Summary
Background
Good physical and mental health are essential for healthy ageing. Holistic mobile health (mHealth) interventions—including at least three components: physical activity, diet, and mental health—could support both physical and mental health and be scaled to the population level. This review aims to describe the characteristics of holistic mHealth interventions and their effects on related behavioural and health outcomes among adults from the general population.
Methods
In this systematic review and meta-analysis, we searched MEDLINE, Embase, Cochrane Central Register of Controlled Trials, PsycINFO, Scopus, China National Knowledge Infrastructure, and Google Scholar (first 200 records). The initial search covered January 1, 2011, to April 13, 2022, and an updated search extended from April 13, 2022 to August 30, 2023. Randomised controlled trials (RCTs) and non-randomised studies of interventions (NRSIs) were included if they (i) were delivered via mHealth technologies, (ii) included content on physical activity, diet, and mental health, and (iii) targeted adults (≥18 years old) from the general population or those at risk of non-communicable diseases (NCDs) or mental disorders. Studies were excluded if they targeted pregnant women (due to distinct physiological responses), individuals with pre-existing NCDs or mental disorders (to emphasise prevention), or primarily utilised web, email, or structured phone support (to focus on mobile technologies without exclusive human support). Data (summary data from published reports) extraction and risk-of-bias assessment were completed by two reviewers using a standard template and Cochrane risk-of-bias tools, respectively. Narrative syntheses were conducted for all studies, and random-effects models were used in the meta-analyses to estimate the pooled effect of interventions for outcomes with comparable data in the RCTs. The study was registered in PROSPERO, CRD42022315166.
Findings
After screening 5488 identified records, 34 studies (25 RCTs and 9 pre-post NRSIs) reported in 43 articles with 5691 participants (mean age 39 years, SD 12.5) were included. Most (91.2%, n = 31/34) were conducted in high-income countries. The median intervention duration was 3 months, and only 23.5% (n = 8/34) of studies reported follow-up data. Mobile applications, short-message services, and mobile device-compatible websites were the most common mHealth delivery modes; 47.1% (n = 16/34) studies used multiple mHealth delivery modes. Of 15 studies reporting on weight change, 9 showed significant reductions (6 targeted on individuals with overweight or obesity), and in 10 studies reporting perceived stress levels, 4 found significant reductions (all targeted on general adults). In the meta-analysis, holistic mHealth interventions were associated with significant weight loss (9 RCTs; mean difference −1.70 kg, 95% CI −2.45 to −0.95; I2 = 89.00%) and a significant reduction in perceived stress levels (6 RCTs; standardised mean difference [SMD] −0.32; 95% CI −0.52 to −0.12; I2 = 14.52%). There were no significant intervention effects on self-reported moderate-to-vigorous physical activity (5 RCTs; SMD 0.21; 95%CI −0.25 to 0.67; I2 = 74.28%) or diet quality scores (5 RCTs; SMD 0.21; 95%CI −0.47 to 0.65; I2 = 62.27%). All NRSIs were labelled as having a serious risk of bias overall; 56% (n = 14/25) of RCTs were classified as having some concerns, and the others as having a high risk of bias.
Interpretation
Findings from identified studies suggest that holistic mHealth interventions may aid reductions in weight and in perceived stress levels, with small to medium effect sizes. The observed effects on diet quality scores and self-reported moderate-to-vigorous physical activity were less clear and require more research. High-quality RCTs with longer follow-up durations are needed to provide more robust evidence. To promote population health, future research should focus on vulnerable populations and those in middle- and low-income countries. Optimal combinations of delivery modes and components to improve efficacy and sustain long-term effects should also be explored.
Funding
National Research Foundation, Prime Minister's Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) Programme and Physical Activity and Nutrition Determinants in Asia (PANDA) Research Programme.
Keywords: Holistic, mHealth intervention, Prevention, Systematic review, Meta-analysis
Research in context.
Evidence before this study
We searched PubMed and PROSPERO up until March 2022 to identify existing systematic reviews and meta-analyses on holistic mHealth interventions (including components targeting physical activity, diet, and mental health) among adults from the general population. We identified several reviews of mHealth interventions for health behaviour changes and mental health. Most targeted individual behaviours or outcomes, or combined physical activity and diet, without addressing aspects of mental health. We identified two reviews focused on holistic mHealth interventions: one involved a holistic Diabetes Prevention Programme delivered via e-health, primarily telehealth consultations and digital video discs (DVDs); the other was a scoping review that identified just four holistic mHealth interventions delivered via mobile applications, of which two were study protocols. Therefore, we conducted a systematic review and meta-analysis to specifically address this research gap.
Added value of this study
This is the first systematic review and meta-analysis to provide a comprehensive overview of holistic mHealth interventions and to evaluate the effectiveness of such interventions on behavioural and health outcomes among adults from the general population. We comprehensively searched the scientific literature and reviewed studies published between January 2011 and August 2023. Our findings demonstrate a rapid growth in the available evidence but also highlight that holistic mHealth interventions primarily involved young and middle-aged participants, with an average age of 40 years old from high-income countries. Mobile applications, short-message services, and mobile device-compatible websites were the top three mHealth delivery modes. Evidence of intervention effectiveness was found for weight loss and perceived stress reduction, with small to medium effect sizes. The evidence for improvements in self-reported moderate-to-vigorous physical activity and diet quality scores was inconclusive. In general, the included studies had low methodological quality and substantial heterogeneity in intervention components.
Implications of all the available evidence
This systematic review and meta-analysis provides important insights into the features and effectiveness of holistic mHealth interventions for adults from the general population, emphasising their potential to enhance physical and mental health. Further work is warranted to strengthen the evidence base in more diverse populations, identify optimal intervention components and features for better efficacy and scalability, and accumulate more high-quality evidence on the long-term effects.
Introduction
Over the past three decades, the global average life expectancy has increased by almost nine years (from 63.6 years in 1990 to 72.8 years in 2019).1 However, not all of these additional years are spent in good physical and mental health. In fact, increased longevity can mean more time spent with poor physical and mental health, with associated significant costs to healthcare systems and societies.2, 3, 4 Further amplifying the burden is the interdependence of physical and mental health; individuals with non-communicable diseases (NCDs) are at high risk of mental disorders, and vice versa.4, 5, 6 Consequently, maintaining both physical and mental health over time and into later stages of life, that is “healthy ageing”, has become a primary objective of public health initiatives.7,8
Promoting healthy ageing is a continuous and lifelong endeavour relevant to individuals across all age groups, including younger and older adults.9 It requires a shift from disease treatment to prevention and a focus on holistic health, including body and mind.9 Adopting healthy lifestyles, especially during early adulthood or before reaching old age, can considerably benefit individuals by supporting the maintenance of a high and stable level of functional capacity.9 Healthy lifestyle behaviours, such as physical activity (PA) and healthy eating, are crucial for the prevention of diseases related to both physical and mental health.3,9,10 Meanwhile, neglecting mental health conditions while promoting healthy lifestyle behaviours may impede these efforts. For example, the presence of mental health conditions, such as depression or anxiety, is associated with engagement in unhealthy lifestyle behaviours (e.g., physical inactivity, poor dietary habits), which can, in turn, increase the risk of developing NCDs and further aggravate mental health conditions.5,11,12 A holistic approach, adding mental health components, such as stress management or mindfulness, to interventions that target PA and diet, may have the potential to address this intertwined challenge. Face-to-face interventions that integrate components of PA, diet, and mental health have been found to promote greater weight reduction compared to interventions that only target PA and diet.13,14 However, healthy ageing is a lifelong process and affects the population as a whole, making traditional face-to-face interventions challenging to apply, especially in countries with limited access to mental health and preventive care services.15, 16, 17
Mobile health (mHealth) offers possible solutions to deliver interventions on population-wide scales using mobile technologies such as smartphones, tablets, and wearables.18 These potentially scalable intervention technologies may enhance operational efficiency and gain more insights into people’s day-to-day real-world behaviours and health outcomes.15, 16, 17 Existing population-level mHealth interventions, such as Singapore’s National Step Challenge, Canada’s Carrot Rewards, or the internationally available Stepthalon, have demonstrated the scalability of this approach.10,19, 20, 21, 22 Further, for the past few years, the COVID-19 pandemic has not only disrupted and altered people’s lifestyles by restricting their opportunities for PA and altering dietary habits, but has also provoked negative mental responses such as heightened symptoms of depression and anxiety, making the implementation of effective interventions more challenging.23 This highlights the timeliness of offering mHealth interventions. Holistic interventions delivered by mobile technologies have also shown potential to aid weight management, encourage the adoption of healthy lifestyles, and improve mental wellbeing, as seen in face-to-face interventions.24, 25, 26
The idea of “holistic”—integrating mental health and modifiable lifestyle factors—is relatively new.4 Despite growing interest, there is no comprehensive and systematic synthesis of the evidence on the characteristics and effectiveness of holistic mHealth interventions in the literature. A systematic review of eHealth interventions using the holistic Diabetes Prevention Programme approach found significant weight loss among individuals with prediabetes after the intervention. However, the review included interventions that were primarily delivered via telehealth consulting or digital video discs (DVDs) and only focused on interventions adopting a specific diabetes programme.27 Another scoping review identified a small number of studies (n = 4) that targeted PA, diet, and sleep: two were study protocols, and one did not consider PA, diet, and sleep as a holistic approach.28 The review focused exclusively on sleep and did not consider any other aspects of mental health. Additionally, it was limited to smartphone-based interventions. Considering the rapid growth of mHealth interventions and existing knowledge gaps on holistic mHealth interventions, this review aims to provide a comprehensive overview of the current evidence for holistic mHealth interventions that cover at least three components: PA, diet, and mental health. The review will focus on describing the characteristics of these interventions and assessing their effects on related behavioural and health outcomes in the general adult population.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis is reported as per the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guidelines.29 The protocol was registered in PROSPERO (CRD42022315166), and further details on the methodology are available here.30
We searched six electronic databases: MEDLINE (via PubMed), Embase, PsycINFO (via Ovid), Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), and China National Knowledge Infrastructure (CNKI) between January 1, 2011, and August 30, 2023. The initial search was conducted from January 1, 2021, to April 13, 2022, with an updated search carried out from April 13, 2022, to August 30, 2023. The decision to limit the review to starting in 2011 was primarily informed by the significant advancements in mobile health technology that coincided with the widespread adoption of smartphones and 4G connectivity. According to a report by the International Telecommunication Union, by the end of 2010, there were approximately 5 billion mobile phone subscriptions globally, and about 90% of the world’s population had access to mobile networks.31,32 Moreover, the World Health Organization (WHO) released a report in 2011 focusing on mHealth, which helped establish the field as a legitimate avenue for health intervention studies.18
The search strategy was based on the following topics: 1) mHealth applications; 2) PA; 3) diet; and 4) mental health. The full search strategy is published elsewhere.30 In addition, we searched the first 200 records on Google Scholar and manually browsed the reference lists of included studies and relevant reviews.
The selection criteria were guided by the population, intervention, comparator group, outcome, and study design (PICOS) framework. More details can be found here.30 Briefly, we included randomised controlled trials (RCTs) and non-randomised studies of interventions (NRSIs) if they 1) recruited either adults aged 18 years or older from the general population or those who were at risk of developing NCDs or mental disorders; 2) had content on PA, diet, and mental health in a single intervention; and 3) were delivered mainly via mHealth technologies (e.g., mobile applications (apps), short message service (SMS), and wearables).
Data analysis
The identified records were uploaded to Covidence, a systematic review software (www.covidence.com), where duplicates were removed. Two reviewers independently conducted title and abstract screening (SZ, CHG) and full-text screening (SZ, SME). Discrepancies were resolved through discussions and involving a third reviewer (SME) during title and abstract screening. Cohen’s Kappa (K), calculated by Covidence, was used to assess consistency between reviewers. During title and abstract screening, the K value was 0.60, and it improved to 0.74 during full-text screening. Data from the included studies were extracted by SZ and checked by CHG. Behaviour change techniques were extracted based on the Behaviour Change Technique Taxonomy version 1 (BCTTv1), with two supplementary categories: personalisation and gamification.33,34 Where applicable, data extraction was supplemented with information from the study protocol papers and/or trial registrations.
We summarised the characteristics of the included studies in tables. Because of the diverse behavioural and health outcomes reported, we grouped them into five domains: anthropometry, PA, diet, mental health, and biomarkers. A narrative approach, vote-counting based on effect direction, was used.35 Data is presented as an effect direction plot to show significant positive, significant negative, or mixed effects (no change). Where multiple measurements were reported within the same outcome domain (i.e., PA intensities and lipid profiles) in one study, these were marked as mixed effects if less than 70% of the measurements reported a consistent direction of effect.35
In the meta-analysis, the pooled effect was estimated for the outcome if it was reported in three or more RCTs: weight change, perceived stress levels, diet quality scores, and moderate-to-vigorous physical activity (MVPA). For consistency, the effect of intervention was estimated based on change scores (from baseline to post-intervention), as most studies reported it in this manner. For studies which did not report change scores but provided baseline and post-intervention values, the standard deviation (SD) of change in score was calculated using the formula: SDE, change = [SD2E, baseline + SD2E, final − (2∗Corr ∗SDE, baseline ∗SDE, final)]∧0.5.36 Conservatively, a correlation value of 0.5 between baseline and post-intervention measurements was assumed.36 When a study had multiple intervention groups, we combined groups that had each received an mHealth intervention to create a single pairwise comparison.
Findings were aggregated based on random effects REML models in Stata/SE 16.0 (Stata Corp. LLC). Intervention effects on weight change were reported as a mean difference (MD), while perceived stress levels, self-reported MVPA, and diet quality scores were expressed as standardised mean difference (SMD) using Hedges’ g to minimise the bias from different measurements.36 Heterogeneity was measured via a Chi-squared test and with the I2 measure. We also performed subgroup analyses for all four outcomes by risk of bias, target population, intervention duration, type of control groups, multiple delivery modes, and human support. Publication bias was only assessed for the weight change outcome using Egger’s test. The trim and fill method was not used because of the limited number of studies for other outcomes. Results were presented as forest plots for each outcome of interest, with the weight (in %) indicating the influence of an individual study on the pooled result. All evaluations were based on a two-sided test with a 5% alpha level.
Risk of bias was assessed using Cochrane assessment tools: Risk of Bias 2 (RoB 2) for RCTs and Risk of Bias In Non-Randomised Studies of Interventions (ROBINS-I) for NRSIs.37,38 The RoB 2 tool for RCTs assesses bias across five domains: the randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcomes, and selection of the reported result.37 The ROBINS-I tool for NRSIs assesses bias across seven domains: confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the results reported.38
For both tools, each domain contains several individual items that were rated as either “Yes/Probably Yes,” “No/Probabaly No,” or “No Information” based on the information available. The overall risk of bias for each study was then classified. For RCTs, the categories were “Low,” “Some Concerns,” or “High.” For NRSIs, the categories were “Low,” “Moderate,” “Serious,” or “Critical.” Two independent reviewers conducted these assessments, and any discrepancies were resolved either through discussion or consultation with a third reviewer.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. SZ, CHG, and SME have access to the dataset. SZ and FMR have final responsibility for the decision to submit for publication.
Results
Study selection
A summary of the screening process is provided in a PRISMA flow diagram (Fig. 1). We obtained 3727 records from six electronic databases and an additional 1761 records from manual citation searches and Google Scholar. After removing duplicates and title/abstract screening, we retrieved 246 records for full-text screening. Following full-text screening, 43 records reporting 34 studies were eligible for data extraction and narrative synthesis; 14 provided sufficient data for inclusion in the meta-analysis.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram. The figure outlines the study selection process for the systematic review and meta-analysis. It describes the number of studies identified through database searches and other methods, the number of studies excluded based on title and abstract screening, and the number of studies excluded after full-text assessment. The reasons for exclusion at each stage are also specified.
Characteristics of the included studies
Description of studies
Table 1 summarises the main characteristics of studies included in this review. Supplementary Table S1 shows the characteristics of individual studies. Of the 34 studies, 26 (76.5%) were published between 2017 and 2023; 25 studies were RCTs and 9 were NRSIs (pre-post design). The studies were mainly conducted in high-income countries, primarily in the United States (n = 10)24,39, 40, 41, 42, 43, 44, 45, 46, 47 and Australia (n = 6).25,48, 49, 50, 51, 52 Only three studies were conducted in middle-income countries, and two were located in Asia: Thailand and Iran.53,54 No identified studies were conducted in low-income countries. Nineteen studies recruited participants from the general adult population,26,38,39,41,48, 49, 50, 51,55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 and 15 studies recruited individuals at risk of NCDs or mental disorders,24,25,42, 43, 44, 45, 46, 47,52, 53, 54,66, 67, 68, 69 particularly participants with overweight or obesity (n = 12).24,25,42,43,45,46,52,53,66, 67, 68, 69 Baseline sample sizes ranged from 15 to 1280 participants, and 17 studies (50.0%) had a sample size of less than 100.26,40, 41, 42,44,45,48, 49, 50, 51,53,55, 56, 57,62,63,69 The 34 studies included a total of 5691 participants with a mean age of 39 years (SD 12.5). Four studies exclusively targeted females (n = 2)40,46 or males (n = 2).25,48 In the remaining 27 studies with reported sex distributions, the percentage of females ranged from 26.0% to 94.3%, with a median of 63.2%.24,26,39,41, 42, 43,45,47,49, 50, 51, 52, 53, 54, 55, 56, 57, 58,60,61,63, 64, 65, 66, 67, 68, 69 Out of the 20 studies that reported education levels, 71.6% of participants had at least a secondary school education.24,25,39,40, 41, 42,45,48,49,51,52,56,57,60,62,63,67, 68, 69, 70
Table 1.
Summary of main characteristics of all included studies.
| Category | Number of studies (%) (n = 34) | Randomised controlled trials (n = 25) | Non-randomised studies of interventions (n = 9) |
|---|---|---|---|
| Publication date | |||
| 2011–2016 | 8 (23.5) | 7 | 1 |
| 2017–2023 | 26 (76.5) | 18 | 8 |
| Study typea | |||
| Pilot study | 16 (47.1) | 10 | 6 |
| Main trial | 18 (52.9) | 16 | 2 |
| Country/setting by incomeb | |||
| High-income country | 31 (91.2) | 23 | 8 |
| Middle-income country | 3 (8.8) | 2 | 1 |
| Low-income country | 0 (0.0) | 0 | 0 |
| Country/setting by region | |||
| Europe | 6 (17.6) | 4 | 2 |
| Asia | 7 (20.6) | 4 | 3 |
| Oceania (Australia and New Zealand) | 8 (23.5) | 8 | 0 |
| North America (United States and Dominican Republic) | 13 (38.2) | 9 | 4 |
| Targeted population | |||
| Adults at risk of non-communicable diseases or mental disorders | 15 (44.1) | 15 | 0 |
| Adults from the general population | 19 (55.9) | 10 | 9 |
| Participant characteristics | |||
| Age (mean ± SD), years | 39.1 ± 12.5 | 40.2 ± 13.0 | 35.5 ± 9.6 |
| Female (%) | 63.2 (n = 27) | 62.7 (n = 21) | 64.9 (n = 6) |
| Male (%) | 36.8 (n = 27) | 37.2 (n = 21) | 35.1 (n = 6) |
| Higher than secondary school (%) | 71.6 (n = 20) | 70.9 (n = 15) | 61.9 (n = 5) |
| Body mass index (mean ± SD), kg/m2 | 30.0 ± 3.8 (n = 18) | 30.0 ± 3.8 (n = 18) | – |
| Intervention duration | |||
| <3 months | 12 (35.3) | 7 | 5 |
| 3–6 months | 22 (64.7) | 18 | 4 |
| Sample size | |||
| <100 | 17 (50.0) | 10 | 7 |
| 100–200 | 11 (32.4) | 10 | 1 |
| >200 | 6 (22.6) | 5 | 1 |
| Mode of delivery | |||
| Single delivery mode | 18 (52.9) | 13 | 5 |
| Multiple delivery modes | 16 (47.1) | 12 | 4 |
| Human support | |||
| With human support | 17 (50.0) | 13 | 4 |
| Without human support | 17 (50.0) | 12 | 5 |
| Theory-based | 17 (50.0) | 13 | 4 |
The study type was self-reported from studies.
Classification by income based on World Bank Country and Leading Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/%20906519-world-bank-country-and-lending-group.
Description of interventions
The core interventions ranged in duration from 7 days to 6 months, with a median of 3 months. Eight studies (23.5%) included post intervention follow-up data at 6, 9, or 12 months (Table 1 and Supplementary Table S1).25,43,44,50,52,59,66,69 Table 2 provides the details of holistic mHealth interventions and control groups. For interventions that recruited adults from the general population, the main focus was mostly on healthy lifestyle or wellness promotion (n = 7),39, 40, 41,48,49,60,61 followed by weight management (n = 3),50,51,57 mental health improvement (n = 3),26,55,65 and NCD prevention (n = 3).56,58,59 Of the 15 studies targeting people at risk of NCDs or mental disorders, 9 interventions were focused on weight management,25,42,43,45,46,52,67, 68, 69 4 on NCD prevention,24,47,53,54 and 2 on mental health improvement.44,66 Apps (n = 27), SMS (n = 11), and mobile device-compatible websites (n = 8) were the top 3 mHealth delivery modes utilised in the interventions, followed by wearables (n = 7) and social media (n = 4). Notably, 16 (47.1%) studies adopted multiple delivery modes, using combinations of apps, wearables, SMS, and/or mobile device-compatible websites.24,25,41,42,45, 46, 47, 48, 49,52,56,57,59,60,62,65 Among the 18 single-mode interventions, 15 were app-based26,39,40,44,50,51,53,55,58,61,63,64,66, 67, 68, 69 and 3 used SMS.43,54,69 SMS provides simple functions like health education content, reminders, and communication (feedback or motivational messages), while apps and websites have more features and functions, such as gamification elements, self-monitoring, and progress tracking.
Table 2.
Descriptions of intervention and control groups.
| Study | Intervention purpose | mHealth delivery mode | mHealth component | Other components | Behaviour change technique category (from BCTTv1)a | Theory | Comparison groups |
|---|---|---|---|---|---|---|---|
| Targeted adults from the general population | |||||||
| Ahtinen 2013 | Stress management | App (Oiva) |
|
Not reported |
|
Acceptance and commitment therapy theory | None |
| Van Drongelen 2014; 2016 | Sleep improvement and fatigue reduction | App (More energy); Project website |
|
Not reported |
|
Not reported | Project website |
| Du 2016 | Healthy lifestyle promotion | App (Fittle) |
|
Not reported |
|
Theory of planned behaviour; Social cognitive theory |
|
| Zhang 2017 | Coronary heart disease prevention | App (Care4Heart); SMS |
|
Not reported |
|
Health belief model | Website links for health information |
| Comulada 2017; 2018 | Healthy lifestyle promotion | App (Ohmage) |
|
Face-to-face support from researchers to review the progress at 3 and 6 months |
|
Not reported | None |
| Ashton 2017 | Healthy lifestyle promotion | Website; App (UP); Wearables (JAWBONE); Social media (Facebook) |
|
Weekly face-to-face support from reseachers and physical education teachers on tailored dietary goals and physical activity; Resistance training band; Dinner disc, a visual guide to assists in controlling the portion size of food |
|
Self-cognitive theory; Self-determination theory |
Wait-list control |
| Baek 2018 | Stress management | App |
|
Not reported |
|
Not reported | None |
| Podina 2018 | Weight management | App (SIGMA); Wearable (Pedometer) |
|
Not reported |
|
Cognitive behavioural therapy’s cognitive ABC model (Antecedents—Beliefs—Consequences) | None |
| Gleen 2019 | Alzheimer’s disease prevention | App (Nuerotrack MHP) |
|
Not reported |
|
Not reported | None |
| Jiang 2019 | Coronary heart disease prevention | App (Care4Heart); SMS |
|
Not reported |
|
Health belief model | None |
| Mhurchu 2019 | Healthy lifestyle promotion | App (OL@-OR @); Website |
|
Not reported |
|
Theoretical domain framework | Sham control: a control version of the OL@-OR@ app for data collection |
| Oftedal 2019 | Healthy lifestyle promotion | App (Balanced); SMS |
|
Participant handbook; Weekly email summaries based on app entries |
|
Self-cognitive theory; Self-regulatory theory |
Wait-list control |
| Brindal 2019 | Weight management | App (MotiMate) |
|
Phone support from registered dietitian and/or psychologists if participants classified as weight gain and/or highly negative mood |
|
Conservation of resources; Self-regulation theory | Sham control: a control version of MotiMate app with limited feedback and no encouraging features |
| Lyzwinski 2019 | Weight management | App (My Student Mindfulness) |
|
Not reported |
|
Not reported |
|
| Torres 2020 | Employee wellness promotion | Wearables (Fitbit); Website (Qualtrics) |
|
Not reported |
|
Not reported | None |
| Bonn 2022 | Healthy lifestyle promotion | App (Health Integrator) |
|
Phone support from a health coach every 4 weeks throughout the intervention |
|
Not reported | Wait-list control |
| Cantisano 2022 | Healthy lifestyle promotion | Social media (WhatsApp, YouTube channel); App (Headspace, Insight timer, Fabulous) |
|
Three sessions of mindful practice via Zoom |
|
Not reported | None |
| Yuan 2022 | Healthy lifestyle promotion | App (My Wellness Coach) |
|
Five people used the app in the group setting led by a health coach; 36 people used the app individually with one onboarding session and one Q&A session |
|
Health belief model; Theory of planned behaviour; Transtheoretical model |
None |
| Wilson 2023 | Cardiovascular disease prevention | App (TeamBuildr) |
|
Not reported |
|
Not reported | Wait-list control |
| Targeted people at risk ofnon-communicable diseases and mental disorders | |||||||
| Napolitano 2013 | Weight management | Social media (Facebook); SMS; Wearables (Pedometer) |
|
Face-to-face support from researchers on weight loss; Weekly support from support buddy to boost self-monitoring compliance |
|
Not reported | Wait-list control |
| Norton 2015; Hartin 2016; Schiwal 2020 | Alzheimer’s disease prevention | App (The Grey Matters); Wearables (Nike monitor); Website |
|
Weekly email or text message support from a health coach (student intern) |
|
Transtheoretical model of change | Wait-list control |
| Block 2015 | Diabetes prevention | Website (Alive-PD); SMS; App (Alive-PD) |
|
Interactive Voice Response technology: personalised phone coaching every 2 weeks; Personalised education materials |
|
Learning theory; Models centreing on cues and triggers; Social cognitive theory; Theory of planned behaviour; Behavioural economics; Positive psychology |
Wait-list control |
| Lin 2015 | Weight management | SMS |
|
Face-to-face support from registered dietitian-physicians (baseline and at 6 months) on weight control plan, feedback, or health status |
|
Health belief model; Transtheoretical model of change; Self-regulation theory |
Standard care: two dietitian visits and one study physician visit; education materials; a digital pedometer |
| Mattila 2016; Jarvela- Reijonen 2018; Jarvela- Reijonen 2020 | Psychological flexibility improvement | App (Oiva) |
|
Not reported |
|
Acceptance and commitment therapy theory | After intervention, one group session about acceptance and commitment therapy and Internet-based lifestyle coaching programme |
| Stahl 2020 | Depression prevention | App (diary-like app) |
|
Weekly phone support from researchers to boost confidence and intrinsic motivation; Printed educational materials |
|
Not reported | Standard care: medical or grief specialty care as needed |
| Puntpanich 2020 | Cardiovascular disease prevention | App (Chicken LOF) | Physical activity, mood, sleep, and diet self-monitoring via a virtual chick | Face-to-face support from physicians at baseline to provide personalised advice on diet and physical activity |
|
Not reported | Standard care: general physical consultation on diet and physical activity |
| Duncan 2020; Fenton 2021 |
Weight management | App (Balanced); Wearables (Fitbit); SMS; Calorie counting platform |
|
Face-to-face support from registered dietitian at baseline to get personalised dietary advice; Body weight scale; Participant handbook; Weekly email summaries |
|
Self-cognitive theory; Self-regulatory theory |
Wait-list control |
| Nezami 2021 | Weight management | App (PATH, Fitbit); Wearable (Fitbit); SMS |
|
Body weight scale |
|
Social cognitive theory | Shame control: the only difference, compared to intervention group was the dietary self-monitoring tool |
| Drew 2021; Young 2021; Drew 2022 | Weight management | Website (SHED-IT); SMS; App (MyFitness Pal) ∗ optional |
|
Phone support from registered psychologists if they had symptom exacerbation or any suicidal ideation (7 times maximum); Programme handbook; Logbook for recoding the progress |
|
Social cognitive theory | Wait-list control |
| Napolitano 2021 | Weight management | App (BeFAB); Social media (Facebook); SMS |
|
Not reported |
|
Not reported | Standard care: educational materials on healthy behaviours and weight monitoring |
| Didehban 2022 | Metabolic syndrome prevention | SMS | Daily messages on physical activity, diet, or stress | Not reported |
|
Not reported | No-intervention |
| Nakata 2022 | Weight management | App (CALO mama Plus) |
|
Not reported |
|
Not reported | Wait-list control |
| Thorgeirsson 2022 | Weight management | App (Sidekick) |
|
Weekly or bi-weekly fitness coaching and monthly nutrition and health education class |
|
Not reported | Standard care: weekly or bi-weekly fitness coaching and monthly nutrition and health education class |
| Jensen 2023 | Weight management | SMS |
|
Face-to-face support from a clinical psychology doctoral student and a clinician at baseline to provide motivational interviewing and skills of weight control and sleep improvement |
|
Not reported | Sham control: the only difference, compared to the intervention group, was the control group did not receive any content on sleep component. |
The numbers in the "Behavior Change Technique Category" column correspond to the categories defined in the Behavior Change Technique Taxonomy version 1 (BCTTv1) with two supplementary categories: personalisation and gamification.
In addition to mHealth, 18 interventions included other components, such as face-to-face human support, printed materials, weighing scales, and email summaries.24,25,40,42, 43, 44, 45,47, 48, 49, 50,52,53,61, 62, 63,68,69 Interventions applied a median of 5 (range 1–9) behaviour change techniques.33,34 Approximately 85% of interventions (n = 29) applied three or more behavioural change techniques.24, 25, 26,39,40,42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52,55, 56, 57, 58, 59, 60, 61, 62, 63, 64,66, 67, 68, 69 The most common techniques employed were feedback and monitoring (n = 23), social support (n = 17), regulation (n = 16), and goals and planning (n = 16). Among the 17 interventions that cited a theoretical grounding,24,25,39,43,45,47, 48, 49, 50,52,55, 56, 57,59,60,63,66 social cognitive theory was the most often used (n = 7), followed by the health belief model (n = 4) and self-regulation theory (n = 3).
The studies included various comparison groups: wait-list control (i.e., receiving the intervention after the active intervention group, n = 10),24,25,42,47, 48, 49,52,61,64,67 standard care (e.g., physician or specialist consultation, n = 5),43,44,46,53,68 sham control (same mHealth intervention with different or limited features, n = 4),45,50,60,69 no-intervention control (n = 1),54 and other controls (e.g., education materials and website links, n = 5).39,51,56,65,66
Outcome measures and effects
Feasibility-related outcomes
In total, 24 studies reported outcomes related to user engagement, adherence, usability, and/or satisfaction, as detailed in Supplementary Table S3. These measurements varied widely among studies, making outcome aggregation challenging. In a subset of five studies, an average of 82% of participants reported being satisfied with or finding the interventions acceptable or helpful.42,46,51,65,67 Conversely, seven studies noted low engagement or adherence,40,43, 44, 45, 46,51,71 with four indicating that these metrics decreased over time.40,42,43,45 For instance, one study reported that 54% of participants accessed the app at least once46; two other studies reported adherence rates of 14%–17% of the intended usage.51,65 In addition, four studies reported significant positive associations between engagement or adherence and behavioural and health outcomes.26,58,71, 72, 73
Behavioural and health outcomes
Among 31 studies that assessed behavioural and health outcomes, most studies (n = 28) measured various outcomes across domains (Supplementary Table S2).24, 25, 26,39,41,42,44, 45, 46, 47, 48, 49, 50, 51, 52, 53,56,58, 59, 60, 61, 62, 63, 64, 65, 66, 67,69 Four studies reported outcomes from all five domains.25,48,52,64 Specifically, 17 studies assessed anthropometric outcomes, such as body weight, body mass index (BMI), and waist circumference.24,25,42,43,45,46,48,50, 51, 52, 53,61,63,64,67, 68, 69 Furthermore, 22 studies measured PA-related outcomes (e.g., different PA intensities),25,26,39,41,44,46, 47, 48, 49, 50, 51, 52,55,56,58, 59, 60,62, 63, 64, 65, 66, 67 20 measured diet-related outcomes (e.g., diet quality scores, fruit and vegetable intake),25,26,39,41,44, 45, 46,48, 49, 50, 51, 52,58,60,62, 63, 64, 65, 66, 67 21 reported mental health outcomes (e.g., stress and depression),25,26,39,41,44,46, 47, 48, 49, 50, 51, 52,55,56,58,59,62, 63, 64, 65,69 and 9 reported biomarker-based outcomes (e.g., blood pressure and lipids).24,25,48,52,53,61,64,66,67
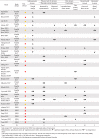
The effect direction plot (Table 3) presents the most frequently reported measures from five outcome domains and their direction of effects. Weight change, the most frequent measure from the anthropometry domain, appeared in 15 studies,24,25,42,43,45,46,48,50, 51, 52, 53,61,64,67,68 with 60% showing significant weight loss favouring the interventions.24,25,42,43,48,61,64,67,68 PA intensity was assessed by 14 studies, using either questionnaires39,42,46,49,51,52,60,62, 63, 64, 65 and/or devices.25,48,52,67 Four studies found significant increases in PA intensity,25,48,63,64 two of which were measured by devices.25,48 For dietary outcomes, 13 studies examined specific food intakes, including vegetable/fruit/snack intake46,48,58,60,64,65,74 and/or diet quality scores.48, 49, 50,52,62,66 Half of the studies reported significant beneficial effects in food intakes,46,48,64,65 while diet quality scores improved only in two studies.49,62 In the mental health domain, perceived stress level was the most common outcome measure (n = 10)39,46,48,51,52,55,56,59,64; four studies reported significant reductions after interventions.26,51,55,64 Lastly, blood lipids were most often measured in the biomarker domain (n = 5),24,48,53,67,74 with two observing significant improvements.24,48
Table 3.
Effect direction plot summarising the direction of intervention impacts in holistic mHealth interventions. The most frequent measures of five outcome domains are shown.
 |
Meta-analysis results
Fourteen RCTs reported results in suitable formats for inclusion in the meta-analysis.24,42,43,45,46,48, 49, 50,52,56,64,66,67,74 Of these studies, 11 studies reported weight change, including a total of 1106 participants (578 intervention, 528 control). Participation in holistic mHealth interventions led to a mean weight loss of 1.70 kg (95% CI −2.45 to −0.95) (Fig. 2). The heterogeneity was substantial, with an I2 of 89%. We did not detect the publication bias based on the Eggers’ test (p = 0.95), and therefore the trim and fill method was not applied. Six studies including 480 individuals (268 intervention, 212 control) showed significant reductions in perceived stress levels (Fig. 3), with small heterogeneity (SMD −0.32; 95% CI −0.52 to −0.12; I2 = 14.52%). The overall effect size of diet quality scores was pooled from five studies, comprising 428 individuals (209 intervention, 219 control) (Fig. 4). Overall, there was no significant effect on diet quality scores with a SMD of 0.21 (95% CI −0.15 to 0.56). Fig. 5 shows a similar result (SMD 0.21; 95% CI −0.25 to 0.67) for self-reported MVPA in a meta-analysis of 5 RCTs involving 330 participants (188 intervention, 142 control).
Fig. 2.
Forest plot of holistic mHealth interventions on weight change (kg). The figure presents a forest plot depicting the effects of eleven holistic mHealth interventions on weight change, measured in mean difference (kg). Participation in holistic mHealth intervention led to a statistically significant weight loss of 1.70 kg (95% CI −2.45 to −0.95). The heterogeneity was substantial with an I2 of 89%. The error bars in the plot signify the 95% confidence interval, providing insights into the range within which the true effect size is expected to lie. Each square within the plot corresponds to the mean difference in weight change observed across different studies. The size of the squares corresponds to the relative weight of each study in the analysis, with smaller squares indicating studies that contribute less significantly to the overall effect size. The hollow diamond represents the overall effect size, accounting for all the studies included in the analysis. The red reference line denotes the absence of an effect. If the error bar or the hollow diamond intersects or crosses the red reference line, it suggests that the observed effect is not statistically significant.
Fig. 3.
Forest plot of holistic mHealth interventions on perceived stress levels. The figure presents a forest plot depicting the effects of six mHealth interventions on perceived stress levels, measured in standardised mean difference (SMD) as Hedges’ g. The interventions led to a statistically significant stress reduction, with small heterogeneity (−0.32; 95% CI −0.52 to −0.12; I2 = 14.52%). The error bars in the plot signify the 95% confidence interval, providing insights into the range within which the true effect size is expected to lie. Each square within the plot corresponds to the SMD in perceived stress levels observed across different studies. The size of the squares corresponds to the relative weight of each study in the analysis, with smaller squares indicating studies that contribute less significantly to the overall effect size. The hollow diamond represents the overall effect size, accounting for all the studies included in the analysis. The red reference line denotes the absence of an effect. If the error bar or the hollow diamond intersects or crosses the red reference line, it suggests that the observed effect is not statistically significant.
Fig. 4.
Forest plot of holistic mHealth interventions on diet quality scores. The figure presents a forest plot depicting the effects of five mHealth interventions on diet quality scores, measured in standardised mean difference (SMD) as Hedges’ g. There was no statistically significant effect on diet quality scores with an overall effect size of 0.21 (95% CI −0.15 to 0.56) and substantial heterogeneity (I2 = 62.27%). The error bars in the plot signify the 95% confidence interval, providing insights into the range within which the true effect size is expected to lie. Each square within the plot corresponds to the SMD in diet quality scores observed across different studies. The size of the squares corresponds to the relative weight of each study in the analysis, with smaller squares indicating studies that contribute less significantly to the overall effect size. The hollow diamond represents the overall effect size, accounting for all the studies included in the analysis. The red reference line denotes the absence of an effect. If the error bar or the hollow diamond intersects or crosses the red reference line, it suggests that the observed effect is not statistically significant.
Fig. 5.
Forest plot of holistic mHealth interventions on self-reported moderate-to-vigorous physical activity (MVPA). The figure presents a forest plot depicting the effects of five mHealth interventions on MVPA, measured in standardised mean difference (SMD) as Hedges’ g. The analysis of self-reported MVPA revealed a non-significant overall effect size (0.21; 95% CI −0.25 to 0.67). Substantial heterogeneity was observed, as indicated by an I2 value of 74.28%. The error bars in the plot signify the 95% confidence interval, providing insights into the range within which the true effect size is expected to lie. Each square within the plot corresponds to the SMD in self-reported MVPA observed across different studies. The size of the squares corresponds to the relative weight of each study in the analysis, with smaller squares indicating studies that contribute less significantly to the overall effect size. The hollow diamond represents the overall effect size, accounting for all the studies included in the analysis. The red reference line denotes the absence of an effect. If the error bar or the hollow diamond intersects or crosses the red reference line, it suggests that the observed effect is not statistically significant.
Subgroup analysis
Studies using wait-list or standard care control groups (9 RCTs; MD −2.00 kg; 95%CI −2.01, −1.99, I2 = 0%) demonstrated greater weight loss as compared to studies using sham controls (2 RCTs; MD 0.86 kg; 95% CI −0.94 to 2.67; I2 = 32.77%). Also, a stronger effect on diet quality scores was observed for interventions targeting adults from the general population (3 RCTs; SMD 0.51; 95% CI 0.18 to 0.85; I2 = 0%) compared to those targeting adults with overweight or obesity (2 RCTs; SMD 0.10; 95% CI −0.36 to 0.16; I2 = 4.54%).
Risk of bias
Figs. 6 and 7 summarise the risk of bias assessments for RCTs and NRSIs, respectively. Information for individual studies is provided in the supplementary document (Supplementary Figs. S1 and S2).
Fig. 6.
Risk-of-bias assessment summary for randomised controlled trials. The figure presents the risk-of-bias assessment summary for 25 randomised controlled trials (RCTs). The green colour indicates a low risk of bias, suggesting that the study is less prone to bias. The yellow colour signifies some concerns, indicating that certain aspects of the study design or implementation may introduce potential biases. The red colour represents a high risk of bias, implying significant methodological limitations that may compromise the validity of the study findings. The figure also includes the percentage of studies associated with each bias domain, providing an understanding of the prevalence and distribution within in included RCTs.
Fig. 7.
Risk-of-bias assessment summary for non-randomised studies of interventions. The figure presents the risk-of-bias assessment summary for 9 non-randomised studies of interventions (NRSIs). The green colour indicates a low risk of bias, suggesting that the study is less susceptible to bias. The yellow colour signifies a moderate risk of bias, indicating that certain aspects of the study design or conduct may introduce potential biases. The red colour represents a serious risk of bias, implying significant methodological limitations that may compromise the validity of the study findings. The blue colour represents a lack of information on bias, indicating that relevant details were not reported or available. The figure also includes the percentage of studies associated with each bias domain, providing an understanding of the prevalence and distribution within in included NRSIs.
Overall, none of the RCTs were rated as having a low risk of bias; 14 RCTs had some concerns24,25,42,43,45,47,48,50,52,53,61,64,67,69; and 11 were rated as having a high risk of bias (Fig. 6).39,44,46,49,51,54,56,60,65,66,68 Most bias resulted from insufficient reporting of details related to the randomisation process (allocation sequence concealment) and selection of the reported result. Additionally, bias often occurred due to deviations from intended interventions (unblinding of participants) and in measurement of outcomes (unblinding of study assessors). The risk of bias for all NRSIs was serious (Fig. 7).26,40,41,55,57, 58, 59,62,63 All included NRSIs were pre-post study designs without control groups, significantly contributing to bias due to confounding, bias in outcome measurement, and in selection of reported outcomes.
Discussion
This is the first systematic review and meta-analysis to synthesise the evidence from 34 studies and provide an overview of the characteristics of holistic mHealth interventions and assess their effectiveness on related behavioural and health outcomes among adults. It is unique in its focus on interventions that address PA, diet, and mental health in an interconnected and holistic manner. Identified interventions primarily involved young and middle-aged participants from high-income countries; applied various delivery modes included apps, SMS, mobile device-compatible websites, and wearables; and covered diverse intervention components and outcomes measures. The narrative syntheses showed that in about half of the studies, a significant intervention effect was found for weight loss. However, other investigated outcomes showed improvement in only a minority of studies. Our meta-analysis results demonstrated that holistic mHealth interventions could be beneficial for weight loss and stress reduction, while non-significant intervention effects were observed for diet quality scores and self-reported MVPA.
In previous reviews, mHealth interventions have been associated with significant weight loss, ranging from 1 kg to 2 kg, which aligns with our findings.75, 76, 77, 78, 79 Similarly, our results on perceived stress levels are consistent with other studies on mHealth mental health interventions, though they tended to show slightly stronger effect sizes, ranging from 0.35 to 0.46.80, 81, 82 Results on MVPA, on the other hand, were inconclusive.19,76,79,83, 84, 85 Of the three reviews specifically reporting MVPA outcomes, two reported moderate effects, while the other reported non-significant results that are similar to ours.19,79,85 Limited evidence was found regarding meta-analyses of mHealth interventions’ impacts on diet quality scores. Scarry et al. reported that two out of five mHealth interventions showed significantly improved diet quality scores,86 whereas in our review, only one out of five studies reported a significant effect. We also observed a non-significant, small-pooled effect of holistic mHealth interventions on diet quality scores in the meta-analysis.
Taken together, our findings appeared to demonstrate less substantial effects on perceived stress levels, self-reported MVPA, and diet quality scores compared to prior reviews. One possible explanation is that each individual intervention component may be less intensive in a holistic intervention compared to interventions focused only on one aspect of health. Nevertheless, greater effects on more distal and multifactorial health outcomes, such as weight change, may still emerge as a result of synergistic effects of various components. It has also been reported that mHealth interventions were less effective in changing behaviours compared to their impacts on anthropometric or biomarker-based outcomes.87 This may be attributable to the short duration of interventions and the challenges of evaluating health behaviours.87 For example, over 90% of included studies evaluated an intervention that was less than 6 months in duration. The lack of long-term effectiveness appears to be one of the most significant limitations of current mHealth interventions.77,82,83 This could be related to the challenge of maintaining participants’ long-term engagement with mHealth interventions, which is important given that higher engagement has been associated with better outcomes.88 Unlike traditional face-to-face interventions, participants engage with mHealth interventions independently, without oversight, they may need intrinsic motivation to sustain their interest.89 In our review, we found studies reported low engagement and a decline in engagement over time. These findings echo previous research describing transient and casual use of apps, and suggest that people may value such interventions with minimal effort.90 Future studies should focus on strategies to boost engagement and sustain the long-term effects of interventions.
Further, evaluating behaviours can be challenging, particularly when considering the various methods for measuring PA and diet. For example, diet can be assessed as frequency of intake, quality, pattern, or certain dietary behaviours. Similarly, PA can be evaluated as frequency, intensity, and duration, using self-reported or devices-based measures. In our meta-analysis, we aimed for data comparability by only combining studies that used similar measurements, such as diet quality scores and self-reported MVPA. Some previous reviews of mHealth interventions combined different PA outcomes, including both self-reported and device-based measures, which is likely to increase the heterogeneity in results.76,83,84 Our effect direction plot (Table 3) reveals that this choice may also affect the significance of outcomes: 2 out of 4 studies using device-based measurements reported significant improvements in PA, while only 2 out of 11 studies replying on self-reported measures showed significant effects. Given these observations, it is plausible that device-based measurements might offer more favourable evaluations of intervention efficacy. To enhance the robustness and comparability of future studies, researchers should consider adopting either device-based measurements or standardised reporting of behavioural outcomes (e.g., MVPA in minutes per week or identical diet quality scores).
Although some researchers have argued that multi-component interventions yield more robust health effects than standalone mHealth interventions, the optimal choices of delivery modes and components in holistic mHealth interventions to improve efficacy remain uncertain.77,79,89 When designing holistic mHealth interventions, it is important to undertake more careful selection and integration based on specific health domains and target populations.89,91 For example, apps are emerging as a dominant mHealth delivery mode due to their ability to provide multiple and complex functions simultaneously, as well as convenient access to participants.92 Some evidence supports the inclusion of wearables (reducing participant burden), SMS (acting as reminders), and a human support component to increase intervention effectiveness.75,89,91 By examining the features of interventions with significant effects on weight change and self-reported MVPA, we found that almost 80% of studies included a human support component. This is consistent with other reviews that suggest effective technology-driven diabetes prevention interventions featured various forms of human support (online, face-to-face, and phone), and mHealth interventions with human-to-human interactions produced better outcomes.75,93,94 However, the necessity of human support raises concerns about scalability, as mHealth interventions that involve human interactions are likely to be more resource-intensive and challenging to scale.75,94 Future research should investigate whether conversational agents or artificial intelligence can provide comparable support and guidance to participants.
Another important aspect of holistic mHealth interventions is the inclusion of a mental health component. We set broad criteria for this component, resulting in the identification of diverse topics. The top three topics included sleep, stress management, and relaxation. Our meta-analysis included 6 stuides focusing on stress, sleep, and/or mood monitoring. Reviews with larger effect sizes for stress primarily emphasised mindfulness or cognitive behavioural therapy approaches.80, 81, 82 Subgroup analyses confirmed that apps based on cognitive behaviour therapy yielded greater effects compared to those that did not.82 Future research could delve deeper into this area, investigating which specific mental health topics are optimal for health outcomes in holistic mHealth interventions.
This review applied a comprehensive search strategy covering six electronic databases, Google Scholar, and manual searches of reference lists from relevant papers over the past 10 years. We also supplemented the intervention descriptions with information from protocol papers and registrations during data extraction. This review emphasises the importance of early prevention and the adoption of healthy lifestyles, recognising that healthy ageing is a continuous and lifelong endeavour.
This study also has several limitations. First, the included studies were quite diverse, with variations in intervention components, mHealth delivery modes, control groups, and outcome measures. This made it challenging to directly compare the studies. Second, only 14 studies were included in the meta-analysis, and there was substantial heterogeneity in weight change, self-reported MVPA, and diet quality scores. Subgroup analyses lacked sufficient power to determine true interaction effects. Third, only immediate post-intervention outcomes were included due to inadequate and unsuitable long-term follow-up data. This highlights the need to evaluate long-term effectiveness in future studies. Lastly, all studies included in the meta-analyses were rated as having some concerns or a high risk of bias, limiting the quality of evidence. Lack of blinding was identified as a key source of bias. Implementing blinding techniques, such as sham procedures, concealing the study hypothesis from participants, or conducting a blinded assessment of outcomes, will improve the quality of evidence.95
Our review highlights the rapid accumulation of evidence in recent years and provides directions for future research on holistic mHealth interventions. First, it is worth noting that the majority of included participants were from high-income countries. Future studies should focus on bridging the evidence gap by including populations with lower socioeconomic status, residing in remote areas or developing countries, who often have limited knowledge of and access to preventive care. Second, given the complexity of holistic mHealth interventions, further research should investigate the intervention components or features that influence intervention efficacy. This may involve examining the optimal dose, frequency, timing, and combination of different delivery modes and components; comparing holistic mHealth interventions to lifestyle (PA and diet) interventions; and exploring the possible role of conversational agents and artificial intelligence to supplement human support and enhance scalability. Lastly, future studies should aim to address the methodological limitations of the current evidence, such as the lack of long-term effectiveness and the need for high-quality evidence.
In summary, this systematic review and meta-analysis synthesised the characteristics of holistic mHealth interventions and suggested that these interventions had beneficial effects on weight loss and perceived stress reduction. The effects on diet quality scores and self-reported MVPA were less clear and require more research, preferably using device-measured assessments. These findings should also be interpreted with caution, however, due to the small number of studies included, substantial heterogeneity, and low methodological quality. While the existing evidence needs to be strengthened, this review provides valuable insights into the characteristics and effectiveness of holistic mHealth interventions in adults from the general population. It highlights the potential for such interventions to improve physical and mental health outcomes and lays the groundwork for future research on the development and scaling of effective holistic mHealth interventions.
Contributors
SZ and FM-R conceived the original research concept for the study. The refinement of this conceptualization was a collaborative effort, with substantial contributions from SME, JLM, OC, AS-S, and TK, ultimately leading to the publication of the protocol. SZ led the development of comprehensive search terms for each relevant database, with essential support from FM-R, SME, and a librarian from the National University of Singapore. SZ and CHG screened titles and abstracts independently, and SME was the third reviewer when there was disagreement. SZ and SME screened the full texts and assessed the risk of bias for the included studies. SZ extracted all the data, with CHG verifying the accuracy and consistency of the data. The meta-analysis was advised by RMvD and performed by SZ under the expert guidance of BCT and FM-R. SZ wrote the first draft. All authors edited and reviewed the final manuscript. SZ, CHG, and SME have accessed and verified the data. SZ and FM-R were responsible for the decision to submit the manuscript for publication.
Data sharing statement
All data used for the study has been included in the manuscript and Supplementary materials.
Declaration of interests
TK is affiliated with the Centre for Digital Health Interventions (CDHI), a joint initiative of the Institute for Implementation Science in Health Care, University of Zürich, the Department of Management, Technology, and Economics at ETH Zürich, and the Institute of Technology Management and School of Medicine at the University of St. Gallen. CDHI is funded in part by CSS, a Swiss health insurer. He is also a cofounder of Pathmate Technologies, a university spin-off company that creates and delivers digital clinical pathways. However, neither CSS nor Pathmate Technologies was involved in this research.
Acknowledgements
This work is conducted as part of the Future Health Technologies programme, which was established collaboratively between ETH Zürich and the National Research Foundation Singapore. It is supported by the National Research Foundation, Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) programme. It is also supported by the PANDA (Physical Activity and Nutrition Determinants in Asia) Research Programme at the Saw Swee Hock School of Public Health, National University of Singapore.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102309.
Appendix ASupplementary data
References
- 1.United Nations. Department of Economic and Social Affairs World population prospects 2022: summary of results. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf
- 2.United Nations. Department of Economic and Social Affairs. Population Division World population ageing, 2019. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report.pdf
- 3.Marcussen L., Marinus J.D. The use of mHealth solutions in active and healthy ageing promotion: an explorative scoping review. J Ideas Health. 2021;4(1):307–320. doi: 10.47108/jidhealth.Vol4.Iss1.92. [DOI] [Google Scholar]
- 4.O’Neil A., Jacka F.N., Quirk S.E., et al. A shared framework for the common mental disorders and non-communicable disease: key considerations for disease prevention and control. BMC Psychiatr. 2015;15(1):15. doi: 10.1186/s12888-015-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngo V.K., Rubinstein A., Ganju V., et al. Grand challenges: integrating mental health care into the non-communicable disease Agenda. PLoS Med. 2013;10(5) doi: 10.1371/JOURNAL.PMED.1001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pryor L., Da Silva M.A., Melchior M. Mental health and global strategies to reduce NCDs and premature mortality. Lancet Public Health. 2017;2(8):e350–e351. doi: 10.1016/S2468-2667(17)30140-8. [DOI] [PubMed] [Google Scholar]
- 7.Kong D., Fu J., Hong Y., Liu S., Luo Y. Link to external site this link will open in a new window. The application and prospect of mobile health (mHealth) in health service for older people living alone in community: a narrative review. Iran J Public Health. 2022;51(4):724–732. doi: 10.18502/ijph.v51i4.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . World Health Organization; 2015. World report on ageing and health.https://apps.who.int/iris/handle/10665/186463 [Google Scholar]
- 9.Nimrod G., Ben-Shem I. Successful aging as a lifelong process. Educ Gerontol. 2015;41(11):814–824. doi: 10.1080/03601277.2015.1050904. [DOI] [Google Scholar]
- 10.Changizi M., Kaveh M.H. Effectiveness of the mHealth technology in improvement of healthy behaviors in an elderly population—a systematic review. mHealth. 2017;3:51. doi: 10.21037/mhealth.2017.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mensah G.A., Collins P.Y. Understanding mental health for the prevention and control of cardiovascular diseases. Glob Heart. 2015;10(3):221. doi: 10.1016/J.GHEART.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein D.J., Benjet C., Gureje O., et al. Integrating mental health with other non-communicable diseases. BMJ. 2019;364:l295. doi: 10.1136/bmj.l295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox T.L., Krukowski R., Love S.J., et al. Stress management–augmented behavioral weight loss intervention for african American women: a pilot, randomized controlled trial. Health Educ Behav. 2013;40(1):78–87. doi: 10.1177/1090198112439411. [DOI] [PubMed] [Google Scholar]
- 14.Mason A.E., Epel E.S., Aschbacher K., et al. Reduced reward-driven eating accounts for the impact of a mindfulness-based diet and exercise intervention on weight loss: data from the SHINE randomized controlled trial. Appetite. 2016;100:86–93. doi: 10.1016/j.appet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K., Varma D.S., Prosperi M. A systematic review of the effectiveness of mobile apps for monitoring and management of mental health symptoms or disorders. J Psychiatr Res. 2018;107:73–78. doi: 10.1016/j.jpsychires.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Faiola A., Papautsky E.L., Isola M. Empowering the aging with mobile health: a mHealth framework for supporting sustainable healthy lifestyle behavior. Curr Probl Cardiol. 2019;44(8):232–266. doi: 10.1016/j.cpcardiol.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Duan Y., Shang B., Liang W., Du G., Yang M., Rhodes R.E. Effects of eHealth-based multiple health behavior change interventions on physical activity, healthy diet, and weight in people with noncommunicable diseases: systematic review and meta-analysis. J Med Internet Res. 2021;23(2) doi: 10.2196/23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Global Observatory for eHealth mHealth: new horizons for health through mobile technologies: second global survey on eHealth. 2011. https://apps.who.int/iris/handle/10665/44607 Published online.
- 19.Mönninghoff A., Kramer J.N., Hess A.J., et al. Long-term effectiveness of mHealth physical activity interventions: systematic review and meta-analysis of randomized controlled trials. J Med Internet Res. 2021;23(4) doi: 10.2196/26699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ang G., Edney S.M., Tan C.S., et al. Physical activity trends among adults in a national mHealth program: a population-based cohort study of 411,528 adults. Am J Epidemiol. 2022;192(3):397–407. doi: 10.1093/aje/kwac193. Published online November 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganesan A.N., Louise J., Horsfall M., et al. International mobile-health intervention on physical activity, sitting, and weight: the stepathlon cardiovascular health study. J Am Coll Cardiol. 2016;67(21):2453–2463. doi: 10.1016/j.jacc.2016.03.472. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell M., White L., Lau E., Leahey T., Adams M.A., Faulkner G. Evaluating the carrot rewards app, a population-level incentive-based intervention promoting step counts across two Canadian provinces: quasi-experimental study. JMIR mHealth uHealth. 2018;6(9) doi: 10.2196/mhealth.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caroppo E., Mazza M., Sannella A., et al. Will nothing Be the same again?: changes in lifestyle during COVID-19 pandemic and consequences on mental health. Int J Environ Res Public Health. 2021;18(16):8433. doi: 10.3390/ijerph18168433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block G., Azar K.M., Romanelli R.J., et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17(10) doi: 10.2196/jmir.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drew R.J., Morgan P.J., Collins C.E., et al. Behavioral and cognitive outcomes of an online weight loss program for men with low mood: a randomized controlled trial. Ann Behav Med. 2021;56(10):1026–1041. doi: 10.1093/abm/kaab109. Published online December 29. [DOI] [PubMed] [Google Scholar]
- 26.Baek J.H., Kim J.H., Oh S., Kim J.Y., Baik S. Smart stress care: usability, feasibility and preliminary efficacy of fully automated stress management application for employees. Psychiatry Investig. 2018;15(10):991–999. doi: 10.30773/pi.2018.08.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joiner K.L., Nam S., Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: a systematic review and meta- analysis. Prev Med. 2017;100:194. doi: 10.1016/J.YPMED.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kankanhalli A., Saxena M., Wadhwa B. Combined interventions for physical activity, sleep, and diet using smartphone apps: a scoping literature review. Int J Med Inform. 2019;123:54–67. doi: 10.1016/J.IJMEDINF.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. Published online March 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng S., Edney S.M., Mair J.L., et al. Holistic mHealth interventions for the promotion of healthy ageing: protocol for a systematic review. BMJ Open. 2023;13(5) doi: 10.1136/bmjopen-2022-066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.International Telecommunication Union The world in 2010: ICT facts and figures. 2010. http://www.itu.int/ITU-D/ict/material/FactsFigures2010.pdf Published online.
- 32.Istepanian R.S.H. Mobile health (m-Health) in retrospect: the known unknowns. Int J Environ Res Public Health. 2022;19(7):3747. doi: 10.3390/ijerph19073747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michie S., Richardson M., Johnston M., et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 34.Dugas M., Gao G., Gordon), Agarwal R. Unpacking mHealth interventions: a systematic review of behavior change techniques used in randomized controlled trials assessing mHealth effectiveness. Digit Health. 2020;6 doi: 10.1177/2055207620905411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boon M.H., Thomson H. The effect direction plot revisited: application of the 2019 Cochrane Handbook guidance on alternative synthesis methods. Res Synth Methods. 2021;12(1):29–33. doi: 10.1002/jrsm.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins J.P.T., Li T., Deeks J.J., editors. Chapter 6: Choosing effect measures and computing estimates of effect. Cochrane handbook for systematic reviews of interventions version 6.4 (updated August 2023) Cochrane; 2023. https://training.cochrane.org/handbook/current/chapter-06 [Google Scholar]
- 37.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/BMJ.L4898. [DOI] [PubMed] [Google Scholar]
- 38.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/BMJ.I4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du H., Venkatakrishnan A., Youngblood G.M., Ram A., Pirolli P. A group-based mobile application to increase adherence in exercise and nutrition programs: a factorial design feasibility study. JMIR mHealth uHealth. 2016;4(1):e4. doi: 10.2196/mhealth.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Comulada W.S., Swendeman D., Koussa M.K., et al. Adherence to self-monitoring healthy lifestyle behaviours through mobile phone-based ecological momentary assessments and photographic food records over 6 months in mostly ethnic minority mothers. Public Health Nutr. 2018;21(4):679–688. doi: 10.1017/S1368980017003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torres E.N., Zhang T. The impact of wearable devices on employee wellness programs: a study of hotel industry workers. Int J Hosp Manag. 2021;93 doi: 10.1016/j.ijhm.2020.102769. [DOI] [Google Scholar]
- 42.Napolitano M.A., Hayes S., Bennett G.G., Ives A.K., Foster G.D. Using facebook and text messaging to deliver a weight loss program to college students: using facebook and text messaging. Obesity. 2013;21(1):25–31. doi: 10.1002/oby.20232. [DOI] [PubMed] [Google Scholar]
- 43.Lin M., Mahmooth Z., Dedhia N., et al. Tailored, interactive text messages for enhancing weight loss among african American adults: the TRIMM randomized controlled trial. Am J Med. 2015;128(8):896–904. doi: 10.1016/j.amjmed.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Stahl S.T., Smagula S.F., Dew M.A., Schulz R., Albert S.M., Reynolds C.F. Digital monitoring of sleep, meals, and physical activity for reducing depression in older spousally-bereaved adults: a pilot randomized controlled trial. Am J Geriatr Psychiatr. 2020;28(10):1102–1106. doi: 10.1016/j.jagp.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nezami B.T., Hurley L., Power J., Valle C.G., Tate D.F. A pilot randomized trial of simplified versus standard calorie dietary self-monitoring in a mobile weight loss intervention. Obesity. 2022;30(3):628–638. doi: 10.1002/oby.23377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Napolitano M.A., Harrington C.B., Patchen L., et al. Feasibility of a digital intervention to promote healthy weight management among postpartum african American/black women. IJERPH. 2021;18(4):2178. doi: 10.3390/ijerph18042178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiwal A., Fauth E.B., Wengreen H., Norton M. The gray matters app targeting health behaviors associated with alzheimer’s risk: improvements in intrinsic motivation and impact on diet quality and physical activity. J Nutr Health Aging. 2020;24(8):893–899. doi: 10.1007/s12603-020-1421-5. [DOI] [PubMed] [Google Scholar]
- 48.Ashton L.M., Morgan P.J., Hutchesson M.J., Rollo M.E., Collins C.E. Feasibility and preliminary efficacy of the ‘HEYMAN’ healthy lifestyle program for young men: a pilot randomised controlled trial. Nutr J. 2017;16(1):2. doi: 10.1186/s12937-017-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oftedal S., Burrows T., Fenton S., Murawski B., Rayward A.B., Duncan M.J. Feasibility and preliminary efficacy of an m-health intervention targeting physical activity, diet, and sleep quality in shift-workers. IJERPH. 2019;16(20):3810. doi: 10.3390/ijerph16203810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brindal E., Hendrie G.A., Freyne J., Noakes M. A mobile phone app designed to support weight loss maintenance and well-being (MotiMate): randomized controlled trial. JMIR mHealth uHealth. 2019;7(9) doi: 10.2196/12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyzwinski L.N., Caffery L., Bambling M., Edirippulige S. The mindfulness app trial for weight, weight-related behaviors, and stress in university students: randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(4) doi: 10.2196/12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duncan M., Fenton S., Brown W., et al. Efficacy of a multi-component m-health weight-loss intervention in overweight and obese adults: a randomised controlled trial. IJERPH. 2020;17(17):6200. doi: 10.3390/ijerph17176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puntpanich S., Taneepanichskul S. Effect of M-health application: “chicken LOF” (low fat in 90 Days) on lipid profile and body composition among dyslipidemia healthcare workers: a randomized controlled trial. TOPHJ. 2020;14(1):341–349. doi: 10.2174/1874944502013010341. [DOI] [Google Scholar]
- 54.Didehban S., Dehdari T., Janani L., Masoudkabir F. Employees’ behaviors concerning metabolic syndrome prevention: a cellphone-based text message education intervention. J Tehran Heart Cent. 2022;16(4):162–168. doi: 10.18502/jthc.v16i4.8602. Published online February 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahtinen A., Mattila E., Välkkynen P., et al. Mobile mental wellness training for stress management: feasibility and design implications based on a one-month field study. JMIR Mhealth Uhealth. 2013;1(2):e11. doi: 10.2196/mhealth.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H., Jiang Y., Nguyen H.D., Poo D.C.C., Wang W. The effect of a smartphone-based coronary heart disease prevention (SBCHDP) programme on awareness and knowledge of CHD, stress, and cardiac-related lifestyle behaviours among the working population in Singapore: a pilot randomised controlled trial. Health Qual Life Outcomes. 2017;15(1):49. doi: 10.1186/s12955-017-0623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Podina I.R., Fodor L.A., Cosmoiu A., Boian R. An evidence-based gamified mHealth intervention for overweight young adults with maladaptive eating habits: study protocol for a randomized controlled trial. Trials. 2017;18(1):592. doi: 10.1186/s13063-017-2340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glenn J., Madero E.N., Gray M., et al. Engagement with a digital platform for multimodal cognitive assessment and multidomain intervention in a Japanese population: pilot, quasi-experimental, longitudinal study. JMIR mHealth uHealth. 2019;7(10) doi: 10.2196/15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang Y., Jiao N., Nguyen H.D., et al. Effect of a mHealth programme on coronary heart disease prevention among working population in Singapore: a single group pretest–post-test design. J Adv Nurs. 2019;75(9):1922–1932. doi: 10.1111/jan.13980. [DOI] [PubMed] [Google Scholar]
- 60.Ni Mhurchu C., Te Morenga L., Tupai-Firestone R., et al. A co-designed mHealth programme to support healthy lifestyles in Māori and Pasifika peoples in New Zealand (OL@-OR@): a cluster-randomised controlled trial. Lancet Digit Health. 2019;1(6):e298–e307. doi: 10.1016/S2589-7500(19)30130-X. [DOI] [PubMed] [Google Scholar]
- 61.Bonn S., Licitra G., Bellocco R., Trolle Lagerros Y. Clinical outcomes among working adults using the health integrator smartphone app: analyses of prespecified secondary outcomes in a randomized controlled trial. J Med Internet Res. 2022;24(3) doi: 10.2196/24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cantisano L.M., Gonzalez-Soltero R., Blanco-Fernández A., Belando-Pedreño N. ePSICONUT: an e-health programme to improve emotional health and lifestyle in university students. Int J Environ Res Public Health. 2022;19(15):9253. doi: 10.3390/ijerph19159253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan N.P., Brooks A.J., Burke M.K., et al. My wellness coach: evaluation of a mobile app designed to promote integrative health among underserved populations. Transl Behav Med. 2022;12(6):752–760. doi: 10.1093/tbm/ibac015. [DOI] [PubMed] [Google Scholar]
- 64.Wilson D., Driller M.W., Johnston B., Gill N.D. A contactless app-based intervention to improve health behaviors in airline pilots: a randomized trial. Am J Prev Med. 2023;64(5):666–676. doi: 10.1016/j.amepre.2022.12.011. [DOI] [PubMed] [Google Scholar]
- 65.van Drongelen A., Boot C.R., Hlobil H., Twisk J.W., Smid T., van der Beek A.J. Evaluation of an mHealth intervention aiming to improve health-related behavior and sleep and reduce fatigue among airline pilots. Scand J Work Environ Health. 2014;40(6):557–568. doi: 10.5271/sjweh.3447. [DOI] [PubMed] [Google Scholar]
- 66.Järvelä-Reijonen E., Karhunen L., Sairanen E., et al. The effects of acceptance and commitment therapy on eating behavior and diet delivered through face-to-face contact and a mobile app: a randomized controlled trial. Int J Behav Nutr Phys Act. 2018;15(1):22. doi: 10.1186/s12966-018-0654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakata Y., Sasai H., Gosho M., et al. A smartphone healthcare application, CALO mama plus, to promote weight loss: a randomized controlled trial. Nutrients. 2022;14(21):4608. doi: 10.3390/nu14214608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thorgeirsson T., Torfadottir J.E., Egilsson E., et al. Randomized trial for weight loss using a digital therapeutic application. J Diabetes Sci Technol. 2022;16(5):1150–1158. doi: 10.1177/19322968211000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jensen C.D., Duraccio K.M., Barnett K.A., et al. A randomized pilot trial of a text messaging intervention for sleep improvement and weight control in emerging adults. Clin Pract Pediatr Psychol. 2023 doi: 10.1037/cpp0000477. Published online April 6. [DOI] [Google Scholar]
- 70.Norton M.C., Clark C.J., Tschanz J.T., et al. The design and progress of a multidomain lifestyle intervention to improve brain health in middle-aged persons to reduce later Alzheimer’s disease risk: the Gray Matters randomized trial. Alzheimers Dement (N Y) 2015;1(1):53–62. doi: 10.1016/j.trci.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Drongelen A., Boot C.R., Hlobil H., Smid T., van der Beek A.J. Process evaluation of a tailored mobile health intervention aiming to reduce fatigue in airline pilots. BMC Public Health. 2016;16:894. doi: 10.1186/s12889-016-3572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartin P.J., Nugent C.D., McClean S.I., et al. The empowering role of mobile apps in behavior change interventions: the gray matters randomized controlled trial. JMIR mHealth uHealth. 2016;4(3):e93. doi: 10.2196/mhealth.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mattila E., Lappalainen R., Välkkynen P., et al. Usage and dose response of a mobile acceptance and commitment therapy app: secondary analysis of the intervention arm of a randomized controlled trial. JMIR mHealth uHealth. 2016;4(3) doi: 10.2196/mhealth.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young M.D., Drew R.J., Kay-Lambkin F., et al. Impact of a self-guided, eHealth program targeting weight loss and depression in men: a randomized trial. J Consult Clin Psychol. 2021;89(8):682–694. doi: 10.1037/ccp0000671. [DOI] [PubMed] [Google Scholar]
- 75.Antoun J., Itani H., Alarab N., Elsehmawy A. The effectiveness of combining nonmobile interventions with the use of smartphone apps with various features for weight loss: systematic review and meta-analysis. JMIR mHealth uHealth. 2022;10(4) doi: 10.2196/35479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mateo G.F., Granado-Font E., Ferré-Grau C., Montaña-Carreras X. Mobile phone apps to promote weight loss and increase physical activity: a systematic review and meta-analysis. J Med Internet Res. 2015;17(11) doi: 10.2196/jmir.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Islam M.M., Poly T.N., Walther B.A., Jack Li Y.C. Use of mobile phone app interventions to promote weight loss: meta-analysis. JMIR mHealth uHealth. 2020;8(7) doi: 10.2196/17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu F., Kong X., Cao J., et al. Mobile phone intervention and weight loss among overweight and obese adults: a meta-analysis of randomized controlled trials. Am J Epidemiol. 2015;181(5):337–348. doi: 10.1093/aje/kwu260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferguson T., Olds T., Curtis R., et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. 2022;4(8):e615–e626. doi: 10.1016/S2589-7500(22)00111-X. [DOI] [PubMed] [Google Scholar]
- 80.Gál É., Ștefan S., Cristea I.A. The efficacy of mindfulness meditation apps in enhancing users’ well-being and mental health related outcomes: a meta-analysis of randomized controlled trials. J Affect Disord. 2021;279:131–142. doi: 10.1016/j.jad.2020.09.134. [DOI] [PubMed] [Google Scholar]
- 81.Eisenstadt M., Liverpool S., Infanti E., Ciuvat R.M., Carlsson C. Mobile apps that promote emotion regulation, positive mental health, and well-being in the general population: systematic review and meta-analysis. JMIR Ment Health. 2021;8(11) doi: 10.2196/31170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Linardon J., Cuijpers P., Carlbring P., Messer M., Fuller-Tyszkiewicz M. The efficacy of app-supported smartphone interventions for mental health problems: a meta-analysis of randomized controlled trials. World Psychiatr. 2019;18(3):325–336. doi: 10.1002/wps.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim H.N., Seo K. Smartphone-based health program for improving physical activity and tackling obesity for young adults: a systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17(1):15. doi: 10.3390/ijerph17010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang M.S.S., Moore K., McGavigan A., Clark R.A., Ganesan A.N. Effectiveness of wearable trackers on physical activity in healthy adults: systematic review and meta-analysis of randomized controlled trials. JMIR mHealth uHealth. 2020;8(7) doi: 10.2196/15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Direito A., Carraça E., Rawstorn J., Whittaker R., Maddison R. mHealth technologies to influence physical activity and sedentary behaviors: behavior change techniques, systematic review and meta-analysis of randomized controlled trials. Ann Behav Med. 2017;51(2):226–239. doi: 10.1007/s12160-016-9846-0. [DOI] [PubMed] [Google Scholar]
- 86.Scarry A., Rice J., O’Connor E.M., Tierney A.C. Usage of mobile applications or mobile health technology to improve diet quality in adults. Nutrients. 2022;14(12):2437. doi: 10.3390/nu14122437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y., Xue H., Huang Y., Huang L., Zhang D. A systematic review of application and effectiveness of mHealth interventions for obesity and diabetes treatment and self-management. Adv Nutr. 2017;8(3):449–462. doi: 10.3945/AN.116.014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karyotaki E., Kleiboer A., Smit F., et al. Predictors of treatment dropout in self-guided web-based interventions for depression: an “individual patient data” meta-analysis. Psychol Med. 2015;45(13):2717–2726. doi: 10.1017/S0033291715000665. [DOI] [PubMed] [Google Scholar]
- 89.Schoeppe S., Alley S., Van Lippevelde W., et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Activ. 2016;13(1):127. doi: 10.1186/s12966-016-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dennison L., Morrison L., Conway G., Yardley L. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res. 2013;15(4):e86. doi: 10.2196/jmir.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qin Y., Wang X (Romy), Namkoong K. A meta-analysis of the overall effect of mHealth physical activity interventions for weight loss and the moderating effect of behavioral change theories, techniques, and mobile technologies. Mob Media Commun. 2022;10(2):337–359. doi: 10.1177/20501579211054929. [DOI] [Google Scholar]
- 92.Sahu M., Grover A., Joshi A. Role of mobile phone technology in health education in Asian and African countries: a systematic review. Int J Electron Healthc. 2014;7(4):269–286. doi: 10.1504/IJEH.2014.064327. Published online August 26. Accessed March 6, 2023. [DOI] [PubMed] [Google Scholar]
- 93.Baumeister H., Reichler L., Munzinger M., Lin J. The impact of guidance on Internet-based mental health interventions — a systematic review. Internet Interv. 2014;1(4):205–215. doi: 10.1016/j.invent.2014.08.003. [DOI] [Google Scholar]
- 94.Mair J., Salamanca-Sanabria A., Frese B., Jakob R., Kowatsch T., Haug S. Effective behavior change techniques in digital health interventions targeting non-communicable diseases: an umbrella review. Ann Behav Med. 2023;57(10):817–835. doi: 10.1093/abm/kaad041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boutron I., Guittet L., Estellat C., Moher D., Hróbjartsson A., Ravaud P. Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PLoS Med. 2007;4(2):e61. doi: 10.1371/journal.pmed.0040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









