ABSTRACT
Hypertension is very common and remains often poorly controlled in patients with chronic kidney disease (CKD). Accurate blood pressure (BP) measurement is the essential first step in the diagnosis and management of hypertension. Dietary sodium restriction is often overlooked, but can improve BP control, especially among patients treated with an agent to block the renin–angiotensin system. In the presence of very high albuminuria, international guidelines consistently and strongly recommend the use of an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker as the antihypertensive agent of first choice. Long-acting dihydropyridine calcium channel blockers and diuretics are reasonable second- and third-line therapeutic options. For patients with treatment-resistant hypertension, guidelines recommend the addition of spironolactone to the baseline antihypertensive regimen. However, the associated risk of hyperkalemia restricts the broad utilization of spironolactone in patients with moderate-to-advanced CKD. Evidence from the CLICK (Chlorthalidone in Chronic Kidney Disease) trial indicates that the thiazide-like diuretic chlorthalidone is effective and serves as an alternative therapeutic opportunity for patients with stage 4 CKD and uncontrolled hypertension, including those with treatment-resistant hypertension. Chlorthalidone can also mitigate the risk of hyperkalemia to enable the concomitant use of spironolactone, but this combination requires careful monitoring of BP and kidney function for the prevention of adverse events. Emerging agents, such as the non-steroidal mineralocorticoid receptor antagonist ocedurenone, dual endothelin receptor antagonist aprocitentan and the aldosterone synthase inhibitor baxdrostat offer novel targets and strategies to control BP better. Larger and longer term clinical trials are needed to demonstrate the safety and efficacy of these novel therapies in the future. In this article, we review the current standards of treatment and discuss novel developments in pathophysiology, diagnosis, outcome prediction and management of hypertension in patients with CKD.
Keywords: chlorthalidone, chronic kidney disease, hypertension, RAS blockade, spironolactone
INTRODUCTION
Hypertension and chronic kidney disease (CKD) commonly coexist and the interrelation between these two pathophysiological states is bidirectional [1, 2]. Persistently high blood pressure (BP) can accelerate the progression of CKD and the progressive decline in the estimated glomerular filtration rate (eGFR) can conversely interfere with the achievement of adequate BP control [2]. The coexistence of uncontrolled hypertension and CKD substantially magnifies the risk of cardiovascular disease, which is the most important cause of morbidity and mortality in patients with CKD [3]. Although clinical trials have failed to demonstrate that intensive BP lowering results in a lower rate of kidney function decline [4–6], interventions to lower BP are generally believed to be effective in attenuating the risk of adverse cardiovascular outcomes and all-cause mortality in the CKD population [7–9].
In this article, we review the current standards of treatment and discuss novel developments in the pathophysiology, diagnosis, outcome prediction and management of hypertension in CKD (Box 1).
TREATMENT STANDARDS
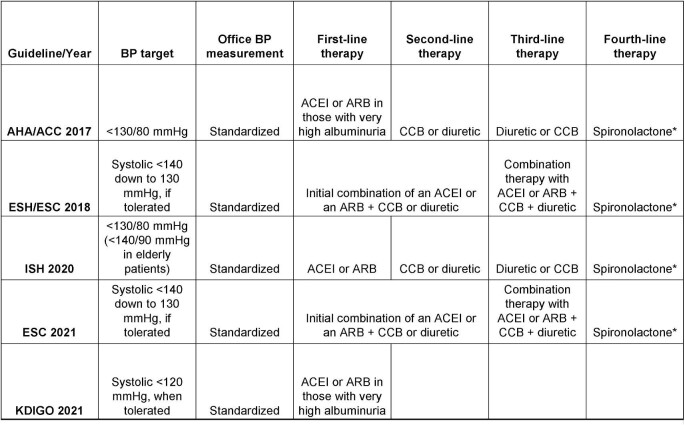
An overview of currently available guidelines for the assessment and management of hypertension in CKD is depicted in Fig. 1. The first critical step is the accurate measurement of office BP (Box 2). Most data that guide our therapeutic decisions are derived from clinical trials that incorporated a standardized BP measurement methodology in the office. As an example, SPRINT (Systolic Blood Pressure Intervention Trial) was a landmark trial demonstrating that among non-diabetic adults at high cardiovascular risk, as compared with <140 mmHg, targeting a systolic BP (SBP) <120 mmHg lowered by 25% the relative risk of cardiovascular morbidity and mortality [9]. The protocol of SPRINT specified a 5-min seated rest period followed by three automated office BP (AOBP) recordings taken without the presence of an observer in a quiet room [9]. A diagnostic test study explored the relation of this research-grade technique to routine office BP in 275 patients with CKD [10]. Compared with routine measurement, research-grade office SBP was 12.7 mmHg lower [10]. However, this comparison provides only an estimate of the mean difference between these two techniques at a population level. The 95% limits of agreement were wide, indicating that individual patients may have differences from routine office SBP ranging from 46.1 mmHg lower up to 20.7 mmHg higher [10]. Therefore, there is no single correction factor to convert a routine BP value into a research-grade BP value [11]. To implement intensive BP-lowering in daily clinical practice, the minimum requirement is the adoption of the research-grade BP measurement methodology in our daily practices.
Figure 1:
Summary of recent guideline recommendations for the assessment and management of hypertension in patients with CKD. *The use of spironolactone as fourth-line therapy is discouraged in patients with eGFR <45 mL/min/1.73 m2 or with a serum potassium concentration of >4.5 mmol/L. AHA/ACC: American Heart Association/American College of Cardiology; ESC: European Sociaty of Cardiology; ESH: European Society of Hypertension; ISH: International Society of Hypertension.
Box 1. “In a nutshell.”
Research grade BP measurement is no longer for research alone but for everyday practice.
Dietary Na restriction can improve BP in individuals and provide low-cost public health benefits.
ACEIs or ARBs remain the first-line choice in pharmacotherapy of hypertension in patients with CKD and very high albuminuria.
Spironolactone is the standard-of-care treatment of resistant hypertension, but the associated risk of hyperkalemia limits its broad utilization in patients with moderate-to-advanced CKD.
The thiazide-like diuretic chlorthalidone is effective in improving BP control in patients with an eGFR <30 mL/min/1.73 m2 and serves as an alternative therapeutic option for managing resistant hypertension in advanced CKD.
Discontinuation of ACEIs or ARBs in patients with advanced and progressive CKD nearing the initiation of dialysis does not result in stabilization of the long-term decline in kidney function.
Newer BP-lowering medications, such as the non-steroidal MRA ocedurenone, the aldosterone synthase inhibitor baxdrostat and the dual endothelin receptor antagonist aprocitentan, are currently under investigation in clinical trials, offering hope for improved BP control with fewer adverse events and better treatment tolerability in the near future.
Box 2. Strategies for the individualization of antihypertensive treatment.
The initiation and intensification of antihypertensive therapy should be guided at least by BP measurements taken under standardized conditions, as recommended by guidelines.
Dietary Na restriction is an important component of management of hypertension, especially among patients with CKD.
Choice of the appropriate antihypertensive agent should take into consideration the presence and severity of albuminuria. In CKD patients with very high albuminuria, in the absence of contraindications, ACEIs or ARBs are recommended as the antihypertensive agents of first choice.
For patients with moderate-to-advanced CKD and resistant hypertension who cannot tolerate add-on therapy with spironolactone, the administration of a potassium-binding polymer can mitigate the risk of hyperkalemia to enable the more persistent use of spironolactone. Whether this strategy results in greater regression of hypertension-related target-organ damage or in improved cardiorenal outcomes is currently unknown.
The thiazide-like diuretic chlorthalidone is an alternative choice for managing resistant hypertension in patients with advanced CKD, but its use requires careful monitoring of BP, serum electrolytes and kidney function for the prevention of adverse events.
In patients who are concomitantly treated with a loop diuretic, chlorthalidone can be administered at a lower starting dose (i.e. 12.5 mg every other day) in the hope of improving BP control with fewer adverse events.
β-blockers are not recommended by guidelines as first-line therapies, but this drug category is useful for the treatment of hypertension is CKD patients with specific indications (i.e. heart failure with reduced ejection fraction or after an acute myocardial infarction).
The diagnosis of hypertension can be improved with the use of ambulatory BP monitoring (ABPM), considered as the reference standard [12]. ABPM is typically performed over 24 h and BP is recorded every 15–20 min during daytime and every 30 min during nighttime [13]. Therefore, a unique advantage of ABPM is that this technique enables the diagnosis of nocturnal hypertension and abnormal diurnal variation of BP (i.e. non-dipping and reverse-dipping BP pattern). These abnormalities in 24-h BP profiles are frequently diagnosed in patients with CKD and are associated with faster progression of kidney injury and increased risk of cardiovascular morbidity and mortality [12]. Home BP monitoring (HBPM) is another method to assess BP outside of the office. In this technique, patients are trained to obtain standardized BP measurements at home (two recordings in the morning and two recordings at bedtime) for at least 3 days, and preferably 7 days, using validated automated BP monitors [14]. In a similar fashion to ABPM, cohort studies showed that 1-week averaged home BP is of superior predictive value as compared with office BP in patients with CKD [15, 16]. However, home BP recording is less reproducible than ABPM and in people with CKD, does not aid in making a diagnosis of masked uncontrolled hypertension (MUCH) [17]. Nevertheless, an advantage of HBPM is also the fact that this technique is more broadly available and can be repeatedly used to monitor the BP-lowering response to antihypertensive therapy over long-term periods of follow-up [14] and therefore surmount therapeutic inertia [18].
The 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend that dietary sodium intake should be restricted to levels <90 mmol of sodium per day as an effective non-pharmacological intervention for the treatment of hypertension in people with CKD [19]. Support for this guidance was provided by an updated Cochrane meta-analysis showing that among patients with CKD, a mean reduction of 73.51 mmol/day in dietary sodium intake is associated with an average reduction of 6.91/3.91 mmHg in office BP and with a 36% reduction in albuminuria [20]. Post hoc analyses of clinical trials showed that dietary sodium restriction enhances the albuminuria-lowering action of renin–angiotensin system (RAS) blockers in patients with albuminuric CKD [21]. Larger and longer-term clinical trials are warranted to elucidate whether these benefits on intermediate endpoints are translated into a long-term improvement in “hard” cardiorenal outcomes. With respect to the dietary potassium intake, a recent open-label, cluster-randomized trial involving 20 995 people who had a history of prior stroke or were 60 years of age or older and had a history of hypertension showed that as compared with regular salt consumption (100% NaCl), the use of a potassium-containing salt substitute (75% NaCl and 25% KCl) lowered by 14% the risk of stroke, by 13% the risk of major adverse cardiovascular events and by 13% the risk of all-cause mortality [22]. However, these results from trials conducted in the general population may not be generalizable to patients with CKD. An earlier systematic review of 11 observational studies incorporating data from 49 573 patients with CKD revealed that the use of diets rich in potassium is not associated with a lower rate of kidney function decline [23]. In contrast, short-term clinical trials showed that among patients with moderate-to-advanced CKD, dietary potassium supplementation raises the risk of hyperkalemia [24].
When BP remains uncontrolled, the administration of antihypertensive therapy is the next step in the management of hypertension. Information with respect to doses, precautions and side effects of most commonly prescribed antihypertensive medications is provided in Table 1. For patients with high BP, CKD and very high albuminuria, the 2021 KDIGO guidelines provide a strong (Level 1B) recommendation that an angiotensin-converting enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB) should be the antihypertensive agent of first choice [19]. The use of RAS blockade as first-line therapy in albuminuric CKD is consistently supported by all major hypertension guidelines on the basis of robust clinical trial evidence [25, 26]. The RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan) trial showed that among 1513 patients with type 2 diabetes (T2D) and albuminuric CKD, losartan improved by 16% the composite outcome of doubling of serum creatinine, end-stage kidney disease (ESKD) or death relative to placebo [27]. In the Irbesartan Diabetic Nephropathy Trial [28], irbesartan was superior to placebo or active treatment with amlodipine in retarding the progression of kidney injury to ESKD in 1715 patients with albuminuric CKD associated with T2D. The AASK (African American Study of Kidney Disease and Hypertension) trial showed that among 1094 African-Americans with hypertensive nephrosclerosis, ramipril provoked relative risk reductions of 22% and 38% in the composite outcome of ≥50% decline in GFR from baseline, ESKD or death as compared with metoprolol and amlodipine, respectively [6]. In contrast, the evidence basis for a kidney protective effect of RAS blockade in non-diabetic patients with CKD and moderately increased albuminuria is less persuasive and the preferential initiation of an ACEI or an ARB as first-line therapy in this setting is not strongly recommended by guidelines [19]. Furthermore, the combination of an ACEI with an ARB is contraindicated. In Veterans Affairs Nephropathy in Diabetes, as compared with monotherapy, the increased risk of hyperkalemia and acute kidney injury with the combination of an ACEI and an ARB led to the premature termination of the trial [29]. The Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints trial also was stopped early, because the addition of the direct renin inhibitor aliskiren to standard treatment with a RAS blocker increased the risk of hyperkalemia and hypotension [30].
Table 1:
Commonly prescribed antihypertensive drugs, usual drug doses, precautions and side effects.
| Drug class and druga | Usual dose | Common side effects | Potential contraindications | Additional considerations |
|---|---|---|---|---|
| ACEIs | ||||
| Lisinopril PerindoprilRamiprilTrandolapril | 10–40 mg/day2–8 mg/day5–10 mg/day0.5–4 mg/day | Cough; angioedema; hyperkalemia; leucopenia; anemia | Hyperkalemia; pregnancy; bilateral renal artery stenosis | First-line antihypertensive agents in patients with severely increased albuminuria |
| ARBs | ||||
| CandesartanIrbesartanLosartanOlmesartanTelmisartanValsartan | 8–32 mg/day75–300 mg/day50–100 mg/day10–40 mg/day40–80 mg/day80–320 mg/day | Cough (less commonly than with ACEIs); angioedema; hyperkalemia; anemia | Hyperkalemia; pregnancy; bilateral renal artery stenosis | First-line antihypertensive agents in patients with severely increased albuminuria |
| Dihydropyridine CCBs | ||||
| AmlodipineFelodipineManidipine | 5–10 mg/day5–10 mg/day10–20 mg/day | Lower-extremity edema; gingival; hypertrophy | Worsening of albuminuria | |
| Non-dihydropyridine CCBs | ||||
| VerapamilDiltiazem | 180–360 mg/day180–360 mg/day | Constipation; gingival hyperplasia | 2nd or 3rd degree heart block | Reduction in albuminuria; increase the levels of calcineurin and mTOR inhibitors; drug interactions (i.e. β-blockers, statins) |
| Thiazide or thiazide-like diuretics | ||||
| HydrochlorothiazideChlorthalidoneMetonazole | 12.5–25 mg/day12.5 mg/day2.5 mg/day | Hyperuricemia; hypercalcemia; hyponatremia; hypokalemia; hyperglycemia | Hyponatremia; hypokalemia; hypercalcemia; volume depletion | The thiazide-like diuretic chlorthalidone is effective in lowering BP in patients with stage 4 CKD and poorly controlled hypertension |
| Loop diuretics | ||||
| FurosemideTorsemide | 40–80 mg/day20 mg/day | Hearing loss; hypokalemia; hypocalcemia; hyponatremia | Volume depletion | Torsemide has better bioavailability and longer elimination half-life as compared with furosemide |
| Steroidal MRAs | ||||
| SpironolactoneEplerenone | 25–50 mg/day50–100 mg/day | Hyperkalemia; metabolic acidosis; gynecomastia | Hyperkalemia | Spironolactone is useful in resistant hypertension as fourth-line therapy |
| β-adrenergic receptor blockers | ||||
| AtenololBisoprololCarvedilolMetoprololNebivolol | 25–100 mg/day2.5–10 mg/day12.5–25 mg twice daily50–100 mg twice daily2.5–10 mg/day | Bradycardia; hyperkalemia; fatigue; depression; sexual dysfunction | Bradycardia; asthma; chronic obstructive pulmonary disease; 2nd or 3rd degree heart block | β-blockers are recommended for the management of hypertension in patients with specific cardiovascular indication for their use |
This is a list of selected medications from each antihypertensive drug category; the use of antihypertensive agents may differ from country to country.
mTOR: mammalian target of rapamycin.
Most patients with CKD require multiple medications to achieve adequate BP control. Accordingly, second-line therapy can include either a long-acting dihydropyridine calcium channel blocker (CCB) or a diuretic [25, 26], with the latter being a more appropriate option for patients with clinical signs or symptoms of volume excess. Third-line therapy in the algorithm completes the combination of a RAS blocker, a dihydropyridine CCB and a diuretic [25, 26]. The use of single-pill combinations is preferable; reducing pill burden simplifies treatment and associates with an improvement in treatment adherence and better BP control rates [31]. With respect to diuretic therapy, higher doses are typically necessary to achieve a therapeutic effect in patients with CKD. Of the loop diuretics, torsemide may be preferable over furosemide, because it can be dosed once daily and its BP effect in people with CKD is similar to twice-daily furosemide [32, 33]. In addition, most of guidelines released over the past years recommend the use of a loop diuretic when the eGFR is <30 mL/min/1.73 m2 [25, 26], because thiazide or thiazide-like diuretics were generally considered as ineffective in patients with advanced CKD. This established therapeutic approach has been recently challenged by the results of the CLICK (Chlorthalidone in Chronic Kidney Disease) trial [34]. In CLICK, 160 patients with stage 4 CKD and uncontrolled hypertension were randomized to receive the thiazide-like diuretic chlorthalidone (at a starting dose of 12.5 mg/day) or placebo for 12 weeks. Relative to placebo, chlorthalidone provoked a reduction of 10.5 mmHg in 24-h ambulatory SBP [34]. This potent BP-lowering effect was paralleled with a placebo-subtracted reduction of 50% in albuminuria, preliminary data supporting a potential cardiorenal protective action of chlorthalidone [34]. However, the use of this agent in advanced CKD requires careful monitoring of the patients for the prevention of adverse events. In CLICK, hypokalemia, reversible deterioration of kidney function, hyperglycemia, orthostatic hypotension, dizziness and hyperuricemia occurred more commonly with chlorthalidone than with placebo, particularly in the subgroup of patients receiving concomitant treatment with a loop diuretic [34]. In such patients, we recommend starting chlorthalidone at a lower dose (i.e. 12.5 mg every other day) in the hope of lowering the risk of adverse events [35].
Patients whose BP remains uncontrolled despite adherence to maximally tolerated doses of a RAS blocker, a CCB and a diuretic fulfill the diagnostic criteria of resistant hypertension [36]. In such patients, the spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2) trial demonstrated that the addition of spironolactone to the baseline antihypertensive regimen is superior to placebo as well as superior to doxazosin or bisoprolol in reducing home SBP over 12 weeks [37]. However, PATHWAY-2 excluded patients with eGFR <45 mL/min/1.73 m2 [37]. Based largely on these clinical trial data, spironolactone is recommended by guidelines as the fourth-line agent for the treatment of resistant hypertension, but the use of spironolactone is discouraged in patients with an eGFR <45 mL/min/1.73 m2 and a serum potassium concentration of >4.5 mmol/L [26].
Despite the fact that the prevalence of resistant hypertension is 2- to 3-fold higher in patients with moderate-to-advanced CKD than in the general population [38–40], the available therapeutic options for this particular subgroup of high-risk patients are few. A 2020 Cochrane meta-analysis showed that among patients with albuminuric CKD, the use of spironolactone in combination with an ACEI or an ARB (or both) is associated with a 2.17-fold higher incidence of hyperkalemia and a 5.14-fold higher risk of gynecomastia [41]. Since hyperkalemia acts as a barrier and limits the broad utilization of spironolactone [42, 43], the AMBER (Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease) trial randomized 295 patients with eGFR ranging from 25 to ≤45 mL/min/1.73 m2 and uncontrolled resistant hypertension to receive spironolactone in addition to double-blind treatment either with the potassium-binding polymer patiromer or with placebo [44]. Patiromer enabled more patients to maintain on spironolactone treatment as compared with placebo. However, even with the simultaneous administration of a potassium-binding polymer, approximately one-third of patients who received spironolactone developed hyperkalemia over 12 weeks of follow-up [44].
Taking into consideration the associated risk of hyperkalemia and the general underutilization of spironolactone, an alternative therapeutic option for the management of resistant hypertension in advanced CKD could be the administration of chlorthalidone. A subgroup analysis of the CLICK trial incorporating data from 113 patients with resistant hypertension at baseline showed that as compared with placebo, chlorthalidone provoked a reduction of 13.9 mmHg in 24-h ambulatory SBP at Week 12 [45]. Unlike spironolactone, the risk of hyperkalemia with chlorthalidone is practically nonexistent. However, as mentioned above, the use of chlorthalidone is also associated with adverse events and requires careful monitoring of BP, serum electrolytes and kidney function [45].
β-blockers are not recommended by guidelines for use as monotherapy or as first-line agents in pharmacotherapy of uncomplicated hypertension [25, 26]. However, this drug category is proven to be efficacious and should be considered for the treatment of hypertension in patients with specific cardiovascular indications for β-blocker use, such as in patients with heart failure with reduced ejection fraction, angina and atrial fibrillation, or after an acute myocardial infarction [46]. Furthermore, β-blockers may be useful for the treatment of resistant hypertension, when spironolactone is either contraindicated or not tolerated [26]. In the aforementioned PATHWAY-2 trial [37], bisoprolol was not as effective as spironolactone, but it was superior to placebo in reducing home BP when added to the background antihypertensive regimen.
NEW DEVELOPMENTS
Pathogenesis
Population-based studies show that the prevalence of hypertension increases in parallel with worsening stage of CKD [39, 47]. These epidemiological data generate the impression that the severity of hypertension travels with the progressive eGFR decline. However, accumulated evidence suggests that albuminuria plays an even more important role. As examples, a cross-sectional study explored the association of 17 risk factors for hypertension with the levels of SBP in 232 US Veterans with CKD. In multivariate models, it was the urinary protein-to-creatinine ratio the factor that was more strongly associated with SBP regardless of the technique of BP measurement [48]. As compared with standardized or routine office recordings, the association between proteinuria and SBP was stronger when hypertension was assessed using ABPM or HBPM. In sharp contrast, eGFR was not an independent determinant of SBP by any technique [48]. A subsequent analysis of 336 US Veterans with or without CKD who underwent 24-h ABPM showed that as compared with the stage of CKD, proteinuria was a stronger determinant of a disrupted circadian BP rhythm [49]. Compared with eGFR decrements, even small increments in the levels of proteinuria had a more dramatic impact on the mean levels of ambulatory BP [49]. The mechanisms through which proteinuria and hypertension are closely interrelated remain unclear. Proteinuria may simply reflect the presence of more severe kidney damage or reflect worse endothelial dysfunction [50].
Diagnosis
MUCH is diagnosed in patients who are being treated for hypertension, when they have a normal office BP but high out-of-office BP [26, 37]. The phenotype of MUCH is identified more commonly in patients with CKD than in the general population [51]. Among 333 US veterans with CKD and a normal office BP, the prevalence of MUCH depended on how hypertension was defined. MUCH was prevalent in 27% of the patients when daytime ambulatory BP ≥135/85 mmHg was used to diagnose hypertension. It was 33% when hypertension was defined as a 24-h ambulatory BP ≥130/80 mmHg and increased to 56% when either daytime ambulatory BP ≥135/85 mmHg or nighttime ambulatory BP ≥120/70 mmHg was used [17]. The prevalence of MUCH is progressively increased with increasing levels of office BP. Patients with repeatedly low BP in the office are unlikely to have MUCH. In contrast, the suspicion of MUCH should be raised when office BP is within the prehypertensive range. Among patients with office SBP of 130–139 mmHg, MUCH is diagnosed in two in three, and among patients with office SBP of 120–129 mmHg MUCH is prevalent in one in three [17]. The accuracy of HBPM in diagnosing MUCH is not superior to the diagnostic accuracy of standardized office BP [17]. ABPM is therefore necessary for the confirmation of the diagnosis of MUCH.
New antihypertensives and comparison of existing antihypertensives
There has been a resurgence in interest to lower BP in people without and with CKD. Additional agents are currently under clinical investigation, offering promise for more effective management of resistant hypertension through blocking unique targets or more safely blocking existing pathways in the future [52] (Box 3).
Box 3. Key developments and future opportunities in pharmacotherapy of hypertension.
Among patients with stage 3b/4 CKD and uncontrolled hypertension, the non-steroidal MRA ocedurenone lowered systolic AOBP at Day 84 with a minimal associated risk of hyperkalemia.
Among patients with resistant hypertension, as compared with placebo, the aldosterone synthase inhibitor baxdrostat lowered unattended automated office SBP in a dose-dependent manner over 12 weeks of treatment. No deaths, serious adverse events and signs of adrenocortical insufficiency were observed over the course of the trial.
In patients with resistant hypertension, the dual endothelin receptor antagonist aprocitentan was superior to placebo in reducing systolic AOBP at Week 4 and this BP-lowering action was sustained at Week 40. Mild-to-moderate edema was the most frequent treatment-related adverse event.
SGLT-2 inhibitors and the non-steroidal MRA finerenone are novel therapies that improve kidney and cardiovascular outcomes in patients with albuminuric CKD. Indirect comparisons show that finerenone provokes a more potent reduction in ambulatory BP as compared with SGLT-2 inhibitors, implying that BP lowering might play a differential role in mediating the cardiorenal protection afforded by these two drug categories.
Published in 2021, a non-steroidal mineralocorticoid receptor antagonist (MRA), ocedurenone (formerly known as KBP-5074), was tested over 12 weeks in 162 patients with stage 3b/4 CKD with uncontrolled hypertension in doses of 0.25 mg and 0.5 mg [53]. The primary endpoint in the phase 2b study of KBP-5074 in subjects with uncontrolled hypertension and advanced chronic kidney disease trial was the change in systolic AOBP from baseline to Week 12. Compared with placebo, a 0.25-mg dose lowered systolic AOBP 7 mmHg [standard error (SE) 3.37, P = .04]. A 0.5-mg dose lowered systolic AOBP 10.2 mmHg (SE 3.32, P = .003). The incidence of mild hyperkalemia (serum potassium concentration ≥5.6 to <6.0 mmol/L) was low and comparable among groups, but the trial is too short to establish safety [53].
In 2022, an aldosterone synthase inhibitor, baxdrostat, was tested over 12 weeks in 248 patients with resistant hypertension in doses of 0.5, 1 and 2 mg [54]. BP averaged 148/88 mmHg. Compared with placebo, in the BrigHTN trial, a 1-mg dose lowered systolic AOBP 8.1 mmHg [95% confidence interval (CI) 2.8–13.5], and a 2-mg dose lowered systolic AOBP 11 mmHg (95% CI 5.5–16.4). Treatment-induced elevations in serum potassium levels were observed in only two patients, but hyperkalemia did not recur after transient withdrawal and re-initiation of active-treatment [54]. However, this trial excluded patients with CKD stage 3b or higher—eGFR was about 85 mL/min/1.73 m2 at baseline—therefore, safety is difficult to establish in this 12-week study.
Published in 2022, a dual endothelin antagonist, aprocitentan, was tested in the parallel-group, phase 3 study with aprocitentan in subjects with resistant hypertension (PRECISION) trial over 4 weeks in patients with resistant hypertension at doses of 12.5 mg and 25 mg [55]. Of the 730 patients enrolled in this trial, only 162 (22.2%) patients had an eGFR <60 mL/min/1.73 m2 at baseline. PRECISION followed a unique trial design that included: (i) a 4-week, double-blind, placebo-controlled, treatment phase; (ii) a 32-week, single-blind, active-treatment phase; and (iii) a 12-week, double-blind, placebo-controlled withdrawal phase. The primary endpoint was the change in systolic AOBP from baseline to 4 weeks. Compared with placebo, a 12.5-mg dose lowered systolic AOBP 3.8 mmHg (95% CI 0.8–6.8), and a 25-mg dose lowered systolic AOBP 3.7 mmHg (95% CI 0.8–6.7) [55]. For the 12.5-mg dose, 24-h ambulatory SBP was lowered by 4.2 (95% CI 2.1–6.2 mmHg) and for the 25-mg dose by 5.9 (95% CI 3.8–7.9 mmHg) [55]. This BP-lowering action was maintained until the completion of the single-blind, active-treatment phase of the trial at Week 40. Notably, subgroup analyses showed numerically greater reductions in standardized office SBP in patients who had very high albuminuria or stage 3–4 CKD [55]. The most frequently reported adverse event was the development of mild-to-moderate edema with aprocitentan, and seven patients stopped treatment with aprocitentan [55]. Given the risk of heart failure with endothelin receptor antagonists [56, 57], longer-term studies are needed to confirm safety, especially with respect to heart failure in people with CKD.
Sodium-glucose co-transporter type 2 (SGLT-2) inhibitors have been initially introduced as hypoglycemic drugs, but it was thereafter discovered that cardiorenal protection is the main therapeutic effect of these agents. A triad of landmark phase 3 clinical trials (canagliflozin and renal events in diabetes with established nephropathy clinical evaluation, dapagliflozin and prevention of adverse outcomes in chronic kidney disease and the study of heart and kidney protection with empagliflozin) demonstrated that SGLT-2 inhibitors safely and effectively attenuate the progression of CKD and improve cardiovascular outcomes in patients with albuminuric CKD, irrespective of the presence or absence of T2D [58–60]. Finerenone, a highly selective non-steroidal MRA, is also proven to be effective in improving cardiorenal outcomes in patients with diabetic kidney disease [61]. In the finerenone in chronic kidney disease and type 2 diabetes: combined FIDELIO-DKD and FIGARO-DKD trial programme analysis pooled analysis of data from 13 026 patients with T2D and a broad spectrum of CKD, as compared with placebo, finerenone retarded the progression of diabetic kidney disease and reduced the risk of hospitalization for heart failure, cardiovascular death and myocardial infarction [62]. Although treatment with finerenone was well tolerated, the risk of hyperkalemia was more common with finerenone than with placebo [62]. Post hoc analyses indicate that the combined therapy with a SGLT-2 inhibitor and finerenone may be superior to either monotherapy by reducing the risk of hyperkalemia [63] in patients who are already receiving standard-of-care treatment with a RAS blocker [64, 65].
Although neither SGLT-2 inhibitors nor finerenone are indicated for their antihypertensive effects, the magnitude and presence of these BP-lowering effects should be noted. In a meta-analysis of seven trials involving 2381 patients with T2D, SGLT-2 inhibitor therapy for 4–12 weeks provoked a placebo-subtracted reduction of 3.61 mmHg in 24-h ambulatory SBP [66]. This effect was similar that seen using ABPM with 12.5-25 mg hydrochlorothiazide [66]. This modest BP-lowering effect of SGLT-2 inhibitors contrasts with the reductions in ambulatory BP seen with finerenone in a recent sub-analysis of the mineralocorticoid receptor antagonist tolerability study–diabetic nephropathy (ARTS-DN) trial. In ARTS-DN, 823 patients with T2D and albuminuric CKD were randomized to placebo or finerenone, administered at doses of 1.25–20 mg once daily in the morning for 90 days [67]. A subset of 240 patients underwent 24-h ABPM at screening, Day 60 and Day 90 [68]. Relative to placebo, the reduction in 24-h ambulatory SBP at Day 90 was 8.3 mmHg with finerenone 10 mg/day, 11.2 mmHg with finerenone 15 mg/day and 9.9 mmHg with finerenone 20 mg/day [68]. This indirect comparison suggests that the BP-lowering properties of SGLT-2 inhibitors and finerenone might substantially differ. Accordingly, the significance of BP lowering as a mediator of the improvement in cardiorenal outcomes also may not be similar for these two novel drug categories.
Renal denervation
Although the interest for device-based treatment of hypertension dampened after the neutral results of the renal denervation in patients with uncontrolled hypertension trial in 2015 [69], more recent studies support the antihypertensive efficacy, tolerability and safety of catheter-based renal denervation [70–72]. Published in 2022, a prespecified analysis of the long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs trial provided evidence in favor of a long-lasting BP-lowering action of this intervention showing that as compared with the sham control procedure, renal denervation provoked a clinically meaningful reduction of 10/5.9 mmHg in 24-h ambulatory BP at 36 months of follow-up [73]. This persistent reduction in ambulatory BP was independent of concomitant use of antihypertensive medications and was not counteracted by increased risk of adverse events [73]. Since sympathetic activity is markedly increased in patients with CKD, there is biologically plausibility that renal denervation may confer an even greater benefit in this particular patient population. Small uncontrolled interventional studies showed remarkable reductions in BP with renal denervation in patients with stage 3–4 CKD, whereas other observational studies suggested that renal denervation is also associated with regression of albuminuria and a slower rate of eGFR decline [72, 74, 75]. Properly designed, sham-controlled clinical trials are needed to demonstrate the safety and efficacy of this intervention in moderate-to-advanced CKD, since patients with eGFR <45 mL/min/1.73 m2 were systematically excluded from the currently available renal denervation trials.
Stopping or continuing RAS inhibitors in advanced CKD
Whether RAS blockers should be continued or stopped in patients with advanced CKD who are nearing the initiation of dialysis remains an area of controversy [76]. In such patients, an earlier observational study suggested that discontinuation of ACEIs or ARBs is associated with better preservation of kidney function [77]. Similarly, a recent nationwide observational study showed that among patients with advanced CKD, stopping RAS inhibitors is associated with a lower absolute risk of initiating dialysis, but higher absolute risks of adverse cardiovascular events and all-cause mortality [78]. A more conclusive answer to this crucial question was provided by the multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease (STOP-ACEi) trial [79]. In this trial, 411 patients with advanced and progressive CKD were randomized either to stop or to continue RAS inhibitor therapy. Over 3 years of follow-up, there was no difference in the rate of eGFR decline between the discontinuation and continuation groups [80]. Although the proportion of patients who progressed to ESKD or initiated kidney replacement therapy did not significantly differ between the two groups, there was a trend to worse outcome in those who discontinued RAS inhibitors (hazard ratio 1.28; 95% CI 0.99–1.65). Therefore, although observational studies favor the intervention of stopping ACEIs or ARBs in advanced CKD, the STOP-ACEi trial showed that discontinuation of RAS blockade does not lead to stabilization of the long-term decline in kidney function and does not delay the initiation of dialysis [80]. In fact, a trend toward earlier dialysis was noted.
SUMMARY
In summary, research-grade BP measurement methodology must move from research to clinics. The diagnosis of hypertension can be also improved when BP is measured outside of the clinic either using HBPM or ABPM. Dietary Na restriction is often overlooked, but effective strategy to manage poorly controlled hypertension. ACEIs and ARBs remain the first-line agents in pharmacotherapy of hypertension in patients with CKD, particularly in those with very high albuminuria [19]. Patients with uncontrolled BP despite adherence to triple therapy with maximally tolerated doses of a RAS blocker, a dihydropyridine CCB and a diuretic have by definition resistant hypertension [36]. In such patients, the addition of spironolactone to the baseline antihypertensive regimen is the pharmacological intervention of choice [26]. Since hyperkalemia is a disadvantage of spironolactone that limits its broad utilization for the management of resistant hypertension in moderate-to-advanced CKD, the thiazide-like diuretic chlorthalidone serves as an alternative therapeutic option in this subgroup of high-risk patients [45]. Chlorthalidone can mitigate the risk of hyperkalemia, enabling in this way the co-administration of spironolactone. However, the combination of chlorthalidone and spironolactone requires careful monitoring of the patients for the prevention of adverse events, such as the episodes of acute kidney injury [35]. Newer BP-lowering medications [53–55], such as the non-steroidal MRA ocedurenone, the aldosterone synthase inhibitor baxdrostat and the dual endothelin receptor antagonist aprocitentan, are at different stages of clinical development, offering promise for more effective BP control in the future. Renal denervation is also anticipated to receive approval by regulatory agencies as an adjunct interventional strategy to medications for patients who select one-time procedures instead of intensified antihypertensive drug therapy.
Contributor Information
Panagiotis I Georgianos, 2nd Department of Nephrology, AHEPA Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Rajiv Agarwal, Division of Nephrology, Department of Medicine, Indiana University School of Medicine and Richard L. Roudebush Veterans Administration Medical Center, Indianapolis, IN, USA.
DATA AVAILABILITY STATEMENT
Not applicable.
CONFLICT OF INTEREST STATEMENT
R.A. reports personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals, Akebia Therapeutics, Boehringer Ingelheim, Eli Lilly, Relypsa, Vifor Pharma and Diamedica; is a member of data safety monitoring committees for Vertex and Chinook; has served as an associate editor of the American Journal of Nephrology and Nephrology Dialysis Transplantation, and has been an author for UpToDate; and has received research grants from the National Institutes of Health and the US Veterans Administration. P.I.G. has nothing to disclose.
FUNDING
R.A. is supported by the National Heart Lung and Blood Institute (grant R01 HL126903).
REFERENCES
- 1. Alencar de Pihno N, Levin A, Fukagawa M et al. Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int 2019;96:983–94. 10.1016/j.kint.2019.04.032 [DOI] [PubMed] [Google Scholar]
- 2. Ku E, Lee BJ, Wei J et al. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis 2019;74:120–31. 10.1053/j.ajkd.2018.12.044 [DOI] [PubMed] [Google Scholar]
- 3. Thompson S, James M, Wiebe N et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol 2015;26:2504–11. 10.1681/ASN.2014070714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klahr S, Levey AS, Beck GJ et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994;330:877–84. 10.1056/NEJM199403313301301 [DOI] [PubMed] [Google Scholar]
- 5. Ruggenenti P, Perna A, Loriga G et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005;365:939–46. 10.1016/S0140-6736(05)71082-5 [DOI] [PubMed] [Google Scholar]
- 6. Wright JT, Bakris G, Greene T et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421–31. 10.1001/jama.288.19.2421 [DOI] [PubMed] [Google Scholar]
- 7. Cheung AK, Rahman M, Reboussin DM et al. Effects of intensive BP control in CKD. J Am Soc Nephrol 2017;28:2812–23. 10.1681/ASN.2017020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewis CE, Fine LJ, Beddhu S et al. Final report of a trial of intensive versus standard blood-pressure control. N Engl J Med 2021;384:1921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright JT, Williamson JD, Whelton PK et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agarwal R. Implications of blood pressure measurement technique for implementation of Systolic Blood Pressure Intervention Trial (SPRINT). J Am Heart Assoc 2017;6:e004536. 10.1161/JAHA.116.004536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Georgianos PI, Agarwal R. Review: automated office BP measures are similar to awake ambulatory BP and lower than other office BP measures. Ann Intern Med 2019;170:JC69. 10.7326/ACPJ201906180-069 [DOI] [PubMed] [Google Scholar]
- 12. Rahman M, Wang X, Bundy JD et al. Prognostic significance of ambulatory BP monitoring in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. J Am Soc Nephrol 2020;31:2609–21. 10.1681/ASN.2020030236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parati G, Stergiou G, O'Brien E et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 2014;32:1359–66. 10.1097/HJH.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 14. Parati G, Stergiou GS, Bilo G et al. Home blood pressure monitoring: methodology, clinical relevance and practical application: a 2021 position paper by the Working Group on Blood Pressure Monitoring and Cardiovascular Variability of the European Society of Hypertension. J Hypertens 2021;39:1742–67. 10.1097/HJH.0000000000002922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agarwal R, Andersen MJ. Blood pressure recordings within and outside the clinic and cardiovascular events in chronic kidney disease. Am J Nephrol 2006;26:503–10. 10.1159/000097366 [DOI] [PubMed] [Google Scholar]
- 16. Agarwal R, Andersen MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006;69:406–11. 10.1038/sj.ki.5000081 [DOI] [PubMed] [Google Scholar]
- 17. Agarwal R, Pappas MK, Sinha AD. Masked uncontrolled hypertension in CKD. J Am Soc Nephrol 2016;27:924–32. 10.1681/ASN.2015030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agarwal R, Bills JE, Hecht TJ et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011;57:29–38. 10.1161/HYPERTENSIONAHA.110.160911 [DOI] [PubMed] [Google Scholar]
- 19. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int 2021;99:S1–87. 10.1016/j.kint.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 20. McMahon EJ, Campbell KL, Bauer JD et al. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev 2021;6:CD010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lambers Heerspink HJ, Holtkamp FA, Parving HH et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int 2012;82:330–7. 10.1038/ki.2012.74 [DOI] [PubMed] [Google Scholar]
- 22. Neal B, Wu Y, Feng X et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med 2021;385:1067–77. 10.1056/NEJMoa2105675 [DOI] [PubMed] [Google Scholar]
- 23. Picard K, Barreto Silva MI, Mager D et al. Dietary potassium intake and risk of chronic kidney disease progression in predialysis patients with chronic kidney disease: a systematic review. Adv Nutr 2020;11:1002–15. 10.1093/advances/nmaa027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gritter M, Wouda RD, Yeung SMH et al. Effects of short-term potassium chloride supplementation in patients with CKD. J Am Soc Nephrol 2022;33:1779–89. 10.1681/ASN.2022020147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–248. [DOI] [PubMed] [Google Scholar]
- 26. Williams B, Mancia G, Spiering W et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018;36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 27. Brenner BM, Cooper ME, de Zeew D et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–9. 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 28. Lewis EJ, Hunsicker LG, Clarke WR et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–60. 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 29. Fried LF, Emanuele N, Zhang JH et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–903. 10.1056/NEJMoa1303154 [DOI] [PubMed] [Google Scholar]
- 30. Parving HH, Brenner BM, McMurray JJ et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367:2204–13. 10.1056/NEJMoa1208799 [DOI] [PubMed] [Google Scholar]
- 31. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension 2010;55:399–407. 10.1161/HYPERTENSIONAHA.109.139816 [DOI] [PubMed] [Google Scholar]
- 32. Ellison DH. Clinical pharmacology in diuretic use. Clin J Am Soc Nephrol 2019;14:1248–57. 10.2215/CJN.09630818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vasavada N, Saha C, Agarwal R. A double-blind randomized crossover trial of two loop diuretics in chronic kidney disease. Kidney Int 2003;64:632–40. 10.1046/j.1523-1755.2003.00124.x [DOI] [PubMed] [Google Scholar]
- 34. Agarwal R, Sinha AD, Cramer AE et al. Chlorthalidone for hypertension in advanced chronic kidney disease. N Engl J Med 2021;385:2507–19. 10.1056/NEJMoa2110730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agarwal R. Spironolactone and chlorthalidone-old drugs, new uses-but approach with caution. Nephrol Dial Transplant 2022;37:407–8. 10.1093/ndt/gfab328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carey RM, Calhoun DA, Bakris GL et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension 2018;72:e53–90. 10.1161/HYP.0000000000000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams B, MacDonald TM, Morant S et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet North Am Ed 2015;386:2059–68. 10.1016/S0140-6736(15)00257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Georgianos PI, Agarwal R. Resistant hypertension in chronic kidney disease (CKD): prevalence, treatment particularities, and research agenda. Curr Hypertens Rep 2020;22:84. 10.1007/s11906-020-01081-x [DOI] [PubMed] [Google Scholar]
- 39. Tanner RM, Calhoun DA, Bell EK et al. Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol 2013;8:1583–90. 10.2215/CJN.00550113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomas G, Xie D, Chen HY et al. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report from the Chronic Renal Insufficiency Cohort study. Hypertension 2016;67:387–96. 10.1161/HYPERTENSIONAHA.115.06487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chung EY, Ruospo M, Natale P et al. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 2020;10:CD007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leon SJ, Whitlock R, Rigatto C et al. Hyperkalemia-related discontinuation of renin-angiotensin-aldosterone system inhibitors and clinical outcomes in CKD: a population-based cohort study. Am J Kidney Dis 2022;80:164–73.e1. 10.1053/j.ajkd.2022.01.002 [DOI] [PubMed] [Google Scholar]
- 43. Wetmore JB, Yan H, Horne L et al. Risk of hyperkalemia from renin-angiotensin-aldosterone system inhibitors and factors associated with treatment discontinuities in a real-world population. Nephrol Dial Transplant 2021;36:826–39. 10.1093/ndt/gfz263 [DOI] [PubMed] [Google Scholar]
- 44. Agarwal R, Rossignol P, Romero A et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet North Am Ed 2019;394:1540–50. 10.1016/S0140-6736(19)32135-X [DOI] [PubMed] [Google Scholar]
- 45. Agarwal R, Sinha AD, Tu W. Chlorthalidone for resistant hypertension in advanced chronic kidney disease. Circulation 2022;146:718–20. 10.1161/CIRCULATIONAHA.122.060167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bangalore S, Messerli FH, Kostis JB et al. Cardiovascular protection using beta-blockers: a critical review of the evidence. J Am Coll Cardiol 2007;50:563–72. 10.1016/j.jacc.2007.04.060 [DOI] [PubMed] [Google Scholar]
- 47. Muntner P, Anderson A, Charleston J et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2010;55:441–51. 10.1053/j.ajkd.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agarwal R, Andersen MJ. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension 2005;46:514–20. 10.1161/01.HYP.0000178102.85718.66 [DOI] [PubMed] [Google Scholar]
- 49. Agarwal R, Light RP. GFR, proteinuria and circadian blood pressure. Nephrol Dial Transplant 2009;24:2400–6. 10.1093/ndt/gfp074 [DOI] [PubMed] [Google Scholar]
- 50. Agarwal R. Caring for individuals with hypertension in CKD, especially those with low education. Kidney Int 2019;96:820–2. 10.1016/j.kint.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 51. Bangash F, Agarwal R. Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol 2009;4:656–64. 10.2215/CJN.05391008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Salvador VD, Bakris GL. Novel antihypertensive agents for resistant hypertension: what does the future hold? Hypertens Res 2022;45:1918–28. 10.1038/s41440-022-01025-9 [DOI] [PubMed] [Google Scholar]
- 53. Bakris G, Pergola PE, Delgado B et al. Effect of KBP-5074 on blood pressure in advanced chronic kidney disease: results of the BLOCK-CKD study. Hypertension 2021;78:74–81. 10.1161/HYPERTENSIONAHA.121.17073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Freeman MW, Halvorsen YD, Marshall W et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med 2023;388:395–405. 10.1056/NEJMoa2213169 [DOI] [PubMed] [Google Scholar]
- 55. Schlaich MP, Bellet M, Weber MA et al. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet North Am Ed 2022;400:1927–37. 10.1016/S0140-6736(22)02034-7 [DOI] [PubMed] [Google Scholar]
- 56. Heerspink HJL, Parving HH, Andress DL et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet North Am Ed 2019;393:1937–47. 10.1016/S0140-6736(19)30772-X [DOI] [PubMed] [Google Scholar]
- 57. Packer M, McMurray JJV, Krum H et al. Long-term effect of endothelin receptor antagonism with bosentan on the morbidity and mortality of patients with severe chronic heart failure: primary results of the ENABLE trials. JACC Heart Fail 2017;5:317–26. 10.1016/j.jchf.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 58. Heerspink HJL, Stefansson BV, Correa-Rotter R et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 59. Herrington WG, Staplin N, Wanner C et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Perkovic V, Jardine MJ, Neal B et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 61. Georgianos PI, Agarwal R. Mineralocorticoid receptor antagonism in chronic kidney disease. Kidney Int Rep 2021;6:2281–91. 10.1016/j.ekir.2021.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Agarwal R, Filippatos G, Pitt B et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022;43:474–84. 10.1093/eurheartj/ehab777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Neuen BL, Oshima M, Agarwal R et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation 2022;145:1460–70. 10.1161/CIRCULATIONAHA.121.057736 [DOI] [PubMed] [Google Scholar]
- 64. Provenzano M, Jongs N, Vart P et al. The kidney protective effects of the sodium-glucose cotransporter-2 inhibitor, dapagliflozin, are present in patients with CKD treated with mineralocorticoid receptor antagonists. Kidney Int Rep 2022;7:436–43. 10.1016/j.ekir.2021.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rossing P, Anker SD, Filippatos G et al. Finerenone in patients with chronic kidney disease and type 2 diabetes by sodium-glucose cotransporter 2 inhibitor treatment: the FIDELITY analysis. Diabetes Care 2022;45:2991–8. 10.2337/dc22-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Georgianos PI, Agarwal R. ambulatory blood pressure reduction with SGLT-2 inhibitors: dose-response meta-analysis and comparative evaluation with low-dose hydrochlorothiazide. Diabetes Care 2019;42:693–700. 10.2337/dc18-2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bakris GL, Agarwal R, Chan JC et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 2015;314:884–94. 10.1001/jama.2015.10081 [DOI] [PubMed] [Google Scholar]
- 68. Agarwal R, Ruilope LM, Ruiz-Hurtado G et al. Effect of finerenone on ambulatory blood pressure in chronic kidney disease in type 2 diabetes. J Hypertens 2023;41:295–302. 10.1097/HJH.0000000000003330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bhatt DL, Kandzari DE, O'Neill WW et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370:1393–401. 10.1056/NEJMoa1402670 [DOI] [PubMed] [Google Scholar]
- 70. Azizi M, Schmieder RE, Mahfoud F et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet North Am Ed 2018;391:2335–45. 10.1016/S0140-6736(18)31082-1 [DOI] [PubMed] [Google Scholar]
- 71. Azizi M, Sanghvi K, Saxena M et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet North Am Ed 2021;397:2476–86. 10.1016/S0140-6736(21)00788-1 [DOI] [PubMed] [Google Scholar]
- 72. Schmieder RE. Renal denervation in patients with chronic kidney disease: current evidence and future perspectives. Nephrol Dial Transplant 2023;38:1089–96. 10.1093/ndt/gfac189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mahfoud F, Kandzari DE, Kario K et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet North Am Ed 2022;399:1401–10. 10.1016/S0140-6736(22)00455-X [DOI] [PubMed] [Google Scholar]
- 74. Ott C, Mahfoud F, Schmid A et al. Improvement of albuminuria after renal denervation. Int J Cardiol 2014;173:311–5. 10.1016/j.ijcard.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 75. Ott C, Mahfoud F, Schmid A et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens 2015;33:1261–6. 10.1097/HJH.0000000000000556 [DOI] [PubMed] [Google Scholar]
- 76. Weir MR, Lakkis JI, Jaar B et al. Use of renin-angiotensin system blockade in advanced CKD: an NKF-KDOQI controversies report. Am J Kidney Diss 2018;72:873–84. 10.1053/j.ajkd.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 77. Ahmed AK, Kamath NS, El Kossi M et al. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010;25:3977–82. 10.1093/ndt/gfp511 [DOI] [PubMed] [Google Scholar]
- 78. Fu EL, Evans M, Clase CM et al. Stopping renin-angiotensin system inhibitors in patients with advanced CKD and risk of adverse outcomes: a nationwide study. J Am Soc Nephrol 2021;32:424–35. 10.1681/ASN.2020050682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bhandari S, Ives N, Brettell EA et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016;31:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bhandari S, Mehta S, Khwaja A et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med 2022;387:2021–32. 10.1056/NEJMoa2210639 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.