Key Points
Question
In patients with suspected poisoning and Glasgow Coma Scale score less than 9, is a conservative airway strategy of withholding intubation associated with a reduction of death, intensive care unit length of stay, and hospital length of stay compared with routine practice?
Findings
In this multicenter, randomized clinical trial that included 225 patients, a strategy of withholding intubation was associated with a significant clinical benefit for the primary end point in the intervention group, with a win ratio of 1.85 and a reduced adverse event risk rate (6% vs 14.7%; absolute risk difference, 8.6%) compared with the control group.
Meaning
Among comatose patients with suspected acute poisoning, a conservative strategy of withholding intubation was associated with a greater clinical benefit for the composite end point of in-hospital death, length of intensive care unit stay, and length of hospital stay.
Abstract
Importance
Tracheal intubation is recommended for coma patients and those with severe brain injury, but its use in patients with decreased levels of consciousness from acute poisoning is uncertain.
Objective
To determine the effect of intubation withholding vs routine practice on clinical outcomes of comatose patients with acute poisoning and a Glasgow Coma Scale score less than 9.
Design, Setting, and Participants
This was a multicenter, randomized trial conducted in 20 emergency departments and 1 intensive care unit (ICU) that included comatose patients with suspected acute poisoning and a Glasgow Coma Scale score less than 9 in France between May 16, 2021, and April 12, 2023, and followed up until May 12, 2023.
Intervention
Patients were randomized to undergo conservative airway strategy of intubation withholding vs routine practice.
Main Outcomes and Measures
The primary outcome was a hierarchical composite end point of in-hospital death, length of ICU stay, and length of hospital stay. Key secondary outcomes included adverse events resulting from intubation as well as pneumonia within 48 hours.
Results
Among the 225 included patients (mean age, 33 years; 38% female), 116 were in the intervention group and 109 in the control group, with respective proportions of intubations of 16% and 58%. No patients died during the in-hospital stay. There was a significant clinical benefit for the primary end point in the intervention group, with a win ratio of 1.85 (95% CI, 1.33 to 2.58). In the intervention group, there was a lower proportion with any adverse event (6% vs 14.7%; absolute risk difference, 8.6% [95% CI, −16.6% to −0.7%]) compared with the control group, and pneumonia occurred in 8 (6.9%) and 16 (14.7%) patients, respectively (absolute risk difference, −7.8% [95% CI, −15.9% to 0.3%]).
Conclusions and Relevance
Among comatose patients with suspected acute poisoning, a conservative strategy of withholding intubation was associated with a greater clinical benefit for the composite end point of in-hospital death, length of ICU stay, and length of hospital stay.
Trial Registration
ClinicalTrials.gov Identifier: NCT04653597
This clinical trial compares the efficacy of intubation withholding vs routine practice on clinical outcomes of comatose patients with acute poisoning and a Glasgow Coma Scale score less than 9.
Introduction
Patients with a decreased level of consciousness may be at risk of aspiration, which can lead to several complications including respiratory distress, pneumonitis, and pneumonia. Acute poisoning caused by alcohol, drugs, or medication is a common nontraumatic reason for a decreased level of consciousness and is often associated with a high rate of intubation.1,2
In contrast with trauma patients, the decision to intubate a patient with suspected acute poisoning, coma, and Glasgow Coma Scale (GCS) score less than 9 is debated.3,4,5 It is estimated that more than 20 000 patients with acute poisoning are intubated each year in the US alone.6 The expected benefit in these patients is to limit the aspiration of stomach contents and subsequent pneumonia. Yet these events are also the main complications from intubation itself. The additional risks of intubation include hemodynamic instability, hypoxia, difficult intubation, and dental injury.7 Despite these trade-offs, there is no study with a high level of evidence to guide the decision to intubate or not among comatose patients with acute poisoning. Prior observational studies have suggested that early intubation may reduce the risk of aspiration, whereas other work found no difference in the risk of aspiration or death.8,9,10,11,12
To address this knowledge gap, the Non-invasive Airway Management of Comatose Poisoned Emergency Patients (NICO) randomized clinical trial tested whether a strategy of withholding intubation in patients with coma due to acute poisoning would improve outcomes compared with routine practice in which the decision of intubation is left to the discretion of the physician.
Methods
Trial Design and Oversight
This was a multicenter, unblinded, randomized, parallel-group trial in which a strategy of withholding tracheal intubation in comatose patients with acute poisoning was compared with that of routine practice. The trial was initiated by the investigators and approved by the institutional review board Comité de Protection des Personnes Ouest VI, Brest, France (2020-A36-33) and the Commission Nationale Informatique et Liberté (DR-2020-279). Due to the decreased level of consciousness, the requirement for informed consent before enrollment was waived. Written consent of the next of kin was sought when available according to French law and delayed written informed consent was obtained as soon as the patient’s state allowed it. The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines, registered at ClinicalTrials.gov (NCT04653597) before initiation, and was overseen by an independent data and safety monitoring board. The trial protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively. Data collected on sites were monitored by clinical research personnel who were independent of the clinical team.
Trial Sites and Patient Population
The trial was conducted at 21 sites, including 20 emergency departments (ED) and 1 intensive care unit (ICU) in France. Among the 20 EDs, 4 included patients recruited in the hospital only and 16 included patients recruited both in hospital and prehospital emergency medical system with an emergency physician present at the scene. Adult patients (age ≥18 years) with a clinical suspicion of acute poisoning and a decreased level of consciousness with a GCS score less than 9 (assessed by the treating physician) were eligible. Patients were excluded if they were known to be pregnant, were incarcerated or involuntarily detained, or had an immediate need for tracheal intubation (defined by signs of respiratory distress, clinical suspicion of any brain injury, seizure, or shock). Patients were also excluded if there was a suspicion of cardiotropic drug poisoning (β-blockers, calcium-channel inhibitors, or angiotensin-converting enzyme inhibitors), or if there was intoxication with a single toxic substance that could be reversed (opioids and benzodiazepines). Details of the trial sites and a complete list of the inclusion and exclusion criteria are provided in Supplement 1.
Randomization and Intervention
Patients were randomly assigned in a 1:1 ratio to the control or the intervention group. Randomization was stratified by hospital and block balanced. The width of the blocks was not communicated to the investigators. Because patients may have been randomized in the emergency ambulance in which access to internet is limited, the randomization process used sealed envelopes. Within each hospital, envelopes were distributed between the ED and the different emergency medical services ambulances. In the intervention group, intubation was withheld unless an emergency intubation criterion was met. These included seizure, respiratory distress (defined by a peripheral oxygen saturation as measured by pulse oximetry <90% that persisted after nasal cannula oxygen therapy), vomiting, and shock (defined by a systolic blood pressure <90 mm Hg that persisted after 1 L of crystalloid fluid resuscitation). In the control group, the decision to intubate was left at the discretion of the treating emergency physician. In the subsequent 4 hours, or up to when the patient had recovered a sufficient level of consciousness defined by a GCS score greater than 8, whichever came first, patients were closely monitored by a nurse or a physician, with control of blood pressure, oxygen saturation as measured by pulse oximetry, heart rate, respiratory rate, and GCS score. After 4 hours, the intervention was defined as complete and the patient was managed according to routine practice, at the discretion of the emergency physician.
In both groups, when performing intubation, physicians were recommended to follow the guidelines of the French Society of Emergency Medicine for rapid sequence intubation.13 These included the use of a hypnotic drug (either etomidate or ketamine) and a paralytic drug (either succinylcholine or rocuronium), and preoxygenation aimed at maintaining peripheral oxygen saturation of 100% for 2 minutes. In patients with hypoxia, noninvasive ventilation was permitted. The choice of direct or video laryngoscopy, and the use of a bougie or stylet, was left to the discretion of the physician. Waveform capnography was recommended to confirm endotracheal tube position. In France, patients who are intubated in the out-of-hospital field are directly transferred to an ICU.
Trial Outcomes
The primary outcome was a hierarchical composite end point of in-hospital death, length of ICU stay, and length of hospital stay, truncated at 28 days. A transfer to a psychiatric ward was considered hospital discharge. Length of stay was prespecifically rounded to the hour.
Secondary outcomes included each component of the primary end point, the proportion of patients receiving mechanical ventilation until hospital discharge (truncated at day 28), the number of days receiving mechanical ventilation until hospital discharge or at day 28 (including noninvasive ventilation), the proportion of patients admitted to ICU, the proportion of patients with rapid-onset pneumonia, and adverse events from intubation. Adverse events from intubation included any peri-intubation occurrence of peripheral oxygen saturation less than 90%, dental trauma, vomiting, cardiac arrest, systolic blood pressure less than 65 mm Hg, esophageal intubation, and Intubation Difficulty Scale (IDS) score of 5 or greater. Diagnostic criteria for pneumonia and detailed IDS score are reported in eTables 1 and 2 in Supplement 3. Total hospital costs and costs consequence analysis were also part of secondary outcomes but are not described in the present analysis. Prior to the completion of recruitment and data analysis, a prespecified analysis of the proportion of first pass failures, which is associated with an increased risk of adverse events, was added to the analysis plan.14,15
Statistical Analysis
In simulation, a minimum sample size of 100 patients in each study group would provide the study with 98% power to detect a difference in primary outcome at the 2-sided 5% significance level.16 Accounting for 10% of patients lost to follow-up and 10% of patients refusing to consent to the continuation of the trial, 240 patients were needed. The assumptions for this sample size calculation are detailed in the statistical analysis plan (Supplement 2). Briefly, based on previous reports, the estimated hospital mortality was 3% in both groups, the mean length of ICU stay was 0 days in the intervention group and 1 day in the control group, and the mean length of hospital stay was 2 days in the intervention group and 4 days in the control group.1,10,17,18 There was no planned interim analysis.
The primary analysis included all randomized patients except those withdrawn because they were opposed to their participation in the trial and those who had protection measures in place (according to French law). A per-protocol analysis was conducted after the further exclusion of patients who failed to meet eligibility criteria or had a major deviation from the protocol (ie, patients in the control group who were intubated without meeting any emergency intubation criteria).
Baseline characteristics of patients were described overall and by group. Categorical data are described as frequencies and percentages, and continuous data are described as means and standard errors or as medians and IQRs.
The primary analysis used the Finkelstein-Schoenfeld method (eTable 3 in Supplement 3) to compare patients along a hierarchy of end points ordered by their clinical importance (death, then ICU length of stay, then hospital length of stay), with each patient in the intervention group being compared with each patient in the control group.16 For each pairwise (patient-to-patient) comparison, a win, loss, or tie was defined hierarchically, based on which patient fared better. The hierarchical composite end point was compared using the Mann-Whitney U test and effect size described with the win ratio, defined as the proportion of wins in the intervention group divided by the proportion of losses, and its associated confidence interval.19 The win odds, which account for ties, were also calculated.20 The test is based on the net wins (wins minus losses) in the intervention group. Variances were calculated using the Dong et al method.20 Win ratios and win odds stratified by site using Mantel-Haenszel weights are also presented.
For binary secondary end point analysis, differences in percentages between groups and associated 95% CIs were calculated using a generalized linear regression mixed model with binomial distribution (logit link) considering each site as a random effect and strategy as a fixed effect. For continuous secondary end points, the rate ratios between groups and associated 2-sided 95% CIs were calculated using generalized linear regression mixed models with negative binomial distribution (log link) considering each site as a random effect and strategy as fixed effects. The proportions of adverse events from intubation (hypoxemia, dental trauma, regurgitation, cardiac arrest, IDS score ≥5, hypotension, or esophageal intubation) were described in each group among the entire population and among patients who were intubated.
Secondary analyses were performed on the per-protocol populations using the same methods. Two supplemental subgroup analyses were performed after the exclusion of patients with a GCS score greater than 6, and one limited to patients who were poisoned with alcohol, benzodiazepines, or γ-hydroxybutyric acid (GHB) or γ-butyrolactone (GBL). An exploratory analysis compared the primary end point and length of ICU stay and length of hospital stay between the patients receiving and not receiving intubation. Unadjusted median differences and 95% CIs of length of ICU stay and length of hospital stay were calculated using the Brookmeyer and Crowley method.21
All the analyses were performed with SAS software version 9.4 (SAS Institute), R package WINS and R software version 4.2.2 (The R Project for Statistical Computing), and Stata software (version 17; StataCorp).22 Two-sided P < .05 was considered to be statistically significant. Confidence intervals for secondary end points comparisons were not adjusted for multiplicity. Full details of the statistical analysis plan are provided in Supplement 2.
Results
Patients
Between May 16, 2021, and April 12, 2023, 237 patients were randomized; 8 were not analyzed because they expressed refusal for data analysis and 4 were not analyzed because they were identified after enrollment as having a protection measure in place, in accordance with French law. The primary analysis population included 225 patients, 116 in the intervention group and 109 in the control group (Figure 1, Table 1). The mean (SD) age was 33 years (IQR, 25-49) and 85 (38%) were female. The median GCS score at inclusion was 6 (IQR, 3-7) and the main toxin was alcohol (67%). Fewer patients were intubated in the intervention group than in the control group (19 patients [16.4%] vs 63 [57.8%], respectively, eTable 4 in Supplement 3). Among 19 patients who were intubated in the intervention group, 16 presented with at least 1 criteria of emergency intubation, including 4 patients within 30 minutes, 8 patients between 30 minutes and 2 hours, and 4 patients between 2 hours and 4 hours after enrollment. Patients were followed up until May 12, 2023.
Figure 1. Recruitment, Randomization, and Analysis of Patients From the NICO Trial.
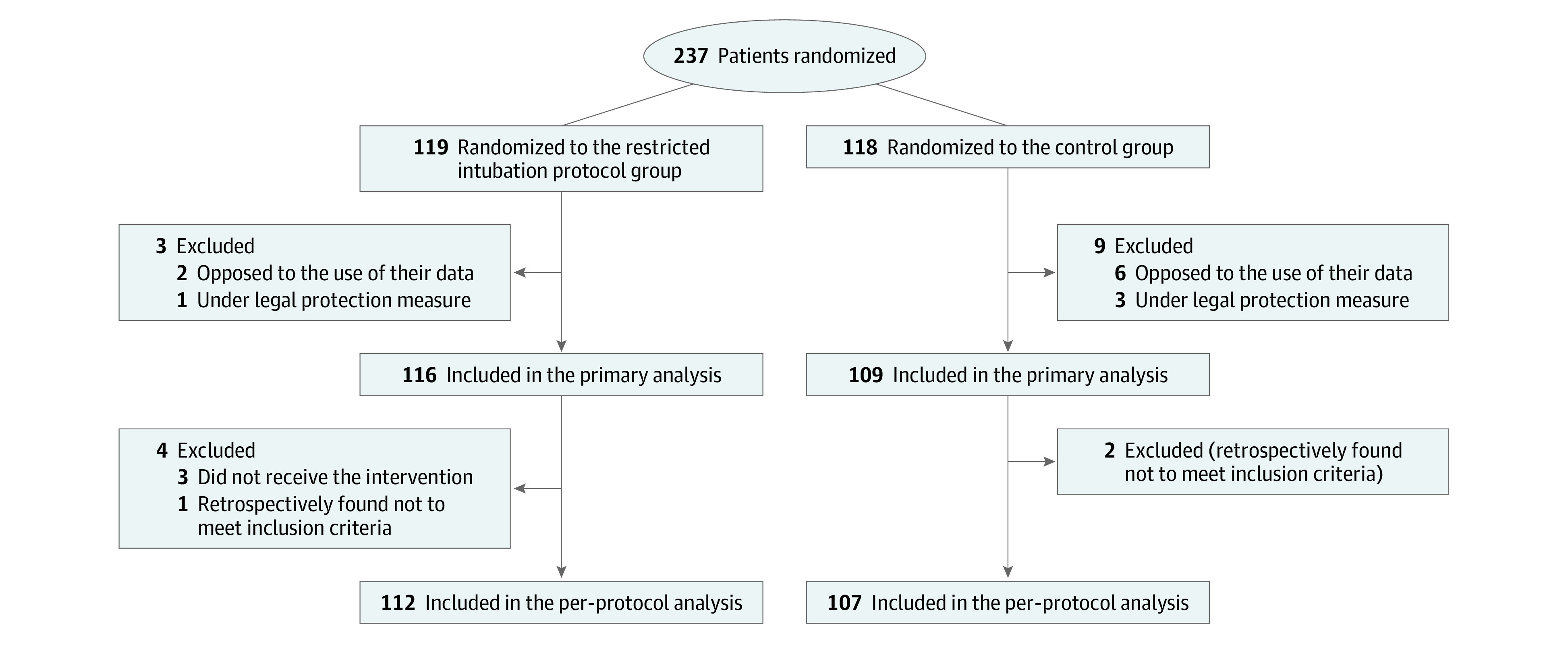
The number of patients screened but not fulfilling all inclusion criteria and no exclusion criteria was not collected. NICO indicates Non-invasive Airway Management of Comatose Poisoned Emergency Patients.
Table 1. Characteristics of the Patients at Baseline in the NICO Trial.
| Characteristic | No. (%) | |
|---|---|---|
| Restricted intubation (n = 116) | Control (n = 109) | |
| Sex | ||
| Female | 46 (39.7) | 39 (35.8) |
| Male | 70 (60.3) | 70 (64.2) |
| Age, median (IQR), y | 33 (25-49) | 34 (26-49) |
| Site of inclusion | ||
| Emergency department | 73 (62.9) | 66 (60.6) |
| Prehospital | 43 (37.1) | 42 (38.5) |
| Intensive care unit | 0 | 1 (0.9) |
| Heart rate, bpm | 85 (18) [n = 114] | 85 (20) [n = 107] |
| Heart rate >100 | 24 (21.1) | 20 (18.7) |
| Blood pressure, mm Hg | n = 115 | n = 106 |
| Systolic, mean (SD) | 113.6 (14.8) | 117.9 (18.9) |
| Systolic ≤100 | 21 (18.3) | 18 (17.0) |
| Diastolic, mean (SD) | 70.3 (13.3) | 70.6 (14.2) [n = 107] |
| Respiratory rate, mean (SD), breaths per min | 17.2 (4.3) [n = 73] | 16.8 (4.4) [n = 72] |
| Respiratory rate ≤12 | 13 (17.8) | 10 (13.9) |
| Peripheral oxygen saturation, median (IQR), % | 97 (95-99) [n = 114] | 97(95-100) [n = 107] |
| Peripheral oxygen saturation <95% | 17 (14.9) | 17 (15.9) |
| Median Glasgow Coma Scale score, median (IQR) | 6 (3-7) | 6 (3-7) |
| Glasgow coma scale score = 3 | 38 (33) | 28 (23) |
| Estimated body mass indexa | 25 (5) [n = 98] | 24 (4) [n = 102] |
| Toxinb | ||
| Alcohol | 79 (68.1) | 71 (65.1) |
| Benzodiazepines | 45 (38.8) | 44 (40.4) |
| Neuroleptic | 25 (19.0) | 31 (28.4) |
| GHB/GBL | 14 (12.1) | 11 (10.1) |
| Crack/cocaine | 13 (11.2) | 6 (5.5) |
| Opioid/heroine | 11 (9.5) | 13 (11.9) |
| Amphetamines | 9 (7.8) | 11 (10.1) |
| Cannabinoid | 9 (7.8) | 6 (5.5) |
| Selective serotonin reuptake inhibitor | 6 (5.2) | 9 (8.3) |
| Tricyclic antidepressant | 6 (5.2) | 9 (8.3) |
| Paracetamol | 5 (4.3) | 4 (3.7) |
| Other | 9 (7.8) | 11 (10.1) |
| Intubation | 19 (16.4) | 63 (57.8) |
Abbreviations: bpm, beats per minute; GHB, γ-hydroxybutyric acid; GBL, γ-butyrolactone; NICO, Non-invasive Airway Management of Comatose Poisoned Emergency Patients.
Calculated as weight in kilograms divided by height in meters squared.
Toxins involved were either suspected, reported by the patient or a relative, or proven by biological analysis. The sum of percentages exceeds 100% because several toxins may be involved.
Primary Outcome
No patients died in either group. Compared with the routine care control group, the intervention strategy of withholding intubation resulted in a lower median length of ICU stay (0 hours [IQR, 0-18.5] vs 24.0 hours [IQR, 0-57.0]; rate ratio [RR], 0.39 [95% CI, 0.24-0.66]) (Table 2). In the intervention group, the median length of hospital stay was 21.5 hours (IQR, 10.5-44.5) compared with 37.0 hours (IQR, 16.0-79.0) in the control group (RR, 0.74 [95% CI, 0.53-1.03]).
Table 2. Components of the Primary Outcome and Secondary Outcomesa.
| Outcome | No. (%) | Value (95% CI) | Absolute difference, percentage points (95% CI)b | |
|---|---|---|---|---|
| Restricted intubation (n = 116) | Control (n = 109) | |||
| Components of the primary outcome | ||||
| In-hospital death | 0 | 0 | NC | NC |
| Intensive care unit admission | 46 (39.7) | 72 (66.1) | OR = 0.23 (0.12 to 0.44) | −29.2 (−41.0 to −17.4) |
| Median length of intensive care unit stay (IQR), h | 0 (0 to 18.5) | 24.0 (0 to 57.0) | RR = 0.39 (0.24 to 0.66) | |
| Median length of hospital stay (IQR), h | 21.5 (10.5 to 44.5) | 37.0 (16.0 to 79.0) | RR = 0.74 (0.53 to 1.03) | |
| Mechanical ventilation | 21 (18.1) | 65 (59.6) | OR = 0.12 (0.06 to 0.24) | −42.5 (−54.1 to −30.9) |
| Additional secondary outcomes | ||||
| Median length of mechanical ventilation (IQR), h | 0 (0 to 0) | 6.0 (0 to 21.0) | RR = 0.21 (0.11 to 0.38) | |
| Occurrence of pneumonia | 8 (6.9) | 16 (14.7) | OR = 0.43 (0.18 to 1.05) | −7.8 (−15.9 to 0.3) |
| Adverse event from intubationc | 7/113 (6.0) [n = 113] | 16/107 (14.7) [n = 107] | OR = 0.37 (0.15 to 0.95) | −8.6 (−16.6 to −0.7) |
| Systolic blood pressure <90 mm Hg | 3 (2.7) | 2 (1.9) | ||
| Peripheral oxygen saturation <90% | 2 (1.8) | 4 (3.7) | ||
| Vomiting | 2 (1.8) | 0 | ||
| Difficult intubation with IDS ≥5 | 1 (0.9) | 14 (13.1) | ||
| Dental trauma | 0 | 2 (1.9) | ||
| Cardiac arrest | 0 | 0 | ||
| Esophageal intubation | 0 | 4 (3.7) | ||
| First pass failure | 1/113 (0.9) | 14/107 (13.1) | OR = 0.06 (0.01 to 0.46) | −12.2 (−18.8 to −5.6) |
Abbreviations: IDS, Intubation Difficulty Scale; NC, not calculated; OR, odds ratio; RR, rate ratio.
All outcomes were truncated at 28 days.
Differences were computed with a generalized mixed linear model with the center as random effect.
The sums of different numbers of adverse events may exceed the number of patients with adverse events because more than 1 adverse event can occur in a patient.
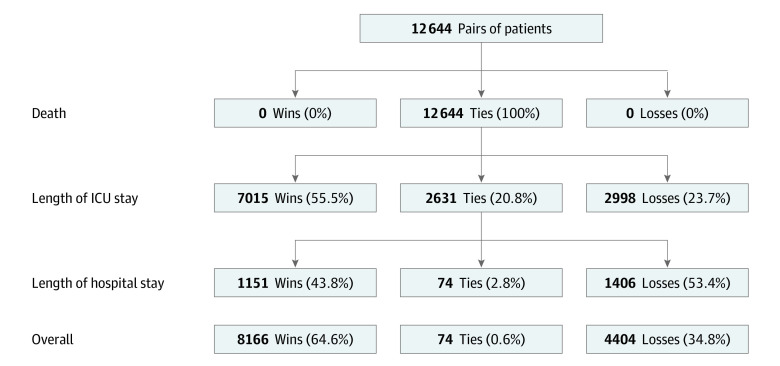
The hierarchical composite primary end point was improved in the intervention compared with the control group, with a win ratio of 1.85 (95% CI, 1.33-2.58; P < .001) and 1.83 (95% CI, 1.29-2.60; P = .001) after stratification by center (Figure 2, Table 3). In the prespecified subgroup analyses, the win ratio was 1.70 (95% CI, 1.10-2.64; P = .02) in patients with a GCS score less than 7 and 1.42 (95% CI, 0.88-2.32; P = .16) in patients intoxicated with alcohol, benzodiazepines, GHB, or GBL.
Figure 2. Distribution of Wins, Ties, and Losses for Patients.
The win ratio denotes the ratio of the proportion of wins to the proportion of losses. The figure shows the distribution of wins, ties, and losses for the 116 patients in the intervention group and 109 patients in the control group (12 644 pairs of patients). Win ratio = total wins/total losses = 8166/4404 = 1.85 (95% CI, 1.33-2.58). ICU indicates intensive care unit.
Table 3. Primary Outcomea.
| Statistic (95% CI) | P valueb | |
|---|---|---|
| Primary analysis | ||
| Win ratio | 1.85 (1.33-2.58) | <.001 |
| Stratified win ratioc | 1.83 (1.29-2.60) | .001 |
| Win odds | 1.85 (1.33-2.56) | <.001 |
| Stratified win oddsc | 1.82 (1.29-2.58) | .001 |
| Per-protocol population | ||
| Win ratio | 1.91 (1.37-2.68) | <.001 |
| Stratified win ratioc | 1.89 (1.32-2.72) | .001 |
| Win odds | 1.90 (1.36-2.66) | <.001 |
| Stratified win oddsc | 1.88 (1.31-2.69) | .001 |
Hierarchical composite of death, length of intensive care unit stay, and length of hospital stay through 28 days. The win ratio denotes the ratio of the total number of wins to the total number of losses. The win odds represents the ratio of the proportion of wins plus 0.5 the proportion of ties to the proportion of losses plus 0.5 the proportion of ties.
See the eMethods in Supplement 3 for a description of the P value calculation.
The 5 centers that had fewer than 5 patients were not included in the stratification (n = 13 patients in total in the primary analysis and 12 patients in total in the per-protocol analysis).
In an exploratory analysis of patients who were intubated (n = 86, including 4 patients who were intubated after initial care during hospital course) vs those who were not (n = 139), there was a clinical benefit for patients who were not intubated, with a win ratio of 12.76 (95% CI, 7.92-20.57) for the hierarchical composite end point.
Secondary Outcomes
When comparing each component of the primary outcome, a lower percentage of patients was admitted to the ICU in the intervention group (39.7% vs 66.1%; absolute risk difference, −29.2 percentage points [95% CI, −41.0 to −17.4) compared with the control group (Table 3). The intervention group had received mechanical ventilation less often (18.1% vs 59.6%; absolute risk difference, −42.5 percentage points [95% CI, −54.1 to −30.9) and experienced fewer adverse events resulting from intubation (6.0% vs 14.7%; absolute risk difference, −8.6 percentage points [95% CI, −16.6 to −0.7]). Pneumonia after intubation occurred in 8 (6.9%) and 16 (14.7%) patients, respectively (absolute risk difference, −7.8 percentage points [95% CI, −15.9 to 0.3]). Analyses in the per-protocol population were similar (eTable 5 in Supplement 3).
Among patients who were intubated (n = 86), there was no difference between groups in the risk of adverse events from intubation (absolute difference, 8.7 percentage points [95% CI, −12.5 to 34.0]) and first pass failure (absolute risk difference, −16.7 percentage points [95% CI, −31.5 to 9.1]). There was no difference in the median length of ICU stay and length of hospital stay (median difference, 0 days [95% CI, −2.0 to 2.1] and −0.2 days [95% CI, −2.3 to 1.9], respectively).
Discussion
In this multicenter, randomized trial, a conservative strategy of withholding intubation in comatose patients with suspected acute poisoning significantly improved the hierarchical composite end point of in-hospital death, length of ICU stay, and length of hospital stay. In the absence of any deaths during the trial, the observed differences in the median length of ICU stay were subsequent to the reduction in ICU admission in the intervention group. The findings of this study have important implications for clinical practice. A conservative strategy can be used to avoid unnecessary intubation in comatose patients after acute poisoning and could lead to a lower risk of adverse events.
In this multicenter trial, more than half of patients were intubated in the control group, illustrating continued clinical equipoise around the decision to intubate patients with a decreased level of consciousness and GCS score of 8 or less from suspected poisoning. Because the trial was unblinded, a Hawthorne effect may have influenced physician behavior and the decision to intubate.23 Prior work that evaluated the indications for intubation in comatose patients from suspected acute poisoning were limited by small sample sizes and narrow study populations. The trial extends prior work by providing randomized evidence to inform guidelines or recommendations as to which patients should be intubated for airway protection.24
There are many caveats around the use of GCS to identify study participants. Although derived and validated among patients with traumatic brain injury, the GCS score is studied to evaluate the risk of adverse events in patients with nontraumatic coma. First, a reduced GCS score was associated with a higher risk of aspiration pneumonia, and that intubation reduced this risk.9 However, the study included patients who were mainly intoxicated by organophosphates, and almost all of whom underwent gastric evacuation. In the present study, no patient was treated with gastric evacuation nor activated charcoal before intubation because it is not routine practice in patients with a decreased level of consciousness in this region. Second, a retrospective study of 209 patients intoxicated with GHB or GBL, including 158 with a GCS score less than 9, reported that withholding intubation was safe, associated with a 2.4% rate of intubation, and a very low risk of severe adverse events.11
There are many strengths to this trial. First, to our knowledge, this is the only randomized trial to date comparing a conservative strategy of withholding intubation vs routine practice among 225 patients. Second, the trial primary outcome addressed more patient-centered end points, while prior work reported only the risk of aspiration pneumonia. The occurrence of pneumonia may not be the main determinant for morbidity and mortality in these patients, and this study allows the assessment of the risk of intubation in patients for whom this procedure could have been avoided. Third, there was an absolute numerical reduction of 7.8% of the risk of pneumonia (relative reduction of 53%). This finding may fill a gap in the existing literature that was controversial and suggested that intubating patients for airway protection to limit the risk of aspiration pneumonia may in fact increase the risk of pneumonia. Fourth, the first pass failure rate was monitored as a validated surrogate for adverse events, and among those intubated, the rate was lower for the intervention group compared with the control group.14,15
Limitations
This trial has several limitations. First, the trial was unblinded and bears the inherent limitations of this design. Second, although the primary end point was a hierarchical composite of death, length of ICU stay, and length of hospital stay, there were no deaths in the study population, and there was no ICU admission in 1 of 5 patients. As such, the main benefit for the composite primary end point is driven by the reduction in ICU length of stay, and the significantly reduced proportion of patients admitted in the ICU. Third, some patients were intubated in the ED or the ICU, and not the prehospital setting. It is possible that differences in intubation location for comatose patients may contribute to the study findings. Fourth, patients were included if there was a suspicion of acute poisoning, which was not ascertained in all patients. However, this pragmatic approach is similar to routine practice when physicians cannot always confirm the cause of coma. Fifth, the use of bougie or videolaryngoscope were not recorded. However, the observed first pass failure was similar to previous trials on intubation.15 Sixth, the GCS was not explicitly designed to guide clinical prediction of the risk of aspiration or need for tracheal intubation.
Conclusions
Among comatose patients with suspected acute poisoning, a conservative strategy of withholding intubation was associated with a greater clinical benefit for the composite end point of in-hospital death, length of ICU stay, and length of hospital stay.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Trial Protocol
Statistical Analysis Plan
eTable 1. Intubation difficulty score
eTable 2. Definition of pneumonia according to the US center of disease control
eTable 3. Calculation method of the hierarchical endpoint (Finkelstein-Schoenfeld method)
eTable 4. Intubation procedures
eTable 5. Secondary outcomes in the per protocol population
eTable 6. Characteristics of patients intubated in the control group
eMethods. P Value Calculation
Data Sharing Statement
References
- 1.Beaune S, Juvin P, Beauchet A, Casalino E, Megarbane B. Deliberate drug poisonings admitted to an emergency department in Paris area: a descriptive study and assessment of risk factors for intensive care admission. Eur Rev Med Pharmacol Sci. 2016;20(6):1174-1179. [PubMed] [Google Scholar]
- 2.Emergency management of poisoning. In: Shannon MW, Borron SW, Burns MJ, eds. Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose. 4th ed. WB Saunders. 2007:13-61. doi: 10.1016/B978-0-7216-0693-4.50007-4 [DOI] [Google Scholar]
- 3.Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007;99(1):18-31. doi: 10.1093/bja/aem128 [DOI] [PubMed] [Google Scholar]
- 4.Geeraerts T, Velly L, Abdennour L, et al. Prise en charge des traumatisés crâniens graves à la phase précoce (24 premières heures). In French. Anesthésie & Réanimation. 2016;2(6):431-453. doi: 10.1016/j.anrea.2016.09.007 [DOI] [Google Scholar]
- 5.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 6.Beauchamp GA, Giffin SL, Horowitz BZ, Laurie AL, Fu R, Hendrickson RG. Poisonings associated with intubation: US National Poison Data System exposures 2000-2013. J Med Toxicol. 2016;12(2):157-164. doi: 10.1007/s13181-015-0528-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russotto V, Myatra SN, Laffey JG, et al. ; INTUBE Study Investigators . Intubation practices and adverse peri-intubation events in critically ill patients from 29 countries. JAMA. 2021;325(12):1164-1172. doi: 10.1001/jama.2021.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montassier E, Le Conte P. Aspiration pneumonia and severe self-poisoning: about the necessity of early airway management. J Emerg Med. 2012;43(1):122-123. doi: 10.1016/j.jemermed.2011.07.036 [DOI] [PubMed] [Google Scholar]
- 9.Eizadi-Mood N, Saghaei M, Alfred S, et al. Comparative evaluation of Glasgow Coma Score and gag reflex in predicting aspiration pneumonitis in acute poisoning. J Crit Care. 2009;24(3):470.e9-470.e15. doi: 10.1016/j.jcrc.2008.08.008 [DOI] [PubMed] [Google Scholar]
- 10.Donald C, Duncan R, Thakore S. Predictors of the need for rapid sequence intubation in the poisoned patient with reduced Glasgow Coma Score. Emerg Med J. 2009;26(7):510-512. doi: 10.1136/emj.2008.064998 [DOI] [PubMed] [Google Scholar]
- 11.van Helmond LPFM, Gresnigt FMJ. Safety of withholding intubation in gamma-hydroxybutyrate- and gamma-butyrolactone-intoxicated coma patients in the emergency department. Eur J Emerg Med. 2020;27(3):223-227. doi: 10.1097/MEJ.0000000000000649 [DOI] [PubMed] [Google Scholar]
- 12.Orso D, Vetrugno L, Federici N, D’Andrea N, Bove T. Endotracheal intubation to reduce aspiration events in acutely comatose patients: a systematic review. Scand J Trauma Resusc Emerg Med. 2020;28(1):116. doi: 10.1186/s13049-020-00814-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quintard H, l’Her E, Pottecher J, et al. Intubation and extubation of the ICU patient. Anaesth Crit Care Pain Med. 2017;36(5):327-341. doi: 10.1016/j.accpm.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 14.Sakles JC, Chiu S, Mosier J, Walker C, Stolz U. The importance of first pass success when performing orotracheal intubation in the emergency department. Acad Emerg Med. 2013;20(1):71-78. doi: 10.1111/acem.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prekker ME, Driver BE, Trent SA, et al. ; DEVICE Investigators and the Pragmatic Critical Care Research Group . Video versus direct laryngoscopy for tracheal intubation of critically ill adults. N Engl J Med. 2023;389(5):418-429. doi: 10.1056/NEJMoa2301601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med. 1999;18(11):1341-1354. doi: [DOI] [PubMed] [Google Scholar]
- 17.Isbister GK, Downes F, Sibbritt D, Dawson AH, Whyte IM. Aspiration pneumonitis in an overdose population: frequency, predictors, and outcomes. Crit Care Med. 2004;32(1):88-93. doi: 10.1097/01.CCM.0000104207.42729.E4 [DOI] [PubMed] [Google Scholar]
- 18.Weiss N, Regard L, Vidal C, et al. Causes of coma and their evolution in the medical intensive care unit. J Neurol. 2012;259(7):1474-1477. doi: 10.1007/s00415-011-6388-z [DOI] [PubMed] [Google Scholar]
- 19.Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33(2):176-182. doi: 10.1093/eurheartj/ehr352 [DOI] [PubMed] [Google Scholar]
- 20.Dong G, Huang B, Verbeeck J, et al. Win statistics (win ratio, win odds, and net benefit) can complement one another to show the strength of the treatment effect on time-to-event outcomes. Pharm Stat. 2023;22(1):20-33. doi: 10.1002/pst.2251 [DOI] [PubMed] [Google Scholar]
- 21.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38(1):29-41. doi: 10.2307/2530286 [DOI] [Google Scholar]
- 22.Cui Y, Huang B. WINS: the R WINS package. Published July 9, 2023. Accessed July 19, 2023. https://cran.r-project.org/web/packages/WINS/index.html
- 23.Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015;351:h4672. doi: 10.1136/bmj.h4672 [DOI] [PubMed] [Google Scholar]
- 24.Lapostolle F, Alhéritière A. To intubate or not intubate, that is still the question! Eur J Emerg Med. 2020;27(5):387-388. doi: 10.1097/MEJ.0000000000000726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Intubation difficulty score
eTable 2. Definition of pneumonia according to the US center of disease control
eTable 3. Calculation method of the hierarchical endpoint (Finkelstein-Schoenfeld method)
eTable 4. Intubation procedures
eTable 5. Secondary outcomes in the per protocol population
eTable 6. Characteristics of patients intubated in the control group
eMethods. P Value Calculation
Data Sharing Statement