Abstract
Background
The Pittsburgh Performance Fatigability Index (PPFI) quantifies the percent decline in cadence using accelerometry during standardized walking tasks. Although PPFI has shown strong correlations with physical performance, the developmental sample was relatively homogenous and small, necessitating further validation.
Methods
Participants from the Study of Muscle, Mobility and Aging (N = 805, age = 76.4 ± 5.0 years, 58% women, 85% White) wore an ActiGraph GT9X on the nondominant wrist during usual-paced 400 m walk. Tri-axial accelerations were analyzed to compute PPFI (higher score = greater fatigability). To evaluate construct and discriminant validity, Spearman correlations (rs) between PPFI and gait speed, Short Physical Performance Battery (SPPB), chair stand speed, leg peak power, VO2peak, perceived fatigability, and mood were examined. Sex-specific PPFI cut-points that optimally discriminated gait speed using classification and regression tree were then generated. Their discriminate power in relation to aforementioned physical performance were further evaluated.
Results
Median PPFI score was 1.4% (25th–75th percentile range: 0%–21.7%), higher among women than men (p < .001). PPFI score was moderate-to-strongly correlated with gait speed (rs = −0.75), SPPB score (rs = −0.38), chair stand speed (rs = −0.36), leg peak power (rs = −0.34) and VO2peak (rs = −0.40), and less strongly with perceived fatigability (rs = 0.28–0.29), all p < .001. PPFI score was not correlated with mood (|rs| < 0.08). Sex-specific PPFI cut-points (no performance fatigability: PPFI = 0%; mild performance fatigability: 0% < PPFI < 3.5% [women], 0% < PPFI < 5.4% [men]; moderate-to-severe performance fatigability: PPFI ≥ 3.5% [women], PPFI ≥ 5.4% [men]) discriminated physical performance (all p < .001), adjusted for demographics and smoking status.
Conclusion
Our work underscores the utility of PPFI as a valid measure to quantify performance fatigability in future longitudinal epidemiologic studies and clinical/pharmaceutical trials.
Keywords: Accelerometry, Fatigue, Gait speed
Greater fatigability often co-occurs with active pathology (1,2), poorer energetic capacity (3,4), as well as functional decline and impairment (5–8), accelerating the pathway to disability (9) and mortality (10). Fatigability also moderates the effect of rehabilitation practice and physical activity interventions, especially in older adults (11). Fatigability is defined as an individual’s propensity to fatigue which can be measured as perception (ie, perceived fatigability) or objectively quantified via performance decrement during a physical task (ie, performance fatigability) (12–14). Although fatigability is highly prevalent in older adults, performance fatigability has been understudied, in part, due to the lack of a sensitive and objective measurement standardized across various walking task protocols (15,16). We filled this gap by developing a sensitive and objective performance fatigability measure using accelerometry, the Pittsburgh Performance Fatigability Index (PPFI), that can be applied during fast- or usual-paced 400 m walks (16).
In the aging literature, performance fatigability is defined as a decrement in performance during a standardized physical task or activity (12,13). Conceptually, PPFI quantifies the percentage of performance decrement during a walking task by comparing the area under the observed cadence-versus-time curve to a hypothetical area that would be observed in the absence of fatigue (ie, sustain the maximal cadence during the entire walk). In our developmental sample of adults aged ≥60 years (N = 63), we found that higher PPFI scores from either fast-paced or usual-paced 400 m walks were associated with slower chair stand speed and 4 m usual gait speed, longer time to complete a 400 m walk, and weaker leg peak power, after adjustment for age, sex, race, weight, height, and smoking status (16). Interestingly, we also revealed women had higher PPFI scores than men did during the fast-paced walk, but not the usual-paced walk (16). However, the developmental sample consisted of relatively healthy and high-functioning older adults, limiting generalizability.
The objectives of this study were to establish PPFI’s construct validity against several physical performance measures (eg, gait speed), leg peak power, cardiorespiratory fitness, and perceived fatigability, as well as PPFI’s discriminant validity against depressive symptomatology and the subdomains of happiness, depression, restless sleep, and loneliness in a large population of older adults with a wide range of physical functioning. Furthermore, for future research and clinical applications, we identified sex- and task-specific PPFI cut-points that most strongly discriminate 400 m usual gait speed. Lastly, we examined the discriminate power of the PPFI cut-points in relation to better versus worse physical performance.
Method
Study Sample
The Study of Muscle, Mobility and Aging (SOMMA) is a large prospective, longitudinal ongoing cohort study to understand how muscle changes as people age (17). A total of 879 community-dwelling older adults (age ≥70 years) with a gait speed of ≥0.6 m/s during a 4 m walk were enrolled between 2019 and 2021 at 2 academic clinical centers: University of Pittsburgh and Wake Forest University School of Medicine. At baseline, participants completed a series of measures over 3 clinic visits that were detailed elsewhere (17). All participants provided written informed consent, and SOMMA was approved by the WIRB-Copernicus Group Institutional Review Board (WCG IRB) as the single IRB for all participating sites.
Pittsburgh Performance Fatigability Index (PPFI)
Participants wore an ActiGraph GT9X (Link) accelerometer (ActiGraph LLC, Pensacola, FL) on their nondominant wrist during a 400 m walk. Raw accelerometer data were collected at a sampling frequency of 80 Hz. Compliance and data quality were addressed via visual examination. During the walk, participants were instructed to complete 10 laps of 20 m one way in an unobstructed long corridor with traffic cones on both ends at their usual pace without overexerting themselves (18). Participants were permitted to use a straight cane during the walk. The time to complete the walk and split times for each lap were recorded in seconds. Immediately after the walk, participants were also asked to rate their perceived exertion using the Borg Rating of Perceived Exertion (RPE, range 6–20) (7).
Raw accelerometer data were processed in R (Version 4.0.3) to calculate PPFI. First, we used the Adaptive Empirical Pattern Transformation (ADEPT) R package (Version 1.2) to estimate raw cadence (steps per second) (19). ADEPT detects and segments strides by iteratively identifying local maxima of correlation function between 5 predefined empirical left-wrist worn accelerometry stride templates provided in the ADEPT package and the observed data. Then, we conducted penalized regression splines to smooth the raw cadence estimates and obtain individual-level smoothed cadence trajectories. Lastly, we scored PPFI using Equation (1) by comparing area under the observed cadence-versus-time curve to a hypothetical area that would be observed in the absence of fatigue (ie, if the participant sustained maximal cadence throughout the entire 400 m walk). We also applied individual weights to (a) minimize the intentional speeding up at the end due to one’s motivation to finish the task and (b) to emphasize the cadence decline that occurred at the beginning of the walk. Participants who completed the usual-paced 400 m walk within 6 minutes exhibited no performance fatigability during the walking task (ie, negligible decline in cadence) and for simplicity were categorized as PPFI = 0%.
| (1) |
where AUCstart→t represents the area under the cadence-versus-time from the beginning of the walk to t = 360 seconds, AUCt→end represents the area under the cadence-versus-time from t to the end of the walk, AUCstart→end represents the area under the cadence-versus-time during the entire walking task, Cadencemax represents the maximum cadence identified during the entire walking task, Total represents the total time (in seconds) to complete the walking task.
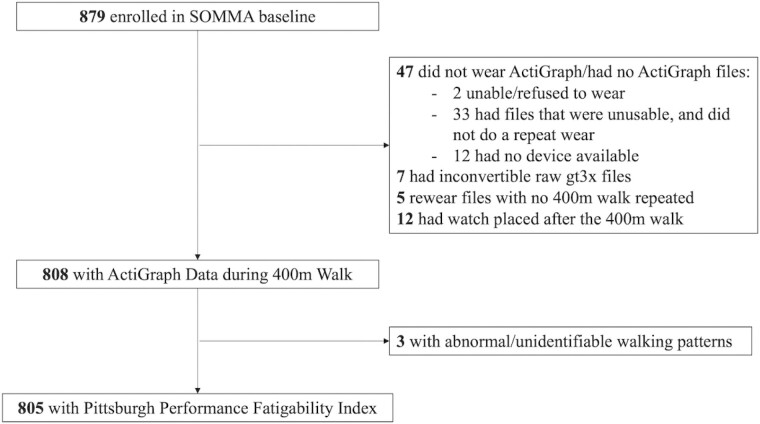
Higher PPFI score indicated more severe performance fatigability. Details on the derivation of PPFI, as well as visual illustrations, can be found elsewhere (16). We computed PPFI scores for 805 participants (92% of the enrolled SOMMA sample; Figure 1).
Figure 1.
Participant flowchart for study inclusion in the current analyses in the Study of Muscle, Mobility and Aging (SOMMA).
Physical Performance Measures
400 m gait speed
As described earlier in the PPFI section, the same usual-paced 400 m walk was used to calculate gait speed. The time to complete the 400 m walk was recorded in seconds using a stopwatch, then it was divided by the total distance walked (ie, 400 m) to obtain usual-paced gait speed (m/s).
Physical function
The Short Physical Performance Battery (SPPB), an objective measure of physical function, consisted of 3 components: a balance battery with side by side, semi-tandem, and tandem positions, a 4 m usual-paced walk, and 5 timed repeated chair stands. Each component was scored 0 (unable to complete) to 4 (best) and a total SPPB score was summed ranging from 0 to 12 with higher score indicating better physical function (20). To gain more insights about lower-extremity function beyond SPPB score, chair stand speed (stands/second) was further analyzed. For participants who were unable to perform chair stands (n = 25), their chair stand speed was set to 5 stands per 60 seconds (= 0.08 stand per second) (21). One participant had missing values for chair stand speed and thus had a missing SPPB score as well.
Leg peak power
Leg peak power was assessed with the Keiser Pneumatic resistance device (A420 model; Keiser Sports Health Equipment, Fresno, CA). First, to measure 1 repetition maximum (1-RM), participants were seated with their leg at a 90° angle and instructed to press their leg as fast as possible through a full range of motion, with a starting resistance of 40 pounds of force. Resistance was gradually increased until the participant reported a Borg RPE of ≥18. Then, after approximately 30 minutes of no physical activity, participants started the peak power testing, 2 trials for each intensity (40%, 50%, 60%, and 70% 1-RM) with 30 seconds of rest between each trial at the same level of resistance and 1 minute of rest between each increase in resistance. Peak power was the maximum power from trials of 40%–70% 1-RM. Approximately 7% of participants (n = 56) did not complete the leg peak power test. Reasons for missingness included (a) equipment, supply, space problem; (b) participants unable to perform due to health problem/safety concern; (c) participants not eligible (eg, significant weakness, pain, or knee issue); (d) missing due to technical issues.
Cardiorespiratory fitness
Cardiopulmonary exercise testing (CPET) was used to measure cardiorespiratory fitness. After a 5-minute treadmill walk at participants’ preferred walking speed (Phase 1), we implemented the symptom-limited peak stage (Phase 2), using a modified Balke protocol, where treadmill speed and incline were increased incrementally until the participant reported volitional exhaustion which was later verified by a respiratory exchange ratio of ≥1.05 and/or RPE of ≥15 (22). Breath-by-breath gas consumption was captured using an Ultima CPX metabolic stress testing system (MGC Diagnostics, Saint Paul, MN). Cardiorespiratory fitness (ie, VO2peak in mL/kg/min) was determined using BreezeSuite software as the highest 30-second average volume of oxygen consumption during the symptom-limited peak stage. A total of 51 participants had missing data for VO2peak. A detailed description of the CPET protocol and exclusion criteria can be found elsewhere (17,22).
Perceived Fatigability Measures
Two perceived fatigability measures were collected in SOMMA. One, the Pittsburgh Fatigability Scale (PFS), a validated 10-item questionnaire for older adults (23,24), was self-administered prior to the first clinic visit. Participants rated (on a scale of 0–5: 0 “no fatigue” to 5 “extreme fatigue”) how much physical fatigue they expected or imagined they would feel immediately after completing each of the 10 tasks/activities. Included activities represented a range of intensity and duration: leisurely walk for 30 minutes, brisk or fast walk for 1 hour, light household activity for 1 hour, heavy gardening or outdoor work for 1 hour, watching television for 2 hours, sitting quietly for 1 hour, moderate- to high-intensity strength training for 30 minutes, participating in a social activity for 1 hour, hosting a social event for 1 hour, and high-intensity activity for 30 minutes. We calculated the PFS Physical score as the sum of responses across the 10 items (range from 0 to 50, higher PFS score = greater perceived fatigability). Scores for participants (n = 13) missing 1–3 PFS items were imputed (25). Six participants had missing PFS Physical scores.
We also assessed perceived fatigability during Phase 3 of the CPET protocol (ie, RPE fatigability) (6,13). Participants walked on a treadmill at a standardized slow pace of 1.5 miles per hour (0.67 m/s) at a 0% grade for 5 minutes. They were asked, immediately after the walk, to rate their perceived exertion using the Borg RPE scale (range 6–20; eg, 6 = no exertion at all, 9 = very light, 11 = light, 13 = somewhat hard, 20 = maximal exertion) (7). Participants (n = 34) had missing values for RPE fatigability because (a) slow speed stage was not performed due to equipment issues or safety concerns that presented at an earlier phase during CPET; or (b) test was incomplete due to medical safety concerns, pain, or inability to maintain the speed.
Self-Reported Mood
Self-reported depressive symptomatology and mood were measured using the 10-item Center for Epidemiologic Studies—Depression Scale (CES-D) and its subdomains of happiness, depression, restless sleep, and loneliness (26). Specifically, participants responded to the following questions from the CES-D on a scale from 0 (ie, rarely or none of the time, <1 day) to 3 (ie, most or all of the time, 5–7 days): During the past 4 weeks, “I was happy,” “I felt depressed,” “My sleep was restless,” and “I felt lonely.”
Potential Confounders
Age at enrollment, sex, race (White, Black, Asian, Native American/Alaskan Native, Native Hawaiian/Pacific Islander, Multiracial, Unknown), and smoking status (current/former/never, with n = 5 missing and modeled as its own group) were obtained from each participant using self-administered questionnaires. Race was binarized for analyses as follows: White; Black, Indigenous, and People of Color (BIPOC). Height (Harpenden stadiometers; Dyved UK) without shoes and weight (balance beam scale) with light clothing were assessed. Multimorbidity (yes/no) included self-reported physician-diagnosed hypertension, diabetes, heart disease (including heart attack, coronary and myocardial infarction), stroke, lung diseases (including chronic obstructive lung disease, chronic bronchitis, asthma, emphysema, and chronic obstructive pulmonary disease), osteoporosis, and arthritis. Fall history was assessed by asking participants, “During the past 12 months, have you fallen and landed on the floor or ground or fallen and hit an object like a table or chair.”
Statistical Analyses
Descriptive characteristics of the final analytical sample (N = 805) were reported as median (25th percentile, 75th percentile), mean ± standard deviation, or frequencies (percentages) in the overall sample and by sex; sample size varied based on missingness for the measure of interests (n = 1 SPPB score and chair stand speed, n = 34 leg peak power, n = 51 VO2peak, n = 6 PFS Physical score, n = 34 RPE fatigability). Comparisons by sex were performed using Mann–Whitney U for non-normally distributed continuous variables, 2-sample t-tests for normally distributed continuous variables, and chi-square tests for categorical variables. Alpha was set to 0.05 and 2-sided p values smaller than .05 were considered significant. All analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria, version 4.0.3).
To assess the construct validity of PPFI, we examined Spearman correlations (rs) between PPFI and physical function, leg peak power, VO2peak, and perceived fatigability measures. To assess the discriminant validity of PPFI, we examined Spearman correlations (rs) between PPFI and CES-D total score and its subdomains of happiness, depression, restless sleep, and loneliness. The strength of correlations was considered as follows: rs ≥ 0.7 strong and 0.3 < rs < 0.7 moderate (27). We also conducted a sensitivity analysis evaluating the aforementioned correlations after excluding participants that used a cane during the 400 m walk (n = 22). Further, we developed classification and regression tree (CART) models using the rpart package (version 4.1) to generate cut-points of PPFI that most strongly discriminated 400 m gait speed. We first grouped participants with PPFI = 0% as their own group. Then, we separated the remaining sample into men and women and randomly split them into training and testing sets (80% vs 20%). Using the training set, we built sex-specific trees by minimizing the least squares criterion with at least 10 observations in a node and performed cross-validation by further randomly partitioning the training set into 10 equally sized mutually exclusive data sets. The final optimal tree was pruned to the most parsimonious tree that was within 1 standard error of the tree with the smallest prediction error (ie, mean square error). Lastly, to examine the discriminant power of the identified PPFI cut-points (the independent variable), we conducted linear regressions to obtain standardized beta coefficients of gait speed, SPPB score, chair stand speed, leg peak power, and VO2peak, adjusted for study site, age, sex, race, height, weight (except for the models with leg peak power and VO2peak), and smoking status. Adjusted means were calculated using generalized linear regression models with the same covariate adjustments.
Results
Participant Characteristics
Participants (N = 805) were 76.4 ± 5.0 years old (range: 70–94), with 58% women and 85% White. Median PPFI score was 1.4% [0%, 2.9%] with a range of 0%–21.7% and a significant difference between women (1.7% [0%, 3.0%]) and men (0.8% [0%, 2.8%]; p < .001). Compared to men, women had slower gait speed, weaker leg peak power, lower VO2peak, and greater perceived fatigability for both PFS and RPE fatigability (all p ≤ .01; Table 1). In addition, women had a higher prevalence of self-reported osteoporosis and arthritis (p ≤ .01), and reported more depressive symptomatology, whereas men had a higher prevalence of self-reported heart diseases (p = .02; Table 1).
Table 1.
Baseline Characteristics of the Sample (N = 805) and Stratified by Sex in the Study of Muscle, Mobility and Aging (SOMMA)*
| Characteristics | All (N = 805) | Women (n = 469) | Men (n = 336) | p Value |
|---|---|---|---|---|
| PPFI score | 1.38 [0, 2.89] | 1.72 [0, 2.97] | 0.82 [0, 2.80] | <.001 |
| Age, years | 76.4 ± 5.0 | 76.4 ± 5.0 | 76.3 ± 5.1 | .60 |
| Race, White | 687 (85.3) | 389 (82.9) | 298 (88.7) | .02 |
| Height, cm | 166.0 ± 9.8 | 159.8 ± 6.3 | 174.6 ± 6.9 | <.001 |
| Weight, kg | 76.3 ± 15.3 | 70.1 ± 13.2 | 84.9 ± 13.8 | <.001 |
| BMI, kg/m2 | 27.6 ± 4.6 | 27.5 ± 4.9 | 27.9 ± 4.2 | .20 |
| Smoking status | .67 | |||
| Current smoker | 25 (3.1) | 16 (3.4) | 9 (2.7) | |
| Former smoker | 331 (41.4) | 188 (40.3) | 143 (42.9) | |
| Never smoker | 444 (55.5) | 263 (56.3) | 181 (54.4) | |
| 400 m gait speed, m/s | 1.05 ± 0.18 | 1.02 ± 0.17 | 1.08 ± 0.17 | <.001 |
| SPPB score, 0–12 | 10.2 ± 1.7 | 10.1 ± 1.7 | 10.3 ± 1.8 | .19 |
| Chair stand speed, stands per second | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | .33 |
| Leg peak power, watts/kg | 4.7 ± 1.7 | 3.9 ± 1.2 | 5.7 ± 1.8 | <.001 |
| VO2peak, mL/kg/min | 20.2 ± 4.9 | 18.7 ± 4.1 | 22.3 ± 5.0 | <.001 |
| PFS Physical score, 0–50 | 15.7 ± 8.6 | 16.8 ± 8.4 | 14.2 ± 8.6 | <.001 |
| RPE fatigability, 6–20 | 8.4 ± 1.9 | 8.5 ± 1.9 | 8.2 ± 1.7 | .01 |
| Hypertension† | 416 (51.7) | 237 (50.5) | 179 (53.3) | .44 |
| Diabetes‡ | 125 (15.6) | 64 (13.7) | 61 (18.3) | .08 |
| Heart diseases‡ | 56 (7.0) | 24 (5.1) | 32 (9.6) | .02 |
| Stroke‡ | 21 (2.6) | 10 (2.1) | 11 (3.3) | .31 |
| Lung disease‡ | 106 (13.2) | 69 (14.7) | 37 (11.1) | .13 |
| Osteoporosis‡ | 140 (17.5) | 129 (27.6) | 11 (3.3) | <.001 |
| Arthritis‡ | 448 (55.9) | 304 (65.1) | 144 (43.1) | <.001 |
| Fall history§ | 223 (27.8) | 142 (30.3) | 81 (24.3) | .06 |
| CES-D, 0–30 | 4.1 ± 3.5 | 4.4 ± 3.5 | 3.7 ± 3.52 | .006 |
Notes: All reported in median (25th percentile, 75th percentile), mean ± SD or n (%). BMI = body mass index; CES-D = Center for Epidemiologic Studies—Depression Scale; PFS = Pittsburgh Fatigability Scale; PPFI = Pittsburgh Performance Fatigability Index; RPE = Borg Rating of Perceived Exertion; SPPB = Short Physical Performance Battery.
*There were n = 1 participant missing SPPB score and chair stand speed, n = 34 participants missing leg peak power, n = 51 missing VO2peak, n = 6 missing PFS Physical score, n = 34 missing RPE fatigability, n = 5 participants missing smoking status.
†Hypertension was classified by systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥80 mmHg.
‡Diabetes and all following health conditions were self-report of physician diagnoses. Heart disease included heart attack or myocardial infarction, heart failure, or atrial fibrillation. Lung diseases included chronic obstructive lung disease, chronic bronchitis, asthma, emphysema, and chronic obstructive pulmonary disease .
§Fall history was self-reported and asked as “During the past 12 months, have you fallen and landed on the floor or ground, or fallen and hit an object like a table or chair?”.
Construct and Discriminant Validity of PPFI
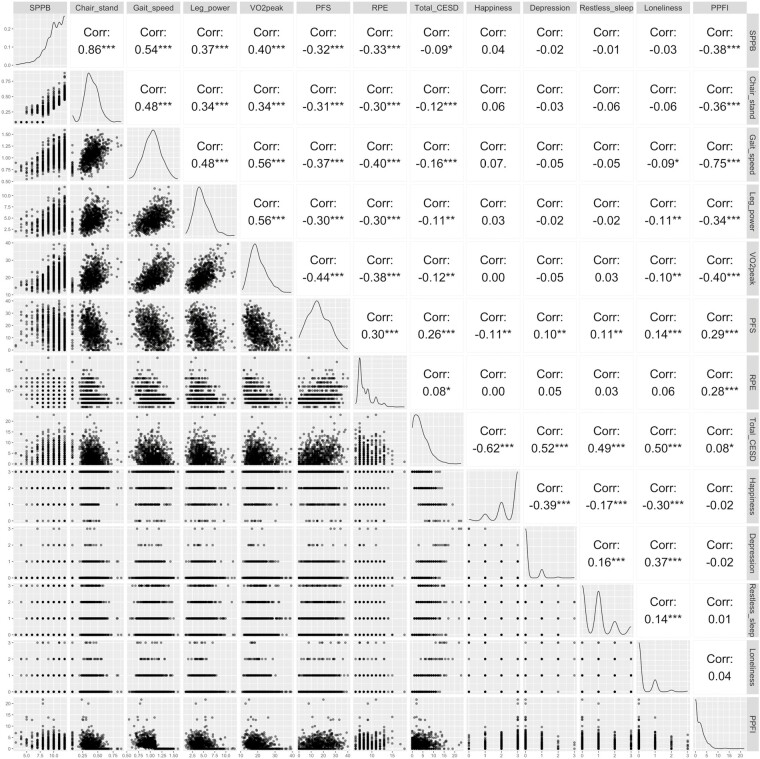
Higher PPFI score was moderate-to-strongly correlated with slower gait speed (rs = −0.75), lower SPPB score (rs = −0.38), slower chair stand speed (rs = −0.36), weaker leg peak power (rs = −0.34), and poorer VO2peak (rs = −0.40), all p < .001 (Figure 2). Higher PPFI score was less strongly correlated with greater perceived fatigability, rs = 0.29, for PFS Physical score and rs = 0.28 for RPE fatigability (Figure 2). Conversely, PPFI score was not correlated with CES-D total score, happiness, depression, restless sleep, and loneliness, all |rs| <0.08 (Figure 2). After excluding 22 participants that used a cane during the 400 m walk, all findings were held (data not shown).
Figure 2.
Spearman correlations between PPFI score and SPPB score, chair stand speed, 400 m gait speed, leg peak power, VO2peak, perceived fatigability, and self-reported mood measures in the Study of Muscle, Mobility and Aging (SOMMA; N = 805). ***p < .001. **p < .01. *p < .05. There were n = 1 participant missing SPPB score and chair stand speed, n = 34 participants missing leg peak power, n = 51 missing VO2peak, n = 6 missing PFS Physical scores, n = 34 missing RPE fatigability. PFS = Pittsburgh Fatigability Scale; PPFI = Pittsburgh Performance Fatigability Index; RPE = Borg Rating of Perceived Exertion; SPPB = Short Physical Performance Battery.
When stratified by sex, higher PPFI score showed higher correlations among men than women with slower gait speed (rs = −0.81 vs −0.68), lower SPPB score (rs = −0.42 vs −0.34), slower chair stand speed (rs = −0.41 vs −0.32), weaker leg peak power (rs = −0.35 vs −0.30), and greater perceived fatigability (rs = 0.31–0.32 vs 0.22–0.23), yet not significant, pinteraction for sex and PPFI > 0.05 (Supplementary Figure 1). Further, the correlation between PPFI score and VO2peak was higher among women (rs = −0.41) than men (rs = −0.35), pinteraction for sex and PPFI > 0.05 (Supplementary Figure 1).
Identified PPFI Cut-Points and Their Discriminant Power
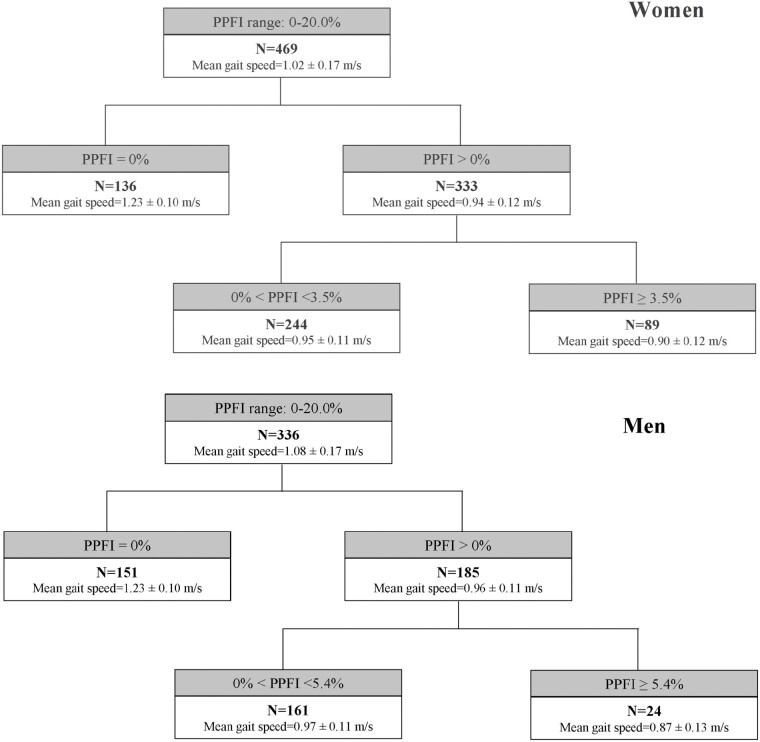
Based on the final optimal tree (Supplementary Figure 2), one split was identified for women and a different one for men among participants with PPFI > 0%. Specifically, there were 136 (29.0%) women and 151 (44.9%) men classified as having no performance fatigability (PPFI = 0%); 244 (52.0%) women and 161 (47.9%) men classified as having mild performance fatigability (0 < PPFI < 3.5% for women and 0 < PPFI < 5.4% for men); and 89 (19.0%) women and 24 (7.1%) men classified as having moderate-to-severe performance fatigability (PPFI ≥ 3.5% for women and PPFI ≥ 5.4% for men). The mean gait speeds were similar by sex within the same PPFI severity stratum (Figure 3).
Figure 3.
Identified sex- and task-specific PPFI cut-points that best discriminate gait speed using the classification and regression tree in the Study of Muscle, Mobility and Aging (SOMMA). PPFI = Pittsburgh Performance Fatigability Index.
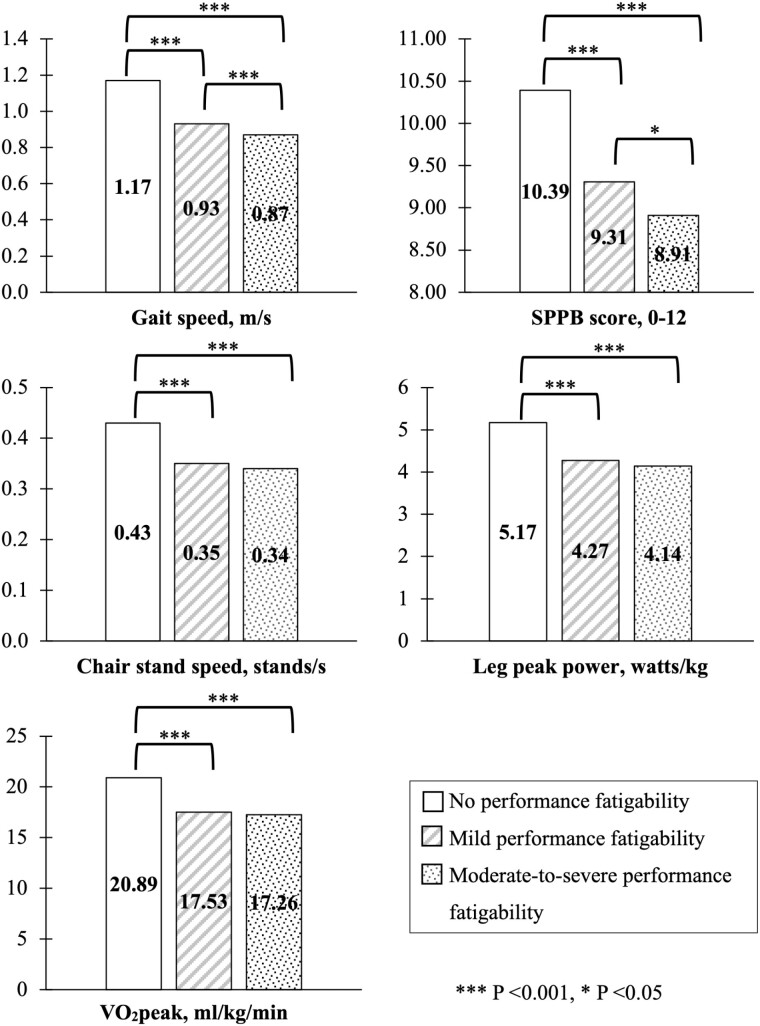
Across the PPFI severity strata, women and men classified as having no performance fatigability had better physical performance (|Standardized Beta| ranging from 0.53-1.71, all p < .001), compared to those with mild or moderate-to-severe performance fatigability (Table S1). Additionally, the magnitude of associations between PPFI severity and physical performance was stronger when comparing those with no performance fatigability versus mild performance fatigability than comparing those with mild performance fatigability versus moderate-to-severe performance fatigability, particularly for gait speed and SPPB scores (Figure 4).
Figure 4.
Adjusted means of gait speed, SPPB score, chair stand speed, leg peak power, and VO2peak across performance fatigability severity based on identified PPFI cut-points in the Study of Muscle, Mobility and Aging (SOMMA; N = 805). Adjusted means were calculated using generalized linear regressions after adjusting for study site, age, sex, race, height, weight (except for the models with leg peak power and VO2peak), and smoking status. Obtained from usual-paced 400 m walk. There were n = 1 participant missing SPPB score and chair stand speed, n = 34 participants missing leg peak power, n = 51 missing VO2peak. PPFI = Pittsburgh Performance Fatigability Index; SPPB = Short Physical Performance Battery.
Discussion
Our findings revealed that PPFI had good construct and discriminant validity. PPFI score was moderate-to-strongly correlated with physical function, leg peak power, and cardiorespiratory fitness, and less strongly with perceived fatigability as perceived and performance fatigability are two distinct constructs (6,14); whereas PPFI score was not correlated with self-reported mood, supporting discriminant validity. Furthermore, the identified sex- and task-specific PPFI cut-points showed strong discriminant power with physical performance. Given that PPFI can be derived from various long-distance walking tasks (16), the CART method provided was used to introduce a standardized analytic approach to determine task-specific PPFI cut-points for future research and clinical applications. Our work underscores the utility of PPFI as a valid measure to quantify performance fatigability in epidemiological research and clinical settings. Ongoing longitudinal data collection in SOMMA will allow us to evaluate the predictive validity of PPFI in future work.
Higher PPFI score was strongly associated with worse physical function, with the strongest association against gait speed, followed by SPPB score. These results are similar to our initial development work for PPFI (16) and to other studies with traditional performance fatigability measures (28,29). For instance, performance fatigability based on a usual-paced 10-minute walk, assessed as the percentage change in average walking speed within the first 2.5 minutes to the average walking speed over the entire 10 minutes, showed a comparable, but weaker correlation with usual gait speed (r = −0.54) than observed in our study (rs = −0.75) (28). Another study measured performance fatigability during a usual-paced 6-minute walk by comparing average speed over 6 minutes to the speed from the first 2 minutes divided by the total distance walked during 6 minutes, and found that performance fatigability was strongly correlated with gait speed (r = −0.67) (29). It was expected that PPFI would have the strongest association with usual-paced gait speed from the 400 m walk, given that it was the same physical task we used to derive PPFI, whereas SPPB score captured physical performance more comprehensively, as the measurement included balance, walking, and lower-extremity strength. Together with evidence from other studies, performance fatigability has been established as an important marker of physical function decline (30,31), reflecting risk of subsequent disability (32), and even mortality (10).
A novel finding in this study was the significant, moderate correlation between lower VO2peak and higher PPFI score. To our knowledge, only one study revealed that higher predicted VO2max from a 6-minute walk was significantly correlated with lower performance fatigability (r = −0.62) among a small sample of 36 women average age 60 years with hip osteoarthritis (33). Additionally, 2 studies found a significant association between VO2peak and perceived fatigability as measured by RPE fatigability (4,21), yet the effect was attenuated after adjusting for health conditions and body composition (4). Our findings confirmed and contributed additional evidence regarding the relation between VO2peak and performance fatigability measured with a novel objective accelerometry-based index—PPFI. Thus, exercise interventions known to improve cardiorespiratory fitness might be a potentially effective intervention to reduce performance fatigability as well.
Furthermore, we found that higher PPFI score was significantly associated with lower leg peak power and slower repeated chair stand. Although no previous studies examined the association between performance fatigability and leg power specifically, it is physiologically and biologically plausible that fatigability severity would be associated with lower-extremity power, given that weaker power is associated with poorer physical performance in older adults (34,35). The significant associations we found between PPFI and leg peak power as well as chair stand corroborate the plausibility that slowing down during a walking task is a manifestation of whole-body fatigue. More importantly, older adults with stronger leg power tend to have more muscle mass (36) and better physical function (37,38) in general, thus they would likely have more energy reserve left after daily activities which could prevent them from being fatigued (9). Although leg peak power represents the product of muscular force and velocity of movement and has been linked to mobility impairment, it was only mildly linked to walking speed (39). Further, neurobiological factors also impact one’s walking strategy and slowing down beyond muscle-related factors like leg peak power (40). Collectively, this may partially explain the weaker association between PPFI and leg peak power observed in this study.
Similar to our previous findings and others examining performance fatigability, PPFI score was less strongly correlated with perceived fatigability measures, implying a similar concept but different underlying constructs between performance and perceived fatigability (6,14,41,42). Perceived fatigability is a self-reported feeling of tiredness or exertion as a function of the duration and intensity of physical tasks/activities (13), which could be influenced by ones’ physical performance, health conditions, mental energy, self-motivation, and other life stressors (12). Whereas performance fatigability is defined and measured as performance decrement during a standardized physical activity (13), which might be less complex and more closely related to physical function and underlying factors contributing to physical performance (14). Better deciphering the differences and connections between performance and perceived fatigability could inform the design of personalized interventions aimed to reduce fatigability.
Noteworthy, we observed stronger correlations between PPFI score and physical performance measures (except for VO2peak) for men compared to women, although not statistically significant. Interestingly, women had a narrower PPFI distribution (median: 1.72%; variance: 4.58) and no statistical difference in gait speed measured during 4 m and 400 m walks (1.02 m/s for both walks, p = .29), whereas men had a wider PPFI distribution (median: 0.82%; variance: 6.60) and in general walked faster during 400 m than 4 m walk (1.08 m/s vs 1.06 m/s, p = .02), which may suggest women and men used different walking strategies across the walking tasks in this study. Although no other studies of older adults have examined sex differences in performance fatigability when using long-distance walks, studies focusing on isometric contractions have found that sex differences in muscle fatigability are task-specific and inconsistent (43). Specifically, women in general exhibited less muscle fatigue during or after exercises like cycling and running than men (44,45), whereas knee extensor muscle fatigability was similar for both older men and women (46). Skeletal muscle physiology may be the primary mechanism behind these population-level observations. Some studies explained a greater loss of voluntary activation for men, compared to women, during isometric fatiguing contractions with the lower limb muscles being the main contributor to the sex difference (47,48). Other studies found that motor unit remodeling and instability of the neuromuscular junction were similar for women and men after the age of ~75–80 years old (49), as well as accumulations of metabolic byproducts caused by adenosine triphosphate deficiency at older age (50), which may altogether lead to no sex differences among older adults. However, biological mechanisms related to whole-body performance fatigability remain unknown. Given that SOMMA collected muscle biopsies, we plan to explore the underlying biological factors of fatigability in future projects in SOMMA. Other epidemiologic studies should also examine sex differences in performance fatigability to better understand its severity in older adults.
By design, PPFI overcomes many methodological issues in existing traditional performance fatigability measures (16). Most importantly, PPFI assesses performance decrement using the entire individual cadence trajectory during a walking task and does not assume when the maximal cadence occurred. Cadence has been identified as a proxy of gait speed (51) and can be reliably estimated from raw accelerometry data without providing additional parameters (eg, step length). Given that studies have shown that step length remains relatively stable in community-dwelling older adults over an in-lab walking task, even after experimentally induced fatigue (52,53), PPFI should not be influenced by step length. However, further evaluation of the application of PPFI among clinical populations with gait abnormalities, such as Parkinson’s patients, is needed. Additionally, we acknowledge possible self-pacing bias during any usual-paced walks, especially in older adults, because participants may deliberately pick a slower speed to begin with in order to avoid fatigue instead of slowing down during the task because of feeling fatigued (12). To minimize the influence of self-pacing, PPFI includes individualized weights to emphasize performance decrement occurs at the beginning of the task. In our study, we found that PPFI score was significantly correlated with RPE queried at the end of the 400 m walk (rs = 0.23, p < .001). Although self-pacing during a usual-paced walk might mask performance fatigability that would be elicited from a more strenuous task, the nuanced performance deterioration that PPFI captures is suggestive of its potential prognostic power to identify individuals with impending mobility decline for early interventions. Thus, PPFI could be utilized in future longitudinal epidemiologic studies to understand meaningful change in performance fatigability and clinical/pharmaceutical trials aimed at reducing fatigability. Collectively, PPFI score is task specific. Future studies extending PPFI to measure performance fatigability using other walking tasks should generate task-specific cut-points with our recommended approach.
Our study has some limitations and strengths. First, we did not collect data to examine the reliability of PPFI to minimize participant burden for an already lengthy study protocol of multiple in-person measurements, as previous studies have established excellent reproducibility of both time to complete 400 m walk and ActiGraph counts (54,55). Thus, we are confident that PPFI is similarly reproducible as these measures were used to calculate PPFI. Second, SOMMA had a large sample of older men and women ranging from 70 to 94 years old, including 23% at the oldest ages (≥80 years old). Our study participants also had a wide range of physical functioning, indicated by gait speeds ranging from 0.55 to 1.59 m/s. Yet, our participants were mostly White, which affects generalizability to other racial/ethnic populations. Third, we excluded participants with missingness for certain measures in their relevant analyses. Based on other nonmissing covariates, these excluded participants in general had lower physical function and slower gait speed (data not shown), compared to participants without any missing data. Thus, we might have lost participants at the lower end of the distribution in terms of physical function who might exhibit more severe fatigability, yielding more conservative relations estimated between PPFI score and correlates of interests in our study.
In conclusion, PPFI has good validity while improving the sensitivity and objectiveness of performance fatigability measured by utilizing accelerometry during a long-distance walk and applying individual weights to account for self-pacing. Efforts to understand the longitudinal changes in performance fatigability and its predictive value to detect mobility disability are warranted.
Supplementary Material
Contributor Information
Yujia (Susanna) Qiao, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Jaroslaw Harezlak, Department of Epidemiology and Biostatistics, School of Public Health-Bloomington, Indiana University, Bloomington, Indiana, USA.
Peggy M Cawthon, San Francisco Coordinating Center, California Pacific Medical Center Research Institute, San Francisco, California, USA; Department of Epidemiology and Biostatistics, School of Medicine, University of California San Francisco, San Francisco, California, USA.
Steven R Cummings, San Francisco Coordinating Center, California Pacific Medical Center Research Institute, San Francisco, California, USA.
Daniel E Forman, Department of Medicine (Divisions of Geriatrics and Cardiology), School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Bret H Goodpaster, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Marquis Hawkins, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Kyle D Moored, Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Barbara J Nicklas, Gerontology and Geriatric Medicine, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA.
Frederico G S Toledo, Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Pamela E Toto, Department of Occupational Therapy, University of Pittsburgh School of Health and Rehabilitation Sciences, Pittsburgh, Pennsylvania, USA.
Adam J Santanasto, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Elsa S Strotmeyer, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Anne B Newman, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Nancy W Glynn, Department of Epidemiology, School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Lewis A Lipsitz, (Medical Sciences Section).
Funding
The Study of Muscle, Mobility and Aging is supported by funding from the National Institute on Aging (AG 059416). Study infrastructure support was funded in part by NIA Claude D. Pepper Older American Independence Centers at University of Pittsburgh (P30 AG024827) and Wake Forest University (P30 AG021332) and the Clinical and Translational Science Institutes, funded by the National Center for Advancing Translational Science, at Wake Forest University (UL1 0TR001420). K.D.M. was supported by the Pittsburgh Epidemiology of Aging Training Program (NIA T32 AG000181).
Conflict of Interest
None.
Author Contributions
Y.Q. and N.W.G. had full access to all of the data for the study and take responsibility for the integrity of the data and accuracy of the data analyses. All authors: interpretation of data, critical revision of manuscript for important intellectual content. All authors read and approved the submitted manuscript.
References
- 1. Qiao Y, Martinez-Amezcua P, Wanigatunga AA, et al. Association between cardiovascular risk and perceived fatigability in mid-to-late life. J Am Heart Assoc. 2019;8:e013049. 10.1161/JAHA.119.013049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carlozzi NE, Boileau NR, Murphy SL, Braley TJ, Kratz AL.. Validation of the Pittsburgh Fatigability Scale in a mixed sample of adults with and without chronic conditions. J Health Psychol. 2021;26:1455–1467. 10.1177/1359105319877448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson CA, Glynn NW, Ferrucci LG, Mackey DC.. Walking energetics, fatigability, and fatigue in older adults: the study of energy and aging pilot. J Gerontol A Biol Sci Med Sci. 2015;70:487–494. 10.1093/gerona/glu146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schrack JA, Wanigatunga AA, Zipunnikov V, Kuo P-L, Simonsick EM, Ferrucci L.. Longitudinal association between energy regulation and fatigability in mid-to-late life. J Gerontol A Biol Sci Med Sci. 2020;75:e74–e80. 10.1093/gerona/glaa011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wanigatunga AA, Simonsick EM, Zipunnikov V, et al. Perceived fatigability and objective physical activity in mid- to late-life. J Gerontol A Biol Sci Med Sci. 2018;73:630–635. 10.1093/gerona/glx181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simonsick EM, Schrack JA, Glynn NW, Ferrucci L.. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–351. 10.1111/jgs.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L.. Fatigued, but not frail: perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc. 2016;64:1287–1292. 10.1111/jgs.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moored KD, Rosso AL, Gmelin T, et al. Life-space mobility in older men: the role of perceived physical and mental fatigability. J Gerontol A Biol Sci Med Sci. 2022;77:2329–2335. 10.1093/gerona/glab286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alexander NB, Taffet GE, Horne FM, et al. Bedside-to-bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 2010;58:967–975. 10.1111/j.1532-5415.2010.02811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glynn NW, Gmelin T, Renner SW, et al. Perceived physical fatigability predicts all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2021;77:837–841. 10.1093/gerona/glab374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jette AM. Toward a common language for function, disability, and health. Phys Ther. 2006;86:726–734. [PubMed] [Google Scholar]
- 12. Eldadah BA. Fatigue and fatigability in older adults. PM R 2010;2:406–413. 10.1016/j.pmrj.2010.03.022 [DOI] [PubMed] [Google Scholar]
- 13. Schrack JA, Simonsick EM, Glynn NW.. Fatigability: a prognostic indicator of phenotypic aging. J Gerontol A Biol Sci Med Sci. 2020;75:e63–e66. 10.1093/gerona/glaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enoka RM, Almuklass AM, Alenazy M, Alvarez E, Duchateau J.. Distinguishing between fatigue and fatigability in multiple sclerosis. Neurorehabil Neural Repair. 2021;35:960–973. 10.1177/15459683211046257 [DOI] [PubMed] [Google Scholar]
- 15. Glynn NW, Qiao YS, Simonsick EM, Schrack JA.. Response to “comment on: fatigability: a prognostic indicator of phenotypic aging”. J Gerontol A Biol Sci Med Sci. 2021;76:e161–e162. 10.1093/gerona/glab058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qiao YS, Harezlak J, Moored KD, et al. Development of a novel accelerometry-based performance fatigability measure for older adults. Med Sci Sports Exerc. 2022;54:1782–1793. 10.1249/MSS.0000000000002966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cummings SR, Newman AB, Coen PM, et al. The Study of Muscle, Mobility and Aging (SOMMA):a unique cohort study about the cellular biology of aging and age-related loss of mobility. J Gerontol A Biol Sci Med Sci. 2023. 10.1093/gerona/glad052 [DOI] [PMC free article] [PubMed]
- 18. Simonsick EM, Newman AB, Visser M, et al. ; Health, Aging and Body Composition Study. Mobility limitation in self-described well-functioning older adults: importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63:841–847. 10.1093/gerona/63.8.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karas M, Stra Czkiewicz M, Fadel W, Harezlak J, Crainiceanu CM, Urbanek JK.. Adaptive empirical pattern transformation (ADEPT) with application to walking stride segmentation. Biostatistics 2019;22:331–347. 10.1093/biostatistics/kxz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 21. Santanasto AJ, Glynn NW, Jubrias SA, et al. Skeletal muscle mitochondrial function and fatigability in older adults. J Gerontol A Biol Sci Med Sci. 2015;70:1379–1385. 10.1093/gerona/glu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moored KD, Qiao YS, Rosso AL, et al. Dual roles of cardiorespiratory fitness and fatigability in the life-space mobility of older adults: The Study of Muscle Mobility and Aging (SOMMA). J Geronotol A Biol Sci Med Sci 2023;78:1392–1401. 10.1093/gerona/glad037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glynn NW, Santanasto AJ, Simonsick EM, et al. The Pittsburgh Fatigability Scale for older adults: development and validation. J Am Geriatr Soc. 2015;63:130–135. 10.1111/jgs.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Renner SW, Bear TM, Brown PJ, et al. Validation of perceived mental fatigability using the Pittsburgh Fatigability Scale. J Am Geriatr Soc. 2021;69:1343–1348. 10.1111/jgs.17017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cooper R, Popham M, Santanasto AJ, Hardy R, Glynn NW, Kuh D.. Are BMI and inflammatory markers independently associated with physical fatigability in old age? Int J Obes. 2019;43:832–841. 10.1038/s41366-018-0087-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- 27. Newman AB, Cauley JA eds. The epidemiology of aging. Springer; 2012. [Google Scholar]
- 28. Schnelle JF, Buchowski MS, Ikizler TA, Durkin DW, Beuscher L, Simmons SF.. Evaluation of two fatigability severity measures in elderly adults. J Am Geriatr Soc. 2012;60:1527–1533. 10.1111/j.1532-5415.2012.04062.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy SL, Kratz AL, Schepens Niemiec SL.. Assessing fatigability in the lab and in daily life in older adults with osteoarthritis using perceived, performance, and ecological measures. J Gerontol A Biol Sci Med Sci. 2017;72:115–120. 10.1093/gerona/glw173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buchowski MS, Simmons SF, Whitaker LE, et al. Fatigability as a function of physical activity energy expenditure in older adults. Age (Omaha) 2013;35:179–187. 10.1007/s11357-011-9338-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pechstein AE, Gollie JM, Guccione AA.. Fatigability and cardiorespiratory impairments in Parkinson’s disease: potential non-motor barriers to activity performance. JFMK 2020;5:78. 10.3390/jfmk5040078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci. 2009;64:76–82. 10.1093/gerona/gln017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Foucher KC, Aydemir B, Huang C-H, Horras M, Chmell SJ.. Aerobic capacity and fatigability are associated with activity levels in women with hip osteoarthritis. J Orthop Res. 2021;39:1236–1244. 10.1002/jor.24856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Winger ME, Caserotti P, Ward RE, et al. Jump power, leg press power, leg strength and grip strength differentially associated with physical performance: the Developmental Epidemiologic Cohort Study (DECOS). Exp Gerontol. 2021;145:111172. 10.1016/j.exger.2020.111172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fielding RA, Rejeski WJ, Blair S, et al. ; LIFE Research Group. The lifestyle interventions and independence for elders study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. 10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reid KF, Pasha E, Doros G, et al. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol. 2014;114:29–39. 10.1007/s00421-013-2728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–467. 10.1046/j.1532-5415.2002.50111.x [DOI] [PubMed] [Google Scholar]
- 38. Reid KF, Fielding RA.. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12. 10.1097/JES.0b013e31823b5f13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuo H-K, Leveille SG, Yen C-J, et al. Exploring how peak leg power and usual gait speed are linked to late-life disability: data from the National Health and Nutrition Examination Survey (NHANES), 1999-2002. Am J Phys Med Rehabil. 2006;85:650–658. 10.1097/01.phm.0000228527.34158.ed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eckert MA. Slowing down: age-related neurobiological predictors of processing speed. Front Neurosci. 2011;5:25. 10.3389/fnins.2011.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Loy BD, Taylor RL, Fling BW, Horak FB.. Relationship between perceived fatigue and performance fatigability in people with multiple sclerosis: a systematic review and meta-analysis. J Psychosom Res. 2017;100:1–7. 10.1016/j.jpsychores.2017.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marrelli K, Cheng AJ, Brophy JD, Power GA.. Perceived versus performance fatigability in patients with rheumatoid arthritis. Front Physiol. 2018;9:1395. 10.3389/fphys.2018.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hunter SK. The relevance of sex differences in performance fatigability. Med Sci Sports Exerc. 2016;48:2247–2256. 10.1249/MSS.0000000000000928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Billaut F, Bishop DJ.. Mechanical work accounts for sex differences in fatigue during repeated sprints. Eur J Appl Physiol. 2012;112:1429–1436. 10.1007/s00421-011-2110-1 [DOI] [PubMed] [Google Scholar]
- 45. Laurent CM, Green JM, Bishop PA, et al. Effect of gender on fatigue and recovery following maximal intensity repeated sprint performance. J Sports Med Phys Fitness. 2010;50:243–253. [PubMed] [Google Scholar]
- 46. Sundberg CW, Kuplic A, Hassanlouei H, Hunter SK.. Mechanisms for the age-related increase in fatigability of the knee extensors in old and very old adults. J Appl Physiol. 2018;125:146–158. 10.1152/japplphysiol.01141.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Russ DW, Kent-Braun JA.. Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol. 2003;94:2414–2422. 10.1152/japplphysiol.01145.2002 [DOI] [PubMed] [Google Scholar]
- 48. Martin PG, Rattey J.. Central fatigue explains sex differences in muscle fatigue and contralateral cross-over effects of maximal contractions. Pflugers Arch. 2007;454:957–969. 10.1007/s00424-007-0243-1 [DOI] [PubMed] [Google Scholar]
- 49. Hepple RT, Rice CL.. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol (Lond). 2016;594:1965–1978. 10.1113/JP270561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Layec G, Trinity JD, Hart CR, et al. Impact of age on exercise-induced ATP supply during supramaximal plantar flexion in humans. Am J Physiol Regul Integr Comp Physiol. 2015;309:R378–R388. 10.1152/ajpregu.00522.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nascimento M de M, Gouveia ER, Gouveia BR, et al. Associations of gait speed, cadence, gait stability ratio, and body balance with falls in older adults. Int J Environ Res Public Health. 2022;19:13926. 10.3390/ijerph192113926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Helbostad JL, Leirfall S, Moe-Nilssen R, Sletvold O.. Physical fatigue affects gait characteristics in older persons. J Gerontol A Biol Sci Med Sci. 2007;62:1010–1015. 10.1093/gerona/62.9.1010 [DOI] [PubMed] [Google Scholar]
- 53. Granacher U, Wolf I, Wehrle A, Bridenbaugh S, Kressig RW.. Effects of muscle fatigue on gait characteristics under single and dual-task conditions in young and older adults. J Neuroeng Rehabil. 2010;7:56. 10.1186/1743-0003-7-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rolland YM, Cesari M, Miller ME, Penninx BW, Atkinson HH, Pahor M.. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52:972–976. 10.1111/j.1532-5415.2004.52267.x [DOI] [PubMed] [Google Scholar]
- 55. Sirard JR, Forsyth A, Oakes JM, Schmitz KH.. Accelerometer test-retest reliability by data processing algorithms: results from the Twin Cities Walking Study. J Phys Act Health. 2011;8:668–674. 10.1123/jpah.8.5.668 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.