This cohort study evaluates the association between psychiatric comorbidities and schizophrenia in children and adolescents with attention-deficit/hyperactivity disorder.
Key Points
Question
Are psychiatric comorbidities in youths with attention-deficit/hyperactivity disorder (ADHD) who are followed up longitudinally associated with higher rates of schizophrenia?
Findings
In this cohort study involving 211 705 children and adolescents with ADHD, patients with psychiatric comorbidities had an approximately 2.1-fold higher risk of being diagnosed with schizophrenia compared with those without comorbidities. The higher risk of subsequent schizophrenia was associated with both a greater number of comorbidities and a variety of individual psychiatric disorders.
Meaning
These findings suggest that many people with ADHD who receive schizophrenia diagnoses have various psychiatric comorbidities at the time of diagnosis and experience various disorders preceding the diagnosis.
Abstract
Importance
The association between attention-deficit/hyperactivity disorder (ADHD) and schizophrenia has received increased attention; however, evidence on the association between psychiatric comorbidities and subsequent schizophrenia in patients with ADHD is limited.
Objective
To investigate the risk of being diagnosed with schizophrenia in children and adolescents with ADHD considering the presence of psychiatric comorbidity.
Design, Setting, and Participants
This was a population-based, retrospective cohort study using the Health Insurance Review and Assessment claims database from January 1, 2007, to December 31, 2019. Participants were children and adolescents aged 5 to 19 years who received an ADHD diagnosis between January 1, 2010, and December 31, 2018, in the nationwide claims data of Korea. Data were analyzed from January 2010 to December 2019.
Interventions or Exposures
The presence of psychiatric comorbidity was assessed from diagnosis records within 1 year before ADHD diagnosis. Comorbidities were further categorized according to the number of comorbidities and specific comorbid disorders.
Main Outcomes and Measures
Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% CIs, examining the association between psychiatric comorbidities and the risk of being diagnosed with schizophrenia. Furthermore, the occurrence of psychiatric comorbidity during the follow-up period was explored among patients without psychiatric comorbidity at baseline.
Results
A total of 211 705 patients with newly diagnosed ADHD were included. A total of 157 272 patients (74.3%) were male, and the age of 5 to 9 years showed the highest distribution (115 081 patients [54.4%]). Patients with psychiatric comorbidity had a significantly higher risk of being diagnosed with schizophrenia than those without (adjusted HR, 2.14; 95% CI, 2.05-2.23). The association between schizophrenia and psychiatric comorbidity became progressively greater with the increasing number of comorbidities. Several individual psychiatric disorders showed an association with development of schizophrenia, with ASD, intellectual disability, tic disorder, depression, and bipolar disorder being the top 5 disorders most associated. Furthermore, 3244 patients (73.8%) without psychiatric comorbidities experienced the emergence of other psychiatric disorders before schizophrenia occurrence.
Conclusions and Relevance
In this retrospective cohort study involving children and adolescents with ADHD, the presence of psychiatric comorbidity in patients with ADHD was associated with an increased risk of being diagnosed with schizophrenia, with an increased risk observed in multiple comorbidities and a wide variety of comorbidities. These findings highlight the significance of assessing and managing psychiatric comorbidities in patients with ADHD to decrease subsequent schizophrenia risk and allow for early intervention.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most common neurodevelopmental disorder in children and adolescents, characterized by impulsivity, hyperactivity, and poor attention.1 There is growing evidence that ADHD is commonly associated with schizophrenia.2,3,4,5,6 One previous systematic review and meta-analysis5 of observational studies demonstrated that ADHD is statistically significantly associated with the incidence of subsequent schizophrenia, with a relative risk of 4.74 (95% CI, 4.11-5.46). Since schizophrenia negatively influences quality of life, including academic functioning, social functioning, and mortality,7 early detection and intervention for schizophrenia for children and adolescents with ADHD have long been considered important.5
To control the risk of developing subsequent schizophrenia among patients with ADHD, understanding the mechanisms and risk factors associated with these 2 disorders is essential. Several previous studies explored the relationship between ADHD and schizophrenia regarding environmental risk factors8 and shared genetic susceptibility.9,10,11 However, studies investigating the influence of psychiatric comorbidity on diagnosis of schizophrenia in patients with ADHD are limited. Psychiatric comorbidity is commonly observed in patients with ADHD.12,13 Although the prevalence rate was varied, approximately 50% to 90% of patients with ADHD have at least 1 other psychiatric comorbid disorder, including depression, bipolar disorder, anxiety, and autism spectrum disorder (ASD).14,15,16,17,18 The presence of psychiatric comorbidity in patients with ADHD has significant implications for symptom severity, functional impairment, and treatment outcomes.13 Furthermore, these psychiatric disorders were independently associated with an increased risk of schizophrenia.19,20,21,22 Moreover, most patients exhibited several clinical symptoms of other psychiatric disorders before schizophrenia occurrence. Therefore, the notion that the presence of psychiatric comorbidity may further be associated with being diagnosed with schizophrenia in this population is plausible. Thus, we aimed to explore the association between ADHD and subsequent schizophrenia and patterns of diagnosis of schizophrenia in children and adolescents with ADHD, focusing on comorbidity.
Methods
Data Sources
We conducted a nationwide population-based retrospective cohort study using the Health Insurance Review and Assessment Service (HIRA) database of South Korea (from January 1, 2007, to December 31, 2019). HIRA is a government-operated agency that evaluates the reimbursement system, which is mandatory for all Korean populations. In this study, we used information about pediatric patients with psychiatric disorders from the HIRA database from 2007 to 2019. The HIRA database contains individual characteristics, including age, sex, and economic vulnerability (ie, approximately 2.8% of the total population who qualify for medical insurance advantage beneficiaries). Clinical data include information billed by health care practitioners, including diagnoses (according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10]), prescriptions, procedures, and devices. This study was approved by the institutional review board of Jeju National University, and the requirement for informed consent was waived as the HIRA database was anonymized. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort study.
Study Participants
Initially, we identified patients aged 5 to 19 years with at least 1 diagnosis record of ADHD (ICD-10: F90) between January 1, 2010, and December 31, 2018. The index date for individual patients was defined as the first date of ADHD diagnosis. We excluded patients with a history of ADHD diagnosis or prescription of stimulants and nonstimulants for ADHD for at least 3 years before the index date to restrict the cohort for new incident cases of ADHD. We subsequently only included those who had no diagnosis record of schizophrenia or psychosis for at least 3 years before the index date. The time frame of 3 years was selected according to previous studies that reported the recovery of schizophrenia or psychosis, measured for at least 2 years after treatment.23,24,25 The detailed codes of diagnosis and medications were summarized in eTable 1 in Supplement 1.
All study populations were followed up from the index date to the first diagnosis date of schizophrenia or end of follow-up (December 31, 2019), whichever occurred earlier. Schizophrenia development was defined according to the ICD-10 code of F2 following ADHD diagnosis.
Association of Being Diagnosed With Schizophrenia With the Psychiatric Comorbidity of ADHD
We defined depression, anxiety disorder, intellectual disability, nonorganic sleep disorder, tic disorder, bipolar disorder, ASD, conduct disorder, obsessive-compulsive disorder, and substance use disorder (SUD) as psychiatric comorbid disorders of ADHD. The detailed ICD-10 codes of psychiatric comorbidities are shown in eTable 2 in Supplement 1. These psychiatric comorbid disorders were selected according to previous studies.12,17,26,27
In this study, we classified patients into 2 groups according to the presence of psychiatric comorbidity within 1 year before the index date. Patients who had at least 1 diagnosis record of these psychiatric disorders within 1 year before the index date belonged to the group with psychiatric comorbidities, and others were categorized into the group without psychiatric disorders. Among those with underlying psychiatric comorbidities, further categorization was conducted according to the number of psychiatric comorbidities and specific psychiatric disorders. The number of psychiatric comorbidities was divided into the following 3 groups: 1 comorbidity, 2 comorbidities, and 3 or more comorbidities. For each psychiatric disorder, patients who had at least 1 diagnostic record of that specific disorder were considered as having that particular disorder. To simultaneously investigate the outcomes of both multiple comorbidities and a specific disorder, patients were additionally categorized into the following 3 groups: 1 comorbidity for each psychiatric disorder, 2 comorbidities, and 3 or more comorbidities.
To estimate hazard ratios (HRs) along with 95% CIs for the association of being diagnosed with schizophrenia with psychiatric comorbidity in patients with ADHD, Cox proportional hazard models (Cox PH models) were used. We considered several potential confounders in schizophrenia development, including sex, age (categorized as 5-9, 10-14, and 15-19 years), insurance type (health insurance or Medicaid), psychiatric hospitalization, and psychotropic medication use. Age, sex, insurance type, and psychiatric hospitalization were assessed at the index date. The use of psychotropic medications (ie, antipsychotics, antidepressants, anxiolytics, anticholinergics, antiepileptics, and lithium) was assessed during the 1-year period before the index date (eTable 3 in Supplement 1). We used 2 models adjusting different potential confounders. Model 1 adjusted sex, age, insurance type, and psychiatric hospitalization as covariates. Model 2 further adjusted for psychotropic medication use as a covariate.
Moreover, we conducted various sensitivity analyses to examine the robustness of main finings. First, to evaluate whether increasing age is associated with the diagnosis of schizophrenia, we performed a subgroup analysis by age group. Second, we performed a subgroup analysis according to the use of methylphenidate (the only approved stimulant for ADHD in South Korea) during the follow-up period. Additionally, we conducted subgroup analyses according to presence of bipolar disorder, ASD, and intellectual disability at baseline period. Third, to further address uncontrolled confounding effects, we performed an analysis using a propensity score (PS)–matched cohort. We used logistic regression to estimate the PS for the probability of having psychiatric comorbidities by including sex, age, health insurance, and health care utilization (overall visit frequency, psychiatric visit frequency, nonpsychiatric hospital visit frequency). Patients with psychiatric comorbidities and those without were matched 1:1 using the estimated PS with a caliper width of 0.2 on the PS scale.
Patterns of Psychiatric Comorbid Diagnosis of Schizophrenia Following ADHD
During the follow-up period, individuals with ADHD might experience various psychiatric comorbidities before the subsequent incidence of schizophrenia. However, in the association analysis, it is difficult to capture the comprehensive longitudinal diagnosis pattern from ADHD to schizophrenia due to the complex trajectories of these disorders. To address this, we used trajectory analysis to examine the progression of psychotic disorders to a schizophrenia diagnosis in patients with ADHD.
We assessed the occurrence of psychiatric comorbid disorders during the period between the index date of ADHD and subsequent schizophrenia to explore the patterns of diagnosis from ADHD to schizophrenia. This analysis exclusively focused on patients who received schizophrenia diagnoses without any preexisting psychiatric comorbidities (patients belonging to the group without psychiatric comorbidities), ensuring that the diagnostic progression was not influenced by prior cumulative diagnoses. During the follow-up period, the first date of diagnosis record for each psychiatric disorder was considered the occurrence of psychiatric comorbidities. The investigation of diagnostic patterns from ADHD to schizophrenia was presented as frequency and incidence rate per 100 person-years. When 2 or more psychiatric disorders occurred simultaneously on the same day, we adjusted weight of incidence by dividing the number of psychiatric comorbid disorders. The diagnostic flow was depicted using a Sankey diagram.
Statistical Analysis
SAS Enterprise Guide 6.1 (SAS Institute) and R software V.3.6.1 (R Project for Statistical Computing) were used for data analyses. The baseline demographics of the patients were presented as numbers and proportions for the 2 groups according to the presence of psychiatric comorbidities. The comparison between these groups was performed using 2-sided χ2 tests with significance set at less than .05. Data were analyzed from January 2010 to December 2019.
Results
We identified a total of 211 705 patients to be involved in our study cohort. The majority were male (157 272 patients [74.3%]), and 115 081 patients (54.4%) were aged 5 to 9 years. Among them, 77 880 (36.8%) had at least 1 diagnosis record of psychiatric disorders within 1 year before the index date, whereas 133 825 (63.2%) did not exhibit any comorbidities (eFigure 1 in Supplement 1; Table 1). Over a mean (SD) follow-up period of 5.57 (2.81) years, 6448 patients (8.28%) with psychiatric comorbidities received schizophrenia diagnoses. Meanwhile, 4396 patients (3.28%) without psychiatric comorbidities received schizophrenia diagnoses during a mean (SD) follow-up of 6.61 (2.71) years. The most common insurance type was health insurance (196 684 patients [92.9%]). A total of 263 patients (0.1%) experienced psychiatric hospitalization within 1 year before the index date. The most used psychotropic medications were anxiolytics (45 730 patients [21.6%]), followed by selective serotonin reuptake inhibitors or serotonin and norepinephrine reuptake inhibitors (SSRIs/SNRIs) (14 861 patients [7.0%]). Patients with psychiatric comorbidities were older, were more likely to be female, and exhibited a greater psychotropic medication use than those without psychiatric comorbidities (these differences were statistically significant). Among diverse psychiatric comorbid disorders, depression (30 091 patients [38.6%]) and anxiety disorder (19 445 patients [25.0%]) were the 2 most common psychiatric disorders.
Table 1. Demographics of Patients With Attention-Deficit/Hyperactivity Disorder (ADHD) According to the Presence of Psychiatric Comorbidities.
| Demographic | Participants, No. (%) | P value | ||
|---|---|---|---|---|
| Total patients (N = 211 705) | ADHD with psychiatric comorbidities (n = 77 880) | ADHD without psychiatric comorbidities (n = 133 825) | ||
| Schizophrenia diagnosis | 10 844 (5.12) | 6448 (8.28) | 4396 (3.28) | NA |
| Follow-up, median (SD), y | 6.31 (2.77) | 5.57 (2.81) | 6.61 (2.71) | NA |
| Sex | ||||
| Male | 157 272 (74.29) | 53 912 (69.22) | 103 360 (77.24) | <.001 |
| Female | 54 433 (25.71) | 23 968 (30.78) | 30 465 (22.76) | |
| Age, y | ||||
| 5-9 | 115 081 (54.36) | 31 647 (40.64) | 83 434 (62.35) | <.001 |
| 10-14 | 64 591 (30.51) | 26 309 (33.78) | 38 282 (28.61) | |
| 15-19 | 32 033 (15.13) | 19 924 (25.58) | 12 109 (9.05) | |
| Insurance type | ||||
| Health insurance | 196 684 (92.90) | 71 636 (91.98) | 125 048 (93.44) | .006 |
| Medicaid | 15 021 (7.10) | 6244 (8.02) | 8777 (6.56) | |
| Psychiatric hospitalizationa | 263 (0.12) | 243 (0.31) | 20 (0.01) | <.001 |
| Psychiatric comorbidities, No.a | NA | |||
| 1 | 56 593 (26.73) | 56 593 (72.67) | NA | NA |
| 2 | 16 563 (7.82) | 16 563 (21.27) | NA | NA |
| ≥3 | 4724 (2.23) | 4724 (6.07) | NA | NA |
| Individual psychiatric comorbid disorderb | ||||
| Depression | 30 091 (14.21) | 30 091 (38.64) | NA | NA |
| Anxiety disorder | 19 445 (9.18) | 19 445 (24.97) | NA | NA |
| Tic disorder | 15 460 (7.30) | 15 460 (19.85) | NA | NA |
| Intellectual disability | 12 440 (5.88) | 12 440 (15.97) | NA | NA |
| Conduct disorder | 10 515 (4.97) | 10 515 (13.50) | NA | NA |
| ASD | 6867 (3.24) | 6867 (8.82) | NA | NA |
| Bipolar disorder | 4358 (2.06) | 4358 (5.60) | NA | NA |
| OCD | 3095 (1.46) | 3095 (3.97) | NA | NA |
| Nonorganic sleep disorder | 2446 (1.16) | 2446 (3.14) | NA | NA |
| SUD | 318 (0.15) | 318 (0.40) | NA | NA |
| Use of psychotropic medicationsa | ||||
| Typical antipsychotics | 1648 (0.78) | 1593 (2.05) | 55 (0.04) | <.001 |
| Atypical antipsychotics | 8624 (4.07) | 8344 (10.71) | 280 (0.21) | <.001 |
| Anticholinergics | 2318 (1.09) | 2253 (2.89) | 65 (0.05) | <.001 |
| Anxiolytics | 45 730 (21.60) | 19 328 (24.82) | 26 402 (19.73) | <.001 |
| SSRIs/SNRIs | 14 861 (7.02) | 13 311 (17.09) | 1550 (1.16) | <.001 |
| TCAs | 976 (0.46) | 799 (1.03) | 177 (0.13) | <.001 |
| MAOIs and others | 2208 (1.04) | 2016 (2.59) | 192 (0.14) | <.001 |
| Antiepileptics | 6866 (3.24) | 4460 (5.73) | 2406 (1.80) | <.001 |
| Lithium | 652 (0.31) | 608 (0.78) | 44 (0.03) | <.001 |
Abbreviations: ASD, autism spectrum disorder; MAOIs, monoamine oxidase inhibitors; NA, not applicable; OCD, obsessive-compulsive disorder; SNRIs, serotonin and norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; SUD, substance use disorder; TCAs, tricyclic antidepressants.
History of psychiatric hospitalization, psychiatric comorbidities, and the use of psychotropic medications were evaluated within 1 year before the index date.
Patients with at least 1 diagnosis record of each disorder were considered to have each disorder.
The results of Cox PH regression analysis for the risk of schizophrenia according to the presence of psychiatric comorbidities in patients with ADHD with and without adjustment for other psychotropic medications are shown in Table 2. In both models, the presence of comorbidities was associated with an increased subsequent schizophrenia risk among patients with ADHD (model 1 HR, 2.51; 95% CI, 2.41-2.61; model 2 HR, 2.14; 95% CI, 2.05-2.23). Furthermore, we observed that male sex, older age, and Medicaid were associated with increased schizophrenia risk. Regarding psychotropic medication use, typical antipsychotics, atypical antipsychotics, anxiolytics, anticholinergics, SSRIs/SNRIs, and antiepileptics showed an increased subsequent schizophrenia risk.
Table 2. Association Between Being Diagnosed With Schizophrenia and Psychiatric Comorbidities in Patients With Attention-Deficit/Hyperactivity Disorder (ADHD).
| Characteristic | HR (95% CI) | |
|---|---|---|
| Model 1a | Model 2b | |
| Sex (reference, male) | 0.93 (0.89-0.97) | 0.91 (0.87-0.95) |
| Age, y (reference, 5-9) | ||
| 10-14 | 1.14 (1.09-1.19) | 1.12 (1.07-1.17) |
| 15-19 | 1.62 (1.55-1.71) | 1.47 (1.40-1.55) |
| Medicaid (reference, health insurance) | 1.86 (1.75-1.97) | 1.86 (1.75-1.97) |
| Psychiatric hospitalization (reference, none) | 1.73 (1.35-2.21) | 1.05 (0.82-1.35) |
| Psychiatric comorbidity (reference, no psychiatric comorbidities) | 2.51 (2.41-2.61) | 2.14 (2.05-2.23) |
| Psychotropic medication use (reference, none) | ||
| Typical antipsychotics | NA | 1.35 (1.19-1.53) |
| Atypical antipsychotics | NA | 1.96 (1.82-2.11) |
| Anticholinergics | NA | 1.41 (1.26-1.58) |
| Anxiolytics | NA | 1.10 (1.05-1.15) |
| SSRIs/SNRIs | NA | 1.26 (1.18-1.34) |
| TCAs | NA | 1.11 (0.93-1.33) |
| MAOIs and others | NA | 0.96 (0.84-1.09) |
| Antiepileptics | NA | 1.26 (1.17-1.37) |
| Lithium | NA | 1.16 (0.98-1.39) |
Abbreviations: HR, hazard ratio; MAOIs, monoamine oxidase inhibitors; NA, not applicable; SNRIs, serotonin and norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants.
Model 1 adjusted sex, age, health insurance, and psychiatric hospitalization as covariates.
Model 2 adjusted sex, age, health insurance, psychiatric hospitalization, and psychotropic medication use as covariates.
The association between being diagnosed with schizophrenia and psychiatric comorbidities according to several studies is depicted in Table 3. With the increasing number of psychiatric comorbidities, patients with psychiatric comorbidities had an increased schizophrenia risk compared with those without psychiatric comorbidities. In model 2, the risk of being diagnosed with schizophrenia was approximately 1.91-fold for 1 comorbidity, 2.94-fold for 2 comorbidities, and 4.26-fold higher for 3 or more comorbidities than those without psychiatric comorbidities. Regarding individual psychiatric comorbidities, the increased risk of subsequent schizophrenia was significantly associated with the presence of all individual comorbidities, excluding SUD. Among them, ASD exhibited the greatest association with schizophrenia risk, with HRs of 2.72 (95% CI, 2.52-2.92) and 2.43 (95% CI, 2.26-2.62) in models 1 and 2, respectively. Moreover, intellectual disability, tic disorder, depression, and bipolar disorder showed a remarkable association, with HRs of 1.83 (95% CI, 1.72-1.95), 1.77 (95% CI, 1.66-1.88), 1.68 (95% CI, 1.60-1.77), and 1.67 (95% CI, 1.53-1.83), respectively. This tendency was consistently observed in analysis that simultaneously considered the number of comorbidities and individual disorder.
Table 3. Association Between Being Diagnosed With Schizophrenia and Psychiatric Comorbidities According to Several Studies.
| Comorbidities | Adjusted HRs (95% CI) | |
|---|---|---|
| Model 1a | Model 2b | |
| Multiple psychiatric comorbidities (reference, none) | ||
| 1 Comorbid disorder | 2.03 (1.94-2.12) | 1.91 (1.83-2.00) |
| 2 Comorbid disorders | 3.54 (3.35-3.75) | 2.94 (2.77-3.13) |
| ≥3 comorbid disorders | 6.53 (6.05-7.04) | 4.26 (3.90-4.65) |
| Individual psychiatric comorbidities | ||
| Depression | 1.82 (1.74-1.91) | 1.68 (1.60-1.77) |
| Anxiety disorder | 1.40 (1.32-1.48) | 1.32 (1.25-1.39) |
| Tic disorder | 2.05 (1.94-2.17) | 1.77 (1.66-1.88) |
| Intellectual disability | 1.91 (1.79-2.03) | 1.83 (1.72-1.95) |
| Conduct disorder | 1.27 (1.18-1.36) | 1.21 (1.12-1.30) |
| ASD | 2.72 (2.52-2.92) | 2.43 (2.26-2.62) |
| Bipolar disorder | 2.22 (2.05-2.40) | 1.67 (1.53-1.83) |
| OCD | 1.87 (1.69-2.07) | 1.64 (1.47-1.82) |
| Nonorganic sleep disorder | 1.57 (1.40-1.77) | 1.40 (1.24-1.58) |
| SUD | 1.22 (0.90-1.65) | 1.11 (0.82-1.52) |
| Individual psychiatric comorbidities (reference, none) | ||
| Depression | 1.98 (1.85-2.12) | 1.90 (1.77-2.03) |
| Anxiety disorder | 1.31 (1.18-1.46) | 1.28 (1.16-1.42) |
| Tic disorder | 2.33 (2.15-2.52) | 2.04 (1.88-2.21) |
| Intellectual disability | 2.35 (2.16-2.56) | 2.29 (2.11-2.49) |
| Conduct disorder | 1.38 (1.23-1.56) | 1.37 (1.22-1.54) |
| ASD | 3.79 (3.41-4.21) | 3.46 (3.11-3.84) |
| Bipolar disorder | 1.67 (1.31-2.13) | 1.57 (1.23-2.00) |
| OCD | 2.92 (2.29-3.72) | 2.46 (1.93-3.14) |
| Nonorganic sleep disorder | 1.05 (0.72-1.52) | 1.04 (0.72-1.50) |
| SUD | 1.51 (0.57-4.02) | 1.52 (0.57-4.05) |
| 2 comorbid disorders | 3.48 (3.29-3.69) | 2.92 (2.75-3.11) |
| ≥3 comorbid disorders | 6.37 (5.91-6.88) | 4.26 (3.90-4.65) |
Abbreviations: ASD, autism spectrum disorder; HR, hazard ratio; OCD, obsessive-compulsive disorder; SUD, substance use disorder.
Model 1 adjusted sex, age, health insurance, and psychiatric hospitalization as covariates.
Model 2 adjusted sex, age, health insurance, psychiatric hospitalization, and psychotropic medication use as covariates.
In subgroup analysis according to age group, methylphenidate use, and the previous presence of bipolar disorder, ASD, and intellectual disability, we showed that the presence of psychiatric disorder was significantly associated with an increased risk of being diagnosed with schizophrenia; the magnitude of this association exhibited an increasing tendency with multiple comorbidities and a wide variety of individual comorbidity (eTable 4, eTable 5, and eTable 6 in Supplement 1). After 1:1 PS matching, we identified 70 590 patients in each group with well-balanced baseline characteristics (eTable 7 and eTable 8 in Supplement 1). Results of analyses using the PS-matched cohort also showed the largely consistent result with our main findings (eTable 9, eTable 10, eTable 11, and eTable 12 in Supplement 1).
Patients who did not have any psychiatric comorbidity still encountered various psychiatric comorbidities before being diagnosed with schizophrenia, as shown in Table 4. Notably, 3244 patients (73.8%) experienced at least 1 psychiatric comorbidity before schizophrenia onset, and this trend was consistently observed across all age groups (5-9 years, 1804 patients [71.8%]; 10-14 years, 1045 patients [77.2%]; 15-19 years, 395 patients [74.3%]). Although the specific comorbidities varied among different age groups, depression emerged as the most common incident psychiatric comorbidity, found in 2023 patients (46.0%), followed by anxiety disorders, found in 1326 patients (30.2%). The longitudinal association between ADHD and schizophrenia, including the occurrence of psychiatric comorbidities, is further illustrated in the Sankey diagram in the Figure; eFigure 2 in Supplement 1; and eTable 13, eTable 14, eTable 15, and eTable 16 in Supplement 2.
Table 4. Incidence of Psychiatric Comorbidities in the Trajectories From Attention-Deficit/Hyperactivity Disorder to Schizophreniaa.
| Comorbidities | Patients, No. (%) | Sum of follow-up duration, y | Incidence rate (per 100 person-years) |
|---|---|---|---|
| All patients (n = 4506) | |||
| No incidence of psychiatric comorbidities | 1152 (26.21) | 2180.47 | 52.83 |
| Incidence of psychiatric comorbidities | 3244 (73.79) | NA | NA |
| Depression | 2023 (46.02) | 8212.54 | 24.63 |
| Anxiety disorder | 1326 (30.16) | 5982.72 | 22.16 |
| Tic disorder | 885 (20.13) | 3242.29 | 27.30 |
| Intellectual disability | 531 (12.08) | 2420.55 | 21.94 |
| Conduct disorder | 737 (16.77) | 3042.79 | 24.22 |
| ASD | 295 (6.71) | 1253.52 | 23.53 |
| Bipolar disorder | 1090 (24.80) | 4570.68 | 23.85 |
| OCD | 238 (5.41) | 1108.09 | 21.48 |
| SUD | 55 (1.25) | 293.62 | 18.73 |
| Aged 5-9 y (n = 2583) | |||
| No incidence of psychiatric comorbidities | 707 (28.16) | 1261.81 | 56.03 |
| Incidence of psychiatric comorbidities | 1804 (71.84) | NA | NA |
| Depression | 1027 (40.90) | 4213.79 | 24.37 |
| Anxiety disorder | 646 (25.73) | 2876.00 | 22.46 |
| Tic disorder | 676 (26.92) | 2487.79 | 27.17 |
| Intellectual disability | 313 (12.47) | 1480.25 | 21.15 |
| Conduct disorder | 465 (18.52) | 2025.13 | 22.96 |
| ASD | 201 (8.00) | 835.35 | 24.06 |
| Bipolar disorder | 552 (21.98) | 2398.58 | 23.01 |
| OCD | 103 (4.10) | 533.12 | 19.32 |
| SUD | 12 (0.48) | 69.00 | 17.39 |
| Aged 10-14 y (n = 1380) | |||
| No incidence of psychiatric comorbidities | 308 (22.76) | 711.49 | 43.29 |
| Incidence of psychiatric comorbidities | 1045 (77.24) | NA | NA |
| Depression | 726 (53.66) | 3007.07 | 24.14 |
| Anxiety disorder | 489 (36.14) | 2366.80 | 20.66 |
| Tic disorder | 191 (14.12) | 680.84 | 28.05 |
| Intellectual disability | 176 (13.01) | 759.94 | 23.16 |
| Conduct disorder | 238 (17.59) | 889.56 | 26.75 |
| ASD | 83 (6.13) | 339.11 | 24.48 |
| Bipolar disorder | 407 (30.08) | 1677.16 | 24.27 |
| OCD | 85 (6.28) | 384.39 | 22.11 |
| SUD | 27 (2.00) | 150.96 | 17.89 |
| Aged 15-19 y (n = 543) | |||
| No incidence of psychiatric comorbidities | 137 (25.75) | 207.17 | 66.13 |
| Incidence of psychiatric comorbidities | 395 (74.25) | NA | NA |
| Depression | 286 (53.76) | 996.77 | 28.69 |
| Anxiety disorder | 198 (37.22) | 742.31 | 26.67 |
| Tic disorder | 27 (5.08) | 76.44 | 35.32 |
| Intellectual disability | 47 (8.83) | 181.54 | 25.89 |
| Conduct disorder | 40 (7.52) | 129.92 | 30.79 |
| ASD | 19 (3.57) | 81.39 | 23.34 |
| Bipolar disorder | 140 (26.32) | 497.35 | 28.15 |
| OCD | 51 (9.59) | 190.90 | 26.72 |
| SUD | 16 (3.01) | 73.65 | 21.72 |
Abbreviations: ASD, autism spectrum disorder; NA, not applicable; OCD, obsessive-compulsive disorder; SUD, substance use disorder.
This analysis is conducted only in patients who received a schizophrenia diagnosis without psychiatric comorbidity at baseline.
Figure. Sankey Diagram Illustrating the Incidence of Psychiatric Comorbidities in the Diagnostic Pattern From Attention-Deficit/Hyperactivity Disorder (ADHD) to Schizophrenia (SCZ).
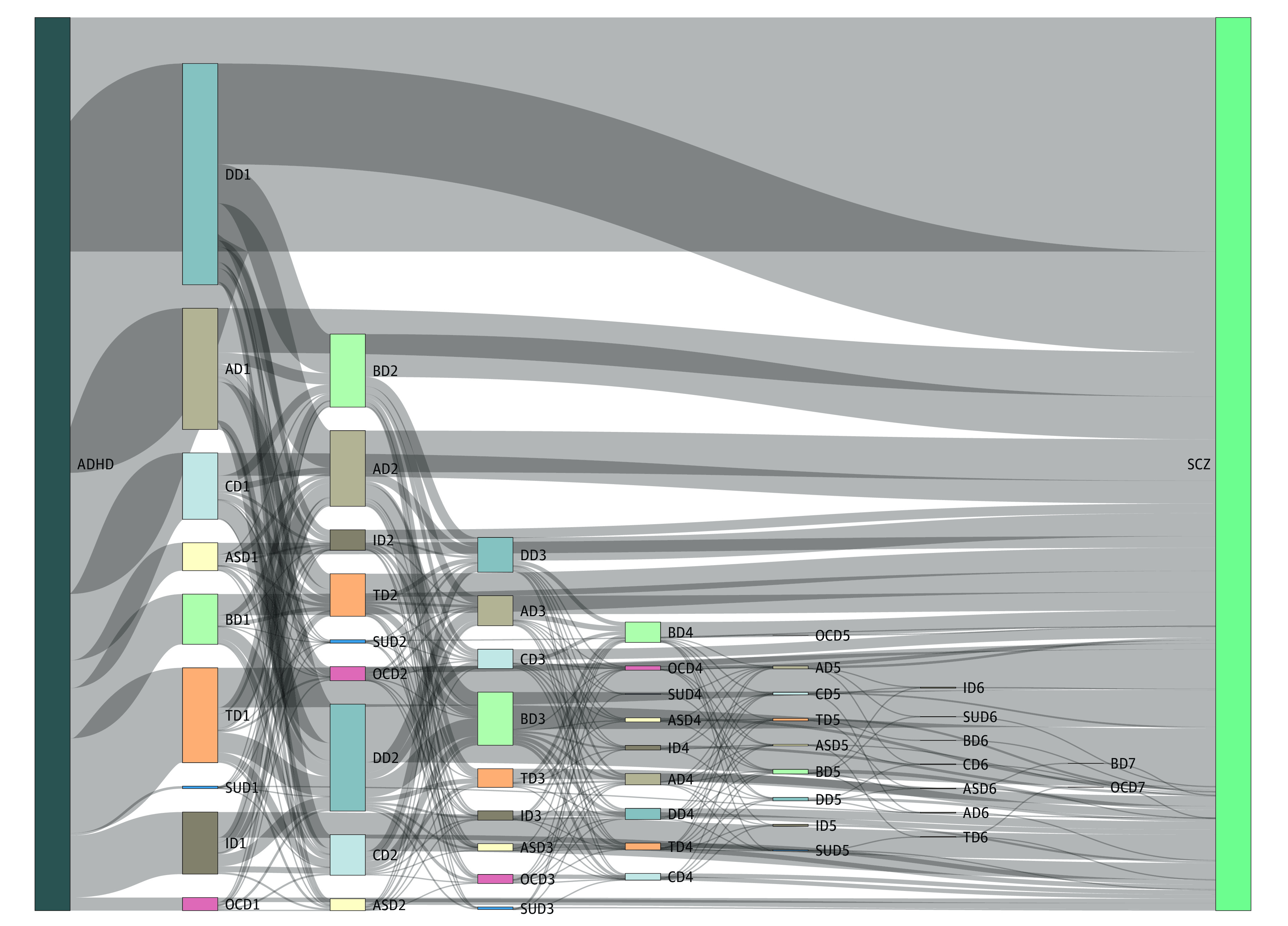
This analysis is specifically conducted in patients who received a schizophrenia diagnosis without any psychiatric comorbidity at baseline. The numbers for each disorder indicate the sequence of comorbidities that occured after the diagnosis of ADHD. AD indicates anxiety disorder; ASD, autism spectrum disorder; BD, bipolar disorder; CD, conduct disorder; DD, depression disorder; ID, intellectual disability; OCD, obsessive-compulsive disorder; SUD, substance use disorder; TD, tic disorder.
Discussion
To our knowledge, this is the first study to investigate schizophrenia risk among children and adolescents with ADHD, with a particular focus on psychiatric comorbidities. Our findings suggested that patients with ADHD with psychiatric comorbidities had a higher risk of being diagnosed with schizophrenia than those without comorbidities. Furthermore, we observed a stepwise increase in schizophrenia risk with an increasing number of comorbidities. Regarding individual psychiatric comorbidities, ASD, intellectual disability, tic disorders, depression, and bipolar disorder showed relatively higher association with the increased schizophrenia risk in patients with ADHD. Additionally, to gain a better understanding of the progression toward schizophrenia in patients without psychiatric comorbidities at baseline, we constructed diagnosis trajectories.
Our results are consistent with previous studies that identified several psychiatric disorders as risk factors for schizophrenia development.28 For example, Maibing et al28 examined schizophrenia risk in pediatrics with a broad range of psychiatric disorders and reported an increased schizophrenia risk in short- and long-term periods. However, since this study primarily focused on the incidence rates of patients with overall psychiatric disorders, it did not address the impact of psychiatric comorbidities in patients with ADHD. Another study2 conducted in Taiwan used the Taiwan national claims database to estimate the risk of psychosis in patients with ADHD and reported approximately 2- to 3-fold higher risk in patients with other psychiatric disorders than those without other disorders. Nevertheless, they did not account for common psychiatric comorbidities, including depression and anxiety, which are frequently observed in patients with ADHD. Furthermore, as most studies focused on the risk factors at baseline, evidence regarding the diagnosis pattern from ADHD to schizophrenia is lacking. Therefore, our study contributes additional evidence regarding the association between several psychiatric comorbidities and subsequent schizophrenia development among children and adolescents with ADHD.
We observed that patients with ADHD with psychiatric comorbidities were older than those without psychiatric comorbidities. Provided that schizophrenia frequently occurs between 10 and 25 years in male patients and between 25 and 35 years in female patients,29 the higher schizophrenia incidence in patients with psychiatric comorbidities seems to be associated with age. However, various models using Cox PH models with age adjustment and subgroup analysis by age group suggested that the presence of psychiatric comorbidities was independently associated with schizophrenia development.
Regarding individual psychiatric comorbidities, ASD, intellectual disability, tic disorder, depression, and bipolar disorder were the top 5 comorbid disorders associated with an increased risk of schizophrenia. These findings are consistent with previous studies that demonstrated a potential positive association between schizophrenia risk and these disorders.2,30,31 For example, Jutla et al2 showed that ASD was a strong risk factor for psychotic symptoms.30 Having a diagnosis of tic disorders and intellectual disability indicated an increased risk of receiving a schizophrenia diagnosis. Yung et al32 conducted a study on predicting psychosis development and reported that a diagnosis of depression and anxiety served as a significant and strong predictor of psychosis. Furthermore, when examining the diagnosis trajectory among patients without psychiatric comorbidities at baseline, these disorders were prominently incident during the follow-up period. These consistent findings across different studies further support the association between these psychiatric comorbidities and increased schizophrenia risk.
However, interestingly, patients with ASD showed a high association of being diagnosed with schizophrenia, but the overall incidence of ASD was relatively low in trajectories analyses. This trend might be associated with the fact that ASD is typically identified early in development compared with other psychiatric disorders.33 Furthermore, in contrast to previous studies, we found no statistically significant association of SUD and schizophrenia, which might be attributed to the small sample size of patients with SUD.
Moreover, we examined other schizophrenia-associated factors in patients with ADHD. Male sex, older age, and Medicaid were robust factors associated with risk for the incidence of schizophrenia in this population, which was consistent with previous findings.34,35,36 Furthermore, we observed that antipsychotic medication use was significantly associated with increased schizophrenia risk. A few studies showed that antipsychotics have the potential to provoke schizophrenia.37 Meanwhile, these drugs may be prescribed for treating severe mental disorders regardless of schizophrenia or psychosis. For instance, the adjuvant uses of antipsychotics with antidepressants have been commonly used for treating resistant depression. The use of anticholinergic medications also showed a consistent association with the increased risk of schizophrenia. Given that anticholinergic medications were commonly used for adverse effects of antipsychotics (such as dystonia and parkinsonism),38 this finding might be associated with the use of antipsychotics. Therefore, further study is needed to investigate the association between anticholinergic use and schizophrenia in patients with ADHD.
Limitations
Several limitations should be considered when interpreting these findings. First, since the diagnosis of ADHD, schizophrenia, and other psychiatric comorbidities were based on diagnosis codes, the possibility of underdiagnosis or overdiagnosis could not be ruled out. Some patients with ADHD have chosen the general health consultation (ICD Z code) due to the social stigma surrounding mental health problems.39 Furthermore, owing to using the claims database, we could not consider the severity or symptoms of ADHD. Instead, psychiatric hospitalization was used as an indirect proxy for assessing symptom severity. Second, despite considering several potential confounders, completely controlling all potential effects of confounders is challenging. However, when adjusting several confounders in Cox PH models and conducting several sensitivity analyses, we observed robust results regarding the associations between psychiatric comorbidities and being diagnosed with schizophrenia. Lastly, psychiatric comorbidities were assessed at baseline, and patients developing signs of these comorbidities during the follow-up period remained in the noncomorbidity group in the analysis. To address this limitation, we analyzed diagnosis trajectories to schizophrenia in patients without psychiatric comorbidities at baseline.
Conclusions
This study suggests that the presence of psychiatric comorbidities was associated with an increased risk of being diagnosed with schizophrenia in children and adolescents with ADHD. Interestingly, although patients had no psychiatric comorbidities during ADHD diagnosis, the occurrence of these disorders was frequently observed before the schizophrenia diagnosis. These findings highlighted the significance of carefully monitoring psychiatric comorbidities in patients with ADHD to effectively mitigate the burden of schizophrenia.
eTable 1. ICD-10 Codes of ADHD and ATC Codes for ADHD Medications
eTable 2. ICD-10 Codes for Psychiatric Comorbid Disorders
eTable 3. ATC Codes for Other Psychotropic Medications
eFigure 1. Flow Chart of Study Population Selection
eTable 4. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis by Age Groups
eTable 5. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis by the Use of Methylphenidate During Follow-Up Period
eTable 6. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis of Patients Without Specific Psychiatric Disorder
eTable 7. Distribution of Patients by the Presence of Psychiatric Comorbidities After Propensity Score Matching
eTable 8. Demographics of ADHD Patients According to the Presence of Psychiatric Comorbidity
eTable 9. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in the PS Matched Cohort
eTable 10. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis by Age Groups in the PS Matched Cohort
eTable 11. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis by the Use of Methylphenidate During Follow-Up Period in the PS Matched Cohort
eTable 12. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis of Patients Without Specific Psychiatric Disorder in the PS Matched Cohort
eFigure 2. Sankey Diagram Illustrating the Incidence of Psychiatric Comorbidities in the Diagnostic Transition From ADHD to Schizophrenia in Patients According to Age Groups
eTable 13. Proportion of Patients in Trajectories From ADHD to Schizophrenia in all Patients
eTable 14. Proportion of Patients in Trajectories From ADHD to Schizophrenia, Aged 5 to 9 Years
eTable 15. Proportion of Patients in Trajectories From ADHD to Schizophrenia, Aged 10 to 14 Years
eTable 16. Proportion of Patients in Trajectories From ADHD to Schizophrenia, Aged 15 to 19 Years
Data Sharing Statement
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 2.Shyu YC, Yuan SS, Lee SY, et al. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: a nationwide population-based study in Taiwan. Schizophr Res. 2015;168(1-2):161-167. doi: 10.1016/j.schres.2015.08.033 [DOI] [PubMed] [Google Scholar]
- 3.Vitiello B, Perez Algorta G, Arnold LE, Howard AL, Stehli A, Molina BSG. Psychotic symptoms in attention-deficit/hyperactivity disorder: an analysis of the MTA database. J Am Acad Child Adolesc Psychiatry. 2017;56(4):336-343. doi: 10.1016/j.jaac.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubino IA, Frank E, Croce Nanni R, Pozzi D, Lanza di Scalea T, Siracusano A. A comparative study of axis I antecedents before age 18 of unipolar depression, bipolar disorder and schizophrenia. Psychopathology. 2009;42(5):325-332. doi: 10.1159/000232975 [DOI] [PubMed] [Google Scholar]
- 5.Nourredine M, Gering A, Fourneret P, et al. Association of attention-deficit/hyperactivity disorder in childhood and adolescence with the risk of subsequent psychotic disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(5):519-529. doi: 10.1001/jamapsychiatry.2020.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Björkenstam E, Pierce M, Björkenstam C, Dalman C, Kosidou K. Attention deficit/hyperactivity disorder and risk for non-affective psychotic disorder: the role of ADHD medication and comorbidity, and sibling comparison. Schizophr Res. 2020;218:124-130. doi: 10.1016/j.schres.2020.01.021 [DOI] [PubMed] [Google Scholar]
- 7.Oh H, Koyanagi A, Kelleher I, DeVylder J. Psychotic experiences and disability: findings from the Collaborative Psychiatric Epidemiology Surveys. Schizophr Res. 2018;193:343-347. doi: 10.1016/j.schres.2017.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linnet KM, Dalsgaard S, Obel C, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160(6):1028-1040. doi: 10.1176/appi.ajp.160.6.1028 [DOI] [PubMed] [Google Scholar]
- 9.Williams NM, Zaharieva I, Martin A, et al. Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. Lancet. 2010;376(9750):1401-1408. doi: 10.1016/S0140-6736(10)61109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamshere ML, Stergiakouli E, Langley K, et al. Shared polygenic contribution between childhood attention-deficit hyperactivity disorder and adult schizophrenia. Br J Psychiatry. 2013;203(2):107-111. doi: 10.1192/bjp.bp.112.117432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owen MJ, O’Donovan MC. Schizophrenia and the neurodevelopmental continuum:evidence from genomics. World Psychiatry. 2017;16(3):227-235. doi: 10.1002/wps.20440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry. 1991;148(5):564-577. doi: 10.1176/ajp.148.5.564 [DOI] [PubMed] [Google Scholar]
- 13.Cumyn L, French L, Hechtman L. Comorbidity in adults with attention-deficit hyperactivity disorder. Can J Psychiatry. 2009;54(10):673-683. doi: 10.1177/070674370905401004 [DOI] [PubMed] [Google Scholar]
- 14.Jensen CM, Steinhausen HC. Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. Atten Defic Hyperact Disord. 2015;7(1):27-38. doi: 10.1007/s12402-014-0142-1 [DOI] [PubMed] [Google Scholar]
- 15.Silva D, Houghton S, Hagemann E, Jacoby P, Jongeling B, Bower C. Child attention deficit hyperactive disorder co morbidities on family stress: effect of medication. Community Ment Health J. 2015;51(3):347-353. doi: 10.1007/s10597-014-9770-3 [DOI] [PubMed] [Google Scholar]
- 16.Yoshimasu K, Barbaresi WJ, Colligan RC, et al. Childhood ADHD is strongly associated with a broad range of psychiatric disorders during adolescence: a population-based birth cohort study. J Child Psychol Psychiatry. 2012;53(10):1036-1043. doi: 10.1111/j.1469-7610.2012.02567.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo JC, Jon DI, Shim SH, et al. Prevalence and comorbidities of attention deficit hyperactivity disorder among adults and children/adolescents in Korea. Clin Psychopharm Neu. 2022;20(2):402-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitner Y. The co-occurrence of autism and attention deficit hyperactivity disorder in children—what do we know? Front Hum Neurosci. 2014;8:268. doi: 10.3389/fnhum.2014.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Häfner H, Löffler W, Maurer K, Hambrecht M, an der Heiden W. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand. 1999;100(2):105-118. doi: 10.1111/j.1600-0447.1999.tb10831.x [DOI] [PubMed] [Google Scholar]
- 20.Chen MH, Tsai SJ, Liang CS, et al. Diagnostic progression to schizophrenia in 35,255 patients with obsessive-compulsive disorder: a longitudinal follow-up study. Eur Arch Psychiatry Clin Neurosci. 2023;273(3):541-551. doi: 10.1007/s00406-021-01361-w [DOI] [PubMed] [Google Scholar]
- 21.Morales-Muñoz I, Palmer ER, Marwaha S, Mallikarjun PK, Upthegrove R. Persistent childhood and adolescent anxiety and risk for psychosis: a longitudinal birth cohort study. Biol Psychiatry. 2022;92(4):275-282. doi: 10.1016/j.biopsych.2021.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selten JP, Lundberg M, Rai D, Magnusson C. Risks for nonaffective psychotic disorder and bipolar disorder in young people with autism spectrum disorder: a population-based study. JAMA Psychiatry. 2015;72(5):483-489. doi: 10.1001/jamapsychiatry.2014.3059 [DOI] [PubMed] [Google Scholar]
- 23.Jääskeläinen E, Juola P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296-1306. doi: 10.1093/schbul/sbs130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warner R. Recovery from schizophrenia and the recovery model. Curr Opin Psychiatry. 2009;22(4):374-380. doi: 10.1097/YCO.0b013e32832c920b [DOI] [PubMed] [Google Scholar]
- 25.Leucht S, Lasser R. The concepts of remission and recovery in schizophrenia. Pharmacopsychiatry. 2006;39(5):161-170. doi: 10.1055/s-2006-949513 [DOI] [PubMed] [Google Scholar]
- 26.Schiweck C, Arteaga-Henriquez G, Aichholzer M, et al. Comorbidity of ADHD and adult bipolar disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;124:100-123. doi: 10.1016/j.neubiorev.2021.01.017 [DOI] [PubMed] [Google Scholar]
- 27.Gnanavel S, Sharma P, Kaushal P, Hussain S. Attention deficit hyperactivity disorder and comorbidity: A review of literature. World J Clin Cases. 2019;7(17):2420-2426. doi: 10.12998/wjcc.v7.i17.2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maibing CF, Pedersen CB, Benros ME, Mortensen PB, Dalsgaard S, Nordentoft M. Risk of schizophrenia increases after all child and adolescent psychiatric disorders: a nationwide study. Schizophr Bull. 2015;41(4):963-970. doi: 10.1093/schbul/sbu119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286-293. doi: 10.1192/bjp.bp.108.060723 [DOI] [PubMed] [Google Scholar]
- 30.Jutla A, Donohue MR, Veenstra-VanderWeele J, Foss-Feig JH. Reported autism diagnosis is associated with psychotic-like symptoms in the Adolescent Brain Cognitive Development cohort. Eur Child Adolesc Psychiatry. 2022;31(7):1-10. doi: 10.1007/s00787-021-01738-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meier SM, Petersen L, Pedersen MG, et al. Obsessive-compulsive disorder as a risk factor for schizophrenia: a nationwide study. JAMA Psychiatry. 2014;71(11):1215-1221. doi: 10.1001/jamapsychiatry.2014.1011 [DOI] [PubMed] [Google Scholar]
- 32.Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60(1):21-32. doi: 10.1016/S0920-9964(02)00167-6 [DOI] [PubMed] [Google Scholar]
- 33.van ’t Hof M, Tisseur C, van Berckelear-Onnes I, et al. Age at autism spectrum disorder diagnosis: a systematic review and meta-analysis from 2012 to 2019. Autism. 2021;25(4):862-873. doi: 10.1177/1362361320971107 [DOI] [PubMed] [Google Scholar]
- 34.Werner S, Malaspina D, Rabinowitz J. Socioeconomic status at birth is associated with risk of schizophrenia: population-based multilevel study. Schizophr Bull. 2007;33(6):1373-1378. doi: 10.1093/schbul/sbm032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olfson M, Ascher-Svanum H, Faries DE, Marcus SC. Predicting psychiatric hospital admission among adults with schizophrenia. Psychiatr Serv. 2011;62(10):1138-1145. doi: 10.1176/ps.62.10.pss6210_1138 [DOI] [PubMed] [Google Scholar]
- 36.Salazar de Pablo G, Studerus E, Vaquerizo-Serrano J, et al. Implementing precision psychiatry: a systematic review of individualized prediction models for clinical practice. Schizophr Bull. 2021;47(2):284-297. doi: 10.1093/schbul/sbaa120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chouinard G, Samaha AN, Chouinard VA, et al. Antipsychotic-induced dopamine supersensitivity psychosis: pharmacology, criteria, and therapy. Psychother Psychosom. 2017;86(4):189-219. doi: 10.1159/000477313 [DOI] [PubMed] [Google Scholar]
- 38.Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356. doi: 10.1002/wps.20567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seo JC, Jon DI, Shim SH, et al. Prevalence and comorbidities of attention deficit hyperactivity disorder among adults and children/adolescents in Korea. Clin Psychopharmacol Neurosci. 2022;20(1):126-134. doi: 10.9758/cpn.2022.20.1.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-10 Codes of ADHD and ATC Codes for ADHD Medications
eTable 2. ICD-10 Codes for Psychiatric Comorbid Disorders
eTable 3. ATC Codes for Other Psychotropic Medications
eFigure 1. Flow Chart of Study Population Selection
eTable 4. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis by Age Groups
eTable 5. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis by the Use of Methylphenidate During Follow-Up Period
eTable 6. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis of Patients Without Specific Psychiatric Disorder
eTable 7. Distribution of Patients by the Presence of Psychiatric Comorbidities After Propensity Score Matching
eTable 8. Demographics of ADHD Patients According to the Presence of Psychiatric Comorbidity
eTable 9. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in the PS Matched Cohort
eTable 10. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis by Age Groups in the PS Matched Cohort
eTable 11. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis by the Use of Methylphenidate During Follow-Up Period in the PS Matched Cohort
eTable 12. Association Between Transitioning to Schizophrenia and Psychiatric Comorbidities in Subgroup Analysis of Patients Without Specific Psychiatric Disorder in the PS Matched Cohort
eFigure 2. Sankey Diagram Illustrating the Incidence of Psychiatric Comorbidities in the Diagnostic Transition From ADHD to Schizophrenia in Patients According to Age Groups
eTable 13. Proportion of Patients in Trajectories From ADHD to Schizophrenia in all Patients
eTable 14. Proportion of Patients in Trajectories From ADHD to Schizophrenia, Aged 5 to 9 Years
eTable 15. Proportion of Patients in Trajectories From ADHD to Schizophrenia, Aged 10 to 14 Years
eTable 16. Proportion of Patients in Trajectories From ADHD to Schizophrenia, Aged 15 to 19 Years
Data Sharing Statement


