Abstract
Pyruvate dehydrogenase (PDH) complex deficiency is a major cause of lactic acidosis and Leigh's encephalomyelopathies in infancy and childhood, resulting in early death in the majority of patients. Most of the molecular defects have been localized in the coding regions of the E1α PDH gene. Recently, we identified a novel mutation of the E1α PDH gene in a patient with an encephalopathy and lactic acidosis. This mutation, located downstream of exon 7, activates a cryptic splice donor and leads to the retention of intronic sequences. Here, we demonstrate that the mutation results in an increased binding of the SR protein SC35. Consistently, ectopic overexpression of this splicing factor enhanced the use of the cryptic splice site, whereas small interfering RNA-mediated reduction of the SC35 protein levels in primary fibroblasts from the patient resulted in the almost complete disappearance of the aberrantly spliced E1α PDH mRNA. Our findings open the exciting prospect for a novel therapy of an inherited disease by altering the level of a specific splicing factor.
Removal of intervening sequences (introns) from precursor messenger RNAs (pre-mRNA) is an essential step in the expression of most metazoan protein-encoding genes, often regulated in a cell-type-specific or developmental manner. Alternative splicing enables the same precursor to give rise to several mRNAs that code for proteins having distinct functions. Thus, the precise selection of 5′ and 3′ splicing sites is a way to generate diversity and may lead to the regulation of gene expression according to tissue type or during development of the organism. The results of a recently published genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays (22) indicate that at least 74% of human multiexon genes are alternatively spliced.
Among the splicing factors involved in splice site choice, members of the SR protein family have been extensively studied (see references 18 and 33 for reviews). SR proteins are characterized by the presence of one or two copies of an RNA recognition motif and a carboxyl-terminal domain rich in arginine and serine residues (RS domain). The RS domain is responsible for specific protein-protein interactions between RS domain-containing proteins (25, 42-44), whereas the RNA recognition motif domain recognizes several classes of specific RNA motifs, including exonic splicing enhancers (ESEs) and intronic splicing enhancers. These sequences have been demonstrated to play a key role in both alternative and constitutive splice site selection (see references 2 and 41 for reviews). This activity is counteracted by that of splicing repressors, such as members of the hnRNP family which can bind RNA in a nonspecific way but also recognize negative regulatory elements known as exonic and intronic splicing silencers (see references 29 and 43 for reviews). Such an antagonism accounts for the ability of SR proteins to influence splice site choice in a concentration-dependent manner (28, 33).
The prevalence of alternative splicing as a general mechanism to control gene expression makes it a privileged target for alterations leading to pathologies. Along this line, up to 50% of point mutations responsible for type 1 neurofibromatosis and ataxia telangiectasia manifest themselves as splicing defects (7). Such mutations are also the cause for other diseases, such as thalassemia, frontotemporal dementia, amyotrophic lateral sclerosis, and spinal muscular atrophy (17). In addition, it has been shown that mutations in splicing regulatory sequences exhibit a variable penetrance depending on the genetic background, suggesting that variations in splicing factor expression could account for this variability (17).
Pyruvate dehydrogenase (PDH) complex deficiency is one of the most common defined genetic defects of mitochondrial energy metabolism (38). Most of the cases of this severe disease, which is responsible for early death in the majority of patients (3), are sporadic and result from a new mutation arising within the germ cells of one of the parents (11, 30, 34). The majority of the molecular defects of the PDH complex have been localized in the E1α subunit-encoding gene at chromosome Xp22.1 (gene symbol PDHA1; MIM 312170), and at least 75 different mutations in the coding region have been reported (31). Two cases of exonic mutations associated with a partial or systematic skipping of the entire E1α PDH exon 6 have also been described (10, 12). Analysis of the silent mutation found in one of the patients has suggested the presence of an exonic splicing enhancer in the middle region of the skipped exon (5).
We have previously described a new case of PDH deficiency explained by a novel intronic mutation of the E1α PDH gene (36). This mutation, located downstream from the normal exon 7 5′ splice site, leads to the major expression of an aberrantly spliced E1α PDH mRNA which results from the activation of a cryptic 5′ splice site and retains 45 nucleotides (nt) of intronic sequences. Scanning intron 7 sequences with the ESE finder program (9) revealed that the mutation strengthens and even creates potential binding sites for members of the SR protein family (36).
In this study, we used both in vitro and in vivo approaches to demonstrate that the intronic mutation in the E1α PDH gene results in an increased binding of the SC35 splicing factor. We also establish the physiological importance of these results, either by ectopic overexpression of a green fluorescent protein (GFP)-SC35 fusion protein or by using small interfering RNAs (siRNAs) to reduce SC35 protein levels in primary cells from the patient. We show that this siRNA strategy allows restoration of normal splicing of the E1α PDH pre-mRNA.
MATERIALS AND METHODS
Plasmid constructs.
PDH sequences (144 bp of exon 7, intron 7, and 69 bp of exon 8) were PCR amplified from cell genomic DNA from either a control or the patient with the following oligonucleotides: exon 7 PDH forward primer (E7-Bam-Fw), AGATGGATCCGAAGCTTACAACATGGAGC; and exon 8 PDH reverse primer (Eco-rev PDH), AGCTGAATTCCCCAGATCTACAATAGGCAG. PCR fragments were purified with the Concert Rapid PCR purification system (Invitrogen) and subcloned at the BamHI and EcoRI restriction sites of the pSpβm3S1 plasmid containing the β-globin cassette (27) to give the βGlo3S1PDH and βGlo3S1PDHmut constructs used for in vitro splicing experiments. The chimeric βGlo-PDH sequences were also inserted into the pcDNA3.1D/V5-His-TOPO vector (Invitrogen) to be used in transfection experiments.
Vectors used to transcribe the RNA probes for binding and cross-linking assays were obtained by inserting double-stranded oligonucleotides containing one copy of either the mutated sequence (5′ AATTCGGTGGGGCCGGAGCCAAGGGATC 3′) or the corresponding wild-type PDH sequence (5′ AATTCGGTGGGGCCGGGGCCAAGGGATC 3′) into the PGEM-2 plasmid (Promega). All constructs were sequenced with the Big Dye terminator kit on a 3100 ABI Prism sequencer (Applied Biosystems).
Cell lines, culture, and conditions.
Primary fibroblastic cells from the patient were classically derived from a skin biopsy specimen. They were cultured in RPMI 1640 medium in the presence of 10% fetal calf serum. HeLa cells were cultured in Dulbecco's modified Eagle medium Nut Mix F12 (HAM) in the presence of 10% calf serum.
Transfection and reverse transcription (RT)-PCR.
HeLa cell transfections with splicing reporter constructs were performed with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Twenty-four hours after transfection, total RNA was purified with RNA-PLUS (Quantum Bioprobe). First-strand cDNA was synthesized from 2 μg of RNA with the Amersham Pharmacia first-strand cDNA synthesis kit. For PCR analyses, 1/15 of the reaction was amplified with Taq polymerase (Invitrogen) and either exon 1b-glo (CACCGAATACAAGCTTACATTTGCTTCTGAC) or the Eco-rev PDH primer. The cycle number was kept to a minimum to maintain linearity. PCR products were separated on a 1.5% agarose gel containing ethidium bromide and visualized under UV light.
Nuclear extract preparation, splicing, and complementation assays.
HeLa cell nuclear extracts were prepared according to Dignam et al. (13). Pre-mRNA were synthesized by in vitro transcription in the presence of 20 U of SP6 (Boehringer) RNA polymerase, 1 μg of the suitable linearized plasmids, and 5 μM [α-32P]UTP (3,000 Ci/mmol) in 25-μl reactions according to the manufacturer's instructions. In vitro transcripts were quantified by Cerenkov counting. Splicing reactions were performed under standard conditions as described previously (28). ASF/SF2 and SC35 proteins used in complementation assays were produced and purified from baculovirus-infected Sf9 cells as described previously (39). Splicing products were analyzed by electrophoresis on 7% denaturing polyacrylamide gels and revealed by autoradiography.
Binding and UV-cross-linking experiments.
Binding experiments with RNA probes immobilized on agarose beads were performed essentially as described by Caputi et al. (4). Substrate RNAs for bead immobilization were synthesized in vitro by using the SP6 Ribomax large-scale RNA production system (Promega). Following incubation in the splicing mix, bound proteins were eluted by addition of sample buffer, heated for 5 min at 90°C, electrophoresed in sodium dodecyl sulfate (SDS)-12% polyacrylamide gels, and electroblotted onto nitrocellulose for 90 min in 10 mM CAPS (pH 11.0) transfer buffer containing 10% ethanol. Antibodies raised against ASF/SF2 and SC35 were kindly provided by J. Stévenin (IGBMC, Illkirch, France).
RNA substrates used in cross-linking experiments were obtained by runoff transcription as described for splicing substrates. Probes (100 fmol) were incubated, either in the absence or in the presence of HeLa S100 cytoplasmic or nuclear extracts (20 μg), in splicing buffer without polyvinyl alcohol for 10 min at 30°C in the presence of 300 ng of purified recombinant SC35 protein. Reaction mixtures (20 μl) were then irradiated for 15 min on ice with UV light with a Stratalinker (Stratagene), digested with RNase A (250 ng) and RNase T1 (100 U) for 45 min at 37°C, and resolved by SDS-12% polyacrylamide gel electrophoresis (PAGE). Dried gels were revealed by autoradiography, and signals were quantified by Phosphorimager analysis.
RNA interference and complementation assays.
The patient's fibroblasts were plated at 2.5 × 105 cells per well in 6-well plates. The next day, 25, 50, or 100 pmol of duplex RNAs (sense strand, AAUCCAGGUCGCGAUCGAAdTdT; Dharmacon) was mixed with 4 μl of Plus reagent and Lipofectamine (Invitrogen) plus 200 μl of OptiMEM medium. Following RNA duplex-lipid complex formation, the mixture was adjusted to 1 ml with OptiMEM and added to the cells. Plates were incubated at 37°C for 6 h before addition of 1 ml of 20% serum. Mock-transfected and siRNA-transfected cells were collected for RNA and protein isolation 48, 72, and 96 h posttransfection. For complementation experiments, 2 μg of pEGFP-hSC35 expression vector (2) was cotransfected with 50 nM siRNAs as described above. Cells were harvested 72 h posttransfection and lysed in Tri-Reagent for total RNA isolation. For protein level analysis, cells were lysed in RIPA-N buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40) in the presence of 5 mM dithiothreitol, 5 mM phenylmethylsulfonyl fluoride, and protease inhibitors (Complete, EDTA-free; Roche).
Determination of expression levels using RT-PCR and Western blot analyses.
First-strand cDNA was synthesized from 5 μg of DNase-treated RNA with the Amersham Pharmacia cDNA synthesis kit. For PCR analyses, 1/10 of the reaction was amplified with Taq polymerase (Invitrogen), and the cycle number was kept to a minimum to maintain linearity. PCR products were separated on 1.5 or 1.0% agarose gels containing ethidium bromide and visualized under UV light. PCR regimes and nucleotidic sequences of the primers used in this study are available upon request. Signals corresponding to both PDH isoforms were quantified by densitometric analyses. Protein samples in Laemmli loading buffer were run on SDS-12% polyacrylamide gel and transferred onto nitrocellulose as described above. Blots were successively probed with monoclonal anti-SC35, anti-ASF/SF2, or antiactin antibodies and revealed by enhanced chemiluminescence.
RESULTS AND DISCUSSION
E1α PDH splicing reporter constructs recapitulate the in vivo situation.
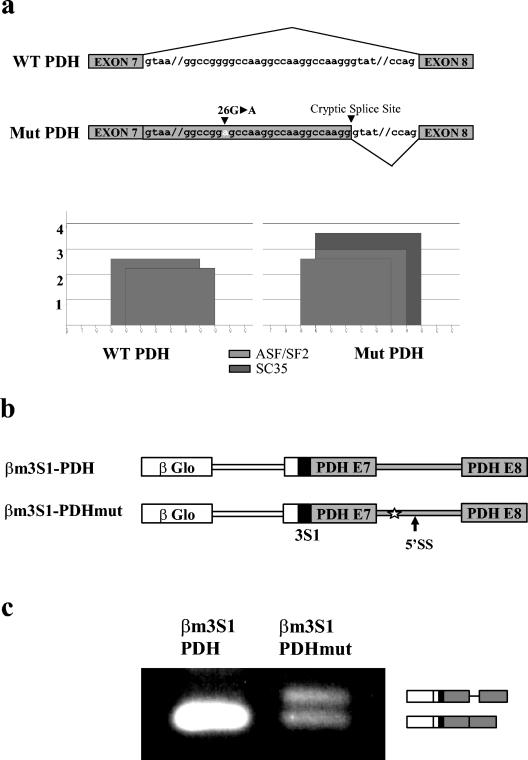
We have previously shown that a G-to-A substitution 26 nt downstream from the exon 7 5′ splice site of the E1α PDH gene (Fig. 1a) is responsible for an aberrant splicing phenotype, which involves a switch from the use of the normal 5′ splice site (intron 7, position 1) to that of a cryptic 5′ splice site (intron 7, position 46 to 47). Using ESE motif prediction tools (9), we have observed that the mutation creates a potential SC35 binding site but also strengthens an overlapping preexisting ASF/SF2 binding motif, slightly upstream from the cryptic splice site (36).
FIG. 1.
β-Globin-PDH splicing reporters recapitulate the in vivo situation in transfected cells. (a) Schematic representation of the region containing the mutation and the cryptic 5′ splice site (arrowheads) within PDH intron 7. The score values of the potential splicing enhancer elements are indicated as histograms. (b) β-Globin-PDH splicing reporters used for ex vivo and in vitro analyses. PDH sequences were inserted immediately downstream from the ASF/SF2 ESE (3S1) in β-globin exon 2. The positions of the mutation (star) and of the cryptic splice site are indicated. (c) Semiquantitative RT-PCR analysis of splicing products in transiently transfected HeLa cells. The position of the fragments corresponding to use of the authentic and cryptic 5′ splice site is shown.
To assess the respective contributions of ASF/SF2 and SC35 in the aberrant splicing of the E1α PDH pre-mRNA, we developed an in vitro splicing system that recapitulates the in vivo situation. Failure of a simple two-exon reporter construct containing exon 7-intron 7-exon 8 of the E1α PDH gene to undergo efficient splicing in vitro prompted us to use an artificial substrate (Fig. 1b) in which PDH sequences, carrying or not carrying the mutation, were inserted downstream of the βm3S1 sequences derived from the human β-globin gene (27) and which already contains a high-affinity ASF/SF2 binding motif (3S1). Similar to what we previously observed for the endogenous PDH mRNAs in cells from a control and the patient (36), transient transfections of these constructs in HeLa cells give rise to a splicing product containing 45 nt of the PDH intronic sequences when the intron sequences are mutated, whereas only the authentic 5′ splice site is used in the case of wild-type PDH sequences (Fig. 1c). In vitro splicing experiments (Fig. 2a) also lead to the same results, as the removal of the mutated PDH intron 7 efficiently occurs through the equal use of the normal and cryptic 5′ splice sites, whereas that of the wild-type PDH intron essentially proceeds through the normal 5′ splice site. Using similar constructs lacking the 3S1 ESE, neither the β-globin nor PDH intronic sequences could be removed in vitro (data not shown), suggesting that the PDH exon 7 splice donor is a weak site which needs upstream enhancer elements to be activated.
FIG. 2.
SC35 but not ASF/SF2 activates the cryptic 5′ splice site in both wild-type and mutated PDH sequences. (a) In vitro splicing experiments performed with βm3S1-PDH (WT) and βm3S1-PDHmut (Mut) in the presence of 30% (lanes 2 and 6), 40% (lanes 3 and 7), and 50% (lanes 4 and 8) HeLa nuclear extracts (NE). Incubation was for 1 h at 30°C, except for controls (CTL, lanes 1 and 5). Splicing reactions were loaded onto a 7% polyacrylamide-8 M urea denaturing gel. The structures of splicing products and intermediates are depicted on the right. (b) Complementation assays with purified recombinant proteins. WT and Mut transcripts were incubated as described for panel a without (lane 2) or in the presence of increasing amounts (100 ng, lanes 3 and 6; 200 ng, lanes 4 and 7; and 300 ng, lanes 5 and 8) of purified recombinant ASF/SF2 (lanes 3 to 5) or SC35 (lanes 6 to 8) splicing factors. The splicing activity of the recombinant ASF/SF2 protein was tested in vitro with the pSpβm3S1 substrate (data not shown).
The E1α PDH intronic mutation strengthens a preexisting SC35 splicing enhancer.
Complementation experiments with nuclear extracts with purified SC35 or ASF/SF2 proteins were conducted to test which protein, SC35 or ASF/SF2, is directly responsible for activation of the 5′ cryptic splice site in the PDH intron 7 (Fig. 2b). While ASF/SF2 clearly has no effect on splice site choice, SC35 significantly activates splicing at the same cryptic site of both the wild-type and mutated PDH intron 7, although at different levels. As expected, the mutated sequences are much more efficient in activating the cryptic site, revealing thereby that the mutation has either strengthened a preexisting SC35 splicing enhancer not selected by the ESE motif prediction tools and/or eliminated a silencer element. Rather consistent with the former hypothesis, reexamination of the potential ESE motifs in the wild-type sequence led to the identification of a low-score SC35 site (2.32) which is converted to a high-score site (3.61) in its mutated counterpart.
To address this point more directly, we first compared the abilities of both SC35 and ASF/SF2 to bind to wild-type and mutated sites. RNAs (37 nt long) containing one copy of the corresponding sequences were coupled to agarose beads and used to affinity purify factors from HeLa cell nuclear extracts. Western blot analysis of the eluted proteins was then performed with polyclonal antibodies raised against these SR proteins. As shown in Fig. 3a, the binding ability of SC35 for the mutated sequence is clearly higher than for the wild-type site, whereas that of ASF/SF2 is not significantly affected by the mutation.
FIG. 3.
SC35 preferentially binds to the mutated PDH intronic splicing enhancer. (a) Probes corresponding to one copy of the wild-type or mutated SC35 splicing enhancer were covalently linked to agarose beads and incubated in HeLa nuclear extracts under splicing conditions. Proteins bounds to the agarose beads were eluted and analyzed by Western blot hybridization with the indicated antibodies. (b) Wild-type and mutated RNA probes were incubated in HeLa nuclear (NE) or cytoplasmic (S100) extracts under splicing conditions without or with 300 ng of purified recombinant SC35 protein. Binding reactions were cross-linked by UV radiation, digested with RNase A and RNase T1, and analyzed by SDS-PAGE. Positions of size marker proteins and SC35 are indicated on the left and on the right, respectively. (c) Cross-linking experiments were performed as described for panel b except that RNA probes were incubated in the presence of SC35 purified protein alone. Signals obtained from independent experiments were quantified by phosphorimager analysis. The mean value of the binding ratio, Mut/WT, ± the standard deviation is indicated on the side (for panel a) or the bottom (for panels b and c) of each panel.
Cross-linking experiments were performed under splicing conditions to establish the profile of proteins able to bind wild-type and mutated sequences. To this end, reactions with 32P-labeled wild-type and mutated RNA probes were exposed to UV light, submitted to RNase digestion, and analyzed by SDS-PAGE. In both HeLa nuclear extracts and S100 cytoplasmic extracts, radiolabeled probes transferred their label predominantly to a doublet of protein species with apparent molecular masses of 47 kDa. While no band migrating at the level of SC35 was detected in S100 extracts, trace amounts of a signal of the expected molecular weight were observed when both probes were incubated in nuclear extracts (Fig. 3b). Since the small amount of nuclear extracts (20 μg) used in the cross-linking assays could account for such a weak signal, and to allow better quantitation of SC35 binding, we then decided to determine the binding efficacy of SC35 to wild-type and mutated sequences under splicing conditions in S100 extracts complemented by purified recombinant SC35 protein. While SC35 was moderately reactive with both sequences and did not attenuate the 47-kDa signals (Fig. 3b), stronger signal is detected with the mutated sequence than with the wild-type sequence. Quantification of the radioactive signals (Fig. 3b) showed that the mutation increases SC35 binding to its target by almost three- to fourfold. Furthermore, cross-linking experiments in the absence of any competing protein (Fig. 3c) also demonstrated a difference of binding of the same order of magnitude between the wild-type and mutated sequences. Although we cannot definitely rule out the possibility that the mutation results in the destabilization of a splicing repressor, it seems more likely that the increased binding of SC35 to the mutated site is responsible for activation of the cryptic splice site within the PDH pre-mRNA.
In the past few years, numerous mutations that affect alternative splicing regulatory elements and that are responsible for different human diseases have been characterized (7, 17, 32). Most of them act by disrupting preexisting enhancer or silencer sequences necessary to ensure accurate splicing and/or maintain the appropriate ratio of natural protein isoforms. To our knowledge, only two well-characterized cases of de novo creation or improvement of regulatory elements resulting in human disease have been reported so far. One concerns the MAPT gene involved in frontotemporal dementia and Parkinsonism linked to chromosome 17, where the N279K mutation strengthens an enhancer sequence specific for the Tra2β splicing factor (15, 21). The second case concerns the survival of motor neuron 2 (SMN2) gene, which is unable to compensate for the inactivation of SMN1 in spinal muscular atrophy because of an exonic mutation that not only disrupts a splicing enhancer specific for ASF/SF2 but also creates a splicing silencer bound by the splicing repressor hnRNP A1 (6, 24). The mutation characterized here therefore constitutes an additional example confirming that the creation of efficient alternative splicing regulatory elements involved in the development of human pathologies may in fact be more common than previously thought.
SC35 siRNAs restore the normal E1α PDH splicing pattern in the patient's cells.
If SC35 plays an important role in activating the PDH intronic 5′ cryptic splice site in vivo, it should be possible to reverse this phenomenon by decreasing SC35 levels. This possibility was next investigated by using siRNAs (16) to reduce the amount of SC35 in primary fibroblasts from the patient. Cells were either mock transfected or transfected with increasing concentrations (25 to 100 nM) of siRNAs raised against SC35, and the corresponding protein levels were assessed by Western blot analysis 48 and 72 h following transfection. A gradual reduction of SC35 levels, directly correlated to the concentration of siRNAs used to transfect the cells, was observed 72 h posttransfection (Fig. 4a), the most dramatic effect being observed in cells treated with 100 nM siRNA. Significant reductions were also observed 48 h following transfection with 50 and 100 nM siRNA (data not shown). Under the same conditions, no significant changes were observed in the expression of ASF/SF2 and β-actin, used as internal controls (Fig. 4a).
FIG. 4.
In vivo depletion of SC35 by RNA interference in the patient's primary fibroblasts restores the use of the PDH authentic 5′ splice site. (a) The patient's cells were transfected with increasing concentrations (25, 50, and 100 nM) of duplex RNAs targeting SC35 coding sequences. Three days after, total protein extracts were prepared, resolved in an SDS-10% PAGE, and analyzed by Western blotting with the monoclonal antibodies indicated on the left. (b) Total RNA purified from the transfected cells was analyzed by RT-PCR with oligonucleotide pairs specific for the indicated mRNAs. The positions of primers allowing discrimination between normally and aberrantly spliced PDH transcripts are depicted on the right. (c) Ectopic expression of a GFP-SC35 fusion protein rescues the effects of the siRNAs. The patient's cells were transiently transfected with a GFP-SC35 expression vector without (GFP-SC35) or with (GFP-SC35 + siRNA) 50 nM duplex RNAs targeting SC35 coding sequences. Three days after, total protein extracts were analyzed by Western blotting with the monoclonal antibodies indicated on the left. (d) Total RNA purified from the transfected cells was analyzed by RT-PCR as described for panel b. Signals obtained from independent experiments were quantified by densitometric analysis and normalized to that of GAPDH (glyceraldehyde-3-phosphate dehydrogenase). The mean values ± standard deviations of the ratios siRNA/control (CTL) (25, 50, and 100 nM), GFP-SC35/CTL, and GFP-SC35 + siRNA/CTL are indicated at the bottom of each panel for SC35 and both PDH mRNAs.
RT-PCR analysis (Fig. 4b) also confirmed that SC35 expression was reduced at the mRNA level. Except for cells treated with 25 nM siRNA, this reduction was quite well correlated with that of the corresponding protein. As already suggested (14), a possible explanation for this is that our SC35 siRNAs also function like microRNAs (miRNAs), repressing SC35 gene expression without a concordant decrease in mRNA stability.
To test the effect of SC35 levels on the splicing profile of PDH primary transcripts expressed in the patient's fibroblasts, we performed RT-PCR experiments with two oligonucleotide pairs, allowing discrimination between splicing of the endogenous E1α PDH mRNAs at the authentic and cryptic 5′ splice sites downstream from exon 7. The results obtained clearly established that siRNA-mediated reduction of SC35 protein levels is correlated with an effective reduction of the aberrantly spliced E1α PDH mRNA isoform (Fig. 4b) and with a parallel increase in the normal mRNA expression level.
In order to definitely establish the role of SC35 in the aberrant PDH splicing, similar analyses were performed in the patient's primary fibroblasts transiently transfected with a GFP-SC35 fusion protein expression vector (1) with or without simultaneous siRNA treatment. As expected, expression of the GFP-SC35 protein at a level similar to that of the endogenous SC35 protein in mock-treated cells (Fig. 4c and d, lane GFP-SC35) induced a significant increase in the aberrantly spliced E1α PDH mRNA level at the expense of the normal mRNA isoform (Fig. 4c and d). In cells simultaneously transfected with the GFP-SC35 vector and the siRNAs (Fig. 4c and d, lane GFP-SC35 + siRNA), expression of the fusion protein was fully knocked down after 72 h of treatment, while that of the endogenous SC35 was still clearly detectable (Fig. 4c); this residual expression was confirmed by RT-PCR analysis of the corresponding mRNAs (Fig. 4d). Given that our siRNAs target the SC35 coding sequences, these observations raised the question of why the GFP-SC35 mRNAs were preferentially degraded. Careful analysis of the GFP sequence revealed a 70% homology (13 matches in 19 nt) between GFP (position 655 to 671 in the pEGFP-C1 sequence) and the SC35 siRNA. As already mentioned, it is expected that such homology is sufficient to trigger translational repression through miRNA activity (14). While endogenous SC35 mRNAs would be subjected to the siRNA activity only, GFP-SC35 mRNAs would be targeted by both the siRNA and miRNA pathways.
In these cells, the expression pattern of the normal and aberrant PDH mRNAs was found to be halfway between that observed in untreated cells and that of cells treated with siRNA alone (Fig. 4b), indicating thereby that the expression of an exogenous SC35 protein is able to rescue the effects of the siRNA in the patient's fibroblasts.
Taken together, these observations establish for the first time that SC35 is directly and specifically involved in a case of genetic disease in a human.
Mutations at the origin of human diseases not only affect the constitutive splice sites (26) but also cause regulatory sequences involved in the control of alternative splicing (19, 41) to produce defective proteins (7, 17). Targeting either the mutated sequences or the factors that bind to them may thus prove to be a potent strategy to correct aberrant splicing. In agreement with this, successful corrections have been accomplished by RNase H inactive 2′-O-methyl and 2′-O-methoxyethyl antisense oligonucleotides, which hybridize to regulatory sequences and prevent their use by the splicing machinery (23, 35, 40). Alternatively, approaches including the overexpression of proteins that regulate splicing of the affected exon (20, 37) and the design of peptide-nucleic acids mimicking SR proteins to stimulate exon inclusion (8) have also been described. Recently, RNA interference targeting hnRNP A1 and A2 proteins has been shown to restore SMN2 exon 7 inclusion in cells expressing a transfected minigene (24). To our knowledge, the results presented here constitute the first report of an RNA interference approach allowing for the correction of an aberrant splicing event and the restoration of a normal expression profile of the mutated gene in primary cells from the patient.
Acknowledgments
We thank J. Stévenin for providing us with ASF/SF2 and SC35 purified proteins and antibodies.
This work was supported by the GIS-Institut des maladies rares, by the Contrat de recherche clinique CRC 03-004 to M.B., and by a grant from the Association pour la Recherche sur le Cancer to J.S. This work was also supported in part by contract QLG2-CT-1999-00660 from the European Union. M.G. was supported by a graduate fellowship from the Ministère Délégué à la Recherche et aux Technologies. M.M. was supported by a grant from the Fédération des maladies orphelines FMO-AFRG.
REFERENCES
- 1.Allemand, E., S. Dokudovskaya, R. Bordonne, and J. Tazi. 2002. A conserved Drosophila transportin-serine/arginine-rich (SR) protein permits nuclear import of Drosophila SR protein splicing factors and their antagonist repressor splicing factor 1. Mol. Biol. Cell 13:2436-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blencowe, B. J. 2000. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem. 25:106-110. [DOI] [PubMed] [Google Scholar]
- 3.Brown, G. K., L. J. Otero, M. LeGris, and R. M. Brown. 1994. Pyruvate dehydrogenase deficiency. J. Med. Genet. 31:875-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caputi, M., A. Mayeda, A. R. Krainer, and A. M. Zahler. 1999. hnRNP A/B proteins are required for inhibition of HIV-1 pre-mRNA splicing. EMBO J. 18:4060-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardozo, A. K., L. De Meirleir, I. Liebaers, and W. Lissens. 2000. Analysis of exonic mutations leading to exon skipping in patients with pyruvate dehydrogenase E1 alpha deficiency. Pediatr. Res. 48:748-753. [DOI] [PubMed] [Google Scholar]
- 6.Cartegni, L., and A. R. Krainer. 2002. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet. 30:377-384. [DOI] [PubMed] [Google Scholar]
- 7.Cartegni, L., S. L. Chew, and A. R. Krainer. 2002. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat. Rev. Genet. 3:285-298. [DOI] [PubMed] [Google Scholar]
- 8.Cartegni, L., and A. R. Krainer. 2003. Correction of disease-associated exon skipping by synthetic exon-specific activators. Nat. Struct. Biol. 10:120-125. [DOI] [PubMed] [Google Scholar]
- 9.Cartegni, L., J. Wang, Z. Zhu, M. Q. Zhang, and A. R. Krainer. 2003. ESE finder: a web resource to identify exonic splicing enhancers. Nucleic Acids Res. 31:3568-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun, K., N. MacKay, R. Petrova-Benedict, A. Federico, A. Fois, D. E. Cole, E. Robertson, and B. H. Robinson. 1995. Mutations in the X-linked E1 alpha subunit of pyruvate dehydrogenase: exon skipping, insertion of duplicate sequence, and missense mutations leading to the deficiency of the pyruvate dehydrogenase complex. Am. J. Hum. Genet. 56:558-569. [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl, H. H., G. K. Brown, R. M. Brown, L. L. Hansen, D. S. Kerr, I. D. Wexler, M. S. Patel, L. De Meirleir, W. Lissens, K. Chun, N. Mackay, and B. H. Robinson. 1992. Mutations and polymorphisms in the pyruvate dehydrogenase E1 α gene. Hum. Mutat. 1:97-102. [DOI] [PubMed] [Google Scholar]
- 12.De Meirleir, L., W. Lissens, C. Benelli, G. Ponsot, I. Desguerre, C. Marsac, D. Rodriguez, J. M. Saudubray, F. Poggi, and I. Liebaers. 1994. Aberrant splicing of exon 6 in the pyruvate dehydrogenase-E1 alpha mRNA linked to a silent mutation in a large family with Leigh's encephalomyelopathy. Pediatr. Res. 36:707-712. [DOI] [PubMed] [Google Scholar]
- 13.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doench, J. G., C. P. Petersen, and P. A. Sharp. 2003. siRNAs can function as miRNAs. Genes Dev. 17:438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Souza, I., P. Poorkaj, M. Hong, D. Nochlin, V. M. Lee, T. D. Bird, and G. D. Schellenberg. 1999. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc. Natl. Acad. Sci. USA 96:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elbashir, S. M., J. Harborth, K. Weber, and T. Tuschl. 2002. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 26:199-213. [DOI] [PubMed] [Google Scholar]
- 17.Faustino, N. A., and T. A. Cooper. 2003. Pre-mRNA splicing and human disease. Genes Dev. 17:419-437. [DOI] [PubMed] [Google Scholar]
- 18.Fu, X. D. 1995. The superfamily of arginine/serine-rich splicing factors. RNA 1:663-680. [PMC free article] [PubMed] [Google Scholar]
- 19.Graveley, B. R. 2000. Sorting out the complexity of SR protein functions. RNA 6:1197-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann, Y., C. L. Lorson, S. Stamm, E. J. Androphy, and B. Wirth. 2000. Htra2-beta 1 stimulates an exonic splicing enhancer and can restore full-length SMN expression to survival motor neuron 2 (SMN2). Proc. Natl. Acad. Sci. USA 97:9618-9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang, Z., H. Tang, N. Havlioglu, X. Zhang, S. Stamm, R. Yan, and J. Y. Wu. 2003. Mutations in tau gene exon 10 associated with FTDP-17 alter the activity of an exonic splicing enhancer to interact with Tra2 beta. J. Biol. Chem. 278:18997-19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, J. M., J. Castle, P. Garrett-Engele, Z. Kan, P. M. Loerch, C. D. Armour, R. Santos, E. E. Schadt, R. Stoughton, and D. D. Shoemaker. 2003. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science 302:2141-2144. [DOI] [PubMed] [Google Scholar]
- 23.Kalbfuss, B., S. A. Mabon, and T. Misteli. 2001. Correction of alternative splicing of tau in frontotemporal dementia and parkinsonism linked to chromosome 17. J. Biol. Chem. 276:42986-42993. [DOI] [PubMed] [Google Scholar]
- 24.Kashima, T., and J. L. Manley. 2003. A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat. Genet. 34:460-463. [DOI] [PubMed] [Google Scholar]
- 25.Kohtz, J. D., S. F. Jamison, C. L. Will, P. Zuo, R. Luhrmann, M. A. Garcia-Blanco, and J. L. Manley. 1994. Protein-protein interactions and 5′-splice-site recognition in mammalian mRNA precursors. Nature 368:119-124. [DOI] [PubMed] [Google Scholar]
- 26.Krawczak, M., J. Reiss, and D. N. Cooper. 1992. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum. Genet. 90:41-54. [DOI] [PubMed] [Google Scholar]
- 27.Labourier, E., E. Allemand, S. Brand, M. Fostier, J. Tazi, and H. M. Bourbon. 1999. Recognition of exonic splicing enhancer sequences by the Drosophila splicing repressor RSF1. Nucleic Acids Res. 27:2377-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labourier, E., H. M. Bourbon, I. Gallouzi, M. Fostier, E. Allemand, and J. Tazi. 1999. Antagonism between RSF1 and SR proteins for both splice site recognition in vitro and Drosophila development. Genes Dev. 13:740-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladd, A. N., and T. A. Cooper. 2001. Finding signals that regulate alternative splicing in the post-genomic era. Genome Biol. 3:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lissens, W., L. De Meirleir, S. Seneca, C. Benelli, C. Marsac, B. T. Poll-The, P. Briones, W. Ruitenbeek, O. van Diggelen, D. Chaigne, V. Ramaekers, and I. Liebaers. 1996. Mutation analysis of the pyruvate dehydrogenase E1 alpha gene in eight patients with a pyruvate dehydrogenase complex deficiency. Hum. Mutat. 7:46-51. [DOI] [PubMed] [Google Scholar]
- 31.Lissens, W., L. De Meirleir, S. Seneca, I. Liebaers, G. K. Brown, R. M. Brown, M. Ito, E. Naito, Y. Kuroda, D. S. Kerr, I. D. Wexler, M. S. Patel, B. H. Robinson, and A. Seyda. 2000. Mutations in the X-linked pyruvate dehydrogenase (E1) alpha subunit gene (PDHA1) in patients with a pyruvate dehydrogenase complex deficiency. Hum. Mutat. 15:209-219. [DOI] [PubMed] [Google Scholar]
- 32.Liu, H. X., L. Cartegni, M. Q. Zhang, and A. R. Krainer. 2001. A mechanism for exon skipping caused by nonsense or missense mutations in BRCA1 and other genes. Nat. Genet. 27:55-58. [DOI] [PubMed] [Google Scholar]
- 33.Manley, J. L., and R. Tacke. 1996. SR proteins and splicing control. Genes Dev. 10:1569-1579. [DOI] [PubMed] [Google Scholar]
- 34.Marsac, C., C. Benelli, I. Desguerre, M. Diry, F. Fouque, L. De Meirleir, G. Ponsot, S. Seneca, F. Poggi, J. M. Saudubray, M. T. Zabot, D. Fontan, and W. Lissens. 1997. Biochemical and genetic studies of four patients with pyruvate dehydrogenase E1 alpha deficiency. Hum. Genet. 99:785-792. [DOI] [PubMed] [Google Scholar]
- 35.Mercatante, D. R., and R. Kole. 2002. Control of alternative splicing by antisense oligonucleotides as a potential chemotherapy: effects on gene expression. Biochim. Biophys. Acta 1587:126-132. [DOI] [PubMed] [Google Scholar]
- 36.Mine, M., M. Brivet, G. Touati, P. Grabowski, M. Abitbol, and C. Marsac. 2003. Splicing error in E1 alpha pyruvate dehydrogenase mRNA caused by novel intronic mutation responsible for lactic acidosis and mental retardation. J. Biol. Chem. 278:11768-11772. [DOI] [PubMed] [Google Scholar]
- 37.Nissim-Rafinia, M., O. Chiba-Falek, G. Sharon, A. Boss, and B. Kerem. 2000. Cellular and viral splicing factors can modify the splicing pattern of CFTR transcripts carrying splicing mutations. Hum. Mol. Genet. 9:1771-1778. [DOI] [PubMed] [Google Scholar]
- 38.Robinson, B. H. 1995. Lactic acidemia (disorders of pyruvate carboxylase, pyruvate dehydrogenase), p. 1479-1499. In C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle (ed.), The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, N.Y.
- 39.Rossi, F., E. Labourier, T. Forne, G. Divita, J. Derancourt, J. F. Riou, E. Antoine, G. Cathala, C. Brunel, and J. Tazi. 1996. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature 381:80-82. [DOI] [PubMed] [Google Scholar]
- 40.Sazani, P., F. Gemignani, S. H. Kang, M. A. Maier, M. Manoharan, M. Persmark, D. Bortner, and R. Kole. 2002. Systemically delivered antisense oligomers upregulate gene expression in mouse tissues. Nat. Biotechnol. 20:1228-1233. [DOI] [PubMed] [Google Scholar]
- 41.Tacke, R., and J. L. Manley. 1999. Determinants of SR protein specificity. Curr. Opin. Cell Biol. 11:358-362. [DOI] [PubMed] [Google Scholar]
- 42.Wu, J. Y., and T. Maniatis. 1993. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 75:1061-1070. [DOI] [PubMed] [Google Scholar]
- 43.Zhu, J., A. Mayeda, and A. R. Krainer. 2001. Exon identity established through differential antagonism between exonic splicing silencer-bound hnRNP A1 and enhancer-bound SR proteins. Mol. Cell 8:1351-1361. [DOI] [PubMed] [Google Scholar]
- 44.Zuo, P., and T. Maniatis. 1996. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 10:1356-1368. [DOI] [PubMed] [Google Scholar]