Abstract
Purpose:
The clinical significance of the p53-abnormal (p53abn) molecular subtype in stage I low-grade endometrioid endometrial carcinoma (EEC) is debated. We aimed to review pathologic and molecular characteristics, and outcomes of stage I low-grade p53abn EEC in a large international cohort.
Experimental Design:
Previously diagnosed stage I p53abn EC (POLE–wild-type, mismatch repair–proficient) low-grade EEC from Canadian retrospective cohorts and PORTEC-1&2 trials were included. Pathology review was performed by six expert gynecologic pathologists blinded to p53 status. IHC profiling, next-generation sequencing, and shallow whole-genome sequencing was performed. Kaplan–Meier method was used for survival analysis.
Results:
We identified 55 stage I p53abn low-grade EEC among 3,387 cases (2.5%). On pathology review, 17 cases (31%) were not diagnosed as low-grade EEC by any pathologists, whereas 26 cases (47%) were diagnosed as low-grade EEC by at least three pathologists. The IHC and molecular profile of the latter cases were consistent with low-grade EEC morphology (ER/PR positivity, patchy p16 expression, PIK3CA and PTEN mutations) but they also showed features of p53abn EC (TP53 mutations, many copy-number alterations). These cases had a clinically relevant risk of disease recurrence (5-year recurrence-free survival 77%), with pelvic and/or distant recurrences observed in 12% of the patients.
Conclusions:
A subset of p53abn EC is morphologically low-grade EEC and exhibit genomic instability. Even for stage I disease, p53abn low-grade EEC are at substantial risk of disease recurrence. These findings highlight the clinical relevance of universal p53-testing, even in low-grade EEC, to identify women at increased risk of recurrence.
Translational Relevance.
Previous studies have reported that a small subset of endometrial carcinomas (EC) have the counterintuitive combination of low-grade (FIGO grade 1–2) endometroid histology and p53-abnormal (p53abn) molecular type. There is a question as to whether these EC are truly low-grade endometrioid or misclassified glandular variants of serous EC, and it is unclear how they behave compared with low-grade endometrioid EC of other molecular types. Here, we demonstrate that a subset of these p53abn EC are morphologically low-grade after stringent pathology review. The IHC and molecular profile of these cases shows features of both endometrioid (patchy p16, ER/PR positivity, and frequent PIK3CA and PTEN alterations) and prototypical p53abn EC (TP53 mutated, high copy-number alterations). Importantly, these tumors have a substantial risk of disease recurrence, supporting recent guidelines recommending all p53abn EC with myometrial invasion be considered high-risk and treated with adjuvant therapy, regardless of stage, grade, and histotype.
Introduction
The Cancer Genome Atlas molecular classification of endometrial carcinomas (EC) provides a reproducible framework for the categorization of EC with important diagnostic, prognostic, and therapeutic implications (1). This molecular classification comprises four genomic subtypes, namely POLE ultramutated (POLEmut), microsatellite instable hypermutated associated with mismatch repair deficiency (MMRd), copy-number–low (CN-L), and copy-number–high (CN-H; ref. 1). The latter subtype is characterized by serous and endometrioid morphology with a high number of somatic copy-number alterations (CNA), a low mutational burden, and frequent TP53 mutations (90%; ref. 1). In clinical practice, cheaper and easier-to-interpret surrogate markers, including targeted POLE sequencing, MMR-, and p53 IHC, are used to molecularly classify EC following the stepwise diagnostic algorithm included in the World Health Organization (WHO) Classification of Female Genital Tumors (2, 3). Using this algorithm, EC with mutant-type p53 expression in the absence of a pathogenic POLE mutation and MMRd are classified as p53-abnormal (p53abn). The p53abn molecular subtype has been repeatedly shown to have poor clinical outcomes, even in patients with stage I disease. Moreover, the p53abn molecular subtype has been shown to benefit from adjuvant chemotherapy (4–9). For these reasons, the ESGO-ESTRO-ESP (2021) and ESMO (2022) clinical guidelines now recommend all patients with p53abn EC with myometrial invasion be considered high-risk, regardless of stage, grade, or histotype, and be treated with chemotherapy with or without radiotherapy (10, 11).
Most p53abn EC are serous or high-grade endometrioid EC (EEC), but other histotypes such as carcinosarcoma, clear cell carcinoma, and uncommonly low-grade (FIGO grade 1–2) EEC are observed (4–7, 12–14). Although this latter combination (low-grade histology and p53abn) is counterintuitive, various publications report 5–15% low-grade EEC in p53abn EC (4–7, 12, 13, 15). It is currently unknown whether these p53abn low-grade EEC have an increased risk of disease recurrence compared with low-grade EEC of other molecular subtypes (specifically NSMP and MMRd), and whether they should be considered high-risk and treated with adjuvant chemotherapy according to the 2021 ESGO-ESTRO-ESP and 2022 EMSO recommendations. Previous studies on nonmolecularly classified ECs have shown adverse clinical outcomes in patients with low-grade EEC with abnormal p53 IHC expression and/or TP53 mutations compared with p53 wild-type tumors (16, 17).
Assessment of the molecular classification is currently recommended for all high-grade and/or advanced-stage EC cases, where the molecular subtype could alter the recommended adjuvant therapy (10). Furthermore, MMR testing is recommended for all EC for Lynch Syndrome screening (10). However, the assessment of the molecular classification in stage I low-grade EEC without other risk factors is debated as these patients generally have excellent clinical outcomes. Only the finding of a p53abn tumor would potentially impact clinical management. In this study, we aimed to pathologically review, molecularly characterize, and assess the clinical outcomes of stage I p53abn EC that were previously morphologically classified as low-grade EEC in our large cohorts.
Materials and Methods
Patient selection
Low-grade (grade 1 and 2) p53abn EEC cases were identified from previously published retrospectively collected Canadian population-based cohorts (n = 2,506; refs. 4–6, 18) and the PORTEC-1 and -2 clinical trials (n = 880; ref. 9) that had undergone molecular classification. The retrospective Canadian cohorts included EC that had previously undergone molecular classification, and where data on adjuvant therapy and clinical outcomes were available with a minimum of 2 years of follow-up. This included historical cohorts (discovery, confirmation, and validation) as well as a recent pan-Canadian research initiative encompassing EC diagnosed in 2016 from 29 cancer centers and community hospitals across Canada (4–6, 18). The design and results of the PORTEC-1 and -2 trials have been previously published (19, 20). In brief, PORTEC-1 included 714 patients with FIGO 2009 stage I grade 1–2 EEC with >50% myometrial invasion or grade 2–3 with <50% myometrial invasion that were randomized (1:1) between post-operative external beam radiotherapy (EBRT) and no adjuvant therapy (19). In PORTEC-2, 427 patients aged >60 years with stage I grade 1–2 EEC with >50% myometrial invasion or grade 3 with ≤50% myometrial invasion, or stage IIA EEC (apart from grade 3 with >50% myometrial invasion) were randomized (1:1) between EBRT or vaginal brachytherapy (VBT; ref. 20). Central pathology review was performed after randomization for PORTEC-1 and -2 to assess histotype, grade, and the presence and extent of lymphovascular space invasion (LVSI). In addition, one representative formalin-fixed, paraffin-embedded (FFPE) tumor block was selected for translational research.
Molecular subgroup assignment for the Canadian and PORTEC cohorts was performed following the WHO 2020 algorithm using targeted sequencing of the exonuclease domain of POLE and IHC staining for mismatch repair proteins and p53 (2). For this study, all FIGO stage I low-grade EEC molecularly classified as p53abn EC were selected. Hence, these were without a pathogenic POLE mutation or MMR-deficiency, as POLEmut-p53abn and MMRd-p53abn “double classifiers” are believed to behave like single classifier POLEmut and MMRd EC (21). Review of p53 IHC was performed by two expert gynecologic pathologists (T. Bosse and C.B. Gilks) to confirm abnormal p53 status and all cases underwent TP53 mutation next-generation sequencing (NGS). Stage I low-grade NSMP EECs (n = 10) from the PORTEC-1 and -2 clinical trials and stage I p53abn serous carcinomas (n = 13) from a prospective clinical cohort (22) were included as a reference set. These reference cases were primarily included for the blinded pathology review, so that the scoring pathologists would not get a hint of the study aim. The reference cases were, therefore, selected for prototypical low-grade endometrioid and serous morphology, as well as the hematoxylin and eosin (H&E) quality. Clinicopathologic parameters, surgical management, adjuvant treatment, and clinical outcomes were available for all cases.
Blinded expert pathologic review
Histotype and FIGO grade (low versus high) of all 55 cases and 23 reference cases were assigned by six expert gynecologic pathologists (J. Carlson, B.E. Howitt, P.P.C. Ip, S.F. Lax, W.G. McCluggage, and N. Singh). They were blinded to the molecular subtype and study aim, and used one representative digital H&E section per case. Prior to the blinded expert pathology review, all H&E slides were reviewed by two expert gynecologic pathologists (T. Bosse and C.B. Gilks, who did not participate in the review procedure) to confirm that they were of sufficient diagnostic quality. This led to the exclusion of three cases. All H&E slides were scanned and uploaded to an online platform; the pathologists were asked to choose one of the following four options for each H&E slide: (i) EEC grade 1–2, (ii) EEC grade 3, (iii) serous EC, or (iv) ambiguous features with discrepancy in architecture and nuclear atypia and would perform IHC before assigning histotype. The pathologists could also leave comments for each case. Although pathologists frequently integrate IHC results when diagnosing EC, the pathology review was performed using H&E slides only to prevent any bias that may be introduced by additional knowledge of biomarkers such as p53.
Molecular analyses
DNA was extracted from FFPE tumor blocks as previously described (4–6, 9). NGS was performed on all cases and controls using the AmpliSeq Cancer Hotspot Panel version 5 (Thermo Fisher Scientific), covering the complete TP53 gene, as well as hotspot sites of frequently mutated genes in EC. The presence of pathogenic mutations was assessed by taking a minimum coverage of 100 reads and variant allele frequency of >10% into account. In addition, shallow whole-genome sequencing (sWGS; single-end, 150 bp, 5 million reads per sample) was performed using the NovaSeq 6000 sequencer (Illumina). Cases were divided into three predefined categories of gains and losses [<5, 5–10, and >10 somatic CNAs (SCNA)] based on visual inspection of the generated relative copy-number plots. In addition, DNA ploidy was predicted on the basis of the relative copy-number plot (23). A detailed description of the copy-number assessment and DNA ploidy, including representative examples, can be found in the data supplement (Supplementary Material and Methods; Supplementary Fig. S1). Both NGS and sWGS were performed centrally at Leiden University Medical Center. Whole slide (PORTEC-1/-2/MST) and tissue microarray (Canadian cohorts) IHC staining for estrogen receptor (ER), progesterone receptor (PR), L1CAM, and p16 was performed on all cases with available material, including the reference set. A 10% cutoff for positivity was used in the assessment of ER, PR, and L1CAM, as this cutoff is frequently used for the assessment of these stains in EC (9, 24–28). p16 IHC was scored as completely negative, patchy positive, or diffusely positive.
Statistical analyses
The median follow-up time was estimated using the reverse Kaplan–Meier method. The Kaplan–Meier method and log-rank test were used to assess differences in the 5-year recurrence-free survival (RFS) of patients with stage I low-grade p53abn EEC compared with patients with stage I low-grade NSMP EEC (n = 453) and stage I p53abn high-grade EEC (n = 25) from the PORTEC-1/-2 trials. Subanalyses were predefined and performed according to the level of agreement on low-grade EEC by the expert pathologists: no pathologists diagnosed low-grade EEC, ≥1 pathologists diagnosed low-grade EEC, and ≥3 diagnosed low-grade EEC. Statistical analyses were performed with SPSS (Statistical Package of Social Science, RRID:SCR_002865) version 25 (IBM) and R (version 3.6.3., https://r-project.org) using the Ggplot2 package. Statistical significance was defined as a two-sided P value of <0.5.
Ethical approval
The PORTEC-1 and PORTEC-2 randomized clinical trials were approved by the ethics committees of all the participating centers. Written informed consent was obtained from all the patients. This study was performed in accordance with the principles of the Declaration of Helsinki.
Data availability
Requests for data sharing with a research proposal should be addressed to the corresponding author within 15 years of publication. Depending on the specific research proposal, the authors will determine when, for how long, for which specific purposes, and under which conditions the requested data can be made available, subject to obtaining ethical consent. Sequencing data are readily available and can be accessed through the Sequencing Read Archive (SRA) database [NCBI BioProject (RRID:SCR_004801) accession number PRJNA1010974].
Results
Clinicopathologic characteristics and expert pathology review
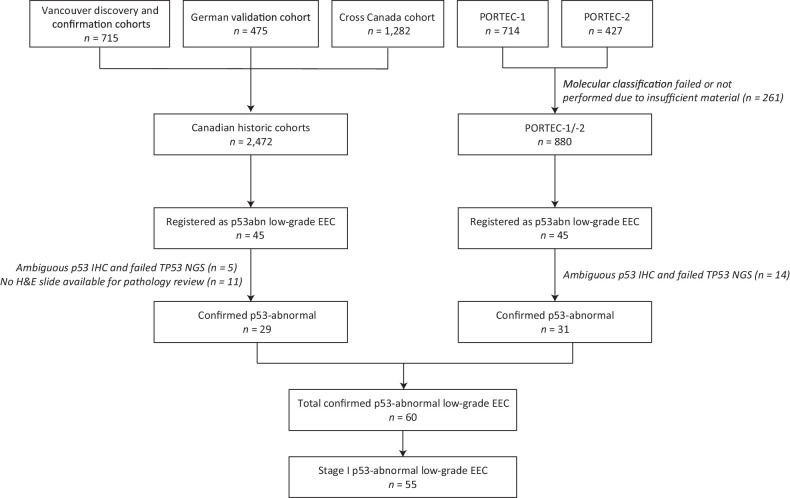
In total, 55 stage I p53abn low-grade EEC met the study inclusion criteria (Fig. 1). Table 1 outlines the clinicopathologic variables of the cohort. Originally, 32 cases (58.2%) were diagnosed as grade 1 EEC and 23 (41.8%) as grade 2 EEC. There were 32 patients (58.2%) with stage IA disease, and 23 patients (41.8%) with stage IB disease. LVSI (any extent) was present in four cases (8.0%). More than half of the patients (n = 28, 50.9%) did not receive any adjuvant therapy. The median follow-up time was 10.2 years [95% confidence interval (CI), 9.6–10.9 years].
Figure 1.
Flowchart of patient selection. Low-grade means grade 1–2.
Table 1.
Patient and tumor characteristics of all stage I p53abn ECs previously classified as low-grade (grade 1–2) endometrioid.
| Total | |
|---|---|
| N = 55 (100%) | |
| Age | |
| Mean (range) | 67 (45–86) |
| Stage | |
| IA | 32 (58.2%) |
| IB | 23 (41.8%) |
| LVSI | |
| Absent | 46 (92.0%) |
| Present | 4 (8.0%) |
| Adjuvant treatment | |
| No adjuvant treatment | 28 (50.9%) |
| EBRT | 12 (21.8%) |
| VBT | 13 (23.6%) |
| CT (+ EBRT) | 2 (3.6%) |
Abbreviation: CT, chemotherapy.
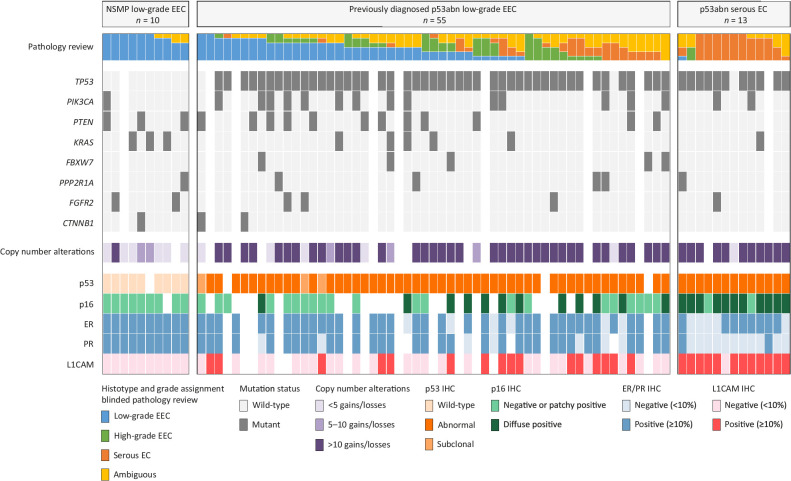
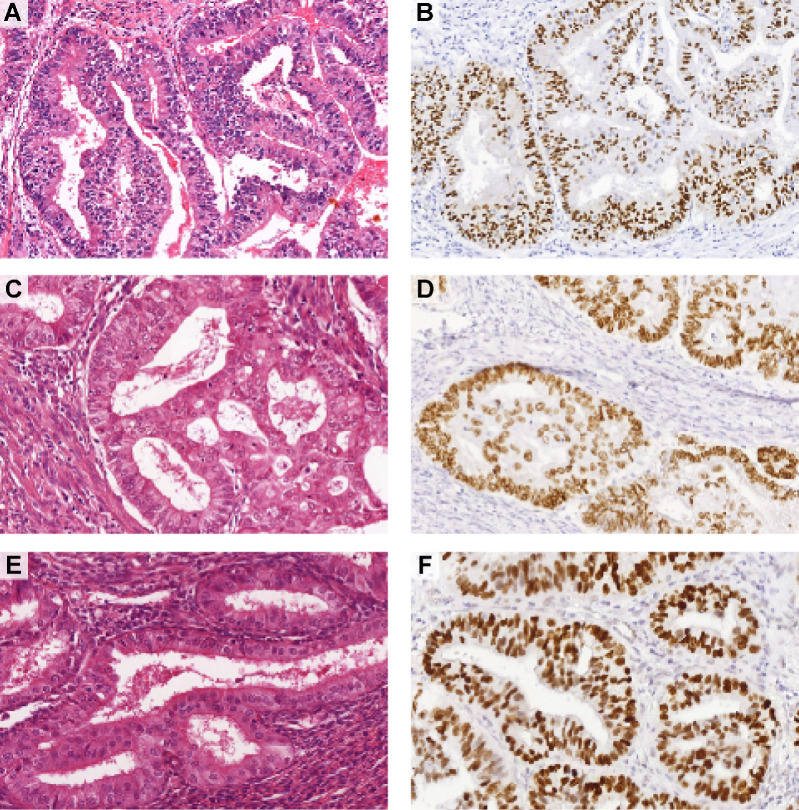
Following H&E pathology review (blinded to p53 status), 17 (30.9%) cases were not diagnosed as low-grade EEC by any of the six pathologists (Fig. 2; Supplementary Fig. S2). In fact, these cases were diagnosed as high-grade EEC (n = 4), serous EC (n = 5), or ambiguous (n = 9) by three or more pathologists. Nevertheless, 38 cases (69.1%) were assigned as low-grade EEC by at least one pathologist, and 26 cases (47.3%) were assigned as low-grade EEC by at least three pathologists. Three representative cases diagnosed as low-grade EEC by ≥3 pathologists are shown in Fig. 3A–F. All low-grade NSMP EEC (n = 10) reference cases were assigned as low-grade EEC by at least four expert pathologists, and 77% (n = 10/13) of the p53abn serous EC reference cases were assigned as serous EC by at least three expert pathologists.
Figure 2.
Molecular landscape of stage I low-grade (grade 1–2) p53abn EECs and controls. Histopathologic and molecular landscape of p53abn ECs previously diagnosed as low-grade endometrioid. Cases are stratified by consensus on a low-grade endometrioid diagnosis after blinded pathology review by six expert gynecologic pathologists. White tiles indicate missing data due to insufficient material or failed analysis. L1CAM, L1 cell adhesion molecule; NSMP, no specific molecular profile.
Figure 3.
Three representative examples of p53abn low-grade (grade 1–2) EEC. H&E slides (A, C, E) and p53 IHC (B, D, F) of three cases diagnosed as low-grade endometrioid by at least three of the expert pathologists upon blinded pathology review. Note the glandular architecture, smooth luminal borders, and presence of only mild to moderate nuclear atypia.
Molecular landscape of the low-grade p53abn EEC
The molecular and IHC landscape of the p53abn low-grade EEC cases, clustered by the degree of agreement on a diagnosis of low-grade EEC by expert pathologists, as well as the NSMP low-grade EEC and p53abn serous EC reference cases is shown in Fig. 2. NGS and sWGS were successfully performed in 87.3% (n = 48/55) and 81.8% (n = 45/55) of the cases, respectively, and both were successful in 91.3% (n = 21/23) of the reference cases. DNA ploidy could be predicted in 53.3% (n = 24/45) of the cases and 42.9% (n = 9/21) of the reference set with successful sWGS-based copy-number plots (Supplementary Fig. S2). Across all p53abn EEC analyzed, a pathogenic TP53 mutation was confirmed in 95.8% (n = 46/48). One of the two cases without a pathogenic TP53 mutation showed subclonal (>10%) abnormal p53 immunoreactivity, whereas the other case showed mutant-type overexpression of p53 in the complete tumor. Other frequently mutated genes were PIK3CA (n = 13/48, 27.1%), PTEN (n = 12/48, 25.0%), KRAS (n = 5/48, 10.4%), FBXW7 (n = 4/48, 8.3%), PPP2R1A (n = 4/48, 8.3%), FGFR2 (n = 3/48, 6.3%), and CTNNB1 (n = 2/48, 2.1%).
Focusing on the 26 p53abn cases diagnosed as low-grade EEC by ≥3 expert pathologists, frequent mutations were identified in PIK3CA and PTEN (n = 8/22, 36.4% for both). In addition, the tumors were predominantly ER (n = 18/19, 94.7%) and PR (n = 16/18, 88.9%) positive, showed negative or patchy immunoreactivity for p16 in the majority of cases (n = 12/14, 85.7%), and showed L1CAM positivity in 27.8% of cases (n = 5/18). In keeping with their abnormal p53 status, 70% had >10 gains and/or losses (n = 14/20) and 10% (n = 2/20) had between 5 and 10 SCNAs. Interestingly, of the remaining four cases with fewer than five SCNAs, two cases showed subclonal abnormal p53 immunoreactivity, of which one also did not have a detectable TP53 mutation. The DNA ploidy could be predicted for 9 cases, of which 6 (66.7%) had an average predicted ploidy between 1.8–2.0 (Supplementary Fig. S3). The H&E and IHC slides, and the relative copy-number plot of one representative case diagnosed as low-grade EEC by ≥3 pathologists are shown in Supplementary Fig. S4. Note the glandular architecture with smooth luminal borders and only mild-to-moderate nuclear atypia.
Cases not assigned as low-grade EEC by any pathologist had PIK3CA and PTEN mutations in 20% (n = 3/15) and 13.3% (n = 2/15) respectively, were predominantly ER/PR positive (n = 13/15, 86.7% for ER and n = 11/14, 78.6% for PR), showed less frequent patchy p16 staining (n = 7/12, 58.3%), and all but one had more than 10 SCNAs (n = 14/15, 93.3%).
The NSMP low-grade EEC reference cases did not have TP53 mutations, showed patchy p16 expression, and ER- and PR-positivity in all cases, and 66.7% (n = 6/9) had less than five SCNAs. The other three NSMP reference cases had 6, 9, and 13 SCNAs, of which the majority comprised gains and losses covering an entire chromosome or chromosomal arm. All four NSMP low-grade EEC for which DNA ploidy could be predicted had a DNA ploidy of 2 (Supplementary Fig. S3). In contrast, the p53abn serous EC reference cases showed a TP53 mutation in all but one case, had predominantly strong diffuse p16 staining (n = 11/13, 84.6%), all but one overexpressed L1CAM, and 91.7% (n = 11/12) had more than 10 SCNAs. In contrast to the NSMP low-grade EEC, all five p53abn serous EC were aneuploid with average predicted DNA ploidies ranging between 4.13–6.0 (Supplementary Fig. S3).
Clinical outcomes of low-grade p53abn EEC
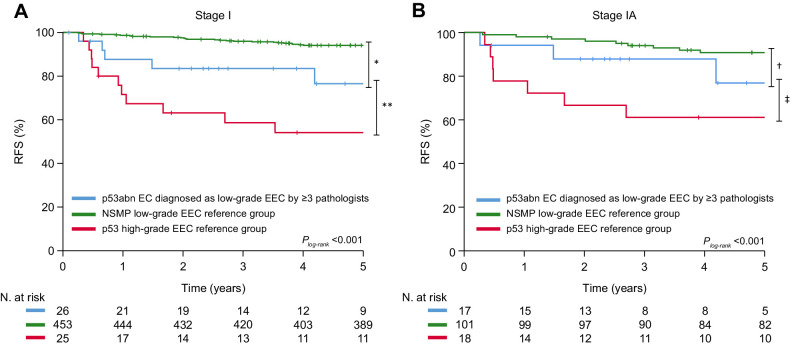
The 5-year RFS of patients with stage I p53abn low-grade EEC (n = 55) was compared with that of patients with stage I NSMP low-grade EEC (n = 453) and stage I p53abn high-grade EEC (n = 25) from the PORTEC-1/-2 trials. Patients with stage I low-grade p53abn EEC had a significantly lower 5-year RFS than those with stage I low-grade NSMP EEC (5-year RFS 69.3% vs. 94.1%, P < 0.001), but a (nonsignificant) higher 5-year RFS than women with stage I high-grade p53abn EEC (5-year RFS 54.1%; P = 0.17; Supplementary Fig. S5A). Analyses based on the level of agreement on low-grade EEC by expert pathologists showed that the 5-year RFS was lowest for cases not diagnosed as low-grade EEC by any pathologist (n = 17, 5-year RFS 61.9%) or by at least one pathologist (n = 38, 5-year RFS 72.8%; Supplementary Fig. S5B and S5C). The 5-year RFS for the cases assigned low-grade by ≥3 expert pathologists was 76.5%, which was significantly lower than that of low-grade NSMP EEC (94.1%; P = 0.003), and nonsignificantly higher than p53abn high-grade EEC (54.1%; P = 0.09; Fig. 4A). A comparable trend was observed when confining to stage IA cases only (Fig. 4B). Adjusted for stage, presence of LVSI, and whether adjuvant treatment was administered, cases diagnosed as low-grade by ≥3 expert pathologists still had a significantly increased risk for recurrence compared with low-grade NSMP (HR, 2.80; 95% CI, 1.03–7.60; P = 0.04). The site of disease recurrence for stage I low-grade p53abn EEC is shown in Table 2. Within the group of cases assigned as low-grade EEC by ≥3 pathologists, 5 (19.2%) patients had a recurrence, including 3 pelvic and 3 distant recurrences (1 of which had both a pelvic and distant recurrence).
Figure 4.
Kaplan–Meier survival curves for 5-year probability recurrence-free for cases assigned low-grade (grade 1–2) by ≥3 expert pathologists and stage I low-grade NSMP EEC and stage I high-grade p53abn EEC controls. Figure 4A shows the Kaplan–Meier survival curves for stage I p53abn low-grade EEC, NSMP low-grade EEC, and p53abn high-grade EEC. Figure 4B includes only stage IA cases. Differences in survival were assessed with the log-rank test: *, P = 0.003; **, P = 0.09; †, P = 0.14; ‡, P = 0.023.
Table 2.
Sites of disease recurrence by agreement on low-grade (grade 1–2) endometrioid morphology.
| N pathologists that assigned low-grade EEC | ||||
|---|---|---|---|---|
| None | ≥ 1 | ≥ 3 | Total | |
| n = 17 (100%) | n = 38 (100%) | n = 26 (100%) | n = 55 (100%) | |
| Overall | 6 (35.3) | 10 (26.3) | 5 (19.2) | 16 (29.1) |
| Recurrence sitea | ||||
| Vaginal | 1 (5.9) | 1 (2.6) | 1 (3.8) | 2 (3.6) |
| Pelvic | 1 (5.9) | 4 (10.5) | 3 (11.5) | 5 (9.1) |
| Distant | 6 (35.3) | 7 (18.4) | 3 (11.5) | 13 (23.6) |
aSome patients had recurrences in multiple sites.
Discussion
In this comprehensive analysis, we undertook a blinded specialist pathology review of a large set of stage I p53abn EC that were previously morphologically diagnosed as low-grade EEC and investigated their molecular landscape and clinical outcomes. Pathologic review showed that some cases were erroneously classified as low-grade EEC. However, 26 cases (47.3%) were confirmed to have low-grade EEC on H&E by at least three of the six pathologists. Importantly, survival analysis showed that these patients, despite having stage I disease, had a substantial risk of disease recurrence with a 5-year RFS of only 77%. Together, these data support universal p53 IHC testing on all stage I EC to identify this rare but clinically relevant subset of stage I p53abn low-grade EEC.
The fact that histologically low-grade (FIGO grade 1–2) endometrioid carcinomas may harbor TP53 mutations has been previously reported and may appear contradictory to some extent. We found 17 cases (31%) that were not diagnosed as low-grade by any of the pathologists, suggesting that a proportion of low-grade p53abn EEC are likely misclassified. In over half of these cases, the majority of pathologists selected ‘ambiguous features with discrepancy in architecture and nuclear atypia and would perform IHC before assigning histotype’. Hence, some of these cases were likely not misclassified as glandular variants of serous EC per se but rather represent high-grade carcinomas with ambiguous morphology. It is important to point out that the reviewing pathologists did not have access to the p53 IHC slides, which, if they had, may have resulted in some of these cases being diagnosed as serous EC. In contrast, 26 cases (47.3%) were diagnosed as low-grade EEC by at least three pathologists. These cases typically showed glandular architecture with smooth luminal borders and absence of striking nuclear atypia and the pathologists did not select the ‘ambiguous’ option and request additional IHC stains. Without universal p53 testing on all EC, it is likely that these cases would not be identified as p53abn, potentially resulting in suboptimal adjuvant treatment.
Several guidelines recommend performing the molecular classification in all high-grade EC and/or advanced-stage cases, where the molecular subtype could significantly alter the adjuvant treatment recommendations (10). Whether the molecular classification should also be undertaken on all stage I low-grade EEC is, however, a topic of debate. Although POLE testing is the main bottleneck, universal p53 testing is also met with some resistance. In a recent study, the potential of selecting cases that require p53 testing based on morphologic features was assessed (29). The authors concluded that selecting cases for p53 IHC testing based on nuclear features was highly sensitive (98.5%) for the detection of EC with abnormal p53 expression. It should be noted that their cases were reviewed by expert gynecologic pathologists who may be more likely to detect subtle features of nuclear atypia compared with the general (gynecologic) pathology community. Therefore, these results are not completely representative of every clinical practice. Furthermore, 95% (n = 40/42) of the cases with abnormal p53 IHC expression were high-grade EEC or non-endometrioid EC. The remaining two cases were grade 1 and grade 2 EEC, for which the reviewing pathologists unanimously requested p53 IHC only in the latter case. It is conceivable that the sensitivity of morphology-based p53 IHC testing in low-grade EEC is considerably lower than the 98.5% described in the total population. In our opinion, performing p53 IHC on all EC provides a more objective and better solution to identify p53abn EC (30).
IHC and molecular profiling was performed on all cases in our study to investigate whether the underlying biology reflected that of “endometrioid” or “serous” carcinogenesis. This investigation could potentially resolve the outstanding issue of how to best classify these EC, as some authors have argued to morphologically classify all p53 EC as serous (31). Our cases diagnosed as low-grade EEC by ≥ 3 pathologists harbored PIK3CA and PTEN mutations in 36%, positive ER and PR staining in 95% and 89% respectively, and patchy p16 expression in 86% of cases. These findings support the low-grade EEC diagnosis and are suggestive of endometrioid-type carcinogenesis (2, 32–35). We confirmed abnormal (“mutation-type”) p53 to reflect the presence of an underlying pathogenic TP53 mutation in 95% of cases. This high agreement between p53 IHC and TP53 mutation status is consistent with previous findings in EC (30, 36). CTNNB1 mutations were infrequently observed, 2% in all cases and 9% in cases diagnosed low-grade EEC by ≥3 pathologists, in line with previous studies reporting low frequencies of co-occurring CTNNB1 and TP53 mutations (1, 9, 16, 37, 38). An interesting finding was that almost all cases diagnosed as low-grade EEC by ≥3 pathologists had >10 SCNAs. Potentially, TP53 mutations can occur as an early driver event in POLE–wild-type and MMR-proficient low-grade EEC, allowing for the accumulation of SCNA. It could be hypothesized that in these cases, we detected p53abn EEC in a (very) early phase of tumor progression, and these cases may progress into, or be associated with, a component of high grade EEC. This may also explain the incidental finding of subclonal abnormal p53 IHC expression. Furthermore, it could be that p53abn low-grade EEC are genomically unstable with many SCNAs but retain a diploid genome. Doubling of the entire genome (whole-genome doubling) is a common feature in chromosomally unstable cancers (39), and has been observed in a subset of EC as well (40). It is conceivable that a higher DNA ploidy, potentially due to whole-genome doubling, affects nuclear atypia, and particularly the size of nuclei. Thus, the presence of a ‘normal’ diploid genome in the context of low-grade EEC may explain the absence of marked nuclear atypia. Taken together, our integrated morpho-molecular findings continue to support the assignment of these tumors as endometrioid rather than serous EC.
In addition to understanding the biological mechanisms at play, we aimed to explore whether stage I p53abn low-grade EEC have an indolent behavior similar to their p53–wild-type counterparts or whether they exhibit more aggressive behavior. Although we found that the RFS was higher with increasing agreement on a low-grade EEC diagnosis, p53abn cases assigned low-grade EEC by ≥3 expert gynecologic pathologists still had a clinically significant risk of recurrence with a 5-year RFS of 77%. Even after correction for stage, presence of LVSI, and adjuvant treatment, these cases had a significantly higher risk of disease recurrence than NSMP low-grade EEC. Despite the limited number of cases, which we are aware of, our findings support consideration of adjuvant therapy. According to the 2021 ESGO-ESTRO-ESP and 2022 ESMO EC guidelines all p53abn EC with myometrial invasion are recommended to be treated with chemoradiotherapy, regardless of stage, grade, or histologic type (10, 11). The evidence behind this recommendation is based on the molecular analyses of the randomized PORTEC-3 trial, which showed significantly improved survival for patients with combined adjuvant chemotherapy and radiotherapy compared with radiotherapy alone (7). However, care should be taken when generalizing these findings to stage I p53abn low-grade EEC, as inclusion in PORTEC-3 was limited to high-risk EC, and only 4% of the p53abn EC in PORTEC-3 were low-grade EEC. To support the discussion on which type of adjuvant treatment these women should ideally receive, in the absence of other clinical evidence, we reviewed the sites of disease of the tumors that recurred. Pelvic and distant recurrences were observed in 12% of the cases assigned low-grade EEC by ≥3 pathologists. Although no firm conclusions can be drawn, these findings tentatively suggest that women with stage I p53abn low-grade EEC may benefit from adjuvant EBRT and/or chemotherapy.
We focused our analysis on the molecular and clinical data of cases diagnosed as low-grade EEC by ≥3 pathologists to limit uncertainty caused by inter-observer variability. In clinical practice, however, it is more likely that only one or two pathologists will look at an individual case. Furthermore, the pathology review was performed by pathologists with a high degree of expertise in gynecologic pathology, limiting the generalizability of our findings. Therefore, the 38 cases (69.1%) diagnosed as low-grade EEC by at least one expert gynecologic pathologist may better reflect real-life clinical practice. Lowering the threshold for a low-grade EEC diagnosis from ≥3 to ≥1 pathologists resulted in a lower RFS (76.5% vs. 72.8%) as well as an increased prevalence of distant recurrences (11.5% vs. 18.4%) in our study. These findings further reinforce the clinical relevance of ancillary p53 testing as opposed to selected testing based on histologic type or presence of nuclear atypia.
In this study, we combined FIGO grade 1 and grade 2 EEC as “low-grade EEC”, in line with the current WHO classification as well as clinical guidelines (2, 10, 11). Furthermore, our study would have been underpowered to identify significant differences between grade 1 and grade 2 EEC due to the limited cohort size. Finally, only one H&E slide was available for each case which potentially hampered proper evaluation of the extent of nuclear atypia needed to upgrade a tumor by one grade. We are aware that this is a limitation of our study, as it would have been informative to know whether our cohort contained FIGO grade 2 cases and whether this was based on the extent of solid growth or architectural grade 1 tumors with marked nuclear atypia. Therefore, it would be interesting to include this level of morphologic detail in future studies investigating the p53abn molecular subgroup.
Given the contradictory findings of prognostically favorable low-grade EEC on morphology and unfavorable p53abn EC molecularly, some have suggested to re-assign these cases as either high-grade endometrioid or even serous EC (17, 31). Thus, integrating p53 IHC results in the final histologic diagnosis, as is recommended for histologic subtyping of other cancers (e.g., ovarian cancer; ref. 41). The importance of the molecular subtype has been endorsed by a recent study that showed no prognostic value of FIGO grade and histologic type in p53abn EC (22). Nevertheless, we believe that the current evidence is too limited to exclude any prognostic value of histologic type and grade within the group of p53abn EC with phenotypic diversity. Pending stronger evidence, we recommend that these tumors be diagnosed as low-grade EEC with the molecular subgroup reported separately.
To our knowledge, this is the largest series of stage I p53abn low-grade EEC to date; however, our study size is still limited. Given their rarity (approximately 1.2% in the Canadian cohorts and 3.5% in the PORTEC-1 and -2 trials, respectively), it is unlikely that these tumors will be prospectively investigated in the future and they will continue to pose a challenge when encountered in clinical practice.
In conclusion, we have undertaken an extensive clinical, IHC, and molecular analysis of a series of cases of p53abn low-grade EEC, and have provided evidence that supports the latest clinical guidelines that recommend adjuvant therapy for this group. We show that a small subset of EC molecularly classified as p53abn (POLE–wild-type and MMR-proficient) have a convincing low-grade endometrioid morphology and is associated with a clinically significant risk of recurrence, even in stage I disease. These findings support the clinical relevance of the EC molecular classification and highlight the importance of universal p53 testing in stage I EC.
Supplementary information
A Supplementary Data (PDF) is available, including a detailed description of the copy-number assessment by shallow whole-genome sequencing. In addition, Supplementary Figures are presented.
Supplementary Material
Supplementary material and methods
Representative relative copy number plots.
Representative examples of cases diagnosed as low-grade EEC by ≤1 pathologists.
Predicted DNA ploidy.
H&E, immunohistochemistry stains and relative copy number plot of a case diagnosed as low-grade EEC by ≥3 pathologists.
Recurrence-free survival by agreement on low-grade endometrioid morphology.
Acknowledgments
The PORTEC-1 and PORTEC-2 randomized clinical trials and subsequent translation work were supported by the Dutch Cancer Society (CKVO 90–01, CTKO 1990–01, 11629 and 12995).
We thank Tessa Rutten (Department of Pathology, Leiden University Medical Center, the Netherlands) for her technical support.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
R.A. Nout reports grants from Dutch Cancer Society, Dutch Research Council, Elekta, Varian, and Accuray outside the submitted work. S.F. Lax reports personal fees from GSK and MSD outside the submitted work. N. Singh reports personal fees from AstraZeneca-MSD and GlaxoSmithKline during the conduct of the study. C.L. Creutzberg reports grants from Dutch Cancer Society during the conduct of the study, as well as grants from Varian outside the submitted work. N. Horeweg reports grants from Dutch Cancer Society during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
A. Jamieson: Conceptualization, writing–original draft. L. Vermij: Conceptualization, formal analysis, writing–original draft. C.J.H. Kramer: Formal analysis. J.J. Jobsen: Writing–review and editing. I. Jürgenliemk-Schulz: Writing–review and editing. L. Lutgens: Writing–review and editing. J.W. Mens: Writing–review and editing. M.A.D. Haverkort: Writing–review and editing. A. Slot: Writing–review and editing. R.A. Nout: Writing–review and editing. J. Oosting: Software, writing–review and editing. J. Carlson: Investigation, writing–review and editing. B.E. Howitt: Investigation, writing–review and editing. P.P.C. Ip: Investigation, writing–review and editing. S.F. Lax: Investigation, writing–review and editing. W.G. McCluggage: Investigation, writing–review and editing. N. Singh: Investigation, writing–review and editing. J.N. McAlpine: Writing–review and editing. C.L. Creutzberg: Writing–review and editing. N. Horeweg: Conceptualization, formal analysis, writing–review and editing. C.B. Gilks: Conceptualization, writing–review and editing. T. Bosse: Conceptualization, writing–review and editing.
References
- 1. Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO Classification of Tumours Editorial Board. WHO Classification of female genital tumors. Lyon: International Agency for Research on Cancer; 2020.
- 3. Vermij L, Smit V, Nout R, Bosse T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology 2020;76:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol 2018;29:1180–8. [DOI] [PubMed] [Google Scholar]
- 5. Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon JS, Melnyk N, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 2015;113:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017;123:802–13. [DOI] [PubMed] [Google Scholar]
- 7. Leon-Castillo A, de Boer SM, Powell ME, Mileshkin LR, Mackay HJ, Leary A, et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol 2020;38:3388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leon-Castillo A, Horeweg N, Peters EEM, Rutten T, Ter Haar N, Smit V, et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol Oncol 2022;164:577–86. [DOI] [PubMed] [Google Scholar]
- 9. Stelloo E, Nout RA, Osse EM, Jurgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res 2016;22:4215–24. [DOI] [PubMed] [Google Scholar]
- 10. Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 2021;31:12–39. [DOI] [PubMed] [Google Scholar]
- 11. Oaknin A, Bosse TJ, Creutzberg CL, Giornelli G, Harter P, Joly F, et al. Endometrial cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:860–77. [DOI] [PubMed] [Google Scholar]
- 12. Momeni-Boroujeni A, Dahoud W, Vanderbilt CM, Chiang S, Murali R, Rios-Doria EV, et al. Clinicopathologic and genomic analysis of TP53-mutated endometrial carcinomas. Clin Cancer Res 2021;27:2613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jamieson A, Thompson EF, Huvila J, Gilks CB, McAlpine JN. p53abn endometrial cancer: understanding the most aggressive endometrial cancers in the era of molecular classification. Int J Gynecol Cancer 2021;31:907–13. [DOI] [PubMed] [Google Scholar]
- 14. Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 2000;88:814–24. [PubMed] [Google Scholar]
- 15. Thompson EF, Huvila J, Jamieson A, Leung S, Lum A, Offman S, et al. Variability in endometrial carcinoma pathology practice: opportunities for improvement with molecular classification. Mod Pathol 2022;35:1974–82. [DOI] [PubMed] [Google Scholar]
- 16. Kurnit KC, Kim GN, Fellman BM, Urbauer DL, Mills GB, Zhang W, et al. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol 2017;30:1032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yano M, Ito K, Yabuno A, Ogane N, Katoh T, Miyazawa M, et al. Impact of TP53 immunohistochemistry on the histological grading system for endometrial endometrioid carcinoma. Mod Pathol 2019;32:1023–31. [DOI] [PubMed] [Google Scholar]
- 18. Jamieson A, Huvila J, Thompson EF, Leung S, Chiu D, Lum A, et al. Variation in practice in endometrial cancer and potential for improved care and equity through molecular classification. Gynecol Oncol 2022;165:201–14. [DOI] [PubMed] [Google Scholar]
- 19. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicenter randomized trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000;355:1404–11. [DOI] [PubMed] [Google Scholar]
- 20. Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomized trial. Lancet 2010;375:816–23. [DOI] [PubMed] [Google Scholar]
- 21. Leon-Castillo A, Gilvazquez E, Nout R, Smit VT, McAlpine JN, McConechy M, et al. Clinicopathological and molecular characterization of 'multiple-classifier' endometrial carcinomas. J Pathol 2020;250:312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vermij L, Jobsen JJ, Leon-Castillo A, Brinkhuis M, Roothaan S, Powell ME, et al. Prognostic refinement of NSMP high-risk endometrial cancers using estrogen receptor immunohistochemistry. Br J Cancer 2023;128:1360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sauer CM, Eldridge MD, Vias M, Hall JA, Boyle S, Macintyre G, et al. Absolute copy number fitting from shallow whole-genome sequencing data. Biorxiv 2021. [Google Scholar]
- 24. Kommoss FK, Karnezis AN, Kommoss F, Talhouk A, Taran FA, Staebler A, et al. L1CAM further stratifies endometrial carcinoma patients with no specific molecular risk profile. Br J Cancer 2018;119:480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Putten LJM, Visser NCM, van de Vijver K, Santacana M, Bronsert P, Bulten J, et al. Added value of estrogen receptor, progesterone receptor, and L1 cell adhesion molecule expression to histology-based endometrial carcinoma recurrence prediction models: an ENITEC collaboration study. Int J Gynecol Cancer 2018;28:514–23. [DOI] [PubMed] [Google Scholar]
- 26. Trovik J, Wik E, Werner HM, Krakstad C, Helland H, Vandenput I, et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicenter trial. Eur J Cancer 2013;49:3431–41. [DOI] [PubMed] [Google Scholar]
- 27. Jongen V, Briet J, de Jong R, ten Hoor K, Boezen M, van der Zee A, et al. Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol Oncol 2009;112:537–42. [DOI] [PubMed] [Google Scholar]
- 28. Mileshkin L, Edmondson R, O'Connell RL, Sjoquist KM, Andrews J, Jyothirmayi R, et al. Phase 2 study of anastrozole in recurrent estrogen (ER)/progesterone (PR) positive endometrial cancer: The PARAGON trial - ANZGOG 0903. Gynecol Oncol 2019;154:29–37. [DOI] [PubMed] [Google Scholar]
- 29. Kang EY, Wiebe NJ, Aubrey C, Lee CH, Anglesio MS, Tilley D, et al. Selection of endometrial carcinomas for p53 immunohistochemistry based on nuclear features. J Pathol Clin Res 2022;8:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vermij L, Leon-Castillo A, Singh N, Powell ME, Edmondson RJ, Genestie C, et al. p53 immunohistochemistry in endometrial cancer: clinical and molecular correlates in the PORTEC-3 trial. Mod Pathol 2022;35:1475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Köbel M. Counterpoint: integration of molecular subtype and histotype/grade into one classification system for endometrial carcinoma. AJSP: Reviews & Reports 2022;27:187–97. [Google Scholar]
- 32. Yemelyanova A, Ji H, Shih Ie M, Wang TL, Wu LS, Ronnett BM. Utility of p16 expression for distinction of uterine serous carcinomas from endometrial endometrioid and endocervical adenocarcinomas: immunohistochemical analysis of 201 cases. Am J Surg Pathol 2009;33:1504–14. [DOI] [PubMed] [Google Scholar]
- 33. Halperin R, Zehavi S, Habler L, Hadas E, Bukovsky I, Schneider D. Comparative immunohistochemical study of endometrioid and serous papillary carcinoma of endometrium. Eur J Gynaecol Oncol 2001;22:122–6. [PubMed] [Google Scholar]
- 34. Alkushi A, Kobel M, Kalloger SE, Gilks CB. High-grade endometrial carcinoma: serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int J Gynecol Pathol 2010;29:343–50. [DOI] [PubMed] [Google Scholar]
- 35. Reid-Nicholson M, Iyengar P, Hummer AJ, Linkov I, Asher M, Soslow RA. Immunophenotypic diversity of endometrial adenocarcinomas: implications for differential diagnosis. Mod Pathol 2006;19:1091–100. [DOI] [PubMed] [Google Scholar]
- 36. Singh N, Piskorz AM, Bosse T, Jimenez-Linan M, Rous B, Brenton JD, et al. p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J Pathol 2020;250:336–45. [DOI] [PubMed] [Google Scholar]
- 37. Ruz-Caracuel I, Lopez-Janeiro A, Heredia-Soto V, Ramon-Patino JL, Yebenes L, Berjon A, et al. Clinicopathological features and prognostic significance of CTNNB1 mutation in low-grade, early-stage endometrial endometrioid carcinoma. Virchows Arch 2021;479:1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Costigan DC, Dong F, Nucci MR, Howitt BE. Clinicopathologic and immunohistochemical correlates of CTNNB1 mutated endometrial endometrioid carcinoma. Int J Gynecol Pathol 2020;39:119–27. [DOI] [PubMed] [Google Scholar]
- 39. Lopez S, Lim EL, Horswell S, Haase K, Huebner A, Dietzen M, et al. Interplay between whole-genome doubling and the accumulation of deleterious alterations in cancer evolution. Nat Genet 2020;52:283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dentro SC, Leshchiner I, Haase K, Tarabichi M, Wintersinger J, Deshwar AG, et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell 2021;184:2239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kobel M, Kang EY. The evolution of ovarian carcinoma subclassification. Cancers 2022;14:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material and methods
Representative relative copy number plots.
Representative examples of cases diagnosed as low-grade EEC by ≤1 pathologists.
Predicted DNA ploidy.
H&E, immunohistochemistry stains and relative copy number plot of a case diagnosed as low-grade EEC by ≥3 pathologists.
Recurrence-free survival by agreement on low-grade endometrioid morphology.
Data Availability Statement
Requests for data sharing with a research proposal should be addressed to the corresponding author within 15 years of publication. Depending on the specific research proposal, the authors will determine when, for how long, for which specific purposes, and under which conditions the requested data can be made available, subject to obtaining ethical consent. Sequencing data are readily available and can be accessed through the Sequencing Read Archive (SRA) database [NCBI BioProject (RRID:SCR_004801) accession number PRJNA1010974].