Abstract
Estrogen is an immunoregulatory agent, in that hormone deprivation increases while 17β-estradiol (E2) administration blocks the inflammatory response; however, the underlying mechanism is still unknown. The transcription factor p65/relA, a member of the nuclear factor κB (NF-κB) family, plays a major role in inflammation and drives the expression of proinflammatory mediators. Here we report a novel mechanism of action of E2 in inflammation. We observe that in macrophages E2 blocks lipopolysaccharide-induced DNA binding and transcriptional activity of p65 by preventing its nuclear translocation. This effect is selectively activated in macrophages to prevent p65 activation by inflammatory agents and extends to other members of the NF-κB family, including c-Rel and p50. We observe that E2 activates a rapid and persistent response that involves the activation of phosphatidylinositol 3-kinase, without requiring de novo protein synthesis or modifying Iκ-Bα degradation and mitogen-activated protein kinase activation. Using a time course experiment and the microtubule-disrupting agent nocodazole, we observe that the hormone inhibits p65 intracellular transport to the nucleus. This activity is selectively mediated by estrogen receptor alpha (ERα) and not ERβ and is not shared by conventional anti-inflammatory drugs. These results unravel a novel and unique mechanism for E2 anti-inflammatory activity, which may be useful for identifying more selective ligands for the prevention of the inflammatory response.
17β-Estradiol (E2) has been shown to oppose the onset of the inflammatory reaction in several animal models of inflammation-associated pathological conditions. A paradigmatic model of this effect is experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis, a chronic inflammatory disease of the central nervous system; low-dose estrogen therapy was shown to prevent the clinical signs and histopathological lesions of the disease (3, 22, 23). In addition, other inflammatory pathological conditions, such as wound healing (1), atherosclerosis (for a review, see reference 18), ischemia (10), uveitis (28), and leukodystrophy (27), were shown to be influenced by E2, which decreased disease susceptibility, severity, and damage. In support of this in vivo evidence, results obtained from cell culture studies have shown that E2 down-regulates the expression of inflammatory genes, such as those coding for the inducible form of nitric oxide synthase (iNOS) or matrix metalloprotease 9, enzymes directly involved in the progression of the inflammatory response, and inhibits the biochemical and morphological activation of macrophages (6, 17, 43). Thus, evidence accumulated so far is uniformly concordant in identifying estradiol as a protective agent against the induction of the inflammatory response.
Estrogens interact with two specific receptors, estrogen receptor (ER) α (ERα) and ERβ, that belong to the superfamily of ligand-activated transcription factors. With ER-knockout mice, it was possible to show that ERα mediates the effects of E2 against immunogenic (31), ischemic (10), and inflammatory (42) insults, thus identifying the specific intracellular mediator of hormone action. The activation of ERs has been shown to involve the regulation of gene transcription as well as the modulation of signal transduction pathways through a nongenomic activity that involves the interaction of ERs with cytoplasmic kinases (29). However, which one of these mechanisms is involved in the immunomodulatory activity of ERs still needs to be elucidated.
Nuclear factor κB (NF-κB) comprises a family of inducible transcription factors that play a key role in the immediate-early process of the inflammatory response. Inactive NF-κB is located in the cytoplasmic compartment in association with the Iκ-Bα inhibitory protein. Upon stimulation by inflammatory signals, such as lipopolysaccharide (LPS), Iκ-Bα is phosphorylated by Iκ-B kinase (IKK) and degraded by the proteasome complex; the dissociation of Iκ-Bα unmasks the nuclear localization sequence of NF-κB, which translocates to the nucleus. Upon binding to its cognate DNA response elements, NF-κB induces the expression of proteins, such as cytokines, chemokines, and cell adhesion molecules, that trigger the inflammatory response. The central role played by NF-κB in several biological pathways that govern immunity suggests that the modulation of NF-κB subcellular localization and activation must be strictly controlled, as dysregulation of these processes may lead to aberrant gene expression (4, 25).
In view of the important role of NF-κB as a central mediator of inflammation, selective inhibitors of NF-κB are efficacious anti-inflammatory agents. Drugs that are presently used to treat inflammatory states may exert some of their functions by inhibiting NF-κB activation through three main mechanisms. (i) The first mechanism is the inhibition of Iκ-Bα phosphorylation and degradation; the nonsteroidal anti-inflammatory drugs (NSAIDs) aspirin, sulfasalazine, and sulindac (45-47), the cyclopentenone prostaglandin 15dPGJ2 (34), and natural compounds, such as resveratrol (19), were shown to inhibit IKK activity, decreasing Iκ-B phosphorylation and degradation. (ii) The second mechanism is the repression of NF-κB activity on inflammatory gene transcription; other members of the nuclear receptor superfamily, namely, the glucocorticoid receptor (GR) and peroxisome proliferator-activated receptor (PPAR) gamma (PPARγ) and PPARα, once activated by their cognate ligands, interfere with NF-κB at the transcriptional level through direct protein-protein interactions with NF-κB or with the basal transcription machinery (8, 30, 32, 37). (iii) The third mechanism is the induction of Iκ-Bα expression; as proposed by Auphan et al. (2), the induction of Iκ-Bα expression by GR leads to NF-κB export from the nucleus and to its subsequent inactivation through its cytoplasmic localization (20, 36).
Because of the key role played by NF-κB in inflammation and because NF-κB is a target of nuclear receptors, we examined whether ER anti-inflammatory activity occurs through the inhibition of NF-κB. Using the RAW 264.7 macrophage cell line and primary cultures of microglia stimulated with inflammatory agents, we demonstrate in this study that E2 inhibits NF-κB activation through a mechanism different from that of conventional NF-κB-inhibiting drugs. In fact, E2 acts by preventing NF-κB nuclear translocation induced by LPS or tumor necrosis factor alpha (TNF-α), without altering IKK activity. We also demonstrate that this mechanism is specifically mediated by ERα through a nongenomic mechanism, thus indicating that the E2-ERα signaling pathway plays a central role in the immediate-early inflammatory response.
MATERIALS AND METHODS
Animals.
This study was conducted according to the guidelines of the institutional animal care committee of the University of Milan. ERα- and ERβ-null mice have a C57BL/6 genetic background. The generation of these animals has been described elsewhere (11).
Reagents.
Unless otherwise specified, chemicals were purchased from Merck (Darmstadt, Germany), and culture media and additives were obtained from Life Technologies-Invitrogen (Paisley, Scotland, United Kingdom). E2, LPS (isotype 0.111:B4), actinomycin D, PD098059, wortmannin, LY294002, indomethacin, dexamethasone, nocodazole (NCZ), WY14,643, ibuprophen, acetylsalicylic acid, raloxifene, tamoxifen, and ICI 182,780 were obtained from Sigma (Milan, Italy).
Cell culture.
RAW 264.7 cells were purchased from the American Type Culture Collection (Manassas, Va.) and grown in Dulbecco minimal essential medium (DMEM)-10% fetal bovine serum (FBS) (Celbio, EuroClone, Milan, Italy) supplemented with 2 g of sodium carbonate/liter, 0.11 g of sodium pyruvate/liter, and streptomycin-penicillin (50,000 IU plus 50 mg per liter) in a humidified 5% CO2-95% air atmosphere at 37°C.
SK-ER3 cells were obtained as described previously (26). SK-ER3 and MCF-7 cells were grown at 37°C in RPMI 1640 medium (Sigma) supplemented with 10% FBS-dextran-coated charcoal (DCC), 1% nonessential amino acids, 5 mM l-glutamine, and streptomycin-penicillin at 37°C in a humidified 5% CO2-95% air atmosphere. For the experiments, cells were incubated in serum-free medium for 6 h, treated with 1 nM E2, and then treated with LPS (50 μg/ml), TNF-α (10 ng/ml), or a cytokine mixture (10 ng of gamma interferon/ml, 10 ng of interleukin 1β/ml, 10 ng of TNF-α/ml, and 10 μg of LPS/ml).
Primary cultures of microglia.
Microglia were isolated from cultures of newborn mouse brains as previously described (43). Briefly, cerebral cortices were isolated from 2-day-old mice and dissociated in 1 mg of DNase (Sigma)/ml. Mixed glial cells were grown in minimal essential medium (MEM)-0.6% glucose supplemented with 20% FBS (Celbio, EuroClone), 1% nonessential amino acids, and streptomycin-penicillin at 37°C in a humidified 5% CO2-95% air atmosphere at a density of 3 × 105 cells/ml. Cells were cultured for 8 to 10 days, and the medium was replaced every 3 days. Two days before experiments, cells were harvested by gentle shaking at 37°C for 90 min and plated on MEM-10% FBS at a density of 2 × 105 cells/ml. After 24 h, the medium was replaced with DMEM-10% FBS DCC. On the next day, serum-free DMEM was added, and cells were incubated for 6 h and then treated as specified in the figure legends.
Chromatin immunoprecipitation.
RAW 264.7 cells were fixed with 1% formaldehyde and lysed in 50 mM Tris-HCl (pH 8)-5 mM EDTA-1% sodium dodecyl sulfate (SDS)-protease inhibitors (Roche, Basel, Switzerland). Extracts were sonicated to shear chromatin. After centrifugation, supernatants were precleared at 4°C for 30 min with salmon sperm DNA-protein A-Sepharose (Amersham Biosciences, Uppsala, Sweden) and then diluted 10-fold in dilution buffer (50 mM Tris-HCl [pH 8], 5 mM EDTA, 200 mM NaCl, 0.5% NP-40) and immunoprecipitated overnight at 4°C with 2 μg of anti-p65 rabbit polyclonal antibody (Santa Cruz, Santa Cruz, Calif.). Immunoprecipitation with rabbit immunoglobulin G (IgG) was performed to evaluate the presence of nonspecific interactions, and aliquots of genomic DNA were analyzed by PCR before immunoprecipitation to normalize for DNA input. Immunocomplexes were collected with 60 μl of salmon sperm DNA-protein A-Sepharose for 30 min at 4°C. Pellets were washed with 20 mM Tris-HCl (pH 8)-2 mM EDTA-0.1% SDS-500 mM NaCl-1% NP-40 and eluted with 1 mM EDTA-10 mM Tris-HCl [pH 8]-2% SDS. DNA-protein cross-linking was reversed overnight at 65°C in 0.2 M NaCl. Proteins were digested for 2 h at 50°C with proteinase K.
Phenol-extracted and ethanol-precipitated DNA was analyzed by semiquantitative PCR with specific primers (MWG Biotech, Ebersberg, Germany) that amplify NF-κB-responsive elements (κB-RE) present in the promoters of the mouse Iκ-Bα (5′-CGC TAA GAG GAA CAG CCT AG-3′ and 5′-CAG CTG GCT GAA ACA TGG C-3′; product, 480 bp), macrophage inflammatory protein 2 (MIP-2) (5′-CAA CAG TGT ACT TAC GCA GAC G-3′ and 5′-CTA GCT GCC TGC CTC ATT CTA C-3′; product, 300 bp), and iNOS (5′-GCA TGA GGA TAC ACC ACA G-3′ and 5′-TGC ATA ACT GTT CCC AAA GG-3′; product, 120 bp) genes. PCR with a Perkin-Elmer thermal cycler 480 was performed with immunoprecipitated or genomic DNA as follows: 95°C for 30 s and then 28 cycles at 94°C for 1 min, 60°C for 45 s, and 72°C for 2 min. As a negative control, PCR was performed with specific primers (5′-TCC CTC TCT CTG TTT GTT CC-3′ and 5′-GCT GAT CCC TGA GTT TGG-3′; product, 300 bp) that amplify the iNOS promoter at a location that does not contain a κB site.
Reverse transcription (RT)-PCR. (i) RNA preparation.
RNA was extracted from RAW 264.7 cells. All samples were resuspended in TRIzol reagent (Invitrogen, Milan, Italy), and RNA was isolated according to the manufacturer's instructions.
(ii) cDNA preparation.
One microgram of RNA was used for cDNA preparation with Moloney murine leukemia virus reverse transcriptase (Promega, Milan, Italy) as previously described (43). Control reactions without the addition of the enzyme were performed for each sample.
(iii) PCR.
PCR was performed with 1 μl of cDNA and 0.4 U of DynaZyme DNA polymerase (Finnzymes Oy, Espoo, Finland). The following primers (MWG Biotech) were used: mouse MIP-2 (5′-CCA GGT CAG TTA GCC TTG-3′ and 5′-TGG CCA GTG AAC TGC GCT G-3′; product, 219 bp), mouse Iκ-Bα (5′-CGT GGC TAG CAA CAT CCA G-3′ and 5′-TCC TCT TGG TCT GAG TCT G-3′; product, 482 bp), and mouse iNOS (5′-TTT GAC CAG AGG ACC CAG AG-3′ and 5′-ATG GCC GAC CTG ATG TTG CC-3′; product, 340 bp). Amplification of the constitutively expressed enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed in parallel to assess RT-PCR efficiency. PCR with a Perkin-Elmer thermal cycler 480 was performed at 95°C for 30 s and then for 28 cycles at 94°C for 1 min, 60°C for 45 s, and 72°C for 2 min.
EMSAs.
RAW 264.7 cells were lysed in 0.4 ml of ice-cold hypotonic lysis buffer 1 (10 mM HEPES [pH 7.8], 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol [DTT], 0.1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], protease inhibitors). After 15 min, 25 μl of 10% NP-40 was added; nuclei were collected at 10,000 × g for 1 min, resuspended in 30 μl of hypertonic extraction buffer (50 mM HEPES [pH 7.8], 50 mM KCl, 300 mM NaCl, 0.1 mM PMSF), and centrifuged at 10,000 × g for 10 min. Supernatants were subjected to electrophoretic mobility shift assays (EMSAs). Single-stranded oligonucleotides (forward, 5′-TGG GGA CTC TCC-3′; complement, 5′-AAG GGA GAG TCC-3′; MWG Biotech) corresponding to the NF-κB binding site in the 5′-flanking region of the mouse iNOS gene were annealed at 95°C for 5 min, filled in with [α-32]dCTP (10 μCi/μl; Amersham, Buckinghamshire, England), deoxynucleoside triphosphates (Pharmacia, Milan, Italy), and the Klenow fragment of DNA polymerase I (Roche, Basel, Switzerland) for 40 min at 37°C, and then purified with a Sephadex G-25 column (Roche). Nuclear proteins (10 μg) were incubated with 10,000 cpm of 32P-labeled NF-κB-specific double-stranded oligonucleotide and poly(dI-dC) (0.1 μg/μl) in EMSA buffer (10 mM Tris-HCl [pH 7.5], 2% glycerol, 0.2 mM EDTA, 0.5 mM DTT, 50 mM NaCl) for 30 min at room temperature and subjected to electrophoresis on a 5% polyacrylamide gel. The gel was dried and autoradiographed.
Immunocytochemical analysis.
RAW 264.7 cells and microglia were grown on glass coverslips in a 24-well plate for 2 days, treated as specified in each figure legend, and then fixed for 10 min with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) (pH 7.5) at room temperature. Cells were washed three times with PBS and incubated for 30 min at room temperature with blocking solution (10% goat serum, 1% bovine serum albumin, 0.5% Tween 20 in PBS). Cells were incubated with a 1:500 dilution of anti-p65 or anti-c-Rel polyclonal antibody (Santa Cruz) or antitubulin monoclonal antibody (Sigma) in 1% goat serum overnight at 4°C. Cells were washed and incubated with the secondary antibody. For immunofluorescence, Alexa-Fluor 488- or 568-conjugated goat anti-rabbit secondary antibody (Molecular Probes, Leiden, The Netherlands) was added and incubated for 60 min at room temperature. Nuclei were counterstained with 1:500,000 Sytox orange (Molecular Probes). Coverslips were mounted in 50% glycerol. p65 immunofluorescence was imaged with a Radiance 2100 confocal laser scanning microscope (Bio-Rad, Milan, Italy) based on an Eclipse TE2000-S microscope (Nikon, Milan, Italy) and operating in the simultaneous-acquisition mode. For light microscopy, cells were incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, Calif.) and stained with avidin-biotin-horseradish peroxidase complex (ABC kit; Vector Laboratories) and 3,3′-diaminobenzidine substrate (Sigma). After immunodetection, coverslips were mounted in 50% glycerol and observed with an Axioscope microscope (Zeiss, Milan, Italy). A semiquantitative analysis of cytoplasmic p65 was obtained by counting 50 cells/coverslip. Experiments were done in duplicate, and cells were counted in a blind manner.
Western blotting.
RAW 264.7 cells were washed with ice-cold PBS and harvested in 0.1 ml of ice-cold hypotonic lysis buffer 2 (10 mM Tris-HCl [pH 7.8], 5 mM MgCl2, 0.3 mM EGTA, 10 mM KCl, 1 mM DTT, 0.1 mM PMSF, protease inhibitors). After 15 min of incubation, 25 μl of NP-40 was added, and the mixture was centrifuged at 10,000 × g for 1 min. The supernatant was the cytosolic extract. The nuclear pellets were collected, resuspended in 30 μl of hypertonic extraction buffer, and centrifuged at 10,000 × g for 10 min. The supernatant was the nuclear extract. The protein contents in cytosolic and nuclear lysates were determined by the Bradford assay (Pierce Chemical Co., Rockford, Ill.). Proteins (20 μg) were separated on an SDS-7.5% polyacrylamide gel and transferred to a Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane (Amersham). The membrane was blocked for 45 min with 5% skim milk in 20 mM Tris (pH 7.5)-150 mM NaCl-0.2% Tween 20. Rabbit polyclonal antibodies for murine p65, p50, and Iκ-Bα (Santa Cruz), for β-actin and histone H1 (Sigma), and for phosphorylated IKKα/β, IKK, phosphorylated extracellular signal-regulated kinase (ERK), and ERK (Cell Signaling, Beverly, Mass.) were applied at 1:500 dilutions and incubated at 4°C overnight. After extensive washing with 20 mM Tris (pH 7.5)-150 mM NaCl-0.2% Tween 20, the secondary antibody was applied at a 1:2,000 dilution and incubated for 60 min. Exposure was carried out with an ECL kit (Amersham).
PI3K assays.
RAW 264.7 cells were lysed in lysis buffer 3 (20 mM Tris [pH 8], 137 mM NaCl, 1% Triton, 2 mM EDTA, 1 mM Na3VO4, 10% glycerol, 1 mM PMSF, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml). Immunoprecipitation was performed with clarified cell lysates (40,000 × g for 35 min at 4°C) and rabbit polyclonal antibody to phosphatidylinositol 3-kinase (PI3K) p85 (Upstate Biotechnology Inc., Lake Placid, N.Y.). Equal amounts of proteins were incubated with anti-p85 antibody overnight at 4°C, followed by 1 h of incubation in the presence of 50 μl of protein A-Sepharose (Amersham Biosciences) at 4°C with mixing. Immunoprecipitates were washed with lysis buffer 3 and with Tris-HCl (pH 7.4) and incubated with phosphatidylinositol (Sigma) in kinase buffer (30 mM HEPES, 30 mM MgCl2, 50 μM ATP, 10 μCi of [γ-32P]ATP) at room temperature for 15 min. The reaction was stopped with 0.1 N HCl. Lipids were extracted with 200 μl of chloroform-methanol (1:1) and separated on potassium oxalate-pretreated thin-layer chromatography (TLC) plates by development with chloroform-methanol-water-ammonium hydroxide (112:88:19:6). After being dried, plates were exposed for autoradiography. Radioactive spots were analyzed with a phosphorimager apparatus (Amersham Biosciences), and the amount of phosphatidylinositol 3,4,5-triphosphate (PIP3) was calculated as a percentage of the total optical density units.
Statistical analysis.
Values are given as means ± standard deviations (SDs). P values were calculated by analysis of variance followed by the Bonferroni test.
RESULTS
Control of p65 DNA binding activity by E2.
In an attempt to discern whether E2 acts by interfering with the transcriptional activity of NF-κB, like glucocorticoids and PPAR ligands, or by inhibiting NF-κB activation upstream of its nuclear activity, like some NSAIDs, we first analyzed the DNA binding activity of NF-κB induced by LPS and examined whether E2 was able to modulate this event. We focused on p65, a member of the NF-κB family which is strictly involved in macrophage activation. Using EMSAs of nuclear extracts from RAW 264.7 macrophages, we observed that the binding of p65 to its DNA-responsive elements induced by LPS was prevented by a 10-min exposure of the cells to E2 prior to the inflammatory stimulus (Fig. 1A, cf. lanes 6 and 3). The addition of a primary antibody against p65 to the reaction further decreased the mobility of the radiolabeled band, confirming the presence of this transcription factor in the DNA-protein complex obtained.
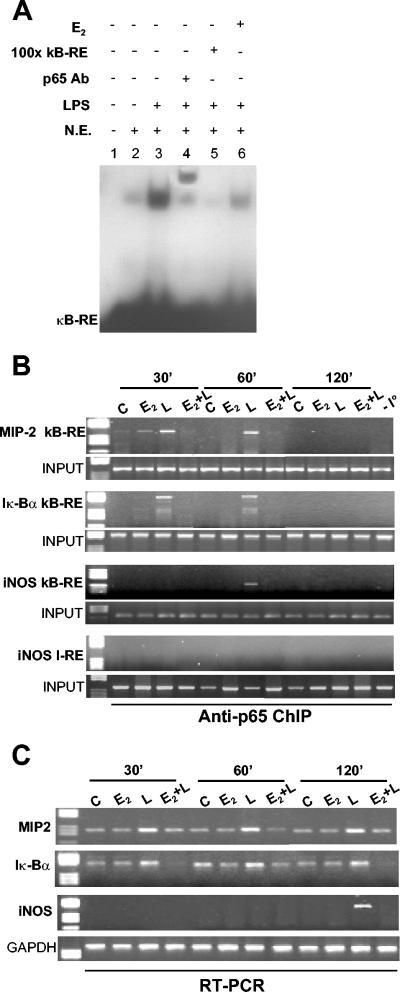
FIG. 1.
p65 DNA binding activity induced by LPS is inhibited by E2. (A) In vitro DNA binding activity of NF-κB. An EMSA was performed with radiolabeled κB-RE incubated with nuclear extracts (N.E.) from RAW 264.7 cells treated with 1 nM E2 for 10 min followed by the addition of LPS for an additional 30 min. For supershift and competition assays, p65 antibody (Ab) (lane 4) and a 100-fold excess ofunlabeled κB-RE (lane 5) were used, respectively. (B) In vivo DNA binding activity of p65. A chromatin immunoprecipitation (ChIP) assay of RAW 264.7 cells was performed in the absence (C) or presence (E2) of 1 nM E2 for 10 min and then with LPS (L and E2+L) at 50 μg/ml for 30, 60, or 120 min. Immunoprecipitated DNA was analyzed by PCR to amplify MIP-2, Iκ-Bα, and iNOS promoters at κB-RE. PCRs performed with genomic DNA before immunoprecipitation (INPUT) or DNA immunoprecipitated with nonspecific IgG (−I°) or with primers that amplify iNOS promoter DNA that does not contain κB-RE (I-RE) are shown. (C) RT-PCR analysis of MIP-2, Iκ-Bα, and iNOS mRNA levels in cells treated as described for panel B. Each experiment was repeated at least three times.
To determine whether E2 also interfered in vivo with the DNA binding activity of p65, we analyzed the occupancy of target gene promoters by p65 and their expression levels by using chromatin immunoprecipitation and RT-PCR assays, respectively. The promoters of the genes encoding Iκ-Bα and MIP-2, a proinflammatory macrophage-derived chemokine, contain a canonical NF-κB sequence; recruitment of p65 to these promoters is extremely fast, being observed immediately after NF-κB entry into the nucleus (35). Consistent with the results of the previous experiment, the LPS-induced recruitment of p65 to the MIP-2 and Iκ-Bα promoters was significantly decreased by E2 added before LPS (Fig. 1B). Similarly, E2 was also able to inhibit p65 binding to the promoter of iNOS, which recruits p65 with slower kinetics (Fig. 1B). The effect of E2 on p65 binding to target gene promoters lasts for a long time, in that we did not observe p65 DNA binding after 120 min of endotoxin stimulation, suggesting that the hormone prevents LPS signaling.
We next analyzed by using RT-PCR assays the levels of Iκ-Bα, MIP-2, and iNOS mRNAs. In the presence of LPS alone, we found a good correlation between p65 recruitment to Iκ-Bα and MIP-2 gene promoters and accumulation of the corresponding mRNAs, indicating that transcription of these early genes does not occur before NF-κB recruitment (Fig. 1C). Consistent with the effect on promoter occupancy, the mRNA levels for both early-response (MIP-2 and Iκ-Bα) and late-response (iNOS) inflammatory genes induced by LPS were decreased by prior exposure to E2 (Fig. 1C). Therefore, deprivation of p65 from inflammatory gene promoters correlates with a lack of induction of the mRNAs for these genes. Again, the hormone induced inhibition and not delay of the endotoxin-induced increases in the levels of the mRNAs for the inflammatory genes (Fig. 1C). Considering that E2 alone does not induce Iκ-Bα gene transcription (Fig. 1C), these results show that the effect of E2 on NF-κB and inflammatory gene transcription is different from that previously described for GR and PPAR ligands, since it involves the inhibition of NF-κB binding to target gene promoters.
E2 prevents NF-κB nuclear accumulation.
To define the process upstream of the location of NF-κB nuclear activity that was modified by the action of E2, we analyzed the subcellular distribution of p65. Immunocytochemical analysis revealed that treatment with E2 plus LPS resulted in the accumulation of p65 in the cytoplasmic compartment, like that in control cells; however, as expected, LPS alone induced p65 nuclear translocation and E2 alone had no effect (Fig. 2A and B). We confirmed these results by using fractionated cellular extracts from macrophages, showing that the nuclear accumulation of p65 induced by LPS was decreased by prior exposure to E2, which resulted in the enrichment of p65 in the cytoplasmic fraction of E2- plus LPS-treated cells (Fig. 2C). The effect of E2 was not restricted to p65. In fact, following inflammatory stimuli, the subcellular distributions of c-Rel (Fig. 2D and E) and p50 (Fig. 2F), two other members of the NF-κB family, were also affected by the hormone. Together, these results show that E2 administration leads to the sequestration of NF-κB transcription factors in the cytoplasm.
FIG. 2.
Cytoplasmic localization of NF-κB induced by E2. (A) Confocal microscopy images of fluorescence immunocytochemical analysis of p65 (green) in RAW 264.7 cells treated with 1 nM E2 for 10 min and with LPS for 30 min. Nuclei were counterstained with Sytox orange (red). ctrl, control. (B) Quantification of the effect of E2 on p65 intracellular localization. The percentage of cells with a cytoplasmic localization of p65 is plotted relative to the total cell number. L, LPS. (C) Western blot analysis of p65, β-actin, and histone H1 in cytoplasmic extracts (Cyt. E.) and nuclear extracts (N. E.) from cells treated as described for panel A. (D) c-Rel subcellular localization. The subcel-lular localization of c-Rel in RAW 264.7 cells treated with 1 nM E2 for 10 min and with LPS at 50 μg/ml for 30 min was assessed by immunocytochemical (ICC) analysis with anti-c-Rel antibody. (E) Quantification of the effect of E2 on c-Rel intracellular localization. The percentage of cells with a cytoplasmic localization of c-Rel is plotted relative to the total cell number. (F) Western blot analysis of p50 in nuclear extracts (N. E.) from RAW 264.7 cells treated with a cytokine mixture (CM) for 30 min and with E2 added before the CM. In panels B and E, values are the means ± SDs of a single experiment performed in triplicate. Single and double asterisks indicate P values of <0.05 in a comparison with the control and in a comparison with LPS, respectively.
E2 uncouples inflammatory signals and NF-κB activation selectively in macrophages.
We next evaluated whether the effect of E2 was specific for macrophages or could be extended to other cell types as well. We assayed primary cultures of microglia, which are the resident macrophages of the brain that regulate its innate immune response to mechanical, chemical, or toxic injuries. Like the results obtained with RAW 264.7 cells, NF-κB nuclear accumulation induced by LPS was prevented by E2 in microglia as well (Fig. 3A), suggesting that the mechanism of hormone action is conserved in macrophages.
FIG. 3.
E2 inhibits NF-κB activation selectively in macrophages. (A) p65 subcellular localization in microglia. The localization of p65 in microglia treated with 1 nM E2 for 10 min and with LPS (50 μg/ml) or TNF-α (10 ng/ml) for 30 min was assessed by immunocytochemical analysis with anti-p65 antibody. ctrl, control. (B and C) p65 subcellular localization in MCF-7 and SK-ER3 cells. The localization of p65 in cells treated with 1 nM E2 for 10 min and with TNF-α (10 ng/ml) for 30 min was assessed by immunocytochemical analysis with anti-p65 antibody. (D) Quantification of the effect of E2 on p65 intracellular localization in the cell types shown in panels A, B, and C. The percentage of cells with a cytoplasmic localization of p65 is plotted relative to the total cell number. Black bars, TNF-α; hatched bars, E2 plus TNF-α. Values are the means ± SDs of a single experiment performed in triplicate. Single and double asterisks indicate P values of <0.05 in a comparison with LPS and in a comparison with E2, respectively.
The inflammatory cytokine TNF-α is known to activate NF-κB to stimulate gene transcription in macrophages as well as in other cell types. Thus, we first used TNF-α to stimulate NF-κB activation in microglia. With this inflammatory stimulus, the inhibitory effect of E2 on NF-κB activation could still be observed, as p65 nuclear translocation induced by TNF-α was not observed with prior exposure to E2 (Fig. 3A and D). We next tested ER-positive MCF-7 and SK-ER3 cell lines of epithelial and neural origins, respectively. Interestingly, the nuclear translocation of NF-κB induced by a 30-min treatment with TNF-α was not affected by E2 administration in either cell line (Fig. 3B to D) Estrogen did not modify p65 localization in any of the cell types used (data not shown).
Since NF-κB nuclear entry correlates with the induction of inflammatory gene transcription, we evaluated whether the effect of E2 on p65 subcellular localization correlates with a reduction in inflammatory gene transcription. When the two compounds were administered at the same time, p65 translocated to the nucleus (Fig. 4A and B) and iNOS mRNA levels increased (Fig. 4C) to an extent similar to that seen with LPS alone; on the other hand, when the incubation time between hormone addition and LPS treatment was increased from 10 min to 4 h, E2 prevented both the p65 nuclear accumulation and the increase in iNOS mRNA levels induced by the endotoxin (Fig. 4A to C). The spatial and temporal correlations between the intracellular localization and the transcriptional activity of p65 thus show that the effect of E2 on inflammatory gene transcription can be ascribed to the cytoplasmic sequestration of NF-κB.
FIG. 4.
Effect of E2 on NF-κB intracellular localization and inflammatory gene transcription. (A) The subcellular localization of p65 in RAW 264.7 cells treated with E2 and with LPS for 30 min was assessed by immunocytochemical analysis with anti-p65 antibody. ctrl, control. (B) Quantification of the E2 activity shown in panel A. The percentage of cells with cytoplasmic p65 is plotted relative to the total cell number. pretreat., pretreatment. Black bars, LPS; hatched bars, E2 plus LPS. Values are the means ± SDs of a single experiment performed in triplicate. An asterisk indicates a P value of <0.05 in a comparison with LPS. (C) RT-PCR analysis of iNOS mRNA levels in RAW 264.7 cells. E2 was added for the indicated times followed by 3 h of treatment with LPS.
Together, these results show that E2 activates a rapid and persistent response selectively in inflammatory cells and that this response modulates intracellular pathways activated in common by inflammatory agents that converge on NF-κB nuclear translocation.
Activation of the PI3K signaling pathway is required for inhibition of NF-κB by E2.
It is well known that the cytoplasmic localization of NF-κB is dependent on Iκ-Bα stability. We therefore examined whether E2 may modify IKK activation induced by LPS and protect Iκ-Bα from degradation. Western blot analysis revealed that E2 did not alter Iκ-Bα degradation induced by LPS (Fig. 5A and B). Accordingly, LPS-induced activation of IKK, as measured by the presence of the phosphorylated form of this kinase in LPS-treated cells, was also not influenced by the action of the hormone (Fig. 5C and D). These results indicate that E2 does not modify the IKK-Iκ-Bα pathway of NF-κB activation, a known target of anti-inflammatory drug activity (47).
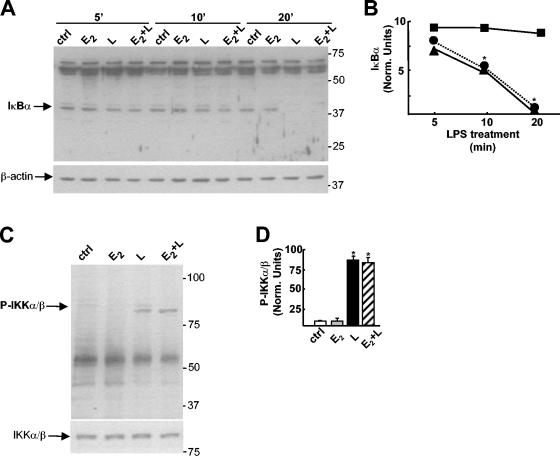
FIG. 5.
The IKK-Iκ-Bα pathway is not modified by E2. (A) Western blot analysis of Iκ-Bα and β-actin proteins in cytoplasmic extracts from RAW 264.7 cells treated with 1 nM E2 for 10 min before treatment with LPS (L) at 50 μg/ml for 5, 10, or 20 min. ctrl, control. (B) Amount of Iκ-Bα calculated as the ratio of the optical density of the Iκ-B band to the optical density of the β-actin band from the Western blot shown in panel A. Symbols: ▪, E2; ▴, LPS; •, E2 plus LPS. Data are from a single representative experiment repeated two times. Norm., normalized. (C) Western blot analysis of phosphorylated IKKα/β (P-IKKα/β) and IKKα/β in total extracts from RAW 264.7 cells treated with 1 nM E2 for 10 min before treatment with LPS at 50 μg/ml for 30 min. (D) Amount of P-IKKα/β calculated as the ratio of the optical density of the P-IKKα/β band to the optical density of the IKKα/β band in three different experiments. White bar, control; gray bar, E2; black bar, LPS; hatched bar, E2 plus LPS. Values are the means ± SDs of three different experiments. An asterisk in panels B and D indicates a P value of <0.05 in a comparison with the control.
The interaction between LPS and its membrane receptors, CD-14 and Toll-like receptor 4, also triggers the activation of mitogen-activated protein kinases (MAPKs), including ERK, and the PI3K pathway. We examined whether E2 was able to interfere with these LPS-induced signaling pathways. MAPK activation by LPS was not inhibited by the hormone, as suggested by the unaltered phosphorylation state of ERK1/2 in cells treated with E2 plus LPS (Fig. 6A) and by the lack of an effect of PD98059, a specific MAPK kinase pharmacological inhibitor, on the subcellular localization of p65 induced by treatment with E2 plus LPS (Fig. 6B and C). In contrast, two specific inhibitors of PI3K, LY294004 and wortmannin, prevented the effect of E2 on p65 cytoplasmic localization (Fig. 6B and C). A direct assessment of the induction of phosphoinositide production by E2 then was obtained by the PI3K assay shown in Fig. 6D: 5 min of incubation with the hormone induced the formation of PIP3, which was present at similar levels following longer incubation times (20 min) with E2 (40% conversion at 5 min compared with 48% conversion after 20 min). When macrophages were exposed to LY294004 before E2 treatment, PIP3 could not be detected, demonstrating that the action of E2 is indeed mediated by the PI3K enzyme.
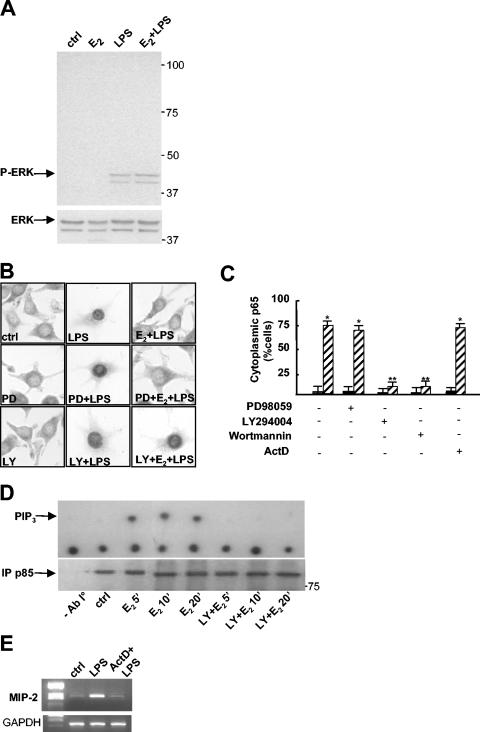
FIG. 6.
A nongenomic, PI3K-dependent pathway is necessary for the action of E2. (A) Western blot analysis of phosphorylated ERK (P-ERK) and ERK proteins in total extracts from RAW 264.7 cells treated with 1 nM E2 for 10 min before treatment with LPS at 50 μg/ml for 30 min. ctrl, control. (B) Subcellular localization of p65. Immunocytochemical analysis with anti-p65 antibody was performed on RAW 264.7 cells treated with PD98059 (50 μM) (PD) or LY294002 (50 μM) (LY) for 1 h, with 1 nM E2 for 10 min, and with LPS at 50 μg/ml for 30 min as indicated. (C) Quantification of E2 activity shown in panel B. The percentage of cells with cytoplasmic p65 is plotted relative to the total cell number. Black bars, LPS; hatched bars, E2 plus LPS. Actinomycin D (5 μg/ml) (ActD) was added 1 h before E2 and LPS. Values are the means ± SDs of a single experiment performed in triplicate. Single and double asterisks indicate P values of <0.05 in a comparison with LPS and in a comparison with E2 plus LPS, respectively. (D) PI3K activity. RAW 264.7 cells were treated in the presence or absence of 50 μM LY294002 for 1 h and then with 1 nM E2 for 5, 10, or 20 min. Cell lysates were immunoprecipitated with anti-p85 antibody (lower panel), and PI3K activity was assayed. The upper panel shows a representative autoradiogram of a thin-layer chromatography analysis of the migration of labeled PIP3. (E) Gene transcription inhibition by actinomycin D. MIP-2 mRNA levels in RAW 264.7 cells treated with actinomycin D (5 μg/ml) for 1 h and then with LPS for 1 h were assessed by RT-PCR.
The rapid formation of lipid secondary messengers by E2 implies a nongenomic mechanism of action. To further substantiate this evidence, we assayed hormone activity in the presence of the transcriptional inhibitor actinomycin D. The effect of E2 on p65 cytoplasmic retention was still observed when cells were preexposed to actinomycin D (Fig. 6C), whose activity resulted, as expected, in the inhibition of LPS-induced gene transcription (Fig. 6E).
Together, these results demonstrate that the action of E2 on LPS signaling occurs through the rapid activation of PI3K-dependent nongenomic signaling pathways without altering the onset of LPS signaling or interfering with Iκ-Bα degradation.
Control of p65 intracellular transport by E2.
The subcellular distribution of NF-κB is a dynamic process of nuclear import and export (39). To understand how E2 affects p65 trafficking, we analyzed the time course of p65 nuclear translocation upon hormone and LPS treatments. The accumulation of p65 in the nucleus occurred after 10 min of LPS stimulation; at this time point, 90% of the cells showed the nuclear immunolocalization of p65 (Fig. 7A). In contrast, in cells treated with E2, the effect of LPS was not observed at any time point analyzed, indicating that the hormone inhibits the translocation of NF-κB to the nucleus. We thus wondered whether the action of E2 could interfere with the transport machinery that moves p65 inside cells. We used the antimitotic drug NCZ, which activates microtubule-dependent intracellular transport and stimulates the nuclear accumulation of NF-κB and other transcription factors (16, 24, 33). Using EMSAs, we showed that NCZ induced p65 DNA binding activity, which was decreased by E2 pretreatment (Fig. 7B). Accordingly, low doses of and short treatments with NCZ induced the perinuclear accumulation of NF-κB, while this effect was not observed in the presence of E2 plus NCZ (Fig. 7C). These results show that E2 limits the effect of NCZ on the translocation of p65 to the nucleus, indicating that the microtubule-associated transport system used by NF-κB is a target of the action of E2 in inflammatory cells.
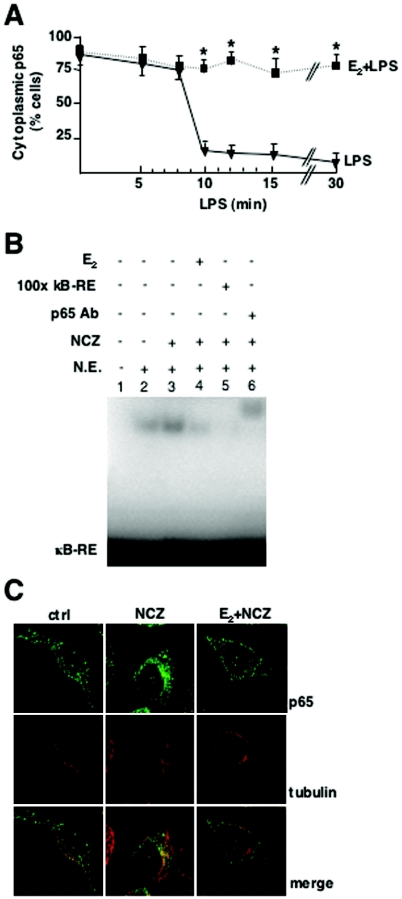
FIG. 7.
Control of p65 intracellular transport by E2. (A) Time-dependent effect of E2 on p65 localization. RAW 264.7 cells were treated with E2 for 10 min and then with LPS for the indicated times. The subcellular distribution of p65 was assessed by immunocytochemical analysis with anti-p65 antibody and by counting of cells with cytoplasmic p65. The percentage of cells with cytoplasmic p65 is plotted relative to the total cell number. Values are the means ± SDs of three independent experiments. An asterisk indicates a P value of <0.05 in a comparison with LPS. (B) In vitro DNA binding assay of NF-kB. An EMSA for radioactively labeled κB-RE was performed with nuclear extracts (N.E.) from RAW 264.7 cells treated with 1 nM E2 for 10 min followed by the addition of 5 μM NCZ for an additional 45 min. For supershift and competition assays, a 100-fold excess of unlabeled κB-RE (lane 5) and p65 antibody (Ab) (lane 6) were used, respectively. (C) Confocal microscopy images of fluorescence immunocytochemical analysis of p65 and α-tubulin in RAW 264.7 cells treated with 1 nM E2 for 10 min and with 5 μM NCZ for 30 min. ctrl, control.
ERα-dependent, E2-specific signaling on NF-κB activation.
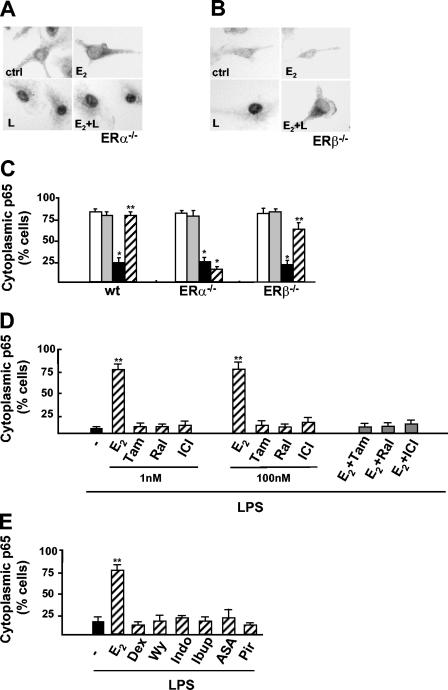
We next evaluated the roles of the two ER isoforms in p65 intracellular localization by using primary cultures of brain macrophages from ERα- or ERβ-null mice. Immunocytochemical analysis revealed that p65 nuclear translocation elicited by LPS occurred in ERα-null macrophages independent of E2 stimulation (Fig. 8A and C). On the other hand, hormone action persisted in ERβ-null microglia in a manner similar to that seen in wild-type cells, and the absence of either ERα or ERβ did not alter the distributions of p65 in untreated and LPS-stimulated cells (Fig. 8B and C). We concluded that the inhibition of p65 intracellular transport by E2 is specifically mediated by ERα. This conclusion is consistent with previous reports from our and other laboratories that showed that ERα exerts anti-inflammatory activity in vivo (31, 42).
FIG. 8.
ERα-dependent E2-specific signaling on NF-κB activation. (A and B) Immunocytochemical analysis with anti-p65 antibody of p65 in primary cultures of microglia extracted from ERα-null (ERα−/−) (A) and ERβ-null (ERβ−/−) mice (B) treated with E2 for 10 min and then with LPS (L) for 30 min. ctrl, control. (C) Quantification of the effect of E2 on the subcellular distribution of p65 in primary cultures of microglia from wild-type (wt), ERα−/−, and ERβ−/− mice shown in panels A and B. The percentage of cells with cytoplasmic p65 is plotted relative to the total cell number. Open bars, untreated cells; gray bars, E2; black bars, LPS; hatched bars, E2 plus LPS. (D) Effect of SERM on the subcellular localization of p65. Immunocytochemical analysis with anti-p65 antibody was performed in RAW 264.7 cells treated for 30 min with LPS alone (black bar) or with a prior 10-min exposure to 1 or 100 nM E2, tamoxifen (Tam), raloxifene (Ral), or ICI 182,780 (ICI) (hatched bars). Competition experiments with ER ligands were also performed by adding 100 nM Tam, Ral, or ICI for 10 min before E2 (gray bars). The percentage of cells with cytoplasmic p65 is plotted relative to the total cell number. (E) Effect of anti-inflammatory drugs. Shown is a quantitative assessment of the cytoplasmic localization of p65 in RAW 264.7 cells treated for 30 min with LPS in the absence (black bar) or presence of 1 nM E2, 1 nM dexamethasone (Dex), 100 μM WY14,643 (Wy), 100 μM indomethacin (Indo), 50 μM ibuprophen (Ibup), 5 μM acetylsalicylic acid (ASA), or 50 μM piroxicam (Pir) (hatched bars); drugs were added for 10 min before a 30-min treatment with LPS. Values are the means ± SDs of three independent experiments. Single and double asterisks indicate P values of <0.05 in a comparison with the control and in a comparison with LPS, respectively.
These data prompted us to evaluate the activities of two selective estrogen receptor modulators (SERMs), tamoxifen and raloxifene, endowed with organ-specific pharmacological profiles. Low (1 nM) and high (100 nM) doses of both SERMs did not mimic the effect of E2 on LPS-induced p65 intracellular localization (Fig. 8D). Furthermore, when assayed in competition experiments, these compounds prevented the effect of E2 on LPS signaling, thus displaying antagonist activity similar to that obtained with the pure antagonist ICI 182,780 (Fig. 8D). These results indicated the lack of an effect of SERMs on p65 activation; in addition, the inhibition of the action of E2 by a receptor antagonist further confirmed the involvement of intracellular receptors in hormone action.
Finally, we evaluated whether NF-κB translocation to the nucleus could be modulated by other known anti-inflammatory agents. We observed that p65 immunolocalization induced by LPS was not modified by dexamethasone, by WY14,643, a ligand of PPARα, or by NSAIDs, such as indomethacin, ibuprofen, acetylsalicylic acid, and piroxicam (Fig. 8E). These results showed that this immediate-early event in LPS signaling is not a target for conventional anti-inflammatory drugs.
Together, these data showed that the prevention of NF-κB activation by E2 is a specific and unique mechanism of action among ER drugs and anti-inflammatory agents.
DISCUSSION
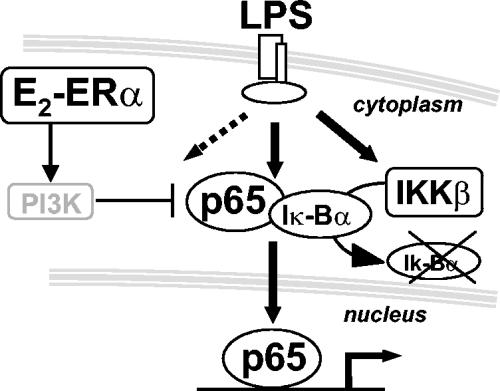
We show here that E2 prevents inflammatory gene transcription induced by inflammatory agents by inhibiting NF-κB intracellular transport, an immediate-early event in the inflammatory signaling cascade. This is a novel mechanism in the control of NF-κB activation that is not shared by conventional anti-inflammatory drugs. In addition, we show that the effect of E2 on NF-κB is mediated by the intracellular receptor ERα through a nongenomic signaling pathway that involves the activation of PI3K (Fig. 9). This is the first report showing an inhibitory effect of E2 on the intracellular transport of proteins toward the nucleus. Other authors previously claimed that E2 inhibits NF-κB-mediated gene transcription (13, 40); we show here that the decreased activity of p65 induced by E2 is due to the cytoplasmic sequestration of this transcription factor.
FIG. 9.
Effect of E2 on NF-κB activation. E2 inhibits the effect of LPS on early inflammatory genes by preventing the intracellular transport of NF-κB without altering the Iκ-B degradation pathway. This mechanism is mediated by the ERα isoform and involves PI3K activation.
NF-κB activation requires the degradation of its inhibitory protein, Iκ-B. NSAIDs that are inhibitors of NF-κB activation mediate this effect by blocking IKK activity and thus suppressing Iκ-Bα phosphorylation and degradation (47). In this way, NF-κB remains inactive in the cytoplasm in association with Iκ-B proteins. In contrast to this mechanism, we observe that inhibition of NF-κB nuclear accumulation by E2 occurs through the blocking NF-κB intracellular transport without affecting Iκ-Bα degradation. Likewise, anti-inflammatory drugs acting on intracellular receptors, such as glucocorticoids and PPAR agonists, do not share a similar mechanism of action, as we show that p65 trafficking induced by LPS is not modified by these drugs. Thus, these data identify intracellular transport as a novel target for pharmacological interventions aimed at preventing inflammation, although little is known at present about the specific interactions between the transport machinery and NF-κB. It has been shown that NF-κB may utilize microtubules as cytoskeletal tracks to massively migrate from the cytoplasm to the nucleus in response to inflammatory signals. In fact, microtubule reorganization induced by antimitotic drugs has been shown to activate the DNA binding activity of NF-κB (5, 33). Iκ-B has been shown to interact with the dynein light chain 1 (Dlc-1), a motor protein that moves proteins toward the nucleus (7); however, it is not understood yet whether NF-κB forms a complex with Iκ-B and Dlc-1 and whether this interaction sequesters NF-κB or contributes to its delivery during activation. By using different cell lines, we observe that the effect of E2 is specific for macrophages; thus, future studies will address the molecular aspects of the regulatory effect of E2 on NF-κB intracellular transport in inflammatory cells.
The action of E2 in inflammation requires the activation of a specific intracellular receptor. In fact, using ERα- and ERβ-null microglia and receptor antagonists, we demonstrate that ERα is necessary for E2 inhibition of NF-κB. These data are consistent with previous reports which showed that the reactivity of macrophages, known to express endogenous ERs, is strongly regulated by E2 (6, 17, 43, 44) and that ERα is necessary for the anti-inflammatory activity of E2 in inflammatory pathologies (1, 10, 14, 31, 42). By showing that ERα mediates the inhibition of NF-κB transport, we thus provide the mechanism to explain this receptor-specific anti-inflammatory activity.
The effect of E2-activated ERα on NF-κB involves a nongenomic signaling pathway, as suggested by the following results: (i) the short time frame (10 min) of hormonal exposure that is needed to prevent LPS-induced p65 translocation; (ii) no alteration of the action of E2 in the presence of transcriptional inhibitors, such as actinomycin D; and (iii) relief of the inhibitory effect of the hormone by PI3K inhibitors. These observations are consistent with the intracellular localization of ERα in macrophages, which we previously reported to reside in the cytoplasm of inflammatory cells as well (41, 43); our results are also in agreement with the involvement of PI3K in the negative regulation of the early phase of LPS signaling (9, 12) on p65 (15) and with the recently described interaction between ER and PI3K in endothelial cells (38). Moreover, we demonstrate that the action of E2 fails when the hormone and LPS are added at the same time, demonstrating that the target of hormone action is the early-phase activation of LPS signaling.
Phosphoinositides in the plasma membrane, including PIP3, play critical roles in regulating the interplay between cytoskeleton dynamics and cell reactivity and shape in response to extracellular signals (21). Interestingly, we show that E2 rapidly stimulates the production of PIP3 in macrophages and that this induction uncouples the inflammatory signaling to the transport system of NF-kB. In light of the widespread biological responses regulated by PIP3, our results suggest that E2 may be a master regulator of intracellular pathways activated at the plasma membrane of inflammatory cells by lipid secondary messengers. This notion also leads us to speculate on whether the action of the hormone on PIP3 signaling pathways may be compromised in inflammatory-based pathologies that do not benefit from E2 action. Future studies will shed more light on these aspects.
The relevant and unique role played by E2 in inflammation may reflect the endocrine activity of this hormone, which could act as a protective agent against inflammatory insults in order to favor pregnancy and grant offspring sustenance during a woman's fertile life. At menopause, the development of chronic degenerative diseases, such as those affecting bone, vessel walls, or the brain, is strictly associated with an increased proinflammatory reaction, indicating that E2 deprivation may indeed facilitate the onset of chronic inflammatory reactions. Since the drugs used to modulate ER activity display both agonist and antagonist profiles, depending on the promoter and cellular contexts, there is a need for the development of SERMs, which should prove to mimic the desired E2 activities while behaving as antagonists of the undesired effects of the hormone. In this study, we show that raloxifene and tamoxifen, two widely prescribed SERMs, display an antagonist profile, suggesting that these molecules may not mimic the anti-inflammatory activity of E2 in vivo.
In summary, the finding of a novel mechanism of E2 action in inflammation supports the unique role of E2 in the control of innate immunity and hints at the development of novel pharmacological tools for the prevention of the inflammatory response.
Acknowledgments
We acknowledge Monica Rebecchi and Samanta Oldoni for skillful technical assistance. We thank Pierre Chambon for helpful discussions and Alessandro Guerini Rocco, Silvia Belcredito, Sabrina Etteri, Simona Caporali, Paolo Sparaciari, Mauro Anglana, Paolo Ciana, Fabio Pellegatta, and Patrizia Risè for technical and critical support.
This research was supported by Telethon (GP0127Y01), European Network of Excellence EMIL and CASCADE, and by Ministero dell'Università e della Ricerca Scientifica (COFIN 58785).
REFERENCES
- 1.Ashcroft, G. S., S. J. Mills, K. Lei, L. Gibbons, M. J. Jeong, M. Taniguchi, M. Burow, M. A. Horan, S. M. Wahl, and T. Nakayama. 2003. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Investig. 111:1309-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auphan, N., J. DiDonato, C. Rosette, A. Hemberg, and M. Karin. 1995. Immunosuppression by glucocorticoids: inhibition of NFκB activity through induction of IκB synthesis. Science 270:286-290. [DOI] [PubMed] [Google Scholar]
- 3.Bebo, B. F., Jr., A. Fyfe-Johnson, K. Adlard, A. G. Beam, A. A. Vandenbark, and H. Offner. 2001. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J. Immunol. 166:2080-2089. [DOI] [PubMed] [Google Scholar]
- 4.Beg, A. A., W. C. Sha, R. T. Bronson, and D. Baltimore. 1995. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 9:2736-2746. [DOI] [PubMed] [Google Scholar]
- 5.Bourgarel-Rey, V., S. Vallee, O. Rimet, S. Champion, D. Braguer, A. Desobry, C. Briand, and Y. Barra. 2001. Involvement of nuclear factor kappaB in c-Myc induction by tubulin polymerization inhibitors. Mol. Pharmacol. 59:1165-1170. [DOI] [PubMed] [Google Scholar]
- 6.Bruce-Keller, A., S. W. Barger, N. I. Moss, J. T. Pham, J. N. Keller, and A. Nath. 2001. Pro-inflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17β-estradiol. J. Neurochem. 78:1515-1524. [DOI] [PubMed] [Google Scholar]
- 7.Crepieux, P., H. Kwon, N. Leclerc, W. Spencer, S. Richard, R. Lin, and J. Hiscott. 1997. IκBα physically interacts with a cytoskeleton-associated protein through its signal response domain. Mol. Cell. Biol. 17:7375-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bosscher, K., M. L. Schmitz, W. Vanden Berghe, S. Plaisance, W. Fiers, and G. Haegeman. 1997. Glucocorticoid-mediated repression of nuclear factor-kappaB-dependent transcription involves direct interference with transactivation. Proc. Natl. Acad. Sci. USA 94:13504-13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz-Guerra, M. J., A. Castrillo, P. Martin-Sanz, and L. Bosca. 1999. Negative regulation by phosphatidylinositol 3-kinase of inducible nitric oxide synthase expression in macrophages. J. Immunol. 162:6184-6190. [PubMed] [Google Scholar]
- 10.Dubal, D. B., H. Zhu, J. Yu, S. W. Rau, P. J. Shughrue, I. Merchenthaler, M. S. Kindy, and P. M. Wise. 2001. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. USA 98:1952-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupont, S., A. Krust, A. Gansmuller, A. Dierich, P. Chambon, and M. Mark. 2000. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277-4291. [DOI] [PubMed] [Google Scholar]
- 12.Fukao, T., M. Tanabe, Y. Terauchi, T. Ota, S. Matsuda, T. Asano, T. Kadowaki, T. Takeuchi, and S. Koyasu. 2002. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 3:875-881. [DOI] [PubMed] [Google Scholar]
- 13.Galien, R., and T. Garcia. 1997. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-κB site. Nucleic Acids Res. 25:342-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garidou, L., S. Laffont, V. Douin-Echinard, C. Coureau, A. Krust, P. Chambon, and J. C. Guery. 2004. Estrogen receptor alpha signaling in inflammatory leukocytes is dispensable for 17beta-estradiol-mediated inhibition of experimental autoimmune encephalomyelitis. J. Immunol. 173:2435-2442. [DOI] [PubMed] [Google Scholar]
- 15.Guha, M., and N. Mackman. 2002. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 277:32124-32132. [DOI] [PubMed] [Google Scholar]
- 16.Gundersen, G. G., and T. A. Cook. 1999. Microtubules and signal transduction. Curr. Opin. Cell Biol. 11:81-94. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi, T., K. Yamada, T. Esaki, E. Muto, G. Chaudhuri, and A. Iguchi. 1998. Physiological concentrations of 17beta-estradiol inhibit the synthesis of nitric oxide synthase in macrophages via a receptor-mediated system. J. Cardiovasc. Pharmacol. 31:292-298. [DOI] [PubMed] [Google Scholar]
- 18.Hodgin, J. B., and N. Maeda. 2002. Minireview: estrogen and mouse models of atherosclerosis. Endocrinology 143:4495-4501. [DOI] [PubMed] [Google Scholar]
- 19.Holmes-McNary, M., and A. S. Baldwin, Jr. 2000. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 60:3477-3483. [PubMed] [Google Scholar]
- 20.Huang, T. T., N. Kudo, M. Yoshida, and S. Miyamoto. 2000. A nuclear export signal in the N-terminal regulatory domain of IκBα controls cytoplasmic localization of inactive NF-κB/IκBα complexes. Proc. Natl. Acad. Sci. USA 97:1014-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Insall, R. H., and O. D. Weiner. 2001. PIP3, PIP2, and cell movement—similar messages, different meanings? Dev. Cell 1:743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito, A., B. F. J. Bebo, A. Matejuk, A. Zamora, M. Silverman, A. Fyfe-Johnson, and H. Offner. 2001. Estrogen treatment down-regulates TNF-α production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J. Immunol. 167:529-542. [DOI] [PubMed] [Google Scholar]
- 23.Jansson, L., T. Olsson, and R. Holmdahl. 1994. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. J. Neuroimmunol. 53:203-207. [DOI] [PubMed] [Google Scholar]
- 24.Jung, Y. J., J. S. Isaacs, S. Lee, J. Trepel, and L. Neckers. 2003. Microtubule disruption utilizes an NFkappa B-dependent pathway to stabilize HIF-1alpha protein. J. Biol. Chem. 278:7445-7452. [DOI] [PubMed] [Google Scholar]
- 25.Klement, J. F., N. R. Rice, B. D. Car, S. J. Abbondanzo, G. D. Powers, P. H. Bhatt, C. H. Chen, C. A. Rosen, and C. L. Stewart. 1996. IκBα deficiency results in a sustained NF-κB response and severe widespread dermatitis in mice. Mol. Cell. Biol. 16:2341-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma, Z. Q., E. Spreafico, G. Pollio, S. Santagati, E. Conti, E. Cattaneo, and A. Maggi. 1993. Activated estrogen receptor mediates growth arrest and differentiation of a neuroblastoma cell line. Proc. Natl. Acad. Sci. USA 90:3740-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda, J., M. T. Vanier, Y. Saito, K. Suzuki, and S. Kunihiko. 2001. Dramatic phenotypic improvement during pregnancy in a genetic leukodystrophy: estrogen appears to be a critical factor. Hum. Mol. Genet. 10:2709-2715. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto, N., M. Mandai, I. Suzuma, K. Suzuma, K. Kobayashi, and Y. Honda. 1999. Estrogen protects against cellular infiltration by reducing the expressions of E-selectin and IL-6 in endotoxin-induced uveitis. J. Immunol. 163:374-379. [PubMed] [Google Scholar]
- 29.Moggs, J. G., and G. Orphanides. 2001. Estrogen receptors: orchestrators of pleiotropic cellular responses. EMBO Rep. 2:775-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nissen, R. M., and K. R. Yamamoto. 2000. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 14:2314-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polanczyk, M., A. Zamora, S. Subramanian, A. Matejuk, D. L. Hess, E. P. Blankenhorn, C. Teuscher, A. A. Vandenbark, and H. Offner. 2003. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am. J. Pathol. 163:1599-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricote, M., A. C. Li, T. M. Willson, C. J. Kelly, and C. K. Glass. 1998. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391:79-82. [DOI] [PubMed] [Google Scholar]
- 33.Rosette, C., and M. Karin. 1995. Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B. J. Cell Biol. 128:1111-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi, A., P. Kapahi, G. Natoli, T. Takahashi, Y. Chen, M. Karin, and M. G. Santoro. 2000. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 403:103-108. [DOI] [PubMed] [Google Scholar]
- 35.Saccani, S., S. Pantano, and G. Natoli. 2001. Two waves of nuclear factor kappaB recruitment to target promoters. J. Exp. Med. 193:1351-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheinman, R. I., P. C. Cogswell, A. K. Lofquist, and A. S. Baldwin, Jr. 1995. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 270:283-286. [DOI] [PubMed] [Google Scholar]
- 37.Scheinman, R. I., A. Guadalberto, C. M. Jewell, J. A. Cidlowski, and A. S. Baldwin. 1995. Characterization of mechanisms involved in transrepression of NF-κB by activated glucocorticoid receptors. Mol. Cell. Biol. 15:943-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simoncini, T., A. H. Moghadam, D. P. Brazil, K. Ley, W. W. Chin, and J. K. Liao. 2000. Interaction of estrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407:538-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tam, W. F., L. H. Lee, L. Davis, and R. Sen. 2000. Cytoplasmic sequestration of Rel proteins by IκBα requires CRM1-dependent nuclear export. Mol. Cell. Biol. 20:2269-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valentine, J. E., E. Kalkhoven, R. White, S. Hoare, and M. G. Parker. 2000. Mutations in the estrogen receptor ligand binding domain discriminate between hormone-dependent transactivation and transrepression. J. Biol. Chem. 275:25322-25329. [DOI] [PubMed] [Google Scholar]
- 41.Vegeto, E., S. Ghisletti, C. Meda, S. Etteri, S. Belcredito, and A. Maggi. 2004. Regulation of the lipopolysaccharide signal transduction pathway by 17beta-estradiol in macrophage cells. J. Steroid Biochem. Mol. Biol. 91:59-66. [DOI] [PubMed] [Google Scholar]
- 42.Vegeto, E., S. Belcredito, S. Etteri, S. Ghisletti, A. Brusadelli, C. Meda, A. Krust, S. Dupont, P. Ciana, P. Chambon, and A. Maggi. 2003. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. USA 100:9614-9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vegeto, E., C. Bonincontro, G. Pollio, A. Sala, S. Viappiani, F. Nardi, A. Brusadelli, B. Viviani, P. Ciana, and A. Maggi. 2001. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J. Neurosci. 21:1809-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vegeto, E., G. Pollio, C. Pellicciari, and A. Maggi. 1999. Estrogen and progesterone induction of survival of monoblastoid cells undergoing TNF-alpha-induced apoptosis. FASEB J. 13:793-803. [DOI] [PubMed] [Google Scholar]
- 45.Wahl, C., S. Liptay, G. Adler, and R. M. Schmid. 1998. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J. Clin. Investig. 101:1163-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto, Y., M. J. Yin, K. M. Lin, and R. B. Gaynor. 1999. Sulindac inhibits activation of the NF-kappaB pathway. J. Biol. Chem. 274:27307-27314. [DOI] [PubMed] [Google Scholar]
- 47.Yin, M. J., Y. Yamamoto, and R. B. Gaynor. 1998. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 396:77-80. [DOI] [PubMed] [Google Scholar]