Abstract
Background:
Arab countries are projecting increase in cancer incidence and mortality; however, there are limited studies that compare the epidemiology of cancer in Arab countries compared with other parts of the world.
Methods:
We used the 2018 Global Cancer Observatory data to compare the age-standardized incidence and mortality estimates in Arab-speaking countries to the rest of the world.
Results:
Rates for incidence and mortality for all cancers in Arab countries were lower than the world's rates but the incidence rates of non-Hodgkin and Hodgkin lymphoma, bladder, breast, and liver cancers were higher. Arab countries generally had higher mortality-to-incidence ratio than the world's ratio. Incidence rates, even in age-specific groups, varied between subregions of Arab countries (the Levant, Arabian Gulf, and Arab African subregions), and Iraq and Egypt, suggesting some common and unique environmental factors and possible ethnic or genetic heritages.
Conclusions:
There are essential scopes for improvements in Arab countries including better treatments to reduce the high mortality-to-incidence ratio, and supporting vaccination programs and antiviral treatments that would prevent the prevalent viral infection–related cancers. The high incidence of several cancers in younger Arabs suggests genetic factors and underlines the importance of genetic epidemiology studies.
Impact:
This study is an essential reference to evaluate and monitor the progress of national cancer initiatives in Arab countries for surveillance and prevention programs and improving clinical management. The study also provides a comprehensive snapshot of cancers in a unique region that could shed light on the interplay of environmental, lifestyle, and genetic risk factors.
Introduction
An estimated 18.1 million new cancer cases (including nonmelanoma skin cancer) and 9.6 million cancer-related deaths occurred globally in 2018 (1, 2). It is expected that the global cancer burden will continue to increase to reach 28.4 million cases in 2040 (3). There are, however, considerable regional variations in incidence and mortality trends in the world attributed to the degree of economic development, and social and lifestyle changes (1). The Arab countries continue to show a rise in cancer incidence with a projected 1.8-fold increase by 2030 (4). Studies from Saudi Arabia (5), Egypt (6), Jordan (7), and Lebanon (8) reported an increasing age-standardized incidence rate (ASIR) during the past 10 years in the five most common cancers. In most Arab countries, cancer is the second cause of premature deaths following cardiovascular disease, except in Lebanon where it is the leading cause of death, while in Saudi Arabia and United Arab Emirates, cancer is one of the top three causes (9).
The total population of the Arab world, referring to the 22 members of the Arab League, has increased from 372.35 million in 2011 to 464.68 million in 2022 to become a larger population than the United States (333.29 million) and the European Union (447.96 million; ref. 10). Arab countries are contributing to the cancer burden worldwide. For example, in addition to the steady increase in cancer incidence (8, 11), Lebanon has the highest ASIR for bladder cancer cases in the world (12); the top 20 countries for bladder cancer ASIR also includes Syria and Egypt. Egypt is also of one of the top contributors to the world's burden of liver cancer incidence and deaths (3). Furthermore, an increase in cancer incidence is expected in the Arab region based on the gradual transition projected between 2000 and 2050 toward an aging population due to reduced fertility and increased life expectancy (13, 14). While the Middle East and Northern Africa (MENA region) is a geopolitical classification, it is mainly composed of Arab countries and shares cultural, economic, and environmental similarities. Arab countries in the MENA region may also share ethnic and genetic heritages. Nevertheless, there has been little effort in interrogating cancer trends and incidences in Arab countries. Such regional population-based information would be instrumental in focusing efforts to decrease cancer burden through systematic implementation of evidence-based interventions for prevention, early diagnosis, and treatment. In this study, we provide a comprehensive analysis of the estimated pattern of cancer incidence and mortality in 2018 in Arab countries using data from the Global Cancer Observatory (GCO) hosted by the International Agency for Research on Cancer (IARC).
Materials and Methods
The global cancer statistics for 2018 from GLOBOCAN 2018 (1, 2) was interrogated for the estimates of incidence and mortality in Arab countries. Data were accessed through the interactive web-based platform, GCO, hosted by the IARC (https://gco.iarc.fr/today). Age-standardized incidence and mortality rates (per 100,000 population), ASIR and ASMR, respectively, are reported in this study and compared between Arab countries, the worldwide rates, and the rates in the United States and Europe. All plots were generated in GraphPad Prism, version 9.4 (GraphPad Software).
Data sources for Arab countries in GLOBOCAN
GLOBOCAN estimates were based on observed rates and other sources for different Arab countries, that were subjected to the different methods developed by GLOBOCAN (2) as described below.
GLOBOCAN Method 1 using observed rates over 10 years from population-based national cancer registries of six Arab countries:
Bahrain Cancer Registry 2003–2012 data
Kuwait Cancer Registry 2003–2012 data
Oman Cancer Registry 2003–2012 data
Saudi Arabia Cancer Registry 2003–2012 data
Jordan Cancer Registry 2003–2012 data
Lebanon Cancer Registry 2007–2016 data
GLOBOCAN Method 2a or 2b using observed rates over less than 10 years were from national cancer registry data of four Arab countries or from local/regional registries of five other Arab countries:
Qatar Cancer Registry 2008–2012 data
Sudan Cancer Registry 2009–2013 data
United Arab Emirates (UAE) Cancer Registry 2013–2015 data
Iraq Cancer Registry 2018 data
Morocco: Casablanca Cancer Registry and Rabat Cancer Registry 2008–2012 data
Tunisia: Sousse Cancer Registry 2003–2007 data and North Tunisia Cancer Registry 2008–2010 data
Algeria: Algiers Cancer Registry 2008–2012 data, Annaba Cancer Registry 2008–2010 data, Batna Cancer Registry 2008–2012 data, Sétif Cancer Registry 2008–2012 data, Sidi-Bel-Abbès Cancer Registry 2010–2012 data, Tizi Ouzou Cancer Registry 2015–2016 data, Tlemcen Cancer Registry 2012–2014 data
Egypt: Aswan Cancer Registry 2009–2010 data, Damietta Cancer Registry 2009–2012 data, and El-Minia Cancer Registry 2009 data
GLOBOCAN Method 9 using observed rates from neighboring countries for four Arab countries:
Somalia: Average of Ethiopia and Kenya rates
Syria: Average of Iraq, Jordan, and Lebanon rates
Mauritania: Rates from Western Africa
Palestine: Average for Arabs from the Israel National Cancer Registry 2008–2012 data and from the Jordan Cancer Registry 2008–2012 data
Data availability
The data generated in this study are available within the article and its supplementary data files. The raw data for incidence and mortality age-adjusted standardized for all ages and different age groups in females and males for each Arab country, subregions of Arab countries, the world, the United States and Europe are shown in Supplementary Table S1. These data were extracted from the GLOBOCAN 2018 database. The large data in Supplementary Table S1 were organized to generate Supplementary Table S2, which can be queried by users to select and visualize the data of interest.
Results
Overall cancer trends in Arab countries
The age-adjusted rate for incidence (ASIR) of all cancers in 2018 in both sexes at all ages in Arab countries in the MENA region (MENA-Arab) was lower than the global ASIR; 131.9 versus 197.9 per 100,000 people, respectively (Supplementary Fig. S1A; Supplementary Tables S1 and S2). Similarly, the age-adjusted rate for mortality (ASMR) was lower than the global ASMR; 83.5 versus 101.1 per 100,000 people (Supplementary Fig. S1A). The mortality-to-incidence ratio (MIR) in 2018 for all cancers in both sexes in most Arab countries was higher compared with the global MIR (Supplementary Fig. S1B). It should be noted that MIR is not a proxy of relative cancer survival (15); however, it allows comparisons across countries and regions for mortality rates in relevance to incidence. The top 15 cancers diagnosed in Arab countries were similar to the global trends but differed in their distribution and their contribution to mortality (Supplementary Fig. S1C).
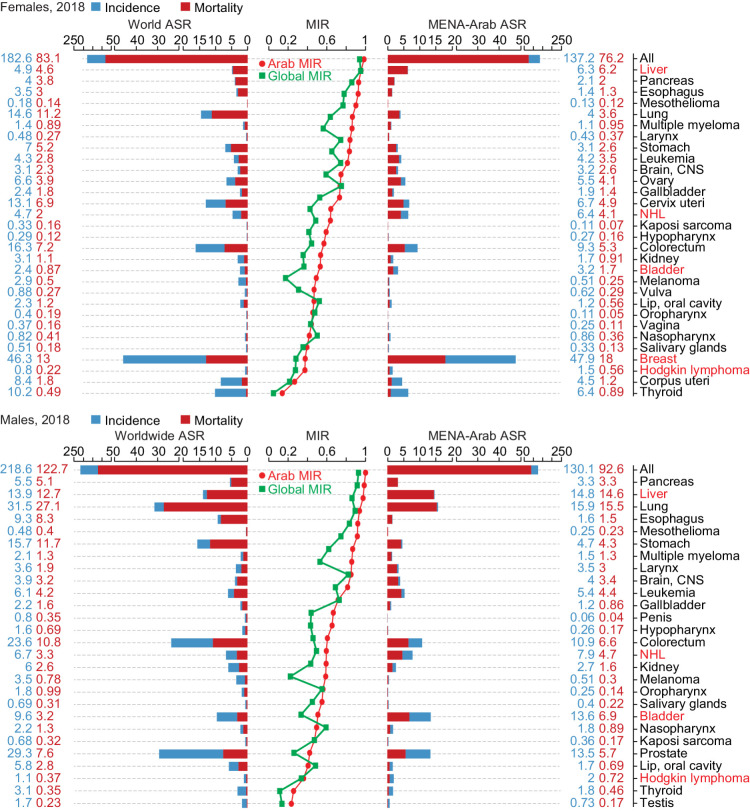
Cancer incidence and mortality in Arab countries remained lower than the worldwide rates when separating the data for females and males, but the MIR was higher than the world MIR for most cancer sites (Fig. 1). The ASIR of non-Hodgkin's lymphoma (NHL) and Hodgkin lymphoma, bladder, breast, and liver cancers were higher than the world rates. As shown in Fig. 2, the incidence of these five cancers varied across the Arab countries, but some subregional trends emerged. The higher ASIR of breast cancer was driven by the Levant region (Lebanon, Syria, Jordan, and Palestine). Bladder cancer ASIR was higher than the world age-standardized rate (ASR) for incidence in females in Lebanon, Syria, Egypt, and Iraq. In males, while the Levant region, Egypt, Tunisia, Libya, and Algeria had higher ASIR for bladder cancer than the world, the Arabian Gulf countries apart from Iraq (Saudi Arabia, Qatar, Kuwait, Oman, UAE, and Bahrain) had similar or lower ASIR.
Figure 1.
Cancer incidence, mortality, and mortality-to-incidence ratio in the Arab region. The MENA-Arab and worldwide ASR for incidence (blue) and mortality (red) for all cancers and each cancer site for all ages in 2018 for females (top) and males (bottom) are shown. The ASR values for incidence and mortality are shown in blue and red, respectively. Cancer sites are ranked according to the mortality-to-incidence ratio (MIR) shown in the middle line graphs; MENA-Arab MIR in red and worldwide MIR in green. Note: Djibouti, Comoros and Yemen were not included due to missing data.
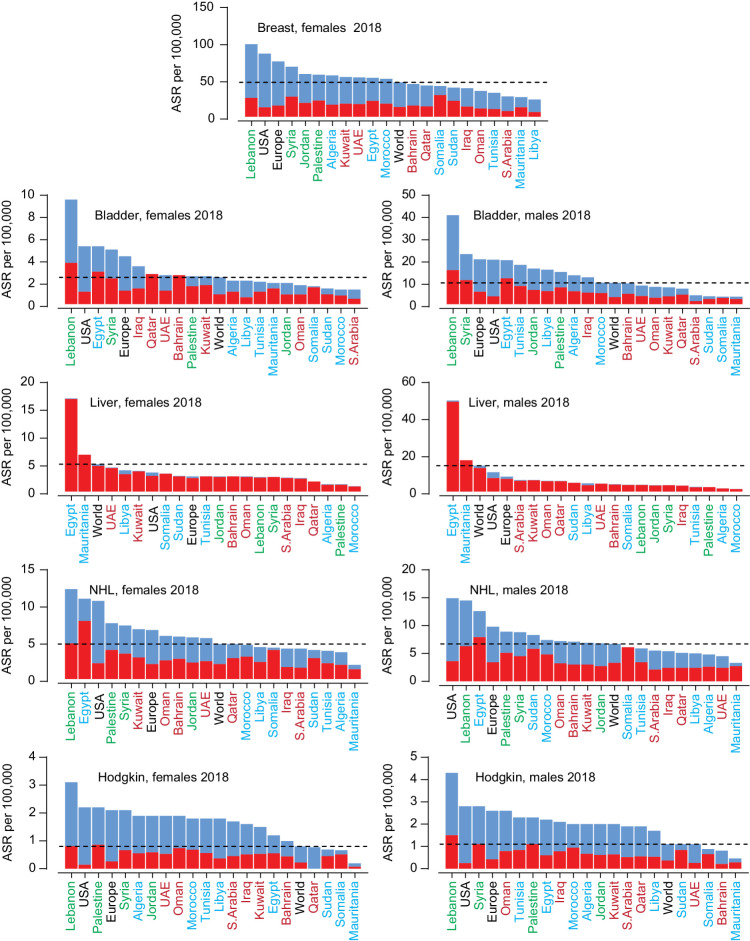
Figure 2.
Incidence and mortality rates of five cancers with higher incidence in the Arab region compared with the worldwide incidence. ASR for incidence (blue) and mortality (red) for Arab countries, United States, Europe, and the world for all ages. The dotted lines mark the world ASR for incidence. Arab countries are labeled as those in the Arabian Gulf (maroon), the Levant (green), and North Africa (blue).
For females and males in Egypt and Mauritania, the incidence of liver cancer was higher than the ASIR in the United States, Europe, and the world (Fig. 2). For NHL in females and males, Northern African Arab countries except Egypt had lower ASIR than the world rate, while the Levant region and half of the Arabian Gulf countries had higher rates. The ASIR of Hodgkin lymphoma in females and males was higher than the world ASIR in most Arab countries. The five cancers with higher ASIR in Arab countries had higher MIR than the worldwide MIR (Supplementary Fig. S2).
Leading incident cancers in females in Arab countries
The top ten leading cancer sites in females in 2018 for all ages varied across the different Arab countries thus, the top 13 cancers were investigated (Supplementary Fig. S3A). The ASIR for all cancers in females was lower than the world ASIR except for Lebanon. Despite some variations, the MIR for the top 13 cancers in females was generally higher in the Arab region compared with the world MIR (Supplementary Fig. S3B). Leading incident cancers in female Arabs included liver cancer in Egypt and Mauritania, lung cancer in the Levant, cervical cancer in Northern African Arab countries excluding Egypt, endometrial cancer (corpus uteri) in the Arabian Gulf and the Levant, breast and colorectal cancer in the Levant, and brain cancer in Iraq, Egypt, and the Levant.
Leading incident cancers in males in Arab countries
Like in females, the top ten leading cancer in males for all ages varied across different Arab countries and the top 13 cancers were investigated to represent all countries (Supplementary Fig. S3C). Apart from Lebanon, the incidence for all cancers in males in 2018 for the rest of Arab countries was lower than the world rate. Like in females, the Arab region had a higher MIR compared with the worldwide MIR (Supplementary Fig. S3D). Leading incident cancers in male Arabs included Egypt, lung cancer in the Levant, brain cancer in the Levant, Iraq and Egypt, colorectal cancer in the Levant and the Arabian Gulf, bladder cancer in the Levant, parts of Northern Africa, and Iraq, and prostate cancer in the Levant and Northern African countries.
Subregional trends within the Arab countries in females and males
The country-specific analyses above suggest that Arab countries may be divided into subregions; the Levant, Iraq, the rest of Arabian Gulf countries (Qatar, Oman, Bahrain, Kuwait, Saudi Arabia, and UAE), Egypt, and the rest of the Northern African countries (Sudan, Libya, Algeria, Tunisia, Morocco, Mauritania, and Somalia). To this end, these Arab subregions were analyzed for the top 10 leading incident cancers and the top 10 leading causes of cancer-related deaths in 2018 (Supplementary Fig. S4). The contribution of cancer types to the total diagnoses varied across the subregions for females and males, suggestion unique genetic, environmental, and/or lifestyle differences. Similarly, the contribution of cancer types to cancer-related deaths also varied across the subregions, which may reflect differences in the subtypes diagnosed and/or patient management.
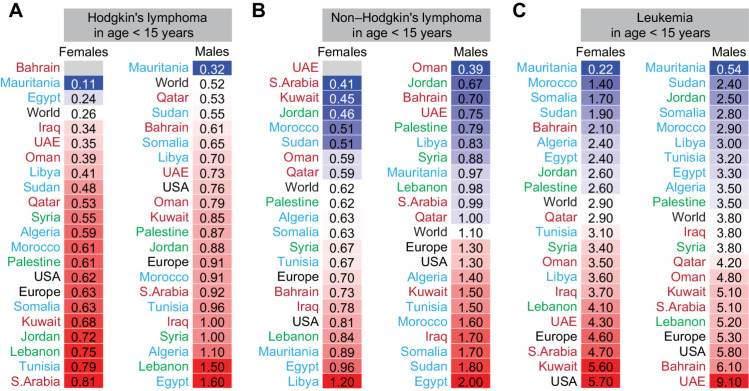
Age-specific incidence of blood cancers
The incidence of blood cancers in many Arab countries was higher than the global rate, particularly at younger age (Supplementary Fig. S5). The incidence of Hodgkin lymphoma in children (Fig. 3A) and adults (35 years and older, Supplementary Fig. S5) was higher than the world ASIR in most Arab countries for both females and males. The ASIR of NHL (Fig. 3B) in children under 15 years of age particularly in females and in Northern African Arab countries was higher than the world rate. In older Arab females and males, the ASIR for NHL in approximately half of the Arab countries was higher than the world ASIR (Supplementary Fig. S5). The incidence of leukemia in children under 15 years in the Arabian Gulf, Syria, and Lebanon was higher than the world ASIR (Fig. 3C). Leukemia in adults (35 years and older) in the Levant had higher incidence than the world rate (Supplementary Fig. S5). Multiple myeloma ASIR in 20- to 54-year-old females and males in more than 50% of Arab countries was higher than the world ASIR (Supplementary Fig. S5).
Figure 3.
Age-specific incidence of blood cancers in Arab countries. ASR for incidence for Hodgkin lymphoma (A), non-Hodgkin's lymphoma (B), and leukemia in females and males (C). Arab countries are labeled as those in the Arabian Gulf (maroon), the Levant (green), and North Africa (blue). The ASR for the world, United States, and Europe are in black font. ASR for specific age groups is shown; for all age groups and for multiple myeloma refer to Supplementary Fig. S5.
Age-specific incidence of smoking-related cancers
The incidence of lung cancer in females was lower than the worldwide ASIR in all Arab countries at all ages (Supplementary Fig. S5), except for 20- to 54-year-old females in Lebanon (Fig. 4A). In 20- to 54-year-old males, the ASIR for lung cancer in Syria, Jordan, Lebanon, Libya, Tunisia, and Morocco was higher than the world rate (Fig. 4A). In line with lung cancer in these countries, the incidence of laryngeal cancer in the 30–54 age group in the Levant for both females and males, and in Libya, Tunisia, and Morocco for males was higher than the world ASIR (Fig. 4B). When considering bladder cancer (Fig. 4C), the higher incidence in Syria, Lebanon, Jordan, and Iraq in females and in males in 12 out of 19 Arab countries for 20–54 years old compared with the world ASIR is also in line with the higher incidence of lung and laryngeal cancers in these countries. These patterns suggest tobacco-related causes. Higher bladder cancer incidence in 55- to 69-year-old females in several Arabian Gulf countries, and in the 20–54 age group in Mauritania for females, Sudan for males, and Egypt for females and males (Supplementary Fig. S5) were at odds with the lower incidence of lung and laryngeal cancers, suggesting other factors such as exposure to chemicals other than tobacco smoke.
Figure 4.
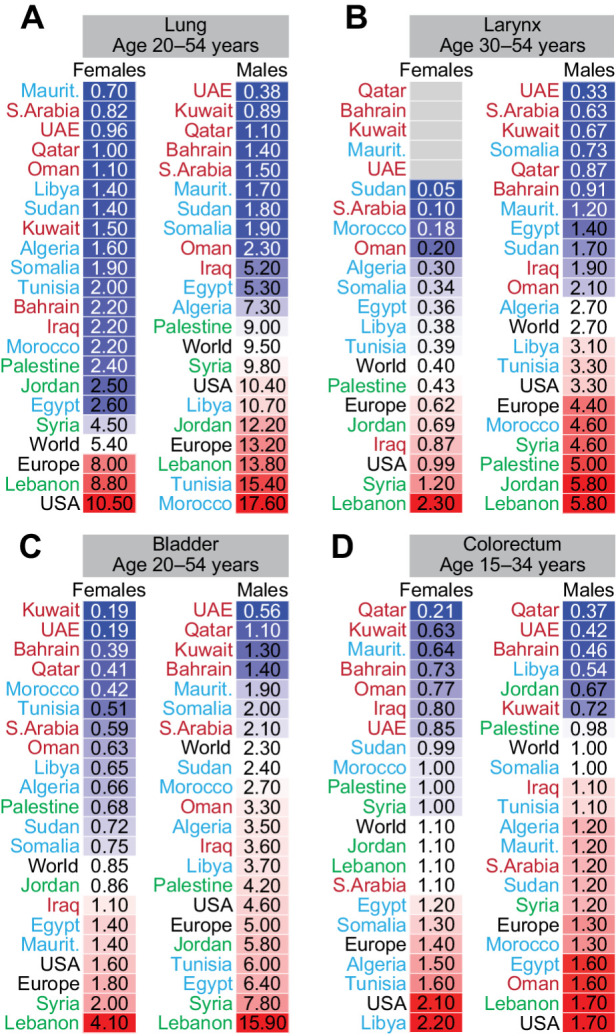
Age-specific incidence of smoking-related cancers in Arab countries. ASR for incidence for cancers of the lung (A), larynx (B), bladder (C), and colorectum (D) in females and males. Arab countries are labeled as those in the Arabian Gulf (maroon), the Levant (green), and North Africa (blue). The ASR for the world, United States, and Europe are in black font. ASR for specific age groups is shown; for all age groups refer to Supplementary Fig. S5.
The ASIR for colorectal cancer in 15–34 years old females and males (Fig. 4D) was higher than the world ASIR in several Northern African Arab countries, Saudi Arabia, Oman, and Iraq. The higher incidence of colorectal cancer at young age (Fig. 4D) and the lower incidence at older age (55 years and older, Supplementary Fig. S5) in Arabs may be because of hereditary factors or reflecting a generational change in diet rather than tobacco or alcohol consumption.
Age-specific incidence of sex-specific cancers
Cervical cancer had an alarming ASIR in 55–69 years old females in four Northern African Arab countries; Libya, Morocco, Somalia, and Mauritania (Fig. 5A). The incidence in Morocco, Somalia, and Mauritania was higher than the world rate in females between 35 and 54 years of age (Supplementary Fig. S5). These trends implicate human papillomavirus (HPV) infections, which is supported by the high ASIR in Northern African Arab countries for vaginal cancer in females, and oropharyngeal, nasopharyngeal, and esophageal cancers in both females and males (Supplementary Fig. S5). Endometrial cancer ASIR in the Levant countries, UAE, Saudi Arabia, and Kuwait was higher than Arab countries in Northern Africa at all ages (Supplementary Fig. S5) and exceeded the world rate in females 55 years and older (Fig. 5B).
Figure 5.
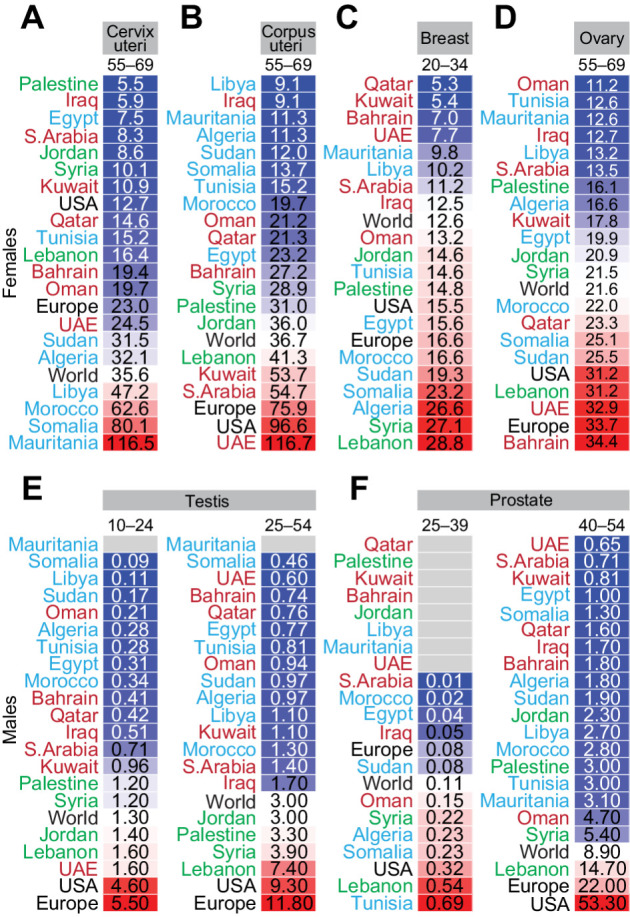
Age-specific incidence of sex-specific cancers in Arab countries. ASR for incidence for sex-specific cancers in females (top) in the cervix uteri (A), corpus uteri (B), breast (C), and ovary (D), and in males (bottom) in the testis (E) and prostate (F). Arab countries are labeled as those in the Arabian Gulf (maroon), the Levant (green), and North Africa (blue). The ASR for the world, United States, and Europe are in black font. ASR for specific age groups is shown; for all age groups and for other sex-specific cancers refer to Supplementary Fig. S5.
The ASIR for breast cancer in 20- to 34-year-old females (Fig. 5C) and 35- to 49-year-old females (Supplementary Fig. S5) was higher than the world ASIR in 11 of 19 Arab countries mainly in the Levant and Northern African Arab countries. The incidence of ovarian cancer in females under 55 years was lower in most Arab countries (17 of 19) than the world ASIR (Supplementary Fig. S5), but higher than the world ASIR in some Arab countries in 55- to 69-year-old females (Fig. 5D). Testicular cancer ASIR was generally lower in Arab countries compared with the world rate, but young adults in the Levant had higher incidence than the world ASIR (Fig. 5E). In men under 40 years, six Arab countries (Oman, Syria, Algeria, Somalia, Lebanon, and Tunisia) had higher incidence of prostate cancer than the world, and the ASIR in Lebanon and Tunisia exceeded the ASIR in the United States (Fig. 5F). In contrast, only Lebanon had higher ASIR for prostate cancer in 40- to 54-year-old men (Fig. 5F), and only Lebanon and Kuwait had higher ASIR for prostate cancer in men aged 55 years and older (Supplementary Fig. S5).
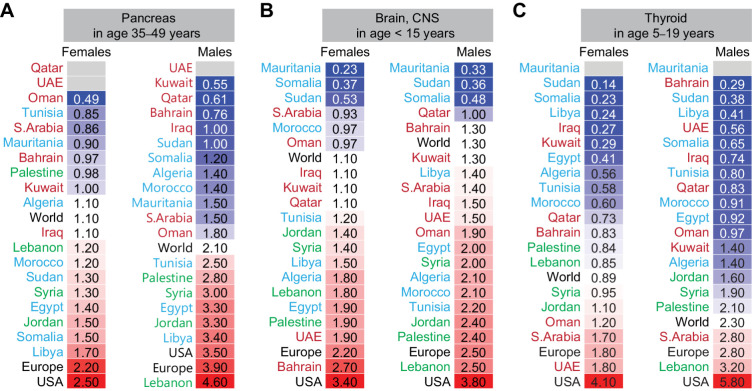
Age-specific incidence of other solid cancers
The incidence of pancreatic cancer in the 35- to 49-year age group was higher than the world ASIR for females in the Levant, Morocco, Sudan, Egypt, Somalia, and Libya, and for males in the Levant, Tunisia, Egypt, and Libya (Fig. 6A). The incidence of brain cancer (Fig. 6B) in Arab children under 15 years was similar or higher than the world ASIR in 13 and 15 of the 19 Arab countries for females and males, respectively. Higher ASIR for brain cancer in the Levant, Iraq, Tunisia, Algeria, and Egypt was also observed in the 15–29 years and 30–54 years groups (Supplementary Fig. S5). The incidence of thyroid cancer in females 5–19 (Fig. 6C) and 20–39 (Supplementary Fig. S5) years old in Saudi Arabia, Kuwait, UAE, Oman, Syria, Jordan, and Lebanon was higher than the world ASIR, whereas for males in these age groups had higher ASIR than the world ASIR only in Saudi Arabia and Lebanon. Thyroid cancer ASIR for over 39 years of age for males in Saudi Arabia, Oman, UAE, Kuwait, Bahrain, Sudan, and Lebanon, and for females in Saudi Arabia, Kuwait, UAE, Lebanon, Jordan, Palestine, Morocco, and Libya was higher than the world ASIR (Supplementary Fig. S5).
Figure 6.
Age-specific incidence of other solid cancers in Arab countries. ASR for incidence for cancers of the pancreas (A), brain, central nervous system (CNS; B), and thyroid (C) in females and males. Arab countries are labeled as those in the Arabian Gulf (maroon), the Levant (green), and North Africa (blue). The worldwide ASR is marked with bold font, United States, and Europe in black font. ASR for specific age groups is shown; for all age groups and other cancer sites refer to Supplementary Fig. S5.
Discussion
The 2018 age-standardized incidence and mortality rates for all cancers combined and at the organ-specific level in both sexes in Arab countries were lower than the global rates except for the higher incidence of Hodgkin, NHL and cancers of the bladder, liver, and breast. The generational and demographical structure in Arab countries cannot be ignored as a factor behind the lower cancer incidence. Older Arabs (55–75 years and older) diagnosed with cancer in 2018 were born between 1943 and 1963 and had a different lifestyle and less affected by modernization and industrialization. Recently, modern lifestyles in the Arab region have started to impact obesity, including childhood obesity, especially in the Arabian Gulf area (16). The transition to western lifestyles in the Arab region may change the profile of cancer incidence in the future. Furthermore, the Arab region showed a delayed decline in infertility compared to the rest of the world, and it is projected that by 2050 there will be several folds increase in the proportion of the population aged above 65 years old (13).
Cancer incidence in Arab countries in 2018 revealed clear trends such as in Northern African Arab countries with high incidence of HPV-related cancers (cervical, vaginal, oropharyngeal, nasopharyngeal, and esophageal cancers), and liver cancer that can be attributed to the high prevalence of the human oncogenic viruses hepatitis B or C virus (HBV or HCV; refs. 17–19). The high prevalence of HCV in Northern Africa may also relate to the higher incidence of NHL in children under 15 years of age in several Northern African Arab countries in 2018. In support, HCV infection has been shown to increase the risk by 14-fold for developing NHL in Egyptian patients (20). HPV and HBV vaccination and treating hepatitis chronic inflammation to avoid liver scarring (cirrhosis), are obvious approaches to reduce the burden of these virus-related cancers. Modeling analysis predicts that high-coverage girls-only HPV vaccination with once or twice lifetime screening can eliminate cervical cancer in low income and lower-middle-income countries including North Africa and the Middle East (21).
Most Arab countries showed higher ASIR in 2018 than the worldwide incidence of Hodgkin's lymphoma in children and adults of both sexes. Although Epstein–Barr virus (EBV) has been suspected to increase the incidence of Hodgkin's lymphoma in developing countries, the percentages of EBV positivity in Hodgkin's lymphoma in several Arab countries such as Saudi Arabia (22), Jordan (23), UAE (24), Tunisia (25), and Syria (26) are not higher than the 40% to 50% rate detected in Western countries (27). While exposure to EBV, particularly in children under 15 years of age, cannot be excluded as a main factor, genetic susceptibility to this type of lymphoma should be considered in countries where EBV infection may not be the major driver as noted in the study from the Saudi Arabia population with high consanguinity (22).
The low incidence of lung, laryngeal, and bladder cancers in females from most Arab countries is in line with their lower prevalence of tobacco use. The maximum age-standardized prevalence estimates of tobacco use in females in year 2000 and year 2018 was 10.1% and 12.6%, respectively, in all Arab countries except for Lebanon which ranked 8th out of the 164 countries listed in the WHO's tobacco use prevalence estimates (29.4% in 2018; ref. 28) and had high ASIR for smoking-related cancer in females. Lung, laryngeal, and bladder cancers had high incidence in 2018 in males in countries with high prevalence tobacco use including Lebanon (tobacco use prevalence 47.3% in 2018), Jordan (55.6% in 2018), Tunisia (48.7% in 2018), and Morocco (29.4% in 2018; ref. 28).
Several Arab countries showed lower than the world's ASIR for lung and laryngeal cancers in 2018 but presented higher ASIR for bladder and/or colorectal cancers suggesting other causes than tobacco use. Unfortunately, there are insufficient studies investigating the genetics and mutational profiles in Arab countries to understand the epidemiology of bladder cancer. Environmental exposures cannot be excluded in bladder cancer, for example contamination of drinking water with halogenated chemical species has contributed to 8.6% of bladder cancer cases in Lebanon (29). The increasing trend in colorectal cancer in young Arab adults (below 50 years old; refs. 30–32) may be driven by the rapid environmental, lifestyle and industrialization changes in the last two decades. The average prevalence of obesity has drastically increased in Arab countries in the past three decades (6.5% in 1975 to 20% in 2016) which has been associated with unique genetic polymorphism in Arabs (33). This increase in obesity might explain the increase of colorectal cancer in younger Arabs given the association of diabetes with higher risk for colorectal cancer (34), including younger women (35).
It has been reported that between 1950 and 2008 the average age at diagnosis of breast cancer in 11 Arab countries (Egypt, Jordan, United Arab Emirates, Kuwait, Lebanon, Oman, Qatar, Saudi Arabia, Sudan, Tunisia, and Yemen) was a decade earlier than in Western countries (36). Most Arab countries showed higher than the world's ASIR for breast cancer in females under 50 years of age, and Arab countries in the Levant region and Northern Africa had higher incidence for breast cancer in females under the age of 35 years than the world's ASIR. While other factors such as reproductive history, taking hormones, and alcohol consumption may increase the risk for breast cancer in young women (37), genetic hereditary may be the main factor (38). This is supported by the higher ASIR for cancers associated with BRCA mutations at younger age, including pancreatic (under 50 years of age), gastric (under 40 years), and prostate cancers (under 40 years), in Arab countries with higher breast cancer ASIR. Several studies on Arabs reported high number of mutations and novel pathogenic mutations in BRCA1 and BRCA2 genes in patients with breast cancer (39–45). A meta-analysis of reported prevalence of BRCA mutations in Arab countries demonstrated that one in five patients with hereditary breast and/or ovarian cancer are likely to carry BRCA mutations, and the Levant region showed higher prevalence of BRCA mutations compared with other Arab countries; however, this meta-analysis had high heterogeneity (46) and there is a need for better genetic epidemiology studies in Arabs. A study from Qatar points to another limitation in our study regarding the genetic risk for certain cancers, such as breast, ovarian, and colorectal cancers because Arabic-speaking countries span across two continents with different ethnic compositions across and possibly within these countries. Whole-genome sequencing of the Qatar Genome Programme cohort consisting of 6,000 individuals across six ancestry groups in Qatar found that 56.4% of the identified BRCA1/BRCA2 variant carriers were in Qataris of Persian origin, and those pathogenic variants were completely absent in Qataris of Arabian Peninsula origin (47). Other limitations in our study include the variation across the MENA region, including Arab countries, in terms of the human development index (HDI), which are associated with different types of cancers at varying magnitudes, and that high-quality cancer registry data are not available for all countries analyzed in GLOBOCAN (1, 2). Nonetheless, it should be noted that the data sources for Arab countries (detailed in the Materials and Methods section) were mainly from population-based national or local cancer registries with similar quality of the overall GLOBOCAN data sources.
Our study of the estimated cancer incidence and mortality rates in Arab countries for 2018 from the GLOBOCAN provides a baseline for future analyses of the next releases of the GLOBOCAN estimates to follow trends over time. We also shed some light on risk factors that may associate with cancers in the Arab countries, particularly based on the analysis of age-standardized rates across different age groups which emphasized the need for more well-designed genetic epidemiologic studies. In addition to the abovementioned ethnic diversities across and within Arab countries, consanguineous marriages which accounts for 35%–50% of marriages in Arab countries (48–50) further underlines the importance of genetic epidemiology studies for the region. In conclusion, much effort is required to identify the genetic and mutational landscapes of the Arab population to better understand genetic risks for cancer and to guide cancer management for reducing cancer burden in this region.
Supplementary Material
Figure S1: Age-standardized rate (ASR) for cancer incidence and mortality, and mortality-to-incidence ratio (MIR) in both sexes in 2018. (A) Comparison of ASR (per 100,000 people) in Arab countries in the MENA region (right) to the worldwide (global) ASR (left) for cancer incidence (blue) and cancer mortality (red) in 2018 for all ages. ASR is shown for all cancers and each cancer site. Cancer sites are ranked according to ASR incidence in the MENA-Arab region. The ASR for incidence and mortality are shown in blue and red, respectively. Cancer sites that are labelled in red had higher incidence and mortality in the MENA-Arab region than the global rates in 2018. (B) ASR for incidence (blue) and mortality (red) for all cancers in 2018 in both sexes for all ages in 186 countries. Countries were ranked according to the mortality-to-incidence ratio (MIR); mortality ASR divided by incidence ASR (black dots). The 22 Arab countries are labelled in red. (C) Top 15 cancer sites for incidence (B) and mortality (C) in both sexes, in 2018 for all ages, in the MENA region and worldwide. The total number of cases is shown in the middle of the donut graphs; the percentage contribution and the number of cases of each cancer site are shown in the legends.
Figure S2: Mortality-to-incidence ratio (MIR) for five cancers with higher incidence in the Arab region. The MIR was calculated as the ratio of ASR for mortality to the ASR for incidence for all ages. The dotted lines mark the worldwide MIR. Arab countries are colored as those in the Arabian Gulf (maroon), the Levant (green) and North Africa (blue).
Figure S3: The top cancer sites in the MENA-Arab region leading incidence or mortality in females or males in 2018 for all ages. The MENA-Arab and worldwide ASR in 2018 for incidence for leading cancer sites for all ages in the MENA-Arab countries (coloured bars) and the worldwide rate for these sites (black bars) for females (A) and males (B). Mortality-to-incidence ratio (MIR) for the leading cancer sites in the MENA-Arab countries and the world for females (C) and males (D). Complete data mortality ASRs are available in Tables S1-S2.
Figure S4: Leading cancers for diagnosis and cancer-related deaths in subregions of the Arab countries. The percentage contribution of cancer sites to all cancers in females or males are shown for the 10 leading sites for cancer diagnoses and mortality in 2018 at all ages in the specified Arab subregions. The total number of diagnoses or deaths are shown under each plot. Complete data for all cancer sites in all subregions, in each country, and data for the USA, Europe and the world are available in Tables S1-S2.
Figure S5: Age-specific incidence of cancers in Arab countries. The ASIR for cancers at different age groups in Arab countries are summarized and compared to the ASIR at the same age groups for the World, USA and Europe. Arab countries are labelled as those in the Arabian Gulf (maroon), the Levant (green) and North Africa (blue). The world, USA and Europe in black font. Each cancer type is shown on a separate page in the following pages organized alphabetically. For all age groups please refer to Supplementary Data Tables S1 and S2.
Table S1: Raw data extracted from GLOBOCAN 2018 dataset for all cancers and each cancer, for all age groups and age-group intervals, for females and males, and for mortality and incidence for Arab countries, the world, USA and Europe. This data was used to generate the pivot table in Table S2 which can be queried. All data in the manuscript was based on this data file.
Table S2: Pivot table which can be queried for cancer incidence and mortality across different age groups in Arab countries and the data for the world, the USA and Europe. The table also includes different subregions in the Arab region. The table is based on the data extracted from the GLOBOCAN 2018 web database supplied in Table S1 in this study.
Acknowledgments
This work was supported by QBRI's Intramural Grant Program, Cycle 5 (IGP5) funding. All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Fares Al-Ejeh and Mariam Al-Muftah. All authors read and approved the final manuscript.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Selected Articles from This Issue, p. 1673
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Authors' Disclosures
No disclosures were reported.
Authors' Contributions
M. Al-Muftah: Conceptualization, resources, data curation, formal analysis, investigation, visualization, methodology, writing–original draft, writing–review and editing. F. Al-Ejeh: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941–53. [DOI] [PubMed] [Google Scholar]
- 3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 4. Arafa MA, Rabah DM, Farhat KH. Rising cancer rates in the Arab world: now is the time for action. East Mediterr Health J 2020;26:638–40. [DOI] [PubMed] [Google Scholar]
- 5. Alqahtani WS, Almufareh NA, Domiaty DM, Albasher G, Alduwish MA, Alkhalaf H, et al. Epidemiology of cancer in Saudi Arabia thru 2010–2019: a systematic review with constrained meta-analysis. AIMS Public Health 2020;7:679–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer incidence in egypt: results of the national population-based cancer registry program. J Cancer Epidemiol 2014;2014:437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdel-Razeq H, Attiga F, Mansour A. Cancer care in Jordan. Hematol Oncol Stem Cell Ther 2015;8:64–70. [DOI] [PubMed] [Google Scholar]
- 8. Shamseddine A, Saleh A, Charafeddine M, Seoud M, Mukherji D, Temraz S, et al. Cancer trends in Lebanon: a review of incidence rates for the period of 2003–2008 and projections until 2018. Popul Health Metr 2014;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021;127:3029–30. [DOI] [PubMed] [Google Scholar]
- 10. The World Bank. World development indicators, population, total - Arab world (ARB). Available from:https://api.worldbank.org/v2/en/indicator/SP.POP.TOTL?downloadformat=excel.
- 11. Khachfe HH, Rahal Z, Sammouri J, Kheil M, Baydoun H, Chatila D, et al. Cancer in lebanon: a review of incidence rates from 2008 to 2015 and projections till 2025. South Asian J Cancer 2020;9:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel) 2020;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hajjar RR, Atli T, Al-Mandhari Z, Oudrhiri M, Balducci L, Silbermann M. Prevalence of aging population in the middle east and its implications on cancer incidence and care. Ann Oncol 2013;24Suppl 7:vii11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Economic and Social Commission for Western Asia (ESCWA). Regional profile of the arab region demographic of ageing: trends, patterns, and prospects into 2030 and 2050; 2017. Available from:https://archive.unescwa.org/sites/www.unescwa.org/files/page_attachments/demographics-ageing-arab-region-final-en_0.pdf p.
- 15. Ellis L, Belot A, Rachet B, Coleman MP. The mortality-to-incidence ratio is not a valid proxy for cancer survival. J Glob Oncol 2019;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farrag NS, Cheskin LJ, Farag MK. A systematic review of childhood obesity in the Middle East and North Africa (MENA) region: prevalence and risk factors meta-analysis. Adv Pediatr Res 2017;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daw MA. Chapter 3.2 - hepatitis C in north Africa (Arabic Maghreb Region). In: Kamal SM, editor. Hepatitis C in Developing Countries: Academic Press; 2018. p57–70. [Google Scholar]
- 18. Daw MA, El-Bouzedi A, MO A, Dau AA, Agnan MM. Hepatitis C virus in north Africa: an emerging threat. Scientific World Journal 2016;2016:7370524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madihi S, Syed H, Lazar F, Zyad A, Benani A. A systematic review of the current hepatitis B viral infection and hepatocellular carcinoma situation in mediterranean countries. Biomed Res Int 2020;2020:7027169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farawela H, Khorshied M, Shaheen I, Gouda H, Nasef A, Abulata N, et al. The association between hepatitis C virus infection, genetic polymorphisms of oxidative stress genes and B-cell non-Hodgkin's lymphoma risk in Egypt. Infect Genet Evol 2012;12:1189–94. [DOI] [PubMed] [Google Scholar]
- 21. Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020;395:575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al-Kuraya K, Narayanappa R, Al-Dayel F, El-Solh H, Ezzat A, Ismail H, et al. Epstein-Barr virus infection is not the sole cause of high prevalence for Hodgkin's lymphoma in Saudi Arabia. Leuk Lymphoma 2006;47:707–13. [DOI] [PubMed] [Google Scholar]
- 23. Sughayer MA, Haddad HA, Al-Yousef RM, El-Khateeb M, Abu-Rass H. Epstein-Barr virus and Hodgkin lymphoma in Jordan. Hematol Oncol Stem Cell Ther 2014;7:85–9. [DOI] [PubMed] [Google Scholar]
- 24. Al-Salam S, John A, Daoud S, Chong SM, Castella A. Expression of Epstein-Barr virus in Hodgkin lymphoma in a population of United Arab Emirates nationals. Leuk Lymphoma 2008;49:1769–77. [DOI] [PubMed] [Google Scholar]
- 25. Dhiab MB, Ziadi S, Saad H, Louhichi T, Trimeche M. Changing patterns in the Epstein-Barr virus (EBV) and Hodgkin lymphoma association in Tunisia. Ann Hematol 2016;95:1537–43. [DOI] [PubMed] [Google Scholar]
- 26. Habeeb R, Al Hafar L, Monem F. EBV Plasma Epstein-Barr Virus (EBV) DNA as a biomarker for diagnosis of EBV-positive Hodgkin Lymphoma in Syria. J Infect Dev Ctries 2021;15:1917–22. [DOI] [PubMed] [Google Scholar]
- 27. Weiss LM. Epstein-Barr virus and Hodgkin's disease. Curr Oncol Rep 2000;2:199–204. [DOI] [PubMed] [Google Scholar]
- 28. Global Health Observatory (GHO) Data. Age-standardized estimates of current tobacco use, tobacco smoking and cigarette smoking (Tobacco control: Monitor). Available from:https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-tobacco-control-monitor-current-tobaccouse-tobaccosmoking-cigarrettesmoking-agestd-tobagestdcurr; 2022.
- 29. Temraz S, Haibe Y, Charafeddine M, Saifi O, Mukherji D, Shamseddine A. The unveiling of a new risk factor associated with bladder cancer in Lebanon. BMC Urol 2019;19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Makhlouf NA, Abdel-Gawad M, Mahros AM, Lashen SA, Zaghloul M, Eliwa A, et al. Colorectal cancer in Arab world: a systematic review. World J Gastrointest Oncol 2021;13:1791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guraya SY. The prevalence and evolving risk factors for colorectal cancer in the Arab world. Biomedical and Pharmacology Journal 2018;11. [Google Scholar]
- 32. Alsanea N, Abduljabbar AS, Alhomoud S, Ashari LH, Hibbert D, Bazarbashi S. Colorectal cancer in Saudi Arabia: incidence, survival, demographics and implications for national policies. Ann Saudi Med 2015;35:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Younes S, Ibrahim A, Al-Jurf R, Zayed H. Genetic polymorphisms associated with obesity in the Arab world: a systematic review. Int J Obes 2021;45:1899–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soltani G, Poursheikhani A, Yassi M, Hayatbakhsh A, Kerachian M, Kerachian MA. Obesity, diabetes and the risk of colorectal adenoma and cancer. BMC Endocr Disord 2019;19:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol 2019;5:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Najjar H, Easson A. Age at diagnosis of breast cancer in Arab nations. Int J Surg 2010;8:448–52. [DOI] [PubMed] [Google Scholar]
- 37. Daly AA, Rolph R, Cutress RI, Copson ER. A review of modifiable risk factors in young women for the prevention of breast cancer. Breast Cancer 2021;13:241–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol 2009;36:237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Troudi W, Uhrhammer N, Sibille C, Dahan C, Mahfoudh W, Bouchlaka Souissi C, et al. Contribution of the BRCA1 and BRCA2 mutations to breast cancer in Tunisia. J Hum Genet 2007;52:915–20. [DOI] [PubMed] [Google Scholar]
- 40. Cherbal F, Salhi N, Bakour R, Adane S, Boualga K, Maillet P. BRCA1 and BRCA2 unclassified variants and missense polymorphisms in Algerian breast/ovarian cancer families. Dis Markers 2012;32:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahfoudh W, Bouaouina N, Ahmed SB, Gabbouj S, Shan J, Mathew R, et al. Hereditary breast cancer in Middle Eastern and North African (MENA) populations: identification of novel, recurrent and founder BRCA1 mutations in the Tunisian population. Mol Biol Rep 2012;39:1037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riahi A, Kharrat M, Ghourabi ME, Khomsi F, Gamoudi A, Lariani I, et al. Mutation spectrum and prevalence of BRCA1 and BRCA2 genes in patients with familial and early-onset breast/ovarian cancer from Tunisia. Clin Genet 2015;87:155–60. [DOI] [PubMed] [Google Scholar]
- 43. Bu R, Siraj AK, Al-Obaisi KA, Beg S, Al Hazmi M, Ajarim D, et al. Identification of novel BRCA founder mutations in Middle Eastern breast cancer patients using capture and Sanger sequencing analysis. Int J Cancer 2016;139:1091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alhuqail AJ, Alzahrani A, Almubarak H, Al-Qadheeb S, Alghofaili L, Almoghrabi N, et al. High prevalence of deleterious BRCA1 and BRCA2 germline mutations in arab breast and ovarian cancer patients. Breast Cancer Res Treat 2018;168:695–702. [DOI] [PubMed] [Google Scholar]
- 45. Abu-Helalah M, Azab B, Mubaidin R, Ali D, Jafar H, Alshraideh H, et al. BRCA1 and BRCA2 genes mutations among high risk breast cancer patients in Jordan. Sci Rep 2020;10:17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abdulrashid K, AlHussaini N, Ahmed W, Thalib L. Prevalence of BRCA mutations among hereditary breast and/or ovarian cancer patients in Arab countries: systematic review and meta-analysis. BMC Cancer 2019;19:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saad M, Mokrab Y, Halabi N, Shan J, Razali R, Kunji K, et al. Genetic predisposition to cancer across people of different ancestries in Qatar: a population-based, cohort study. Lancet Oncol 2022;23:341–52. [DOI] [PubMed] [Google Scholar]
- 48. Al-Ghanim KA. Consanguineous marriage in the Arab societies. J Psychol Clin Psychiatry 2020;11:166‒8. [Google Scholar]
- 49. Rahman S, Zayed H. Breast cancer in the GCC countries: a focus on BRCA1/2 and non-BRCA1/2 genes. Gene 2018;668:73–6. [DOI] [PubMed] [Google Scholar]
- 50. Abedalthagafi MS. Precision medicine of monogenic disorders: lessons learned from the Saudi human genome. Front Biosci (Landmark Ed) 2019;24:870–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Age-standardized rate (ASR) for cancer incidence and mortality, and mortality-to-incidence ratio (MIR) in both sexes in 2018. (A) Comparison of ASR (per 100,000 people) in Arab countries in the MENA region (right) to the worldwide (global) ASR (left) for cancer incidence (blue) and cancer mortality (red) in 2018 for all ages. ASR is shown for all cancers and each cancer site. Cancer sites are ranked according to ASR incidence in the MENA-Arab region. The ASR for incidence and mortality are shown in blue and red, respectively. Cancer sites that are labelled in red had higher incidence and mortality in the MENA-Arab region than the global rates in 2018. (B) ASR for incidence (blue) and mortality (red) for all cancers in 2018 in both sexes for all ages in 186 countries. Countries were ranked according to the mortality-to-incidence ratio (MIR); mortality ASR divided by incidence ASR (black dots). The 22 Arab countries are labelled in red. (C) Top 15 cancer sites for incidence (B) and mortality (C) in both sexes, in 2018 for all ages, in the MENA region and worldwide. The total number of cases is shown in the middle of the donut graphs; the percentage contribution and the number of cases of each cancer site are shown in the legends.
Figure S2: Mortality-to-incidence ratio (MIR) for five cancers with higher incidence in the Arab region. The MIR was calculated as the ratio of ASR for mortality to the ASR for incidence for all ages. The dotted lines mark the worldwide MIR. Arab countries are colored as those in the Arabian Gulf (maroon), the Levant (green) and North Africa (blue).
Figure S3: The top cancer sites in the MENA-Arab region leading incidence or mortality in females or males in 2018 for all ages. The MENA-Arab and worldwide ASR in 2018 for incidence for leading cancer sites for all ages in the MENA-Arab countries (coloured bars) and the worldwide rate for these sites (black bars) for females (A) and males (B). Mortality-to-incidence ratio (MIR) for the leading cancer sites in the MENA-Arab countries and the world for females (C) and males (D). Complete data mortality ASRs are available in Tables S1-S2.
Figure S4: Leading cancers for diagnosis and cancer-related deaths in subregions of the Arab countries. The percentage contribution of cancer sites to all cancers in females or males are shown for the 10 leading sites for cancer diagnoses and mortality in 2018 at all ages in the specified Arab subregions. The total number of diagnoses or deaths are shown under each plot. Complete data for all cancer sites in all subregions, in each country, and data for the USA, Europe and the world are available in Tables S1-S2.
Figure S5: Age-specific incidence of cancers in Arab countries. The ASIR for cancers at different age groups in Arab countries are summarized and compared to the ASIR at the same age groups for the World, USA and Europe. Arab countries are labelled as those in the Arabian Gulf (maroon), the Levant (green) and North Africa (blue). The world, USA and Europe in black font. Each cancer type is shown on a separate page in the following pages organized alphabetically. For all age groups please refer to Supplementary Data Tables S1 and S2.
Table S1: Raw data extracted from GLOBOCAN 2018 dataset for all cancers and each cancer, for all age groups and age-group intervals, for females and males, and for mortality and incidence for Arab countries, the world, USA and Europe. This data was used to generate the pivot table in Table S2 which can be queried. All data in the manuscript was based on this data file.
Table S2: Pivot table which can be queried for cancer incidence and mortality across different age groups in Arab countries and the data for the world, the USA and Europe. The table also includes different subregions in the Arab region. The table is based on the data extracted from the GLOBOCAN 2018 web database supplied in Table S1 in this study.
Data Availability Statement
The data generated in this study are available within the article and its supplementary data files. The raw data for incidence and mortality age-adjusted standardized for all ages and different age groups in females and males for each Arab country, subregions of Arab countries, the world, the United States and Europe are shown in Supplementary Table S1. These data were extracted from the GLOBOCAN 2018 database. The large data in Supplementary Table S1 were organized to generate Supplementary Table S2, which can be queried by users to select and visualize the data of interest.