Abstract
The intestinal microbiota is considered to be a forgotten organ in human health and disease. It maintains intestinal homeostasis through various complex mechanisms. A significant body of research has demonstrated notable differences in the gut microbiota of patients with gastrointestinal tumours compared to healthy individuals. Furthermore, the dysregulation of gut microbiota, metabolites produced by gut bacteria, and related signal pathways can partially explain the mechanisms underlying the occurrence and development of gastrointestinal tumours. Therefore, this article summarizes the latest research progress on the gut microbiota and gastrointestinal tumours. Firstly, we provide an overview of the composition and function of the intestinal microbiota and discuss the mechanisms by which the intestinal flora directly or indirectly affects the occurrence and development of gastrointestinal tumours by regulating the immune system, producing bacterial toxins, secreting metabolites. Secondly, we present a detailed analysis of the differences of intestinal microbiota and its pathogenic mechanisms in colorectal cancer, gastric cancer, hepatocellular carcinoma, etc. Lastly, in terms of treatment strategies, we discuss the effects of the intestinal microbiota on the efficacy and toxic side effects of chemotherapy and immunotherapy and address the role of probiotics, prebiotics, FMT and antibiotic in the treatment of gastrointestinal tumours. In summary, this article provides a comprehensive review of the pathogenic mechanisms of and treatment strategies pertaining to the intestinal microbiota in patients with gastrointestinal tumours. And provide a more comprehensive and precise scientific basis for the development of microbiota-based treatments for gastrointestinal tumours and the prevention of such tumours.
Introduction
Gastrointestinal tumours are common malignant tumours worldwide. Hepatocellular carcinoma (HCC) (8.3%) has the highest mortality rate among gastrointestinal tumours, followed by gastric cancer (GC) (7.7%), colorectal cancer (CRC) (5.8%), and oesophageal cancer (EC) (5.5%), etc. At the same time, colorectal cancer has the highest incidence rate (6.0%) among gastrointestinal tumours, followed by gastric cancer (5.6%), hepatocellular carcinoma (4.7%), and oesophageal cancer (3.1%), etc. [1]. Increasing evidence suggests that gastrointestinal cancers are caused by multiple factors, such as immune system disorders, heavy alcohol consumption, smoking, obesity, diet, environmental factors, and intestinal microbiota dysbiosis [2]. The intestines are a complex environment in which bacteria, fungi, and viruses coexist. The total number of microorganisms in the intestines may be as high as 100 trillion, approximately 10 times the number of human cells in the entire body [3]. The intestinal microbiota plays a major role in the maintenance of human health and the development of diseases. An increasing number of studies have shown that the intestinal microbiota is involved in regulating intestinal function, protecting the intestinal mucosa from pathogens within the intestines, and preventing pathogenic bacteria from escaping from the intestines into other tissues [4] The intestinal microbiota is also closely related to many diseases such as metabolic syndrome [5], depression [6], and tumours [7]. As 16S rDNA sequencing and metagenomics technology rapidly develop, accumulating evidence suggests that the intestinal microbiota is closely related to gastrointestinal tumours. Dysbiosis of the intestinal microbiota leads to increased inflammation and activation of carcinogenic pathways, thereby promoting tumour development. Additionally, metabolites produced by the intestinal microbiota, such as short-chain fatty acids (SCFAs) and bile acids, can regulate the proliferation, apoptosis, and immune functions of gastrointestinal tumour cells, thus influencing tumorigenesis. Furthermore, the intestinal microbiota can enhance the efficacy of chemotherapy and immunotherapy, while also reducing their toxic side effects [8, 9].
In this review, we have summarized the latest literatures and comprehensively explored the pathogenic mechanisms of the gut microbiota and its metabolites in gastrointestinal tumours. This provides a more comprehensive and precise scientific basis for the development of microbiota-based tumours therapies and personalized tumours treatment strategies. Firstly, we introduce the composition and biological functions of the gut microbiota, and discuss the impact of gut microbiota on the oncogenesis and development of gastrointestinal tumours through metabolic pathways, immune pathways, and secretion of protein toxins. We then systematically describe the effects of the gut microbiota and its metabolites on digestive system tumours such as colorectal cancer, gastric cancer, liver cancer, and oesophageal cancer, in order to have a comprehensive understanding of the pathogenic mechanisms. At last, we discuss the effects of the intestinal microbiota on the efficacy and toxic side effects of chemotherapy and immunotherapy and address the role of probiotics, prebiotics, FMT and antibiotic in the treatment of gastrointestinal tumours.
Composition and function of the intestinal microbiota
Composition of the intestinal microbiota
The intestinal microbiota refers to the collection of various microorganisms that inhabit the human intestine, and is primarily composed of bacteria, fungi, viruses, and other microorganisms [3]. Bacteria are the major members of the intestinal microbiota, with the predominant phyla being Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, and the predominant genera including Bacteroides, Bifidobacterium, Clostridium, Lactobacillus, Prevotella, Ruminococcus, Streptococcu, among others. These bacteria account for more than 90% of the total intestinal microbiota [10]. Based on their functions, the various bacteria within the intestinal microbiota can be categorised as probiotic species, opportunistic pathogens, and pathogenic bacteria. Probiotics beneficial microorganisms that contribute to host health, and include Bifidobacterium, Lactobacillus, Butyricicoccus, Roseburia, and more. They facilitate food digestion to produce SCFAs, synthesise vitamins, and enhance immune function, thus playing a crucial role in maintaining gut health [11]. Opportunistic pathogens primarily include Escherichia coli, Streptococcus (Enterococcus), and Veillonella. Under normal circumstances, opportunistic pathogens are harmless to their human hosts. However, when immune defences are compromised, or other factors lead to their overgrowth, they can cause intestinal infections, inflammation, food poisoning, and other conditions by secreting toxins [12]. Although the majority of the intestinal microbiota consists of probiotic species and opportunistic pathogens, approximately 10% of the intestinal microbiota comprises pathogenic bacteria, including Proteus, Salmonella, and Clostridium difficile. These pathogenic bacteria can promote the development of gastrointestinal diseases and tumours by secreting harmful metabolites and toxins, disrupting the intestinal mucosal barrier, and inhibiting immune cell function [13, 14]. In summary, maintaining a balanced intestinal microbiota is crucial for preventing intestinal-related diseases and maintaining optimal health.
Function of the intestinal microbiota
The intestinal microbiota is an important component of the complex human microecosystem and plays a vital role in regulating metabolism, protecting the intestinal barrier, and modulating the immune system [3, 15]. Studies have shown that bacteria such as Roseburia, Eubacterium rectale, Faecalibacterium prausnitzii, Clostridium, and Butyricicoccus can metabolise indigestible carbohydrates, such as cellulose, hemicellulose, resistant starch, pectin, oligosaccharides, and lignin, into SCFAs [16]. The SCFAs mainly include acetate, propionate and butyrate. Butyrate is the main energy source for colon epithelial cells and can inhibit over-proliferation of CRC cells, Escherichia coli, Salmonella, and other pathogens, thus suppressing the development of CRC [17, 18]. The intestinal microbiota also plays a role in vitamin synthesis. Lactic acid bacteria, Roseburia, and Bifidobacterium synthesise vitamins such as biotin, thiamine, cobalamin, riboflavin, vitamin B, and K [19].
The intestinal barrier is a physical barrier that lines the intestinal mucosa and prevents the entry of harmful substances and microorganisms, thereby maintaining intestinal homeostasis [20]. Beneficial bacteria within the intestinal microbiota localise to the surface of the intestinal tract, forming a physical barrier that prevents the attachment and proliferation of harmful bacteria. This competitive inhibition mechanism reduces the abundance of harmful bacteria and diminishes the damage that they could cause to the intestinal mucosa [21]. Some probiotics produce antimicrobial substances such as antimicrobial peptides and organic acids that can directly kill harmful bacteria and prevent them from invading the intestinal mucosa [22]. The intestinal microbiota helps maintain normal function of the intestinal mucosa by producing SCFAs, which promote intestinal cell proliferation and repair [20]. Additionally, the intestinal microbiota can influence the production of adhesive proteins and mucus by mucosal cells, thereby strengthening the protective layer of the intestinal barrier [23].
The intestinal microbiota plays a critical role in the development of the host immune system. Studies have shown that the genus Clostridium can promote the generation of regulatory T (Treg) cells in the gut, inhibit overactivation of the immune system, and maintain immune balance [24, 25]. Chuang et al. demonstrated that germ-free mice exhibited decreased immunity, as well as a significant decrease in the numbers of plasma cells and T cells and IgA levels in the intestine. Moreover, colonisation with segmented filamentous bacteria can restore the number of T cells in the intestine of germ-free mice [26]. However, the intestinal microbiota is not static, and dysbiosis of the intestinal microbiota due to disruption of homeostasis within the host's internal environment or a decline in immune defences is closely associated with the occurrence and development of gastrointestinal inflammation, mental and psychological diseases, metabolic diseases and malignancies [27–29]. Increasing evidence suggest that the intestinal flora and its products are closely related to gastrointestinal tumours [30–32]. The intestinal microbiota can damage host cells and the intestinal mucosa and influence tumour occurrence, development, and prognosis through secretion of metabolites, modulation of immune cell function, and production of toxins [33–35].
The effects of the intestinal microbiota and its products on gastrointestinal tumours
In recent years, there has been increasing interest in the role of the intestinal microbiota and its products in the occurrence and development of gastrointestinal tumours. The production of metabolites such as bile acids, trimethylamine oxide (TMAO) and SCFAs by the intestinal microbiota promotes the development of gastrointestinal tumours. Additionally, the intestinal microbiota and its products can influence the occurrence and development of gastrointestinal tumours through immune pathways, and toxin secretion pathways [30, 32, 34, 36, 37]. Therefore, in-depth research on the effects of the intestinal microbiota and its products on gastrointestinal tumours, as well as the underlying mechanisms, is of great significance for the prevention, diagnosis, and treatment of such tumours.
Metabolite pathways
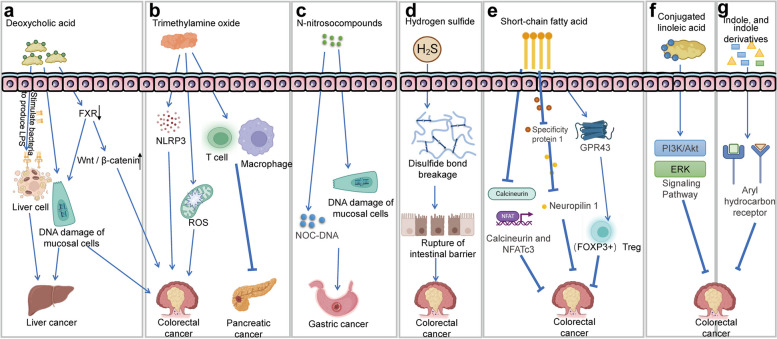
The interaction between the intestinal microbiota and gastrointestinal tumours is mainly mediated by metabolites. Studying the relationship between intestinal microbiota metabolites and gastrointestinal tumours helps reveal the mechanisms by which the intestinal microbiota regulates gastrointestinal tumour development (Fig. 1, Table 1). Pathogenic gut bacteria promote the occurrence and development of gastrointestinal tumours by producing harmful metabolites such as bile acids, TMAO, N-nitrosocompounds (NOCs), and hydrogen sulphide (H2S), which damage normal cellular DNA, activate intracellular tumorigenic signalling pathways, and promote the release of inflammatory factors [35]. However, beneficial intestinal bacteria inhibit the occurrence and development of gastrointestinal tumours through the production of beneficial metabolites such as SCFAs, conjugated linoleic acid (CLA), indole, and indole derivatives, which suppress tumorigenic signalling pathways, inhibit the release of inflammatory factors, and decrease the proliferation of pathogenic bacteria and cancer cells.
Fig. 1.
Metabolite pathways. Abbreviations: LPS Lipopolysaccharides, TLR4 Toll-like receptor 4, FXR Farnesoid X receptor, DCA Deoxycholic acid, NFATc3 Nuclear factor of activated T cells 3, FOXP3 Forkhead box P3, GPCR G protein-coupled receptor, ERK Extracellular signal-regulated kinase, PI3K/Akt Phosphatidylinositol 3-kinase/Protein kinase B, IL Interleukin
Table 1.
Pathogenic mechanism of intestinal microbiota metabolites in gastrointestinal tumours
| Metabolite type | Related mechanisms | Reference |
|---|---|---|
| Dichloroacetic acid (DCA) | DCA induces DNA damage in liver cells and intestinal mucosal cells. Additionally, DCA reduces the activation of functional farnesoid X receptor (FXR) signalling in CRC cells, thereby promoting the development of colon cancer | [38, 39] |
| DCA | DCA stimulates Gram-negative bacteria to produce more lipopolysaccharides (LPS), which enter the liver, thereby activating the Toll-like receptor 4 (TLR4) pathway and promoting the occurrence and development of liver cancer | [40] |
| Trimethylamine oxide (TMAO) | TMAO promotes vascular inflammation by activating the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome. Additionally, TMAO can stimulate the production of reactive oxygen species (ROS) in the mitochondria | [41, 42] |
| TMAO | Delivery of TMAO intraperitoneally or via a dietary choline supplement to orthotopic pancreatic cancer bearing mice reduced the tumour growth and associated with an immunostimulatory tumour-associated macrophage phenotype and activated effector T cell response in the tumour microenvironment | [43] |
| N-nitrosocompounds (NOCs) | NOCs can damage the DNA of mucosal cells, simultaneously generating NOC-DNA adducts that impair the cell's self-repair mechanisms. This disruption leads to dysregulation of cell proliferation and differentiation, ultimately resulting in carcinogenesis | [44–46] |
| Hydrogen sulfide(H2S) | H2S can induce DNA damage in normal cells while reducing disulfide bonds in the intestinal epithelial mucus network. This ultimately leads to the breakdown of the intestinal mucosal barrier, exposing the intestinal epithelium to bacteria and toxins, resulting in inflammation | [47, 48] |
| Short-chain fatty acids (SCFAs) | SCFAs can inhibit the excessive proliferation of colorectal cancer cells and gut pathogenic bacteria such as Escherichia coli and Salmonella | [18] |
| SCFAs | SCFAs help inhibit the proliferation of colorectal tumour cells by inhibiting the activation of calcineurin and nuclear factor 3 (NFATc3) of activated T cells | [49] |
| SCFAs | Butyrate-mediated activation of GPR109A could upregulate anti-inflammatory effector molecules IL-10 and Aldh1a in colonic DCs and macrophages, which promoted the differentiation of IL-10-producing CD4 T cells and Tregs, and inhibited the development of IL17-producing T cells, thereby inhibiting the occurrence of CRC | [50] |
| SCFAs | Acetate and propionate could induce forkhead box P3 (FOXP3 +) Tregs in a GPR43-dependent manner to protect CRC | [51, 52] |
| Conjugated linoleic acid (CLA) | CLA helps inhibit the PI3K/Akt and ERK signalling cascades, inducing apoptosis in tumour cells | [53] |
| Indole, and indole derivatives | Indole, and indole derivatives are the major ligands for the aryl hydrocarbon receptor (AhR). They can activate AhR, thereby maintaining intestinal homeostasis, inhibiting pathogen infection, and improving symptoms of colitis | [54, 55] |
Abbreviations: CRC Colorectal cancer, TNF Tumour necrosis factor, NF-κB Nuclear factor-kappa B, ERK Extracellular signal-regulated kinase, PI3K/Akt Phosphatidylinositol 3-kinase/Protein kinase B, TLR4 Toll-like receptor 4, IL Interleukin, TGF-β Transforming Growth Factor-beta, Bcl-2 B-cell lymphoma 2
Intestinal microbiota (e.g., Bacteroides fragilis, Bacteroides vulgatus, Clostridium perfringens, Eubacterium, Lactobacillus, and Bifidobacterium) metabolize primary bile acids to secondary bile acids by 7α-dehydroxylation [35]. After entering the liver through the portal vein, deoxycholic acid (DCA), a derivative of secondary bile acids, induces DNA damage in liver cells and intestinal mucosal cells [56]. DCA also dissolves epithelial tissue to stimulate bacteria to produce more lipopolysaccharide (LPS) that enters the liver, thereby activating the Toll-like receptor 4 (TLR4) pathway and promoting the development of HCC [40]. Mechanistically, DCA promotes the growth of CRC cells by inducing the activation of the epidermal growth factor receptor (EGFR) and Wingless/integrated (Wnt) signalling pathways [57, 58]. Additionally, in CRC, DCA reduces activation of functional farnesoid X receptor (FXR) signalling in CRC cells, thereby promoting the occurrence of CRC [38, 39]. Recent studies have shown that a lack of FXR not only impairs intestinal and hepatic circulation and bile acid production but also enhances the canonical Wnt/β-catenin signalling pathways, thereby promoting DNA damage [35, 59].
TMAO is a metabolite of trimethylamine (TMA), which is primarily produced by Clostridium anaerobes (phylum Clostridia) and Facultative Anaerobic Enterobacteriaceae (phylum Proteobacteria) during the digestion of foods rich in choline and carnitine, such as meat, eggs, and fish [60, 61]. Although there is no direct evidence suggesting that TMAO directly promotes the occurrence and development of gastrointestinal tumours, numerous studies have shown that TMAO can induce inflammation and oxidative stress. TMAO promotes vascular inflammation by activating the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome and can also stimulate reactive oxygen species (ROS) production in mitochondria [41, 42]. Clinical studies have shown that plasma TMAO levels are positively correlated with CRC [62]. However, Gauri et al. found that TMAO enhances antitumour immunity in pancreatic cancer (PC). Delivery of TMAO to orthotopic PC–bearing mice intraperitoneally or via a dietary choline supplement reduced tumour growth and was associated with an immunostimulatory tumour-associated macrophage phenotype and activated effector T cell response in the tumour microenvironment (TME) [43]. However, the precise mechanism by which TMAO promotes tumorigenesis is still unclear and requires further exploration.
NOCs are a group of compounds containing nitroso groups, including N-nitrosamines and N-nitrosamides. NOCs are potent carcinogens and have been associated with the occurrence and development of gastrointestinal tumours, such as GC and CRC. In the gastrointestinal tract, nitrite undergoes nitrosation to form NOCs. The high concentration of NOCs can damage colon mucosal cell DNA and generate NOC-DNA adducts, resulting in loss of the self-repair ability in damaged cells, leading to cell proliferation and differentiation disorders, and ultimately inducing carcinogenesis [44–46].
Sulphate-reducing bacteria (SRB) generate H2S by metabolising sulphate in food and other sulphur-containing compounds, such as taurine and cysteine [63, 64]. Extensive research has shown that the levels of H2S in the faeces of gastrointestinal tumour patients are significantly higher than those found in the faeces of healthy individuals, indicating that H2S plays a role in the pathogenesis of gastrointestinal tumours [65, 66]. H2S has been found to reduce disulfide bonds in the mucin network of intestinal epithelium, leading to disruption of the intestinal mucosal barrier and exposing the intestinal epithelium to bacteria and toxins, ultimately triggering inflammation [47]. Additionally, Matias et al. demonstrated that H2S can induce DNA damage in normal cells, in a process that may be mediated by free radicals [48]. Interestingly, some studies have suggested that H2S can protect and rebuild the damaged mucus layer, thereby preventing inflammation [67, 68]. Further research is needed to explore the mechanisms by which H2S affects the development of CRC, as it remains controversial whether H2S acts as a promoting or inhibiting factor.
Beneficial metabolites SCFAs are a type of organic acid produced by the metabolism of dietary fibre and undigested carbohydrates by the intestinal microbiota, mainly including acetic acid, propionic acid, and butyric acid [69]. These SCFAs are primarily produced by Bacteroides, Coprococcus comes, Anaerostipes spp, Eubacterium rectale, Faecalibacterium prausnitzii, and Roseburia spp.[70]. SCFAs inhibit the development of gastrointestinal tumours through a variety of mechanisms, such as inhibiting the proliferation of pathogenic bacteria and cancer cells, inducing cancer cell apoptosis, and suppressing inflammatory responses. Research has shown that SCFAs can inhibit excessive proliferation of pathogenic intestinal bacteria and the growth of CRC cells [18]. SCFAs serve as the main energy source for colon cells, promote the proliferation of colon epithelial cells, protect the intestinal mucosa, and help prevent CRC [71]. SCFAs help inhibit protein acetylation and inhibit the proliferation of CRC cells by inhibiting activation of calcineurin and nuclear factor of activated T cells 3 (NFATc3) [49]. Butyrate could diminish the expression of neuropilin 1 by suppressing the transactivation of Specificity protein 1 (Sp1) to inhibit the angiogenesis, metastasis, and survival of CRC cells, and butyrate could trigger the CRC cells apoptosis by activating the signalling of Wnt [51]. Activation of G protein-coupled receptors (GPCRs) inhibits the development of colonic inflammation and CRC, while SCFAs can act as ligands for certain GPCRs. There are three main types of GPCRs associated with SCFAs: GPR109A, GPR43 and GPR41 [72]. Mechanistically, Singh et al. found that butyrate-mediated activation of GPR109A could upregulate anti-inflammatory effector molecules IL-10 and Aldh1a in colonic DCs and macrophages, which promoted the differentiation of IL-10-producing CD4 T cells and Tregs, and inhibited the development of IL17-producing T cells, thereby inhibiting the occurrence of CRC [50]. Acetate and propionate could induce forkhead box P3 (FOXP3 +) Tregs in a GPR43-dependent manner to protect CRC [51, 52]. In addition, SCFAs promote IL-22 production by CD4 T cells and ILCs through GPR41 and inhibition of histone deacetylase, thereby alleviating colorectal inflammation [73].. Additionally, Kobayashi et al. showed that in SCFAs enhance the cytotoxicity of cisplatin against HCC cells by regulating the G protein-coupled receptor 41 signalling pathway [74].
In addition to SCFAs, beneficial metabolites such as CLA, indole, and indole derivatives can also inhibit the occurrence and development of gastrointestinal tumours. CLA is an isomer of LA, and linoleic acid can be converted into CLA by Lactobacilli and Bifidobacterium. Research has shown that CLA can inhibit tumour cell apoptosis and proliferation [75–77]. Mechanistically, CLA can suppress the cascade connection of Phosphatidylinositol 3-kinase/Protein kinase B (PI3K/Akt) and Extracellular signal-regulated kinase (ERK) signalling pathway, induce cell apoptosis, and inhibit the cell cycle in cancer cell lines [53]. Trans-10, cis-12 CLA induces colon cancer cell apoptosis by inducing ROS production and endoplasmic reticulum stress [76]. Indole and its derivatives are produced by Bacteroides, Enterococcus, and Lactobacillus via tryptophan metabolism [78]. Studies have shown that indole and its derivatives are major ligands for aryl hydrocarbon receptor (AhR) that induce AhR activation, thereby maintaining intestinal homeostasis, inhibiting pathogen infection, and improving colitis symptoms [54, 55].
In summary, metabolites mediate the interaction between the intestinal microbiota and gastrointestinal tumours. Pathogenic bacteria damage normal cellular DNA, activate oncogenic signalling pathways, and promote the release of pro-inflammatory factors by producing harmful metabolites such as TMAO, NOCs, and H2S, thereby driving tumour development. Probiotics produce beneficial metabolites such as SCFAs, CLA, and indole that inhibit cancer signalling pathways, reduce the release of inflammatory factors, and suppress the growth of pathogenic bacteria and cancer cells, thereby inhibiting tumour development. Therefore, further research on the relationship among metabolites, the intestinal microbiota, and tumour development is expected to provide new insights into the treatment of gastrointestinal tumours.
Immune pathway
The TME is a specialized and complex biological environment that affects the growth, invasion, and metastasis of tumour cells. Many types of immune cells are present in the TME, including T cells, B cells, macrophages, natural killer (NK) cells, adipocytes, fibroblasts, and myeloid-derived suppressor cells (MDSCs). However, increasing evidence suggests that microorganisms are also major components of certain TMEs [79]. The gut microbiome, as an important participant in the gut environment, is involved in immune regulation and related to the tumour immune response [80, 81]. Moreover, the gut microbiome imbalance may promote or inhibit tumour development by reshaping the TME and altering the immune response [33].
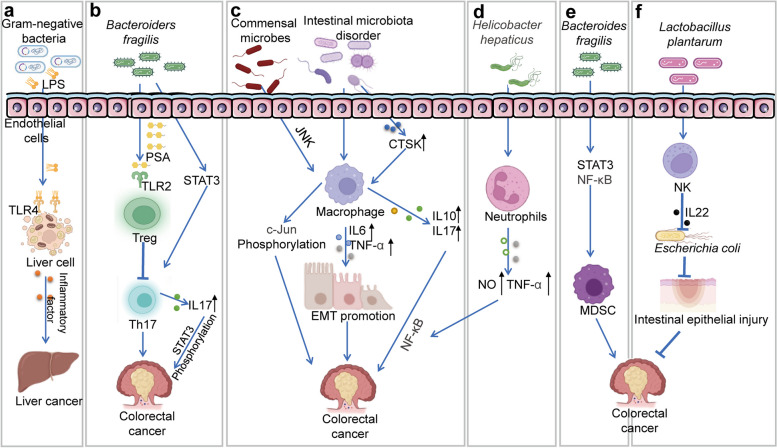
The gut microbiome has a bidirectional regulatory effect on the TME. The gut microbiome affects the occurrence and development of tumours by regulating the functions of various immune cell types (Fig. 2). LPS is a component of the cell wall of gram-negative bacteria in the gut and binds to TLR4 on the surface of liver cells, activating the NF-κB signalling pathway and promoting immune inflammatory reactions, leading to the release of a large number of pro-inflammatory cytokines that promote the occurrence and development of HCC [82]. Liu et al. demonstrated that TLR2 mRNA and protein expression are significantly upregulated in colorectal tumours. Furthermore, TLR2 promotes CRC cell proliferation by activating the PI3K/Akt and NF-κB signal pathways [83]. Additionally, in the gastrointestinal tract, LPS stimulates intestinal epithelial cells through the TLR pathway, induces phosphorylation of IL-1 receptor-associated kinase and mitogen-activated protein kinase (MAPK), promotes IL-8 expression, and induces inflammation [84]. Additionally, enterotoxigenic Bacteroides fragilis activates Th17 cells through the signal transduction and transcription factor 3 (STAT3) signalling pathway. Th17 cells release IL-17, which promotes STAT3 phosphorylation in tumour cells, thereby promoting tumour development [85]. Lactobacillus rhamnosus increases Treg activation, and Tregs suppress the occurrence and development of breast tumours by promoting apoptosis [86]. These findings suggest that some intestinal microbiota affect tumour development by regulating the function and quantity of T cell subsets.
Fig. 2.
Immune pathways. Abbreviations: LPS Lipopolysaccharides, PSA Polysaccharide A, TNF Tumour necrosis factor, NF-κB Nuclear factor-kappa B, FadA, Fusobacterium Nucleatum Adhesin A, BFT Bacteroides fragilis toxin, TLR2/4 Toll-like receptor 2/4, Treg Regulatory T cell, IL-10/17/6/22 Interleukin-10/17/6/22, Th17 Helper T cell 17, STAT3 Signal transduction and transcription factor 3, EMT Epithelial-mesenchymal transition, CTSK Cathepsin K, SAPK Stress-activated protein kinase, NO Nitric oxide, MDSC Myeloid-derived suppressor cell, NK Natural killer cell
Intestinal microbiota also influences the occurrence and development of tumours by affecting tumour-associated macrophages (TAMs) in the TME. Wan et al. found that an intestinal microbiota imbalance leads to TAM activation, which subsequently promotes the secretion of IL-6 and TNF-α. IL-6 and TNF-α accelerate the development of CRC by promoting epithelial–mesenchymal transition (EMT) formation [87]. The intestinal microbiota imbalance also induces secretion of cathepsin K by tumour cells, which activates macrophages through the mammalian target of rapamycin pathway. Cathepsin K stimulates macrophages to secrete IL-10 and IL-17, which promote CRC cell invasion and metastasis through the NF-κB signalling pathway [88]. Additionally, some commensal microbiota stimulates macrophages to promote CRC cell proliferation by activating the stress-activated protein kinase signalling pathway and increasing c-Jun phosphorylation in CRC cells [89].
In addition to macrophages and T cells, intestinal microbiota influences the occurrence and development of tumours through neutrophils, NK cells, and MDSCs. Studies have shown that Helicobacter hepaticus activates neutrophils, which increase the levels of nitric oxide (NO) and TNF-α, and promote CRC development by activating the NF-κB signalling pathway [90]. Erik et al. reported that Bacteroides fragilis directly promote the occurrence of colon tumours by activating MDSCs through STAT3 and NF-κB signalling pathways [85]. Takuya et al. showed that Lactobacillus plantarum-induced NK cells secrete IL-22, which protects against enterotoxigenic Escherichia coli-induced intestinal epithelial barrier damage [91]. In summary, certain intestinal microbiota promotes or inhibits the occurrence of tumours by affecting various immune cells in the TME.
Bacterial toxin pathway
Increasing evidence suggests that pathogenic bacteria induce host cell DNA damage by producing protein toxins or inducing inflammation through interference of apoptosis and cell proliferation, thereby promoting tumour development (Fig. 3) [34]. Cytotoxic dilatoxin (Cdts) are a group of heat-labile protein exotoxins secreted by more than 30 pathogenic gram-negative bacteria [92]. Cdt enters cells through endocytosis, and exerts its cytotoxic effects in the nucleus [93]. Moreover, Cdt induces low levels of single-stranded DNA breaks and high levels of double-stranded DNA breaks (DSBs) in host cells [94]. When the degree of DNA damage exceeds the ability of host cells to repair, cell death or carcinogenic mutations occur. In addition to Cdt, colibactin secreted by B2-group Escherichia coli is also associated with DSB formation [95]. Escherichia coli promotes crosslinking between DDR, single-stranded DNA breaks, and DNA in eukaryotic cells by inducing DSBs [96], which affects the cell cycle and leads to apoptosis or senescence [97].
Fig. 3.
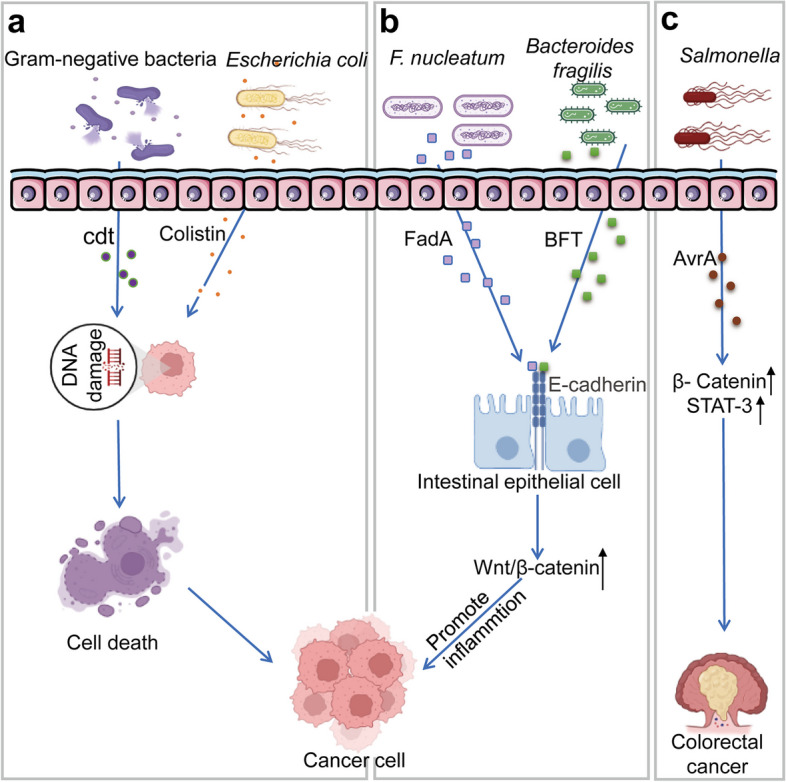
Bacterial toxin pathways. Abbreviations: Cdt Cytolethal distending toxin, FadA Fusobacterium nucleatum adhesin A, BFT Bacteroides fragilis toxin, AvrA Avirulence protein A, STAT3 Signal transduction and transcription factor 3
In addition to causing DNA damage, bacterial genotoxins regulate signalling pathways, thereby promoting tumour initiation and progression. A bacterial cell surface adhesin expressed by Fusobacterium nucleatum known as Fusobacterium nucleatum adhesin A (FadA), and Bacteroides fragilis toxin secreted by enterotoxigenic Bacteroides fragilis, when combined with host intestinal epithelial cell E-cadherin, regulate the canonical Wnt/β-catenin signalling pathway. As a result, the expression of Wnt pathway-related transcription factors, oncogenes, and inflammation-related genes increases, leading to increased intestinal epithelial cell proliferation, barrier disruption, and promotion of mucosal inflammation and carcinogenesis [13, 98]. Lu et al. showed that avirulence protein A (AvrA), a bacterial effector protein produced by Salmonella strains, promotes tumorigenesis through sustained activation of β-catenin and upregulation of STAT-3 [99, 100].
Imbalances in the intestinal microbiota and the occurrence and development of gastrointestinal tumours
Research has shown that dysbiosis of the intestinal microbiota is closely associated with the occurrence and development of gastrointestinal tumours such as CRC, HCC and GC. The intestinal microbiota maintains intestinal stability and health. However, when the intestinal microbiota becomes imbalanced, as characterised by changes in composition or abnormal fluctuations in quantity (Table 2), these changes may increase the risk of developing gastrointestinal tumours. An imbalance in the intestinal microbiota may promote the occurrence and development of gastrointestinal tumours through secretion of toxic metabolites, regulation of immune responses, and secretion of protein toxins (Table 3).
Table 2.
Abundance differences and prognosis of different bacterial communities in gastrointestinal tumours
| Type | Intestinal microbiota | Reference |
|---|---|---|
| Colorectal cancer | In the gut of colorectal cancer patients, the abundance of Oral Peptostreptococcus, Proteus, Fusobacterium nucleatum, Escherichia coli fragilis and Enterococcus faecalis was high, while the abundance of Rothia, Clostridium, Faecal Bacillus and Bifidobacterium was generally reduced | [101, 102] |
| Colorectal cancer | Significant enrichment of Fusobacterium nucleatum could serve as a biomarker for colorectal cancer (AUC: 0.8) and were associated with a poor prognosis | [103] |
| Colorectal cancer | 11 metabolite markers (2-hydroxybutyric acid, gamma-aminobutyric acid, L-alanine, L-aspartic acid, norvaline, ornithine, oxoadipic acid, oxoglutaric acid, palmitoleic acid, phenylacetic acid and pimelic acid) and 6 bacterial species (Fusobacterium nucleatu, Peptostreptococcus anaerobius, Peptostreptococcus micros, Ruminiclostridium inulinivorans, Eikenella corrodens and Xanthomonas. perforin) achieved a higher AUC of 0.9417 | [104] |
| Colorectal cancer | Bacillus fragilis and faecal bacteria were positively correlated with prognosis. Fusobacterium nucleatum and Bacteroides fragilis were negatively correlated with prognosis.a | [105] |
| Gastric cancer | The aerobic bacteria (streptococci and enterococci) and facultative anaerobes (Escherichia, Enterobacter and Streptococcus) in gastric cancer patients increased significantly after gastrectomy because of the increase of intestinal oxygen and the translocation of oral flora | [32] |
| Gastric cancer | After chemotherapy, the PFS of gastric cancer patients with increased R. faeces abundance was longer | [106] |
| Hepatocellular Carcinoma | Butyrate-producing bacteria decreased significantly, such as Ruminococcus, Oscillibacter, Faecalibacterium, Clostridium IV and Coprococcus, while there was an increase in Klebsiella and Haemophilus that produce Lipopolysaccharides | [107] |
| Hepatocellular Carcinoma | The abundance of Blautia was positively correlated with progression-free survival and overall survival in liver cancer patients undergoing chemotherapy | [108] |
| Hepatocellular Carcinoma | Significant enrichment of Bacteroides, Lachnospiracea incertae sedis, and Clostridium XIVa in liver cancer patients was associated with poor clinical prognosis | [109] |
| Oesophageal cancer | The abundance of Streptococcus, Prevobacter, Clostridium, Velococcus and Lactobacillus increased in patients with oesophageal cancer | [110] |
| Oesophageal cancer | Streptococcus and Prevotella are biomarkers of poor prognosis in oesophageal cancer | [111] |
| Pancreatic Cancer | Studies have shown that the abundance of Veillonella atypica, Fusobacterium nucleatum/hwasookii and Alloscardovia omnicolens in pancreatic cancer tissues and stool samples is significantly increased | [112] |
| Pancreatic Cancer | The poor prognosis of pancreatic cancer was associated with the Fusobacterium | [113] |
Abbreviations: AUC Area under the curve
Table 3.
Different roles and possible mechanisms of different bacterial communities in gastrointestinal tumours
| Type | Main related intestinal microbiota | Related mechanisms | Reference |
|---|---|---|---|
| Colorectal Cancer | Bacteria that produce short chain fatty acids (SCFAs) | SCFAs help inhibit protein acetylation and inhibit the proliferation of CRC cells by inhibiting activation of calcineurin and nuclear factor of activated T cells 3 (NFATc3) | [49] |
| Colorectal Cancer | Fusobacterium nucleatum and enterotoxigenic Bacteroides fragilis | Fusobacterium nucleatum secretes FadA and enterotoxigenic Bacteroides fragilis secretes BFT to regulate the Canonical Wnt/β-catenin signalling pathway, causing intestinal inflammation and damaging the intestinal mucosa, inducing the occurrence of CRC. Additionally, cytotoxic dilation toxins can induce cell DNA damage, interfere with cell cycle and apoptosis | [13, 94, 98] |
| Colorectal Cancer | Intestinal microbiota imbalance | Dysbiosis of intestinal microbiota leads to activation of tumour-associated macrophages (TAMs), promoting secretion of IL-6 and TNF-α, which accelerates the development of CRC by inducing epithelial-mesenchymal transition (EMT) | [87] |
| Hepatocellular Carcinoma | Lipopolysaccharides (LPS) in the cell wall of Gramnegative bacteria | LPS binds to TLR4 on liver cells, activating the NF-κB signalling pathway, which in turn activates the immune inflammatory response and promotes the release of a large number of inflammatory factors, ultimately accelerating the development of Hepatocellular Carcinoma | [82] |
| Hepatocellular Carcinoma | Clostridium | Clostridium promote the breakdown of primary bile acids into secondary bile acids. The derivative of secondary bile acid (DCA) induces DNA damage in liver cells and intestinal mucosal cells | [112] |
| Oesophageal cancer | LPS in the cell wall of Gramnegative bacteria | TLR4 expression is significantly increased in the oesophageal cells of patients with oesophageal cancer. LPS binds to TLR4 and activates the NF-κB pathway, promoting the formation of oesophageal cancer. Meanwhile, LPS directly affects the function of the lower oesophageal sphincter, indirectly promoting the development of oesophageal cancer | [114] |
| Oesophageal cancer | Fusobacterium nucleatum | Infection with Fusobacterium nucleatum can induce increased expression of high-mobility group box 1 protein (HMGB1), thus promoting the proliferation of oesophageal cancer cell lines | [115, 116] |
| Pancreatic Cancer |
Microbe-associated molecular patterns (MAMPs) such as lipopolysaccharide and peptidoglycan |
The binding of Toll-like receptors to MAMPs and damage-associated molecular patterns (DAMPs) activates the NF-κB and MAPK pathways, resulting in excessive secretion of pro-inflammatory cytokines that promote the occurrence and development of pancreatic cancer | [117–119] |
Abbreviations: CRC Colorectal cancer, TNF Tumour necrosis factor, NF-κB Nuclear factor-kappa B, FadA Fusobacterium Nucleatum Adhesin A, BFT Bacteroides fragilis toxin, TLR4 Toll-like receptor 4, IL Interleukin, MAPK Mitogen-activated protein kinase
Colorectal cancer
CRC is one of the most common malignant tumours, ranking second in global cancer deaths in 2018 [1]. Compared with healthy individuals, microbiota in CRC patients is primarily composed of pathogenic bacteria associated with metabolic disorders, while the abundance of probiotics, such as butyrate-producing bacteria, is reduced [101]. Oral Peptostreptococcus, Proteus, Fusobacterium nucleatum, Escherichia coli fragilis, and Enterococcus faecalis are high in CRC patients, while the abundance of Rothia, Clostridium, Feacal Bacillus, and Bifidobacterium is generally reduced in CRC patients [101, 102]. Combining metabolic products and bacterial markers can more accurately distinguish CRC patients from healthy individuals. Yu et al. established a random forest model by combining 11 metabolites and six bacterial species, obtaining an area under the curve (AUC) of 0.9417 [104].
In CRC, pathogenic bacteria in the gut mainly promote tumour development through three pathways: regulating immune responses, secreting metabolites, and producing bacterial toxins. In terms of the immune pathway, dysbiosis of intestinal microbiota activates TAMs, which promotes the secretion of IL-6 and TNF-α. In turn, IL-6 and TNF-α accelerate CRC development by promoting EMT [87]. Helicobacter hepaticus activates neutrophils to produce NO and TNF-α, which activate the NF-κB signalling pathway and promote CRC development [90]. Additionally, Bacteroides fragilis directly promotes the development of colorectal tumours through activation of MDSCs via STAT3 and NF-κB signalling pathways [85, 120]. In terms of the genotoxin pathway, Fusobacterium nucleatum produces Fusobacterium nucleatum adhesin A, while enterotoxigenic Bacteroides fragilis secretes Bacteroides fragilis toxin. These toxins bind to E-cadherin on intestinal epithelial cells and regulate the canonical Wnt/β-catenin signalling pathway, causing inflammation, disrupting the intestinal mucosa, and inducing CRC [13, 98]. Cdt induces DNA damage in intestinal cells, interferes with the cell cycle, and inhibits apoptosis [94]. In terms of the metabolite pathway, the metabolites produced by intestinal microbiota also influence the onset and development of CRC. Dysbiosis of intestinal microbiota leads to the production of secondary bile acids that cause DNA damage by generating pro-oxidant molecules such as ROS and nitrogen-containing substances [121]. Furthermore, DCA reduces activation of the FXR signalling pathway in CRC cells, thereby promoting tumour development [38, 39]. SCFAs help inhibit protein acetylation and inhibit the proliferation of CRC cells by inhibiting activation of calcineurin and nuclear factor of activated T cells 3 (NFATc3) [49].
Gastric cancer
GC is one of the most common malignant tumours worldwide, and its mortality ranks fourth [1]. With the development of high-throughput technologies and metagenomic sequencing, and 16S rDNA sequencing, significant differences have been found in intestinal microbiota between GC patients and healthy individuals. After surgery, because of increased oxygen in the gut and translocation of oral microbial communities, there is a significant increase in obligate aerobes (Streptococci and Enterococci) and facultative anaerobes (Escherichia, Enterobacter, and Streptococcus) in GC patients [122–124]. The stomach and intestine are directly connected in the human body. The abundance of multiple dominant components of the intestinal microbiota that are associated with CRC, such as Fusobacterium nucleatum, Atopobium parvulum, and Escherichia coli, is significantly increased in post-gastrectomy patients [122]. Moreover, these patients have a twofold increased risk of CRC compared with patients with other types of cancer [125–127]. Post-gastrectomy patients exhibit increased proliferation of the rectal mucosa, and levels of the risk biomarker for CRC, macrophage migration inhibitory factor (MIF), continues to rise within three years after surgery [128]. Additionally, research has demonstrated that dysbiosis of the intestinal microbiota in post-gastrectomy patients mediates changes in certain intestinal metabolites and metabolic pathways, thereby promoting CRC tumorigenesis [122, 129, 130]. For instance, dysbiosis of the intestinal microbiota in GC patients can indirectly promote CRC tumorigenesis and development through increased secondary bile acid production and decreased short-chain fatty acid production [129].Moreover, in the gastrointestinal tract, nitrite undergoes nitrosation to form NOCs, which can damage DNA in mucosal cells. It also can generate NOC-DNA adduct, which makes damaged cells lose the ability of DNA self-repair, leading to cellular proliferation and differentiation disorders, creating an inflammatory environment and ultimately inducing carcinogenesis [44].
Hepatocellular carcinoma
HCC is the third leading cause of cancer death [1]. Compared with healthy individuals, HCC patients show a significant decrease in bacteria that produce butyrate, such as Ruminococcus, Oscillibacter, Faecalibacterium, Clostridium IV, and Coprococcus, while bacteria that produce LPS, such as Klebsiella and Haemophilus, increase.
The gut and liver are anatomically and physiologically connected, and thus the relationship between the gut and liver is referred to as the gut–liver axis [37]. Dysbiosis of intestinal microbiota can lead to intestinal mucosal damage. Large amounts of bacterial metabolites and intestinal microbiota itself can be transferred to the liver through the portal vein, inducing inflammation and promoting the occurrence and development of HCC [131, 132]. LPS, also known as endotoxin, is a complex polysaccharide molecule that is present on the outer membrane of bacteria. It is primarily found in gram-negative bacteria, such as Escherichia coli and Salmonella [133]. Moreover, when intestinal microbiota dysbiosis occurs, LPS can be translocated to the liver. LPS translocation directly recognizes TLR4 on liver cells and activates NF-κB, leading to enhanced tumour cell proliferation [134]. Additionally, intestinal microbiota dysbiosis promotes the metabolism of primary bile acids into secondary bile acids, such as DCA and lithocholic acid (LCA), which cause liver cell damage and promote HCC [135]. Jang et al. demonstrated that intestinal microbiota, such as Clostridium, promote the breakdown of primary bile acids into secondary bile acids. The derivative of DCA induces DNA damage in liver cells and intestinal mucosal cells, thereby promoting the development of HCC [136]. The liver serves as the metabolic centre for choline, which aids in lipid transport within liver cells and prevents abnormal lipid accumulation in the liver. Choline deficiency is often associated with liver steatosis [37]. Firmicutes and Proteobacteria are capable of metabolizing choline into TMA, which is then transported to the liver and converted into TMAO[60, 61]. TMAO can induce inflammatory responses and oxidative stress, thereby promoting the development of HCC [41, 42, 137]. In summary, the intestinal microbiota promotes the occurrence and progression of HCC through various pathways such as intestinal mucosal damage, LPS translocation, bile acid conversion, and abnormal choline metabolism.
Oesophageal cancer
EC is the eighth most common type of cancer worldwide and the sixth leading cause of cancer deaths [1]. Microbiota diversity and richness in patients with oesophageal squamous cell carcinoma (ESCC) and postoperative patients are significantly lower than those in healthy individuals, and the abundance of Streptococcus, Prevotella, Clostridium, Veillonella, and Lactobacillus increases in EC patients. PICRUSt results showed that ESCC patients had mainly enriched pathways related to cysteine, monosaccharide, and starch metabolism, while the levels of fatty acids, SCFAs, tryptophan, and β-alanine metabolism were lower [110]. In EC, intestinal pathogenic bacteria mainly promote tumour occurrence by regulating immune response pathways. In terms of immune pathways, TLR4 increases in oesophageal epithelial cells of oesophageal cancer patients. And due to the dysbiosis of intestinal flora, LPS binds to TLR4 and activates the NF-κB pathway, promoting the formation of oesophageal adenocarcinoma. LPS directly affects the function of the oesophageal lower oesophageal sphincter, indirectly promoting the development of oesophageal adenocarcinoma [114]. Additionally, enrichment of Fusobacterium in EC patients negatively correlates to patient survival [110]. Fusobacterium induces the expression of proinflammatory cytokines in epithelial cells, including IL-6 and IL-8, which may contribute to dynamic crosstalk between tumour cells and cancer-associated fibroblasts (CAF) in the ESCC TME [115]. Fusobacterium infection also induces the expression of high-mobility group box 1 (HMGB1) protein, which may lead to increased proliferation of ESCC cells [116].
Pancreatic cancer
PC is currently the seventh leading cause of cancer-related death [1]. The abundance of Veillonella atypica, Fusobacterium nucleatum/hwasookii, and Alloscardovia omnicolens significantly increases in PC tissues and faecal samples [112]. The detection rate of genus Clostridium in PC tissues is 8.8%, and Clostridium is associated with a poor prognosis [113, 138]. The liver and pancreas are directly connected to the intestine through the bile duct and pancreatic duct, allowing harmful intestinal microbiota components and metabolites to reach the pancreas via the "intestine-liver-pancreas axis," thereby promoting the development of PC [139]. These findings indicate that intestinal flora in PC patients do not promote the occurrence or development of PC through direct actions, but through chronic low-level activation of the immune system and continuous tumour inflammation, thereby promoting the occurrence of PC [117, 118, 140]. TLRs are a family of pattern recognition receptors (PRR) expressed on the vast majority of immune cells. When intestinal flora is imbalanced, TLRs recognize various microbe-associated molecular patterns (MAMP), such as LPS and peptidoglycans. In addition, TLRs are also activated by damage-associated molecular patterns (DAMP). As downstream targets of these patterns, the NF-κB and MAPK signalling pathways are activated, ultimately leading to excessive secretion of proinflammatory cytokines and further recruitment of proinflammatory cells, thereby promoting the occurrence and development of PC [117–119]. Additionally, blocking TLR activation inhibits the interaction between NF-κB and MAPK signalling pathways, and directly inhibits activation of TLR4 and TLR7[141]. Pushalkar et al. showed that, in a mouse model of PC, depletion of the intestinal microbiota was associated with immunogenic reprogramming of the pancreatic TME, including a reduction in myeloid-derived suppressor cells, an increase in M1 macrophage differentiation, and an increase in CD4 + T cell Th1 differentiation and CD8 + T cell activation [139]. In addition, the deletion of homologous trimer type 1 collagen in the pancreatic cancer gene engineering mouse model changed the tumours microbiome, increased T cell infiltration, enhanced anti PD-1 treatment, and ultimately inhibited tumour growth [142].
Interactions between intestinal microbiota and anticancer therapies
With the maturation of microbial therapeutic technologies such as oral probiotics/prebiotics preparations and FMT, numerous studies have shown that the intestinal microbiota and its products can influence the efficacy and toxicity of chemotherapy and immunotherapy in gastrointestinal tumours [8, 143].
The impact of the intestinal microbiota on chemotherapy efficacy and toxicity
Chemotherapy involves using chemotherapeutic agents to treat cancer. These drugs not only have cytotoxic effects toward cancer cells, but also damage normal cells, leading to toxic side effects such as nausea, vomiting, diarrhoea, hair loss, and immunosuppression. In recent years an increasing number of studies have indicated that the intestinal microbiota can influence the efficacy and toxic side effects of chemotherapy drugs [8, 143]. Research has shown that the intestinal microbiota and its products can enhance the effectiveness of chemotherapy. For example, Lactobacillus and its metabolites can enhance the therapeutic effect of 5-fluorouracil on colon cancer and reverse 5-fluorouracil resistance [144, 145]. Butyrate can promote the efficacy of oxaliplatin by regulating CD8 T cell function in the TME [146]. Synergistic effects have been observed between butyrate and irinotecan, in that butyrate reduces the half maximal inhibitory concentration (IC50) of irinotecan [147]. The anti-tumour efficacy of oxaliplatin and cisplatin is reduced in germ-free mice or mice treated with antibiotics [148]. In addition, the intestinal microbiota and its products can significantly alleviate the toxic side effects of chemotherapy. Numerous studies have shown that the combination of various probiotics and prebiotics can improve chemotherapy-induced mucositis, reduce the frequency of diarrhoea and abdominal pain, and lower the incidence of postoperative complications such as pneumonia, infections and anastomotic leakage [149–151]. Consistent results have also been observed in animal experiments, where mice with CRC that received probiotics showed reduced upregulation of pro-inflammatory cytokines and less 5-fluorouracil induced colonic mucositis [152]. The intestinal microbiota can also influence the toxicity of chemotherapeutic drugs. SN-38 is the active metabolite of irinotecan. The hepatic glucuronidation enzyme can convert the active SN-38 into the inactive SN-38-G, thereby reducing the gastrointestinal toxicity of the drug. However, gut bacteria can secrete β-glucuronidase, which converts SN-38-G back into SN-38, leading to diarrhoea [153, 154]. Therefore, the use of β-glucuronidase inhibitors can alleviate irinotecan-induced toxic side effects [155]. In conclusion, the intestinal microbiota plays an important role in improving chemotherapy efficacy and reducing toxic side effects. It is anticipated that future research will further elucidate the mechanisms underlying the interactions between the intestinal microbiota and chemotherapeutic drugs, providing more precise and effective treatment strategies to improve the quality of life and therapeutic outcomes of chemotherapy patients.
The response of the intestinal microbiota to immunotherapy for gastrointestinal tumours
In addition to chemotherapy, immune checkpoint inhibitors (ICIs) are also an important therapeutic strategy in the treatment of gastrointestinal tumours. ICIs are drugs that restore the immune system's ability to attack tumour cells by inhibiting immune checkpoint function. Numerous studies have shown that the intestinal microbiota can modulate anti-tumour immunity and affect the efficacy of cancer immunotherapy [9]. The intestinal microbiota can improve the efficacy of ICIs by reshaping the TME and modulating immune cell function within the TME, and the combination of the intestinal microbiota and ICIs can reduce the occurrence of immune-related adverse events (irAEs) [9, 143]. Multiple studies support this notion. In a murine model of CpG island methylator phenotype (CIMP) CRC, infection with Enterotoxic Fragile Bacteroides promoted the recruitment of IFN-γ-producing CD8 T cells, thereby enhancing the efficacy of anti-programmed cell death ligand 1 (Anti PD-L1) therapy [156]. Takeshi et al. also showed that a combination of 11 bacterial strains can enhance the anti-tumour efficacy of ICIs and promote the proliferation of IFN-γ-producing CD8 T cells [157]. Marie et al. demonstrated that tumour cells in antibiotic-treated and germ-free mice had no response to CTLA blockade, but the efficacy of anti–CTLA-4 therapy was improved after oral administration of Enterotoxic Fragile Bacteroides, which activated CD4 T cells and promoted dendritic cell maturation [158]. Gopalakrishnan et al. found that patients who responded to PD-1 treatment had a higher abundance of Clostridium in the intestine, as well as exhibiting more T cells within the tumour and higher levels of circulating T cells that kill abnormal cells. Conversely, patients with higher levels of Bifidobacterium had higher levels of Treg cells, myeloid-derived suppressor cells, and a weakened cytokine response, leading to inhibition of the anti-tumour immune response [159]. The intestinal microbiota can synthesise and transform a large number of small, molecular metabolites, which can then diffuse from the intestine to the body, influencing local and systemic anti-tumour immune responses and enhancing ICI efficacy. Disruption of gut barrier function by ICI therapy can lead to an increase in the intestinal microbiota and metabolite translocation. Adenosine is a purine metabolite produced by Bifidobacterium mucosum and Bifidobacterium pseudolongum. Adenosine translocation can activate anti-tumour T cells and enhance ICI efficacy [160]. Synergism between SCFAs and ICIs is currently an area of intense research interest. SCFAs can improve the efficacy of ICI therapy by directly promoting the cytotoxic effects of CD8 T cells, regulating tumour signalling pathways, and reducing the expression of inflammatory factors [9, 161, 162]. In recent years, some studies have indicated that FMT may influence ICI efficacy. Bertrand et al. found that transplantation of faeces from tumour patients who responded to ICI therapy into tumour-bearing mice improved the efficacy of ICI therapy [163]. ICI therapy, which activates the patient's own immune system to attack tumour cells, may also induce the irAEs, for example by inducing immune overactivation and intestinal inflammation. These adverse reactions may limit treatment effectiveness and tolerability. Studies have shown that modulation of the intestinal microbiota can alleviate irAEs associated with ICI therapy, improve treatment tolerance, and enhance treatment efficacy[164]. In patients receiving anti–PD-1 therapy, the abundance of Bacteroides was positively correlated with colitis resistance, while Ruminococcus abundance was associated with a favourable clinical response and reduced incidence of colitis [165]. Furthermore, a meta-analysis and bioinformatics analysis of four published datasets on ICI-treated tumours conducted by John et al. showed that the Actinobacteria phylum and the Lachnospiraceae/Ruminococcaceae families of Firmicutes were associated with a favourable clinical response and a lower incidence of colitis, while Streptococceae spp. were associated with significant irAEs [166]. Sun et al. demonstrated that oral administration of lactobacillus can enhance the expression levels of tight junction protein zona occludens protein 1 (ZO-1) and mucoprotein2 in the colonic mucosa of mice with colitis. This reduces the severity of inflammation and restores intestinal epithelial barrier function[167]. Renga et al. found that indole-3-carboxaldehyde (3-IAld), directly delivered in the intestine, activated the AhR/IL-22 pathway for epithelial barrier function and immune homeostasis during colitis. In addition, 3-IAld also can increase the expression of ZO-1, thereby reducing the severity of inflammation and restoring intestinal epithelial barrier function[168]. In summary, the intestinal microbiota and its products can enhance the efficacy of chemotherapy and immunotherapy for gastrointestinal tumours, while mitigating the adverse reactions caused by treatment. However, current research on the role of gut microbiota and its metabolites in mitigating adverse reactions caused by ICI therapy is primarily focused on exploring correlations, with a lack of in-depth mechanistic studies. Therefore, further mechanistic studies can be considered as a future research direction.
Modulation of the intestinal microbiota as a therapeutic strategy
In-depth study of the mechanisms by which the intestinal flora regulates gastrointestinal tumours has attracted considerable attention in recent years regarding microbial treatment strategies such as oral probiotics, prebiotics formulations, FMT and antibiotic. These approaches aim to regulate intestinal microbiota balance, enhance immune responses, and promote the production of beneficial metabolic products. These effects could potentially play a positive role in the treatment and prevention of gastrointestinal tumours (Table 4) [169, 170].
Table 4.
Therapeutic potential and health benefits of probiotics, prebiotics and FMT
| Type | Probiotics/Prebiotics/FMT/ Antibiotic | Treatment | Reference |
|---|---|---|---|
|
Postoperative patients with colorectal cancer (Placebo group, n = 80 Probiotics group, n = 84) |
Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium lactis, Saccharomyces boulardiisi |
The probiotics group reduced the incidence of postoperative pneumonia, surgical site infection and anastomotic leakage |
[171] |
|
Postoperative patients with colorectal cancer (Placebo group, n = 30 Probiotics group, n = 30) |
Bifidobacterium ongum, Lactobacillus acidophilus, Enterococcus faecalis |
Probiotic therapy reduced the incidence of diarrhea and bacteremia in colorectal cancer patients | [172] |
|
Colorectal model mice (5-fluorouraci group, n = 12 Lcr35group, n = 12 LaBi group, n = 12) |
Lcr35(Lactobacillus casei variety rhamnosus) LaBi (Lactobacillus acidophilus, Bifidobacterium bifidum) |
Oral administration of probiotics Lcr35 and LaBi in mice can improve chemotherapy induced intestinal mucositis and inhibit the pro-inflammatory factor TNF in a mouse model- α、 IL-1 β Upregulation of IL-6 | [173] |
|
Patients with colorectal cancer, n = 34 (Placebo group, n = 15 Treatment group, n = 19) |
Lactobacillus rhamnosus GG, Bifidobacterium lactis Bb12 and inulin enriched with oligofructose | Compared with the placebo group, the content of interferon gamma in the treatment group was higher than that in the placebo group | [174] |
|
Colorectal model mice, n = 20 (Control group, n = 10 GOS group, n = 10) |
Galacto-oligosaccharides (GOS) | Compared with the control group, the GOS group had fewer colorectal tumours, increased Bifidobacteria and short-chain fatty acids and decreased pro-inflammatory flora | [175] |
|
Colorectal model mice, n = 16 (Control group, n = 8 Lactulos group, n = 8) |
Lactulose | Compared with the control group, the number of colorectal tumours in Lactulose group was less, the degree of inflammation and fibrosis was less, the abundance of Lactobacillus intestinalis, Lactobacillus murinus and Bacteroides caecimuris was significantly increased, and the abundance of Mucispirillum schaedleri was significantly decreased | [176] |
|
Colorectal model mice, n = 20 (Control group: FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin), n = 10 Treatment group: FOLFOX + FMT, n = 10) |
FMT | FMT alleviated diarrhea and intestinal mucositis caused by FOLFOX treatment, and restored the damaged faecal-intestinal microflora | [177] |
|
Patients with gastric cancer undergoing chemotherapy, n = 24 (Control group: Autologous FMT, n = 12 Treatment group: Allogeneic FMT, healthy donor, n = 12) |
FMT | FMT from healthy obese donors improved disease control rate and survival rate in patients with metastatic cancer | [178] |
|
Colorectal model mice, n = 39 (Control group, n = 20 Metronidazole group, n = 19) |
Metronidazole | The treatment with metronidazole reduced the abundance of Clostridium and suppressed tumour cell proliferation and tumour growth in a CRC xenograft mouse model | [179] |
|
liver cancer (primary liver cancer cases, n = 1195 matched controls, n = 4640) |
Antibiotic | Ever-use of prescription antibiotics was associated with a slightly increased risk of liver cancer, compared to non-use (OR = 1.22, 95% CI = 1.03–1.45) | [180] |
Abbreviations: FMT, Faecal Microbiota Transplantation; TNF, Tumour necrosis factor; IL, Interleukin
Probiotics as a treatment target
Probiotics are a group of active microorganisms that colonise the gastrointestinal tract and are beneficial to the host. They primarily impact the development of gastrointestinal tumours by inhibiting the growth of carcinogenic bacteria, regulating immune function, and protecting mucosal barrier function [169, 181]. Bacillus, through the secretion of Bacillus lipopeptides, inhibit colonisation of the gastrointestinal tract by Staphylococcus aureus [182]. Lactobacillus reuteri ferments glycerol to produce reuterin, which directly inhibits Clostridium difficile activity [183]. Raffaella et al. demonstrated that cell-free supernatants from Lactobacilli can directly inhibit the activity of pathogenic bacteria. Additionally, the auto-aggregation and co-aggregation properties of Lactobacilli promote their colonisation of the gastrointestinal tract while reducing colonisation by pathogenic bacteria [184, 185]. Therefore, probiotics can inhibit the colonization of pathogenic bacteria, reduce the risk of intestinal infections and inflammation, and prevent the occurrence of CRC.
Probiotics have a regulatory effect on the immune system, improving intestinal immune responses, promoting immune cell differentiation, and inhibiting inflammatory responses.. Ma et al. demonstratde that Lactiplantibacillus plantarum-12, through the inhibition of NF-κB signalling, alleviated the cancer burden and inflammation in mouse model of AOM/DSS-induced CRC [186]. Similarly, Liu et al. conducted study showing that Lactobacillus fermentum ZS40 reduced cancer burden and inflammation in AOM/DSS-induced CRC mouse models by suppressing the expression of inflammatory cytokines TNF-α and IL-1β, as well as key proteins IκBα and p65 in the NF-κB signal pathway [187]. Lactobacillus rhamnosus GG (LGG) decreased tumour burden in the murine gut cancer models by a CD8 T-cell-dependent manner [188]. Liotti et al. demonstrated that LGG restrains the angiogenic potential of colorectal carcinoma cells by activating formyl peptide receptor 1 [189].
An intact intestinal barrier protects the intestines from damage caused by toxins and pathogens. Studies have shown that strains such as Lactobacillus rhamnosus GG and Lactobacillus plantarum ZJ617 can stimulate mucin production and upregulate the expression of tight junction proteins (claudin-1, occludin, occludens-1, occludens-2) to restore the damaged intestinal mucosal barrier [190–193]. The studies conducted by Oh et al. and Ma et al. revealed that Lactobacillus gasseri 505 and Lactiplantibacillus plantarum-12 can upregulate the expression of tight junction proteins (claudin-1, occludin, and ZO-1) in tumour tissues of AOM/DSS-induced CRC mouse models [186, 194]. Probiotics have emerged as a viable microbial therapeutic tool. In recent years, oral probiotic preparations have become an important strategy in the treatment of gastrointestinal tumours. Kotzampassi et al. administered Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium lactis, and Saccharomyces boulardii capsules to patients with CRC (n = 124) and found that, compared with the placebo group, patients in the probiotic group had reduced postoperative pneumonia, surgical site infection, and anastomotic leakage rates, as well as shorter hospital stays [171]. Another study, by Yang et al., showed that the combination of Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis reduced the incidence of diarrhoea and bacteraemia in patients with CRC (n = 60) [172]. In a mouse model of CRC (n = 36), oral administration of the probiotics Lcr35 (Lactobacillus casei variety rhamnosus) and LaBi (Lactobacillus acidophilus and Bifidobacterium bifidum) improved chemotherapy-induced (5-fluorouracil) intestinal mucositis and suppressed upregulation of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 [173]. In conclusion, probiotic therapy is an important treatment strategy in the management of gastrointestinal tumours.
Prebiotics as therapeutic targets
Prebiotics are substances that promote the growth of intestinal probiotics and enhance their activity. They are not degraded by human digestive enzymes and are fermented by the microbiota in the gastrointestinal tract, providing nutritional and energy support for the growth and metabolic activities of probiotics [195]. Prebiotics can promote probiotic colonisation and proliferation, inhibit colonisation by pathogenic bacteria, exert anti-inflammatory effects, and modulate the immune system [196]. Common prebiotics include fructans, galacto-oligosaccharides (GOS), and lactulose [196]. Fructans include fructo-oligosaccharides and inulin. Fructans are beneficial dietary fibres that contribute to increased faecal bulk, alleviate constipation, and promote digestion. Consumption of inulin-type fructans can promote Bifidobacterium growth and enhance satiety [197]. The systematic review conducted by Mazraeh et al. suggested that inulin can improve the viscosity of feces of CRC patients after chemotherapy and increase the content of butyrate in their feces [198]. Moreover, fructans can stimulate cytokine release. Monika et al. demonstrated that, compared with the placebo group, CRC patients who consumed a combination of Lactobacillus rhamnosus GG, Bifidobacterium lactis Bb12, and inulin enriched with oligofructose had increased levels of IFN-γ in their peripheral blood [174]. GOS is an oligosaccharide composed of galactose molecules that can promote Bifidobacterium growth [199]. Javier et al. found that, compared with the control group, rats in the GOS group had fewer colorectal tumours, increased levels of Bifidobacterium and SCFAs, and decreased abundance of pro-inflammatory microbiota components[175]. Additionally, Qamar et al. demonstrated that the combined application of galactooligosaccharides (GOS) and inulin in AOM/DSS-induced CRC mouse models can effectively reduce the occurrence of aberrant crypt foci and promote the production of SCFAs [200]. Lactulose, a galactose-fructose disaccharide, is derived from lactose by heating or isomerisation. Hiraishi et al. showed that, compared with the control group, mice in the lactulose group had fewer colorectal tumours, lower degrees of inflammation and fibrosis, significantly increased abundance of Lactobacillus intestinalis, Lactobacillus murinus and Bacteroides caecimuris, and significantly decreased abundance of Mucispirillum schaedleri [176]. Currently, prebiotics are added to various food products such as biscuits, candies, yogurts, and infant formulas [201]. Increasing the intake of prebiotics in one’s daily diet can support gut health and help prevent and improve certain Symptoms of gastrointestinal tumours.
Faecal microbiota transplantation as a therapeutic strategy.
FMT is a treatment method that involves the transfer of beneficial microbiota from faeces from a healthy individual to the intestines of a patient to restore the balance of the intestinal microbiota and improve treatment efficacy in gastrointestinal cancer patients [202]. According to the 2013 Guidelines for the Treatment of Clostridium difficile, FMT has been approved as a clinical method for treating recurrent Clostridium difficile infection [203]. In recent years, there has been growing interest in the potential of FMT to treat gastrointestinal cancers and other diseases [202]. FMT can improve intestinal mucosal barrier function and alleviate intestinal mucosal inflammation. Additionally, FMT can restore disruption of the intestinal microecological balance in patients, increasing the abundance of beneficial microbiota components and inhibiting the growth of harmful microbiota components. In a mouse model of CRC (n = 20), FMT alleviated diarrhoea and intestinal mucositis caused by FOLFOX (5-fluorouracil, leucovorin, and oxaliplatint) treatment and restored disrupted faecal-intestinal microbiota composition [177] Nicolien et al. treated 24 GC patients undergoing chemotherapy with FMT and found that allogenic FMT (from healthy donors, n = 12) improved disease control and survival rates in patients with GC and metastatic cancer compared with autologous FMT (n = 12) [178]. FMT has shown promise in treating gastrointestinal tumours, but it is important to note that this therapy also carries potential risks and hazards. Potential risks of FMT include transmission of infectious diseases, adverse reactions, and cross-infections due to improper performance of the procedure. Some case reports have documented infections following FMT, including norovirus gastroenteritis [204], Escherichia coli bacteraemia [205] and cytomegalovirus infection [206]. Therefore, when performing FMT, individualised treatment plans should be formulated, and strict adherence to operating protocols is necessary.
Antibiotic as a therapeutic strategy
Antibiotics are natural compounds produced by microorganisms such as bacteria or fungi. Antibiotics can inhibit the growth and reproduction of bacteria and also interfere with cell growth [207]. Numerous studies have shown that antibiotics have the potential to promote apoptosis, antiproliferation, and anti-metastasis in gastrointestinal tumours, making them a potential treatment option [207]. Bullman et al. demonstrated that treatment with metronidazole reduced the abundance of Clostridium and suppressed tumour cell proliferation and tumour growth in a CRC xenograft mouse model [179]. Similarly, Zackular et al. found that treatment with metronidazole, streptomycin, and vancomycin significantly reduced tumour numbers in an AOM/DSS-induced CRC mouse model [208]. In addition, Zhou et al. showed that salinomycin induced apoptosis in cisplatin-resistant CRC cells through the accumulation of reactive oxygen species [209]. However, an increasing body of evidence suggests that while antibiotics can eliminate pathogenic bacteria, they also disrupt the probiotics, leading to gut dysbiosis and promoting the development of gastrointestinal tumours. Clinical evidence has shown that antibiotic treatment reduces the efficacy of immune checkpoint inhibitors and patient survival rates [210, 211]. A case–control study also indicated an increased risk of cancer among individuals who received antibiotic treatment compared to those who did not (OR = 1.22, 95% CI = 1.03–1.45), although the relationship between antibiotic dosage and cancer risk remains uncertain [180]. Corty et al. demonstrated that antibiotic use in PC patients undergoing chemotherapy increased the risk of anaemia, thrombocytopenia, leukopenia, neutropenia, and gastrointestinal adverse events [212]. In summary, antibiotics are a double-edged sword. While they can be used to treat gastrointestinal tumours, they may also disrupt the gut microbiota and promote the development of such tumours. Therefore, it is important to use antibiotics selectively, aiming to eliminate pathogens while minimizing disruption to the normal gut microbiota, in order to reduce side effects and improve treatment efficacy.
Future directions and Conclusion
As genomics technology continues to advance, the correlation between the intestinal microbiota and gastrointestinal tumours has become increasingly clear. However, a series of issues still need to be addressed. Numerous studies have identified elements of the intestinal microbiota as potential early screening biomarkers for gastrointestinal tumours. Yet, most of these studies employed different sequencing methods and had small sample sizes (< 100), making it difficult to obtain consistent results. Therefore, it is necessary to establish a multicentre, multi-omics, standardised, and large-scale intestinal microbiota dataset to develop more accurate, reliable, and sensitive early screening methods. Next, the ways in which microbiota influence tumour development through metabolites, immune regulation, and changes in the TME still require in-depth study. Leveraging a combination of genomics, transcriptomics, metabolomics, proteomics, and basic experimental approaches will help uncover the molecular mechanisms underlying the relationship between microbial communities and gastrointestinal tumours. In addition, the development and optimisation of personalised treatment strategies are crucial future research directions. Although the concept of personalised microbiota-related treatments has been proposed, a series of studies is still required to determine the optimal treatment methods, dosage, and time windows. Long-term clinical observation and follow-up are also necessary to assess the effects and safety of personalised treatment. In summary, future research should focus on the complex interactions between the intestinal microbiota and gastrointestinal tumours, as well as exploring methods and norms for personalised microbiota-related therapies. Interdisciplinary collaboration and advanced technological approaches are required to address these issues and challenges.
The intestinal microbiota plays a crucial role in gastrointestinal tumours, and its composition and function are essential for maintaining intestinal health and preventing tumorigenesis. Gastrointestinal tumours include CRC, GC, PC, EC, and more. The intestinal microbiota differs according to tumour type, and dysbiosis of the intestinal microbiota promotes gastrointestinal tumour development through secretion of harmful metabolites, alteration of immune system regulation, and secretion of protein toxins. The intestinal microbiota has also received much attention as a potential therapeutic target for gastrointestinal tumours. the interaction between the intestinal microbiota and anticancer treatments is a key research focus, as the intestinal microbiota and its metabolites can enhance the effectiveness of gastrointestinal tumour chemotherapy and immunotherapy while reducing the incidence and severity of adverse reactions. Additionally, Oral probiotic and prebiotic formulations can suppress tumour growth through restoration of intestinal homeostasis, alleviation of mucosal inflammation, protection of the intestinal barrier, and regulation of the immune system. FMT, an emerging treatment strategy, improves tumour treatment efficacy by transplanting the intestinal microbiota from healthy donors to patients. In conclusion, the intestinal microbiota plays a crucial role in gastrointestinal tumours, and an in-depth understanding of its composition, function, and mechanism will provide important breakthroughs in the development of individualised treatment strategies, as well as the prevention, treatment and early screening of gastrointestinal tumours.
Acknowledgements
These images are original, but some of the image materials are sourced from the BioRender tool. We thank Emily Crow, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Authors’ contributions
Conception and design: Lizhou Jia and Jikai He. (II) Administrative support: Wei Zhao, Yanqiang Huang and Lizhou Jia. (III) Collection and assembly of data: Jikai He. (IV) Manuscript writing: All authors. (V) Final approval of manuscript: All authors. All authors have read and approved the final manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China (82060438), Natural Science Foundation of Inner Mongolia Autonomous Region (2021MS08113, 2021MS08150, 2022MS08031, 2022LHMS08019), Inner Mongolia Autonomous Region Science and Technology Plan Project (2020GG0273, 2022YFSH0018), Bayannur city Science and Technology Plan Project (K202025), Inner Mongolia Medical University Joint Project(YKD2021LH072, YKD2021LH097), Inner Mongolia Autonomous Region Health Science and Technology Plan Project (202202402, 202201625, 202202405, 202201282).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest regarding the publication of this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jikai He and Haijun Li contributed equally to this work.
Contributor Information
Yanqi Liu, Email: 20010019@immu.edu.cn.
Lizhou Jia, Email: jlz@immu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021;71(3):209–49. 10.3322/caac.21660. [DOI] [PubMed]
- 2.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–60.e16. doi: 10.1053/j.gastro.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14(7):685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulbright LE, Ellermann M, Arthur JC. The microbiome and the hallmarks of cancer. PLoS Pathog. 2017;13(9):e1006480. doi: 10.1371/journal.ppat.1006480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He M, Shi B. Gut microbiota as a potential target of metabolic syndrome: the role of probiotics and prebiotics. Cell Biosci. 2017;7:54. doi: 10.1186/s13578-017-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce-Keller AJ, Salbaum JM, Berthoud HR. Harnessing Gut Microbes for Mental Health: Getting From Here to There. Biol Psychiatry. 2018;83(3):214–223. doi: 10.1016/j.biopsych.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng C, Bai C, Brown TD, Hood LE, Tian Q. Human Gut Microbiota and Gastrointestinal Cancer. Genomics Proteomics Bioinformatics. 2018;16(1):33–49. doi: 10.1016/j.gpb.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023;20(7):429–452. doi: 10.1038/s41571-023-00766-x. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Yuan X, Wang M, He Z, Li H, Wang J, et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. 2022;15(1):47. doi: 10.1186/s13045-022-01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdollahi-Roodsaz S, Abramson SB, Scher JU. The metabolic role of the gut microbiota in health and rheumatic disease: mechanisms and interventions. Nat Rev Rheumatol. 2016;12(8):446–455. doi: 10.1038/nrrheum.2016.68. [DOI] [PubMed] [Google Scholar]
- 11.Ventura M, Turroni F, Motherway MO, MacSharry J, van Sinderen D. Host-microbe interactions that facilitate gut colonization by commensal bifidobacteria. Trends Microbiol. 2012;20(10):467–476. doi: 10.1016/j.tim.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Qiu P, Ishimoto T, Fu L, Zhang J, Zhang Z, Liu Y. The Gut Microbiota in Inflammatory Bowel Disease. Front Cell Infect Microbiol. 2022;12:733992. doi: 10.3389/fcimb.2022.733992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33(9):496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Kuziel GA, Rakoff-Nahoum S. The gut microbiome. Curr Biol. 2022;32(6):R257–R264. doi: 10.1016/j.cub.2022.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76(3):473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science (New York, NY) 2017;357(6351):570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24(2):160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Cui W, Li X, Yang H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front Immunol. 2021;12:761981. doi: 10.3389/fimmu.2021.761981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y, Kamada N, Moon JJ. Oral nanomedicine for modulating immunity, intestinal barrier functions, and gut microbiome. Adv Drug Deliv Rev. 2021;179:114021. doi: 10.1016/j.addr.2021.114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardoso MH, Meneguetti BT, Oliveira-Júnior NG, Macedo MLR, Franco OL. Antimicrobial peptide production in response to gut microbiota imbalance. Peptides. 2022;157:170865. doi: 10.1016/j.peptides.2022.170865. [DOI] [PubMed] [Google Scholar]
- 23.Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–2243. doi: 10.1136/gutjnl-2020-322260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 25.Yamashiro Y. Gut Microbiota in Health and Disease. Ann Nutr Metab. 2017;71(3–4):242–246. doi: 10.1159/000481627. [DOI] [PubMed] [Google Scholar]
- 26.Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149(7):1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science (New York, NY) 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 28.Mou Y, Du Y, Zhou L, Yue J, Hu X, Liu Y, et al. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front Immunol. 2022;13:796288. doi: 10.3389/fimmu.2022.796288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 30.Nikolaieva N, Sevcikova A, Omelka R, Martiniakova M, Mego M, Ciernikova S. Gut Microbiota-MicroRNA Interactions in Intestinal Homeostasis and Cancer Development. Microorganisms. 2022;11(1). 10.3390/microorganisms11010107. [DOI] [PMC free article] [PubMed]
- 31.Phipps O, Quraishi MN, Dickson EA, Steed H, Kumar A, Acheson AG, et al. Differences in the On- and Off-Tumor Microbiota between Right- and Left-Sided Colorectal Cancer. Microorganisms. 2021;9(5). 10.3390/microorganisms9051108. [DOI] [PMC free article] [PubMed]
- 32.Erawijantari PP, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Influence of gastrectomy for gastric cancer treatment on faecal microbiome and metabolome profiles. Gut. 2020;69(8):1404–1415. doi: 10.1136/gutjnl-2019-319188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchta Rosean C, Bostic RR, Ferey JCM, Feng TY, Azar FN, Tung KS, et al. Preexisting Commensal Dysbiosis Is a Host-Intrinsic Regulator of Tissue Inflammation and Tumor Cell Dissemination in Hormone Receptor-Positive Breast Cancer. Cancer Res. 2019;79(14):3662–3675. doi: 10.1158/0008-5472.Can-18-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiorentini C, Carlini F, Germinario EAP, Maroccia Z, Travaglione S, Fabbri A. Gut Microbiota and Colon Cancer: A Role for Bacterial Protein Toxins? International journal of molecular sciences. 2020;21(17). 10.3390/ijms21176201. [DOI] [PMC free article] [PubMed]
- 35.Liu T, Song X, Khan S, Li Y, Guo Z, Li C, et al. The gut microbiota at the intersection of bile acids and intestinal carcinogenesis: An old story, yet mesmerizing. Int J Cancer. 2020;146(7):1780–1790. doi: 10.1002/ijc.32563. [DOI] [PubMed] [Google Scholar]
- 36.Fang Y, Yan C, Zhao Q, Xu J, Liu Z, Gao J, et al. The roles of microbial products in the development of colorectal cancer: a review. Bioengineered. 2021;12(1):720–735. doi: 10.1080/21655979.2021.1889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohtani N, Kawada N. Role of the Gut-Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatology communications. 2019;3(4):456–470. doi: 10.1002/hep4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ocvirk S, O'Keefe SJD. Dietary fat, bile acid metabolism and colorectal cancer. Semin Cancer Biol. 2021;73:347–355. doi: 10.1016/j.semcancer.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Yin Y, Wang M, Gu W, Chen L. Intestine-specific FXR agonists as potential therapeutic agents for colorectal cancer. Biochem Pharmacol. 2021;186:114430. doi: 10.1016/j.bcp.2021.114430. [DOI] [PubMed] [Google Scholar]
- 40.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. Journal of the American Heart Association. 2017;6(9). 10.1161/jaha.117.006347. [DOI] [PMC free article] [PubMed]
- 42.Li T, Chen Y, Gua C, Li X. Elevated Circulating Trimethylamine N-Oxide Levels Contribute to Endothelial Dysfunction in Aged Rats through Vascular Inflammation and Oxidative Stress. Front Physiol. 2017;8:350. doi: 10.3389/fphys.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirji G, Worth A, Bhat SA, El Sayed M, Kannan T, Goldman AR, et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Science immunology. 2022;7(75):eabn0704. 10.1126/sciimmunol.abn0704. [DOI] [PMC free article] [PubMed]
- 44.Koeppel M, Garcia-Alcalde F, Glowinski F, Schlaermann P, Meyer TF. Helicobacter pylori Infection Causes Characteristic DNA Damage Patterns in Human Cells. Cell Rep. 2015;11(11):1703–1713. doi: 10.1016/j.celrep.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 45.Toh JWT, Wilson RB. Pathways of Gastric Carcinogenesis, Helicobacter pylori Virulence and Interactions with Antioxidant Systems, Vitamin C and Phytochemicals. International journal of molecular sciences. 2020;21(17). 10.3390/ijms21176451. [DOI] [PMC free article] [PubMed]
- 46.Han S, Zhuang J, Wu Y, Wu W, Yang X. Progress in Research on Colorectal Cancer-Related Microorganisms and Metabolites. Cancer management and research. 2020;12:8703–8720. doi: 10.2147/cmar.S268943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ijssennagger N, van der Meer R, van Mil SWC. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol Med. 2016;22(3):190–199. doi: 10.1016/j.molmed.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Attene-Ramos MS, Wagner ED, Gaskins HR, Plewa MJ. Hydrogen sulfide induces direct radical-associated DNA damage. Molecular cancer research : MCR. 2007;5(5):455–459. doi: 10.1158/1541-7786.Mcr-06-0439. [DOI] [PubMed] [Google Scholar]
- 49.Zagato E, Pozzi C, Bertocchi A, Schioppa T, Saccheri F, Guglietta S, et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat Microbiol. 2020;5(3):511–524. doi: 10.1038/s41564-019-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 51.Mirzaei R, Afaghi A, Babakhani S, Sohrabi MR, Hosseini-Fard SR, Babolhavaeji K, et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2021;139:111619. 10.1016/j.biopha.2021.111619. [DOI] [PubMed]
- 52.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science (New York, NY) 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim EJ, Kang IJ, Cho HJ, Kim WK, Ha YL, Park JH. Conjugated linoleic acid downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. J Nutr. 2003;133(8):2675–2681. doi: 10.1093/jn/133.8.2675. [DOI] [PubMed] [Google Scholar]
- 54.Metidji A, Omenetti S, Crotta S, Li Y, Nye E, Ross E, et al. The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity. 2018;49(2):353–62.e5. doi: 10.1016/j.immuni.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Islam J, Sato S, Watanabe K, Watanabe T, Ardiansyah, Hirahara K, et al. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. The Journal of nutritional biochemistry. 2017;42:43–50. 10.1016/j.jnutbio.2016.12.019. [DOI] [PubMed]
- 56.Quante M, Bhagat G, Abrams JA, Marache F, Good P, Lee MD, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21(1):36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee HY, Crawley S, Hokari R, Kwon S, Kim YS. Bile acid regulates MUC2 transcription in colon cancer cells via positive EGFR/PKC/Ras/ERK/CREB, PI3K/Akt/IkappaB/NF-kappaB and p38/MSK1/CREB pathways and negative JNK/c-Jun/AP-1 pathway. Int J Oncol. 2010;36(4):941–953. doi: 10.3892/ijo_00000573. [DOI] [PubMed] [Google Scholar]
- 58.Cao H, Xu M, Dong W, Deng B, Wang S, Zhang Y, et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int J Cancer. 2017;140(11):2545–2556. doi: 10.1002/ijc.30643. [DOI] [PubMed] [Google Scholar]
- 59.Fu T, Coulter S, Yoshihara E, Oh TG, Fang S, Cayabyab F, et al. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell. 2019;176(5):1098–112.e18. doi: 10.1016/j.cell.2019.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martínez-del Campo A, Bodea S, Hamer HA, Marks JA, Haiser HJ, Turnbaugh PJ, et al. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. mBio. 2015;6(2). 10.1128/mBio.00042-15. [DOI] [PMC free article] [PubMed]
- 61.Zhu Y, Jameson E, Crosatti M, Schäfer H, Rajakumar K, Bugg TD, et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci USA. 2014;111(11):4268–4273. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu R, Wang Q. Towards understanding brain-gut-microbiome connections in Alzheimer's disease. BMC Syst Biol. 2016;10 Suppl 3(Suppl 3):63. 10.1186/s12918-016-0307-y. [DOI] [PMC free article] [PubMed]
- 63.Kimura H. Production and physiological effects of hydrogen sulfide. Antioxid Redox Signal. 2014;20(5):783–793. doi: 10.1089/ars.2013.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blachier F, Beaumont M, Kim E. Cysteine-derived hydrogen sulfide and gut health: a matter of endogenous or bacterial origin. Curr Opin Clin Nutr Metab Care. 2019;22(1):68–75. doi: 10.1097/mco.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 65.O'Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramasamy S, Singh S, Taniere P, Langman MJ, Eggo MC. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am J Physiol Gastrointest Liver Physiol. 2006;291(2):G288–G296. doi: 10.1152/ajpgi.00324.2005. [DOI] [PubMed] [Google Scholar]
- 67.Motta JP, Flannigan KL, Agbor TA, Beatty JK, Blackler RW, Workentine ML, et al. Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflamm Bowel Dis. 2015;21(5):1006–1017. doi: 10.1097/mib.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 68.Wallace JL, Motta JP, Buret AG. Hydrogen sulfide: an agent of stability at the microbiome-mucosa interface. Am J Physiol Gastrointest Liver Physiol. 2018;314(2):G143–G149. doi: 10.1152/ajpgi.00249.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Cheuk-Hay Lau H, Cheng WY, Yu J. Gut microbiome in colorectal cancer: Clinical diagnosis and treatment. Genomics Proteomics Bioinformatics. 2022 doi: 10.1016/j.gpb.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 71.Saito K, Kuroda A, Arima S, Kawataki M, Tanaka H. Arrhythmogenic right ventricular dysplasia with left ventricular involvement: report of a case. Heart Vessels Suppl. 1990;5:62–64. [PubMed] [Google Scholar]
- 72.Chen Y, Chen YX. Microbiota-Associated Metabolites and Related Immunoregulation in Colorectal Cancer. Cancers. 2021;13(16). 10.3390/cancers13164054. [DOI] [PMC free article] [PubMed]
- 73.Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11(1):4457. doi: 10.1038/s41467-020-18262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kobayashi M, Mikami D, Uwada J, Yazawa T, Kamiyama K, Kimura H, et al. A short-chain fatty acid, propionate, enhances the cytotoxic effect of cisplatin by modulating GPR41 signaling pathways in HepG2 cells. Oncotarget. 2018;9(59):31342–54. 10.18632/oncotarget.25809. [DOI] [PMC free article] [PubMed]
- 75.Ip C, Ip MM, Loftus T, Shoemaker S, Shea-Eaton W. Induction of apoptosis by conjugated linoleic acid in cultured mammary tumor cells and premalignant lesions of the rat mammary gland. Cancer Epidemiol Biomarkers Prev. 2000;9(7):689–696. [PubMed] [Google Scholar]
- 76.Pierre AS, Minville-Walz M, Fèvre C, Hichami A, Gresti J, Pichon L, et al. Trans-10, cis-12 conjugated linoleic acid induced cell death in human colon cancer cells through reactive oxygen species-mediated ER stress. Biochem Biophys Acta. 2013;1831(4):759–768. doi: 10.1016/j.bbalip.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Nava Lauson CB, Tiberti S, Corsetto PA, Conte F, Tyagi P, Machwirth M, et al. Linoleic acid potentiates CD8(+) T cell metabolic fitness and antitumor immunity. Cell Metab. 2023;35(4):633–50.e9. doi: 10.1016/j.cmet.2023.02.013. [DOI] [PubMed] [Google Scholar]
- 78.Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019;178(4):795–806.e12. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science (New York, NY). 2018;360(6391). 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed]
- 81.Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, et al. Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell. 2017;32(5):654–68.e5. doi: 10.1016/j.ccell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 83.Liu YD, Ji CB, Li SB, Yan F, Gu QS, Balic JJ, et al. Toll-like receptor 2 stimulation promotes colorectal cancer cell growth via PI3K/Akt and NF-κB signaling pathways. Int Immunopharmacol. 2018;59:375–383. doi: 10.1016/j.intimp.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 84.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126(4):1054–1070. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 85.Thiele Orberg E, Fan H, Tam AJ, Dejea CM, Destefano Shields CE, Wu S, et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2017;10(2):421–433. doi: 10.1038/mi.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, et al. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2014;135(3):529–540. doi: 10.1002/ijc.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wan G, Xie M, Yu H, Chen H. Intestinal dysbacteriosis activates tumor-associated macrophages to promote epithelial-mesenchymal transition of colorectal cancer. Innate Immun. 2018;24(8):480–489. doi: 10.1177/1753425918801496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li R, Zhou R, Wang H, Li W, Pan M, Yao X, et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019;26(11):2447–2463. doi: 10.1038/s41418-019-0312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, Kundu P, Seow SW, de Matos CT, Aronsson L, Chin KC, et al. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis. 2012;33(6):1231–1238. doi: 10.1093/carcin/bgs137. [DOI] [PubMed] [Google Scholar]
- 90.Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, et al. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci USA. 2009;106(4):1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70(4):631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gargi A, Reno M, Blanke SR. Bacterial toxin modulation of the eukaryotic cell cycle: are all cytolethal distending toxins created equally? Front Cell Infect Microbiol. 2012;2:124. doi: 10.3389/fcimb.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cortes-Bratti X, Chaves-Olarte E, Lagergård T, Thelestam M. Cellular internalization of cytolethal distending toxin from Haemophilus ducreyi. Infect Immun. 2000;68(12):6903–6911. doi: 10.1128/iai.68.12.6903-6911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fedor Y, Vignard J, Nicolau-Travers ML, Boutet-Robinet E, Watrin C, Salles B, et al. From single-strand breaks to double-strand breaks during S-phase: a new mode of action of the Escherichia coli Cytolethal Distending Toxin. Cell Microbiol. 2013;15(1):1–15. doi: 10.1111/cmi.12028. [DOI] [PubMed] [Google Scholar]
- 95.Nougayrède JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science (New York, NY) 2006;313(5788):848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 96.Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A, et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science (New York, NY). 2019;363(6428). 10.1126/science.aar7785. [DOI] [PMC free article] [PubMed]
- 97.Guerra L, Cortes-Bratti X, Guidi R, Frisan T. The biology of the cytolethal distending toxins. Toxins (Basel) 2011;3(3):172–190. doi: 10.3390/toxins3030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11–22. doi: 10.1016/j.critrevonc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 99.Lu R, Wu S, Zhang YG, Xia Y, Zhou Z, Kato I, et al. Salmonella Protein AvrA Activates the STAT3 Signaling Pathway in Colon Cancer. Neoplasia. 2016;18(5):307–316. doi: 10.1016/j.neo.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu R, Bosland M, Xia Y, Zhang YG, Kato I, Sun J. Presence of Salmonella AvrA in colorectal tumor and its precursor lesions in mouse intestine and human specimens. Oncotarget. 2017;8(33):55104–15. 10.18632/oncotarget.19052. [DOI] [PMC free article] [PubMed]
- 101.Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66(1):70–78. doi: 10.1136/gutjnl-2015-309800. [DOI] [PubMed] [Google Scholar]
- 102.Thomas AM, Manghi P, Asnicar F, Pasolli E, Armanini F, Zolfo M, et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med. 2019;25(4):667–678. doi: 10.1038/s41591-019-0405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X, Zhu X, Cao Y, Fang JY, Hong J, Chen H. Fecal Fusobacterium nucleatum for the diagnosis of colorectal tumor: A systematic review and meta-analysis. Cancer Med. 2019;8(2):480–491. doi: 10.1002/cam4.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coker OO, Liu C, Wu WKK, Wong SH, Jia W, Sung JJY, et al. Altered gut metabolites and microbiota interactions are implicated in colorectal carcinogenesis and can be non-invasive diagnostic biomarkers. Microbiome. 2022;10(1):35. doi: 10.1186/s40168-021-01208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei Z, Cao S, Liu S, Yao Z, Sun T, Li Y, et al. Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients' survival? A pilot study on relevant mechanism. Oncotarget. 2016;7(29):46158–72. 10.18632/oncotarget.10064. [DOI] [PMC free article] [PubMed]
- 106.Li N, Bai C, Zhao L, Sun Z, Ge Y, Li X. The Relationship Between Gut Microbiome Features and Chemotherapy Response in Gastrointestinal Cancer. Front Oncol. 2021;11:781697. doi: 10.3389/fonc.2021.781697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68(6):1014–1023. doi: 10.1136/gutjnl-2017-315084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iida N, Mizukoshi E, Yamashita T, Terashima T, Arai K, Seishima J, et al. Overuse of antianaerobic drug is associated with poor postchemotherapy prognosis of patients with hepatocellular carcinoma. Int J Cancer. 2019;145(10):2701–2711. doi: 10.1002/ijc.32339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang H, Ren Z, Gao X, Hu X, Zhou Y, Jiang J, et al. Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in HBV-related hepatocellular carcinoma. Genome Med. 2020;12(1):102. doi: 10.1186/s13073-020-00796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li D, He R, Hou G, Ming W, Fan T, Chen L, et al. Characterization of the Esophageal Microbiota and Prediction of the Metabolic Pathways Involved in Esophageal Cancer. Front Cell Infect Microbiol. 2020;10:268. doi: 10.3389/fcimb.2020.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, Lin Z, Lin Y, Chen Y, Peng XE, He F, et al. Streptococcus and Prevotella are associated with the prognosis of oesophageal squamous cell carcinoma. J Med Microbiol. 2018;67(8):1058–1068. doi: 10.1099/jmm.0.000754. [DOI] [PubMed] [Google Scholar]
- 112.Kartal E, Schmidt TSB, Molina-Montes E, Rodríguez-Perales S, Wirbel J, Maistrenko OM, et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut. 2022;71(7):1359–1372. doi: 10.1136/gutjnl-2021-324755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mitsuhashi K, Nosho K, Sukawa Y, Matsunaga Y, Ito M, Kurihara H, et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget. 2015;6(9):7209–20. 10.18632/oncotarget.3109. [DOI] [PMC free article] [PubMed]
- 114.Zhou J, Sun S, Luan S, Xiao X, Yang Y, Mao C, et al. Gut Microbiota for Esophageal Cancer: Role in Carcinogenesis and Clinical Implications. Front Oncol. 2021;11:717242. doi: 10.3389/fonc.2021.717242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karakasheva TA, Lin EW, Tang Q, Qiao E, Waldron TJ, Soni M, et al. IL-6 Mediates Cross-Talk between Tumor Cells and Activated Fibroblasts in the Tumor Microenvironment. Cancer Res. 2018;78(17):4957–4970. doi: 10.1158/0008-5472.Can-17-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bui FQ, Johnson L, Roberts J, Hung SC, Lee J, Atanasova KR, et al. Fusobacterium nucleatum infection of gingival epithelial cells leads to NLRP3 inflammasome-dependent secretion of IL-1β and the danger signals ASC and HMGB1. Cell Microbiol. 2016;18(7):970–981. doi: 10.1111/cmi.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Q, Jin M, Liu Y, Jin L. Gut Microbiota: Its Potential Roles in Pancreatic Cancer. Front Cell Infect Microbiol. 2020;10:572492. doi: 10.3389/fcimb.2020.572492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zambirinis CP, Pushalkar S, Saxena D, Miller G. Pancreatic cancer, inflammation, and microbiome. Cancer J. 2014;20(3):195–202. doi: 10.1097/ppo.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9(1):57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 120.Peng C, Ouyang Y, Lu N, Li N. The NF-κB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front Immunol. 2020;11:1387. doi: 10.3389/fimmu.2020.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009;15(27):3329–3340. doi: 10.3748/wjg.15.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palleja A, Kashani A, Allin KH, Nielsen T, Zhang C, Li Y, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016;8(1):67. doi: 10.1186/s13073-016-0312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Celiker H. A new proposed mechanism of action for gastric bypass surgery: Air hypothesis. Med Hypotheses. 2017;107:81–89. doi: 10.1016/j.mehy.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 124.Ilhan ZE, DiBaise JK, Isern NG, Hoyt DW, Marcus AK, Kang DW, et al. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. Isme j. 2017;11(9):2047–2058. doi: 10.1038/ismej.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eom BW, Lee HJ, Yoo MW, Cho JJ, Kim WH, Yang HK, et al. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol. 2008;98(2):106–110. doi: 10.1002/jso.21027. [DOI] [PubMed] [Google Scholar]
- 126.Saito S, Hosoya Y, Togashi K, Kurashina K, Haruta H, Hyodo M, et al. Prevalence of synchronous colorectal neoplasms detected by colonoscopy in patients with gastric cancer. Surg Today. 2008;38(1):20–25. doi: 10.1007/s00595-007-3567-8. [DOI] [PubMed] [Google Scholar]
- 127.Watanabe M, Kochi M, Fujii M, Kaiga T, Mihara Y, Funada T, et al. Dual primary gastric and colorectal cancer: is the prognosis better for synchronous or metachronous? Am J Clin Oncol. 2012;35(5):407–410. doi: 10.1097/COC.0b013e318218585a. [DOI] [PubMed] [Google Scholar]
- 128.Kant P, Sainsbury A, Reed KR, Pollard SG, Scott N, Clarke AR, et al. Rectal epithelial cell mitosis and expression of macrophage migration inhibitory factor are increased 3 years after Roux-en-Y gastric bypass (RYGB) for morbid obesity: implications for long-term neoplastic risk following RYGB. Gut. 2011;60(7):893–901. doi: 10.1136/gut.2010.230755. [DOI] [PubMed] [Google Scholar]
- 129.Hull MA, Markar SR, Morris EJA. Cancer risk after bariatric surgery - is colorectal cancer a special case? Nat Rev Gastroenterol Hepatol. 2018;15(11):653–654. doi: 10.1038/s41575-018-0070-1. [DOI] [PubMed] [Google Scholar]
- 130.Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes Surg. 2017;27(4):917–925. doi: 10.1007/s11695-016-2399-2. [DOI] [PubMed] [Google Scholar]
- 131.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15(7):397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14(9):527–539. doi: 10.1038/nrgastro.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Afroz R, Tanvir EM, Tania M, Fu J, Kamal MA, Khan MA. LPS/TLR4 Pathways in Breast Cancer: Insights into Cell Signalling. Curr Med Chem. 2022;29(13):2274–2289. doi: 10.2174/0929867328666210811145043. [DOI] [PubMed] [Google Scholar]
- 134.French SW, Oliva J, French BA, Li J, Bardag-Gorce F. Alcohol, nutrition and liver cancer: role of Toll-like receptor signaling. World J Gastroenterol. 2010;16(11):1344–1348. doi: 10.3748/wjg.v16.i11.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Payne CM, Weber C, Crowley-Skillicorn C, Dvorak K, Bernstein H, Bernstein C, et al. Deoxycholate induces mitochondrial oxidative stress and activates NF-kappaB through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis. 2007;28(1):215–222. doi: 10.1093/carcin/bgl139. [DOI] [PubMed] [Google Scholar]
- 136.Jang ES, Yoon JH, Lee SH, Lee SM, Lee JH, Yu SJ, et al. Sodium taurocholate cotransporting polypeptide mediates dual actions of deoxycholic acid in human hepatocellular carcinoma cells: enhanced apoptosis versus growth stimulation. J Cancer Res Clin Oncol. 2014;140(1):133–144. doi: 10.1007/s00432-013-1554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68(2):359–370. doi: 10.1136/gutjnl-2018-316307. [DOI] [PubMed] [Google Scholar]
- 138.Maekawa T, Fukaya R, Takamatsu S, Itoyama S, Fukuoka T, Yamada M, et al. Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem Biophys Res Commun. 2018;506(4):962–969. doi: 10.1016/j.bbrc.2018.10.169. [DOI] [PubMed] [Google Scholar]
- 139.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018;8(4):403–416. doi: 10.1158/2159-8290.Cd-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med. 2015;212(12):2077–2094. doi: 10.1084/jem.20142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209(9):1671–1687. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou Y, Zhang L, Xie J, Han J. Type I collagen homotrimers: A potential cancer-specific interventional target. 2022;1(2):e16. 10.1002/mog2.16.
- 143.Fernandes MR, Aggarwal P, Costa RGF, Cole AM, Trinchieri G. Targeting the gut microbiota for cancer therapy. Nat Rev Cancer. 2022;22(12):703–722. doi: 10.1038/s41568-022-00513-x. [DOI] [PubMed] [Google Scholar]
- 144.An J, Ha EM. Combination Therapy of Lactobacillus plantarum Supernatant and 5-Fluouracil Increases Chemosensitivity in Colorectal Cancer Cells. J Microbiol Biotechnol. 2016;26(8):1490–1503. doi: 10.4014/jmb.1605.05024. [DOI] [PubMed] [Google Scholar]
- 145.An J, Ha EM. Lactobacillus-derived metabolites enhance the antitumor activity of 5-FU and inhibit metastatic behavior in 5-FU-resistant colorectal cancer cells by regulating claudin-1 expression. J Microbiol. 2020;58(11):967–977. doi: 10.1007/s12275-020-0375-y. [DOI] [PubMed] [Google Scholar]
- 146.He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021;33(5):988–1000.e7. doi: 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 147.Encarnação JC, Pires AS, Amaral RA, Gonçalves TJ, Laranjo M, Casalta-Lopes JE, et al. Butyrate, a dietary fiber derivative that improves irinotecan effect in colon cancer cells. J Nutr Biochem. 2018;56:183–192. doi: 10.1016/j.jnutbio.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 148.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science (New York, NY) 2013;342(6161):967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang Y, Xia Y, Chen H, Hong L, Feng J, Yang J, et al. The effect of perioperative probiotics treatment for colorectal cancer: short-term outcomes of a randomized controlled trial. Oncotarget. 2016;7(7):8432–40. 10.18632/oncotarget.7045. [DOI] [PMC free article] [PubMed]
- 150.Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014;33(5):761–767. doi: 10.1016/j.clnu.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 151.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science (New York, NY) 2017;357(6356):1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mi H, Dong Y, Zhang B, Wang H, Peter CCK, Gao P, et al. Bifidobacterium Infantis Ameliorates Chemotherapy-Induced Intestinal Mucositis Via Regulating T Cell Immunity in Colorectal Cancer Rats. Cell Physiol Biochem. 2017;42(6):2330–2341. doi: 10.1159/000480005. [DOI] [PubMed] [Google Scholar]
- 153.Cheng KW, Tseng CH, Tzeng CC, Leu YL, Cheng TC, Wang JY, et al. Pharmacological inhibition of bacterial β-glucuronidase prevents irinotecan-induced diarrhea without impairing its antitumor efficacy in vivo. Pharmacol Res. 2019;139:41–49. doi: 10.1016/j.phrs.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 154.Yamamoto M, Kurita A, Asahara T, Takakura A, Katono K, Iwasaki M, et al. Metabolism of irinotecan and its active metabolite SN-38 by intestinal microflora in rats. Oncol Rep. 2008;20(4):727–730. [PubMed] [Google Scholar]
- 155.Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science (New York, NY) 2010;330(6005):831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.DeStefano Shields CE, White JR, Chung L, Wenzel A, Hicks JL, Tam AJ, et al. Bacterial-Driven Inflammation and Mutant BRAF Expression Combine to Promote Murine Colon Tumorigenesis That Is Sensitive to Immune Checkpoint Therapy. Cancer Discov. 2021;11(7):1792–1807. doi: 10.1158/2159-8290.Cd-20-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 158.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (New York, NY) 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (New York, NY) 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science (New York, NY) 2020;369(6510):1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- 161.Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A, et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. 2021;12(1):4077. doi: 10.1038/s41467-021-24331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Santoni M, Piva F, Conti A, Santoni A, Cimadamore A, Scarpelli M, et al. Re: Gut Microbiome Influences Efficacy of PD-1-based Immunotherapy Against Epithelial Tumors. Eur Urol. 2018;74(4):521–522. doi: 10.1016/j.eururo.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 163.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (New York, NY) 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 164.Dora D, Bokhari SMZ, Aloss K, Takacs P, Desnoix JZ, Szklenárik G, et al. Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy. International journal of molecular sciences. 2023;24(3). 10.3390/ijms24032769. [DOI] [PMC free article] [PubMed]
- 165.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2019;30(12):2012. doi: 10.1093/annonc/mdz224. [DOI] [PubMed] [Google Scholar]
- 166.McCulloch JA, Davar D, Rodrigues RR, Badger JH, Fang JR, Cole AM, et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med. 2022;28(3):545–556. doi: 10.1038/s41591-022-01698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sun S, Xu X, Liang L, Wang X, Bai X, Zhu L, et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front Immunol. 2021;12:777665. doi: 10.3389/fimmu.2021.777665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Renga G, Nunzi E, Pariano M, Puccetti M, Bellet MM, Pieraccini G, et al. Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite. Journal for immunotherapy of cancer. 2022;10(3). 10.1136/jitc-2021-003725. [DOI] [PMC free article] [PubMed]
- 169.Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene. 2020;39(26):4925–4943. doi: 10.1038/s41388-020-1341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li HY, Zhou DD, Gan RY, Huang SY, Zhao CN, Shang A, et al. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients. 2021;13(9). 10.3390/nu13093211. [DOI] [PMC free article] [PubMed]
- 171.Kotzampassi K, Stavrou G, Damoraki G, Georgitsi M, Basdanis G, Tsaousi G, et al. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind. Placebo-Controlled Study World J Surg. 2015;39(11):2776–2783. doi: 10.1007/s00268-015-3071-z. [DOI] [PubMed] [Google Scholar]
- 172.Gong J, Bai T, Zhang L, Qian W, Song J, Hou X. Inhibition effect of Bifidobacterium longum, Lactobacillus acidophilus, Streptococcus thermophilus and Enterococcus faecalis and their related products on human colonic smooth muscle in vitro. PLoS ONE. 2017;12(12):e0189257. doi: 10.1371/journal.pone.0189257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yeung CY, Chan WT, Jiang CB, Cheng ML, Liu CY, Chang SW, et al. Amelioration of Chemotherapy-Induced Intestinal Mucositis by Orally Administered Probiotics in a Mouse Model. PLoS ONE. 2015;10(9):e0138746. doi: 10.1371/journal.pone.0138746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Roller M, Clune Y, Collins K, Rechkemmer G, Watzl B. Consumption of prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis has minor effects on selected immune parameters in polypectomised and colon cancer patients. Br J Nutr. 2007;97(4):676–684. doi: 10.1017/s0007114507450292. [DOI] [PubMed] [Google Scholar]
- 175.Fernández J, Moreno FJ, Olano A, Clemente A, Villar CJ, Lombó F. A Galacto-Oligosaccharides Preparation Derived From Lactulose Protects Against Colorectal Cancer Development in an Animal Model. Front Microbiol. 2018;9:2004. doi: 10.3389/fmicb.2018.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hiraishi K, Zhao F, Kurahara LH, Li X, Yamashita T, Hashimoto T, et al. Lactulose Modulates the Structure of Gut Microbiota and Alleviates Colitis-Associated Tumorigenesis. Nutrients. 2022;14(3). 10.3390/nu14030649. [DOI] [PMC free article] [PubMed]
- 177.Chang CW, Lee HC, Li LH, Chiang Chiau JS, Wang TE, Chuang WH, et al. Fecal Microbiota Transplantation Prevents Intestinal Injury, Upregulation of Toll-Like Receptors, and 5-Fluorouracil/Oxaliplatin-Induced Toxicity in Colorectal Cancer. International journal of molecular sciences. 2020;21(2). 10.3390/ijms21020386. [DOI] [PMC free article] [PubMed]
- 178.de Clercq NC, van den Ende T, Prodan A, Hemke R, Davids M, Pedersen HK, et al. Fecal Microbiota Transplantation from Overweight or Obese Donors in Cachectic Patients with Advanced Gastroesophageal Cancer: A Randomized, Double-blind, Placebo-Controlled. Phase II Study Clin Cancer Res. 2021;27(13):3784–3792. doi: 10.1158/1078-0432.Ccr-20-4918. [DOI] [PubMed] [Google Scholar]
- 179.Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science (New York, NY) 2017;358(6369):1443–1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang B, Hagberg KW, Chen J, Sahasrabuddhe VV, Graubard BI, Jick S, et al. Associations of antibiotic use with risk of primary liver cancer in the Clinical Practice Research Datalink. Br J Cancer. 2016;115(1):85–89. doi: 10.1038/bjc.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science (New York, NY). 2021;371(6536). 10.1126/science.abc4552. [DOI] [PMC free article] [PubMed]
- 182.Piewngam P, Zheng Y, Nguyen TH, Dickey SW, Joo HS, Villaruz AE, et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature. 2018;562(7728):532–537. doi: 10.1038/s41586-018-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mills JP, Rao K, Young VB. Probiotics for prevention of Clostridium difficile infection. Curr Opin Gastroenterol. 2018;34(1):3–10. doi: 10.1097/mog.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zawistowska-Rojek A, Kośmider A, Stępień K, Tyski S. Adhesion and aggregation properties of Lactobacillaceae strains as protection ways against enteropathogenic bacteria. Arch Microbiol. 2022;204(5):285. doi: 10.1007/s00203-022-02889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Campana R, van Hemert S, Baffone W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017;9:12. doi: 10.1186/s13099-017-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ma F, Sun M, Song Y, Wang A, Jiang S, Qian F, et al. Lactiplantibacillus plantarum-12 Alleviates Inflammation and Colon Cancer Symptoms in AOM/DSS-Treated Mice through Modulating the Intestinal Microbiome and Metabolome. Nutrients. 2022;14(9). 10.3390/nu14091916. [DOI] [PMC free article] [PubMed]
- 187.Liu J, Wang S, Yi R, Long X, Zhao X. Effect of Lactobacillus fermentum ZS40 on the NF-κB signaling pathway in an azomethane-dextran sulfate sodium-induced colon cancer mouse model. Front Microbiol. 2022;13:953905. doi: 10.3389/fmicb.2022.953905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Owens JA, Saeedi BJ, Naudin CR, Hunter-Chang S, Barbian ME, Eboka RU, et al. Lactobacillus rhamnosus GG Orchestrates an Antitumor Immune Response. Cell Mol Gastroenterol Hepatol. 2021;12(4):1311–1327. doi: 10.1016/j.jcmgh.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liotti F, Marotta M, Sorriento D, Pagliuca C, Caturano V, Mantova G, et al. Probiotic Lactobacillus rhamnosus GG (LGG) restrains the angiogenic potential of colorectal carcinoma cells by activating a proresolving program via formyl peptide receptor 1. Mol Oncol. 2022;16(16):2959–2980. doi: 10.1002/1878-0261.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cui Y, Liu L, Dou X, Wang C, Zhang W, Gao K, et al. Lactobacillus reuteri ZJ617 maintains intestinal integrity via regulating tight junction, autophagy and apoptosis in mice challenged with lipopolysaccharide. Oncotarget. 2017;8(44):77489–99. 10.18632/oncotarget.20536. [DOI] [PMC free article] [PubMed]
- 191.Wang L, Cao H, Liu L, Wang B, Walker WA, Acra SA, et al. Activation of epidermal growth factor receptor mediates mucin production stimulated by p40, a Lactobacillus rhamnosus GG-derived protein. J Biol Chem. 2014;289(29):20234–20244. doi: 10.1074/jbc.M114.553800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9(3):804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 193.Martín R, Chamignon C, Mhedbi-Hajri N, Chain F, Derrien M, Escribano-Vázquez U, et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci Rep. 2019;9(1):5398. doi: 10.1038/s41598-019-41738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Oh NS, Lee JY, Kim YT, Kim SH, Lee JH. Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut microbes. 2020;12(1):1785803. doi: 10.1080/19490976.2020.1785803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 196.Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl Microbiol Biotechnol. 2022;106(2):505–521. doi: 10.1007/s00253-021-11646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hiel S, Bindels LB, Pachikian BD, Kalala G, Broers V, Zamariola G, et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am J Clin Nutr. 2019;109(6):1683–1695. doi: 10.1093/ajcn/nqz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mazraeh R, Azizi-Soleiman F, Jazayeri S, Noori SMA. Effect of inulin-type fructans in patients undergoing cancer treatments: A systematic review. Pakistan journal of medical sciences. 2019;35(2):575–80. 10.12669/pjms.35.2.701. [DOI] [PMC free article] [PubMed]
- 199.Guarino MPL, Altomare A, Emerenziani S, Di Rosa C, Ribolsi M, Balestrieri P, et al. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients. 2020;12(4). 10.3390/nu12041037. [DOI] [PMC free article] [PubMed]
- 200.Qamar TR, Syed F, Nasir M, Rehman H, Zahid MN, Liu RH, et al. Novel Combination of Prebiotics Galacto-Oligosaccharides and Inulin-Inhibited Aberrant Crypt Foci Formation and Biomarkers of Colon Cancer in Wistar Rats. Nutrients. 2016;8(8). 10.3390/nu8080465. [DOI] [PMC free article] [PubMed]
- 201.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. 2019;8(3). 10.3390/foods8030092. [DOI] [PMC free article] [PubMed]
- 202.Chen D, Wu J, Jin D, Wang B, Cao H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int J Cancer. 2019;145(8):2021–2031. doi: 10.1002/ijc.32003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kaźmierczak-Siedlecka K, Daca A, Fic M, van de Wetering T, Folwarski M, Makarewicz W. Therapeutic methods of gut microbiota modification in colorectal cancer management - fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut microbes. 2020;11(6):1518–1530. doi: 10.1080/19490976.2020.1764309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol. 2013;108(8):1367. doi: 10.1038/ajg.2013.164. [DOI] [PubMed] [Google Scholar]
- 205.Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn's disease and recurrent Clostridium difficile infection. J Crohns Colitis. 2014;8(3):252–253. doi: 10.1016/j.crohns.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 206.Hohmann EL, Ananthakrishnan AN, Deshpande V. Case Records of the Massachusetts General Hospital. Case 25–2014. A 37-year-old man with ulcerative colitis and bloody diarrhea. N Engl J Med. 2014;371(7):668–75. 10.1056/NEJMcpc1400842. [DOI] [PubMed]
- 207.Gao Y, Shang Q, Li W, Guo W, Stojadinovic A, Mannion C, et al. Antibiotics for cancer treatment: A double-edged sword. J Cancer. 2020;11(17):5135–5149. doi: 10.7150/jca.47470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zackular JP, Baxter NT, Chen GY, Schloss PD. Manipulation of the Gut Microbiota Reveals Role in Colon Tumorigenesis. mSphere. 2016;1(1). 10.1128/mSphere.00001-15. [DOI] [PMC free article] [PubMed]
- 209.Zhou J, Li P, Xue X, He S, Kuang Y, Zhao H, et al. Salinomycin induces apoptosis in cisplatin-resistant colorectal cancer cells by accumulation of reactive oxygen species. Toxicol Lett. 2013;222(2):139–145. doi: 10.1016/j.toxlet.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 210.Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol. 2019;5(12):1774–1778. doi: 10.1001/jamaoncol.2019.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Huang XZ, Gao P, Song YX, Xu Y, Sun JX, Chen XW, et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology. 2019;8(12):e1665973. doi: 10.1080/2162402x.2019.1665973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Corty RW, Langworthy BW, Fine JP, Buse JB, Sanoff HK, Lund JL. Antibacterial Use Is Associated with an Increased Risk of Hematologic and Gastrointestinal Adverse Events in Patients Treated with Gemcitabine for Stage IV Pancreatic Cancer. Oncologist. 2020;25(7):579–584. doi: 10.1634/theoncologist.2019-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.