Abstract
The causes of male infertility can vary. Lifestyles, environmental factors, stressful conditions, and socio-economic conditions are significant factors. Diet plays a crucial role in improving a man's reproductive capacity. The appropriate diet should be diverse and ensure the intake of all the necessary nutrients to enhance sperm quality. The Mediterranean diet, which includes high amounts of vegetables and fruits rich in detoxifying and antioxidant substances, as well as polyphenols, flavonoids, carotenoids, and microelements, especially when consumed with organic foods and a lower carbohydrate regimen, are the key aspects addressed in this study. The objective of this research was to modify the diets of 50 subfertile men by providing them with a specific nutritional plan. This plan included consuming 80% organic foods, introducing whole grains and low glycemic load options, eliminating refined carbohydrates, consuming green leafy vegetables and red fruits daily, reducing or eliminating dairy products, consuming primarily grass-fed meat and wild caught seafood, eliminating saturated fats in favor of healthy fats like olive oil, avocado, and nuts. After three months of adhering to the low-carb food plan, testosterone levels significantly increased, while sperm DNA fragmentation decreased in a subgroup of individuals who reduced their carbohydrate intake by 35%.
Keywords: Mediterranean diet, Organic low-carb diet, Pollution, Male infertility, Testosterone, Sperm DNA fragmentation, SCD test
Graphical abstract
Highlights
-
•
Mediterranean diet has a beneficial effect on male fertility.
-
•
Organic food increase the beneficial effects of the Mediterranean diet.
-
•
Low-carb organic Mediterranean diet reduces sperm DNA fragmentation.
-
•
Low-carb organic Mediterranean diet increases testosterone levels.
-
•
Organic Mediterranean diet can counteract the prooxidant effect of pollutants.
1. Introduction
Couple infertility is defined as the inability to conceive after more than a year of unprotected sexual intercourse in the absence of other reproductive pathologies (Barratt et al., 2017). The incidence of this condition is increasing, to the extent that it affects 8–12% of couples globally, with the male factor contributing approximately 50% (Agarwal et al., 2021). There can be numerous causes of male infertility. In addition to subjective factors, unhealthy lifestyles and exposure to environmental toxins can also impact a man's reproductive capacity. In particular, continuous exposure to endocrine disruptors chemicals (EDCs) (Yilmaz et al., 2020), a highly diverse group of molecules that includes synthetic chemicals used in industrial solvents/lubricants and their by-products, can lead to adverse reproductive outcomes such as decreased sperm quality and quantity, as well as testicular damage (Chiang et al., 2017; Selvaraju et al., 2021; Fathi Najafi et al., 2015; Pironti et al., 2021, 2023). Unhealthy lifestyle factors, such as smoking cigarettes, alcohol consumption, drug use, coffee intake, lack of sleep, exposure to electromagnetic fields, psychological stress, and poor dietary practices (Durairajanayagam, 2018), as well as being overweight or obese, are more commonly associated with male infertility. These factors can cause hormonal imbalances, as well as direct changes to sperm function and molecular composition (Palmer et al., 2012; Bellastella et al., 2019; Hammoud et al., 2008). In this regard, testicular steroidogenesis plays a fundamental role in production of testosterone and maintaining germ cell development. These functions are supported by two types of somatic cells found in the testes: Leydig cells and Sertoli cells (SCs). Leydig cells are responsible for the production of testosterone.The level of testosterone undergoes dynamic changes during different stages of development. It increases towards the end of fetal life, decreases after birth until puberty, and then increases again. Testosterone exerts its effects through both classical and non-classical mechanisms (Shah et al.). Additionally, it plays a role in the hormonal control of apoptosis in the testis (Sinha Hikim and Swerdloff, 1999). Apoptosis is one of the factors contributing to sperm DNA fragmentation. Low testosterone levels, in combination with other factors such as high levels of reactive oxygen species (ROS), particularly in obese men, are associated with elevated levels of DNA fragmentation index (DFI). (Kahn and Brannigan, 2017).
Generally, when it comes to dietary aspects and eating habits, especially for couples trying to conceive, there is still little attention from clinicians and a lack of clear guidelines. However, there is no doubt that the Mediterranean diet is universally recognized as the most beneficial for maintaining overall health. This diet is characterized by a high consumption of vegetables, fruits, olive oil, grains, dairy products, and nuts, with very low intake of red meat and moderate consumption of fish and wine. Presently, all the most important and influential scientific societies in the world recommend the Mediterranean diet as the ideal dietary profile for maintaining a healthy state and reducing the incidence of major chronic diseases (Guasch-Ferré and Willet, 2021). When it comes to the effects of this diet on sperm quality and male fertility, there are only a few observational studies reported in the literature, and only two randomized controlled trials (RCTs) showing improvements in seminal parameters. One of these studies, conducted by Montano et al., even took place in highly polluted areas and showed improvement in seminal redox status as well (Cao et al., 2022; Caruso et al., 2020; Montano et al., 2021). Recent advancements in understanding the molecular basis of the Mediterranean diet's effects have focused primarily on cardiovascular disease, but also discuss other related diseases. These advancements have reviewed the composition of the Mediterranean diet and the latest developments in evaluating its effects on a genomic, epigenomic (DNA methylation, histone modifications, microRNAs, and other emerging regulators), transcriptomic (selected genes and whole transcriptome), metabolomic, and metagenomic level.
In the literature, a review of the clinical effects of this dietary style and its biochemical and molecular effects has also been reported, highlighting its benefits on human health, particularly its anti-inflammatory, antioxidant, and anti-atherosclerotic effects. Recently, new knowledge on the relationship between epigenetics and gut microbiota has also been added to these effects (Tuttolomondo et al., 2019). It is important to note that adherence to this type of diet, characterized by high intake of vegetables, fruits, and seafood, which are rich in detoxifying and antioxidant substances, can be useful in balancing the production of reactive oxygen species (ROS) and epigenetic alterations caused by environmental contaminants (Chung, 2017; Vanduchova et al., 2019; Chang et al., 2018; Jamalan et al., 2016; Tedesco et al., 2020; Huetos et al., 2019; Ricci et al., 2019; Karayiannis et al., 2017a; Montano et al., 2017; Montano et al., 2022). The consumption of typical foods of the Mediterranean diet is characterized by the consumption of organic foods, which are free of pesticides that have negative effects on human health (Kim et al., 2017; Roeleveld and Bretveld, 2008). Organic foods have higher concentrations of antioxidants and trace elements (Yu-Han Chiu et al., 2016; Barański et al., 2014; Ribes-Moya et al., 2020; Hallmann et al., 2019; Benbrook et al., 2013; Palupi et al., 2012; Średnicka-Tober et al., 2016a), increased levels of omega-3 fatty acids in organic dairy products (Prandini et al., 2009), and higher concentrations of polyunsaturated fatty acids such as omega-3, as well as improved profiles of saturated fatty acids such as linoleic acid and palmitoleic acid in organic meat (Ribas-Agusti et al., 2019; Średnicka-Tober et al., 2016b). Therefore, the higher content of bioactive compounds in organic food compared to conventional ones represents an additional safeguard against the effects of environmental pollutants and helps maintain good general and reproductive health (Hurtado-Barroso et al., 2017a; Vigar et al., 2019a; Montano et al., 2022). However, data suggests that adherence to a “healthy” diet that is rich in certain nutrients such as omega-3 fatty acids, antioxidants (vitamin E, vitamin C, β-carotene, selenium, zinc, and lycopene), and other vitamins (vitamin D and folate), while low in saturated and trans fatty acids, can help improve some semen quality parameters, particularly those related to sperm motility, which is one of the most important parameters for fertility (Chiu et al., 2015; Safarinejad et al., 2010; Salas-Huetos et al., 2019a). Additionally, it may also improve sperm DNA fragmentation index (Karayiannis et al., 2017b). Sperm cells are particularly sensitive to endogenous and exogenous stress, including oxidative stress, which is the final effect of a series of intracellular events triggered by insult (Gallo et al., 2020; Montano et al., 2018; Bergamo et al., 2016). Sperm DNA is also an excellent indicator of male fertility, as even if other sperm parameters may be normal, high DNA fragmentation can be the main cause of undiagnosed/unexplained infertility. Furthermore, pollutants seem to have DNA damage as their primary effect, thus any antioxidant dietary intervention is important for maintaining genomic integrity (Rubes et al., 2005; Bosco et al., 2018; Aitken et al., 2009).
In this study, we focused on the organic Mediterranean diet, particularly on a low-carb regimen. Apart from promoting weight loss, this regimen is effective in stabilizing blood sugar levels and has beneficial effects such as reducing the risk of type 2 diabetes and metabolic syndrome (Westman and Yancy, 2020). In general, low-carb diets limit the intake of carbohydrates to less than 130 g per day or less than 45% of total calorie intake, while favoring the consumption of low-glycemic index carbohydrates. The main hypothesis behind low-carb approaches is that reducing insulin, a critical hormone that promotes fat storage, improves cardiometabolic function and induces weight loss (O'Neill, 2020). Studies have shown that a moderately low-carb diet can be beneficial for the heart, as long as the protein and fat come from healthy sources (O'Neill, 2020). Higher-carb foods such as fruits, starchy vegetables, and whole grains can also be included in these diets in moderation (O'Neill, 2020).
2. Material and methods
2.1. Subjects
The nutritional aspects of 50 men who turned to a pre-conception diet, with an age ranging between 35 and 45, were evaluated from November 2020 to October 2021.
2.2. Measures
Adherence to the Mediterranean diet (MD) was evaluated using a 15-item food frequency questionnaire, which has already been validated to assess adherence to the Mediterranean dietary pattern among Italian adults, scoring from 0 (minimal adherence) to 9 (maximal adherence) (Biasini et al., 2021). Anthropometric measurements were taken for the male participants, and only those with a body mass index (BMI) ranging from 20 to 24 were included in the evaluation. The selection criteria for the EcoFoodFertility project, a human biomonitoring study investigating the impact of pollutants, diet, and lifestyle on reproductive and overall health, led to the exclusion of patients who were smokers, habitual drinkers, obese, underweight, had varicocele, or had chronic pathologies. Further details about this project can be found at https://www.ecofoodfertility.it/(accessed on July 12, 2022). All methods employed in this study were conducted in accordance with the World Medical Association Code of Ethics Guidelines (Declaration of Helsinki) and regulations. The baseline characteristics of the study population are presented in Table 1.
Table 1.
Characteristics of the study population.
| Variables | mean ± SEM |
|---|---|
| Age (yr) | 40,2 ± 0,9 |
| Body measurements | |
| Weight (kg) | 75,5 ± 1,3 |
| Height (mt) | 1,8 ± 0,01 |
| BMI (kg/m2) | 23,8 ± 0,3 |
| Adherence to the Mediterranean Diet score (on a 0–9 scale) | 3,02 ± 0,2 |
| Semen parameters | |
| Volume (ml) | 2,6 ± 0,2 |
| Sperm concentration (106/ml) | 32,9 ± 4,9 |
| Total motility (%) | 31,03 ± 4,9 |
| Progressive motility (%) | 10,15 ± 1,4 |
| Cell with normal morphology (%) | 3,38 ± 0,3 |
Age, body measurements, adherence to the Mediterranean Diet score and semen parameters, including spermiogram variables, are reported as mean ± SEM. SEM = standard error of the mean.
2.3. Material & methods
After obtaining informed consent, a group of 50 individuals were assigned a Mediterranean diet regimen consisting of the following precise nutritional guidelines:
Consumption of 80% organic foods.
Daily intake of whole grains and foods with a low glycemic index.
Breakfast included whole grain bread with a protein source like eggs, wild salmon, nuts, and red fruits. Alternatively, oats in the form of pancakes or porridge were also allowed as a breakfast option.
Dairy products were eliminated or reduced, with some exceptions for goat cheeses.
Daily consumption of fermented foods such as yogurt or kefir.
Daily consumption of red fruits.
Daily consumption of 3 portions of green leafy vegetables.
Daily consumption of 30 g of nuts.
Consumption of legumes at least 3–4 times a week.
Consumption of wild-caught seaweed at least 5–6 times a week, including oily fish 2–3 times.
Elimination of processed meat.
Consumption of grass-fed or organic meat 3–4 times a week.
Consumption of 8–10 eggs per week.
Fruit consumption limited to a maximum of 300 g per day.
Consumption of cruciferous vegetables at least 4–5 times a week.
Elimination of packaged products.
Frequent use of spices such as ginger, turmeric, coriander, rosemary, basil, garlic, onion, and parsley.
A subgroup of 20 participants out of the initial 50 were given additional instructions to reduce their carbohydrate intake to 35% of their daily caloric intake.
The participants adhered to the diet for a period of 3 months before undergoing a testosterone test and Sperm DNA fragmentation test.
2.4. Blood analysis
The participants underwent blood chemistry tests at their designated laboratory.
2.5. DNA fragmentation index (DFI)
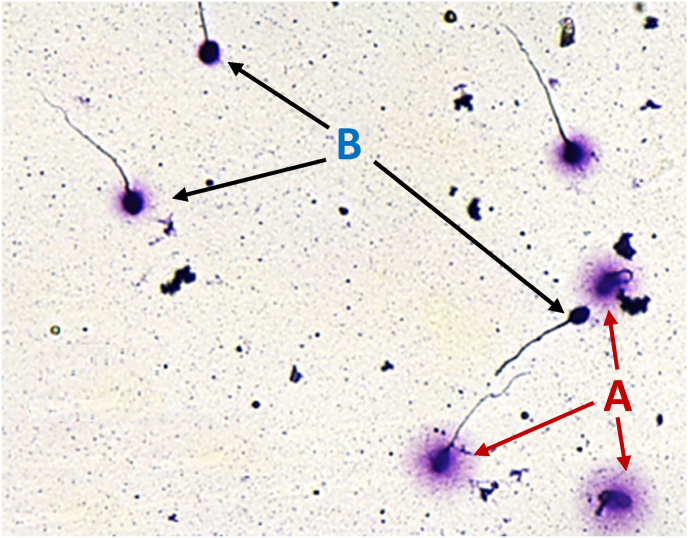
Participants were instructed to abstain from sexual activity for a minimum of two days and a maximum of five days before providing a semen sample on-site. The DFI analysis was then conducted at their designated laboratory using the Sperm Chromatin Dispersion (SCD) test (Fernández et al., 2003; Bosco et al., 2018). In brief, the semen samples were diluted in a 1 × phosphate buffered saline solution (PBS Gibco, Invitrogen) with a pH of 7.4 to avoid overlap of sperm DNA halos. Semen samples with a sperm concentration below 10 × 106/mL were not diluted. A portion of the semen sample was embedded in low melting point agarose and spread onto an agarose pretreated slide using the Halosperm® kit (Halotech DNA SL Spain). A total of 500 spermatozoa were observed for each sample using brightfield microscopy (Nikon Ci-L) and categorized based on the size of the sperm DNA dispersion halo. Five different patterns of sperm DNA halos were observed: large and medium, categorized as non-fragmented DNA, and small, absent halos, and degraded spermatozoa, categorized as fragmented. The DNA fragmentation index (DFI) values were calculated as the percentage of fragmented nuclei relative to the total number of cells. Fig. 3 illustrates the interpretation of the sperm chromatin dispersion test (SCD) and was captured using a Gigabit Camera (Basler Ace ACA780-75 GC) connected to the microscope.
Fig. 3.
Interpretation of the SCD test. Interation of the SCD test: Spermatozoa that are not fragmented are denoted as A, while spermatozoa with fragmented DNA are denoted as B.
3. Data analysis
The data from each group are presented as the mean ± SEM. Statistical significance was determined using an unpaired two-tailed t-test, with values considered significantly different if p < 0.05. P < 0.05; *P < 0.005. The unpaired two-samples t-test is utilized to compare the means of two independent groups and determine if there is a significant difference between them.
4. Results
In total, the dietary habits of 50 male participants were assessed, along with the impact of dietary changes on their testosterone levels and Sperm DNA fragmentation. An examination of their eating habits revealed a consumption of low-quality proteins, excessive intake of high glycemic index and refined carbohydrates, as well as sweet foods. Moreover, they exhibited limited concern for food quality, rarely reading food labels, and rarely purchasing organic products. Many participants consumed excessive amounts of coffee and processed foods, including dairy products.
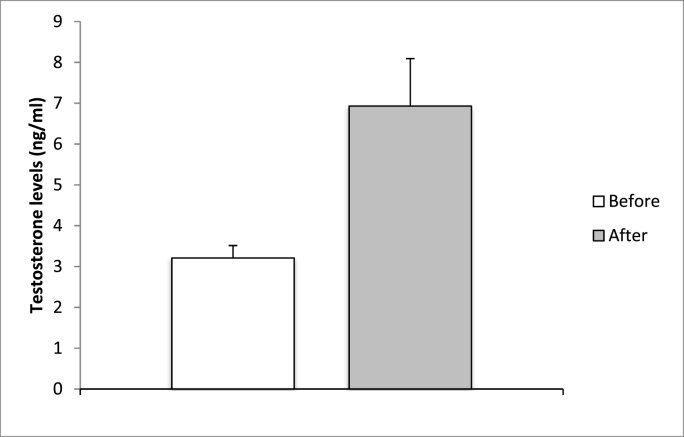
Following nutritional counseling and the implementation of a personalized dietary plan, it was observed that individuals who modified their diet by reducing refined sugars and increasing their consumption of whole grains, fresh vegetables, and legumes, while avoiding packaged foods and dairy products, experienced a noteworthy increase in testosterone levels (p < 0.05, n = 30) (Fig. 1).
Fig. 1.
Evaluation of testosterone levels following a 3-month period of dietary modifications. The “Before” value represents the baseline level before commencing the diet, while the “After” value indicates the level after 3 months of diet. The statistical analysis revealed a significant difference (p = 0.011; p < 0.05). The initial testosterone level was 3.2 ± 0.3, whereas after the diet it increased to 6.92 ± 1.16.
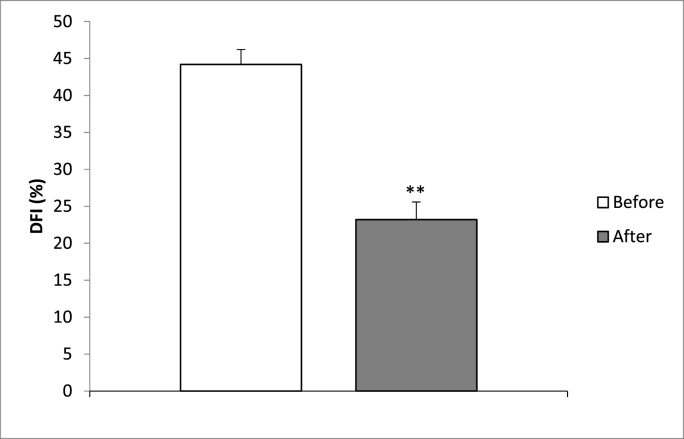
Simultaneously, the group of men who adhered to a diet comprising approximately 50% carbohydrate intake for 3 months (n = 20), whereby their antioxidant intake was increased through daily consumption of red fruits and a minimum of 3 portions of fresh vegetables per day, while avoiding packaged foods and eliminating dairy products, demonstrated a notable reduction in sperm DNA fragmentation (p < 0.005) (n = 20) (Fig. 2).
Fig. 2.
Evaluation of DFI levels after 3 months of dietary intervention. “Before” represents the baseline value prior to initiating the diet, while “After” signifies the value after 3 months of adhering to the prescribed dietary plan (p = 0.001; p < 0.005). The initial DFI level was recorded as 44.2 ± 3.02, whereas after the intervention, it declined to 23.2 ± 3.57.
5. Discussion
The present study demonstrates the beneficial effects of a diet rich in antioxidant and detoxifying foods, polyunsaturated fats, and whole grains on two male fertility parameters: testosterone levels and sperm DNA fragmentation. Our findings indicate that men who adhere to the Mediterranean diet and reduce their carbohydrate intake experience lower sperm DNA fragmentation and increased testosterone levels. Previous studies have also shown an inverse correlation between serum testosterone levels and energy intake of carbohydrates and proteins (Volek et al., 1985). Glucose loading has been found to reduce total and free testosterone levels, potentially due to changes in insulin resistance (Caronia et al., 2013). Long-term changes in protein and carbohydrate intake can impact testosterone metabolism and indirectly affect weight and visceral adiposity. The authors propose two hypotheses: 1) that testosterone levels might decrease as a result of insulin's inhibitory effect on hepatic SHBG production; 2) the reduction in testosterone might be influenced by peripheral signals to the central nervous system, such as leptin, which could impact the HPG (hypothalamic-pituitary-gonadal) axis and lead to a disruption in communication. Both insulin and leptin play crucial roles in transmitting information to the brain regarding energy reserves, consequently affecting hormonal balance.
Another study conducted on young athletes in 2017 (Wilson et al., 2020) showed that following a ketogenic diet led to increased total testosterone levels compared to a Western-style diet. This could be attributed to the higher concentration of cholesterol in a ketogenic diet, which serves as a precursor for sex hormone production.
High levels of Reactive oxygen species (ROS) are responsible for a significant percentage of male infertility cases, in fect they can damage sperm DNA, membranes, and proteins (Agarwal and Said, 2005). Lifestyle factors such as obesity, stress, alcohol, tobacco, environmental factors like heat and pollution, and inflammation increase the risk of ROS generation (Tremellen, 2008). When the amount of ROS exceeds the sperm's ability to scavenge them, oxidative stress occurs, resulting in impaired sperm motility, membrane damage, DNA sperm damage and even apoptosis (Gharagozloo and Aitken, 2011; Sharma and Agarwal, 1996; Agarwal et al., 2008; Lee et al., 1997). Spermatozoa are highly susceptible to oxidative damage owing to the abundance of polyunsaturated fatty acids (PUFA), which pose a high risk of lipid peroxidation (Wathes et al., 2007). Furthermore, during the maturation process of sperm, there is a decrease in cytoplasmic content (Wathes et al., 2007; Flesch and Gadella, 2000), resulting in reduced levels of enzymes and antioxidants required to counteract reactive oxygen species (ROS) production and repair ROS-induced nuclear and mitochondrial DNA damage (Lee et al., 1997).
It is crucial to consume vitamins and antioxidants through diet to protect sperm from oxidative damage. Seminal plasma contains enzymatic antioxidants such as SOD, catalase, and glutathione peroxidase (Mennella and Jones, 1980; Zini et al., 1993; Vernet et al., 2004), as well as non-enzymatic antioxidants like vitamin C, vitamin E, carotenoids, and flavonoids (Gharagozloo and Aitken, 2011). Non-enzymatic antioxidants can be obtained through dietary intake and act synergistically with enzymatic antioxidants to eliminate ROS.
Certain foods and nutrients have stronger associations with fertility benefits. It is essential to maintain a healthy weight and pay attention to nutrition to promote good sperm quality (Oostingh et al., 2017). Diets high in refined carbohydrates and sugar have negative effects on sperm health, including reduced motility, morphology, and count (Cutillas-Tolin et al., 2019; Danielewicz et al., 2018). A Mediterranean-type diet, which includes fruits, fish, vegetables, legumes, whole grains, healthy fats, and oils, has been shown to improve male fertility (Guasch-Ferré and Willet, 2021; Salas-Huetos et al., 2019b). Increasing the consumption of green leafy vegetables and cruciferous vegetables aids liver function and has anti-inflammatory effects (Gaskins et al., 2012). Protein intake also plays a role in sperm morphology and motility, and improving the oxidative state can be achieved by decreasing carbohydrate intake and increasing protein intake (Ajuogu et al., 2020; Xia et al., 2015; Harris et al., 2011).
DNA fragmentation is a significant factor in male infertility (Nassan et al., 2018). High levels of DNA fragmentation decrease the chances of natural conception or success with procedures such as intrauterine insemination and in vitro fertilization. Minimizing DNA fragmentation is crucial, as oxidative stress is the primary cause. Antioxidants can protect against oxidative stress and potentially improve conception rates. Fruits and vegetables are rich in antioxidants, such as vitamin C, vitamin A, β-carotene, polyphenols, minerals like potassium and magnesium, folate, and fiber.
There is a direct correlation between antioxidant status and the generation of reactive oxygen species (ROS) in spermatozoa (Ross et al., 2010). The Mediterranean diet, which is rich in antioxidants and essential vitamins for sperm function, such as coenzyme Q-10, plays a crucial role in sperm motility by facilitating movement and energy release in sperm. Vitamins B aid in liver detoxification and the elimination of free radicals, promoting healthy sperm growth and assisting in protein synthesis and division. Folate (vitamin B9) and vitamin B12, concentrated in the sperm head, provide essential nutrients for sperm production and survival (Azizollahi et al., 2013). Folate deficiency, commonly found in green leafy vegetables, is associated with apoptosis through p53. Folate-deficient cells have significantly increased lipid peroxidation indices, activating a redox-sensitive transcription factor, NF-κB, responsible for reactive oxygen species-mediated apoptosis (Murphy et al., 2011). Vitamin C, abundant in citrus fruits, is necessary for the development of healthy sperm cells (Akmal et al., 2006). The Mediterranean diet, rich in essential fatty acids, is crucial for sperm health, especially omega-3 fatty acids like docosahexaenoic acid (DHA). DHA, found mainly in the sperm tail, is associated with motility and the sperm's ability to fertilize the oocyte. Low levels of DHA are linked to an increase in abnormal sperm forms (Aksoy et al., 2006). Additionally, various phytochemical compounds found in the Mediterranean diet have been found to interact with contaminants, offering protection against their harmful effects through mechanisms such as scavenging ROS, chelation of toxic metals, anti-inflammatory action, and epigenetic up-regulation of detoxifying genes or enzymes (Chung, 2017; Vanduchova et al., 2019; Chang et al., 2018; Jamalan et al., 2016; Tedesco et al., 2020; Montano et al., 2022). Adhering to a diet rich in antioxidants can help improve semen quality and counteract the negative impact of contaminants, including dangerous pesticides and phthalates (Montano et al., 2021; Kelly, 2004). Organic food, known for its higher content of bioactive compounds and lower levels of nitrates, Cadmium, pesticides, fertilizer, and pollutants, has been shown to improve various health outcomes, including fertility treatment, overweight and obesity reduction, prevention of pre-eclampsia in pregnancy, eczema in infants, some cancers, diabetes, and other noncommunicable diseases (Hurtado-Barroso et al., 2019; Liang et al., 2019; Glibowski, 2020; Baudry et al., 2018; Chiu et al., 2018; Sun et al., 2018; Bradman et al., 2015a; Curl et al., 2019a). The lower morbidity associated with organic food consumption is believed to be due to reduced exposure to pesticide residues and increased intake of antioxidants; however, more research is needed to draw a definitive conclusion (Hurtado-Barroso et al., 2019). A large-scale randomized clinical trial comparing the effects of organic and conventional products on human health, lasting more than 4 weeks, has not yet been conducted. Recommendations have been made to conduct such studies to provide solid evidence on the benefits of organic food (Glibowski, 2020; Hurtado-Barroso et al., 2017b; Vigar et al., 2019b).
Given that enhancing male fertility requires addressing lifestyle factors (Humaidan et al., 2022), the findings presented in this study underscore the positive effects of dietary variation and the incorporation of organic foods on male fertility. Organic foods, which accounted for approximately 80% of the participants' diet, have been found to enhance the antioxidant capacity of the Mediterranean diet. Furthermore, studies have indicated that an organic diet is associated with decreased levels of urinary pesticide analytes, including glyphosate, a widely used herbicide (Bradman et al., 2015b; Curl et al., 2019b; Fagan et al., 2020). However, investigations on the protective effects of organic food against pollutant toxicity are still lacking, although there are studies that report the effects of single substances with the ability to detoxify the body from environmental pollutants (Chung, 2017; Vanduchova et al., 2019; Chang et al., 2018; Jamalan et al., 2016; Tedesco et al., 2020).
6. Conclusions
The male contribution to a couple's fertility is important and the findings of this study underscore the importance of dietary variation and the inclusion of organic foods in achieving this goal. Specifically, adhering to a pre-conception Mediterranean diet that is low in carbohydrates and high in legumes, whole grains, and green leafy vegetables, along with consuming 80% organic foods, was associated with improved testosterone levels and reduced sperm DNA fragmentation. Although this study has a limited sample size and is currently being expanded, it emphasizes the significance of consuming quality food for physical and psychological well-being, and suggests that it may serve as an achievable measure of human resilience against environmental insults.
CRediT authorship contribution statement
Veronica Corsetti: Conceptualization, Investigation, Methodology, Software. Tiziana Notari: Visualization, Investigation. Luigi Montano: Conceptualization, Data curation, Writing – original draft, preparation, Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Professor A.G. Marangoni
Contributor Information
Tiziana Notari, Email: dr.notari@gmail.com.
Luigi Montano, Email: l.montano@aslsalerno.it.
Data availability
The data that has been used is confidential.
References
- Agarwal A., Said T.M. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005;95(4):503–507. doi: 10.1111/j.1464-410X.2005.05328.x. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Makker K., Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am. J. Reprod. Immunol. 2008;59(1):2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Baskaran S., Parekh N., Cho C.L., Henkel R., Vij S., Arafa M., Kumar M., Selvam P., Shah R. Male infertility. Lancet. 2021 Jan 23;397(10271):319–333. doi: 10.1016/S0140-6736(20)32667-2. Epub 2020 Dec 10. [DOI] [PubMed] [Google Scholar]
- Aitken R.J., De Iuliis G.N., McLachlan R.I. Biological and clinical significance of DNA damage in male germ line. Int. J. Androl. 2009;32:46–56. doi: 10.1111/j.1365-2605.2008.00943.x. [DOI] [PubMed] [Google Scholar]
- Ajuogu P.K., Al-Aqbi M.A., Hart R.A., Wolden M., Smart N.A., McFarlane J.R. The effect of dietary protein intake on factors associated with male infertility: a systematic literature review and meta-analysis of animal clinical trials in rats. Nutr. Health. 2020 Mar;26(1):53–64. doi: 10.1177/0260106019900731. Epub 2020 Jan 28. [DOI] [PubMed] [Google Scholar]
- Akmal M., Qadri J.Q., Al-Waili N.S., Thangal S., Haq A., Saloom K.Y. Improvement in human semen quality after oral supplementation of vitamin C. J. Med. Food. 2006;9(3):440–442. doi: 10.1089/jmf.2006.9.440. [DOI] [PubMed] [Google Scholar]
- Aksoy Y., Aksoy H., Altinkaynak K., Aydin H.R., Ozkan A. Sperm fatty acid composition in subfertile men. Prostaglandins Leukot. Essent. Fatty Acids. 2006 Aug;75(2):75–79. doi: 10.1016/j.plefa.2006.06.002. Epub 2006 Aug 8. [DOI] [PubMed] [Google Scholar]
- Azizollahi G., Azizollahi S., Babaei H., Kianinejad M., Baneshi M.R., Nematollahi-mahani S.N. Effects of supplement therapy on sperm parameters, protamine content and acrosomal integrity of varicocelectomized subjects. J. Assist. Reprod. Genet. 2013 Apr;30(4):593–599. doi: 10.1007/s10815-013-9961-9. Epub 2013 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barański M., Srednicka-Tober D., Volakakis N., Seal C., Sanderson R., Stewart G.B., Benbrook C., Biavati B., Markellou E., Giotis C., Gromadzka-Ostrowska J., Rembiałkowska E., Skwarło-Sońta K., Tahvonen R., Janovská D., Niggli U., Nicot P., Leifert C. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: a systematic literature review and meta-analyses. Br. J. Nutr. 2014;112(5) doi: 10.1017/S0007114514001366. 794–81. 10.107/S0007114514001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt C.L.R., Björndahl L., De Jonge C.J., et al. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum. Reprod. Update. 2017;23:660–661. doi: 10.1093/humupd/dmx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry J., Assmann K.E., Touvier M., Alles B., Seconda L., Latino-Martel P., Ezzedine K., Galan P., Hercberg S., Lairon D., Kesse-Guyot E. Association of frequency of organic food consumption with cancer risk: findings from the NutriNet-sante prospective cohort study. JAMA Intern. Med. 2018;178:1597–1606. doi: 10.1001/jamainternmed.2018.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellastella G., Menafra D., Puliani G., Colao A., Savastano S. Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. How much does obesity affect the male reproductive function? Int. J. Obes. Suppl. 2019 Apr;9(1):50–64. doi: 10.1038/s41367-019-0008-2. Epub 2019 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook C.M., Butler G., Latif M.A., Leifert C., Davis D.R. Organic production enhances milk NutritionalQuality by shifting fatty acid composition: a United States–wide, 18-month study. PLoS One. 2013 Dec 9;8(12) doi: 10.1371/journal.pone.0082429. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamo P., Volpe M.G., Lorenzetti S., Mantovani A., Notari T., Cocca E., Cerullo S., Di Stasio M., Cerino P., Luigi Montano L. Human semen as an early, sensitive biomarker of environmental exposure of healthy men living in highly polluted areas: a pilot biomonitoring study of trace elements in blood and semen and relationship with sperm quality and RedOx status. Reprod. Toxicol. 2016;66:1–9. doi: 10.1016/j.reprotox.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Biasini B., Rosi A., Menozzi D., Scazzina F. Adherence to the mediterranean diet in association with self-perception of diet sustainability, anthropometric and sociodemographic factors: a cross-sectional study in Italian adults. Nutrients. 2021 Sep 20;13(9):3282. doi: 10.3390/nu13093282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco L., Notari T., Ruvolo G., Roccheri M.C., Martino C., Chiappetta R., Carone D., Lo Bosce G., Carrillo L., Raimondo S., Guglielmino A., Montano L. Sperm DNA fragmentation: an early and reliable marker of air pollution. Environ. Toxicol. Pharmacol. 2018 Mar;58:243–249. doi: 10.1016/j.etap.2018.02.001. Epub 2018 Feb 7. [DOI] [PubMed] [Google Scholar]
- Bradman A., Quiros-Alcala L., Castorina R., Schall R.A., Camacho J., Holland N.T., Barr D.B., Eskenazi B. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ. Health Perspect. 2015;123:1086–1093. doi: 10.1289/ehp.1408660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A., Quiros-Alcala L., Castorina R., Schall R.A., Camacho J., Holland N.T., Barr D.B., Eskenazi B. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ. Health Perspect. 2015;123:1086–1093. doi: 10.1289/ehp.1408660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L.L., Chang J.J., Wang S.J., Li Y.H., Yuan M.Y., Wang G.F., Su P.Y. The effect of healthy dietary patterns on male semen quality: a systematic review and metaanalysis. Asian J. Androl. 2022;24:549–557. doi: 10.4103/aja202252. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caronia L.M., Dwyer A.A., Hayden D., Amati F., Pitteloud N., Hayes F.J. Abrupt decrease in serum testosterone levels after an oral glucose load in men: implications for screening for hypogonadism. Clin. Endocrinol. 2013;78(2):291–296. doi: 10.1111/j.1365-2265.2012.04486.x. [DOI] [PubMed] [Google Scholar]
- Caruso P., Caputo M., Cirillo P., Scappaticcio L., Longo M., Maiorino M.I., Bellastella G., Esposito K. Effects of Mediterranean diet on semen parameters in healthy young adults: a randomized controlled trial. Minerva Endocrinol. 2020 Dec;45(4):280–287. doi: 10.23736/S0391-1977.20.03362-3. [DOI] [PubMed] [Google Scholar]
- Chang S.K., Alasalvar C., Shahidi F. Superfruits: phytochemicals, antioxidant efficacies, and health effects - a comprehensive review. Crit. Rev. Food Sci. Nutr. 2018 Jan 23:1–25. doi: 10.1080/10408398.2017.1422111. [DOI] [PubMed] [Google Scholar]
- Chiang C., Mahalingam S., Flaws J. Environmental contaminants affecting fertility and somatic health. Semin. Reprod. Med. 2017;35:241–249. doi: 10.1055/s-0037-1603569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y.H., Afeiche M.C., Gaskins A.J., Williams P.L., Petrozza J.C., Tanrikut C., Hauser R., Chavarro J.E. Fruit and vegetable intake and their pesticide . residues in relation to semen quality among men from a fertility clinic. Hum. Reprod. 2015;30:1342–1351. doi: 10.1093/humrep/dev064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y.-H., Williams P.L., Gillman M.W., Gaskins A.J., Mínguez-Alarcon L., Souter I., Toth T.L., Ford J.B., Hauser R., Chavarro J.E. Association between pesticide residue intake from consumption of fruits and vegetables and pregnancy outcomes among women undergoing infertility treatment with assisted reproductive technology. JAMA Intern. Med. 2018;178:17–26. doi: 10.1001/jamainternmed.2017.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung R.T. Detoxification effects of phytonutrients against environmental toxicants and sharing of clinical experience on practical applications. Environ. Sci. Pollut. Res. Int. 2017 Apr;24(10):8946–8956. doi: 10.1007/s11356-015-5263-3. [DOI] [PubMed] [Google Scholar]
- Curl C.L., Porter J., Penwell I., Phinney R., Ospina M., Calafat A.M. Effect of a 24-week randomized trial of an organic produce intervention on pyrethroid and organophosphate pesticide exposure among pregnant women. Environ. Int. 2019;132 doi: 10.1016/j.envint.2019.104957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl C.L., Porter J., Penwell I., Phinney R., Ospina M., Calafat A.M. Effect of a 24-week randomized trial of an organic produce intervention on pyrethroid and organophosphate pesticide exposure among pregnant women. Environ. Int. 2019;132 doi: 10.1016/j.envint.2019.104957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutillas-Tolin A., Adoamnei E., Navarrete-Munoz E.M., Vioque J., Monino-Garcia M., Jorgensen N., Chavarro J.E., Mendiola J., Torres-Cantero A.M. Adherence to diet quality indices in relation to semen quality and reproductive hormones in young men. Hum. Reprod. 2019;34:1866–1875. doi: 10.1093/humrep/dez157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielewicz A., Przybylowicz K.E., Przybylowicz M. Dietary patterns and poor semen quality risk in men: a cross-sectional study. Nutrients. 2018;10:1162. doi: 10.3390/nu10091162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durairajanayagam D. Lifestyle causes of male infertility. Arab J Urol. 2018 Mar;16(1):10–20. doi: 10.1016/j.aju.2017.12.004. Published online 2018 Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J., Bohlen L., Patton S., Klein K. Organic diet intervention significantly reduces urinary glyphosate levels in U.S. children and adults. Environ. Res. 2020 Oct;189 doi: 10.1016/j.envres.2020.109898. Epub 2020 Aug 11. [DOI] [PubMed] [Google Scholar]
- Fathi Najafi T., Latifnejad Roudsari R., Namvar F., Ghavami Ghanbarabadi V., Hadizadeh Talasaz Z., Esmaeli M. Air pollution and quality of sperm: a meta-analysis. Iran. Red Crescent Med. J. 2015 Apr 25;17(4) doi: 10.5812/ircmj.17(4)2015.26930. eCollection 2015 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández J.L., Muriel L., Rivero M.T., Goyanes V., Vazquez R., Alvarez J.G. The sperm chr omatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J. Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- Flesch F.M., Gadella B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertil- ization. Biochim. Biophys. Acta. 2000;1469:197–235. doi: 10.1016/s0304-4157(00)00018-6. [DOI] [PubMed] [Google Scholar]
- Gallo A., Boni R., Tosti E. Gamete quality in a multistressor environment. Environ. Int. 2020 May;138 doi: 10.1016/j.envint.2020.105627. Epub 2020 Mar 6. [DOI] [PubMed] [Google Scholar]
- Gaskins A.J., Colaci D.S., Mendiola J., Swan S.H., Chavarro J.E. Dietary patterns and semen quality in young men. Hum. Reprod. 2012;27:2899–2907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharagozloo P., Aitken R.J. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum. Reprod. 2011;26(7):1628–1640. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- Glibowski Paweł. Organic food and health. Rocz. Panstw. Zakl. Hig. 2020;71(2):131–136. doi: 10.32394/rpzh.2020.0110. [DOI] [PubMed] [Google Scholar]
- Guasch-Ferré M., Willet W.C. The Mediterranean diet and health: a comprehensive overview. J. Intern. Med. 2021 Sep;290(3):549–566. doi: 10.1111/joim.13333. Epub 2021 Aug 23. [DOI] [PubMed] [Google Scholar]
- Hallmann E., Marszałek K., Lipowski J., Jasińska U., Kazimierczak R., Średnicka-Tober D., Rembiałkowska E. Polyphenols and carotenoids in pickled bell pepper from organic and conventional production. Food Chem. 2019 Apr 25;278:254–260. doi: 10.1016/j.foodchem.2018.11.052. [DOI] [PubMed] [Google Scholar]
- Hammoud A.O., Gibson M., Peterson C.M., Meikle A.W., Carrell D.T. Impact of male obesity on infertility: a critical review of the current literature. Fertil. Steril. 2008 Oct;90(4):897–904. doi: 10.1016/j.fertnstert.2008.08.026. 1; 2; 3. [DOI] [PubMed] [Google Scholar]
- Harris I.D., Fronczak C., Roth L., Meacham R.B. Fertility and the aging male. Rev. Urol. 2011;13(4):e184–e190. [PMC free article] [PubMed] [Google Scholar]
- Huetos A.S., Babio N., Carrel D.T., Bullo’M, Salvadò J.S. Adherence to the Mediterranean diet is positively associated with sperm motility: a cross-sectionale analysis. Sci Sci Rep. 2019;9(1):3389. doi: 10.1038/s41598-019-39826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humaidan P., Haahr T., Povlsen B.B., Kofod L., Laursen R.J., Alsbjerg B., Elbaek H.O., Esteves S.C. The combined effect of lifestyle intervention and antioxidant therapy on sperm DNA fragmentation and seminal oxidative stress in IVF patients: a pilot study. Int. Braz J. Urol. 2022 Jan-Feb;48(1):131–156. doi: 10.1590/S1677-5538.IBJU.2021.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Barroso S., Tresserra-Rimbau A., Vallverdu-Queralt A., Lamuela-Raventos R.M. Organic food and the impact on human health. Crit. Rev. Food Sci. Nutr. 2017;59:704–714. doi: 10.1080/10408398.2017.1394815. [DOI] [PubMed] [Google Scholar]
- Hurtado-Barroso S., Tresserra-Rimbau A., Vallverdu-Queralt A., Lamuela-Raventos R.M. Organic food and the impact on human health. Crit. Rev. Food Sci. Nutr. 2017;59:704–714. doi: 10.1080/10408398.2017.1394815. [DOI] [PubMed] [Google Scholar]
- Hurtado-Barroso S., Tresserra-Rimbau A., Vallverdú-Queralt A., Lamuela-Raventós R.M. Organic food and the impact on human health. Crit. Rev. Food Sci. Nutr. 2019;59:704–714. doi: 10.1080/10408398.2017.1394815. [DOI] [PubMed] [Google Scholar]
- Jamalan M., Ghaffari M.A., Hoseinzadeh P., Hashemitabar M., Zeinali M. Human sperm quality and metal toxicants: protective effects of some flavonoids on male reproductive function. Int J Fertil Steril. 2016 Jul-Sep;10(2):215–223. doi: 10.22074/ijfs.2016.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B.E., Brannigan R.E. Obesity and male infertility. Curr. Opin. Urol. 2017;27 doi: 10.1097/MOU.0000000000000417. 000–000. [DOI] [PubMed] [Google Scholar]
- Karayiannis D., Kontogianni M.D., Mendorou C., Douka L., Mastrominas M. Yiannakouris N Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum. Reprod. 2017;32(1):215–222. doi: 10.1093/humrep/dew288. [DOI] [PubMed] [Google Scholar]
- Karayiannis D., Kontogianni M.D., Mendorou C., Douka L., Mastrominas M., Yiannakouris N. Association between adherence to the Mediterranean diet and semen quality parameters in male part f couples attempting fertility. Hum. Reprod. 2017;32:215–222. doi: 10.1093/humrep/dew288. [DOI] [PubMed] [Google Scholar]
- Kelly F.J. Dietary antioxidants and environmental stress. Proc. Nutr. Soc. 2004 Nov;63(4):579–585. doi: 10.1079/pns2004388. 27. [DOI] [PubMed] [Google Scholar]
- Kim K.H., Kabir E., Jahan S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017 Jan 1;575:525–535. doi: 10.1016/j.scitotenv.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Lee J., Richburg J.H., Younkin S.C., Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138(5):2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- Liang J., Liang X., Cao P., Wang X., Gao P., Ma N., Li N., Xu H. A preliminary investigation of naturally occurring aluminum in grains, vegetables, and fruits from some areas of China and dietary intake assessment. J. Food Sci. 2019;84:701–710. doi: 10.1111/1750-3841.14459. [DOI] [PubMed] [Google Scholar]
- Mennella M.R., Jones R. Properties of spermatozoal superoxide dismutase and lack of involve- ment of superoxides in metal-ion-catalysed lipid-peroxidation and reactions in semen. Biochem. J. 1980;191(2):289–297. doi: 10.1042/bj1910289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano L., Porciello G., Crispo A., Lorenzetti S., Raimondo S., Ubaldi S., Caputo M. The role of the Mediterranean diet on sperm morphology in healthy men living in polluted area (EcoFoodFertility project) Reprod. Toxicol. 2017;72:45. doi: 10.1016/j.reprotox.2017.06.170. [DOI] [Google Scholar]
- Montano L., Bergamo P., Andreassi M.G., Lorenzetti S. 2018. The Role of Human Semen as an Early and Reliable Tool of Environmental Impact Assessment on Human Health. Full Chapter in Final Book Title & ISBN: Spermatozoa - Facts and Perspectives, " 978-1-78923-171-7. InTechOpen June 13th. [DOI] [Google Scholar]
- Montano L., Ceretti E., Donato F., Bergamo P., Zani C., Viola G.C.V., Notari T., Pappalardo S., Zani D., Ubaldi S., Bollati V., Consales C., Leter G., Trifuoggi M., Amoresano A., Lorenzetti S., Fast Study group Effects of a lifestyle change intervention on semen quality in healthy young men living in highly polluted areas in Italy: the FASt randomized controlled trial. Eur Urol Focus. 2021;8:351–359. doi: 10.1016/j.euf.2021.01.017. [DOI] [PubMed] [Google Scholar]
- Montano L., Maugeri A., Volpe M.G., Micali S., Mirone V., Mantovani A., Navarra M., Piscopo M. Mediterranean diet as a shield against male infertility and cancer risk induced by environmental pollutants: a focus on flavonoids. Int. J. Mol. Sci. 2022 Jan 29;23(3):1568. doi: 10.3390/ijms23031568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L.E., Mills J.L., Molloy A.M., Qian C., Carter T.C., Strevens H., Wide-Swensson D., Giwercman A., Levine R.J. Folate and vitamin B12 in idiopathic male infertility. Asian J. Androl. 2011 Nov;13(6):856–861. doi: 10.1038/aja.2011.96. Epub 2011 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan F.L., Chavarro J.E., Tanrikut C. Diet and men's fertility: does diet affect sperm quality? Fertil. Steril. 2018;110:570–577. doi: 10.1016/j.fertnstert.2018.05.025. [DOI] [PubMed] [Google Scholar]
- O'Neill B.J. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2020 Oct;27(5):301–307. doi: 10.1097/MED.0000000000000569. [DOI] [PubMed] [Google Scholar]
- Oostingh E.C., Steegers-Theunissen R.P., de Vries J.H., Laven J.S., Koster M.P. Strong adherence to a healthy dietary pattern is associated with better semen quality, especially in men with poor semen quality. Fertil. Steril. 2017;107(4):916–923.e2. doi: 10.1016/j.fertnstert.2017.02.103. Epub 2017 Mar 11. [DOI] [PubMed] [Google Scholar]
- Palmer N.O., Bakos H.W., Fullston T., Lane M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis. 2012 Oct 1;2(4):253–263. doi: 10.4161/spmg.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palupi E., Jayanegara A., Ploeger A., Kahl J. Comparison of nutritional quality between conventional andorganic dairy products: a meta-analysis. J. Sci. Food Agric. 2012;92:2774–2781. doi: 10.1002/jsfa.5639. [DOI] [PubMed] [Google Scholar]
- Pironti C., Ricciardi M., Motta O., Miele M., Proto A., Montano L. Microplastics in the environment: intake through the food web, human exposure and toxicological effects. Toxics. 2021;9:224. doi: 10.3390/toxics9090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pironti C., Notarstefano V., Ricciardi M., Motta O., Giorgini E., Montano L. First evidence of microplastics in human urine, a preliminary study of intake in the human body. Toxics. 2023;11:40. doi: 10.3390/toxics11010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prandini A., Sigolo S., Piva G. Conjugated linoleic acid (CLA) and fatty acid composition of milk, curd and Grana Padano cheese in conventional and organic farming systems. J. Dairy Res. 2009 Aug;76(3):278–282. doi: 10.1017/S002202990900409936–38. [DOI] [PubMed] [Google Scholar]
- Ribas-Agusti A., Diaz I., Sarraga C., Garcia-Regueiro J.A., Castellari M. Nutritional properties of organic and conventional beef meat at retail. J. Sci. Food Agric. 2019;99(9):4218–4225. doi: 10.1002/jsfa.9652. [DOI] [PubMed] [Google Scholar]
- Ribes-Moya A.M., Adalid A.M., Raigón M.D., Hellín P., Fita A., Rodríguez-Burruezo A. Variation in flavonoids in a collection of peppers (Capsicum sp.) under organic and conventional cultivation: effect of the genotype, ripening stage, and growing system. J. Sci. Food Agric. 2020 Jan 6 doi: 10.1002/jsfa.1024532. [DOI] [PubMed] [Google Scholar]
- Ricci E., Bravi F., Noli S., Ferrari S., De Cosmi V., La Vecchia I., Cavadini M., La Vecchia C., Parazzini F. Mediterranean diet and the risk of poor semen quality: cross-sectional analysis of men referring to an Italian Fertility Clinic. Andrology. 2019 Mar;7(2):156–162. doi: 10.1111/andr.12587. [DOI] [PubMed] [Google Scholar]
- Roeleveld N., Bretveld R. The impact of pesticides on male fertility. Curr. Opin. Obstet. Gynecol. Jun 2008;20(3):229–233. doi: 10.1097/GCO.0b013e3282fcc334. [DOI] [PubMed] [Google Scholar]
- Ross C., Morriss A., Khairy M., Khalaf Y., Braude P., Coomarasamy A., El-Toukhy T. A systematic review of the effect of oral antioxidants on male infertility. Reprod. Biomed. Online. 2010 Jun;20(6):711–723. doi: 10.1016/j.rbmo.2010.03.008. Epub 2010 Mar 10. [DOI] [PubMed] [Google Scholar]
- Rubes J., Selevan S.G., Evenson D.P., Zudova D., Vozdova M., Zudova Z., Robbins W.A., Perreault S.D. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum. Reprod. Oct 2005;20(10):2776–2783. doi: 10.1093/humrep/dei122. [Epub Jun 24, 2005] [DOI] [PubMed] [Google Scholar]
- Safarinejad M.R., Hosseini S.Y., Dadkhah F., Asgari M.A. Relationship of omega-3 and omega-6 fatty acids with semen characteristics, and anti-oxidant status of seminal plasma: a comparison between fertile and infertile men. Clin. Nutr. 2010 Feb;29(1):100–105. doi: 10.1016/j.clnu.2009.07.008. Epub 2009 Aug 8. [DOI] [PubMed] [Google Scholar]
- Salas-Huetos A., James E.R., Aston K.I., Jenkins T.G., Carrell D.T. Diet and sperm quality: nutrients, foods and dietary patterns. Reprod. Biol. 2019;19:219–224. doi: 10.1016/j.repbio.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Salas-Huetos A., Babio N., Carrell D.T., Bulló M., Salas-Salvadó J. Adherence to the Mediterranean diet is positively associated with sperm motility: a cross-sectional analysis. Sci. Rep. 2019;9(1):3389. doi: 10.1038/s41598-019-39826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraju V., Baskaran S., Agarwal A., Henkel R. Environmental contaminants and male infertility: effects and mechanisms. Andrologia. 2021 Feb;53(1) doi: 10.1111/and.13646. Epub 2020 May. [DOI] [PubMed] [Google Scholar]
- Shah W, Khan R, Shah B, Khan A, Dil S, Liu W, Wen J and Jiang X. The molecular mechanism of sex hormones on Sertoli cell development and proliferation. Front. Endocrinol. 12:648141. doi: 10.3389/fendo.2021.648141. Feb. [DOI] [PMC free article] [PubMed]
- Sharma R.K., Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48(6):835–850. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim Amiya P., Swerdloff Ronald S. Hormonal and genetic control of germ cell apoptosis in the testis. Rev. Reprod. 1999;4:38–47. doi: 10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- Średnicka-Tober D., Barański M., Seal C.J., Sanderson R., Benbrook C., Steinshamn H., Gromadzka-Ostrowska J., Rembiałkowska E., Skwarło-Sońta K., Eyre M., Cozzi G., Larsen M.K., Jordon T., Niggli U., Sakowski T., Calder P.C., Burdge G.C., Sotiraki S., Stefanakis A., Stergiadis S., Yolcu H., Chatzidimitriou E., Butler G., Stewart G., Leifert C. Higher PUFA and n-3 PUFA, conjugated linoleic acid, α-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: a systematic literature review and meta- and redundancy analyses. Br. J. Nutr. 2016;115(6):1043–1060. doi: 10.1017/S0007114516000349. 10-1017/S0007114516000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Średnicka-Tober D., Barański M., Seal C., Sanderson R., Benbrook C., Steinshamn H., Gromadzka-Ostrowska J., Rembiałkowska E., Skwarło-Sońta K., Eyre M., Cozzi G., Krogh Larsen M., Jordon T., Niggli U., Sakowski T., Calder P.C., Burdge G.C., Sotiraki S., Stefanakis A., Yolcu H., Stergiadis S., Chatzidimitriou E., Butler G., Stewart G., Leifert C. Composition differences between organic and conventional meat: a systematic literature review and meta-analysis. Br. J. Nutr. 2016;115:994–1011. doi: 10.1017/S0007114515005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Liu B., Du Y., Snetselaar L.G., Sun Q., Hu F.B., Bao W. Inverse association between organic food purchase and diabetes mellitus in US adults. Nutrients. 2018;10:1877. doi: 10.3390/nu10121877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco I., Russo M., Moccia S., Cervellera C., Spagnuolo C., Carbone V., Minasi P., Montano L., Verze P., Capece M., MironeV, Baraschino A., De Rosa M., Volpe M.G., Russo G.L. Protective effect of curcumin towards cadmium and polycyclic aromatic hydrocarbons toxicities: the EcoNutraPrevention Project. Nutr. Metabol. Cardiovasc. Dis. 2020;30(3):240. doi: 10.1016/j.numecd.2019.12.036. [DOI] [Google Scholar]
- Tremellen K. Oxidative stress and male infertility—a clinical perspective. Hum. Reprod. Update. 2008;14(3):243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A., Simonetta I., Daidone M., Mogavero A., Ortello A., Pinto A. Metabolic and vascular effect of the mediterranean diet. 31Int J Mol Sci. 2019 Sep 23;20(19):4716. doi: 10.3390/ijms20194716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanduchova A., Anzenbacher P., Anzenbacherova E. Isothiocyanate from broccoli, sulforaphane, and its properties. J. Med. Food. 2019 Feb;22(2):121–126. doi: 10.1089/jmf.2018.0024. Epub 2018 Oct 27. [DOI] [PubMed] [Google Scholar]
- Vernet P., Aitken R.J., Drevet J.R. Antioxidant strategies in the epididymis. Mol. Cell. Endocrinol. 2004;216(1–2):31–39. doi: 10.1016/j.mce.2003.10.069. [DOI] [PubMed] [Google Scholar]
- Vigar V., Myers S., Oliver C., Arellano J., Robinson S., Leifert C. A systematic review of organic versus conventional food consumption: is there a measurable benefit onHuman health. Nutrients. 2019;12(1) doi: 10.3390/nu12010007. pii: E7. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigar V., Myers S., Oliver C., Arellano J., Robinson S., Leifert C. A systematic review of organic versus conventional food consumption: is there a measurable benefit onHuman health. Nutrients. 2019;12(1) doi: 10.3390/nu12010007. pii: E7. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volek J.S., Kraemer W.J., Bush J.A., Incledon T., Boetes M. Testosterone and cortisol in relationship to dietary nutrients and resistance exercise. J. Appl. Physiol. 1985;82(1):49–54. doi: 10.1152/jappl.1997.82.1.49. 1997 Jan. [DOI] [PubMed] [Google Scholar]
- Wathes D.C., Abayasekara D.R.E., Aitken R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007;77:190–201. doi: 10.1095/biolreprod.107.060558. [DOI] [PubMed] [Google Scholar]
- Westman E.C., Yancy W.S., Jr. Using a low-carbohydrate diet to treat obesity and type 2 diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2020 doi: 10.1097/MED.0000000000000565. [DOI] [PubMed] [Google Scholar]
- Wilson J.M., Lowery R.P., Roberts M.D., Sharp M.H., Joy J.M., Shields K.A., Partl J.M., Volek J.S., D'Agostino D.P. Effects of ketogenic dieting on body composition, strength, power, and hormonal profiles in resistance training men. J. Strength Condit Res. 2020 Dec;34(12):3463–3474. doi: 10.1519/JSC.0000000000001935. [DOI] [PubMed] [Google Scholar]
- Xia W., Chiu Y.H., Williams P.L., Gaskins A.J., Toth T.L., Tanrikut C., Hauser R., Chavarro J.E. Men's meat intake and treatment outcomes among couples undergoing assisted reproduction. Fertil. Steril. 2015 Oct;104(4):972–979. doi: 10.1016/j.fertnstert.2015.06.037. Epub 2015 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz B., Terecia H., Sandal S., Kelestimur F. Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020 Mar;21(1):127–147. doi: 10.1007/s11154-019-09521-z. [DOI] [PubMed] [Google Scholar]
- Yu-Han Chiu Y.H., Gaskins A.J., Pls William, Mendiola J., Jørgensen N., Levine H., Hauser R., Swan S.H., Chavarro J.E. Intake of fruits and vegetables with low to moderate pesticide residues is positively associated with semen-quality parameters among young healthy men. J. Nutr. 2016 May;146(5):1084–1092. doi: 10.3945/jn.115.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini A., de Lamirande E., Gagnon C. Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int. J. Androl. 1993;16(3):183–188. doi: 10.1111/j.1365-2605.1993.tb01177.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.