Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by progressive and irreversible airflow obstruction with abnormal lung function. Because its pathogenesis involves multiple aspects of oxidative stress, immunity and inflammation, apoptosis, airway and lung repair and destruction, the clinical approach to COPD treatment is not further updated. Therefore, it is crucial to discover a new means of COPD diagnosis and treatment. COPD etiology is associated with complex interactions between environmental and genetic determinants. Numerous genes are involved in the pathogenic process of this illness in research samples exposed to hazardous environmental conditions. Among them, Long non-coding RNAs (lncRNAs) have been reported to be involved in the molecular mechanisms of COPD development induced by different environmental exposures and genetic susceptibility encounters, and some potential lncRNA biomarkers have been identified as early diagnostic, disease course determination, and therapeutic targets for COPD. In this review, we summarize the expression profiles of the reported lncRNAs that have been reported in COPD studies related to environmental risk factors such as smoking and air pollution exposure and provided an overview of the roles of those lncRNAs in the pathogenesis of the disease.
Keywords: Chronic Obstructive Pulmonary Disease (COPD), Long non-coding RNAs (lncRNAs), Environmental exposure, Air pollution, Cigarette smoke
1. Introduction
With the climate crisis, aging population, deepening industrialization, and changes in lifestyles around the world, people in both developing and developed countries are plagued by chronic respiratory diseases [1,2]. One of the most frequent chronic respiratory disorders is chronic obstructive pulmonary disease (COPD). As of 2019, researchers predicted that over 391 million persons aged 30–79 globally have COPD, with a global incidence of 10.3 % [3]. In 2019, COPD was the third leading cause of death globally [4]. In China, the incidence and mortality rates of COPD are also high, making further research into COPD an urgent task for researchers [5,6].
Long non-coding RNAs (lncRNAs) are a class of linear RNA molecules that are longer than 200 nucleotides and have little to no capacity to code for proteins. Their structure is comparable to that of mRNA (with a 5′ cap structure and a polyA tail at the 3′ end in certain lncRNAs) [7,8]. Researchers' understanding of lncRNAs has increasingly expanded as high-throughput sequencing technologies and bioinformatics platforms have advanced. Growing evidence reveals that lncRNAs can influence gene expression at several levels, including epigenetic, transcriptional, and post-transcriptional regulation [[7], [8], [9], [10], [11]].
With the deepening of research, the presence of lncRNAs has been found in the plasma, serum, sputum, bronchial lavage fluid, and lung tissue of COPD patients [[12], [13], [14], [15]]. Increasing evidence suggests that lncRNAs may become a new generation of biomarkers for COPD and have profound research significance. In this paper, we evaluated the lncRNAs engaged in COPD-related research that were produced by environmental variables such as smoking and air pollution, and we discussed their implications in the pathogenesis of COPD.
2. Overview and clinical management of COPD
Smoking and air pollution are the primary environmental causes of COPD, which is a disease that is brought on by environmental and genetic risk factors [3]. Simply calling it COPD may seem too general, as the abnormalities exhibited by COPD may be due to multiple pathophysiological factors [16] and have multiple endogenous phenotypes and clinical presentations [17]. It is currently believed that at least several mechanisms are involved in the pathogenesis of COPD, including inflammatory responses, oxidative stress, and abnormal apoptosis of lung structural cells [18]. (Fig. 1). The activation of immune cells (Table 1) observed in various clinical trials of COPD and the abnormal apoptosis of lung structural cells all confirm this point. These mechanisms involve multiple signaling pathways, accelerating the natural physiological decline of the lungs that should occur with age, leading to persistent and irreversible airway obstruction [19].
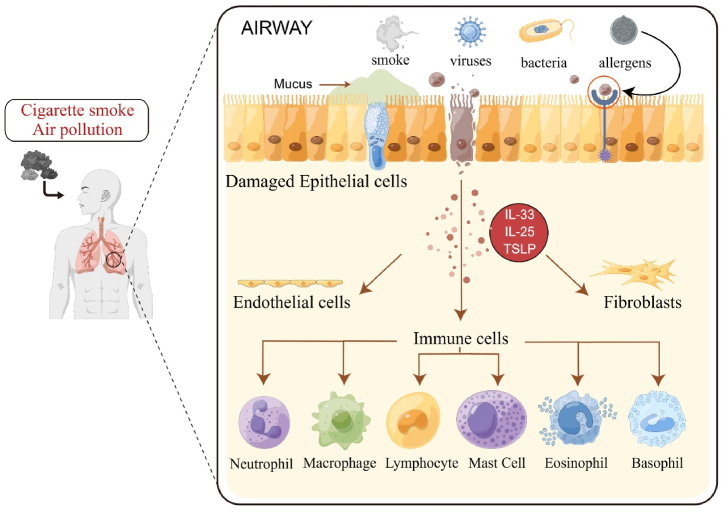
Fig. 1.
Diagrammatic representation of COPD etiology and pathogenesis. Risk factors for COPD include smoking and air pollution, and so on. When irritants and toxicants are inhaled, the structural cells of the lung, such as epithelial cells, fibroblasts, and endothelial cells, get activated. When bronchial epithelial cells are damaged, alarm molecules (TSLP, IL-33, IL-25) are released, activating a variety of immune cells, endothelial cells, and fibroblasts. These cells release inflammatory mediators, which attract other inflammatory cells such as neutrophils, macrophages, and lymphocytes to the exposed area, resulting in persistent airway inflammation.
Table 1.
Activation of immune cells in COPD patients.
| Inflammatory Cells | Expression | Location | Downstream Targets | References |
|---|---|---|---|---|
| Neutrophils | + | COPD patients' bronchoalveolar lavage (BAL) fluid and sputum | CXCL8 | [17] |
| Macrophages | + | COPD patients' sputum, BAL fluid, and lung parenchyma | cytokines (TNF-α), chemotactic factors [CXCL1, CXCL8, CCL2, LTB4] and ROS | [30] |
| Lymphocytes (CD8+ and CD4+) | + | COPD patients' lung parenchyma, bronchi, and bronchioles | IL-17A and IL-22 | [31,32] |
| Mast Cells | + | human lung parenchyma | VEGF-A and FGF-2 | [33,34] |
| Eosinophils | + | Stable COPD patients' airways and BAL fluid | VEGF-A, FGF-2, TNF-α, GM-CSF, nerve growth factor (NGF), and CXCL8 | [35,36] |
| Basophils | + | COPD patient's lung tissue | VEGF-A and ANGPT1 | [35,37] |
Despite the heterogeneity of COPD, progressive and irreversible airflow limitation with abnormal lung function is a common feature of COPD [20,21].Clinical diagnosis approaches have not altered considerably as a result of this trait, and still rely nearly exclusively on post-bronchodilator spirometry, which is the most often used diagnostic strategy [22]. Although widely used, this approach also has its limitations, including insensitivity to early pathological changes, underutilization, frequent misinterpretation, and inability to predict symptoms. In addition, most of the treatment strategies for COPD use palliative care, that is, to directly improve airflow limitation. Inhaled medications, which include bronchodilators and corticosteroids, are the backbone of COPD management [23], especially in the acute exacerbation of COPD [24]. However, relieving airway limitation in AECOPD patients by drugs remains difficult. Furthermore, the side effects of these treatments must not be overlooked, such as the frequent use of inhaled corticosteroids, which increases the risk of osteoporosis, pneumonia, immunosuppression, and infection [25,26], considerably lowering patients' quality of life.
Alpha-1 antitrypsin deficiency which is the only known genetic cause of COPD [27]. It is worth mentioning that AAT intensive therapy is also a treatment method for COPD. Currently available intensive AAT therapies include lifestyle modification, asthma and COPD drug management, oxygen therapy, influenza vaccine and pneumococcal vaccine combination, and polysaccharides [28]. The only particular medication that has been approved for AAT-deficient patients who have symptoms of respiratory illness is augmentation therapy with intravenous pure AAT [29]. However, considering the cost of treatment and the limitations of the applicable population, intensive treatment with AAT has not been widely used.
Therefore, we need new biomarkers to assess the progression of COPD, identify and diagnose high-risk patients for treatment, and develop specific targeted drugs to improve the condition of COPD patients. Fortunately, with the development of molecular biology and the deepening understanding of lncRNAs, developing targeted drugs for treating COPD from the perspective of lncRNAs is no longer just a pipe dream.
3. The expression of lncRNA in COPD samples under different environmental risk factors
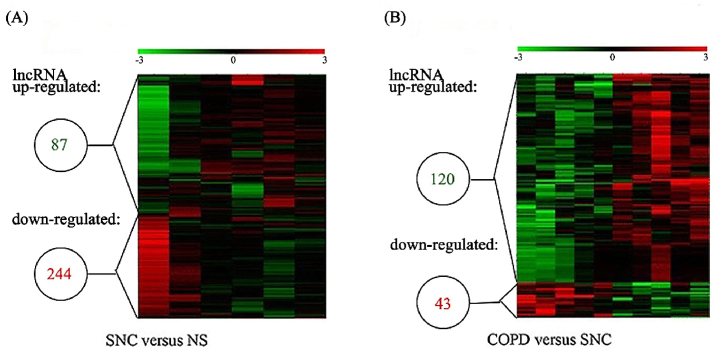
COPD is a complex disease characterized by hereditary and environmental risk factors. Cigarette smoking (CS) and air pollution are the most prevalent environmental pathogenic causes among the multiple environmental impacts [38]. Exposure to Cigarette smoke (CSE) is the major risk factor for induction of COPD. In a genome-wide lncRNA expression analysis study [39,40], RNA was extracted from lung tissues (all with lung cancer) from nonsmokers without COPD(NS), smokers without COPD(SNC), and smokers with COPD, and compared using microarray analysis, and the results were compared. It was found that compared to non-COPD smokers, COPD patients had 120 lncRNAs with high expression and 43 lncRNAs with low expression, with some lncRNAs possibly implicated in the control of the expression of cellular mediators and signaling pathways relevant to immune system alterations in COPD. (Fig. 2).
Fig. 2.
Heat maps displaying the differential expression and hierarchical clustering of lncRNAs in (A) smokers with no COPD (SNC) compared with non-smokers without COPD (NS) and in (B) COPD patients compared with SNC. Reprinted with permission from Ref. [39]. Copyright (2015) Springer Link.
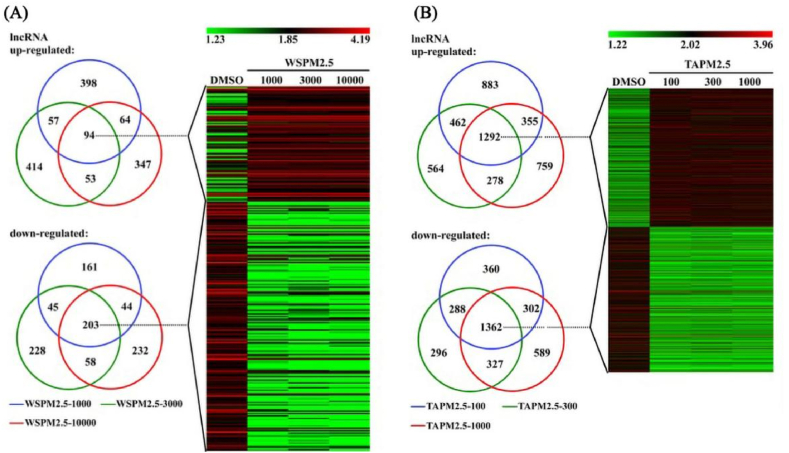
Smoking causes COPD in most patients, but it is now recognized that around 30 % of all COPD cases in the world occur in never-smokers [41]. The impact of air pollution cannot be ignored. Similar to CS-induced lung disease, air pollution also causes inflammation and oxidative stress. However, air pollution can cause more bronchial involvement, with less change in emphysema [42], whereas CS particles have a higher deposition rate in the lungs, which also explains why smoking is associated with emphysema [43]. In response to air pollution, researchers explored the effect of traffic-related PM2.5 (TAPM2.5) and wood smoke particles (WSPM2.5) on the expression of lncRNA in human bronchial epithelial cells (HBECs) and performed microarray analysis on the HBECs [44]. They discovered that in cells treated with WSPM2.5 and TAPM2.5, respectively, 94 and 1292 lncRNAs were upregulated and 203 and 1362 lncRNAs were downregulated in comparison to the control group. (Fig. 3). By comparing the differences in lncRNA expression between the two, we may be able to identify more different pathological responses in COPD patients under different environmental exposure conditions, which may lead to better-targeted treatments.
Fig. 3.
Heat maps displaying the differential expression and hierarchical clustering of lncRNAs in (A) WSPM2.5 and (B) TAPM2.5 concentrations stimulation. Reprinted with permission from Ref. [44]. Copyright (2018) Portland Press.
4. The role of lncRNA in COPD pathology under different environmental risk factors
Whether in vitro or clinical studies, researchers have found that many functional lncRNAs interact with COPD-related genes and perform multiple physiological functions involved in the pathogenesis of COPD through multiple pathways, including inflammatory response [[45], [46], [47], [48], [49]], oxidative stress [48,50,51] and apoptosis [45,48,[52], [53], [54]], etc. However, their functions in COPD disease progression under different environmental risk factors remain to be elucidated.
4.1. The role of lncRNA in the pathogenesis of CS-induced COPD
4.1.1. Cs-induced COPD inflammatory response and the role of lncRNA in it
COPD is a classic inflammatory disease, and as mentioned earlier, one of its pathogenetic features is the activation of inflammatory cells in the alveoli, including increased numbers of macrophages, neutrophils, and eosinophils. Typically, when inhaling harmful particles such as CS, inflammatory cells will enter the respiratory tract and lungs, leading to varying degrees of inflammation [55]. Although it is unclear how CSE affects the mechanisms of inflammation generation in COPD and what associations exist, CSE-induced changes in the expression of some lncRNAs have been found to be associated with the dysregulation of various immune cells in the lung, suggesting that CSE can act as a driving factor for lncRNA regulation of inflammation and play an indelible role.
LASI lncRNA expression is upregulated in the airways of CSE macaques and COPD smokers, which is associated with high mucus expression and mucus cell proliferation in severe COPD [56]. Mucus is the reaction product of innate and adaptive inflammatory responses made by the organism at the airway level to combat toxic gases and particles and is a complex viscoelastic gel [57]. The number and size of bronchial mucus glands, the amount of mucus in the airway epithelium, the proliferative activity of epithelial cells, and the development of squamous cell chemosis are all increased as a result of the infiltration of inflammatory cells into lung tissue and their involvement in the damage and repair of lung structural cells in COPD. The inflammatory exudate containing mucus obstructs the airway lumen and the repair process of the tissue thickens the wall, thus narrowing the diameter of the conducting airway [58], which is consistent with airflow limitation in COPD patients. lnc-IL7R expression is reduced in association with airway epithelial cell inflammation, suggesting impaired lung function, the pathogenesis of COPD, and predicting COPD progression [59].
It has now been shown that LINC00612 and HOXA-AS2 are significantly downregulated in COPD lung tissue. Regulation of HPMECs proliferation through Notch1 is involved in the pathogenesis of COPD, including CSE-induced apoptosis, inflammation, and oxidative stress in endothelial cells [48,60]. Most critically, Notch signaling has been linked to smoking and COPD and is essential for lung homeostasis, damage, and repair [61].
It is well documented that the lncRNA-miRNA-mRNA axis is important in the development of COPD. LncRNAs act as sponges for miRNAs and influence gene expression by competitively binding them. As a ceRNA for miR18a-5p, serum CASC2 was much lower in COPD patients than in non-COPD smokers, and in 16HBE cells, overexpression of miR-18a-5p reversed CASC2's effects on apoptosis and inflammation [62]. Meanwhile, the expression of CCAT1 was significantly increased in CS-induced HBE cells [63], which has piqued the interest of researchers to explore deeper mechanisms between CCAT1 and COPD, CCAT1 was highly expressed in HBE cells from COPD patients treated with CSE, and CCAT1 knockdown alleviated the inflammatory response of HBE cells [13]. An abnormal increase of MIR155HG was also found in lung tissues of smokers. In smoking-associated COPD HPMECs, MIR155HG upregulates the miR-128-5p/BRD4 axis to promote apoptosis and inflammatory responses [54]. There are many more examples of the same, which are summarized in Table 2.
Table 2.
lncRNAs associated with COPD.
| LncRNA | Expression | Location | Action | Targeted Pathway | Downstream Targets | References |
|---|---|---|---|---|---|---|
| ANRIL | Down | plasma of AECOPD patients | Anti-inflammatory | – | TNF, IL1B, IL17A, CXCL8 | [12] |
| CCAT1 | Up | lungs of COPD patients and CSE-treated HBE cells | MircoRNA sponging and epigenetic modification | miR-152-3p | – | [13] |
| GAS5 | Up | Human lung fibroblast cell line | Involved in the regulation of pyroptosis | miR-223-3p | NLRP3 | [15] |
| Down | lung of mice exposed to CS | Suppress airway inflammation and fibroblast activation | miR-217-5p | PTEN | [64] | |
| H19 | Up | quadriceps of FFMI patients with COPD | Susceptibility to low FFMI | – | MYOD1 | [65,66] |
| HOTAIR | Up | lungs of COPD patients and CSE-treated HPVEC | aggravate the apoptosis of CSE HPVEC | – | DNMT1 | [67] |
| Up | male BALB/c mice exposed to CS and CSE-treated HBE cells | – | – | IL6, CXCL8, CDH2, VIM, ACTA2, CDH1 | [68] | |
| IL7R | Down | serum from COPD patients |
Protected against cellular senescence and apoptosis | – | EZH2 recruitment | [52,59,69] |
| LASI | Up | CS-exposed macaques and airways of COPD smokers | Increase the mucoinflammatory response | – | secretory mucin MUC5AC. and inflammatory factors, ICAM-1, and IL-6 |
[56,70] |
| LUCAT1 | Up | serum from COPD patients |
Promoted CSE-induced 16HBE cells apoptosis and inhibited proliferation | miR- 181a- 5p | Wnt/β-catenin | [71,72] |
| MEG3 | Up | lungs of COPD patients and CSE-treated HBE cells | Induces apoptosis and inflammation | Apoptosis | IL1B, IL6, TNF | [73] |
| MHC-R | Up | lungs of rats exposed to air pollution PM | Regulate the immune activities of DCs | – | GATA3 | [74] |
| MIAT | Up | lung of mice exposed to CS | Knockdown of MIAT attenuates CS-induced airway remodeling | miR-29c-3p | HIF3A | [53] |
| TUG1 | Up | lungs and sputum from smokers and non-smokers with COPD | Inhibits inflammation and airway remodeling | – | DUSP6 | [75] |
| TGFB1 treated BEAS-2B and HFL1 cells | Inhibits cell proliferation | – | ACTA2, FN1 | [14] | ||
| SNHG5 | Down | COPD tissues and CSE-treated HBE cells | Repress cell apoptosis, proliferation, and inflammation | miR-132 | PTEN | [76] |
| LINC00612 | Down | COPD tissues and CSE-treated HBE cells | Repress cell apoptosis, inflammation, and oxidative stress | miR-31-5p | Notch1 | [48] |
| HOXA-AS2 | Down | COPD tissues and CSE-treated HBE cells | Repress cell apoptosis | – | Notch1 | [60] |
| MIR155HG | Up | Lung tissues of smokers without or with COPD | Contributed to the apoptosis and inflammation | miR-128-5p | BRD4 | [54] |
| Nqo1-AS1 | Up | The cytoplasm of mouse alveolar epithelium | Attenuated CS-induced oxidative stress | – | Nqo1 | [50] |
| LINC00987 | Up | COPD tissues | Repress cell apoptosis, inflammation, and oxidative stress | let-7b-5p | SIRT1 | [51] |
| PVT1 | Up | peripheral blood mononuclear cells (PBMCs), and serum of COPD patients | – | miR-146a | – | [77] |
| RP11-86H7.1 | Up | TRAPM2.5 exposed HBE cells | – | miR-9-5p | NFKB1/NF-κB | [47] |
| NEAT1 | Up | plasma of COPD patients | – | miR-193a | – | [78] |
4.1.2. Cs-induced COPD oxidative stress and the role of lncRNA in it
CS contains a large number of harmful chemicals, including high concentrations of free radicals and oxidants [79,80]. These high concentrations of free radicals and exogenous oxidants are released in the respiratory tract and bronchial epithelium, producing large amounts of reactive oxygen species (ROS). While this chemical has the advantage of being involved as a signaling component in maintaining intracellular homeostasis [81] but excessive intracellular accumulation may lead to oxidative imbalance, altered dynamic homeostasis, and subsequent cell death. Oxidative stress occurs when the production of ROS exceeds the levels allowed by the body's antioxidant defense mechanisms [82]. An association between oxidative stress and the skeletal muscle dysfunction common in COPD patients was proposed in an earlier study [79], that is, it may be caused by an imbalance in the redox environment of skeletal muscle. This suggests, in part, that the imbalance in the redox environment in COPD patients extends beyond the diseased lung and is likely to extend throughout the body.
Vascular endothelial growth factor (VEGF) may also be downregulated by oxidative stress [83]. Notably, it is frequently overexpressed in chronic inflammatory conditions [84], which is connected to a decline in lung function and the death of structural lung cells such alveolar endothelial cells [85].
As research progresses, the role of lncRNAs in oxidative stress in COPD is gradually being discussed. Among them, lncRNA Nqo1-AS1 was shown to lessen CS-induced oxidative stress by enhancing Nqo1 expression and boosting Nqo1 mRNA stability through antisense pairing with Nqo1 3′UTR because of its particular relationship with Nqo1, a multifunctional antioxidant enzyme that is essential for preventing oxidative damage to cells [50]. It has also been mentioned that LINC00612 [48], and LINC00987 [51]. However, unfortunately, there is a lack of data on the effects of COPD pathogenesis in vivo, which needs to be explored in more depth.
4.1.3. Cs-induced COPD in apoptosis and remodeling of lung structural cells and role of lncRNA in it
Among the diseases related to the lung, apoptosis is often considered to be related to emphysema and plays a crucial role in the course of COPD patients. It is inextricably linked to inflammation and oxidative stress. In CS-induced COPD samples, the initiation of apoptosis in lung structures is often accompanied by an inflammatory response and oxidative stress [67,86]. Low or short exposure to CS induces apoptosis, while high or prolonged exposure can lead to necrosis [87]. Although it is unclear from current research what role apoptosis has in the pathophysiology of COPD, it has been demonstrated that several lncRNAs are crucial in the imbalance between apoptosis and remodeling of lung structural cells in COPD samples exposed to CS. Bcl-2 is a widely accepted anti-apoptotic regulator, and it has been shown that reduced Bcl-2/Bax induces apoptosis through the release of cytoplasmic C from mitochondria release of cytoplasmic cytosolic C causes apoptosis [88]. Researchers discovered apoptosis in HPVEC cells from emphysema mice models and HPVEC from CSE-induced cells that had their expression of Bcl-2 downregulated and their levels of DNMT1, Bax, and cleaved cysteinase 3 upregulated [67]. Meanwhile, HOTAIR expression levels were upregulated in the lungs of COPD patients and CSE-induced HPVEC. In mice, HOTAIR knockdown could reduce emphysema and apoptosis through DNMT1-mediated hypermethylation of the Bcl-2 promoter.
The long non-coding RNA (lncRNA) growth arrest specific 5 (GAS5) is a well-known tumor suppressor linked to cell development and demise in a number of disorders. lncRNA GAS5 stimulates COPD inflammation by concentrating on the miR-223-3p/NLRP3 axis [15]. MIAT binds to miR-29c-3p and upregulates hypoxia-inducible factor 3α (HIF3A), which is involved in CS-induced EMT and COPD airway remodeling [53]. Knockdown of OIP5-AS1 promotes cell recovery from CSE injury by stimulating cell viability, regulating apoptosis, and alleviating inflammatory conditions via miR-410-3p and IL-13 [86]. By targeting the miR-18a-5p/IGF1 axis [62], overexpression of CASC2 may reduce inflammation and apoptosis in bronchial epithelial cells. These results suggest, at least in part, that CSE-induced dysregulation of lncRNAs contributes to the development of COPD by aberrantly regulating downstream targets, leading to an imbalance between apoptosis and replenishment of lung structural cells.
4.2. The role of lncRNA in the pathogenesis of air pollution-induced COPD
However, not all smokers develop COPD, and at least 20–30 % of COPD patients never smoke [89], which may be due to a variety of factors, but it is undeniable that air pollution must account for a large part of them. The pathogenic consequences of air pollution on the lung are comparable to those of CS in that they are mediated by inflammatory pathways and include oxidative stress and apoptosis of lung tissues once more [90]. Harmful airborne particles or gases are a major culprit in inducing COPD, especially when exposed to higher concentrations of particulate matter (PM), especially PM2.5, and are strongly associated with increased COPD prevalence and respiratory dysfunction [91].
As hazardous particles enter the airway, they initially make contact with bronchial epithelial cells. This causes alterations in the bronchial epithelial phenotype and prompts the release of significant amounts of cytokines and inflammatory mediators from these cells. This release sets off a chain of inflammatory events that worsens lung damage and airway inflammation. lncRNA RP11-86H7.1 promotes TRAPM2's inflammatory response. Since forced expression of lncRNARP11-86H7.1 decreases the production of miR-9-5p, which releases NFKB1 and maintains the activation of NF–B, 5 stimulated bronchial epithelial cells by functioning as a ceRNA for miR-9-5p [47]. With the upregulation of PM2.5 concentration in the air, lncRNA IL7R is exposed in normal cells, and although it is not known whether the persistent oxidative stress in COPD patients is involved in this response, it is possible to obtain the result that its expression is negatively correlated with the concentration of emphysema and PM2.5 [52]. Additionally, air pollution-exposed rats' lung tissue had considerably higher levels of the lncRNA MHC-R, with particular expression in immune-related cells, antigen presentation, and adaptive immune response [74].
Given the limited number of studies on the association between air pollution-induced lncRNAs and COPD, the mechanisms by which the environment affects differential lncRNA expression in COPD patients are unclear. However, as demonstrated above by the differential expression profiles of lncRNAs in air pollution34, this is a neglected area that needs to be explored by more researchers.
4.3. The role of lncRNA in AECOPD
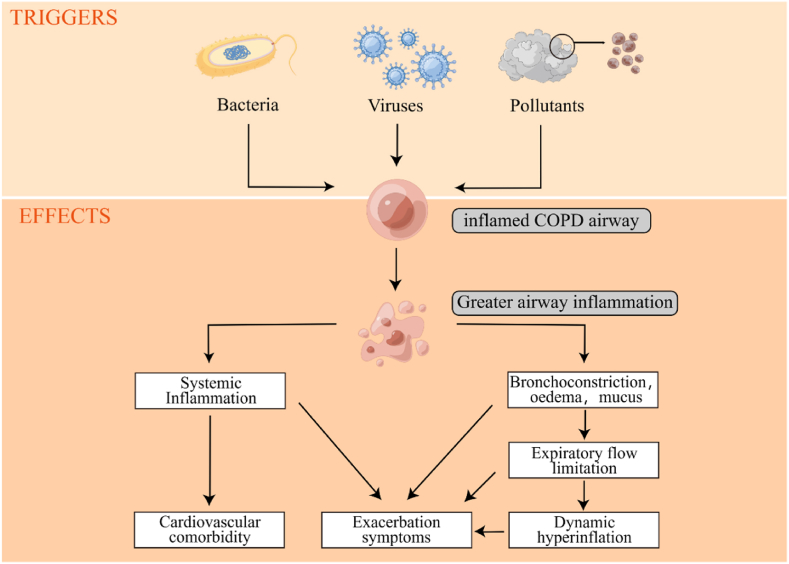
Acute exacerbations of COPD(AECOPD) are known as exacerbations, and such episodes account for a large proportion of patient morbidity and mortality [92]. (Fig. 4). They have a prognosis that is comparable to many stage III or IV solid organ cancers and are linked to rapid loss of lung function, long-term quality of life degradation, and these symptoms [93]. However, the definition of acute exacerbations is sometimes ambiguous, and their intensity is determined more by the location of therapy than by the severity of a physiologic illness that underlies it. Chronic airway respiratory diseases, COPD and asthma have some degree of commonality in their course progression. And in terms of clinical practice, lnc-PVT1 has an excellent ability to differentiate between patients with severe asthma and non-severe asthma [94] The researchers studied lnc-PVT1 in relation to the commonalities between asthma and COPD and found that it can also differentiate COPD patients from HCs (healthy controls) and AECOPD patients from stable COPD patients [77].
Fig. 4.
Triggers of COPD exacerbations and associated pathophysiological changes leading to increased exacerbation symptoms.
In addition, similar to lnc-PVT1, lncRNAs involved in COPD disease course by targeting mRNAs are lnc-NEAT1 [78], whose expression is positively correlated with disease severity, while the expression of the miRNAs they target is inversely associated to the GOLD stage and inflammatory cytokine levels. Although LncRNA ANRIL was also used to predict disease susceptibility and risk of acute exacerbation in COPD, the difference was that lncRNA ANRIL expression was inversely linked with pro-inflammatory cytokines in AECOPD patients and stable COPD patients, and with GOLD stage in AECOPD patients, but not in stable COPD patients [12]. These findings suggest that these reported lncRNAs may be used not only to detect the severity of COPD but also as possible therapeutic targets for COPD.
5. The role of lncRNA in genetic susceptibility to COPD
The chronic obstructive pulmonary disease itself is a highly heterogeneous disease with complex and diverse etiologies. Studies have found that approximately 40 % of the variability in airflow limitation and up to 60 % of the risk of smoking-related COPD is associated with genetic variation [95]. Numerous genetic variations that affect COPD risk, decreased lung function, and other COPD-related traits have been discovered through genetic association studies [96]. Other pulmonary features are linked to these genetic variations. These genetic variations have been linked to various pulmonary and non-pulmonary features, indicating a genetic foundation for part of the COPD heterogeneity that significantly affects the overall risk of developing the disease [97].
Alpha-1 antitrypsin deficiency (AATD) was the first genetic risk factor for chronic obstructive pulmonary disease (COPD) described [98] and is inherited in an autosomal co-dominant fashion. The alpha-1 antitrypsin molecule is a serine protease inhibitor that is predominantly produced in the liver. Its most important physiologic functions are the protection of pulmonary tissue from aggressive proteolytic enzymes and regulation of pulmonary immune processes [99]. At present, some researchers have reviewed the role of lncRNA before and after intensive treatment of AATD [100].
However, as research has progressed, COPD has been considered to be a self-induced disease resulting from external stimuli in genetically susceptible individuals. Recent studies have shown the upregulation of p16 expression, a marker of cellular senescence, in CSE-stimulated endothelial progenitor cells (EPC) and in COPD patients [101]. Cellular senescence is involved in the development of COPD, i.e. we can deduce the role of lncRNA in genetic susceptibility to COPD by reversing the genes associated with cellular senescence. IL7R is associated with cellular senescence-associated genes p16INK4a and p21CIP1/WAF1, and surprisingly Lnc-IL7R levels are decreased in COPD patients, whereas higher lnc-IL7R expression can prevent cellular senescence and apoptosis, while lower lnc-IL7R expression exacerbated PM2.5-treated cellular damage [69]. (Fig. 5).
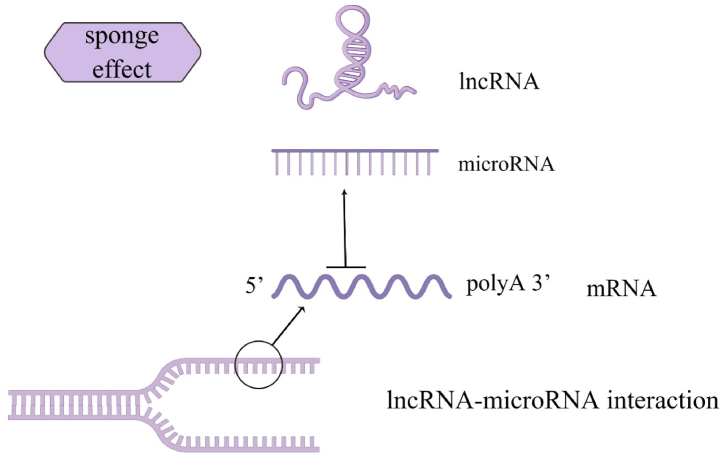
Fig. 5.
MicroRNAs may be captured by lncRNAs like a sponge. By attaching to these microRNAs, they stop microRNAs from attaching to their target mRNAs, eliminating post-transcriptional control.
6. Summary
A complex illness, COPD is brought on by hereditary and environmental risk factors. Few studies, however, have been able to identify the pathophysiology and susceptibility of COPD. The diagnosis of lung illness is receiving more attention as a result of COVID-19's continued and widespread occurrence. One of the most common comorbidities of COVID-19 disease progression is COPD [102]. Clinical symptoms of COPD vary, and it might be challenging to identify any apparent patterns. Determining the pathophysiology of COPD and developing precise diagnosis techniques and preventative plans are therefore crucial and urgent concerns for the present and the future.
Numerous in vitro and in vivo studies have demonstrated that environmental exposures like CS and air pollutants can disrupt the expression of lncRNAs in various samples from COPD and non-COPD patients, interfering with their regulation of COPD-related genes in crucial signaling pathways in COPD pathogenesis, including the imbalance between inflammatory cell entry into the lung, oxidative stress, and apoptosis as well as remodeling of lung structures. Yet, while having comparable environmental risk factors, each individual may experience clinical symptoms differently because of their individuality and genetic predisposition.
In conclusion, lncRNA is a molecule with far-reaching effects and has an important position in transcriptional regulation. Unfortunately, however, their relationship with COPD lacks more literature, and more studies revealing the biological functions of lncRNAs in COPD or revealing the pathogenesis of COPD would generate more scientific interest among researchers.
Financial support
This work supported by the Key Technologies Research and Development Program of Anhui Province (No. 2022e07020040), Independent Innovation Borrowing and Replenishment Project of Hefei Science and Technology Bureau (No. J2020Y09), Open Fund of Key Laboratory of Anti-Inflammatory and Immune Medicine, Ministry of Education (No. KFJJ-2023-01)
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Jing Xie: Writing – review & editing, Writing – original draft. Yongkang Wu: Visualization, Investigation. Qing Tao: Investigation. Hua Liu: Supervision. Jingjing Wang: Investigation. Chunwei Zhang: Investigation. Yuanzhi Zhou: Investigation. Chengyan Wei: Investigation. Yan Chang: Supervision, Resources. Yong Jin: Writing – review & editing, Supervision, Resources. Zhen Ding: Writing – review & editing, Supervision, Resources, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yan Chang, Email: yychang@ahmu.edu.cn.
Yong Jin, Email: jinyong@ahmu.edu.cn.
Zhen Ding, Email: imdzh@163.com.
References
- 1.Martins P., Rosado-Pinto J., do Céu Teixeira M., Neuparth N., Silva O., Tavares H., Spencer J.L., Mascarenhas D., Papoila A.L., Khaltaev N., Annesi-Maesano I. Under-report and underdiagnosis of chronic respiratory diseases in an African country. Allergy. 2009;64:1061–1067. doi: 10.1111/j.1398-9995.2009.01956.x. [DOI] [PubMed] [Google Scholar]
- 2.Bai J., Zhao Y., Yang D., Ma Y., Yu C. Secular trends in chronic respiratory diseases mortality in Brazil, Russia, China, and South Africa: a comparative study across main BRICS countries from 1990 to 2019. BMC Publ. Health. 2022;22:91. doi: 10.1186/s12889-021-12484-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeloye D., Song P., Zhu Y., Campbell H., Sheikh A., Rudan I. NIHR RESPIRE Global Respiratory Health Unit, Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir. Med. 2022;10:447–458. doi: 10.1016/S2213-2600(21)00511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global health estimates Leading causes of death. 2023. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death
- 5.Wang C., Xu J., Yang L., Xu Y., Zhang X., Bai C., Kang J., Ran P., Shen H., Wen F., Huang K., Yao W., Sun T., Shan G., Yang T., Lin Y., Wu S., Zhu J., Wang R., Shi Z., Zhao J., Ye X., Song Y., Wang Q., Zhou Y., Ding L., Yang T., Chen Y., Guo Y., Xiao F., Lu Y., Peng X., Zhang B., Xiao D., Chen C.-S., Wang Z., Zhang H., Bu X., Zhang X., An L., Zhang S., Cao Z., Zhan Q., Yang Y., Cao B., Dai H., Liang L., He J. China Pulmonary Health Study Group, Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–1717. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhou M., Wang H., Zeng X., Yin P., Zhu J., Chen W., Li X., Wang L., Wang L., Liu Y., Liu J., Zhang M., Qi J., Yu S., Afshin A., Gakidou E., Glenn S., Krish V.S., Miller-Petrie M.K., Mountjoy-Venning W.C., Mullany E.C., Redford S.B., Liu H., Naghavi M., Hay S.I., Wang L., Murray C.J.L., Liang X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Statello L., Guo C.-J., Chen L.-L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bridges M.C., Daulagala A.C., Kourtidis A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021;220 doi: 10.1083/jcb.202009045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.K F., M Jt. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172 doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukherjee N., Calviello L., Hirsekorn A., de Pretis S., Pelizzola M., Ohler U. Integrative classification of human coding and noncoding genes through RNA metabolism profiles. Nat. Struct. Mol. Biol. 2017;24:86–96. doi: 10.1038/nsmb.3325. [DOI] [PubMed] [Google Scholar]
- 11.Arman K., Dalloul Z., Bozgeyik E. Emerging role of microRNAs and long non-coding RNAs in COVID-19 with implications to therapeutics. Gene. 2023;861 doi: 10.1016/j.gene.2023.147232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge J., Geng S., Jiang H. Long noncoding RNAs antisense noncoding RNA in the INK4 locus (ANRIL) correlates with lower acute exacerbation risk, decreased inflammatory cytokines, and mild GOLD stage in patients with chronic obstructive pulmonary disease. J. Clin. Lab. Anal. 2019;33 doi: 10.1002/jcla.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zong D., Liu X., Li J., Long Y., Ouyang R., Chen Y. LncRNA-CCAT1/miR-152-3p is involved in CSE-induced inflammation in HBE cells via regulating ERK signaling pathway. Int Immunopharmacol. 2022;109 doi: 10.1016/j.intimp.2022.108818. [DOI] [PubMed] [Google Scholar]
- 14.Tang W., Shen Z., Guo J., Sun S. Screening of long non-coding RNA and TUG1 inhibits proliferation with TGF-β induction in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016;11:2951–2964. doi: 10.2147/COPD.S109570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mo R., Li J., Chen Y., Ding Y. lncRNA GAS5 promotes pyroptosis in COPD by functioning as a ceRNA to regulate the miR-223-3p/NLRP3 axis. Mol. Med. Rep. 2022;26:219. doi: 10.3892/mmr.2022.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabe K.F., Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389:1931–1940. doi: 10.1016/S0140-6736(17)31222-9. [DOI] [PubMed] [Google Scholar]
- 17.J Ae, M Wj, S E., W Gm. Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Research. 2019;8 doi: 10.12688/f1000research.18411.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demedts I.K., Demoor T., Bracke K.R., Joos G.F., Brusselle G.G. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 2006;7:53. doi: 10.1186/1465-9921-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogelmeier C.F., Criner G.J., Martinez F.J., Anzueto A., Barnes P.J., Bourbeau J., Celli B.R., Chen R., Decramer M., Fabbri L.M., Frith P., Halpin D.M.G., López Varela M.V., Nishimura M., Roche N., Rodriguez-Roisin R., Sin D.D., Singh D., Stockley R., Vestbo J., Wedzicha J.A., Agusti A. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur. Respir. J. 2017;49 doi: 10.1183/13993003.00214-2017. [DOI] [PubMed] [Google Scholar]
- 20.Holloway R.A., Donnelly L.E. Immunopathogenesis of chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2013;19:95–102. doi: 10.1097/MCP.0b013e32835cfff5. [DOI] [PubMed] [Google Scholar]
- 21.Brusselle G.G., Joos G.F., Bracke K.R. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- 22.Şerifoğlu İ., Ulubay G. The methods other than spirometry in the early diagnosis of COPD. Tuberk Toraks. 2019;67:63–70. doi: 10.5578/tt.68162. [DOI] [PubMed] [Google Scholar]
- 23.Labaki W.W., Rosenberg S.R. Chronic obstructive pulmonary disease. Ann. Intern. Med. 2020;173:ITC17–ITC32. doi: 10.7326/AITC202008040. [DOI] [PubMed] [Google Scholar]
- 24.Guo P., Li R., Piao T.H., Wang C.L., Wu X.L., Cai H.Y. Pathological mechanism and targeted drugs of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2022;17:1565–1575. doi: 10.2147/COPD.S366126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janson C., Lisspers K., Ställberg B., Johansson G., Gutzwiller F.S., Mezzi K., Mindeholm L., Bjerregaard B.K., Jorgensen L., Larsson K. Osteoporosis and fracture risk associated with inhaled corticosteroid use among Swedish COPD patients: the ARCTIC study. Eur. Respir. J. 2021;57 doi: 10.1183/13993003.00515-2020. [DOI] [PubMed] [Google Scholar]
- 26.Price D., Yawn B., Brusselle G., Rossi A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim. Care Respir. J. 2013;22:92–100. doi: 10.4104/pcrj.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillai S.G., Ge D., Zhu G., Kong X., Shianna K.V., Need A.C., Feng S., Hersh C.P., Bakke P., Gulsvik A., Ruppert A., Lødrup Carlsen K.C., Roses A., Anderson W., Rennard S.I., Lomas D.A., Silverman E.K., Goldstein D.B. ICGN Investigators, A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cazzola M., Stolz D., Rogliani P., Matera M.G. α1-Antitrypsin deficiency and chronic respiratory disorders. Eur. Respir. Rev. 2020;29 doi: 10.1183/16000617.0073-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonelli A.R., Brantly M.L. Augmentation therapy in alpha-1 antitrypsin deficiency: advances and controversies. Ther. Adv. Respir. Dis. 2010;4:289–312. doi: 10.1177/1753465810373911. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki K., van Eeden S.F. Lung macrophage phenotypes and functional responses: role in the pathogenesis of COPD. Int. J. Mol. Sci. 2018;19:E582. doi: 10.3390/ijms19020582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams M., Todd I., Fairclough L.C. The role of CD8 + T lymphocytes in chronic obstructive pulmonary disease: a systematic review. Inflamm. Res. 2021;70:11–18. doi: 10.1007/s00011-020-01408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saetta M., Di Stefano A., Turato G., Facchini F.M., Corbino L., Mapp C.E., Maestrelli P., Ciaccia A., Fabbri L.M. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998;157:822–826. doi: 10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- 33.Soltani A., Ewe Y.P., Lim Z.S., Sohal S.S., Reid D., Weston S., Wood-Baker R., Walters E.H. Mast cells in COPD airways: relationship to bronchodilator responsiveness and angiogenesis. Eur. Respir. J. 2012;39:1361–1367. doi: 10.1183/09031936.00084411. [DOI] [PubMed] [Google Scholar]
- 34.Boesiger J., Tsai M., Maurer M., Yamaguchi M., Brown L.F., Claffey K.P., Dvorak H.F., Galli S.J. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of fc epsilon receptor I expression. J. Exp. Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jogdand P., Siddhuraj P., Mori M., Sanden C., Jönsson J., Walls A.F., Kearley J., Humbles A.A., Kolbeck R., Bjermer L., Newbold P., Erjefält J.S. Eosinophils, basophils and type 2 immune microenvironments in COPD-affected lung tissue. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00110-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tashkin D.P., Wechsler M.E. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:335–349. doi: 10.2147/COPD.S152291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Paulis A., Prevete N., Fiorentino I., Rossi F.W., Staibano S., Montuori N., Ragno P., Longobardi A., Liccardo B., Genovese A., Ribatti D., Walls A.F., Marone G. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J. Immunol. 2006;177:7322–7331. doi: 10.4049/jimmunol.177.10.7322. [DOI] [PubMed] [Google Scholar]
- 38.Mannino D.M., Buist A.S. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 39.Bi H., Zhou J., Wu D., Gao W., Li L., Yu L., Liu F., Huang M., Adcock I.M., Barnes P.J., Yao X. Microarray analysis of long non-coding RNAs in COPD lung tissue. Inflamm. Res. 2015;64:119–126. doi: 10.1007/s00011-014-0790-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H., Sun D., Li D., Zheng Z., Xu J., Liang X., Zhang C., Wang S., Wang J., Lu W. Long non-coding RNA expression patterns in lung tissues of chronic cigarette smoke induced COPD mouse model. Sci. Rep. 2018;8:7609. doi: 10.1038/s41598-018-25702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodríguez García C., Ruano-Ravina A., Pérez Ríos M., Martín Gisbert L., Varela-Lema L., Candal-Pedreira C., Represas-Represas C., Rey-Brandariz J., Valdés-Cuadrado L., Agustí A. Clinical characteristics of chronic obstructive pulmonary disease in never-smokers: a systematic review. Respir. Med. 2023;214 doi: 10.1016/j.rmed.2023.107284. [DOI] [PubMed] [Google Scholar]
- 42.Aghaeimeybodi F., Samadzadeh G., Haji Safari Z., Nouri S., Talebi H.R., Shahcheraghi S.H. Comparison of chronic obstructive pulmonary diseases induced by wood smoke and tobacco smoke. Tanaffos. 2021;20:268–276. [PMC free article] [PubMed] [Google Scholar]
- 43.Nicolaou L., Checkley W. Differences between cigarette smoking and biomass smoke exposure: an in silico comparative assessment of particulate deposition in the lungs. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Zheng M., Pu J., Zhou Y., Hong W., Fu X., Peng Y., Zhou W., Pan H., Li B., Ran P. Identification of abnormally expressed lncRNAs induced by PM2.5 in human bronchial epithelial cells. Biosci. Rep. 2018;38 doi: 10.1042/BSR20171577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen P., Jiang P., Chen J., Yang Y., Guo X. XIST promotes apoptosis and the inflammatory response in CSE-stimulated cells via the miR-200c-3p/EGR3 axis. BMC Pulm. Med. 2021;21:215. doi: 10.1186/s12890-021-01582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M., Liu Y., Zhang Y., Zhang L. LncRNA LOC729178 acts as a sponge of miR-144-3p to mitigate cigarette smoke extract-induced inflammatory injury via regulating PHLPP2 in 16HBE cells. J. Mol. Histol. 2021;52:437–447. doi: 10.1007/s10735-021-09972-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhao J., Pu J., Hao B., Huang L., Chen J., Hong W., Zhou Y., Li B., Ran P. LncRNA RP11-86H7.1 promotes airway inflammation induced by TRAPM2.5 by acting as a ceRNA of miRNA-9-5p to regulate NFKB1 in HBECS. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-68327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo J., Li L., Hu D., Zhang X. LINC00612/miR-31-5p/Notch1 Axis regulates apoptosis, inflammation, and oxidative stress in human pulmonary microvascular endothelial cells induced by cigarette smoke extract. Int. J. Chronic Obstr. Pulm. Dis. 2020;15:2049–2060. doi: 10.2147/COPD.S255696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei J., Zhang Y., Lu S., Wang J. Long non-coding RNA NNT-AS1 regulates proliferation, apoptosis, inflammation and airway remodeling of chronic obstructive pulmonary disease via targeting miR-582-5p/FBXO11 axis. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110326. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H., Guan R., Zhang Z., Li D., Xu J., Gong Y., Chen X., Lu W. LncRNA nqo1-AS1 attenuates cigarette smoke-induced oxidative stress by upregulating its natural antisense transcript Nqo1. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.729062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y., Chen J., Chen W., Liu L., Dong M., Ji J., Hu D., Zhang N. LINC00987 ameliorates COPD by regulating LPS-induced cell apoptosis, oxidative stress, inflammation and autophagy through let-7b-5p/SIRT1 Axis. Int. J. Chronic Obstr. Pulm. Dis. 2020;15:3213–3225. doi: 10.2147/COPD.S276429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee K.-Y., Ho S.-C., Sun W.-L., Feng P.-H., Lin C.-W., Chen K.-Y., Chuang H.-C., Tseng C.-H., Chen T.-T., Wu S.-M. Lnc-IL7R alleviates PM2.5-mediated cellular senescence and apoptosis through EZH2 recruitment in chronic obstructive pulmonary disease. Cell Biol. Toxicol. 2022 doi: 10.1007/s10565-022-09709-1. [DOI] [PubMed] [Google Scholar]
- 53.Gu W., Wang L., Deng G., Gu X., Tang Z., Li S., Jin W., Yang J., Guo X., Li Q. Knockdown of long noncoding RNA MIAT attenuates cigarette smoke-induced airway remodeling by downregulating miR-29c-3p-HIF3A axis. Toxicol. Lett. 2022;357:11–19. doi: 10.1016/j.toxlet.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Song J., Wang Q., Zong L. LncRNA MIR155HG contributes to smoke-related chronic obstructive pulmonary disease by targeting miR-128-5p/BRD4 axis. Biosci. Rep. 2020;40 doi: 10.1042/BSR20192567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barnes P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017;131:1541–1558. doi: 10.1042/CS20160487. [DOI] [PubMed] [Google Scholar]
- 56.Manevski M., Devadoss D., Long C., Singh S.P., Nasser M.W., Borchert G.M., Nair M.N., Rahman I., Sopori M., Chand H.S. Increased expression of LASI lncRNA regulates the cigarette smoke and COPD associated airway inflammation and mucous cell hyperplasia. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.803362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pangeni R., Meng T., Poudel S., Sharma D., Hutsell H., Ma J., Rubin B.K., Longest W., Hindle M., Xu Q. Airway mucus in pulmonary diseases: muco-adhesive and muco-penetrating particles to overcome the airway mucus barriers. Int. J. Pharm. 2023;634 doi: 10.1016/j.ijpharm.2023.122661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cerveri I., Brusasco V. Revisited role for mucus hypersecretion in the pathogenesis of COPD. Eur. Respir. Rev. 2010;19:109–112. doi: 10.1183/09059180.00002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bamodu O.A., Wu S.-M., Feng P.-H., Sun W.-L., Lin C.-W., Chuang H.-C., Ho S.-C., Chen K.-Y., Chen T.-T., Tseng C.-H., Liu W.-T., Lee K.-Y. lnc-IL7R expression reflects physiological pulmonary function and its aberration is a putative indicator of COPD. Biomedicines. 2022;10:786. doi: 10.3390/biomedicines10040786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou A.-Y., Zhao Y.-Y., Zhou Z.-J., Duan J.-X., Zhu Y.-Z., Cai S., Chen P. Microarray analysis of long non-coding RNAs in lung tissues of patients with COPD and HOXA-AS2 promotes HPMECs proliferation via Notch1. Int. J. Chronic Obstr. Pulm. Dis. 2020;15:2449–2460. doi: 10.2147/COPD.S259601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zong D., Ouyang R., Li J., Chen Y., Chen P. Notch signaling in lung diseases: focus on Notch1 and Notch3. Ther. Adv. Respir. Dis. 2016;10:468–484. doi: 10.1177/1753465816654873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu P., Zhang H., Zeng H., Meng Y., Gao H., Zhang M., Zhao L. LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis. Ther. Adv. Respir. Dis. 2021;15 doi: 10.1177/17534666211028072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu L., Xu H., Luo F., Liu X., Lu X., Yang Q., Xue J., Chen C., Shi L., Liu Q. Epigenetic silencing of miR-218 by the lncRNA CCAT1, acting via BMI1, promotes an altered cell cycle transition in the malignant transformation of HBE cells induced by cigarette smoke extract. Toxicol. Appl. Pharmacol. 2016;304:30–41. doi: 10.1016/j.taap.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 64.Du Y., Ding Y., Shi T., He W., Feng J., Mei Z., Chen X., Feng X., Zhang X., Jie Z. Long noncoding RNA GAS5 attenuates cigarette smoke-induced airway remodeling by regulating miR-217-5p/PTEN axis. Acta Biochim. Biophys. Sin. 2022;54:1–9. doi: 10.3724/abbs.2022074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis A., Lee J.Y., Donaldson A.V., Natanek S.A., Vaidyanathan S., Man W.D.-C., Hopkinson N.S., Sayer A.A., Patel H.P., Cooper C., Syddall H., Polkey M.I., Kemp P.R. Increased expression of H19/miR-675 is associated with a low fat-free mass index in patients with COPD. J Cachexia Sarcopenia Muscle. 2016;7:330–344. doi: 10.1002/jcsm.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng Z.-H., Wu D.-M., Fan S.-H., Zhang Z.-F., Chen G.-Q., Lu J. Upregulation of miR-675-5p induced by lncRNA H19 was associated with tumor progression and development by targeting tumor suppressor p53 in non-small cell lung cancer. J. Cell. Biochem. 2019;120:18724–18735. doi: 10.1002/jcb.29182. [DOI] [PubMed] [Google Scholar]
- 67.Dai Z., Liu X., Zeng H., Chen Y. Long noncoding RNA HOTAIR facilitates pulmonary vascular endothelial cell apoptosis via DNMT1 mediated hypermethylation of Bcl-2 promoter in COPD. Respir. Res. 2022;23:356. doi: 10.1186/s12931-022-02234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia H., Xue J., Xu H., Lin M., Shi M., Sun Q., Xiao T., Dai X., Wu L., Li J., Xiang Q., Tang H., Bian Q., Liu Q. Andrographolide antagonizes the cigarette smoke-induced epithelial-mesenchymal transition and pulmonary dysfunction through anti-inflammatory inhibiting HOTAIR. Toxicology. 2019;422:84–94. doi: 10.1016/j.tox.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 69.Wu S.-M., Feng P.-H., Chuang H.-C., Ho S.-C., Fan Chung K., Chen K.-Y., Wu G.-S., Chen T.-T., Tseng C.-H., Liu W.-T., Lee K.-Y. Impaired lnc-IL7R modulatory mechanism of Toll-like receptors is associated with an exacerbator phenotype of chronic obstructive pulmonary disease. Faseb. J. 2020;34:13317–13332. doi: 10.1096/fj.202000632R. [DOI] [PubMed] [Google Scholar]
- 70.Manevski M., Devadoss D., Long C., Singh S.P., Nasser M.W., Borchert G.M., Nair M.N., Rahman I., Sopori M., Chand H.S. Corrigendum: increased expression of LASI LncRNA regulates the cigarette smoke and COPD associated airway inflammation and mucous cell hyperplasia. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.988069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vierbuchen T., Agarwal S., Johnson J.L., Galia L., Lei X., Stein K., Olagnier D., Gaede K.I., Herzmann C., Holm C.K., Heine H., Pai A., O'Hara Hall A., Hoebe K., Fitzgerald K.A. The lncRNA LUCAT1 is elevated in inflammatory disease and restrains inflammation by regulating the splicing and stability of NR4A2. Proc. Natl. Acad. Sci. U. S. A. 2023;120 doi: 10.1073/pnas.2213715120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao S., Lin C., Yang T., Qian X., Lu J., Cheng J. Expression of long non-coding RNA LUCAT1 in patients with chronic obstructive pulmonary disease and its potential functions in regulating cigarette smoke extract-induced 16HBE cell proliferation and apoptosis. J. Clin. Lab. Anal. 2021;35 doi: 10.1002/jcla.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song B., Ye L., Wu S., Jing Z. Long non-coding RNA MEG3 regulates CSE-induced apoptosis and inflammation via regulating miR-218 in 16HBE cells. Biochem. Biophys. Res. Commun. 2020;521:368–374. doi: 10.1016/j.bbrc.2019.10.135. [DOI] [PubMed] [Google Scholar]
- 74.He F., Wang N., Yu X., Zheng Y., Liu Q., Chen Q., Pu J., Li N., Zou W., Li B., Ran P. GATA3/long noncoding RNA MHC-R regulates the immune activity of dendritic cells in chronic obstructive pulmonary disease induced by air pollution particulate matter. J. Hazard Mater. 2022;438 doi: 10.1016/j.jhazmat.2022.129459. [DOI] [PubMed] [Google Scholar]
- 75.Gu W., Yuan Y., Wang L., Yang H., Li S., Tang Z., Li Q. Long non-coding RNA TUG1 promotes airway remodelling by suppressing the miR-145-5p/DUSP6 axis in cigarette smoke-induced COPD. J. Cell Mol. Med. 2019;23:7200–7209. doi: 10.1111/jcmm.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen Q., Zheng J., Wang X., Hu W., Jiang Y., Jiang Y. LncRNA SNHG5 regulates cell apoptosis and inflammation by miR-132/PTEN axis in COPD. Biomed. Pharmacother. 2020;126 doi: 10.1016/j.biopha.2020.110016. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y., Lyu X., Wu X., Yu L., Hu K. Long non-coding RNA PVT1, a novel biomarker for chronic obstructive pulmonary disease progression surveillance and acute exacerbation prediction potentially through interaction with microRNA-146a. J. Clin. Lab. Anal. 2020;34 doi: 10.1002/jcla.23346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ming X., Duan W., Yi W. Long non-coding RNA NEAT1 predicts elevated chronic obstructive pulmonary disease (COPD) susceptibility and acute exacerbation risk, and correlates with higher disease severity, inflammation, and lower miR-193a in COPD patients. Int. J. Clin. Exp. Pathol. 2019;12:2837–2848. [PMC free article] [PubMed] [Google Scholar]
- 79.Langen R.C.J., Korn S.H., Wouters E.F.M. ROS in the local and systemic pathogenesis of COPD. Free Radic. Biol. Med. 2003;35:226–235. doi: 10.1016/s0891-5849(03)00316-2. [DOI] [PubMed] [Google Scholar]
- 80.Korytina G.F., Akhmadishina L.Z., Aznabaeva Y.G., Kochetova O.V., Zagidullin N.Sh, Kzhyshkowska J.G., Zagidullin S.Z., Viktorova T.V. Associations of the NRF2/KEAP1 pathway and antioxidant defense gene polymorphisms with chronic obstructive pulmonary disease. Gene. 2019;692:102–112. doi: 10.1016/j.gene.2018.12.061. [DOI] [PubMed] [Google Scholar]
- 81.Fisher A.B. Redox signaling across cell membranes. Antioxidants Redox Signal. 2009;11:1349–1356. doi: 10.1089/ars.2008.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 83.Kanazawa H., Yoshikawa J. Elevated oxidative stress and reciprocal reduction of vascular endothelial growth factor levels with severity of COPD. Chest. 2005;128:3191–3197. doi: 10.1378/chest.128.5.3191. [DOI] [PubMed] [Google Scholar]
- 84.Postma D.S., Timens W. Remodeling in asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006;3:434–439. doi: 10.1513/pats.200601-006AW. [DOI] [PubMed] [Google Scholar]
- 85.Tuder R.M., Zhen L., Cho C.Y., Taraseviciene-Stewart L., Kasahara Y., Salvemini D., Voelkel N.F., Flores S.C. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am. J. Respir. Cell Mol. Biol. 2003;29:88–97. doi: 10.1165/rcmb.2002-0228OC. [DOI] [PubMed] [Google Scholar]
- 86.Hao W., Lin F., Shi H., Guan Z., Jiang Y. Long non-coding RNA OIP5-AS1 regulates smoke-related chronic obstructive pulmonary disease via targeting micro RNA -410-3p/IL-13. Bioengineered. 2021;12:11664–11676. doi: 10.1080/21655979.2021.2000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng Y., Zhao Y., Chen Y., Cai S., Chen P. PECAM EMPs regulate apoptosis in pulmonary microvascular endothelial cells in COPD by activating the Akt signaling pathway. Tob. Induc. Dis. 2022;20:40. doi: 10.18332/tid/146959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang C., Youle R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang I.A., Jenkins C.R., Salvi S.S. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir. Med. 2022;10:497–511. doi: 10.1016/S2213-2600(21)00506-3. [DOI] [PubMed] [Google Scholar]
- 90.Berend N. Contribution of air pollution to COPD and small airway dysfunction. Respirology. 2016;21:237–244. doi: 10.1111/resp.12644. [DOI] [PubMed] [Google Scholar]
- 91.Liu S., Zhou Y., Liu S., Chen X., Zou W., Zhao D., Li X., Pu J., Huang L., Chen J., Li B., Liu S., Ran P. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax. 2017;72:788–795. doi: 10.1136/thoraxjnl-2016-208910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ko F.W., Chan K.P., Hui D.S., Goddard J.R., Shaw J.G., Reid D.W., Yang I.A. Acute exacerbation of COPD. Respirology. 2016;21:1152–1165. doi: 10.1111/resp.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Celli B.R., Fabbri L.M., Aaron S.D., Agusti A., Brook R., Criner G.J., Franssen F.M.E., Humbert M., Hurst J.R., O'Donnell D., Pantoni L., Papi A., Rodriguez-Roisin R., Sethi S., Torres A., Vogelmeier C.F., Wedzicha J.A. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am. J. Respir. Crit. Care Med. 2021;204:1251–1258. doi: 10.1164/rccm.202108-1819PP. [DOI] [PubMed] [Google Scholar]
- 94.Austin P.J., Tsitsiou E., Boardman C., Jones S.W., Lindsay M.A., Adcock I.M., Chung K.F., Perry M.M. Transcriptional profiling identifies the long noncoding RNA plasmacytoma variant translocation (PVT1) as a novel regulator of the asthmatic phenotype in human airway smooth muscle. J. Allergy Clin. Immunol. 2017;139:780–789. doi: 10.1016/j.jaci.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agustí A., Melén E., DeMeo D.L., Breyer-Kohansal R., Faner R. Pathogenesis of chronic obstructive pulmonary disease: understanding the contributions of gene-environment interactions across the lifespan. Lancet Respir. Med. 2022;10:512–524. doi: 10.1016/S2213-2600(21)00555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho M.H., Hobbs B.D., Silverman E.K. Genetics of chronic obstructive pulmonary disease: understanding the pathobiology and heterogeneity of a complex disorder. Lancet Respir. Med. 2022;10:485–496. doi: 10.1016/S2213-2600(21)00510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silverman E.K. Genetics of COPD. Annu. Rev. Physiol. 2020;82:413–431. doi: 10.1146/annurev-physiol-021317-121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strange C. Alpha-1 antitrypsin deficiency associated COPD. Clin. Chest Med. 2020;41:339–345. doi: 10.1016/j.ccm.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 99.Köhnlein T., Welte T. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation, diagnosis, and treatment. Am. J. Med. 2008;121:3–9. doi: 10.1016/j.amjmed.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 100.Smith S.G.J., Greene C.M. Long non-coding RNA expression in alpha-1 antitrypsin deficient monocytes pre- and post-AAT augmentation therapy. Noncoding RNA. 2023;9:6. doi: 10.3390/ncrna9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He Z., Peng H., Gao M., Liang G., Zeng M., Zhang X. p300/Sp1-Mediated high expression of p16 promotes endothelial progenitor cell senescence leading to the occurrence of chronic obstructive pulmonary disease. Mediat. Inflamm. 2021;2021 doi: 10.1155/2021/5599364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chattopadhyay S., Malayil L., Kaukab S., Merenstein Z., Sapkota A.R. The predisposition of smokers to COVID-19 infection: a mini-review of global perspectives. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e17783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.