Key Points
Question
Does adding sintilimab, an IgG4 monoclonal antibody that binds to programmed cell death 1 (PD-1), to first-line chemotherapy improve survival for patients with previously untreated advanced gastric and gastroesophageal junction adenocarcinoma, both overall and in the subset of patients with the PD ligand 1 (PD-L1) combined positive score (CPS) of 5 or more?
Findings
In this randomized clinical trial of 650 patients, from 62 centers in China, with previously untreated advanced gastric or gastroesophageal junction adenocarcinoma, the combination of sintilimab with chemotherapy compared with placebo with chemotherapy significantly improved overall survival in all randomized patients (median, 15.2 vs 12.3 months) and in patients with a CPS of 5 or more (median, 18.4 vs 12.9 months).
Meaning
Among patients with previously untreated advanced gastric or gastroesophageal junction adenocarcinoma, adding sintilimab to chemotherapy significantly improved overall survival, compared with placebo with chemotherapy, among all randomized patients and in patients with a CPS of 5 or more.
Abstract
Importance
Gastric and gastroesophageal junction cancers are diagnosed in more than 1 million people worldwide annually, and few effective treatments are available. Sintilimab, a recombinant human IgG4 monoclonal antibody that binds to programmed cell death 1 (PD-1), in combination with chemotherapy, has demonstrated promising efficacy.
Objective
To compare overall survival of patients with unresectable locally advanced or metastatic gastric or gastroesophageal junction cancers who were treated with sintilimab with chemotherapy vs placebo with chemotherapy. Also compared were a subset of patients with a PD ligand 1 (PD-L1) combined positive score (CPS) of 5 or more (range, 1-100).
Design, Setting, and Participants
Randomized, double-blind, placebo-controlled, phase 3 clinical trial conducted at 62 hospitals in China that enrolled 650 patients with unresectable locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma between January 3, 2019, and August 5, 2020. Final follow-up occurred on June 20, 2021.
Interventions
Patients were randomized 1:1 to either sintilimab (n = 327) or placebo (n = 323) combined with capecitabine and oxaliplatin (the XELOX regimen) every 3 weeks for a maximum of 6 cycles. Maintenance therapy with sintilimab or placebo plus capecitabine continued for up to 2 years.
Main Outcomes and Measures
The primary end point was overall survival time from randomization.
Results
Of the 650 patients (mean age, 59 years; 483 [74.3%] men), 327 were randomized to sintilimab plus chemotherapy and 323 to placebo plus chemotherapy. Among the randomized patients, 397 (61.1%) had tumors with a PD-L1 CPS of 5 or more; 563 (86.6%) discontinued study treatment and 388 (59.7%) died; 1 patient (<0.1%) was lost to follow-up. Among all randomized patients, sintilimab improved overall survival compared with placebo (median, 15.2 vs 12.3 months; stratified hazard ratio [HR], 0.77 [95% CI, 0.63-0.94]; P = .009). Among patients with a CPS of 5 or more, sintilimab improved overall survival compared with placebo (median, 18.4 vs 12.9 months; HR, 0.66 [95% CI, 0.50-0.86]; P = .002). The most common grade 3 or higher treatment-related adverse events were decreased platelet count (sintilimab, 24.7% vs placebo, 21.3%), decreased neutrophil count (sintilimab, 20.1% vs placebo, 18.8%), and anemia (sintilimab, 12.5% vs placebo, 8.8%).
Conclusions and Relevance
Among patients with unresectable locally advanced or metastatic gastric and gastroesophageal junction adenocarcinoma treated with first-line chemotherapy, sintilimab significantly improved overall survival for all patients and for patients with a CPS of 5 or more compared with placebo.
Trial Registration
ClinicalTrials.gov Identifier: NCT03745170
This randomized clinical trial evaluates whether sintilimab plus chemotherapy vs placebo plus chemotherapy improved overall survival in patients in China with unresectable, locally advanced, recurrent, or metastatic gastric or gastroesophageal junction adenocarcinoma.
Introduction
Gastric and gastroesophageal junction cancer is the fifth most common cancer and the fourth most common cause of cancer mortality worldwide.1 Approximately 44% of all gastric cancer is diagnosed in China and approximately 49% of gastric cancer deaths occur in China.1
The prognosis for advanced gastric or gastroesophageal junction cancer is poor and few treatments exist.2,3 The median overall survival is approximately 1 year.4,5,6,7 In the early 21st century, fluoropyrimidine-based plus platinum-based chemotherapy was the first-line treatment for unresectable advanced or metastatic ERBB2 (formerly HER2)–negative gastric or gastroesophageal junction adenocarcinoma, both in China and globally.2,3,4,5,6,7,8,9 However, recently, immune checkpoint inhibitor therapy targeting programmed death 1 (PD-1) or its ligand (PD-L1) has demonstrated efficacy for gastric and gastroesophageal junction adenocarcinomas.10,11 PD-L1 expression on tumor cells and tumor-associated immune cells (measured as a combined positive score [CPS]) is a potential biomarker to identify patients likely to respond to immune checkpoint inhibitors.10,12
Sintilimab is a recombinant, fully human IgG4 anti–PD-1 monoclonal antibody, administered intravenously, with greater affinity for human PD-1 than the immune checkpoint inhibitors nivolumab and pembrolizumab.13 The ORIENT-16 randomized trial evaluated whether sintilimab combined with chemotherapy improved overall survival compared with placebo plus chemotherapy in patients in China with unresectable, locally advanced, recurrent, or metastatic gastric or gastroesophageal junction adenocarcinoma.
Methods
Trial Design and Oversight
This was a randomized, double-blind, phase 3 clinical trial conducted at 62 hospitals in China in accordance with the Good Clinical Practice Guidelines. The trial protocol (Supplement 1) was approved by independent ethics committees at each study site. All patients provided written informed consent prior to enrollment. Enrollment occurred from January 3, 2019, to August 5, 2020, and the final follow-up occurred on June 20, 2021. The study protocol is provided in Supplement 1 and the statistical analysis plan in Supplement 2. In the study protocol and statistical analysis plan, reference to PD-L1 is used interchangeably with CPS greater than or equal to 5.
Participants
Eligible patients were at least 18 years of age, with histologically confirmed unresectable locally advanced, or metastatic gastric or gastroesophageal junction adenocarcinoma. Patients with any degree of PD-L1 expression, characterized using the CPS score, were potentially eligible. Additional inclusion criteria included at least 1 measurable or evaluable lesion per the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and adequate hematologic, hepatic, and renal function. Patients previously treated with adjuvant or neoadjuvant chemotherapy, or radiotherapy, were eligible if their disease reoccurred at least 6 months after their last treatment (Figure 1 and Table 1).
Figure 1. Patient Flow in the ORIENT-16 Trial.
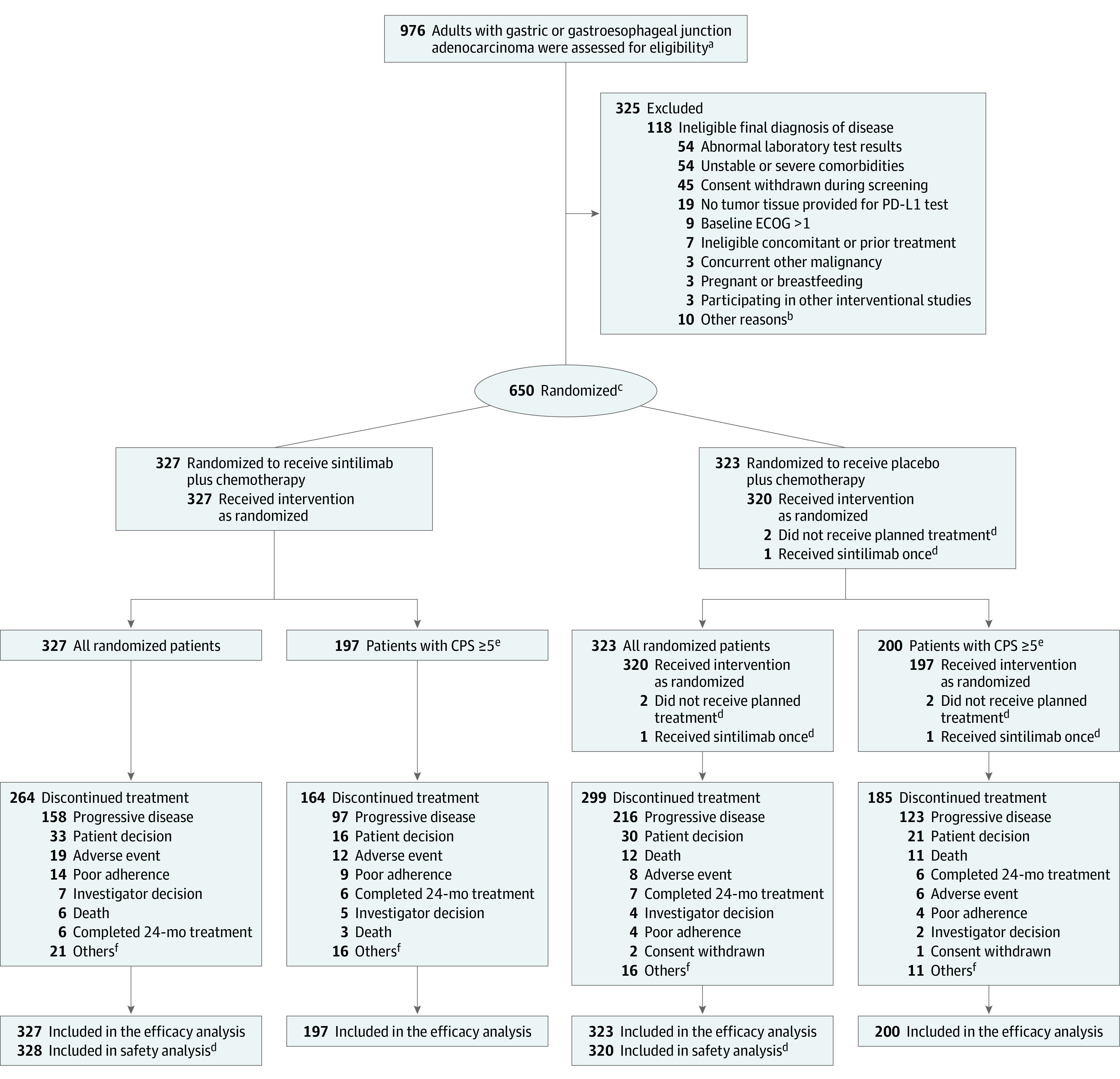
aThe total number of patients approached for participation in the trial was not recorded.
bOther reasons included a screening period exceeding 28 days or having an ineligible condition.
cPatients were randomized in a 1:1 ratio and stratified according to Eastern Cooperative Oncology Group performance status score (0 or 1), liver metastasis (yes or no), and programmed cell death ligand 1 (PD-L1) expression (combined positive score [CPS], <10 or ≥10). See Table 1 footnotes for more information. One patient who was assigned with 2 randomization numbers was counted as 1.
dFor the safety analysis, 1 patient who received 1 dose of sintilimab was analyzed in the sintilimab group, and 2 patients who did not receive planned treatment were excluded.
ePD-L1 expression was measured by CPS, which was defined as the number of PD-L1 staining cells (tumor cells, lymphocytes and macrophages) divided by the total number of viable tumor cells present in the sample multiplied by 100. The maximum score is defined as 100 when the calculation exceeds 100. A CPS of 5 or more was defined as PD-L1 positive.
fOther reasons included clinical progression, maximum benefit reached for patients who became eligible to undergo radical surgery after study treatment, and dose interruption up to the maximum duration of 12 weeks.
Table 1. Baseline Characteristics of Study Patients.
| CPS ≥5a | All randomized | CPS <5a | ||||
|---|---|---|---|---|---|---|
| Sintilimab + chemotherapy (n = 197) | Placebo + chemotherapy (n = 200) | Sintilimab + chemotherapy (n = 327) | Placebo + chemotherapy (n = 323) | Sintilimab + chemotherapy (n = 130) | Placebo + chemotherapy (n = 123) | |
| Age, y | ||||||
| Median (IQR) | 62 (56-67) | 61 (55-67) | 62 (55-67) | 60 (52-67) | 62 (54-68) | 58 (51-66) |
| <65 | 125 (63.5) | 124 (62.0) | 206 (63.0) | 209 (64.7) | 81 (62.3) | 85 (69.1) |
| ≥65 | 72 (36.5) | 76 (38.0) | 121 (37.0) | 114 (35.3) | 49 (37.7) | 38 (30.9) |
| Sex | ||||||
| Male | 149 (75.6) | 149 (74.5) | 253 (77.4) | 230 (71.2) | 104 (80.0) | 81 (65.9) |
| Female | 48 (24.4) | 51 (25.5) | 74 (22.6) | 93 (28.8) | 26 (20.0) | 42 (34.1) |
| Weightb | ||||||
| <60 kg | 89 (45.2) | 105 (52.5) | 159 (48.6) | 169 (52.3) | 70 (53.8) | 64 (52.0) |
| ECOG performance statusc | ||||||
| 0 (fully active) | 59 (29.9) | 60 (30.0) | 89 (27.2) | 91 (28.2) | 30 (23.1) | 31 (25.2) |
| 1 (restricted in physically strenuous activity but ambulatory) | 138 (70.1) | 140 (70.0) | 238 (72.8) | 232 (71.8) | 100 (76.9) | 92 (74.8) |
| Primary tumor location at initial diagnosis | ||||||
| Gastric | 161 (81.7) | 167 (83.5) | 266 (81.3) | 263 (81.4) | 105 (80.8) | 96 (78.0) |
| Gastroesophageal junction | 36 (18.3) | 33 (16.5) | 60 (18.3) | 60 (18.6) | 24 (18.5) | 27 (22.0) |
| Otherd | 0 | 0 | 1 (0.3) | 0 | 1 (0.8) | 0 |
| Disease status at enrollment | ||||||
| Locally advanced | 21 (10.7) | 13 (6.5) | 28 (8.6) | 23 (7.1) | 7 (5.4) | 10 (8.1) |
| Metastatic | 176 (89.3) | 186 (93.0) | 299 (91.4) | 299 (92.6) | 123 (94.6) | 113 (91.9) |
| Othere | 0 | 1 (0.5) | 0 | 1 (0.3) | 0 | 0 |
| Site of metastases | ||||||
| Liver | 78 (39.6) | 87 (43.5) | 127 (38.8) | 128 (39.6) | 49 (37.7) | 41 (33.3) |
| Peritoneum | 42 (21.3) | 37 (18.5) | 65 (19.9) | 63 (19.5) | 23 (17.7) | 26 (21.1) |
| PD-L1 expression (CPS)a | ||||||
| CPS ≥10 | 146 (74.1) | 142 (71.0) | 146 (44.6) | 142 (44.0) | 0 | 0 |
| Prior radical gastrectomy | 28 (14.2) | 23 (11.5) | 58 (17.7) | 58 (18.0) | 30 (23.1) | 35 (28.5) |
Abbreviations: CPS, combined positive score; ECOG, Eastern Cooperative Oncology Group.
See Figure 1 legend footnote e for programmed cell death ligand 1 (PD-L1) CPS calculations.
Sintilimab administered at 3 mg/kg for body weight less than 60 kg and 200 mg for 60 kg or more.
The Eastern Cooperative Oncology Group performance status score 5-point scale is commonly used for selection of appropriate patients in clinical trials, which defines 0 as fully active, able to carry on all predisease performance without restriction, and defines 1 as restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature.
One patient had tumors located at gastric and gastroesophageal junction separately.
One patient had local recurrence.
Exclusion criteria included prior systemic treatment for advanced or metastatic gastric or gastroesophageal junction adenocarcinoma; known ERBB2-positive status; and active, known, or suspected autoimmune disease.
Randomization and Masking
Eligible patients received chemotherapy and were randomly assigned (1:1) to also receive either sintilimab or placebo using an interactive web response system with block size randomly selected from 4 or 6 and stratified according to ECOG PS score (0 or 1), liver metastasis (yes or no), and PD-L1 expression (CPS, <10 or ≥10). Patients, investigators, and the study team were masked to treatment allocation.
Treatment
Sintilimab (3 mg/kg for body weight <60 kg, 200 mg for ≥60 kg) or placebo was administered intravenously in combination with chemotherapy (XELOX, capecitabine 1000 mg/m2 orally twice a day for days 1-14 and oxaliplatin 130 mg/m2 intravenously) every 3 weeks for a maximum of 6 cycles, followed by maintenance therapy with sintilimab or placebo plus capecitabine, continuing at the same dose. Treatment continued until progressive disease, intolerable toxicity, initiation of new antitumor therapy, patient request, or any other investigator-determined reason for discontinuation occurred or for a maximum of 24 months, whichever occurred first.
Assessment
Tumors were assessed with computed tomographic (CT) scans or magnetic resonance imaging (MRI) at baseline, every 6 weeks from week 1 to week 48 and subsequently every 12 weeks until disease progression or a new antitumor therapy was initiated, whichever occurred first, in accordance with the RECIST version 1.1 guidelines.14 Safety assessments continued from the first dose to 90 days after treatment discontinuation. Treatment-related adverse events connected to any drug and immune-related adverse events (defined as an adverse event connected to sintilimab with potential immunologic etiology)15 were assessed and recorded. After safety assessments were completed, survival was assessed every 60 days.
PD-L1 expression was measured using the CPS at a central laboratory (Covance, Shanghai, China) using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies) to measure the expression of PD-L1 on tumor cells and immune cells combined. CPS (range, 0-100; maximum score, 100 despite higher scores) was defined at baseline as the number of PD-L1 stained cells (tumor cells, lymphocytes, and macrophages) divided by the number of viable tumor cells present in the sample, multiplied by 100. Tissue from either primary or metastatic lesions by biopsy or resection were used for PD-L1 testing. The CPS of 5 or more was defined as the threshold for PD-L1 positivity.10
Primary Outcome
The primary end points were overall survival, defined as time from randomization until death from any cause in all randomized patients and in the subset of patients with a CPS of 5 or more (Sections 2.2 and 3.1 in Supplement 2). After enrollment completion (August 5, 2020), the primary PD-L1–positive population was amended from patients with a CPS of 10 or more to 5 or more (on June 11, 2021) prior to when investigators began reviewing study outcomes. This change was prompted by the results of the CheckMate 64910 study, which demonstrated that the efficacy of another PD-L1 inhibitor, nivolumab, for patients with advanced gastric or gastroesophageal junction adenocarcinoma and a CPS of 5 or more. This amendment was discussed with the regulatory agency and accepted by China’s National Medical Products Administration before the database lock (August 3, 2021) and unblinding by the independent data monitoring committee for the prespecified interim efficacy analysis (performed on August 15, 2021, eAppendix 1 in Supplement 3).
Secondary Outcomes
Secondary end points included progression-free survival, defined as the time from randomization to the date of first documented radiological tumor progression or death; the radiological objective response rate, defined as the proportion of patients whose best overall response was a complete or partial response; radiological disease control rate, defined as the proportion of patients whose best overall response was a complete response, partial response, or stable disease; and duration of response, defined as the time from the first response until radiological disease progression or death (ascertained by relevant certificate of death) from any cause, whichever occurred first.
Adverse Events
Adverse events were classified by treating clinicians according to the Medical Dictionary for Regulatory Activities and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 5.0).
Sample Size Calculation
The determination of sample size aimed to provide statistical power to detect superiority in overall survival among all randomized patients and patients with a CPS of 5 or more. The calculation assumed a hazard ratio (HR) for overall survival of 0.75 (median, 14.7 months for sintilimab vs 11 months for placebo) in all randomized patients and 0.7 (median, 15.7 months for sintilimab vs 11 months for placebo) for patients with a CPS of 5 or more. These assumptions were based on prior studies16,17,18 and were regarded as the minimal clinically meaningful improvement based on consensus among study investigators. Based on these assumptions and under the exponential model, 515 overall survival events provided 90% power for all randomized patients and 287 overall survival events provided 85% power for patients with CPS of 5 or more at the final analysis with a 2-sided α of .05. Six hundred fifty patients would be needed, assuming an enrollment duration of 18 months and study duration of 46 months with a 0.5% censoring rate per month. The proportion of patients with a CPS of 5 or more was assumed to be 56.2% (ie, 365), and there was no enrollment cap based on CPS status.
Statistical Analysis
An interim efficacy analysis and final analysis were planned. The interim efficacy analysis of overall survival among patients with a CPS of 5 or more and all randomized patients would be performed when at least 70% of the total planned overall survival events had occurred in both populations (ie, 201 in patients with CPS ≥5 and 361 in all randomized patients). Type I errors were controlled using a fixed-sequence-testing approach and the O’Brien-Fleming boundary based on the Lan-DeMets approach. The original protocol and statistical analysis plan were not clear about designating the primary analysis time point. However, the primary analysis time point considered the interim efficacy analysis as the primary overall survival result if the efficacy boundaries were crossed.
The overall type I error was controlled at a 2-sided .05 level for hypothesis testing of the overall survival primary outcome and the key progression-free-survival secondary outcome by using a fixed-sequence testing method. Overall survival among patients with a CPS of 5 or more was tested first and, if statistically significant, was followed by a test of overall survival in all randomized patients (see Section 3.1 of Supplement 2). Statistical testing of the progression-free survival secondary end point was conducted sequentially among patients with a CPS of 5 or more and all randomized patients using a 2-sided significance level of .05 only if the primary efficacy end points were statistically significant in both populations. Analyses of secondary end points other than progression-free survival were not adjusted for multiple comparisons and should be considered exploratory.
Overall and progression-free survival were analyzed according to treatment group at randomization (ie, based on the intention-to-treat statistical analysis principle, see Sections 3.6 and 7.1 in Supplement 2). Patients were censored at the last date of survival for overall survival and at the last tumor imaging assessment for progression-free survival. Objective response rate, disease control rate, and duration of response were analyzed for all randomized patients who had at least 1 measurable tumor lesion at baseline (eAppendix 2 in Supplement 3). Patients with no postbaseline tumor imaging assessment were considered nonresponders for the objective response and disease control rates. In the statistical analysis plan, the term intention to treat had 2 different meanings in different sections: (1) the intention-to-treat set referred to conducting statistical analyses according to each patient’s randomized treatment assignment and (2) the intention-to-treat population referred to randomized patients regardless of their CPS status according to their group assignment. Safety was assessed in all randomized patients who received at least 1 dose of the investigational drug or placebo (safety analysis set; see Section 3.6 in Supplement 2).
A stratified log-rank test was used to compare overall survival between groups. The HRs and corresponding 2-sided 95% CIs were estimated using a stratified Cox proportional hazards model. For all stratified analyses, the stratification factors were ECOG PS (0 vs 1), liver metastasis (present vs absent), and CPS (<10 vs ≥10). The median overall survival and the corresponding 2-sided 95% CI were estimated using the Kaplan-Meier method. Progression-free survival and duration of response were analyzed using the same method as overall survival. Sensitivity analyses for overall and progression-free survival were carried out as specified in the statistical analysis plan.
The by-treatment group objective response rate and disease control rate were reported along with corresponding 2-sided 95% CIs, which were estimated using normal approximation. The rate differences between the treatment groups and corresponding 2-sided 95% CIs were calculated using the Miettinen-Nurminen methods.19
Prespecified subgroup analyses by age (<65 years or ≥65 years), sex (male or female), weight (<60 kg or ≥60 kg), ECOG score (0 or 1), PD-L1 expression level (CPS, <10 or ≥10, <5 or ≥5, <1 or ≥1, tumor proportion scores [TPSs] <10% or ≥10%, <5% or ≥5%, and <1% or ≥1%), hepatic metastasis (yes or no), tumor site (gastric, or gastroesophageal junction), types of disease (locally progressive, or metastatic), and previous radical surgery (yes or no) were performed for both overall and progression-free survival. The subgroup factors of PD-L1 expression (CPS, <1 or ≥1, <5 or ≥5) were not included for the subgroup analyses of patients with a CPS of 5 or more. For each subgroup, the between-treatment group HRs and corresponding 2-sided 95% CIs were estimated using an unstratified Cox proportional hazards model. A 2-sided P value for the interaction effect between subgroup factors and treatment was calculated.
A post hoc analysis was also conducted using a stratified Cox proportional hazards model and stratified log-rank test to compare between-group differences for overall and progression-free survival in the subgroup of patients (130 in the sintilimab and 123 in the placebo groups) with a CPS of less than 5.
The number of overall survival events was monitored in a blinded manner by the investigators. At the cutoff date for the interim efficacy analysis (June 20, 2021), a total of 220 and 388 overall survival events had occurred in patients with a CPS of 5 or more and all randomized patients, respectively. The independent data monitoring committee confirmed that the primary end point for all randomized patients and for those with a CPS of 5 or more were met at the interim efficacy analysis.
All statistical analyses were conducted using SAS version 9.4 or higher (SAS Institute Inc).
Results
Enrollment and Patient Characteristics
Of 976 patients assessed for eligibility, 650 were randomized; 327 (50.3%) to sintilimab and 323 (49.7%) to placebo. A total of 197 patients (60.2%) in the sintilimab group and 200 (61.9%) in the placebo group had tumors expressing PD-L1 with a CPS of 5 or more; 648 patients (99.7%) received at least 1 dose of sintilimab (n = 328 [50.5%]) or placebo (n = 320 [49.2%]) plus chemotherapy. For the safety analysis, 1 patient in the placebo group who received 1 dose of sintilimab was analyzed in the sintilimab group, and 2 patients in the placebo group who did not receive treatment were excluded from the safety analysis data set. Patient recruitment and disposition is summarized in Figure 1.
Baseline characteristics are shown in Table 1 and eTable 1 in Supplement 3. Overall, 120 patients (18.5%) had gastroesophageal junction adenocarcinoma; 598 (92.0%) had metastatic cancer; and 470 (72.3%) had an ECOG PS of 1.
At the data cutoff for the interim efficacy analysis, the median follow-up was 18.8 months (IQR, 13.8-22.6); treatment had been discontinued by 264 patients (80.7%) in the sintilimab group and 299 (92.6%) in the placebo group. The most common reasons for treatment discontinuation in the sintilimab and placebo groups, respectively, were progressive disease (48.3% vs 66.9%) and patient decision to stop treatment (10.1% vs 9.3%). The median treatment duration was 6.1 months (IQR, 3.4-11.7 months) in the sintilimab group and 5.5 months (IQR, 2.8-9.7 months) in the placebo group. Subsequent antitumor treatment after discontinuation of study treatment was received by 119 of 327 patients (36.4%) in the sintilimab group and 153 of 323 patients (47.4%) in the placebo group. The most common subsequent antitumor treatment in both groups was additional systemic therapy: 109 patients (33.3%) in the sintilimab group and 150 (46.4%) in the placebo group. Twenty-two patients (6.7%) in the sintilimab group and 34 (10.5%) in the placebo group received subsequent immunotherapy (eTable 2 in Supplement 3).
Primary Outcome
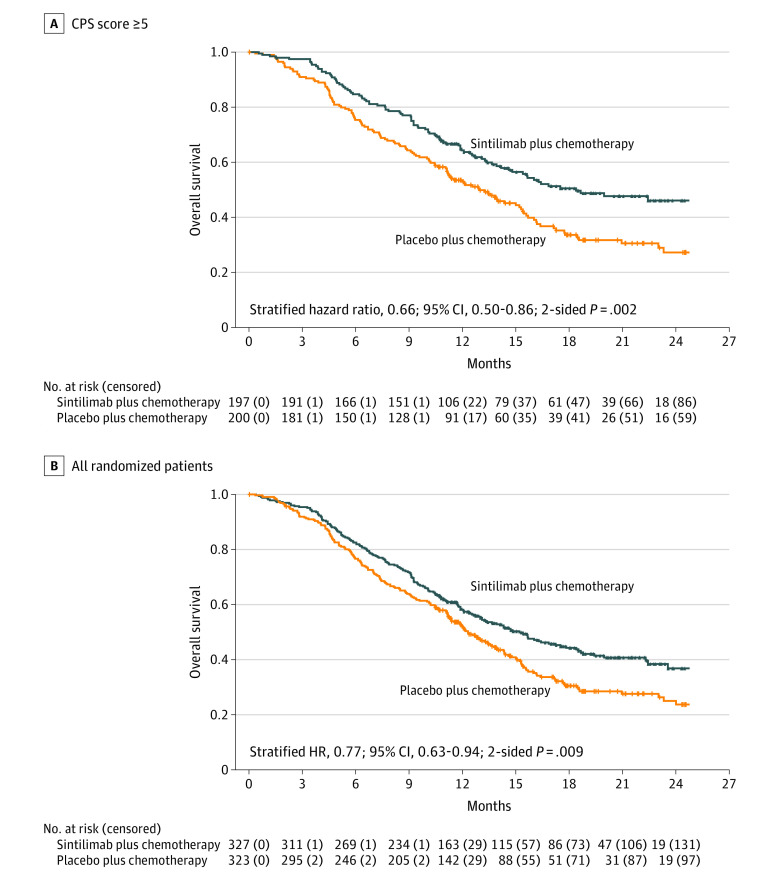
For patients with a CPS of 5 or more, the median overall survival was 18.4 months in the sintilimab group and 12.9 months in the placebo group (stratified HR, 0.66 [95% CI, 0.50-0.86]; 2-sided P = .002; Figure 2A). In all randomized patients, the median overall survival was 15.2 months in the sintilimab group and 12.3 months in the placebo group (stratified HR, 0.77 [95% CI, 0.63-0.94]; 2-sided P = .009; Figure 2B).
Figure 2. Overall Survival.
For patients with a combined positive score (CPS) of 5 or more, the median follow-up for overall survival was 19.4 (IQR, 14.1-23.2) months for sintilimab plus chemotherapy and 18.9 (IQR, 13.8-24.5) months for placebo plus chemotherapy; the median overall survival was 18.4 months vs 12.9 months. For all randomized patients, median follow-up for overall survival was 19.3 (IQR, 14.1-22.4) months for sintilimab plus chemotherapy and 18.1 (IQR, 13.5-22.7) months for placebo plus chemotherapy; median overall survival was 15.2 months vs 12.3 months. See Figure 1 legend footnote e for programmed cell death ligand 1 CPS calculations. A CPS of 5 or more was defined as PD-L1 positive.
Secondary Outcomes
The median progression-free survival in patients with a CPS of 5 or more was 7.7 months for the sintilimab group vs 5.8 months for the placebo group, with a stratified HR of 0.63 (95% CI, 0.49-0.81; 2-sdied P < .001; eFigure 1A in Supplement 3). The median progression-free survival in all randomized patients was 7.1 months for the sintilimab group vs 5.7 months for the placebo group, with a stratified HR of 0.64 (95% CI, 0.52-0.77; 2-sided P < .001; eFigure 1B in Supplement 3).
For patients with a CPS of 5 or more, the objective response rate was 63.6% in the sintilimab group and 49.4% in the placebo group (rate difference, 13.9% [95% CI, 3.6% to 24.3%]; 2-sided P = .008; eTable 3 in Supplement). The disease control rate was 90.1% in the sintilimab group and 84.3% in the placebo group (rate difference, 5.2% [95% CI, –2.4% to 12.8%]; 2-sided P = .18). The median duration of response was 9.8 months in the sintilimab group and 7.1 months in the placebo group (stratified HR, 0.62 [95% CI, 0.42 to 0.91]; 2-sided P = .01; eFigure 2A in Supplement 3). Among all randomized patients, the overall response rate was 58.2% in the sintilimab group vs 48.4% in the placebo group, with a difference between groups of 9.6% (95% CI, 1.3 to 17.9; 2-sided P = .02). The disease control rate was 87.7% vs 84.3% (rate difference, 3.7% [95% CI, –2.5% to 10.0%]; 2-sided P = .24) and the median duration of response was 9.8 months vs 7.0 months, for the sintilimab and placebo groups (stratified HR, 0.57 [95% CI, 0.42 to 0.77]; 2-sided P < .001), respectively (eFigure 2B in Supplement 3).
Sensitivity Analyses
Sensitivity analyses were performed with an unstratified log-rank test (unstratified HR for patients with CPS ≥5, 0.64 [95% CI, 0.49-0.84]; 2-sided P = .001, and for all randomized patients, 0.74 [95% CI, 0.61-0.90]; 2-sdied P = .003). Analysis of patients who had received protocol-assigned treatment did not change the primary result in the intention-to-treat group (stratified HR for patients with CPS ≥5, 0.66 [95% CI, 0.50-0.86]; 2-sided P = .002; for all patients, 0.76 [95% CI, 0.62-0.93]; 2-sided P = .008; eTable 4 in Supplement 3). Sensitivity analysis for progression-free survival using a log-rank test with multiple imputation of missing data also did not change study results (eTable 5 in Supplement 3).
Subgroup Analyses
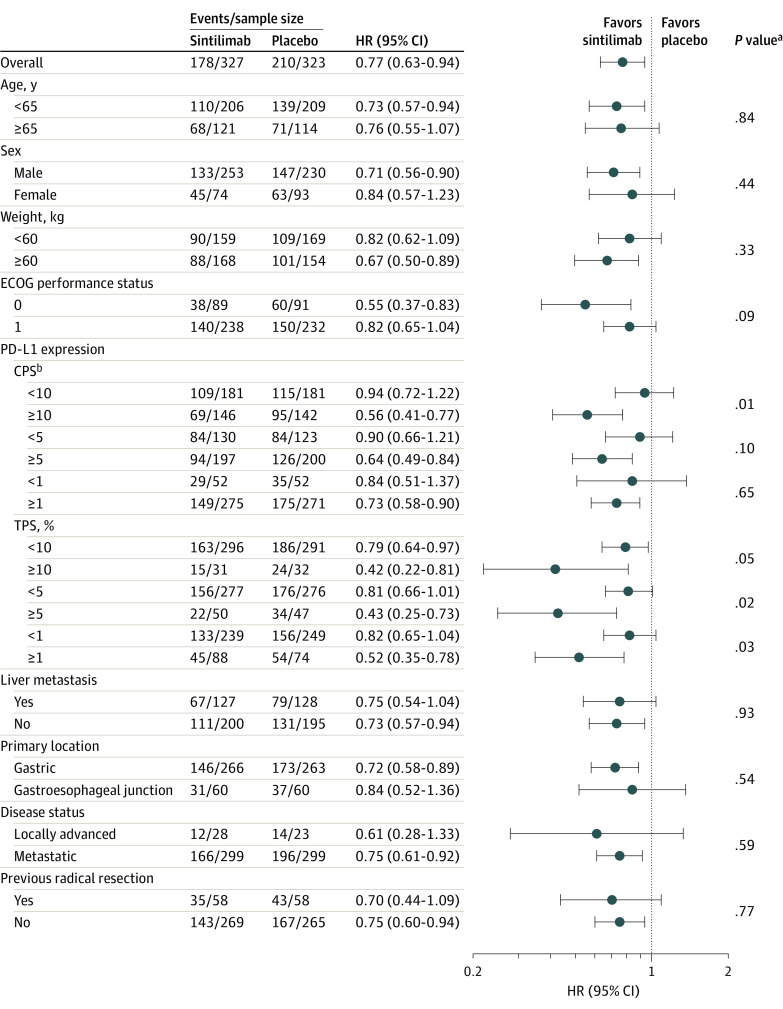
HRs for overall survival favored the sintilimab group vs the placebo group across multiple prespecified subgroups in patients with a CPS of 5 or more (eFigure 3 in Supplement 3) and in all randomized patients (Figure 3). Interaction P values were .10 or less between PD-L1 expression level (except for CPS threshold of 1) and treatment group. In patients with a CPS of 5 or more and less than 5, the HRs for overall survival between the sintilimab group and placebo group were 0.64 (95% CI, 0.49-0.84) and 0.90 (95% CI 0.66-1.21), respectively, with an interaction P value of .10 (Figure 3). Similarly, HRs for progression-free survival favored the sintilimab group across most subgroups (eFigure 4 in Supplement 3). Interaction testing revealed no important significant interactions between the subgroup factors and treatment group.
Figure 3. Subgroup Plot for Prespecified Subgroup Analyses of Overall Survival in All Randomized Patients at the Interim Efficacy Analysis.
aInteraction P values.
bSee Figure 1 legend footnote e for programmed cell death ligand 1 (PD-L1) combined positive score (CPS) calculations. A CPS of 5 or more was defined as PD-L1 positive.
ECOG indicates Eastern Cooperative Oncology Group; HR, hazard ratio; TPS, tumor proportion score.
Adverse Events
Treatment-related adverse events of any grade were reported by 319 of 328 patients (97.3%) treated with sintilimab plus chemotherapy and 308 of 320 patients (96.3%) treated with placebo plus chemotherapy (Table 2). Grade 3 or higher treatment-related adverse events occurred in 196 patients (59.8%) in the sintilimab plus chemotherapy group and 168 (52.5%) in the placebo plus chemotherapy group, with the most common being thrombocytopenia (81 of 328 [24.7%] vs 68 of 320 [21.3%]), decreased neutrophil count (66 of 328 [20.1%] vs 60 of 320 [18.8%]), and anemia (41 of 328 [12.5%] vs 28 of 320 [8.8%]). Treatment-related adverse events leading to any drug discontinuation and serious treatment-related adverse events are summarized in eTables 6 and 7 in Supplement 3. There were 6 deaths (1.8%) in the sintilimab group and 2 (0.6%) in the placebo group (both attributed to decreased platelet count) that were considered treatment related (eTable 8 in Supplement 3). Of the 6 deaths in the sintilimab group, 2 were determined related to sintilimab only (1 patient each attributed to cystitis and immune-mediated lung disease), 3 to both sintilimab and chemotherapy (1 patient each due to pneumonitis, immune-mediated hepatitis, and myelosuppression), and 1 to chemotherapy only (due to decreased neutrophil count, white blood cell count, and platelet count). Most immune-related adverse events as assessed by investigators were grade 1 or 2.
Table 2. Adverse Events in Safety Population at the Interim Analysis.
| Sintilimab plus chemotherapy (n = 328) | Placebo plus chemotherapy (n = 320) | |||
|---|---|---|---|---|
| ≥Grade 3 | All grades | ≥Grade 3 | All grades | |
| Any treatment-related adverse eventsa | 196 (59.8) | 319 (97.3) | 168 (52.5) | 308 (96.3) |
| Treatment-related adverse events in 15% or more of treated patients in either groupb | ||||
| Platelet count decreased | 81 (24.7) | 217 (66.2) | 68 (21.3) | 209 (65.3) |
| Neutrophil count decreased | 66 (20.1) | 195 (59.5) | 60 (18.8) | 177 (55.3) |
| White blood cell count decreased | 25 (7.6) | 176 (53.7) | 22 (6.9) | 172 (53.8) |
| Anemia | 41 (12.5) | 153 (46.6) | 28 (8.8) | 148 (46.3) |
| Nausea | 11 (3.4) | 138 (42.1) | 3 (0.9) | 120 (37.5) |
| Vomiting | 12 (3.7) | 131 (39.9) | 6 (1.9) | 127 (39.7) |
| Elevated aspartate aminotransferase | 4 (1.2) | 108 (32.9) | 1 (0.3) | 99 (30.9) |
| Elevated alanine aminotransferase increased | 2 (0.6) | 71 (21.6) | 1 (0.3) | 61 (19.1) |
| Decreased appetite | 2 (0.6) | 69 (21.0) | 1 (0.3) | 63 (19.7) |
| Hypothyroidism | 0 | 58 (17.7) | 0 | 30 (9.4) |
| Hyperbilirubinemia | 8 (2.4) | 56 (17.1) | 4 (1.3) | 60 (18.8) |
| Palmar-plantar erythrodysesthesia syndrome | 11 (3.4) | 56 (17.1) | 12 (3.8) | 51 (15.9) |
| Asthenia | 5 (1.5) | 52 (15.9) | 4 (1.3) | 48 (15.0) |
| Hypoesthesia | 2 (0.6) | 50 (15.2) | 2 (0.6) | 36 (11.3) |
| Any immune-related adverse events | 35 (10.7) | 114 (34.8) | 11 (3.4) | 68 (21.3) |
| Immune-related adverse events in 2% or more of patients in either groupc | ||||
| Hypothyroidism | 0 | 45 (13.7) | 0 | 21 (6.6) |
| Hyperthyroidism | 0 | 20 (6.1) | 0 | 6 (1.9) |
| Amylase increased | 1 (0.3) | 17 (5.2) | 2 (0.6) | 9 (2.8) |
| Rash | 0 | 16 (4.9) | 0 | 3 (0.9) |
| Aspartate aminotransferase increased | 0 | 11 (3.4) | 0 | 6 (1.9) |
| Blood thyroid stimulating hormone increased | 0 | 9 (2.7) | 0 | 6 (1.9) |
| Lipase increased | 4 (1.2) | 9 (2.7) | 1 (0.3) | 4 (1.3) |
| Platelet count decreased | 4 (1.2) | 7 (2.1) | 2 (0.6) | 4 (1.3) |
Adverse events were classified according to Medical Dictionary for Regulatory Activities and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0 (range, 1-5 [1, mild; 2, moderate; 3, severe; 4, life-threatening; and 5, death]).
Treatment-related adverse events to any drug occurring in 15% or more of patients in either group are shown in descending order of frequency in the sintilimab plus chemotherapy group.
Immune-related adverse events (related to sintilimab with potential immunologic etiology) assessed by investigators occurring in 2% or more of patients in either group are listed in descending order.
Post Hoc Outcomes
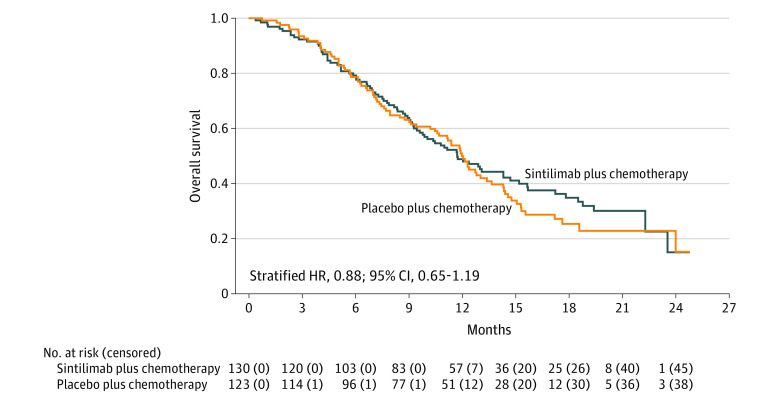
The median overall survival was 11.7 months in the sintilimab group compared with 12.0 months in the placebo group for patients with a CPS of less than 5 (Figure 4), with a stratified HR of 0.88 (95% CI, 0.65-1.19). The median progression-free survival was 7.0 months vs 5.6 months (eFigure 5 in Supplement 3), with a stratified HR of 0.66 (95% CI, 0.49-0.89).
Figure 4. Post Hoc Overall Survival at the Interim Efficacy Analysis in Patients With a Combined Positive Score Less Than 5.
The median follow-up for overall survival was 18.8 (IQR, 13.7-20.7) months for sintilimab plus chemotherapy and 17.4 (IQR, 13.1-20.3) months for placebo plus chemotherapy; the median overall survival was 11.7 months vs 12.0 months.
See Figure 1 legend footnote e for programmed cell death ligand 1 (PD-L1) combined positive score (CPS) calculations. A CPS of 5 or more was defined as PD-L1 positive.
Discussion
Among patients with previously untreated advanced gastric or gastroesophageal junction adenocarcinoma, adding sintilimab to standard chemotherapy was associated with significantly improved overall survival and progression-free survival compared with placebo plus standard chemotherapy. Our results showed a statistically significant benefit of sintilimab for the primary end points of overall survival at the interim efficacy analysis among patients with a CPS of 5 or more and in all randomized patients irrespective of baseline CPS score.
Since 2021, the combination of a PD-1 inhibitor and chemotherapy has become a global standard of care for patients with advanced gastric or gastroesophageal junction adenocarcinoma and is integrated into treatment guidelines20 based on the results of the CheckMate 649 study.10 This study randomly assigned patients in a 1:1:1 ratio to receive nivolumab plus chemotherapy (capecitabine and oxaliplatin or leucovorin, fluorouracil, and oxaliplatin), nivolumab plus ipilimumab, or chemotherapy alone and the primary end points were overall or progression-free survival in patients with a CPS of 5 or more. The results of CheckMate 649 showed a significant improvement in overall and progression-free survival with nivolumab plus chemotherapy vs chemotherapy alone in patients with a CPS of 5 or more and all randomized patients. The present trial was a randomized, double-blind, placebo-controlled, study conducted in China with a single chemotherapy option based on current practice guidelines, in which patients were assigned (1:1) to receive chemotherapy with or without sintilimab, an alternative anti-PD-1 antibody to nivolumab, the drug used in CheckMate649. Similar to the CheckMate 649 study, the present study found that the magnitude of improvement in overall survival with the addition of immunotherapy to standard chemotherapy was greater for patients with higher PD-L1 expression. Neither the study reported herein nor CheckMate 649 demonstrated a statistically significant survival benefit in the subgroup of patients with a CPS less than 5.
The safety profile was similar among patients in the sintilimab and placebo groups, with the same most common adverse events (incidence ≥30%) including decreased platelet count, decreased neutrophil count, decreased white blood cell count, anemia, nausea, vomiting, and increased aspartate aminotransferase. The overall safety profile of sintilimab was comparable with that reported for other PD-1 inhibitors combined with first-line chemotherapy in patients with gastric or gastroesophageal junction adenocarcinoma and other solid tumors, with the most common adverse events comprising hematological and gastrointestinal toxicities.10,12 No new or unanticipated serious adverse events were observed.
Limitations
This study had several limitations. First, this was a multicenter study performed in China and the generalizability of the results to other populations is unclear. Second, a CPS threshold of 10 was a randomization stratification factor, but the primary PD-L1 positive population was later revised to patients with a CPS of 5 or more based on the results of the CheckMate 649 study, which used this threshold to evaluate an alternative PD-1 inhibitor, nivolumab.10 Third, microsatellite instability analysis was not performed, although microsatellite instability was previously shown to be associated with longer overall survival in patients with advance gastric cancer who received a PD-1 inhibitor plus chemotherapy. Fourth, tumor responses were not assessed by a blinded independent central review committee, which may have introduced bias in ascertainment of progression-free survival. Fifth, the protocol and statistical analysis plan lacked clarity in the description of some parameters, including the time point of the primary outcome and testing of the primary outcomes in the entire cohort and in patients with a CPS of 5 or more.
Conclusion
Among patients with unresectable locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma, sintilimab combined with first-line chemotherapy significantly improved survival for all patients and for patients with a CPS of 5 or more compared with placebo combined with first-line chemotherapy.
Trial Protocol
Trial Protocol Amendments
Statistical Analysis Plan
eAppendix 1. History of the statistical analysis plan finalization, IDMC meeting, and interim efficacy analysis
eAppendix 2. Definition of measurable tumor lesion at baseline
eTable 1. Additional baseline disease characteristics
eTable 2. Subsequent anticancer therapy at interim efficacy analysis
eTable 3. Antitumour activity at interim efficacy analysis
eTable 4. Sensitivity analysis on overall survival at interim efficacy analysis
eTable 5. Sensitivity analysis on progression-free survival at interim efficacy analysis
eTable 6. Treatment-related adverse events leading to treatment discontinuation at interim efficacy analysis
eTable 7. Treatment-related serious adverse events at interim efficacy analysis
eTable 8. Treatment-related adverse events leading to death at interim efficacy analysis
eFigure 1. Kaplan-Meier plot of progression-free survival at interim efficacy analysis
eFigure 2. Kaplan-Meier curve of duration of confirmed response at the interim efficacy analysis
eFigure 3. Subgroup plot for subgroup analyses of overall survival in patients with CPS ≥5 at the interim efficacy analysis
eFigure 4. Subgroup plot for subgroup analyses of progression-free survival at the interim efficacy analysis
eFigure 5. Post hoc Kaplan-Meier plot of progression-free survival per investigator’s assessment in patients with PD-L1 combined positive score (CPS) <5 at the interim efficacy analysis
Nonauthor Collaborators. The ORIENT-16 Investigators
Data Sharing Statement
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Muro K, Van Cutsem E, Narita Y, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(1):19-33. doi: 10.1093/annonc/mdy502 [DOI] [PubMed] [Google Scholar]
- 3.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee . Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38-v49. doi: 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- 4.Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(11):1467-1482. doi: 10.1016/S1470-2045(17)30566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs CS, Shitara K, Di Bartolomeo M, et al. ; RAINFALL Study Group . Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420-435. doi: 10.1016/S1470-2045(18)30791-5 [DOI] [PubMed] [Google Scholar]
- 6.Lordick F, Kang YK, Chung HC, et al. ; Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators . Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490-499. doi: 10.1016/S1470-2045(13)70102-5 [DOI] [PubMed] [Google Scholar]
- 7.Shah MA, Bang YJ, Lordick F, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. 2017;3(5):620-627. doi: 10.1001/jamaoncol.2016.5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(2):167-192. doi: 10.6004/jnccn.2022.0008 [DOI] [PubMed] [Google Scholar]
- 9.Wang FH, Shen L, Li J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39(1):10. doi: 10.1186/s40880-019-0349-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27-40. doi: 10.1016/S0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiravand Y, Khodadadi F, Kashani SMA, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022;29(5):3044-3060. doi: 10.3390/curroncol29050247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571-1580. doi: 10.1001/jamaoncol.2020.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Fei K, Jing H, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs. 2019;11(8):1443-1451. doi: 10.1080/19420862.2019.1654303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 15.Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(4):387-405. doi: 10.6004/jnccn.2022.0020 [DOI] [PubMed] [Google Scholar]
- 16.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20(4):666-673. doi: 10.1093/annonc/mdn717 [DOI] [PubMed] [Google Scholar]
- 17.Luo HY, Xu RH, Wang F, et al. Phase II trial of XELOX as first-line treatment for patients with advanced gastric cancer. Chemotherapy. 2010;56(2):94-100. doi: 10.1159/000305256 [DOI] [PubMed] [Google Scholar]
- 18.Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26(1):141-148. doi: 10.1093/annonc/mdu472 [DOI] [PubMed] [Google Scholar]
- 19.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4(2):213-226. doi: 10.1002/sim.4780040211 [DOI] [PubMed] [Google Scholar]
- 20.Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(2):167-192. doi: 10.6004/jnccn.2022.0008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Trial Protocol Amendments
Statistical Analysis Plan
eAppendix 1. History of the statistical analysis plan finalization, IDMC meeting, and interim efficacy analysis
eAppendix 2. Definition of measurable tumor lesion at baseline
eTable 1. Additional baseline disease characteristics
eTable 2. Subsequent anticancer therapy at interim efficacy analysis
eTable 3. Antitumour activity at interim efficacy analysis
eTable 4. Sensitivity analysis on overall survival at interim efficacy analysis
eTable 5. Sensitivity analysis on progression-free survival at interim efficacy analysis
eTable 6. Treatment-related adverse events leading to treatment discontinuation at interim efficacy analysis
eTable 7. Treatment-related serious adverse events at interim efficacy analysis
eTable 8. Treatment-related adverse events leading to death at interim efficacy analysis
eFigure 1. Kaplan-Meier plot of progression-free survival at interim efficacy analysis
eFigure 2. Kaplan-Meier curve of duration of confirmed response at the interim efficacy analysis
eFigure 3. Subgroup plot for subgroup analyses of overall survival in patients with CPS ≥5 at the interim efficacy analysis
eFigure 4. Subgroup plot for subgroup analyses of progression-free survival at the interim efficacy analysis
eFigure 5. Post hoc Kaplan-Meier plot of progression-free survival per investigator’s assessment in patients with PD-L1 combined positive score (CPS) <5 at the interim efficacy analysis
Nonauthor Collaborators. The ORIENT-16 Investigators
Data Sharing Statement