Abstract
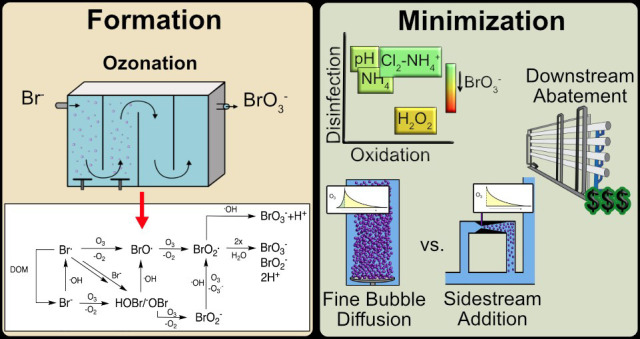
Ozone is a commonly applied disinfectant and oxidant in drinking water and has more recently been implemented for enhanced municipal wastewater treatment for potable reuse and ecosystem protection. One drawback is the potential formation of bromate, a possible human carcinogen with a strict drinking water standard of 10 μg/L. The formation of bromate from bromide during ozonation is complex and involves reactions with both ozone and secondary oxidants formed from ozone decomposition, i.e., hydroxyl radical. The underlying mechanism has been elucidated over the past several decades, and the extent of many parallel reactions occurring with either ozone or hydroxyl radicals depends strongly on the concentration, type of dissolved organic matter (DOM), and carbonate. On the basis of mechanistic considerations, several approaches minimizing bromate formation during ozonation can be applied. Removal of bromate after ozonation is less feasible. We recommend that bromate control strategies be prioritized in the following order: (1) control bromide discharge at the source and ensure optimal ozone mass-transfer design to minimize bromate formation, (2) minimize bromate formation during ozonation by chemical control strategies, such as ammonium with or without chlorine addition or hydrogen peroxide addition, which interfere with specific bromate formation steps and/or mask bromide, (3) implement a pretreatment strategy to reduce bromide and/or DOM prior to ozonation, and (4) assess the suitability of ozonation altogether or utilize a downstream treatment process that may already be in place, such as reverse osmosis, for post-ozone bromate abatement. A one-size-fits-all approach to bromate control does not exist, and treatment objectives, such as disinfection and micropollutant abatement, must also be considered.
Keywords: bromate, ozonation, human carcinogen, dissolved organic matter
Introduction
Ozone is applied as a disinfectant and oxidant during drinking water treatment, with hundreds of full-scale treatment plants worldwide.1 As interest in water reuse and ecological protection of waterways grows in the United States and Europe, ozonation is increasingly included in advanced wastewater treatment processes for the oxidation of micropollutants by ozone and hydroxyl radicals (•OH).1,2 During ozonation of bromide-containing waters, oxidation of bromide by ozone and •OH leads to bromate formation, which is a human and ecological health concern.
This Critical Review of the current knowledge of bromate will cover the following aspects: (i) toxicity of bromate, (ii) sources of bromide in natural waters and wastewaters, (iii) analytical methods for bromate determination, (iv) kinetics and mechanisms of bromate formation, (v) theoretical and empirical modeling of bromate formation, and (vi) bromate mitigation strategies implemented before, during, and after ozonation.
Toxicological Aspects of Bromate
Bromate was classified as a possible human carcinogen in the 1990s and is regulated to a maximum contaminant level of 10 μg/L by several regulatory agencies.3,4 This stringent limit is based on toxicological studies conducted on rodents5−7 and subsequent risk assessments conducted by the U.S. Environmental Protection Agency (USEPA) and the World Health Organization (WHO).8,9 These studies have shown that exposure to bromate may result in cancer in kidneys, thyroid, and testicular mesothelium of rats.7 Although bromate is regulated as a probable genotoxic carcinogen, there is evidence of a nongenotoxic mode of action.10,11 Genotoxic effects have been shown to result from oxidative damage to DNA at relatively high levels of exposure to bromate, whereas nongenotoxic effects, including apoptosis (cell death) and mutation, can result at lower bromate concentrations.10 Studies have also shown the reduction of bromate to bromide in simulated gastric solutions, suggesting that regulatory limits might be set conservatively low.12 The strict human health standard for bromate has limited the applicability of ozone for disinfection/oxidation in both water and enhanced wastewater treatment.
Bromate has also demonstrated potential ecological impacts. Lethal concentrations (LC50) of bromate in the range of 31–2258 mg/L for different fish species have been reported.13,14 With a safety factor of 10, a long-term bromate exposure limit of 3 mg/L was proposed to protect the most sensitive aquatic organisms.13 Bromate exposure tests with Ceriodaphnia dubia resulted in a more stringent acute and chronic standard of 50 μg/L.15,16
Bromide Occurrence and Sources
Bromide is naturally occurring in geological structures such as limestone, granite, and shale, at concentrations ranging between 0.3 and 24 mg/kg.17 Typically, bromide in surface water results from geogenic sources,17 but other sources include seawater intrusion and anthropogenic sources.18,19 Anthropogenic sources of bromide include industrial point discharges, municipal waste incinerators, landfills, chemical plants, coal-fired power plants, private swimming pools, and hydraulic fracturing.19−22 Median bromide levels in drinking water sources (groundwaters and surface waters) were in the range of 30–80 μg/L on the basis of U.S. surveys; however, concentrations have been observed in the range of hundreds of microgram to milligrams per liter.23,24
A bromide concentration threshold of ∼100 μg/L was proposed for drinking water disinfection with ozone to avoid a violation of the drinking water standard for bromate.25 This is only a rough estimate, because bromate formation largely depends on other treatment goals and the water matrix.26 In a specific water source, the bromide threshold level may be significantly different, and therefore, site specific tests should be performed.26 Bromide levels in wastewater effluents can be significantly higher, with median levels reported around 230 μg/L,23 and higher levels of ≤50 mg/L have been reported.22 The elevated bromide levels in wastewaters magnify the challenges pertaining to bromate control during ozonation for potable reuse applications and ecosystem protection scenarios. A full summary of bromide occurrence is outside the scope of this review; however, this topic has been evaluated in numerous previous studies.17,20,22−24,27−32
Bromate Measurement
Bromate is primarily analyzed by ion chromatography with conductivity detection (IC-CD) with method reporting limits (MRL) of 4–5 μg/L.33−37 Detection of bromate by postcolumn reactions followed by ultraviolet (UV) measurement (IC-PCR) avoids interference from chloride and sulfate and increases sensitivity. An MRL of ≲1 μg/L is possible with this approach.38−40 Other methods use ion chromatography coupled with inductively coupled plasma mass spectrometry (IC-ICP-MS), (tandem) mass spectrometry (IC-MS and IC-MS/MS), or liquid chromatography-tandem triple quadrupole mass spectrometry (LC-MS/MS).40−44 MS-based methods have submicrogram per liter MRLs. For laboratory studies on bromate formation, or monitoring in practice, IC-PCR or IC/LC-MS methods should be applied to have MRLs far below the drinking water standard of 10 μg/L. There has been limited success for online bromate measurements. One approach, which utilizes fluorescence detection of trifluoperazine (TFP), has been examined and showed promise; however, further long-term experience is needed with such systems.45,46
For sample collection, oxidant quenching should be carried out to avoid continuing bromate formation during storage. Quenching agents, such as indigo trisulfonate, thiosulfate, sulfite, buten-3-ol, or cinnamic acid, can be applied.42,47 Proper preservation and the proper storage temperature (<6 °C) result in holding times of approximately one month without sample deterioration.34
Bromate Formation during Ozonation
Overview of Pathways
Ozone Reactions
Acidic solutions containing ozone and bromide were investigated in the 1940s with the goal of measuring Br2 formation.48 This reaction was confirmed at circumneutral pH with the formation of HOBr/OBr–, which is the hydrolysis product of Br2.49,50 This is the first step in bromate formation with ozone (eq 1):
| 1 |
Two second-order rate constants for reaction 1 were reported: k = 160 M–1 s–1 at 20 °C49 or k = 258 M–1 s–1 at 25 °C.50 With these moderate second-order rate constants, calculated half-life times of bromide are in the range of 2–4 min for an ozone concentration of 1 mg/L. Therefore, for disinfection processes with a substantial ozone exposure, significant extents of OBr– can be formed. During oxidation of micropollutants, often no or low ozone residual concentrations are present and therefore OBr– formation will be minor.22
OBr– is in equilibrium with HOBr with a pKa of 8.851 (eq 2):
| 2 |
This high pKa of HOBr is crucial for bromate formation with ozone, because HOBr reacts very slowly with ozone (k ≤ 10–2 M–1 s–149), whereas OBr– has a moderate reactivity (k = 100 M–1 s–1 at 20 °C49) (eq 3):
| 3 |
In addition to reaction 3, the attack of ozone on OBr– can also proceed through a second faster reaction, which is a reduction of OBr– back to bromide (k = 330 M–1 s–1 at 20 °C49) (eq 4):
| 4 |
Paradoxically, this is a reductive process occurring during ozonation. Similar reactions occur during the ozonation of OCl– and Mn2+.52,53
The last reaction en route to bromate is an oxidation of bromite (BrO2–), which has been suggested to be an oxygen-transfer reaction (k > 105 M–1 s–1 at 20 °C49) (eq 5):
| 5 |
However, more recently it was demonstrated that reaction 5 proceeds via an electron transfer (k = 8.9 × 104 M–1 s–1 at 25 °C54) (eq 6):
| 6 |
Bromine dioxide (BrO2•) undergoes a self-reaction and a disproportionation54,55 according to eqs 7 and 8:
| 7 |
| 8 |
An overall second-order rate constant from bromine dioxide to bromate has been estimated as k = 5 × 107 M–1 s–1 at 10 °C.54
Hydroxyl Radical Reactions
In the original studies on bromate formation, the role of •OH was not considered.49 However, it can play a major role at various levels of bromate formation during ozonation.56
Bromide can be oxidized by •OH to bromine radicals (Br•) in a two-step reaction with an equilibrium57 (eqs 9 and 10):
| 9 |
| 10 |
The second-order rate constant for reaction 9 is 1.06 × 1010 M–1 s–1;58 however, due to the equilibrium character of eqs 9 and 10, an overall second-order rate constant of k = 1.1 × 109 M–1 s–1 for the net reaction of bromide to Br• can be estimated for bromide concentrations of ≤1 mg/L59 (eq 11):
| 11 |
One of the main sinks of Br• is its reaction with bromide, with a second-order rate constant (k) of ≈1010 M–1 s–1 for the forward and 6.6 × 103 M–1 s–1 for the reverse reaction1 (eq 12):
| 12 |
Br2•– can lead to the formation of Br2 over several equilibria and disproportionation reactions,1 and Br2 is then hydrolyzed to HOBr under typical water treatment conditions.51 This pathway is also crucial for bromate formation in systems with only •OH (without ozone) as was demonstrated with γ-radiolysis experiments.60,61 Because HOBr/OBr– is a decisive intermediate, bromate is not formed in UV-based advanced oxidation in the presence of hydrogen peroxide (H2O2) (e.g., UV/H2O2), because H2O2 reduces HOBr/OBr– back to bromide. This is not the case for ozone-based advanced oxidation (see Hydrogen Peroxide).62 Also, BrOH•–, which is formed from the reaction of •OH with Br– (eq 9), reacts with bromide, leading to Br2•– and the ensuing reactions.1,57
HOBr/OBr–, which is formed from the oxidation of bromide with ozone or •OH, can further react with •OH with second-order rate constants of 2 × 109 M–1 s–1 (kOH,HOBr) and 4.5 × 109 M–1 s–1 (kOH,OBr–)63 (eqs 13 and 14):
| 13 |
| 14 |
Bromine monoxide (BrO•) can also be formed from the reaction of hypobromite with carbonate radicals (k = 4.3 × 107 M–1 s–163). This reaction is relevant, because of the higher steady-state concentrations of carbonate radicals compared to that of •OH.64
BrO• undergoes disproportionation with the formation of hypobromite and bromite (k = 4.9 × 109 M–1 s–163) (eq 15):
| 15 |
Bromite, which also reacts with ozone (see above), can react further with •OH to afford bromine dioxide (BrO2•) (k = 1.9 × 109 M–1 s–163) (eq 16):
| 16 |
An analogues reaction (eq 16) also occurs with the carbonate radical (k = 1.1 × 108 M–1 s–163). Bromine dioxide can then react further to afford bromate according to reactions 7 and 8 or with •OH (k = 2 × 109 M–1 s–165) (eq 17):
| 17 |
Bromine Radical Reactions
Bromine radicals (see eqs 9 and 10) can undergo the following reactions: (i) oxidation by ozone, (ii) reaction with bromide, and (iii) reaction with dissolved organic matter (DOM).
-
(i)On the basis of a combination of γ-radiolysis and tailored ozonation experiments, it was estimated by kinetic modeling that Br• reacts with ozone to afford bromine monoxide (BrO•) with a k of ≈1.5 × 108 M–1 s–161 (eq 18), the same product that is also formed by eqs 13 and 14:
Bromine monoxide then disproportionates according to eq 15. This pathway can lead to bromate during the O3/H2O2 advanced oxidation process (AOP).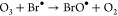
18 -
(ii)
According to eq 12, Br• is in equilibrium with Br2•– as a potential sink. Figure S1 shows the Br•/Br2•– equilibrium concentration ratios as a function of bromide concentration. For a bromide concentration of 1 μM (80 μg/L), ∼87% is present as Br• and ∼13% as Br2•–. The Br•/Br2•– concentration ratio is ∼1 at 500 μg/L bromide. Therefore, for low to moderate bromide levels, Br• will always dominate and therefore contribute to further reactions with ozone.
-
(iii)
Another important sink for Br• is DOM, with second-order rate constants (k) in the range of 1.4–4.2 × 108 MC–1 s–1 for DOM isolates and real waters.66,67 Experiments with a preozonated DOM isolate showed that the second-order rate constant with Br• did not change significantly, which implies a constant consumption of Br• by DOM during ozonation.67
Reactions of Br• with organic compounds proceed mainly by electron transfer,66−68 shown for DOM in eq 19:
| 19 |
It has been demonstrated that a minor fraction may proceed by an addition of bromine to the ensuing brominated products.67
On the basis of the second-order rate constants for the reactions of Br• with ozone and DOM, the initial fraction of Br• reacting with either constituent can be calculated as a function of the specific ozone dose (milligrams of O3 per milligram of DOC) (eq 20 and Figure 1):
| 20 |
Figure 1.
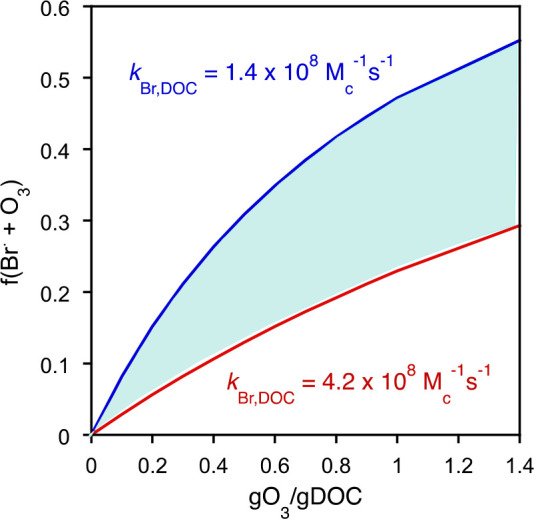
Fraction of the reaction of ozone with the bromine radical as a function of the specific ozone dose (the initial ozone dose is taken for the calculations). The blue and red curves represent the lower and higher limits, respectively, of the second-order rate constant for the reaction of Br• with DOM. The colored area represents the range of the fraction f(Br• + O3) (eq 20) for the lower or higher second-order rate constants for the Br•–DOM reaction (second-order rate constants were obtained from ref (66)) (DOC = 5 mg/L).
Figure 1 shows that for typically applied specific ozone doses (0.2–1.0 g of O3/g of DOC), the initial fraction of Br• reacting with ozone can account for ≤50% (DOC = 5 mg/L).
Bromate Formation Mechanism
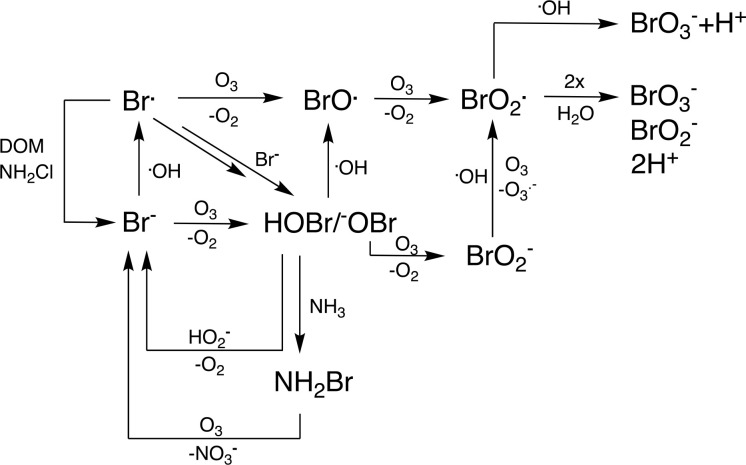
On the basis of the reactions discussed above, a bromate formation mechanism including both ozone and •OH can be compiled (Figure 2).
Figure 2.
Simplified mechanism for bromate formation during ozonation of bromide-containing waters. Adapted from and expanded on the basis of a previous study.1 Reactions of HOBr/OBr– with hydrogen peroxide and ammonia are also included.
It is evident from Figure 2 that bromate formation is a complex reaction mechanism occurring during ozonation, because of the relevance of both ozone and •OH at most reaction steps. The mechanism is compiled on the basis of several key papers47,49,56,59,61,69−72 and was also discussed in a previous publication.1 This reaction mechanism was previously categorized into three main pathways. (i) The direct–direct (O3) pathway consists of ozone-controlled bromate formation. (ii) The direct–indirect (•OH) pathway comprises an oxidation of bromide to HOBr/OBr– by ozone followed by a further oxidation by •OH. (iii) The indirect–indirect pathway consists of a •OH-dominated pathway.73 Even though this approach can help as an orientation in the mechanism, it neglects that most reaction steps depend on the •OH/O3 concentration ratio and the corresponding second-order rate constants. This ratio is water specific and depends on the specific ozone dose. To overcome this problem, the Rct concept was developed, which allows for the determination of the •OH/O3 concentration ratio by a relatively simple procedure.74−77 This approach has also been applied to determine the ozonation transformation products of micropollutants.78,79 During ozonation, typical concentration ratios (Rct) of •OH and O3 are on the order of 10–6–10–9. Figure 3 shows the fractions of reactions proceeding by O3 or •OH for various bromine species (Br–, HOBr/OBr–, and BrO2–) as a function of Rct.
Figure 3.
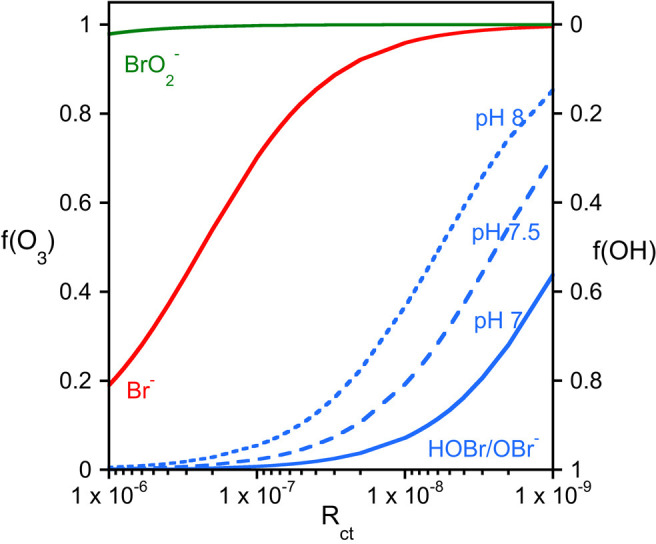
Fractions of reactions of Br–, HOBr/OBr–, and BrO2– occurring with ozone or •OH as a function of Rct in the range of 10–6–10–9 (•OH/O3 concentration ratio). Note that the X-axis and the second Y-axis are reversed.
Figure 3 illustrates that during the initial phase (second range) of ozonation (Rct values as high as 10–680), reactions of •OH with bromide and HOBr/OBr– dominate over ozone reactions. For the later phases of ozonation (Rct > 10–7), bromide oxidation occurs mainly by ozone and, depending on the pH, the fraction of the further reaction of HOBr/OBr– with ozone making up between 10% and 80%. For bromite, ozone always outcompetes •OH for the further oxidation to bromate.
Water Quality Considerations
Role of pH
pH plays a decisive role in bromate formation; first and most importantly, it is crucial for ozone chemistry. At low pH, ozone is more stable and the formation of •OH is slow, which is demonstrated by 40 times lower Rct values at pH 6 than at pH 9.1,74 Such conditions are ideal for disinfection because high levels of ozone exposure are achieved with limited formation of •OH. Under such conditions, mainly HOBr is formed from the reaction of ozone with bromide, and it is not further oxidized by ozone due to the low reactivity of HOBr with ozone (see above). Even though the oxidation of HOBr by •OH has a reasonable second-order rate constant, its oxidation by this pathway is slow because of the low transient •OH concentrations at low pH.47,74 The effect of pH on bromate formation has been demonstrated (e.g., during the ozonation of Seine River water with a bromide concentration of 60 μg/L) for which bromate concentrations of 4 and 9 μg/L were obtained at pH 6 (Rct = 2.9 × 10–9) and pH 8 (Rct = 9.0 × 10–9), respectively, for an ozone exposure of 10 mg L–1 min–1.47 pH depression can be applied as a bromate mitigation strategy during disinfection with ozone; at lower pH values, a better disinfection efficiency can be achieved with a lower level of bromate formation (see Mitigation).
Temperature
Temperature affects bromate formation at two levels: (i) faster reactions of bromide and transient bromine species50 with ozone and •OH and (ii) faster decomposition of ozone.74 The only information about the effect of temperature on the oxidation of bromine species is related to the oxidation of bromide by ozone, with second-order rate constants of 258 and 97 M–1 s–1 at 25 and 5 °C, respectively.50 The effect of temperature on Rct is quite significant with an approximately 10-fold increase from 6.0 × 10–9 to 8.5 × 10–8 from 5 to 35 °C, respectively, for the ozonation of Lake Zurich water at pH 8. For a given ozone exposure, this leads to much higher level of bromate formation at higher temperatures, if the enhanced inactivation of microorganisms at higher temperatures is not considered.81 Therefore, a temperature correction for disinfection should be implemented for ozone dosage control to achieve an ozone exposure, which guarantees a certain inactivation of target organisms.82 This approach helps to save on ozone production and mitigates bromate formation. The overall benefit is difficult to predict, because only a few activation energies of the involved reactions (bromate formation, ozone decay, and inactivation of microorganisms) are known.
Dissolved Organic Matter
The effect of DOM on bromate formation is threefold: (i) quenching transient bromine species, (ii) scavenging ozone and •OH, and (iii) influencing the Rct.
-
(i)
HOBr can react with DOM moieties, such as phenols, β-dicarbonyl compounds, and amines.47,51,83,84 Except for the amines, ozone also reacts quickly with such HOBr-quenching moieties, and therefore, they will not persist to react with HOBr.1,85 It has been shown that the concentration of Br(+I) (sum of HOBr and bromamines) remains fairly constant during ozonation.47 The reactivities of organic bromamines that might be present are 2–3 orders of magnitude lower with phenolic moieties than with HOBr, and therefore, the formation of bromoorganic compounds is also not expected from this pathway.84
-
(ii)
Scavenging of ozone and •OH is a major factor affecting ozonation processes. However, because ozonation has a certain oxidation/disinfection target, typical ozone doses are adapted to DOC concentrations to compensate for the oxidant demand. In this context, the specific ozone dose (milligrams of O3 per milligram of DOC) is decisive for bromate formation. It has been demonstrated during wastewater ozonation that bromate formation is initiated at specific ozone doses of ≳0.5 mg of O3/mg of DOC.22 Under these conditions, the ozone residual is high enough for reactions with bromide and Br•, and therefore, bromate can be formed.
-
(iii)
The effect of DOM type on Rct is probably the most important factor influencing bromate formation. In a study of 12 groundwaters and lake waters, it was shown that the Rct values vary over 2 orders of magnitude.75 Part of this effect is also due to varying carbonate levels (see the next section). Nevertheless, this shows that the indirect effect of DOM on bromate formation can be very significant.
Carbonate Alkalinity
Carbonate/bicarbonate reacts moderately with •OH to afford carbonate/bicarbonate radicals.86,87 This reaction can influence bromate formation on two levels, (i) reactions of carbonate radical with bromine species and (ii) quenching of •OH, thereby influencing Rct.
-
(i)
•OH scavenging by carbonate depends on the pH (kOH,HCO3– = 8.5 × 106 M–1 s–1; kOH,CO32– = 3.9 × 108 M–1 s–1)86,87 and the DOC concentration, because DOM is typically the main •OH scavenger during the ozonation of real waters. Basically, carbonate scavenging affects the Rct, and this in turn has a significant effect on bromate formation (see above). It has been shown during the ozonation of Lake Zurich water by varying the carbonate levels at pH 8 (15 °C) from 0 to 2.5 mM that Rct decreases from 1.25 × 10–7 to 1.5 × 10–8.74 The influence of such changes on bromate formation is difficult to assess, because of the role of carbonate radicals as oxidants, which increase, while Rct decreases. More systematic studies are necessary to assess these counteracting effects.
-
(ii)
It has been reported that carbonate radicals can react with bromide with a second-order rate constant of <5 × 105 M–1 s–1:88
| 21 |
This reaction is in equilibrium with the back reaction with second-order rate constants of 2 × 106 and 1 × 106 M–1 s–1 for carbonate and bicarbonate, respectively:88
| 22 |
| 23 |
Because the bicarbonate concentration in natural waters is typically in the millimolar range, the first-order rate constant for the back reaction will be orders of magnitude higher than that for the forward reaction, and therefore, oxidation of bromide by carbonate radical is negligible.
Oxidation of OBr– by the carbonate radical occurs with a second-order rate constant of 4.3 × 107 M–1 s–1:86
| 24 |
This reaction seems to be relevant, because significant differences in bromate formation in the absence and presence of carbonate have been observed during the ozonation of bromide-containing waters under standardized oxidant conditions.56
Modeling
Mechanistic Models
Kinetic models for the prediction of bromate formation during ozonation are set up by a combination of all of the relevant chemical equations with the corresponding rate constants in an equation system that may contain 100–200 reactions. Available codes such as Kintecus can be used to solve such coupled differential equations.89
Bromate modeling is one of the most challenging endeavors in environmental oxidation chemistry. The challenges are related to the roles of ozone and •OH at various levels of the bromate formation pathway and also require modeling of the complex ozone chemistry in aquatic systems.
Kinetic Modeling of Ozone Decomposition
Even though rate constants for the inorganic reactions involved in ozone decomposition and the ensuing •OH formation are available in the literature, there are two main challenges:
-
(i)
Second-order rate constants for individual ozone decomposition reactions have been measured individually by different researchers, and large differences can be expected between different laboratories.1 Therefore, ozone decomposition modeling has a high level of uncertainty.
-
(ii)
The DOM can react with ozone and •OH with second-order rate constants that vary from one type of DOM to another.1,75 Furthermore, the fraction of promotion and inhibition of the radical chain reaction upon reaction of •OH with DOM is not a priori known and has to be determined by fitting procedures.76,90
On the basis of these factors, ozone modeling in real waters has high levels of uncertainty. Several attempts to kinetically model ozone decomposition were made, but fitting of some of the rate constants was typically necessary to match the ozone evolution.91,92 Transient •OH formation was not even assessed in these models, and therefore, there is only limited application of such modeling exercises in real systems.
To overcome these inherent problems with ozone modeling, the experimentally determined ratios of the concentrations of •OH and O3 discussed above, Rct has been applied to model bromate formation under well-defined conditions during ozonation and advanced oxidation with O3/H2O2. This enabled a fitting of the second-order rate constant for the reaction of O3 with Br• (k = 1.5 × 108 M–1 s–1).61
Kinetic Modeling of Bromate Formation
Modeling of bromate formation was established first for ozonation systems in which •OH radicals were scavenged. Such models can be set up with only a few kinetic equations and were successfully applied for a trend analysis of bromate formation for varying parameters in ultrapurified water.18 If both O3 and •OH are included in such bromate formation models, >40 reactions are needed.56,61,94 In addition, DOM may play an important role in quenching transient bromine species, such as HOBr and Br•.47,66,72 Similar to the ozone decomposition models, this approach also has the inherent problem of second-order rate constants that were determined by different research groups. One case in point is the reaction of ozone with bromide, for which the two values in the literature differ by a factor of 1.6 [160 and 258 M–1 s–1 (see above)].49,50 This example for a second-order rate constant that can be easily determined illustrates clearly the challenges of bromate formation models. Furthermore, if the >40 equations for bromate formation during ozonation of bromide-containing water are combined with the ozone decomposition chemistry, the level of uncertainty increases even more.
Nevertheless, kinetic bromate formation models can still yield useful information related to (i) relative bromate formation for changing water quality parameters (pH, ammonium, alkalinity, etc.), (ii) the contribution of a certain pathway to bromate formation, (iii) estimation of unknown rate constants for the reactions of transient bromine species with O3 and •OH, and (iv) planning of tailored experiments to elucidate well-defined partial reaction systems. Bromate formation modeling has been extensively performed to support mechanistic studies for which relative changes in bromate are important and can be translated into mitigation strategies at full scale.56,94
Empirical Models
Since the early 1990s, nonmechanistic, empirical correlations have been applied to model bromate formation during ozonation of water and wastewater.95,96 These can be generally categorized into three model types: linear regression, multilinear regression (MLR), and models based on artificial neural networks (ANNs).26,97−107Table S1 provides a list of various models, including their corresponding boundary conditions.
Applying real world data to several different models demonstrated a large variability, which is an inherent weakness of empirical models.106 At their best, they were able to predict the trend of bromate formation with varying water quality parameters; however, the inaccuracy of the predicted bromate concentration was large. It appears that these models are highly water specific and should be used with caution, and not without prior model validation.
Mitigation
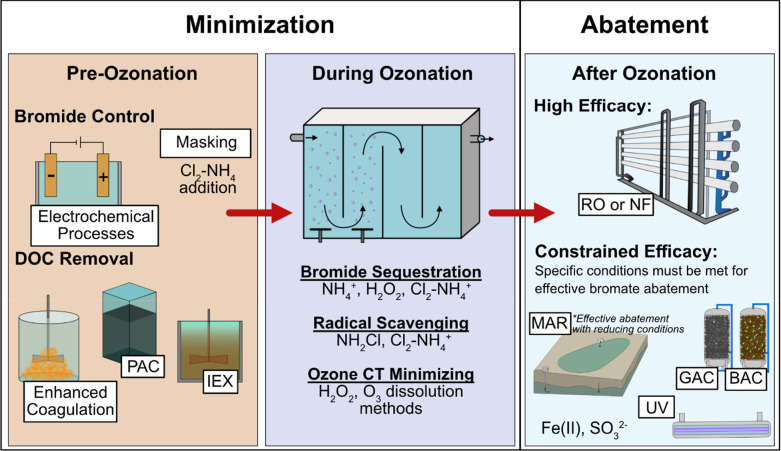
Bromate control is challenging because of the need for micropollutant abatement and/or disinfection by ozone and/or •OH, which in turn leads to the formation of bromate. Several strategies can be applied before, during, or after ozonation to minimize the level of bromate in finished waters while maintaining the treatment goals (Figure 4). Strategies applied before or during ozonation aim to minimize the formation of bromate, whereas post-ozonation treatments focus on the abatement of bromate.
Figure 4.
Overview of bromate mitigation strategies during pretreatment, ozonation, and post-treatment.
Bromate Minimization before Ozonation
Pretreatment strategies include processes located before ozonation aimed at bromide or DOC removal (Figure 4).
Bromide Removal
Electrochemical processes can remove ≤35% of bromide from natural waters in laboratory batch and continuous flow systems.108,109 During the process, bromide is oxidized to bromine, which could potentially lead to formation of brominated compounds. Nevertheless, this may be offset by minimized brominated compound formation associated with lower bromide levels during oxidative post-treatment processes. Because bromate formation during ozonation is roughly proportional to the initial bromide concentration, this approach could partially mitigate bromate. However, for electrochemical processes, up-scaling and cost/energy effectiveness lead to a limited applicability of this process in full-scale systems.110
A more promising bromide sequestration approach is the sequential addition of chlorine and NH4+,111 and due to the multiple bromate suppression mechanisms that occur both before and during ozonation, it is described in greater detail in subsequent sections (see Ammonium- and Chloramine-Based Approaches).
DOC Removal
Treatments for DOC removal may inadvertently reduce bromate formation by decreasing the ozone demand to achieve target ozone exposures. This may be particularly relevant for waters with higher concentrations of DOC, such as wastewater.
Anion exchange resin has been demonstrated to remove ≤50–60% of bromide and DOC from natural waters.112,113 Generally, bromate minimization occurred primarily from DOC rather than bromide removal.114 During pilot-scale testing, magnetic ion exchange pretreatment removed 30% of the influent DOC and reduced the ozone dose requirements by 15–25% to meet CT requirements, which subsequently reduced the level of bromate formation by 35%.115 Despite these promising results, this approach has not been readily implemented in full for bromate control.
Pretreatment by powdered activated carbon (PAC) can remove DOC. As a pretreatment step, it was shown to reduce the bromate yield at relatively large PAC doses (50–100 mg/L, >40% DOC removal), most likely due to the smaller doses of ozone needed to meet target micropollutant abatement.96 However, for PAC doses in the range of 10–20 mg/L, an increased bromate yield was observed, potentially due to changes in electron-donating capacity of the DOM.96 Because this behavior is not fully understood, more tests are needed before a broader application of this method will be possible.
DOC removal can also be achieved during enhanced coagulation, with optimal conditions based on coagulant type, pH, hydraulic conditions, etc.116 In a study of three wastewaters, enhanced coagulation with 10–30 mg/L ferric chloride removed 10–47% of the DOC, which subsequently reduced the ozone dose by a similar percentage to meet treatment objectives.117 This reduction in the applied ozone dose would likely reduce the level of bromate formation; however, further evaluation is necessary.
Bromate Minimization during Ozonation
There are several methods for minimizing bromate formation during ozonation, including reactor design and operation and chemical interventions (Table 1).
Table 1. Summary of Chemical Bromate Control Strategies.
| method | bromate minimization mechanism | bromate minimization efficiency | disinfection efficiency | oxidation efficiency | feasibility |
|---|---|---|---|---|---|
| pH depression | shifting HOBr/OBr– equilibrium, decreasing Rct (increased O3 stability) | pH 8 to 6 in drinking water, 50–91%47,121 | enhanced (stabilized ozone) | potentially diminished for O3 recalcitrant micropollutants (lower Rct) | expensive, requires storage of caustic chemicals, not applicable for medium/high-alkalinity water |
| NH3 addition | HOBr quenching (NH2Br formation) | 42–73% (surface water, pH 8, 100–900 μg of NH3-N/L)47,121,124,125 | unaltered from conventional ozonation | unaltered from conventional ozonation | •OH pathway not affected, removal of excess ammonium in biological postfiltration |
| preformed NH2Cl | decreasing Rct (radical scavenging), HOBr and Br• quenching | 68–87% (wastewater, 1–5 mg of NH2Cl as Cl2/L)130 | unaltered, although lower levels of ozone exposures have been demonstrated | potentially diminished for O3 recalcitrant micropollutants (lower Rct) | monochloramine must be produced on site, removal of excess ammonium in biological postfiltration |
| chlorine–ammonium | bromide sequestration (NH2Br formation), HOBr quenching | 44–94% (surface water, 0.25–1.0 mg/L Cl2 and 100–500 μg of NH3-N/L)124−126 | unaltered from ozone alone or slightly enhanced | unaltered from ozone alone | formation of chlorinated/brominated DBPs |
| H2O2 | reduced lifetime of ozone, reaction with HOBr | –130% to 60% (surface water, 0.5–1.5 mol of H2O2/mol of O3);135 −50% to 67% (wastewater, 0.14–4.2 mol of H2O2/mol of O3)96,136,137 | diminished (low level of or no O3 exposure) | enhanced (increased level of radical production) | residual H2O2 removal in biological postfiltration |
Process Design and Operation
Because reactor hydraulics should approach plug flow for efficient disinfection and oxidation, there is limited room for changes. However, one factor that can influence bromate formation is the ozone mass-transfer (i.e., dissolution of gaseous ozone in water) method and design.118,119 There are two major methods for ozone mass transfer: (i) fine bubble diffusion (FBD) and (ii) addition of a concentrated ozone solution through a sidestream. Alternative ozone injection methods, such as injection through membranes or as micro/nanobubbles, have been developed, though their ability to minimize bromate formation has not yet been evaluated.
-
(i)
In FBD systems, the first chamber of an ozone contactor is used for ozone gas–liquid transfer (Figure 5a). The ozone exposure (CT) in this dissolution zone (i.e., dissolution CT) is not included in regulatory disinfection credit (i.e., compliance CT). Therefore, the FBD chamber may contribute to bromate formation without any regulatory disinfection credit. When a treatment plant operates at design flow, the residence time in the FBD chamber can be minimized (often <2 min), which minimizes bromate formation in the ozone-transfer zone. However, during routine operation, flow rates can range from 25% to 60% of the design flow rate, resulting in longer contact times in the mass-transfer zone and thus higher levels of ozone exposure, leading to higher levels of bromate formation (2–7-fold in a pilot study).118 Multiple contactor systems can take contactors out of service to minimize the residence time and the corresponding bromate production in the ozone mass-transfer zone.118 This aspect of FBD should be considered when designing an ozonation process.
-
(ii)
In sidestream systems, ozone gas is added to a sidestream water flow (10–20%) using venturi or static mixers and subsequently blended with the main water flow rate (80–90%) to achieve a target ozone dose entering the disinfection zone (Figure 5b),82,118 which can minimize the dissolution zone CT compared to that of FBD systems. However, a nearly 5-fold larger ozone dose than in FBD is required in the sidestream to meet target dosages after blending. Hence, the residence time of the sidestream can influence the bromate concentration in the mainstream. Sidestream residence times increased bromate levels from 3–6 μg/L at 5 s to 40–140 μg/L at a residence time of >30 s.118 Therefore, for the minimization of ozone decomposition and bromate formation, design guidance recommends that the residence time in the sidestream should not exceed 5 s, although time allowance for gas/liquid mass transfer should also be considered.118
Figure 5.
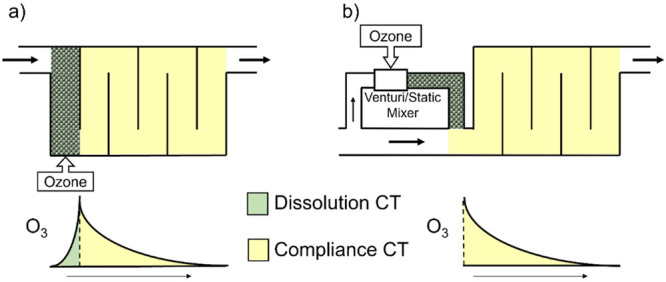
Comparisons of dissolution CT and compliance CT for different ozone mass-transfer systems: (a) conventional fine bubble diffuser and (b) sidestream addition.
pH Depression
Decreasing the pH influences both ozone stability and bromate formation (Table 1). A lower pH minimizes the amount of OBr– available for oxidation by ozone (see Figure 2). However, because both HOBr and OBr– are oxidized by •OH with similar second-order rate constants (eqs 13 and 14), another major benefit of a decrease in pH is increased ozone stability with a lower transient •OH concentration and a lower Rct.47 A decrease in pH from 8 to 6 typically results in a 50–60% decrease in the level of bromate formation in drinking water,47,120 though a bromate minimization of >90% has also been demonstrated.121 This approach allows for bromate minimization while meeting disinfection objectives. However, the chemical costs associated with pH depression, and subsequently increasing pH downstream, are in the range of 2–9 times higher than for ozone generation, depending on the water quality.121 Because of this, pH depression is impractical for waters with moderate to high alkalinity, such as many drinking waters and wastewaters. Additionally, the decreased level of •OH generation caused by pH depression should also be considered, as it may be counterproductive to a desired oxidation of micropollutants.96
Ammonium- and Chloramine-Based Approaches
The literature refers to ammonium-based strategies as “ammonia”/“NH3” rather than “ammonium”/“NH4+”. It should be noted that we are choosing to henceforth refer to these strategies as “ammonium”, as this is the applied form. Ammonium addition suppresses bromate formation by forming monobromamine, NH2Br, from hypobromous acid and ammonia (k = 5.5–7.5 × 107 M–1 s–1):84,122
| 25 |
preventing HOBr from being further oxidized to bromate. NH2Br is oxidized by ozone to nitrate, releasing bromide again (k = 40 M–1 s–1):123
| 26 |
While the second-order rate constant for the reaction between hypobromous acid and ammonia is high, it should be noted that the reaction is between the two nonionic species. With pKa values of 8.8 and 9.3 for HOBr/OBr– and NH4+/NH3, respectively, neither can be the dominant species simultaneously. With the pH in the general range for drinking water or wastewater (6–8), the apparent second-order rate constant decrease by 1–2 orders of magnitude compared to the species specific second-order rate constant in eq 25.122 While an increasing pH can increase the rate of NH2Br formation, this leads to a lower ozone stability and potentially offsets the benefit of ammonium addition due to larger ozone doses required to meet disinfection objectives.121 Regardless, in surface waters at pH ∼8, the level of bromate was decreased by 40–73% (Table 1 and Table S2) across a wide range of ozone doses with ∼200 μg of NH4+-N/L.47,121,124,125 Because of the fast consumption of HOBr by ammonia, reaction 25 dominates the consumption of HOBr at 200 μg of NH4+-N/L (k′ = 28 s–1) over its further reaction with ozone (k′ ≈ 3.2 × 10–4 s–1 at 1 mg/L O3) and •OH (k′ ≈ 5 × 10–4 s–1 for an Rct of 10–8) by orders of magnitude.
Bromate initiated by the oxidation of bromide with •OH is not efficiently mitigated by ammonium addition because HOBr is only a minor product of this pathway. Hence, the efficiency of ammonium addition for bromate mitigation depends strongly on the water characteristics and the importance of the main bromide oxidation pathway. This is also illustrated by the fact that bromate formation cannot be completely suppressed by ammonium addition, with often a maximum mitigation to 50–70%.121,124 Because ammonium addition minimizes bromate through sequestration of bromine and not through the alteration of ozone and/or •OH exposure, the efficiency of ozone for either disinfection or oxidation is not affected (Table 1).
The chlorine–ammonium process was developed to provide enhanced bromate control beyond what is possible with ammonium addition alone.120,125,126 In this process, chlorine is added upstream of ozonation to oxidize bromide to HOBr (k = 1550 M–1 s–1):
| 27 |
Five to seven minutes of chlorine contact before ammonium addition is typical, though contact times as short as 1 min have been investigated.120,125,126 Ammonium is then added to form NH2Br prior to ozonation (reaction 25). Via the formation of NH2Br prior to ozonation, the chlorine–ammonium process masks bromine and, in contrast to ammonium addition, can suppress bromate formation initiated by both O3 and •OH. The level of bromate formation has been demonstrated to be reduced by 44–94%120,124−126 depending on the treatment conditions (Table 1 and Table S3).
In addition to HOBr formation and quenching, the chlorine–ammonium process affects ozone performance in several ways. Preoxidation with chlorine decreases the ozone consumption rate and the level of •OH formation by altering the DOM.126 This was also confirmed at pilot- and full-scale, where a decreased ozone demand and decay rate were observed, largely due to chlorine preoxidation, with a significantly increased level of ozone exposure at the same ozone dose.125 Additionally, monochloramine is formed by the reaction of residual HOCl with NH3. It is a weak •OH scavenger, which can partly suppress •OH reactions and stabilize dissolved ozone (k = 5.2–5.7 × 108 M–1 s–1):127,128
| 28 |
In one study, there was no difference in bromate formation for prechlorination contact times 1 or 5 min prior to ammonium addition. This is an indication that monochloramine alone may also play an important role in bromate suppression.120
On the basis of this observation, the options and limitations of NH2Cl for bromate mitigation were investigated. To this end, it has been demonstrated that the ammonium–chlorine processes, in which mostly NH2Cl is formed prior to ozonation, has a bromate mitigation effect similar to that of the chlorine–ammonium process. In this configuration, bromide will not be masked as bromamine prior to ozonation. Instead, bromochloramine can be formed during ozonation (k = 2.86 × 105 M–1 s–1):129
| 29 |
However, similar to the addition of ammonium, this does not quench the bromine radical pathway. In a comparative study, the ammonium–chlorine process reduced the level of bromate formation from 17 to 3–4 μg/L compared to a value of 2 μg/L for the chlorine–ammonium process.120 Nevertheless, the ammonium–chlorine process was selected for bromate control because it resulted in a lower level of formation of trihalomethanes (THMs).
As a variant of the ammonium–chlorine process, preformed monochloramine has been added to wastewater to control bromate formation;99 5 mg/L NH2Cl (as Cl2) (∼130 μM NH2Cl) could reduce the level of bromate formation by ≤92%, depending on the specific ozone dose (Table 1 and Table S4). This dose is larger than what is commonly utilized in drinking water [1–2 mg of NH2Cl/L (as Cl2) (∼15–30 μM NH2Cl)]; however, the purpose of NH2Cl addition is not disinfection of the distribution system but mitigation of bromate. Similar to ammonium addition, an optimum monochloramine concentration exists beyond which bromate minimization cannot be further enhanced.81,126 Monochloramine minimizes bromate formation by several mechanisms: (i) quenching of •OH (see above), (ii) formation of bromochloramine, and (iii) quenching of Br•.
-
(i)As an •OH scavenger, monochloramine should stabilize ozone decay in drinking water. However, a certain increase in the ozone decay rate was observed in the presence of monochloramine both in river water and during water reuse.130 This may be due to the reaction of monochloramine with ozone (k = 26 M–1 s–1):52
The •OH scavenging of NH2Cl not only mitigates bromate formation but also may reduce the rate of oxidation of ozone-resistant compounds, such as 1,4-dioxane.130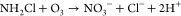
30 -
(ii)Even though the formation of bromochloramine from the reaction of NH2Cl and Br– is often mentioned as a mitigation effect for bromate, this is not very likely. The apparent second-order rate constant at circumneutral pH for the formation of bromochloramine from the reaction of NH2Cl with Br– is low (k = 1.4 × 10–1 M–1 s–1):132
For a NH2Cl concentration of 5 mg/L as Cl2 (∼130 μM NH2Cl), an ozone concentration of 1 mg/L, and an Rct of 10–8, the fractions of Br– reacting with NH2Cl, O3, and •OH are 0.3%, 94%, and 5.7%, respectively. This shows clearly that reaction 31 is not an efficient sink for bromide.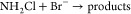
31 -
(iii)
It has been shown that Br• reacts with a k of 4.4 × 109 M–1 s–1 with NH2Cl.67 An addition of 15 μM NH2Cl (∼1 mg/L as Cl2) can reduce the contribution of the O3–Br• reaction (eq 18) from 8–15% to 2–4%, and therefore, the NH2Cl–Br• and NH2Cl–HOBr reactions contribute roughly equally to the reduction of the level of bromate formation.67
Hydrogen Peroxide
The addition of hydrogen peroxide (H2O2) during ozonation leads to an advanced oxidation process (AOP), which can maintain micropollutant abatement and mitigate bromate formation compared to conventional ozonation with the same ozone dose.61,96
Ozone reacts only with HO2– (eq 32), which is present in only a minor fraction at neutral pH (pKa,H2O2 = 11.6).133 The reaction of O3 with HO2– (k = 5.5 × 106 M–1 s–1; kapp,pH7 = 140 M–1 s–1) produces •OH with a yield of ∼50% through a complex mechanism, which is discussed elsewhere (eq 32).1,55,133
| 32 |
O3/H2O2 can influence bromate formation on two levels: (i) reduction of the lifetime of ozone and (ii) quenching of HOBr.134
-
(i)
Enhanced transformation of O3 to •OH in the O3/H2O2 process results in a shift toward the formation of Br• (eqs 9 and 10). Under these conditions, Br• will primarily react with DOM back to bromide (eq 19) due to the resulting shorter lifetime and lower transient concentration of O3.
-
(ii)
Reduction of HOBr by H2O2 (k = 7.6 × 108 M–1 s–1) proceeds by eq 33:62
| 33 |
with an apparent second-order rate constant of ∼2 × 104 M–1 s–1 at pH 7. For a H2O2 concentration of 1 mg/L, the half-life of HOBr is ∼1 s at pH 7.
The performance of H2O2 for bromate suppression is quite variable (Table 1 and Table S5); an 85% decrease and a 110% increase in the level of bromate formation have been reported in bench testing and in full-scale systems relative to conventional ozonation.96,135−137 These differences are largely due to different operational conditions, with either constant ozone doses or constant ozone residual, for conventional ozonation and the O3/H2O2 process, respectively, although the water matrix can also impact the efficacy of H2O2.137 If a constant ozone dose is applied for the two processes, the level of ozone exposure decreases in the O3/H2O2 process and therefore the level of bromate formation decreases. For a constant ozone residual, the ozone exposures for the two processes are similar, while in the O3/H2O2 process, the level of •OH exposure increases, which leads to a higher level of bromate formation. Therefore, O3/H2O2 should not be used for treatment objectives that include disinfection of bacteria and protozoa due to the necessity to maintain an ozone residual. A significant inactivation of viruses can still be with the O3/H2O2 process, despite there being no measurable CT.138 This is caused by the high second-order rate constants for virus inactivation with ozone.81,139
Several novel approaches to ozone contactor design have been developed to maximize •OH exposure while minimizing bromate formation in the presence of H2O2 by multiple smaller doses (for more details, see section S.1 of the Supporting Information).110,134,140,141 A serial O3/H2O2/LP UV process approach allowed for application of optimized ozone doses and demonstrated minimal bromate formation while achieving significant abatement of micropollutants.142
Alternative Chemical Control Strategies
Preoxidation processes with various oxidants, such as chlorine, chlorine dioxide, and permanganate, may affect ozone and •OH chemistry, and thus potentially bromate formation, through changes in DOM properties. Typically, this leads to a higher ozone stability and allows the partial mitigation of bromate formation, while a certain disinfection target can be reached.125,143 Heterogenous catalytic ozonation in which a catalyst, such as metal oxides (e.g., FeOOH), is added to enhance ozone transformation to •OH144 have been demonstrated to reduce the level of bromate formation by ≤91%.145 However, there are numerous issues related to heterogeneous processes and large doses of catalysts are required, which have prevented full-scale applications so far.110 In a preliminary study, a very low level of bromate formation with effective abatement of micropollutants was achieved by ozonation in the activated sludge reactor instead of the clarified secondary effluent.146
Post-Treatment for Bromate Abatement
Abatement of bromate downstream of ozone treatment has largely proven to be unsuccessful, with the exception of high-pressure membrane treatment such as reverse osmosis (RO) and nanofiltration (NF). It has been demonstrated that 96–97% of bromate can be removed in pilot- and full-scale RO systems after ozonation,147,148 whereas NF membranes have been demonstrated to remove 45–77% with rejection increasing at high pH and ionic strength and decreasing in the presence of DOM.149 This is especially relevant in the context of wastewater reuse that typically has DOC concentrations higher than those of natural waters. However, RO and NF may not be viable options or cost-effective for some systems (i.e., inland communities) employing upstream ozone, but RO and NF could be particularly useful for an integrated process train with multiple water quality goals, if both ozone and RO are necessary for potential regulatory requirements (e.g., California’s Draft Criteria for Direct Potable Reuse).150 Other post-ozone treatment processes have demonstrated limited success in the abatement of bromate, such as granular activated carbon and biofiltration,151−163 ion exchange,164−170 managed aquifer recharge,171−176 ferrous iron and sulfite,177−180 and UV irradiation.181−183
Practical Considerations
A one-size-fits-all approach to bromate mitigation is difficult to achieve. Absolute bromate concentrations will depend on both the water matrix composition, including bromide levels, and treatment goals. Only once bromate levels are demonstrated with a particular water and particular treatment conditions can a mitigation strategy be chosen for application. An approach for making such decisions is suggested in Figure 6. Outside of approaches that can be taken by the utility, an additional option worth mentioning is bromide source control. In the case in which there is a known bromide point source, elimination via diverting this waste stream, or eliminating bromide via treatment prior to discharge, could lead to reduced bromide levels entering the treatment facility and thus a reduced level of bromate formation.22 This approach is not feasible for most drinking water utilities but could be considered, if necessary, for wastewater treatment facilities.
Figure 6.
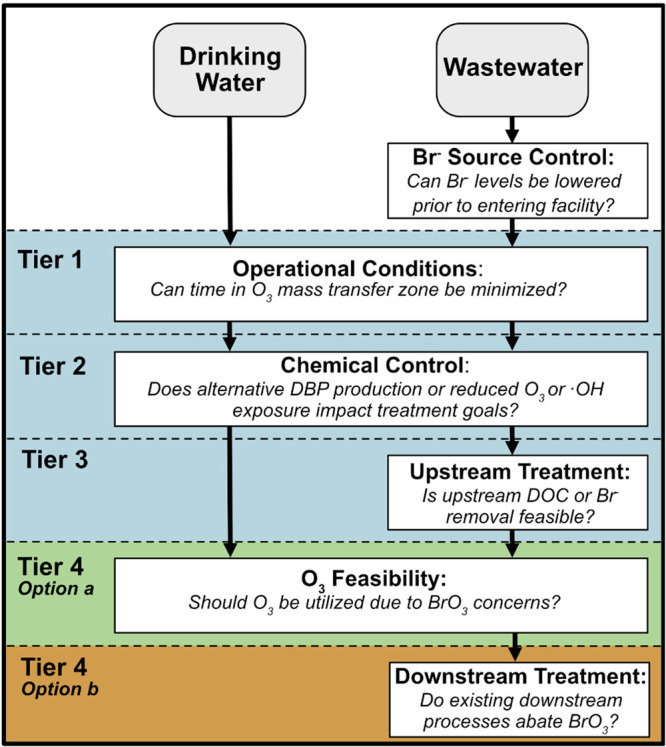
Tiered approach for the assessment of bromate control strategies based on the results of this work. In the blue area, the focus should primarily be on removing bromide from entering the ozonation process and on minimizing bromate formation through optimizing ozone dissolution, adding specific chemicals that sequester bromide, and/or disrupting bromate formation reactions. In the green area, if these solutions are not viable, then the question of whether ozone should be used should be assessed. The brown area shows options and limitations of downstream treatment for bromate abatement.
Tier One
Ideally, operational conditions affecting the extent of ozone transfer versus regulatory CT should first be considered, for both drinking water and wastewater treatment approaches. The ozone exposure should be minimized in portions of the contactor where no ozone CT credit is measured or assigned. This can be achieved by the selection (e.g., FBD and SSI) and optimization of the dissolution method (e.g., distributed ozone diffusion and reduction of sidestream residence time), which can reduce the level of bromate formation and overall cost.
Tier Two
If bromate levels are still increased after the optimization of operational conditions, a chemical control during ozonation should be considered. The chemical strategy of choice should be based on the specific treatment objectives for the ozone process, as many of the chemical strategies can affect ozone and •OH exposures. In these cases, practitioners are often challenged with balancing treatment goals with bromate formation. For instance, if ozone is implemented for disinfection purposes, it is not appropriate to utilize hydrogen peroxide as a bromate control strategy as it greatly reduces the level of ozone exposure (CT). However, this strategy would still allow for micropollutant oxidation. Furthermore, addition of chloramine or hydrogen peroxide may require the management of residuals during downstream treatment processes, such as biofiltration. Table 1 provides an overview of the different chemical addition strategies.
Tier Three
If bromate levels are still an issue, then upstream treatment minimizing either bromide or DOC, such as enhanced coagulation or PAC, could be considered. However, on the basis of the inconclusive results for different DOC removal options, such an approach should be used with caution. Preliminary bench- or pilot-scale testing with the specific source water and specific upstream treatment should be evaluated to demonstrate the extent, if any, of bromate mitigation. Such upstream treatments, if successful, also have the added benefit of reducing the size of the ozone doses necessary to achieve treatment goals, particularly in wastewater. This could potentially lower overall ozone costs, although this analysis is outside the scope of this work. DOC removal is less relevant for drinking water treatment due to the lower DOC levels compared to those of wastewater.
Tier Four
A majority of post-ozonation bromate abatement strategies are generally ineffective; therefore, relying on such an approach for bromate mitigation is not recommended. A universal promising approach to the abatement of bromate appears to be RO; however, it is cost-prohibitive if it is not already utilized for other treatment objectives and is not typically realistic for drinking water treatment facilities. The lack of post-ozonation bromate abatement options highlights the necessity of focusing efforts on minimizing bromate formation during ozonation. However, abstaining from an ozone-based process altogether may be the most feasible option for a challenging matrix. In these cases, alternate treatment processes may be desirable depending on the treatment objectives. For example, If disinfection and micropollutant abatement are desired, the AOP UV/H2O2 system may be an option,60,110 whereas if treatment is solely targeting micropollutant abatement, activated carbon (GAC, PAC, etc.) could be utilized.184,185
Research Needs
This Critical Review has highlighted several current research needs, which are particularly important considering the continued interest in using ozone for drinking water treatment or enhanced wastewater treatment for potable reuse, irrigation, or ecosystem protection:
Better mechanistic understanding of the mitigation strategies based on chlorine and ammonia, including remeasurement of some of the second-order rate constants.
Better mechanistic understanding of bromate mitigation during heterogeneous ozonation in the presence of metal oxides.
Further development of analytical methods for online determination of bromate concentrations to adapt treatment for changing water qualities.
Process optimization to better control the balance between treatment objectives and bromate formation (i.e., multiple-point peroxide addition, multistage mass transfer, and novel ozone-transfer systems).
Standardized reporting of ozone and •OH exposures during bromate control studies, including determination methods. This would allow a better evaluation of the trade-offs between bromate control and other treatment objectives (i.e., oxidation and/or disinfection).
Further evaluations of the ability of upstream treatments that aim to remove DOC to minimize bromate formation during ozonation. Certain upstream treatments, such as enhanced coagulation, may allow for several water quality changes that can impact bromate formation, such as DOC concentration, pH, and alkalinity reduction.
Determinations for the need to manage chemical residuals, i.e., chloramine and hydrogen peroxide, for downstream treatment processes, such as biofiltration.
Field demonstration for the reduction of the level of bromate in managed aquifer recharge systems for potable reuse applications.
Acknowledgments
This review was funded by The Water Research Foundation Project Number 5035: Impact of Bromate Control Measures on Ozone Oxidation/Disinfection and Downstream Treatment Processes in Potable Reuse.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c00538.
Details on Br•/Br2•– concentrations as a function of bromide; summary of bromate formation models; and water quality data demonstrating the performance of ammonium, chlorine–ammonia, preformed monochloramine, and hydrogen peroxide for bromate control (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- von Sonntag C.; von Gunten U.. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; IWA Publishing, 2012. 10.2166/9781780400839 [DOI] [Google Scholar]
- Oneby M. A.; Bromley C. O.; Borchardt J. H.; Harrison D. S. Ozone treatment of secondary effluent at U.S. municipal wastewater treatment plants. Ozone Sci. Eng. 2010, 32, 43–55. 10.1080/01919510903482780. [DOI] [Google Scholar]
- Stage 1 Disinfectants and Disinfection Byproduct Rule (Stage 1 DBPR) 63 FR 69390. U.S. Environmental Protection Agency, 1998; 63, No. (241), .
- Guidelines for Drinking Water Quality, 4th ed. (incorporating the first and second addenda); World Health Organization: Geneva, 2022. [PubMed] [Google Scholar]
- Kurokawa Y.; Aoki S.; Matsushima Y.; Takamura N.; Imazawa T.; Hayashi Y. Dose-response studies on the carcinogenicity of potassium bromate in F344 rats after long-term oral administration. J. Natl. Cancer Inst. 1986, 77, 977–982. [PubMed] [Google Scholar]
- Kurokawa Y; Takayama S; Konishi Y; Hiasa Y; Asahina S; Takahashi M; Maekawa A; Hayashi Y Long-term in vivo carcinogenicity tests of potassium bromate, sodium hypochlorite, and sodium chlorite conducted in Japan. Environ. Health Perspect. 1986, 69, 221–235. 10.1289/ehp.8669221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelo A. B.; George M. H.; Kilburn S. R.; Moore T. M.; Wolf D. C. Carcinogenicity of potassium bromate administered in the drinking water to male B6C3F1 mice and F344/N rats. Toxicol. Pathol. 1998, 26, 587–594. 10.1177/019262339802600501. [DOI] [PubMed] [Google Scholar]
- Health Risk Assessment/Characterization of the Drinking Water Disinfection Byproduct Bromate. U.S. Environmental Protection Agency, 1998.
- Bromate in Drinking Water; World Health Organization: Geneva, 2003. [Google Scholar]
- Bull R. J.; Cotruvo J. A. Nongenotoxic mechanisms involved in bromate-induced cancer in rats. J. Am. Water Works Assoc 2013, 105, E709–E720. 10.5942/jawwa.2013.105.0155. [DOI] [Google Scholar]
- Kolisetty N.; Delker D. A.; Muralidhara S.; Bull R. J.; Cotruvo J. A.; Fisher J. W.; Cummings B. S. Changes in mRNA and protein expression in the renal cortex of male and female F344 rats treated with bromate. Arch. Toxicol. 2013, 87, 1911–1925. 10.1007/s00204-013-1052-2. [DOI] [PubMed] [Google Scholar]
- Cotruvo J. A.; Keith J. D.; Bull R. J.; Pacey G. E.; Gordon G. Bromate reduction in simulated gastric juice. J. Am. Water Works Assoc. 2010, 102, 77–86. 10.1002/j.1551-8833.2010.tb11341.x. [DOI] [Google Scholar]
- Hutchinson T. H.; Hutchings M. J.; Moore K. W. A review of the effects of bromate on aquatic organisms and toxicity of bromate to oyster (Crassostrea gigas) embryos. Ecotoxicol. Environ. Saf. 1997, 38, 238–243. 10.1006/eesa.1997.1584. [DOI] [PubMed] [Google Scholar]
- Butler R.; Godley A.; Lytton L.; Cartmell E. Bromate environmental contamination: Review of impact and possible treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 193–217. 10.1080/10643380590917888. [DOI] [Google Scholar]
- Santiago S.Study to support the derivation of environmental quality standard for bromate ecotoxicity of sodium bromate on reproduction of Ceriodaphnia dubia and on other freshwater organisms. 2015. https://www.ecotoxcentre.ch/projects/risk-assessment/ecotoxicological-assessment-of-bromate (accessed 2022-08-15).
- Ecotox Centre of Switzerland. Proposals for Quality Criteria for Surface Waters. 2015. https://www.ecotoxcentre.ch/expert-service/quality-criteria/quality-criteria-for-surface-waters (accessed 2022-08-15).
- Flury M.; Papritz A. Bromide in the Natural Environment: Occurrence and Toxicity. J. Environ. Qual 1993, 22, 747–758. 10.2134/jeq1993.00472425002200040017x. [DOI] [Google Scholar]
- von Gunten U.; Hoigné J. Factors controlling the formation of bromate during ozonation of bromide-containing waters. J. Water Supply: Res. Technol.—AQUA 1992, 41, 299–304. [Google Scholar]
- Winid B. Bromine and water quality- Selected aspects and future perspectives. Appl. Geochem. 2015, 63, 413–435. 10.1016/j.apgeochem.2015.10.004. [DOI] [Google Scholar]
- Mctigue N. E.; Cornwell D. A.; Graf K.; Brown R. Occurrence and consequences of increased bromide in drinking water sources. J. Am. Water Works Assoc 2014, 106, E492–E508. 10.5942/jawwa.2014.106.0141. [DOI] [Google Scholar]
- Harkness J. S.; Dwyer G. S.; Warner N. R.; Parker K. M.; Mitch W. A.; Vengosh A. Iodide, bromide, and ammonium in hydraulic fracturing and oil and gas wastewaters: Environmental implications. Environ. Sci. Technol. 2015, 49, 1955–1963. 10.1021/es504654n. [DOI] [PubMed] [Google Scholar]
- Soltermann F.; Abegglen C.; Götz C.; von Gunten U. Bromide Sources and Loads in Swiss Surface Waters and Their Relevance for Bromate Formation during Wastewater Ozonation. Environ. Sci. Technol. 2016, 50 (18), 9825–9834. 10.1021/acs.est.6b01142. [DOI] [PubMed] [Google Scholar]
- Westerhoff P.Occurrence Survey of Bromide and Iodide in Water Supplies. Water Research Foundation Project 4711. 2022. https://www.waterrf.org/research/projects/occurrence-survey-bromide-and-iodide-water-supplies (accessed 2022-08-15). [Google Scholar]
- Obolensky A.; Singer P. C.; Shukairy H. M. Information Collection Rule Data Evaluation and Analysis to Support Impacts on Disinfection By-Product Formation. J. Environ. Eng. 2007, 133, 53–63. 10.1061/(ASCE)0733-9372(2007)133:1(53). [DOI] [Google Scholar]
- Siddiqui M. S.; Amy G. L. Factors Affecting DBP Formation During Ozone-Bromide Reactions. J. Am. Water Works Assoc. 1993, 85, 63–72. 10.1002/j.1551-8833.1993.tb05922.x. [DOI] [Google Scholar]
- Ozekin K.; Amy G. L. Threshold Levels for Bromate Formation in Drinking Water. Ozone Sci. Eng. 1997, 19, 323–337. 10.1080/01919519708547296. [DOI] [Google Scholar]
- Regli S.; Chen J.; Messner M.; Elovitz M. S.; Letkiewicz F. J.; Pegram R. A.; Pepping T. J.; Richardson S. D.; Wright J. M. Estimating Potential Increased Bladder Cancer Risk Due to Increased Bromide Concentrations in Sources of Disinfected Drinking Waters. Environ. Sci. Technol. 2015, 49, 13094–13102. 10.1021/acs.est.5b03547. [DOI] [PubMed] [Google Scholar]
- Amy G.; Siddiqui M.; Zhai W.; Debroux J.; Odem W.. Survey of bromide in drinking water and impacts on DBP formation. Water Research Foundation Project 825; 1994. https://www.waterrf.org/research/projects/survey-bromide-drinking-water-and-impacts-dbp-formation.
- Lundström U.; Olin Å. Bromide concentration in Swedish precipitation, surface and ground waters. Water Res. 1986, 20, 751–756. 10.1016/0043-1354(86)90099-0. [DOI] [Google Scholar]
- Magazinovic R. S.; Nicholson B. C.; Mulcahy D. E.; Davey D. E. Bromide levels in natural waters: its relationship to levels of both chloride and total dissolved solids and the implications for water treatment. Chemosphere 2004, 57, 329–335. 10.1016/j.chemosphere.2004.04.056. [DOI] [PubMed] [Google Scholar]
- Davis S. N.; Fabryka-Martin J. T.; Wolfsberg L. E. Variations of bromide in potable ground water in the United States. Ground Water 2004, 42, 902–909. 10.1111/j.1745-6584.2004.t01-8-.x. [DOI] [PubMed] [Google Scholar]
- von Gunten U.; Salhi E. Bromate in drinking water: A problem in Switzerland?. Ozone Sci. Eng. 2003, 25, 159–166. 10.1080/01919510390481487. [DOI] [Google Scholar]
- Wagner H. P.; Pepich B. V.; Hautman D. P.; Munch D. J. Performance evaluation of a method for the determination of bromate in drinking water by ion chromatography (EPA Method 317.0) and validation of EPA Method 324.0. J. Chromatogr. A 2000, 884, 201–210. 10.1016/S0021-9673(99)01277-7. [DOI] [PubMed] [Google Scholar]
- Wagner H. P.; Pepich B. V.; Hautman D. P.; Munch D. J.. US EPA Method 317: Determination of inorganic oxyhalide disinfection by-products in drinking water using ion chromatography with the addition of a postcolumn reagent for trace bromate analysis. 2001. [DOI] [PubMed]
- Wagner H. P.; Pepich B. V.; Hautman D. O.; Munch D. J.; Salhi E.; von Gunten U.. US EPA Method 326.0 Determination of Inorganic Oxyhalide Disinfection By-Products in Drinking Water Using Ion Chromatography Incorporating the Addition of a Suppressor Acidified Postcolumn Reagent for Trace Bromate Analysis Revision 1.0. 2002.
- Wagner H. P.; Pepich B. V.; Pohl C.; Srinivasan K.; De Borba B.; Lin R.; Munch D. J.. US EPA Method 302.0 : Determination of Bromate in Drinking Water Using Two-Dimensional Ion Chromatography With Suppressed Conductivity Detection. 2009.
- Pfaff J.US EPA Method 300.1. Revision 1.0: Determination of Inorganic Anions in Drinking Water by Ion Chromatography. 1993.
- Salhi E.; von Gunten U. Simultaneous determination of bromide, bromate and nitrite in low ug/L levels by ion chromatography without sample pretreatment. Water Res. 1999, 33, 3239–3244. 10.1016/S0043-1354(99)00053-6. [DOI] [Google Scholar]
- Weinberg H. S.; Yamada H. Post-Ion-Chromatography Derivatization for the Determination of Oxyhalides at Sub-PPB Levels in Drinking Water. Anal. Chem. 1998, 70, 1–6. 10.1021/ac970651m. [DOI] [PubMed] [Google Scholar]
- Creed J. T.; Brockhoff C. A.; Martin T. D.. US EPA Method 321.8: Determination of bromate in drinking waters by ion chromatography inductively coupled plasma-mass spectrometry. 1997.
- Snyder S. A.; Vanderford B. J.; Rexing D. J. Trace analysis of bromate, chlorate, iodate, and perchlorate in natural and bottled waters. Environ. Sci. Technol. 2005, 39, 4586–4593. 10.1021/es047935q. [DOI] [PubMed] [Google Scholar]
- Shah A. D.; Liu Z. Q.; Salhi E.; Hofer T.; Werschkun B.; von Gunten U. Formation of disinfection by-products during ballast water treatment with ozone, chlorine, and peracetic acid: influence of water quality parameters. Environ. Sci. Water Res. Technol. 2015, 1, 465–480. 10.1039/C5EW00061K. [DOI] [Google Scholar]
- Young T. R.; Cheng S.; Li W.; Dodd M. C. Rapid, high-sensitivity analysis of oxyhalides by non-suppressed ion chromatography-electrospray ionization-mass spectrometry: application to ClO4–, ClO3–, ClO2–, and BrO3– quantification during sunlight/chlorine advanced oxidation. Environ. Sci. Water Res. Technol. 2020, 6, 2580–2596. 10.1039/D0EW00429D. [DOI] [Google Scholar]
- Zaffiro A. D.; Pepich B. V.; Slingsby R. W.; Pohl J.; Pohl C. A.; Munch D. J.. US EPA Method 557: Determination of Haloacetic Acids, Bromate, and Dalapon in Drinking Water by Ion Chromatography Electrospray Ionization Tandem Mass Spectrometry (IC-ESI-MS/MS). 2009.
- Ohtomo T.; Yatabe R.; Tanaka Y.; Kato J.; Igarashi S. Fluorescence Detection-FIA for ppb Levels of Bromate with Trifluoperazine. J. Flow Injection Anal. 2009, 26, 127–131. [Google Scholar]
- Fujioka T.; Boivin S.; Takeuchi H. Online monitoring of bromate in treated wastewater: implications for potable water reuse. Environ. Sci.: Water Res. Technol. 2022, 8, 2034–2039. 10.1039/D1EW00634G. [DOI] [Google Scholar]
- Pinkernell U.; von Gunten U. Bromate minimization during ozonation: Mechanistic considerations. Environ. Sci. Technol. 2001, 35, 2525–2531. 10.1021/es001502f. [DOI] [PubMed] [Google Scholar]
- Taube H. Reactions in Solutions Containing O3, H2O2, H+ and Br–. The Specific Rate of the Reaction O3 + Br–. J. Am. Chem. Soc. 1942, 64, 2468–2474. 10.1021/ja01262a072. [DOI] [Google Scholar]
- Haag W. R.; Hoigné J. Ozonation of Bromide-Containing Waters: Kinetics of Formation of Hypobromous Acid and Bromate. Environ. Sci. Technol. 1983, 17, 261–267. 10.1021/es00111a004. [DOI] [Google Scholar]
- Liu Q.; Schurter L. M.; Muller C. E.; Aloisio S.; Francisco J. S.; Margerum D. W. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. Inorg. Chem. 2001, 40, 4436–4442. 10.1021/ic000919j. [DOI] [PubMed] [Google Scholar]
- Heeb M. B.; Criquet J.; Zimmermann-Steffens S. G.; von Gunten U. Oxidative treatment of bromide-containing waters: Formation of bromine and its reactions with inorganic and organic compounds - A critical review. Water Res. 2014, 48, 15–42. 10.1016/j.watres.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Haag W. R.; Hoigné J. Ozonation of water containing chlorine or chloramines. Reaction products and kinetics. Water Res. 1983, 17, 1397–1402. 10.1016/0043-1354(83)90270-1. [DOI] [Google Scholar]
- Reisz E.; Leitzke A.; Jarocki A.; Irmscher R.; von Sonntag C. Permanganate formation in the reactions of ozone with Mn(II): a mechanistic study. J. Water Supply Res. Technol. 2008, 57, 451–464. 10.2166/aqua.2008.091. [DOI] [Google Scholar]
- Odeh I. N.; Nicoson J. S.; Huff Hartz K. E.; Margerum D. W. Kinetics and mechanisms of bromine chloride reactions with bromite and chlorite ions. Inorg. Chem. 2004, 43, 7412–7420. 10.1021/ic048982m. [DOI] [PubMed] [Google Scholar]
- Fischbacher A.; Löppenberg K.; von Sonntag C.; Schmidt T. C. A New Reaction Pathway for Bromite to Bromate in the Ozonation of Bromide. Environ. Sci. Technol. 2015, 49, 11714–11720. 10.1021/acs.est.5b02634. [DOI] [PubMed] [Google Scholar]
- von Gunten U.; Hoigné J. Bromate formation during ozonation of bromide-containing waters: Interaction of Ozone and Hydroxyl Radical Reactions. Environ. Sci. Technol. 1994, 28, 1234–1242. 10.1021/es00056a009. [DOI] [PubMed] [Google Scholar]
- Klaning U.; Wolff T. Laser Flash Photolysis of HClO, ClO–, HBrO, and BrO– in Aqueous Solution. Reactions of Cl– and Br– Atoms. Ber. Bunsen-Ges. 1985, 89, 243–245. 10.1002/bbpc.19850890309. [DOI] [Google Scholar]
- Zehavi D.; Rabani J. The oxidation of aqueous bromide ions by hydroxyl radicals. A pulse radiolytic investigation. J. Phys. Chem. 1972, 76, 312–319. 10.1021/j100647a006. [DOI] [Google Scholar]
- von Gunten U.; Hoigné J.. Ozonation of bromide-containing waters: Bromate formation through ozone and hydroxyl radicals. In Disinfection By-Products in Water Treatment; Minear R. A., Amy G. L., Eds.; CRC Press: Boca Raton, FL, 1996; pp 187–206. [Google Scholar]
- Symons J. M.; Zheng M. C. H. H. Technical Note: Does hydroxyl radical oxidize bromide to bromate?. J. Am. Water Works Assoc 1997, 89, 106–109. 10.1002/j.1551-8833.1997.tb08246.x. [DOI] [Google Scholar]
- von Gunten U.; Oliveras Y. Advanced Oxidation of Bromide-Containing Waters: Bromate Formation Mechanisms. Environ. Sci. Technol. 1998, 32, 63–70. 10.1021/es970477j. [DOI] [Google Scholar]
- von Gunten U.; Oliveras Y. Kinetics of the reaction between hydrogen peroxide and hypobromous acid: Implication on water treatment and natural systems. Water Res. 1997, 31, 900–906. 10.1016/S0043-1354(96)00368-5. [DOI] [Google Scholar]
- Buxton G. V.; Dainton F. Radical and Molecular Yields in the γ-Radiolysis of Water. V. The Sodium Hypobromite System. Proc. R. Soc. A 1968, 304, 441–447. 10.1098/rspa.1968.0096. [DOI] [Google Scholar]
- Canonica S.; Kohn T.; Mac M.; Real F. J.; Wirz J.; von Gunten U. Photosensitizer method to determine rate constants for the reaction of carbonate radical with organic compounds. Environ. Sci. Technol. 2005, 39, 9182–9188. 10.1021/es051236b. [DOI] [PubMed] [Google Scholar]
- Field R. J.; Raghavan N. V.; Brummer J. G. A pulse radiolysis investigation of the reactions of BrO2· with Fe(CN)64-, Mn(II), phenoxide ion, and phenol. J. Phys. Chem. 1982, 86, 2443–2449. 10.1021/j100210a040. [DOI] [Google Scholar]
- Lei Y.; Lei X.; Westerhoff P.; Tong X.; Ren J.; Zhou Y.; Cheng S.; Ouyang G.; Yang X. Bromine Radical (Br• and Br2•-) Reactivity with Dissolved Organic Matter and Brominated Organic Byproduct Formation. Environ. Sci. Technol. 2022, 56, 5189–5199. 10.1021/acs.est.2c00549. [DOI] [PubMed] [Google Scholar]
- Lim S.; Barrios B.; Minakata D.; von Gunten U. Reactivity of Bromine Radical with Dissolved Organic Matter Moieties and Monochloramine: Effect on Bromate Formation during Ozonation.. Environ. Sci. Technol. 2023, 10.1021/acs.est.2c07694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y.; Lei X.; Yu Y.; Li K.; Li Z.; Cheng S.; Ouyang G.; Yang X. Rate Constants and Mechanisms for Reactions of Bromine Radicals with Trace Organic Contaminants. Environ. Sci. Technol. 2021, 55, 10502–10513. 10.1021/acs.est.1c02313. [DOI] [PubMed] [Google Scholar]
- Song R.Ozone-Bromide-NOM interactions in water treatment. Ph.D. Dissertation, University of Illinois at Urbana-Champaign, Urbana, IL, 1996. [Google Scholar]
- von Gunten U.; Bruchet A.; Costentin E. Bromate formation in advanced oxidation processes. J. Am. Water Works Assoc 1996, 88, 53–65. 10.1002/j.1551-8833.1996.tb06571.x. [DOI] [Google Scholar]
- Westerhoff P.; Song R.; Amy G.; Minear R. Numerical Kinetic Models for Bromide Oxidation To Bromine And Bromate. Water Res. 1998, 32, 1687–1699. 10.1016/S0043-1354(97)00287-X. [DOI] [Google Scholar]
- Westerhoff P.; Song R.; Amy G.; Minear R. NOM’s role in bromine and bromate formation during ozonation. J. Am. Water Works Assoc 1998, 90, 82–94. 10.1002/j.1551-8833.1998.tb08380.x. [DOI] [Google Scholar]
- Song R.; Westerhoff P.; Minear R.; Amy G. Bromate minimization during ozonation. J. Am. Water Works Assoc 1997, 89, 69–78. 10.1002/j.1551-8833.1997.tb08243.x. [DOI] [Google Scholar]
- Elovitz M. S.; von Gunten U.; Kaiser H. P. Hydroxyl radical/ozone ratios during ozonation processes. II. The effect of temperature, pH, alkalinity, and DOM properties. Ozone Sci. Eng. 2000, 22, 123–150. 10.1080/01919510008547216. [DOI] [Google Scholar]
- Elovitz M. S.; von Gunten U.; Kaiser H.-P.. The Influence of Dissolved Organic Matter Character on Ozone Decomposition Rates and Rct. Natural Organic Matter and Disinfection By-Products; American Chemical Society: Washington, DC, 2000; pp 248–269. 10.1021/bk-2000-0761.ch016 [DOI] [Google Scholar]
- Elovitz M. S.; von Gunten U. Hydroxyl radical/ozone ratios during ozonation processes. I. The Rct concept. Ozone Sci. Eng. 1999, 21, 239–260. 10.1080/01919519908547239. [DOI] [Google Scholar]
- Yong E. L.; Lin Y. P. Incorporation of initiation, promotion and inhibition in the Rct concept and its application in determining the initiation and inhibition capacities of natural water in ozonation. Water Res. 2012, 46, 1990–1998. 10.1016/j.watres.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Gulde R.; Clerc B.; Rutsch M.; Helbing J.; Salhi E.; McArdell C. S.; von Gunten U. Oxidation of 51 micropollutants during drinking water ozonation: Formation of transformation products and their fate during biological post-filtration. Water Res. 2021, 207, 117812. 10.1016/j.watres.2021.117812. [DOI] [PubMed] [Google Scholar]
- Gulde R.; Rutsch M.; Clerc B.; Schollee J.; von Gunten U.; McArdell C. S. Formation of transformation products during ozonation of secondary wastewater effluent and their fate in post-treatment: From laboratory- to full-scale. Water Res. 2021, 200, 117200. 10.1016/j.watres.2021.117200. [DOI] [PubMed] [Google Scholar]
- Buffle M.-O.; Schumacher J.; Meylan S.; Jekel M.; von Gunten U. Ozonation and Advanced Oxidation of Wastewater: Effect of O3 Dose, pH, DOM and HO• Scavengers on Ozone Decomposition and HO• Generation. Ozone Sci. Eng. 2006, 28, 247–259. 10.1080/01919510600718825. [DOI] [Google Scholar]
- Morrison C. M.; Hogard S.; Pearce R.; Gerrity D.; von Gunten U.; Wert E. C. Ozone disinfection of waterborne pathogens and their surrogates: A critical review. Water Res. 2022, 214, 118206. 10.1016/j.watres.2022.118206. [DOI] [PubMed] [Google Scholar]
- Kaiser H. P.; Koster O.; Gresch M.; Perisset P. M. J.; Jaggi P.; Salhi E.; von Gunten U. Process Control for Ozonation Systems: A Novel Real-Time Approach. Ozone Sci. Eng. 2013, 35, 168–185. 10.1080/01919512.2013.772007. [DOI] [Google Scholar]
- Dickenson E. R. V.; Summers R. S.; Croué J. P.; Gallard H. Haloacetic acid and trihalomethane formation from the chlorination and bromination of aliphatic β-Dicarbonyl acid model compounds. Environ. Sci. Technol. 2008, 42, 3226–3233. 10.1021/es0711866. [DOI] [PubMed] [Google Scholar]
- Heeb M. B.; Kristiana I.; Trogolo D.; Arey J. S.; von Gunten U. Formation and reactivity of inorganic and organic chloramines and bromamines during oxidative water treatment. Water Res. 2017, 110, 91–101. 10.1016/j.watres.2016.11.065. [DOI] [PubMed] [Google Scholar]
- Houska J.; Salhi E.; Walpen N.; von Gunten U. Oxidant-reactive carbonous moieties in dissolved organic matter: Selective quantification by oxidative titration using chlorine dioxide and ozone. Water Res. 2021, 207, 117790. 10.1016/j.watres.2021.117790. [DOI] [PubMed] [Google Scholar]
- Buxton G. V.; Elliot A. J. Rate constant for reaction of hydroxyl radicals with bicarbonate ions. Int. J. Radiat. Appl. Instrumentation. Part 1986, 27, 241–243. 10.1016/1359-0197(86)90059-7. [DOI] [Google Scholar]
- Buxton G. V.; Greenstock C. L.; Helman W. P.; Ross A. B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals in Aqueous Solutions. J. Phys. Chem. Ref. Data 1988, 17, 513–5486. 10.1063/1.555805. [DOI] [Google Scholar]
- Matthew B. M.; Anastasio C. A chemical probe technique for the determination of reactive halogen species in aqueous solution: Part 1 - Bromide solutions. Atmos. Chem. Phys. 2006, 6, 2423–2437. 10.5194/acp-6-2423-2006. [DOI] [Google Scholar]
- Ianni J. C.Kintecus, ver. 6.51; 2018.
- Staehelin J.; Hoigné J. Decomposition of Ozone in Water in the Presence of Organic Solutes Acting as Promoters and Inhibitors of Radical Chain Reactions. Environ. Sci. Technol. 1985, 19, 1206–1213. 10.1021/es00142a012. [DOI] [PubMed] [Google Scholar]
- Tomiyasu H.; Fukutomi H.; Gordon G. Kinetics and Mechanism of Ozone Decomposition in Basic Aqueous Solution. Inorg. Chem. 1985, 24, 2962–2966. 10.1021/ic00213a018. [DOI] [Google Scholar]
- Westerhoff P.; Song R.; Amy G.; Minear R. Applications of ozone decomposition models. Ozone Sci. Eng. 1997, 19, 55–73. 10.1080/01919519708547318. [DOI] [Google Scholar]
- Westerhoff P.; Song R.; Amy G.; Minear R. Numerical kinetic models for bromide oxidation to bromine and bromate. Water Res. 1998, 32, 1687–1699. 10.1016/S0043-1354(97)00287-X. [DOI] [Google Scholar]
- Li W. T.; Cao M. J.; Young T.; Ruffino B.; Dodd M.; Li A.-M.; Korshin G. Application of UV absorbance and fluorescence indicators to assess the formation of biodegradable dissolved organic carbon and bromate during ozonation. Water Res. 2017, 111, 154–162. 10.1016/j.watres.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Soltermann F.; Abegglen C.; Tschui M.; Stahel S.; von Gunten U. Options and limitations for bromate control during ozonation of wastewater. Water Res. 2017, 116, 76–85. 10.1016/j.watres.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Siddiqui M.; Amy G.; Ozekin K.; Westerhoff P. Empirically and Theoretically-Based Models for Predicting Brominated Ozonated by-Products. Ozone: Sci. Eng. 1994, 16, 157–178. 10.1080/01919519408552419. [DOI] [Google Scholar]
- Aljundi I. H. Bromate formation during ozonation of drinking water: A response surface methodology study. Desalination 2011, 277, 24–28. 10.1016/j.desal.2011.03.090. [DOI] [Google Scholar]
- Moslemi M.; Davies S. H.; Masten S. J. Empirical modeling of bromate formation during drinking water treatment using hybrid ozonation membrane filtration. Desalination 2012, 292, 113–118. 10.1016/j.desal.2012.02.015. [DOI] [Google Scholar]
- Song R.; Donohoe C.; Minear R.; Westerhoff P.; Ozekin K.; Amy G. Empirical Modeling of Bromate Formation During Ozonation of Bromide-Containing Waters. Water Res. 1996, 30, 1161–1168. 10.1016/0043-1354(95)00302-9. [DOI] [Google Scholar]
- Galey C.; Sohn J.; Amy G.; Cavard J.. Modeling Bromate Formation at the Full Scale: A Comparison of Three Ozonation Plants. Amererican Water Works Association Water Quality and Technology Conference. 1997.
- Sohn J.; Amy G.; Cho J.; Lee Y.; Yoon Y. Disinfectant decay and disinfection by-products formation model development: Chlorination and ozonation by-products. Water Res. 2004, 38, 2461–2478. 10.1016/j.watres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Legube B.; Parinet B.; Gelinet K.; Berne F.; Croue J. P. Modeling of bromate formation by ozonation of surface waters in drinking water treatment. Water Res. 2004, 38, 2185–2195. 10.1016/j.watres.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Van Der Helm A. W. C.; Smeets P. W. M. H.; Baars E. T.; Rietveld L. C.; Van Dijk J. C. Modeling of ozonation for dissolved ozone dosing. Ozone Sci. Eng. 2007, 29, 379–389. 10.1080/01919510701573400. [DOI] [Google Scholar]
- Mizuno T.; Tsuno H.; Yamada H. A simple model to predict formation of bromate ion and hypobromous acid/hypobromite ion through hydroxyl radical pathway during ozonation. Ozone Sci. Eng. 2007, 29, 3–11. 10.1080/01919510601093806. [DOI] [Google Scholar]
- Jarvis P.; Parsons S. A.; Smith R. Modeling bromate formation during ozonation. Ozone Sci. Eng. 2007, 29, 429–442. 10.1080/01919510701643732. [DOI] [Google Scholar]
- Civelekoglu G.; Yigit N. O.; Diamadopoulos E.; Kitis M. Prediction of bromate formation using multi-linear regression and artificial neural networks. Ozone Sci. Eng. 2007, 29, 353–362. 10.1080/01919510701549327. [DOI] [Google Scholar]
- Kimbrough D. E.; Suffet I. H. Electrochemical removal of bromide and reduction of THM formation potential in drinking water. Water Res. 2002, 36, 4902–4906. 10.1016/S0043-1354(02)00210-5. [DOI] [PubMed] [Google Scholar]
- Kimbrough D. E.; Suffet I. H. Electrochemical process for the removal of bromide from California state project water. J. Water Supply Res. Technol.—AQUA 2006, 55, 161–167. 10.2166/aqua.2006.0002. [DOI] [Google Scholar]
- von Gunten U. Oxidation Processes in Water Treatment: Are We on Track?. Environ. Sci. Technol. 2018, 52, 5062–5075. 10.1021/acs.est.8b00586. [DOI] [PubMed] [Google Scholar]
- Haag W. R.; Hoigné J.; Bader H. Improved ammonia oxidation by ozone in the presence of bromide ion during water treatment. Water Res. 1984, 18, 1125–1128. 10.1016/0043-1354(84)90227-6. [DOI] [Google Scholar]
- Hsu S.; Singer P. C. Removal of bromide and natural organic matter by anion exchange. Water Res. 2010, 44, 2133–2140. 10.1016/j.watres.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Singer P. C.; Schneider M.; Edwards-Brandt J.; Budd G. C. MIEX for removal of DBP precursors: Pilot-plant findings. J. Am. Water Work. Assoc. 2007, 99, 128–139. 10.1002/j.1551-8833.2007.tb07913.x. [DOI] [Google Scholar]
- Johnson C. J.; Singer P. C. Impact of a magnetic ion exchange resin on ozone demand and bromate formation during drinking water treatment. Water Res. 2004, 38, 3738–3750. 10.1016/j.watres.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Wert E. C.; Edwards-Brandt J. C.; Singer P. C.; Budd G. C. Evaluating magnetic ion exchange resin (MIEX)® pretreatment to increase ozone disinfection and reduce bromate formation. Ozone Sci. Eng. 2005, 27, 371–379. 10.1080/01919510500250754. [DOI] [Google Scholar]
- Sun Y.; Zhou S.; Chiang P.-C.; Shah K. J. Evaluation and optimization of enhanced coagulation process: Water and energy nexus. Water-Energy Nexus 2019, 2, 25–36. 10.1016/j.wen.2020.01.001. [DOI] [Google Scholar]
- Wert E. C.; Gonzales S.; Dong M. M.; Rosario-Ortiz F. L. Evaluation of enhanced coagulation pretreatment to improve ozone oxidation efficiency in wastewater. Water Res. 2011, 45, 5191–5199. 10.1016/j.watres.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Wert E. C.; Lew J.; Rakness K. L. Effect of ozone dissolution systems on ozone exposure and bromate formation. J. Am. Water Works Assoc. 2017, 109, E302–E312. 10.5942/jawwa.2017.109.0048. [DOI] [Google Scholar]
- Rakness K. L.; Hunter G.; Lew J.; Mundy B.; Wert E. C. Design Considerations for Cost-Effective Ozone Mass Transfer in Sidestream Systems. Ozone Sci. Eng. 2018, 40, 159–172. 10.1080/01919512.2018.1424532. [DOI] [Google Scholar]
- Liang S.; Yun T. I.; Krasner S. W.; Yates R. S. Evaluation of Emerging Bromate Control Strategies. Water Pract. Technol. 2010, 5 (3), wpt2010064. 10.2166/wpt.2010.064. [DOI] [Google Scholar]
- Williams M. D.; Coffey B. M.; Krasner S. W. Evaluation of pH and ammonia for controlling bromate during Cryptosporidium disinfection. J. Am. Water Works Assoc. 2003, 95, 82–93. 10.1002/j.1551-8833.2003.tb10476.x. [DOI] [Google Scholar]
- Wajon J. E.; Morris J. C. Rates of Formation of N-Bromo Amines in Aqueous Solution. Inorg. Chem. 1982, 21, 4258–4263. 10.1021/ic00142a030. [DOI] [Google Scholar]
- Haag W. R.; Hoigné J. Kinetics and products of the reactions of ozone with various forms of chlorine and bromine in water. Ozone Sci. Eng. 1984, 6, 103–114. 10.1080/01919518408551009. [DOI] [Google Scholar]
- Ikehata K.; Wang L.; Nessl M. B.; Komor A. T.; Cooper W. J.; McVicker R. R. Effect of Ammonia and Chloramine Pretreatment during the Ozonation of a Colored Groundwater with Elevated Bromide. Ozone Sci. Eng. 2013, 35, 438–447. 10.1080/01919512.2013.815105. [DOI] [Google Scholar]
- Wert E. C.; Neemann J. J.; Johnson D.; Rexing D.; Zegers R. Pilot-scale and full-scale evaluation of the chlorine-ammonia process for bromate control during ozonation. Ozone Sci. Eng. 2007, 29, 363–372. 10.1080/01919510701552883. [DOI] [Google Scholar]
- Buffle M.-O.; Galli S.; von Gunten U. Enhanced Bromate Control during Ozonation: The Chlorine-Ammonia Process. Environ. Sci. Technol. 2004, 38, 5187–5195. 10.1021/es0352146. [DOI] [PubMed] [Google Scholar]
- Gleason J. M.; McKay G.; Ishida K. P.; Mezyk S. P. Temperature dependence of hydroxyl radical reactions with chloramine species in aqueous solution. Chemosphere 2017, 187, 123–129. 10.1016/j.chemosphere.2017.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskrebyshev G. A.; Huie R. E.; Neta P. Radiolytic Reactions of Monochloramine in Aqueous Solutions. J. Phys. Chem. A 2003, 107, 7423–7428. 10.1021/jp030198k. [DOI] [Google Scholar]
- Gazda M.; Margerum D. W. Reactions of Monochloramine with Br2, Br3–, HOBr, OBr–: Formation of Bromochloramines. Inorg. Chem. 1994, 33, 118–123. 10.1021/ic00079a022. [DOI] [Google Scholar]
- Pearce R.; Hogard S.; Buehlmann P.; Salazar-Benites G.; Wilson C.; Bott C. Evaluation of preformed monochloramine for bromate control in ozonation for potable reuse. Water Res. 2022, 211, 118049. 10.1016/j.watres.2022.118049. [DOI] [PubMed] [Google Scholar]
- Luh J.; Mariñas B. J. Kinetics of bromochloramine formation and decomposition. Environ. Sci. Technol. 2014, 48, 2843–2852. 10.1021/es4036754. [DOI] [PubMed] [Google Scholar]
- Staehelin J.; Hoigné J. Decomposition of Ozone in Water: Rate of Initiation by Hydroxide Ions and Hydrogen Peroxide. Environ. Sci. Technol. 1982, 16, 676–681. 10.1021/es00104a009. [DOI] [Google Scholar]
- Merle T.; Pronk W.; von Gunten U. MEMBRO3X, a novel combination of a membrane contactor with advanced oxidation (O3/H2O2) for simultaneous micropollutant abatement and bromate minimization. Environ. Sci. Technol. Lett. 2017, 4, 180–185. 10.1021/acs.estlett.7b00061. [DOI] [Google Scholar]
- Yu J.; Wang Y.; Wang Q.; Wang Z.; Zhang D.; Yang M. Implications of bromate depression from H2O2 addition during ozonation of different bromide-bearing source waters. Chemosphere 2020, 252, 126596. 10.1016/j.chemosphere.2020.126596. [DOI] [PubMed] [Google Scholar]
- Hübner U.; Zucker I.; Jekel M. Options and limitations of hydrogen peroxide addition to enhance radical formation during ozonation of secondary effluents. J. Water Reuse Desalin. 2015, 5, 8–16. 10.2166/wrd.2014.036. [DOI] [Google Scholar]
- Lee Y.; Gerrity D.; Lee M.; Gamage S.; Pisarenko A.; Trenholm R. A.; Canonica S.; Snyder S. A.; von Gunten U. Organic Contaminant Abatement in Reclaimed Water by UV/H2O2 and a Combined Process consisting of O3/H2O2 followed by UV/H2O2: Prediction of abatement efficiency, energy consumption, and byproduct formation. Environ. Sci. Technol. 2016, 50 (7), 3809–3819. 10.1021/acs.est.5b04904. [DOI] [PubMed] [Google Scholar]
- Gamage S.; Gerrity D.; Pisarenko A. N.; Wert E. C.; Snyder S. A. Evaluation of Process Control Alternatives for the Inactivation of Escherichia coli, MS2 Bacteriophage, and Bacillus subtilis Spores during Wastewater Ozonation. Ozone Sci. Eng. 2013, 35, 501–513. 10.1080/01919512.2013.833852. [DOI] [Google Scholar]
- Wolf C.; von Gunten U.; Kohn T. Kinetics of Inactivation of Waterborne Enteric Viruses by Ozone. Environ. Sci. Technol. 2018, 52, 2170–2177. 10.1021/acs.est.7b05111. [DOI] [PubMed] [Google Scholar]
- Bowman R. H.HiPOx Advanced Oxidation of TBA and MTBE in Groundwater. In Contaminated Soils, Sediments, and Water: Science in the Real World; Calabrese E. J., Kostecki P. T., Dragun J., Eds.; Springer US, 2005; pp 299–213. 10.1007/0-387-23079-3_19 [DOI] [Google Scholar]
- Bourgin M.; Borowska E.; Helbing J.; Hollender J.; Kaiser H.-P.; Kienle C.; McArdell C. S.; Simon E.; von Gunten U. Effect of operational and water quality parameters on conventional ozonation and the advanced oxidation process O3/H2O2: Kinetics of micropollutant abatement, transformation product and bromate formation in a surface water. Water Res. 2017, 122, 234–245. 10.1016/j.watres.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Lekkerkerker-Teunissen K.; Knol A. H.; van Altena L. P.; Houtman C. J.; Verberk J. Q. J. C.; van Dijk J. C. Serial ozone/peroxide/low pressure UV treatment for synergistic and effective organic micropollutant conversion. Sep. Purif. Technol. 2012, 100, 22–29. 10.1016/j.seppur.2012.08.030. [DOI] [Google Scholar]
- Antoniou M. G.; Sichel C.; Andre K.; Andersen H. R. Novel pre-treatments to control bromate formation during ozonation. J. Hazard. Mater. 2017, 323, 452–459. 10.1016/j.jhazmat.2016.03.041. [DOI] [PubMed] [Google Scholar]
- Legube B. Catalytic ozonation: a promising advanced oxidation technology for water treatment. Catal. Today 1999, 53, 61–72. 10.1016/S0920-5861(99)00103-0. [DOI] [Google Scholar]
- Li W.; Lu X.; Xu K.; Qu J.; Qiang Z. Cerium incorporated MCM-48 (Ce-MCM-48) as a catalyst to inhibit bromate formation during ozonation of bromide-containing water: Efficacy and mechanism. Water Res. 2015, 86, 2–8. 10.1016/j.watres.2015.05.052. [DOI] [PubMed] [Google Scholar]
- De Franceschi L.; Heiniger B.; Murillo A.; Mende K.; Baumgarten S.; Bornemann C.; Fischer F.; Hachenberg M.; Taudien Y.; Kolisch G.. A bromate-free solution to remove micropollutants. IOA Conference and Exhibition. 2022.
- Gyparakis S.; Diamadopoulos E. Formation and reverse osmosis removal of bromate ions during ozonation of groundwater in coastal areas. Sep. Sci. Technol. 2007, 42, 1465–1476. 10.1080/01496390701290011. [DOI] [Google Scholar]
- Van Der Hoek J. P.; Rijnbende D. O.; Lokin C. J. A.; Bonne P. A. C.; Loonen M. T.; Hofman J. A. M. H. Electrodialysis as an alternative for reverse osmosis in an integrated membrane system. Desalination 1998, 117, 159–172. 10.1016/S0011-9164(98)00086-1. [DOI] [Google Scholar]
- Lin D.; Liang H.; Li G. Factors affecting the removal of bromate and bromide in water by nanofiltration. Environ. Sci. Pollut. Res. 2020, 27, 24639–24649. 10.1007/s11356-019-06002-3. [DOI] [PubMed] [Google Scholar]
- Marron E. L.; Mitch W. A.; von Gunten U.; Sedlak D. L. A Tale of Two Treatments: The Multiple Barrier Approach to Removing Chemical Contaminants during Potable Water Reuse. Acc. Chem. Res. 2019, 52, 615–622. 10.1021/acs.accounts.8b00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui M.; Zhai W.; Amy G.; Mysore C. Bromate ion removal by activated carbon. Water Res. 1996, 30, 1651–1660. 10.1016/0043-1354(96)00070-X. [DOI] [Google Scholar]
- Kirisits M. J.; Snoeyink V. L.; Kruithof J. C. The reduction of bromate by granular activated carbon. Water Res. 2000, 34, 4250–4260. 10.1016/S0043-1354(00)00189-5. [DOI] [Google Scholar]
- Bourgin M.; Beck B.; Boehler M.; Borowska E.; Fleiner J.; Salhi E.; Teichler R.; von Gunten U.; Siegrist H.; McArdell C. S. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. 10.1016/j.watres.2017.10.036. [DOI] [PubMed] [Google Scholar]
- Zimmermann S. G.; Wittenwiler M.; Hollender J.; Krauss M.; Ort C.; Siegrist H.; von Gunten U. Kinetic assessment and modeling of an ozonation step for full-scale municipal wastewater treatment: Micropollutant oxidation, by-product formation and disinfection. Water Res. 2011, 45, 605–617. 10.1016/j.watres.2010.07.080. [DOI] [PubMed] [Google Scholar]
- Hollender J.; Zimmermann S. G.; Koepke S.; Krauss M.; McArdell C. S.; Ort C.; Singer H.; von Gunten U.; Siegrist H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. 10.1021/es9014629. [DOI] [PubMed] [Google Scholar]
- Bao M. L.; Griffini O.; Santianni D.; Barbieri K.; Burrini D.; Pantani F. Removal of bromate ion from water using granular activated carbon. Water Res. 1999, 33, 2959–2970. 10.1016/S0043-1354(99)00015-9. [DOI] [Google Scholar]
- Huang W. J.; Chen L. Y. Assessing the effectiveness of ozonation followed by GAC filtration in removing bromate and assimilable organic carbon. Environ. Technol. 2004, 25, 403–412. 10.1080/09593332508618461. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Q.; Wu Q. P.; Zhang J. M.; Yang X. H. Removal of bromide and bromate from drinking water using granular activated carbon. J. Water Health 2015, 13, 73–78. 10.2166/wh.2014.084. [DOI] [PubMed] [Google Scholar]
- Asami M.; Aizawa T.; Morioka T.; Nishijima W.; Tabata A.; Magara Y. Bromate removal during transition from new granular activated carbon (GAC) to biological activated carbon (BAC). Water Res. 1999, 33, 2797–2804. 10.1016/S0043-1354(98)00504-1. [DOI] [Google Scholar]
- Chen W.-f.; Zhang Z. Y.; Li Q.; Wang H. Y. Adsorption of bromate and competition from oxyanions on cationic surfactant-modified granular activated carbon (GAC). Chem. Eng. J. 2012, 203, 319–325. 10.1016/j.cej.2012.07.047. [DOI] [Google Scholar]
- Kirisits M. J.; Snoeyink V. L. Reduction of bromate in a BAC filter. J. Am. Water Works Assoc. 1999, 91, 74–84. 10.1002/j.1551-8833.1999.tb08684.x. [DOI] [Google Scholar]
- Kirisits M. J.; Snoeyink V. L.; Inan H.; Chee-Sanford J. C.; Raskin L.; Brown J. C. Water quality factors affecting bromate reduction in biologically active carbon filters. Water Res. 2001, 35, 891–900. 10.1016/S0043-1354(00)00334-1. [DOI] [PubMed] [Google Scholar]
- Kirisits M. J.; Snoeyink V. L.; Chee-Sanford J. C.; Daugherty B. J.; Brown J. C.; Raskin L. Effects of operating conditions on bromate removal efficiency in BAC filters. J. Am. Water Works Assoc. 2002, 94, 182–193. 10.1002/j.1551-8833.2002.tb09462.x. [DOI] [Google Scholar]
- Matos C. T.; Velizarov S.; Reis M. A. M.; Crespo J. G. Removal of bromate from drinking water using the ion exchange membrane bioreactor concept. Environ. Sci. Technol. 2008, 42, 7702–7708. 10.1021/es801176f. [DOI] [PubMed] [Google Scholar]
- Wiśniewski J. A.; Kabsch-Korbutowicz M. Bromate removal in the ion-exchange process. Desalination 2010, 261, 197–201. 10.1016/j.desal.2010.03.029. [DOI] [Google Scholar]
- Chubar N. I.; Samanidou V. F.; Kouts V. S.; Gallios G. G.; Kanibolotsky V. A.; Strelko V. V.; Zhuravlev I. Z. Adsorption of fluoride, chloride, bromide, and bromate ions on a novel ion exchanger. J. Colloid Interface Sci. 2005, 291, 67–74. 10.1016/j.jcis.2005.04.086. [DOI] [PubMed] [Google Scholar]
- Mestri S.; Dogan S.; Tizaoui C. Bromate Removal from Water Using Ion Exchange Resin: Batch and Fixed Bed Column Performance. Ozone: Sci. Eng. 2023, 45, 291–304. 10.1080/01919512.2022.2114420. [DOI] [Google Scholar]
- Naushad M.; Alothman Z. A.; Khan M. R.; Wabaidur S. M. Removal of Bromate from Water Using De-Acidite FF-IP Resin and Determination by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Clean - Soil, Air, Water 2013, 41, 528–533. 10.1002/clen.201200461. [DOI] [Google Scholar]
- Wïsniewski J. A.; Kabsch-Korbutowicz M.; Łakomska S. Removal of bromate ions from water in the processes with ion-exchange membranes. Sep. Purif. Technol. 2015, 145, 75–82. 10.1016/j.seppur.2015.03.004. [DOI] [Google Scholar]
- Chen R.; Yang Q.; Zhong Y.; Li X.; Liu Y.; Li X.-M.; Du W.-X.; Zeng G.-M. Sorption of trace levels of bromate by macroporous strong base anion exchange resin: Influencing factors, equilibrium isotherms and thermodynamic studies. Desalination 2014, 344, 306–312. 10.1016/j.desal.2014.04.001. [DOI] [Google Scholar]
- Hübner U.; Kuhnt S.; Jekel M.; Drewes J. E. Fate of bulk organic carbon and bromate during indirect water reuse involving ozone and subsequent aquifer recharge. J. Water Reuse Desalin. 2016, 6, 413–420. 10.2166/wrd.2015.222. [DOI] [Google Scholar]
- Wang F.; van Halem D.; Ding L.; Bai Y.; Lekkerkerker-Teunissen K.; van der Hoek J. P. Effective removal of bromate in nitrate-reducing anoxic zones during managed aquifer recharge for drinking water treatment: Laboratory-scale simulations. Water Res. 2018, 130, 88–97. 10.1016/j.watres.2017.11.052. [DOI] [PubMed] [Google Scholar]
- Hijnen W. A. M.; Jong R.; Van Der Kooij D. Bromate removal in a denitrifying bioreactor used in water treatment. Water Res. 1999, 33, 1049–1053. 10.1016/S0043-1354(98)00306-6. [DOI] [Google Scholar]
- Wang F.; Salgado V.; van der Hoek J. P.; van Halem D. Bromate reduction by iron(II) during managed aquifer recharge: A laboratory-scale study. Water (Basel, Switz.) 2018, 10, 370. 10.3390/w10040370. [DOI] [Google Scholar]
- Demirel S.; Uyanik I.; Yurtsever A.; çelikten H.; Uçar D. Simultaneous bromate and nitrate reduction in water using sulfur-utilizing autotrophic and mixotrophic denitrification processes in a fixed bed column reactor. Clean - Soil, Air, Water 2014, 42, 1185–1189. 10.1002/clen.201300475. [DOI] [Google Scholar]
- Chairez M.; Luna-Velasco A.; Field J. A.; Ju X.; Sierra-Alvarez R. Reduction of bromate by biogenic sulfide produced during microbial sulfur disproportionation. Biodegradation 2010, 21, 235–244. 10.1007/s10532-009-9296-5. [DOI] [PubMed] [Google Scholar]
- Listiarini K.; Tor J. T.; Sun D. D.; Leckie J. O. Hybrid coagulation-nanofiltration membrane for removal of bromate and humic acid in water. J. Membr. Sci. 2010, 365, 154–159. 10.1016/j.memsci.2010.08.048. [DOI] [Google Scholar]
- Gordon G.; Gauw R. D.; Emmert G. L.; Walters B. D.; Bubnis B. Chemical Reduction Methods for Bromate Ion Removal. J. Am. Water Works Assoc. 2002, 94, 91–98. 10.1002/j.1551-8833.2002.tb09410.x. [DOI] [Google Scholar]
- Qiao J.; Feng L.; Dong H.; Zhao Z.; Guan X. Overlooked Role of Sulfur-Centered Radicals during Bromate Reduction by Sulfite. Environ. Sci. Technol. 2019, 53, 10320–10328. 10.1021/acs.est.9b01783. [DOI] [PubMed] [Google Scholar]
- Siddiqui M.; Amy G.; Ozekin K.; Zhai W.; Westerhoff P. Alternative strategies for removing bromate. J. Am. Water Works Assoc. 1994, 86, 81–96. 10.1002/j.1551-8833.1994.tb06263.x. [DOI] [Google Scholar]
- Farkas L.; Klein F. S. On the photo-chemistry of some ions in solution. J. Chem. Phys. 1948, 16, 886–893. 10.1063/1.1747027. [DOI] [Google Scholar]
- Siddiqui M. S.; Amy G. L.; McCollum L. J. Bromate destruction by UV irradiation and electric arc discharge. Ozone Sci. Eng. 1996, 18, 271–290. 10.1080/01919519608547330. [DOI] [Google Scholar]
- Peldszus S.; Andrews S. A.; Souza R.; Smith F.; Douglas I.; Bolton J.; Huck P. M. Effect of medium-pressure UV irradiation on bromate concentrations in drinking water, a pilot-scale study. Water Res. 2004, 38, 211–217. 10.1016/j.watres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Altmann J.; Ruhl A. S.; Zietzschmann F.; Jekel M. Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res. 2014, 55, 185–193. 10.1016/j.watres.2014.02.025. [DOI] [PubMed] [Google Scholar]
- Ruhl A. S.; Zietzschmann F.; Hilbrandt I.; Meinel F.; Altmann J.; Sperlich A.; Jekel M. Targeted testing of activated carbons for advanced wastewater treatment. Chem. Eng. J. 2014, 257, 184–190. 10.1016/j.cej.2014.07.069. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.