Abstract
The transmembrane glycoprotein adhesion molecule CD146 is overexpressed in a wide variety of cancers. Through molecular imaging, a specific biomarker’s expression and distribution can be viewed in vivo non-invasively. Radionuclide-labeled monoclonal antibodies or relevant fragments that target CD146 may find potential applications in cancer imaging, thereby offering tremendous value in cancer diagnosis, staging, prognosis evaluation, and prediction of drug resistance. This review discusses the recent developments of CD146-targeted molecular imaging via nuclear medicine, especially in malignant melanoma, brain tumor, lung cancer, liver cancer, breast cancer, and pancreatic cancer. Many studies have proved that CD146 targeting may present a promising strategy for cancer theranostics.
Keywords: CD146, molecular imaging, radionuclide, immunoPET, cancer
Graphical Abstract
Radionuclide-labeled monoclonal antibodies or relevant fragments that target CD146 may find potential applications in cancer imaging, thereby offering tremendous value in cancer diagnosis, staging, prognosis evaluation, and prediction of drug resistance. This review discusses the recent developments of CD146-targeted molecular imaging via nuclear medicine, especially in malignant melanoma, brain tumor, lung cancer, liver cancer, breast cancer, and pancreatic cancer. Many studies have proved that CD146 targeting may present a promising strategy for cancer theranostics.
The transmembrane glycoprotein adhesion molecule CD146 is overexpressed in a wide variety of cancers. CD146 expression constitutes a marker of poor prognosis and correlates with cancer progression. Through molecular imaging, a specific biomarker's expression and distribution can be viewed in vivo non-invasively. Radionuclide-labeled monoclonal antibodies or relevant fragments that target CD146 may find potential applications in cancer imaging, thereby offering tremendous value in cancer diagnosis, staging, prognosis evaluation, and prediction of drug resistance. This review discusses the recent developments of CD146-targeted molecular imaging via nuclear medicine, especially in malignant melanoma, brain tumor, lung cancer, liver cancer, breast cancer, and pancreatic cancer. Many studies have proved that CD146 targeting may present a promising strategy for cancer theranostics.
A cluster of differentiation 146 (CD146), also known as MUC18 or melanoma adhesion molecule (MCAM), was first identified in melanoma in 1987[1]. CD146-coded gene resides in subband 3 of long-arm region 2 of human chromosome 11 (11q23.3)[2]. The CD146 glycoprotein adhesion molecule has a molecular weight of 113 kDa and is transmembrane in nature[3]. There is an important role for CD146 protein in regulating vascular permeability, cell adhesion, leukocyte migration, angiogenesis, and epithelial-mesenchymal transition (EMT)[4]. EMT cells undergo significant morphological changes as epithelial cell adhesion molecules are reduced in expression. As a result, when these cells infiltrate blood vessels, they develop into circulating tumor cells with invasion and migration abilities[5]. CD146 can also up-regulate the expression levels of CD31 and vascular endothelial growth factor (VEGF), thus promoting tumor angiogenesis and metastasis[6]. A high CD146 expression level in different types of cancer is associated with poor prognosis and progression of cancer[5a, 7]. In recent years, a number of studies have found that CD146 is overexpressed in many types of cancer, including melanoma, breast cancer, liver cancer, lung cancer, pancreatic cancer, prostate cancer, ovarian cancer, and kidney cancer among others. This finding highlights the crucial role of CD146 in cancer development and metastasis.
Molecular imaging technology combines molecular biology with in vivo imaging to reflect the changes in physiological and pathological functions, as well as the expression and distribution of a specific molecule at the molecular, cellular, and organ levels. Molecular imaging based on nuclear medicine is a reproducible, safe, and non-invasive in vivo evaluation technique, which has been widely used for diagnosing, staging, predicting prognosis, and evaluating the efficacy of various cancer treatments [8]. In nuclear medicine, radiotracers are combinations of radioactive atoms and chemicals that target metabolic processes. These radionuclide images are created using positron emission tomography (PET) or single photon emission computed tomography (SPECT), usually in conjunction with a CT scan. ImmunoPET imaging takes advantages of PET’s high sensitivity and antibody’s high affinity and specificity to achieve non-invasive evaluation of targeted antigens in vivo[9]. In the following sections, we will review the applications of CD146-targeted molecular imaging and nuclide therapy using nuclear medicine in common cancers, to find more evidence to confirm the application prospect and value of radionuclide-labeled monoclonal antibodies targeting CD146 or its fragments in tumor imaging.
1. Molecular mechanism of CD146 in cancer
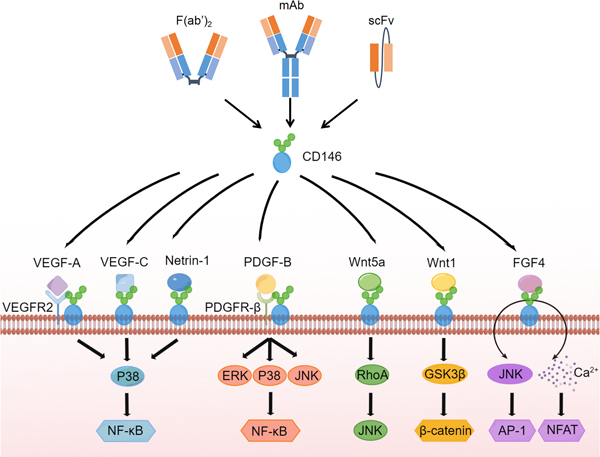
CD146 is necessary for VEGF-induced VEGFR-2 phosphorylation and AKT/p38 mitogen-activated protein kinases (MAPKs) activation, in order to promote endothelial cell migration and the formation of microvessels (Figure 1)[10]. CD146 proteins regulate pro-angiogenic genes like interleukin-8 (IL-8), intercellular cell adhesion molecule-1 (ICAM-1), and matrix metallopeptidase 9 (MMP9) when activated by tumor secretions[11]. CD146 binds to Wnt5a activating the c-Jun N-terminal kinase-planar cell polarity (JNK-PCP) pathway and downregulating the expression of β-catenin [12]. In addition, by binding directly to Wnt1, CD146 activates fibroblasts via the canonical Wnt/β-catenin pathway. Proliferation of fibroblasts and production of extracellular matrix are inextricably linked to this interaction[13]. By silencing or deleting CD146, HUVEC proliferation, migration, and tube formation is inhibited, as is VEGFR2, p38, and extracellular regulated extracellular regulated protein kinases 1/2 (ERK 1/2)[14]. Synergistic effect of fibroblast growth factors (FGF) signaling-dependent ERK activation and CD146-dependent JNK and nuclear factor of activated T cells (NFAT) activation ensure that CD146+ cells simultaneously upregulate AP-1 and NFAT transcription during organ morphogenesis[15]. CD146 protein is essential for E-cadherin-to-N-cadherin switch during transforming growth factor-β (TGF-β) signaling-induced EMT[16]. A heterophilic ligand interacts with CD146 to mediate homotypic cell adhesion[17].
Figure 1.
CD146 associated antibodies and signaling pathways.
CD146 is overexpressed in many types of cancer, including melanoma, brain tumor, lung cancer, hepatocellular carcinoma, breast cancer, renal cell carcinoma, pancreatic cancer, prostate cancer, gastrointestinal cancer, uterine and adnexal tumors, and other cancer types. Many studies on the mechanism of CD146 in cancer have been carried out.
1.1. Melanoma
CD146 is frequently found to overexpress on the surface of metastatic and advanced human melanoma cells[18]. In response to VEGF, CD146 deficiency alters the activation of focal adhesion kinase (FAK) and decreases the expression of VEGFR-2/Ve-cadherin[19]. The VEGF-induced pathway and tumor angiogenesis are both impaired in CD146 knockout mice, resulting in the inhibition of VEGF-induced VEGFR-2 phosphorylation and AKT/p38 MAPKs/nuclear factor kappa-B (NF-κB) activation[20]. Malignant melanoma is promoted by CD146, a novel receptor for S100A8/A9, by activating NF-κB and forming ROS[18]. Since CD146 is involved in the regulation of inhibitor of DNA binding-1 (Id-1) and activating transcription factor-3 (ATF-3) expressions, it therefore contributes to the development of melanoma metastases[21]. Invasion and metastasis are putatively mediated by laminin-421, which is a primary ligand for CD146[22]. By activating the platelet-activating factor receptor (PAFR), protease-activated receptor 1 (PAR1) mediates the expression of CD146. In addition to mediating the adhesion of melanoma cells to microvascular endothelial cells, the PAR1-PAFR-MUC18 pathway also modulates transendothelial migration and metastatic retention of melanoma cells[23]. β−1,3-Galactosyl- O-Glycosyl-Glycoprotein β−1,6- N-Acetylglucosaminyltransferase 3 (GCNT3) is an upstream regulator of CD146 protein. A novel S100A8/A9 receptor, GCNT3, favorably glycosylates the MCAM receptor and extends its half-life in melanoma cells, further increasing S100A8/A9-mediated cellular motility[24]. Endothelins upregulate CD146 in an AKT and ERK/MEK-dependent, but CREB-independent manner, thus promoting the invasion and metastasis of melanoma[25].
1.2. Brain tumor
There is a high expression of CD146 in glioma stem cells by regulating the cell cycle. Most differentiated cells in the G0/G1 phase are arrested in the cell cycle when CD146 is expressed ectopically in parental glioma cells[26]. CD146 expression is statistically significant correlated with lower disease-free survival and overall survival in glioblastomas[27].
1.3. Lung cancer
Poor prognosis is associated with CD146 expression in surgically treated non-small-cell lung cancer (NSCLC)[28]. The expression of EMT markers (i.e., epithelial cadherin, vimentin, and snail) is influenced by CD146, as well as the phosphorylation of AKT. Thus, the migration and proliferation of pulmonary large cell neuroendocrine carcinoma cells may be regulated by CD146[29]. Stemness and migration of epidermal growth factor receptor-tyrosine kinase inhibitors-resistant (EGFR-TKI-resistant) cells are influenced by CD146. Therefore, CD146 can be a potential target for treating and preventing lung cancers which are resistant to EGFR-TKIs and their subsequent metastases[30]. As a result of CD146-mediated chemosensitivity, mitochondrial 37S ribosomal protein 1/ATP-binding cassette subfamily C member 1 (MRP1/ABCC1) and the PI3K pathway are upregulated through SOX2-dependent signaling[31].
1.4. Hepatocellular carcinoma (HCC)
CD146 is highly elevated in HCC cells as a result of positive regulation by Yes-associated protein. The transcriptional and translational activities of c-Jun/c-Fos are initiated by CD146. In addition, eukaryotic initiation factor 4E is bound to c-Jun/c-Fos mRNA through AKT activation, which is promoted by CD146[32]. HCC patients with CD146 are at a high risk of metastasis and a poor prognosis. It is possible that CD146-induced EMT, IL-8 upregulation, and STAT1 downregulation contribute to EMT, but further investigation is required to determine the exact mechanism[33]. Cluster of differentiation 166 (CD166) and CD146 are both important to maintain the transformative phenotype of HCC cells. CD166 positively regulates CD146 by inhibiting ubiquitin E3 ligases Smurf1 and β-transducin repeats-containing proteins through PI3K/AKT and c-Raf/MEK/ERK signaling in HCC cells[34].
1.5. Breast cancer
EMT in breast cancer is associated with CD146. In breast cancer, CD146 has also been linked to poor prognosis and tumor aggressiveness. Moreover, in receptor-specific negative breast cancer subtypes, such as triple-negative breast cancer (TNBC), CD146 is associated with tumors of higher grade and poorer prognosis. On the other hand, CD146 downregulation is associated with less aggressive phenotypes[35]. During breast cancer progression, aberrant promoter methylation may control CD146 gene expression[36]. S100A8/A9 binds to CD146 and accelerates breast cancer growth and metastasis. A novel transcription factor regulated by S100A8/A9-CD146 has been identified as ETV4. Breast cancer metastases are facilitated by CD146-mediated ETV4 activation through upregulation of ZEB1[37]. Through phospholipase D1, GTPases of the Ral family regulate multi-vesicular body homeostasis while tuning the biogenesis and secretion of pro-metastatic cancer extracellular vehicles (EVs). CD146 levels in EVs are reduced by RalA and RalB, enabling EVs to target the lungs and develop metastatic lesions there. RalA, RalB, and CD146 are indicators of poor prognosis in breast cancer patients[38]. Notch1 positively associates with CD146. Patients with basal-like/TNBC and high levels of Notch1 as well as CD146 have poor survival, particularly if they have received chemotherapy[39].
Interestingly, CD146 also acts as a tumor suppressor in breast carcinoma. Breast carcinomas rarely express CD146, but overexpression results in more cohesive cell growth and smaller tumors in nude mice[40]. CD146 suppresses breast cancer progression by targeting the CD44-downstream signaling pathway that regulates neovascularization and cancer cell motility[41]. SM3A regulates neuronilin-1-mediated PTEN-dependent FOXO 3a activation, which stimulates CD146 expression and suppresses breast cancer growth[42]. CD146 promotes tamoxifen resistance in breast cancer cells by causing EMT, reducing ER expression, and triggering AKT[43].
1.6. Renal cell carcinoma (RCC)
Clear cell RCC (ccRCC) patients exhibiting CD146 overexpression have poor prognoses. CD146 can be an independent prognostic factor. Among the co-expressed genes of CD146 are those related to differentiation of Th1 and Th2 cells, differentiation of Th17 cells, and migration of leukocytes across endothelium. Immune infiltration, immunomodulators, and chemokines are strongly correlated with the expression and methylation status of CD146. Furthermore, CD146 expression is linked to the sensitivity and resistance of renal cancer cell lines to certain drugs[44]. The expression level of CD146 gene can serve as an indicator for disease recurrence in cystic RCC (cRCC)[45]. Compared with other cancers and healthy tissues, the increased expression of CD146 in ccRCC vessels may be related to the induction of VEGF[46]. Wnt/β-catenin signaling is activated by insulin-like growth factor binding protein 4 (IGFBP-4), which induces CD146 expression in human RCC cells[47].
1.7. Pancreatic cancer
Pancreatic cancer progression is facilitated by a decrease in CD146 expression in cancer-associated fibroblasts (CAFs)[48]. Pancreatic cancer cells migrate in vitro more readily when they interact with the enhancer-DNA of CD146 and the myeloid ecotropic viral integration site (MEIS)[49]. There is a subset of CD34+ cells that express endothelial cell markers, such as CD146, when they differentiate. Angiogenesis-mediated tumor metastasis is further enhanced by the presence of the latter cell type in newly formed vessels[50].
1.8. Prostate cancer
Hypermethylation of the CD146 gene is often found in advanced prostate cancer[51]. Prostate cancer malignancy is associated with a lack of predominant cytoplasmic membrane expression of CD146[52]. A human prostate cancer cell with ectopic expression of CD146 is more likely to metastasize[53]. In vivo, increased expression of CD146 stimulates prostate tumorigenesis by promoting proliferation, activating the AKT survival pathway, and increasing prostate cancer’s angiogenic ability[6].
1.9. Gastrointestinal cancer
Activation of CD146 results in loss of E-cadherin and the increase in nuclear β-catenin and vimentin expression in mesenchymal cells. A poor prognosis may be associated with CD146 in gastric cancer because it may promote EMT and progression[54]. SRY-box transcription factor 18 (SOX18) triggers gastric cancer metastasis through the activation of CD146 and CCL7[55].
CD146 expression is correlated with histological grade, Duke’s stage, and liver metastasis. It is possible that CD146 is a biomarker for the postoperative liver metastases[56]. CAFs play an important role in colorectal cancer (CRC) progression, and their presence indicates poor prognosis in CRC patients. Pericryptal Lepr-lineage cells proliferate to generate CD146+ CAFs, which act as tumor-promoting immune cells in colorectal cancer. Mechanistically, CD146 enhances NF-κB-IL34/CCL8 signaling to promote macrophage chemotaxis when it interacts with interleukin-1 receptor 1[57]. A study has found that cancer stemness and tumorigenesis are promoted by the reduction of CD146 expression in colorectal cancer[58]. By regulating SPDL1 and CD146, myocardin-related transcription factor B (MRTFB) suppresses colorectal cancer development[59].
1.10. Uterine and adnexal tumors
CD146 is overexpressed in ovarian cancer[60]. The presence of CD146 may indicate ovarian carcinomas’ malignant potential. Increased signaling in anti-apoptosis, proliferation, survival pathways, and angiogenesis may be associated with ovarian cancer progression[61]. In serious ovarian carcinomas and epithelial ovarian cancers, CD146 expression is an independent prognostic factor for survival[62]. In ovarian cancer cells, CD146 protects them from apoptosis and promotes their invasion and metastasis by regulating the Rho signaling pathway[63]. Through a reduction in proliferation, aerobic glycolysis (metabolism) and angiogenesis, CD146 may suppress in vivo tumorigenesis and malignant progression of ovarian carcinoma cells, likely through the suppression of the PI3K-AKT signaling pathway[64]. A positive and significant correlation exists between CD146 expression and the pathological subtype of cervical cancer, as well as the degree of myometrial invasion in endometrial cancer. Cervical and endometrial cancers contain CD146 in the majority of blood vessels, suggesting that CD146 facilitates the metastasis of these cancers through the blood vessels[65].
1.11. Other cancer types
As nasopharyngeal carcinoma (NPC) develops, CD146 expression is reduced, but increased expression is necessary for metastatic growth This suggests that CD146 can act as both a tumor suppressor like TGF-β as well as a metastasis promoter for NPC[66]. A knockdown of CD146 with short hairpin RNA or treatment with an anti-CD146 polyclonal antibody can effectively inhibit neuroblastoma cell growth. They are halted by increased apoptosis via focal adhesion kinase and/or nuclear factor-kappa B signaling[67]. CD146 promotes osteosarcoma (OS) progression by mediating pro-tumoral and angiogenic effects[68].
The expression of CD146 is related to many transcription factors. Most mechanism of CD146 in tumor depends on AKT and ERK pathways, which mediate the adhesion of tumor cells to microvascular endothelial cells and promote metastasis. In addition, CD146 is associated with the cell cycle of tumor cells and drug resistance, even the mechanism is not particularly clear. Interestingly, a dual role of CD146 has been found in some tumors, for example, CD146 acts as both tumor suppressor like TGF-β and a metastasis promoter in breast cancer and nasopharyngeal carcinoma[66]. In short, the mechanism of CD146 needs more investigation.
2. CD146-targeted nuclear medicine imaging
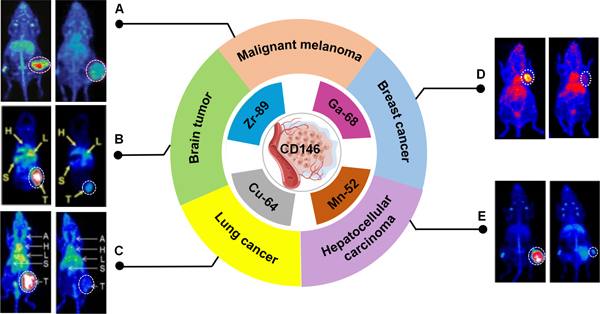
At present, CD146 targeted imaging is based on immunoPET by using radiolabeled CD146-targeted antibodies, including anti-CD146 monoclonal antibody (mAb), F(ab’)2 fragment, and scFv. Following standard procedures, antibodies are conjugated with appropriate chelating agents and radiolabeled with radionuclides. Serial PET/CT or SPECT/CT imaging is then performed in tumor models[35]. CD146 expression has been assessed via imaging in malignant melanoma, brain tumor, lung cancer, hepatocellular carcinoma, and breast cancer among other cancer types (Figure 2).
Figure 2. CD146-targeted imaging via nuclear medicine.
(A) 89Zr-Df-YY146 (left) and 89Zr-Df-IgG PET (right) imaging enable visualize CD146-expressing A375 xenografts clearly[69]. (B) PET images of 64Cu-NOTA-YY146 in mice containing U87MG (left) and those are preinjected with a blocking dose of unlabeled antibody (right)[27]. (C)PET Images of tumor-bearing mice derived from lung cancer. H460 (left) and H522 (right) tumors have shown the highest and lowest tracer accumulation, respectively[70]. (D) 64Cu-NOTA-YY146 PET imaging in MDA-MB-435 (left) and pre-blocking (right) tumor models[35]. (E) PET images of mice bearing HepG2 and mice pre-injected with a blocking dose of YY146[71]. There is a white dashed circle indicating the tumor’s location.
2.1. Breast cancer
Breast cancer accounts for the highest morbidity and the second highest cancer mortality in women[72]. Breast cancer patients with CD146 positive disease have a poor prognosis, especially if they have high-grade, estrogen receptor (ER) negative, progesterone receptor (PR) negative, and/or TNBC[73]. Moreover, CD146 is overexpressed in about 63.9% of TNBC patients [74]. Patients with primary breast cancer that overexpresses CD146 have significantly reduced overall survival, relapse-free survival, and distant metastasis-free survival[75]. Therefore, by evaluating the difference in expression of CD146, breast cancer can be diagnosed, staged, prognosticated, efficaciously evaluated, and drug-resistant evaluated.
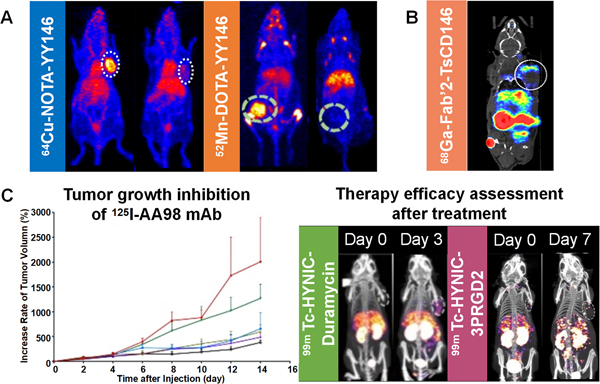
Ferreira et al.[76] labeled YY146 with manganese-52 (52Mn) radionuclide to construct a radiotracer 52Mn-DOTA-YY146 (Figure 3A). In MDA-MB-435 tumor model, the uptake of 52Mn-DOTA-YY146 increased gradually over time. On the other hand, the uptake was low in MCF-7 tumor due to the low expression of CD146. Li et al.[77] performed immunoPET imaging after administering 64Cu-NOTA-YY146 in an orthotopic breast cancer model. A maximum uptake of 14.7 ± 1.75 %ID/g was observed in MDA-MB-435 tumor model. This value was comparable to the MCF-7 group but was statistically significantly different from the non-specific 64Cu-NOTA-IgG control groups (P < 0.001). Furthermore, the uptake of 64Cu-NOTA-YY146 in the MDA-MB-435 lung metastatic tumor group was significantly higher than the MCF-7 tumor group (P < 0.001, Figure 3A). This indicates that the tumor uptake of YY146 is closely related to the expression level of CD146 in orthotopic, subcutaneous, and metastatic breast cancer models. YY146 can also be used as an immunoPET probe to non-invasively assess the distribution and expression of CD146. Thus, CD146-targeted imaging may provide benefits in clinical diagnosis, staging, prognosis prediction, and drug resistance assessment of breast cancer. It may also advance the clinical development of individualized treatment plans.
Figure 3. Theranostic application of three different molecules targeting CD146.
(A) Imaging of YY146 mAb labeled with 64Cu and 52Mn in a breast cancer model[35, 76]. (B) PET imaging of malignant melanoma mouse model injected with 68Ga Fab’2-TsCD146[1]. (C) Treatment of hepatocellular carcinoma by intratumoral injection of 125I-AA98 and subsequent therapy efficacy assessment by 99mTc-HYNIC-duramycin and 99mTc-HYNIC-3PRGD2 via SPECT/CT imaging[78].
2.2. Malignant melanoma
Malignant melanoma (MM) is a tumor produced by melanocytes and occurs mostly on the skin. CD146 was first found in melanoma cells. While CD146 is considered a marker of MM, it is generally not expressed in benign moles [79]. Therapeutic or imaging agents targeting CD146 may have higher specificity, lower toxicity, and better therapeutic efficacy than current conventional treatment modalities. Nollet et al.[1] developed antibodies that recognize the extracellular domain of CD146 and evaluated their ability to identify CD146 expressing cancer cells. These antibodies are known as TsCD146 mAbs (antiCD146 monoclonal antibodies) and Fab’2 fragments from TsCD146 mAb coupled with gallium-68 (68Ga). From the study, PET imaging of nude mice injected with 68Ga Fab’2-TsCD146 showed that the tumor could be visualized with radiolabeled Fab’2 fragments (Figure 3B). As compared with the IgG control group, the size of the tumor in the treatment group was significantly reduced by 50% after 46 days post injection. This finding suggests that the antibody fragments not only facilitate immunoPET imaging, but also exert some therapeutic effects. It is possible for cancer cells to release microparticles into the bloodstream of patients, and these microparticles contain many proteins from the mother cells. The authors found that this probe could detect CD146-positive tumor particles in the plasma of MM patients. The number of CD146-positive particles was also significantly higher in MM patients, and thus the new antibody could be used to detect CD146-positive tumors through liquid biopsies, a minimally invasive procedure that has gained a lot of attention in the clinical field. Wei et al.[69] generated a radiotracer (89Zr-DFO-YY146) by coupling anti-CD146 mAb (YY146) with zirconium-89 (89Zr) radionuclide using the deferoxamine (DFO) chelator. ImmunoPET imaging was performed on MM, and after 72 h, radiotracer uptake was obvious in CD146 overexpressing MM lesions with a statistically significant difference compared to the non-specific imaging agent 89Zr-DFO-IgG (P < 0.01). This finding demonstrates that PET imaging of 89Zr-DFO-YY146 can be used to assess the CD146 status in MM. All of the above studies indicate that CD146, as a targeting marker, can greatly improve the clinical management of MM.
2.3. Brain Tumor
According to the World Health Organization (WHO), glioblastoma (GBM) is the most common primary malignancy of the central nervous system (CNS) and has a median survival time of only 8 months [80]. Researchers have recently discovered that CD146 is overexpressed in high-grade gliomas and thus may serve as a viable therapeutic target.
Hernandez et al.[81] evaluated CD146 targeting in vivo using immunoPET imaging agent 89Zr-DFO-YY146 in athymic nude mice bearing subcutaneous U87MG or U251 tumors. In CD146-overexpressing U87MG mice, 89Zr-DFO-YY146 showed persistent tumor uptake with high specificity. A peak tumor uptake was achieved at 48 h after imaging agent injection. This result was statistically significantly different from the U251 mouse tumor model that has low CD146 expression (P < 0.05). In CD146 blocking experiments with U87MG-bearing mice, the authors corroborated that 89Zr-DFO-YY146 is highly specific toward CD146 expressing cells. Their results also validated the accuracy of PET imaging data. The tumor uptake values declined significantly in U87MG-bearing mice given YY146 at 50 mg/kg (P < 0.05). Since macromolecules cannot cross an intact blood-brain barrier (BBB), the effectiveness of antibody-based imaging and treatment in some brain diseases is greatly reduced. However, due to a disrupted BBB in GBM, copper-64 (64Cu) radionuclides coupled with 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA)-labeled YY146 (64Cu-NOTA-YY146) could pass through and accumulate persistently in U87MG tumors. A peak tumor uptake was reached at 48 h after injection of the imaging agent. Comparing with U87MG tumors, CD146-negative U251 tumors showed significantly lower tumor uptake (P < 0.001), while U87MG tumor uptake value was 37.3 times higher than surrounding normal brain tissue[27]. This demonstrates that radiotracer accumulation is highly associated with CD146 expression. Therefore, the expression level of CD146 in GBM can be evaluated noninvasively through immunoPET imaging.
2.4. Lung Cancer
Lung cancer has the second highest disease incidence (about 12.42%) and the highest mortality rate (about 21.67%) among all cancers [72]. More than 78% of patients have local or diffuse metastases at the time of initial diagnosis and most of them have a poor prognosis [28a]. To increase lung cancer survival rates, it is necessary to diagnose the disease early and treat it promptly. There is an urgent requirement for new approaches for early detection, staging, efficacy monitoring, and prognosis prediction of lung cancer. Several studies have reported that CD146 is closely related to the progression, metastasis, and prognosis of lung cancer[28, 31, 82]. Sun et al.[70] coupled YY146 with NOTA and radiolabeled with 64Cu. ImmunoPET imaging of 64Cu-NOTA-YY146 was performed in six subcutaneous xenograft tumor models of lung cancer with differing CD146 expression levels (A549, NCI-H522, NCI-H358, HCC4006, H23, and NCI-H460). The results showed that 64Cu-NOTA-YY146 has excellent targeting abilities and accumulates rapidly and persistently with high specificity toward CD146-expressing tumors. There was a positive correlation between 64Cu-NOTA-YY146 tumor uptake and CD146 expression levels (r2 = 0.98, P < 0.01), with the highest uptake value of 20.1±2.86 %ID/g in the H460 model that has an overexpression of CD146 proteins. On the other hand, only 4.10±0.98 %ID/g was found in the H522 model, which has a low CD146 expression. Further development of 64Cu-NOTA-YY146 may allow for improved stratification of lung cancer patients and enhanced monitoring of therapeutic response. Malignant cells are known to acquire metastatic potential and apoptotic resistance through EMT. Upon overexpression of CD146, epithelial markers are downregulated and mesenchymal markers are upregulated. CD146 has also been recognized as an ideal biomarker for lung cancer. By first performing in vitro assays, YY146 was determined to be well suited for CD146 immunoPET imaging in two models of intrapulmonary metastasis (H460 and H358) of non-small-cell lung cancer. Intrapulmonary metastases in the H460 model showed rapid, sustained, and specific radiotracer uptake, while reaching a peak uptake value at 48 hours after agent injection. The uptake was approximately 13.85 ± 1.07 %ID/g (n = 4), which was statistically significantly different from the control H358 model that exhibits a low CD146 expression (6.08 ± 0.73 %ID/g, P < 0.05)[83]. The above studies suggest that CD146-targeted immunoPET imaging not only non-invasively evaluates the differential expression of CD146 in primary lung cancer, but also enables the early detection of intrapulmonary metastases.
2.5. Hepatocellular carcinoma
The most common primary malignant tumor of the liver in adults is hepatocellular carcinoma (HCC). HCC ranks as the fifth leading cause of cancer death among men (about 6.34%) and seventh among women (about 3.52%)[84]. Metastasis and recurrence of HCC are the main causes of reduced patient survival, and around half of the patients diagnosed with advanced HCC cannot be resected or transplanted[85]. Previous research has shown that the expression of CD146 is significantly increased in liver cancer tissues and is an indicator of disease development, metastasis, and poor prognosis[86]. CD146 is a novel tumor marker for HCC and acts as a potential target for the diagnosis and treatment of HCC[32].
Hernandez et al.[71] constructed a radiotracer (89Zr-DFO-YY146) by conjugating 89Zr radionuclide to YY146 via the chelating agent DFO. After the administration of 89Zr-DFO-YY146, a statistically significant difference was found among the CD146-overexpressing HepG2 subcutaneous graft tumor model, Huh7 tumor group, and HepG2 blocking or low CD146 expression group (P < 0.001). Therefore, CD146 is a suitable target for noninvasive diagnosis and evaluation of HCC.
AA98 mAb is a promising mAb that acts against CD146 and has inhibitory effects on angiogenesis and tumor growth. Zhou et al.[78] labeled AA98 with iodine-125 (125I) and injected intratumorally to treat HCC xenograft lesions. To evaluate the treatment efficacy of this radioligand, apoptosis-targeted imaging with 99mTc-HYNIC-duramycin and angiogenesis-targeted imaging with 99mTc-HYNIC-3PRGD2 were employed (Figure 3C). The high dose group (200 μCi 125I-AA98 mAb) demonstrated the highest relative inhibitory rates of tumor (69.5%) and apoptotic index (11.8 ± 3.8%), indicating the potential of 125I-AA98 mAb to treat CD146-expressing HCC. In conclusion, CD146-targeted molecular imaging and therapy may bring tremendous value in the detection, prognosis prediction, and management of hepatic malignancies.
2.6. Other cancer types
This deadly disease occurs when the mesothelium, a protective layer surrounding most of the body’s internal organs, undergoes malignant transformation. Epithelioid mesothelioma, sarcomatoid mesothelioma, and mixed mesothelioma are the most common types. Studies of mesothelioma tissue microarrays revealed that CD146 is overexpressed in more than 80% of epithelioid and sarcomatous mesotheliomas, but not in normal mesothelium[87]. An et al.[88] selected several human scFvs from phage antibody libraries that are highly binding to the surface epitopes on mesothelioma cells that are clinically related to mesothelioma. They used technetium-99m (99mTc)-labeled anti-CD146 scFvs to detect tumor cells in mesothelioma organ xenografts in vivo using SPECT/CT. It was found that anti-CD146 M1 scFv accumulates more readily in mesothelioma xenografts than the control scFv at a higher level than surrounding soft tissues, thus demonstrating the potential utility using noninvasive imaging and immunotherapy to treat specific diseases[87].
CD146 has revealed distinguished performance in nuclear medicine imaging while many different forms of antibodies against CD146 have been studied (Table 1). To be consistent with the half-life of the whole antibody, most studies selected nuclides with relatively long half-lives, such as 52Mn, 64Cu, and 89Zr. However, some studies used 68Ga, which should be proper for Fab’2 fragments with a shorter half-life in the blood. Generally, the nuclide with a short half-life is more suitable for clinical use. Therefore, future research could tend to focus on antibody fragments using short half-life nuclides, whereas there are few studies about radionuclide therapy.
Table 1.
Different nuclear medicine tracers targeting CD146
| Types of cancer | Radionuclides | Imaging agents | Tumor models | References |
|---|---|---|---|---|
| Breast cancer | 52Mn | 52Mn-DOTA-YY146 | MDA-MB-435 orthotopic breast cancer | [76] |
| 64Cu | 64Cu-DOTA-YY146 | MDA-MB-435 orthotopic breast cancer | [35] | |
| Malignant melanoma | 68Ga | 68Ga-Fab’2 TsCD146 | C8161 xenograft melanoma | [1] |
| 89Zr | 89Zr-DFO-YY146 | A375 subcutaneous melanoma | [69] | |
| Brain tumor | 89Zr | 89Zr-DFO-YY146 | U87MG subcutaneous brain tumor | [81] |
| 64Cu | 64Cu-NOTA-YY146 | U87MG subcutaneous brain tumor | [27] | |
| Lung cancer | 64Cu | 64Cu-NOTA-YY146 | H460 subcutaneous lung cancer | [70] |
| 64Cu | 64Cu-NOTA-YY146 | H460 intrapulmonary metastasis model | [83] | |
| Hepatocellular carcinoma | 89Zr | 89Zr-DFO-YY146 | HepG2 subcutaneous liver tumor | [71] |
| Epithelioid mesothelioma | 99mTc | 99mTc-anti-CD146 scFv | Mesothelioma xenograft | [87] |
3. Concluding remarks and future directions
CD146 is overexpressed in a variety of malignancies and is closely associated with tumor aggressiveness, metastasis, poor prognosis, and drug resistance. CD146, therefore, is highly attractive for imaging and therapy of cancer. CD146-targeted mAbs or their fragments can be utilized in cancer immunoimaging to aid in the diagnosis, staging, and prognosis prediction of tumors, as well as provide a novel clinical imaging approach. CD146 is also a predictive marker of tumor angiogenesis. Consequently, CD146-targeted imaging has been explored to monitor the progression of angiogenesis reperfusion in vivo and track the recovery in mouse hindlimb ischemia models[89].
Some studies have used relatively long half-life nuclides to label antibodies targeted by CD146, including 89Zr, 64Cu, and 52Mn, which is consistent with the circulating time of antibodies in vivo. However, for clinical applications, short half-life nuclides and faster-circulating antibody fragments seem to be more popular and promising. A study has labeled Fab’2 antibody fragment targeting CD146 with a shorter half-life nuclide 68Ga. Although the antibody fragment still has the defect of insufficient tumor accumulation, this suggests that we should make more improvements in the future.
In terms of cancer theranostic applications, YY146 labeled with therapeutic radionuclides, e.g., lutetium-177 (177Lu), may represent a novel treatment for patients who have developed tolerance toward conventional therapies. Our research group has used 177Lu labeled anti-Trop2 and CD38-targeting monoclonal antibodies to treat pancreatic cancer and lymphoma, and confirmed that 177Lu-labeled antibodies have good efficacy in xenograft tumor models. Therefore, it is likely that 177Lu-labeled CD146-targeted antibody will also have good prospects but further studies should be performed[90]. Antibody-based imaging agents facilitate higher tumor uptake owing to their slow pharmacokinetics and long circulation time in vivo. On the other hand, the fast blood clearance of radiolabeled antibody fragments provides a better signal-to-noise ratio, resulting in higher quality images. The coupling of short half-life nucleophiles (e.g., 68Ga) necessitates same day immunoPET imaging, which may increase the likelihood for clinical implementation. Although current studies have been limited to small animal experiments, CD146 targeting proves to be a feasible strategy for cancer theranostics and holds great promise in furthering personalized precision medicine.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (82171970, 81871385), the Beijing Science Foundation for Distinguished Young Scholars (JQ21025), the Beijing Municipal Science & Technology Commission (Z221100007422027), the Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (BMU2022PY006), the University of Wisconsin - Madison, and the National Institutes of Health (P30 CA014520 and T32 CA009206).
Biographies

Qi Yang received the B.S. degree in Anesthesiology from Central South University, Hunan, China, in 2021. She is currently working toward the M.S. degree in Nuclear Medicine in the First Hospital of Peking University, Beijing, China. Her research interests include tumor-related immunoPET imaging and the integration of diagnosis and treatment.

Lei Kang received his MD and PhD degrees in Medical Imaging from Peking University in 2010 and he is now an associate professor, associate chief physician, and the vice director of the nuclear medicine department of Peking University First Hospital. He developed several radiolabeled antibodies, peptides, and nucleotides for the in vivo theranostics of malignant tumors for future clinical translation. He has published 107 papers (H-index 27), which include 80 papers (48 SCI-indexed articles, total impact factors 352) as first or corresponding authors (including co-authors). His articles have been published in Advanced Science, Nat Commun, Proc Natl Acad Sci, J Clin Invest, J Am Chem Soc, J Nucl Med, and Eur J Nucl Med Mol I. His studies have been supported by more than 18 grants from NSFC, the Chinese Education Ministry, Beijing Science, and Technology Commission. He has been awarded by Higher School Natural Science Outstanding Achievement Second Prize, Beijing Distinguished Youth Scientist, Beijing Nova Scientist, Xiaolun Youth Science Award, 2019 SNMMI CMIIT (Center for Molecular Imaging Innovation & Translation) YIA First Place, Hope Star Award of Chinese Nuclear Medicine Society and Peking University Outstanding Doctoral Dissertation. He has been granted 1 patent from US and 5 patents from China. He has given more than 30 oral presentations and posters at top international academic conferences, such as SNMMI and EANM annual meetings.

Weibo Cai received his BS degree from Nanjing University in 1995 and a PhD degree in Chemistry from the University of California at San Diego in 2004. After three years of post-doctoral training at the Molecular Imaging Program at Stanford University, he joined the University of Wisconsin - Madison as a Biomedical Engineering Cluster Hire in February 2008. Dr. Cai is currently a Vilas Distinguished Achievement Professor with joint appointment in the Departments of Radiology and Medical Physics, as well as affiliation with Materials Science & Engineering, and Pharmaceutical Sciences. Prof. Cai is currently a member of the UW Carbone Cancer Center (UWCCC), UW Stem Cell & Regenerative Medicine Center (UW-SCRMC), UW Cardiovascular Research Center (UW-CVRC), and the UW Institute for Clinical and Translational Research (UW-ICTR). Prof. Cai is currently the Editor-in-Chief of the American Journal of Nuclear Medicine and Molecular Imaging, and the Journal of Nanobiotechnology (Impact Factor 9.429). In addition, he is also an Associate Editor for the European Journal of Nuclear Medicine and Molecular Imaging (Impact Factor 10.057).
Footnotes
Conflict of Interest
Weibo Cai is a scientific advisor, stockholder, and grantee of Focus-X Therapeutics, Inc.; a consultant and grantee of Actithera, Inc.; a consultant of Rad Source Technologies, Inc.; a scientific advisor of Portrai, Inc.; and a scientific advisor and stockholder rTR Technovation Corporation. All other authors declare no conflict of interest.
References
- 1.Nollet M, Stalin J, Moyon A, Traboulsi W, Essaadi A, Robert S, Malissen N, Bachelier R, Daniel L, Foucault-Bertaud A, Gaudy-Marqueste C, Lacroix R, Leroyer AS, Guillet B, Bardin N, Dignat-George F, Blot-Chabaud M, Oncotarget 2017, 8, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stalin J, Nollet M, Dignat-George F, Bardin N, Blot-Chabaud M, Antibodies (Basel) 2017, 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Yan X, Cancer Lett. 2013, 330, 2. [DOI] [PubMed] [Google Scholar]
- 4.Joshkon A, Heim X, Dubrou C, Bachelier R, Traboulsi W, Stalin J, Fayyad-Kazan H, Badran B, Foucault-Bertaud A, Leroyer AS, Bardin N, Blot-Chabaud M, Biomedicines 2020, 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.aHyun KA, Koo GB, Han H, Sohn J, Choi W, Kim SI, Jung HI, Kim YS, Oncotarget 2016, 7, 17; [DOI] [PMC free article] [PubMed] [Google Scholar]; bYu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S, Science 2013, 339, 6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu GJ, Wu MW, Wang C, Liu Y, J. Urol. 2011, 185, 4. [DOI] [PubMed] [Google Scholar]
- 7.Kebir A, Harhouri K, Guillet B, Liu JW, Foucault-Bertaud A, Lamy E, Kaspi E, Elganfoud N, Vely F, Sabatier F, Sampol J, Pisano P, Kruithof EK, Bardin N, Dignat-George F, Blot-Chabaud M, Circ. Res 2010, 107, 1. [DOI] [PubMed] [Google Scholar]
- 8.Anderson CJ, Lewis JS, Philos Trans A Math Phys Eng Sci 2017, 375, 2107. [DOI] [PubMed] [Google Scholar]
- 9.aAlcantara D, Leal MP, Garcia-Bocanegra I, Garcia-Martin ML, Front Chem 2014, 2; [DOI] [PMC free article] [PubMed] [Google Scholar]; bKang L, Li C, Rosenkrans ZT, Engle JW, Wang R, Jiang D, Xu X, Cai W, J Nucl Med 2021, 62, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang T, Zhuang J, Duan H, Luo Y, Zeng Q, Fan K, Yan H, Lu D, Ye Z, Hao J, Feng J, Yang D, Yan X, Blood 2012, 120, 11. [DOI] [PubMed] [Google Scholar]
- 11.Zheng C, Qiu Y, Zeng Q, Zhang Y, Lu D, Yang D, Feng J, Yan X, Int. J. Biochem. Cell Biol 2009, 41, 11. [DOI] [PubMed] [Google Scholar]
- 12.Ye Z, Zhang C, Tu T, Sun M, Liu D, Lu D, Feng J, Yang D, Liu F, Yan X, Nat Commun 2013, 4. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Luo Y, Teng X, Wu Z, Li M, Xu D, Wang Q, Wang F, Feng J, Zeng X, Yan X, Protein Cell 2018, 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu T, Zhang C, Yan H, Luo Y, Kong R, Wen P, Ye Z, Chen J, Feng J, Liu F, Wu JY, Yan X, Cell Res. 2015, 25, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Q, Zhang J, Wang X, Liu Y, He R, Liu X, Wang F, Feng J, Yang D, Wang Z, Meng A, Yan X, Nat Commun 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y, Zhang H, Xiong C, Liu Z, Xu Q, Feng J, Zhang J, Wang Z, Yan X, Cancer Lett. 2018, 430. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JP, Bar-Eli M, Jansen B, Markhof E, Int. J. Cancer 1997, 73, 5. [DOI] [PubMed] [Google Scholar]
- 18.Ruma IM, Putranto EW, Kondo E, Murata H, Watanabe M, Huang P, Kinoshita R, Futami J, Inoue Y, Yamauchi A, Sumardika IW, Youyi C, Yamamoto K, Nasu Y, Nishibori M, Hibino T, Sakaguchi M, Clin. Exp. Metastasis 2016, 33, 6. [DOI] [PubMed] [Google Scholar]
- 19.Jouve N, Bachelier R, Despoix N, Blin MG, Matinzadeh MK, Poitevin S, Aurrand-Lions M, Fallague K, Bardin N, Blot-Chabaud M, Vely F, Dignat-George F, Leroyer AS, Int. J. Cancer 2015, 137, 1. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Q, Wu Z, Duan H, Jiang X, Tu T, Lu D, Luo Y, Wang P, Song L, Feng J, Yang D, Yan X, Protein Cell 2014, 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zigler M, Villares GJ, Dobroff AS, Wang H, Huang L, Braeuer RR, Kamiya T, Melnikova VO, Song R, Friedman R, Alani RM, Bar-Eli M, Cancer Res. 2011, 71, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa T, Wondimu Z, Oikawa Y, Gentilcore G, Kiessling R, Egyhazi Brage S, Hansson J, Patarroyo M, Matrix Biol. 2014, 38. [DOI] [PubMed] [Google Scholar]
- 23.Melnikova VO, Balasubramanian K, Villares GJ, Dobroff AS, Zigler M, Wang H, Petersson F, Price JE, Schroit A, Prieto VG, Hung MC, Bar-Eli M, J. Biol. Chem 2009, 284, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumardika IW, Youyi C, Kondo E, Inoue Y, Ruma IMW, Murata H, Kinoshita R, Yamamoto KI, Tomida S, Shien K, Sato H, Yamauchi A, Futami J, Putranto EW, Hibino T, Toyooka S, Nishibori M, Sakaguchi M, Oncol. Res 2018, 26, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams B, Schneider RJ, Jamal S, Melanoma Res. 2014, 24, 4. [DOI] [PubMed] [Google Scholar]
- 26.Yawata T, Higashi Y, Kawanishi Y, Nakajo T, Fukui N, Fukuda H, Ueba T, J. Neurooncol 2019, 144, 1. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Hernandez R, Rao J, Yin L, Qu Y, Wu J, England CG, Graves SA, Lewis CM, Wang P, Meyerand ME, Nickles RJ, Bian XW, Cai W, Proc. Natl. Acad. Sci. U. S. A 2015, 112, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.a Zhang X, Wang Z, Kang Y, Li X, Ma X, Ma L, Clin. Transl. Oncol 2014, 16, 2; [DOI] [PubMed] [Google Scholar]; b Oka S, Uramoto H, Chikaishi Y, Tanaka F, Anticancer Res. 2012, 32, 3. [PubMed] [Google Scholar]
- 29.Piao Y, Guo H, Qu Z, Zheng B, Gao Y, Oncol. Lett 2019, 17, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang F, Wang J, Wang X, Wei N, Liu H, Zhang X, Clin. Respir. J 2019, 13, 1. [DOI] [PubMed] [Google Scholar]
- 31.Tripathi SC, Fahrmann JF, Celiktas M, Aguilar M, Marini KD, Jolly MK, Katayama H, Wang H, Murage EN, Dennison JB, Watkins DN, Levine H, Ostrin EJ, Taguchi A, Hanash SM, Cancer Res. 2017, 77, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Tang X, Weng W, Qiao Y, Lin J, Liu W, Liu R, Ma L, Yu W, Yu Y, Pan Q, Sun F, Oncogene 2015, 34, 47. [DOI] [PubMed] [Google Scholar]
- 33.Jiang G, Zhang L, Zhu Q, Bai D, Zhang C, Wang X, J. Exp. Clin. Cancer Res 2016, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang X, Chen X, Xu Y, Qiao Y, Zhang X, Wang Y, Guan Y, Sun F, Wang J, Cell. Signal 2015, 27, 9. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Kang L, Fan K, Ferreira CA, Becker KV, Huo N, Liu H, Yang Y, Engle JW, Wang R, Xu X, Jiang D, Cai W, Bioconjug. Chem 2021, 32, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudzik P, Trojan SE, Ostrowska B, Lasota M, Dulińska-Litewka J, Laidler P, Kocemba-Pilarczyk KA, Acta Biochim. Pol 2019, 66, 4. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Sumardika IW, Tomonobu N, Kinoshita R, Inoue Y, Iioka H, Mitsui Y, Saito K, Ruma IMW, Sato H, Yamauchi A, Murata H, Yamamoto KI, Tomida S, Shien K, Yamamoto H, Soh J, Futami J, Kubo M, Putranto EW, Murakami T, Liu M, Hibino T, Nishibori M, Kondo E, Toyooka S, Sakaguchi M, Neoplasia 2019, 21, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghoroghi S, Mary B, Larnicol A, Asokan N, Klein A, Osmani N, Busnelli I, Delalande F, Paul N, Halary S, Gros F, Fouillen L, Haeberle AM, Royer C, Spiegelhalter C, André-Grégoire G, Mittelheisser V, Detappe A, Murphy K, Timpson P, Carapito R, Blot-Chabaud M, Gavard J, Carapito C, Vitale N, Lefebvre O, Goetz JG, Hyenne V, eLife 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng D, Liang YK, Xiao YS, Wei XL, Lin HY, Wu Y, Bai JW, Chen M, Zhang GJ, Int. J. Cancer 2020, 147, 2. [DOI] [PubMed] [Google Scholar]
- 40.Shih IM, J. Pathol 1999, 189, 1. [DOI] [PubMed] [Google Scholar]
- 41.Ouhtit A, Abdraboh ME, Hollenbach AD, Zayed H, Raj MHG, Cell Commun Signal 2017, 15, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mishra R, Thorat D, Soundararajan G, Pradhan SJ, Chakraborty G, Lohite K, Karnik S, Kundu GC, Oncogene 2015, 34, 12. [DOI] [PubMed] [Google Scholar]
- 43.Liang YK, Zeng D, Xiao YS, Wu Y, Ouyang YX, Chen M, Li YC, Lin HY, Wei XL, Zhang YQ, Kruyt FA, Zhang GJ, Cancer Lett. 2017, 386. [DOI] [PubMed] [Google Scholar]
- 44.Lv Z, Feng HY, Tao W, Li HZ, Zhang X, Front. Oncol 2021, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng G, Fang F, Liu C, Zhang F, Huang H, Pu C, Int. Urol. Nephrol 2012, 44, 6. [DOI] [PubMed] [Google Scholar]
- 46.Wragg JW, Finnity JP, Anderson JA, Ferguson HJ, Porfiri E, Bhatt RI, Murray PG, Heath VL, Bicknell R, Cancer Res. 2016, 76, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueno K, Hirata H, Majid S, Tabatabai ZL, Hinoda Y, Dahiya R, Int. J. Cancer 2011, 129, 10. [DOI] [PubMed] [Google Scholar]
- 48.Zheng B, Ohuchida K, Chijiiwa Y, Zhao M, Mizuuchi Y, Cui L, Horioka K, Ohtsuka T, Mizumoto K, Oda Y, Hashizume M, Nakamura M, Tanaka M, Mol. Carcinog 2016, 55, 11. [DOI] [PubMed] [Google Scholar]
- 49.von Burstin J, Bachhuber F, Paul M, Schmid RM, Rustgi AK, Mol. Carcinog 2017, 56, 3. [DOI] [PubMed] [Google Scholar]
- 50.Vizio B, Biasi F, Scirelli T, Novarino A, Prati A, Ciuffreda L, Montrucchio G, Poli G, Bellone G, J. Transl. Med 2013, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu JW, Nagpal JK, Jeronimo C, Lee JE, Henrique R, Kim MS, Ostrow KL, Yamashita K, van Criekinge V, Wu G, Moon CS, Trink B, Sidransky D, Prostate 2008, 68, 4. [DOI] [PubMed] [Google Scholar]
- 52.Wu GJ, Wu MW, Wang SW, Liu Z, Qu P, Peng Q, Yang H, Varma VA, Sun QC, Petros JA, Lim SD, Amin MB, Gene 2001, 279, 1. [DOI] [PubMed] [Google Scholar]
- 53.Wu GJ, Peng Q, Fu P, Wang SW, Chiang CF, Dillehay DL, Wu MW, Gene 2004, 327, 2. [DOI] [PubMed] [Google Scholar]
- 54.Liu WF, Ji SR, Sun JJ, Zhang Y, Liu ZY, Liang AB, Zeng HZ, Int. J. Mol. Sci 2012, 13, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J, Dang Y, Feng W, Qiao C, Liu D, Zhang T, Wang Y, Tian D, Fan D, Nie Y, Wu K, Xia L, Oncogene 2020, 39, 33. [DOI] [PubMed] [Google Scholar]
- 56.Tian B, Zhang Y, Li N, Cancer Biother. Radiopharm 2013, 28, 6. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi H, Gieniec KA, Lannagan TRM, Wang T, Asai N, Mizutani Y, Iida T, Ando R, Thomas EM, Sakai A, Suzuki N, Ichinose M, Wright JA, Vrbanac L, Ng JQ, Goyne J, Radford G, Lawrence MJ, Sammour T, Hayakawa Y, Klebe S, Shin AE, Asfaha S, Bettington ML, Rieder F, Arpaia N, Danino T, Butler LM, Burt AD, Leedham SJ, Rustgi AK, Mukherjee S, Takahashi M, Wang TC, Enomoto A, Woods SL, Worthley DL, Gastroenterology 2022, 162, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu D, Du L, Chen D, Ye Z, Duan H, Tu T, Feng J, Yang Y, Chen Q, Yan X, Oncotarget 2016, 7, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kodama T, Marian TA, Lee H, Kodama M, Li J, Parmacek MS, Jenkins NA, Copeland NG, Wei Z, Proc. Natl. Acad. Sci. U. S. A 2019, 116, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou P, Xiong T, Chen J, Li F, Qi T, Yuan J, Oncol. Lett 2019, 17, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu GJ, Dickerson EB, Taiwan. J. Obstet. Gynecol 2014, 53, 4. [DOI] [PubMed] [Google Scholar]
- 62.Onisim A, Vlad C, Simon I, Dina C, Achimas Cadariu P, J. BUON 2019, 24, 3. [PubMed] [Google Scholar]
- 63.Wu Z, Wu Z, Li J, Yang X, Wang Y, Yu Y, Ye J, Xu C, Qin W, Zhang Z, Tumour Biol. 2012, 33, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu GJ, Adv. Exp. Med. Biol 2021, 1330. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Zhang J, Wang Z, Lu D, Feng J, Yang D, Chen X, Yan X, Oncol. Lett 2013, 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin JC, Chiang CF, Wang SW, Wang WY, Kwan PC, Wu GJ, Asian Pac. J. Cancer Prev 2014, 15, 1. [DOI] [PubMed] [Google Scholar]
- 67.Obu S, Umeda K, Ueno H, Sonoda M, Tasaka K, Ogata H, Kouzuki K, Nodomi S, Saida S, Kato I, Hiramatsu H, Okamoto T, Ogawa E, Okajima H, Morita K, Kamikubo Y, Kawaguchi K, Watanabe K, Iwafuchi H, Yagyu S, Iehara T, Hosoi H, Nakahata T, Adachi S, Uemoto S, Heike T, Takita J, Cancer Sci. 2021, 112, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lei X, Wang K, Wang W, Jin H, Gu W, Chen Z, Wang W, Gao K, Wang H, Cancer Cell Int. 2021, 21, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei W, Jiang D, Ehlerding EB, Barnhart TE, Yang Y, Engle JW, Luo QY, Huang P, Cai W, Adv Sci (Weinh) 2019, 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun H, England CG, Hernandez R, Graves SA, Majewski RL, Kamkaew A, Jiang D, Barnhart TE, Yang Y, Cai W, Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hernandez R, Sun H, England CG, Valdovinos HF, Ehlerding EB, Barnhart TE, Yang Y, Cai W, Theranostics 2016, 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siegel RL, Miller KD, Fuchs HE, Jemal A, CA: A Cancer Journal for Clinicians 2021, 71, 1. [DOI] [PubMed] [Google Scholar]
- 73.Zabouo G, Imbert AM, Jacquemier J, Finetti P, Moreau T, Esterni B, Birnbaum D, Bertucci F, Chabannon C, Breast Cancer Res 2009, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Kruijff IE, Timmermans AM, den Bakker MA, Trapman-Jansen A, Foekens R, Meijer-Van Gelder ME, Oomen-de Hoop E, Smid M, Hollestelle A, van Deurzen CHM, Foekens JA, Martens JWM, Sleijfer S, Cancers (Basel) 2018, 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.aLiang YK, Zeng YS Xiao Y.Wu, Ouyang YX, Chen M, Li YC, Lin HY, Wei XL, Zhang YQ, Kruyt FA, Zhang GJ, Cancer Lett 2017, 386; [DOI] [PubMed] [Google Scholar]; bTripathi SC, Fahrmann JF, Celiktas M, Aguilar M, Marini KD, Jolly MK, Katayama H, Wang H, Murage EN, Dennison JB, Watkins DN, Levine H, Ostrin EJ, Taguchi A, Hanash SM, Cancer Research 2017, 77, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferreira CA, Kang L, Li C, Kamkaew A, Barrett KE, Aluicio-Sarduy E, Yang Y, Engle JW, Jiang D, Cai W, Am. J. Cancer Res 2021, 11, 4. [PMC free article] [PubMed] [Google Scholar]
- 77.Li C, Kang L, Fan K, Ferreira CA, Becker KV, Huo N, Liu H, Yang Y, Engle JW, Wang R, Xu X, Jiang D, Cai W, Bioconjug Chem 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou J, Hu P, Si Z, Tan H, Qiu L, Zhang H, Fu Z, Mao W, Cheng D, Shi H, Front Bioeng Biotechnol 2019, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lei X, Guan CW, Song Y, Wang H, Cancer Cell Int 2015, 15, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wirsching HG, Galanis E, Weller M, Handb Clin Neurol 2016, 134. [DOI] [PubMed] [Google Scholar]
- 81.Hernandez R, Sun H, England CG, Valdovinos HF, Barnhart TE, Yang Y, Cai W, Mol. Pharm 2016, 13, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kristiansen G, Yu Y, Schlüns K, Sers C, Dietel M, Petersen I, Anal. Cell. Pathol 2003, 25, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.England CG, Jiang D, Hernandez R, Sun H, Valdovinos HF, Ehlerding EB, Engle JW, Yang Y, Huang P, Cai W, Mol. Pharm 2017, 14, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siegel RL, Miller KD, Fuchs HE, Jemal A, CA Cancer J Clin 2022, 72, 1. [DOI] [PubMed] [Google Scholar]
- 85.Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O’Neil B, Poon R, Schwartz L, Tepper J, Yao F, Haller D, Mooney M, Venook A, J Clin Oncol 2010, 28, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qiao Y, Qian Y, Wang J, Tang X, Oncol Lett 2016, 11, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bidlingmaier S, He J, Wang Y, An F, Feng J, Barbone D, Gao D, Franc B, Broaddus VC, Liu B, Cancer Res. 2009, 69, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.An F, Drummond DC, Wilson S, Kirpotin DB, Nishimura SL, Broaddus VC, Liu B, Mol. Cancer Ther 2008, 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferreira CA, Hernandez R, Yang Y, Valdovinos HF, Engle JW, Cai W, Mol. Pharm 2018, 15, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.aKang L, Li C, Rosenkrans ZT, Huo N, Chen Z, Ehlerding EB, Huo Y, Ferreira CA, Barnhart TE, Engle JW, Wang R, Jiang D, Xu X, Cai W, Adv Sci (Weinh) 2021, 8, 10; [DOI] [PMC free article] [PubMed] [Google Scholar]; bLi C, Liu J, Yang X, Yang Q, Huang W, Zhang M, Zhou D, Wang R, Gong J, Miao Q, Kang L, Yang J, Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 1. [DOI] [PubMed] [Google Scholar]