method. In addition, we extracted the following method-specific items: the usage of methods to reduce extreme weights (i.e., weight stabilization93 and truncation80) for studies using IPTW and the off-policy evaluation94 (OPE, Box 1) method used for studies using RL. Finally, if a study estimated the treatment effect both by adjusting for baseline confounding and by adjusting for baseline confounding and TTC, we also collected these different estimates.
Quality of reporting
To assess the quality of reporting (QOR) of the included studies, two reviewers (JS and WK) independently judged the reporting of the components of the target trial framework11 and the causal assumptions (Box 1). Conflicts regarding the QOR assessment were resolved by consensus in regular sessions between the two reviewers.
Target trial components
The ‘target trial framework’, introduced by Hernán and Robins11, consists of seven main components. We judged the reporting of five of these: eligibility criteria, treatment strategies, follow-up period, outcome and analysis plan (Table 2). We scored the analysis plan component as ‘reported’ if one could reproduce the modeling given the required data. For studies using RL, we judged the ‘treatment strategies’ and ‘outcome’ components as ‘reported’ if the definitions of the used action space and reward were reported, respectively. We split the follow-up period component into three subcomponents: time-zero (or ‘index date’), end of follow-up, and time resolution (i.e., the time steps in which the treatment level is considered the same)95. We split the analysis plan component into specific subcomponents depending on the CI method used (Supplementary Table 9). We scored the target trial components that are split in subcomponents as ‘reported, ‘partially reported’ and ‘not reported’ if all, some, or none of the subcomponents were reported, respectively. Leading questions used to judge whether each target trial subcomponent was reported can be found in Supplementary Table 9. We did not evaluate QOR for the components of ‘assignment procedures’ and ‘causal contrast of interest’, as no variability among the studies was expected. This is due to the fact that (except during clinical trials) ICU physicians typically have full knowledge of the treatments being administered to patients, resulting in an unblinded assignment of the modeled treatment. Additionally, the primary objective in CI studies using observational data is typically to compare the effect of actual treatment adherence (per-protocol effect), rather than the effect of being assigned to a specific treatment regime at baseline (intention-to-treat effect).
Table 2.
Components of the target trial framework included in the reporting quality assessment with examples for the ICU setting.
| Component | Subcomponent | Included for QOR assessment | ICU example |
|---|---|---|---|
| Eligibility criteria | – | ✓ | Individuals aged 65 years or older admitted to the ICU meeting Sepsis-3 criteria upon admission. |
| Treatment strategies | – | ✓ | 1. Liberal fluid therapy (administration of intravenous fluid boluses during the first several hours of treatment) 2. Restrictive fluid therapy (Intravenous fluid boluses during ICU stay in only in case of severe hypoperfusion) |
| Assignment procedures | – | Unblinded, random assignment to one of the treatment strategies. | |
| Follow-up period | Start of follow-up | ✓ | Time of ICU admission. |
| End of follow-up | ✓ | Death, ICU discharge, or loss to follow-up, whichever occurs first. | |
| Time resolution | ✓ | Hourly weights were applied to adjust for the impact of time-varying confounders on the hourly risk of adhering to one of the two treatment strategies. | |
| Outcome | – | ✓ | All- cause mortality. |
| Causal contrasts of interest | – | Per-protocol effect. | |
| Analysis plana | ✓ | Per-protocol effect will be estimated adjusting for pre- and postbaseline confounders by a marginal structural model using IPTW.b |
aThe analysis plan component is subdivided in a specific set of subcomponents depending on the modeling strategy used, these are summarized in Supplementary Table 9.
bThis description would not be considered as sufficient reporting of the analysis plan component, but simply serves as an example.
Causal assumptions
The task of CI relies on strong assumptions, including conditional exchangeability, positivity, and consistency (referred to collectively as ‘causal assumptions’, Box 1). Violations of these assumptions result in biased estimates and therefore it is crucial to acknowledge them and, if possible, assess potential violations. A study’s QOR regarding causal assumptions was scored using three levels of increasingly good reporting quality: (1) no mention of the assumption, (2) mention of the assumption, and (3) attempted examination of potential assumption violations. For the conditional exchangeability assumption, two types of attempts were differentiated: ‘indirect approaches’11 and ‘bias analyses’71. The examination of the distribution of the (stabilized) IPT weights was considered as a method to assess potential positivity assumption violations. As there are currently no approaches to check for consistency assumption violations, merely mentioning the consistency assumption (level 2) was considered the highest level of reporting quality.
Evidence synthesis
We tabulated extracted study items for each study individually and grouped by CI method used. QOR results concerning the target trial components and the causal assumptions are summarized as percentages using bar charts, and results of the QOR assessment for each study individually were presented in a long format table. For the reporting of the target trial components, we made separate tables for each group of studies that used a specific CI method. The collected treatment effect estimates reported by studies that estimated the treatment effect both by adjusting for baseline confounding and by adjusting for baseline and TTC, were plotted as point estimates and corresponding 95% confidence intervals.
Supplementary information
Acknowledgements
We acknowledge and thank all authors of the included articles who responded to our emails and were open for fruitful discussions, in particular Sébastien Bailly, Johan Steen and Zach Shahn. We thank Wichor Bramer for assisting in generating the search strings. No funding was granted for this study.
Author contributions
J.S., J.K., J.v.B., D.G., M.R. and M.v.G. designed the study. J.S. (with help from a medical librarian) developed the search strings. J.S. and W.K. extracted the data and performed the quality assessment. J.S. analyzed the data and wrote the first draft of the manuscript. All authors revised the manuscript and approved the final version of the submitted manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. J.S. acts as the guarantor.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. Jeremy Labrecque is supported by Veni grant 09150162010213 from the Netherlands Organisation for Scientific Research (NWO) and the Netherlands Organisation for Health Research and Development (ZonMW). The authors declare no further competing financial or non-financial interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41746-023-00961-1.
References
- 1.Komorowski, M. Artificial intelligence in intensive care: are we there yet? Intensive Care Med. 45, 1298–1300 (2019). [DOI] [PubMed]
- 2.Yoon, J. H., Pinsky, M. R. & Clermont, G. Artificial intelligence in critical care medicine. Crit. Care26, 75 (2022). [DOI] [PMC free article] [PubMed]
- 3.Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 2019;25:44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- 4.van de Sande D, van Genderen ME, Huiskens J, Gommers D, van Bommel J. Moving from bytes to bedside: a systematic review on the use of artificial intelligence in the intensive care unit. Intensive Care Med. 2021;47:750–760. doi: 10.1007/s00134-021-06446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komorowski, M. Clinical Management of Sepsis can be Improved by Artificial Intelligence: Yes. Intensive Care Medicine (Springer, 2020). [DOI] [PubMed]
- 6.Hernán MA, Hsu J, Healy B. A second chance to get causal inference right: a classification of data science tasks. Chance. 2019;32:42–49. doi: 10.1080/09332480.2019.1579578. [DOI] [Google Scholar]
- 7.Fleuren LM, et al. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 2020;46:383–400. doi: 10.1007/s00134-019-05872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smit JM, Krijthe JH, van Bommel J. The future of artificial intelligence in intensive care: moving from predictive to actionable AI. Intensive Care Med. 2023;49:1114–1116. doi: 10.1007/s00134-023-07102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prosperi M, et al. Causal inference and counterfactual prediction in machine learning for actionable healthcare. Nat. Mach. Intell. 2020;2:369–375. doi: 10.1038/s42256-020-0197-y. [DOI] [Google Scholar]
- 10.Hernán, M. & Robins, J. Causal Inference: What If (Boca Raton: Chapman & Hall/CRC., 2020).
- 11.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol. 2016;183:758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robins, J. & Hernan, M. Estimation of the causal effects of time-varying exposures. In Longitudinal Data Analysis (eds. Fitzmaurice, G., Davidian, M., Verbeke, G. & Molenberghs, G.) 553–599 (Chapman and Hall/CRC Press: New York, 2009).
- 13.Mansournia MA, Etminan M, Danaei G, Kaufman JS, Collins G. Handling time varying confounding in observational research. BMJ. 2017;359:1–6. doi: 10.1136/bmj.j4587. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 15.Karim ME, Tremlett H, Zhu F, Petkau J, Kingwell E. Dealing with treatment-confounder feedback and sparse follow-up in longitudinal studies: application of a marginal structural model in a multiple sclerosis cohort. Am. J. Epidemiol. 2021;190:908–917. doi: 10.1093/aje/kwaa243. [DOI] [PubMed] [Google Scholar]
- 16.Daniel RM, Cousens SN, De Stavola BL, Kenward MG, Sterne JAC. Methods for dealing with time-dependent confounding. Stat. Med. 2013;32:1584–1618. doi: 10.1002/sim.5686. [DOI] [PubMed] [Google Scholar]
- 17.Naimi AI, Cole SR, Kennedy EH. An introduction to g methods. Int. J. Epidemiol. 2017;46:756–762. doi: 10.1093/ije/dyw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schisterman EF, Cole SR, Platf RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14:300–306. doi: 10.1097/01.EDE.0000042804.12056.6C. [DOI] [PubMed] [Google Scholar]
- 20.Robins JM. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math. Model. 1986;7:1393–1512. doi: 10.1016/0270-0255(86)90088-6. [DOI] [Google Scholar]
- 21.Sutton, R. S. & Barto, A. G. Reinforcement Learning: An Introduction (MIT press, 2018).
- 22.Liu, S. et al. Reinforcement learning for clinical decision support in critical care: comprehensive review. J. Med. Internet Res. 22, e18477 (2020). [DOI] [PMC free article] [PubMed]
- 23.Torres, L. K. et al. Attributable mortality of acute respiratory distress syndrome: a systematic review, meta-analysis and survival analysis using targeted minimum loss-based estimation. Thorax10.1136/thoraxjnl-2020-215950 (2021). [DOI] [PMC free article] [PubMed]
- 24.van der Laan MJ, Rubin D. Targeted maximum likelihood learning. Int. J. Biostat. 2006;2:1–38. [Google Scholar]
- 25.Johnson AEW, et al. MIMIC-III, a freely accessible critical care database. Sci. Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martucci G, et al. Transfusion practice in patients receiving VV ECMO (PROTECMO): a prospective, multicentre, observational study. Lancet. Respir. Med. 2023;11:245–255. doi: 10.1016/S2213-2600(22)00353-8. [DOI] [PubMed] [Google Scholar]
- 27.Morzywołek P, et al. Timing of dialysis in acute kidney injury using routinely collected data and dynamic treatment regimes. Crit. Care. 2022;26:365. doi: 10.1186/s13054-022-04252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahn, Z., Shapiro, N.I., Tyler, P. D., Talmor, D. & Lehman, L.-W. H. Fluid-limiting treatment strategies among sepsis patients in the ICU: A retrospective causal analysis. Crit. Care24, 62, (2020). [DOI] [PMC free article] [PubMed]
- 29.Shahn Z, Lehman L-WH, Mark RG, Talmor D, Bose S. Delaying initiation of diuretics in critically ill patients with recent vasopressor use and high positive fluid balance. Br. J. Anaesth. 2021;127:569–576. doi: 10.1016/j.bja.2021.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Shahn Z, et al. Effects of aggressive and conservative strategies for mechanical ventilation liberation. J. Crit. Care. 2023;76:154275. doi: 10.1016/j.jcrc.2023.154275. [DOI] [PubMed] [Google Scholar]
- 31.Urner M, et al. Venovenous extracorporeal membrane oxygenation in patients with acute covid-19 associated respiratory failure: comparative effectiveness study. BMJ. 2022;377:1–9. doi: 10.1136/bmj-2021-068723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, et al. Estimating the causal effect of low tidal volume ventilation on survival in patients with acute lung injury. J. R. Stat. Soc. Ser. C. Appl. Stat. 2011;60:475–496. doi: 10.1111/j.1467-9876.2010.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarnell CJ, et al. Oxygenation thresholds for invasive ventilation in hypoxemic respiratory failure: a target trial emulation in two cohorts. Crit. Care. 2023;27:67. doi: 10.1186/s13054-023-04307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Precup, D., Sutton, R. S. & Singh, S. P. Eligibility traces for off-policy policy evaluation. In Proceedings of the Seventeenth International Conference on Machine Learning. 759–766 (ACM, 2000).
- 35.Hanna, J., Stone, P. & Niekum, S. Bootstrapping with models: Confidence intervals for off-policy evaluation. In Proceedings of the AAAI Conference on Artificial Intelligence, 31 (AAAI, 2017).
- 36.Le, H., Voloshin, C. & Yue, Y. Batch policy learning under constraints. In International Conference on Machine Learning 3703–3712 (PMLR, 2019).
- 37.Jiang, N. & Li, L. Doubly robust off-policy value evaluation for reinforcement learning. in International Conference on Machine Learning 652–661 (PMLR, 2016).
- 38.Gottesman, O. et al. Evaluating Reinforcement Learning Algorithms in Observational Health Settings. Preprint at http://arxiv.org/abs/1805.12298 (2018).
- 39.Althoff MD, et al. Noninvasive ventilation use in critically Ill patients with acute asthma exacerbations. Am. J. Respir. Crit. Care Med. 2020;202:1520–1530. doi: 10.1164/rccm.201910-2021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dupuis, C. et al. Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: a multicenter cohort study of the OUTCOMEREA network. PLoS One16, e0255644 (2021). [DOI] [PMC free article] [PubMed]
- 41.Moromizato T, Sakaniwa R, Tokuda Y, Taniguchi K, Shibuya K. Intravenous methylprednisolone pulse therapy and the risk of in-hospital mortality among acute COVID-19 patients: nationwide clinical cohort study. Crit. Care. 2023;27:1–11. doi: 10.1186/s13054-023-04337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohbe H, et al. Early enteral nutrition for cardiogenic or obstructive shock requiring venoarterial extracorporeal membrane oxygenation: a nationwide inpatient database study. Intensive Care Med. 2018;44:1258–1265. doi: 10.1007/s00134-018-5319-1. [DOI] [PubMed] [Google Scholar]
- 43.Peng, C. et al. Impact of early tracheostomy on clinical outcomes in trauma patients admitted the to intensive care unit: a retrospective causal analysis. J. Cardiothorac. Vasc. Anesth. 10.1053/j.jvca.2022.12.022 (2022). [DOI] [PubMed]
- 44.Truche A-S, et al. Continuous renal replacement therapy versus intermittent hemodialysis in intensive care patients: impact on mortality and renal recovery. Intensive Care Med. 2016;42:1408–1417. doi: 10.1007/s00134-016-4404-6. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, et al. Protocol for the development of a reporting guideline for causal and counterfactual prediction models in biomedicine. BMJ Open. 2022;12:1–5. doi: 10.1136/bmjopen-2021-059715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterne JA, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:1–7. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickerman BA, García-Albéniz X, Logan RW, Denaxas S, Hernán MA. Avoidable flaws in observational analyses: an application to statins and cancer. Nat. Med. 2019;25:1601–1606. doi: 10.1038/s41591-019-0597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthews AA. Target trial emulation: applying principles of randomised trials to observational studies. BMJ. 2020;39:1199–1236. doi: 10.1136/bmj-2022-071108. [DOI] [PubMed] [Google Scholar]
- 49.Hernán MA, Wang W, Leaf DE. Target trial emulation: a framework for causal inference from observational data. JAMA. 2022;328:2446–2447. doi: 10.1001/jama.2022.21383. [DOI] [PubMed] [Google Scholar]
- 50.Groenwold RHH. Trial emulation and real-world evidence. JAMA Netw. Open. 2021;4:e213845–e213845. doi: 10.1001/jamanetworkopen.2021.3845. [DOI] [PubMed] [Google Scholar]
- 51.Ioannou GN, et al. COVID-19 vaccination effectiveness against infection or death in a National U.S. Health Care System a target trial emulation study. Ann. Intern. Med. 2022;175:352–361. doi: 10.7326/M21-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffman KL, et al. Comparison of a target trial emulation framework vs Cox regression to estimate the association of corticosteroids with COVID-19 mortality. JAMA Netw. open. 2022;5:e2234425. doi: 10.1001/jamanetworkopen.2022.34425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uehara, M., Shi, C. & Kallus, N. A review of off-policy evaluation in reinforcement learning. Preprint at https://arxiv.org/abs/2212.06355 (2022).
- 54.Watkins, C. J. C. H. & Dayan, P. Q-learning. Mach. Learn. 8, 279–292 (1992).
- 55.Nemati, S., Ghassemi, M. M. & Clifford, G. D. Optimal medication dosing from suboptimal clinical examples: a deep reinforcement learning approach. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2978–2981. 10.1109/EMBC.2016.7591355 (2016). [DOI] [PubMed]
- 56.Padmanabhan, R., Meskin, N. & Haddad, W. M. Reinforcement learning-based control for combined infusion of sedatives and analgesics. in 2017 4th International Conference on Control, Decision and Information Technologies (CoDIT) 505–509 (IEEE, 2017).
- 57.Padmanabhan R, Meskin N, Haddad WM. Closed-loop control of anesthesia and mean arterial pressure using reinforcement learning. Biomed. Signal Process. Control. 2015;22:54–64. doi: 10.1016/j.bspc.2015.05.013. [DOI] [Google Scholar]
- 58.Peine, A. et al. Development and validation of a reinforcement learning algorithm to dynamically optimize mechanical ventilation in critical care. npj Digit. Med. 4, 32 (2021). [DOI] [PMC free article] [PubMed]
- 59.Sinzinger ED, Moore B. Sedation of simulated ICU patients using reinforcement learning based control. Int. J. Artif. Intell. Tools. 2005;14:137–156. doi: 10.1142/S021821300500203X. [DOI] [Google Scholar]
- 60.Chakraborty B, Murphy S, Strecher V. Inference for non-regular parameters in optimal dynamic treatment regimes. Stat. Methods Med. Res. 2010;19:317–343. doi: 10.1177/0962280209105013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, R. et al. The Effect of Loop Diuretics on 28-Day Mortality in Patients With Acute Respiratory Distress Syndrome. Front. Med. 8, 740675 (2021). [DOI] [PMC free article] [PubMed]
- 62.Bailly S, et al. Failure of empirical systemic antifungal therapy in mechanically ventilated critically ill patients. Am. J. Respir. Crit. Care Med. 2015;191:1139–1146. doi: 10.1164/rccm.201409-1701OC. [DOI] [PubMed] [Google Scholar]
- 63.Brower RG, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 64.Arabi YM, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 65.Chakraborty, B. & Moodie, E. E. M. Statistical Methods for Dynamic Treatment Regimes: Reinforcement Learning, Causal Inference, and Personalized Medicine (Springer New York, 2013).
- 66.Li X, Young JG, Toh S. Estimating effects of dynamic treatment strategies in pharmacoepidemiologic studies with time-varying confounding: a primer. Curr. Epidemiol. Rep. 2017;4:288–297. doi: 10.1007/s40471-017-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hernán MA, Lanoy E, Costagliola D, Robins JM. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin. Pharmacol. Toxicol. 2006;98:237–242. doi: 10.1111/j.1742-7843.2006.pto_329.x. [DOI] [PubMed] [Google Scholar]
- 68.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. doi: 10.1097/00001648-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 69.Tennant PWG, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. Int. J. Epidemiol. 2021;50:620–632. doi: 10.1093/ije/dyaa213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson JW. Diagnostics for confounding of time-varying and other joint exposures. Epidemiology. 2016;27:859–869. doi: 10.1097/EDE.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Der Weele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-Value. Ann. Intern. Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 72.Petersen ML, Porter KE, Gruber S, Wang Y, Van Der Laan MJ. Diagnosing and responding to violations in the positivity assumption. Stat. Methods Med. Res. 2012;21:31–54. doi: 10.1177/0962280210386207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gottesman O, et al. Guidelines for reinforcement learning in healthcare. Nat. Med. 2019;25:16–18. doi: 10.1038/s41591-018-0310-5. [DOI] [PubMed] [Google Scholar]
- 74.Sauer CM, et al. Systematic review and comparison of publicly available ICU data sets-a decision guide for clinicians and data scientists. Crit. Care Med. 2022;50:e581–e588. doi: 10.1097/CCM.0000000000005517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levine, S., Kumar, A., Tucker, G. & Fu, J. Offline reinforcement learning: tutorial, review, and perspectives on open problems. Preprint at https://arxiv.org/abs/2005.01643 (2020).
- 76.Kumar A, Zhou A, Tucker G, Levine S. Conservative q-learning for offline reinforcement learning. Adv. Neural Inf. Process. Syst. 2020;33:1179–1191. [Google Scholar]
- 77.Kaushik, P., Kummetha, S., Moodley, P. & Bapi, R. S. A conservative q-learning approach for handling distribution shift in sepsis treatment strategies. Preprint at https://arxiv.org/abs/2203.13884 (2022).
- 78.Kondrup F, et al. Towards safe mechanical ventilation treatment using deep offline reinforcement learning. Proc. AAAI Conf. Artif. Intell. 2023;37:15696–15702. [Google Scholar]
- 79.Kaufman JS. Marginalia: Comparing adjusted effect measures. Epidemiology. 2010;21:490–493. doi: 10.1097/EDE.0b013e3181e00730. [DOI] [PubMed] [Google Scholar]
- 80.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am. J. Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clare PJ, Dobbins TA, Mattick RP. Causal models adjusting for time-varying confounding - a systematic review of the literature. Int. J. Epidemiol. 2019;48:254–265. doi: 10.1093/ije/dyy218. [DOI] [PubMed] [Google Scholar]
- 82.Farmer RE, et al. Application of causal inference methods in the analyses of randomised controlled trials: a systematic review. Trials. 2018;19:1–14. doi: 10.1186/s13063-017-2381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wallace MP, Moodie EEM. Doubly-robust dynamic treatment regimen estimation via weighted least squares. Biometrics. 2015;71:636–644. doi: 10.1111/biom.12306. [DOI] [PubMed] [Google Scholar]
- 84.Wallace, M. P., Moodie, E. E. M. & Stephens, D. A. Dynamic treatment regimen estimation via regression-based techniques: Introducing R package reg. J. Stat. Softw. 80, 1–20 (2017).
- 85.Zhang Z, Zheng B, Liu N. Individualized fluid administration for critically ill patients with sepsis with an interpretable dynamic treatment regimen model. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-74906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma P, et al. Individualized resuscitation strategy for septic shock formalized by finite mixture modeling and dynamic treatment regimen. Crit. Care. 2021;25:1–16. doi: 10.1186/s13054-021-03682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lal A, et al. Development and verification of a digital twin patient model to predict specific treatment response during the first 24 h of sepsis. Crit. Care Explor. 2020;2:e0249. doi: 10.1097/CCE.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lederer DJ, et al. Control of confounding and reporting of results in causal inference studies. Ann. Am. Thorac. Soc. 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 89.Maslove DM, Leisman DE. Causal inference from observational data: new guidance from pulmonary, critical care, and sleep journals. Crit. Care Med. 2019;47:1–2. doi: 10.1097/CCM.0000000000003531. [DOI] [PubMed] [Google Scholar]
- 90.Smit, J. et al. Answering ‘What If?’ in the intensive care unit: a protocol for a systematic review and critical appraisal of methodology. PROSPEROhttps://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022324014 (2022).
- 91.Tricco AC, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 92.von Elm E, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu S, et al. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Heal. J. Int. Soc. Pharmacoeconomics Outcomes Res. 2010;13:273–277. doi: 10.1111/j.1524-4733.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gottesman, O. et al. Interpretable off-policy evaluation in reinforcement learning by highlighting influential transitions. In Proceedings of the 37th International Conference on Machine Learning (ACM, 2020).
- 95.Lange T, Rod NH. Commentary: causal models adjusting for time-varying confounding -please send more data. Int. J. Epidemiol. 2019;48:265–267. doi: 10.1093/ije/dyy300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information.