Abstract
BACKGROUND
The glycemic control of automated insulin delivery (AID) systems in outpatient children and adolescents with type 1 diabetes (T1D) has not been systematically evaluated.
PURPOSE
To evaluate the efficacy and safety of AID systems in children and adolescents in outpatient settings.
DATA SOURCES
PubMed, Embase, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov were searched until 4 May 2023. This study was registered with PROSPERO (2023, CRD42023395252).
STUDY SELECTION
Randomized controlled trials that compared AID systems with conventional insulin therapy in outpatient children and adolescents with T1D and reported continuous glucose monitoring outcomes were selected.
DATA EXTRACTION
Percent time in range (TIR) (3.9–10 mmol/L), time below range (TBR) (<3.9 mmol/L), and time above range (TAR) (>10 mmol/L) were extracted. Data were summarized as mean differences (MDs) with 95% CIs.
DATA SYNTHESIS
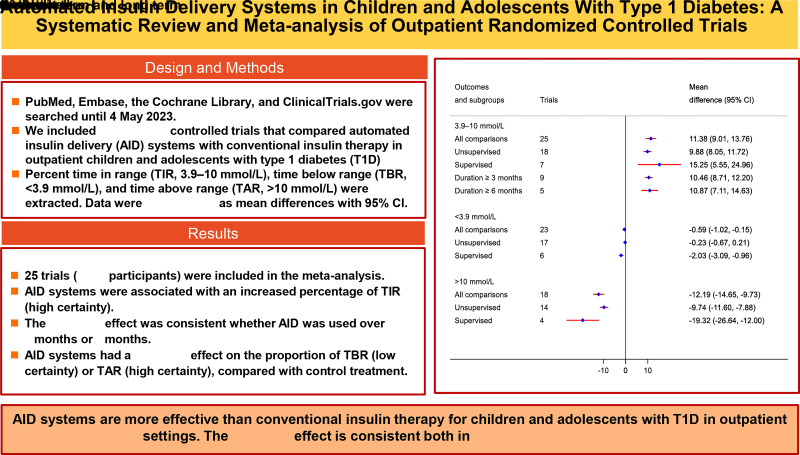
Twenty-five trials (1,345 participants) were included in the meta-analysis. AID systems were associated with an increased percentage of TIR (MD, 11.38% [95% CI 9.01–13.76], P < 0.001; high certainty). The favorable effect was consistent whether AID was used over 3 months (10.46% [8.71–12.20]) or 6 months (10.87% [7.11–14.63]). AID systems had a favorable effect on the proportion of TBR (−0.59% [−1.02 to −0.15], P = 0.008; low certainty) or TAR (−12.19% [−14.65 to −9.73], P < 0.001; high certainty) compared with control treatment.
LIMITATIONS
Substantial heterogeneity was observed in most analyses.
CONCLUSIONS
AID systems are more effective than conventional insulin therapy for children and adolescents with T1D in outpatient settings. The favorable effect is consistent both in the short term and long term.
Graphical Abstract
Introduction
Type 1 diabetes (T1D) is a chronic autoimmune disease characterized by insulin deficiency and resultant hyperglycemia (1). According to the International Diabetes Federation Diabetes Atlas, an estimated 1.21 million children and adolescents aged <20 years have T1D, with 149,500 new cases diagnosed annually worldwide in 2021 (2). The American Diabetes Association now recommends that glycated hemoglobin (HbA1c) level be kept at <7% (3), which corresponds to a time in range (TIR) >65–75% as measured by continuous glucose monitoring (4,5). Despite increased use of insulin pump therapy and continuous glucose monitoring, <10% of children and adolescents aged ≤17 years in the U.S. reach the international target (6). T1D in children often leads to a high management burden and reduced quality of life for the whole family (7).
Automated insulin delivery (AID), a novel technology, is a hybrid closed-loop system that automatically administers an insulin dose based on glucose sensor readings and a dosing algorithm (8,9). Three previous meta-analyses have concluded that the AID systems could improve glucose control compared with conventional insulin pump therapy in outpatients with T1D, but most of the included trials recruited adults or mixed populations (10–12). Another meta-analysis showed that AID systems were superior to the standard sensor-augmented pump treatment of T1D in children and adolescents, but most of the trials included were in inpatient settings, and some were not randomized (13). Finally, a recent meta-analysis summarized evidence from published trials AID systems in children and adolescents with T1D (14). In recent years, some important randomized controlled trials (RCTs) assessing AID systems in young people were also published. Therefore, we performed a meta-analysis of AID systems for glycemic control in children and adolescents with T1D compared with conventional insulin therapy in outpatient settings.
Research Design and Methods
Data Sources and Searches
We conducted this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (15). The protocol was registered with the International prospective register of systematic reviews (PROSPERO) (2023, CRD42023395252 [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=395252]). We searched for literature published in PubMed, Embase, and the Cochrane Central Register of Controlled Trials and gray literature from ClinicalTrials.gov until 4 May 2023. Keywords included “type 1 diabetes,” “artificial pancreas,” “closed loop,” “children,” and “adolescent.” The detailed search strategy is provided in the Supplementary Material. Our search was restricted to articles published in English. Additionally, we identified references by searching the reference lists of included studies and relevant reviews.
Study Selection
We included RCTs in children and adolescents aged ≤25 years with T1D seen in the outpatient setting, irrespective of trial design (parallel or crossover) or timing of intervention (24 h or overnight), which compared AID systems with conventional insulin therapy. The outpatient setting was defined as the participant’s home, hotel, diabetes camp, or research house. The conventional insulin therapy included multiple daily insulin injection (MDI), continuous subcutaneous insulin infusion (CSII), and sensor-augmented pump (SAP) with or without low glucose suspend. We excluded studies with very short intervention periods (<3 days). Of note, only studies reporting continuous glucose monitoring outcomes were included.
The primary outcome was the percentage of TIR, i.e., percentage of the total duration of the intervention that blood glucose was within target range (3.9–10 mmol/L). Secondary outcomes included time below range (TBR) (<3.9 mmol/L), TBR (<3.0 mmol/L), time above range (TAR) (>10 mmol/L), TAR (>13.9 mmol/L), severe hypoglycemic event (as defined in each individual study), and diabetic ketoacidosis.
Data Extraction and Quality Assessment
Two authors reviewed the titles and abstracts independently to identify eligible studies that met prespecified inclusion criteria and extracted the data. When consensus was lacking, a third reviewer was consulted. Study characteristics (e.g., year of publication, study design, sample size), intervention and comparator characteristics, patient characteristics (e.g., age, sex, diabetes duration, baseline HbA1c), and outcomes were extracted. The risk of bias of RCTs was assessed using the Cochrane Collaboration’s tool (16). If the study reported both 24-h and overnight results, we extracted both results. The quality of evidence for each outcome was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework (17).
Data Synthesis and Analysis
We conducted DerSimonian and Laird random-effects meta-analysis when data were available for at least two studies (18). TIR, TBR, and TAR were analyzed as mean differences (MDs) with 95% CIs. We only performed narrative descriptive synthesis for severe hypoglycemic events and diabetic ketoacidosis. Medians were assumed to equal means, and SD was calculated as the interquartile range/1.35, which was recommended by the Cochrane Collaboration (16). We combined data from both parallel and crossover trials. If crossover trials did not report the mean and SE of the paired differences, we planned a priori to analyze all studies using group means and SDs, assuming a correlation coefficient of 0.5. For studies comparing both dual-hormone and single-hormone AIDs with conventional insulin therapy in a three-way crossover design, we included two comparisons (dual-hormone vs. control and single-hormone vs. control) in the meta-analysis. Statistical heterogeneity among the studies was assessed using χ2 test and I2 statistics. I2 values of 25%, 50%, and 75% have been suggested to be indicators of low, moderate, and high heterogeneity, respectively (19). Publication bias was assessed using a funnel plot for the primary outcome and Egger test (20,21), where P < 0.05 indicates the presence of publication bias.
For the primary outcome and two secondary outcomes (TBR [<3.9 mmol/L] and TAR [>10 mmol/L]), we conducted a prespecified subgroup analysis based on timing of the intervention (24 h vs. overnight) and mean age (<14 vs. ≥14 years) and conducted a post hoc subgroup analysis based on setting (unsupervised home vs. supervised diabetes camp and hotel), AID system (single vs. dual hormone), study design (parallel vs. crossover), study duration (<1 month vs. >1 month), and comparator (SAP vs. CSII/MDI). The P value for the difference was calculated using random-effects meta-regression, and a difference between the estimates of these subgroups was considered significant if Pinteraction < 0.10 (22). All the analysis were performed with Stata 17 statistical software.
Data and Resource Availability
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Results
Characteristics of Included Studies
The systematic literature search initially identified 1,038 records. After excluding duplicates and irrelevant articles, 90 articles were evaluated in full text for eligibility (Supplementary Fig. 1). Finally, 25 RCTs (1,345 participants) were included in the present meta-analysis (full reference citations are available in the Supplementary Material). Ten trials were parallel, and the remaining 15 trials were crossover. All the studies were published except one, which was gray literature from ClinicalTrials.gov. In 22 trials, a single-hormone AID system was assessed, mostly compared with SAP. Two trials assessed a dual-hormone AID system by comparing it with CSII. The remaining study was a three-way crossover trial, which assessed dual-hormone AID, single-hormone AID, and CSII.
In two studies assessing AID compared with SAP, the control treatment comprised SAP combined with low glucose suspend. Among trials evaluating single-hormone AID systems, four used the Diabetes Assistant platform, four used the Florence configuration, four used the Control-IQ system, two used the MiniMed 670G, two used Florence M or CamAPS FX configuration, and the remainder used other systems, mixed systems, or did not provide relevant details. In terms of setting, six trials were conducted in a diabetes camp, one was conducted in a hotel, and 18 were conducted in participants’ home. At baseline, participant mean age ranged from 3.9 to 17 years, and HbA1c ranged from 7.3 to 10.6%. Characteristics of individual studies are summarized in Supplementary Table 1. Most examined items were assessed as low or unclear risk except blinding. Because of the nature of the intervention, blinding for participants and personnel seemed to be impracticable. The detailed risk-of-bias assessment is available in Supplementary Table 2. The quality of evidence for each outcome was rated following the GRADE framework (Supplementary Table 3).
Primary Outcome
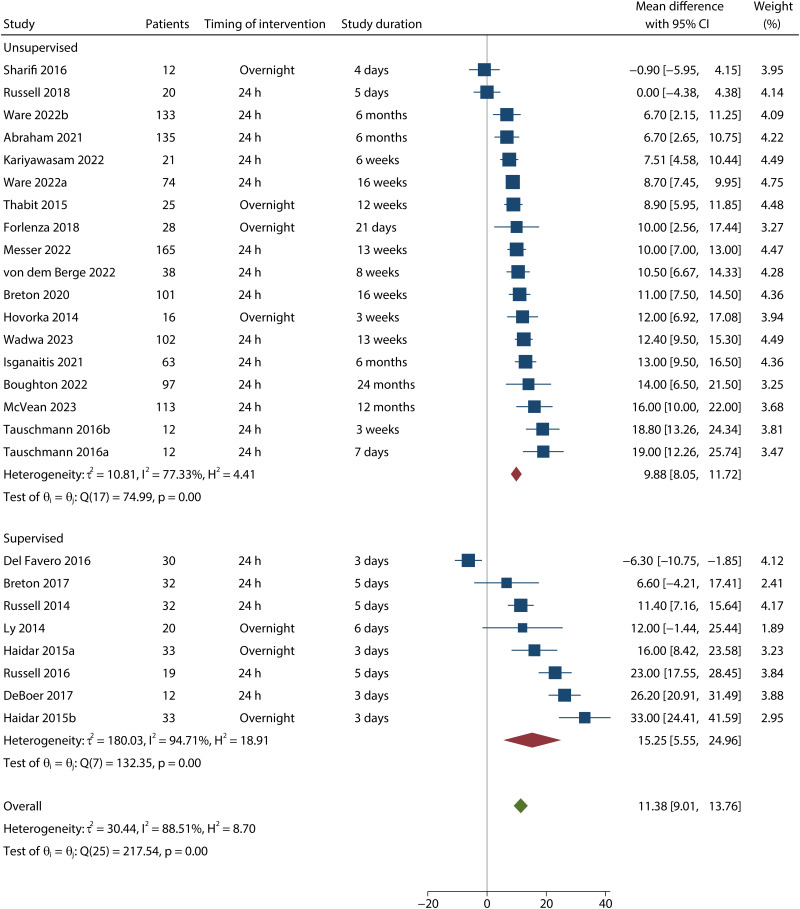
All meta-analysis results are presented as pooled effect estimates for AID systems versus conventional insulin therapy. Twenty-six comparisons from 25 studies with 1,345 participants were pooled for the primary outcome of TIR. Compared with conventional insulin therapy, use of AID was associated with an increased percentage of time (164 additional minutes) in target range (MD 11.38% [95% CI 9.01–13.76], P < 0.001; high certainty) (Fig. 1). There was high statistical heterogeneity (I2 = 89%). Effect sizes of individual studies ranged from an MD of −6.30 to 33.00%.
Figure 1.
Forest plot for TIR (3.9–10 mmol/L) by study included in the meta-analysis. Full reference citations are available in the Supplementary Material.
The primary outcomes stratified by type of AID system, timing of intervention, study design, mean age, study duration, and comparator are shown in Table 1. Of note, use of AID had a significant favorable effect on TIR in all subgroups. The favorable effect was consistent when AID was used over 3 months (nine trials, MD 10.45% [95% CI 8.71–12.20]) or 6 months (five trials, MD 10.87% [7.11–14.63]). AID systems seem to have a greater improvement in TIR in supervised settings (seven trials, MD 15.25% [5.55–24.96]) compared with unsupervised settings (18 trials, MD 9.88% [8.05–11.72]), but the test for interaction was not significant (Pinteraction = 0.156).
Table 1.
Subgroup analyses for primary and secondary outcomes
| Subgroup | Comparisons, n | RD (95% CI) | P | I2, % | P interaction |
|---|---|---|---|---|---|
| TIR (3.9–10.0 mmol/L) | |||||
| All comparisons | 26 | 11.38 (9.01–13.76) | <0.001 | 89 | |
| Setting | |||||
| Unsupervised | 18 | 9.88 (8.05–11.72) | <0.001 | 77 | 0.156 |
| Supervised | 8 | 15.25 (5.55–24.96) | 0.002 | 95 | |
| Study design | |||||
| Parallel | 10 | 10.74 (8.97–12.51) | <0.001 | 37 | 0.370 |
| Crossover | 16 | 12.01 (8.32–15.70) | <0.001 | 93 | |
| Hormone | |||||
| Dual | 3 | 22.00 (10.30–33.69) | <0.001 | 92 | 0.048 |
| Single | 23 | 10.12 (7.83–12.42) | <0.001 | 87 | |
| Timing of intervention* | |||||
| 24 h | 19 | 11.14 (8.49–13.78) | <0.001 | 89 | 0.793 |
| Overnight | 18 | 14.79 (10.96–18.62) | <0.001 | 87 | |
| Study duration, months | |||||
| <1 | 14 | 12.81 (6.71–18.90) | <0.001 | 93 | 0.235 |
| ≥1 | 12 | 9.95 (8.63–11.28) | <0.001 | 49 | |
| Mean age, years | |||||
| <14 | 18 | 11.45 (8.53–14.37) | <0.001 | 90 | 0.655 |
| ≥14 | 8 | 11.29 (6.94–15.63) | <0.001 | 83 | |
| Comparator | |||||
| SAP | 8 | 10.32 (7.20–13.43) | <0.001 | 89 | 0.569 |
| CSII or MDI | 15 | 13.38 (7.11–19.65) | <0.001 | 91 | |
| Mixed | 3 | 12.01 (9.27–14.76) | <0.001 | 42 | |
| TBR (<3.9 mmol/L) | |||||
| All comparisons | 24 | −0.59 (−1.02 to −0.15) | 0.008 | 86 | |
| Setting | |||||
| Unsupervised | 17 | −0.23 (−0.67 to 0.21) | 0.308 | 87 | 0.004 |
| Supervised | 7 | −2.03 (−3.09 to −0.96) | <0.001 | 52 | |
| Study design | |||||
| Parallel | 10 | −0.45 (−0.86 to −0.03) | 0.034 | 77 | 0.400 |
| Crossover | 14 | −0.87 (−1.64 to −0.11) | 0.025 | 85 | |
| Hormone | |||||
| Dual | 3 | −1.98 (−2.94 to −1.02) | <0.001 | 0 | 0.063 |
| Single | 21 | −0.42 (−0.86 to 0.03) | 0.064 | 86 | |
| Timing of intervention* | |||||
| 24 h | 18 | −0.63 (−1.05 to −0.21) | 0.003 | 79 | 0.913 |
| Overnight | 13 | −0.65 (−1.46 to 0.17) | 0.121 | 86 | |
| Study duration, months | |||||
| <1 | 13 | −1.04 (−1.87 to −0.20) | 0.015 | 68 | 0.186 |
| ≥1 | 11 | −0.28 (−0.81 to 0.24) | 0.294 | 91 | |
| Mean age, years | |||||
| <14 | 17 | −0.46 (−0.93 to 0.01) | 0.054 | 84 | 0.769 |
| ≥14 | 7 | −0.79 (−1.61 to 0.03) | 0.060 | 75 | |
| Comparator | |||||
| SAP | 13 | −0.52 (−1.13 to 0.10) | 0.098 | 88 | 0.227 |
| CSII or MDI | 8 | −1.12 (−2.02 to −0.22) | 0.014 | 58 | |
| Mixed | 3 | −0.59 (−1.02 to −0.15) | 0.334 | 30 | |
| TAR (>10 mmol/L) | |||||
| All comparisons | 19 | −12.19 (−14.65 to −9.73) | <0.001 | 83 | |
| Setting | |||||
| Unsupervised | 14 | −9.74 (−11.60 to −7.88) | <0.001 | 64 | 0.005 |
| Supervised | 5 | −19.32 (−26.64 to −12.00) | <0.001 | 85 | |
| Study design | |||||
| Parallel | 7 | −9.41 (−11.88 to −6.95) | <0.001 | 47 | 0.153 |
| Crossover | 12 | −14.04 (−17.78 to −10.30) | <0.001 | 88 | |
| Hormone | |||||
| Dual | 3 | −18.31 (−28.24 to −8.38) | <0.001 | 87 | 0.121 |
| Single | 16 | −11.21 (−13.68 to −8.73) | <0.001 | 81 | |
| Timing of intervention* | |||||
| 24 h | 14 | −12.03 (−14.76 to −9.30) | <0.001 | 85 | 0.098 |
| Overnight | 10 | −17.34 (−22.90 to −11.78) | 0.002 | 83 | |
| Study duration, months | |||||
| <1 | 9 | −17.14 (−21.89 to −12.38) | <0.001 | 76 | 0.002 |
| ≥1 | 10 | −8.67 (−10.24 to −7.11) | <0.001 | 48 | |
| Mean age, years | |||||
| <14 | 13 | −12.46 (−15.50 to −9.43) | <0.001 | 86 | 0.765 |
| ≥14 | 6 | −11.64 (−16.29 to −6.99) | <0.001 | 75 | |
| Comparator | |||||
| SAP | 11 | −11.97 (−15.21 to −8.73) | <0.001 | 85 | 0.704 |
| CSII or MDI | 6 | −13.22 (−19.51 to −6.93) | <0.001 | 85 | |
| Mixed | 2 | −11.88 (−18.63 to −5.13) | 0.001 | 73 |
RD, risk difference.
If the study reported both 24-h and overnight results, both results were extracted.
We repeated the meta-analysis for the primary outcome using only one comparison from the study that used a three-way crossover design, and the result was almost unchanged. Additionally, we performed a post hoc sensitivity analysis by excluding studies that enrolled participants aged >18 years, and the primary outcome was also consistent (20 trials, MD 11.56% [95% CI 8.85–14.28]). There was no significant publication bias among the studies based on Egger test that compared AID with conventional insulin therapy (P = 0.201), and the funnel plot did not show evidence of publication bias visually (Supplementary Fig. 2).
Secondary Outcomes
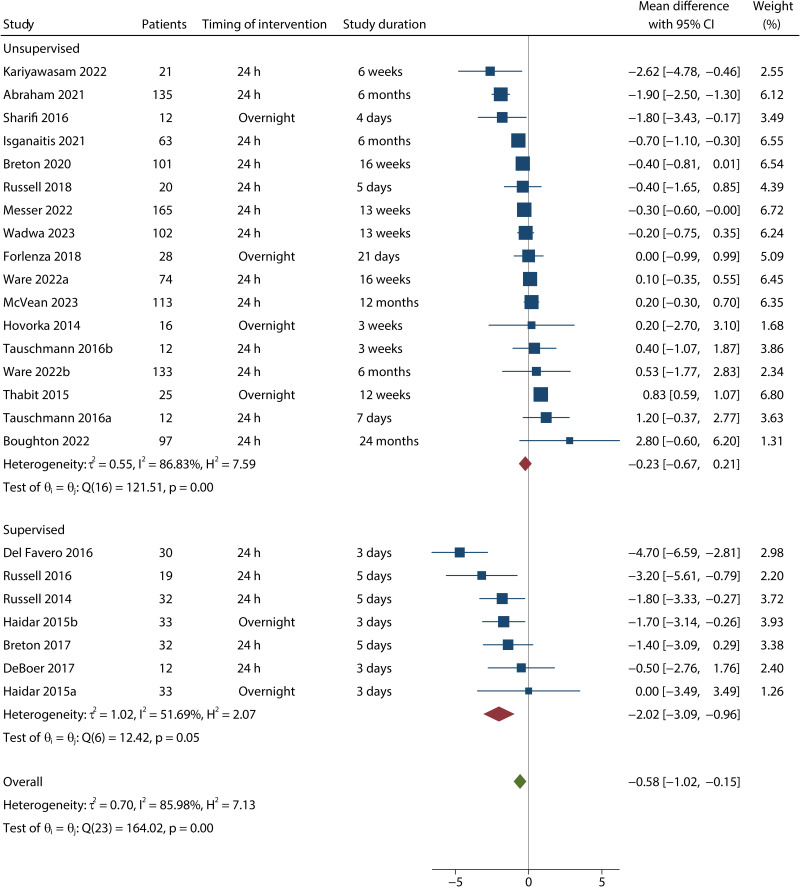
Twenty-four comparisons with 1,287 participants were pooled for TBR (<3.9 mmol/L). TBR (<3.9 mmol/L) was 0.59% (95% CI −0.59 to −0.15, P = 0.008; low certainty) lower for AID systems compared with conventional insulin therapy (Fig. 2). AID systems showed improvement in TBR (<3.9 mmol/L) in supervised settings (six trials, MD −2.03% [−3.09 to −0.96], P < 0.001) but not in unsupervised settings (17 trials, MD −0.23% [−0.67 to 0.21], P = 0.308), and the subgroup difference was significant (Pinteraction = 0.004). The favorable effect of AID use for TBR (<3.9 mmol/L) over conventional insulin therapy was significant in dual-hormone, short-term, and 24-h subgroups, but not in single-hormone, long-term, and overnight subgroups. Twenty-one comparisons with 1,051 participants were pooled for TBR (<3.0 mmol/L). There was no difference in TBR (<3.0 mmol/L) between AID and conventional insulin therapy (MD −0.09% [−0.19 to 0.01], P = 0.088, I2 = 82%; low certainty).
Figure 2.
Forest plot for TBR (<3.9 mmol/L) by study included in the meta-analysis. Full reference citations are available in the Supplementary Material.
Nineteen comparisons with 1,032 participants were pooled for TAR (>10 mmol/L). TAR (>10 mmol/L) was 12.19% (95% CI −14.65 to −9.73, P < 0.001; high certainty) shorter by AID use compared with conventional insulin therapy. Use of AID had a significant favorable effect on TAR (>10 mmol/L) in all subgroups. AID systems had a greater improvement in TAR (>10 mmol/L) in supervised settings (four trials, MD −19.32% [−26.64 to −12.00], P < 0.001) compared with unsupervised settings (14 trials, MD −9.74% [−11.60 to −7.88], P < 0.001) (Pinteraction = 0.005). Long-term study duration was associated with lower improvement in TAR (>10 mmol/L) compared with short-term (Pinteraction = 0.002). Twelve comparisons with 824 participants were pooled for TAR (>13.9 mmol/L), and AID showed favorable effects compared with conventional insulin therapy (MD −4.14% [−6.29 to −1.99], P < 0.001, I2 = 92%; low certainty).
Most trials reported no serious adverse effects. Episodes of severe hypoglycemia were mentioned in six studies (23–28), and there was no distinct difference between AID systems and conventional insulin therapy. Only four diabetic ketoacidosis events were reported in three trials (23,28,29), all of which occurred in the AID groups.
Discussion
This meta-analysis included 25 randomized crossover trials involving 1,345 patients and provided an overview of glycemic control of AID systems compared with conventional insulin therapy in outpatient settings in children and adolescents with T1D. The use of AID systems resulted in an 11.38% (95% CI 9.01–13.76) increased TIR, equivalent to 164 min/day. This finding was also verified by its effect on TAR (176 min less than control treatment) and TBR (8 min less). The primary outcome was robust and consistent in all sensitivity analyses performed. This favorable effect was observed consistently when the AID was used continuously over 3 and 6 months, which was not analyzed in previous studies. Most trials reported no serious adverse events, such as severe hypoglycemia and diabetic ketoacidosis.
This study also suggested greater improvement in TIR for dual-hormone compared with single-hormone AID systems. The characteristics of different AID systems and conventional insulin treatments have been reviewed comprehensively in three reviews (9,30,31). A dual-hormone system has been shown to be superior to a single-hormone system in improving nocturnal glucose control in children and adolescents with T1D in one three-way crossover trial (32). However, dual-hormone systems have only been tested for 3–5 days with very close supervision (diabetes camp) in a small number of children. Future, free-range studies are required in which dual-hormone and single-hormone AID systems are compared directly with each other in children and adolescents.
AID systems reduced TBR only by 0.59% compared with conventional insulin therapy (P = 0.008); however, significant heterogeneity was present across trials. The subgroup analysis showed that the reduction was significant in dual-hormone systems and supervised settings but not in single-hormone systems and unsupervised settings. This finding contrasts with previous meta-analyses of AID use that did not have age limitations (10,12) but is consistent with a recent meta-analysis focused on young people (14). More trials of dual-hormone AID in young people are needed.
Despite heterogeneity in interventions and comparators used, our systematic review provides a valid and up-to-date overview about the use of AID in children and adolescents. Previous meta-analyses of AID in all age-groups showed favorable effects both overnight and over a 24-h period, but the longest follow-up was 12 weeks (10,12). The favorable effect was also evident in a subgroup analysis for the pediatric population (12), which was consistent with our meta-analysis. However, our study included more trials, performed comprehensive assessment, and had greater reliability. Furthermore, Karageorgiou et al. (13) analyzed the effectiveness of AID in the nonadult population (aged <18 years), and the results suggested that AID systems are superior to standard SAP treatment for T1D. However, only 5 of 19 included studies were in outpatient settings, and 4 trials were not randomized. The effect of AID systems in children and adolescents was examined in a recent systematic review and meta-analysis of 26 RCTs (915 participants) (14). However, one-half of the trials included had a follow-up duration of <5 days, and the validity and clinical interpretation were undermined by methodological decisions regarding the analysis of crossover trials, date extraction of overnight glucose control, and inadequate subgroup analysis (14).
Initially, we used a correlation coefficient of 0.5 to calculate the mean and SE of the paired differences if there was no reporting in crossover trials. A post hoc validation for the correlation coefficient was done using a method recommended by the Cochrane Collaboration (33), which yielded a value of 0.8 for TIR in the largest crossover trial (27). Therefore, the result of our meta-analysis was more conservative.
Our study has several strengths. The meta-analysis followed the PRISMA guidelines and a protocol that was registered with PROSPERO. We conducted a comprehensive search of multiple databases and included all available RCTs of AID compared with conventional insulin therapy. Risk of bias for included trials was assessed using a valid methodological tool, and quality of evidence for each outcome was evaluated using GRADE. Subgroup analyses were performed to explain heterogeneity, and sensitivity analyses were conducted to examine the robustness of the results.
We also acknowledge some limitations. First, the sample size was small in most trials, which reduced the precision of effect estimates. Second, most included trials were considered at high risk of performance bias because of infeasibility of blinding patients and physicians to the allocation assignments. Third, statistical assumptions were made in this study. We retrieved means and SDs from medians and IQRs, respectively, which would be most problematic within the secondary analysis of TBR (<3.0 mmol/L) and TAR (>13.9 mmol/L). Additionally, a correlation coefficient of 0.5 was assumed to calculate the mean and SE of the paired differences. Fourth, heterogeneity was high in most analyses, which could be attributed to differences in study design, duration of intervention, continuous glucose monitoring systems, AID algorithms, insulin pumps, and persisting impact of human factors. Finally, the results of this meta-analysis might not apply to some clinically relevant subgroups, such as those with increased hypoglycemia burden, hypoglycemia unawareness, and high HbA1c. While some studies have investigated the association between AID use and HbA1c (34,35) and hypoglycemia unawareness (36), further studies are warranted to fully clarify these relationships.
In conclusion, this systematic review and meta-analysis shows that AID systems are more effective than conventional insulin therapy for children and adolescents with T1D in outpatient settings. AID systems increase TIR both in short-term and long-term intervention.
This article contains supplementary material online at https://doi.org/10.2337/figshare.23700345.
Article Information
Funding. This work was supported by the National Natural Science Foundation of China (72074011).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.Z. and L.G. interpreted the data and wrote the first draft of the manuscript. B.Z. and Q.Y. coded the statistical analysis, figures, and supplementary material. B.Z. and H.J. completed the literature review and extracted the data. B.Z. and F.S. conceived and designed the study. All authors reviewed and revised subsequent drafts of the manuscript and approved the final version. B.Z. and F.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
This work was supported by the National Natural Science Foundation of China (72074011).
Footnotes
See accompanying article, p. 2126.
References
- 1. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet 2018;391:2449–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. International Diabetes Federation . IDF Diabetes Atlas, 10th edition. Brussels, Belgium, International Diabetes Foundation, 2021. Accessed 13 March 2023. Available from https://diabetesatlas.org/atlas/tenth-edition/
- 3. American Diabetes Association . 13. Children and Adolescents: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S163–S182 [DOI] [PubMed] [Google Scholar]
- 4. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21:81–85 [DOI] [PubMed] [Google Scholar]
- 6. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kimbell B, Lawton J, Boughton C, Hovorka R, Rankin D. Parents’ experiences of caring for a young child with type 1 diabetes: a systematic review and synthesis of qualitative evidence. BMC Pediatr 2021;21:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haidar A, Smaoui MR, Legault L, Rabasa-Lhoret R. The role of glucagon in the artificial pancreas. Lancet Diabetes Endocrinol 2016;4:476–479 [DOI] [PubMed] [Google Scholar]
- 9. Moon SJ, Jung I, Park CY. Current advances of artificial pancreas systems: a comprehensive review of the clinical evidence. Diabetes Metab J 2021;45:813–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang Z, Liu M, Tao J, Li C, Zou F, Zhang W. Efficacy and safety of closed-loop insulin delivery versus sensor-augmented pump in the treatment of adults with type 1 diabetes: a systematic review and meta-analysis of randomized-controlled trials. J Endocrinol Invest 2022;45:471–481 [DOI] [PubMed] [Google Scholar]
- 12. Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 13. Karageorgiou V, Papaioannou TG, Bellos I, et al. Effectiveness of artificial pancreas in the non-adult population: a systematic review and network meta-analysis. Metabolism 2019;90:20–30 [DOI] [PubMed] [Google Scholar]
- 14. Michou P, Gkiourtzis N, Christoforidis A, Kotanidou EP, Galli-Tsinopoulou A. The efficacy of automated insulin delivery systems in children and adolescents with type 1 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2023;199:110678. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ, John Wiley & Sons, 2011 [Google Scholar]
- 17. Guyatt GH, Oxman AD, Vist GE, et al.; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 19. von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol 2015;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008;61:991–996 [DOI] [PubMed] [Google Scholar]
- 22. Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Health 2019;7:192–198 [Google Scholar]
- 23. Boughton CK, Allen JM, Ware J, et al.; CLOuD Consortium . Closed-loop therapy and preservation of C-peptide secretion in type 1 diabetes. N Engl J Med 2022;387:882–893 [DOI] [PubMed] [Google Scholar]
- 24. Massachusetts General Hospital . The insulin-only bionic pancreas bridging study- pediatric transitional study. In: ClinicalTrials.gov. Bethesda, MD, National Library of Medicine, 2018. NLM Identifier: NCT04112069. Accessed 13 March 2023. Available from https://clinicaltrials.gov/show/NCT04112069
- 25. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014;371:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ware J, Allen JM, Boughton CK, et al.; KidsAP Consortium . Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med 2022;386:209–219 [DOI] [PubMed] [Google Scholar]
- 28. Ware J, Boughton CK, Allen JM, et al.; DAN05 Consortium . Cambridge hybrid closed-loop algorithm in children and adolescents with type 1 diabetes: a multicentre 6-month randomised controlled trial. Lancet Digit Health 2022;4:e245–e255 [DOI] [PubMed] [Google Scholar]
- 29. Isganaitis E, Raghinaru D, Ambler-Osborn L, et al.; iDCL Trial Research Group . Closed-loop insulin therapy improves glycemic control in adolescents and young adults: outcomes from the international diabetes closed-loop trial. Diabetes Technol Ther 2021;23:342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bakhtiani PA, Zhao LM, El Youssef J, Castle JR, Ward WK. A review of artificial pancreas technologies with an emphasis on bi-hormonal therapy. Diabetes Obes Metab 2013;15:1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phillip M, Nimri R, Bergenstal RM, et al. Consensus recommendations for the use of automated insulin delivery technologies in clinical practice. Endocr Rev 2023;44:254–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haidar A, Legault L, Matteau-Pelletier L, et al. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:595–604 [DOI] [PubMed] [Google Scholar]
- 33. Higgins JP, Altman DG, Gøtzsche PC, et al.; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garg SK, Grunberger G, Weinstock R, et al.; Adult and Pediatric MiniMed HCL Outcomes 6-month RCT: HCL versus CSII Control Study Group . Improved glycemia with hybrid closed-loop versus continuous subcutaneous insulin infusion therapy: results from a randomized controlled trial. Diabetes Technol Ther 2023;25:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Messer LH, Buckingham BA, Cogen F, et al. Positive impact of the bionic pancreas on diabetes control in youth 6-17 years old with type 1 diabetes: a multicenter randomized trial. Diabetes Technol Ther 2022;24:712–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abitbol A, Rabasa-Lhoret R, Messier V, et al. Overnight glucose control with dual- and single-hormone artificial pancreas in type 1 diabetes with hypoglycemia unawareness: a randomized controlled trial. Diabetes Technol Ther 2018;20:189–196 [DOI] [PubMed] [Google Scholar]