Abstract
Aim:
Ravulizumab and eculizumab are complement C5 inhibitors approved for the treatment of atypical hemolytic uremic syndrome (aHUS). Ravulizumab requires less frequent infusions than eculizumab, which may reduce treatment burden. This study investigated patients' treatment preferences and the impact of both treatments on patient and caregiver quality of life.
Materials & methods:
Two surveys were conducted (one for adult patients with aHUS and one for caregivers of pediatric patients with aHUS) to quantitatively assess treatment preference and the patient- and caregiver-reported impact of ravulizumab and eculizumab on quality of life. Patients were required to have a diagnosis of aHUS, to be currently receiving treatment with ravulizumab and to have received prior treatment with eculizumab. Participants were recruited via various sources: the Alexion OneSource™ patient support program, the Rare Patient Voice recruitment agency, the aHUS Foundation and directly via a clinician involved in the study.
Results:
In total, 50 adult patients (mean age: 46.5 years) and 16 caregivers of pediatric patients (mean age: 10.1 years) completed the surveys. Most adult patients (94.0%) and all caregivers reported an overall preference for ravulizumab over eculizumab; infusion frequency was one of the main factors for patients when selecting their preferred treatment. Fewer patients reported disruption to daily life and the ability to go to work/school due to ravulizumab infusion frequency (4.0% and 5.7%, respectively) than eculizumab infusion frequency (72.0% and 60.0%), with similar results for caregivers.
Conclusion:
Adult patients and caregivers of pediatric patients indicated an overall preference for ravulizumab than eculizumab for the treatment of aHUS, driven primarily by infusion frequency. This study contributes to the emerging real-world evidence on the treatment impact and preference in patients with aHUS.
Keywords: aHUS, atypical hemolytic uremic syndrome, caregiver preference, eculizumab, patient-reported outcomes, patient preference, quality of life, ravulizumab, treatment preference
Plain language summary
What is this article about?
Atypical hemolytic uremic syndrome, also called aHUS, is a rare disease that causes kidney damage. Ravulizumab and eculizumab are medications given as intravenous (IV) infusions to treat people who have aHUS. Ravulizumab does not have to be taken as often as eculizumab. In this research, the investigators made surveys to ask people which medication they preferred to treat aHUS and how the medications affected their lives. Those who took part were either adult patients with aHUS, or people who were caring for a child with aHUS. Patients had experience being treated with both ravulizumab and eculizumab.
What were the results?
The survey responses showed that most people preferred ravulizumab over eculizumab. One of the main reasons they preferred ravulizumab was that they did not have to take ravulizumab as often as eculizumab, and their answers showed that this led to less disruption to their daily lives and their work/school attendance.
What do the results mean?
These findings should help doctors, patients and caregivers to decide whether ravulizumab or eculizumab is best for the patient.
Tweetable abstract
Surveys of patients and caregivers of pediatric patients with #aHUS showed an overall preference for ravulizumab over eculizumab, driven primarily by infusion frequency. Ravulizumab had a more positive impact on quality of life than eculizumab.
Atypical hemolytic uremic syndrome (aHUS) is a rare disease that manifests as a thrombotic microangiopathy characterized by uncontrolled complement activation leading to the development of microthrombi and organ damage, particularly in the kidneys [1–3]. A diagnosis of aHUS is considered upon the presentation of microangiopathic hemolytic anemia, thrombocytopenia and renal impairment after the other clinical causes of thrombotic microangiopathy, including thrombotic thrombocytopenic purpura and Shiga toxin-producing Escherichia coli hemolytic uremic syndrome, have been excluded [1,4]. Early diagnosis is important to initiate effective treatment [5,6]. Terminal complement C5 inhibition is considered the standard of care for the treatment of aHUS [1].
Eculizumab, a complement C5 inhibitor, has been the standard of care for patients with aHUS since its approval, initially in the USA in 2011 [7,8]. Eculizumab has since been approved in other regions such as the European Union [9–11]. The efficacy and safety profile of eculizumab has been demonstrated in previous research [5,12–15]. The maintenance dosing frequency for eculizumab is every 2 weeks for patients weighing 10 kg or more [7]. Ravulizumab (10 mg/ml), first approved in the USA in 2019 for the treatment of aHUS, was engineered from eculizumab to achieve a reduced maintenance dosing frequency of 4–8 weeks, depending on body weight [16,17]. In clinical trials, ravulizumab was an effective treatment for adult and pediatric patients with aHUS and was associated with an acceptable safety profile [18–21]. An indirect treatment comparison of eculizumab and ravulizumab using aHUS clinical trial data demonstrated no apparent differences in efficacy between the two treatments [22]. Further, the 100 mg/ml formulation of ravulizumab, approved in the USA in 2020, enabled shorter overall infusion time per year (3.3–10.4 h) than the original formulations of ravulizumab (13.0–24.7 h) and eculizumab (15.2–52.0 h) [23,24].
There is a need for real-world studies to compare the impact of these treatments on quality of life, particularly the impact associated with the reduced administration frequency of ravulizumab. Additionally, there is a need to understand patients' and caregivers' treatment preferences and the factors that influence those preferences to enable clinicians to make informed treatment recommendations. The objectives of this study were (1) to evaluate preference for ravulizumab or eculizumab in adult patients with aHUS and caregivers of pediatric patients with aHUS who have experience with both treatments, and (2) to understand the impact of the disease and treatments on patient and caregiver quality of life.
Materials & methods
Study design
This study used web-based surveys to quantitatively assess preference for ravulizumab or eculizumab and treatment impact of ravulizumab versus eculizumab. Two surveys, one for adult patients with aHUS and one for caregivers of pediatric patients with aHUS, were developed following methodological best practices (Supplementary Text 1).
The final surveys assessed demographics and health information, aHUS disease and management, preference for ravulizumab or eculizumab, the impact of each treatment on aspects of quality of life, time involved in receiving infusions and overall time lost owing to treatment (Supplementary Table 1). Content included in the online surveys is provided in Supplementary Text 2. Qualtrics XM software (Provo, UT, USA) was used to collect survey data. The target sample size was 50 participants per cohort (adult patients and caregivers).
This study was conducted in compliance with the Declaration of Helsinki and the International Council for Harmonisation guidelines. Informed consent was obtained from each participant before enrollment into the study and study materials were approved by the WCG institutional review board (approval reference no. 1297161).
Study population
The cohorts were adult patients (aged 18 years or older) with aHUS, and primary caregivers or the legal parents/guardians (aged 18 years or older) of pediatric patients (aged 1 month to 17 years) with aHUS. Participants were recruited via the Alexion OneSource™ patient support program, the Rare Patient Voice recruitment agency, the aHUS Foundation and directly via a clinician involved in the study. To be eligible for inclusion, patients were required to have either a clinician-confirmed diagnosis or a medical document or communication showing evidence of aHUS that could be verified by the study investigators, and to have previously received eculizumab followed by three or more doses of ravulizumab as treatments for aHUS.
Survey administration
Participants eligible for inclusion were contacted by study personnel to be granted access to the web-based survey via a link that was unique to them and the survey could be completed on a computer or mobile device. This approach was taken to prevent unauthorized access to the study materials and participation. The survey was open for responses between 27 May and 10 December 2021. All data collected were de-identified, with participants identified using an assigned number. All documents and electronic files were stored securely, and all identifiers were removed from the data at the time of study completion.
Statistical analysis
Adult patient and caregiver data were analyzed separately, and no statistical comparisons were made between these cohorts. Categorical variables were described by their frequency and the percentage of each response. Missing data were minimal as the web-based surveys required responses to proceed, therefore, the data were analyzed as observed and any missing data arising for any other reasons were not included in percentage calculations, nor were missing data imputed. Quantitative variables were described using their frequency, mean, standard deviation (SD), and minimum and maximum values. Target sample sizes were based on general principles of ideal minimum numbers of participants needed to detect statistical differences between participant experience with ravulizumab versus prior treatment with eculizumab. However, the final sample size was based on availability in this rare population.
For statistical comparisons, statistical tests were two-sided using a significance level of p < 0.05. Owing to small sample sizes, all presented p-values are at the nominal level. For questions that examined treatment preference on a 5-point Likert scale (from ‘strongly prefer ravulizumab’ to ‘strongly prefer eculizumab’), the binomial test was used to compare the proportion of those who indicated any preference for ravulizumab versus those who indicated either ‘no preference’ or any preference for eculizumab. For the analysis of responses to parts of the surveys that assessed the impact of ravulizumab and eculizumab on quality of life, responses on the 5-point Likert scale were combined into ‘not at all/a little bit’, ‘somewhat’ and ‘quite a bit/very much’ to assist with analyses given the small sample sizes. Given that patients received both treatments, a repeated measures comparison of treatment impact was performed with the McNemar–Bowker test of symmetry or paired t-tests, as appropriate for the variable type. Survey responses of “not applicable” were excluded from the analyses. All statistical analyses of survey data were performed with SAS version 9.4 or higher (SAS Institute, Inc., NC, USA).
If sample size was sufficient (prespecified as n = 30), additional subgroup analyses were performed for patients who received the 100 mg/ml formulation of ravulizumab separately from patients who received the 10 mg/ml formulation of ravulizumab for the treatment preference and treatment impact portions of the surveys.
Results
Survey respondents
A total of 355 individuals in the USA were screened between 13 December 2020 and 22 November 2021, and 133 were eligible for inclusion. The most common reasons for exclusion were that the patient had not received both eculizumab and ravulizumab, or that the patient in the caregiver cohort was aged 18 years or older. Of the eligible participants, 76 provided informed consent and suitable confirmation of aHUS diagnosis, and 66 non-duplicate surveys were completed (49.6% response rate, calculated as the number of completed surveys as a proportion of the 133 participants eligible for inclusion). The recruitment target of 50 adult patients was achieved, while recruitment for the caregiver cohort halted at 16 completed surveys despite efforts to attain the target sample size of 50.
Study population
Table 1 lists the demographics of survey respondents and pediatric patients. Adult patients, caregivers and pediatric patients were predominantly female (80.0%, 93.8% and 62.5%, respectively), White (92.0%, 100% and 100%, respectively), and non-Hispanic/non-Latino (94.0%, 100% and 100%, respectively). The mean (SD) age (at the time of enrollment) of adult patients was 46.5 (13.9) years and the mean age of pediatric patients was 10.1 (4.8) years. Most adult patients (60.0%) and caregivers (81.3%) had a college degree or higher; 42.0% of adult patients and 43.8% of caregivers were in full-time employment.
Table 1. . Adult patient, caregiver and caregiver-reported pediatric patient demographics.
| Demographic variable | Adult patients (n = 50) | Caregivers (n = 16) | Pediatric patients (n = 16) |
|---|---|---|---|
| Sex, n (%) Female Male |
40 (80.0) 10 (20.0) |
15 (93.8) 1 (6.3) |
10 (62.5) 6 (37.5) |
| Age†, years Mean (SD) Range |
46.5 (13.9) 19–71 |
43.1 (9.6) 29–57 |
10.1 (4.8) 2–16 |
| Ethnicity, n (%) Hispanic/Latino Not Hispanic/not Latino |
3 (6.0) 47 (94.0) |
0 (0.0) 16 (100) |
0 (0.0) 16 (100) |
| Race, n (%) White Black/African American Other‡ |
46 (92.0) 3 (6.0) 1 (2.0) |
16 (100) 0 (0.0) 0 (0.0) |
16 (100) 0 (0.0) 0 (0.0) |
| Highest education completed§, n (%) Not yet of school age Elementary school Middle school High school Did not complete high school High school diploma or General Educational Development Some college, certification program or currently enrolled College, technical college or university degree Graduate degree |
1 (2.0) 4 (8.0) 15 (30.0) 19 (38.0) 11 (22.0) |
0 (0.0) 0 (0.0) 3 (18.8) 8 (50.0) 5 (31.3) |
3 (18.8) 6 (37.5) 3 (18.8) 4 (25.0) |
| Current work status, n (%) Employed full-time¶ Employed part-time# Homemaker Student Retired Disabled Unemployed |
21 (42.0) 7 (14.0) 8 (16.0) 6 (12.0) 9 (18.0) 4 (8.0) 2 (4.0) |
7 (43.8) 3 (18.8) 7 (43.8) 0 (0.0) 0 (0.0) 0 (0.0) 1 (6.3) |
Represents the reported participant age at the time of participation.
The full text response to ‘other’ was “Mexican”.
Elementary school = kindergarten to 5th grade, middle school = 6th to 8th grade, high school = 9th to 10th grade.
40 h per week or more.
Fewer than 40 h per week.
Percentages may not sum to 100 owing to rounding, and because multiple answers could be selected for current work status.
SD: Standard deviation.
Disease and management characteristics are presented in Table 2. The participant-reported mean (range) number of years since aHUS diagnosis was 6.8 (1–48) years for adult patients, and 6.1 (1–14) years for pediatric patients. For adult patients, the mean (SD) length of treatment with eculizumab and ravulizumab was 46.9 (33.4) months and 12.9 (6.0) months, respectively. For pediatric patients, caregivers reported that the mean (SD) length of treatment with eculizumab and ravulizumab was 46.5 (32.1) months and 13.6 (5.7) months, respectively.
Table 2. . Disease and management characteristics as reported by adult patients and by caregivers on behalf of pediatric patients.
| Demographic variable | Adult patients (n = 50) | Pediatric patients (n = 16) |
|---|---|---|
| Years since diagnosis Mean (SD) Range |
6.8 (7.5) 1–48 |
6.1 (3.8) 1–14 |
| Trigger Infection Severe hypertension Autoimmune disease Pregnancy or childbirth None Other† Unsure |
11 (22.0) 2 (4.0) 1 (2.0) 9 (18.0) 2 (4.0) 12 (24.0) 13 (26.0) |
6 (37.5) 0 (0.0) 0 (0.0) 0 (0.0) 4 (25.0) 2 (12.5) 4 (25.0) |
| Length of eculizumab treatment, months Mean (SD) Range |
46.9 (33.4) 2–133 |
46.5 (32.1) 3–119 |
| Site of last eculizumab treatment Hospital (inpatient) Hospital (outpatient) Clinic Home |
0 (0.0) 10 (20.0) 24 (48.0) 16 (32.0) |
1 (6.3) 6 (37.5) 4 (25.0) 5 (31.3) |
| Length of ravulizumab treatment, months‡ Mean (SD) Range |
12.9 (6.0) 0–25 |
13.6 (5.7) 4–27 |
| Site of last ravulizumab treatment Hospital (inpatient) Hospital (outpatient) Clinic Home |
0 (0.0) 8 (16.0) 28 (56.0) 14 (28.0) |
1 (6.3) 6 (37.5) 4 (25.0) 5 (31.3) |
| Current ravulizumab formulation 100 mg/ml 10 mg/ml Unsure |
39 (78.0) 8 (16.0) 3 (6.0) |
12 (75.0) 2 (12.5) 2 (12.5) |
| Venous access port previously implanted for eculizumab Yes No |
17 (34.0) 33 (66.0) |
10 (62.5) 6 (37.5) |
| Venous access port currently implanted for ravulizumab Yes No |
15 (30.0) 35 (70.0) |
8 (50.0) 8 (50.0) |
Participant free-text responses mentioned medication use, transplantation, surgery, pulmonary hemorrhages, pneumonia, pregnancy, infections, stress and vaccination.
Length of treatment was calculated using the difference between the dates of the first and last treatments as reported by participants. The minimum value of 0 months on ravulizumab may be caused by a participant adding the same date for both responses.
SD: Standard deviation.
For eculizumab infusions, 34.0% and 62.5% of adult and pediatric patients, respectively, had a venous access port previously implanted. For ravulizumab infusions, 30.0% and 50.0% of adult and pediatric patients, respectively, had venous access ports currently implanted. Overall, 78.0% of adult patients and 75.0% of pediatric patients were receiving the 100 mg/ml formulation of ravulizumab at the time of the study.
Treatment preference
In total, 47 of 50 adult patients (94.0%) and all caregivers reported an overall preference for ravulizumab over ‘no preference’ or eculizumab (p < 0.001; Figure 1). For specific factors that may influence patients' treatment preferences, adult patients preferred ravulizumab over ‘no preference’ or eculizumab. When considering the frequency of infusions, all adult patients preferred ravulizumab. There was also a strong preference for ravulizumab over ‘no preference’ and eculizumab regarding the ability to travel/go on vacation, the benefit to quality of life, infusion duration and the ability to plan social activities (all p < 0.001 vs preference for eculizumab or ‘no preference’). For caregivers, the benefit to their quality of life, the benefit to the pediatric patient's quality of life, the ability to travel/go on vacation, the frequency of infusions and the ability to plan social activities were the five factors with the highest proportions of participants reporting preference for ravulizumab over ‘no preference’ or eculizumab (all p < 0.001).
Figure 1. . Treatment preference between ravulizumab and prior eculizumab treatment by factor for (A) adult patients and (B) caregivers of pediatric patients.
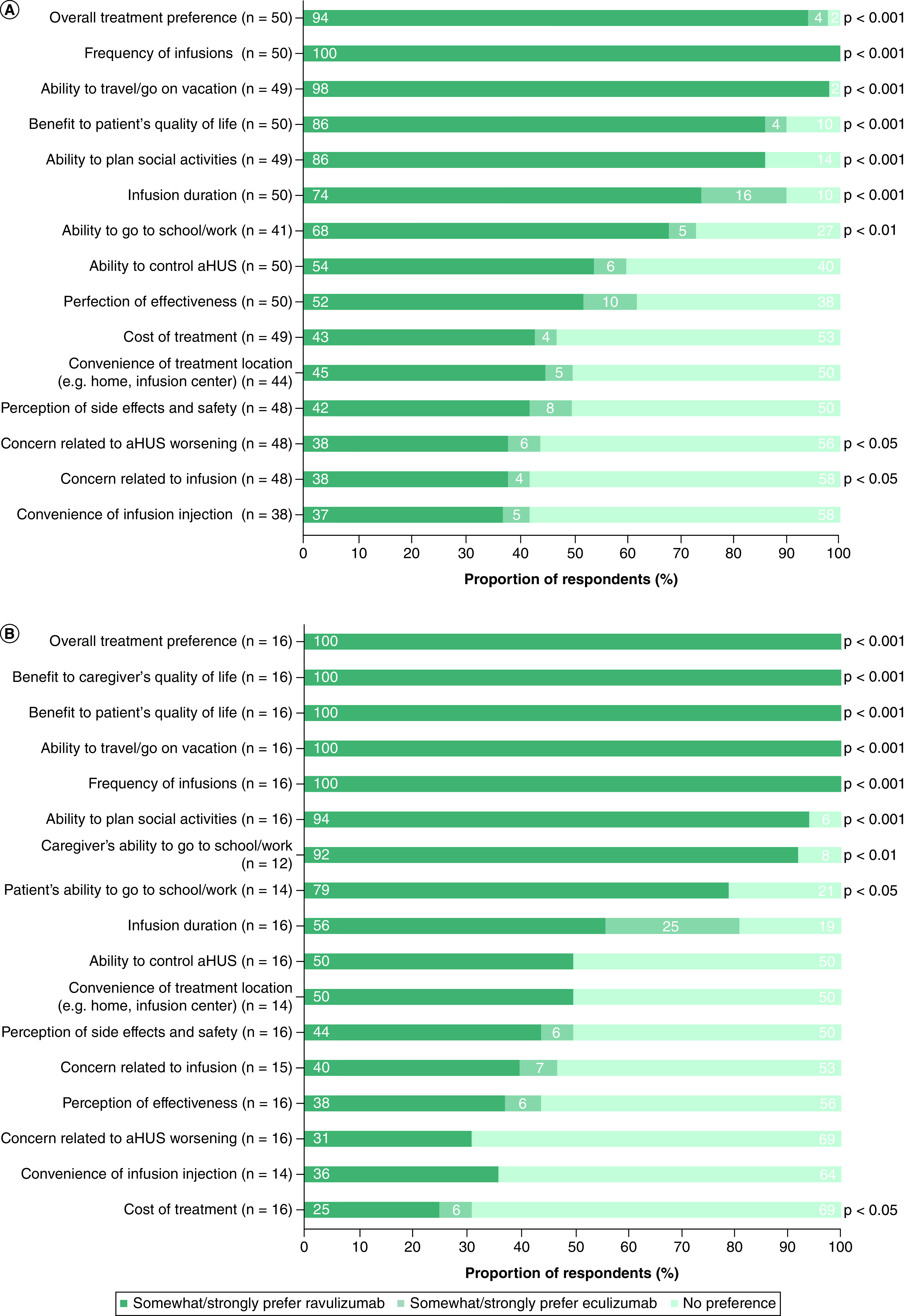
For survey items relating to concerns (e.g. concern related to infusion), respondents indicated which treatment they preferred to overcome their concern. The binomial test was used to compare the proportion of those who indicated any preference for ravulizumab versus those who indicated either ‘no preference’ or any preference for eculizumab.
aHUS: Atypical hemolytic uremic syndrome.
The factors that participants most frequently ranked in their top five considerations when deciding their overall treatment preference are shown in Figure 2. The frequency of infusions, the ability to control aHUS and the benefit to the patient's quality of life were the three most important factors when selecting a preferred treatment in both cohorts.
Figure 2. . Frequencies of participant responses when asked to select up to five most-important factors when deciding overall preference between treatments for (A) adult patients and (B) caregivers of pediatric patients.
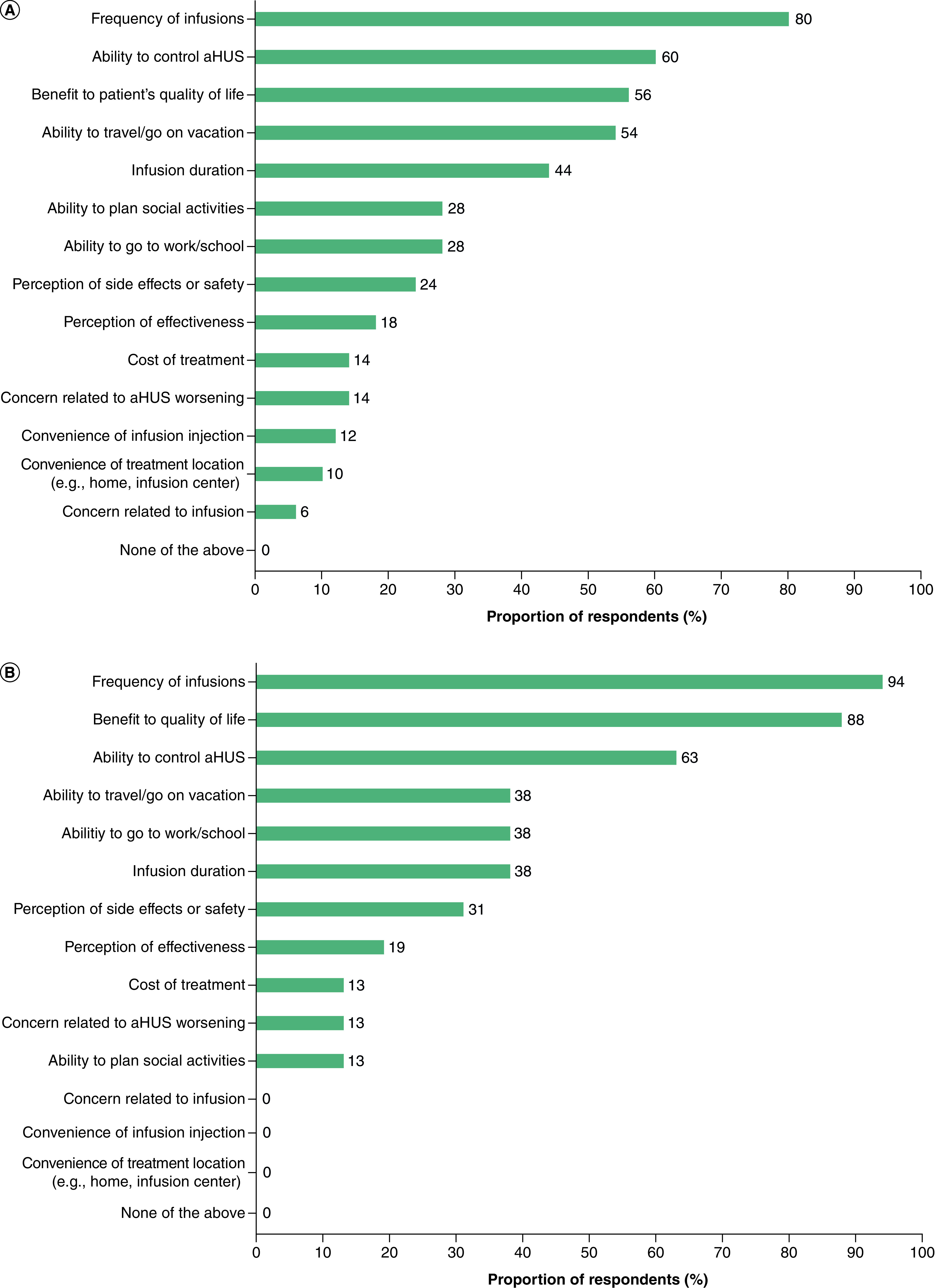
Participants were asked to select up to five factors that were most important to them when deciding their overall preference between prior eculizumab treatment and ravulizumab.
aHUS: Atypical hemolytic uremic syndrome.
Treatment impact
Among adult patients, markedly fewer responded that ravulizumab infusion frequency disrupted their daily lives or impacted their ability to go to work/school at least somewhat (4.0% and 5.7%, respectively) versus eculizumab (72.0 and 60.0%, respectively; p < 0.001; Figure 3). More participants responded that they were able to enjoy life ‘quite a bit/very much’ while receiving ravulizumab (91.8%) than while receiving eculizumab (66.0%; p < 0.05). The majority of adult patients responded ‘quite a bit/very much’ when asked if they were able to talk to their doctor or nurse as often as they would have liked while receiving ravulizumab or eculizumab (81.6 and 87.5%, respectively; p = 0.392). When considering treatment effectiveness and side effects, the distribution of adult responses was similar between both ravulizumab and eculizumab.
Figure 3. . Comparison of impacts of ravulizumab and prior eculizumab treatment reported by adult patients (n = 50).
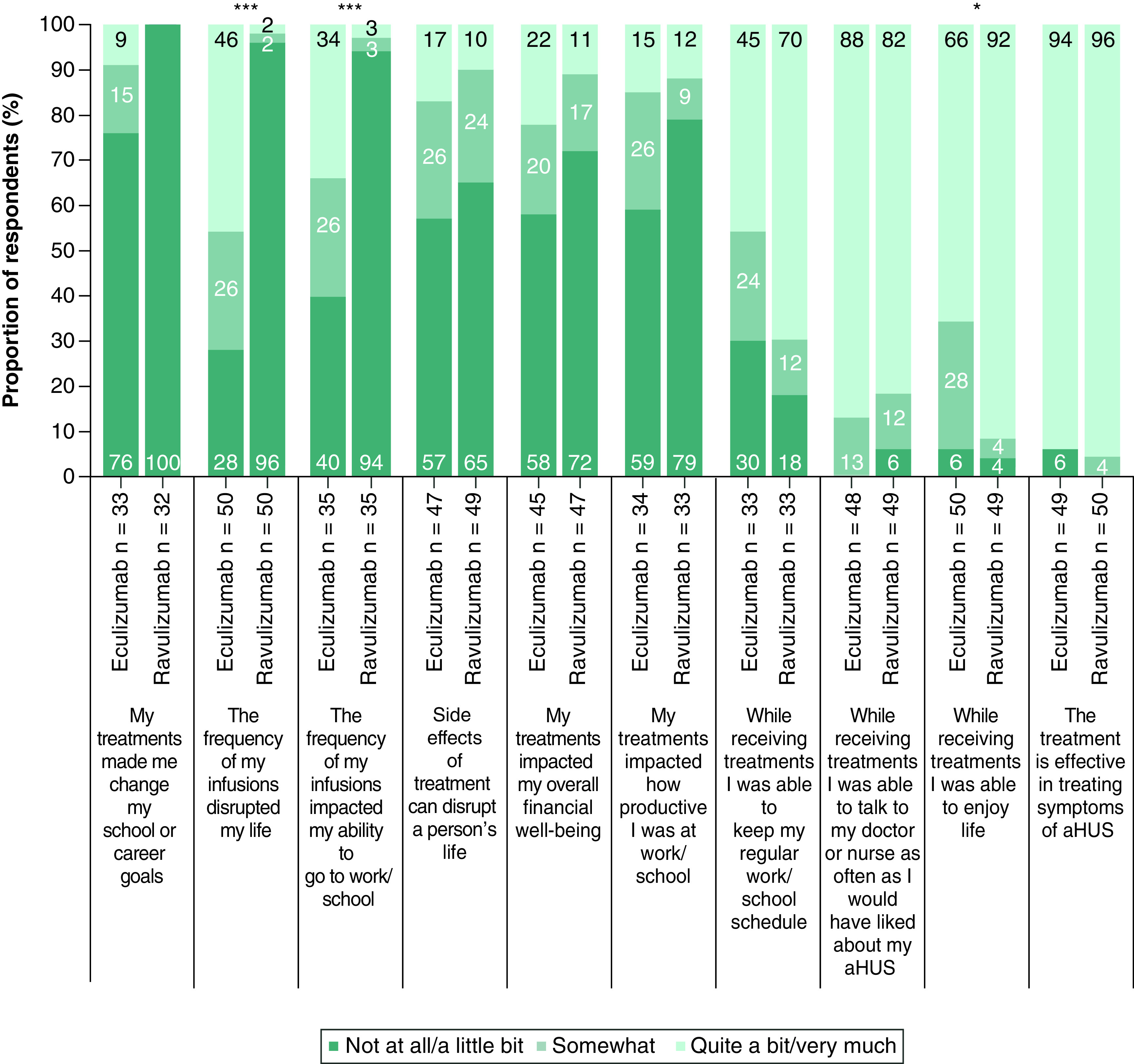
For each statement, participants were asked to select how much they agreed based on their own experience. Bar labels show the survey questions verbatim and have been grouped by negative (bar groups 1–6) and positive (bar groups 7–10) statements. It is acknowledged as a possible limitation that respondents may interpret ‘impact’ differently and not according to the intended directionality. Percentages may not sum to 100 owing to rounding.
aHUS: Atypical hemolytic uremic syndrome.
*p < 0.05; ***p < 0.001.
Greater proportions of caregivers reported little to no disruption to their daily lives (p < 0.01) and impact on their (p < 0.05) or their child's (p < 0.05) ability to go to work/school as a result of the infusion frequency of ravulizumab versus eculizumab (Figure 4). Across these three factors, the infusion frequency of ravulizumab was associated with a minimal negative impact on daily life and the ability to go to work/school, while the infusion frequency of eculizumab was associated with a greater impact on daily life compared with ravulizumab. The majority of caregivers felt that their families were able to enjoy life with ravulizumab or eculizumab (93.8% and 87.5%, respectively, responded quite a bit/very much) and that they were able to talk to a healthcare professional as often as they would have liked (62.5% and 81.3%). All caregivers perceived ravulizumab and eculizumab to be effective for the treatment of aHUS.
Figure 4. . Comparison of impacts of ravulizumab and prior eculizumab treatment reported by caregivers of pediatric patients (n = 16).
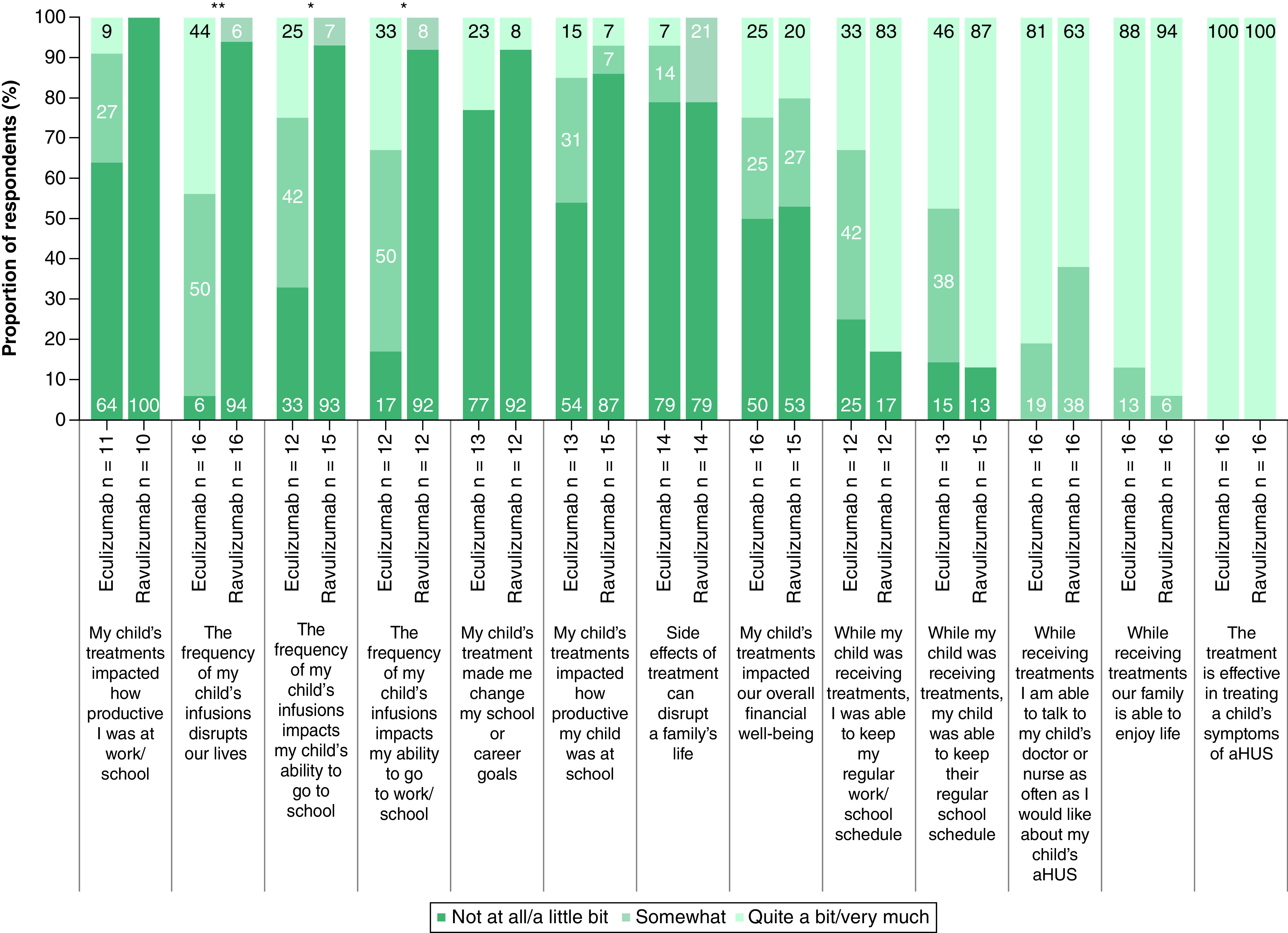
For each statement, participants were asked to select how much they agree based on their own experience. Bar labels show the survey questions verbatim and have been grouped by negative (bar groups 1–8) and positive (bar groups 9–13) statements. It is acknowledged as a possible limitation that respondents may interpret ‘impact’ differently and not according to the intended directionality. Percentages may not sum to 100 owing to rounding.
aHUS: Atypical hemolytic uremic syndrome.
*p < 0.05; **p < 0.01.
When participants were asked to indicate the impact of the treatments on productivity at work/school, the ability to maintain work/school schedules and goals and financial well-being, eculizumab and ravulizumab were generally rated similarly by both cohorts.
The sample size for adult patients was sufficient to allow subgroup analysis of factors related to treatment impact while receiving the 100 mg/ml formulation of ravulizumab compared with eculizumab (n = 39). The results of this analysis were consistent with the non-subgroup analysis with the exception of the survey item assessing the ability of patients to maintain their work/school schedule, with a larger proportion of patients responding that they could maintain their work/school schedule quite a bit/very much when receiving the 100 mg/ml formulation of ravulizumab (73.1%) versus eculizumab (46.2%; p < 0.05).
Time lost to treatment
Adult patients reported that they required less time each month to manage their aHUS when receiving treatment with ravulizumab (mean [SD], 2.8 [4.4] h) versus eculizumab (5.4 [6.7] h; p < 0.01; Table 3). For caregivers, the mean (SD) time needed each month to help manage aHUS was similar between ravulizumab (1.7 [3.5] h) and eculizumab (3.3 [6.2] h; p > 0.05). For both cohorts, there was no clear difference in the mean number of hours needed per treatment session for ravulizumab versus eculizumab.
Table 3. . Patient- and caregiver-reported hours needed per treatment session and hours needed per month to manage aHUS.
| Survey item |
Eculizumab |
Ravulizumab |
p-value |
|---|---|---|---|
| Adult patient response | (n = 50) | (n = 50) | |
| Time needed per treatment session, h n† Mean (SD) |
49 3.5 (2.5) |
48 3.2 (2.3) |
.1886 |
| Time needed per month to manage aHUS, h n† Mean (SD) |
49 5.4 (6.7) |
49 2.8 (4.4) |
.0014 |
| Caregiver response | (n = 16) | (n = 16) | |
|---|---|---|---|
| Caregiver time needed per treatment session, h Mean (SD) |
6.3 (3.8) |
4.4 (3.2) |
.1063 |
| Caregiver time needed per month to manage aHUS, h Mean (SD) |
3.3 (6.2) |
1.7 (3.5) |
.3203 |
| Patient time needed per treatment session, h Mean (SD) |
5.3 (3.5) |
4.9 (2.9) |
.7193 |
| Patient time needed per month to manage aHUS, h Mean (SD) |
2.9 (6.1) |
1.7 (3.5) |
.4369 |
Values that were clear outliers (possible misunderstanding of the question/response requirement) were removed.
Time (h) needed per treatment session included time taken to travel for/receive treatment. Time (h) needed per month to manage aHUS excluded time needed to receive treatment. The p-value compares the proportion of paired responses between eculizumab and ravulizumab using paired t-tests.
aHUS: Atypical hemolytic uremic syndrome; SD: Standard deviation.
Discussion
This study used a survey that was developed using methodological best practices to understand patient and caregiver perspectives on two approved treatments for aHUS. Results demonstrated a patient and caregiver preference for ravulizumab over eculizumab. To our knowledge, prior to this study, the only published patient survey that examined patient satisfaction with complement C5 inhibitor treatment for aHUS focused solely on eculizumab [25]. In our study, the frequency of treatment infusions, impact on quality of life and the ability to control aHUS were the most important factors for patients when selecting their preferred treatment. This concurs with the findings of a previous discrete-choice experiment assessing utility values associated with aHUS-related attributes, which showed that a reduced infusion frequency was preferable among a sample of the general population [26].
In our study, participants indicated that the impacts of both ravulizumab and eculizumab on daily life and productivity were mostly positive, but there were several notable differences in patients' treatment preferences. The ability to travel or go on vacation, the ability to plan social activities and the benefits of treatment on both patient and caregiver quality of life were important factors that patients and caregivers considered when selecting ravulizumab over eculizumab. It should be noted that over 40% of the participants were in full-time employment, and at least 60% had a college degree or higher qualification. Most adult patients and caregivers indicated that the infusion frequency of ravulizumab did not substantially restrict their, or the pediatric patients’, ability to go to work or school. It is likely that the reduced infusion frequency of ravulizumab contributes to greater patient freedom to participate in daily activities with minimal restrictions. Participants were less concerned about the impact of the infusion frequency of ravulizumab versus eculizumab on daily life and the ability to go to work or school. The time lost per treatment session did not differ between eculizumab and ravulizumab; this may be because the majority of patients received the 100 mg/ml dose, which has an estimated infusion duration that is comparable with eculizumab (depending on body weight) [23].
Importantly, adult patients and caregivers reported that they were able to enjoy their lives when receiving either treatment. This could be an indication of the effectiveness of the treatments, in line with previous studies showing that patients receiving either treatment exhibited improvements in Functional Assessment of Chronic Illness Therapy – Fatigue Scale scores [5,14,20,27] and US time trade-off index in the EQ-5D questionnaire for eculizumab [14,28]. Almost all patients indicated that both treatments were effective for their aHUS. This is consistent with previous literature showing that the efficacy of ravulizumab was consistent with eculizumab [22,29], and that ravulizumab and eculizumab were associated with acceptable safety profiles [5,12–15,18–21].
Patient advocacy groups recognize that eculizumab is a transformative therapy, but note that there are some concerns surrounding treatment costs and side effects [11]. Meningococcal infectionis a treatment-related risk associated with complement C5 inhibitors that has been assessed in multiple long-term, real-world studies of eculizumab [15,30]. For ravulizumab, no meningococcal infections were reported in a 2-year analysis of clinical trials in both adult and pediatric patients [31]. Our study demonstrated that side effects were not a substantial concern for the majority of patients and caregivers. Up to 25% of participants reported that treatment affected their overall financial well-being ‘quite a bit’ or ‘very much’. However, participants in this study were not asked whether they had health insurance. Previous research has shown a cost benefit and a reduction in lost productivity with ravulizumab versus eculizumab treatment [32,33]. There may be some concern that the reduced infusion frequency of ravulizumab reduces the contact time between patients and healthcare providers. However, in our study, participants reported minimal perceived differences in their ability to talk to healthcare providers when receiving ravulizumab compared with eculizumab.
There is ongoing discussion regarding the optimal duration of complement C5 inhibitor treatment [34,35]. The decision to discontinue treatment should be based on a thorough assessment of risks and patients should be closely monitored [34–36]. Additionally, given that treatment for aHUS can often be long term [37], patient preference for treatment and the impact of treatment on the daily lives of patients and caregivers are important considerations.
A limitation of this study is that the target sample size of 50 caregivers was not achieved, likely owing to aHUS being a rare disease and the relatively recent approval of ravulizumab for the treatment of aHUS, thus limiting the power of statistical analyses for caregiver responses. There may be factors relating to the responsibilities of caregivers that limit their ability to participate in research, or that prompted a reluctance to participate. Another limitation is that the participant sample consisted predominantly of White and non Hispanic/Latino individuals, females and those educated to a college level or higher, limiting the generalizability of the findings to the wider population. However, for adults, the proportion of White patients in the current study sample (92.0%) was similar to that reported among 516 patients in the global aHUS registry (90.5%), while the proportions of White pediatric patients differed (100% of the current study sample vs 80.6% in the global aHUS registry). The proportions of adult and pediatric patients who were female in the current study (80.0% and 62.5%, respectively) is higher than in the registry (61.6% and 40.3%, respectively) [38]. The lack of diversity could have arisen from the use of online surveys, which may have been challenging to access for participants with more limited time or resources, including participants from lower socioeconomic backgrounds. A potential solution would be to recruit participants in-person in the clinical setting during a routine visit and collect data via paper copies of surveys administered by personnel in clinicians' offices. Providing the survey and study materials in other languages, such as Spanish, may have resulted in a more diverse distribution of ethnicities among the participant sample. Furthermore, there could be a recall bias associated with between-treatment comparisons given that the participants had more recent experience with ravulizumab than eculizumab. The key strengths of this study were that it captured the real-world experience of participants who had received ravulizumab and eculizumab outside of the clinical trial setting and that the surveys were developed using a robust development process that was based on clinical expert, patient and caregiver input using industry best practices.
Conclusion
Patients and caregivers had a positive perception of both eculizumab and ravulizumab. Most participants indicated preference for ravulizumab over eculizumab for the treatment of aHUS, and ravulizumab was shown to have a more positive effect on patient and caregiver quality of life than eculizumab. This preference was primarily driven by the reduced infusion frequency of ravulizumab, allowing patients more freedom. These results add to the emerging body of real-world evidence on treatment impact and treatment preferences of patients with aHUS.
Summary points
This study was the first to assess patient and caregiver preferences between ravulizumab and eculizumab for the treatment of atypical hemolytic uremic syndrome (aHUS).
The majority of adult patients (94.0%) and all caregivers of pediatric patients with aHUS reported an overall preference for ravulizumab over eculizumab.
Ravulizumab allows less frequent infusions than eculizumab, and the majority of participants identified infusion frequency as one of the most important factors when selecting their preferred treatment.
Fewer patients reported disruption to daily life and work/school attendance due to ravulizumab infusion frequency (4.0% and 5.7%, respectively) than eculizumab infusion frequency (72.0% and 60.0%).
Ravulizumab was shown to have a more positive effect on patient and caregiver quality of life than eculizumab.
Both ravulizumab and eculizumab were considered to be effective in treating symptoms of aHUS by most adult patients (96% and 94%, respectively) and all caregivers of pediatric patients.
Adult patients reported that they required less time each month to manage aHUS when receiving ravulizumab (mean, 2.8 h) versus eculizumab (mean, 5.4 h).
The strengths of this study were that it captured real-world experience with ravulizumab and eculizumab outside of the clinical trial setting, the participants were recruited through a range of sources in an effort to achieve a representative participant sample, and the surveys were developed based on industry best practices and a robust development process using the input of clinical experts, patients and caregivers of pediatric patients.
The limitations of this study were that the sample size of caregivers of pediatric patients was limited, the diversity of the patient sample may be limited and there may have been a recall bias associated with between-treatment comparisons given that the participants had more recent experience with ravulizumab than eculizumab.
Overall, patients and caregivers had a positive perception of both ravulizumab and eculizumab, but most participants preferred ravulizumab over eculizumab for the treatment of aHUS.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2023-0036
Author contributions
All authors provided substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data for the work. All authors contributed to the drafting of the work or revising it critically for important intellectual content. All authors provided final approval of the version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial & competing interests disclosure
This study was sponsored and funded by Alexion, AstraZeneca Rare Disease, Boston, MA, US. TJ Mauch has served on the advisory board and speakers bureau for Alexion and was a site principal investigator on research funded by Alexion, AstraZeneca Rare Disease. MR Chladek was an employee of Clinical Outcomes Solutions at the time of the study, which received a fee from Alexion, AstraZeneca Rare Disease to design and conduct this research, and is currently an employee of IQVIA Inc. S Cataland received research funding and consulting fees from Alexion, AstraZeneca Rare Disease, Sanofi Genzyme and Takeda. S Chaturvedi has served on the advisory boards of Alexion, AstraZeneca Rare Disease, argenx, Sanofi Genzyme, Sobi, Takeda and UCB, and has served as a consultant for Sanofi Genzyme. BP Dixon has served as a consultant for Alexion, AstraZeneca Rare Disease and Apellis Pharmaceuticals. K Garlo, C Gawteyger and Y Wang are employees of Alexion, AstraZeneca Rare Disease and own stock/options in Alexion, AstraZeneca Rare Disease. I Tomazos was an employee of Alexion, AstraZeneca Rare Disease at the time of the study and owned/owns stock/options in Alexion, AstraZeneca Rare Disease. He is now an employee of PTC Therapeutics, Inc. A Java serves on the scientific advisory boards of Alexion, AstraZeneca Rare Disease and Novartis Pharmaceuticals, and serves as a consultant for Chinook Therapeutics and principal investigator for Apellis Pharmaceuticals. J Leguizamo has served on speaker bureaus for AbbVie, Alexion, AstraZeneca Rare Disease, Apellis Pharmaceuticals, AstraZeneca, Epizyme, Janssen, Pharmacyclics and Sanofi Genzyme, and has served as a member of advisory boards for AbbVie and Genmab. Lucy Lloyd-Price, TP Phan and T Symonds are employees of Clinical Outcomes Solutions, which received a fee from Alexion, AstraZeneca Rare Disease to design and conduct this research, and to perform statistical analyses and interpret the data. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Ethics approval was provided by the WCG institutional review board (approval reference no. 1297161).
Information pertaining to writing assistance
Medical writing support was provided by L Bratton of Oxford PharmaGenesis, Oxford, UK, and was funded by Alexion, AstraZeneca Rare Disease, Boston, MA, USA.
Data availability statement
The data underlying this article cannot be shared publicly owing to the proprietary nature of this research and the rarity of the condition. The data will be shared on reasonable request to the corresponding author. Supplementary data are available at Journal of Comparative Effectiveness Research online.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Goodship TH, Cook HT, Fakhouri F et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 91, 539–551 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Fakhouri F, Zuber J, Fremeaux-Bacchi V, Loirat C. Hemolytic uremic syndrome. Lancet 390, 681–696 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Raina R, Krishnappa V, Blaha T et al. Atypical hemolytic-uremic syndrome: an update on pathophysiology, diagnosis, and treatment. Ther. Apher. Dial. 23, 4–21 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Asif A, Nayer A, Haas CS. Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment with eculizumab. J. Nephrol. 30, 347–362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenbaum LA, Fila M, Ardissino G et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 89, 701–711 (2016). [DOI] [PubMed] [Google Scholar]; • Evidence from this phase 2 study supports the safety and efficacy of eculizumab in pediatric patients with atypical hemolytic uremic syndrome (aHUS).
- 6.Walle JV, Delmas Y, Ardissino G, Wang J, Kincaid JF, Haller H. Improved renal recovery in patients with atypical hemolytic uremic syndrome following rapid initiation of eculizumab treatment. J. Nephrol. 30, 127–134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration. Eculizumab (SOLIRIS) prescribing information (2019). https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125166s431lbl.pdf
- 8.US Food and Drug Administration. Approval of eculizumab (SOLIRIS) for the treatment of atypical hemolytic uremic syndrome (aHUS) (2011). https://www.accessdata.fda.gov/drugsatfda_docs/bla/2011/125166Orig1s172-2.pdf
- 9.European Medicines Agency. Eculizumab (SOLIRIS) summary of product characteristics (2018). https://www.ema.europa.eu/en/documents/product-information/soliris-epar-product-information_en.pdf
- 10.European Medicines Agency. EU/3/09/653: public summary of positive opinion for orphan designation of eculizumab for the treatment of atypical hemolytic uremic syndrome (aHUS) (2015). https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/09/653-public-summary-positive-opinion-orphan-designation-eculizumab-treatment-atypical-hemolytic_en.pdf
- 11.Raina R, Grewal MK, Radhakrishnan Y et al. Optimal management of atypical hemolytic uremic disease: challenges and solutions. Int. J. Nephrol. Renovasc. Dis. 12, 183–204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legendre CM, Licht C, Muus P et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Licht C, Greenbaum LA, Muus P et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of Phase II studies. Kidney Int. 87, 1061–1073 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fakhouri F, Hourmant M, Campistol JM et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am. J. Kidney Dis. 68, 84–93 (2016). [DOI] [PubMed] [Google Scholar]; • This phase 2 trial demonstrated improvement in hematological, renal and quality of life outcomes in adult patients with aHUS treated with eculizumab.
- 15.Rondeau E, Cataland SR, Al-Dakkak I, Miller B, Webb NJA, Landau D. Eculizumab safety: five-year experience from the Global Atypical Hemolytic Uremic Syndrome Registry. Kidney. Int. Rep. 4, 1568–1576 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Ravulizumab (ULTOMIRIS) prescribing information (2021). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761108s012lbl.pdf
- 17.Sheridan D, Yu ZX, Zhang Y et al. Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PLoS One. 13, e0195909 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbour T, Scully M, Ariceta G et al. Long-term efficacy and safety of the long-acting complement C5 inhibitor ravulizumab for the treatment of atypical hemolytic uremic syndrome in adults. Kidney. Int. Rep. 6, 1603–1613 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rondeau E, Scully M, Ariceta G et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naive to complement inhibitor treatment. Kidney Int. 97, 1287–1296 (2020). [DOI] [PubMed] [Google Scholar]; • A phase 3 study demonstrating the efficacy and safety of ravulizumab in adult patients with aHUS.
- 20.Ariceta G, Dixon BP, Kim SH et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in pediatric patients with atypical hemolytic uremic syndrome naive to complement inhibitor treatment. Kidney Int. 100, 225–237 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, Adams B, Aris AM et al. The long-acting C5 inhibitor, ravulizumab, is efficacious and safe in pediatric patients with atypical hemolytic uremic syndrome previously treated with eculizumab. Pediatr. Nephrol. 36, 889–898 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An analysis of the safety and efficacy of switching to ravulizumab in a cohort of pediatric patients who were previously treated with eculizumab. Renal and hematological parameters were stable, and there were no unexpected safety concerns.
- 22.Tomazos I, Hatswell AJ, Cataland S et al. Comparative efficacy of ravulizumab and eculizumab in the treatment of atypical hemolytic uremic syndrome: an indirect comparison using clinical trial data. Clin. Nephrol. 97, 361–372 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An indirect treatment comparison between ravulizumab and eculizumab in patients with aHUS. Findings showed no differences in patient outcomes between treatments.
- 23.Dixon BP, Sabus A. Ravulizumab 100 mg/ml formulation reduces infusion time and frequency, improving the patient and caregiver experience in the treatment of atypical hemolytic uremic syndrome. J. Clin. Pharm. Ther. 47, 1081–1087 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Alexion, AstraZeneca Rare Disease. Alexion receives FDA approval for new advanced formulation of ULTOMIRIS® (ravulizumab-cwvz) with significantly reduced infusion time (2020). https://media.alexion.com/node/22871/pdf
- 25.Kimman ML, Rotteveel AH, Wijsenbeek M et al. Development and pretesting of a questionnaire to assess patient experiences and satisfaction with medications (PESaM Questionnaire). Patient. 10, 629–642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams K, Aggio D, Chen P, Anokhina K, Lloyd AJ, Wang Y. Utility values associated with atypical hemolytic uremic syndrome-related attributes: a discrete choice experiment in five countries. Pharmacoeconomics 39, 901–912 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenbaum LA, Licht C, Nikolaou V et al. Functional assessment of fatigue and other patient-reported outcomes in patients enrolled in the Global aHUS Registry. Kidney. Int. Rep. 5, 1161–1171 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • A longitudinal study of Global aHUS Registry data that showed improvements in patient-reported outcomes, including fatigue, following treatment with eculizumab in patients with aHUS.
- 28.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann. Med. 33, 337–343 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Bernuy-Guevara C, Chehade H, Muller YD et al. The inhibition of complement system in formal and emerging indications: results from parallel one-stage pairwise and network meta-analyses of clinical trials and real-life data studies. Biomedicines. 8, 355 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Socie G, Caby-Tosi MP, Marantz JL et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical hemolytic uremic syndrome: 10-year pharmacovigilance analysis. Br. J. Haematol. 185, 297–310 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • A pharmacovigilance analysis demonstrating the long-term safety of eculizumab in patients with aHUS and paroxysmal nocturnal hemoglobulinuria, using over 28 518 patient-years of data.
- 31.Dixon B, Madris-Aris AD, Adams B et al. Two-year efficacy and safety of ravulizumab in adults and children with atypical hemolytic uremic syndrome (aHUS): analysis of two phase 3 studies. Blood 138, 769 (2021). [Google Scholar]
- 32.Wang Y, Johnston K, Popoff E et al. A US cost-minimization model comparing ravulizumab versus eculizumab for the treatment of atypical hemolytic uremic syndrome. J. Med. Econ. 23, 1503–1515 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Levy AR, Chen P, Johnston K, Wang Y, Popoff E, Tomazos I. Quantifying the economic effects of ravulizumab versus eculizumab treatment in patients with atypical hemolytic uremic syndrome. J. Med. Econ. 25, 1–16 (2022). [DOI] [PubMed] [Google Scholar]; •• This study compared the productivity implications of treatment with ravulizumab versus eculizumab for patients with aHUS. Lost productivity costs associated with treatment with ravulizumab were lower than with eculizumab.
- 34.Ariceta G. Optimal duration of treatment with eculizumab in atypical hemolytic uremic syndrome (aHUS)-a question to be addressed in a scientific way. Pediatr. Nephrol. 34, 943–949 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Laurence J. Defining treatment duration in atypical hemolytic uremic syndrome in adults: a clinical and pathological approach. Clin. Adv. Hematol. Oncol. 18, 221–230 (2020). [PubMed] [Google Scholar]
- 36.Chaturvedi S, Dhaliwal N, Hussain S et al. Outcomes of a clinician-directed protocol for discontinuation of complement inhibition therapy in atypical hemolytic uremic syndrome. Blood. Adv. 5, 1504–1512 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fakhouri F, Fila M, Hummel A et al. Eculizumab discontinuation in children and adults with atypical hemolytic-uremic syndrome: a prospective multicenter study. Blood 137, 2438–2449 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Licht C, Ardissino G, Ariceta G et al. The global aHUS registry: methodology and initial patient characteristics. BMC Nephrol. 16, 207 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


