Abstract
Liquid crystal monomers (LCMs) are a family of synthetic organic chemicals applied in the liquid crystal displays (LCDs) of various electric and electronic products (e-products). Due to their unique properties (i.e., persistence, bioaccumulative potential, and toxicity) and widespread environmental distributions, LCMs have attracted increasing attention across the world. Recent studies have focused on the source, distribution, fate, and toxicity of LCMs; however, a comprehensive review is scarce. Herein, we highlighted the persistence and bioaccumulation potential of LCMs by reviewing their physical–chemical properties. The naming rules were suggested to standardize the abbreviations regarding LCMs. The sources and occurrences of LCMs in different environmental compartments, including dust, sediment, soil, leachate, air and particulate, human serum, and biota samples, were reviewed. It is concluded that the LCMs in the environment mainly originate from the usage and disassembly of e-products with LCDs. Moreover, the review of the potential recycling and removal technologies regarding LCMs from waste LCD panels suggests that a combination of natural attenuation and physic-chemical remediation should be developed for LCMs remediations in the future. By reviewing the health risks and toxicity of LCMs, it is found that a large gap exists in their toxicity and risk to organisms. The fate and toxicity investigation of LCMs, and further investigations on the effects on the human exposure risks of LCMs to residents, especially to occupational workers, should be considered in the future.
Keywords: Liquid crystal monomers, Occurrences, Removal, Human exposure, Toxicity
Graphical abstract
Highlights
-
•
The current trends of the levels and profiles for LCMs in the environment were examined.
-
•
Potential recycling and removal technologies regarding LCMs from waste LCD panels were evaluated.
-
•
The health risks and toxicity of LCMs were clarified.
-
•
The limitations in the existing information and possible recommendations for future research directions and perspectives are highlighted.
1. Introduction
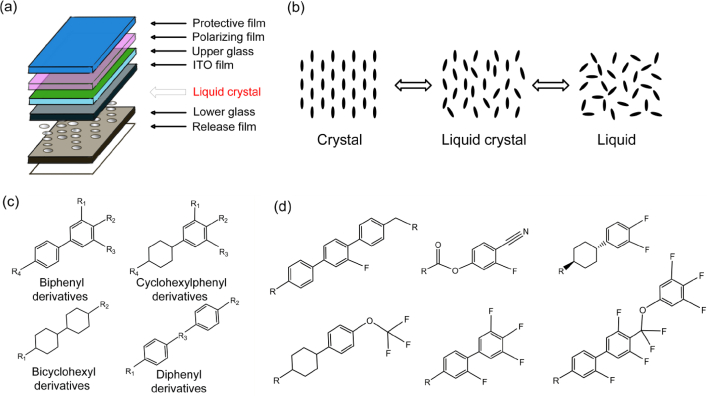
Liquid crystal monomers (LCMs) are a category of synthesized organic chemicals, which are broadly applied in various electric and electronic products with liquid crystal displays (LCDs), such as TVs, computers, and smartphones, due to the transformation between liquid and solid phases at specific temperatures (Fig. 1a and b) [1]. It has been reported that 0.5 mg/cm2 of liquid crystal material was used in a typical LCD screen [2]. It was estimated that approximately 500 tons of LCMs are produced for the production of LCD panels annually [3]. By 2021, the demand for LCMs used in LCD panels was estimated to increase to about 1,300 tons [4]. Structurally, most LCMs consist of a diphenyl backbone with functional groups, such as cyano, fluorine, chlorine, or bromine (Fig. 1c) [2]. In addition, the biphenyls can be sub-divided into biphenyls (BAs), cyanobiphenyls (CBAs), and fluorinated biphenyls (FBAs) and corresponding analogues (Fig. 1d) [5].
Fig. 1.
Schematic of a liquid crystal display (a) and the Phase transitions of liquid crystal (b), chemical structures of LCMs (c) (R indicates fluoro, alkoxyl, cyclohexyl, alkyl, hydroxyl, carboxyl, alkynyl, and cyano groups) and the structures of typical fluorinated LCMs (d).
Recently, LCMs are attracting increasing attention due to their persistent, bio-accumulative, and toxic properties [6]. With the rapid update and iteration of electronic products, the number of LCD panels being scrapped continues to increase. The total amount of LCMs contained in the LCD panels of discarded TVs and computers can reach 214 tons each year, including 126 tons of BAs and 88 tons of FBAs [5]. However, LCMs are quite readily released from the base materials into the surrounding environment during the use and dismantling of LCDs. A recent study has shown that about 1.07–107 kg/year of LCMs were released from waste TV/computer LCD panels into various environmental compartments at a global scale [5]. As expected, they were widely detected in various environmental and human matrices, including dust [[5], [6], [7], [8], [9]], soil [10], sediments [[11], [12], [13]], leachate [14], gas, particular [15], and even in aquatic organisms [16] and humans [17]. Although Simon-Hettich et al. considered ten liquid crystal compounds and their mixture were safe to aquatic model organisms such as daphnia and algae at the highest achievable concentration, a more comprehensive toxicity assessment of LCMs is urgently needed since the toxicity of compounds varies greatly between compounds as well as species [18]. Su et al. reported the toxicological data showing that LCM mixtures extracted from the smartphone LCD panels affected gene expression and caused mutations in chicken embryonic hepatocytes [6]. Because of the growing concerns about environmental fate and behavior, there have been a number of recent studies on environmental occurrence, fate, toxicity, and even human exposure to LCMs [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17],[19], [20], [21], [22]]. However, a literature survey is needed to investigate the occurrence, recycling and removal, health risks, and toxicity of LCMs in order to identify challenges and propose directions for future research.
Accordingly, the aim of the current work is to provide a critical overview of LCMs based on articles from 2019 to 2023 found in “Web of Science” to (1) review the physicochemical properties of LCMs and examine the current trends of the levels and profiles of LCMs in the environmental matrix; (2) evaluate the potential recycling and removal technologies regarding LCMs from waste LCD panels; (3) clarify the health risks and toxicity of LCMs; and (4) highlight the limitations in the existing information and make possible recommendations for future research directions and perspectives.
2. Naming rules for LCMs
LCMs consist of different structures and most of them have the backbone structure of cyclohexyl phenyl, bicyclohexyl phenyl, biphenyl, terphenyl, cyclohexyl biphenyl, or bicyclohexyl derivatives (Fig. 1c). Diverse central bridge groups or linkages and functional groups have been introduced into the backbone structures of LCMs to satisfy the complex and diverse requirements of LCDs [23]. Different naming rules of LCMs were used by different teams which are not convenient for comparison between studies. For example, 2′,3,4,5-tetrafluoro-4″-propyl-1,1':4′,1″-terphenyl (CASRN: 205806-87-7) was called OLEM-6 by Wang et al. [14], TeFPrT by Cheng et al. [7], and TePT by Zhu et al. [8]. There are various LCMs categories at present with complex structures, so it is highly recommended to establish a systematic and standardized naming rule for LCMs. Su et al. provided a specific guideline in previous studies as follows: the abbreviation starts with biphenyl (B) entity first and is followed by other functional groups; the center bridge of “difluoro methyl” is represented as “CF2”, and the number of carbon atoms of terminal alkyl chains is represented by the Arabic numerals [16]. Detailed information and abbreviations of functional groups are summarized in Table 1. Based on this criterion, 2′,3,4,5-tetrafluoro-4″-propyl-1,1':4′,1″-terphenyl can be called 3teFT. Considering a coverage of 1,173 abbreviations of LCMs covered, the abbreviations shown in Su et al.’s database are suitable to be used as the standard for future research [16].
Table 1.
Abbreviations of the functional groups in LCMs [16].
| Letter | Group | Letter | Group | Letter | Group |
|---|---|---|---|---|---|
| A | Allyl | Ac | Acetyl | Acr | Acrylate |
| AO | Allyloxy | b | Bis-/Bi- | B | Biphenyl |
| Ba | Benzoic acid | Be | Butenyl | BeO | Butenyl-oxy |
| Br | Bromo | Buo | Butoxy | Bzo | Benzoate |
| C | Cyan | CaA | Carboxylic acid | CF2 | Difluoromethyl |
| cH | Cyclohexane | cHe | Cyclohexenyl | cP | Cyclopentyl |
| cPr | Cyclopropyl | d | Di- | Da | Dianhydrohrxitol |
| Do | Dioxane | Dx | Dioxabicyclo[2.2.2]octane | E | Ether |
| Et | Ethyl | EtO | Ethoxy | F | Fluoro |
| hFa | Tetrahydrofuran | Hi | 2, 3-dihydro-1H-indene | HeO | Hexyloxy |
| i | Iso- | L | Chloro | Me | Methyl |
| MeO | Methoxy | OH | Hydroxyl | OO | Octyloxy |
| P | Perfluoro | P | Phenyl | Pa | Phenylacetylene/Phenylethynyl |
| Pe | Propenyl | Pen | Pentene | Ph | Phenol |
| Pi | Phenyliminomethyl | PO | Phenoxy | PrO | Propyloxy |
| Py | Tetrahydro-2H-pyran-yl | Pym | Pyrimidine | Pyr | Pyridine |
| Q | Quaterphenyl | r | Rectus- | s | Siniter- |
| t | Tris-/Tri- | T | Terphenyl | Te | Tetra- |
| Tx | Trioxabicyclo[2.2.2]octane | V | Vinyl | VO | Vinyloxy |
3. Physical–chemical properties of LCMs
The physical–chemical properties are correlated with the environmental fate, occurrence, distribution, and transformation of organic compounds. To date, only a few studies reported the physical–chemical properties of LCMs. Su et al. estimated the physicochemical properties of 362 currently produced LCMs, including octanol–water partition coefficients (log Kow), air–water partition coefficients (log Kaw), bioconcentration factors (BCF), octanol-air partition coefficients (log Koa), atmospheric oxidation half-lives (AO1/2), degradation half-lives (t1/2) in water, soil, and sediment (fugacity) by EPI (Estimation Programs Interface) Suite software from U.S. Environmental Protection Agency (U.S. EPA) [6].
After that, the database was further expanded to 1,173 LCMs, and their Arctic contamination and bioaccumulation potential (ACBAP) was also predicted in 2022 (Fig. 2) [16]. Based on the screening criteria [24], all 1,173 LCMs were classified as persistent and bioaccumulative (P&B) chemicals. 1,096 out of 1,173 LCMs were predicted with log Kow values of >5, indicating great possibilities to adsorb to sediments and to bioaccumulate in organisms. It was estimated that 674 and 263 LCMs have a BCF of >1,000 and >5,000, respectively, suggesting bioaccumulation potential of LCMs. Besides, 766 LCMs were classified as potentially persistent chemicals with t1/2,w values of ≥60 d in an aqueous environment. By the combined criteria of log Kow > 5, log Kaw > −5 and < −1, and t1/2, w ≥ 60 d, 476 LCMs were classified as P&B chemicals. It is worth noting that 473 out of 476 P&B LCMs were structurally fluorinated, consistent with some halogenated chemicals [25]. In addition, among these chemicals, 66 LCMs were predicted to be potentially very persistent and very bioaccumulative (vPvB) chemicals by the combined criteria of log Kow > 5, log Kaw > −5 and < −1, BCF > 5000 and t1/2, w ≥ 180 d. Moreover, 320 LCMs were classified as potential ACBAP by the combined criteria of log Koa + log Kaw ≥ 3.5, log Koa ≥ 6, 0.5 ≥ log Kaw ≥ −7, and log Kaw ≤ −1.78 × log Koa + 14.56. Liu et al. explored the mechanisms of heterogeneous hydroxyl radical (OH) oxidation of two typical LCMs, 1-ethyl-4-(4-(4-propylcyclohexyl)phenyl)benzene (EPPB, CAS No. 107949-21-3) and 4″-ethyl-2′-fluoro-4-propyl-1,1′:4′,1″-terphenyl (EFPT, CAS No. 95759-44-7) onto ammonium sulfate particles, suggesting that the atmospheric lifetime of two LCMs is up to 25−38 days, much longer than the previously predicted lifetime (<1 day) [21]. The results suggested that the LCMs have the potential of more permanent persistence in the air than predicted as well as long-range transport. Besides, the atmospheric fate and risk assessment of canonical LCMs analysis based on computational analysis further supported that these target LCMs had the potential of atmospheric persistence and long-range transport [26]. Due to the similar chemical structure to PBDEs and PCBs, it is reasonable to take the potential of their persistent, long-range environmental transport, bioaccumulative and toxic effects as a reference potential for LCMs [[27], [28], [29], [30]]. It has been reported that PBDEs and PCBs such as 2,2′,4,4′-tetra-bromodiphenyl ether (BDE-47) and 2,3,3′,4,4′,5′-hexa-chlorobiphenyl (CB-157) are persistent in the environment and have a strong potential to bioaccumulate in organisms [27].
Fig. 2.
Persistence and bioaccumulation characteristics of 1,173 LCMs (Log Kow [octanol–water partition coefficient] indicate tendency to adsorb to sediments and to bioaccumulate; BCF is an estimate of bioaccumulation potential; Kaw describes air–water partitioning; Compounds with log Kaw > −5 and < −1 have long-range transport potential; t1/2,w indicates the biodegradation half-life in water; Chemicals with log Kow > 5, log Kaw > −5 and < −1, and t1/2, w ≥ 60 d are classified as potential P&B; Chemicals with log Kow > 5, log Kaw > −5 and < −1, BCF > 5000 and t1/2, w ≥ 180 d are classified as potential vPvB; Chemicals with log Koa + log Kaw ≥ 3.5, log Koa ≥ 6, 0.5 ≥ log Kaw ≥ −7, and log Kaw ≤ −1.78 × log Koa + 14.56 are classified as potential ACBAP) [16].
Recently, there have also been experimental tests of the physical and chemical properties of LCMs. Based on the gas chromatography-retention time approach, Feng et al. measured log Koa values for 21 target LCMs, which vary from 7.60 to 13.13 [31]. Zhu et al. reported log Kow values of 39 LCMs by the shake-flask method, and the measured values generally ranged from 4.94 to 7.62 [32]. Compared with the experimentally measured value, the log Kow values of some LCMs might be underestimated by the EPI Suite software. For example, for LCMs with benzene or cyclohexane rings (e.g., 4-[4-[4-(4-ethylcyclohexyl)cyclohexyl]phenyl]-1,2-difluorobenzene [2bcHdFB, CAS No.139195-63-4]), the log Kow values increased to three or more times than predicted, indicating that clear difference exists between experimental and predicted Kow values [32].
4. Environmental pollution of LCMs in different phases
The earliest report on LCMs’ environmental pollution was the investigation of dust from indoor environment (including student dormitory, canteen, campus building, et al.) from Nanjing, China, with concentration ranging from 0.13 to 2,213 ng/g (17 out of 33 LCMs) in 2019 [6]. Since then, LCMs have been reported in environmental matrix associated with electronic waste recycling plants and landfills (Table 2) [[6], [7], [8], [9], [10], [11]]. For example, Dubocq et al. applied integrated screening analysis to dust samples collected in 2019 or 2016 around Örebro County in Sweden and reported that three LCMs were detectable in analyzed samples [33]. Another study conducted also in Sweden demonstrated that 3 of 13 LCMs were detected in indoor dust samples with the concentration of ΣLCMs ranging from 63 to 430 ng/g [34]. Zhu et al. investigated 46 LCMs (including 39 FBAs and 7 BAs) in e-waste dust samples by a dedicated target analysis [8]. Briefly, 34 of 39 FBAs (ranging from 225 to 976,000 ng/g with median concentrations of 18,500 ng/g) and 6 of 7 BAs (ranging from 29.8 to 269,000 ng/g with median concentrations of 3,470 ng/g) were detected in the LCD disassembly area in Yichun, China [8]. In addition, 55 target LCMs were selected for their quantification in dust from an e-waste recycling area in Central China [7]. LCMs were heavily detected in the indoor dust from LCD dismantling workshop, outdoor dust, and industry park outdoor dust, with median concentrations of 67,400, 11,000, and 6,950 ng/g, respectively. Sediment concentrations of LCMs were also observed from rivers around LCM/LCD manufacturers, exhibiting a high mean concentration value of 26.1 ng/g dry weight (dw), followed by 1.15 and 0.076 ng/g dw from e-waste recycling site areas and Taihu Lake [9]. Jin et al. first provided the data of LCMs in landfill leachate from Hong Kong (ΣLCMs = 1,120 ng/L) and Shenzhen (ΣLCMs = 409 ng/L), China, which might also be emitted from the e-wastes [10]. Most LCMs are considered semi-volatile organic compounds (SVOCs), and thus air is regarded as the significant transport media for LCMs migrating from e-waste recycling sites to their surrounding environment [[35], [36], [37]]. Recently, the release of LCMs during the dismantling of waste LCD panels and their gas-particle partitioning behaviors were investigated [11]. The median level of total atmospheric concentrations (in the gas and particle phases) was 204,000 pg/m3, with concentrations ranging from 68,800 to 385,000 pg/m3. These results further suggest the significance of uncovering the atmospheric behavior of LCMs.
Table 2.
Statistical summary of LCM concentrations in the environment.
| Sample type | Sample description | Concentration |
Number of compounds | Sample year | Region or country | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Range | Median | Mean | ||||||
| Dust | Indoor dust (ng/g) | – | – | – | 12 | 2016, 2019 | Örebro, Sweden | [33] |
| Indoor dust (ng/g) | 63–430 | – | – | 13 | Sweden | [34] | ||
| Indoor dust (ng/g dw) | 0.13−2,213 | – | – | 33 | 2018 | Nanjing, China | [6] | |
| Indoor dust (ng/g) | 17.3–529 | 41.6 | 87.2 | 60 | 2017 | China | [15] | |
| Outdoor dust (ng/g) | ND–441 | 94.7 | 113 | |||||
| Indoor dust from cybercafés (ng/g) | 4.40–2,540 | 106 | 326 | 2021 | Tianjin, China | |||
| Indoor dust from phone repair stores (ng/g) | 2.37–991 | 171 | 240 | |||||
| LCD e-waste indoor dust (ng/g) | 225−976,000 | 18,500 | 82,000 | 39 (FBA) | 2020 | Yichun, China | [8] | |
| Non-LCD e-waste indoor dust (ng/g) | 292−18,500 | 2,300 | 3,290 | |||||
| LCD e-waste indoor dust (ng/g) | 29.8−269,000 | 3,470 | 18,700 | 7 (BAs) | ||||
| Non-LCD e-waste indoor dust (ng/g) | 45.3−3,210 | 411 | 643 | |||||
| Sediment | Sediments from rivers around LCM/LCD manufactures (ng/g dw) | 0.032–554 | – | 26.1 | 39 | 2021 | Jiangsu, China | [9] |
| Sediments from Taihu Lake (ng/g dw) | 0.033–0.193 | – | 0.076 | Taihu Lake, China | ||||
| Sediments from rivers around e-waste recycling site areas (ng/g dw) | – | – | 1.15 | Taizhou, China | ||||
| Sediments from rivers around LCM/LCD manufactures (ng/g dw) | 31.0−1,603 | – | – | 1,173 (Suspect screening) | Jiangsu, China | [16] | ||
| Sediments from Taihu Lake (ng/g dw) | 30.9 | – | – | Taihu lake, China | ||||
| Sediments from rivers around e-waste recycling site areas (ng/g dw) | 291 | – | – | Taizhou, China | ||||
| Sediments from Pearl River Estuary (ng/g dw) | 0.90–31.1 | – | – | 39 | 2018 | Pearl River Estuary, China | [17] | |
| Leachate | Leachate (ng/L) | 1,120 | – | – | 39 | 2021 | Hong Kong, China | [10] |
| 409 | – | – | Shenzhen, China | |||||
| Dust | Indoor dust from LCD e-waste workshop (ng/g) | 21,600−354,000 | 67,400 | 97,800 | 55 | 2020 | Central China | [7] |
| Outdoor dust from non-LCD e-waste workshop (ng/g) | 4,720−38,300 | 11,000 | 12,600 | |||||
| Industry park outdoor dust (ng/g) | 3,450−15,300 | 6,950 | 7,310 | |||||
| Reference indoor dust from resident areas (ng/g) | 54−1,050 | 303 | 399 | |||||
| Skin wipes | Hand wipes from dismantling workers (ng/m2) | 9,980−1,197,000 | 46,100 | 129,000 | ||||
| Forehead wipes from dismantling workers (ng/m2) | 13,200−2,071,000 | 62,100 | 244,000 | |||||
| Reference wipes from residents (ng/m2) | 1,350−22,000 | 4,160 | 6,030 | |||||
| Serum | Serum samples from dismantling workers (ng/mL) | 3.9–26.3 | 8.0 | – | 2015 | South China | ||
| 7.78–276 | 35.2 | 51.9 | 60 | [12] | ||||
| Serum samples from reference population (ng/mL) | 3.16–28.5 | 9.59 | 11.3 | |||||
| Air and particulate | E-waste recycling industrial park indoor air (gaseous) (pg/m3) | 66,200−243,000 | 158,000 | – | 93 | 2020 | South China | [11] |
| E-waste recycling industrial park indoor air (particulate) (pg/m3) | 2,690−142,000 | 46,900 | – | |||||
| Soil | Agricultural zone (ng/g) | ND−250 | 0.64 | 12.9 | 39 | 2019 | South China | [13] |
| Commercial zone (ng/g) | ND−24.0 | 0.49 | 5.23 | |||||
| Residential zone (ng/g) | ND−7.65 | 0.63 | 3.30 | |||||
| Industrial zone (ng/g) | ND−8.73 | 0.91 | 2.48 | |||||
| Scenic zone (ng/g) | ND−1.71 | 0.25 | 0.77 | |||||
| Biota | Invertebrates (ng/g ww) | ND−7.36 | 0.13 | – | 15 | – | Shen Zhen, China | [14] |
| Fish (ng/g ww) | ND−29.7 | 9.5 | – | |||||
ND, not detected; dw, dry weight; ww, wet weight.
Recently, the presence of LCMs in real environmental media, such as sediment, indoor and outdoor dust, and soil, has also been observed. For example, the contamination of 39 LCMs was investigated by Tao et al. in 45 surface sediment samples from the Peral River Estuary (PRE) [17]. The target LCMs were widespread in the sediment from the PRE, with levels ranging from 0.90 to 31.1 ng/g dw. Zhang et al. first monitored the polluted levels of 60 LCMs in dust (both indoor and outdoor) collected across China, with median levels of 41.6, 94.7, 106, and 171 ng/g, respectively [15]. Urban soils are considered sinks of organic pollutants originating from human activities [38,39]. In addition to dust and sediment samples, Li et al. analyzed the LCM concentration in surface soil samples from agriculture, scenic, industrial, commercial, and residential zones by target and suspect analysis [13]. Target LCMs were detected from 0.774 to 12.9 ng/g dw in surface soils from these five different urban functional zones, and 51 of 1,173 suspect LCMs (with 69 chemical structures) were preliminarily identified in soils.
Additionally, the bioaccumulation of LCMs was also observed in the biota samples. For instance, 16 LCMs employed in organic light-emitting diode displays were first detected in wild aquatic invertebrates and fishes, ranging from ND to 7.36 ng/g ww (median: 0.13 ng/g ww) and from ND to 29.7 ng/g ww (median: 9.5 ng/g ww), respectively [14]. Compared to the flame retardants, such as novel brominated flame retardants (NBFRs), concentrations of LCMs (ΣLCMs) in dust, sediment, and soil samples were less than those of ΣNBFRs [6,40,41]. The relatively lower levels of LCMs may be the reason that the applications of most LCMs are confined to electronic equipment. However, the sources of new brominated flame retardants are more extensive. These studies contribute to the direct evidence of LCMs in environmental samples and therefore demonstrate that LCMs have widespread pollution in the environment.
5. Recycling and removal of LCMs from waste LCD panels
Although LCMs play a crucial role in LCD devices, the relative content of LCMs components in LCD panels was low [42,43]. The presence of organic materials (including LCMs, polarizing film, etc.) might interfere with the process of recycling indium from the waste LCD panels due to the difficulty of separating organic materials from ITO film [44]. Several methods, e.g., solvent extraction [45] and supercritical fluid extraction [46], were employed and investigated to recover and recycle LCMs in LCD panels.
For recycling the valuable LCMs from waste LCD panels, Zhu et al. developed a method to dissolve LCD screens based on the organic solvent (acetone) with ultrasonic wave. The recovery rate of LCMs was more than 50%, but the collection could not satisfy the requirement of purity for LCDs [46]. Based on the supercritical fluid technology, LCMs could be extracted with high purity and stability and used as raw materials for LCDs [47]. However, the cost of supercritical equipment is relatively high and could not be operated on a large scale [48]. Improving the recovery efficiency and purity of LCMs from waste LCD panels can not only save the production cost of LCMs, but also reduce the entry of LCMs into the environment during the process of production or disposal and reduce environmental health risks.
Incineration [49] and pyrolysis [50] have been tried to directly remove the LCMs in LCD panels without extraction. Incineration is a widely used method for disposing of LCMs in LCD panels due to its simple operation, high efficiency, and economic benefits. Studies found that PCBs could be yielded during the thermal decomposition of chlorinated paraffins (CPs), and PCBs start to decompose at temperatures higher than 800 °C [51]. Similar to CPs, the hazardous by-products (such as PAHs, CH4, C2H6, and CO) produced during the combustion process of LCMs could be a new source of environmental pollutants [49,52]. Guo and co-authors developed a device for the harmless heat treatment of waste LCD panels, which could ensure the full pyrolysis of the LCMs into organic gases in the pyrolysis furnace and then realize the harmless treatment of organic gases by increasing temperature of incinerator to 1,000 °C [53,54]. Zhang et al. found that LCMs could be well separated in pyrolysis process under 873 K, 40 min, and 2 L/min N2 flow rate, while the oil and even the gas pyrolysis products could be reused as energy and fuel [3]. In comparison to the recycling methods, removing LCMs by pyrolysis technology has low environmental costs, although it cannot provide recycled LCMs [43,55,56].
6. Possible removal and degradation methods of LCMs from the environment
Most of the LCMs identified with P&B characteristics were fluorine-, alkynyl- or biphenyl derivatives, with p-π or π conjugated structure between fluorine atoms or alkynyl groups and the phenyl ring increasing the chemical structure stability [6]. After entering the environment, these LCMs will exhibit less susceptibility to microbial degradation and chemical reactions. To date, there are rarely developed methods or strategies for reducing LCMs in environmental matrices, which is critically important and extremely urgent. Jin and co-authors developed a synchronized oxidation-adsorption Fenton technology to remove the typical LCM 4-[difluoro(3,4,5-trifluorophenoxy)methyl]-3,5-difluoro-4′-propylbiphenyl in landfill leachate [57]. This paper provides a possibility to remove LCMs from environmental matrices. However, the highly fluorine-substituted transformation products and a single target analyte make the broad applicability and environmental friendliness of the technology on LCMs worrisome [57]. Due to the similar chemical structure to PCBs and PBDEs, other removal technologies for the traditional halogenated organic pollutants, such as PCBs and PBDEs, can be considered for the removal of LCMs from the environment.
Different technologies, including adsorption [58], thermal degradation [59], electrochemical degradation [60], photodegradation [61], biodegradation [62], and phytoremediation [63], have been investigated and applied to remediate the contamination of traditional organic contaminants (Fig. 3). As an effective physic-chemical technology, adsorption technology has been used for volatile organic compounds (VOCs) abatement and removal of organic pollutants from impaired water for many years [58]. Various adsorption materials (e.g., activated carbons, zeolites, hyper crosslinked polymeric resins, and metal-organic frameworks) have been synthesized and studied to improve the adsorption capacity, stability, and safety to decrease the investment and operating cost, and to optimize regeneration cycle performance [64,65]. Since 1968, various liquid crystal materials have been designed to meet the specific requirements of LCDs [66], which has challenged scientists to develop suitable absorbents for LCMs.
Fig. 3.
Treatment technologies for removing traditional halogenated organic pollutants from environmental matrices.
Photochemical degradation of persistent halogenated organics has been well investigated [61]. The photo-transformation process of PBDEs has attracted extensive research interest, and the photodegradation kinetics and mechanisms of PBDEs in different natural and artificial mediums under various ambient conditions have been well summarized by Pan et al. [67]. Su and co-authors reported the photolytic degradation products of BDE-209 by natural sunlight irradiation, and observed a significantly decreasing toxicity by examining mRNA expression of 27 dioxin-responsive genes following exposure of chicken embryonic hepatocytes (CEH) to the degradation products [68]. Owing to their special optical properties and uses, LCMs need to be optically stable [69], which might lead to photodegradation not suitable for the removal of LCMs from contaminated environmental matrices, or require very harsh reaction conditions such as more efficient photocatalysts, intense ultraviolet light, etc.
As mentioned above, hazardous by-products might be produced during the incineration process, resulting in new environmental pollutants [49]. Although the harmless treatment of organic pollutants can be achieved by increasing the temperature in the incinerator [51], their high investment cost and energy consumption make it unnecessary to use them for LCMs removal because of the relatively lower concentration of LCMs in the environmental matrices [10].
The electrochemical technique has the characteristics of mild reaction, high efficiency, low cost, and high environmental compatibility, which has been used to treat organic halides [70]. Zhou et al. found that the electrochemical removal of chlorobenzene and p-chlorophenol followed an apparent first-order kinetic reaction [[71], [72], [73]]. The mechanisms of the electrochemical degradation in dichlorobiphenyls were investigated by Muthukrishnan et al., and the ease of reduction in dichlorobiphenyls followed the order by ortho-Cl, para-Cl, and meta-Cl [74]. Matsunaga et al. found that the presence of aromatic radical-anion mediators (e.g., biphenyl and naphthalene) could promote the rate of reduction of polychlorobiphenyls [75]. Numerous technologies and developments regarding the electrocatalytic reduction of persistent chemicals in various solvents have been comprehensively highlighted and reviewed by Martin and co-workers, which provides a theoretical basis for the electrochemical degradation of LCMs [76]. Notably, the LCM molecules align along the electric field, resulting in a significant electrooptic effect. This might be helpful for the desorption of LCMs from particles, and enhance the electro-remediation process [66,77].
As an efficient natural attenuation, bacterial degradation was widely investigated for the removal of organic halides from the contaminated environmental matrices. A 108-week long-term dechlorination of PCBs by microbial was conducted in Taihu Lake sediment microcosms, and 84.8% of parent PCBs declined [78]. He et al. observed that hepta-BDE and octa-BDE could be produced by S.multivorans culture exposed to deca-BDE [79]. Anaerobic dichlorination of syn- and anti-Dechlorane Plus was investigated in sewage sludge, and monohydrodechlorinated products were produced after 7 days [80]. Anaerobic and aerobic bacteria degradation of pollutants are the two main pathways occurring in the environment. For example, for highly halogenated PBDEs and PCBs, the anaerobic transformation process involves reductive dehalogenation, and oxidative degradation carried out under aerobic conditions is responsible for the degradation of lower halogenated congeners [[81], [82], [83], [84]].
Phytoremediation is an effective, low-cost, environmentally friendly, and promising technology for the treatment of soil contaminated with organic pollutants [85]. Different plant species have been studied for POPs remediation. Ancona and co-authors selected a specific poplar (Populus spp.) clone to promote organic pollutant degradation, and significant removal of PCBs was observed after 420 days [86]. The combination of Italian ryegrass (Lolium multiflorum) and green garden waste biochar was used to remove the PCBs from contaminated soil, and 85% of the total PCBs load was removed in the vegetated soil amended [87]. Thermal processing and further phytoremediation with zucchini (Cucurbita pepo L. cv ‘Black Beauty’) were employed to decrease the PCBs concentrations in sediment admixtures by Urbaniak and co-authors, and a 51%–81% reduction for PCB concentrations compared to untreated control was observed by thermal treated at 300 °C [88].
Considering the complex composition of LCMs and their potential persistence and stability, it is impossible to recommend a single removal method for all LCMs. With growing environmental concerns, efficient, low-cost, environmental friendly, and large-scale treatment methods should be developed for LCMs remediations in the future. Due to the advantages of low-cost, large-scale employment and technical feasibility, natural attenuation (such as phytoremediation, photodegradation, and biodegradation) is a key approach to the remediation of contaminated environmental matrices. Nevertheless, for persistent organic contaminants, natural attenuation requires many years, and might remain incomplete and produce toxic products. The unclear mechanism and different natural conditions complicate their large-scale and in situ application [77]. It seems to be promising to establish a combination of natural attenuation and physic-chemical remediation.
7. Health risks and toxicity of LCMs
Contaminated environmental matrices have the potential to cause exposure risk of LCMs to occupational workers. Limited studies have been conducted on the external and internal exposure risk to LCMs [7]. Significantly higher concentrations of LCMs were observed on the skin of hand and forehead samples from the e-waste dismantling occupational workers, with median concentrations of 46,100 and 62,100 ng/m2, respectively, than that from residents (median concentrations of 4,160 ng/m2) [7]. Cheng et al. first reported the occurrence of 29 LCMs in the serum of elderly occupational workers (≥60 years old), with range total concentrations of 7.78–276 ng/mL (median: 35.2 ng/mL) [12]. These results indicated a high occupational exposure risk of these compounds to the dismantling workers. We recently used EpiKuits 3D-Human Skin Equivalents (3D-HSE) to comprehensively evaluate the percutaneous penetration potential of typical LCMs, further exploring the occupational dermal exposure risks [89].
A recent study evaluated the hazardous effects of 1,431 commercially available LCMs by quantitative structure–property relationship (QSPR) models, suggesting potential adverse effects on human health or aquatic ecosystems [90]. As mentioned above, LCMs’ presence was identified in various environmental matrices [5,[7], [8], [9],11], which raised emerging public health concerns. Previous research put great emphasis on the toxicological effect of heavy metals and polymers in LCDs, while LCMs’ adverse outcomes were rarely demonstrated [91,92]. Global LCMs’ producers claimed that LCMs are harmless to living organisms concluded from rat acute toxicity and bacterial mutagenic test [93,94]. Su et al. reported LCMs’ bioaccumulation and persistence ability, with an alternation potential in embryonic hepatocytes transcription profiles certified [6]. The significantly dysregulated genes were typically enriched in lipid metabolism, xenobiotic detoxification, and hormone signaling pathways, which implied LCMs’ negative effect on energy homeostasis, redox balance, and cell cycle. The researcher further compared the genotoxicity of LCMs with dioxin-like compounds (DLCs) and brominated flame retardants (BFRs), and proposed LCMs’ toxigenic mechanisms as a competitive binding with certain enzyme targets (e.g., transthyretin, aryl hydrocarbon receptor). In another eukaryotic study, two mammalian cell lines were exposed to various types of LCMs, with a strong correlation between functional group composition and cell viability observed [95]. For example, compounds with fluorophenyl groups posed minimal toxicity, while cholesteric liquid crystal showed lethal consequences in human corneal epithelial cells. Recently, Zhao et al. reported that the peroxisome proliferator-activated receptor gamma (PPARγ) was significantly antagonized by target LCMs for the first time by a combination of in silico simulation and in vitro assay validation along with omics integration analysis [96]. Other nuclear receptors, such as glucocorticoid receptor (GR) and estrogen receptor (ER), were also examined in this study and reported to be negative in the agonist test [96].
Apart from in vitro tests, researchers have been investigating LCMs’ ecotoxicological effects with in vivo approaches. Weber et al. tested the adverse biological outcomes in mice exposed to 3-β-Chlorosteroids, a group of liquid crystalline products heavily used and released from LCD industries [97]. LCMs were also found to be responsible for the induced acute toxicity and upregulated antioxidant defense capacity in aquatic species [98]. An et al. determined levels of antioxidant enzymes in LCMs-exposed catfish [19], and found significant upregulation and dose–response induction effects for most antioxidative components such as catalase (CAT), superoxide dismutase (SOD) and selenium-dependent glutathione peroxidase (Se-GPx), with global oxidative stress observed at dose equals to 20 μg LCM/g fish. Though toxic effects and mechanisms remain largely unclear, many LCMs have been categorized and restricted as toxic chemicals according to EPA (Table 3) [98].
Table 3.
Reported biological effects provoked by LCMs.
| Study Type | Result | Reference | |
|---|---|---|---|
| In vivo (mice) | No difference in body weight and food intake; No indication of cell damage in the stomach, spleen, liver, or kidney; LCMs were greatly absorbed by the intestinal tract. |
[97] | |
| In vivo (catfish) | Significant upregulation of antioxidant enzymes (CAT, SOD, and Se-GPx); Global oxidative stress exhibited when the dosage level exceeded 20 μg LCM/g fish. |
[19] | |
| In vitro (fluorescent staining assays) | LCMs exposure induced cell death in 3T3 fibroblast and HCEC; LCM with fluorophenyl group showed minimal acute toxicity; Cholesteric LCM posed a lethal threat to HCEC only. |
[95] | |
| In vitro (PCR array) | Upregulation FC | CYP1A4: 2.38–14.4 | [6] |
| FGF19: 2.68–4.47 | |||
| LBFABP: 1.77–5.94 | |||
| Downregulation FC | PDK4:1.56–2.68 | ||
| CRYAB: −1.71 to −3.63 | |||
| IGF1: −1.57 to −3.32 | |||
| In vitro (bacterial viability assay) | LCMs mixture’s viability ratio | THRSP: −1.57 to −2.85 | [99] |
| E. coli: 1.01 ± 0.02 | |||
| S. aureus: 0.97 ± 0.02 | |||
| E. coli death ratio | B. atrophaeus: 0.94 ± 0.03 | ||
| CPCI: 0.913 | |||
| CsPFO: 0.854 | |||
FC, fold change; CAT, catalase; SOD, superoxide dismutase; Se-GPx, selenium-dependent glutathione peroxidase; CPCI, cetylpyridinium chloride; CsPFO, cesium pentadecafluorooctanoate; HCEC, human corneal epithelial.
Christopher et al. observed that several lyotropic chromonic LCMs exhibited limited adverse effects on prokaryotic species’ viability, while another two surfactant-based LCMs posed a deadly threat to E. coli. [99] It was suspected that LCMs can impair bacteria by the inhibition of germination and spores’ growth, denaturation of membrane protein, and induction of cell lysis [99].
In this review, we managed to calculate and predict 33 LCMs’ potential toxicological effects with Ecological Structure Activity Relationships (ECOSAR) Predictive Model (Table 4) [100]. The selection of these LCMs is based on the environmental occurrence, human relevance, and potential toxic effects reported previously [9,96,101]. Acute toxic endpoints were investigated for three aquatic species as well as rats. Generally, most LCMs showed quite moderate lethality in comparison with previously reported environmental pollutants (Perfluorooctanesulfonic acid [PFOS]: 154–251 mg/kg rat; 2,3,7,8-Tetrachlorodibenzo-p-dioxin [TCDD]: 115–275 μg/kg rabbit; 2,3,3′,4,4′,5,5′-Heptachlorobiphenyl [PCB 189]: 3 mg/kg guinea pig). Various species exhibited quite toxic tolerance and sensitivity distinctions, which suggested aquatic organisms are more likely to be influenced. It is also worth noting that LCM-9, featured with a trifluoromethoxy group, demonstrated quite a noticeable lethal effect among all organisms. Trifluoromethoxy functional group was utilized in drug design as methyl oxidative protector (Celecoxib), thus LCM with trifluoromethoxy group is of emerging ecotoxicological concerns and study values [102]. Developmental toxicity and mutagenicity were further concluded. Among the 33 studied LCMs, 82% showed positive developmental toxicity, and 18% exhibited concerning mutagenicity induction potential. The results provided an insightful overview of LCMs’ adverse health effects, which are of future validation research interest.
Table 4.
Acute toxicity endpoints of 33 LCMs calculated by ECOSAR.a
| Abbreviation | CASRN | Name | Pimephales promelas | Daphnia magna | Tetrahymena pyriformis | Rattus norvegicus | DTPV | DTPR | MPV | MPR |
|---|---|---|---|---|---|---|---|---|---|---|
| LCM-1 | NA | 1,4-dibutoxy-2,3-difluorobenzene | 1.81 | 3.26 | 1.65 | 499.89 | 0.50 | Negative | 0.31 | Negative |
| LCM-2 | 116020-44-1 | 1-(4-propylcyclohexyl)-4-vinylcyclohexane | 0.18 | 0.72 | 0.33 | 7,577.54 | 0.86 | Positive | N/A | N/A |
| LCM-3 | 97398-80-6 | 1-methoxy-4-(4-propylcyclohexyl)cyclohexane | 2.59 | 1.64 | 0.79 | 2,341.7 | 0.47 | Negative | −0.03 | Negative |
| LCM-4 | 279246-65-0 | 1-(prop-1-enyl)-4-(4-propylcyclohexyl)cyclohexane | 0.22 | 0.62 | 0.21 | 6,480.22 | 0.86 | Positive | N/A | N/A |
| LCM-5 | 174350-05-1 | 1-ethoxy-2,3-difluoro-4-(4-propylcyclohexyl)benzene | 1.1 | 0.43 | 0.51 | 515.38 | 0.41 | Negative | 0.44 | Negative |
| LCM-6 | 64835-63-8 | 4-methyl-4′-pentylbiphenyl | 0.27 | 0.21 | 0.77 | 3,073.79 | 0.85 | Positive | N/A | N/A |
| LCM-7 | 157248-24-3 | 1-ethoxy-2,3-difluoro-4-(4-propylphenyl)benzene | 0.24 | 0.1 | 0.87 | 266.89 | 0.46 | Negative | 0.46 | Negative |
| LCM-8 | NA | 1-butoxy-2,3-difluoro-4-(4-methylphenyl)benzene | 0.26 | 0.12 | 0.71 | 385.01 | 0.43 | Negative | 0.39 | Negative |
| LCM-9 | 650634-92-7 | 4-(4-ethylcyclohexyl)-4'-(trifluoromethoxy)biphenyl | 7.57E-03 | 8.19E-02 | N/A | 5.01 | 0.97 | Positive | 0.31 | Negative |
| LCM-10 | 155041-85-3 | 1-methyl-4-(4-(4-vinylcyclohexyl) cyclohexyl) benzene | 0.17 | 0.18 | 0.17 | 4,437.81 | 1.01 | Positive | N/A | N/A |
| LCM-11 | NA | 1,2,3-trifluoro-4-methoxy-5-(4-(4-propylcyclohexyl) cyclohex yl)benzene | 4.29E-02 | 0.52 | 0.0787 | 455.68 | 0.69 | Positive | 0.73 | Positive |
| LCM-12 | 303186-20-1 | 4-[difluoro(3,4,5-trifluorophenoxy) methyl]-3,5-difluoro-4′-ropylbiphenyl | 2.78E-03 | 0.13 | N/A | 165.2 | N/A | N/A | 0.25 | Negative |
| LCM-13 | 84656-75-7 | 1-methyl-4-(4-(4-propylcyclohexyl) cyclohexyl)benzene | 0.12 | 0.4 | 0.0588 | 2,045.18 | 0.96 | Positive | N/A | N/A |
| LCM-14 | 1690317-23-7 | 4-[difluoro(2-methyl-3,4,5-trifluorophenoxy) methyl]-3,5-difluoro-4′-propylbiphenyl | 1.59E-03 | 0.11 | N/A | 146.41 | N/A | N/A | 0.34 | Negative |
| LCM-15 | 431947-34-1 | 2,3-difluoro-1-methoxy-4-(4-(4-propylcyclohexyl) cyclohexyl)benzene | 6.28E-02 | 0.22 | 0.11 | 442.72 | 0.62 | Positive | 0.29 | Negative |
| LCM-16 | 84540-37-4 | 1-ethyl-4-(4-(4-propylcyclohexyl)phenyl)benzene | 2.09E-02 | 0.14 | 0.0889 | 15.68 | 0.96 | Positive | N/A | N/A |
| LCM-17 | 323178-01-4 | 4-ethoxy-4'-(4-ethylcyclohexyl)-2,3-difluorobiphenyl | 1.04E-02 | 9.20E-02 | 0.2 | N/A | 0.56 | Positive | 0.50 | Negative |
| LCM-18 | 123560-48-5 | 1-ethoxy-2,3-difluoro-4-(4-(4-propylcyclohexyl) cyclohexyl)benzene | 2.13E-02 | 0.33 | 0.0768 | 453.46 | 0.57 | Positive | 0.31 | Negative |
| LCM-19 | 95759-44-7 | 4″-ethyl-2′-fluoro-4-propyl-1,1':4′,1″-terphenyl | 8.73E-03 | 4.11E-02 | 0.34 | 282.25 | 0.50 | Positive | 0.22 | Negative |
| LCM-20 | NA | 1-ethoxy-2,3-difluoro-4-(4-(4- (prop-1-enyl)cyclohexyl)cyclohexyl) benzene | 2.71E-02 | 0.11 | 0.0675 | 470 | 0.63 | Positive | 0.30 | Negative |
| LCM-21 | 189750-98-9 | 4-ethoxy-2,3-difluoro-4'-(4-propylcyclohexyl)biphenyl | 2.18E-02 | 8.07E-02 | 0.15 | N/A | 0.57 | Positive | 0.52 | Positive |
| LCM-22 | 473257-14-6 | 2,3-difluoro-1-propoxy-4-(4-(4- propylcyclohexyl)cyclohexyl)benzene | 2.23E-02 | 0.3 | 0.0557 | 485.12 | 0.58 | Positive | 0.16 | Negative |
| LCM-23 | 473257-15-7 | 1-(4-(4-butylcyclohexyl)cyclohexyl)-4-ethoxy-2,3-difluorobenzene | 1.84E-02 | 0.32 | 0.0557 | 479.12 | 0.58 | Positive | 0.15 | Negative |
| LCM-24 | NA | 1-(4-(4-butylcyclohexyl)cyclohex-1-enyl)-4-ethoxy-2,3-difluorobenzene | 2.60E-02 | 0.45 | N/A | 747.4 | 0.67 | Positive | 0.32 | Negative |
| LCM-25 | 825633-75-8 | 4-butyl-4″-ethyl-2′-fluoro-1,1':4′,1″-terphenyl | 5.36E-03 | 3.66E-02 | 0.21 | 175.67 | 0.51 | Positive | 0.24 | Negative |
| LCM-26 | 139195-63-4 | 3,4-difluoro-4'-[4′-ethyl-1,1′- bi(cyclohexyl)-4-yl]biphenyl | 2.04E-03 | 8.93E-02 | 0.0406 | N/A | 0.75 | Positive | 0.36 | Negative |
| LCM-27 | NA | 4-[difluoro(3,4,5-trifluorophenoxy) methyl]-3,5-difluoro-4'-[(5-propyl tetrahydro-2H-pyran)-yl]-biphenyl | 5.17E-04 | 7.85E-02 | N/A | N/A | N/A | N/A | 0.61 | Positive |
| LCM-28 | 524709-77-1 | 4-trifluoromethoxy-3,5-difluoro -2′-fluoro-4"-(4-propylcyclohexyl)-1,1':4′,1″-terphenyl | 2.30E-04 | 2.94E-02 | N/A | N/A | 0.96 | Positive | 0.54 | Positive |
| LCM-29 | 303186-36-9 | 4-[difluoro(3,4,5-trifluorophenoxy) methyl]-3,5-difluoro-2′-fluoro-4″-propyl-1,1':4′,1″-terphenyl | 1.86E-04 | 2.03E-02 | N/A | N/A | N/A | N/A | 0.38 | Negative |
| LCM-30 | 1700444-88-7 | 4-[difluoro(2-methyl-3,4,5-trifluorophenoxy) methyl]-3,5-difluoro-4'-[(5-ethyltetrahydro-2H-pyran)-yl]-biphenyl | 4.81E-04 | 6.92E-02 | N/A | N/A | N/A | N/A | 0.56 | Positive |
| LCM-31 | 119990-81-7 | 3,4-difluoro-4'-[4′-propyl-1,1′-bi(cyclohexyl)-4-yl]biphenyl | 1.25E-03 | 8.00E-02 | 0.0294 | N/A | 0.75 | Positive | 0.34 | Negative |
| LCM-32 | 119990-82-8 | 3,4-difluoro-4'-[4′-butyl-1,1′-bi(cyclohexyl)-4-yl]biphenyl | 7.60E-04 | 8.14E-02 | 0.0213 | N/A | 0.76 | Positive | 0.33 | Negative |
| LCM-33 | 136609-96-6 | 3,4-difluoro-4'-[4′-pentyl-1,1′-bi(cyclohexyl)-4-yl]biphenyl | 1.15E-03 | 7.33E-02 | 0.0154 | N/A | 0.64 | Positive | 0.35 | Negative |
Pimephales promelas, Pimephales promelas LC50 (96 h) Predicted Value (mg/L); Daphnia magna, Daphnia magna LC50 (96 h) Predicted Value (mg/L); Tetrahymena pyriformis, Tetrahymena pyriformis LC50 (96 h) Predicted Value (mg/L); Rattus norvegicus, Rattus norvegicus LC50 (96 h) Predicted Value (mg/L); DTPV, Developmental Toxicity Predicted Value; DTPR, Developmental Toxicity Predicted Result; MPV, Mutagenicity Predicted Value; MPR, Mutagenicity Predicted Result; N/A, Not Applicable.
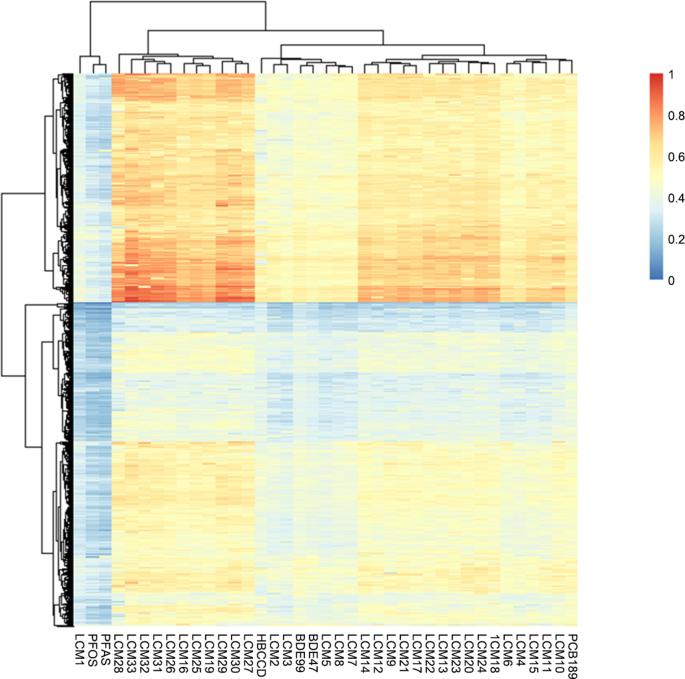
To investigate the underlying toxigenic mechanisms, we further employed high-throughput virtual screen to fish protein/enzymatic targets which LCMs potentially bind with (Fig. 4). Over a thousand protein candidates were screened, and unique clustering patterns were observed. Gene Set Enrichment Analysis (GSEA) results suggested proteins in the heat area (red region) were heavily enriched in signaling transduction, lipid metabolism, nucleotide catabolism, and stimulus response, which may provide a mechanism interpretation for the induced adverse outcomes by LCMs exposure (Fig. 5).
Fig. 4.
Normalized docking scores of 33 LCMs along with several environmental pollutants calculated by docking energy [96].
Fig. 5.
Gene Set Enrichment Analysis (GSEA) for LCMs potential targets derived from virtual screen result.
Besides, the metabolism of LCM is of great research interest. Up to date, limited research investigated the biodegradation and detoxification of LCM either in vivo or in vitro. Wang et al. examined the metabolites of selected LCM in human and other animal’s liver microsomes, with over 20 metabolites identified and structurally elucidated [103]. The result suggested that dealkylation, H-abstraction, and hydroxylation reactions are the dominant pathways involved in LCM biotransformation. However, some metabolites were pinpointed as potentially toxic compounds according to ECOSAR prediction models (developmental toxicity and mutagenicity), which implied that LCM biotransformation might be a toxicity reactivation process. Therefore, researchers are encouraged to further focus on the adverse health outcomes after the metabolism of LCM to better assess the ecological and human safety of LCMs.
In total, the results suggested that despite the trace ambient presence and weak acute toxicity, LCMs still pose a threat of exhibiting chronic toxicity by elevating oxidative stress, inducing metabolic dysregulation, and impairing epigenome maintenance [6]. There is a knowledge gap to fill on the systematical investigation of LCMs’ chronic adverse effects and relevant biochemical mechanisms.
8. Research prospects
To the best of our knowledge, there are more than 1,173 LCMs used in industrial production. However, only a few were tried to be detected in the environment matrixes. The gaps between the determination and production come from the manufacturers’ confidentiality of the “formulas”. In the future, more analytical methods need to be implemented for suspect-targeted and non-targeted high-throughput screening. On the other hand, we appeal to the management department to urge enterprises to provide the list to the maximum extent to control the ecological and health risks caused by the contamination of LCMs.
The physicochemical properties of most LCMs are obtained by model calculations (e.g., EPI Suite software from US EPA). These ideal data calculated by model calculations have great uncertainty confirmed by limited experimental test results. Therefore, more experimental testing of LCMs’ physicochemical properties should be encouraged. One of the key limitations is that the standard products of LCMs that are being used are not available to environmental chemists.
In terms of toxicity test of LCMs, the gap between the calculated values from the model and the experimental test data, especially the in vivo experiment, also exists. More toxicological effects of more species need to be conducted. Furthermore, the lack of a list of LCMs in commission may result in the neglect of some hazardous LCMs.
Based on the existing environmental pollution level and known toxic effects of LCMs, it is imperative to address LCMs in the environment matrixes. However, for some matrixes with high pollution levels, such as the dust and particulates in the e-waste dismantling areas and residual sludge in the sewage treatment plant, appropriate and efficient disposal technology should be considered. For example, gas phase LCMs should be recovered with adsorbent, and dust and sludge containing LCMs should be disposed by incineration. Considering that many LCMs are environmentally persistent chemicals, technical reserves for their environmental remediation should be considered. For example, the applicability of existing halogenated persistent chemical remediation technologies, such as microbial reduction and dehalogenation, to halogenated LCMs should be examined.
Author contributions
Z.P.C.: conceptualization, writing–original draft, funding acquisition; S.H.Z., H.J.S. and H.D.Z.: writing–original draft; G.Y.S. and M.L.F.: supervision, writing–review & editing; L.W.: supervision, writing–review & editing, funding acquisition.
Declaration of competing interests
The authors declare no conflicts of interests.
Acknowledgments
This work was funded by the National Key Research and Development Project of China (2022YFC3703203), the National Natural Science Foundation of China (42207484), and Ministry of Education of China (T2017002).
Footnotes
Given his role as an Editor, Lei Wang had no involvement in the peer-review of this article and has no access to information regarding its peer-review.
Contributor Information
Guanyong Su, Email: sugy@njust.edu.cn.
Mingliang Fang, Email: mlfang@fudan.edu.cn.
Lei Wang, Email: wang2007@nankai.edu.cn.
References
- 1.Rajak P., Nath L.K., Bhuyan B. Liquid crystals: an approach in drug delivery. Indian J. Pharmaceut. Sci. 2019;81(1):11–21. [Google Scholar]
- 2.Martin R., Simon-Hettich B., Becker W. 2004. Safe Recovery of Liquid Crystal Displays (LCDs) in Compliance with WEEE, International Congress and Exhibition on Electronics Goes Green 2004+Berlin, GERMANY, 2004; Berlin, GERMANY; pp. 147–150. [Google Scholar]
- 3.Zhang L., Wu B., Chen Y., Xu Z. Treatment of liquid crystals and recycling indium for stripping product gained by mechanical stripping process from waste liquid crystal display panels. J. Clean. Prod. 2017;162:1472–1481. [Google Scholar]
- 4.Large TFT LCD panel supply and demand worldwide 2015−2021. https://www.statista.com/statistics/883811/worldwide-tft-lcd-display-supply-demand/ (2 October)
- 5.Liang X., Xie R., Zhu C., Chen H., Shen M., Li Q., Du B., Luo D., Zeng L. Comprehensive identification of liquid crystal monomers-biphenyls, cyanobiphenyls, fluorinated biphenyls, and their analogues-in waste LCD panels and the first estimate of their global release into the environment. Environ. Sci. Technol. 2021;55(18):12424–12436. doi: 10.1021/acs.est.1c03901. [DOI] [PubMed] [Google Scholar]
- 6.Su H.J., Shi S.B., Zhu M., Crump D., Letcher R.J., Giesy J.P., Su G.Y. Persistent, bioaccumulative, and toxic properties of liquid crystal monomers and their detection in indoor residential dust. Proc. Natl. Acad. Sci. U.S.A. 2019;116(52):26450–26458. doi: 10.1073/pnas.1915322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Z., Shi Q., Wang Y., Zhao L., Li X., Sun Z., Lu Y., Liu N., Su G., Wang L., et al. Electronic-waste-driven pollution of liquid crystal monomers: environmental occurrence and human exposure in recycling industrial parks. Environ. Sci. Technol. 2022;56(4):2248–2257. doi: 10.1021/acs.est.1c04621. [DOI] [PubMed] [Google Scholar]
- 8.Zhu M., Shen M., Liang X., Chen H., Zhu C., Du B., Luo D., Lan S., Feng Z., Zeng L. Identification of environmental liquid-crystal monomers: a class of new persistent organic pollutants-fluorinated biphenyls and analogues-emitted from E-waste dismantling. Environ. Sci. Technol. 2021;55(9):5984–5992. doi: 10.1021/acs.est.1c00112. [DOI] [PubMed] [Google Scholar]
- 9.Su H., Shi S., Zhu M., Li J., Su G. Liquid crystal monomers (LCMs) in sediments: method validation and detection in sediment samples from three typical areas. Environ. Sci. Technol. 2021;55(4):2336–2345. doi: 10.1021/acs.est.0c06427. [DOI] [PubMed] [Google Scholar]
- 10.Jin Q., Tao D., Lu Y., Sun J., Lam C.H., Su G., He Y. New insight on occurrence of liquid crystal monomers: a class of emerging e-waste pollutants in municipal landfill leachate. J. Hazard Mater. 2022:423. doi: 10.1016/j.jhazmat.2021.127146. [DOI] [PubMed] [Google Scholar]
- 11.Shen M., Feng Z., Liang X., Chen H., Zhu C., Du B., Li Q., Zeng L. Release and gas-particle partitioning behavior of liquid crystal monomers during the dismantling of waste liquid crystal display panels in E-waste recycling facilities. Environ. Sci. Technol. 2022;56(5):3106–3116. doi: 10.1021/acs.est.1c07394. [DOI] [PubMed] [Google Scholar]
- 12.Li Y., Zhang T., Cheng Z., Zhang Q., Yang M., Zhao L., Zhang S., Lu Y., Sun H., Wang L. Direct evidence on occurrence of emerging liquid crystal monomers in human serum from E-waste dismantling workers: implication for intake assessment. Environ. Int. 2022;169:107535. doi: 10.1016/j.envint.2022.107535. [DOI] [PubMed] [Google Scholar]
- 13.Li R., Ren K., Su H., Wei Y., Su G. Target and suspect analysis of liquid crystal monomers in soil from different urban functional zones. Sci. Total Environ. 2022;854:158408. doi: 10.1016/j.scitotenv.2022.158408. [DOI] [PubMed] [Google Scholar]
- 14.Wang J., Nan J., Li M., Yuan G., Zhao Y., Dai J., Zhang K. First evidence of contamination in aquatic organisms with organic light-emitting materials. Environ. Sci. Technol. Lett. 2022;9(9):739–746. [Google Scholar]
- 15.Zhang S., Yang M., Li Y., Wang Y., Lu Y., Cheng Z., Sun H. Occurrence, distribution, and human exposure of emerging liquid crystal monomers (LCMs) in indoor and outdoor dust: a nationwide study. Environ. Int. 2022;164:107295. doi: 10.1016/j.envint.2022.107295. [DOI] [PubMed] [Google Scholar]
- 16.Su H., Ren K., Li R., Li J., Gao Z., Hu G., Fu P., Su G. Suspect screening of liquid crystal monomers (LCMs) in sediment using an established database covering 1173 LCMs. Environ. Sci. Technol. 2022;56(12):8061–8070. doi: 10.1021/acs.est.2c01130. [DOI] [PubMed] [Google Scholar]
- 17.Tao D.Y., Jin Q.Q., Ruan Y.F., Zhang K., Jin L.J., Zhan Y.T., Su G.Y., Wu J.X., Leung K.M.Y., Lam P.K.S., et al. Widespread occurrence of emerging E-waste contaminants-Liquid crystal monomers in sediments of the Pearl River Estuary, China. J. Hazard Mater. 2022:437. doi: 10.1016/j.jhazmat.2022.129377. [DOI] [PubMed] [Google Scholar]
- 18.Simon-Hettich B., Broschard T.H., Becker W., Takeuchi H., Saito H., Ohnishi H., Takatsu H., Naemura S., Kobayashi K. Ecotoxicological properties of liquid-crystal compounds. J. Soc. Inf. Disp. 2001;9:307–312. [Google Scholar]
- 19.An R., Li Y., Niu X., Yu H. Responses of antioxidant enzymes in catfish exposed to liquid crystals from E-waste. Int. J. Environ. Res. Publ. Health. 2008;5(2):99–103. doi: 10.3390/ijerph5020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y., Zhang X., Li C., Zhao Y., Zhang Y.N., Qu J. Atmospheric persistence and toxicity evolution for fluorinated biphenylethyne liquid crystal monomers unveiled by in silico methods. J. Hazard Mater. 2022;424(Pt B):127519. doi: 10.1016/j.jhazmat.2021.127519. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q.F., Liggio J., Wentzell J., Lee P., Li K., Li S.M. Atmospheric OH oxidation chemistry of particulate liquid crystal monomers: an emerging persistent organic pollutant in air. Environ. Sci. Technol. Lett. 2020;7(9):646–652. [Google Scholar]
- 22.Li J., Su G., Letcher R.J., Xu W., Yang M., Zhang Y. Liquid crystal monomers (LCMs): a new generation of persistent bioaccumulative and toxic (PBT) compounds? Environ. Sci. Technol. 2018;52(9):5005–5006. doi: 10.1021/acs.est.8b01636. [DOI] [PubMed] [Google Scholar]
- 23.Brown G.H.J. Liquid crystals-The chameleon chemicals. Chem. Educ. 1983;60:900–905. [Google Scholar]
- 24.Gobas F.A.P.C., de Wolf W., Burkhard L.P., Verbruggen E., Plotzke K. Revisiting bioaccumulation criteria for POPs and PBT assessments. Integr. Environ. Assess. Manag. 2009;5(4):624–637. doi: 10.1897/IEAM_2008-089.1. [DOI] [PubMed] [Google Scholar]
- 25.Howard P.H., Muir D.C.G. Identifying new persistent and bioaccumulative organics among chemicals in commerce. Environ. Sci. Technol. 2010;44(7):2277–2285. doi: 10.1021/es903383a. [DOI] [PubMed] [Google Scholar]
- 26.Li C., Huang Y., Zhang X., Zhao Y., Huo Y. Atmospheric fate and risk investigation of typical liquid crystal monomers. Acs Sustain Chem Eng. 2021;9(9):3600–3607. [Google Scholar]
- 27.United Nations Environment Programme . United Nations Environment Program; Geneva, Switzerland: 2009. Stockholm Convention on Persistent Organic Pollutants (POPs), Adoption of Amendments of Annexes A, B and C. [Google Scholar]
- 28.Hites R.A. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ. Sci. Technol. 2004;38(4):945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 29.Darnerud P.O., Eriksen G.S., Johannesson T., Larsen P.B., Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 2001;109:49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wit C.A. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46(5):583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- 31.Feng J.J., Sun X.F., Zeng E.Y. Measurement of octanol-air partition coefficients for liquid crystals based on gas chromatography-retention time and its implication in predicting long-range transport potential. Chemosphere. 2021;282:131109. doi: 10.1016/j.chemosphere.2021.131109. [DOI] [PubMed] [Google Scholar]
- 32.Zhu M., Su H., Bao Y., Li J., Su G. Experimental determination of octanol-water partition coefficient (K(OW)) of 39 liquid crystal monomers (LCMs) by use of the shake-flask method. Chemosphere. 2022;287(Pt 4):132407. doi: 10.1016/j.chemosphere.2021.132407. [DOI] [PubMed] [Google Scholar]
- 33.Dubocq F., Karrman A., Gustavsson J., Wang T. Comprehensive chemical characterization of indoor dust by target, suspect screening and nontarget analysis using LC-HRMS and GC-HRMS. Environ. Pollut. 2021:276. doi: 10.1016/j.envpol.2021.116701. [DOI] [PubMed] [Google Scholar]
- 34.Berner-Branzell Filip F.E., Emma Overgaard, Isabel Häggblom. Thesis; 2020. The Presence of Liquid Crystal Monomers in House Dust and Public Environments. [Google Scholar]
- 35.Li Y.-F., Qiao L.-N., Ren N.-Q., Sverko E., Mackay D., Macdonald R.W. Decabrominated diphenyl ethers (BDE-209) in Chinese and global air: levels, gas/particle partitioning, and long-range transport: is long-range transport of BDE-209 really governed by the movement of particles? Environ. Sci. Technol. 2017;51(2):1035–1042. doi: 10.1021/acs.est.6b05395. [DOI] [PubMed] [Google Scholar]
- 36.Yang M., Qi H., Jia H.-L., Ren N.-Q., Ding Y.-S., Ma W.-L., Liu L.-Y., Hung H., Sverko E., Li Y.-F. Polybrominated diphenyl ethers in air across China: levels, compositions, and gas-particle partitioning. Environ. Sci. Technol. 2013;47(15):8978–8984. doi: 10.1021/es4022409. [DOI] [PubMed] [Google Scholar]
- 37.Tian M., Chen S.-J., Wang J., Zheng X.-B., Luo X.-J., Mai B.-X. Brominated flame retardants in the atmosphere of E-waste and rural sites in Southern China: seasonal variation, temperature dependence, and gas-particle partitioning. Environ. Sci. Technol. 2011;45(20):8819–8825. doi: 10.1021/es202284p. [DOI] [PubMed] [Google Scholar]
- 38.Caballero-Casero N., Castro G., Bastiaensen M., Gys C., van Larebeke N., Schoeters G., Covaci A. Identification of chemicals of emerging concern in urine of Flemish adolescents using a new suspect screening workflow for LC-QTOF-MS. Chemosphere. 2021;280:130683. doi: 10.1016/j.chemosphere.2021.130683. [DOI] [PubMed] [Google Scholar]
- 39.Mihajlović I., Miloradov M.V., Fries E. Application of Twisselmann extraction, SPME, and GC-MS to assess input sources for organophosphate esters into soil. Environ. Sci. Technol. 2011;45(6):2264–2269. doi: 10.1021/es103870f. [DOI] [PubMed] [Google Scholar]
- 40.Ling S.Y., Zhou S.Q., Tan J.Q., Lu C., Fu M.R., Peng C., Zhang W., Hu S.Q., Lin K.F., Zhou B.S. Brominated flame retardants (BFRs) in sediment from a typical e-waste dismantling region in Southern China: occurrence, spatial distribution, composition profiles, and ecological risks. Sci. Total Environ. 2022;824:153813. doi: 10.1016/j.scitotenv.2022.153813. [DOI] [PubMed] [Google Scholar]
- 41.Li W.L., Liu L.Y., Zhang Z.F., Song W.W., Huo C.Y., Qiao L.N., Ma W.L., Li Y.F. Brominated flame retardants in the surrounding soil of two manufacturing plants in China: occurrence, composition profiles and spatial distribution. Environ. Pollut. 2016;213:1–7. doi: 10.1016/j.envpol.2016.01.092. [DOI] [PubMed] [Google Scholar]
- 42.Ma E., Zhang C., Bai J., Wang J. 2016. A Green Method for Recycling Materials from Liquid Crystal Display Panel; p. 8. 8. [Google Scholar]
- 43.Chen Y., Zhang L., Xu Z. Vacuum pyrolysis characteristics and kinetic analysis of liquid crystal from scrap liquid crystal display panels. J. Hazard Mater. 2017;327:55–63. doi: 10.1016/j.jhazmat.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Wang R., Xu Z. Pyrolysis characteristics and pyrolysis products separation for recycling organic materials from waste liquid crystal display panels. J. Hazard Mater. 2016;302:45–56. doi: 10.1016/j.jhazmat.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 45.Nie E., Luo X.Z., Zheng Z., Sheng M. Treatment of liquid crystal and recovery of indium from liquid crystal display. Chinese J. Environ. Eng. 2008;2(9):1251–1254. [Google Scholar]
- 46.Zhu H.B., Wang Y.L., Liu G.F., Niu H.L., Cao L.L. Liquid crystal recovery and performance test from wasted liquid crystal display screens. Res. Environ. Sci. 2013;26(3):300–306. [Google Scholar]
- 47.Zhu H., Wang Y., Liu G. Study on method of recycling liquid crystal from waste LCD based on supercritical CO2 fluid technology. Adv. Mater. Res. 2012;479–481:2165–2170. [Google Scholar]
- 48.Zhu H. Hefei university of technology; Hefei: 2013. Research on Method of Recycling Liquid Crystal from Wasted LCDs. [Google Scholar]
- 49.Chien Y.-C., Shih P.-H. Emission of polycyclic aromatic hydrocarbons on the combustion of liquid crystal display components. J. Environ. Eng. 2006;132(9):1028–1033. [Google Scholar]
- 50.Lu R., Ma E., Xu Z. Application of pyrolysis process to remove and recover liquid crystal and films from waste liquid crystal display glass. J. Hazard Mater. 2012;243:311–318. doi: 10.1016/j.jhazmat.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 51.Xin S., Gao W., Wang Y., Jiang G. Identification of the released and transformed products during the thermal decomposition of a highly chlorinated paraffin. Environ. Sci. Technol. 2018;52(17):10153–10162. doi: 10.1021/acs.est.8b01729. [DOI] [PubMed] [Google Scholar]
- 52.Guo Y.W., Wang S.T., Liu J.Y., Qiao Q., Liang J.J. Preliminary study on heat treatment weight loss characteristics and corresponding pyrolysis products of LC. Res. Environ. Sci. 2009;22(9):1074–1078. [Google Scholar]
- 53.Guo Y., Liu J., Qiao Q. 2009. Method for Harmless Heat Treatment of Waste Liquid Crystal Display. [Google Scholar]
- 54.Guo Y., Liu J., Qiao Q. 2009. Waste Liquid Crystal Innocent Treatment Device. [Google Scholar]
- 55.Zhang L., Chen Y., Xu Z. Controllable Formation of carbon fiber in pyrolysis process of liquid crystals from waste LCD panels and indium recovery by vacuum in situ reduction with carbon fiber. Acs Sustain Chem Eng. 2018;6(1):541–550. [Google Scholar]
- 56.Zhang L., Wu B., Chen Y., Xu Z. Energy and valuable resource recovery from waste liquid crystal display panels by an environment-friendly technological process: pyrolysis of liquid crystals and preparation of indium product. J. Clean. Prod. 2017;162:141–152. [Google Scholar]
- 57.Jin Q., Zhan Y., Tao D., Wang T., Khim J.S., He Y. Removing emerging e-waste pollutant DTFPB by synchronized oxidation-adsorption Fenton technology. J. Hazard Mater. 2023;445:130587. doi: 10.1016/j.jhazmat.2022.130587. [DOI] [PubMed] [Google Scholar]
- 58.Duan S. Beijing Forestry University; Beijing: 2020. Preparation of Polycyclic Polymer and Study on the Adsorption Efficiency of Brominated Flame Retardant from Water. [Google Scholar]
- 59.Sang Y., He L., Yu W., Ma F., Zhu L., Jiao Y., Gu Q. Charring behaviors and their influence of organic contaminated soil during thermal treatment. Chinese J. Environ. Eng. 2021;15(7):2181–2190. [Google Scholar]
- 60.Martinez-Huitle C.A., Hernandez F., Ferro S., Antonio M., Alfaro Q., de Battisti A.P. Electrochemical oxidation: an alternative for the wastewater treatment with organic pollutants agents. Afinidad. 2006;62(521):26–34. [Google Scholar]
- 61.Fang L., Huang J., Yu G. Photochemical degradation of polybrominated diphenyl ethers. Prog. Chem. 2008;20(7–8):1180–1186. [Google Scholar]
- 62.Gaur N., Narasimhulu K., PydiSetty Y. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018;198:1602–1631. [Google Scholar]
- 63.Feng N.-X., Yu J., Zhao H.-M., Cheng Y.-T., Mo C.-H., Cai Q.-Y., Li Y.-W., Li H., Wong M.-H. Efficient phytoremediation of organic contaminants in soils using plant-endophyte partnerships. Sci. Total Environ. 2017;583:352–368. doi: 10.1016/j.scitotenv.2017.01.075. [DOI] [PubMed] [Google Scholar]
- 64.Li X.Q., Zhang L., Yang Z.Q., Wang P., Yan Y.F., Ran J.Y. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: a review. Sep. Purif. Technol. 2020:235. [Google Scholar]
- 65.Jiang N., Shang R., Heijman S.G.J., Rietveld L.C. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: a review. Water Res. 2018;144:145–161. doi: 10.1016/j.watres.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 66.Geelhaar T., Griesar K., Reckmann B. 125 years of liquid crystals—a scientific revolution in the home. Angew. Chem. Int. Ed. 2013;52(34):8798–8809. doi: 10.1002/anie.201301457. [DOI] [PubMed] [Google Scholar]
- 67.Pan Y., Tsang D.C.W., Wang Y., Li Y., Yang X. The photodegradation of polybrominated diphenyl ethers (PBDEs) in various environmental matrices: kinetics and mechanisms. Chem. Eng. J. 2016;297:74–96. [Google Scholar]
- 68.Su G., Letcher R.J., Crump D., Farmahin R., Giesy J.P., Kennedy S.W. Sunlight irradiation of highly brominated polyphenyl ethers generates polybenzofuran products that alter dioxin-responsive mRNA expression in chicken hepatocytes. Environ. Sci. Technol. 2016;50(5):2318–2327. doi: 10.1021/acs.est.5b04939. [DOI] [PubMed] [Google Scholar]
- 69.Kawamoto H. The history of liquid-crystal displays. Proc. IEEE. 2002;90(4):460–500. [Google Scholar]
- 70.Zhang M., Shi Q., Song X., Wang H., Bian Z. Recent electrochemical methods in electrochemical degradation of halogenated organics: a review. Environ. Sci. Pollut. Res. 2019;26(11):10457–10486. doi: 10.1007/s11356-019-04533-3. [DOI] [PubMed] [Google Scholar]
- 71.Zhou M., Wu Z., Wang D. Study on electrocatalytic degradation of biorefractory aromatic compounds:Effect of structure on degradation reactivity. J. Zhejiang Univ. 2003;37(1):74–77. 103. [Google Scholar]
- 72.Zhou M., Wu Z., Wang D. Advanced electrochemical oxidation process for treatment of biorefractory wastewater containing typical aromatic compounds. Environ. Sci. 2003;24(2):121–124. [PubMed] [Google Scholar]
- 73.Zhou M.H., Wu Z.C., Wang D.H. Electrocatalytic degradation of phenol in acidic and saline wastewater. J. Environ. Sci. Health - Part A Toxic/Hazard. Subst. Environ. Eng. 2002;37(7):1263–1275. doi: 10.1081/ese-120005985. [DOI] [PubMed] [Google Scholar]
- 74.Muthukrishnan A., Boyarskiy V., Sangaranarayanan M.V., Boyarskaya I. Mechanism and regioselectivity of the electrochemical reduction in polychlorobiphenyls (PCBs): kinetic analysis for the successive reduction of chlorines from dichlorobiphenyls. J. Phys. Chem. C. 2012;116(1):655–664. [Google Scholar]
- 75.Matsunaga A., Yasuhara A. Dechlorination of PCBs by electrochemical reduction with aromatic radical anion as mediator. Chemosphere. 2005;58(7):897–904. doi: 10.1016/j.chemosphere.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 76.Martin E.T., McGuire C.M., Mubarak M.S., Peters D.G. Electroreductive remediation of halogenated environmental pollutants. Chem. Rev. 2016;116(24):15198–15234. doi: 10.1021/acs.chemrev.6b00531. [DOI] [PubMed] [Google Scholar]
- 77.Sredlova K., Cajthaml T. Recent advances in PCB removal from historically contaminated environmental matrices. Chemosphere. 2022;287(Pt 1):132096. doi: 10.1016/j.chemosphere.2021.132096. [DOI] [PubMed] [Google Scholar]
- 78.Xu L., Liu S., Tang Y., Han X., Wang Y., Fu D., Qin Q., Xu Y. Long-term dechlorination of polychlorinated biphenyls (PCBs) in Taihu Lake sediment microcosms: identification of new pathways, PCB-driven shifts of microbial communities, and insights into dechlorination potential. Environ. Sci. Technol. 2022;56(2):938–950. doi: 10.1021/acs.est.1c06057. [DOI] [PubMed] [Google Scholar]
- 79.He J., Robrock K.R., Alvarez-Cohen L. Microbial reductive debromination of polybrominated diphenyl ethers (PBDEs) Environ. Sci. Technol. 2006;40(14):4429–4434. doi: 10.1021/es052508d. [DOI] [PubMed] [Google Scholar]
- 80.Sverko E., McCarry B., McCrindle R., Brazeau A., Pena-Abaurrea M., Reiner E., Anne Smyth S., Gill B., Tomy G.T. Evidence for anaerobic dechlorination of Dechlorane Plus in sewage sludge. Environ. Sci. Technol. 2015;49(23):13862–13867. doi: 10.1021/acs.est.5b03550. [DOI] [PubMed] [Google Scholar]
- 81.Fan J., Jia Y., Xu D., Ye Z., Zhou J., Huang J., Fu Y., Shen C. Anaerobic condition induces a viable but nonculturable state of the PCB-degrading Bacteria Rhodococcus biphenylivorans TG9. Sci. Total Environ. 2021;764:142849. doi: 10.1016/j.scitotenv.2020.142849. [DOI] [PubMed] [Google Scholar]
- 82.Pathiraja G., Egodawatta P., Goonetilleke A., Te'o V.S.J. Effective degradation of polychlorinated biphenyls by a facultative anaerobic bacterial consortium using alternating anaerobic aerobic treatments. Sci. Total Environ. 2019;659:507–514. doi: 10.1016/j.scitotenv.2018.12.385. [DOI] [PubMed] [Google Scholar]
- 83.Robrock K.R., Korytár P., Alvarez-Cohen L. Pathways for the anaerobic microbial debromination of polybrominated diphenyl ethers. Environ. Sci. Technol. 2008;42(8):2845–2852. doi: 10.1021/es0720917. [DOI] [PubMed] [Google Scholar]
- 84.Chitsaz M., Fennell D.E., Rodenburg L.A. Sources of polychlorinated biphenyls to Upper Hudson River sediment post-dredging. Chemosphere. 2020;259:127438. doi: 10.1016/j.chemosphere.2020.127438. [DOI] [PubMed] [Google Scholar]
- 85.Arslan M., Imran A., Khan Q.M., Afzal M. Plant-bacteria partnerships for the remediation of persistent organic pollutants. Environ. Sci. Pollut. Res. 2017;24(5):4322–4336. doi: 10.1007/s11356-015-4935-3. [DOI] [PubMed] [Google Scholar]
- 86.Ancona V., Barra Caracciolo A., Grenni P., Di Lenola M., Campanale C., Calabrese A., Uricchio V.F., Mascolo G., Massacci A. Plant-assisted bioremediation of a historically PCB and heavy metal-contaminated area in Southern Italy. N. Biotech. 2017;38:65–73. doi: 10.1016/j.nbt.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Hayat A., Hussain I., Soja G., Iqbal M., Shahid N., Syed J.H., Yousaf S. Organic and chemical amendments positively modulate the bacterial proliferation for effective rhizoremediation of PCBs-contaminated soil. Ecol. Eng. 2019;138:412–419. [Google Scholar]
- 88.Urbaniak M., Baran A., Lee S., Kannan K. Utilization of PCB-contaminated Hudson River sediment by thermal processing and phytoremediation. Sci. Total Environ. 2020;738:139841. doi: 10.1016/j.scitotenv.2020.139841. [DOI] [PubMed] [Google Scholar]
- 89.Zhang S., Cheng Z., Yang M., Guo Z., Zhao L., Baqar M., Lu Y., Wang L., Sun H. Percutaneous penetration of liquid crystal monomers (LCMs) by in vitro three-dimensional human skin Equivalents: possible mechanisms and implications for human dermal exposure risks. Environ. Sci. Technol. 2023;57(11):4454–4463. doi: 10.1021/acs.est.2c07844. [DOI] [PubMed] [Google Scholar]
- 90.Feng J.-J., Sun X.-F., Zeng E.Y. Predicted health and environmental hazards of liquid crystal materials via quantitative structure-property relationship modeling. J. Hazard Mater. 2023;446:130592. doi: 10.1016/j.jhazmat.2022.130592. [DOI] [PubMed] [Google Scholar]
- 91.Sakai Y., Sakai T. A risk analysis of the liquid crystal display industry. J. Risk Res. 2007;10(6):871–882. [Google Scholar]
- 92.Savvilotidou V., Hahladakis J.N., Gidarakos E. Determination of toxic metals in discarded liquid crystal displays (LCDs) Resour. Conserv. Recycl. 2014;92:108–115. [Google Scholar]
- 93.Werner B.S.-H.,B., Hoenicke P. 2002. Toxicological and Ecotoxicological Investigations of Liquid Crystals. [Google Scholar]
- 94.Lakatos A.I. Introduction. J. Soc. Inf. Disp. 2000;8(1):1. 1. [Google Scholar]
- 95.Luk Y.Y., Campbell S.F., Abbott N.L., Murphy C.J. Non-toxic thermotropic liquid crystals for use with mammalian cells. Liq. Cryst. 2004;31(5):611–621. [Google Scholar]
- 96.Zhao H., Li C., Naik M.Y., Wu J., Cardilla A., Liu M., Zhao F., Snyder S.A., Xia Y., Su G., Fang M. Liquid crystal monomer: a potential PPARγ antagonist. Environ. Sci. Technol. 2023;57(9):3758–3771. doi: 10.1021/acs.est.2c08109. [DOI] [PubMed] [Google Scholar]
- 97.Weber N., Richter K.D. 3 beta-Chlorosteroids: artefacts and possible contaminants in food and feed--toxicological effects in mice. Food Chem. Toxicol. 1994;32(4):297–303. doi: 10.1016/0278-6915(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 98.EPA, U. S. 2010. Life-Cycle Assessment of Desktop Computer Displays: Summary of Results. [Google Scholar]
- 99.Woolverton C.J., Gustely E., Li L.F., Lavrentovich O.D. , Liquid crystal effects on bacterial viability. Liq. Cryst. 2005;32(4):417–423. [Google Scholar]
- 100.Reuschenbach P., Silvani M., Dammann M., Warnecke D., Knacker T. ECOSAR model performance with a large test set of industrial chemicals. Chemosphere. 2008;71(10):1986–1995. doi: 10.1016/j.chemosphere.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 101.Su H., Shi S., Zhu M., Crump D., Letcher R.J., Giesy J.P., Su G. Persistent, bioaccumulative, and toxic properties of liquid crystal monomers and their detection in indoor residential dust. Proc. Natl. Acad. Sci. U. S. A. 2019;116(52):26450–26458. doi: 10.1073/pnas.1915322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yale H.L. The trifluoromethyl group in medical chemistry. J. Med. Pharmaceut. Chem. 1959;1(2):121–133. doi: 10.1021/jm50003a001. [DOI] [PubMed] [Google Scholar]
- 103.Wang X., Yang R., Zhang J., Chen X., Feng Y., Niu Y., Shao B. Metabolic profiling of the fluorinated liquid-crystal monomer 1-ethoxy-2,3-difluoro-4-(trans-4-propylcyclohexyl)benzene. Sci. Total Environ. 2023;860:160448. doi: 10.1016/j.scitotenv.2022.160448. [DOI] [PubMed] [Google Scholar]