Abstract
Background
Cardiovascular disease is the most frequent cause of death in people with early stages of chronic kidney disease (CKD), and the absolute risk of cardiovascular events is similar to people with coronary artery disease. This is an update of a review first published in 2009 and updated in 2014, which included 50 studies (45,285 participants).
Objectives
To evaluate the benefits and harms of statins compared with placebo, no treatment, standard care or another statin in adults with CKD not requiring dialysis.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 4 October 2023. Studies in the Register are identified through searches of CENTRAL, MEDLINE, EMBASE, conference proceedings, the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov. An updated search will be undertaken every three months.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs that compared the effects of statins with placebo, no treatment, standard care, or other statins, on death, cardiovascular events, kidney function, toxicity, and lipid levels in adults with CKD (estimated glomerular filtration rate (eGFR) 90 to 15 mL/min/1.73 m2) were included.
Data collection and analysis
Two or more authors independently extracted data and assessed the study risk of bias. Treatment effects were expressed as mean difference (MD) for continuous outcomes and risk ratios (RR) for dichotomous benefits and harms with 95% confidence intervals (CI). The risk of bias was assessed using the Cochrane risk of bias tool, and the certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
We included 63 studies (50,725 randomised participants); of these, 53 studies (42,752 participants) compared statins with placebo or no treatment. The median duration of follow‐up was 12 months (range 2 to 64.8 months), the median dosage of statin was equivalent to 20 mg/day of simvastatin, and participants had a median eGFR of 55 mL/min/1.73 m2. Ten studies (7973 participants) compared two different statin regimens. We were able to meta‐analyse 43 studies (41,273 participants). Most studies had limited reporting and hence exhibited unclear risk of bias in most domains.
Compared with placebo or standard of care, statins prevent major cardiovascular events (14 studies, 36,156 participants: RR 0.72, 95% CI 0.66 to 0.79; I2 = 39%; high certainty evidence), death (13 studies, 34,978 participants: RR 0.83, 95% CI 0.73 to 0.96; I² = 53%; high certainty evidence), cardiovascular death (8 studies, 19,112 participants: RR 0.77, 95% CI 0.69 to 0.87; I² = 0%; high certainty evidence) and myocardial infarction (10 studies, 9475 participants: RR 0.55, 95% CI 0.42 to 0.73; I² = 0%; moderate certainty evidence). There were too few events to determine if statins made a difference in hospitalisation due to heart failure. Statins probably make little or no difference to stroke (7 studies, 9115 participants: RR 0.64, 95% CI 0.37 to 1.08; I² = 39%; moderate certainty evidence) and kidney failure (3 studies, 6704 participants: RR 0.98, 95% CI 0.91 to 1.05; I² = 0%; moderate certainty evidence) in people with CKD not requiring dialysis.
Potential harms from statins were limited by a lack of systematic reporting. Statins compared to placebo may have little or no effect on elevated liver enzymes (7 studies, 7991 participants: RR 0.76, 95% CI 0.39 to 1.50; I² = 0%; low certainty evidence), withdrawal due to adverse events (13 studies, 4219 participants: RR 1.16, 95% CI 0.84 to 1.60; I² = 37%; low certainty evidence), and cancer (2 studies, 5581 participants: RR 1.03, 95% CI 0.82 to 1.30; I² = 0%; low certainty evidence). However, few studies reported rhabdomyolysis or elevated creatinine kinase; hence, we are unable to determine the effect due to very low certainty evidence. Statins reduce the risk of death, major cardiovascular events, and myocardial infarction in people with CKD who did not have cardiovascular disease at baseline (primary prevention). There was insufficient data to determine the benefits and harms of the type of statin therapy.
Authors' conclusions
Statins reduce death and major cardiovascular events by about 20% and probably make no difference to stroke or kidney failure in people with CKD not requiring dialysis. However, due to limited reporting, the effect of statins on elevated creatinine kinase or rhabdomyolysis is unclear. Statins have an important role in the primary prevention of cardiovascular events and death in people who have CKD and do not require dialysis.
Editorial note: This is a living systematic review. We will search for new evidence every three months and update the review when we identify relevant new evidence. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Keywords: Adult; Humans; Creatinine; Hydroxymethylglutaryl-CoA Reductase Inhibitors; Hydroxymethylglutaryl-CoA Reductase Inhibitors/adverse effects; Myocardial Infarction; Myocardial Infarction/prevention & control; Renal Dialysis; Renal Insufficiency, Chronic; Renal Insufficiency, Chronic/complications; Renal Insufficiency, Chronic/therapy; Rhabdomyolysis; Rhabdomyolysis/chemically induced; Rhabdomyolysis/drug therapy; Stroke; Stroke/drug therapy; Systematic Reviews as Topic
Plain language summary
Statins can help reduce risk of death in people with chronic kidney disease who do not need dialysis
What is the issue? Adults with chronic kidney disease (CKD) have a high risk of cardiovascular events, with elevated serum cholesterol and triglycerides, a factor that contributes to cardiovascular disease. Statin therapy helps to lower the level of bad cholesterol (low‐density lipoprotein) and has cardiovascular protective effects beyond cholesterol reduction. For people not needing dialysis, statin therapy has been shown to reduce death and cardiovascular events. Still, studies in this population have shown unclear effects on stroke, kidney failure, and harms such as muscle damage (rhabdomyolysis).
What did we do? We looked at 62 studies published before 4 October 2023 concerning statins in over 50,000 people with CKD who did not need dialysis treatment. This review is a living systematic review. A search for new evidence will be conducted every three months, and the review will be updated accordingly.
What did we find?
We found that compared to placebo, statin therapy reduced death and major heart‐related events, with one in 13 people receiving statin therapy avoiding heart‐related events and one in 26 people avoiding death. Statin therapy probably had little or no effect on stroke and kidney failure (when people would benefit from dialysis or a kidney transplant). The benefits of statin therapy were also evident in patients with CKD but not heart disease. Statins have some potential harms; however, we found there was probably no effect on cancer, liver function or withdrawing from treatment due to adverse events. There was limited reporting of muscle damage in the studies.
Studies did not identify a preferred type or dose of statin in treating people with CKD not requiring dialysis.
Conclusions Statins decrease death, major cardiovascular events, and myocardial infarction in people with moderate CKD. Limited data related to treatment toxicity resulted in uncertain effects.
Editorial note: Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Summary of findings
Summary of findings 1. Statin therapy versus placebo or standard of care for people with chronic kidney disease not requiring dialysis.
| Statin therapy versus placebo or standard of care for people with chronic kidney disease not requiring dialysis | ||||||
| Patient or population: people with chronic kidney disease not requiring dialysis Setting: primary and tertiary care Intervention: statin therapy Comparison: placebo or standard of care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or standard of care | Risk with statin therapy | |||||
| Major cardiovascular events (MACE) follow up: mean 46 months | 6 per 1,000 | 5 per 1,000 (4 to 5) | RR 0.72 (0.66 to 0.79) | 36156 (14) | ⊕⊕⊕⊕ HIGH | Statin therapy reduces major cardiovascular events |
| Death follow up: mean 40 months | 3 per 1,000 | 2 per 1,000 (2 to 3) | RR 0.83 (0.73 to 0.96) | 34978 (13) | ⊕⊕⊕⊕ HIGH | Statin therapy reduces death |
| Rhabdomyolysis follow up: mean 64 months | 1 per 1,000 | 2 per 1,000 (0 to 59) | RR 3.07 (0.13 to 75.37) | 2618 (2) | ⊕⊝⊝⊝ VERY LOW 1 2 | The evidence is very uncertain about the effect of statin therapy on rhabdomyolysis |
| Myocardial infarction follow up: mean 39 months | 3 per 1,000 | 2 per 1,000 (1 to 2) | RR 0.55 (0.42 to 0.73) | 9475 (10) | ⊕⊕⊕⊝ MODERATE 2 | Statin therapy probably reduces myocardial infarction |
| Stroke follow up: mean 40 months | 13 per 1,000 | 8 per 1,000 (5 to 14) | RR 0.64 (0.37 to 1.08) | 9158 (7) | ⊕⊕⊕⊝ MODERATE 3 | Statin therapy probably results in little to no difference in stroke |
| Kidney failure follow up: mean 34 months | 2 per 1,000 | 2 per 1,000 (2 to 2) | RR 0.98 (0.91 to 1.05) | 6704 (3) | ⊕⊕⊕⊝ MODERATE 2 | Statin therapy probably results in little to no difference in kidney failure |
| Hospitalisation due to heart failure follow up: 54 months | 75 per 1,000 | 53 per 1,000 (28 to 99) | RR 0.70 (0.37 to 1.32) | 579 (1) | ⊕⊕⊝⊝ LOW 1 4 5 | Statin therapy may result in little to no difference in hospitalisation due to heart failure |
| *The risk in the intervention group (and its 95% CI) is based on the baseline risk of outcomes from observational studies (and its 95%CI) . The relevant studies in the Summary of Findings Table are listed below: Major cardiovascular events and death ‐ Go 2004 Rhabdomyolysis ‐ based on the assumed risk of outcomes in the comparison group Myocardial infarction ‐ Meisinger 2006 Stroke and hospitalisation due to heart failure ‐ Bansal 2017 Kidney failure ‐ Dalrymple 2011 CI: Confidence interval; RR: Risk ratio; MACE: Major Adverse Cardiac Events | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Very few events
2 Inadequate sequence generation/ generation of comparable groups, resulting in potential for selection bias
3 Effect estimates were on both side of the null
4 Inadequate/lack of blinding of participants and personnel, resulting in potential for performance bias, Inadequate concealment of allocation during randomisation process, resulting in potential for selection bias,
5 Only one study
Background
Description of the condition
The incidence and prevalence of chronic kidney disease (CKD) are increasing, with a global prevalence estimated at around 10% (Global Burden of Disease CKD Collaboration 2020). CKD is a known risk factor for cardiovascular disease (CVD) (Go 2004). Overall cardiovascular deaths are more common than progression to kidney failure in patients with CKD (Gargiulo 2015). For patients with severe kidney disease on long‐term dialysis, 39% of deaths are attributable to cardiovascular causes (USRDS 2019). People with moderate CKD not on dialysis experience risks of death or complications from CVD equivalent to people with heart disease (Go 2004; Henry 2002).
Description of the intervention
HMG‐CoA reductase inhibitors, known as statins, are a commonly prescribed class of cholesterol‐lowering medications used in the primary prevention of atherosclerotic CVD and secondary prevention for those who have developed CVD (Sidebottom 2020). Ezetimibe is another cholesterol‐lowering medication class which has been examined in combination with statins in clinical trials (SHARP 2010).
How the intervention might work
Many traditional and non‐traditional cardiovascular risk factors are prevalent in people with CKD (Muntner 2005; Shilpak 2005). The majority (60%) of people with CKD have elevated serum cholesterol and triglycerides levels and an even higher proportion in those with nephrotic syndrome (Harris 2002). Abnormal lipid levels may contribute to the development of CVD and the initiation and progression of CKD (Drueke 2001; Schaeffner 2003). In the Atherosclerosis Risk in Communities Study (ARIC) and Physicians Health study, elevated lipid levels were associated with lower kidney function (Manjunath 2003). Apart from their lipid‐lowering function, it is plausible that statin use may result in improved kidney function by decreasing urinary protein excretion and inflammation and reducing fibrosis of tubular cells (Kasiske 1988). Ezetimibe prevents intestinal cholesterol absorption through inhibition of the synthetic 2‐azetidinone agent at the brush border of the small intestine (StatPearls Textbook), while statins could reduce kidney disease progression and CVD incidence in people with kidney dysfunction (Massy 2001). Clinical studies in people with CVD have shown that statins safely reduce the five‐year incidence of death or major cardiovascular events by about 20% (Baigent 2005).
Why it is important to do this review
In previous versions of this review published in 2009 and 2014, statins proportionally reduced the risk of death and major cardiovascular events in people with CKD. However, the risks of stroke, adverse events (muscle or liver damage), and kidney failure in these studies were uncertain due to few reported events (Palmer 2014). The previous version of this review relied on the post‐hoc analysis of larger general population studies. Additional studies have been published, warranting a review update. This review has transitioned to a living systematic review. Clinical practice guidelines recommend using statins with or without ezetimibe for people with CKD, not on dialysis (CARI 2013; KDIGO 2013). The Caring for Australian and New ZealandeRs with Kidney Impairment (CARI) Guideline is being updated, and this review will form the underlying evidence review for guideline recommendations.
Objectives
To evaluate the benefits and harms of statin therapy compared with placebo, standard care without statin therapy, or another different statin in adults with CKD who were not on dialysis. A secondary objective is to maintain the currency of the evidence using a living systematic review approach.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) that evaluated the benefits and harms of statins in adults with CKD who were not on dialysis. The first period of randomised cross‐over studies was included. We excluded studies of fewer than eight weeks duration as these studies were unlikely to enable detection of death or cardiovascular outcomes related to statin therapy (Briel 2006).
Types of participants
Inclusion criteria
We included studies enrolling adults with CKD (estimated glomerular filtration rate (eGFR) 90 to 15 mL/min/1.73 m2) (defined and staged according to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines) (KDIGO 2012) who were not on dialysis, including people with persistent urine abnormalities (proteinuria or albuminuria) or structural kidney disease with normal kidney function. Studies were included irrespective of whether participants had CVD at baseline.
Exclusion criteria
Studies of adults with kidney failure treated with dialysis (peritoneal dialysis or haemodialysis) and recipients of a kidney transplant were excluded. Other Cochrane Reviews have specifically reviewed these populations (Palmer 2013; Palmer 2014a).
Types of interventions
We included studies that compared statins with placebo, standard care without statin therapy, or another statin. We excluded studies where a statin was compared with a different lipid‐lowering strategy, including fibrate therapy and ezetimibe.
Types of outcome measures
Standardised Outcomes in NephroloGy (SONG) is currently developing a core outcome set for CKD. This finalised core outcome set will be incorporated in updated versions of this review.
Primary outcomes
Major cardiovascular events
Death
Rhabdomyolysis
Secondary outcomes
Cardiovascular death
Myocardial infarction (MI) (fatal and non‐fatal)
Stroke (fatal and non‐fatal)
Hospitalisation due to heart failure
Kidney failure
Doubling of serum creatinine (SCr)
Acute kidney injury (AKI)
Elevated creatine kinase
Elevated liver enzymes
Withdrawal due to adverse events
Cancer
Onset of diabetes
Revascularisation procedure
End of treatment creatinine clearance (CrCl) or GFR (any measure)
End of treatment proteinuria
-
Serum lipid levels
Total cholesterol
Low‐density lipoprotein (LDL) cholesterol
High‐density lipoprotein (HDL) cholesterol
Triglycerides
Fatigue
Life participation
Memory loss
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies to 4 October 2023 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, and a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Living systematic review considerations
An updated search of the Cochrane Kidney and Transplant Register of Studies will be undertaken every three months.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Contacting relevant individuals/organisations seeking information about unpublished or incomplete studies.
Grey literature sources (e.g. abstracts, dissertations and theses) in addition to those already included in the Cochrane Kidney and Transplant Register of Studies will be searched.
Living systematic review considerations
References lists of newly identified studies from updated searches were checked, and relevant individuals and organisations were contacted if appropriate. Grey literature sources screening will not be periodically undertaken during the living evidence surveillance period.
Data collection and analysis
Selection of studies
The following processes were used in the current update and previous versions of the review. The search strategy described was used to obtain titles and abstracts of studies relevant to the review. Title and abstracts were screened independently by two authors (BC and DJT), who discarded studies that were not eligible. However, studies and reviews thought to include relevant data or information on studies were retained initially for full‐text review. Two authors (BC and DJT) independently assessed retrieved abstracts and the full text, where necessary, to determine which studies satisfied the inclusion criteria. Disagreements between authors were resolved in consultation with a third author (SP).
Living systematic review considerations
The 2023 review update undertook study selection in Covidence (https://www.covidence.org/). Two independent authors (BC and DJT) immediately screened the retrieved citations from three monthly searches. Disagreements were resolved in consultation with a third author (SP).
Data extraction and management
Data extraction was carried out independently by authors (DJT, BC) using Robot Reviewer (https://www.robotreviewer.net/). Data extraction was checked independently by authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped. Data were obtained from all relevant reports, including long‐term follow‐up data if the attrition rate was less than 80% of randomised participants. Any discrepancies between published versions were highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the Cochrane risk of bias assessment tool (Higgins 2022) (Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
For dichotomous outcomes (major cardiovascular events, death, rhabdomyolysis, kidney failure, doubling of SCr, onset of diabetes, revascularisation procedure, memory loss, elevated liver enzymes, elevated creatine kinase, withdrawal due to adverse events, and cancer), results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (lipid parameters, proteinuria, kidney function (CrCl or eGFR), fatigue, and life participation), the mean difference (MD) was used, or the standardised mean difference (SMD) where different scales were used for outcome measurement.
Unit of analysis issues
Cross‐over RCTs were analysed using only the data from the first period. Studies with multiple treatment arms were included. When considering these studies, we tried to combine all relevant intervention groups into a single group when appropriate, i.e. high‐ and low‐dose statin, to allow a single pairwise comparison. If appropriate, we compared the intervention arms against one another. It was planned that cluster RCTs were not included in the meta‐analysis and were analysed and reported separately. However, no cluster RCTs were included in the review.
Dealing with missing data
Any further information required from the original author was requested, and relevant information was included in the review. Evaluation of important numerical data, such as screened or randomised patients and intention‐to‐treat, as‐treated, and per‐protocol population, was performed. Attrition rates, such as drop‐outs, losses to follow‐up, and withdrawals, were investigated, including the balance between treatment groups. Issues of missing data and imputation methods (e.g. last observation carried forward) were critically appraised (Higgins 2022).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies likely due to statistical heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I² values was as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depended on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P value from the Chi² test or a CI for I²) (Higgins 2022).
Meta‐regression for age and GFR was undertaken for outcomes with more than 10 studies in which meta‐analysis demonstrated evidence of substantial or considerable heterogeneity.
Assessment of reporting biases
To assess potential bias from small‐study effects, we constructed funnel plots for the log risk ratio in individual studies against the standard error of the risk ratio. Statistical assessment of funnel plot asymmetry with the Egger regression test (Harbord 2006) using R (R Core Team 2013 ‐ http://www.R-project.org/) to assess publication bias if required or sufficient data is available).
Data synthesis
Data were pooled using the random‐effects model, but the fixed‐effect model was also used to ensure the robustness of the model chosen and susceptibility to outliers.
The anticipated absolute effects have been calculated from baseline risks reported in published observational studies that examine the incidence of cardiovascular outcomes, death, and kidney failure in people with CKD (Bansal 2017; Dalrymple 2011; Go 2004; Meisinger 2006). These studies were selected as they were large multi‐national observational studies conducted on people with CKD that may be generalisable to the populations in the identified RCTs in this review. When no published data was available, the assumed risk of the outcomes was given by the event rates in the comparison group.
Living systematic review considerations
All included studies in future review updates will undergo data extraction and critical appraisal by two authors (BC and DJT) independently, using Robot Reviewer (https://www.robotreviewer.net/) as a supportive tool. Whenever new evidence (i.e. studies, data or other information) relevant to the review is identified, we will extract the data and assess the risk of bias as appropriate. We will wait until the accumulating evidence changes one or more of the following components of the review before incorporating it and re‐publishing the review.
The findings of one or more primary outcomes
The credibility (e.g. GRADE rating) of one or more primary outcomes
New settings, populations, interventions, comparisons or outcomes were examined
New serious adverse events.
Formal sequential meta‐analysis approaches will not be used for updated meta‐analyses.
Subgroup analysis and investigation of heterogeneity
For outcomes with more than 10 studies, we planned to conduct subgroup analyses to explore potential sources of heterogeneity in modifying estimates of the effects of statins if substantial or considerable heterogeneity or significant heterogeneity (P < 0.1) was present in the primary analysis. We planned subgroup analyses according to participant, intervention, or study‐related characteristics when subgroups contained four or more independent studies (statin type, statin dose (equivalent to simvastatin, baseline total cholesterol (< 230 mg/dL versus ≥ 230 mg/dL), age (≤ 55 years versus > 55 years), the proportion with diabetes (≥ 20% versus < 20%), presence or absence of CVD (primary versus secondary prevention), or adequacy of allocation concealment. Additionally, post‐hoc analyses were conducted on the impact of baseline kidney function on the following outcomes: kidney failure, doubling of SCr, end‐of‐treatment kidney function, and end‐of‐treatment proteinuria.
We conducted meta‐regression using a mixed‐effects model with the Metafor package for R (R Core Team 2013) for primary outcomes with more than 10 studies and substantial or considerable heterogeneity. Age and kidney function were considered covariates for meta‐regression.
Sensitivity analysis
The following sensitivity analyses were considered to explore the influence of the following factors on effect size.
Repeating the analysis, excluding unpublished studies
Repeating the analysis taking into account the risk of bias
Repeating the analysis, excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, the language of publication, source of funding (industry versus other), and country.
However, there were insufficient data for sensitivity analyses for unpublished studies, risk of bias, the language of publication, and country.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in the 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2022a). The 'Summary of findings' tables also include an overall grading of the evidence‐related main outcome using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of the within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, the precision of effect estimates and risk of publication bias (Schünemann 2022b). We presented the following outcomes in the 'Summary of findings' tables.
Major cardiovascular events
Death
Rhabdomyolysis
Cardiovascular death
MI
Stroke
Hospitalisation due to heart failure
Kidney failure
Methods for future updates
Living systematic review considerations
The conduct and production of this living systematic review will be overseen by the CARI Guidelines Living Evidence Working Group and the Statins in CKD Guideline Working Group. We will review the scope and methods when updating the review in light of potential changes in the topic area or new evidence (e.g. when additional comparisons, interventions, subgroups, outcomes, or new review methods become available). At every three‐month search, we will consider the necessity for the review to be living. By assessing the questions' ongoing relevance to decision‐makers and determining if continuing uncertainty in the evidence is present, we will determine whether further relevant research is likely to change recommendations for clinical practice via the CARI Guidelines Statins in CKD Living Guideline.
Results
Description of studies
The following section contains broad descriptions of the studies considered in this review. For further details on each individual study, please see Included studies; Excluded studies; Studies awaiting classification; Ongoing studies.
Results of the search
Previous versions of this Cochrane review (Navaneethan 2009; Palmer 2014) identified 47 studies (39,738 participants) compared statin therapy with placebo or no treatment, and three studies (5547 participants) compared a statin with another statin (Table 2).
1. List of included studies according to Cochrane review update.
2023 review update
For this update, we searched the Cochrane Kidney and Transplant Register of Studies (4 October 2023) and identified 134 new reports. Thirteen new studies (22 reports) were included, 20 (50 reports) were excluded, and one ongoing study was identified. One new study is awaiting classification (recently completed; no data available). We also identified 61 new reports of existing included studies and studies awaiting classification.
We reassessed and reclassified seven ongoing studies: three studies have been included (Ohsawa 2015; PLANET I 2006; PLANET II 2006); four studies were excluded (Mose 2014a; Mose 2014b; Weinstein 2013; Zinellu 2012); and one study has been moved to awaiting classification as no data have been published (NCT00768638). Eighteen previously excluded studies were deleted because they were not randomised (4), enrolled the wrong population (4), or did not use statins (10).
A total of 63 studies were included (336 reports, 50,725 randomised participants), 32 were excluded, two are awaiting classification, and there is one ongoing study (Figure 1).
1.
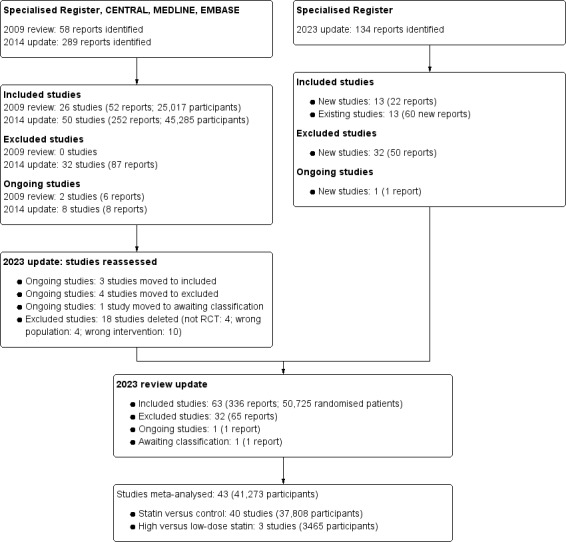
Flow diagram showing study selection
Included studies
Details are provided in Included studies.
Fifty‐three studies (42,752 randomised participants) compared statin therapy to placebo or no treatment. Nine studies compared high to low‐dose statin (7851 randomised participants), and one study (122 randomised participants) compared continuous‐release versus intermittent‐release statin. Of the 63 included studies, 11 studies provided post hoc data for subgroups of 31,315 adults with CKD within larger studies that compared statins with placebo or no treatment (4S 1993; AFCAPS/TexCAPS 1997; ALLIANCE 2000; ASCOT‐LLA 2003; CARDS 2003; HPS 2002; JUPITER 2007; LIPS 2001; MEGA 2004; PPP 1992; SAGE 2004). Two comparisons provided post hoc data for 5428 adults comparing two statin regimens (IDEAL 2004; TNT 2004). Overall, 43 studies (41,273 participants) contributed to the meta‐analyses.
Statin versus placebo or no treatment
Studies varied in sample size (median 55 participants; range 14 to 3107 participants). Seven comparisons that included more than 1000 participants (4S 1993; ASCOT‐LLA 2003; HPS 2002; JUPITER 2007; MEGA 2004; PPP 1992; SHARP 2010).
The median statin dose (equivalent to simvastatin) was 20 mg (range 5 to 80 mg/day). Twelve studies used simvastatin, 10 studies used atorvastatin, nine studies used pravastatin, nine studies used fluvastatin, four studies used pitavastatin, four studies used rosuvastatin, two studies used lovastatin, one study used cerivastatin, one study used simvastatin with ezetimibe (SHARP 2010). One study used simvastatin or pravastatin (Scanferla 1991) (Table 3).
2. Studies classified by type of statin therapy.
| Atorvastatin (dose) | Cerviastatin (dose) | Fluvastatin (dose) | Lovastatin (dose) | Pitavastatin (dose) | Pravastatin (dose) | Rosuvastatin (dose) | Simvastatin (dose) | Simvastatin and ezetimibe (dose) |
| ALLIANCE 2000 (10 mg/d) | Nakamura 2002 (0.15 mg/d) | Buemi 2000 (40 mg/d) | AFCAPS/TexCAPS 1997 (20 mg/d) | Nakamura 2005 (10 mg/d) | Aranda Arcas 1994 (20 mg/d) | Abe 2011c (5 mg/d) | 4S 1993 (20 mg/d) | SHARP 2010 (20 mg/d and 10 mg/d) |
| ASCOT‐LLA 2003 (10 mg/d) | Cha 2015 (20 mg/d) | Lam 1995 (20 mg/d) | Nakamura 2006 (10 mg/d) | ASUCA 2013 (mean 12.5 mg/d) | JUPITER 2007 (20 mg/d) | Dummer 2008 (20 mg/d) | ||
| Bianchi 2003 (40 mg/d) | Di Lullo 2005 (80 mg/d) | Ohsawa 2015 (20 mg/d) | Imai 1999 (mean 7.5 mg/d) | Sawara 2008 (2.5 mg/d) | Fassett 2010 (10 mg/d) | |||
| CARDS 2003 (10 mg/d) | ESPLANADE 2010 (80 mg/d) | Tokunaga 2008 (40 mg/d) | Lee 2002 (10 mg/d) | Verma 2005 (10 mg/d) | Fried 2001 (10 mg/d) | |||
| Goicoechea 2006 (20 mg/d) | Gheith 2002 (20 mg/d) | MEGA 2004 (15 mg/d) | Hommel 1992 (15 mg/d) | |||||
| Inukai 2011 (10 mg/d) | Lintott 1995 (40 mg/d) | Mori 1992 (10 mg/d) | HPS 2002 (40 mg/d) | |||||
| LORD 2006 (10 mg/d) | LIPS 2001 (20 mg/d) | PPP 1992 (40 mg/d) | Nielsen 1993 (15 mg/d) | |||||
| Masajtis‐Zagajewska 2018 (20 mg/d) | Samuelsson 2002 (40 mg/d) | PREVEND IT 2000 (40 mg/d) | Panichi 2006a (40 mg/d) | |||||
| Renke 2010a (40 mg/d) | Yasuda 2004 (20 mg/d) | Scanferla 1991 (10 mg/d) | Rayner 1996 (25 mg/d) | |||||
| Stegmayr 2005 (10 mg/d) | Zhang 1995 (20 mg/d) | Scanferla 1991 (10 mg/d) | ||||||
| SHARP 2010 (20 mg/d with ezetimibe 10 mg/d) | ||||||||
| Thomas 1993 (40 mg/d) | ||||||||
| Tonolo 1997 (20 mg/d) | ||||||||
| UK‐HARP‐I 2005 (20 mg/d) | ||||||||
| High‐dose statin versus low‐dose statin | ||||||||
| PANDA 2011 (80 mg/d versus 10 mg/d) | PLANET I 2006 (40 mg/d versus 10 mg/d) | |||||||
| TNT 2004 (80 mg/d versus 10 mg/d) | PLANET II 2006 (40 mg/d versus 10 mg/d) | |||||||
The median follow‐up duration was 12 months (range 2 to 66 months). Studies reporting death outcomes that could be included in meta‐analyses had a median follow‐up duration of 44 months (range 5 to 65 months).
Although three studies enrolled participants with established acute or stable coronary artery disease (4S 1993; ALLIANCE 2000; LIPS 2001), 12 studies excluded participants with clinical coronary artery disease (Abe 2011c; AFCAPS/TexCAPS 1997; CARDS 2003; Fried 2001; Imai 1999; JUPITER 2007; Lam 1995; MEGA 2004; Nakamura 2002; Nakamura 2005; Ohsawa 2015; SHARP 2010). Median baseline LDL cholesterol was 143.9 mg/dL (range 108.5 to 189 mg/dL) (3.72 mmol/L; range 2.80 to 4.89 mmol/L)
Ten studies included only participants who had diabetes (Abe 2011c; CARDS 2003; Hommel 1992; Inukai 2011; Lam 1995; Mori 1992; Nakamura 2005; Nielsen 1993; Tonolo 1997; Zhang 1995); and people with diabetes were excluded from eight studies (Bianchi 2003; Gheith 2002; Lee 2002; Nakamura 2002; Nakamura 2006; Ohsawa 2015; Panichi 2006a; Renke 2010a). Stegmayr 2005 combined outcome data for adults not on dialysis with those on dialysis; data from this study were not included in the meta‐analyses.
Effects of statins on adverse events data from SHARP 2010 could not be included in analyses because disaggregated data for 6247 adults with CKD were not available.
The Pravastatin Pooling Project (PPP 1992) was a pooled analysis of three large data sets of participants with kidney impairment who were included in three major statin studies (LIPID 1998; Sacks 1996; Shepherd 1995) conducted in the general population and were included as a single comparison.
High versus low dose statin
PANDA 2011 and TNT 2004 compared two doses of atorvastatin, while PLANET I 2006 and PLANET II 2006 compared low‐ and high‐dose rosuvastatin. The median follow‐up was 24 months (range 12 to 58.8 months). Meta‐analysis was undertaken when two or more studies were available.
Continuous release versus intermittent release statin
Yi 2014 compared continuous‐release statin (20 mg/day) administered in the morning with intermittent‐release statin (20 mg/day) administered in the evening for two months. The study included 122 participants, with a mean LDL cholesterol of 140.5 ± 28.3 mg/day (3.63 ± 0.73 mmol/L).
Statin versus another statin
PLANET I 2006 and PLANET II 2006 compared rosuvastatin and atorvastatin
IDEAL 2004 compared simvastatin with atorvastatin
Abe 2015 compared rosuvastatin with pitavastatin
Ikeda 2012 compared pravastatin with rosuvastatin
Kimura 2012 compared pitavastatin with pravastatin
SAGE 2004 compared atorvastatin with pravastatin.
Sample sizes ranged from 28 to 3107 participants. The median follow‐up was 12 months (range 12 to 57.6 months). Baseline LDL cholesterol levels ranged from 123.6 to 150.8 mg/dL (3.20 to 3.90 mmol/L). Meta‐analysis was not possible because three or more studies were not available for each comparison.
Excluded studies
See Excluded studies.
Thirty‐two studies did not meet our eligibility criteria for the following reasons.
Wrong intervention (11 studies): did not compare a statin with placebo or standard of care without a statin (Almquist 2012; IMPROVE‐IT 2008; Ishimitsu 2014; Kouvelos 2013; NCT03543774; Suzuki 2013a; UK‐HARP‐II 2006; Weinstein 2013; Yasmeen 2015; Yasuda 2010; Zinellu 2012)
No comparator: one study (ESTABLISH 2004)
Wrong study design: three studies (Mose 2014a; Mose 2014b; Siddiqi 2011)
Wrong population: eight studies (Almukhtar 2021; Fotso 2021; Mackie 2008; Mohammadi 2023; PACTR202110707328144; Vasin 2021; Watanabe 2022; Yen 2022)
Unclear duration or less than eight weeks: nine studies (Dogra 2005; Dogra 2007; Golper 1987; Golper 1989; Ott 2008; Paulsen 2010; Schmieder 2003; Torraca 2006; Van Dijk 2001).
Risk of bias in included studies
Seven studies comparing statins with placebo or no treatment were conducted according to published protocols, outcomes were adjudicated by a committee, specified outcomes were reported, and analyses were conducted using intention‐to‐treat methods (4S 1993; HPS 2002; JUPITER 2007; PPP 1992; PREVEND IT 2000; SHARP 2010; UK‐HARP‐I 2005). In placebo or no treatment‐controlled studies, adverse events were reported in 33 studies (63%) and systematically evaluated in 17 studies (33%). See Included studies for detailed information on study risk of bias assessment.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
Random sequence generation was low risk in 15 of the included studies, and the remaining 48 studies were judged to have an unclear risk of bias.
Allocation concealment
Allocation concealment was low risk in 19 studies, and the remaining 44 studies were judged to have an unclear risk of bias.
In studies that examined the primary outcomes, six of 17 studies were low risk for random sequence generation, and nine of 17 were low risk for allocation concealment.
Blinding
Performance bias
Participants and personnel were blinded in 27 studies and considered low risk, and 24 studies were open‐label and hence high risk, with the remaining 12 studies did not report blinding and were judged to have an unclear risk of bias.
Detection bias
Sixteen blinded outcome assessors and four studies were high risk with no blinding of outcome assessors. The remaining 43 studies were judged to have an unclear risk of bias.
In studies that reported the primary outcome, nine of 17 studies were blinded study participants and personnel, and outcome assessors.
Incomplete outcome data
Completeness of outcome reporting was applied in 26 of the included studies, and 18 included studies exhibited a high risk of incomplete outcome reporting. The remaining 19 studies did not provide sufficient information to permit judgement and were judged to have an unclear risk of bias. Ten of the 17 studies that reported the primary outcomes exhibited a low risk of attrition bias.
Selective reporting
Selective outcome reporting occurred in four studies, and only four studies exhibited a low risk of selective outcome reporting. The remaining 55 studies had unclear reporting, with studies not reporting a protocol or clinical registry number. Only two studies that reported the primary outcomes of the review exhibited a low risk of selective reporting.
Other potential sources of bias
The risk of bias due to sources of funding was high in 14 studies, 21 studies exhibited a low risk of any other bias, while in 28 studies the risk of bias was unclear. Nine studies that reported the primary outcomes of the review exhibited a low risk for other potential sources of bias.
Effects of interventions
See: Table 1
Statin therapy versus placebo
The main findings of the analysis are presented in Table 1.
Primary outcomes
Compared to placebo, statin therapy reduces major cardiovascular events (Analysis 1.1 (14 studies, 36,156 participants): RR 0.72, 95% CI 0.66 to 0.79; I² = 39%; high certainty evidence) and death (Analysis 1.2 (13 studies, 34,978 participants): RR 0.83, 95% CI 0.73 to 0.96; I² = 53%; high certainty evidence).
1.1. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 1: Major cardiovascular events
1.2. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 2: Death
Statins had uncertain effects on rhabdomyolysis due to study limitations and very serious imprecision (Analysis 1.3 (2 studies, 2618 participants): RR 3.07, 95% CI 0.13 to 75.37; very low certainty).
1.3. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 3: Rhabdomyolysis
Secondary outcomes
Cardiovascular endpoints
Compared to placebo, statin therapy probably reduces cardiovascular death (Analysis 1.4 (8 studies, 19,112 participants): RR 0.77, 95% CI 0.69 to 0.87; I² = 0; moderate certainty evidence) and MI (Analysis 1.5 (10 studies, 9475 participants): RR 0.55, 95% CI 0.42 to 0.73; I² = 0%; moderate certainty evidence).
1.4. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 4: Cardiovascular death
1.5. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 5: Myocardial infarction
Statin therapy probably has little or no effect on stroke (Analysis 1.6 (7 studies, 9115 participants): RR 0.64, 95% CI 0.37 to 1.08; I² = 39%; moderate certainty evidence) but probably decreases revascularisation procedures (Analysis 1.7 (3 studies, 4156): RR 0.62, 95% CI 0.47 to 0.82; I² = 0%; moderate certainty evidence), and may have no difference on hospitalisation due to heart failure (Analysis 1.8 (1 study, 579 participants): RR 0.70, 95% CI 0.37, to 1.32; low certainty evidence).
1.6. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 6: Stroke
1.7. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 7: Revascularisation procedure
1.8. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 8: Hospitilsation due to heart failure
Kidney endpoints
Compared to placebo, statin therapy probably has no protective kidney effect with little or no effect on kidney failure (Analysis 1.9 (3 studies, 6704 participants): RR 0.98, 95% CI 0.91 to 1.05, I² = 0%; moderate certainty evidence), and doubling of SCr (Analysis 1.10 (1 study, 6245 participants): RR 0.90, 95% CI 0.79 to 1.02; moderate certainty evidence).
1.9. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 9: Kidney failure
1.10. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 10: Doubling serum creatinine
Statin therapy probably improves kidney function (CrCl or eGFR) (Analysis 1.11 (18 studies, 4213 participants): MD 1.83, 95% CI 0.27 to 3.39 mL/min; I² = 17%; moderate certainty evidence). No difference was found with the use of measured CrCl compared to eGFR (test for subgroup differences: Chi² = 3.11, df = 1 (P = 0.08), I² = 67.8%).
1.11. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 11: End of treatment kidney function
Compared to placebo, statin therapy may lower proteinuria (Analysis 1.12 (7 studies, 356 participants): MD ‐0.47 g/24 hours, 95% CI ‐0.75 to ‐0.19; I² = 81%; low certainty evidence) with substantial heterogeneity.
1.12. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 12: End of treatment proteinuria
Safety endpoints
Statin therapy may have little to no effect on elevated liver enzymes (Analysis 1.13 (7 studies, 7991 participants): RR 0.76, 95% CI 0.39 to 1.50; I² = 0%; low certainty evidence), withdrawal due to adverse events (Analysis 1.14 (13 studies, 4219 participants): RR 1.10, 95% CI 0.83 to 1.48; I² = 37%; low certainty evidence), cancer (Analysis 1.15 (2 studies, 5581 participants): RR 1.03, 95% CI 0.82 to 1.30; I² = 0%; low certainty evidence), and onset of diabetes (Analysis 1.16) (1 study, 3267 participants): RR 1.03, 95%CI 0.71 to 1.50; low certainty evidence).
1.13. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 13: Elevated liver enzymes
1.14. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 14: Withdrawal due to adverse events
1.15. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 15: Cancer
1.16. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 16: Onset of diabetes
Compared to placebo, the effect of statin therapy on elevated creatinine kinase was unclear because of serious study limitations and very serious imprecision (Analysis 1.17 (7 studies, 4514 participants): RR 0.84, 95% CI 0.20 to 3.48; I2 = 0%; very low certainty evidence).
1.17. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 17: Elevated creatine kinase
Fatigue, life participation, and memory loss were not reported by any of the included studies.
Lipid endpoints
Statins compared to placebo probably lowers total serum cholesterol (Analysis 1.18 (27 studies, 2234 participants): MD ‐50.26 mg/dL, 95%CI ‐64.34 to ‐36.17 mg/dL (MD ‐1.3 mmol/L, 95% CI ‐1.67 to ‐0.97 mmol/L); I² = 96%; moderate certainty evidence), may decrease LDL cholesterol (Analysis 1.19 (24 studies, 2183 participants): MD ‐43.84 mg/dL, 95%CI ‐52.56 to ‐35.12 mg/dL (MD ‐1.14 mmol/L, 95% CI ‐1.37 to 0.91 mmol/L); I² = 92%; low certainty evidence) and triglycerides (Analysis 1.20 (20 studies, 1186 participants): MD ‐26.02 mg/dL, 95%CI ‐40.99 to ‐11.06 mg/dL (MD ‐0.29 mmol/L, 95% CI ‐0.45 to ‐1.22 mmol/L); I² = 82%; low certainty evidence), and may increase HDL cholesterol (Analysis 1.21 (22 studies, 1374 participants): MD 2.92 mg/dL, 95% CI ‐0.03 to 5.88 mg/dL (MD 0.08 mmol/L, 95% CI 0.0001 to 0.15 mmol/L); I² = 81%; low certainty evidence). Analyses of treatment effects on serum lipids showed evidence for substantial heterogeneity (I² > 80%) for all outcomes.
1.18. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 18: Total cholesterol
1.19. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 19: LDL cholesterol
1.20. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 20: Triglycerides
1.21. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 21: HDL cholesterol
No other secondary outcomes were reported in the included studies.
Subgroup analyses
Cardiovascular disease
When we limited analyses to studies in which CVD was an exclusion criterion at baseline, effect modification was not evident for death (Analysis 1.22: test for subgroup differences: Chi² = 1.40, df = 1 (P = 0.24), I² = 28.5%). However, the test of subgroup differences indicated potential effect modification for major cardiovascular events, but it was considered not important as the direction of the effect estimates were in the same direction, indicating a benefit of therapy and cross‐over of the 95% CIs (Analysis 1.23: test for subgroup differences: Chi² = 2.71, df = 1 (P = 0.10), I² = 63.0%).
1.22. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 22: Subgroup analysis (cardiovascular disease): death
1.23. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 23: Subgroup analysis (cardiovascular disease): major cardiovascular events
Statin dose
When comparing < 20 mg/day simvastatin equivalent with ≥ 20 mg/day simvastatin equivalent, effect modification was evident for major cardiovascular events (Analysis 1.24: test for subgroup differences: Chi² = 10.27, df = 1 (P = 0.001), I² = 90.3%) but was not important as the effect estimates were in the same direction and overlap of the 95% CIs. Test for subgroup differences found no evidence of effect modification for statin dose for the outcomes of death (Analysis 1.25), withdrawal due to adverse effects (Analysis 1.26), total cholesterol (Analysis 1.27), LDL cholesterol (Analysis 1.28), HDL cholesterol (Analysis 1.29), or triglycerides (Analysis 1.30).
1.24. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 24: Subgroup analysis (statin dose): major cardiovascular death
1.25. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 25: Subgroup analysis (statin dose): death
1.26. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 26: Subgroup analysis (statin dose): withdrawal due to adverse events
1.27. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 27: Subgroup analysis (statin dose): total cholesterol
1.28. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 28: Subgroup analysis (statin dose): LDL cholesterol
1.29. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 29: Subgroup analysis (statin dose): HDL cholesterol
1.30. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 30: Subgroup analysis (statin dose): triglycerides
Age
Comparing age (< 55 years versus ≥ 55 years), a test of subgroup difference was significant for total cholesterol (Analysis 1.31: test for subgroup differences: Chi² = 2.91, df = 1 (P = 0.09), I² = 65.6%). However, effect modification was not considered important because the subgroup effect estimates were in the same direction and overlap of the 95% CIs. Other outcomes did not demonstrate any effect modification (withdrawal due to adverse effects (Analysis 1.32), LDL cholesterol (Analysis 1.33), HDL cholesterol (Analysis 1.34), or triglycerides (Analysis 1.35)).
1.31. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 31: Subgroup analysis (age): total cholesterol
1.32. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 32: Subgroup analysis (age): withdrawal due to adverse events
1.33. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 33: Subgroup analysis (age): LDL cholesterol
1.34. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 34: Subgroup analysis (age): HDL cholesterol
1.35. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 35: Subgroup analysis (age): triglycerides
Diabetes mellitus
Effect modification was examined for DM (< 20% versus ≥ 20%) and was evident for withdrawal due to adverse events (Analysis 1.36: test for subgroup differences: Chi² = 3.60, df = 1 (P = 0.06), I² = 72.2%) but not for death (Analysis 1.37), major cardiovascular death (Analysis 1.38), total cholesterol (Analysis 1.39), LDL cholesterol (Analysis 1.40) HDL cholesterol (Analysis 1.41), or triglycerides (Analysis 1.42).
1.36. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 36: Subgroup analysis (diabetes mellitus): withdrawal due to adverse events
1.37. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 37: Subgroup analysis (diabetes mellitus): death
1.38. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 38: Subgroup analysis (diabetes mellitus): major cardiovascular death
1.39. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 39: Subgroup analysis (diabetes mellitus): total cholesterol
1.40. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 40: Subgroup analysis (diabetes mellitus): LDL cholesterol
1.41. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 41: Subgroup analysis (diabetes mellitus): HDL cholesterol
1.42. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 42: Subgroup analysis (diabetes mellitus): triglycerides
Baseline cholesterol
When baseline cholesterol at study entry was ≥ 230 mg/dL compared to < 230 mg/dL, test for subgroup differences were found for the outcomes, total cholesterol (Analysis 1.43: test for subgroup differences: Chi² = 4.47, df = 1 (P = 0.03), I² = 77.6%) and LDL cholesterol (Analysis 1.44: test for subgroup differences: Chi² = 10.02, df = 1 (P = 0.002), I² = 90.0%). The subgroup differences were not considered important because the subgroups are in the same direction and the 95% CI overlap. For the outcomes of withdrawal due to adverse events (Analysis 1.45), HDL cholesterol (Analysis 1.46), and triglycerides (Analysis 1.47), no effect modification was evident.
1.43. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 43: Subgroup analysis (baseline cholesterol): total cholesterol
1.44. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 44: Subgroup analysis (baseline cholesterol): LDL cholesterol
1.45. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 45: Subgroup analysis (baseline cholesterol): withdrawal due to adverse events
1.46. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 46: Subgroup analysis (baseline cholesterol): HDL cholesterol
1.47. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 47: Subgroup analysis (baseline cholesterol): triglycerides
Allocation concealment
We assessed if allocation concealment resulted in effect modification and found that for the outcome of death, a test of subgroup differences was significant (Analysis 1.48: test for subgroup differences: Chi² = 5.70, df = 1 (P = 0.02), I² = 82.5%). Studies with high or unclear risk of bias indicated little to no effect (5 studies, 2838 participants: RR 0.98 95% CI 0.82 to 1.16), while studies with appropriate allocation concealment demonstrated a benefit of statin therapy (8 studies, 32,142 participants: RR 0.77, 95% CI 0.70 to 0.84). Furthermore, subgroup differences were demonstrated in total cholesterol (Analysis 1.49: test for subgroup differences: Chi² = 4.83, df = 1 (P = 0.03), I² = 79.3%), but the subgroup effect estimates are in the same direction and the 95% CI overlapped and hence considered not important. No effect modification was evident for major cardiovascular events (Analysis 1.50) and triglycerides (Analysis 1.51).
1.48. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 48: Subgroup analysis (allocation concealment): death
1.49. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 49: Subgroup analysis (allocation concealment): total cholesterol
1.50. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 50: Subgroup analysis (allocation concealment): major cardiovascular events
1.51. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 51: Subgroup analysis (allocation concealment): triglycerides
Baseline kidney function
A post hoc analysis was undertaken to examine the influence of baseline kidney function on the outcome end of treatment kidney function. We found that there was no effect modification evident (Analysis 1.52: test for subgroup differences: Chi² = 0.07, df = 1 (P = 0.79), I² = 0%). Studies with lower kidney function at baseline (< 55 mL/min/1.73 m2)demonstrated little or no effect on (5 studies, 3207 participants: MD 1.56 mL/min/1.73 m2, 95% CI ‐3.02 to 6.15), but studies with higher kidney function at baseline (≥ 55 mL/min/1.73 m2)indicated that there might be a small increase in kidney function at the end of treatment (12 studies, 963 participants: MD 2.24 mL/min/1.73 m2, 95% CI 0.30 to 4.17).
1.52. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 52: Subgroup analysis (kidney function): end of treatment kidney function
Sensitivity analyses
We excluded SHARP 2010 from the meta‐analysis for primary outcomes because it added ezetimibe to statin therapy. We found no differences for major cardiovascular events and death. The summary treatment estimates were essentially unchanged (Analysis 1.53: RR 0.70, 95% CI 0.63 to 0.77) (Analysis 1.54: RR 0.80, 95% CI 0.70 to 0.90).
1.53. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 53: Sensitivity analysis (excluding SHARP): major cardiovascular events
1.54. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 54: Sensitivity analysis (excluding SHARP): death
Excluding studies with industry funding, we found that in studies with non‐industry funding or where funding was not reported, major cardiovascular events were decreased with statin therapy compared to placebo (Analysis 1.55), similar to the meta‐analysis with all included studies (Analysis 1.1). However, for death, studies with no industry funding or where funding was not reported, statins compared to placebo made no difference (Analysis 1.56), unlike the full analysis that found statins decreased death compared to placebo. Although the effect estimates direction indicates a decrease in death, the exclusion of studies may decrease the precision of the finding, and hence the finding should be interpreted with caution. There was insufficient data to examine the effect on rhabdomyolysis.
1.55. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 55: Sensitivity analysis (funding): major cardiovascular events
1.56. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 56: Sensitivity analysis (funding): death
Excluding large studies from the analysis (N > 3500) did not change the direction and size of the effect estimates for the primary outcomes, major cardiovascular events and death (data not shown).
The following sensitivity analyses were considered but not undertaken.
Repeating the analysis excluding unpublished studies (due to limited unpublished studies)
Repeating analysis taking account of risk of bias (due to limited low risk of bias studies)
Repeating the analysis using the following filters: diagnostic criteria, language of publication, and country.
Meta‐regression
Considerable heterogeneity (I2 = 53%) was found for the primary outcome of death. Subgroup analyses to explore heterogeneity among the studies found that kidney function at baseline accounted for 100% of the observed heterogeneity in the meta‐analysis for death. Additionally, subgroup analysis indicated that baseline kidney function (98.8% of heterogeneity explained), the proportion with diabetes (99.6%), and adequacy of allocation concealment (100%) modified the observed risks of withdrawal due to adverse events. These findings were exploratory and should be interpreted with caution.
Assessment of publication bias
Publication bias was assessed for all outcomes listed in Table 1, which included more than 10 studies. The funnel plots for the outcomes of major cardiovascular events (Figure 4), death (Figure 5), and MI (Figure 6) were symmetrical and indicated little concern regarding small study effects. No further statistical testing was undertaken to assess publication bias for these outcomes.
4.

Funnel plot of comparison: 1 Statins versus placebo or no treatment, outcome: 1.1 Major cardiovascular events.
5.

Funnel plot of comparison: 1 Statins versus placebo or no treatment, outcome: 1.2 Death.
6.

Funnel plot of comparison: 1 Statins versus placebo or no treatment, outcome: 1.5 Myocardial infarction.
Statin therapy versus different statin therapy
PLANET I 2006 and PLANET II 2006 compared low‐ and high‐dose rosuvastatin and atorvastatin and found both therapies were well tolerated, with few events to determine the difference between primary outcome death and secondary outcomes, doubling of SCr and AKI.
IDEAL 2004 compared simvastatin with atorvastatin, Abe 2015 compared rosuvastatin with pitavastatin, Ikeda 2012 compared pravastatin with rosuvastatin, Kimura 2012 compared pitavastatin with pravastatin, and SAGE 2004 compared atorvastatin with pravastatin. Sample sizes ranged from 28 to 3107 participants. The median follow‐up was 12 months (range 12 to 58.8 months). Baseline LDL cholesterol levels ranged from 3.05 to 3.9 mmol/L. Meta‐analysis was not possible because there was insufficient data for each comparison. It was unclear which statin therapy was the most effective and safe from these studies. IDEAL 2004 reported the primary outcome of major cardiovascular events,
Secondary outcomes reported in the identified studies included LDL cholesterol, HDL cholesterol, total cholesterol, triglyceride, MDA‐LDL, non‐HDL cholesterol, SCr, cystine C, urinary albumin/creatinine ratio, hs‐CRP, HbA1c, eGFR, adverse events, the cost to lower LDL cholesterol by 10 mg/dL, onset of diabetes, time to first occurrence of a major coronary event, any chronic heart disease event, any cardiovascular event, urinary protein excretion, kidney failure, AKI, and serum uric acid.
High versus low dose statin
PANDA 2011 and TNT 2004 compared two doses of atorvastatin, and PLANET I 2006 and PLANET II 2006 compared low‐ and high‐dose rosuvastatin.
Primary outcomes
High‐dose statin compared to low‐dose statin may have little or no effect on major cardiovascular events (Analysis 2.1 (2 studies, 3226 participants): RR 1.17, 95% CI 0.25 to 5.55; I2 = 59%; low certainty evidence), and death (Analysis 2.2 (3 studies, 3465 participants): RR 1.05, 95% CI 0.29 to 3.83; I2 = 56%; low certainty evidence). No events of rhabdomyolysis were reported.
2.1. Analysis.

Comparison 2: High dose versus low dose statin, Outcome 1: Major cardiovascular events
2.2. Analysis.

Comparison 2: High dose versus low dose statin, Outcome 2: Death
Secondary outcomes
TNT 2004 (3107 participants) reported high‐dose statin may decrease stroke (Analysis 2.3: RR 0.67, 95% CI 0.50 to 0.89) and hospitalisation due to heart failure (Analysis 2.4: RR 0.55, 95% CI 0.39 to 0.77; low certainty evidence) compared to low‐dose statin. High‐dose statin, compared to low‐dose statin, probably has little or no effect on serious adverse events (Analysis 2.5 (2 studies, 358 participants): RR 1.12, 95% CI 0.73 to 1.72; I2 = 0%; moderate certainty evidence), but there was limited data on AKI and doubling of SCr to determine if high‐dose statin made a difference. However, high‐dose statins may increase elevated liver enzymes (Analysis 2.6: RR 20.67, 95% CI 2.79 to 153.14; low certainty evidence) compared to low‐dose statins. PLANET I 2006 (223 participants) reported high‐ versus low‐dose rosuvastatin may improve change in total cholesterol and LDL cholesterol at 12 months, but there was no difference in change in HDL cholesterol and change in triglycerides. No other secondary outcomes were reported in the included studies.
2.3. Analysis.

Comparison 2: High dose versus low dose statin, Outcome 3: Stroke
2.4. Analysis.

Comparison 2: High dose versus low dose statin, Outcome 4: Hospitilisation due to heart failure
2.5. Analysis.

Comparison 2: High dose versus low dose statin, Outcome 5: Serious adverse events
2.6. Analysis.

Comparison 2: High dose versus low dose statin, Outcome 6: Elevated liver enzyme
Continuous release versus intermittent release statin
Yi 2014 (122 participants) compared continuous‐release to intermittent‐release statin (20 mg/day). No primary outcomes of major cardiovascular events, death, or rhabdomyolysis were reported. There were few withdrawals due to adverse events in the study. Continuous‐release versus intermittent‐release statin may have little or no effect on total cholesterol, LDL cholesterol, and HDL cholesterol, but may increase triglycerides. No other secondary outcomes were reported.
There was insufficient data to perform a meta‐analysis for this comparison.
Discussion
Summary of main results
This update confirmed findings from the previous 2014 review (Palmer 2014). Compared to placebo, statins reduced the risk of death by 20% and major cardiovascular events by 28% in people with CKD not needing dialysis. Statins probably reduce the risk of MI by nearly half, but the effects on stroke and hospitalisation due to heart failure remain uncertain. Statins decreased death, major cardiovascular events, and MI in people with (secondary prevention) and without CVD (primary prevention).
Considering the estimated baseline risk of people with CKD not needing dialysis (Table 1), statins given to 1000 people with CKD for one year might be expected to prevent 32 major cardiovascular events, 10 deaths from any cause, and seven non‐fatal or fatal MIs. There was limited reporting of adverse effects of statins in people with CKD not needing dialysis. Withdrawal due to adverse effects probably occurs more frequently among those with lower kidney function or pre‐existing diabetes, suggesting that these people may be more at risk of treatment‐related toxicity. However, there was uncertainty regarding the effect on rhabdomyolysis due to the few events reported and no reporting of patient‐important outcomes such as fatigue, life participation, and memory loss.
Data comparing different statins were sparse in patients with CKD. One study reported a benefit of high‐dose statins in reducing stroke and heart failure events compared to low‐dose statins, but a further examination of this finding is required. Additionally, there was limited reporting of safety in studies comparing high‐ versus low‐dose statins.
Overall completeness and applicability of evidence
Based on current evidence, statins probably make little to no difference to CKD progression, as treatment effects were similar to placebo with more than 2000 events. However, statins probably have a slight benefit on kidney function and may reduce proteinuria due to the pleiotropic effects of statins, but the clinical importance of these outcomes is unclear. Nearly two‐thirds of the included studies did not systematically evaluate and report adverse events, such as rhabdomyolysis and elevated liver enzymes. There was no reporting of patient‐important outcomes, such as fatigue, memory loss, and life participation.
SHARP 2010 assessed ezetimibe (a drug that lowers cholesterol absorption in the intestine) combined with simvastatin. There was insufficient data to determine if treatment effects differed between combination therapy and treatment with a statin alone. There was limited data that could be used to determine the most effective and safe type of statin or dose of statin in the CKD population. Furthermore, it was unclear if treatment benefits for statins depended on treatment‐related reductions in serum cholesterol or by another mechanism of action, as there were insufficient number of studies that reported both cardiovascular and death outcomes and changes in cholesterol levels with treatment.
Quality of the evidence
Overall, the quality of the evidence in clinical outcomes (death, cardiovascular events, kidney failure) for shared decision‐making for statin with or without ezetimibe is high to moderate certainty evidence. However, the data for patient‐important outcomes, such as rhabdomyolysis is very low or not reported for fatigue, life participation and memory loss.
Current data on the effects of statins in people with earlier stages of kidney disease relating to the primary outcomes of death and cardiovascular events were derived from post hoc subgroup analyses from major studies in larger populations (4S 1993; AFCAPS/TexCAPS 1997; ALLIANCE 2000; CARDS 2003; HPS 2002; JUPITER 2007; LIPS 2001; MEGA 2004; PPP 1992; PREVEND IT 2000; SHARP 2010). Data for treatment effects in people with CKD derived from post hoc analyses of larger studies may be less reliable (Boutron 2010). The heterogeneity observed in our review was not explained by prespecified or post hoc studies of people with CKD, as the effects estimates in all studies were similar.
Potential biases in the review process
This review is reported using standardised Cochrane methods and the PRISMA checklist (Liberati 2009). It includes a highly sensitive electronic search of the Cochrane Kidney and Transplant's Specialised Registry of Studies, designed by an Information Specialist. Despite applying these best practice approaches, potential biases may exist in the review process. Incomplete reporting of outcomes in the identified studies limited the power to detect differences among interventions for important outcomes. For example, data for treatment effects on stroke and hospitalisation due to heart failure suggest a potential for benefit, but the small number of events resulted in imprecision in the effect estimate. This incomplete reporting of outcomes may also limit the statistical power to detect heterogeneity. However, the analysis of most outcomes exhibits low to moderate heterogeneity, indicating that the meta‐analysis was appropriate. Additionally, we could not include data for people with CKD not on dialysis from Stegmayr 2005 for death and cardiovascular death. Reported data for these outcomes combined results for people on dialysis with those at earlier stages of kidney disease not on dialysis; separate unpublished data for earlier stages of kidney disease were not available.
Agreements and disagreements with other studies or reviews
This update confirmed our earlier review findings that statins reduce serious cardiovascular events and death in people with CKD not requiring dialysis (Palmer 2014). The finding that statins proportionally reduce cardiovascular death by 20% is similar to the prospective meta‐analysis in broader populations of people who had or were at risk for CVD, which showed that statins lowered lipids to reduce coronary‐related death at a rate of 20% (Baigent 2005). People with CKD risk cardiovascular events equivalent to those with existing coronary artery disease (Foley 1998). The absolute benefits of statins in people with CKD are similar to people with a 20% or more 10‐year absolute cardiovascular risk, for whom statins are recommended (NICE 2014).
We also found that statins reduced death by approximately 28%, similar to the 10% proportional reduction in broader populations (Baigent 2005). The inclusion of studies conducted in people with CKD without clinically evident CVD in this review (Abe 2011c; Masajtis‐Zagajewska 2018; Ohsawa 2015) reaffirmed the previous finding that statins have a role in the primary prevention of death and major cardiovascular events (Palmer 2014). The beneficial effect of statins on cardiovascular end‐points observed in our review may be potentially explained by either cholesterol‐dependent or cholesterol‐independent effects or both. In addition to the well‐documented association between cholesterol‐lowering and cardiovascular risk reduction in non‐CKD populations (Baigent 2005), statins may modulate cardiovascular risk by decreasing inflammation, enhancing endothelial function, inhibiting smooth muscle proliferation, exerting direct anti‐thrombotic properties and stabilising pre‐existing atherosclerotic plaque (Kinlay 2003; Robinson 2005; Sotiriou 2000). Similar mechanisms may underpin the beneficial actions of statins on the progression of kidney disease, although other cholesterol‐independent renoprotective actions such as inhibition of renal cell proliferation, anti‐fibrotic effects, suppression of macrophage recruitment, anti‐oxidation, and down‐regulation of inflammatory cytokines, may also contribute (Campese 2005; Keane 2000).
Notably, the finding that statins significantly reduce adverse cardiovascular outcomes in people with earlier stages of CKD contrasts with a similar systematic review and meta‐analysis we have conducted of studies in people with advanced kidney disease on dialysis (Palmer 2013). Statin therapy in people with advanced CKD on dialysis has little or no effect on major cardiovascular events (RR 0.95, 95% CI 0.88 to 1.03), death (any cause) (RR 0.96, 95% CI 0.90 to 1.02), cardiovascular death (RR 0.94, 95% CI 0.84 to 1.06), or MI (RR 0.87, 95% CI 0.71 to 1.07) with uncertain effects on liver or muscle function or cancer, despite a similar reduction in cholesterol levels. Similarly, an individual patient‐level meta‐analysis of 28 RCTs found that the effects of statins decreased with declining kidney function, with patients on dialysis receiving little to no benefit from statins (Herrington 2016).
The KDIGO Lipids in CKD guideline recommends statins for patients 50 years or older and an eGFR ≥ 60 mL/min/1.73 m2 "For patients 50 years of age and older and eGFR < 60 mL/min/1.73 m2 not needing dialysis, a statin with or without ezetimibe" (KDIGO 2013). Additionally, CARI Guidelines (CARI 2013) recommend using statins with or without ezetimibe in patients with early CKD (stages 1 to 3) to reduce the risk of atherosclerotic disease. Our analysis confirms the benefits of statins in people with CKD (including primary prevention of death and cardiovascular events) and supports the present guidelines. However, there is limited data on the incremental benefit of ezetimibe combined with statins in people with CKD.
Authors' conclusions
Implications for practice.
Moderate‐to‐high‐quality evidence currently supports the widespread use of statins for people with CKD not on dialysis to prevent death and cardiovascular events, including people without existing CVD. Statins reduce death and cardiovascular events, including MI, although effects on the risk of stroke were less certain. Treating 1000 people with CKD not on dialysis may prevent 32 major cardiovascular events and 10 deaths over one year. Statins have uncertain effects on the progression of CKD and cannot be recommended to slow the progression of CKD. Currently, the toxicity of statins for people with CKD is poorly characterised in studies, and this needs to be acknowledged when commencing treatment.
Implications for research.
Available data confirms the benefit of statins in reducing lipid levels and improving death and cardiovascular death in people with CKD not needing dialysis. Future research, including post‐marketing surveillance, is required to monitor the safety of statins in people with CKD, with a focus on risks of muscle or liver damage associated with longer‐term treatment. A co‐ordinated approach to post‐marketing surveillance might be considered.
Currently, the available evidence depends largely on post hoc analyses from larger studies. Future prospective studies of primary prevention in people with moderate to severe CKD (eGFR 15 to 60 mL/min/1.73 m²), including a detailed assessment of treatment toxicity, are warranted. PLANET I 2006 and PLANET II 2006 have demonstrated that the type of statin may have different effects on kidney function, including proteinuria. A network meta‐analysis to rank the efficacy of the type of statin is warranted. Additionally, future prospective studies comparing statins combined with ezetimibe with statins alone in people with moderate to severe CKD are warranted because of a lack of certainty regarding the benefits and harms of therapy from the several small published trials. Finally, further examination of the type of statin should be considered.
Feedback
Review conclusions (2009 review),
Summary
Navaneethan SD et al (Navaneethan 2009¹) in their systematic review titled ‘HMG CoA reductase inhibitors (statins) for people with chronic kidney disease (CKD) not requiring dialysis’ came to the conclusion that “statins cause a significant reduction in the risk of all‐cause mortality and cardiovascular mortality in CKD patients who are not requiring dialysis.” We feel that this claim requires reassessment based on the following reasons.
For the outcome all‐cause mortality, the review included 21 ‘individual’ studies. The results of this outcome were driven (99%) by a pooling of three individual studies (LIPID², CARE³ and WOSCOPS⁴). These three trials were included as one entry (PPP 2004⁵) in the forest plot (analysis 1.1). The pooling of these three trials is incorrect in a meta‐analysis as it gives improper weighting to the data. Inappropriate pooling of data as one study can negatively impact the weight and precision that would be apparent had these trials been entered as separate studies. The review explained that the reason for pooling the trials is that they could not get the data in a separate form. This however, is not a sufficient reason to pool the trials together as one. Also, the review references the subgroup analysis of CARE, where the individual data is reported separately. We would like to know the attempts that were made to obtain individual trial data and see this in an updated review.
Secondly, we are concerned with how studies were identified and included in this review. While the PPP makes up 99% of the all‐cause mortality data, we are aware of many other statin trials with mortality data. For example, the Heart Protection Study (HPS 2003) was included in non‐fatal CV events (analysis 1.3), but not for all‐cause or cardiovascular mortality. We are unclear as to the efforts that were made to obtain subgroup data on kidney disease patients from all of the statin trials published. We have the same concern as above with respect to those studies that were excluded from this review. Figure 1 provides a flow diagram of the number of trials excluded, however the authors do not describe in detail the reasons for exclusion.
Third, the PPP publication separates individuals into normal, mild and moderate kidney disease (GFR 30‐59.99 mL/min/1.73m²). According to this paper, the total amount of patients that would meet the inclusion criteria of this Cochrane review (that is moderate kidney disease) would be 4,491, instead of the 16,824 that were actually analysed. This means that there were approximately an extra ~12,000 patients included in this review that who not actually meet the inclusion criteria of moderate kidney disease. We would like to know what efforts were made to extract this data and what process was done to see if the extra 12,000 patients fit the other inclusion criteria of this Cochrane review (i.e. some form of kidney disease, but normal renal function, elevated baseline serum creatinine). Upon review of two of the individual trials, we could not find any indication for inclusion of the patient data in this Cochrane review based on what was reported in the publications.
With respect to serious adverse events, this review does not specifically address all of the potential harms associated with statins. The Cholesterol Treatment Trialists’ (CTT) collaboration⁶ report an increased risk of hemorrhagic stroke with statin use, with high dose vs. low dose and compared to control. This increase although not statistically significant, is a concern of statin use that should be addressed when considering the benefit and risk of this class of medications.
Two of the three trials included (LIPID and CARE) were secondary prevention trials. That is, patients whom had already experienced an event (e.g. myocardial infarction, stroke). The benefit of statins in secondary prevention, irrespective of CKD has already been well established. The authors do outline that they were including patients with or without coronary artery disease, however the bigger question that remains unanswered is there any benefit of statins in a primary prevention patient with CKD. The SHARP⁷ trial aimed to answer this question in a dialysis and non‐dialysis population, and found no reduction in all‐cause mortality or cardiovascular mortality. Including both primary and secondary prevention trials together can bias the end result in favour of treatment for the entire population, when in fact the benefit may only exist in secondary prevention patients. Given the above issues, we cannot be certain of the benefit that is claimed for statins in non‐dialysis CKD patients.
We hope this provides some constructive feedback for the next review. We look forward to hearing from you.
Sincerely,
Megan Harbin, BSc.Pharm, Pharmacy Resident
Anthony Amadio, BSc.Pharm, ACPR, PharmD Student
Aaron Tejani, BSc.Pharm, PharmD
The authors of this letter have no known conflicts of interest to declare.
References:
Navaneethan SD, Pansini F, Perkovic V, Manno C, Pellegrini F, Johnson DW, Craig JC, Strippoli GFM. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No.: CD007784. DOI: 10.1002/14651858.CD007784.
Anonymous. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group.[see comment]. New England Journal of Medicine 1998;339(19): 1349–57.
Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G, Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Annals of Internal Medicine 2003;138 (2):98–104.
Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolaemia. West of Scotland Coronary Prevention Study Group. New England Journal of Medicine 1995; 333(20):1301–7
Tonelli M, et al. Effect of Pravastatin on Cardiovascular Events in People with Chronic Kidney Disease. Circulation 2004;110:1557‐1563.
Baigent C, Blackwell L, Emberson J et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376(9753):1670‐1681.
Baigent C, Landray MJ, Reith C, et al, on behalf of the SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus Registryibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet 2011; 377: 2181–92.
Reply
Dear Ms.Harbin,
We like to thank you for your careful review of our Cochrane systematic review titled‐ HMG CoA reductase inhibitors for people with chronic kidney disease not requiring dialysis published in 2009. Please see our responses below which answers your specific concerns:
Pooling of Pravastatin Pooling Project with other studies: We obtained additional unpublished data for the Pravastatin pooling project from Dr. Tonelli (email communication by Dr. Strippoli). As discussed in the review, we attempted to obtain individual trial data but without success. An updated systematic review that examined the use of statins in patients with varying severity of kidney disease was published in 2012 (Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Ann Intern Med. 2012 Aug 21;157(4):263‐75). This review included additional studies and reported results separately for those who were on dialysis, received renal transplant and those who had non‐dialysis dependent CKD. Cochrane reviews get updated every 2‐3 years to include any new studies that gets published. Update of our 2009 Cochrane review is in press (to be published in late 2013) and clarify some other issues pointed out by you.
Inclusion of other studies with CKD sub‐groups: Thanks for pointing out this. We have included the Heart Protection Study and other trials that provided data relating to those with CKD in the updated review (Palmer SC et al. Annals of Internal Medicine 2012) and the upcoming Cochrane Review. In the updated Cochrane review (in Press), reasons for exclusion were provided in Figure 1.
Confirmation of CKD: We reported the total number of patients included in the studies and agree that not all participants had CKD and only 4491/19700 patients in the Pravastatin Pooling Project had CKD. We did not have access to the original data for this study precluding us to assess whether others had any other forms of kidney disease (such as dialysis or renal transplant). These could be addressed by an individual patient‐level meta‐analysis that could be considered by other investigators in future.
Inclusion of stroke as an adverse event: We extracted details relating to adverse events from all individual studies. Even though few trials reported increased incidence of stroke in dialysis population, there was no increased risk for this subgroup (RR, 0.61 [CI, 0.38 to 0.98]) (Palmer SC et al. Annals of Internal Medicine 2012). These data were not included in the 2009 Cochrane Review but will be available in the update Cochrane review.
Statins for primary vs. secondary prevention: This important issue that has been discussed in the literature (Erickson et al. J Am Coll Cardiol. 2013 Mar 26;61(12):1250‐8 and Massy et al. Kidney Int. 2013 Sep;84(3):451‐6 and Updhyay et al Ann Intern Med. 2012 Aug 21;157(4):251‐62.). CKD population have significant CV disease burden with higher coronary events (Tonelli M et al. Lancet 2012 Sep 1;380(9844):807‐14) arguing for the use of statins as a primary prevention measure. As you pointed out, SHARP trial reported a statistically significant reduction in atherosclerotic events (primary outcome measure) but no significant reduction in the individual secondary end‐points. Given the limited data available relating to primary and secondary prevention with statins in CKD, primary and secondary prevention trials were pooled together in our review.
In summary, we agree with some of the issues raised by you and your team members. We have added additional details suggested by you in the updated Cochrane review (in press) and in the paper published in Annals of Internal Medicine (Palmer SC et al. 2012). However, we do have several unanswered questions that might be addressed in future clinical trials to better treat our patients. Once again, thank you for the constructive feedback.
Sincerely,
Sankar Navaneethan, MD, MPH,
Suetonia Palmer, MBChB, PhD
Giovanni Strippoli MD, MM, MPH, PhD.
Contributors
Megan Harbin, BSc.Pharm, Pharmacy Resident
Anthony Amadio, BSc.Pharm, ACPR, PharmD Student
Aaron Tejani, BSc.Pharm, PharmD
Sankar Navaneethan, MD, MPH,
Suetonia Palmer, MBChB, PhD
Giovanni Strippoli MD, MM, MPH, PhD.
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2023 | Amended | Minor amendment to Plain Language Summary |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 29 November 2023 | New search has been performed | New search |
| 29 November 2023 | New citation required but conclusions have not changed | Thirteen new included studies added |
| 8 July 2014 | Amended | Sources of external support amended |
| 7 July 2014 | Amended | Declarations of interests amended |
| 10 June 2014 | Feedback has been incorporated | Feedback on original review incorporated |
| 9 May 2014 | New citation required and conclusions have changed | New studies included |
| 9 May 2014 | New search has been performed | Methods updated, new authors, new search preformed |
| 4 March 2012 | Amended | Author added (SP) and contact details updated. |
| 1 March 2012 | New search has been performed | Updated search to February 2012. 24 new trials included (14,803 additional participants). Results and conclusions updated. Conclusions generally unchanged. |
| 13 May 2009 | Amended | Contact details updated. |
| 3 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We acknowledge the contributions of study authors (Drs Baigent, Tonnelli, Stegmayr, Lins, Thomas, Zhang, Van Dijk, Kosch and Nakamura) who provided data about their studies upon request. We would like to thank the referees for their editorial advice during the preparation of this review. We also thank the Cochrane Kidney and Transplant Group's Tess Cooper for her help in co‐ordinating the review, Narelle Willis and editing this review, and Ruth Mitchell and Gail Higgins for assistance in the development of search strategies.
We also wish to thank Sankar Navaneethan, Jorgen Hegbrant, Vlado Perkovic, Francesca Pansini, Carlo Manno and Fabio Pellegrini who contributed to the design, quality assessment, data collection, entry, analysis and interpretation and writing of the previous version of the review (Navaneethan 2009; Palmer 2014).
We wish to thank the peer reviewers for their comments and feedback: Arlene C Crisostomo, MD (St Luke's Medical Center‐Quezon City Philippines); JL Mehta, MD, PhD (University of Arkansas for Medical Sciences, Little Rock, AR); Anna Mathew (Associate Professor of Medicine, McMaster University); Anil K Agarwal MD. (Professor, University of California San Francisco, Fresno and Chief of Medicine, VA Central California Health Care System); Maristela Böhlke (Postgraduate Program in Health and Behavior, Catholic University of Pelotas, Pelotas, Brazil); Nishank Jain, MD, MPH (Associate Professor of Medicine, University of Arkansas for Medical Sciences, Little Rock, AR, USA); Coronado‐Daza Jorge (Universidad de Cartagena, Colombia/Nefrología y Diálisis SAS, Colombia)
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 3. Living systematic review protocol
| Systematic review section | Methods considerations specific to living systematic reviews | Comments |
| Title | HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis | The title of the review will remain the same, with no indication that the review has been changed to a living review |
| What's New | New events including will be highlighted in the 'What's new' table at the beginning of the review. This table will highlight all updates of the living systematic review to identify changes in currency of the review. | Events may include, new citation: conclusions not changed etc, new citation: conclusions changed etc. |
| Abstract | Editorial note: This is a living systematic review. We search for new evidence every three months and update the review when we identify relevant new evidence. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review. | We will include the following statement in the abstract |
| Plain language summary | Editorial note: Please refer to the Cochrane Database of Systematic Reviews for the current status of this review. | We will include the following statement in the plain language summary |
| Methods: Search methods for identification of studies | ||
| Electronic sources | An updated search of the Cochrane Kidney and Transplant Register of Studies will be undertaken every 3 months | |
| Searching other sources | No proposed change – maintain Cochrane Kidney and Transplant Registry of studies current handsearching | |
| Methods: Data collection analysis | ||
| Selection of studies | We will immediately screen any new citations retrieved by three monthly searches. Additionally, moderated crowd sourcing platforms may be used to support screening of the literature, for example Cochrane Crowd. | |
| Inclusion criteria | No proposed changes | |
| Data extraction and management, Assessment of risk of bias of included studies | No proposed changes | |
| Measurement of treatment effect, unit of analysis issues, dealing with missing data | No proposed changes | |
| Assessment of heterogeneity, Assessment of reporting biases | No proposed changes | |
| Data synthesis |
Decide when to incorporate new evidence All included studies future review updates will undergo data extraction and critical appraisal by two authors (BC and DJT) independently, using Robot Reviewer (https://www.robotreviewer.net/) as supportive tool. Whenever new evidence (meaning studies, data or other information) relevant to the review is identified, we will extract the data and assess risk of bias, as appropriate. We will wait until the accumulating evidence changes one or more of the following components of the review before incorporating it and re‐publishing the review:
Adjustments for repeated meta‐analyses Formal sequential meta‐analysis approaches will not be used for updated meta‐analyses. |
|
| Subgroup analysis and investigation of heterogeneity, Sensitivity analysis, Summary of Findings Table | Summary of findings tables will be developed in GRADEPro and will be exported to Review Manager. | |
| Methods for future updates | The conduction and production of this living systematic review will be overseen by the CARI Guidelines Living Evidence Working Group and the Statins in CKD Guideline Work Group. We will review the scope and methods when updating the review, in the light of potential changes in the topic area or new evidence. For example, additional comparisons, interventions, subgroups, outcomes, or new review methods available. At every three monthly search, we will consider the necessity for the review to be living. By assessing the questions ongoing relevance to decision‐makers and by determining if continuing uncertainty in the evidence is presence, and whether further relevant research is likely to change recommendations for clinical practice via the CARI Guidelines Statins in CKD Living Guideline. | |
Data and analyses
Comparison 1. Statins versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Major cardiovascular events | 14 | 36156 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.66, 0.79] |
| 1.2 Death | 13 | 34978 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.73, 0.96] |
| 1.3 Rhabdomyolysis | 2 | 2618 | Risk Ratio (IV, Random, 95% CI) | 3.07 [0.13, 75.37] |
| 1.4 Cardiovascular death | 8 | 19112 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.69, 0.87] |
| 1.5 Myocardial infarction | 10 | 9475 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.42, 0.73] |
| 1.6 Stroke | 7 | 9115 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.37, 1.08] |
| 1.7 Revascularisation procedure | 3 | 4156 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.47, 0.82] |
| 1.8 Hospitilsation due to heart failure | 1 | 579 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.37, 1.32] |
| 1.9 Kidney failure | 3 | 6704 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.91, 1.05] |
| 1.10 Doubling serum creatinine | 1 | 6245 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.79, 1.02] |
| 1.11 End of treatment kidney function | 18 | 4213 | Mean Difference (IV, Random, 95% CI) | 1.83 [0.27, 3.39] |
| 1.11.1 CrCl [mL/min] | 7 | 319 | Mean Difference (IV, Random, 95% CI) | 5.19 [0.58, 9.79] |
| 1.11.2 eGFR [mL/min/1.73 m²] | 11 | 3894 | Mean Difference (IV, Random, 95% CI) | 1.01 [0.46, 1.57] |
| 1.12 End of treatment proteinuria | 7 | 356 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.75, ‐0.19] |
| 1.13 Elevated liver enzymes | 7 | 7991 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.39, 1.50] |
| 1.14 Withdrawal due to adverse events | 13 | 4219 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.83, 1.48] |
| 1.15 Cancer | 2 | 5581 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.82, 1.30] |
| 1.16 Onset of diabetes | 1 | 3267 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.71, 1.50] |
| 1.17 Elevated creatine kinase | 7 | 4514 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.20, 3.48] |
| 1.18 Total cholesterol | 27 | 2234 | Mean Difference (IV, Random, 95% CI) | ‐50.26 [‐64.34, ‐36.17] |
| 1.19 LDL cholesterol | 24 | 2183 | Mean Difference (IV, Random, 95% CI) | ‐43.84 [‐52.56, ‐35.12] |
| 1.20 Triglycerides | 20 | 1186 | Mean Difference (IV, Random, 95% CI) | ‐26.02 [‐40.99, ‐11.06] |
| 1.21 HDL cholesterol | 22 | 1374 | Mean Difference (IV, Random, 95% CI) | 2.92 [‐0.03, 5.88] |
| 1.22 Subgroup analysis (cardiovascular disease): death | 12 | 34857 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.73, 0.88] |
| 1.22.1 No cardiovascular disease | 4 | 13462 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.55, 0.90] |
| 1.22.2 Cardiovascular disease | 8 | 21395 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.76, 0.89] |
| 1.23 Subgroup analysis (cardiovascular disease): major cardiovascular events | 14 | 36156 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.66, 0.79] |
| 1.23.1 No cardiovascular disease | 5 | 13766 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.46, 0.79] |
| 1.23.2 Cardiovascular disease | 9 | 22390 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.71, 0.81] |
| 1.24 Subgroup analysis (statin dose): major cardiovascular death | 14 | 36156 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.66, 0.79] |
| 1.24.1 < 20 mg/day simvastatin equivalent | 4 | 3630 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.37, 0.64] |
| 1.24.2 ≥ 20 mg/day simvastatin equivalent | 10 | 32526 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.74, 0.81] |
| 1.25 Subgroup analysis (statin dose): death | 12 | 34857 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.73, 0.88] |
| 1.25.1 < 20 mg/day simvastatin equivalent | 4 | 9869 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.68, 0.89] |
| 1.25.2 ≥ 20 mg/day simvastatin equivalent | 8 | 24988 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.71, 0.92] |
| 1.26 Subgroup analysis (statin dose): withdrawal due to adverse events | 12 | 4189 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.82, 1.64] |
| 1.26.1 < 20 mg/day simvastatin equivalent | 5 | 250 | Risk Ratio (IV, Random, 95% CI) | 2.66 [0.61, 11.65] |
| 1.26.2 ≥ 20 mg/day simvastatin equivalent | 7 | 3939 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.77, 1.51] |
| 1.27 Subgroup analysis (statin dose): total cholesterol | 27 | 2234 | Mean Difference (IV, Random, 95% CI) | ‐50.26 [‐64.34, ‐36.17] |
| 1.27.1 < 20 mg/day simvastatin equivalent | 15 | 510 | Mean Difference (IV, Random, 95% CI) | ‐41.16 [‐51.24, ‐31.07] |
| 1.27.2 ≥ 20 mg/day simvastatin equivalent | 12 | 1724 | Mean Difference (IV, Random, 95% CI) | ‐60.28 [‐84.29, ‐36.27] |
| 1.28 Subgroup analysis (statin dose): LDL cholesterol | 24 | 2183 | Mean Difference (IV, Random, 95% CI) | ‐43.84 [‐52.56, ‐35.12] |
| 1.28.1 < 20 mg/day simvastatin equivalent | 12 | 433 | Mean Difference (IV, Random, 95% CI) | ‐39.42 [‐51.98, ‐26.87] |
| 1.28.2 ≥ 20 mg/day simvastatin equivalent | 12 | 1750 | Mean Difference (IV, Random, 95% CI) | ‐47.69 [‐59.55, ‐35.84] |
| 1.29 Subgroup analysis (statin dose): HDL cholesterol | 22 | 1374 | Mean Difference (IV, Random, 95% CI) | 2.92 [‐0.03, 5.88] |
| 1.29.1 < 20 mg/day simvastatin equivalent | 12 | 433 | Mean Difference (IV, Random, 95% CI) | 2.77 [1.18, 4.36] |
| 1.29.2 ≥20 mg/dL simvastatin equivalent | 10 | 941 | Mean Difference (IV, Random, 95% CI) | 3.33 [‐2.31, 8.97] |
| 1.30 Subgroup analysis (statin dose): triglycerides | 20 | 1186 | Mean Difference (IV, Random, 95% CI) | ‐26.02 [‐40.99, ‐11.06] |
| 1.30.1 < 20 mg/day simvastatin equivalent | 10 | 398 | Mean Difference (IV, Random, 95% CI) | ‐38.43 [‐66.72, ‐10.15] |
| 1.30.2 ≥ 20 mg/day simvastatin equivalent | 10 | 788 | Mean Difference (IV, Random, 95% CI) | ‐15.23 [‐29.18, ‐1.28] |
| 1.31 Subgroup analysis (age): total cholesterol | 27 | 2234 | Mean Difference (IV, Random, 95% CI) | ‐50.26 [‐64.34, ‐36.17] |
| 1.31.1 < 55 years | 14 | 1572 | Mean Difference (IV, Random, 95% CI) | ‐38.28 [‐45.93, ‐30.63] |
| 1.31.2 ≥ 55 years | 13 | 662 | Mean Difference (IV, Random, 95% CI) | ‐60.48 [‐84.81, ‐36.15] |
| 1.32 Subgroup analysis (age): withdrawal due to adverse events | 12 | 4189 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.82, 1.64] |
| 1.32.1 < 55 years | 6 | 1111 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.55, 2.58] |
| 1.32.2 ≥ 55 years | 6 | 3078 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.86, 1.80] |
| 1.33 Subgroup analysis (age): LDL cholesterol | 24 | 2183 | Mean Difference (IV, Random, 95% CI) | ‐43.84 [‐52.56, ‐35.12] |
| 1.33.1 < 55 years | 10 | 1203 | Mean Difference (IV, Random, 95% CI) | ‐39.06 [‐50.84, ‐27.28] |
| 1.33.2 ≥ 55 years | 14 | 980 | Mean Difference (IV, Random, 95% CI) | ‐46.80 [‐58.28, ‐35.33] |
| 1.34 Subgroup analysis (age): HDL cholesterol | 22 | 1374 | Mean Difference (IV, Random, 95% CI) | 2.92 [‐0.03, 5.88] |
| 1.34.1 < 55 years | 9 | 657 | Mean Difference (IV, Random, 95% CI) | 2.97 [1.49, 4.45] |
| 1.34.2 ≥ 55 years | 13 | 717 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐2.60, 8.01] |
| 1.35 Subgroup analysis (age): triglycerides | 20 | 1186 | Mean Difference (IV, Random, 95% CI) | ‐26.02 [‐40.99, ‐11.06] |
| 1.35.1 < 55 years | 6 | 228 | Mean Difference (IV, Random, 95% CI) | ‐33.45 [‐44.75, ‐22.15] |
| 1.35.2 ≥ 55 years | 14 | 958 | Mean Difference (IV, Random, 95% CI) | ‐23.37 [‐43.72, ‐3.02] |
| 1.36 Subgroup analysis (diabetes mellitus): withdrawal due to adverse events | 13 | 4219 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.84, 1.60] |
| 1.36.1 < 20% with DM | 8 | 3541 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.72, 1.32] |
| 1.36.2 ≥ 20% with DM | 5 | 678 | Risk Ratio (IV, Random, 95% CI) | 1.47 [1.10, 1.98] |
| 1.37 Subgroup analysis (diabetes mellitus): death | 13 | 34980 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.73, 0.88] |
| 1.37.1 < 20% with DM | 7 | 26672 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.62, 0.86] |
| 1.37.2 ≥ 20% with DM | 6 | 8308 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.77, 0.96] |
| 1.38 Subgroup analysis (diabetes mellitus): major cardiovascular death | 14 | 36156 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.66, 0.79] |
| 1.38.1 < 20% with DM | 9 | 27014 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.56, 0.77] |
| 1.38.2 ≥ 20% with DM | 5 | 9142 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.69, 0.83] |
| 1.39 Subgroup analysis (diabetes mellitus): total cholesterol | 27 | 2234 | Mean Difference (IV, Random, 95% CI) | ‐50.26 [‐64.34, ‐36.17] |
| 1.39.1 < 20% with DM or not reported | 15 | 1759 | Mean Difference (IV, Random, 95% CI) | ‐55.16 [‐77.63, ‐32.68] |
| 1.39.2 ≥ 20% with DM | 12 | 475 | Mean Difference (IV, Random, 95% CI) | ‐43.89 [‐51.36, ‐36.43] |
| 1.40 Subgroup analysis (diabetes mellitus): LDL cholesterol | 24 | 2179 | Mean Difference (IV, Random, 95% CI) | ‐43.84 [‐52.56, ‐35.12] |
| 1.40.1 < 20% with DM or not reported | 14 | 1749 | Mean Difference (IV, Random, 95% CI) | ‐42.19 [‐55.12, ‐29.26] |
| 1.40.2 ≥ 20% with DM | 10 | 430 | Mean Difference (IV, Random, 95% CI) | ‐46.09 [‐53.60, ‐38.58] |
| 1.41 Subgroup analysis (diabetes mellitus): HDL cholesterol | 22 | 1374 | Mean Difference (IV, Random, 95% CI) | 2.92 [‐0.03, 5.88] |
| 1.41.1 < 20% with DM or not reported | 13 | 973 | Mean Difference (IV, Random, 95% CI) | 2.87 [‐1.19, 6.92] |
| 1.41.2 ≥ 20% with DM | 9 | 401 | Mean Difference (IV, Random, 95% CI) | 2.25 [‐0.43, 4.93] |
| 1.42 Subgroup analysis (diabetes mellitus): triglycerides | 20 | 1186 | Mean Difference (IV, Random, 95% CI) | ‐26.02 [‐40.99, ‐11.06] |
| 1.42.1 < 20% with DM or not reported | 11 | 769 | Mean Difference (IV, Random, 95% CI) | ‐25.71 [‐37.17, ‐14.24] |
| 1.42.2 ≥ 20% with DM | 9 | 417 | Mean Difference (IV, Random, 95% CI) | ‐26.53 [‐68.03, 14.97] |
| 1.43 Subgroup analysis (baseline cholesterol): total cholesterol | 27 | 2234 | Mean Difference (IV, Random, 95% CI) | ‐50.26 [‐64.34, ‐36.17] |
| 1.43.1 < 230 mg/dL | 16 | 1758 | Mean Difference (IV, Random, 95% CI) | ‐37.10 [‐43.11, ‐31.08] |
| 1.43.2 ≥ 230 mg/dL | 11 | 476 | Mean Difference (IV, Random, 95% CI) | ‐68.21 [‐96.42, ‐40.01] |
| 1.44 Subgroup analysis (baseline cholesterol): LDL cholesterol | 24 | 2183 | Mean Difference (IV, Random, 95% CI) | ‐43.84 [‐52.56, ‐35.12] |
| 1.44.1 < 230 mg/dL | 14 | 1735 | Mean Difference (IV, Random, 95% CI) | ‐34.01 [‐40.83, ‐27.19] |
| 1.44.2 ≥ 230 mg/dL | 10 | 448 | Mean Difference (IV, Random, 95% CI) | ‐58.87 [‐72.67, ‐45.07] |
| 1.45 Subgroup analysis (baseline cholesterol): withdrawal due to adverse events | 13 | 4219 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.84, 1.60] |
| 1.45.1 < 230 mg/dL | 7 | 1798 | Risk Ratio (IV, Random, 95% CI) | 1.27 [0.69, 2.32] |
| 1.45.2 ≥ 230 mg/dL | 6 | 2421 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.77, 1.37] |
| 1.46 Subgroup analysis (baseline cholesterol): HDL cholesterol | 22 | 1374 | Mean Difference (IV, Random, 95% CI) | 2.92 [‐0.03, 5.88] |
| 1.46.1 < 230 mg/dL | 12 | 926 | Mean Difference (IV, Random, 95% CI) | 2.41 [0.98, 3.84] |
| 1.46.2 ≥ 230 mg/dL | 10 | 448 | Mean Difference (IV, Random, 95% CI) | 4.01 [‐2.45, 10.46] |
| 1.47 Subgroup analysis (baseline cholesterol): triglycerides | 20 | 1186 | Mean Difference (IV, Random, 95% CI) | ‐26.02 [‐40.99, ‐11.06] |
| 1.47.1 < 230 mg/dL | 11 | 755 | Mean Difference (IV, Random, 95% CI) | ‐22.34 [‐31.33, ‐13.35] |
| 1.47.2 ≥ 230 mg/dL | 9 | 431 | Mean Difference (IV, Random, 95% CI) | ‐35.24 [‐64.04, ‐6.44] |
| 1.48 Subgroup analysis (allocation concealment): death | 13 | 34980 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.73, 0.88] |
| 1.48.1 Low RoB | 8 | 32142 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.70, 0.84] |
| 1.48.2 High/Unclear RoB | 5 | 2838 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.82, 1.16] |
| 1.49 Subgroup analysis (allocation concealment): total cholesterol | 27 | 2234 | Mean Difference (IV, Random, 95% CI) | ‐50.26 [‐64.30, ‐36.21] |
| 1.49.1 Low RoB | 5 | 1083 | Mean Difference (IV, Random, 95% CI) | ‐32.67 [‐39.88, ‐25.47] |
| 1.49.2 High/Unclear RoB | 22 | 1151 | Mean Difference (IV, Random, 95% CI) | ‐53.66 [‐70.93, ‐36.39] |
| 1.50 Subgroup analysis (allocation concealment): major cardiovascular events | 14 | 36156 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.66, 0.79] |
| 1.50.1 Low RoB | 9 | 31287 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.69, 0.81] |
| 1.50.2 High/Unclear RoB | 5 | 4869 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.45, 0.79] |
| 1.51 Subgroup analysis (allocation concealment): triglycerides | 20 | 1186 | Mean Difference (IV, Random, 95% CI) | ‐26.02 [‐40.99, ‐11.06] |
| 1.51.1 Low RoB | 4 | 345 | Mean Difference (IV, Random, 95% CI) | ‐30.22 [‐42.66, ‐17.78] |
| 1.51.2 High/Unclear RoB | 16 | 841 | Mean Difference (IV, Random, 95% CI) | ‐25.27 [‐44.38, ‐6.16] |
| 1.52 Subgroup analysis (kidney function): end of treatment kidney function | 17 | 4170 | Mean Difference (IV, Random, 95% CI) | 1.57 [0.25, 2.88] |
| 1.52.1 < 55 mL/min | 5 | 3207 | Mean Difference (IV, Random, 95% CI) | 1.56 [‐3.02, 6.15] |
| 1.52.2 ≥ 55 mL/min | 12 | 963 | Mean Difference (IV, Random, 95% CI) | 2.24 [0.30, 4.17] |
| 1.53 Sensitivity analysis (excluding SHARP): major cardiovascular events | 13 | 29909 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.63, 0.77] |
| 1.54 Sensitivity analysis (excluding SHARP): death | 12 | 28733 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.70, 0.90] |
| 1.55 Sensitivity analysis (funding): major cardiovascular events | 5 | 4126 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.40, 0.72] |
| 1.55.1 Non‐industry funding/not reported | 5 | 4126 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.40, 0.72] |
| 1.56 Sensitivity analysis (funding): death | 5 | 4249 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.49, 1.08] |
| 1.56.1 Non industry funding/not reported | 5 | 4249 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.49, 1.08] |
| 1.57 Sensitivity analysis (funding): withdrawal due to adverse events | 9 | 1267 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.71, 2.17] |
| 1.57.1 Non industry funding/not reported | 9 | 1267 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.71, 2.17] |
| 1.58 Sensitvitiy analysis (funding): total cholesterol | 22 | 1118 | Mean Difference (IV, Random, 95% CI) | ‐54.42 [‐71.98, ‐36.87] |
| 1.58.1 Non‐industry funding/not reported | 22 | 1118 | Mean Difference (IV, Random, 95% CI) | ‐54.42 [‐71.98, ‐36.87] |
| 1.59 Sensitivity analysis (funding): LDL cholesterol | 19 | 1071 | Mean Difference (IV, Random, 95% CI) | ‐47.85 [‐57.72, ‐37.98] |
| 1.59.1 Non‐industry funding/not reported | 19 | 1071 | Mean Difference (IV, Random, 95% CI) | ‐47.85 [‐57.72, ‐37.98] |
| 1.60 Sensitivy analysis (funding): HDL cholesterol | 19 | 1098 | Mean Difference (IV, Random, 95% CI) | 3.08 [‐0.29, 6.45] |
| 1.60.1 Non‐industry funding/not reported | 19 | 1098 | Mean Difference (IV, Random, 95% CI) | 3.08 [‐0.29, 6.45] |
| 1.61 Sensitivity analysis (funding): triglycerides | 17 | 845 | Mean Difference (IV, Random, 95% CI) | ‐27.28 [‐44.25, ‐10.31] |
| 1.61.1 Non‐industry funding/not reported | 17 | 845 | Mean Difference (IV, Random, 95% CI) | ‐27.28 [‐44.25, ‐10.31] |
1.57. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 57: Sensitivity analysis (funding): withdrawal due to adverse events
1.58. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 58: Sensitvitiy analysis (funding): total cholesterol
1.59. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 59: Sensitivity analysis (funding): LDL cholesterol
1.60. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 60: Sensitivy analysis (funding): HDL cholesterol
1.61. Analysis.

Comparison 1: Statins versus placebo or no treatment, Outcome 61: Sensitivity analysis (funding): triglycerides
Comparison 2. High dose versus low dose statin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Major cardiovascular events | 2 | 3226 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.25, 5.55] |
| 2.2 Death | 3 | 3465 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.29, 3.83] |
| 2.3 Stroke | 1 | 3107 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.50, 0.89] |
| 2.4 Hospitilisation due to heart failure | 1 | 3107 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.39, 0.77] |
| 2.5 Serious adverse events | 2 | 358 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.73, 1.72] |
| 2.6 Elevated liver enzyme | 1 | 3107 | Risk Ratio (M‐H, Random, 95% CI) | 20.67 [2.79, 153.14] |
| 2.7 eGFR [mL/min/1.73 m2] | 1 | 105 | Mean Difference (IV, Random, 95% CI) | ‐11.00 [‐19.49, ‐2.51] |
2.7. Analysis.

Comparison 2: High dose versus low dose statin, Outcome 7: eGFR [mL/min/1.73 m2]
Characteristics of studies
Characteristics of included studies [ordered by study ID]
4S 1993.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Reported outcomes
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation by sequential assignment of allocation numbers for which the pre‐packaged test medication had been prepared according to the randomisation code. Patients who qualified for the study on the basis of a previous MI were assigned allocation numbers beginning with the lowest allocation number available for each respective centre; patients who qualified on the basis of angina only were assigned allocation numbers starting with the highest number available. Randomisation was made in separate blocks for each centre. The block size was unknown to the investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The Scandinavian Simvastatin Survival Study (4S) was a multicentre, double‐blind (triple‐blind because patients, investigators and study administration were unaware of patients’ drug assignment), randomised, placebo‐controlled study with simvastatin |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 4S was a multicentre, double‐blind (triple‐blind because patients, investigators and study administration were unaware of patients’ drug assignment), randomised, placebo‐controlled study with simvastatin |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for in the analysis; all data analysed by intention‐to‐treat (ITT) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Funding: supported by a grant from Merck Research Laboratories, Rahway, NJ |
Abe 2011c.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
Cointerventions
|
|
| Outcomes | Reported outcomes
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: A computer‐generated list was used for randomization. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Around 97% of enrolled participants completed the study and were analysed. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement from publications |
| Other bias | Low risk | Study appears free of other biases |
Abe 2015.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
Cointerventions
|
|
| Outcomes | Reported outcomes
|
|
| Notes | Additonal information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was undertaken by an independent investigator. Unclear what methods, i.e.. random number tables were used. |
| Allocation concealment (selection bias) | Low risk | The details of the assignment were maintained by four independent investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement from publications. Unclear if ITT was undertaken |
| Selective reporting (reporting bias) | Low risk | All outcomes in the registry are reported |
| Other bias | Unclear risk | Funding declared: "Grant from AstraZeneca (No financial support was received for implementation of this study)" |
AFCAPS/TexCAPS 1997.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | AFCAPS/TexCAPS was a double blind, randomised, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators, Steering Committee members, and those providing participant care, monitoring or managing data, or adjudicating end points were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details of study withdrawals provided, all analyses were based on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | 'Mr Cook is an employee of Merck & Co Inc, which markets lovastatin'. 'This study was funded by an Amgen fellowship grant.' |
ALLIANCE 2000.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficent information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up details reported, ITT analysis performed for all randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Support: This study was sponsored initially by Parke‐Davis and later by Pfizer Inc. Editorial support was provided by Mr Steve Dobson and Dr Shirley Smith at Envision Pharma Ltd and funded by Pfizer Inc. |
Aranda Arcas 1994.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Reported outcomes
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding: insufficient information to permit judgement |
ASCOT‐LLA 2003.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Reported outcomes
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators submitted all information relevant to any potential endpoints to the coordinating centre for central review of endpoints by the endpoint committee, who were unaware of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding: insufficient information to permit judgement |
ASUCA 2013.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
Cointerventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis undertaken has been reported but only < 80% included in the primary outcome analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes in the protocol are reported |
| Other bias | Low risk | Declaration of interest/disclosures: No conflict of interest Funding: Department of EBM Research Institute of Advancement of Clinical and Translational Science Kyoto University Hospital with an unrestricted grant from Pfizer Japan. |
Bianchi 2003.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Controlled, prospective, open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding: Insufficient information to permit judgement |
Buemi 2000.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding: Insufficient information to permit judgement |
CARDS 2003.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Addtional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Within every centre, the local investigator sequentially randomly assigned eligible patients to study treatment (either placebo or atorvastatin 10 mg daily) from a block of drugs that had been prepackaged for every centre by Pfizer, according to a computer‐generated randomisation code |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, pharmacists, study administrators, and patients were unaware of the randomisation code throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up was minimal, all analyses were ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Funding: this study was funded in part by the UK Department of Health and Diabetes UK |
Cha 2015.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
Cointerventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Norvatis role is unclear in the design, analysis and decision to present these findings |
Di Lullo 2005.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding: insufficient information to permit judgement |
Dummer 2008.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Washout period: 4 weeks Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomised, placebo‐controlled, double‐blind, cross‐over clinical study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for; 2 lost to follow‐up during 1st washout period |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Funding: financial support was received from the research Promotion Fund of the Hospital de Clinicas de Porto Alegre (FIPE‐HCPA) The BIOLAB Pharma Laboratory provide the blinded simvastatin and placebo tablets |
ESPLANADE 2010.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 175/186 (94%) completed study; ITT not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Funding: supported by a grant from Regione Lombardia, Direzione Generale Sanita`, Milan (Italy) and Novartis Farma S.p.A., Varese (Italy). Novartis Pharma also supplied the benazepril (Cibacen), valsartan (Tareg), and fluvastatin (Lescol) tablets required for study conduction. Novartis Pharma had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. |
Fassett 2010.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 49/60 (82%) completed study; ITT not conducted |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Funding: supported by a grant from the Clifford Craig Medical Research Trust |
Fried 2001.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by an unblinded pharmacist using a randomly generated list |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficent information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant drop‐outs at 1 year and 2 year follow‐up; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Funding: supported by a grant from Merck |
Gheith 2002.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Funding: financially supported by the research unit of the Mansoura University |
Goicoechea 2006.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients in the control group were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding: not reported |
Hommel 1992.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/21 patients were withdrawn from the study; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding: tablets supplied by Merck Sharpe and Dohme |
HPS 2002.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Subgroup analysis of larger study conducted in general population Funding: funded by the UK Medical Research Council, the British Heart Foundation, Merck & Co (manufacturers of simvastatin), and Roche Vitamins Ltd (manufacturers of the vitamins). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A central randomisation service was telephoned which enabled the coordinating centre to conduct a final check of eligibility prior to randomisation, and to balance the randomisation with respect to important patient characteristics (in particular, eligibility criteria and other major prognostic factors) using a minimisation algorithm |
| Allocation concealment (selection bias) | Low risk | A central randomisation service was telephoned which enabled the coordinating centre to conduct a final check of eligibility prior to randomisation, and to balance the randomisation with respect to important patient characteristics (in particular, eligibility criteria and other major prognostic factors) using a minimisation algorithm |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes ‐ outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding: funded by the UK Medical Research Council, the British Heart Foundation, Merck & Co (manufacturers of simvastatin), and Roche Vitamins Ltd (manufacturers of the vitamins). |
IDEAL 2004.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study medication assigned via a central interactive voice response system (ClinPhone, Nottingham, England) |
| Allocation concealment (selection bias) | Low risk | Allocation numbers assigned in blocks of 24. Allocation was balanced by centre; no other stratification was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An end‐point classification committee blindly reviewed reports on potential end‐points and adjudicated outcomes at regular meetings. All reports were first screened by an independent centre (Inveresk, Raleigh, NC) for blinding of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of randomised and analysed not reported but all analyses based on the ITT principle including all randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Funding: Drs Lindahl and Fayyad are Pfizer employees. All other authors except Dr Holdaas have received Honoraria from Pfizer Inc. as steering committee members of IDEAL. This study was sponsored by Pfizer Inc. |
Ikeda 2012.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
Cointerventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% of the randomised patients were analysed as part of the intention‐to‐treat population |
| Selective reporting (reporting bias) | High risk | No registry or protocol reported; no full‐text study published (2023) |
| Other bias | Unclear risk | Declaration of interest/disclosures: Not reported. Funding declared: Not reported." |
Imai 1999.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 62 participants were registered for the study. Five participants who did not conform to the criteria were excluded from the study after the study was completed. There were 57 patients entered into the analysis; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding: Insufficient information to permit judgement |
Inukai 2011.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
Cointerventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 100% of the randomised patients were analysed as part of the ITT population |
| Selective reporting (reporting bias) | Unclear risk | Comment: no registry or protocol reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
JUPITER 2007.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Reported outcomes
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed using an interactive voice‐response system and stratified according to centre |
| Allocation concealment (selection bias) | Low risk | Randomisation performed using an interactive voice‐response system and stratified according to centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomised, double‐blind, placebo‐controlled, multi‐centre study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All reported primary end‐points that occurred to 30 March 2008 were adjudicated on the basis of standardised criteria by an independent end‐point committee unaware of randomised treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up was minimal, all primary analyses performed on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Funding: the JUPITER trial was supported by Astra‐Zeneca; Dr. Cressman is an employee of AstraZeneca |
Kimura 2012.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
Cointerventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Around 88% of enrolled participants completed the study and were analysed. Participants were lost to follow‐up because of study exclusions |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
| Other bias | Low risk | Funding: unrestricted research grant from Kowa Pharmaceutical Co Ltd. |
Lam 1995.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Single blinded study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All subjects accounted for in the main analysis; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Lee 2002.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/63 (%) patients excluded; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Study appears free of other biases |
Lintott 1995.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Funding: Dr. Tony Cutten of Sandoz Pharma (New Zealand) for his support of this study |
LIPS 2001.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Patients were allocated to treatment in the order in which they were enrolled into the study at each centre according to medication pack numbers using block randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not reported; ITT performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Funding: this study was supported by an industry grant (Novartis) |
LORD 2006.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | A clinical research pharmacist independent of the trial used computer generated random numbers to conduct the randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/37 patients were excluded; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Study appears free of other biases |
Masajtis‐Zagajewska 2018.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Washout period
Control group
Cointerventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% of the randomised patients were analysed as part of the ITT population |
| Selective reporting (reporting bias) | Unclear risk | Comment: No registry or protocol reported |
| Other bias | Low risk | Study appears free of other biases |
MEGA 2004.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomly assigned either diet or diet plus pravastatin by computerised randomisation by the permuted‐block method |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All endpoints were reviewed strictly by the endpoint committee, without knowledge of treatment allocations, and additional information obtained from the physician as needed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 102 participants lost to follow‐up; statistical analyses done by ITT |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Study appears free of other biases |
Mori 1992.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nakamura 2002.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Addtional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nakamura 2005.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelope method |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nakamura 2006.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelope method |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nielsen 1993.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not all patients accounted for in the analysis; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Funding: this study was supported by Novo Nordisk Research Foundation, the Danish Medical Research Council, and Merck Sharp & Dohme |
Ohsawa 2015.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
Co‐interventions
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% of the randomised patients were analysed as part of the intention‐to‐treat population |
| Selective reporting (reporting bias) | High risk | Some outcomes listed in the registry have not been reported (hs‐CRP, L‐FABP, Ambulatory BP monitoring, PWV, central blood pressure) |
| Other bias | Low risk | Study appears free of other biases |
PANDA 2011.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (high dose)
Intervention group 2 (low dose)
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information Funding: We acknowledge support from the Manchester NIHR Biomedical Research Centre and Manchester Academic Health Sciences Centre (MAHSC). The study was funded by an unrestricted grant from Pfizer UK which markets atorvastatin. The sponsors were allowed to comment on the manuscript but they had no right of veto over any of its contents. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All adverse events and outcomes were assessed by an investigator who was blind to patient status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 111/119 (93%) included in analysis of kidney function; ITT not conducted |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Study appears free of other biases |
Panichi 2006a.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficent information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficent information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data analysers were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant drop‐outs, treatment group (8/28), control group (12/27); ITT not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Study appears free of other biases |
PLANET I 2006.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
Intervention group 3
Co‐interventions or additional treatments
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was produced with Global Randomisation System software (AstraZeneca) |
| Allocation concealment (selection bias) | Low risk | Quote: "All study drugs were in identical capsules, and supplied in identical bottles labelled appropriately so as to maintain the allocation masking" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All study personnel were blinded to the allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: About 92% of the randomised patients were analysed as part of the intention‐to‐treat population |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes listed in the trial registry are reported in the study. |
| Other bias | High risk | Funding: Provided by AstraZenca The management strategy while important may not be sufficient to avoid bias. Quote: "The study was overseen by a steering committee, including non‐voting members from the sponsor. The steering committee oversaw the design of the study, the conduct of the trial, and the management and analysis of all data. The sponsor was involved in the design of the study, in the collection and analysis of data, and in writing the report." Conflicts: authors being employees or having stock options in the company |
PLANET II 2006.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
Intervention group 3
Co‐interventions or additional treatments
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Trial registration: NCT00296400 Protocol registration/published: published Ethics: Declaration of Helsinki and Good Clinical Practice guidelines. Ethics committees and institutional review boards approved the research protocol. All patients gave written informed consent before starting. Declaration of interest/disclosures: N.M.A.I. and M.J.P. report no conflicts of interest. D.d.Z. is consultant for and received honoraria (to employer) from AbbVie, Astellas, Eli‐Lilly, Chemocentryx, Fresenius and Janssen. H.‐H.P. is consultant for and received honoraria from AbbVie, Novartis and Astra Zeneca. H.J.L.H. is consultant for AbbVie, Astellas, Astra Zeneca, Boehringer Ingelheim, Fresenius, Janssen and Merck and has a policy that all honoraria are paid to his employer. The manuscript or portions thereof are not under consideration by another journal or electronic publication and have not been previously published. Funding declared: Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: The randomisation sequence was produced with Global Randomisation System software (AstraZeneca) |
| Allocation concealment (selection bias) | Low risk | Quote: "All study drugs were in identical capsules, and supplied in identical bottles labelled appropriately so as to maintain the allocation masking" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All study personnel were blinded to the allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Insufficient information to permit judgement from publications |
| Selective reporting (reporting bias) | High risk | Comment: Secondary outcomes of the PLANET II trial are not reported in the publications |
| Other bias | High risk | Funding: Provided by AstraZenca The management strategy while important may not be sufficient to avoid bias. Quote: "The study was overseen by a steering committee, including non‐voting members from the sponsor. The steering committee oversaw the design of the study, the conduct of the trial, and the management and analysis of all data. The sponsor was involved in the design of the study, in the collection and analysis of data, and in writing the report." Conflicts: authors being employees or having stock options in the company |
PPP 1992.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated allocation numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT performed; analyses in this article were not prespecified in the original Pravastatin Pooling Project (PPP) protocol but were specified before examining information on kidney function and were undertaken on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Funding: The CARE, LIPID, and WOSCOPS studies and this substudy on kidney disease are investigator‐initiated studies funded by Bristol‐Myers Squibb. Statistical analyses were performed at Wake Forest University, Winston‐Salem, NC, independently of the sponsor. The authors had unlimited access to the data used in this analysis. The sponsor is entitled to comment on manuscripts before submission, and the authors may consider these comments, but the rights to publication reside contractually with the investigators. The sponsor maintained information on adverse events and other trial data, as required by federal regulation |
PREVEND IT 2000.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed in blocks of 20 based on computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Subjects meeting the randomisation criteria were allocated to a treatment number in the order in which they entered the randomised study period |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent endpoint committee reviewed all end points. Committee members had no knowledge of the subject’s treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 10% withdrawals in treatment and control arms; all analyses were performed on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Study appears free of other biases |
Rayner 1996.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Patients were alternatively allocated to treatment and control groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/17 terminated study and were not included in analyses; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | This study was supported by Logos Pharntaceuticals, South Africa |
Renke 2010a.
| Study characteristics | ||
| Methods |
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Unclear risk | Not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/14 (86%) completed protocol; ITT not conducted |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Funding: supported by grant from the Committee for Scientific Research through the Medical University of Gdansk (ST‐4 and W‐80). The authors thank Pfizer Polska and Adamed for providing drugs. The drug providers and sponsors had no involvement in the study design, patient recruitment, analysis, interpretation of data, writing of the report, or the decision to submit the report for publication |
SAGE 2004.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics (CKD sub‐study)
|
|
| Interventions | Intervention group 1
Intervention group 2
Co‐interventions or additional treatments
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All Holter tests were analysed in a blinded fashion by an experienced physician at Covance Central Diagnostics (Reno, Nev)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 96% of participants in main trial had eGFR readings, with 48% having CKD |
| Selective reporting (reporting bias) | High risk | Comment: the primary outcome has not been reported in the CKD substudy |
| Other bias | High risk | Funding: Pzifer Conflicts: Authors employees of Pzifer at the time of publication Other: posthoc analysis of larger trial (no test for subgroup differences undertaken) |
Samuelsson 2002.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Funding: supported in part by a research grant from AstraZeneca, Molndal, Sweden, and resources of the Oklahoma Medical Research Foundation, Oklahoma City, OK, USA |
Sawara 2008.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant number of drop‐outs; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Scanferla 1991.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
SHARP 2010.
| Study characteristics | ||
| Methods |
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information Funding: funded by Merck/Schering‐Plough Pharmaceuticals (North Wales, PA, USA), with additional support from the Australian National Health Medical Research Council, the British Heart Foundation, and the UK Medical Research Council. SHARP was initiated, conducted, and interpreted independently of the principal study funder |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated by local study laptop computer with minimised randomisation |
| Allocation concealment (selection bias) | Low risk | Local laptop computer that was synchronised regularly with central database and double‐dummy treatment to ensure blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy 2 x 2 factorial design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Central adjudication by trained clinicians who were masked to study treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analyses; ITT conducted |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Funding: funded by Merck/Schering‐Plough Pharmaceuticals (North Wales, PA, USA), with additional support from the Australian National Health Medical Research Council, the British Heart Foundation, and the UK Medical Research Council. SHARP was initiated, conducted, and interpreted independently of the principal study funder |
Stegmayr 2005.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by means of a telephone call to the study data centre where sealed envelopes were drawn |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients analysed; ITT performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Study appears free of other biases |
Thomas 1993.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/30 patients did not complete the study; ITT not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Comment: Funders role in the study design, conduct and analysis is not clear. |
TNT 2004.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Randomisation code was prepared by a central unit |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An endpoint committee reviewed (blinded) all potential primary and secondary endpoints to adjudicate the endpoint designation. The committee members who performed patient endpoint review were not investigators or sub‐investigators in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up details were presented in the primary publication and ITT analysis was conducted; interim analyses for the TNT study began after a median 3 year follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Funding: TNT trial (NCT00327691) was funded by Pfizer Inc (New York, NY, USA) |
Tokunaga 2008.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Tonolo 1997.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts not reported; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
UK‐HARP‐I 2005.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1
Intervention group 2
Intervention group 3
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimised randomisation used to balance the treatment groups |
| Allocation concealment (selection bias) | Low risk | Randomisation by telephone to the Clinical Trial Service Unit |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | All events were coded centrally according to a standard protocol. Otherwise unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 442/448 patients completed follow up; ITT performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | Study appears free of other biases |
Verma 2005.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/91 were excluded; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Yasuda 2004.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/88 patients excluded from the study; ITT not performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Yi 2014.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fixed‐allocation (1:1) block‐randomization method. The size of each block was 4 patients." Comment: Not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Each placebo was prepared in the same color and shape as the corresponding drugs so that patients and physicians could not differentiate them" Comment: The study referral institutions completed the random assignment of patients. All clinicians and patients remained blinded until the end of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT analysis undertaken for outcomes. With 96% included in the analysis, but only 78% completing the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: No registry or protocol reported |
| Other bias | Unclear risk | Comment: Unclear role of the sponsor Trial registration: Not reported. Protocol registration/published: Not reported. Declaration of interest/disclosures: Not reported. Funding declared: Not reported." |
Zhang 1995.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group
Control group
|
|
| Outcomes | Outcomes reported
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
ADPKD: autosomal dominant polycystic kidney disease; ACEi: angiotensin‐converting enzyme inhibitors; AKI: acute kidney injury; ALT: alanine aminotransferase; AMI: acute myocardial infarction; Apo: apolipoprotein; ARB: angiotensin receptor blockers; AST: aspartate aminotransferase; BP: blood pressure; BSA: body surface area; CABG: coronary artery bypass graft; CHD: congestive heart disease; CHF: congestive heart failure; CKD: chronic kidney disease; CrCl: creatinine clearance; CRP: C‐reactive protein; CV: cardiovascular; CVA: cardiovascular accident; DM: diabetes mellitus; ECG: electrocardiograph; eGFR: estimated glomerular filtration rate; ESA: Erythropoietin stimulating agent; GN: glomerulonephritis; DBP: diastolic blood pressure; ESKD: end‐stage kidney disease; FSGS: focal segmental glomerulosclerosis; GFR: glomerular filtration rate; GN: glomerulonephritis; Hb: haemoglobin; HbA1c: glycated haemoglobin; HDL: high‐density lipoprotein; HRY: hormone‐replacement therapy; IgA: immunoglobulin A; IMN: idiopathic membranous nephropathy; ITT: intention‐to‐treat; IV: intravenous; KRT: kidney replacement therapy; LDL: low‐density lipoprotein; MDA: malondialdehyde‐modified; MDRD: modification of diet in renal disease; MI: myocardial infarction; MPGN: membranoproliferative glomerulonephritis; NSAID: nonsteroidal anti‐inflammatory drug; NYHA: New York Heart Association; PTCA: percutaneous transluminal coronary angiography; SBP: systolic blood pressure; SCr: serum creatinine; TC: total cholesterol; TIA: transient ischaemic attack; TSAT: transferrin saturation; TSH: thyroid‐stimulating hormones; UACR: urinary albumin‐creatinine ratio; UAE: urinary albumin excretion; ULN: upper limit of normal; UPCR: urinary protein‐creatinine ratio; UTI: urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almquist 2012 | Wrong intervention |
| Almukhtar 2021 | Wrong population |
| Dogra 2005 | Short duration |
| Dogra 2007 | Short duration |
| ESTABLISH 2004 | Wrong comparator: no control for participants with CKD |
| Fotso 2021 | Wrong population |
| Golper 1987 | Duration not reported |
| Golper 1989 | Short duration |
| IMPROVE‐IT 2008 | Wrong intervention |
| Ishimitsu 2014 | Wrong intervention |
| Kouvelos 2013 | Wrong intervention |
| Mackie 2008 | Wrong population: children (< 18 years) |
| Mohammadi 2023 | Wrong population |
| Mose 2014a | Wrong study design |
| Mose 2014b | Wrong study design |
| NCT03543774 | Wrong intervention |
| Ott 2008 | Short duration |
| PACTR202110707328144 | Wrong population |
| Paulsen 2010 | Short duration |
| Schmieder 2003 | Short duration |
| Siddiqi 2011 | Wrong study design |
| Suzuki 2013a | Wrong intervention |
| Torraca 2006 | Short duration |
| UK‐HARP‐II 2006 | Wrong intervention |
| Van Dijk 2001 | Short duration |
| Vasin 2021 | Wrong population |
| Watanabe 2022 | Wrong population |
| Weinstein 2013 | Wrong intervention |
| Yasmeen 2015 | Wrong intervention |
| Yasuda 2010 | Active intervention |
| Yen 2022 | Wrong population |
| Zinellu 2012 | Wrong intervention |
CKD ‐ chronic kidney disease; RCT ‐ randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
NCT00768638.
| Methods | Randomised, parallel‐group, double‐blind |
| Participants | Inclusion criteria
|
| Interventions | Intervention group 1
Intervention group 2
|
| Outcomes | Proteinuria at 6 months |
| Notes |
NCT02863185.
| Methods | Randomised, parallel‐group, open‐label controlled trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group 1
Intervention group 2
|
| Outcomes | Primary outcomes
Secondary outcome
|
| Notes | Follow‐up 24 months |
ACEi: angiotensin‐converting enzyme inhibitor; AKI: acute kidney injury; ARB: angiotensin receptor blocker; BP: blood pressure; CKD: chronic kidne disease; HbA1c: glycolated haemoglobin; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein; MDRD: Modification of Diet on Renal Disease
Characteristics of ongoing studies [ordered by study ID]
NCT05870007.
| Study name | Atorvastatin and alkali therapy in patients with autosomal dominant polycystic kidney disease |
| Methods | Parallel, open‐label RCT |
| Participants | Country: Taiwan Inclusion criteria: ADPKD; ≥ 18 years |
| Interventions | Intervention group
Control group
|
| Outcomes | Change in kidney function: SCr; eGFR; BUN; potassium Change in liver function: ALT; AST; total bilirubin; prothrombin time; creatine phosphokinase Changes in muscle injury marker Changes in muscle tenderness BP |
| Starting date | Estimated: May 2023 |
| Contact information | Not available |
| Notes | Not yet recruiting |
ADPKD: autosomal‐dominant polycystic kidney disease; ALT: alanine aminotransferase; BP: blood pressure; BUN: blood urea nitrogen; CRP: C‐reactive protein; eGFR: estimated glomerular filtration rate; RCT: randomised controlled trial; SCr: serum creatinine
Differences between protocol and review
We did not include studies with follow‐up < eight weeks duration (excluding data from Van Dijk 2001) in the 2014 review update. The updated review has included some outcomes, including a new primary outcome of rhabdomyolysis, and secondary outcomes of onset of diabetes, revascularization procedures, fatigue, life participation, and memory loss. Additionally, post hoc analyses were conducted on the impact of baseline kidney function on the following outcomes: kidney failure, doubling serum creatinine, end‐of‐treatment kidney function, and end‐of‐treatment proteinuria. These outcomes were identified as critical and important by the CARI Statins in CKD living guideline Work Group (www.cariguidelines.org).
This update includes some new methods relevant to living systematic reviews, which are described in the Methods and Appendix 3 (Living systematic review protocol).
Contributions of authors
David J Tunnicliffe: Data extraction, analysis and interpretation, drafting the updated manuscript, final approval of version to be published
Suetonia C Palmer: Analysis and interpretation, drafting the updated manuscript, final approval of version to be published
Brydee A Cashmore: Data extraction, analysis and interpretation, writing the final manuscript, final approval of version to be published
Valeria M Saglimbene: Analysis and interpretation, writing the final manuscript, final approval of version to be published
Kelly Lambert: Analysis and interpretation, writing the final manuscript, final approval of version to be published
Rathika Krishnasamy: Analysis and interpretation, writing the final manuscript, final approval of version to be published
David W Johnson: Analysis and interpretation of data, writing the final manuscript, final approval of version to be published
Jonathan Craig: Concept and design, analysis and interpretation of data, writing the final manuscript, final approval of version to be published
Giovanni FM Strippoli: Concept and design of the review, data extraction, analysis and interpretation of data, writing the final manuscript, final approval of version to be published
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
David Tunnicliffe: no relevant interests were disclosed
Suetonia Palmer: no relevant interests were disclosed
Brydee A Cashmore: no relevant interests were disclosed
Varleria M Saglimbene: no relevant interests were disclosed
Kelly Lambert: no relevant interests were disclosed
Rathika Krishnasamy: Baxter Healthcare Corporation (Independent Contractor ‐ Other), BAXTER HEALTHCARE (Independent Contractor ‐ Other), Amgen (Travel), Baxter Healthcare Corporation (Grant / Contract)
Jonathan Craig: no relevant interests were disclosed
David Johnson: Boehringer Ingelheim (Independent Contractor ‐ Other), Amgen (Travel), Ono (Independent Contractor ‐ Consultant), Vifor Fresenius Medical Care Renal Pharma Ltd. (Independent Contractor ‐ Consultant), AWAK (Independent Contractor ‐ Consultant), BAXTER HEALTHCARE (Independent Contractor ‐ Consultant), AstraZeneca (Independent Contractor ‐ Consultant), Bayer (Independent Contractor ‐ Consultant), Baxter Healthcare Corporation (Independent Contractor ‐ Consultant)
Giovanni Strippoli: no relevant interests were disclosed
Edited (no change to conclusions)
References
References to studies included in this review
4S 1993 {published data only}
- Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation 2001;104(25):3046-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baseline serum cholesterol and treatment effect in the Scandinavian Simvastatin Survival Study (4S). Lancet 1995;345(8960):1274-5. [MEDLINE: ] [PubMed] [Google Scholar]
- Berg K, Dahlén G, Christophersen B, Cook T, Kjekshus J, Pedersen T. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival Study. Clinical Genetics 1997;52(5):254-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. American Journal of Kidney Diseases 2007;49(3):373-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Crea F, Monaco C, Lanza GA, Maggi E, Ginnetti F, Cianflone D, et al. Inflammatory predictors of mortality in the Scandinavian Simvastatin Survival Study. Clinical Cardiology 2002;25(10):461-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Design and baseline results of the Scandinavian Simvastatin Survival Study of patients with stable angina and/or previous myocardial infarction. American Journal of Cardiology 1993;71(5):393-400. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Faergeman O, Kjekshus J, Cook T, Pyorälä K, Wilhelmsen L, Thorgeirsson G, et al. Differences in the treatment of coronary heart disease between countries as revealed in the Scandinavian Simvastatin Survival Study (4S). European Heart Journal 1998;19(10):1531-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gerdes LU, Gerdes C, Kervinen K, Savolainen M, Klausen IC, Hansen PS, et al. The apolipoprotein Ɛ4 allele determines prognosis and the effect on prognosis of simvastatin in survivors of myocardial infarction: a substudy of the Scandinavian simvastatin survival study. Circulation 2000;101(12):1366-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Haffner SM, Alexander CM, Cook TJ, Boccuzzi SJ, Musliner TA, Pedersen TR, et al. Reduced coronary events in simvastatin-treated patients with coronary heart disease and diabetes or impaired fasting glucose levels: subgroup analyses in the Scandinavian Simvastatin Survival Study. Archives of Internal Medicine 1999;159(22):2661-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Haffner SM. The Scandinavian Simvastatin Survival Study (4S) subgroup analysis of diabetic subjects: implications for the prevention of coronary heart disease. Diabetes Care 1997;20(4):469-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Herman WH, Alexander CM, Cook JR, Boccuzzi SJ, Musliner TA, Pedersen TR, et al. Effect of simvastatin treatment on cardiovascular resource utilization in impaired fasting glucose and diabetes. Findings from the Scandinavian Simvastatin Survival Study. Diabetes Care 1999;22(11):1771-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Huskey J, Lindenfeld J, Cook T, Kjekshus J, Pedersen T. Effect of simvastatin on kidney function loss in patients with coronary heart disease [abstract no: SU-FC137]. Journal of the American Society of Nephrology 2007;18(Abstracts):98A-9A. [CENTRAL: CN-00708811] [Google Scholar]
- Huskey J, Lindenfeld J, Cook T, Targher G, Kendrick J, Kjekshus J, et al. Effect of simvastatin on kidney function loss in patients with coronary heart disease: findings from the Scandinavian Simvastatin Survival Study (4S). Atherosclerosis 2009;205(1):202-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Johannesson M, Jonsson B, Kjekshus J, Olsson AG, Pedersen TR, Wedel H. Cost effectiveness of simvastatin treatment to lower cholesterol levels in patients with coronary heart disease. Scandinavian Simvastatin Survival Study Group. New England Journal of Medicine 1997;336(5):332-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jonsson B, Cook JR, Pedersen TR. The cost-effectiveness of lipid lowering in patients with diabetes: results from the 4S trial. Diabetologia 1999;42(11):1293-301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jönsson B, Johannesson M, Kjekshus J, Olsson AG, Pedersen TR, Wedel H. Cost-effectiveness of cholesterol lowering. Results from the Scandinavian Simvastatin Survival Study (4S). European Heart Journal 1996;17(7):1001-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Miettinen TA, Gylling H, Strandberg T, Sarna S. Baseline serum cholestanol as predictor of recurrent coronary events in subgroup of Scandinavian simvastatin survival study. Finnish 4S Investigators. BMJ 1998;316(7138):1127-30. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen TA, Gylling H, Subgroup analysis of Scandinavian Simvastatin Survival Study (4S). Blood glucose and the metabolism of cholesterol in coronary patients with and without simvastatin treatment. Clinica Chimica Acta 2007;379(1-2):53-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Miettinen TA, Pyorälä K, Olsson AG, Musliner TA, Cook TJ, Faergeman O, et al. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Survival Study (4S). Circulation 1997;96(12):4211-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pedersen TR, Berg K, Cook TJ, Faergeman O, Haghfelt T, Kjekshus J, et al. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian Simvastatin Survival Study. Archives of Internal Medicine 1996;156(18):2085-92. [MEDLINE: ] [PubMed] [Google Scholar]
- Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atherosclerosis Supplements 2004;5(3):81-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pedersen TR, Kjekshus J, Berg K, Olsson AG, Wilhelmsen L, Wedel H, et al. Cholesterol lowering and the use of healthcare resources. Results of the Scandinavian Simvastatin Survival Study. [Erratum appears in Circulation 1996 Aug 15;94(4):849]. Circulation 1996;93(10):1796-802. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pedersen TR, Kjekshus J, Pyorälä K, Olsson AG, Cook TJ, Musliner TA, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S). American Journal of Cardiology 1998;81(3):333-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pedersen TR, Olsson AG, Faergeman O, Kjekshus J, Wedel H, Berg K, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998;97(15):1453-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pedersen TR, Wilhelmsen L, Faergeman O, Strandberg TE, Thorgeirsson G, Troedsson L, et al. Follow-up study of patients randomized in the Scandinavian Simvastatin Survival Study (4S) of cholesterol lowering. American Journal of Cardiology 2000;86(3):257-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pyorälä K, Ballantyne CM, Gumbiner B, Lee MW, Shah A, Davies MJ, et al. Reduction of cardiovascular events by simvastatin in nondiabetic coronary heart disease patients with and without the metabolic syndrome: subgroup analyses of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 2004;27(7):1735-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pyorälä K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S).[Erratum appears in Diabetes Care 1997 Jun;20(6):1048]. Diabetes Care 1997;20(4):614-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Strandberg TE, Pyorälä K, Cook TJ, Wilhelmsen L, Faergeman O, Thorgeirsson G, et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet 2004;364(9436):771-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wilhelmsen L, Pyorälä K, Wedel H, Cook T, Pedersen T, Kjekshus J. Risk factors for a major coronary event after myocardial infarction in the Scandinavian Simvastatin Survival Study (4S). Impact of predicted risk on the benefit of cholesterol-lowering treatment. European Heart Journal 2001;22(13):1119-27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Abe 2011c {published data only}
- Abe M, Maruyama N, Okada K, Matsumoto S, Matsumoto K, Soma M. Effects of lipid-lowering therapy with rosuvastatin on kidney function and oxidative stress in patients with diabetic nephropathy. Journal of Atherosclerosis & Thrombosis 2011;18(11):1018-28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Abe 2015 {published data only}
- Abe M, Maruyama N, Maruyama T, Okada K, Soma M. A trial of pitavastatin versus rosuvastatin for dyslipidemia in chronic kidney disease. Journal of Atherosclerosis & Thrombosis 2015;22(12):1235-47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
AFCAPS/TexCAPS 1997 {published data only}
- Clearfield M, Downs JR, Weis S, Whitney EJ, Kruyer W, Shapiro DR, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): efficacy and tolerability of long-term treatment with lovastatin in women. Journal of Womens Health & Gender-Based Medicine 2001;10(10):971-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Clearfield M, Whitney EJ, Weis S, Downs JR, Shapiro DR, Stein EA, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): baseline characteristics and comparison with USA population. Journal of Cardiovascular Risk 2000;7(2):125-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Clearfield MB, Weis SE, Willis JM, Vasenius KA, McConathy WJ. Lability of serum low-density lipoprotein cholesterol levels during screening in subgroup of Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) cohort. Journal of the American Osteopathic Association 2002;102(7):377-84. [MEDLINE: ] [PubMed] [Google Scholar]
- Cui Y, Watson DJ, Girman CJ, Shapiro DR, Gotto AM, Hiserote P, et al. Effects of increasing high-density lipoprotein cholesterol and decreasing low-density lipoprotein cholesterol on the incidence of first acute coronary events (from the Air Force/Texas Coronary Atherosclerosis Prevention Study). American Journal of Cardiology 2009;104(6):829-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Downs JR, Beere PA, Whitney E, Clearfield M, Weis S, Rochen J, et al. Design & rationale of the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). American Journal of Cardiology 1997;80(3):287-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Downs JR, Clearfield M, Tyroler HA, Whitney EJ, Kruyer W, Langendorfer A, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TEXCAPS): additional perspectives on tolerability of long-term treatment with lovastatin. American Journal of Cardiology 2001;87(9):1074-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279(20):1615-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gotto AM Jr, Boccuzzi SJ, Cook JR, Alexander CM, Roehm JB, Meyer GS, et al. Effect of lovastatin on cardiovascular resource utilization and costs in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). AFCAPS/TexCAPS Research Group. American Journal of Cardiology 2000;86(11):1176-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. American Journal of Kidney Diseases 2010;55(1):42-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ALLIANCE 2000 {published data only}
- Isaacsohn JL, Davidson MH, Hunninghake D, Singer R, McLain R, Black DM. Aggressive Lipid-Lowering Initiation Abates New Cardiac Events (ALLIANCE)-rationale and design of atorvastatin versus usual care in hypercholesterolemic patients with coronary artery disease. American Journal of Cardiology 2000;86(2):250-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP, et al. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. American Journal of Kidney Diseases 2009;53(5):741-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Koren MJ, Davidson MH. Aggressive treatment with atorvastatin is associated with improvement in renal function in managed care patients with coronary heart disease: the ALLIANCE study [abstract no: S-FC-018]. In: 4th World Congress of Nephrology. 19th International Congress of the International Society of Nephrology (ISN); 2007 Apr 21-25; Rio de Janeiro, Brazil. 2007:44.
- Koren MJ, Hunninghake DB. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the ALLIANCE study. Journal of the American College of Cardiology 2004;44(9):1772-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mullins CD, Rattinger GB, Kuznik A, Koren MJ. Cost-effectiveness of intensive atorvastatin treatment in high-risk patients compared with usual care in a postgeneric statin market: economic analysis of the Aggressive Lipid-lowering Initiation Abates New Cardiac Events (ALLIANCE) study. Clinical Therapeutics 2008;30 Pt 2:2204-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aranda Arcas 1994 {published data only}
- Aranda Arcas JL, Sanchez R, Guijarro C, Araque A, Pulido F, Praga M, et al. Effect of pravastatin on hypercholesterolemia associated with proteinuria [Efecto de la pravastatina en la hipercolesterolemia asociada a proteinuria]. Anales de Medicina Interna 1994;11(11):523-7. [MEDLINE: ] [PubMed] [Google Scholar]
ASCOT‐LLA 2003 {published data only}
- Gupta A, Chang C, Collier D, Dahlof B, Poulter N, Sever P, et al. The relationship between statin therapy and progression of renal damage among 10305 hypertensive patients randomised in the ASCOT-lipid-lowering arm (LLA) [abstract no: O86]. In: British Renal Society Conference; 2013 May 14-May 16; Manchester, UK. 2013.
- Gupta A, Chang C, Collier D, Dahlof B, Poulter N, Sever P, et al. The relationship between statin therapy and progression of renal damage among 10305 hypertensive patients randomised in the ASCOT-lipid-lowering arm (LLA) [abstract]. In: Renal Association & British Renal Society Joint Meeting; 2011 Jun 6-9 ; Birmingham, UK. 2011:1043.
- Gupta A, Chang CL, Collier D, Dahlof B, Poulter NR, Sever PS. The relationship between statin therapy and progression of renal damage among 10305 hypertensive patients randomised in the Ascot-Lipid-Lowering Arm (LLA) [abstract]. Atherosclerosis Supplements 2011;12(1):158-9. [EMBASE: 70805024] [Google Scholar]
- Gupta AK, Chang CL, Dahlof B, Poulter NR, Sever PS. Cardiovascular and all-cause mortality outcomes among hypertensive patients with moderate renal dysfunction in the ASCOT-LLA, and its extended follow-up [abstract no: 5A.1]. Journal of Human Hypertension 2011;25(10):633-4. [EMBASE: 70537932] [Google Scholar]
- Gupta AK, Chang CL, Dahlof B, Poulter NR, Sever PS. Cardiovascular and all-cause mortality outcomes among hypertensive patients with moderate renal dysfunction in the ASCOT-LLA, and its extended follow-up [abstract no: P1480]. European Heart Journal 2011;32:220. [EMBASE: 70533871] [Google Scholar]
- Lindgren P, Buxton M, Kahan T, Poulter NR, Dahlof B, Sever PS, et al. Cost-effectiveness of atorvastatin for the prevention of coronary and stroke events: an economic analysis of the Anglo-Scandinavian Cardiac Outcomes Trial--lipid-lowering arm (ASCOT-LLA). European Journal of Cardiovascular Prevention & Rehabilitation 2005;12(1):29-36. [MEDLINE: ] [PubMed] [Google Scholar]
- Sever PS, Chang CL, Gupta AK, Whitehouse A, Poulter NR. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the U.K. European Heart Journal 2011;32(20):2525-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Drugs 2004;64 Suppl 2:43-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361(9364):1149-58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sever PS, Poulter NR, Dahlof B, Wedel H, Beevers G, Caulfield M, et al. The Anglo-Scandinavian Cardiac Outcomes Trial lipid lowering arm: extended observations 2 years after trial closure. European Heart Journal 2008;29(4):499-508. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sever PS, Poulter NR, Dahlof B, Wedel H, Collins R, Beevers G, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial--lipid-lowering arm (ASCOT-LLA). Diabetes Care 2005;28(5):1151-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sever PS, Poulter NR, Dahlof B, Wedel H. Antihypertensive therapy and the benefits of atorvastatin in the Anglo-Scandinavian Cardiac Outcomes Trial: lipid-lowering arm extension. Journal of Hypertension 2009;27(5):947-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ASUCA 2013 {published data only}
- Kasahara M, Ueshima K, Koya D, Babazono T, Sato T, Imamoto M, et al. Effects of atorvastatin on renal function in patients with dyslipidemia and chronic kidney disease: design of the ASsessment of clinial usefulness in CKD patients with atorvastatin (ASUCA) trial [abstract no: FR-PO194]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):413A. [Google Scholar]
- Kimura G, Kasahara M, Ueshima K, Tanaka S, Yasuno S, Fujimoto A, et al. Effects of atorvastatin on renal function in patients with dyslipidemia and chronic kidney disease: assessment of clinical usefulness in CKD patients with atorvastatin (ASUCA) trial. Clinical & Experimental Nephrology 2017;21(3):417-24. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G, Ueshima K, Kashahara M, Tanaka S, Yasuno S, Fujimoto A, et al. Effects of atorvastatin on renal function in patients with dyslipidemia and chronic kidney disease: ASsessment of clinical usefulness in CKD patients with atorvastatin (ASUCA) trial [abstract no: SA-O1086]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):5B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara Y, Yasuno S, Kasahara M, Ueshima K, Nakao K. The association between the control of LDL-cholesterol level and renal function in CKD patients with hyperlipidemia [abstract no: MP147]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii482. [EMBASE: 617290474] [Google Scholar]
- Kuwabara Y, Yasuno S, Kasahara M, Ueshima K, Nakao K. The association between uric acid levels and renal function of CKD patients with hyperlipidemia: a sub-analysis of the ASUCA trial. Clinical & Experimental Nephrology 2020;24(5):420-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueshima K, Kasahara M, Koya D, Babazono T, Sato T, Imamoto M, et al. Effects of atorvastatin on renal function in patients with dyslipidemia and chronic kidney disease: rationale and design of the ASsessment of clinical Usefulness in CKD patients with Atorvastatin (ASUCA) trial. Clinical & Experimental Nephrology 2013;17(2):211-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bianchi 2003 {published data only}
- Bianchi S, Bigazzi R, Caiazza A, Campese VM. A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease [Erratum in: Am J Kidney Dis. 2004 Jan;43(1):193]. American Journal of Kidney Diseases 2003;41(3):565-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Buemi 2000 {published data only}
- Buemi M, Allegra A, Corica F, Aloisi C, Giacobbe M, Pettinato G, et al. Effect of fluvastatin on proteinuria in patients with immunoglobulin A nephropathy. Clinical Pharmacology & Therapeutics 2000;67(4):427-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CARDS 2003 {published data only}
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). American Journal of Kidney Diseases 2009;54(5):810-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364(9435):685-96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Rapid emergence of effect of atorvastatin on cardiovascular outcomes in the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetologia 2005;48(12):2482-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hitman GA, Colhoun H, Newman C, Szarek M, Betteridge DJ, Durrington PN, et al. Stroke prediction and stroke prevention with atorvastatin in the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetic Medicine 2007;24(12):1313-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Neil HA, DeMicco DA, Luo D, Betteridge DJ, Colhoun HM, Durrington PN, et al. Analysis of efficacy and safety in patients aged 65-75 years at randomization: Collaborative Atorvastatin Diabetes Study (CARDS). Diabetes Care 2006;29(11):2378-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Newman CB, Szarek M, Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, et al. The safety and tolerability of atorvastatin 10 mg in the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetes & Vascular Disease Research 2008;5(3):177-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Raikou M, McGuire A, Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, et al. Cost-effectiveness of primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes: results from the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetologia 2007;50(4):733-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Soedamah-Muthu SS, Colhoun HM, Thomason MJ, Betteridge DJ, Durrington PN, Hitman GA, et al. The effect of atorvastatin on serum lipids, lipoproteins and NMR spectroscopy defined lipoprotein subclasses in type 2 diabetic patients with ischaemic heart disease. Atherosclerosis 2003;167(2):243-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thomason MJ, Colhoun HM, Livingstone SJ, Mackness MI, Betteridge DJ, Durrington PN, et al. Baseline characteristics in the Collaborative AtoRvastatin Diabetes Study (CARDS) in patients with Type 2 diabetes. Diabetic Medicine 2004;21(8):901-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cha 2015 {published data only}
- Cha JJ, Kim K, Min HS, Ghee J, Kim YJ, Lee EY, et al. Effect of fluvastatin treatment on proteinuria in diabetic patients with chronic kidney disease [abstract no: TH-PO656]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):239a. [EMBASE: 641102077] [Google Scholar]
Di Lullo 2005 {published data only}
- Di Lullo L, Addesse R, Comegna C, Firmi G, Galderisi C, Iannacci GR, et al. Effects of fluvastatin treatment on lipid profile, C-reactive protein trend, and renal function in dyslipidemic patients with chronic renal failure. Advances in Therapy 2005;22(6):601-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Di Lullo L, Polito P. Effects of fluvastatin treatment on lipid profile, inflammatory status and renal function in dyslipidemic patients with chronic renal failure [abstract no: S-PO-0543]. In: 4th World Congress of Nephrology.19th International Congress of the International Society of Nephrology (ISN); 2007 Apr 21-25; Rio de Janeiro, Brazil. 2007:195.
Dummer 2008 {published data only}
- Dummer CD, Thome' FS, Zingano B, Lindoso A, Veronese FV. Acute effect of simvastatin on inflammation and oxidative stress in chronic kidney disease. Journal of Nephrology 2008;21(6):900-8. [MEDLINE: ] [PubMed] [Google Scholar]
ESPLANADE 2010 {published data only}
- Remuzzi G. Effects of add-on fluvastatin therapy in patients with chronic proteinuric nephropathy on dual RAS blockade: esplanade trial [abstract no: F-PO1221]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):389A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Tonelli M, Loriga G, Motterlini N, Rubis N, et al. Effects of add-on fluvastatin therapy in patients with chronic proteinuric nephropathy on dual renin-angiotensin system blockade: the ESPLANADE Trial. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(11):1928-38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fassett 2010 {published data only}
- Fassett RG, Coombes JS, Packham D, Fairley KF, Kincaid-Smith P. Effect of pravastatin on kidney function and urinary protein excretion in autosomal dominant polycystic kidney disease. Scandinavian Journal of Urology & Nephrology 2010;44(1):56-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fried 2001 {published data only}
- Fried LF, Forrest KY, Ellis D, Chang Y, Silvers N, Orchard TJ. Lipid modulation in insulin-dependent diabetes mellitus: effect on microvascular outcomes. Journal of Diabetes & its Complications 2001;15(3):113-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gheith 2002 {published data only}
- Gheith O, Saad M, Sobh M. Nephrotic dyslipidemia, harvest of treatment [abstract no: MP085]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:258. [CENTRAL: CN-00509210]
- Gheith O, Sobh M, Gazaren S, Ahmed H, El Baz M. Study the effect of treatment of nephrotic dyslipidemia on renal function, fat deposits and scarring in patients with persistent nephrotic syndrome [abstract no: M182]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):100. [CENTRAL: CN-00520338] [DOI] [PubMed] [Google Scholar]
- Gheith OA, Sobh MA, Mohamed Kel-E, El-Baz M, El-Husseini F, Gazarin SS, et al. Impact of treatment of dyslipidemia on renal function, fat deposits and scarring in patients with persistent nephrotic syndrome. Nephron 2002;91(4):612-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goicoechea 2006 {published data only}
- Goicoechea M, Vinuesa SG, Lahera V, Cachofeiro V, Gomez-Campdera F, Vega A, et al. Effects of atorvastatin on inflammatory and fibrinolytic parameters in patients with chronic kidney disease. Journal of the American Society of Nephrology 2006;17(12 Suppl 3):S231-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hommel 1992 {published data only}
- Hommel E, Andersen P, Gall MA, Nielsen F, Jensen B, Rossing P, et al. Plasma lipoproteins and renal function during simvastatin treatment in diabetic nephropathy. Diabetologia 1992;35(5):447-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HPS 2002 {published data only}48489393
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361(9374):2005-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004;363(9411):757-67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Emberson JR, Ng LL, Armitage J, Bowman L, Parish S, Collins R. N-terminal Pro-B-type natriuretic peptide, vascular disease risk, and cholesterol reduction among 20,536 patients in the MRC/BHF heart protection study. Journal of the American College of Cardiology 2007;49(3):311-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360(9326):23-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360(9326):7-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R. Cost-effectiveness of simvastatin in people at different levels of vascular disease risk: economic analysis of a randomised trial in 20,536 individuals. Lancet 2005;365(9473):1779-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. Journal of Vascular Surgery 2007;45(4):645-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: a randomised placebo-controlled trial [ISRCTN48489393]. BMC Medicine 2005;3:6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
IDEAL 2004 {published data only}
- Holme I, Cater NB, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, et al. Lipoprotein predictors of cardiovascular events in statin-treated patients with coronary heart disease. Insights from the Incremental Decrease In End-points Through Aggressive Lipid-lowering Trial (IDEAL). Annals of Medicine 2008;40(6):456-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holme I, Fayyad R, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, et al. Cardiovascular outcomes and their relationships to lipoprotein components in patients with and without chronic kidney disease: results from the IDEAL trial. Journal of Internal Medicine 2010;267(6):567-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008;117(23):3002-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, et al. Design and baseline characteristics of the Incremental Decrease in End Points through Aggressive Lipid Lowering study. American Journal of Cardiology 2004;94(6):720-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial [Erratum appears in JAMA. 2005 Dec 28;294(24):3092]. JAMA 2005;294(19):2437-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ikeda 2012 {published data only}
- Ikeda H, Ura Y, Nakayama M. Rosuvastatin reduces urinary protein excretion in patients with chronic glomerulonephritis compared to the pravastatin use: a crossover trial [abstract no: SA-PO274]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):699A. [Google Scholar]
Imai 1999 {published data only}
- Imai Y, Suzuki H, Saito T, Tsuji I, Abe K, Saruta T. The effect of pravastatin on renal function and lipid metabolism in patients with renal dysfunction with hypertension and hyperlipidemia. Pravastatin and Renal Function Research Group. Clinical & Experimental Hypertension (New York) 1999;21(8):1345-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Inukai 2011 {published data only}
- Inukai K, Iuchi T, Sumita T, Ito D, Ikebukuro K, Imai K, et al. Combination therapy with angiotensin receptor blocker (ARB) plus atorvastatin reduced proteinuria in type 2 diabetic patients with diabetic nephropathy. Therapeutic Research 2011;32(7):947-53. [EMBASE: 362412882] [Google Scholar]
JUPITER 2007 {published data only}
- Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. New England Journal of Medicine 2009;360(18):1851-61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New England Journal of Medicine 2008;359(21):2195-207. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ridker PM, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Khurmi NS, et al. Baseline characteristics of participants in the JUPITER trial, a randomized placebo-controlled primary prevention trial of statin therapy among individuals with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein. American Journal of Cardiology 2007;100(11):1659-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. Journal of the American College of Cardiology 2010;55(12):1266-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kimura 2012 {published data only}
- Kimura S, Inoguchi T, Yokomizo H, Maeda Y, Sonoda N, Takayanagi R. Randomized comparison of pitavastatin and pravastatin treatment on the reduction of urinary albumin in patients with type 2 diabetic nephropathy. Diabetes, Obesity & Metabolism 2012;14(7):666-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lam 1995 {published data only}
- Lam KS, Cheng IK, Janus ED, Pang RW. Cholesterol-lowering therapy may retard the progression of diabetic nephropathy. Diabetologia 1995;38(5):604-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parving HH. Cholesterol-lowering therapy may retard the progression of diabetic nephropathy. Diabetologia 1996;39(3):367-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee TM, Lin MS, Tsai CH, Chang NC. Add-on and withdrawal effect of pravastatin on proteinuria in hypertensive patients treated with AT receptor blockers. Kidney International 2005;68(2):779-87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lee TM, Su SF, Tsai CH. Effect of pravastatin on proteinuria in patients with well-controlled hypertension. Hypertension 2002;40(1):67-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lintott 1995 {published data only}
- Lintott CJ, Scott RS, Bremer JM, Shand BI. Fluvastatin for dyslipoproteinemia, with or without concomitant chronic renal insufficiency. American Journal of Cardiology 1995;76(2):97-101A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
LIPS 2001 {published data only}
- Arampatzis CA, Goedhart D, Serruys PW, Saia F, Lemos PA, Feyter P. Fluvastatin reduces the impact of diabetes on long-term outcome after coronary intervention--a Lescol Intervention Prevention Study (LIPS) substudy. American Heart Journal 2005;149(2):329-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lemos PA, Serruys PW, Feyter P, Mercado NF, Goedhart D, Saia F, et al. Long-term fluvastatin reduces the hazardous effect of renal impairment on four-year atherosclerotic outcomes (a LIPS substudy). American Journal of Cardiology 2005;95(4):445-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serruys PW, De Feyter PJ, Benghozi R, Hugenholtz PG, Lesaffre E. The Lescol(R) Intervention Prevention Study (LIPS): a double-blind, placebo-controlled, randomized trial of the long-term effects of fluvastatin after successful transcatheter therapy in patients with coronary heart disease. International Journal of Cardiovascular Interventions 2001;4(4):165-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Serruys PW, Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;287(24):3215-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
LORD 2006 {published data only}
- Fassett R, Robertson I, Ball M, Geraghty D, Sharman J, Coombes J. A randomised trial assessing the effects of atorvastatin on arterial stiffness in chronic kidney disease [abstract no: SU193]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009. [CENTRAL: CN-01912394]
- Fassett RG, Ball MJ, Robertson IK, Geraghty DP, Coombes JS. Atorvastatin and the progression of chronic kidney disease: the LORD Trial [abstract no: F-PO1919]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):543A. [CENTRAL: CN-00773725] [Google Scholar]
- Fassett RG, Ball MJ, Robertson IK, Geraghty DP, Coombes JS. Baseline serum lipids and renal function in chronic kidney disease patients entering the LORD trial. International Journal of Clinical Pharmacology & Therapeutics 2006;44(11):580-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fassett RG, Ball MJ, Robertson IK, Geraghty DP, Coombes JS. The Lipid lowering and Onset of Renal Disease (LORD) Trial: a randomized double blind placebo controlled trial assessing the effect of atorvastatin on the progression of kidney disease. BMC Nephrology 2008;9:4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassett RG, Coombes JS, Purse LM, Watts M, Chugg KL, Geraghty DP. Enrolment in a clinical trial improves renal function [abstract no: SU-PO1057]. Journal of the American Society of Nephrology 2003;14(Nov):768A. [CENTRAL: CN-00583896] [Google Scholar]
- Fassett RG, Oliver K, Summers MJ, Smith M, Anderson LM, Coombes JS. Lipid lowering therapy and cognitive function in patients with chronic kidney disease [abstract no: PUB623]. Journal of the American Society of Nephrology 2005;16:917A. [CENTRAL: CN-00636136] [Google Scholar]
- Fassett RG, Robertson I, Ball MJ, Geraghty DP, Coombes JS. Atorvastatin slows the progression of chronic kidney disease in patients with inflammation [abstract no: TH-PO204]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):158A. [Google Scholar]
- Fassett RG, Robertson I, Ball MJ, Geraghty DP, Coombes JS. Relationships between physical activity and nutrition with kidney function in CKD patients [abstract no: FR-PO1383]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):432A. [Google Scholar]
- Fassett RG, Robertson I, Ball MJ, Geraghty DP, Coombes JS. Relationships between physical activity and nutrition with quality of life in CKD patients [abstract no: SA-PO2441]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):680A. [Google Scholar]
- Fassett RG, Robertson I, Healy HG, Geraghty DP, Ball MJ, Cardinal JW, et al. Effect of atorvastatin on NGAL and cystatin C in chronic kidney disease: a post-hoc analysis of the LORD trial [abstract no: SA-PO2320]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):641A. [DOI] [PubMed] [Google Scholar]
- Fassett RG, Robertson IK, Ball MJ, Geraghty DP, Cardinal JW, Coombes JS. Effects of atorvastatin on NGAL and cystatin C in chronic kidney disease: a post hoc analysis of the LORD trial. Nephrology Dialysis Transplantation 2012;27(1):182-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fassett RG, Robertson IK, Ball MJ, Geraghty DP, Coombes JS. Effect of atorvastatin on kidney function in chronic kidney disease: a randomised double-blind placebo-controlled trial. Atherosclerosis 2010;213(1):218-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fassett RG, Robertson IK, Ball MJ, Geraghty DP, Coombes JS. Effects of atorvastatin on biomarkers of inflammation in chronic kidney disease. Clinical Nephrology 2014;81(2):75-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fassett RG, Robertson IK, Ball MJ, Geraghty DP, Sharman JE, Coombes JS. Effects of atorvastatin on arterial stiffness in chronic kidney disease: a randomised controlled trial. Journal of Atherosclerosis & Thrombosis 2010;17(3):235-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fassett RG, Robertson IK, Ball MJ, Geraghty DP. Atorvastatin slows the progression of CKD in patients with inflammation [abstract no: 066]. Nephrology 2011;16(Suppl 1):40-1. [EMBASE: 70532414] [Google Scholar]
- Fassett RG, Robertson IK, Geraghty DP, Ball MJ, Burton NW, Coombes JS. Physical activity levels in patients with chronic kidney disease entering the LORD trial. Medicine & Science in Sports & Exercise 2009;41(5):985-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fassett RG, Robertson IK, Geraghty DP, Ball MJ, Coombes JS. Dietary intake of patients with chronic kidney disease entering the LORD trial: adjusting for underreporting. Journal of Renal Nutrition 2007;17(4):235-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fassett RG, Swanson CE, Sharma S, White A, Singh G. Atorvastatin improves kidney function in chronic kidney disease patients undertaking high intensity exercise [abstract no: 067]. Nephrology 2011;16(Suppl 1):41. [PMID: 70532415] [Google Scholar]
- Summers MJ, Oliver KR, Coombes JS, Fassett RG. Effect of atorvastatin on cognitive function in patients from the Lipid Lowering and Onset of Renal Disease (LORD) trial. Pharmacotherapy: The Journal of Human Pharmacology & Drug Therapy 2007;27(2):183-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Masajtis‐Zagajewska 2018 {published data only}
- Masajtis-Zagajewska A, Nowicki M. Effect of atorvastatin on iron metabolism regulation in patients with chronic kidney disease - a randomized double blind crossover study. Renal Failure 2018;40(1):700-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
MEGA 2004 {published data only}
- Ishikawa T, Mizuno K, Nakaya N, Ohashi Y, Tajima N, Kushiro T, et al. The relationship between the effect of pravastatin and risk factors for coronary heart disease in Japanese patients with hypercholesterolemia. Circulation Journal 2008;72(10):1576-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) Study Group. Design and baseline characteristics of a study of primary prevention of coronary events with pravastatin among Japanese with mildly elevated cholesterol levels. Circulation Journal 2004;68(9):860-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mizuno K, Nakaya N, Ohashi Y, Tajima N, Kushiro T, Teramoto T, et al. Usefulness of pravastatin in primary prevention of cardiovascular events in women: analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA study). Circulation 2008;117(4):494-502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006;368(9542):1155-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nakamura H, Mizuno K, Ohashi Y, Yoshida T, Hirao K, Uchida Y, et al. Pravastatin and cardiovascular risk in moderate chronic kidney disease. Atherosclerosis 2009;206(2):512-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nakamura H. Primary prevention of cardiovascular diseases among hypercholesterolemic Japanese with a low dose of pravastatin. Atherosclerosis Supplements 2007;8(2):13-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Matsuyama Y, Ohashi Y. Estimation of treatment effect adjusting for treatment changes using the intensity score method: application to a large primary prevention study for coronary events (MEGA study). Statistics in Medicine 2008;27(10):1718-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teramoto T, Ohashi Y, Nakaya N, Yokoyama S, Mizuno K, Nakamura H, et al. Practical risk prediction tools for coronary heart disease in mild to moderate hypercholesterolemia in Japan: originated from the MEGA study data. Circulation Journal 2008;72(10):1569-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Nakaya N, Mizuno K, Ohashi Y, Tajima N, Kushiro T, et al. Risk factors for stroke and lipid-lowering effect of pravastatin on the risk of stroke in Japanese patients with hypercholesterolemia: analysis of data from the MEGA Study, a large randomized controlled trial. Journal of the Neurological Sciences 2009;284(1-2):72-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yoshida M, Matsuyama Y, Ohashi Y. Estimation of treatment effect adjusting for dependent censoring using the IPCW method: an application to a large primary prevention study for coronary events (MEGA study). Clinical Trials 2007;4(4):318-28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mori 1992 {published data only}
- Mori Y, Tsuruoka A. Effect of pravastatin on microalbuminuria in patients with non-insulin-dependent diabetes mellitus. Journal of the Japan Diabetes Society 1992;35(3):265-8. [EMBASE: 22147775] [Google Scholar]
- Mori Y, Yokoyama J, Tsuruoka A, Ikeda Y. Effect of pravastatin on microalbuminuria in patients with non-insulin-dependent diabetes mellitus - a randomized control study. Jikeikai Medical Journal 1992;39(4):341-8. [EMBASE: 23068032] [Google Scholar]
Nakamura 2002 {published data only}
- Nakamura T, Ushiyama C, Hirokawa K, Osada S, Inoue T, Shimada N, et al. Effect of cerivastatin on proteinuria and urinary podocytes in patients with chronic glomerulonephritis. Nephrology Dialysis Transplantation 2002;17(5):798-802. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 2005 {published data only}
- Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Osada S, Koide H. Effect of pitavastatin on urinary liver-type fatty acid-binding protein levels in patients with early diabetic nephropathy. Diabetes Care 2005;28(11):2728-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 2006 {published data only}
- Nakamura T, Sugaya T, Kawagoe Y, Suzuki T, Inoue T, Node K. Effect of pitavastatin on urinary liver-type fatty-acid-binding protein in patients with nondiabetic mild chronic kidney disease. American Journal of Nephrology 2006;26(1):82-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nielsen 1993 {published data only}
- Nellemann B, Gormsen LC, Dollerup J, Schmitz O, Mogensen CE, Rasmussen LM, et al. Simvastatin reduces plasma osteoprotegerin in type 2 diabetic patients with microalbuminuria. Diabetes Care 2007;30(12):3122-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nielsen S, Schmitz O, Moller N, Porksen N, Klausen IC, Alberti KG, et al. Renal function and insulin sensitivity during simvastatin treatment in type 2 (non-insulin-dependent) diabetic patients with microalbuminuria. Diabetologia 1993;36(10):1079-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ohsawa 2015 {unpublished data only}
- Ohsawa M, Tamura K, Wakui H, Kanaoka T, Azushima K, Uneda K, et al. Effects of pitavastatin add-on therapy on chronic kidney disease with albuminuria and dyslipidemia. Lipids in Health & Disease 2015;14:161. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PANDA 2011 {published data only}ISRCTN58196433
- Narayanan RP, Gittins M, Siddals KW, Oliver RL, Hudson JE, White A, et al. Atorvastatin administration is associated with dose-related changes in IGF bioavailability. European Journal of Endocrinology 2013;168(4):543-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rutter MK, Davies RR, Cruickshank JK, Prais HR, Gittins M, Roberts C, et al. Protection Against Nephropathy in Diabetes with Atorvastatin (PANDA): trial results [abstract no: 1207]. Diabetologia 2009;52(Suppl 1):S466. [EMBASE: 70068693] [Google Scholar]
- Rutter MK, Prais HR, Charlton-Menys V, Gittins M, Roberts C, Davies RR, et al. Protection Against Nephropathy in Diabetes with Atorvastatin (PANDA): a randomized double-blind placebo-controlled trial of high- vs. low-dose atorvastatin. Diabetic Medicine 2011;28(1):100-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Soran H, Liu Y, Adam S, Siahmansur T, Ho JH, Schofield JD, et al. A comparison of the effects of low- and high-dose atorvastatin on lipoprotein metabolism and inflammatory cytokines in type 2 diabetes: results from the Protection Against Nephropathy in Diabetes with Atorvastatin (PANDA) randomized trial. Journal of Clinical Lipidology 2018;12(1):44-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Panichi 2006a {published data only}
- Panichi V, Mantuano E, Paoletti S, Santi S, Manca Rizza G, Cutrupi S, et al. Effect of simvastatin on plasma asymmetric dimethylarginine concentration in patients with chronic kidney disease. Journal of Nephrology 2008;21(1):38-44. [MEDLINE: ] [PubMed] [Google Scholar]
- Panichi V, Paoletti S, Manca-Rizza G, Taccola D, Innocenti M, Casto G, et al. Effect of simvastatin therapy on inflammatory markers and lipids in pre-dialysis patients [abstract no: SP167]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v75. [CENTRAL: CN-00678037] [Google Scholar]
- Panichi V, Paoletti S, Mantuano E, Manca-Rizza G, Filippi C, Santi S, et al. In vivo and in vitro effects of simvastatin on inflammatory markers in pre-dialysis patients. Nephrology Dialysis Transplantation 2006;21(2):337-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PLANET I 2006 {published data only}
- Idzerda NM, Pena MJ, Parving HH, Zeeuw D, Heerspink HJ. Proteinuria and cholesterol reduction are independently associated with less renal function decline in statin-treated patients; a post hoc analysis of the PLANET trials. Nephrology Dialysis Transplantation 2019;34(10):1699-706. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroonen M, Stevens J, De Zeeuw D, Heerspink HJ. Association between individual cholesterol and albuminuria response and exposure to atorvastatin or rosuvastatin [abstract no: FR-PO253]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):500. [EMBASE: 633769807] [Google Scholar]
- Zeeuw D, Anzalone DA, Cain VA, Cressman MD, Heerspink HJ, Molitoris BA, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. The Lancet Diabetes & Endocrinology 2015;3(3):181-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PLANET II 2006 {unpublished data only}
- Idzerda NM, Pena MJ, Parving HH, Zeeuw D, Heerspink HJ. Proteinuria and cholesterol reduction are independently associated with less renal function decline in statin-treated patients; a post hoc analysis of the PLANET trials. Nephrology Dialysis Transplantation 2019;34(10):1699-706. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroonen M, Stevens J, De Zeeuw D, Heerspink HJL. Association between individual cholesterol and albuminuria response and exposure to atorvastatin or rosuvastatin [abstract no: FR-PO253]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):500. [EMBASE: 633769807] [Google Scholar]
- Zeeuw D, Anzalone DA, Cain VA, Cressman MD, Heerspink HJ, Molitoris BA, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. The Lancet Diabetes & Endocrinology 2015;3(3):181-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PPP 1992 {published data only}
- A coronary primary prevention study of Scottish men aged 45-64 years: trial design. The West of Scotland Coronary Prevention Study Group. Journal of Clinical Epidemiology 1992;45(8):849-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baseline risk factors and their association with outcome in the West of Scotland Coronary Prevention Study. The West of Scotland Coronary Prevention Study Group. American Journal of Cardiology 1997;79(6):756-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Batty GD, Wang Y, Brouilette SW, Shiels P, Packard C, Moore J, et al. Socioeconomic status and telomere length: the West of Scotland Coronary Prevention Study. Journal of Epidemiology & Community Health 2009;63(10):839-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 2007;369(9556):107-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Byington RP, Davis BR, Plehn JF, White HD, Baker J, Cobbe SM, et al. Reduction of stroke events with pravastatin: the Prospective Pravastatin Pooling (PPP) Project. Circulation 2001;103(3):387-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Caro J, Klittich W, McGuire A, Ford I, Norrie J, Pettitt D, et al. The West of Scotland coronary prevention study: economic benefit analysis of primary prevention with pravastatin. BMJ 1997;315(7122):1577-82. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J, Klittich W, McGuire A, Ford I, Pettitt D, Norrie J, et al. International economic analysis of primary prevention of cardiovascular disease with pravastatin in WOSCOPS. West of Scotland Coronary Prevention Study. European Heart Journal 1999;20(4):263-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Computerised record linkage: compared with traditional patient follow-up methods in clinical trials and illustrated in a prospective epidemiological study. The West of Scotland Coronary Prevention Study Group. Journal of Clinical Epidemiology 1995;48(12):1441-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cui J, Forbes A, Kirby A, Marschner I, Simes J, West M, et al. Parametric conditional frailty models for recurrent cardiovascular events in the lipid study. Clinical Trials 2008;5(6):565-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- DeFilippis AP, Bansal S, Blumenthal RS. Long-term follow-up of the West of Scotland Coronary Prevention Study. New England Journal of Medicine 2008;358(2):194-5. [MEDLINE: ] [PubMed] [Google Scholar]
- Design, rational, and baseline characteristics of the Prospective Pravastatin Pooling (PPP) project--a combined analysis of three large-scale randomized trials: Long-term Intervention with Pravastatin in Ischemic Disease (LIPID), Cholesterol and Recurrent Events (CARE), and West of Scotland Coronary Prevention Study (WOSCOPS). American Journal of Cardiology 1995;76(12):899-905. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ford I, Murray H, Packard CJ, Shepherd J, MacFarlane PW, Cobbe SM, et al. Long-term follow-up of the West of Scotland Coronary Prevention Study. New England Journal of Medicine 2007;357(15):1477-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 2002;51(5):1596-600. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Glasziou PP, Irwig L, Heritier S, Simes RJ, Tonkin A, LIPID Study Investigators. Monitoring cholesterol levels: measurement error or true change? Annals of Internal Medicine 2008;148(9):656-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Mellies MJ, Sacks FM, Moye LA, Howard BV, Howard WJ, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation 1998;98(23):2513-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hague W, Forder P, Simes J, Hunt D, Tonkin A, LIPID Investigators. Effect of pravastatin on cardiovascular events and mortality in 1516 women with coronary heart disease: results from the Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) study. American Heart Journal 2003;145(4):643-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation 1998;97(15):1440-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- LIPID Study Group. Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up.[Erratum appears in Lancet 2002 Nov 2;360(9343):1430]. Lancet 2002;359(9315):1379-87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Moye LA, Sacks FM, Johnstone DE, Timmis G, Mitchell J, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Annals of Internal Medicine 1998;129(9):681-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Liakishev AA. Long-term follow-up of the West of Scotland Coronary Prevention Study. Kardiologiia 2007;47(12):65-6. [MEDLINE: ] [PubMed] [Google Scholar]
- Lowe G, Rumley A, Norrie J, Ford I, Shepherd J, Cobbe S, et al. Blood rheology, cardiovascular risk factors, and cardiovascular disease: the West of Scotland Coronary Prevention Study.[Erratum appears in Thromb Haemost 2001 May;85(5):946]. Thrombosis & Haemostasis 2000;84(4):553-8. [MEDLINE: ] [PubMed] [Google Scholar]
- Macfarlane PW, Norrie J, WOSCOPS Executive Committee. The value of the electrocardiogram in risk assessment in primary prevention: experience from the West of Scotland Coronary Prevention Study. Journal of Electrocardiology 2007;40(1):101-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McEvoy JW, Margey R, Blake GJ. Long-term follow-up of the West of Scotland Coronary Prevention Study. New England Journal of Medicine 2008;358(2):193-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nestel PJ, Baghurst K, Colquhoun DM, Simes RJ, Mehalski K, White HD, et al. Relation of diet to cardiovascular disease risk factors in subjects with cardiovascular disease in Australia and New Zealand: analysis of the Long-Term Intervention with Pravastatin in Ischaemic Disease trial. American Journal of Clinical Nutrition 2005;81(6):1322-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. New England Journal of Medicine 2000;343(16):1148-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Keech A, Sacks FM, Cobbe SM, Tonkin A, Byington RP, et al. Safety and tolerability of pravastatin in long-term clinical trials: prospective Pravastatin Pooling (PPP) Project. Circulation 2002;105(20):2341-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. New England Journal of Medicine 1998;339(19):1349-57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Reid IR, Hague W, Emberson J, Baker J, Tonkin A, Hunt D, et al. Effect of pravastatin on frequency of fracture in the LIPID study: secondary analysis of a randomised controlled trial. Long-term Intervention with Pravastatin in Ischaemic Disease. Lancet 2001;357(9255):509-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. New England Journal of Medicine 1996;335(14):1001-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sacks FM, Pfeffer MA, Moye' L, Brown LE, Hamm P, Cole TG, et al. Rationale and design of a secondary prevention trial of lowering normal plasma cholesterol levels after acute myocardial infarction: the Cholesterol and Recurrent Events trial (CARE).[erratum appears in Am J Cardiol 1992 Feb 15;69(5):574]. American Journal of Cardiology 1991;68(15):1436-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sacks FM, Tonkin AM, Shepherd J, Braunwald E, Cobbe S, Hawkins CM, et al. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: the Prospective Pravastatin Pooling Project. Circulation 2000;102(16):1893-900. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003;108(4):414-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sattar N, Scherbakova O, Ford I, O'Reilly DS, Stanley A, Forrest E, et al. Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of Scotland coronary prevention study. Diabetes 2004;53(11):2855-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Screening experience and baseline characteristics in the West of Scotland Coronary Prevention Study. The WOSCOPS Study Group. West of Scotland Coronary Prevention Study. American Journal of Cardiology 1995;76(7):485-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. New England Journal of Medicine 1995;333(20):1301-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shepherd J, Gaw A, West of Scotland Coronary Prevention Study Group. The anatomy of a clinical trial. The West of Scotland Coronary Prevention Study. Medical Principles & Practice 2002;11 Suppl 2:17-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shepherd J. The West of Scotland Coronary Prevention Study: a trial of cholesterol reduction in Scottish men. American Journal of Cardiology 1995;76(9):113C-7C. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Simes J, Furberg CD, Braunwald E, Davis BR, Ford I, Tonkin A, et al. Effects of pravastatin on mortality in patients with and without coronary heart disease across a broad range of cholesterol levels. The Prospective Pravastatin Pooling project. European Heart Journal 2002;23(3):207-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Simes RJ. Prospective meta-analysis of cholesterol-lowering studies: the Prospective Pravastatin Pooling (PPP) Project and the Cholesterol Treatment Trialists (CTT) Collaboration. American Journal of Cardiology 1995;76(9):122C-6C. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Soderberg S, Colquhoun D, Keech A, Yallop J, Barnes EH, Pollicino C, et al. Leptin, but not adiponectin, is a predictor of recurrent cardiovascular events in men: results from the LIPID study. International Journal of Obesity 2009;33(1):123-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stewart RA, North FM, Sharples KJ, Simes RJ, Tonkin AM, White HD, et al. Differences in cardiovascular mortality between Australia and New Zealand according to socioeconomic status: findings from the Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study. New Zealand Medical Journal 2008;121(1269):11-23. [MEDLINE: ] [PubMed] [Google Scholar]
- Stewart RA, North FM, West TM, Sharples KJ, Simes RJ, Colquhoun DM, et al. Depression and cardiovascular morbidity and mortality: cause or consequence? European Heart Journal 2003;24(22):2027-37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stewart RA, Sharples KJ, North FM, Menkes DB, Baker J, Simes J. Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. The LIPID Study Investigators. Archives of Internal Medicine 2000;160(20):3144-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Streja L, Packard CJ, Shepherd J, Cobbe S, Ford I, WOSCOPS Group. Factors affecting low-density lipoprotein and high-density lipoprotein cholesterol response to pravastatin in the West Of Scotland Coronary Prevention Study (WOSCOPS). American Journal of Cardiology 2002;90(7):731-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- The effects of pravastatin on hospital admission in hypercholesterolemic middle-aged men: West of Scotland Coronary Prevention Study. Journal of the American College of Cardiology 1999;33(4):909-15. [MEDLINE: ] [PubMed] [Google Scholar]
- Tonelli M, Isles C, Craven T, Tonkin A, Pfeffer MA, Shepherd J, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005;112(2):171-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tonelli M, Isles C, Curhan GC, Tonkin A, Pfeffer MA, Shepherd J, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation 2004;110(12):1557-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tonelli M, Jose P, Curhan G, Sacks F, Braunwald E, Pfeffer M, et al. Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. BMJ 2006;332(7555):1426. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli M, Keech A, Shepherd J, Sacks F, Tonkin A, Packard C, et al. Effect of pravastatin in people with diabetes and chronic kidney disease. Journal of the American Society of Nephrology 2005;16(12):3748-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tonelli M, Moye L, Sacks F, Coles T, Curhan G. Effect of pravastatin on loss of renal function in patients with CKD [abstract no: F-P0996]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):257A-8A. [CENTRAL: CN-00448025] [DOI] [PubMed] [Google Scholar]
- Tonelli M, Moye L, Sacks F, Curhan G. Pravastatin is effective for secondary prevention of cardiovascular events in patients with chronic renal insufficiency (CRI) [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):252A. [CENTRAL: CN-00448024] [Google Scholar]
- Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC, Cholesterol and Recurrent Events Trial Investigators. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. Journal of the American Society of Nephrology 2003;14(6):1605-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G, Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Annals of Internal Medicine 2003;138(2):98-104. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G, Cholesterol and Recurrent Events (CARE) Trial Investigators. Biomarkers of inflammation and progression of chronic kidney disease. Kidney International 2005;68(1):237-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tonelli M, Sacks F, Pfeffer M, Lopez-Jimenez F, Jhangri GS, Curhan G. Effect of pravastatin on blood pressure in people with cardiovascular disease. Journal of Human Hypertension 2006;20(8):560-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tsubokura M, Kami M. Long-term follow-up of the West of Scotland Coronary Prevention Study. New England Journal of Medicine 2008;358(2):193-4. [MEDLINE: ] [PubMed] [Google Scholar]
- Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 2001;104(25):3052-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- West MJ, Nestel PJ, Kirby AC, Schnabel R, Sullivan D, Simes RJ, et al. The value of N-terminal fragment of brain natriuretic peptide and tissue inhibitor of metalloproteinase-1 levels as predictors of cardiovascular outcome in the LIPID study. European Heart Journal 2008;29(7):923-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- West MJ, White HD, Simes RJ, Kirby A, Watson JD, Anderson NE, et al. Risk factors for non-haemorrhagic stroke in patients with coronary heart disease and the effect of lipid-modifying therapy with pravastatin. Journal of Hypertension 2002;20(12):2513-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- West of Scotland Coronary Prevention Study: identification of high-risk groups and comparison with other cardiovascular intervention trials. Lancet 1996;348(9038):1339-42. [MEDLINE: ] [PubMed] [Google Scholar]
- White HD, Simes RJ, Anderson NE, Hankey GJ, Watson JD, Hunt D, et al. Pravastatin therapy and the risk of stroke. New England Journal of Medicine 2000;343(5):317-26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zito F, Lowe GD, Rumley A, McMahon AD, Humphries SE, WOSCOPS Study Group West of Scotland Coronary Prevention Study. Association of the factor XII 46C>T polymorphism with risk of coronary heart disease (CHD) in the WOSCOPS study. Atherosclerosis 2002;165(1):153-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PREVEND IT 2000 {published data only}
- Asselbergs FW, Diercks GF, Hillege HL, Boven AJ, Janssen WM, Voors AA, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation 2004;110(18):2809-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Asselbergs FW, Roon AM, Hillege HL, Jong PE, Gans RO, Smit AJ, et al. Effects of fosinopril and pravastatin on carotid intima-media thickness in subjects with increased albuminuria. Stroke 2005;36(3):649-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Asselbergs FW, Harst P, Roon AM, Hillege HL, Jong PE, Gans RO, et al. Long-term effects of pravastatin and fosinopril on peripheral endothelial function in albuminuric subjects. Atherosclerosis 2008;196(1):349-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Atthobari J, Asselbergs FW, Boersma C, Vries R, Hillege HL, Gilst WH, et al. Cost-effectiveness of screening for albuminuria and subsequent treatment with an ACE-inhibitor; a pharmaco-economic analysis [abstract no: TH-FC169]. Journal of the American Society of Nephrology 2005;16:36A. [CENTRAL: CN-00583782] [Google Scholar]
- Atthobari J, Asselbergs FW, Boersma C, Vries R, Hillege HL, Gilst WH, et al. Cost-effectiveness of screening for albuminuria with subsequent fosinopril treatment to prevent cardiovascular events: A pharmacoeconomic analysis linked to the prevention of renal and vascular endstage disease (PREVEND) study and the prevention of renal and vascular endstage disease intervention trial (PREVEND IT). Clinical Therapeutics 2006;28(3):432-44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Atthobari J, Brantsma AH, Gansevoort RT, Visser ST, Asselbergs FW, Gilst WH, et al. The effect of statins on urinary albumin excretion (UAE) and glomerular filtration rate (GFR): a randomized controlled trial (RCT) and observational cohort study [abstract no: F-FC151]. Journal of the American Society of Nephrology 2005;16:71A. [CENTRAL: CN-00583784] [Google Scholar]
- Atthobari J, Brantsma AH, Gansevoort RT, Visser ST, Asselbergs FW, Gilst WH, et al. The effect of statins on urinary albumin excretion and glomerular filtration rate: results from both a randomized clinical trial and an observational cohort study. Nephrology Dialysis Transplantation 2006;21(11):3106-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bello AK, Zeeuw D, El Nahas M, Brantsma AH, Bakker SJ, Jong PE, et al. Impact of weight change on albuminuria in the general population. Nephrology Dialysis Transplantation 2007;22(6):1619-27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brouwers FP, Asselbergs FW, Hillege HL, Boer RA, Gansevoort RT, Veldhuisen DJ, et al. Long-term effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria: ten years of follow-up of Prevention of Renal and Vascular End-stage Disease Intervention Trial (PREVEND IT). American Heart Journal 2011;161(6):1171-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- De Jong MA, Eisenga MF, Ballegooijen AJ, Beulens JW, Vervloet MG, Navis G, et al. Fibroblast growth factor 23 and new-onset chronic kidney disease in the general population: the Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Nephrology Dialysis Transplantation 2021;36(1):121-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diercks GF, Janssen WM, Boven AJ, Bak AA, Jong PE, Crijns HJ, et al. Rationale, design, and baseline characteristics of a trial of prevention of cardiovascular and renal disease with fosinopril and pravastatin in nonhypertensive, nonhypercholesterolemic subjects with microalbuminuria (the Prevention of REnal and Vascular ENdstage Disease Intervention Trial [PREVEND IT]). American Journal of Cardiology 2000;86(6):635-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Geluk CA, Asselbergs FW, Hillege HL, Bakker SJ, Jong PE, Zijlstra F, et al. Impact of statins in microalbuminuric subjects with the metabolic syndrome: a substudy of the PREVEND Intervention Trial. European Heart Journal 2005;26(13):1314-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pouwels KB, Visser ST, Hak E. Effect of pravastatin and fosinopril on recurrent urinary tract infections. Journal of Antimicrobial Chemotherapy 2013;68(3):708-14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wal RM, Gansevoort RT, Harst P, Boomsma F, Thijs Plokker HW, Veldhuisen DJ, et al. Predictors of angiotensin-converting enzyme inhibitor-induced reduction of urinary albumin excretion in nondiabetic patients. Hypertension 2006;48(5):870-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- de Wal RM, Harst P, Gerritsen WB, Horst F, Plokker TH, Gansevoort RT, et al. Plasma matrix metalloproteinase-9 and ACE-inhibitor-induced improvement of urinary albumin excretion in non-diabetic, microalbuminuric subjects. Journal of the Renin-Angiotensin-Aldosterone System 2007;8(4):177-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Harst P, Asselbergs FW, Hillege HL, Voors AA, Veldhuisen DJ, Gilst WH. Effect of fosinopril treatment on serum C-reactive protein levels in patients with microalbuminuria. American Journal of Cardiology 2008;102(2):223-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rayner 1996 {published data only}
- Rayner B, Byrne M, Zyl-Smit R. A clinical trial comparing the treatment of hypercholesterolaemia with simvastatin and diet versus diet alone in patients with idiopathic membranous nephropathy: a preliminary report [abstract]. Kidney International 1993;43:1183. [Google Scholar]
- Rayner BL, Byrne M, Zyl-Smit R. A clinical trial comparing the treatment of hypercholesterolaemia with simvastatin (sv) and diet versus diet alone in patients with idiopathic membranous nephropathy (IMN) [abstract]. In: 12th International Congress of Nephrology; 1993 Jun 13-18; Jerusalem, Israel. 1993:76.
- Rayner BL, Byrne M, Zyl-Smit R. Treatment of hyperlipidaemia with simvastatin (SV) and diet, vs diet alone in idiopathic membranous nephropathy (IMN) [abstract]. Nephrology Dialysis Transplantation 1994;9(7):963. [CENTRAL: CN-00261011] [Google Scholar]
- Rayner BL, Byrne MJ, Zyl Smit R. A prospective clinical trial comparing the treatment of idiopathic membranous nephropathy and nephrotic syndrome with simvastatin and diet, versus diet alone. Clinical Nephrology 1996;46(4):219-24. [MEDLINE: ] [PubMed] [Google Scholar]
Renke 2010a {published data only}
- Renke M, Tylicki L, Rutkowski P, Neuwelt A, Larczynski W, Zietkiewicz M, et al. Atorvastatin improves tubular status in non-diabetic patients with chronic kidney disease - placebo controlled, randomized, cross-over study. Acta Biochimica Polonica 2010;57(4):547-52. [MEDLINE: ] [PubMed] [Google Scholar]
SAGE 2004 {published data only}
- Deedwania P, Stone PH, Bairey Merz CN, Cosin-Aguilar J, Koylan N, Luo D, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the Study Assessing Goals in the Elderly (SAGE). Circulation 2007;115(6):700-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Deedwania PC, Stone P, Fayyad R, Laskey R, Wilson DJ. Improvement in renal function with intensive statin therapy in older patients without increased muscle symptoms: the SAGE trial [abstract no: SA-PO2762]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):743A. [Google Scholar]
- Deedwania PC, Stone PH, Fayyad RS, Laskey RE, Wilson DJ. Improvement in renal function and reduction in serum uric acid with intensive statin therapy in older patients: a post hoc analysis of the SAGE trial. Drugs & Aging 2015;32(12):1055-65. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deedwania PC. Effect of aggressive versus moderate lipid-lowering therapy on myocardial ischemia: the rationale, design, and baseline characteristics of the Study Assessing Goals in the Elderly (SAGE). American Heart Journal 2004;148(6):1053-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Samuelsson 2002 {published data only}
- Alaupovic P, Attman PO, Knight-Gibson C, Mulec H, Weiss L, Samuelsson O. Effect of fluvastatin on apolipoprotein-defined lipoprotein subclasses in patients with chronic renal insufficiency. Kidney International 2006;69(10):1865-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Samuelsson O, Attman PO, Knight-Gibson C, Mulec H, Weiss L, Alaupovic P. Fluvastatin improves lipid abnormalities in patients with moderate to advanced chronic renal insufficiency. American Journal of Kidney Diseases 2002;39(1):67-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sawara 2008 {published data only}
- Sawara Y, Takei T, Uchida K, Ogawa T, Yoshida T, Tsuchiya K, et al. Effects of lipid-lowering therapy with rosuvastatin on atherosclerotic burden in patients with chronic kidney disease. Internal Medicine 2008;47(17):1505-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sawara Y, Takei T, Uchida K, Tsuchiya K, Nitta K. Effects of lipid-lowering therapy with rosuvastatin on atherosclerotic burden in patients with chronic kidney disease [abstract no: SA166]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009. [CENTRAL: CN-01912388] [DOI] [PubMed]
Scanferla 1991 {published data only}
- Scanferla F, Toffoletto PP, Roncali D, Bazzato G. Associated effect of hepatic hydroxymethylglutaryl coenzyme A reductase + angiotensin converting enzyme inhibitors on the progression of renal failure in hypertensive subjects. American Journal of Hypertension 1991;4(10 Pt 1):868-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SHARP 2010 {published data only}54137607
- Baigent C, Landray M. Study of Heart and Renal Protection (SHARP): Does lowering cholesterol prevent major vascular events in patients with chronic kidney disease? Final Protocol (Version 5: 12th July 2005). https://www.ctsu.ox.ac.uk/files/download_protocol_en_v5.pdf (accessed 5 October 2023).
- Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377(9784):2181-92. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent C, Landry M. Study of Heart and Renal Protection (SHARP). Kidney International - Supplement 2003;63(84):S207-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Butler CR, O'Hare AM. Considerations in applying the results of randomized controlled clinical trials to the care of older adults with kidney disease in the clinical setting: the SHARP trial. Advances in Chronic Kidney Disease 2016;23(1):29-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Haynes R, Lewis D, Emberson J, Reith C, Agodoa L, Cass A, et al. Effects of lowering LDL cholesterol on progression of kidney disease. Journal of the American Society of Nephrology 2014;25(8):1825-33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R, SHARP Collaborative Group. Relevance of renal diagnosis to prognosis of chronic kidney disease: results from the Study of Heart and Renal Protection (SHARP) [abstract no: TH-PO327]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):170A. [Google Scholar]
- Herrington W, Emberson J, Staplin N, Blackwell L, Fellstrom B, Walker R, et al. The effect of lowering LDL cholesterol on vascular access patency: post hoc analysis of the Study of Heart and Renal Protection. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(5):914-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington W, Staplin N, Judge PK, Mafham M, Emberson J, Haynes R, et al. Evidence for reverse causality in the association between blood pressure and cardiovascular risk in patients with chronic kidney disease. Hypertension 2017;69(2):314-22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington WG, SHARP Collaborative Group. The effect of lowering LDL-cholesterol with simvastatin plus ezetimibe on vascular access patency: results from the Study of Heart and Renal Protection (SHARP) [abstract no: TH-PO762]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):275A. [Google Scholar]
- Judge PK, Herrington WG. The relevance of systolic blood pressure to vascular disease in chronic kidney disease patients with and without vascular disease: observations from the Study of Heart and Renal Protection (SHARP) [abstract no: TH-PO586]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):221-2A. [EMBASE: 641101613] [Google Scholar]
- Kent S, Schlackow I, Lozano-Kuhne J, Reith C, Emberson J, Haynes R, et al. What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease? BMC Nephrology 2015;16:65. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprea-Montealegre JA, Staplin N, Herrington WG, Haynes R, Emberson J, Baigent C, et al. Apolipoprotein B, triglyceride-rich lipoproteins, and risk of cardiovascular events in persons with CKD. Clinical Journal of the American Society of Nephrology: CJASN 2020;15(1):47-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafham M, Staplin N, SHARP Collaborative Group. Prognostic utility of estimated albumin excretion rate (eAER) in chronic kidney disease (CKD): results from the Study of Heart and Renal Protection (SHARP) [abstract no: FR-PO903]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):578A. [Google Scholar]
- Mafham MM, Staplin N, Emberson J, Haynes R, Herrington W, Reith C, et al. Prognostic utility of estimated albumin excretion rate in chronic kidney disease: results from the Study of Heart and Renal Protection. Nephrology Dialysis Transplantation 2018;33(2):257-64. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova B, Schlackow I, Herrington W, Lozano-Kuhne J, Kent S, Emberson J, et al. Cost-effectiveness of simvastatin plus ezetimibe for cardiovascular prevention in CKD: results of the Study of Heart and Renal Protection (SHARP). American Journal of Kidney Diseases 2016;67(4):576-84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova BN, Lozano-Kuehne JP, Schlackow I, Herrington WG, SHARP Collaborative Group. Cost-effectiveness of lowering LDL-cholesterol in chronic kidney disease: results from the Study of Heart and Renal Protection (SHARP) [abstract no: FR-PO177]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):409A. [Google Scholar]
- Morton RL, Cass A, Mihaylova BN. The impact of chronic kidney disease on household income: does health affect wealth? [abstract no: SA-PO729]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):795-6A. [EMBASE: 641103963] [Google Scholar]
- Morton RL, Schlackow I, Staplin N, Gray A, Cass A, Haynes R, et al. Impact of educational attainment on health outcomes in moderate to severe CKD. American Journal of Kidney Diseases 2016;67(1):31-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton RL, Schlackow I, Staplin N, Gray A, Cass A, Haynes R, et al. Impact of educational attainment on health outcomes in moderate-to-severe chronic kidney disease [abstract no: FP329]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii178. [EMBASE: 72206749] [Google Scholar]
- Reith C, Staplin N, Herrington WG, Stevens W, Emberson J, Haynes R, et al. Effect on non-vascular outcomes of lowering LDL cholesterol in patients with chronic kidney disease: results from the Study of Heart and Renal Protection. BMC Nephrology 2017;18(1):147. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith CA, Staplin N, Herrington WG. Effect of lowering LDL-cholesterol with simvastatin plus ezetimibe on non-vascular outcomes in patients with chronic kidney disease (CKD): results from the Study of Heart and Renal Protection (SHARP) [abstract no: FR-PO564]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):488A. [CENTRAL: 641103416] [Google Scholar]
- Reith CA, SHARP Collaborative Group. Effect of lowering LDL-cholesterol with simvastatin plus ezetimibe on infection in patients with chronic kidney disease: results from the Study of Heart and Renal Protection (SHARP) [abstract no: SA-PO220]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):686A. [Google Scholar]
- SHARP Collaborative Group. SHARP: an international randomized placebo-controlled trial of lipid-lowering in chronic kidney disease. 1-year safety and efficacy experience [abstract no: SU-PO1044]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):817A. [CENTRAL: CN-00691495] [Google Scholar]
- Schlackow I, Kent S, Herrington W, Emberson J, Haynes R, Reith C, et al. Cost-effectiveness of lipid lowering with statins and ezetimibe in chronic kidney disease. Kidney International 2019;96(1):170-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp Collaborative Group. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. American Heart Journal 2010;160(5):785-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Staplin N, Haynes R, Herrington WG, Reith C, Cass A, Fellstrom B, et al. Smoking and adverse outcomes in patients with CKD: the Study of Heart and Renal Protection (SHARP). American Journal of Kidney Diseases 2016;68(3):371-80. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staplin N, SHARP Collaborative Group. The effects of cigarette smoking in patients with chronic kidney disease (CKD): results from the Study of Heart and Renal Protection (SHARP) [abstract no: SA-PO158]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):660A. [Google Scholar]
- Storey B. Relevance of LDL cholesterol and C-reactive protein to cardiovascular risk among patients with chronic kidney disease - results from the Study of Heart and Renal Protection [abstract no: TH-OR116]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):29A. [PMID: 641102804] [Google Scholar]
- Storey BC, Staplin N, Haynes R, Reith C, Emberson J, Herrington WG, et al. Lowering LDL cholesterol reduces cardiovascular risk independently of presence of inflammation. Kidney International 2018;93(4):1000-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukkar L, Gallagher M, Jardine M, Reith C, Cass A, Walker R. The SHARP-ER study: design of the extended follow-up of the SHARP study cohort [abstract no: 235]. Nephrology 2015;30(Suppl 3):80. [EMBASE: 71996024] [Google Scholar]
- Sukkar L, Talbot B, Jun M, Dempsey E, Walker R, Hooi L, et al. Protocol for the Study of Heart and Renal Protection-extended review: additional 5-year follow-up of the Australian, New Zealand, and Malaysian SHARP cohort. Canadian Journal of Kidney Health and Disease 2019;6:2054358119. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot B, Cass A, Walker R, Hooi L, Jardine M, Jun M, et al. Comparing survival in patients with chronic kidney disease across three countries – results from the study of heart and renal protection-extended review. Nephrology 2022;28(1):36-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot B, Sukkar L, Smyth B, Jun M, Jardine M, Cass A, et al. Comparing survival in patients with chronic kidney disease across three countries - results from the study of Heart and Renal Protection-extended review (SHARP-ER) [abstact no: Sun-119]. Kidney International Reports 2020;5(3 Suppl):S250. [EMBASE: 2005255667] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot B, Sukkar L, Smyth B, Jun M, Jardine MJ, Cass A, et al. Cause of death in patients on renal replacement therapy varies across Australia, New Zealand, and Malaysia-results from the study of heart and renal protection-extended review (SHARP-ER) [abstract no: FR-PO834]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):639. [EMBASE: 633735077] [Google Scholar]
Stegmayr 2005 {published data only}
- Holmberg B, Brannstrom M, Bucht B, Crougneau V, Dimeny E, Ekspong A, et al. Safety and efficacy of atorvastatin in patients with severe renal dysfunction. Scandinavian Journal of Urology & Nephrology 2005;39(6):503-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stegmayr BG, Brannstrom M, Bucht S, Crougneau V, Dimeny E, Ekspong A, et al. Low-dose atorvastatin in severe chronic kidney disease patients: a randomized, controlled endpoint study. Scandinavian Journal of Urology & Nephrology 2005;39(6):489-97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stegmayr BG, Brannstrom M, Bucht S, Dimeny E, Ekspong A, Granroth B, et al. Safety and efficacy of atorvastatin in patients with impaired renal function [abstract]. In: 38th Congress. European Renal Association. European Dialysis and Transplantation Association; 2001 Jun 24-27; Vienna, Austria. 2001:134. [CENTRAL: CN-00447841]
- Stegmayr BG, Nasstrom BG, Brannstrom M, Bucht S, Dimeny E, Gosch J, et al. Safety and efficacy of atorvastatin in patients with severe renal dysfunction [abstract no: A0828]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):161A. [CENTRAL: CN-00447842] [Google Scholar]
- Stegmayr BG, Nasstrom BG, Brannstrom M, Bucht S, Dimeny E, Granroth B, et al. Safety and efficacy of atorvastatin in patients with severe renal dysfunction [abstract no: A1356]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):268A. [CENTRAL: CN-00583406] [Google Scholar]
Thomas 1993 {published data only}
- Thomas ME, Harris KP, Ramaswamy C, Hattersley JM, Wheeler DC, Varghese Z, et al. Simvastatin therapy for hypercholesterolemic patients with nephrotic syndrome or significant proteinuria. Kidney International 1993;44(5):1124-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TNT 2004 {published data only}
- Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. New England Journal of Medicine 2007;357(13):1301-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Deedwania P, Barter P, Carmena R, Fruchart JC, Grundy SM, Haffner S, et al. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet 2006;368(9539):919-28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Deedwania PC, Bittner V, Wenger NK, Shepherd J, Wun CC, Breazna A, et al. Improvement in estimated glomerular filtration rate with atorvastatin in patients with metabolic syndrome: The treating to new targets (TNT) study [abstract no: TH-PO140]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):142A. [Google Scholar]
- Ho JE, Waters DD, Kean A, Wilson DJ, DeMicco DA, Breazna A, et al. Relation of improvement in estimated glomerular filtration rate with atorvastatin to reductions in hospitalizations for heart failure (from the Treating to New Targets [TNT] study). American Journal of Cardiology 2012;109(12):1761-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Waters DD, DeMicco DA, Breazna A, Bittner V, Greten H, et al. Comparison of effectiveness of atorvastatin 10 mg versus 80 mg in reducing major cardiovascular events and repeat revascularization in patients with previous percutaneous coronary intervention (post hoc analysis of the Treating to New Targets [TNT] Study). American Journal of Cardiology 2008;102(10):1312-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008;117(23):3002-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Khush KK, Waters DD, Bittner V, Deedwania PC, Kastelein JJ, Lewis SJ, et al. Effect of high-dose atorvastatin on hospitalizations for heart failure: subgroup analysis of the Treating to New Targets (TNT) study. Circulation 2007;115(5):576-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kostis JB, Breazna A, Deedwania PC, LaRosa JC. The benefits of intensive lipid lowering in patients with stable coronary heart disease with normal or high systolic blood pressure: an analysis of the Treating to New Targets (TNT) study. Journal of Clinical Hypertension 2008;10(5):367-76. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa JC, Grundy SM, Kastelein JJ, Kostis JB, Greten H. Safety and efficacy of Atorvastatin-induced very low-density lipoprotein cholesterol levels in Patients with coronary heart disease (a post hoc analysis of the treating to new targets [TNT] study). American Journal of Cardiology 2007;100(5):747-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. New England Journal of Medicine 2005;352(14):1425-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mark DB, Knight JD, Cowper PA, Davidson-Ray L, Anstrom KJ. Long-term economic outcomes associated with intensive versus moderate lipid-lowering therapy in coronary artery disease: results from the Treating to New Targets (TNT) Trial. American Heart Journal 2008;156(4):698-705. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ong KL, Waters DD, Fayyad R, Vogt L, Melamed S, DeMicco DA, et al. Relationship of high-density lipoprotein cholesterol with renal function in patients treated with atorvastatin. Journal of the American Heart Association 2018;7(2):e007387. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SJ, Waters DD, Barter P, Kastelein JJ, Shepherd J, Wenger NK, et al. Intensive lipid-lowering with atorvastatin for secondary prevention in patients after coronary artery bypass surgery. Journal of the American College of Cardiology 2008;51(20):1938-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care 2006;29(6):1220-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shepherd J, Bittner V, Wenger NK, Deedwania PC, Wun CC, Breazna A, et al. Improved in estimated glomerular filtration rate with atorvastatin predicts reduced cardiovascular risk: the treating to new targets (TNT) study [abstract no: F-PO1118]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):370A. [Google Scholar]
- Shepherd J, Breazna A, Deedwania PC, LaRosa JC, Wenger NK, Messig M, et al. Relation between change in renal function and cardiovascular outcomes in atorvastatin-treated patients (from the Treating to New Targets [TNT] Study). American Journal of Cardiology 2016;117(8):1199-205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, et al. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clinical Journal of The American Society of Nephrology: CJASN 2007;2(6):1131-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, et al. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease: the TNT (Treating to New Targets) study. Journal of the American College of Cardiology 2008;51(15):1448-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shepherd J, Kastelein JP, Bittner VA, Carmena R, Deedwania PC, Breazna A, et al. Intensive lipid lowering with atorvastatin in patients with coronary artery disease, diabetes, and chronic kidney disease. Mayo Clinic Proceedings 2008;83(8):870-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Shepherd J, Wenger NK, Wilson DJ. Intensive lipid lowering with atorvastatin is associated with a significant improvement in renal function: The Treating to New Targets (TNT) study [abstract no: S-FC-019]. In: 4th World Congress of Nephrology. 19th International Congress of the International Society of Nephrology (ISN); 2007 Apr 21-25; Rio de Janeiro, Brazil. 2007:44.
- Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin is associated with significant cardiovascular benefits in patients with and without chronic kidney disease: the Treating to New Targets (TNT) study [abstract no: SA-PO1121]. Journal of the American Society of Nephrology 2006;17(Abstracts):809A. [CENTRAL: CN-00653729] [Google Scholar]
- Shepherd J, Wu C, Zuckerman AL, Wilson DJ, TNT Steering Committee. Serum uric acid as a predictor of cardiovascular risk: the treating to new targets (TNT) study [abstract no: F-PO1955]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):552A. [CENTRAL: CN-00776228] [Google Scholar]
- Shepherd J, Wun C, Wilson DJ, Zuckerman AL. On-treatment changes in estimated GFR predict response to atorvastatin in patients with coronary heart disease: the treating to new targets (TNT) study [abstract no: TH-PO872]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):306A. [CENTRAL: CN-00775650] [Google Scholar]
- Shepherd J, Wun C, Zuckerman AL, Wilson DJ, TNT Steering Committee. High dose atorvastatin reduces serum uric acid: a post-hoc analysis of the Treating to New Targets (TNT) study [abstract no: PUB512]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):926A. [CENTRAL: CN-00775061] [Google Scholar]
- Waters DD, Guyton JR, Herrington DM, McGowan MP, Wenger NK, Shear C. Treating to New Targets (TNT) Study: does lowering low-density lipoprotein cholesterol levels below currently recommended guidelines yield incremental clinical benefit? American Journal of Cardiology 2004;93(2):154-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Waters DD, LaRosa JC, Barter P, Fruchart JC, Gotto AM Jr, Carter R, et al. Effects of high-dose atorvastatin on cerebrovascular events in patients with stable coronary disease in the TNT (treating to new targets) study. Journal of the American College of Cardiology 2006;48(9):1793-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Waters DD. Clinical insights from the treating to new targets trial. Progress in Cardiovascular Diseases 2009;51(6):487-502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wenger NK, Lewis SJ, Welty FK, Herrington DM, Bittner V. Beneficial effects of aggressive low-density lipoprotein cholesterol lowering in women with stable coronary heart disease in the Treating to New Targets (TNT) study. Heart 2008;94(4):434-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tokunaga 2008 {published data only}
- Tokunaga M, Tamura M, Kabashima N, Serino R, Shibata T, Matsumoto M, et al. Beneficial effects of pitavastatin on albuminuria and renal function in essential hypertensive patients with chronic kidney disease [abstract no: TH-PO875]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):307A. [CENTRAL: CN-00773767] [Google Scholar]
Tonolo 1997 {published data only}
- Tonolo G, Ciccarese M, Brizzi P, Puddu L, Secchi G, Calvia P, et al. Reduction of albumin excretion rate in normotensive microalbuminuric type 2 diabetic patients during long-term simvastatin treatment. Diabetes Care 1997;20(12):1891-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
UK‐HARP‐I 2005 {published data only}
- Baigent C, Landray M, Leaper C, Altmann P, Armitage J, Baxter A, et al. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. American Journal of Kidney Diseases 2005;45(3):473-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Baigent C, UK-HARP Steering Committee. Efficacy and safety of simvastatin and safety of low-dose aspirin among patients with chronic kidney disease: final results of the first UK-heart and renal protection (UK-HARP-I) study [abstract no: SA-P0841]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):437A. [CENTRAL: CN-00444305] [Google Scholar]
Verma 2005 {published data only}
- Verma A, Gordon N, Ranganna KM, Reddy RS, Verma M. Rosuvastatin reduces high sensitivity-C reactive protein and the decline in renal function in hyperlipidemic patients with chronic kidney disease [abstract no: SA-PO134]. Journal of the American Society of Nephrology 2004;15(Oct):329A. [CENTRAL: CN-00583418] [Google Scholar]
- Verma A, Ranganna KM, Reddy RS, Verma M, Gordon NF. Effect of rosuvastatin on C-reactive protein and renal function in patients with chronic kidney disease. American Journal of Cardiology 2005;96(9):1290-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yasuda 2004 {published data only}
- Yasuda G, Kuji T, Hasegawa K, Ogawa N, Shimura G, Ando D, et al. Safety and efficacy of fluvastatin in hyperlipidemic patients with chronic renal disease. Renal Failure 2004;26(4):411-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yi 2014 {published data only}
- Yi YJ, Kim HJ, Jo SK, Kim SG, Song YR, Chung W, et al. Comparison of the efficacy and safety profile of morning administration of controlled-release simvastatin versus evening administration of immediate-release simvastatin in chronic kidney disease patients with dyslipidemia. Clinical Therapeutics 2014;36(8):1182-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 1995 {published data only}
- Zhang A, Vertommen J, Van Gaal L, De Leeuw I. Effects of pravastatin on lipid levels, in vitro oxidizability of non-HDL lipoproteins and microalbuminuria in IDDM patients. Diabetes Research & Clinical Practice 1995;29(3):189-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Almquist 2012 {published data only}
- Almquist T, Jacobson SH, Lins PE, Farndale RW, Hjemdahl P. Effects of lipid-lowering treatment on platelet reactivity and platelet-leukocyte aggregation in diabetic patients without and with chronic kidney disease: a randomized trial. Nephrology Dialysis Transplantation 2012;27(9):3540-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Almquist T, Jacobson SH, Lins RL, Hjemdahl P. Effects of lipid-lowering treatment on inflammatory markers in diabetic patients with chronic kidney disease [abstract no: TH-PO506]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):213A. [Google Scholar]
- Almquist T, Jacobson SH, Mobarrez F, Nasman P, Hjemdahl P. Lipid-lowering treatment and inflammatory mediators in diabetes and chronic kidney disease. European Journal of Clinical Investigation 2014;44(3):276-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Almquist T, Mobarrez F, Jacobson SH, Wallen H, Hjemdahl P. Effects of lipid-lowering treatment on circulating microparticles in patients with diabetes mellitus and chronic kidney disease. Nephrology Dialysis Transplantation 2016;31(6):944-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Almquist TC, Jacobson SH, Lins PE, Farndale RW, Hjemdahl P. Effects of lipid-lowering treatment on platelet reactivity and platelet-leukocyte aggregation in diabetic patients with chronic kidney disease- a randomized trial [abstract no: TH-PO516]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):231A. [DOI] [PubMed] [Google Scholar]
Almukhtar 2021 {published data only}
- Almukhtar HM, Faisal IM, Merkhan MM. Acute effect of atorvastatin in comparison with rosuvastatin on glucose homeostasis in hypercholesteremic patients. Current Topics in Pharmacology 2021;25:25‐34. [EMBASE: 2013766821] [Google Scholar]
Dogra 2005 {published data only}
- Dogra GK, Watts GF, Chan DC, Stanton K. Statin therapy improves brachial artery vasodilator function in patients with Type 1 diabetes and microalbuminuria. Diabetic Medicine 2005;22(3):239-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dogra 2007 {published data only}
- Dogra G, Irish A, Chan D, Watts G. A randomized trial of the effect of statin and fibrate therapy on arterial function in CKD. American Journal of Kidney Diseases 2007;49(6):776-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dogra S, Kanganas C, Chan D, Irish A, Watts G. Effect of statin and fibrate therapy on vascular function in chronic kidney disease (CKD) [abstract no: 160]. Nephrology 2005;10(Suppl 3):A422. [CENTRAL: CN-00774612] [Google Scholar]
ESTABLISH 2004 {published data only}
- Dohi T, Miyauchi K, Okazaki S, Yokoyama T, Tamura H, Kojima T, et al. Long-term impact of mild chronic kidney disease in patients with acute coronary syndrome undergoing percutaneous coronary interventions. Nephrology Dialysis Transplantation 2011;26(9):2906-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dohi T, Miyauchi K, Okazaki S, Yokoyama T, Yanagisawa N, Tamura H, et al. Early intensive statin treatment for six months improves long-term clinical outcomes in patients with acute coronary syndrome (Extended-ESTABLISH trial): a follow-up study. Atherosclerosis 2010;210(2):497-502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dohi T, Miyauchi K, Okazaki S, Yokoyama T, Yanagisawa N, Tamura H, et al. Higher baseline LDL-C levels amplify the short-term benefit of early intensive statin treatment in acute coronary syndrome. Journal of Atherosclerosis & Thrombosis 2011;18(1):42-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation 2004;110(9):1061-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fotso 2021 {published data only}
- Fotso Soh J, Beaulieu S, Trepiccione F, Linnaranta O, Torres-Platas G, Platt RW, et al. A double-blind, randomized, placebo-controlled pilot trial of atorvastatin for nephrogenic diabetes insipidus in lithium users. Bipolar Disorders 2021;23(1):66‐75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Golper 1987 {published data only}
- Golper TA, Illingworth DR, Bennett WM. Correction of nephrotic hypercholesterolemia with the HMG-CoA reductase inhibitor lovastatin [abstract]. In: 10th International Congress of Nephrology; 1987 Jul 26-31; London, UK. 1987:64. [CENTRAL: CN-00583909]
- Golper TA, Illingworth DR, Bennett WM. Effective and safe short term improvement in nephrotic hypercholesterolemia with the HMG CO-A reductase inhibitor mevinolin [abstract]. Kidney International 1987;31(1):200. [CENTRAL: CN-00774455] [Google Scholar]
Golper 1989 {published data only}
- Golper TA, Illingworth DR, Morris CD, Bennett WM. Lovastatin in the treatment of multifactorial hyperlipidemia associated with proteinuria. American Journal of Kidney Diseases 1989;13(4):312-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IMPROVE‐IT 2008 {published data only}
- Azar RR, Badaoui G, Sarkis A, Azar M, Aydanian H, Harb S, et al. Effect of ezetimibe/atorvastatin combination on oxidized low density lipoprotein cholesterol in patients with coronary artery disease or coronary artery disease equivalent. American Journal of Cardiology 2010;106(2):193-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Blazing MA, Giugliano RP, Cannon CP, Musliner TA, Tershakovec AM, White JA, et al. Evaluating cardiovascular event reduction with ezetimibe as an adjunct to simvastatin in 18,144 patients after acute coronary syndromes: final baseline characteristics of the IMPROVE-IT study population. American Heart Journal 2014;168(2):205-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation 2015;132(13):1224-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bohula EA, Morrow DA, Giugliano RP, Blazing MA, He P, Park JG, et al. Atherothrombotic risk stratification and ezetimibe for secondary prevention. Journal of the American College of Cardiology 2017;69(8):911-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bohula EA, Wiviott SD, Giugliano RP, Blazing MA, Park JG, Murphy SA, et al. Prevention of stroke with the addition of ezetimibe to statin therapy in patients with acute coronary syndrome in IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation 2017;136(25):2440-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. New England Journal of Medicine 2015;372(25):2387-97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cannon CP, Giugliano RP, Blazing MA, Harrington RA, Peterson JL, Sisk CM, et al. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. American Heart Journal 2008;156(5):826-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalan R, Spinar J, et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: Results From IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation 2018;137(15):1571-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Giugliano RP, Wiviott SD, Blazing MA, De Ferrari GM, Park JG, Murphy SA, et al. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiology 2017;2(5):547-55. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato ET, Cannon CP, Blazing MA, Bohula E, Guneri S, White JA, et al. Efficacy and safety of adding ezetimibe to statin therapy among women and men: insight from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Journal of the American Heart Association 2017;6(11):e006901. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SA, Cannon CP, Blazing MA, Giugliano RP, White JA, Lokhnygina Y, et al. Reduction in total cardiovascular events with ezetimibe/simvastatin post-acute coronary syndrome: the IMPROVE-IT Trial. Journal of the American College of Cardiology 2016;67(4):353-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Peto R, Emberson J, Landray M, Baigent C, Collins R, Clare R, et al. Analyses of cancer data from three ezetimibe trials. New England Journal of Medicine 2008;359(13):1357-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pokharel Y, Chinnakondepalli K, Vilain K, Wang K, Mark DB, Davies G, et al. Impact of ezetimibe on the rate of cardiovascular-related hospitalizations and associated costs among patients with a recent acute coronary syndrome: results from the IMPROVE-IT Trial (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation: Cardiovascular Quality & Outcomes 2017;10(5):e003201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Simon TG, Corey KE, Cannon CP, Blazing M, Park JG, O'Donoghue ML, et al. The nonalcoholic fatty liver disease (NAFLD) fibrosis score, cardiovascular risk stratification and a strategy for secondary prevention with ezetimibe. International Journal of Cardiology 2018;270:245-52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanifer JW, Charytan DM, White J, Lokhnygina Y, Cannon CP, Roe MT, et al. Benefit of ezetimibe added to simvastatin in reduced kidney function. Journal of the American Society of Nephrology 2017;28(10):3034-43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishimitsu 2014 {published data only}
- Ishimitsu T, Ohno E, Ueno Y, Onoda S, Nagase A, Ohira T, et al. Effects of atorvastatin and ezetimibe on endothelial function in dyslipidemic patients with chronic kidney disease. Clinical & Experimental Nephrology 2014;18(5):704-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kouvelos 2013 {published data only}
- Kouvelos GN, Arnaoutoglou EM, Matsagkas MI, Kostara C, Gartzonika C, Bairaktari ET, et al. Effects of rosuvastatin with or without ezetimibe on clinical outcomes in patients undergoing elective vascular surgery: results of a pilot study. Journal of Cardiovascular Pharmacology & Therapeutics 2013;18(1):5-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kouvelos GN, Arnaoutoglou EM, Milionis HJ, Raikou VD, Papa N, Matsagkas MI. The effect of adding ezetimibe to rosuvastatin on renal function in patients undergoing elective vascular surgery. Angiology 2015;66(2):128-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mackie 2008 {published data only}
- Mackie F, Rosenberg A, Harmer J, Kainer G, Celermajer D. HMG CoA reductase inhibitors - effects on endothelial function and lipoprotein levels in children with chronic kidney disease [abstract no: P053]. Pediatric Nephrology 2008;23:1610. [Google Scholar]
Mohammadi 2023 {published data only}
- Mohammadi Kebar S, Atighi E, Hosseninia S, Babapour B. Preventive effects of nicorandil and atorvastatin in contrastinduced nephropathy in patients with renal dysfunction undergoing coronary artery angiography: a double blind, randomized, controlled clinical trial. Iranian Journal of Kidney Diseases 2023;17(4):205‐14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mose 2014a {published data only}
- Mose FH, Larsen T, Jensen JM, Bech JN, Pedersen E. Effect of atorvastatin on renal NO availability and tubular function in patients with stage II-III chronic kidney disease and type 2 diabetes [abstract no: TH-PO447]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):204A. [DOI] [PubMed] [Google Scholar]
- Mose FH, Larsen T, Jensen JM, Hansen AB, Bech JN, Pedersen EB. Effect of atorvastatin on renal NO availability and tubular function in patients with stage II-III chronic kidney disease and type 2 diabetes. Scandinavian Journal of Clinical & Laboratory Investigation 2014;74(1):8-19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mose 2014b {published data only}
- Larsen T, Jensen J, Bech J, Pedersen E, Mose F. Effect of atorvastatin on renal NO availability and tubular function in patients with non-diabetic, stage II-III chronic kidney disease [abstract no: MP219]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i367. [EMBASE: 71076128] [Google Scholar]
- Mose FH, Larsen T, Jensen JM, Bech JN, Pedersen E. Increased NO activity during statin treatment in patients with non-diabetic nephropathy [abstract no: SA-PO169]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):673A. [Google Scholar]
- Mose FH, Larsen T, Jensen JM, Hansen AB, Bech JN, Pedersen EB. Effects of atorvastatin on systemic and renal NO dependency in patients with non-diabetic stage II-III chronic kidney disease. British Journal of Clinical Pharmacology 2014;78(4):789-99. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03543774 {published data only}
- Lan DT. Lipid-lowering therapies in Vietnamese chronic kidney disease population (VietCKD) [Lipid-lowering regimes improve oxidative stress, tryptophan degradation in hypercholesterolemia chronic kidney disease patients]. www.clinicaltrials.gov/show/NCT03543774 (first received 1 June 2018).
Ott 2008 {published data only}
- Ott C, Schlaich MP, Schmidt BM, Titze SI, Schaufele T, Schmieder RE. Rosuvastatin improves basal nitric oxide activity of the renal vasculature in patients with hypercholesterolemia. Atherosclerosis 2008;196(2):704-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PACTR202110707328144 {published data only}
- Impact of ATOrvastatin REload on the Prevention of Contrast-INduced Nephropathy in patients on Chronic Statin Therapy: a prospective randomized trial (ATORE-CIN) [Impact of atorvastatin reload on the prevention of contrast-induced nephropathy]. https://trialsearch.who.int/Trial2.aspx?TrialID=PACTR202110707328144 (first posted 16 September 2021).
Paulsen 2010 {published data only}
- Paulsen L, Matthesen SK, Bech JN, Starklint J, Pedersen EB. Acute effects of atorvastatin on glomerular filtration rate, tubular function, blood pressure, and vasoactive hormones in patients with type 2 diabetes. Journal of Clinical Pharmacology 2010;50(7):816-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmieder 2003 {published data only}
- Schmieder RE, Schneider MP, Delles C, Oehmer S, John S. Evidence for the lipid independent effects of statins on endothelial function [abstract no: T245]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):359. [CENTRAL: CN-00447626] [Google Scholar]
Siddiqi 2011 {published data only}
- Siddiqi L, Joles JA, Oey PL, Blankestijn PJ. Atorvastatin reduces sympathetic activity in patients with chronic kidney disease. Journal of Hypertension 2011;29(11):2176-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Suzuki 2013a {published data only}
- Suzuki H, Takenaka T, Okada H, Inoue T. Comparative efficacy and adverse effects of the addition of ezetimibe to statin versus statin titration in chronic disease (CKD) patients [abstract no: SA-PO161]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):672A. [Google Scholar]
- Suzuki H, Watanabe Y, Kumagai H, Shuto H. Comparative efficacy and adverse effects of the addition of ezetimibe to statin versus statin titration in chronic kidney disease patients. Therapeutic Advances in Cardiovascular Disease 2013;7(6):306-15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Torraca 2006 {published data only}
- Torraca S, Nazzaro P, Visciano B, Pisani A, Di Micco L, Caputo DL, et al. Effects of high doses of atorvastatin (ATO) on renal dynamics and vascular reactivity in patients with chronic renal failure (CRF) [abstract no: MP272]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv390. [CENTRAL: CN-00774679] [Google Scholar]
UK‐HARP‐II 2006 {published data only}
- Landray M, Baigent C, Leaper C, Adu D, Altmann P, Armitage J, et al. The second United Kingdom Heart and Renal Protection (UK-HARP-II) Study: a randomized controlled study of the biochemical safety and efficacy of adding ezetimibe to simvastatin as initial therapy among patients with CKD. American Journal of Kidney Diseases 2006;47(3):385-95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Landray M, Baigent C, Leaper C, UK-HARP Steering Committee. Biochemical safety and efficacy of co-administration of ezetimibe and simvastatin among patients with chronic kidney disease: the second UK-heart and renal protection (UK-HARP-II) study [abstract no: M383]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):119-20. [CENTRAL: CN-00446268] [Google Scholar]
Van Dijk 2001 {published data only}
- Dijk MA, Kamper AM, Veen S, Souverijn JH, Blauw GJ. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 2001;16(11):2152-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vasin 2021 {published data only}
- Vasin A, Mironova O, Fomin V. The role of short-term, high-dose atorvastatin for prevention of contrast-induced acute kidney injury (CI-AKI) in patients with cardiovascular diseases undergoing computed tomography with intravenous contrast administration. Nephrology Dialysis Transplantation 2021;36(Suppl 1):i274. [EMBASE: 635918386] [Google Scholar]
Watanabe 2022 {published data only}
- Watanabe M, Aonuma K, Murohara T, Okumura Y, Morimoto T, Okada S, et al. Prevention of contrast-induced nephropathy after cardiovascular catheterization and intervention with high-dose strong statin therapy in Japan - The PREVENT CINC-J Study. Circulation Journal 2022;86(9):1455‐63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weinstein 2013 {published data only}
- Weinstein DL, Williams LA, Carlson DM, Kelly MT, Burns KM, Setze CM, et al. A randomized, double-blind study of fenofibric acid plus rosuvastatin compared with rosuvastatin alone in stage 3 chronic kidney disease [Erratum appears in Clin Ther. 2013 Nov;35(11):1862]. Clinical Therapeutics 2013;35(8):1186-98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yasmeen 2015 {published data only}
- Yasmeen G, Dawani ML, Mahboob T. Association of high-density lipoprotein cholesterol with improvement of endothelial dysfunction recovery in renovascular disease. Iranian Journal of Kidney Diseases 2015;9(1):39-45. [MEDLINE: ] [PubMed] [Google Scholar]
Yasuda 2010 {published data only}
- Yasuda G, Ando D, Hirawa N, Umemura S. Effects of atorvastatin versus probucol on low-density lipoprotein subtype distribution and renal function in hyperlipidemic patients with nondiabetic nephropathy. Renal Failure 2010;32(6):680-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yen 2022 {published data only}
- Yen CL, Fan PC, Lee CC, Chen JJ, Kuo G, Tu YR, et al. Association of low-density lipoprotein cholesterol levels during statin treatment with cardiovascular and renal outcomes in patients with moderate chronic kidney disease. Journal of the American Heart Association 2022;11(19):e027516. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zinellu 2012 {published data only}
- Zinellu A, Sotgia S, Loriga G, Deiana L, Satta AE, Carru C. Oxidative stress improvement is associated with increased levels of taurine in CKD patients undergoing lipid-lowering therapy. Amino Acids 2012;43(4):1499-507. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zinellu A, Sotgia S, Mangoni AA, Sanna M, Satta AE, Carru C. Impact of cholesterol lowering treatment on plasma kynurenine and tryptophan concentrations in chronic kidney disease: relationship with oxidative stress improvement. Nutrition Metabolism & Cardiovascular Diseases 2015;25(2):153-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zinellu A, Sotgia S, Mangoni AA, Sotgiu E, Ena S, Satta AE, et al. Effect of cholesterol lowering treatment on plasma markers of endothelial dysfunction in chronic kidney disease. Journal of Pharmaceutical & Biomedical Analysis 2016;129:383-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zinellu A, Sotgia S, Pisanu E, Loriga G, Deiana L, Satta AE, et al. LDL S-homocysteinylation decrease in chronic kidney disease patients undergone lipid lowering therapy. European Journal of Pharmaceutical Sciences 2012;47(1):117-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zinellu A, Sotgia S, Sotgiu E, Assaretti S, Baralla A, Mangoni AA, et al. Cholesterol lowering treatment restores blood global DNA methylation in chronic kidney disease (CKD) patients. Nutrition Metabolism & Cardiovascular Diseases 2017;27(9):822-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00768638 {published data only}
- Agharazii M. The renal protective effects of low-dose and high-dose atorvastatin in patients with glomerular disease and proteinuria: a randomized controlled double blinded study. clinicaltrials gov/ct2/show/NCT00768638 (first received 8 October 2008).
NCT02863185 {published data only}
- An WS. Effect of pitavastatin on erythrocyte membrane fatty acid contents in patients with chronic kidney disease. clinicaltrials.gov/show/NCT02863185 (first received 11 August 2016).
References to ongoing studies
NCT05870007 {published data only}
- Atorvastatin and alkali therapy in patients with autosomal dominant polycystic kidney disease [Atorvastatin and alkali therapy in patients with autosomal dominant polycystic kidney disease, a pilot trial for safety and feasibility]. https://clinicaltrials.gov/show/NCT05870007 (first posted 23 May 2023).
Additional references
Baigent 2005
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins [Erratum in: Lancet. 2005 Oct 15-21;366(9494):1358] [Erratum in: Lancet. 2008 Jun 21;371(9630):2084]. Lancet 2005;366(9493):1267-78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bansal 2017
- Bansal N, Katz R, Robinson-Cohen C, Odden MC, Dalrymple L, Shlipak MG, et al. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiology 2017;2(3):314-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2010
- Boutron I, Dutton S, Ravaud P, Altman DG. Reporting and interpretation of randomized controlled trials with statistically nonsignificant results for primary outcomes. JAMA 2010;303(20):2058-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Briel 2006
- Briel M, Schwartz GG, Thompson PL, Lemos JA, Blazing MA, Es GA, et al. Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: a meta-analysis of randomized controlled trials. JAMA 2006;295(17):2046-56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Campese 2005
- Campese VM, Nadim MK, Epstein M. Are 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors renoprotective? Journal of the American Society of Nephrology 2005;16 Suppl 1:S11-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CARI 2013
- Johnson DW, Atai E, Chan CM, Phoon RKS, Scott C, Toussaint ND, Turner GL, Usherwood T, Wiggins KJ. KHA-CARI Guideline: Early chronic kidney disease: Detection, prevention and management. Nephrology 2013;18(5):340-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dalrymple 2011
- Dalrymple LS, Katz R, Kesetenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. Journal General Internal Medicine 2011;26(4):379-85. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drueke 2001
- Drueke TB, Nguyen Khoa T, Massy ZA, Witko-Sarsat V, Lacour B, Descamps-Latscha B. Role of oxidized low-density lipoprotein in the atherosclerosis of uremia. Kidney International - Supplement 2001;78:S114-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foley 1998
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. American Journal of Kidney Diseases 1998;32(5 Suppl 3):S112-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gargiulo 2015
- Gargiulo R, Suhail F, Lerma EV. Cardiovascular disease and chronic kidney disease. Disease-a-Month 2015;61(9):403-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Global Burden of Disease CKD Collaboration 2020
- Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020;395(10225):709-33. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Go 2004
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New England Journal of Medicine 2004;351(13):1296-305. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harris 2002
- Harris K, Thomas M, Short C, Moore R. Assessment of the efficiency of treatment of dyslipidemia in renal outpatients. Journal of Nephrology 2002;15(3):263-9. [MEDLINE: ] [PubMed] [Google Scholar]
Henry 2002
- Henry RM, Kostense PJ, Bos G, Dekker JM, Nijpels G, Heine RJ, et al. Mild renal insufficiency is associated with increased cardiovascular mortality: the Hoorn Study. Kidney International 2002;62(4):1402-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herrington 2016
- Herrington WG, Emberson J, Mihaylova B, Blackwell L, Reith C, Solbu MD, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. The Lancet Diabetes & Endocrinology 2016;4(10):829-39. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Kasiske 1988
- Kasiske BL, O'Donnell MP, Garvis WJ, Keane WF. Pharmacologic treatment of hyperlipidemia reduces glomerular injury in rat 5/6 nephrectomy model of chronic renal failure. Circulation Research 1988;62(3):367-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2012
- Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, et al. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplments 2013;3(1):1-150. [EMBASE: 369856107] [Google Scholar]
KDIGO 2013
- Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney International Supplement 2013;3:259-305. [DOI: 10.1038/kisup.2013.27] [DOI] [Google Scholar]
Keane 2000
- Keane WF. The role of lipids in renal disease: future challenges. Kidney International - Supplement 2000;75:S27-31. [MEDLINE: ] [PubMed] [Google Scholar]
Kinlay 2003
- Kinlay S, Selwyn AP. Effects of statins on inflammation in patients with acute and chronic coronary syndromes. American Journal of Cardiology 2003;91(4A):9-13B. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche GC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
LIPID 1998
- Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. New England Journal of Medicine 1998;339(19):1349-57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manjunath 2003
- Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. Journal of American College of Cardiology 2003;41(1):47-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Massy 2001
- Massy ZA, Guijarro C. Statins: effects beyond cholesterol lowering. Nephrology Dialysis Transplantation 2001;16(9):1738-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meisinger 2006
- Meisigner C, Döring A, Löwel H. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. European Heart Journal 2006;27(10):1245-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Muntner 2005
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. Journal of the American Society of Nephrology 2005;16(2):529-38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Clinical Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. https://www.nice.org.uk/guidance/cg181 (accessed 11 October 2023).
Palmer 2013
- Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Nigwekar SU, et al. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database of Systematic Reviews 2013, Issue 9. Art. No: CD004289. [DOI: 10.1002/14651858.CD004289.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2014a
- Palmer SC, Navaneethan SD, Craig JC, Perkovic V, Johnson DW, Nigwekar SU, et al. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD005019. [DOI: 10.1002/14651858.CD005019.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
R Core Team 2013
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing 2013;Vienna, Austria:URL http://www.R-project.org/.
Robinson 2005
- Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction. A meta-regression analysis. Journal of the American College of Cardiology 2005;46(10):1855-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sacks 1996
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. New England Journal of Medicine 1996;335(14):1001-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schaeffner 2003
- Schaeffner ES, Kurth T, Curhan GC, Glynn RJ, Rexrode KM, Baigent C, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. Journal of the American Society of Nephrology 2003;14(8):2084-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2022a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. www.training.cochrane.org/handbook.
Schünemann 2022b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Shepherd 1995
- Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. New England Journal of Medicine 1995;333(20):1301-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shilpak 2005
- Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA 2005;293(14):1737-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sidebottom 2020
- Sidebottom AC, Vacquier MC, Jensen JC, Bradley SM, Knickelbine T, Strauss C, et al. Trends in prevalence of guideline‐based use of lipid‐lowering therapy in a large health system. Clinical Cardiology 2020;43(6):560-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sotiriou 2000
- Sotiriou CG, Cheng JW. Beneficial effects of statins in coronary artery disease--beyond lowering cholesterol. Annals of Pharmacotherapy 2000;34(12):1432-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
StatPearls Textbook
- Omeed Sizar, Ali Nassereddin, Raja Talati. StatPearls - Ezetimibe [Internet]. Treasure Island Florida: StatPearls Publishing, 2023. [PubMed] [Google Scholar]
USRDS 2019
- USRDS. US Renal Data System 2018 Annual Data Report. Chapter 4. Cardiovascular disease in patients with CKD. American Journal of Kidney Diseases 2019;73(3 (Suppl 1)):S79-98. [DOI: 10.1053/j.ajkd.2018.12.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Navaneethan 2009
- Navaneethan SD, Pansini F, Perkovic V, Manno C, Pellegrini F, Johnson DW, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007784. [DOI: 10.1002/14651858.CD007784] [DOI] [PubMed] [Google Scholar]
Palmer 2014
- Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Hegbrant J, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD007784. [DOI: 10.1002/14651858.CD007784.pub2] [DOI] [PubMed] [Google Scholar]
Strippoli 2008
- Strippoli GF, Navaneethan SD, Johnson D, Perkovic V, Pellegrini F, Nicolucci A, et al. Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ 2008;336(7645):645-51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


