Abstract
Background:
Glyphosate is the most commonly used herbicide worldwide and has been implicated in the development of certain hematologic cancers. Although mechanistic studies in human cells and animals support the genotoxic effects of glyphosate, evidence in human populations is scarce.
Objectives:
We evaluated the association between lifetime occupational glyphosate use and mosaic loss of chromosome Y (mLOY) as a marker of genotoxicity among male farmers.
Methods:
We analyzed blood-derived DNA from 1,606 farmers years of age in the Biomarkers of Exposure and Effect in Agriculture study, a subcohort of the Agricultural Health Study. mLOY was detected using genotyping array intensity data in the pseudoautosomal region of the sex chromosomes. Cumulative lifetime glyphosate use was assessed using self-reported pesticide exposure histories. Using multivariable logistic regression, we estimated odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between glyphosate use and any detectable mLOY (overall mLOY) or mLOY affecting of cells (expanded mLOY).
Results:
Overall, mLOY was detected in 21.4% of farmers, and 9.8% of all farmers had expanded mLOY. Increasing total lifetime days of glyphosate use was associated with expanded mLOY [highest vs. lowest quartile; (95% CI: 1.00, 3.07), ] but not with overall mLOY; the associations with expanded mLOY were most apparent among older ( years of age) men [ (95% CI: 1.13, 4.67), ], never smokers [ (95% CI: 1.04, 5.21), ], and nonobese men [ (95% CI: 0.99, 4.19), ]. Similar patterns of associations were observed for intensity-weighted lifetime days of glyphosate use.
Discussion:
High lifetime glyphosate use could be associated with mLOY affecting a larger fraction of cells, suggesting glyphosate could confer genotoxic or selective effects relevant for clonal expansion. As the first study to investigate this association, our findings contribute novel evidence regarding the carcinogenic potential of glyphosate and require replication in future studies. https://doi.org/10.1289/EHP12834
Introduction
Glyphosate is a broad-spectrum, post-emergent herbicide used in both agricultural and nonagricultural settings worldwide.1 Since it was first registered for use in 1974, glyphosate has become the most widely applied agricultural pesticide and the second most commonly used home and garden pesticide in the United States.1,2 Agricultural use of glyphosate has increased dramatically since the mid-1990s, largely attributed to the introduction of genetically modified glyphosate-resistant crops, such as soybeans and corn.1 Biomonitoring studies suggest substantial exposure to glyphosate among farmers and other occupations involving pesticide use or production.3,4 Recent data from the National Health and Nutrition Examination Survey also indicate widespread exposure in the general population, with 81% of U.S. adults and children years of age having detectable levels of glyphosate in their urine.5
Glyphosate has been linked to a number of adverse health effects6 and was classified as “probably carcinogenic in humans” (Group 2A) by the International Agency for Research on Cancer (IARC) in 2015, on the basis of limited evidence for an association with non-Hodgkin lymphoma (NHL) in humans, sufficient evidence of carcinogenicity in laboratory animals, and strong mechanistic evidence of genotoxicity and oxidative stress in human cells and animals.7,8 However, assessments of the carcinogenic potential of glyphosate by public health and regulatory bodies remain controversial, especially in light of the conflicting epidemiologic evidence to date.9 Positive associations between occupational glyphosate use and hematologic cancers, particularly NHL and its subtypes, have been reported by some,10–14 but not all,15,16 case–control studies. A recently updated analysis of the Agricultural Health Study (AHS), a prospective cohort of pesticide applicators in Iowa and North Carolina, found a suggestive association between high lifetime glyphosate use and increased risk of acute myeloid leukemia (AML) but not NHL or other cancers.17 Furthermore, a pooled analysis of the AHS and two other agricultural cohorts in Europe reported an association with elevated risk of diffuse large B-cell lymphoma, a common NHL subtype.18 Given the widespread use of glyphosate, investigations of intermediate end points related to cancer development, such as markers of genotoxicity, may provide novel and more timely evidence for future evaluations of glyphosate carcinogenicity.19 Although numerous in vitro and animal studies support the genotoxic potential of glyphosate,8 there is a paucity of research among human populations other than a few relatively small studies evaluating glyphosate exposure in relation to markers of DNA or chromosomal damage.20–22
Mosaic chromosomal alterations (mCAs) are large structural somatic mutations, such as chromosomal gains, deletions, and copy-neutral loss of heterozygosity, that can occur in a subset of cells within an individual.23,24 The clonal expansion of cells harboring such mutations results in genetic mosaicism, which has been linked to elevated risks of hematologic and other malignancies.24–28 Among men, mosaic loss of chromosome Y (mLOY) is the most frequently detected mCA in circulating leukocytes.29,30 mLOY prevalence increases with age, affecting 2.5% and 43.6% of men at 40 and 70 years of age, respectively, in the UK Biobank.29 Although evidence is inconclusive, mLOY has been previously associated with hematologic cancers,31–34 and more recently, with certain solid tumors,35–38 cardiovascular disease,39 and Alzheimer’s disease.40 It has also been suggested that mCAs affecting a larger fraction of cells may have a greater impact on health outcomes.24 Beyond age and genetic susceptibility, cigarette smoking has been consistently identified as a major risk factor for mLOY,29,36,41–44 whereas evidence regarding other modifiable factors (e.g., alcohol consumption) is more limited.42 There is also a growing body of research evaluating environmental and occupational exposures as potential contributors to mLOY, including several recent studies that reported associations with outdoor air pollution45 and arsenic exposure46 in the general population and exposures to polycyclic aromatic hydrocarbons and metals among coke oven workers.47,48 To our knowledge, no studies have investigated glyphosate exposure in relation to mLOY as a marker of genotoxicity and possible early indicator of future cancer risk. To this end, we evaluated the association between occupational glyphosate use and mLOY among a large sample of male farmers in the Biomarkers of Exposure and Effect in Agriculture (BEEA) study, a subcohort of the AHS.
Methods
Study Design and Population
The BEEA study is a molecular epidemiologic study nested within the AHS. Details of the design and methodology of the AHS and BEEA have been described elsewhere.49,50 Briefly, the AHS is a large prospective cohort that enrolled licensed private pesticide applicators (mostly farmers; ) and their spouses () from Iowa and North Carolina and commercial pesticide applicators () from Iowa between 1993 and 1997.49 Male private pesticide applicators in the AHS cohort were eligible for BEEA if they were years of age, resided in Iowa or North Carolina, had no blood clotting disorder (e.g., hemophilia), and had not been diagnosed with cancer (other than nonmelanoma skin cancer) at the time of BEEA recruitment and enrollment in 2010–2017. In addition, BEEA participants must have completed questionnaires administered at AHS enrollment (1993–1997) and during the AHS Phase 2 (1999–2003) and Phase 3 (2005–2010) follow-up interviews.50 The BEEA study protocol was approved by institutional review boards at the National Cancer Institute (NCI), the University of Iowa, and Westat (Rockville, Maryland), and all participants provided written informed consent.
Blood Collection and Genotyping
At the BEEA enrollment home visit, biospecimens, including blood, urine, and (since October 2015) buccal cells, were collected from each participant by a trained phlebotomist and were shipped and aliquoted for long-term storage as previously described.50 Consistent with previous studies examining risk factors for mLOY41,42,45–48 and given the possible link between glyphosate exposure and hematologic cancers, our study assessed mLOY in blood. Among the 1,681 participating BEEA farmers, 1,660 had available stored whole blood or buffy coat samples for genomic DNA extraction. Notably, the majority () of participants had whole blood samples available, except for a small number participating in certain substudies within BEEA for which only buffy coat samples were available.51,52 For those with both sample types available, whole blood was preferentially selected for this investigation because it was available for most participants.
DNA extraction was performed based on a standard protocol using the automated QIAsymphony system (Qiagen) at the NCI’s Cancer Genomics Research Laboratory (CGR). Genotyping was performed on extracted DNA samples using the Illumina Infinium Global Screening Array (GSA) version 2.0 plus Multi-Disease BeadChips (GSAMD-24v2-0; Illumina Inc.) at the CGR. Standard quality control metrics were generated to assess genotype completion rates, sample contamination, sex concordance (i.e., identified as male based on X chromosome heterozygosity or, if conflicted with reported sex, the Y chromosome was examined), and presence of any unexpected replicates. Seven participants had samples with failed DNA extraction, low genotype call rates (), or contamination rates of and were excluded from further analysis, leaving 1,653 participants assessed for mLOY.
Detection of mLOY
The detection of mLOY in blood-derived DNA was performed using a pseudoautosomal region (PAR)-based detection approach initially developed for the assessment of autosomal mCAs28,53,54 and later adapted for detecting mLOY.29 Previous studies of mLOY relied on total signal intensity of array-genotyped single nucleotide polymorphisms in the male-specific region of chromosome Y [i.e., log R ratio (LRR)-based approaches],36–43,45–48 whereas this improved approach uses both normalized total signal intensity (LRR; logged ratio of observed probe intensity to expected intensity) in the PAR, as well as phased B-allele frequency (BAF; a normalized measure of allelic intensity ratio of two alleles) deviation for improved sensitivity to detect mLOY.29 Given the sequence homology of the PAR between the X and Y chromosomes and the rarity of chromosome X mosaicism in males,55 mosaic alterations in chromosome Y can be determined based on phased allelic imbalances in genotyping intensities of paternal vs. maternal alleles at heterozygous sites in the PAR.29
The computational approach used to ascertain mLOY from the genotyping intensity data has been described previously28,29,53 and briefly below. First, we converted the raw intensity data from the GSA (.idat; two files per sample for the red and green channels) to genotype call files (.gtc) using Illumina’s AutoConvert Software; LRR and BAF values were generated during this conversion. Genotype phasing was performed using the Eagle2 algorithm to estimate haplotypes.56 Mosaic events were then detected using the MOsaic CHromosomal Alterations (MoChA) pipeline (https://github.com/freeseek/mocha), which involves a three-state hidden Markov model to identify phased BAF deviations.28,53 The detection of mCAs on the Y chromosome was based on array-genotyped variants in two PAR regions, PAR1 and PAR2 (including 627 and 109 markers, respectively); however, in practice, mLOY is primarily detected through PAR1, which is much larger than PAR2.29 The PAR mCA calls were post-processed to distinguish event types, with mLOY determined based on a size of Mb and relative copy number , taking into consideration of the BAF deviation estimation for those with relative copy number . Cellular fractions (percentage of cells harboring mLOY) were estimated based on BAF deviations,28,53 and “expanded mLOY” indicative of greater clonal expansion was defined as mLOY present in of cells.24
Glyphosate Exposure Assessment
Information on lifetime occupational use of glyphosate was obtained from questionnaires administered at AHS enrollment and two follow-up telephone interviews (Phases 2 and 3), as well as at the BEEA enrollment interview during the home visit for biospecimen collection. At AHS enrollment, participants were asked to report whether they have ever personally mixed or applied specific pesticides, including glyphosate, during their lifetime, and if so, the number of days in an average year and the number of years each pesticide was used. Subsequently, each of the AHS follow-up questionnaires collected updated information on pesticide use since enrollment or the previous interview, and the BEEA questionnaire collected information on pesticide use in the 12 months preceding the interview/blood collection. In addition to frequency and duration of pesticide use, participants also provided details on pesticide application practices and personal protective equipment use in each interview.
Combining responses from all four questionnaires, two metrics were created to characterize cumulative lifetime exposure to glyphosate. First, we calculated total lifetime days of glyphosate use by multiplying the number of days glyphosate was used per year by the number of years used (reported at AHS enrollment and in subsequent questionnaires for each intervening time interval up to and including BEEA enrollment) and summing the usage across questionnaires. Second, using pesticide-specific information reported on each questionnaire, we calculated intensity-weighted lifetime days of glyphosate use by multiplying total lifetime days of glyphosate use by an exposure intensity score that incorporated mixing or loading glyphosate, application method, repairing application equipment, and use of gloves and other personal protective equipment, and summing the values across questionnaires. This intensity score was estimated using an algorithm previously developed for the AHS, which assigned weights to each of the factors listed above based on what was known about their influence on pesticide exposure from exposure biomonitoring studies and published literature.57 In our analyses, total lifetime days and intensity-weighted lifetime days of glyphosate use were each categorized into quartiles based on the distribution among farmers with available data. After excluding those with missing data on lifetime days of glyphosate use () from the 1,653 BEEA farmers assessed for mLOY, the final analytic data set for this investigation consisted of 1,606 farmers.
Statistical Analysis
We estimated prevalence of mLOY according to cellular fraction among all farmers and across age groups. Descriptive statistics were also computed to examine distributions of basic participant characteristics, including demographic, lifestyle, and medical factors (reported on the BEEA enrollment questionnaire), overall and by mLOY status.
We performed multivariable logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between lifetime occupational glyphosate use (quartiles of total lifetime days or intensity-weighed lifetime days of use) and a) overall mLOY (any detectable mLOY; yes vs. no) or b) expanded mLOY (cellular fraction ; yes vs. no). We also conducted additional analyses evaluating associations according to cellular fraction ( vs. no mLOY and vs. no mLOY). All models were adjusted for established or implicated risk factors for mLOY, including age (continuous; years), (to account for the nonlinear association between age and mLOY), cigarette smoking status and pack-years (tertiles) of smoking (never, former and pack-years, former and pack-years, former and pack-years, current and pack-years, current and pack-years, current and pack-years), alcohol consumption (0, 1–2, 3–6, drinks in the past 7 d), body mass index (BMI; , , , ), history of diabetes (no, yes), and history of hypertension/heart disease (no, yes),42 as well as study design-related variables [state (Iowa, North Carolina) and source of DNA (whole blood, buffy coat)]. Models were also adjusted for any other pesticide whose use was correlated with glyphosate use (Spearman correlation coefficient based on total lifetime days or intensity-weighted lifetime days of use, as in previous analyses in the AHS and BEEA17,58); the only pesticide meeting this criterion was 2,4-dichlorophenoxyacetic acid (2,4-D), another widely used herbicide. Linear trends across quartiles were assessed by modeling quartile-specific median values of total lifetime days or intensity-weighted lifetime days of glyphosate use as a continuous variable.
To investigate potential effect modification by factors previously associated with mLOY, we conducted stratified analyses to estimate ORs and 95% CIs within subgroups defined by age ( y, y), smoking status [never, ever (former or current)], and BMI [nonobese (), obese ()], with the hypothesis that the associations would be stronger among farmers who may be more susceptible to mLOY (i.e., older, ever smokers, and nonobese). We also conducted stratified analyses by state of residence (Iowa, North Carolina) to investigate whether potential differences in unmeasured factors across study site or geographic location impacted our results. In addition, we evaluated statistical evidence of multiplicative interaction by creating a cross-product term between glyphosate use (quartile-specific medians) and each of the potential effect modifiers and assessing its statistical significance using a likelihood-ratio test.
We conducted several sensitivity analyses to evaluate the robustness of our findings, including restricting analyses to participants of European ancestry () or participants whose DNA was extracted from whole blood samples (), to remove any potential influence of including participants with other genetic ancestry or other sample type on the results. Furthermore, to investigate the potential impact of duration of sample storage on our results, we performed analyses additionally adjusting for year of blood sample collection. We also ran models additionally adjusted for alcohol consumption at AHS enrollment (1993–1997) to assess potential confounding by longer-term exposure to alcohol.
Statistical analyses were performed using SAS (version 9.4; SAS Institute Inc.). All tests were two-sided, and statistical significance was evaluated at .
Results
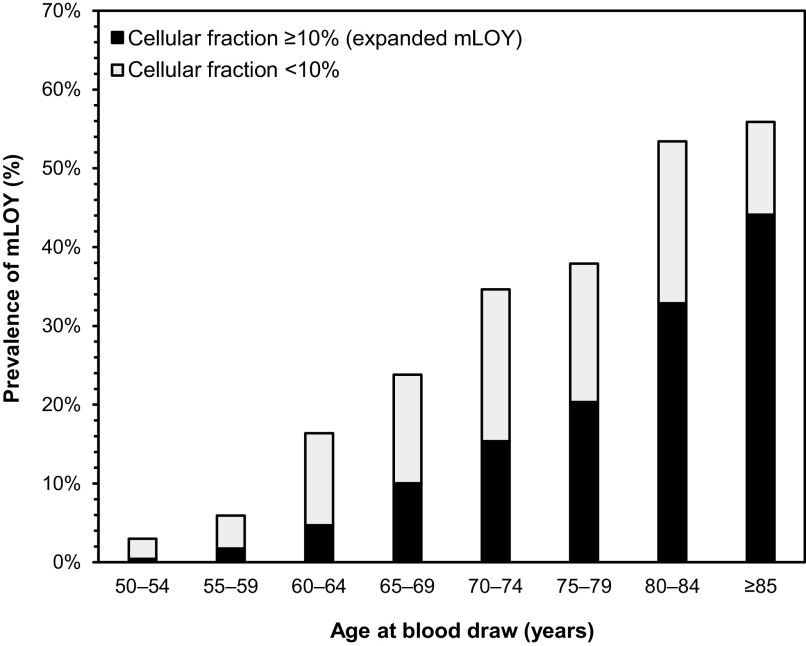
Overall, mLOY was detected in 343 (21.4%) of the 1,606 male farmers included in this analysis (age y, range: 50–96 y). Among farmers with mLOY, the proportion of affected cells ranged from 1.4% to 76.2%, and nearly half (; 9.8% of all participants) had mLOY present in of cells (i.e., expanded mLOY). As shown in Figure 1 and Table S1, the prevalence of mLOY increased with age, ranging from 3.0% among those 50–54 years of age to 55.9% among those years of age; the corresponding prevalence were 0.4% and 44.1%, respectively, for expanded mLOY, which accounted for a larger proportion of overall mLOY with advancing age.
Figure 1.
Age-specific prevalence of mosaic loss of chromosome Y according to cellular fraction among male farmers in the Biomarkers of Exposure and Effect in Agriculture study. The corresponding numerical data for this figure are shown in Table S1. Note: mLOY, mosaic loss of chromosome Y.
Table 1 presents characteristics of BEEA participants overall and by mLOY status. The majority of participants were of European ancestry (99%), resided in Iowa (77%), and had DNA extracted from whole blood (93%). Compared with those without mLOY, farmers with mLOY were older and more likely to be smokers, drink less alcohol, have lower BMI, and have a history of diabetes or hypertension/heart disease. After adjustment for age and simultaneously for other variables, current (vs. never) smoking, regardless of pack-years, was positively associated with both overall (; 95% CI: 1.74, 5.96) and expanded (; 95% CI: 1.42, 6.87) mLOY; we also observed a pattern of increasing ORs across tertiles of pack-years among former smokers and higher ORs among all tertiles of pack-years for current smokers (Table S2). In addition, we noted a suggestive inverse association with expanded mLOY for higher BMI, particularly class II/III obesity [ vs. (reference) ; (95% CI: 0.24, 1.14); Table S2].
Table 1.
Selected characteristics of male farmers in the BEEA study, overall and according to mLOY status.
| Characteristic | Overall () | No mLOY () | mLOY () | -Valuea |
|---|---|---|---|---|
| Age (y) | ||||
| Median (IQR) | 64 (58–72) | 62 (56–69) | 72 (66–77) | |
| Genetic ancestry | ||||
| European | 1,593 (99) | 1,252 (99) | 341 (99) | 1.00 |
| Otherb | 13 (1) | 11 (1) | 2 (1) | |
| State | ||||
| Iowa | 1,237 (77) | 984 (78) | 253 (74) | 0.11 |
| North Carolina | 369 (23) | 279 (22) | 90 (26) | |
| Source of DNA | ||||
| Whole blood | 1,500 (93) | 1,178 (93) | 322 (94) | 0.69 |
| Buffy coat | 106 (7) | 85 (7) | 21 (6) | |
| Smoking status and pack-yearsc | ||||
| Never | 950 (60) | 770 (62) | 180 (54) | 0.001 |
| Former (tertile) | ||||
| T 1 () | 187 (12) | 155 (12) | 32 (10) | |
| T 2 () | 188 (12) | 147 (12) | 41 (12) | |
| T 3 () | 186 (12) | 126 (10) | 60 (18) | |
| Current (tertile) | ||||
| T 1 () | 24 (2) | 17 (1) | 7 (2) | |
| T 2 () | 23 (1) | 16 (1) | 7 (2) | |
| T 3 () | 23 (1) | 16 (1) | 7 (2) | |
| Alcohol consumption (servings/wk)d | ||||
| 0 | 798 (50) | 609 (48) | 189 (55) | 0.045 |
| 1–2 | 292 (18) | 228 (18) | 64 (19) | |
| 3–6 | 247 (15) | 200 (16) | 47 (14) | |
| 269 (17) | 226 (18) | 43 (13) | ||
| BMI () | ||||
| 252 (16) | 178 (14) | 74 (22) | 0.004 | |
| 25 to | 716 (45) | 567 (45) | 149 (43) | |
| 30 to | 441 (27) | 353 (28) | 88 (26) | |
| 197 (12) | 165 (13) | 32 (9) | ||
| History of diabetes | ||||
| No | 1,369 (85) | 1,088 (86) | 281 (82) | 0.05 |
| Yes | 237 (15) | 175 (14) | 62 (18) | |
| History of hypertension/heart disease | ||||
| No | 763 (48) | 621 (49) | 142 (41) | 0.01 |
| Yes | 843 (52) | 642 (51) | 201 (59) | |
Note: Data are presented as frequencies and percentages [ (%)] unless otherwise specified. BEEA, Biomarkers of Exposure and Effect in Agriculture; BMI, body mass index; IQR, interquartile range; mLOY, mosaic loss of chromosome Y; T, tertile.
-Value for difference between participants with and without mLOY, calculated using the Wilcoxon rank-sum test for age, Fisher’s exact test for genetic ancestry, and chi-square test for all other variables.
Including African ( African ancestry), African-European (50% to African and 20% to European ancestry), and admixed African (50% to African and of each of European and Asian ancestries).
Numbers do not sum up to the total owing to missing data on pack-years for 22 former and 3 current smokers.
Number of servings of alcoholic beverages in the past 7 d. One serving of an alcoholic beverage was defined as 12 fluid oz (355 ml) of beer, 5 fluid oz (148 ml) of wine, or 1.5 fluid oz (44 ml) of hard liquor.
In fully adjusted models, we observed a positive association with expanded mLOY for increasing total lifetime days of use [highest vs. lowest quartile; (95% CI: 1.00, 3.07), ; Table 2]. A similar association was observed between intensity-weighted lifetime days of use and expanded mLOY, although with only suggestive evidence of an exposure–response trend [ (95% CI: 1.00, 3.06), ; Table 2]. These fully adjusted associations with expanded mLOY showed similar patterns but were slightly stronger compared with those observed in minimally adjusted models with age, , state of residence, source of DNA, smoking, and BMI as the only covariates (Table S3). Similar associations with expanded mLOY were also observed when mLOY with cellular fraction were excluded from the analysis (i.e., expanded mLOY vs. no mLOY; Table S4). In contrast, we observed little evidence of an association between lifetime occupational glyphosate use and overall mLOY in fully adjusted models, except in the second (vs. lowest) quartile of intensity-weighted lifetime days of use [ (95% CI: 1.01, 2.09); Table 2]. No associations were observed with overall mLOY in minimally adjusted models (Table S3) or with mLOY affecting of cells compared with no mLOY (Table S4).
Table 2.
Associations between lifetime occupational glyphosate use and overall or expanded mLOY among male farmers in the BEEA study.
| Glyphosate use | Overall mLOYa | Expanded mLOYb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI)c | d | OR (95% CI)c | d | ||||||
| Total lifetime days (quartile) | |||||||||
| Q 1 (0–26) | 402 | 300 | 102 | 1.00 (Ref) | 0.47 | 351 | 51 | 1.00 (Ref) | 0.03 |
| Q 2 () | 402 | 306 | 96 | 1.21 (0.85, 1.73) | 363 | 39 | 1.01 (0.62, 1.65) | ||
| Q 3 () | 403 | 327 | 76 | 1.22 (0.83, 1.79) | 371 | 32 | 1.23 (0.72, 2.07) | ||
| Q 4 () | 399 | 330 | 69 | 1.23 (0.81, 1.89) | 363 | 36 | 1.75 (1.00, 3.07) | ||
| Intensity-weighted lifetime days (quartile) | |||||||||
| Q 1 (0–1,341) | 401 | 303 | 98 | 1.00 (Ref) | 0.58 | 352 | 49 | 1.00 (Ref) | 0.07 |
| Q 2 () | 400 | 301 | 99 | 1.45 (1.01, 2.09) | 358 | 42 | 1.30 (0.80, 2.13) | ||
| Q 3 () | 401 | 327 | 74 | 1.19 (0.80, 1.77) | 372 | 29 | 1.03 (0.59, 1.77) | ||
| Q 4 () | 400 | 328 | 72 | 1.29 (0.85, 1.98) | 362 | 38 | 1.75 (1.00, 3.06) | ||
Note: BEEA, Biomarkers of Exposure and Effect in Agriculture; BMI, body mass index; CI, confidence interval; mLOY, mosaic loss of chromosome Y; OR, odds ratio; Q, quartile; Ref, reference.
Any detectable mLOY (yes vs. no).
mLOY affecting of cells [yes vs. no (cellular fraction or no mLOY)].
Calculated using a logistic regression model adjusted for age (years; continuous), , state of residence (Iowa, North Carolina), source of DNA (whole blood, buffy coat), smoking status and pack-years (never, former/tertile 1, former/tertile 2, former/tertile 3, current/tertile 1, current/tertile 2, current/tertile 3, missing), alcohol consumption (0, 1–2, 3–6, servings in the past 7 d), BMI (, , , ), history of diabetes (no, yes), history of hypertension/heart disease (no, yes), and lifetime occupational use of 2,4-dichlorophenoxyacetic acid (quartiles of total lifetime days or intensity-weighted lifetime days; missing as a separate category).
Calculated by modeling quartile-specific median values of total lifetime days or intensity-weighted lifetime days of glyphosate use as a continuous variable.
In stratified analyses, the positive association between total lifetime days of glyphosate use and expanded mLOY was most prominent among farmers years of age [highest vs. lowest quartile; (95% CI: 1.13, 4.67), ], never smokers [ (95% CI: 1.04, 5.21), ], and those with BMI [ (95% CI: 0.99, 4.19), ]; however, we did not detect statistically significant interactions between glyphosate use and age, smoking status, or BMI (Table 3). Furthermore, analysis stratified by state of residence showed similar magnitudes of associations between total lifetime days of glyphosate use and expanded mLOY among farmers living in Iowa and North Carolina (highest vs. lowest quartile; and 1.96, respectively). Stratified analyses for the associations between intensity-weighted lifetime days of glyphosate use and expanded mLOY (Table S5) revealed similar patterns as those observed for total lifetime days of use.
Table 3.
Associations between total lifetime days of occupational glyphosate use and expanded mLOY (cellular fraction ) among male farmers in the BEEA study, stratified by age group, smoking status, BMI, or state of residence.
| Subgroup | Total lifetime days of glyphosate use | a | b | |||
|---|---|---|---|---|---|---|
| Q 1 | Q 2 | Q 3 | Q 4 | |||
| Age group | ||||||
| y | ||||||
| Cases/total () | 11/218 | 12/263 | 12/303 | 12/305 | ||
| OR (95% CI)c | 1.00 (Ref) | 1.15 (0.46, 2.87) | 1.32 (0.52, 3.33) | 1.32 (0.47, 3.68) | 0.64 | 0.54 |
| y | ||||||
| Cases/total () | 40/184 | 27/139 | 20/100 | 24/94 | ||
| OR (95% CI)c | 1.00 (Ref) | 1.04 (0.57, 1.90) | 1.26 (0.65, 2.47) | 2.30 (1.13, 4.67) | 0.01 | |
| Smoking status | ||||||
| Never smokers | ||||||
| Cases/total () | 23/229 | 21/230 | 18/256 | 20/235 | ||
| OR (95% CI)c | 1.00 (Ref) | 1.23 (0.61, 2.50) | 1.34 (0.64, 2.81) | 2.32 (1.04, 5.21) | 0.04 | 0.24 |
| Ever smokers | ||||||
| Cases/total () | 28/173 | 18/172 | 14/147 | 16/164 | ||
| OR (95% CI)c | 1.00 (Ref) | 0.79 (0.39, 1.60) | 1.04 (0.47, 2.26) | 1.43 (0.63, 3.24) | 0.25 | |
| BMI | ||||||
| (nonobese) | ||||||
| Cases/total () | 30/254 | 25/234 | 20/230 | 26/250 | ||
| OR (95% CI)c | 1.00 (Ref) | 1.03 (0.55, 1.93) | 1.45 (0.73, 2.86) | 2.04 (0.99, 4.19) | 0.03 | 0.15 |
| (obese) | ||||||
| Cases/total () | 21/148 | 14/168 | 12/173 | 10/149 | ||
| OR (95% CI)c | 1.00 (Ref) | 0.94 (0.42, 2.12) | 0.98 (0.41, 2.34) | 1.15 (0.44, 3.04) | 0.73 | |
| State of residence | ||||||
| Iowa | ||||||
| Cases/total () | 39/312 | 29/312 | 24/328 | 20/285 | ||
| OR (95% CI)c | 1.00 (Ref) | 1.08 (0.61, 1.92) | 1.23 (0.67, 2.26) | 1.62 (0.80, 3.30) | 0.16 | 0.53 |
| North Carolina | ||||||
| Cases/total () | 12/90 | 10/90 | 8/75 | 16/114 | ||
| OR (95% CI)c | 1.00 (Ref) | 0.82 (0.29, 2.31) | 1.17 (0.37, 3.66) | 1.96 (0.69, 5.52) | 0.11 | |
Note: BEEA, Biomarkers of Exposure and Effect in Agriculture; BMI, body mass index; CI, confidence interval; mLOY, mosaic loss of chromosome Y; OR, odds ratio; Q, quartile; Ref, reference.
Calculated by modeling quartile-specific median values of total lifetime days of glyphosate use as a continuous variable.
Calculated using likelihood-ratio test comparing models with and without a multiplicative interaction term between total lifetime days of glyphosate use (quartile-specific medians) and age group, smoking status, BMI, or state of residence.
Calculated using a logistic regression model adjusted for age (years; continuous), , state of residence (Iowa, North Carolina), source of DNA (whole blood, buffy coat), smoking status and pack-years (never, former/tertile 1, former/tertile 2, former/tertile 3, current/tertile 1, current/tertile 2, current/tertile 3, missing), alcohol consumption (0, 1–2, 3–6, servings in the past 7 d), BMI (, , , ), history of diabetes (no, yes), history of hypertension/heart disease (no, yes), and lifetime occupational use of 2,4-dichlorophenoxyacetic acid (quartiles of total lifetime days; missing as a separate category).
Our main results, including the positive association between lifetime glyphosate use and expanded mLOY, remained essentially unchanged in sensitivity analyses restricted to farmers of European ancestry (Table S6) or those with DNA extracted from whole blood samples (Table S7), and in analyses with additional adjustment for year of blood sample collection (Table S8) or alcohol consumption at AHS enrollment (Table S9).
Discussion
In this large study of male farmers with detailed pesticide exposure histories and genotype data, we found that although lifetime occupational glyphosate use was not associated with mLOY overall, it was positively associated with mLOY affecting of cells (expanded mLOY) and that this association was most apparent among farmers who were years of age, never smokers, or nonobese. To our knowledge, this is the first investigation of mLOY in an agricultural population and the first study to evaluate glyphosate exposure in relation to mLOY as a biomarker of genotoxicity, which has been identified by IARC as a key characteristic of carcinogens.59,60 Although future studies are needed to confirm the observed associations, our findings for glyphosate add to the limited literature on occupational and environmental exposures as contributors to mLOY, the most common acquired chromosomal alteration in men, and provide novel mechanistic evidence supporting the potential carcinogenicity of this widely used herbicide.
Owing to differences in population characteristics (e.g., age distribution and smoking characteristics) and methods used to detect mLOY, it is difficult to compare frequency estimates across studies. However, results from our study using an improved and more sensitive detection approach suggest that mLOY is common among BEEA participants, affecting approximately one-fifth of this population of middle-aged and older male farmers of predominantly European ancestry. This is similar to the prevalence of mLOY recently reported in the UK Biobank using a similar PAR-based detection (20%)29 and higher than most other studies of older adults using less sensitive LRR-based approaches ().36,41,42,45 The associations we observed with mLOY for established risk factors, particularly increasing age and smoking, are consistent with those reported in various other populations.29,36,39,41–47 Notably, the magnitude of the association between high lifetime glyphosate use and expanded mLOY in our study (highest vs. lowest quartile; ; Table 2) is within the range observed for former (vs. never) smoking (OR ) but weaker than for current smoking (OR ) in most other studies that evaluated associations with mLOY36,39,41,44,46; current smoking was also strongly associated with mLOY in our study (OR for both overall and expanded mLOY; Table S2). For comparison, each year increase in age was associated with a increase in odds of mLOY both in our study [ (95% CI: 1.10, 1.14); Table S2] and in a pooled analysis of three prospective cohort studies [ (95% CI: 1.12, 1.15)].36
Few studies have assessed the potential genotoxic effects of glyphosate exposure in humans.20–22 A study in Ecuador found evidence of increased DNA damage, as determined by strand breaks using the comet assay, among 24 community residents exposed to glyphosate through aerial spraying compared with 21 unexposed control residents living farther away.20 Similarly, in a larger study conducted in areas with aerial glyphosate spraying in Colombia ( across three separate populations), increased chromosomal damage, as indicated by micronuclei formation in lymphocytes, was detected within the same individuals 5 d post-spraying compared with before spraying in each population.21 Another study in Ecuador ( exposed and 90 control study participants) assessing chromosomal aberrations by karyotyping blood collected 2 y after the last aerial spraying reported no effects.22 Besides genotoxicity, there is also accumulating evidence linking glyphosate exposure to oxidative stress, another key characteristic of carcinogens, in both agricultural61–63 and general64,65 populations. In particular, we previously observed elevated concentrations of urinary 8-hydroxy-2′-deoxyguanosine, a biomarker of oxidative DNA damage, among a subset of farmers with more recent glyphosate use and higher urinary glyphosate levels in the BEEA study.63 It should however be noted that most of these previous human mechanistic studies of glyphosate relied on residential proximity-based exposure assessment20–22 or urinary markers that are only indicative of recent exposures,62–65 whereas our investigation used detailed, longitudinally reported information from multiple questionnaires over time to assess cumulative lifetime use of glyphosate. Moreover, unlike previous studies focusing on relatively short-term biomarkers of genotoxicity or other end points, our findings for mLOY, a presumably more stable indicator for long-term health,66 may be more relevant to the potential long-lasting effects of glyphosate on the development of cancer or other aging-related chronic diseases.
Although mLOY is identified as a structural chromosomal aberration and thus a biomarker of genotoxicity,60 it has also been suggested as an indicator of genomic instability in blood and other tissues.29 Our finding of an association between high lifetime glyphosate use and expanded mLOY, but not overall mLOY, provides evidence suggesting that glyphosate exposure could confer a selective advantage contributing to increased clonal expansion of cells bearing these genomic alterations.60 Recently, a study among Bangladeshi men also reported a stronger positive association between environmental exposure to arsenic, an established carcinogenic metal, and mLOY affecting a larger percentage of cells, supporting mLOY as a marker of carcinogen-induced genomic instability.46 In addition, the presence of mLOY in leukocytes may also reflect impaired immune function, as suggested by epidemiologic studies that observed associations between mLOY and alterations in blood cell counts,24,43,44 with potential implications for immunosurveillance in cancer.67 Taken together, our findings for mLOY provide new insights into potential biologic mechanisms through which glyphosate may contribute to genomic instability, which is another key characteristic of carcinogens (beyond genotoxicity and oxidative stress) with more limited evidence in relation to glyphosate exposure.8,68
Our findings corroborate those of in vitro and animal experiments that generally support the ability of glyphosate to induce genotoxic effects,6,8,68 including studies that have shown increased chromosomal aberrations in bone marrow cells of male rodents treated with glyphosate compared with control rodents69,70; however, none of these studies specifically assessed mLOY. Notably, as a potential indicator of cancer susceptibility, mLOY has been demonstrated to promote clonal hematopoiesis and leukemogenesis in mice.71 It has also been associated with altered hematologic parameters (e.g., reduced erythrocyte and elevated leukocyte and platelet counts)43,44 and both hematologic31–34 and nonhematologic35–38 malignancies in humans, with possibly stronger associations for mLOY affecting a larger fraction of cells.24,31,33 Although the specific roles of mLOY in carcinogenesis remain to be elucidated, it is an indicator of genomic instability that could be a potential mechanism underlying previously observed associations between glyphosate use and certain hematologic cancers. Notably, glyphosate use was positively associated with risk of AML, but not NHL, in the AHS17 and with NHL or its subtypes in other studies.10–14,18
Results from our stratified analyses further suggest that high lifetime glyphosate use may be more strongly associated with expanded mLOY among certain subpopulations. The stronger association observed among older farmers is not surprising given the increasing prevalence of mLOY, particularly expanded mLOY, with advancing age.24,29 It is also consistent with the hypothesis that an individual’s DNA repair capacity and ability to maintain a stable genome decline with age,72 possibly rendering older men more susceptible to the effects of cumulative exposure to glyphosate. Cigarette smoking, a strong risk factor for mLOY, did not appear to synergistically interact with glyphosate use in the association with expanded mLOY (). However, the strong association observed among never smokers, including a clear exposure–response relationship, confirms the robustness of our findings in the absence of confounding by smoking. Although our analyses adjusted for a metric incorporating both smoking status and pack-years of smoking, potential residual confounding related to timing, duration, intensity, or other characteristics of cigarette use could not be ruled out and may influence associations among former and current smokers.36 It is also possible that the effects of glyphosate exposure were obscured by those of smoking, a presumably much stronger risk factor for mLOY, which may explain the weaker association we observed in ever compared with never smokers. Interestingly, we observed a more prominent association between high lifetime glyphosate use and expanded mLOY among nonobese than obese farmers. Similar to our study, several others have also reported a statistically significant or suggestive inverse association between BMI (or obesity) and mLOY.39,42,44,46 However, it remains unclear whether and how lower adiposity may contribute to mLOY occurrence or serve as a proxy for other factors related to mLOY, especially given conflicting evidence regarding the impact of obesity on genomic stability in humans.73 Furthermore, although the toxicokinetics of glyphosate are not well understood, it is possible that body weight influences metabolism or excretion of glyphosate following exposure,4 which may partly explain the different magnitudes of associations with mLOY by obesity status; however, this hypothesis remains to be tested in future investigations.
A major strength of our study was the availability of detailed information on the use of glyphosate and other specific pesticides collected longitudinally across several decades. This allowed us to assess cumulative lifetime exposure to glyphosate while adjusting for correlated pesticide use. In addition, we were able to evaluate associations with both total lifetime days and intensity-weighted lifetime days (which incorporates information on factors potentially influencing exposure intensity); the consistent patterns observed for both metrics further strengthened our study findings. Another strength was the large sample size compared with most previous human mechanistic studies of glyphosate exposure, which permitted analyses that uncovered an important association with the smaller subset of mLOY (i.e., expanded mLOY), as well as stratified analyses that revealed stronger associations among certain subgroups. Finally, by assessing mLOY using the PAR-based approach incorporating both LRR and phased BAF data, we were able to detect mLOY with improved sensitivity compared with previous studies using other methods.29
Our study also has several limitations. First, given the cross-sectional nature of the study design, we could not evaluate the relationship between glyphosate use and initial development of mLOY, which was assessed in blood samples collected at a single point in time. However, as noted above, glyphosate exposure was based on longitudinally reported information across each farmer’s lifetime and was obtained prior and up to the time of biospecimen collection, which mitigates recall bias and provides some support for the potential temporal relationship with mLOY. Second, our analyses relied on glyphosate use reported by participants, which may be subject to nondifferential exposure misclassification, although previous studies in the AHS have demonstrated reasonable accuracy and reliability of self-reported lifetime pesticide use74,75 and significant correlations of questionnaire-assessed exposures and intensity metrics with pesticide biomarkers.76 Third, although our analyses adjusted for established and implicated risk factors for mLOY, as well as study design-related variables and any other pesticide correlated with glyphosate use, potential residual confounding by unmeasured/unknown exposures or factors related to both glyphosate use and mLOY could not be ruled out. Fourth, the results of our study consisting of predominantly non-Hispanic White male farmers of European ancestry may not be generalizable to other populations. Notably, differences in mLOY prevalence by race/ethnicity and genetic ancestry have been observed in the UK Biobank (e.g., lower prevalence among Black or Asian compared with White men),36,42,44 although it remains unclear whether associations between potential carcinogens and mLOY may differ by these characteristics. Future studies including more racially and ethnically diverse agricultural populations, as well as individuals who are not occupationally exposed to glyphosate (i.e., individuals in the general population), are needed to confirm whether our findings are more widely applicable to other populations. Finally, although our study focused on mLOY as the outcome of interest, we had limited power to evaluate other mCAs, particularly those affecting the autosomes, which are much less frequently detected than mLOY but have also been associated with increased cancer risk.24,77 Future research with larger samples or analyses pooling data across studies may provide a better understanding of the potential relationships between glyphosate exposure and different forms of mCAs.
In conclusion, our study contributes new evidence that high lifetime use of glyphosate among older male farmers may be positively associated with mLOY, particularly mLOY affecting a larger fraction of cells, suggesting that glyphosate may confer genotoxic or selective effects relevant for clonal expansion. Given the current understanding about mLOY as a potential indicator of genotoxicity and genomic instability, our findings provide biologic plausibility and shed light on possible underlying mechanisms for previously observed associations between glyphosate use and certain hematologic cancers. Future research is needed to confirm our novel findings and to further explore specific mechanisms or biomarkers through which glyphosate exposure may contribute to mLOY, other mCAs, and cancer risk.
Supplementary Material
Acknowledgments
We thank A. Miller, K. Torres, S. Woodruff, and M. Dunn (Westat, Rockville, Maryland) and A. Taylor (Information Management Services, Rockville, Maryland) for study coordination and data management. We gratefully acknowledge the participants of the Biomarkers of Exposure and Effect in Agriculture study who made this work possible.
This work was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI) (Z01 CP 010119) and the National Institute of Environmental Health Sciences (Z01 ES 049030). We acknowledge the research contributions of the Cancer Genomics Research Laboratory for their expertise, execution, and support of this research in the areas of project planning, wet laboratory processing of specimens, and bioinformatics analysis of generated data. This project has been funded in part with federal funds from the NCI/NIH, under NCI contract no. 75N910D00024. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Benbrook CM. 2016. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28(1):3, PMID: , 10.1186/s12302-016-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atwood D, Paisley-Jones C. 2017. Pesticides Industry Sales and Usage: 2008–2012 Market Estimates. Washington, DC: U.S. Environmental Protection Agency. https://www.epa.gov/sites/default/files/2017-01/documents/pesticides-industry-sales-usage-2016_0.pdf [accessed 16 September 2022]. [Google Scholar]
- 3.Gillezeau C, van Gerwen M, Shaffer RM, Rana I, Zhang L, Sheppard L, et al. 2019. The evidence of human exposure to glyphosate: a review. Environ Health 18(1):2, PMID: , 10.1186/s12940-018-0435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly A, Coggins MA, Koch HM. 2020. Human biomonitoring of glyphosate exposures: state-of-the-art and future research challenges. Toxics 8(3):60, PMID: , 10.3390/toxics8030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ospina M, Schütze A, Morales-Agudelo P, Vidal M, Wong LY, Calafat AM. 2022. Exposure to glyphosate in the United States: data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int 170:107620, PMID: , 10.1016/j.envint.2022.107620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marino M, Mele E, Viggiano A, Nori SL, Meccariello R, Santoro A. 2021. Pleiotropic outcomes of glyphosate exposure: from organ damage to effects on inflammation, cancer, reproduction and development. Int J Mol Sci 22(22):12606, PMID: , 10.3390/ijms222212606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, et al. 2015. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol 16(5):490–491, PMID: , 10.1016/S1470-2045(15)70134-8. [DOI] [PubMed] [Google Scholar]
- 8.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2017. Some organophosphate insecticides and herbicides. IARC Monogr Eval Carcinog Risks Hum 112:1–464, PMID: , https://publications.iarc.fr/549 [accessed 16 September 2022].31829533 [Google Scholar]
- 9.Kogevinas M. 2019. Probable carcinogenicity of glyphosate. BMJ 365:l1613, PMID: , 10.1136/bmj.l1613. [DOI] [PubMed] [Google Scholar]
- 10.McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, et al. 2001. Non-Hodgkin’s lymphoma and specific pesticide exposures in men: cross-Canada study of pesticides and health. Cancer Epidemiol Biomarkers Prev 10(11):1155–1163, PMID: . [PubMed] [Google Scholar]
- 11.Hardell L, Eriksson M, Nordstrom M. 2002. Exposure to pesticides as risk factor for non-Hodgkin’s lymphoma and hairy cell leukemia: pooled analysis of two Swedish case-control studies. Leuk Lymphoma 43(5):1043–1049, PMID: , 10.1080/10428190290021560. [DOI] [PubMed] [Google Scholar]
- 12.De Roos AJ, Zahm SH, Cantor KP, Weisenburger DD, Holmes FF, Burmeister LF, et al. 2003. Integrative assessment of multiple pesticides as risk factors for non-Hodgkin’s lymphoma among men. Occup Environ Med 60(9):e11, PMID: , 10.1136/oem.60.9.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson M, Hardell L, Carlberg M, Akerman M. 2008. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int J Cancer 123(7):1657–1663, PMID: , 10.1002/ijc.23589. [DOI] [PubMed] [Google Scholar]
- 14.Pahwa M, Beane Freeman LE, Spinelli JJ, Blair A, McLaughlin JR, Zahm SH, et al. 2019. Glyphosate use and associations with non-Hodgkin lymphoma major histological sub-types: findings from the North American Pooled Project. Scand J Work Environ Health 45(6):600–609, PMID: , 10.5271/sjweh.3830. [DOI] [PubMed] [Google Scholar]
- 15.Orsi L, Delabre L, Monnereau A, Delval P, Berthou C, Fenaux P, et al. 2009. Occupational exposure to pesticides and lymphoid neoplasms among men: results of a French case-control study. Occup Environ Med 66(5):291–298, PMID: , 10.1136/oem.2008.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocco P, Satta G, Dubois S, Pili C, Pilleri M, Zucca M, et al. 2013. Lymphoma risk and occupational exposure to pesticides: results of the Epilymph study. Occup Environ Med 70(2):91–98, PMID: , 10.1136/oemed-2012-100845. [DOI] [PubMed] [Google Scholar]
- 17.Andreotti G, Koutros S, Hofmann JN, Sandler DP, Lubin JH, Lynch CF, et al. 2018. Glyphosate use and cancer incidence in the Agricultural Health Study. J Natl Cancer Inst 110(5):509–516, PMID: , 10.1093/jnci/djx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leon ME, Schinasi LH, Lebailly P, Beane Freeman LE, Nordby KC, Ferro G, et al. 2019. Pesticide use and risk of non-Hodgkin lymphoid malignancies in agricultural cohorts from France, Norway and the USA: a pooled analysis from the AGRICOH consortium. Int J Epidemiol 48(5):1519–1535, PMID: , 10.1093/ije/dyz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward EM. 2018. Glyphosate use and cancer incidence in the Agricultural Health Study: an epidemiologic perspective. J Natl Cancer Inst 110(5):446–447, PMID: , 10.1093/jnci/djx247. [DOI] [PubMed] [Google Scholar]
- 20.Paz-y-Miño C, Sánchez ME, Arévalo M, Muñoz MJ, Witte T, De-la-Carrera GO, et al. 2007. Evaluation of DNA damage in an Ecuadorian population exposed to glyphosate. Genet Mol Biol 30(2):456–460, 10.1590/S1415-47572007000300026. [DOI] [Google Scholar]
- 21.Bolognesi C, Carrasquilla G, Volpi S, Solomon KR, Marshall EJ. 2009. Biomonitoring of genotoxic risk in agricultural workers from five Colombian regions: association to occupational exposure to glyphosate. J Toxicol Environ Health A 72(15–16):986–997, PMID: , 10.1080/15287390902929741. [DOI] [PubMed] [Google Scholar]
- 22.Paz-y-Miño C, Muñoz MJ, Maldonado A, Valladares C, Cumbal N, Herrera C, et al. 2011. Baseline determination in social, health, and genetic areas in communities affected by glyphosate aerial spraying on the northeastern Ecuadorian border. Rev Environ Health 26(1):45–51, PMID: , 10.1515/reveh.2011.007. [DOI] [PubMed] [Google Scholar]
- 23.Machiela MJ, Chanock SJ. 2013. Detectable clonal mosaicism in the human genome. Semin Hematol 50(4):348–359, PMID: , 10.1053/j.seminhematol.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zekavat SM, Lin SH, Bick AG, Liu A, Paruchuri K, Wang C, et al. 2021. Hematopoietic mosaic chromosomal alterations increase the risk for diverse types of infection. Nat Med 27(6):1012–1024, PMID: , 10.1038/s41591-021-01371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machiela MJ. 2019. Mosaicism, aging and cancer. Curr Opin Oncol 31(2):108–113, PMID: , 10.1097/CCO.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs KB, Yeager M, Zhou W, Wacholder S, Wang Z, Rodriguez-Santiago B, et al. 2012. Detectable clonal mosaicism and its relationship to aging and cancer. Nat Genet 44(6):651–658, PMID: , 10.1038/ng.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurie CC, Laurie CA, Rice K, Doheny KF, Zelnick LR, McHugh CP, et al. 2012. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat Genet 44(6):642–650, PMID: , 10.1038/ng.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loh PR, Genovese G, Handsaker RE, Finucane HK, Reshef YA, Palamara PF, et al. 2018. Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature 559(7714):350–355, PMID: , 10.1038/s41586-018-0321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson DJ, Genovese G, Halvardson J, Ulirsch JC, Wright DJ, Terao C, et al. 2019. Genetic predisposition to mosaic Y chromosome loss in blood. Nature 575(7784):652–657, PMID: , 10.1038/s41586-019-1765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo X, Dai X, Zhou T, Wang H, Ni J, Xue J, et al. 2020. Mosaic loss of human Y chromosome: what, how and why. Hum Genet 139(4):421–446, PMID: , 10.1007/s00439-020-02114-w. [DOI] [PubMed] [Google Scholar]
- 31.Wiktor A, Rybicki BA, Piao ZS, Shurafa M, Barthel B, Maeda K, et al. 2000. Clinical significance of Y chromosome loss in hematologic disease. Genes Chromosomes Cancer 27(1):11–16, PMID: , . [DOI] [PubMed] [Google Scholar]
- 32.Zhang LJ, Shin ES, Yu ZX, Li SB. 2007. Molecular genetic evidence of Y chromosome loss in male patients with hematological disorders. Chin Med J (Engl) 120(22):2002–2005, PMID: , 10.1097/00029330-200711020-00012. [DOI] [PubMed] [Google Scholar]
- 33.Wong AK, Fang B, Zhang L, Guo X, Lee S, Schreck R. 2008. Loss of the Y chromosome: an age-related or clonal phenomenon in acute myelogenous leukemia/myelodysplastic syndrome? Arch Pathol Lab Med 132(8):1329–1332, PMID: , 10.5858/2008-132-1329-LOTYCA. [DOI] [PubMed] [Google Scholar]
- 34.Chapiro E, Antony-Debre I, Marchay N, Parizot C, Lesty C, Cung HA, et al. 2014. Sex chromosome loss may represent a disease-associated clonal population in chronic lymphocytic leukemia. Genes Chromosomes Cancer 53(3):240–247, PMID: , 10.1002/gcc.22134. [DOI] [PubMed] [Google Scholar]
- 35.Noveski P, Madjunkova S, Sukarova Stefanovska E, Matevska Geshkovska N, Kuzmanovska M, Dimovski A, et al. 2016. Loss of Y chromosome in peripheral blood of colorectal and prostate cancer patients. PLoS One 11(1):e0146264, PMID: , 10.1371/journal.pone.0146264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou W, Machiela MJ, Freedman ND, Rothman N, Malats N, Dagnall C, et al. 2016. Mosaic loss of chromosome Y is associated with common variation near TCL1A. Nat Genet 48(5):563–568, PMID: , 10.1038/ng.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machiela MJ, Dagnall CL, Pathak A, Loud JT, Chanock SJ, Greene MH, et al. 2017. Mosaic chromosome Y loss and testicular germ cell tumor risk. J Hum Genet 62(6):637–640, PMID: , 10.1038/jhg.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loftfield E, Zhou W, Yeager M, Chanock SJ, Freedman ND, Machiela MJ. 2019. Mosaic Y loss is moderately associated with solid tumor risk. Cancer Res 79(3):461–466, PMID: , 10.1158/0008-5472.CAN-18-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haitjema S, Kofink D, van Setten J, van der Laan SW, Schoneveld AH, Eales J, et al. 2017. Loss of Y chromosome in blood is associated with major cardiovascular events during follow-up in men after carotid endarterectomy. Circ Cardiovasc Genet 10(4):e001544, PMID: , 10.1161/CIRCGENETICS.116.001544. [DOI] [PubMed] [Google Scholar]
- 40.Dumanski JP, Lambert JC, Rasi C, Giedraitis V, Davies H, Grenier-Boley B, et al. 2016. Mosaic loss of chromosome Y in blood is associated with Alzheimer disease. Am J Hum Genet 98(6):1208–1219, PMID: , 10.1016/j.ajhg.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumanski JP, Rasi C, Lönn M, Davies H, Ingelsson M, Giedraitis V, et al. 2015. Smoking is associated with mosaic loss of chromosome Y. Science 347(6217):81–83, PMID: , 10.1126/science.1262092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loftfield E, Zhou W, Graubard BI, Yeager M, Chanock SJ, Freedman ND, et al. 2018. Predictors of mosaic chromosome Y loss and associations with mortality in the UK Biobank. Sci Rep 8(1):12316, PMID: , 10.1038/s41598-018-30759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terao C, Momozawa Y, Ishigaki K, Kawakami E, Akiyama M, Loh PR, et al. 2019. GWAS of mosaic loss of chromosome Y highlights genetic effects on blood cell differentiation. Nat Commun 10(1):4719, PMID: , 10.1038/s41467-019-12705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin SH, Loftfield E, Sampson JN, Zhou W, Yeager M, Freedman ND, et al. 2020. Mosaic chromosome Y loss is associated with alterations in blood cell counts in UK Biobank men. Sci Rep 10(1):3655, PMID: , 10.1038/s41598-020-59963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong JYY, Margolis HG, Machiela M, Zhou W, Odden MC, Psaty BM, et al. 2018. Outdoor air pollution and mosaic loss of chromosome Y in older men from the Cardiovascular Health Study. Environ Int 116:239–247, PMID: , 10.1016/j.envint.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demanelis K, Delgado DA, Tong L, Jasmine F, Ahmed A, Islam T, et al. 2022. Somatic loss of the Y chromosome is associated with arsenic exposure among Bangladeshi men. Int J Epidemiol 52(4):1035–1046, PMID: , 10.1093/ije/dyac176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Bai Y, Wu X, Li G, Wei W, Fu W, et al. 2020. Polycyclic aromatic hydrocarbons exposure and their joint effects with age, smoking, and TCL1A variants on mosaic loss of chromosome Y among coke-oven workers. Environ Pollut 258:113655, PMID: , 10.1016/j.envpol.2019.113655. [DOI] [PubMed] [Google Scholar]
- 48.Bai Y, Guan X, Wei W, Feng Y, Meng H, Li G, et al. 2021. Effects of polycyclic aromatic hydrocarbons and multiple metals co-exposure on the mosaic loss of chromosome Y in peripheral blood. J Hazard Mater 414:125519, PMID: , 10.1016/j.jhazmat.2021.125519. [DOI] [PubMed] [Google Scholar]
- 49.Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. 1996. The Agricultural Health Study. Environ Health Perspect 104(4):362–369, PMID: , 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofmann JN, Beane Freeman LE, Lynch CF, Andreotti G, Thomas KW, Sandler DP, et al. 2015. The Biomarkers of Exposure and Effect in Agriculture (BEEA) study: rationale, design, methods, and participant characteristics. J Toxicol Environ Health A 78(21–22):1338–1347, PMID: , 10.1080/15287394.2015.1091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shearer JJ, Beane Freeman LE, Liu D, Andreotti G, Hamilton J, Happel J, et al. 2019. Longitudinal investigation of haematological alterations among permethrin-exposed pesticide applicators in the Biomarkers of Exposure and Effect in Agriculture study. Occup Environ Med 76(7):467–470, PMID: , 10.1136/oemed-2018-105559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sauvé JF, Locke SJ, Josse PR, Stapleton EM, Metwali N, Altmaier RW, et al. 2020. Characterization of inhalable endotoxin, glucan, and dust exposures in Iowa farmers. Int J Hyg Environ Health 228:113525, PMID: , 10.1016/j.ijheh.2020.113525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loh PR, Genovese G, McCarroll SA. 2020. Monogenic and polygenic inheritance become instruments for clonal selection. Nature 584(7819):136–141, PMID: , 10.1038/s41586-020-2430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vattathil S, Scheet P. 2016. Extensive hidden genomic mosaicism revealed in normal tissue. Am J Hum Genet 98(3):571–578, PMID: , 10.1016/j.ajhg.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou W, Lin SH, Khan SM, Yeager M, Chanock SJ, Machiela MJ. 2021. Detectable chromosome X mosaicism in males is rarely tolerated in peripheral leukocytes. Sci Rep 11(1):1193, PMID: , 10.1038/s41598-020-80948-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loh PR, Danecek P, Palamara PF, Fuchsberger C, Reshef YA, Finucane HK, et al. 2016. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet 48(11):1443–1448, PMID: , 10.1038/ng.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coble J, Thomas KW, Hines CJ, Hoppin JA, Dosemeci M, Curwin B, et al. 2011. An updated algorithm for estimation of pesticide exposure intensity in the Agricultural Health Study. Int J Environ Res Public Health 8(12):4608–4622, PMID: , 10.3390/ijerph8124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shearer JJ, Sandler DP, Andreotti G, Murata K, Shrestha S, Parks CG, et al. 2021. Pesticide use and kidney function among farmers in the Biomarkers of Exposure and Effect in Agriculture study. Environ Res 199:111276, PMID: , 10.1016/j.envres.2021.111276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, et al. 2016. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect 124(6):713–721, PMID: , 10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith MT, Guyton KZ, Kleinstreuer N, Borrel A, Cardenas A, Chiu WA, et al. 2020. The key characteristics of carcinogens: relationship to the hallmarks of cancer, relevant biomarkers, and assays to measure them. Cancer Epidemiol Biomarkers Prev 29(10):1887–1903, PMID: , 10.1158/1055-9965.EPI-19-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Souza Espindola Santos A, Parks CG, Senna MM, de Carvalho LVB, Meyer A. 2021. Exposure to pesticides and oxidative stress in Brazilian agricultural communities. Biomarkers 26(6):539–547, PMID: , 10.1080/1354750X.2021.1933593. [DOI] [PubMed] [Google Scholar]
- 62.Sidthilaw S, Sapbamrer R, Pothirat C, Wunnapuk K, Khacha-Ananda S. 2022. Effects of exposure to glyphosate on oxidative stress, inflammation, and lung function in maize farmers, Northern Thailand. BMC Public Health 22(1):1343, PMID: , 10.1186/s12889-022-13696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang VC, Andreotti G, Ospina M, Parks CG, Liu D, Shearer JJ, et al. 2023. Glyphosate exposure and urinary oxidative stress biomarkers in the Agricultural Health Study. J Natl Cancer Inst 115(4):394–404, PMID: , 10.1093/jnci/djac242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eaton JL, Cathey AL, Fernandez JA, Watkins DJ, Silver MK, Milne GL, et al. 2022. The association between urinary glyphosate and aminomethyl phosphonic acid with biomarkers of oxidative stress among pregnant women in the PROTECT birth cohort study. Ecotoxicol Environ Saf 233:113300, PMID: , 10.1016/j.ecoenv.2022.113300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makris KC, Efthymiou N, Konstantinou C, Anastasi E, Schoeters G, Kolossa-Gehring M, et al. 2022. Oxidative stress of glyphosate, AMPA and metabolites of pyrethroids and chlorpyrifos pesticides among primary school children in Cyprus. Environ Res 212(pt B):113316, PMID: , 10.1016/j.envres.2022.113316. [DOI] [PubMed] [Google Scholar]
- 66.Fukami M, Miyado M. 2022. Mosaic loss of the Y chromosome and men’s health. Reprod Med Biol 21(1):e12445, PMID: , 10.1002/rmb2.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forsberg LA. 2017. Loss of chromosome Y (LOY) in blood cells is associated with increased risk for disease and mortality in aging men. Hum Genet 136(5):657–663, PMID: , 10.1007/s00439-017-1799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rana I, Nguyen PK, Rigutto G, Louie A, Lee J, Smith MT, et al. 2023. Mapping the key characteristics of carcinogens for glyphosate and its formulations: a systematic review. Chemosphere 339:139572, PMID: , 10.1016/j.chemosphere.2023.139572. [DOI] [PubMed] [Google Scholar]
- 69.Bolognesi C, Bonatti S, Degan P, Gallerani E, Peluso M, Rabboni R, et al. 1997. Genotoxic activity of glyphosate and its technical formulation Roundup. J Agric Food Chem 45(5):1957–1962, 10.1021/jf9606518. [DOI] [Google Scholar]
- 70.Prasad S, Srivastava S, Singh M, Shukla Y. 2009. Clastogenic effects of glyphosate in bone marrow cells of Swiss albino mice. J Toxicol 2009:308985, PMID: , 10.1155/2009/308985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Q, Zhao L, Yang Y, Li S, Liu Y, Chen C. 2022. Mosaic loss of chromosome Y promotes leukemogenesis and clonal hematopoiesis. JCI Insight 7(3):e153768, PMID: , 10.1172/jci.insight.153768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vijg J, Suh Y. 2013. Genome instability and aging. Annu Rev Physiol 75:645–668, PMID: , 10.1146/annurev-physiol-030212-183715. [DOI] [PubMed] [Google Scholar]
- 73.Setayesh T, Nersesyan A, Mišík M, Ferk F, Langie S, Andrade VM, et al. 2018. Impact of obesity and overweight on DNA stability: few facts and many hypotheses. Mutat Res Rev Mutat Res 777:64–91, PMID: , 10.1016/j.mrrev.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Hoppin JA, Yucel F, Dosemeci M, Sandler DP. 2002. Accuracy of self-reported pesticide use duration information from licensed pesticide applicators in the Agricultural Health Study. J Expo Anal Environ Epidemiol 12(5):313–318, PMID: , 10.1038/sj.jea.7500232. [DOI] [PubMed] [Google Scholar]
- 75.Blair A, Tarone R, Sandler D, Lynch CF, Rowland A, Wintersteen W, et al. 2002. Reliability of reporting on life-style and agricultural factors by a sample of participants in the Agricultural Health Study from Iowa. Epidemiology 13(1):94–99, PMID: , 10.1097/00001648-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 76.Thomas KW, Dosemeci M, Coble JB, Hoppin JA, Sheldon LS, Chapa G, et al. 2010. Assessment of a pesticide exposure intensity algorithm in the Agricultural Health Study. J Expo Sci Environ Epidemiol 20(6):559–569, PMID: , 10.1038/jes.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin SH, Brown DW, Rose B, Day F, Lee OW, Khan SM, et al. 2021. Incident disease associations with mosaic chromosomal alterations on autosomes, X and Y chromosomes: insights from a phenome-wide association study in the UK Biobank. Cell Biosci 11(1):143, PMID: , 10.1186/s13578-021-00651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.