Abstract
Background:
Findings on the associations between prenatal PFAS exposures and offspring adiposity are inconsistent. Whether such associations may extend to adolescence is especially understudied.
Objectives:
We investigated associations of prenatal PFAS exposures with offspring adiposity and body composition at 16–20 years of age.
Methods:
We studied 545 mother–child pairs in the prospective prebirth cohort Project Viva (Boston, Massachusetts). We measured six PFAS (PFOA, PFOS, PFNA, PFHxS, EtFOSAA, and MeFOSAA) in maternal early pregnancy (, range: 5.7–19.6 wk) plasma samples. At the late adolescence visit ( y, range: 15.9–20.0 y), we obtained anthropometric measures and assessed body composition using bioelectrical impedance analysis and dual-energy X-ray absorptiometry. We examined associations of individual PFAS with obesity [i.e., age- and sex-specific body mass index (BMI) percentile] and adiposity and body composition using multivariable Poisson and linear regression models, respectively. We assessed PFAS mixture effects using Bayesian kernel machine regression (BKMR) and quantile g-computation. We used fractional-polynomial models to assess BMI trajectories (at 3–20 years of age) by prenatal PFAS levels.
Results:
Thirteen percent () of the children had obesity in late adolescence. After multivariable adjustment, higher prenatal PFAS concentrations were associated with higher obesity risk [e.g., 1.59 (95% CI: 1.19, 2.12), 1.24 (95% CI: 0.98, 1.57), and 1.49 (95% CI: 1.11, 1.99) times the obesity risk per doubling of PFOS, PFOA, and PFNA, respectively]. BKMR showed an interaction between PFOA and PFOS, where the positive association between PFOS and obesity was stronger when PFOA levels were lower. Each quartile increment of the PFAS mixture was associated with 1.52 (95% CI: 1.03, 2.25) times the obesity risk and 0.52 (95% CI: , 1.06) higher BMI. Children with higher prenatal PFOS, EtFOSAA, and MeFOSAA concentrations had higher rates of BMI increase starting from 9–11 years of age.
Discussion:
Prenatal PFAS exposures may have obesogenic effects into late adolescence. https://doi.org/10.1289/EHP12597
Introduction
Per- and polyfluoroalkyl substances (PFAS) are synthetic chemicals that are persistent in the environment and resistant to biodegradation.1 Long-chain PFAS, such as perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), perfluorononanoate (PFNA), and perfluorohexane sulfonate (PFHxS), can be detected in over 95% of the U.S. population owing to widespread exposure and long biological half-lives in the human body.2 PFAS are endocrine-disrupting chemicals that can alter hormones and other signaling molecules of the endocrine system,3 and studies have shown their potential obesogenic effects in adults.4 PFAS may also be an early life risk factor for childhood and adolescent obesity, the prevalence of which has nearly quadrupled in the past four decades in the United States and reached an all-time high of 19.7% in 2017–2020.5,6 Maternal PFAS can cross the placenta during pregnancy and lead to continuous fetal exposures,7 and these exposures can affect the metabolism, growth, and development of fetuses and may predispose individuals to long-term metabolic health.3,8,9 However, the extent to which prenatal PFAS exposures may influence offspring adiposity is unclear.
Previous studies investigating the associations between prenatal PFAS exposures and offspring adiposity have reported mixed results, with possible adverse, null, and even protective effects.10–28 Limitations of some studies included relying solely on anthropometric measures [e.g., body mass index (BMI), skinfold thickness, and waist circumference] without directly assessing body composition, having limited follow-up periods with measures collected only in childhood (i.e., before 12 years of age), or analyzing PFAS individually without accounting for the mixture effect. To our knowledge, no study has examined the associations of prenatal exposure to PFAS mixtures with both adiposity and body composition measures in late adolescence (i.e., 16- to 20-y olds). The National Academies of Sciences, Engineering, and Medicine recently concluded there is “inadequate or insufficient evidence” on the effects of PFAS on childhood and adolescent adiposity and suggested this as “an area worthy of future study.”29 In addition, many previous studies observed sex-specific effects of prenatal PFAS on offspring adiposity.11,13–15,23,24 Male and female children differ biologically in body composition (e.g., lean mass vs. fat mass), especially when going through puberty.30–32 Assessing body composition in late adolescence, when most individuals have completed puberty, may help improve our understanding of the sex-specific, intergenerational effects of PFAS.
To address these gaps, we used data from a prebirth cohort from eastern Massachusetts to examine the prospective associations of prenatal PFAS exposures with adiposity and body composition at 16–20 years of age. We used Bayesian kernel machine regression (BKMR) and quantile g-computation to investigate PFAS mixture effects. We hypothesized that a) prenatal PFAS exposures, both individually and as mixtures, were associated with higher adiposity measures in late adolescence; and b) the associations differed by child sex and types of adiposity and body composition measures. In a post hoc analysis, we also examined how prenatal PFAS exposures may affect BMI trajectories from early childhood to late adolescence.
Methods
Study Design and Population
Participants were from Project Viva, a prospective prebirth cohort. The detailed methods for recruitment and follow-up have been provided previously in the cohort profile.15 From April 1999 to July 2002, we enrolled pregnant women seen for prenatal care at eight eastern Massachusetts urban and suburban practices of Atrius Harvard Vanguard Medical Associates. Women were excluded from enrollment if they had multiple gestations or gestational at enrollment, were unable to answer questions in English, or planned to move away from the study area prior to delivery.
Of the 2,128 mother–child pairs in Project Viva, 555 pairs had data on maternal early pregnancy PFAS and child obesity at the late adolescence visit. After excluding 7 children whose older siblings were also in this study and 3 with missing covariates, 545 pairs were included in the main analysis (Figure S1; age at the late adolescence visit: y; range: 15.9–20.0 y). In a post hoc analysis of BMI trajectories, we included 1,156 children who had data on prenatal PFAS and BMI measures from at least one follow-up visit since early childhood [ for early childhood (age range: 2.9–6.1 y), 876 for mid-childhood (age range: 6.6–10.9 y), 829 for early adolescence (age range: 11.9–16.6 y), and 558 for late adolescence (age range: 15.4–20.0 y)].
Institutional review boards of participating institutions approved the study protocols. At enrollment, we obtained written informed consent from pregnant women. At each follow-up visit, we obtained written informed consent from mothers and either assent (if ) or signed consent (if ) from their participating child.
Exposures: Prenatal PFAS
We collected maternal plasma samples at study enrollment ( gestation; range: 5.7–19.6 wk gestation) and stored them in PFAS-free cryovial tubes in liquid nitrogen freezers. We sent aliquots to the Division of Laboratory Sciences at the Centers for Disease Control and Prevention (CDC) for analysis. Detailed analytic methods have been previously described.33 This analysis included six PFAS that were detected in of samples: a) PFOA, b) PFOS, c) PFNA, d) PFHxS, e) 2-(-ethyl-perfluorooctane sulfonamido) acetate (EtFOSAA), and f) 2-(-methyl-perfluorooctane sulfonamido) acetate (MeFOSAA). Perfluorooctane sulfonamide (PFOSA; 10% detected) and perfluorodecanoate (PFDA; 45% detected) were excluded owing to their low detection frequencies. We imputed values below the limit of detection (LOD) as the LOD divided by the square root of 2. Table 1 shows the raw distribution, the LOD, and the number of samples below the LOD for each included PFAS.
Table 1.
Distributions of prenatal PFAS levels, LOD for each PFAS, and number (%) of samples below LOD for Project Viva participants included in this analysis ().
| PFAS (ng/mL) | Minimum | Percentile | Maximum | LOD (ng/mL) | Samples [ (%)] | ||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | |||||
| PFOS | 5.30 | 18.00 | 24.70 | 33.80 | 134.00 | 0.20 | 0 (0.0) |
| PFOA | 0.80 | 3.90 | 5.40 | 7.60 | 49.30 | 0.10 | 0 (0.0) |
| PFHxS | 0.07 | 1.50 | 2.30 | 3.60 | 43.20 | 0.10 | 6 (1.1) |
| PFNA | 0.07 | 0.50 | 0.70 | 0.90 | 3.40 | 0.10 | 7 (1.3) |
| EtFOSAA | 0.07 | 0.70 | 1.10 | 1.80 | 19.40 | 0.10 | 3 (0.6) |
| MeFOSAA | 0.10 | 1.20 | 1.90 | 2.90 | 29.70 | 0.10 | 0 (0.0) |
Note: Samples were collected between April 1999 and July 2002. We imputed PFAS concentrations below the LOD with the LOD divided by the square root of 2. Distributions of PFAS levels in this table are after imputation and before -transformation. Distributions of PFAS levels after -transformation are provided in Table S2. Histograms that show the distributions of these PFAS before and after -transformation are provided in Figure S2. EtFOSAA, 2-(-ethyl-perfluorooctane sulfonamido) acetate; LOD, limit of detection; MeFOSAA, 2-(-methyl-perfluorooctane sulfonamido) acetate; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
Outcomes: Adiposity and Body Composition Measures in Late Adolescence
During the late adolescence visit (conducted between July 2017 and September 2021), trained research assistants collected adiposity and body composition measures. We measured weight and bioimpedance using a Tanita Scale (TBF-300A; Tanita), height using a stadiometer (Shorr Height Board; Shorr Productions), waist and hip circumferences using a Gulick II measuring tape (Performance Health), and subscapular and triceps skinfold thicknesses using the Holtain Caliper (Holtain). We also conducted 3-min open-air whole-body dual-energy X-ray absorptiometry (DXA) scans in a subset of participants using Hologic model Discovery A (Hologic). Participants who could not attend in-person visits at our study site, met exclusion criteria (e.g., exceeding 450 lb (), being pregnant, or having specific contraindications), or refused the DXA scan did not receive a DXA scan. Of the 545 children included in our analysis, 439 received DXA scans (Figure S1). Bioelectrical impedance analysis (BIA) and DXA both provided data on percentage fat, fat mass, and lean mass, and DXA additionally provided data on trunk fat mass. Methods for obtaining consistent and accurate measures have been previously published.34
We calculated BMI as weight (in kilograms) divided by height (in meters) squared, calculated age- and sex-specific BMI percentiles and -scores using the CDC Growth Charts,35 and defined obesity as percentile (reference: percentile). We calculated BIA and DXA fat and lean mass indices as fat mass (in kilograms) and lean mass (in kilograms) divided by height (in meters) squared, and DXA trunk fat mass index as trunk fat mass (in kilograms) divided by height (in meters) squared. We derived the ratio of waist-to-hip circumferences, the sum of subscapular and triceps skinfold thicknesses, and the ratio of subscapular-to-triceps skinfold thicknesses, consistent with our prior work and other studies of child adiposity.36–38
We classified measures into four domains: a) obesity; b) overall adiposity, including BMI, BMI -score, sum of subscapular and triceps skinfold thicknesses, percentage fat, and fat mass indices; c) central adiposity, including waist circumference, waist-to-hip circumference ratio, subscapular-to-triceps skinfold thickness ratio, and trunk fat mass index; and d) lean mass, including lean mass indices.
Covariates
On interviews and questionnaires at enrollment, mothers self-reported their date of birth (which we used to derive age), race and ethnicity, prepregnancy weight and height, educational level, marital status, household income, pregnancy smoking status, and parity. We calculated maternal prepregnancy BMI as self-reported weight (in kilograms) divided by measured height (in meters) squared and classified BMI as normal or underweight (), overweight (25 to ), and obesity ().
We derived the Dietary Approaches to Stop Hypertension (DASH) diet scores39 based on self-reported dietary intake from the food frequency questionnaire administered at enrollment.40 We measured maternal albumin levels using plasma samples collected at enrollment (i.e., the same early pregnancy samples used to measure prenatal PFAS levels). We defined urbanicity as the proportion of urban land use within a radius of the mother’s residential address during pregnancy, using nationwide data from the Multi-Resolution Land Characteristics Consortium 2001 National Land Cover Dataset.41 We calculated the proportion of urban land use based on methods developed by Yanosky et al.42 and derived urbanicity scores.
Statistical Analyses
We separately examined the distributions and Spearman correlation coefficients of both prenatal PFAS concentrations and late adolescence adiposity and body composition measures. We compared maternal and child characteristics by child obesity status (yes vs. no) at the late adolescence visit, as well as PFAS levels by select maternal and child characteristics. We used linear regression and Poisson regression models with robust variance estimates43 to examine associations of prenatal PFAS with continuous adiposity measures and obesity risk, respectively. Given that PFAS levels were heavily right-skewed (Figure S2, upper panels), following the recommendations by Choi et al.,44 we explored analytic models, including linear, -transformed, and quadratic forms, and compared their Akaike information criterion (AIC) (Table S1). Based on the AIC results and to improve model interpretability (i.e., changes in the outcomes per doubling of PFAS levels), we -transformed the PFAS concentrations (Figure S2, lower panels) for subsequent analyses. The distributions of each -transformed PFAS can be found in Table S2.
We used BKMR45,46 and quantile g-computation47 to examine the mixture effects of PFAS on selected late adolescence adiposity (i.e., BMI and obesity risk) and body composition measures (i.e., DXA measures). We chose to use both methods based on their statistical capabilities and interpretations. BKMR allows for the estimation of complex exposure–response relationships and interactions between exposures,45 whereas quantile g-computation estimates the parameters of a joint, marginal structural model that quantifies the average effects of simultaneously changing all exposures.47
Specifically, we used BKMR to estimate45,46: a) associations of each PFAS with the outcomes, while holding all other five PFAS at their 50th percentile; b) associations of pairs of PFAS with the outcomes, while holding all other four PFAS at their 50th percentile; and c) overall effects of PFAS mixtures on the outcomes, by estimating the differences in the outcomes when all six PFAS levels simultaneously change from the 10th to 90th percentile (in 10-percentile point increments) in comparison with when all six PFAS are held at their 50th percentile. We used probit BKMR for the binary outcome of obesity risk. We fitted four parallel Markov chain Monte Carlo (MCMC) chains using the “kmbayes_parallel” function in the R package “bkmrhat” (version 1.1.3).48 Each chain comprised 25,000 iterations, including 12,500 burn-in iterations, and we enabled component-wise variable selection. We investigated model convergence by inspecting the trace plots, autocorrelation plots, density plots, and the Gelman–Rubin convergence statistics. All estimated parameters exhibited good convergence across the MCMC chains. We estimated the posterior inclusion probabilities (PIPs) to assess the relative importance of individual PFAS exposures in the overall mixture effects.49 Although no well-defined PIP threshold exists, prior research often considers a as indicative of importance.50,51
We used quantile g-computation to assess the impact of increasing all six PFAS levels by one quartile on the outcomes.47 This method produces a fixed positive or negative weight for each exposure based on the direction of the independent effect. Positive and negative weights, respectively, add up to 1, representing the proportion of the overall effect and the relative importance of each mixture component within each direction. In figures showing positive and negative weights, the shading of bars represents the overall effect size within each direction, with darker shades indicating a larger effect compared with lighter shades. We used linear models for the continuous outcomes and logistic models for the binary outcome of obesity risk in the quantile g-computation analyses.
We defined confounders as covariates associated with the exposures and the outcomes but not on the potential pathway based on a priori knowledge. We included the following covariates in the regression models and mixture analyses: maternal age at enrollment (continuous), self-reported race and ethnicity [non-Hispanic white; non-Hispanic black; others (including Hispanic or Latina/o, Asian or Pacific Islander, American Indian or Alaskan Native, other race, do not know, or more than one race), which were grouped together owing to their small sample sizes], prepregnancy BMI (normal or underweight; overweight; obesity), educational level (college graduate: yes; no), marital status (married or cohabiting: yes; no), parity (nulliparous: yes; no), and pregnancy smoking status (never smoker; former smoker; smoked during pregnancy). Figure S3 provides the directed acyclic graph (DAG) that outlines the hypothesized relationships between the covariates in this analysis. We included maternal education level and marital status as indicators for socioeconomic status (SES), given that previous studies have consistently linked SES with PFAS exposures52,53 and parental SES with childhood adiposity54,55; we did not include household income in the analysis owing to missingness of this variable (; Table 2). We considered maternal race and ethnicity as a potential confounder owing to structural and social factors leading to differences in maternal PFAS exposure levels and childhood and adolescence obesity prevalence among different racial and ethnic groups.56–58 We conducted stratified analyses to examine effect modification by child sex (male vs. female). We included product terms (i.e., ) in the multivariable-adjusted regression models to derive -values for interaction and considered a two-sided as potential effect modification.
Table 2.
Characteristics of Project Viva mothers and children included in this analysis ().
| Variables, categorical and continuous [ (%) or median (IQR)]a | Overall | Children with obesity at the late adolescence visitb | |
|---|---|---|---|
| No | Yes | ||
| 545 | 472 (87) | 73 (13) | |
| Maternal characteristics | |||
| Age at enrollment (y) | 32.7 (29.7–35.9) | 32.9 (29.8–36.0) | 31.4 (26.4–34.6) |
| Race and ethnicity | |||
| Non-Hispanic white | 379 (70) | 348 (74) | 31 (42) |
| Non-Hispanic black | 75 (14) | 58 (12) | 17 (23) |
| Others | 91 (17) | 66 (14) | 25 (34) |
| Prepregnancy BMI category | |||
| Normal or underweight () | 343 (63) | 326 (69) | 17 (23) |
| Overweight (25 to ) | 128 (23) | 98 (21) | 30 (41) |
| Obesity () | 74 (14) | 48 (10) | 26 (36) |
| College graduate | |||
| No | 144 (26) | 106 (22) | 38 (52) |
| Yes | 401 (74) | 366 (78) | 35 (48) |
| Married or cohabiting | |||
| No | 46 (8) | 29 (6) | 17 (23) |
| Yes | 499 (92) | 443 (94) | 56 (77) |
| Household income | |||
| No | 178 (35) | 144 (33) | 34 (54) |
| Yes | 324 (65) | 295 (67) | 29 (46) |
| Missing | 43 | 33 | 10 |
| Pregnancy smoking status | |||
| Never smoker | 392 (72) | 344 (73) | 48 (66) |
| Former smoker | 98 (18) | 88 (19) | 10 (14) |
| Smoked during pregnancy | 55 (10) | 40 (8) | 15 (21) |
| Nulliparous | |||
| No | 278 (51) | 236 (50) | 42 (58) |
| Yes | 267 (49) | 236 (50) | 31 (42) |
| Early pregnancy albumin (g/dL) | 6.0 (5.1–6.9) | 6.0 (5.1–6.9) | 5.8 (5.1–6.6) |
| Missing | 20 | 15 | 5 |
| Early pregnancy DASH diet score | 24.0 (21.0–28.0) | 25.0 (21.0–29.0) | 22.0 (18.0–25.0) |
| Missing | 23 | 16 | 7 |
| Urbanicity score | |||
| Q 1 (5.31–624.8) | 108 (20) | 98 (21) | 10 (14) |
| Q 2 (626.0–856.6) | 108 (20) | 99 (21) | 9 (13) |
| Q 3 (859.9–998.4) | 107 (20) | 90 (19) | 17 (24) |
| Q 4 (999.4–1,075.4) | 106 (20) | 90 (19) | 16 (22) |
| Q 5 (1,075.8–1,111.1) | 108 (20) | 88 (19) | 20 (28) |
| Missing | 8 | 7 | 1 |
| PFAS concentration (ng/mL) | |||
| PFOS | 24.7 (18.0–33.8) | 23.8 (17.6–32.6) | 29.9 (21.7–37.2) |
| PFOA | 5.4 (3.9–7.6) | 5.3 (3.7–7.6) | 5.7 (4.6–7.6) |
| PFHxS | 2.3 (1.5–3.6) | 2.3 (1.5–3.5) | 2.4 (1.8–3.6) |
| PFNA | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) | 0.7 (0.5–1.0) |
| EtFOSAA | 1.1 (0.7–1.8) | 1.0 (0.7–1.7) | 1.4 (0.8–2.2) |
| MeFOSAA | 1.9 (1.2–2.9) | 1.8 (1.2–3.0) | 2.1 (1.6–2.8) |
| Child characteristics | |||
| Sex | |||
| Female | 273 (50) | 240 (51) | 33 (45) |
| Male | 272 (50) | 232 (49) | 40 (55) |
| Birth weight (g) | 3,543.0 (3,203.0–3,883.0) | 3,543.0 (3,203.0–3,883.0) | 3,487.0 (3,203.0–3,855.0) |
| Gestational age (wk) | 39.9 (38.9–40.6) | 39.9 (38.9–40.6) | 39.9 (39.0–40.7) |
| BMI category at the late adolescence visitb | |||
| Normal or underweight | 403 (74) | 403 (85) | 0 (0) |
| Overweight | 69 (13) | 69 (15) | 0 (0) |
| Obesity | 73 (13) | 0 (0) | 73 (100) |
| Age at the late adolescence visit (y) | 17.4 (17.2–17.9) | 17.5 (17.2–17.9) | 17.3 (17.2–17.8) |
Note: Pregnant women were enrolled between April 1999 and July 2002, and the late adolescence visit was conducted between July 2017 and September 2021. Participants with missing values for maternal race, ethnicity, college graduate, or pregnancy smoking status were excluded from the analyses (Figure S1). BMI, body mass index; DASH, Dietary Approaches to Stop Hypertension; EtFOSAA, 2-(-ethyl-perfluorooctane sulfonamido) acetate; IQR, interquartile range; MeFOSAA, 2-(-methyl-perfluorooctane sulfonamido) acetate; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; Q, quintile.
Percentages for categorical variables may not add up to 100% owing to rounding. For categorical variables with missing values, missing values were not included in the denominators when deriving percentages for known values.
Obesity was defined as percentile for age and sex based on the Centers for Disease Control and Prevention Growth Charts. Overweight was defined as and percentile. Normal or underweight was defined as percentile.
We observed an association between prenatal PFAS exposures and late adolescence BMI. In our previous study, however, prenatal PFAS exposures were associated with mid-childhood ( y) BMI in female children, but not with early childhood ( y) BMI.15 We thus hypothesized that the effects of prenatal PFAS on BMI may strengthen as the child grows older and performed a post hoc analysis to model BMI trajectories. We categorized each prenatal PFAS into higher vs. lower levels by median split and used fractional-polynomial prediction plots to model BMI trajectories from early childhood to late adolescence (overall and stratified by sex) by prenatal PFAS levels.
We performed sensitivity analyses to examine the robustness of our findings. First, we excluded maternal prepregnancy BMI from the multivariable-adjusted models because it may be on the pathway between prenatal PFAS and child adiposity/body composition given the years-long half-life of long-chain PFAS in blood.59 Second, albumin serves as a biomarker for pregnancy plasma volume expansion60 and is the primary binding site for plasma PFAS.61,62 Considering that pregnancy plasma volume may confound the associations between maternal PFAS levels and child health outcomes,63,64 we additionally adjusted for maternal plasma albumin to account for the impacts of hemodynamics. Third, we additionally adjusted for maternal early pregnancy DASH diet score to examine the potential impact of diet quality on the associations. The DASH diet promotes the consumption of fruits, vegetables, whole grains, and low-fat dairy, while reducing the intake of foods high in saturated fats, cholesterol, and sodium, and limiting sugar-sweetened beverages and sweets.65 Previous research has shown that the DASH diet is associated with lower PFAS concentrations in adults,66 and better maternal adherence to the DASH diet during pregnancy is associated with lower risk of overweight and obesity in late-childhood.67 Fourth, we additionally adjusted for urbanicity score, given that participants resided in both urban and suburban communities and because residence location may be a potential confounder associated with both PFAS exposures68 and child adiposity.69,70 We categorized urbanicity scores into quintiles, consistent with our previous study.71 Finally, because mothers included in this analysis showed different characteristics than those excluded, we used stabilized inverse probability weighting to address the potential impact of selection bias on the associations.72 The covariates used to derive the predicted probability weights included all the covariates in Table S3. We used multiple imputation by chained equations (10 imputations and each with 10 iterations) to impute missing covariates in the weight prediction models.73
We used R (version 4.2.1; R Development Core Team) packages “bkmrhat” (version 1.1.3)46,48 for BKMR and “gqcomp” (version 2.9.0)47 for quantile g-computation analyses. We created the DAG using DAGitty (dagitty.net).74 We conducted all other analyses using Stata (version 17.0; StataCorp).
Results
Descriptive Results
Table 2 provides the characteristics of the mother–child pairs included in this analysis. Of the mothers, 70% () identified as non-Hispanic white and 14% () as non-Hispanic black, 37% () had overweight or obesity before pregnancy, 74% () graduated from college, and 49% () were nulliparous. The median [interquartile range (IQR)] age at the late adolescence visit was 17.4 (17.2–17.9) y. Children with obesity (13%; ), compared with those without obesity (87%; ), had mothers who were younger and had lower educational levels, household income, and early pregnancy DASH diet scores. These mothers were also less likely to be non-Hispanic white, married or cohabiting, and nulliparous and more likely to have prepregnancy overweight or obesity, smoked during pregnancy, and lived in areas with higher urbanicity scores. Most prenatal PFAS (except PFNA) levels were higher in children with obesity. PFAS levels were higher in mothers who were younger or did not graduate from college; there were no consistent differences in PFAS levels by other maternal and child characteristics (Table S4). Most maternal and child characteristics were comparable between pairs included in the main analysis () vs. excluded (), except that included mothers were more likely to be college graduates and have a household income of (Table S3).
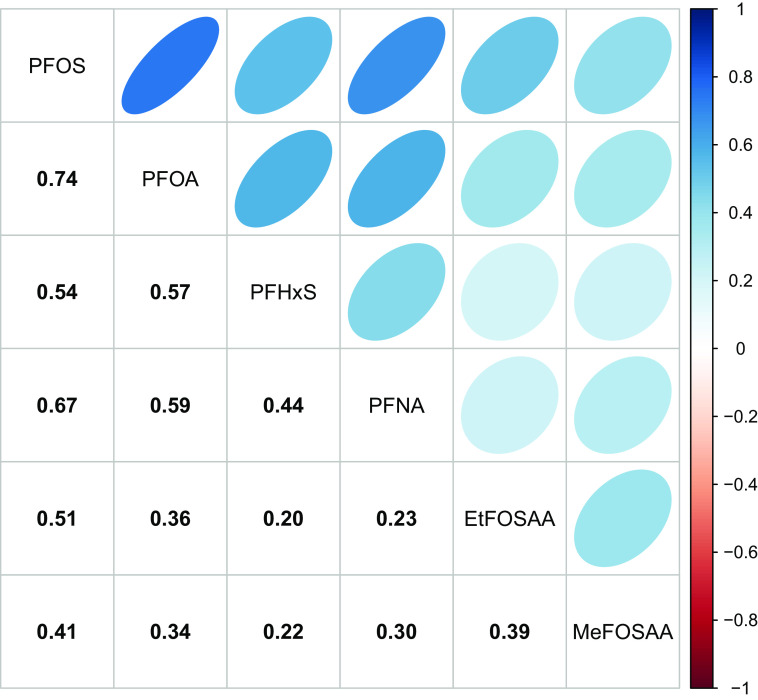
Prenatal PFAS were moderately to strongly correlated, with correlation coefficients () ranging from 0.20 (PFHxS and EtFOSAA) to 0.74 (PFOS and PFOA) (Figure 1). Late adolescence adiposity and body composition measures showed different degrees of correlation (Figure S4). Table S5 provides the distributions and sample size of each adiposity and body composition measure overall and by child sex. Female adolescents had higher percentage fat, fat mass index, and trunk fat mass index, whereas male adolescents had higher lean mass index.
Figure 1.
Spearman correlation matrix for PFAS measured in maternal plasma samples collected in early pregnancy (). Color intensity is proportional to the correlation coefficients. Samples were collected between April 1999 and July 2002. PFAS concentrations below the LOD (provided in Table 1) were imputed using the LOD divided by the square root of 2. Note: EtFOSAA, 2-(-ethyl-perfluorooctane sulfonamido) acetate; LOD, limit of detection; MeFOSAA, 2-(-methyl-perfluorooctane sulfonamido) acetate; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
Single Pollutant Models: Individual Prenatal PFAS
Table 3 shows the associations of individual prenatal PFAS with adiposity measures. In general, PFOS and EtFOSAA showed stronger associations (based on effect sizes) with adiposity measures than other PFAS. In the unadjusted models, both PFOS and EtFOSAA were associated with multiple adiposity outcomes across all domains (i.e., obesity, overall adiposity, central adiposity, and lean mass). However, associations were attenuated after multivariable adjustment. After adjustment, each doubling of PFOS was associated with a 1.59 [95% confidence interval (CI): 1.19, 2.12] times the risk of obesity (Figure 2A), 0.74 (95% CI: 0.15, 1.33) higher BMI (Figure S5A), 0.13 (95% CI: 0.01, 0.25) higher BMI -score, and 0.36 (95% CI: 0.05, 0.66) higher BIA lean mass index. EtFOSAA was not associated with any adiposity measure after adjustment.
Table 3.
Associations (estimates and 95% confidence intervals) of individual prenatal PFAS levels with adiposity and body composition measures in late adolescence in Project Viva.
| Adiposity and body composition measures (units) | Models | PFOS | PFOA | PFHxS | PFNA | EtFOSAA | MeFOSAA |
|---|---|---|---|---|---|---|---|
| Obesity | |||||||
| Obesitya | Unadjusted | 1.59 (1.23, 2.05) | 1.26 (1.00, 1.58) | 1.07 (0.88, 1.30) | 1.19 (0.90, 1.58) | 1.27 (1.06, 1.51) | 1.15 (0.94, 1.41) |
| Adjustedb | 1.59 (1.19, 2.12) | 1.24 (0.98, 1.57) | 1.14 (0.93, 1.40) | 1.49 (1.11, 1.99) | 1.16 (0.97, 1.39) | 1.13 (0.90, 1.42) | |
| Overall adiposity | |||||||
| BMI () | Unadjusted | 1.05 (0.42, 1.68) | 0.44 (, 1.05) | 0.13 (, 0.54) | 0.12 (, 0.69) | 0.66 (0.24, 1.07) | 0.38 (, 0.86) |
| Adjustedb | 0.74 (0.15, 1.33) | 0.31 (, 0.90) | 0.25 (, 0.62) | 0.43 (, 0.96) | 0.38 (, 0.77) | 0.20 (, 0.64) | |
| BMI -score | Unadjusted | 0.19 (0.06, 0.31) | 0.07 (, 0.19) | 0.04 (, 0.12) | 0.01 (, 0.12) | 0.13 (0.04, 0.21) | 0.09 (, 0.19) |
| Adjustedb | 0.13 (0.01, 0.25) | 0.03 (, 0.15) | 0.05 (, 0.13) | 0.06 (, 0.17) | 0.08 (, 0.16) | 0.06 (, 0.16) | |
| Sum of subscapular and triceps skinfold thicknesses (mm) | Unadjusted | 1.84 (, 3.74) | 0.73 (, 2.55) | (, 0.98) | 0.43 (, 2.14) | 1.67 (0.41, 2.92) | 0.59 (, 2.04) |
| Adjustedb | 1.31 (, 3.22) | 0.51 (, 2.38) | 0.12 (, 1.29) | 0.86 (, 2.55) | 1.17 (, 2.41) | 0.41 (, 1.82) | |
| BIA percentage fat | Unadjusted | 1.18 (, 2.47) | 0.67 (, 1.90) | (, 0.63) | (, 0.91) | 1.08 (0.23, 1.94) | 0.34 (, 1.32) |
| Adjustedb | 0.72 (, 2.01) | 0.48 (, 1.76) | 0.00 (, 0.80) | 0.16 (, 1.31) | 0.68 (, 1.53) | 0.08 (, 1.03) | |
| DXA percentage fat | Unadjusted | 0.85 (, 2.01) | 0.38 (, 1.49) | (, 0.24) | (, 0.98) | 0.88 (0.11, 1.65) | 0.08 (, 0.97) |
| Adjustedb | 0.48 (, 1.66) | 0.12 (, 1.28) | (, 0.32) | 0.26 (, 1.29) | 0.59 (, 1.37) | (, 0.81) | |
| BIA fat mass index () | Unadjusted | 0.63 (0.14, 1.12) | 0.32 (, 0.79) | (, 0.29) | (, 0.42) | 0.47 (0.14, 0.79) | 0.16 (, 0.53) |
| Adjustedb | 0.41 (, 0.88) | 0.25 (, 0.71) | 0.08 (, 0.37) | 0.18 (, 0.59) | 0.28 (, 0.59) | 0.03 (, 0.38) | |
| DXA fat mass index () | Unadjusted | 0.61 (0.10, 1.11) | 0.23 (, 0.72) | (, 0.19) | 0.00 (, 0.45) | 0.48 (0.14, 0.81) | 0.15 (, 0.53) |
| Adjustedb | 0.34 (, 0.83) | 0.08 (, 0.56) | (, 0.24) | 0.19 (, 0.62) | 0.29 (, 0.61) | 0.04 (, 0.40) | |
| Central adiposity | |||||||
| Waist circumference (cm) | Unadjusted | 1.80 (0.26, 3.34) | 0.58 (, 2.06) | 0.16 (, 1.13) | (, 0.88) | 1.37 (0.35, 2.39) | 0.42 (, 1.60) |
| Adjustedb | 1.09 (, 2.59) | (, 1.48) | 0.25 (, 1.18) | 0.04 (, 1.38) | 0.82 (, 1.80) | 0.08 (, 1.20) | |
| Waist-to-hip circumference ratio () | Unadjusted | 0.62 (, 1.38) | 0.41 (, 1.14) | (, 0.44) | (, 0.31) | 0.54 (0.03, 1.04) | 0.34 (, 0.92) |
| Adjustedb | 0.31 (, 1.08) | 0.03 (, 0.79) | (, 0.33) | (, 0.48) | 0.34 (, 0.84) | 0.23 (, 0.80) | |
| Subscapular-to-triceps skinfold thickness ratio () | Unadjusted | 4.08 (0.61, 7.54) | 3.53 (0.24, 6.81) | 0.73 (, 2.91) | 1.88 (, 4.99) | 2.58 (0.30, 4.87) | (, 2.41) |
| Adjustedb | 2.71 (, 6.25) | 3.23 (, 6.70) | 0.73 (, 2.92) | 1.72 (, 4.88) | 1.69 (, 4.00) | (, 1.95) | |
| DXA trunk fat mass index () | Unadjusted | 0.33 (0.07, 0.59) | 0.17 (, 0.43) | (, 0.12) | 0.03 (, 0.26) | 0.24 (0.07, 0.42) | 0.07 (, 0.27) |
| Adjustedb | 0.19 (, 0.44) | 0.07 (, 0.32) | (, 0.13) | 0.12 (, 0.34) | 0.15 (, 0.31) | 0.01 (, 0.20) | |
| Lean mass | |||||||
| BIA lean mass index () | Unadjusted | 0.46 (0.15, 0.77) | 0.13 (, 0.43) | 0.15 (, 0.35) | 0.16 (, 0.43) | 0.21 (0.01, 0.42) | 0.23 (, 0.46) |
| Adjustedb | 0.36 (0.05, 0.66) | 0.08 (, 0.38) | 0.17 (, 0.36) | 0.26 (, 0.53) | 0.12 (, 0.32) | 0.18 (, 0.40) | |
| DXA lean mass index () | Unadjusted | 0.45 (0.09, 0.80) | 0.10 (, 0.44) | 0.16 (, 0.37) | 0.01 (, 0.32) | 0.23 (, 0.47) | 0.27 (0.00, 0.55) |
| Adjustedb | 0.25 (, 0.61) | (, 0.33) | 0.19 (, 0.40) | 0.10 (, 0.40) | 0.11 (, 0.34) | 0.20 (, 0.46) | |
Note: Pregnant women were enrolled between April 1999 and July 2002, and the late adolescence visit was conducted between July 2017 and September 2021. Estimates show differences in continuous values of adiposity and body composition measures or relative risks of obesity (yes vs. no) per doubling of PFAS levels ( to 545). for obesity, 545 for BMI, 545 for BMI -score, 534 for sum of subscapular and triceps skinfold thicknesses, 544 for BIA percentage fat, 439 for DXA percentage fat, 543 for BIA fat mass index, 439 for DXA fat mass index, 544 for waist circumference, 544 for waist-to-hip circumference ratio, 534 for subscapular-to-triceps skinfold thickness ratio, 439 for DXA trunk fat mass index, 543 for BIA lean mass index, and 439 DXA lean mass index. BIA, bioelectrical impedance analysis; BMI, body mass index; DXA, dual-energy X-ray absorptiometry; EtFOSAA, 2-(-ethyl-perfluorooctane sulfonamido) acetate; MeFOSAA, 2-(-methyl-perfluorooctane sulfonamido) acetate; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
Obesity was defined as percentile for age and sex based on the Centers for Disease Control and Prevention Growth Charts (reference is percentile).
Models were adjusted for maternal age at enrollment, race and ethnicity, prepregnancy BMI, educational level, marital status, parity, and pregnancy smoking status.
Figure 2.
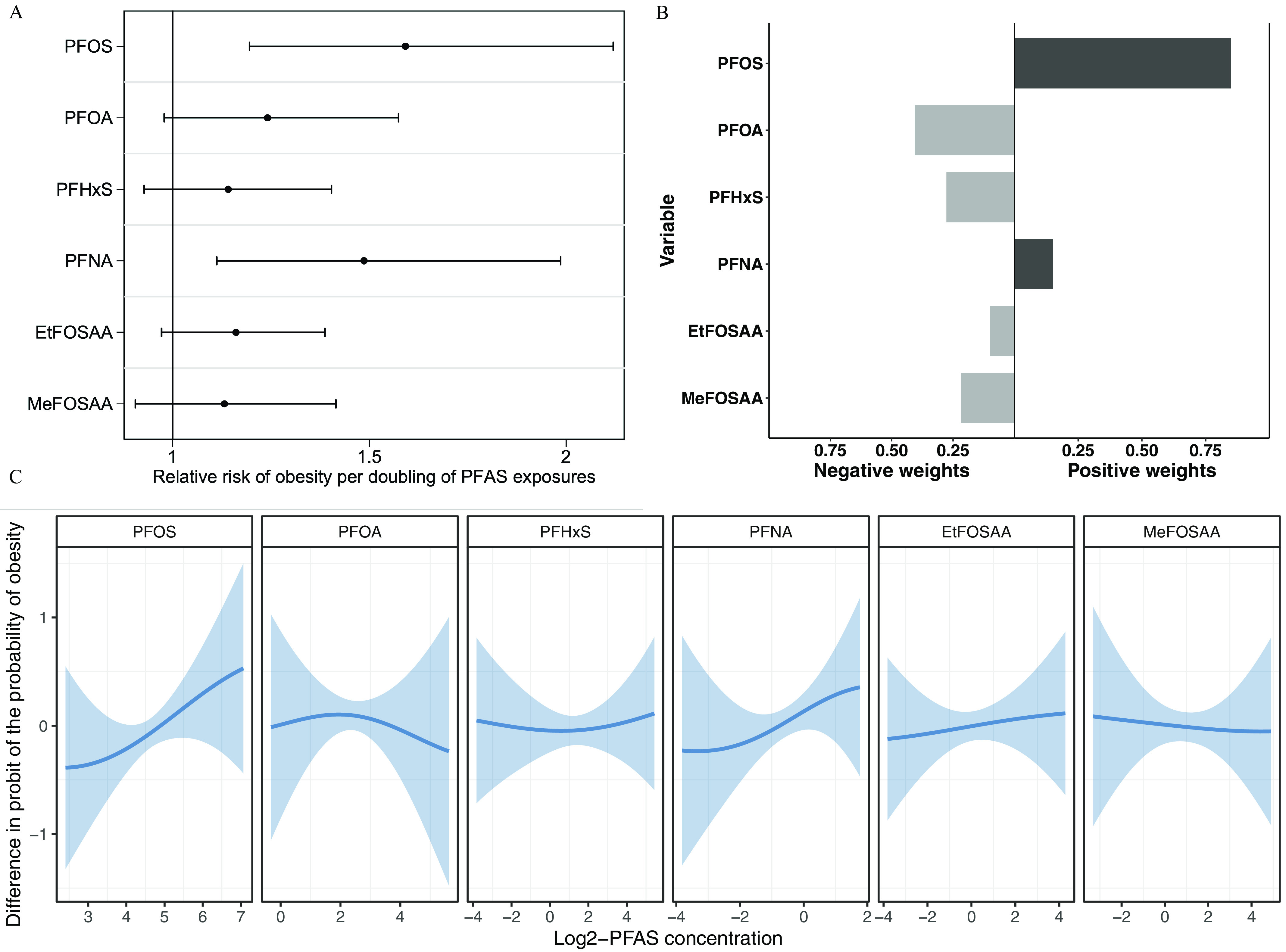
Multivariable-adjusted associations between individual prenatal PFAS and obesity in late adolescence estimated using (A) Poisson regression with robust variance estimates, (B) quantile g-computation, and (C) Bayesian kernel machine regression (). (A) shows the relative risks and 95% confidence intervals of obesity per doubling of individual PFAS levels [estimates are provided in Table 3 (adjusted)]. (B) shows the weights for each PFAS that correspond to the proportion of the overall effect of all PFAS on obesity in the positive or negative direction (positive and negative weights, respectively, add up to 1, representing the proportion of the overall effect and the relative importance of each mixture component within each direction; the shading of bars represents the overall effect size within each direction, with darker shades indicating a larger effect compared with lighter shades; estimates are provided in Table S8). (C) shows the estimates and 95% credible intervals of PFAS with obesity risk when all other PFAS are fixed at their 50th percentile (percentile values for -transformed PFAS levels are provided in Table S2). Obesity was defined as percentile for age and sex based on the Centers for Disease Control and Prevention Growth Charts (reference is percentile). Participants were from the Project Viva prebirth cohort. Pregnant women were enrolled between April 1999 and July 2002, and the late adolescence visit was conducted between July 2017 and September 2021. Models were adjusted for maternal age at enrollment, race and ethnicity, prepregnancy BMI, educational level, marital status, parity, and pregnancy smoking status. Note: BMI, body mass index; EtFOSAA, 2-(-ethyl-perfluorooctane sulfonamido) acetate; MeFOSAA, 2-(-methyl-perfluorooctane sulfonamido) acetate; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
The other four PFAS did not show consistent associations with adiposity measures. For PFOA: the associations between PFOA and adiposity measures were all positive. PFOA was associated with higher subscapular-to-triceps skinfold thickness ratio in the unadjusted models, but this association disappeared after multivariable adjustment. For PFHxS: PFHxS was not associated with any adiposity measures, and multiple effect estimates were even negative; however, it showed positive associations with lean mass measures. For PFNA: PFNA was not associated with any adiposity measures in the adjusted models; however, unlike the other four PFAS, most associations of PFNA with adiposity measures were strengthened after multivariable adjustment, including qualitative changes in associations with BIA and DXA percentage fat, BIA fat mass index, and waist circumference. After adjustment, each doubling of PFNA was associated with a 1.49 (95% CI: 1.11, 1.99) times the risk of obesity (Figure 2A). For MeFOSAA: MeFOSAA was associated with higher DXA lean mass index in the unadjusted models, but not after multivariable adjustment.
Mixture Analyses: Overall Prenatal PFAS Mixtures
As the PFAS mixture increased from the 10th to the 90th percentile, obesity risk and BMI monotonically increased (Figure 3; Table S6). PFOS () was the most important contributor to the overall mixture effect on obesity risk, followed by PFNA (), PFOA (), and other PFAS (). PFOS was also the most important contributor to the overall mixture effect on BMI (), followed by PFOA () and other PFAS (all ) (Table S7). Using quantile g-computation, for each quartile increment of the PFAS mixture, obesity risk was 1.52 (95% CI: 1.03, 2.25) times higher, and BMI was 0.52 (95% CI: , 1.06) higher (Table S8).
Figure 3.
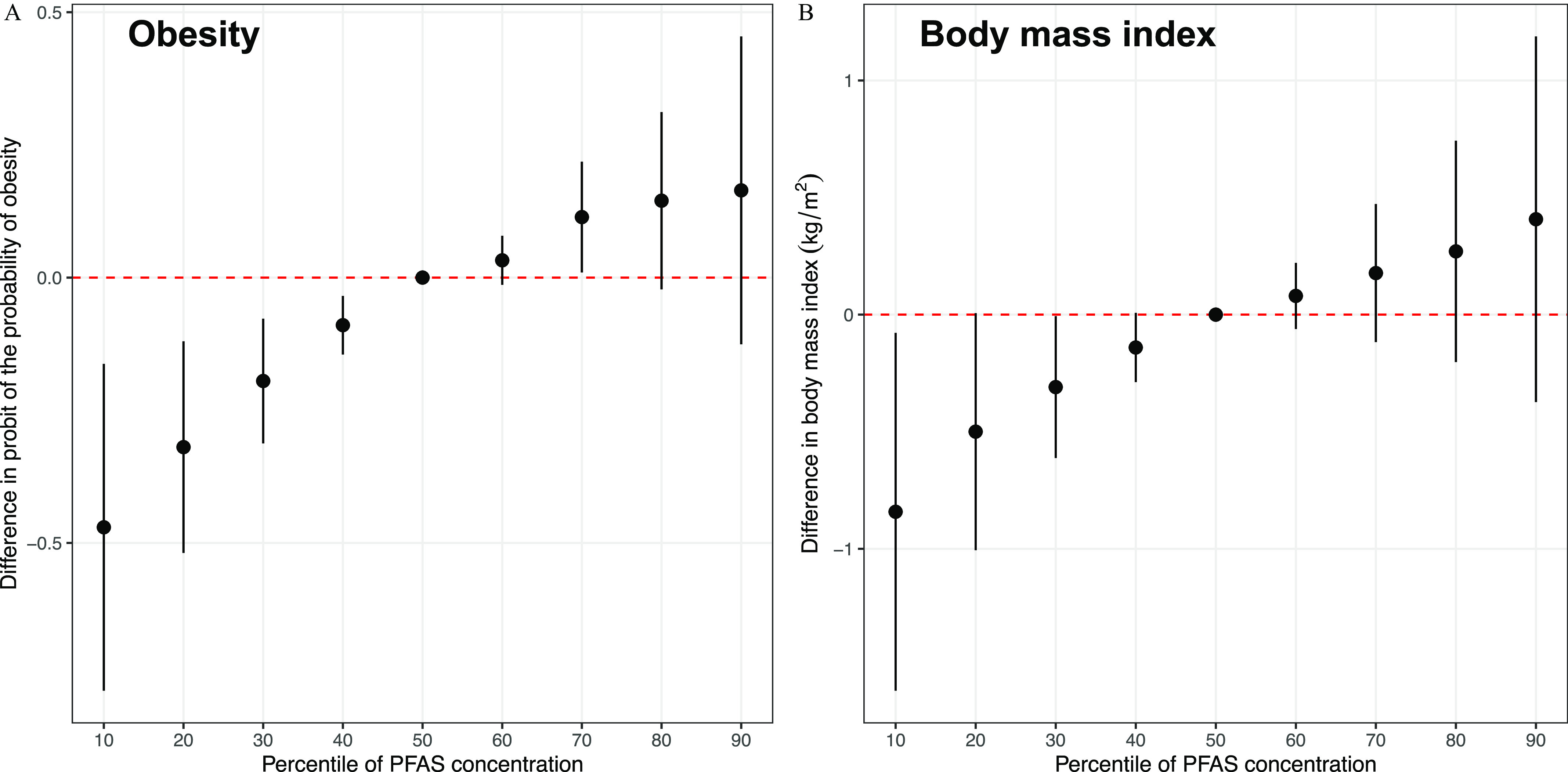
Overall effects (estimates and 95% credible intervals) of prenatal PFAS mixtures estimated by the differences in (A) probit of the probability of obesity and (B) BMI () in late adolescence when all PFAS are in their 10th to 90th percentile (with an interval of 10 percentile) as compared with when they are in their 50th percentile estimated using Bayesian kernel machine regression (). Percentile values for -transformed PFAS levels are provided in Table S2. Estimates are provided in Table S6. Obesity was defined as percentile for age and sex based on the Centers for Disease Control and Prevention Growth Charts (reference is percentile). Participants were from the Project Viva prebirth cohort. Pregnant women were enrolled between April 1999 and July 2002, and the late adolescence visit was conducted between July 2017 and September 2021. Models were adjusted for maternal age at enrollment, race/ethnicity, prepregnancy BMI, educational level, marital status, parity, and pregnancy smoking status. Note: BMI, body mass index; PFAS, per- and polyfluoroalkyl substances.
As the PFAS mixture increased from the 10th to the 90th percentile, DXA percentage fat (Figure S6A), fat mass index (Figure S6B), trunk fat mass index (Figure S6C), and lean mass index (Figure S6D) also increased monotonically (Table S6). PFOS has the largest PIPs for DXA percentage fat and fat mass index, whereas PFOA had the largest PIPs for DXA trunk fat mass index and lean mass index. However, all PIPs for the six PFAS on the DXA outcomes were , indicating modest contributions to the mixture effects (Table S7). Using quantile g-computation, for each quartile increment of the PFAS mixture, DXA percentage fat was 0.20 (95% CI: , 1.27) higher, fat mass index was 0.18 (95% CI: , 0.62) higher, trunk fat mass index was 0.10 (95% CI: , 0.33) higher, and lean mass index was 0.24 (95% CI: , 0.55) higher (Table S8).
Mixture Analyses: Individual Prenatal PFAS
Quantile g-computation showed that PFOS and PFNA contributed positive weights (sum of ) to the mixture effect on obesity risk and the other four PFAS contributed negative weights (sum of ) (Figure 2B, Table S8). BKMR similarly showed that PFOS and PFNA were associated with higher obesity risk, whereas the other four PFAS did not seem to be independently associated with obesity risk (Figure 2C).
Quantile g-computation showed that PFOS, PFHxS, and EtFOSAA contributed positive weights (sum of ) to the mixture effect on BMI and the other three PFAS contributed negative weights (sum of ) (Figure S5B, Table S8). BKMR showed that PFOS and EtFOSAA were associated with higher BMI, PFOA was associated with lower BMI, and the other three PFAS did not seem to be independently associated with BMI (Figure S5C).
Comparisons of the associations of individual prenatal PFAS with DXA percentage fat, fat mass index, trunk fat mass index, and lean mass index, as estimated using quantile g-computation and BKMR, are provided in Figure S7 through Figure S10, respectively. Consistent with the linear regression results, in quantile g-computation and BKMR, PFOS and EtFOSAA generally showed positive associations with all DXA outcomes, whereas PFHxS showed negative associations with DXA percentage fat, fat mass index, and trunk fat mass index, but a positive association with DXA lean mass index. The associations of PFOA with DXA measures showed more inconsistencies when comparing linear regression, BKMR, and quantile g-computation models. For example, although the linear regression model showed no association between PFOA and DXA lean mass index (Table 3), both quantile g-computation and BKMR showed negative associations (Figure S10).
Mixture Analyses: Interactions between Pairs of Prenatal PFAS
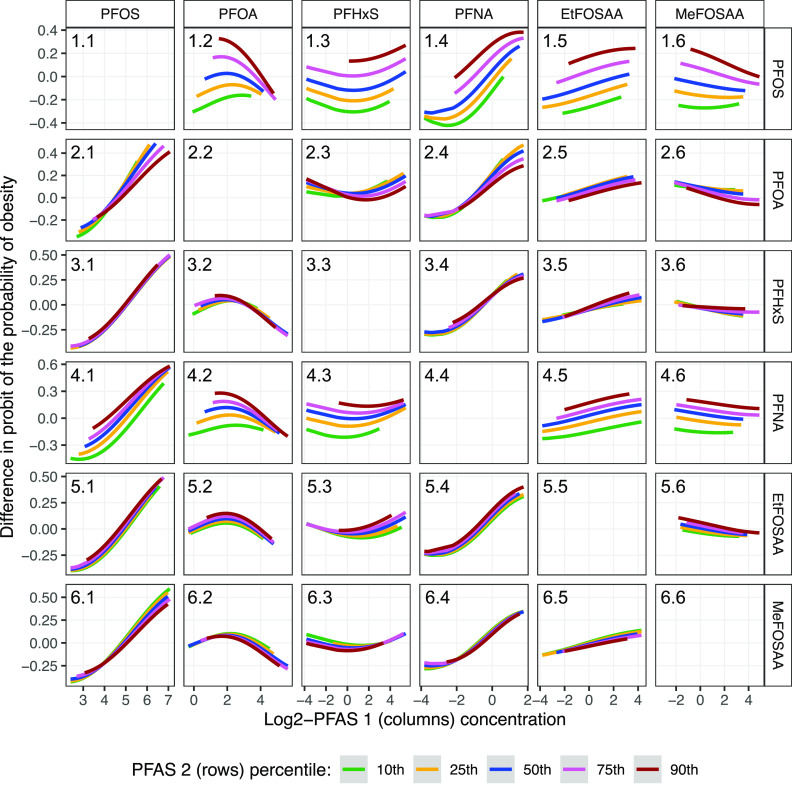
BKMR showed a potential interaction between PFOS and PFOA on obesity risk (Figure 4; Excel Table S1): the positive association between PFOS and obesity was stronger when PFOA levels were lower (Panel 2.1), and there was a negative association between PFOA and obesity risk when PFOS levels were higher (Panel 1.2); both associations were in a dose-dependent fashion. When we looked at BMI as the outcome (Figure S11), the positive association between PFOS and BMI was still stronger with lower PFOA levels (Panel 2.1), but the negative association between PFOA and BMI was consistent across different PFOS levels (Panel 1.2). We did not observe other interactions between pairs of PFAS on obesity risk (Figure 4) or BMI (Figure S11 and Excel Table S2). We also did not observe interactions between pairs of PFAS on DXA percentage fat (Figure S12 and Excel Table S3), fat mass index (Figure S13 and Excel Table S4), trunk fat mass index (Figure S14 and Excel Table S5), or lean mass index (Figure S15 and Excel Table S6).
Figure 4.
Associations (estimates) between prenatal PFAS 1 concentrations (columns) and obesity risk (defined as percentile for age and sex; reference is percentile) in late adolescence by levels (10th, 25th, 50th, 75th, and 90th percentiles) of prenatal PFAS 2 concentration (rows) when all other PFAS are fixed at their 50th percentile estimated using Bayesian kernel machine regression (). Percentile values for -transformed PFAS levels are provided in Table S2. Estimates are provided in Excel Table S1. Participants were from the Project Viva prebirth cohort. Pregnant women were enrolled between April 1999 and July 2002, and the late adolescence visit was conducted between July 2017 and September 2021. Models were adjusted for maternal age at enrollment, race and ethnicity, prepregnancy BMI, educational level, marital status, parity, pregnancy smoking status. Note: BMI, body mass index; EtFOSAA, 2-(-ethyl-perfluorooctane sulfonamido) acetate; MeFOSAA, 2-(-methyl-perfluorooctane sulfonamido) acetate; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
Analyses of Prenatal PFAS with BMI Trajectories
Fractional-polynomial prediction plots showed that children’s BMI trajectories differed by prenatal PFOS, EtFOSAA, and MeFOSAA levels (Figure 5). BMI trajectories by prenatal PFOS levels overlapped until children were y old, after which the trajectories began to diverge, i.e., children with higher (vs. lower) prenatal PFOS exposures had a higher rate of BMI increase starting from 11 years of age. At 20 years of age, children exposed to higher prenatal PFOS had a significantly higher BMI. Similarly, BMI trajectories by prenatal EtFOSAA and MeFOSAA levels also began to diverge when children were . After then, children with higher prenatal EtFOSAA or MeFOSAA exposures had a higher rate of BMI increase. BMI trajectories did not differ by prenatal PFOA, PFHxS, or PFNA levels.
Figure 5.
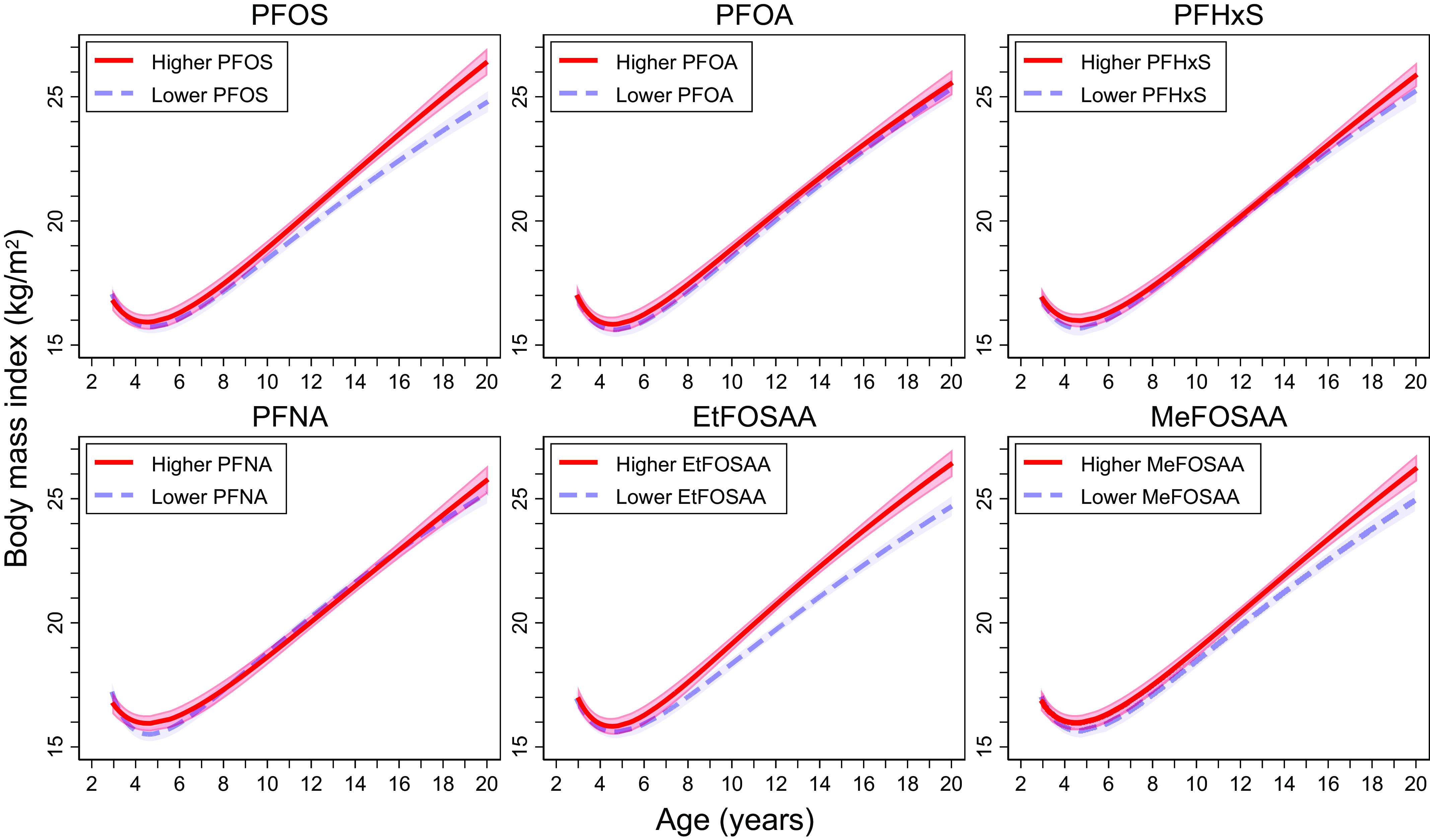
Fractional-polynomial prediction plots (estimates and 95% confidence intervals) showing the associations of age and BMI across childhood and adolescence by maternal PFAS levels (). “Higher” was defined as PFAS levels above the population median, and “lower” was defined as PFAS levels below the population median. Median levels were for PFOS, for PFOA, for PFHxS, for PFNA, for EtFOSAA, and for MeFOSAA. These levels are slightly different from the ones in Table 1 due to the different analytic sample for the main analysis () vs. this post hoc analysis (). Participants were from the Project Viva prebirth cohort. Pregnant women were enrolled between April 1999 and July 2002, and the late adolescence visit was conducted between July 2017 and September 2021. Note: BMI, body mass index; EtFOSAA, 2-(-ethyl-perfluorooctane sulfonamido) acetate; MeFOSAA, 2-(-methyl-perfluorooctane sulfonamido) acetate; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
Sex-Stratified Analyses
Table S9 shows the associations of prenatal PFAS with adiposity and body composition measures by child sex (male vs. female). Most associations did not significantly differ by sex (i.e., ), but we observed two consistent patterns. First, EtFOSAA showed stronger associations with adiposity and body composition measures in females. EtFOSAA was associated with all overall adiposity measures, waist circumference, waist-to-hip circumference ratio, and BIA lean mass index only in females (). Second, PFHxS was associated with higher BMI -score and BIA and DXA lean mass indices only in males (), suggesting that the association between PFHxS and BMI -score in males was likely driven by lean mass.
We observed sex-specific BMI trajectories by prenatal PFOS, EtFOSAA, and MeFOSAA levels (Figure S16). For PFOS, the trajectories diverged earlier for females (at years of age) vs. males (at years of age). For EtFOSAA, the trajectories diverged at years of age for females but were largely overlapped for males (with a slight divergence at 10–11 but reconvergence was seen at 16–17 years of age). For MeFOSAA, the trajectories overlapped for females but diverged for males at years of age.
Sensitivity Analyses
After removing maternal prepregnancy BMI from the multivariable-adjusted models, the associations of PFOS, PFOA, and EtFOSAA with adiposity and body composition easures were consistently stronger (Table S10, model 2). PFOS was now additionally associated with higher BIA and DXA fat mass indices, waist circumference, DXA trunk fat mass index, and DXA lean mass index. Further, EtFOSAA was now additionally associated with obesity risk, all overall adiposity measures, waist circumference, and DXA trunk fat mass index. The associations of PFOS, PFOA, and PFNA with adiposity and body composition measures were also generally strengthened after further adjusting for maternal early pregnancy albumin (Table S10, model 3). Results were consistent after we additionally adjusted for early pregnancy DASH diet scores (Table S10, model 4) or urbanicity scores (Table S10, model 5). Associations also did not markedly change when we used stabilized inverse probability weighting to account for potential selection bias, except that PFHxS was now additionally associated with higher BIA and DXA lean mass indices and that PFNA was now associated with higher BIA lean mass index (Table S11).
Discussion
In this study, we found that higher concentrations of select prenatal PFAS were associated with higher obesity risk and greater adiposity during late adolescence. The prenatal PFAS mixture was also associated with higher obesity risk, BMI, and DXA measures at 16–20 years of age in a dose-dependent fashion.
Prenatal PFAS exposures may have long-lasting obesogenic effects on offspring. Previous research has produced inconsistent results, with some studies finding positive associations and others finding no associations.11–27 For example, Braun et al. found in the Health Outcomes and Measures of the Environment (HOME) Study (in Cincinnati, Ohio) that higher second-trimester serum PFOA was associated with greater adiposity at 8 ()14 and 12 years of age (),23 whereas Andersen et al. found in the Danish National Birth Cohort (in Denmark) that cord blood plasma PFOA was not associated with BMI at 7 years of age ().12 A recent review synthesized these inconsistent findings and highlighted that most prior studies also had shorter follow-up periods and assessed adiposity in childhood only (i.e., before 12 years of age).28 Our study thus contributes to the limited body of research related to the long-term associations of prenatal PFAS exposures on adiposity and body composition measures (objectively measured via BIA and DXA) 16–20 y later. Our study is also among the first to analyze PFAS mixtures. In addition to the clear associations in single pollutant models, the mixture analyses using BKMR and quantile g-computation provided mostly consistent findings, supporting the long-term obesogenic effects of prenatal PFAS.
Potential Mechanisms
Several mechanisms may underlie the effects of prenatal PFAS exposures on child adiposity, as described in review articles by Braun3 and Heindel and Blumberg.75 PFAS can cross the placental barrier during pregnancy, leading to direct in utero exposure. The efficiency of transplacental transfer for PFAS depends on their chemical structure, such as carbon chain lengths.76 Certain PFAS, such as PFOA and PFNA, can accumulate in cord blood and the fetus owing to the lower metabolic capacity of the fetus compared with the pregnant woman.7,76,77 Maternal PFAS can affect both maternal and infant thyroid hormones, which are critical for regulating fetal and postnatal growth.78,79 PFAS can also bind and activate peroxisome proliferator-activated receptors (PPARs), which regulate lipid metabolism, placental functions, and fetal and postnatal growth.80 Given that PPARs are involved in adipogenesis, adipocyte physiology, and energy regulation, their binding to PFAS may alter developmental adipogenesis and adipocyte programming, leading to long-term obesogenic effects.25 In vitro studies have shown that PFAS promote adipocyte differentiation and up-regulate the expression of lipid-related proteins, including and .81–83 PFAS may also alter lipid metabolism, gene expressions associated with lipid metabolism, and DNA methylation status, potentially contributing to obesogenic effects.84–86 Further research is needed to fully understand and establish the underlying biological pathways.
Sex-Specific Associations
Many previous studies have observed sex-specific associations of prenatal PFAS with child adiposity.11,13–15,23,24 However, no consistent patterns were observed, and all but one study used adiposity measures at of age (i.e., before or during puberty). Children undergo significant, sex-specific, and age-varying changes in body composition during puberty, for example, males gain more lean mass, females gain more fat mass, and both experience changes in fat distribution.87 These changes may reveal different programming effects of PFAS on adiposity measures before vs. after puberty. Our study, building on previous findings in Project Viva, which investigated prenatal PFAS and early- and mid-childhood adiposity,15 offers insights into sex-differences. For example, although the associations between prenatal PFAS and lean mass did not differ by sex at of age in our previous study,15 they were much stronger in males at of age in the present study. In the one study that measured postpubertal adiposity,11 Halldorsson et al. found that third-trimester serum PFOA was associated with higher BMI and waist circumference at 20 years of age in females (but not in males) among 665 Danish young adults. Consistent with this finding, we found that most associations between prenatal PFOA and adiposity measures were stronger in females. In our study, EtFOSAA also showed stronger associations with adiposity measures in females.
As endocrine-disrupting chemicals, PFAS can interact with estrogen receptors and affect circulating estrogen concentrations, potentially explaining the stronger effects of these PFAS on adiposity in females.23,88,89 A previous study in the Avon Longitudinal Study of Parents and Children (ALSPAC; in Avon, UK) also found positive associations of prenatal PFOA, PFOS, and PFHxS with testosterone levels in females at 15 years of age (), possibly involving alterations in cholesterol metabolism through activation and changes in testosterone conversion in adipose tissue through activation.90 In addition, studies have associated PFAS exposures with other sex hormones (e.g., estradiol, follicle-stimulating hormone, luteinizing hormone) and insulin-like growth factor-1,91–94 which play crucial roles in growth and sexual maturation and may contribute to sex-differences in adipose tissue development and adiposity. We also observed stronger associations between PFHxS and lean mass measures in males. Previous studies have suggested that PFAS may inhibit androgen receptor activity and reduce testosterone levels,93,95,96 which, if anything, should not promote lean mass. The underlying mechanisms for these male-specific associations remain unclear and warrants future investigations.
Potential Interactions and Overall Effects of Prenatal PFAS
We observed a potential PFOA–PFOS interaction on obesity risk. In vitro studies have reported similar PFOA–PFOS interactions. For example, Rodea-Palomares et al. found strong antagonistic interactions between PFOA and PFOS in a bioluminescent cyanobacterial toxicity test.97 Carr et al. observed a similar PFOA–PFOS interaction in transiently transfected COS-1 reporter models using a mixing ratio based on serum PFAS levels in U.S. National Health and Nutrition Examination Survey (NHANES) participants.98 To our knowledge, no in vivo or human studies have investigated similar interactions. Our finding of the PFOA–PFOS interaction should be interpreted with caution, particularly because we did not observe such interactions for BMI and DXA measures.
We observed a potential protective effect of PFOA on obesity when PFOS levels were higher. This aligns with findings from ALSPAC (in Avon, UK), where Hartman et al. found that higher second-trimester serum PFOA was associated with lower BMI, waist circumference, and DXA trunk and total body fat at 9 years of age in females whose mothers had higher educational status (these mothers also had higher PFOS levels) ().18 Notably, inverse associations for PFOS with these adiposity outcomes were also observed in the same subgroup. Although Hartman et al. did not specifically investigate PFOA and PFOS interactions, the inverse association observed for both PFOA and PFOS in mothers with higher educational status may be attributed to the higher levels of other PFAS, rather than education itself, in line with our study findings. The protective effect of PFOA on obesity observed in our study may also result from unmeasured confounding by social and lifestyle factors linked to higher maternal PFOA levels and lower child adiposity. In addition, it is also important to note the contrasting associations of PFOA with adiposity measures in single pollutant models (i.e., linear and Poisson regression) vs. mixture analyses (i.e., BKMR and quantile g-computation). PFOA consistently showed associations with higher adiposity measures in single pollutant models in our study. However, PFOA was either not associated with these outcomes (e.g., DXA percentage fat) or associated with lower measures (e.g., BMI, DXA fat mass index, DXA lean mass index) in the BKMR models, or it contributed negative weights to the overall mixture effects (e.g., BMI, DXA lean mass index) in the quantile g-computation models. The mechanisms underlying the protective effects of prenatal PFOA and the discrepancies between models are unclear and warrant further investigation.
We also observed linear, dose-dependent associations of PFAS mixtures with obesity, BMI, and DXA measures (Figure 2; Figure S6 and Table S8). In contrast, Zhang et al. recently found in 206 mother–child pairs in the Laizhou Wan Birth Cohort in China that a mixture of 10 cord blood PFAS was inversely associated with child adiposity at 7 years of age.27 Yet, their study used the weighted quantile sum approach that assumed directional homogeneity and was not able to examine the potential pairwise interactions of PFAS. More studies are needed to understand the possible long-term obesogenic effects of prenatal PFAS mixtures and potential interactions between individual PFAS.
Trajectory Analyses
Trajectory analyses demonstrated that the effects of some prenatal PFAS on BMI may become stronger as children grow older. In our previous study, we found stronger effects of prenatal PFAS on mid-childhood ( y) BMI than early childhood ( y) BMI.15 Our trajectory analyses, which included BMI data collected from all Project Viva research visits since early childhood, showed that children exposed to higher PFOS, EtFOSAA, and MeFOSAA prenatally had higher rates of BMI increase after puberty. In the HOME Study (), Braun et al. reported associations of second-trimester PFAS with postnatal BMI trajectories. Different from our results, they found that prenatal PFOS and PFHxS were consistently associated with higher BMI in the first 12 y of life (i.e., no divergent in trajectories by PFAS levels) and that PFOA was associated with BMI trajectories.24 The HOME Study did not include BMI measures after 12 years of age. To our knowledge, no other studies examined how prenatal PFAS exposure is associated with BMI trajectories. Because obesity in adolescence (vs. childhood) persists more strongly into adulthood,99 findings from this trajectory analysis underscore the possible persistent impact of prenatal PFAS exposures on adulthood obesity. Future research should investigate how prenatal PFAS mixtures affect the trajectories of BMI and other adiposity and body composition measures, as well as the impact of postnatal PFAS on these trajectories.
Limitations and Strengths
Our study has limitations. First, residual or unmeasured confounding exists in observational studies. To minimize confounding, we carefully selected and included a comprehensive set of confounders in the analyses, and we additionally adjusted for maternal hemodynamics biomarker, diet quality scores, and urbanicity scores in the sensitivity analyses. Second, a considerable number of children did not have prenatal PFAS exposure or outcome measures in late adolescence, which might have led to selection bias. Yet, most maternal and child characteristics were similar between those included vs. excluded, and the stabilized inverse probability weighting analysis showed that selection bias, if any, did not substantially affect the observed associations. Third, our study population consisted mostly of non-Hispanic white participants, which may limit the generalizability of our findings to more diverse populations, taking into account the documented racial and ethnic differences in PFAS exposure levels and the prevalence of overweight and obesity in children and adolescents.56–58 Fourth, we measured PFAS levels in early pregnancy plasma samples and were thus not able to investigate the cumulative effects of PFAS exposures during the entire gestational period. Finally, PFAS levels were measured from plasma samples collected between 1999 and 2002. Although our results provide insights into the impacts of PFAS exposures at historical levels, they might not reflect current exposure levels or health effects associated with present-day exposures in the U.S. population for certain long-chain PFAS, particularly PFOS and PFOA, which have decreased since the early 2000s.100 Nevertheless, levels of PFAS such as PFNA and PFHxS have not decreased significantly, and our study’s PFAS levels still overlap with levels in recent NHANES cycles. For example, the median PFNA level in our study is , equivalent to the 75th percentile for the overall population (both sexes combined) in 2017–2018 NHANES; similarly, our study’s median PFHxS is , which is only slightly higher than the 75th percentile (i.e., ) in the same NHANES sample.101
Our study has several strengths. First, we used data from Project Viva, one of the largest and longest-running U.S. prebirth cohorts, to investigate the prospective, dose–response associations of prenatal PFAS exposures with a comprehensive set of adiposity and body composition measures at 16–20 years of age. Direct body composition measures, such as the ones from BIA and DXA, allowed us to better understand the sex-specific effects observed in previous studies and to parse out whether the associations were driven by fat or lean mass. Second, all outcome measures were collected at in-person visits using research standard procedures by trained staff, which minimized measurement error. Third, we used two mixture methods, BKMR and quantile g-computation, to examine the effects of PFAS mixtures. Both methods provided comparable results. Fourth, we were able to use BMI data collected at four Project Viva research visits from early childhood to late adolescence to model BMI trajectories and examine how prenatal PFAS exposures affect these trajectories.
Conclusions
In summary, our findings suggested long-lasting () obesogenic effects of prenatal PFAS exposures and observed the potential interactions of PFAS and the overall effects of coexposure to PFAS mixtures on obesity risk. Future studies are warranted to replicate these PFAS interactions and overall effects in larger cohorts or consortia [such as the Environmental influences on Child Health Outcomes (ECHO) program, a consortium of 69 pediatric cohorts in the United States, many of which collected data on early life PFAS exposures102 and adiposity70] and examine the pathways underlying these intergenerational associations.
Supplementary Material
Acknowledgments
Funding sources: The Project Viva study is supported by grants from the National Institutes of Health [NIH; R01HD034568 (EO and MH), UG3OD023286, and R24ES030894, all to E.O.]. A.F.F. is supported by a grant from the NIH (R01ES030101). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders or the publishers.
References
- 1.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: , 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol 45(19):8037–8045, PMID: , 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 3.Braun JM. 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13(3):161–173, PMID: , 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi W, Clark JM, Timme-Laragy AR, Park Y. 2020. Per- and polyfluoroalkyl substances and obesity, type 2 diabetes and non-alcoholic fatty liver disease: a review of epidemiologic findings. Toxicol Environ Chem 102(1–4):1–36, PMID: , 10.1080/02772248.2020.1763997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fryar CD, Carroll MD, Afful J. 2020. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2017–2018. NCHS Health E-Stats. https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/obesity-child.htm [accessed 4 November 2022]. [Google Scholar]
- 6.Ogden CL, Fryar CD, Martin CB, Freedman DS, Carroll MD, Gu Q, et al. 2020. Trends in obesity prevalence by race and Hispanic origin—1999–2000 to 2017–2018. JAMA 324(12):1208–1210, PMID: , 10.1001/jama.2020.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma D, Lu Y, Liang Y, Ruan T, Li J, Zhao C, et al. 2022. A critical review on transplacental transfer of per- and polyfluoroalkyl substances: prenatal exposure levels, characteristics, and mechanisms. Environ Sci Technol 56(10):6014–6026, PMID: , 10.1021/acs.est.1c01057. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. 2008. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1):61–73, PMID: , 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillman MW. 2005. Developmental origins of health and disease. N Engl J Med 353(17):1848–1850, PMID: , 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frigerio G, Ferrari CM, Fustinoni S. 2023. Prenatal and childhood exposure to per-/polyfluoroalkyl substances (PFASs) and its associations with childhood overweight and/or obesity: a systematic review with meta-analyses. Environ Health 22(1):56, PMID: , 10.1186/s12940-023-01006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, et al. 2012. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect 120(5):668–673, PMID: , 10.1289/ehp.1104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen CS, Fei C, Gamborg M, Nohr EA, Sørensen TIA, Olsen J. 2013. Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. Am J Epidemiol 178(6):921–927, PMID: , 10.1093/aje/kwt057. [DOI] [PubMed] [Google Scholar]
- 13.Høyer BB, Ramlau-Hansen CH, Vrijheid M, Valvi D, Pedersen HS, Zviezdai V, et al. 2015. Anthropometry in 5- to 9-year-old Greenlandic and Ukrainian children in relation to prenatal exposure to perfluorinated alkyl substances. Environ Health Perspect 123(8):841–846, PMID: , 10.1289/ehp.1408881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, et al. 2016. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: the HOME Study. Obesity (Silver Spring) 24(1):231–237, PMID: , 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mora AM, Oken E, Rifas-Shiman SL, Webster TF, Gillman MW, Calafat AM, et al. 2017. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ Health Perspect 125(3):467–473, PMID: , 10.1289/EHP246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsen M, Grandjean P, Weihe P, Steuerwald U, Oulhote Y, Valvi D. 2017. Early-life exposures to persistent organic pollutants in relation to overweight in preschool children. Reprod Toxicol 68:145–153, PMID: , 10.1016/j.reprotox.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Iñiguez C, Martinez D, et al. 2017. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the Spanish INMA Birth Cohort Study. Environ Health Perspect 125(9):097018, PMID: , 10.1289/EHP1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartman TJ, Calafat AM, Holmes AK, Marcus M, Northstone K, Flanders WD, et al. 2017. Prenatal exposure to perfluoroalkyl substances and body fatness in girls. Child Obes 13(3):222–230, PMID: , 10.1089/chi.2016.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauritzen HB, Larose TL, Øien T, Sandanger TM, Odland JØ, van de Bor M, et al. 2018. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: a prospective cohort study. Environ Health 17(1):9, PMID: , 10.1186/s12940-017-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gyllenhammar I, Diderholm B, Gustafsson J, Berger U, Ridefelt P, Benskin JP, et al. 2018. Perfluoroalkyl acid levels in first-time mothers in relation to offspring weight gain and growth. Environ Int 111:191–199, PMID: , 10.1016/j.envint.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Zhang X, Zhao Y, Lu W, Wu J, Zhao S, et al. 2019. Prenatal exposure to perfluorobutanesulfonic acid and childhood adiposity: a prospective birth cohort study in Shanghai, China. Chemosphere 226:17–23, PMID: , 10.1016/j.chemosphere.2019.03.095. [DOI] [PubMed] [Google Scholar]
- 22.Martinsson M, Nielsen C, Björk J, Rylander L, Malmqvist E, Lindh C, et al. 2020. Intrauterine exposure to perfluorinated compounds and overweight at age 4: a case–control study. PLoS One 15(3):e0230137, PMID: , 10.1371/journal.pone.0230137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Li N, Papandonatos GD, Calafat AM, Eaton CB, Kelsey KT, et al. 2020. Exposure to per- and polyfluoroalkyl substances and adiposity at age 12 years: evaluating periods of susceptibility. Environ Sci Technol 54(24):16039–16049, PMID: , 10.1021/acs.est.0c06088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun JM, Eliot M, Papandonatos GD, Buckley JP, Cecil KM, Kalkwarf HJ, et al. 2021. Gestational perfluoroalkyl substance exposure and body mass index trajectories over the first 12 years of life. Int J Obes (Lond) 45(1):25–35, PMID: , 10.1038/s41366-020-00717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloom MS, Commodore S, Ferguson PL, Neelon B, Pearce JL, Baumer A, et al. 2022. Association between gestational PFAS exposure and children’s adiposity in a diverse population. Environ Res 203:111820, PMID: , 10.1016/j.envres.2021.111820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Wosu AC, Fleisch AF, Dunlop AL, Starling AP, Ferrara A, et al. 2023. Associations of gestational perfluoroalkyl substances exposure with early childhood BMI z-scores and risk of overweight/obesity: results from the ECHO cohorts. Environ Health Perspect 131(6):067001, PMID: , 10.1289/EHP11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, Lei X, Zhang Y, Shi R, Zhang Q, Gao Y, et al. 2022. Prenatal exposure to per- and polyfluoroalkyl substances and childhood adiposity at 7 years of age. Chemosphere 307(pt 4):136077, PMID: , 10.1016/j.chemosphere.2022.136077. [DOI] [PubMed] [Google Scholar]
- 28.Lee YJ, Jung HW, Kim HY, Choi YJ, Lee YA. 2021. Early-life exposure to per- and poly-fluorinated alkyl substances and growth, adiposity, and puberty in children: a systematic review. Front Endocrinol (Lausanne) 12:683297, PMID: , 10.3389/fendo.2021.683297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Academies of Sciences, Engineering, and Medicine. 2022. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 30.Shah B, Tombeau Cost K, Fuller A, Birken CS, Anderson LN. 2020. Sex and gender differences in childhood obesity: contributing to the research agenda. BMJ Nutr Prev Health 3(2):387–390, PMID: , 10.1136/bmjnph-2020-000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang E, Varghese M, Singer K. 2018. Gender and sex differences in adipose tissue. Curr Diab Rep 18(9):69, PMID: , 10.1007/s11892-018-1031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bredella MA. 2017. Sex differences in body composition. Adv Exp Med Biol 1043:9–27, PMID: , 10.1007/978-3-319-70178-3_2. [DOI] [PubMed] [Google Scholar]
- 33.Kato K, Basden BJ, Needham LL, Calafat AM. 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218(15):2133–2137, PMID: , 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 34.Louer AL, Simon DN, Switkowski KM, Rifas-Shiman SL, Gillman MW, Oken E. 2017. Assessment of child anthropometry in a large epidemiologic study. J Vis Exp 120:54895, 10.3791/54895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2002. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 246:1–190, PMID: . [PubMed] [Google Scholar]
- 36.Mueller NT, Zhang M, Rifas-Shiman SL, Oken E, Hivert MF, Chavarro J. 2021. Mode of delivery, type of labor, and measures of adiposity from childhood to teenage: Project Viva. Int J Obes (Lond) 45(1):36–44, PMID: , 10.1038/s41366-020-00709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamin-Neelon SE, Bai J, Østbye T, Neelon B, Pate RR, Crainiceanu C. 2020. Physical activity and adiposity in a racially diverse cohort of US infants. Obesity (Silver Spring) 28(3):631–637, PMID: , 10.1002/oby.22738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boeke CE, Oken E, Kleinman KP, Rifas-Shiman SL, Taveras EM, Gillman MW. 2013. Correlations among adiposity measures in school-aged children. BMC Pediatr 13(1):99, PMID: , 10.1186/1471-2431-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulay AP, Rifas-Shiman SL, Oken E, Perng W. 2018. Associations of the Dietary Approaches to Stop Hypertension (DASH) diet with pregnancy complications in Project Viva. Eur J Clin Nutr 72(10):1385–1395, PMID: , 10.1038/s41430-017-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. 2004. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol 14(10):754–762, PMID: , 10.1016/j.annepidem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 41.MRLC (Multi-Resolution Land Characteristics). n.d. Multi-Resolution Land Characteristics (MRLC) Consortium. https://www.mrlc.gov/ [accessed 9 September 2023].
- 42.Yanosky JD, Paciorek CJ, Schwartz J, Laden F, Puett R, Suh HH. 2008. Spatio-temporal modeling of chronic PM10 exposure for the Nurses’ Health Study. Atmos Environ (1994) 42(18):4047–4062, PMID: , 10.1016/j.atmosenv.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou G. 2004. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706, PMID: , 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 44.Choi G, Buckley JP, Kuiper JR, Keil AP. 2022. Log-transformation of independent variables: must we? Epidemiology 33(6):843–853, PMID: , 10.1097/EDE.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508, PMID: , 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bobb JF, Claus Henn B, Valeri L, Coull BA. 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17(1):67, PMID: , 10.1186/s12940-018-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect 128(4):047004, PMID: , 10.1289/EHP5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keil A. 2022. bkmrhat: parallel chain tools for Bayesian kernel machine regression. https://CRAN.R-project.org/package=bkmrhat [accessed 14 April 2023].
- 49.Barbieri MM, Berger JO. 2004. Optimal predictive model selection. Ann Stat 32(3):870–897, 10.1214/009053604000000238. [DOI] [Google Scholar]
- 50.Eick SM, Barr DB, Brennan PA, Taibl KR, Tan Y, Robinson M, et al. 2023. Per- and polyfluoroalkyl substances and psychosocial stressors have a joint effect on adverse pregnancy outcomes in the Atlanta African American Maternal–Child cohort. Sci Total Environ 857(pt 2):159450, PMID: , 10.1016/j.scitotenv.2022.159450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cathey AL, Eaton JL, Ashrap P, Watkins DJ, Rosario ZY, Vélez Vega C, et al. 2021. Individual and joint effects of phthalate metabolites on biomarkers of oxidative stress among pregnant women in Puerto Rico. Environ Int 154:106565, PMID: , 10.1016/j.envint.2021.106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buekers J, Colles A, Cornelis C, Morrens B, Govarts E, Schoeters G. 2018. Socio-economic status and health: evaluation of human biomonitored chemical exposure to per- and polyfluorinated substances across status. Int J Environ Res Public Health 15(12):2818, PMID: , 10.3390/ijerph15122818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyrrell J, Melzer D, Henley W, Galloway TS, Osborne NJ. 2013. Associations between socioeconomic status and environmental toxicant concentrations in adults in the USA: NHANES 2001–2010. Environ Int 59:328–335, PMID: , 10.1016/j.envint.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 54.Woo JG, Dolan LM, Morrow AL, Geraghty SR, Goodman E. 2008. Breastfeeding helps explain racial and socioeconomic status disparities in adolescent adiposity. Pediatrics 121(3):e458–e465, PMID: , 10.1542/peds.2007-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shrewsbury V, Wardle J. 2008. Socioeconomic status and adiposity in childhood: a systematic review of cross-sectional studies 1990–2005. Obesity (Silver Spring) 16(2):275–284, PMID: , 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
- 56.Ogden CL, Carroll MD, Fakhouri TH, Hales CM, Fryar CD, Li X, et al. 2018. Prevalence of obesity among youths by household income and education level of head of household—United States 2011–2014. MMWR Morb Mortal Wkly Rep 67(6):186–189, PMID: , 10.15585/mmwr.mm6706a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boronow KE, Brody JG, Schaider LA, Peaslee GF, Havas L, Cohn BA. 2019. Serum concentrations of PFASs and exposure-related behaviors in African American and non-Hispanic white women. J Expo Sci Environ Epidemiol 29(2):206–217, PMID: , 10.1038/s41370-018-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding N, Karvonen-Gutierrez CA, Zota AR, Mukherjee B, Harlow SD, Park SK. 2023. The role of exposure to per- and polyfluoroalkyl substances in racial/ethnic disparities in hypertension: results from the Study of Women’s Health Across the Nation. Environ Res 227:115813, PMID: , 10.1016/j.envres.2023.115813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. 2021. Temporal trends of concentrations of per- and polyfluoroalkyl substances among adults with overweight and obesity in the United States: results from the Diabetes Prevention Program and NHANES. Environ Int 157:106789, PMID: , 10.1016/j.envint.2021.106789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faupel-Badger JM, Hsieh CC, Troisi R, Lagiou P, Potischman N. 2007. Plasma volume expansion in pregnancy: implications for biomarkers in population studies. Cancer Epidemiol Biomarkers Prev 16(9):1720–1723, PMID: , 10.1158/1055-9965.EPI-07-0311. [DOI] [PubMed] [Google Scholar]
- 61.D’eon JC, Simpson AJ, Kumar R, Baer AJ, Mabury SA. 2010. Determining the molecular interactions of perfluorinated carboxylic acids with human sera and isolated human serum albumin using nuclear magnetic resonance spectroscopy. Environ Toxicol Chem 29(8):1678–1688, PMID: , 10.1002/etc.204. [DOI] [PubMed] [Google Scholar]
- 62.Forsthuber M, Kaiser AM, Granitzer S, Hassl I, Hengstschläger M, Stangl H, et al. 2020. Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environ Int 137:105324, PMID: , 10.1016/j.envint.2019.105324. [DOI] [PubMed] [Google Scholar]
- 63.Savitz DA. 2014. Invited commentary: interpreting associations between exposure biomarkers and pregnancy outcome. Am J Epidemiol 179(5):545–547, PMID: , 10.1093/aje/kwt314. [DOI] [PubMed] [Google Scholar]
- 64.Savitz DA. 2007. Guest editorial: biomarkers of perfluorinated chemicals and birth weight. Environ Health Perspect 115(11):A528–A529, PMID: , 10.1289/ehp.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. 1997. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 336(16):1117–1124, PMID: , 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 66.Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. 2020. Dietary characteristics associated with plasma concentrations of per- and polyfluoroalkyl substances among adults with pre-diabetes: cross-sectional results from the Diabetes Prevention Program Trial. Environ Int 137:105217, PMID: , 10.1016/j.envint.2019.105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen LW, Aubert AM, Shivappa N, Bernard JY, Mensink-Bout SM, Geraghty AA, et al. 2021. Maternal dietary quality, inflammatory potential and childhood adiposity: an individual participant data pooled analysis of seven European cohorts in the ALPHABET consortium. BMC Med 19(1):33, PMID: , 10.1186/s12916-021-01908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurwadkar S, Dane J, Kanel SR, Nadagouda MN, Cawdrey RW, Ambade B, et al. 2022. Per- and polyfluoroalkyl substances in water and wastewater: a critical review of their global occurrence and distribution. Sci Total Environ 809:151003, PMID: , 10.1016/j.scitotenv.2021.151003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson JA III, Johnson AM. 2015. Urban–rural differences in childhood and adolescent obesity in the United States: a systematic review and meta-analysis. Child Obes 11(3):233–241, PMID: , 10.1089/chi.2014.0085. [DOI] [PubMed] [Google Scholar]
- 70.Bekelman TA, Dabelea D, Ganiban JM, Law A, McGovern Reilly A, Althoff KN, et al. 2021. Regional and sociodemographic differences in average BMI among US children in the ECHO program. Obesity (Silver Spring) 29(12):2089–2099, PMID: , 10.1002/oby.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bottino CJ, Rifas-Shiman SL, Kleinman KP, Oken E, Redline S, Gold D, et al. 2012. The association of urbanicity with infant sleep duration. Health Place 18(5):1000–1005, PMID: , 10.1016/j.healthplace.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Austin PC, Stuart EA. 2015. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 34(28):3661–3679, PMID: , 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. 2011. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 20(1):40–49, PMID: , 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. 2016. Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int J Epidemiol 45(6):1887–1894, PMID: , 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 75.Heindel JJ, Blumberg B. 2019. Environmental obesogens: mechanisms and controversies. Annu Rev Pharmacol Toxicol 59(1):89–106, PMID: , 10.1146/annurev-pharmtox-010818-021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang T, Sun H, Lin Y, Qin X, Zhang Y, Geng X, et al. 2013. Distribution of poly- and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ Sci Technol 47(14):7974–7981, PMID: , 10.1021/es400937y. [DOI] [PubMed] [Google Scholar]
- 77.Gützkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G. 2012. Placental transfer of perfluorinated compounds is selective—a Norwegian Mother and Child sub-cohort study. Int J Hyg Environ Health 215(2):216–219, PMID: , 10.1016/j.ijheh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Kato S, Itoh S, Yuasa M, Baba T, Miyashita C, Sasaki S, et al. 2016. Association of perfluorinated chemical exposure in utero with maternal and infant thyroid hormone levels in the Sapporo cohort of Hokkaido Study on the Environment and Children’s Health. Environ Health Prev Med 21(5):334–344, PMID: , 10.1007/s12199-016-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Preston EV, Webster TF, Claus Henn B, McClean MD, Gennings C, Oken E, et al. 2020. Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the Project Viva cohort: a mixtures approach. Environ Int 139:105728, PMID: , 10.1016/j.envint.2020.105728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szilagyi JT, Avula V, Fry RC. 2020. Perfluoroalkyl substances (PFAS) and their effects on the placenta, pregnancy, and child development: a potential mechanistic role for placental peroxisome proliferator-activated receptors (PPARs). Curr Environ Health Rep 7(3):222–230, PMID: , 10.1007/s40572-020-00279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu S, Yang R, Yin N, Wang YL, Faiola F. 2019. Environmental and human relevant PFOS and PFOA doses alter human mesenchymal stem cell self-renewal, adipogenesis and osteogenesis. Ecotoxicol Environ Saf 169:564–572, PMID: , 10.1016/j.ecoenv.2018.11.064. [DOI] [PubMed] [Google Scholar]
- 82.Watkins AM, Wood CR, Lin MT, Abbott BD. 2015. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol Cell Endocrinol 400:90–101, PMID: , 10.1016/j.mce.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 83.Yamamoto J, Yamane T, Oishi Y, Kobayashi-Hattori K. 2015. Perfluorooctanoic acid binds to peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation in 3T3-L1 adipocytes. Biosci Biotechnol Biochem 79(4):636–639, PMID: , 10.1080/09168451.2014.991683. [DOI] [PubMed] [Google Scholar]
- 84.Fletcher T, Galloway TS, Melzer D, Holcroft P, Cipelli R, Pilling LC, et al. 2013. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int 57–58:2–10, PMID: , 10.1016/j.envint.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 85.Sen P, Qadri S, Luukkonen PK, Ragnarsdottir O, McGlinchey A, Jäntti S, et al. 2022. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J Hepatol 76(2):283–293, PMID: , 10.1016/j.jhep.2021.09.039. [DOI] [PubMed] [Google Scholar]
- 86.Kobayashi S, Azumi K, Goudarzi H, Araki A, Miyashita C, Kobayashi S, et al. 2017. Effects of prenatal perfluoroalkyl acid exposure on cord blood IGF2/H19 methylation and ponderal index: the Hokkaido Study. J Expo Sci Environ Epidemiol 27(3):251–259, PMID: , 10.1038/jes.2016.50. [DOI] [PubMed] [Google Scholar]
- 87.Loomba-Albrecht LA, Styne DM. 2009. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes 16(1):10–15, PMID: , 10.1097/med.0b013e328320d54c. [DOI] [PubMed] [Google Scholar]
- 88.Shi Z, Zhang H, Ding L, Feng Y, Xu M, Dai J. 2009. The effect of perfluorododecanonic acid on endocrine status, sex hormones and expression of steroidogenic genes in pubertal female rats. Reprod Toxicol 27(3–4):352–359, PMID: , 10.1016/j.reprotox.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 89.Benninghoff AD, Bisson WH, Koch DC, Ehresman DJ, Kolluri SK, Williams DE. 2011. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol Sci 120(1):42–58, PMID: , 10.1093/toxsci/kfq379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maisonet M, Calafat AM, Marcus M, Jaakkola JJK, Lashen H. 2015. Prenatal exposure to perfluoroalkyl acids and serum testosterone concentrations at 15 years of age in female ALSPAC study participants. Environ Health Perspect 123(12):1325–1330, PMID: , 10.1289/ehp.1408847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez -Espinosa MJ, Mondal D, Armstrong BG, Eskenazi B, Fletcher T. 2016. Perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6–9 years of age: a cross-sectional analysis within the C8 Health Project. Environ Health Perspect 124(8):1269–1275, PMID: , 10.1289/ehp.1509869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vested A, Ramlau -Hansen CH, Olsen SF, Bonde JP, Kristensen SL, Halldorsson TI, et al. 2013. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect 121(4):453–458, PMID: , 10.1289/ehp.1205118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joensen UN, Veyrand B, Antignac JP, Blomberg Jensen M, Petersen JH, Marchand P, et al. 2013. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum Reprod 28(3):599–608, PMID: , 10.1093/humrep/des425. [DOI] [PubMed] [Google Scholar]
- 94.Tsai MS, Lin CY, Lin CC, Chen MH, Hsu SHJ, Chien KL, et al. 2015. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. Int J Hyg Environ Health 218(5):437–443, PMID: , 10.1016/j.ijheh.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Tarapore P, Ouyang B. 2021. Perfluoroalkyl chemicals and male reproductive health: do PFOA and PFOS increase risk for male infertility? Int J Environ Res Public Health 18(7):3794, PMID: , 10.3390/ijerph18073794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petersen MS, Halling J, Jørgensen N, Nielsen F, Grandjean P, Jensen TK, et al. 2018. Reproductive function in a population of young Faroese men with elevated exposure to polychlorinated biphenyls (PCBs) and perfluorinated alkylate substances (PFAS). Int J Environ Res Public Health 15(9):1880, PMID: , 10.3390/ijerph15091880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodea-Palomares I, Leganés F, Rosal R, Fernández-Piñas F. 2012. Toxicological interactions of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) with selected pollutants. J Hazard Mater 201–202:209–218, PMID: , 10.1016/j.jhazmat.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 98.Carr CK, Watkins AM, Wolf CJ, Abbott BD, Lau C, Gennings C. 2013. Testing for departures from additivity in mixtures of perfluoroalkyl acids (PFAAs). Toxicology 306:169–175, PMID: , 10.1016/j.tox.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simmonds M, Llewellyn A, Owen CG, Woolacott N. 2016. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev 17(2):95–107, PMID: , 10.1111/obr.12334. [DOI] [PubMed] [Google Scholar]
- 100.ATSDR (Agency for Toxic Substances and Disease Registry). 2022. PFAS in the US population. https://www.atsdr.cdc.gov/pfas/health-effects/us-population.html [accessed 4 November 2022].
- 101.National Center for Environmental Health. 2021. Fourth National Report on Human Exposure to Environmental Chemicals. https://stacks.cdc.gov/view/cdc/105345 [accessed 14 April 2023].
- 102.Buckley JP, Barrett ES, Beamer PI, Bennett DH, Bloom MS, Fennell TR, et al. 2020. Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) program. J Expo Sci Environ Epidemiol 30(3):397–419, PMID: , 10.1038/s41370-020-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.