Abstract
Solid tumors are complex entities that actively shape their microenvironment to create a supportive environment for their own growth. Angiogenesis and immune suppression are two key characteristics of this tumor microenvironment. Despite attempts to deplete tumor blood vessels using antiangiogenic drugs, extensive vessel pruning has shown limited efficacy. Instead, a targeted approach involving the judicious use of drugs at specific time points can normalize the function and structure of tumor vessels, leading to improved outcomes when combined with other anticancer therapies. Additionally, normalizing the immune microenvironment by suppressing immunosuppressive cells and activating immunostimulatory cells has shown promise in suppressing tumor growth and improving overall survival. Based on these findings, many studies have been conducted to normalize each component of the tumor microenvironment, leading to the development of a variety of strategies. In this review, we provide an overview of the concepts of vascular and immune normalization and discuss some of the strategies employed to achieve these goals.
Subject terms: Cancer microenvironment, Cancer immunotherapy
Vascular-immune modulation: a new frontier in cancer therapy
New insights into vascular and immune normalization strategies show potential for improving cancer therapy outcomes. In this review, authors discuss the current state of research on vascular and immune normalization in cancer treatment, highlighting the intricate relationship between these two processes. Vascular normalization aims to restore the integrity of tumor blood vessels, improving oxygenation and drug delivery, while immune normalization focuses on modulating the tumor immune microenvironment to suppress immunosuppression and recruit anti-tumoral immune cells. Various methods for achieving vascular normalization include targeting VEGF signaling, Ang-Tie signaling, oncogenic signaling in cancer cells, and even CD4 + T-cells. Strategies for immune normalization involve modulating the myeloid compartment, enhancing effector T cell function through cancer vaccines or immune checkpoint blockade, and using oncolytic viruses to target tumor cells. Combining these approaches may yield even greater efficacy in cancer treatment.
Introduction
Cancer is characterized by the uncontrolled growth and spread of cells harboring genetic or epigenetic abnormalities1. Viewing cancer solely as a collection of malignant cells fails to capture the intricate processes occurring within tumors. Increasing evidence supports the notion that cancers should be regarded as disorganized “pseudo-organs” engaged in constant interactions with their surrounding milieu2. From this, the concept of the tumor microenvironment (TME) has emerged. The TME encompasses not only the tumor cells but also the neighboring cellular and structural components of the tumor, including immune cells, blood vessels, stromal cells such as fibroblasts, and extracellular matrix (ECM)3,4. Furthermore, the TME does not exhibit a fixed phenotype, instead varying substantially with tumor type, host factors, and disease progression and showing dynamism even within an individual host. Within the TME, complex interactions often give rise to conditions of hypoxia, low pH, and elevated interstitial fluid pressure (IFP), fostering an immunosuppressive environment that facilitates tumor progression, metastasis, and resistance to anticancer therapies5–7. For instance, hypoxia, a hallmark of the TME of various cancer types, can attenuate the efficacy of chemotherapy and radiotherapy8,9, trigger the secretion of immune-suppressive cytokines, and promote the recruitment and proliferation of immune-regulatory cell populations10. Numerous studies have focused on targeting factors contributing to these effects to augment the effectiveness of anticancer therapies.
Initial attempts to target these factors proved inefficient and, in some instances, even exacerbated tumor progression11,12. Notably, the widespread use of antiangiogenic therapy (AAT), initially developed to eliminate tumor blood vessels, often results in excessive vessel pruning, aggravating hypoperfusion and hypoxia, thus enhancing tumor aggressiveness11. These observations suggest that, rather than depletion, a strategy of normalization may yield superior outcomes12–14. Additionally, there is growing recognition of the crucial role played by the immune microenvironment in tumor progression and therapy response. Strategies aimed at normalizing the tumor immune microenvironment (TIME) have gained much attention as potential therapeutic interventions15. These approaches encompass modulation of immune checkpoints, recruitment and activation of effector immune cells, and suppression of immunosuppressive cell populations, with the ultimate goal of enhancing antitumor immune responses and restoring immune surveillance against cancer15. In this review, we categorize current normalization approaches into two main groups: vascular normalization and TIME normalization. We discuss the characteristic features of the TME associated with each strategy and provide a concise overview of ongoing research targeting these strategies.
Vascular normalization
Background
The rapid growth of solid tumors necessitates an increased supply of oxygen and nutrients. To meet this demand, tumors stimulate the sprouting of blood vessels from preexisting vessels, that is, angiogenesis2,16–18. Tumor angiogenesis arises from an imbalance between proangiogenic and antiangiogenic factors, often favoring the former14,19. Consequently, the vasculature that forms within tumors is heterogeneous and poorly perfused, comprising abnormal and leaky blood vessels17,19,20. Blood flow through this irregular vasculature is stagnant and variable, leading the tumor environment to be characterized by hypoxia, low pH, and elevated IFP21–23. Moreover, in certain solid tumors, cancer-associated fibroblasts (CAFs) and their production of ECM components exert compressive forces on tumor vessels, exacerbating the hypoxia24. This TME facilitates the recruitment and expansion of immune-suppressive cell populations25 and pathological CAFs26–28, hampers the cytotoxic activity of tumor-infiltrating effector T cells, enables immune evasion by cancer cells8,29, promotes metastasis30, and diminishes the efficacy of radiotherapy31, chemotherapy32, and immunotherapy33.
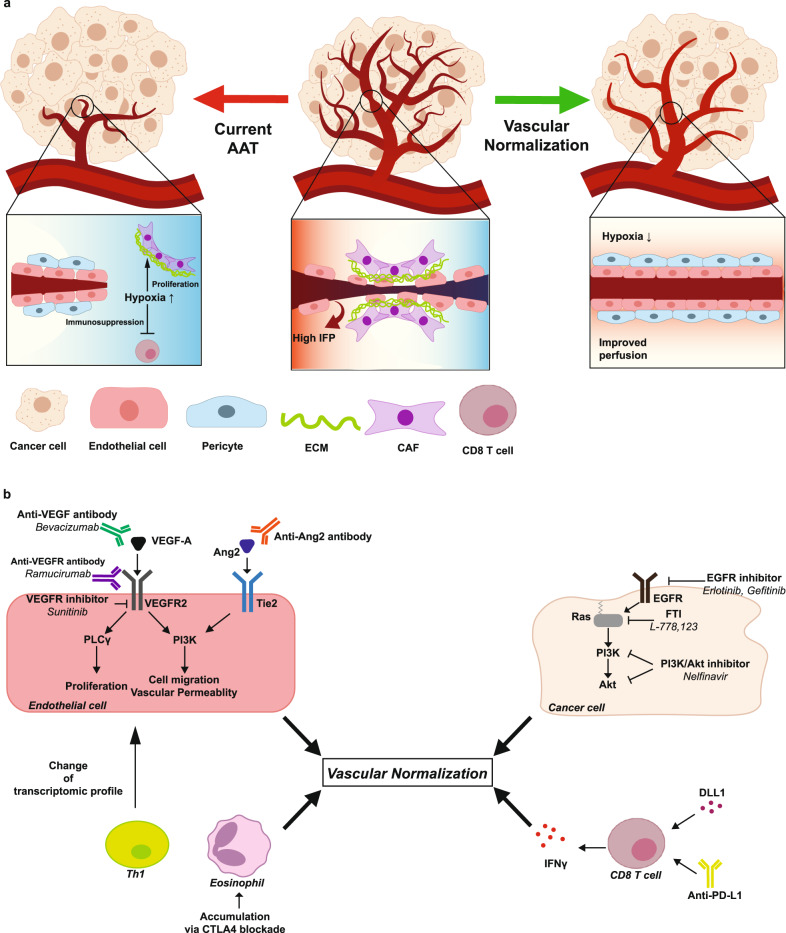
Initially, AATs were developed with the intention of inhibiting tumor growth by disrupting the blood supply and oxygen delivery to tumors34,35. However, the use of AATs alone was not successful at combating cancer, exhibiting promising results only when combined with other agents, including chemotherapeutics36,37. These unexpected outcomes were termed the “AAT paradox“21, and the concept of “vascular normalization” was proposed by Rakesh K. Jain in 2001 to explain this phenomenon38. The vascular normalization hypothesis holds that the structure and function of tumor vessels can be restored by employing low doses of AATs within a specific time frame. This restoration process improves oxygen delivery and enhances the penetration of therapeutic drugs39–41. Although both conventional AAT-induced vessel pruning and vascular normalization result in overall decreased vessel density, the underlying mechanisms differ substantially23. Conventional AAT reduces vessel density through vessel depletion, whereas vascular normalization improves vessel function, manifested by enhanced vessel perfusion, increased pericyte coverage, and reduced hypoxia23,42,43 (Fig. 1a).
Fig. 1. Vascular normalization: Restoring vascular integrity to enhance oxygenation and therapeutic drug delivery in tumor vasculature.
a Comparison of vascular normalization with current therapies using AATs. Tumors exhibit abnormal vasculature, characterized by irregularly shaped endothelial cells, sparse pericyte coverage, and compression by tumor cells and their extracellular matrices, resulting in poorly perfused vessels (middle panel). Current antiangiogenic therapies, mainly aimed at depleting the tumor vasculature, often lead to excessive vessel pruning, causing hypoxia and limited drug delivery (left panel). Vascular normalization strategies aim to restore vascular integrity, improving oxygenation and enhancing the delivery of therapeutic drugs (right panel). b Various vascular normalization methods. Vascular normalization can be induced by targeting VEGF signaling or Ang–Tie signaling in endothelial cells or oncogenic signaling in cancer cells. Immune checkpoint blockade can induce vascular normalization through the function of CD8+ or CD4 + T cells and the accumulation of eosinophils. AAT, antiangiogenic therapy; IFP, interstitial fluid pressure; CAF, cancer-associated fibroblast; ECM, extracellular matrix; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; PLCγ, phospholipase γ; PI3K, phosphoinositide 3-kinase; Ang2, angiopoietin 2; EGFR, epidermal growth factor receptor.
One of the notable advantages of vascular normalization is its potential for synergizing with other anticancer treatments, including chemotherapy44,45, radiotherapy46, and immunotherapy2,47,48. For instance, a phase II clinical trial in HER2-negative breast cancer patients demonstrated that the combination of bevacizumab (an anti- vascular endothelial growth factor (VEGF)-A antibody) with adriamycin/cyclophosphamide/paclitaxel chemotherapy exhibited vascular normalization effects and was associated with tumor regression44. In another phase II clinical trial, bevacizumab showed synergistic effects with lomustine, a chemotherapeutic agent, in patients with recurrent glioblastoma (GBM)49.
Achieving vascular normalization poses certain challenges. The optimal dose of AAT and the timing of effective normalization, known as the “normalization window,” are tightly limited and vary between individuals, which presents obstacles to its clinical application21. Recent research has unveiled various approaches to induce vascular normalization, ranging from targeting the well-known VEGF signaling pathway to utilizing helper T cells (Fig. 1b). In the next section, we will introduce some of the numerous targets that can be exploited for vascular normalization.
VEGF signaling remains the major target in vascular normalization
Many solid tumors overexpress factors involved in the VEGF signaling pathway, a key feature in their pathological angiogenesis50,51. Targeting the ligands or receptors within this pathway has demonstrated vascular normalization effects in various types of cancer. VEGF-A is one of the best-studied ligands in the context of tumor angiogenesis. Initial attempts to target this VEGF ligand involved the use of A4.6.1, a murine anti-human VEGF IgG. A4.6.1 demonstrated the capability to normalize tumor vasculature in different cancer models, including colorectal cancer, glioblastoma, and melanoma xenografts52. Furthermore, this antibody enhanced the effectiveness of chemotherapy and radiation therapy when used in combination with each modality53.
Another notable example is bevacizumab (Avastin, Genentech/Roche), a humanized monoclonal anti-VEGF IgG that has received regulatory approval from the US Food and Drug Administration (FDA) for first-line treatment of metastatic colorectal cancer, non-small-cell lung cancer (NSCLC), metastatic renal cell carcinoma, and recurrent GBM in combination with other anticancer therapies54. Studies utilizing bevacizumab have demonstrated vascular normalization effects in melanoma, breast carcinoma, ovarian carcinoma, and GBM39,55,56. Many studies highlighting the synergistic effects of vascular normalization with other therapies have been based on the use of bevacizumab39 (Fig. 1b).
Targeting the VEGF receptor (VEGFR) itself has also been explored57. Among the three reported VEGFRs (VEGFR-1, VEGFR-2, VEGFR-3) in mammals, VEGFR-2, whose signaling is triggered by VEGF-A binding, is associated with pathologic angiogenesis51,58. Blocking VEGFR-2 activity results in increased oxygenation through vascular normalization in various tumor models. For instance, DC101, a rat monoclonal antibody specific to mouse VEGFR-2, induced vascular normalization not only in GBM but also in lung, breast, and colorectal cancer46,59. Moreover, this antibody appeared to enhance drug delivery into tumors in breast and colon carcinomas60. Another antibody targeting VEGFR-2 is ramucirumab (Cyramza, ImClone Systems), which shares a similar mechanism with DC101 and may possess the potential for vascular normalization, although limited studies have been conducted on it61 (Fig. 1b).
VEGFR-2 functions as a receptor tyrosine kinase (RTK), regulating downstream molecules such as phospholipase γ (PLCγ) and growth factor receptor-bound protein 2 (Grb2)62. Several inhibitors have been developed to target the kinase activity of VEGFR-2 and suppress tumor growth. Sunitinib (Sutene, Pfizer), a type I tyrosine kinase inhibitor that binds to the ATP-binding site of the receptor and thereby blocks ATP hydrolysis, is one example. Sunitinib efficiently increases tumor oxygenation in a murine squamous cell cancer model (SCC VII)63 and reduces tumor IFP in an orthotopic human glioma model64 (Fig.1b). Notably, in the latter model, sunitinib also improves the delivery of the chemotherapeutic drug temozolomide (Temodar, Merck) into the tumor.
Targeting Ang–Tie signaling restores abnormal tumor vasculature
Another promising target for vascular normalization is the angiopoietin (Ang)–Tie pathway. Among the Ang ligands, Ang2 is often upregulated during tumor angiogenesis. Binding of Ang2 to the Tie2 receptor induces pericyte detachment, vessel regression, and hypoxia65,66. Importantly, Ang2 plays a role in the early stages of tumorigenesis, and in Ang2-knockout (KO) mice, the tumor vessel phenotype resembles that of mature blood vessels, indicating that targeting the Ang–Tie pathway can achieve vascular normalization65,67.
Concomitant targeting of Ang2 and VEGFR has demonstrated vascular normalization effects in several tumor models46. For instance, dual inhibition of VEGFR and Ang2 using cediranib (Recentin, AstraZeneca) in combination with an anti-Ang2-neutralizing antibody or simultaneous targeting of both pathways with a bispecific antibody resulted in an extended normalization window in orthotopic GBM models, leading to prolonged survival68,69 (Fig. 1b).
Furthermore, the simultaneous activation of Tie2 and inhibition of Ang2 using an antibody capable of triggering Tie2 phosphorylation through clustering of Ang2 has normalized tumor vessels in orthotopic GBM, subcutaneous Lewis lung carcinoma, and spontaneous mammary cancer models70,71. These findings suggest that targeting aberrant blood vessels in tumors by modulating the Ang–Tie pathway and the VEGF pathway may be a promising strategy for restoring the functionality of the tumor vascular bed.
Targeting notch signaling normalizes tumor vasculature through the action of immune cells
The evolutionarily conserved Notch signaling pathway plays crucial roles in vascular development. Notch signaling begins at four transmembrane receptors (Notch 1–4) activated by five transmembrane ligands (Jagged 1, Jagged 2, Delta-like [DLL] 1, DLL3, DLL4)23,72. Among these ligands, DLL1 and DLL4 have been identified as key regulators of tumor angiogenesis, making them promising therapeutic targets23,73. However, it is important to note that targeting DLL4 has shown several significant adverse effects74,75. Long-term DLL4 blockade can lead to the development of vascular neoplasms, while persistent activation of DLL4 has been associated with T-cell acute lymphoblastic leukemia23,76,77.
In contrast, targeting DLL1 has shown promising results. Zhang et al. demonstrated that DLL1 overexpression in E0771 breast cancer models and LAP0297 lung cancer models induced vascular normalization, characterized by a more uniform distribution of functional blood vessels throughout the tumor23 (Fig.1b). Additionally, DLL1 overexpression correlated with the normalization of the tumor immune profile, as it polarized tumor-associated macrophages (TAMs) from an immunosuppressive M2-like phenotype to a proinflammatory M1-like phenotype and activated CD8 + T cells. Notably, in vivo depletion of CD8 + T cells or deficiency of interferon-ɣ (IFN-ɣ) nullified the normalization effect observed with DLL1 overexpression, suggesting that CD8 + T cells may be responsible for the normalization of blood vessels and that immune normalization and vascular normalization are interconnected phenomena.
Oncogenic signaling in cancer cells is an alternative target for vascular normalization
Uncontrolled growth, a hallmark characteristic of cancer, often arises due to genetic alterations in oncogenes, tumor suppressor genes, and stability genes17,78. Many signaling pathways, including those upstream of VEGF or other angiogenic factors, are affected by such genetic alterations. One well-known oncogenic signaling pathway that impacts angiogenesis is the epidermal growth factor (EGF)–epidermal growth factor receptor (EGFR)–Ras–phosphoinositide 3-kinase (PI3K)–Akt pathway79 (Fig. 1b). Therefore, targeting the components of this pathway can regulate angiogenesis. For example, targeting EGFR with erlotinib (Tarceva, Genentech) induces vascular normalization by reducing the expression of hypoxia-inducible factor-1α (HIF-1α) and VEGF in head and neck squamous cell carcinoma (HNSCC) xenograft models80.
Another potential target is H-Ras, which is involved in angiogenesis through the activation of downstream kinases81. To become activated and oncogenically transformed, H-Ras requires prenylation by a farnesyltransferase. Inhibiting these posttranslational modifications with farnesyltransferase inhibitors (FTIs) suppresses the growth of Ras-transformed cells82. In glioma xenografts, inhibition of Ras prenylation with FTIs has been shown to increase oxygenation, indicating vascular normalization83,84.
Indirect inhibition of the Akt pathway has also demonstrated vessel-normalizing effects. The HIV protease inhibitor nelfinavir (Viracept, Pfizer) induces a decrease in VEGF secretion and an increase in oxygenation, similar to the vascular normalization effects observed with bevacizumab85.
Qayum et al. demonstrated that vascular normalization can be achieved in HT1080 tumors and spontaneously arising tumors in MMTV-neu mice by targeting EGFR, Ras, PI3K, or Akt86. Specifically, they used gefitinib (Iressa, AstraZeneca) to target EGFR, FTI L-778,123 to target Ras, and nelfinavir to target PI3K and Akt. This strategy effectively increased tumor oxygenation, and the normalization window in this study extended from Day 5 after treatment until the end of the study.
The HGF/c-Met pathway, upregulated in various cancers87–89, is associated with negative prognostic outcomes90. Activation of this pathway promotes cell proliferation and secretion of angiogenic factors, including VEGF ligands. HGF/c-Met signaling synergizes with VEGF signaling to promote angiogenesis and confer resistance against AATs91–93. Therefore, targeting the HGF/c-Met pathway has promise for normalizing tumor vasculature92. Moreover, HGF’s inhibitory effect on dendritic cell function can lead to the differentiation of immunosuppressive regulatory T cells, again highlighting the interconnectedness between vascular and immune normalization94.
CD4 + T cells can induce vascular normalization
As mentioned above, vascular normalization and immune normalization are closely interconnected concepts. Bioinformatic analyses have revealed correlations between gene expression features associated with vascular normalization and those related to immunostimulatory pathways95. Additionally, studies have demonstrated increased vessel permeability and pericyte deficiency in CD4-KO or T-cell receptor (TCR)-KO mice, indicating the crucial roles played by CD4 + T cells in vascular normalization. The adoptive transfer of CD4 + T cells reduces hypoxia in E0771 xenograft models, and immune checkpoint blockade (ICB) induces vascular normalization95,96.
Activated CD4 + T-cells colocalize with tumor-associated endothelial cells, causing transcriptomic alterations in these cells. This was confirmed in CD4 + T-cell KO mouse models, which had increased VEGF-A ligand and Ang1/Ang2 ratio, indicating vascular dysfunction97. Additionally, the extracellular matrices of tumor-associated cells were disrupted in the absence of CD4 + T cells95. Although the specific molecular mechanism is not fully elucidated, these findings make it clear that CD4 + T cells induce vascular normalization via various pathways. Among the diverse CD4 + T cells, Th1 cells and their IFN-γ secretion were the major CD4 + T-cell subtypes associated with vascular normalization. CTLA4 blockade promotes vessel normalization via the accumulation of eosinophils in breast cancer98. Additionally, in colorectal cancer, anti-PD-L1 therapy induces tumor vascular normalization via CD8 + T cells, which is antagonized by CD4 + T cells99 (Fig. 1b).
Other strategies that normalize the tumor vasculature
Other than the aforementioned approaches, several strategies have been explored to normalize aberrant tumor vasculature. One such strategy involves the use of nanocarriers that deliver nitric oxide (NO), a molecule known to regulate angiogenesis and maintain vascular homeostasis100. Delivery of NO using nanocarriers normalizes tumor vasculature and improves the efficacy of anticancer therapies, particularly in hepatocellular carcinoma101. Low-dose NO delivery through this approach can create an immunostimulatory environment, highlighting the intertwined nature of vascular and immune normalization101.
Targeting stromal cells, including CAFs and the ECM produced by them, represents another avenue to alleviate solid stress, which can enhance blood perfusion and improve drug delivery24,102. Additionally, physical methods such as low-dose gamma radiation or the use of oxygen microbubble delivery with ultrasound have also been explored as vascular normalization strategies103,104. Additionally, an emerging strategy for vascular normalization involves the use of microRNAs (miRNAs) that are associated with angiogenesis105.
Normalization of the immune microenvironment
Background
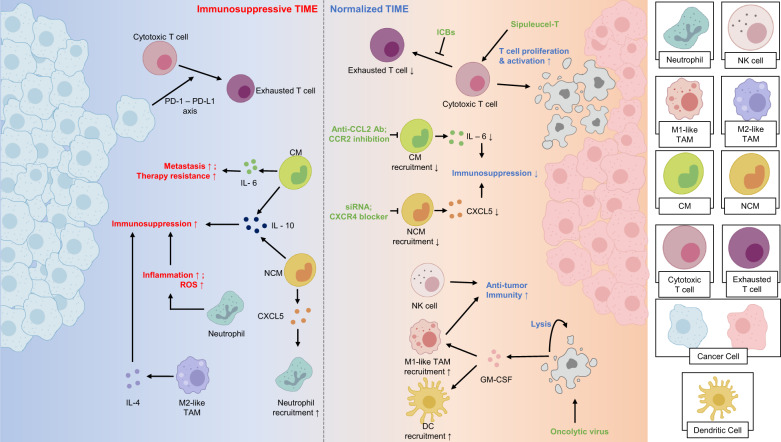
In many cancer types, tumors mold the TME into an immunosuppressive state characterized by defects in antigen presentation machinery and the recruitment of immunosuppressive cells. These features are often observed in the TME of solid tumors and are influenced by factors such as hypoxia and high interstitial fluid pressure (IFP)106. For example, hypoxia leads to increased activation of hypoxia-inducible factor-1 (HIF-1) and vascular endothelial growth factor-A (VEGF-A), which in turn result in the expression of inhibitory checkpoints in CD8 + T cells, reduced functionality of dendritic cells, and the recruitment and polarization of myeloid cells into immunosuppressive phenotypes25,107–109. The high IFP in solid tumors stimulates CAFs to produce transforming growth factor-beta (TGF-β), which promotes the differentiation and proliferation of regulatory T helper cells (Tregs), induces the generation of protumorigenic myeloid cell phenotypes, and accelerates desmoplasia2,110 (Fig. 2).
Fig. 2. Immune normalization: Modulating the tumor immune microenvironment to suppress immunosuppression and recruit antitumoral immune cells.
Tumors employ mechanisms to enhance immunosuppression, including recruiting immunosuppressive leukocytes and suppressing the function of antitumoral immune cells. Chemotaxis of classical monocytes (CMs) is facilitated by the CCL2–CCR2 axis, and these CMs secrete cytokines such as IL-6, that promote tumor metastasis and therapy resistance. Tumors secrete CX3CL1, recruiting nonclassical monocytes (NCMs) that, in turn, can recruit neutrophils via the CXCL5–CXCR2 axis. Both CMs and NCMs secrete immunosuppressive cytokines, including IL-10. M2-like TAMs further suppress the antitumoral immune response by secreting cytokines such as IL-4. Immune checkpoints employed by tumors dampen the function of effector T cells. Strategies for immune normalization include inhibiting monocyte recruitment using anti-CCL2 antibodies/siRNAs, CXCR4 blockers, or CCR2 inhibition. Enhancing effector T-cell function can be achieved through cancer vaccines or immune checkpoint blockade (ICB). Additionally, oncolytic viruses can target tumor cells, inducing their lysis and recruiting antitumoral immune cells. NK, natural killer; CM, classical monocyte; NCM, nonclassical monocyte; TAM, tumor-associated macrophage; ROS, reactive oxygen species; ICB, immune checkpoint blockade; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Normalization of the immune cell profile within the TME is defined differently by different research groups. Sanmamed et al. describe normalization in the context of immunotherapy, categorizing strategies into “enhancement therapies” that activate a general systemic immune response and “normalization therapies” that target specific dysfunctional immune responses111. In this review, we will use a broader definition, considering the transition of the TME from an immunosuppressive microenvironment to a tumor-suppressing phenotype as normalization (Fig. 2).
Given the promising results of immunotherapy against various cancers, several anticancer therapies harnessing the immune system have been developed. Therefore, we will explore some of these methods, including immune checkpoint blockade, reprogramming of myeloid cell populations, and cancer vaccines.
Immune checkpoint blockade restores the function of antitumoral immune cells
CD8 + T cells are immune cells that play a crucial role in exerting antitumoral effects, primarily by eliminating tumor cells through mechanisms such as perforin/granzyme secretion or FasL/TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis2. Upon activation, CD8 + T cells also upregulate immune checkpoint molecules, including cytotoxic T lymphocyte antigen-4 (CTLA-4)112. CTLA-4 binds to the same ligands as B7 costimulatory receptors with higher affinity, resulting in reduced T-cell proliferation and diminished secretion of inflammatory cytokines113. Another important immune checkpoint molecule is programmed death-1 (PD-1), which is expressed on activated T cells. PD-1 binds to its ligands PD-L1 or PD-L2, which are expressed on various cells within the TME, leading to T-cell inhibition114. Current immunotherapies targeting these “exhaustion” mechanisms by blocking CTLA-4 or PD-1 signaling can reverse the exhaustion of effector T cells and enhance their antitumor activity115 (Fig. 2).
Several other immune checkpoint molecules have been identified, and some of them are being evaluated as potential therapeutic targets. One such molecule is lymphocyte activation gene-3 (LAG-3), which is expressed on Tregs. LAG-3high Tregs secrete immunosuppressive cytokines, and blockade of LAG-3 signaling using an anti-LAG-3 antibody slows tumor growth. The blockade of immune checkpoint molecules represents a promising strategy in immunotherapy, aiming to unleash the full potential of the immune system against tumors.
Myeloid cells are key targets in normalizing the immune environment
The myeloid cell population within the TME is highly heterogeneous and has a role in modulating tumor progression116–118. However, the significance of myeloid cells in immunotherapy is often underestimated, and current approaches targeting these cells have shown limited effectiveness119.
Monocytes are myeloid cells that can be classified into two subtypes, classical and nonclassical120. Classical monocytes (CD14 + CD16- in humans, Ly6Chi CCR2+ in mice) have been generally associated with protumoral functions, including promoting cancer metastasis and increasing therapy resistance121,122. Strategies targeting the recruitment of classical monocytes in mice through CCR2 inhibition or the use of anti-CCL2 antibodies have shown promise in creating an antitumoral TME and reducing therapeutic resistance, respectively122,123. One limitation of this approach is that termination of anti-CCL2 treatment in certain tumors has led to accelerated tumor metastasis and worse outcomes due to increased IL-6 within the tumors124 (Fig. 2).
Nonclassical monocytes, defined as CD14- CD16+ in humans and Ly6Clo CX3CR1+ in mice, can enhance resistance to anticancer therapy. AATs can induce tumors to secrete CX3CL1, which increases the recruitment of nonclassical monocytes. These monocytes can enhance the infiltration of neutrophils into the TME through the CXCL5–CXCR2 axis117,118. Both nonclassical monocytes and neutrophils exert protumoral functions by secreting immunosuppressive cytokines such as IL-10. Several methods targeting these cells, such as the use of siRNAs or CXCR4 blockers, have shown promising results118 (Fig. 2).
The function of monocytes in the TME is context-dependent, and they have also been reported to play antitumoral roles in certain circumstances. For example, infiltration of classical monocytes into KPC tumors, a murine pancreatic ductal adenocarcinoma (PDAC) model, has led to a reduction in tumor fibrosis, increasing the efficacy of chemotherapy125. Nonclassical monocytes can inhibit metastasis and activate natural killer (NK) cells, contributing to cancer immunosurveillance126,127 (Fig. 2).
Neutrophils are another myeloid cell population that can exert protumoral functions within the TME. Recruited neutrophils can promote tumorigenesis by facilitating inflammation or releasing reactive oxygen species. They promote tumor proliferation, angiogenesis, and metastasis through diverse mechanisms120 (Fig. 2). Therefore, reducing the neutrophil population or inhibiting their function within the TME may help create an immune environment favorable for anticancer treatments. In HER2-negative gastric cancers, which exhibit a limited response to current therapies, increased infiltration and protumoral activities of neutrophils and nonclassical monocytes have been observed, highlighting the potential of targeting these cell types as novel therapeutic approaches128. Emerging evidence has demonstrated the significant role of neutrophil extracellular traps (NETs) in promoting tumor metastasis, further underscoring the criticality of targeting myeloid cells within the TME129,130. To fully exploit the therapeutic potential of the monocyte and neutrophil populations within the TME, a comprehensive understanding of their context-dependent functions is needed, and detailed reviews of their important properties have been provided by Jeong et al.120.
Macrophages are another myeloid cell population that deserves attention. High infiltration of macrophages has been associated with a negative cancer prognosis131. TAMs are highly heterogeneous and can be divided into two subtypes, although these definitions are oversimplified: inflammatory M1-like TAMs and immunosuppressive M2-like TAMs. The TME promotes the polarization of TAMs toward a more M2-like phenotype through hypoxia or signaling by immunosuppressive cytokines, including IL-4132. This makes TAMs attractive TME targets. For example, the chemokine CCL2 recruits CCR2+ monocytes, which differentiate into macrophages, and blocking CCR2 has prevented immune suppression in some preclinical models, improving chemotherapeutic efficacy133. Since M2-like TAMs, not M1-like TAMs, drive immune suppression, inhibiting polarization toward an M2-like phenotype is another potential mechanism for successful treatment. Targeting the metabolic pathways or essential transcription factors of TAMs has demonstrated antitumoral effects in various mouse models134 (Fig. 2).
Myeloid cells can transform into malignant cells, leading to acute myeloid leukemia (AML) or chronic myeloid leukemia (CML)135,136. AML is characterized by the infiltration of malignant hematopoietic cells into various tissues, including bone marrow and blood137. While conventional chemotherapy is commonly used for AML, emerging research suggests that immunotherapies targeting surface molecules, chimeric antigen receptor (CAR) T-cell therapy, and cancer vaccines could be strategies for AML treatment138. CML is a slower-developing blood cancer similar to AML139. Studies investigating the effects of immunotherapy for CML have explored immunostimulatory treatments such as tyrosine kinase inhibitors, IFN-γ therapy, and ICBs140. Since this review focuses on solid cancers, we will not delve further into blood cancers.
Recruitment of antitumoral immune cells normalizes the protumoral immune profile
Modulating the immune profile of the TME by recruiting antitumoral immune cells can create a favorable environment for cancer treatment. One approach to achieve this is through the use of oncolytic viruses (OVs). OVs are either genetically modified or naturally occurring viruses that selectively replicate in cancer cells to kill the tumor without harming normal cells141. Talimogene laherparepvec (T-VEC, Imlygic) is a recombinant herpes simplex virus (HSV)-1 engineered to express human granulocyte–macrophage colony-stimulating factor (GM-CSF). It replicates within tumor cells, causing their lysis and the release of GM-CSF. This recruitment of GM-CSF promotes the maturation of dendritic cells (DCs) and macrophages, establishing a robust antitumor immune response142 (Fig. 2).
Inflammatory cytokines can also be utilized to recruit antitumoral immune cells. Interferon-α (IFN-α), one of the first cytokines approved for cancer therapy, upregulates MHC class I expression, induces caspase-dependent apoptosis, and attenuates tumor angiogenesis143. Although IFN-α treatment has shown effectiveness as a single agent, its effects are enhanced when combined with other anticancer drugs144. Another cytokine, interleukin-2 (IL-2), can recruit antitumoral immune cells. High-dose IL-2 treatment has demonstrated promising results in metastatic renal cell carcinoma and melanoma145,146.
Physical methods can be employed to recruit immune cells. For example, mild photothermal therapy has been shown to effectively increase the infiltration of lymphocytes into tumors and enhance antitumoral T-cell activity147.
Adoptive transfer of engineered immune cells is a promising normalization method in blood cancers
Forming an antitumoral immune environment can also be achieved through the adoptive transfer of autologous immune cells that have been isolated, engineered, and expanded in advance to generate durable antitumor immune responses148. One example of this approach is CAR T-cell therapy, which involves transferring T cells engineered with synthetic receptors capable of recognizing tumor-associated antigens (TAAs) to kill tumor cells149. CAR T cells utilize cytotoxic pathways similar to those employed by effector CD8 + T cells, including the secretion of perforin and granzymes and the activation of apoptotic pathways through death receptors150. CAR T-cell therapy targeting the CD19 molecule on B cells has demonstrated effectiveness in eradicating lymphoma151.
Although CAR T-cell therapy has shown promising results, it also has limitations. In many solid tumors, CAR T-cell therapy exhibits low efficacy due to limited infiltration152. However, considering the overall advantages and effectiveness of CAR T-cell therapy, efforts to improve its performance in solid tumors have been pursued through various approaches, such as expressing CCR2 or overexpressing heparanase (HPSE)153,154.
Cancer vaccines boost the function of antitumoral immune cells
Cancer vaccines are designed to enhance antitumor immune responses to tumor-associated antigens (TAAs) by modulating the antigen-presenting function of antigen-presenting cells (APCs) and establishing durable antitumor memory155. Sipuleucel-T (Provenge, Dendreon) is an FDA-approved therapeutic cancer vaccine used for prostate cancer. It is produced by culturing autologous peripheral-blood mononuclear cells (PBMCs) and activating them with engineered antigens containing the TAA prostatic acid phosphatase (PAP)156. Treatment with Sipuleucel-T prior to prostate cancer surgery has been shown to enhance T-cell proliferation, activation, and infiltration.
Cancer vaccines are expected to synergize with immune checkpoint inhibitors (ICBs) since their ultimate goal is to enhance adaptive immunity157. Additionally, cancer vaccines have the potential to induce long-lasting antitumor efficacy by triggering immunological memory. The efficacy of cancer vaccines can be compromised by various factors within the TME, such as the local accumulation of immunosuppressive myeloid cells and Tregs, as well as downregulation of antigen expression and alterations in the antigen processing pathway155.
The immunogenic cell death and STING pathways are promising targets for immune normalization
Another approach to normalize the immune microenvironment is through controllable lytic cell death, which induces immunogenic cell death (ICD)158. Controllable ICD pathways, such as necroptosis, ferroptosis, and pyroptosis, trigger the release or surface expression of damage-associated molecular patterns (DAMPs), which can function as adjuvants or danger signals to enhance the immune response against tumors159. Several anticancer agents currently used in the clinic, including such chemotherapeutic agents as oxaliplatin, induce hallmarks of ICD, suggesting their potential as vaccines for inducing protective anticancer immune responses160,161.
A second method of immune normalization is targeting the STING pathway, which plays a crucial role in the antitumoral response of CD8 + T cells in several cancer models162. Notably, the expression of STING in endothelial cells has been correlated with better prognosis in human colon and breast cancer163. Targeting the STING pathway not only helps normalize the immune environment but also promotes vascular normalization. Specifically, intratumoral STING activation normalizes the tumor vasculature through type I IFN signaling and CD8 + T cell activity163.
Conclusions
Vascular normalization holds promise as an explanation for the synergistic effects observed when combining AATs with chemotherapy and immunotherapy, which are not as effective when used alone. There is still debate about the actual benefits of this strategy. For instance, in cerebral tumors, vascular normalization may restore the low permeability of the brain vasculature, potentially hindering the delivery of therapeutic agents and reducing the effectiveness of other treatments164. Similarly, vascular normalization with bevacizumab in xenograft models of ovarian and esophageal cancer leads to decreased uptake of antibodies, highlighting the need for careful consideration when combining AATs with other therapies165. Nonetheless, there is ongoing discussion about whether the dose of bevacizumab used in that trial was too high or appropriate. These debates underscore the necessity for clear guidelines that recommend an effective dose, timing, and duration of treatment that will enhance blood vessel function while minimizing adverse effects.
Another challenge is the concept of the normalization window, which varies significantly between patients and poses a significant obstacle to clinical application. Therefore, the development of strategies aimed at improving blood vessel function, rather than relying solely on existing drugs, may be necessary.
In relation to immune normalization, the heterogeneity of immune cell populations in the TME plays a crucial role in the response to anticancer therapies166,167 (Fig. 2). Recent advancements in single-cell omics technologies have provided insights into this diversity168, but our understanding is far from complete. Moreover, even cell types that were previously thought to be well defined are composed of heterogeneous subsets. For example, the current classifications of M1-like and M2-like TAMs and myeloid-derived suppressor cells (MDSCs) may oversimplify the sophisticated phenotypes/functions of these cells within tumors. Therefore, to better normalize the TIME, a deeper understanding of the functions of diverse immune cell subtypes is needed.
Many immune cell types exhibit context-dependent functions in tumors, meaning that the same subtype may have different effects depending on the tumor type. Inflammatory immune cells, for instance, are often considered beneficial cells that target tumor cells. However, in PDAC, the well-known inflammation has been shown to enhance desmoplasia and accelerate cancer cell proliferation169. Therefore, considering the diverse roles of immune cells in various cancer types is crucial for harnessing the immense potential of immune cells within the TME to combat the tumor.
As mentioned above, vascular normalization and immune normalization are intertwined strategies, each strategy impacting the other. Vascular normalization improves vessel function, thereby influencing the immune cell population in the TME. Conversely, immune cells can also induce vascular normalization. Based on these findings, several clinical trials evaluating the safety and efficacy of AATs in combination with immunotherapies have been conducted in various tumor types (Table 1). Targeting CAFs not only affects vascular normalization but also impacts immune cells, potentially enhancing the effects of immunotherapies. Some reports suggest that the effects of CAF-targeting antibodies are mediated by CD8 + T cells, further highlighting the close relationship between vascular and immune normalization170.
Table 1.
List of clinical trials combining antiangiogenic therapies and immunotherapies.
| Tumor Type | Clinical Trial ID | AAT | IT | Other therapies included in the combinationa |
|---|---|---|---|---|
| Malignant Solid Tumor | NCT02857920 | Bevacizumab | Allogenic NK therapy | No |
| Hepatocellular carcinoma/Bilary tract cancer | NCT04518852 | Sorafenib | PD-1 mAbb | Yes |
| NCT04273100 | Lenvatinib | PD-1 mAbb | Yes | |
| NCT05313282 | Apatinib | Camrelizumab | Yes | |
| NCT05775159 |
Bevacizumab Lenvatinib |
MEDI5752 | Yes | |
| NCT05665348 | Bevacizumab |
Atezolizumab Ipilimumab |
No | |
| NCT05750030 | Bevacizumab | Atezolizumab | Yes | |
| NCT04191889 | Apatinib | Camrelizumab | Yes | |
| NCT05052099 | Bevacizumab |
Atezolizumab RP3 |
No | |
| NCT05733598 | Bevacizumab | Atezolizumab | Yes | |
| NCT03937830 | Bevacizumab |
Durvalumab Tremelimumab |
Yes | |
| NCT02519348 | Bevacizumab |
Durvalumab Tremelimumab |
Yes | |
| NCT05359861 | Bevacizumab |
Atezolizumab SRF388 |
No | |
| Ovarian cancer | NCT04928583 | Bevacizumab | Oregovomab | Yes |
| NCT02759588 | Bevacizumab | GL-ONC1 | No | |
| NCT03197584 | Bevacizumab | Avelumab | Yes | |
| NCT05281471 | Bevacizumab | Olvimulogene nanivacirepvec | Yes | |
| Gastrointestinal cancer | NCT04069273 | Ramucirumab | Pembrolizumab | Yes |
| Lung cancer | NCT05360979 | Recombinant human endostatin | Envafolimab | Yes |
| NCT05529355 | Endostar | Envafolimab | Yes | |
| NCT04459078 | Apatinib | Camrelizumab | Yes | |
| NCT03280563 | Bevacizumab | Atezolizumab | Yes | |
| NCT05781308 | Bevacizumab | Atezolizumab | No | |
| NCT05782764 | Endostar | ICBb | Yes | |
| NCT04303130 | Endostar | Camrelizumab | No | |
| NCT03169738 | Bevacizumab | Avelumab | Yes | |
| NCT02039674 | Bevacizumab | Pembrolizumab | Yes | |
| Pancreatic cancer | NCT05298020 | Endostar | Envafolimab | Yes |
| NCT03136406 | Bevacizumab | Severalc | Yes | |
| NCT03329248 | Bevacizumab | Avelumab | Yes | |
| NCT03387098 | Bevacizumab | Several | Yes | |
| NCT03193190 | Bevacizumab | Several | Yes | |
| Breast cancer | NCT04877821 | Anlotinib | Sintilimab | No |
| NCT03395899 | Bevacizumab | Atezolizumab | Yes | |
| NCT0518006 | Bevacizumab | Atezolizumab | No | |
| NCT03175666 | Bevacizumab | Avelumab | Yes | |
| NCT04739670 | Bevacizumab | Atezolizumab | Yes | |
| NCT03387085 | Bevacizumab | Severalc | Yes | |
| Colorectal cancer | NCT03169777 | Bevacizumab | Severalc | Yes |
| NCT04262687 | Bevacizumab | Pembrolizumab | Yes | |
| NCT03950154 | Bevacizumab | PD-1-T cell | Yes | |
| NCT04017455 | Bevacizumab | Atezolizumab | No | |
| NCT05733611 | Bevacizumab | Atezolizumab | Yes | |
| NCT04527068 | Bevacizumab | Tripleitriumab | No | |
| NCT04194359 | Bevacizumab | Sintilimab | No | |
| Blood cancer | NCT03169790 | Bevacizumab | Avelumab | Yes |
| NCT03167177 | Bevacizumab | Avelumab | Yes | |
| Nasopharyngeal cancer | NCT03813394 | Bevacizumab | Pembrolizumab | No |
| GBM | NCT04952571 | Bevacizumab | Camrelizumab | No |
| Skin cancer | NCT03167164 | Bevacizumab | Avelumab | Yes |
| NCT03387111 | Bevacizumab | Several | Yes | |
| Urothelial cancer | NCT03197571 | Bevacizumab | Avelumab | Yes |
| Head and Neck Squamous Cell Carcinoma | NCT03193190 | Bevacizumab | Avelumab | Yes |
AAT anti-angiogenic therapies; IT immunotherapy.
Clinical trials are included if the trial title mentions a combination of AAT and IT. Resource: http://clinicaltrials.gov.
aOther therapies include chemotherapy and transarterial chemoembolization.
bSpecific therapeutics used are not clarified.
cMore than two types of immunotherapies were utilized.
Therefore, instead of pursuing a single strategy, targeting both vascular and immune normalization simultaneously may yield even greater efficacy. Studies evaluating the effects of different combinations of strategies are warranted to fully explore the potential benefits of this approach.
Acknowledgements
This work was supported by the National Research Foundation of Korea [grant RS-2023–00208004 to KJ], by the Bio & Medical Technology Development Program of the National Research Foundation funded by the Ministry of Science & ICT [NRF-2021M3A9I4026318 to KJ], by grant 800–20220113 from Seoul National University [to KJ], by grant no 0320220050 and grant no 2620200020 from the SNUH Research Fund [to KJ], and by the Hyundai Motor Chung Mong-Koo Foundation [to YC]. We also extend our gratitude to all members of the Jung Lab.
Author contributions
K.J. conceived the concept. Y.C. and K.J. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zane L, Sharma V, Misteli T. Common features of chromatin in aging and cancer: cause or coincidence? Trends Cell Biol. 2014;24:686–694. doi: 10.1016/j.tcb.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaaf MB, Garg AD, Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018;9:115. doi: 10.1038/s41419-017-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Hyeon DY, et al. Proteogenomic landscape of human pancreatic ductal adenocarcinoma in an Asian population reveals tumor cell-enriched and immune-rich subtypes. Nat. Cancer. 2023;4:290–307. doi: 10.1038/s43018-022-00479-7. [DOI] [PubMed] [Google Scholar]
- 5.Ganss R, Arnold B, Hämmerling GJ. Mini-review: Overcoming tumor-intrinsic resistance to immune effector function. Eur. J. Immunol. 2004;34:2635–2641. doi: 10.1002/eji.200425474. [DOI] [PubMed] [Google Scholar]
- 6.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015;15:669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 8.Munn LL, Jain RK. Vascular regulation of antitumor immunity. Science. 2019;365:544–545. doi: 10.1126/science.aaw7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suwa T, Kobayashi M, Nam J-M, Harada H. Tumor microenvironment and radioresistance. Exp. Mol. Med. 2021;53:1029–1035. doi: 10.1038/s12276-021-00640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin JD, Seano G, Jain RK. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu. Rev. Physiol. 2019;81:505–534. doi: 10.1146/annurev-physiol-020518-114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whatcott CJ, Han H, Von Hoff DD. Orchestrating the tumor microenvironment to improve survival for patients with pancreatic cancer: normalization, not destruction. Cancer J. 2015;21:299–306. doi: 10.1097/PPO.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng J, Gao P. Toward normalization of the tumor microenvironment for cancer therapy. Integr. Cancer Ther. 2019;18:1534735419862352. doi: 10.1177/1534735419862352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 15.Binnewies M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Invest. 2013;123:3190–3200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg, Robert A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 2009;19:329–337. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 21.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat. Rev. Immunol. 2011;11:702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 23.Zhang N, et al. DLL1 orchestrates CD8+ T cells to induce long-term vascular normalization and tumor regression. Proc. Natl. Acad. Sci. USA. 2021;118:e2020057118. doi: 10.1073/pnas.2020057118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noman MZ, et al. Hypoxia: a key player in antitumor immune response. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiology-Cell Physiol. 2015;309:C569–C579. doi: 10.1152/ajpcell.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira BA, et al. CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trends Cancer. 2019;5:724–741. doi: 10.1016/j.trecan.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Costa A, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–479.e410. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Yang D, Liu J, Qian H, Zhuang Q. Cancer-associated fibroblasts: from basic science to anticancer therapy. Exp. Mol. Med. 2023;55:1322–1332. doi: 10.1038/s12276-023-01013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abou Khouzam R, et al. Tumor hypoxia regulates immune escape/invasion: influence on angiogenesis and potential impact of hypoxic biomarkers on cancer therapies. Front. Immunol. 2021;11:613114. doi: 10.3389/fimmu.2020.613114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sørensen BS, Horsman MR. Tumor hypoxia: impact on radiation therapy and molecular pathways. Front. Oncol. 2020;10:562. doi: 10.3389/fonc.2020.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosse JP, Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med. Chem. 2008;8:790–797. doi: 10.2174/187152008785914798. [DOI] [PubMed] [Google Scholar]
- 33.Jayaprakash P, Vignali PDA, Delgoffe GM, Curran MA. Hypoxia reduction sensitizes refractory cancers to immunotherapy. Annu. Rev. Med. 2022;73:251–265. doi: 10.1146/annurev-med-060619-022830. [DOI] [PubMed] [Google Scholar]
- 34.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 35.Kim KJ, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 36.Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc. Natl Acad. Sci. USA. 2013;110:18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388:518–529. doi: 10.1016/S0140-6736(15)01088-0. [DOI] [PubMed] [Google Scholar]
- 38.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat. Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 39.Goel S, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao XC, et al. Inhibition of tumor angiogenesis and tumor growth by the DSL domain of human Delta-like 1 targeted to vascular endothelial cells. Neoplasia. 2013;15:815–825. doi: 10.1593/neo.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang JP, et al. Overexpression of Notch ligand Dll1 in B16 melanoma cells leads to reduced tumor growth due to attenuated vascularization. Cancer Lett. 2011;309:220–227. doi: 10.1016/j.canlet.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Tolaney SM, et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc. Natl Acad. Sci. USA. 2015;112:14325–14330. doi: 10.1073/pnas.1518808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol. Cancer Ther. 2008;7:3670–3684. doi: 10.1158/1535-7163.MCT-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, et al. Improving immune–vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018;18:195–203. doi: 10.1038/nri.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol. 2018;15:310–324. doi: 10.1038/nrclinonc.2018.9. [DOI] [PubMed] [Google Scholar]
- 49.Taal W, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–953. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 50.Rak JW, St Croix BD, Kerbel RS. Consequences of angiogenesis for tumor progression, metastasis and cancer therapy. Anti-Cancer Drug. 1995;6:3–18. doi: 10.1097/00001813-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Kerbel RS. Tumor angiogenesis. N. Engl. J. Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan F, et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc. Natl Acad. Sci. USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wildiers H, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Brit. J. Cancer. 2003;88:1979–1986. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Co. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 55.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 2018;15:325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dings RP, et al. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin. Cancer Res. 2007;13:3395–3402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Min H-Y, Lee H-Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022;54:1670–1694. doi: 10.1038/s12276-022-00864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 59.Chauhan, V. P. et al. in Nano-Enabled Medical Applications 279-311 (Jenny Stanford Publishing, 2020).
- 60.Tong RT, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 61.Wu J-b, Tang Y-l, Liang X-h. Targeting VEGF pathway to normalize the vasculature: an emerging insight in cancer therapy. OncoTargets. Ther. 2018;11:6901–6909. doi: 10.2147/OTT.S172042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010;13:1–14. doi: 10.1007/s10456-009-9160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Batra S, Matsumoto S, Hyodo F, Mitchell J, Krishna MC. MRI assessment of angiogenesis inhibitor sunitinib’s influence on tumor oxygenation to identify an optimal chemoradiotherapeutic window. Int. J. Radiat. Oncol. 2009;75:S43. [Google Scholar]
- 64.Zhou Q, Gallo JM. Differential effect of sunitinib on the distribution of temozolomide in an orthotopic glioma model. Neuro-Oncology. 2009;11:301–310. doi: 10.1215/15228517-2008-088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends. Mol. Med. 2011;17:347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 66.Holash J, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 67.Nasarre P, et al. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer Res. 2009;69:1324–1333. doi: 10.1158/0008-5472.CAN-08-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peterson TE, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl Acad. Sci. 2016;113:4470–4475. doi: 10.1073/pnas.1525349113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kloepper J, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc. Natl Acad. Sci. 2016;113:4476–4481. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han S, et al. Amelioration of sepsis by TIE2 activation induced vascular protection. Sci. Transl. Med. 2016;8:335ra355–335ra355. doi: 10.1126/scitranslmed.aad9260. [DOI] [PubMed] [Google Scholar]
- 71.Park J-S, et al. Normalization of Tumor Vessels by Tie2 Activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell. 2016;30:953–967. doi: 10.1016/j.ccell.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 72.Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat. Rev. Immunol. 2013;13:427–437. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 73.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat. Rev. Cancer. 2007;7:327–331. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 74.Yan M, Plowman GD. Delta-like 4/Notch signaling and its therapeutic implications. Clin. Cancer Res. 2007;13:7243–7246. doi: 10.1158/1078-0432.CCR-07-1393. [DOI] [PubMed] [Google Scholar]
- 75.Patel NS, et al. Up-Regulation of Endothelial Delta-like 4 Expression Correlates with Vessel Maturation in Bladder Cancer. Clin. Cancer Res. 2006;12:4836–4844. doi: 10.1158/1078-0432.CCR-06-0285. [DOI] [PubMed] [Google Scholar]
- 76.Yan M, et al. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:E6–E7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- 77.Yan X-Q, et al. A novel Notch ligand, Dll4, induces T-cell leukemia/lymphoma when overexpressed in mice by retroviral-mediated gene transfer. Blood. 2001;98:3793–3799. doi: 10.1182/blood.v98.13.3793. [DOI] [PubMed] [Google Scholar]
- 78.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat. Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 79.Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cerniglia GJ, et al. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS One. 2009;4:e6539. doi: 10.1371/journal.pone.0006539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serban D, Leng J, Cheresh D. H-Ras regulates angiogenesis and vascular permeability by activation of distinct downstream effectors. Circ. Res. 2008;102:1350–1358. doi: 10.1161/CIRCRESAHA.107.169664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cohen LH, et al. Inhibitors of prenylation of Ras and other G-proteins and their application as therapeutics. Biochem. Pharmacol. 2000;60:1061–1068. doi: 10.1016/s0006-2952(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 83.Cohen-Jonathan E, et al. The farnesyltransferase inhibitor L744,832 reduces hypoxia in tumors expressing activated H-ras. Cancer Res. 2001;61:2289–2293. [PubMed] [Google Scholar]
- 84.Delmas C, et al. The farnesyltransferase inhibitor R115777 reduces hypoxia and matrix metalloproteinase 2 expression in human glioma xenograft. Clin. Cancer Res. 2003;9:6062–6068. [PubMed] [Google Scholar]
- 85.Pore N, et al. Nelfinavir down-regulates hypoxia-inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006;66:9252–9259. doi: 10.1158/0008-5472.CAN-06-1239. [DOI] [PubMed] [Google Scholar]
- 86.Qayum N, et al. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Res. 2009;69:6347–6354. doi: 10.1158/0008-5472.CAN-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma PC, Maulik G, Christensen J, Salgia R. C-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22:309–325. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- 88.Lee J-H, et al. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19:4947–4953. doi: 10.1038/sj.onc.1203874. [DOI] [PubMed] [Google Scholar]
- 89.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 90.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat. Rev. Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 91.Xin X, et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am. J. Pathol. 2001;158:1111–1120. doi: 10.1016/S0002-9440(10)64058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shojaei F, et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70:10090–10100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 93.Cascone T, et al. The HGF/c-MET Pathway Is a Driver and Biomarker of VEGFR-inhibitor Resistance and Vascular Remodeling in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2017;23:5489–5501. doi: 10.1158/1078-0432.CCR-16-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benkhoucha M, et al. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+ Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA. 2010;107:6424–6429. doi: 10.1073/pnas.0912437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tian L, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Palma M, Jain RK. CD4+ T cell activation and vascular normalization: two sides of the same coin? Immunity. 2017;46:773–775. doi: 10.1016/j.immuni.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 97.Ong T, et al. Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit. Care Med. 2010;38:1845–1851. doi: 10.1097/CCM.0b013e3181eaa5bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng X, et al. CTLA4 blockade promotes vessel normalization in breast tumors via the accumulation of eosinophils. Int. J. Cancer. 2020;146:1730–1740. doi: 10.1002/ijc.32829. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y, et al. Vascular normalization was associated with colorectal tumor regression upon anti-PD-L1 combinational therapy. J. Immunol. Res. 2023;2023:5867047. doi: 10.1155/2023/5867047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walford G, Loscalzo J. Nitric oxide in vascular biology. J. Thromb. Haemost. 2003;1:2112–2118. doi: 10.1046/j.1538-7836.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- 101.Sung Y-C, et al. Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies. Nat, Nanotechnol. 2019;14:1160–1169. doi: 10.1038/s41565-019-0570-3. [DOI] [PubMed] [Google Scholar]
- 102.Papageorgis P, et al. Tranilast-induced stress alleviation in solid tumors improves the efficacy of chemo- and nanotherapeutics in a size-independent manner. Sci. Rep. 2017;7:46140. doi: 10.1038/srep46140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klug F, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 104.Ho YJ, et al. Normalization of tumor vasculature by oxygen microbubbles with ultrasound. Theranostics. 2019;9:7370–7383. doi: 10.7150/thno.37750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matejuk A, Collet G, Nadim M, Grillon C, Kieda C. MicroRNAs and tumor vasculature normalization: impact on anti-tumor immune response. Arch. Immunol. Ther. Exp. 2013;61:285–299. doi: 10.1007/s00005-013-0231-4. [DOI] [PubMed] [Google Scholar]
- 106.Drake, C. G., Jaffee, E. & Pardoll, D. M. In Advances in Immunology Vol. 90 51-81 (Academic Press, 2006). [DOI] [PubMed]
- 107.Voron T, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gabrilovich DI, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 109.Gabrilovich D, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 110.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat. Rev. Cancer. 2012;12:210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 111.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fritz JM, Lenardo MJ. Development of immune checkpoint therapy for cancer. J. Exp. Med. 2019;216:1244–1254. doi: 10.1084/jem.20182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sobhani N, et al. CTLA-4 in regulatory T cells for cancer immunotherapy. Cancers. 2021;13:1440. doi: 10.3390/cancers13061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am. J. Cancer Res. 2020;10:727. [PMC free article] [PubMed] [Google Scholar]
- 115.LV B, et al. Immunotherapy: reshape the tumor immune microenvironment. Front. Immunol. 2022;13:844142. doi: 10.3389/fimmu.2022.844142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jahchan NS, et al. Tuning the tumor myeloid microenvironment to fight cancer. Front. Immunol. 2019;10:1611. doi: 10.3389/fimmu.2019.01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jung K, et al. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. J. Clin. Invest. 2017;127:3039–3051. doi: 10.1172/JCI93182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jung K, et al. Targeting CXCR4-dependent immunosuppressive Ly6C(low) monocytes improves antiangiogenic therapy in colorectal cancer. Proc. Natl Acad. Sci. USA. 2017;114:10455–10460. doi: 10.1073/pnas.1710754114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ries CH, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 120.Jeong J, Suh Y, Jung K. Context drives diversification of monocytes and neutrophils in orchestrating the tumor microenvironment. Front. Immunol. 2019;10:1817. doi: 10.3389/fimmu.2019.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qian B-Z, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kalbasi A, et al. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2017;23:137–148. doi: 10.1158/1078-0432.CCR-16-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanford DE, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 Axis. Clin. Cancer Res. 2013;19:3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bonapace L, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 125.Long KB, et al. IFNγ and CCL2 cooperate to redirect tumor-infiltrating monocytes to degrade fibrosis and enhance chemotherapy efficacy in pancreatic carcinoma. Cancer Discov. 2016;6:400–413. doi: 10.1158/2159-8290.CD-15-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hanna RN, et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985–990. doi: 10.1126/science.aac9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kubo H, Mensurado S, Gonçalves-Sousa N, Serre K, Silva-Santos B. Primary tumors limit metastasis formation through induction of IL15-mediated cross-talk between patrolling monocytes and NK cells. Cancer Immunol. Res. 2017;5:812–820. doi: 10.1158/2326-6066.CIR-17-0082. [DOI] [PubMed] [Google Scholar]
- 128.Jeong J, et al. Tumor-Infiltrating neutrophils and non-classical monocytes may be potential therapeutic targets for HER2(negative) Gastric Cancer. Immune Netw. 2021;21:e31. doi: 10.4110/in.2021.21.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kwak S-B, et al. Tumor regionalization after surgery: Roles of the tumor microenvironment and neutrophil extracellular traps. Exp. Mol. Med. 2022;54:720–729. doi: 10.1038/s12276-022-00784-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rayes, R. F. et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis-promoting effects. JCI Insight4, 10.1172/jci.insight.128008 (2019). [DOI] [PMC free article] [PubMed]
- 131.Yuan ZY, Luo RZ, Peng RJ, Wang SS, Xue C. High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. Onco. Targets Ther. 2014;7:1475–1480. doi: 10.2147/OTT.S61838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J. Clin. Invest. 2016;126:3672–3679. doi: 10.1172/JCI84427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nywening TM, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–662. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Petty, A. J., Owen, D. H., Yang, Y. & Huang, X. Targeting Tumor-Associated Macrophages in Cancer Immunotherapy. Cancers (Basel)13, 10.3390/cancers13215318 (2021). [DOI] [PMC free article] [PubMed]
- 135.Kim J, et al. Novel endogenous endoplasmic reticulum transmembrane protein SURF4 suppresses cell death by negatively regulating the STING-STAT6 axis in myeloid leukemia. Cancer Commun. (Lond.) 2023;43:395–399. doi: 10.1002/cac2.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Woo SY, et al. Role of reactive oxygen species in regulating 27-hydroxycholesterol-induced apoptosis of hematopoietic progenitor cells and myeloid cell lines. Cell Death Dis. 2022;13:916. doi: 10.1038/s41419-022-05360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N. Engl. J. Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 138.Beyar-Katz O, Gill S. Novel approaches to acute myeloid leukemia immunotherapy. Clin. Cancer Res. 2018;24:5502–5515. doi: 10.1158/1078-0432.CCR-17-3016. [DOI] [PubMed] [Google Scholar]
- 139.Faderl S, et al. The biology of chronic myeloid leukemia. N. Engl. J. Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 140.Ilander M, Hekim C, Mustjoki S. Immunology and immunotherapy of chronic myeloid leukemia. Curr. Hematol. Malig. Rep. 2014;9:17–23. doi: 10.1007/s11899-013-0190-1. [DOI] [PubMed] [Google Scholar]
- 141.Cao G-D, et al. The oncolytic virus in cancer diagnosis and treatment. Front. Oncol. 2020;10:1786. doi: 10.3389/fonc.2020.01786. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 142.Zheng M, Huang J, Tong A, Yang H. Oncolytic viruses for cancer therapy: barriers and recent advances. Molecular Therapy - Oncolytics. 2019;15:234–247. doi: 10.1016/j.omto.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the treatment of cancer. J. Interf. Cytok. Res. 2019;39:6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kirkwood JM, Resnick GD, Cole BF. Efficacy, safety, and risk-benefit analysis of adjuvant interferon alfa-2b in melanoma. Semin. Oncol. 1997;24:S16–S23. [PubMed] [Google Scholar]
- 145.Klapper JA, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Davar D, et al. High-dose interleukin-2 (HD IL-2) for advanced melanoma: a single center experience from the University of Pittsburgh Cancer Institute. J. Immunother. Cancer. 2017;5:74. doi: 10.1186/s40425-017-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang L, et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat. Commun. 2019;10:4871. doi: 10.1038/s41467-019-12771-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Benmebarek, M. R. et al. Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells. Int. J. Mol. Sci. 20 (2019). [DOI] [PMC free article] [PubMed]
- 151.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Safarzadeh Kozani, P. et al. Recent Advances in Solid Tumor CAR-T Cell Therapy: Driving Tumor Cells From Hero to Zero? Front. Immunol. 13, 10.3389/fimmu.2022.795164 (2022). [DOI] [PMC free article] [PubMed]
- 153.Moon EK, et al. Expression of a Functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human t cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Caruana I, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 2015;21:524–529. doi: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat. Rev. Cancer. 2021;21:360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 156.Higano CS, et al. Sipuleucel-T. Nat. Rev. Drug Discov. 2010;9:513–514. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- 157.Ali OA, Lewin SA, Dranoff G, Mooney DJ. Vaccines combined with immune checkpoint antibodies promote cytotoxic t-cell activity and tumor eradication. Cancer Immunol. Res. 2016;4:95–100. doi: 10.1158/2326-6066.CIR-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Choi M, et al. Immunogenic cell death in cancer immunotherapy. BMB Rep. 2023;56:275–286. doi: 10.5483/BMBRep.2023-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krysko DV, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 160.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 161.Vanmeerbeek I, et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology. 2020;9:1703449. doi: 10.1080/2162402X.2019.1703449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Demaria O, et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl Acad. Sci. USA. 2015;112:15408–15413. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yang H, et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J. Clin. Invest. 2019;129:4350–4364. doi: 10.1172/JCI125413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ribatti D. Vascular normalization: a real benefit? Cancer Chemother. Pharm. 2011;68:275–278. doi: 10.1007/s00280-011-1683-z. [DOI] [PubMed] [Google Scholar]
- 165.Arjaans M, et al. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res. 2013;73:3347–3355. doi: 10.1158/0008-5472.CAN-12-3518. [DOI] [PubMed] [Google Scholar]
- 166.Cassier PA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 167.Papadopoulos KP, et al. First-in-human study of AMG 820, a monoclonal anti-colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin. Cancer Res. 2017;23:5703–5710. doi: 10.1158/1078-0432.CCR-16-3261. [DOI] [PubMed] [Google Scholar]
- 168.Kim C, Lee H, Jeong J, Jung K, Han B. MarcoPolo: a method to discover differentially expressed genes in single-cell RNA-seq data without depending on prior clustering. Nucleic Acids Res. 2022;50:e71–e71. doi: 10.1093/nar/gkac216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang T, Ren Y, Yang P, Wang J, Zhou H. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Cell Death Dis. 2022;13:897. doi: 10.1038/s41419-022-05351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim DK, et al. PD-L1-directed PlGF/VEGF blockade synergizes with chemotherapy by targeting CD141+ cancer-associated fibroblasts in pancreatic cancer. Nat. Commun. 2022;13:6292. doi: 10.1038/s41467-022-33991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]