Summary
Disparities in social determinants of health (SDOH) play a significant role in causing health inequities globally. The physical environment, including housing and workplace environment, can increase the prevalence and spread of fungal infections. A number of professions are associated with increased fungal infection risk and are associated with low pay, which may be linked to crowded and sub-optimal living conditions, exposure to fungal organisms, lack of access to quality health care, and risk for fungal infection. Those involved and displaced from areas of armed conflict have an increased risk of invasive fungal infections. Lastly, a number of fungal plant pathogens already threaten food security, which will become more problematic with global climate change. Taken together, disparities in SDOH are associated with increased risk for contracting fungal infections. More emphasis needs to be placed on systematic approaches to better understand the impact and reducing the health inequities associated with these disparities.
Keywords: Social determinants of health, Fungal infections, Working conditions, Health care access, Structural conflict
Introduction
Despite efforts to improve access to quality health care, health inequities persist globally. Many factors influence disparities in health outcomes, including race, ethnic background, sex, gender identity, and sexual orientation, among others. For example, compared to white Americans, data shows that Black, Indigenous, and people of color (BIPOC) throughout the U.S. experience higher rates of morbidity and mortality from a diverse spectrum of chronic health conditions, including diabetes mellitus, hypertension, heart disease, cancer, obesity, asthma, mental health disorders, and acute conditions, such as infections.1,2 The ongoing coronavirus disease 2019 (COVID-19) pandemic provides a stark illustration of how underlying health inequities can be amplified by superimposed events like pandemics, leading to further increase in disease burden and health disparities.3,4
SDOH refer to non-medical factors that shape individuals' health outcomes and well-being. They encompass a broad range of influences and systems that impact the circumstances of people's everyday lives, including working conditions, income, housing, access to affordable and quality health services, early childhood development, education, job security, food security, social inclusion, and structural conflict.5 In this context, social and economic factors including education, employment, and income have a significant impact on our health behaviors and access to quality health care.6 These factors are further influenced and shaped by biased economic policies, development agendas, social norms, social policies, and political systems.
Globally, significant health inequities exist both within and between countries, contributing to wide gaps in life expectancy. Average life expectancy ranges from 52 years in Sierra Leone and the Central African Republic to 84 years in Japan and Hong Kong—a gap of 32 years.7 Even within a country, there can be striking differences in life expectancy.8 For instance, in Scotland, the average life expectancy for males in the less advantaged parts of Glasgow is 66 years compared to 82 years in more advantaged areas.9 Globally, children from the lowest income households are twice as likely to die by the age of 5 years as children born into the highest income households.9
Invasive fungal infections (IFIs) occur primarily in individuals with underlying immunocompromising conditions such as human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), such as those with hematologic malignancies, solid organ transplant recipients, or those critically ill in the intensive care unit (ICU),10, 11, 12, 13, 14, 15 and are overrepresented in males.16 While genetic and immunologic factors as well as long-term chemotherapy in individuals with cancer may play a role in the increased risk for certain IFIs in some racial and ethnic groups, such as with coccidioidomycosis in individuals with Black African or Filipino descent,2 it is largely believed that risk factors for IFIs are predominantly influenced by SDOH and differences in exposure to them.17 However, this relationship is still not fully understood.
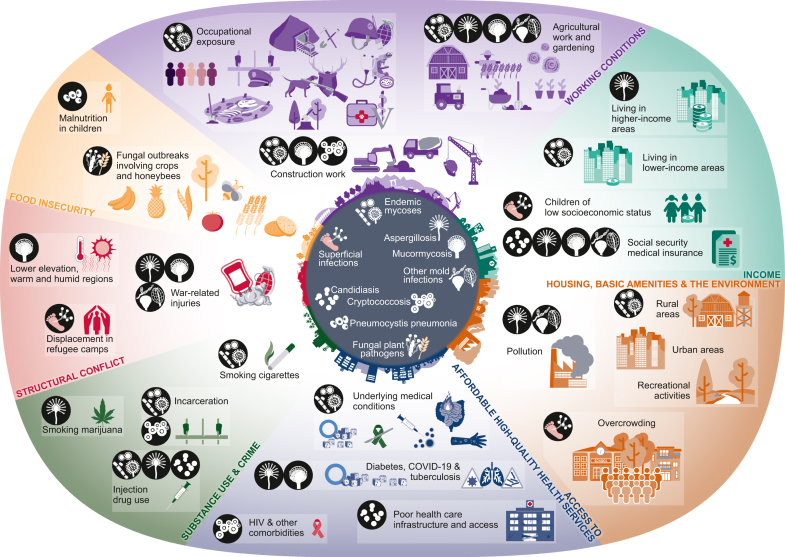
Here, we review the literature examining how SDOH, using examples suggested by the World Health Organization (WHO)5 (Table 1), impact the risk for fungal infections (Fig. 1).
Table 1.
Examples of social determinants of health as defined by the World Health Organization.5
| Examples of social determinants of health |
|---|
| Income and social protection |
| Education |
| Unemployment and Job Insecurity |
| Working conditions |
| Food insecurity |
| Housing, basic amenities, and the environment |
| Substance use, crime, and incarceration |
| Early childhood development |
| Social inclusion and non-discrimination |
| Structural conflict |
| Access to affordable health services of decent quality |
Fig. 1.
Social determinants of health and fungal infections.
Methods
Search strategy and selection criteria
Data for this Review were identified by searches of MEDLINE, PubMed, Google Scholar, and references from relevant articles using the search terms “fungal infections”, “invasive fungal infections”, “superficial fungal infections”, “occupation”, “income”, “housing”, “healthcare access”, “incarceration”, “substance use”, “structural conflict”, and “food insecurity”. We selected relevant case reports, case series, or other studies describing cases or outbreaks of fungal infections that were related to SDOH and published in English. Searches were conducted from database inception until 14 June 2023.
Role of the funding source
This study was unfunded.
Social determinants of health and fungal infections
Working conditions
Occupational factors and associated working conditions represent one of the key SDOH. Occupational exposures that increase the hazard of inhalation of fungal propagules, such as construction and agricultural work, have been shown to increase the risk for mycoses caused by dimorphic fungi in endemic regions of the U.S.18, 19, 20, 21, 22 Multiple studies have found an association between occupational exposure and outbreaks of coccidioidomycosis infection, including work on military bases23,24 or during military training exercises,25,26 employment in prisons,27 excavation at archeological sites,28, 29, 30 construction work,31, 32, 33 agricultural farm work,34 combating wildland fires,18 and work in cotton mills.35
Similarly, outbreaks of occupational histoplasmosis have been linked to work at an agricultural processing plant in Nebraska, U.S.,20 exposure to Histoplasma conidium in an air conditioning unit at a medical school campus,36 and work at a landfill and repair of a bridge in Illinois, U.S.37 In Alberta, Canada, an outbreak of histoplasmosis was associated with renovation of a golf course, during which soil was disturbed.38 A large outbreak associated with aerosol-generating tasks in caves occurred among workers in La Habana province in Cuba39 and among workers in the Dominican Republic who removed large amounts of bat guano from tunnels without proper respiratory protection.40
Occupational exposure to Blastomyces spp. has been reported as well. One study evaluating forestry workers in Minnesota and Wisconsin, U.S., found a high prevalence of previous subclinical infection.21 Other workers in endemic areas who may be at increased risk for blastomycosis include veterinarians,41 pathologists,42 or other laboratory workers.43 In 2023, an outbreak at a Michigan paper mill resulted in more than 100 cases of blastomycosis and at least one death.44 Two cases that occurred in Colorado, U.S., were associated with relocation of infected prairie dogs.45
Paracoccidioides brasiliensis can cause paracoccidioidomycosis, which most commonly occurs in countries in South America and mainly affects young and middle-aged men who work outdoors as farmers, miners, and hunters.46,47 One outbreak of paracoccidioidomycosis involving eight individuals in Rio de Janeiro, Brazil was thought to be due to deforestation and earth removal during construction of a highway.22 Infections due to Talaromyces marneffei, which is endemic to Southeast Asia, have also been associated with soil exposure from agricultural work and farming.48
Although Aspergillus spp. are ubiquitous in the environment and humans inhale at least several hundred Aspergillus conidia daily,49, 50, 51 high-inoculum exposures have been associated with allergic pulmonary manifestations52 and long-term changes to the adaptive immune environment.53 Occupations such as mining that involve excavation, drilling or tunneling pose a significant risk as these activities often expose workers to high concentrations of fungal propagules in the dust generated during these operations.
Occupational exposure to aflatoxin in poultry workers in Portugal has been associated with Aspergillus section Flavi contaminating indoor air in workspaces.54 Moreover, there have been rare cases of invasive lung infections after occupational exposures to Aspergillus, even in immunocompetent persons, such as from agricultural work55 and gardening.56 Cryptococcosis has been associated with outdoor occupations, such as construction and landscaping,57 and mucormycosis with dust exposure in a warehouse,58 construction59 and after farm work accidents.60 Entomophthoramycosis, which is caused by infections from fungi belonging to the order Entomophthorales, can occur following direct inoculation such as during farm work.61
Individuals in certain occupations may be prone to soft tissue fungal infections acquired through direct, traumatic inoculation. Sporotrichosis, caused by Sporothrix spp. has been associated with work in mines,62 forestry and tree nursery work,63, 64, 65 armadillo hunting in Uruguay and Brazil,66,67 and veterinary work.68 Feline sporotrichosis, caused by Sporothrix brasiliensis, is endemic in Brazil and neighboring countries and is a risk for veterinarians, animal caretakers, and even the general public. Infection can occur following a scratch or bite from an infected cat, although even touching an infected cat can result in infection.69 Infections from fungi causing mycetoma and chromoblastomycosis have been linked to farm work, agricultural work, or other outdoor manual labor.47,70 In endemic regions such as Sudan, fungi causing mycetoma (eumycetoma) is often found to disproportionately affect manual workers, including farmers, laborers, and herdsmen.71 Additionally, students in limited resource areas who frequently walk to schools without appropriate footwear or barefoot are also at higher risk.72 Both chromoblastomycosis and mycetoma can lead to significant disfigurement and disability which can cause considerable psychological trauma, stigma, and social isolation.73, 74, 75
Direct inoculation through gardening has been implicated as the cause of subcutaneous mucormycosis76 and mycetoma caused by Acremonium spp.77 Pythiosis, an infection caused by Pythium insidiosum, has been associated with agricultural work.78 A number of fungi causing traumatic keratitis from fungus-contaminated plant material, including Fusarium spp., Aspergillus spp., Chrysosporium spp., and Curvularia spp., has also been related to agricultural work.79,80
In the U.S., work that poses the greatest risk for occupational exposure to fungi employ a high percentage of BIPOC individuals, such as landscapers, groundskeepers, nursery workers, farm workers, agricultural workers, and construction workers.81 Notably, occupations associated with increased risk for fungal exposures and infection predominantly represent the low-wage sector82,83 as these workers are often unable to advocate for better working conditions or adhere to public health recommendations to prevent fungal diseases due to lack of support from supervisors. Workers who are employed in such conditions are often exposed to high rates of occupational hazards, inadequate ventilation, limited access to proper sanitation and hygiene facilities, and a lack of proper workwear and personal protective equipment (PPE).
Thus, there is a clear association between a range of fungal infections, occupations, working conditions, and the workers’ socioeconomic background.
Income
Income, another key SDOH, is closely related to working conditions, as discussed above, as well as housing, neighborhood conditions, and access to health care. In the U.S., life expectancy has been shown to increase with income.84
In 2019, of 35.5 million hospitalizations in the U.S., those living in areas with the lowest quartile median income had disproportionately high hospitalization rates. IFIs overall were 1.2 times more likely to be diagnosed in individuals living in a Q1 postal code (median household income < US$48,000) compared to those in a Q4 postal code (median household income ≥ US$82,000), driven by cryptococcosis (twice as likely), and histoplasmosis (1.7 times more likely). Only aspergillosis was diagnosed more frequently in Q4 postal codes compared to Q1 postal codes, possibly related to higher rates of autoimmune diseases, hematologic disorders, and access to advanced cancer treatment and procedures such as organ transplants among hospitalized patients living in higher income areas. IFIs were also more common in hospitalizations where Medicaid was billed compared to hospitalizations where private insurance was billed.85
Although poorly studied on a global level, individuals belonging to lower socioeconomic groups have also been shown to be at greater risk for superficial fungal infections. For example, in a case series of consecutive patients diagnosed with tinea capitis in India, 90% of cases occurred in children younger than 15 years and 75% of cases among individuals who belonged to lower income socioeconomic groups.86 In Nigeria, among schoolchildren diagnosed with superficial fungal infections, infection was more likely to occur in children from families of lower socioeconomic status, with 66% of cases reported, whereas those from higher socioeconomic status were overrepresented (57%) in the control group.87 In the U.S., lower socioeconomic status has been shown to be associated with increased risk of onychomycosis.88
Housing, basic amenities, and the environment
The residential environment, encompassing both housing conditions and community characteristics, exerts a substantial influence on individuals well-being and health outcomes. In most settings the physical built environment has a significant impact on public health and health outcomes, as it influences factors such as physical activity levels, access to healthy food, good quality indoor air, and water, social interactions, medical services, and overall safety and well-being.
Currently, three billion people—40% of the global population—lack facilities at home to wash their hands with soap and water, and nearly half of schools and 43% of healthcare settings lack these amenities.9 In addition, over 90% of people globally breathe outdoor air with pollution levels exceeding WHO air quality guideline values, which contributes to over 7 million deaths annually.9 Even within industrialized Western countries, the residential environment is a major determinant of health outcomes. In western countries, for instance, high levels of crime and violence, high concentrations of convenience food outlets, lack of grocery stores, limited access to fresh, affordable, and nutritious food, and high concentrations of liquor stores and tobacco products correlate strongly with poor physical and mental health outcomes.89, 90, 91, 92 Higher social vulnerability has also been shown to be associated with unsafe housing environments.93
There is a relationship between population density and risk for developing an IFI. Rates of histoplasmosis tend to be higher in individuals who live in rural lower income areas, as previously discussed. There is likely an interplay between living in rural areas, lower income, and environmental as well as occupational exposures to fungi.85,94 Conversely, some IFIs are diagnosed more frequently in urban areas, including aspergillosis, coccidioidomycosis, and pneumocystosis.85,95 Aspergillosis and pneumocystosis may be diagnosed more frequently in urban areas due to the risk factors for this infection, such as hematologic malignancy, immunosuppressive conditions, transplantation, and closer proximity to tertiary medical centers treating patients with these conditions. Outbreaks of IFIs, particularly aspergillosis, have been associated with construction work in the vicinity of hospitals, which can aerosolize conidia,96 further contributing to the risk for IFIs from aerosolized conidia in urban areas.
Globally, higher rates of dermatophytoses have been observed in areas with higher population density or overcrowded areas such as in homes and schools. In Iran, higher rates of tinea capitis were associated with larger families and larger class size in schools.97 Similarly, among school-age children, sharing a bed with more than three people was associated with higher rates of superficial fungal infections of the skin in southern Tanzania.98 Poor living conditions were associated with higher rates of superficial fungal infections in South Western Nigeria,87 and in another study in Nigeria, diagnosis of tinea capitis occurred more frequently in areas of frequent interaction with soil and animals.99
In India, which represents the country with the highest reported rate of mucormycosis, studies have shown that some individuals acquired COVID-19-associated mucormycosis (CAM) at home in their bedrooms, where higher Mucorales spore counts were measured in the rooms of individuals convalescing from COVID-19 infection compared to homes of individuals not affected by CAM.100,101
In addition to invasive infection, outdoor and indoor air pollution in residential environments, including high concentrations of fungal pathogens, has a significant adverse effect on human health by enhancing the production of allergens.102 Stachybotrys chartarum growth can occur on water-damaged areas of buildings and has been associated with acute idiopathic pulmonary hemorrhage in infants, although this association is controversial.103 Some fungal propagules contain pro-inflammatory and pro-allergenic compounds which can cause allergic response. For example, Aspergillus fumigatus contains eighty allergic proteins capable of inducing IgE responses, Alternaria spp. contains twelve, and Cladosporium herbarum eight allergens.104 It is estimated that 6% of the general population and 20–30% of allergy-predisposed individuals are allergic to environmental fungi.105 In addition, an estimated eight to ten percent of individuals with cystic fibrosis experience allergic bronchopulmonary aspergillosis.104 Lastly, Exophiala dermatitidis colonizes the respiratory tract of up to nineteen percent of individuals with cystic fibrosis106,107 and can cause infections as well.108
Besides the residential environment, recreational activities can contribute to fungal exposure and infection. For instance, outbreaks of blastomycosis infections have been associated with environmental exposure during recreational activities. One study in Wisconsin, U.S. documented a high incidence of blastomycosis infection associated with visitation to rivers or associated waterways, suspected to reflect recreational exposure.109 Another cluster was documented among two school groups who visited an environmental camp in northern Wisconsin, with soil at a beaver pond near the camp thought to be the cause.110 Lastly, overcrowded housing may have contributed to a large outbreak of blastomycosis in Wisconsin clustered among households which primarily affected people with Hmong ethnicity, for whom larger average household sizes have been documented.111 In addition, an outbreak of Cryptococcus gattii occurred on Vancouver Island in British Columbia, Canada. C. gattii was cultured from a number of environmental samples, with the highest concentration occurring at a park where almost half of those involved in the outbreak had visited within the prior 12 months.112
Access to affordable and high-quality health services
A lack of timely access to affordable health services is associated with worse health outcomes. Living far from a health care center, fear of deportation if living in a county without documentation, high medical costs, or lack of understanding of how to access health care are among the more common reasons why individuals may lack access to high-quality health care. In the U.S., adults without health insurance are less likely to receive preventive services for chronic medical conditions that predispose to IFI such as poorly controlled diabetes mellitus, especially in the setting of widespread glucocorticoid use,113 cardiovascular disease, and cancer care.114 Conversely, health insurance is associated with improved access to health services and basic clinical services,115,116 increased rates of detection of diabetes mellitus, lower rates of depression, and reduced financial strain.116 Globally, approximately half of the population lacks access to health care, with an additional 100 million driven into extreme poverty by the cost of health care.117 Additionally, many communities globally affected by communicable diseases such as neglected tropical diseases (NTDs) are of low socioeconomic status and lack access to health services.118 Studies have shown that expanding health coverage decreases health disparities,119,120 and it is likely that health disparities are lower in settings with universal access to health care compared to settings that lack health care access for all.
Higher incidence of histoplasmosis has been associated with underlying medical conditions such as poorly controlled diabetes mellitus, autoimmune conditions such as rheumatoid arthritis, inflammatory bowel disease, psoriasis, and solid organ or hematopoietic stem cell transplant.94 Poorly controlled diabetes mellitus is a well-known risk factor for a number of IFIs, including mucormycosis.85,121 Severe COVID-19 infection, especially in the setting of widespread glucocorticoid use,113 has emerged as another major risk factor for mucormycosis121 and invasive aspergillosis,12 including a large mucormycosis outbreak during the COVID-19 delta wave in India.121,122 In one study, access to advanced COVID-19 treatment—including supplemental oxygen, remdesivir therapy, and ICU admission–was associated with lower CAM risk in resource-limited settings.123 There is also a clear association between prior pulmonary tuberculosis with cavitation and subsequent chronic pulmonary aspergillosis, which is a significant health problem in tuberculosis-endemic settings.124
Recently, an outbreak of fungal meningitis among patients who received procedures under epidural anesthesia at two clinics has been reported in Matamoros, Mexico. Fusarium solani species complex has been identified as the causative pathogen, although the exact source is still unclear.125 Many of these affected individuals were from the U.S. and involved in medical tourism–seeking lower-cost procedures with shorter waiting times. Increased risk for candidemia has been associated with poor health care infrastructure and access in lower income countries.126 Overcrowded intensive care units, insufficient air conditioning/ventilation systems, and lack of efficient infection control services predispose individuals from lower income countries to a greater risk for hospital and ICU outbreaks of IFIs, as recently shown for COVID-19-associated pulmonary aspergillosis, CAM, and CNS fusariosis outbreaks in Mexico,123,127,128 as well as invasive Candida infections caused by fluconazole resistant C. parapsilosis129 and C. auris.130 Cryptococcosis is associated with advanced HIV infection as well as other medical comorbidities such as diabetes mellitus, corticosteroid use, and malignancy.131,132 Improved primary prevention, affordable access to early diagnosis, and treatment of chronic medical conditions would likely reduce the risk for IFIs.
Substance use, crime, and incarceration
Substance use has been associated with a number of IFIs. Injection drug use (IDU) is a common cause of bacterial and, less frequently, fungal endocarditis. The latter is mainly caused by Candida spp., followed by Aspergillus spp. and rarely Histoplasma spp. or other endemic fungi,133 and mucormycosis.134, 135, 136 Cigarette smoking has been associated with an increased risk for coccidioidomycosis,137,138 paracoccidioidomycosis,139 and cryptococcosis57. Smoking marijuana has been linked to invasive pulmonary aspergillosis, possibly due to Aspergillus-contaminated leaves from the Cannabis sativa plant.140, 141, 142 In one study, persons who use cannabis were 3.5 times more likely to develop an IFI compared to non-cannabis users.141 Electronic cigarette use or vaping has been associated with invasive pulmonary aspergillosis infection143 and Candida albicans growth in the gingiva of the oral cavity.144
Multiple studies have shown increased risk for coccidioidomycosis infection for incarcerated individuals in areas endemic for Coccidioides spp., such as in the Central Valley in California145, 146, 147 as well as state prisons in other regions in California.148 Those who are incarcerated are more likely to be men and BIPOC individuals as Black or African American men between ages 20 to 34 have the highest rate of incarceration in the U.S.149 In addition, compared to the general population, incarcerated individuals are more likely to have high blood pressure, asthma, cancer, arthritis, and infectious diseases such as tuberculosis, HIV, and hepatitis C infection.150, 151, 152
Structural conflict
Structural conflict such as armed conflict and/or the occupation by foreign armed forces can result in the displacement of populations, loss of income and shelter, the destruction of social networks and physical infrastructure, and violence and human rights abuses, exposing underlying inequities in SDOH. For instance, in Afghanistan, thousands of individuals were injured by unexploded ordinance associated with the ongoing war. One study showed that the vast majority were men and boys, with most injuries occurring in children playing and tending to animals, and adults engaged in economically necessary activities such as farming and traveling.153
In a study of combat casualties in Afghanistan during a 5-year period, it was estimated that IFIs accounted for up to 13% of associated morbidity and mortality. Risk factors included sustaining a dismounted blast injury, experiencing a traumatic transfemoral amputation, and requiring large-volume blood transfusions.154 Higher risk for IFIs has also been shown to be related to lower elevations, warmer temperatures, and greater isothermality, such as in southern Afghanistan where molds grew from 61% of wound cultures.155 Mucorales are the most frequent cause of fungal infections complicating combat-related injuries, followed by Aspergillus spp. and Fusarium spp.156,157 In addition, species not commonly seen in sinopulmonary mucormycosis are more common in theaters of war, such as Apophysomyces spp., Saksenaea spp., and Lichtheimia spp.158 Treatment of combat related IFIs can be particularly challenging, often requiring extensive debridement159 due to imperfect penetration of antifungals into wounds and often limited medical infrastructure in conflict zones.160 Lastly, the ongoing conflict in Sudan has led to the suspension of activities at the Mycetoma Research Center in Khartoum, the only institution in the world that specializes in the treatment of this disease. Thousands of patients now lack access to treatment.161
People displaced from structural conflict are also at an increased risk for fungal infections. For instance, superficial dermatophytoses were common among displaced Rohingya in a refugee camp in Bangladesh,162 refugees from camps in Sudan,163 Syrian refugees in a refugee camp in Jordan,164 and refugees settling in the U.S.165
Food insecurity
Food insecurity is defined as a household-level economic or social condition of limited or uncertain access to adequate food.166 Globally, between 702 and 828 million people–approximately 10% of the global population–were affected by hunger in 2021. This number grew by 150 million since the start of the COVID-19 pandemic, with global conflict, climate extremes, economic instability, and growing inequality also contributing.167 In the U.S., 13.5 million households were food insecure at some point in 2021.
Food insecurity and malnutrition can be a predisposing factor for IFIs. As an example, Pneumocystis jirovecii infection has been associated with low body weight and protein deficiency in children.168
In addition, fungal plant pathogens pose a significant threat to food security and contribute to the US$220 billion estimated annual crop losses caused by pathogens and other pests.169 For instance, the plant fungus Claviceps purpurea can contaminate rye, wheat, and other cereals and cause ergot, which can manifest in a diverse array of symptoms in humans, including tremors, delusions, seizures, muscle spasms, and hallucinations. The plant pathogen Phytophthora infestans causes an estimated US$6 billion in potato losses and management costs annually.170 Pyricularia oryzae is a major rice pathogen, causing 10–35% of loss to harvests, and Puccinia graminis can cause up to 70% of wheat crop losses. Numerous Fusarium spp. are pathogenic to a diverse array of fruits and vegetables,171 causing up to 50% yield losses in fruit and vegetable crops such as bananas, pineapples, lentils, tomatoes, and peas.172 Nosema spp. have been implicated in colony collapse disorder and declining honeybee populations, which has had a significant impact on crops pollinated by the honeybee.173,174 Overall, fungi destroy a third of all food crops annually, resulting in significant economic loss and contributing to global poverty.175 A significant fungal outbreak involving one or more crops could have devastating consequences for the global food supply, which would most impact those from lower income settings. Lastly, although fungicides may help prevent crop loss, they can also lead to the emergence of antifungal resistance, as has been shown against all classes of antifungal drugs.176,177
Interventions to reduce the risk for fungal infections
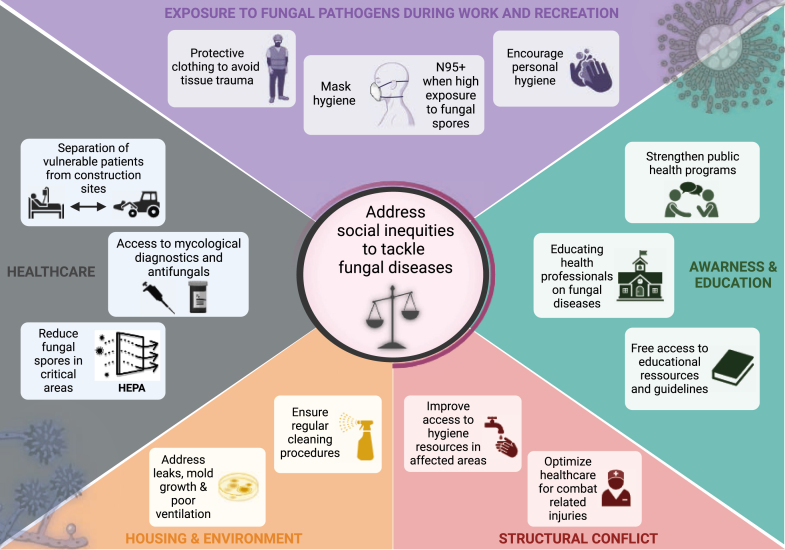
Reducing the risk for fungal infections involves addressing underlying disparities in SDOH that increase the risk for acquiring these infections. Some of these interventions may be applied generally, while specific interventions may be needed to target individual risk factors. Social inequities, such as poverty, limited access to food and health care, inadequate housing, and educational disparities can contribute to an increased risk for developing fungal infections, and addressing these iniquities is multi-faceted (Fig. 2).
Fig. 2.
Strategies to decrease risk of fungal infections by SDOH.
Susceptible individuals may develop IFIs after traumatic inoculation of fungal propagules in wounds. Thus, at-risk individuals should avoid tissue trauma when working in surroundings likely to have high concentrations of fungal propagules, such as in soil. PPE such as protective clothing and gloves can help to protect the skin from contact with mycoses that can potentially cause infection.178
In addition, addressing housing issues such as leaks, mold growth, or poor ventilation can also decrease the risk for acquiring a fungal infection, and this is particularly important for immunocompromised individuals. Mitigating actions to reduce the risk for developing fungal infections during construction work may help reduce outbreaks in hospitals. Such actions may include educating construction workers and hospital staff on preventative strategies to reduce dust production during construction activities, implementing physical barriers to minimize dust spread, and implementing filter systems to reduce fungal burden in the air. High-efficiency particular air (HEPA) filters, for example, have been proven to efficiently filter particles with a diameter of more than 0.3 μm179 and can prevent airborne IFIs in critical areas, such as stem cell transplantation units.180 The implementation and enforcement of well-funded laws and regulations are crucial to address the discussed issues. Governments, along with regulatory bodies, have a responsibility to ensure their implementation and that strong labor laws and protections exist to protect workers from fungal infections. Stakeholders, including professional associations, tenants, landlords and private sector industries such as housing and agriculture, should prioritize these concerns by conducting regular checkups and air quality testing to safeguard the well-being of residents and workers. Employers must uphold the highest occupational safety and health standards, minimizing hazards through the provision of necessary mitigation strategies. These actions are vital in creating healthier and safer living and working environments for individuals and communities.
Access to affordable and quality health care is critical for individuals with underlying conditions associated with increased susceptibility to IFIs. In addition, health care institutions should have access to appropriate fungal diagnostics and antifungal treatment. Unfortunately, these resources are not equitably distributed globally.181 For example, 90% of health care institutions in Europe but only 70% of health care institutions in the Asia/Pacific region reported access to echinocandins to treat IFIs such as candidiasis and aspergillosis.182,183 Access to antifungals for the treatment of implantation mycoses, such as eumycetoma, actinomycetoma, cutaneous sporotrichosis, and chromoblastomycosis, continues to be problematic in lower and middle-income countries globally.184 Inequities in antifungal drug agents and diagnostics can lead to suboptimal management of IFIs in resource limited settings.
Awareness and education on the signs and symptoms of fungal infections is important for both the general population as well as health care workers, to ensure timely and effective management of these infections. Several guidelines, such as those published on the management of cryptococcosis,185,186 mucormycosis,187 endemic mycoses,188 and rare mold infections189 offer guidance on the management of these infections, including in resource-limited settings. In addition, in the oncology setting recommendations on how to avoid excessive fungal exposure have been published.190 Still, there remains a lack of guidelines for neglected fungal tropical diseases such as mycetoma and chromoblastomycosis, and the guidelines that currently exist need to be widely available and freely accessible to healthcare workers around the globe. More research should be done on IFIs associated with occupational health hazards and combat injuries, including better tracking of these fungal infections so those who manage these injuries are better able to diagnose and offer appropriate antifungal treatment. Lastly, although a number of surveillance networks already exist to monitor existing communicable and non-communicable diseases, including for emerging pathogens,191, 192, 193 more widespread global surveillance of fungi is warranted to mitigate the impact of these pathogens on the health of those most at risk.
Discussion
SDOH play a significant role in global health inequity and are associated with increased risk for developing superficial and IFIs, as discussed in this review (Fig. 1). This is particularly true for working conditions, where the risk for developing an infection from fungi is highly associated with certain types of work, including construction, farming, landscaping, and other agricultural work. Lower income is related with increased risk for certain IFIs globally and, along with more crowded housing, is associated with risk for superficial fungal infections globally. Lack of access to quality health care, and the underlying medical conditions associated with it, is associated with increased risk for IFIs. Structural and armed conflicts are associated with risk for IFIs following traumatic injury, and displacement of people fleeing conflict is associated with superficial and likely subcutaneous fungal infections. Finally, several fungal plant pathogens pose a significant threat to food security, and this threat is expected to intensify with the progression of global climate change194,195 and the subsequent weather phenomenon will likely increase the vulnerability of communities to natural disaster globally.196, 197, 198, 199, 200, 201 These global changes are expected to predominantly affect those who are already at an increased risk for fungal infections due to inequities in SDOH. Of note, the current published literature likely significantly underrepresents the association between SDOH and IFIs outside of the U.S., where the risks likely mirror those found within the U.S.
To decrease the morbidity and mortality associated with fungal infections, more research needs to be dedicated to implementing interventions that can help decrease the acquisition of fungal infections in those most at risk. The gaps in evidence need to be addressed and a more systematic approach to understanding and addressing the impact of SDOH on IFIs globally–with a specific focus on low-resource settings–needs to be implemented. Well-funded regulations that hold employers, landlords, and companies accountable could help address SDOH, if they are enforced. In addition, more emphasis needs to be placed on decreasing the health inequities between low-income and high-income countries and within higher-income countries such as the U.S.
Outstanding questions
An important question is how to best provide surveillance of fungal infections globally, particularly in resource-limited settings where the prevalence of fungal infections is likely grossly underestimated. Another challenge is how to capture data on SDOH at a global level so the association between SDOH and fungal infections can be better understood. From a programmatic level, what role do agencies such as the WHO play in monitoring SDOH and fungal diseases and the association between the two?
Contributors
JDJ and MH conceived and designed the study. JDJ, JP, and MH wrote the initial draft. RS, MO, and ME produced figures. SW, RS, DS, ME, CDR, HS, OAC, GRT, and DPK provided critical comments. All authors read and approved the final manuscript.
Declaration of interests
Conflict of Interest and Sources of Funding: JDJ received research funding from Astellas, F2G, and Pfizer—all outside of the submitted work. JP has received speakers’ fees from Gilead Sciences, Pfizer, Swedish Orphan Biovitrum, Associated of Cape Cod, served at advisor boards for Gilead Sciences and Pfizer and holds stocks of Novo Nordisk and AbbVie Inc—all outside of the submitted work. RS received speaker fees and travel support from Pfizer—all outside of the submitted work. DS received speaker fees from Pfizer—all outside of the submitted work. OAC reports grants or contracts from BMBF, Cidara, EU-DG RTD (101 037 867), F2G, Gilead, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; Consulting fees from Abbvie, AiCuris, Biocon, Cidara, Gilead, IQVIA, Janssen, Matinas, MedPace, Menarini, Moderna, Molecular Partners, MSG-ERC, Noxxon, Octapharma, Pfizer, PSI, Scynexis, Seres; Honoraria for lectures from Abbott, Abbvie, Al-Jazeera Pharmaceuticals/Hikma, Gilead, Grupo Biotoscana/United Medical/Knight, ISHAM Working Group, MedScape, MedUpdate, Merck/MSD,Noscendo, Pfizer, Shionogi, streamedup!; Payment for expert testimony from Cidara; Participation on a Data Safety Monitoring Board or Advisory Board from Boston Strategic Partners, Cidara, IQVIA, Janssen, MedPace, PSI, Pulmocide, Shionogi, The Prime Meridian Group; A patent at the German Patent and Trade Mark Office (DE 10 2021 113 007.7); Stocks from CoRe Consulting, EasyRadiology; Other interests from Wiley. GRT received research and consulting fees from Astellas, Cidara, F2G, Mayne, Melinta, Mundipharma, and served on the DRC for Pfizer—all outside of the submitted work. DPK received honoraria and research support from Gilead Sciences, Merck, United Medical, and Astellas Pharma. He received consultant fees from Astellas Pharma, Amplyx Pharmaceuticals, Ciadara Therapeutics, Mayne Pharma, and is a member of the Data Review Committee of Cidara Therapeutics, AbbVie, Scynexis, and the Mycoses Study Group–all outside of the submitted work. MH received research funding from Gilead, Astellas, Euroimmune, MSD, IMMY, Mundipharma, Scynexis, and Pfizer—all outside of the submitted work. All authors declare no conflict of interest.
Contributor Information
Jeffrey D. Jenks, Email: jeffrey.jenks@duke.edu.
Martin Hoenigl, Email: hoeniglmartin@gmail.com.
References
- 1.Centers for Disease Control and Prevention Minority health: Racism and health. https://www.cdc.gov/minorityhealth/racism-disparities/index.html Available at:
- 2.Jenks J.D., Aneke C.I., Al-Obaidi M.M., et al. Race and ethnicity: risk factors for fungal infections? PLoS Pathog. 2023;19(1) doi: 10.1371/journal.ppat.1011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi W.Y. Mortality rate of patients with COVID-19 based on underlying health conditions. Disaster Med Public Health Prep. 2021:1–6. doi: 10.1017/dmp.2021.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maness S.B., Merrell L., Thompson E.L., Griner S.B., Kline N., Wheldon C. Social determinants of health and health disparities: COVID-19 exposures and mortality among African American people in the United States. Public Health Rep. 2021;136(1):18–22. doi: 10.1177/0033354920969169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Social determinants of health. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 Available at:
- 6.Hood C.M., Gennuso K.P., Swain G.R., Catlin B.B. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50(2):129–135. doi: 10.1016/j.amepre.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Freeman T., Gesesew H.A., Bambra C., et al. Why do some countries do better or worse in life expectancy relative to income? An analysis of Brazil, Ethiopia, and the United States of America. Int J Equity Health. 2020;19(1):202. doi: 10.1186/s12939-020-01315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geronimus A.T., Bound J., Waidmann T.A., Hillemeier M.M., Burns P.B. Excess mortality among blacks and whites in the United States. N Engl J Med. 1996;335(21):1552–1558. doi: 10.1056/NEJM199611213352102. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization It's time to build a fairer, healthier world for everyone, everywhere. https://cdn.who.int/media/docs/default-source/world-health-day-2021/health-equity-and-its-determinants.pdf?sfvrsn=6c36f0a5_1&download=true Available at:
- 10.Jenks J.D., Nam H.H., Hoenigl M. Invasive aspergillosis in critically ill patients: review of definitions and diagnostic approaches. Mycoses. 2021;64:1002. doi: 10.1111/myc.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 2017;3(4) doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoenigl M., Seidel D., Sprute R., et al. COVID-19-associated fungal infections. Nat Microbiol. 2022;7:1127. doi: 10.1038/s41564-022-01172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoenigl M., Salmanton-García J., Egger M., et al. Guideline adherence and survival of patients with candidaemia in Europe: results from the ECMM Candida III multinational European observational cohort study. Lancet Infect Dis. 2023;23:751. doi: 10.1016/S1473-3099(22)00872-6. [DOI] [PubMed] [Google Scholar]
- 14.Pappas P.G., Alexander B.D., Andes D.R., et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50(8):1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 15.Rajasingham R., Smith R.M., Park B.J., et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17(8):873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M., Hoenigl M., Thompson G.R., 3rd, Carvalho A., Jenks J.D. Let's talk about sex characteristics-As a risk factor for invasive fungal diseases. Mycoses. 2022;65(6):599–612. doi: 10.1111/myc.13449. [DOI] [PubMed] [Google Scholar]
- 17.Sipsas N.V., Kontoyiannis D.P. Occupation, lifestyle, diet, and invasive fungal infections. Infection. 2008;36(6):515–525. doi: 10.1007/s15010-008-8129-5. [DOI] [PubMed] [Google Scholar]
- 18.Laws R.L., Jain S., Cooksey G.S., et al. Coccidioidomycosis outbreak among inmate wildland firefighters: California, 2017. Am J Ind Med. 2021;64(4):266–273. doi: 10.1002/ajim.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson D., Ebisu K., Wu X., Basu R. A review of coccidioidomycosis in California: exploring the intersection of land use, population movement, and climate change. Epidemiol Rev. 2019;41(1):145–157. doi: 10.1093/epirev/mxz004. [DOI] [PubMed] [Google Scholar]
- 20.Outbreak of histoplasmosis among industrial plant workers--Nebraska, 2004. MMWR Morb Mortal Wkly Rep. 2004;53(43):1020–1022. [PubMed] [Google Scholar]
- 21.Vaaler A.K., Bradsher R.W., Davies S.F. Evidence of subclinical blastomycosis in forestry workers in northern Minnesota and northern Wisconsin. Am J Med. 1990;89(4):470–476. doi: 10.1016/0002-9343(90)90378-q. [DOI] [PubMed] [Google Scholar]
- 22.do Valle A.C.F., Marques de Macedo P., Almeida-Paes R., Romão A.R., Lazéra M.D.S., Wanke B. Paracoccidioidomycosis after highway construction, Rio de Janeiro, Brazil. Emerg Infect Dis. 2017;23(11):1917–1919. doi: 10.3201/eid2311.170934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith C.E. U.S. Army; Washington, DC: 1598. Preventative medicine in world war II. [Google Scholar]
- 24.Willet F.M., Weiss A. Coccidioidomycosis in southern California: report of a new endemic area with a review of 100 cases. Ann Intern Med. 1945;23(3):349–375. [Google Scholar]
- 25.Crum N., Lamb C., Utz G., Amundson D., Wallace M. Coccidioidomycosis outbreak among United States Navy SEALs training in a Coccidioides immitis-endemic area--Coalinga, California. J Infect Dis. 2002;186(6):865–868. doi: 10.1086/342409. [DOI] [PubMed] [Google Scholar]
- 26.Standaert S.M., Schaffner W., Galgiani J.N., et al. Coccidioidomycosis among visitors to a Coccidioides immitis-endemic area: an outbreak in a military reserve unit. J Infect Dis. 1995;171(6):1672–1675. doi: 10.1093/infdis/171.6.1672. [DOI] [PubMed] [Google Scholar]
- 27.de Perio M.A., Niemeier R.T., Burr G.A. Coccidioides exposure and coccidioidomycosis among prison employees, California, United States. Emerg Infect Dis. 2015;21(6):1031–1033. doi: 10.3201/eid2106.141201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werner S.B., Pappagianis D. Coccidioidomycosis in Northern California. An outbreak among archeology students near Red Bluff. Calif Med. 1973;119(3):16–20. [PMC free article] [PubMed] [Google Scholar]
- 29.Werner S.B., Pappagianis D., Heindl I., Mickel A. An epidemic of coccidioidomycosis among archeology students in northern California. N Engl J Med. 1972;286(10):507–512. doi: 10.1056/NEJM197203092861003. [DOI] [PubMed] [Google Scholar]
- 30.Perera P., Stone S. Coccidioidomycosis in workers at an archeologic site-dinosaur national monument, Utah, June-July 2001. Ann Emerg Med. 2002;39(5):566–569. doi: 10.1067/mem.2002.123550. [DOI] [PubMed] [Google Scholar]
- 31.Sondermeyer Cooksey G.L., Wilken J.A., McNary J., et al. Dust exposure and coccidioidomycosis prevention among solar power farm construction workers in California. Am J Public Health. 2017;107(8):1296–1303. doi: 10.2105/AJPH.2017.303820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings K.C., McDowell A., Wheeler C., et al. Point-source outbreak of coccidioidomycosis in construction workers. Epidemiol Infect. 2010;138(4):507–511. doi: 10.1017/S0950268809990999. [DOI] [PubMed] [Google Scholar]
- 33.Nicas M. A point-source outbreak of coccidioidomycosis among a highway construction crew. J Occup Environ Hyg. 2018;15(1):57–62. doi: 10.1080/15459624.2017.1383612. [DOI] [PubMed] [Google Scholar]
- 34.McCurdy S.A., Portillo-Silva C., Sipan C.L., Bang H., Emery K.W. Risk for coccidioidomycosis among hispanic farm workers, California, USA, 2018. Emerg Infect Dis. 2020;26(7):1430–1437. doi: 10.3201/eid2607.200024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gehlbach S.H., Hamilton J.D., Conant N.F. Coccidioidomycosis. An occupational disease in cotton mill workers. Arch Intern Med. 1973;131(2):254–255. doi: 10.1001/archinte.131.2.254. [DOI] [PubMed] [Google Scholar]
- 36.Luby J.P., Southern P.M., Jr., Haley C.E., Vahle K.L., Munford R.S., Haley R.W. Recurrent exposure to histoplasma capsulatum in modern air-conditioned buildings. Clin Infect Dis. 2005;41(2):170–176. doi: 10.1086/430907. [DOI] [PubMed] [Google Scholar]
- 37.Huhn G.D., Austin C., Carr M., et al. Two outbreaks of occupationally acquired histoplasmosis: more than workers at risk. Environ Health Perspect. 2005;113(5):585–589. doi: 10.1289/ehp.7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson H., Honish L., Taylor G., et al. Histoplasmosis cluster, golf course, Canada. Emerg Infect Dis. 2006;12(1):163–165. doi: 10.3201/eid1201.051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández Andreu C.M., Martínez Machín G., Illnait Zaragozi M.T., Perurena Lancha M.R., González L. [Outbreaks of occupational acquired histoplasmosis in La Habana province] Rev Cubana Med Trop. 2010;62(1):68–72. [PubMed] [Google Scholar]
- 40.Armstrong P.A., Beard J.D., Bonilla L., et al. Outbreak of severe histoplasmosis among tunnel workers-Dominican Republic, 2015. Clin Infect Dis. 2018;66(10):1550–1557. doi: 10.1093/cid/cix1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsey D.T. Blastomycosis in a veterinarian. J Am Vet Med Assoc. 1994;205(7):968. [PubMed] [Google Scholar]
- 42.Larson D.M., Eckman M.R., Alber R.L., Goldschmidt V.G. Primary cutaneous (inoculation) blastomycosis: an occupational hazard to pathologists. Am J Clin Pathol. 1983;79(2):253–255. doi: 10.1093/ajcp/79.2.253. [DOI] [PubMed] [Google Scholar]
- 43.Baum G.L., Lerner P.I. Primary pulmonary blastomycosis: a laboratory-acquired infection. Ann Intern Med. 1970;73(2):263–265. doi: 10.7326/0003-4819-73-2-263. [DOI] [PubMed] [Google Scholar]
- 44.Tumin R. 2023. 1 dead and nearly 100 sickened in fungal OUtbreak at paper mill. The New York Times. [Google Scholar]
- 45.Blastomycosis acquired occupationally during prairie dog relocation--Colorado, 1998. MMWR Morb Mortal Wkly Rep. 1999;48(5):98–100. [PubMed] [Google Scholar]
- 46.Maluf M.L., Pereira S.R., Takahachi G., Svidzinski T.I. [Prevalence of paracoccidioidomycosis infection determined by sorologic test in donors' blood in the Northwest of Paraná, Brazil] Rev Soc Bras Med Trop. 2003;36(1):11–16. doi: 10.1590/s0037-86822003000100003. [DOI] [PubMed] [Google Scholar]
- 47.Minotto R., Bernardi C.D., Mallmann L.F., Edelweiss M.I., Scroferneker M.L. Chromoblastomycosis: a review of 100 cases in the state of Rio Grande do Sul, Brazil. J Am Acad Dermatol. 2001;44(4):585–592. doi: 10.1067/mjd.2001.112220. [DOI] [PubMed] [Google Scholar]
- 48.Chariyalertsak S., Sirisanthana T., Supparatpinyo K., Praparattanapan J., Nelson K.E. Case-control study of risk factors for Penicillium marneffei infection in human immunodeficiency virus-infected patients in northern Thailand. Clin Infect Dis. 1997;24(6):1080–1086. doi: 10.1086/513649. [DOI] [PubMed] [Google Scholar]
- 49.Chazalet V., Debeaupuis J.P., Sarfati J., et al. Molecular typing of environmental and patient isolates of Aspergillus fumigatus from various hospital settings. J Clin Microbiol. 1998;36(6):1494–1500. doi: 10.1128/jcm.36.6.1494-1500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodley J.M., Clayton Y.M., Hay R.J. Environmental sampling for aspergilli during building construction on a hospital site. J Hosp Infect. 1994;26(1):27–35. doi: 10.1016/0195-6701(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 51.Hospenthal D.R., Kwon-Chung K.J., Bennett J.E. Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: lack of correlation. Med Mycol. 1998;36(3):165–168. [PubMed] [Google Scholar]
- 52.Sabino R., Veríssimo C., Viegas C., et al. The role of occupational Aspergillus exposure in the development of diseases. Med Mycol. 2019;57(Supplement_2):196–205. doi: 10.1093/mmy/myy090. [DOI] [PubMed] [Google Scholar]
- 53.Lauruschkat C.D., Etter S., Schnack E., et al. Chronic occupational mold exposure drives expansion of Aspergillus-Reactive type 1 and type 2 T-helper cell responses. J Fungi (Basel) 2021;7(9) doi: 10.3390/jof7090698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viegas S., Veiga L., Malta-Vacas J., et al. Occupational exposure to aflatoxin (AFB₁) in poultry production. J Toxicol Environ Health. 2012;75(22-23):1330–1340. doi: 10.1080/15287394.2012.721164. [DOI] [PubMed] [Google Scholar]
- 55.Murthy J.M., Sundaram C., Prasad V.S., Purohit A.K., Rammurti S., Laxmi V. Sinocranial aspergillosis: a form of central nervous system aspergillosis in south India. Mycoses. 2001;44(5):141–145. doi: 10.1046/j.1439-0507.2001.00643.x. [DOI] [PubMed] [Google Scholar]
- 56.Zuk J.A., King D., Zakhour H.D., Delaney J.C. Locally invasive pulmonary aspergillosis occurring in a gardener: an occupational hazard? Thorax. 1989;44(8):678–679. doi: 10.1136/thx.44.8.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hajjeh R.A., Conn L.A., Stephens D.S., et al. Cryptococcosis: population-based multistate active surveillance and risk factors in human immunodeficiency virus-infected persons. Cryptococcal Active Surveillance Group. J Infect Dis. 1999;179(2):449–454. doi: 10.1086/314606. [DOI] [PubMed] [Google Scholar]
- 58.He J., Sheng G., Yue H., Zhang F., Zhang H.L. Isolated pulmonary mucormycosis in an immunocompetent patient: a case report and systematic review of the literature. BMC Pulm Med. 2021;21(1):138. doi: 10.1186/s12890-021-01504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saul S.R., Sandra A., Magnotti M. A patient with newly diagnosed diabetes presenting with sino-orbital mucormycosis. Case Reports. 2016:E41–E45. [Google Scholar]
- 60.Lambert D., Nerot C., Huguenin A., et al. [Post-traumatic mucormycosis due to Lichtheimia corymbifera: three case reports] J Mycol Med. 2014;24(4):345–350. doi: 10.1016/j.mycmed.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 61.Gugnani H.C. Entomophthoromycosis due to conidiobolus. Eur J Epidemiol. 1992;8(3):391–396. doi: 10.1007/BF00158574. [DOI] [PubMed] [Google Scholar]
- 62.Quintal D. Sporotrichosis infection on mines of the Witwatersrand. J Cutan Med Surg. 2000;4(1):51–54. doi: 10.1177/120347540000400113. [DOI] [PubMed] [Google Scholar]
- 63.Coles F.B., Schuchat A., Hibbs J.R., et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136(4):475–487. doi: 10.1093/oxfordjournals.aje.a116521. [DOI] [PubMed] [Google Scholar]
- 64.Powell K.E., Taylor A., Phillips B.J., et al. Cutaneous sporotrichosis in forestry workers. Epidemic due to contaminated Sphagnum moss. JAMA. 1978;240(3):232–235. [PubMed] [Google Scholar]
- 65.Hajjeh R., McDonnell S., Reef S., et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis. 1997;176(2):499–504. doi: 10.1086/514070. [DOI] [PubMed] [Google Scholar]
- 66.Alves S.H., Boettcher C.S., Oliveira D.C., et al. Sporothrix schenckii associated with armadillo hunting in Southern Brazil: epidemiological and antifungal susceptibility profiles. Rev Soc Bras Med Trop. 2010;43(5):523–525. doi: 10.1590/s0037-86822010000500010. [DOI] [PubMed] [Google Scholar]
- 67.Rodrigues A.M., Bagagli E., de Camargo Z.P., Bosco Sde M. Sporothrix schenckii sensu stricto isolated from soil in an armadillo's burrow. Mycopathologia. 2014;177(3-4):199–206. doi: 10.1007/s11046-014-9734-8. [DOI] [PubMed] [Google Scholar]
- 68.Reed K.D., Moore F.M., Geiger G.E., Stemper M.E. Zoonotic transmission of sporotrichosis: case report and review. Clin Infect Dis. 1993;16(3):384–387. doi: 10.1093/clind/16.3.384. [DOI] [PubMed] [Google Scholar]
- 69.Gremião I.D.F., Martins da Silva da Rocha E., Montenegro H., et al. Guideline for the management of feline sporotrichosis caused by Sporothrix brasiliensis and literature revision. Braz J Microbiol. 2021;52(1):107–124. doi: 10.1007/s42770-020-00365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maiti P.K., Ray A., Bandyopadhyay S. Epidemiological aspects of mycetoma from a retrospective study of 264 cases in West Bengal. Trop Med Int Health. 2002;7(9):788–792. doi: 10.1046/j.1365-3156.2002.00915.x. [DOI] [PubMed] [Google Scholar]
- 71.Hounsome N., Hassan R., Bakhiet S.M., et al. Role of socioeconomic factors in developing mycetoma: results from a household survey in Sennar State, Sudan. PLoS Negl Trop Dis. 2022;16(10) doi: 10.1371/journal.pntd.0010817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fahal A., Mahgoub el S., El Hassan A.M., Abdel-Rahman M.E. Mycetoma in the Sudan: an update from the mycetoma research centre, university of Khartoum, Sudan. PLoS Negl Trop Dis. 2015;9(3) doi: 10.1371/journal.pntd.0003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fahal A.H. Mycetoma: a global medical and socio-economic dilemma. PLoS Negl Trop Dis. 2017;11(4) doi: 10.1371/journal.pntd.0005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emmanuel P., Dumre S.P., John S., Karbwang J., Hirayama K. Mycetoma: a clinical dilemma in resource limited settings. Ann Clin Microbiol Antimicrob. 2018;17(1):35. doi: 10.1186/s12941-018-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santos D., de Azevedo C., Vicente V.A., et al. The global burden of chromoblastomycosis. PLoS Negl Trop Dis. 2021;15(8) doi: 10.1371/journal.pntd.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costa A.R., Porto E., Tayah M., et al. Subcutaneous mucormycosis caused by Mucor hiemalis Wehmer f. luteus (Linnemann) Schipper 1973. Mycoses. 1990;33(5):241–246. doi: 10.1111/myc.1990.33.5.241. [DOI] [PubMed] [Google Scholar]
- 77.Guevara D., Wongkittiroch K., Goodman M., Singh A., Weiss E., Glick B. Acremonium mycetoma: a case report and discussion. Cutis. 2011;88(6):293–295. [PubMed] [Google Scholar]
- 78.Krajaejun T., Sathapatayavongs B., Pracharktam R., et al. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin Infect Dis. 2006;43(5):569–576. doi: 10.1086/506353. [DOI] [PubMed] [Google Scholar]
- 79.Klotz S.A., Penn C.C., Negvesky G.J., Butrus S.I. Fungal and parasitic infections of the eye. Clin Microbiol Rev. 2000;13(4):662–685. doi: 10.1128/cmr.13.4.662-685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas P.A., Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19(3):210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 81.U.S. Bureau of Labor Statistics Labor force statistics from the current population survey. 11. Employed persons by detailed occupation, sex, race, and hispanic or latino ethnicity. https://www.bls.gov/cps/cpsaat11.htm Available at:
- 82.International Labour Organization Global Wage Report. 2020-21. Wages and minimum wages in the time of COVID-19. https://www.ilo.org/wcmsp5/groups/public/---dgreports/---dcomm/---publ/documents/publication/wcms_762534.pdf Available at:
- 83.U.S. Bureau of Labor Statistics Median weekly earnings of full-time wage and salary workers by detailed occupation and sex. https://www.bls.gov/cps/cpsaat39.pdf Available at:
- 84.Chetty R., Stepner M., Abraham S., et al. The association between income and life expectancy in the United States, 2001-2014. JAMA. 2016;315(16):1750–1766. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rayens E., Rayens M.K., Norris K.A. Demographic and socioeconomic factors associated with fungal infection risk, United States, 2019. Emerg Infect Dis. 2022;28(10):1955–1969. doi: 10.3201/eid2810.220391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singal A., Rawat S., Bhattacharya S.N., Mohanty S., Baruah M.C. Clinico-myocological profile of tinea capitis in North India and response to griseofulvin. J Dermatol. 2001;28(1):22–26. doi: 10.1111/j.1346-8138.2001.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 87.Olutoyin O.O., Onayemi O., Gabriel A.O. Risk factors associated with acquiring superficial fungal infections in school children in South Western Nigeria: a comparative study. Afr Health Sci. 2017;17(2):330–336. doi: 10.4314/ahs.v17i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moseley I., Ragi S.D., Ouellette S., Rao B. Onychomycosis in underrepresented groups: an all of us database analysis. Arch Dermatol Res. 2023;315(3):647–651. doi: 10.1007/s00403-022-02413-4. [DOI] [PubMed] [Google Scholar]
- 89.van Erpecum C.L., van Zon S.K.R., Bültmann U., Smidt N. The association between the presence of fast-food outlets and BMI: the role of neighbourhood socio-economic status, healthy food outlets, and dietary factors. BMC Publ Health. 2022;22(1):1432. doi: 10.1186/s12889-022-13826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jones-Webb R., Karriker-Jaffe K.J. Neighborhood disadvantage, high alcohol content beverage consumption, drinking norms, and drinking consequences: a mediation analysis. J Urban Health. 2013;90(4):667–684. doi: 10.1007/s11524-013-9786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schleicher N.C., Johnson T.O., Fortmann S.P., Henriksen L. Tobacco outlet density near home and school: associations with smoking and norms among US teens. Prev Med. 2016;91:287–293. doi: 10.1016/j.ypmed.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee J.G., Henriksen L., Rose S.W., Moreland-Russell S., Ribisl K.M. A systematic review of neighborhood disparities in point-of-sale tobacco marketing. Am J Public Health. 2015;105(9):e8–e18. doi: 10.2105/AJPH.2015.302777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hollar T.L., Melo A.F., Maitland K., Cuenca S., Chung E. Social vulnerability and safe building Recertification violations in Miami, Florida, 2013-2018. Am J Public Health. 2022;112(8):1217–1220. doi: 10.2105/AJPH.2022.306890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Armstrong P.A., Jackson B.R., Haselow D., et al. Multistate epidemiology of histoplasmosis, United States, 2011-2014. Emerg Infect Dis. 2018;24(3):425–431. doi: 10.3201/eid2403.171258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grehn C., Eschenhagen P., Temming S., Düesberg U., Neumann K., Schwarz C. Urban life as risk factor for aspergillosis. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alvarez-Moreno C.A., Combariza J.F. Risk of invasive fungal infections during hospital construction: how to minimize its impact in immunocompromised patients. Curr Opin Infect Dis. 2019;32(4):322–329. doi: 10.1097/QCO.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 97.Bassiri Jahromi S., Khaksar A.A. Aetiological agents of tinea capitis in Tehran (Iran) Mycoses. 2006;49(1):65–67. doi: 10.1111/j.1439-0507.2005.01182.x. [DOI] [PubMed] [Google Scholar]
- 98.Chikoi R., Nyawale H.A., Mghanga F.P. Magnitude and associated risk factors of superficial skin fungal infection among primary school children in southern Tanzania. Cureus. 2018;10(7) doi: 10.7759/cureus.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ayanbimpe G.M., Taghir H., Diya A., Wapwera S. Tinea capitis among primary school children in some parts of central Nigeria. Mycoses. 2008;51(4):336–340. doi: 10.1111/j.1439-0507.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 100.Ghosh A.K., Singh R., Reddy S., et al. Evaluation of environmental Mucorales contamination in and around the residence of COVID-19-associated mucormycosis patients. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.953750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prakash H., Singh S., Rudramurthy S.M., et al. An aero mycological analysis of Mucormycetes in indoor and outdoor environments of northern India. Med Mycol. 2020;58(1):118–123. doi: 10.1093/mmy/myz031. [DOI] [PubMed] [Google Scholar]
- 102.Eguiluz-Gracia I., Mathioudakis A.G., Bartel S., et al. The need for clean air: the way air pollution and climate change affect allergic rhinitis and asthma. Allergy. 2020;75(9):2170–2184. doi: 10.1111/all.14177. [DOI] [PubMed] [Google Scholar]
- 103.Dyląg M., Spychała K., Zielinski J., Łagowski D., Gnat S. Update on stachybotrys chartarum-black mold perceived as toxigenic and potentially pathogenic to humans. Biology. 2022;11(3) doi: 10.3390/biology11030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carsin A., Romain T., Ranque S., et al. Aspergillus fumigatus in cystic fibrosis: an update on immune interactions and molecular diagnostics in allergic bronchopulmonary aspergillosis. Allergy. 2017;72(11):1632–1642. doi: 10.1111/all.13204. [DOI] [PubMed] [Google Scholar]
- 105.Horner W.E., Helbling A., Salvaggio J.E., Lehrer S.B. Fungal allergens. Clin Microbiol Rev. 1995;8(2):161–179. doi: 10.1128/cmr.8.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Horré R., Schaal K.P., Siekmeier R., Sterzik B., de Hoog G.S., Schnitzler N. Isolation of fungi, especially Exophiala dermatitidis, in patients suffering from cystic fibrosis. A prospective study. Respiration. 2004;71(4):360–366. doi: 10.1159/000079640. [DOI] [PubMed] [Google Scholar]
- 107.Kirchhoff L., Olsowski M., Rath P.M., Steinmann J. Exophiala dermatitidis: key issues of an opportunistic fungal pathogen. Virulence. 2019;10(1):984–998. doi: 10.1080/21505594.2019.1596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haase G., Skopnik H., Kusenbach G. Exophiala dermatitidis infection in cystic fibrosis. Lancet. 1990;336(8708):188–189. doi: 10.1016/0140-6736(90)91721-l. [DOI] [PubMed] [Google Scholar]
- 109.Baumgardner D.J., Buggy B.P., Mattson B.J., Burdick J.S., Ludwig D. Epidemiology of blastomycosis in a region of high endemicity in north central Wisconsin. Clin Infect Dis. 1992;15(4):629–635. doi: 10.1093/clind/15.4.629. [DOI] [PubMed] [Google Scholar]
- 110.Klein B.S., Vergeront J.M., Weeks R.J., et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314(9):529–534. doi: 10.1056/NEJM198602273140901. [DOI] [PubMed] [Google Scholar]
- 111.Roy M., Benedict K., Deak E., et al. A large community outbreak of blastomycosis in Wisconsin with geographic and ethnic clustering. Clin Infect Dis. 2013;57(5):655–662. doi: 10.1093/cid/cit366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kidd S.E., Hagen F., Tscharke R.L., et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proc Natl Acad Sci U S A. 2004;101(49):17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel) 2021;7(4) doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ayanian J.Z., Weissman J.S., Schneider E.C., Ginsburg J.A., Zaslavsky A.M. Unmet health needs of uninsured adults in the United States. JAMA. 2000;284(16):2061–2069. doi: 10.1001/jama.284.16.2061. [DOI] [PubMed] [Google Scholar]
- 115.McWilliams J.M., Zaslavsky A.M., Meara E., Ayanian J.Z. Impact of Medicare coverage on basic clinical services for previously uninsured adults. JAMA. 2003;290(6):757–764. doi: 10.1001/jama.290.6.757. [DOI] [PubMed] [Google Scholar]
- 116.Baicker K., Taubman S.L., Allen H.L., et al. The Oregon experiment--effects of Medicaid on clinical outcomes. N Engl J Med. 2013;368(18):1713–1722. doi: 10.1056/NEJMsa1212321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.World Health Organization World Bank and WHO: half the world lacks access to essential health services, 100 million still pushed into extreme poverty because of health expenses. https://www.who.int/news/item/13-12-2017-world-bank-and-who-half-the-world-lacks-access-to-essential-health-services-100-million-still-pushed-into-extreme-poverty-because-of-health-expenses Available at:
- 118.Ochola E.A., Karanja D.M.S., Elliott S.J. The impact of Neglected Tropical Diseases (NTDs) on health and wellbeing in sub-Saharan Africa (SSA): a case study of Kenya. PLoS Negl Trop Dis. 2021;15(2) doi: 10.1371/journal.pntd.0009131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goldman A.L., McCormick D., Haas J.S., Sommers B.D. Effects of the ACA's health insurance marketplaces on the previously uninsured: a quasi-experimental analysis. Health Aff. 2018;37(4):591–599. doi: 10.1377/hlthaff.2017.1390. [DOI] [PubMed] [Google Scholar]
- 120.Serakos M., Wolfe B. The ACA: impacts on health, access, and employment. Forum Health Econ Policy. 2016;19(2):201–259. doi: 10.1515/fhep-2015-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rudramurthy S.M., Hoenigl M., Meis J.F., et al. ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries. Mycoses. 2021;64(9):1028–1037. doi: 10.1111/myc.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoenigl M., Seidel D., Carvalho A., et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022;3:e543. doi: 10.1016/S2666-5247(21)00237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chowdhary A., Gupta N., Wurster S., et al. Multimodal analysis of the COVID-19-associated mucormycosis outbreak in Delhi, India indicates the convergence of clinical and environmental risk factors. Mycoses. 2023;66(6):515–526. doi: 10.1111/myc.13578. [DOI] [PubMed] [Google Scholar]
- 124.Bongomin F. Post-tuberculosis chronic pulmonary aspergillosis: an emerging public health concern. PLoS Pathog. 2020;16(8) doi: 10.1371/journal.ppat.1008742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith D.J., Gold J.A.W., Chiller T., et al. Update on outbreak of fungal meningitis among U.S. Residents who received epidural anesthesia at two clinics in Matamoros, Mexico. Clin Infect Dis. 2023 doi: 10.1093/cid/ciad570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaur H., Chakrabarti A. Strategies to reduce mortality in adult and neonatal candidemia in developing countries. J Fungi (Basel) 2017;3(3) doi: 10.3390/jof3030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hoenigl M., Jenks J.D., Egger M., et al. Treatment of Fusarium infection of the central nervous system: a review of past cases to guide therapy for the ongoing 2023 outbreak in the United States and Mexico. Mycopathologia. 2023 doi: 10.1007/s11046-023-00790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soriano M.C., Narváez-Chávez G., López-Olivencia M., Fortún J., de Pablo R. Inhaled amphotericin B lipid complex for prophylaxis against COVID-19-associated invasive pulmonary aspergillosis. Intensive Care Med. 2022;48(3):360–361. doi: 10.1007/s00134-021-06603-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Daneshnia F., de Almeida Júnior J.N., Ilkit M., et al. Worldwide emergence of fluconazole-resistant Candida parapsilosis: current framework and future research roadmap. Lancet Microbe. 2023;4:e470. doi: 10.1016/S2666-5247(23)00067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rajni E., Singh A., Tarai B., et al. Open Forum Infectious Diseases; 2021. A high frequency of Candida auris blood stream infections in COVID-19 patients admitted to intensive care units, North-western India: a case control study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Henao-Martínez A.F., Gross L., McNair B., et al. Risk factors for cryptococcal meningitis: a single United States center experience. Mycopathologia. 2016;181(11-12):807–814. doi: 10.1007/s11046-016-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khan Z.U. Smoking, melanization, and cryptococcosis: is there a connection? J Clin Microbiol. 2006;44(3):1207. doi: 10.1128/JCM.44.3.1207.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thompson G.R., III, Jeffrey D.J., Baddley J.W., Lewis J.S., II, et al. Fungal endocarditis: pathophysiology, epidemiology, clinical presentation, diagnosis, and management. Clin Microbiol Rev. 2023;36(3) doi: 10.1128/cmr.00019-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Meyerowitz E.A., Sanchez S., Mansour M.K., Triant V.A., Goldberg M.B. Isolated cerebral mucormycosis in immunocompetent adults who inject drugs: case reports and systematic review of the literature. Open Forum Infect Dis. 2020;7(12) doi: 10.1093/ofid/ofaa552. ofaa552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hazama A., Galgano M., Fullmer J., Hall W., Chin L. Affinity of mucormycosis for basal ganglia in intravenous drug users: case illustration and review of literature. World Neurosurg. 2017;98:872.e1–872.e3. doi: 10.1016/j.wneu.2016.11.130. [DOI] [PubMed] [Google Scholar]
- 136.Woods K.F., Hanna B.J. Brain stem mucormycosis in a narcotic addict with eventual recovery. Am J Med. 1986;80(1):126–128. doi: 10.1016/0002-9343(86)90062-8. [DOI] [PubMed] [Google Scholar]
- 137.Rosenstein N.E., Emery K.W., Werner S.B., et al. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995-1996. Clin Infect Dis. 2001;32(5):708–715. doi: 10.1086/319203. [DOI] [PubMed] [Google Scholar]
- 138.Leake J.A., Mosley D.G., England B., et al. Risk factors for acute symptomatic coccidioidomycosis among elderly persons in Arizona, 1996-1997. J Infect Dis. 2000;181(4):1435–1440. doi: 10.1086/315400. [DOI] [PubMed] [Google Scholar]
- 139.dos Santos W.A., da Silva B.M., Passos E.D., Zandonade E., Falqueto A. [Association between smoking and paracoccidioidomycosis: a case-control study in the State of Espírito Santo, Brazil] Cad Saúde Pública. 2003;19(1):245–253. doi: 10.1590/s0102-311x2003000100027. [DOI] [PubMed] [Google Scholar]
- 140.Kagen S.L. Aspergillus: an inhalable contaminant of marihuana. N Engl J Med. 1981;304(8):483–484. doi: 10.1056/NEJM198102193040812. [DOI] [PubMed] [Google Scholar]
- 141.Benedict K., Thompson G.R., 3rd, Jackson B.R. Cannabis use and fungal infections in a commercially insured population, United States, 2016. Emerg Infect Dis. 2020;26(6):1308–1310. doi: 10.3201/eid2606.191570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thompson G.R., 3rd, Tuscano J.M., Dennis M., et al. A microbiome assessment of medical marijuana. Clin Microbiol Infect. 2017;23(4):269–270. doi: 10.1016/j.cmi.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 143.Kupelian C., Kim A., Vijayan V. E-cigarette or vaping product use-associated lung injury complicated by pulmonary aspergillosis. Cureus. 2021;13(12) doi: 10.7759/cureus.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alanazi H., Semlali A., Chmielewski W., Rouabhia M. E-cigarettes increase Candida albicans growth and modulate its interaction with gingival epithelial cells. Int J Environ Res Public Health. 2019;16(2) doi: 10.3390/ijerph16020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pappagianis D. Coccidioidomycosis in California state correctional institutions. Ann N Y Acad Sci. 2007;1111:103–111. doi: 10.1196/annals.1406.011. [DOI] [PubMed] [Google Scholar]
- 146.Lee L.A., Yuan J., Vugia D., Wheeler C., Chapnick R., Mohle-Boetani J. Increased coccidioidomycosis among inmates at a California prison: initial investigation in 2005 to 2006. J Correct Health Care. 2017;23(3):347–352. doi: 10.1177/1078345817716451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Benedict K., Purfield A.E., Mohle-Boetani J., Wheeler C., Park B.J. Awareness and environmental exposures related to coccidioidomycosis among inmates at two California prisons, 2013. J Correct Health Care. 2016;22(2):157–163. doi: 10.1177/1078345816635577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wheeler C., Lucas K.D., Derado G., et al. Risk stratification with coccidioidal skin test to prevent valley fever among inmates, California, 2015. J Correct Health Care. 2018;24(4):342–351. doi: 10.1177/1078345818792679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.U.S. Department of Justice Figure 2. U.S. Incarceration rates by race and sex. https://nij.ojp.gov/media/image/19511 Available at:
- 150.Binswanger I.A., Krueger P.M., Steiner J.F. Prevalence of chronic medical conditions among jail and prison inmates in the USA compared with the general population. J Epidemiol Community Health. 2009;63(11):912–919. doi: 10.1136/jech.2009.090662. [DOI] [PubMed] [Google Scholar]
- 151.Dumont D.M., Brockmann B., Dickman S., Alexander N., Rich J.D. Public health and the epidemic of incarceration. Annu Rev Public Health. 2012;33:325–339. doi: 10.1146/annurev-publhealth-031811-124614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Spaulding A.C., Seals R.M., Page M.J., Brzozowski A.K., Rhodes W., Hammett T.M. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS One. 2009;4(11) doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bilukha O.O., Brennan M., Woodruff B.A. Death and injury from landmines and unexploded ordnance in Afghanistan. JAMA. 2003;290(5):650–653. doi: 10.1001/jama.290.5.650. [DOI] [PubMed] [Google Scholar]
- 154.Rodriguez C.J., Ganesan A., Shaikh F., et al. Combat-related invasive fungal wound infections. Mil Med. 2022;187(Supplement_2):34–41. doi: 10.1093/milmed/usab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tribble D.R., Rodriguez C.J., Weintrob A.C., et al. Environmental factors related to fungal wound contamination after combat trauma in Afghanistan, 2009-2011. Emerg Infect Dis. 2015;21(10):1759–1769. doi: 10.3201/eid2110.141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tribble D.R., Ganesan A., Rodriguez C.J. Combat trauma-related invasive fungal wound infections. Curr Fungal Infect Rep. 2020;14(2):186–196. doi: 10.1007/s12281-020-00385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ganesan A., Shaikh F., Bradley W., et al. Classification of trauma-associated invasive fungal infections to support wound treatment decisions. Emerg Infect Dis. 2019;25(9):1639–1647. doi: 10.3201/eid2509.190168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Walsh T.J., Hospenthal D.R., Petraitis V., Kontoyiannis D.P. Necrotizing mucormycosis of wounds following combat injuries, natural disasters, burns, and other trauma. J Fungi (Basel) 2019;5(3) doi: 10.3390/jof5030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reischies F., Hoenigl M. The role of surgical debridement in different clinical manifestations of invasive aspergillosis. Mycoses. 2014;57(Suppl 2):1–14. doi: 10.1111/myc.12224. [DOI] [PubMed] [Google Scholar]
- 160.Akers K.S., Rowan M.P., Niece K.L., et al. Antifungal wound penetration of amphotericin and voriconazole in combat-related injuries: case report. BMC Infect Dis. 2015;15:184. doi: 10.1186/s12879-015-0918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Menjibar D. 2023. The global struggle against mycetoma is paralyzed by the conflict in Sudan. El Pais.https://english.elpais.com/international/2023-06-28/the-global-struggle-against-mycetoma-is-paralyzed-by-the-conflict-in-sudan.html Available at: [Google Scholar]
- 162.Khan S.S., Padovese V., Maurer T.A., et al. A skin disease and needs assessment analysis of the displaced Rohingya population in the Kutupalong refugee camp, Bangladesh. Clin Exp Dermatol. 2020;45(8):1051–1054. doi: 10.1111/ced.14310. [DOI] [PubMed] [Google Scholar]
- 163.Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps) Int J Dermatol. 2019;58(11):1341–1349. doi: 10.1111/ijd.14619. [DOI] [PubMed] [Google Scholar]
- 164.Saikal S.L., Ge L., Mir A., et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34(2):419–425. doi: 10.1111/jdv.15909. [DOI] [PubMed] [Google Scholar]
- 165.Ching A.H., Tay T., Brown B., Mohareb A.M., Sethi A., Annamalai A., et al. Dermatologic Conditions of adult refugees followign resettlement in the United States, 2015 to 2018. J Migr Health. 2023;31 doi: 10.1016/j.jmh.2023.100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.U.S. Department of Agriculture Definitions of food security. https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-u-s/definitions-of-food-security/ Available at:
- 167.Food and Agriculture Organization of the United Nations The state of food security and nutrition in the world 2022. https://www.fao.org/3/cc0639en/cc0639en.pdf Available at:
- 168.Hughes W.T., Price R.A., Sisko F., et al. Protein-calorie malnutrition. A host determinant for Pneumocystis carinii infection. Am J Dis Child. 1974;128(1):44–52. doi: 10.1001/archpedi.1974.02110260046008. [DOI] [PubMed] [Google Scholar]
- 169.Singh B.K., Delgado-Baquerizo M., Egidi E., et al. Climate change impacts on plant pathogens, food security and paths forward. Nat Rev Microbiol. 2023:1–17. doi: 10.1038/s41579-023-00900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fry W.E., Birch P.R., Judelson H.S., et al. Five reasons to consider Phytophthora infestans a Reemerging pathogen. Phytopathology. 2015;105(7):966–981. doi: 10.1094/PHYTO-01-15-0005-FI. [DOI] [PubMed] [Google Scholar]
- 171.Arie T. Fusarium diseases of cultivated plants, control, diagnosis, and molecular and genetic studies. J Pestic Sci. 2019;44(4):275–281. doi: 10.1584/jpestics.J19-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Perincherry L., Lalak-Kańczugowska J., Stępień Ł. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins. 2019;11(11) doi: 10.3390/toxins11110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Godfray H.C., Mason-D'Croz D., Robinson S. Food system consequences of a fungal disease epidemic in a major crop. Philos Trans R Soc Lond B Biol Sci. 2016;371(1709) doi: 10.1098/rstb.2015.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fisher M.C., Henk D.A., Briggs C.J., et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484(7393):186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Almeida F., Rodrigues M.L., Coelho C. The still underestimated problem of fungal diseases worldwide. Front Microbiol. 2019;10:214. doi: 10.3389/fmicb.2019.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fisher M.C., Alastruey-Izquierdo A., Berman J., et al. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol. 2022;20(9):557–571. doi: 10.1038/s41579-022-00720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bastos R.W., Rossato L., Goldman G.H., Santos D.A. Fungicide effects on human fungal pathogens: cross-resistance to medical drugs and beyond. PLoS Pathog. 2021;17(12) doi: 10.1371/journal.ppat.1010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.U.S. Department of Labor . Occupational Safety and Health Administration; 2023. A brief guide to mold in the workplace. SHIB 03-10-10 A brief guide to mold in the Workplace–English.osha.gov Available at: [Google Scholar]
- 179.Sehulster L., Chinn R.Y., Cdc, Hicpac Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the healthcare infection control practices advisory committee (HICPAC) MMWR Recomm Rep (Morb Mortal Wkly Rep) 2003;52(RR-10):1–42. [PubMed] [Google Scholar]
- 180.Nihtinen A., Anttila V.J., Richardson M., Meri T., Volin L., Ruutu T. The utility of intensified environmental surveillance for pathogenic moulds in a stem cell transplantation ward during construction work to monitor the efficacy of HEPA filtration. Bone Marrow Transplant. 2007;40(5):457–460. doi: 10.1038/sj.bmt.1705749. [DOI] [PubMed] [Google Scholar]
- 181.World Health Organization WHO model list of essential medicines–22nd list, 2021. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 Available at:
- 182.Salmanton-Garcia J., Au W.Y., Hoenigl M., et al. The current state of laboratory mycology in Asia/Pacific: a survey from the European confederation of medical mycology (ECMM) and international society for human and animal mycology (ISHAM) Int J Antimicrob Agents. 2023;61(3) doi: 10.1016/j.ijantimicag.2023.106718. [DOI] [PubMed] [Google Scholar]
- 183.Salmanton-Garcia J., Hoenigl M., Gangneux J.P., et al. The current state of laboratory mycology and access to antifungal treatment in Europe: a European Confederation of Medical Mycology survey. Lancet Microbe. 2023;4(1):e47–e56. doi: 10.1016/S2666-5247(22)00261-0. [DOI] [PubMed] [Google Scholar]
- 184.Milani B., Dagne D.A., Choi H.L., Schito M., Stone H.A. Diagnostic capacities and treatment practices on implantation mycoses: results from the 2022 WHO global online survey. PLoS Negl Trop Dis. 2023;17(6) doi: 10.1371/journal.pntd.0011443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chang C.C., Hall V., Cooper C., et al. Consensus guidelines for the diagnosis and management of cryptococcosis and rare yeast infections in the haematology/oncology setting, 2021. Intern Med J. 2021;51(Suppl 7):118–142. doi: 10.1111/imj.15590. [DOI] [PubMed] [Google Scholar]
- 186.Perfect J.R., Dismukes W.E., Dromer F., et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cornely O.A., Alastruey-Izquierdo A., Arenz D., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thompson G.R., 3rd, Le T., Chindamporn A., et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European confederation of medical mycology in cooperation with the international society for human and animal mycology. Lancet Infect Dis. 2021;21(12):e364–e374. doi: 10.1016/S1473-3099(21)00191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hoenigl M., Salmanton-García J., Walsh T.J., et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European confederation of medical mycology in cooperation with the international society for human and animal mycology and the American society for microbiology. Lancet Infect Dis. 2021;21(8):e246–e257. doi: 10.1016/S1473-3099(20)30784-2. [DOI] [PubMed] [Google Scholar]
- 190.Ariza-Heredia E.J., Kontoyiannis D.P. Our recommendations for avoiding exposure to fungi outside the hospital for patients with haematological cancers. Mycoses. 2014;57(6):336–341. doi: 10.1111/myc.12167. [DOI] [PubMed] [Google Scholar]
- 191.Centers for Disease Control and Prevention Division of preparedness and emerging infections (DPEI) https://www.cdc.gov/ncezid/dpei/index.html Available at:
- 192.Ammon A., Faensen D. [Surveillance of infectious diseases at the EU level] Bundesgesundheitsblatt Gesundheitsforsch Gesundheitsschutz. 2009;52(2):176–182. doi: 10.1007/s00103-009-0759-y. [DOI] [PubMed] [Google Scholar]
- 193.Nkengasong J.N., Maiyegun O., Moeti M. Establishing the Africa centres for disease control and prevention: responding to Africa's health threats. Lancet Glob Health. 2017;5(3):e246–e247. doi: 10.1016/S2214-109X(17)30025-6. [DOI] [PubMed] [Google Scholar]
- 194.Sharpe I., Davison C.M. A scoping review of climate change, climate-related disasters, and mental disorders among children in low- and middle-income countries. Int J Environ Res Public Health. 2022;19(5) doi: 10.3390/ijerph19052896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Birkmann J., Jamshed A., McMillan J.M., et al. Understanding human vulnerability to climate change: a global perspective on index validation for adaptation planning. Sci Total Environ. 2022;803 doi: 10.1016/j.scitotenv.2021.150065. [DOI] [PubMed] [Google Scholar]
- 196.Gray L. Social determinants of health, disaster vulnerability, severe and morbid obesity in adults: triple Jeopardy? Int J Environ Res Public Health. 2017;14(12) [Google Scholar]
- 197.Raduszynski T., Numada M. Measure and spatial identification of social vulnerability, exposure and risk to natural hazards in Japan using open data. Sci Rep. 2023;13(1):664. doi: 10.1038/s41598-023-27831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fan J., Huang G. Are women more vulnerable to flooding than men in an aging Japanese society? Int J Environ Res Public Health. 2023;20(2) doi: 10.3390/ijerph20021299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cornelius A.P., Char D.M., Doyle C., et al. Disparities in disaster healthcare: a review of past disasters. Am J Disaster Med. 2022;17(2):171–184. doi: 10.5055/ajdm.2022.0431. [DOI] [PubMed] [Google Scholar]
- 200.Pyda J., Patterson R.H., Caddell L., et al. Towards resilient health systems: opportunities to align surgical and disaster planning. BMJ Glob Health. 2019;4(3) doi: 10.1136/bmjgh-2019-001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wood E., Frazier T. Disasters, community vulnerability, and poverty: the intersection between economics and emergency management. J Emerg Manag. 2021;19(3):227–233. doi: 10.5055/jem.0563. [DOI] [PubMed] [Google Scholar]