Abstract
OBJECTIVES
The concept of non-inferiority is widely adopted in randomized trials comparing transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR). However, uncertainty exists regarding the long-term outcomes of TAVR, and non-inferiority may be difficult to assess. We performed a systematic review and meta-analysis of randomized trials comparing TAVR and SAVR, with a specific emphasis on the non-inferiority margin for 5-year all-cause mortality.
METHODS
A systematic search was applied to 3 electronic databases. Randomized trials comparing TAVR and SAVR were included. Bayesian methods were implemented to evaluate the posterior probability of non-inferiority at different trial non-inferiority margins under either a vague, Cauchy, or a literature-based prior. Primary outcomes were 5-year actuarial all-cause mortality, and the probability of non-inferiority at various transformed trial non-inferiority margins. Secondary outcomes were long-term survival and 1- and 2-year actuarial survival.
RESULTS
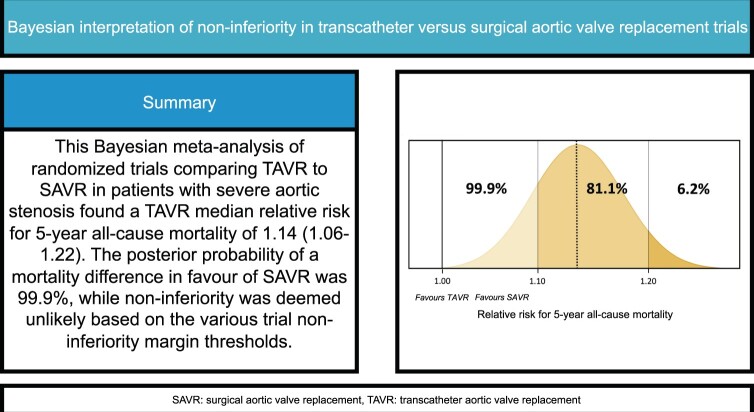
Eight trials (n = 8698 patients) were included. Kaplan–Meier-derived 5-year survival was 61.6% (95% CI 59.8–63.5%) for TAVR, and 63.7% (95% CI 61.9–65.6%) for SAVR. Six trials (n = 6370 patients) reported all-cause mortality at 5-year follow-up. Under a vague prior, the posterior median relative risk for all-cause mortality of TAVR was 1.14, compared to SAVR (95% credible interval 1.06–1.22, probability of relative risk <1.00 = 0.01%, I2 = 0%). Similar results in terms of point estimate and uncertainty measures were obtained using frequentist methods. Based on the various trial non-inferiority margins, the results of the analysis suggest that non-inferiority at 5 years is no longer likely.
CONCLUSIONS
It is unlikely that TAVR is still non-inferior to SAVR at 5 years in terms of all-cause mortality.
Keywords: Transcatheter aortic valve replacement, Surgical aortic valve replacement, Bayesian inference, Survival, Non-inferiority
The non-inferiority design has been applied in several trials evaluating transcatheter valve interventions.
INTRODUCTION
The non-inferiority design has been applied in several trials evaluating transcatheter valve interventions. The rationale of transcatheter techniques is to reduce surgical trauma and associated morbidity (i.e. a lower periprocedural risk) without compromising the primary outcome (which should be non-inferior to the standard procedure) [1]. For transcatheter aortic valve replacement (TAVR), several perceived advantages comprise a reduced complication rate, faster recovery, and an improved quality of life [2]. In TAVR trials, TAVR has been weighed against surgical aortic valve replacement (SAVR) for non-inferiority regarding the primary outcome, which has been defined as mortality (usually with the composite incorporation of stroke, sometimes in combination with rehospitalization). Consistently, these randomized controlled trials (RCTs) have found TAVR to be non-inferior to SAVR for the primary outcome during the first years [2–4]. However, at 5 years, RCTs comparing TAVR to SAVR have found a ‘numerically’ increased long-term all-cause mortality rate in TAVR patients. Still, these findings have lacked statistical significance in the individual studies.
Several meta-analyses have attempted to pool these RCTs, either at various timepoints or by use of commendable reconstructed time-to-event landmark analyses [5–7]. Although these extensive analyses provided important insights, these approaches lacked the possibility to evaluate non-inferiority and may not accurately reflect the cumulative all-cause mortality rate.
The Bayesian framework provides the opportunity to incorporate prior evidence from literature into the data and allows the estimation of the posterior probability at any given treatment effect threshold [8–11]. Particularly the latter feature of Bayesian inference seems applicable to the estimation of the posterior probability of the non-inferiority margin in pooled analyses of RCTs.
Therefore, the objective of the current study is to systematically review and meta-analyse current RCTs evaluating long-term all-cause mortality of TAVR and SAVR under the Bayesian framework, and to estimate the posterior probabilities of the various trial non-inferiority margins at 5 years.
MATERIALS AND METHODS
Design and protocol registration
The current study was designed as a systematic review and Bayesian meta-analysis and registered in the PROSPERO database (CRD42022379520, dated 8 December 2022) [12], adhering to the PRIMA2020 statement [13]. Furthermore, we followed the Bayesian reporting guidelines as proposed by Ferreira et al. [14] (Supplementary Material, Checklist).
Search strategy, eligibility criteria and study inclusion
A systematic search was applied to 3 electronic databases PubMed (i.e. PubMed Central and MEDLINE), Embase and the Cochrane Library (from conception until 26 October 2023, Supplementary Material S1). Inclusion criteria were: trials evaluating patients randomized to TAVR and SAVR and reporting on all-cause mortality during long-term follow-up (maximum follow-up 5 years). Studies were excluded when reporting results exclusively on patients undergoing TAVR through a non-transfemoral approach. If the same cohort was reported at different timepoints, the most recent report of that cohort was included in the current analysis.
Selection process
The selection process was performed by 2 authors independently (Samuel Heuts and Michal Kawczynski). Studies were screened based on title and abstract for eligibility, after which eligible full-texts were evaluated for final inclusion. The openly available web-based app ‘Rayyan’ was used for this semi-automated selection process [15].
Data extraction
Data extraction was performed by the first 2 authors (Samuel Heuts and Michal Kawczynski). Extracted data comprised: trial design, year, period, duration of follow-up, non-inferiority margin (either absolute or relative), number of patients, age, sex, risk scores, prevalence of peripheral arterial disease and cerebrovascular disease, type of prosthesis, approach and all-cause mortality at 1, 2 and 5 years. In case of disagreement, the senior author (Andrea Gabrio) was consulted.
Outcomes, non-inferiority margins, non-inferiority assessment and measures
The primary outcome was all-cause mortality at the 5-year actuarial timepoint. ‘Long-term survival’ was also evaluated as a secondary time-to-event outcome, based on individual patient data derived from Kaplan–Meier curves (IPD-KM). Other secondary outcomes were 1- and 2-year all-cause mortality.
For the Bayesian analyses, we studied the non-inferiority margins deduced from the included studies. The principle outcome measure was relative risk (RR, defined as the ratio of the probability for an event occurring in the exposure group versus the probability of the event in the non-exposure group) at 5 years. The non-inferiority margins of the various trials reporting on 5-year outcomes were formulated for the primary outcome. This primary outcome ranged from all-cause mortality (PARTNER 1A [16] and CoreValve US [17]), to a composite of all-cause mortality and disabling stroke (SURTAVI [18] and PARTNER 2A [19]), or a composite of all-cause mortality, disabling stroke, and valve-related rehospitalization (PARTNER 3 [20]). To formulate the non-inferiority margins as RRs, the all-cause mortality rate in the SAVR group at 5 years (in percentages) was used as baseline in every separate trial reporting at 5 years. Derived from the 5-year SAVR all rate and the non-inferiority absolute risk margins, an RR margin for non-inferiority could be calculated, as proposed previously [1]. As such, we assumed a similar RR non-inferiority for all-cause mortality as for the other end-points incorporated in the composite outcome.
The following non-inferiority margins were calculated (Supplementary Material S2): PARTNER 1A (RR <1.12), PARTNER 2A (RR <1.20—PARTNER 2A only defined a non-inferiority RR, and not an ARD), PARTNER 3 (RR <1.22), CoreValve US (RR <1.15), and SURTAVI (RR <1.23). Of note, the NOTION trial was a superiority trial and did therefore not specify a non-inferiority margin. The posterior probabilities of these non-inferiority margins, of ‘any’ TAVR all-cause mortality benefit (i.e. RR <1.0), and of a <10% RR difference (RR <1.10) were estimated using Bayesian inference.
In many Bayesian non-inferiority trial analyses, including the SURTAVI trial [21], non-inferiority is met if the posterior probability of the non-inferiority margin exceeds >97.1% [18]. Therefore, this posterior probability threshold will be applied to determine if non-inferiority was met or not met in the current pooled analysis.
Bayesian outcomes were expressed as the mean log of the relative risk (log RR) with corresponding 95% credible intervals (CrI), assuming a normal probability distribution (Supplementary Material S3). For clinical interpretation, the mean log RRs were converted to median RRs with 95% CrIs. The results of the frequentist sensitivity analyses were reported as RRs and 95% confidence intervals (CI). The analysis of the time-to-event data was expressed in hazard ratios (HRs).
Risk of bias in individual studies
Risk of bias was assessed by the first 2 authors (Samuel Heuts and Michal Kawczynski) using the Revised Cochrane Risk of Bias Tool for Randomized trials (RoB 2.0) [22].
Statistical analysis
To illustrate the statistical difficulty in the assessment of long-term survival differences of therapies that have time-dependent survival outcomes (i.e. crossing of curves), we first performed an IPD-KM curve analysis, as previously proposed by Liu et al. and Barili et al. [5, 6, 23]. The obtained curves were tested for Schoenfeld residuals and adapted accordingly. HR results from these curves were obtained using frequentist approaches.
For the primary outcome, we studied the 5-year actuarial timepoint as provided by the all-cause mortality event rates (absolute or relative) and total study population of the individual studies. Therefore, our primary meta-analysis was conducted under a Bayesian framework based on the log RR scale, assuming a normally distributed vague prior (log RR μ 0 and σ 2, resembling frequentist analyses with minimal prior information), a prior with a Cauchy distribution (location 0, scale 1/ = 0.707) [24], and a literature-based prior. This literature-based prior was derived from the contemporary nationwide German propensity-matched GARY registry [25], and provided a μ of 0.324 and σ of 0.045 on the log RR scale (Supplementary Material S4 presents prior selection and rationale). For sensitivity purposes, this literature-based prior was down-weighted to 75% and 50%. A Bayesian averaged model (instead of either a fixed or random effects model) was applied to report the eventual effect size [24, 26]. Heterogeneity between studies was assessed using the I2-metric and τ [27]. As part of sensitivity analyses, various secondary frequentist meta-analytical methods with different assumptions for heterogeneity were employed (i.e. DerSimonian–Laird, Sidik–Jonkman and restricted maximum likelihood), as proposed previously [27].
For Bayesian analyses, Markov-Chain Monte-Carlo sampling was applied, incorporating 3 chains with 10 000 saved iterations per chain. We used JASP to model these parameters (‘JASP’ program, JASP team, 2023, version 0.17.1, for Mac, Amsterdam University, Amsterdam, Netherlands) [24] and R studios (R Foundation for Statistical Analysis, Vienna, Austria), using the ‘meta’ and ‘dmetar’ software packages.
Risk of bias across studies
Publication bias was assessed visually using funnel plots of the primary outcomes (5-year mortality). Additionally, Egger’s test was performed for which P < 0.05 was considered statistically significant.
Certainty of evidence
The certainty of the evidence for the primary and secondary outcomes was estimated based on the four-step approach as advocated by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group [28], performed by 2 independent authors (Samuel Heuts and Michal Kawczynski).
RESULTS
Study inclusion
The predefined search yielded 1882 hits, of which n = 857 were excluded because of duplication and ineligibility by automation tools. Consequently, 1025 studies were screened based on title and abstract, and 35 studies underwent full-text assessment, after which 8 were included for the final analysis (reasons for exclusion in Supplementary Material S5).
Study characteristics
The 8 studies included in the final analysis reporting on the longest follow-up of the study cohorts were published between 2015 and 2023 (PARTNER 1A [16], PARTNER 2A [19], PARTNER 3 [20], CoreValve US [17], SURTAVI [18], EVOLUT-LR [29], NOTION [30], UK-TAVI [31]), evaluating patients operated on between 2007 and 2018. Table 1 presents the study characteristics.
Table 1:
Trial characteristics
| Trial | Author | Year | Period | Follow-up (years) | Design | Initial NI margina | Final analysis | Risk (low/intermediate/high) |
|---|---|---|---|---|---|---|---|---|
| PARTNER 1A [16] | Mack et al. | 2015 | 2007–2009 | 5 | Non-inferiority | ARD 7.5% | ITT | High |
| PARTNER 2A [19] | Makkar et al. | 2020 | 2011–2013 | 5 | Non-inferiority | RR 1.20 | ITT | Intermediate |
| PARTNER 3 [20] | Mack et al. | 2021 | 2016–2017 | 5 | Non-inferiority | ARD 6.0% | AT | Low |
| CoreValve US [17] | Gleason et al. | 2018 | 2011–2012 | 5 | Non-inferiority | ARD 7.5% | AT | High |
| SURTAVI [18] | Van Mieghem et al. | 2022 | 2012–2016 | 5 | Non-inferiority | ARD 7.0%b | mITT | Intermediate |
| EVOLUT-LR [29] | Forrest et al. | 2023 | 2016–2018 | 3 | Non-inferiority | ARD 6.0%b | AT | Low |
| NOTION [30] | Thyregod et al. | 2018 | 2009–2013 | 5c | Superiority | NA | ITT | >70 years of age |
| UK-TAVI [31] | UK-TAVI trial investigators | 2022 | 2014–2018 | 1 | Non-inferiority | ARD 5.0% | ITT | >70 years of age |
For the composite end point.
Using Bayesian inference.
The maximum reported follow-up of the NOTION trial is 8 years, but the 5-year analysis was included for the current study corresponding to the other trials’ follow-up.
ARD: absolute risk difference; AT: as-treated; ITT: intention to treat; mITT: modified ITT; NA: not applicable; NI: non-inferiority; RR: relative risk.
Patient characteristics
In total, 8698 patients were included in this analysis (TAVR n = 4443, SAVR n = 4255). Patient characteristics, including surgical risk estimations, are presented in Table 2.
Table 2:
Patient characteristics
| Trial | Intervention | Patients (n) analyzed | Age (years) | Sex (female) | STS score | PAD | CVD |
|---|---|---|---|---|---|---|---|
| PARTNER 1A [16] | TAVR | 348 | 84 (7) | 147 (42%) | 11.8 (3.3) | 149 (43%) | 96 (29%) |
| SAVR | 351 | 85 (6) | 153 (43%) | 11.7 (3.5) | 142 (42%) | 87 (27%) | |
| PARTNER 2A [19] | TAVR | 1011 | 82 (7) | 463 (46%) | 5.8 (2.1) | 282 (28%) | 325 (32%) |
| SAVR | 1021 | 82 (7) | 461 (45%) | 5.8 (1.9) | 336 (33%) | 317 (31%) | |
| PARTNER 3 [45] | TAVR | 496 | 73 (6) | 32% | 1.9 (0.7) | 6.9% | 3.4% |
| SAVR | 454 | 74 (6) | 29% | 1.9 (0.6) | 7.3% | 5.1% | |
| CoreValve US [17] | TAVR | 391 | 83 (7) | 184 (47%) | 7.3 (3.0) | 159 (41%) | 97 (25%) |
| SAVR | 359 | 83 (6) | 171 (48%) | 7.5 (3.3) | 150 (42%) | 90 (25%) | |
| SURTAVI [18] | TAVR | 864 | 80 (6) | 366 (42%) | 4.4 (1.5) | 266 (31%) | 151 (18%) |
| SAVR | 796 | 80 (6) | 358 (45%) | 4.5 (1.6) | 238 (30%) | 130 (16%) | |
| EVOLUT-LR [46] | TAVR | 730 | 74 (6) | 266 (36.4%) | 2.0 (0.2) | 54 (8%) | 74 (10%) |
| SAVR | 684 | 74 (6) | 233 (34.1%) | 2.0 (0.2) | 56 (8%) | 82 (12%) | |
| NOTION [30] | TAVR | 145 | 79 (5) | 67 (46%) | 2.9 (1.6) | 6 (4%) | 24 (17%) |
| SAVR | 135 | 79 (5) | 64 (47%) | 3.1 (1.7) | 9 (7%) | 22 (16%) | |
| UK-TAVI [31] | TAVR | 458 | NRa | 211 (46%) | 2.6 [2.0–3.5] | 40 (9%) | 75 (16%) |
| SAVR | 455 | NRa | 213 (47%) | 2.7 [2.0–3.5] | 35 (8%) | 67 (15%) |
Continuous data are reported as mean and (standard deviation), except in UK-TAVI, where STS score is reported by median and [25th–75th percentile].
Study reported categorized age groups.
AT: as-treated, CVD: cerebrovascular disease, ITT: intention to treat, mITT: modified ITT; PAD: peripheral arterial disease, SAVR: surgical aortic valve replacement; STS: Society of Thoracic Surgeons, TAVR: transcatheter aortic valve replacement.
Procedural characteristics and approaches
Patients enrolled in these RCTs underwent conventional SAVR. Patients in the TAVR groups either received a balloon-expendable valve prosthesis [16, 19, 20] or a self-expandable valve prosthesis [17, 18, 29, 30]. UK-TAVI allowed balloon-expendable valve, self-expandable valve and mechanically expandable valves [31] (Supplementary Material S6).
Risk of bias
Detailed information regarding the assessment of risk of bias is presented in Supplementary Material S7. The assessment suggests differing study quality, ranging from ‘low’ to ‘high’ risk of bias. This was especially due to missing outcomes at 5 years and deviation from intended interventions.
Long-term survival based on individual patient data derived from Kaplan–Meier curves
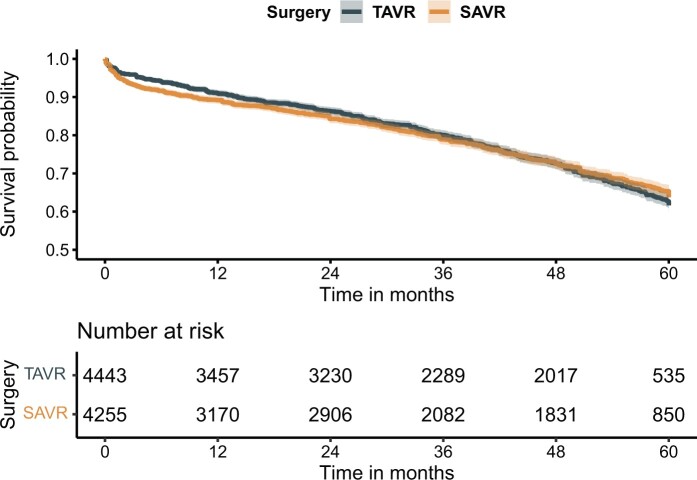
To illustrate the statistical difficulty in the assessment of pooled long-term survival differences of therapies that have time-dependent survival outcomes, we started with the IPD-KM analysis, in which 8698 patients were included (Fig. 1). Based on the Kaplan–Meier data, 5-year survival was 61.6% (95% CI 59.8–63.5%) for TAVR and 63.7% (95% CI 61.9–65.6%) for SAVR. There was strong visual evidence for violation of the proportional hazard assumption (Schoenfeld residuals P < 0.001), but the HR for TAVR all-cause mortality risk was 1.02 (95% CI 0.94–1.11). Based on visual assessment of the acquired KM curves and experience from literature [5, 6], landmark analyses were performed (Supplementary Material S8, HR trend in Supplementary Material S9, GRADE certainty of evidence; ‘low’). However, this approach does not account for lives lost prior to the 40-month period, and the difference between the cumulative all-cause mortality rate therefore remains unknown.
Figure 1:
Overall individual-patient data derived from Kaplan–Meier curves survival analysis. Frequentist HR: 1.02 [0.94–1.11], P = 0.60. HR: hazard ratio; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement.
Pooled 5-year actuarial all-cause mortality
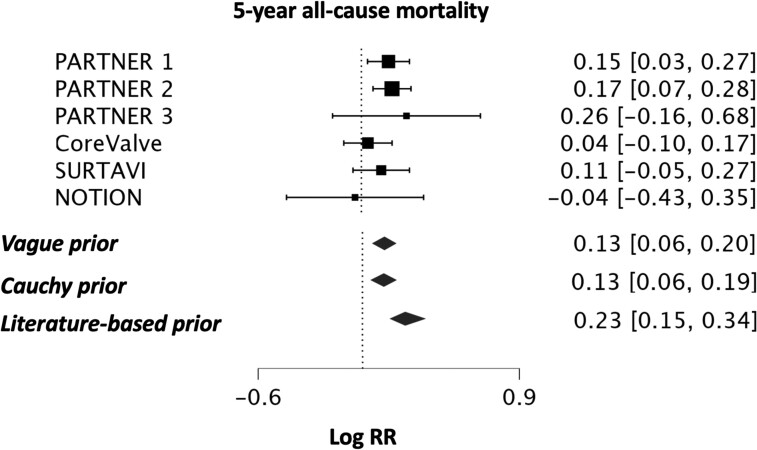
The primary outcome was 5-year all-cause mortality, which was analysed primarily under the Bayesian framework, using all 6 trials reporting on the outcome (n = 6370 patients) [16–20, 30]. Under a vague prior, the posterior mean log RR was 0.13 (TAVR versus SAVR, 95% CrI 0.06–0.20) corresponding to an estimated posterior median RR of 1.14 (TAVR versus SAVR, 95% CrI 1.06–1.22, GRADE certainty of evidence; ‘moderate’, Fig. 2). In a Bayesian sensitivity analysis under a Cauchy prior (median RR 1.13, 95% CrI 1.06–1.22) and by use of frequentist meta-analytical methods (DerSimonian–Laird, Sidik–Jonkman, restricted maximum likelihood, all: RR 1.14, 95% CI 1.07–1.21, P < 0.001), the robustness of these results was confirmed. Heterogeneity was absent or considered unlikely (I2 = 0%, mean τ = 0.084, 0.083, and 0.147, respectively, for the normal, Cauchy and literature-based prior).
Figure 2:
Bayesian meta-analysis of 5-year all-cause mortality under a vague prior, a prior with a Cauchy distribution and a literature-based prior. Effect size is expressed as the log of the RR, assuming a normal probability distribution. RR: relative risk; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement.
Additionally, under a literature-based prior, derived from the matched GARY registry [25], the posterior averaged mean log RR for TAVR 5-year all-cause mortality was 0.23 compared to SAVR ([95% CrI 0.15–0.34], estimated median RR of 1.26 [95% CrI 1.16–1.41]). This informative prior was down-weighted to 75% and 50%, providing similar results (Table 3).
Table 3:
Posterior probabilities of surgical aortic valve replacement 5-year benefit at different relative risk thresholds, in relation to previously established non-inferiority margins
| Posterior median RR | 95% CrI | RR < 1.0 | RR < 1.10 | RR < 1.12 | RR < 1.15 | RR < 1.20 | RR <1.22 | RR < 1.23 | |
|---|---|---|---|---|---|---|---|---|---|
| (Any benefit [%]) | (<10% relative increase [%]) | (PARTNER 1A NI margin [%]) | (CoreValve US NI margin (%]) | (PARTNER 2A NI margin [%]) | (PARTNER 3 NI margin [%]) | (SURTAVI NI margin [%]) | |||
| Primary analyses | |||||||||
| Vague prior | 1.14 | 1.06–1.22 | <0.1 | 18.9 | 35.2 | 63.8 | 93.8 | 97.7 | 98.7 |
| Cauchy prior | 1.13 | 1.06–1.22 | <0.1 | 19.7 | 36.2 | 64.9 | 94.1 | 97.9 | 98.8 |
| Literature-based prior (100%) | 1.26 | 1.16–1.41 | <0.1 | 0.3 | 0.9 | 3. | 15.5 | 24.5 | 29.8 |
| Secondary analyses with down-weighting of the literature-based prior to adjust for the observational character of the GARY registry | |||||||||
| Literature-based prior (down-weighted to 75%) | 1.19 | 1.11–1.31 | <0.1 | 2.6 | 7.8 | 20.3 | 59.2 | 37.6 | 79.7 |
| Literature-based prior (down-weighted to 50%) | 1.17 | 1.09–1.26 | <0.1 | 5.1 | 12.9 | 34.6 | 88.4 | 89.4 | 92.7 |
See Supplementary Material S2 for the rationale of the relative risk non-inferiority thresholds.
CrI: credible interval; NI: non-inferiority; GARY: German Aortic Valve Registry; NI: non-inferiority; RR: relative risk.
Pooled 1- and 2-year actuarial all-cause mortality
As secondary outcomes, 1-year (8 studies, n = 8698) and 2-year (6 studies, n = 7505) all-cause mortalities were analysed under a vague prior (Supplementary Material S10) [16–20, 29].
Posterior probabilities of 5-year all-cause mortality
Under the vague and literature-based priors, the posterior probability of any TAVR all-cause mortality benefit at 5 years (i.e. RR <1.0) was <0.1% (Fig. 3 for the vague prior, Table 3 for the other priors and sensitivity analyses). Furthermore, the posterior probability of a > 10% relative increase in mortality of TAVR at 5 years was 81.1% and 99.7% for the vague and literature-based prior. Figure 3 presents these full posterior probabilities graphically in a grid plot for the analysis under a vague prior.
Figure 3:
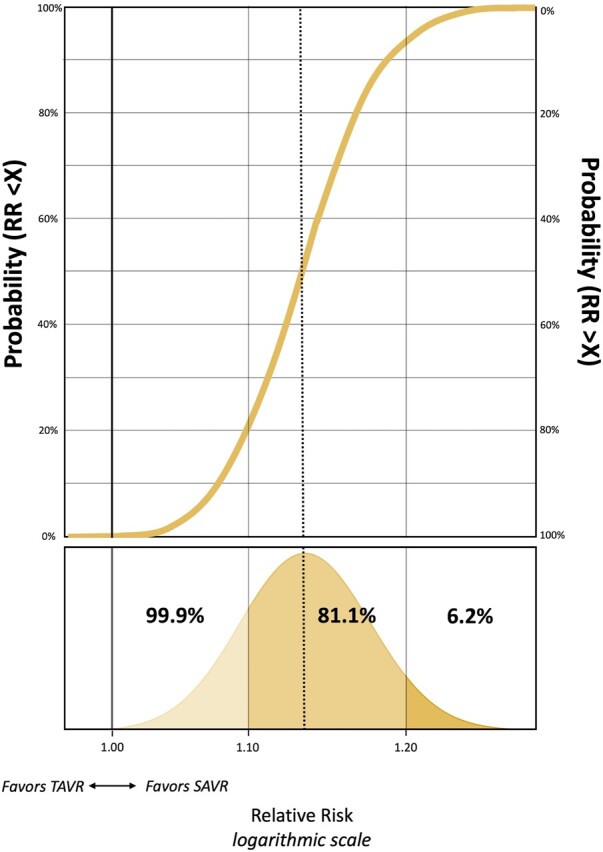
Full posterior probability distribution of a 5-year actuarial all-cause mortality difference in favour of SAVR under a vague prior in grid a plot. The golden curve represents the posterior probability at various RR thresholds, as reflected by the Bell curve in the lower part of the figure. RR: relative risk; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement.
Non-inferiority margins
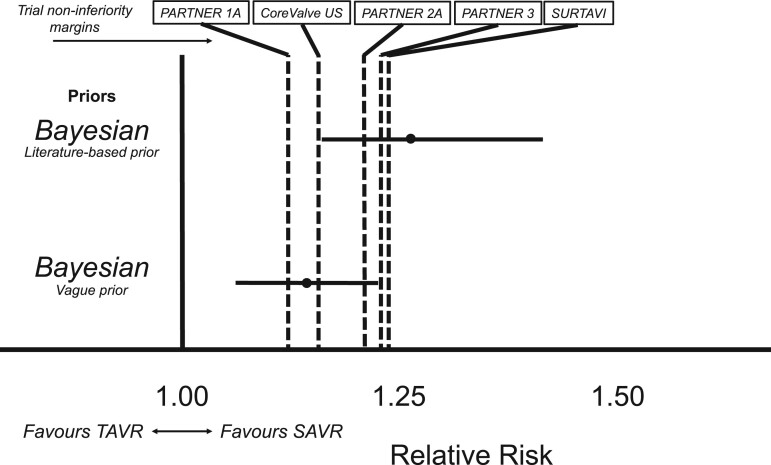
Non-inferiority was considered met if the posterior probability of the non-inferiority margin exceeded 97.1%, as previously specified [21]. Under a vague prior, the 5-year posterior probability of non-inferiority was 35.2% at the PARTNER 1A non-inferiority margin (RR <1.12) [16], 63.8% at the CoreValve US non-inferiority margin (RR <1.15) [17], and 93.8% at the PARTNER 2A non-inferiority margin [19] (RR <1.20, Supplementary Material S2 and Table 3). These findings imply non-inferiority was not met for these trial-specific non-inferiority thresholds. However, non-inferiority was met for the PARTNER 3 margin (RR <1.23), for which the posterior probability exceeded 97.1%, as it was 97.7% [18], and for the SURTAVI margin (98.7% posterior probability of RR <1.23) [20]. Of note, as the lower bound of the 95% CI does not cross the RR of 1.0, while the upper bound does not cross the non-inferiority margin, these findings imply TAVR to be ‘non-inferior’ while at the same time being ‘inferior’, as previously described [1].
Under the literature-based prior, the posterior probabilities for the non-inferiority margins of RR <1.12 (PARTNER 1A), <1.15 (CoreValve US), <1.20 (PARTNER 2A), <1.22 (PARTNER 3) and <1.23 (SURTAVI) were 0.9%, 3.2%, 15.5%, 24.5% and 29.8%, respectively (Table 3). These results suggest that non-inferiority was no longer met for any of the PARTNER 1A, CoreValve US, PARTNER 2A, PARTNER 3 and SURTAVI non-inferiority thresholds when incorporating prior evidence from literature, even when this prior was down-weighted to 50% (Table 3). These observations and corresponding non-inferiority margins are graphically presented in Fig. 4.
Figure 4:
Relative risk distributions for 5-year all-cause mortality using different priors, at different non-inferiority margins. Using the Bayesian approach under a vague prior, TAVR is non-inferior and at the same time inferior [1] to SAVR at 5 years when applying the PARTNER 3 (RR <1.22) and SURTAVI (RR <1.23) non-inferiority margins, while TAVR is no longer non-inferior to SAVR using the PARTNER 1, CoreValve US, PARTNER and 2 non-inferiority margins. Finally, when incorporating prior knowledge from a large registry (the literature-based prior) [25], TAVR is also no longer non-inferior to SAVR at the PARTNER 3 and SURTAVI non-inferiority margins, also when this prior is down-weighted to 50% (Table 3). The dotted lines represent the median RR. The solid horizontal lines represent the 95% CrI. The interrupted vertical lines represent the various non-inferiority margins for the separate trials reporting at 5 years (importantly, NOTION was a superiority trial). See Supplementary Material S2 for the clarification of RR non-inferiority thresholds. See Fig. 2 for the point estimate results under the different priors. CrI: credible interval; RR: relative risk; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement.
Publication bias
There was no indication for publication bias, both visually and statistically (Egger’s test P = 0.811, Supplementary Material S11).
DISCUSSION
The current study is the first to analyse the pooled randomized evidence of TAVR versus SAVR studies under the Bayesian framework. Furthermore, we incorporated the most recent trial results, including the 5-year follow-up of the PARTNER 3 trial [20]. We estimated the difference in all-cause mortality between TAVR and SAVR at 5 years and found convincing evidence for a SAVR survival benefit at that timepoint, and non-inferiority was deemed unlikely. Based on these findings, TAVR may no longer be non-inferior to SAVR regarding all-cause mortality at 5-year follow-up, irrespective of surgical risk.
In frequentist statistics, non-inferiority is met in case of statistical significance (and given the one-sided testing, usually as P < 0.025). In this Bayesian analysis, a posterior probability of >97.1% at the non-inferiority margin was considered as meeting the non-inferiority criterion. This posterior probability is in agreement with the SURTAVI trial, a non-inferiority trial analysed under the Bayesian framework [18, 21]. Therefore, based on our pooled data, non-inferiority was not met for the margins as specified by PARTNER 1A, CoreValve US and PARTNER 2A (RR <1.12, <1.15 and <1.20, respectively). Of note, the SURTAVI and PARTNER 3 trials specified an absolute risk non-inferiority margin of 7.0% and 6.0%, respectively [18, 20], which translate to an RR margin of 1.22 and 1.23 (a 22% and 23% RR difference). In our analysis under a vague prior, the posterior probability at a threshold of RR <1.22 was 97.7% and of RR <1.23, 98.7%, implying non-inferiority was met for the SURTAVI and PARTNER 3 margins. However, it is questionable whether such wide non-inferiority margins are appropriate in intermediate and low-risk patients, given the excellent outcomes of SAVR in these cohorts [32–34]. Such excellent outcomes were recently reconfirmed in an extensive analysis of the Society of Thoracic Surgeons database by Thourani and colleagues, applying the RCTs’ inclusion criteria to low-risk SAVR patients [34].
The external validity and generalizability of results from RCTs are increasingly questioned, as >70% of contemporary cardiovascular trials exhibit significant disparities between the included patients and the typical patient population undergoing the intervention [35, 36]. Moreover, the RCTs included in the current study—with selected populations—also had high deviation rates of assigned treatment, loss to follow-up, and missing data, which may have favoured TAVR and affected internal validity [37]. Some of these limitations may be mitigated by the evaluation of real-world data, derived from registries. Indeed, registry data facilitate a real-world evaluation of a treatment’s outcome across all patient categories but are hampered by other forms of selection bias [38]. Although one can never truly control for selection bias, several methods have been proposed to improve group balance, such as propensity score matching. Therefore, we derived our literature-based prior from the GARY registry [25]. This contemporary German all-comer registry for TAVR and SAVR patients reported 5-year survival and performed propensity score matching to reduce bias. By incorporating the prior evidence derived from the GARY registry data in the pooled results from the included RCTs, Bayesian analyses were performed under this prior and found decisive evidence in favour of a SAVR survival benefit at 5 years. In addition, this analysis also rejected non-inferiority of TAVR at the PARTNER 3 and SURTAVI non-inferiority margin (24.5% and 29.5% posterior probability of a TAVR benefit at these non-inferiority margins).
Based on our analyses, we consider non-inferiority for the outcome of all-cause mortality at 5 years to be unlikely. However, whether this SAVR survival benefit outweighs the potential advantages of TAVR in the short term, remains a matter of subjectivity and should be thoroughly discussed between physicians and patients. Furthermore, the interpretation of the posterior probability of the non-inferiority margin may be a matter of subjectivity. When the non-inferiority margin is smaller (i.e. for PARTNER 1A, CoreValve US, and PARTNER 2A), TAVR is clearly no longer non-inferior to SAVR in terms of all-cause mortality. Only with the incorporation of prior evidence, non-inferiority was not met for the PARTNER 3 and SURTAVI margin (RR <1.22 and RR <1.23), while it was not met under the vague prior (the most unbiased prior, focusing solely on the pooled results). Whether this is a result of true non-inferiority, or an unreasonably wide non-inferiority margin, remains to be determined. As such, our findings should be considered in that context, and are meant to stimulate the reader to consider the various aspects of the non-inferiority design, and whether they are appropriate or not in a study.
Explanations
We found an important survival benefit of SAVR at 5 years, for which several explanations can be hypothesized. Indeed, the long-term durability of TAVR, in terms of valve longevity and patient survival, remains uncertain [4]. The prevalence of paravalvular regurgitation and pacemaker implantation was not studied in the current analysis. Still, mild paravalvular regurgitation has been related to an increased risk of mortality at 5 years [39, 40]. Furthermore, the high rates of permanent pacemaker implantations in TAVR patients [2] (up to 24.6% in contemporary low-risk randomized TAVR trials at 4 years [41]) have also been associated with reduced long-term survival [42]. In addition, bioprosthetic valve thrombosis seems to occur more in patients undergoing TAVR as compared to SAVR [20], which may have further clinical implications.
Implications
Current European guidelines recommend TAVR in patients aged above 75 years, or in patients with a high surgical risk (class I) [43]. American guidelines value SAVR and (transfemoral) TAVR even equally between the ages of 65–80 years—regardless of surgical risk—and prefer TAVR over SAVR when age is above 80 years, or life expectancy is below 10 years (both class I) [44].
Based on the findings of the current analysis, we hypothesize life expectancy to be the most important factor to take into consideration when evaluating the approach for aortic valve replacement. The estimated posterior survival difference at 5 years (mean relative difference of 14%) is clinically meaningful and should be weighed against the potential advantages of TAVR in the shorter term. Furthermore, we should be cautious to equate high-risk ‘young’ patients to high-risk ‘older’ patients, as survival was markedly decreased at 5 years in the overall studied higher-risk populations. In that light, SAVR may still be the most valid option in younger operable patients (<75 years of age), even at higher surgical risk thresholds. These considerations should be weighted heavily in the (shared) decision-making and informed consent process. Furthermore, we should proceed with caution when extending the indication for TAVR to younger patients at low operative risk.
Limitations
Six of 8 included trials have currently reported their results at or beyond 5 years. As the 5-year actuarial timepoint comprised our primary outcome, potentially important survival data of trials prior to this timepoint were not incorporated. This is illustrated by the 4-year results of the Evolut-LR trial, which were recently published in a research letter [41]. In this letter, all-cause mortality was not reported separately, and could therefore not be incorporated in the IPD-KM meta-analysis. For this IPD-KM analysis, the 3-year results were used [29].
Future studies will need to assess the all-cause mortality data at this timepoint when the complete follow-up analyses of these trials are published. Furthermore, the various trials studied different risk populations, and the results may therefore not be generalizable to 1 specific risk subgroup. In addition, the non-inferiority margins were primarily specified in the studies reporting at 1 year. Still, by converting the absolute risk margins to RR margins, we have corrected for this potential limitation. Of note, these margins were specified for the primary ‘composite’ outcome, which included mortality, but also morbidity and other outcomes such as stroke and/or rehospitalization. As such, we assumed that the newly formulated RR non-inferiority margin could be applied equally to all-cause mortality as to the other end-points incorporated in the composite outcome, such as disabling stroke (SURTAVI and PARTNER 2), or disabling stroke and valve-related rehospitalization (PARTNER 3). Still, although we applied non-inferiority margins derived from the included trials, the non-inferiority concept is independent of the statistical methods used, and the margin is open to discussion.
As illustrated above, the incorporated prior can have a marked effect on the results. We have opted to use the GARY-registry as literature-based prior because several attempts to reduce bias were undertaken in this analysis, but the choice of prior remains a heavily debated topic and may be considered subjective as well. In that light, the analyses under the vague prior (assuming no difference) are the least biased methods to evaluate the trials’ results and ensure internal validity of the analysis, while the analyses under the literature-based prior can facilitate external validity and generalizability of our results.
We focused on all-cause mortality, but other important factors were not studied, such as stroke, paravalvular regurgitation and permanent pacemaker implantation.
Several studies had high deviation rates of assigned treatment, loss to follow-up, and missing data. However, these limitations may have even favoured TAVR, as deviation of assigned treatment favours the study intervention in the intention-to-treat analysis of a non-inferiority study [1, 37]. Finally, the devices applied in the studies reporting the longest follow-up may be considered outdated.
CONCLUSION
In the current pooled analysis of most contemporary TAVR versus SAVR RCTs reporting on long-term outcomes, we evaluated long-term all-cause mortality under the Bayesian framework and deemed TAVR non-inferiority to be unlikely at 5 years in terms of all-cause mortality.
Supplementary Material
Glossary
ABBREVIATIONS
- CI
Confidence intervals
- CrI
Credible intervals
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HRs
Hazard ratios
- IPD-KM
Individual patient data derived from Kaplan–Meier curves
- log RR
Log of the relative risk
- RCTs
Randomized controlled trials
- RR
Relative risk
- SAVR
Surgical aortic valve replacement
- TAVR
Transcatheter aortic valve replacement
Contributor Information
Samuel Heuts, Department of Cardiothoracic Surgery, Maastricht University Medical Centre+, Maastricht, Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, Netherlands.
Michal J Kawczynski, Department of Cardiothoracic Surgery, Maastricht University Medical Centre+, Maastricht, Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, Netherlands.
Peyman Sardari Nia, Department of Cardiothoracic Surgery, Maastricht University Medical Centre+, Maastricht, Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, Netherlands.
Jos G Maessen, Department of Cardiothoracic Surgery, Maastricht University Medical Centre+, Maastricht, Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, Netherlands.
Giuseppe Biondi-Zoccai, Department of Medical-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Rome, Italy; Mediterranea Cardiocentro, Napoli, Italy.
Andrea Gabrio, Department of Methodology and Statistics, Maastricht University, Maastricht, Netherlands.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: Peyman Sardari Nia reports consultancy agreements with NeoChord, Edwards Lifesciences, Medtronic, Abbott and Fujifilm, not related to this work. Giuseppe Biondi-Zoccai has consulted for Amarin, Balmed, Cardionovum, Crannmedical, Endocore Lab, Eukon, Innovheart, Guidotti, Meditrial, Microport, Opsens Medical, Replycare, Teleflex, Terumo and Translumina, not related to this work. All other authors report no conflict of interest.
DATA AVAILABILITY
Coding is available through https://github.com/samuelheuts/BayesianSAVRTAVR.
Author contributions
Samuel Heuts: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Visualization; Writing—original draft; Writing—review & editing. Michal Kawczynski: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Visualization; Writing—original draft; Writing—review & editing. Peyman Sardari Nia: Conceptualization; Supervision; Writing—review & editing. Jos G. Maessen: Conceptualization; Supervision; Writing—review & editing. Giuseppe Biondi-Zoccai: Supervision; Validation; Writing—review & editing. Andrea Gabrio: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing—review & editing.
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks Tomislav Kopjar, Roman Gottardi and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Leung JT, Barnes SL, Lo ST, Leung DY.. Non-inferiority trials in cardiology: what clinicians need to know. Heart 2020;106:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad Y, Howard JP, Arnold AD, Madhavan MV, Cook CM, Alu M. et al. Transcatheter versus surgical aortic valve replacement in lower-risk and higher-risk patients: a meta-analysis of randomized trials. Eur Heart J 2023;44:836–52. [DOI] [PubMed] [Google Scholar]
- 3. Siemieniuk RA, Agoritsas T, Manja V, Devji T, Chang Y, Bala MM. et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: systematic review and meta-analysis. BMJ 2016;354:i5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boskovski MT, Nguyen TC, McCabe JM, Kaneko T.. Outcomes of transcatheter aortic valve replacement in patients with severe aortic stenosis: a review of a disruptive technology in aortic valve surgery. JAMA Surg 2020;155:69–77. [DOI] [PubMed] [Google Scholar]
- 5. Barili F, Freemantle N, Musumeci F, Martin B, Anselmi A, Rinaldi M. et al. ; Latin European Alliance of CardioVascular Surgical Societies (LEACSS) and with the endorsement of the Latin American Association of Cardiac and Endovascular Surgery (LACES), LEACSS members are the Italian Society of Cardiac Surgery (FB FM MR MdM AP), the Portuguese Society of Cardiac Surgery (MSU), the French Society of Cardiac Surgery (JFV, AA) and the Spanish Society of Cardiac Surgery (JRR) Institutions. Five-year outcomes in trials comparing transcatheter aortic valve implantation versus surgical aortic valve replacement: a pooled meta-analysis of reconstructed time-to-event data. Eur J Cardiothorac Surg 2022;61:977–87. [DOI] [PubMed] [Google Scholar]
- 6. Barili F, Freemantle N, Pilozzi Casado A, Rinaldi M, Folliguet T, Musumeci F. et al. Mortality in trials on transcatheter aortic valve implantation versus surgical aortic valve replacement: a pooled meta-analysis of Kaplan-Meier-derived individual patient data. Eur J Cardiothorac Surg 2020;58:221–9. [DOI] [PubMed] [Google Scholar]
- 7. Kheiri B, Osman M, Bakhit A, Radaideh Q, Barbarawi M, Zayed Y. et al. Meta-analysis of transcatheter aortic valve replacement in low-risk patients. Am J Med 2020;133:e38–e41. [DOI] [PubMed] [Google Scholar]
- 8. Yarnell CJ, Abrams D, Baldwin MR, Brodie D, Fan E, Ferguson ND. et al. Clinical trials in critical care: can a Bayesian approach enhance clinical and scientific decision-making? Lancet Respir Med 2021;9:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zampieri FG, Casey JD, Shankar-Hari M, Harrell FE Jr, Harhay MO.. Using Bayesian methods to augment the interpretation of critical care trials. An overview of theory and example reanalysis of the alveolar recruitment for acute respiratory distress syndrome trial. Am J Respir Crit Care Med 2021;203:543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ.. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet 2004;364:583–91. [DOI] [PubMed] [Google Scholar]
- 11. Sutton AJ, Abrams KR.. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res 2001;10:277–303. [DOI] [PubMed] [Google Scholar]
- 12. Pepper DJ, Sun J, Welsh J, Cui X, Suffredini AF, Eichacker PQ.. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta-analysis. Crit Care 2016;20:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M. et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev 2012;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferreira D, Barthoulot M, Pottecher J, Torp KD, Diemunsch P, Meyer N.. A consensus checklist to help clinicians interpret clinical trial results analysed by Bayesian methods. Br J Anaesth 2020;125:208–15. [DOI] [PubMed] [Google Scholar]
- 15. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM. et al. ; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477–84. [DOI] [PubMed] [Google Scholar]
- 17. Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS. et al. ; CoreValve U.S. Pivotal High Risk Trial Clinical Investigators. 5-Year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol 2018;72:2687–96. [DOI] [PubMed] [Google Scholar]
- 18. Van Mieghem NM, Deeb GM, Sondergaard L, Grube E, Windecker S, Gada H. et al. ; SURTAVI Trial Investigators. Self-expanding transcatheter vs surgical aortic valve replacement in intermediate-risk patients: 5-year outcomes of the SURTAVI randomized clinical trial. JAMA Cardiol 2022;7:1000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG. et al. ; PARTNER 2 Investigators. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med 2020;382:799–809. [DOI] [PubMed] [Google Scholar]
- 20. Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P. et al. ; PARTNER 3 Investigators. Transcatheter aortic-valve replacement in low-risk patients at five years. N Engl J Med 2023; [DOI] [PubMed] [Google Scholar]
- 21. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, SURTAVI Investigators et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–31. [DOI] [PubMed] [Google Scholar]
- 22. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 23. Liu N, Zhou Y, Lee JJ.. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2021;21:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berkhout SW, Haaf JM, Gronau QF, Heck DW, Wagenmakers EJ.. A tutorial on Bayesian model-averaged meta-analysis in JASP. Behav Res Methods 2023. 10.3758/s13428-023-02093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beyersdorf F, Bauer T, Freemantle N, Walther T, Frerker C, Herrmann E. et al. ; GARY Executive Board. Five-year outcome in 18 010 patients from the German Aortic Valve Registry. Eur J Cardiothorac Surg 2021;60:1139–46. [DOI] [PubMed] [Google Scholar]
- 26. Morey R, Rouder J. BayesFactor 0.9.12-4.2, 2018. http://cranr-projectorg/web/packages/BayesFactor/indexhtml.
- 27. Hammond NE, Myburgh J, Seppelt I, Garside T, Vlok R, Mahendran S. et al. Association between selective decontamination of the digestive tract and in-hospital mortality in intensive care unit patients receiving mechanical ventilation: a systematic review and meta-analysis. JAMA 2022;328:1922–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P. et al. ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B. et al. ; Low Risk Trial Investigators. 3-Year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol 2023;81:1663–74. [DOI] [PubMed] [Google Scholar]
- 30. Thyregod HGH, Ihlemann N, Jorgensen TH, Nissen H, Kjeldsen BJ, Petursson P. et al. Five-year clinical and echocardiographic outcomes from the Nordic Aortic Valve Intervention (NOTION) randomized clinical trial in lower surgical risk patients. Circulation 2019;139:2714–23. [DOI] [PubMed] [Google Scholar]
- 31. Toff WD, Hildick-Smith D, Kovac J, Mullen MJ, Wendler O, Mansouri A. et al. ; UK TAVI Trial Investigators. Effect of transcatheter aortic valve implantation vs surgical aortic valve replacement on all-cause mortality in patients with aortic stenosis: a randomized clinical trial. JAMA 2022;327:1875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thourani VH, Suri RM, Gunter RL, Sheng S, O'Brien SM, Ailawadi G. et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg 2015;99:55–61. [DOI] [PubMed] [Google Scholar]
- 33. Johnston DR, Mahboubi R, Soltesz EG, Artis AS, Roselli EE, Blackstone EH. et al. ; Cleveland Clinic Aortic Valve Center Collaborators. Redefining "low risk": outcomes of surgical aortic valve replacement in low-risk patients in the transcatheter aortic valve replacement era. J Thorac Cardiovasc Surg 2023;165:591–604 e3. [DOI] [PubMed] [Google Scholar]
- 34. Thourani VH, Habib R, Szeto WY, Sabik JF, Romano JC, MacGillivray TE. et al. Survival following surgical aortic valve replacement in low-risk patients: a contemporary trial benchmark. Ann Thorac Surg 2023:S0003-4975(23)01064-0. [DOI] [PubMed] [Google Scholar]
- 35. Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J.. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 2015;16:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Averitt AJ, Weng C, Ryan P, Perotte A.. Translating evidence into practice: eligibility criteria fail to eliminate clinically significant differences between real-world and study populations. NPJ Digit Med 2020;3:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barili F, Brophy JM, Ronco D, Myers PO, Uva MS, Almeida RMS. et al. ; International Evidence Grading Research Initiative Targeting Transparency and Quality (INTEGRITTY). Risk of bias in randomized clinical trials comparing transcatheter and surgical aortic valve replacement: a systematic review and meta-analysis. JAMA Netw Open 2023;6:e2249321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krumholz HM. Registries and selection bias: the need for accountability. Circ Cardiovasc Qual Outcomes 2009;2:517–8. [DOI] [PubMed] [Google Scholar]
- 39. Laakso T, Laine M, Moriyama N, Dahlbacka S, Airaksinen J, Virtanen M. et al. Impact of paravalvular regurgitation on the mid-term outcome after transcatheter and surgical aortic valve replacement. Eur J Cardiothorac Surg 2020;58:1145–52. [DOI] [PubMed] [Google Scholar]
- 40. Okuno T, Tomii D, Heg D, Lanz J, Praz F, Stortecky S. et al. Five-year outcomes of mild paravalvular regurgitation after transcatheter aortic valve implantation. EuroIntervention 2022;18:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B. et al. ; Evolut Low Risk Trial Investigators. 4-Year outcomes of patients with aortic stenosis in the evolut low risk trial. J Am Coll Cardiol 2023;82:2163–5. [DOI] [PubMed] [Google Scholar]
- 42. Pompeu Sá M, Jacquemyn X, Sun T, Van den Eynde J, Tasoudis O, Erten O. et al. Late outcomes of permanent pacemaker implantation after TAVR: meta-analysis of reconstructed time-to-event data. J Soc Cardiovasc Angio Inter 2022;1;370:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. BeyersdorfF, Vahanian A, Praz F, Milojevic M, Baldus S, Bauersachs J. et al. ; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2021;60:727–800. [DOI] [PubMed] [Google Scholar]
- 44. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F. et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 45. Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK. et al. ; PARTNER 3 Investigators. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol 2021;77:1149–61. [DOI] [PubMed] [Google Scholar]
- 46. Forrest JK, Deeb GM, Yakubov SJ, Rovin JD, Mumtaz M, Gada H. et al. 2-Year outcomes after transcatheter versus surgical aortic valve replacement in low-risk patients. J Am Coll Cardiol 2022;79:882–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Coding is available through https://github.com/samuelheuts/BayesianSAVRTAVR.