Abstract
Background
This review examined the role of artificial intelligence (AI) in the diagnosis and management of rotator cuff tears (RCTs).
Methods
A literature search was conducted in October 2023 using PubMed (MEDLINE), SCOPUS, and EMBASE databases, included only peer-reviewed studies. Relevant articles on AI technology in RCTs. A critical analysis of the relevant literature was conducted.
Results
AI is transforming RCTs management through faster and more precise identification and assessment using algorithms that facilitate segmentation, quantification, and classification of the RCTs across various imaging modalities. Precise algorithms focusing on preoperative factors to assess RCTs reparability have been developed for personalized treatment planning and outcome prediction. AI also aids in exercise classification and promotes patient adherence during at-home physiotherapy. Despite promising advancements, challenges in data quality and symptom integration persist. Future research should include refining AI algorithms, expanding their integration into various imaging techniques, and exploring their roles in postoperative care and surgical decision-making.
Conclusions
AI-driven solutions improve diagnostic accuracy and have the potential to influence treatment planning and postoperative outcomes through the automated RCTs analysis of medical imaging. Integration of high-quality datasets and clinical symptoms into AI models can enhance their reliability. Current AI algorithms can also be refined, integrated into other imaging techniques, and explored further in surgical decision-making and postoperative care.
Keywords: Artificial intelligence, Rotator cuff tears, Diagnostic imaging, Machine learning, Personalized medicine, Deep learning
Abbreviations
- AI
Artificial Intelligence
- AUC
Area Under the Curve
- CAD
Computer-Aided Diagnosis
- CNN
Convolutional Neural Network
- DSC
Dice Similarity Coefficient
- KNN
K-Nearest Neighbors
- MRA
Magnetic Resonance Arthrography
- MCID
Minimal Clinically Important Difference
- MRI
Magnetic Resonance Imaging
- RCTs
Rotator Cuff Tears
- SCB
Substantial Clinical Benefit
- USG-Net:
Ultrasound Scanning-Guide Network
Author contributions
AVG and KH contributed to search design, performed the search and data extraction, and in content writing and editing along with KM. Additionally, KH and KM were responsible for document formatting and referencing. AVG and KH crafted the final draft and managing the submission process, with all authors reviewing and endorsing the manuscript's ultimate version.
1. 1- Introduction
Rotator cuff tears (RCTs) are a frequent orthopedic condition that affects diverse demographics, including professional athletes and the elderly.1 The incidence of RCTs in the general population has been researched extensively, and genetic and family susceptibility is associated with this condition.2,3 Several risk factors for RCTs have also been identified, including smoking and acromion morphology.4, 5, 6 The socioeconomic burden of RCTs is significant because of the high prevalence of the condition and the associated costs of diagnosis, treatment, and rehabilitation.7
The rotator cuff is susceptible to a wide spectrum of injuries, ranging from asymptomatic partial-thickness tears to debilitating full-thickness tears.8,9 Accurate diagnosis, differentiation of tear severity, and personalized treatment planning are essential to ensure successful recovery.10,11 Diagnosing and assessing the severity of RCTs is complicated because it involves a combination of clinical presentation, physical examination, and medical imaging.10,12
The standard diagnostic method involves using various radiological techniques, such as magnetic resonance imaging (MRI), ultrasound, and X-rays, which are complemented by the specialized expertise of radiologists and orthopedic surgeons.12, 13, 14 MRI is considered the gold standard for rotator cuff imaging. It is widely used for evaluation because of its high sensitivity for detecting full- and partial-thickness tears and for accurate identification of location and extent.13,15,16 Although X-rays are useful for identifying massive irreparable RCTs by observing humeral head migration and arthropathy,17 they have limited sensitivity and specificity, especially in cases involving partial and smaller full-thickness tears.18 Ultrasound and MRI have been shown to have similar accuracies in detecting RCTs in certain cases.12,19 Although these techniques have significantly increased the accuracy of diagnosis, a certain level of variability persists, contingent on the proficiency of healthcare providers and distinct characteristics of each case.12,20
The introduction of artificial intelligence (AI), a technological frontier currently reshaping the landscape of medical diagnostics, is paramount to understanding its role in addressing the complexities associated with RCTs. AI is a promising solution to the challenges in diagnosing and treating this widespread musculoskeletal condition. The remarkable capabilities of pattern recognition, image analysis, and predictive modeling herald a paradigm shift in our approach to managing rotator cuff pathology.21,22 AI applications can excel in four areas of musculoskeletal imaging analysis: segmentation, classification, lesion detection, and non-interpretive tasks.23
This study provides a comprehensive exploration of the evolving role of AI in the diagnosis and management of RCTs. The objectives encompass AI's integration of AI into diverse imaging modalities for accurate RCTs diagnosis, its potential to personalize treatment planning by predicting outcomes, and its impact on rehabilitation and progress monitoring. This article also addresses current challenges and limitations and outlines future research avenues to fully exploit the potential of AI. The general objective is to highlight the transformative potential of AI in improving patient care and reshaping orthopedic practice related to RCTs.
2. Materials and methods
A comprehensive literature search was conducted in October 2023, encompassing the PubMed (MEDLINE), SCOPUS, and EMBASE databases, focusing only on peer-reviewed articles. The primary objective of this search was to identify relevant studies on the use of AI technology in RCTs. Following this, a rigorous critical analysis was conducted, with the goal of creating a narrative review.
Integration of AI into diagnostic imaging modalities for RCTs.
2.1. MRI imaging
MRI is a crucial tool for diagnosing RCTs because of its exceptional soft-tissue contrast and lack of invasiveness. A network meta-analysis showed that high-field magnetic resonance arthrography (MRA) is the most effective method for identifying RCTs, followed by low-field MRA, high-field MRI, high-frequency ultrasound, low-field MRI, and low-frequency ultrasound.24 However, diagnosing RCTs can be time-consuming and challenging, particularly for certain conditions, such as intratendinous tears.25
2.2. AI models and algorithms in MRI
Recent advancements in machine learning (ML) models have yielded significant improvements in the accuracy and efficiency of detecting and classifying RCTs using MRI scans. These state-of-the-art AI models excel in processing extensive image datasets, enabling the precise identification of tear locations and severity. A series of studies have investigated the application of AI for detecting rotator cuff pathology through MRI, collectively showing remarkable diagnostic performance.26, 27, 28, 29, 30 These AI models consistently achieved impressive diagnostic performance, often surpassing an area under the curve (AUC) value of 0.8, as documented in a systematic review by Zhan et al.31 Notably, among the evaluated models, k-nearest neighbors (KNN) and the Capsule Network (CapsNet)-based Computer-Aided Diagnosis (CAD) system emerged as the top performers in two separate studies.31
One study used a convolutional neural network (CNN) on shoulder MRI scans to diagnose RCTs and applied a weighted linear combination layer to handle sequential information and weighted cross-entropy loss to address class imbalance. This resulted in a diagnostic accuracy of 87 % and M-AUC score of 97 %, outperforming human annotators with a diagnostic accuracy of 76 % and M-AUC score of 81 %.32
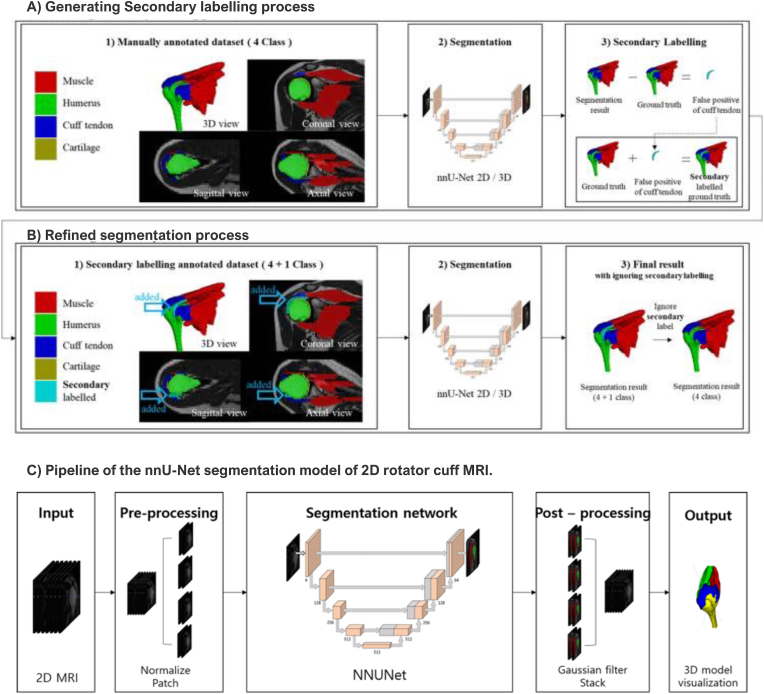
AI-based segmentation, quantification, and classification models represent a valuable avenue for comprehensive analysis of rotator cuff injuries.23 Beyond the mere identification of injury presence, these AI-driven methodologies enhance the granularity and depth of RCTs evaluation, thereby promising more effective diagnostics and treatment planning in this clinical domain.23 Kim et al. used a CNN to automate semantic segmentation on 2D MRI images of RCTs, helping to visualize 3D models of related anatomic structures. This method accelerated manual segmentation for 3D reconstruction of MRI in RCTs. The Dice Similarity Coefficient (DSC) was used to evaluate segmentation quality. The study achieved high DSC scores for cuff tendon (81 % ± 10 %), muscle (86 % ± 9 %), bone (98 % ± 1 %), and cartilage (81 % ± 15 %) (Fig. 1).3.3
Fig. 1.
Example of ground truth creation and pipeline of the no new net (nnU-Net) segmentation process of rotator cuff MRI. A) Secondary labeling enhances segmentation accuracy by addressing false positives. B) Refined segmentation training further improves the results. C) The diagram depicts the nnU-Net model pipeline for 2D rotator cuff MRI. Adapted from Fig. 1, Fig. 3 in Kim H, Shin K, Kim H et al. (2022) Can Deep Learning Reduce the Time and Effort Required for Manual Segmentation in 3D Reconstruction of MRI in Rotator Cuff Tears? PLoS One. https://doi.org/10.1371/journal.pone.0274075. Licensed under CC BY.
Another deep learning model has been developed for automated quantification and classification of a full-thickness posterosuperior RCTs from MR images, including the calculation of tear width and retraction. The model classifies tears based on their patterns, extensions, and retractions. The accuracy of tear retraction calculation was 6.6 mm ± 6.5 mm, compared to the interrater variability of manual segmentations, which was 3.8 mm ± 3.6 mm23.
2.3. Ultrasound imaging
Ultrasound is another diagnostic tool that can identify RCTs, although it may have lower sensitivity and specificity than MRI.34 Several studies have examined the potential of AI for detecting and classifying various rotator cuff pathologies using ultrasound images. AI, including deep learning models and feature extraction techniques, such as gray-level co-occurrence matrices, can enhance the diagnosis of several pathological conditions of the shoulder. Different AI approaches, from traditional CNN models to innovative end-to-end models, such as SMART-CA, demonstrate promising diagnostic capabilities.31
2.4. AI models and algorithms in ultrasound
In one study, five pretrained deep models (VGG19, InceptionV3, Xception, ResNet50, and DenseNet121) were used through transfer learning to develop an approach to automatically classify and fine-tune RCTs and provide visualization of tear location. Bayesian optimization was employed to optimized the hyperparameters, and the CNN models were trained and tested. The use of Grad-CAM in the learning process of ultrasound images helped identify critical features (Fig. 2). Notably, DenseNet121 emerged as the top performer, achieving 88 % accuracy, 94 % sensitivity, 84 % specificity, and an AUC score of 0.832.35
Fig. 2.
Methodology featuring pre-trained models optimized using Bayesian techniques, along with their predictions, complemented by Grad-CAM heatmap images for identifying rotator cuff tears (Figure reproduced with permission from Ho TT, Kim G-T, Kim T et al. (2022) Classification of rotator cuff tears in ultrasound images using deep learning models. Med Biol Eng Comput 60:1269–1278. https://doi.org/10.1007/s11517-022-02502-6.
A novel end-to-end fully CNN, denoted as the Segmentation Model Adopting a pRe-trained Classification Architecture (SMART-CA), was developed to diagnose the locations of RCTs using ultrasound images. SMART-CA can improve the segmentation accuracy by extracting distinct features that cannot be extracted using a normal encoder. The SMART-CA algorithm has demonstrated enhanced precision, recall, and DSC accuracy in RCT segmentation of normal and severe speckle noise ultrasound images (Fig. 3).36
Fig. 3.
Structure of SMART-CA Training. SMART-CA comprises three key components: a pretrained encoder, a trainable encoder, and a decoder. (a) The pretrained encoder was initially trained to address the classification task and determine the presence of rotator cuff tears (RCTs) in ultrasound (US) images. Subsequently, (b) the trainable encoder and decoder undergo training for the segmentation task, which focuses on precisely localizing the RCT. From Lee K, Kim JY, Lee MH et al. (2021) Imbalanced loss-integrated deep-learning-based ultrasound image analysis for diagnosis of rotator cuff tear. Sensors 21:1–20. https://doi.org/10.3390/s21062214. with CC BY permission.
Another study developed the novel concept of an ultrasound scanning-guide network (USG-Net).37 This is an innovative deep-learning-based approach designed to precisely identify RCTs using a dataset comprising multidimensional ultrasound images from 80 patients. USG-Net detects RCTs and offers real-time guidance to users, ensuring precise placement of the ultrasound probe for optimal image acquisition. Preliminary findings confirm the efficacy of this refined scanning-guide algorithm, underscoring its ability to facilitate real-time probe localization and enhance the acquisition of diagnostically valuable ultrasound images.37
2.5. Radiographic imaging
The use of AI in the analysis of X-ray images for diagnosing RCTs is supported in the potential connection between radiographic measurements and rotator cuff pathology. Yu et al. hypothesized that the relationship between AI, lateral acromion angle, and critical shoulder angle are relevant in the diagnosis of RCTs.38 However, only a few studies have used AI algorithms to detect RCTs using X-rays.
2.6. AI models and algorithms in radiographic images
Iio et al. developed a deep-learning algorithm to detect RCTs on shoulder radiographs. The algorithm was trained on 2803 images and classified as intact or with varying degrees of tears. The algorithm's performance had an AUC of 0.82, a high sensitivity of 91 %, a negative predictive value of 93 %, and a low negative likelihood ratio of 0.16. For full-thickness tears, the sensitivity and negative predictive value were 95 % and 96 %, respectively. In contrast, for partial-thickness tears, the sensitivity and negative predictive values were 78.9 % and 96.2 %, respectively.39 Another CNN architecture was implemented using TensorFlow and Keras for X-ray image processing. The results revealed significantly enhanced accuracy in diagnosing RCTs using this ML-based approach, with an accuracy of 80 % for full-thickness tears and 88 % for partial-thickness tears, surpassing the accuracy of physician-based diagnosis, which were 73 % and 60 % for full-thickness and partial-thickness tears, respectively.40
3. Personalized treatment planning and outcome prediction
AI has the potential to improve decision-making, postoperative care, and rehabilitation. Recent data have shown promise in personalized treatment planning and the prediction of outcomes in patients with RCTs. Various clinical parameters have been investigated to improve the treatment planning and prognosis.
3.1. RCTs reparability
Preoperative factors of RCT reparability have been investigated, including RCT size, arthropathy, superior migration of the humeral head, fatty infiltration of the supraspinatus and infraspinatus muscles, and supraspinatus muscle atrophy. All these factors have been found to be correlated with the reparability of large-to-massive RCTs.41 AI algorithms can incorporate these and other factors to provide personalized predictions of reparability.
A study developed a fully convolutional deep learning algorithm to analyze supraspinatus muscle atrophy by quantifying the occupation ratio of the supraspinatus muscle within the fossa on MRI images. The results shown high pixel-wise accuracy, with 0.998 ± 0.073 in the fossa region and 0.9988 ± 0.065 in the muscle region. Additionally, DSC demonstrated a strong performance, with 0.9718 ± 0.012 in the fossa region and 0.9463 ± 0.047 in the muscle region.42
Ro et al. evaluated the fatty infiltration of the supraspinatus muscle in sagittal MRI images using an automated Otsu thresholding technique and a CNN trained based on the VGG19 network. The evaluation was performed with high accuracy, sensitivity, and specificity, with DSC values of 0.97 for the supraspinatus fossa and 0.94 for the muscle. The relative area difference was 0.07 for the fossa and 2.03 for the muscle. These results indicate a high degree of similarity between the fatty infiltration assessment by clinicians and AI analysis (Fig. 4).43
Fig. 4.
Fatty infiltration analysis of the supraspinatus muscle using a deep learning and computer-assisted approach on MRI data. This figure shows outlier cases regarding occupation ratio and fatty infiltration, revealing a noteworthy negative correlation between the two variables. Some cases displayed elevated occupation ratios and fatty infiltrations (A), whereas others exhibited comparatively lower values (B). Adapted from Ro K, Kim JY, Park H et al. (2021) Deep-learning framework and computer assisted fatty infiltration analysis for the supraspinatus muscle in MRI. Sci Rep 11:. https://doi.org/10.1038/s41598-021-93026-w. Under CCBY.
3.2. Postoperative outcome prediction
The success of RCT repair surgery is contingent on multiple patient-related, pathology-related, and surgical technical factors. Potty et al. trained an ML model using preoperative patient information, such as demographic data, comorbidities, RCT characteristics, tissue quality, and fixation implant details, to generate predicted postoperative American Shoulder and Elbow Surgeons scores for individual patients. The results showed that the algorithm successfully predicted 67 % of the 12-month postoperative outcomes within the minimal clinically important difference (MCID) threshold, and 84 % fell within the substantial clinical benefit (SCB) range.44 Similarly, Allaart et al. proposed a study protocol for gathering all possible factors that may impact the risk of retear from retrospective multicenter data, to incorporate more than 1000 patients globally to ultimately develop an ML algorithm that can predict the risk of retear in patients undergoing rotator cuff repair surgery.45
4. Advancements on rehabilitation and postoperative exercise monitoring
Identifying and addressing barriers to physiotherapy adherence requires accurate and objective measurement.46 Patient-reported registers may have low completion rates and bias.47 AI-driven solutions could benefit patient engagement and rehabilitation outcomes in at-home shoulder physiotherapy.47
Boyer et al.47 evaluated the effectiveness of ML methods in detecting and classifying inertial data gathered during both in-clinic and at-home shoulder physiotherapy exercises. Using a smartwatch, the authors collected inertial data from 42 patients who underwent rotator cuff injury rehabilitation. A two-stage ML approach was used to identify non-exercise data and classify exercises.47 The results showed that a patient-specific approach with engineered features achieved the highest performance in distinguishing physiotherapy exercises from non-exercise activity during in-clinic sessions, and incorporating non-exercise data in training further enhanced the classifier performance.
The best accuracy for classifying individual in-clinic exercises was 0.903 (area under the receiver operating characteristic), using a patient-specific method with deep neural network model-derived features.47 Grouping exercises by motion type also improved the exercise classification. For at-home data, out-of-distribution detection achieved a similar performance when non-exercise data were included in the algorithm training. These results may be beneficial depending on the data quality, including non-exercise data in algorithm training, enhanced exercise detection, and a patient-specific approach.47
5. Challenges and limitations in AI implementation
The integration of AI into the diagnosis and treatment of RCTs presents different challenges and constraints. One of the main challenges is the complexity of diagnosing RCTs, which relies on clinical assessment, functional testing, and various imaging modalities.48 To achieve reliable diagnoses and increase the utility in clinical decision-making processes, AI models must be able to synthesize and interpret this diverse information beyond the structural diagnosis of RCTs, particularly clinical symptoms,49 demographic data, surgical features, and rehabilitation protocols.
Another important constraint is the substantial diversity in the manifestation and severity of RCTs among patients. Variables such as tear size, location, and presence of concomitant injuries exhibit substantial heterogeneity.50 Consequently, AI models must adapt to this variability and tailor their recommendations to individual cases to provide personalized management strategies. Additionally, the effectiveness of AI models in diagnosing RCTs is contingent on the quality and accessibility of the data. The acquisition of precise and comprehensive datasets is essential for the training and validation of AI algorithms.22 However, procuring such datasets can be challenging owing to the limited availability of high-quality imaging data and standardized outcome metrics.51
6. Future avenues and directions for research
The advancement of AI algorithms in diagnosing RCTs has shown promising results; however, there is still room for improvement. Future research should focus on developing more sophisticated AI algorithms capable of analyzing complex datasets and delivering more precise and reliable diagnoses. This could enhance the overall accuracy of RCT diagnosis. Additionally, there is potential for expanding the scope of AI applications within the field of RCTs, including integrating current AI algorithms into other imaging techniques, such as ultrasound. This could revolutionize the diagnosis of RCTs and provide clinicians with a broader toolkit for accurate assessments.
Moreover, integrating AI into routine clinical practice to diagnose and manage RCTs is a promising research area. Future research will involve overcoming potential barriers to implementation and devising strategies for seamless AI integration into daily clinical workflow. There is also the potential for developing predictive models, investigating its potential in surgical decision-making processes, and exploring the role of AI in postoperative care and rehabilitation.
7. Conclusions
AI is revolutionizing the diagnosis and management of RCTs, demonstrating its potential in various aspects of care. AI-based solutions can provide more precise and efficient diagnostic, aid in personalized treatment planning, and predict postoperative outcomes. AI algorithms automate segmentation and quantification of RCTs in imaging, incorporating some preoperative factors to predict reparability, clinical outcomes, and potentially enhance rehabilitation through improved exercise classification and patient adherence during at-home physiotherapy. However, high-quality standardized datasets and the incorporation of clinical symptoms and other demographics into current AI models are essential for reliable and clinically relevant diagnoses. Future research should focus on refining AI algorithms, integrating them into other imaging techniques, and exploring their potential in postoperative care and surgical decision-making. AI holds the promise of personalized and effective care for patients with this prevalent orthopedic condition. However, it should be approached with caution and should not replace the expertise of healthcare professionals.
Ethics approval
The research described in this study involving literature review and analysis did not involve human participants, animal subjects, or identifiable personal data. Therefore, ethical approval was not applicable for this research.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Patient's consent
Not applicable.
Declaration of competing interest
The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Acknowledgments
Not applicable.
References
- 1.Arner J.W., Provencher M.T., Bradley J.P., Millett P.J. Evaluation and management of the contact athlete's shoulder. J Am Acad Orthop Surg. 2022 doi: 10.5435/jaaos-d-20-01374. Published online. [DOI] [PubMed] [Google Scholar]
- 2.Longo U.G., Candela V., Berton A., et al. Genetic basis of rotator cuff injury: a systematic review. BMC Med Genet. 2019 doi: 10.1186/s12881-019-0883-y. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dabija D.I., Gao C., Edwards T.L., Kuhn J.E., Jain N.B. J Shoulder Elbow Surg; 2017. Genetic and Familial Predisposition to Rotator Cuff Disease: A Systematic Review. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgarten K.M., Gerlach D., Galatz L.M., et al. Clin Orthop Relat Res; 2010. Cigarette Smoking Increases the Risk for Rotator Cuff Tears. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J.R., Ryu K.J., Hong I.T., Kim B.K., Kim J.H. Can a high acromion index predict rotator cuff tears? Int Orthop. 2012 doi: 10.1007/s00264-012-1499-4. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyffeler R.W., Meyer D.C. Acromion and glenoid shape: why are they important predictive factors for the future of our shoulders? EFORT Open Rev. 2017 doi: 10.1302/2058-5241.2.160076. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto A., Takagishi K., Osawa T., et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010 doi: 10.1016/j.jse.2009.04.006. Published online. [DOI] [PubMed] [Google Scholar]
- 8.Mihata T., Morikura R., Hasegawa A., et al. Partial-thickness rotator cuff tear by itself does not cause shoulder pain or muscle weakness in baseball players. Am J Sports Med. 2019 doi: 10.1177/0363546519878141. Published online. [DOI] [PubMed] [Google Scholar]
- 9.Fucentese S.F., Roll AL von, Pfirrmann C.W.A., Gerber C., Jost B. Evolution of nonoperatively treated symptomatic isolated full-thickness supraspinatus tears. J Bone Joint Surg. 2012 doi: 10.2106/jbjs.i.01286. Published online. [DOI] [PubMed] [Google Scholar]
- 10.Morag Y., Jacobson J.A., Miller B.S., Maeseneer M De, Girish G., Jamadar D.A. MR imaging of rotator cuff injury: what the clinician needs to know. Radiographics. Published online. 2006 doi: 10.1148/rg.264055087. [DOI] [PubMed] [Google Scholar]
- 11.Minagawa H., Yamamoto N., Abe H., et al. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: from mass-screening in one village. J Orthop. 2013;10(1):8–12. doi: 10.1016/j.jor.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jesus JO de, Parker L., Frangos A.J., Nazarian L.N. American Journal of Roentgenology; 2009. Accuracy of MRI, MR Arthrography, and Ultrasound in the Diagnosis of Rotator Cuff Tears: A Meta-Analysis. Published online. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan N.S., Ahluwalia A., Sharma Y.P., Thakur L. A prospective comparative study of high resolution ultrasound and MRI in the diagnosis of rotator cuff tears in a tertiary hospital of north India. Pol J Radiol. 2016 doi: 10.12659/pjr.897830. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A., Thukral C.L., Gupta K., Singh M.I., Lata S., Arora R.K. Role and correlation of high resolution ultrasound and magnetic resonance imaging in evaluation of patients with shoulder pain. Pol J Radiol. 2017;82:410–417. doi: 10.12659/PJR.901540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juel N.G., Natvig B. Shoulder diagnoses in secondary care, a one year cohort. BMC Muscoskel Disord. 2014 doi: 10.1186/1471-2474-15-89. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutten M.J.C.M., Spaargaren G.J., loon T van, Malefijt MC. de W., Kiemeney L.A., Jager G.J. Eur Radiol; 2009. Detection of Rotator Cuff Tears: The Value of MRI Following Ultrasound. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith C., Dattani R., Deans V.M., Drew S. 2010. The Sourcil Sign: A Useful Finding on Plain X-Ray? Shoulder Elbow. Published online. [DOI] [Google Scholar]
- 18.Wallny T., Wagner U., Prange S.R., Schmitt O., Reich H. Evaluation of chronic tears of the rotator cuff by ultrasound. Journal of Bone and Joint Surgery - British. 1999;ume doi: 10.1302/0301-620x.81b4.0810675. Published online. [DOI] [PubMed] [Google Scholar]
- 19.Park B.K., Hong S., Jeong W.K. Effectiveness of ultrasound in evaluation of fatty infiltration in rotator cuff muscles. Clin Orthop Surg. 2020 doi: 10.4055/cios.2020.12.1.76. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis J. Rotator cuff tendinopathy/subacromial impingement syndrome: is it time for a new method of assessment? Br J Sports Med. 2009 doi: 10.1136/bjsm.2008.052183. Published online. [DOI] [PubMed] [Google Scholar]
- 21.Fritz B., Fritz J. Artificial intelligence for MRI diagnosis of joints: a scoping review of the current state-of-the-art of deep learning-based approaches. Skeletal Radiol. 2022;51(2):315–329. doi: 10.1007/s00256-021-03830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Familiari F., Galasso O., Massazza F., et al. Int J Environ Res Public Health; 2022. Artificial Intelligence in the Management of Rotator Cuff Tears. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber S. 2021. Deep Learning Based Fully Automatic Quantification of Rotator Cuff Tears from MRI [Masters Thesis]https://api.semanticscholar.org/CorpusID:250921014 [Google Scholar]
- 24.Liu F., Dong J., Shen W.J., Kang Q., Zhou D., Xiong F. Detecting rotator cuff tears: a network meta-analysis of 144 diagnostic studies. Orthop J Sports Med. 2020;8(2) doi: 10.1177/2325967119900356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim K.C., Lee W.Y., Shin H.D., Joo Y.B., Han S.C., Chung H.J. Repair integrity and functional outcomes of arthroscopic repair for intratendinous partial-thickness rotator cuff tears. J Orthop Surg. 2019 doi: 10.1177/2309499019847227. Published online. [DOI] [PubMed] [Google Scholar]
- 26.Key S., Demir S., Gurger M., et al. ViVGG19: novel exemplar deep feature extraction-based shoulder rotator cuff tear and biceps tendinosis detection using magnetic resonance images. Med Eng Phys. 2022;110 doi: 10.1016/j.medengphy.2022.103864. [DOI] [PubMed] [Google Scholar]
- 27.Lin C.C., Wang C.N., Ou Y.K., Fu J. Combined image enhancement, feature extraction, and classification protocol to improve detection and diagnosis of rotator-cuff tears on MR imaging. Magn Reson Med Sci. 2014;13(3):155–166. doi: 10.2463/mrms.2013-0079. [DOI] [PubMed] [Google Scholar]
- 28.Yao J., Chepelev L., Nisha Y., Sathiadoss P., Rybicki F.J., Sheikh A.M. Evaluation of a deep learning method for the automated detection of supraspinatus tears on MRI. Skeletal Radiol. 2022;51(9):1765–1775. doi: 10.1007/s00256-022-04008-6. [DOI] [PubMed] [Google Scholar]
- 29.Sezer A., Sezer H.B. Capsule network-based classification of rotator cuff pathologies from MRI. Comput Electr Eng. 2019;80 [Google Scholar]
- 30.Hahn S., Yi J., Lee H.J., et al. Image quality and diagnostic performance of accelerated shoulder MRI with deep learning-based reconstruction. Am J Roentgenol. 2022;218(3):506–516. doi: 10.2214/AJR.21.26577. [DOI] [PubMed] [Google Scholar]
- 31.Zhan H., Teng F., Liu Z., et al. Artificial intelligence aids detection of rotator cuff pathology: a systematic review. Arthrosc J Arthrosc Relat Surg. 2023:1–12. doi: 10.1016/j.arthro.2023.06.018. Published online. [DOI] [PubMed] [Google Scholar]
- 32.Kim M., Park H.M., Kim J.Y., Kim S.H., Van Hoeke S., De Neve W. In: Proceedings of Machine Learning Research. DV F., F J., J K., et al., editors. ML Research Press; 2020. MRI-Based diagnosis of rotator cuff tears using deep learning and weighted linear combinations; pp. 292–308.https://www.scopus.com/inward/record.uri?eid=2-s2.0-85114795544&partnerID=40&md5=338f06369ae89aba3b95039a6061bc95 126. [Google Scholar]
- 33.Kim H., Shin K., Kim H., et al. Can deep learning Reduce the time and Effort required for manual segmentation in 3D reconstruction of MRI in rotator cuff tears? PLoS One. 2022 doi: 10.1371/journal.pone.0274075. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanjare H.A., Panwar J. Accuracy of rotator cuff tears and tendinosis diagnoses on shoulder ultrasound performed by a short-experienced operator. American Journal of Sonography. 2018 doi: 10.25259/ajs-22-2018. Published online. [DOI] [Google Scholar]
- 35.Ho T.T., Kim G.T., Kim T., Choi S., Park E.K. Classification of rotator cuff tears in ultrasound images using deep learning models. Med Biol Eng Comput. 2022;60(5):1269–1278. doi: 10.1007/s11517-022-02502-6. [DOI] [PubMed] [Google Scholar]
- 36.Lee K., Kim J.Y., Lee M.H., Choi C.H., Hwang J.Y. Imbalanced loss-integrated deep-learning-based ultrasound image analysis for diagnosis of rotator-cuff tear. Sensors. 2021;21(6):1–20. doi: 10.3390/s21062214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K., Yang J., Lee M.H., Chang J.H., Kim J.Y., Hwang J.Y. In: Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) W L., D Q., T. F P., S S., L S., editors. Springer Science and Business Media Deutschland GmbH; 2022. USG-net: deep learning-based ultrasound scanning-guide for an orthopedic sonographer; pp. 23–32. 13437 LNCS. [DOI] [Google Scholar]
- 38.Yu M., Zhu X., Zhao S., et al. 2020. Correlation of Multiple Acromion Morphological Parameters on Radiographs in a Chinese Population. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iio R., Ueda D., Matsumoto T., et al. Deep learning-based screening tool for rotator cuff tears on shoulder radiography. J Orthop Sci. 2023 doi: 10.1016/j.jos.2023.05.004. Published online May. [DOI] [PubMed] [Google Scholar]
- 40.Cho Y., Jalics A., Lv D., et al. ACM International Conference Proceeding Series. Association for Computing Machinery; 2021. Predicting rotator cuff tear severity using radiographic images and machine learning techniques; pp. 237–241. [DOI] [Google Scholar]
- 41.Kuptniratsaikul V., Laohathaimongkol T., Umprai V., Yeekian C., Prasathaporn N. Pre-operative factors correlated with arthroscopic reparability of large-to-massive rotator cuff tears. BMC Muscoskel Disord. 2019;20(1):1–9. doi: 10.1186/s12891-019-2485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J.Y., Ro K., You S., et al. Development of an automatic muscle atrophy measuring algorithm to calculate the ratio of supraspinatus in supraspinous fossa using deep learning. Comput Methods Progr Biomed. 2019;182 doi: 10.1016/j.cmpb.2019.105063. [DOI] [PubMed] [Google Scholar]
- 43.Ro K., Kim J.Y., Park H., et al. Deep-learning framework and computer assisted fatty infiltration analysis for the supraspinatus muscle in MRI. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-93026-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potty A.G., Potty A.S.R., Maffulli N., et al. Approaching artificial intelligence in orthopaedics: predictive analytics and machine learning to prognosticate arthroscopic rotator cuff surgical outcomes. J Clin Med. 2023;12(6) doi: 10.3390/jcm12062369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allaart L.J.H., Spanning S.V., Lafosse L., et al. Developing a machine learning algorithm to predict probability of retear and functional outcomes in patients undergoing rotator cuff repair surgery: protocol for a retrospective, multicentre study. BMJ Open. 2023;13(2) doi: 10.1136/bmjopen-2022-063673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burns D., Boyer P., Razmjou H., Richards R., Whyne C. Adherence patterns and dose response of physiotherapy for rotator cuff pathology: longitudinal cohort study. JMIR Rehabil Assist Technol. 2021;8(1) doi: 10.2196/21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyer P., Burns D., Whyne C. Evaluation of at-home physiotherapy. Bone Joint Res. 2023;12(3):165–177. doi: 10.1302/2046-3758.123.BJR-2022-0126.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pietroski A., Burdick G.B., Warren J.W., Franovic S., Muh S.J. Patient-reported outcomes measurements information system (PROMIS) upper extremity and pain interference do not significantly predict rotator cuff tear dimensions. JSES Int. 2022 doi: 10.1016/j.jseint.2021.10.003. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain N.B., Fan R., Higgins L.D., Kuhn J.E., Ayers G.D. Does my patient with shoulder pain have a rotator cuff tear? A predictive model from the row cohort. Orthop J Sports Med. 2018 doi: 10.1177/2325967118784897. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khatri C., Ahmed I., Parsons H., et al. The natural history of full-thickness rotator cuff tears in randomized controlled trials: a systematic review and meta-analysis. Am J Sports Med. 2018 doi: 10.1177/0363546518780694. Published online. [DOI] [PubMed] [Google Scholar]
- 51.Ai-ping T., Liu F., Zhou D., He T., Yan C., Klar R.M. 2018. Effectiveness of 3-Dimensional Shoulder Ultrasound in the Diagnosis of Rotator Cuff Tears. Medicine. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]