Abstract
Objective: It remains unclear whether balanced solutions improve patient-centred outcomes in critically ill patients overall and whether the treatment effect is heterogeneous, with evidence that some populations of patients may be helped and others harmed. To provide the most up-to-date and comprehensive assessment of the totality of the evidence, we will perform an ongoing living systematic review with aggregated and individual patient data meta-analysis (IPDMA) comparing the use of balanced solutions with saline in critically ill adults.
Design: Living systematic review using aggregated and individual patient data from randomised controlled trials.
Data sources: We will conduct annual searches of MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials. gov, the Australian New Zealand Clinical Trials Registry (ANZCTR), Japan’s University Hospital Medical Information Network (UMIN) Center, and the Brazilian Registry of Clinical Trials (ReBEC). The first search was completed on 1 March 2022 and will be repeated annually. Authors of eligible trials will be invited to provide individual data for the IPDMA. The initial analysis will use all data received up to 30 June 2022.
Review methods: We will include randomised controlled trials in adults treated in an intensive care unit that allocated individuals or clusters of patients to a balanced crystalloid solution or 0.9% saline for intravenous fluid therapy. Studies that used colloids as part of the intervention or that recruited only elective surgical patients will be excluded. The primary endpoint will be in-hospital mortality. The key secondary endpoint will be survival at longest follow-up for each trial. Data will be synthesised using both a random effect Bayesian meta-analysis and using hierarchical Bayesian models for individual patient data.
Discussion: The use of balanced crystalloid solutions may reduce mortality and improve other outcomes in some critically ill patients. We will assess the totality of current and future evidence by performing an ongoing living systematic review with aggregated data and IPDMA.
Protocol registration: CRD42022299282.
Fluid use is an obligatory component of the management of critically ill patients.1,2 Intravenous fluids are used to maintain intravascular volume, for hydration via maintenance fluids and as the carrier fluids for intravenous drugs. Saline (ie, 0.9% sodium chloride) has traditionally been the most commonly used fluid in intensive care units (ICUs).2 Saline is a hyperosmolar fluid with a much higher chloride concentration than human plasma, which, having a strong ion difference of zero, can induce a hyperchloraemic acidosis and may adversely affect organ function and patient-centred outcomes.1,3
Balanced (“low chloride”) solutions have been developed as alternatives to mitigate the potential adverse effects of the high chloride concentration of saline.1,4 By replacing chloride with another rapidly metabolised anion (eg, lactate, acetate or gluconate), balanced solutions have a positive strong ion difference and do not induce hyperchloraemia or acidosis. However, these replacement anions also have intrinsic properties that may be relevant to homeostasis and could potentially produce adverse effects of their own. The most common balanced fluids currently used include compound sodium lactate (lactated Ringer’s or Hartmann’s solution), acetated Ringer’s solution, and Plasma-Lyte 148 which mimic plasma tonicity and the plasma concentration of several ions.1,4
The comparative effectiveness of balanced solutions compared with saline in patients treated in ICUs has been evaluated in large investigator-initiated randomised controlled trials with particular focus on mortality and occurrence of acute kidney injury.5, 6, 7, 8, 9, 10, 11 Three such studies used a cluster design: the Saline v Plasma-Lyte 148 for ICU fluid Therapy (SPLIT) trial;5 the isotonic Solution Administration Logistical Testing (SALT),6 and the Isotonic Solutions and Major Adverse Renal Events Trial (SMART).7 Of those trials, only SMART provided evidence that, overall, balanced solutions may be preferred to saline. SMART included 15 082 patients and used the Major Adverse Kidney Events within 30 days (MAKE30) composite of death, doubling of creatinine, and need for new renal replacement therapy (RRT) as its primary outcome. A total of 1139 patients in the balanced solution group (14.3%) had a major adverse kidney event within 30 days, compared with 1211 (15.4%) in the saline group (odds ratio [OR], 0.91; 95% CI, 0.84-0.99; P = 0.04). However, the results were inconclusive for hospital mortality.7
Two other individually randomised trials comparing Plasma-Lyte 148 with 0.9% saline have recently been published.9, 10, 11 The Balanced Solutions in Intensive Care Study (BaSICS) was a large pragmatic blinded parallel group factorial randomised controlled trial comparing saline with Plasma-Lyte 148 in 11 000 critically ill patients in Brazil.8,9 The Plasma-Lyte 148 v saline study (PLUS) trial was a blinded, parallel group randomised controlled trial which also compared saline with Plasma-Lyte 148 in 5037 critically ill patients in Australia and New Zealand.10 Both trials reported no significant effect of the choice of fluid on the primary endpoint of 90-day mortality and on relevant secondary endpoints, including need for RRT. An updated aggregate data meta-analysis including the PLUS and BaSICS data reported that the relative risk for 90-day mortality for balanced solutions compared with saline was 0.96 (95% CI, 0.91–1.01). The same study reported a Bayesian posterior probability of 89.5% that the use of balanced solutions reduced mortality by some amount.11
These large clinical trials vary regarding inclusion and exclusion criteria, blinding, and type of balanced fluid administered. They are also likely to reflect differences in fluid administration practices based on their patient populations, health care systems and local clinical practice. Therefore, as a more precise estimate of effect size for types of fluids has the potential to affect many critically ill patients around the world, an individual patient data meta-analysis will provide valuable and robust data to guide clinical management in addition to the aggregated data meta-analysis.12 Living systematic reviews and meta-analyses are a recently proposed method to regularly update evidence as new trial data are reported, thereby improving the efficiency with which evidence is updated as new trial data are produced.12, 13, 14, 15 After its first iteration, yearly updates of the results can be produced with maximum efficiency.15 The use of individual patients’ data for meta-analysis also has important advantages compared with the use of aggregated data, including:
-
•
re-analysis of the original trial data to confirm the published results of primary trials;
-
•
provision of more information than is available in a triallevel meta-analysis and, hence, increased precision of estimates;
-
•
increased power to explore treatment effects in subgroups;
-
•
more appropriate adjustment for potential confounders or moderators of effects without the risk of ecological bias, as in aggregate data meta-regression; and
-
•
better comparison across different study designs with different units of randomisations (ie, individuals, clusters of patients).
Objectives
The primary objective of this meta-analysis is to assess whether the use of balanced solutions, compared with saline, for fluid therapy in the ICU is associated with lower in-hospital mortality.
Other objectives will be to assess the impact of using balanced solutions compared with saline in ICUs on:
-
•
survival until longest follow-up available;
-
•
proportion of patients newly treated with RRT; and
-
•
length-of-stay in the ICU and hospital.
Furthermore, we will conduct exploratory mechanistic analyses that may aid in interpreting the effects of balanced solutions compared with saline on the outcomes listed above, including changes in serum creatinine, arterial pH, serum sodium, chloride and bicarbonate concentration over time.
Finally, the IPDMA will compare the effects the use of a balanced solution in the ICU on hospital mortality, and the proportion of patients treated with new RRT in those admitted to the ICU in the following subgroups:
-
•
patients with and without sepsis;
-
•
patients admitted to the ICU with traumatic brain injury versus those admitted without acute traumatic brain injury. Alternatively, this analysis will be performed stratifying patients in three groups (traumatic brain injury, non-traumatic acute brain injury, and no brain injury);
-
•
subgroups based on baseline chloride concentration;
-
•
subgroups based on baseline arterial pH;
-
•
patients who received no saline before enrolment versus those who received 1–999 mL versus those who received 1000 mL or more;
-
•
effects on women compared with men; and
-
•
patients enrolled in cluster randomised versus individually randomised trials.
Methods
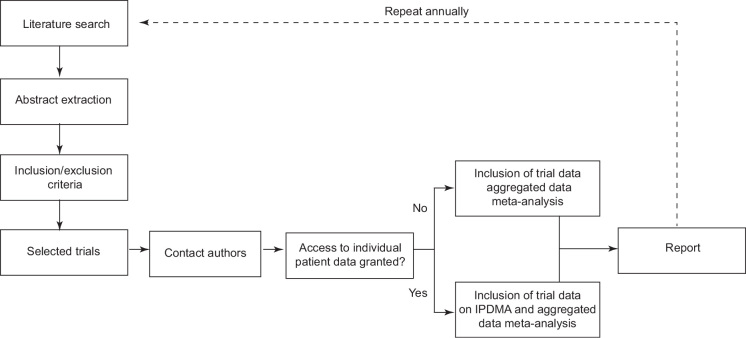
An overview of this living systematic review and meta-analysis is shown in Figure 1. We will follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P).16 The final report will adhere to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Individual Participant Data (PRISMA-IPD).17
Figure 1.
Description of living systematic review
IPDMA = individual patient data meta-analysis.
Eligibility criteria
We will consider only randomised controlled trials that meet the following criteria:
-
•
study population includes adults treated in an ICU, defined as patients aged ≥ 16 years being treated in an ICU that can provide invasive mechanical ventilation and advanced organ support to an individual patient for an unlimited period of time;
-
•
random allocation of individuals or clusters of patients to administration of a balanced crystalloid solution or saline; and
-
•
study fluid (balanced or saline) is to be administered in the ICU for the duration of the ICU stay. For studies with a primary outcome of landmark mortality, study fluid (balanced or saline) is to be administered in the ICU until the time of landmark mortality, which must be at a minimum of 28 days. Studies that truncated the use of study fluid in the ICU before 28 days will not be included. We will not consider trials that meet the following criteria:
-
•
trials that use colloid as part of the intervention (eg, trials that compared colloids in saline to colloid in balanced solutions);
-
•
trials limited to elective surgery patients;
-
•
trials in which loss to follow-up, excluding loss due to withdrawal of consent, exceeds 10% by hospital discharge;
-
•
trials that do not report hospital mortality; and
-
•
trials in which the study fluids were used for fluid bolus therapy only.
Participants
All participants enrolled in trials that are eligible will be included.
Definition of intervention
We will define as a balanced solution any crystalloid solution in which the sodium concentration exceeds the chloride concentration by at least 15 mmol/L. Examples include Hartmann’s solution, lactated and acetated Ringer’s solutions, Plasma-Lyte A and Plasma-Lyte 148. The active comparator will be 0.9% sodium chloride (normal saline, saline).
Information sources and trial identification
The first iteration of this living meta-analysis will include trials identified in a search that is up to date on 1 March 2022; on that date, we will perform or update computerised searches in MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials.gov, the Australian New Zealand Clinical Trials Registry (ANZCTR), Japan’s University Hospital Medical Information Network (UMIN) Center, and the Brazilian Registry of Clinical Trials (ReBEC). We will also check references from included manuscripts for other potentially eligible trials and contact experts in the field.
Search strategy and selection of trials
The following search strategy will be used, and results will be filtered to include only clustered or individually randomised trials: “Balanced crystalloid solutions OR balanced salt solutions OR buffered crystalloids OR lactated Ringer’s OR Hartmann’s OR Plasma-Lyte OR crystalloid OR normal saline OR 0.9% sodium chloride OR isotonic saline OR saline”. Both text words and their respective MeSH terms will be used. This search is similar to the one used for the recent aggregate data meta-analysis. Only studies reported in English will be considered, with no restriction on date of publication.
Two investigators will independently screen all potentially eligible trials based on review of titles and abstracts, followed by full review of reports of trials identified as likely to be eligible. Disagreements will be resolved by consensus or adjudication by a third investigator. Investigators will be excluded from screening any trial in which they were involved in any way.
Data extraction and data collection
For aggregated data, studies from the search will be imported to a reference management software. At least two investigators will perform a systematic data extraction using a dedicated form. Disagreements will be resolved by consensus or adjudication by a third investigator. Reviewers cannot extract data of any trials in which they were involved in any way. Principal investigators of the selected trials will be invited to submit patient-level data to be included in the IPDMA. Trials will be added to the aggregated data meta-analysis regardless of the trial author’s decision to provide patient-level data to be included in the IPDMA.
For the first iteration, data will be locked using all data received by 30 June 2022. Thereafter, the search will be repeated on a yearly basis and new trials added if they meet the above eligibility criteria. Investigators will provide de-identified data from all enrolled patients including all data available on the domains shown in Table 1. We will make at least two attempts to assure that trialists receive an invitation to submit data for the IPDMA. For those who agree to submit patient-level data, a formal data-sharing agreement will be agreed and signed between the relevant parties and the IPDMA coordinating centre. Data will be shared online in a secure environment in a manner that complies with all applicable data security and privacy laws and regulations. Data will be stored solely for the purpose of the IPDMA.
Table 1.
Data to be collected from included trials
| Domains | Data |
|---|---|
| Baseline (pre-randomisation) | Age, sex or gender, ethnicity Days from trial initiation (first enrolment) to randomisation Enrolling ICU code or cluster identification Days from ICU admission to randomisation Type of ICU admission (medical, elective surgery, non-elective surgery) Presence of specific conditions at enrolment:
Laboratory values at enrolment (arterial blood pH, base excess, serum chloride, serum potassium, serum creatinine concentrations) |
| Randomisation | Treatment allocation (balanced solution or saline) Type of balanced solution used |
| Follow-up (daily or whenever available) | Laboratory values of serum creatinine, chloride, bicarbonate, pH, base excess Organ support use:
|
| Outcomes | Vital status at last follow-up Days from enrolment until ICU discharge Days from enrolment until hospital discharge Treatment with and duration of new treatment with RRT Treatment with and duration of treatment with mechanical ventilation |
ICU = intensive care unit; RRT = renal replacement therapy.
Data will be harmonised following the principles outlined on Table 2. Investigators will provide raw data using their format of choice. Data will be used to populate a main dataset, which will be used for analysis.
Table 2.
Variable harmonisation
| Variable | Description |
|---|---|
| Trial information | |
| Trial name | The name of the trial |
| Design | Cluster or individual randomisation |
| Cluster (for cluster trials) | The cluster name (or period) for cluster trials |
| Enrolling site (for individual randomisation trials) | A code for enrolling site, used for hierarchical modeling — it will be anonymised before use |
| Intervention | Balanced solution used in the intervention group; if more than one type was used, this will be labelled as “mixed” |
| Baseline patient data | |
| Age | Patient age at enrolment (in years) |
| Enrolment arm | Whether patient was enrolled to receive balanced solutions or 0.9% saline. For cluster trials, we will consider the same group that was used for the trial primary analysis. This is important, for example, in trials with multiple crossovers for patients that remained in the ICU for a long period. Should this occur, we will attribute as arm, for that given patient, the same arm that was used for the trial primary analysis |
| Admission type | Type of ICU admission (medical, elective surgery, non-elective surgery). This will be collected as reported in each trial. As a general rule, elective surgery will be defined as any surgical procedure scheduled for more than 24 hours. Trauma admissions will be defined as medical, or non-elective surgery if admitted to the ICU from the operating or recovery room |
| Days from ICU admission to enrolment | The number of days from the patient’s ICU admission to trial enrolment. If in hours, it will be converted to days |
| Sepsis | Presence of sepsis at enrolment, as defined by each study. The definition used for each trial will be noted and reported in the Online Appendix of the resulting manuscript |
| Traumatic brain injury | Presence of traumatic brain injury, as defined by each trial, at enrolment |
| Non-traumatic acute brain injury | Presence of a diagnostic code on the case report form compatible with subarachnoid haemorrhage, ischaemic stroke, intracerebral haemorrhage, mass lesion due to tumour, post-cardiac arrest encephalopathy, and central nervous system infection |
| Use of vasopressors at enrolment | Use of any dose of continuous vasopressors (eg, norepinephrine, epinephrine, dopamine, phenylephrine, angiotensin II) at enrolment |
| Dose of norepinephrine at enrolment | Dose of norepinephrine at enrolment. This will be preferable reported in mg/kg/min as a continuous variable. It may also be alternatively reported as categories, especially when values are below or above 0.3 mg/kg/min |
| Use of dobutamine, dopamine, milrinone or levosimendan enrolment | Whether the patient was receiving each of the mentioned inotropes, at any dose, at enrolment. Dose will not be recorded |
| Mechanical ventilation at enrolment | Whether the patient was receiving invasive or non-invasive mechanical ventilation at enrolment |
| Fluid use in the 24 hours before enrolment | Whether the patient received any volume of fluid therapy in the 24 hours before enrolment (yes or no), defined as any prescription of fluid for either volume expansion or maintenance, as recorded in the case report form |
| Volume of 0.9% saline before enrolment | Volume of 0.9% saline used in the 24 hours before enrolment |
| Volume of balanced solution before enrolment | Volume of balanced solution (Hartmann’s solution, lactated and acetated Ringer’s solutions, Plasma-Lyte A and Plasma-Lyte 148) used in the 24 hours before enrolment |
| Renal replacement therapy | Whether the patient was receiving any type of RRT before enrolment, regardless of modality (intermittent, continuous etc) |
| Laboratory values at enrolment | If available, we will collect the laboratory values of pH, base excess, sodium, chloride, and creatinine closest to enrolment. A window of up to 12 hours will be allowed in case laboratory values are drawn after randomisation |
| Daily data (on all days data are available) | |
| Use of mechanical ventilation | Whether the patient was treated with mechanical ventilation on that day. If the number of hours of mechanical ventilation is available, we will consider any day when the patient spent more than 12 hours on mechanical ventilation as one day of mechanical ventilation |
| Use of vasopressors | Whether the patient was treated with vasopressors for any period, regardless of dose, during that day |
| Use of inotrope | Whether the patient was treated with inotropes for any period, regardless of dose, during that day |
| Use of RRT | Whether the patient was treated with RRT on that day. This counts for any renal replacement method (continuous or intermittent) |
| Laboratory values at enrolment | If available, we will collect daily laboratory values of pH, base excess, sodium, chloride, and creatinine closest to enrolment. If more than one measurement is available for the same day, we will use the worst value: lowest pH, lowest base excess, highest sodium level and highest chloride level |
| Days from enrolment until last follow-up | Number of days between enrolment and last follow-up, in days |
| Outcomes | |
| Vital status at last follow-up | Whether the patient was alive or dead at last follow-up |
| Days from enrolment until ICU discharge | Number of days between trial enrolment and first discharge from ICU |
| Days from enrolment until hospital discharge | Number of days between trial enrolment and first discharge from hospital |
| Treatment and duration of RRT | Number of days the patient was treated with RRT. This is the number of days between first RRT and the last session. All days between RRT sessions, in the case of intermittent RRT, will be considered as days treated with RRT |
| Treatment with and duration of mechanical ventilation | Number of days the patient spent treated with mechanical ventilation during the ICU stay. We will use data as reported on each trial. If available, we will harmonise information assuming that any day the patient spent more than 12 hours on mechanical ventilation counts as a full day on mechanical ventilation. This is especially important during the weaning phase and for patients with tracheostomy. If information is not available, we will consider it as reported on each trial |
ICU = intensive care unit; RRT = renal replacement therapy.
Evidence quality and bias assessment
We will use the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach to assess overall evidence quality from the included studies.18 Bias will be assessed using Cochrane Collaborators tools.19 Publication bias will be assessed by inspection of funnel plots and use of the Egger test; assessment of heterogeneity using funnel plot for aggregated meta-analysis will only be performed if at least ten studies are included.20
Data synthesis and statistical analysis
Baseline information of all included patients stratified by group (balanced v saline) will be displayed in a table, with data being reported as frequencies and percentages (for counts), and median and interquartile range or mean and standard deviation (SD), as appropriate, for continuous variables. Additionally, information displaying patient features enrolled on each trial will be presented in an Online Appendix.
We will perform both aggregate and individual patient meta-analyses. Results of aggregated meta-analysis will be displayed for comparison with IPDMA data to assess the potential for bias due to the absence of trials for which we do not obtain patient-level data. An aggregate data meta-analysis will be performed only for primary endpoint (hospital mortality) and proportion of patients newly treated with RRT. This analysis will be performed under a Bayesian framework considering random effects. Cluster studies will have their sample size corrected for their interclass correlation coefficient.
Before conducting the main analysis, anonymised data from each individual trial will be re-analysed for primary and secondary endpoints following the analysis plan described in the primary study publication. This is to ensure that findings are consistent with published results — any discrepancies will be resolved with the trial’s principal investigator to ensure data have not been corrupted in the transfer to the master database. Subsequently, a one-stage meta-analysis approach will be used by first combining the data and then analysing the combined data according to the pre-specified statistical analysis plan agreed by the steering committee. The statistical analysis plan will be published before any analysis is started; the principles of the analysis are outlined below.
All endpoints will be analysed under a Bayesian framework. The model for the primary endpoint (hospital mortality) will be a Bayesian hierarchical model adjusted for the intervention of interest (balanced solution v saline). There will be two layers in the hierarchical model, the ICU (or cluster) nested within trial. The prior for the intervention will be set as a neutral regularising normal prior (mean, 0 [SD, 0.355]; probability masses, 0.95 [odds ratio, 0.5–2.0). Sensitivity analyses will consider different priors to be decided by the steering committee before the data analysis. Sensitivity priors will include both optimistic and pessimistic priors.21 A sensitivity analysis using a frequentist model will also be conducted.
Survival regression models for the key secondary endpoint will be made using a parametric survival model following parametric (Weibull distribution) or semi-parametric (Cox) models (depending on which better fits the data) adjusted for the intervention of interest and the hierarchical effects, similar to the analysis for the primary outcome. The proportion of patients treated with RRT will be assessed similarly to the main endpoint, using a Bayesian hierarchical logistic model. Continuous endpoints will be assessed considering linear or count models, as appropriate. Our primary analysis will be based on complete cases.
Subgroup analyses
Subgroup analyses will be performed exclusively for individual patient data for hospital mortality and new treatment with RRT. Subgroups of special interest may be analysed in more detail after investigation of heterogeneity of treatment effects.17 Further analysis will follow a separate pre-approved statistical analysis plan. Additional subgroups may be considered in future iterations should new trials identify populations in which treatment effects may differ. For subgroup analyses, we will use data from any trial for which the subgroup classification can be made. Planned subgroups for the first iteration are as follows and are subject to data availability:
-
•
patients with sepsis, as defined by each trial versus those without sepsis;
-
•patients with TBI, as defined by each trial versus those without TBI:
-
►secondarily, this analysis will also consider the effects under three dummy-coded categorical groups — non-traumatic acute brain injury versus TBI versus nonacute brain injury. We will consider acute brain injury to exist in patients admitted to the ICU following a cerebrovascular event (ischaemic or haemorrhagic stroke), subarachnoid haemorrhage, cerebral venous thrombosis, acute intracranial mass effect due to tumour, cardiac arrest-related hypoxic-ischaemic encephalopathy, or others, depending on availability of data;
-
►
-
•
baseline plasma chloride concentration stratified in three groups (low, < 100 mmol/L; normal, 100–110 mmol/L; and high, > 110 mmol/L).
-
•
arterial pH, in four groups (severe acidaemia, pH < 7.20; mild acidaemia, pH 7.20–7.34; normal, pH 7.35–7.45; alkalaemia, pH > 7.45);
-
•
volume of saline received before enrolment — this will be stratified into three groups (no previous saline received; 1–999 mL saline received; or ≥ 1000 mL saline received);
-
•
treatment effect in women versus men, using sex as reported in each trial; and
-
•
patients recruited to cluster randomised versus individual randomisation trials.
Management and coordination
The project will be managed by the steering committee. The secretariat will be based at the HCor Research Institute in São Paulo, Brazil. If any study investigator requests it, their individual data can be removed entirely from individual analyses after written notification to the steering committee; however, data will be retained for manuscripts that have already been published or submitted. The IPDMA will use existing data from trials. Data storage and management will be done as described above.
Publication policy
Publications will be in the name of the Balanced vErsus Saline Trialists Living Systematic review and Meta-analysis (BEST-Living) Collaboration. Each manuscript will have a writing committee, which will include all steering committee members who fulfil the International Committee of Medical Journal Editors (ICMJE) authorship criteria. The first manuscript will be led by Fernando Zampieri, with Simon Finfer as senior and corresponding author. In addition to this protocol, we plan to make the statistical analysis plan freely available before the data analysis is performed, and it will be updated yearly as required. Any subsequent report will be authored by all members of the steering committee who meet the ICMJE authorship criteria.
Discussion
We present the protocol for a living systematic review with aggregate and IPDMA to assess the effects of use of balanced solutions compared with saline for fluid therapy in ICUs. This IPDMA will provide the highest available evidence to assess the comparative risks and benefits of balanced solutions compared with saline in ICU patients.
This analysis will provide data to determine the impact of fluid choice on important outcomes in critically ill patients, not limited to mortality. Given that the difference between various crystalloid solutions is likely to be small, this IPDMA will increase power to detect a small but important difference. As millions of people are treated in ICUs each year, small differences in outcomes and costs may be important to patients, clinicians and health care planners. Existing data suggest a potential benefit of balanced solutions in patients with sepsis,22 and potential harm in those with TBI.9 The inclusion of a large number of patients compared with previous individual studies is a major advantage that will allow us to investigate these, and other, possible subgroup effects.
Acknowledgments
Acknowledgements:
This research was completed during the tenure of a Health Research Council of New Zealand Clinical Practitioner Research Fellowship held by Paul Young. The Medical Research Institute of New Zealand is supported by Independent Research Organisation Funding from the Health Research Council of New Zealand. John Myburgh and Naomi Hammond are supported by National Health and Medical Research Council (NHMRC) Investigator Grants. Simon Finfer is supported by an NHMRC Practitioner Fellowship. This study is not externally funded but received institutional support by HCor-Hospital do Coração, Brazil and the George Institute for Global Health, Australia.
Authors’ contribution:
FGZ, ABC and SF are guarantors of this study. This individual patient data meta-analysis (IPDMA) was conceived by SF, FGZ, FRM, NH and PY. All other authors were invited to join the steering committee and helped design and draft the protocol. LPD, FGZ, and GLDT developed the statistical methods which were agreed by all authors. All authors reviewed and approved the final draft.
Steering Committee:
The members of the steering committee for the Balanced Fluid Trialists Collaboration are Fernando G Zampieri, Alexandre B Cavalcanti, Gian-Luca Di Tanna, Lucas P Damiani, Naomi Hammond, Flavia R Machado, Sharon Micallef, John Myburgh, Todd W Rice, Matthew W Semler, Paul J Young, and Simon Finfer (Chair).
Competing interests
FGZ reports receiving grants for investigator-initiated trial from Ionis Pharmaceuticals (USA) and logistics support from Baxter Hospitalar (Brazil) for the BaSICS trial. SF / JM / NH / SM received unrestricted grants from Baxter and CSL (Paid to their institution).
References
- 1.Myburgh J.A., Mythen M.G. Resuscitation fluids. N Engl J Med. 2013;369:1243–1251. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 2.Finfer S., Liu B., Taylor C., et al. SAFE TRIPS Investigators. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yunos N.M., Bellomo R., Hegarty C., et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 4.PlasmaLyte Rizoli S. J Trauma. 2011;70(Suppl):S17–S18. doi: 10.1097/TA.0b013e31821a4d89. [DOI] [PubMed] [Google Scholar]
- 5.Young P., Bailey M., Beasley R., et al. SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701–1710. doi: 10.1001/jama.2015.12334. Erratum in: JAMA 2015; 314: 2570. [DOI] [PubMed] [Google Scholar]
- 6.Semler M.W., Wanderer J.P., Ehrenfeld J.M., et al. SALT Investigators and the Pragmatic Critical Care Research Group; SALT Investigators. Balanced crystalloids versus saline in the intensive care unit. The SALT randomized trial. Am J Respir Crit Care Med. 2017;195:1362–1372. doi: 10.1164/rccm.201607-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semler M.W., Self W.H., Wanderer J.P., et al. SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zampieri F.G., Azevedo L.C.P., Corrêa T.D., et al. BaSICS Investigators and the BRICNet. Study protocol for the Balanced Solution versus Saline in Intensive Care Study (BaSICS): a factorial randomised trial. Crit Care Resusc. 2017;19:175–182. [PubMed] [Google Scholar]
- 9.Zampieri F.G., Machado F.R., Biondi R.S., et al. BaSICS investigators and the BRICNet members. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021;326:1–12. doi: 10.1001/jama.2021.11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finfer S., Micallef S., Hammond N., et al. PLUS Study Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Balanced multielectrolyte solution versus saline in critically ill adults. N Engl J Med. 2022;386:815–826. doi: 10.1056/NEJMoa2114464. [DOI] [PubMed] [Google Scholar]
- 11.Hammond N.E., Zampieri F.G., Di Tanna G.L., et al. Balanced crystalloids versus saline in critically ill adults: a systematic review with meta-analysis. NEJM Evid. 2022 doi: 10.1056/EVIDoa2100010. [DOI] [PubMed] [Google Scholar]
- 12.Riley R.D., Lambert P.C., Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340 doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 13.Siemieniuk R.A., Bartoszko J.J., Ge L., et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2980. Erratum in: BMJ 2021; 373: n967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott J.H., Turner T., Clavisi O., et al. Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidler A.L., Hunter K.E., Cheyne S., et al. A guide to prospective meta-analysis. BMJ. 2019;367 doi: 10.1136/bmj.l5342. [DOI] [PubMed] [Google Scholar]
- 16.Shamseer L., Moher D., Clarke M., et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350 doi: 10.1136/bmj.g7647. Erratum in: BMJ 2016; 354: i4086. [DOI] [PubMed] [Google Scholar]
- 17.Stewart L.A., Clarke M., Rovers M., et al. PRISMA-IPD Development Group Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313:1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne J.A.C., Savovi J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20.Sterne J.A., Sutton A.J., Ioannidis J.P., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343 doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 21.Zampieri F.G., Casey J.D., Shankar-Hari M., et al. Using Bayesian methods to augment the interpretation of critical care trials. an overview of theory and example reanalysis of the alveolar recruitment for acute respiratory distress syndrome trial. Am J Respir Crit Care Med. 2021;203:543–552. doi: 10.1164/rccm.202006-2381CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown R.M., Wang L., Coston T.D., et al. Balanced crystalloids versus saline in sepsis. A secondary analysis of the SMART clinical trial. Am J Respir Crit Care Med. 2019;200:1487–1495. doi: 10.1164/rccm.201903-0557OC. [DOI] [PMC free article] [PubMed] [Google Scholar]