Abstract
A one-step transformation to produce 8,9-dihydrocannabidiol (H2CBD) and related “neocannabinoids” via controlled Friedel-Crafts reactions is reported. Experimental and computational studies probing the mechanism of neocannabinoid synthesis from cyclic allylic alcohol and substituted resorcinol reaction partners provide understanding of the kinetic and thermodynamic factors driving regioselectivity for the reaction. Herein, we present a reaction scope for neocannabinoid synthesis including the production of both normal and abnormal isomers under both kinetic and thermodynamic control. Discovery and optimization of this one-step protocol between various allylic alcohols and resorcinol derivatives is discussed and supported with density-functional theory (DFT) calculations.
Graphical Abstract
INTRODUCTION
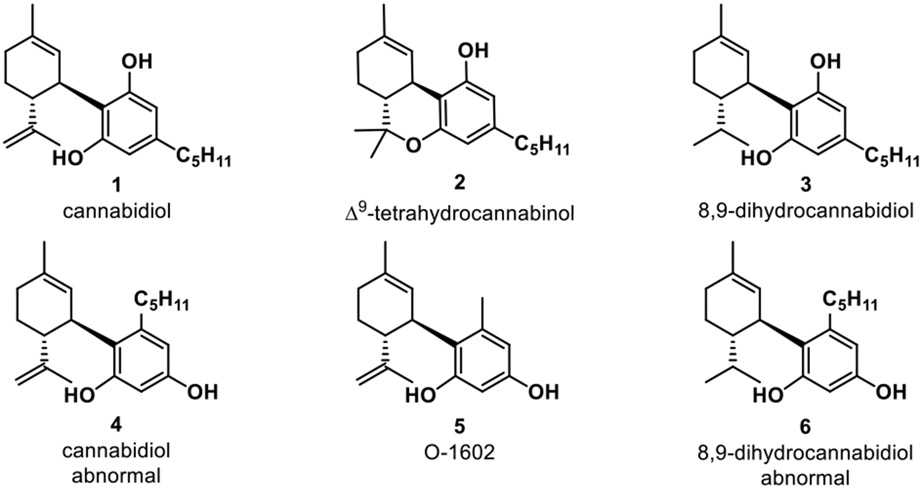
Cannabidiol (CBD) 1 (Figure 1) was discovered in 1940 and is one of more than 100 identified cannabinoids in cannabis plants; together, CBD and tetrahydrocannabinol (THC) 2 account for almost half of the plant–s extract.1,2 Although CBD has shown promise in therapeutic uses for Alzheimer’s disease,3 Parkinson’s disease,3 epilepsy,3 and cancer,3 its propensity for intramolecular cyclization to generate the psychoactive and DEA Schedule I-controlled substance THC 2 have limited its utility and potential.4,5 Furthermore, marketing of CBD for consumption carries the risk of illicit use in the production of THC analogues in analogy to challenges associated with pseudoephedrine-to-methamphetamine.6,7 These concerns have highlighted the potential of the synthetic cannabinoid 8,9-dihydrocannabidiol (H2CBD) 3 to abrogate these concerns due to saturation of the exocyclic isoprene moiety, thereby preventing electrophilic cyclization to THC.6 Thus, H2CBD poses no legal issues as it is fully synthetic, can be scaled up from readily available starting materials that do not require cultivation of cannabis or hemp, and poses no concern for the generation of THC. Such molecules could provide a path toward cannabinoid-based therapeutics without psychotropic effects to prevent drug abuse liabilities.
Figure 1.
Natural and synthetic cannabinoids.
In parallel studies involving CBD and abnormal cannabidiol 4 (Abn-CBD) it was found that their affinities for CB1 and CB2 receptors were substantially different.8,9 It was then discovered that Abn-CBD and synthetic analogs (e.g. 5, O-1602) have binding affinities to the orphaned cannabinoid receptors GPR18 and GPR55, two receptors which are responsible for effects of cannabinoids independent of CB1 and CB2.9 Abnormal CBD 4 is a CBD regioisomer in which the terpene subunit is positioned ortho to the n-pentyl substituent (vs. the para relationship present in “normal” CBD 1), a structural difference for binding to other targets in future analogs. Further structure-activity relationship (SAR) studies show that the substitution of the alkyl chain from Abn-CBD, the relative position of aromatic groups, and sites with the ability to form hydrogen-bonds are important for binding to GPR18 and GPR55.9,10 These findings suggest that synthetic investigations towards unnatural cannabinoid derivatives, herein referred to as “neocannabinoids,” including abnormal H2CBD 6 can greatly benefit therapeutic applications.11,13
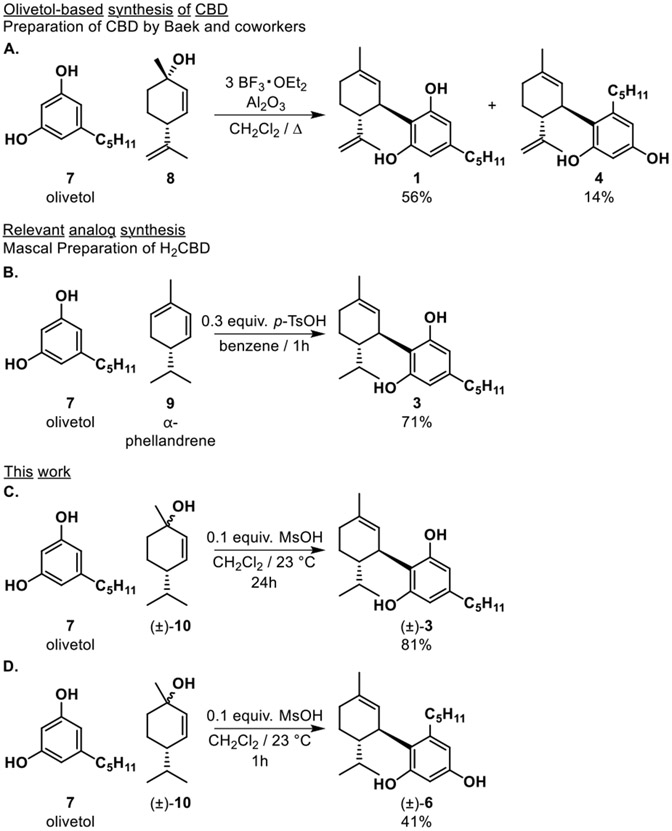
A number of routes for the chemical synthesis of CBD and related molecules have been devised (Scheme 1).14-17 The most efficient approach leverages Friedel-Crafts alkylation of olivetol 7 and chiral allylic alcohols such as menthadienol 8 under mild acidic conditions affording CBD through an SN1’ mechanism (Scheme 1A).15,18 These reactions typically deliver exclusive diastereoselectivity wherein the olivetol substituent favors a trans orientation relative to the isopropyl group. Notably these reactions generally lack regioselectivity between the (“abnormal”) 4 and (“normal”) 1 isomers. Production of the generally undesired abnormal CBD isomer is often a complication in the synthetic production of CBD via the Friedel-Crafts route and can be further complicated by unwanted cycloetherification when using Brønsted acid catalysis. To address this issue, Baek and coworkers reported the synthesis of CBD 1 in 56% isolated yield along with 14% abnormal CBD isomer 4 using BF3•OEt2 on alumina (Al2O3) as a Lewis acid promoter.15,19 Other modes of entry to synthetic CBD include the use of olivetol derivatives to control the site of reaction by removable “blocking group” strategies (e.g. ester or halogens), thereby inhibiting abnormal product formation.15 This method, which mimics the biosynthesis of CBD, generally involves multiple synthetic steps to access CBD in good yields.5
Scheme 1.
Synthesis of CBD isomers and dihydro-CBD regioisomers
The challenge in controlling regioselectivity of CBD synthesis without cyclization to THC birthed the expansion into the synthesis of relevant analogs. The racemic, reduced version of CBD (H2CBD) 3 displays similar pharmacological properties to CBD and does not require chiral pool starting materials making it a promising synthetic target. Importantly, synthetic generation of H2CBD provides room for reaction manipulation and optimization to control regioselectivity without the concern of unintended THC production. This prompted our efforts to develop a better understanding of the kinetic and thermodynamic factors that dictate regioselectivity in this reaction.
Herein, we describe our efforts toward an efficient and regioselective synthesis of normal and abnormal neocannabinoids. In this study, we confirmed that regioselectivity is largely controlled by both kinetic and thermodynamic considerations. Our findings were aligned with computational studies, thereby providing a model for a range of reaction partners and product distributions.
RESULTS AND DISCUSSION
We began our investigation by screening numerous Lewis and Brønsted acids to initiate allylic cation chemistry by mimicking the effects of BF3•OEt2 as reported in the literature.20-22 Previously, Mascal and coworkers obtained normal CBD 3 in 71% yield by para-toluenesulfonic acid (p-TsOH) acid-catalyzed addition to α-phellandrene 9 (Scheme 1B).6,15 In an effort to match this efficiency, we considered the well-established Friedel-Crafts alkylation/cationic pathway using olivetol 7 and allylic alcohol 10 to access H2CBD isomers 3 and 6 (Scheme 1C-D). Our catalyst scope included various Lewis acids including Sc(III)-, La(III)-, Eu(III)-, Yb(III)- triflates, p-TsOH and methanesulfonic acid (MsOH), as well as combinations of both Lewis and Brønsted acids. Metal triflates were tested over multiple time points with varying catalyst loadings and temperatures. All conditions screened provided a mixture of normal H2CBD 3 and abnormal H2CBD 6 (Table 1); in many cases, the bis-addition product 11 was also observed; reactions conducted at higher temperatures (40 °C) provided only cyclized products.23-25 La(III)- and Yb(III)- triflates provided the highest conversion to H2CBD 3 after 24 h, but also led to increased production of bis-addition product 11 which was ultimately a significant drawback for use of metal triflates. p-TsOH and MsOH both performed well in the reaction and optimally in CH2Cl2, although use of p-TsOH led to solubility issues. Thus, optimal reaction conditions were found using MsOH as catalyst. The friendliness of MsOH as a liquid with low corrosivity and toxicity in comparison to the other Brønsted acids made it very easy to work with.26
Table 1.
Acid screening scope for Friedel-Crafts reactions
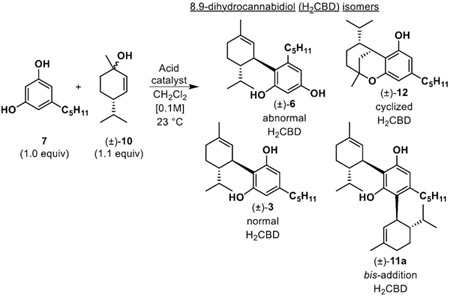
| ||||||
|---|---|---|---|---|---|---|
| Entry | Acid | Amt. | Time (h) |
Conversiona | (±)- 6b |
(±)- 3b |
| 1 | La(OTf)3 | 20 mol% | 1 | 35% | 10% | 25% |
| 2 | 24 | 78% | 4% | 52% | ||
| 3 | Yb(OTf)3 | 20 mol% | 1 | 35% | 10% | 25% |
| 4 | 24 | 75% | 9% | 47% | ||
| 5 | MsOH | 10 mol% | 1 | 90% | 41%b | 39% |
| 6 | 24 | 99% | 0% | 81%b | ||
| 7 | p-TsOH | 10 mol% | 1 | 78% | 30% | 36% |
| 8 | 24 | 91% | 8% | 63% | ||
Conversion measured by 1H NMR analysis.
Isolated yield after silica gel chromatography.
The MsOH-catalyzed reaction was optimized by evaluating catalyst loading, temperature, and time. In these studies, we found that initially the reaction generated roughly a 1:1 ratio of normal H2CBD 3 to abnormal derivative 6 as well as bis-addition product 11 as a side product. However, we observed greater conversion to H2CBD as determined by 1H NMR analysis at a 24 h reaction time. Allowing the reaction to proceed beyond 24 h increased the amount of cycloetherification product 12; accordingly, optimal reaction conditions to generate H2CBD were 0.1 equiv. of MsOH at room temperature for 24 h (Table 1, entry 6). However, we believed that the apparent conversion from abnormal to normal isomers suggested that production of 6 could be a kinetic process and by terminating the reaction at an earlier timepoint, we could increase selectivity. Indeed, we were able to obtain abnormal H2CBD 6 (rr = 1:1) in 41% isolated yield after 1 h reaction time (Table 1, entry 5), in contrast to an 81% isolated yield of normal H2CBD 3 after 24 h.
We next tested the abnormal to normal equilibration hypothesis with a time study by treating pure abnormal isomer 6 with catalytic amounts of MsOH in CH2Cl2. We observed that the abnormal product does in fact equilibrate to the normal isomer 3 in as quickly as 1 h, with full consumption of 6 observed over a longer time period albeit with concomitant emergence of byproducts 11 and 12 (Table 2). Further screening was also conducted with p-TsOH and camphorsulfonic acid (CSA) as catalysts (not shown), but these acids exhibited poor solubility in CH2Cl2 and produced greater amounts of side products. Previous work by Crombie and coworkers showed that reactions of olivetol with electrophiles such as (+)-trans-car-2-ene epoxide25 and 927 in the presence of p-TsOH also afforded both abnormal and normal cannabinoids at early timepoints, with an “interconversion” of abnormal to normal occurring with elevated temperatures.28 Consistent with our findings, it was noted that in this process the presumably kinetically favored abnormal cannabinoid transforms to the more thermodynamically stable normal cannabinoid.27
Table 2.
Equilibration of abnormal to normal H2CBD by in situ 1H NMR analysis
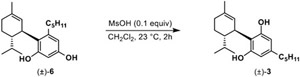
| |||
|---|---|---|---|
| time (h) | (±)-6 | (±)-3 | (±)-11 and (±)-12 |
| 1 | 40% | 60% | 0 |
| 2 | 10% | 90% | 0 |
| 6 | 2% | 74% | 24% |
| 24 | 0% | 50% | 50% |
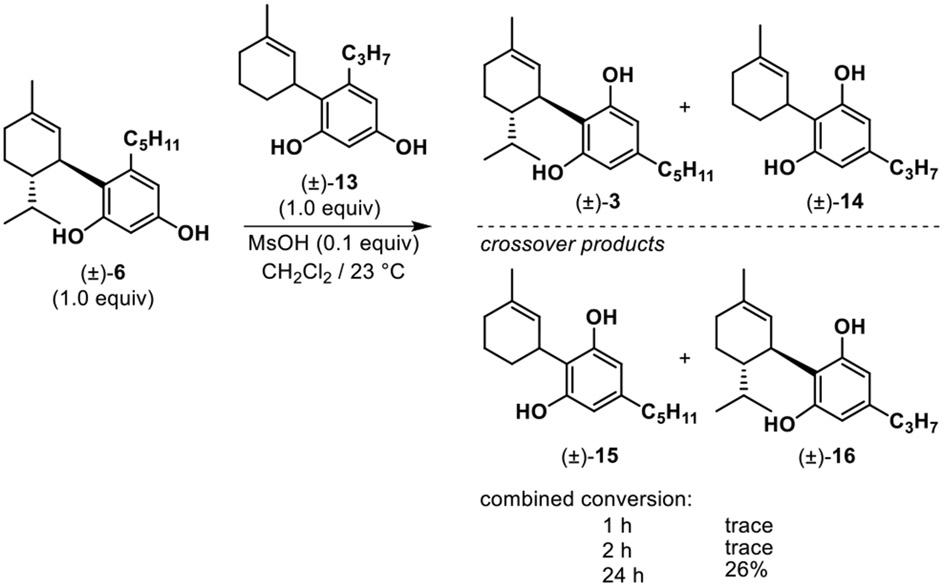
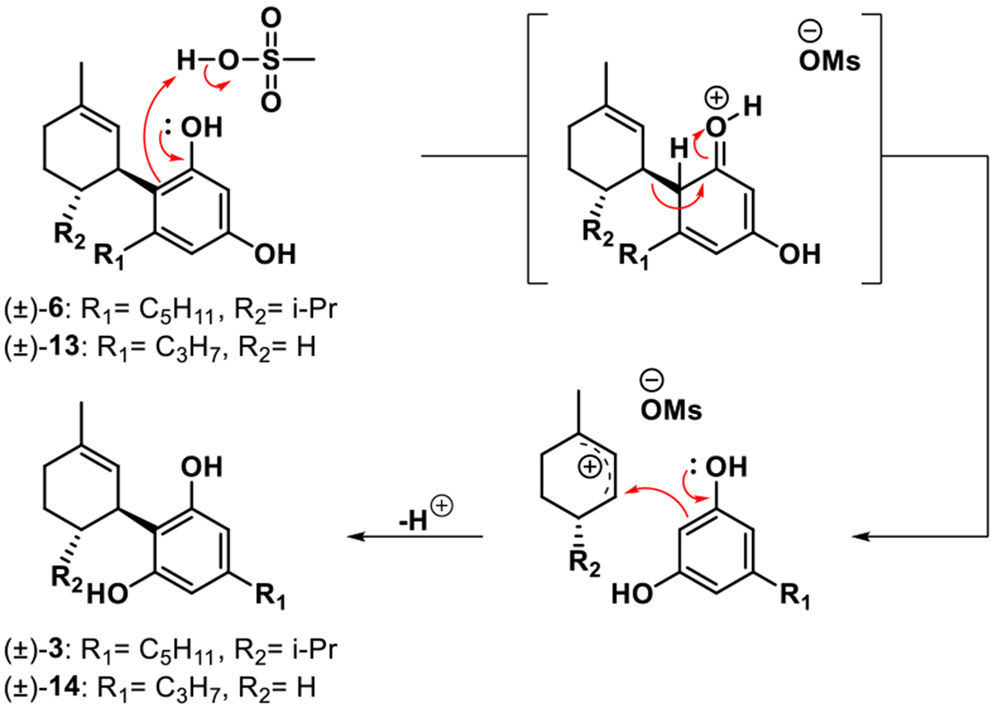
Further probing of a potential dissociative equilibration process was performed in a crossover experiment to refine mechanistic understanding. The experiment shown in Scheme 2 was designed using two different abnormal cannabinoids, 6 and 13, which were treated under the same acidic conditions. Abnormal cannabinoid isomer 13 was prepared using divarinol and 1-methylcyclohex-2-en-1-ol as reaction partners. In this experiment, we expected equilibration from abnormal to normal cannabinoid e.g. 6 to 3 and 13 to 14, as well as crossover products 15 and 16, assuming that equilibration proceeds by disassociation to regenerate the allylic cation and resorcinol partner (Scheme 3). Using a combination of in situ 1H NMR and HPLC-ELSD/MS analysis, we monitored the ratios of the pure abnormal cannabinoids 6/13, their corresponding normal isomers 3/14, and expected crossover products 15/16 (Scheme 2). As expected, we observed products 3 and 14 after 1 h, with only trace amounts of 15 and 16 detected; after 24 h, we obtained a considerable amount (26%) of crossover products.29 Overall, this data confirmed that the generation of 3 from 6 (cf. Table 2) likely proceeds via an intermolecular retro-Friedel Crafts/Friedel-Crafts process30 and not an intramolecular rearrangement pathway.
Scheme 2.
Crossover experiment between abnormal cannabinoids 6 and 13 and tracking formation of crossover products
Scheme 3.
Proposed mechanism for equilibration of abnormal to normal neocannabinoids 6 and 13 to 3 and 14
We next sought to prepare a small library of allylic alcohols for neocannabinoid synthesis. We envisioned a scope of allylic alcohols varying in substituents at the 4-position to determine the steric effects of normal vs. abnormal addition in terms of both kinetic and thermodynamic control. Five reagents (10-10f) were chosen to represent a gradual increase in sterics progressing from no substituents (10c) to the bulky tert-butyl substituted partner (10f). Likewise, selection of the resorcinol reagent also varied in both sterics and alkyl chain length. We chose orcinol (7a), olivetol (7), and 5-(1,1-dimethylheptyl) resorcinol (7b) as three reaction partners as shown in Table 3.
Table 3.
Kinetic vs. thermodynamic study of various neocannabinoids with experimental selectivity ratios and DFT-calculated energies
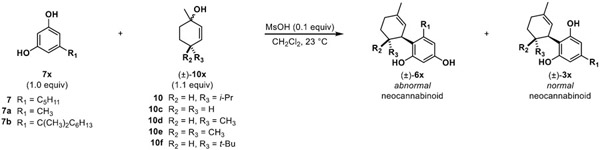
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Resorcinol 7x |
Allylic alcohol 10x |
(r.r.), 1 h (3x:6x)a |
(r.r.), 24 h (3x:6x)a |
Isolated product (±)-3x |
% Yield isolated (±)-3x, 24 h |
Computational results | |
| K3/k6 b | ΔG (kcal/mol)c | |||||||
| 1 | 7a | 10c | (1:3) | (1:1) | 3ac | 9% | 0.48 | 1.3 |
| 2 | 7a | 10d | (2:5) | (3:4) | 3ad | 37% | 0.24 | 1.7 |
| 3 | 7a | 10e | (1:10) | (3:10) | 3ae | 16% | 0.48 | 5.9 |
| 4 | 7a | 10 | (2:3) | (11:1) | 3a | 47% | 1.20 | 4.8 |
| 5 | 7a | 10f | (5:1) | (9:1) | 3af | 51% | 0.34 | 4.0 |
| 6 | 7 | 10c | (7:10) | (1:1) | 3c | 43% | 0.67 | 1.0 |
| 7 | 7 | 10d | (1:1) | (5:1) | 3d | 56% | 0.85 | 4.0 |
| 8 | 7 | 10e | (1:4) | (1:3) | 3e | 18% | 0.51 | 2.1 |
| 9 | 7 | 10 | (11:10) | (1:0) | 3 | 81% | 0.49 | 1.7 |
| 10 | 7 | 10f | (27:1) | (25:1) | 3f | 75% | 2.34 | 1.7 |
| 11 | 7b | 10c | (1:0) | (1:0) | 3bc | 98% | 4.52 | 7.3 |
| 12 | 7b | 10d | (1:0) | (1:0) | 3bd | 98% | 8.80 | 9.3 |
| 13 | 7b | 10e | (1:0) | (1:0) | 3be | 72% | 206.01 | 11.4 |
| 14 | 7b | 10 | (1:0) | (1:0) | 3b | 79% | 49.72 | 10.8 |
| 15 | 7b | 10f | (1:0) | (1:0) | 3bf | 56% | 117.65 | 11.9 |
Ratio determined by HPLC/ELSD/MS and 1H NMR analysis.
, where ΔΔG = G(6x‡) – G(3x‡), T = 298.15 K, and G(3/6x‡) is the Boltzmann-averaged free energy.
ΔG = G(6x) – G(3x), where G(3/6x) is the Boltzmann-averaged free energy.
For all reagent pairings, we tracked the ratio of isomers generated at both 1 and 24 h by both HPLC and 1H NMR analyses. We found that in almost all cases employing orcinol 7a (Table 3, entries 1-4), the abnormal neocannabinoid 6ac-6a was enriched within the first hour. The tert-butyl allylic alcohol (10f, Table 3, entry 5) was the only example where the normal neocannabinoid (3af) was the major product at the 1 h timepoint. The kinetic preference for the abnormal isomers can also be attributed to favorable electrostatics (Lowdin charges) between the ortho (relative to the resorcinol alkyl chain) carbons and the cationic partner as compared to the para carbons (see Figure S1 in the Supporting Information). Overall, we observed that the ratio of normal:abnormal (3ac-3af:6ac-6af) increases over time, consistent with our observation of a thermodynamic equilibration process. In order to rationalize these outcomes, the systems were studied computationally (Orca, r2SCAN-3C/CPCM(CH2Cl2))31-35 to understand the kinetic and thermodynamic factors driving the 1 h/24 h ratios observed (Table 3 and Supporting Information). Indeed, our calculations generally support the premise that with increasing bulk at the 4-position of the allylic alcohol partner, the normal product is increasingly thermodynamically favored (ΔG in Table 3), a likely consequence of the added steric strain introduced between the R2/R3 substituents and the R1 sidechain. The computations suggest a kinetic preference for the abnormal isomer in 3(6)ac-3(6)ae and 3(6)af with k3/k6 ratios less than 1.
Even for 3/6a, the observed ratio of 1.2 is close to unity. These thermodynamic and reactivity trends also held for reactions with the same set of allylic alcohols and olivetol 7 (Table 3, entries 6-10). It should be noted that the key difference in orcinol vs. olivetol is the alkyl chain (pentyl vs. methyl group); this change did impact the results for obtaining high ratios of normal to abnormal neocannabinoids. Using orcinol as a reagent, lower yields obtained may be attributed to competition between normal, abnormal, bis-addition, and cyclized products. In addition, we found that use of allylic alcohol 10e led to lower yields of neocannabinoid monoadducts (cf. Table 3, entries 3 and 8), largely due to more sluggish reactivity of this partner.
We next hypothesized that an increase in steric bulk on the resorcinol alkyl chain could further drive initial formation of the normal neocannabinoid isomer.29 This hypothesis led us to evaluate the bulky 5-(1,1-dimethylheptyl) resorcinol partner 7b. Using this resorcinol partner, we observed formation of the normal neocannabinoid as the major isomer at 1 h with all allylic alcohols employed which we posited was due to increased steric strain imposed by the dimethyl heptyl chain (3bc-3bf). DFT calculations also confirmed that the normal isomers were significantly more stable as reaction products and were also kinetically preferred.
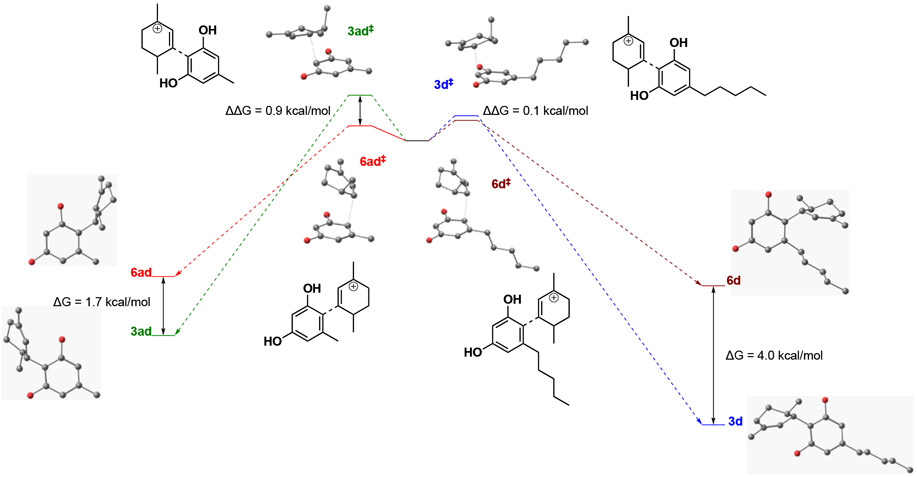
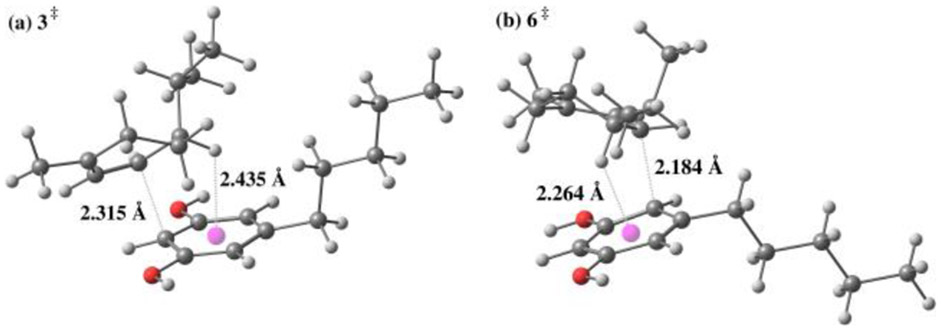
Overall, the experimental and computational findings shown in Table 3 support a mechanism that initially produces the abnormal neocannabinoid derivative when there is less steric bulk surrounding the cation intermediate and the normal neocannabinoid in cases of greater steric bulk. This mechanistic correlation can be visualized by the select examples shown in Figure 2 outlining the computed differences in Gibbs free energy of the corresponding abnormal and normal isomer transition states. We also noticed that many transition states leading to both normal and abnormal isomers displayed stabilization imparted by C-H- π interactions36 (see Supporting Information). Figure 3 shows examples of global minimum transition states highlighting these interactions enroute to normal H2CBD isomer 3 and abnormal isomer 6. As shown in Figure 2, the difference in the alkyl chain of products 3ad and 6ad vs. 3d and 6d show that the energy needed to kinetically form abnormal product 6ad is preferred in comparison to the energy needed to form abnormal isomer 6d. By leveraging this mechanism and terminating the reaction at different timepoints, we can favor production of either normal or abnormal neocannabinoids based on steric considerations. As previously described, efficient synthesis of abnormal analogues could be a valuable asset in cannabinoid therapeutics; Table 4 highlights examples of abnormal isomers accessed in moderate yields by terminating the reaction at a 1 h timepoint.
Figure 2.
Qualitative energy diagrams for neocannabinoid isomers 3ad, 6ad, 3d, and 6d with the corresponding 3D models. The positions of isomer pairs on the y-axis are a guide for the eyes only and are not meant to represent absolute energies.
Figure 3.
Global minimum transition state structures for normal (a, 3‡) and abnormal (b, 6‡) isomers highlighting stabilization imparted by C-H π interactions. The pink sphere is the center of the resorcinol aryl ring.
Table 4.
Optimized syntheses of abnormal neocannabinoid derivatives
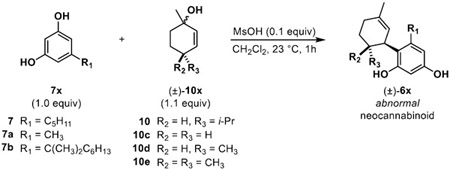
| ||||
|---|---|---|---|---|
| Entry | Resorcinol 7x |
Allylic alcohol 10x |
Isolated product (±)-6x |
% Isolated yield (±)-6x |
| 1 | 7a | 10c | 6ac | 35% |
| 2 | 7a | 10d | 6ad | 50% |
| 3 | 7a | 10e | 6ae | 33% |
| 4 | 7a | 10 | 6a | 40% |
| 5 | 7 | 10c | 6c | 50% |
| 6 | 7 | 10d | 6d | 37% |
| 7 | 7 | 10e | 6e | 24% |
| 8 | 7 | 10 | 6 | 41% |
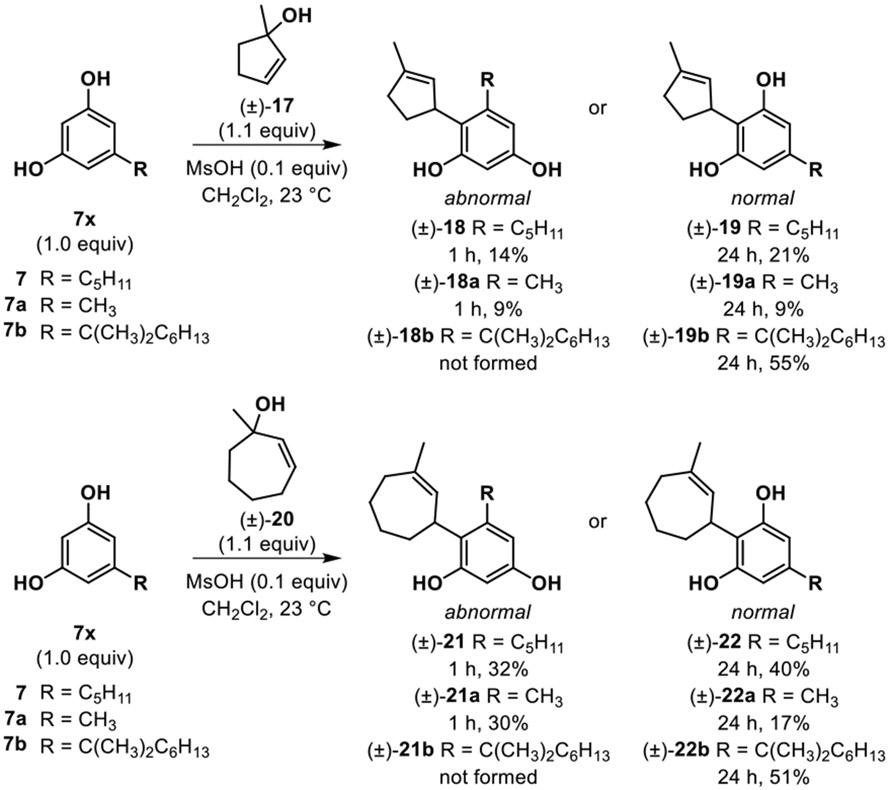
As a final study, we sought to test whether the condition-dependent regioselective reactions could also be applied to access neocannabinoids with alternative, unnatural ring sizes for the terpenoid fragment. Accordingly, we synthesized both 5- and 7-member cyclic allylic alcohols 17 and 20, respectively, and subjected them to the general reaction conditions shown in Scheme 4. These reactions were found to tolerate each ring size wherein both abnormal and normal neocannabinoids were generated and isolated, with bis-addition byproducts generated using allylic alcohols 17 and 20. When tracking these reactions, using orcinol and olivetol we again observed that abnormal products (18, 18a, 21, 21a) were produced in competition with the normal isomers (19, 19a, 22, 22a) and over time produced more normal isomer due to product equilibration. Use of 5-(1,1,-dimethylheptyl) resorcinol as reaction partner again supported the idea that steric bulk inhibits formation of abnormal products (18b, 21b) where only the normal neocannabinoid product was observed with use of both 5- and 7-member cyclic allylic alcohols.
Scheme 4.
Neocannabinoid synthesis from alternate cyclic allylic alcohols
CONCLUSIONS
Herein, we report the synthesis of diverse neocannabinoids using controlled Friedel-Crafts reactions for construction of either the abnormal or normal isomer. We have demonstrated the scope and limitations of this process over 35 examples to produce synthetic unnatural analogs of both normal and abnormal cannabidiol (CBD). Improved understanding of the kinetic and thermodynamic properties for the process can be leveraged in the future towards predictive neocannabinoid synthesis. Moreover, our ongoing and future studies will continue to investigate the power of neocannabinoids as therapeutics and in targeted medicinal chemistry applications which will be the subject of future publications from our laboratories.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Norman Lee of the Boston University Chemical Instrumentation Center for HRMS data and analyses. We thank CBDQure Inc. for funding the initial phases of the project and the National Institutes of Health (NIH) (R35 GM 118173) for additional financial support. We also thank Nick Wangtz (Shanghai Xishite Biosciences Co., Ltc.) for a generous donation of divarinol. We also thank the National Science Foundation (NSF) for support of NMR (CHE-0619339) and MS (CHE-0443618) facilities at Boston University. Computational work reported on in this paper was performed on the Shared Computing Cluster (SCC) which is administered by Boston University’s Research Computing Services.
ABBREVIATIONS
- CBD
cannabidiol
- THC
tetrahydrocannabinol
- H2CBD
8,9-dihydrocannabidiol
- Abn-CBD
abnormal cannabidiol, DFT density-functional theory
- GPR
G-protein coupled receptor
- NMR,
nuclear magnetic resonance
- HPCL
high-performance liquid chromatography
Footnotes
The authors declare the following competing financial interest(s): J.A.P, Jr. A.B, A. M., R.T., J.M., and L.E.B are inventors on a provisional patent application describing the synthesis of neocannabinoid isomers.
REFERENCES
- (1).Adams R; Hunt M; Clark JH; Clark JH; Loewe DS Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. J. Am. Chem. Soc 1940, 62, 196–200. [Google Scholar]
- (2).Atalay S; Jarocka-karpowicz I; Skrzydlewskas E Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants. 2020, 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Pisanti S; Malfitano AM; Ciaglia E; Lamberti A; Ranieri R; Cuomo G; Abate M; Faggiana G; Proto MC; Fiore D; Laezza C; Bifulco M Cannabidiol: State of the Art and New Challenges for Therapeutic Applications. J. Pharm. Thera 2017, 175, 133–150. [DOI] [PubMed] [Google Scholar]
- (4).Ben-Shabat S; Hanuš LO; Katzavian G; Gallily R New Cannabidiol Derivatives: Synthesis, Binding to Cannabinoid Receptor, and Evaluation of Their Anti-inflammatory Activity. J. Med. Chem 2006, 49, 1113–1117. [DOI] [PubMed] [Google Scholar]
- (5).Nelson KM; Bisson J; Singh G; Graham JG; Chen SN; Friesen JB; Dahlin JL; Niemitz M; Walters MA; Pauli GF The Essential Medicinal Chemistry of Cannabidiol (CBD). J. Med. Chem 2020, 63, 12137–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Mascal M; Hafezi N; Wang D; Hu Y; Serra G; Dallas ML; Spencer JPE Synthetic, Non-Intoxicating 8,9-Dihydrocannabidiol for the Mitigation of Seizures. Sci. Rep 2019, 9, 7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bloemendal VRLJ; van Hest JCM; Rutjes FPJT Synthetic Pathways to Tetrahydrocannabinol (THC): An Overview. Org. Biomol. Chem 2020, 18, 3203–3215. [DOI] [PubMed] [Google Scholar]
- (8).Leite R; Carlini EA; Lander N; Mechoulam R Anticonvulsant Effects of the (−) and (+) Isomers of Cannabidiol and Their Dimethylheptyl Homologs. Pharmacology. 1982, 24, 141–146. [DOI] [PubMed] [Google Scholar]
- (9).Ashton CJ, The Atypical Cannabinoid O-1602: Targets, Actions, and the Central Nervous System. Cent. Nerv. Syst. Agents. Med. Chem 2012, 12, 233–239. [DOI] [PubMed] [Google Scholar]
- (10).Johns DG; Behm DJ; Walker DJ; Ao Z; Shapland EM; Daniels DA; Riddick M; Dowell S; Staton PC; Green P; Shabon U; Bao W; Aiyar N; Yue TL; Brown AJ; Morrison AD; Douglas SA The Novel Endocannabinoid Receptor GPR55 Is Activated by Atypical Cannabinoids but Does Not Mediate Their Vasodilator Effects. Br. J. Pharmacol 2007, 152, 825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hanuš LO; Meyer SM; Muñoz E; Taglialatela-Scafati O; Appendino G Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep 2016, 33, 1357–1392. [DOI] [PubMed] [Google Scholar]
- (12).Jentsch NG; Zhang X; Magolan J Efficient Synthesis of Cannabigerol, Grifolin, and Piperogalin via Alumina-Promoted Allylation. J. Nat. Prod 2020, 83, 2587–2591. [DOI] [PubMed] [Google Scholar]
- (13).Kearney S; Gangano A; Navaratne P; Barrus D; Rehrauer K; Reid T-E; Roitberg A; Ghiviriga I; Cunningham C; Gamage T; Grenning A Axially Chiral Cannabinoids: Design, Synthesis, and Cannabinoid Receptor Affinity. J. Am. Chem. Soc 2023, 145, 13581–13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lago-Fernandez A; Redondo V; Hernandez-Folgado L; Figuerola-Asencio L; Jagerovic N New Methods for the Synthesis of Cannabidiol Derivatives, in: Methods Enzymol., Academic Press Inc 2017, 593, 237–257. [DOI] [PubMed] [Google Scholar]
- (15).Pirrung MC Synthetic Access to Cannabidiol and Analogs as Active Pharmaceutical Ingredients. J. Med. Chem 2020, 63, 12131–12136. [DOI] [PubMed] [Google Scholar]
- (16).Anand R; Cham PS; Gannedi V; Sharma S; Kumar M; Singh R; Vishwakarma RA; Singh PP Stereoselective Synthesis of Nonpsychotic Natural Cannabidiol and Its Unnatural/Terpenyl/Tail-Modified Analogues. J. Org. Chem 2022, 87, 4489–1498. [DOI] [PubMed] [Google Scholar]
- (17).Grimm JAA; Zhou H; Properzi R; Leutzsch M; Bistoni G; Nienhaus J; List B Catalytic Asymmetric Synthesis of Cannabinoids and Menthol from Neral. Nature. 2023, 615, 634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Maiocchi A; Barbieri J; Fasano V; Passarella D Stereoselective Synthetic Strategies to (−)-Cannabidiol. ChemistrySelect. 2022, 7, e202202400. [Google Scholar]
- (19).Baek S-H; Srebnik M; Mechoulam R Boron Triflouride Etherate on Alimina - a Modified Lewis Acid Reagent. Tet. Lett 1985, 26, 1083–1086. [Google Scholar]
- (20).Gaoni Y; Mechoulam R Hashish—VII: The Isomerization of Cannabidiol to Tetrahydrocannabinols. Tetrahedron. 1966, 22, 1481–1488. [Google Scholar]
- (21).Petrzilka T; Haefliger W; Sikemeier C Synthese von Haschisch-Inhaltsstoffen. 4. Mitteilung. Helv. Chim. Acta 1969, 52, 1102–1134. [Google Scholar]
- (22).Bloemendal VRLJ; Spierenburg B; Boltje TJ; van Hest JCM; Rutjes FPJT One-Flow Synthesis of Tetrahydrocannabinol and Cannabidiol Using Homo- and Heterogeneous Lewis Acids. J. Flow. Chem 2021, 11, 99–105. [Google Scholar]
- (23).Crombie L; Crombie WML, Cannabinoid Bis-Homologues: Miniaturised Synthesis and GLC Study. Phytochemistry. 1975, 14, 213–220. [Google Scholar]
- (24).Appendino G; Gibbons S; Giana A; Pagani A; Grassi G; Stavri M; Smith E; Rahman MM Anti-bacterial Cannabinoids from Cannabis Sativa : A Structure–Activity Study. J. Nat. Prod 2008, 71, 1427–1430. [DOI] [PubMed] [Google Scholar]
- (25).Nguyen GN; Jordan EN; Kayser O Synthetic Strategies for Rare Cannabinoids Derived from Cannabis Sativa. J. Nat. Prod 2022, 85, 1555–1568. [DOI] [PubMed] [Google Scholar]
- (26).Kulkarni P. Methane Sulphonic Acid Is Green Catalyst in Organic Synthesis. Orient. J. Chem 2015, 31, 447–451. [Google Scholar]
- (27).Crombie L; Crombie WML; Firth DF Terpenylations Using (R)-(−)-α-Phellandrene. Synthesis of the (3S,4R)-8,9-Dihydro-o- and -p-Cannabidiols, Their Iso-THC’s, and the Natural Dihydrochalcone (3S,4R)-(+)-Linderatin. J. Chem. Soc., Perkin Trans. 1 1988, 5, 1251–1253. [Google Scholar]
- (28).Crombie L; Crombie WML; Jamieson SV; Palmer CJ Acid-Catalysed Terpenylations of Olivetol in the Synthesis of Cannabinoids. J. Chem. Soc., Perkin Trans. 1 1988, 5, 1243. [Google Scholar]
- (29).Razdan RK; Dalzell HC; Handrick GR Hashish. X. Simple One-Step Synthesis of (−)-.DELTA. 1-Tetrahydrocannabinol (THC) from p-Mentha-2,8-Dien-1-Ol and Olivetol. J. Am. Chem. Soc 1974, 96, 5860–5865. [DOI] [PubMed] [Google Scholar]
- (30).Iwata T; Kawano R; Fukami T; Shindo M Retro-Friedel-Crafts-Type Acidic Ring-Opening of Triptycenes: A New Synthetic Approach to Acenes. Chem. Eur. J 2022, 28, e202104160. [DOI] [PubMed] [Google Scholar]
- (31).Neese F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci 2012, 2, 73–78. [Google Scholar]
- (32).Neese F. Software Update: The ORCA Program System, Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci 2018, 8, e1327. [Google Scholar]
- (33).Neese F; Wennmohs F; Becker U; Riplinger C The ORCA Quantum Chemistry Program Package. J. Chem. Phys 2020, 152, 224108. [DOI] [PubMed] [Google Scholar]
- (34).Neese F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci 2022, 12, e1606. [Google Scholar]
- (35).Grimme S; Hansen A; Ehlert S; Mewes J-M R2SCAN-3c: A “Swiss Army Knife” Composite Electronic Structure Method. J. Chem. Phys 2021, 154, 064103. [DOI] [PubMed] [Google Scholar]
- (36).Hobza P. Stacking Interactions. Phys. Chem. Chem. Phys 2008, 10, 2581–2583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.