Abstract
Background
Pancreatoduodenectomy (PD) is associated with significant postoperative morbidity. Surgeons should have a sound understanding of the potential complications for consenting and benchmarking purposes. Furthermore, preoperative identification of high-risk patients can guide patient selection and potentially allow for targeted prehabilitation and/or individualized treatment regimens. Using a large multicentre cohort, this study aimed to calculate the incidence of all PD complications and identify risk factors.
Method
Data were extracted from the Recurrence After Whipple’s (RAW) study, a retrospective cohort study of PD outcomes (29 centres from 8 countries, 2012–2015). The incidence and severity of all complications was recorded and potential risk factors for morbidity, major morbidity (Clavien–Dindo grade > IIIa), postoperative pancreatic fistula (POPF), post-pancreatectomy haemorrhage (PPH) and 90-day mortality were investigated.
Results
Among the 1348 included patients, overall morbidity, major morbidity, POPF, PPH and perioperative death affected 53 per cent (n = 720), 17 per cent (n = 228), 8 per cent (n = 108), 6 per cent (n = 84) and 4 per cent (n = 53), respectively. Following multivariable tests, a high BMI (P = 0.007), an ASA grade > II (P < 0.0001) and a classic Whipple approach (P = 0.005) were all associated with increased overall morbidity. In addition, ASA grade > II patients were at increased risk of major morbidity (P < 0.0001), and a raised BMI correlated with a greater risk of POPF (P = 0.001).
Conclusion
In this multicentre study of PD outcomes, an ASA grade > II was a risk factor for major morbidity and a high BMI was a risk factor for POPF. Patients who are preoperatively identified to be high risk may benefit from targeted prehabilitation or individualized treatment regimens.
Our multicentre study of pancreatoduodenectomy outcomes calculated the incidence and severity of all recorded complications (all classified using Clavien–Dindo and international definitions). Overall morbidity, major morbidity, pancreatic fistula, postoperative haemorrhage and 90-day mortality rates were 53 per cent, 17 per cent, 11 per cent, 6 per cent and 4 per cent, respectively. A high BMI and a high ASA grade correlated with the adverse perioperative outcomes studied.
Introduction
Pancreatoduodenectomy (PD) remains the only curative-intent treatment option for fit patients with a resectable pancreatic head adenocarcinoma (PDAC), ampullary adenocarcinoma (AA) or distal cholangiocarcinoma (CC). It is a major operation that is associated with high morbidity1 and mortality2 rates. Cancer recurrence is common after PD, particularly in patients with PDAC, and only around one in five achieves 5-year survival3,4.
Due to the complexities of the resection, several general and procedure-specific complications may occur after PD. Pancreatic surgeons must have a sound understanding of the incidence of these, as this will guide the consenting process and allow them to benchmark their own complication rates when auditing. The preoperative identification of high-risk patients allows for targeted prehabilitation and/or individualized treatment regimens, which may lead to subtle gains. For example, selected patients might benefit from an intensive preoperative diet and exercise plan5, and others might benefit from neoadjuvant chemotherapy6. While the latter is not currently recommended in those with resectable disease, high-risk patients who may have their adjuvant treatment delayed or omitted as a result of a serious complication may stand to benefit from this approach6.
Several studies7,8 have recently reported on the procedure-specific outcomes of PD, but no large studies have compiled a robust complication profile. Using a large multicentre cohort, this study aimed to calculate the incidence and severity of all PD complications and identify risk factors for overall morbidity, major morbidity, postoperative pancreatic fistula (POPF), post-pancreatectomy haemorrhage (PPH) and 90-day mortality.
Methods
Data were extracted from the Recurrence After Whipple’s (RAW) study (clinicaltrials.gov identifier: NCT04596865). This study was approved by North West–Greater Manchester South Research Ethics Committee (20/NW/0397) and adhered to the standards laid down in the Declaration of Helsinki (revised 2013). The RAW study included patients that underwent PD for histologically confirmed PDAC, AA or distal CC at one of 29 participating centres between 1 June 2012 and 31 May 2015. The study involved 19 centres from the UK, three from Spain, two from Italy, and one from Australia, Austria, Mexico, Pakistan and Sudan (see Supplementary material for full details). The end date of 31 May 2015 was selected so that 5-year follow-up data were available for all included patients. However, the current study did not utilize the 5-year follow-up data as it focussed on perioperative outcomes.
Each participating unit collected data from physical and electronic patient records and uploaded this onto a purpose-built electronic REDCap database (v11.0.3, Nashville, TN, USA). Details of the following were collected: patient demographics, co-morbidities, preoperative imaging and staging, neoadjuvant therapy, preoperative blood results, type of PD, postoperative management and complications, histology results, and adjuvant treatment. Specific data were collected on the following complications: postoperative pancreatic fistula (POPF), bile leak, gastro-jejunal (G-J) anastomotic leak, PPH, delayed gastric emptying (DGE), acute kidney injury, cardiac arrhythmia, chest infection, cholangitis, chyle leak, Clostridium difficile infection, ileus, intra-abdominal collection, liver abscess, myocardial infarction, pancreatic necrosis, pancreatitis, portal vein/superior mesenteric vein thrombosis, sepsis of unknown origin, splenic vein thrombosis, surgical site infection (SSI), urinary tract infection, deep vein thrombosis and pulmonary embolism (Supplementary material).
G-J leak was categorized as grade A (no change to patient management), grade B (requiring active therapeutic intervention other than surgery) or grade C (requiring reoperation). Postoperative pancreatitis was diagnosed on imaging only; serum amylase/lipase levels were not used for this purpose. All other complications were diagnosed based on predefined clinical and/or radiological criteria. An unplanned return to theatre was defined as any emergency reoperation within the index admission. An unplanned readmission was defined as any emergency presentation within 30 days of discharge that included at least one overnight stay.
The patients were compared according to binary groupings: complications versus no complications, major morbidity (at least one Clavien–Dindo grade ≥ IIIa complication) versus no major morbidity, POPF versus no POPF, PPH versus no PPH, 90-day mortality versus no 90-day mortality.
Statistical methods
Categorical data are presented as frequency counts and associated percentages, and continuous data are presented as mean (s.d.) or median with interquartile range (i.q.r.). Means were compared using Student’s t-test, distributions using the Mann–Whitney U test, and percentages using Pearson’s χ2 test or Fisher’s exact test. Following the univariable tests, each of the outcomes in turn was fitted using logistic regression to all the key demographic variables (age, sex), baseline co-morbidities (diabetes, cardiovascular disease, respiratory disease), key risk groups (ASA grade, preoperative nodes on CT) and salient procedural features (classic Whipple versus pylorus-preserving approach, anastomosis type). P < 0.05 was considered significant. Analyses were performed using Microsoft Excel (v2103, Redmond, WA, USA) and GraphPad Prism (v9.3.1, San Diego, CA, USA).
Results
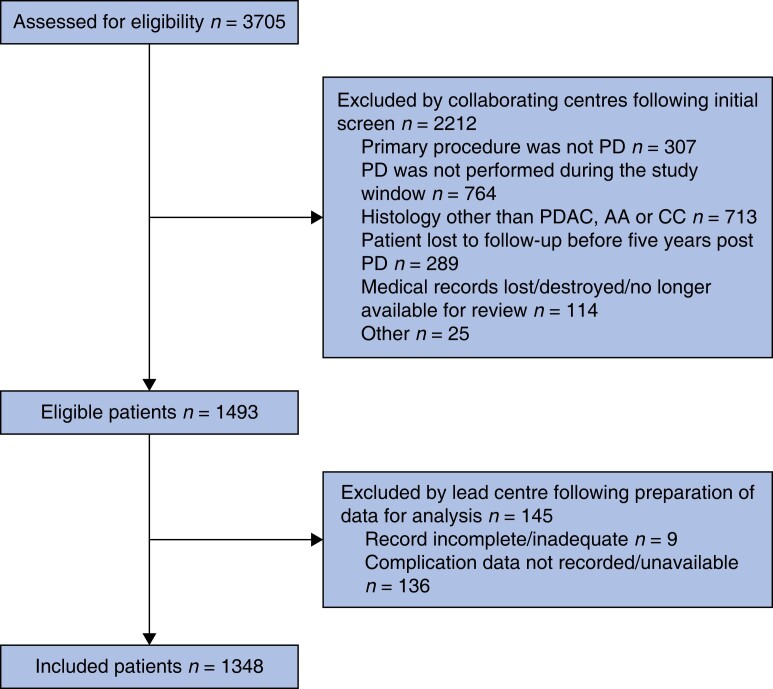
A total of 3705 patient records were assessed for eligibility and 2212 were excluded as they did not meet the inclusion criteria (Fig. 1). Nine records were removed as they were incomplete and 136 records were removed as they did not include data on complications. The final analysis included 1348 patients. Table 1 displays the demographics, preoperative, intraoperative and postoperative details of those included. The mean patient age was 66 years (s.d.: 9.8 years), and 42 per cent (n = 587) were female. The mean BMI was 25.5 kg/m2 (s.d.: 4.4 kg/m2) and the ASA grade was > II in 34 per cent (n = 467) of cases. A classic Whipple was performed in 49 per cent (n = 660) of patients and 51 per cent (n = 685) underwent a pylorus-preserving (PPPD) approach. A pancreato-jejunostomy (P-J) was fashioned in 81 per cent (n = 1064) of patients and 19 per cent (n = 246) received a pancreato-gastrostomy (P-G). The median length of stay was 13 days (i.q.r.: 10–20 days) and 6 per cent (n = 74) of patients had an unplanned urgent reintervention. The 30-day readmission rate was 10 per cent (n = 134) and the 90-day mortality rate was 4 per cent (n = 51). Regarding postoperative histology, 792 (59 per cent), 363 (27 per cent) and 192 (14 per cent) patients had PDAC, AA and CC, respectively.
Fig. 1.
Cohort flow diagram. AA, ampullary carcinoma; CC, cholangiocarcinoma; PD, pancreatoduodenectomy; PDAC, pancreatic ductal adenocarcinoma
Table 1.
Demographic, preoperative, operative and postoperative details
| Total no. of patients included | 1348 | |
| Age (years), mean (s.d.) | 66.0 (9.8) | |
| Female gender | 587 (42.4) | |
| BMI (kg/m2), mean (s.d.) | 25.5 (4.4) | Unknown/not recorded: 561 (40.5) |
| Preoperative co-morbidities | ||
| Diabetes | 277 (20.6) | Unknown/not recorded: 38* |
| Cardiovascular | 590 (42.6) | |
| Respiratory | 142 (10.5) | |
| Preoperative biliary stent | 875 (63.3) | Unknown/not recorded: 2* |
| Neoadjuvant chemotherapy received | 61 (4.6) | |
| Preoperative blood tests, median (i.q.r.) | ||
| Bilirubin (µmol/l) | 42 (10-52) | Unknown/not recorded: 2 (0.1) |
| Albumin (g/l) | 10 (32-42) | Unknown/not recorded: 100 (7.4) |
| Neutrophils (×109/l) | 2.8 (3.7-6.5) | Unknown/not recorded: 28 (2.1) |
| Lymphocytes (×109/l) | 1.2 (1.3-2.5) | Unknown/not recorded: 28 (2.1) |
| ASA grade > II | 467 (33.7) | Unknown/not recorded: 116* |
| Positive nodes on preoperative CT | 324 (27.7) | Unknown/not recorded: 177* |
| Type of PD performed | Classic Whipple: 660 (49.1) Pylorus-preserving PD: 685 (50.9) |
Unknown/not recorded: 3* |
| Pancreatic anastomosis | P-J: 1064 (81.2) P-G: 246 (18.8) |
Unknown/not recorded: 38* |
| Concomitant venous resection | 205 (15.5) | Unknown/not recorded: 28* |
| Concomitant arterial resection | 25 (1.9) | Unknown/not recorded: 29* |
| Intraoperative blood transfusion | 164 (18.1) | Unknown/not recorded: 442* |
| Unplanned return to theatre | 74 (5.5) | |
| Length of stay (days), median (i.q.r.) | 10 (10-20) | Unknown/not recorded: 70 (5.2) |
| 30-day unplanned readmission | 134 (10.0) | Unknown/not recorded: 5 (0.4) |
| 90-day mortality | 51 (4.0) | |
| Postoperative histology | ||
| PDAC | 792 (58.8) | |
| AA | 364 (27.0) | |
| CC | 192 (14.2) |
Values are n (%) unless otherwise indicated. AA, ampullary adenocarcinoma; CC, cholangiocarcinoma; CT, computed tomography; HDU, high dependency unit; PD, pancreatoduodenectomy; PDAC, pancreatic ductal adenocarcinoma; P-G, pancreato-gastrostomy; P-J, pancreato-jejunostomy. *Not included in percentages.
A total of 1340 complications were reported; 72 per cent (n = 968) were Clavien–Dindo grade I–II, 18 per cent (n = 240) were grade III, 7 per cent (n = 79) were grade IV, and 4 per cent (n = 53) were grade V (Table 2). Postoperative pancreatic fistula (excluding biochemical leaks), PPH, chyle leak, bile leak and G-J leak affected 8 per cent (n = 108), 6 per cent (n = 84), 4 per cent (n = 47), 3 per cent (n = 44) and 2 per cent (n = 20), respectively. Other notable complications included intra-abdominal collection (160; 12 per cent), SSI (115; 9 per cent) and chest infection (96; 7 per cent). In total, 720 patients (53 per cent) experienced at least one complication. When patients who experienced a complication were compared to those who did not (Table 3), the mean BMI was higher in the former (25.9 versus 25.0 kg/m2, P = 0.003), as was the number of patients with preoperative cardiovascular disease (47 per cent versus 40 per cent, P = 0.006) or an ASA grade > II (32 per cent versus 24 per cent, P = 0.002). The median preoperative serum albumin was lower in those who experienced morbidity (38 versus 39 g/l, P = 0.004). A higher proportion of patients who experienced complications had undergone a classic Whipple (versus PPPD, 53 per cent versus 44 per cent, P < 0.0001) or a P-G (versus P-J, 21 per cent versus 15 per cent, P < 0.0001). The histological diagnosis was similar between the groups that developed complications and the groups that did not; PDAC (54 per cent versus 59 per cent, P = 0.06), AA (29 per cent versus 27 per cent, P = 0.2) and CC (16 per cent versus 14 per cent, P = 0.3).
Table 2.
The postoperative complications recorded classified by their Clavien–Dindo grade
| Postoperative complications n (%) | Incidence by Clavien–Dindo grade | ||||||
|---|---|---|---|---|---|---|---|
| I | II | IIIa | IIIb | IVa | IVb | V | |
| Postoperative pancreatic fistula: 108 (15.6) | 68 | 91 | 22 | 14 | 5 | 5 | 5 |
| Biochemical leak: 102 (7.6) | |||||||
| Grade B and grade C POPF: 108 (8.0) | |||||||
| Grade B: 85 | |||||||
| Grade C: 23 | |||||||
| Bile leak: 44 (3.3) | 12 | 9 | 8 | 7 | 3 | 2 | 3 |
| Grade A: 13 | |||||||
| Grade B: 18 | |||||||
| Grade C: 13 | |||||||
| Gastrojejunal leak: 20 (1.5) | 2 | 8 | 2 | 5 | 1 | 0 | 2 |
| Grade A: 6 | |||||||
| Grade B: 8 | |||||||
| Grade C: 6 | |||||||
| Postpancreatectomy haemorrhage: 84 (6.2) | 11 | 21 | 14 | 17 | 7 | 3 | 11 |
| Grade A: 17 | |||||||
| Grade B: 40 | |||||||
| Grade C: 27 | |||||||
| Delayed gastric emptying: 167 (12.4) | 50 | 97 | 8 | 9 | 0 | 2 | 1 |
| Grade A: 73 | |||||||
| Grade B: 59 | |||||||
| Grade C: 35 | |||||||
| Acute kidney injury: 33 (2.4) | 10 | 9 | 0 | 0 | 8 | 2 | 4 |
| Cardiac arrhythmia: 32 (2.4) | 8 | 19 | 0 | 1 | 3 | 0 | 1 |
| Chest infection: 96 (7.1) | 10 | 70 | 3 | 0 | 11 | 1 | 1 |
| Cholangitis: 6 (0.4) | 0 | 5 | 0 | 0 | 1 | 0 | 0 |
| Chyle leak: 47 (3.5) | 24 | 17 | 6 | 0 | 0 | 0 | 0 |
| Clostridium difficile infection: 9 (0.7) | 0 | 9 | 0 | 0 | 0 | 0 | 0 |
| Ileus: 37 (2.7) | 15 | 20 | 0 | 2 | 0 | 0 | 0 |
| Intra-abdominal collection: 160 (11.9) | 21 | 64 | 52 | 16 | 2 | 1 | 4 |
| Liver abscess: 13 (1.0) | 1 | 6 | 6 | 0 | 0 | 0 | 0 |
| Myocardial infarction: 3 (0.2) | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| Pancreatic necrosis: 2 (0.1) | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pancreatitis: 5 (0.4) | 2 | 2 | 0 | 0 | 1 | 0 | 0 |
| PV/SMV thrombosis: 16 (1.2) | 1 | 6 | 1 | 3 | 1 | 0 | 4 |
| Sepsis of unknown origin: 19 (1.4) | 1 | 13 | 0 | 0 | 4 | 0 | 1 |
| Splenic vein thrombosis: 3 (0.2) | 0 | 2 | 0 | 0 | 0 | 0 | 1 |
| Surgical site infection: 115 (8.5) | 52 | 57 | 4 | 1 | 1 | 0 | 0 |
| Urinary tract infection: 20 (1.5) | 1 | 19 | 0 | 0 | 0 | 0 | 0 |
| Deep vein thrombosis: 6 (0.4) | 0 | 5 | 0 | 1 | 0 | 0 | 0 |
| Pulmonary embolism: 15 (1.1) | 4 | 10 | 0 | 0 | 0 | 0 | 1 |
| Other complication: 177 (13.1) | 34 | 79 | 16 | 21 | 9 | 4 | 14 |
| Sum of complications (n = 1340) by Clavien–Dindo grade | 328 (24.5%) | 640 (47.8%) | 142 (10.6%) | 98 (7.3%) | 59 (4.4%) | 20 (1.5%) | 53 (4.0%) |
PV, portal vein; SMV, superior mesenteric vein.
Table 3.
Univariable analysis: comparing patients by selected outcomes
| Variable | Any complication (n = 720) | No complication (n = 628) | P |
|---|---|---|---|
| Age (years), mean (s.d.) | 66.4 (9.6) | 65.5 (10.1) | 0.103 |
| Age ≥80 years | 46 (6.4) | 36 (5.7) | 0.649 |
| Female sex | 301 (41.8) | 286 (45.5) | 0.169 |
| BMI (kg/m2), mean (s.d.) | 25.9 (4.5) | 25.0 (4.2) | 0.0028* |
| BMI ≥30 kg/m2 | 82 (17.7) | 40 (11.1) | 0.010* |
| Preoperative co-morbidities | |||
| Diabetes | 144 (20.0) | 133 (21.2) | 0.593 |
| Cardiovascular | 340 (47.2) | 250 (39.8) | 0.006* |
| Respiratory | 86 (11.9) | 56 (8.9) | 0.071 |
| Preoperative biliary stent | 471 (65.4) | 404 (64.3) | 0.700 |
| Preoperative blood tests, median (i.q.r.) | |||
| Bilirubin, µmol/l | 20 (44) | 21 (41) | 0.800 |
| Albumin, g/l | 38 (12) | 39 (9) | 0.004* |
| Neutrophils ×109/l | 4.9 (2.7) | 4.9 (3.0) | 0.649 |
| Lymphocytes ×109/l | 1.8 (1.2) | 1.8 (1.1) | 0.298 |
| ASA grade > II | 214 (32.3) | 138 (24.2) | 0.002* |
| Positive nodes on preoperative CT | 176 (27.5) | 148 (27.8) | 0.948 |
| Classic Whipple versus PPPD | 382 (53.1) | 278 (44.3) | 0.0015* |
| P-J anastomosis versus P-G | 553 (76.2) | 511 (81.4) | 0.004* |
| Variable | Major morbidity (n = 228) | No major morbidity (n = 1120) | P |
|---|---|---|---|
| Age (years), mean (s.d.) | 66.0 (9.6) | 66.0 (9.9) | 0.905 |
| Age ≥80 years | 13 (5.7) | 69 (6.2) | 0.880 |
| Female sex | 96 (42.1) | 491 (43.8) | 0.660 |
| BMI (kg/m2), mean (s.d.) | 25.5 (3.9) | 25.5 (4.9) | 0.990 |
| BMI ≥30 kg/m2 | 21 (13.8) | 100 (14.9) | 0.801 |
| Preoperative co-morbidities | |||
| Diabetes | 46 (20.2) | 231 (20.6) | 0.929 |
| Cardiovascular | 101 (44.3) | 489 (43.7) | 0.884 |
| Respiratory | 21 (9.2) | 121 (10.8) | 0.554 |
| Preoperative biliary stent | 141 (61.8) | 734 (65.5) | 0.288 |
| Preoperative blood tests, median (i.q.r.) | |||
| Bilirubin, µmol/l | 19 (52) | 21 (41) | 0.573 |
| Albumin, g/l | 37 (13) | 38 (10) | 0.456 |
| Neutrophils ×109/l | 5.0 (2.7) | 4.9 (2.9) | 0.650 |
| Lymphocytes ×109/l | 1.8 (1.4) | 1.8 (1.1) | 0.463 |
| ASA grade > II | 81 (39.3) | 271 (26.4) | 0.0003* |
| Positive nodes on preoperative CT | 56 (27.9) | 268 (27.6) | 0.931 |
| Classic Whipple versus PPPD | 123 (54.0) | 537 (47.9) | 0.110 |
| P-J anastomosis versus P-G | 176 (77.2) | 888 (79.3) | 0.477 |
| Variable | Grade B/C POPF (n = 142) | No grade B/C POPF (n = 1206) | P |
|---|---|---|---|
| Age (years), mean (s.d.) | 65.6 (10.5) | 66.0 (9.8) | 0.595 |
| Age ≥80 years | 11 (7.7) | 71 (5.9) | 0.355 |
| Female sex | 45 (31.7) | 542 (44.9) | 0.003* |
| BMI (kg/m2), mean (s.d.) | 27.1 (4.5) | 25.3 (4.3) | 0.0002* |
| BMI ≥30 kg/m2 | 21 (20.1) | 100 (13.8) | 0.070 |
| Preoperative co-morbidities | |||
| Diabetes | 23 (16.2) | 254 (21.1) | 0.119 |
| Cardiovascular | 71 (50.0) | 519 (43.0) | 0.128 |
| Respiratory | 21 (14.8) | 121 (10.0) | 0.084 |
| Preoperative biliary stent | 95 (66.9) | 780 (64.7) | 0.643 |
| Preoperative blood tests, median (i.q.r.) | |||
| Bilirubin (µmol/l) | 19 (54) | 21 (42) | 0.992 |
| Albumin (g/l) | 37 (11) | 38 (10) | 0.828 |
| Neutrophils (×109/l) | 4.9 (3.1) | 4.9 (2.7) | 0.831 |
| Lymphocytes (×109/l) | 1.9 (1.35) | 1.8 (1.35) | 0.195 |
| ASA grade >II | 51 (37.8) | 301 (27.4) | 0.0152* |
| Positive nodes on preoperative CT | 35 (27.3) | 289 (27.7) | 1.00 |
| Classic Whipple versus PPPD | 76 (53.5) | 584 (48.5) | 0.287 |
| P-J anastomosis versus P-G | 111 (78.7) | 953 (81.5) | 0.425 |
| Variable | PPH (n = 84) | No PPH (n = 1264) | P |
|---|---|---|---|
| Age (years), mean (s.d.) | 65.0 (10.0) | 66.0 (9.8) | 0.330 |
| Age ≥80 years | 3 (3.6) | 79 (6.3) | 0.477 |
| Female sex | 36 (42.9) | 551 (43.6) | 0.910 |
| BMI (kg/m2), mean (s.d.) | 25.5 (3.9) | 25.5 (4.4) | 0.898 |
| BMI ≥30 kg/m2 | 9 (14.5) | 112 (14.7) | 1.00 |
| Preoperative co-morbidities | |||
| Diabetes | 11 (13.1) | 266 (21.1) | 0.094 |
| Cardiovascular | 30 (35.7) | 560 (44.3) | 0.140 |
| Respiratory | 6 (7.1) | 136 (10.8) | 0.361 |
| Preoperative biliary stent | 48 (57.1) | 827 (65.5) | 0.125 |
| Preoperative blood tests, median (i.q.r.) | |||
| Bilirubin (µmol/l) | 33.5 (122.5) | 20 (40) | 0.0219* |
| Albumin (g/l) | 36 (11.5) | 38 (10) | 0.474 |
| Neutrophils (×109/l) | 5.0 (2.7) | 4.9 (2.8) | 0.707 |
| Lymphocytes (×109/l) | 1.8 (1.4) | 1.8 (1.1) | 0.985 |
| ASA grade > II | 35 (44.3) | 317 (27.5) | 0.002* |
| Positive nodes on preoperative CT | 30 (37.5) | 294 (26.9) | 0.0515 |
| Classic Whipple versus PPPD | 48 (57.8) | 612 (48.5) | 0.113 |
| P-J anastomosis versus P-G | 60 (71.4) | 1004 (81.9) | 0.0211* |
| Variable | 90-day mortality (n = 51) | Alive at 90 days (n = 1297) | P |
|---|---|---|---|
| Age (years), mean (s.d.) | 69.0 (10.6) | 65.8 (9.8) | 0.0219* |
| Age ≥80 years | 6 (11.8) | 76 (5.9) | 0.122 |
| Female sex | 22 (43.1) | 565 (43.6) | 1.00 |
| BMI (kg/m2), mean (s.d.) | 25.5 (5.0) | 25.5 (4.4) | 0.929 |
| BMI ≥30 kg/m2 | 6 (11.8) | 115 (14.5) | 0.452 |
| Preoperative co-morbidities | |||
| Diabetes | 15 (29.4) | 262 (20.2) | 0.114 |
| Cardiovascular | 26 (51.0) | 564 (43.5) | 0.315 |
| Respiratory | 2 (3.9) | 140 (10.8) | 0.160 |
| Preoperative biliary stent | 31 (60.8) | 844 (65.2) | 0.551 |
| Preoperative blood tests, median (i.q.r.) | |||
| Bilirubin (µmol/l) | 17 (39) | 21 (43) | 0.287 |
| Albumin (g/l) | 35 (11) | 38 (10) | 0.233 |
| Neutrophils (×109/l) | 5.1 (3.5) | 4.9 (2.7) | 0.706 |
| Lymphocytes (×109/l) | 1.8 (0.8) | 1.8 (1.2) | 0.896 |
| ASA grade > II | 18 (40.0) | 334 (28.2) | 0.093 |
| Positive nodes on preoperative CT | 16 (35.6) | 308 (27.4) | 0.236 |
| Classic Whipple versus PPPD | 25 (49.0) | 635 (49.1) | 1.00 |
| P-J anastomosis versus P-G | 43 (89.6) | 1021 (80.9) | 0.185 |
Values are n (%) unless otherwise indicated. Major morbidity includes any Clavien–Dindo grade ≥ IIIa complication. Statistical methods: Student’s t-test: age, BMI, Fisher’s exact test: sex, co-morbidities, preoperative biliary stent, ASA grade, positive nodes on preoperative CT, classic Whipple versus PPPD, P-J versus P-G, Mann–Whitney U test: blood tests. Where data were missing (Table 1), patients were excluded from the relevant subanalysis. CR-POPF, clinically relevant postoperative pancreatic fistula; PD, pancreatoduodenectomy; P-G, pancreato-gastrostomy; PPH, post-pancreatectomy haemorrhage; P-J, pancreato-jejunostomy; PP, pylorus preserving. *Denotes statistical significance.
A total of 228 patients (17 per cent) experienced a Clavien–Dindo grade ≥ IIIa complication. This group were more often ASA grade > II (45 per cent versus 36 per cent, P = 0.0006). Patients with POPF were more often male (68 per cent versus 55 per cent, P = 0.003) or ASA grade > II (38 per cent versus 27 per cent, P = 0.02) and had a higher mean BMI (27.1 versus 25.3 kg/m2, P = 0.0002). Those who experienced PPH had a higher median preoperative serum bilirubin (34 versus 20 µmol/l, P = 0.02), were more often ASA grade > II (44 per cent versus 26 per cent, P = 0.002) and were more likely to have received a P-G (29 per cent versus 18 per cent, P = 0.02). Patients who died within 90 days were significantly older (mean difference: 3.1 years, P = 0.02) but no other risk factors were identified. Among the major morbidity group, the numbers of patients with AA (33 per cent versus 27 per cent, P = 0.07) and CC (18 per cent versus 14 per cent, P = 0.1) were like that of the entire cohort. PDAC was less common among those who developed serious complications (49 per cent versus 59 per cent, P = 0.04).
Results from the multivariable analyses are displayed in Table 4. Factors associated with higher complication rate were increasing BMI (OR: 1.1, P = 0.007), ASA grade > II (OR: 2.2, P < 0.0001) and a classic Whipple procedure (OR: 1.2, P = 0.01). Only ASA grade > II correlated with major morbidity (OR: 2.2, P < 0.0001) and only increasing BMI (OR: 1.1, P = 0.001) correlated with POPF. ASA grade > II (OR: 2.5, P = 0.002) and positive nodes on preoperative imaging (OR: 2.1, P = 0.01) were associated with an increased risk of PPH. Preoperative diabetes (OR: 0.4, P = 0.045) and a P-J anastomosis (OR: 0.5, P = 0.03) were associated with a decreased risk of PPH. Interestingly, none of the studied variables had a significant relationship with 90-day mortality.
Table 4.
Multivariable analysis: comparing patients by selected outcomes
| Variable | Any complication OR (s.d.) | P |
|---|---|---|
| Age | 1.009 (0.008) | 0.261 |
| Female sex (versus male) | 0.918 (0.146) | 0.589 |
| BMI | 1.054 (0.020) | 0.007* |
| Preoperative diabetes | 0.772 (0.157) | 0.203 |
| Preoperative cardiovascular disease | 1.017 (0.170) | 0.918 |
| Preoperative respiratory disease | 1.596 (0.449) | 0.097 |
| ASA grade > II | 2.208 (0.404) | <0.00001* |
| Positive nodes on preoperative CT | 0.835 (0.149) | 0.313 |
| Classic Whipple (versus PPPD) | 1.589 (0.259) | 0.005* |
| P-J anastomosis (versus P-G) | 0.742 (0.154) | 0.150 |
| Variable | Major morbidity OR (s.d.) | P |
|---|---|---|
| Age | 0.991 (0.010) | 0.385 |
| Female sex (versus male) | 1.036 (0.202) | 0.856 |
| BMI | 1.005 (0.023) | 0.826 |
| Preoperative diabetes | 0.972 (0.238) | 0.907 |
| Preoperative cardiovascular disease | 0.839 (0.180) | 0.412 |
| Preoperative respiratory disease | 0.544 (0.188) | 0.079 |
| ASA grade > II | 2.159 (0.429) | <0.00001* |
| Positive nodes on preoperative CT | 1.220 (0.269) | 0.365 |
| Classic Whipple (versus PPPD) | 1.245 (0.258) | 0.290 |
| P-J anastomosis (versus P-G) | 1.155 (0.280) | 0.552 |
| Variable | Grade B/C POPF OR (s.d.) | P |
|---|---|---|
| Age | 1.005 (0.013) | 0.671 |
| Female sex (versus male) | 0.763 (0.181) | 0.255 |
| BMI | 1.093 (0.028) | 0.001* |
| Preoperative diabetes | 0.611 (0.189) | 0.111 |
| Preoperative cardiovascular disease | 1.087 (0.274) | 0.739 |
| Preoperative respiratory disease | 1.269 (0.428) | 0.480 |
| ASA grade > II | 1.096 (0.273) | 0.712 |
| Positive nodes on preoperative CT | 1.600 (0.401) | 0.061 |
| Classic Whipple (versus PPPD) | 0.819 (0.201) | 0.414 |
| P-J anastomosis (versus P-G) | 1.072 (0.315) | 0.813 |
| Variable | PPH OR (s.d.) | P |
|---|---|---|
| Age | 0.983 (0.014) | 0.224 |
| Female sex (versus male) | 1.032 (0.291) | 0.911 |
| BMI | 1.002 (0.032) | 0.954 |
| Preoperative diabetes | 0.397 (0.183) | 0.045* |
| Preoperative cardiovascular disease | 0.638 (0.203) | 0.158 |
| Preoperative respiratory disease | 0.392 (0.242) | 0.129 |
| ASA grade > II | 2.470 (0.709) | 0.002* |
| Positive nodes on preoperative CT | 2.065 (0.603) | 0.013* |
| Classic Whipple (versus PPPD) | 1.718 (0.511) | 0.069 |
| P-J anastomosis (versus P-G) | 0.510 (0.155) | 0.027* |
| Variable | 90-day mortality OR (s.d.) | P |
|---|---|---|
| Age | 1.029 (0.025) | 0.242 |
| Female sex (versus male) | 1.436 (0.608) | 0.393 |
| BMI | 1.007 (0.049) | 0.889 |
| Preoperative diabetes | 1.307 (0.636) | 0.583 |
| Preoperative cardiovascular disease | 1.140 (0.519) | 0.774 |
| Preoperative respiratory disease | 0.317 (0.329) | 0.268 |
| ASA grade > II | 1.043 (0.470) | 0.925 |
| Positive nodes on preoperative CT | 1.969 (0.863) | 0.122 |
| Classic Whipple (versus PPPD) | 1.193 (0.523) | 0.687 |
| P-J anastomosis (versus P-G) | 2.488 (1.626) | 0.163 |
Major morbidity includes any Clavien-Dindo grade ≥ IIIa complication. Where data were missing (Table 1), patients were excluded from the relevant subanalysis. CR-POPF, clinically relevant postoperative pancreatic fistula; PD, pancreatoduodenectomy; P-G, pancreato-gastrostomy; PPH, post-pancreatectomy haemorrhage; P-J, pancreato-jejunostomy; PP, pylorus preserving. *Denotes statistical significance.
Discussion
This study described the complications experienced by a large cohort of patients who underwent PD for PDAC, AA or distal CC. While prior multicentre studies have been carried out with similar patient numbers, few have used strict diagnostic criteria and few have included only patients with a histologically confirmed cancer9. The patient demographics and postoperative outcomes of the present study were comparable to that of the current literature10–12.
The incidence of POPF in the current study was 8 per cent, lower than the 10–35 per cent observed in most series10,11,13,14. The lower observed incidence among the RAW cohort could reflect the fact that only patients with a histologically confirmed cancer were included and that most of them had PDAC (59 per cent). Indeed, PDAC patients tend to have a firmer pancreas compared to those with AA or CC15,16. Similar to Lovasik et al., this study observed that patients with a high BMI more often experienced POPF17. This may be because patients with a high BMI had a higher parenchymal fat content. This study did not observe a relationship between POPF and a P-J anastomosis, preoperative biliary drainage or preoperative diabetes, while Williamsson et al. found that a P-J was a risk factor and that both preoperative biliary drainage and preoperative diabetes were protective for POPF18.
PPH is one of the most common causes of reoperation and death after PD2. The reported incidence is between 4 and 14 per cent9,11, comparable to the current study (6 per cent). PPH was the leading cause of perioperative death among the RAW cohort, and, as previously described19, preoperative diabetes was a protective factor for PPH.
Similar to other published series20, the RAW patients who experienced morbidity had a significantly higher BMI than those who did not. Patients with a high BMI are likely to have a worse baseline fitness level; are often challenging to ventilate, which can increase the risk of respiratory and anaesthetic complications; and present technical challenges from a surgical point of view. In a recent meta-analysis by You et al., patients with a BMI ≥ 25 kg/m2 were compared to those with a BMI < 25 kg/m2. The former were found to have longer operation times, increased intraoperative blood loss, higher rates of POPF, DGE and SSI, and a longer hospital stay21.
The ASA impact on outcomes after pancreatic surgery is well documented22,23. The present study found that ASA grade > II patients were more than twice as likely to develop complications, major morbidity or PPH. As such, one should consider the additional risks when offering PD to patients in this group, especially if they are elderly or have a high BMI.
A classic PD was found to be more common among those who experienced complications. Data in the literature are conflicting and several studies have shown that the operative approach does not significantly affect perioperative outcomes24,25.
A P-G anastomosis was associated with higher rates of overall morbidity and PPH, as described in many studies in the literature26,27. Several other studies have found no advantage of one type of reconstruction compared to the other28,29.
The preoperative identification of patients who are at high-risk for adverse perioperative outcomes is important for their management. ‘Prehabilitation’ is the concept of enhancing general health and well-being in high-risk patients prior to surgery30. Interventions could be multimodal and could include activities such as a structured exercise programme or a patient-centred dietary plan5. Prehabilitation programmes aim to help patients ‘weather the storm’ of an operation and reduce the morbidity associated with major surgery. Although evidence of their effectiveness in improving PD outcomes is limited, recent studies have highlighted the potential benefits that prehabilitation programmes can provide5. A recent survey of UK pancreatic surgeons suggested that around half of British centres offer a prehabilitation programme to PD patients, but there was little consistency in what was offered31. As further evidence emerges, it is likely that consensus guidelines will be formulated that will advise what should be offered and to whom. The preoperative identification of high-risk patients may help identify those who have the most to gain from prehabilitation.
Patients who are preoperatively deemed to be high risk may wish to reconsider the treatment to be received, as serious complications can affect suitability for adjuvant treatment6. While neoadjuvant treatment is not routinely offered to patients with resectable disease in many centres, a subset of patients (for example, those with a high BMI or ASA > II) might benefit from a tailored treatment approach. In high-risk individuals, a course of neoadjuvant therapy would ensure that a course of systemic therapy is delivered (regardless of the postoperative course).
This study had several weaknesses and biases due to its retrospective nature, and practice has evolved since the study inclusion period. While a robust data set has been produced, this was not complete. As is inevitable with large multicentre studies, the larger high-volume centres provided more cases than the smaller low-volume centres. Data for the intraoperative period, such as main pancreatic duct diameter and parenchyma texture, which are known for their association with POPF, were not collected and it was not possible to compare the cases from the different collaborating centres as the data set was fully anonymized.
In this multicentre study of patients who underwent PD for malignancy, the major morbidity rate was 17 per cent and the perioperative mortality rate was 4 per cent. A high BMI and an ASA grade > II were associated with POPF and major morbidity, respectively. Patients who fall into these subgroups should be made aware of the additional risks they face. The preoperative identification of high-risk patients is important as this group may benefit from a tailored treatment approach—for example, targeted prehabilitation or neoadjuvant chemotherapy.
Collaborators
1David Sheridan, 1Mark Puckett, 1Matthew G. Browning, 3Carolina González-Abós, 4Nair Fernandes, 4Elsa Garcia Moller, 4Cristina Dopazo Taboada, 5Rupaly Pande, 5Jameel Alfarah, 6Samik Bandyopadhyay, 6Ahmed Abdelrahim, 6Ayesha Khan, 7Caitlin Jordan, 7Jonathan R. E. Rees, 8Harry Blege, 9William Cambridge, 9Olga White, 10Sarah Blacker, 10Jessie Blackburn, 10Casie Sweeney, 11Daniel Field, 11Mohammed Gouda, 12Ruben Bellotti, 13Hytham K. S. Hamid, 14Hassan Ahmed, 15Catherine Moriarty, 15Louise White, 15Mark Priestley, 15Kerry Bode, 15Judith Sharp, 15Rosie Wragg, 15Beverley Jackson, 15Samuel Craven, 16Matyas Fehervari, 16Madhava Pai, 16Laith Alghazawi, 16Anjola Onifade, 17Julliette Ribaud, 17Ashitha Nair, 17Michael Mariathasan, 17Niamh Grayson, 18Stephanos Pericleous, 18Krishna Patel, 18Conrad Shaw, 18Nolitha Morare, 18Mohamad Khish Zaban, 19Joseph Doyle, 21Alan Guerrero, 21Andre Moguel, 21Carlos Chan, 22Michael Jones, 22Edward Buckley, 22Nasreen Akter, 22Kyle Treherne, 23Gregory Gordon, 24Daniel Hughes, 24Tomas Urbonas, 25Gioia Brachini, 25Roberto Caronna, 25Piero Chirletti, 26Teresa Perra, 27Nurul Nadhirah Abd Kahar, 27Thomas Hall, 27Nabeegh Nadeem, 28Shoura Karar, 28Ali Arshad, 29Adam Yarwood, 29Mohammed Hammoda, 30Maria Artigas, 30Sandra Paterna-López
Collaborator affiliations
1University Hospitals Plymouth NHS Trust, Plymouth, UK, 2University of Muenster, Muenster, Germany, 3Hospital Clínic de Barcelona, Barcelona, Spain, 4Hospital Universitari Vall d’Hebron, Barcelona, Spain, 5University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK, 6East Lancashire Hospitals NHS Trust, Blackburn, UK, 7University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK, 8University Hospital Coventry & Warwickshire, Coventry, UK, 9NHS Lothian, Edinburgh, UK, 10Royal Surrey NHS Foundation Trust, Guildford, UK, 11Hull University Teaching Hospitals NHS Trust, Hull, UK, 12Medical University of Innsbruck, Innsbruck, Austria, 13Ibn Sina Specialized Hospital, Khartoum, Sudan, 14Shaukat Khanum Memorial Cancer Hospital, Lahore, Pakistan, 15Leeds Teaching Hospitals NHS Trust, Leeds, UK, 16Imperial College Healthcare NHS Trust, London, UK, 17King’s College Hospital NHS Foundation Trust, London, UK, 18Royal Free London NHS Foundation Trust, London, UK, 19The Royal Marsden NHS Foundation Trust, London, UK, 20Monash Medical Centre, Melbourne, Australia, 21Salvador Zubiran National Institute of Health Sciences and Nutrition, Mexico City, Mexico, 22Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK, 23Nottingham University Hospitals NHS Trust, Nottingham, UK, 24Oxford University Hospitals NHS Foundation Trust, Oxford, UK, 25Policlinico Umberto I University Hospital Sapienza, Rome, Italy, 26Azienda Ospedaliero Universitaria di Sassari, Sassari, Italy, 27Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK, 28University Hospital Southampton NHS Foundation Trust, Southampton, UK, 29Swansea Bay University Health Board, Swansea, UK, 30Hospital Universitario Miguel Servet, Zaragoza, Spain, 31University of Plymouth, Plymouth, UK
Supplementary Material
Acknowledgements
The authors would like to thank all those who contributed to the Recurrence After Whipple’s (RAW) study. The preliminary findings of this study were presented on 23 September 2022 at the AUGIS Annual Scientific Meeting (Aberdeen, UK). The findings of this study will be presented at the ASGBI Congress 2023 (Harrogate, UK).
Contributor Information
Thomas B Russell, Department of HPB Surgery, University Hospitals Plymouth NHS Trust, Plymouth, UK.
Peter L Labib, Department of HPB Surgery, University Hospitals Plymouth NHS Trust, Plymouth, UK.
Jemimah Denson, Department of HPB Surgery, University Hospitals Plymouth NHS Trust, Plymouth, UK.
Adam Streeter, Department of Medical Statistics, University of Muenster, Muenster, Germany; Department of Medical Statistics, University of Plymouth, Plymouth, UK.
Fabio Ausania, Department of HPB Surgery, Hospital Clínic de Barcelona, Barcelona, Spain.
Elizabeth Pando, Department of HPB Surgery, Hospital Universitari Vall d’Hebron, Barcelona, Spain.
Keith J Roberts, Department of HPB Surgery, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK.
Ambareen Kausar, Department of HPB Surgery, East Lancashire Hospitals NHS Trust, Blackburn, UK.
Vasileios K Mavroeidis, Department of HPB Surgery, University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK; Department of HPB Surgery, The Royal Marsden NHS Foundation Trust, London, UK.
Gabriele Marangoni, Department of HPB Surgery, University Hospital Coventry & Warwickshire, Coventry, UK.
Sarah C Thomasset, Department of HPB Surgery, NHS Lothian, Edinburgh, UK.
Adam E Frampton, Department of HPB Surgery, Royal Surrey NHS Foundation Trust, Guildford, UK.
Pavlos Lykoudis, Department of HPB Surgery, Hull University Teaching Hospitals NHS Trust, Hull, UK.
Manuel Maglione, Department of HPB Surgery, Medical University of Innsbruck, Innsbruck, Austria.
Nassir Alhaboob, Department of HPB Surgery, Ibn Sina Specialized Hospital, Khartoum, Sudan.
Hassaan Bari, Department of HPB Surgery, Shaukat Khanum Memorial Cancer Hospital, Lahore, Pakistan.
Andrew M Smith, Department of HPB Surgery, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Duncan Spalding, Department of HPB Surgery, Imperial College Healthcare NHS Trust, London, UK.
Parthi Srinivasan, Department of HPB Surgery, King’s College Hospital NHS Foundation Trust, London, UK.
Brian R Davidson, Department of HPB Surgery, Royal Free London NHS Foundation Trust, London, UK.
Ricky H Bhogal, Department of HPB Surgery, The Royal Marsden NHS Foundation Trust, London, UK.
Daniel Croagh, Department of HPB Surgery, Monash Medical Centre, Melbourne, Australia.
Ismael Dominguez, Department of HPB Surgery, Salvador Zubiran National Institute of Health Sciences and Nutrition, Mexico City, Mexico.
Rohan Thakkar, Department of HPB Surgery, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK.
Dhanny Gomez, Department of HPB Surgery, Nottingham University Hospitals NHS Trust, Nottingham, UK.
Michael A Silva, Department of HPB Surgery, Oxford University Hospitals NHS Foundation Trust, Oxford, UK.
Pierfrancesco Lapolla, Department of HPB Surgery, Policlinico Umberto I University Hospital Sapienza, Rome, Italy.
Andrea Mingoli, Department of HPB Surgery, Policlinico Umberto I University Hospital Sapienza, Rome, Italy.
Alberto Porcu, Department of HPB Surgery, Azienda Ospedaliero Universitaria di Sassari, Sassari, Italy.
Nehal S Shah, Department of HPB Surgery, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
Zaed Z R Hamady, Department of HPB Surgery, University Hospital Southampton NHS Foundation Trust, Southampton, UK.
Bilal A Al-Sarrieh, Department of HPB Surgery, Swansea Bay University Health Board, Swansea, UK.
Alejandro Serrablo, Department of HPB Surgery, Hospital Universitario Miguel Servet, Zaragoza, Spain.
Somaiah Aroori, Department of HPB Surgery, University Hospitals Plymouth NHS Trust, Plymouth, UK.
RAW Study Collaborators:
Somaiah Aroori, Peter L Labib, Thomas B Russell, Adam Streeter, Jemimah Denson, David Sheridan, Mark Puckett, Matthew G Browning, Fabio Ausania, Carolina Gonzalez-Abos, Elizabeth Pando, Nair Fernandes, Elsa Garcia Moller, Cristina Dopazo Taboada, Keith J Roberts, Rupaly Pande, Jameel Alfarah, Ambareen Kausar, Samik Bandyopadhyay, Ahmed Abdelrahim, Ayesha Khan, Vasileios K Mavroeidis, Caitlin Jordan, Jonathan R E Rees, Gabriele Marangoni, Harry Blege, Sarah C Thomasset, William Cambridge, Olga White, Adam E Frampton, Sarah Blacker, Jessie Blackburn, Casie Sweeney, Pavlos Lykoudis, Daniel Field, Mohammed Gouda, Manuel Maglione, Ruben Bellotti, Nassir Alhaboob, Hytham K S Hamid, Hassaan Bari, Hassan Ahmed, Andrew M Smith, Catherine Moriarty, Louise White, Mark Priestley, Kerry Bode, Judith Sharp, Rosie Wragg, Beverley Jackson, Samuel Craven, Duncan Spalding, Matyas Fehervari, Madhava Pai, Laith Alghazawi, Anjola Onifade, Parthi Srinivasan, Julliette Ribaud, Ashitha Nair, Michael Mariathasan, Niamh Grayson, Brian R Davidson, Stephanos Pericleous, Krishna Patel, Conrad Shaw, Nolitha Morare, Mohamad Khish Zaban, Ricky H Bhogal, Joseph Doyle, Vasileios K Mavroeidis, Daniel Croagh, Ismael Dominguez, Alan Guerrero, Andre Moguel, Carlos Chan, Rohan Thakkar, Michael Jones, Edward Buckley, Nasreen Akter, Kyle Treherne, Dhanny Gomez, Gregory Gordon, Michael A Silva, Daniel Hughes, Tomas Urbonas, Pierfrancesco Lapolla, Andrea Mingoli, Gioia Brachini, Roberto Caronna, Piero Chirletti, Alberto Porcu, Teresa Perra, Nehal S Shah, Nurul Nadhirah Abd Kahar, Thomas Hall, Nabeegh Nadeem, Zaed Z R Hamady, Shoura Karar, Ali Arshad, Bilal Al-Sarrieh, Adam Yarwood, Mohammed Hammoda, Alejandro Serrablo, Maria Artigas, and Sandra Paterna-López
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Thomas Russell (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing—original draft, Writing—review & editing), Peter Labib (Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Writing—review & editing), Jemimah Denson (Supervision, Writing—review & editing), Adam Streeter (Formal analysis, Supervision, Writing—review & editing), Fabio Ausania (Writing—review & editing), Elizabeth Pando (Writing—review & editing), Keith Roberts (Writing—review & editing), Ambareen Kausar (Writing—review & editing), Vasileios Mavroeidis (Writing—review & editing), Gabriele Marangoni (Writing—review & editing), Sarah Thomasset (Writing—review & editing), Adam Frampton (Writing—review & editing), Pavlos Lykoudis (Writing—review & editing), Manuel Maglione (Writing—review & editing), Nassir Alhaboob (Writing—review & editing), Hassaan Bari (Writing—review & editing), Andrew Smith (Writing—review & editing), Duncan Spalding (Writing—review & editing), Parthi Srinivasan (Writing—review & editing), Brian Davidson (Writing—review & editing), Ricky Bhogal (Writing—review & editing), Daniel Croagh (Writing—review & editing), Ismael Dominguez (Writing—review & editing), Rohan Thakkar (Writing—review & editing), Dhanny Gomez (Writing—review & editing), Michael Silva (Writing—review & editing), Pierfrancesco Lapolla (Writing—review & editing), Andrea Mingoli (Writing—review & editing), Alberto Porcu (Writing—review & editing), Nahal Shah (Writing—review & editing), Zaed Hammady (Writing—review & editing), Bilal Al-Sarireh (Writing—review & editing), Alejandro Serrablo (Writing—review & editing), and Somaiah Aroori (Conceptualization, Supervision, Validation, Visualization, Writing—review & editing).
References
- 1. Chen L, Peng L, Wang C, Li SC, Zhang M. New score for prediction of morbidity in patients undergoing open pancreaticoduodenectomy. J Int Med Res 2021;49:3000605211001984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Narayanan S, Martin AN, Turrentine FE, Bauer TW, Adams RB, Zaydfudim VM. Mortality after pancreaticoduodenectomy: assessing early and late causes of patient death. J Surg Res 2018;231:304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luu AM, Braumann C, Belyaev O, Janot-Matuschek M, Rudolf H, Praktiknjo M et al. Long-term survival after pancreaticoduodenectomy in patients with ductal adenocarcinoma of the pancreatic head. Hepatobiliary Pancreat Dis Int 2020;20:271–278 [DOI] [PubMed] [Google Scholar]
- 4. Sánchez Acedo P, Herrera Cabezón J, Zazpe Ripa C, Tarifa Castilla A. Survival, morbidity and mortality of pancreatic adenocarcinoma after pancreaticoduodenectomy with a total mesopancreas excision. Rev Esp Enferm Dig 2019;111:609–614 [DOI] [PubMed] [Google Scholar]
- 5. Bundred JR, Kamarajah SK, Hammond JS, Wilson CH, Prentis J, Pandanaboyana S. Prehabilitation prior to surgery for pancreatic cancer: a systematic review. Pancreatology 2020;20:1243–1250 [DOI] [PubMed] [Google Scholar]
- 6. Russell TB, Labib PL, Bowles M, Aroori S. Serious complications of pancreatoduodenectomy correlate with lower rates of adjuvant chemotherapy: would high-risk patients benefit from neoadjuvant therapy? Eur J Surg Oncol 2023;49:142–149 [DOI] [PubMed] [Google Scholar]
- 7. El Nakeeb A, Askar W, Atef E, Hanafy EE, Sultan AM, Salah T et al. Trends and outcomes of pancreaticoduodenectomy for periampullary tumors: a 25-year single-center study of 1000 consecutive cases. World J Gastroenterol 2017;23:7025–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karim SAM, Abdulla KS, Abdulkarim QH, Rahim FH. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): cross-sectional study. Int J Surg 2018;52:383–387 [DOI] [PubMed] [Google Scholar]
- 9. Russell TB, Aroori S. Procedure-specific morbidity of pancreatoduodenectomy: a systematic review of incidence and risk factors. ANZ J Surg 2022;92:1347–1355 [DOI] [PubMed] [Google Scholar]
- 10. Williamsson C, Rystedt J, Andersson B. An analysis of gender differences in treatment and outcome of periampullary tumours in Sweden—a national cohort study. HPB (Oxford) 2021;23:847–853 [DOI] [PubMed] [Google Scholar]
- 11. Bassi C, Marchegiani G, Giuliani T, Di Gioia A, Andrianello S, Zingaretti CC et al. Pancreatoduodenectomy at the Verona Pancreas Institute: the evolution of indications, surgical techniques, and outcomes: a retrospective analysis of 3000 consecutive cases. Ann Surg 2022;276:1029–1038 [DOI] [PubMed] [Google Scholar]
- 12. Giuliani T, Marchegiani G, Di Gioia A, Amadori B, Perri G, Salvia R et al. Patterns of mortality after pancreatoduodenectomy: a root cause, day-to-day analysis. Surgery 2022;172:329–335 [DOI] [PubMed] [Google Scholar]
- 13. Ke Z, Cui J, Hu N, Yang Z, Chen H, Hu J et al. Risk factors for postoperative pancreatic fistula: analysis of 170 consecutive cases of pancreaticoduodenectomy based on the updated ISGPS classification and grading system. Medicine (Baltimore) 2018;97:e12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu S-J, Shen S-L, Li S-Q, Hu W-J, Hua Y-P, Kuang M et al. Risk factors and outcomes of postoperative pancreatic fistula after pancreatico-duodenectomy: an audit of 532 consecutive cases. BMC Surg 2015;15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eshmuminov D, Schneider MA, Tschuor C, Raptis DA, Kambakamba P, Muller X et al. Systematic review and meta-analysis of postoperative pancreatic fistula rates using the updated 2016 international study group pancreatic fistula definition in patients undergoing pancreatic resection with soft and hard pancreatic texture. HPB (Oxford) 2018;20:992–1003 [DOI] [PubMed] [Google Scholar]
- 16. Mavroeidis VK, Russell TB, Clark J, Adebayo D, Bowles M, Briggs C et al. Pancreatoduodenectomy for suspected malignancy: nonmalignant histology confers increased risk of serious morbidity. Ann R Coll Surg Engl 2022;105:446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lovasik BP, Kron P, Clavien P-A, Petrowsky H, Kooby DA. Pancreatectomy and body mass index: an international evaluation of cumulative postoperative complications using the comprehensive complications index. HPB (Oxford) 2019;21:1761–1772 [DOI] [PubMed] [Google Scholar]
- 18. Williamsson C, Stenvall K, Wennerblom J, Andersson R, Andersson B, Tingstedt B. Predictive factors for postoperative pancreatic fistula—a Swedish nationwide register-based study. World J Surg 2020;44:4207–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Izumo W, Higuchi R, Yazawa T, Uemura S, Shiihara M, Yamamoto M. Evaluation of preoperative risk factors for postpancreatectomy hemorrhage. Langenbecks Arch Surg 2019;404:967–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marchegiani G, Crippa S, Perri G, Rancoita PMV, Caravati A, Belfiori G et al. Surgery for intraductal papillary mucinous neoplasms of the pancreas: preoperative factors tipping the scale of decision-making. Ann Surg Oncol 2022;29:3206–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. You L, Zhao W, Hong X, Ma L, Ren X, Shao Q et al. The effect of body mass index on surgical outcomes in patients undergoing pancreatic resection: a systematic review and meta-analysis. Pancreas 2016;45:796–805 [DOI] [PubMed] [Google Scholar]
- 22. Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R et al. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg 2011;254:702–708 [DOI] [PubMed] [Google Scholar]
- 23. Wiltberger G, Muhl B, Benzing C, Atanasov G, Hau H-M, Horn M et al. Preoperative risk stratification for major complications following pancreaticoduodenectomy: identification of high-risk patients. Int J Surg 2016;31:33–39 [DOI] [PubMed] [Google Scholar]
- 24. Hüttner FJ, Fitzmaurice C, Schwarzer G, Seiler CM, Antes G, Büchler MW et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev 2016;2:CD006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diener MK, Fitzmaurice C, Schwarzer G, Seiler CM, Hüttner FJ, Antes G et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev 2014;11:CD006053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lyu Y, Li T, Cheng Y, Wang B, Chen L, Zhao S. Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: an up-to-date meta-analysis of RCTs applying the ISGPS (2016) criteria. Surg Laparosc Endosc Percutan Tech 2018;28:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keck T, Wellner UF, Bahra M, Klein F, Sick O, Niedergethmann M et al. Pancreatogastrostomy versus pancreatojejunostomy for RECOnstruction after PANCreatoduodenectomy (RECOPANC, DRKS 00000767): perioperative and long-term results of a multicenter randomized controlled trial. Ann Surg 2016;263:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang W, Zhang Z, Gu C, Liu Q, Liang Z, He W et al. The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: a network meta-analysis of randomized control trials. Int J Surg 2018;57:111–116 [DOI] [PubMed] [Google Scholar]
- 29. Cheng Y, Briarava M, Lai M, Wang X, Tu B, Cheng N et al. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction for the prevention of postoperative pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst Rev 2017;9:CD012257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Durrand J, Singh SJ, Danjoux G. Prehabilitation. Clin Med (Lond) 2019;19:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russell TB, Murphy P, Tanase A, Sen G, Aroori S. Results from a UK-wide survey: the nutritional assessment and management of pancreatic resection patients is highly variable. Eur J Clin Nutr 2022;76:1038–1040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.