Abstract
Background:
The adjuvant 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX or FLOX) have been the standard of care for resected colon cancer patients since 2004. Herein, we examined the change of outcomes over a ten-year period in stage III colon cancer patients who received this regimen.
Patients and Methods:
Individual patient data from the ACCENT database was used to compare the outcomes in an older (1998–2003) and newer (2004–2009) treatment eras of patients with stage III colon cancer who received adjuvant FOLFOX or FLOX. Outcomes were compared between the two groups by multivariate Cox proportional hazards model adjusting for age, gender, performance score, T stage, N stage, tumor sidedness, and histological grade.
Results:
A total of 6501 stage III patients, who received adjuvant FOLFOX or FLOX in six randomized trials, were included in the analysis. Patients enrolled in the newer era experienced statistically significantly improved time-to-recurrence (three-year rate, 76.1% versus 73.0%; adjusted hazard ratio [HRadj]=0.83, [95% CI, 0.74–0.92], P=0.0008), disease-free survival (DFS, three-year rate, 74.7% versus 72.3%; HRadj=0.88 [0.79–0.98], P=0.024), survival after recurrence (SAR, median time, 27.0 versus 17.7 months; HRadj=0.65 (0.57–0.74), P<0.0001), and overall survival (OS, five-year rate, 80.9% versus 75.7%; HRadj=0.78 (0.69–0.88), P<0.0001). The improved outcomes remained in patients diagnosed at 45 years or older, low-risk patients (T1–3 and N1), left colon, pMMR, BRAF, and KRAS wildtype tumors.
Conclusion:
Improved outcomes were observed in stage III colon cancer patients enrolled in clinical trials who received adjuvant FOLFOX/FLOX therapy in 2004 or later compared to older era. Prolonged SAR calls for re-validation of three-year DFS as the surrogate endpoint of OS in adjuvant clinical trials, and re-evaluation of optimal follow-up of OS to confirm trial findings based on DFS endpoints.
Clinical Trials Numbers:
NCT00079274; NCT00096278; NCT00004931; NCT00275210; NCT00265811; NCT00112918
Keywords: colon cancer, adjuvant, FOLFOX, stage III, disease-free survival, overall survival
Introduction
Patients with stage III colon cancer are a heterogeneous group with a wide range of five-year overall survival (OS) rates [1, 2]. In 2004, the MOSAIC trial investigators noted improved disease-free survival (DFS) with the addition of oxaliplatin to 5-fluorouracil (5-FU) and leucovorin (LV) (FOLFOX). The three-year DFS rate was 72.2% in the FOLFOX group compared to 65.3% in the 5-FU/LV group [3]. Ten-year follow-up confirmed the observed OS benefit of adding oxaliplatin in stage III colon cancer [4]. Hence, the combination of oxaliplatin and 5-FU/LV or capecitabine (i.e. FOLFOX or CAPOX) became, and has remained, the standard of care for stage III colon cancer after surgical resection [4, 5]. Using the ACCENT (Adjuvant Colon Cancer Endpoints) database, Shi et al. previously demonstrated an increase in survival after recurrence and overall survival over time of patients with stage II/III colon cancer who were treated with 5-FU-based regimen [6]. The same question was asked when standard of care shifted to 5-FU/LV+oxaliplatin with multiple newer therapeutic options available for advanced and recurrent diseases [7]. We now have nearly a decade of experience since 5-FU plus oxaliplatin regimens supplanted 5-FU plus LV as the standard of care in stage III disease, leading us to examine the change of outcomes over a ten-year period of patients with stage III colon cancer who had received oxaliplatin in addition to 5-FU plus LV adjuvant therapy.
Methods and Patients
This analysis focused on stage III colon cancer patients from six adjuvant trials included in the ACCENT database who were treated with 5-FU/LV+oxaliplatin (Appendix Table 1). Patients randomized to trial arms that included any biologics were excluded. In this report, we use “FOLFOX” as an inclusive term to encompass the variations. Approval to perform the analysis was granted by the Mayo Clinic Investigational Review Board (IRB). IRBs at individual treatment sites approved patient enrollment at their institutions in the individual trials at the time that these trials were conducted. In the current analysis, the patient enrollment period was dichotomized into two cohorts: older (1998–2003) versus newer (2004–2009) eras. The cut-off points were chosen to reflect the evolution of therapy and the introduction of biologic agents around 2004.
Outcomes included time to recurrence (TTR), DFS, SAR, and OS. DFS was defined as the time from randomization to recurrence or death from any cause. TTR was defined as time from randomization to disease recurrence, with death without recurrence censored at the time of death. SAR was defined as time from first documented recurrence to death from any cause. OS was defined as time from randomization to death from any cause. To control for potential confounding effects, all analyses were adjusted for patient pretreatment characteristics: age, gender, performance score, T stage, N stage, tumor sidedness, and histologic grade. The distribution of time-to-event outcomes were estimated using the adjusted Kaplan-Meier methods and compared between the two eras by multivariate Cox proportional hazards modeling. All analyses were conducted using two-sided tests with a significance level of 0.05.
Results
A total of 6501 patients with stage III colon cancer enrolled in six adjuvant trials and received FOLFOX as their only adjuvant treatment. Two trials (n=1532 [24%] patients) and four trials (n=4969 [76%] patients) were conducted in older (1998–2003) and newer (2004–2009) time, respectively (Table 1, Appendix Table 1). Overall, 1793 relapsed, 222 died without recurrence, and 4483 were alive without any recurrence with a median follow-up of 6.6 years.
Table 1.
Patient demographics and disease characteristics.
| Enrollment Time Era | |||
|---|---|---|---|
| 1998–2003 (N =1532) | 2004–2009 (N =4969) | P-value | |
|
| |||
| Age Group, N (%) | 0.06201 | ||
| <45 | 171 (11.2%) | 589 (11.9%) | |
| 45–65 | 912 (59.5%) | 3075 (61.9%) | |
| >65 | 449 (29.3%) | 1305 (26.3%) | |
| Gender, N (%) | 0.09351 | ||
| Female | 680 (44.4%) | 2327 (46.8%) | |
| Male | 852 (55.6%) | 2642 (53.2%) | |
| Performance Score, N (%) | 0.00871 | ||
| Missing | 4 | 75 | |
| 0 | 1293 (84.6%) | 3998 (81.7%) | |
| 1 | 235 (15.4%) | 896 (18.3%) | |
| T-Stage, N (%) | 0.00381 | ||
| Missing | 2 | 14 | |
| T1/2 | 191 (12.5%) | 656 (13.2%) | |
| T3 | 1157 (75.6%) | 3553 (71.7%) | |
| T4 | 182 (11.9%) | 746 (15.1%) | |
| N-Stage, N (%) | 0.00351 | ||
| Missing | 1 | 0 | |
| N1 | 993 (64.9%) | 3017 (60.7%) | |
| N2 | 538 (35.1%) | 1952 (39.3%) | |
| Total Evaluated Lymph Nodes, N (%) | <.00011 | ||
| Missing | 10 | 1284 | |
| <12 | 594 (39.0%) | 882 (23.9%) | |
| 12+ | 928 (61.0%) | 2803 (76.1%) | |
| Tumor Sidedness, N (%) | 0.01661 | ||
| Missing | 43 | 1044 | |
| Right Colon | 589 (39.6%) | 1497 (38.1%) | |
| Transverse | 144 (9.7%) | 301 (7.7%) | |
| Left Colon | 756 (50.8%) | 2127 (54.2%) | |
| Differentiation Grade, N (%) | 0.00061 | ||
| Missing | 34 | 58 | |
| Low Grade (Grade 1–2) | 1227 (81.9%) | 3820 (77.8%) | |
| High Grade (Grade 3/4/Anaplastic) | 271 (18.1%) | 1091 (22.2%) | |
| MMR Status, N (%) | 0.00011 | ||
| Missing | 644 | 1831 | |
| dMMR | 139 (15.7%) | 342 (10.9%) | |
| pMMR | 749 (84.3%) | 2796 (89.1%) | |
| KRAS Status, N (%) | 0.35271 | ||
| Missing | 880 | 1530 | |
| MT | 221 (33.9%) | 1231 (35.8%) | |
| WT | 431 (66.1%) | 2208 (64.2%) | |
| BRAF Status, N (%) | 0.01591 | ||
| Missing | 581 | 1478 | |
| MT | 123 (12.9%) | 356 (10.2%) | |
| WT | 828 (87.1%) | 3135 (89.8%) | |
Chi-Square p-value;
Kruskal-Wallis p-value;
Patient characteristics
Patient characteristics by newer versus older era were included in Table 1. Patients enrolled in the newer (versus older) era were more likely to have T4, N2 disease, a performance score of 1, higher tumor grade, and left-sided tumors with a moderate increase in proportion (<4.5%, P<0.02). However, much more patients in the newer era had ≥12 lymph nodes (LNs) examined compared to the older era (76.1% versus 61.0%, P<0.0001). Among patients with available molecular marker data, fewer patients enrolled in the newer (versus older) time era had DNA mismatch repair deficient (dMMR, 10.9% versus 15.7%, P=0.0001) and BRAF mutant (10.2% versus 12.9%, P=0.0159) tumors.
Outcomes
Figure 1 and Table 2 include detailed comparisons of outcomes between patients treated in two time eras. A statistically significant improvement of both DFS (adjusted three-year rate, 74.7% versus 72.3%, P=0.0235) and TTR (adjusted three-year rate, 76.1% versus 73.0%, P=0.0008) was observed in stage III colon cancer patients who received adjuvant FOLFOX in the newer versus older time era. Furthermore, patients enrolled in 2004 and afterward experienced longer survival after disease recurrence (SAR) compared to patients enrolled before 2004, with an adjusted median SAR increase from 17.7 to 27.0 months (P<0.0001). The gains in DFS, TTR, and especially SAR translated into OS improvement with a 5.2% absolute increase in adjusted five-year OS rate (80.9% versus 75.7%, P<0.0001) compared to patients treated in the older era.
Figure 1.
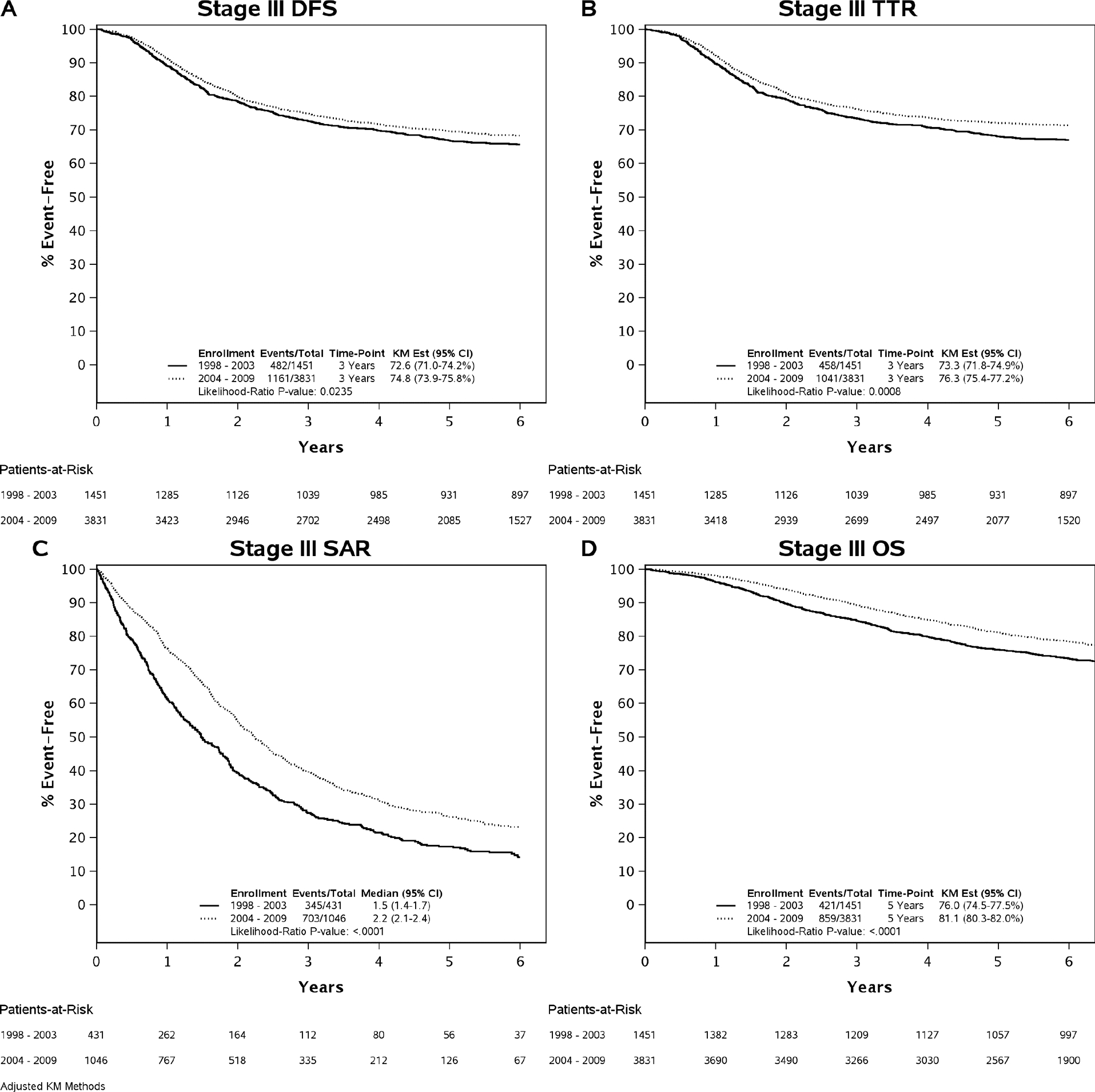
Comparing Clinical Outcomes between Newer and Old Era Trials. Abbreviations: KM=Kaplan-Meier; DFS=Disease-Free Survival; TTR=Time-to-Recurrence; SAR=Survival after Recurrence; OS=Overall Survival.
Table 2.
Multivariate Model results
| DFS (N =5282, Events=1643) |
TTR (N=5282, Events=1499) |
SAR (N=1477, Events=1048) |
OS (N=5282, Events=1280) |
|||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P -value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P -value | Hazard Ratio (95% CI) | P -value | |
|
| ||||||||
| Enrollment | 0.02351 | 0.00081 | <.00011 | <.00011 | ||||
| 1998–2003 | Reference | Reference | Reference | Reference | <.00012 | |||
| 2004–2009 | 0.88 (0.79–0.98) | 0.02232 | 0.83 (0.74–0.92) | 0.00072 | 0.65 (0.57–0.74) | <.00012 | 0.78 (0.69–0.88) | |
| Gender | 0.00111 | 0.02741 | 0.20461 | <.00011 | ||||
| Female | Reference | Reference | Reference | Reference | <.00012 | |||
| Male | 1.18 (1.07–1.30) | 0.00112 | 1.12 (1.01–1.24) | 0.02772 | 1.08 (0.96–1.22) | 0.20522 | 1.25 (1.12–1.40) | |
| Performance Score | 0.00301 | 0.03991 | 0.01331 | <.00011 | ||||
| 0 | Reference | Reference | Reference | Reference | <.00012 | |||
| 1 | 1.20 (1.07–1.35) | 0.00252 | 1.14 (1.01–1.30) | 0.03732 | 1.21 (1.04–1.40) | 0.01182 | 1.35 (1.18–1.54) | |
| T-Stage (Grouped) | <.00011 | <.00011 | 0.00081 | <.00011 | ||||
| T1/2 | 0.26 (0.21–0.32) | <.00012 | 0.22 (0.17–0.28) | <.00012 | 0.61 (0.44–0.84) | 0.00272 | 0.28 (0.21–0.36) | <.00012 |
| T3 | 0.53 (0.47–0.60) | <.00012 | 0.50 (0.44–0.56) | <.00012 | 0.79 (0.68–0.91) | 0.00142 | 0.54 (0.48–0.62) | <.00012 |
| T4 | Reference | Reference | Reference | Reference | ||||
| N-Stage | <.00011 | <.00011 | 0.00011 | <.00011 | ||||
| N1 | Reference | Reference | Reference | Reference | ||||
| N2 | 2.08 (1.88–2.29) | <.00012 | 2.24 (2.02–2.48) | <.00012 | 1.28 (1.13–1.45) | 0.00012 | 2.09 (1.87–2.34) | <.00012 |
| Age Group | 0.00621 | 0.47431 | 0.00661 | <.00011 | ||||
| <45 | 0.81 (0.68–0.96) | 0.01522 | 0.91 (0.76–1.09) | 0.30282 | 0.78 (0.62–0.97) | 0.02462 | 0.63 (0.52–0.77) | <.00012 |
| 45–65 | 0.85 (0.76–0.95) | 0.00352 | 0.94 (0.84–1.06) | 0.29412 | 0.81 (0.71–0.93) | 0.00242 | 0.72 (0.64–0.81) | <.00012 |
| >65 | Reference | Reference | Reference | Reference | ||||
| Tumor Sidedness | 0.04611 | 0.15821 | <.00011 | <.00011 | ||||
| Left Colon | Reference | Reference | Reference | Reference | ||||
| Right Colon | 1.14 (1.03–1.27) | 0.01302 | 1.11 (1.00–1.24) | 0.05442 | 1.61 (1.41–1.83) | <.00012 | 1.35 (1.20–1.52) | <.00012 |
| Transverse | 1.07 (0.90–1.29) | 0.44392 | 1.05 (0.87–1.27) | 0.63682 | 1.35 (1.08–1.70) | 0.00932 | 1.19 (0.97–1.46) | 0.09742 |
| Differentiation Grade | 0.02001 | 0.02971 | <.00011 | 0.00341 | ||||
| Low Grade (Grade 1–2) | Reference | Reference | Reference | Reference | ||||
| High Grade (Grade 3/4/Anaplastic) | 1.15 (1.02–1.29) | 0.01882 | 1.14 (1.01–1.29) | 0.02812 | 1.35 (1.17–1.55) | <.00012 | 1.21 (1.07–1.38) | 0.00302 |
Type 3 likelihood-ratio p-value;
Covariate Wald p-value;
Analysis by patient risk defined by T and N stage
There are significant interaction effects between risk groups (low: T1–3 N1 versus high: T4 and/or N2) and time eras for DFS (Pinteraction=0.042), TTR (Pinteraction=0.022), and OS (Pinteraction=0.033), except SAR (Pinteraction=0.51). Among low-risk stage III patients treated with FOLFOX, the improvement in DFS, TTR, and SAR was observed in the newer era compared to older era (adjusted three-year DFS rate=85.8% versus 81.1%; adjusted three-year recurrence rate, 87.0% versus 82.0%; adjusted median SAR=36.3 versus 22.6 months, P≤0.01). Finally, gains among the low-risk group in DFS, TTR, and SAR translated into an increased OS (adjusted five-year rate=89.3% versus 83.9%; P=0.0005). On the other hand, among high-risk stage III patients, only SAR showed significant improvement for those enrolled in the newer era compared to the older era (adjusted median time: 24.4 versus 14.8 months; P<0.0001).
Subgroup analysis by other factors
In addition to analysis by patient risk, Figure 2 shows comparisons of DFS, TTR, SAR, and OS in newer versus older era trials in subpopulations defined by age group, tumor sidedness, and mutation status with interaction P values. An improved SAR in patients in newer versus older era trials was consistently observed among all subgroups by age, tumor sidedness, and KRAS and BRAF mutation status, except for dMMR tumors (likely due to small sample size) (Figure 2).
Figure 2.
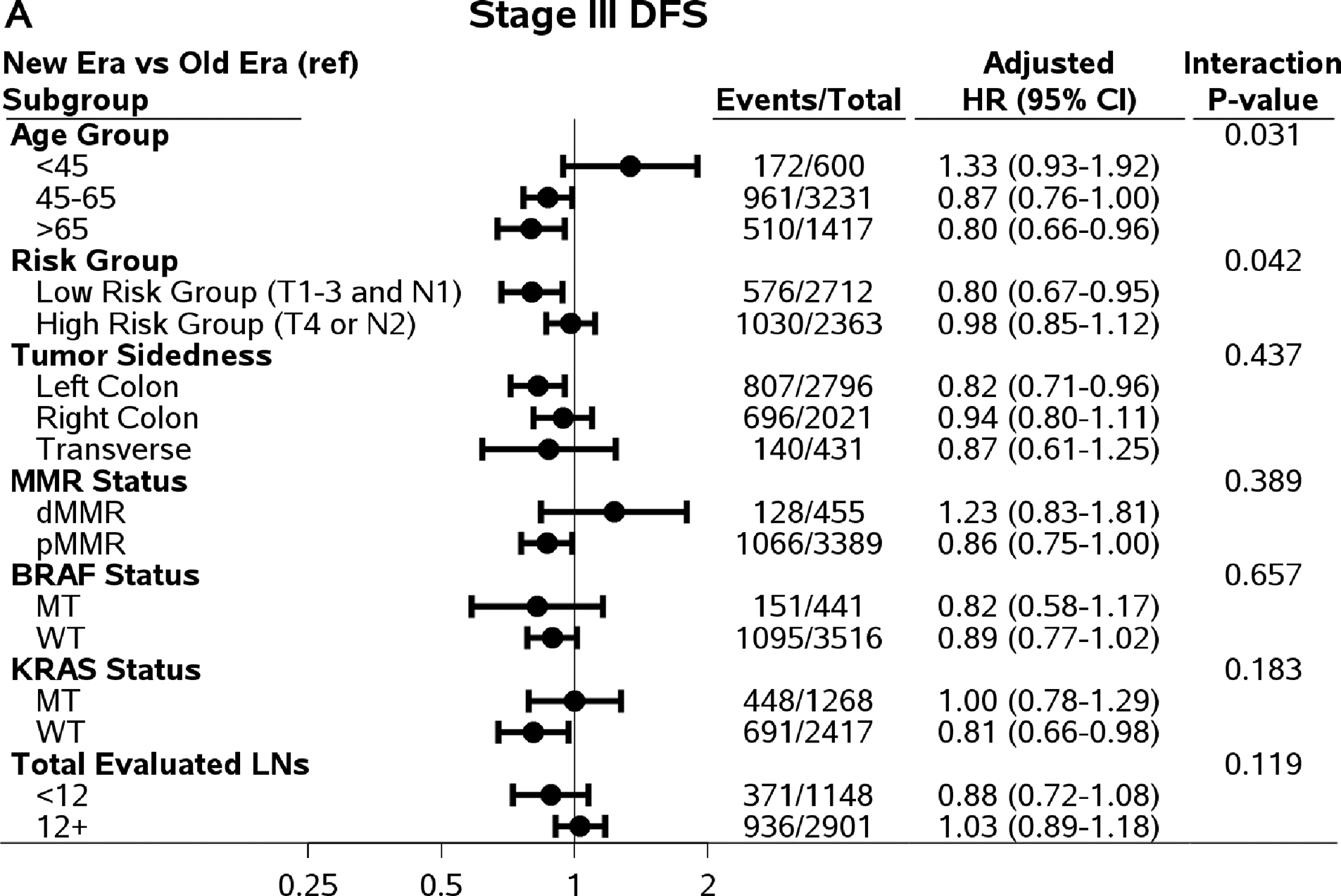
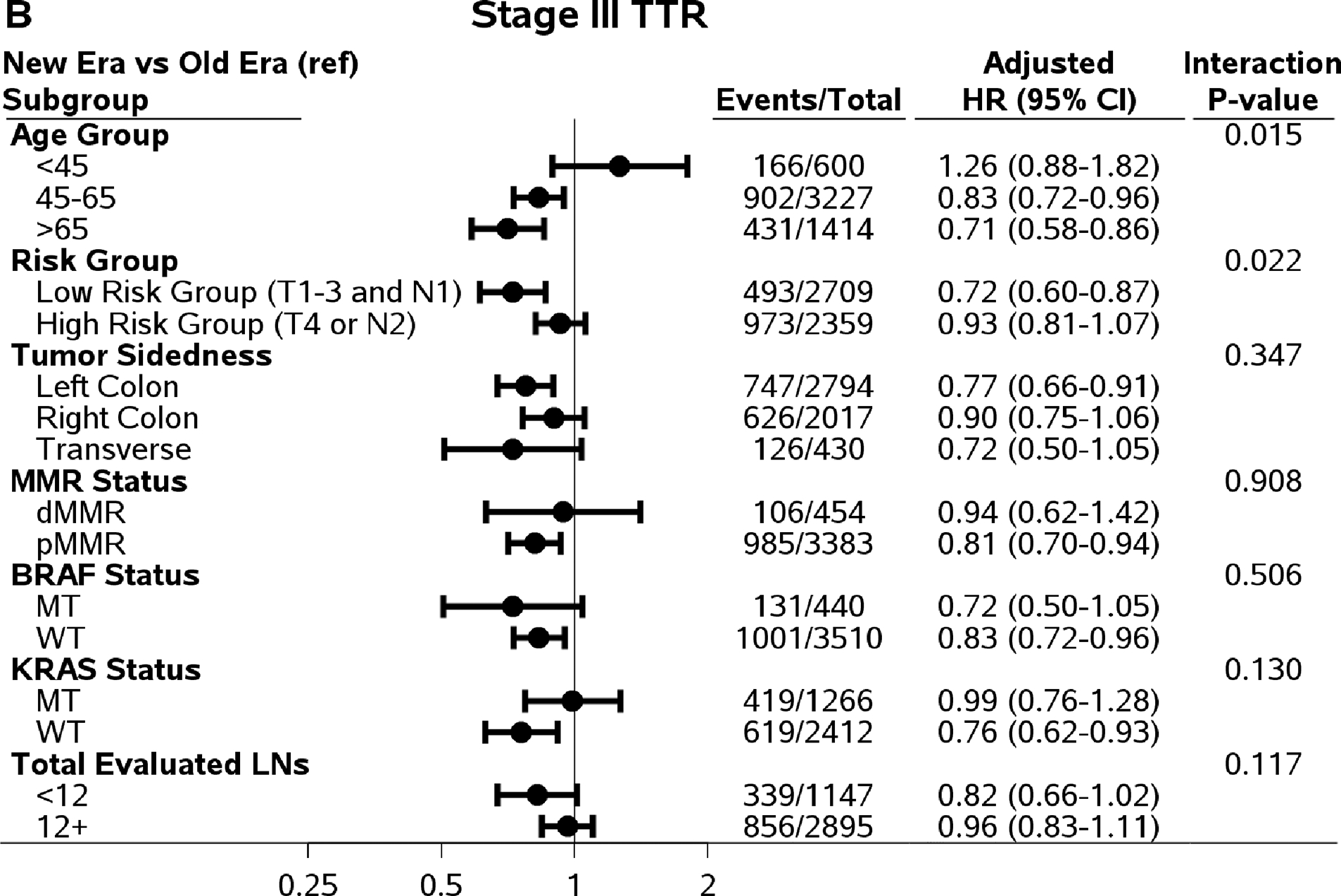
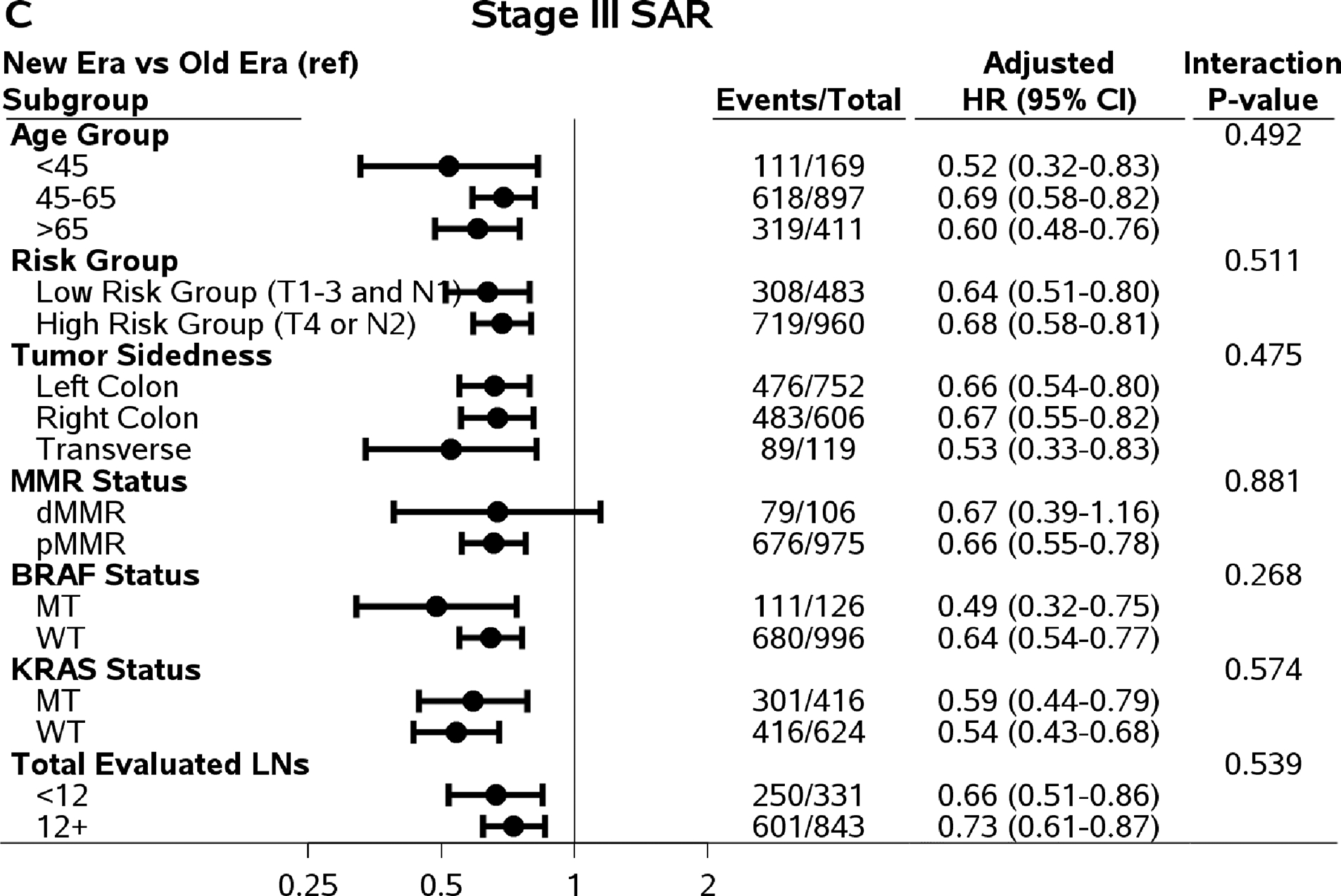
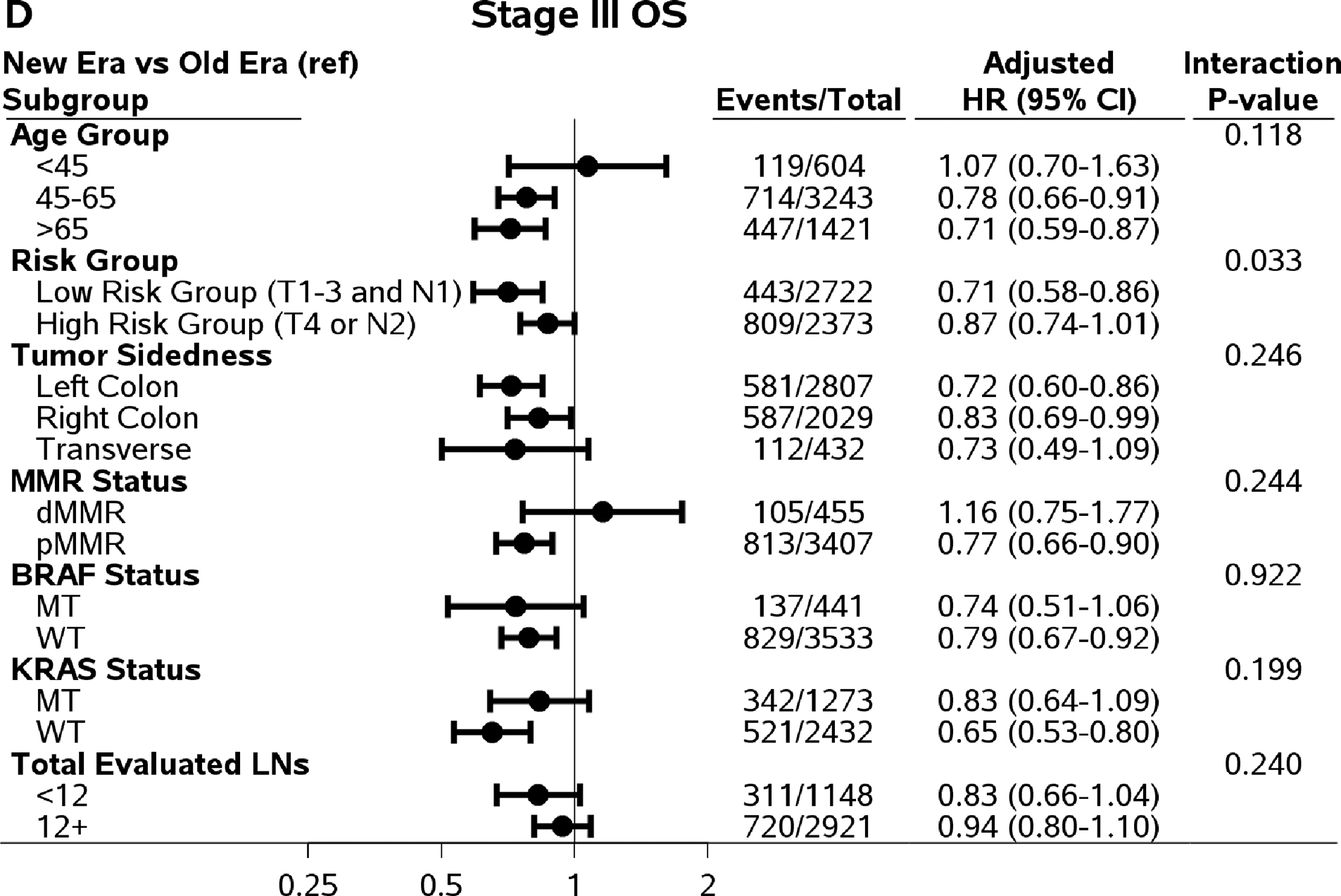
Comparing outcomes between time eras (newer versus old) by subpopulations. Abbreviations: DFS=Disease-Free Survival; TTR=Time-to-Recurrence; SAR=Survival after Recurrence; OS=Overall Survival; HR=Hazard Ratio; MT=Mutant Type; WT=Wild Type.
For DFS and TTR, only the interaction effect between age and time era reached statistical significance (Pinteraction=0.031 and 0.015, respectively). The increased three-year DFS, TTR, and five-year OS rates in newer versus older era remained among patients who were 45 years or older, not among patients <45 years of age (Figure 2). Although the interaction effects did not reach statistical significant level, the improvements in DFS, TTR, and OS remained in left-colon cancer, pMMR tumors, BRAF WT and KRAS WT tumors. It is worth noting that among patients with adequate number of LNs examined (12+), only SAR showed significant improvement (27.1 versus 19.0 months of median SAR) comparing patients treated in newer to older time era. The differences in three-year DFS (0%, 95% CI, −4.1 to 4.1%) and the difference in three-year TTR (0.5%, 95% CI, −3.6 to 4.6%) rates were small comparing those in patients treated in newer vs older time era.
Discussion
In the present study, we examined the potential change in long-term outcomes over time among stage III colon cancer patients who received adjuvant fluorouracil, leucovorin, and oxaliplatin chemotherapy after curative surgical resection. The use of a 5-FU plus oxaliplatin regimen not only is the current standard of care but also serves as the control treatment for testing novel regimens in adjuvant clinical trials. Hence, updated estimates of outcomes are critical to facilitate better communications with patients regarding treatment benefits, given potential toxicity in treatment decision-making, as well as assist the optimal design for ongoing and future adjuvant trials (e.g., sample size/power and follow-up duration considerations). Overall, we observed significant improvement in outcomes. These findings remained after adjusting for key biologic and clinical prognostic factors, suggesting a shift in biology may not drive this difference.
The three-year DFS rate was updated to 74.7% (95% CI, 73.4–76.1%) comparing post-2004 vs. pre-2004 era. This rate was consistent with the contemporary findings reported by IDEA collaboration–75.5% (95% CI, 74.4–76.7%) in six months of FOLFOX arm [8]. The subgroup analyses showed that the improvements in DFS (TTR) were more profound in elderly (65+ years old), low-risk (T1–3 N1), left-colon, KRAS and BRAF wildtype tumors (HRadjusted < 0.85, P<0.05). Although only four of six studies supplied data on LNs examined, we noticed that more patients in older era had <12 LNs examined compared to those in post-2004 era (39% versus 24%). This may suggest higher risk of under-staging of N2 disease and/or residual nodal disease in older than newer time era. The prognosis of patients with <12 (versus ≥12) LNs examined was inferior (three-year DFS rate=70.9% versus 74.6%; P=0.0025). This may partially contribute to the greater recurrence in the older time era in overall population. Furthermore, the improved DFS (and TTR) remained in low risk-group (T1–3 N1) but diminished in high-risk group (T4 or N2), potentially supporting the under-staging phenomenon. When the multivariable model included LNs examined as an additional covariate, the HRadjusted was attenuated for all outcomes (see Appendix Table 5). Furthermore, the complete mesocolic excision has been considered as the standard of care in many European countries, as well as the enhanced surgical support, which may contribute to improved outcomes.
There was a 5.2% (95% CI, 2.2–8.2%) absolute increase in five-year OS and about 22% reduction in the risk of death. This improvement could be largely driven by the prolongation in SAR. The updated median SAR of 27.0 months in post-2004 era is similar to the OS noted in patients with an initial diagnosis of metastatic disease [9]. Further exploration in the relative mortality rate after recurrence over time showed a delay in the time to reach the mortality rate peak after recurrence (Appendix Figure 2). More importantly, this finding was consistent regardless of patient clinical, pathological, and molecular marker status. This could be a strong indication of increase in post-recurrence hepatic resection [10], especially the access to palliative therapy involving biologic (e.g., bevacizumab and cetuximab) or immune-agents after recurrence. The prolonged SAR and OS can have two implications regarding adjuvant trial design and conduct. Firstly, an extended follow-up is needed to obtain sufficient number of deaths (i.e. statistical power) to demonstrate a treatment effect on OS. This can be shown by a hypothetical trial design considering OS as the primary endpoint with similar sample size using newer versus older era benchmark OS estimates (Appendix Table 3). Secondly, the prolonged SAR in patients treated with standard of care regimen (which will be the control arm in future trial testing novel agents) may reduce the correlation between DFS and OS endpoints, as suggested by De Gramont et al [11]. This raises the question whether three-year DFS remains a validated surrogate endpoint of five-year OS with the shifts in the choice of control arm as well as the baseline estimates in these endpoints.
Another interesting finding is that of the impact of age on outcomes. Although the benefits from fluoropyrimidine are well established in elderly patients [12], the doublet with oxaliplatin showed mixed results for benefit in different meta-analyses [13, 14]. The OS improvements in patients over 65 years of age in the newer compared to older era trials may reflect the advances we have made in the supportive care of these patients (e.g., increased medical and surgical support), permitting them to benefit more from doublet chemotherapy. Similar to high-risk (T4 or N2) patients, outcomes did not show improvement comparing newer versus older time era in early onset patients (age <45). This might give hints of different risk for early-onset patients.
We acknowledge that there are several limitations. The dose and delivery schedule of the FOLFOX regimen and its variations in the included trials have evolved over time, especially with increased dose of 5-FU, comparing mFOLFOX6 (newer era) to FOLFOX4 (both eras) and FLOX (older era) (Appendix Table 2). By examining the dose intensity data reported in the original publications of included trials (see Appendix Table 1), there was a trend that the treatment completeness, especially for 5-FU, was higher in post-2004 trials. This may indicate the greater experience with the regimen and better toxicity management, despite the increased dose. Restricted to FOLFOX4 only, the results are consistent with the overall population (Appendix Figure 1). Additional analysis with regimen included as a covariate did not find differences in outcomes between variations of FOLFOX treatment (Appendix Table 4). Therefore, the heterogeneity in dose and delivery schedule of the therapy may not bias the results.
There are imbalances in several clinical and pathological characteristics between older and newer era patient cohorts. However, after adjusting for these factors, the improvements in outcomes remained. Two of the newer era trials (N0147 and PETACC8; both testing efficacy of cetuximab) amended their design to restrict the randomization to patients with KRAS-wild type tumors only. However, in the N0147 study, post-amendment patients with mutant KRAS tumor were still followed up for outcomes and included in the current analyses. Further sensitivity analyses were conducted among patients with KRAS wild-type tumors, and results showed consistent findings in outcomes over time (Figure 1).
In conclusion, we observed improved DFS, TTR, SAR, and OS in patients with stage III colon cancer treated with oxaliplatin and 5-FU/LV adjuvant therapy in the newer versus the older trial era. Adherence to sufficient lymph nodes examinations is continuously recommended for accurate diagnosis that provides better treatment decision-making. Prolonged SAR calls for re-validation of three-year DFS as the surrogate endpoint of OS in adjuvant clinical trials, and re-evaluation of optimal follow-up of OS to confirm trial findings based on DFS endpoints.
Supplementary Material
Appendix Figure 1. Comparing outcomes between time eras (newer versus old) among patients receiving FOLFOX4 only
Appendix Figure 2 Mortality rate by time from recurrence (among patients with relapse)
Footnotes: Risk of death in each six-month interval following recurrence among patients remaining alive at the start of each interval
Key Message:
Improved outcomes were observed in stage III colon cancer patients enrolled in clinical trials who received adjuvant FOLFOX/FLOX therapy in 2004 or later compared to older era. The results call for re-validation of three-year DFS as the surrogate endpoint of OS in adjuvant clinical trials, and re-evaluation of optimal follow-up of OS to confirm trial findings based on DFS endpoints.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number U10CA180882. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health [grant number: U10CA180882]
Footnotes
Disclosure
The authors have declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gill S, Loprinzi CL, Sargent DJ et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004; 22: 1797–1806. [DOI] [PubMed] [Google Scholar]
- 2.Gunderson LL, Jessup JM, Sargent DJ et al. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol 2010; 28: 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre T, Boni C, Mounedji-Boudiaf L et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–2351. [DOI] [PubMed] [Google Scholar]
- 4.Andre T, de Gramont A, Vernerey D et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol 2015; 33: 4176–4187. [DOI] [PubMed] [Google Scholar]
- 5.Kuebler JP, Wieand HS, O’Connell MJ et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007; 25: 2198–2204. [DOI] [PubMed] [Google Scholar]
- 6.Shi Q, Andre T, Grothey A et al. Comparison of outcomes after fluorouracil-based adjuvant therapy for stages II and III colon cancer between 1978 to 1995 and 1996 to 2007: evidence of stage migration from the ACCENT database. J Clin Oncol 2013; 31: 3656–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel A, Hofheinz RD, Kubicka S, Arnold D. Treatment decisions in metastatic colorectal cancer - Beyond first and second line combination therapies. Cancer Treat Rev 2017; 59: 54–60. [DOI] [PubMed] [Google Scholar]
- 8.Grothey A, Sobrero AF, Shields AF et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med 2018; 378: 1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venook AP, Niedzwiecki D, Lenz HJ et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017; 317: 2392–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopetz S, Chang GJ, Overman MJ et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27: 3677–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Gramont A, Hubbard J, Shi Q et al. Association between disease-free survival and overall survival when survival is prolonged after recurrence in patients receiving cytotoxic adjuvant therapy for colon cancer: simulations based on the 20,800 patient ACCENT data set. J Clin Oncol 2010; 28: 460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sargent DJ, Goldberg RM, Jacobson SD et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001; 345: 1091–1097. [DOI] [PubMed] [Google Scholar]
- 13.Haller DG, O’Connell MJ, Cartwright TH et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol 2015; 26: 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCleary NJ, Meyerhardt JA, Green E et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013; 31: 2600–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1. Comparing outcomes between time eras (newer versus old) among patients receiving FOLFOX4 only
Appendix Figure 2 Mortality rate by time from recurrence (among patients with relapse)
Footnotes: Risk of death in each six-month interval following recurrence among patients remaining alive at the start of each interval


