Abstract
Smith-Lemli-Opitz syndrome is an autosomal recessive disorder that arises from mutations in the gene DHCR7, which encodes the terminal enzyme of cholesterol biosynthesis, leading to decreased production of cholesterol and accumulation of the cholesterol precursor, 7-dehydrocholesterol, and its oxysterol metabolites. The disorder displays a wide range of neurodevelopmental defects, intellectual disability, and behavioral problems. However, an in-depth study on the temporal changes of gene expression in the developing brains of SLOS mice has not been done before. In this work, we carried out the transcriptomic analysis of whole brains from WT and Dhcr7-KO mice at four-time points through postnatal day 0. First, we observed the expected downregulation of the Dhcr7 gene in the Dhcr7-KO mouse model, as well as gene expression changes of several other genes involved in cholesterol biosynthesis throughout all time points. Pathway and GO term enrichment analyses revealed affected signaling pathways and biological processes that were shared amongst time points and unique to individual time points. Specifically, the pathways important for embryonic development, including Hippo, Wnt, and TGF-β signaling pathways are the most significantly affected at the earliest time point, E12.5. Additionally, neurogenesis-related GO terms were enriched in earlier time points, consistent with the timing of development. Conversely, pathways related to synaptogenesis, which occurs later in development compared to neurogenesis, are significantly affected at the later time points, E16.5 and PND0, including the cholinergic, glutamatergic, and GABAergic synapses. The impact of these transcriptomic changes and enriched pathways is discussed in the context of known biological phenotypes of SLOS.
1. Introduction
Smith-Lemli-Opitz syndrome (SLOS, OMIM #270400) is caused by genetic mutations in the DHCR7 gene [1–5], which encodes the terminal enzyme of cholesterol biosynthesis, 7-dehydrocholesterol reductase (DHCR7, EC 1.3.1.21), leading to decreased levels of cholesterol and increased levels of the cholesterol precursor, 7-dehydrocholesterol (7-DHC), other sterol intermediates, and oxysterol metabolites [6–8]. Clinically, the disorder manifests a wide variety of phenotypes, including multiple congenital malformations (structural abnormalities and functional defects across several organ systems), developmental delay, and cognitive impairment [9]. Specifically in the CNS, anatomical abnormalities have been observed in SLOS patients, ranging from microcephaly to enlarged ventricles and hypoplasia of the corpus callosum, and in the most severe cases, holoprosencephaly [6, 10–12].
Development of the CNS is a carefully orchestrated and regulated process involving the interplay of many different signaling pathways. Within the CNS, cholesterol plays an essential role as a precursor to neurosteroids and steroid hormones, a major component of plasma membranes, myelin, and lipid rafts, and an important modulator of developmental signaling pathways, such as the Sonic hedgehog pathway [13–16]. Thus, disruption of cholesterol synthesis profoundly impacts many cellular processes in brain development, given the numerous biological functions of cholesterol. In addition, the cholesterol pool in the brain is especially unique due to the blood-brain barrier preventing cholesterol uptake from the circulation [17]. This means that cholesterol metabolism must be tightly regulated in a spatiotemporal manner during neurodevelopment to supply the high metabolic needs of cells during neurogenesis, as cells proliferate, migrate, differentiate, and undergo programmed cell death [17, 18]. While there is limited maternal-fetal transfer during early development in mice, de novo cholesterol synthesis becomes critical in the CNS after the formation of the blood-brain barrier around E10-11, while cortical neurogenesis begins around E12.5 and peaks around E15.5.
The development of genetically engineered mouse models of SLOS has made it possible to study the molecular mechanisms underlying the disorder at embryonic stages. In this study, we utilize a commercially available SLOS mouse model, Dhcr7-KO (Ex8), in which the exon VIII coding region of Dhcr7 has been deleted, leading to a truncated gene product [19]. The mouse model replicates the known biochemical defect of cholesterol synthesis [20] and exhibits developmental abnormalities in the lung, cleft palate, and bladder [19]. Homozygous pups die on the first day of birth due to an uncoordinated suck and failure to feed.
Transcriptomic studies of SLOS models have been previously reported in the literature [20–22]. Waage-Baudet et al. carried out the first transcriptomic study of the hindbrains of the same Dhcr7-KO SLOS mouse model, but on the 129/SvEv background, at gestational day 14 (E14) using the Affymetrix microarray analysis [21]. Hierarchical clustering of the differentially expressed genes identified alterations in genes involved in cholesterol homeostasis, cell cycle control and apoptosis, neurodifferentiation, embryogenesis, and, of particular interest, axon guidance. Dysregulated axon guidance was believed to be partly responsible for the abnormal hippocampal development that was previously reported in these mice [23]. In another study using a human-derived induced pluripotent stem cell (iPSC) model of SLOS that exhibited aberrant neural differentiation, Francis et al. determined that several transcriptional networks related to neural differentiation, cadherin-associated signaling, and kinase signaling were affected when comparing control iPSCs to SLOS iPSCs. In addition, they found overall downregulation of Wnt/β-catenin signaling (CAV1, CDH1, SNAI2), suggesting a role of defective Wnt signaling in the aberrant iPSC differentiation phenotype in SLOS. [22]
In a recent study from our lab, we found that Dhcr7-KO leads to a premature neurogenesis phenotype (with decreased proliferation and increased differentiation) of neural precursors in both a SLOS mouse model and in SLOS patient-derived neural progenitor cells (NPCs) and eventual thinning of cortical layers in embryonic mouse brains [20]. This mechanism was found to be mediated by glucocorticoid receptor and the neurotrophin kinase, TrkB. RNA sequencing analysis of human SLOS NPCs revealed gene expression changes related to neural precursor proliferation and differentiation, including those involved in MAPK and Ras signaling. While many transcriptomics experiments have been performed to investigate the pathophysiological mechanism of SLOS, no study has examined the temporal changes in gene expression and biological pathways throughout the embryonic developmental stages.
In this chapter, we discuss the results of an RNA sequencing analysis of whole brains [24] in a well-established SLOS mouse model, Dhcr7-KO mice, at E12.5, E14.5, E16.5, and PND0 in comparison with matching wild-type (WT) mice. Differentially expressed genes and significantly affected signaling pathways and biological processes were identified at each time point. Meta-analysis of all four time points highlighted shared and unique biological pathways at distinct developmental stages.
2. Methods and Materials
2.1. Chemicals
2-methylbutane was purchased from Thermo Fisher Scientific (Grand Island, New York). RNeasy Lipid Tissue Mini Kit was purchased from Qiagen (Germantown, Maryland).
2.2. Animals
Animal studies were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Washington Institutional Animal Care and Use Committee. C57BL/6J and transgenic heterozygous mice with a null mutation for Dhcr7 (Ex8) mice were purchased from Jackson Laboratories (Bar Harbor, Maine; catalog #007453). Mice were housed in an animal care facility with a standard 12-hour light and dark cycle and fed an ad libitum commercial rodent chow diet. Heterozygous Dhcr7 (Ex8) mice were mated overnight, where the day after time-mating was designated embryonic day 0.5 (E0.5). Heterozygous mating produced Dhcr7+/+ (WT), Dhcr7+/− (Het), and Dhcr7−/− (KO) offspring at roughly the expected Mendelian 1:2:1 ratio. Animals were genotyped using PCR as described previously [19]. Brains were harvested from mice at E12.5, E14.5, E16.5 (n=3 per genotype), and PND0 (n=4 per genotype) time points. For the embryonic time points, the pregnant dam was anesthetized under CO2 and decapitated. For the PND0 time point, neonates were removed from cages after birth, anesthetized on ice, and decapitated. Whole brains were excised under the dissection scope in ice-cold PBS and frozen in pre-cooled 2-methylbutane to be stored at −80°C until further processing steps.
2.3. RNA Isolation
E12.5, E14.5, and E16.5 whole brains and PND0 half brains (cut along the midsagittal plane) were processed for RNA sequencing. RNA was isolated using the Qiagen Lipid RNeasy Kit following the manufacturer’s protocol. RNA quality was assessed with a NanoDrop One spectrophotometer (Thermo Scientific), and only samples with appropriate purity (260/280 ratio > 2.0 and 260/230 ratio > 2.0) were used for analysis. Gel electrophoresis was also used to check for DNA contamination and RNA integrity.
2.4. RNA Sequencing (Novogene)
Samples were sent to Novogene Co. Ltd (Sacramento, CA) for total RNA sequencing on their Illumina platform. RNA sample quality control was further assessed, and the mRNA library was prepared with additional polyA enrichment, RNA fragmentation, and cDNA transcription steps. Libraries were sequenced with 150 bp paired-end reads on the Illumina NovaSeq 6000 Sequencing System with a minimum sequencing read depth of 6 Gb of raw data per sample.
2.5. Data Analysis
Data analysis was performed using a combination of Linux, R code, and various open-source online tools. We used an in-house-built Python script that streamlines raw data processing steps, utilizing zipped raw sequence read files to conserve disk memory space. hisat2 is used to align sequence reads to the reference mouse genome (mm10), where greater than 95% sequence alignment was achieved [25]. samtools sort and featureCounts are then used to count reads to genomic features (annotation file VM25) [26, 27]. The resulting sequence count data are input into DESeq2 (using R/Bioconductor) for differential expression analysis [28]. DESeq2 uses a generalized linear model with negative binomial distribution to estimate dispersion and the Wald significance test. Independent filtering step removes genes with very low counts. For pathway analysis, we utilized iPathwayGuide and Database for Annotation, Visualization, and Interpretation (DAVID) [29–32]. For both iPathwayGuide and DAVID, we used a list of all measured genes specific for each time point as the reference background lists.
2.6. Data Availability
Raw RNA-seq data has been deposited in NCBI’s Gene Expression Omnibus (GEO) and is available with the GEO Accession Number: GSE247566.
3. Results
3.1. Differential Expression Analysis
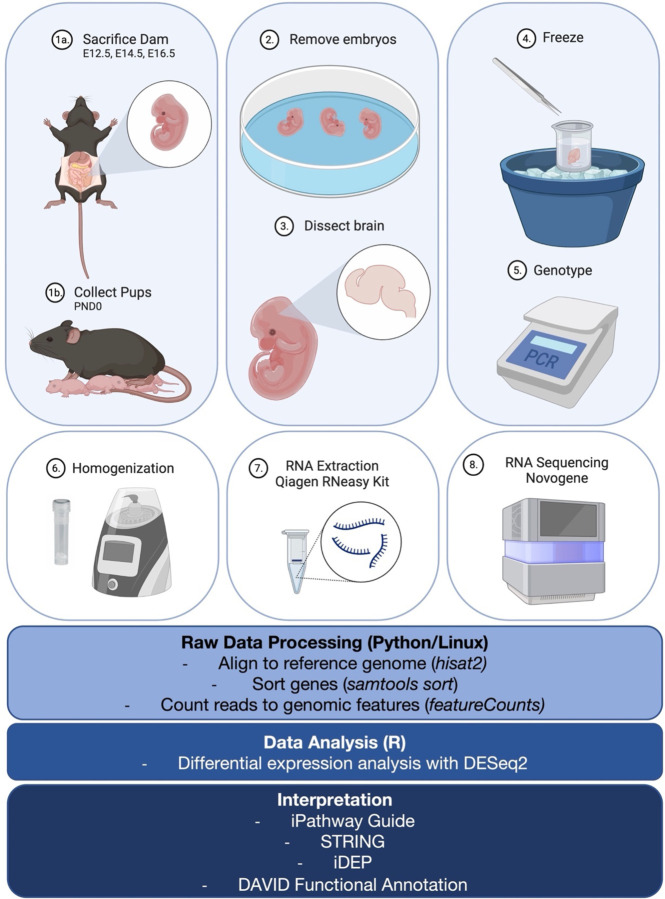
RNA sequencing (RNAseq) of whole mouse brains was performed, and gene expression profiles were compared between WT and Dhcr7-KO mice at three embryonic time points (E12.5, E14.5, E16.5) and one postnatal time point (PND0). RNAseq steps are outlined in Figure 1, including brain dissections, RNA extraction and sequencing, and data analysis.
Figure 1.
Diagram of RNA sequencing workflow. Steps for sample preparation (brain tissue collection from mouse embryos or neonates), RNA extraction, RNA sequencing, and data analysis are outlined. Created with BioRender.com
When considering all the WT and KO samples together in one principal component analysis (PCA) bi-plot (Supplementary Figure S1A), there is separation and clustering of the four groups according to time points, indicating that there are differences in the transcriptome at each time point that make each cluster distinct from the others. The PCA bi-plots constructed for each individual time point (Supplementary Figure S1B) more clearly demonstrate the separation between WT and KO samples.
To examine gene expression differences between the WT and KO conditions at the various time points, we performed differential expression analysis using DESeq2 in R. The number of differentially expressed genes (DEGs) for each time point, using an adjusted p-value (or false discovery rate, FDR) threshold of 0.05, are summarized in Table 1, including the number of total measured genes used as a reference background for pathway analysis. In summary, E12.5 had 647 differentially expressed genes (213 up-regulated in Dhcr7-KO compared to control, 434 down-regulated), E14.5 had 279 genes (129 up-regulated, 150 down-regulated), E16.5 had 645 genes (391 up-regulated, 254 down-regulated), and PND0 had 919 genes (391 up-regulated, 528 down-regulated). In Figure 2, significant DEGs (using thresholds: adjusted p-value (FDR) ≤ 0.05 and absolute fold change ≥ 1.2) are visually represented on volcano plots displaying the magnitude of change on the y-axis and statistical significance on the x-axis. The complete list of DEGs is available in Supplementary Table S1. The low number of measured genes for E14.5 resulted from the independent filtering step in DESeq2, which removes features with very low read counts that have low power to be detected as significant due to high dispersion.
Table 1.
Summary of differentially expressed genes, with an adjusted p-value threshold (FDR ≤ 0.05), or an adjusted p-value threshold and a fold change threshold (|FC| ≥ 1.2), and the total number of genes with measured expression (after DESeq2 filtering of genes with an extreme count outlier and low mean normalized counts).
| Time Point | Number of DEGs (FDR ≤ 0.05) | Number of DEGs (FDR ≤ 0.05 and |FC| ≥ 1.2) | Total Number of Measured Genes |
|---|---|---|---|
| E12.5 | 647 (Up: 213; Down: 434) | 644 (Up: 212; Down: 432) | 17386 |
| E14.5 | 279 (Up: 129; Down: 150) | 42 (Up: 29; Down: 13) | 6559 |
| E16.5 | 645 (Up: 391; Down: 254) | 470 (Up: 297, Down: 173) | 11674 |
| PND0 | 919 (Up: 391; Down: 528) | 844 (Up: 369; Down: 515) | 13145 |
Figure 2.
Volcano plots highlighting differentially expressed genes (shown in red) filtered by adjusted p-value ≤0.05 (shown in blue) and fold change ≥1.2 (shown in green). The complete list of DEGs is available in Supplementary Table S1.
Created with R/Enhanced Volcano.
Interestingly, most DEGs were not overlapping between time points, as seen in Figure 3, suggesting that there are differences in the genes and pathways being affected at specific times throughout development. Only nine DEGs were found to be shared between all four groups. Three that were down-regulated (Abca1, Dhcr7, Srebf1) and six up-regulated (Aacs, Fdft1, Hmgcs1, Nsdhl, Sqle, Stmn4). Besides Stmn4, the rest of the genes are all directly involved in different aspects of sterol metabolism, such as efflux transport (Abca1), transcriptional regulation (Srebf1), and various enzymes along the synthetic pathway. As expected in this Dhcr7-KO mouse model, the gene encoding for Dhcr7 was the most down-regulated gene transcript, with the largest fold changes across all four time points and lowest p-values. However, the other observed changes, such as up-regulation of key enzymes involved in cholesterol biosynthesis, are likely compensatory mechanisms that are transcriptionally regulated by the low cellular cholesterol levels in Dhcr7-KO mice.
Figure 3.
UpSet plot for meta-analysis of differentially expressed genes from all four time points. Bar plot displaying log fold-change values for the nine shared genes from all four time points and table of gene annotations.
Created by iPathwayGuide and R/ggplot2.
3.2. Pathway Analysis and Gene Ontology (GO) Term Enrichment
For genes included in pathway analysis, we used a significance threshold of an adjusted p-value (FDR) ≤ 0.05. Pathway analysis is used to identify which pathways are over-represented in a given set of genes beyond pure random chance. iPathwayGuide uses their proprietary “Impact Analysis” that factors in two probabilities, pORA (over-representation) and pAcc (perturbation) to compute statistical significance and is modelled after the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways [31–33]. It should be noted that we did not apply p-value adjustment for the pathway analysis results. Many pathways were significantly affected at individual time points. The top 10 KEGG pathways significantly affected at each time point are shown in Figure 4, and additional significantly altered pathways are available in Supplementary Table S2. Pathway results are visualized with bubble plots, which are ranked by p-value. The horizontal position on the plot designates the ratio of differentially expressed genes to the total number of genes associated with the specific pathway, while the size of the bubble designates the number of differentially expressed genes and the color designates the p-value. Interestingly, some of the top results in the earliest time point (E12.5) include important signaling pathways for embryonic development (Hippo, Wnt, and TGF-β). In contrast, later time points include pathways related to neurotransmission (synaptic vesicle cycle and different types of synapses, including cholinergic, glutamatergic, and GABAergic synapses).
Figure 4.
Top 10 enriched pathways from pathway analysis using iPathwayGuide. Bubble plots are ordered by p-value, and display pathway name and gene ratio, where the size of the bubble designates the number of differentially expressed genes, and the color designates the p-value.
* COVID-19 removed from E14.5, E16.5, and PND0 time points.
Created by R/ggplot2.
We also performed a GO enrichment analysis to identify which GO terms for biological processes are significantly over-represented in the given set of genes [34, 35]. The top 10 GO biological process terms enriched at each time point are shown in Supplementary Figure S2. Like pathway analysis, we found many of the top terms related to sterol synthesis, development, and synaptic signaling. To verify these pathway analysis results, we used another online tool, the Database for Annotation, Visualization, and Integrated Discovery (DAVID), for comparison [29,30]. The DAVID pathway analysis and GO enrichment analysis results are shown in Supplementary Figure S3 and Supplementary Figure S4 and were consistent with iPathwayGuide. Though GO terms tend to have much larger gene sets than KEGG pathways, the significantly affected biological processes are in line with the significantly affected pathways revealed by iPathwayGuide. The complete list of significantly enriched GO terms for biological processes is available in Supplementary Table S3.
3.3. Pathway Meta-Analysis of All Time Points
The meta-analysis feature in iPathwayGuide enables cross-comparison of the four time points, as shown via the UpSet plot in Figure 5. Steroid biosynthesis (mmu:00100) is the only pathway significantly affected at all time points, with a p-value significance threshold set at 0.05. This is a similarly expected result as the shared DEGs. Figure 6 shows the genes associated with the steroid biosynthesis pathway and their log fold-changes for each time point. This list of genes includes transcripts different from the nine shared DEGs (from Figure 3), including Lss, Msmo1, Cyp51, Sc5d, Dhcr24, and Tm7sf2. In this case, Dhcr7 is the only down-regulated gene, while all the other steroid synthesis-related genes are up-regulated.
Figure 5.
UpSet plot for meta-analysis of shared pathway hits from all time points with some specific pathways listed. All pathway results are available in Supplementary Table S2.
No p-value correction for multiple hypothesis testing was applied for pathway hits.
Created by iPathwayGuide.
Figure 6.
Bar plots of log fold-changes for DEGs in the steroid biosynthesis pathway (mmu:00100) for time points where this pathway had a p-value ≤ 0.05. DEGs are ordered by absolute LFC.
In Figure 5, we have highlighted pathways that are commonly affected at two or more time points. For example, the cell adhesion molecules pathway (mmu:04515) is the only other pathway shared between all embryonic time points, E12.5-E16.5 (Figure 7). L1cam is the only gene consistently up-regulated at those three time points. The ribosome pathway (mmu:03010) was significantly affected in E14.5, E16.5, and PND0 groups, and the associated genes are broken down for each time point in Error! Reference source not found.. Many of the genes in this pathway encode ribosomal proteins either in the small or large subunit. However, there is little overlap between the ribosomal DEGs at these three time points. It is also interesting to note that while all the DEGs in this pathway are down-regulated for E14.5 and E16.5 time points, all the DEGs except for one are up-regulated at PND0. This result could indicate a defect in translation at the ribosome level and in protein synthesis, while the switch in the direction of fold-changes highlights the temporal aspect of regulation.
Figure 7.

Bar plots of log fold-changes for DEGs in the tight junction (mmu:04530) and cell adhesion molecules pathways (mmu:04515) for time points where these pathways had a p-value ≤ 0.05.
Both cholinergic (mmu:04725) and GABAergic synapse pathways (mmu:04727) were significantly affected at the later E16.5 and PND0 time points ( Figure). For both, most genes are up-regulated at E16.5 and down-regulated at PND0, although the DEGs specific to the pathway differ between the time points. Gene expression changes in neurotransmitter signaling pathways could suggest defects in neurotransmission in SLOS, which will be discussed in the next section. Another related pathway, the glutamatergic synapse (mmu:04724), was shared between E12.5 and E16.5 time points (Figure 10), along with the axon guidance (mmu:04360) and neuroactive ligand-receptor interaction pathways (mmu:04080). Similar to the cholinergic and GABAergic synapse pathways, DEGs were found to be mainly up-regulated at the E16.5 time point. GABA is the primary inhibitory neurotransmitter, and glutamate is the main excitatory neurotransmitter in the mammalian CNS, suggesting that both inhibitory and excitatory neurotransmission were affected.
Figure 10.

Bar plots of log fold-changes for DEGs in the axon guidance (mmu:04360), glutamatergic synapse (mmu:04724), and neuroactive ligand-receptor interaction pathways (mmu:04080) for time points where these pathways had a p-value ≤ 0.05.
3.4. Unique Pathways for Individual Time Points
In addition to the pathways commonly enriched at multiple time points, some are uniquely enriched at individual time points. We expect to observe significant genes and pathways that are specific to the different stages of development. Thus, it makes sense that amongst earlier time points, there were pathways more closely related to neurogenesis and early embryonic development of the CNS. In contrast, later time points had a greater emphasis on neuronal maturation and synaptic signaling.
E12.5
Amongst the pathways significantly affected at E12.5 (Error! Reference source not found.4), three are related to embryogenesis, including Hippo, Wnt, and TGF-β pathways. All three are well-known, evolutionarily conserved signaling pathways crucial for mammalian embryonic development. DEGs for these three pathways are shown in an interaction network in Figure 11, where lines denote different types of interactions between genes and colors represent the direction of fold change. There is some overlap between the genes within these three related pathways. Most of the DEGs are down-regulated, which could indicate that inhibited signaling within these pathways are underlying some of the structural and functional CNS abnormalities present in the SLOS phenotype.
Figure 11.

Interaction networks of DEGs in unique signaling pathways that are significantly affected at each time point (p-value ≤ 0.05). Up-regulated genes are shown in red, and down-regulated genes are in blue.
E14.5
For E14.5 (mid-neurogenesis), two related pathways, cell cycle (mmu:04110) and cellular senescence (mmu:04218), were found to be among those significantly affected. Both pathways are related to cell cycle control, an important element of embryonic development and neuronal differentiation. Cyclin-dependent kinases (CDKs) are key regulatory enzymes in both pathways [36], and CDK1 (the major CDK enzyme that controls cell cycle) and CDK4 gene transcripts were both found to be down-regulated, as seen by the interaction network in Figure 11. This finding is interesting, considering the critical role of apoptosis in neurogenesis.
E16.5
E16.5 had the largest number of unique pathways significantly affected among all the time points, with 31 pathways with a p-value ≤ 0.05. Among these, the synaptic vesicle cycle (mmu:04721) and two pathways related to cAMP/AMPK signaling (mmu:04024 and mmu:04152) were particularly interesting (Figure 11). Synaptic vesicles mediate the release of neurotransmitters into the synaptic cleft between synapses, and this affected pathway is related to the other synapse-associated pathways that were shared between several time points. Similar to those pathways, the DEGs for synaptic vesicle cycle were mostly up-regulated at E16.5. For cAMP/AMPK signaling pathways, there appears to be a link between these two pathways and their role in cellular homeostasis [37]. While most DEGs involved were up-regulated, dysregulation of cAMP/AMPK signaling could likely affect downstream physiological processes, including metabolism (various biosynthetic pathways), cell fate, and gene transcription.
PND0
For PND0, we found many of the DEGs in significantly affected pathways to be down-regulated. In general, at this time point, there were more down-regulated genes than up-regulated ones, as seen in the asymmetrical volcano plot of DEGs (Error! Reference source not found.). Among the significantly affected pathways, growth hormone, secretion, and action (mmu:04935) and phospholipase D signaling pathways (mmu:04072) were of particular interest, as seen in Figure 11.
4. Discussion
Individuals with SLOS show a wide range of severity in phenotype across the many affected organ systems [9], and this applies to the neurological phenotype seen in the CNS. Neurological defects manifest as structural abnormalities [6, 10–12], developmental delay, intellectual disability, and behavioral problems, as well as frequent co-occurrence of autism spectrum disorder (ASD) [38, 39].
Cortical neurogenesis occurs over the span of several days, from approximately E11 to E17 [40]. De novo cholesterol synthesis turns on around E10-E11 to supply the large cholesterol needs of neurogenesis [41]. The time points within our study span these neurogenesis stages. We and others have reported that loss of DHCR7 leads to decreased proliferation and premature neurogenesis in both human and mouse NPCs [20, 22]. Francis et al. found that defective Wnt signaling may be responsible for such a phenotype, while our work suggested that activation of glucocorticoid receptor (GR) by an oxysterol metabolite may be the critical signaling pathway. However, these two findings are not mutually exclusive because activation of GR has been found to inhibit Wnt/β-catenin signaling [42, 43]. Wnt signaling is critical for basic developmental processes such as cell-fate specification, progenitor proliferation, and cell division [44]. At E12.5, Wnt9b (large fold-change), Wnt4, and Wnt5b are all down-regulated, as well as Wnt receptors, low-density lipoprotein receptor-related protein 5 (Lrp5) and Frizzled class receptor (Frz7 and Frz2). These transcriptomics results support the important role of Wnt signaling in the decreased proliferation and increased neurogenesis phenotype of SLOS NPCs.
Hippo and TGF-β signaling pathways are the other two significantly affected pathways at E12.5 that are critical for embryonic development. The Hippo signaling pathway controls organ size in embryonic development, which could be related to the commonly observed microcephaly phenotype in SLOS [9]. The signaling cascade consists of MST1 and MST2 kinases and their cofactors. In response to high cell density, activation of the pathway leads to cell apoptosis that prevents organ size overgrowth. At low cell density, when the pathway is inactivated, there are transcriptional factors that promote cell growth and proliferation. The TGF-β signaling pathway is involved in many different cellular functions, including proliferation, apoptosis, differentiation, and migration that are regulated by TGF-β family members. Most of the genes in Hippo and TGF-β signaling pathways are down-regulated, consistent with the decreased proliferation of Dhcr7-KO NPCs reported previously [20].
E14.5 is in the middle of cortical neurogenesis as NPCs differentiate into neurons that migrate to the outer layers of the neocortex while a proliferating pool of NPCs is also maintained [45, 46]. Programmed cell death, or apoptosis, plays a critical role in regulating neuronal precursors and pruning inefficient synapses [40]. Thus, it is not surprising to observe that cell cycle and cellular senescence were among the most significantly affected pathways at this time point.
It is important to note that GABAergic synapse, glutamatergic synapse, and cholinergic synapse pathways are significantly affected at later time points. Although previous work used mouse cortical precursors to demonstrate premature neurogenesis of excitatory neurons [20], the current results indicate that the formation of all types of neurons may be affected similarly by the loss of Dhcr7 function. Indeed, an impaired response of neurons to glutamate [47] and aberrant development of the serotonergic system [23] have been reported previously.
At E16.5, the axon guidance pathway was found to be significantly upregulated. This is consistent with previous work by Jiang et al., which showed increased axon and dendrite formation in Dhcr7-KO mouse hippocampal neurons relative to WT [48].
Ribosome pathway was found to be significantly affected at E14.5, E16.5, and PND0, but mostly downregulated at E14.5 and E16.5 and upregulated at PND0. This is a novel pathway that has not been indicated in previous studies. A recent paper showed that reduced cholesterol levels block the proliferation of erythroid precursors by inhibiting ribosome biogenesis [49]. Although neural precursors are a different cell type than erythroid precursors, a similar mechanism could likely be in play for regulating the proliferation and differentiation of neural precursors. The role of cholesterol in regulating ribosome biogenesis in the brain and the significance of this pathway in neurodevelopment are worthy of further investigation in future studies.
Because SLOS patients are known to display autistic behavior, we examined whether the DEGs observed in Dhcr7-KO mouse brains contain known genes involved in autism by comparing them with the SFARI autism gene database. Indeed, we found 69, 39, 75, and 114 autism-related genes at E12.5, E14.5, E16.5, and PND0, respectively (Figure 12). Among these genes, only DHCR7 was shared by all time points. Genes shared by three groups include FAT1, CACNA1B, KCNQ2, KCTD13, HERC2, STXBP1, LDLR, and TSPAN7. Additional genes shared by at least two time points are shown in Figure 12.
Figure 12.
Venn diagram of DEGs shared with SFARI autism gene database at each time point.
5. Conclusion
We have performed transcriptomic analysis of whole brains from Dhcr7-KO and WT mice at three embryonic time points and one postnatal time point. Comparative analysis of differentially expressed genes and pathway analysis shows the altered regulation of different physiological processes that could be related to the neurological phenotype in SLOS. Early time points suggest downregulation of genes and pathways involved in embryonic development, such as Hippo, Wnt, and TGF-β, and later time points suggest universal defective neuronal synapse pathways. In addition, the ribosome was found to be another novel pathway affected by deficient cholesterol biosynthesis at three-time points. Thus, disruption of cholesterol synthesis through genetic mutation of Dhcr7 has deleterious and concerted effects beyond those expected in simply sterol and lipid homeostasis.
Supplementary Material
Figure 8.
Bar plots of log fold-changes for DEGs in the ribosome pathway (mmu:03010) for time points where this pathway had a p-value ≤ 0.05.
Figure 9.
Bar plots of log fold-changes for DEGs in the cholinergic (mmu:04725) and GABAergic synapse pathways (mmu:04727) for time points where these pathways had a p-value ≤ 0.05.
Acknowledgements
We would like to sincerely thank James W. MacDonald and Theo K. Bammler for their knowledge and assistance with iPathway Guide through the University of Washington EDGE Center.
Funding Statement
This work was supported by grants from the National Institutes of Health: R01 HD092659 (L.X.), Pharmacological Sciences Training Program (T32 GM007750), the NIEHS funded EDGE Center (P30ES007033), and Institute of Translational Health Sciences TL1 Program (TL1 TR002318) from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fitzky B.U., et al. , Mutations in the Delta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc Natl Acad Sci U S A, 1998. 95(14): p. 8181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wassif C.A., et al. , Mutations in the human sterol delta7-reductase gene at 11q12–13 cause Smith-Lemli-Opitz syndrome. Am J Hum Genet, 1998. 63(1): p. 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tint G.S., et al. , Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med, 1994. 330(2): p. 107–13. [DOI] [PubMed] [Google Scholar]
- 4.Yu H., et al. , Spectrum of ∆7-dehydrocholesterol reductase mutations in patients with the Smith–Lemli–Opitz (RSH) syndrome. Hum Mol Genet, 2000. 9(9): p. 1385–1391. [DOI] [PubMed] [Google Scholar]
- 5.Jira P.E., et al. , Smith-Lemli-Opitz syndrome and the DHCR7 gene. Ann Hum Genet, 2003. 67(Pt 3): p. 269–80. [DOI] [PubMed] [Google Scholar]
- 6.Porter F.D. and Herman G.E., Malformation syndromes caused by disorders of cholesterol synthesis. J Lipid Res, 2011. 52(1): p. 6–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thurm A., et al. , Development, behavior, and biomarker characterization of Smith-Lemli-Opitz syndrome: an update. J Neurodev Disord, 2016. 8: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tint G.S., et al. , Markedly increased tissue concentrations of 7-dehydrocholesterol combined with low levels of cholesterol are characteristic of the Smith-Lemli-Opitz syndrome. J Lipid Res, 1995. 36(1): p. 89–95. [PubMed] [Google Scholar]
- 9.Kelley R.I. and Hennekam R.C., The Smith-Lemli-Opitz syndrome. J Med Genet, 2000. 37(5): p. 321–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marion R.W., et al. , Computed tomography of the brain in the Smith-Lemli-Opitz syndrome. J Child Neurol, 1987. 2(3): p. 198–200. [DOI] [PubMed] [Google Scholar]
- 11.Dang Do A.N., et al. , Spontaneously regressing brain lesions in Smith-Lemli-Opitz syndrome. Am J Med Genet A, 2018. 176(2): p. 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee R.W., et al. , Brain magnetic resonance imaging findings in Smith-Lemli-Opitz syndrome. Am J Med Genet A, 2013. 161A(10): p. 2407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter J.A., Young K.E., and Beachy P.A., Cholesterol modification of hedgehog signaling proteins in animal development. Science, 1996. 274(5285): p. 255–9. [DOI] [PubMed] [Google Scholar]
- 14.Saher G., et al. , High cholesterol level is essential for myelin membrane growth. Nat Neurosci, 2005. 8(4): p. 468–75. [DOI] [PubMed] [Google Scholar]
- 15.Mauch D.H., et al. , CNS synaptogenesis promoted by glia-derived cholesterol. Science, 2001. 294(5545): p. 1354–7. [DOI] [PubMed] [Google Scholar]
- 16.Koudinov A.R. and Koudinova N.V., Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J, 2001. 15(10): p. 1858–60. [DOI] [PubMed] [Google Scholar]
- 17.Dietschy J.M. and Turley S.D., Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res, 2004. 45(8): p. 1375–97. [DOI] [PubMed] [Google Scholar]
- 18.Funfschilling U., et al. , Critical Time Window of Neuronal Cholesterol Synthesis during Neurite Outgrowth. J Neurosci, 2012. 32(22): p. 7632–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzky B.U., et al. , 7-Dehydrocholesterol–dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Opitz/RSH syndrome. Journal of Clinical Investigation, 2001. 108(6): p. 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hideaki Tomita K.M.H., Herron Josi M, Li Amy, Baggett David W, Xu Libin, 7-Dehydrocholesterol-derived oxysterols cause neurogenic defects in Smith-Lemli-Opitz syndrome. eLife, 2022. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waage-Baudet H., et al. , Immunohistochemical and microarray analyses of a mouse model for the smith-lemli-opitz syndrome. Dev Neurosci, 2005. 27(6): p. 378–96. [DOI] [PubMed] [Google Scholar]
- 22.Francis K.R., et al. , Modeling Smith-Lemli-Opitz syndrome with induced pluripotent stem cells reveals a causal role for Wnt/beta-catenin defects in neuronal cholesterol synthesis phenotypes. Nat Med, 2016. 22(4): p. 388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waage-Baudet H., et al. , Abnormal serotonergic development in a mouse model for the Smith-Lemli-Opitz syndrome: implications for autism. Int J Dev Neurosci, 2003. 21(8): p. 451–9. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z., Gerstein M., and Snyder M., RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet, 2009. 10(1): p. 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D., et al. , Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol, 2019. 37(8): p. 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., et al. , The Sequence Alignment/Map format and SAMtools. Bioinformatics, 2009. 25(16): p. 2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Y., Smyth G.K., and Shi W., featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics, 2014. 30(7): p. 923–30. [DOI] [PubMed] [Google Scholar]
- 28.Love M.I., Huber W., and Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol, 2014. 15(12): p. 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman B.T., et al. , DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res, 2022. 50(W1): p. W216–W221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dennis G., et al. , DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biology, 2003. 4(5). [PubMed] [Google Scholar]
- 31.Donato M., et al. , Analysis and correction of crosstalk effects in pathway analysis. Genome Res, 2013. 23(11): p. 1885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Draghici S., et al. , A systems biology approach for pathway level analysis. Genome Res, 2007. 17(10): p. 1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanehisa M. and Goto S., KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res, 2000. 28(1): p. 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gene Ontology C., et al. , The Gene Ontology knowledgebase in 2023. Genetics, 2023. 224(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashburner M., et al. , Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet, 2000. 25(1): p. 25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brantley S.E. and Di Talia S., Cell cycle control during early embryogenesis. Development, 2021. 148(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aslam M. and Ladilov Y., Emerging Role of cAMP/AMPK Signaling. Cells, 2022. 11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tierney E., et al. , Behavior phenotype in the RSH/Smith-Lemli-Opitz syndrome. Am J Med Genet, 2001. 98(2): p. 191–200. [DOI] [PubMed] [Google Scholar]
- 39.Sikora D.M., et al. , The near universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz syndrome. Am J Med Genet A, 2006. 140(14): p. 1511–8. [DOI] [PubMed] [Google Scholar]
- 40.Chen V.S., et al. , Histology Atlas of the Developing Prenatal and Postnatal Mouse Central Nervous System, with Emphasis on Prenatal Days E7.5 to E18.5. Toxicol Pathol, 2017. 45(6): p. 705–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sisecioglu M., et al. , A compendium of expression patterns of cholesterol biosynthetic enzymes in the mouse embryo. J Lipid Res, 2015. 56(8): p. 1551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olkku A. and Mahonen A., Calreticulin mediated glucocorticoid receptor export is involved in beta-catenin translocation and Wnt signalling inhibition in human osteoblastic cells. Bone, 2009. 44(4): p. 555–65. [DOI] [PubMed] [Google Scholar]
- 43.Zhou H., et al. , Endothelial cell-glucocorticoid receptor interactions and regulation of Wnt signaling. JCI Insight, 2020. 5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teo J.L. and Kahn M., The Wnt signaling pathway in cellular proliferation and differentiation: A tale of two coactivators. Adv Drug Deliv Rev, 2010. 62(12): p. 1149–55. [DOI] [PubMed] [Google Scholar]
- 45.Dehay C. and Kennedy H., Cell-cycle control and cortical development. Nat Rev Neurosci, 2007. 8(6): p. 438–50. [DOI] [PubMed] [Google Scholar]
- 46.Molyneaux B.J., et al. , Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci, 2007. 8(6): p. 427–37. [DOI] [PubMed] [Google Scholar]
- 47.Wassif C.A., et al. , Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith--Lemli--Opitz syndrome. Hum Mol Genet, 2001. 10(6): p. 555–64. [DOI] [PubMed] [Google Scholar]
- 48.Jiang X.S., et al. , Activation of Rho GTPases in Smith-Lemli-Opitz syndrome: pathophysiological and clinical implications. Hum Mol Genet, 2010. 19(7): p. 1347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Z., et al. , Fine-Tuning of Cholesterol Homeostasis Controls Erythroid Differentiation. Adv Sci (Weinh), 2022. 9(2): p. e2102669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw RNA-seq data has been deposited in NCBI’s Gene Expression Omnibus (GEO) and is available with the GEO Accession Number: GSE247566.