Abstract
Background and aims:
There are limited data regarding fibrosis progression in biopsy-proven nonalcoholic fatty liver disease (NAFLD), between people with type 2 diabetes mellitus (T2DM) versus people without T2DM. We assessed the time to fibrosis progression in people with T2DM versus people without T2DM in a large, multicenter, study of people with NAFLD who had paired liver biopsies.
Methods:
This study included 447 adult participants (64% female) with NAFLD who had paired liver biopsies >1 year apart. Liver histology was systematically assessed by a central pathology committee blinded to clinical data. The primary outcome was the cumulative incidence of a ≥1-stage increase in fibrosis, compared between participants with T2DM versus participants without T2DM.
Results:
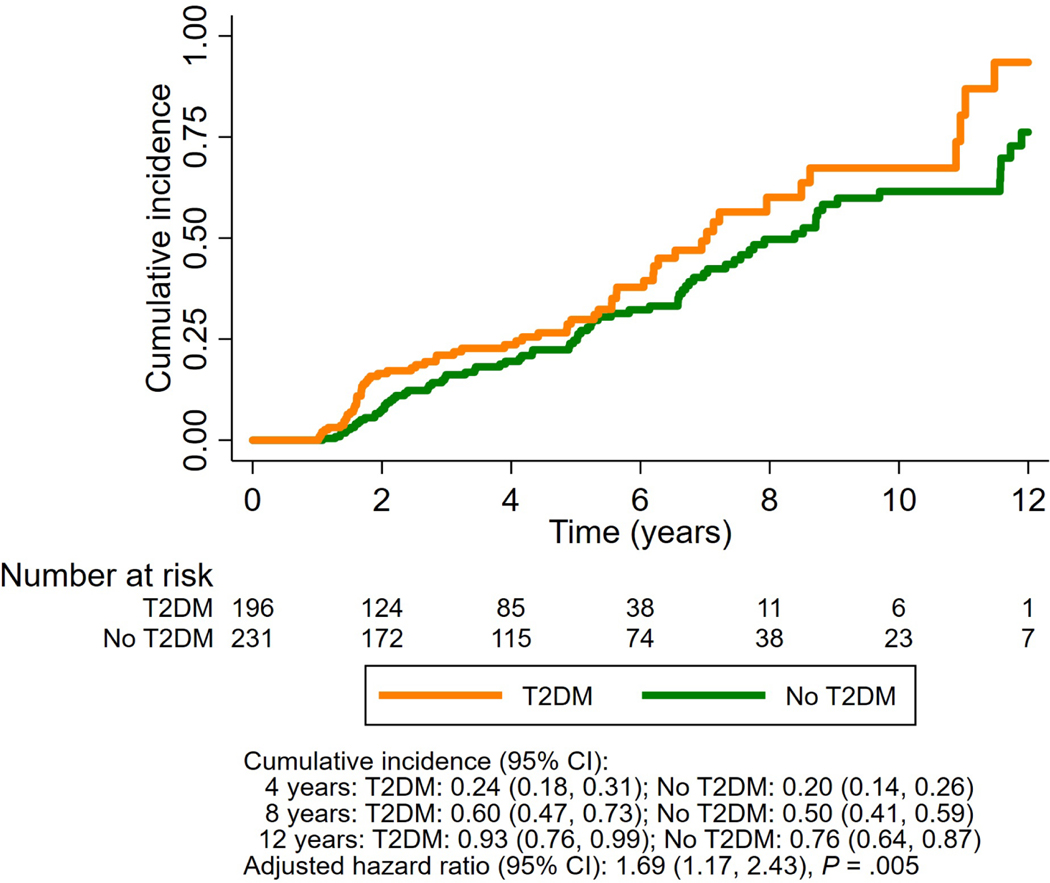
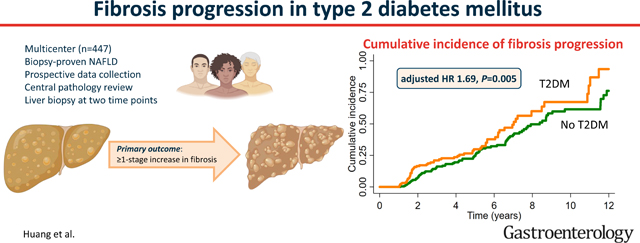
The mean (±SD) age and BMI were 50.9 (±11.5) years and 34.7 (±6.3) kg/m2, respectively. The median (IQR) time between biopsies was 3.3 (1.8–6.1) years. Participants with T2DM had a significantly higher cumulative incidence of fibrosis progression at 4-years (24% versus 20%), 8-years (60% versus 50%), and 12-years (93% versus 76%), P=0.005. Using a multivariable Cox proportional hazards model adjusted for multiple confounders, T2DM remained an independent predictor of fibrosis progression (adjusted hazard ratio 1.69, 95%CI 1.17 – 2.43, P=0.005). The cumulative incidence of fibrosis regression by ≥1 stage was similar between participants with T2DM versus participants without T2DM, (P=0.24).
Conclusion:
In this large, multicenter cohort study of well-characterized participants with NAFLD and paired liver biopsies, we demonstrate that fibrosis progresses faster in participants with T2DM compared to participants without T2DM. These data have important implications for clinical practice and trial design.
Keywords: Nonalcoholic steatohepatitis, NAFLD, cirrhosis, type 2 diabetes mellitus
Graphical Abstract
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) affects a third of the global adult population and is one of the fastest-growing causes of liver-related morbidity and mortality 1–5. NAFLD encompasses nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH), the inflammatory form of NAFLD that may progress to advanced fibrosis and hepatocellular carcinoma 6–10. The risk of all-cause and liver-related mortality in NAFLD increases substantially with each increment in the fibrosis stage, and individuals with advanced fibrosis are at the highest risk of hepatic decompensation and death 11–14. Concurrent with the obesity epidemic, up to 10% of the global population has type 2 diabetes mellitus (T2DM), more than a third of individuals with T2DM have NASH, and around one in six harbors advanced fibrosis 15, 16. In general, liver fibrosis progresses by one stage over seven years for individuals with NASH, but the time to fibrosis progression in people with T2DM versus people without T2DM is unknown 17.
While several studies have described an association between T2DM and fibrosis progression 18–22, the time to fibrosis progression in biopsy-proven NAFLD, compared between people with T2DM versus people without T2DM has not been systematically assessed. Therefore, we conducted a large, multicenter cohort study within the NASH Clinical Research Network (CRN) consortium to examine the time to fibrosis progression, and time to fibrosis regression, between people with and without T2DM who had available paired liver biopsies.
METHODS
Study design
This study included adult participants with NAFLD who had paired liver biopsies that were at least one year apart, recruited at eight sites across the United States as part of the ongoing National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) sponsored NASH CRN consortium. This multi-center study included participants from the non-interventional registries of the NASH CRN consortium (NAFLD Database Study Phases 1, 2, and 3) and participants from the placebo arms of the PIVENs (NCT00063622) and FLINT (NCT01265498) trials 23, 24. A total of 447 well-characterized participants who underwent serial liver biopsy assessments at two distinct time points were included. All participants provided written informed consent, and the study was approved by the institutional review board at each participating site and the data coordinating center.
Inclusion and exclusion criteria
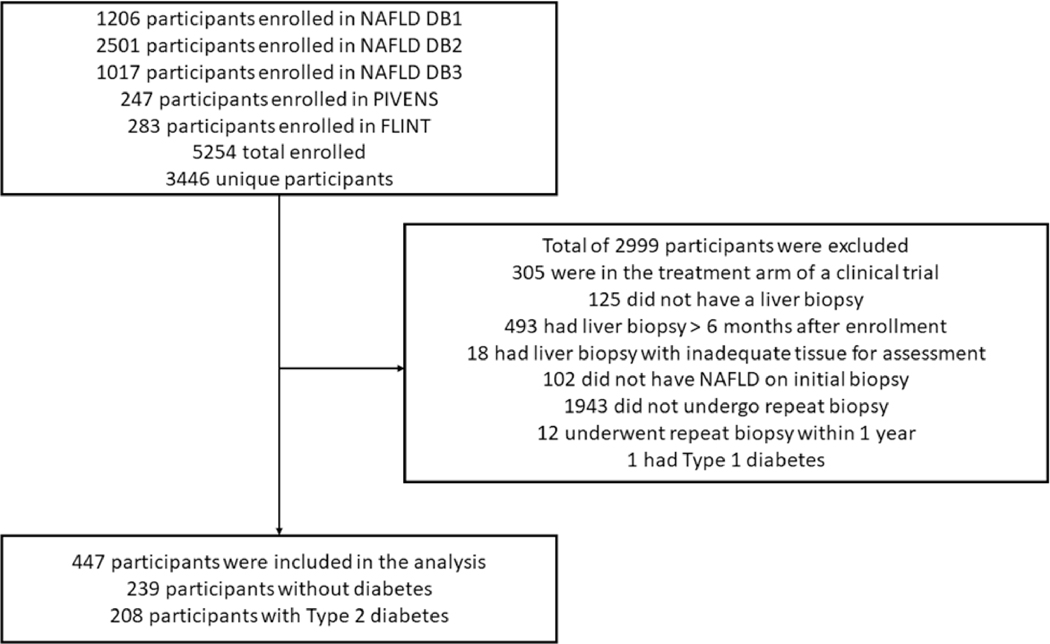
Participants ≥18 years of age with biopsy-proven NAFLD and written informed consent were included. Participants were included if they underwent a liver biopsy, had laboratory and physical measurements within six months of the liver biopsy, and underwent a subsequent liver biopsy more than one year after the first liver biopsy. Participants were excluded if they were enrolled in the treatment arm of a clinical trial, had type 1 diabetes mellitus, had liver disease other than NAFLD, had an Alcohol Use Disorders Identification Test questionnaire suggestive of unhealthy alcohol use25, had received a liver transplant, or had hepatocellular carcinoma.
Clinical and laboratory data
Clinical and laboratory data were obtained at baseline (enrollment) and prospectively at 48-week intervals in a protocol-mandated manner. Clinical and laboratory data were also recorded at the time of any liver biopsies. The presence of T2DM at baseline was based on the clinical practice recommendations from the American Diabetes Association and included any of the following criteria: HbA1c ≥ 6.5%; fasting plasma glucose ≥ 126 mg/dL (7.0 mmol/L); plasma glucose ≥ 200 mg/dL (11.1 mmol/L); medical diagnosis of T2DM or use of medications to treat T2DM 26, while the metabolic syndrome was defined based on ATP III criteria (having at least 3 of the following 5 factors: impaired fasting glucose ≥110 mg/dL; waist circumference ≥88 cm in women, ≥102 cm in men, triglycerides ≥150 mg/dL, high-density lipoprotein cholesterol <50 mg/dL in women, 40 mg/dL in men, systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg 27.
Histological assessment
All participants underwent a liver biopsy at baseline, followed by subsequent liver biopsies at time points determined by the standard of care; in participants with more than two biopsies, the latest biopsy was compared to the first biopsy. Liver histology assessment for grade and stage was conducted per NASH-CRN protocol as a consensus review of glass slides for each feature of the NAS score and stage. The biopsy specimens were examined by the NASH CRN central pathology committee, which comprised of at least three pathologists during each assessment. Pathologists were unaware of clinical data or the sequence of the biopsy at the time of review. H&E and trichrome stained slides were reviewed for NAS components (steatosis, lobular activity, and ballooning degeneration) and fibrosis stage per NASH-CRN criteria and practice 28. Separately, a diagnosis of NASH, borderline NASH, NAFLD not NASH, or not NAFLD was made based on recognition of the distinctive features of steatohepatitis independent of the NAS. 29.
Outcome measures
The primary outcome was the cumulative incidence of a ≥1-stage increase in fibrosis, compared between participants with T2DM versus participants without T2DM. The secondary outcome was the cumulative incidence of a ≥1-stage decrease in fibrosis, compared between participants with T2DM versus participants without T2DM.
Statistical analyses
Descriptive statistics of participant demographic, laboratory, histological, and imaging characteristics at baseline were presented and dichotomized by the presence of T2DM at baseline. Baseline categorical variables were compared with chi-square, and continuous variables were compared using a t-test or Wilcoxon two-sample test where appropriate. Survival analysis was conducted to evaluate time-to-fibrosis progression. The Cox proportional hazards model was used to evaluate the hazards ratio (HR) for fibrosis progression, and fibrosis regression, between participants with T2DM at baseline versus participants without T2DM at baseline, adjusted for age, gender, BMI, Hispanic ethnicity, and baseline fibrosis stage. Participants with fibrosis stage 4 at baseline were excluded from the analysis for fibrosis progression since there can be no further progression measured with the NASH CRN scoring system, and participants with fibrosis stage 0 at baseline were excluded from the analysis for fibrosis regression since there can be no further regression. The fibrosis progression rate was defined by the increase in fibrosis stage over time between biopsies (years) and compared between participants with T2DM at baseline versus participants without T2DM at baseline using linear regression, adjusted for age, gender, ethnicity/race, body mass index, and baseline fibrosis stage 30. In the analysis for fibrosis progression rate, participants with fibrosis regression on the latest biopsy were censored. The proportion of participants who progressed from fibrosis stage 0–2 at baseline to advanced fibrosis (stage 3–4) on the latest liver biopsy was compared between participants with T2DM at baseline versus those without T2DM at baseline using the chi-squared test. The Cox proportional hazards model was used to evaluate the HR for fibrosis progression, and fibrosis regression, between the top quartile of HbA1c values versus the others (decided a priori). Multiple sensitivity/subgroup analyses were performed, these included comparing the participants in the current study with participants in the wider NASH-CRN who did not receive a second biopsy; determining the association between progression in non-invasive tests and the presence of T2DM; including only participants with a biopsy specimen length ≥ 15 mm; excluding participants who developed incident T2DM after enrollment into the study; and adjusting for center effects, participation in a clinical trial, and the impact of medication classes on fibrosis progression. Statistical significance was defined as a two-tailed P value of ≤0.05. Analyses were conducted with the use of SAS software, version 9.4 (SAS Institute), Stata software, version 15.1 (StataCorp), and GraphPad Prism.
RESULTS
Characteristics of the study population
A total of 3,446 participants were enrolled between October 2004 through March 2022 (Figure 1), including 83 participants who were enrolled in the placebo arm of the PIVENs trial23 and 142 who were enrolled in the placebo arm of the FLINT trial, before applying inclusion and exclusion criteria 24. After applying inclusion and exclusion criteria (Figure 1), a total of 447 adult participants with NAFLD (64% female) and available paired liver biopsies were included in this study. The final study cohort included 65 and 82 participants from the placebo arms of the PIVENS and FLINT trials, respectively. The mean (±SD) age and BMI were 50.9 (±11.5) years and 34.7 (±6.3) kg/m2, respectively. Most participants (85%) were White, and 10% were Hispanic. The number of participants with baseline fibrosis stage 0, 1, 2, 3, and 4 was 93, 116, 103, 115, and 20, respectively. The median (IQR) time between biopsies was 3.3 (1.8–6.1) years.
Figure 1.
Study flow diagram
Participants with T2DM at baseline were older (53.0 years versus 49.1 years, P<0.001), had a greater proportion of females (70% versus 58%, P=0.006), had higher BMI (35.6 kg/m2 versus 33.9 kg/m2, P=0.005), were more likely to have the metabolic syndrome (74% versus 61%, P=0.006), had higher NAS (5.0 versus 4.5, P=0.003), had a higher proportion of definite or borderline NASH (79% versus 57%, P<0.001), had a greater proportion with advanced fibrosis (stage 3–4) (39% versus 22%, P<0.001) on initial biopsy, and had a shorter median [IQR] time between biopsies (2.8 [1.7–5.5] years versus 3.9 [2.0–6.9] years, P=0.001) compared to participants without T2DM.
Progression and regression of fibrosis
The transitions of the fibrosis stages are summarized in Supplemental Table 1. Overall, 151 participants (35%) experienced fibrosis progression, 194 participants (43%) had no change in the fibrosis stage, and 102 participants (23%) had fibrosis regression. A greater proportion of participants with T2DM progressed from stage 0–2 fibrosis at baseline to advanced fibrosis (stage 3–4) versus participants without T2DM (26.0% versus 14.1%, P=0.008) despite a shorter median (IQR) time between biopsies (2.8 [1.7–5.5] years versus 3.9 [1.7–5.5] years, P=0.001). There was no difference in the proportion with regression from advanced fibrosis (stage 3–4) to stage 0–2 fibrosis between participants with T2DM versus participants without T2DM (27% versus 22%, P=0.52).
Fibrosis progression in participants with T2DM versus participants without T2DM
Among participants with baseline fibrosis stage 0 or 1, the mean (SD) fibrosis progression rate was higher in participants with T2DM versus participants without T2DM (+0.23 [0.39] stages per year versus +0.16 [0.26] stages per year, P=0.048), after adjustment for age, gender, ethnicity/race, body mass index, and baseline fibrosis stage.
Participants with T2DM at baseline had a significantly higher cumulative incidence of fibrosis progression by ≥1 stage at 4-years (24% [95% CI 18–31] versus 20% [95% CI 14–26]), 8-years (60% [95% CI 47–73] versus 50% [95% CI 41–59]) and 12-years (93% [95% CI 76–99] versus 76% [CI 64–87]), P=0.005 after adjustment for age, gender, ethnicity/race, body mass index, and baseline fibrosis stage, compared with participants without T2DM at baseline (Figure 2).
Figure 2.
Cumulative incidence of fibrosis progression in nonalcoholic fatty liver disease, among participants with T2DM versus participants without T2DM
Abbreviation: T2DM, type 2 diabetes mellitus
Association of T2DM with fibrosis progression
In unadjusted analysis, T2DM at baseline was associated with fibrosis progression (HR 1.45, 95% CI 1.04 – 2.03, P=0.03) (Table 2). After multivariable adjustment for age, gender, BMI, race/ethnicity, and baseline fibrosis stage, the presence of T2DM at baseline remained statistically significant and an independent predictor of fibrosis progression (adjusted HR 1.69, 95% CI 1.17 – 2.43, P=0.005). HbA1c ≥ 7.0% was not associated with fibrosis progression in both unadjusted and multivariable-adjusted analyses (Supplemental Table 2).
Table 2.
Predictors of (A) fibrosis progression and (B) fibrosis regression
| (A) Predictors of fibrosis progression | Hazard Ratio (95% CI) | P-value |
| T2DM, unadjusted | 1.45 (1.04 – 2.03) | 0.03 |
| T2DM, adjusted for age and sex | 1.42 (1.00 – 2.00) | 0.05 |
| T2DM, adjusted for age, sex, BMI, and Hispanic race/ethnicity | 1.52 (1.07 – 2.17) | 0.02 |
| T2DM, adjusted for age, sex, BMI, Hispanic race/ethnicity, and baseline fibrosis stage | 1.69 (1.17 – 2.43) | 0.005 |
| (B) Predictors of fibrosis regression | Hazard Ratio (95% CI) | P-value |
| T2DM, unadjusted | 1.15 (0.77 – 1.71) | 0.49 |
| T2DM, adjusted for age and sex | 1.36 (0.90 – 2.07) | 0.15 |
| T2DM, adjusted for age, sex, BMI, and Hispanic race/ethnicity | 1.38 (0.90 – 2.11) | 0.14 |
| T2DM, adjusted for age, sex, BMI, Hispanic race/ethnicity, and baseline fibrosis stage | 1.29 (0.84 – 1.99) | 0.24 |
Abbreviation: T2DM, type 2 diabetes mellitus; BMI, body mass index
Fibrosis regression in participants with T2DM versus participants without T2DM
The cumulative incidence of fibrosis regression by ≥1 stage was similar between participants with T2DM versus participants without T2DM at 4-, 8-, and 12-years (P=0.24) (Supplemental Figure 1). The presence of T2DM was not a predictor of fibrosis regression, both in unadjusted and multivariable-adjusted analyses (Table 2).
Subgroup and sensitivity analyses
Sensitivity analyses were conducted after excluding 69 participants without T2DM at baseline who developed incident T2DM after study enrollment. T2DM remained an independent predictor for fibrosis progression, after adjustment for age, gender, BMI, Hispanic ethnicity, and baseline fibrosis stage (adjusted HR 1.73, 95% CI 1.13 – 2.65, P=0.01), but was not a predictor of fibrosis regression (Supplemental Table 3). The cumulative incidence of fibrosis progression by ≥1 stage remained higher in participants with T2DM versus participants without T2DM (Supplemental Figure 2). The cumulative incidence of fibrosis regression by ≥1 stage remained similar between groups (Supplemental Figure 3). There was a lower proportion of participants with T2DM that were enrolled in clinical trials, compared to those without T2DM (26% versus 39%, P=0.002). However, the association between T2DM and fibrosis progression remained consistent even after adjustment for center and clinical trial participation (Supplemental Table 4). Participants in the wider NASH-CRN cohort without a follow-up biopsy had similar demographics to the participants that were included in the current study (with a follow-up biopsy), but were more likely to have cirrhosis (stage 4 fibrosis) and were less likely to have a definite diagnosis of NASH on the baseline biopsy (Supplemental Table 5). Analysis of the wider NASH-CRN cohort (inclusive of participants with and without a follow-up liver biopsy) revealed a higher rate of progression in non-invasive tests between participants with T2DM versus those without T2DM (Supplemental Table 6). Fibrosis progression was associated with an increase in FIB-4 and a decrease in platelets, but not aspartate aminotransferase, alanine aminotransferase, or HbA1c (Supplemental Table 7).
We performed a sensitivity analysis among participants with biopsy specimen length ≥ 15 mm, and determined similar findings to the main analysis (Supplemental Table 8). All participants receive lifestyle and dietary advice, and we provided data on the proportion that received Vitamin E, thiazolidinediones, and glucagon-like peptide-1 receptor agonists between the two groups in Supplemental Table 9. The association of T2DM with fibrosis progression remained consistent after adjusting for medication classes (Supplemental Table 10). Among participants with fibrosis progression, there was a reduction in the NAS and steatosis scores, but not in lobular inflammation or ballooning scores (Supplemental Table 11).
DISCUSSION
Main findings
In this large, multicenter center study of well-characterized participants with paired liver biopsies within the NASH CRN consortium, we determined that fibrosis progresses faster in participants with T2DM versus participants without T2DM. The 4-year (24% versus 20%), 8-year (60% versus 50%), and 12-year (93% versus 76%) cumulative incidences of fibrosis progression were significantly higher in participants with T2DM versus participants without T2DM. Among participants with baseline fibrosis stage 0 or 1, the fibrosis progression rate was significantly higher in participants with T2DM versus participants without T2DM. T2DM remained a significant predictor of fibrosis progression, even after adjustment for age, gender, BMI, race/ethnicity, and baseline fibrosis stage. These findings remained consistent in sensitivity analyses excluding participants who developed incident T2DM after study enrollment. By contrast, the cumulative incidence of fibrosis progression was similar between participants with T2DM versus participants without T2DM, which may be related to the fact that the majority of participants with T2DM had adequate T2DM control. In addition, the NASH-CRN protocol was designed with a focus on determining fibrosis progression, and may not be optimal for identifying regression 28.
These data have important implications. The faster time to fibrosis progression in people with T2DM should be taken into consideration when designing NASH therapeutic trials and underscores the importance of ensuring comparable proportions of participants with T2DM in treatment and control arms 31. While the incidence of fibrosis progression in participants with T2DM was significantly higher than in those without T2DM, the absolute difference was modest, which may be related to the fact that most of the study participants with T2DM had adequate glycemic control. Care providers should emphasize the importance of lifestyle measures and good glycemic control to people with T2DM.
In context with current literature
Liver fibrosis has been established as the major determinant of outcomes in people with NAFLD 11, 12, 32, 33. A landmark prospective study of 1,773 people with NAFLD demonstrated an increased risk of liver-related complications and death among those with advanced fibrosis (stage 3–4), highlighting the need to detect and prevent fibrosis progression 13. A study of 1,770 people with T2DM who underwent vibration-controlled transient elastography [VCTE] by the M probe determined that 17% had a liver stiffness measurement suggestive of advanced fibrosis (defined as ≥9.6 kPa in this study) 34. A study of 501 people aged ≥50 years with T2DM characterized by elastography (magnetic resonance elastography in 83% and VCTE in the others) determined that the prevalence of advanced fibrosis was 14% 35. Another study of people with T2DM performed a follow-up VCTE after 3 years and determined that 4% with baseline liver stiffness measurement <10 kPA had a liver stiffness measurement ≥10 kPa on follow-up 36. Although these studies demonstrated an association between T2DM and fibrosis progression, the time to fibrosis progression between people with T2DM versus people without T2DM was previously unknown. 18, 21, 37. In addition, previous paired biopsy studies had modest numbers18, 21, or depended on non-invasive tests or ICD-codes as surrogate measures for fibrosis37. The current study fills this knowledge gap and demonstrates that T2DM is associated with a significantly higher cumulative incidence of fibrosis progression, possibly related to the stimulating effect of hyperinsulinemia and high glucose levels on hepatic stellate cells 38.
Strengths and limitations
This is the first study reporting the time to fibrosis progression in people with T2DM versus people without T2DM. Its strengths include prospective data collection, multicenter study design, large sample size, and well-characterized participants with serial, centrally read liver biopsies. However, it is not without limitations. The sampling variability of liver biopsy may affect the classification of fibrosis stage. Histologic staging may underestimate the changes that are present and more sensitive quantification of histology by image analysis may show additional changes between people with and without T2DM. The reporting of fibrosis stages may have been susceptible to misclassification due to the sampling variability of liver biopsy, although previous studies reporting sampling variability were exacerbated by observational variability 39. We did not calculate the collagen proportionate area, which may have added further granularity to the results. The median time between biopsies was relatively short (3.3 years) given the slow rate of disease progression in NAFLD, therefore, studies with longer follow-ups may be required. The cumulative incidence of fibrosis progression and regression might differ by fibrosis stage, but the sample size was insufficient to meaningfully analyze the time to fibrosis progression stratified by each fibrosis stage. However, we adjusted for the baseline fibrosis stage in our analyses. The analysis for fibrosis progression rate was based on previous studies of fibrosis progression in the literature 30, however, we limited the reporting of fibrosis progression rate to participants with stage 0 or 1 fibrosis. The majority of participants with T2DM had an HbA1c consistent with adequate glycemic control, and we speculate that a larger cohort of participants may be necessary to determine the role of different levels of glycemic control on fibrosis progression and regression. We excluded participants who had been randomized to the active treatment arm of the completed PIVENS or FLINT trials. However, we did not exclude participants in active treatment arms of clinical trials conducted outside of the NASH CRN, which may have introduced some degree of bias. We acknowledge that fibrosis progression and regression may not always be a linear process, with paired biopsy studies revealing that histological improvement may be followed by worsening, and vice-versa 40. The current analysis was based on irregular biopsy intervals and may represent an oversimplification 40. While we determined that the demographic characteristics of included participants in this study were similar to those within the NASH CRN without a follow-up biopsy, there may have been some degree of selection bias, as care providers may have been less likely to request a liver biopsy in people who were assessed to have an improvement. Future studies that perform protocolled interval biopsies may be helpful.
Conclusion
In this large, multicenter cohort study of well-characterized participants with NAFLD and paired liver biopsies, we demonstrate that fibrosis progresses faster in people with T2DM compared to people without T2DM. T2DM remained a strong and independent predictor for fibrosis progression, even after adjustment for multiple confounders. The faster fibrosis progression in people with T2DM should be taken into consideration when designing therapeutic trials and underscores the unmet need for efficacious therapies in this high-risk group.
Supplementary Material
Table 1.
Baseline characteristics of participants with paired biopsies, stratified by the presence of T2DM at baseline
| Variable | Overall (N=447) | No T2DM (N=239) | T2DM (N=208) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age — yr | 50.9 (11.5) | 49.1 (12.1) | 53.0 (10.4) | <0.001 |
| Sex — no. (%) | 0.006 | |||
| Male | 163 (36%) | 101 (42%) | 62 (30%) | |
| Female | 284 (64%) | 138 (58%) | 146 (70%) | |
| Race — no. (%) | 0.09 | |||
| White | 381 (85%) | 210 (88%) | 171 (82%) | |
| Other or not reported | 66 (15%) | 29 (12%) | 37 (18%) | |
| Hispanic ethnic group | 0.15 | |||
| Yes | 46 (10%) | 20 (8%) | 26 (13%) | |
| No | 401 (90%) | 219 (92%) | 182 (88%) | |
| Body-mass index | ||||
| Mean (SD) | 34.7 (6.3) | 33.9 (6.1) | 35.6 (6.4) | 0.005 |
| Distribution — no. (%) | 0.005 | |||
| <25 | 13 (3%) | 10 (4%) | 3 (1%) | |
| 25 to <30 | 91 (20%) | 61 (26%) | 30 (14%) | |
| 30 to <35 | 168 (38%) | 80 (33%) | 88 (42%) | |
| ≥35 | 175 (39%) | 88 (37%) | 87 (42%) | |
| Median time between biopsies (IQR) — yr | 3.3 (1.8, 6.1) | 3.9 (2.0, 6.9) | 2.8 (1.7, 5.5) | 0.001 |
| Comorbidities— no. (%) | ||||
| Hypertension | 256 (57%) | 108 (45%) | 148 (71%) | <0.001 |
| Coronary artery disease | 19 (4%) | 7 (3%) | 12 (6%) | 0.16 |
| Metabolic syndrome | 298 (67%) | 145 (61%) | 153 (74%) | 0.006 |
| Baseline histologic features | ||||
| NAFLD activity score | 4.8 (1.6) | 4.5 (1.6) | 5.0 (1.6) | 0.003 |
| Steatosis score | 0.12 | |||
| 0 | 11** (2%) | 3 (1%) | 8 (4%) | |
| 1 | 149 (33%) | 86 (36%) | 63 (30%) | |
| 2 | 161 (36%) | 79 (33%) | 82 (39%) | |
| 3 | 126 (28%) | 71 (30%) | 55 (26%) | |
| Lobular inflammation score | 0.17 | |||
| 0 | 2 (<1%) | 2 (1%) | 0 (0%) | |
| 1 | 205 (46%) | 117 (49%) | 88 (42%) | |
| 2 | 179 (40%) | 93 (39%) | 86 (41%) | |
| 3 | 61 (14%) | 27 (11%) | 34 (16%) | |
| Ballooning score | <0.001 | |||
| 0 | 111 (25%) | 78 (33%) | 33 (16%) | |
| 1 | 142 (32%) | 76 (32%) | 66 (32%) | |
| 2 | 194 (43%) | 85 (36%) | 109 (52%) | |
| Fibrosis stage – mean (SD) | 1.7 (1.2) | 1.4 (1.2) | 2.0 (1.1) | <0.001 |
| Stage 0 | 93 (21%) | 70 (29%) | 23 (11%) | <0.001 |
| Stage 1 | 116 (26%) | 64 (27%) | 52 (25%) | |
| Stage 2 | 103 (23%) | 51 (21%) | 52 (25%) | |
| Stage 3 | 115 (26%) | 46 (19%) | 69 (33%) | |
| Stage 4 | 20 (4%) | 8 (3%) | 12 (6%) | |
| NASH diagnosis | <0.001 | |||
| Definite/borderline NASH | 301 (67%) | 137 (57%) | 164 (79%) | |
| NAFLD, not NASH | 146 (33%) | 102 (43%) | 44 (21%) | |
| Biopsy specimen length — mm | 20.0 (9.5) | 18.7 (8.8) | 21.5 (9.9) | 0.002 |
| Laboratory results – Median (IQR) | ||||
| ALT — U/L | 63 (44, 94) | 66.0 (47, 96) | 60.5 (40, 92) | 0.08 |
| AST — U/L | 44 (32, 67) | 43 (32, 69) | 45 (31, 64) | 0.50 |
| ALP — U/L | 79 (65, 96) | 79 (67, 94) | 78 (65, 99) | 0.98 |
| Total bilirubin — mg/dl | 0.6 (0.4, 0.8) | 0.7 (0.5, 0.9) | 0.5 (0.4, 0.8) | <0.001 |
| INR – mean (SD) | 1.02 (0.15) | 1.01 (0.09) | 1.02 (0.19) | 0.45 |
| Serum creatinine — mg/dl, mean (SD) | 0.83 (0.18) | 0.87 (0.19) | 0.78 (0.17) | <0.001 |
| eGFR — ml/min/1.73 m2 | 93.5 (79.6, 104.7) | 91.7 (78.7, 104.1) | 95.6 (81.2, 105.3) | 0.19 |
| Serum albumin — g/dl | 4.3 (4.1, 4.6) | 4.3 (4.1, 4.6) | 4.3 (4.1, 4.6) | 0.75 |
| Platelets per μL | 242 (203, 287) | 245 (204, 287) | 238 (201, 287) | 0.56 |
| FIB-4 | 1.2 (0.8, 1.7) | 1.1 (0.8, 1.7) | 1.3 (0.9, 1.8) | 0.03 |
| HbA1c — % | 6.0 (5.5, 6.7) | 5.6 (5.3, 5.9) | 6.8 (6.2, 7.4) | <0.001 |
| Fasting glucose – mg/dL | 100.0 (89.0, 118.0) | 93.0 (86.0, 101.0) | 118.0 (98.0, 143.0) | <0.001 |
| Fasting insulin – ng/mL | 19.0 (13.6, 29.0) | 18.3 (13.0, 25.4) | 20.0 (14.2, 32.9) | 0.03 |
| HOMA-IR | 4.7 (3.3, 7.8) | 4.2 (3.0, 5.9) | 5.9 (3.8, 10.8) | <0.001 |
| Total cholesterol - mg/dL | 188.0 (163.0, 220.0) | 194.0 (173.0, 222.0) | 179.0 (158.0, 215.0) | 0.002 |
| HDL cholesterol – mg/dL | 41.0 (35.0, 50.0) | 41.0 (34.0, 51.0) | 41.0 (35.0, 49.0) | 0.65 |
| LDL cholesterol – mg/dL | 115.0 (90.0, 141.0) | 121.0 (97.0, 147.0) | 106.0 (83.0, 135.0) | <0.001 |
| Triglycerides – mg/dL | 152.0 (109.0, 211.0) | 148.0 (105.0, 208.0) | 160.5 (111.5, 218.5) | 0.08 |
Values are medians (IQR) unless otherwise noted. Percentages may not total 100 because of rounding. Data were missing for the following variables: metabolic syndrome (N=4), biopsy length (N=1), insulin (N=3), HOMA-IR (N=3), HDL cholesterol (N=1), LDL cholesterol (N=9).
Abbreviations: T2DM, type 2 diabetes mellitus; INR, international normalized ratio; IQR interquartile range; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Race and ethnic group were reported by the participant.
Body-mass index is the weight in kilograms divided by the square of the height in meters. A body-mass index of less than 25 indicates normal weight, of 25 to less than 30 overweight, 30 to less than 35 obese, and 35 or above morbid obesity.
The NAFLD activity score was assessed on a scale of 0 to 8, with higher scores indicating more severe disease; the components of this measure are steatosis (assessed on a scale of 0 to 3), lobular inflammation (assessed on a scale of 0 to 3), and hepatocellular ballooning (assessed on a scale of 0 to 2).
The fibrosis stage was assessed on a scale of 0 to 4, with higher scores indicating more severe fibrosis and stage 4 defining cirrhosis.
The eGFR was calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration formula.
Among these 11 participants, 8 had advanced fibrosis (stage 3 or 4) and a diagnosis of borderline or definite NASH; 1 participant had F1 fibrosis and a diagnosis of definite NASH; and 2 participants had F2 fibrosis and a diagnosis of borderline NASH.
Acknowledgements
Members of the Nonalcoholic Steatohepatitis Clinical Research Network Adult Clinical Centers
Cleveland Clinic Foundation, Cleveland, OH: Daniela Allende, MD; Annette Bellar, MSLA; Jaividhya Dasarathy, MD; Srinivasan Dasarathy, MD; Nicole Welch, MD; Rahul Yerrapothu
Duke University Medical Center, Durham, NC: Mustafa Bashir, MD; Anna Mae Diehl, MD; Cynthia Guy, MD; Mariko Kopping, MS, RD; Dawn Piercy, MS, FNP; Ayako Suzuki, MD, PhD; Naglaa Tawadrou
Indiana University School of Medicine, Indianapolis, IN: Naga Chalasani, MD; Mandy Cruz, RN; Oscar W. Cummings, MD; Lisa Garrison, RN; Samer Gawrieh, MD; Niharika Samala, MD; Raj Vuppalanchi, MD
Saint Louis University, St Louis, MO: Danielle Carpenter, MD; Theresa Cattoor, RN; Janet Freebersyser, RN; Brent A. Neuschwander-Tetri, MD
Liver Institute Northwest, Seattle, WA: Pannapat Angkanaworakul; Achashman Berihun; Andrew Buysse; Theresa Dorrian; Breanna Gulati, ARNP; Kris V. Kowdley, MD; Kevin Liu; Sandra Misic; Adam Sohal, MD; Joseph Vuong
University of California San Diego, San Diego, CA: Veeral Ajmera, MD; Cynthia Behling, MD, PhD; Rohit Loomba, MD, MHSc; Egbert Madamba; Michael S. Middleton, MD, PhD; Lisa Richards, NP; Seema Singh; Claude Sirlin, MD
University of California San Francisco, San Francisco, CA: Ryan Gill, MD, PhD; Bilal Hameed, MD; Remilekun Awe
University of Southern California, Los Angeles, CA: Daisy Olvera, BA; Norah Terrault, MD, MPH; Liyun Yuan, MD, PhD
University of Washington Medical Center, Seattle, WA: Matthew Yeh, MD, PhD
Virginia Commonwealth University, Richmond, VA: Somaya Albhaisi, MBBCh, MD; Amon Asgharpour, MD; Sherry Boyett, RN, BSN; Melissa J. Contos, MD; Velimir AC Luketic, MD; Arun J. Sanyal, MD; Jolene Schlosser, RN, BSN; Mohammad S. Siddiqui, MD
Resource Centers
National Cancer Institute, Bethesda, MD: David E. Kleiner, MD, PhD
Data Coordinating Center, Johns Hopkins University, Bloomberg School of Public Health, Baltimore, MD: Peggy Adamo, BS; Patricia Belt, BS; Jeanne M. Clark, MD, MPH; Jennifer M. DeSanto, RN, BSN, MS; Jill Meinert; Laura Miriel, BS; Emily P. Mitchell, MPH, MBA; Carrie Shade, BA; Jacqueline Smith, AA; Michael Smith, BS; Alice Sternberg, ScM; James Tonascia, PhD; Mark L. Van Natta, MHS; Annette Wagoner; Laura A. Wilson, ScM; Tinsay Woreta, MD, MPH; Katherine P. Yates, ScM
Grant support:
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061713, U01DK061718, U01DK061728, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U24DK061730). Additional support is received from the National Center for Advancing Translational Sciences (NCATS) (grants UL1TR000439, UL1TR000436, UL1TR000006, UL1TR000448, UL1TR000100, UL1TR000004, UL1TR000423, UL1TR002649). This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute. The PIVENS trial (NCT00063622) was conducted by the NASH CRN and supported in part by Takeda Pharmaceuticals North America through a Cooperative Research and Development Agreement with the NIDDK. The vitamin E and matching placebo for the PIVENS trial were provided by Pharmavite through a Clinical Trial Agreement with the NIH. The FLINT trial (NCT01265498) was conducted by the NASH CRN and supported in part by a Collaborative Research and Development Agreement (CRADA) between NIDDK and Intercept Pharmaceuticals.
R.L. receives funding support from NIAAA (U01AA029019), NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378, R01DK124318), NHLBI (P01HL147835), and DOD PRCRP (W81XWH-18–2-0026).
D.H. receives funding support from the Singapore Ministry of Health’s National Medical Research Council under its NMRC Research Training Fellowship (MOH-000595–01).
Disclosures
RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
DQH has served as a consultant for Gilead Sciences and Eisai.
AMD has been a consultant for Allergan, Alderya Therapeutics, Aria Pharmaceutics, Casma, Filcitrine, Generon, Gilead, Glympse Bio, Merk, Novartis, Pfizer, RAPT Therapeutics and SunBio Pharmaceuticals. In addition, her institution receives research grants from Allergan, Astra Zeneca, Boerhinger Ingelheim, Celgene, Enyo, Excella Health, Galmed Pharmaceuticals, Gilead, Glympse Bio, Hanmi, Intercept, Inventiva, Madrigal Pharmaceuticals, Merk, NGM Biopharmaceuticals, Novo Nordisk, Novartis, and Poxel.
NT has institutional grant support from Gilead Sciences, Glaxo-Smith-Kline, Helio Health, Roche-Genetech and DURECT Corp. She has served as consultant for Moderna. BANT Advisor or consultant: Akero, Alimentiv, Allergan, Allysta, Alnylam, Amgen, Arrowhead, Axcella, Boehringer Ingelheim, BMS, Coherus, Cymabay, Durect, Enanta, Fortress, GSK, Genfit, Gilead, Glympse, Hepeon, High Tide, HistoIndex, Innovo, Intercept, Ionis, LG Chem, Lipocine, Madrigal, Medimmune, Merck, Mirum, NGM, NovoNordisk, Novus Therapeutics, pH-Pharma, Sagimet, Target RWE, Theratechnologies, 89Bio; Stock options: HepGene; Institutional research grants: Allergan, BMS, Celgene, Cirius, Enanta, Genfit, Gilead, HighTide, Intercept, Inventiva, Madrigal, NGM
Abbreviations:
- NASH
nonalcoholic steatohepatitis
- NAFLD
nonalcoholic fatty liver disease
- T2DM
type 2 diabetes mellitus
Footnotes
LAW, CB, DEK, KVK, SD, MA, NC, BAN, AJS, JT declare no conflicts of interest.
Transcript profiling: Not applicable
Writing assistance: Not applicable
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data transparency statement:
Data will not be made publicly available.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021;184:2537–2564. [DOI] [PubMed] [Google Scholar]
- 3.Paik JM, Golabi P, Younossi Y, et al. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology 2020;72:1605–1616. [DOI] [PubMed] [Google Scholar]
- 4.Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2022;7:851–861. [DOI] [PubMed] [Google Scholar]
- 5.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023. DOI: 10.1097/HEP.0000000000000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686–90. [DOI] [PubMed] [Google Scholar]
- 7.Tan DJH, Ng CH, Lin SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol 2022;23:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: Past, present and future. J Hepatol 2022;76:1362-1378. [DOI] [PubMed] [Google Scholar]
- 9.Huang DQ, Singal AG, Kono Y, et al. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab 2022;34:969–977.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan DJH, Setiawan VW, Ng CH, et al. Global burden of liver cancer in males and females: Changing etiological basis and the growing contribution of NASH. Hepatology 2023;77:1150–1163. [DOI] [PubMed] [Google Scholar]
- 11.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor RS, Taylor RJ, Bayliss S, et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020;158:1611–1625.e12. [DOI] [PubMed] [Google Scholar]
- 13.Sanyal AJ, Van Natta ML, Clark J, et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med 2021;385:1559-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng CH, Lim WH, Hui Lim GE, et al. Mortality Outcomes by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2023;21:931–939.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol 2019;71:793–801. [DOI] [PubMed] [Google Scholar]
- 16.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 17.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643–54.e1–9; quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–55. [DOI] [PubMed] [Google Scholar]
- 19.Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012;56:943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced Liver Fibrosis Is Common in Patients With Type 2 Diabetes Followed in the Outpatient Setting: The Need for Systematic Screening. Diabetes Care 2021;44:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams LA, Sanderson S, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005;42:132–8. [DOI] [PubMed] [Google Scholar]
- 22.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–21. [DOI] [PubMed] [Google Scholar]
- 23.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 26.2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S13–s27. [DOI] [PubMed] [Google Scholar]
- 27.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- 28.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 29.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis 2012;32:3–13. [DOI] [PubMed] [Google Scholar]
- 30.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349:825–32. [DOI] [PubMed] [Google Scholar]
- 31.Loomba R, Ratziu V, Harrison SA. Expert Panel Review to Compare FDA and EMA Guidance on Drug Development and Endpoints in Nonalcoholic Steatohepatitis. Gastroenterology 2022;162:680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng CH, Lim WH, Hui Lim GE, et al. Mortality Outcomes by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagstrom H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. Journal of Hepatology 2017;67:1265–1273. [DOI] [PubMed] [Google Scholar]
- 34.Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016;65:1359–68. [DOI] [PubMed] [Google Scholar]
- 35.Ajmera V, Cepin S, Tesfai K, et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HW, Wong GL, Kwok R, et al. Serial Transient Elastography Examinations to Monitor Patients With Type 2 Diabetes: A Prospective Cohort Study. Hepatology 2020;72:1230–1241. [DOI] [PubMed] [Google Scholar]
- 37.Noureddin N, Noureddin M, Singh A, et al. Progression of Nonalcoholic Fatty Liver Disease-Associated Fibrosis in a Large Cohort of Patients with Type 2 Diabetes. Dig Dis Sci 2022;67:1379–1388. [DOI] [PubMed] [Google Scholar]
- 38.Paradis V, Perlemuter G, Bonvoust F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology 2001;34:738–44. [DOI] [PubMed] [Google Scholar]
- 39.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898–906. [DOI] [PubMed] [Google Scholar]
- 40.Ratziu V, Sanyal A, Harrison SA, et al. Cenicriviroc Treatment for Adults With Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology 2020;72:892–905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will not be made publicly available.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.