Abstract
Background:
Polycystic ovary syndrome (PCOS) is a common endocrinological disorder associated with abdominal obesity (AO) and some reproductive complications including low pregnancy rate. Embryo-endometrium cross-talk has a key role in successful embryo implantation and subsequent normal pregnancy rate. The primary objective of this study is to evaluate the decidualization potential of endometrial stromal cells (ESCs) using the embryo condition media (ECM) collected from PCOS patients with AO, compared to ECM of those patients without AO.
Materials and Methods:
In this experimental study, we measured the capacity of ECM collected from PCOS patients with or without AO for decidualization induction in healthy ESCs after coculture. A total number of 53 embryos from 40 couples belonging to PCOS with AO, PCOS without AO, nonPCOS with AO, and nonPCOS without AO patients, were included in our study. The embryosof four groups were single-cultured up to the blastocyst stage. Their ECM (45λ/well) were pooled and added to healthy ESCs monolayer culture media to investigate their effects on decidualization potential via gene (PRL, IGFBP1, IL1-β, HOXA10, IL-6 and TNF-α) and protein (PRL, IGFBP1, IL1-β) expression analysis and ESCs migration assay.
Results:
The morphological analysis, migration assay (P≤0.0321), protein (P≤0.0139) and gene expression analysis showed PCOS with AO accounted for the highest gene (PRL, IGFBP1, IL1-β, HOXA10, IL-6, TNF-α) and protein markers (PRL, IGFBP1, IL1-β) (P≤0.05). NonPCOS individuals without AO had the lowest level of both gene and protein decidualization markers (P≤0.05).
Conclusion:
Considering decidualization as an inflammatory process, a higher level of decidualization markers was associated with a higher inflammatory status created by AO and PCOS, separately. Inflammation may disrupt the process of inflammatory to anti-inflammatory phase required for prevention of pregnancy loss, this could explain the high rate of abortion in these cases.
Keywords: Abdominal Obesity, Decidualization, Polycystic Ovary Syndrome, Stromal Cells, Supernatant
Introduction
In recent years, polycystic ovary syndrome (PCOS) diverted the attention to the reproductive-age women, who suffer of this disorder with a 4-21% prevalence rate (1). PCOS has been considered a heterogenic endocrine and metabolic disorder with pathological characteristics includes hyperandrogenemia (HA), anovulation, polycystic ovary disease, obesity, insulin resistance (IR), and infertility. This syndrome has been recognized as a low-grade chronic inflammatory disease, but the exact mechanism of this association has not been fully understood. It has been demonstrated that both natural and assisted reproductive technology (ART) cycles in PCOS patients are challenging due to low pregnancy rate, reduced live birth rate and high miscarriage rate even after a high-quality embryos transfer in ART plans (2). It seems that other pathological complications, including endometrial insufficiency, may contribute to the pathogenesis of infertility disorders (3).
Recent evidence shows that IR, HA and obesity play an essential role in the PCOS pathogenesis and its consequences (4). Obesity contributes to one of the most critical complications among PCOS women, in terms of their reproduction (5). Obesity (body mass index, BMI>30) affects 61-76% of these patients (6). A cohort study in more than 9500 ART cycles indicates that obesity may lead to embryonic implantation failure, which is caused by an impaired endometrial receptivity (7). In PCOS patients, the presence of metabolic disorders, inflammation and endocrine abnormalities, the same features that we observe in obesity, play a significant role in ovulation function, oocyte quality, endometrial receptivity and miscarriage rate (8). The implantation disorder and abortion occurrence in these patients indicate that the cross-talk between the embryo and the endometrium is probably disturbed.
Naturally, successful embryo implantation depends on a receptive endometrium, the presence of a goodquality blastocyst, and synchronization of embryoendometrium cross-talk. In addition, before the embryo’s implantation, there is a communication between the uterus and the blastocyst that occurs during a specific period of time in which implantation is possible (9).
There is an evidence that the embryo-endometrium cross-talk is carried out through the secretion of various factors by an embryo (10). Previous studies showed that interleukins, immunosuppressive factors and growth factors can be isolated from the embryo condition media (ECM) (11). In order to induce the expression of integrins, endometrial leukemia inhibitory factor, and other inflammatory factors that improve the rate of implantation via facilitating embryo-endometrium cross-talk (12).
Endometrial receptivity, a physiological changes complex, provides an appropriate environment for the different phases of an embryo implantation, including the opposition, attachment, invasion, and decidualization. In humans, decidualization occurs routinely during the latter half of each menstrual cycle and disappears in the absence of an embryo in the endometrium (13). Decidualization is a morphological and functional change of stromal cells under ovarian steroidal hormones (estrogen; E2 and progesterone; P4) secretion to prepare these cells to accept the embryo (14). Prolactin (PRL) and insulin like growth factor binding protein 1 (IGFBP-1) are two specific markers of decidualization process (15). In addition, homeboxA10 (HOXA10) is an essential inducer factor for promoting decidualization (16). Induction of decidualization requires the expression of inflammatory cytokines and low-grade inflammation such as interleukin 1-beta (IL-1β) (17), tumor necrosis factor alpha (TNF-α) (17), and IL-6 (18). It has been shown that an excessive inflammation in the implantation process can enhance the decidualization process (19).
Considering that in ART, the embryo is cultured until the blastocyst stage on day 5, the cross-talk between the uterus and the embryo may be lost. For this purpose, Goto et al. (20) evaluated a new method called stimulation of endometrium embryo transfer (SEET). In their experiment, ECM from a fresh cycle was frozen and then injected into the uterus of patients before blastocyst transfer, that led to an increase in the pregnancy rate. Therefore, this approach may help us to define the embryo capacity for decidualization induction in endometrial stromal cells (ESCs) which required for embryo implantation.
The implantation rate following a blastocyst transfer has been around 4-55% worldwide, and it has remained constant over the last decades (21). A higher rate of live birth in a blastocyst transfer procedure in comparison with the cleavage stage transfer, placed it in the first line of ART. Accordingly, the embryo- endometrial cross talk lack can be accounted for main reason of a low implantation rate after in vitro embryo transfer (22). An impaired decidualization creates a series of reproductive disorders e.g., miscarriage, implantation and various pregnancy complications (13).
The exact mechanism by which the PCOS and abdominal obesity (AO) may affect the outcome of both natural and ART cycles is not yet well understood. Therefore, in the present study, we intend to investigate the independent roles of PCOS or/and obesity on the implantation rate of embryos which is characterized by ESCs decidualization process.
Materials and Methods
This experimental study was approved by the Ethical Committee of Royan Institute, Tehran, Iran (IR.ACECR.ROYAN.REC.1400.004). All volunteer participants submitted their written informed consent. All volunteer participants were invited among aged 25-35 infertile couples who had referred to the Royan Institute Tehran, Iran, between 1st May until 1st December in 2021.
For isolating ESCs, we had to invite healthy married fertile women who were referred to the Royan Clinic, Tehran, Iran, for a diagnostic laparoscopy for pelvic pain without any uterine abnormality. After describing the aim and procedure of our study, of those who were volunteering to take part in this trial expressed their willingness and provided their self-signed consent for participation.
Study population and group design
Totally, 53 infertile couples, including PCOS affected and normal oogenesis women participated in this experimental study. According to the Rotterdam criteria, 25 women diagnosis a PCOS affected. The normal group included 28 normal oogenesis women who were placed in the ART indication due to their husbands’ infertility. Different plans, including in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI) and frozen embryo transfer (FET) were considered for them. The participants were divided into four subgroups: the PCOS patients with AO (waist/hip ratio ≥0.80) named as PCOS+- AO+ group. The PCOS patients without AO named as PCOS+- AO– group. Non-PCOS patients with AO considered as PCOS--AO+ group, and non-PCOS patients without AO were as PCOS--AO- group. We designed two supplementary groups, ESCs treatment neither ECM nor induction medium [control induction (CI)] and ESCs which were treated with only induction medium [control supernatant (CS)]. The participants who suffered an obvious sign of systemic inflammatory diseases, including diabetes, hypertension, hypothyroidism, hyperthyroidism, ankylosing spondylitis (AS), gout, rheumatoid arthritis, scleroderma, and systemic lupus erythematosus were excluded of this study. Also, patients without at least three months before the start of the present study anti-inflammatory drug consumption history were included Ibuprofen, Diclofenac and Mefenamic acid made this dug box.
Embryo condition media preparation
Ovarian hyperstimulation and embryo freeze transfer protocol
Our patients underwent standard ovarian stimulation protocol, according to age ≤36 years (23). For this aim, during the first 6 days of their menstrual cycle, 150 IU of the recombinant follicle-stimulating hormone (follitropin-b, MSD, Ballerup, Denmark) was injected daily. On day 2 or 3, controlled ovarian stimulation (COS) was performed until ovulation induction. Through the serial vaginal ultrasonography monitoring, on day 6, 0.25 mg dosage of GnRH antagonist (Ganirelix-Orgalutranw; MSD, Ballerup, Denmark) was used in a daily injection. When the patients had at least 2-3 retrievable follicles with an average diameter of ≥13 mm, 6500IU dosage of the human chorionic gonadotrophin (hCG, Ovitrelle; Merk Serono, Hellerup, Denmark) was used to induce final oocyte maturation. The “Freeze all strategy” was performed for the high-risk ovarian hyper stimulation syndrome (OHSS) patients who had more than 15 follicles. Our patients who had <15 follicles and/or estradiol level <3000, received a 10000 IU hCG. Oocytes retrieved was performed 36-38 hours after hCG injection using a standard ultrasonically guided follicular puncture. Subsequently, these oocytes underwent an IVF/ICSI process.
The vitrification and warming were performed according to the previously protocols (24). For this aim the 3rd of embryos culture, high quality ones (≥8 cells and ≤25% fragmentation) were incubated for 5-15 minutes at the room temperature (RT) in the equilibrium solution. Then, the embryos were placed 1 minutes at RT in the vitrification solution.
For warming, the Cryotop was taken out of liquid nitrogen and the embryos were exposed for 50-60 seconds at 37.0°C to the thawing solution. Then, the embryos were transferred into the dilution solution (0.5 mol/L sucrose) for 3 minutes at RT. After which, the embryos were converted to another solution (0.25 mol/L sucrose) for 3 minutes at RT. The warmed embryos were washed four or five times in washing solution (VitROwash medium supplemented with 20% Albuminal-5) and then cultured in SAGE-1 stepTM (ORIGIO® CULTURE MEDIA, Denmark) until day 5. After 48 hours culture, the embryos were transferred to mother’s uterus in the FET cycle and the ECM residual were collected and stored at -080°C until use. Our study participants included 53 embryos from 40 infertile couples (25 PCOS and 28 normal oogenesis) who were subdivided into patients with or without AO. It is as follows: PCOS+-AO+ (n=12), PCOS+-AO- (n=13), PCOS--AO+ (n=13) and PCOS--AO- (n=15).
Endometrial stromal cells isolation and in vitro culture
The tissue biopsies of healthy participants were obtained during the secretory phase of the menstrual cycle. The collected biopsies were transferred to a medium for transfer containing phosphate-buffered saline (PBS, Gibco, USA) (0.8 g NaCl, 1.44 g Na3HPO4, 0.2 g KCl, and 0.24 g KH2PO4), 2% pen/strep (Gibco, USA) and 1% anti-fungal (Gibco, USA). Then, tissue biopsies were washed in the PBS three to four times under the sterile condition in a petry dish (Falcon, USA). After mechanical digestion, the collected tissue biopsies (≈1 mm3) were transferred to an enzymatic digestion medium (0.0125 g of collagenase type IV, Gibco, USA), dissolved in 5 ml of Dulbecco’s modified eagle medium nutrient mixture F-12 (DMEM-F12, Gibco, USA), then incubated at 37°C in a water bath. The digested samples were filtered through 100, and 40 μm mesh filter, respectively. The final filtered cells were centrifuged at 1200 rpm for 20 minutes. The sediment was suspended in 1 ml of DMEM-F12 medium (1.2 g DMEM-F12, 0.24 g NaHCO3, 0.005 g penicillin, and 0.006 g streptomycin) containing 15% fetal bovine serum (FBS, Gibco, USA). Primary ESCs were cultured up to first passage, due to long term in vitro culture can alter their decidualization potential (25). After first passage, 70000 cells/150λ were transferred to each 24-well dish (Falcon).
Decidualization induction of endometrial stromal cells
To induce in vitro decidualization conditions in the ESCs, in each well of 24 wells, 200 μL of induction medium including DMEM-F12+FBS 2%+8-Br-cyclic adenosine monophosphate (cAMP, 0.5 mM, Sigma) and medroxyprogesterone acetate (MPA, 1 μM; Sigma) were added and incubated at 37ºC, 5% CO2 for 48 hours. The ESCs morphological change during 48 hours after decidualization induction was recorded by light microscopy and was including appearance of rounded, epithelioid‐like cells.
Culture of induced endometrial stromal cells with the embryo condition media
The primary induced ESCs were cultured with the ECM collected from different experimental groups [PCOS+-AO+ (n=12), PCOS+-AO– (n=13), PCOS--AO+ (n=13) and PCOS-- AO- (n=15)]. For this aim, 150 μL/well of pooled ECM (30% dilution: 30% ECM+70% DMEM-F12 with 10% FBS) was added per well in 24 wells and incubated for 12 hours (26).
Evaluation of decidualization capacity in cultured induced cells with the embryo condition media
For all groups, the ECM was removed after a 12 hours exposure of the ESCs with the ECM and replaced by the media culture (DMEM-F12 supplemented with 10% FBS). Then, the collected condition media were stored at -80°C until evaluation by Enzyme linked immunosorbent assay (ELISA). The concentration of two decidualization biomarkers PRL (M06L1H1, ng/ml, Monokit Prolactin ELISA Kit inc., Iran), and IGFBP-1 (ZB-OEH543211103-21, ng/ml, Zellbio assay kit Inc., Germany), as well as pro-inflammatory cytokine, IL1-β (HL1-0222002, pg/ml, Biotech assay kit Inc., Iran) was evaluated using commercial ELISA kits, according to the manufacturer’s protocol.
Assessment of migratory capacity in endometrial stromal cells cultured with the embryo condition media
The wound healing assay was performed according to our previous study (23) with some modification due to ECM limitation in this study for this aim, we used 24 well plate for performing a modified wound healing assay to investigate the migration capacity of ESCs. Then 70.000 cells were seeded into each well of 24 well plate. A wound was created by a sterile christal pipette tip on the centre of each well. Subsequently, the ESCs were treated with 0.1 mg/ml of mitomycin C (Sigma) to prevent cell proliferation for 2 hours, followed by washing samples twice with 1% PBS. Then, 200 μL DMEM-F12+10% FBS were added to each well. The ESCs migration capacity was evaluated at 0, 6, 12, 18, and 24 hours after wounding. The grove width was measured at each time point, using Image J software following the below formula:
Measuring of narrowing furrow width in each time point=The width of the grove at 0 time-The width of grove width at each time point
Evaluation of decidualization gene markers expression in endometrial stromal cells treated with embryo condition media
ESCs were stored in RNA protect solution (Cat. No. 76526, Qiagen, Germany) at -80°C. After thawing, ESCs were centrifuged and total RNA was extracted using RNeasy kit (Qiagen, Germany) according to manufacturer’s instructions. RNA quantity was assessed using “Nanodrop One” spectrophotometer (Thermo Scientific™, Germany) Subsequently, 11 μg RNA was converted into cDNA per manufacturer’s Reverse Transcription kit instruction (SMO-BIO, Taiwan).
All primers were designed by using Primer-BLAST software (NCBI Primer-BLAST website, Table 1). Primers were assessed for specificity, dimers and splice variant detection if applicable. Quantitative polymerase chain reactions (PCRs) were conducted using SYBR Green (Thermo Scientific™) on StepOnePlus™ Real- Time PCR System (Thermo Scientific™), according to the manufacturer’s instructions in strips. Samples reactions were done in duplicate. For each reaction, 2 μl cDNA and 8 μl master mix was used.
Table 1.
Primer sequence for decidualization markers
|
| ||
|---|---|---|
| Gene | Primer sequence (5′-3′) | PCR product (bp) |
|
| ||
| PRL | F: TCTGTATCATCTGGTCAC | 133 |
| R: ATGAACCTGGCTGACTAT | ||
| IGFBP1 | F: GCCCTGCCGAATAGAACTC | 136 |
| R: GTCTCACACTGTCTGCTG | ||
| IL-1β | F: CTGTCCTGCGTGTTGAAAGA | 180 |
| R: TTCTGCTTGAGAGGTGCTGA | ||
| HOXA10 | F: ACAAGCACACCACAATTCTC | 161 |
| R: AATCCAAACAATGTCTCCCTTC | ||
| IL-6 | F: AGGAGACTTGCCTGGTGAAA | 180 |
| R: CAGGGGTGGTTATTGCATCT | ||
| TNF-α | F: CCTCTCTCTAATCAGCCCTCTG | 212 |
| R: GAGGACCTGGGAGTAGATGAG | ||
|
| ||
PCR; Polymerase chain reaction.
Analyse data comparative cycle threshold (CT) method as a fold change and normalize with GAPDH as a reference gene.
RNA was extracted from ESCs which were cultured for 2 days in induction medium supplemented with cAMP and MPA and next 12 hours with ECM. followed by culture in ECM from four patients and two control groups, qRT-PCR was conducted to evaluate PRL, IGFBP1, IL1-β, HOXA10, IL-6, and TNF-α expression among the studied groups.
Statistical analysis
In this research, SPSS version 26 Software (IBM, Germany) was used for statistical analysis. Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as number (%). The normality of the investigated variables was checked with the Shapiro-Wilk test. In case of normality, the one-way ANOVA test and Tukey’s post hoc test, and in case of non-normality, Kruskal-Varis and Conover’s post hoc test were used, and the significance level of P<0.05 was considered as statistically significant. All qPCR results were analysed using the 2-ΔΔCT, as a relative expression method. Chi-square was used to test the possible differences in pregnancy rate among the studied groups. All graphs were drawn using the GraphPad Prism version 9 Software (GraphPad, San Diego, CA).
Results
Our study participants included 25 PCOS and 28 normal oogenesis subdivided into patients with or without AO. Analysis of BMI showed no significant differences between the studied groups (Table 2).
Table 2.
Comparison of the patient’s characteristics among four studied groups
|
| |||||
|---|---|---|---|---|---|
| Characteristics | PCOS+-AO+ (n=12) | PCOS+-AO– (n=13) | PCOS--AO+ (n=13) | PCOS--AO- (n=15) | P value of ANOVA |
|
| |||||
| Age (Y) | 27.4 ± 3.2B | 30.1 ± 2.3AB | 30.8 ± 3.1A | 28.3 ± 4.1AB | 0.025 |
| BMI (kg/m2) | 28.2 ± 3.7 | 28.1 ± 3.9 | 27.3 ± 2.8 | 25.2 ± 3.5 | 0.066 |
| WHR (cm/cm) | 0.85 ± 0.05A | 0.75 ± 0.04B | 0.82 ± 0.05A | 0.74 ± 0.05B | <0.001 |
| Neck circumstance (cm) | 35.9 ± 2.7A | 34.1 ± 1.8AB | 34.4 ± 2.0A | 32.2 ± 1.0B | <0.001 |
| Wiest/Neck (cm/cm) | 2.54 ± 0.17 | 2.35 ± 0.14 | 2.47 ± 0.18 | 2.33 ± 0.17 | 0.002 |
| Arm circumstance (cm) | 30.1 ± 2.9 | 30.5 ± 2.6 | 30.4 ± 3.0 | 28.6 ± 3.2 | 0.270 |
| Wiest/Arm (cm/cm) | 3.03 ± 0.20 | 2.64 ± 0.20 | 2.81 ± 0.20 | 2.65 ± 0.19 | <0.001 |
| TSH (μl/ml) | 2.5 (1.2-3.9) | 2.2 (1.3-3.2) | 2.6 (1.3-3.7) | 1.8 (1.5-2.3) | |
| Previews ART cycles | 1 | 0 | 0 | 0 | |
| Pregnancy rate | 12 (42) | 13 (33) | 13 (54) | 15 (44) | 0.769 |
| Abortion rate | 12 (17) | 13 (15) | 13 (0) | 15 (13) | 0.517 |
|
| |||||
Data is represented as mean ± SD or n (%) or mean (minimum-maximum). PCOS; Polycystic ovary syndrome, AO; Abdominal obesity, BMI; Body mass index, WHR; Waist/hip ratio, TSH; Thyroid stimulating hormone, and ART; Assisted reproductive technology. Similar superscripts indicate a statistically significant difference (P≤0.001).
Decidualization induction in endometrial stromal cells cultured
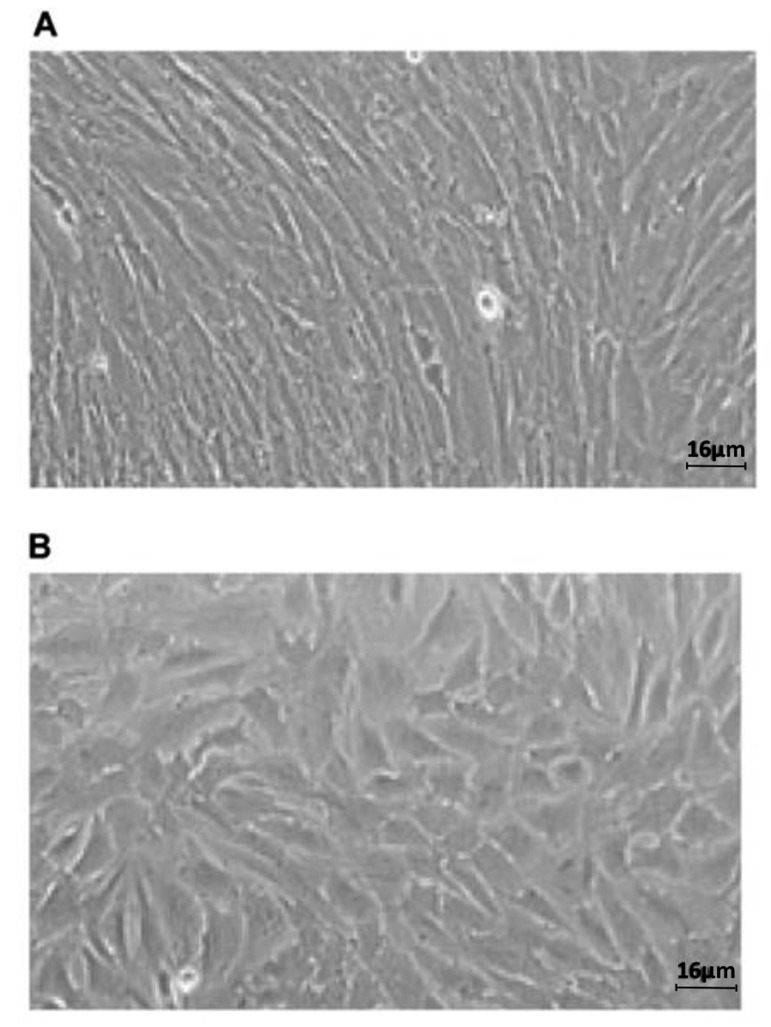
After decidualization induction, the morphological changes of ESCs cells were recorded by light microscopy and visualized (Fig .1).
Fig 1.
Morphological change of ESCs from 24 hours to 48 hours after induction with MPA and cAMP. A. Before decidualization and B. After decidualization (scale bar: 16 μm). ESC; Endometrial stromal cells, cAMP; Cyclic adenosine monophosphate, and MPA; Medroxyprogesterone acetate.
Evaluation of decidualization protein markers
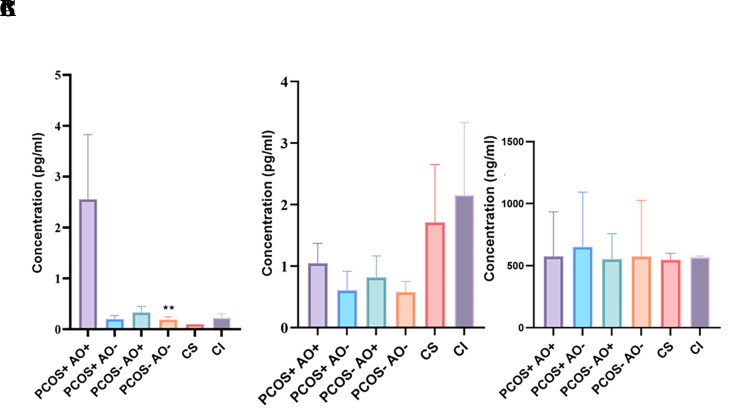
The concentration of secreted IL1-β, PRL and IGFBP-1 proteins in condition media of ESCs cultured by ECM for 12 hours was measured using ELISA kit and is illustrated in Figure 2. Results showed considerably higher secretion level of PRL (P≤0.0139) in PCOS+-AO+ group (2.6 ± 3.8) in comparison with the PCOS+-AO- (0.20 ± 0.19), PCOS- -AO+ (0.33 ± 0.32), PCOS--AO- (0.19 ± 0.16), CS (0.10 ± 0.00), and CI (0.23 ± 0.23) groups. However, levels of IL1-β, and IGFBP-1 were not significantly different among our groups (Fig .2).
Fig 2.
Results of ELISA for quantification of secreted A. PRL, B. IL1-β, and C. IGFBP1 proteins in the ECM from different studied groups by One-Way ANOVA statistical test. PRL; Prolactin, IL1- β; Interluekin 1 beta, IGFBP1; Insulin like growth factor binding protein 1, ECM; Embryo condition media, PCOS; Polycystic ovary syndrome, AO; Abdominal obesity, +; with (PCOS or AO), -; without (PCOS or AO), CI; Control induction, CS; Control supernatant. Stars show a statistically significant difference with PCOS+- AO+ group (**; P<0.01).
Calculating the migration capacity of endometrial stromal cells cocultured with embryo supernatant in different groups
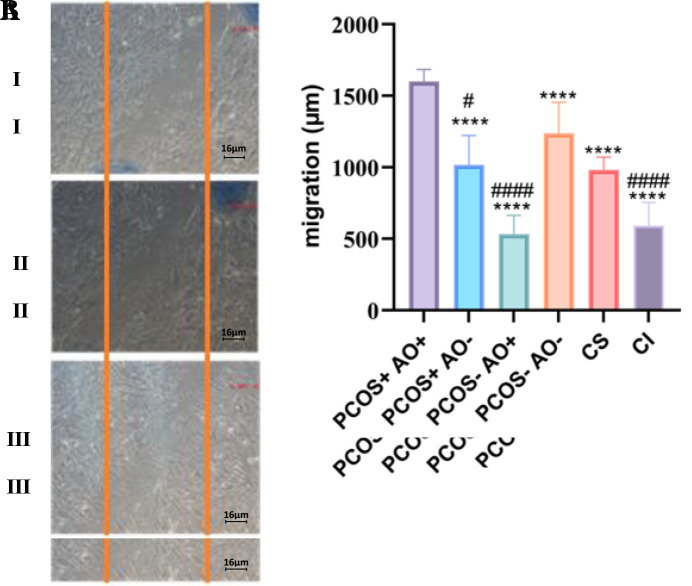
The result of cell migration assay through scratch assay demonstrated that the highest migration capacity was belonged to the PCOS+-AO+ group (1600 μm ± 83.3), and the lowest migration capacity belonged to the PCOS-- AO+ group (532.2 μm ± 132.8). The calculated migration in other groups were PCOS+-AO- group (1017 μm ± 205.3), PCOS--AO- group (1237 μm ± 216.9), CI group (590.2 μm ± 164.7), and CS group (982.2 μm ± 88.3) (P≤0.0321, Fig .3).
Fig 3.
Cell migration assay for ESCs treated with ECM collected from the conditioned media of blastocysts culture from study groups by One-Way ANOVA statistical test. A. Migration of endometrial stromal cells treated with ECM at 6 hours (I), 12 hours (II) and 18 hours (III). B. Migration results of decidualization endometrial stromal cells 18 hours after scratching. PCOS; Polycystic ovary syndrome, AO; Abdominal obesity, +; With (PCOS or AO), -; Without (PCOS or AO), ESC; Endometrial stromal cells, ECM; Embryo condition media, CI; Control induction, CS; Control supernatant. Stars show a statistically significant difference with PCOS+-AO+ group (****; P<0.0001). Hashtags show a statistically significant difference with PCOS--AO- group (#; P=0.0001 and ####; P=0.0001). Bar graphs show the calculated migration of ESCs.
Gene expression analysis
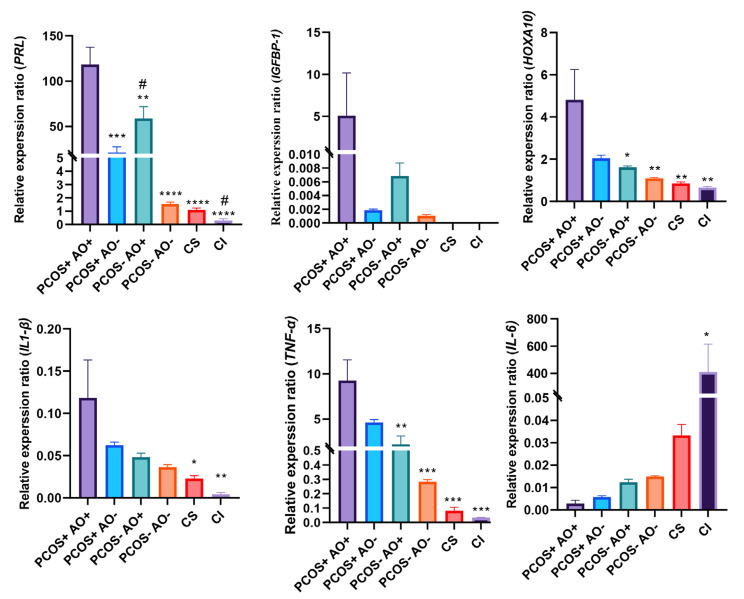
The qRT-PCR results were illustrated in Figure 4. Analysis of decidualization markers showed a significant highest expression of PRL in the PCOS+- AO+ group among our groups (P≤0.009). Moreover, the PCOS--AO+ group showed significantly higher PRL expression level than the PCOS--AO– group (P=0.0128). The same trend was observed for IGFBP-1 gene, but the differences were not statistically significant due to high variation among the individuals in the PCOS+-AO+ group. Moreover, the PCOS+-AO+ group showed significantly highest expression of level HOXA10 in comparison with among our groups (P≤0.0248). These results show higher decidualization properties in the PCOS+-AO+ group.
Analysis of decidualization related genes showed highest expression level of IL-1β in the PCOS+-AO+ group among our groups, while this difference was significant in comparison with the CS (P=0.013), and CI groups (P=0.0095). The expression level of TNF-α was significantly highest in the PCOS+-AO+ group among our groups, including PCOS--AO+ group (P=0.0039), PCOS--AO- group (P=0.0005), CS group (P=0.0004), and CI group (P=0.0004). The lowest expression level of IL-6 was belonged to the PCOS+- AO+ group (0.0029 ± 0.0025) in comparison with other groups. The CI group (409.4 ± 354.5) showed a significant highest expression level of IL-6 among our groups (P<0.05, Table 2).
Fig 4.
Results of qRT-PCR of genes related to decidualization and inflammation in the studied groups by One-Way ANOVA statistical test. qRT-PCR; Quantitative reverse transcription polymerase chain reaction, PCOS; Polycystic ovary syndrome, AO; Abdominal obesity, +; With (PCOS or AO), -; Without (PCOS or AO), CI; Control induction, and SC; Control supernatant. Stars (*) show a statistically significant difference with the PCOS+-AO+ group (*; P<0.05, **; P<0.01, ***; P<0.001, and ****; P<0.0001). Hashtags (#) show a statistically significant difference with the PCOS--AO- group (#; P<0.05). Obese PCOS Patients Have Higher Decidualization Potential 73
Discussion
In this study, the role of AO in the PCOS patients was investigated. Recently, it was suggested that the ECM can be used to evaluate the capacity of embryo to induce decidualization representing the in vitro embryo implantation potential (27). Accordingly, ECM samples were obtained of PCOS patients, with and without of AO, and also healthy fertile women to evaluate the potential of decidualization induction in the primary ESCs.
In the present study, we showed that the markers of ECMinduced decidualization in PCOS+-AO+ were significantly increased compared to other groups. Accordingly, decidualization induction by the ECM from the PCOS+-AO+ group caused a change in the morphology of ESCs, as well as a significant increase in the concentration of secreted PRL, although there is no significant difference between IGFBP-1, and IL-1β proteins secretion between groups. Moreover, we observed higher expression of PRL, IGFBP-1, IL-1β, TNF-α, and HOXA10 genes in ESCs from different groups. Nonetheless, the expression of IL- 6, as an acute phase response stimulator, was decreased in the PCOS+-AO+ group, along with the highest rate of migration capacity which was observed in cultured decidualized ESCs with their ECM.
Notably, it has been demonstrated that PCOS patients have a larger number of retrieved oocytes in comparison with normal oogenesis individuals. The PCOS patients experience a lower live birth rate and also, a higher spontaneous abortion rates (28). It was suggested that, disrupted decidualization process, endometrial receptivity and subsequent blastocyst implantation may be involved in these outcomes.
Based on the contribution of proinflammatory cytokines in the etiology of PCOS, it was suggested that this disease can be considered as an inflammatory disease (29). Evidences show that HA and IR, two main complications of syndrome, may play a role in inflammation induction in women with PCOS (30). Moreover, obesity is a known condition that activates inflammatory pathways in cells and tissue. It has been shown that the triglyceride storage process is disturbed in the PCOS patients with increase of free fatty acids which could lead to a state of IR. Inflammation cytokines can lead to IR by disrupting the insulin post receptor signalling pathway. The relationship between HA, IR, and chronic inflammation is still not fully understood (31).
While a chronic inflammation may disrupt fertility related process in healthy women, a low level acute endometrial inflammation is necessary for successful embryo implantation (19). It has been showed that an inflammatory response in the uterine mucosa is required prior to blastocyst invasion (32). Therefore, Proinflammatory cytokines can produce by multiple source including embryo secretory profile, maternal immune cells such as natural killer cells (NKc), macrophages, dendritic cells, and ESCs (33).
Therefore, the ESCs can produce pro-inflammatory cytokines including TNF-α and IL-1 at this critical stage. Similar studies have been observed that PCOS patients have higher level of TNF-α and IL-1 in comparison with non-PCOS patients (34). It has been suggested that the early detection of such cytokines in the endometrium may be considered as markers of an implantation competency (19). In the present study, we observed that IL-6 level decreased significantly in the PCOS+-AO+ group. Scheller et al. (35) reported that IL-6 seems to possess both pro inflammatory and anti-inflammatory properties. The association of IL-6 levels with PCOS susceptibilities has been investigated in several studies (36). However, the results were controversial, failing to find a clear role of IL-6 in pathogenesis of PCOS.
In this study, the PCOS+-AO+ group showed the highest expression of decidualization markers. It has been explained that, inflammatory process is required for improvement of decidualization and implantation within days of conception (19). Accordingly, all the decidualization markers investigated in this study also has been considered as inflammatory markers in previous study. It seems that pro inflammatory cytokines are enriched in a PCOS associated AO conditions which can lead to higher induction of decidualization in the PCOS+-AO+ group in comparison with other groups.
The association of increasing decidualization markers and endometrial receptivity has been demonstrated in other studies (37). Our result show that embryos collected from PCOS patients have higher potency for a decidualization induction in healthy endometrial receptivity. Zhao et al. (38) reported that IR and hyperinsulinemia (HI), two main complication of PCOS, may exacerbate the endometrial receptivity. Our results including a higher expression of level decidualization markers in the ESCs co-cultured with ECM was consistent with this report. Therefore, we may conclude that the ECM collected from the PCOS+-AO+ group, may increase the endometrial decidualization and subsequent endometrial receptivity. Due to an increased endometrium receptivity in these patients, even suboptimal embryos may likely implant, which subsequently may result a higher abortion rate (39). In the other hand, It has been shown that a pre-inflammatory response followed by an anti-inflammatory phase is necessary for successful in mammalian embryo implantation (40). We suggest that the uterine microenvironment may switch disruptively from an inflammatory to an anti-inflammatory phase in the PCOS+-AO+ women. We can propose that higher abortion rate in PCOS patients may arise from these two possible hypotheses. In the present study, abortion rate was evaluated but it was not significant that it seems because of our participants number limitation or due to freeze embryo transfer indication. However, several studies shown that abortion rate was higher in these patients (39).
Controversially, several studies showed that endometrial receptivity potential can be decreased in PCOS patients due to metabolic alterations, inflammatory events, and some abnormally expressed endometrial molecular markers (38). In these studies, the decidualization capacity of endometrial cells which obtained from PCOS patients was not intended. Also, we did not examine the decidualization phenotype of ESCs collected from our PCOS patients then, we don't have a clear view of the ESCs of these PCOS patients. Further studies with large population are needed to clarify the decidualization capacity of ESCs collected from the PCOS patients under treatment of their ECM.
Conclusion
Our results showed that the collected embryos from PCOS patients with AO have elevated level of inflammatory mediators leading to the higher potential for decidualization induction in the healthy ESCs. This result may be employed for future therapeutic approach in which the increased decidualization capacity and receptivity of ESCs are intended.
Acknowledgments
The authors gratefully thanks from the IVF, Embryo Biotechnology and Molecular labs staffs of the Royan Institute who provided assisted for collecting our data. This work was supported by the Royan Research and Technology Foundation. Authors have no competing interests to declare.
Authors’ Contributions
Z.Sh., S.T.; Conducted the experiments, Analyzed and Illustrated the results, and Wrote the first draft of the manuscript. P.E.Y.; Designed the study, Verified the analytical methods, Supervised the findings, and Critically revised the manuscript. N.N.; Helped design the study, Verify the analytical methods, Supervise the findings, and Critically revised the manuscript. M.H., T.M.; Designed the project and provided clinical advices. All authors read and approved the final manuscript.
References
- 1.Deswal R, Narwal V, Dang A, Pundir CS. The prevalence of polycystic ovary syndrome: a brief systematic review. J Hum Reprod Sci. 2020;13(4):261–271. doi: 10.4103/jhrs.JHRS_95_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xue Z, Li J, Feng J, Han H, Zhao J, Zhang J, et al. Research progress on the mechanism between polycystic ovary syndrome and abnormal endometrium. Front Physiol. 2021;12:788772–788772. doi: 10.3389/fphys.2021.788772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil Steril. 2018;110(5):794–809. doi: 10.1016/j.fertnstert.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Wen YX, Mai QY. Impact of metabolic disorders on endometrial receptivity in patients with polycystic ovary syndrome. Exp Ther Med. 2022;23(3):221–221. doi: 10.3892/etm.2022.11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah D, Patil M. In: Impact of polycystic ovary, metabolic syndrome and obesity on women health.Springer. Genazzani AR, Ibáñez L, Milewicz A, Shah D, editors. Springer; 2021. Infertility management in lean versus obese PCOS; pp. 105–127. [Google Scholar]
- 6.Barrea L, Frias-Toral E, Verde L, Ceriani F, Cucalón G, Garcia- Velasquez E, et al. PCOS and nutritional approaches differences between lean and obese phenotype. Metabol Open. 2021;12(3):100123–100123. doi: 10.1016/j.metop.2021.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellver J, Rossal LP, Bosch E, Zúñiga A, Corona JT, Meléndez F, et al. Obesity and the risk of spontaneous abortion after oocyte donation. Fertil Steril. 2003;79(5):1136–1140. doi: 10.1016/s0015-0282(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 8.Singh R, Kaur S, Yadav S, Bhatia S. Gonadotropins as pharmacological agents in assisted reproductive technology and polycystic ovary syndrome. Trends Endocrinol Metab. 2023;34(4):194–215. doi: 10.1016/j.tem.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Ochoa-Bernal MA, Fazleabas AT. Physiologic events of embryo implantation and decidualization in human and non-human primates. Int J Mol Sci. 2020;21(6):1973–1973. doi: 10.3390/ijms21061973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sehring J, Beltsos A, Jeelani R. Human implantation: the complex interplay between endometrial receptivity, inflammation, and the microbiome. Placenta. 2022;117:179–186. doi: 10.1016/j.placenta.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Badri Seighaldehi O, Mohseni Kouchesfahani H, Nasiri N, Shahverdi M, Hezavehei M, Shahverdi A. New findings in noninvasive assessment of embryo quality. Journal of Cell and Tissue (JCT) 2021;12(3):206–219. [Google Scholar]
- 12.Diedrich K, Fauser B, Devroey P, Griesinger G. The role of the endometrium and embryo in human implantation. Hum Reprod Update. 2007;13(4):365–377. doi: 10.1093/humupd/dmm011. [DOI] [PubMed] [Google Scholar]
- 13.Brosens JJ, Bennett PR, Abrahams VM, Ramhorst R, Coomarasamy A, Quenby S, et al. Maternal selection of human embryos in early gestation: insights from recurrent miscarriage. Semin Cell Dev Biol. 2022;131:14–24. doi: 10.1016/j.semcdb.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358(2):155–165. doi: 10.1016/j.mce.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura I, Asada H, Maekawa R, Tanabe M, Lee L, Taketani T, et al. Induction of IGFBP-1 expression by cAMP is associated with histone acetylation status of the promoter region in human endometrial stromal cells. Endocrinology. 2012;153(11):5612–5621. doi: 10.1210/en.2012-1420. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Lu J, Wu J, Jiang R, Guo C, Tang Y, et al. HOXA10 co-factor MEIS1 is required for the decidualization in human endometrial stromal cell. J Mol Endocrinol. 2020;64(4):249–258. doi: 10.1530/JME-19-0100. [DOI] [PubMed] [Google Scholar]
- 17.Snegovskikh V, Foyouzi N, Schatz F, Buhimschi C, Buhimschi I, Funai E, et al. IL-1 stimulates TNF production by human first trimester decidual stromal cells: Implications for implantation. Am J Obstet Gynecol. 2005;193(Suppl 6):S175–S175. [Google Scholar]
- 18.Pathare ADS, Hinduja I, Mahadik RC. Basic aspects of endometrial receptivity in PCOS patients. Mol Biol Rep. 2022;49(2):1519–1528. doi: 10.1007/s11033-021-06976-9. [DOI] [PubMed] [Google Scholar]
- 19.Sieg W, Kiewisz J, Podolak A, Jakiel G, Woclawek-Potocka I, Lukaszuk J, et al. Inflammation-related molecules at the maternalfetal interface during pregnancy and in pathologically altered endometrium. Curr Issues Mol Biol. 2022;44(9):3792–3808. doi: 10.3390/cimb44090260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goto S, Kadowaki T, Hashimoto H, Kokeguchi S, Shiotani M. Stimulation of endometrium embryo transfer (SEET): injection of embryo culture supernatant into the uterine cavity before blastocyst transfer can improve implantation and pregnancy rates. Fertil Steril. 2007;88(5):1339–1343. doi: 10.1016/j.fertnstert.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Liu S, Lv Q. Single blastocyst stage versus single cleavage stage embryo transfer following fresh transfer: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;267:11–17. doi: 10.1016/j.ejogrb.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Franasiak JM, Alecsandru D, Forman EJ, Gemmell LC, Goldberg JM, Llarena N, et al. A review of the pathophysiology of recurrent implantation failure. Fertil Steril. 2021;116(6):1436–1448. doi: 10.1016/j.fertnstert.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Nasiri N, Babaei S, Moini A, Eftekhari-Yazdi P. Controlling semiinvasive activity of human endometrial stromal cells by inhibiting NF-kB signaling pathway using aloe-emodin and aspirin. J Reprod Infertil. 2021;22(4):227–240. doi: 10.18502/jri.v22i4.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fathi R, Valojerdi MR, Yazdi PE, Ebrahimi B, Alipour H, Hassani F. Development of 4-cell mouse embryos after re-vitrification. Cryobiology. 2012;64(1):23–26. doi: 10.1016/j.cryobiol.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Jividen K, Movassagh MJ, Jazaeri A, Li H. Two methods for establishing primary human endometrial sromal cells from hysterectomy specimens. J Vis Exp. 2014;(87):51513–51513. doi: 10.3791/51513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, Lucas ES, et al. Uterine selection of human embryos at implantation. Sci Rep. 2014 Feb 6;4:3894–3894. doi: 10.1038/srep03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siristatidis CS, Sertedaki E, Karageorgiou V, Vaidakis D. Endometrial injection of embryo culture supernatant for subfertile women in assisted reproduction. Cochrane Database Syst Rev. 2020;8(8):CD013063–CD013063. doi: 10.1002/14651858.CD013063.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan IF, Lim AJ, Indran IR, Kramer MS, Yong EL. Reproductive outcomes of women with polycystic ovarian syndrome following invitro fertilization—a meta-analysis and systematic review. Fertility & Reproduction. 2019;1(4):193–201. [Google Scholar]
- 29.Regidor PA, Mueller A, Sailer M, Gonzalez Santos F, Rizo JM, Egea FM. Chronic inflammation in PCOS: the potential benefits of specialized pro-resolving lipid mediators (SPMs) in the improvement of the resolutive response. Int J Mol Sci. 2020;22(1):384–384. doi: 10.3390/ijms22010384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dabravolski SA, Nikiforov NG, Eid AH, Nedosugova LV. Obese PCOS Patients Have Higher Decidualization Potential 75 Int J Fertil Steril, Vol 18, No 1, January-March 2024 Starodubova AV, Popkova TV, et al.Mitochondrial dysfunction and chronic inflammation in polycystic ovary syndrome. Int J Mol Sci. 2021;22(8):3923–3923. doi: 10.3390/ijms22083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Rourke RW. Inflammation in obesity-related diseases. Surgery. 2009;145(3):255–259. doi: 10.1016/j.surg.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson SA, Moldenhauer LM, Green ES, Care AS, Hull ML. Immune determinants of endometrial receptivity: a biological perspective. Fertil Steril. 2022;117(6):1107–1120. doi: 10.1016/j.fertnstert.2022.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, Shen HH, Qin XY, Li MQ. The metabolic characteristic of decidual immune cells and their unique properties in pregnancy loss. Immunol Rev. 2022;308(1):168–186. doi: 10.1111/imr.13085. [DOI] [PubMed] [Google Scholar]
- 34.Blumenfeld Z. The possible practical implication of high CRP levels in PCOS. Clin Med Insights Reprod Health. 2019;13:1179558119861936–1179558119861936. doi: 10.1177/1179558119861936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The proand anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 36.Eser B, Islimye Taskin M, Hismiogullari AA, Aksit H, Bodur AS. The effects of IL-1A and IL-6 genes polymorphisms on gene expressions, hormonal and biochemical parameters in polycystic ovary syndrome. J Obstet Gynaecol. 2017;37(3):358–362. doi: 10.1080/01443615.2016.1256966. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Huang X, Mor G, Liao A. Epigenetic modifications working in the decidualization and endometrial receptivity. Cell Mol Life Sci. 2020;77(11):2091–2101. doi: 10.1007/s00018-019-03395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao J, Chen Q, Xue X. An Update on the progress of endometrial receptivity in women with polycystic ovary syndrome. Reprod Sci. 2022;29(8):2136–2144. doi: 10.1007/s43032-021-00641-z. [DOI] [PubMed] [Google Scholar]
- 39.Sun YF, Zhang J, Xu YM, Cao ZY, Wang YZ, Hao GM, et al. High BMI and Insulin resistance are risk factors for spontaneous abortion in patients with polycystic ovary syndrome undergoing assisted reproductive treatment: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2020;11:592495–592495. doi: 10.3389/fendo.2020.592495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T, Zhu W, Zhao Y, Cheung WC, Liu Y, Chen X, et al. Early transient suppression of immune checkpoint proteins T-cell immunoglobulin mucin-3 and programmed cell death-1 in peripheral blood lymphocytes after blastocyst transfer is associated with successful implantation. Fertil Steril. 2020;114(2):426–435. doi: 10.1016/j.fertnstert.2019.12.022. [DOI] [PubMed] [Google Scholar]