Abstract
Background:
High-risk alcohol use is a common surgical risk factor. Stopping or reducing alcohol use in the weeks before and after surgery could improve surgical health and outcomes. The purpose of this study was to evaluate the feasibility and acceptability of two interventions that address high-risk alcohol use in the context of surgery.
Methods:
Participants included patients scheduled for elective surgeries at an academic health system in the Midwestern United States. Recruitment took place by phone and text. Participants were included if they were 18 – 75 years-old, scheduled for elective surgeries, and scored ≥ 5 on the Alcohol Use Disorders Identification Test-Consumption tool. Participants were randomized to either a low-intensity intervention, Brief Advice (10-minute phone-based psychoeducation plus feedback session) or a higher-intensity intervention, Health Coaching (two 45-minute sessions including education, feedback, motivational interviewing and goal setting). Assessments took place at baseline, 1-, and 4-month follow-ups. Alcohol biomarkers were collected the day of surgery.
Results:
The final study sample included (n=51) participants randomized to Brief Advice and Health Coaching conditions. Participants in both conditions rated interventions as satisfactory and personally relevant. Trial retention was high (86.3%) at 4 months. Attrition was significantly higher in Brief Advice (n=6) relative to Health Coaching (n=1). Average weekly alcohol use decreased 50%-60% between baseline and follow-ups in both conditions. Biomarkers corroborated self-report.
Conclusions:
The trial demonstrated intervention feasibility and acceptability. Alcohol use changed in expected directions. The next steps include a randomized controlled trial to test intervention efficacy in reducing alcohol use and surgical complications.
INTRODUCTION
As many as 1 in 4 Americans undergoing surgery meet criteria for high-risk alcohol use.(1) High-risk alcohol use prior to surgery is often defined as > 2 drinks/day, or a score of ≥ 5 on the Alcohol Use Disorders Identification Test Consumption screening tool (AUDIT-C) prior to surgery.(2,3) High-risk alcohol use is one of the most common surgical risk factors,(1,4) and is associated with an increased risk of infections, wound complications, pulmonary complications, and prolonged hospital stays following elective surgical procedures.(1-3,5-7) Heavy drinking (4 drinks/day), is linked to three-fold increase in the likelihood of death after surgery.(1,8) Every additional point scored on the AUDIT-C alcohol screening tool increases the expected number of surgical complications by 29%.(5) These alcohol-associated adverse surgical outcomes are not specific to certain surgeries or subpopulations; instead, they are evident across heterogeneous patients and surgery types.(3,6)
Alcohol use is recognized as a central surgical risk factor by the American Society of Anesthesiologists(9) and American College of Surgeons,(10) yet elective surgical patients are rarely offered alcohol-focused education, intervention, or treatment referrals prior to surgery.(1,11,12) This gap is a missed opportunity because the pathophysiological effects of high-risk alcohol use are reversible with as little as 2 to 4 weeks of alcohol abstinence prior to surgery.(13-16) Preoperative alcohol interventions can successfully increase preoperative alcohol abstinence and reduce surgical complications.(16) However, the only rigorously evaluated intervention protocol is for those with alcohol use disorder and requires weekly on-site supervised disulfiram administration.(17) A more scalable program that addresses the full spectrum of high-risk alcohol use could reach the broader population at increased risk for complications.(13)
Research suggests alcohol-focused interventions at the time of surgery are acceptable to patients and surgery may uniquely facilitate alcohol cessation and other behavior change(18). However, research also indicates feasibility challenges in terms of identifying participants early enough in the preoperative period to screen, enroll, and deliver interventions(19). Research also suggests heterogeneity in patient preferences in terms of time commitment and intervention modality, with some patients preferring brief, advice-driven approaches and others preferring more intensive one-on-one meetings with a clinician.(20) Likewise, surgical health providers indicate brief advice is more feasible in busy real-world surgical clinics however they would be willing to refer patients to counseling-based interventions if they were available, similar to the processes in place existing presurgical health optimization programs (20-22).
Given the dearth of scalable behavioral interventions that address high-risk alcohol use among elective surgical patients,(23) this pilot randomized clinical trial used a two-group parallel design to examine the feasibility and acceptability of two preoperative alcohol interventions of varying intensities delivered prior to surgery. This pilot study was designed to prepare for a fully-powered sequential multiple phase randomized trial (SMART), which is clinical trial design that enables evaluation of a sequence of treatments of various intensities and modalities over time. (24). Outcomes included recruitment feasibility, intervention engagement, participant satisfaction, and intervention fidelity. We also describe longitudinal alcohol use and surgical outcomes across the two study conditions. We hypothesized both conditions would be acceptable to participants and that Health Coaching would lead to greater reductions in alcohol use.
MATERIALS & METHODS
Setting
Participants were recruited from Michigan Medicine, a large academic health system in the Midwestern United States. All study procedures received approval from the institutional review board at Michigan Medicine. The clinical trial was registered April 24, 2019 (NCT03929562).
Recruitment and participants
Participant enrollment took place from August, 2019 through March, 2021. Recruitment and enrollment was paused from April 2020 to July 2020 because of COVID-19-related restrictions on elective and non-emergent surgeries. Past research cites numerous barriers to preoperative alcohol interventions including difficulties identifying and engaging eligible patients and lack of time and expertise for screening and intervention in busy preoperative settings.(19,20,25) Thus this study sought to overcome these barriers through novel recruitment strategies and remote intervention delivery. Recruitment took place over the phone and through text messages. Research staff conducted weekly, automated, operating room schedule and chart reviews to identify patients who may be eligible for the trial. To be included patients had to be; 1) scheduled for elective or semi-elective surgery in the next 35 - 120 days in select subspecialties (Plastic, Knee/hip arthroplasy, Minimally Invasive, Endocrine, Gynecology, Urology, Colorectal, or Hepatobiliary); 2) receiving regional or general anesthesia, 3) aged 18-75, and 4) had positive or unknown alcohol use in their social history in the electronic health record. We excluded the lowest acuity surgeries by excluding regional anesthesia cases. All other levels of surgical acuity were considered within each surgical specialty. This range of acuity and surgical types were chosen for inclusion because alcohol-associated surgical complications occur across heterogeneous surgical types and acuity.(6,26) Patients were mailed a letter informing them they may be eligible for a research study, contacted via phone and text, and invited to participate in a brief (~5 min) computerized screening survey.
Participants were eligible for the trial if they met all inclusion criteria and scored ≥ 5 on the AUDIT-C for past 3-months alcohol use, assessed by research staff. A score of ≥ 5 prior to surgery is associated with adverse surgical outcomes.(3,27,28) Exclusion screening criteria included: (1) inability to provide voluntary informed consent for any reason (including incompetency); (2) substantial cognitive impairment as evidenced by lack of orientation to person, place, or time or lack of ability to repeat back and answer screening questions; (3) evidence of psychotic symptoms; (4) inability to read or understand English; (5) undergoing surgeries/procedures that require local anesthesia only.
Trial Design and Randomization
This study used a two-group parallel trial design with stratified computer-generated randomization based on sex and AUDIT-C score (Low 5-7; High 8-12). A statistical analyst generated randomization assignments. Research staff were blinded to study assignment. Sample size was selected to provide sufficient information on study protocols, trial feasibility, intervention acceptability, and participant satisfaction for a future randomized controlled trial.
Intervention Conditions
The two study conditions included: a) Health Coaching, a two-session intervention that included feedback on alcohol use, health education, motivational interviewing (MI), and goal setting, and b) Brief Advice, a 10-minute intervention that included feedback on alcohol use and surgical health education. Both interventions sought to help patients reduce or stop alcohol use for 4 weeks prior and 6 weeks after surgery. Brief advice was classified as the comparator condition given its lower intensity.
Intervention content, timing, and modality was informed by theory, literature, and qualitative research conducted by the study team. Qualitative findings reflected patient and health care provider preferences for flexible, remote intervention delivery, with some patients wanting brief informative sessions (i.e. Brief Advice) and others wanting lengthier counseling (i.e. Health Coaching).(20) Based on qualitative data,(20) all elements of interventions emphasized acute risks of alcohol use in the context of physical and surgical health rather than chronic health problems or addiction framing.
Interventionist Training and Supervision:
Both interventions were delivered by a “Health Coach” who was a master’s level social worker. The Health Coach was provided with intervention manuals and content guide for both conditions. The Health Coach attended an 8-hour MI certification training (needed to deliver Health Coaching condition), and took part in monthly supervision with a clinical psychologist (author: ACF) to receive feedback on MI fidelity as well as overall session content fidelity throughout the trial. The same master’s level clinician delivered both study conditions for staffing consistency.
Health Coaching.
Participants in the Health Coaching condition received two 45-minute sessions 4 weeks and 2 weeks prior to surgery, respectively. The Health Coaching condition was based on principles of models of collaborative care, MI,(29) and the Health Belief Model.(30,31) Participants had a choice of completing sessions in person or via teleconferencing (after the COVID-19 pandemic only teleconferencing was offered). Prior to the session participants received a 4-page feedback and education guide by email. Session one focused on risks of preoperative alcohol use and session two focused on postoperative alcohol use. Health education components focused on the link between alcohol use and surgical outcomes (e.g wound complications) rather than on other risks of alcohol use (e.g. dependence) to encourage a conversation around stopping alcohol use for surgery. The Health Coach presented information on reducing or abstaining from alcohol in the 4 weeks before their procedure and 6 weeks after to reduce their risk of surgical and healing complications, using an MI communication style. The Health Coach worked with participants to set a goal for reducing or stopping alcohol use if they were ready to do so using MI.
The second Health Coaching session was similar in structure to session one, but with a focus on the risks of alcohol use after surgery, including healing difficulties. In this session, participants were able to report what progress they had made on their goals, discuss barriers, and engage in goal setting and planning related to the 6 weeks after surgery.
Brief Advice.
Brief Advice was a 10-minute educational and feedback intervention delivered 4 weeks prior to surgery. The session included a 2-page infographic e-mailed to participants prior to the session. The session included a verbal review of the participants’ current alcohol use (from baseline surveys), educational information about alcohol and surgical health, and advice to stop alcohol use for 4 weeks prior to surgery and 6 weeks after surgery. Treatment resources and alcohol withdrawal information were included in a separate pamphlet. Participants had the opportunity to ask questions.
Assessments
Participants completed a baseline assessment (approximately 5 weeks prior to surgery), a 1-month follow-up assessment (approximately 1 week prior to surgery), and a 4-month follow-up assessment (approximately 3 months after surgery). All assessments were computerized and completed online via secure personalized links. Brief Advice and Health Coaching session participants completed an acceptability and satisfaction assessment immediately after each session. Participants received $30 for completing the baseline assessment, $50 for the 1-month follow up, $50 for the 4-month follow-up, and $30 and $40 for completing the intervention/post-session assessment for Brief Advice and Health Coaching respectively.
Measures
Feasibility.
The primary feasibility measures were: 1) percent of patients eligible for participation; 2) percent of eligible patients who consent and are randomized (>40% based on past literature)(23,25,32); 3) percent of participants who complete intervention sessions (>80%), and 5) percent of participants who complete follow-up assessments (>80%).
Acceptability.
Acceptability of the two interventions was assessed immediately after each session using Likert-type scales ranging from 1 to 4. Participants rated their session satisfaction (1= ‘Very dissatisfied’ and 4 = ‘Very satisfied’), whether the session was personally relevant (1 = ‘No, definitely not’ and 4 = ‘Yes, definitely’), and if they would recommend the session to another patient like themselves (1 = ‘No, definitely not’ and 4 = ‘Yes, definitely’). A score of ≥ 3 on each item met the threshold for ‘acceptability.’
Intervention Fidelity.
Intervention content fidelity was assessed using a post-session checklist. A research assistant listened to each session recording after completion and completed the content fidelity checklist. Treatment fidelity was evaluated using the revised Motivational Interviewing Treatment Integrity coding manual version 4.2 (MITI 4.2)(33), The MITI 4.2 includes global ratings (overall session) and therapist behavior counts (e.g., questions, affirmations). A random selection of session recordings (n = 23; 17 HC, 6 BI) were rated by 4 expert raters, with 8 of these sessions (4 HC, 4 BI) double-coded to establish inter-rater reliability using a two-way absolute single-measures intra-class correlation (ICC; 34).(34)
Self-Reported Alcohol Use.
Change in alcohol use was measured at baseline and follow-up using the AUDIT-C(35) and the Timeline Follow Back (TLFB).(36) The three-item AUDIT-C assessed alcohol quantity and frequency with an overall score ranging from 0 to 12, with higher scores reflecting higher levels of alcohol use. We used an online version of the TLFB, using a calendar format on which participants self-reported the number of standard drinks consumed on each day. The TLFB yielded measures of average drinks per week and percent days abstinent. Time frames for assessment included past 3-months for baseline and final follow-up, and past 1-month for 1 month follow-up.
Alcohol Biomarkers.
Phosphatidylethanol (PEth) and ethyl glucoronide (EtG) biomarkers were collected by surgical clinic staff on the day of surgery. PEth is a direct alcohol biomarker that detects alcohol use in the past 2-4 weeks.(37-39) The cut-off for positive PEth was > 20 ng/ml. Ethyl glucoronide (EtG), detects alcohol use in the past 3-4 days.(40) Cut-offs for positive EtG was < 500 ng/ml.
Surgical Outcomes.
All surgical outcomes were ascertained through electronic health record review including ICU admission, length of stay, and 30-day postoperative mortality. Postoperative complications are defined as any of the following conditions recorded in the electronic medical record during and/or in the 30-day period following the index procedure: surgical site infection, pneumonia, pulmonary embolism, urinary tract infection, stroke or CVA, cardiac arrest requiring CPR, myocardial infarction, postoperative RBC transfusion, DVT requiring therapy, postoperative sepsis and severe sepsis/septic shock, C-difficile, anastomotic leak, ileus requiring NG tube or NPO. Postoperative events are defined as any of the following occurring within 30 days following the performance of the index procedure: Presentation to Emergency Department (ED) or Urgent care for a complaint related to the index procedure, readmission, unplanned return to the operating room.
Postoperative complications and events were abstracted from the medical record by emulating the data abstraction processes of the Michigan Surgical Quality Collaborative (MSQC), a surgery data registry for the state of Michigan which is commonly used to report outcomes and healthcare quality among Michigan Hospitals. Training for chart abstraction following the MSQC model was provided to study team members by a licensed nurse abstractor from the MSQC registry. After training, retrospective chart review was conducted from July 1 – July 28th, 2022 by a clinically knowledgeable healthcare professional (MT), supervised by AH and AF. Additional details relating to the surgical outcome definitions of the variables above may be found on the MSQC website.(41) The study team made no modification to the MSQC abstraction method for the purposes of this study.
Mental Health.
Depression and anxiety in the past 7 days were assessed using the Promis-29 anxiety and depression subscales.(42) These 4-item scales assess anxiety (e.g. My worried overwhelmed me… ) and depression (e.g. I felt worthless…) using a 5-point Likert-type response scale ranging from 1 (Never) to 5 (Always). Responses were summed to create a total score for each subscale which could range from 4 to 20 with higher numbers indicating higher levels of anxiety or depression.
Statistical methods
All continuous and categorical measures were evaluated for their distributional characteristics. Alcohol use over time and surgical outcomes are reported using descriptive statistics (means, standard deviation, percent change) given small sample size precluding between group comparisons. Analyses examining differences in baseline variables, acceptability scores, and attrition analysis used t-tests, Kruskal-Wallis H test, Fisher’s exact test, and Χ2 tests as appropriate. For acceptability analysis, we compared Brief Advice acceptability ratings to the averaged acceptability item score across the Health Coaching sessions 1 and 2 (after finding no significant difference between the two session ratings). Statistical significance was set at a two-sided p < 0.05 for all testing. Analyses used all available observations.
RESULTS
Feasibility
In total, staff attempted to contact 2928 individuals by phone or text message, of whom 1433 were assessed for eligibility, and n = 122 were eligible (8.5% of those assessed for eligibility). Among those who were eligible, 53% (n=65) consented and completed baseline assessment. However,14 participants became ineligible after randomization, predominantly due to clinics canceling all elective surgeries early in the COVID-19 pandemic. The final sample included 51 participants randomized to Brief Advice (n = 26) and Health Coaching (n = 25). There were no significant differences in demographics or alcohol use variables between study conditions at baseline (Table 1).
Table 1.
Participant, surgery, and baseline characteristics by study condition
| Total Baseline Sample N = 51 |
Brief Advice N = 26 |
Health Coaching N = 25 |
Baseline Group differences |
|
|---|---|---|---|---|
| N (%) | N (%) | N (%) | p-value | |
| Sex at birth (female) | 28 (54.9%) | 14 (53.8%) | 14 (56.0%) | 0.88 |
| Age (M,SD) | 47.7 (14.1) | 46.1 (13.9) | 49.4 (14.4) | 0.33 |
| Hispanic Ethnicity | 7 (13.7%) | 4 (15.4%) | 3 (12.0%) | 0.73 |
| Race | ||||
| White | 44 (86.3%) | 24 (92.3%) | 20 (80.0%) | 0.20a |
| Black | 2 (3.9%) | 0 (0.0%) | 2 (8.0%) | |
| Asian | 2 (3.9%) | 1 (3.8%) | 1 (4.0%) | |
| American Indian/Alaska Native | 2 (3.9%) | 0 (0.0%) | 2 (8.0%) | |
| Other Race | 2 (3.9%) | 1 (3.8%) | 1 (4.0%) | |
| Marital Status | ||||
| Married | 28 (54.9%) | 16 (61.5%) | 12 (48.0%) | 0.33 |
| Not Married | 23 (45.1%) | 10 (38.5%) | 13 (52.0%) | |
| Highest Education | 0.68b | |||
| Some high school | 1 (2.0%) | 0(0.0%) | 1 (4.0%) | |
| High school/GED | 5 (9.8%) | 3 (11.5%) | 2 (8.0%) | |
| Some college | 10 (19.6%) | 4 (15.4%) | 6 (24.0%) | |
| College graduate | 26 (51.0%) | 15 (57.7%) | 11 (44.0%) | |
| Post college | 9 (17.7%) | 4 (15.4%) | 5 (20.0%) | |
| Surgical stay | 0.44 | |||
| Inpatient | 10 (17.7%) | 4 (11.5%) | 6 (24.0%) | |
| Outpatient | 41 (80.4%) | 22 (84.6%) | 19 (76.0%) | |
| Surgical Service | -- | |||
| Plastic | 23 (45.1%) | 11 (42.3%) | 12 (48.0%) | |
| Orthopedic | 13 (25.5%) | 6 (23.1%) | 7 (28.0%) | |
| Minimally Invasive | 7 (13.7%) | 5 (19.2%) | 2 (8.0%) | |
| Endocrine | 2 (3.9%) | 1 (3.8%) | 1 (4.0%) | |
| Gynecology | 2 (3.9%) | 1 (3.8%) | 1 (4.0%) | |
| Urology | 2 (3.9%) | 1 (3.8%) | 1 (4.0%) | |
| Colorectal | 1 (2.0%) | 1 (3.8%) | 0 (0.0%) | |
| Hepatobiliary | 1 (2.0%) | 0 (0.0%) | 1 (4.0%) | |
| Audit-C score (M, SD) | 6.3 (1.5) | 6.4 (1.4) | 6.2 (1.5) | 0.48 |
| Average weekly alcohol consumption (M, SD) | 12.3 (10.5) | 13.8 (12.5) | 10.7 (7.8) | 0.45 |
| Percent days abstinent (M, SD) | 50.3 (33.0) | 47.8 (33.8) | 52.8 (32.8) | 0.42 |
| Percent weeks exceeding moderate drinking (M, SD) | 49.0 (39.2) | 54.1 (39.6) | 43.7 (38.9) | 0.33 |
| Depression | 6.7 (3.7) | 7.5 (4.1) | 6.0 (3.1) | 0.14 |
| Anxiety | 7.5 (4.2) | 7.7 (4.4) | 7.3 (4.1) | 0.76 |
White vs all other groups
College graduate vs non-college graduate
In terms of trial retention, 46 participants (90.2%) completed the 1-month follow-up, and 44 participants (86.3%) completed the 4-month follow-up. In terms of intervention completion, 23 participants (88.4%) completed the Brief Advice session, and 24 participants (96%) completed both Health Coaching sessions. We examined study attrition (withdrawal or lost to follow-up) by age, sex, race/ethnicity, baseline AUDIT-C score, and inpatient/outpatient surgical procedure groups. A higher proportion of participants in Brief Advice (n = 6) leaving the trial or lost to follow-up relative to Health Coaching (n = 1).
Treatment Fidelity and Integrity
MI reliability was good to excellent on global ratings (94% of ratings identical or within 1 on 1-5 Likert scale) and the therapist behaviors of giving information, questions, simple and complex reflections, seeking collaboration and emphasizing autonomy (ICCs from 0.46 to 0.95). Significantly more MI consistent behaviors (simple and complex reflections, seeking collaboration and emphasizing autonomy) were evident in the Health Coaching sessions (M = 16.8; SD = 8.49) than the Brief Advice sessions [M = 1.16; SD = 1.47 ]; t (21) = 7.30, p < .0001).
Acceptability.
All participants were mostly or very satisfied with the Brief Advice and Health Coaching sessions and indicated session content was personally relevant (Table 2). All Health Coaching participants indicated they would recommend sessions to a patient like themselves. For Brief Advice, 21 out of 23 participants (91.3%) indicated they would recommend the session to a patient like themselves.
Table 2.
Acceptability ratings and for Brief Advice and Health Coaching sessions
| Brief Advice (N = 23) |
Health Coaching, Session 1 (N=24) |
Health Coaching, Session 2 (N=24) |
Kruskal- Wallis H- Test* |
|
|---|---|---|---|---|
| N (%) | N (%) | N (%) | test statistic | |
| In an overall, general sense, how satisfied were you with the session? | ||||
| Very dissatisfied (1) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Mildly dissatisfied (2) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Mostly satisfied (3) | 8 (34.8%) | 4 (16.7%) | 1 (4.2%) | |
| Very Satisfied (4) | 15 (65.2%) | 20 (83.3%) | 23 (95.8%) | |
| Average rating (M, SD) | 3.7 (0.5) | 3.8 (0.4) | 4.0 (0.2) | X2(1)=2.8ns |
| Do you feel that the information provided to you was relevant to you personally? | ||||
| No, definitely not (1) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| No, I don’t think so (2) | 1 (4.4%) | 0 (0.0%) | 0 (0.0%) | |
| Yes, I think so (3) | 8 (34.8%) | 10 (41.7%) | 5 (21.7%) | |
| Yes, definitely (4) | 14 (60.9%) | 14 (58.3%) | 18 (78.3%) | |
| Average rating (M, SD) | 3.6 (0.6) | 3.6 (0.5) | 3.8 (0.4) | X2(1)=0.7ns |
| Would you recommend a session such as this to other patients like yourself? | ||||
| No, definitely not (1) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| No, I don’t think so (2) | 2 (8.7%) | 0 (0.0%) | 0 (0.0%) | |
| Yes, I think so (3) | 6 (26.1%) | 6 (26.1%) | 6 (25.0%) | |
| Yes, definitely (4) | 15 (65.2%) | 17 (73.9%) | 18 (75.0%) | |
| Average rating (M, SD) | 3.6 (0.7) | 3.7 (0.4) | 3.8 (0.4) | X2(1)=0.3ns |
Note. Kruskall-Wallis compared Brief Advice with the item-level average across the two Health Coaching sessions. Health Coaching Sessions 1 and 2 ratings were not significantly different.
ns= not significant
Alcohol Use.
The percent decrease in AUDIT-C score from baseline to 1-month follow-up was 49.7% in the Brief Advice condition and 57.2% in the Health Coaching condition (Table 3 and Supplemental Table 1). At 4-months the percent decrease was 43.1% for Brief Advice and 41.8% for Health Coaching. The percent decrease in average weekly alcohol use from baseline to 1-month was 50.9% for the Brief Advice condition and 56.6% for the Health Coaching condition. At 4-months the percent decrease was 59.2% for Brief Advice, and 60.1% for Health Coaching. Percent days abstinent increased from baseline to follow-up in both conditions. Biomarker results are presented in Table 4. PEth tests were conducted for 28 of 51 participants (55.0%) of which 14 (50.0%) were positive. EtG tests were conducted for 31 of 51 participants (60.1%) and none were positive. Only 1 PEth test was positive when a participant self-reported abstinence (based on AUDIT-C and TLFB data). Nine participants had negative biomarkers but self-reported alcohol use.
Table 3:
Changes in alcohol use in Brief Advice and Health Coaching conditions over time.
| Baseline (N=51) |
1-Month Follow Up (N=46) |
4-Month Follow Up (N=44) |
Change from Baseline to 1-month |
Change from Baseline to 4-month |
|
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | % | % | |
| Audit-C | |||||
| Brief Advice | 6.4 (1.4) | 3.2 (2.6) | 3.7 (2.0) | −49.7% | −43.1% |
| Health Coaching | 6.2 (1.5) | 2.7 (2.2) | 3.6 (2.5) | −57.2% | −41.8% |
| Average weekly alcohol consumption (in standard drinks) | |||||
| Brief Advice | 13.8 (12.5) | 6.8 (8.2) | 5.6 (6.4) | −50.9% | −59.2% |
| Health Coaching | 10.7 (7.8) | 4.7 (7.1) | 4.3 (5.3) | −56.6% | −60.1% |
| Percent days abstinent | |||||
| Brief Advice | 47.8 (33.8) | 67.6 (35.6) | 70.6 (28.8) | 41.3% | 42.0% |
| Health Coaching | 52.8 (32.8) | 77.8 (31.4) | 78.6 (25.6) | 47.3% | 59.0% |
Table 4.
Surgical Outcomes and Alcohol Biomarkers in Brief Advice and Health Coaching conditions.
| Total Sample N = 51 |
Brief Advice N = 26 |
Health Coaching N = 25 |
|
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Surgical Outcomes (30 days after surgery) | |||
| Postoperative complications (≥ 1) | 5 (9.8%) | 2 (7.7%) | 3 (12.0%) |
| Postoperative ICU admission | 1 (2.0%) | 0 (0.0%) | 1 (4.0%) |
| Postoperative event† | 12 (23.5%) | 5 (3.8%) | 7 (12.0%) |
| Length of Stay, in days (Mean, SD) | 0.9 (1.4) | 0.8 (1.1) | 1.0 (1.8) |
| Postoperative Mortality | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Alcohol Biomarkers * | |||
| Phosphatidylethanol | |||
| Positive | 14 (50%) | 8 (57.1%) | 6 (42.9%) |
| Negative | 14 (50%) | 6 (42.9%) | 8 (57.1%) |
| Missing | 23 | 12 | 11 |
| Ethyl Glucuronide | |||
| Positive | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Negative | 31 (100.0%) | 16 (100.0%) | 15 (100.0%) |
| Missing | 20 | 11 | 9 |
Biomarker Percentages calculated using the total number of available biomarkers as the denominator; Phosphotidylethanol = 28; Ethyl Glucuronide = 31.
Postoperative events defined as presentation to ED or Urgent care for a complaint related to the index procedure, readmission, or unplanned return to the operating room.
Surgical Outcomes.
In the study sample, 5 participants (9.8%) experienced 1 or more surgical complications in the 30 days after surgery (Table 4), including 2 participants in the Brief Advice condition, and 3 participants in the Health Coaching condition. There was 1 ICU admission (2.0%) in Health Coaching condition. There were 12 (23.5%) postoperative events (e.g. readmission, ED visits), 5 in Brief Advice, and 7 in Health Coaching. The average length of stay was 0.9 days overall, 0.8 days in Brief Advice and 1.0 in Health Coaching. No deaths occurred.
DISCUSSION
This study demonstrated feasibility and acceptability of two alcohol interventions of varying intensities prior to elective surgery. Participant satisfaction was high for both interventions. However, the higher rate of attrition in Brief Advice condition relative to Health Coaching suggests engagement and satisfaction may have been lower in this condition. Alcohol use changed in expected directions between baseline and follow-ups. The study was feasible despite the COVID-19 pandemic. Recruitment and refusal rates were comparable to similar trials in the literature.(25,32) Among those screened, the eligibility rate was 8.5%, and more than half of eligible patients consented to participate. The percentage consented was higher than other brief intervention studies in surgical settings,(25,32) but lower than brief intervention studies in other healthcare settings.(43) Intervention participation and trial retention were above 80%, exceeding thresholds for trial feasibility.
This pilot study adds to an emerging body of research literature on behavioral alcohol interventions among elective surgical patients.(18,23,25) Preoperative alcohol interventions are ideally delivered several weeks or even months prior to surgery.(44) Historically, preoperative alcohol interventions trials have had difficulty identifying eligible patients, screening, and providing alcohol interventions in the narrow window of time between scheduling and surgery, with recruitment of eligible patients as low as 34% (19,25) However, we were able to reach a large number of patients and recruit 52% of those eligible after screening by monitoring operating room schedules daily in the electronic health record and recruiting by phone/text five or more weeks prior to surgery. In the early phases of trial planning, we determined that surgical clinics at the study site were too numerous, dispersed, and fast-paced to effectively recruit and deliver interventions in person. While we were not able to reach all patients, this increased our recruitment pool and recruitment success dramatically and facilitated timely intervention delivery. These findings support the feasibility of a future fully-powered SMART study which will incorporate lessons learned from this pilot, streamline enrollment processes, and use both in person and remote recruitment.
To our knowledge this study is the first preoperative intervention trial to collect alcohol biomarkers. Biomarker collection proved challenging. Many surgical clinic staff were unwilling to fulfill lab orders or incorporate them into their workflow, particularly if the orders were not signed by the surgeon of record. In total, 61% of the sample had one or more alcohol biomarker collected on the day of surgery. The available biomarker data largely corroborated self-reported alcohol use. In only 1 of 28 cases PEth, which detects alcohol use up to 4 weeks prior, was positive when the participant self-reported abstinence. This result suggests that self-reported alcohol use is valid prior to surgery, as has been widely demonstrated in other settings.(40,45,46) Furthermore, self-report also provided more granular alcohol use information (e.g. drinks per day), and captured 9 cases of reported alcohol use not detected by biomarkers.
The acceptability ratings of Brief Advice and Health Coaching were high, which is consistent with the broader literature indicating perioperative alcohol interventions are viewed as helpful, novel, and relevant.(25,47,48) Remote intervention delivery did not appear to detract from participant experience. Sessions were delivered by phone (Brief Advice) or video conferencing (Health Coaching). In a real-world setting remote delivery of interventions is highly scalable particularly with recent rapid increases in telehealth for substance use and behavioral health.(49) In fact, remote delivery could potentially increase value and quality of care by allowing multiple surgical departments to pool resources and hire specialized social work or nursing staff whose sole role was delivering alcohol and other behavioral health interventions. Such a remote delivery model has been effectively implemented for other surgical health optimization programs prior to surgery.(21) This study used a master’s level clinician for intervention delivery, however Brief Advice could be delivered by any clinical staff regardless of degree type. Health Coaching requires training in MI, which is widely disseminated and offered to healthcare providers nation-wide.
While, this study did not examine the relative efficacy of Brief Advice vs Health Coaching, the reported change in alcohol use did not differ greatly across conditions. However, a fully-powered trial is needed to evaluate these findings. If the two approaches are equivalent, the lower resource intervention, i.e. Brief Advice, would be recommended given lower resource intensity. Cost data in future research could further illuminate relative differences in cost to inform final recommendations regarding optimal clinical approaches. The next step in this research includes evaluating intervention efficacy, timing, and cost using a SMART study design to determine which intervention approach is most effective and for whom. A SMART is an adaptive randomized trial design that allows multiple randomizations at different time points based on intervention response and non-response.(50) It is uniquely suited to comparing multiple intervention strategies and tailoring adaptive intervention strategies to address individual’s needs in real time, such as increasing intervention dose, or switching strategies for those who do not initially respond.(51)
This study has several limitations. The data do not provide information about intervention efficacy. The sample size enabled feasibility and acceptability testing and comparison of Health Coaching and Brief Advice. The study had a 4-month follow-up and was not powered to detect differences in alcohol use or surgical outcomes. We collected alcohol biomarkers to corroborate self-report but failed to obtain them for the full sample. This study was conducted within a single health system and the sample is predominantly white and non-Hispanic. The COVID-19 pandemic emerged mid-trial and influenced study feasibility.
In conclusion, this study examined two preoperative alcohol interventions of varying intensities, which were scalable, feasible, and acceptable for elective surgical care. Future research should evaluate the efficacy of these two interventions relative to a control condition in reducing alcohol use and surgical outcomes in an adequately powered randomized controlled trial.
Supplementary Material
Figure 1.
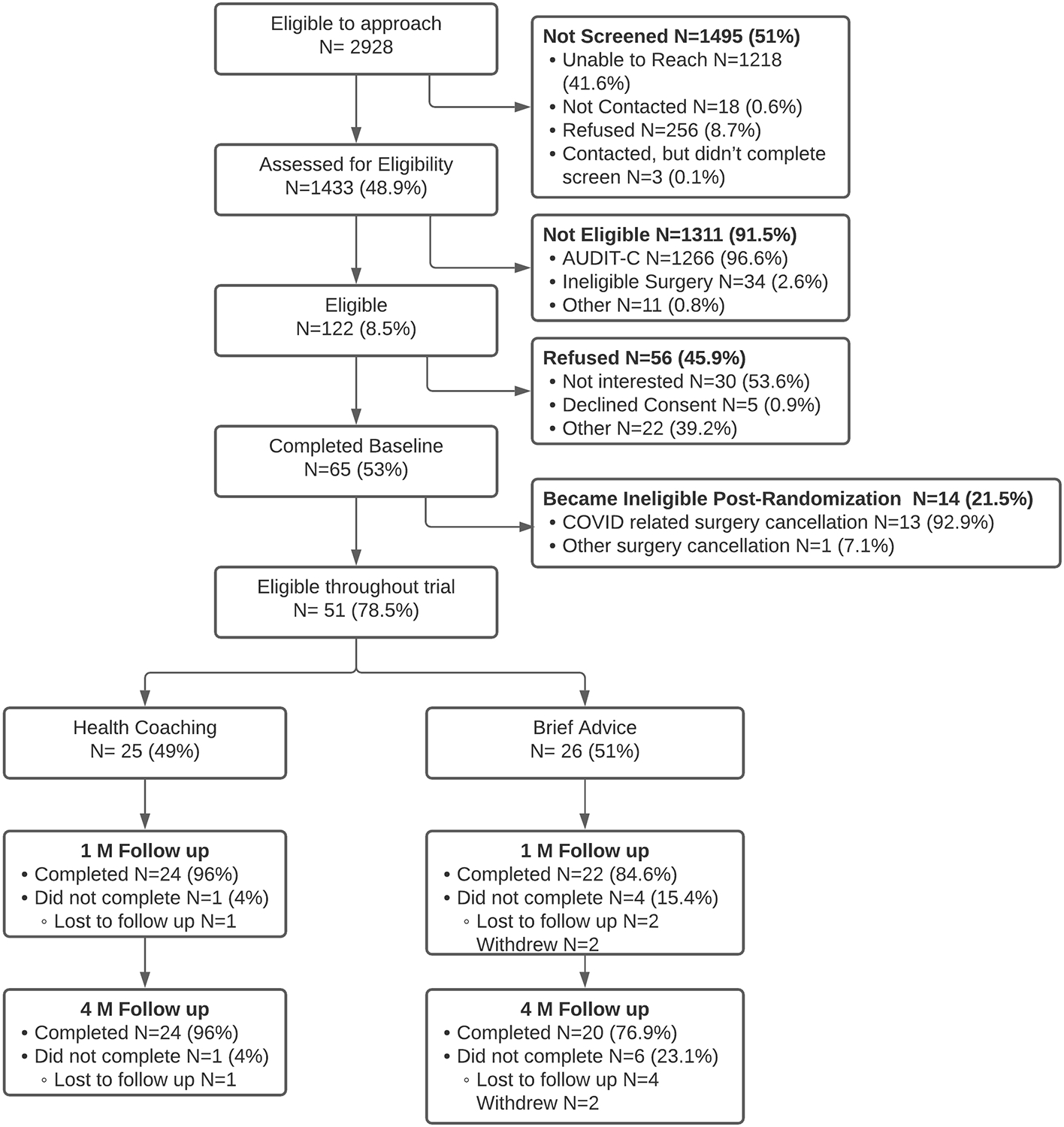
Participant Flowchart
FUNDING/FINANCIAL SUPPORT
Dr. Fernandez’s effort on this study was supported by a career development award from the National Institute of Alcohol Abuse and Alcoholism (K23AA023869). This award also supported participant incentives, study coordinator effort (Lyndsay Chapman), and research assistant effort (Tom Ren), and other study materials. Dr. Baxley’s effort was supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship in Mental Illness Research and Treatment, the San Francisco VA Health Care System, and the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center.
Footnotes
COI/DISCLOSURES
The authors have no related conflicts of interest to declare
REFERENCES
- 1.Harris AHS, Frey MS, Debenedetti AF, Bradley KA. Alcohol Misuse Prevalence and Associations with Post-Operative Complications in US Surgical Patients : A Review. Open J Surg. 2008;(2):50–8. [Google Scholar]
- 2.Rubinsky AD, Bishop MJ, Maynard C, Henderson WG, Hawn MT, Harris AH, et al. Postoperative risks associated with alcohol screening depend on documented drinking at the time of surgery. Drug Alcohol Depend. 2013. Oct 1;132(3):521–7. [DOI] [PubMed] [Google Scholar]
- 3.Bradley KA, Rubinsky AD, Sun H, Bryson CL, Bishop MJ, Blough DK, et al. Alcohol screening and risk of postoperative complications in male VA patients undergoing major non-cardiac surgery. J Gen Intern Med. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khuri SF, Henderson WG, Daley J, Jonasson O, Jones RS, Campbell D a, et al. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg [Internet]. 2008. Aug [cited 2013 Nov 12];248(2):329–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18650645 [DOI] [PubMed] [Google Scholar]
- 5.Harris AH, Reeder R, Ellerbe L, Bradley KA, Rubinsky AD, Giori NJ. Preoperative alcohol screening scores: association with complications in men undergoing total joint arthroplasty. J bone Jt surgeryAmerican Vol. 2011. Feb 16;93(4):321–7. [DOI] [PubMed] [Google Scholar]
- 6.Eliasen M, Grønkjær M, Skov-Ettrup LS, Mikkelsen SS, Becker U, Tolstrup JS, et al. Preoperative Alcohol Consumption and Postoperative Complications: A Systematic Review and Meta-analysis. Ann Surg [Internet]. 2013. May 31 [cited 2013 Nov 7];258(6). Available from: http://www.ncbi.nlm.nih.gov/pubmed/23732268 [DOI] [PubMed] [Google Scholar]
- 7.Tonnesen H, Petersen KR, Hojgaard L, Stokholm KH, Nielsen HJ, Knigge U, et al. Postoperative morbidity among symptom-free alcohol misusers. Lancet. 1992. Aug 8;340(8815):334–7. [DOI] [PubMed] [Google Scholar]
- 8.Delgado-Rodriguez M, Gomez-Ortega A, Mariscal-Ortiz M, Palma-Perez S, Sillero-Arenas M. Alcohol drinking as a predictor of intensive care and hospital mortality in general surgery: a prospective study. Addiction [Internet]. 2003. May [cited 2013 Nov 6];98(5):611–6. Available from: http://doi.wiley.com/10.1046/j.1360-0443.2003.00353.x [DOI] [PubMed] [Google Scholar]
- 9.American Society of Anesthesiologists. ASA Physical Status Classification System. 2014. [Google Scholar]
- 10.American College of Surgeons. Strong for Surgery [Internet]. [cited 2020 Aug 5]. Available from: https://www.facs.org/quality-programs/strong-for-surgery/about
- 11.Aarvold A, Crofts T. Missed opportunities? Management of patients with alcohol problems in a surgical ward. Health Bull (Raleigh) [Internet]. 2002. Jan;60(1):55–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12664770 [PubMed] [Google Scholar]
- 12.Tonnesen H, Faurschou P, Ralov H, Molgaard-Nielsen D, Thomas G, Backer V. Risk reduction before surgery. The role of the primary care provider in preoperative smoking and alcohol cessation. BMC Health Serv Res. 2010. May 12;10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez AC, Claborn KR, Borsari B. A systematic review of behavioural interventions to reduce preoperative alcohol use. Drug Alcohol Rev [Internet]. 2015. Sep [cited 2018 Nov 26];34(5):508–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26120973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egholm JW, Pedersen B, Møller AM, Adami J, Juhl CB, Tønnesen H. Perioperative alcohol cessation intervention for postoperative complications. Cochrane Database Syst Rev [Internet]. 2018. Nov 8 [cited 2018 Nov 12];(11). Available from: http://doi.wiley.com/10.1002/14651858.CD008343.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tønnesen H, Egholm JW, Oppedal K, Lauritzen JB, Madsen BL, Pedersen B. Patient education for alcohol cessation intervention at the time of acute fracture surgery: study protocol for a randomised clinical multi-centre trial on a gold standard programme (Scand-Ankle). BMC Surg [Internet]. 2015. May 1 [cited 2018 Mar 21];15:52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25925742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oppedal K, Møller A, Pedersen B, Tønnesen H. Preoperative alcohol cessation prior to elective surgery. Cochrane database Syst Rev. 2012;(7):CD008343. [DOI] [PubMed] [Google Scholar]
- 17.Fuller RK, Branchey L, Brightwell DR, Derman RM, Emrick CD, Iber FL, et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA [Internet]. 1986. Sep 19 [cited 2014 May 15];256(11):1449–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3528541 [PubMed] [Google Scholar]
- 18.Vahr Lauridsen S, Thomsen T, Thind P, Tønnesen H. STOP smoking and alcohol drinking before OPeration for bladder cancer (the STOP-OP study), perioperative smoking and alcohol cessation intervention in relation to radical cystectomy: study protocol for a randomised controlled trial. [cited 2018 Mar 26]; Available from: https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-017-2065-6?site=trialsjournal.biomedcentral.com [DOI] [PMC free article] [PubMed]
- 19.Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643–9. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez AC, Guetterman TC, Borsari B, Mello MJ, Mellinger JL, Tonnesen H, et al. Gaps in Alcohol Screening and Intervention Practices in Surgical Healthcare: A Qualitative Study. J Addict Med. 2020;15(2):113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Englesbe MJ, Grenda DR, Sullivan JA, Derstine BA, Kenney BN, Sheetz KH, et al. The Michigan Surgical Home and Optimization Program is a scalable model to improve care and reduce costs. Surg (United States). 2017. Jun 1;161(6):1659–66. [DOI] [PubMed] [Google Scholar]
- 22.Englesbe MJ, Lussiez AD, Friedman JF, Sullivan JA, Wang SC. Starting a Surgical Home. Ann Surg. 2015; [DOI] [PubMed] [Google Scholar]
- 23.Fernandez AC, Claborn KR, Borsari B. A Systematic Review of Behavioral Interventions to Reduce Preoperative Alcohol Use. Drug Alcohol Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almirall D, Compton SN, Gunlicks-Stoessel M, Duan N, Murphy SA. Designing a pilot sequential multiple assignment randomized trial for developing an adaptive treatment strategy. Stat Med [Internet]. 2012. Jul 30 [cited 2022 Aug 1];31(17):1887–902. Available from: https://pubmed.ncbi.nlm.nih.gov/22438190/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snowden C, Lynch E, Avery L, Haighton C, Howel D, Mamasoula V, et al. Preoperative behavioural intervention to reduce drinking before elective orthopaedic surgery: the PRE-OP BIRDS feasibility RCT. Health Technol Assess [Internet]. 2020. [cited 2021 Jul 14];24(12):1. Available from: /pmc/articles/PMC7086307/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley KA, Rubinsky AD, Sun H, Bryson CL, Bishop MJ, Blough DK, et al. Alcohol screening and risk of postoperative complications in male VA patients undergoing major non-cardiac surgery. J Gen Intern Med. 2011. Feb;26(2):162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley KA, Rubinsky AD, Sun H, Blough DK, Tønnesen H, Hughes G, et al. Prevalence of alcohol misuse among men and women undergoing major noncardiac surgery in the Veterans Affairs health care system. Surgery [Internet]. 2012. Jul [cited 2013 Oct 2];152(1):69–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22503319 [DOI] [PubMed] [Google Scholar]
- 28.Rubinsky AD, Sun H, Blough DK, Maynard C, Bryson CL, Harris AH, et al. AUDIT-C alcohol screening results and postoperative inpatient health care use. J Am Coll Surg. 2012. Mar;214(3):296–305.e1. [DOI] [PubMed] [Google Scholar]
- 29.Miller WR, Rollnick S. Motivational Interviewing: Preparing people for change. 2nd ed. New York: Guilford; 2002. [Google Scholar]
- 30.Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions [Internet]. Vol. 31, Annual Review of Public Health. Annu Rev Public Health; 2010. [cited 2020 Aug 3]. p. 399–418. Available from: https://pubmed.ncbi.nlm.nih.gov/20070207/ [DOI] [PubMed] [Google Scholar]
- 31.Janz NK, Becker MH. The Health Belief Model: A Decade Later. Heal Educ Behav [Internet]. 1984. Mar 4 [cited 2020 Aug 2];11(1):1–47. Available from: http://journals.sagepub.com/doi/10.1177/109019818401100101 [DOI] [PubMed] [Google Scholar]
- 32.Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol [Internet]. 2006. [cited 2013 Oct 2];41(6):643–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16905552 [DOI] [PubMed] [Google Scholar]
- 33.Moyers TB, Rowell LN, Manuel JK, Ernst D, Houck JM. The Motivational Interviewing Treatment Integrity Code (MITI 4): Rationale, preliminary reliability and validity. J Subst Abuse Treat [Internet]. 2016. Jun 1 [cited 2021 Dec 16];65:36. Available from: /pmc/articles/PMC5539964/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallgren KA. Computing Inter-Rater Reliability for Observational Data: An Overview and Tutorial. Tutor Quant Methods Psychol [Internet]. 2012. Feb 1 [cited 2021 Dec 16];8(1):23. Available from: /pmc/articles/PMC3402032/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med [Internet]. 1998. Sep 14 [cited 2014 Mar 13];158(16):1789–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9738608 [DOI] [PubMed] [Google Scholar]
- 36.Sobell LC, Sobell MB. Timeline Follow-Back. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial And Biochemical Methods [Internet]. Totowa, NJ: Humana Press; 1992. [cited 2017 Jan 25]. p. 41–72. Available from: http://link.springer.com/10.1007/978-1-4612-0357-5_3 [Google Scholar]
- 37.Schrock A, Thierauf-Emberger A, Schurch S, Weinmann W. Phosphatidylethanol (PEth) detected in blood for 3 to 12 days after single consumption of alcohol-a drinking study with 16 volunteers. Int J Legal Med. 2017. Jan;131(1):153–60. [DOI] [PubMed] [Google Scholar]
- 38.Afshar M, Burnham EL, Joyce C, Clark BJ, Yong M, Gaydos J, et al. Cut-Point Levels of Phosphatidylethanol to Identify Alcohol Misuse in a Mixed Cohort Including Critically Ill Patients. Alcohol Clin Exp Res [Internet]. 2017. Oct [cited 2019 Jun 4];41(10):1745–53. Available from: http://doi.wiley.com/10.1111/acer.13471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cherrier MM, Shireman LM, Wicklander K, Yeung W, Kooner P, Saxon AJ, et al. Relationship of Phosphatidylethanol Biomarker to Self-Reported Alcohol Drinking Patterns in Older and Middle-Age Adults. Alcohol Clin Exp Res. 2020. Dec 1;44(12):2449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.RZ L, AM B, HB M. Alcohol biomarkers in applied settings: recent advances and future research opportunities. Alcohol Clin Exp Res [Internet]. 2010. [cited 2021 Aug 18];34(6):955–67. Available from: https://pubmed.ncbi.nlm.nih.gov/20374219/ [DOI] [PubMed] [Google Scholar]
- 41.Clinical Data Abstraction – Michigan Surgical Quality Collaborative [Internet]. [cited 2022 Aug 5]. Available from: https://msqc.org/member-resources/clinical-data-abstraction/
- 42.Kroenke K, Yu Z, Wu J, Kean J, Monahan PO. Operating Characteristics of PROMIS Four-Item Depression and Anxiety Scales in Primary Care Patients with Chronic Pain. Pain Med [Internet]. 2014. Nov 1 [cited 2022 Jun 28];15(11):1892–901. Available from: https://academic.oup.com/painmedicine/article/15/11/1892/1835664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez AC, Waller R, Walton MA, Bonar EE, Ignacio RV., Chermack ST, et al. Alcohol use severity and age moderate the effects of brief interventions in an emergency department randomized controlled trial. Drug Alcohol Depend [Internet]. 2019. Jan [cited 2019 May 28];194:386–94. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0376871618308019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tonnesen H, Nielsen PR, Lauritzen JB, Moller AM. Smoking and alcohol intervention before surgery: evidence for best practice. Br J Anaesth. 2009. Mar;102(3):297–306. [DOI] [PubMed] [Google Scholar]
- 45.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: State of the science and challenges for research [Internet]. Vol. 98, Addiction. Blackwell Publishing Ltd; 2003. [cited 2020 Jul 19]. p. 1–12. Available from: https://onlinelibrary.wiley.com/doi/full/10.1046/j.1359-6357.2003.00586.x [DOI] [PubMed] [Google Scholar]
- 46.D GN, K A, A SN, C J, A M. Consistency between self-reported alcohol consumption and biological markers among patients with alcohol use disorder - A systematic review. Neurosci Biobehav Rev [Internet]. 2021. May 1 [cited 2021 Aug 18];124:370–85. Available from: https://pubmed.ncbi.nlm.nih.gov/33581224/ [DOI] [PubMed] [Google Scholar]
- 47.Pedersen B, Alva-Jørgensen P, Raffing R, Tønnesen H. Fractures and alcohol abuse - patient opinion of alcohol intervention. Open Orthop J [Internet]. 2011. Jan 7 [cited 2019 Aug 13];5:7–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21464911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauridsen SV, Thomsen T, Kaldan G, Lydom LN, Tønnesen H. Smoking and alcohol cessation intervention in relation to radical cystectomy: a qualitative study of cancer patients’ experiences. BMC Cancer [Internet]. 2017. Dec 25 [cited 2019 Feb 8];17(1):793. Available from: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-017-3792-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin LA, Fernandez AC, Bonar EE. Telehealth for Substance-Using Populations in the Age of Coronavirus Disease 2019: Recommendations to Enhance Adoption [Internet]. Vol. 77, JAMA Psychiatry. American Medical Association; 2020. [cited 2021 May 25]. p. 1209–10. Available from: https://www.samhsa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy SA, Lynch KG, Oslin D, McKay JR, TenHave T. Developing adaptive treatment strategies in substance abuse research. Drug and Alcohol Dependence. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med [Internet]. 2007. May [cited 2018 Mar 28];32(5 Suppl):S112–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17466815 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


