Abstract
A central goal in neuroscience is the development of a comprehensive mapping between structural and functional brain features, which facilitates mechanistic interpretation of brain function. However, the interpretability of structure-function brain models remains limited by a lack of biological detail. Here, we characterize human structural brain networks weighted by multiple white matter microstructural features including total intra-axonal cross-sectional area and myelin content. We report edge-weight-dependent spatial distributions, variance, small-worldness, rich club, hubs, as well as relationships with function, edge length, and myelin. Contrasting networks weighted by the total intra-axonal cross-sectional area and myelin content of white matter tracts, we find opposite relationships with functional connectivity, an edge-length-independent inverse relationship with each other, and the lack of a canonical rich club in myelin-weighted networks. When controlling for edge length, networks weighted by either fractional anisotropy, radial diffusivity, or neurite density show no relationship with whole-brain functional connectivity. We conclude that the co-utilization of structural networks weighted by total intra-axonal cross-sectional area and myelin content could improve our understanding of the mechanisms mediating the structure-function brain relationship.
Keywords: Connectomics, Network neuroscience, Structural connectivity, Functional connectivity, White matter, Myelin, Microstructure-weighted imaging
Author Summary
For computational network models to provide mechanistic links between brain structure and function, they must be informed by networks in which edge weights quantify structural features relevant to brain function. Here, we characterized several weighted structural networks capturing multiscale features of white matter connectivity including total intra-axonal cross-sectional area and myelin density. We describe these networks in terms of edge weight distribution, variance, and network topology, as well as their relationships with each other, edge length, and function. Overall, these findings support the joint use of structural networks weighted by the total intra-axonal cross-sectional area and myelin content of white matter tracts in structure-function models. This thorough characterization serves as a benchmark for future investigations of weighted structural brain networks.
INTRODUCTION
The quest to relate human structural and functional brain networks spans the spectrum of spatial scale and repertoire of data modalities absolutely. At the macroscale, the human brain can be modeled as an anatomical network of discrete neuronal populations (nodes) interconnected by white matter fibers (edges) (Sporns, 2011). Coordinated spatiotemporal patterns of neuronal activity unfolding upon this structural backbone are fine-tuned by white matter microstructure (Hodgkin & Huxley, 1952; Huxley & Stämpfli, 1949; Moore et al., 2020; Pumphrey & Young, 1938) and form the basis of cognition and behavior (Biswal et al., 1995; Greicius et al., 2003; Hampson et al., 2006; Liégeois et al., 2019; S. M. Smith et al., 2009; van den Heuvel et al., 2009). Increasingly, MRI facilitates in vivo measurement of multiscale properties of both brain structure (e.g., Alexander et al., 2019; Drakesmith et al., 2019; Jeurissen et al., 2019; Mancini et al., 2020) and function (e.g., Finn et al., 2019; Friston, 2011; Gordon et al., 2017; Liu et al., 2022). Diffusion MRI streamline tractography and resting-state functional MRI are often respectively used to estimate structural and functional connectivity (SC and FC) networks. Network science provides a framework to bring these fundamentally different substrates into a common space where their features can be quantified (Fornito et al., 2016; Sporns, 2010; Suárez et al., 2020) and used to probe the mechanisms mediating human brain function (e.g., Cabral et al., 2017; Fornito et al., 2015).
SC network edges can be weighted by a range of MRI-derived metrics quantifying white matter microstructural features relevant to brain function, including voxel-level estimates of tissue diffusivity (e.g., Caeyenberghs et al., 2016), neurite density (H. Zhang et al., 2012), axon diameter distributions (Alexander et al., 2010; Assaf et al., 2008), myelin content (Heath et al., 2018; Mancini et al., 2020), and the g-ratio (ratio of inner/outer diameters of myelinated axons) (Stikov et al., 2011, 2015), as well as tract/bundle-level measures of axonal cross-sectional area (Daducci, Dal Palù, et al., 2015; R. E. Smith et al., 2015). Subsets of these metrics have been investigated using a microstructure-weighted connectomics approach (Boshkovski et al., 2021; Caeyenberghs et al., 2016; Deligianni et al., 2016; Frigo et al., 2020; Mancini et al., 2018; Messaritaki et al., 2021; Schiavi et al., 2020; van den Heuvel et al., 2010; van den Heuvel & Sporns, 2011; F. C. Yeh et al., 2016). We aim to extend this work by providing a comprehensive assessment of the fundamental characteristics of a range of standard and state-of-the-art weighted structural brain networks, including a network weighted by myelin.
The networks considered here can be grouped into two classes: those computed with tractometry (Bells et al., 2011) and those computed directly from the streamline weights in a tractogram, that is, streamline-specific. We consider three examples of the latter: (a) the number of streamlines (NoS); and two methods that optimize the streamline weights in a tractogram to increase specificity for white matter structural features; (b) spherical-deconvolution informed filtering of tractograms (SIFT2) (R. E. Smith et al., 2015); and (c) convex optimization modeling for microstructure informed tractography (COMMIT) (Daducci et al., 2013; Daducci, Dal Palù, et al., 2015). SIFT2 and COMMIT were designed to overcome known limitations of streamline counts (Girard et al., 2014; Jones, 2010; Jones et al., 2013). While the edge weights in all three networks generally capture white matter features relevant to connection strength, SIFT2 and COMMIT more specifically quantify the total intra-axonal cross-sectional area of white matter tracts (henceforth referred to as edge caliber). To date, COMMIT and SIFT2 have not been compared to NoS with uniform connection density (Frigo et al., 2020; Schiavi et al., 2020; C. H. Yeh et al., 2016). Thus, it remains unclear how the edge weights themselves affect network topology.
In contrast, tractometry allows network edge weights to be derived from any volumetric brain image that is coregistered to the tractogram. This increase in methodological flexibility comes at the expense of anatomical specificity. Tractometry is unable to resolve the separate contributions of individual fiber populations to the aggregate value of a voxel. Given that an estimated 90% of white matter voxels at typical diffusion MRI resolutions (∼2 mm) contain multiple fiber populations (Jeurissen et al., 2012), the quantitative link between white matter microstructure and essentially all tractometry-derived edge weights is biased by partial volume effects.
In this work, tractometry is combined with a diffusion tensor model (Basser, 1995; Basser et al., 1994) to derive networks weighted by FA (fractional anisotropy) and RD (radial diffusivity), which respectively quantify the degree of diffusion anisotropy (i.e., directional dependence) and diffusion magnitude perpendicular to the major axis. The crossing fiber problem described above is also known to limit the ability of diffusion tensor models to quantify white matter features (De Santis et al., 2014; Tournier et al., 2011). Additional tractometry networks examined here include a network weighted by ICVF (intracellular volume fraction) computed with NODDI (neurite orientation dispersion and density imaging) (H. Zhang et al., 2012), as well as a network weighted by the longitudinal relaxation rate R1 (1/T1) derived from a quantitative T1 map. The edge weights in this network are myelin-weighted, as R1 has been shown to correlate with histology-derived myelin content (Mancini et al., 2020; Mottershead et al., 2003).
This characterization of weighted structural brain networks is carried out as follows: (a) within-network features of edge weight distribution and variance; (b) edgewise relationships with FC, edge length, and myelin (R1); and (c) topological features of small-worldness, rich club, and network hubs. Importantly, uniform binary connectivity is enforced across all weighted network variants, that is, the underlying binary connectivity map is identical. This allows the edge weights themselves to drive the characterization.
RESULTS
In 50 healthy adults (27 men; 29.54 ± 5.62 years; 47 right-handed), structural brain networks were estimated from multi-shell diffusion MRI data with probabilistic tractography. Each subject’s structural network was used to compute eight SC networks (Table 1) in which edges were weighted by NoS, SIFT2, COMMIT, FA, RD, ICVF, R1, and LoS (edge length computed as the mean length of streamlines). NoS, SIFT2, COMMIT, and LoS correspond to streamline-specific metrics, whereas networks weighted by FA, RD, ICVF, and R1 were computed using tractometry. The edge weights in NoS, SIFT2, and COMMIT networks were normalized by node volume. Additionally, a static FC network was derived for each subject by zero-lag Pearson cross-correlation of nodewise resting-state time series. Unless otherwise stated, all results shown correspond to networks parcellated with the Schaefer-400 cortical atlas (Schaefer et al., 2018) and include 14 subcortical nodes.
Table 1. .
Summary of structural network weights
| Short name | Long name | Method | Data source | Interpretation |
|---|---|---|---|---|
| LoS | Length of streamlines | Streamline-specific | Diffusion MRI | Mean length of the streamlines connecting two nodes |
| NoS | Number of streamlines | Streamline-specific | Diffusion MRI | Number of streamlines connecting two nodes; connection strength |
| SIFT2 | Spherical-deconvolution informed filtering of tractograms | Streamline-specific | Diffusion MRI | Fiber density from spherical deconvolution summed across streamlines; connection strength |
| COMMIT | Convex optimization modeling for microstructure informed tractography | Streamline-specific | Diffusion MRI | Total intra-axonal cross-sectional area summed across streamlines; connection strength |
| R1 | Longitudinal relaxation rate | Tractometry | Multimodal MRI (diffusion + relaxometry) | R1 = 1/T1; index of tissue myelin content |
| FA | Fractional anisotropy | Tractometry | Diffusion MRI | Diffusion directional dependence |
| RD | Radial diffusivity | Tractometry | Diffusion MRI | Diffusion perpendicular to the principal axis |
| ICVF | Intracellular volume fraction | Tractometry | Diffusion MRI | Neurite density |
Structural Brain Networks Vary in the Distribution of Their Edge Weights
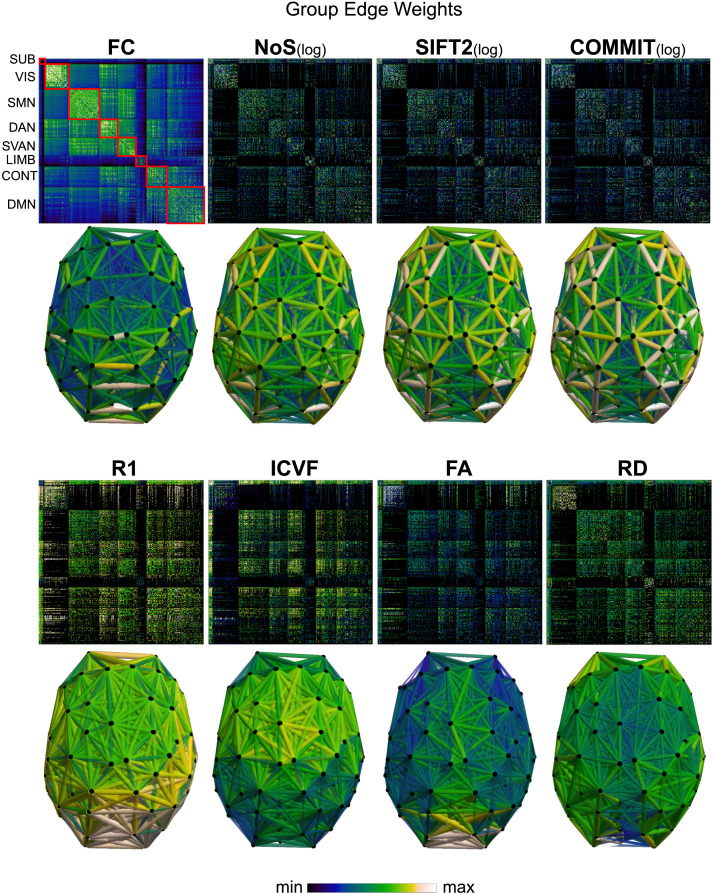
Group-level networks weighted by NoS, SIFT2, and COMMIT show spatially distributed patterns of high-magnitude edge weights and noticeably accentuate within-module connectivity (Figure 1). Modules correspond to the seven-canonical resting-state networks (Yeo et al., 2011) plus the subcortex. These patterns are hallmarks of FC networks and are observed in the FC network shown here. The contrast between high- and low-magnitude edge weights is most evident in COMMIT. By comparison, the spatial variation of edge weight distribution in the tractometry networks is smoother with more pronounced regional concentrations. R1 is highest in the edges connecting the visual module to itself and to the rest of the brain; and lowest within the subcortex and between the subcortical and limbic modules. The surface plot shows the highest concentration of R1 in the white matter projections of posterior cortical regions.
Figure 1. .
Edge weight spatial distribution. Connectivity matrices of group-level edge weights for FC (functional connectivity), NoS (number of streamlines), SIFT2 (spherical-deconvolution informed filtering of tractograms), COMMIT (convex optimization modeling for microstructure informed tractography), R1 (longitudinal relaxation rate), ICVF (intracellular volume fraction), FA (fractional anisotropy), RD (radial diffusivity), and LoS (mean length of streamlines). Each network is composed of 414 nodes as defined by the Schaefer-400 cortical parcellation and 14 subcortical ROIs. Nodes are grouped into the canonical resting-state modules (Yeo et al., 2011) plus the subcortex: SUB (subcortex), VIS (visual), SMN (somatomotor), DAN (dorsal attention), SVAN (salience ventral attention), LIMB (limbic), CONT (control), and DMN (default mode). 3D cortical surfaces (shown below) of group-level edge weights in the Schaefer-100 parcellation generated with BrainNet Viewer (Xia et al., 2013). Edge diameter and color indicate weight magnitude. The edge weights in NoS, SIFT2, and COMMIT networks were log10 transformed for visualization.
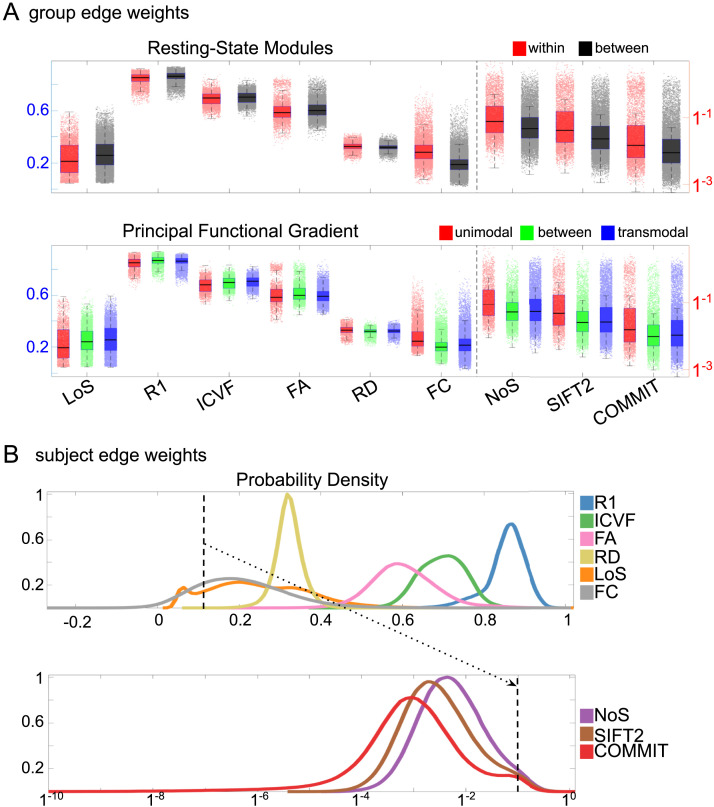
Group-level edge weight distributions are summarized with respect to two important organizational patterns of brain function (Figure 2A): within and between resting-state modules (Yeo et al., 2011); and along the principal functional gradient (Margulies et al., 2016). NoS, SIFT2, and COMMIT mirror FC in both plots with greater edge weight magnitude within module, especially within unimodal modules. R1, ICVF, FA, and RD generally mirror LoS with the reverse trend: higher between module and lowest in unimodal modules. This suggests that tractometry-derived networks may be influenced by edge length to a greater extent.
Figure 2. .
Edge weight distribution. (A) Distribution of group-level edge weights binned by (top) within- and between-module; (bottom) unimodal, transmodal, and between. Unimodal is defined as the VIS and SMN modules. Transmodal is defined as the DMN, CONT, DAN, and SVAN modules. (B) Probability density of pooled subject-level edge weight distributions. R1, ICVF, FA, RD, LoS, and FC are shown on a linear x-axis (top), and NoS, SIFT2, and COMMIT are shown on a logarithmic x-axis (bottom). All networks were normalized to the range [0, 1] by dividing by the subject-level max for visualization.
Subject-level edge weight distributions in R1, ICVF, FA, and RD are near normal and network-specific (Figure 2B). They differ in both the magnitude (R1 > ICVF > FA > RD) and the dynamic range (FA and ICVF > R1 and RD) of their edge weights. In contrast, NoS, SIFT2, and COMMIT distributions are highly skewed and tend to be much lower in magnitude (dashed line). This effect is greatest in COMMIT, suggesting that the optimization performed by COMMIT exerts a stronger scaling effect than SIFT2. These results support the conclusion that the structural networks considered here quantify subsets of white matter features that are at least partially nonoverlapping.
Edge Weights in Streamline-Specific Networks Are More Variable
Edge weight variance was quantified using the quartile coefficient of dispersion (CQD) because of its robustness to outliers and skewed data. The CQD is computed from the first and third quartiles as the following: CQD = (Q3 − Q1)/(Q3 + Q1).
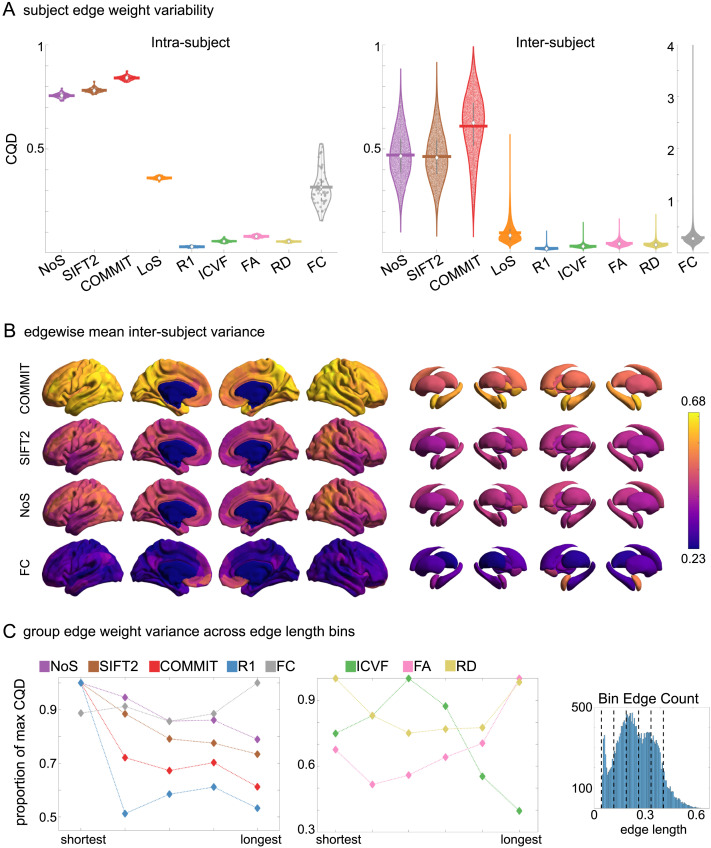
Intra-subject variance is roughly twofold greater in NoS, SIFT2, and COMMIT relative to LoS and FC; and an order of magnitude greater than R1, ICVF, FA, and RD in all subjects (Figure 3A). COMMIT is the highest overall. Subjects are more tightly clustered in all weighted SC networks, relative to FC: intra-subject CQD values span roughly a fourfold greater range in FC. This suggests that individual diversity of functional connectivity is not necessarily reflected in the variability of their structural networks. These patterns are repeated for inter-subject variance. However, FC shows a small subset of highly variable edges with roughly fourfold greater CQD than the maximum values observed in COMMIT; that is, the most subject-specific connections are functional. The very low edge weight variability in R1, ICVF, FA, and RD is in part due to the widespread blurring effect (partial voluming) resulting from the tractometry computation.
Figure 3. .
Edge weight variability. Variability is quantified using the coefficient of quartile dispersion (CQD). (A) Violin distributions of intra-subject (left) and inter-subject (right) edge weight variance. Colored data points respectively correspond to individual subjects (N = 50) and edges (N = 8,549). (B) Surface projections of edgewise mean inter-subject variance for cortical nodes in the Schaefer-400 parcellation (left) and 14 subcortical nodes (right). Cortical and subcortical surfaces were respectively generated with BrainSpace (Vos de Wael et al., 2020) and ENIGMA toolboxes (Larivière et al., 2021). (C) The proportion of within-network max CQD is shown across edge length bins for FC, NoS, SIFT2, COMMIT, and R1 (left), as well as ICVF, FA, and RD (middle). Edge weights are grouped into six bins according to edge length, as illustrated by the histogram (right). The edges of bins 1–5 were linearly spaced of width, w. The edges of the final bin were of width 3w.
In general, inter-subject edge weight variance is more spatially distributed in SC networks relative to FC (Figure 3B). COMMIT shows the highest mean CQD over the entire cortex and subcortex. NoS, SIFT2, and COMMIT all show lateral-medial and posterior-anterior cortical gradients. Mean CQD in FC shows the highest concentration in medial inferior frontal cortex and, to a lesser extent, the expected pattern of high variance in association cortex. The most variable subcortical regions include the hippocampus, amygdala, and accumbens.
Many features of brain networks (e.g., connection probability, weight magnitude) are known to vary with edge length. Here, we examined the relationship between edge weight variability and edge length by computing the CQD within subsets of group-level edge weights binned according to their edge length (Figure 3C). Edge weight variance in NoS, SIFT2, COMMIT, and R1 is highest in the shortest edges and decreases with edge length. ICVF roughly follows the same pattern. FA and RD instead show the highest variability in the longest edges. Overall, the edge weights in streamline-specific SC networks (NoS, SIFT2, and COMMIT) show greater contrast both within and across subjects. SC networks show network-dependent relationships between edge weight variance and edge length. Shorter edges are more variable in myelin- and connection-strength-weighted networks, and longer edges are more variable in networks with edge weights derived from a diffusion tensor model.
To complement the above results, a supplemental analysis was performed using intraclass correlation to quantify edge weight variance within each edge weight (Supporting Information Figure S9).
Opposing Correlations with Function in Connection-Strength- and Myelin-Weighted Networks
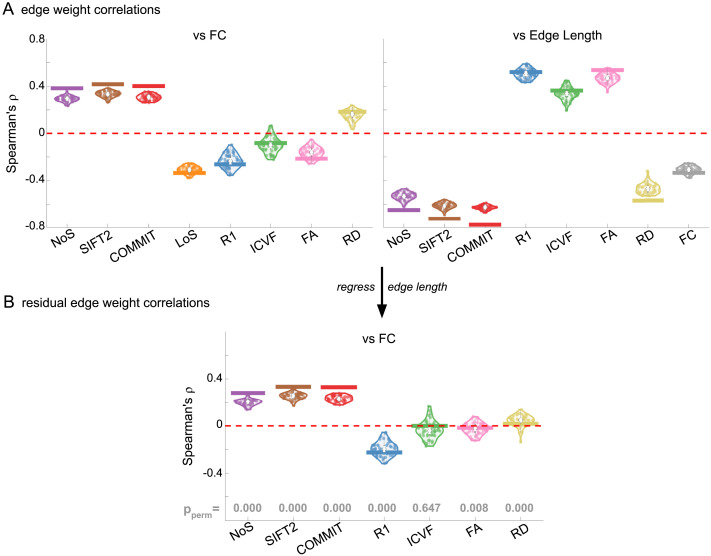
Shifting to inter-network edge weight relationships shows that SC networks are differentially related to FC (Figure 4A). Importantly, we also see that all brain networks (SC and FC) are strongly and differentially related to edge length at the subject and group levels. Correlations with edge length are negative for NoS, SIFT2, COMMIT, RD, and FC; and positive for R1, ICVF, and FA. Correlation magnitude is strongest in group-level COMMIT (ρ ≈ −0.8). To account for this strong obscuring effect, we recomputed correlations using residual edge weights following linear regression of edge length (Figure 4B). NoS, SIFT2, and COMMIT remain positively associated (group-level ρ ≈ 0.35) and R1 remains negatively associated with FC (group-level ρ ≈ −0.22). Correlation magnitude was reduced following linear regression of edge length in all cases. ICVF, FA, and RD are reduced to 0, suggesting that they may not be useful in modeling whole-brain FC. These results support the idea that R1-weighted SC networks provide complementary information to NoS, SIFT2, and COMMIT about the brain structure-function relationship.
Figure 4. .
Edge weight correlations with FC and edge length. (A) Violin distributions of edgewise Spearman’s rank correlations of all networks with FC (left) and edge length (right). (B) Violin distributions of edgewise Spearman’s rank correlations of residual edge weights in all networks with residual edge weights in FC. Residual edge weights were computed by linear regression of edge length. Colored data points and bars respectively indicate subject-level and group-level correlations. Pperm gives the one-sided p value obtained from permutation testing (Supporting Information Figure S7).
Edge Caliber and Myelin Content Are Inversely Related
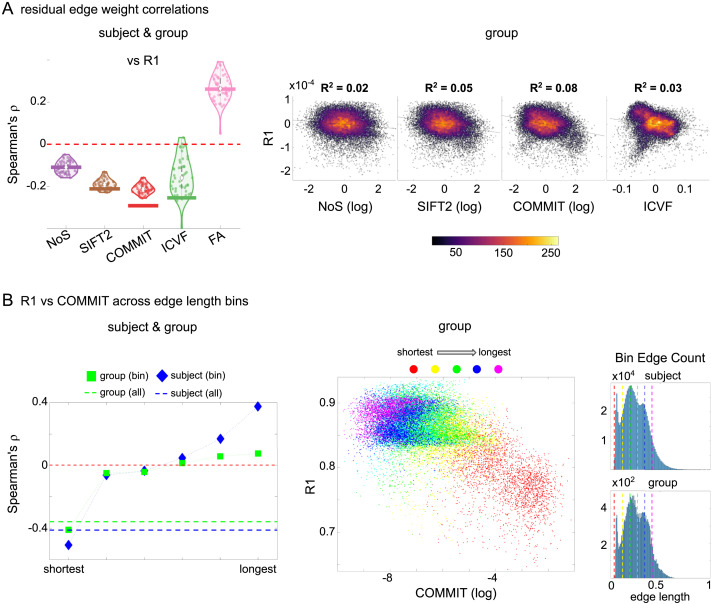
Here, we ask how R1, which we refer to as the myelin-weighted network, is related to the connection-strength-weighted network COMMIT. Edge-length regressed residual edge weights in NoS, SIFT2, and COMMIT show a negative association with R1 residuals for all subjects and at the group level, which is strongest in COMMIT (group-level ρ ≈ −0.29) (Figure 5A). This suggests an edge-length-independent inverse relationship between white matter structural features related to connection strength and myelin content.
Figure 5. .
The myelin-dependence of structural brain networks. (A) Violin distributions (left) of edgewise Spearman’s rank correlations with the myelin-weighted network R1. Residual edge weights are compared following linear regression of edge length. Colored data points and bars respectively indicate subject-level and group-level correlations. Heat scatterplots (right) of group-level residual edge weights in R1 as a function of NoS (left), SIFT2 (left middle), COMMIT (right middle), and ICVF (right). The best fit linear curve is shown in black, and R2 (coefficient of determination) is reported. Data color indicates density. Permutation testing provided a one-sided p value of Pperm = 0.000 for all edgewise correlations (Supporting Information Figure S8). (B) Line plot (left) of edgewise Spearman’s rank correlation of edge weights in R1 versus COMMIT across edge length bins. Group-level and subject-level are respectively shown in green and blue. The square and diamond markers connected by dotted lines show binned correlation values, and the horizontal dashed green and blue lines mark the correlation values for all edges pooled together. Scatterplot (middle) of group-level edge weights in R1 as a function of COMMIT with data points colored by bin identity. Histograms (right) illustrating subject- and group-level edge length bins.
Computing correlations of edge weights (not residuals) within edge length bins allows the inverse relationship between R1 and COMMIT to be traced to the shortest edges of the network (group ρ ≈ −0.40, subject ρ ≈ −0.50). As edge length increases, this relationship is reduced to 0, then becomes strongly positive in the longest subject-level edges (ρ ≈ 0.39). The scatterplot of group-level R1 versus COMMIT (middle) shows decreasing COMMIT and increasing R1 with increasing edge length. All together, these results support an inverse relationship between the edge caliber and myelin content of a given white matter tract. This can be partly explained by the differential dependence of these structural features on edge length: longer tracts tend to be more myelinated with lower total intra-axonal cross-sectional area. However, this relationship is robust to controlling for edge length supporting an intrinsic dependence between these white matter features.
In addition, we show that our R1-weighted network corresponds well with a previously reported (Boshkovski et al., 2021) R1-weighted structural connectome (Supporting Information Figure S13).
Divergent Small-Worldness, Hubness, and Rich Club in Weighted Structural Networks
In this final section, we apply network analysis tools (Rubinov & Sporns, 2010) based on graph theory (Fornito et al., 2013; Sporns, 2018) to group-level weighted SC networks. This facilitates high-level interpretation of general features of network communication such as integrative versus segregative processing and the economy of network organization. Although the high material and metabolic cost of brain tissue naturally tends to favor local connectivity (high clustering), short overall network path length is achieved through a small number of relatively expensive long-range connections (Bullmore & Sporns, 2012). These edges and the nodes they interlink form a densely connected network core known as the rich club (van den Heuvel & Sporns, 2011). While the general proclivity for high local clustering gives rise to segregated functional modules, the rich-club nodes act as network communication hubs supporting inter-modular integration (Collin et al., 2014; de Reus & van den Heuvel, 2014; Griffa & van den Heuvel, 2018; Kim & Min, 2020; van den Heuvel & Sporns, 2013). Thus, small-world network topology (high clustering and low path length) (Bassett & Bullmore, 2006, 2017) supports both integrative and segregative processing at a minimum of wiring cost, and the underlying scaffold of hub brain regions tends to show high centrality, low path length (high closeness), and low clustering (van den Heuvel et al., 2010).
Here, we report normalized small-worldness, normalized rich-club curves, and nodal hubness (Figure 6). Normalized small-worldness (S) is computed as the quotient of normalized measures of clustering coefficient (C/Cnull) and path length (L/Lnull).
Figure 6. .
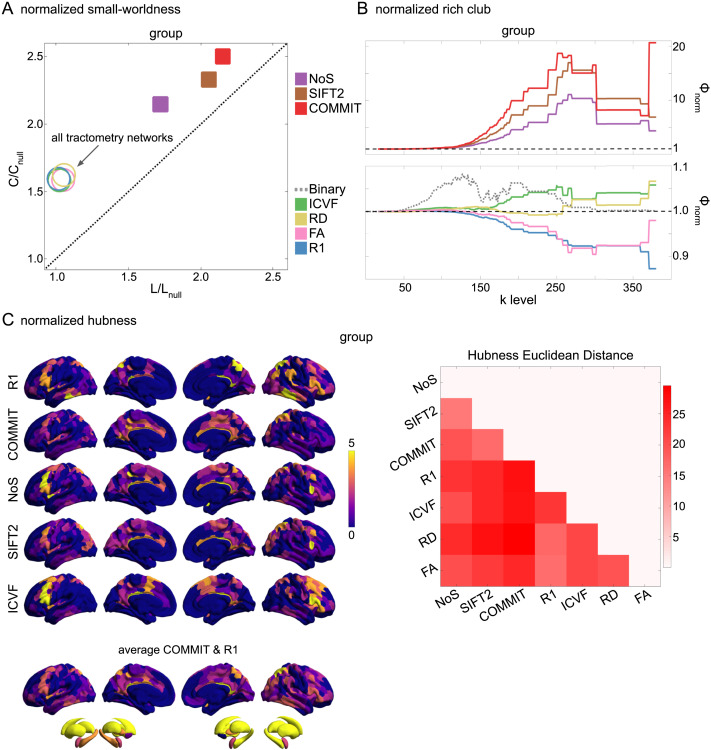
Group-level network topology. (A) Small-worldness was estimated in all structural networks: clustering coefficient was normalized within each node, averaged across nodes (C/Cnull), then plotted as a function of normalized characteristic path length (L/Lnull). Topology measures averaged across 50 degree- and strength-preserving null networks were used for normalization. Networks above the identity line (dotted black) are characterized by the small-world attribute. Tractometry networks are indicated by the arrow. (B) Normalized rich-club curves are shown for COMMIT, NoS, and SIFT2 (top), as well as ICVF, RD, FA, and R1 (bottom). A single binary network (dotted gray line) is also shown (bottom) as binary connectivity was uniform across weighted networks. The normalized rich-club coefficient (ϕnorm) was computed across the range of degree (k) and normalized against 1,000 null networks (degree-preserving for binary and degree- and strength-preserving for weighted networks). A ϕnorm value > 1 (horizontal dashed black lines) over a range of k indicates the presence of a rich club. (C) Nodewise hubness scores are projected onto Schaefer-400 cortical and 14-ROI subcortical surfaces. Scores (0–5) were computed for each node as +1 point for all nodes in top 20% strength, betweenness, closeness, and eigenvector centrality, as well as bottom 20% clustering coefficient. The matrix (right) shows the Euclidean distance between all pairs of nodal hubness vectors.
All group-level weighted SC networks show the normalized small-world property (S > 1) of higher clustering and lower path length than would be expected by chance (Figure 6A). Small-worldness is highest in COMMIT (S ≈ 2.5) and lowest in R1, ICVF, FA, and RD (S ≈ 1.6). In contrast, all weighted SC networks did not show a canonical rich club (Figure 6B). Relative to the tractometry and binary SC networks, the normalized rich-club coefficient (ϕnorm) was much higher in magnitude in NoS, SIFT2, and COMMIT. A rich club was detected in these networks across a large range of degree (k) levels (150 < k < 300). ϕnorm was maximal at k ≈ 265 in COMMIT. A rich club was also detected across a similar range of k levels in ICVF and across k in the range [250, 300] for RD, albeit with much lower magnitude ϕnorm. However, no clear rich club was observed in R1 or FA. In fact, the rich-club curves for these networks are roughly symmetric about the ϕnorm = 1 line relative to COMMIT. A densely connected core was of course recovered in all weighted SC networks (uniform binary connectivity), but these results suggest that its interconnecting edges were consistently weaker than would be expected by chance in R1 and FA. By comparison, a rich club was observed in the binary SC network across the very large range of k [50, 300]. This supports two important concepts: (1) SC network edge weights can provide an additional layer of information useful for refining the topology of binary SC. (2) Different methods for computing SC network edge weights yield diverse network topology.
Weighted SC networks show network-dependent spatial topology of hubness scores (Figure 6C). The COMMIT and R1 averaged surface shows prominent hubs distributed throughout the brain including the frontoparietal network. Nearly all of the subcortex showed a hubness score of 4 or greater in all networks. The Euclidean distance between hubness score vectors (right) was lower for COMMIT and SIFT2 than for either network with NoS. Of the streamline-specific networks, NoS was more similar to both R1 and IVCF. Overall, these results illustrate the considerable impact that edge weighting can have on network topology.
DISCUSSION
Structure-function brain models provide a flexible framework for investigating the mechanistic relationship between human brain structure and function in vivo, yet the interpretability of these models is currently limited by a lack of biological detail. Here, we assemble a thorough characterization of structural brain networks weighted by a range of quantitative MRI metrics capturing the macro- and microscopic features of white matter tracts. Notable trends included the following: (a) greater edge weight contrast and skewed (heavy-tailed) distributions in the streamline-specific networks NoS, SIFT2, and COMMIT; (b) whole-brain correlations with FC in networks weighted by connection strength (positive) and myelin (negative) that were robust to controlling for edge length; (c) whole-brain inverse relationships with myelin for networks weighted by connection strength and neurite density independent of edge length; and (d) the absence of a rich club in R1 and FA networks. All weighted SC networks showed a strong spatial dependence and small-world architecture. Collectively, these results support the overall conclusion that SC networks weighted by edge caliber (e.g., SIFT2 and COMMIT) and myelin (e.g., R1) can be used to quantify nonoverlapping subsets of white matter structural features related to FC, supporting their joint utilization in modeling function.
Interpretable Measures of Connection Strength Provided by COMMIT and SIFT2
A principal goal of this work is to identify what, if any, advantage over NoS is provided by the global optimization methods SIFT2 and COMMIT. NoS has previously been used to inform the strength of interregional coupling in computational models of function (e.g., Honey et al., 2009). However, important limitations restrict model interpretation. Besides suffering from a range of biases related to the position, size, shape, and length of white matter tracts (Girard et al., 2014), NoS varies as a function of tracking parameters, limiting its specificity for white matter structural features (Jones, 2010; Jones et al., 2013).
SIFT2 and COMMIT reportedly restore the quantitative link between connectome edge weights and white matter structural features related to connection strength. COMMIT and SIFT2 solve for the effective cross-sectional area (i.e., signal fraction) of each streamline using different approaches. COMMIT uses the global diffusion signal to optimize these values, whereas SIFT2 seeks to fit the streamline density throughout the white matter to the fiber densities estimated using spherical deconvolution. Thus, while both methods rely on the simplifying assumption that streamline features are invariant along their length, SIFT2 additionally requires that the estimates of fiber density derived from the fiber orientation distribution (FOD) are biologically accurate.
These networks also differ in the computation of their edge weights: SIFT2 is computed as the simple sum of streamline weights, whereas COMMIT is computed as the length-weighted sum of streamline weights. Indeed, our analysis methods do not permit us to make strong claims as to the relationship between these methodological differences and our observed results; however, we do show that both SIFT2 and COMMIT display comparable but not identical fundamental characteristics to NoS. This supports the use of SIFT2 or COMMIT in place of NoS as a measure of connection strength, which brings with it improved biological interpretability.
Myelin Complements Connection Strength in Predicting FC
Despite the differences between COMMIT, SIFT2, and NoS, our results indicate that their edge weights show roughly equivalent positive correlations with FC over the whole brain. R1 was negatively correlated with FC. Significant evidence indicates a link between cerebral myelin and FC, including a relationship between intracortical myelin and FC (Huntenburg et al., 2017; Wang et al., 2019); the prediction of cognition (Bells et al., 2017; Caeyenberghs et al., 2016) and FC-derived components (Messaritaki et al., 2021) using myelin-sensitive metrics; and a relationship between damaged myelin sheaths and greater conduction delays in multiple sclerosis (Sorrentino et al., 2022). At the cellular level, myelin contributes to conduction velocity (Huxley & Stämpfli, 1949), metabolic support (Nave & Werner, 2014), and plasticity (Gibson et al., 2018), all of which could be argued to support brain function. Myelin plasticity in particular can be described in terms of “activity-dependence,” whereby an increase in the functional activity of a given circuit stimulates cellular signaling cascades, promoting greater myelination (Fields, 2015; Mount & Monje, 2017). Coupled with our results, this complex mix of functional roles supports the idea that structure-function models will be improved by integrating measures of myelin and connection strength.
An Opposing Relationship With Edge Length for Edge Caliber and Myelin Content
When controlling for edge length, we found an inverse relationship between R1 and COMMIT over the whole brain in all subjects and at the group level. This suggests that the aggregate g-ratio (ratio of inner/outer diameters of myelinated axons) of a white matter tract may increase with edge caliber. At the cellular level, the diameter of an axon and the thickness of its myelin sheath show nearly a linear relationship over a broad range of smaller diameter axons; this relationship becomes increasingly nonlinear as axon diameter increases (Berthold et al., 1983; Hildebrand & Hahn, 1978). In general, increasing axon diameter tends to outpace increasing myelin thickness, that is, the g-ratio tends to increase with increasing axon caliber (Hildebrand & Hahn, 1978). Our findings suggest that this cellular-level principle may extend to the systems level: Increases in edge caliber tend to outpace changes in the myelin content, resulting in a concomitant increase in the g-ratio of white matter tracts.
We localized the inverse relationship between R1 and COMMIT to the shortest edges, suggesting that the g-ratio was the highest in the shortest connections. This result is supported by a previous imaging study showing the highest g-ratio in “local” connections (Mancini et al., 2018). In general, we found that R1 increased and COMMIT decreased with increasing edge length, which aligns with previously reported results of higher R1 and fewer streamlines for the white matter connections between transmodal regions (Boshkovski et al., 2021). Both of these trends fit well with theories of brain wiring economy in which the energetic cost of maintaining biological material increases with connection length (Bullmore & Sporns, 2012). This natural pressure acts to reduce the total axonal volume of longer white matter bundles. Increasing the myelin content of longer tracts comes at a cost as well, but this may be at least partially offset as increasing myelin content reduces the total membrane surface area along which expensive electrochemical gradients must be maintained (Bullmore & Sporns, 2012). However, a cost-benefit analysis of the energetics of myelination concluded that the energetic cost of myelin maintenance outweighs any savings on action potentials (Harris & Attwell, 2012). This suggests that higher myelination of longer edges may be better explained as a mechanism to provide trophic support (Nave & Werner, 2014) to vital interregional connections (van den Heuvel et al., 2012) or to reduce conduction delays.
Edge Weight Variance Decreases With Edge Length in Most Weighted Structural Networks
White matter features related to myelin content, connection strength, and neurite density tend to become more consistent across tracts as tract length increases. Greater variability in the weights of the shortest connections could result from a higher proportion of false positive streamlines influencing these edge weights. For SIFT2 and COMMIT, streamline weight computation becomes increasingly unstable with decreasing length as fewer voxels contribute to the fit. However, this result could also be explained more generally by contrasting the roles of shorter and longer connections in the brain. Shorter white matter tracts connect brain regions near each other in space, such as within the same module. Just as we might expect the characteristics of smaller roads and streets (e.g., width, building materials, markings, signs, sidewalks) to vary by neighborhood and city, we might also expect the morphology of shorter white matter connections to change as the functional specialization of any given region or module changes. On the other hand, longer tracts (i.e., the freeways of the brain) may overlap more in both their functional role and their morphological features relative to shorter connections, hence lower edge weight variability. Breaking with the above pattern, FA and RD showed the highest edge weight variance in the longest connections. Given that structural measures derived using a voxel-wise diffusion tensor model are particularly sensitive to the white matter “architectural paradigm” (Jones et al., 2013), these results suggest that white matter features related to fiber orientation and geometry actually diverge with increasing tract length. Note that we are unable to say decisively whether the edge weight variance measured in these structural and functional brain networks corresponds to true signal or noise. The inclusion of scan-rescan data (e.g., as in Amico & Goñi, 2018) could support stronger conclusions as to the source of this variability.
The Absence of a Rich Club in Structural Networks Weighted by R1 and FA
Group-level R1 and FA did not show a normalized weighted rich club for any degree k. Higher myelination in the white matter tracts connecting rich-club nodes has previously been reported (Collin et al., 2014); however, methodological differences limit comparability. A rich club has previously been reported in FA-weighted networks using similar methods to ours (van den Heuvel & Sporns, 2011). The source of this disagreement could potentially be attributed to differences in our tractography algorithm, parcellation, or null network computation.
In weighted rich-club detection, the identification of a densely connected core is independent of edge weight (depends only on node degree), but the designation of this subnetwork as a rich club requires that it contains a higher-than-chance proportion of the strongest edges from the full network. Indeed, this is the case over a broad range of degree k for COMMIT. Over the same range of k, the normalized rich-club curves for R1 and FA are inverted about the threshold value of 1 with respect to COMMIT. This implies that the subnetwork found at a given k in this range contains edges that tend to show higher COMMIT and lower R1 edge weights than expected by chance. We previously showed edgewise inverse correlations between R1 and COMMIT that were robust to controlling for edge length. We also showed that R1 and FA are positively correlated under these same conditions. In this light, it is not surprising that the edges connecting rich-club nodes tend to show opposite trends in R1- and FA-weighting with respect to COMMIT. Nonetheless, it is possible that the lack of a rich club in our myelin-weighted network is an artifact of tractometry. Future work will attempt to replicate this result using myelin-weighted networks computed with a different methodology (Schiavi et al., 2022).
Replication Across Parcellation Resolution and in a Second Dataset
In this report, we have chosen to feature data in the Schaefer-400 cortical parcellation plus 14 subcortical nodes. However, there is little consensus on the best brain atlas, and the optimal choice likely depends on the specifics of your data and the question being investigated. In a supplementary analysis, we replicated our results across 100- to 900-node Schaefer cortical atlases. We found that residual edgewise correlations with FC (Supporting Information Figure S1) and R1 (Supporting Information Figure S2), as well as normalized rich club and normalized small-worldness (Supporting Information Figure S3), were robust to parcellation resolution. In contrast, the spatial topography of high-hubness brain regions appears qualitatively dependent on parcellation granularity, although further analyses would be necessary to draw stronger conclusions (Supporting Information Figure S4).
An independent multimodal dataset was also used to replicate the main SC results, including the residual edgewise correlations with R1 and the relationship between R1 and COMMIT across edge length bins (Supporting Information Figure S5), as well as all network topology results (Supporting Information Figure S6).
Limitations
Streamline tractography is known to suffer from several important biases, including both false positive and false negative streamlines, which can influence downstream analyses (Maier-Hein et al., 2017; Schilling et al., 2019; Sotiropoulos & Zalesky, 2019; Zalesky et al., 2016). Through probabilistic tractography, we opted to minimize false negatives while maximizing false positives. This allowed us to implement careful streamline- and edge-filtering strategies in postprocessing to address this known bias. Still, without a ground truth, we cannot quantify the extent to which we were successful in mitigating this issue, nor can we guarantee that we did not erroneously filter true positive streamlines or edges. All processing and filtering methods were consistent and network density was uniform across weighted structural networks. Thus, any major tractography bias should be as homogeneous as possible across networks.
Tractometry-derived brain networks suffer from widespread partial volume effects due to crossing and kissing fibers in a majority of white matter voxels. The net effect of this bias is well understood and is apparent in our results and previous work (De Santis et al., 2014; Schiavi et al., 2022). Nonetheless, this method was included here, as our goal was to characterize widely used structural connectivity methods. New techniques for reducing this bias are currently being developed; these techniques allow for the estimation of tract-specific microstructural features (e.g., Barakovic, Girard, et al., 2021; Barakovic, Tax, et al., 2021; De Santis et al., 2016; Leppert et al., 2021, 2023; Schiavi et al., 2022).
We were unable to assess repeatability in this work as we did not have scan-rescan data. However, reproducibility has already been assessed for NODDI (Chung et al., 2016; Lehmann et al., 2021), MP2RAGE-derived T1 maps (Marques et al., 2010), diffusion-tractography-based structural connectivity (Bonilha et al., 2015), as well as COMMIT and SIFT2 tractogram filtering (Koch et al., 2022). The reproducibility of the tractometry features (R1, FA, RD, ICVF) will mainly depend on these previous steps and the accuracy of the spatial alignment of the multimodal data.
In this work, we have attempted to thoroughly examine the fundamental properties of a wide range of standard and state-of-the-art metrics for quantifying white matter brain structure. However, the scope of possible methods and their respective variants is too broad to treat thoroughly in a single body of work. In particular, track-weighted imaging (Calamante, 2017; Calamante et al., 2010, 2012) and fixel-based analysis (Dhollander et al., 2021; Raffelt et al., 2015, 2017) provide state-of-the-art solutions to the challenge of quantifying white matter structural features in the presence of crossing fibers.
Conclusion
We presented a thorough characterization of weighted SC networks. Overall, our findings support the joint use of SC networks weighted by connection strength and myelin in predicting FC. In particular, using the COMMIT or SIFT2 algorithms to quantify connection strength shows promise to improve model interpretability relative to NoS. Beyond R1, there is a wide array of myelin-sensitive metrics that could be used to compute useful myelin-weighted networks. The integration of this microstructure-weighted connectivity approach into structure-function models will advance the mechanistic interpretation of both the function and the dysfunction of the living human brain.
MATERIALS AND METHODS
Data Acquisition and Preprocessing
Multimodal MRI data were collected in 50 healthy volunteers at 3 Tesla on a Siemens Magnetom Prisma-Fit scanner equipped with a 64-channel head coil as follows:
T1-weighted (T1w) anatomical: 3D magnetization-prepared rapid gradient-echo sequence (MP-RAGE; 0.8 mm isotropic; TR = 2,300 ms; TE = 3.14 ms; TI = 900 ms; iPAT = 2; partial Fourier = 6/8).
Multi-shell diffusion-weighted imaging (DWI): 2D pulsed gradient spin-echo echo-planar imaging sequence consisting of three shells with b-values 300, 700, and 2,000 s/mm2 and diffusion directions 10, 40, and 90, respectively (1.6 mm isotropic; TR = 3,500 ms, TE = 64.40 ms; multiband factor = 3); b0 images were also acquired with reverse phase encoding direction to facilitate distortion correction of DWI data.
7 min of resting-state functional MRI: multiband accelerated 2D-BOLD gradient echo echo-planar sequence (3 mm isotropic; TR = 600 ms, TE = 30 ms; mb factor = 6; flip angle = 52°). Two spin-echo images with AP and PA phase encoding were additionally acquired (3 mm isotropic; TR = 4,029 ms; TE = 48 ms; flip angle = 90°).
Quantitative TI relaxometry data were acquired with a 3D-MP2RAGE sequence (Marques et al., 2010) (0.8 mm isotropic; TR = 5,000 ms, TE = 2.9 ms, TI1 = 940 ms, T12 = 2,830 ms; iPAT = 3; partial Fourier = 6/8). This was used to compute a T1 map that was sampled to estimate the edge weights in R1 (1/T1) networks (myelin-weighted).
The multimodal processing pipeline micapipe (Cruces et al., 2022) (https://micapipe.readthedocs.io/) was used to preprocess diffusion, anatomical, and functional images. T1w images were deobliqued, reoriented to standard neuroscience orientation (LPI), corrected for intensity nonuniformity (Tustison et al., 2010), intensity normalized, and skull stripped. Subcortical segmentations were performed with FSL FIRST (Jenkinson et al., 2012; Patenaude et al., 2011) and tissue types were classified using FSL FAST (Y. Zhang et al., 2001). A five-tissue-type image segmentation was generated for anatomically constrained tractography (R. E. Smith et al., 2012). Cortical surface segmentations were generated with FreeSurfer 6.0 (Dale et al., 1999; Fischl, Sereno, & Dale, 1999; Fischl, Sereno, Tootell, & Dale, 1999).
Diffusion preprocessing was performed in native DWI space using tools from MRtrix3 (Tournier et al., 2012, 2019) and proceeded in the following sequence: (1) image denoising (Cordero-Grande et al., 2019; Veraart, Fieremans, et al., 2016; Veraart, Novikov, et al., 2016); (2) two b = 0 s/mm2 volumes with reverse phase encoding were used to correct for susceptibility distortion, head motion, and eddy currents via FSL’s eddy and TOPUP tools (Andersson et al., 2003; Andersson & Sotiropoulos, 2016; S. M. Smith et al., 2004); and (3) B1+ bias-field correction (Tustison et al., 2010). These preprocessed data were used to estimate multi-shell and multi-tissue response functions for constrained spherical-deconvolution (Christiaens et al., 2015; Dhollander et al., 2016, 2019; Jeurissen et al., 2014) followed by intensity normalization. Nonlinear registration was performed with ANTs (Avants et al., 2008) to coregister anatomical images to DWI space.
Resting-state fMRI preprocessing entailed discarding the first five TRs, reorientation (LPI), motion correction by registering all volumes to the mean, and distortion correction using main phase and reverse phase field maps. Nuisance signal was removed using an ICA-FIX (Salimi-Khorshidi et al., 2014) classifier and by spike regression using motion outlier outputs from FSL (Jenkinson et al., 2012). Volumetric time series were averaged for boundary-based registration (Greve & Fischl, 2009) to native FreeSurfer space and mapped to individual surfaces using trilinear interpolation. Spatial smoothing (Gaussian, FWHM = 10 mm) was applied to native-surface and template-mapped cortical time series.
Tractography and Microstructural Metrics
To estimate structural connectomes, anatomically constrained tractography (R. E. Smith et al., 2012) was performed on the normalized white matter FOD image using the probabilistic algorithm iFOD2 (Tournier et al., 2010). Tractograms of 5 million streamlines were generated by seeding the gray-white matter interface using the following parameters: max length = 400, min length = 10, angle = 22.5, step = 0.5, cutoff = 0.06, backtrack, crop_at_gmwmi (gray-matter-white-matter interface). These tractograms were filtered in a two-stage process: (1) A temporary whole-brain connectome weighted by NoS was computed then decomposed into its composite streamlines to derive a new tractogram in which any streamline that failed to connect two gray matter ROIs in the temporary connectome was excluded. This “streamline-filtering” step typically resulted in approximately a 5% decrease in the size of the tractogram (∼250,000 streamlines removed) and was undertaken to ensure that these erroneous streamlines did not affect the COMMIT model. Streamline-filtered tractograms were used to compute NoS and were used as inputs to both the SIFT2 and the COMMIT models. COMMIT was run using a Stick-Zeppelin-Ball forward model and default settings (see https://github.com/daducci/COMMIT). (2) Any streamline with a COMMIT weight < 1e−12 (machine precision 0) was interpreted as a false positive and filtered from the tractogram. This streamline-level COMMIT-filtering step typically resulted in greater than a 90% decrease in the size of the tractogram, with most containing between ∼300,000 and 600,000 streamlines. COMMIT-filtered tractograms were used not only in the computation of COMMIT, but in all tractometry networks as well. This additional filtering step was performed on COMMIT streamline weights only (not SIFT2) to reduce the impact of false positive streamlines in tractometry networks as much as possible.
In a supplemental analysis, the COMMIT streamline weights were additionally used in the computation of edge weights in tractometry-derived networks by performing a COMMIT-weighted average of a given tractometry metric (e.g., FA) over streamlines for each node pair (Supporting Information Figures S10–S12).
Construction of Weighted Structural Networks
The streamline-specific SC networks were computed in the following manner: (1) NoS as the summed streamline count; (2) LoS as the mean streamline length; (3) SIFT2 as the sum of SIFT2 streamline weights; and (4) COMMIT as the length-weighted sum of COMMIT streamline weights as in Schiavi et al. (2020). Explicitly, edgewise entries in COMMIT-weighted networks were computed as the following:
where αij is the edge weight between nodes i and j; ij is the mean streamline length; Nij is the number of streamlines; is the COMMIT weight of streamline k; and lk is its length. Edge weights in NoS, SIFT2, and COMMIT were normalized by node volume.
SC networks weighted by FA, RD, ICVF (H. Zhang et al., 2012), and R1 were derived using multimodal tractometry (Bells et al., 2011). Streamline weights were computed by (1) coregistering the tractogram and desired image and (2) sampling the voxel-level aggregate value along the length of each streamline. Edge weights were computed as the median along each streamline and the mean across streamlines by node pair. Voxel-wise measures of FA and RD were computed with a diffusion tensor model (Basser et al., 1994) and ICVF by applying the NODDI multicompartment model (H. Zhang et al., 2012) to preprocessed DWI data (Daducci, Canales-Rodríguez, et al., 2015).
The 400-node Schaefer (Schaefer et al., 2018) cortical parcellation is used in all results. Subcortical ROIs corresponded to 7 bilateral regions (14 nodes): amygdala, thalamus, caudate, accumbens, putamen, hippocampus, and pallidum. A single static, zero-lag FC network was derived by product-moment pairwise Pearson cross-correlation of node-averaged time series. FC network edge weights were Fisher Z-transformed.
Connectome Post-processing
COMMIT-weighted networks were used to filter all other weighted structural networks at the edge level. This was chosen as COMMIT-weighted networks had the lowest connection density to start, and all nonzero COMMIT edges were also nonzero in all other SC networks. All SC networks were thresholded at the edge level within subject by (1) setting edges = 0 in all weighted SC networks if that edge had a COMMIT weight < 1e−12 and (2) applying a 50% uniform threshold mask to facilitate group-consensus averaging. This minimized differences in binary structural network density across subjects and enforced a uniform binary connectivity map across weighted SC networks at the group level and within subject. Group-level networks were computed as the subject-wise mean at each edge excluding zero-valued edges.
Network Analysis
Network analysis was performed using tools (Rubinov & Sporns, 2010) based on graph theory (Fornito et al., 2013; Sporns, 2018). Measures of clustering coefficient and path length were normalized against 50 degree- and strength-preserving null networks. Clustering coefficient was normalized within node then averaged across nodes to obtain a scalar value per network. The following weight (Wij) to length (Lij) transform was used in path length computation: Lij = −log(Wij). Weighted rich-club curves were normalized against 1,000 degree- and strength-preserving null networks. The edges in all degree- and strength-preserving null networks were rewired 1e6 times total, and the strength sequence was approximated using simulated annealing. Rich-club curves were normalized in binary networks against 1,000 degree-preserving null networks in which each edge was rewired 100 times. All edge rewiring followed the Maslov-Sneppen rewiring model (Maslov & Sneppen, 2002). Similar to van den Heuvel et al. (2010), hubness scores (0–5) were computed as 1 point for all nodes showing top 20% strength, betweenness, closeness, or eigenvector centrality; and lowest 20% clustering coefficient.
Permutation Testing
Statistical significance for the edgewise correlation of residual edge weights in NoS, SIFT2, COMMIT, and R1 with FC (Supporting Information Figure S7), as well as all connection-strength-weighted networks with R1 (Supporting Information Figure S8), was quantified using permutation testing as described in the Supporting Information. One-sided p values are reported in the main text figures as pperm.
ACKNOWLEDGMENTS
Data collection at the McConnell Brain Imaging Centre was supported by Brain Canada and Healthy Brains for Healthy Lives.
DATA AVAILABILITY
These data are available for download (https://portal.conp.ca/dataset?id=projects/mica-mics; Royer et al., 2022b). See Royer et al. (2022a) and Cruces et al. (2022) for full details of data acquisition and processing. All data processing and analysis code is openly available at https://github.com/TardifLab/Weighted-SC-Networks (Nelson, 2023).
SUPPORTING INFORMATION
Supporting information for this article is available at https://doi.org/10.1162/netn_a_00330.
AUTHOR CONTRIBUTIONS
Mark Cameron Nelson: Conceptualization; Formal analysis; Methodology; Project administration; Software; Validation; Visualization; Writing – original draft; Writing – review & editing. Jessica Royer: Data curation; Software; Writing – review & editing. Wen Da Lu: Data curation; Methodology; Writing – review & editing. Ilana R. Leppert: Methodology; Writing – review & editing. Jennifer Campbell: Methodology; Writing – review & editing. Simona Schiavi: Methodology; Writing – review & editing. Hannah Jin: Conceptualization; Methodology; Writing – review & editing. Shahin Tavakol: Data curation. Reinder Vos de Wael: Data curation; Software. Raul Rodriguez-Cruces: Software. G. Bruce Pike: Writing – review & editing. Boris Bernhardt: Funding acquisition; Writing – review & editing. Alessandro Daducci: Methodology; Software; Writing – review & editing. Bratislav Misic: Conceptualization; Methodology; Supervision; Writing – review & editing. Christine Tardif: Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
FUNDING INFORMATION
Boris Bernhardt, NSERC-Discovery, Award ID: 1304413, DGECR-2018-00216. Boris Bernhardt, CIHR, Award ID: FDN-154298, PJT-174995. Boris Bernhardt, SickKids New Investigator, Award ID: NI17-039. Boris Bernhardt, Azrieli Center for Autism Research, Montreal Neurological Institute. Boris Bernhardt, Brain Canada Azrieli Future Leaders. Boris Bernhardt, Tier-2 Canada Research Chairs program. Christine Tardif, Fonds de Recherche du Québec – Santé, Research Scholar Junior 1, Award ID: 280246 and 266479. Christine Tardif, Healthy Brains for Healthy Lives, Award ID: 1c-ll-13. Christine Tardif, NSERC-Discovery, Award ID: 1304413, RGPIN-2018-05176.
Supplementary Material
TECHNICAL TERMS
- Streamline:
The contiguous set of 3D points produced by a tractography algorithm and representing the reconstructed trajectory of a white matter pathway.
- Tractography:
A method or algorithm that applies some model to diffusion MRI data to reconstruct a 3D visual representation of the white matter pathways of the brain.
- Tractometry:
A method for mapping the voxel-level values of a volumetric brain map onto the streamlines of a tractogram.
- Tractogram:
The 3D image representing the collection of streamlines reconstructed by a tractography algorithm.
- Edge weight:
The value assigned to the inter-nodal connections of a network that describes an explicit feature of each connection such as physical length or connection strength.
- Partial volume effect:
A “smoothing” or “blurring” imaging artifact whereby the data corresponding to sub-voxel-level features are lost or averaged as a result of the limited resolution of an imaging system.
- Small-worldness:
A topological network feature characterized by a low number of edges or connections separating distal nodes (short path length) alongside high connectivity between neighboring nodes (high local clustering).
- Rich club:
The tendency for highly connected (high degree) nodes to be more densely connected among themselves than nodes of a lower degree.
- Graph theory:
The study of graphs, which are mathematical structures used to model pairwise relationships between objects.
- Characteristic path length:
The average shortest path length between all pairs of nodes in the network.
- Clustering coefficient:
The fraction of triangles around an individual node, that is, the fraction of a node’s neighbors that are also neighbors with each other.
- Maslov-Sneppen rewiring:
An iterative rewiring process used to randomize the connections of an empirical network while preserving network size, connection density, and degree distribution.
Supporting Information
REFERENCES
- Alexander, D. C., Dyrby, T. B., Nilsson, M., & Zhang, H. (2019). Imaging brain microstructure with diffusion MRI: Practicality and applications. NMR in Biomedicine, 32(4), e3841. 10.1002/nbm.3841, [DOI] [PubMed] [Google Scholar]
- Alexander, D. C., Hubbard, P. L., Hall, M. G., Moore, E. A., Ptito, M., Parker, G. J. M., & Dyrby, T. B. (2010). Orientationally invariant indices of axon diameter and density from diffusion MRI. NeuroImage, 52(4), 1374–1389. 10.1016/j.neuroimage.2010.05.043, [DOI] [PubMed] [Google Scholar]
- Amico, E., & Goñi, J. (2018). The quest for identifiability in human functional connectomes. Scientific Reports, 8(1), 8254. 10.1038/s41598-018-25089-1, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, J. L. R., Skare, S., & Ashburner, J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. NeuroImage, 20(2), 870–888. 10.1016/S1053-8119(03)00336-7, [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. R., & Sotiropoulos, S. N. (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–1078. 10.1016/j.neuroimage.2015.10.019, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf, Y., Blumenfeld-Katzir, T., Yovel, Y., & Basser, P. J. (2008). AxCaliber: A method for measuring axon diameter distribution from diffusion MRI. Magnetic Resonance in Medicine, 59(6), 1347–1354. 10.1002/mrm.21577, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants, B. B., Epstein, C. L., Grossman, M., & Gee, J. C. (2008). Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12(1), 26–41. 10.1016/j.media.2007.06.004, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakovic, M., Girard, G., Schiavi, S., Romascano, D., Descoteaux, M., Granziera, C., Jones, D. K., Innocenti, G. M., Thiran, J.-P., & Daducci, A. (2021). Bundle-specific axon diameter index as a new contrast to differentiate white matter tracts. Frontiers in Neuroscience, 15, 646034. 10.3389/fnins.2021.646034, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakovic, M., Tax, C. M. W., Rudrapatna, U., Chamberland, M., Rafael-Patino, J., Granziera, C., Thiran, J. P., Daducci, A., Canales-Rodríguez, E. J., & Jones, D. K. (2021). Resolving bundle-specific intra-axonal T2 values within a voxel using diffusion-relaxation tract-based estimation. NeuroImage, 227, 117617. 10.1016/j.neuroimage.2020.117617, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser, P. J. (1995). Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine, 8(7), 333–344. 10.1002/nbm.1940080707, [DOI] [PubMed] [Google Scholar]
- Basser, P. J., Mattiello, J., & LeBihan, D. (1994). Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance, Series B, 103(3), 247–254. 10.1006/jmrb.1994.1037, [DOI] [PubMed] [Google Scholar]
- Bassett, D. S., & Bullmore, E. (2006). Small-world brain networks. Neuroscientist, 12(6), 512–523. 10.1177/1073858406293182, [DOI] [PubMed] [Google Scholar]
- Bassett, D. S., & Bullmore, E. T. (2017). Small-world brain networks revisited. Neuroscientist, 23(5), 499–516. 10.1177/1073858416667720, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bells, S., Cercignani, M., Deoni, S., & Assaf, Y. (2011). “Tractometry”—Comprehensive multi-modal quantitative assessment of white matter along specific tracts. Proceedings of the International Society for Magnetic Resonance in Medicine, 19(2009), 678. https://cds.ismrm.org/protected/11MProceedings/PDFfiles/678.pdf [Google Scholar]
- Bells, S., Lefebvre, J., Prescott, S. A., Dockstader, C., Bouffet, E., Skocic, J., Laughlin, S., & Mabbott, D. J. (2017). Changes in white matter microstructure impact cognition by disrupting the ability of neural assemblies to synchronize. Journal of Neuroscience, 37(34), 8227–8238. 10.1523/JNEUROSCI.0560-17.2017, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold, C. H., Nilsson, I., & Rydmark, M. (1983). Axon diameter and myelin sheath thickness in nerve fibres of the ventral spinal root of the seventh lumbar nerve of the adult and developing cat. Journal of Anatomy, 136(Pt. 3), 483–508. [PMC free article] [PubMed] [Google Scholar]
- Biswal, B., Zerrin Yetkin, F., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. 10.1002/mrm.1910340409, [DOI] [PubMed] [Google Scholar]
- Bonilha, L., Gleichgerrcht, E., Fridriksson, J., Breedlove, J. L., Rorden, C., Nesland, T., Paulus, W., Helms, G., & Focke, N. K. (2015). Reproducibility of the structural brain connectome derived from diffusion tensor imaging. PLOS ONE, 10(9), e0135247. 10.1371/journal.pone.0135247, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshkovski, T., Kocarev, L., Cohen-Adad, J., Mišić, B., Lehéricy, S., Stikov, N., & Mancini, M. (2021). The R1-weighted connectome: Complementing brain networks with a myelin-sensitive measure. Network Neuroscience, 5(2), 358–372. 10.1162/netn_a_00179, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore, E., & Sporns, O. (2012). The economy of brain network organization. Nature Reviews Neuroscience, 13(5), 336–349. 10.1038/nrn3214, [DOI] [PubMed] [Google Scholar]
- Cabral, J., Kringelbach, M. L., & Deco, G. (2017). Functional connectivity dynamically evolves on multiple time-scales over a static structural connectome: Models and mechanisms. NeuroImage, 160, 84–96. 10.1016/j.neuroimage.2017.03.045, [DOI] [PubMed] [Google Scholar]
- Caeyenberghs, K., Metzler-Baddeley, C., Foley, S., & Jones, D. K. (2016). Dynamics of the human structural connectome underlying working memory training. Journal of Neuroscience, 36(14), 4056–4066. 10.1523/JNEUROSCI.1973-15.2016, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamante, F. (2017). Track-weighted imaging methods: Extracting information from a streamlines tractogram. Magnetic Resonance Materials in Physics, Biology and Medicine, 30(4), 317–335. 10.1007/s10334-017-0608-1, [DOI] [PubMed] [Google Scholar]
- Calamante, F., Tournier, J. D., Jackson, G. D., & Connelly, A. (2010). Track-density imaging (TDI): Super-resolution white matter imaging using whole-brain track-density mapping. NeuroImage, 53(4), 1233–1243. 10.1016/j.neuroimage.2010.07.024, [DOI] [PubMed] [Google Scholar]
- Calamante, F., Tournier, J. D., Smith, R. E., & Connelly, A. (2012). A generalised framework for super-resolution track-weighted imaging. NeuroImage, 59(3), 2494–2503. 10.1016/j.neuroimage.2011.08.099, [DOI] [PubMed] [Google Scholar]
- Christiaens, D., Reisert, M., Dhollander, T., Sunaert, S., Suetens, P., & Maes, F. (2015). Global tractography of multi-shell diffusion-weighted imaging data using a multi-tissue model. NeuroImage, 123, 89–101. 10.1016/j.neuroimage.2015.08.008, [DOI] [PubMed] [Google Scholar]
- Chung, A. W., Seunarine, K. K., & Clark, C. A. (2016). NODDI reproducibility and variability with magnetic field strength: A comparison between 1.5 T and 3 T. Human Brain Mapping, 37(12), 4550–4565. 10.1002/hbm.23328, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin, G., Sporns, O., Mandl, R. C. W., & van den Heuvel, M. P. (2014). Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cerebral Cortex, 24(9), 2258–2267. 10.1093/cercor/bht064, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Grande, L., Christiaens, D., Hutter, J., Price, A. N., & Hajnal, J. V. (2019). Complex diffusion-weighted image estimation via matrix recovery under general noise models. NeuroImage, 200, 391–404. 10.1016/j.neuroimage.2019.06.039, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruces, R. R., Royer, J., Herholz, P., Larivière, S., Vos de Wael, R., Paquola, C., Benkarim, O., Park, B.-Y., Degré-Pelletier, J., Nelson, M. C., DeKraker, J., Leppert, I. R., Tardif, C., Poline, J.-B., Concha, L., & Bernhardt, B. C. (2022). Micapipe: A pipeline for multimodal neuroimaging and connectome analysis. NeuroImage, 263, 119612. 10.1016/j.neuroimage.2022.119612, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daducci, A., Canales-Rodríguez, E. J., Zhang, H., Dyrby, T. B., Alexander, D. C., & Thiran, J. P. (2015). Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. NeuroImage, 105, 32–44. 10.1016/j.neuroimage.2014.10.026, [DOI] [PubMed] [Google Scholar]
- Daducci, A., Dal Palù, A., Lemkaddem, A., & Thiran, J.-P. (2013). A convex optimization framework for global tractography. In Proceedings of the 10th International Symposium on Biomedical Imaging (pp. 524–527). IEEE. 10.1109/ISBI.2013.6556527 [DOI] [Google Scholar]
- Daducci, A., Dal Palù, A., Lemkaddem, A., & Thiran, J. P. (2015). COMMIT: Convex optimization modeling for microstructure informed tractography. IEEE Transactions on Medical Imaging, 34(1), 246–257. 10.1109/TMI.2014.2352414, [DOI] [PubMed] [Google Scholar]
- Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. 10.1006/nimg.1998.0395, [DOI] [PubMed] [Google Scholar]
- de Reus, M. A., & van den Heuvel, M. P. (2014). Simulated rich club lesioning in brain networks: A scaffold for communication and integration? Frontiers in Human Neuroscience, 8, 647. 10.3389/fnhum.2014.00647, [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis, S., Assaf, Y., Jeurissen, B., Jones, D. K., & Roebroeck, A. (2016). T1 relaxometry of crossing fibres in the human brain. NeuroImage, 141, 133–142. 10.1016/j.neuroimage.2016.07.037, [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis, S., Drakesmith, M., Bells, S., Assaf, Y., & Jones, D. K. (2014). Why diffusion tensor MRI does well only some of the time: Variance and covariance of white matter tissue microstructure attributes in the living human brain. NeuroImage, 89, 35–44. 10.1016/j.neuroimage.2013.12.003, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligianni, F., Carmichael, D. W., Zhang, G. H., Clark, C. A., & Clayden, J. D. (2016). NODDI and tensor-based microstructural indices as predictors of functional connectivity. PLOS ONE, 11(4), e0153404. 10.1371/journal.pone.0153404, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhollander, T., Clemente, A., Singh, M., Boonstra, F., Civier, O., Duque, J. D., Egorova, N., Enticott, P., Fuelscher, I., Gajamange, S., Genc, S., Gottlieb, E., Hyde, C., Imms, P., Kelly, C., Kirkovski, M., Kolbe, S., Liang, X., Malhotra, A., … Caeyenberghs, K. (2021). Fixel-based analysis of diffusion MRI: Methods, applications, challenges and opportunities. NeuroImage, 241, 118417. 10.1016/j.neuroimage.2021.118417, [DOI] [PubMed] [Google Scholar]
- Dhollander, T., Mito, R., Raffelt, D., & Connelly, A. (2019). Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. Proceedings of the International Society of Magnetic Resonance in Medicine, 27, 555. https://www.researchgate.net/publication/331165168_Improved_white_matter_response_function_estimation_for_3-tissue_constrained_spherical_deconvolution [Google Scholar]
- Dhollander, T., Raffelt, D., & Connelly, A. (2016). Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. ISMRM Workshop on Breaking the Barriers of Diffusion MRI, 35, 1–2. https://www.researchgate.net/publication/307863133 [Google Scholar]
- Drakesmith, M., Harms, R., Rudrapatna, S. U., Parker, G. D., Evans, C. J., & Jones, D. K. (2019). Estimating axon conduction velocity in vivo from microstructural MRI. NeuroImage, 203, 116186. 10.1016/j.neuroimage.2019.116186, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, R. D. (2015). A new mechanism of nervous system plasticity: Activity-dependent myelination. Nature Reviews Neuroscience, 16(12), 756–767. 10.1038/nrn4023, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, E. S., Huber, L., Jangraw, D. C., Molfese, P. J., & Bandettini, P. A. (2019). Layer-dependent activity in human prefrontal cortex during working memory. Nature Neuroscience, 22(10), 1687–1695. 10.1038/s41593-019-0487-z, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B., Sereno, M. I., & Dale, A. M. (1999). Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. NeuroImage, 9(2), 195–207. 10.1006/nimg.1998.0396, [DOI] [PubMed] [Google Scholar]
- Fischl, B., Sereno, M. I., Tootell, R. B. H., & Dale, A. M. (1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8(4), 272–284. , [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito, A., Zalesky, A., & Breakspear, M. (2013). Graph analysis of the human connectome: Promise, progress, and pitfalls. NeuroImage, 80, 426–444. 10.1016/j.neuroimage.2013.04.087, [DOI] [PubMed] [Google Scholar]
- Fornito, A., Zalesky, A., & Breakspear, M. (2015). The connectomics of brain disorders. Nature Reviews Neuroscience, 16(3), 159–172. 10.1038/nrn3901, [DOI] [PubMed] [Google Scholar]
- Fornito, A., Zalesky, A., & Bullmore, E. T. (2016). Fundamentals of brain network analysis. Elsevier. 10.1016/C2012-0-06036-X [DOI] [Google Scholar]
- Frigo, M., Deslauriers-Gauthier, S., Parker, D., Ismail, A. A. O., Kim, J. J., Verma, R., & Deriche, R. (2020). Diffusion MRI tractography filtering techniques change the topology of structural connectomes. Journal of Neural Engineering, 17(6), 065002. 10.1088/1741-2552/abc29b, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity, 1(1), 13–36. 10.1089/brain.2011.0008, [DOI] [PubMed] [Google Scholar]
- Gibson, E. M., Geraghty, A. C., & Monje, M. (2018). Bad wrap: Myelin and myelin plasticity in health and disease. Developmental Neurobiology, 78(2), 123–135. 10.1002/dneu.22541, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard, G., Whittingstall, K., Deriche, R., & Descoteaux, M. (2014). Towards quantitative connectivity analysis: Reducing tractography biases. NeuroImage, 98, 266–278. 10.1016/j.neuroimage.2014.04.074, [DOI] [PubMed] [Google Scholar]
- Gordon, E. M., Laumann, T. O., Gilmore, A. W., Newbold, D. J., Greene, D. J., Berg, J. J., Ortega, M., Hoyt-Drazen, C., Gratton, C., Sun, H., Hampton, J. M., Coalson, R. S., Nguyen, A. L., McDermott, K. B., Shimony, J. S., Snyder, A. Z., Schlaggar, B. L., Petersen, S. E., Nelson, S. M., & Dosenbach, N. U. F. (2017). Precision functional mapping of individual human brains. Neuron, 95(4), 791–807. 10.1016/j.neuron.2017.07.011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, 100(1), 253–258. 10.1073/pnas.0135058100, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve, D. N., & Fischl, B. (2009). Accurate and robust brain image alignment using boundary-based registration. NeuroImage, 48(1), 63–72. 10.1016/j.neuroimage.2009.06.060, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffa, A., & van den Heuvel, M. P. (2018). Rich-club neurocircuitry: Function, evolution, and vulnerability. Dialogues in Clinical Neuroscience, 20(2), 121–132. 10.31887/DCNS.2018.20.2/agriffa, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson, M., Driesen, N. R., Skudlarski, P., Gore, J. C., & Constable, R. T. (2006). Brain connectivity related to working memory performance. Journal of Neuroscience, 26(51), 13338–13343. 10.1523/JNEUROSCI.3408-06.2006, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, J. J., & Attwell, D. (2012). The energetics of CNS white matter. Journal of Neuroscience, 32(1), 356–371. 10.1523/JNEUROSCI.3430-11.2012, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath, F., Hurley, S. A., Johansen-Berg, H., & Sampaio-Baptista, C. (2018). Advances in noninvasive myelin imaging. Developmental Neurobiology, 78(2), 136–151. 10.1002/dneu.22552, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand, C., & Hahn, R. (1978). Relation between myelin sheath thickness and axon size in spinal cord white matter of some vertebrate species. Journal of the Neurological Sciences, 38(3), 421–434. 10.1016/0022-510X(78)90147-8, [DOI] [PubMed] [Google Scholar]
- Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology, 117(4), 500–544. 10.1113/jphysiol.1952.sp004764, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey, C. J., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J. P., Meuli, R., & Hagmann, P. (2009). Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences, 106(6), 2035–2040. 10.1073/pnas.0811168106, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntenburg, J. M., Bazin, P. L., Goulas, A., Tardif, C. L., Villringer, A., & Margulies, D. S. (2017). A systematic relationship between functional connectivity and intracortical myelin in the human cerebral cortex. Cerebral Cortex, 27(2), 981–997. 10.1093/cercor/bhx030, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley, A. F., & Stämpfli, R. (1949). Evidence for saltatory conduction in peripheral myelinated nerve fibres. Journal of Physiology, 108(3), 315–339. 10.1113/jphysiol.1949.sp004335, [DOI] [PubMed] [Google Scholar]
- Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W., & Smith, S. M. (2012). FSL. NeuroImage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015, [DOI] [PubMed] [Google Scholar]
- Jeurissen, B., Descoteaux, M., Mori, S., & Leemans, A. (2019). Diffusion MRI fiber tractography of the brain. NMR in Biomedicine, 32(4), e3785. 10.1002/nbm.3785, [DOI] [PubMed] [Google Scholar]
- Jeurissen, B., Leemans, A., Tournier, J. D., Jones, D. K., & Sijbers, J. (2012). Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Human Brain Mapping, 34(11), 2747–2766. 10.1002/hbm.22099, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen, B., Tournier, J. D., Dhollander, T., Connelly, A., & Sijbers, J. (2014). Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage, 103, 411–426. 10.1016/j.neuroimage.2014.07.061, [DOI] [PubMed] [Google Scholar]
- Jones, D. K. (2010). Challenges and limitations of quantifying brain connectivity in vivo with diffusion MRI. Imaging in Medicine, 2(3), 341–355. 10.2217/iim.10.21 [DOI] [Google Scholar]
- Jones, D. K., Knösche, T. R., & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage, 73, 239–254. 10.1016/j.neuroimage.2012.06.081, [DOI] [PubMed] [Google Scholar]
- Kim, D.-J., & Min, B.-K. (2020). Rich-club in the brain’s macrostructure: Insights from graph theoretical analysis. Computational and Structural Biotechnology Journal, 18, 1761–1773. 10.1016/j.csbj.2020.06.039, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, P. J., Girard, G., Brügger, J., Cadic-Melchior, A. G., Beanato, E., Park, C.-H., Morishita, T., Wessel, M. J., Pizzolato, M., Canales-Rodríguez, E. J., Fischi-Gomez, E., Schiavi, S., Daducci, A., Piredda, G. F., Hilbert, T., Kober, T., Thiran, J.-P., & Hummel, F. C. (2022). Evaluating reproducibility and subject-specificity of microstructure-informed connectivity. NeuroImage, 258, 119356. 10.1016/j.neuroimage.2022.119356, [DOI] [PubMed] [Google Scholar]
- Larivière, S., Paquola, C., Park, B., Royer, J., Wang, Y., Benkarim, O., Vos de Wael, R., Valk, S. L., Thomopoulos, S. I., Kirschner, M., Lewis, L. B., Evans, A. C., Sisodiya, S. M., McDonald, C. R., Thompson, P. M., & Bernhardt, B. C. (2021). The ENIGMA toolbox: Multiscale neural contextualization of multisite neuroimaging datasets. Nature Methods, 18(7), 698–700. 10.1038/s41592-021-01186-4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, N., Aye, N., Kaufmann, J., Heinze, H. J., Düzel, E., Ziegler, G., & Taubert, M. (2021). Longitudinal reproducibility of neurite orientation dispersion and density imaging (NODDI) derived metrics in the white matter. Neuroscience, 457, 165–185. 10.1016/j.neuroscience.2021.01.005, [DOI] [PubMed] [Google Scholar]
- Leppert, I. R., Andrews, D. A., Campbell, J. S. W., Park, D. J., Pike, G. B., Polimeni, J. R., & Tardif, C. L. (2021). Efficient whole-brain tract-specific T1 mapping at 3T with slice-shuffled inversion-recovery diffusion-weighted imaging. Magnetic Resonance in Medicine, 86(2), 738–753. 10.1002/mrm.28734, [DOI] [PubMed] [Google Scholar]
- Leppert, I. R., Bontempi, P., Rowley, C. D., Campbell, J. S., Nelson, M. C., Schiavi, S., Pike, G. B., Daducci, A., & Tardif, C. L. (2023). Dual-encoded magnetization transfer and diffusion imaging and its application to tract-specific microstructure mapping. arXiv:2303.03449. 10.48550/arXiv.2303.03449 [DOI] [Google Scholar]
- Liégeois, R., Li, J., Kong, R., Orban, C., Van De Ville, D., Ge, T., Sabuncu, M. R., & Yeo, B. T. T. (2019). Resting brain dynamics at different timescales capture distinct aspects of human behavior. Nature Communications, 10(1), 2317. 10.1038/s41467-019-10317-7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. Q., Vázquez-Rodríguez, B., Spreng, R. N., Bernhardt, B. C., Betzel, R. F., & Misic, B. (2022). Time-resolved structure-function coupling in brain networks. Communications Biology, 5(1), 532. 10.1038/s42003-022-03466-x, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein, K. H., Neher, P. F., Houde, J.-C., Côté, M.-A., Garyfallidis, E., Zhong, J., Chamberland, M., Yeh, F.-C., Lin, Y.-C., Ji, Q., Reddick, W. E., Glass, J. O., Chen, D. Q., Feng, Y., Gao, C., Wu, Y., Ma, J., Renjie, H., Li, Q., … Descoteaux, M. (2017). The challenge of mapping the human connectome based on diffusion tractography. Nature Communications, 8(1), 1349. 10.1038/s41467-017-01285-x, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini, M., Giulietti, G., Dowell, N., Spanò, B., Harrison, N., Bozzali, M., & Cercignani, M. (2018). Introducing axonal myelination in connectomics: A preliminary analysis of g-ratio distribution in healthy subjects. NeuroImage, 182, 351–359. 10.1016/j.neuroimage.2017.09.018, [DOI] [PubMed] [Google Scholar]
- Mancini, M., Karakuzu, A., Cohen-Adad, J., Cercignani, M., Nichols, T. E., & Stikov, N. (2020). An interactive meta-analysis of MRI biomarkers of myelin. eLife, 9, e61523. 10.7554/eLife.61523, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies, D. S., Ghosh, S. S., Goulas, A., Falkiewicz, M., Huntenburg, J. M., Langs, G., Bezgin, G., Eickhoff, S. B., Castellanos, F. X., Petrides, M., Jefferies, E., & Smallwood, J. (2016). Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences, 113(44), 12574–12579. 10.1073/pnas.1608282113, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, J. P., Kober, T., Krueger, G., van der Zwaag, W., Van de Moortele, P. F., & Gruetter, R. (2010). MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage, 49(2), 1271–1281. 10.1016/j.neuroimage.2009.10.002, [DOI] [PubMed] [Google Scholar]
- Maslov, S., & Sneppen, K. (2002). Specificity and stability in topology of protein networks. Science, 296(5569), 910–913. 10.1126/science.1065103, [DOI] [PubMed] [Google Scholar]
- Messaritaki, E., Foley, S., Schiavi, S., Magazzini, L., Routley, B., Jones, D. K., & Singh, K. D. (2021). Predicting MEG resting-state functional connectivity from microstructural information. Network Neuroscience, 5(2), 477–504. 10.1162/netn_a_00187, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, S., Meschkat, M., Ruhwedel, T., Trevisiol, A., Tzvetanova, I. D., Battefeld, A., Kusch, K., Kole, M. H. P., Strenzke, N., Möbius, W., de Hoz, L., & Nave, K.-A. (2020). A role of oligodendrocytes in information processing. Nature Communications, 11(1), 5497. 10.1038/s41467-020-19152-7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottershead, J. P., Schmierer, K., Clemence, M., Thornton, J. S., Scaravilli, F., Barker, G. J., Tofts, P. S., Newcombe, J., Cuzner, M. L., Ordidge, R. J., McDonald, W. I., & Miller, D. H. (2003). High field MRI correlates of myelin content and axonal density in multiple sclerosis: A post-mortem study of the spinal cord. Journal of Neurology, 250(11), 1293–1301. 10.1007/s00415-003-0192-3, [DOI] [PubMed] [Google Scholar]
- Mount, C. W., & Monje, M. (2017). Wrapped to adapt: Experience-dependent myelination. Neuron, 95(4), 743–756. 10.1016/j.neuron.2017.07.009, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave, K.-A., & Werner, H. B. (2014). Myelination of the nervous system: Mechanisms and functions. Annual Review of Cell and Developmental Biology, 30, 503–533. 10.1146/annurev-cellbio-100913-013101, [DOI] [PubMed] [Google Scholar]
- Nelson, M. C. (2023). Weighted SC networks, GitHub. https://github.com/TardifLab/Weighted-SC-Networks [Google Scholar]
- Patenaude, B., Smith, S. M., Kennedy, D. N., & Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage, 56(3), 907–922. 10.1016/j.neuroimage.2011.02.046, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumphrey, R. J., & Young, J. Z. (1938). The rates of conduction of nerve fibres of various diameters in cephalopods. Journal of Experimental Biology, 15(4), 453–466. 10.1242/jeb.15.4.453 [DOI] [Google Scholar]
- Raffelt, D., Smith, R., Ridgway, G., Tournier, J. D., Vaughan, D., Rose, S., Henderson, R., & Connelly, A. (2015). Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. NeuroImage, 117, 40–55. 10.1016/j.neuroimage.2015.05.039, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffelt, D., Tournier, J. D., Smith, R., Vaughan, D., Jackson, G., Ridgway, G., & Connelly, A. (2017). Investigating white matter fibre density and morphology using fixel-based analysis. NeuroImage, 144, 58–73. 10.1016/j.neuroimage.2016.09.029, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer, J., Rodríguez-Cruces, R., Tavakol, S., Larivière, S., Herholz, P., Li, Q., Vos de Wael, R., Paquola, C., Benkarim, O., Park, B., Lowe, A. J., Margulies, D., Smallwood, J., Bernasconi, A., Bernasconi, N., Frauscher, B., & Bernhardt, B. C. (2022a). An open MRI dataset for multiscale neuroscience. Scientific Data, 9(1), 569. 10.1038/s41597-022-01682-y, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer, J., Rodríguez-Cruces, R., Tavakol, S., Larivière, S., Herholz, P., Li, Q., Vos de Wael, R., Paquola, C., Benkarim, O., Park, B., Lowe, A. J., Margulies, D., Smallwood, J., Bernasconi, A., Bernasconi, N., Frauscher, B., & Bernhardt, B. C. (2022b). MICA-MICs: A dataset for microstructure-informed connectomics [Data set]. https://portal.conp.ca/dataset?id=projects/mica-mics [Google Scholar]
- Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. 10.1016/j.neuroimage.2009.10.003, [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi, G., Douaud, G., Beckmann, C. F., Glasser, M. F., Griffanti, L., & Smith, S. M. (2014). Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. NeuroImage, 90, 449–468. 10.1016/j.neuroimage.2013.11.046, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, A., Kong, R., Gordon, E. M., Laumann, T. O., Zuo, X.-N., Holmes, A. J., Eickhoff, S. B., & Yeo, B. T. T. (2018). Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cerebral Cortex, 28(9), 3095–3114. 10.1093/cercor/bhx179, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavi, S., Lu, P., Weigel, M., Lutti, A., Jones, D. K., Kappos, L., Granziera, C., & Daducci, A. (2022). Bundle myelin fraction (BMF) mapping of different white matter connections using microstructure informed tractography. NeuroImage, 249, 118922. 10.1016/j.neuroimage.2022.118922, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavi, S., Petracca, M., Battocchio, M., El Mendili, M. M., Paduri, S., Fleysher, L., Inglese, M., & Daducci, A. (2020). Sensory-motor network topology in multiple sclerosis: Structural connectivity analysis accounting for intrinsic density discrepancy. Human Brain Mapping, 41(11), 2951–2963. 10.1002/hbm.24989, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling, K. G., Daducci, A., Maier-Hein, K., Poupon, C., Houde, J. C., Nath, V., Anderson, A. W., Landman, B. A., & Descoteaux, M. (2019). Challenges in diffusion MRI tractography—Lessons learned from international benchmark competitions. Magnetic Resonance Imaging, 57, 194–209. 10.1016/j.mri.2018.11.014, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, R. E., Tournier, J. D., Calamante, F., & Connelly, A. (2012). Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage, 62(3), 1924–1938. 10.1016/j.neuroimage.2012.06.005, [DOI] [PubMed] [Google Scholar]
- Smith, R. E., Tournier, J. D., Calamante, F., & Connelly, A. (2015). SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. NeuroImage, 119, 338–351. 10.1016/j.neuroimage.2015.06.092, [DOI] [PubMed] [Google Scholar]
- Smith, S. M., Fox, P. T., Miller, K. L., Glahn, D. C., Fox, P. M., Mackay, C. E., Filippini, N., Watkins, K. E., Toro, R., Laird, A. R., & Beckmann, C. F. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31), 13040–13045. 10.1073/pnas.0905267106, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E. J., Johansen-Berg, H., Bannister, P. R., De Luca, M., Drobnjak, I., Flitney, D. E., Niazy, R. K., Saunders, J., Vickers, J., Zhang, Y., De Stefano, N., Brady, J. M., & Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl. 1), 208–219. 10.1016/j.neuroimage.2004.07.051, [DOI] [PubMed] [Google Scholar]
- Sorrentino, P., Petkoski, S., Sparaco, M., Troisi Lopez, E., Signoriello, E., Baselice, F., Bonavita, S., Pirozzi, M. A., Quarantelli, M., Sorrentino, G., & Jirsa, V. (2022). Whole-brain propagation delays in multiple sclerosis, a combined tractography-magnetoencephalography study. Journal of Neuroscience, 42(47), 8807–8816. 10.1523/JNEUROSCI.0938-22.2022, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos, S. N., & Zalesky, A. (2019). Building connectomes using diffusion MRI: Why, how and but. NMR in Biomedicine, 32(4), e3752. 10.1002/nbm.3752, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns, O. (2010). Networks of the brain. MIT Press. 10.7551/mitpress/8476.001.0001 [DOI] [Google Scholar]
- Sporns, O. (2011). The human connectome: A complex network. Annals of the New York Academy of Sciences, 1224(1), 109–125. 10.1111/j.1749-6632.2010.05888.x, [DOI] [PubMed] [Google Scholar]
- Sporns, O. (2018). Graph theory methods: Applications in brain networks. Dialogues in Clinical Neuroscience, 20(2), 111–120. 10.31887/DCNS.2018.20.2/osporns, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stikov, N., Campbell, J. S. W., Stroh, T., Lavelée, M., Frey, S., Novek, J., Nuara, S., Ho, M.-K., Bedell, B. J., Dougherty, R. F., Leppert, I. R., Boudreau, M., Narayanan, S., Duval, T., Cohen-Adad, J., Picard, P.-A., Gasecka, A., Côté, D., & Pike, G. B. (2015). In vivo histology of the myelin g-ratio with magnetic resonance imaging. NeuroImage, 118, 397–405. 10.1016/j.neuroimage.2015.05.023, [DOI] [PubMed] [Google Scholar]
- Stikov, N., Perry, L. M., Mezer, A., Rykhlevskaia, E., Wandell, B. A., Pauly, J. M., & Dougherty, R. F. (2011). Bound pool fractions complement diffusion measures to describe white matter micro and macrostructure. NeuroImage, 54(2), 1112–1121. 10.1016/j.neuroimage.2010.08.068, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez, L. E., Markello, R. D., Betzel, R. F., & Misic, B. (2020). Linking structure and function in macroscale brain networks. Trends in Cognitive Sciences, 24(4), 302–315. 10.1016/j.tics.2020.01.008, [DOI] [PubMed] [Google Scholar]
- Tournier, J.-D., Calamante, F., & Connelly, A. (2010). Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. ISMRM, 88(2003), 2010. https://cds.ismrm.org/protected/10MProceedings/PDFfiles/1670_4298.pdf [Google Scholar]
- Tournier, J. D., Calamante, F., & Connelly, A. (2012). MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology, 22(1), 53–66. 10.1002/ima.22005 [DOI] [Google Scholar]
- Tournier, J. D., Mori, S., & Leemans, A. (2011). Diffusion tensor imaging and beyond. Magnetic Resonance in Medicine, 65(6), 1532–1556. 10.1002/mrm.22924, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier, J. D., Smith, R., Raffelt, D., Tabbara, R., Dhollander, T., Pietsch, M., Christiaens, D., Jeurissen, B., Yeh, C.-H., & Connelly, A. (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 202, 116137. 10.1016/j.neuroimage.2019.116137, [DOI] [PubMed] [Google Scholar]
- Tustison, N. J., Avants, B. B., Cook, P. A., Zheng, Y., Egan, A., Yushkevich, P. A., & Gee, J. C. (2010). N4ITK: Improved N3 bias correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. 10.1109/TMI.2010.2046908, [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P., Kahn, R. S., Goñi, J., & Sporns, O. (2012). High-cost, high-capacity backbone for global brain communication. Proceedings of the National Academy of Sciences, 109(28), 11372–11377. 10.1073/pnas.1203593109, [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P., Mandl, R. C. W., Stam, C. J., Kahn, R. S., & Hulshoff Pol, H. E. (2010). Aberrant frontal and temporal complex network structure in schizophrenia: A graph theoretical analysis. Journal of Neuroscience, 30(47), 15915–15926. 10.1523/JNEUROSCI.2874-10.2010, [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P., & Sporns, O. (2011). Rich-club organization of the human connectome. Journal of Neuroscience, 31(44), 15775–15786. 10.1523/JNEUROSCI.3539-11.2011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P., & Sporns, O. (2013). An anatomical substrate for integration among functional networks in human cortex. Journal of Neuroscience, 33(36), 14489–14500. 10.1523/JNEUROSCI.2128-13.2013, [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P., Stam, C. J., Kahn, R. S., & Hulshoff Pol, H. E. (2009). Efficiency of functional brain networks and intellectual performance. Journal of Neuroscience, 29(23), 7619–7624. 10.1523/JNEUROSCI.1443-09.2009, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart, J., Fieremans, E., & Novikov, D. S. (2016). Diffusion MRI noise mapping using random matrix theory. Magnetic Resonance in Medicine, 76(5), 1582–1593. 10.1002/mrm.26059, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart, J., Novikov, D. S., Christiaens, D., Ades-Aron, B., Sijbers, J., & Fieremans, E. (2016). Denoising of diffusion MRI using random matrix theory. NeuroImage, 142, 394–406. 10.1016/j.neuroimage.2016.08.016, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos de Wael, R., Benkarim, O., Paquola, C., Lariviere, S., Royer, J., Tavakol, S., Xu, T., Hong, S.-J., Langs, G., Valk, S., Misic, B., Milham, M., Margulies, D. S., Smallwood, J., & Bernhardt, B. C. (2020). BrainSpace: A toolbox for the analysis of macroscale gradients in neuroimaging and connectomics datasets. Communications Biology, 3(1), 103. 10.1038/s42003-020-0794-7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P., Kong, R., Kong, X., Liégeois, R., Orban, C., Deco, G., van den Heuvel, M. P., & Yeo, B. T. T. (2019). Inversion of a large-scale circuit model reveals a cortical hierarchy in the dynamic resting human brain. Science Advances, 5(1), eaat7854. 10.1126/sciadv.aat7854, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, M., Wang, J., & He, Y. (2013). BrainNet Viewer: A network visualization tool for human brain connectomics. PLOS ONE, 8(7), e68910. 10.1371/journal.pone.0068910, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, C. H., Smith, R. E., Liang, X., Calamante, F., & Connelly, A. (2016). Correction for diffusion MRI fibre tracking biases: The consequences for structural connectomic metrics. NeuroImage, 142, 150–162. 10.1016/j.neuroimage.2016.05.047, [DOI] [PubMed] [Google Scholar]
- Yeh, F. C., Badre, D., & Verstynen, T. (2016). Connectometry: A statistical approach harnessing the analytical potential of the local connectome. NeuroImage, 125, 162–171. 10.1016/j.neuroimage.2015.10.053, [DOI] [PubMed] [Google Scholar]
- Yeo, B. T. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., Roffman, J. L., Smoller, J. W., Zöllei, L., Polimeni, J. R., Fisch, B., Liu, H., & Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. 10.1152/jn.00338.2011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky, A., Fornito, A., Cocchi, L., Gollo, L. L., van den Heuvel, M. P., & Breakspear, M. (2016). Connectome sensitivity or specificity: Which is more important? NeuroImage, 142, 407–420. 10.1016/j.neuroimage.2016.06.035, [DOI] [PubMed] [Google Scholar]
- Zhang, H., Schneider, T., Wheeler-Kingshott, C. A., & Alexander, D. C. (2012). NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage, 61(4), 1000–1016. 10.1016/j.neuroimage.2012.03.072, [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Brady, M., & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. 10.1109/42.906424, [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
These data are available for download (https://portal.conp.ca/dataset?id=projects/mica-mics; Royer et al., 2022b). See Royer et al. (2022a) and Cruces et al. (2022) for full details of data acquisition and processing. All data processing and analysis code is openly available at https://github.com/TardifLab/Weighted-SC-Networks (Nelson, 2023).