Abstract
BACKGROUND:
Nitric oxide (NO) has been identified as a signaling molecule generated during β-adrenergic receptor stimulation in the heart. Furthermore, a role for NO in triggering spontaneous Ca2+ release via S-nitrosylation of CaMKIIδ (Ca2+/calmodulin kinase II delta) is emerging. NO donors are routinely used clinically for their cardioprotective effects on the heart, but it is unknown how NO donors modulate the proarrhythmic CaMKII to alter cardiac arrhythmia incidence. We test the role of S-nitrosylation of CaMKIIδ at the Cysteine-273 inhibitory site and cysteine-290 activating site in cardiac Ca2+ handling and arrhythmogenesis before and during β-adrenergic receptor stimulation.
METHODS:
We measured Ca2+-handling in isolated cardiomyocytes from C57BL/6J wild-type (WT) mice and mice lacking CaMKIIδ expression (CaMKIIδ-KO) or with deletion of the S-nitrosylation site on CaMKIIδ at cysteine-273 or cysteine-290 (CaMKIIδ-C273S and -C290A knock-in mice). Cardiomyocytes were exposed to NO donors, S-nitrosoglutathione (GSNO; 150 μM), sodium nitroprusside (200 μM), and β-adrenergic agonist isoproterenol (100 nmol/L).
RESULTS:
Both WT and CaMKIIδ-KO cardiomyocytes responded to isoproterenol with a full inotropic and lusitropic Ca2+ transient response as well as increased Ca2+ spark frequency. However, the increase in Ca2+ spark frequency was significantly attenuated in CaMKIIδ-KO cardiomyocytes. The protection from isoproterenol-induced Ca2+ sparks and waves was mimicked by GSNO pretreatment in WT cardiomyocytes but lost in CaMKIIδ-C273S cardiomyocytes. When GSNO was applied after isoproterenol, this protection was not observed in WT or CaMKIIδ-C273S but was apparent in CaMKIIδ-C290A. In Langendorff-perfused isolated hearts, GSNO pretreatment limited isoproterenol-induced arrhythmias in WT but not CaMKIIδ-C273S hearts, while GSNO exposure after isoproterenol sustained or exacerbated arrhythmic events.
CONCLUSIONS:
We conclude that prior S-nitrosylation of CaMKIIδ at cysteine-273 can limit subsequent β-adrenergic receptor–induced arrhythmias, but that S-nitrosylation at cysteine-290 might worsen or sustain β-adrenergic receptor–induced arrhythmias. This has important implications for the administration of NO donors in the clinical setting.
Keywords: calcium, heart, nitric oxide
Novelty and Significance.
What Is Known?
Calcium flux is altered in cardiac cells exposed to nitric oxide donors and other conditions associated with protein nitrosylation.
The activity of CaMKII (Ca2+/calmodulin kinase II), a cardiac signaling kinase associated with proarrhythmic pathways, is mediated by nitrosylation.
What New Information Does This Article Contribute?
We show that cardiac CaMKII expression is necessary for increased calcium leak in myocytes treated with nitric oxide donors.
Using novel mouse models that resist CaMKII nitrosylation, we demonstrate that proarrhythmic calcium leak can be prolonged OR inhibited depending on the site of CaMKII nitrosylation.
Finally, we show that the C273 nitrosylation site on CaMKII protects against arrhythmias, both in vivo and ex vivo.
Previous groups have demonstrated that conditions favoring protein nitrosylation can alter myocyte and whole heart calcium handling. However, there is controversy on the direction of the effect, as nitric oxide donors have shown both positive and negative effects on the development of cardiac arrhythmias. Here, we show that nitrosylation of the cardiac kinase CaMKII at 2 distinct sites (C273 and C290) are intergral to nitrosylation-mediated calcium mishandling. Moreover, the 2 sites have opposing effects, as nitrosylation at C273 inhibits the kinase and prevents arrhythmias, while C290 nitrosylation prolongs CaMKII activity and worsens arrhythmic phenotypes in cells and isolated hearts. These findings link CaMKII to nitrosylative stress and suggest future clinical directions focussed on cardiac CaMKII inhibition to prevent arrhythmia.
Meet the First Author, see p 965
The ability of the heart to rapidly enhance output is mediated in part by stimulation of β-adrenergic receptors (β-ARs), which trigger increased release of intracellular Ca2+ from the sarcoplasmic reticulum (SR) and accelerated reuptake within cardiomyocytes.1,2 Excessive β-AR stimulation can lead to arrhythmias3 and heart failure4; therefore, an understanding of the downstream signaling pathways that lead to pathological Ca2+ handling is vital. Nitric oxide (NO) is a gaseous signaling molecule that can alter cardiac function5 and is produced endogenously within cardiomyocytes following β-AR stimulation.6 NO-releasing drugs (donors) have been used clinically for over a century for their protective actions on heart function7; however, the direct effects of NO on cardiomyocytes are still under investigation. NO can exert positive inotropic effects due to protein modification by S-nitrosylation,8 where NO covalently attaches to cysteine (cysteine) residues and alters protein activity.9 NO exposure (either endogenously produced or exogenously applied) has been regarded as cardioprotective in the context of ischemia-reperfusion, due to the S-nitrosylation of cardiac proteins.10
Recently, evidence has demonstrated that endogenous NO production is linked to increased spontaneous release of Ca2+ from the SR during β-adrenergic stimulation.11–13 These observations challenge the cardioprotective role of NO, as spontaneous Ca2+ leak is arrhythmogenic.14 The function of several cardiac Ca2+ handling proteins is reported to be modulated by S-nitrosylation, including the RyR2 (ryanodine receptor type 2), LTCC (L-type Ca2+ channel), and SERCA (SR Ca2+ ATPase).15 Interestingly, NO can alter the frequency of Ca2+ sparks in cardiomyocytes following β-AR stimulation in either a positive or negative manner,16 although the detailed mechanism by which NO can both enhance and reduce Ca2+ spark frequency is not clearly understood.
An emerging target for cardiac NO is the CaMKIIδ (CaM [Ca2+/calmodulin]–dependent kinase II delta),11,13,17 a nodal regulator of cardiac Ca2+ handling.18 The regulatory domain of CaMKIIδ contains 2 S-nitrosylation sites that alter its activity.19 S-nitrosylation at cysteine-290, following initial activation by Ca2+/CaM, causes autonomous activation of the kinase, consistent with the observation that NO exposure can enhance CaMKIIδ activity and increase Ca2+ sparks.11,13,20 In contrast, S-nitrosylation at cysteine-273 inhibits CaMKIIδ by preventing activation by Ca2+/CaM,19 suggesting a dual role for NO in mediating CaMKIIδ activity that may be alternately protective or pathological depending on intracellular conditions.
β-AR stimulation increases spontaneous Ca2+ release from RyR2 in a CaMKIIδ-dependent manner.4 β-AR stimulation also increases endogenous NO production in cardiomyocytes,13 which is necessary for the enhancement of Ca2+ sparks.11 We therefore hypothesized that arrhythmogenic activity at the cellular and whole heart levels would be prolonged by NO after β-adrenergic stimulation due to S-nitrosylation of the cysteine-290 site on CaMKIIδ. Further, we hypothesized that exposure to NO before β-AR stimulation would lead to S-nitrosylation of the cysteine-273 site on CaMKIIδ, reducing β-AR induced Ca2+ mishandling. Here, we tested the role of NO and CaMKIIδ on altered Ca2+ handling in the context of β-AR stimulation in isolated mouse cardiomyocytes and Langendorff-perfused mouse hearts from transgenic mice lacking expression of CaMKIIδ or the S-nitrosylation sites at cysteine-273 (inhibitory) or cysteine-290 (activating).
METHODS
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Mouse Models
Experiments were performed using 12- to 16-week-old male and female mice with 4 genotypes: C57BL/6J wild-type (WT), knockout mice with deletion of CaMKIIδ (CaMKIIδ-KO), and novel knock-in mouse models with a single mutation of the cysteine-273 or cysteine-290 S-nitrosylation sites on CaMKIIδ (CaMKIIδ-C273S and CaMKIIδ-C290A). The CaMKIIδ-C290A knock-in mice were generated by the UC Davis Mouse Biology Core and have been described previously.20 CaMKIIδ-C273S animals were generated using CRISPR/Cas9 genome editing at the Australian Phenomics Facility (Australian National University, Australia). For all mouse resources described here and materials listed below, please see the Major Resources Table in the Supplemental Material.
Protein Expression
The ventricles were dissected and snap-frozen in liquid nitrogen for protein analysis as previously described.21 CaMKIIδ expression was assessed using a primary antibody against CaMKIIδ (1:5000, Thermo Fisher PA5-22168) and GAPDH (1:10000, GeneTex GTX627408) for a loading control. Blots were then incubated with secondary mouse or rabbit antibodies conjugated with horse-radish peroxidase (1:10 000; Thermo Fisher 31430, 31460), visualized by chemiluminescence detection with Super-signal West Pico (Thermo Fisher), and imaged using a Syngene gel doc system.
Heart Fibrosis
Ventricles were dissected above the apex of the heart and fixed in 4% formalin (Sigma) for 24 hours. The fixed tissue was then transferred to PBS for 24 hours, followed by 30% sucrose in PBS for 48 hours. Masson trichrome staining of sections was performed by the Histology Unit at University of Otago. Slides were then scanned on an Aperio Slide Scanner (Leica) under 40× magnification. Blue target pixels (representing collagen) were then identified. Percent collagen is the sum of all regions from sample sections of target pixels divided by total pixels.
Measurement of NO Concentration
The amount of NO released following addition of NO donor S-nitrosoglutathione (GSNO) to our experimental buffer (Krebs-Ringer HEPES buffer; see below) was measured using an Apollo 1000 Free Radical analyser with an isoproterenol-NOPF100 NO microsensor (1 mm; World Precision Instruments). The sensor was calibrated with S-nitroso-N-Acetyl-D,L-Penicillamine (Toronto Research) in 0.1 M CuCl2 solution.22 Data were acquired using a Powerlab 2/25 and recorded in LabChart 8.1 (ADInstruments, New Zealand).
Calcium Imaging
Freshly isolated cardiomyocytes were loaded with 2 μM Fluo-4-AM (Thermo Fisher) for 20 minutes at room temperature, followed by wash and de-esterification for 30 minutes. Cardiomyocytes were field stimulated at 0.5 Hz for 30 s to establish a steady state before recording Ca2+ transients in line-scan mode (2 ms/line, 0.15×0.15 µm pixel size). Ca2+ sparks and the occurrence of waves was measured under quiescent conditions (30 seconds after termination of 0.5 Hz pacing). Total SR Ca2+ content was determined at the end of each experiment with a rapid 20-mM caffeine exposure in Ca2+-free Krebs-Ringer HEPES buffer following a 30-second train of 0.5 Hz stimulations. Representative images have been chosen as illustrative examples of the results.
Isolated Heart Function
Mouse hearts were excised and arrested in Ca2+-free buffer before being cannulated via the aorta and Langendorff-perfused. Baseline data were recorded for 10 minutes, followed by drug infusion for 10 minutes. Ventricular arrhythmic events from the LV pressure trace were evaluated and classified as outlined in Figure S1 and Table S1. The different types of arrhythmias observed were classified by an arrhythmia score, which indicated severity of the arrhythmias (Table S2).
ECG Recordings
ECGs were recorded from mice under 1.5% to 2% isoflurane. ECG measurements were acquired from lead II connected to a Powerlab and recorded in LabChart 8.1(ADInstruments). Recordings were made for 10 minutes with the last 5 minutes used for analysis performed in LabChart.
Data Analysis
All individual data points are shown for cardiomyocyte Ca2+ and isolated heart parameters along with the mean±SEM. For results reported in the text, the data are mean±SD. Isolated heart perfusion data were analyzed on laboratory Chart 8.1 (ADInstruments). Statistical analysis was performed using Prism 10 (GraphPad) and RStudio (Posit; McNemar test only). Numeric data (n>10) were analyzed for normality using Shapiro-Wilk test. Paired data (drug responses) with a normal distribution were analyzed using paired 2-tailed Student t test. Non-normally distributed data or n<10 were analyzed using a nonparametric alternative test (Wilcoxon matched-pairs signed rank test or a Friedman test). For group comparisons (and SR content), an unpaired t test or an ordinary 1-way ANOVA (Tukey multiply comparisons test) was used for normally distributed data, while a Mann-Whitney test or Kruskal-Wallis, was used for nonparametric data sets. Differences in the fraction of cardiomyocytes displaying Ca2+ waves were determined by a λ2 test (between groups) or a McNemar test (paired data). Values where P≤0.05 were considered statistically significant.
RESULTS
NO Donor Does Not Alter Baseline Ca2+ Transient Properties
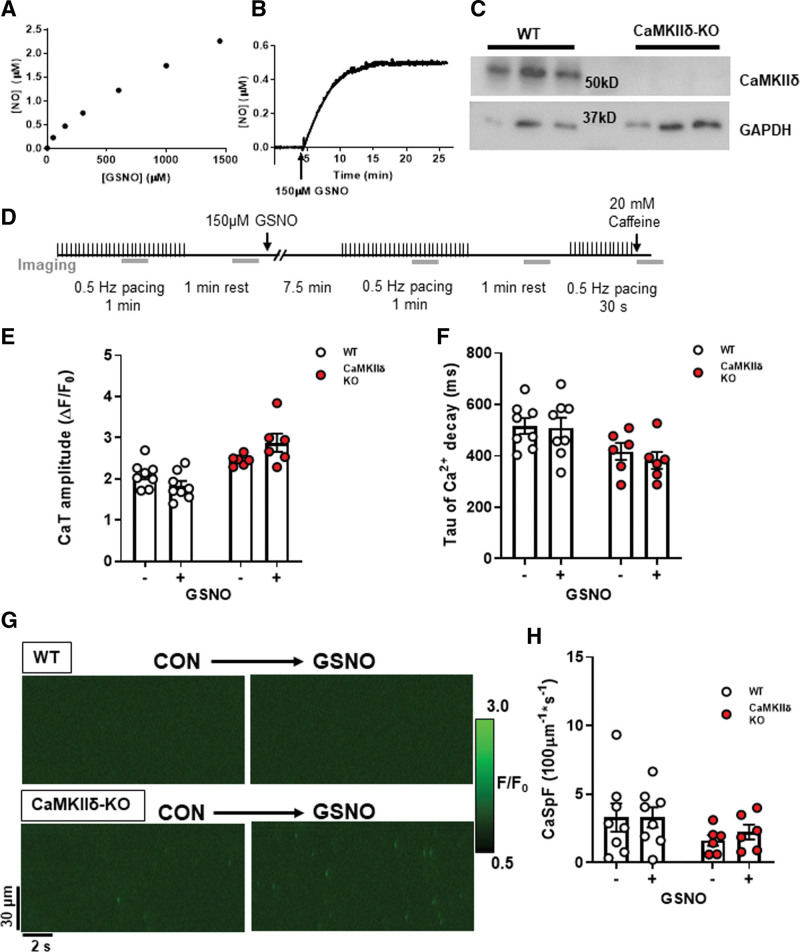
To induce S-nitrosylation of target proteins, we used the NO donor GSNO, which spontaneously releases NO into the perfusate (Figure 1A). [NO] peaked in Krebs-Ringer HEPES buffer at 0.54±0.06 μM after 10 minutes and remained stable over the duration of our experimental timeframe (Figure 1B). Ventricular cardiomyocytes were isolated from WT and CaMKIIδ-KO mice, which had undetectable expression of CaMKIIδ (Figure 1C). The cardiomyocytes were exposed to 150 μM GSNO according to the protocol outlined in Figure 1D. There were no observed effects of GSNO on Ca2+ transient amplitude (Figure 1E; control versus GSNO WT: P=3.1×10−1, KO: P=9.4×10−2) or time constant Tau of Ca2+ decay (Figure 1F; control versus GSNO WT: P=9.5×10−1, KO: P=1.6×10−1) in either WT or CaMKIIδ-KO myocytes. We also measured Ca2+ sparks in unpaced cardiomyocytes with confocal line-scan imaging (Figure 1G). We observed no effect of GSNO on Ca2+ spark frequency in cardiomyocytes of either genotype (Figure 1H; control versus GSNO WT: 5.5×10−1, KO: P=6.3×10−2). There was also no effect of GSNO on SR Ca2+ content (Figure S2). Taken together, our data show that at the baseline low frequency stimulation used here, there is no major difference in electrically evoked or spontaneous Ca2+ transients in WT versus CaMKIIδ-KO, nor does exposure to GSNO alone alter these properties.
Figure 1.
Cardiomyocyte Ca2+ transients and sparks with nitric oxide donor S-nitrosoglutathione (GSNO). A, Nitric oxide (NO) liberated from increasing GSNO concentrations in buffer was measured using a NO electrode. B, The kinetics of NO release following the addition of 150 μM GSNO to buffer. C, Western blot of ventricle tissue samples from wild-type (WT) and CaMKIIδ (Ca2+/calmodulin kinase II delta) KO mice (n=3 hearts) showing loss of CaMKIIδ protein expression in CaMKIIδ-KO vs WT hearts (with GAPDH loading controls). D, WT or CaMKIIδ-KO cardiomyocytes were treated with 150 μM GSNO and paced at 0.5 Hz. There was no change in Ca2+ transient amplitude (E) or decay kinetics (F). In quiescent cardiomyocytes, Ca2+ sparks were measured from line-scan images (G), and there was also no effect of GSNO on Ca2+ spark frequency in either WT or CaMKIIδ-KO cardiomyocytes (H; WT: n=8 cells, N=3 hearts; CaMKIIδ-KO: n=8 cells, N=3 hearts).
CaMKIIδ-KO Cardiomyocytes Produce Fewer Ca2+ Sparks During β-Adrenergic Stimulation
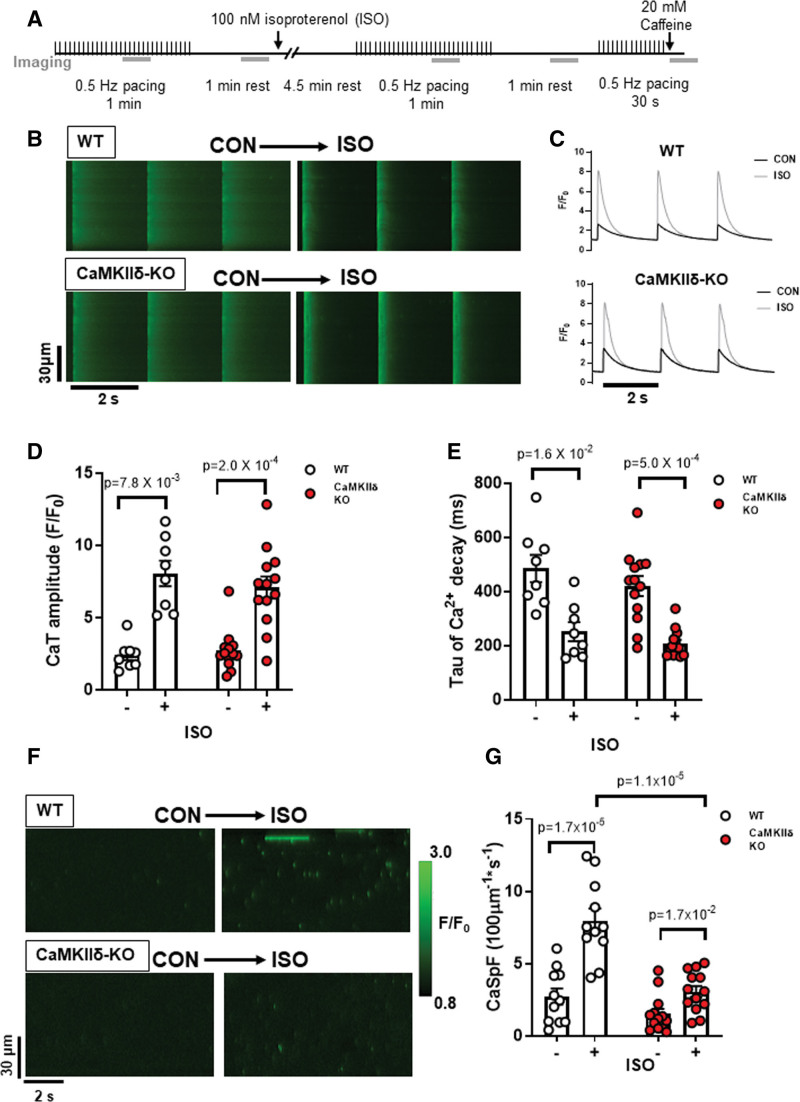
Next we increased myocyte Ca2+ transients with exposure to 100 nmol/L isoproterenol, which is expected to strongly promote CaMKIIδ activation (Figure 2A). Representative confocal line-scans from Fluo-4-AM loaded cardiomyocytes isolated from WT and CaMKIIδ-KO hearts are shown in Figure 2B. Fluo-4 fluorescence was normalized to baseline (F0) to determine the Ca2+ transient characteristics in WT and CaMKIIδ-KO cardiomyocytes during isoproterenol exposure (Figure 2C). Isoproterenol induced a 3-fold increase in Ca2+ transient amplitude (Figure 2D), while Ca2+ transient decay was twice as fast as in control conditions in both WT and CaMKIIδ-KO cardiomyocytes (Figure 2E). The SR Ca2+ content, as measured by peak Ca2+ release after caffeine application (Figure S2), was not significantly increased by isoproterenol for either mouse genotype (WT: P=8.1×10−2; KO: P=1.5×10−1) and did not differ between mouse genotypes (CON: P=3.8×10−1; isoproterenol: P=5.5×10−5); P =. isoproterenol increased Ca2+ spark frequency (WT: P=1.7×10−5; CaMKIIδ-KO: P=1.7×10−2) and amplitude (WT control 0.66±0.49 ΔF/F0 versus isoproterenol 1.21±0.61 ΔF/F0 P=2.0×10−3; CaMKIIδ-KO control 0.58±0.15 ΔF/F0 versus isoproterenol 0.83±0.29 ΔF/F0: P=2.6×10−2) in both WT and CaMKIIδ-KO cardiomyocytes; however, the increase in spark frequency was significantly attenuated in the CaMKIIδ-KO cardiomyocytes (P=1.1×10−5; Figure 2F and 2G), and there was a trend toward a smaller Ca2+ spark amplitude (P=7.2×10−2). Thus, while CaMKIIδ deletion did not prevent the effect of isoproterenol on overall Ca2+ transient properties, it limited spontaneous Ca2+ release during β-AR stimulation.
Figure 2.
Cardiomyocyte Ca2+ transient and spark properties in response to β-adrenergic agonist isoproterenol. A, Experimental protocol used for Ca2+ imaging, the gray bars represent when line-scan images were acquired. Representative line-scans during pacing (B) and resultant Ca2+ transients (C) from a WT and CaMKIIδ (Ca2+/calmodulin kinase II delta)-KO cardiomyocyte stimulated at 0.5 Hz under control conditions and with 100 nmol/L ISO. ISO increased Ca2+ transient amplitude (D) and accelerated decay (E) in both WT and CaMKIIδ-KO cardiomyocytes to a similar degree (WT n=8 cells, N=2 hearts; CaMKIIδ-KO n=13 cells, N=4 hearts). F, Representative line-scans from a WT and CaMKIIδ-KO cardiomyocyte show an increase in the number of Ca2+ sparks following exposure to ISO. Mean data show the ISO-induced increase in Ca2+ spark frequency (CaSpF) was attenuated in the CaMKIIδ-KO cardiomyocytes (G; WT: n=11 cells, N=2 hearts; CaMKIIδ-KO: n=13 cells, N=4 heats).
The cysteine-273 Site on CaMKIIδ Attenuates SR Ca2+ Release in Response to NO and Isoproterenol
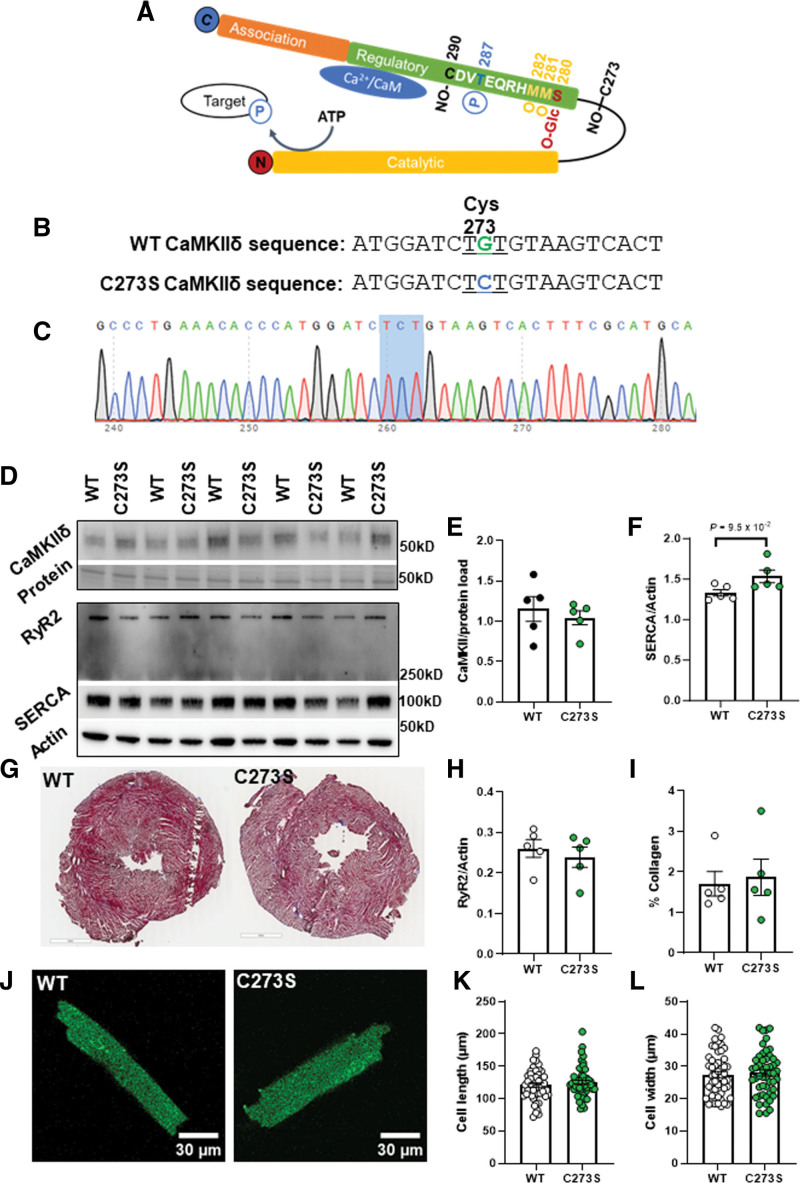
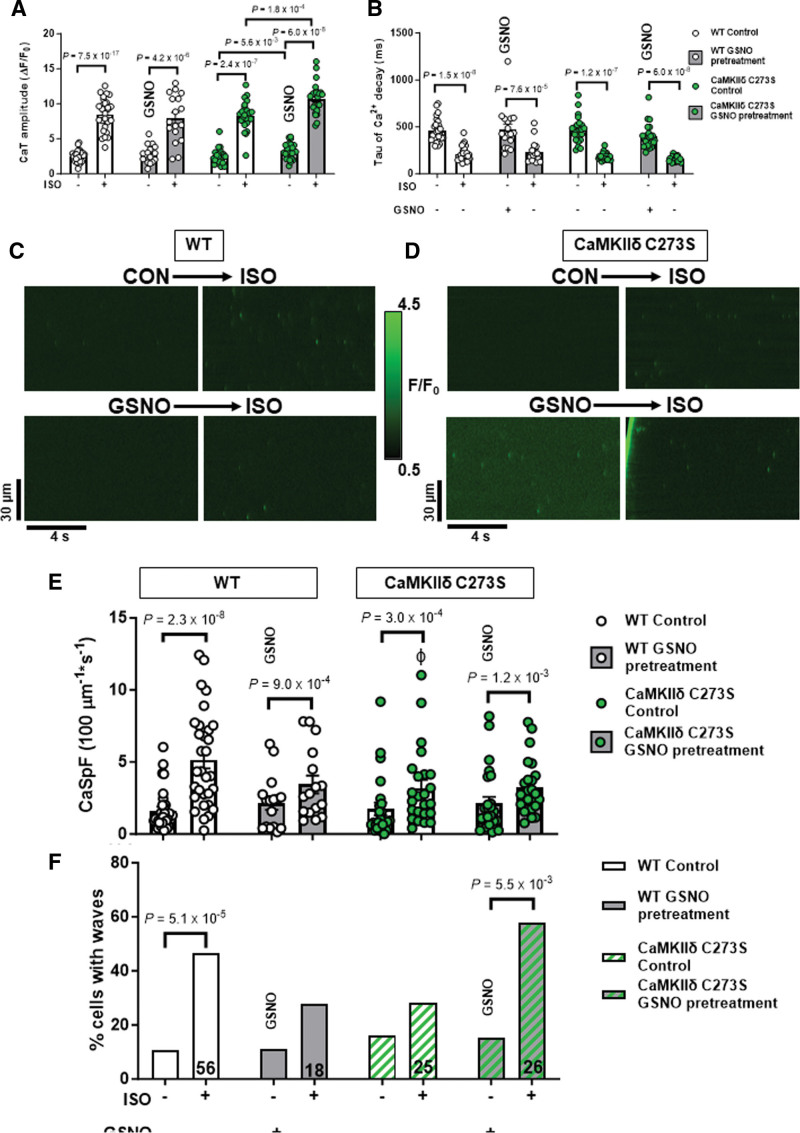
We previously showed that S-nitrosylation of the cysteine-273 site of CaMKIIδ in vitro can prevent activation of the kinase in response to increased Ca2+/CaM.19 We hypothesized that this mechanism might protect myocytes from the development of arrhythmogenic events by suppressing CaMKIIδ activity during transient periods of NO stress. To test this hypothesis, we generated knock-in mice that lack the inhibitory S-nitrosylation site (CaMKIIδ-C273S; Figure 3A through 3C). The CaMKIIδ-C273S mice had normal CaMKIIδ, RyR2 and SERCA2A expression in the ventricle (Figure 3D through 3H; WT versus C273S CaMKIIδ: P=5.5×10−1, RyR2: P=5.5×10−1, SERCA2A: 9.5×10−2). There was no evidence of ventricular fibrosis (Figure 3G and 3I; P=6.9×10−1) or cardiomyocyte hypertrophy (Figure 3J through 3L; cell length: P=5.9×10−1, cell width: P=6.9×10−1) in the CaMKIIδ-C273S mouse hearts compared with WT. Isolated ventricular myocytes from WT and CaMKIIδ-C273S mice were treated with 150 μM GSNO for 7 minutes immediately before wash-in of 100 nmol/L isoproterenol without GSNO. Preincubation of WT cardiomyocytes with GSNO had no effect on baseline Ca2+ transient amplitude (Figure 4A; WT: P=4.3×10−1); however, there was a slight increase in Ca2+ transient amplitude in CaMKIIδ-C273S cardiomyocytes (P=5.6×10−3). Time constant of [Ca2+]i decay was not affected by GNSO to cardiomyocytes in normal control Krebs-Ringer HEPES buffer (Figure 4B; WT: P=9.2×10−1; CaMKIIδ-C273S: P=2.5×10−3, not significant with a false discovery rate using a 2-stage step-up Benjamini, Krieger, and Yekutieli) relative. Moreover, WT cardiomyocytes pretreated with GSNO showed a typical Ca2+ transient response to isoproterenol, with Ca2+ transient amplitude (P=5.6×10−1) and decay (P=9.2×10−1) similar in magnitude to control WT cardiomyocytes not subject to GSNO pretreatment (Figure 4A and 4B). Notably, cardiomyocytes from CaMKIIδ-C273S mice pretreated with GSNO had larger isoproterenol-induced Ca2+ transients compared with CaMKIIδ-C273S cardiomyocytes not pretreated with GSNO (Figure 4A, bar 6 versus bar 8; P=1.8×10−4). These data indicate that genetic ablation of the inhibitory C273 S-nitrosylation site increases the effects of CaMKIIδ on SR Ca2+ release in the presence of NO and is consistent with our hypothesis that S-nitrosylation of the C273 site is protective in limiting CaMKIIδ activation and its effects on RyR2-related Ca2+ transient properties.
Figure 3.
Characterization of the CaMKIIδ (Ca2+/calmodulin kinase II delta)-C273S knock-in mouse model. Schematic of the CaMKIIδ monomer with the residue positions of various posttranslational modifications within the regulatory domain including S-nitrosylation (NO-), phosphorylation (P), oxidation (O), and O-GlcNAc modification, which are known to alter CaMKII activity (A). A mouse model was generated using CRISPR/cas9 causing a single point mutation in the cystine codon at position 273, resulting in replacement with a serine residue which cannot be S-nitrosylated (B). Example of a CaMKIIδ-C273S offspring confirmed with genotyping of ear notch (C). Protein expression in ventricular tissue (N=5 hearts) was measured using Western blots (D) for CaMKIIδ (E), SERCA (SR Ca2+ ATPase; F), RyR (ryanodine receptor type; H), and normalized to actin or protein load. Ventricular fibrosis was measured in fixed and stained tissue (G) by quantifying collagen content (I). Representative cardiomyocytes are shown in J for measurement of cell length (K) and width (L), wild type (WT): n=52, N=7 hearts; CaMKIIδ-C273S: n=50, N=8 hearts.
Figure 4.
Pretreatment with S-nitrosoglutathione (GSNO) enhances Ca2+ transient response to isoproterenol in CaMKIIδ (Ca2+/calmodulin kinase II delta)-C273S cardiomyocytes. Ca2+ transients from wild-type (WT) and CaMKIIδ C273S cardiomyocytes from were recorded at 0.5 Hz under control conditions and with 100 nmol/L ISO or with pretreatment of 150 μM GSNO before ISO exposure. Mean Ca2+ transient amplitude (A) and decay (B) data in response to ISO with control KRH buffer (white bars; WT: n=28 cells, N=14 hearts; CaMKIIδ-C273S: n=24 cells, N=14 hearts) or when pretreated with GSNO (gray bars; WT: n=18 cells, N=7 hearts; CaMKIIδ-C273S: n=24, N=9 hearts). Representative line-scans from quiescent WT (C) and CaMKIIδ-C273S (D) cardiomyocytes under control conditions and with ISO or with pretreatment of GSNO before ISO exposure. Mean Ca2+ spark frequency (E) data are shown for cardiomyocytes in response to ISO with control buffer (white bars; WT: n=31 cells, N=14 hearts; CaMKIIδ-C273S: n=24 cells, N=9 hearts) or when pretreated with GSNO (gray bars; WT: n=16 cells, N=7 hearts; CaMKIIδ-C273S: n=25 cells, N=9 hearts). Percentage of cardiomyocytes exhibiting Ca2+ waves in quiescent WT and CaMKIIδ-C273S cardiomyocytes (F). ɸP<0.05 compared with WT ISO, aP=0.0002 vs CaMKIIδ-C273S ISO, bP<0.0001 vs WT ISO with GSNO pretreatment.
Representative line-scans to detect spontaneous Ca2+ leak with GSNO pretreatment followed by isoproterenol are shown for WT (Figure 4C) and CaMKIIδ-C273S (Figure 4D) cardiomyocytes. Pretreatment with GSNO did not alter baseline Ca2+ spark frequency for either WT (P=5.4×10−1) or CaMKIIδ-C273S (P=3.1×10−1) cardiomyocytes (Figure 4E). Isoproterenol increased the frequency of Ca2+ sparks in both WT and CaMKIIδ-C273S cardiomyocytes. However, this isoproterenol-induced increase was attenuated in WT cardiomyocytes pretreated with GSNO (Figure 4E, bar 3 to 4: 2.6±2.0-fold versus 1 to 2: 4.6±4.3-fold; P=6.0×10−2). In the C273S myocytes pretreatment did not alter the isoproterenol-induced increase in Ca2+ sparks (Figure 4E, bar 7 to 8: 3.4±4.8-fold versus 5 to 6: 2.9-fold; P=6.6×10−1), consistent with our hypothesis that the inhibitory effect of S-nitrosylation at cysteine-273 was expected to be lost.
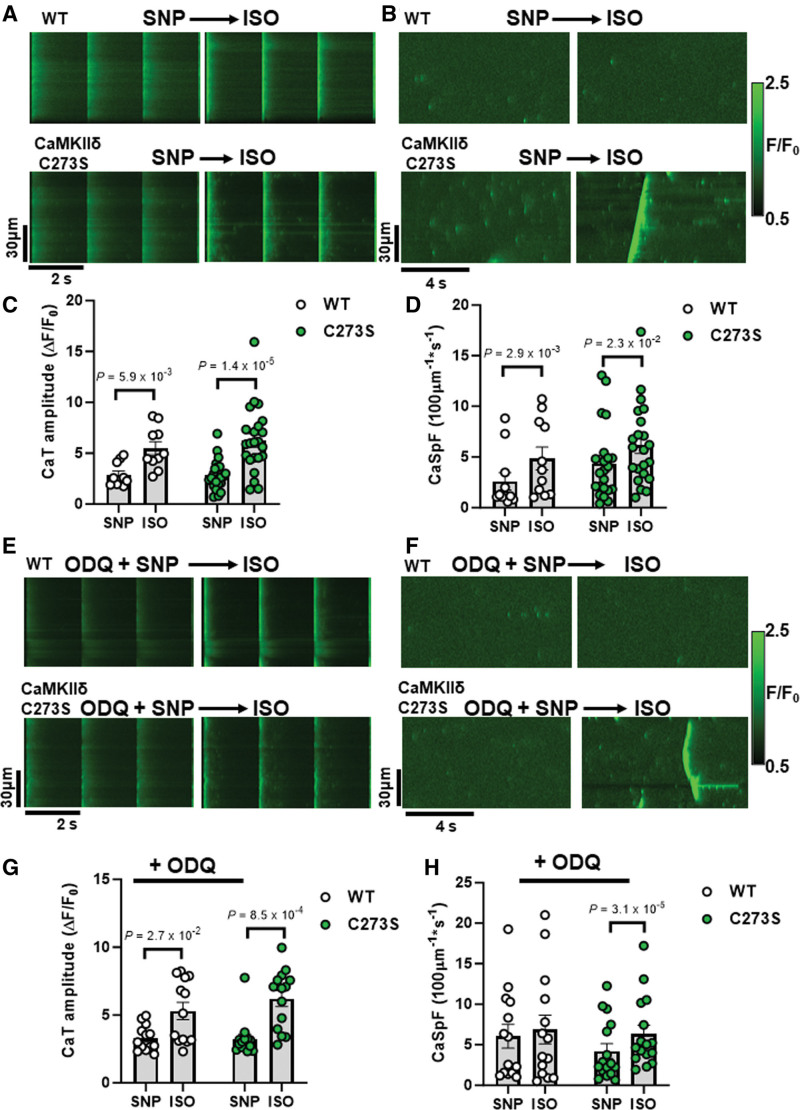
To further interrogate potential arrhythmogenic signaling, we quantified larger propagating proarrhythmic Ca2+ release events known as Ca2+ waves. Isoproterenol induced an increase in Ca2+ waves in WT cardiomyocytes, but in WT, this effect was suppressed by GSNO pretreatment (Figure 4F, left; P=2.5×10−1). In contrast, CaMKIIδ-C273S cardiomyocytes pretreated with GSNO robustly increased isoproterenol-induced Ca2+ waves (Figure 4F, right), suggesting that the inhibitory cysteine-273 S-nitrosylation site is important for preventing Ca2+ waves that can trigger action potentials and arrhythmias. We repeated the experiments with sodium nitroprusside (SNP), a NO donor used in clinical settings, which demonstrated a similar attenuation of Ca2+ sparks in the WT cardiomyocytes (as for GSNO pretreatment) in the presence of sGC (soluble guanylyl cyclase) inhibitor ODQ. This SNP-mediated reduction in Ca2+ sparks was lost in the CaMKIIδ-C273S cardiomyocytes, without altering the Ca2+ transient response to isoproterenol (Figure 5A through 5D). We infer that the SNP (and GSNO)–mediated protection against isoproterenol-induced arrhythmogenic SR Ca release events occurs primarily via S-nitrosylation of CaMKIIδ-C273 rather than through an sGC-dependent pathway.
Figure 5.
Pretreatment with clinical NO donor sodium nitroprusside (SNP) and sGC (soluble guanylyl cyclase) inhibitor elicits similar protection against isoproterenol-induced Ca2+ sparks. Ca2+ transients (A) and sparks (B) from wild-type (WT) and CaMKIIδ (Ca2+/calmodulin kinase II delta)-C273S cardiomyocyte were recorded at the end of a 7-minute incubation with SNP (200 μM) followed by wash-in of 100 nmol/L ISO. Mean Ca2+ transient amplitude and spark frequency data are shown in C and D, respectively (WT: n=10 cells, N=3 hearts; CaMKIIδ-C273S: n=23 cells, N=6 hearts). The same experiments were performed following a pretreatment with sGC inhibitor ODQ (10 μM; 20 minutes incubation) with representative line-scans (E and F) and mean data (G and H) plotted for WT (n=13 cells, N=3 hearts) and CaMKIIδ-C273S (n=14 cells, N=4 hearts) cardiomyocytes. ISO indicates isoproterenol.
NO Treatment After β-AR Activation Does Not Alter Ca2+ Transients or Sparks
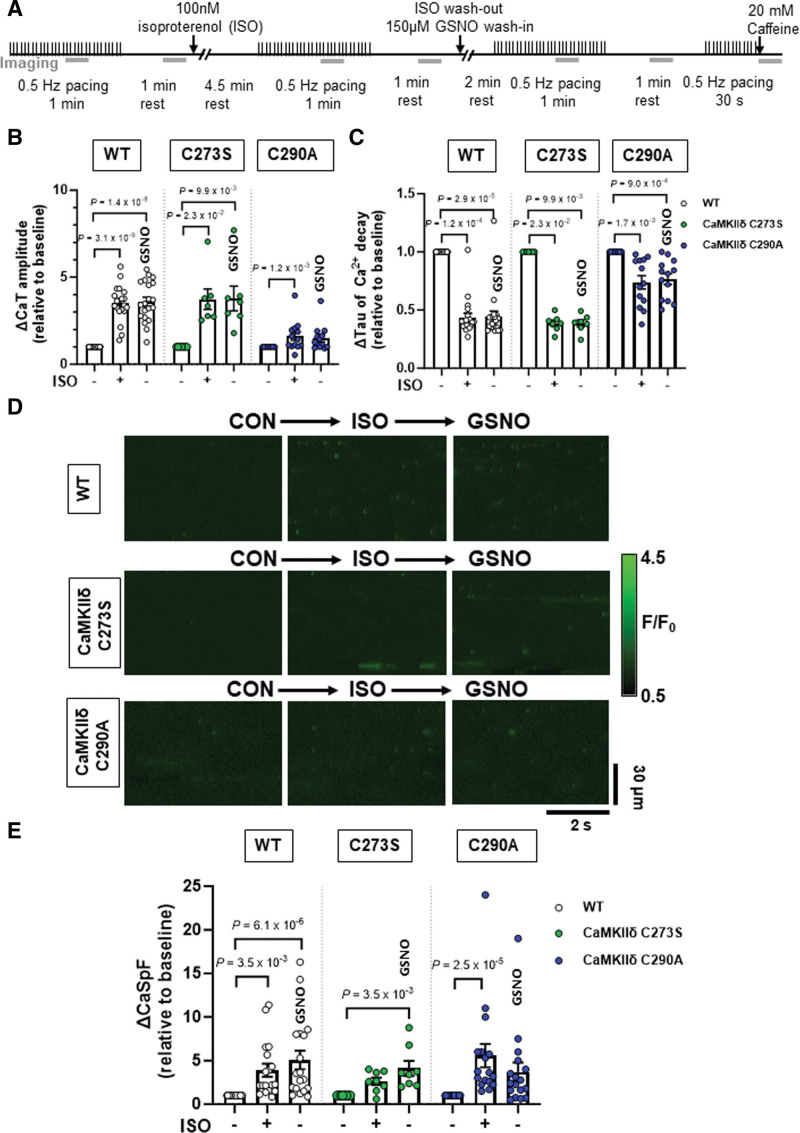
Previous literature has demonstrated a role for autonomous activation of CaMKIIδ by S-nitrosylation at cysteine-29019 and enhanced Ca2+ release events mediated by CaMKII.13 Due to the position of the cysteine-290 in the CaMKIIδ regulatory domain adjacent to the Thr-287 autophosphorylation site, the S-nitrosylation site is unlikely to be available under basal conditions when the kinase is autoinhibited by regulatory domain binding to the catalytic domain.19 Therefore, we tested the effect of GSNO following isoproterenol treatment when the CaMKIIδ activation state is increased in cardiomyocytes.23 We also examined the effects of isoproterenol and GSNO on ventricular myocytes isolated from CaMKIIδ-C290A knock-in mice, in addition to WT and CaMKIIδ-C273S animals.
Cardiomyocytes underwent wash-in of 100 nmol/L isoproterenol for 5 minutes followed by washout with control buffer, or buffer containing 150 μM GSNO. Ca2+ transients were measured at baseline, during isoproterenol wash-in, and following washout with GSNO buffer (Figure 6A). Isoproterenol increased Ca2+ transient amplitude and accelerated decay in WT and CaMKIIδ-C273S cardiomyocytes as expected, and these effects were sustained during washout in the presence of GSNO (Figure 6B and 6C, white and green symbols). Similar Ca2+ transient results were observed in CaMKIIδ-C290A cardiomyocytes; however, the Ca2+ transient amplitude increase was not sustained with GSNO (Figure 6C, blue symbols, P=2.3×10−1).
Figure 6.
Effects of S-nitrosoglutathione (GSNO) after ISO on Ca2+ transients and sparks in wild-type (WT) or CaMKIIδ (Ca2+/calmodulin kinase II delta)-C273S and -C290A cardiomyocytes. WT, CaMKIIδ-C273S, and -C290A myocytes were stimulated at 0.5 Hz and exposed to 100 nmol/L ISO, then following ISO exposure myocytes were exposed to 150-μM GSNO. Experimental protocol used for Ca2+ imaging, the gray bars represent when line-scan images were acquired (A). Mean Ca2+ transient amplitude (B) and decay (C) data are shown for cardiomyocytes in response to ISO and washout with GSNO (WT: n=20 cells, N=9 hearts; CaMKIIδ C273S: n=7 cells, N=5 hearts; CaMKIIδ-C290A: n=13 cells, N=5 hearts). Representative line-scans from quiescent WT, CaMKIIδ-C273S and -C290A cardiomyocytes under indicated conditions (D). Mean Ca2+ spark frequency data (E) are shown for cardiomyocytes in response to ISO with washout with GSNO (WT: n=18 cells, N=9 hearts; CaMKIIδ C273S: n=8 cells, N=5 hearts; CaMKIIδ-C290A: n=17 cells, N=5 hearts). All data are normalized to baseline control before addition of ISO. ISO indicates isoproterenol.
When spontaneous Ca2+ release was measured in quiescent cardiomyocytes (Figure 6D), isoproterenol resulted in an increase in the Ca2+ spark frequency (Figure 6E) for both WT and C290A genotypes. Surprisingly, this was not observed for C273S myocytes (P=1.4×10−3) until the addition of GSNO. Addition of GSNO during the isoproterenol washout prevented Ca2+ spark frequency from returning to baseline in both the WT and CaMKIIδ-C273S myocytes, while increased Ca2+ spark frequency was not maintained in myocytes from the CaMKIIδ-C290A mice during the isoproterenol washout, despite the presence of GSNO. These data are consistent with the hypothesis that isoproterenol-induced CaMKIIδ activation regulates Ca2+ handling in myocytes, and subsequent exposure to GSNO causes S-nitrosylation at the cysteine-290 site, thereby prolonging the enhancement of CaMKIIδ activity and Ca2+ sparks.
Knock-In CaMKIIδ-C273S Mice Have Impaired Cardiac Function and Altered ECG Characteristics at 12 Weeks of Age
The myocyte Ca2+ spark and wave data (Figure 4) suggest that the CaMKIIδ-C273 S-nitrosylation site might offer basal protection from stress associated with sympathetic activation of β-adrenergic receptors (ie, that is lost in CaMKIIδ-C273S mice). To test whether there is chronic cardiac adaptation in terms of cardiac function, we measured these parameters in anesthetized WT and CaMKIIδ-C273S mice at 12 weeks of age (Table S3). The echocardiography data indicated unaltered body weight, heart rate, and septal thickness between the groups. However, both end diastolic and systolic volumes of the LV were significantly increased in the CaMKIIδ-C273S mice, with reduced fractional shortening and ejection fraction. The ratio of early to late ventricular filling velocities was also significantly impaired in the CaMKIIδ-C273S hearts. Taken together, these data show that the lack of 1 S-nitrosylation site in the CaMKIIδ-C273S mice results in a modest reduction in both systolic and diastolic function, even in the absence of a significant cardiac challenge.
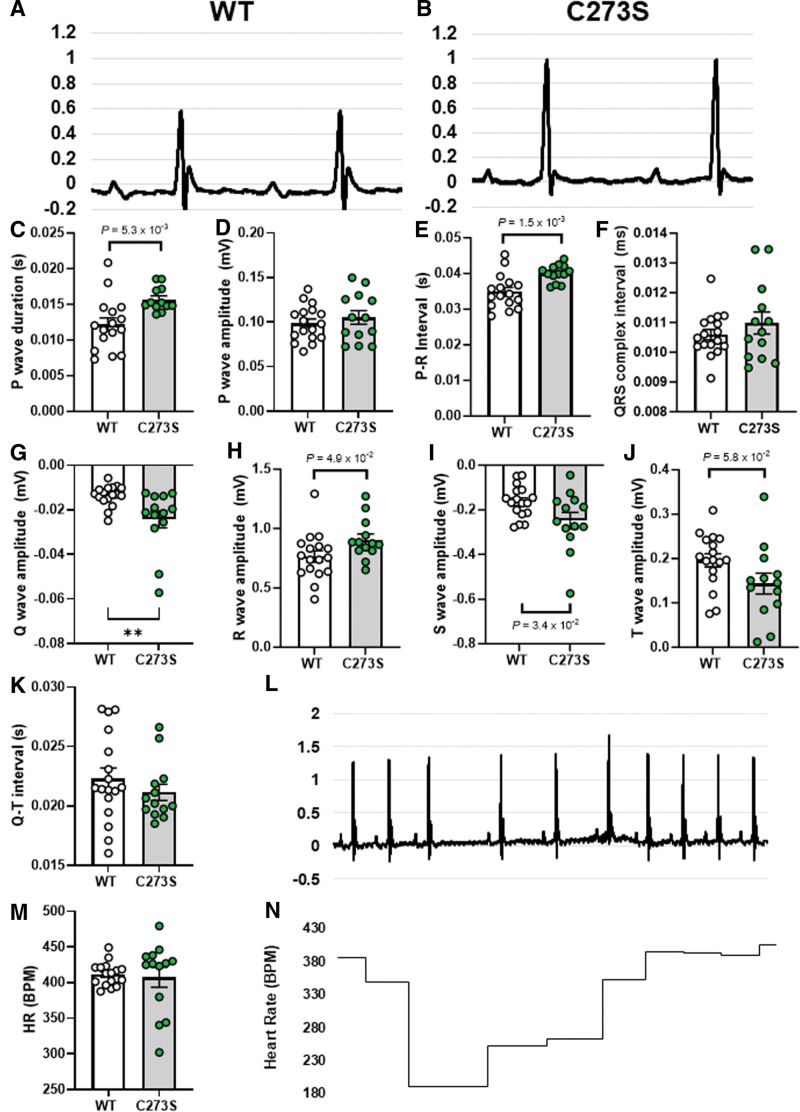
We also measured conduction characteristics through the hearts of anesthetized WT (Figure 7A) and CaMKIIδ-C273S (Figure 7B) using ECG. Even at 12 weeks of age, we observed significant differences in a number of ECG parameters (Figure 7C through 7K) for the CaMKIIδ-C273S mice, including prolongation of P wave duration and P-R interval, as well as increased Q, R, and S wave amplitudes. There was also a trend toward increased T wave amplitude in the CaMKIIδ-C273S mice (P=5.8×10−2). In addition to these baseline alterations to conduction, we observed spontaneous arrhythmic events in the ECG traces of the CaMKIIδ-C273S mice. Figure 7L shows an example of periodic spontaneous variability in heart rate, which was observed in several of the CaMKIIδ-C273S mice but none of the WT mice (Figure 7M), leading to occasional periods of bradycardia (Figure 7N). These arrhythmic events were excluded from our analysis of ECG parameters (Figure 7C through 7K), as this would have greatly exaggerated the differences in these values. Nonetheless, our observations that conduction is altered and spontaneous arrhythmic events are occurring in the CaMKIIδ-C273S mice suggest that the cysteine-273 site on CaMKIIδ is playing a protective role in the heart.
Figure 7.
ECG characteristics for wild-type (WT) and CaMKIIδ (Ca2+/calmodulin kinase II delta)-C273S mice. Representative electrocardiograms from a WT (A) and CaMKIIδ-C273S (B) mouse under anesthetic and mean data for waveform amplitudes and durations (C through K). N=13 to 17 per group. ECG traces for some of the CaMKIIδ-C273S mice showed spontaneous arrhythmic events (L). These events led to variability in total heart rate (HR) (M) and acute periods of bradycardia (N).
NO Mediates Arrhythmogenic Response to β-AR Stress in Langendorff-Perfused Mouse Hearts
Our observation that GSNO pretreatment could suppress isoproterenol-induced Ca2+ sparks in isolated cardiomyocytes, motivated us to test the arrhythmogenic consequences at the whole heart level using Langendorff-perfused mouse hearts (Figure S3A). To ensure that the GSNO treatment was sufficient to induce S-nitrosylation during Langendorff-perfusion, we snap-froze hearts after perfusion and used a modified biotin switch assay to measure total S-nitrosylation (Figure S3B). Hearts that received GSNO after isoproterenol had a significant increase in total S-nitrosylation compared with control (Figure S3C, red versus white bar; P=9.1×10−3), and isoproterenol only (Figure S3C, red versus gray bar; P=1.0×10−2). This effect was partially reversed when GSNO was followed by 10-minute isoproterenol perfusion (Figure S3C, white versus blue bar; P=1.2×10−1).
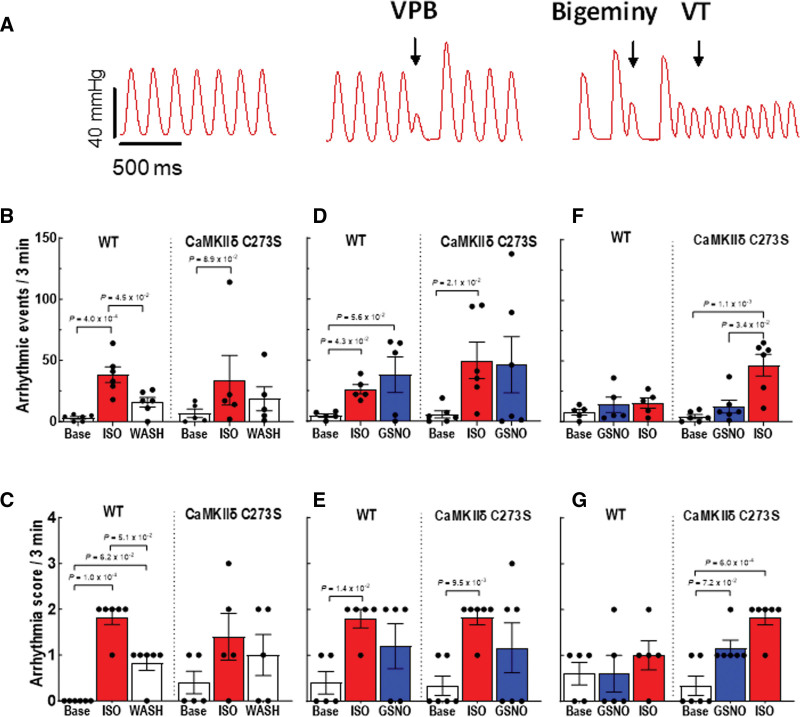
We observed different types of arrhythmias in the isolated hearts treated with isoproterenol and GSNO (Figure 8A). Treatment with 100 nmol/L isoproterenol induced a significant increase in total arrhythmic events (Figure 8B) and arrhythmia score (Figure 8C) in the WT hearts. After isoproterenol washout, arrhythmic events remained significantly higher in the WT but not in the CaMKIIδ-C273S hearts. The arrhythmia score also remained elevated above baseline in WT hearts even after isoproterenol washout, indicating that some isoproterenol-induced sensitization of the hearts persisted 10 minutes after washout. We then repeated the experiment with GSNO added to the perfusate during the isoproterenol washout (analogous to Figure 6) to test whether GSNO would stabilize the arrhythmic phenotype after the isoproterenol stress. GSNO treatment did not seem to prolong the increase in arrhythmias during the isoproterenol washout phase for both WT (P=5.8×10−2) and CaMKIIδ-C273S hearts (P=3.9×10−1; Figure 8D). However, arrhythmia score remained significantly increased above baseline after isoproterenol treatment in the CaMKIIδ-C273S hearts (Figure 8E). These data are consistent with our hypothesis that isoproterenol-mediated stress activates CaMKIIδ to induce cardiac arrhythmia and that S-nitrosylation of activated CaMKIIδ (at cysteine-290) can prolong this activation state, independent of cysteine-273 availability.
Figure 8.
Pretreatment with S-nitrosoglutathione (GSNO) prevents ISO-induced arrhythmias in Langendorff-perfused hearts from wild-type (WT), but not CaMKIIδ (Ca2+/calmodulin kinase II delta)-C273S, mice. Arrhythmias were measured in Langendorff-perfused hearts following protocols outlined in Figure 7A. A, Examples of arrhythmic events observed in the isolated hearts. We measured (B) quantity of arrhythmic events and (C) arrhythmia score during ISO perfusion only, (D) quantity of arrhythmic events and (E) arrhythmia score during ISO pretreatment before GSNO, and (F) quantity of arrhythmic events and (G) arrhythmia score during GSNO pretreatment before ISO. White bar=buffer, red bar=ISO, blue bar=GSNO. n=5 to 6 hearts per group. ISO indicates isoproterenol.
Our myocyte data showed that GSNO treatment before isoproterenol exposure could limit arrhythmogenic Ca2+ wave activity in myocytes from WT but not CaMKIIδ-C273S mice (Figure 4F). To test this at the whole heart level, we exposed the hearts to GSNO first (10 minutes) before administration of isoproterenol. Strikingly, WT mice were protected from isoproterenol-induced arrhythmic events (or increases in arrhythmia score; Figure 8F and 8G), but this protection was not observed in the CaMKIIδ-C273S knock-in mice, which lack the S-nitrosylation site that limits CaMKIIδ activation (Figure 8F). In the CaMKIIδ-C273S mice, GSNO alone was sufficient to enhance the number of arrhythmic events (Figure 8G), a response that could be mediated through cysteine-290, which promotes autonomous CaMKIIδ activation and would be unopposed in the absence of the inhibitory cysteine-273 site. These data demonstrate that the cysteine-273 site on CaMKIIδ is critical to the protective effect of GSNO pretreatment with respect to isoproterenol-induced arrhythmic response in the heart.
Discussion
One main finding of this study was that NO donors promote CaMKIIδ-dependent spontaneous SR Ca2+ release following β-AR stimulation in cardiomyocytes and ventricular arrhythmias in the intact heart. Conversely, pretreatment of cardiomyocytes or hearts with NO donors before isoproterenol exposure limited the β-AR-induced increase in spontaneous Ca2+ release events and ventricular arrhythmias. Furthermore, this protective effect of NO pretreatment was mediated by the cysteine-273 site on CaMKIIδ since it was attenuated when this site was mutated and not available for S-nitrosylation. Exposure to GSNO did not alter baseline Ca2+ handling or heart function. Notably, pretreatment with GSNO conferred protection against β-AR stimulation of pathological Ca2+ leak and arrhythmias without compromising β-AR induced gain of function (enhanced Ca2+ transient amplitude and decay rate). These data provide a new understanding of how NO treatment influences β-AR signaling and cardiac function with both protective and proarrhythmic consequences depending on the timing of NO exposure.
While the β-AR signaling system is a vital arm of the fight-or-flight response responsible for increasing cardiac output on demand, activation of the pathway can lead to aberrant Ca2+ release4,11,12,17 and trigger arrhythmias.24 Activation of NOS (nitric oxide synthase) has been identified as a downstream signaling mechanism of β-AR stimulation, as endogenous NO levels increase following cardiomyocyte isoproterenol exposure13,17 and inhibition of NOS prevents an increase in Ca2+ leak with isoproterenol.11,13,17 It has been proposed that NOS is activated following β-AR stimulation by a pathway independent of protein kinase A, apparently involving Epac and Akt (protein kinase B).11 Here, we found that isoproterenol increased both Ca2+ spark frequency and amplitude in isolated cardiomyocytes, exposure of cardiomyocytes to GSNO alone under baseline conditions had no effect on triggered or untriggered Ca2+ events (Figures 2 and 4). This result was in contrast to the findings that GSNO (or SNP) increased Ca2+ sparks and arrhythmogenic waves in cardiomyocytes and was mediated by CaMKII nitrosylation and activation (rather than direct S-nitrosylation of RyR2).11,13 As we have shown here, the directional effect of GSNO on CaMKII-dependent signaling is highly dependent on the basal state of CaMKII activation and whether Cys273 or Cys290 is the prime mediator, and that can depend on time, pacing rate, oxidative stress, Na+, and Ca2+ levels. That, and species differences25 could readily explain baseline differences in basal GSNO effects. Indeed, cardiac CaMKIIδ activity modulates the function of many myocyte targets,18,26,27 including ion channels associated with arrhythmias and excitation-contraction coupling such as RyR2,28 SERCA (via phospholamban29) and LTCC.30
Under healthy resting conditions, CaMKIIδ is largely but not completely autoinhibited and becomes progressively activated when intracellular Ca2+ levels rise and Ca2+/CaM is bound to the regulatory domain.18 Several posttranslational modifications31–33 of CaMKIIδ, including S-nitrosylation,19 enable an autonomously active form of the kinase that is associated with pathological Ca2+ mishandling and can be driven by high oxidative stress32 or hyperglycemia.33 The degree of CaMKIIδ S-nitrosylation is increased in cardiomyocytes treated with either GSNO13 or isoproterenol17 as determined by immunoprecipitation of CaMKIIδ and probing with anti-S-nitrosylation antibodies. However, this method is not able to discriminate between the 2 cysteine residues within the regulatory domain of CaMKIIδ that have opposite effects on CaMKIIδ activity.19 S-nitrosylation at cysteine-290 causes autonomous activation of the kinase, consistent with the observation that NO exposure can enhance CaMKIIδ activity and increase Ca2+ sparks.11,13 Conversely, S-nitrosylation at cysteine-273 inhibits CaMKIIδ by preventing Ca2+/CaM binding,19 suggesting a dual role for NO in mediating CaMKIIδ activity.
When cardiomyocytes are stimulated at 0.5 Hz, CaMKIIδ activation can be low23; therefore, the cysteine-290 residue within the regulatory region of CaMKIIδ may not be accessible for S-nitrosylation to induce autonomous activation.19 Stimulation of cardiomyocytes at a physiological rate (6–8 Hz in mice) would likely increase the sensitivity of the cells to the NO and CaMKIIδ-mediated effects reported here in isolated cardiomyocytes; however, physiological rates are obtained in the isolated whole hearts and exposure to GSNO alone caused an increase in arrhythmia score in CaMKIIδ-C273S hearts (Figure 8G). Under the baseline conditions for isolated cardiomyocytes used in this study, we propose that GSNO pretreatment of cardiomyocytes results in S-nitrosylation of CaMKIIδ at cysteine-273, which can prevent Ca2+/CaM dependent CaMKIIδ activation when subsequently stimulated with β-AR agonist isoproterenol. We found that pretreatment with GSNO prevented an isoproterenol-dependent increase in Ca2+ spark frequency and amplitude (Figure 4), which is known to be induced by CaMKIIδ-mediated phosphorylation of RyR2.4 However, we cannot rule out attenuation of Ca2+ cycling of other Ca2+ handling proteins, such as SERCA34 or LTCC35 by direct S-nitrosylation.
Interestingly, our novel CaMKIIδ-C273S animals showed a trend toward increased expression of SERCA compared with WT littermates (Figure 3), despite their relatively young age and lack of a directed cardiac challenge. This observation could be consistent with the C273S mutant mice undergoing compensation to increase SR Ca2+ uptake, potentially in response to increased baseline CaMKIIδ activation and enhanced calcium leak during diastole. Future studies in older mice or after a significant cardiac challenge (ex. AngII pump implantation) might show a more pronounced shift in SERCA expression. Moreover, we acknowledge that CaMKII is subject to oxidation,33 and new evidence suggests that cysteine residues on CaMKIIδ may be targets for oxidative stress.36,37 Moreover, conditions of oxidative stress that would be conducive to CaMKIIδ activation would also alter both the mechanism and rate of thiol nitrosylation.38 Another possibility for future consideration is that, during chronic cardiac stress, nitrosylation events on CaMKII are replaced with more stable oxidative modifications (ex. disulfide bonds). Therefore, our generation of knock-in mice that either lack the cysteine-273 or cysteine-290 sites, potentially used in conjunction with existing cellular or mouse models that lack the oxidation-sensitive methionine residues on CaMKII, will be valuable tools to further investigate the effect of NO and ROS-mediated CaMKIIδ activity on cardiac function in both normal and pathological settings.39
NO donors (eg, nitroglycerin, SNP) used for their vasodilatory actions have been administered clinically for almost a century, initially for angina pectoris and later for myocardial infarction.7 NO donors are considered cardioprotective due in part to the ability of NO donors to mimic the benefits of ischemic preconditioning.40 However, we found GSNO or SNP pretreatment suppressed isoproterenol-induced cardiac arrhythmias. When the order of treatment was reversed in the isolated hearts, we found that NO donors administered after isoproterenol could enhance the number of arrhythmias, suggesting that NO donors should be used with caution when β-AR stress is elevated. Conversely, our data demonstrate that a well-timed acute dose or low-dose chronic treatment with an NO donor (potentially paired with a CaMKII inhibitor) could be a powerful antiarrhythmic strategy in the clinic. Importantly, our data demonstrate that the effects of NO donors on isoproterenol-induced Ca2+ sparks persist even in the presence of ODQ, a soluble guanylyl cyclase inhibitor. Guanylyl cyclase is known to play a role in mediating the inotropic effects of NO in myocytes,41 and our findings suggest that CaMKII nitrosylation works independently of guanylyl cyclase, though the 2 pathways may be complimentary in modulating myocytes function. These findings present new evidence for dual and opposing effects of NO signaling in the whole heart with regard to triggered arrhythmias.
ARTICLE INFORMATION
Acknowledgments
We thank Dr Julie Bossuyt for advise during the course of this project.
Author Contributions
Experiments were performed in the laboratory of J.R. Erickson, D.M. Bers, and M.J. Kohr within the Department of Physiology at the University of Otago, the Department of Pharmacology at the University of California, Davis, and the Department of Environmental Health and Engineering at Johns Hopkins Bloomberg School of Public Health, respectively. J.R. Erickson and D.M. Bers are responsible for the conception of the work and interpretation of the results. A.S. Power, E.U. Asamudo, L.P.I. Worthington, C.C. Alim, O.V. Ebenebe, R.E. Parackal, and R.S. Wallace contributed to study design, performed and analyzed experiments, prepared figures, and drafted the article. J. Heller Brown contributed to the interpretation of the work and revised the article. All authors contributed to critical revisions of the article.
Sources of Funding
This work was funded by Marsden Project grants UOO1707 and UOO2108 awarded to J.R. Erickson, National Institutes of Health (NIH) grants R01-HL142282 and P01-HL141084 awarded to D.M. Bers, and NIH Grant R01-HL145459 to J. Heller Brown.
Disclosures
None.
Supplemental Material
Expanded Materials and Methods
Tables S1–S3
Figures S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- β-AR
- β-adrenergic receptor
- Akt
- protein kinase B
- CaM
- Ca2+/calmodulin
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- CaMKIIδ-KO
- mice lacking the delta isoform of CaMKII
- GSNO
- S-nitrosoglutathione
- LTCC
- L-type Ca2+ channel
- NO
- nitric oxide
- NOS
- nitric oxide synthase
- RyR2
- ryanodine receptor type 2
- SERCA
- SR Ca2+ ATPase
- sGC
- soluble guanylyl cyclase
- SNP
- sodium nitroprusside
- SR
- sarcoplasmic reticulum
- WT
- wild type
A.S. Power and E.U. Asamudo contributed equally as co-first authors.
For Sources of Funding and Disclosures, see page 1054.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.123.323571.
REFERENCES
- 1.Ginsburg KS, Bers DM. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J Physiol. 2004;556:463–480. doi: 10.1113/jphysiol.2003.055384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983;258:464–471. doi: 10.1016/S0021-9258(18)33279-4 [PubMed] [Google Scholar]
- 3.Jelinek M, Wallach C, Ehmke H, Schwoerer AP. Genetic background dominates the susceptibility to ventricular arrhythmias in a murine model of beta-adrenergic stimulation. Sci Rep. 2018;8:2312. doi: 10.1038/s41598-018-20792-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm M, Ling H, Willeford A, Pereira L, Gray CB, Erickson JR, Sarma S, Respress JL, Wehrens XH, Bers DM, et al. CaMKIIdelta mediates beta-adrenergic effects on RyR2 phosphorylation and SR Ca(2+) leak and the pathophysiological response to chronic beta-adrenergic stimulation. J Mol Cell Cardiol. 2015;85:282–291. doi: 10.1016/j.yjmcc.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, Losano G. Nitric oxide and cardiac function. Life Sci. 2007;81:779–793. doi: 10.1016/j.lfs.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 6.Kanai AJ, Mesaros S, Finkel MS, Oddis CV, Birder LA, Malinski T. Beta-adrenergic regulation of constitutive nitric oxide synthase in cardiac myocytes. Am J Physiol. 1997;273:C1371–C1377. doi: 10.1152/ajpcell.1997.273.4.C1371 [DOI] [PubMed] [Google Scholar]
- 7.Parratt JR. Nitroglycerin--the first one hundred years: new facts about an old drug. J Pharm Pharmacol. 1979;31:801–809. [PubMed] [Google Scholar]
- 8.Gonzalez DR, Fernandez IC, Ordenes PP, Treuer AV, Eller G, Boric MP. Differential role of S-nitrosylation and the NO-cGMP-PKG pathway in cardiac contractility. Nitric Oxide. 2008;18:157–167. doi: 10.1016/j.niox.2007.09.086 [DOI] [PubMed] [Google Scholar]
- 9.Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (S)NO signals: translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4 [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–296. doi: 10.1161/CIRCRESAHA.109.209452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira L, Bare DJ, Galice S, Shannon TR, Bers DM. beta-Adrenergic induced SR Ca(2+) leak is mediated by an Epac-NOS pathway. J Mol Cell Cardiol. 2017;108:8–16. doi: 10.1016/j.yjmcc.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dries E, Santiago DJ, Johnson DM, Gilbert G, Holemans P, Korte SM, Roderick HL, Sipido KR. Calcium/calmodulin-dependent kinase II and nitric oxide synthase 1-dependent modulation of ryanodine receptors during beta-adrenergic stimulation is restricted to the dyadic cleft. J Physiol. 2016;594:5923–5939. doi: 10.1113/JP271965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez DA, Fernandez-Tenorio M, Ogrodnik J, Niggli E. NO-dependent CaMKII activation during beta-adrenergic stimulation of cardiac muscle. Cardiovasc Res. 2013;100:392–401. doi: 10.1093/cvr/cvt201 [DOI] [PubMed] [Google Scholar]
- 14.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy E, Kohr M, Sun J, Nguyen T, Steenbergen C. S-nitrosylation. a radical way to protect the heart. J Mol Cell Cardiol. 2012;52:568–577. doi: 10.1016/j.yjmcc.2011.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziolo MT, Katoh H, Bers DM. Positive and negative effects of nitric oxide on Ca(2+) sparks: influence of beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2001;281:H2295–H2303. doi: 10.1152/ajpheart.2001.281.6.H2295 [DOI] [PubMed] [Google Scholar]
- 17.Curran J, Tang L, Roof SR, Velmurugan S, Millard A, Shonts S, Wang H, Santiago D, Ahmad U, Perryman M, et al. Nitric oxide-dependent activation of CaMKII increases diastolic sarcoplasmic reticulum calcium release in cardiac myocytes in response to adrenergic stimulation. PLoS One. 2014;9:e87495. doi: 10.1371/journal.pone.0087495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson JR. Mechanisms of CaMKII activation in the heart. Front Pharmacol. 2014;5:59. doi: 10.3389/fphar.2014.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erickson JR, Nichols CB, Uchinoumi H, Stein ML, Bossuyt J, Bers DM. S-Nitrosylation induces both autonomous activation and inhibition of calcium/calmodulin-dependent protein kinase II delta. J Biol Chem. 2015;290:25646–25656. doi: 10.1074/jbc.M115.650234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alim CC, Ko CY, Hernandez JM, Shen EY, Baidar S, Chen-Izu Y, Bers DM, Bossuyt J. Nitrosylation of cardiac CaMKII at Cys290 mediates mechanical afterload-induced increases in Ca(2+) transient and Ca(2+) sparks. J Physiol. 2022;600:4865–4879. doi: 10.1113/JP283427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels LJ, Wallace RS, Nicholson OM, Wilson GA, McDonald FJ, Jones PP, Baldi JC, Lamberts RR, Erickson JR. Inhibition of calcium/calmodulin-dependent kinase II restores contraction and relaxation in isolated cardiac muscle from type 2 diabetic rats. Cardiovasc Diabetol. 2018;17:89. doi: 10.1186/s12933-018-0732-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley SA, Steinert JR. Characterisation and comparison of temporal release profiles of nitric oxide generating donors. J Neurosci Methods. 2015;245:116–124. doi: 10.1016/j.jneumeth.2015.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erickson JR, Patel R, Ferguson A, Bossuyt J, Bers DM. Fluorescence resonance energy transfer-based sensor Camui provides new insight into mechanisms of calcium/calmodulin-dependent protein kinase II activation in intact cardiomyocytes. Circ Res. 2011;109:729–738. doi: 10.1161/CIRCRESAHA.111.247148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira L, Cheng H, Lao DH, Na L, van Oort RJ, Brown JH, Wehrens XH, Chen J, Bers DM. Epac2 mediates cardiac beta1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia. Circulation. 2013;127:913–922. doi: 10.1161/CIRCULATIONAHA.12.148619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards AG, Louch WE. Species-dependent mechanisms of cardiac arrhythmia: a cellular focus. Clin Med Insights Cardiol. 2017;11:1179546816686061. doi: 10.1177/1179546816686061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegyi B, Bers DM, Bossuyt J. CaMKII signaling in heart diseases: emerging role in diabetic cardiomyopathy. J Mol Cell Cardiol. 2019;127:246–259. doi: 10.1016/j.yjmcc.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 28.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 29.Tada M, Inui M, Yamada M, Kadoma M, Kuzuya T, Abe H, Kakiuchi S. Effects of phospholamban phosphorylation catalyzed by adenosine 3’:5’-monophosphate- and calmodulin-dependent protein kinases on calcium transport ATPase of cardiac sarcoplasmic reticulum. J Mol Cell Cardiol. 1983;15:335–346. doi: 10.1016/0022-2828(83)91345-7 [DOI] [PubMed] [Google Scholar]
- 30.Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994;267:H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982 [DOI] [PubMed] [Google Scholar]
- 31.Lai Y, Nairn AC, Gorelick F, Greengard P. Ca2+/calmodulin-dependent protein kinase II: identification of autophosphorylation sites responsible for generation of Ca2+/calmodulin-independence. Proc Natl Acad Sci USA. 1987;84:5710–5714. doi: 10.1073/pnas.84.16.5710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bencsik P, Kupai K, Giricz Z, Gorbe A, Huliak I, Furst S, Dux L, Csont T, Jancso G, Ferdinandy P. Cardiac capsaicin-sensitive sensory nerves regulate myocardial relaxation via S-nitrosylation of SERCA: role of peroxynitrite. Br J Pharmacol. 2008;153:488–496. doi: 10.1038/sj.bjp.0707599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozmaritsa N, Christ T, Van Wagoner DR, Haase H, Stasch JP, Matschke K, Ravens U. Attenuated response of L-type calcium current to nitric oxide in atrial fibrillation. Cardiovasc Res. 2014;101:533–542. doi: 10.1093/cvr/cvt334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hegyi B, Fasoli A, Ko CY, Van BW, Alim CC, Shen EY, Ciccozzi MM, Tapa S, Ripplinger CM, Erickson JR, et al. CaMKII serine 280 O-GlcNAcylation links diabetic hyperglycemia to proarrhythmia. Circ Res. 2021;129:98–113. doi: 10.1161/CIRCRESAHA.120.318402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocco-Machado N, Lai L, Kim G, He Y, Luczak ED, Anderson ME, Levine RL. Oxidative stress-induced autonomous activation of the calcium/calmodulin-dependent kinase II involves disulfide formation in the regulatory domain. J Biol Chem. 2022;298:102579. doi: 10.1016/j.jbc.2022.102579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kharitonov VG, Sundquist AR, Sharma VS. Kinetics of nitrosation of thiols by nitric oxide in the presence of oxygen. J Biol Chem. 1995;270:28158–28164. doi: 10.1074/jbc.270.47.28158 [DOI] [PubMed] [Google Scholar]
- 39.Jesus ICG, Mesquita TRR, Monteiro ALL, Parreira AB, Santos AK, Coelho ELX, Silva MM, Souza LAC, Campagnole-Santos MJ, Santos RS, et al. Alamandine enhances cardiomyocyte contractility in hypertensive rats through a nitric oxide-dependent activation of CaMKII. Am J Physiol Cell Physiol. 2020;318:C740–C750. doi: 10.1152/ajpcell.00153.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879 [DOI] [PubMed] [Google Scholar]
- 41.Cawley SM, Kolodziej S, Ichinose F, Brouckaert P, Buys ES, Bloch KD. sGCalpha1 mediates the negative inotropic effects of NO in cardiac myocytes independent of changes in calcium handling. Am J Physiol Heart Circ Physiol. 2011;301:H157–H163. doi: 10.1152/ajpheart.01273.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegyi B, Borst JM, Bailey LRJ, Shen EY, Lucena AJ, Navedo MF, Bossuyt J, Bers DM. Hyperglycemia regulates cardiac K(+) channels via O-GlcNAc-CaMKII and NOX2-ROS-PKC pathways. Basic Res Cardiol. 2020;115:71. doi: 10.1007/s00395-020-00834-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol. 2007;293:C1073–C1081. doi: 10.1152/ajpcell.00586.2006 [DOI] [PubMed] [Google Scholar]
- 44.Curtis MJ, Walker MJ. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc Res. 1988;22:656–665. doi: 10.1093/cvr/22.9.656 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.