Abstract
In Saccharomyces cerevisiae cells exhibiting high-affinity glucose transport, the glucose consumption rate at extracellular concentrations above 10 mM was only half of the zero trans-influx rate. To determine if this regulation of glucose transport might be a consequence of intracellular free glucose we developed a new method to measure intracellular glucose concentrations in cells metabolizing glucose, which compares glucose stereoisomers to correct for adhering glucose. The intracellular glucose concentration was 1.5 mM, much higher than in most earlier reports. We show that for the simplest model of a glucose carrier, this concentration is sufficient to reduce the glucose influx by 50%. We conclude that intracellular glucose is the most likely candidate for the observed regulation of glucose import and hence glycolysis. We discuss the possibility that intracellular glucose functions as a primary signal molecule in these cells.
The first step of glycolysis in the yeast Saccharomyces cerevisiae involves the transport of glucose (or some other sugar) from the external medium across the plasma membrane. It has been suggested that the transport step exerts a high level of control on the glycolytic flux in this yeast (6, 18). It has also been proposed that the transport system, as a component of a glucose-sensing complex, may be directly involved in the initial sensing of glucose by the yeast cell (6, 42). Two members of the hexose transporter family, SNF3 and RGT2, have recently been implicated in the sensing of glucose for the regulation of expression of some HXT (named HXT for hexose transporter) genes (29). It is unclear, however, whether the glucose is sensed extracellularly or intracellularly. If the glucose is sensed inside the cell then the transporters may have an indirect role not only in the generation of the initial glucose signal but also in the maintenance of such a signal (32, 44).
In S. cerevisiae, glucose transport is extremely complex. At least 17 putative HXT genes have been identified (24, 33), and even the basic experimental procedures for measuring and interpreting glucose transport kinetics are a topic of continuous debate (11, 44). It is generally agreed that glucose transport in S. cerevisiae is a facilitated diffusion process (6, 15, 25, 26). Facilitated diffusion carriers are specific carriers that transport solutes down a concentration gradient (23, 39). This means that effective transport is only attained if there is removal of the intracellular product, in this case glucose by the hexose kinases. Most studies on transport kinetics in yeast have been concerned with zero trans-influx experiments, where the initial rate of radiolabeled glucose uptake is measured, assuming no feedback from metabolism given the short time scale applied (7, 39, 45).
We have observed that under some conditions, the predicted activity of the transporter based on zero trans-influx kinetics is higher than the actual glucose consumption rate (reference 41 and this study). In steady state, however, the in vivo activity of the transporter necessarily equals the glucose consumption rate. An explanation for the difference between the zero trans-influx and steady-state glucose consumption rates should involve the interaction of metabolism with the transporter, which by definition is omitted when the zero trans-influx rate is measured. The feedback may be carried out by some metabolites or some proteins that may interact with the transporter protein. Glucose-6-phosphate and ATP have been suggested to exert an effect on the glucose transport activity (1, 16, 35, 37), although their role remains controversial and direct evidence is absent (30, 46). Also, proteins have been implied to affect the activity of the transporter directly, such as the hexose kinases (7) and the GGS1/TPS1 subunit of the trehalose synthase complex (42).
Considering glucose transport as a facilitated diffusion process, however, the most straightforward feedback mechanism would be a significant concentration of internal free glucose. Feedback by accumulation of intracellular glucose has already explained the kinetic phenotype of energetically compromised cells (46) and that of a triple hexose kinase mutant (38). In wild-type yeast cells under standard conditions, however, the capacity for removal of intracellular glucose has been taken to be in excess over that of transport itself (18). Consequently, the intracellular glucose concentration in wild-type cells consuming glucose has been taken to be very, if not negligibly, low (6, 10, 18, 28, 35). This idea was supported when attempts to measure the intracellular glucose found very low concentrations (0 to 0.4 mM) in the cell (3, 10, 28). Some groups have even hypothesized that in yeast a direct coupling between transport and phosphorylation of glucose is operative (37, 43; see also reference 10), as it is in the phosphotransferase system in many bacteria (31). There are some reports, however, where significant concentrations of intracellular glucose have been measured (13, 20, 28).
We have developed a new method for measuring the intracellular glucose concentration in cells metabolizing glucose. We found that, depending on the conditions, the internal free glucose concentration could be as high as 2 to 3 mM. We discuss the implications of our results with respect to the role of transport in the control of glycolytic flux and in glucose signalling.
MATERIALS AND METHODS
Materials.
Reagents were obtained from the following sources. High specific activity d-[U-14C]glucose was from Amersham International, l-[1-3H]glucose was from New England Nuclear, growth medium constituents were from Difco, and all other chemicals were from Sigma Chemical Co. and were of reagent grade or better. The labelled sugars were dried down and resuspended immediately prior to use.
Strain and growth conditions.
In all experiments the S. cerevisiae diploid strain X2180 was used. Cells were grown on 2% (wt/vol) glucose as previously described (45), harvested in early exponential phase (optical density at 600 nm [OD600] of 0.2 [repressed cells]) or at glucose exhaustion (OD600 of 4 [derepressed cells]), and resuspended in 0.1 M phosphate buffer at pH 6.5 to a final cell density of 10% (wet weight/vol).
Measurement of glucose consumption rate and zero trans-influx rate.
Cells were incubated with stirring in a vessel thermostated at 30°C. An equal volume of prewarmed glucose solution in 0.1 M phosphate buffer (pH 6.5) was added to the cells to obtain the desired concentration of glucose and a cell density of 5% (wet weight/vol). To measure glycolytic fluxes, 0.1 ml of cell suspension was quenched in 0.1 ml of 10% trichloroacetic acid at time intervals of 1 min for at least 15 min and vortexed at room temperature, after which the samples were analyzed for glucose by NADH-linked enzymatic analysis according to the method of Bergmeyer (5).
The zero trans-influx measurements were carried out, as described previously (45), under exactly the same conditions as the glucose consumption experiments. The data were fitted to a one-component-uptake system by using computer-assisted nonlinear regression with explicit weighting (Enzfitter). Due to a numerical error in the protein determination in the previous study, the rates in this study are approximately two times the erroneous values for this strain reported in the previous study (45).
Protein concentrations were determined by the method of Lowry, with bovine serum albumin as a standard (27).
Measurement of internal free glucose concentration.
To measure the internal free glucose concentration, cells (5 ml) were incubated and glucose was added as described above for the measurement of the glucose consumption rate. Five minutes after the addition of glucose, 0.1 ml of carrier-free l-[3H]glucose and d-[14C]glucose was added to final specific radioactivities of 0.5 to 11 Bq · nmol−1 and 0.2 to 5 Bq · nmol−1, respectively. Samples of 2 ml were quenched at regular time intervals into 4 ml of methanol at −40°C. A sample of 50 to 100 μl was taken to determine the specific activities of [14C] and [3H]. The cells were washed three times in 4 ml of 60% (vol/vol) methanol by centrifugation for 5 min at the lowest possible temperature (≤−10°C) and then extracted at −40°C by the addition of 0.5 ml of 5% perchloric acid in 60% methanol. The volume of the extract was determined with an automatic pipette and was always around 650 μl. The extract was centrifuged, and 100 μl of the supernatant was loaded onto a high-performance liquid chromatography (HPLC) column (Biorad Aminex 87H, mobile phase 5 mM sulfuric acid, pump speed 0.5 ml/min, 21°C). Fractions of 30 s (0.25 ml) were collected and counted by liquid scintillation counting. The tritium was used as a marker for the fractions containing glucose. The internal free glucose concentration was determined as the 14C counts in the glucose fractions, corrected for the (tritium-based) carryover. Intracellular concentrations were calculated assuming that 1 mg of protein corresponds to 3.75 μl of intracellular water (13, 21a, 34).
RESULTS AND DISCUSSION
Measuring intracellular glucose concentrations in cells metabolizing glucose.
A reliable assay for intracellular glucose requires at least two features. First, during the manipulations, the cells should be metabolically inactive but, for obvious reasons, still intact. Second, the assay has to include a correction for the carryover of extracellular glucose. In the literature, some attempts to measure internal free glucose concentrations in a yeast cell suspension consuming glucose have been described. It remains unclear whether these methods result in an effective inactivation of metabolic activity during the filtration step, especially during the transfer of the filter into the quenching solution.
Complete quenching of metabolic activity during the manipulations can be achieved by using the −40°C methanol quench technique developed by De Koning and Van Dam (13). The methanol does not permeabilize the cells and prevents freezing of the cell suspension, which enables the cells to be washed at a very low temperature. This method has been used by De Koning and Van Dam (13) and by Thevelein and coworkers (20) to measure intracellular glucose concentrations, and both groups found significant glucose levels.
Given the need to correct for the carryover, other methods have included extensive washing (3) or some estimation of the carryover (10). However, apart from inclusion of medium in the pellet or filter, another significant contribution to carryover of extracellular glucose is expected to be glucose that binds nonspecifically to the cell wall or periplasmic space and cannot be easily washed away. In the case of maltose transport an apparent low-affinity component was an artifact caused by such nonspecific binding of maltose (4). The measurements of intracellular glucose by De Koning and Van Dam (13) and by Thevelein and coworkers (20) were not corrected at all for such carryover of extracellular glucose. In this paper we show that correction for carryover is very important even after three washes.
Our method is based on the −40°C methanol quench technique but does include a correction for the carryover of extracellular glucose caused by inclusion of medium and nonspecific binding. Rather than using the classical solution of [3H]inuline to correct for carryover (10, 40), we noted that d-glucose, and not l-glucose, is taken up by yeast cells (17). We used l-[3H]glucose to determine the carryover, and d-[14C]glucose equilibrated with the intracellular metabolite pools was used to estimate the internal free glucose concentration.
To separate the 14C counts into glucose and nonglucose counts, the metabolites in the extracts were separated by HPLC. l-glucose elutes at exactly the same time as d-glucose, and the tritiated counts could therefore be used as a marker for the fractions containing glucose (Fig. 1).
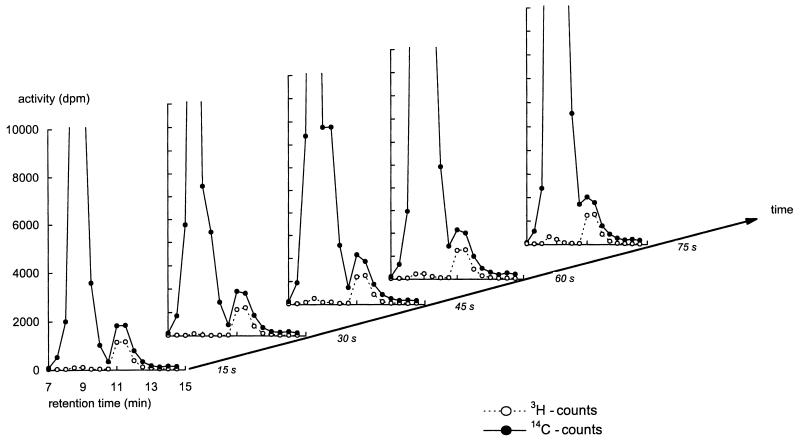
FIG. 1.
Time series of HPLC chromatograms of extracts obtained after addition of l-[3H]glucose and d-[14C]glucose to d-glucose-consuming yeast cells exhibiting high-affinity d-glucose transport. The extracellular glucose concentration at the time of label addition was 13.3 mM. The cells were quenched in −40°C methanol at the indicated times after addition of label. The extracts and chromatograms were obtained as described in Material and Methods.
Figure 1 shows a series of chromatograms from extracts obtained from derepressed cells in (pseudo) steady state consuming glucose at different times after addition of l-[3H]glucose and d-[14C]glucose. After the addition of label to these cells, several samples were taken in time to check complete equilibration of 14C label with the intracellular glucose pool. Glucose leaves the column at a retention time of around 11 min, as evident from the tritium peak. At shorter retention times, metabolites of glucose such as glucose-6-phosphate and fructose-1,6-bisphosphate eluted, confirmed by the absence of tritium label in this peak.
The 14C counts in the chromatograms have been normalized by the 14C/3H ratio added to the cells. The difference between the 14C and 3H labels in the glucose peaks of Fig. 1 should therefore represent the intracellular glucose. This intracellular glucose concentration was found to be 1.5 mM, which is significantly higher than previously reported for these conditions.
Judging from the overlap with the tritium label, more than 50% of the glucose peak derived from extracellular glucose. This demonstrates the importance of the correction for adhering glucose, even after our three washes. In time, the peak of the charged metabolites broadened and penetrated the glucose peak (Fig. 1, t = 75 s). Pyruvate is known to elute close to glucose in our HPLC system. To correct for this peak overlap, we used the 3H/14C ratio in the fraction with the highest tritium counts (i.e., the fraction where only glucose was expected). This correction only significantly affected the calculation of the intracellular glucose concentration at the longer incubation times, which were merely done to check equilibration of radiolabel. Under all conditions examined so far, radiolabel equilibration with the intracellular glucose pool was achieved within 15 s (see Fig. 1 and 2). At such a short time scale, the contribution of labelled pyruvate to the 14C signal is insignificant (see also below).
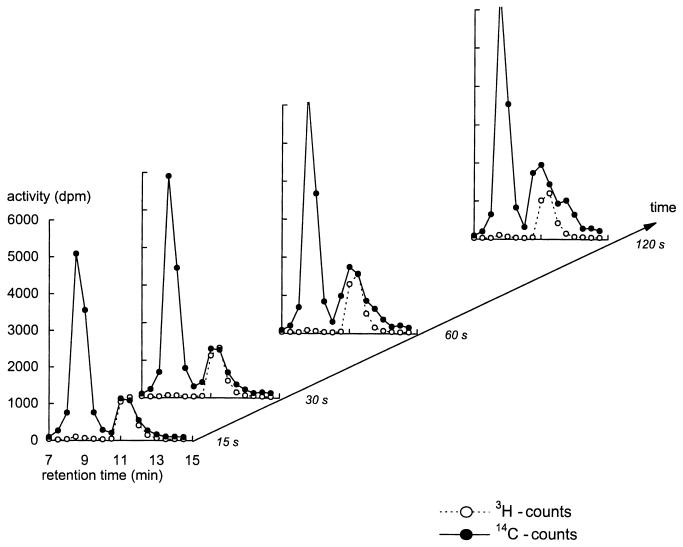
FIG. 2.
Time series of HPLC chromatograms of extracts obtained after addition of l-[3H]glucose and d-[14C]glucose to d-glucose-consuming yeast cells exhibiting low-affinity d-glucose transport. The extracellular glucose concentration at the time of label addition was 9.4 mM. See legend to Fig. 1 and Materials and Methods for details.
For the results shown in Fig. 2, the experiment of Fig. 1 was repeated but with glucose-repressed cells that have a low-affinity glucose transport system. All the 14C counts at the glucose (tritium) peak could be attributed to extracellular glucose rather than to intracellular glucose. Under these conditions, the intracellular glucose contribution was insignificant compared to the extracellular glucose carried over. As the incubation progressed some peaks appeared mixed with the 14C glucose peak. These peaks may reflect metabolic products that became labelled over time, one of which could be pyruvate. The chromatograms after 15 and 30 s, however, show that equilibration of the (very small) intracellular glucose pool was achieved before the other peaks appeared.
Steady-state glucose consumption rate versus net glucose transport rate.
When the extracellular glucose concentration was at or below the affinity of the transport system, the intracellular glucose concentration was below the detection limit of the assay (less than 0.1 mM) (Table 1). The intracellular glucose concentration was significant when the extracellular glucose concentration was above the affinity of the transport system, i.e., as the carrier approached saturation. We have measured the glucose consumption rate under the same conditions as were used to measure the internal glucose concentrations.
TABLE 1.
Comparison of the steady-state glucose consumption rate with the zero trans-influx kinetics of glucose transport and the intracellular glucose concentration in derepressed and repressed cellsa
| Extracellular glucose concn (mM) | Vmax (nmol · min−1 · mg of protein−1) | Km (mM) | Calculated zero transinflux rate (nmol · min−1 · mg of protein−1) | Measured intracellular glucose concn (mM) | Measured glucose consumption rate (nmol · min−1 · mg of protein−1) | Calculated glucose consumption rate (nmol · min−1 · mg of protein−1) |
|---|---|---|---|---|---|---|
| Derepressed cells | ||||||
| 13.3 | 536 ± 12 | 1.7 ± 0.1 | 475 ± 10 | 1.5 ± 0.1 | 220 ± 16 | 233 |
| 34 | 536 ± 12 | 1.7 ± 0.1 | 511 ± 11 | 1.6 ± 0.1 | 245 ± 18 | 261 |
| Repressed cells | ||||||
| 9.4 | 614 ± 38 | 55 ± 5 | 90 ± 8 | <0.1 | 145 ± 16 | 88 |
| 36 | 614 ± 38 | 55 ± 5 | 243 ± 15 | <0.1 | 420 ± 38 | 240 |
| 255 | 614 ± 38 | 55 ± 5 | 503 ± 31 | 2.7 ± 0.5 | 568 ± 55 | 481 |
Values are means ± standard deviations. Values for Vmax and Km were measured as described in Walsh et al. (45). Estimation of the glucose consumption rate was based on the assumption that the facilitated diffusion carrier is symmetrical, and estimates were obtained by using the rate equation detailed in Appendix.
In the derepressed cells the glucose consumption rate was significantly lower than the zero trans-influx rate (Table 1). This fits qualitatively with a model of a facilitated diffusion carrier that is inhibited by its product, in this case intracellular glucose. This analysis can be extended to ascertain if the concentration of intracellular glucose is of sufficient magnitude, quantitatively, to account for the reduction in the rate of transport. If one assumes that the facilitated diffusion carrier is symmetrical (23, 39) and that substrate dissociation does not control the flux through the carrier, one may calculate the steady-state glucose consumption rate from the extracellular glucose concentration, the intracellular glucose concentration, and the zero trans-influx kinetics (see equation A1 of Appendix for explanation).
In the case of the derepressed cells described above, the calculated glucose consumption rate was very similar to the measured rate. Clearly, in derepressed cells consuming glucose, the intracellular glucose concentration is of sufficient magnitude to reduce the flux through the carrier by 50% (see Table 1). Further, the high-affinity transport system present in these cells is close to saturation at 13.3 and 34 mM extracellular glucose so it is not surprising that both the glucose consumption rates and the intracellular glucose concentrations were similar at these extracellular glucose concentrations (Table 1). Carryover of extracellular glucose, however, should differ greatly between these two cases, strongly suggesting that our correction for carryover was effective.
A similar quantitative analysis with repressed cells exhibiting low-affinity transport was more problematic. With these cells the errors in the prediction of the glucose consumption rate are large because of the low affinity of the transporter for glucose (in this study the Km was approximately 55 mM). Small errors in the determination of the transport kinetics have a disproportional effect on the predicted glucose consumption rate. At the highest glucose concentration (255 mM) the measured intracellular glucose concentration of 2.7 ± 0.5 mM (mean ± standard deviation) should have little effect on the consumption rate because the affinity of this transporter is so low. As shown in Table 1, at this glucose concentration the calculated zero trans-influx rate, the measured glucose consumption rate, and the predicted glucose consumption rate were all similar. Furthermore, the low affinity of this carrier system and the relatively high cell density meant it was impossible to achieve even a pseudo steady state in the glucose consumption experiments, as performed, at concentrations below the Km of the carrier. Interestingly, in repressed cells with low-affinity uptake, the glucose consumption rate appeared to accelerate in the first few minutes of the experiment (data not shown). This may indicate that the low-affinity transporter is sensitive to (positive) effectors other than glucose or that glucose has a cooperative effect, which may lead to an underestimation of the low-affinity uptake capacity in a zero trans-influx experiment. Indeed, the glucose consumption rate, in these cells, is higher than the zero trans-influx rate at the lower glucose concentrations (Table 1). Certainly if the kinetics of low-affinity uptake in a wild-type strain are compared to those in a strain expressing only the low-affinity transporter (HXT1), the apparent Km in the wild-type cells is between 30 to 50 mM while in a strain expressing only HXT1 the Km is over 100 mM (32). A similar comparison between the kinetics of high-affinity uptake in a wild-type strain and those in a strain only expressing the high-affinity carrier (HXT7) showed no significant difference (reference 32 and unpublished observations). This suggests that there is additional regulation of the low-affinity carrier that is not required for high-affinity uptake.
Possible consequences of a significant intracellular glucose concentration for control of glycolysis and glucose sensing.
If glucose transport was only slowed down by its product, intracellular glucose, and the intracellular glucose concentration was negligible, then the transport step should be blind to metabolic signals from metabolism and, hence, should completely control flux (21). This may be the case for low-affinity transport where the intracellular glucose concentration was far below the supposed Km of the transporter for intracellular glucose. Cells exhibiting high-affinity transport, however, contained significant concentrations of intracellular glucose when the extracellular glucose concentration was high relative to the Km of the transporter. Under these conditions, the activity of the transport system appears to be in excess and might not have a high level of control on the glycolytic flux (see also reference 41).
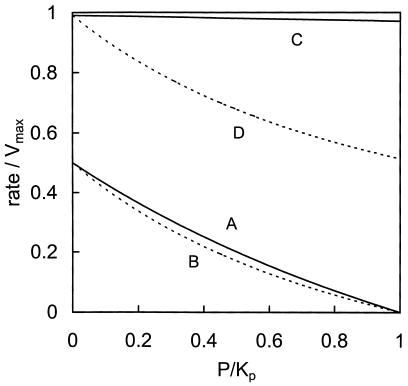
The driving force for a facilitated diffusion process is a concentration gradient, and a 10-fold smaller concentration of internal glucose compared to the external glucose concentration may seem insignificant. A specific feature of a facilitated diffusion carrier, however, as opposed to an ordinary reversible Michaelis-Menten type of enzyme, is the fact that substrate and product may not directly compete for the same binding site (39). The consequences of that can be seen in Fig. 3, where the rate of an ordinary reversible Michaelis-Menten type of enzyme, with one substrate and one product, is compared to the rate of a symmetrical facilitated diffusion carrier as a function of the product concentration. At a half-saturating substrate concentration, the Michaelis-Menten enzyme (Fig. 3, line A) and the transporter (Fig. 3, line B) were similarly sensitive to the product concentration. At nearly saturating substrate concentrations, the relatively small amount of product could not compete with the large amount of substrate in the case of the Michaelis-Menten enzyme (Fig. 3, line C), where the binding site is simultaneously accessible to substrate and product. Under the same condition, however, this type of transporter was sensitive to the product concentration. Net glucose uniport requires the carrier to be loaded with glucose when it moves inward and to be mainly empty when it moves back out. When the intracellular glucose concentration exceeds the binding constant for glucose, most outward-bound carriers also carry glucose. An important consequence is that the observation that there was less than 0.4 mM glucose inside the cells when there was 100 mM glucose outside (3) cannot be taken as evidence that the transport step was insensitive to the intracellular glucose concentration and that the transporter completely controlled glycolytic flux (18). Moreover, it is not the concentration of internal glucose that determines the control of the transporter but rather the relative sensitivities of the transporter and the rest of metabolism to the internal glucose concentration (see reference 2 for more detail).
FIG. 3.
Comparison of the sensitivities to product concentration between an enzyme exhibiting reversible Michaelis-Menten kinetics and a symmetrical facilitated diffusion carrier. P is the product concentration, which is normalized to its affinity constant for the protein, Kp. The rates of the enzymes are normalized to the forward Vmax. The equilibrium constant is set to 1 for both enzymes. For the transporter, Ki was set to 0.91 (see Appendix). Solid lines indicate the Michaelis Menten enzyme; dotted lines indicate the transporter. The substrate concentration S was either set at the affinity constant Ks, for the enzyme towards the substrate (A and B) or at 100 times the affinity constant (C and D), i.e., S/Ks = 1 or 100, respectively.
Conversely, the simplest model based on our data is that the activity of high-affinity transport is regulated by the intracellular glucose concentration. The proposed effects of ATP and glucose-6-phosphate on the glucose uptake activity may then be explained by an indirect effect via the phosphorylation capacity of the hexokinases and glucokinase on the intracellular glucose concentration, which will subsequently affect the net transport rate. Other effectors of the hexose-phosphorylating enzymes, like trehalose-6-phosphate, may also play such an indirect role in regulation of the transport activity (8, 19).
Free glucose is a candidate signal molecule.
Intracellular glucose, in contrast to glucose-6-phosphate, has not often been considered as a signalling molecule for glucose repression and other glucose-triggered regulatory events, probably because of its supposed low concentration. Our finding that its concentration can be quite significant suggests that its possible signalling function should be reassessed. In glucose-induced regulatory responses such as glucose repression, the hexose kinases are involved to various degrees (14, 36). On the other hand, the HXT-encoded transporters have been shown to affect glucose signalling (32, 44).
The reciprocal roles of the hexose transporters on the one hand and the sugar kinases on the other in generation and maintenance of several glucose signals implicate intracellular glucose as a possible signalling molecule, being the only metabolite that should be directly and inversely affected by both processes. For galactose induction, it has been shown that intracellular galactose concentration was the signal (47).
Two glucose transport homologs, RGT2 and SNF3, have been proposed to act as sensors of high and low extracellular glucose concentrations, respectively (29). It is not clear, however, whether SNF3 and RGT2 sense extracellular glucose and transduce the signal via the C-terminal domain typical for these two proteins (9, 12, 29) or whether they respond to an internal signal, possibly via an interaction with this C-terminal domain. It is also conceivable that SNF3 and RGT2 bind glucose extracellularly and are at the same time receptive to an internal signal, possibly intracellular glucose. Such a mechanism would integrate signals from outside and inside the yeast cell. It would explain the need for glucose transport in glucose signalling and the inability of maltose-derived metabolites (among which is internal glucose) to trigger the same responses as glucose does. The availability of a reliable assay for intracellular glucose should enable evaluation of the role of intracellular glucose in glucose signalling.
ACKNOWLEDGMENTS
We thank Mike Schepper and Louis Hartog for help with the HPLC and Barbara M. Bakker for fruitful discussions and critical reading of the manuscript.
This work was in part funded by grant no. CHRX-CT93-0265 of the Human Capital and Mobility program of the European Union and grant no. BIO4 CT950107 of the BIOTECH program of the European Union. We also acknowledge the financial assistance of the Foundation for Chemical Research (SON), which is subsidized by the Netherlands Organization for Scientific Research (NWO), and the Netherlands Association of Biotechnological Research Centers (ABON).
Appendix
The rate equation of a facilitated diffusion carrier can be described by a Haldane type of equation (23, 39). When a symmetrical carrier is assumed, e.g., when the rate constants for association and dissociation of glucose are independent of whether the binding site is facing inside or outside and the rate constants for transmembrane movement of the empty or loaded carrier are independent of the direction of movement, the rate equation reads:
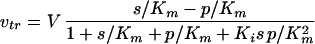 |
A1 |
where s and p are the extracellular and the intracellular glucose concentrations, respectively. V is the maximal transport activity, Km the Michaelis-Menten constant for glucose, and Ki is the so called “interactive constant” (22).
The magnitude of the interactive constant Ki depends on the relative mobility of the bound and unbound carrier, assuming that transmembrane movement is much slower than substrate association and dissociation (22). Rewriting Kotyk’s original rate equation in the form of equation A1, it can be derived that:
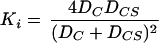 |
A2 |
where DCS and DC are the mobilities of the carrier with and without glucose, respectively (in s−1). Since it was measured that DCS = 1.87DC (22), it follows that Ki = 0.91.
REFERENCES
- 1.Azam F, Kotyk A. Glucose 6-phosphate as regulator of monosaccharide transport in baker’s yeast. FEBS Lett. 1969;2:333–335. doi: 10.1016/0014-5793(69)80057-8. [DOI] [PubMed] [Google Scholar]
- 2.Bakker B M, Michels P A M, Westerhoff H V. Control of the glycolytic flux in Trypanosoma brucei: why control can shift suddenly. In: Westerhoff H V, Snoep J L, Sluse F E, Wijker J E, Kholodenko B N, editors. BioThermoKinetics of the living cell—1996. Amsterdam, The Netherlands: BioThermoKinetics Press; 1996. pp. 136–142. [Google Scholar]
- 3.Becker J U, Betz A. Membrane transport as controlling pacemaker of glycolysis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972;274:584–597. doi: 10.1016/0005-2736(72)90205-2. [DOI] [PubMed] [Google Scholar]
- 4.Benito B, Lagunas R. The low-affinity component of Saccharomyces cerevisiae maltose transport is an artifact. J Bacteriol. 1992;174:3065–3069. doi: 10.1128/jb.174.9.3065-3069.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmeyer H U. Methods of enzymatic analysis. Weinheim, Germany: Verlag Chemie; 1974. [Google Scholar]
- 6.Bisson L F, Coons D M, Kruckenerg A L, Lewis D A. Yeast sugar transporters. Crit Rev Biochem Mol Biol. 1993;28:259–308. doi: 10.3109/10409239309078437. [DOI] [PubMed] [Google Scholar]
- 7.Bisson L F, Fraenkel D G. Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1983;80:1730–1734. doi: 10.1073/pnas.80.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blazquez M A, Lagunas R, Gancedo C, Gancedo J M. Trehalose-6-phosphate, a new regulator of yeast glycolysis that inhibits hexokinase. FEBS Lett. 1993;329:51–54. doi: 10.1016/0014-5793(93)80191-v. [DOI] [PubMed] [Google Scholar]
- 9.Celenza J L, Marshall-Carlson L, Carlson M. The yeast SNF3 gene encodes a glucose transporter homologous to the mammalian protein. Proc Natl Acad Sci USA. 1988;85:2130–2134. doi: 10.1073/pnas.85.7.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifton D, Walsh R B, Fraenkel D G. Functional studies of yeast glucokinase. J Bacteriol. 1993;175:3289–3294. doi: 10.1128/jb.175.11.3289-3294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coons D C, Boulton R B, Bisson L F. Computer-assisted nonlinear regression analysis of the multicomponent glucose uptake kinetics of Saccharomyces cerevisiae. J Bacteriol. 1995;177:3251–3258. doi: 10.1128/jb.177.11.3251-3258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coons D M, Vagnoli P, Bisson L F. The C-terminal domain of Snf3p is sufficient to complement the growth defect of snf3 null mutants in Saccharomyces cerevisiae: SNF3 functions in glucose recognition. Yeast. 1997;13:9–20. doi: 10.1002/(SICI)1097-0061(199701)13:1<9::AID-YEA51>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 13.De Koning W, Van Dam K. A method for the determination of changes of glycolytic metabolites in yeast on a subsecond time scale using extraction at neutral pH. Anal Biochem. 1992;204:118–123. doi: 10.1016/0003-2697(92)90149-2. [DOI] [PubMed] [Google Scholar]
- 14.De Winde J H, Crauwels M, Hohmann S, Thevelein J M, Winderinckx J. Differential requirement of the yeast sugar kinases for sugar sensing in establishing the catabolite-repressed state. Eur J Biochem. 1997;241:633–643. doi: 10.1111/j.1432-1033.1996.00633.x. [DOI] [PubMed] [Google Scholar]
- 15.Fuhrmann G F, Voelker B, Sander S, Potthast M. Kinetic analysis and simulation of glucose transport in plasma membrane vesicles of glucose-repressed and derepressed Saccharomyces cerevisiae cells. Experientia. 1989;45:1018–1023. doi: 10.1007/BF01950152. [DOI] [PubMed] [Google Scholar]
- 16.Galazzo J L, Bailey J E. Fermentation pathway kinetics and metabolic flux control in suspended and immobilized Saccharomyces cerevisiae. Enzyme Microb Technol. 1990;12:162–172. [Google Scholar]
- 17.Gamo F J, Moreno E, Lagunas R. The low-affinity component of the glucose-transport system in Saccharomyces cerevisiae is not due to passive diffusion. Yeast. 1995;11:1393–1398. doi: 10.1002/yea.320111407. [DOI] [PubMed] [Google Scholar]
- 18.Gancedo C, Serrano R. Energy-yielding metabolism. In: Rose A H, Harrison J S, editors. The yeast—1989. London, United Kingdom: Academic Press; 1989. pp. 205–259. [Google Scholar]
- 19.Hohmann S, Bell W, Neves M J, Valckx D, Thevelein J M. Evidence for trehalose-6-phosphate-dependent and trehalose-6-phosphate-independent mechanisms in the control of sugar influx into yeast glycolysis. Mol Microbiol. 1996;20:981–991. doi: 10.1111/j.1365-2958.1996.tb02539.x. [DOI] [PubMed] [Google Scholar]
- 20.Hohmann S, Neves M J, De Koning W, Alijo R, Ramos J, Thevelein J M. The growth and signalling defects of the ggs1 (fdp1/byp1) deletion mutant on glucose are suppressed by a deletion of the gene encoding hexokinase PII. Curr Genet. 1993;23:281–289. doi: 10.1007/BF00310888. [DOI] [PubMed] [Google Scholar]
- 21.Kacser H, Burns J A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- 21a.Kattevilder, A. Personal communication.
- 22.Kotyk A. Mobility of the free and of the loaded monosaccharide carrier in Saccharomyces cerevisiae. Biochim Biophys Acta. 1967;135:112–119. doi: 10.1016/0005-2736(67)90013-2. [DOI] [PubMed] [Google Scholar]
- 23.Kotyk A. Kinetic studies of transport in yeast. Methods Enzymol. 1989;174:567–591. [Google Scholar]
- 24.Kruckeberg A L. The hexose transporter family of Saccharomyces cerevisiae. Arch Microbiol. 1997;166:283–292. doi: 10.1007/s002030050385. [DOI] [PubMed] [Google Scholar]
- 25.Lagunas R. Sugar transport in Saccharomyces cerevisiae. FEMS Microbiol Rev. 1993;104:229–242. doi: 10.1016/0378-1097(93)90598-v. [DOI] [PubMed] [Google Scholar]
- 26.Lang J M, Cirillo P. Glucose transport in a kinaseless Saccharomyces cerevisiae mutant. J Bacteriol. 1987;169:2932–2937. doi: 10.1128/jb.169.7.2932-2937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowry O H, Roseborough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Neves M-J, Francois J. On the mechanism by which a heat shock induces trehalose accumulation in Saccharomyces cerevisiae. Biochem J. 1992;288:859–864. doi: 10.1042/bj2880859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozcan S, Dover J, Rosenwald A G, Wolfl S, Johnston M. Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene-expression. Proc Natl Acad Sci USA. 1996;93:12428–12432. doi: 10.1073/pnas.93.22.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perea J, Gancedo G. Glucose transport in a glucosephosphate isomeraseless mutant of Saccharomyces cerevisiae. Curr Microbiol. 1978;1:209–211. [Google Scholar]
- 31.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate—carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reifenberger E, Boles E, Ciriacy M. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem. 1997;245:324–333. doi: 10.1111/j.1432-1033.1997.00324.x. [DOI] [PubMed] [Google Scholar]
- 33.Reifenberger E, Freidel K, Ciriacy M. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol Microbiol. 1995;16:157–167. doi: 10.1111/j.1365-2958.1995.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 34.Richard P, Teusink B, Hemker M B, Van Dam K, Westerhoff H V. Sustained oscillations in free energy state and hexose phosphates in yeast. Yeast. 1996;12:731–740. doi: 10.1002/(SICI)1097-0061(19960630)12:8%3C731::AID-YEA961%3E3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 35.Rizzi M, Theobald U, Querfurth E, Rohrhirsch T, Baltes M, Reuss M. In vivo investigation of glucose transport in Saccharomyces cerevisiae. Biotechnol Bioeng. 1996;49:316–327. doi: 10.1002/(SICI)1097-0290(19960205)49:3<316::AID-BIT10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 36.Rose M, Albig W, Entian K-D. Glucose repression in Saccharomyces cerevisiae is directly associated with hexose phosphorylation by hexokinases PI and PII. Eur J Biochem. 1991;199:511–518. doi: 10.1111/j.1432-1033.1991.tb16149.x. [DOI] [PubMed] [Google Scholar]
- 37.Schuddemat J, Van den Broek P J A, Van Stevenick J. The influence of ATP on sugar uptake mediated by the constitutive glucose carrier of Saccharomyces cerevisiae. Biochim Biophys Acta. 1988;937:81–87. doi: 10.1016/0005-2736(88)90229-5. [DOI] [PubMed] [Google Scholar]
- 38.Smits H P, Smits G J, Postma P W, Walsh M C, Van Dam K. High-affinity glucose uptake in Saccharomyces cerevisiae is not dependent on the presence of glucose-phosphorylating enzymes. Yeast. 1996;12:438–447. doi: 10.1002/(SICI)1097-0061(199604)12:5%3C439::AID-YEA925%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 39.Stein W D. Transport and diffusion across cell membranes. London, United Kingdom: Academic Press; 1986. [Google Scholar]
- 40.Ter Kuyle B H, Opperdoes F R. Glucose-uptake by Tryponosoma brucei—rate-limiting steps in glycolysis and regulation of the glycolytic flux. J Biol Chem. 1991;266:857–862. [PubMed] [Google Scholar]
- 41.Teusink B, Walsh M C, Van Dam K, Gustafsson L, Westerhoff H V. The extent to which the glycolytic flux in Saccharomyces cerevisiae is controlled by the glucose transport system varies with the extracellular glucose concentration. In: Westerhoff H V, Snoep J L, Sluse F E, Wijker J E, Kholodenko B N, editors. BioThermoKinetics of the living cell—1996. Amsterdam, The Netherlands: BioThermoKinetics Press; 1996. pp. 417–421. [Google Scholar]
- 42.Thevelein J M, Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- 43.Van Steveninck J. The mechanism of transmembrane glucose transport in yeast: evidence for phosphorylation-associated transport. Arch Biochem Biophys. 1969;130:244–252. doi: 10.1016/0003-9861(69)90030-7. [DOI] [PubMed] [Google Scholar]
- 44.Walsh M C, Scholte M, Valkier J, Smits H P, van Dam K. Glucose sensing and signalling properties in Saccharomyces cerevisiae require the presence of at least two members of the glucose transporter family. J Bacteriol. 1996;178:2593–2597. doi: 10.1128/jb.178.9.2593-2597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh M C, Smits H P, Scholte M, van Dam K. The affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J Bacteriol. 1994;176:953–958. doi: 10.1128/jb.176.4.953-958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh M C, Smits H P, Van Dam K. Respiratory inhibitors affect incorporation of glucose into Saccharomyces cerevisiae cells, but not the activity of glucose transport. Yeast. 1994;10:1553–1558. doi: 10.1002/yea.320101204. [DOI] [PubMed] [Google Scholar]
- 47.Yano K. Galactose dependent reversible interaction of Gal3p with Gal80p in the induction pathway of Gal4p-activated genes of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94:1721–1726. doi: 10.1073/pnas.94.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]