Abstract
Background
Presbycusis/Age-related hearing loss is a sensorineural hearing loss caused by age-related deterioration of the auditory system that poses a risk to the physical and mental health of older people, including social and cognitive decline. It is also associated with frailty, falls and depression. There are currently no specific medications for the treatment of presbycusis, and early detection and intervention are key to its prevention and management. Traditional Chinese medicine interventions may offer opportunities in the prevention and treatment of presbycusis, but there is no relevant review.
Methods
Literature searches was conducted using PubMed, Cochrane Library, Web of Science, and China National Knowledge Infrastructure (CNKI) databases for review articles, research articles, clinical trials, meta-analyses, and case studies in animal models and clinical trials.
Results
We summarized the pathological mechanisms associated with presbycusis, related to genetic factors, environment, lifestyle, and molecular mechanisms related to oxidative stress, mitochondrial dysfunction, and inflammatory pathways. It is suggested that traditional Chinese medicine interventions may offer opportunities in the prevention and treatment of presbycusis using active ingredients of herbs or formulas, acupuncture, and exercise such as Tai Chi Chuan or Ba Duan Jin. The active ingredients of herbs or formulas may exert ear protection through Nrf2-mediated antioxidant pathways, NF-kB and NLRP3-related anti-inflammatory signaling, and regulation of autophagy.
Conclusions
Here, we review the pathogenetic factors and pathological mechanisms involved in presbycusis, as well as traditional Chinese medicine interventions and treatments, with the aim of providing a new perspective for the prevention and treatment of hearing loss in the elderly and further improving their quality of life.
Keywords: Traditional Chinese medicine, Age-related hearing loss, Presbycusis, Mechanism
1. Introduction
The World Report on Hearing 2021 estimates that more than 1.5 billion people already have varying degrees of hearing loss, which could increase to 2.5 billion by 2050. The global prevalence of moderate or greater hearing loss increases with age, affecting more than 58 % of adults over 60 years of age, and the prevalence of some degree of hearing loss among males (5.6 %) is slightly higher than that among females (5.5 %) [1]. Although this sensory disorder is not life threatening in the older population, it affects the psychosocial well-being and quality of life of individuals. Increasing evidence links falls, social isolation and depression, and cognitive impairment with loss of hearing [[2], [3], [4]]. Recent studies have identified age-related hearing loss (ARHL) as a high-risk factor for cognitive decline, including dementia and Alzheimer's disease (AD) [[5], [6], [7]]. It therefore represents a significant social and economic burden that is increasing with current demographic changes.
Presbycusis, also known as ARHL, is a sensorineural hearing loss (SNHL) caused by degeneration of the auditory system with increasing age [8]. It is a progressive, bilateral, symmetrical hearing loss, and its hearing curve is mostly slope-shaped at higher frequencies and sometimes flat [9]. Historical studies have shown that there are six pathological types of presbycusis: sensory, neural, metabolic, cochlear conductive, mixed, and indeterminate. These involve alterations in many auditory structures, including degeneration of cochlear hair cells, vascular striae atrophy, and degeneration of the auditory nerve [10,11]. Until now, the etiology of presbycusis remains unclear. Some studies showed that oxidative stress, apoptosis, mtDNA mutations and autophagy are involved in the progression of presbycusis [[12], [13], [14]].
Up until recently, there were no therapeutic treatments available to save the dying cochlear hair cells and SGNs or to replenish these cells once they had been lost. To prevent or reverse the presbycusis, however, great efforts have been undertaken to find innovative therapies. Due to its lengthy history of preventing and postponing age-related disorders, Traditional Chinese Medicine (TCM) is seen as a potential resource. The primary causes of presbycusis and how it is treated by TCM will be outlined in this review, which will serve as a resource or inspiration for future studies on presbycusis and TCM.
2. Methodology
The literature search was conducted using PubMed, Cochrane Library, Web of Science, and China National Knowledge Infrastructure (CNKI) databases, using the keywords "presbycusis, " "age-related hearing loss, " "age-related deafness, " "aging, " and "ageing, " "traditional Chinese medicine, " "traditional medicine, " "herbal medicine, " "medicinal plants, " "plants, " "ethnopharmacology, " and "ethnomedicine ". Boolean operators ‘OR’ and ‘AND’ were used to narrow and target the search. The inclusion criteria were as follows: articles published in the last 15 years for review articles, research articles, clinical trials, meta-analyses, and case studies.
3. Pathogenetic factors and pathological mechanisms of presbycusis
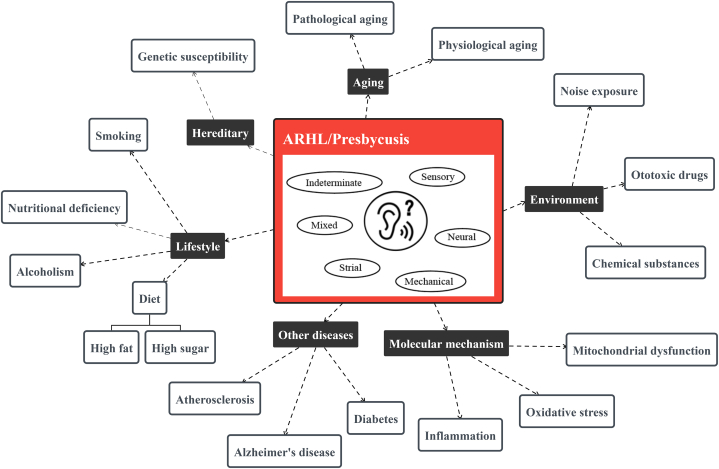
The occurrence and development of presbycusis are multifactorial. In addition to age-related degeneration, other contributing factors, such as genetic factors, the environment, lifestyle, and diseases of other organ systems, can accelerate the progression of presbycusis (Fig. 1).
Fig. 1.
Influencing factors involved in the pathogenesis of presbycusis.
3.1. Genetic factors for presbycusis
Hearing loss is genetically heterogeneous, and there are approximately 1000 different pathogenic genes, of which 140 have been shown to cause human deafness [15,16]. The genetic architecture of age-related hearing impairment (ARHI) has recently been revealed by genome-wide association analysis, which found that carriers of rare ARHI variants were at higher risk according to the ARHI Genetic Risk Score (GRS), and that rare variants were more likely to develop severe ARHI than combinations of common GRS variants [17]. Genetic factors can also influence hearing loss among middle-aged and elderly in the middle and high frequencies, with heritability of approximately 40–55 % for PTA and better ear hearing level at 2, 4, 8 and 12.5 kHz in 358 twins and 1 triplet (mean age 51.55 years) studied [18]. In addition, many new epigenetic markers have been identified that contribute to further understanding the pathological mechanisms underlying presbycusis. In a clinical study, elevated levels of methylation at the CpG site of Cadherin 23 gene were found to correlate with ARHI in the peripheral blood of 50 women aged 50–75 years with presbycusis [19]. Animal experiments have shown that deletion of Ceacam16 accelerates age-related tectorial membrane degeneration in the mouse cochlea, and the extent of this degeneration depends on the genetic background [20]. Hearing loss is a common symptom in individuals harboring inherited mitochondrial DNA (mtDNA) mutations. In the inner ear of mitochondrial mutator mice (Polgmut/mut), mtDNA deletions accumulate significantly with increasing age [21]. BAK1 gene expression and BAK1/BCL2 ratio in peripheral blood may be biomarkers for Iranian presbycusis [22]. CCR3 and GILZ genes may be biomarkers for Chinese presbycusis subjects by our previous research [23]. The relationship between miRNAs and presbycusis was discovered by Jianguo Tang's research group. They summarized the following miRNAs as miRNA-183, miRNA-181a, miR-34a, Let-7 family, miR-29b/SIRT1/PGC-1α,95 and 60 miRNAs may be important for the presbycusis [13].
3.2. Environmental factors for presbycusis
Multiple genetic factors are inextricably linked to the risk of various diseases, and environmental factors exacerbate this risk. A previous study showed that presbycusis and noise-induced hearing loss are determined by the interaction between environmental and genetic factors [24]. The risk of hearing loss in different work environments is related to the level of noise exposure and hearing protective equipment, with the highest risk of hearing loss in industry, shipbuilding, construction, military, and farming [25]. A study based on a Canadian population(aged 30–100)found that long-term noise exposure increased the severity of presbycusis and prevalence of tinnitus [26]. Continuous exposure of 2-month-old C57BL/6J mice to noise resulted in increased auditory thresholds at 6 months of age, morphological damage to the spiral ganglion neurons (SGNs) of the cochlea, and a reduction in the number of banded synapses in the inner hair cells [27].
Some ototoxic medications are potentially associated with the incidence of presbycusis and disease progression. A population-based longitudinal study showed that older adult participants taking loop diuretics or nonsteroidal anti-inflammatory drugs had an increased risk and severity of hearing loss over a 10-year period [28]. It has also been shown that hearing loss in Fischer 344/NHsd rats exposed to cisplatin worsens with age [29]. In addition, there are potentially ototoxic chemicals in some workplaces, such as occupational exposure to organic solvents or metallic elements, which can lead to varying degrees of hearing loss [30]. A study of 1117 workers exposed to solvents (xylene, styrene, a mixture of n-hexane and toluene) alone or in combination with noise found that exposure to organic agents exacerbated noise-induced hearing loss and that co-exposure to both greatly increased the incidence of hearing loss [31]. An experiment exposing rats to noise or styrene found that noise and SGNs might aggravate presbycusis [32]. Manganese, a neurotoxic element associated with age-related diseases, was found to accelerate presbycusis in young adult mice in studies in which it mediated ototoxicity, a mechanism of action associated with neurodegenerative lesions in mouse SGNs caused by impaired c-Ret [33].
3.3. Lifestyle factors for presbycusis
Lifestyle is also a major factor that influences presbycusis. Healthy dietary patterns can help reduce the risk of hearing loss [34]. However, bad food habits, such as the consumption of high-sugar or fatty foods, smoking, and alcohol abuse, may drive the progression of presbycusis. Compared with non-smokers or former smokers, hearing loss in smokers is further aggravated, and there is a need for tight control of low-density lipoprotein cholesterol [35]. A population-based cross-sectional analysis revealed that the dietary intake of cholesterol and monounsaturated fats may be considered as potential risk factors for hearing loss among older adults [36]. High-fat dietary intake is one of the main triggers of atherosclerosis, and poorer hearing may be due to lower cochlear blood flow in elderly patients with carotid atherosclerosis which was speculated in a cross-sectional cohort study [37]. A prospective cohort study in southern Italy observed that sugar-rich beverages (fruit juices) increased serum triglyceride levels, which may affect hearing function [38]. In a study on the association between presbycusis and nutrition, a higher intake of riboflavin, niacin, and retinol in 4742 participants was negatively correlated with the prevalence of presbycusis [39]. Smoking may aggravate presbycusis in patients with diabetes [40]. Therefore, healthy eating habits, such as reduced sugar and oil consumption, should be maintained, and malpractices, such as smoking and alcohol consumption, should be reduced to reduce the incidence of presbycusis.
3.4. Molecular mechanisms and related pathways of generation and progression for presbycusis
During the pathological process of presbycusis, cochlear dysfunction includes sensory cell loss, atrophy of the stria vascularis, and loss of the SGNs, with associated mechanisms involving oxidative stress, mitochondrial dysfunction and inflammation [41].
3.4.1. Correlation of oxidative stress and mitochondrial dysfunction with presbycusis
Oxidative stress is thought to be an important contributor to aging. Nicotinamide adenine dinucleotide phosphate (NADPH) is an important cofactor in the antioxidant system. NADPH oxidase (NOX) acts as an important source of ROS, and NOX activation can lead to presbycusis, whereas deficiency of the NOX subunit p22phox prevents age-related lesions of the auditory system [42].
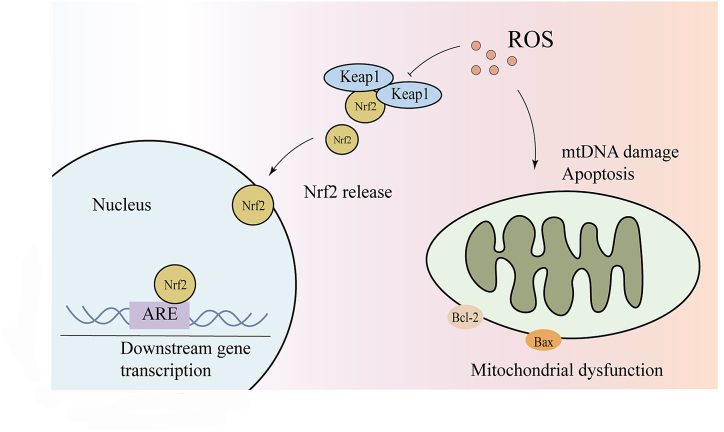
ROS are one of the stressors induced by nuclear factor-E2-related factor 2 (Nrf2), a key transcription factor that mediates the oxidative stress signaling pathway and plays a critical role in maintaining redox homeostasis and preventing oxidative damage (Fig. 2). Nrf2 has been reported to be localized mainly in the inner and outer hair cells and supporting cells of the organ of Corti, as well as in the vestibular sensory epithelium. Nrf2 immunoreactivity is reduced in the cochlear tissue of older individuals [43]. During presbycusis progression, Nrf2 exerts a protective effect by upregulating the expression of antioxidant enzymes. The number of hair and spiral ganglion cells of Nrf2 knockout mice is significantly reduced compared to that in wild-type mice with age [44]. A study based on a candidate gene approach showed that increased levels of oxidative stress, accompanied by insufficient Cx26 expression and dysregulation of the Nrf2/ARE pathway in the cochlea, are the causes of presbycusis [45]. The Keap1-Nrf2 system is a key defense mechanism for cellular and biological responses to redox abnormalities and contributes to the prevention and mitigation of physiological aging and age-related diseases [46]. At 12 months of age, Keap1 knockout mice had more intact cochlear tissue morphology at the apical and middle turns, higher expression levels of some Nrf2 target genes than WT mice, and reduced accumulation of oxidative stress markers (4-HNE and 8-OHdG), suggesting that Nrf2 activation caused by Keap1 knockout is the key to attenuating presbycusis [47]. It has been revealed that mtDNA damage, apoptosis, and degeneration are involved in the development of central presbycusis and that activation of Nrf2-mediated antioxidant signaling maintains mtDNA integrity and delays aging [48]. It has also been suggested that deuterated oxygen supplementation slows metabolism and reduces endogenous oxidative stress, as evidenced by the reduced activity of the Nrf2/HO-1/glutathione axis [49].
Fig. 2.
Nrf2-mediated oxidative stress and mitochondrial dysfunction as part of the pathogenesis of presbycusis.
Mitochondria are the main source and site of ROS production, and their structure, properties, and functions are altered during aging [50]. Cochlear aging involves mitochondrial redox imbalance and mtDNA damage are involved in the cochlear aging process [51]. Increased mitochondrial reactive ROS levels promote cochlear cell senescence and contribute to the accelerated onset and development of presbycusis [52].
Isocitrate dehydrogenase (IDH) 2 plays a key role in protecting mitochondria from oxidative stress. Loss of IDH2 accelerated hearing loss in 24-month-old mice, exhibiting increased oxidative DNA damage with apoptosis, severe SGNs and hair cell loss, and decreased mitochondrial oxygen consumption in HEI-OC1 cells with IDH2 gene knockdown [53]. Furthermore, a decrease in nicotinamide adenine dinucleotide (NAD+) levels during senescence leads to a cellular and mitochondrial decline [54]. Sirt3 activates oxidative metabolism by deacetylating several mitochondrial enzymes. CMP-Neu5Ac hydroxylase-null mice exhibit presbycusis, and the mechanism of action may be related to Sirt3 downregulation, which in turn regulates ROS-induced oxidative damage and mitochondrial dysfunction [55].
3.4.2. Autophagy of progression for presbycusis
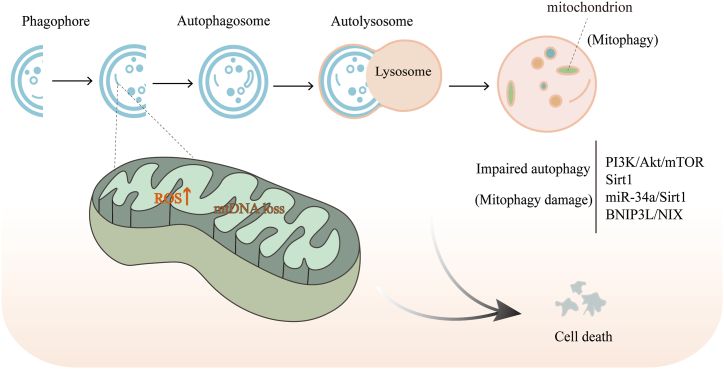
Research on the auditory system associated with aging has shown that mitochondrial damage caused by mtDNA deletion and increased ROS levels can be ameliorated by the autophagic pathway [56]. Autophagic dysfunction in the progression of aging has been extensively studied, particularly mitophagy (Fig. 3), which can maintain mitochondrial homeostasis by removing dysfunctional mitochondria from cells [57]. An imbalance between mitophagy and mitochondrial biogenesis can lead to oxidative stress and cochlear hair cell damage during aging. The marked loss of mitochondrial biogenesis and mitophagy in the cochlea with increasing age has emerged as a potential mechanism for age-related auditory system dysfunction [58,59]. Age-related defects in autophagy may lead to increased apoptosis of SGNs [60]. Relevant studies have shown that miR-34a expression is upregulated in the aging cochlea and that the activation of miR-34a inhibits ATG9A, impairs autophagic flux, and promotes cell death, whereas an appropriate increase in autophagy can reduce inner ear damage and prevent or delay presbycusis [61,62]. The PI3K/Akt/mTOR pathway is an important pathway that regulates cochlear autophagy in the inner ear and mediates cell aging [63]. It has also been shown that Sirt1 acts as an important regulator of autophagy in presbycusis, and its activation can exert a slowing effect on presbycusis by restoring autophagy [14].
Fig. 3.
Impaired autophagy (mitochondrial autophagy) in the pathological process of presbycusis, associated with increased ROS and mtDNA deletion.
3.4.3. Correlation of inflammation with presbycusis
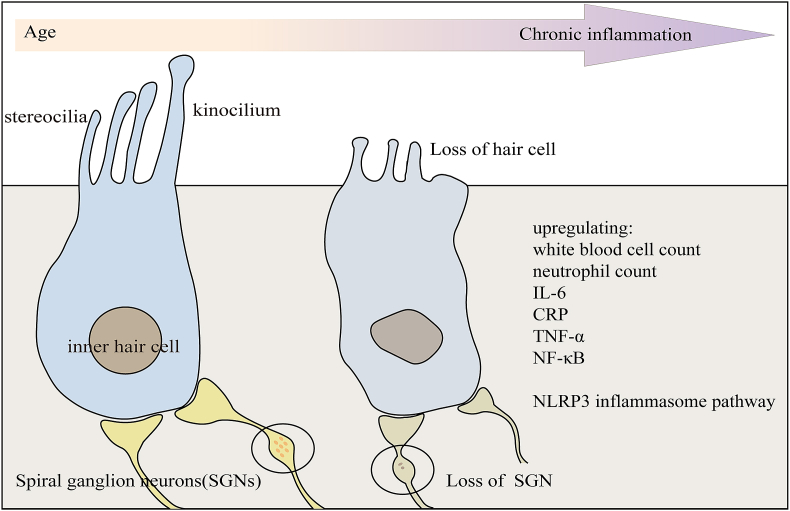
Aging is also strongly associated with chronic low-grade inflammation, and the phenomenon of “chronic inflammation”, which worsens with age, plays a key role in the pathogenesis of presbycusis [64] (Fig. 4). A cross-sectional analysis of a cohort study showed a significant correlation between inflammatory markers, such as white blood cell count, neutrophil count, interleukin-6, c-reactive protein levels, and hearing thresholds in elderly individuals [65]. Another cohort study showed that the signaling cascade of TNF-α was also associated with presbycusis [66]. Evidence of chronic inflammation has been found in aging cochlea, providing a new strategy for preventing and treating presbycusis [64]. This inflammatory process is a phenomenon of immune senescence, and there is evidence that the transcription factor nuclear factor kappa-B (NF-κB) is upregulated in aging cochlea, and that the NF-κB interacting molecules TNF-α and prostaglandin-endoperoxide synthase 2 are also expressed in the spiral ligaments and vascular striae of the lateral wall, suggesting that inflammation and immune responses are regulated in the aging process of cochlear in presbycusis mice [67]. Activated levels of caspase-1, Interleukin-1β, Interleukin-18 and nucleotide-binding structural domain-like receptor protein 3 (NLRP3) were elevated in the inner ear of aged mice compared to young mice; activation of NLRP3 contributes to the assembly of inflammatory vesicles and facilitates subsequent inflammatory events in the cochlea of aging mice [68].
Fig. 4.
Increased loss of cochlear inner ear hair cells and SGNs with advancing age.
Presbycusis is associated with increased oxidative stress, inflammation, and autophagic stress. SAMP8 mice show a range of premature manifestations characterized by oxidative damage, chronic inflammation, and reduced mitochondrial complex activity, which are similar to the changes observed in human presbycusis [69]. Overexpression of glucose-6-phosphate dehydrogenase triggers hypo-inflammation and acts as a positive regulator of the inflammatory response, thereby protecting the cochlea from ROS-derived oxidative damage [70]. Deficiency of insulin-like growth factor, which causes presbycusis, controls inflammation and oxidative stress, and plays a potential role in presbycusis-related mechanisms [71].
The above literature shows that the pathological mechanisms underlying presbycusis are complex and heterogeneous. Oxidative stress is present throughout the pathology of presbycusis and can occur in conjunction with mitochondrial dysfunction, abnormal autophagy, and inflammation. Crosstalk between mechanisms such as ROS-induced mtDNA damage and inflammation due to oxidative damage is also important in presbycusis.
4. Research progress in therapeutic approaches for presbycusis
Presently, hearing aids [[72], [73], [74]], and cochlear implants [[75], [76], [77]] are effective means of improving hearing and quality of life in older people. However, only a small proportion of people continue using hearing aids, mainly due to its economic burden, issues with comfort, equipment maintenance and social stigma [78,79]. Similarly, the high cost of cochlear implants severely limits their affordability, with market penetration rates of approximately 20 % in developed countries and less than 1 % in developing countries [80]. In addition, there are clinical trials evaluating the ameliorative effects of water-soluble coenzyme Q10 formulation on presbycusis [81,82]. Animal studies have shown that istradefylline, a small molecule antagonist of adenosine A2A receptor, improved cochlear hair cell survival in C57BL/6J mice [83]. The combination of antioxidants effectively lowered the auditory brainstem response (ABR) threshold in presbycusis mice [84]. Lecithin improves age-related hearing loss by protecting mitochondrial function in the rat cochlea [85]. However, there are no specific drugs available for the treatment of presbycusis.
5. TCM possibilities for prevention and treatment of presbycusis
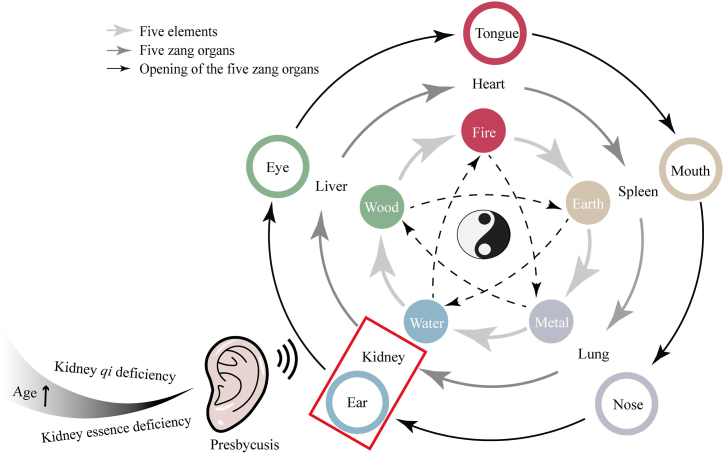
TCM has been used in Chinese culture and history for millennia [86], and the basic theories and treatments of TCM are used throughout the development of a wide range of diseases. According to TCM, the kidney store essence. Kidney essence is the basic substance that maintains the activities of the human body and plays an important role in all life processes. The ears are the opening orifices of the kidney and are nourished by kidney essence and qi(energy), which are associated with hearing sensitivity. If the kidney essence is full and well-nourished, the hearing will be sensitive, and the resolution will be high. In contrast, in kidney essence deficiency, the marrow lacks nourishment, resulting in hearing loss and tinnitus.
With an increase in age, kidney qi and essence are gradually insufficient, leading to malnourishment of the ears and subsequently, hearing loss. Consequently, kidney essence failing to nourish the ears contributes to presbycusis (Fig. 5). According to one study, the correlation between presbycusis and kidney deficiency in TCM from a metabolic perspective was positively correlated with the score of kidney deficiency in TCM, which may be associated with glutathione metabolism, amino acid metabolism, glucose metabolism, N-methyl-d-aspartic acid receptor (NMDA) receptors, γ-aminobutyric acid (GABA) receptors, and other related material bases [87].
Fig. 5.
A schematic diagram of the development of presbycusis based on the fundamentals of TCM: the Kidney stores essence and opens into the ears. Deficiency of kidney qi and essence with increasing age is one of the causes of presbycusis.
5.1. The action of Chinese medicine formulas and specific application
Chinese medicine formulas interventions for the treatment of presbycusis have been confirmed in several experiments in vitro and in vivo with different mechanisms in TCM (Table 1.).
Table 1.
Prevention and treatment of presbycusis with TCM.
| Approach | Experiment evidence | Name | Objects (Gender; Age; Intervention) | Mechanism | Refs |
|---|---|---|---|---|---|
| Formula | In vivo | Erlong Zuoci Decoction | C57BL/6J mice; Male, 3 m; fed | anti-apoptotic(mRNA and protein of p53, Bak↓) | [88] |
| Erlong Zuoci Pill | GM-induced cochlear basilar membrane of kunming mice (P2–P3) | Reduction of GM-induced ototoxicity (cochlear hair cells loss ↓) | [89] | ||
| Zuogui Pill | Dgal-induced C57BL/6 mice; Male; fed | hearing threshold levels of 8 kHz and 16 kHz ↓ | [90] | ||
| Jian Er preparation | C57BL/6J mice; half male and female; drinking tap water containing TCM | Anti-oxidant (ABR thresholds from 8 to 48 KHz ↓, MDA contents in cochlear tissue, auditory cortex and liver ↓) | [91] | ||
| Jian Er preparation | C57BL/6J mice; half male and female; drinking tap water containing TCM | Anti-oxidation, anti-apoptotic (lipid peroxidation and MDA levels, Cyt-C and caspase-3 mRNA expression ↓) | [92] | ||
| Fucong Decoction | DBA/2J mice; half male and female; 4w; orally | Prevent the process of hair cell death (ABR threshold ↓, survival rate of cochlea hair cells↑; protein of prestin↑) | [93] | ||
| In vitro | Erlong Zuoci | H2O2-induced HEI-OC1 cell | Anti-aging, anti-inflammatory (SA-β-gal activity ↓, proteins of p-P53, P21 and p-Stat3 ↓, p-ERK↑) | [94] | |
| Erlong Zuoci Pill | HEI-OC1 cell transfected with a miR-34a mimic | Regulate autophagy and apoptosis (protein of miR-34a, ac-p53, cleaved caspase 3, LC3Ⅱ and p62↓, SIRT1↑) | [95] | ||
| Yiqi congming Decoction | HEI-OC1 cells | Modulate apoptosis (ΔΨM↑, γH2AX↓, cleaved caspase-3↓) | [96] | ||
| Extract | In vivo | KRG | C57BL/6 mice; Male; 3 m; drinking water containing KRG | Modulate mitochondrial apoptotic (Bcl-xL↑, Cyt-C↓) | [97] |
| Ginkgo biloba extract (EGb761) | SD rat; Male and female; 12 m; drinking water contain EGb761 | Anti-oxidation, anti-apoptotic (ATP ↑, protein of Bax, caspase-3/7 and caspase 9↓, Bcl-xL ↑) | [98] | ||
| Polyphenols | SD rat; 3, 6, 12, 18 24 m; female; mixture of polyphenols in the drinking water | Anti-oxidation (improved ASSR and tone-burst ABR auditory thresholds; ROS, RNS, superoxide anions and nitrotyrosine↓; caspase-3, caspase-9, annexin-V, p53, 8-OHdG and Bax↓, Bcl-2, ATP↑) |
[[99], [100], [101]] | ||
| Compounds | Resveratrol (PubChem CID: 445154) | C57BL/6 mice; 1–3 m, 12–16 m; dietary supplementation with Resveratrol | ABR threshold shifts at 4, 8 and 16 kHz↓, loss of outer hair cells in the apical turn↓ | [102] | |
| Apocynin (PubChem CID: 2214) | D-gal induced SD rat; Male; 1 m; intraperitoneally administered | Anti-apoptotic (protein of 8-OHdG, p-p47phox, TNFα, UCP2, Cyt-C and cleaved-caspase 3↓, activity of NADPH, H2O2↓, T-SOD and GSH-Px ↑, levels of ATP and MMP ↑) | [103] | ||
| In vitro | Ursolic Acid (PubChem CID: 64945) | H2O2-induced HEI-OC1 cell | Anti-oxidation (activity of MDA↓, CAT and GPX↑) | [104] | |
| Resveratrol (PubChem CID: 445154) | H2O2-induced HEI-OC1 cell | Anti-apoptotic (Ac-p53↓, 4HNE↓, 5-fluorouracil↓, miR-34a↓) | [102] | ||
| Acupuncture | Clinic | 126 senile SNHL patients; 60–69 years old; Acupuncture points: Tinggong (SI 19), Tinghui (GB 2), and Yifeng (SJ 17), acupoint injection and auricular point pressing | Improve pure tone hearing threshold and tinnitus | [105] |
↑, upgrade; ↓, downgrade.
5.1.1. The action of tonify the kidney
The view that presbycusis should be treated from a "Kidney" perspective has been taken seriously by many scholars. The Erlong Zuoci (ELZC) formula is commonly used in TCM clinics to treat hearing loss due to kidney deficiency and consists of Liu Wei Di Huang (LWDH) plus Radix Bupleuri and Magnetitum. A classical kidney tonic formula, LWDH improves or cures aging-related diseases [106]. In presbycusis mice, ELZC could lower hearing threshold levels and reduce SGN damage, and this mechanism of action may be related to the P53/Bak-mediated apoptosis pathway [88]. In vitro culture of newborn mouse cochlear spiracles revealed that ELZC had a protective effect against gentamicin-induced ototoxic damage, and that its disassembled formulation, LWDH, was a major formula in protecting cochlear hair cells [89]. Network pharmacological analysis showed that ELZC contains compounds with antioxidant and anti-inflammatory biological activities that cross-target biological processes, including cell proliferation, immune response, and inflammatory response, and may improve aging, inflammation, and synaptic connectivity in mouse auditory cells (HEI-OC1) through the c-Jun N-terminal kinase/signal transducer and activator of transcription 3 and extracellular regulated protein kinases cascade signaling pathways [94]. In age-induced SNHL, autophagic activity decreases with increasing age, and upregulation of autophagy promotes the survival of senescent inner ear hair cells and slows the degeneration of auditory cells [107]. miR-34a overexpression caused HEI-OC1 auditory cell death and autophagosome accumulation, and ELZC pills regulated autophagy through the SIRT1/p53 signaling pathway to inhibit apoptosis, thus exerting otoprotective effect [95].
Additionally, another classic formula to tonify kidney essence is called Zuo Gui Pill, which is modified from LWDH, also attenuates cognitive decline with the effect of protecting against d-galactose-induced hearing impairment [90].
5.1.2. The action of circulate blood
The Chinese herbal formula Jian Er, which tonifies the kidney and strengthens the spleen to circulates blood and opens the orifices, significantly improves hearing thresholds, and reduces malondialdehyde content in the cochlea and auditory cortex of presbycusis model mice, which is related to the antioxidant effect of the formula [91]. Further studies showed that it also reduced the number of cochlear basement membrane hair cells with SGNs damage and inhibited caspase-mediated mitochondrial apoptotic pathways in the same model mouse [92]. Fucong Decoction could activate blood to resolve stasis. In animal experiment, it effectively prevented and delayed the development of presbycusis by upregulating prestin to improve the survival of mouse cochlear hair cells [93].
5.1.3. The action of tonify qi of the spleen and stomach to ascend yang
Yi-Qi Cong-Ming decoction, which tonifies qi of the spleen and stomach to ascend yang, exerted a protective effect against presbycusis by alleviating oxidative stress-induced auditory hair cell death by regulating DNA damage and apoptosis [96].
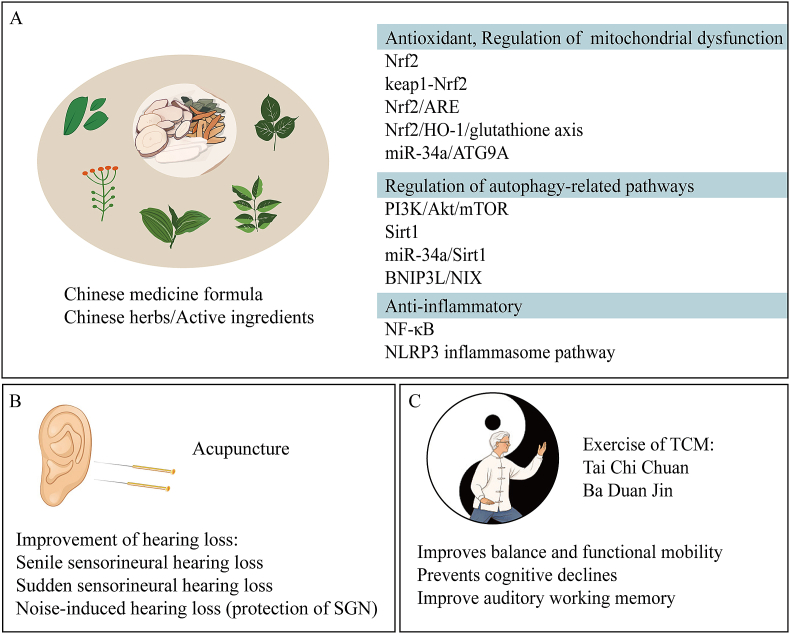
The results from the above-mentioned studies show that most herbal formulations have been studied in vivo and in vitro, and the specific mechanisms of effective intervention in presbycusis mainly include regulation of oxidative damage, autophagy and apoptosis (Table 1.) (Fig. 6 A). However, Chinese medicine formulas are composed of a variety of Chinese herbs in strict compliance with the principles of formulas and contain complex chemical components with multi-level and multi-targeted pharmacological effects, and the quality control of the formulas is yet to be optimized. Therefore, the study of the pharmacology of herbal compound prescriptions needs to be encouraged.
Fig. 6.
TCM intervention in presbycusis. (A) TCM compound/herbs and their active ingredients can intervene in presbycusis by exerting antioxidant, autophagy-regulating and anti-inflammatory effects. (B) Acupuncture has an improving effect on hearing loss by stimulating acupuncture points. (C) Tai Chi Chuan or Ba Duan Jin can improve balance and functional mobility as well as improve auditory working memory.
5.2. The mechanisms of Chinese herbs and active ingredients
In addition to Chinese medicine formulas, some traditional oriental herbs and their active ingredients have shown beneficial effects in the treatment of presbycusis (Table 1.).
5.2.1. Ginsenosides
Ginsenosides are the main active constituents of ginseng. One study found that red ginseng delayed early onset hearing loss and progressive vestibular dysfunction in C57BL/6 mice with increasing age, partly by regulating the mitochondrial apoptotic pathway [97].
5.2.2. Ginkgo biloba extract
Similarly, the antioxidant properties of Ginkgo biloba extract are widely used in neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease [108]. Ginkgo biloba leaf extract EGb761 exerts beneficial effects on presbycusis by increasing ATP levels and downregulating the expression of pro-apoptotic proteins (Caspase-3, Caspase-7) in the aging rat cochlea [98].
5.2.3. Polyphenols
Polyphenols are important natural products that are widely found in herbal medicines such as Veratrum grandiflorum and curcuminoids [109]. It has been shown that a mixture of polyphenols (tannic acid, resveratrol, quercitine, rutin, gallic acid and morin) can significantly improve the auditory steady state response and tone-burst ABR auditory thresholds in rats [99]. It was further observed that the levels of ROS and reactive nitrogen species in the rat cochlea increased with age and decreased after polyphenol treatment, and that SOD and GPx enzyme activities were restored [100]. The polyphenol mixture also inhibited age-related apoptotic signaling by reducing oxidative stress in the rat cochlea, which was mainly associated with the blockade of pro-apoptotic Bax and p53 and the activation of anti-apoptotic Bcl2 [101].
5.2.4. Ursolic acid
Cornus officinalis also attenuates inner ear dysfunction, and ursolic acid (PubChem CID: 64945), its main active ingredient, has a protective effect against H2O2-induced HEI-OC1 cell damage, which is associated with the inhibition of lipid peroxidation and modulation of the activities of antioxidant enzymes catalase (CAT) and glutathione peroxidase (GSH-Px) [104].
5.2.5. Resveratrol
Resveratrol (PubChem CID: 445154) is a potent compound found in many foods and oriental herbs. It acts as a Sirt1 activator, protects against miR-34a overexpression-induced HEI-OC1 cell death, and reduces hearing threshold changes and hair cell loss in C57BL/6 mice, which is largely dependent on the miR-34a/SIRT1/p53-mediated apoptotic pathway [102].
5.2.6. Apocynin
Apocynin (PubChem CID: 2214) has been reported as a nitrogen oxides (NOX) inhibitor present in the leaves of Apocynum venetum [110], and is an active component of Picrorrhiza kurroa [111]. In D-gal-induced rats, the snail ventral nucleus (VCN) exhibited oxidative damage, mitochondrial dysfunction, and apoptosis. Apocynin (PubChem CID: 2214) attenuated NOX-related oxidative stress and mitochondrial DNA damage by inhibiting NOX activity, H2O2 levels, DNA damage biomarker 8-OHdG expression, and mtDNA deletion in the VCN, and increasing total superoxide dismutase and GSH-Px activity, thereby partially blocking caspase-3-dependent apoptosis and effectively preventing or delaying the development of central presbycusis [103].
The above studies suggest that single herbs and their active ingredients have considerable potential for the prevention and treatment of presbycusis, with relevant mechanisms focusing on antioxidant and anti-apoptotic effects (Table 1.) (Fig. 6 A).
5.3. The application of acupuncture
Acupuncture, a traditional Chinese treatment method, can be used to prevent or treat various diseases, such as neurological and metabolic disorders, by stimulating the relevant acupuncture points on the body surface through needle insertion and rotation [112]. According to case reports, patients with hearing loss showed significant improvements in hearing after receiving acupuncture treatment [113,114]. Clinical studies have shown that the combination of acupuncture and medical therapy is effective in treating sudden deafness. A randomized controlled trial observed differences in the efficacy of the three approaches for the treatment of senile SNHL and found that combined treatment was superior to acupuncture and oral mecobalamin alone [105]. Another clinical trial showed that a combination of acupuncture and Western medicine combined therapy (WMCT) was a more effective intervention for sudden SNHL than WMCT alone [115]. In addition to the clinical trials mentioned above, an experimental animal study found that electroacupuncture intervention could promote hearing recovery after noise exposure by increasing SGN density.
The above study shows that acupuncture treatment for presbycusis is less commonly reported than for sudden deafness (Table 1.) (Fig. 6 B). On the one hand, it may be that acupuncture may be less effective in chronic conditions (age-related degenerative lesions) than in acute lesions. On the other hand, given the body constitution of the older adults and the complex pathogenesis, the variable efficacy is closely related to the absence of complete treatment rules based on basic principles, as well as different treatment cycles.
5.4. Exercise of TCM: Tai chi Chuan, Ba Duan Jin
Clinical studies have revealed that group audiological rehabilitation, exercise intervention, and social contact can improve presbycusis and associated isolation it brings [116]. Popular traditional Chinese mind-body exercises including Tai Chi Chuan and Ba Duan Jin practices can improve balance and enhance the quality of life in older adults [117,118]. In a study on the rehabilitation of children with congenital SNHL, Tai Chi Chuan and conventional exercise training improved balance and functional mobility [119]. Moreover, numerous clinical studies have confirmed that Tai Chi Chuan and/or Ba Duan Jin are effective exercises for preventing cognitive decline in the elderly [[120], [121], [122]]. Due to the higher risk of falls in people with presbycusis, sequential cognitive and exercise training improves auditory working memory [123].
There are no reports of traditional exercise in presbycusis. However, it has become influential in age-related diseases. The positive physical and psychological effects of these traditional exercise methods have been validated in clinical trials (Fig. 6 C), thereby promoting physical and mental health and enhancing the overall physical fitness of the elderly population.
6. Conclusions, implications and future perspectives
6.1. The conclusion of the review
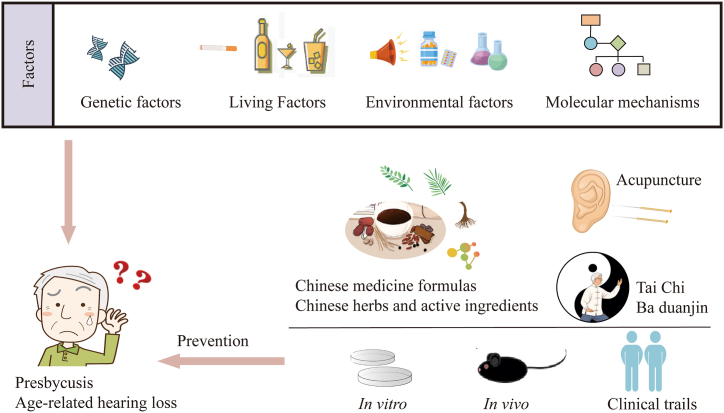
We have reviewed the pathogenetic factors associated with presbycusis, related to genetic factors, environment, lifestyle, and molecular mechanisms related to oxidative stress, mitochondrial dysfunction, and inflammatory pathways. Traditional Chinese medicine interventions could offer opportunities in the prevention and treatment of presbycusis using active ingredients of herbs or formulas, acupuncture, and exercise such as Tai Chi Chuan or Ba Duan Jin. Fig. 7 illustrates the overview of the article.
Fig. 7.
The overview of the article.
Given the intricate pathogenesis of presbycusis, Chinese medicine may focus on a variety of pathways and targets to coordinate the disease's progression from various angles. This could contribute to the disease's pathological progression in a variety of ways and offer a fresh perspective on presbycusis intervention and treatment. This article searched for the effects of Chinese herbal compounds or herbs and their active ingredients on oxidative stress and its resulting mtDNA damage, mitochondrial dysfunction, relevant targets or pathways including Nrf2, keap1-Nrf2, Nrf2/ARE, Nrf2/HO-1/glutathione axis, autophagy-related pathways such as miR-34a/ATG9A, PI3K/Akt/mTOR, Sirt1, BNIP3L/NIX, miR-34a/Sirt1, and the inflammation-related NF-κB, NLRP3 inflammatory vesicle pathway. By acting synergistically on multiple targets and pathways, TCM can maintain the redox status, regulate mitochondrial function, modulate autophagy, reduce inflammation, and ultimately improve presbycusis.
Although the role of acupuncture in presbycusis has rarely been reported, it is commonly used to treat age-related diseases. In TCM theory, most elderly people are believed to have deficiency of heathy qi (weak immune system in modern medicine), acupuncture can be used as a method to reinforce or tonify the body based on individualized pattern identification. With advances in biotechnology, the mechanism of action of acupuncture needs to be further elucidated. In clinical practice, the effectiveness of acupuncture is closely related to the acupuncture points, needling technique, treatment period, and individualized body constitution. Therefore, more basic research and clinical trials are needed at a later stage to establish and improve standardized acupuncture treatment protocols, thereby providing evidence for the potential mechanisms of action of acupuncture in the treatment of presbycusis.
Traditional exercise methods are beneficial for rehabilitation and healthcare. As classic examples of the "integration of martial arts and medicine", Tai Chi Chuan and Ba Duan Jin act to balance between yin and yang, maintain the free flow of qi within meridians and collaterals. They are characterized by movements (upper-lower coordination, opening, and closing) of the limbs, twisting and turning, flexion and extension, blowing and breathing (opening with breathing out and closing with breathing in, opening with breathing in and closing with breathing out), to achieve the purpose of strengthening the body.
6.2. The strengths and limitations of the review
The retrieval approach might not be sufficient because the database only contains information in English and Chinese. The majority of research on the prevention and treatment of presbycusis with TCM now comes from in vivo and in vitro studies, which have limits for examining the formula effects of presbycusis. Additionally, the molecular pathways behind the therapeutic benefits of this prescription are yet unknown. In order to completely identify the possible targets and pertinent signaling pathways involved, more investigation using standardized and rigorous clinical trials is therefore required in order to uncover the underlying process.
6.3. The implications and future perspectives of the review
Presbycusis, an age-related chronic illness, has a substantial influence on social and public health due to the tendency of an aging population. First and foremost, we should protect our ears by living a healthy lifestyle that includes avoiding loud noises, using ototoxic medications and chemicals, quitting smoking, drinking alcohol, and using nutritional supplements (vitamins) whenever feasible. Second, effective intervention strategies for hearing loss in elderly people need to be further explored through a thorough, individualized assessment, as the pathophysiological mechanisms involved in presbycusis have not yet been fully elucidated, combined with the complex and varied clinical presentations of presbycusis.
The Chinese medicine prescription is made up of a wide variety of traditional Chinese herbs, and the total thing comprises different substances that work together to regulate the body. On the one hand, holistic approach of TCM is one of the fundamental qualities; it not only aids in the treatment of illnesses but also functions as conditioning and has fewer negative effects. TCM, on the other hand, is advantageous for illness prevention and has a positive impact on sustaining health. Additionally, the cost of modern therapies, such as the choice of hearing aids and cochlear implants, is a significant burden for less fortunate households or developing countries. Acupuncture and exercises such as Tai Chi Chuan or Ba Duan Jin present an economical approach to prevent and treat presbycusis among the elderly population.
Presbycusis needs more attention as the population ages more rapidly. In order to lessen the negative impacts of presbycusis on the elderly's quality of life, cognitive function, mood, behavior, and social activities, TCM has certain benefits in its treatments and prevention.
Funding statement
This work was supported by grants from Shanghai Municipal Commission of Health and Family Planning, NO. ZY (2021–2023)-0208, NO. 202340284 and the National Natural Science Foundation of China (81774020).
Institutional review Board statement
Not applicable.
Informed consent statement
Not applicable.
Data availability statement
No data was used for the review described in the article.
CRediT authorship contribution statement
Li Yan: Writing - original draft, Methodology, Data curation. Yan Huo: Writing - original draft, Methodology, Data curation. Jianrong Shi: Supervision, Project administration, Funding acquisition, Conceptualization. Yang Dong: Writing - review & editing, Methodology, Conceptualization. Hongsheng Tan: Writing - review & editing, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank anonymous reviewers for the helpful comments. We would like to thank Editage [https://www.editage.cn/] for editing and reviewing this manuscript for the English language.
Contributor Information
Yang Dong, Email: dongyang@shutcm.edu.cn.
Hongsheng Tan, Email: tanhs@shsmu.edu.cn.
References
- 1.Chadha S., Kamenov K., Cieza A. The world report on hearing. Bull. World Health Organ. 2021;99(4) doi: 10.2471/blt.21.285643. 242 242A 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiam N.T., Li C., Agrawal Y. Hearing loss and falls: a systematic review and meta-analysis. Laryngoscope. 2016;126(11):2587–2596. doi: 10.1002/lary.25927. [DOI] [PubMed] [Google Scholar]
- 3.Mick P., Kawachi I., Lin F.R. The association between hearing loss and social isolation in older adults. Otolaryngol. Head Neck Surg. 2014;150(3):378–384. doi: 10.1177/0194599813518021. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford B.R., Brewster K., Golub J.S., Kim A.H., Roose S.P. Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am. J. Psychiatr. 2018;175(3):215–224. doi: 10.1176/appi.ajp.2017.17040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An S., Jo E., Jun S.B., Sung J.E. Effects of cochlear implantation on cognitive decline in older adults: a systematic review and meta-analysis. Heliyon. 2023;9(9) doi: 10.1016/j.heliyon.2023.e19703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chern A., Golub J.S. Age-related hearing loss and dementia. Alzheimer Dis. Assoc. Disord. 2019;33(3):285–290. doi: 10.1097/WAD.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su P., Hsu C.C., Lin H.C., Huang W.S., Yang T.L., Hsu W.T., et al. Age-related hearing loss and dementia: a 10-year national population-based study. Eur. Arch. Oto-Rhino-Laryngol. 2017;274(5):2327–2334. doi: 10.1007/s00405-017-4471-5. [DOI] [PubMed] [Google Scholar]
- 8.Gates G.A., Mills J.H., Presbycusis Lancet. 2005;366(9491):1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- 9.Bowl M.R., Dawson S.J. Age-related hearing loss. Cold Spring Harb. Perspect. Med. 2019;9(8):a033217. doi: 10.1101/cshperspect.a033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuknecht H.F. Further observations on the pathology of presbycusis. Arch. Otolaryngol. 1964;80:369–382. doi: 10.1001/archotol.1964.00750040381003. [DOI] [PubMed] [Google Scholar]
- 11.Schuknecht H.F., Gacek M.R. Cochlear pathology in presbycusis. Ann. Otol. Rhinol. Laryngol. 1993;102(1 Pt 2):1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- 12.Tawfik K.O., Klepper K., Saliba J., Friedman R.A. Advances in understanding of presbycusis. J. Neurosci. Res. 2020;98(9):1685–1697. doi: 10.1002/jnr.24426. [DOI] [PubMed] [Google Scholar]
- 13.Hu W., Wu J., Jiang W., Tang J. MicroRNAs and presbycusis. Aging Dis. 2018;9(1):133–142. doi: 10.14336/AD.2017.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang J., Xiong H., Ou Y., Yang H., Xu Y., Chen S., et al. SIRT1 protects cochlear hair cell and delays age-related hearing loss via autophagy. Neurobiol. Aging. 2019;80:127–137. doi: 10.1016/j.neurobiolaging.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Ingham N.J., Pearson S.A., Vancollie V.E., Rook V., Lewis M.A., Chen J., et al. Mouse screen reveals multiple new genes underlying mouse and human hearing loss. PLoS Biol. 2019;17(4) doi: 10.1371/journal.pbio.3000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delmaghani S., El-Amraoui A. Inner ear gene therapies take off: current promises and future challenges. J. Clin. Med. 2020;9(7):2309. doi: 10.3390/jcm9072309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivarsdottir E.V., Holm H., Benonisdottir S., Olafsdottir T., Sveinbjornsson G., Thorleifsson G., et al. The genetic architecture of age-related hearing impairment revealed by genome-wide association analysis. Commun. Biol. 2021;4(1):706. doi: 10.1038/s42003-021-02224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan H., Zhang D., Liang Y., Xu C., Wu Y., Tian X., et al. Heritability of age-related hearing loss in middle-aged and elderly Chinese: a population-based twin study. Ear Hear. 2019;40(2):253–259. doi: 10.1097/AUD.0000000000000610. [DOI] [PubMed] [Google Scholar]
- 19.Bouzid A., Smeti I., Chakroun A., Loukil S., Gibriel A.A., Grati M., et al. CDH23 methylation status and presbycusis risk in elderly women. Front. Aging Neurosci. 2018;10:241. doi: 10.3389/fnagi.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodyear R.J., Cheatham M.A., Naskar S., Zhou Y., Osgood R.T., Zheng J., et al. Accelerated age-related degradation of the tectorial membrane in the ceacam16(betagal/betagal) null mutant mouse, a model for late-onset human hereditary deafness DFNB113. Front. Mol. Neurosci. 2019;12:147. doi: 10.3389/fnmol.2019.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M.J., Haroon S., Chen G.D., Ding D., Wanagat J., Liu L., et al. Increased burden of mitochondrial DNA deletions and point mutations in early-onset age-related hearing loss in mitochondrial mutator mice. Exp. Gerontol. 2019;125 doi: 10.1016/j.exger.2019.110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falah M., Najafi M., Houshmand M., Farhadi M. Expression levels of the BAK1 and BCL2 genes highlight the role of apoptosis in age-related hearing impairment. Clin. Interv. Aging. 2016;11:1003–1008. doi: 10.2147/CIA.S109110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong Y., Li M., Liu P., Song H., Zhao Y., Shi J. Genes involved in immunity and apoptosis are associated with human presbycusis based on microarray analysis. Acta Otolaryngol. 2014;134(6):601–608. doi: 10.3109/00016489.2014.880795. [DOI] [PubMed] [Google Scholar]
- 24.Momi S.K., Wolber L.E., Fabiane S.M., MacGregor A.J., Williams F.M. Genetic and environmental factors in age-related hearing impairment. Twin Res. Hum. Genet. 2015;18(4):383–392. doi: 10.1017/thg.2015.35. [DOI] [PubMed] [Google Scholar]
- 25.Lie A., Skogstad M., Johannessen H.A., Tynes T., Mehlum I.S., Nordby K.C., et al. Occupational noise exposure and hearing: a systematic review. Int. Arch. Occup. Environ. Health. 2016;89(3):351–372. doi: 10.1007/s00420-015-1083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jafari Z., Copps T., Hole G., Kolb B.E., Mohajerani M.H. Noise damage accelerates auditory aging and tinnitus: a Canadian population-based study. Otol. Neurotol. 2020;41(10):1316–1326. doi: 10.1097/MAO.0000000000002848. [DOI] [PubMed] [Google Scholar]
- 27.Fetoni A.R., Pisani A., Rolesi R., Paciello F., Viziano A., Moleti A., et al. Early noise-induced hearing loss accelerates presbycusis altering aging processes in the cochlea. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.803973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joo Y., Cruickshanks K.J., Klein B.E.K., Klein R., Hong O., Wallhagen M.I. The contribution of ototoxic medications to hearing loss among older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75(3):561–566. doi: 10.1093/gerona/glz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bielefeld E.C. Age-related hearing loss patterns in Fischer 344/NHsd rats with cisplatin-induced hearing loss. Hear. Res. 2013;306:46–53. doi: 10.1016/j.heares.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Campo P., Morata T.C., Hong O. Chemical exposure and hearing loss. Dis. Mon. 2013;59(4):119–138. doi: 10.1016/j.disamonth.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sliwinska-Kowalska M., Zamyslowska-Szmytke E., Szymczak W., Kotylo P., Fiszer M., Wesolowski W., et al. Exacerbation of noise-induced hearing loss by co-exposure to workplace chemicals. Environ. Toxicol. Pharmacol. 2005;19(3):547–553. doi: 10.1016/j.etap.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Campo P., Venet T., Rumeau C., Thomas A., Rieger B., Cour C., et al. Impact of noise or styrene exposure on the kinetics of presbycusis. Hear. Res. 2011;280(1–2):122–132. doi: 10.1016/j.heares.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Ohgami N., Yajima I., Iida M., Li X., Oshino R., Kumasaka M.Y., et al. Manganese-mediated acceleration of age-related hearing loss in mice. Sci. Rep. 2016;6 doi: 10.1038/srep36306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallagher N.E., Patterson C.C., Neville C.E., Yarnell J., Ben-Shlomo Y., Fehily A., et al. Dietary patterns and hearing loss in older men enrolled in the Caerphilly Study. Br. J. Nutr. 2019;121(8):877–886. doi: 10.1017/S0007114519000175. [DOI] [PubMed] [Google Scholar]
- 35.Kim H., Lee J.J., Moon Y., Park H.Y. Longitudinal pure-tone threshold changes in the same subjects: analysis of factors affecting hearing. Laryngoscope. 2019;129(2):470–476. doi: 10.1002/lary.27478. [DOI] [PubMed] [Google Scholar]
- 36.Gopinath B., Flood V.M., Teber E., McMahon C.M., Mitchell P. Dietary intake of cholesterol is positively associated and use of cholesterol-lowering medication is negatively associated with prevalent age-related hearing loss. J. Nutr. 2011;141(7):1355–1361. doi: 10.3945/jn.111.138610. [DOI] [PubMed] [Google Scholar]
- 37.Croll P.H., Bos D., Vernooij M.W., Arshi B., Lin F.R., Baatenburg de Jong R.J., et al. Carotid atherosclerosis is associated with poorer hearing in older adults. J. Am. Med. Dir. Assoc. 2019;20(12):1617–1622.e1. doi: 10.1016/j.jamda.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 38.Sardone R., Lampignano L., Guerra V., Zupo R., Donghia R., Castellana F., et al. Relationship between inflammatory food consumption and age-related hearing loss in a prospective observational cohort: results from the salus in apulia study. Nutrients. 2020;12(2):426. doi: 10.3390/nu12020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim T.S., Chung J.W. Associations of dietary riboflavin, niacin, and retinol with age-related hearing loss: an analysis of Korean national health and nutrition examination survey data. Nutrients. 2019;11(4):896. doi: 10.3390/nu11040896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bae S.H., Kwak S.H., Choi J.Y., Jung J. Synergistic effect of smoking on age-related hearing loss in patients with diabetes. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-75880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Z.G., Feng T.N., Xie Y., Zhang X.Y., Du J., Tian R., et al. Prognosis and rescue therapy for sepsis-related severe thrombocytopenia in critically ill patients. Cytokine. 2020;136:7. doi: 10.1016/j.cyto.2020.155227. [DOI] [PubMed] [Google Scholar]
- 42.Rousset F., Nacher-Soler G., Coelho M., Ilmjarv S., Kokje V.B.C., Marteyn A., et al. Redox activation of excitatory pathways in auditory neurons as mechanism of age-related hearing loss. Redox Biol. 2020;30 doi: 10.1016/j.redox.2020.101434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosokawa K., Hosokawa S., Ishiyama G., Ishiyama A., Lopez I.A. Immunohistochemical localization of Nrf2 in the human cochlea. Brain Res. 2018;1700:1–8. doi: 10.1016/j.brainres.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoshino T T.K., Nishimura B., Tanaka S., Nakayama M., Ishii T., Warabi E., Yanagawa T., Shimizu R., Yamamoto M., Hara A. Protective role of Nrf2 in age-related hearing loss and gentamicin ototoxicity. Biochem. Biophys. Res. Commun. 2011;415(1):94–98. doi: 10.1016/j.bbrc.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Fetoni A.R., Zorzi V., Paciello F., Ziraldo G., Peres C., Raspa M., et al. Cx26 partial loss causes accelerated presbycusis by redox imbalance and dysregulation of Nfr2 pathway. Redox Biol. 2018;19:301–317. doi: 10.1016/j.redox.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98(3):1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oishi T., Matsumaru D., Ota N., Kitamura H., Zhang T., Honkura Y., et al. Activation of the NRF2 pathway in Keap1-knockdown mice attenuates progression of age-related hearing loss. npj Aging Mech. Dis. 2020;6(1):14. doi: 10.1038/s41514-020-00053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Zhao X., Hu Y., Sun H., He Z., Yuan J., et al. Age-associated decline in Nrf2 signaling and associated mtDNA damage may be involved in the degeneration of the auditory cortex: implications for central presbycusis. Int. J. Mol. Med. 2018;42 doi: 10.3892/ijmm.2018.3907. 3371-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou S., Chen P., He J., Chen J., Zhang J., Mammano F., et al. Dietary intake of deuterium oxide decreases cochlear metabolism and oxidative stress levels in a mouse model of age-related hearing loss. Redox Biol. 2022;57 doi: 10.1016/j.redox.2022.102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han C., Someya S. Mouse models of age-related mitochondrial neurosensory hearing loss. Mol. Cell. Neurosci. 2013;55:95–100. doi: 10.1016/j.mcn.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H., Tang J. The role of mitochondria in age-related hearing loss. Biogerontology. 2014;15(1):13–19. doi: 10.1007/s10522-013-9475-y. [DOI] [PubMed] [Google Scholar]
- 52.Rivas-Chacon L.D.M., Martinez-Rodriguez S., Madrid-Garcia R., Yanes-Diaz J., Riestra-Ayora J.I., Sanz-Fernandez R., et al. Role of oxidative stress in the senescence pattern of auditory cells in age-related hearing loss. Antioxidants. 2021;10(9):1497. doi: 10.3390/antiox10091497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White K., Kim M.J., Han C., Park H.J., Ding D., Boyd K., et al. Loss of IDH2 accelerates age-related hearing loss in male mice. Sci. Rep. 2018;8(1):5039. doi: 10.1038/s41598-018-23436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 55.Kwon D.N., Park W.J., Choi Y.J., Gurunathan S., Kim J.H. Oxidative stress and ROS metabolism via down‐regulation of sirtuin 3 expression in Cmah‐null mice affect hearing loss. Aging. 2015;7(8):579–592. doi: 10.18632/aging.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He Z.H., Li M., Fang Q.J., Liao F.L., Zou S.Y., Wu X., et al. FOXG1 promotes aging inner ear hair cell survival through activation of the autophagy pathway. Autophagy. 2021;17(12):4341–4362. doi: 10.1080/15548627.2021.1916194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran M., Reddy P.H. Defective autophagy and mitophagy in aging and Alzheimer's disease. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.612757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Youn C.K., Jun Y., Jo E.R., Cho S.I. Age-related hearing loss in C57bl/6J mice is associated with mitophagy impairment in the central auditory system. Int. J. Mol. Sci. 2020;21(19):7202. doi: 10.3390/ijms21197202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh J., Youn C.K., Jun Y., Jo E.R., Cho S.I. Reduced mitophagy in the cochlea of aged C57BL/6J mice. Exp. Gerontol. 2020;137 doi: 10.1016/j.exger.2020.110946. [DOI] [PubMed] [Google Scholar]
- 60.Liu H., Li F., Li X., Wu Q., Dai C. Rapamycin ameliorates age-related hearing loss in C57BL/6J mice by enhancing autophagy in the SGNs. Neurosci. Lett. 2022;772 doi: 10.1016/j.neulet.2022.136493. [DOI] [PubMed] [Google Scholar]
- 61.Pang J., Xiong H., Lin P., Lai L., Yang H., Liu Y., et al. Activation of miR-34a impairs autophagic flux and promotes cochlear cell death via repressing ATG9A: implications for age-related hearing loss. Cell Death Dis. 2017;8(10) doi: 10.1038/cddis.2017.462. e3079-e3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong H., Pang J., Min X., Ye Y., Lai L., Zheng Y. miR-34a/ATG9A/TFEB signaling modulates autophagy in cochlear hair cells and correlates with age-related hearing loss. Neuroscience. 2022;491:98–109. doi: 10.1016/j.neuroscience.2022.03.033. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y., Lv Z., Liu Y., Cao H., Yang J., Wang B. PIN1 protects hair cells and auditory HEI-OC1 cells against senescence by inhibiting the PI3K/Akt/mTOR pathway. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/9980444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watson N., Ding B., Zhu X., Frisina R.D. Chronic inflammation - inflammaging - in the ageing cochlea: a novel target for future presbycusis therapy. Ageing Res. Rev. 2017;40:142–148. doi: 10.1016/j.arr.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verschuur C.A., Dowell A., Syddall H.E., Ntani G., Simmonds S.J., Baylis D., et al. Markers of inflammatory status are associated with hearing threshold in older people: findings from the Hertfordshire Ageing Study. Age Ageing. 2012;41(1):92–97. doi: 10.1093/ageing/afr140. [DOI] [PubMed] [Google Scholar]
- 66.Uchida Y., Sugiura S., Ueda H., Nakashima T., Ando F., Shimokata H. The association between hearing impairment and polymorphisms of genes encoding inflammatory mediators in Japanese aged population. Immun. Ageing. 2014;11(1):18. doi: 10.1186/s12979-014-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uraguchi K., Maeda Y., Takahara J., Omichi R., Fujimoto S., Kariya S., et al. Upregulation of a nuclear factor-kappa B-interacting immune gene network in mice cochleae with age-related hearing loss. PLoS One. 2021;16(10) doi: 10.1371/journal.pone.0258977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi X., Qiu S., Zhuang W., Yuan N., Wang C., Zhang S., et al. NLRP3-inflammasomes are triggered by age-related hearing loss in the inner ear of mice. Am. J. Transl. Res. 2017;9(12):5611–5618. PMID: 29312513. [PMC free article] [PubMed] [Google Scholar]
- 69.Menardo J., Tang Y., Ladrech S., Lenoir M., Casas F., Michel C., et al. Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid. Redox Signal. 2012;16(3):263–274. doi: 10.1089/ars.2011.4037. [DOI] [PubMed] [Google Scholar]
- 70.Bermudez-Munoz J.M., Celaya A.M., Hijazo-Pechero S., Wang J., Serrano M., Varela-Nieto I. G6PD overexpression protects from oxidative stress and age-related hearing loss. Aging Cell. 2020;19(12) doi: 10.1111/acel.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez-de la Rosa L., Lassaletta L., Calvino M., Murillo-Cuesta S., Varela-Nieto I. The role of insulin-like growth factor 1 in the progression of age-related hearing loss. Front. Aging Neurosci. 2017;9:411. doi: 10.3389/fnagi.2017.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romanet P., Guy M., Allaert F.A. Clinical study on the efficacy, acceptance, and safety of hearing aids in patients with mild to moderate presbyacusis. Panminerva Med. 2018;60(3):92–100. doi: 10.23736/S0031-0808.18.03447-X. [DOI] [PubMed] [Google Scholar]
- 73.Sacco G., Gonfrier S., Teboul B., Gahide I., Prate F., Demory-Zory M., et al. Clinical evaluation of an over-the-counter hearing aid (TEO First(R)) in elderly patients suffering of mild to moderate hearing loss. BMC Geriatr. 2016;16:136. doi: 10.1186/s12877-016-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu X., Ren Y., Wang Q., Li B., Wu H., Huang Z., et al. Factors associated with the efficiency of hearing aids for patients with age-related hearing loss. Clin. Interv. Aging. 2019;14:485–492. doi: 10.2147/CIA.S190651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clark J.H., Yeagle J., Arbaje A.I., Lin F.R., Niparko J.K., Francis H.W. Cochlear implant rehabilitation in older adults: literature review and proposal of a conceptual framework. J. Am. Geriatr. Soc. 2012;60(10):1936–1945. doi: 10.1111/j.1532-5415.2012.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lenarz M., Sonmez H., Joseph G., Buchner A., Lenarz T. Cochlear implant performance in geriatric patients. Laryngoscope. 2012;122(6):1361–1365. doi: 10.1002/lary.23232. [DOI] [PubMed] [Google Scholar]
- 77.Eshraghi A.A., Rodriguez M., Balkany T.J., Telischi F.F., Angeli S., Hodges A.V., et al. Cochlear implant surgery in patients more than seventy-nine years old. Laryngoscope. 2009;119(6):1180–1183. doi: 10.1002/lary.20182. [DOI] [PubMed] [Google Scholar]
- 78.Michels T.C., Duffy Mt R.D.J. Hearing loss in adults: differential diagnosis and treatment. Am. Fam. Physician. 2019;100(2):98–108. PMID: 31305044. [PubMed] [Google Scholar]
- 79.McCormack A., Fortnum H. Why do people fitted with hearing aids not wear them? Int. J. Audiol. 2013;52(5):360–368. doi: 10.3109/14992027.2013.769066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeng F.G. Challenges in improving cochlear implant performance and accessibility. IEEE Trans. Biomed. Eng. 2017;64(8):1662–1664. doi: 10.1109/TBME.2017.2718939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salami A., Mora R., Dellepiane M., Manini G., Santomauro V., Barettini L., et al. Water-soluble coenzyme Q10 formulation (Q-TER(R)) in the treatment of presbycusis. Acta Otolaryngol. 2010;130(10):1154–1162. doi: 10.3109/00016481003727590. [DOI] [PubMed] [Google Scholar]
- 82.Guastini L., Mora R., Dellepiane M., Santomauro V., Giorgio M., Salami A. Water-soluble coenzyme Q10 formulation in presbycusis: long-term effects. Acta Otolaryngol. 2011;131(5):512–517. doi: 10.3109/00016489.2010.539261. [DOI] [PubMed] [Google Scholar]
- 83.Shin M., Pandya M., Espinosa K., Telang R., Boix J., Thorne P.R., et al. Istradefylline mitigates age-related hearing loss in C57bl/6J mice. Int. J. Mol. Sci. 2021;22(15) doi: 10.3390/ijms22158000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heman-Ackah S.E., Juhn S.K., Huang T.C., Wiedmann T.S. A combination antioxidant therapy prevents age-related hearing loss in C57BL/6 mice. Otolaryngol. Head Neck Surg. 2010;143(3):429–434. doi: 10.1016/j.otohns.2010.04.266. [DOI] [PubMed] [Google Scholar]
- 85.Seidman M.D., Khan M.J., Tang W.X., Quirk W.S. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol. Head Neck Surg. 2002;127(3):138–144. doi: 10.1067/mhn.2002.127627. [DOI] [PubMed] [Google Scholar]
- 86.Cheung F. TCM: made in China. Nature. 2011;480(7378):S82–S83. doi: 10.1038/480S82a. [DOI] [PubMed] [Google Scholar]
- 87.Dong Y., Ding Y., Liu P.Z., Song H.Y., Zhao Y.P., Li M., et al. Investigation of the material basis underlying the correlation between presbycusis and kidney deficiency in traditional Chinese medicine via GC/MS metabolomics, evid. Based complement. Alternat. Med. 2013 doi: 10.1155/2013/762092. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong Y., Guo C.R., Ding Y., Zhang Y., Song H.Y., Peng Y.T., et al. Effects of Erlong Zuoci decoction on the age-related hearing loss in C57BL/6J mice. J. Ethnopharmacol. 2016;181:59–65. doi: 10.1016/j.jep.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 89.Dong Y., Cao B.Y., Wang J., Ding D.L., Han Z.F., Shi J.R. Effects of Erlong Zuoci pill and its disassembled prescriptions on gentamicin-induced ototoxicity model in vitro. Chin. J. Integr. Med. 2010;16(3):258–263. doi: 10.1007/s11655-010-0258-x. [DOI] [PubMed] [Google Scholar]
- 90.Zhao Y.J., Yan K., Chen S.M., He Z.Y., Fang Y.D., Li L., et al. [Effects of Zuogui Pill on cognitive decline and hearing loss in mice induced by D-galactose] Chin J Ger. 2017;37(12):2864–2866. https://kns.cnki.net/kcms2/article/abstract?v=JxCH2R2Ogom_BaxN02Ehgn3wCA4XSZtn1DV9NaMSbCkpkdgcpq-l7ntFi3khUsX2oV9ZAVdhh8Sx5BvcjXrsJ7V3qLJJI_nIIqAQr--z8gZI6WmjS-kws_utVItwxpZqxwn8h3aao6dz5fArIP1a2g==&uniplatform=NZKPT&language=CHS.Chinese [Google Scholar]
- 91.Xuan W.J., Tang J.B., Chen Z., Wei Y.L., Ding D.L. [Protecting effects of jian'erji on age-related hearing loss of C57BL/6J mice] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36(10):1247–1251. PMID: 30641015. Chinese. [PubMed] [Google Scholar]
- 92.Xuan Y., Ding D., Xuan W., Huang L., Tang J., Wei Y., et al. A traditional Chinese medicine compound (Jian Er) for presbycusis in a mouse model- Reduction of apoptosis and protection of cochlear sensorineural cells and hearing. Int. J. Mol. Med. 2018;6(6):127–135. PMCID: PMC6936738. [PMC free article] [PubMed] [Google Scholar]
- 93.Li S.L., Wu K.Y., Zhang Y. [Fucong Decoction on up-regulating the expression of prestin in the outer hair cells of the cochlea on the deafness of DBA/2J mice with age-related hearing loss] Chin J Tradit Chin Med Pharm. 2018;33(11):5223–5227. https://kns.cnki.net/kcms2/article/abstract?v=JxCH2R2OgontCgoM5H7n8TPd4f7R0p5xr2e51MtkLov4g_24WrVCMf7FZ7MPBtdncMLKta4uOtXJn9QKnDxtesNmjGq27IZZXgJwRxobsbWC1VzZhBGITSIr2b2TQpl2wgSDbmQkGEjR5jZD5U51iw==&uniplatform=NZKPT&language=CHS.Chinese [Google Scholar]
- 94.Liu Q., Li N., Yang Y., Yan X., Dong Y., Peng Y., et al. Prediction of the molecular mechanisms underlying erlong Zuoci treatment of age-related hearing loss via network pharmacology-based analyses combined with experimental validation. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.719267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gong Y.C., Li N., Wang Y.R., Yan X.R., Dong Y., Liiu Q., et al. [Effects of Erlong Zuoci Pills on autophagy and apoptosis of HEI-OC1 cells by regulating miR-34a] Chin J Tradit Chin Med Pharm. 2022;37(2):740–744. https://kns.cnki.net/kcms2/article/abstract?v=JxCH2R2OgoliAOuIa_iERnPmZkHLU-J_PSugCZnR0iVeOT4H3Dt2dNfw0WJIL7vjnd8Obs1O5i1sixyLVZl5KafBqVGNCn7Vqns6qeBd700i7FkW4UwNHjoeVNLkV__C7YxhkH_ZPXcnIEnQBgfm0Q==&uniplatform=NZKPT&language=CHS.Chinese [Google Scholar]
- 96.Yang Y.F., Yan X.R., Wu R.X., Li N., Chu M., Dong Y., et al. Network pharmacology and experimental evidence reveal the protective mechanism of Yi-Qi Cong-Ming decoction on age-related hearing loss. Pharm. Biol. 2022;60(1):1478–1490. doi: 10.1080/13880209.2022.2101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tian C., Kim Y.J., Lim H.J., Kim Y.S., Park H.Y., Choung Y.H. Red ginseng delays age-related hearing and vestibular dysfunction in C57BL/6 mice. Exp. Gerontol. 2014;57:224–232. doi: 10.1016/j.exger.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 98.Nevado J., Sanz R., Sanchez-Rodriguez C., Garcia-Berrocal J.R., Martin-Sanz E., Gonzalez-Garcia J.A., et al. Ginkgo biloba extract (EGb761) protects against aging-related caspase-mediated apoptosis in rat cochlea. Acta Otolaryngol. 2010;130(10):1101–1112. doi: 10.3109/00016481003713657. [DOI] [PubMed] [Google Scholar]
- 99.Sanz-Fernandez R., Sanchez-Rodriguez C., Granizo J.J., Durio-Calero E., Martin-Sanz E. Accuracy of auditory steady state and auditory brainstem responses to detect the preventive effect of polyphenols on age-related hearing loss in Sprague-Dawley rats. Eur. Arch. Oto-Rhino-Laryngol. 2016;273(2):341–347. doi: 10.1007/s00405-015-3551-7. [DOI] [PubMed] [Google Scholar]
- 100.Sanchez-Rodriguez C., Martin-Sanz E., Cuadrado E., Granizo J.J., Sanz-Fernandez R. Protective effect of polyphenols on presbycusis via oxidative/nitrosative stress suppression in rats. Exp. Gerontol. 2016;83:31–36. doi: 10.1016/j.exger.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 101.Sanchez-Rodriguez C., Cuadrado E., Riestra-Ayora J., Sanz-Fernandez R. Polyphenols protect against age-associated apoptosis in female rat cochleae. Biogerontology. 2018;19(2):159–169. doi: 10.1007/s10522-018-9747-7. [DOI] [PubMed] [Google Scholar]
- 102.Xiong H., Pang J., Yang H., Dai M., Liu Y., Ou Y., et al. Activation of miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis: implications for age-related hearing loss. Neurobiol. Aging. 2015;36(4):1692–1701. doi: 10.1016/j.neurobiolaging.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 103.Du Z.D., Yu S., Qi Y., Qu T.F., He L., Wei W., et al. NADPH oxidase inhibitor apocynin decreases mitochondrial dysfunction and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging model in rats. Neurochem. Int. 2019;124:31–40. doi: 10.1016/j.neuint.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 104.HyeonHeeYu, Hur J.-M., Seo S.-J., Moon H.-D., Kim H.-J., Park R.-K., et al. Protective effect of ursolic acid from Cornus officinalis on the hydrogen peroxide-induced damage of HEI-OC1 auditory cells. Am. J. Chin. Med. 2009;37:735–746. doi: 10.1142/S0192415X0900720X. [DOI] [PubMed] [Google Scholar]
- 105.Mo W., Pei J., Wang J., Chu W., Liu M., Li H., et al. [Comprehensive therapy for senile sensorineural hearing loss: a randomized controlled trial] Zhongguo Zhen Jiu. 2018;38(6):604–608. doi: 10.13703/j.0255-2930.2018.06.009.Chinese. [DOI] [PubMed] [Google Scholar]
- 106.Wang P., Lv H., Zhang A., Sun H., Yan G., Han Y., et al. Improved ultra-performance liquid chromatography with electrospray ionization quadrupole-time-of-flight high-definition mass spectrometry method for the rapid analysis of the chemical constituents of a typical medical formula: liuwei Dihuang Wan. J. Sep. Sci. 2013;36(21–22):3511–3516. doi: 10.1002/jssc.201300742. [DOI] [PubMed] [Google Scholar]
- 107.Guo L., Cao W., Niu Y., He S., Chai R., Yang J. Autophagy regulates the survival of hair cells and spiral ganglion neurons in cases of noise, ototoxic drug, and age-induced sensorineural hearing loss. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.760422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh S.K., Srivastav S., Castellani R.J., Plascencia-Villa G., Perry G. Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics. 2019;16(3):666–674. doi: 10.1007/s13311-019-00767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J., Hu J., Chen X., Lei X., Feng H., Wan F., et al. Traditional Chinese medicine monomers: novel strategy for endogenous neural stem cells activation after stroke. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.628115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiong Q., Fan W., Tezuka Y., Adnyana I.K., Stampoulis P., Hattori M., et al. Hepatoprotective effective of apocynum venetum and its active constituents. Planta Med. 2000;66(2):127–133. doi: 10.1055/s-2000-11135. [DOI] [PubMed] [Google Scholar]
- 111.Pandey A., Kour K., Bani S., Suri K.A., Satti N.K., Sharma P., et al. Amelioration of adjuvant induced arthritis by apocynin. Phytother Res. 2009;23(10):1462–1468. doi: 10.1002/ptr.2803. [DOI] [PubMed] [Google Scholar]
- 112.Wen J., Chen X., Yang Y., Liu J., Li E., Liu J., et al. Acupuncture medical therapy and its underlying mechanisms: a systematic review. Am. J. Chin. Med. 2021;49(1):1–23. doi: 10.1142/S0192415X21500014. [DOI] [PubMed] [Google Scholar]
- 113.Dong Q., Zhang Y., Wu Q., Hu H., Gao H. Acupuncture for hearing loss: a case report. Acupunct. Med. 2022;40(3):284–286. doi: 10.1177/09645284221076509. [DOI] [PubMed] [Google Scholar]
- 114.Kim M.H., Kwak J.Y., Choi I. Acupuncture as a salvage treatment for sudden sensorineural hearing loss, Acupunct. Med. 2022;40(3):278–280. doi: 10.1177/09645284221076513. [DOI] [PubMed] [Google Scholar]
- 115.Zhang X.C., Xu X.P., Xu W.T., Hou W.Z., Cheng Y.Y., Li C.X., et al. Acupuncture therapy for sudden sensorineural hearing loss: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0125240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jones C.A., Siever J., Knuff K., Van Bergen C., Mick P., Little J., et al. Walk, Talk and Listen: a pilot randomised controlled trial targeting functional fitness and loneliness in older adults with hearing loss. BMJ Open. 2019;9(4) doi: 10.1136/bmjopen-2018-026169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu H., Frank A. Tai chi as a balance improvement exercise for older adults: a systematic review. J. Geriatr. Phys. Ther. 2010;33(3):103–109. PMID: 21155504. [PubMed] [Google Scholar]
- 118.Bao X., Qiu Q.X., Shao Y.J., Quiben M., Liu H. Effect of sitting Ba-Duan-Jin exercises on balance and quality of life among older adults: a preliminary study. Rehabil. Nurs. 2020;45(5):271–278. doi: 10.1097/rnj.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 119.Cetin S.Y., Erel S., Bas Aslan U. The effect of Tai Chi on balance and functional mobility in children with congenital sensorineural hearing loss. Disabil. Rehabil. 2020;42(12):1736–1743. doi: 10.1080/09638288.2018.1535629. [DOI] [PubMed] [Google Scholar]
- 120.Tao J., Liu J., Egorova N., Chen X., Sun S., Xue X., et al. Increased hippocampus-medial prefrontal cortex resting-state functional connectivity and memory function after Tai chi chuan practice in elder adults. Front. Aging Neurosci. 2016;8:25. doi: 10.3389/fnagi.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tao J., Chen X., Egorova N., Liu J., Xue X., Wang Q., et al. Tai Chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Sci. Rep. 2017;7 doi: 10.1038/srep41581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tao J., Chen X., Liu J., Egorova N., Xue X., Liu W., et al. Tai chi chuan and baduanjin mind-body training changes resting-state low-frequency fluctuations in the frontal lobe of older adults: a resting-state fMRI study. Front. Hum. Neurosci. 2017;11:514. doi: 10.3389/fnhum.2017.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bruce H., Lai L., Bherer L., Lussier M., St-Onge N., Li K.Z.H. The effect of simultaneously and sequentially delivered cognitive and aerobic training on mobility among older adults with hearing loss. Gait Posture. 2019;67:262–268. doi: 10.1016/j.gaitpost.2018.10.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the review described in the article.