Abstract
Membrane protein homeostasis is fine-tuned by the cellular pathways for vacuolar degradation and recycling, which ultimately facilitate plant growth and cell–environment interactions. The endosomal sorting complex required for transport (ESCRT) machinery plays important roles in regulating intraluminal vesicle (ILV) formation and membrane protein sorting to vacuoles. We previously showed that the plant-specific ESCRT component FYVE DOMAIN PROTEIN REQUIRED FOR ENDOSOMAL SORTING1 (FREE1) performs multiple functions in plants, although the underlying mechanisms remain elusive. In this study, we performed a suppressor screen of the FREE1-RNAi mutant and identified and characterized 2 suppressor of free1 (sof) mutants in Arabidopsis (Arabidopsis thaliana). These mutants, sof10 and sof641, result in a premature stop codon or a missense mutation in AT5G10370, respectively. This gene was named DEAH and RING domain-containing protein as FREE1 suppressor 1 (DRIF1). DRIF1 has a homologous gene, DRIF2, in the Arabidopsis genome with 95% identity to DRIF1. The embryos of drif1 drif2 mutants arrested at the globular stage and formed enlarged multivesicular bodies (MVBs) with an increased number of ILVs. DRIF1 is a membrane-associated protein that coordinates with retromer component sorting nexin 1 to regulate PIN-FORMED2 recycling to the plasma membrane. Altogether, our data demonstrate that DRIF1 is a unique retromer interactor that orchestrates FREE1-mediated ILV formation of MVBs and vacuolar sorting of membrane proteins for degradation in plants.
DRIF1, a unique evolutionary FREE1 suppressor, negatively regulates intraluminal vesicle formation in multivesicular bodies and the sorting of membrane proteins for degradation in plants.
IN A NUTSHELL.
Background: Cells maintain the balance and stability of membrane proteins through vacuolar degradation and recycling, which is crucial for plant growth and interactions with the environment. Previously, we characterized an endosomal sorting complex required for transport protein called FYVE DOMAIN PROTEIN REQUIRED FOR ENDOSOMAL SORTING 1 (FREE1) in Arabidopsis thaliana. FREE1 performs multiple functions in plant cells, such as regulation of organelle biogenesis and vacuolar degradation of membrane proteins. Via a forward genetic screening approach using the FREE1-RNAi mutant line, we recently identified and characterized 2 suppressor of free1 (sof) mutants with causal genes called BRO1-DOMAIN PROTEIN AS FREE1 SUPPRESSOR and RESURRECTION1.
Questions: What are the underlying mechanisms and regulators of FREE1-mediated functions in plants?
Findings: In this genetic screen, we further identified 2 sof mutants, sof10 and sof641. These mutants had mutations in a protein called DEAH AND RING DOMAIN-CONTAINING PROTEIN AS FREE1 SUPPRESSOR 1 (DRIF1). We showed that DRIF1 has a close homolog, DRIF2, in the Arabidopsis genome. The embryos of drif1 drif2 double mutants arrested at the globular stage and formed enlarged multivesicular bodies with increased numbers of intraluminal vesicles. DRIF1 coordinates with SORTING NEXIN 1 to regulate PIN-FORMED2 recycling to the plasma membrane. Overall, through a combination of cellular, biochemical, and genetic approaches, our study revealed DRIF1 functions in orchestrating FREE1-mediated intraluminal vesicle formation of multivesicular bodies and vacuolar sorting of membrane proteins for degradation in plants.
Next steps: Future studies will aim to understand the mechanisms underlying the formation of enlarged multivesicular bodies with increased intraluminal vesicles in drif1 drif2 double mutants, and how DRIF regulate SORTING NEXIN 1-dependent membrane protein recycling to the plasma membrane in response to multiple environmental cues.
Introduction
Eukaryotic cells regulate the homeostasis of proteins on the plasma membrane (PM) such as receptors, transporters, and channels through cellular pathways to facilitate plant development, intercellular communication, and environmental sensing (Piper and Luzio 2007; MacGurn et al. 2012; Luschnig and Vert 2014). Thus, the fine-tuning of membrane protein abundance through selective vacuolar/lysosomal degradation and/or recycling are important processes in organismal development, especially for sessile plants. In the plant endomembrane system, ubiquitinated and other internalized PM proteins are firstly received by the trans-Golgi network (TGN)/early endosome and then transported to the vacuole for degradation via sequestration into the intraluminal vesicles (ILVs) of the multivesicular bodies (MVBs)/prevacuolar compartments (PVCs)/late endosome. Alternatively, cargoes can be recycled back to the PM through retromer components (Reyes et al. 2011). Defects in these processes can lead to abnormal cellular function and aberrant protein accumulation (Jaillais et al. 2007; Gao et al. 2014).
The formation of ILVs that sort ubiquitinated proteins and their subsequent fusion with vacuoles/lysosomes for degradation is facilitated by the endosomal sorting complex required for transport (ESCRT) machinery, which resides on the endosome membrane, including ESCRT-0, -Ⅰ, -Ⅱ, -Ⅲ, and vacuolar protein sorting-associated protein 4 complex (Hurley and Emr 2006; Raiborg and Stenmark 2009; Gao et al. 2017; Isono and Kalinowska 2017). Plants contain most ESCRT subunit orthologs, except for ESCRT-0 and Mvb12 orthologs in ESCRT-Ⅰ (Mayers et al. 2011). In plants, unique ESCRT components have evolved for sorting proteins for degradation such as the unique-plant FYVE domain protein required for endosomal sorting 1 (FREE1/FYVE1), which binds phosphatidylinositol-3-phosphate and interacts with the ESCRT-Ⅰ component Vps23 (Gao et al. 2014). Functional loss of FREE1 in Arabidopsis showed a seedling lethal phenotype and fragmented vacuoles, which results from ILV formation defects that block the vacuolar degradation of endocytosed PM proteins (Gao et al. 2014). FREE1 can also participate in the autophagic pathway through the interaction with autophagic regulator SH3 domain-containing protein 2 (Zhuang et al. 2013; Gao et al. 2015). In addition to its role in endosomal sorting, FREE1 can also play a transcriptional function in the nucleus to inhibit abscisic acid (ABA) signaling (Li et al. 2019). The multiple functions of FREE1 involved in both endosomal and nonendosomal processes indicate the crosstalk between different pathways, as well as the unique and diverse mechanisms plants evolved in regulating FREE1 functions.
In order to elucidate the multiple roles of FREE1, we have developed a forward genetic screen to identify suppressor of free1 (sof) mutants that suppressed the seedling lethal phenotype of FREE1-RNAi transgenic plants (Zhao et al. 2015). Through this strategy, the plant-specific ESCRT regulator BRo1-domain protein As FREE1 suppressor (BRAF) and a negative regulator RESURRECTION1 (RST1) of the endomembrane trafficking pathway were identified and characterized (Shen et al. 2018; Zhao et al. 2019). In this study, we identified the sof10 and sof641 mutants as suppressors of FREE1 in Arabidopsis, which resulted from a premature stop codon or a missense mutation, respectively, in AT5G10370, named as DEAH and RING domain-containing protein as FREE1 suppressor 1 (DRIF1). We demonstrated that the DRIF1, together with the homolog DRIF2, is essential for embryo development. Enlarged MVBs with increased number of ILVs were observed in embryo cells of drif1 drif2 compared to wild-type (WT). DRIF1 is a membrane-associated protein that colocalizes and interacts with the retromer component sorting nexin 1 (SNX1), to regulate the recycling of membrane-localized PIN-FORMED (PIN) proteins. Our work defines the plant-specific protein DRIF1 as a retromer component interactor that regulates FREE1-mediated ILV formation of MVBs and is thus required for the vacuolar sorting and degradation of membrane proteins in plants.
Results
sof10 and sof641 can rescue the seedling lethality of FREE1-RNAi
To elucidate the molecular basis of multiple roles of FREE1 in regulating membrane protein transport and organelle biogenesis in plants, we performed a suppressor screen using a dexamethasone (DEX)-inducible ProDEX:FREE1-RNAi mutant as a starting material (Zhao et al. 2015). Seedlings of FREE1-RNAi transgenic plants died under DEX induction (Gao et al. 2014). To start the suppressor screen, seeds of FREE1-RNAi plants were mutagenized with ethyl methanesulfonate (EMS). M2 seeds were sown on MS plates with DEX to identify surviving seedlings, which were named sof (Zhao et al. 2015). To confirm the screened M2 mutants, M3 seeds collected from individual M2 plants were screened on MS plates with 10μmol/L DEX for subsequent phenotypic studies (Supplemental Fig. S1A).
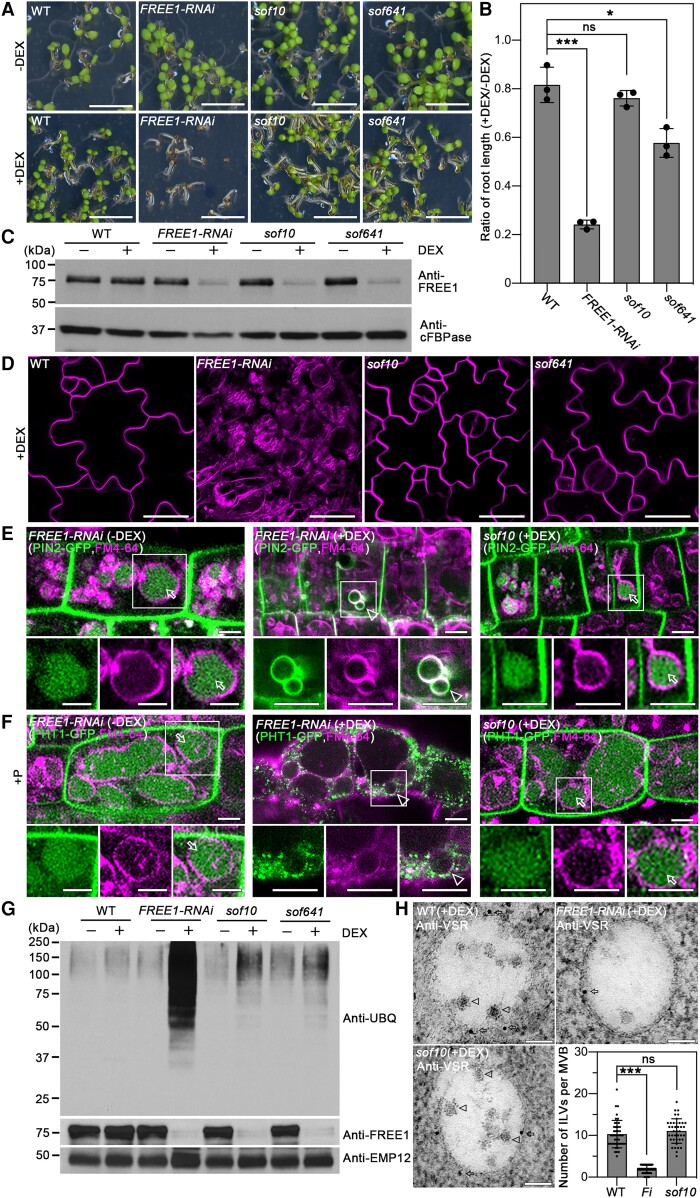
After growth on medium with DEX, the sof10 and sof641 seedlings exhibited a viable growth phenotype, which suppressed the lethal phenotype of the FREE1-RNAi seedlings (Fig. 1A). Quantification of the root length confirmed that the sof10 and sof641 seedlings showed a suppressed root growth inhibition compared to the FREE1-RNAi, although the root length of sof641 was reduced compared with WT (Fig. 1B; Supplemental Fig. S1B). This result suggests that the sof10 and sof641 mutations partially restored the phenotype of FREE1-RNAi. In addition, FREE1 protein was barely detectable in the sof10 and sof641 mutants under DEX induction (Fig. 1C), suggesting that sof10 and sof641 are involved in the FREE1-related pathway and the suppressed phenotype is not caused by disruption of the RNAi process.
Figure 1.
The sof10 mutant converts the seedling lethality of FREE1-RNAi. A) The lethal phenotype of DEX-inducible FREE1-RNAi is rescued in the sof10 and sof641 mutants. M3 seeds were sown on MS plates without DEX (−DEX) or with 10 μm DEX (+DEX) and grown for 7 d before phenotypic analysis. Scale bar, 5 mm. B) Quantification of the root length ratio for 7-d-old seedlings of WT, FREE1-RNAi, sof10, and sof641 mutants grown on MS with (+) or without (−) DEX as shown in A). Data are shown as mean ± Sd, and results from 3 individual experiments are plotted. For each genotype from each independent experiment, at least 20 seedlings were measured. ***P < 0.001, *P < 0.05, ns, no significance in 1-way ANOVA followed by Turkey's multiple test. For each experiment, n = 16 roots. C)sof10 and sof641 mutants show reduced FREE1 protein levels as FREE1-RNAi. Total proteins were extracted from 7-d-old seedlings of WT, FREE1-RNAi, sof10, and sof641 grown on MS with (+) or without (−) DEX, followed by western blot analysis using FREE1 antibodies. The cytosolic protein cFBPase was used as a loading control. D) The sof10 and sof641 mutants recover vacuolar defects compared with FREE1-RNAi when grown on MS with DEX (+DEX). The 7-d-old seedlings from indicated genotypes were stained with 4 μm FM4-64 dye for 3 h, and the labeled tonoplast was visualized by confocal imaging analysis in magenta. Scale bars, 25 μm. E) The mislocalization of auxin efflux carrier PIN2-GFP is converted in sof10 mutant. Seven-day-old seedlings of PIN2-GFP/FREE1-RNAi or PIN2-GFP/sof10 with or without DEX induction were stained with FM4-64 for the tonoplast and visualized after 6 h dark treatment. Arrows and arrowheads indicate vacuolar and tonoplast localized GFP signals, respectively. Images in white squared boxes are shown by separated channels (from left to right: GFP, RFP, and merged). Scale bars, 5 μm. F) The mislocalization of phosphate transporter PHT1-GFP is reversed in sof10 mutant. Seven-d-old seedlings of PHT1-GFP/FREE1-RNAi or PHT1-GFP/sof10 with or without DEX were stained with FM4-64 and incubated in +P liquid medium for 3 h dark treatment before visualization. Arrows and arrowheads indicate vacuolar and tonoplast localized GFP signals, respectively. Images in white squared boxes are shown by separated channels (from left to right: GFP, RFP, and merged). Scale bars, 5 μm. G) The sof10 and sof641 mutants convert the ubiquitin conjugates accumulation phenotype in FREE1-RNAi. Membrane proteins extracted from indicated 7-d-old seedlings grown on MS with (+) or without (−) DEX were subjected to immunoblot analysis with UBQ and FREE1 antibodies. EMP12 antibody was used as loading control. H) The formation of ILVs in MVB is restored in sof10 mutants. Ultrathin sections were prepared from HPF/FS roots of WT, FREE1-RNAi, and sof10 grown on MS with (+) DEX, followed by immunogold labeling using VSR antibodies. Arrows and arrowheads indicate the gold particles and ILVs, respectively. The number of ILVs per MVB were statistically analyzed, and individual data points were plotted with mean ± Sd ***P < 0.001, ns, no significance in 1-way ANOVA followed by Turkey's multiple test. For each sample, n = 40. Scale bars, 100 nm. FREE1-RNAi, ProDEX:FREE1-RNAi.
Moreover, the sof10 mutants also rescued the defect of central vacuole morphology in FREE1-RNAi seedlings. Both FM4-64 staining and tonoplast marker YFP-vesicle-associated membrane protein 711 (VAMP711) showed morphological recovery of the large central vacuole compared to the numerous fragmented vacuoles in FREE1-RNAi (Fig. 1D; Supplemental Fig. S1C). The route of protein transport to lytic vacuole was also partially recovered in the sof10 with a fraction of the soluble cytosolic cargo spL-RFP transported into the vacuole, compared to the secretion in FREE1-RNAi plants (Supplemental Fig. S1D).
FREE1 functions to sort ubiquitinated membrane cargo into the vacuole lumen for degradation. Loss of function of FREE1 in free1 mutant and DEX-induced FREE1-RNAi plants resulted in the accumulation of ubiquitinated membrane proteins (Gao et al. 2014). Through a set of experiments, we demonstrated that the sof10 mutant could revert the phenotype of membrane protein homeostasis in FREE1-RNAi plants. The auxin efflux carrier, PIN2-GFP, has been shown to undergo ubiquitination and transport through ESCRT-dependent endocytic pathways to the vacuole for degradation (Geldner et al. 2003; Leitner et al. 2012; Gao et al. 2014). In WT plants, PIN2-GFP is mainly localized to the PM in a polarized manner. When plants were incubated in the dark for 6 h to stabilize fluorescent-tagged reporters in the vacuole (Tamura et al. 2003), vacuolar accumulation of PIN2-GFP was observed in control plants (PIN2-GFP/FREE1-RNAi without DEX induction). In DEX-treated FREE1-RNAi plants, dark treatment did not cause substantially vacuolar accumulation of PIN2-GFP; instead, internalized PIN2-GFP signals were found on the tonoplast (Fig. 1E). The vacuolar accumulation of PIN2-GFP in sof10 was restored as in WT compared with in FREE1-RNAi plant roots (Fig. 1E), indicating that these proteins were transported to the vacuole for degradation. The PHOSPHATE TRANSPORTER 1 (PHT1)-GFP displayed similar mislocalization on the tonoplast in FREE1-RNAi mutant, whereas the vacuolar degradation phenotypes were restored in sof10 mutants (Fig. 1F) (Bayle et al. 2011; Lin et al. 2013; Cardona-López et al. 2015).
An immunoblot analysis with antiubiquitin antibody (anti-UBQ) was performed for membrane proteins extracted from FREE1-RNAi and sof10 plants to confirm whether the mutant restored the defect of FREE1-RNAi plants in sorting ubiquitinated membrane cargo for vacuolar degradation. We detected a high level of ubiquitin signals of different molecular weights in membrane fractions isolated from DEX-treated FREE1-RNAi plants, while the accumulation of ubiquitinated proteins was largely restored to similar levels comparable to WT in the sof mutants (Fig. 1G).
To analyze the recovery phenotype at ultrastructural levels, we performed a transmission electron microscopy (TEM) analysis of ultrathin sections of high-pressure frozen and freeze substituted (HPF/FS) samples to study the morphology of MVB. Our analyses revealed that in FREE1-RNAi root cells, MVBs/PVCs labeled by the vacuolar sorting receptor (VSR) antibodies were almost empty and lacked visible ILVs. By contrast, root cells of sof10 had typical MVB with ILVs structures, comparable with WT plants (Fig. 1H). Statistical analysis of ILV numbers per MVB further supported our observation that in sof10, the number of ILVs in MVBs is restored as in WT, compared to MVBs in FREE1-RNAi (Fig. 1H).
The sof10 and sof641 mutations affect DRIF1 protein
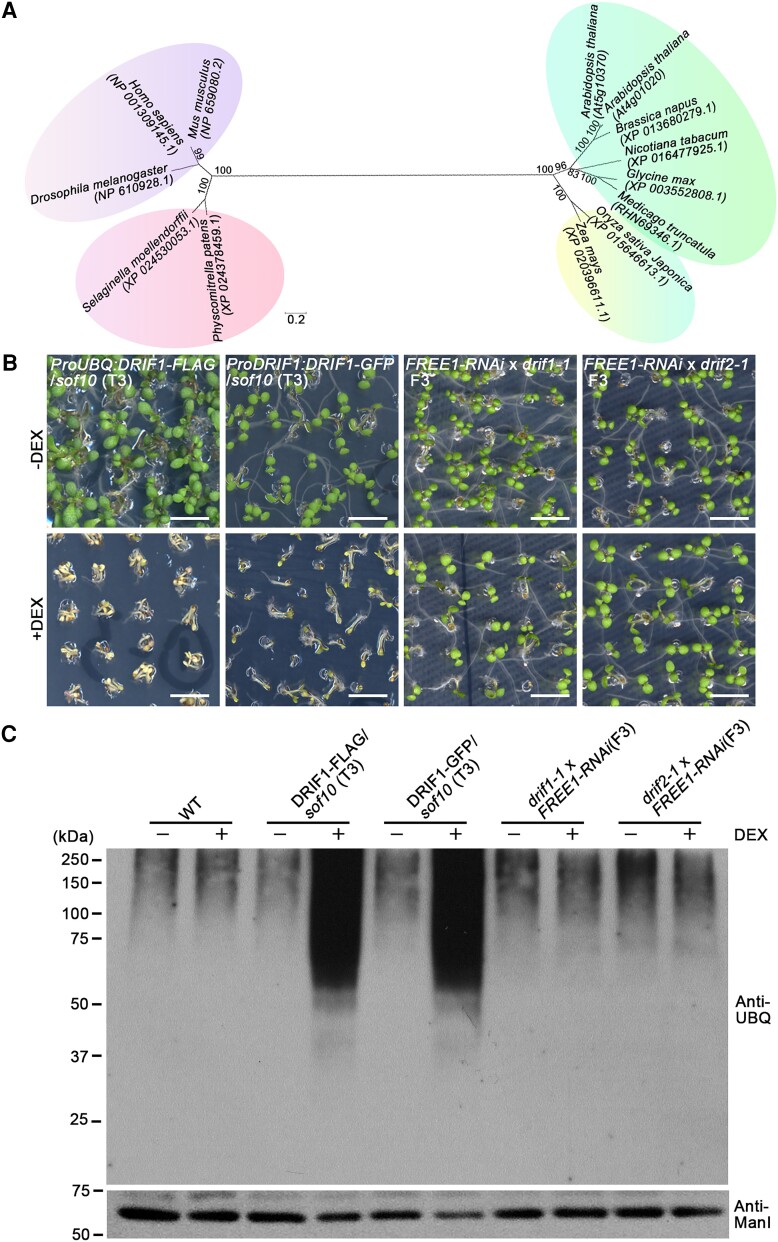
To identify the causal genes in the sof10 and sof641 mutants, we performed next generation sequencing (NGS). We established outcross populations of sof mutants with Ler ecotype and then self-pollinated to generate F2 populations. The F2 population with a segregation ratio of 3/16 was used to create a DNA library and sequenced following previously described workflow (Zhao et al. 2015). NGS mapping identified a specific peak in sof10 and sof641 located on chromosome 5 (Supplemental Fig. S2A), and fine mapping identified a C-to-T transition and a G-to-A transition in the third exon of AT5G10370 for sof10 and sof641, respectively (Supplemental Fig. S2, B and C). The C-to-T transition generated a premature stop codon (Gln996-to-stop), while the G-to-A transition caused a change in a conserved amino acid (Asp787-to-Asn) (Supplemental Fig. S2B to S2D). The AT5G10370 gene encodes a DEAH and RING domain-containing protein, whose biological function remains unknown. We use the name DEAH and RING domain-containing protein as FREE1 suppressor (DRIF) hereafter, according to the FREE1 suppressor phenotype.
To understand the phylogenetic relationships of this protein in plants and other eukaryotes, we searched and compared the sequences of putative homologs in different species. Based on phylogenetic analysis results, the homologs are widespread in plants, where they form a subgroup in dicotyledonous plants (Fig. 2A). Proteins in other species outside the vascular plants group have low sequence similarity, and the DRIF protein thus appears to be a plant-specific protein (Supplemental Fig. S3). The Arabidopsis genome contains 2 putative gene copies, which we have termed DRIF1 and DRIF2 (AT4G01020), encoding proteins with 95% identity to each other.
Figure 2.
The sof10 and sof641 mutants affect DRIF1 protein. A) Phylogenetic analysis of DRIF1 homologs by neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The tree is drawn to scale, with the computed evolutionary distance shown as branch length. Major groupings are indicated by different colors. Note that the DRIF1 protein has a homolog DRIF2 in Arabidopsis. Scale bar, 0.2 substitutions per site. B) Complementation of sof10 with indicated fusion proteins or phenotype of indicated genotype mutants on MS plates with (+) or without (−) DEX. The seedling phenotype of sof10 could be complemented by ProUBQ:DRIF1-FLAG and ProDRIF1:DRIF1-GFP. Note that both drif1-1 and drif2-1 T-DNA insertions could revert the lethal phenotype of FREE1-RNAi seedlings in DEX treatment. Scale bars, 5 mm. C) Analysis of ubiquitin conjugates in indicated genotypes. Membrane proteins were extracted from 7-d-old seedlings of WT and various complementary lines as indicated grown on MS with (+) or without (−) DEX, followed by immunoblot analysis with UBQ antibody. Note that the lines exhibited a lethal phenotype in the DEX treatment also accumulated ubiquitinated membrane proteins at higher levels than the lines that exhibited a survival phenotype as shown in B). Anti-ManI antibody was used as loading control.
Next, we obtained the T-DNA insertional mutants for DRIF1 and DRIF2 termed drif1-1 and drif2-1, respectively (Supplemental Fig. S4, A and B). Both of the insertional sites are in exons (Supplemental Fig. S4A) and lead to the inactivation of DRIF1 and DRIF2, respectively, as confirmed by reverse transcription quantitative PCR (RT-qPCR) assays (Supplemental Fig. S4C). To confirm the expression of DRIF1 and DRIF2 proteins in these mutants, we performed immunoblot analysis using antibodies raised against synthetic peptides (P1 to P6) corresponding to different regions of DRIF1 and DRIF2 (Supplemental Fig. S5A). Total proteins extracted from Arabidopsis protoplasts expressing the GFP fusions of DRIF1 N-terminus (NT) (1 to 943 amino acids, DRIF1-NT-GFP), DRIF1 C-terminus (944 to 1,775 amino acids, DRIF1-CT-GFP), DRIF2 NT (1 to 955 amino acids, DRIF2-NT-GFP), or DRIF2 C-terminus (956 to 1,787 amino acids, DRIF2-CT-GFP) were used to test the specificity and cross-reactivity of the antibodies (Supplemental Fig. S5, B and C).
The DRIF1 P1 antibody can detect both the endogenous DRIF1 and the DRIF1-NT-GFP fusion proteins (Supplemental Fig. S5D). Similarly, the DRIF2 P6 antibody can also detect the endogenous DRIF2 and the DRIF2-CT-GFP fusion proteins (Supplemental Fig. S5E). Predicted structural modeling of DRIF1 with highlighted domains and mutated residues is also shown (Supplemental Fig. S5F). Next, we analyzed the protein levels of DRIF1 and DRIF2 in T-DNA insertion mutants using these antibodies. P1 could detect both DRIF1 and DRIF2 proteins, as indicated by the detection of a small amount of the remaining proteins in drif1-1 T-DNA mutants. P6 specifically recognizes DRIF2 as no target protein was detected in drif2-1 T-DNA mutants (Supplemental Fig. S6). Taken together, these results suggest that drif2-1 and likely drif1-1 are loss-of-function mutants that affect DRIF transcription and protein expression, respectively.
To confirm the effect of DRIF1 mutations in sof10 and sof641, we next performed complementation studies by introducing ProUBQ:DRIF1-FLAG and ProDRIF1:DRIF1-GFP into sof10 plants. Homozygous T3 seedlings displayed a lethal phenotype under DEX treatment compared with the original survival phenotype of sof10 plants (Fig. 2B). In addition, both the drif1-1 FREE1-RNAi and drif2-1 FREE1-RNAi homozygous seedlings showed a viable phenotype in DEX treatment compared with the original lethal phenotype of FREE1-RNAi (Fig. 2B). Meanwhile, decreased FREE1 transcript levels were observed in drif1-1 FREE1-RNAi and drif2-1 FREE1-RNAi homozygous backgrounds upon DEX treatment, as demonstrated by RT-qPCR analysis (Supplemental Fig. S7). Interestingly, seedling lethal phenotype under DEX treatment is associated with the accumulated ubiquitinated membrane proteins (Fig. 2C). Intriguingly, we failed to identify any viable double-homozygous line in 24 progenies of drif1(−/−) free1(+/−) and 18 progenies of drif2(−/−) free1(+/−) (Supplemental Fig. S8), suggesting the possibility that the suppressor activity of sof10 and sof641 may rely on the residual FREE1. Altogether, the genetic complementation and recapitulation results suggest that loss of function of DRIF1 is responsible for recovering the lethal phenotype of FREE1-RNAi.
DRIF1 and DRIF2 depletion arrests embryo development and induces MVB enlargement
When grown under normal long-day conditions, we did not find any growth difference between the single homozygous drif1 or drif2 mutant and WT plants. Considering that single drif1 or drif2 mutants restore the lethal phenotype of FREE1-RNAi upon DEX treatment, a dosage-dependent effect rather than merely redundant function of DRIF proteins as FREE1 suppressor may play in this scenario. In order to further clarify how DRIF1 and DRIF2 affect MVB formation and membrane protein homeostasis, we crossed these 2 single mutants, and the progenies were subject to PCR-based genotyping to identify double homozygous mutant plants. Interestingly, the mutant plants drif1(+/−) drif2(−/−) and drif1(−/−) drif2(+/−) showed no obvious phenotype. However, no double homozygous mutant plants were identified after the analysis of 51 progenies from self-pollinated drif1(+/−) drif2(−/−) plants, which yielded 17 drif1(+/+) drif2(−/−) plants and 34 drif1(+/−) drif2(−/−) plants. Similar results were obtained from 49 progenies from self-pollinated drif1(−/−) drif2(+/−) plants (Supplemental Table S1).
To test whether the lethality takes place during the gametophytic development, reciprocal experiments in testing the segregation pattern of the insertional mutant alleles were performed. We analyzed the efficiency of mutation transmission through male and female gametes by performing 2 sets of reciprocal crosses between WT and drif1(+/−) drif2(−/−) or drif1(−/−) drif2(+/−) plants. Normal transmission efficiency of the drif1 and drif2 mutations through pollen and ovules was observed in the subsequent progeny, suggesting that the double mutations do not affect female or male gamete formation (Supplemental Table S2). A χ2-test of the observed ratios of progeny allele frequencies was conducted to test whether homozygous double mutants were embryonic lethal (Supplemental Table S1). Indeed, the statistical analysis suggests that lethality may occur during embryo development rather than during gametophytic development.
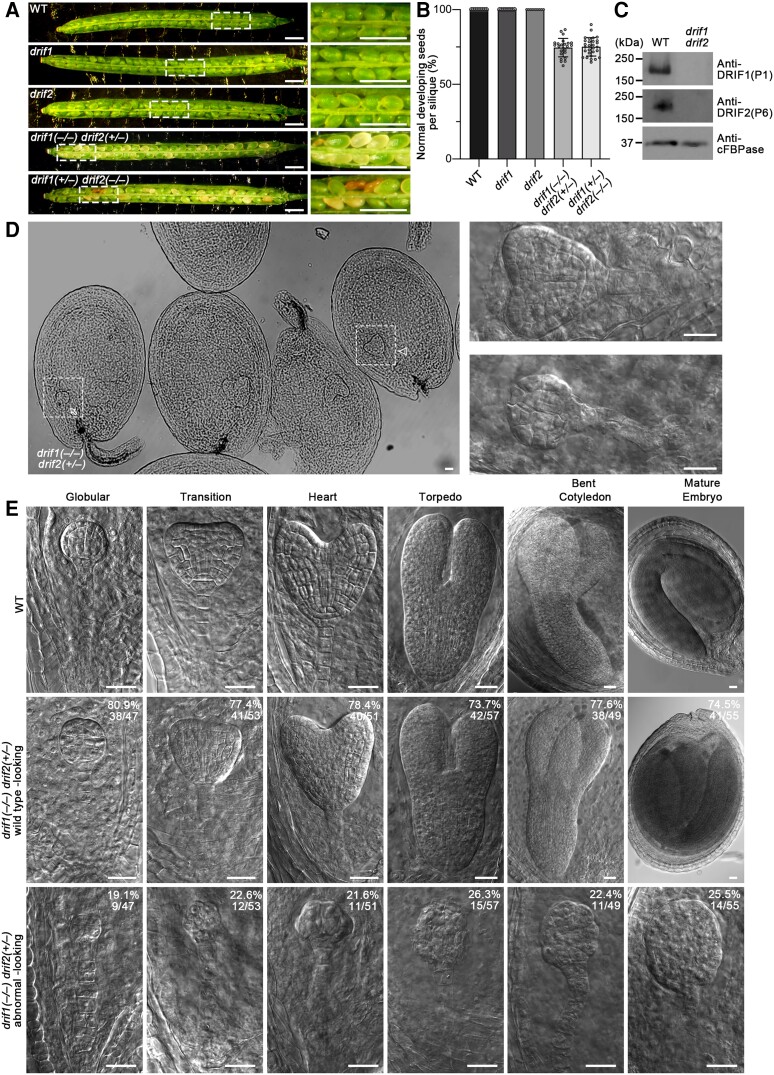
We then dissected the siliques to analyze the seeds from these plants. We found that 74.6% ± 6.2% (n = 24 siliques) of the seeds from drif1(−/−) drif2(+/−) plants and 75.1% ± 6.1% (n = 29 siliques) of the seeds from drif1(+/−) drif2(−/−) plants were normal, whereas 100% of the seeds in WT plants and single drif1 and drif2 mutants had normal appearance (Fig. 3, A and B). The abnormal seeds in mutant siliques are whitish and shrunken (Fig. 3A). The immunoblot analysis of proteins extracted from these abnormal seeds and WT seeds using P1 and P6 antibodies indicated that the abnormal seeds are drif1(−/−) drif2(−/−) double homozygous mutants (Fig. 3C).
Figure 3.
Embryos of drif1 drif2 mutants are arrested at the globular stage. A) Seed development phenotype in siliques of indicated genotypes viewed under a stereomicroscope. Images in the white dashed boxes are enlarged on the right panels. Note the whitish and shrunken seeds. Scale bars, 1 mm. B) Quantification of percentage of normal developing seeds in progenies of the indicated genotypes. For each genotype, siliques from 3 individual plants were analyzed with all data points plotted with mean ± Sd For WT, n = 9; for drif1, n = 8; for drif2, n = 9; for drif1(−/−) drif2(+/−), n = 24; and for drif1(+/−) drif2(−/−), n = 29 siliques. C) Immunoblot analysis of DRIF1 and DRIF2 proteins in WT and drif1 drif2 mutant seeds. D) Heart stage embryos in progenies of drif1(−/−) drif2(+/−) plants. Details of embryos in the white dashed boxes are enlarged and shown on the right panels. Arrow and arrowhead indicate examples of the normal developing heart stage embryo and the abnormal embryo, respectively, with corresponding enlargements shown on right panels. Scale bars, 25 μm. E) Embryos at different stages in progenies of WT and drif1(−/−) drif2(+/−) plants. Up panel shows WT embryos. Middle and low panels show WT-looking embryos and abnormal embryos in progenies of drif1(−/−) drif2(+/−) plants, respectively. Values and percentages indicate the number and percentages of representative embryos from different stages segregated from the drif1(−/−) drif2(+/−) plants. Scale bars, 25 μm.
In order to investigate the function of the DRIF1 and DRIF2 proteins in embryo development, we examined the embryogenesis in double homozygous mutants (Fig. 3D). In ovaries isolated from drif1(−/−) drif2(+/−) mutant, differences between the double mutant and WT-looking developing embryos became apparent at the globular stage, when the development of 19.1% (n = 47) of embryos was delayed and still at the 8-cell stage (Fig. 3E). From the transition stage onward, the difference became much more obvious. At the mature stage, about 25.5% (n = 55) of the embryos showed abnormality and were arrested at the globular stage without differentiating any polar axis and displaying loose borders (Fig. 3E). In contrast, WT-looking embryos become mature (Fig. 3E). These observations suggest that DRIF1 and DRIF2 are essential for embryo development.
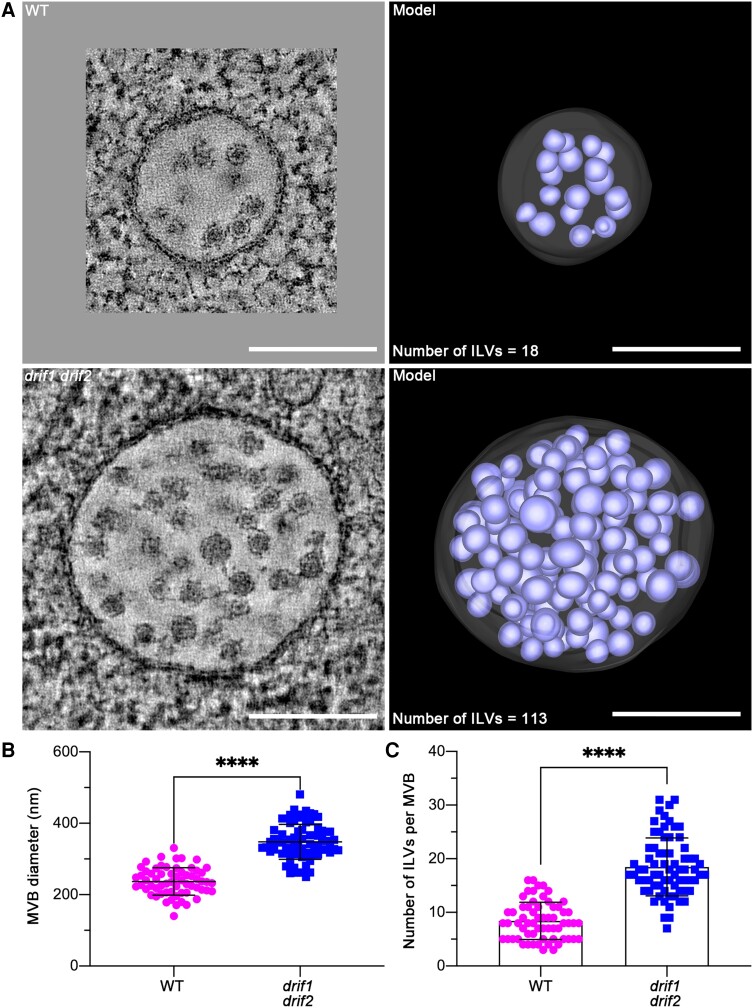
To detect whether the lack of both DRIF1 and DRIF2 affects morphologies of subcellular compartments, we further performed HPF/FS and analyzed embryos of WT and double homozygous mutants. We found that the morphology of Golgi and TGN in drif1 drif2 embryos was relatively normal (Supplemental Fig. S9), while the structures of MVBs were dramatically changed (Fig. 4A; Supplemental Fig. S9). The diameter of MVBs in the double mutant embryos was significantly larger (P < 0.0001), with mean diameter 348 ± 49 nm (n = 72 MVBs), compared with WT, with mean diameter 237 ± 38 nm (n = 62 MVBs) (Fig. 4B). Meanwhile, 2D TEM images indicated that double mutant MVBs contained more ILVs compared to those of the WT (drif1 drif2, 18 ± 5 ILVs/MVB section, n = 72 MVBs; WT, 8 ± 3 ILVs/MVB section, n = 62 MVBs) (Fig. 4C). Furthermore, based on the 3D model of whole MVB in WT and double mutant embryo, the total number of ILVs in WT is 18, while it is 113 in double mutant (Fig. 4A). All these data indicate that DRIF1 and DRIF2 affect the size of MVBs and the formation of ILVs.
Figure 4.
Electron tomography (ET) analysis of MVBs in embryos of WT and drif1 drif2 mutant. A) Representative tomographic slices (left panel) from 3 serial sections (300-nm thick) with corresponding 3D ET models (right panel) revealed the morphology of MVBs in the embryos of WT and drif1 drif2 mutant, respectively.Scale bars, 200 nm. B and C) Quantification of MVB structural features (MVB diameter and number of ILVs per MVB) from 2D TEM images of WT and drif1 drif2 mutant embryos, respectively, shown in A). Mean ± Sd with all data points plotted. For WT, n = 62 MVBs from at least 20 cells. For drif1 drif2, n = 72 MVBs from at least 20 cells. ****P < 0.0001, ns, no significance in Student's t-test.
Dysfunction of the DRIF1 and DRIF2 proteins affects PIN recycling
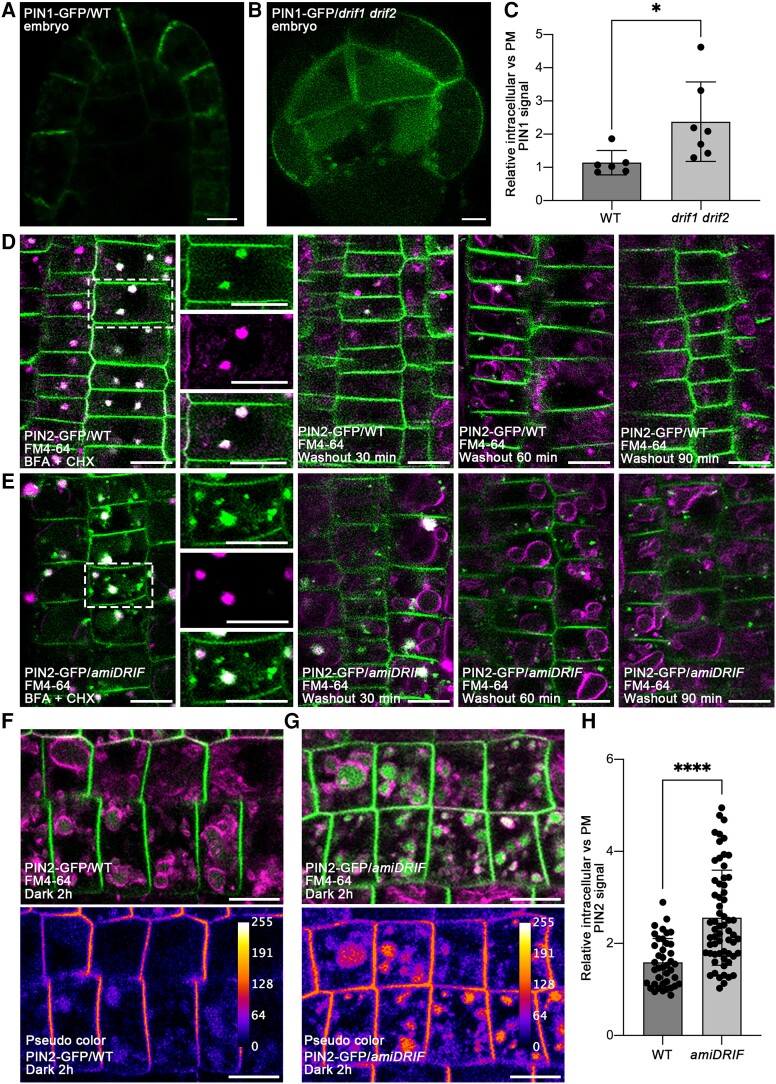
PIN proteins are auxin efflux carriers with a fundamental role in patterning processes during embryogenesis (Möller and Weijers 2009; Lau et al. 2012). PIN function is dependent on its intracellular localization, which is typically polarized. Based on the observation that the drif1 drif2 double mutant has defects in embryo development potentially caused by defects in MVB homeostasis, we further investigated the effects of DRIF1 and DRIF2 on the polar localization of PIN proteins. To do so, we crossed PIN1-GFP into drif1(−/−) drif2(+/−) and investigated the generated drif1 drif2 double homozygous embryos. In contrast to the polar distribution of PIN1-GFP mainly in epidermal cells in the WT embryos, double mutant embryos displayed a disrupted PIN1-GFP polar localization reflected by pronounced intracellular punctate foci and vacuolar GFP signals (Fig. 5, A and B). The statistical analysis of the relative intracellular vs PM PIN1-GFP signals further supports our observed results (Fig. 5C).
Figure 5.
Dysfunction of the DRIF1 and DRIF2 proteins affects PIN proteins recycling and degradation. A and B) The polar localization of PIN1-GFP in WT A) or drif1 drif2B) embryos. Bars, 5 μm. C) Quantification of relative intracellular versus PM-localized PIN1-GFP signals in WT and drif1 drif2 embryos shown in A) and B). For each genotype, data from 6 embryos were used for quantification and statistical analysis. Mean ± Sd with all data points plotted. *P < 0.05 in Student's t-test. D to E) The DRIF1 and DRIF2 proteins affect PIN2-GFP recycling. Five-day-old WT or amiDRIF seedlings were pretreated with 50 μm CHX for 60 min and then stained with 4 μm FM4-64 in the presence of 50 μm BFA and 50 μm CHX for 5 min, followed by transferred into liquid medium containing 50 μm BFA and 50 μm CHX for 60 min. BFA-induced aggregations of PIN2-GFP in WT D) and amiDRIF mutant E) seedlings were examined after BFA treatment (D/E, left panels). BFA washout was performed by transferring seedlings to fresh liquid medium with 50 μm CHX. The subcellular localization of PIN2-GFP was examined at the indicated time points after washout. PIN2-GFP recycling to the PM is arrested upon BFA removal in amiDRIF mutant E) compared with WT D) (as indicated by 30, 60, and 90 min washout) (D/E, right panels). Images in the white dashed boxes are shown by separated channels (from top to bottom: GFP, RFP, and merged). Note the defects of polar recycling in amiDRIF background. Bars, 10 μm. F to G) The PM-localized PIN2-GFP accumulated in the vacuole of amiDRIFG) compared with WT F) after 2 h dark treatment. Vacuoles are outlined with FM4-64 dye (4 μm) after 2 h uptake in dark. Pseudo color image was used for PIN2-GFP intensity visualization. The calibration bar on up-right corner indicates the level of intensity from min to max. Bars, 10 μm. H) Quantification of relative intracellular versus PM-localized PIN2-GFP signals in WT and amiDRIF background. Mean ± Sd with all data points plotted. Forty and 62 cells from at least 3 individual plants were used for quantification and statistical analysis in WT and amiDRIF, respectively. ****P < 0.0001 in Student's t-test.
Since it is challenging to investigate the recycling of PIN proteins from the TGN to the PM in the abolished drif1 drif2 embryos, we applied artificial microRNAs (amiRNAs) targeting both DRIF1 and DRIF2. To test the knock down efficiency and specificity, amiDRIF or amiR D1s, the previously reported amiRNA that targeted ADP-ribosylation factor (ARF) D1A and D1B (Niu et al. 2022), were cotransformed into Arabidopsis protoplasts with empty vector, ARFD1A-GFP or GFP. The immunoblot analysis results showed that the protein level of DRIF1 was dramatically decreased, while the ARFD1A or the GFP proteins remained similar to that of control, demonstrating the good efficiency and specificity of amiDRIF (Supplemental Fig. S10A to S10C).
The amiRNA was then used to generate transgenic plants termed amiDRIF. Results from both immunoblot and RT-qPCR analysis confirmed the reduced proteins and transcripts in amiDRIF (Supplemental Fig. S11A to S11C). The endosomal cycling of PIN proteins relies on the ADP-ribosylation factor GTP-exchange factor GNOM, which is sensitive to brefeldin A (BFA), a fungal toxin that reversibly inhibits vesicle trafficking (Geldner et al. 2001, 2003; Cherfils and Melancon 2005). BFA treatment and washout accompanied with the protein biosynthesis inhibitor cycloheximide (CHX) were then performed to investigate the possible role of DRIF proteins in the recycling of PIN2 proteins from the TGN to the PM (Wang et al. 2013; Jásik and Schmelzer 2014; Naramoto et al. 2014; Doyle et al. 2015; Mao et al. 2016). After 1 h BFA treatment accompanied with FM4-64 staining in the presence of CHX, in WT roots, the PIN2-GFP accumulated intracellularly as BFA bodies, colocalized with the endocytic tracer FM4-64 (Fig. 5D). However, only a portion of PIN2-GFP colocalized with FM4-64 in amiDRIF lines, while additional puncta did not, suggesting that the latter were unrelated to BFA bodies (Fig. 5E). In addition, BFA washout experiments showed that as expected BFA bodies had rapidly disappeared and the PM localization was restored in the WT (Fig. 5D). However, in amiDRIF lines, the recovery of the PM signal was less pronounced, and more PIN2-GFP signals remained in puncta (Fig. 5E).
To further characterize the punctate structures, an immunolabeling analysis using marker antibodies for Golgi, TGN, MVB/PVC, and tonoplast was performed, showing that the remaining puncta in amiDRIF lines colocalized with the MVB/PVC marker anti-VSR (Supplemental Fig. S12). All these data indicate that the polar recycling of PIN2-GFP from endosomal compartments to the apical PM requires DRIF proteins.
Consistent with the observation of pronounced intracellular punctate foci of PIN1 in drif1 drif2 double homozygous embryos, a portion of PIN2-GFP abnormally accumulated in MVB/PVC in amiDRIF mutant cells at steady-state prior to the treatment (Supplemental Fig. S13). We next investigated the vacuolar trafficking of PIN2-GFP by incubating plants in darkness with the uptake of the endocytic tracer FM4-64. We found that in amiDRIF plants, the vacuolar accumulation of PIN2-GFP was preferentially enhanced after dark treatments for 2 h in FM4-64 dye labeled vacuolar, compared with the small amount PIN2-GFP signals in intracellular parts in WT (Fig. 5, F and G). Accordingly, the statistical analysis of the relative intracellular vs PM PIN2-GFP signals supported the observation that a significant proportion accumulated in the vacuoles of amiDRIF seedlings in comparison to the WT (Fig. 5H). Interestingly, the endocytic uptake of FM4-64 was not significantly altered in amiDRIF mutants (Supplemental Fig. S14). Collectively, these results suggest that the endosomal recycling of PIN2-GFP back to the PM is impaired, resulting in increased vacuolar traffic in amiDRIF mutant.
DRIF1 colocalizes and interacts with SNX1
To investigate the pathway in which DRIF1 functions to regulate PM proteins recycling, we further investigated the membrane distribution and subcellular localization of DRIF1 protein. The raised antibody P1 was used to probe the distribution of DRIF1 in cellular fractions. DRIF1 was detected in both soluble and membrane fractions (Supplemental Fig. S15). In addition, treatment of membrane fractions with alkali or high-salt buffer was able to partially solubilize DRIF1, but not the integral membrane protein endomembrane protein 12 (EMP12), suggesting that DRIF1 is membrane-associated (Supplemental Fig. S15).
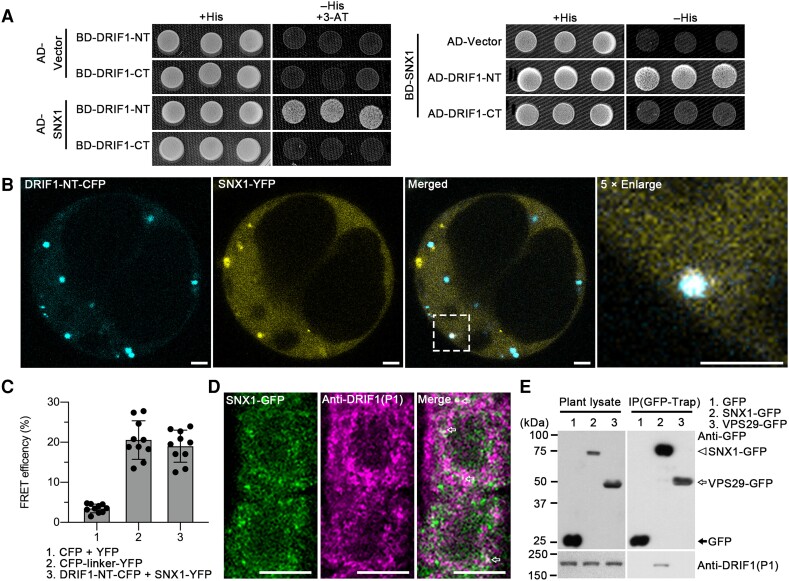
We next performed a yeast 2-hybrid (Y2H) screen using the Universal Arabidopsis Normalized Library and NT of DRIF1 (1 to 943 amino acids) as the bait. In this screen, we identified SNX1 as a potential interactor and validated the interaction of full length SNX1 with DRIF1-NT (1 to 943 amino acids) but not DRIF1-CT (944 to 1,775 amino acids) (Fig. 6A; Supplemental Fig. S16A). We also confirmed the interaction between DRIF1 and SNX1 using acceptor photobleaching fluorescence resonance energy transfer (FRET-AB) between DRIF1-NT-CFP and SNX1-YFP (Fig. 6, B and C). We further performed immunolabeling analysis using the DRIF1 (P1) antibody in SNX1-GFP seedlings, showing that DRIF1 partially colocalized with SNX1-GFP (Fig. 6D).
Figure 6.
The NT of DRIF1 interacts with the retromer complex component SNX1. A) Y2H analysis of the binary interactions of full-length SNX1 with DRIF1-NT (1 to 943 amino acids) and DRIF1-CT (944 to 1775 amino acids). Transformed yeast cells were grown on synthetic complete medium + His (SD/−Leu/−Trp) as a transformation control, -His (SD/−His/−Leu/−Trp) for protein interaction. Two millimolar 3-AT were added to suppress BD autonomous-activation. NT (1 to 943 amino acids of DRIF1); CT, C-terminus (944 to 1775 amino acids of DRIF1); SD, synthetic defined bases; BD, binding domain. B and C) Fluorescence resonance energy transfer (FRET) analysis shows interaction between DRIF1-NT-CFP and SNX1-YFP in vivo. Arabidopsis protoplasts transiently expressing DRIF1-NT (1 to 943 amino acids)-CFP and full-length SNX1-YFP fusions were subjected to photobleaching and FRET analysis. Images of DRIF1-NT-CFP and SNX1-YFP as well as 5× enlarged images in dashed box before FRET are shown B). FRET efficiency was quantified by using the acceptor photobleaching approach C). FRET efficiency was calculated as FRETeff = (Dpost−Dpre)/Dpost, where Dpre and Dpost stand for the donor intensities before and after acceptor bleaching, respectively. For each group, 10 individual protoplasts were used for FRET efficiency quantification and statistical analysis. Mean ± Sd with all data points plotted. Bar, 25 μm. NT (1 to 943 amino acids of DRIF1). D) Partial colocalization of SNX1-GFP with DRIF1 (arrows) in plant. Five-day-old SNX1-GFP seedlings were subjected to PFA fixation for subsequent immunolabeling with DRIF1 (P1) antibody and confocal imaging analysis. Bar, 10 μm. E) IP assay shows association of SNX1 with DRIF1 in plant. GFP, SNX1-GFP, or VPS29-GFP transgenic plants were subjected to total protein extraction and IP with GFP-trap, followed by immunoblotting analysis with indicated antibodies.
To test the possible interaction between DRIF1 and SNX1 in plant, we next performed whole plant-based immunoprecipitation (IP) assay using total lysates extracted from 5-d-old Arabidopsis SNX1-GFP seedlings. Detected by DRIF1 P1 antibody, the full length DRIF1 was immunoprecipitated with SNX1-GFP but not with the GFP control or the core retromer component VPS29-GFP, indicating the association of DRIF1 with SNX1 (Fig. 6E). The plant retromer complex consists of SNX1-SNX2 complex and the separate core retromer VPS35-VPS29-VPS26 trimer (Simonetti and Cullen 2018; Ivanov and Robinson 2020). We next tested the possible interaction between DRIF1 and other retromer components; however, results from both Y2H assay and the Arabidopsis plant system biology dark-type culture (PSBD) cell-based IP showed that DRIF1 did not interact with the core retromer (VPS26, VPS29, and VPS35) nor SNX2 proteins (Supplemental Figs. S16A and S17). In addition, direct interaction was not detected between DRIF1 and Arabidopsis ESCRT-I components Vps23A and Vps23B (Supplemental Fig. S16B).
Considering the defects in PIN family protein recycling and lytic degradation in amiDRIF, which are similar to those seen in the mutants of retromer complex components snx1 and vps29 (Kleine-Vehn et al. 2008; Nodzyński et al. 2013), we next investigated the genetic interaction between DRIF1 and components of the retromer complex. We analyzed the phenotypes of drif mutants in sucrose-limited conditions. We found it was similar to the retromer complex mutants; a portion of the drif1-1, drif2-1, and amiDRIF seedlings arrested growth after cotyledon formation in the absence of sucrose (Supplemental Fig. S18A). Quantification of the growth arrest phenotype indicated that more than half (drif1-1, 56%, n = 78; drif2-1, 62%, n = 72) of the single mutants exhibited growth inhibition when germinated on sucrose-limited conditions, and in amiDRIF, the percentage was 88% (n = 77), compared with only 6% in WT (n = 74) (Supplemental Table S3). In addition, conditional growth arrest was fully rescued by sucrose application (Supplemental Fig. S18B).
DRIF proteins regulate the subcellular localization of SNX1
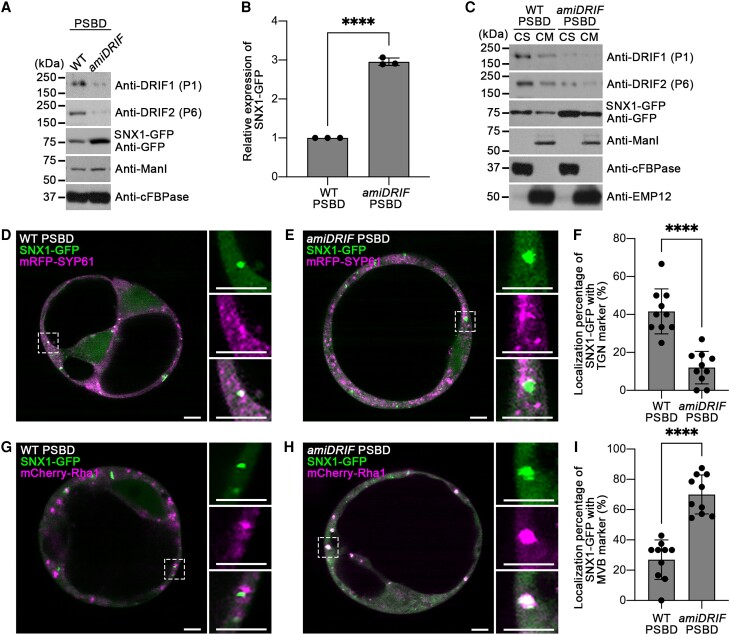
To further address the functional relationship between DRIF proteins and SNX1, the protein level of SNX1 in WT and amiDRIF was analyzed. We found that the level of SNX1-GFP in total protein extracted from Arabidopsis PSBD cells expressing amiDRIF was significantly higher than WT cells (Fig. 7, A and B), indicating a role of DRIF proteins in regulating SNX1 protein level in Arabidopsis cells. Further immunoblot analysis showed increased distribution of SNX1-GFP in both cell soluble (CS) and cell membrane (CM) fractions in Arabidopsis PSBD cells expressing amiDRIF compared with WT PSBD cells (Fig. 7C).
Figure 7.
DRIF proteins regulate the protein level and subcellular localization of SNX1. A) Immunoblot analysis of SNX1-GFP protein. Arabidopsis protoplasts transiently expressing SNX1-GFP or SNX1-GFP and amiDRIF were subjected to total protein extraction, followed by immunoblot analysis with indicated antibodies. P1 and P6 antibodies were applied to detect DRIF1 and DRIF2 proteins, respectively. Anti-cFBPase and anti-ManI antibodies were used as loading controls to exclude the possibility of nonspecific effects of the microRNA knock down, respectively. B) Quantification analysis of protein level of SNX1-GFP. The relative percentages of band intensity of the WT and amiDRIF were calculated and normalized to the anti-cFBPase loading control. The normalized band intensity of SNX1-GFP in WT Arabidopsis PSBD was defined as 1. Three independent immunoblots (n =3) were used for the quantification. Mean ± Sd with all data points plotted, ****P < 0.0001 in Student's t-test. C) Immunoblot analysis of SNX1-GFP distribution in CS and CM fractions. The CS and CM fractions were prepared from Arabidopsis protoplasts transiently expressing SNX1-GFP or SNX1-GFP and amiDRIF. P1 and P6 antibodies were applied to detect DRIF1 and DRIF2 proteins, respectively. GFP antibody was used to detect transiently expressed SNX1-GFP protein. Anti-ManI was used to exclude the possibility of nonspecific effects of the microRNA knock down. Anti-cFBPase and anti-EMP12 were used as loading controls for CS and CM fractions, respectively. D and E) Localization of SNX1-GFP and TGN marker mRFP-SYP61 in Arabidopsis protoplasts D) and Arabidopsis protoplasts expressing amiDRIFE). Images in white squared boxes are shown by separated channels (from top to bottom: GFP, RFP, and merged). Scale bars, 5 μm. F) Quantification of localization percentage of SNX1-GFP with TGN marker mRFP-SYP61. Similar results were observed in 2 independent experiments, and 10 individual protoplasts were used for quantification and statistical analysis. Mean ± Sd with all data points plotted, ****P < 0.0001 in Student's t-test. G and H) Localization of SNX1-GFP and MVB marker mCherry-Rha1 in Arabidopsis protoplasts G) and Arabidopsis protoplasts expressing amiDRIFH). Images in white squared boxes are shown by separated channels (from top to bottom: GFP, RFP, and merged). Scale bars, 5 μm. I) Quantification of localization percentage of SNX1-GFP with MVB marker mCherry-Rha1. Similar results were observed in 2 independent experiments, and 10 individual protoplasts were used for quantification and statistical analysis. Mean ± Sd with all data points plotted, ****P < 0.0001 in Student's t-test.
We then analyzed the subcellular localization of SNX1-GFP puncta with organelle markers in WT Arabidopsis PSBD cells and Arabidopsis PSBD cells expressing amiDRIF (Fig. 7, D to I). The colocalization percentage of SNX1-GFP puncta with TGN was significantly decreased (Fig. 7, D to F) in Arabidopsis PSBD cells expressing amiDRIF compared to WT Arabidopsis PSBD cells, while the colocalization percentage of SNX1-GFP with MVB was increased (Fig. 7, G to I). Similar results were observed in Arabidopsis WT leaf protoplasts and amiDRIF plant-derived leaf protoplasts (Supplemental Fig. S19). Taken together, these results suggest that DRIF proteins may regulate the protein level and TGN-MVB distribution of SNX1.
Discussion
Cellular functions including hormone signal perception, environmental responses, nutrient uptake as well as intracellular communication are facilitated by PM proteins. The ESCRT machinery plays a fundamental role in the sorting of ubiquitinated membrane proteins, thus regulating the homeostasis of PM localized membrane proteins (Raiborg and Stenmark 2009).
Through the sof mutant screen strategy (Zhao et al. 2015), we previously identified the regulators BRAF and RST1 (Shen et al. 2018; Zhao et al. 2019). BRAF negatively regulates ILV formation in MVBs through the competition with FREE1 for binding to the ESCRT-Ⅰ component Vps23 (Shen et al. 2018). Different from the observation of BRAF, the Y2H analysis showed no direct interaction between DRIF1 and Arabidopsis Vps23 homologs Vps23A and Vps23B (Supplemental Fig. S16B), suggesting a distinct mechanism of DRIF proteins from BRAF. In addition, it was reported that RST1 may be involved in the FREE1-independent membrane protein vacuolar sorting pathway that also plays a negative role in vacuolar transport (Zhao et al. 2019), as a significant overaccumulation of RST1 was observed in free1 and FREE1-RNAi plants. Taken together, the study of BRAF, RST1, and DRIF proteins indicates the diverse mechanisms derived from the screen. Investigation of different suppressors recalls the multiple functions of the plant-unique FREE1 and the unique mechanisms the plants have evolved in the regulation of membrane proteins degradation.
Internalized proteins can be recycled from endosomes back to the PM to escape degradation (Rodriguez-Furlan et al. 2019). In plants, the retrograde transport can possibly be regulated by the SNX1 (Kleine-Vehn et al. 2008; Salanenka et al. 2018). SNXs have been reported to localize at the TGN and MVB in Arabidopsis root cells and protoplasts (Jaillais et al. 2006; Niemes et al. 2010; Pourcher et al. 2010). The correct localization of SNX proteins is important for cellular homeostasis as demonstrated by the interaction of SNX1 with microtubule-associated protein CYTOPLASMIC LINKER ASSOCIATED PROTEIN (CLASP). In clasp-1 mutant, the endosomal SNX1 association is decreased, leading to the PIN2-GFP accumulation in the lumen of lytic vacuole rather than being recycled (Ambrose et al. 2013).
Similarly, in the absence of the 1-phosphatidylinositol-3-phosphate 5-kinase FORMATION OF APLOID AND BINUCLEATE CELLS1A, the SNX1 was released from the endosome membrane and showed a cytoplasmic distribution, leading to the disruption of basal localized PIN proteins in young cortical cells (Hirano et al. 2015). Recent studies have also characterized the function of SNX proteins in the recycling of specific PM transporters in the TGN, facilitating their redirection to the PM. SNX1 and IRON-REGULATED TRANSPORTER1 (IRT1) can partially colocalize in a subpopulation of TGN. Dysfunction of SNX1 perturbs IRT1 retrograde transport back to the PM and finally leads to IRT1 vacuolar degradation (Ivanov et al. 2014). Interestingly, the endosome-localized FREE1/FYVE1 might coordinate with the SNX1 in facilitating the IRT1 recycling (Barberon et al. 2014; Brumbarova et al. 2015). These findings intriguingly suggest the importance of further revealing regulators of FREE1- and SNX1-mediated membrane proteins hemostasis.
Indeed, our data suggest DRIF1 as a possible negative regulator of FREE1 in ILVs formation of MVBs. In addition, DRIF1 can interact with SNX1, and dysfunction of DRIF proteins further affects the recycling and degradation of PIN proteins as indicated by the abnormal accumulation of PIN proteins in the intracellular compartments that are colocalized with MVB/PVC marker. Since SNX1 was previously shown to localize in MVB/PVC (Jaillais et al. 2006; Pourcher et al. 2010), SNX1 is thus likely to colocalize with the PIN2-positive puncta in MVB/PVC and the DRIF1–SNX1 or DRIF2–SNX1 interaction may play a role in membrane protein recycling by retrieving them from degradation. Further colocalization study of accumulated PIN2 puncta with SNX1 in amiDRIF would broaden the understanding of DRIF1–SNX1 or DRIF2–SNX1 in membrane protein recycling.
In addition, the sof10 mutant recovered the vacuolar transport of soluble cargo spL-RFP in FREE1-RNAi, suggesting the possibility that DRIF1–SNX1 or DRIF2–SNX1 also functions in vacuolar transport of soluble cargos. Considering the coordinated function of FREE1/FYVE1, DRIF1, and SNX1, it will be interesting in future study to investigate the membrane protein recycling and MVB-mediated vacuolar sorting in double mutants of SNX1 and FREE1 (such as snx1 FREE1-RNAi).
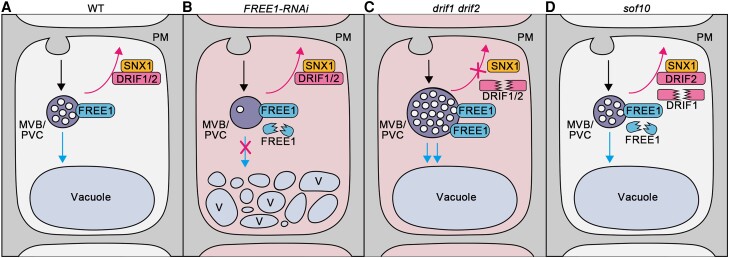
As illustrated in our working model, in WT plants, ubiquitinated membrane proteins are sorted into ILVs of MVBs/PVCs for degradation with the function of FREE1 and recycled back to the PM with the proper function of DRIF1 or DRIF2 and SNX1 interactors (Fig. 8A). In the FREE1-RNAi mutants, the decrease of FREE1 protein levels leads to defects in ILV formation and thus vacuolar degradation. Meanwhile, the DRIF1–SNX1 or DRIF2–SNX1 interaction maintains the normal recycling of membrane proteins, thus leading to the lethal phenotype of the FREE1-RNAi mutant (Fig. 8B). Dysfunction of DRIF proteins (as in drif1 drif2) blocks membrane protein recycling and induced the enlarged MVBs/PVCs with increased number of ILVs, which finally enhances the degradation of membrane proteins in the vacuole (Fig. 8C). Finally, in the sof10 mutant, the decreased protein levels of DRIF1 and FREE1 compromise the defects observed in FREE1-RNAi, suggesting the suppressor function of DRIF1 in FREE1-mediated membrane protein sorting (Fig. 8D).
Figure 8.
Working model of DRIF proteins function in regulating membrane protein recycling and degradation. The endocytosed PM proteins are subjected to vacuolar degradation (blue arrow) and/or saved from degradation by recycling from MVB/PVC (magenta arrow). A) In WT plants, the membrane proteins are ubiquitinated and sorted into ILVs of MVBs/PVCs for degradation (blue arrow) with the normal function of FREE1 and recycling back to PM (magenta arrow) with the proper function of DRIF1 or DRIF2 and SNX1 interactor. B) In the FREE1-RNAi lethal cells (indicated by red background), the decreased FREE1 proteins level leads to the defects of ILVs formation in MVBs/PVCs and vacuolar degradation (indicated by a magenta cross on blue arrow), while the DRIF1- or DRIF2-SNX1 interaction maintains the normal recycling of membrane proteins (magenta arrow). C) In the drif1 drif2 double mutants, the recycling of membrane proteins was blocked (indicated by a magenta cross on magenta arrow) and induced enlarged MVBs/PVCs with increased number of ILVs, which finally enhanced the degradation of membrane proteins into vacuole (blue arrows). The drif1 drif2 double mutant is embryo lethal (indicated by red background). D) In the sof10 mutant, dysfunction of DRIF1 partially blocks the recycling of membrane proteins (magenta arrow) and recovers the normal morphology and ILVs of MVBs/PVCs as well as the vacuolar degradation (blue arrow) of membrane proteins, resulting in the recovery of the lethal phenotype of FREE1-RNAiB). Therefore, it is proposed that the DRIF1 is an important regulator of the FREE1-mediated vacuolar sorting pathway for membrane proteins in Arabidopsis.
DRIF1 or DRIF2 mutants cannot suppress a free1 null mutant (Supplemental Fig. S8), suggesting that the suppressor activity of DRIF1 or DRIF2 may rely on residual FREE1 levels. As proposed in the working model (Fig. 8), DRIF1–SNX1 or DRIF2–SNX1 interaction may maintain the normal recycling of membrane proteins, and the degradation of membrane protein pathways requires the function of FREE1. In the sof10 mutant, dysfunction of DRIF1 partially blocks the recycling of membrane proteins and enhances the degradation of membrane proteins into vacuole, which requires the function of residual FREE1, thus recovered the lethal phenotype of FREE1-RNAi.
Gene duplication often contributes to the functional redundancy of daughter genes; thus, mutation of one of the duplicates is often phenotypically invisible (Bouché and Bouchez 2001). This phenomenon is also observed for DRIF1 and DRIF2 because a loss of function of either gene led to no discernible phenotypes under normal growth conditions. However, drif1 or drif2 mutation alone can suppress the FREE1-RNAi lethal phenotype (Fig. 2B), indicating that these 2 genes might not be fully functionally redundant. This observation could possibly be explained by the gene dosage-dependent effect of DRIF proteins on growth, of which the expression level of DRIF might be strictly controlled. In this scenario, the decreased DRIF protein level caused by single mutation of drif1 or drif2 can suppress the FREE1-RNAi lethal phenotype, whereas double mutant exhibited abnormal embryo development.
Collectively, we proposed that plants might have evolved unique features related to the tight regulation of membrane protein homeostasis, in order to modulate different cellular pathways in response to cellular or environmental changes. However, it remains unclear why the enlarged MVBs and increased ILVs occur in drif1 drif2 and how the DRIF proteins regulate the SNX1-dependent membrane protein recycling to PM. In addition, there are several conserved domains such as helicase-related domains and a RING domain in DRIF1 with unknown functions. Interestingly, the D787N mutation in sof641 is not in one of these domains (Supplemental Fig. S5F), and the sof10 mutant would be missing a RING domain located at the very C-terminus of the DRIF1 protein (amino acids 1,564 to 1,612), suggesting its potential function in regulating gene transcription under certain environmental conditions. Such multiple functions of DRIF1 would meet the diverse functions of FREE1 in both MVB-mediated vacuolar sorting and the nucleus-related transcriptional modulation of ABA signaling (Gao et al. 2014; Li et al. 2019). Future studies on the identification and characterization of additional sof mutants will shed light on our understanding of regulators for ESCRT-mediated ILVs formation of MVBs and vacuolar sorting pathways in plants.
Materials and methods
Arabidopsis FREE1-RNAi suppressor screen and sof identification
Seeds of pTA7002-ProDEX:FREE1-RNAi transgenic plants were mutagenized with EMS, and the mutant pool was established as previously described (Zhao et al. 2015). Around 40,000 seeds corresponding to 10,000 mutagenized M1 seeds were grown on the MS plates with 3% (w/v) sucrose. DEX was added (10 μm) to the MS, and seedlings were grown for 5 d to screen and determine a survival phenotype, as suppressors of free1 (sof). M3 seeds were collected individually for selected M2 seedlings planting in soil and then grown on MS plates supplied with 10 μm DEX and 50 mg/mL hygromycin, and 7-d-old M3 seedlings were subjected to phenotypic confirmation.
The whole-genome sequence-based mapping was described previously (Zhao et al. 2015). The sof lines sof10 and sof641 were firstly outcrossed with the Landsberg erecta (Ler). For each line, the F2 population was grown on MS plates with 3% (w/v) sucrose supplemented with 10 μm DEX and hygromycin (50 mg/mL). Surviving sof seedlings were pooled for a genomic DNA preparation using DNeasy Plant Mini Kit (QIAGEN), followed by library preparation and sequencing on a HiSeq2000 (Illumina) to generate 100-bp pair-end reads, yielding more than 15-fold genome coverage. The single-nucleotide polymorphism (SNP) frequency was identified through mapping to the Arabidopsis (Arabidopsis thaliana) Col-0 genome (TAIR10) by SAMTOOLS software (Li et al. 2009) and then plotted using SHOREmap (Schneeberger et al. 2009) on chromosomal location based on 461,070 SNP markers. Relatively reliable loci were filtered with consensus quality >20 (error rate, 1%) and with total depth >5. Only EMS-induced C/G to T/A transitions were considered as further candidates.
Phylogenetic analysis
The computed homologs of DRIF1 protein sequences were obtained from NCBI and aligned using ClustalW (Larkin et al. 2007). Evolutionary analyses were conducted by using the Neighbor-Joining method in MEGA7 (Kumar et al. 2016) (Saitou and Nei 1987). The phylogenetic tree was illustrated as radiation format rooted on the Physcomitrium patens group. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and were in the units of the number of amino acid substitutions per site. The alignment and tree files are provided as Supplemental Files 1 and 2.
Plant materials and growth conditions
The Arabidopsis T-DNA insertion lines of drif1-1 (SALK_122385C) and drif2-1 (SALK_088782) were obtained from NASC. Genome-sequencing data of chimeric reads with both T-DNA and genome DNA sequences were used for the confirmation of the drif1-1 and drif2-1 single lines. For homozygous mutants’ confirmation, PCR-based genotyping was performed using gene and T-DNA-specific primers. Double mutants were generated by crossing the single mutants, followed by selecting the desired genotypes by PCR-based genotyping. To generate the transgenic plants, all of the resulting constructs were transformed into Agrobacterium (Agrobacterium tumefaciens) GV3101 and then introduced into WT Col-0 or indicated mutants by floral dip method (Clough and Bent 1998).
For plants growth, seeds were first surface-sterilized and grown on plates with full MS (pH 5.7) with 1% sucrose (w/v) and 0.8% (w/v) agar at 22 °C under a long-day condition with a 16 h light (120 s−1 m−2 light intensity provided by white LED lights)/8 h dark photoperiod. If seedlings require treatment with DEX, 10 μm DEX (30 mm stock in ethanol) was added directly in the medium.
Plasmid construction
For generating tagged DRIF1 plasmid for transgenic plants of ProUBQ:DRIF1-FLAG and ProDRIF1:DRIF1-GFP, the coding sequence was amplified from cDNA without stop codon and recombined together with ProUBQ or ProDRIF1 and FLAG tag or GFP tag using NEBuilder HiFi DNA Assembly Cloning Kit into the pBI121 (Clontech) vectors. The artificial microRNAs targeting both DRIF1 and DRIF2 was designed by Web microRNA Designer (WMD3), and the corresponding Arabidopsis MIR319a precursor was generated and cloned from pRS300 (Addgene) plasmid, followed by restriction cloning by EcoRI and BamHI into desired binary vector pBI121 for transformation. To generate the constructs used for transient expression in Arabidopsis protoplasts, the encoded cDNAs of the corresponding genes were amplified and then cloned into the pDONR/Zeo vector, followed by recombination using modified pBI221 vectors containing CFP, GFP, and YFP under the UBQ10 promoter by Gateway cloning (Invitrogen). All constructs were confirmed by Sanger sequencing. Primers and constructs used in this study are listed in Supplemental Table S4.
Chemical treatment
For phosphate treatment, 5-d-old PHT1-GFP seedlings were treated with 1 mm KH2PO4 (+P) in liquid ½-MS. For FM4-64 uptake experiments, 4 μm FM4-64 was used with 5 min incubation time and was washed twice before observation.
Experiment of BFA treatment and washout accompanied with CHX was performed as described previously (Wang et al. 2013; Jásik and Schmelzer 2014). Briefly, 5-d-old PIN2-GFP/WT or PIN2-GFP/amiDRIF seedlings were pretreated with 50 μm CHX for 60 min. Seedlings were then stained with 4 μm FM4-64 with 50 μm BFA and 50 μm CHX for 5 min, followed by treated in 50 μm BFA and 50 μm CHX for 60 min. For BFA washout, seedlings were transferred to fresh medium with 50 μm CHX and observed at indicated time points.
Antibody production
Synthetic peptides (GeneScript) corresponding to different regions of DRIF1 and DRIF2 were used for raising polyclonal antibodies in rabbits at the Laboratory Animal Services Center of The Chinese University of Hong Kong. Final serums were affinity-purified using CnBr-activated Sepharose 4B (Sigma-Aldrich) column conjugated with the peptides. FREE1, EMP12, ManI, SYP61, VSR, and V-PPase antibodies were homemade or gifted antibodies as previously described (Sanderfoot et al. 2001; Li et al. 2002; Tse et al. 2004; Gao et al. 2012; Jia et al. 2013; Gao et al. 2014). The usage concentration of the primary antibodies was described in related sections. Additional primary antibodies used were anti-cFBPase (Agrisera, AS04043) with 1:5,000 dilution, anti-UBQ (Santa Cruz, P4D1) with 1:1,000 dilution, and anti-GFP (Chromotek, 029762) with 1:5,000 dilution for immunoblots.
Immunolabeling
Immunolabeling of seedling roots was carried out as described previously (Friml et al. 2003; Paciorek et al. 2006; Sauer et al. 2006) with modifications. Briefly, 4-d-old seedlings were fixed by 4% (w/v) paraformaldehyde (PFA, Sigma) in PBS solution, followed by cell wall digestion using 2% (w/v) Driselase (Sigma) in 1 × PBS for 60 min. For the permeation of root tips, 3% (v/v) NP-40 plus DMSO (Sigma) in 1 × PBS solution was used for 60 min. The root tips were then blocked with 3% (w/v) BSA for 60 min. The primary antibodies were used in a concentration of 4 μg/mL for overnight at 4 °C. For the incubation of secondary antibody, Alexa Fluor 568 goat antirabbit IgG (Invitrogen) was used in a concentration of 2 μg/mL for 180 min at 37 °C.
Protein extraction and immunoblot analysis
For Arabidopsis seedlings samples, 0.1 g materials were grounded to powder in liquid nitrogen and homogenized in 100 μL protein extraction buffer containing 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1% (v/v) Triton X-100, and 1× complete protease inhibitor cocktail (PIC, Roche). Total lysates were centrifuged at 10,000 g for 15 min at 4 °C; supernatant protein samples were boiled in SDS sample loading dye at 95 °C, followed by SDS-PAGE and immunoblot analysis using specified antibodies. All uncropped immunoblots were provided in Supplemental Figs. S20 to S22.
For CS and CM fraction isolation, the 7-d-old Arabidopsis seedlings were ground in ice-cold extraction buffer (40 mm HEPES-KOH at pH 7.5, 1 mm EDTA, 10 mm KCl, 0.4 m sucrose, and 1× complete PIC), followed by centrifugation at 600 × g for 3 min to remove large cellular debris; the supernatant was further centrifuged at 100,000 × g for 60 min at 4 °C. The supernatant was soluble fraction. The pellet was washed twice and used as the membrane fraction.
Transient expression assays
The transient expression in Arabidopsis was performed as described previously (Miao and Jiang 2007; Shen et al. 2014). The Arabidopsis suspension cultured cells were subcultured every 5 d and grown at 25 °C, 130 rpm in the dark. Usually the 5-d-old Arabidopsis PSB-D suspension cells were digested by enzyme solution (1% [w/v] cellulase “ONOZUKA” RS, 0.05% [w/v] pectinase, and 0.2% [w/v] Driselase in 25 mL TEX buffer) for around 2 h. The TEX buffer is composed of 4.3 g/L MS salts, 0.4 m sucrose, 500 mg/L MES hydrate, 750 mg/L CaCl2·2H2O, 250 mg/L NH4NO3, and pH 5.7. Then, electroporation buffer (0.4 m sucrose, 2.4 g/L HEPES, 6 g/L KCl, and 600 mg/L CaCl2·2H2O, pH 7.2) was used to wash the cells twice to get rid of enzyme solution and prepare the cells for electroporation with desired plasmids. The protoplasts were then incubated in protoplast culture medium (4.3 g/L MS salts, 0.4 m sucrose, 500 mg/L MES hydrate, 750 mg/L CaCl2·2H2O, and 250 mg/L NH4NO3, pH 5.7) for 10–16 h prior to confocal imaging analysis or protein extraction.
The transient expression in Arabidopsis leaf protoplast was performed as described previously (Yoo et al. 2007; Wu et al. 2009). Leaves from 3- to 5-wk-old plants were collected, and abaxial epidermal surfaces were peeling off before subjected to enzyme digestion (1.5% [w/v] cellulase “ONOZUKA” R10, 0.4% [w/v] macerozyme R10, 0.4 m mannitol, 10 mm CaCl2, 20 mm KCl, 0.1% [w/v] BSA, and 20 mm MES, pH 5.7). Protoplasts were washed by W5 solution (154 mm NaCl, 125 mm CaCl2, 5 mm KCl, 5 mm glucose, and 2 mm MES, pH 5.7) and resuspended in MMG solution (0.4 m mannitol, 15 mm MgCl2, and 4 mm MES, pH 5.7) to a final concentration of 2 to 5 × 105 cells/mL. Around 2 to 5 × 104 cells in MMG were mixed with PEG-calcium transfection solution (20% to 40% [w/v] PEG4000, 0.2 m mannitol, and 100 mm CaCl2) and desired plasmids for transformation. Cells were incubated in W5 for 16 h before confocal imaging analysis.
Co-IP
For Co-IP assays of plants, 5-d-old seedlings were freeze grounded to powder and homogenized in IP buffer (50 mm Tris-HCl pH 7.4, 150 mm NaCl, 1 mm MgCl2, 20% [v/v] glycerol, 0.2% [v/v] NP-40, 1× PIC from Roche). After centrifugation at 10,000 × g for 15 min at 4 °C, the supernatants were incubated with 10 μL GFP-Trap magnetic beads (ChromoTek) on a top-to-end rotator kept at 4 °C for 4 h. The beads were then washed 5 times with 1 mL ice-cold washing buffer (50 mm Tris-HCl pH 7.4, 150 mm NaCl, 1 mm MgCl2, 20% [v/v] glycerol, 0.02% [v/v] NP-40) and then eluted by boiling in reducing SDS sample buffer. Samples were separated by SDS-PAGE for subsequent immunoblot analysis using specific antibodies.
For Co-IP of transiently expressed Arabidopsis protoplasts, cells were first diluted 5-fold with 250 mm NaCl and harvested by centrifugation at 700 × g for 3 min, followed by cells lysis using 1 mL syringe in IP lysis buffer (50 mm Tris-HCl pH 7.4, 150 mm NaCl, 1 mm MgCl2, 20% [v/v] glycerol, 0.2% [v/v] NP-40, 1× PIC from Roche). After incubating on ice for 30 min, centrifugation was used at 600 × g for 3 min to remove intact cells and large cellular debris. The supernatant total cell extracts were then centrifuged at 10,000 × g for 15 min at 4 °C to obtain the supernatant used for GFP-Trap magnetic beads (ChromoTek) binding for 4 h at 4 °C in a rotator. The following beads washing and immunoblot steps were the same as previously described for plants.
Confocal microscopy and FRET
Confocal microscopy images were acquired using the 63× water lens of Leica SP8 laser scanning confocal system or a 93× oil lens of the Leica Stellaris 8 confocal. For collecting GFP, RFP, mCherry, or FM4-64 signals, the settings were performed according to the manufacturer's instruction. Confocal images were generally acquired with 1,024 × 1,024 pixels with average line number setting as 2 and pinhole at 1 Airy unit. The excitation wavelengths/collection bandwidths are 488 nm/500–550 nm for GFP and 560 nm/580–640 nm for FM4-64 and mRFP. A sequential acquisition mode was used for collecting fluorescent images in the observation of these fluorescent proteins. Images were processed with ADOBE PHOTOSHOP software as well as ImageJ software. FRET analysis was performed on a Leica SP8 confocal system using 405 and 514 nm laser according to the manufacturer's instructions. Briefly, a region of interest of YFP was bleached to less than 10% of its initial intensity, and CFP donor fluorescence was imaged before and after bleaching. FRET efficiency was quantified by using the acceptor photobleaching approach. FRET efficiency was calculated as FRETeff = (Dpost−Dpre)/Dpost, where Dpre and Dpost stand for the donor intensities before and after acceptor bleaching, respectively. At least 10 individual protoplasts were used for FRET efficiency quantification and statistical analysis.
HPF/FS and TEM analysis
For high-pressure freezing of Arabidopsis seedling root samples, root tips of 5-d-old seedlings were cut into 0.1 m sucrose prepared in advance and immediately frozen in a high-pressure freezer (Leica EM ICE). The following freeze substitution steps were performed in dry acetone with 0.1% (w/v) uranyl acetate at −85 °C in a freeze-substitution unit (Leica EM AFS). Samples were infiltrated with 33% (v/v), 66% (v/v), and 100% Lowicryl HM20, and then embedding and ultraviolet polymerization were performed stepwise at −20 °C. For immunogold labeling of the samples, anti-VSR antibody was used at a concentration of 40 μg/mL. Goat antirabbit 10 nm gold-coupled secondary antibody (Electron Microscopy Sciences, 25109) was used at a 1:50 dilution.
For high-pressure freezing of Arabidopsis seed samples, developing seeds dissected from siliques were loaded in sample holders filled with 0.1 m sucrose and immediately frozen in a high-pressure freezer (Leica EM ICE), followed by subsequent freeze substitution in dry acetone containing 0.2% (w/v) uranyl acetate and 0.2% glutaraldehyde (w/v) at −85 °C for 120 h and then slowly warming to −50 °C during a period of 2 d. After several acetone rinses, these samples were infiltrated with Lowicryl HM20 during 72 h with slowly increased concentration from 5% (v/v) to 100% (v/v) and polymerized at −50 °C under UV light for 48 h.
TEM examination was performed by using an 80 kV Hitachi H-7650 TEM (Hitachi High-Technologies Corporation, Japan) supplied with a charge-coupled device camera.
Electron tomography, 3D reconstruction, and modeling
The procedures of electron tomography were described previously (Cui et al. 2019; Cao et al. 2022). Three continuous 300-nm thick sections of embryos were cut and recorded using a Tecnai F20 electron microscope (Thermo Fisher Scientific, USA) operated at 200 kV. For each grid, a tilt image stack (81 images) from +60° to −60° with 1.5° intervals was collected, and then the second tilt image stack was collected by rotating the grid with 90°. Dual-axis tomograms were calculated from pairs of image stacks with the etomo program of the IMOD software package (version 4.9.13, bio3d.colorado.edu), and 3D models were generated using the 3dmod program.
Y2H analysis
For the screening of Arabidopsis cDNA library, the Mate and Plate normalized Universal Arabidopsis Library (Clontech) in yeast (Saccharomyces cerevisiae) strain Y187 was used. The bait pGBKT7 vector (Clontech) containing GAL4 BD and NT of DRIF1 (1-943a.a.) was constructed by Gateway (Invitrogen) and then transformed into AH109 strain and mated with the Y187 cells expressing the Mate & Plate Library made from 11 Arabidopsis tissues. To identify bait and prey interaction, the mixed strain combination was plated on synthetic drop-out (SD/−Leu−Trp−His) medium, and plasmids from β-galactosidase (β-gal) positive colonies were sequenced and selected for further analysis. In total, about 574 positive colonies were sequenced, and interactions supported by more than 3 colonies were selected for further analysis, while SNX1 was one of the selected interactions.
For the pair verification, the corresponding genes used were amplified from cDNA and cloned into the pGBKT7 or pGADT7 vectors (Clontech). Plasmids of each pair were cotransformed into the yeast AH109 strain and grown on SD medium at 30 °C for 3 d. Positive colonies were further picked and grown on SD medium lacking His, Trp, Leu (SD−3), or SD−3 with 2 mm 3-amino-1,2,4-triazole (3-AT) for 2 d at 30 °C. The experiments were performed at least twice independently.
RT-qPCR
Total RNA was extracted from sample using Rneasy PowerPlant Kit (QIAGEN) and was reverse transcribed using iScript Reverse Transcription Supermix for RT-qPCR. Transcript levels were normalized using ACTIN2 (AT3G18780) as a reference. For each sample, 3 technical replicates were performed. Three biological replicates were performed using distinct samples. Data were analyzed using 2−ΔΔCT method (Livak and Schmittgen 2001). Primer sequences used in this study are listed in Supplemental Table S4.
Homology modeling of DRIF1
Structure model of DRIF1 was obtained using the latest version of AlphaFold machine learning approach AlphaFold2 (Jumper et al. 2021). Images were generated using PyMol (http://www.pymol.org/pymol) (Schrödinger and DeLano 2020).
Statistical analysis
The sample numbers and replicates for each experiment are indicated in the corresponding figure legends. For quantification analysis, mean ± Sd was plotted. One-way ANOVA followed by Turkey's multiple test or t-test was used when data met criteria for parametric analysis. Significance levels are *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Statistical data are provided in Supplemental Data Set 1.
Accession numbers
The Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are DRIF1 (AT5G10370), DRIF2 (AT4G01020), FREE1 (AT1G20110), and SNX1 (AT5G06140). GenBank accession numbers for proteins used in the phylogenetic analysis are indicated in the related figures.
Supplementary Material
Acknowledgments
We thank Lorenzo Frigerio (University of Warwick) for sharing transgenic plants expressing spL-RFP and Nam-Hai Chua (The Rockefeller University) for the binary vector pTA7002.
Contributor Information
Ying Zhu, School of Life Sciences, Centre for Cell & Developmental Biology and State Key Laboratory of Agrobiotechnology, The Chinese University of Hong Kong, Shatin, Hong Kong, China.
Qiong Zhao, School of Life Sciences, East China Normal University, Shanghai 200062, China.
Wenhan Cao, School of Life Sciences, Centre for Cell & Developmental Biology and State Key Laboratory of Agrobiotechnology, The Chinese University of Hong Kong, Shatin, Hong Kong, China; State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou 311300, China.
Shuxian Huang, School of Life Sciences, Centre for Cell & Developmental Biology and State Key Laboratory of Agrobiotechnology, The Chinese University of Hong Kong, Shatin, Hong Kong, China.
Changyang Ji, School of Life Sciences, Centre for Cell & Developmental Biology and State Key Laboratory of Agrobiotechnology, The Chinese University of Hong Kong, Shatin, Hong Kong, China.
Wenxin Zhang, School of Life Sciences, Centre for Cell & Developmental Biology and State Key Laboratory of Agrobiotechnology, The Chinese University of Hong Kong, Shatin, Hong Kong, China.
Marco Trujillo, RWTH Aachen University, Institute for Biology 3, Aachen 52074, Germany.
Jinbo Shen, State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Hangzhou 311300, China.
Liwen Jiang, School of Life Sciences, Centre for Cell & Developmental Biology and State Key Laboratory of Agrobiotechnology, The Chinese University of Hong Kong, Shatin, Hong Kong, China; Institute of Plant Molecular Biology and Agricultural Biotechnology, The Chinese University of Hong Kong, Shatin, Hong Kong, China; CUHK Shenzhen Research Institute, Shenzhen 518057, China.
Author contributions
Y.Z., J.S., and L.J. designed the research. Y.Z., W.C., S.H., C.J., and W.Z. performed experiments. Y.Z., J.S., and Q.Z. generated materials. M.T. performed DRIF1 homology modeling. Y.Z., J.S., and L.J. analyzed the data. Y.Z., J.S., and L.J. wrote the manuscript with comments from all authors.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. sof mutant screen and confirmation of suppressor phenotype.
Supplemental Figure S2. Next-generation sequencing (NGS) analysis of sof10 and sof641 mutants.
Supplemental Figure S3. Multiple sequence alignment of DRIF1 amino acid sequences in Arabidopsis and other nonvascular plant species.
Supplemental Figure S4. Characterization of DRIF1 and DRIF2 T-DNA insertion mutants.
Supplemental Figure S5. Characterization of DRIF1 and DRIF2 antibodies and homology modeling of DRIF1.
Supplemental Figure S6. Expression of DRIF1 and DRIF2 in T-DNA insertion mutants.
Supplemental Figure S7. The relative FREE1 transcript levels in drif-reverted FREE1-RNAi seedlings after DEX treatment.
Supplemental Figure S8. PCR-based genotyping of progenies of drif1(−/−) free1(+/−) and drif2(−/−) free1(+/−).
Supplemental Figure S9. Ultrastructural analysis of selective organelles in wild-type and drif1 drif2 mutant embryos.
Supplemental Figure S10. Analysis of efficiency and specificity of DRIF amiRNA in Arabidopsis protoplasts.
Supplemental Figure S11. Characterization of amiDRIF plants.
Supplemental Figure S12. Abnormal accumulation of PIN2-GFP signals in MVB/PVC in amiDRIF mutants.
Supplemental Figure S13. PIN2-GFP signals accumulated in MVB/PVC at steady-state in amiDRIF mutants.
Supplemental Figure S14. Internalization of FM4-64 was not affected in amiDRIF plants.
Supplemental Figure S15. Biochemical characterization of DRIF1 protein.
Supplemental Figure S16. DRIF1 interacted with neither Vps26, Vps29, Vps35, SNX2 proteins nor Vps23 homologs.
Supplemental Figure S17. Immunoprecipitation (IP) assay shows no interaction of DRIF1 with the core retromer components nor SNX2 proteins.
Supplemental Figure S18. Analysis of conditional growth phenotypes in the absence of exogenous sucrose.
Supplemental Figure S19. DRIF proteins regulate the subcellular localization of SNX1.
Supplemental Figure S20. Uncropped images of immunoblots shown in Figs. 1 to 3.
Supplemental Figure S21. Uncropped images of immunoblots shown in Figs. 6 and 7.
Supplemental Figure S22. Uncropped images of immunoblots shown in Supplemental Figures.
Supplemental Table S1. Progeny of drif1(+/−) drif2(−/−) and drif1(−/−) drif2(+/−).
Supplemental Table S2. Gametophytic transmission of drif1 and drif2 alleles determined by reciprocal crosses.
Supplemental Table S3. Quantification of conditional growth phenotype.
Supplemental Table S4. Primers used in this study.
Supplemental Data Set 1. Statistical data.
Supplemental File 1. Alignment used for phylogenetic analysis.
Supplemental File 2. Tree file from phylogenetic analysis.
Funding
This work has been supported by grants from the Research Grants Council of Hong Kong (AoE/M-05/12, CUHK14101219, C4033-19E, C4002-20W, C4002-21EF, C2009-19G, C2003-22WF, R4005-18 and Senior Research Fellow Scheme SRFS2122-4S01), the Chinese University of Hong Kong (CUHK) Research Committee, and CAS-Croucher Funding Scheme for Joint Laboratories to L.J.; the National Natural Science Foundation of China (32170342 and 31970181), the Zhejiang Provincial Natural Science Foundation of China (LR20C020001), the Fundamental Research Funds for the Provincial Universities of Zhejiang (2020KJ001), and the 111 Project (D18008) to J.S.; and the DFG Heisenberg Program to M.T.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Ambrose C, Ruan Y, Gardiner J, Tamblyn LM, Catching A, Kirik V, Marc J, Overall R, Wasteneys GO. CLASP Interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev Cell. 2013:24(6):649–659. 10.1016/j.devcel.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Barberon M, Dubeaux G, Kolb C, Isono E, Zelazny E, Vert G. Polarization of IRON-REGULATED TRANSPORTER 1 (IRT1) to the plant-soil interface plays crucial role in metal homeostasis. Proc Natl Acad Sci U S A. 2014:111(22):8293–8298. 10.1073/pnas.1402262111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle V, Arrighi J-F, Creff A, Nespoulous C, Vialaret J, Rossignol M, Gonzalez E, Paz-Ares J, Nussaume L. Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell. 2011:23(4):1523–1535. 10.1105/tpc.110.081067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché N, Bouchez D. Arabidopsis gene knockout: phenotypes wanted. Curr Opin Plant Biol. 2001:4(2):111–117. 10.1016/S1369-5266(00)00145-X [DOI] [PubMed] [Google Scholar]
- Brumbarova T, Bauer P, Ivanov R. Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci. 2015:20(2):124–133. 10.1016/j.tplants.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Cao W, Li Z, Huang S, Shi Y, Zhu Y, Lai MN, Lok PL, Wang X, Cui Y, Jiang L. Correlation of vacuole morphology with stomatal lineage development by whole-cell electron tomography. Plant Physiol. 2022:188(4):2085–2100. 10.1093/plphys/kiac028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-López X, Cuyas L, Marín E, Rajulu C, Irigoyen ML, Gil E, Puga MI, Bligny R, Nussaume L, Geldner N. ESCRT-III-associated protein ALIX mediates high-affinity phosphate transporter trafficking to maintain phosphate homeostasis in Arabidopsis. Plant Cell. 2015:27(9):2560–2581. 10.1105/tpc.15.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherfils J, Melancon P. On the action of brefeldin A on Sec7-stimulated membrane-recruitment and GDP/GTP exchange of Arf proteins. Biochem Soc Trans. 2005:33(4): 635–638. 10.1042/BST0330635 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998:16(6):735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Cui Y, Cao W, He Y, Zhao Q, Wakazaki M, Zhuang X, Gao J, Zeng Y, Gao C, Ding Y. A whole-cell electron tomography model of vacuole biogenesis in Arabidopsis root cells. Nat Plants. 2019:5(1):95–105. 10.1038/s41477-018-0328-1 [DOI] [PubMed] [Google Scholar]
- Doyle SM, Haeger A, Vain T, Rigal A, Viotti C, Łangowska M, Ma Q, Friml J, Raikhel NV, Hicks GR. An early secretory pathway mediated by GNOM-LIKE 1 and GNOM is essential for basal polarity establishment in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2015:112(7):E806–E815. 10.1073/pnas.1424856112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Benková E, Mayer U, Palme K, Muster G. Automated whole mount localisation techniques for plant seedlings. Plant J. 2003:34(1):115–124. 10.1046/j.1365-313X.2003.01705.x [DOI] [PubMed] [Google Scholar]
- Gao C, Luo M, Zhao Q, Yang R, Cui Y, Zeng Y, Xia J, Jiang L. A unique plant ESCRT component, FREE1, regulates multivesicular body protein sorting and plant growth. Curr Biol. 2014:24(21):2556–2563. 10.1016/j.cub.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Gao C, Yu CK, Qu S, San MWY, Li KY, Lo SW, Jiang L. The Golgi-localized Arabidopsis endomembrane protein12 contains both endoplasmic reticulum export and Golgi retention signals at its C terminus. Plant Cell. 2012:24(5):2086–2104. 10.1105/tpc.112.096057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Zhuang X, Cui Y, Fu X, He Y, Zhao Q, Zeng Y, Shen J, Luo M, Jiang L. Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc Natl Acad Sci U S A. 2015:112(6):1886–1891. 10.1073/pnas.1421271112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Zhuang X, Shen J, Jiang L. Plant ESCRT complexes: moving beyond endosomal sorting. Trends Plant Sci. 2017:22(11):986–998. 10.1016/j.tplants.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003:112(2):219–230. 10.1016/S0092-8674(03)00003-5 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001:413(6854):425–428. 10.1038/35096571 [DOI] [PubMed] [Google Scholar]
- Hirano T, Munnik T, Sato MH. Phosphatidylinositol 3-phosphate 5-kinase, FAB1/PIKfyve kinase mediates endosome maturation to establish endosome-cortical microtubule interaction in Arabidopsis. Plant Physiol. 2015:169(3):1961–1974. 10.1104/pp.15.01368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006:35(1):277–298. 10.1146/annurev.biophys.35.040405.102126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono E, Kalinowska K. ESCRT-dependent degradation of ubiquitylated plasma membrane proteins in plants. Curr Opin Plant Biol. 2017:40:49–55. 10.1016/j.pbi.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Ivanov R, Brumbarova T, Blum A, Jantke A-M, Fink-Straube C, Bauer P. SORTING NEXIN1 is required for modulating the trafficking and stability of the Arabidopsis IRON-REGULATED TRANSPORTER1. Plant Cell. 2014:26(3):1294–1307. 10.1105/tpc.113.116244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov R, Robinson DG. EMAC, retromer, and VSRs: do they connect? Protoplasma. 2020:257(6):1725–1729. 10.1007/s00709-020-01543-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Fobis-Loisy I, Miege C, Rollin C, Gaude T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature. 2006:443(7107):106–109. 10.1038/nature05046 [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miege C, Gaude T. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell. 2007:130(6):1057–1070. 10.1016/j.cell.2007.08.040 [DOI] [PubMed] [Google Scholar]
- Jásik J, Schmelzer E. Internalized and newly synthesized Arabidopsis PIN-FORMED2 pass through brefeldin A compartments: a new insight into intracellular dynamics of the protein by using the photoconvertible fluorescence protein Dendra2 as a tag. Mol Plant. 2014:7(10):1578–1581. 10.1093/mp/ssu052 [DOI] [PubMed] [Google Scholar]
- Jia T, Gao C, Cui Y, Wang J, Ding Y, Cai Y, Ueda T, Nakano A, Jiang L. ARA7 (Q69L) expression in transgenic Arabidopsis cells induces the formation of enlarged multivesicular bodies. J Exp Bot. 2013:64(10):2817–2829. 10.1093/jxb/ert125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A. Highly accurate protein structure prediction with AlphaFold. Nature. 2021:596(7873):583–589. 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Leitner J, Zwiewka M, Sauer M, Abas L, Luschnig C, Friml J. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci U S A. 2008:105(46):17812–17817. 10.1073/pnas.0808073105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016:33(7):1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R. Clustal W and Clustal X version 2.0. Bioinformatics. 2007:23(21):2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lau S, Slane D, Herud O, Kong J, Jürgens G. Early embryogenesis in flowering plants: setting up the basic body pattern. Annu Rev Plant Biol. 2012:63(1):483–506. 10.1146/annurev-arplant-042811-105507 [DOI] [PubMed] [Google Scholar]
- Leitner J, Petrášek J, Tomanov K, Retzer K, Pařezová M, Korbei B, Bachmair A, Zažímalová E, Luschnig C. Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc Natl Acad Sci U S A. 2012:109(21):8322–8327. 10.1073/pnas.1200824109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics. 2009:25(16):2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li Y, Zhao Q, Li T, Wei J, Li B, Shen W, Yang C, Zeng Y, Rodriguez PL. The plant ESCRT component FREE1 shuttles to the nucleus to attenuate abscisic acid signalling. Nat Plants. 2019:5(5):512. 10.1038/s41477-019-0400-5 [DOI] [PubMed] [Google Scholar]
- Li Y-B, Rogers SW, Tse YC, Lo SW, Sun SS, Jauh G-Y, Jiang L. BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments. Plant Cell Physiol. 2002:43(7):726–742. 10.1093/pcp/pcf085 [DOI] [PubMed] [Google Scholar]
- Lin W-Y, Huang T-K, Chiou T-J. NITROGEN LIMITATION ADAPTATION, a target of microRNA827, mediates degradation of plasma membrane–localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell. 2013:25(10):4061–4074. 10.1105/tpc.113.116012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001:25(4):402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Luschnig C, Vert G. The dynamics of plant plasma membrane proteins: PINs and beyond. Development. 2014:141(15):2924–2938. 10.1242/dev.103424 [DOI] [PubMed] [Google Scholar]
- MacGurn JA, Hsu P-C, Emr SD. Ubiquitin and membrane protein turnover: from cradle to grave. Annu Rev Biochem. 2012:81(1):231–259. 10.1146/annurev-biochem-060210-093619 [DOI] [PubMed] [Google Scholar]
- Mao H, Nakamura M, Viotti C, Grebe M. A framework for lateral membrane trafficking and polar tethering of the PEN3 ATP-binding cassette transporter. Plant Physiol. 2016:172(4):2245–2260. 10.1104/pp.16.01252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers JR, Fyfe I, Schuh AL, Chapman ER, Edwardson JM, Audhya A. ESCRT-0 assembles as a heterotetrameric complex on membranes and binds multiple ubiquitinylated cargoes simultaneously. J Biol Chem. 2011:286(11):9636–9645. 10.1074/jbc.M110.185363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Jiang L. Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat Protoc. 2007:2(10):2348–2353. 10.1038/nprot.2007.360 [DOI] [PubMed] [Google Scholar]
- Möller B, Weijers D. Auxin control of embryo patterning. Cold Spring Harb Perspect Biol. 2009:1(5):a001545. 10.1101/cshperspect.a001545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramoto S, Otegui MS, Kutsuna N, De Rycke R, Dainobu T, Karampelias M, Fujimoto M, Feraru E, Miki D, Fukuda H. Insights into the localization and function of the membrane trafficking regulator GNOM ARF-GEF at the Golgi apparatus in Arabidopsis. Plant Cell. 2014:26(7):3062–3076. 10.1105/tpc.114.125880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemes S, Langhans M, Viotti C, Scheuring D, Yan SW, Jiang M, Hillmer L, Robinson S, Pimpl DG. Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J. 2010:61(1):107–121. 10.1111/j.1365-313X.2009.04034.x [DOI] [PubMed] [Google Scholar]
- Niu F, Ji C, Liang Z, Guo R, Chen Y, Zeng Y, Jiang L. ADP-ribosylation factor D1 modulates Golgi morphology, cell plate formation, and plant growth in Arabidopsis. Plant Physiol. 2022:190(2):1199–1213. 10.1093/plphys/kiac329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodzyński T, Feraru MI, Hirsch S, De Rycke R, Niculaes C, Boerjan W, Van Leene J, De Jaeger G, Vanneste S, Friml J. Retromer subunits VPS35A and VPS29 mediate prevacuolar compartment (PVC) function in Arabidopsis. Mol Plant. 2013:6(6):1849–1862. 10.1093/mp/sst044 [DOI] [PubMed] [Google Scholar]
- Paciorek T, Sauer M, Balla J, Wiśniewska J, Friml J. Immunocytochemical technique for protein localization in sections of plant tissues. Nat Protoc. 2006:1(1):104–107. 10.1038/nprot.2006.16 [DOI] [PubMed] [Google Scholar]
- Piper RC, Luzio JP. Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr Opin Cell Biol. 2007:19(4):459–465. 10.1016/j.ceb.2007.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcher M, Santambrogio M, Thazar N, Thierry A-M, Fobis-Loisy I, Miège C, Jaillais Y, Gaude T. Analyses of sorting nexins reveal distinct retromer-subcomplex functions in development and protein sorting in Arabidopsis thaliana. Plant Cell. 2010:22(12):3980–3991. 10.1105/tpc.110.078451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009:458(7237):445–452. 10.1038/nature07961 [DOI] [PubMed] [Google Scholar]
- Reyes FC, Buono R, Otegui MS. Plant endosomal trafficking pathways. Curr Opin Plant Biol. 2011:14(6):666–673. 10.1016/j.pbi.2011.07.009 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Furlan C, Minina EA, Hicks GR. Remove, recycle, degrade: regulating plasma membrane protein accumulation. Plant Cell. 2019:31(12):2833–2854. 10.1105/tpc.19.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987:4(4):406–425. 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- Salanenka Y, Verstraeten I, Löfke C, Tabata K, Naramoto S, Glanc M, Friml J. Gibberellin DELLA signaling targets the retromer complex to redirect protein trafficking to the plasma membrane. Proc Natl Acad Sci U S A. 2018:115(14):3716–3721. 10.1073/pnas.1721760115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV. Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol Biol Cell. 2001:12(12):3733–3743. 10.1091/mbc.12.12.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Paciorek T, Benková E, Friml J. Immunocytochemical techniques for whole-mount in situ protein localization in plants. Nat Protoc. 2006:1(1):98–103. 10.1038/nprot.2006.15 [DOI] [PubMed] [Google Scholar]
- Schneeberger K, Ossowski S, Lanz C, Juul T, Petersen AH, Nielsen KL, Jørgensen J-E, Weigel D, Andersen SU. SHOREmap: simultaneous mapping and mutation identification by deep sequencing. Nat Methods. 2009:6(8):550–551. 10.1038/nmeth0809-550 [DOI] [PubMed] [Google Scholar]
- Schrödinger L, DeLano W. PyMOL. The PyMOL molecular graphics system, version 22020.
- Shen J, Fu J, Ma J, Wang X, Gao C, Zhuang C, Wan J, Jiang L. Isolation, culture, and transient transformation of plant protoplasts. Curr Protoc Cell Biol. 2014:63(1):2.8.1–2.8.17. 10.1002/0471143030.cb0208s63 [DOI] [PubMed] [Google Scholar]
- Shen J, Zhao Q, Wang X, Gao C, Zhu Y, Zeng Y, Jiang L. A plant Bro1 domain protein BRAF regulates multivesicular body biogenesis and membrane protein homeostasis. Nat Commun. 2018:9(1):3784. 10.1038/s41467-018-05913-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti B, Cullen PJ. Endosomal sorting: architecture of the retromer coat. Curr Biol. 2018:28(23):R1350–R1352. 10.1016/j.cub.2018.10.040 [DOI] [PubMed] [Google Scholar]
- Tamura K, Shimada T, Ono E, Tanaka Y, Nagatani A, Higashi SI, Watanabe M, Nishimura M, Hara-Nishimura I. Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J. 2003:35(4):545–555. 10.1046/j.1365-313X.2003.01822.x [DOI] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell. 2004:16(3):672–693. 10.1105/tpc.019703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yan X, Chen Q, Jiang N, Fu W, Ma B, Liu J, Li C, Bednarek SY, Pan J. Clathrin light chains regulate clathrin-mediated trafficking, auxin signaling, and development in Arabidopsis. Plant Cell. 2013:25(2):499–516. 10.1105/tpc.112.108373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F-H, Shen S-C, Lee L-Y, Lee S-H, Chan M-T, Lin C-S. Tape-Arabidopsis sandwich-a simpler Arabidopsis protoplast isolation method. Plant Methods. 2009:5(1):16. 10.1186/1746-4811-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-D, Cho Y-H, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007:2(7):1565–1572. 10.1038/nprot.2007.199 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Gao C, Lee P, Liu L, Li S, Hu T, Shen J, Pan S, Ye H, Chen Y, et al. Fast-suppressor screening for new components in protein trafficking, organelle biogenesis and silencing pathway in Arabidopsis thaliana using DEX-inducible FREE1-RNAi plants. J Genet Genomics. 2015:42(6):319–330. 10.1016/j.jgg.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Shen J, Gao C, Cui Y, Wang Y, Cui J, Cheng L, Cao W, Zhu Y, Huang S, et al. RST1 is a FREE1 suppressor that negatively regulates vacuolar trafficking in Arabidopsis. Plant Cell. 2019:31(9):2152–2168. 10.1105/tpc.19.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Wang H, Lam SK, Gao C, Wang X, Cai Y, Jiang L. A BAR-domain protein SH3P2, which binds to phosphatidylinositol 3-phosphate and ATG8, regulates autophagosome formation in Arabidopsis. Plant Cell. 2013:25(11):4596–4615. 10.1105/tpc.113.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.