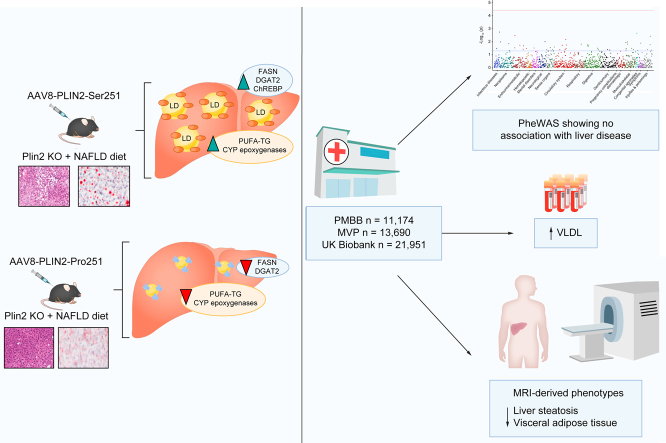
Abstract
Background & Aims
Non-alcoholic fatty liver disease (NAFLD) is characterised by the accumulation of lipid droplets (LDs) within hepatocytes. Perilipin 2 (PLIN2) is the most abundant protein in hepatic LDs and its expression correlates with intracellular lipid accumulation. A recently discovered PLIN2 coding variant, Ser251Pro (rs35568725), was found to promote the accumulation of small LDs in embryonic kidney cells. In this study, we investigate the role of PLIN2-Ser251Pro (PLIN2-Pro251) on hepatic LD metabolism in vivo and research the metabolic phenotypes associated with this variant in humans.
Methods
For our animal model, we used Plin2 knockout mice in which we expressed either human PLIN2-Pro251 (Pro251 mice) or wild-type human PLIN2-Ser251 (Ser251 mice) in a hepatocyte-specific manner. We fed both cohorts a lipogenic high-fat, high-cholesterol, high-fructose diet for 12 weeks.
Results
Pro251 mice were associated with reduced liver triglycerides (TGs) and had lower mRNA expression of fatty acid synthase and diacylglycerol O-acyltransferase-2 compared with Ser251 mice. Moreover, Pro251 mice had a reduction of polyunsaturated fatty acids-TGs and reduced expression of epoxygenase genes. For our human study, we analysed the Penn Medicine BioBank, the Million Veteran Program, and UK Biobank. Across these databases, the minor allele frequency of PLIN2-Pro251 was approximately 5%. There was no association with the clinical diagnosis of NAFLD, however, there was a trend toward reduced liver fat in PLIN2-Pro251 carriers by MRI-spectroscopy in UK Biobank subjects.
Conclusions
In mice lacking endogenous Plin2, expression of human PLIN2-Pro251 attenuated high-fat, high-fructose, high-cholesterol, diet-induced hepatic steatosis compared with human wild-type PLIN2-Ser251. Moreover, Pro251 mice had lower polyunsaturated fatty acids-TGs and epoxygenase genes expression, suggesting less liver oxidative stress. In humans, PLIN2-Pro251 is not associated with NAFLD.
Impact and Implications
Lipid droplet accumulation in hepatocytes is the distinctive characteristic of non-alcoholic fatty liver disease. Perilipin 2 (PLIN2) is the most abundant protein in hepatic lipid droplets; however, little is known on the role of a specific polymorphism PLIN2-Pro251 on hepatic lipid droplet metabolism. PLIN2-Pro251 attenuates liver triglycerides accumulation after a high-fat-high-glucose-diet. PLIN2-Pro251 may be a novel lipid droplet protein target for the treatment of liver steatosis.
Keywords: NAFLD, Lipid droplets, Perilipin, DGAT2, triglycerides
Graphical abstract
Highlights
-
•
NAFLD is characterised by the accumulation of lipid droplets within hepatocytes.
-
•
Lipid droplets are active storage organelles with a unique architecture.
-
•
PLIN2 plays an important role in the formation and stability of lipid droplets and lipophagy.
-
•
The PLIN2-Pro251 variant has been associated with reduced plasma levels of triglycerides and VLDLs.
-
•
The PLIN2-Pro251 variant reduced liver triglycerides and lipid droplet accumulation.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic and heterogenous liver condition characterised by the presence of lipid droplets (LDs) in more than 5% of the hepatocytes.1 NAFLD affects approximately 25% of the adult population worldwide.2 This condition can gradually progress into non-alcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and hepatocellular carcinoma.3 There is currently no effective treatment for NAFLD, and as a result, this condition has become one of the most common causes of cirrhosis, liver disease-related mortality, and liver transplantation.4 As the progression of NAFLD is associated with the accumulation of hepatic LDs and dysregulated glucose metabolism, understanding the specific mechanisms underlying LD accumulation is key to develop novel diagnostic and therapeutic strategies.3
Triglycerides (TGs) that accumulate in hepatocytes are stored in LDs. LDs are active, storage organelles with a unique architecture consisting of a hydrophobic core of neutral lipids (predominantly cholesterol esters and TGs) enveloped in a phospholipid monolayer. In this monolayer lie heterogeneous proteins and enzymes responsible for neutral lipid synthesis and metabolism.5 Perilipin 2 (PLIN2) is the most abundant hepatic protein in the LD phospholipid monolayer.6,7 PLIN2 plays an important role in the formation and stability of LDs and lipophagy,[7], [8], [9], [10], [11] and is associated with increased long-chain ceramides12 that impair insulin signalling.9,11,13
Previous studies showed that gene variants affecting liver TG metabolism were associated with a high risk of developing hepatocellular carcinoma irrespective of severe fibrosis.14,15
Recently, a human PLIN2 missense variant characterised by a serine-to-proline substitution at position 251 (Ser251Pro; rs35568725) was shown to have an impact on LD structure and lipid metabolism. The PLIN2-Ser251Pro (PLIN2-Pro251) variant was found to promote LD accumulation in a range of cell types, including macrophages, human embryonic kidney 293 cells, and hepatic cells (HuH7 cells).16,17 By contrast, there are conflicting results regarding the effect of PLIN2-Pro251 on cellular TG accumulation.16,17 In humans, the PLIN2-Pro251 variant has been associated with reduced plasma levels of TGs and VLDLs.16 However, the molecular role of PLIN2-Pro251 in LD metabolism is not well understood.
In the current study, we developed an experimental mouse model to investigate the biologic role of human PLIN2-Pro251 on hepatic lipid metabolism. We found that the PLIN2-Pro251 variant reduced liver TGs and LD accumulation compared to the wild-type human PLIN2-Ser251. Concurrently, we analysed the Penn Medicine BioBank (PMBB), the Million Veteran Program (MVP) and UK Biobank (UKB), three large and well-validated databases, to determine the metabolic phenotypes associated with PLIN2-Pro251. Our analyses revealed that this variant is not associated with NAFLD and data from the UKB also showed that PLIN2-Pro251 carriers have reduced liver fat compared with non-carriers. Our results offer insights into the role of PLIN2-Pro251 in hepatocellular LD homeostasis and provide a basis for future investigations.
Materials and methods
Animal model
For our study, we used 8–10-week-old male Plin2 KO-mice (Supplementary material).9 (Male mice were used to interrogate the effect of Pro251 on lipid droplet biology, as female mice were relatively protected from steatosis in our pilot study of 12 weeks of high-fat, high-fructose, high-cholesterol [FFC] feeding.) Mice were fed with chow diet until they were 8 weeks old. Thereafter, we administered a retro-orbital injection of adeno-associated virus utilising a thyroxine-binding globulin promoter to direct the expression of either the PLIN2-Pro251 polymorphism (Pro251 mice) or the wild-type PLN2-Ser251 (Ser251 mice) in the liver (Fig. S1A–D).
All injected mice were divided into two dietary groups: the first group (FFC group) was given 12 weeks of ad libitum access to an FFC diet (40% kcal fat, 20% kcal fructose, 2% cholesterol); the second group (Chow group) was given 12 weeks ad libitum access to a chow diet (Fig. S1E).18
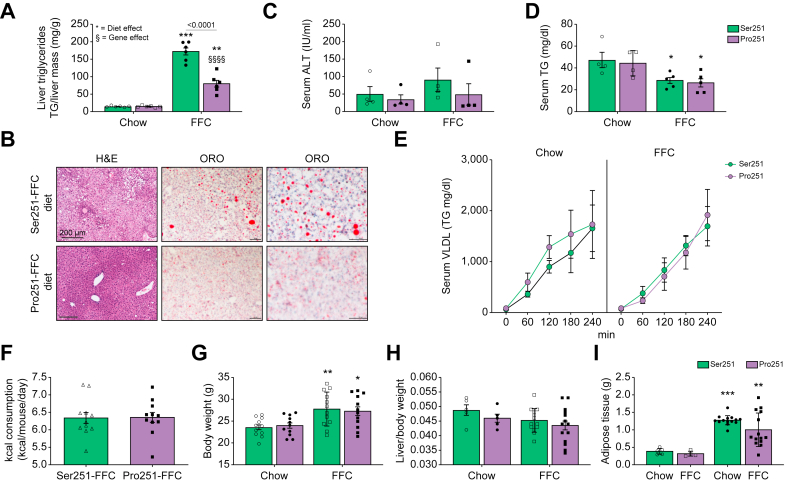
Fig. 1.
Impact of Pro251 on hepatic and metabolic parameters in mice after 12-week FFC diet.
Hepatic TG levels (A), representative histologic images of H&E-stained and Oil Red O stained (B), hepatic ALT (C), serum TG levels (D), serum VLDL (E), calories consumed (F), body weights (G), liver/body weight ratio (H), adipose weight (I). Statistical analyses were performed using a two-tailed unpaired t-test or one-way ANOVA with Tukey’s post-hoc test. ∗Statistical difference between diets, ∗p <0.05; ∗∗p <0.005; ∗∗∗p <0.0005; ∗∗∗∗p <0.0001. §Statistical difference between genotypes, §p <0.05; §§p <0.005; §§§p <0.0005; §§§§p <0.0001. ALT, alanine aminotransferase; FFC, high-fat, high-fructose, high-cholesterol; TG, triglyceride; VLDL, very low density lipoprotein.
Biochemical and lipidomic analysis
Plasma samples for biochemical assays were obtained by centrifuging blood in a lithium heparin tube at 2,000 × g at 4 °C for 10 min. Liver samples were prepared by adding 6 μl 2:1 ethanol (EtOH):30% potassium hydroxide solution per mg of liver tissue, vortexing, then incubating in a 60 °C water bath overnight. Subsequently, 1.08 volumes of 1 MgCl2 were added to the liver digest, before it was vortexed to a milky consistency, and placed on ice for 10 min. The liver digest was then centrifuged at 18,000 × g at room temperature for 30 min, and the resulting supernatant was used for biochemical assays.
Plasma alanine aminotransferase (ALT) activity was measured using a Stanbio ALT/SGPT Liqui-UV Test kit (EKF Diagnostics, TX, USA). Plasma and liver TG levels were measured using a Stanbio Triglycerides LiquiColor Test Mono (EKF Diagnostics, TX, USA). Plasma levels of non-esterified fatty acid (NEFA) were measured using a Wako HR Series NEFA-HR 2 test kit (Fujifilm, Japan). All biochemical assays were analysed using Infinite 200 PRO plate reader (Tecan Trading AG, Switzerland). Fasting blood glucose was measured using the Accu-Chek Nano Glucose Meter (Roche Diabetes Care, Inc., New York, NY, USA).
To estimate hepatic VLDL production, a kinetic experiment was performed. Mice were fasted for 4 h (from 07:00 to 11:00) then injected with 1 g/kg poloxamer 407 (Sigma-Aldrich, St. Louis, MO, USA) that blocks the lipolysis of TGs. Tail vein blood was collected at time 0 and 1, 2, and 4 h after injection, measuring TGs by colorimetric assay and thereby enabling estimation of VLDL secretion rate.
Lipidomics
Tissue preparation for lipids extraction
Sections of approximately 10 mg of frozen tissues were cut on a tile kept in dry ice with a new blade kept in dry ice. The tissue was added to a microcentrifuge tube with 0.6 ml 80% methanol (MeOH) and 20 μl on internal standard mix (1:1, SPLASH® LIPIDOMIX #330707 and Ceramide/Sphingoid Internal Standard Mixture I #LM6002, both from Avanti Polar Lipids, Alablaster, AL, USA).
Liquid chromatography-high resolution mass spectrometry for lipids
Metabolites were separated using an Ascentis Express C18, 2.1 × 150 mm 2.7 μm column (Sigma-Aldrich) on an UltiMate 3000 HPLC system. The metabolites were eluted on a 0.4 m/min flow-rate gradient using Solvent A (4:6 v/v water:acetonitrile, 0.1% formic acid, 10 mM ammonium formate) and Solvent B (1:9 v/v acetonitrile:isopropanol, 0.1% formic acid, 10 mM ammonium formate). The gradient was as follows: 10% B at 0 min, 10% B at 1 min, 40% B at 4 min, 75% B at 12 min, 99% B at 21 min, 99% B at 24 min, 10% B at 24.5 min, 10% at 30 min. Separations were performed at 55 °C.
For the lipidomic and ceramide analyses see the Supplementary material.
Histology
Dissected liver samples were placed in a cartridge, fixed in 10% buffered formalin overnight, transferred to 70% EtOH, and paraffin embedded. Paraffin sections were stained with H&E. To visualise lipid deposition, liver sections frozen in cryoprotectant media were stained with Oil Red O. Sectioning and staining of the liver were performed by the Molecular Pathology and Imaging Core at the University of Pennsylvania. Slides were visualised under a bright field with Nikon 80i microscope (Nikon Instruments, Melville, NY, USA) and interpreted by a liver pathologist (EEF). Images were captured with a Nikon DS-Qi1MC camera (Nikon Instruments).
Western blot
Tissue protein lysates were prepared by homogenising liver, adipose, or muscle tissue in a RIPA buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 5 mM EDTA, 1% v/v NP-40, 0.5% w/v sodium deoxycholate, 0.1% v/v SDS, 50 mM sodium fluoride) containing cOmplete Protease Inhibitor tablets and PhosStop phosphatase inhibitor tablets (Roche Diagnostics, Germany). Protein concentrations were determined using a Pierce BCA protein assay kit (Thermo-Fisher Scientific). Proteins were then resolved on a NuPAGE 4–12% Bis-Tris gel (Invitrogen, CA, USA) by electrophoresis, and transferred onto a nitrocellulose membrane by electroblotting. Blots were analysed using ImageJ software (NIH). Data were normalised to glyceraldehyde 3-phosphate dehydrogenase protein levels.
Quantitative PCR
Liver tissue samples stored in RNAlater solution (Invitrogen, Lithuania) were solubilised using a Bio-Gen PRO 200 homogeniser (PRO Scientific, CT, USA). mRNA was extracted from the homogenate using a PureLINK RNA Mini Kit (Invitrogen, CA, USA). The mRNA samples were treated with Amplification Grade DNase I (Invitrogen, CA, USA), then MultiScribe reverse transcriptase (Applied Biosystems, CA) to produce cDNA. Quantitative real-time PCR (qPCR) was performed with the cDNA on a StepOnePlus PCR system (Applied Biosystems, CA, USA) or Taqman 7900 (Thermo-Fisher Scientific) using gene-specific primers and SYBR Select Master Mix (Applied Biosystems, Lithuania). Data were normalised to mRNA levels of ribosomal protein, large, P0 (36b4) reference gene.
Glucose and insulin tolerance test
For glucose tolerance tests (GTTs), mice were fasted for 6 h and then intraperitoneally injected with PBS supplemented with 20% glucose at a dosage of 10 ml/kg body weight. Blood glucose levels were measured at time 0 and 15, 30, 60, 120, and 180 min after injection with an ACCU-CHEK Inform II glucometer (Roche Diabetes Care, IN, USA). For the insulin tolerance test (ITT), animals were fasted for 5 h before 0.75 U/kg of human insulin was intraperitoneally administered. Tail blood glucose was measured at time 0 and 15, 30, 60, and 90 min after injection with a glucometer (Lifescan, Inc., Milipitas, CA, USA). Mice were allowed to recover and resume their diets after completion of these tests.
Metabolic monitoring
Mice were individually placed in the Oxymax Lab Animal Monitoring System (Columbus Instruments, Columbus, OH, USA). Following a 24-h acclimation period, oxygen consumption, carbon dioxide production, and locomotor activity were determined. The respiratory exchange ratio (RER) was calculated by dividing the volume of carbon dioxide produced by the volume of oxygen consumed (VCO2/VO2). Whole body fat and lean mass determinations were performed using the Eco-MRI-100 system (Echo MRI; Houston, TX, USA).
RNA-seq
mRNA samples for RNA-seq analysis were prepared from liver tissue homogenates using a PureLINK RNA Mini Kit (Invitrogen, CA, USA). Libraries were prepared by the Next Generation Sequencing Core at the University of Pennsylvania. Total RNA quantity and quality were assayed with an Agilent 2100 Bioanalyzer instrument using the RNA 6000 Nano Kit (Agilent Technologies). Libraries were prepared at Next Generation Sequencing Core at the University of Pennsylvania using TruSeq Stranded mRNA HT Sample Prep Kit (Illumina) as per standard protocol in the kit’s sample prep guide. Libraries were assayed for size using the DNA 1000 kit of an Agilent 2100 Bioanalyzer (Agilent Technologies) and quantified using the KAPA Library Quantification Kit for Illumina platforms (KAPABiosystems). The 100-bp single-read sequencing of multiplexed samples was performed on an Illumina HiSeq 4000 sequencer. Illumina’s bcl2fastq version 2.20.0.422 software was used to convert bcl to fastq files.
The Penn Medicine Biobank
The PMBB is an institutional resource of the University of Pennsylvania Health System. This database includes DNA, blood, and tissue samples from over 60,000 patients. Samples were obtained under a single umbrella Institutional Review Board protocol that permits DNA genotyping and sequencing, biomarker assays, access to electronic health record (EHR) phenotype data, and permission to recontact. PMBB participants were genotyped using the Illumina Global Screening Array v.2.0 and further imputed using the TOPMed Imputation Server. For the phenome-wide association study (PheWAS), ICD-9 and ICD-10 codes were used to assign traits to each patient according to their records, and a genome-wide association study for each was run using the imputed genome-wide genetic data. Subsequently, the results for rs35568725 were retrieved to determine its association with clinical phenotypes. For our study, we screened the genome-wide genotype data for over 20,000 White and Black participants.
Million Veteran Program
The US Department of Veterans Affairs (VA) provides care to approximately nine million veterans. The MVP is a VA database of genomic and phenotypic information.19,20 Launched in 2011 and supported by the Veterans Health Administration Office of Research and Development in the United States, the MVP largely comprises White, Black, and Hispanic male veteran participants and contains both clinical and genetic data. Clinical data were prospectively collected and combined with prior health record data from the VA EHR, and the VA Corporate Data Warehouse. The association of cardiometabolic traits for PLIN2-Pro251 among MVP participants was tested under an additive logistic model and was corrected for age, sex, and the first 10 ethnicity-specific principal components. The present study was approved by the VA Central Institutional Review Board and by the Corporal Michael J. Crescenz VA Medical Center and by the University of Pennsylvania Institutional Review Board.
The United Kingdom Biobank
The UKB is a population-based cohort study, which recruited 502,505 UK volunteers between 2006 and 2010 (age range between 37 and 73 years). Genome-wide genotyping was performed using the UKB Axiom Array and additional imputation was performed using the TOPMed reference panel. The UKB was approved by the Northwest Multi-Center Research Ethics Committee. Details of the methods, data availability, and access procedures for UKB are publicly disclosed on its website (http://www.ukbiobank.ac.uk). A total of 1,419 EHR-derived broad PheWAS codes were available for approximately 400,000 White British individuals. For liver fat analysis and adipose tissue analysis we used UKB MRI-derived phenotypes. For our study, association analyses were performed using the logistic regression test implemented in plink, including age, sex, and 10 ancestry-informative principal components as covariates.
Data analysis
Statistical analyses for the in vivo model were performed using GraphPad Prism 8.2 (GraphPad, San Diego, CA, USA). All data were presented as mean ± standard error of measurement (SEM). Statistical analysis was performed using either a t test or one-way ANOVA with Tukey’s multiple comparisons test. To test for any independent associations linear or logistic regressions were performed. Statistical significance was determined at p <0.05. The following programs were used to analyse our collected data: R version 4.0.2 (R Foundation for Statistical Computing; Vienna, Austria), SPSS Statistics version 26 (IBM; Armonk, NY, USA) and Prism version 8 (GraphPad, La Jolla, CA, USA). We calculated the Bonferroni corrected/adjusted p value, by dividing the original α-value (0.05) by the number of analyses on the dependent variable.
Results
PLIN2-Pro251 expression attenuates FFC-induced hepatic steatosis compared with PLIN2-Ser251
After 12 weeks of the FFC diet, we found that Pro251 mice had a significantly lower hepatic TG level compared with Ser251 mice (Fig. 1A and B). In addition, the liver histology showed a shift in LD organisation with increasing microvesicular patterns in Pro251 mice compared with Ser251, without evidence of inflammation or fibrosis in either genotype (Fig. 1B and Fig. S1E). Moreover, FFC-fed Pro251 and Ser251 mice showed similar liver ALT levels, serum TG levels, and hepatic VLDL-TG secretion (Fig. 1C–E). Pro251 and Ser251 mice also had similar caloric consumption, increase in body weight, liver to body weight ratio, and adipose tissue weight (Fig. 1F–I).
PLIN2-Pro251 has a role in liver lipid remodelling
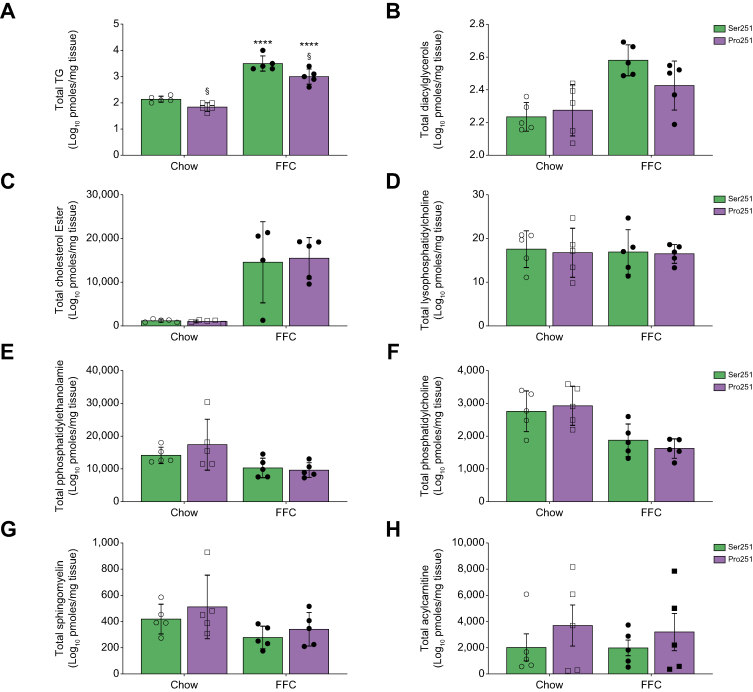
Given the changes observed with overall hepatic TG content and reduction in steatosis in Pro251 mice, we quantified the specific lipid species that contribute to liver LD composition. We performed a targeted shotgun comparison of hepatic lipids obtained from liver lysates of Pro251 and Ser251 mice. The lipidomic analysis demonstrated that Pro251 mice had a decrease in total hepatic TG species compared with Ser251 mice (Fig. 2A), confirming our biochemical measurements. In addition, we found a trend towards a reduction of total hepatic diacylglycerols (DGs) in Pro251 compared with Ser251 mice (Fig. 2B). We did not find any difference in total hepatic levels of cholesterol esters, lysophosphatidylcholine, phosphatidylethanolamine, phosphatidylcholine, and sphingomyelin in both genotypes (Fig. 2C–G); however, we found a trend towards increase in acyl-CoA (Fig. 2H).
Fig. 2.
Liver lipidomic analysis.
Total triglycerides (TG) (A), diacylglycerols (DG) (B), total cholesterol ester (C), total lysophosphatidilcholine (D), total phosphatidylethanolamine (E), total phosphatidylcholine (F), total sphingomyelin (G) and acylcarnitine (H). Statistical analyses were performed using a two-tailed unpaired t-test or one-way ANOVA with Tukey’s post-hoc test. ∗Statistical difference between diets, ∗p <0.05; ∗∗p <0.005; ∗∗∗p <0.0005; ∗∗∗∗p <0.0001. §Statistical difference between genotypes, §p <0.05; §§p <0.005; §§§p <0.0005; §§§§p <0.0001. FFC, high-fat, high-fructose, high-cholesterol.
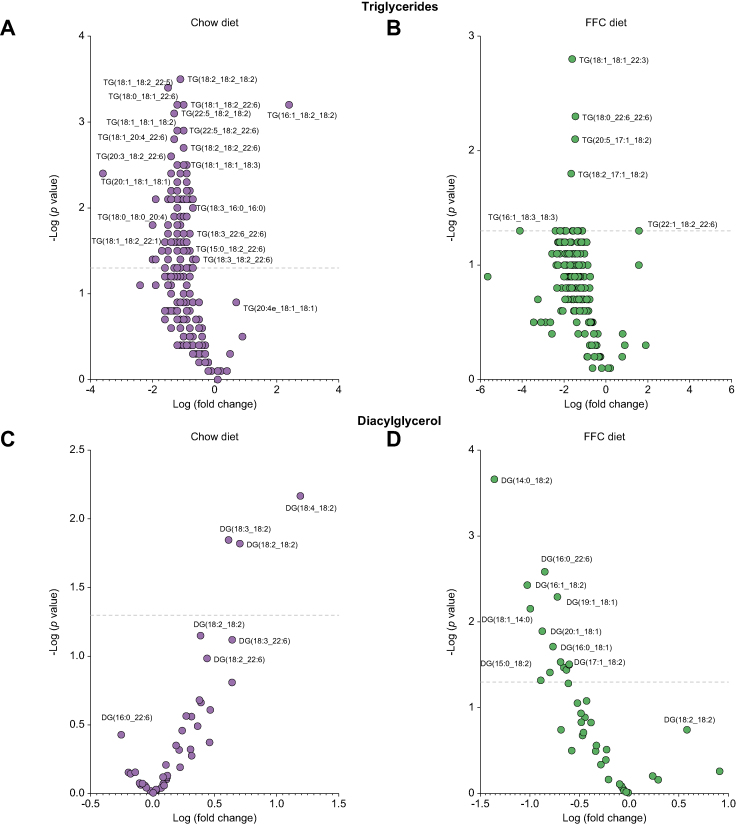
A detailed analysis of the lipid species demonstrated a reduction in the quantity of polyunsaturated-fatty-acids-TG (PUFA-TG) in Pro251 mice (Fig. 3A and B and Table S1). In both diets, Pro251 mice had a significant reduction in TG (18:1_18:1_22:3), TG (18:0_22:6_22:6), TG (20:5_17:1_18:2), TG (18:2_17:1_18:2), TG (16:0_14:0_18:1) TG (18:0_16:0_16:0), and TG (18:1_14:0_14:0) (Fig. 3B and Table S1). Interestingly, we found that in the Chow group, there was an increase in PUFA-DG in Pro251 mice; whereas, in the FFC group, Pro251 mice had a reduction in DG containing saturated and monounsaturated fatty acids compared with Ser251 mice (Fig. 3C and D). With regard to other lipids, Pro251 mice fed an FFC diet also had a trend towards increase in PC containing α-linolenic acid PC (18:3e_18:0) and lower (albeit non statistically significant) levels of PE containing docosapentaenoic acid PE (18:1_22:5) and linoleic acid (18:1_18:2) compared with Ser251.
Fig. 3.
Volcano plot showing the log-fold changes of lipids in Pro251 mice.
Liver triglycerides in chow diet (A) and FFC diet (B); liver diacylglycerols (C) and (D). The line marks the Bonferroni significant cut-off. FFC, high-fat, high-fructose, high-cholesterol; TG, triglyceride.
Having previously established that ceramides are also present in the LD fraction,12,21 we analysed the distribution of several serum and hepatic sphingolipids in Pro251 and Ser251 mice using mass spectrometry (Fig. S2A–H). The overall patterns of serum and hepatic sphingolipids – both upstream (i.e. dihydrosphingosine) and downstream (i.e. sphingosine) in the ceramide metabolic pathway – were similar in both genotypes. In the FFC-diet group, Pro251 mice had lower levels of C20 compared with Ser251 mice. These observations are important as higher C20 ceramide is positively associated with cardiometabolic risk.22
Expression of PLIN2-Pro251 downregulates expression of genes associated with fatty acid synthesis and epoxygenase P450 pathway
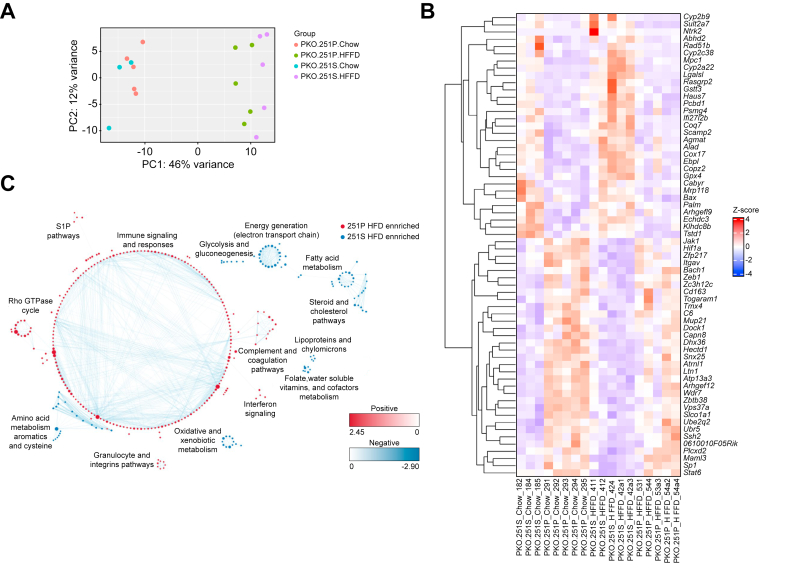
To gain insight into the molecular mechanisms of PLIN2-Pro251, we investigated the effect of this variant on hepatic lipids. First, we performed RNA-seq in the liver samples of our mice in both diet groups. The principal component analysis showed a clear separation between both FFC and Chow groups and slight separation between both genotypes (Pro251 and Ser251) in the FFC group (Fig. 4A). With regard to the differentially expressed genes (DEGs), we found 46 genes that were significantly downregulated and 30 genes that were significantly upregulated in the Pro251 mice compared with the Ser251 mice. Among downregulated genes, gene enrichment analysis identified several significantly altered metabolic pathways, including epoxygenase P450 pathway (Cyp2b13, Cyp2b9, Cyp2a22, and Cyp3a59), fatty acid metabolism (Mogat1, Fasn, and Hsd17b12), and glutathione metabolism pathway (Gstm3 and Gstt1). With regard to the upregulated genes, gene enrichment analysis showed altered pathways involved in immune signalling and responses (Ikbkg, Junb, Egr1, Fos, and Fosb) and a gene involved in the fatty acyl-CoA synthesis that controls mitochondrial respiratory capacity (Acsbg1) (Fig. 4B and C).
Fig. 4.
Liver differentially expressed genes in Pro251 and Ser251.
Principal component analysis showing a clear separation between the FFC and chow group and slight separation between both genotypes (Pro251 and Ser251) in the FFC group (A). Each dot represents data from individual mice (A); a heatmap depicts 36 DEGs that were significantly changed in the Pro251 compared with Ser251 in the FFC group (B); 13 enrichment pathways identified using GSEA and Cytoscape (C). DEGs, differentially expressed genes; FFC, high-fat, high-fructose, high-cholesterol.
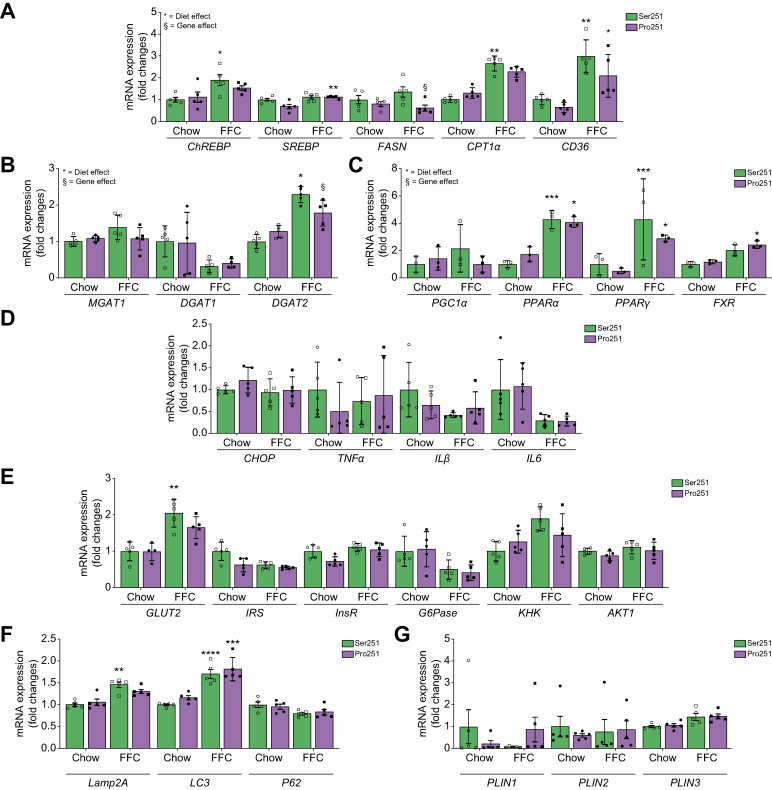
We validated these findings using qPCR of representative genes. In the FFC group, hepatic mRNA levels of lipogenic genes were upregulated in both Pro251 and Ser251 mice. However, compared with Ser251 mice, fatty acid synthase (Fasn) and diacylglycerol O-acyltransferase 2 (Dgat2) expression were significantly downregulated in Pro251 mice, indicating that they had less fatty acid synthesis and less conversion of diacylglycerol and fatty acyl-CoA to TGs (Fig. 5A and B). However, we did not observe any differences in DGAT2 protein expression between Ser251 and Pro251 mice (data not shown). We did not find significant changes in mRNA expression of peroxisome proliferator-activated receptor alpha or gamma in both genotypes (Fig. 5C) or in genes associated with inflammation, autophagy, cellular stress, or glucose metabolism (Fig. 5D–F). Moreover, there was no significant difference in Plin1, Plin3 mRNA, and protein expression between Pro251 and Ser251 mice (Fig. 5G and Fig. S3A–C).
Fig. 5.
De novo lipogenesis gene expression measured by qPCR mice on chow and FFC diets.
(A) lipogenesis gene expression measured by qPCR in mice on chow and FFC diets (B), MGAT, DGAT1, DGAT2 gene expression measured by qPCR in mice on chow and FFC diets (C), inflammatory response gene expression measured by qPCR in mice on chow and FFC diets (D), glucose metabolism genes expression measured by qPCR in mice on chow and FFC diets (E), autophagy gene expression measured by qPCR in mice on chow and FFC diets (F), PLIN1, PLIN2, and PLIN3 gene expression by qPCR in mice on chow and FFC diets (G). Statistical analyses were performed using a two-tailed unpaired t-test or one-way ANOVA with Tukey’s post-hoc test. ∗p <0.05; ∗∗p <0.005; ∗∗∗p <0.0005; ∗∗∗∗p <0.0001. §Statistical difference between genotypes, §p <0.05; §§p <0.005; §§§p <0.0005; §§§§p <0.0001. DGAT, diacylglycerol acyltransferase; FFC, high-fat, high-fructose, high-cholesterol; MGAT, monoacylglycerol acyltransferase; PLIN, perilipin; qPCR, quantitative real-time PCR.
PLIN2-Pro251 has no effect on glucose tolerance or insulin sensitivity
To determine the effect of PLIN 2-Pro251 on insulin sensitivity, we performed an intraperitoneal GTT and ITT. We found no differences in fasting blood glucose (Fig. S4A), GTT or ITT (Fig. S4B and C) between the genotypes. In addition, we did not find differences in serum NEFA (a surrogate measure of adipose tissue insulin sensitivity) between the two genotypes (Fig. S4D).
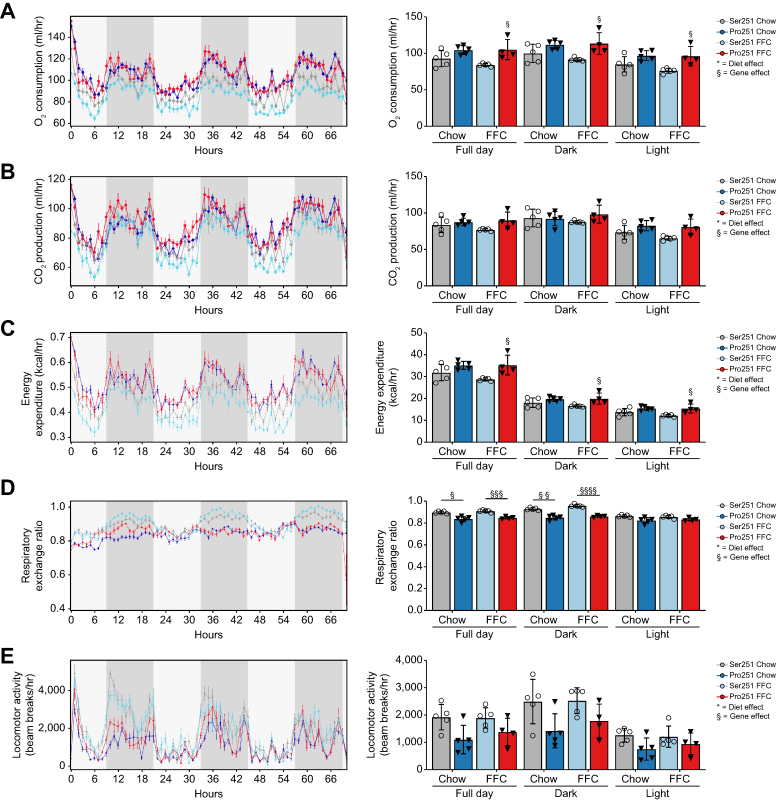
PLIN2-Pro251 expression increases energy expenditure
To evaluate the effect of the PLIN2-Pro251 on metabolic phenotypes, we measured energy expenditure with indirect calorimetry (Fig. 6). Throughout the day, Pro251 mice had increased O2 consumption, CO2 production and energy expenditure compared with Ser251 mice (Fig. 6A–C). We also measured the RER which equals VCO2/VO2). RER is an index of fuel oxidation: a RER value of 0.7 indicates fat oxidation, whereas a value of 1 indicates carbohydrate oxidation. Pro251 mice had significantly reduced RER values compared with Ser251 mice, in the dark period. Notably, in Pro251 mice, RER levels were approximately 0.8, suggesting that a mix of fat and carbohydrates were utilised as fuel. By contrast, the RER of Ser251 mice was close to 0.9, indicating that more carbohydrates were utilised as fuel (Fig. 6D).
Fig. 6.
Impact of Pro251 on metabolic phenotype.
Pro251 had positive effect on metabolic phenotyping in both diets (A–C and E). Pro251 had a decrease in respiratory exchange ratio in full day and dark (D). Statistical analyses were performed using a two-tailed unpaired t-test or one-way ANOVA with Tukey’s post-hoc test. ∗Statistical difference between diets, ∗p <0.05; ∗∗p <0.005; ∗∗∗p <0.0005; ∗∗∗∗p <0.0001. §Statistical difference between genotypes, §p <0.05; §§p <0.005; §§§p <0.0005; §§§§p <0.0001. FFC, high-fat, high-fructose, high-cholesterol.
PLIN2-Pro251 was not associated with NAFLD and metabolic diseases in humans
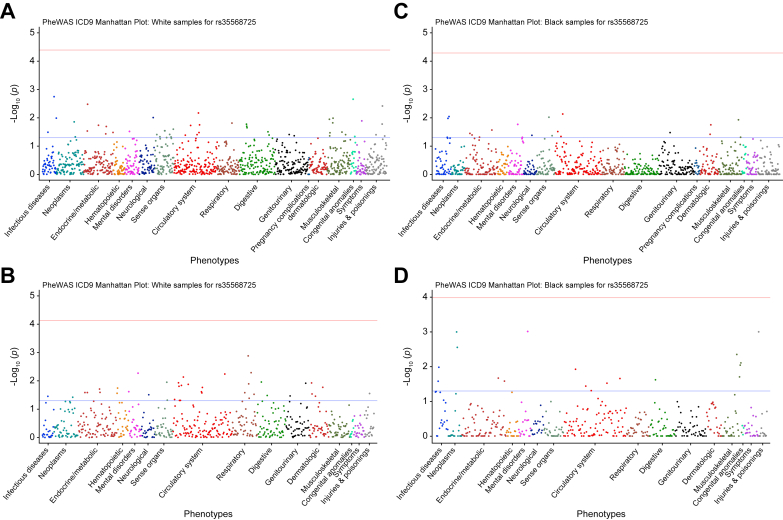
Data for 11,174 participants were available in the PMBB. The minor allele frequency of PLIN2-Pro251 was approximately 5% in White participants and approximately 2% in Black participants. We identified a total of 296 ICD-10 code diagnoses of NAFLD and 10,878 controls. The PheWAS showed no association between homozygous PLIN2-Pro251 and diagnosis of NAFLD or other severe liver diseases (Fig. 7A–D).
Fig. 7.
Multivariable PheWAS of the effect of Pro251 on clinical phenotypes.
Multivariable PheWAS of the effect of Pro251 on clinical phenotypes for White population (A and B). Multivariable PheWAS of the effect of Pro251 on clinical phenotypes for African American population (C and D). The line marks the Bonferroni significant cut-off. PheWAS, phenome-wide association study.
Similarly, in the MVP, the minor allele frequency of PLIN2-Pro251 was approximately 5% in White participants and approximately 2% in Black, Hispanic, and Asian participants (n = 13,690) (Table S2). We further analysed the MVP dataset to identify whether there were associations between homozygous PLIN2-Pro251, NAFLD, and metabolic diseases. We did not find any association between PLIN2-Pro251 carriers and increased risk of NAFLD or other severe liver diseases (Table 1). In addition, we found that homozygous PLIN2-Pro251 had a trend towards a higher risk of cardiomyopathy and type 2 diabetes compared with non-carriers. However, these associations were not significant when adjusted with the Bonferroni correction (Table S3).
Table 1.
Impact of the variant on phenotypes related to more severe liver disease.
| Phenotype | Beta | SE | p value |
|---|---|---|---|
| HCC | 1.933e-01 | 1.383e-01 | 0.16226 |
| Cirrhosis | 2.389e-01 | 1.562e-01 | 0.12624 |
Multivariable analyses adjusted for age, sex, BMI, and PC1-10.
HCC, hepatocellular carcinoma.
In the UKB, the minor allele frequency of PLIN2-Pro251 was again approximately 5%. We further examined this dataset to determine the phenotypic and metabolic profile of PLIN2-Pro251 carriers (n = 21,951.9) compared with non-carriers (n = 439,038). We analysed data from 30,359 UKB participants who had MRI-derived imaging (adjusted for age and BMI). Notably, we found that homozygous PLIN2-Pro251 carriers had a trend towards reduced liver fat percentage (β = -0.15; SE = 0.08; p = 0.05) (Table 2); these data are especially notable as they are consistent with the results of our mouse model. Our analysis (adjusted for age and BMI) of the UKB also showed that homozygous PLIN2-Pro251 carriers were associated with a significant reduction in visceral adipose tissue (β = -0.04; SE = 0.02; p = 0.04) and a trend towards increase in liver iron (β = 1.7; SE = 0.8; p = 0.05) (Table 2). We repeated the analysis to investigate the liver phenotype of the PLIN2-Pro251 by adjusting for age and BMI in males and females. We found that the PLIN2-Pro251 was associated with a trend towards a decrease in MRI-PDFF in males (β = -0.2; SE = 0.1; p = 0.06) but not in females (Table 2). In addition, there was a significant association between PLIN2-Pro251 and decrease in visceral adipose tissue in all individuals. However, this association was not significant when considering males and females separately (Table S4). Moreover, these patients had increased levels of large and very large VLDL particles compared with non-carriers. The sex stratification of the lipidomic profile suggested a difference in the male group compared with female, confirming an increase in lipid secretion, and suggesting a reduction in lipid accumulation in the liver (Fig. S5A–C).
Table 2.
Association between PLIN2-Pro251 and MRI-PDFF liver phenotype in the UKB adjusted for age and BMI.
| Phenotype | Beta | SE | p value |
|---|---|---|---|
| All participants (N = 30,359) | |||
| Liver volume | -5.251e-03 | 3.848e-03 | 0.17245 |
| Subcutaneous fat volume | -0.0539815 | 0.0374349 | 0.149308 |
| Visceral adipose tissue | -0.0481373 | 0.0238025 | 0.0431 |
| MRI-PDFF | -0.1570789 | 0.0819436 | 0.055258 |
| Liver iron | 1.726799 | 0.876434 | 0.048818 |
| HCC | 1.933e-01 | 1.383e-01 | 0.16226 |
| Cirrhosis |
2.389e-01 |
1.562e-01 |
0.12624 |
|
Females (n = 14,645) | |||
| Liver volume | -7.612e-03 | 4.861e-03 | 0.117 |
| Subcutaneous fat volume | -0.0614021 | 0.0559731 | 0.272 |
| Visceral adipose tissue | -0.0349735 | 0.0246701 | 0.156 |
| MRI-PDFF | -0.0591458 | 0.1072458 | 0.581 |
| Liver iron | 1.633119 | 1.191840 | 0.170 |
| HCC | 3.262e-01 | 2.322e-01 | 0.160 |
| Cirrhosis |
3.105e-01 |
2.593e-01 |
0.231 |
|
Males (n = 15,714) | |||
| Liver volume | -1.922e-04 | 5.979e-03 | 0.974 |
| Subcutaneous fat volume | -0.0705943 | 0.0484526 | 0.145 |
| Visceral adipose tissue | -3.518e-02 | 3.945e-02 | 0.372 |
| MRI-PDFF | -0.226995 | 0.124209 | 0.067 |
| Liver iron | 1.948e+00 | 1.288e+00 | 0.130 |
| HCC | 1.500e-01 | 1.636e-01 | 0.359 |
| Cirrhosis | 0.168310 | 0.211089 | 0.425 |
Multivariable analyses adjusted for age, sex, BMI, and PC1-10. Values in bold denote statistical significance.
HCC, hepatocellular carcinoma; PDFF, proton density fat fraction.
We also tested the additive model in the MVP and in the UKB and we did not find any significant association between PNPL2-Pro251 allele and risk of liver disease and lipidomic profile (data not shown).
Discussion
Our study shows that after 12 weeks of an FFC diet, hepatocyte-specific expression of human PLIN2-Pro251 in mice lacking Plin2 is protective against macrosteatosis and liver TG accumulation. These improvements in metabolism were in part derived from higher energy expenditure, as evidenced by calorimetry data showing that Pro251 mice had an increase in O2 consumption and RER. We found that Pro251 mice exhibited a trend towards increased acylcarnitine levels and a 4.4-fold increase in Acsbg1 (Acyl-CoA Synthetase, Bubblegum Family, member 1) gene expression (adjusted p = 0.045). Acylcarnitines are produced through the conjugation of acyl-CoAs with carnitine, which facilitates the transport of long-chain fatty acids across the inner mitochondrial membrane for β-oxidation. Recently, it has been demonstrated that acylcarnitine is involved in energy mobilisation and stimulates energy expenditure. Acsbg1-dependent fatty acyl-CoA synthesis plays a crucial role in controlling mitochondrial respiratory capacity and participates in the oxidation of long-chain saturated and unsaturated fatty acids. Consequently, the increased expression of Acsbg1 and the trend towards elevated acylcarnitine levels might have an impact on increasing energy expenditure.23,24 Additional results from our lipidomic data also revealed that Pro251 mice have 47% less liver TG accumulation and a trend towards a decrease in liver DG compared with Ser251 mice. Moreover, the liver lipidomic composition of Pro251 mice showed a reduction in PUFA-TGs compared with Ser251 mice, indicating that there was less accumulation of PUFAs in the hepatic LD lipid core of Pro251 mice compared with Ser251 mice. Congruent with these data were our RNA-seq data which demonstrated reduced expression of genes involved in the epoxygenase pathway, thus supporting the hypothesis that there is less oxidative stress and less PUFA peroxidation in Pro251 mice compared with Ser251 mice. It is also possible that Pro251 mice produce fewer oxidised eicosanoids compared with Ser251 mice, thereby leading to an accumulation of PUFA species in the cytosol.25 The reduction in PUFA-TGs that we observed in Pro251 mice is consistent with recent evidence showing that PUFAs redistribute into the LD core during oxidative stress.25,26
In accordance with previous literature, we noticed an increase in gene expression associated with de novo lipogenesis, in both Ser251 and Pro251 mice on the FFC diet.27 However, mice expressing the PLIN2-Pro251 had less increase in lipogenic genes compared with Ser251 mice. In particular, Pro251 mice on the FFC diet had reduced mRNA expression of Dgat2 and Fasn which are genes that have significant roles in de novo lipogenesis.28 However, we did not observe a significant reduction in DGAT2 protein expression.
The mechanisms linking PLIN2-Pro251 to the downregulation of mRNA expression of Dgat2 and Fasn are not clear. The downregulation of Dgat2 gene expression might reduce the gene expression of Fasn, resulting in a reduction of de novo lipogenesis and ultimately in less accumulation of liver TGs. This hypothesis is consistent with previous literature. A recent paper has shown that liver specific Dgat2-KO (knockout) mice have reduced expression of genes associated with de novo lipogenesis and especially a downregulation of Fasn. This was associated with a reduction in liver TG accumulation.27 There is also evidence illustrating that DGAT1/DGAT2 double KO cells treated with oleic acid accumulate less TG and fewer LDs than wild-type control cells.29 This same study also demonstrated that DGAT1/DGAT2 double KO cells, when treated with oleic acid, showed reduced accumulation of PLIN2 protein. Interestingly, this occurred despite these cells maintaining PLIN2 mRNA levels similar to those found in wild-type cells. This suggests a potential genetic interaction between DGAT2 and PLIN2.29 PLIN2 protein has two structural domains: (1) the N-terminal domain that contains amphipathic alpha-helices that modulate the binding of the protein to lipid droplets; and (2) the C-terminal four-helix bundle domain that interacts with membranes and other proteins. With regard to the latter, the substitution of a serine-to-proline residue in position aa 251 observed in PLIN2-Pro251 might disrupt the hydrogen bonding of the PLIN2 protein peptide backbone and induce a kink in the helix, altering the organisation of the four-helix bundle (Fig. S6). This alteration to the C-terminal four-helix bundle domain might affect the phosphorylation capacity of PLIN2-Pro251 and inhibit its interaction with other proteins. Further studies are warranted to gain a better understanding of the role of PLIN2 and its interaction with other proteins.
Our analysis of three independent biobank datasets of diverse, international populations encompasses the largest study to date of PLIN2-Pro251. We found that this variant is rare with an overall prevalence of 2% in Black, Hispanic, and Asian populations and 5% in White populations. Our PheWAS analyses revealed that individuals homozygous for this gene variant exhibit a mixed metabolic risk profile but no association with a clinical diagnosis of NAFLD or more severe liver diseases. Interestingly, we observed trends toward lower liver fat as diagnosed by magnetic resonance spectroscopy and lower subcutaneous and visceral adiposity in all individuals and in males, but not in females. This suggests that the influence of the PLIN2-Pro251 variant on hepatic steatosis may be secondary to its effect on visceral fat accumulation, rather than a direct effect on hepatic lipid metabolism. It is noteworthy that the present experimental mouse model, which specifically expressed PLIN2-Pro251 in the liver in a full Plin2 KO context, could not address this question. Conversely, we detected trends toward higher risk of cardiometabolic disorders such as cardiomyopathy, ischaemic heart disease, and type 2 diabetes, as well as increased risk of large and very-large circulating VLDL particles. These results are consistent with those of our mouse model, where we saw a trend towards an increase in VLDL liver secretion in Pro251 mice compared with Ser251 mice.
The findings of our human data analyses appear to be in contrast with two previous clinical studies finding that PLIN2-Pro251 was significantly associated with NASH, with an allelic odds ratio of 2.9817 and that a cohort of Italian women with PLIN2-Pro251 and obesity-associated metabolic alterations had significantly lower insulin levels, respectively.30 Nevertheless, we note that both these studies involved small cohorts and that the association between PLIN2-Pro251 and NASH was based on only three homozygous carriers of this genetic variant.
In conclusion, our study suggests that PLIN2-Pro251 protects against hepatic steatosis through the downregulation of lipid synthetic and epoxygenase genes, and the alteration of LD and liver lipids. Counterintuitively, this genetic variant also appears to increase cardiometabolic risk perhaps through the promotion of VLDL production. As there is presently very limited knowledge and understanding of how liver lipid species confer cardiometabolic risk, further research is warranted including an extension of the feeding duration to investigate the trajectory and durability of our findings on lipid and glucose metabolism. More broadly, our study confirms that a comprehensive understanding of the biology of LDs in hepatocytes and other liver cells is necessary to comprehend the molecular mechanisms and heterogeneity of NAFLD and other steatotic diseases.
Financial support
This work was supported by the USA AASLD Afdhal/McHutchison LIFER Award (ES); USA NIH T32-DK007742 (JD); USA NIH 1R01AA026302 (RC). CVS is supported by the NRW Rueckkehr Programme of the Ministry of Culture and Science of the German State of North Rhine-Westphalia, Germany (Europe). MG is supported by the Burroughs Wellcome Fund (USA).
Authors’ contributions
Conceptualisation: ES, RC. Methodology: ES, RC. Formal analysis: ES. Investigation: ES. Data collection: ES. Data curation: YS, SJ, DGB, CL, EEF, JLD, DC, CAM. Software: CVS, BEH, MV. Validation: ES, CVS, BEH, MV. Formal analysis: CVS, BEH, MV. Visualisation: YS, SJ, DGB, CL, EEF, JLD, DC, CAM, KTC, JTB, NJH, DEK, KMC, PST, JAL, JH, SB, MG, MCP. Writing – original draft: ES. Writing – review and editing: all authors. Project administration: ES. Resources: DJR, RC. Supervision: DJR, RC. Funding acquisition: ES, RC.
Data availability statement
Research data that documents, supports and validates the research findings will be made available after the main findings from the final research data set have been accepted for publication.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors would like to thank Dr Kenton Woodard for the generation of the liver-specific vector (Penn Vector Core and Gene Therapy Program, University of Pennsylvania); Dr Jonathan Schug and Dr John Tobias for expert technical support. Special thanks also to the Center for Molecular Studies in Digestive and Liver Diseases (NIH P30 DK050306) and its core facilities (Molecular Pathology and Imaging Core, Molecular Biology).
This research has been conducted using the UK Biobank Resource under Application Number 70653. UK biobank data was accessed by C.V.S and D.J.R.. Copyright © 2023, NHS England. Re-used with the permission of the NHS England and/or UK Biobank. All rights reserved. This work uses data provided by patients and collected by the NHS as part of their care and support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100902.
Supplementary data
The following are the supplementary data to this article:
:
References
- 1.Scorletti E., Carr R.M. A new perspective on NAFLD: focusing on lipid droplets. J Hepatol. 2022;76:934–945. doi: 10.1016/j.jhep.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Paik J.M., Henry L., De Avila L., Younossi E., Racila A., Younossi Z.M. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun. 2019;3:1459–1471. doi: 10.1002/hep4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl A.M., Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 4.Sherif Z.A., Saeed A., Ghavimi S., Nourie S.-M., Laiyemo A.O., Brim H., et al. Global epidemiology of nonalcoholic fatty liver disease and perspectives on US minority populations. Dig Dis Sci. 2016;61:1214–1225. doi: 10.1007/s10620-016-4143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiam A.R., Farese R.V., Jr., Walther T.C. The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol. 2013;14:775–786. doi: 10.1038/nrm3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr R.M., Ahima R.S. Pathophysiology of lipid droplet proteins in liver diseases. Exp Cell Res. 2016;340:187–192. doi: 10.1016/j.yexcr.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najt C.P., Senthivinayagam S., Aljazi M.B., Fader K.A., Olenic S.D., Brock J.R.L., et al. Liver-specific loss of Perilipin 2 alleviates diet-induced hepatic steatosis, inflammation, and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2016;310:G726–G738. doi: 10.1152/ajpgi.00436.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr R.M., Dhir R., Mahadev K., Comerford M., Chalasani N.P., Ahima R.S. Perilipin staining distinguishes between steatosis and nonalcoholic steatohepatitis in adults and children. Clin Gastroenterol Hepatol. 2017;15:145–147. doi: 10.1016/j.cgh.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr R.M., Peralta G., Yin X., Ahima R.S. Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby A.E., Bales E., Orlicky D.J., McManaman J.L. Perilipin-2 deletion impairs hepatic lipid accumulation by interfering with sterol regulatory element-binding protein (SREBP) activation and altering the hepatic lipidome. J Biol Chem. 2016;291:24231–24246. doi: 10.1074/jbc.M116.759795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McManaman J.L., Bales E.S., Orlicky D.J., Jackman M., MacLean P.S., Cain S., et al. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res. 2013;54:1346–1359. doi: 10.1194/jlr.M035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams B., Correnti J., Oranu A., Lin A., Scott V., Annoh M., et al. A novel role for ceramide synthase 6 in mouse and human alcoholic steatosis. FASEB J. 2018;32:130–142. doi: 10.1096/fj.201601142R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai Y., Boyle S., Varela G.M., Caron E., Yin X., Dhir R., et al. Effects of perilipin 2 antisense oligonucleotide treatment on hepatic lipid metabolism and gene expression. Physiol Genomics. 2012;44:1125–1131. doi: 10.1152/physiolgenomics.00045.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann J.P., Romeo S., Valenti L. Lipid droplets as the genetic nexus of fatty liver. Liver Int. 2022;42:2594–2596. doi: 10.1111/liv.15460. [DOI] [PubMed] [Google Scholar]
- 15.Bianco C., Jamialahmadi O., Pelusi S., Baselli G., Dongiovanni P., Zanoni I., et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol. 2021;74:775–782. doi: 10.1016/j.jhep.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magne J., Aminoff A., Perman Sundelin J., Mannila M.N., Gustafsson P., Hultenby K., et al. The minor allele of the missense polymorphism Ser251Pro in perilipin 2 (PLIN2) disrupts an alpha-helix, affects lipolysis, and is associated with reduced plasma triglyceride concentration in humans. FASEB J. 2013;27:3090–3099. doi: 10.1096/fj.13-228759. [DOI] [PubMed] [Google Scholar]
- 17.Faulkner C.S., White C.M., Shah V.H., Jophlin L.L. A single nucleotide polymorphism of PLIN2 is associated with nonalcoholic steatohepatitis and causes phenotypic changes in hepatocyte lipid droplets: a pilot study. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865 doi: 10.1016/j.bbalip.2020.158637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz M., Bukong T.N., Csak T., Saha B., Park J.-K., Ambade A., et al. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J Transl Med. 2015;13:193. doi: 10.1186/s12967-015-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaziano J.M., Concato J., Brophy M., Fiore L., Pyarajan S., Breeling J., et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214–223. doi: 10.1016/j.jclinepi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Klarin D., Damrauer S.M., Cho K., Sun Y.V., Teslovich T.M., Honerlaw J., et al. Genetics of blood lipids among ∼300,000 multi-ethnic participants of the Million Veteran Program. Nat Genet. 2018;50:1514–1523. doi: 10.1038/s41588-018-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correnti J., Lin C., Brettschneider J., Kuriakose A., Jeon S., Scorletti E., et al. Liver-specific ceramide reduction alleviates steatosis and insulin resistance in alcohol-fed mice. J Lipid Res. 2020;61:983–994. doi: 10.1194/jlr.RA119000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tippetts T.S., Holland W.L., Summers S.A. The ceramide ratio: a predictor of cardiometabolic risk. J Lipid Res. 2018;59:1549–1550. doi: 10.1194/jlr.C088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simcox J., Geoghegan G., Maschek J.A., Bensard C.L., Pasquali M., Miao R., et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab. 2017;26:509–522.e6. doi: 10.1016/j.cmet.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanno T., Nakajima T., Kawashima Y., Yokoyama S., Asou H.K., Sasamoto S., et al. Acsbg1-dependent mitochondrial fitness is a metabolic checkpoint for tissue T(reg) cell homeostasis. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.109921. [DOI] [PubMed] [Google Scholar]
- 25.Bailey A.P., Koster G., Guillermier C., Hirst E.M.A., MacRae J.I., Lechene C.P., et al. Antioxidant role for lipid droplets in a stem cell niche of Drosophila. Cell. 2015;163:340–353. doi: 10.1016/j.cell.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers S., Gui L., Kovalenko A., Zoni V., Carpentier M., Ramji K., et al. Triglyceride lipolysis triggers liquid crystalline phases in lipid droplets and alters the LD proteome. J Cell Biol. 2022;221 doi: 10.1083/jcb.202205053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gluchowski N.L., Gabriel K.R., Chitraju C., Bronson R.T., Mejhert N., Boland S., et al. Hepatocyte deletion of triglyceride-synthesis enzyme acyl CoA: diacylglycerol acyltransferase 2 reduces steatosis without increasing inflammation or fibrosis in mice. Hepatology. 2019;70:1972–1985. doi: 10.1002/hep.30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strable M.S., Ntambi J.M. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45:199–214. doi: 10.3109/10409231003667500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S., Zou F., Diao Z., Zhang S., Deng Y., Zhu X., et al. Perilipin 2 and lipid droplets provide reciprocal stabilization. Biophys Rep. 2019;5:145–160. [Google Scholar]
- 30.Sentinelli F., Capoccia D., Incani M., Bertoccini L., Severino A., Pani M.G., et al. The perilipin 2 (PLIN2) gene Ser251Pro missense mutation is associated with reduced insulin secretion and increased insulin sensitivity in Italian obese subjects. Diabetes Metab Res Rev. 2016;32:550–556. doi: 10.1002/dmrr.2751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
:
Data Availability Statement
Research data that documents, supports and validates the research findings will be made available after the main findings from the final research data set have been accepted for publication.