Abstract
Cartilage regeneration remains difficult due to a lack of blood vessels. Degradation of the extracellular matrix (ECM) causes cartilage defects, and the ECM provides the natural environment and nutrition for cartilage regeneration. Until now, collagen hydrogels are considered to be excellent material for cartilage regeneration due to the similar structure to ECM and good biocompatibility. However, collagen hydrogels also have several drawbacks, such as low mechanical strength, limited ability to induce stem cell differentiation, and rapid degradation. Thus, there is a demanding need to optimize collagen hydrogels for cartilage regeneration. In this review, we will first briefly introduce the structure of articular cartilage and cartilage defect classification and collagen, then provide an overview of the progress made in research on collagen hydrogels with chondrocytes or stem cells, comprehensively expound the research progress and clinical applications of collagen‐based hydrogels that integrate inorganic or organic materials, and finally present challenges for further clinical translation.
Keywords: Cartilage Regeneration, Collagen, Hydrogel, Stem Cells
This review provided an overview of the progress made in research on collagen hydrogels with chondrocytes or stem cells, comprehensively covered the research progress and clinical applications of collagen‐based hydrogels that integrated inorganic or organic materials.
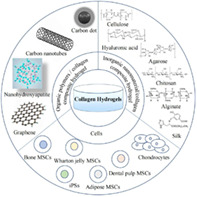
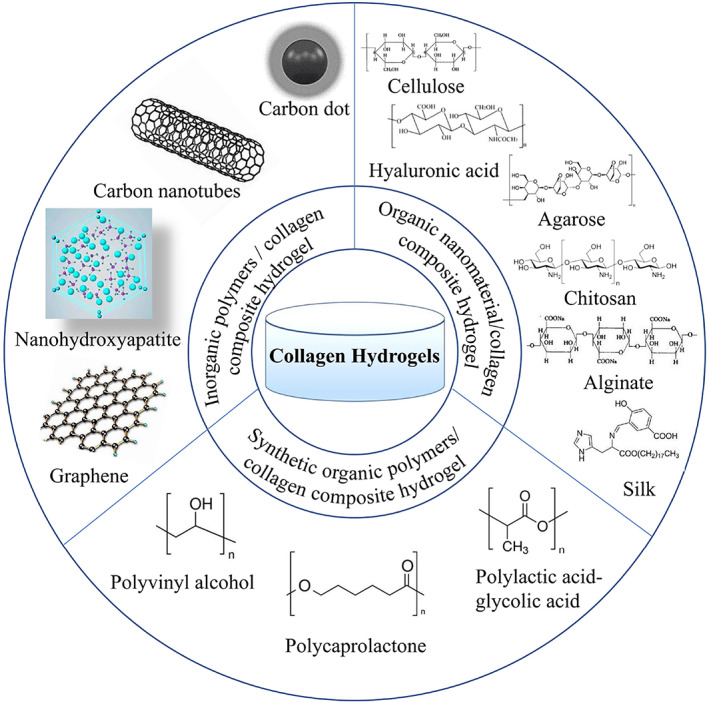
Introduction
Cartilage defects are very common and can cause joint pain, effusion, and limited function, resulting in a significant medical burden. Cartilage tissues are classified as elastic cartilage, fibrocartilage, and hyaline cartilage. Unlike elastic and fibrous cartilage, articular cartilage acts as hyaline cartilage with an ECM composed of type II collagen (COL II) fibrils (15%–25%), proteoglycans (5%–10%), and water (5%–10%). 1 However, different from other hyaline cartilage, articular cartilage is composed of four parts: the superficial zone (SZ), the middle zone (MZ), the deep zone (DZ), the calcified zone (CZ) (Figure 1A). 2 The number and orientation of the cells in each layer of the structure are different in articular cartilage; also mature articular cartilage has no blood supply and the subchondral bone is porous and sparse (Figure 1B). 3 Being an avascular tissue, mature articular cartilage hardly recovers itself if it is not treated properly, cartilage defects may penetrate deep into the subchondral bone and ultimately require joint replacement. 4 There are numerous classifications of cartilage defects, for example, Noyes and Stabler Methods, 5 histological classification, 6 International Osteoarthritis Cartilage Histopathology Assessment System (OOCHAS), 6 and International Cartilage Repair Society grading system (ICRS). 7 The most widely used is Outerbridge classification system (Figure 1C,D). 8 Traditionally, cartilage defects have been managed clinically in a number of ways, that include non‐steroidal anti‐inflammatory drugs (NSAIDs) and physical fixation, and surgical strategies like osteotomy, arthroscopic polished arthroplasty, autologous osteochondral grafting, microfracture, autologous chondrocyte implantation (ACI), matrix‐assisted autologous chondrocyte transplantation (MACT). In the short term, these treatments provide pain relief, but in the long term, the results are not satisfactory because ACI, MACT, and microfractures often lead to fibrocartilage proliferation rather than hyaline cartilage regeneration. Therefore, the most optimal treatment option available for cartilage defects remains inconclusive.
Fig. 1.
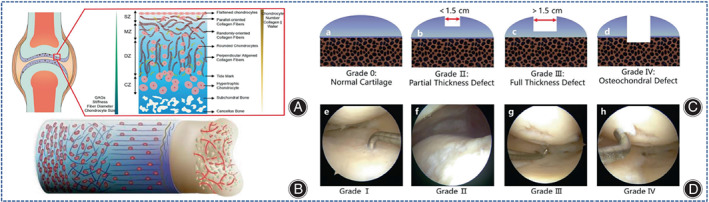
Simulation structure of the osteochondral unit. (A) Schematic diagram of the structure of the cartilage and subchondral bone of the knee joint. From top to bottom, the superficial zone (SZ) (10%–20%) consists mainly of flattened chondrocytes arranged in parallel with the highest concentration of collagen; the middle zone (MZ) (40%–60%) consists mainly of randomly distributed chondrocytes and collagen fibers; the deep zone (DZ) (30%–40%) consists mainly of spherical chondrocytes and neatly arranged collagen fibers around them; the calcified zone (CZ) is mainly composed of subchondral bone and cancellous bone. From top to bottom, the number of chondrocytes, collagen, and water was gradually reduced, whereas glycosaminoglycan (GAG), structural stiffness, and chondrocyte size were progressively increased. (Figures are adapted with permission from Zhou et al. 2 ) (B) Illustration of differences in the number and orientation of cells in cartilage and in the arrangement of extracellular matrix fibers at the osteochondral interface, as seen in subchondral bone with a vascularized porous structure and the avascular feature of cartilage. (Figures are adapted with permission from Ansari et al. 3 ) (C) Outerbridge classification system of articular cartilage. (a) Grade 0 represents “normal cartilage.” (b) Grade II represents “partial thickness defect” with a size of less than 1.5 cm in the cartilage layer. (c) Grade III represents a “total defect” with a diameter greater than 1.5 cm in the cartilage layer. (d) Grade IV represents “osteochondral defect,” where both cartilage and subchondral bone layers are damaged. (Figures are adapted with permission from Zhou et al. 2 ) (D) Clinical arthroscopic images of human cartilage from grade I to grade IV (e–h). (Figures are adapted with permission from Zhou et al. 2 ).
As we all know, collagen is one of the most studied biopolymers in medicine. The disadvantages of collagen are its weak mechanical properties and low thermal stability. To solve this problem for its clinical application, collagen can be combined with other biomaterials to make collagen‐based hydrogels. Collagen‐based hydrogels are used for cardiovascular disease, dental restoration, neurological disorders, bone defects, cartilage regeneration, wound healing, and tendon injuries. In recent years, cartilage tissue engineering (CTE) has been recognized as the most promising option for the therapy of cartilage defects due to imitating a three‐dimensional (3D) ECM microenvironment resulting in the regeneration of cartilage. The desirable material should have not only the physical properties for cell proliferation, but also the chemical properties to regulate cell differentiation. Hydrogels have highly hydrated properties similar in composition and structure to ECM, and have gained wide applications as CTE materials. Hydrogels are also injectable and shield host cells from mechanical damage during the injection process. In additional, some hydrogels have fast gelation properties, which significantly reduces operation time. Materials used for CTE mainly include natural polymers (collagen, chitosan, silk proteins, hyaluronic acid, alginates), as well as synthetic polymers (polycaprolactone (PCL), polyglycolic acid (PLGA), polyethylene glycol (PEG)). In general, natural polymer hydrogels have superior cytocompatibility, but inferior mechanical properties, while synthetic polymer hydrogels have better mechanical strength but weak cytocompatibility. Therefore, it is challenging to invent hydrogel materials that meet all the needs of cartilage regeneration. Collagen is one of the natural biomaterials that exist in a variety of animal tissues, and they are cytocompatibility, bioadaptive, toxicity‐free, and degradable. 9 , 10 , 11 Notably, collagen also possesses good hemostatic properties by promoting platelet aggregation and plasma coagulation. In addition, specific acid sequence in the collagen structure provides many adhesion sites for cells, which enables the delivery of chondrocytes or stem cells for chondrocyte derivatization and ECM composition. Furthermore, increases in collagen content can boost the swelling rate of hydrogels. 12 Many studies had shown that collagen hydrogels are the most prospective material for cartilage regeneration. 10 , 13 Although collagen is a major component of natural connective tissue, in the absence of covalent cross‐linking, collagen hydrogels have weak mechanical properties. It is not conducive to regenerate stiffer tissues. To overcome these defects, cross‐linking of collagen hydrogels increases the stiffness of the matrix and stimulates differentiation of mesenchymal stem cells (MSC) into the osteogenic spectrum best at Young's modulus above 25 kPa. 14 In addition to the strength of the crosslink or the presence of polyfunctional groups, the crosslink density is also a major factor affecting the physical properties of collagen hydrogels. As well, blending with other materials (inorganic nanomaterials and organic polymers) is also required to enhance the properties of collagen hydrogels to fit the different needs of CTE. 15 The reader can consult the comprehensive reviews by Thoniyot et al. and Tozzi et al. 16 , 17 and Fathi‐Achachelouei et al., 18 which provide an overview of examples of collagen hydrogels combined with other materials to enhance their stiffness.
The aim of this review is to systematically present recent advances and application of collagen‐based hydrogels for CTE (Figure 2). We first briefly introduce the structure of articular cartilage, cartilage defects classification and the collagen, and summarize the developments in the study of pure collagen hydrogels as scaffolds loaded with chondrocytes and stem cells in detail. The applications of hybrid collagen‐based hydrogels for cartilage repair are then highlighted. Finally, the status and challenges of these collagen hydrogel‐based cartilage repairs are summarized, and the perspectives of its development are discussed.
Fig. 2.
Strategies for collagen‐based hydrogels in cartilage tissue engineering.
Collagen
As we all know, collagen is the most abundant protein in animals, accounting for 25%–35% of the total protein content. 19 The most common triplet in collagen is Gly‐Pro‐Hyp, which accounts for about 10.5%. The secondary structure of collagen consists of (Gly‐X‐Y)n, the C‐terminus is associated with the triple helix structure and the N‐terminus regulates the diameter of collagen fibers (Figure 3A). 19 The non‐helical ends are connected by covalent cross‐linking and the C=O groups and hydroxyl groups on the main chain of Hyp residues provide multiple anchoring points for water molecules (Figure 3B). 20 In the collagen structure, the right triple helix structure (tertiary structure) is composed of two A1 chains and one A2 chain (Figure 3C). 19 The tertiary structure of collagen can autonomously assemble into well‐ordered collagen fibers (quaternary structure) (Figure 3D). 19 Type I collagen (COL I) hydrogel is very effective for cartilage regeneration. 21 , 22 , 23 , 24 Notably, COL II hydrogels containing chondrocytes produce more cartilage‐specific matrix proteins than COL I hydrogels containing chondrocytes. 10 However, COL II tends to promote fibrocartilage rather than hyaline cartilage regeneration, this significantly limits its clinical application. In fact, if the induction of fibrocartilage regeneration by COL II can be addressed, then it is better to use COL II rather than COL I.
Fig. 3.
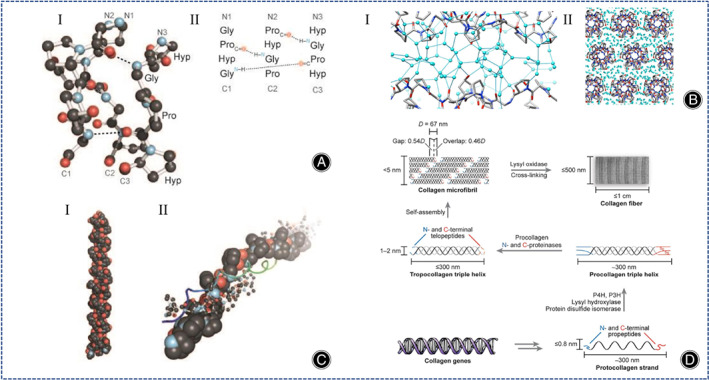
(A) (I) Schematic diagram of the ball‐and‐stick structure of collagen triple helix. (II) Molecular formula of the triple helix structure of collagen. (Figures are adapted with permission from Shoulders and Raines 19 ) (B) (I) The lateral arrangement of the triple helix of the 9‐peptide collagen structure, the gaps are filled with water molecules and the triple helix structure is separated from each other. (II) The main chain C=O group and hydroxyl group of Hyp residues provide multiple anchoring points for water molecules. (Figures are adapted with permission from Bella 20 ) (C) (I) Schematic diagram of the triple helix structure of collagen. (II) Model of the spatial arrangement of the components between the collagen triple helix structures. (Figures are adapted with permission from Shoulders and Raines 19 ) (D) Illustration of the biosynthetic pathway of collagen fibrils. Post‐translational modification and self‐assembly of collagen genes increase the size and structural complexity of collagen fibrils, and oxidation of lysine side chains leads to spontaneous formation of hydroxy lysylpyridine and lysylpyrine crosslinks. (Figures are adapted with permission from Shoulders and Raines 19 ).
Collagen Hydrogel
Collagen Hydrogel
In 2009, Erggelet et al. first published the cell‐free matrix cartilage regeneration technique, which is derived from MACT and belongs to matrix‐induced autologous cartilage regeneration (MACR). 25 Collagen is often used as a matrix for MACR because their gap structure can provide guidance for cell migration and their chemotactic properties can selectively recruit cells.
Cell‐free collagen hydrogel has been shown to be effective in the treatment of cartilage defects smaller than 1 cm2. Later, the researchers treated patients with cartilage defects of 3.71 ± 1.93 cm2 with small arthrotomy implants of cell‐free COL I hydrogel and found promising short‐term results. OtaŠeviČ et al. used a combination of basal microfracture and cell‐free COL I hydrogel to repair knee cartilage with a mean defect area of 8 cm2, the MOCART score at 18‐month follow‐up was 74.67 ± 14.08. 26 The migration of chondrocytes from healthy cartilage through the matrix into the collagen hydrogel is the key to the success of this approach. Although studies have confirmed the presence of migrating chondrocytes in cell‐free collagen hydrogels, the process of chondrocyte migration and the factors that influence cell settlement are not fully understood. Nachtsheim et al. developed an in vitro model in which cell‐free COL I hydrogels were placed on a collagen matrix containing human chondrocytes, and after a period of cultivation simulating the in vivo environment, it was found that chondrocytes existed in all cell‐free COL I hydrogels, but the chondrocytes mainly spread in the bottom layer of the collagen hydrogels, and the difference in the distribution of the chondrocyte population might be influenced by the mechanical properties of collagen hydrogels, such as the sensitivity strain rate. 27
Although encouraging results have been obtained for the treatment of cartilage defects in animal models and clinical patients with cell‐free collagen hydrogels, recently Horbert et al. found that cell‐containing collagen hydrogels were more effective than cell‐free collagen hydrogels for cartilage defects. 28 However, the presence of a mild immune response is a problem that cannot be ignored.
Pure Collagen Hydrogels Loaded with Cells
Combining hydrogels with cells offer a promising approach for repairing cartilage defects. On the other hand, collagen has been used as a cell‐loaded CTE material due to the ability to modulate cellular immune responses and has a high density of cellular adhesion sites.
Pure Collagen Hydrogels Loaded with Chondrocytes
Pure collagen hydrogels containing chondrocytes for cartilage defects belong to the MACT technique. Earlier, Ochi et al. successfully repaired cartilage defects in the human knee using COL I hydrogel loaded with autologous chondrocytes. 29 To enable more efficient cell settlement in hydrogels, researchers have developed 3D printing hydrogel. A previous report indicated that the survival rate of chondrocytes remained high after printing a collagen hydrogel with a chondrocyte density of 10 million cells mL−1. 30 However, the cell density in CTE is typically between 100 and 100 million cells mL−1. Recently, Diamantides et al. found collagen hydrogels had the best storage modulus and viscosity at a cell density of 100 million cells mL−1, 14‐day culture results showed no harmful effects of the printing process on cell viability or function, indicating that the printability of collagen hydrogels not only related to rheology, but also increases with the increase of cell density. 31
One issue that must be considered is the influence that the physical properties of collagen hydrogels can have on chondrocyte metabolism. For example, hardness (Young's modulus >20 kPa) hydrogels are more appropriate for chondrocyte regeneration. Ogura et al. exploited chondrocyte‐loaded collagen hydrogels and demonstrated that chondrocyte metabolic function is modulated by hydrostatic pressure (HP), deviatoric stress (DS), and stress loading time, with HP contributing to the stimulation of ECM synthesis by chondrocytes and DS possibly effecting the catabolism of ECM. 32 Dong et al. synthesized three different DS of photo‐crosslinked COL I (CM) by modifying the amino group of COL I with methacrylic anhydride (MA), and then used CM to synthesize a hydrogel whose degradation rate could be adjusted by modifying the DS of CM, and the contractility of the CM hydrogel could also be adjusted to promote the synthesis of cartilage‐specific matrices. 33
Another thought‐provoking issue is the dedifferentiation of chondrocytes in chondrocyte‐containing collagen hydrogels. Chondrocyte dedifferentiation occurs during the 2D expansion phase of chondrocyte culture in vitro. Typically, autologous human serum (HS) is added to the culture matrix to induce chondrocyte redifferentiation, whereas in most cases, supplementation with HS causes chondrocytes to differentiate into fibrocartilage rather than hyaline cartilage. Jeyakumar et al. added hyperacute serum (HAS) and platelet‐rich plasma (PRP) to chondrocyte‐containing collagen hydrogels and found that the addition of PRP exceeding 14 days decreased IL‐6 secretion and increased platelet derived growth factor (PDGF) production by chondrocytes (p < 0.05) and enhanced gene expression of COL II A1 and SOX9 (p < 0.05), whereas addition of HAS enhanced the generation of COL I A1. 34 Similarly, Mohd et al. demonstrated that the incorporation of Pseudomonas aeruginosa aqueous extract (SCAE) into chondrocyte collagen‐containing hydrogels stimulated chondrocyte proliferation, decreased chondrocyte dedifferentiation, and contributed to the synthesis of cartilage matrix. 35
To overcome the limited source of autologous chondrocytes, Lim et al. added human nasal septum‐derived chondrocytes (hNCs) to a COL I hydrogel and treated cartilage defects in rats. 36 Dasargyri et al. used COL I hydrogel enriched with a high density of fetal chondrocytes to culture cartilage tissue in vitro, but the disadvantage of this novel material is the characteristic of auto‐contraction. 37
Pure Collagen Hydrogel Loaded with Stem Cells
In CTE, stem cells have induced differentiation and have become a good substitute for autologous chondrocytes in recent years. Collagen hydrogels interact with bone mesenchymal stem cells (BMSCs) to boost cellular signal transduction as well as production of ECM. In addition to promoting the synthesis of ECM, these natural materials can maintain the phenotype of chondrocytes. It is not certain which source of stem cells is better suited for cartilage regeneration. Earlier, Wakitani et al. synthesized injectable collagen‐based hydrogels by embedding BMSCs into calf skin COL I for the treatment of cartilage defects in rabbits, and showed that COL I promotes the differentiation of BMSCs into chondrocytes. 38 Sakura et al. added induced pluripotent stem cells (iPSs) to collagen hydrogel and introduced differentiation of iPSs, and after 8 weeks green fluorescent protein (GFP) immunohistochemistry demonstrated cartilage repair by iPSs. 39 Similarly, Elizabeth et al. reported that iPSs‐loaded collagen hydrogels were a promising CTE hydrogel. 40 Fabre et al. recently employed a microscopic mass model to research the chondrogenic differentiation potential of bone, adipose, dental pulp (DP), and Wharton jelly (WJ)‐derived MSCs. and found that only BMSCs showed chondrogenic induction, concluding that BMSCs are most beneficial for cartilage regeneration. 41 In CTE, besides pluripotent stem cells (iPSs) and bone mesenchymal stem cells (BMSCs), other stem cells also exist, such as synovial mesenchymal stem cells (SMSCs) and human umbilical cord blood mesenchymal stem cells (hUCB‐MSCs). The origin of SMSCs was unclear and was generally believed to be derived from synovium, type B synovial lining, or infrapatellar pad. It has been shown that the amount of SMSCs in the knee joint cavity was increased approximately 100‐fold in patients with OA. 42 Therefore, the concentration of SMSCs may be positively correlated with the degree of joint damage. Lee et al. found that SMSCs had a greater chondrocyte differentiation capacity compared to BMSCs in vitro. 43 A large number of studies have shown that the use of SMSCs to treat cartilage injuries may be feasible in the future. 44 , 45 However, no clinical studies have been reported on the use of SMSCs for cartilage regeneration. The lack of clinical evidence makes it difficult to draw conclusions about the efficacy and safety of SMSCs for cartilage regeneration. hUCB‐MSCs have a higher chondrogenic potential compared to BMSCs. Ibrahim et al. demonstrated that differentiation of hUCB‐MSCs into chondrocytes was possible in vitro. 46 Park et al. repaired critical sized full thickness cartilage defects in mice joints with hydrogel‐loaded hUCB‐MSCs and showed that the combination of hUCB‐MSCs and 4% hyaluronic acid hydrogel was most effective in repairing cartilage defects in vivo. 47 Park et al. found that undifferentiated hUCB‐MSCs (undiff‐MSCs) was more favorable for cartilage repair compared to chondrogenic pre‐differentiated hUCB‐MSCs (chondro‐MSCs) in rat model, which indicated that chondrogenic pre‐differentiation of hUCB‐MSCs did not enhance cartilage repair. 48 Gómez‐Leduc et al. considered hypoxia as a key parameter for chondrogenic differentiation of hUCB‐MSCs in type I/III collagen sponges. 49 Therefore, hUCB‐MSCs can be considered as a promising MSC source for cartilage regeneration.
The technical difficulty of treating cartilage defects with stem cell‐containing collagen hydrogels lies in the efficient differentiation of stem cells into hyaline chondrocytes. The chondrogenic differentiation of BMSCs depends on the type and properties of the hydrogel, the presence of soluble bioactive factors and external factors. 10 The properties of hydrogels affect the cell‐matrix interaction and thus the differentiation of BMSCs, and collagen‐based hydrogels can provide a natural environment for the differentiation of BMSCs into chondrocytes. Wang et al. designed and synthesized three groups of collagen hydrogels based on articular cartilage ECM from rabbits of all ages. The research results indicated that all three groups of hydrogels could stimulate chondrogenesis in BMSCs, but the neonatal group synthesized the most cartilage matrix in BMSCs resulted in severe matrix calcification, while the adult group synthesized the least cartilage matrix in BMSCs had the least calcification, and overall the juvenile group collagen hydrogels were the most effective. 50 Wu et al. demonstrated that heterologous collagen hydrogels were more capable of cartilage regeneration than decellularized porcine articular cartilage (DPC) microparticles in an experimental model of knee osteoarthropathy in rabbits. 51 Stem cell multiplication and differentiation are influenced by the physical properties of collagen hydrogels, including stiffness, 3D pore size and pore topology form. Chen et al. designed a collagen‐based hydrogel containing stromal cell‐derived factor 1 (SDF‐1) possessing channels in both horizontal and vertical directions and evaluated the effect of the hydrogel on in vivo osteochondral regeneration and in vitro MSC migration, found that the mechanical properties of the randomly oriented hydrogel were inferior to those of the radially oriented hydrogel, but both favored cell proliferation. 52 Yang and Li et al. prepared two collagen‐based hydrogels with different structures but similar physical and chemical properties and found that in the absence of growth factors, the collagen hydrogel with a fibrous network structure better promoted the differentiation of BMSCs and reduced the calcification of ECM; however, chondrocyte hypertrophy induced by BMSCs was present in both structural hydrogels. 24 Yang and Tang et al. prepared three kinds of different hydrogels (hyaluronic acid methacrylate hydrogel, self‐assembled collagen hydrogel, self‐assembled collagen hydrogel cross‐linked with kynurenine), and showed that the early collagen hydrogel were strong in cartilage induction, but the later self‐assembled collagen hydrogel, self‐assembled hydrogel cross‐linked with kynurenine collagen hydrogels were severely contracted resulting in insufficient space for cell proliferation within the hydrogel. In comparison, the continued production of cartilage‐associated matrix is facilitated by the stable structure of hyaluronic acid methacrylate hydrogels (Figure 4A). 13
Fig. 4.
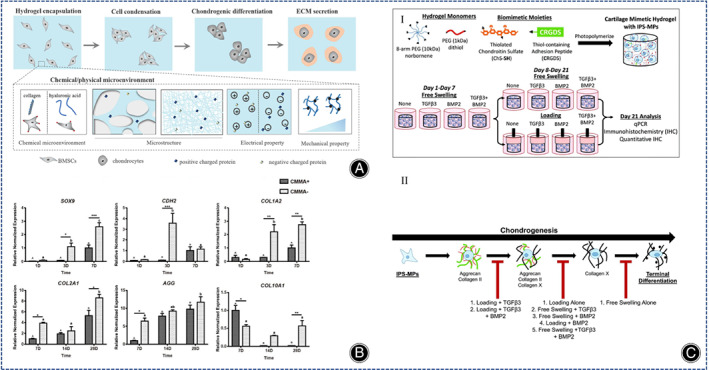
(A) Schematic representation of chondrogenic differentiation of BMSCs. (Figures are adapted with permission from Yang et al. 13 ). (B) Gene expressions of SOX9, CDH2 and COL I A2 in the early stage (days 1, 3 and 7) and COL II A1, AGG and COL 10 A1 in the later stage (days 7, 14 and 28) were quantified separately. All gene expressions were normalized to housekeeping gene GAPDH. The data are reported as the mean ± SEM of experiments (n = 8). At each time point, the significant differences between the two groups are presented as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001. Within each experiment group, time points not connected by the same letter (capital letters for CMMA+ group and lowercase letters for CMMA− group) are significantly different (p < 0.05). (Figures are adapted with permission from Wang et al. 55 ). (C) (I) iPS‐MP was encapsulated in a hydrogel made of multilayer polyethylene norbornene macromolecules and chondroitin and CRGDS. Then they were cultured under free conditions without growth factors, TGFβ3, BMP2, TGFβ3, and BMP2 or under dynamic pressurized conditions, respectively. Proliferation was performed first under dynamic pressurized conditions for 7 days and then at 1 h intervals per day. On day 21, iPS‐MP differentiation was analyzed by qPCR, IHC, and quantitative IHC. II) Chondrogenic differentiation of iPS‐MPS in proposed cartilage hydrogels with different growth factors and pressurized conditions. (Figures are adapted with permission from Aisenbrey et al. 40 ).
Soluble bioactive factors are favorable for the synthesis of ECM and the derivatization of BMSCs. The most recent studied soluble factor is the transforming growth factor TGF‐β, which is a recognized bioactive factor for inducing chondrocyte formation, but their immune rejection and unexpected side effects (such as carcinogenesis, cartilage hypertrophy and production of an X‐type collagen‐rich matrix and expression of osteogenic marker genes) limit their clinical application. Gene editing enables BMSCs to produce cartilage growth factor autonomously and durably. Recently, the transcription factor SOX9 has been used to induce chondrogenesis in BMSCs by gene delivery via adenovirus and significantly reduced the level of chondro‐hypertrophy in vitro culture. 53 Weissenberger et al. induced cartilage regeneration with different degrees of hypertrophy in human mesenchymal stem cells (hMSCs) in collagen hydrogels by adenovirus‐encoded genes for the SOX9, TGF‐β1 and BMP2. TGF‐β1 resulted in a significant rise in COL II A1 expression compared to SOX9 and BMP2, and BMP2 induced a remarkable increase in the hypertrophy marker gene alkaline phosphatase (ALP) more than TGF‐β1. 54
The selective addition of other bioactive factors such as n‐cadherin, autologous PRP, COL II, and microRNAs (miRNAs) are also able to stimulate cartilage regeneration in collagen hydrogels loaded with BMSCs. Wang et al. showed that the early cartilage differentiation of MSCs was significantly slower in the presence of n‐calcine mucin antibodies, and the later collagen microenvironment counteracted the inhibitory effect of n‐calcine mucin antibodies on n‐calcine mucin, suggesting that collagen hydrogels further promoted the late differentiation of BMSCs (Figure 4B). 55 Fang et al. showed that PRP stimulated cell proliferation and hyaline cartilage formation through the TGF‐β1/SMAD signaling pathway, while inhibiting the synthesis of the fibrocartilage marker gene COL I. 56 Rajagopal et al. reported cartilage differentiation in BMSCs with mir‐140‐activated collagen hydrogels, which consistently provided mir‐140, reduced COL I A1 synthesis, and promoted hyaline cartilage production. 57
External factors closely affect the cartilage differentiation of BMSCs in stem cell‐loaded collagen hydrogels, such as mechanical stimulation, negative electrical microenvironment, etc. Mechanical stimulation, including dynamic compressive and shear stresses, can mimic the natural joint loading environment and facilitate cartilage formation for BMSCs (Figure 4C). 40 Yang et al. prepared three collagen hydrogels with different electrical properties and observed that the secretion of COL II was high in collagen hydrogels containing a higher negative charge. 58 Notably, it was recently shown that hypoxia facilitates hMSCs differentiation towards cartilage and tendon, 59 however, there were no such studies in cartilage regeneration with stem cell‐loaded collagen hydrogels.
In summary, the main stem cells currently used for cartilage repair are mesenchymal stem cells of various origins, among which bone mesenchymal stem cells are the most common. The collagen hydrogels loaded with cells have the advantage of being non‐cytotoxic and can repair cartilage defects to regenerative cartilage. However, low mechanical strength of collagen hydrogels may lead to cell death during the squeezing process.
Inorganic Nanomaterial/Collagen Composite Hydrogel
Nano Hydroxyapatite/Collagen Composite Hydrogel
Cartilage are complex biomineralized layered structures containing nanohydroxyapatite (nHA) and collagen. As a natural inorganic component with good biocompatibility and bio‐integration, hydroxyapatite is often used as a tissue engineering material for cartilage and bone regeneration, with more applications in bone regeneration. Hydroxyapatite itself is hard, therefore, its addition to collagen hydrogels can improve the hardness of collagen hydrogels. Hydroxyapatite releases calcium and phosphate ions, which have an effect on certain mineralization signaling pathways. Previous studies have revealed that adding of hydroxyapatite nanoparticles in moderate amounts to hydrogels increased the Young's modulus of hydrogels and decelerated the degradation rate. 60 Martincic et al. found lower infection rates in hydroxyapatite collagen hydrogels than alginate agarose hydrogels in the treatment of knee cartilage injuries. 61 The biomimetic mineralization approach is the assembly of nHA onto COL I, a strategy that mimics natural bone at the molecular level. Mg2+ facilitates the binding of nHA and collagen. 62 However, the drawback of most current hydroxyapatite/collagen hydrogels remains the weak mechanical properties, mainly caused by too rapid degradation of collagen and insufficient mechanical strength. Previous studies have shown that ribose can stabilize the collagen matrix by non‐enzymatic glycosylation of collagen. 63 Mg2+/HA/collagen hydrogels cross‐linked with ribose have higher enzyme resistance and histocompatibility, 64 and non‐cytotoxicity. 65 Although these studies have made significant progress, the relevant mechanisms are not precisely understood. Dellaquila et al. synthesized ribose cross‐linked Mg2+/HA/collagen hydrogels and systematically investigated their properties by a full factorial design of experiment and found that glycosylation of collagen increased the swelling and porosity of hydrogels, while the degradation rate of composite hydrogels was influenced by the hydroxyapatite content and ribose content. 66
With the discovery of the role of subchondral bone in articular surface injuries, the focus of research has shifted to mimicking the structure of subchondral bone during the repair process. These scaffolds are assembled as double or triple‐layered scaffolds to accommodate the simultaneous regeneration of cartilage and bone, depending on the structural characteristics of the osteochondral bone. Yudoh et al. 67 manufactured an autologous cartilage complex with hydroxyapatite, proteoglycan, and COL II, which showed that hydroxyapatite‐containing material was superior to the same material without hydroxyapatite, and that hydroxyapatite improved the viscoelasticity of the autologous cartilage material. The hydroxyapatite/collagen composite hydrogel had a gradient structure that induced BMSCs differentiate into cartilage tissue. Fan et al. synthesized a porous hydrogel that hydroxyapatite nanoparticles were filled in the pores of the hydrogel, and the different content of hydroxyapatite nanoparticles in different layers of the hydrogel could lead to different differentiation of BMSCs in the different layers of the hydrogel (Figure 5A). 68 Yan et al. used hydroxyapatite/COL I and PLGA‐PEG‐PLGA hydrogel to make porous and gradient mineralized hydrogels to address the cartilage defects in rabbit femoral condyles, and stimulated BMSCs encapsulated in the hydrogel with electromagnetic fields (EMF), showing that EMF enhanced the cartilage repair properties of the hydrogels by coordinating PI3K/AKT/mTOR and Wnt1/LRP6/β‐associated protein signaling pathways (Figure 5B). 69 Thus, carbon nanoparticles are able to provide more mechanical strength to collagen hydrogels and even achieve cartilage and bone repair at the same time.
Fig. 5.
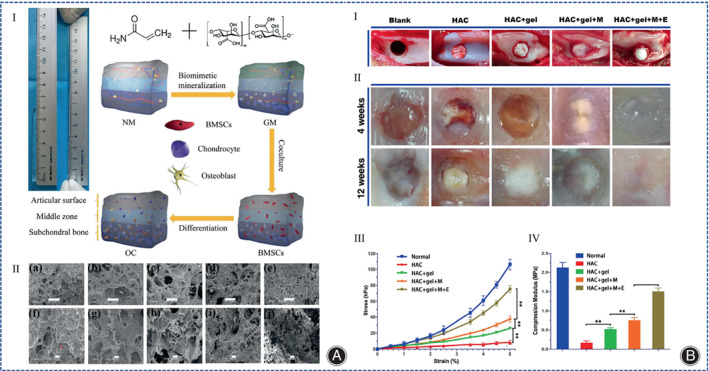
(A) (I) Optical photograph of gradient mineralized (GM) hydrogel and illustration of the whole experimental procedure. (II) SEM image of the hydrogel: group (a, f) represent non‐mineralized (NM) hydrogel; group (b, g) represent 5% mineralized (M) hydrogel; group (c, h) represent 10% M hydrogel; (d, i) group represent 15% M hydrogel; group (e, j) represent GM hydrogels. The scale in group (a–e) is 500 μm. The scale in group (f–j) is 100 μm. (Figures are adapted with permission from Fan et al. 68 ) (B) (I) Images of the appearance of cartilage repair under different treatment methods. (II) Appearance showing healing of osteochondral defects in all groups after 4 to 12 weeks of treatment. This regenerated cartilage in the EMF group was closest to normal cartilage. (III) Strain–stress curves of regenerated cartilage tissue with representative points at 0.5% strain intervals. (IV) The compressive modulus was calculated at 5% strain, and the highest modulus in the EMF group indicates that the hydrogel has excellent mechanical properties. Data are shown as mean ± standard deviation (n = 5). # indicates no statistically significant difference compared to the group shown, * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001. (Figures are adapted with permission from Yan et al. 69 ).
Carbon Nanomaterial/Collagen Composite Hydrogel
Currently, the common carbon nanomaterials in CTE are graphene, carbon nanotubes (CNTs), and carbon dot nanoparticles (CD NPs). Among them, graphene family is the oldest 2D carbon nanomaterial, which was first isolated in 2004. 70 The oxygen‐containing functional groups enhance the hydrophilicity and adsorption of graphene, so graphene oxide has good biocompatibility. An X‐ray microimaging‐based analysis of porous hydrogels showed that amino‐functionalized graphene as a nano‐crosslinker promoted the formation of uniform pore size and increased the interconnected microporous structure in collagen hydrogels. For hydrogels containing MSCs, the role of growth factors that induce chondrogenic differentiation is significant. To maintain the release of growth factors in the hydrogel, a second carrier needs to be added to the hydrogel, and graphene is a good choice because it not only improves the mechanical properties of the hydrogel, but also promotes the proliferation and differentiation of hMSCs by adsorbing and releasing bioactive factors. Zhou et al. found that graphene oxide tablets released transforming growth factor β3 (TGF‐β3) more effectively than the addition of exogenous TGF‐β3 without the need for repeated TGF‐β3 supplementation. 71
CNTs have a diameter of about 10–100 nm, and they have superior thermodynamic, electrical, and adsorption properties, so they are expected to be used for CTE. CNTs are commonly used as materials to increase the hardness of hydrogels, and it can change the rheological properties of the polymer matrix. CNTs can be classified into single‐walled carbon nanotubes (SWCNT) and multi‐walled carbon nanotubes (MWCNT). Mao et al. studied the cellular uptake of SWCNT using a 3D cell culture system and showed better proliferation of bovine articular chondrocytes in SWCNT/collagen composite hydrogels compared to collagen hydrogels (Figure 6A). 72 Surface modification of SWCNT with ‐COOH but not ‐PEG has been shown to increase the expression of COL II A1 in SWCNT collagen composite hydrogels, suggesting that the charging of SWCNT by ‐COOH may regulate the metabolic activity of chondrocytes. 73 Similarly, Wang et al. used fish collagen and SWCNT‐COOH to prepare COL/SWCNT‐COOH hydrogels with the addition of TGF‐1, which were superior to pure collagen hydrogels for cartilage regeneration. 74 Among multilayer carbon nanotubes MWCNT, vertically aligned carbon nanotubes (VACNT) have been shown to be very promising in cartilage regeneration (Figure 6B). 75 Earlier, nanostructured scaffolds of MWCNT combined with poly‐L‐lactic acid (PLLA), which is highly stiff and conducive to the chondrogenic differentiation of MSCs, were fabricated using electrostatic spinning technology. 76 Recently, Henao et al. used sodium riboflavin phosphate to modify multilayer carbon nanotubes and cross‐link them with collagen to synthesize photosensitive collagen nanocomposites, and the results showed that MWCNT‐riboflavin (MWFMN) nanoparticles improved the structural and thermal properties of the collagen matrix. 77
Fig. 6.
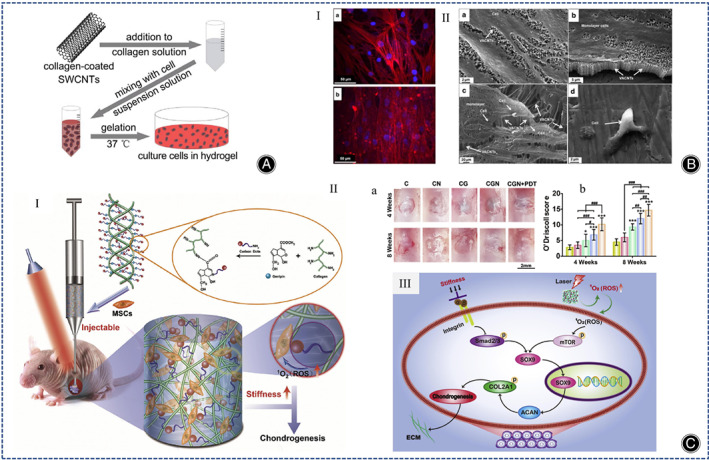
(A) Illustration of the synthesis of SWCNT/collagen composite hydrogels. (Figures are adapted with permission from Mao et al. 72 ) (B) (I) Fluorescence microscopy images of Actin (red) and DAPI (blue) of human chondrocytes (scale bar is 50μm). (a) Chondrocytes in superhydrophilic VACNT; (b) chondrocytes on the membrane. (II) SEM images of chondrocytes after 5 days of culture. (a, b) Characterization of human chondrocytes in superhydrophilic VACNT membranes and (c) VACNT/PMMA membranes; (d) chondrocytes on membranes with arrows pointing to chondrocytes and VACNTs. (Figures are adapted with permission from Antonioli et al. 75 ) (C) (I) Schematic diagram of the preparation process of collagen‐genipin‐carbon dot (CGN) nanocomposite hydrogel. (II) PDT promotes cartilage regeneration after CGN implantation. (a, b) Macroscopic images of cartilage tissue engineering (a) and O'Driscoll score (b). (Scale bar is 2 mm) (C represents collagen, CN represents collagen mixed with CD NPs, CG represents collagen cross‐linked with genipin, CGN represents collagen cross‐linked with genipin and CD NPs, and CGN + PDT represents CGN after 3 min of 808 nm laser irradiation at a power density of 5.6 mW/cm2). (III) Schematic diagram of the signaling pathways of mechanotransduction and laser irradiation. (Figures are adapted with permission from Lu et al. 80 ).
CD NPs have low cytotoxicity and good biocompatibility and can be applied as nanomaterials to enhance the mechanical stability of collagen hydrogels. Many researchers have shown that CD NPs can be applied as photosensitizers for photodynamic therapy (PDT), generating reactive oxygen species (ROS). 78 , 79 Lu et al. used genipin to combine collagen with CD NPs to develop a novel injectable hydrogel, and the CD NPs enhanced the stiffness of the collagen hydrogel, reduced degradation rate of collagen, and promoted cartilage regeneration in BMSCs by generating ROS under light (Figure 6C). 80 The same strategy to increase the stiffness of collagen hydrogels as well as to increase the production of ROS is the incorporation of cadmium selenide (CdSe) quantum dots into collagen hydrogels using ginepin. 81 In summary, both CD NPs, graphene, CNTs, and hydroxyapatite are able to enhance the physical properties of collagen hydrogels and favorably promote cartilage regeneration.
Organic Polymers/Collagen Composite Hydrogel
Natural Organic Polymers/Collagen Composite Hydrogel
Cellulose/Collagen Composite Hydrogel
Cellulose, a natural hydrophilic polymer, has gained great attention in the biomedical field. Because of excellent characteristics, such as high tensile strength, modulus of elasticity (130–150 GPa), biocompatibility, and biodegradability (Figure 7A). 82 Cellulose is mainly divided into cellulose nano‐objects, cellulose microcrystals, and cellulose microprimary fibers. Cellulose nano‐objects include cellulose nanocrystals (CNCs) as well as cellulose nanofibers (CNFs). Cellulose nano‐objects have larger surface area and more uniform particle size distribution, which are more suitable for synthesizing hydrogels. Torabizadeh et al. compared the effects of CNCs and CNFs on physical properties and cellular properties of collagen hydrogels. As far as physical properties of collagen hydrogels, both CNFs and CNCs optimized the physical properties of collagen hydrogels (reduced gelation time, increased compressive strength, enhanced injectability, increased swelling rate, and reduced degradability), but the effect of CNCs was greater in terms of cellular properties. CNFs were harmless to fibroblasts, whereas CNCs improved cell viability. 83 Liu et al. studied the characteristics of CNCs/collagen hydrogels with different pore orientations (horizontal, random, vertical, multi‐structured), hydrogels arranged horizontally with the largest stiffness and energy dissipation capacity, and multi‐structure hydrogels exhibited a larger storage modulus compared to conventional hydrogels with random pore (Figure 7B). 84 Acidic collagen is able to bind to chemical cross‐linking agents. Cellulose is easily surface modified, so the modified cellulose can be combined with collagen hydrogel and the properties of collagen hydrogel can be changed. Yang et al. mixed collagen solution with dialdehyde carboxymethylcellulose (DCMC) in a biphasic manner and showed that the DCMC/collagen hydrogel facilitated cell adhesion and proliferation. 85 Unfortunately, above experiments were performed in fibroblast cell lines and not in chondrogenic cells. Later, Zhang et al. incorporated aldehyde‐functionalized CNCs (a‐CNCs) into collagen hydrogels (Figure 7C). Compared with CNCs/collagen hydrogels without Schiff base bonds, the shear stress of a‐CNCs/collagen hydrogels were lower and more favorable for encapsulating MSCs, and a‐CNCs/collagen hydrogels can be performed by 18‐gauge needle subcutaneous injection, which is expected to deliver MSCs to the site of cartilage defects by injection. 86
Fig. 7.
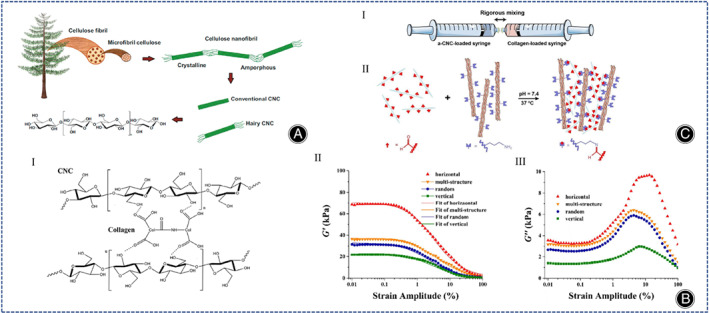
(A) Illustration of the structure of cellulose from natural resources to the molecular level. (Figures are adapted with permission from Culica et al. 82 ) (B) (I) Molecular structure diagram of CNCs cross‐linked with the collagen network within the hydrogel. Strain amplitude dependence of G' (II) and G" (III) of hydrogels with different pore orientations at a constant frequency of 1 Hz (Figures are adapted with permission from Liu et al. 84 ) (C) (I) Neutralized a‐CNC suspension and collagen solution were loaded in separate syringes and mixed rigorously with a joint connector. (II) The reaction of aldehyde groups on the a‐CNC surface with primary amine groups of collagen yields imine cross‐links. (Figures are adapted with permission from Zhang et al. 86 ).
Hyaluronic Acid/Collagen Composite Hydrogel
Hyaluronic acid (HA) is one of the main components of the ECM of cartilage and as glycosaminoglycans (GAGs) it has good water retention capacity and binds to various cell surface receptors. However, the hydrophilic and polyanionic properties of hyaluronic acid inhibit cell adhesion. The lack of adhesion sites is detrimental to the connection between nascent cells. Synergistic mechanical effect between collagen and HA enhance the hardness of the complex. In addition, collagen has self‐assembly characteristics, and sulfur‐HA (HA‐SH) can be used to synthesize injectable hydrogels. The injectable composite hydrogels provide an excellent environment for in vivo cell growth and have been extensively studied and developed for CTE (Figure 8A). 87 Previous studies have reported that injectable HA/collagen composite hydrogel relieved collagen contraction and promoted chondrocyte adhesion and proliferation. 88 , 89 , 90 The physicochemical properties of HA/collagen composite hydrogel facilitate cartilage differentiation. Yang et al. showed that the chemical and physical microenvironments (microstructure, mechanical properties, electrical properties, protein adsorption) all affect the cartilage regeneration of BMSCs. 13
Fig. 8.
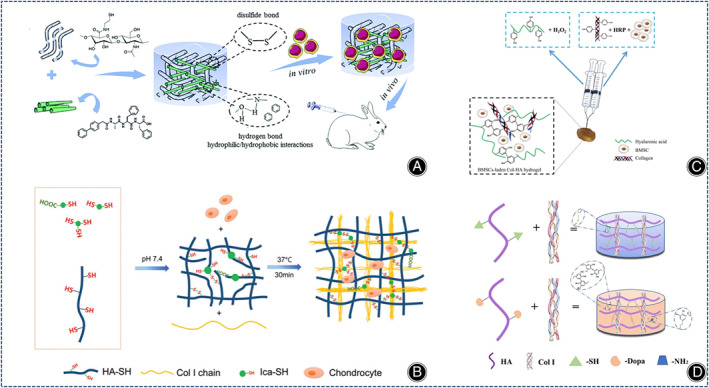
(A) Illustration of the structure of a novel injectable bionic hydrogel with covalent/non‐covalent network and in vivo tests under the skin of rabbits. (Figures are adapted with permission from Wang et al. 87 ) (B) Illustration of the preparation of injectable thioglycoside‐functionalized HA/Col hydrogels. (Figures are adapted with permission from Liu et al. 93 ) (C) Illustration of the preparation of BMSC‐loaded injectable Col‐HA hydrogels. (Figures are adapted with permission from Zhang et al. 95 ) (D) Illustration of the preparation of HD‐C hydrogels. (Figures are adapted with permission from Li et al. 100 ).
Different cross‐linking methods can also affect the performance of collagen hydrogels. Kong et al. designed and prepared three COL I/HA hydrogels and found that chondrocytes in HA‐sNHS/COL I (HSC) and HA‐CHO/COL I (HCC) produced more ECM, concluding that the chemical cross‐linking method mainly affected the ECM secretion of chondrocytes and had no associated with chondrocyte differentiation. 91 Gao et al. prepared different types of collagen‐based hydrogels by varying the ratios of COL I and modified hyaluronic acid (HACHO) and chondroitin sulfate (CS‐ADH), and found that C8CH hydrogels (4:1:1 volume ratio of COL I, HA‐CHO and CS‐ADH) had the best biological properties. 92
Icariin (Ica) promotes cartilage regeneration in MSCs. Yang et al. showed that Ica‐HA/collagen hydrogels is more effective in promoting cartilage regeneration in mice than HA/collagen hydrogels. 88 To increase the content of Ica, Ica is often chemically incorporated into hydrogels by chemical crosslinking of methacrylate‐modified Ica (Ica‐MA). However, because Ica‐MA is cytotoxic, new methods need to be developed. Liu et al. prepared thioglycoside‐functionalized HA/collagen hydrogels with high Ica content by self‐assembly of collagen molecules and disulfide bonding between thiol groups, which promoted chondrocyte proliferation, cartilage ECM secretion, and, most importantly, reduced the cytotoxicity of Ica compared with HA/collagen hydrogels (Figure 8B). 93
Enzymatic cross‐linking reactions enable rapid hydrogel formation under physiological conditions. Ren et al. invented a maleimide‐modified bionic collagen‐based hydrogel using matrix metalloproteinase (MMP) as a cross‐linking agent, which contained both Arg‐Gly‐Asp (RGD) sequences and functional peptide sequences and was able to induce the differentiation of BMSCs into cartilage and inhibit their hypertrophic phenotypic differentiation. 94 Zhang et al. used hydrogen peroxide (H2O2) and horseradish peroxidase (HRP) to synthesize a COL I tyramine (COL I‐TA) complex hyaluronan tyramine (HA‐TA) injectable hydrogel loaded with BMSCs and TGF‐β1, providing an advantageous microenvironment for chondrogenic differentiation of BMSCs (Figure 8C). 95 Also using COL I and HA‐TA, Schwab et al. synthesized novel enzymatic cross‐linked 3D printed hydrogels that had a more uniform distribution of collagen protofibrils with the ability to directionally regulate cell alignment and migration, allowing 3D printed collagen hydrogels to resemble natural cartilage tissue more. 96 Kim et al. synthesized a composite collagen hydrogel using recombinant human transglutaminase 4 (rhTG‐4), rhTG‐4 was able to increase the stiffness of the composite hydrogel and induce the production of biomolecules that facilitate the differentiation of synovial mesenchymal stem cells (SDSCs) in rabbits in vivo. 97
Yao et al. synthesized an injectable hydrogel using HA‐SH, hyaluronic acid, and COL I, which promoted cartilage matrix production and hyaline cartilage‐related gene expression by encapsulating exogenous chondrocytes. 98 These hydrogels containing exogenous tissue cells increased the chances of an immune response. 99 Injectable engineered bionic cartilage matrix hydrogels that recruit stem cells can solve this challenge. Li et al. synthesized dopamine‐containing collagen‐based hydrogels (HD‐C) using COL I and hyaluronic acid derivatives (HA‐DN). HD‐C hydrogels with tighter pore structure, higher mechanical properties and water retention significantly promoted the proliferation of rabbit bone marrow stem cells (rBMSCs) and the synthesis of cartilage matrix through a potentially functional protein adsorption mechanism (Figure 8D). 100
Chemically cross‐linked initiators or catalysts may be harmful to the cells encapsulated in the hydrogels. Therefore, to meet the demand for cell encapsulation in CTE, new methods need to be explored to prepare hydrogels with better physical properties and biocompatibility. Yao et al. prepared injectable biomimetic double self‐crosslinked HA‐SH‐Mal‐HA (HSMSSA)/COL I hydrogels that enhanced the expression of genes related to hyaline cartilage formation and multi‐proteoglycan secretion. 98 Gao et al. prepared self‐crosslinked injectable hydrogels using COL I and N‐hydroxysulfosuccinate‐activated hyaluronic acid (HA‐sNHS), and showed that chondrocytes in the 32% DS hydrogel secreted more specific cartilage matrix. 101 Wang et al. created an injectable bionic hydrogel that overcame the immunogenicity of animal‐derived collagen, and the bionic composite hydrogel improved chondrocyte proliferation compared to HA‐SH hydrogel. 87
In conclusion, the current research direction for HA/collagen hydrogels is focusing on both overcoming the shortcomings of cell adhesion ability and avoiding the toxicity of chemical cross‐linking agents to cells.
Agarose/Collagen Composite Hydrogels
Agarose is a linear polysaccharide extracted from red algae. Agarose is biocompatible, has strong mechanical properties and maintains round cell morphology. Research on agarose has centered on enhancing biological properties and reducing cellular immunotoxicity. On the one hand, agarose is biologically inert. The biological properties of agarose can be improved by covalent modification, but covalent modification is very time‐consuming and cytotoxic. Another approach is the addition of collagen, where the two components interact to form both the biological function of natural ECM and the tunable mechanical properties. Ulrich et al. showed that the addition of agarose to COL I hydrogels greatly improved their elastic modulus and reduced cell migration. 102 Later studies showed that the typical concentration of agarose to achieve mechanical stability in dynamic compression was equal to or higher than 2% w/v. 103 Cambria et al. mixed two concentrations of COL I with 2% wt/vol agarose showing that agarose‐collagen hybrid hydrogels were superior to pure agarose hydrogels. This result is identical to previous studies of collagen hydrogels mixed with lower concentrations of agarose. 104
Another study on agarose showed variable results on host cell immune rejection by agarose. Rahfoth et al. 105 demonstrated that embedding of rabbit allogeneic chondrocytes in agarose hydrogel did not produce an immune rejection reaction. However, Kawabe and Yoshinao showed the contrary result, they transplanted cartilage into rabbits and after 2~3 weeks the transplanted cartilage started to degenerate, partly due to humoral immune response and partly due to cytotoxicity. 106 Recently, Perrier et al. comparatively studied the responses of mice to collagen hydrogels and agarose hydrogels, the result showed that collagen hydrogels triggered a weak inflammatory response, whereas agarose hydrogels had no inflammatory response (Figure 9). 107
Fig. 9.
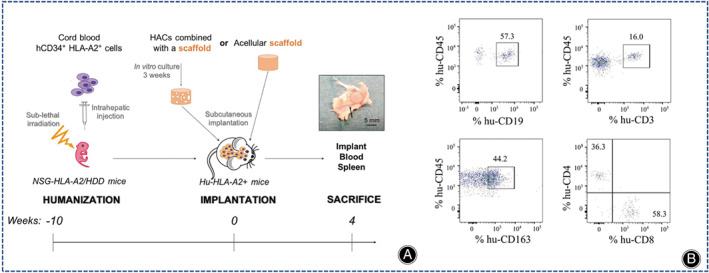
(A) Experimental flow diagram. Black shaded circles represent cartilage structures on the skin and black arrows point to capillaries. (B) Results of flow cytometric analysis of hCD34+ hematopoietic stem cells 10 weeks after inoculation. (Figures are adapted with permission from Perrier‐Groult et al. 107 ).
Chitosan/Collagen Composite Hydrogel
Chitosan is a polyatomic polysaccharide that is found in insects, crustaceans. It is synthesized from chitin by deacetylation reaction. Chitosan is widely used in tissue engineering owing to it being non‐toxic, antifungal, antibacterial, as well as having good biocompatibility. The biological properties are similar to those of GAG in articular cartilage, chitosan has been commonly utilized to create materials that mimic cartilage. However, using only chitosan, the mechanical strength is not enough. Modification of natural hydrogels by blending or cross‐linking has been reported to improve their properties. 108 The addition of chitosan to collagen not only improved the mechanical properties and reduced the degradation rate of the composite hydrogel, but also enhanced the cell attachment and cell proliferation.
The development of multilayer hydrogels is an effective strategy to model the multilayered structure of osteochondral tissue. Chitosan, collagen, and nano‐hydroxyapatite were combined in a bionic way to form a multilayer hydrogel, and these nanocomposite hydrogels were promising strategies for regenerating articular cartilage tissue. 109 , 110 ATDC5 chondrocytes can survive well in this multilayer hydrogel without growth factors. 109 , 110 Chitosan can be easily modified. 9 Modification of chitosan by natural chemical cross‐linking agents optimizes the conjugation of chitosan to collagen. For example, tannins and genipin were used as crosslinkers of chitosan and collagen for cartilage regeneration. 111 Choi et al. used photopolymerizable chitosan (methacrylated glycol chitosan, MeGC), COL II and riboflavin (RF) to synthesize injectable modified chitosan collagen hydrogels under visible blue light (VBL), avoiding the disadvantages associated with ultraviolet (UV) irradiation and toxic initiators, and this hydrogel system enhanced cell‐matrix interactions through the interaction of integrin α10 with COL II and was able to support the proliferation of pericytes and mesenchymal stem cells and the production of cartilage matrix (Figure 10A). 112 Recently, Shah et al. prepared collagen‐chitosan hydrogels for the first time using dual cross‐linkers (tannic acid and genipin) (Figure 10B), but this hydrogel has only been used for corneal repair and skin regeneration, and has not been used for CTE. 113
Fig. 10.
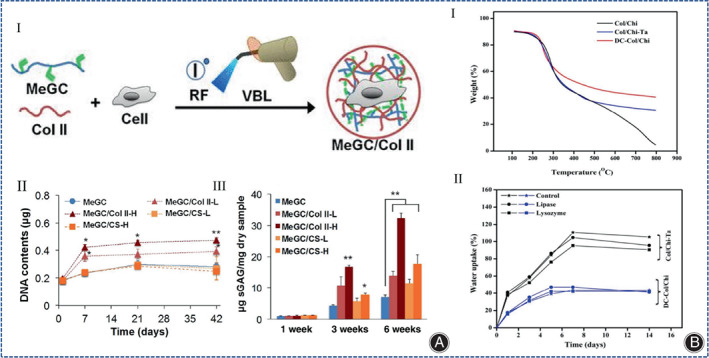
(A) (I) Schematic diagram of the preparation method of injectable modified chitosan collagen hydrogels. (II) Increase of DNA contents during culture period is correlated with cell proliferation, and this was analyzed using Picogreen assay (n = 3; *p < 0.05 and **p < 0.01 compared with MeGC). (III) Quantification of sGAG content as performed by DMB assay at 1, 3, and 6 weeks in culture and the value was then further normalized to the dry weight of each sample to determine chondrogenic potential of hydrogels (n = 3; *p < 0.05 and **p < 0.01 compared with MeGC). (Figures are adapted with permission from Choi et al. 112 ) (B) (I) TGA of Col/Chi, Col/Chi‐Ta and DC‐Col/Chi hydrogels. (II) Swelling studies of Col/Chi ‐Ta and DC‐Col/Chi hydrogels. (Figures are adapted with permission from Shah et al. 113 ).
Alginate/Collagen Composite Hydrogel
Alginate belongs to water‐soluble linear polysaccharides, copolymers of β‐D‐mannate (M) and α‐L‐glutamate (G) were randomly arranged to form poly‐GG, poly‐MG, and poly‐MM fragments linked by 1,4‐glycosidic bonds. Alginates are easily cross‐linked with divalent cations (Ca2+) (Figure 11A). 9 Therefore, changing the calcium concentration can adjust the hardness of alginate‐based complexes. Since there are no cell attachment sites or specific receptors in alginate, and collagen has more integrin structural domains, alginate can be used in combination with collagen to synthesize cell culture scaffolds in CTE. More than a decade ago, in a study of cartilage reconstruction in rats, Zheng et al. demonstrated that alginate collagen hydrogel (CAH) had superior cytocompatibility to genipin cross‐linked collagen hydrogel (CGH). 114 Later, Jahanbakhsh et al. used alginate collagen hydrogels for in vitro cartilage regeneration of MSCs and concluded that the use of hydrostatic pressure (HP) (5 MPa) on COL I ‐encapsulated MSCs had the potential to replace the role of TGF‐β (Figure 11B). 115 The important effect of HP on cartilage regeneration in MSCs was also demonstrated by Ledo et al. In their design of a COL I and alginate interpenetrating polymer network (IPN), the hardness of IPN was varied by changing the number of calcium carbonate nanoparticles, while the number of ligands was varied by changing the weight ratio of sodium alginate and collagen, and the hydrogel was stiffer and more similar to a real ECM than a biologically inert polymer hydrogel in the presence of cell adhesion ligands (Figure 11C). 116 Another method for the improvement of cell adhesion of alginate collagen hydrogels is the enzymatic cross‐linking method. Saghati et al. synthesized a phenolic alginate collagen hydrogel and investigated the effect of this collagen‐based hydrogel on the chondrogenic differentiation of human amniotic mesenchymal stem cells and found that collagen increased the elasticity of the composite hydrogel by 10% and also improved the survival of human amniotic mesenchymal stem cells in the composite hydrogel. 117
Fig. 11.
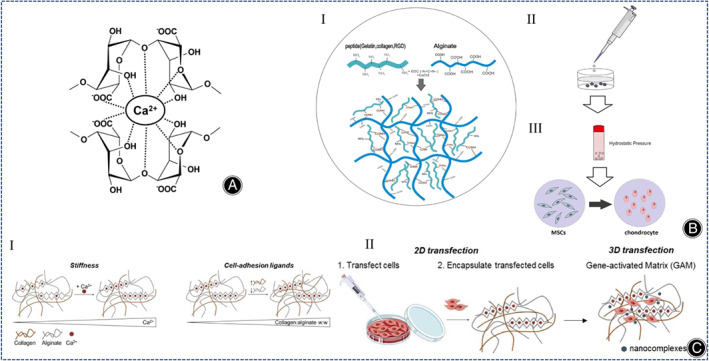
(A) Calcium ion cross‐linking of sodium alginate. (Figures are adapted with permission from Li et al. 9 ) (B) (I) Modification of alginate structures with proteins and peptides having amine groups. (II) Illustration of MSC encapsulation. (III) Induction of chondrogenesis in alginate medium under IHP. (Figures are adapted with permission from Jahanbakhsh et al. 115 ) (C) (I) The mechanical properties of IPN are regulated by the concentration of cross‐linker and the ratio of polymer. IPN shows brown collagen fibers and gray alginate polymer chains, with calcium ions in the sodium alginate network (sawtooth structure) shown as red dots. (II) Schematic diagram of the experimental protocol of the transfection assay. (Figures are adapted with permission from Ledo et al. 116 ).
Silk/Collagen Composite Hydrogel
Silk fibroin (SF) is a natural fibronectin composed of 18 amino acids, of which Gly, Ala, and Se account for more than 80%, and these amino acids are linked by peptide bonds. Remarkably, the amino acid composition gives SF satisfactory biological properties and the hierarchical structure of SF contributes to its excellent mechanical properties and degradability. SF can be made into hydrogels by various methods. However, the poor mechanical properties and low cell affinity of SF limit the further application of SF in cell culture. Collagen/SF hybrid hydrogels are a promising material for CTE. 118 Due to the poor interaction between collagen and SF, the composite hydrogels exhibit poor mechanical strength and rapid degradation. Ultrasound sonication is an eco‐friendly technology that can regulate SF degradation, and has been used to induce gelation of SF to achieve uniform cell encapsulation prior to gelation. 119 Long et al. invented a silk collagen (COL) composite hydrogel (COL+SF(S)) and evaluated its ability to regenerate cartilage in rabbit knee defects, producing intact articular hyaline cartilage after 6 months in the absence of any exogenous growth factors (Figure 12). 120
Fig. 12.
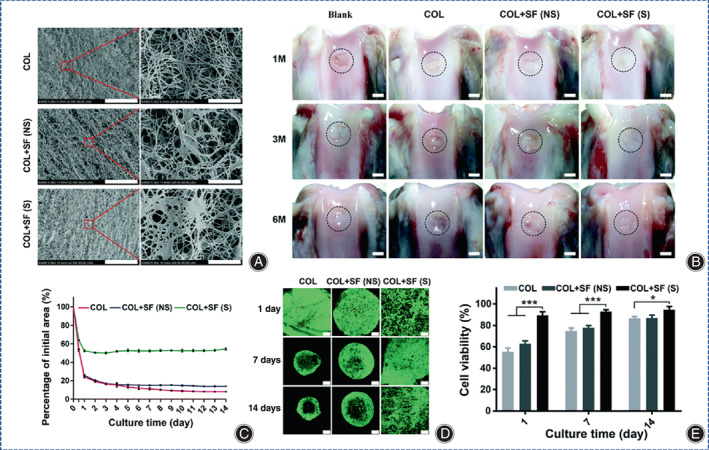
(A) SEM images of ultrasonically treated filamentous protein, COL, COL+SF(NS) and COL+SF(S) hydrogels (scale bar on the left images is 20 mm; scale bar on the right images is 2 mm). (B) Macroscopic appearance of cartilage defects in rabbits treated untreated (blank controls) or with COL, COL+SF (NS), and COL+S (S) hydrogels containing BMSCs at a density of 5 × 106 mL−1 at 1, 3, and 6 months postoperatively, surrounded by dashed lines of regenerated cartilage tissue (scale bar is 2 mm). (C) Quantitative analysis of sGAG/DNA synthesis in COL, COL+SF(NS) and COL+SF(S) hydrogels containing BMSCs in the absence of exogenous chondrogenic growth factors at days 1, 7, and 14 in vitro, data are shown as mean ± SD of experimental specimens (n = 3). (* represents p < 0.05, ** represents p < 0.01, *** represents p < 0.00). (D) The viability and growth state of BMSCs in different hydrogel microenvironments using software, FDA/PI staining: live cells were stained green and dead cells were stained red (scale bar is 250 μm). (E) A line graph of the initial area percentage of BMSCs within COL, COL+SF(NS) and COL+SF(S) hydrogels in the absence of exogenous chondrogenic growth factors and the culture time of the cells at 1–14 days of in vitro culture. (Figures are adapted with permission from Long et al. 120 ).
A material with similar properties to SF is silk glue (SG), but most CTE uses SF instead of SG because of the immunogenic character of SG. However, recent studies have shown no significant difference in the immunogenicity of SF and SG. 121 In addition, several studies have shown that SG is a good performing biomaterial because it is beneficial for mesenchymal cells. 122 Hector et al. synthesized 3D printable composite hydrogels with stiffness close to that of natural cartilage using cocoons and rat COL I. SG was preserved by using physical solubilization, a process that ensures the integrity of the structure of COL I, more than two‐fold increase in stiffness of collagen/SG hydrogels compared to pure collagen hydrogels, and the method is more friendly to BMSC cell culture and bioprinting as it does not require toxic chemicals, additional cross‐linking agents, or complex physical methods. 12
Synthetic Organic Polymers/Collagen Composite Hydrogels
Polyvinyl Alcohol/Collagen Composite Hydrogels
Polyvinyl alcohol (PVA) belongs to the synthetic biomolecular material. PVA hydrogels are rapidly developing in cartilage regeneration because of their high swelling, porosity, and viscoelasticity. However, its poor biocompatibility and microstructure impede its further application. Pure PVA hydrogels lack cell adhesion sites and, like many synthetic polymer hydrogels, are biologically inert. PVA hydrogels can be physically cross‐linked. As well, Huang et al. produced a novel magnetic nanohydrogel using Fe3O4, PVA, and COL II in an optimal ratio of 5:95:5. Thus, PVA combined with collagen can be used in different ratios to synthesize new composite hydrogels with different functions for cartilage regeneration. 123 Xie et al. utilized 3D printing to synthesize a novel hybrid PVA collagen hydrogel that mimics the properties of natural cartilage and provides mechanical support in which the collagen scaffold allows the hydrogel to integrate with the surrounding natural cartilage. 124
Polycaprolactone/Collagen Composite Hydrogels
Polycaprolactone (PCL) is a synthetic polymer, which has certain rigidity, good compatibility with polymer materials, can also be used as a modifier for other polymers, and is widely used in biomedical field. And yet, disadvantages such as low hardness, few cell binding sites, poor bioactivity, and poor hydrophilicity limit its further promotion. PCL/collagen composite hydrogels exhibit good biochemical properties and cytocompatibility in bone tissue engineering, 125 corneal tissue engineering, 126 artificial blood vessels, 127 wound healing, 128 and nerve repair, 129 but not in CTE.
Polylactic Acid‐Glycolic Acid/Collagen Composite Hydrogels
Polylactic acid‐glycolic acid (PLGA), which is polymerized from lactic acid and hydroxyacetic acid, is a degradable polymeric organic compound with good biocompatibility, non‐toxicity, and good film‐forming properties. Lamparelli et al. synthesized injectable PLGA microcarriers (PLGA‐MCs)‐ collagen hydrogels containing hTFG‐β1and performed in vitro culture of hBMSCs and found that the immunomodulatory activity of the cells was mainly related to the commitment rather than the synthetic environment, providing a fresh direction for the application of injectable collagen hydrogels. 130
Conclusion and Prospects
This review describes recent advances in the characterization, synthesis, and application of collagen‐based hydrogels for CTE. Overall, collagen‐based hydrogels, as analogous polymers of natural ECM, can be used as cell‐loaded materials for cartilage repair and has shown good results in vivo and in vitro tests. Due to the similarity to natural ECM, collagen‐based hydrogels have the advantages of low immunogenicity, good biocompatibility, and biodegradability, while the disadvantages are their weak mechanical strength and their inability to induce cell differentiation well. In addition, the effectiveness of cartilage damage repair is influenced by the synthesis of ECM, and how to synthesize hydrogels with similar properties to natural cartilage is the key to hydrogel design. Despite the advantages of collagen hydrogels, their weak mechanical strength, and high shrinkage limit their use in CTE and also affect their clinical usability. To alleviate these problems, several strategies have been developed to improve the mechanical properties of collagen hydrogels by combining them with other materials or by physical or chemical modifications that are more favorable for their application in CTE. In CTE, several therapeutic strategies exist. Chondrocytes can be encapsulated in a collagen hydrogel to fill the cartilage defect site. BMSCs can also be encapsulated in a collagen‐based hydrogel to induce differentiation of BMSCs into chondrocytes. In the clinic, for early cartilage defects in the knee joint, collagen‐based hydrogel can be injected into the cartilage defect site through arthroscopy originally. Therefore, collagen‐based hydrogel has a good application prospect in the clinic.
In the future, there are several issues to be considered to synthesize desirable collagen hydrogels as well as to accelerate clinical translation. (1) How to obtain collagen in a more efficient and less contaminated way to overcome the potential immunogenicity as well as the risk of virus transmission. (2) How to organically combine collagen and other natural or synthetic polymers to form a CTE material capable of inducing long‐term growth of hyaline chondrocytes. (3) How to truly achieve organic integration of tissue‐engineered cartilage with subchondral bone and even with surrounding tissues. (4) How to protect the loaded cells during the injection or printing of collagen‐based hydrogels. (5) How to facilitate the batch preparation of collagen‐based hydrogels, which is one of the key topics for cartilage regeneration materials to be widely used in clinical applications. (6) How to develop 3D printing of collagen‐based hydrogels for cartilage defects to achieve precise treatment. (7) How to establish a precise and reliable clinical diagnosis standard, efficacy assessment principle, and risk control mechanism to achieve precision medicine.
Author Contributions
Conceptualization, supervision—Jianguo Liu, Chunsheng Xiao, Dongsong Li, Bo Cai; Fund Acquisition—Dongsong Li ; Original draft preparation—Lihui Sun, Yan Xu, Yu Han; Review and editing—Jing Cui, Zheng Jing, Dongbo Li; All authors have read and agreed to the published version of the manuscript.
Funding Information
Jilin Scientific and Technological Development Program (20230204077YY).
Conflict of Interest
The authors declare no conflict of interest.
Ethics Statement
Not applicable.
Contributor Information
Dongsong Li, Email: lidongsong@jlu.edu.cn.
Bo Cai, Email: bobo96693@163.com.
References
- 1. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–468. 10.1177/1941738109350438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou L, Gjvm VO, Malda J, Stoddart MJ, Lai Y, Richards RG, et al. Innovative tissue‐engineered strategies for Osteochondral defect repair and regeneration: current progress and challenges. Adv Healthc Mater. 2020;9(23):e2001008. 10.1002/adhm.202001008 [DOI] [PubMed] [Google Scholar]
- 3. Ansari S, Khorshidi S, Karkhaneh A. Engineering of gradient osteochondral tissue: from nature to lab. Acta Biomater. 2019;87:41–54. 10.1016/j.actbio.2019.01.071 [DOI] [PubMed] [Google Scholar]
- 4. Correa D, Lietman SA. Articular cartilage repair: current needs, methods and research directions. Semin Cell Dev Biol. 2017;62:67–77. 10.1016/j.semcdb.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 5. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505–513. 10.1177/036354658901700410 [DOI] [PubMed] [Google Scholar]
- 6. Acebes C, Roman‐Blas JA, Delgado‐Baeza E, Palacios I, Herrero‐Beaumont G. Correlation between arthroscopic and histopathological grading systems of articular cartilage lesions in knee osteoarthritis. Osteoarthr Cartil. 2009;17(2):205–212. 10.1016/j.joca.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 7. Gomoll AH, Minas T. The quality of healing: articular cartilage. Wound Repair Regen. 2014;22(Suppl 1):30–38. 10.1111/wrr.12166 [DOI] [PubMed] [Google Scholar]
- 8. Slattery C, Kweon CY. Classifications in brief: outerbridge classification of chondral lesions. Clin Orthop Relat Res. 2018;476(10):2101–2104. 10.1007/s11999.0000000000000255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L, Yu F, Zheng L, Wang R, Yan W, Wang Z, et al. Natural hydrogels for cartilage regeneration: modification, preparation and application. J Orthop Translat. 2019;17:26–41. 10.1016/j.jot.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irawan V, Sung TC, Higuchi A, Ikoma T. Collagen scaffolds in cartilage tissue engineering and relevant approaches for future development. Tissue Eng Regen Med. 2018;15(6):673–697. 10.1007/s13770-018-0135-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miao Z, Lu Z, Wu H, Liu H, Li M, Lei D, et al. Collagen, agarose, alginate, and Matrigel hydrogels as cell substrates for culture of chondrocytes in vitro: a comparative study. J Cell Biochem. 2018;119(10):7924–7933. 10.1002/jcb.26411 [DOI] [PubMed] [Google Scholar]
- 12. Sanz‐Fraile H, Amoros S, Mendizabal I, Galvez‐Monton C, Prat‐Vidal C, Bayes‐Genis A, et al. Silk‐reinforced collagen hydrogels with raised multiscale stiffness for mesenchymal cells 3D culture. Tissue Eng Part A. 2020;26(5–6):358–370. 10.1089/ten.TEA.2019.0199 [DOI] [PubMed] [Google Scholar]
- 13. Yang J, Tang Z, Liu Y, Luo Z, Xiao Y, Zhang X. Comparison of chondro‐inductivity between collagen and hyaluronic acid hydrogel based on chemical/physical microenvironment. Int J Biol Macromol. 2021;182:1941–1952. 10.1016/j.ijbiomac.2021.05.188 [DOI] [PubMed] [Google Scholar]
- 14. Engler AJ, Sen S, Sweeney HL, Discher DE, Engler AJ, Sen S, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. [DOI] [PubMed] [Google Scholar]
- 15. Ding X, Li G, Zhang P, Jin E, Xiao C, Chen X. Injectable self‐healing hydrogel wound dressing with cysteine‐specific on‐demand dissolution property based on tandem dynamic covalent bonds. Adv Funct Mater. 2021;31(19):2011230. 10.1002/adfm.202011230 [DOI] [Google Scholar]
- 16. Thoniyot P, Tan MJ, Karim AA, Young DJ, Loh XJ. Nanoparticle‐hydrogel composites: concept, design, and applications of these promising, multi‐functional materials. Adv Sci. 2015;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gianluca T, Arianna DM, Antero O, Marta R. Composite hydrogels for bone regeneration. Materials. 2016;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fathi‐Achachelouei M, Knopf‐Marques H, Ribeiro da Silva CE, Barthes J, Bat E, Tezcaner A, et al. Use of nanoparticles in tissue engineering and regenerative medicine. Front Bioeng Biotechnol. 2019;7:113. 10.3389/fbioe.2019.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. 10.1146/annurev.biochem.77.032207.120833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bella J. Collagen structure: new tricks from a very old dog. Biochem J. 2016;473(8):1001–1025. 10.1042/BJ20151169 [DOI] [PubMed] [Google Scholar]
- 21. Schneider U, Rackwitz L, Andereya S, Siebenlist S, Fensky F, Reichert J, et al. A prospective multicenter study on the outcome of type I collagen hydrogel‐based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee. Am J Sports Med. 2011;39(12):2558–2565. 10.1177/0363546511423369 [DOI] [PubMed] [Google Scholar]
- 22. Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, et al. Treatment of a full‐thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone‐marrow stromal cells. Osteoarthr Cartil. 2007;15(2):226–231. 10.1016/j.joca.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 23. Huang BJ, Hu JC, Athanasiou KA. Cell‐based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. 10.1016/j.biomaterials.2016.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang J, Li Y, Liu Y, Li D, Zhang L, Wang Q, et al. Influence of hydrogel network microstructures on mesenchymal stem cell chondrogenesis in vitro and in vivo. Acta Biomater. 2019;91:159–172. 10.1016/j.actbio.2019.04.054 [DOI] [PubMed] [Google Scholar]
- 25. Erggelet C, Endres M, Neumann K, Morawietz L, Ringe J, Haberstroh K, et al. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell‐free polymer‐based implants. J Orthop Res. 2009;27(10):1353–1360. 10.1002/jor.20879 [DOI] [PubMed] [Google Scholar]
- 26. OtaŠeviČ T, ValiŠ P, Rouchal M, NovÁk J, Repko M, ŠprlÁkovÁ‐PukovÁ A. Two‐year results of modified AMIC technique for treatment of cartilage defects of the knee. Acta Chir Orthop Traumatol Cech. 2020;87(3):167–174. [PubMed] [Google Scholar]
- 27. Nachtsheim J, Dursun G, Markert B, Stoffel M. Chondrocyte colonisation of a tissue‐engineered cartilage substitute under a mechanical stimulus. Med Eng Phys. 2019;74:58–64. 10.1016/j.medengphy.2019.09.022 [DOI] [PubMed] [Google Scholar]
- 28. Horbert V, Xin L, Foehr P, Brinkmann O, Bungartz M, Burgkart RH, et al. In vitro analysis of cartilage regeneration using a collagen type I hydrogel (CaReS) in the bovine cartilage punch model. Cartilage. 2019;10(3):346–363. 10.1177/1947603518756985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage‐like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84(4):571–578. 10.1302/0301-620x.84b4.11947 [DOI] [PubMed] [Google Scholar]
- 30. Rhee S, Puetzer JL, Mason BN, Reinhart‐King CA, Bonassar LJ. 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater Sci Eng. 2016;2(10):1800–1805. 10.1021/acsbiomaterials.6b00288 [DOI] [PubMed] [Google Scholar]
- 31. Diamantides N, Dugopolski C, Blahut E, Kennedy S, Bonassar LJ. High density cell seeding affects the rheology and printability of collagen bioinks. Biofabrication. 2019;11(4):045016. 10.1088/1758-5090/ab3524 [DOI] [PubMed] [Google Scholar]
- 32. Ogura T, Minas T, Tsuchiya A, Mizuno S. Effects of hydrostatic pressure and deviatoric stress on human articular chondrocytes for designing neo‐cartilage construct. J Tissue Eng Regen Med. 2019;13(7):1143–1152. 10.1002/term.2863 [DOI] [PubMed] [Google Scholar]
- 33. Dong L, Liu Q, Gao Y, Jia H, Dai W, Guo L, et al. The effect of collagen hydrogels on chondrocyte behaviors through restricting the contraction of cell/hydrogel constructs. Regen Biomater. 2021;8(4):rbab030. 10.1093/rb/rbab030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jeyakumar V, Niculescu‐Morzsa E, Bauer C, Lacza Z, Nehrer S. Redifferentiation of articular chondrocytes by Hyperacute serum and platelet rich plasma in collagen type I hydrogels. Int J Mol Sci. 2019;20(2):316. 10.3390/ijms20020316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohd Yunus MH, Shuid AN, Busra MF, Chua KH, Abdul Ghafar N, Abd RR. The effect of Stichopus chloronotus aqueous extract on human osteoarthritis articular chondrocytes in three‐dimensional collagen type I hydrogel in vitro. Sains Malaysiana. 2019;48(8):1671–1683. 10.17576/jsm-2019-4808-13 [DOI] [Google Scholar]
- 36. Lim MH, Jeun JH, Kim DH, Park SH, Kim SJ, Lee WS, et al. Evaluation of collagen gel‐associated human nasal septum‐derived chondrocytes as a clinically applicable injectable therapeutic agent for cartilage repair. Tissue Eng Regen Med. 2020;17(3):387–399. 10.1007/s13770-020-00261-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dasargyri A, Reichmann E, Moehrlen U. Bio‐engineering of fetal cartilage for in utero spina bifida repair. Pediatr Surg Int. 2020;36(1):25–31. 10.1007/s00383-019-04573-3 [DOI] [PubMed] [Google Scholar]
- 38. Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell‐based repair of large, full‐thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76(4):579–592. 10.2106/00004623-199404000-00013 [DOI] [PubMed] [Google Scholar]
- 39. Uto S, Nishizawa S, Takasawa Y, Asawa Y, Fujihara Y, Takato T, et al. Bone and cartilage repair by transplantation of induced pluripotent stem cells in murine joint defect model. Biomedical Res. 2013;34(6):281–288. 10.2220/biomedres.34.281 [DOI] [PubMed] [Google Scholar]
- 40. Aisenbrey EA, Bilousova G, Payne K, Bryant SJ. Dynamic mechanical loading and growth factors influence chondrogenesis of induced pluripotent mesenchymal progenitor cells in a cartilage‐mimetic hydrogel. Biomater Sci. 2019;7(12):5388–5403. 10.1039/c9bm01081e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fabre H, Ducret M, Degoul O, Rodriguez J, Perrier‐Groult E, Aubert‐Foucher E, et al. Characterization of different sources of human MSCs expanded in serum‐free conditions with quantification of Chondrogenic induction in 3D. Stem Cells Int. 2019;2019:2186728. 10.1155/2019/2186728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, et al. Synovial fluid‐derived mesenchymal stem cells increase after intra‐articular ligament injury in humans. Rheumatology. 2008;47(8):1137–1143. [DOI] [PubMed] [Google Scholar]
- 43. Lee WJ, Hah YS, Ock SA, Lee JH, Jeon RH, Park JS, et al. Cell source‐dependent in vivo immunosuppressive properties of mesenchymal stem cells derived from the bone marrow and synovial fluid of minipigs. Exp Cell Res. 2015;333(2):273–288. [DOI] [PubMed] [Google Scholar]
- 44. Fang W, Sun ZT, Chen X, Han B, Vangsness CT. Synovial fluid mesenchymal stem cells for knee arthritis and cartilage defects: a review of the literature. Journal of Knee Surgery. 2020;34:1476–1485. [DOI] [PubMed] [Google Scholar]
- 45. Bekbolsynov D. Synovial membrane derived mesenchymal stem cells in hyaluronic acid scaffold transplantation for regeneration of cartilage defects. 2012.
- 46. Ibrahim AM, Elgharabawi NM, Makhlouf MM, Ibrahim OY. Chondrogenic differentiation of human umbilical cord blood‐derived mesenchymal stem cells in vitro. Microsc Res Tech. 2015;78‐8:667–675. [DOI] [PubMed] [Google Scholar]
- 47. Park YB, Song M, Lee CH, Kim JA, Ha CW. Cartilage repair by human umbilical cord blood‐derived mesenchymal stem cells with different hydrogels in a rat model. J Orthop Res. 2015;33(11):1580–1586. [DOI] [PubMed] [Google Scholar]
- 48. Park Y‐B, Ha C‐W, Kim J‐A, Kim S, Park Y‐G. Comparison of undifferentiated versus Chondrogenic predifferentiated mesenchymal stem cells derived from human umbilical cord blood for cartilage repair in a rat model. Am J Sports Med. 2019;47(2):451–461. 10.1177/0363546518815151 [DOI] [PubMed] [Google Scholar]
- 49. Gómez‐Leduc T, Desancé M, Hervieu M, Legendre F, Ollitrault D, Vienne CD, et al. Hypoxia is a critical parameter for Chondrogenic differentiation of human umbilical cord blood mesenchymal stem cells in type I/III collagen sponges. Int J Mol Sci. 2017;18:1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang X, Lu Y, Wang W, Wang Q, Liang J, Fan Y, et al. Effect of different aged cartilage ECM on chondrogenesis of BMSCs in vitro and in vivo. Regen Biomater. 2020;7(6):583–595. 10.1093/rb/rbaa028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sevastianov VI, Basok YB, Kirsanova LA, Grigoriev AM, Kirillova AD, Nemets EA, et al. A comparison of the capacity of mesenchymal stromal cells for cartilage regeneration depending on collagen‐based injectable biomimetic scaffold type. Life. 2021;11(8):756. 10.3390/life11080756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen P, Tao J, Zhu S, Cai Y, Mao Q, Yu D, et al. Radially oriented collagen scaffold with SDF‐1 promotes osteochondral repair by facilitating cell homing. Biomaterials. 2015;39:114–123. 10.1016/j.biomaterials.2014.10.049 [DOI] [PubMed] [Google Scholar]
- 53. Weissenberger M, Weissenberger MH, Gilbert F, Groll J, Evans CH, Steinert AF. Reduced hypertrophy in vitro after chondrogenic differentiation of adult human mesenchymal stem cells following adenoviral SOX9 gene delivery. BMC Musculoskelet Disord. 2020;21(1):109. 10.1186/s12891-020-3137-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weissenberger M, Weissenberger MH, Wagenbrenner M, Heinz T, Reboredo J, Holzapfel BM, et al. Different types of cartilage neotissue fabricated from collagen hydrogels and mesenchymal stromal cells via SOX9, TGFB1 or BMP2 gene transfer. PloS One. 2020;15(8):e0237479. 10.1371/journal.pone.0237479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Y, Xiao Y, Long S, Fan Y, Zhang X. Role of N‐cadherin in a niche‐mimicking microenvironment for Chondrogenesis of mesenchymal stem cells In vitro. ACS Biomater Sci Eng. 2020;6(6):3491–3501. 10.1021/acsbiomaterials.0c00149 [DOI] [PubMed] [Google Scholar]
- 56. Fang D, Jin P, Huang Q, Yang Y, Zhao J, Zheng L. Platelet‐rich plasma promotes the regeneration of cartilage engineered by mesenchymal stem cells and collagen hydrogel via the TGF‐beta/SMAD signaling pathway. J Cell Physiol. 2019;234:15627–15637. 10.1002/jcp.28211 [DOI] [PubMed] [Google Scholar]
- 57. Rajagopal K, Arjunan P, Marepally S, Madhuri V. Controlled differentiation of mesenchymal stem cells into hyaline cartilage in miR‐140‐activated collagen hydrogel. Cartilage. 2021;13(2_suppl):571S–581S. 10.1177/19476035211047627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang J, Xiao Y, Tang Z, Luo Z, Li D, Wang Q, et al. The negatively charged microenvironment of collagen hydrogels regulates the chondrogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J Mater Chem B. 2020;8(21):4680–4693. 10.1039/d0tb00172d [DOI] [PubMed] [Google Scholar]
- 59. Govoni M, Muscari C, Bonafe F, Morselli PG, Cortesi M, Dallari D, et al. A brief very‐low oxygen tension regimen is sufficient for the early chondrogenic commitment of human adipose‐derived mesenchymal stem cells. Adv Med Sci. 2021;66(1):98–104. 10.1016/j.advms.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 60. Jung HG, Lee D, Lee SW, Kim I, Kim Y, Jang JW, et al. Nanoindentation for monitoring the time‐variant mechanical strength of drug‐loaded collagen hydrogel regulated by hydroxyapatite nanoparticles. ACS Omega. 2021;6(13):9269–9278. 10.1021/acsomega.1c00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martincic D, Mekac J, Drobnic M. Survival rates of various autologous chondrocyte grafts and concomitant procedures. A prospective single‐center study over 18 years. Cell Transplant. 2019;28(11):1439–1444. 10.1177/0963689719861922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Menale C, Campodoni E, Palagano E, Mantero S, Erreni M, Inforzato A, et al. Mesenchymal stromal cell‐seeded biomimetic scaffolds as a factory of soluble RANKL in rankl‐deficient osteopetrosis. Stem Cells Transl Med. 2019;8(1):22–34. 10.1002/sctm.18-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shankar KG, Gostynska N, Montesi M, Panseri S, Sprio S, Kon E, et al. Investigation of different cross‐linking approaches on 3D gelatin scaffolds for tissue engineering application: a comparative analysis. Int J Biol Macromol. 2017;95:1199–1209. 10.1016/j.ijbiomac.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 64. Krishnakumar GS, Gostynska N, Campodoni E, Dapporto M, Montesi M, Panseri S, et al. Ribose mediated crosslinking of collagen‐hydroxyapatite hybrid scaffolds for bone tissue regeneration using biomimetic strategies. Mater Sci Eng C Mater Biol Appl. 2017;77:594–605. 10.1016/j.msec.2017.03.255 [DOI] [PubMed] [Google Scholar]
- 65. Roy R, Boskey AL, Bonassar LJ. Non‐enzymatic glycation of chondrocyte‐seeded collagen gels for cartilage tissue engineering. J Orthop Res. 2008;26(11):1434–1439. 10.1002/jor.20662 [DOI] [PubMed] [Google Scholar]
- 66. Dellaquila A, Campodoni E, Tampieri A, Sandri M. Overcoming the design challenge in 3D biomimetic hybrid scaffolds for bone and Osteochondral regeneration by factorial design. Front Bioeng Biotechnol. 2020;8:743. 10.3389/fbioe.2020.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yudoh K, Kumai T, Yui N. Self‐assembled artificial cartilage‐hydroxyapatite conjugate for combined articular cartilage and subchondral bone repair. Osteoarthr Cartil. 2019;27:27. 10.1016/j.joca.2019.02.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fan Z, Chen Z, Zhang H, Nie Y, Xu S. Gradient mineralized and porous double‐network hydrogel effectively induce the differentiation of BMSCs into Osteochondral tissue in vitro for potential application in cartilage repair. Macromol Biosci. 2021;21(3):e2000323. 10.1002/mabi.202000323 [DOI] [PubMed] [Google Scholar]
- 69. Yan J, Liu C, Tu C, Zhang R, Tang X, Li H, et al. Hydrogel‐hydroxyapatite‐monomeric collagen type‐I scaffold with low‐frequency electromagnetic field treatment enhances osteochondral repair in rabbits. Stem Cell Res Ther. 2021;12(1):572. 10.1186/s13287-021-02638-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chimene D, Alge DL, Gaharwar AK. Two‐dimensional nanomaterials for biomedical applications: emerging trends and future prospects. Adv Mater. 2015;27(45):7261–7284. 10.1002/adma.201502422 [DOI] [PubMed] [Google Scholar]
- 71. Zhou M, Lozano N, Wychowaniec JK, Hodgkinson T, Richardson SM, Kostarelos K, et al. Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019;96:271–280. 10.1016/j.actbio.2019.07.027 [DOI] [PubMed] [Google Scholar]
- 72. Mao H, Kawazoe N, Chen G. Cellular uptake of single‐walled carbon nanotubes in 3D extracellular matrix‐mimetic composite collagen hydrogels. J Nanosci Nanotechnol. 2014;14(3):2487–2492. 10.1166/jnn.2014.8526 [DOI] [PubMed] [Google Scholar]
- 73. Chahine NO, Collette NM, Thomas CB, Genetos DC, Loots GG. Nanocomposite scaffold for chondrocyte growth and cartilage tissue engineering: effects of carbon nanotube surface functionalization. Tissue Eng Part A. 2014;20(17–18):2305–2315. 10.1089/ten.TEA.2013.0328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang J, He C, Cheng N, Yang Q, Chen M, You L, et al. Bone marrow stem cells response to collagen/single‐wall carbon nanotubes‐COOHs nanocomposite films with transforming growth factor Beta 1. J Nanosci Nanotechnol. 2015;15(7):4844–4850. 10.1166/jnn.2015.9844 [DOI] [PubMed] [Google Scholar]
- 75. Antonioli E, Lobo AO, Ferretti M, Cohen M, Marciano FR, Corat EJ, et al. An evaluation of chondrocyte morphology and gene expression on superhydrophilic vertically‐aligned multi‐walled carbon nanotube films. Mater Sci Eng C Mater Biol Appl. 2013;33(2):641–647. 10.1016/j.msec.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 76. Holmes B, Castro NJ, Li J, Keidar M, Zhang LG. Enhanced human bone marrow mesenchymal stem cell functions in novel 3D cartilage scaffolds with hydrogen treated multi‐walled carbon nanotubes. Nanotechnology. 2013;24(36):365102. 10.1088/0957-4484/24/36/365102 [DOI] [PubMed] [Google Scholar]
- 77. Henao‐Holguin LV, Cornejo‐Santiago E, Rojas‐Montoya ID, Gracia‐Mora J, Bernad‐Bernad MJ. MWCNT‐riboflavin nanocomposite for collagen crosslinking: a green approach. Mater Chem Phys. 2020;241:241. 10.1016/j.matchemphys.2019.122361 [DOI] [Google Scholar]
- 78. Hua XW, Bao YW, Wu FG. Fluorescent carbon quantum dots with intrinsic nucleolus‐targeting capability for nucleolus imaging and enhanced cytosolic and nuclear drug delivery. ACS Appl Mater Interfaces. 2018;10(13):10664–10677. 10.1021/acsami.7b19549 [DOI] [PubMed] [Google Scholar]
- 79. Jouffroy M, Armspach D, Matt D, Osakada K, Takeuchi D. Synthesis of optically active polystyrene catalyzed by Monophosphine Pd complexes. Angew Chem Int Ed Engl. 2016;55(29):8367–8370. 10.1002/anie.201603191 [DOI] [PubMed] [Google Scholar]
- 80. Lu Z, Liu S, Le Y, Qin Z, He M, Xu F, et al. An injectable collagen‐genipin‐carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials. 2019;218:119190. 10.1016/j.biomaterials.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 81. Zheng L, Liu S, Cheng X, Qin Z, Lu Z, Zhang K, et al. Intensified stiffness and photodynamic provocation in a collagen‐based composite hydrogel drive Chondrogenesis. Adv Sci. 2019;6(16):1900099. 10.1002/advs.201900099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Culica ME, Chibac‐Scutaru AL, Mohan T, Coseri S. Cellulose‐based biogenic supports, remarkably friendly biomaterials for proteins and biomolecules. Biosens Bioelectron. 2021;182:113170. 10.1016/j.bios.2021.113170 [DOI] [PubMed] [Google Scholar]
- 83. Torabizadeh F, Fadaie M, Mirzaei E, Sadeghi S, Nejabat GR. Tailoring structural properties, mechanical behavior and cellular performance of collagen hydrogel through incorporation of cellulose nanofibrils and cellulose nanocrystals: a comparative study. Int J Biol Macromol. 2022;219:438–451. 10.1016/j.ijbiomac.2022.08.006 [DOI] [PubMed] [Google Scholar]
- 84. Liu D, Dong X, Liu H, Zhao Y, Qi M. Effect of pore orientation on shear viscoelasticity of cellulose nanocrystal/collagen hydrogels. J Appl Polym Sci. 2020;138(7):49856. 10.1002/app.49856 [DOI] [Google Scholar]
- 85. Yang H, Shen L, Bu H, Li G. Stable and biocompatible hydrogel composites based on collagen and dialdehyde carboxymethyl cellulose in a biphasic solvent system. Carbohydr Polym. 2019;222:114974. 10.1016/j.carbpol.2019.114974 [DOI] [PubMed] [Google Scholar]
- 86. Zhang S, Huang D, Lin H, Xiao Y, Zhang X. Cellulose nanocrystal reinforced collagen‐based nanocomposite hydrogel with self‐healing and stress‐relaxation properties for cell delivery. Biomacromolecules. 2020;21(6):2400–2408. 10.1021/acs.biomac.0c00345 [DOI] [PubMed] [Google Scholar]
- 87. Wang Q, Li X, Wang P, Yao Y, Xu Y, Chen Y, et al. Bionic composite hydrogel with a hybrid covalent/noncovalent network promoting phenotypic maintenance of hyaline cartilage. J Mater Chem B. 2020;8(20):4402–4411. 10.1039/d0tb00253d [DOI] [PubMed] [Google Scholar]
- 88. Yang J, Liu Y, He L, Wang Q, Wang L, Yuan T, et al. Icariin conjugated hyaluronic acid/collagen hydrogel for osteochondral interface restoration. Acta Biomater. 2018;74:156–167. 10.1016/j.actbio.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 89. Li X, Teng Y, Liu J, Lin H, Fan Y, Zhang X. Chondrogenic differentiation of BMSCs encapsulated in chondroinductive polysaccharide/collagen hybrid hydrogels. J Mater Chem B. 2017;5(26):5109–5119. 10.1039/C7TB01020F [DOI] [PubMed] [Google Scholar]
- 90. Chen Y, Sui J, Wang Q, Yin Y, Liu J, Wang Q, et al. Injectable self‐crosslinking HA‐SH/Col I blend hydrogels for in vitro construction of engineered cartilage. Carbohydr Polym. 2018;190:57–66. 10.1016/j.carbpol.2018.02.057 [DOI] [PubMed] [Google Scholar]
- 91. Kong W, Gao Y, Liu Q, Dong L, Guo L, Fan H, et al. The effects of chemical crosslinking manners on the physical properties and biocompatibility of collagen type I/hyaluronic acid composite hydrogels. Int J Biol Macromol. 2020;160:1201–1211. 10.1016/j.ijbiomac.2020.05.208 [DOI] [PubMed] [Google Scholar]
- 92. Gao Y, Li K, Guo L, Fan H, Fan Y, Zhang X. Fabrication of biomimetic hydrogel for chondrocyte delivery. Mater Lett. 2020;258:258. 10.1016/j.matlet.2019.126660 [DOI] [Google Scholar]
- 93. Liu Y, Yang J, Luo Z, Li D, Lu J, Wang Q, et al. Development of an injectable thiolated icariin functionalized collagen/hyaluronic hydrogel to promote cartilage formation in vitro and in vivo. J Mater Chem B. 2019;7(17):2845–2854. 10.1039/c9tb00211a [DOI] [PubMed] [Google Scholar]
- 94. Ren Y, Zhang H, Qin W, Du B, Liu L, Yang J. A collagen mimetic peptide‐modified hyaluronic acid hydrogel system with enzymatically mediated degradation for mesenchymal stem cell differentiation. Mater Sci Eng C Mater Biol Appl. 2020;108:110276. 10.1016/j.msec.2019.110276 [DOI] [PubMed] [Google Scholar]
- 95. Zhang Y, Cao Y, Zhao H, Zhang L, Ni T, Liu Y, et al. An injectable BMSC‐laden enzyme‐catalyzed crosslinking collagen‐hyaluronic acid hydrogel for cartilage repair and regeneration. J Mater Chem B. 2020;8(19):4237–4244. 10.1039/d0tb00291g [DOI] [PubMed] [Google Scholar]
- 96. Schwab A, Helary C, Richards RG, Alini M, Eglin D, D'Este M. Tissue mimetic hyaluronan bioink containing collagen fibers with controlled orientation modulating cell migration and alignment. Mater Today Bio. 2020;7:100058. 10.1016/j.mtbio.2020.100058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim JK, Bae HC, Ro DH, Lee S, Lee MC, Han HS. Enhancement of cartilage regeneration of synovial stem cells/hydrogel by using Transglutaminase‐4. Tissue Eng Part A. 2021;27(11–12):761–770. 10.1089/ten.TEA.2020.0271 [DOI] [PubMed] [Google Scholar]
- 98. Yao Y, Wang P, Li X, Xu Y, Lu G, Jiang Q, et al. A di‐self‐crosslinking hyaluronan‐based hydrogel combined with type I collagen to construct a biomimetic injectable cartilage‐filling scaffold. Acta Biomater. 2020;111:197–207. 10.1016/j.actbio.2020.05.007 [DOI] [PubMed] [Google Scholar]
- 99. Zhai M, Zhu Y, Yang M, Mao C. Human mesenchymal stem cell derived exosomes enhance cell‐free bone regeneration by altering their miRNAs profiles. Adv Sci. 2020;7(19):2001334. 10.1002/advs.202001334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li Z, Cao H, Xu Y, Li X, Han X, Fan Y, et al. Bioinspired polysaccharide hybrid hydrogel promoted recruitment and chondrogenic differentiation of bone marrow mesenchymal stem cells. Carbohydr Polym. 2021;267:118224. 10.1016/j.carbpol.2021.118224 [DOI] [PubMed] [Google Scholar]
- 101. Gao Y, Liu Q, Kong W, Wang J, He L, Guo L, et al. Activated hyaluronic acid/collagen composite hydrogel with tunable physical properties and improved biological properties. Int J Biol Macromol. 2020;164:2186–2196. 10.1016/j.ijbiomac.2020.07.319 [DOI] [PubMed] [Google Scholar]
- 102. Ulrich TA, Jain A, Tanner K, MacKay JL, Kumar S. Probing cellular mechanobiology in three‐dimensional culture with collagen‐agarose matrices. Biomaterials. 2010;31(7):1875–1884. 10.1016/j.biomaterials.2009.10.047 [DOI] [PubMed] [Google Scholar]
- 103. Anderson DE, Johnstone B. Dynamic mechanical compression of chondrocytes for tissue engineering: a critical review. Front Bioeng Biotechnol. 2017;5:76. 10.3389/fbioe.2017.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cambria E, Brunner S, Heusser S, Fisch P, Hitzl W, Ferguson SJ, et al. Cell‐laden agarose‐collagen composite hydrogels for Mechanotransduction studies. Front Bioeng Biotechnol. 2020;8:346. 10.3389/fbioe.2020.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rahfoth B, Weisser J, Sternkopf F, Aigner T, von der Mark K, Bräuer R. Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthr Cartil. 1998;6(1):50–65. 10.1053/joca.1997.0092 [DOI] [PubMed] [Google Scholar]
- 106. Kawabe N, Yoshinao M. The repair of full‐thickness articular cartilage defects. Immune responses to reparative tissue formed by allogeneic growth plate chondrocyte implants. Clin Orthop Relat Res. 1991;268:279–293. [PubMed] [Google Scholar]
- 107. Perrier‐Groult E, Peres E, Pasdeloup M, Gazzolo L, Duc Dodon M, Mallein‐Gerin F. Evaluation of the biocompatibility and stability of allogeneic tissue‐engineered cartilage in humanized mice. PloS One. 2019;14(5):e0217183. 10.1371/journal.pone.0217183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Huang Z, Feng Q, Yu B, Li S. Biomimetic properties of an injectable chitosan/nano‐hydroxyapatite/collagen composite. Mater Sci Eng C. 2011;31(3):683–687. 10.1016/j.msec.2010.12.014 [DOI] [Google Scholar]
- 109. Korpayev S, Kaygusuz G, Sen M, Orhan K, Oto C, Karakecili A. Chitosan/collagen based biomimetic osteochondral tissue constructs: a growth factor‐free approach. Int J Biol Macromol. 2020;156:681–690. 10.1016/j.ijbiomac.2020.04.109 [DOI] [PubMed] [Google Scholar]
- 110. Korpayev S, Toprak O, Kaygusuz G, Sen M, Orhan K, Karakecili A. Regulation of chondrocyte hypertrophy in an osteochondral interface mimicking gel matrix. Colloids Surf B Biointerfaces. 2020;193:111111. 10.1016/j.colsurfb.2020.111111 [DOI] [PubMed] [Google Scholar]
- 111. Sionkowska A, Kaczmarek B, Lewandowska K. Modification of collagen and chitosan mixtures by the addition of tannic acid. J Mol Liq. 2014;199:318–323. 10.1016/j.molliq.2014.09.028 [DOI] [Google Scholar]
- 112. Choi B, Kim S, Lin B, Wu BM, Lee M. Cartilaginous extracellular matrix‐modified chitosan hydrogels for cartilage tissue engineering. ACS Appl Mater Interfaces. 2014;6(22):20110–20121. 10.1021/am505723k [DOI] [PubMed] [Google Scholar]
- 113. Shah R, Stodulka P, Skopalova K, Saha P. Dual crosslinked collagen/chitosan film for potential biomedical applications. Polymers. 2019;11(12):2094. 10.3390/polym11122094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zheng L, Sun J, Li B, Zhou W, Fan H, Zhang X. Comparative study of collagen hydrogels modified in two ways using the model of ectopic cartilage construction with diffusion‐chamber in immunocompetent host. J Appl Biomater Funct Mater. 2014;12(1):41–47. 10.5301/JABFM.2012.9340 [DOI] [PubMed] [Google Scholar]
- 115. Jahanbakhsh A, Nourbakhsh MS, Bonakdar S, Shokrgozar MA, Haghighipour N. Evaluation of alginate modification effect on cell‐matrix interaction, mechanotransduction and chondrogenesis of encapsulated MSCs. Cell Tissue Res. 2020;381(2):255–272. 10.1007/s00441-020-03216-7 [DOI] [PubMed] [Google Scholar]
- 116. Ledo AM, Vining KH, Alonso MJ, Garcia‐Fuentes M, Mooney DJ. Extracellular matrix mechanics regulate transfection and SOX9‐directed differentiation of mesenchymal stem cells. Acta Biomater. 2020;110:153–163. 10.1016/j.actbio.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Saghati S, Rahbarghazi R, Baradar Khoshfetrat A, Moharamzadeh K, Tayefi Nasrabadi H, Roshangar L. Phenolated alginate‐collagen hydrogel induced chondrogenic capacity of human amniotic mesenchymal stem cells. J Biomater Appl. 2021;36(5):789–802. 10.1177/08853282211021692 [DOI] [PubMed] [Google Scholar]
- 118. Yuan T, Li Z, Zhang Y, Shen K, Zhang X, Xie R, et al. Injectable ultrasonication‐induced silk fibroin hydrogel for cartilage repair and regeneration. Tissue Eng Part A. 2021;27(17‐18):1213–1224. [DOI] [PubMed] [Google Scholar]
- 119. Serodio R, Schickert SL, Costa‐Pinto AR, Dias JR, Granja PL, Yang F, et al. Ultrasound sonication prior to electrospinning tailors silk fibroin/PEO membranes for periodontal regeneration. Mater Sci Eng C Mater Biol Appl. 2019;98:969–981. 10.1016/j.msec.2019.01.055 [DOI] [PubMed] [Google Scholar]
- 120. Long S, Huang D, Ma Z, Shi S, Xiao Y, Zhang X. A sonication‐induced silk‐collagen hydrogel for functional cartilage regeneration. J Mater Chem B. 2022;10(26):5045–5057. 10.1039/d2tb00564f [DOI] [PubMed] [Google Scholar]
- 121. Liu B, Song YW, Jin L, Wang ZJ, Pu DY, Lin SQ, et al. Silk structure and degradation. Colloids Surf B Biointerfaces. 2015;131:122–128. 10.1016/j.colsurfb.2015.04.040 [DOI] [PubMed] [Google Scholar]
- 122. Lamboni L, Gauthier M, Yang G, Wang Q. Silk sericin: a versatile material for tissue engineering and drug delivery. Biotechnol Adv. 2015;33(8):1855–1867. 10.1016/j.biotechadv.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 123. Huang J, Liang Y, Huang Z, Xiong J, Wang D. Preparation, characterization, and biological testing of novel magnetic nanocomposite hydrogels. ACS Omega. 2020;5(17):9733–9743. 10.1021/acsomega.9b04080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Xie J, Wang W, Zhao R, Lu W, Chen L, Su W, et al. Fabrication and characterization of microstructure‐controllable COL‐HA‐PVA hydrogels for cartilage repair. J Mater Sci Mater Med. 2021;32(9):100. 10.1007/s10856-021-06577-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Song Y, Choi JH, Tumursukh N‐E, Kim NE, Jeon GY, Kim SE, et al. Macro‐ and microporous polycaprolactone/duck's feet collagen scaffold fabricated by combining facile phase separation and particulate leaching techniques to enhance osteogenesis for bone tissue engineering. J Biomater Sci Polym Ed. 2022;33(8):1025–1042. 10.1080/09205063.2022.2036933 [DOI] [PubMed] [Google Scholar]
- 126. Hu Y, Feng B, Zhang W, Yan C, Yao Q, Shao C, et al. Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp Ther Med. 2019;17(5):3717–3726. 10.3892/etm.2019.7387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen D, Zhu T, Fu W, Zhang H. Electrospun polycaprolactone/collagen nanofibers cross‐linked with 1‐ethyl‐3‐(3‐dimethylaminopropyl) carbodiimide/N‐hydroxysuccinimide and genipin facilitate endothelial cell regeneration and may be a promising candidate for vascular scaffolds. Int J Nanomedicine. 2019;14:2127–2144. 10.2147/IJN.S192699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ghorbani M, Nezhad‐Mokhtari P, Ramazani S. Aloe vera‐loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int J Biol Macromol. 2020;153:921–930. 10.1016/j.ijbiomac.2020.03.036 [DOI] [PubMed] [Google Scholar]
- 129. Entekhabi E, Haghbin Nazarpak M, Shafieian M, Mohammadi H, Firouzi M, Hassannejad Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J Biomed Mater Res A. 2021;109(3):300–312. 10.1002/jbm.a.37023 [DOI] [PubMed] [Google Scholar]
- 130. Lamparelli EP, Lovecchio J, Ciardulli MC, Giudice V, Dale TP, Selleri C, et al. Chondrogenic commitment of human bone marrow mesenchymal stem cells in a perfused collagen hydrogel functionalized with hTGF‐beta1‐releasing PLGA microcarrier. Pharmaceutics. 2021;13(3):399. 10.3390/pharmaceutics13030399 [DOI] [PMC free article] [PubMed] [Google Scholar]


